- 1School of Metallurgical Engineering, Xi’an University of Architecture and Technology, Xi’an, China
- 2Department of Pediatric Orthopaedics, Xin Hua Hospital Affiliated to Shanghai Jiao Tong University School of Medicine, Shanghai, China
- 3State Key Laboratory of Metal Matrix Composites, School of Material Science and Engineering, Shanghai Jiao Tong University, Shanghai, China
- 4Affiliated Hospital of Youjiang Medical University for Nationalities, Baise, China
- 5Chengsteel Group Co., Ltd., HBIS Group Co., Ltd., Chengde, China
Depending on the requirements of specific applications, implanted materials including metals, ceramics, and polymers have been used in various disciplines of medicine. Titanium and its alloys as implant materials play a critical role in the orthopedic and dental procedures. However, they still require the utilization of surface modification technologies to not only achieve the robust osteointegration but also to increase the antibacterial properties, which can avoid the implant-related infections. This article aims to provide a summary of the latest advances in surface modification techniques, of titanium and its alloys, specifically in biomedical applications. These surface techniques include plasma spray, physical vapor deposition, sol-gel, micro-arc oxidation, etc. Moreover, the microstructure evolution is comprehensively discussed, which is followed by enhanced mechanical properties, osseointegration, antibacterial properties, and clinical outcomes. Future researches should focus on the combination of multiple methods or improving the structure and composition of the composite coating to further enhance the coating performance.
Introduction
With the growing maturity of medical technologies, it is found that implanting biomaterials into the human body is an excellent way to treat some of the orthopedic and dental diseases (Lausmaa et al., 1990; Ohthuki et al., 1999). The commonly used metallic biomaterials are titanium (Ti) and its alloys (Wang et al., 2009; Guo et al., 2013; Jemat et al., 2015; Hafeez et al., 2019), 316L stainless steel (Singh et al., 2018), and cobalt-based alloys (Wang et al., 2014). Apart from these, shape memory alloys like magnesium (Mg) (Kirkland et al., 2010), NiTi (Bansiddhi et al., 2008; Wang et al., 2016, Wang et al., 2018; Liu et al., 2020a, b), and tantalum (Ta) are also potential candidates in biomedical applications (Balla et al., 2010). For the first time Ti was discovered in the 1790s (Chouirfa et al., 2019). Nowadays, due to its high specific strength, strong corrosion resistance, and excellent biocompatibility (Jemat et al., 2015; Niinomi et al., 2016; Shi et al., 2017; Rabadia et al., 2018, 2019; Ran et al., 2018; Hafeez et al., 2020; Wang L. et al., 2020), titanium and its alloys have been widely used in the biomedical field (Wang et al., 2017), among which Ti-6Al-4V alloy applications account for more than 50% (Hu et al., 2012; Ding et al., 2016; Zhang et al., 2017). Although their beneficial properties (Matter and Burch, 1990), titanium and its alloys are considered as inert metals and cannot properly stimulate the proliferation of osteoblasts and bone cells (Zhu et al., 2016; Xiao et al., 2017; Souza et al., 2019). In addition, most of the failures are caused by implant-related infections, thus, lots of researches have been focused on improving the antibacterial ability of titanium implants (Yousefi et al., 2017; Ding et al., 2019; Liu et al., 2019; Wang et al., 2020). The exposed titanium alloy cannot resist the wear triggered by the relative movement between the implant and the bone, and external force and body fluid immersion will cause the disappearance of the passive film on the titanium alloy surface, resulting in a decrease in its corrosion performance (Zhang and Chen, 2019). The above problems can be solved by improving the surface properties of titanium and its alloys. Therefore, various surface modification methods have been used to improve the biological function, wear, and corrosion resistance of implants. In the last decade, coatings have been using in multiple applications to modify the surface of implants and, in some cases, to create new surfaces with exceptional properties that are very different from uncoated materials (Zhong, 1999, 2001; Wang et al., 2015; Wang et al., 2017; Gu et al., 2019). Furthermore, many studies proved that surface modifications techniques can minimize the bacterial adhesion on the implant substrate. They can also inhibit the biofilm formation and provide the effective bacterial removal, thus improving the performance of implanted biomaterials (Asri et al., 2017; Awad et al., 2017; Ahn et al., 2018; Zhang et al., 2020).
This review thematically focuses on surface modifications technologies such as plasma spray, plasma immersion ion implantation (PIII), plasma immersion ion implantation and deposition (PIII&D), physical vapor deposition (PVD), chemical vapor deposition (CVD), sol-gel, and micro-arc oxidation (MAO) methods. These methods are divided into two major parts: physical modification and chemical modification techniques. In the chemical methods, the surface is dipped into chemically active solutions, while in physical methods the surface is exposed to highly energetic charges or other physical species like a flame, plasma, etc. Certain technologies can have the involvement of multiple physical and chemical processes. Thus, it is impossible to strictly separate physical and chemical methods. The classification mainly depends on the main idea behind each technology. Moreover, this article summarizes the osteogenic and antibacterial properties achieved through surface technologies on Ti-based implant materials from these two aspects and provides a comprehensive incite to improve the surface techniques to manufacture the modern implant materials with improved properties. Figure 1 shows all the surface treatment methods along with their pros and cons.
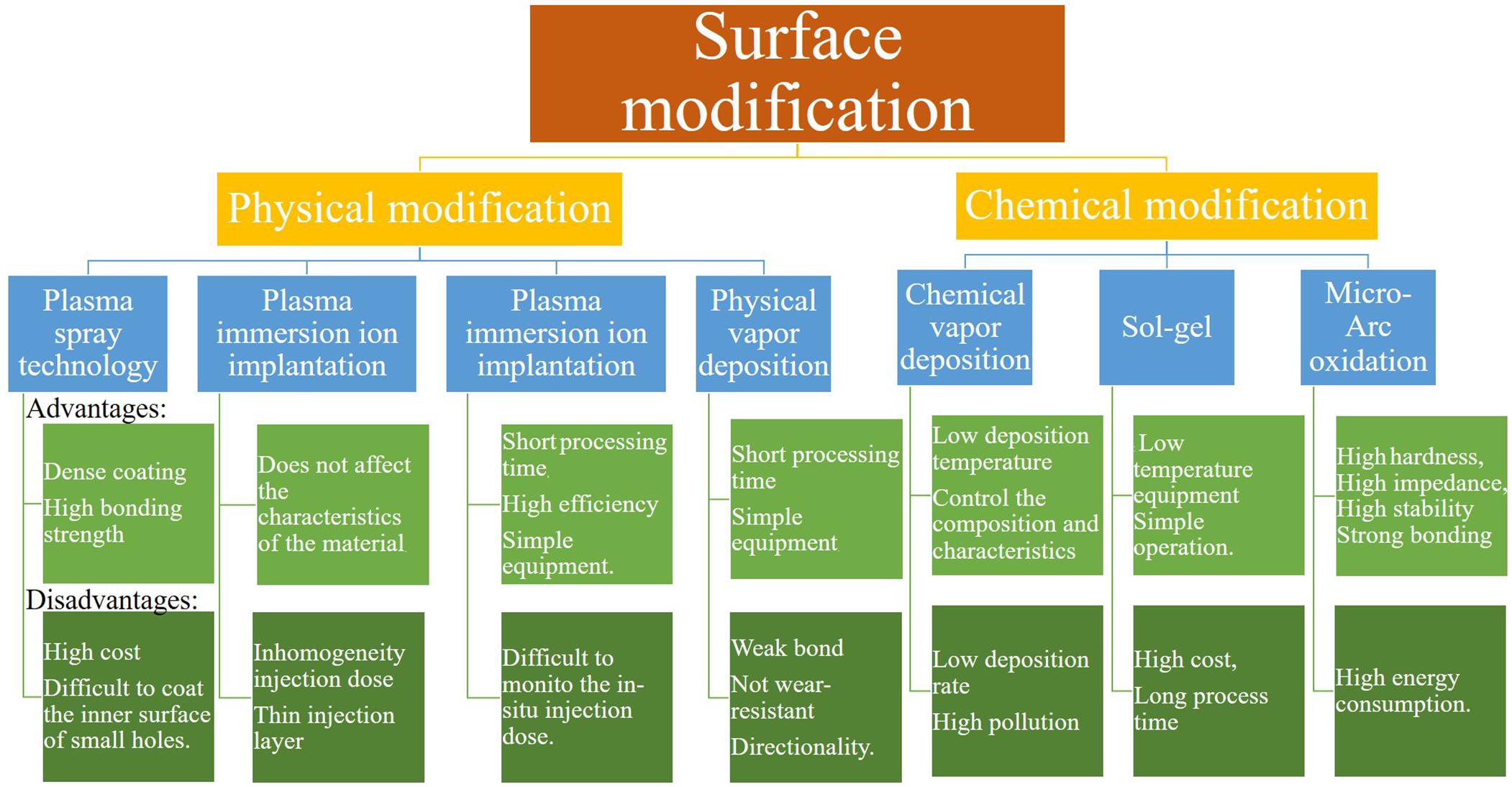
Figure 1. Surface treatment methods with their advantages, disadvantages, and applications (Vahabzadeh et al., 2015; Wang et al., 2015; Asri et al., 2017; Azari et al., 2019; Chouirfa et al., 2019; Kaur and Singh, 2019; Souza et al., 2019; Thangavel et al., 2019; Youn et al., 2019; Xia et al., 2020).
Physical Modification
The main idea of the physical modification method in Ti-based alloys is to treat and change the surface ultrastructure, and these methods include plasma spray technology, PIII, PIII&D, and PVD. The physical modification method is relatively cheap, and the preparation method and mechanism are simple. Correspondingly, the bonding force of the coating is weak, and it is slightly insufficient in the preparation of complex samples. Table 1 gives a comparison of the main results of different physical methods.
Plasma Spray Technology
Plasma spray technology is a thermal spraying technique using plasma arc as the heat source, and it has been widely used to form coatings with excellent physical, chemical, and mechanical properties (Karthikeyan et al., 1997; Shaw et al., 2000), especially in the biomedical field. As shown in Figure 2, many parameters involved in this method, which can potentially affect the microstructure and properties of coatings, among them porosity is the most significant factor which determines the coating quality.
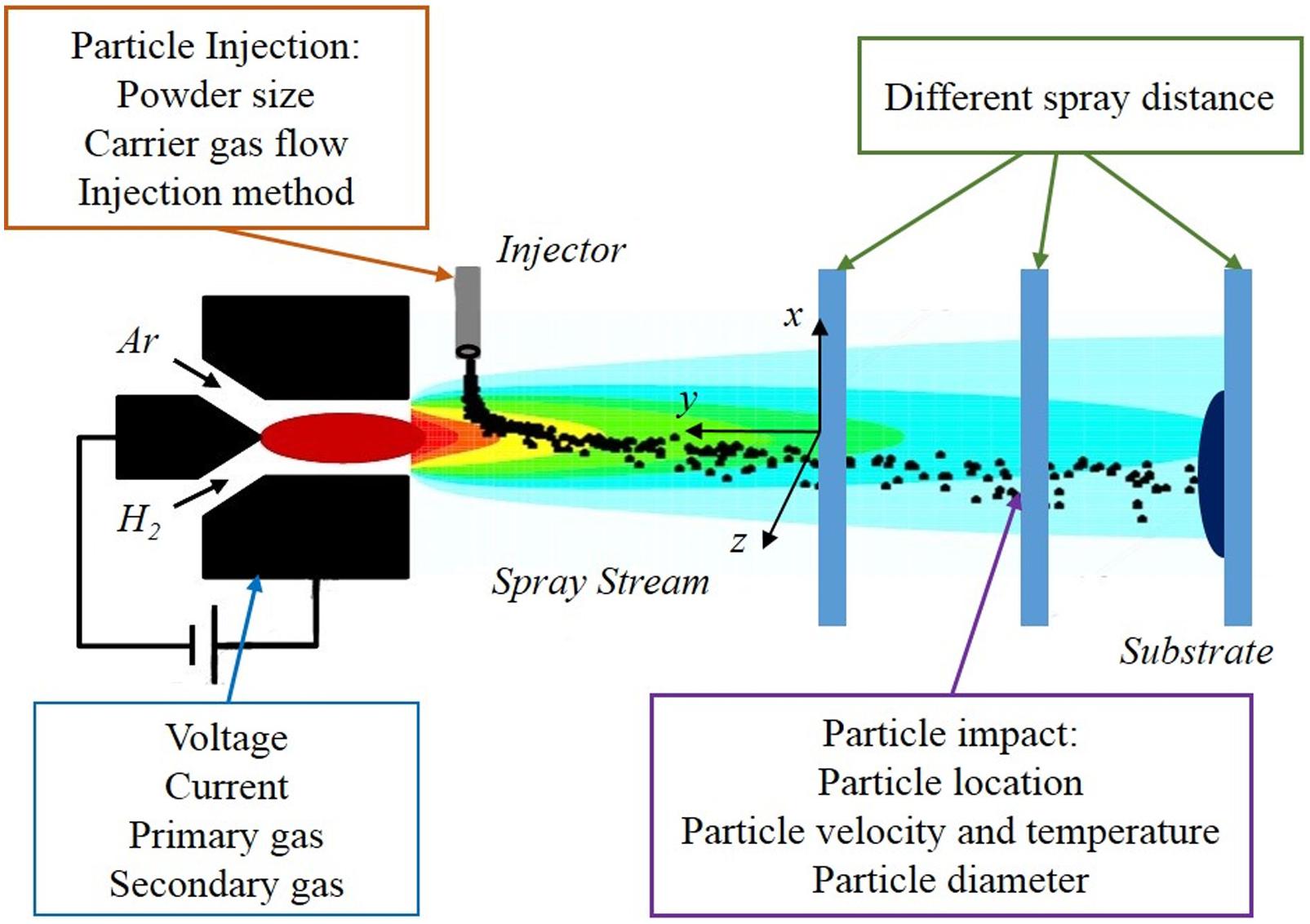
Figure 2. Related parameters and important variables of plasma spraying. Reproduced from Zhu et al. (2020) with permission.
Hydroxyapatite (HA) coating is used to improve osteoconductivity and enhances osseointegration. Kotian et al. (2017) analyzed the production of HA coatings on Ti and Ti-6Al-4V under different plasma atmospheres. They proved that the atmosphere has a substantial influence on the composition, crystallinity, and the formation of microcracks of HA-coated implants. In order to obtain high-quality coatings, researchers need to control the temperature of the plasma gas to reduce microcracks. Besides, the atmosphere with argon and nitrogen gases showed the highest degree of crystallinity. Furthermore, according to Liu Y.-C. et al. (2020), a new vapor-induced pore-forming atmospheric plasma spraying (VIPF-APS) technique has great potential for producing bioactive porous HA coating which enhances osteoblast attachment and differentiation. Apart from the plasma spray technology, other strategies have been considered to improve the overall performance of the coating. Meanwhile, a new double-layer HA/Al2O3-SiO2 coating was put forward by Ebrahimi et al. (2018), compared to monolayer HA, it has improved cell behavior and biocompatibility. Vahabzadeh et al. (2015) and Cao et al. (2019) doped Sr (Mg and Sr) into HA coating. In Figure 3, the formation of steroids is evident in the Sr-HA coating, which indicates that the bone regeneration of the Sr-HA coating is accelerated compared to uncoated Ti and HA coating implants. As for (Mg, Sr)-HA, on the fifth day, the visible cell adhesion prove its good biocompatibility on the surface of the coating, and it also showed high bonding strength. In another study, MgO, Ag2O, and gradient HA were mixed in order to improve the biological and antibacterial properties (Ke et al., 2019). This novel method improves osseointegration and decreases the possibility of failure due to loosening or infection. Besides, Otsuka et al. (2016) clarified that due to the acceleration of dissolution at the interface, the delamination life of the HA coating immersed in the simulated body fluid (SBF) is shortened. Therefore, the delamination behavior of extracorporeal circulation should be considered to extend the service life of HA coatings.
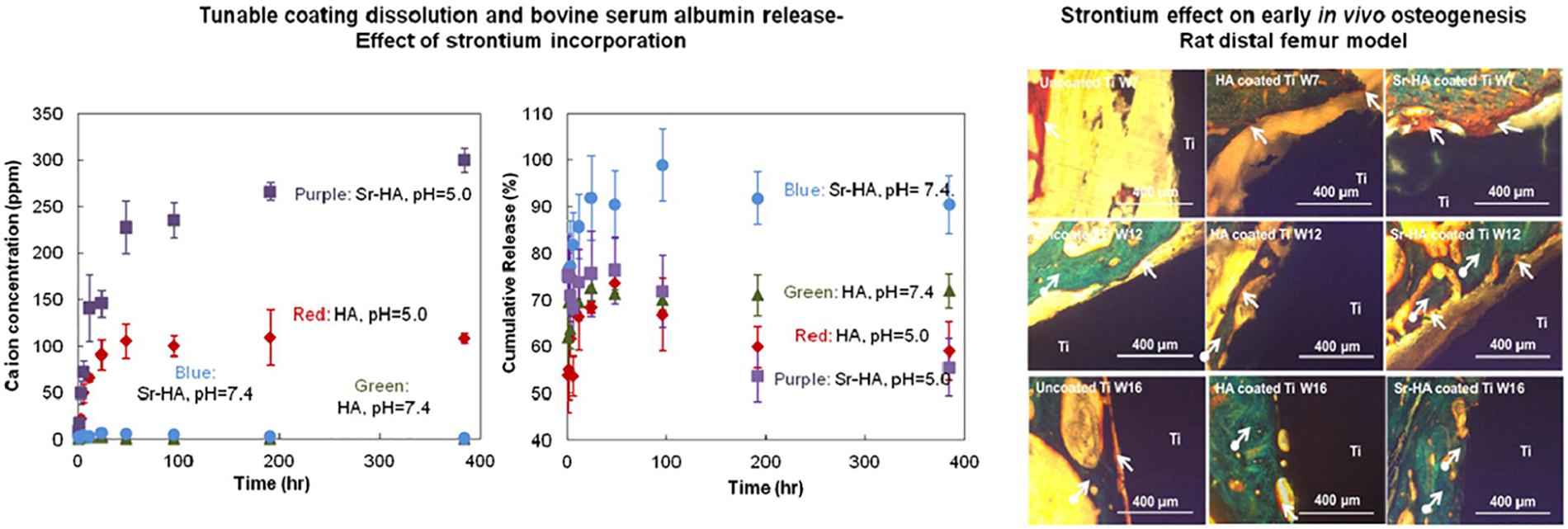
Figure 3. The evaluation of stability and new bone formation of plasma sprayed HA coating. Reproduced from Vahabzadeh et al. (2015) with permission.
Researchers investigated the composite coatings for a decade, trying to improve the tribological behavior of implants, Ganapathy et al. (2015) prepared Al2O3 −40 wt.%8 YSZ on the biomedical grade Ti-6Al-4V alloy used for total joint prosthetic components through plasma spray. Another method, combined with the mutual effect of ceramics and metallic materials, have been investigated by Veerachamy et al. (2018). According to their research, Al2O3 +13 wt.% TiO2/-YSZ BL can be deemed as a suitable coating on Ti-6Al–4V because of its high antibacterial activity and superior cell compatibility. Furthermore, the bioactive glass-ceramic coating named M2 coating (including CaO–MgO–SiO2) on Ti-6Al-4V alloy has shown good performance in vitro. In order to figure out its performance in osteogenesis and osseointegration, Zhang et al. (2019) implanted it into rabbits, it was verified that the M2 coated Ti-6Al-4V was provided with the better biological performance in vivo and could probable replace HA coating to repair load bearing bone implants. Many new coating materials have received extensive attention. For instance, tricalcium magnesium silicate is recommended as a new coating, which has almost the same thermal expansion properties as Ti-6Al-4V, also it has the potential to enhance the corrosion and biological behavior of permanent metallic implants (Maleki-Ghaleh et al., 2015). At the same time, other metal elements with excellent bio-properties like tantalum (Kuo et al., 2019), have been deposited on titanium alloy implants.
Plasma spray technology provides a cost-effective, straightforward, and reliable approach to prepare coatings on titanium alloys. The gas atmosphere and temperature of plasma spraying will affect the thermal stress and crystallinity of the coating, which will affect the osteogenic activity and other properties. On the one hand, as the conventional coating material, HA needs to be upgraded by improving the production process or with doping new elements. On the other hand, novel coatings like metal composites should be considered. Although it was initially found that plasma sprayed TiO2 and ZrO2 coatings have good biological activity and biocompatibility, the related mechanisms still need to be further explored. In addition, the plasma spraying temperature is extremely high, and the coating encounters large thermal stresses. Special attention should be paid to the bonding force between the coating and the substrate. Also, it still requires some improvement in the preparation of coatings on small and special-shaped workpieces.
Plasma Immersion Ion Implantation
Since the PIII technique enables to embed a great variety of elements into the near-surface region of the various substrates, it offers unique advantages for surface modification technologies of biomaterials (Lin et al., 2019). The most valuable feature of PIII is that the concentration and depth distribution of the implanted ions in the substrate can be strictly controlled by adjusting the implantation parameters (Jin et al., 2014). In addition, it has been demonstrated that it can enhance the hardness, corrosion resistance, wear resistance, bioactivity, and antibacterial properties of biomaterials (Chen et al., 2020).
As the most common surface coating in Ti-based alloys, TiO2 has attracted attention in the PIII method. PIII method and optical emission spectrometry (OES) was used to produce TiO2, which has the potential to improve the osseointegration of implants due to its super hydrophilicity (Lin et al., 2019). Shiau et al. (2019) and Chen et al. (2020) investigated the parameters of O-PIII respectively, the former proved that the applied voltage during O-PIII treatment promote blood-clotting and platelet activation, as shown in Figure 4, the latter indicates that the use of higher doses of oxygen ions can improve osteocytic differentiation and osseointegration of dental Ti implants in vivo. In addition to O-PIII, nitrogen plasma immersion ion implantation (N-PIII), carbon plasma immersion ion implantation (C-PIII), etc. were also widely used in fabricating coatings. Nitrogen was incorporated into TiO2 coatings by N-PIII, which could effectively reduce the viability of bacteria in visible light (Zheng et al., 2020). Different from N-PIII, C-PIII was used to preparing coatings with increased mechanical properties and corrosion resistance (Shanaghi and Chu, 2019a). Unfortunately, it could also release the Ni element of NiTi alloys in the SBF solution (Shanaghi and Chu, 2019b).
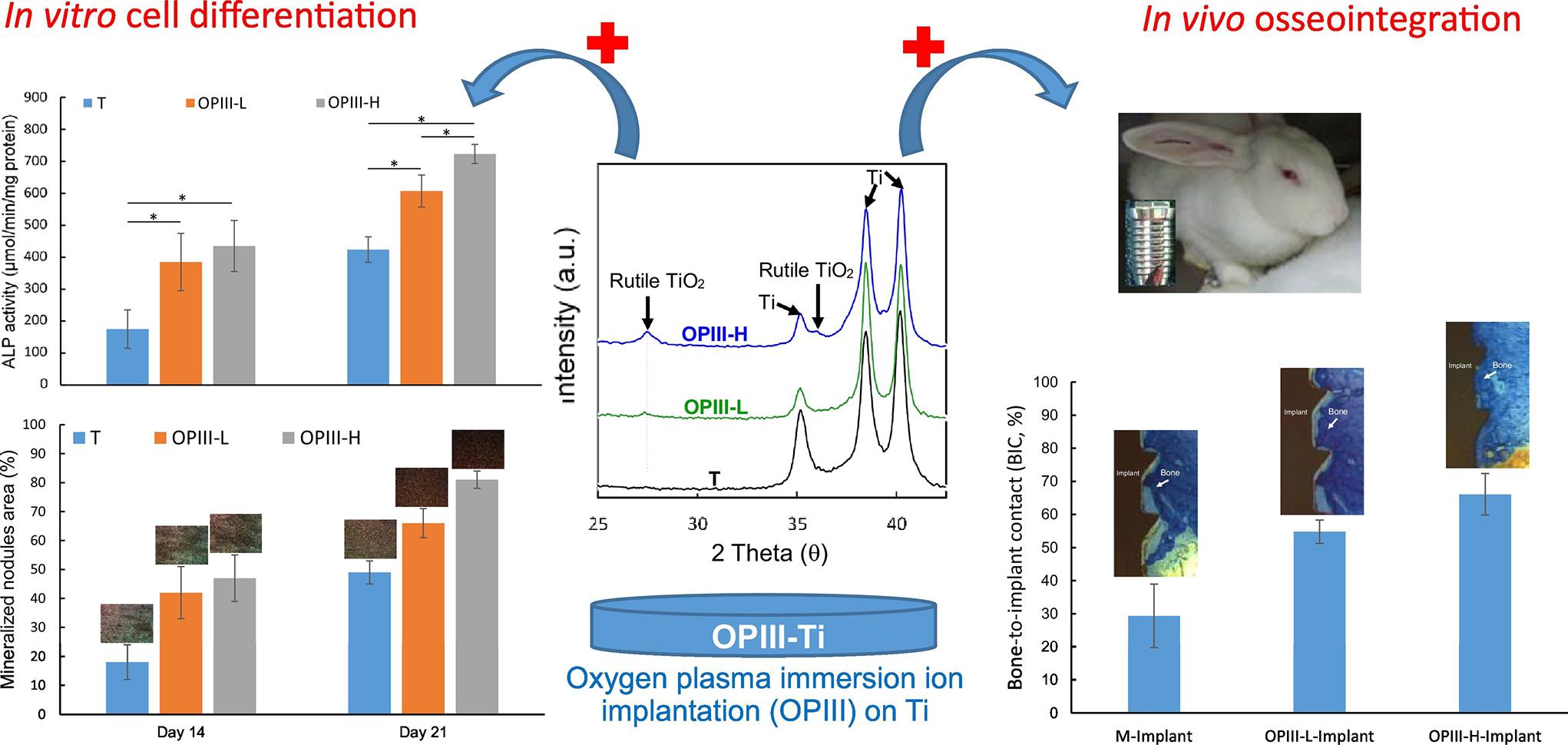
Figure 4. The illustration of the presence of rutile phase TiO2, which enhances the osteocytic differentiation and osseointegration of dental Ti implants in vivo. Reproduced from Chen et al. (2020) with permission.
Besides, the TiN thin film can be formed on Ti-6Al-4V by N-PIII method (Huang et al., 2019), which could positively affect the surface hardness, corrosion resistance, cell responses, and antibacterial adhesion. Furthermore, Xu et al. (2015) added Ag into TiN films as an antibacterial agent, which has good cytocompatibility and retains the required mechanical properties. The Zn-implanted Ti exhibits excellent osteogenic activity and partly antibacterial effect. It is worth noting that the depth profile of zinc in CP-Ti resembles a Gaussian distribution (Jin et al., 2014). Interestingly, Yu et al. (2017) developed dual Zn/Mg ion co-implanted titanium (Zn/Mg-PIII). Zinc is considered as an important and necessary trace element for bone metabolism and production, also Mg plays a critical role in the adhesion of osteoblasts and osteoblasts to orthopedic implants. Thus, due to the beneficial combination of Zn/Mg, the Zn/Mg-PIII implants present good osteoinductivity, pro-angiogenic and antibacterial effects and as shown in Figure 5, these implants can increase the rate of osseointegration and maintain biomechanical fixation.
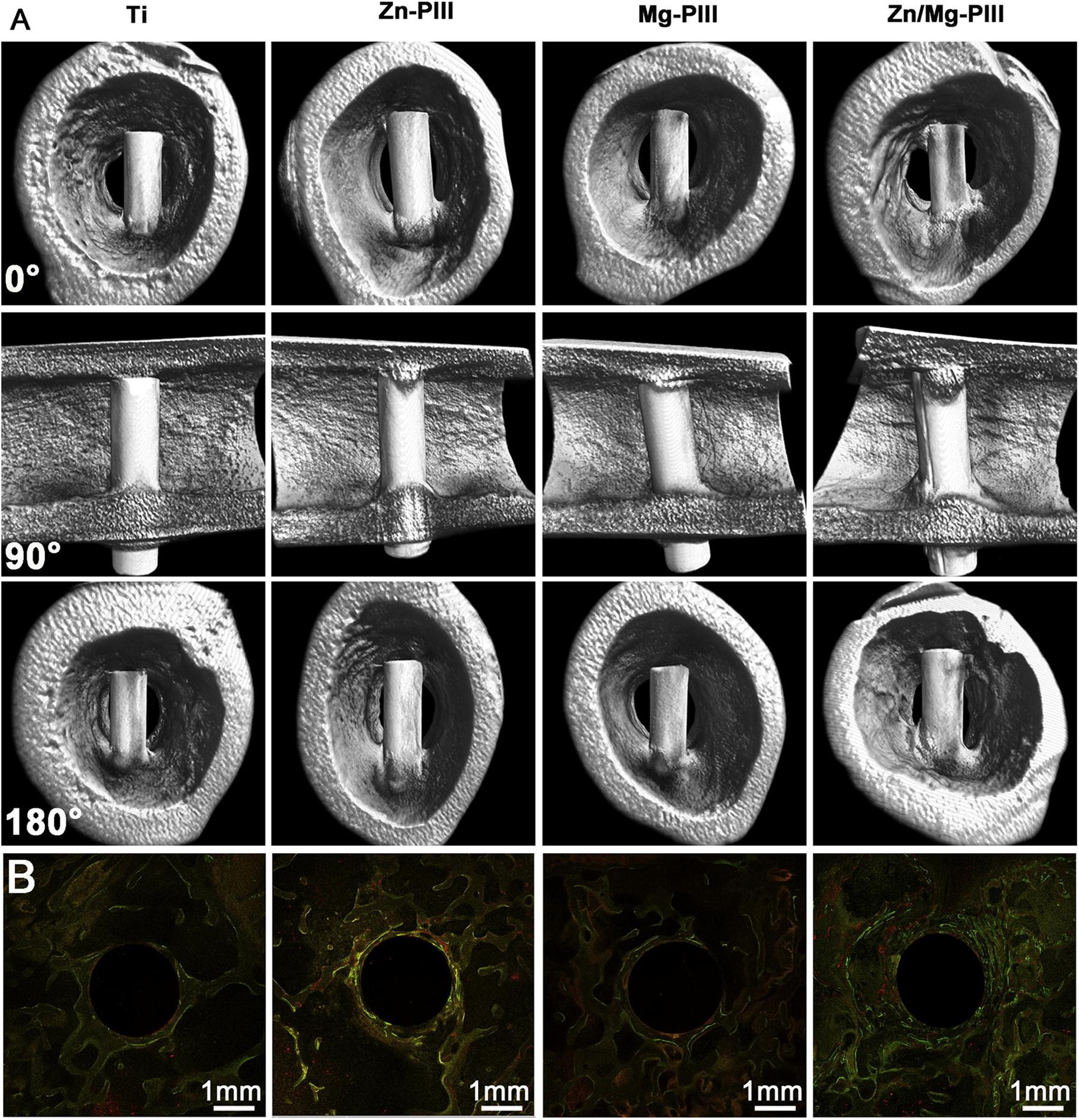
Figure 5. Twelve weeks after implantation, newly formed around Zn/Mg-PIII implants and its sequential fluorescent labeling images. Reproduced from Yu et al. (2017) with permission. (A) Micro-CT 3D images of new bone formation around various implants in rabbit femur. (B) Sequential fluorescent labeling images of newly-formed bone around various implants in rabbit femoral condylar: Alizarin red S (red), tetracycline (yellow), Calcein (green).
In summary, with the ability to control the concentration and depth distribution of implanted ions, PIII shows the potential to implant single or multiple metal ions according to demand. Cell differentiation and osseointegration can be enhanced by injecting designated oxygen, nitrogen, or carbon ions. Additionally, O-PIII, N-PIII, C-PIII, etc. could contribute to essential elements for biocompatibility. Therefore, future research should focus on the procedures to achieve reasonable implantation of multiple metal ions by adjusting the PIII process parameters and to reduce the cytotoxicity caused by metal ion release.
Plasma Immersion Ion Implantation and Deposition
The PIII&D method, invented in 1987 by Conrad et al. (1987), it has become a routine surface modification method. It has the advantage to levitate the retained dose levels that were limited by the sputtering because of ion implantation. Therefore, using PIII&D with relatively low cost, a three-dimensional film with strong adhesion, thick and without stress is possible to be produced (Yang et al., 2007). The schematic process of PIII&D was shown in Figure 6.
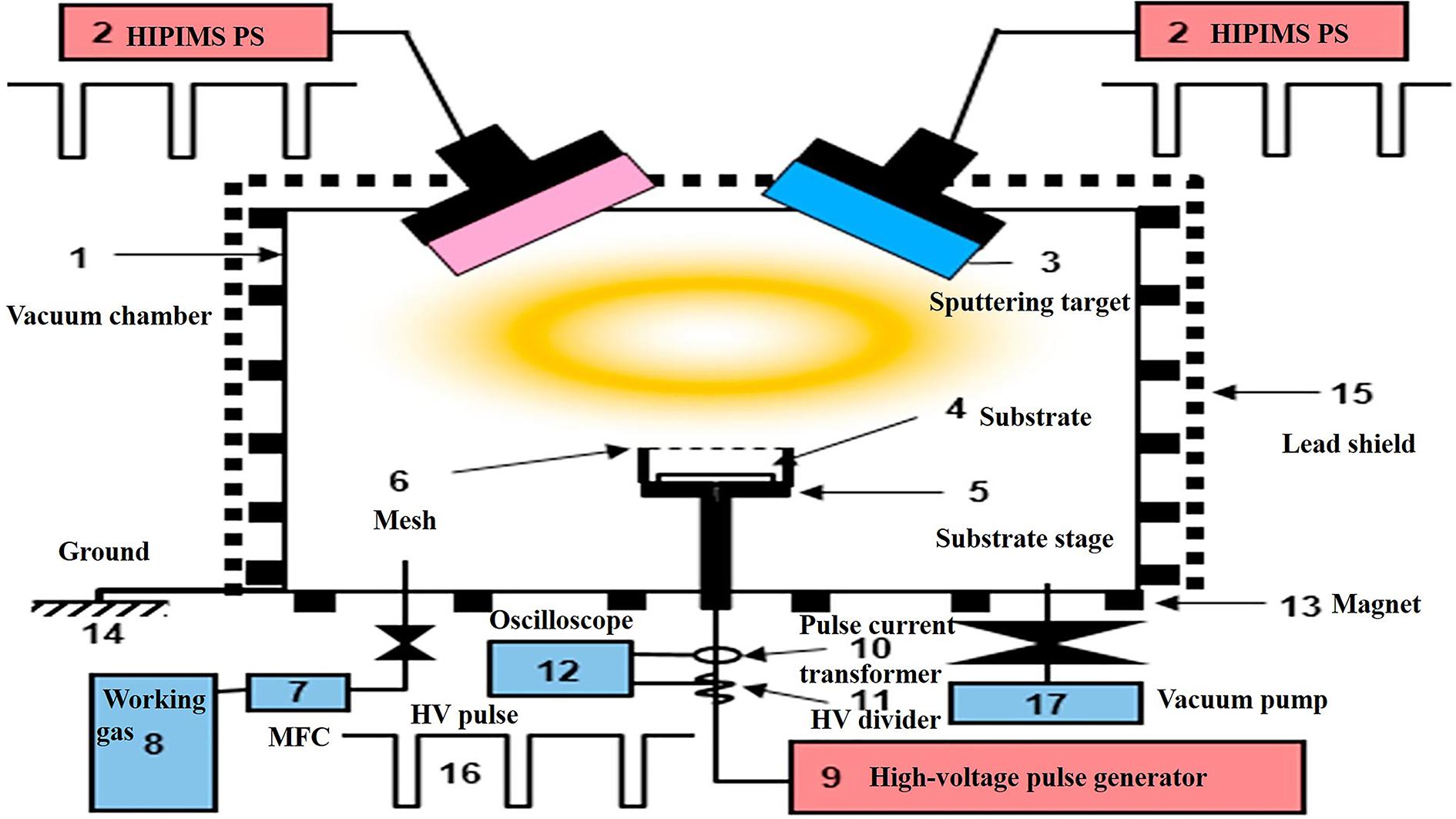
Figure 6. A schematic representation of the PIII&D instrument. Reproduced from Hwang et al. (2019) with permission.
Facing a crucial problem namely thrombosis, blood-contacting biomaterials need to form an interface between the material and blood. Yang et al. (2007) modified the surface characteristics of biomaterial with the functional inorganic films of Ti–O, a-C: N:H, and Si–N synthesized using PIII-D, which can prevent the platelet adhesion/activation. Later in 2013, Ca film deposition has been completed on Ti for the applications of osseointegration in artificial components (Ueda et al., 2013), which is resulted in the formation of a well adherent Ca film. PIII&D was also used to improve the cell response to titanium, Mg–Ag PIII&D treated Ti not only can inhibit the adhesion and proliferation of Escherichia coli bacteria but also promote the initial adhesion and alkaline phosphatase (ALP) expression of MG63 cells (Cao et al., 2014). Concurrently, an excellent compromise between the biocompatibility and cytotoxicity of incorporated metals (like Cu, Mn, etc.) is still required. Copper, a trace element that also exists in the human tissues, has a well-known antimicrobial activity. Hempel et al. (2014) testified that Cu doped and coated Ti can prevent and treat implant-associated infections. It’s worth noting that the surface of overdosed Cu-bearing Ti exhibits negative biocompatibilities (Yu et al., 2016), except for the Cu coating. Yu et al. (2017) investigated a stable Mn ion release on Ti, showing significantly enhanced osteogenesis-related gene expressions and providing a better understanding of relationships between the doped element and biological properties which are caused by additive induction. Aim to solve the bio-inertness of Ti, Ta-implanted entangled porous titanium (EPT) was constructed by the PIII&D method (Wang et al., 2016). As shown in Figure 7, compared with Ca-implanted EPTs, Ta-implanted EPTs shows more stable and continuous effects in the long-term utilization. Another study has deposited zirconium oxide nanostructured coating on the Ti-6Al-4V surface to improve the tribological properties (Saleem et al., 2017). Apart from these coatings, the penetration of nitrogen ions can also be used to support the stability of phospholipid artificial membranes (SLBs) with enhanced biocompatibility (Cisternas et al., 2020). Targeting improvement on the corrosion resistance and prolong the lifespan of Ti, carbon film deposition was accomplished by using a PIII&D system. Santos et al. (2019) verified the desirable properties of carbon films as coating, it can protect the titanium alloy tubes and also can provide new ideas in biology.
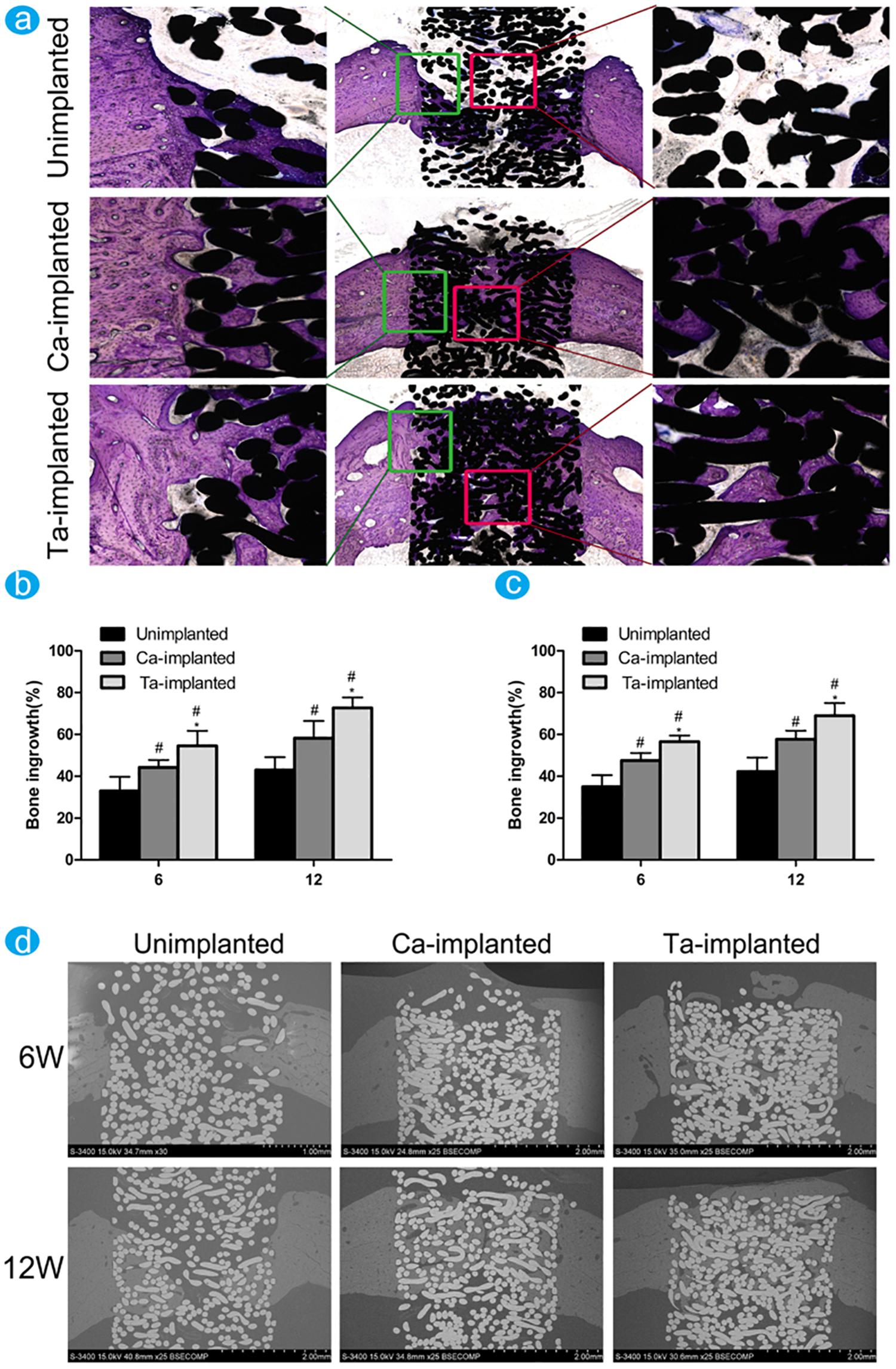
Figure 7. The difference of new bone ingrowth was evaluated through histological observation and histomorphological measurement. Reproduced from Wang et al. (2016) with permission. (a) Undecalcified sections of samples were stained with toluidine blue at 12 weeks. The percentage of new bone ingrowth and pores in different EPT implants measured from toluidine blue staining (b) and back scattered SEM images (c) at 6 and 12 weeks. (d) The back scattered SEM images of new bone around and inside pores of EPT implants at 6 and 12 weeks.
In summary, PIII&D technologies are widely used to form metal coatings on titanium and its alloys. Injecting metal ions into the surface of the Ti substrate through the PIII&D technology, since the metal phase tends to act as an anode to release metal ions, the antibacterial properties of the material can be improved. The PIII&D method generally deposits a single metal element on the titanium substrate, but the balance between toxicity and biocompatibility must be considered. PIII&D technology overcomes the apparent linearity problem of other physical deposition methods, and it is suitable for surface modification of complex-shaped workpieces but its biological safety should also be scrutinized. In the future, the deposition of multiple metal elements or carbon nanomaterials must be investigated to further enhance the biocompatibility of the coatings.
Physical Vapor Deposition
Physical vapor deposition implies a physical coating strategy, involving the evaporation of solid metal under the vacuum environment and depositing it on a conductive substrate (Hauschild et al., 2015). Generally, vacuum evaporation, ion plating, and sputter coating, etc. are among the main methods of PVD. Among them, magnetron sputtering technology has been extensively studied and it results in the formation of high-quality films over a large area and at a relatively low substrate temperature (Nemati et al., 2018; Hamdi et al., 2019).
In the biomedical field, TiN coating concurrent with its favorable biocompatibility can be used as a desirable blood-contacting material. Prachar et al. (2015) compared the properties of TiN with ZrN on pureTi, Ti-6Al-4V, and Ti35Nb6Ta titanium alloys. It was confirmed that TiN has higher cell colonization than ZrN. Furthermore, their color solves the issue of aesthetics in oral implantology because the color of these coatings prevents Ti visibility through the gingiva. Hussein et al. (2020) deposited TiN on Ti20Nb13Zr via cathodic arc PVD. The coated alloys show better corrosion protection behavior in both SBF and the artificial saliva medium. Wu et al. (2019) used the high-power impulse magnetron sputtering (HiPIMS) method which has high peak current and maximum power to deposit TiN on TiAl6V4. The 110 A deposited coating exhibits the highest cell viability. However, the biocompatibility of surface-modified Ti alloys mainly depends on the nitrogen content of the film, therefore in the work of Nemati et al. (2018), TixNy are applied to Ti-6Al-4V substrates as thin films. They controlled the nitrogen partial pressure and prepared samples under a mixed atmosphere of Ar and N2. Greater mechanical properties, corrosion resistance, and biocompatibility occurred with the upgrade of the N/Ti ratio. In the work of Bahi et al. (2020), two types of coatings were studied: TiN as the top layer, while the upper layer of the others was TiO2 with two different oxygen content. The TiN presents the best tribological performance in the multilayered film condition when its surface slide against the bovine cortical bone. Some researchers (Cui et al., 2019) found that compared to TiN and ZrN coatings, partial replacement of Ti atoms with Zr provides excellent wear resistance and fracture toughness. The TiZrN graded coating which was prepared by Cui et al. (2019) is suitable for artificial joint applications that can endure large loads and resist serious wear conditions. Besides, Hauschild et al. (2015) put the Ag-coated cementless stem into the canine model and showed osseous integration in vivo, where toxic side effects did not appear. Afterward, Ag-doped NiTi (NiTi/Ag) coatings were prepared on pure Ti substrates by Thangavel et al. (2019). The NiTi/Ag coating with 3 at. % Ag showed the highest cell viability of human dermal fibroblast neonatal cells and showed a well-grown actin filament network. YSZ was deposited onto the titanium substrate in the study of Kaliaraj et al. (2016) unfortunately this coating couldn’t inhibit bacterial growth but it could enhance the blood protein adhesion. Tantalum pentoxide nanotubes (Ta2O5 NTs) were prepared on biomedical grade Ti-6Al-4V alloy by Sarraf et al. (2017), results of the SBF tests exhibited that on the first day of immersion, a bone-like apatite layer has already formed on the coating of the nanotubular array, indicating the importance of nanotubular configuration for in vitro biological activity.
Nowadays, multi-layered coatings with prominent osseointegration properties and mechanical strength have become focused research areas. In this respect, HA bioceramics have good biocompatibility but the mechanical strength is weak. Hence, Hamdi et al. (2019) prepared triple-layered HA/Al2O3/TiO2 coating on Ti-6Al-4V alloys. In this work, HA plays a critical role in biocompatibility, while others improve the corrosion behavior of the substrate, which prevents the entry of active ions from body fluids onto the surface. Chen et al. (2019) deposit a novel bio-functional bilayer coating consist of calcium phosphate and magnesium (CaP-Mg) on Ti. The CaP coating could inhibit the releasement of Mg, while the alkaline environment caused by Mg degradation has the potential to reduce the bacterial viability. Furthermore, BCP as a kind of CaP is the mixture of β-TCP, Behera et al. (2020) proved that BCP-TiO2 film can be beneficial to improve the biological performance of implants. Nowadays, the development of amorphous carbon (a-C) films has received a lot of attention, Liu et al. (2020) successfully deposited Zr/a-C gradient multilayer films (GMFs), it comprised of three distinct layers. Zr/a-C GMFs modified Ti shows upgraded wettability, enhancing the proliferation and adhesion of osteoblast cells.
To sum up, PVD is used as a mature method to form a nearly perfect adhering layer of materials, which does not disintegrate nor affects the surface topography and shows good tribological properties. Due to the mismatch of the coefficient of thermal expansion between the coating and the substrate, their bonding force is weak, which limits the applications of this type of coating. At present, the transition layer or gradient coating deposition methods are generally used in order to reduce the mismatch of the crystal lattice and thermal stress between the coating and the substrate, thereby enhancing its binding force. TiN coatings with different element doped compositions seem to be the further research direction at present. It is necessary to consider the cell cytotoxicity, adhesion, activity, and antibacterial properties of the newly designed coating composition. In addition, multi-layered coatings could provide proper performance, while researchers should rationally design multi-layer structures to maximize their respective advantages and avoid the possible adverse effects.
Chemical Modification
The chemical modification changes the chemical properties of the carrier surface to produce specific interactions between cell surface molecules, which not only affect the cell surface properties but also cause closely related changes in the internal structure and function of cells. Chemical modifiers are relatively complex in preparation mechanisms and they are expensive. Current research focuses on composition control, multilayer structure design, multi-scale coatings, or coatings with novel surface morphologies. Table 2 gives a comparison of the main findings of different chemical methods.
Chemical Vapor Deposition
Chemical vapor deposition represents a coating method to form a thin film layer on the substrate surface by chemical reaction of one or several vapor compounds or elements that contain the final film elements (Marsh et al., 2010). It has been used in the inorganic synthetic chemistry to prepare inorganic materials like carbon nanotubes, graphene, TiO2, etc. (Somani et al., 2006), the final product can be carefully controlled, both quantitatively and qualitatively. The facts have shown that the technology is very successful in industrial applications. However, their applications on titanium alloy substrate for biomedical surface modification is still limited.
Chemical vapor deposition methods are mainly used for complex workpieces and inner hole coating. Coatings prepared by the CVD method usually show high osteogenic activity, which has a certain potential for orthopedic applications. Giavaresi et al. (2003) used a metal-organic chemical vapor deposition (MOCVD) method to prepare the titanium oxide layer on pure titanium. Ti/MOCVD exhibited higher ALP activity than the control group, which means it has a higher potential for bone implantation. Subsequently, Du et al. (2016) successfully deposited Si-doped TiO2 nanowires on the TiSi2 layer by atmospheric pressure chemical vapor deposition (APCVD). It is not only showing higher hydrophilic activities but also has great importance in the field of doping. Related to the previous works, Xu et al. (2016) grafted a thin graphitic C3N4 (g-C3N4) layer on aligned TiO2 nanotube arrays (TiNT) by CVD. The binary nanocomposite coating shows excellent bactericidal efficiency. Glycidyl methacrylate (GMA) is a chemically versatile reagent through a ring-opening reaction (Mao and Gleason, 2004; Kang et al., 2014). Hence, in the research of Park et al. (2015), dot-patterned GMA-coated titanium implants notably displayed higher ALP activities, while displayed increased protein adsorption and higher calcium deposition. Furthermore, based on the previous study, Youn et al. (2019) added recombinant human bone morphogenic protein-2 (rhBMP2) as osteoinductive agents on GMA-coated titanium. From in vitro analysis, they found its good osteogenic activity without any cytotoxicity. Scarce information exists on the effect of amino group plasma-enhanced CVD on nerve regeneration. Hence, Zhao et al. (2018) introduced the amino group onto the titanium disk. Although, it exhibited the best cell attachment performance, it inhibited the expression of the key growth factors like glial cell-derived neurotrophic factor (GDNF) and neurotrophin nerve growth factor (NGF) in vitro, at least within a week. Tantalum coating on porous Ti-6Al-4V scaffold was investigated by Li et al. (2013), they found the better bone ingrowth within the coated scaffolds, indicating the potential for orthopedics. Interestingly, Ji et al. (2016) compared the adhesion of Streptococcus mutans on polished titanium (control group), magnetron sputtering titanium, and plasma nitriding modified titanium samples. There is not any clear difference between the treated samples and the control group. Gu et al. (2018) indicated the influence of heat treatment on the bonding strength and osteoinductive activity of monolayer graphene sheets with the antibacterial and osteoinductive properties.
In summary, the utilization of the CVD methods is not as common as the physical methods mentioned earlier. It may be because of its high reaction temperature that leads to a low deposition rate, also in this method the gas source and exhaust gas have certain toxicity, which may be harmful to the subsequent implantation process. Despite this, the coatings made by CVD usually have good quality, and their purity and density can be controlled. It has been used in industries like electronics, automobiles, aviation, and aerospace. However, vapor deposition equipment is more expensive, and some processes have higher film forming temperatures, which may adversely affect the structure of the substrate. In addition, some process line-of-sight film forming methods are more difficult to form on small shaped parts and need to be improved. In the future, the preparation of copolymer and inorganic coatings by CVD should be studied in depth to form a bacteriostatic surface.
Sol-Gel
The Sol-gel technique is widely implemented to produce multifarious oxide films. This kind of method has the following advantages: simple fabrication environment, reliability of the consuming equipment, high uniformity of films, and utilization of different sizes of the substrate (Hench and West, 1990). The main factor that affects the sol-gel method is pH, chemical equilibrium, substrate-precursor interface, time, etc. (Wang and Bierwagen, 2009). Figure 8 is the schematic representation of sol-gel.
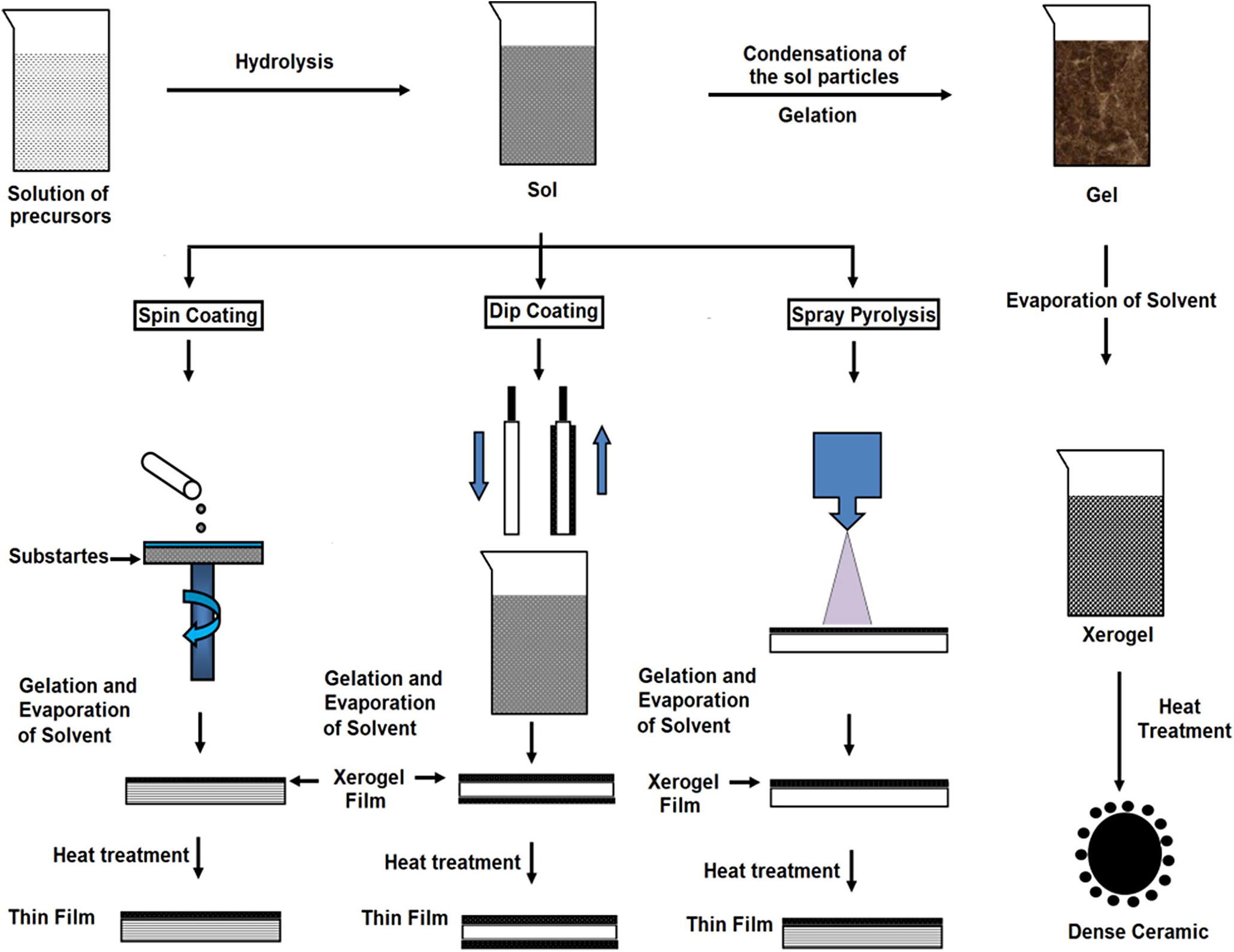
Figure 8. Schematic representation of sol-gel. Reproduced from Kaliyannan et al. (2020) with permission.
Ti, the dominant material for orthopedic application now, maybe impairing physical integrity like change its hardness and flexural modulus after sol-gel treatment. In order to solve this problem, Greer et al. (2016) evaluated coatings properties at different annealing temperatures and concluded that although the ductility decreased, 500°C was the optimal annealing temperature. TiO2 coatings have good physical properties as follows: high surface hardness, good wear resistance, low friction coefficient, and excellent corrosion resistance. Çomaklı et al. (2018) compared TiO2 films produced by sol-gel and successive ionic layer adsorption and reaction (SILAR) methods, the former exhibited better wear and corrosion resistance than the latter. Titania containing silver was deposited on TiSi alloys and commercially pure titanium (CP-Ti) by Horkavcova et al. (2017) and Yetim (2017), respectively. The results showed no cytotoxicity and excellent corrosion resistance, which means these materials are potential candidates for orthopedic application. Moreover, Ziabka et al. (2020) confirmed that this coating can be used in the veterinary treatment of bone fractures. In addition, doping with silver, TiO2 often forms a double-layer coating with HA. As mentioned before, the use of HA promotes bone formation, and in addition, proper chemical homogeneity can be obtained through sol-gel technology (Domínguez-Trujillo et al., 2018). Mohammed Hussein and Talib Mohammed (2019) prepared bilayer TiO2/HA coating, which has good corrosion protection with advanced crystallization and nano-scale homogeneous surface morphology. To enhance the adhesion strength of HA coatings sintered at low temperatures, Robertson et al. (2019) formed titania nanotubes through anodization. Azari et al. (2019) did further research and produced a functionally graded HA-TiO2 on Ti-6Al-4V alloy substrate and improved the adhesion and cohesion of the single-layer coating. Meanwhile, other strategies have been adopted to deal with the shortcomings of HA. Zinc substituted hydroxyapatite/bismuth (Zn-HA/Bi-HA) biphasic coatings were fabricated through sol-gel and dip-coating processes by Bi et al. (2020), which exhibited the most positive effect on osteoblast proliferation.
Moreover, bio-inert ceramics like silicon dioxide and zirconia attracts a wide range of attention because of their stability in the human body. It also exhibits excellent load-bearing capacity and high cell viability. Lee et al. (2017) made zirconia-coated porous-Ti (Z-P-Ti) by the hydrothermal method, and then the sol-gel method was used. Among the samples, Z-P-Ti_55 (Ti samples with 55 wt.% additions of NaCl) exhibited excellent load-bearing capacity and high cell viability. Figure 9 is the mechanism of interaction between Z-P-Ti and the cell surface. Sandblasting Al2O3 combined with ZrO2 sol-gel layer was obtained by Lubas et al. (2018), providing a stable bond. Romero-Gavilan et al. (2018) prepared silica hybrid sol-gel coating (35M35G30T) on Ti, it can adsorb a large number of complement proteins. These proteins are involved in maintaining cell renewal, healing, proliferation and regeneration, and many other processes, which might be related to their intrinsic bioactivity. The addition of strontium (Sr) could affect their interactions with cells and proteins. Thus, Romero-Gavilan et al. (2019) applied a silica-hybrid sol-gel network doped with SrCl2 as a coating on Ti. In in vitro analysis, the coating containing Sr is more abundant in proteins involved in the coagulation process. Besides, the gene expression of ALP and TGFβ was enhanced in the MC3T3-E1 cells.
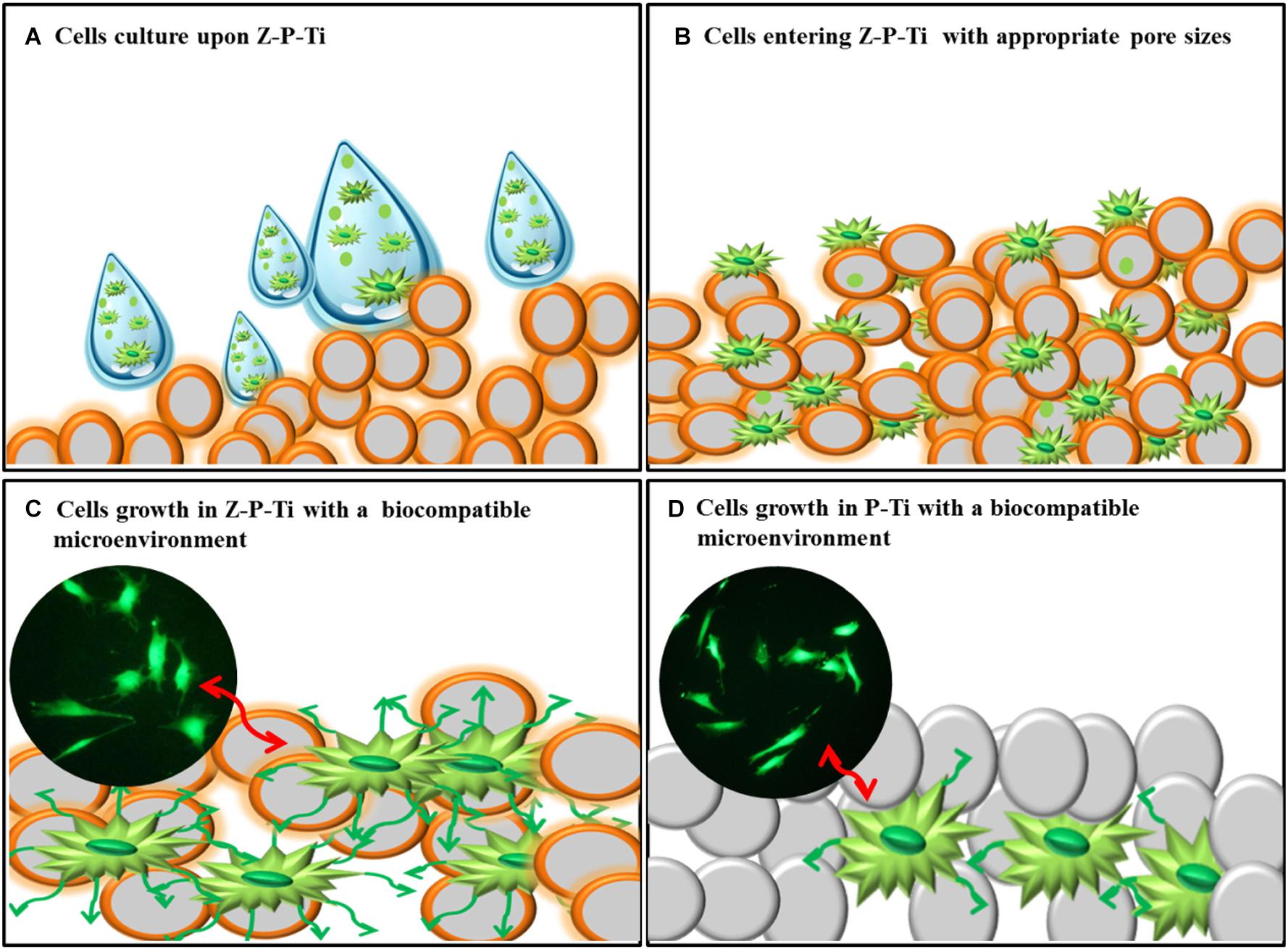
Figure 9. The mechanism of interaction between Z-P-Ti and the cell surface. Reproduced from Lee et al. (2017) with permission. (A) Cells culture upon Z-P-Ti. (B) Cells entering Z-P-Ti with appropriate pore sizes. (C) Cells growth in Z-P-Ti with biocompatible microenvironment. (D) Cells growth in P-Ti with biocompatible microenvironment.
Recently, organic-inorganic composite coatings have also received a lot of attention, which is a suitable candidate for metallic prosthetic equipment. Catauro et al. (2018) synthesized coating from a multicomponent solution. Higher vitality of cells seeded on the coated samples was recorded and the higher HA nucleation was detected on the CP-Ti surface after soaking in SBF, which was also happened in the research of Aghajanian et al. (2019). They coated the porous titanium surface with forsterite/poly-3-hydroxybutyrate (P3HB) nano-biocomposite, this coating inhibited the excessive pH increment of the SBF. Moreover, Palla-Rubio et al. (2019) found that different amounts of chitosan and tetraethyl orthosilicate (TEOS) could modulate silicon release in hybrid silica-chitosan coatings, which plays a critical role in osteoregeneration. Based on the former research, Ballarre et al. (2020) added gentamicin to the chitosangelatin/silica, aimed at extending the bioactive effect. Based on, the sol consists of ZrO2, TiO2, Li+, and polyethylene glycol (PEG), Alcázar et al. (2019) evaluated the biocompatibility of hybrid coatings and found that the modified titanium surfaces have higher cellular growth. El hadad et al. (2020) have developed a new hybrid nanocomposite coating based on organofunctional alkoxysilanes precursors and phosphorus precursors, which prove that the presence of phosphorus at the molecular level can lead to the enhancement of biocompatibility. Simultaneously, Garcia-Casas et al. (2019) also deemed that an intermediate quantity of organophosphate showed the ability to enhance the mineralization of the substrate, which is why it was considered as the most suitable candidate for metallic prosthetic equipment. The drawbacks of pure HA were overcome by adding multi-minerals with the combination of PSSG polymer as hydroxyapatite/sorbitol sebacate glutamate (MHAP/PSSG) composite (Pan et al., 2019). Interestingly, Melatonin (MLT), used primarily to regulate the circadian rhythm and its role in bone regeneration and inflammation has been studied. Cerqueira et al. (2020) used sol-gel coatings as a release agent for MLT on a titanium substrate, they found that it didn’t improve the ALP activity, but has the potential in the activation and development of pathways. Based on the sol-gel coating, Toirac et al. (2020) added two different fungicides (fluconazole and anidulafungin) directly both of them exhibited anti-fungal properties.
At present, more research is conducted on the composition control of the sol-gel method than the process parameter control. These sol-gel coatings greatly enhance the corrosion protection and the migration of the metal matrix, thereby reducing the incidence of prosthesis rejection. Like other thermal deposition methods, it needs to consider the impact of thermal effects, so its current clinical use is subject to certain restrictions. There are extensive studies on the preparation of titanium dioxide, bio-inert ceramics, and organic-inorganic composite layers. For the titanium dioxide coating, the performance can be improved by doping other elements or improving the structural design of the composite coating with HA. For organic-inorganic composite coatings, in the future, it is possible to assess the comprehensive biocompatibility experiments by increasing the types of coating raw materials and adjusting the ratio.
Micro-Arc Oxidation
Micro-arc oxidation, is developed based on anodizing technology. The MAO process mainly relies on the matching adjustment of the electrolyte and the electrical parameters. The process is done under the instantaneous high temperature and high pressure generated by the arc discharge, on the surface of Al, Mg, Ti, and other valve metals and their alloys. A modified ceramic coating produced by MAO is mainly composed of base metal oxides and supplemented with electrolyte components (Han et al., 2003; Li et al., 2004). It has the advantages of simple process, small area, strong processing capacity, high production efficiency, suitable for large industrial production, environmental protection, etc. (Liu et al., 2015; Wang et al., 2015).
According to the principle of plasma-electrolytic oxidation, MAO can create a macro-porous and firmly adherent TiO2 film on the Ti substrate, which got a lot of attention. Some organic substances coated on the layer can make a balance between antibacterial and cell compatibility (He et al., 2018). Additionally, bioactive elements, like B, Ag, Ca, and Sr can be incorporated with TiO2 coating to enhance its bioactivity and biological properties. Huang et al. (2016, 2018) prepared boron-incorporated TiO2 coating (B-TiO2 coating) and Cu-containing TiO2 coatings successively. Specifically, the change of the chemical properties of the surface of B-TiO2 coating and release of B ions from its surface is believed to be the main reason for the improvement of ALP activity and cell differentiation. In the latter research, although the incorporation of Cu did not change the surface morphology and roughness, it still improved the macrophage-mediated osteogenesis and sterilization ability (Figure 10). Zhang et al. (2020) also fabricated Cu-TiO2 through a one-step MAO in a solution containing ethylene diamine tetraacetic acid cupric disodium (Na2CuEDTA) that has a two-layered coating consisting of TiO2 and porous Ca, P-rich outer layer containing nano-sized HA crystals. Subsequently, they investigated the enhanced antibacterial property and osteogenic activity of Zn-TiO2 coating fabricated by one step MAO method (Zhang et al., 2019). This structure improved the proliferation and differentiation of osteoblasts and slightly enhanced the antibacterial capability relative to its relatively higher Cu content. Ag-incorporated TiO2 coatings were prepared by Lv et al. (2019), the resultant film exhibits significantly improved antibacterial capability and bone-forming capacity with the increase of Ag2O nanoparticles in the electrolyte, also it has a slightly upgraded cytotoxicity behavior relative to polished Ti substrate. Li et al. (2020) incorporated Ca and Sr, which are good for bone reconstruction, into the MAO coating. This coating has a highly porous and super-hydrophilic layered structure, which showed excellent promoting effects in the proliferation of human bone marrow-derived mesenchymal stem cells (hBMSC). It is also a good way to combine MAO with other processes to improve the performance of the coating. Therefore, Tang et al. (2020) prepared BaTiO3 on the surface of TiO2 produced by MAO through a hydrothermal reaction. In the early period after bone implantation, the piezoelectric effect of this coating may play a positive role in bone growth and bone integration. High energy shot peening (HESP) pretreatment can be used to enhance the stability and bioactivity of the TiO2 coatings fabricated by MAO, Shen et al. (2020) used this method to increase the effective doping of Ca & P elements on the surfaces. Novel “cortex-like” coatings were investigated by Li et al. (2017, 2018), they have studied a macro/micro/nano triple hierarchical structure and micro/nano dual-scale structured TiO2 coating on Ti. Results proved that the “cortex-like” structure significantly promotes the cell adhesion, diffusion, and differentiation and increases the matrix mineralization. The graphic abstract and schematic diagram of the “cortex-like” TiO2 were shown in Figure 11.
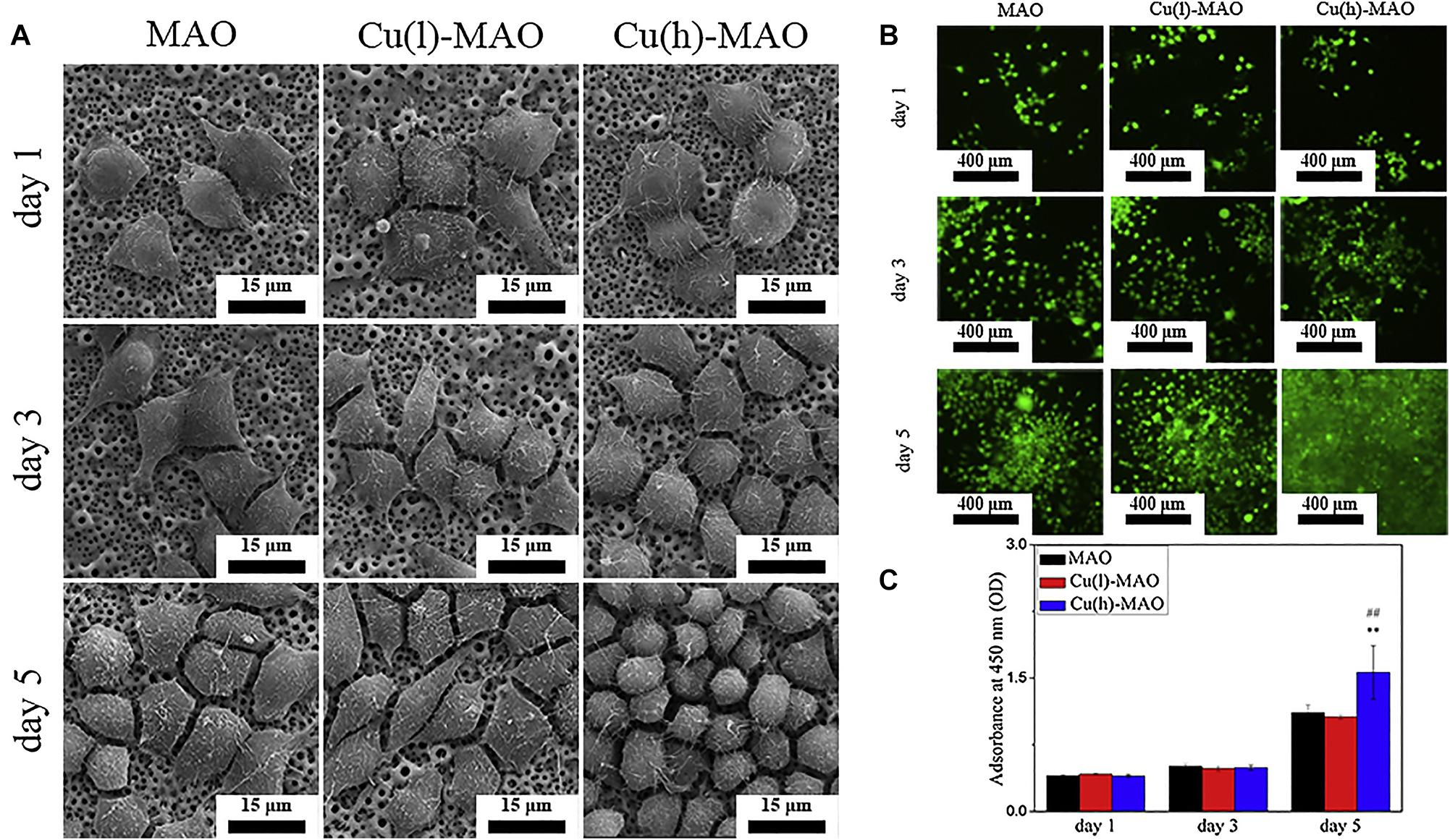
Figure 10. (A) The morphology of macrophages cultured on the surface of various materials for 1, 3, and 5 days. (B) Calcein-AM staining and (C) CCK-8 results show that Cu(h)-MAO surface promotes the proliferation of macrophages. Reproduced from Huang et al. (2018) with permission.
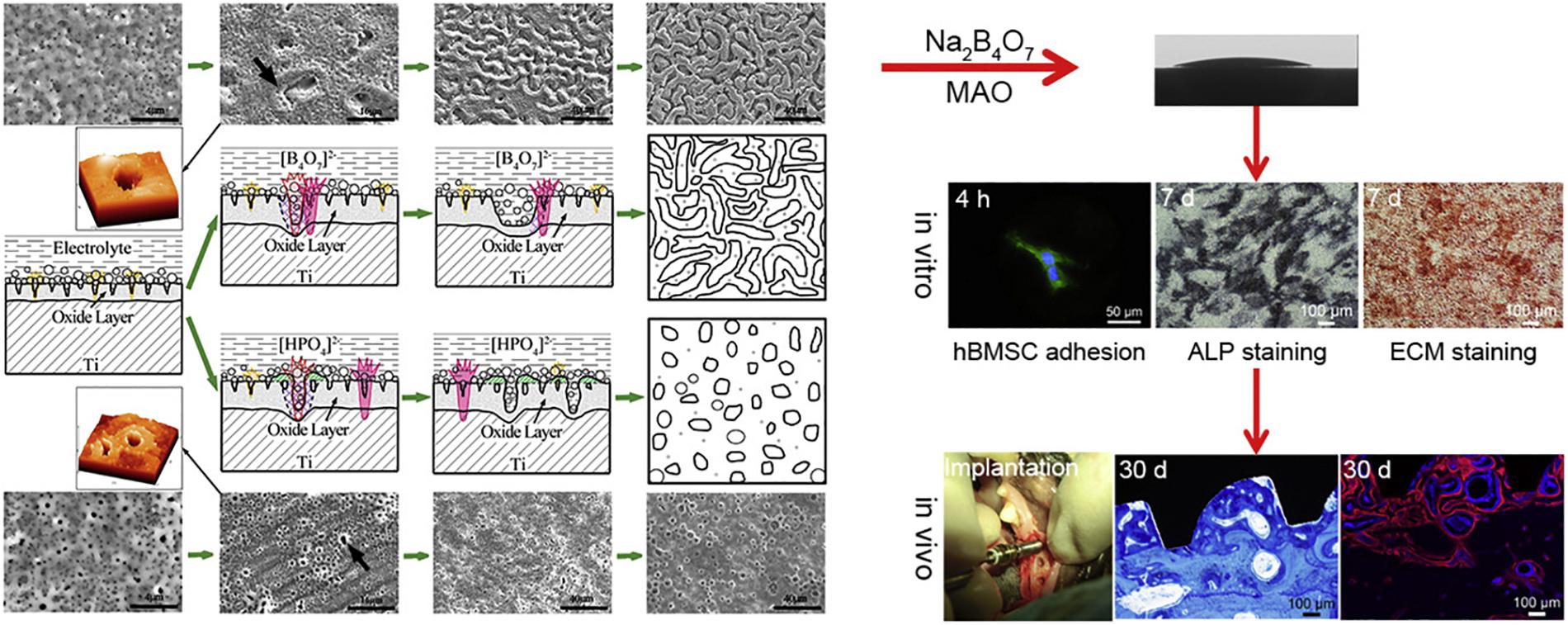
Figure 11. The forming mechanism and biological performance evaluation of the micro-arc oxidized TiO2 coatings with the “cortex-like” structure and the “volcanolike” structure. Reproduced from Li et al. (2018) with permission.
The incorporation of Ca and P species into the TiO2 surfaces can cause the biocompatible compound formation. Thus, plenty of studies focused on obtaining HA-containing coating on titanium and its alloys. Karbowniczek et al. (2017) proved that in the electrolyte containing disodium hydrogen phosphate and calcium acetate hydrate, with the Ca/P ratio of 2/1 the coated Ti6Al7Nb alloy achieved the best combination of bioactivity and mechanical properties. Through a two-step method, it is also possible to produce an oxide layer with micro-pores and bio-active elements by MAO on a surface with macro-porosity (Costa et al., 2020). Similarly, Durdu et al. (2018) combined thermal evaporation-physical vapor deposition (TE-PVD) and MAO. In addition to higher hydrophilicity, the uniform and dense apatite distribution were observed on the Ag-incorporated coatings. Sedelnikova et al. (2017) deposited wollastonite-calcium phosphate (WeCaP) on the pure titanium, revealing the identical dependencies of coating thickness variation, surface roughness, and adhesion strength with process voltage. Interestingly, calcium-rich waste eggshell was used to produce HA coating on Ti-6Al-4V, which is in good agreement with that of bone.
As a hot spot technology for surface modification, MAO was used in lots of research schemes including the preparation of titanium dioxide and HA layers. The enhanced surface hydrophilicity of the porous coating prepared by the MAO method can stimulate the interaction between the implant and the surrounding biological environment, and it also brings excellent antibacterial properties due to the presence of metal ions. Although the anodic oxidation technology is convenient and economical, its bonding strength with the titanium matrix needs to be further improved. In future research, in addition to combining with other preparation methods, the structural design of the coating should be developed, such as the multi-level structure designs, multi-scale coating, or coating with novel surface morphology.
Conclusion
Titanium and its alloys are the most commonly used materials for permanent implants, especially in application with direct contact with the bone, teeth, and bodily fluid. Numerous techniques exist to modify titanium and its alloys surfaces, their different mechanisms, procedure, and targets were listed in this review and with the goal for further clarification of how to choose the corresponding surface modification process and to select its optimum parameters for different demands.
This article reviews the main physical and chemical surface modification techniques for Ti related biomaterials, such as plasma spray, PIII, PIII&D, PVD, CVD, sol-gel, and MAO. Although these methods have been applied in practice and achieved some results, they still have some deficiencies, like the bonding strength still needs to be improved, the influence of thermal effects is eliminated, and how to compromise between toxicity and biological performance, etc. Future studies must be focused on designing the basic new methods or the combination of a variety of surface modification methods to play a synergistic effect and combine their advantages to conquer the deficiencies. On the other hand, the structure and composition of the composite coating can be tailored in order to achieve excellent biomedical performance.
Author Contributions
TX and SA wrote the main part of the manuscript. SL, JL, and YT greatly contributed to the physical methods parts. SA made major contributions particularly in planning the tables. TX, SA, and XS made significant contribution to the revision stage. TX, XS, LL, and BZ prepared and formulated the references. All the authors contributed to the article and approved the submitted version.
Funding
This research was funded by the National Natural Science Foundation of China (Nos. 51671152 and 51874225), the Industrialization Project of Shaanxi Education Department (18JC019), and the funding 2020ZDLGY13-10 and 2020KJRC0048.
Conflict of Interest
LL and BZ were employed by the company Chengsteel Group Co., Ltd., HBIS Group Co., Ltd.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We sincerely thank Ning Ma, Qingge Wang, and Wei Liu from Xi’an University of Architecture and Technology for data analysis of this article.
References
Aghajanian, A. H., Bigham, A., Khodaei, M., and Hossein Kelishadi, S. (2019). Porous titanium scaffold coated using forsterite/poly-3-hydroxybutyrate composite for bone tissue engineering. Surf. Coat. Technol. 378:124942. doi: 10.1016/j.surfcoat.2019.124942
Ahn, T. K., Lee, D. H., Kim, T. S., Jang, G. C., Choi, S., Oh, J. B., et al. (2018). Modification of Titanium Implant and Titanium Dioxide for Bone Tissue Engineering. Adv. Exp. Med. Biol. 1077, 355–368. doi: 10.1007/978-981-13-0947-2_19
Alcázar, J. C. B., Lemos, R. M. J., Conde, M. C. M., Chisini, L. A., Salas, M. M. S., Noremberg, B. S., et al. (2019). Preparation, characterization, and biocompatibility of different metal oxide/PEG-based hybrid coating synthesized by sol–gel dip coating method for surface modification of titanium. Progr. Organic Coat. 130, 206–213. doi: 10.1016/j.porgcoat.2019.02.007
Asri, R. I. M., Harun, W. S. W., Samykano, M., Lah, N. A. C., Ghani, S. A. C., Tarlochan, F., et al. (2017). Corrosion and surface modification on biocompatible metals: a review. Mater. Sci. Eng. C Mater. Biol. Appl. 77, 1261–1274. doi: 10.1016/j.msec.2017.04.102
Awad, N. K., Edwards, S. L., and Morsi, Y. S. (2017). A review of TiO2 NTs on Ti metal: electrochemical synthesis, functionalization and potential use as bone implants. Mater. Sci. Eng. C Mater. Biol. Appl. 76, 1401–1412. doi: 10.1016/j.msec.2017.02.150
Azari, R., Rezaie, H. R., and Khavandi, A. (2019). Investigation of functionally graded HA-TiO2 coating on Ti–6Al–4V substrate fabricated by sol-gel method. Ceramics Int. 45, 17545–17555. doi: 10.1016/j.ceramint.2019.05.317
Bahi, R., Nouveau, C., Beliardouh, N. E., Ramoul, C. E., Meddah, S., and Ghelloudj, O. (2020). Surface performances of Ti-6Al-4V substrates coated PVD multilayered films in biological environments. Surf. Coat. Technol. 385:125412. doi: 10.1016/j.surfcoat.2020.125412
Balla, V. K., Bose, S., Davies, N. M., and Bandyopadhyay, A. (2010). Tantalum—A bioactive metal for implants. JOM 62, 61–64. doi: 10.1007/s11837-010-0110-y
Ballarre, J., Aydemir, T., Liverani, L., Roether, J. A., Goldmann, W. H., and Boccaccini, A. R. (2020). Versatile bioactive and antibacterial coating system based on silica, gentamicin, and chitosan: improving early stage performance of titanium implants. Surf. Coat. Technol. 381:125138. doi: 10.1016/j.surfcoat.2019.125138
Bansiddhi, A., Sargeant, T., Stupp, S. I., and Dunand, D. (2008). Porous NiTi for bone implants: a review. Acta Biomater. 4, 773–782. doi: 10.1016/j.actbio.2008.02.009
Behera, R. R., Das, A., Hasan, A., Pamu, D., Pandey, L. M., and Sankar, M. R. (2020). Effect of TiO2 addition on adhesion and biological behavior of BCP-TiO2 composite films deposited by magnetron sputtering. Mater. Sci. Eng. C 114:111033. doi: 10.1016/j.msec.2020.111033
Bi, Q., Song, X., Chen, Y., Zheng, Y., Yin, P., and Lei, T. (2020). Zn-HA/Bi-HA biphasic coatings on Titanium: fabrication, characterization, antibacterial and biological activity. Colloids Surf. B Biointerf. 189:110813. doi: 10.1016/j.colsurfb.2020.110813
Cao, H., Cui, T., Jin, G., and Liu, X. (2014). Cellular responses to titanium successively treated by magnesium and silver PIII&D. Surf. Coat. Technol. 256, 9–14. doi: 10.1016/j.surfcoat.2013.11.006
Cao, L., Ullah, I., Li, N., Niu, S., Sun, R., Xia, D., et al. (2019). Plasma spray of biofunctional (Mg. Sr)-substituted hydroxyapatite coatings for titanium alloy implants. J. Mater. Sci. Technol. 35, 719–726. doi: 10.1016/j.jmst.2018.10.020
Catauro, M., Bollino, F., and Papale, F. (2018). Surface modifications of titanium implants by coating with bioactive and biocompatible poly (ε-caprolactone)/SiO2 hybrids synthesized via sol–gel. Arab. J. Chem. 11, 1126–1133. doi: 10.1016/j.arabjc.2015.02.010
Cerqueira, A., Romero-Gavilán, F., Araújo-Gomes, N., García-Arnáez, I., Martinez-Ramos, C., Ozturan, S., et al. (2020). A possible use of melatonin in the dental field: protein adsorption and in vitro cell response on coated titanium. Mater Sci. Eng. C 2020:111262. doi: 10.1016/j.msec.2020.111262
Chen, C.-S., Chang, J.-H., Srimaneepong, V., Wen, J.-Y., Tung, O.-H., Yang, C.-H., et al. (2020). Improving the in vitro cell differentiation and in vivo osseointegration of titanium dental implant through oxygen plasma immersion ion implantation treatment. Surf. Coat. Technol. 399:126125. doi: 10.1016/j.surfcoat.2020.126125
Chen, L., Yan, X., Tan, L., Zheng, B., Muhammed, F. K., Yang, K., et al. (2019). In vitro and in vivo characterization of novel calcium phosphate and magnesium (CaP-Mg) bilayer coated titanium for implantation. Surf. Coat. Technol. 374, 784–796. doi: 10.1016/j.surfcoat.2019.06.023
Chouirfa, H., Bouloussa, H., Migonney, V., and Falentin-Daudre, C. (2019). Review of titanium surface modification techniques and coatings for antibacterial applications. Acta Biomater. 83, 37–54. doi: 10.1016/j.actbio.2018.10.036
Cisternas, M., Bhuyan, H., Retamal, M. J., Casanova-Morales, N., Favre, M., Volkmann, U. G., et al. (2020). Study of nitrogen implantation in Ti surface using plasma immersion ion implantation & deposition technique as biocompatible substrate for artificial membranes. Mater. Sci. Eng. C Mater. Biol. Appl. 113:111002. doi: 10.1016/j.msec.2020.111002
Çomaklı, O., Yazıcı, M., Kovacı, H., Yetim, T., Yetim, A. F., and Çelik, A. (2018). Tribological and electrochemical properties of TiO2 films produced on Cp-Ti by sol-gel and SILAR in bio-simulated environment. Surf. Coat. Technol. 352, 513–521. doi: 10.1016/j.surfcoat.2018.08.056
Conrad, J. R., Radtke, J. L., Dodd, R. A., Worzala, F. J., and Tran, N. C. (1987). Plasma source ion-implantation technique for surface modification of materials. J. Appl. Phys. 62, 4591–4596. doi: 10.1063/1.339055
Costa, A. I., Sousa, L., Alves, A. C., and Toptan, F. (2020). Tribocorrosion behaviour of bio-functionalized porous Ti surfaces obtained by two-step anodic treatment. Corrosion Sci. 166:108467. doi: 10.1016/j.corsci.2020.108467
Cui, W., Cheng, J., and Liu, Z. (2019). Bio-tribocorrosion behavior of a nanocrystalline TiZrN coating on biomedical titanium alloy. Surf. Coat. Technol. 369, 79–86. doi: 10.1016/j.surfcoat.2019.04.036
Ding, Z., Fan, Q., and Wang, L. (2019). A review on friction stir processing of titanium alloy: characterization, method, microstructure, properties. Metallurgical Mater. Trans. B 50, 2134–2162. doi: 10.1007/s11663-019-01634-9
Ding, Z., Zhang, C., Xie, L., Zhang, L.-C., Wang, L., and Lu, W. (2016). Effects of Friction Stir Processing on the Phase Transformation and Microstructure of TiO2-Compounded Ti-6Al-4V Alloy. Metallurgical Mater. Trans. A 47, 5675–5679. doi: 10.1007/s11661-016-3809-8
Domínguez-Trujillo, C., Peón, E., Chicardi, E., Pérez, H., Rodríguez-Ortiz, J. A., Pavón, J. J., et al. (2018). Sol-gel deposition of hydroxyapatite coatings on porous titanium for biomedical applications. Surf. Coat. Technol. 333, 158–162. doi: 10.1016/j.surfcoat.2017.10.079
Du, J., Li, X., Li, K., Gu, X., Qi, W., and Zhang, K. (2016). High hydrophilic Si-doped TiO2 nanowires by chemical vapor deposition. J. Alloys Compounds 687, 893–897. doi: 10.1016/j.jallcom.2016.06.182
Durdu, S., Aktug, S. L., Korkmaz, K., Yalcin, E., and Aktas, S. (2018). Fabrication, characterization and in vitro properties of silver-incorporated TiO2 coatings on titanium by thermal evaporation and micro-arc oxidation. Surf. Coat. Technol. 352, 600–608. doi: 10.1016/j.surfcoat.2018.08.050
Ebrahimi, N., Zadeh, A. S. A. H., Vaezi, M. R., and Mozafari, M. (2018). A new double-layer hydroxyapatite/alumina-silica coated titanium implants using plasma spray technique. Surf. Coat. Technol. 352, 474–482. doi: 10.1016/j.surfcoat.2018.08.022
El hadad, A. A., García-Galván, F. R., Mezour, M. A., Hickman, G. J., Soliman, I. E., Jiménez-Morales, A., et al. (2020). Organic-inorganic hybrid coatings containing phosphorus precursors prepared by sol–gel on Ti6Al4V alloy: electrochemical and in-vitro biocompatibility evaluation. Prog. Organic Coat. 148:105834. doi: 10.1016/j.porgcoat.2020.105834
Ganapathy, P., Manivasagam, G., Rajamanickam, A., and Natarajan, A. (2015). Wear studies on plasma-sprayed Al2O3 and 8mole% of Yttrium-stabilized ZrO2 composite coating on biomedical Ti-6Al-4V alloy for orthopedic joint application. Int. J. Nanomed. 10(Suppl. 1), 213–222. doi: 10.2147/IJN.S79997
Garcia-Casas, A., Aguilera-Correa, J. J., Mediero, A., Esteban, J., and Jimenez-Morales, A. (2019). Functionalization of sol-gel coatings with organophosphorus compounds for prosthetic devices. Colloids Surf. B Biointerf. 181, 973–980. doi: 10.1016/j.colsurfb.2019.06.042
Giavaresi, G., Giardino, R., Ambrosio, L., Battiston, G., Gerbasi, R., Fini, M., et al. (2003). In vitro biocompatibility of titanium oxide for prosthetic devices nanostructured by low pressure metal-organic chemical vapor deposition. Int. J. Artif. Organs. 26, 774–780. doi: 10.1177/039139880302600811
Greer, A. I., Lim, T. S., Brydone, A. S., and Gadegaard, N. (2016). Mechanical compatibility of sol-gel annealing with titanium for orthopaedic prostheses. J. Mater. Sci. Mater. Med. 27:21. doi: 10.1007/s10856-015-5611-3
Gu, H., Ding, Z., Yang, Z., Yu, W., Zhang, W., Lu, W., et al. (2019). Microstructure evolution and electrochemical properties of TiO2/Ti-35Nb-2Ta-3Zr micro/nano-composites fabricated by friction stir processing. Mater. Design 169:107680. doi: 10.1016/j.matdes.2019.107680
Gu, M., Lv, L., Du, F., Niu, T., Chen, T., Xia, D., et al. (2018). Effects of thermal treatment on the adhesion strength and osteoinductive activity of single-layer graphene sheets on titanium substrates. Sci. Rep. 8:8141. doi: 10.1038/s41598-018-26551-w
Guo, Y., Chen, D., Cheng, M., Lu, W., Wang, L., and Zhang, X. (2013). The bone tissue compatibility of a new Ti35Nb2Ta3Zr alloy with a low Young’s modulus. Int. J. Mol. Med. 31, 689–697. doi: 10.3892/ijmm.2013.1249
Hafeez, N., Liu, J., Wang, L., Wei, D., Tang, Y., Lu, W., et al. (2020). Superelastic response of low-modulus porous beta-type Ti-35Nb-2Ta-3Zr alloy fabricated by laser powder bed fusion. Addit. Manufact. 34:101264. doi: 10.1016/j.addma.2020.101264
Hafeez, N., Liu, S., Lu, E., Wang, L., Liu, R., Lu, W., et al. (2019). Mechanical behavior and phase transformation of β-type Ti-35Nb-2Ta-3Zr alloy fabricated by 3D-Printing. J. Alloys Compounds 790, 117–126. doi: 10.1016/j.jallcom.2019.03.138
Hamdi, D. A., Jiang, Z.-T., No, K., Rahman, M. M., Lee, P.-C., Truc, L. N. T., et al. (2019). Biocompatibility study of multi-layered hydroxyapatite coatings synthesized on Ti-6Al-4V alloys by RF magnetron sputtering for prosthetic-orthopaedic implant applications. Appl. Surf. Sci. 463, 292–299. doi: 10.1016/j.apsusc.2018.08.157
Han, Y., Hong, S.-H., and Xu, K. (2003). Structure and in vitro bioactivity of titania-based films by micro-arc oxidation. Surf. Coat. Technol. 168, 249–258. doi: 10.1016/s0257-8972(03)00016-1
Hanawa, T. (2019). Titanium-tissue interface reaction and its control with surface treatment. Front. Bioeng. Biotechnol. 7:170. doi: 10.3389/fbioe.2019.00170
Hauschild, G., Hardes, J., Gosheger, G., Stoeppeler, S., Ahrens, H., Blaske, F., et al. (2015). Evaluation of osseous integration of PVD-silver-coated hip prostheses in a canine model. Biomed. Res. Int. 2015:292406. doi: 10.1155/2015/292406
He, Y., Zhang, Y., Shen, X., Tao, B., Liu, J., Yuan, Z., et al. (2018). The fabrication and in vitro properties of antibacterial polydopamine-LL-37-POPC coatings on micro-arc oxidized titanium. Colloids Surf. B Biointerf. 170, 54–63. doi: 10.1016/j.colsurfb.2018.05.070
Hempel, F., Finke, B., Zietz, C., Bader, R., Weltmann, K. D., and Polak, M. (2014). Antimicrobial surface modification of titanium substrates by means of plasma immersion ion implantation and deposition of copper. Surf. Coat. Technol. 256, 52–58. doi: 10.1016/j.surfcoat.2014.01.027
Horkavcova, D., Novak, P., Fialova, I., Cerny, M., Jablonska, E., Lipov, J., et al. (2017). Titania sol-gel coatings containing silver on newly developed TiSi alloys and their antibacterial effect. Mater. Sci. Eng. C Mater. Biol. Appl. 76, 25–30. doi: 10.1016/j.msec.2017.02.137
Hu, Y., Cai, K., Luo, Z., Zhang, Y., Li, L., Lai, M., et al. (2012). Regulation of the differentiation of mesenchymal stem cells in vitro and osteogenesis in vivo by microenvironmental modification of titanium alloy surfaces. Biomaterials 33, 3515–3528. doi: 10.1016/j.biomaterials.2012.01.040
Huang, H.-H., Shiau, D.-K., Chen, C.-S., Chang, J.-H., Wang, S., Pan, H., et al. (2019). Nitrogen plasma immersion ion implantation treatment to enhance corrosion resistance, bone cell growth, and antibacterial adhesion of Ti-6Al-4V alloy in dental applications. Surf. Coat. Technol. 365, 179–188. doi: 10.1016/j.surfcoat.2018.06.023
Huang, Q., Elkhooly, T. A., Liu, X., Zhang, R., Yang, X., Shen, Z., et al. (2016). SaOS-2 cell response to macro-porous boron-incorporated TiO2 coating prepared by micro-arc oxidation on titanium. Mater. Sci. Eng. C Mater. Biol. Appl. 67, 195–204. doi: 10.1016/j.msec.2016.05.051
Huang, Q., Li, X., Elkhooly, T. A., Liu, X., Zhang, R., Wu, H., et al. (2018). The Cu-containing TiO2 coatings with modulatory effects on macrophage polarization and bactericidal capacity prepared by micro-arc oxidation on titanium substrates. Colloids Surf. B Biointerf. 170, 242–250. doi: 10.1016/j.colsurfb.2018.06.020
Hussein, M. A., Adesina, A. Y., Kumar, A. M., Sorour, A. A., Ankah, N., and Al-Aqeeli, N. (2020). Mechanical, in-vitro corrosion, and tribological characteristics of TiN coating produced by cathodic arc physical vapor deposition on Ti20Nb13Zr alloy for biomedical applications. Thin. Solid Films 709:138183. doi: 10.1016/j.tsf.2020.138183
Hwang, S., Lim, S. H., and Han, S. (2019). Highly adhesive and bioactive Ti–Mg alloy thin film on polyether ether ketone formed by PIII&D technique. Appl. Surf. Sci. 471, 878–886. doi: 10.1016/j.apsusc.2018.12.080
Jemat, A., Ghazali, M. J., Razali, M., and Otsuka, Y. (2015). Surface Modifications and Their Effects on Titanium Dental Implants. Biomed. Res. Int. 2015:791725. doi: 10.1155/2015/791725
Ji, M. K., Lee, M. J., Park, S. W., Lee, K., Yun, K. D., Kim, H. S., et al. (2016). Evaluation of antibacterial activity of titanium surface modified by PVD/PACVD process. J. Nanosci. Nanotechnol. 16, 1656–1659. doi: 10.1166/jnn.2016.11924
Jin, G., Cao, H., Qiao, Y., Meng, F., Zhu, H., and Liu, X. (2014). Osteogenic activity and antibacterial effect of zinc ion implanted titanium. Colloids Surf. B Biointerf. 117, 158–165. doi: 10.1016/j.colsurfb.2014.02.025
Kaliaraj, G. S., Bavanilathamuthiah, M., Kirubaharan, K., Ramachandran, D., Dharini, T., Viswanathan, K., et al. (2016). Bio-inspired YSZ coated titanium by EB-PVD for biomedical applications. Surf. Coat. Technol. 307, 227–235. doi: 10.1016/j.surfcoat.2016.08.039
Kaliyannan, G. V., Palanisamy, S. V., Priyanka, E. B., Thangavel, S., Sivaraj, S., and Rathanasamy, R. (2020). Investigation on sol-gel based coatings application in energy sector – A review. Mater. Today Proc. doi: 10.1016/j.matpr.2020.03.484
Kang, B.-J., Kim, H., Lee, S. K., Kim, J., Shen, Y., Jung, S., et al. (2014). Umbilical-cord-blood-derived mesenchymal stem cells seeded onto fibronectin-immobilized polycaprolactone nanofiber improve cardiac function. Acta Biomater. 10, 3007–3017. doi: 10.1016/j.actbio.2014.03.013
Karbowniczek, J., Muhaffel, F., Cempura, G., Cimenoglu, H., and Czyrska-Filemonowicz, A. (2017). Influence of electrolyte composition on microstructure, adhesion and bioactivity of micro-arc oxidation coatings produced on biomedical Ti6Al7Nb alloy. Surf. Coat. Technol. 321, 97–107. doi: 10.1016/j.surfcoat.2017.04.031
Karthikeyan, J., Berndt, C., Tikkanen, J., Reddy, S., and Herman, H. (1997). Plasma spray synthesis of nanomaterial powders and deposits. Mater. Sci. Eng. A 238, 275–286. doi: 10.1016/s0921-5093(96)10568-2
Kaur, M., and Singh, K. (2019). Review on titanium and titanium based alloys as biomaterials for orthopaedic applications. Mater. Sci. Eng. C Mater. Biol. Appl. 102, 844–862. doi: 10.1016/j.msec.2019.04.064
Ke, D., Vu, A. A., Bandyopadhyay, A., and Bose, S. (2019). Compositionally graded doped hydroxyapatite coating on titanium using laser and plasma spray deposition for bone implants. Acta Biomater. 84, 414–423. doi: 10.1016/j.actbio.2018.11.041
Kirkland, N., Lespagnol, J., Birbilis, N., and Staiger, M. (2010). A survey of bio-corrosion rates of magnesium alloys. Corrosion Sci. 52, 287–291. doi: 10.1016/j.corsci.2009.09.033
Kotian, R., Rao, P. P., and Madhyastha, P. (2017). X-ray diffraction analysis of hydroxyapatite-coated in different plasma gas atmosphere on Ti and Ti-6Al-4V. Eur. J. Dent. 11, 438–446. doi: 10.4103/ejd.ejd_100_17
Kuo, T.-Y., Chin, W.-H., Chien, C.-S., and Hsieh, Y.-H. (2019). Mechanical and biological properties of graded porous tantalum coatings deposited on titanium alloy implants by vacuum plasma spraying. Surf. Coat. Technol. 372, 399–409. doi: 10.1016/j.surfcoat.2019.05.003
Lausmaa, J., Kasemo, B., and Mattsson, H. (1990). Surface spectroscopic characterization of titanium implant materials. Appl. Surf. Sci. 44, 133–146. doi: 10.1016/0169-4332(90)90100-e
Lee, H., Liao, J.-D., Sivashanmugan, K., Liu, B. H., Weng, S.-L., Juang, Y.-D., et al. (2017). Dual properties of zirconia coated porous titanium for a stiffness enhanced bio-scaffold. Mater. Design 132, 13–21. doi: 10.1016/j.matdes.2017.06.053
Li, L.-H., Kong, Y.-M., Kim, H.-W., Kim, Y.-W., Kim, H.-E., Heo, S.-J., et al. (2004). Improved biological performance of Ti implants due to surface modification by micro-arc oxidation. Biomaterials 25, 2867–2875. doi: 10.1016/j.biomaterials.2003.09.048
Li, X., Wang, L., Yu, X., Feng, Y., Wang, C., Yang, K., et al. (2013). Tantalum coating on porous Ti6Al4V scaffold using chemical vapor deposition and preliminary biological evaluation. Mater. Sci. Eng. C Mater. Biol. Appl. 33, 2987–2994. doi: 10.1016/j.msec.2013.03.027
Li, Y., Wang, W., Duan, J., and Qi, M. (2017). A super-hydrophilic coating with a macro/micro/nano triple hierarchical structure on titanium by two-step micro-arc oxidation treatment for biomedical applications. Surf. Coat. Technol. 311, 1–9. doi: 10.1016/j.surfcoat.2016.12.065
Li, Y., Wang, W., Liu, H., Lei, J., Zhang, J., Zhou, H., et al. (2018). Formation and in vitro/in vivo performance of “cortex-like” micro/nano-structured TiO2 coatings on titanium by micro-arc oxidation. Mater. Sci. Eng. C Mater. Biol. Appl. 87, 90–103. doi: 10.1016/j.msec.2018.02.023
Li, Y., Wang, W., Yu, F., Wang, D., Guan, S., Li, Y., et al. (2020). Characterization and cytocompatibility of hierarchical porous TiO2 coatings incorporated with calcium and strontium by one-step micro-arc oxidation. Mater. Sci. Eng. C Mater. Biol. Appl. 109:110610. doi: 10.1016/j.msec.2019.110610
Lin, Z., Li, S.-J., Sun, F., Ba, D.-C., and Li, X.-C. (2019). Surface characteristics of a dental implant modified by low energy oxygen ion implantation. Surf. Coat. Technol. 365, 208–213. doi: 10.1016/j.surfcoat.2018.09.003
Liu, D., Yang, T., Ma, H., and Liang, Y. (2020). The microstructure, bio-tribological properties, and biocompatibility of titanium surfaces with graded zirconium incorporation in amorphous carbon bioceramic composite films. Surf. Coat. Technol. 385:125391. doi: 10.1016/j.surfcoat.2020.125391
Liu, S., Han, S., Zhang, L., Chen, L.-Y., Wang, L., Zhang, L., et al. (2020a). Strengthening mechanism and micropillar analysis of high-strength NiTi–Nb eutectic-type alloy prepared by laser powder bed fusion. Composites Part B Eng. 200:108358. doi: 10.1016/j.compositesb.2020.108358
Liu, S., Liu, J., Wang, L., Ma, R. L.-W., Zhong, Y., Lu, W., et al. (2020b). Superelastic behavior of in-situ eutectic-reaction manufactured high strength 3D porous NiTi-Nb scaffold. Scripta Mater. 181, 121–126. doi: 10.1016/j.scriptamat.2020.02.025
Liu, Y.-C., Lin, G.-S., Lee, Y.-T., Huang, T.-C., Chang, T.-W., Chen, Y.-W., et al. (2020). Microstructures and cell reaction of porous hydroxyapatite coatings on titanium discs using a novel vapour-induced pore-forming atmospheric plasma spraying. Surf. Coat. Technol. 393:125837. doi: 10.1016/j.surfcoat.2020.125837
Liu, W., Cheng, M., Wahafu, T., Zhao, Y., Qin, H., Wang, J., et al. (2015). The in vitro and in vivo performance of a strontium-containing coating on the low-modulus Ti35Nb2Ta3Zr alloy formed by micro-arc oxidation. J. Mater. Sci. Mater. Med. 26:203. doi: 10.1007/s10856-015-5533-0
Liu, W., Liu, S., and Wang, L. (2019). Surface modification of biomedical titanium alloy: micromorphology, microstructure evolution and biomedical applications. Coatings 9:249. doi: 10.3390/coatings9040249
Lubas, M., Jasinski, J. J., Jelen, P., and Sitarz, M. (2018). Effect of ZrO 2 sol-gel coating on the Ti 99.2 – Porcelain bond strength investigated with mechanical testing and Raman spectroscopy. J. Mol. Struct. 1168, 316–321. doi: 10.1016/j.molstruc.2018.04.086
Lv, Y., Wu, Y., Lu, X., Yu, Y., Fu, S., Yang, L., et al. (2019). Microstructure, bio-corrosion and biological property of Ag-incorporated TiO2 coatings: influence of Ag2O contents. Ceramics Int. 45, 22357–22367. doi: 10.1016/j.ceramint.2019.07.265
Maleki-Ghaleh, H., Hafezi, M., Hadipour, M., Nadernezhad, A., Aghaie, E., Behnamian, Y., et al. (2015). Effect of tricalcium magnesium silicate coating on the electrochemical and biological behavior of Ti-6Al-4V Alloys. PLoS One 10:e0138454. doi: 10.1371/journal.pone.0138454
Mao, Y., and Gleason, K. K. (2004). Hot filament chemical vapor deposition of poly (glycidyl methacrylate) thin films using tert-butyl peroxide as an initiator. Langmuir 20, 2484–2488. doi: 10.1021/la0359427
Marsh, E. P., Quick, T., Uhlenbrock, S., and Kraus, B. (2010). Deposition Systems, ALD Systems, CVD Systems, Deposition Methods, ALD Methods and CVD Methods. U.S. Patent No:US20100075037A1. Washington, DC: U.S. Patent and Trademark Office.
Matter, P., and Burch, H. (1990). Clinical experience with titanium implants, especially with the limited contact dynamic compression plate system. Arch. Orthopaedic Trauma Surg. 109, 311–313. doi: 10.1007/bf00636167
Mohammed Hussein, S., and Talib Mohammed, M. (2019). Pure and bilayer sol-gel nanolayers derived on a novel Ti surface for load bearing applications. Mater. Today Proc. 18, 2217–2224. doi: 10.1016/j.matpr.2019.07.001
Nemati, A., Saghafi, M., Khamseh, S., Alibakhshi, E., Zarrintaj, P., and Saeb, M. R. (2018). Magnetron-sputtered TixNy thin films applied on titanium-based alloys for biomedical applications: composition-microstructure-property relationships. Surf. Coat. Technol. 349, 251–259. doi: 10.1016/j.surfcoat.2018.05.068
Niinomi, M., Liu, Y., Nakai, M., Liu, H., and Li, H. (2016). Biomedical titanium alloys with Young’s moduli close to that of cortical bone. Regen. Biomater. 3, 173–185. doi: 10.1093/rb/rbw016
Ohthuki, C., Osaka, A., Iida, H., and Ohta, K. (1999). Biocompatible Titanium Implant. U.S. Patent No: US 6544288 B2. Washington, DC: U.S. Patent and Trademark Office.
Otsuka, Y., Kawaguchi, H., and Mutoh, Y. (2016). Cyclic delamination behavior of plasma-sprayed hydroxyapatite coating on Ti-6Al-4V substrates in simulated body fluid. Mater. Sci. Eng. C Mater. Biol. Appl. 67, 533–541. doi: 10.1016/j.msec.2016.05.058
Palla-Rubio, B., Araujo-Gomes, N., Fernandez-Gutierrez, M., Rojo, L., Suay, J., Gurruchaga, M., et al. (2019). Synthesis and characterization of silica-chitosan hybrid materials as antibacterial coatings for titanium implants. Carbohydr. Polym. 203, 331–341. doi: 10.1016/j.carbpol.2018.09.064
Pan, J., Prabakaran, S., and Rajan, M. (2019). In-vivo assessment of minerals substituted hydroxyapatite / poly sorbitol sebacate glutamate (PSSG) composite coating on titanium metal implant for orthopedic implantation. Biomed. Pharmacother. 119:109404. doi: 10.1016/j.biopha.2019.109404
Park, S. W., Lee, D., Lee, H. R., Moon, H. J., Lee, B. R., Ko, W. K., et al. (2015). Generation of functionalized polymer nanolayer on implant surface via initiated chemical vapor deposition (iCVD). J. Colloid Interf. Sci. 439, 34–41. doi: 10.1016/j.jcis.2014.10.018
Prachar, P., Bartakova, S., Brezina, V., Cvrcek, L., and Vanek, J. (2015). Cytocompatibility of implants coated with titanium nitride and zirconium nitride. Bratisl. Lek. Listy. 116, 154–156. doi: 10.4149/bll_2015_031
Rabadia, C. D., Liu, Y. J., Chen, L. Y., Jawed, S. F., Wang, L. Q., Sun, H., et al. (2019). Deformation and strength characteristics of Laves phases in titanium alloys. Mater. Design 179:107891. doi: 10.1016/j.matdes.2019.107891
Rabadia, C. D., Liu, Y. J., Wang, L., Sun, H., and Zhang, L. C. (2018). Laves phase precipitation in Ti-Zr-Fe-Cr alloys with high strength and large plasticity. Mater. Design 154, 228–238. doi: 10.1016/j.matdes.2018.05.035
Ran, R., Liu, Y., Wang, L., Lu, E., Xie, L., Lu, W., et al. (2018). α? Martensite and amorphous phase transformation mechanism in tinbtazr alloy incorporated with tio2 particles during friction stir processing. Metallurgical Mater. Trans. A 49, 1986–1991. doi: 10.1007/s11661-018-4577-4
Robertson, S. F., Bandyopadhyay, A., and Bose, S. (2019). Titania nanotube interface to increase adhesion strength of hydroxyapatite sol-gel coatings on Ti-6Al-4V for orthopedic applications. Surf. Coat. Technol. 372, 140–147. doi: 10.1016/j.surfcoat.2019.04.071
Romero-Gavilan, F., Araujo-Gomes, N., Garcia-Arnaez, I., Martinez-Ramos, C., Elortza, F., Azkargorta, M., et al. (2019). The effect of strontium incorporation into sol-gel biomaterials on their protein adsorption and cell interactions. Colloids Surf. B Biointerf. 174, 9–16. doi: 10.1016/j.colsurfb.2018.10.075
Romero-Gavilan, F., Araujo-Gomes, N., Sanchez-Perez, A. M., Garcia-Arnaez, I., Elortza, F., Azkargorta, M., et al. (2018). Bioactive potential of silica coatings and its effect on the adhesion of proteins to titanium implants. Colloids Surf. B Biointerfaces 162, 316–325. doi: 10.1016/j.colsurfb.2017.11.072
Saleem, S., Ahmad, R., Ayub, R., Ikhlaq, U., Jin, W., and Chu, P. K. (2017). Investigation of nano-structured Zirconium oxide film on Ti6Al4V substrate to improve tribological properties prepared by PIII&D. Appl. Surf. Sci. 394, 586–597. doi: 10.1016/j.apsusc.2016.09.091
Santos, N. M., Mariano, S. F. M., and Ueda, M. (2019). Carbon films deposition as protective coating of titanium alloy tube using PIII&D system. Surf. Coat. Technol. 375, 164–170. doi: 10.1016/j.surfcoat.2019.03.083
Sarraf, M., Razak, B. A., Nasiri-Tabrizi, B., Dabbagh, A., Kasim, N. H. A., Basirun, W. J., et al. (2017). Nanomechanical properties, wear resistance and in-vitro characterization of Ta2O5 nanotubes coating on biomedical grade Ti-6Al-4V. J. Mech. Behav. Biomed. Mater. 66, 159–171. doi: 10.1016/j.jmbbm.2016.11.012
Sedelnikova, M. B., Komarova, E. G., Sharkeev, Y. P., Tolkacheva, T. V., Khlusov, I. A., Litvinova, L. S., et al. (2017). Comparative investigations of structure and properties of micro-arc wollastonite-calcium phosphate coatings on titanium and zirconium-niobium alloy. Bioact. Mater. 2, 177–184. doi: 10.1016/j.bioactmat.2017.01.002
Shanaghi, A., and Chu, P. K. (2019a). Enhancement of mechanical properties and corrosion resistance of NiTi alloy by carbon plasma immersion ion implantation. Surf. Coat. Technol. 365, 52–57. doi: 10.1016/j.surfcoat.2018.04.027
Shanaghi, A., and Chu, P. K. (2019b). Investigation of corrosion mechanism of NiTi modified by carbon plasma immersion ion implantation (C-PIII) by electrochemical impedance spectroscopy. J. Alloys Compounds 790, 1067–1075. doi: 10.1016/j.jallcom.2019.03.272
Shaw, L. L., Goberman, D., Ren, R., Gell, M., Jiang, S., Wang, Y., et al. (2000). The dependency of microstructure and properties of nanostructured coatings on plasma spray conditions. Surf. Coat. Technol. 130, 1–8. doi: 10.1016/s0257-8972(00)00673-3
Shen, X., Ping, L., Wang, L., Liu, C., Liu, J., and Deng, Z. (2020). Improving the stability and bioactivity of micro-arc oxidized calcium phosphate/titania porous coatings by high energy shot peening pretreatment. Ceramics Int. 46, 2041–2048. doi: 10.1016/j.ceramint.2019.09.183
Shi, Q., Qian, Z., Liu, D., and Liu, H. (2017). Surface modification of dental titanium implant by layer-by-layer electrostatic self-assembly. Front. Physiol. 8:574. doi: 10.3389/fphys.2017.00574
Shiau, D.-K., Yang, C.-H., Sun, Y.-S., Wu, M.-F., Pan, H., and Huang, H.-H. (2019). Enhancing the blood response and antibacterial adhesion of titanium surface through oxygen plasma immersion ion implantation treatment. Surf. Coat. Technol. 365, 173–178. doi: 10.1016/j.surfcoat.2018.05.029
Singh, D., Singh, R., Boparai, K., Farina, I., Feo, L., and Verma, A. K. (2018). In-vitro studies of SS 316 L biomedical implants prepared by FDM, vapor smoothing and investment casting. Composites Part B Eng. 132, 107–114. doi: 10.1016/j.compositesb.2017.08.019
Somani, P. R., Somani, S. P., and Umeno, M. (2006). Planer nano-graphenes from camphor by CVD. Chem. Phys. Lett. 430, 56–59. doi: 10.1016/j.cplett.2006.06.081
Souza, J. C. M., Sordi, M. B., Kanazawa, M., Ravindran, S., Henriques, B., Silva, F. S., et al. (2019). Nano-scale modification of titanium implant surfaces to enhance osseointegration. Acta Biomater. 94, 112–131. doi: 10.1016/j.actbio.2019.05.045
Tang, Y., Wu, C., Tian, P., Zhao, K., and Wu, Z. (2020). Fabrication and induced mineralization of bio-piezoelectric ceramic coating on titanium alloys. Ceramics Int. 46, 4006–4014. doi: 10.1016/j.ceramint.2019.10.040
Thangavel, E., Dhandapani, V. S., Dharmalingam, K., Marimuthu, M., Veerapandian, M., Arumugam, M. K., et al. (2019). RF magnetron sputtering mediated NiTi/Ag coating on Ti-alloy substrate with enhanced biocompatibility and durability. Mater. Sci. Eng. C Mater. Biol. Appl. 99, 304–314. doi: 10.1016/j.msec.2019.01.099
Toirac, B., Garcia-Casas, A., Cifuentes, S. C., Aguilera-Correa, J. J., Esteban, J., Mediero, A., et al. (2020). Electrochemical characterization of coatings for local prevention of Candida infections on titanium-based biomaterials. Progr. Organic Coat. 146:105681. doi: 10.1016/j.porgcoat.2020.105681
Ueda, M., Oliveira, R. M., Rossi, J. O., Mello, C. B., Rangel, R. C. C., and Vieira, M. S. (2013). Improvements of plasma immersion ion implantation (PIII) and deposition (PIII&D) processing for materials surface modification. Surf. Coat. Technol. 229, 97–104. doi: 10.1016/j.surfcoat.2012.06.057
Vahabzadeh, S., Roy, M., Bandyopadhyay, A., and Bose, S. (2015). Phase stability and biological property evaluation of plasma sprayed hydroxyapatite coatings for orthopedic and dental applications. Acta Biomater. 17, 47–55. doi: 10.1016/j.actbio.2015.01.022
Veerachamy, S., Hameed, P., Sen, D., Dash, S., and Manivasagam, G. (2018). Studies on mechanical, biocompatibility and antibacterial activity of plasma sprayed nano/micron ceramic bilayered coatings on Ti-6Al-4V alloy for biomedical application. J. Nanosci. Nanotechnol. 18, 4515–4523. doi: 10.1166/jnn.2018.15332
Wang, D., and Bierwagen, G. P. (2009). Sol–gel coatings on metals for corrosion protection. Progr. Organic Coat. 64, 327–338. doi: 10.1016/j.porgcoat.2008.08.010
Wang, L., Lu, W., Qin, J., Zhang, F., and Zhang, D. (2009). Influence of cold deformation on martensite transformation and mechanical properties of Ti–Nb–Ta–Zr alloy. J. Alloys Compounds 469, 512–518. doi: 10.1016/j.jallcom.2008.02.032
Wang, L., Qu, J., Chen, L., Meng, Q., Zhang, L.-C., Qin, J., et al. (2015). Investigation of Deformation Mechanisms in β-Type Ti-35Nb-2Ta-3Zr Alloy via FSP leading to surface strengthening. Metallurg. Mater. Trans. A 46, 4813–4818. doi: 10.1007/s11661-015-3089-8
Wang, Y., Yu, H., Chen, C., and Zhao, Z. (2015). Review of the biocompatibility of micro-arc oxidation coated titanium alloys. Mater. Design 85, 640–652. doi: 10.1016/j.matdes.2015.07.086
Wang, L., Wang, C., Zhang, L. C., Chen, L., Lu, W., and Zhang, D. (2016). Phase transformation and deformation behavior of NiTi-Nb eutectic joined NiTi wires. Sci. Rep. 6:23905. doi: 10.1038/srep23905
Wang, Q., Qiao, Y., Cheng, M., Jiang, G., He, G., Chen, Y., et al. (2016). Tantalum implanted entangled porous titanium promotes surface osseointegration and bone ingrowth. Sci. Rep. 6:26248. doi: 10.1038/srep26248
Wang, L., Wang, Y., Huang, W., Liu, J., Tang, Y., Zhang, L., et al. (2020). Tensile and superelastic behaviors of Ti-35Nb-2Ta-3Zr with gradient structure. Mater. Design 194:108961. doi: 10.1016/j.matdes.2020.108961
Wang, Q., Zhou, P., Liu, S., Attarilar, S., Ma, R. L., Zhong, Y., et al. (2020). Multi-scale surface treatments of titanium implants for rapid osseointegration: a review. Nanomaterials 10:1244. doi: 10.3390/nano10061244
Wang, L., Xie, L., Lv, Y., Zhang, L.-C., Chen, L., Meng, Q., et al. (2017). Microstructure evolution and superelastic behavior in Ti-35Nb-2Ta-3Zr alloy processed by friction stir processing. Acta Mater. 131, 499–510. doi: 10.1016/j.actamat.2017.03.079
Wang, L., Xie, L., Zhang, L.-C., Chen, L., Ding, Z., Lv, Y., et al. (2018). Microstructure evolution and superelasticity of layer-like NiTiNb porous metal prepared by eutectic reaction. Acta Mater. 143, 214–226. doi: 10.1016/j.actamat.2017.10.021
Wang, S., Yang, C., Ren, L., Shen, M., and Yang, K. (2014). Study on antibacterial performance of Cu-bearing cobalt-based alloy. Mater. Lett. 129, 88–90. doi: 10.1016/j.matlet.2014.05.020
Wu, W.-Y., Chan, M.-Y., Hsu, Y.-H., Chen, G.-Z., Liao, S.-C., Lee, C.-H., et al. (2019). Bioapplication of TiN thin films deposited using high power impulse magnetron sputtering. Surf. Coat. Technol. 362, 167–175. doi: 10.1016/j.surfcoat.2019.01.106
Xia, C., Ma, X., Zhang, X., Li, K., Tan, J., Qiao, Y., et al. (2020). Enhanced physicochemical and biological properties of C/Cu dual ions implanted medical titanium. Bioact. Mater. 5, 377–386. doi: 10.1016/j.bioactmat.2020.02.017
Xiao, M., Chen, Y. M., Biao, M. N., Zhang, X. D., and Yang, B. C. (2017). Bio-functionalization of biomedical metals. Mater. Sci. Eng. C Mater. Biol. Appl. 70(Pt 2), 1057–1070. doi: 10.1016/j.msec.2016.06.067
Xu, J., Li, Y., Zhou, X., Li, Y., Gao, Z. D., Song, Y. Y., et al. (2016). Graphitic C3 N4 -Sensitized TiO2 nanotube layers: a visible-light activated efficient metal-free antimicrobial platform. Chemistry 22, 3947–3951. doi: 10.1002/chem.201505173
Xu, R., Yang, X., Jiang, J., Li, P., Zhang, X., Wu, G., et al. (2015). Effects of silver plasma immersion ion implantation on the surface characteristics and cytocompatibility of titanium nitride films. Surf. Coat. Technol. 279, 166–170. doi: 10.1016/j.surfcoat.2015.08.033
Yang, P., Huang, N., Leng, Y., Wan, G., Zhao, A., Chen, J., et al. (2007). Functional inorganic films fabricated by PIII(-D) for surface modification of blood contacting biomaterials: fabrication parameters, characteristics and antithrombotic properties. Surf. Coat. Technol. 201, 6828–6832. doi: 10.1016/j.surfcoat.2006.09.014
Yetim, T. (2017). An investigation of the corrosion properties of Ag-doped TiO 2 -coated commercially pure titanium in different biological environments. Surf. Coat. Technol. 309, 790–794. doi: 10.1016/j.surfcoat.2016.10.084
Youn, Y. H., Lee, S. J., Choi, G. R., Lee, H. R., Lee, D., Heo, D. N., et al. (2019). Simple and facile preparation of recombinant human bone morphogenetic protein-2 immobilized titanium implant via initiated chemical vapor deposition technique to promote osteogenesis for bone tissue engineering application. Mater. Sci. Eng. C Mater. Biol. Appl. 100, 949–958. doi: 10.1016/j.msec.2019.03.048
Yousefi, M., Dadashpour, M., Hejazi, M., Hasanzadeh, M., Behnam, B., de la Guardia, M., et al. (2017). Anti-bacterial activity of graphene oxide as a new weapon nanomaterial to combat multidrug-resistance bacteria. Mater. Sci. Eng. C Mater. Biol. Appl. 74, 568–581. doi: 10.1016/j.msec.2016.12.125
Yu, L., Jin, G., Ouyang, L., Wang, D., Qiao, Y., and Liu, X. (2016). Antibacterial activity, osteogenic and angiogenic behaviors of copper-bearing titanium synthesized by PIII&D. J. Mater. Chem. B 4, 1296–1309. doi: 10.1039/c5tb02300a
Yu, L., Tian, Y., Qiao, Y., and Liu, X. (2017). Mn-containing titanium surface with favorable osteogenic and antimicrobial functions synthesized by PIII&D. Colloids Surf. B Biointerfaces 152, 376–384. doi: 10.1016/j.colsurfb.2017.01.047
Yu, Y., Jin, G., Xue, Y., Wang, D., Liu, X., and Sun, J. (2017). Multifunctions of dual Zn/Mg ion co-implanted titanium on osteogenesis, angiogenesis and bacteria inhibition for dental implants. Acta Biomater. 49, 590–603. doi: 10.1016/j.actbio.2016.11.067
Zhang, C., Ding, Z., Xie, L., Zhang, L.-C., Wu, L., Fu, Y., et al. (2017). Electrochemical and in vitro behavior of the nanosized composites of Ti-6Al-4V and TiO2 fabricated by friction stir process. Appl. Surf. Sci. 423, 331–339. doi: 10.1016/j.apsusc.2017.06.141
Zhang, L. C., and Chen, L. Y. (2019). A review on biomedical titanium alloys: recent progress and prospect. Adv. Eng. Mater. 21:1215. doi: 10.1002/adem.201801215
Zhang, L.-C., Chen, L.-Y., and Wang, L. (2020). Surface modification of titanium and titanium alloys: technologies, developments, and future interests. Adv. Eng. Mater. 22:1258. doi: 10.1002/adem.201901258
Zhang, X., Peng, Z., Lu, X., Lv, Y., Cai, G., Yang, L., et al. (2020). Microstructural evolution and biological performance of Cu-incorporated TiO2 coating fabricated through one-step micro-arc oxidation. Appl. Surf. Sci. 508:144766. doi: 10.1016/j.apsusc.2019.144766
Zhang, M., Pu, X., Chen, X., and Yin, G. (2019). In-vivo performance of plasma-sprayed CaO-MgO-SiO2-based bioactive glass-ceramic coating on Ti-6Al-4V alloy for bone regeneration. Heliyon 5:e02824. doi: 10.1016/j.heliyon.2019.e02824
Zhang, X., Li, C., Yu, Y., Lu, X., Lv, Y., Jiang, D., et al. (2019). Characterization and property of bifunctional Zn-incorporated TiO2 micro-arc oxidation coatings: the influence of different Zn sources. Ceramics Int. 45, 19747–19756. doi: 10.1016/j.ceramint.2019.06.228
Zhao, J., Guo, Y., Lan, A., Luo, W., Wang, X., Fu, L., et al. (2018). The effect of amino plasma-enhanced chemical vapor deposition-treated titanium surface on Schwann cells. J. Biomed. Mater. Res. A 106, 265–271. doi: 10.1002/jbm.a.36167
Zheng, L., Qian, S., and Liu, X. Y. (2020). Induced antibacterial capability of TiO2 coatings in visible light via nitrogen ion implantation. Trans. Nonferrous Metals Soc. China 30, 171–180. doi: 10.1016/S1003-6326(19)65189-7
Zhong, S.-P. (1999). Method of Providing a Substrate with a Bio-Active/Biocompatible Coating. U.S. Patent No: US5869127A. Washington, DC: U.S. Patent and Trademark Office.
Zhong, S.-P. (2001). Hybrid Coating for Medical Devices. U.S. Patent No: US-6179817-B1. Washington, DC: U.S. Patent and Trademark Office.
Zhu, C., Lv, Y., Qian, C., Qian, H., Jiao, T., Wang, L., et al. (2016). Proliferation and osteogenic differentiation of rat BMSCs on a novel Ti/SiC metal matrix nanocomposite modified by friction stir processing. Sci. Rep. 6:38875. doi: 10.1038/srep38875
Zhu, J., Wang, X., Kou, L., Zheng, L., and Zhang, H. (2020). Prediction of control parameters corresponding to in-flight particles in atmospheric plasma spray employing convolutional neural networks. Surf. Coat. Technol. 394:125862. doi: 10.1016/j.surfcoat.2020.125862
Keywords: titanium, surface modification, implant materials, osteogenesis properties, antibacterial function
Citation: Xue T, Attarilar S, Liu S, Liu J, Song X, Li L, Zhao B and Tang Y (2020) Surface Modification Techniques of Titanium and its Alloys to Functionally Optimize Their Biomedical Properties: Thematic Review. Front. Bioeng. Biotechnol. 8:603072. doi: 10.3389/fbioe.2020.603072
Received: 05 September 2020; Accepted: 07 October 2020;
Published: 11 November 2020.
Edited by:
Lechun Xie, Wuhan University of Technology, ChinaReviewed by:
Liang-Yu Chen, Jiangsu University of Science and Technology, ChinaPeng Fu, Liaocheng University, China
Jiuxiao Li, Shanghai University of Engineering Sciences, China
Copyright © 2020 Xue, Attarilar, Liu, Liu, Song, Li, Zhao and Tang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shifeng Liu, bGl1c2hpZmVuZzY2QHhhdWF0LmVkdS5jbg==; Jia Liu, bGl1amlhMDExMUBsaXZlLmNu; Yujin Tang, dGFuZ3l1amluMTk2N0BsNjMuY29t
†These authors have contributed equally to this work
 Tong Xue
Tong Xue Shokouh Attarilar
Shokouh Attarilar Shifeng Liu1*
Shifeng Liu1* Jia Liu
Jia Liu