
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Bioeng. Biotechnol. , 19 November 2020
Sec. Cell and Gene Therapy
Volume 8 - 2020 | https://doi.org/10.3389/fbioe.2020.598466
This article is part of the Research Topic Non-Viral Gene Therapy Systems for Orthopaedics View all 6 articles
 Benjamin Gantenbein1,2*†‡
Benjamin Gantenbein1,2*†‡ Shirley Tang3†‡
Shirley Tang3†‡ Julien Guerrero1,2†‡
Julien Guerrero1,2†‡ Natalia Higuita-Castro4‡
Natalia Higuita-Castro4‡ Ana I. Salazar-Puerta4‡
Ana I. Salazar-Puerta4‡ Andreas S. Croft1,2‡
Andreas S. Croft1,2‡ Amiq Gazdhar5‡
Amiq Gazdhar5‡ Devina Purmessur3*†‡
Devina Purmessur3*†‡Viral carrier transport efficiency of gene delivery is high, depending on the type of vector. However, viral delivery poses significant safety concerns such as inefficient/unpredictable reprogramming outcomes, genomic integration, as well as unwarranted immune responses and toxicity. Thus, non-viral gene delivery methods are more feasible for translation as these allow safer delivery of genes and can modulate gene expression transiently both in vivo, ex vivo, and in vitro. Based on current studies, the efficiency of these technologies appears to be more limited, but they are appealing for clinical translation. This review presents a summary of recent advancements in orthopedics, where primarily bone and joints from the musculoskeletal apparatus were targeted. In connective tissues, which are known to have a poor healing capacity, and have a relatively low cell-density, i.e., articular cartilage, bone, and the intervertebral disk (IVD) several approaches have recently been undertaken. We provide a brief overview of the existing technologies, using nano-spheres/engineered vesicles, lipofection, and in vivo electroporation. Here, delivery for microRNA (miRNA), and silencing RNA (siRNA) and DNA plasmids will be discussed. Recent studies will be summarized that aimed to improve regeneration of these tissues, involving the delivery of bone morphogenic proteins (BMPs), such as BMP2 for improvement of bone healing. For articular cartilage/osteochondral junction, non-viral methods concentrate on targeted delivery to chondrocytes or MSCs for tissue engineering-based approaches. For the IVD, growth factors such as GDF5 or GDF6 or developmental transcription factors such as Brachyury or FOXF1 seem to be of high clinical interest. However, the most efficient method of gene transfer is still elusive, as several preclinical studies have reported many different non-viral methods and clinical translation of these techniques still needs to be validated. Here we discuss the non-viral methods applied for bone and joint and propose methods that can be promising in clinical use.
Non-viral gene therapy holds great premises as it is assumed to be less toxic for the host and much safer in terms of gene delivery compared to viral vectors (NIH Report, 2002; Kaiser, 2007).
Generally, gene transfer approaches in clinical trials are much less common than clinical trials in general that may involve drug testing (Figure 1). In the clinical trial register (clinicaltrials.gov accessed on 9-October-2020) there were 5,013 (60%) studies reported on “general bone diseases,” 1,034 (11%) on the “hip”-joint, 600 (7%) studies on “rotator cuff,” 1,002 (12%) studies on “tendon” repair, 337 (5%) studies on “intervertebral disk degeneration” (IVD), and 379 (5%) studies on cartilage repair (“cartilage”) (Figure 1). However, with the additional mesh-terms “gene delivery” OR “viral gene therapy” combined with the afore-mentioned orthopedic “specialties” 289 studies were identified for “bone,” only two for the “tendon” and five were found for “IVD” and none for “cartilage” (inlet, Figure 1). Finally, “non-viral” AND “gene delivery” resulted in “zero” studies in all fields of orthopedics. This fact reflects the current situation of non-viral gene delivery trials in this field. One reason might be that the search for new gene therapies, which target certain tissues and cells, has become more cumbersome due to increased levels of regulation (Boissier and Bessis, 1997; Evans et al., 2006, 2012). Many of the recently developed products have not been translated into the clinics, for which many reasons have been identified. One important aspect is safety. The risks and the acceptance of viral gene transfer methods experienced have been affected by sudden patient deaths, such as the examples of Jesse Gelsinger and Joli Mohr (Wilson, 2009; Yarborough and Sharp, 2009). Thus, non-viral gene therapy seems an attractive alternative to viral gene delivery and is an new and emerging field being applied to regenerative medicine. It offers a safer approach to viral vectors with lack of immunogenicity and host genome integration. However, pre-clinical application of such technologies to the musculoskeletal field is still limited.
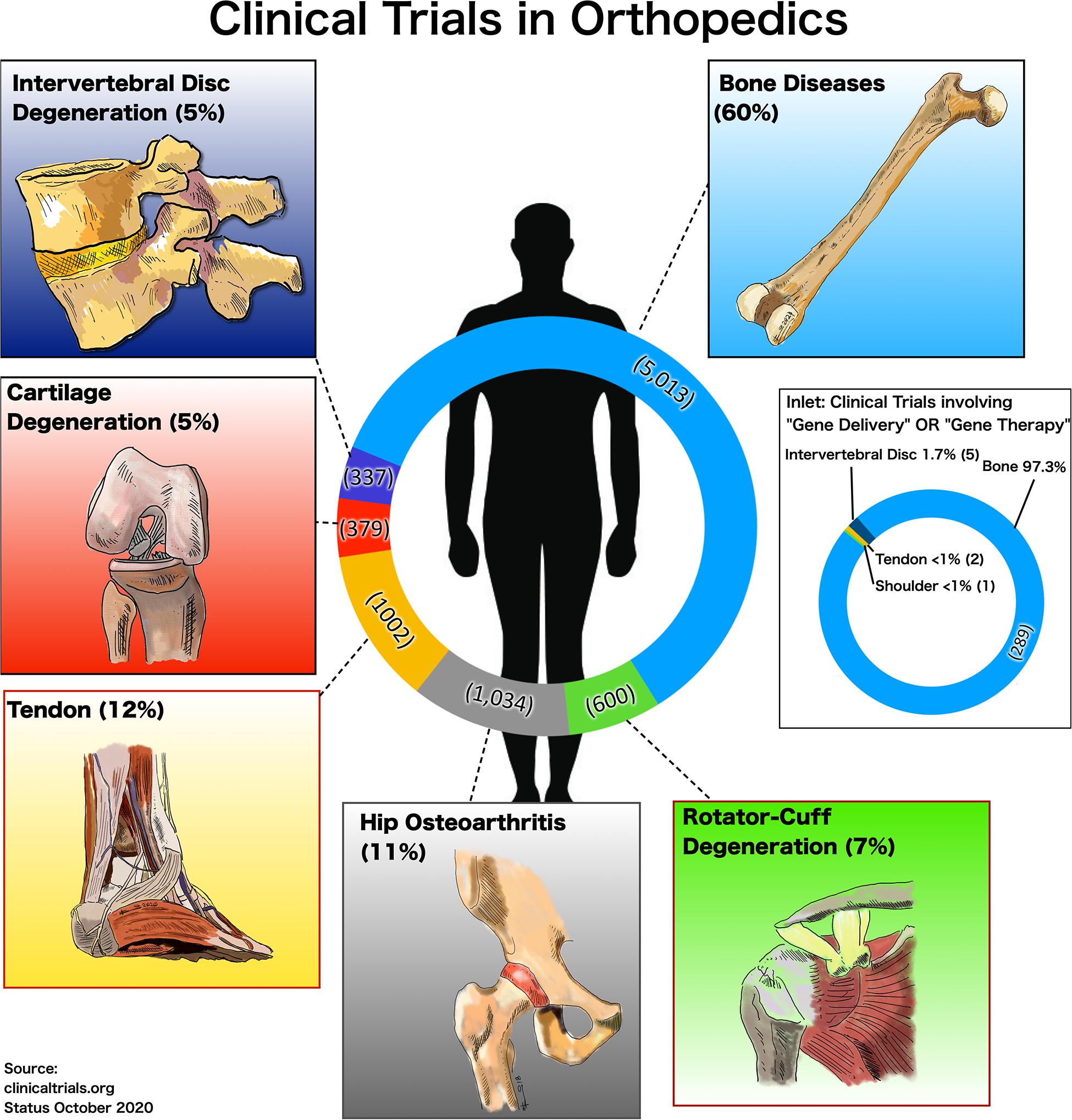
Figure 1. Number and percentages of clinical trials accessed on the 9-October-2020 at ClinicalTrials.gov for different fields in orthopedics, and for inlet limiting the search for the search terms “gene delivery” OR “viral gene therapy” AND the respective area in orthopedics, i.e., “general bone diseases,” “hip,” “tendon,” “cartilage,” and “intervertebral disk degeneration.”
Many of the alternate approaches are less efficient than viral delivery systems (NIH Report, 2002; Pranatharthiharan et al., 2013; O’Reilly et al., 2015) and due to necessary optimization that is required increases developmental costs exponentially as the product approaches market release (Epstein, 1991; Evans et al., 2012). Another current challenge lies in the experimental designs of clinical trials, which, if not properly planned or randomized, produce doubtful conclusions. As for clinical trials, it needs to be mentioned and clarified if appropriate placebo controls were considered in the original experimental set-up (NIH Report, 2002; Wilson, 2009). In the absence of properly designed controls, it may be impossible to determine whether observed toxicity is due to an underlying disease or the use of a specific vector.
In orthopedic research there are a number of significant health burdens that urgently warrant better therapeutic solutions. In addition to bone metabolic diseases, this also includes problematic musculoskeletal degenerative pathologies of cartilage, tendons, and ligaments, as well as the intervertebral disks (IVDs) of the spine. It has been identified that osteoarthritis (OA) (Wittenauer et al., 2013) and low back pain (LBP) caused by degenerative changes in the IVD are two of the significant global clinical problems to be tackled in the future (GBD 2017 Disease and Injury Incidence and Prevalence Collaborators, 2018). With an increasing elderly population, the demand for joint-replacement surgeries has risen exponentially. For many of the degenerated joints, whether due to aging, genetic predisposition, or trauma, pure mechanical implant solutions exist until now. These do not necessarily take into account the natural tissue properties. Here, in particular in the field of early prevention, non-viral gene therapy could become highly relevant in the near future and is the focus of this review. Here we evaluate promising in vitro and in vivo non-viral methods being utilized and more specifically in cartilage, the intervertebral disk and bone and gaps/areas that need to be addressed to move these non-viral strategies forward.
Gene delivery in general may involve the packaging of DNA or RNA in so-called “vectors” but can also be delivered naked (Patil et al., 2019). Generally, one can classify methods according to the approach to overcome the cell’s phosphobilayer membrane: There are “carrier-free” methods that use physical penetration (e.g., electroporation, gene gun, laser, microinjection) or there are methods that use so-called “carriers,” in which DNA or RNA is packed into lipo-philic particles, so-called liposomes, or similar (Figure 2). A distinction can also be made between methods that use fluorescence to monitor the success of the gene transfer or methods that lack this practical feature to monitor the efficiency (Patil et al., 2019). There are several commercial suppliers offering kits that pack DNA or RNA into liposomes and then transfect cells in vitro (Figure 2). However, the success of these transfections and duration of the changes may be extremely dependent on the cell-type and the vectors. In some cases, a short over-expression of particular genes is even a warranted side-effect. The advantages of non-viral gene therapy are the fact that the effects are not long-lived. In the following sections, we will briefly introduce the different methods.
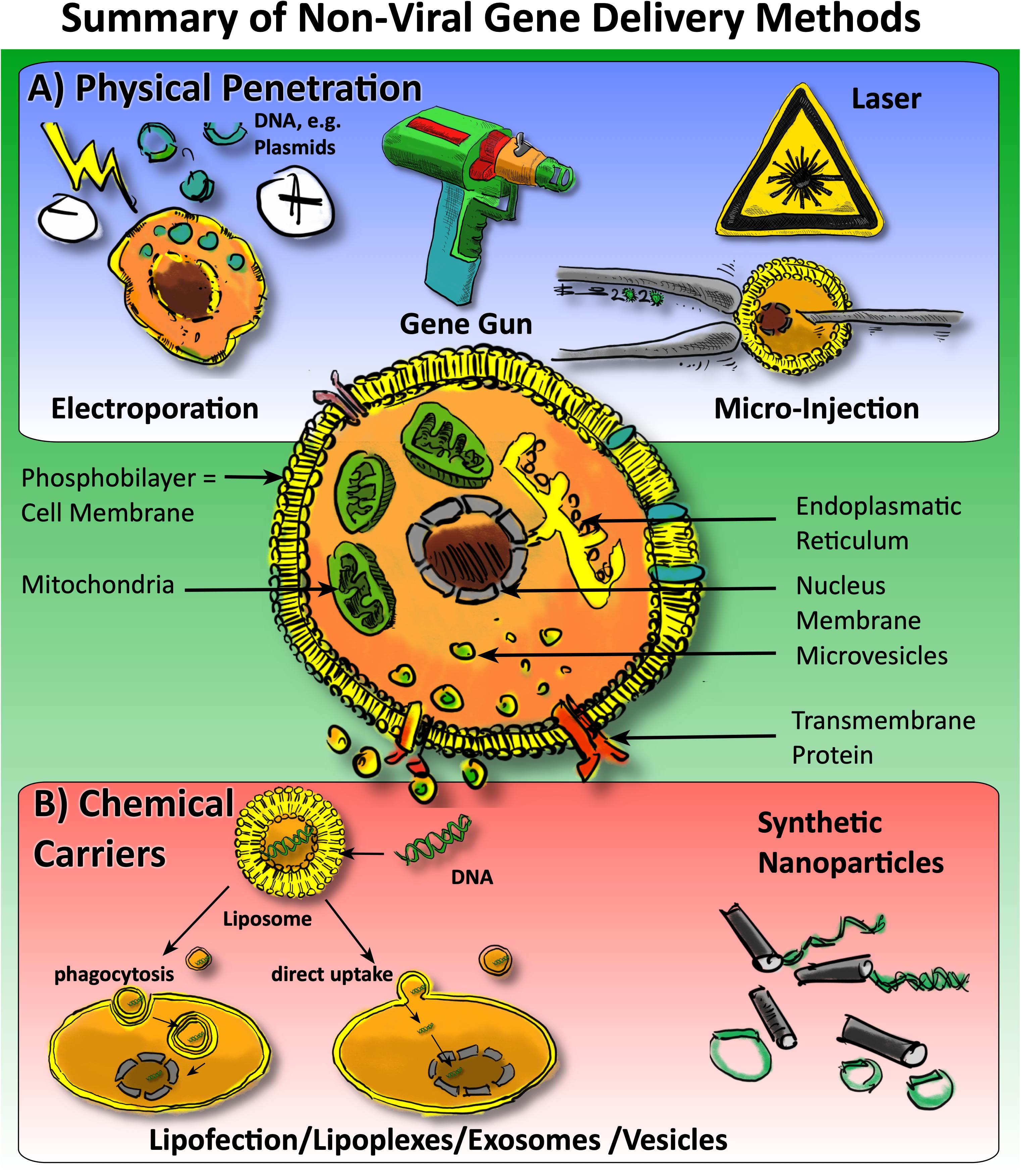
Figure 2. Overview of non-viral approaches for gene delivery to cells in orthopedics. One may generally categorize the methods into (A) physical penetration methods (in blue panel) versus (B) chemical carriers, i.e., methods involving carriers such as lipofection, micro vesicles, and EVs and, the usage of nanoparticles.
Lipofection via liposomes or lipoplexes has been widely utilized to deliver genetic cargo to cells in vitro. This method involves encapsulating pDNA, siRNA, or MicroRNA in spheroids with hydrophilic polar head groups and hydrophobic tails, similar to the structure of the cell membrane (Felgner et al., 1987; Torchilin, 2005). One of the earliest and popular lipofection systems involved cationic lipid, N-[1-(2,3-dioleyloxy)propyl]-N,N, N-trimethylammonium chloride (DOTMA). However, due to initial limitations associated with non-specific protein binding (Lv et al., 2006), more recent iterations have included modifications such as neutral helper lipids to reduce cytotoxicity and to improve the efficiency of the transfection (Gao and Hui, 2001; Dabkowska et al., 2012). These improvements led to the development of lipid nanoparticles, which are formed from cationic lipids with both neutral helper lipids and ionizable cationic amino head groups (Wheeler et al., 1999). These systems can readily form complexes with large nucleic acid constructs and have many advantages such as efficient in vitro delivery, biodegradability and the option to tune as well as to functionalize them as described in Table 1. Yet the efficiency of in vivo transfection is more limited with these lipid-based methods, as is the optimization of formulations for mass manufacturing. In orthopedics, lipid-based vectors for non-viral gene delivery have been widely utilized (Table 1) such as for treatments for osteoporosis, arthritis, and the IVD.
Electroporation (electro-permeabilization) is a physical method based on the application of high voltage pulses for a short duration to facilitate cellular uptake of nucleic acids or drugs. The concept of electroporation was pioneered by Neumann et al. (1982), and since then it has become a standard method of in vitro transfection due to its low cost and safety (Wong and Neumann, 1982). Optimized electric pulses increase the permeability of the cell membrane through which nucleic acid or drug can enter the cell, once the pulses are terminated the cell membrane rapidly recovers and closes (Gowrishankar et al., 1999; Somiari et al., 2000; Gehl, 2003; Glover et al., 2005; Mehier-Humbert and Guy, 2005; Liu et al., 2006; Al-Dosari and Gao, 2009; Boukany et al., 2011; Guo and Huang, 2012; Mellott et al., 2013; Wang et al., 2013; Song et al., 2015; Tschon et al., 2016; Tsuchiya et al., 2017; Vroomen et al., 2017; Kawai et al., 2018; Melancon et al., 2018; Shapiro et al., 2018; Shi J. et al., 2018; Tang W. et al., 2019; Bono et al., 2020) (Table 1). Over the years, electroporation has also been applied for in vivo application, with most applications for preclinical models in skin (Jafari et al., 2018; Pasquet et al., 2018), lung (Gazdhar et al., 2006; Gazdhar et al., 2007) heart (Ayuni et al., 2010; Hargrave et al., 2014; Sugrue et al., 2020) diaphragm (Beshay et al., 2009), liver (Heller et al., 1996; Kobayashi et al., 2003), tumor (Goepfert et al., 2011), cornea (Zhou and Dean, 2007), retina (Matsuda and Cepko, 2004; Lirong et al., 2014), brain (Inoue and Krumlauf, 2001; De Fry et al., 2010; Nomura et al., 2016), artery and muscle (Zhang et al., 2001; Molnar et al., 2004; Tavakoli et al., 2006; Sokołowska and Błachnio-Zabielska, 2019).
In vivo electroporation is dependent on various parameters. Therefore, studies have been conducted over to optimize the electrical impulse protocol (voltage, number, and type of pulses), estimation of the interval between the injection of therapeutics and the delivery of electrical pulses, electrode geometry and tissue properties to increase the efficiency of electroporation (Satkauskas et al., 2012; Haberl et al., 2013; Shi B. et al., 2018; Hyder et al., 2020). The mechanism of electroporation mediated nucleic acid and drug delivery is still under investigation. However, detailed research shows that it is a multistep process and involves (i) permeabilization of the plasma membrane under the influence of an electric field, (ii) migration of the DNA/drug toward membrane by electrophoretic forces (iii) and translocation across the membrane. Importantly the mechanisms studied in vitro cannot be exactly transferred for in vivo electroporation. However, it is agreed that under the influence of an electric filed the cell membrane is being electropermeabilized, which leads to electrophoretically driven migration of nucleic acids and drugs through the target tissue. Therefore, high voltage (HV) and low voltage (LV) pulses have been studied, and their effects have been tested for electropermeabilization.
Various electrodes are used depending on the target site and are of different size shapes and made of different materials. Most commonly, the electrodes are made of stainless steel, copper, titanium, and they differ in their electrical conductivity, price, and corrosion (Rebersek et al., 2014). For the clinical purpose, electrodes made of stainless steel and titanium are used. Recent recommendations suggest using electrodes with a gallium core so that they can absorb the heat generated and thus protect the tissue (Kotnik et al., 2001; Arena et al., 2013). The most commonly used electrodes are either plate electrodes or needle array electrodes. Furthermore, nanochannel-based electroporation has been reported for various applications in Orthopedic research (Boukany et al., 2011; Geng and Lu, 2013; Wang and Lee, 2013; Xie et al., 2013; Gao et al., 2014; Chang et al., 2016; Gallego-Perez et al., 2016, 2017; Shi J. et al., 2018) (Table 1).
Extracellular Vesicles (EVs) are cell-derived, lipid membrane enclosed nanoscale particles capable of packaging proteins, lipids, and genetic cargo such as DNA and various RNAs as summarized by O’Brien et al. (2020). They are used for intercellular communication and are excreted by nearly all cells in the body leading to their isolation from most bodily fluids including blood, urine, saliva, amniotic and synovial fluids via ultracentrifugation (Simpson et al., 2008; Andaloussi et al., 2013; Properzi et al., 2013; De Jong et al., 2014; Mulcahy et al., 2014; Lamichhane et al., 2015; Yáñez-Mó et al., 2015; Tkach and Théry, 2016; Maas et al., 2017; Xie et al., 2017; Diomede et al., 2018; Li et al., 2018; van Niel et al., 2018; Marolt Presen et al., 2019; Pizzicannella et al., 2019; Trubiani et al., 2019). Historically, they have been categorized into three main classes mainly based on particle size and biogenesis: Exosomes (40–120 nm) via endolysosomal pathway, Microvesicles/Microparticles (50–1,000 nm) via budding from plasma membrane, and Apoptotic bodies (1–5,000 nm) via blebbing from plasma membrane (Andaloussi et al., 2013; Rilla et al., 2019). However, overlap in the size of these vesicular bodies along with their heterogeneous population, has resulted in interchangeability between the nomenclature (Kowal et al., 2016; Tkach et al., 2017). Thus, micro-vesicles and exosomes will be referred to as EVs in this review.
In general, EVs consist of a lipid bilayer membrane composed of tetraspanins (CD9, CD63, CD81, CD82), integrins, and cell-specific receptors for cell-to-cell communication and internal cargo as described in Wu et al. (2019). Their composition allows for the transmission of proteins, bioactive lipids, and genes, which can alter the function and phenotype of target cells (Andaloussi et al., 2013). Besides, different surface molecules can facilitate ligand-receptor signaling for targeting, adhesion, and fusion to the recipient cell (Boere et al., 2018). Cell-derived EVs can be engineered to carry exogenous genes as a non-viral delivery system as described by Gallego-Perez et al. (2017) via generating EVs from autologous mice fibroblasts and reprogramming them with a cocktail of exogenous of transcription factors into neuronal and endothelial cells. Furthermore, MSC-derived EVs have received growing interest due to their therapeutic potential for joint diseases such as OA and periprosthetic infections, and further characterization of specific therapeutic genetic factors will produce EVs with enhanced regenerative potential (Wu et al., 2019; Rüwald et al., 2020). Thus, these EVs can be engineered both via modification of genetic cargo (electroporation, lipofectamine, siRNA, etc.) or alteration of the EV surface proteins for desired targeting and gene delivery, as summarized in two recent reviews (Sutaria et al., 2017; Mentkowski et al., 2018). EVs offer benefits over conventional delivery systems such as polymers and liposomal systems in terms of stability, immunogenicity, and biocompatibility. Since EVs are generated from innate cells of the body, their size and membrane composition allow for avoidance of degradation in vivo through pathways such as lysosomal degradation, endosomal pathway, phagocytosis, or degradation by macrophages as reviewed in Ha et al. (2016). Their small size allows for long term systemic delivery along with the ability to cross the blood-brain barrier and deliver genetic cargo directly into target cell cytosol with high efficiency (Kooijmans et al., 2012; Tran et al., 2015). As EVs are generated from almost all cell types, they are abundant in quantity and can be derived from desired cell types to contain surface markers for cell-specific targeting. They also have advantages over cell therapy due to decreased immunogenicity compared to parent cells because of lower trans-membrane MHC proteins and longer shelf life (Ong and Wu, 2015). Despite these advantages, there are some technical and biological challenges still associated with EVs.
Firstly, there are many underexplored areas in EV research, such as their population heterogeneity, differences in isolation methods, and reproducibility, as described in O’Brien et al. (2020). Heterogeneity in EVs can differ between sample to sample as well as within batches due to differences between cell types, culture conditions, and lack of determining specific biomarkers (Nolte-’t Hoen and Wauben, 2012). Isolation methods also vary amongst the field, resulting in heterogeneously isolated EVs with inconsistent naming conventions and make reproducibility difficult (Malda et al., 2016). Besides, their small size also poses disadvantages, as there may be undesired systemic circulation of the generated EV throughout the body. Contradictory findings have also been observed demonstrating the complex nature of EVs such as MSC-derived EVs that both inhibit and promote tumor growth, although EVs themselves do not exhibit the ability to form tumors (Zhu et al., 2012; Bruno et al., 2013).
Current EV related research has primarily focused on MSC derived exosomes, and many are in clinical trials for the treatment and repair of soft tissues (Mendt et al., 2019). Very recently, a systematic review has been conducted on the application of EV to regenerate cartilage using MSCs (To et al., 2020). In these models, all studies that involved MSC-EVs reported less loss of cartilage with the implementation of EVs compared to placebo (To et al., 2020). MicroRNA delivery using EVs has also been a large area of interest. It has shown effects on cell migration, angiogenesis, cell proliferation, and osteogenic differentiation of target cells as summarized in O’Brien et al. (2020). Current research on exosomes/EVs is focused on innate EVs without engineering and their treatment of target cells/tissue. In terms of gene delivery using EVs for orthopedic tissues, this is an unexplored area of research. Thus, EVs demonstrate significant therapeutic potential for non-viral gene delivery due to their intrinsic biocompatibility, low immunogenicity/cytotoxicity, stability, diverse cargo, and engineering capacity. However, there is more elucidation desired before EVs can be used as a gene delivery vehicle in the clinical setting.
Synthetic polymers, both degradable and non-degradable, have several characteristics that make them suitable for gene vector delivery, including biocompatibility, low immunogenicity, high affinity for nucleic acids, improved stability in biological fluids, and the ability to be engineered to mediate cellular entry and endosomal escape (e.g., via hydrophobic modifications) (Anderson and Shive, 1997; Prokop et al., 2002; Pack et al., 2005; Patil et al., 2019). Moreover, their tunable properties and molecular flexibility enable functionalization with specific targeting moieties to favor cell-specific uptake, or conjugation with fusion tags to confirm successful gene delivery (Eliyahu et al., 2005; Guo and Huang, 2012; Foldvari et al., 2016; Patil et al., 2019). Cationic synthetic polymers such as polyamidoamine (PAMAM) dendrimers, polyethyleneimine (PEI), poly[2-(dimethylamino)ethyl methacrylate] (PDMAEMA), poly-Lysine, and polyamidoamine-epichlorohydrin (PAAE), have been widely used for gene delivery applications due to their positive charge, which facilitates genetic cargo loading mediated by their electrostatic interaction with the negatively charged nucleic acids, as well as cellular uptake (De Laporte et al., 2006; Basarkar and Singh, 2007; Patil et al., 2019). Although polycationic-based vectors, such as PEI and PAMAM dendrimers have shown to be effective vehicles for siRNA and miRNA delivery, their highly positive charge may lead to non-specific interactions with the negatively charged phospholipid membrane of circulating cells after systemic delivery (Guo and Huang, 2012; Patil et al., 2019). Cationic polymers can also be modified to modulate their binding strength to the genetic material to achieve successful nucleic acid transfer while still providing viable protection from enzymatic degradation (Jones et al., 2013). The stability of these synthetic polymers can be significantly influenced by their molecular weight, where small complexes with lower molecular weight can be more unstable under physiological conditions, resulting in molecular cargo unpacking, degradation, and clearance (Su et al., 2012). As these small complexes require higher concentrations to achieve adequate gene regulation, if not correctly stabilized, they can aggregate and form larger complexes that can accumulate overtime in organs such as the lung and liver, which significantly impacts cell/tissue function and leads to higher toxicity (Su et al., 2012). A similar phenomenon can be observed for synthetic polymers with higher molecular weight (>25 KDa) (Su et al., 2012). This issue can be addressed by introducing specific surface modifications, such as a PEG-conjugation, to improve steric stabilization and reduce unwanted interactions with salts and other charged or neutral particles present in the circulation (Pack et al., 2005; Su et al., 2012; Jones et al., 2013). Polymers, on the other hand, can help to overcome these limitations by preventing accumulation of the carrier as the genetic material is delivered. For this type of polymer, size and degradation rate can be optimized to favor rapid intracellular delivery (Ditto et al., 2009).
Viral vectors have developed into the gold standard for modulating gene expression in vivo thanks to their high transfection efficiency and ability to bypass endocytosis to enter the cytosol, especially when compared to synthetic transfection methods such as lipo/polyplex-based carriers (Ziello et al., 2010). However, although promising to obtained stable (when using adeno-associated viruses) or transient (when using adenoviruses) transfection of cells, viral vectors present significant limitations due to the persistent risk of triggering immune reactions which hinders the ability for redosing, limited size of the molecular cargo due to capsid size restrictions, and potential biosafety concerns for clinical applications (Daya and Berns, 2008; Joshi et al., 2017). To overcome these limitations many non-viral physical and chemical/biological transfection methods have been developed (e.g., electroporation-based approaches, synthetic nanocarriers, and electro exosomes/EVs) (Wu et al., 2013). However, some of these methods are still limited for example by low stability in biological fluids for synthetic nanocarriers, low transfection efficiency and electro-toxicity for some electroporation-based methods such as bulk electroporation since the entire cell surface is exposed to a high-intensity electric field, and nanocarrier (i.e., gold/tungsten) toxicity for biolistic transfection methods (Al-Dosari and Gao, 2009; Boukany et al., 2011; Wang and Lee, 2013). Nanochannel-based electroporation approaches have emerged as a potent tool to circumvent these limitations. In this type of technology, nanochannel membranes are used to focus a high-intensity electric field applied to the cell membrane, where only the cells in contact with the nanochannels are porated, and the electric field is only applied to a very small portion of the cell membrane equivalent to the area of the nanochannel. This feature improves cell viability and leads to a larger transmembrane potential with enhanced transfection efficiencies and closer control over molecular cargo transfer with a highly deterministic transfection profile, compared to the stochastic profile observed when using bulk electroporation (Boukany et al., 2011; Gallego-Perez et al., 2016). More recently, Gallego-Perez et al. (2017) have used the same governing physical principles to enable transfection of tissues in vivo via Tissue Nano-Transfection to induce direct cell reprogramming for regenerative applications (Gallego-Perez et al., 2017). Table 1 provides an overview of advantages and limitations for several widely used non-viral gene delivery techniques, such as electroporation (Gehl, 2003; Wells, 2004; Glover et al., 2005; Lin et al., 2005; Liu et al., 2005, 2006; Mehier-Humbert and Guy, 2005; O’Brien and Lummis, 2006; Al-Dosari and Gao, 2009; Boukany et al., 2011; Tzeng et al., 2011; Guo and Huang, 2012; Mellott et al., 2013; Wang et al., 2013; Ding et al., 2014; Song et al., 2015; Das et al., 2016; Tschon et al., 2016; Tsuchiya et al., 2017; Vroomen et al., 2017; Kawai et al., 2018; Melancon et al., 2018; Shapiro et al., 2018; Shi J. et al., 2018; Pomatto et al., 2019; Tang S. et al., 2019; Bono et al., 2020), Nanochannel-based electroporation (Boukany et al., 2011; Geng and Lu, 2013; Wang and Lee, 2013; Xie et al., 2013; Gao et al., 2014; Gallego-Perez et al., 2016; Chang et al., 2016; Gallego-Perez et al., 2017; Shi J. et al., 2018), Sonoporation (Mehier-Humbert and Guy, 2005; Sheyn et al., 2008a; Al-Dosari and Gao, 2009; Wang et al., 2013; Balmayor and van Griensven, 2015; Kawai et al., 2018; Bono et al., 2020), Biolistic gene delivery (Gene gun) (Kitagawa et al., 2003; Zhu et al., 2004; Al-Dosari and Gao, 2009; Su et al., 2012; Wang et al., 2013; Bono et al., 2020), engineered EVs (microvesicles and exosomes) (Andaloussi et al., 2013; De Jong et al., 2014; Mulcahy et al., 2014; Lamichhane et al., 2015; Yáñez-Mó et al., 2015; Tkach and Théry, 2016; Maas et al., 2017; Xie et al., 2017; Diomede et al., 2018; Li et al., 2018; van Niel et al., 2018; Marolt Presen et al., 2019; Pizzicannella et al., 2019; Trubiani et al., 2019).
Articular cartilage degeneration is a severe pathology and affects about three out of 10 people worldwide (Evans and Robbins, 1999; Wittenauer et al., 2013). There is an increase in interest to deliver gene therapy to the cartilage to rescue or activate remaining chondrocytes or to drive MSCs toward chondrocytes (Evans and Robbins, 1999; Huizinga, 1999; Burstein, 2001; Im, 2016). The clinical problem is that hyaline cartilage cannot be easily regrown ex vivo, although the chondrocytes can be expanded after isolation. However, the quality of the matrix that these cells produce differs from native tissue and with inferior biomechanical properties (Gelse et al., 2003). Most methods that have been proposed so far involve the removal of chondrocytes and the ex vivo cell expansion, and then in a second step, the cells will be treated with non-viral gene delivery approaches, such as TGFβ, other Bone Morphogenic Proteins (BMPs), or other anabolic genes such as insulin-like growth factor-1 (IGF-1) (Saraf and Mikos, 2006). It has also been shown that autologous chondrocytes seem challenging for successful transfections and other cell sources as adipose or bone-marrow-derived MSCs may be more promising (Heyde et al., 2007). Addressing anti-inflammatory pathways by incorporation of IL-10 or similar cytokines has been tested with promising results (Khoury et al., 2006).
Lipids were successfully used in a three-step method to achieve high efficiency of transfection by combining permeabilization of primary cells with a mild detergent, by association of pDNA with a polycationic (poly-L-lysine) core covalently linked to a receptor-ligand (transferrin) and addition of cationic liposomes (Goomer et al., 2001). Transfection efficiencies using lipofection reached 40% after 36 h (Stöve et al., 2002). Gene delivery for tissue, which is rich in GAGs, collagens, and other extracellular matrices (ECM) components seems particularly challenging for in vivo delivery of DNA. Noteworthy, non-viral gene delivery with FITC-labeled chondrocyte-affinity peptide (CAP) conjugated PEI/DNA particles was investigated in a rabbit knee joint OA-model (Pi et al., 2011). These authors found that by using the CAP-motive that the integration of the PEI/DNA was much more efficient than with placebo. Many more studies were undertaken based on in vitro primary cultures (Odabas et al., 2013; Raftery et al., 2016) using rabbit or bovine-derived chondrocytes or even patient-derived chondrocytes. Recently, chondrogenic differentiation was induced from induced pluripotent stem cells (iPSC) using non-viral mini-circle vectors (Rim et al., 2020). The various approaches for cartilage repair to treat rhematoid arthritis (RA) were recently summarized by Pirmardvand Chegini et al. (2018). Here, mainly anti-inflammatory genes like IL-1, IL-6, and IL-10 were influenced by vector transfer. A prominent inducer for the regeneration of cartilage, i.e., SOX9, delivered in non-viral approach has been shown as a promising strategy (Song and Park, 2020). Also, here a wide range of studies used liposome-based methods to transfect primary chondrocytes and MSCs (Goomer et al., 2001; Stöve et al., 2002; Sun et al., 2009).
Recently, Gonzalez-Fernandez et al. (2017) found that if MSCs were transfected with different gene carriers that the morphology of MSCs was highly influenced by the application of different categories of vectors. Generally, studies tried to modulate and activate gene expression of differentiated chondrocytes and/or MSCs. Target genes of interest were SOX9 and collagen type X among others. It was shown that gold-nanoparticles were found to be very efficient to transfer genes to cartilage (Pirmardvand Chegini et al., 2018).
Nucleofection through electroporation (EP) has been applied successfully on primary chondrocytes in a high throughput format (Haag et al., 2009). Earlier electroporation has been evaluated in cartilage by Mir et al. (2005) among other tissues to test regenerative effects in cartilage. A more systematic comparison to address whether local administration versus systemic gene electrotransfer (ET) could be more successful would be to apply anti-inflammatory plasmids (Khoury et al., 2006). They found in a mouse OA-model that intra-muscular application of ET was more efficient than intra-articular ET, which is unexpected, given the local administration of the vector to the site of action.
Extracellular vesicles were used successfully to thrive differentiation of MSCs toward chondrocytes in vitro and in vivo (see also chapter on EVs) (To et al., 2020). It has been shown that cell-derived EVs are involved in the pathogenesis of OA, playing important roles in antigen presentation, inflammation, angiogenesis, cell–cell signal communication, thrombosis, and articular cartilage ECM degradation (Fu et al., 2018; Rilla et al., 2019). It could be shown that even up-regulation of autophagy is involved in the release of EVs in bovine and human degenerated chondrocytes (Rosenthal et al., 2015). It also has been shown that their specific interactions exist between the ECM proteins of articular cartilage and matrix EV’s proteins (Wu et al., 1992). In chondrocytes (but also for osteoblasts and tenocytes) EVs play a key role in the induction of matrix mineralization, these are called matrix vesicles (MVs) (Anderson, 2003). Thus, MVs are involved in the onset of calcification in painful OA-joints (Jubeck et al., 2008). Chondrocytes have been proven in vitro to transfer EVs to MSCs in co-culture (Kim et al., 2019). On the other hand, EVs from MSCs activate chondrocytes and lead to an improved ECM (Kim et al., 2019). It was further shown experimentally that cellular proximity was needed to induce EV-associated regenerative effects. Thus, EVs seem to be the perfect vehicle to transfer DNA or RNA as these have been proven to exist naturally, and some do even contain miRNA (Lin et al., 2018).
To summarize, there were many studies conducted in the area of cartilage repair (∼700 in PubMed, starting from 1986 to the present). Unsolved issues concern how EVs interact with components of the ECM of cartilage. It seems clear that hyaluronic acid (HA) and GAGs, such as chondroitin sulfate, are involved in the regulation of EVs and MVs activity (Rilla et al., 2019). Of great interest in the field of cartilage repair is the ability of EVs to transfer bioactive cargo between cells and influence phenotype and behavior directly upon uptake (Gerlach and Griffin, 2016). The EV-mediated delivery of active contents, including cytoplasmic and membrane proteins as well as nucleic acids, and in particular miRNA sequences, has been demonstrated (Gerlach and Griffin, 2016; Rilla et al., 2019). Of specific interest is HA, which interacts via CD44 receptor, and thus could be used as a potential non-viral gene delivery system for chondrocytes. In vivo, particular challenges persist in overcoming the barriers of GAG and other ECM components to reach the chondrocytes with EVs or other non-viral vectors.
The IVD is the largest avascular and aneural organ in the human body. It is a joint between adjacent vertebrae in the spinal column and facilitates flexion, extension, and rotation of the spine while relying on the diffusion of nutrients through the cartilage endplate of the vertebral body (Urban et al., 2004; Heuer et al., 2008). As a consequence of the avascular nature of this tissue, the healthy mature disk is relatively acellular; few cells existing within a dense ECM of proteoglycans and collagen (Humzah and Soames, 1988). During aging and degeneration, there is a decline in matrix biosynthesis and cellularity, together with an increase in catabolism and inflammation resulting in a loss of IVD structure/function (Antoniou et al., 1996; Le Maitre et al., 2007). These changes create a hostile microenvironment for regenerative strategies that focus on restoring structure and function to the joint while reducing the underlying mechanisms of disease. This, together with logistical and regulatory challenges, pose significant barriers to the success of therapeutic strategies for the IVD, specifically: (i) the lack of continuous drug delivery systems, (ii) reduced sustained cell viability in the hostile microenvironment of the IVD or (iii) regulatory and safety hurdles in the case of viral gene editing that permanently integrates with host DNA which may cause off-target mutations. Current biological strategies for disk repair to date have focused on growth factors, anti-inflammatory drugs, stem cell therapy (adult mesenchymal and iPSC) and viral gene delivery (Sakai et al., 2006; Orozco et al., 2011; Gorth et al., 2014; Hodgkinson et al., 2019) with limited long-term efficacy and safety due to many of the barriers stated above. Non-viral gene delivery strategies for treating the degenerate and painful IVD are receiving increasing attention given their potential for sustained effects on the innate IVD cell phenotype of interest in situ; however, this is still an emerging field with relevant studies discussed below, described in Table 2 and categorized based on their mode of delivery.
Lipid-based gene delivery systems were amongst the first non-viral methods used to investigate the effects of gene transfection on IVD cells. Morrey et al. (2008) screened several lipid-based non-viral agents for gene delivery in human degenerative IVD cells in vitro focusing on efficiency, safety, and optimal dose. Out of the seventeen agents assessed, they identified “LT1” as the most efficient and least toxic when compared to other lipid-based agents. When culture medium without antibiotics, buffers, and amino acids was used, including hyaluronidase pre- and post-transfection, these changes to the transfection protocol increased efficiency while maintaining viability. Yet, when compared to the adenoviral associated gene delivery controls, LT1 transfection was significantly less efficient than viral delivery, warranting a need for further optimization of these transfection methods.
Lipofectamine has been used to transfect nucleus pulposus cells with either DNA plasmid vectors as well as small interfering RNAs (siRNAs) in vitro. To determine the potential of siRNAs to knockdown gene expression in nucleus pulposus cells isolated from rats and human patients with scoliosis, these cells were co-transfected with reporter luciferase plasmid Firefly and its corresponding siRNA using lipofectamine (Kakutani et al., 2006). The expression of Firefly luciferase was reduced by 94.7 and 93.7% in rat and human nucleus pulposus cells respectively. This demonstrates successful knockdown of “Firefly luciferase” that was maintained for 2 weeks, however, significant decreases in nucleus pulposus cell proliferation were observed compared to the fibroblast control and inhibitory effects of knockdown disappeared by 3 weeks. To investigate the effect of siRNAs on silencing a relevant target associated with disk degeneration, Sudo and Minami (2011) transfected rabbit nucleus pulposus cells with Caspase 3 siRNA in vitro and in vivo using lipofectamine or “invivofectamine” reagent complex, respectively. Significant decreases in apoptosis in vitro and suppression of degenerative changes as observed on MRI and histologically were noted in vivo with non-viral delivery of Caspase 3 siRNA. In addition to the non-viral delivery of siRNAs, lipofectamine has been used in vitro to transfect ovine nucleus pulposus cells with a plasmid vector containing human telomerase reverse transcriptase (hTERT) to examine effects on cellular lifespan (Chung et al., 2007). hTERT significantly increased telomerase activity, lifespan, and collagen I and II expression relative to vector controls, however, karyotype instability suggested further studies are necessary to validate the safety of this strategy.
Lipid-based vectors such as liposomes have been used to transfect multiple siRNAs into cells in vitro and in vivo. Transfection of liposomal siRNA for Caspase 3 and A Disintegrin and Metalloproteinase with Thrombospondin motifs-5 (ADAMTS5) was first optimized in a human hepatocellular carcinoma cell line in vitro followed by injection of Caspase 3 and ADAMTS5 siRNA alone or in synergy into a rabbit IVD puncture model (Banala et al., 2019). The liposomal siRNA formulations for Caspase 3, including the combined synergy groups, were able to limit IVD degeneration in vivo as demonstrated by MRI and histopathology with the limited effect of ADAMTS5 siRNA treatment alone suggesting that the ADAMTS5 siRNA was ineffective at suppressing ADAMTS5 expression. The studies described above highlight the potential of lipid-based transfection and vector-based systems to deliver genes and gene targets successfully to IVD cells in vitro and in vivo, however, given the limitations associated with transfection efficiency and the few gene targets that have been assessed so far, warrants further optimization of these methods with a diverse array of gene candidates.
Synthetic polymer-based gene vectors are attractive alternatives for non-viral gene delivery when compared to viral vectors as they demonstrate low immunogenicity, have tunable structural and surface components, and can be synthesized on a large scale at relatively low-cost (Pack et al., 2005). A limited number of studies have explored the potential of such polymer-based non-viral gene delivery systems to treat IVD cells in vitro and in vivo. Feng et al. (2015) developed an elegant system to therapeutically deliver pDNA by combining cationic block polymers polyethyleneglycol (PEG)-block-poly (N-[N-(2-aminoethyl)-2-aminoehtyl]aspartamide) [PEG-b-PAsp(DET)] and poly(N-isopropylacrylamide)-block-PAsp(DET) [PNIPAM-b-PAsp(DET)], which they termed “mixed polyplex micelles” (MPMs). These MPMs demonstrated high resistance to nuclease activity and protein absorption including significantly higher gene transfection efficiency in nucleus pulposus cells when compared with single block polymers [PEG-b-PAsp(DET)] in vitro and in vivo. Furthermore, when MPMs were loaded with heme oxygenase-1 (HO-1), an anti-oxidant and anti-inflammatory, and used to treat nucleus pulposus cells previously stimulated with IL-1β in vitro, decreases in matrix metalloproteinase 3 (MMP3) and cyclo-oxygenase-2 (COX-2) were observed. These effects were reproduced in an IVD degeneration rat tail model where MPMs loaded with HO-1 were more effective an decreasing the inflammatory response and restoring glycosaminoglycans (GAG) when compared to the single block polymer loaded vectors. The authors of this study went on to develop new synthetic polymer-based non-viral gene delivery systems for treating IVD degeneration. One involved nano-sized polyplexes that self-assemble into a double-shell structure, which are then encapsulated in biodegradable nano-spheres and co-injected with nanofibrous spongy microspheres, providing a two-stage delivery system with both temporal control and highly efficient delivery of pDNA (Feng et al., 2017). This system was used to successfully deliver the gene encoding anti-fibrotic agent, orphan nuclear receptor 4A1 (NR4A1) to the IVD in vivo, and limit fibrosis in a rat tail model of disk degeneration. In a more recent study, Feng et al. (2018) developed an injectable MMP-degradable hydrogel encapsulating MMP-responsive polyplex micelles for continuous and bioresponsive delivery microRNA-29 to limit fibrosis and reduce degeneration in an in vivo rabbit puncture model of IVD degeneration. These polyplex non-viral systems described above highlight the potential of synthetic polymers to successfully deliver genes of interest to the degenerate IVD using a variety of small animal models (rat and rabbit) and gene targets with high efficiency and low cytotoxicity. The next steps could include longer-term studies (>12 months) and scaling-up to relevant larger animal models of IVD degeneration such as the sheep, goat, or dog.
Physical methods for non-viral gene delivery offer a safe and feasible way for transfecting large quantities of cells in vitro. Studies by Bucher et al. used Nucleofector technology to electroporate human MSCs with growth factor differentiation factor 5 (GDF5) to transplant these cells in a degenerate bovine IVD organ culture model (Bucher et al., 2013). Monolayer cultures of transfected MSCs expressed GDF5 for up to 3 weeks. When GDF5 transfected MSCs were seeded in alginate beads, key IVD markers ACAN, SOX9, and KRT19 were up-regulated in these cells compared to untransfected cells. When GDF5 transfected MSCs were injected within a PEG hydrogel suspension into the bovine IVD organ culture papain degeneration model, a partial recovery of GAG/DNA was observed after seven days. In a more recent study, May et al. (2017) have used the Neon transfection system to validate parameters of voltage, number and duration of pulses for electroporation mediated gene transfer in bovine and human IVD cells. They determined successful transfection (≥47% efficiency) of commercially available plasmid pCMV6-AC-GFP by flow cytometry with a protocol of two pulses of 1400V for 20ms in bovine and human nucleus pulposus and annulus fibrosus cells. The effect of transfecting GDF6 was examined using this protocol and system; however, due to potential limitations with the specific GDF6 plasmid used, no increase in ECM proteins could be observed. Tang et al. used this same Neon transfection system to examine the effect of electroporating developmental transcription factor Brachyury into human nucleus pulposus cells from cadavers and patients undergoing surgery for low back pain in 3D in vitro culture (Tang S. et al., 2019). In this study, significant increases in Brachyury were observed up to 4 weeks, together with improvements in IVD phenotypic markers FOXF1, KRT19, and SOX9 and decreases in inflammatory/catabolic/pain markers IL1−β, IL-6, NGF, and MMP-13 compared to transfected sham vector control cells. Besides, significant increases in glycosaminoglycan accumulation were observed, suggesting that Brachyury was able to reprogram degenerate nucleus pulposus cells to a healthier pro-anabolic phenotype, however, since some effects appeared transient, further optimization of the protocol was deemed necessary.
The studies described above highlight the potential and feasibility of using bulk electroporation to deliver genes to IVD cells non-virally. An alternative physical method that has been investigated is Microbubble-Enhanced Ultrasound Gene Therapy. GFP and firefly luciferase reporter plasmids were mixed with microbubbles of ultrasonography contrast agent and injected into the IVDs of rat tails in vivo (Nishida et al., 2006). Therapeutic ultrasound was applied to the surface of inserted disks, and the IVD was isolated at 1, 3, 6, 12, and 24 weeks post-injection. Transgene expression was observed up to 24 weeks in the IVD however, overall declined with time suggesting that, while a potentially promising method, further validation of this technique may be necessary.
Physical non-viral transfection of pDNA is an attractive method for delivering genes of interest to the IVD. Electroporation shows promise for in vitro gene delivery, however, a direct translation of this method for use in vivo in relevant animal models of disk degeneration is more challenging, and this is where other physical techniques such as ultrasound could be used.
The therapeutic potential of exosomes and EVs is a new and emerging field. With respect to the IVD, exosomes derived from both human MSCs and nucleus pulposus cells have been shown to promote ECM biosynthesis and enhance IVD phenotypic markers when co-cultured with either nucleus pulposus cells or MSCs, respectively (Lu et al., 2017). A recent study by Cheng et al. (2018) has demonstrated the potential of MSC derived exosomes to deliver specific endogenous cargo in the form of microRNAs to nucleus pulposus cells in vitro and in vivo suggesting that exosomes could be engineered to deliver specific exogenous pDNA to IVD cells as a method of non-viral gene delivery.
Identifying non-viral gene delivery systems for the treatment of IVD degeneration is a research priority given the potential of gene therapy-based approaches to regenerate the IVD using discogenic growth factors, RNA interference/silencing and transcription factors and limitations associated with the use of viral vectors. While this is still a growing area for the IVD, the studies described above highlight the clinical applicability and relevance of these methods as safe and efficacious alternatives to viruses that warrant further investigation.
Bone tissue has the ability to repair and regenerate itself. Nonetheless, this capacity may be reduced or completely lost depending on the size of the defect (aka. critical size defect) or by the presence of specific disease states. They were going further from a healthy state of bone tissue results in clinical cases with an increase in morbidity and mortality (Vajgel et al., 2014). In this context, bone grafts are widely applied in a wide array of clinical settings to augment or induce bone regeneration and repair. Therapies currently used, such as allografts and autografts, involve numerous practical and clinical problems.
On the one hand, allografts have enhanced osteoinductivity and are relatively abundant in supply; nevertheless, they involve the potential risk to transmit disease. On the other hand, autografts are still considered as the “gold standard” for bone regeneration, as they can provide all the needed osteogenic components for bone repair. However, pain and morbidity at the donor site, a limited amount of available tissue, but also prolonged surgery are the main problems now facing this clinical approach. Nowadays, besides bone autografts and allografts, regenerative procedures are more focused on bone tissue engineering as an alternative using ceramics, polymers, and growth factors (Dimitriou et al., 2011). In combination with those scaffolds and biomaterials, factors inducing osteogenesis have been used to accelerate bone healing (Pereira et al., 2020). Many teams designed excellent delivery systems for growth factors; however, recombinant growth factors are expensive and onerous to produce (De Witte et al., 2018). Moreover, in an in vivo setting, high doses must be injected/administered to address the issues related to the brief half-life of the growth factors (Balmayor and van Griensven, 2015). In summary, we can say that protein delivery systems are still paved with many challenges, while gene therapy may provide a more suitable alternative.
Non-viral gene delivery/transfer is often performed using pDNA These circular, small, double-stranded DNA structures are stable, can be readily produced in bacteria and customized with a variety of different promoters (Gill et al., 2009). To be transcribed by the recipient cell, the pDNA has to reach the cell’s nucleus, and several barriers have to be overcome for this to occur. First of all, body clearance (in vivo) and degradation must be limited. Secondly, to be efficient, the pDNA has to cross both cell and nuclear membranes to enter the nucleus. Thirdly, the pDNA has to be released from any possible transfection complexes (Dang and Leong, 2006; Smith, 2008). To be efficient, a non-viral gene delivery is dependent on; (I) the DNA sequence, (II) preparation of the construct, (III) purification from bacterial expansion, (IV) the chosen transfection method, (V) the recipient cell type, and (VI) the cell cycle phase the recipient cells are in Table 3.
To deliver biologics to the bone fracture site to repair bone defects, gene therapy using gene vectors offers an attractive alternative method. At the delivery site, the target genes induce the production of potent growth factors (e.g., endogenous BMPs, VEGF) (Curtin et al., 2015), which is more efficient than exogenous delivery of recombinant proteins. Additionally, gene therapy induces in situ osteoblast differentiation, enhances osteoinduction via the expression of growth factors, and facilitates mineralized matrix production (Luo et al., 2005). Recently, non-viral gene delivery vectors, including lipids, peptides, dendrimers, and cationic polymers have been proposed as alternative strategies for gene delivery. This renewed interest is mainly attributed to their many advantages, such as the absence of endogenous virus recombination, their low immunogenicity, and tunable construction and easy fabrication (Pack et al., 2005; Mintzer and Simanek, 2009; Guo and Huang, 2012). Futhermore, many of these non-viral gene vectors have been used in clinical trials, combined with or without biomaterials (Li et al., 2016). In the following section, we summarize the most commonly used non-viral gene vectors and highlight their potential applications (Lechardeur and Lukacs, 2002; Ramamoorth and Narvekar, 2015) or more advanced ones (Pack et al., 2005; Mintzer and Simanek, 2009; Guo and Huang, 2012).
The most commonly used lipid-based delivery systems, e.g., FuGENETM and Lipofactamine 2000TM, have been widely used in research for several years due to their high and stable transfection efficiencies and commercially availability. On one hand, we can notably cite FuGENE6, which was used to transfer the gene TGF-β1, an osteoinductive growth factor into osteoblasts (Macdonald et al., 2007). After transfection, the osteoblasts demonstrated superior cell proliferation in comparison to cells treated with equivalent levels of recombinant TGF-β1 added to the culture medium. These results highlighted the advantages and efficiency of gene delivery instead of exogenous delivery of growth factors for bone tissue engineering (Macdonald et al., 2007). Lipofectamine 2000-based formulations have been used to deliver the oligonucleotide antimiR-138 to bone-marrow derived stromal cells (BMSCs) to form stem cell “patches.” When these sheets are applied to freeze-dried allograft bone, this induces massive bone regeneration with good vascularisation (Yan et al., 2014). Another example of lipid-based non-viral gene delivery system are the two molecules 1,2-dioleoyl-3-trimethylammonium propane (DOTAP)-2-dioleoyl-sn-glycerol-3-phosphatidylethanolamine and DOTAP-cholesterol. These two were used to deliver β-galactosidase plasmid to human and mouse osteoblastic cell lines (MG63 and MC3T3-E1, respectively). To increase the expression and efficiency of this delivery system, transferrin was incorporated into the system. The results demonstrated that this method had a higher efficiency in osteoblastic cell lines than in a human melanoma cell line (aka. 294T cell line). It also revealed a high correlation between lipid formulation, transfection activity, DNA dose, and charge ratios of the complexes (Oliveira et al., 2009; Yan et al., 2014).
Lipid-mediated gene transfer was one of the earliest strategies applied in gene therapy (Dwivedi et al., 2012), and positively charged liposomes were the first non-viral delivery vectors used in clinical trials (Li et al., 2015). Most of the time, to initiate bone progenitor cell differentiation and newly formed bone ossification, strategies have been focused on the delivery of genes encoding TGF-β and BMPs (Winn et al., 2005; Guo-ping et al., 2010). Another approach can be to target directly the master gene of bone differentiation (aka. runt-related transcription factor 2, RUNX2) with DNA plasmid encoding transcription factor RUNX2 loaded into liposomes and covalently immobilized onto polycaprolactone (PCL) nanofibers (Monteiro et al., 2014). Using BMSCs results showed that cells cultured with this setup showed a higher total protein synthesis and enhanced levels of metabolic activity (Table 3). However, even though liposome-based gene delivery was one of the first methods used to introduce exogenous DNA into eukaryotic cells, this method is not widespread in other fields like bone tissue engineering. This is possibly due to the involvement of cationic liposomes (lipoplexes), which are cytotoxic at higher concentrations (Tachibana et al., 2002; Madeira et al., 2010). For this reason, liposomes associated with scaffolds as a combined system should be used to deliver genes in a cell-controlled and spatially localized manner, for efficient bone tissue engineering applications.
Synthetic polymers can be also used as non-viral gene carriers as they can be endocytosed by cells. A variety of molecules that can differ in chemical composition, 3D architecture, weight, side-chain length, size, and branching, or even density, are available (Park et al., 2006). Most polymers described in the literature for gene therapy are cationic (aka. with a positive charge) with mainly amines groups (Santos et al., 2011). These positive groups interact with the negatively charged phosphate groups present in the DNA sequence and after association form condensed structures called polyplexes.
PEI, one of the first and most successful polyplexes used as non-viral gene vectors (Pack et al., 2005), was first introduced in 1995 both in vitro and in vivo (Boussif et al., 1995). PEI as a non-viral vector has several critical advantages over viral vectors; (I) it is less cytotoxic, (II) less immunogenic, (III) there are no carcinogenic concerns, (IV) it induces transient gene expression, and (V) it is safe for clinical use (Pack et al., 2005). Additionally, PEI has a high transfer efficiency (Akinc et al., 2005; Deng et al., 2009; Schafer et al., 2010) due to a phenomenon known as “proton sponge effect” (Benjaminsen et al., 2013). The transfection efficiencies are comparable with viral gene delivery agents (Abdallah et al., 1996). Numerous publications have highlighted the branched 25 kDa PEI polymer as the most widely utilized gene transfer agent (Huang et al., 2005a,b; Ali and Mooney, 2008) and as a “gold standard” (aka. positive control) across in vitro studies (Park, 2009). In brief, PEI combined with pDNA as polyplex have properties that can be changed by merely altering the PEI amines/DNA phosphates ratio. Higher ratios of PEI to pDNA usually result in higher transfection efficiencies, but the downside is an increased cytotoxicity (Boussif et al., 1995; Godbey et al., 1999). To optimize the use of polyplexes for gene transfer for bone tissue engineering applications, a balance between efficiency and cytotoxicity must be reached (Tierney et al., 2012).
To achieve the above, collagen scaffolds can be used to incorporate the complex branched PEI (25 kDa) with pDNA (Elangovan et al., 2014). The use of gene-activated scaffolds (with pPDGF-β) in a calvarial defect rat model, favored cell attachment and promoted cell proliferation in vitro. It was also described to promote osteogenesis (osteoinduction and osteoconduction) and demonstrated superior tissue regeneration when compared to empty scaffold and empty calvarial defect groups. Another documented polyplex is the combination of PEI (branched, 25 kDa)/pBMP-2, in association with a poly(ε-caprolactone) scaffold. This combination was applied to initiate in vitro differentiation of myoblasts (Reckhenrich et al., 2012). With optimized gene doses, cells increased the secretion of osteocalcin and osteopontin compared to the control group, demonstrating transdifferentiation of C2C12 cells into the osteoblastic lineage.
As a last example of polyplex, we can cite the advanced system consisting of dural plasmids, polyethyleneglycol (PEG)-block-catiomer (PEG-b-P[Asp-(DET)]) and a CaP-cement scaffold. This system has a high bio-compatibility rate with plasmids encoding osteogenic factors, activin receptor-like kinase 6 (caALK6) together with RUNX2 (Itaka et al., 2007). With this delivery system, osteogenic differentiation was enhanced compared to PEI or FuGENE6 (Itaka et al., 2007). Another study used branched PEI (25 KDa) with siRNA or miRNA to create complexes encapsulated within the PEG hydrogel, to deliver nucleic acids directly in situ. The goals of this study were to guide stem cells through osteogenic lineage with localized and sustained RNA release (Nguyen et al., 2014).
Natural polymers have been used due to their lower cytotoxicity and enhanced biocompatibility compared to synthetic polymers. Chitosan is one of the most studied natural polymers in bone tissue engineering (Raftery et al., 2013). Biodegradable and biocompatible, chitosan is formed by deacetylating chitin and can be used as a gel or as micro/nanoparticles (Moreira et al., 2009; Garcia-Fuentes and Alonso, 2012) to form complexes with pDNA. Compared with liposomes, the transfection efficiency of chitosan is always a little bit lower (comparable to naked DNA), but it is significantly less toxic than liposomes and easy to work with (Corsi et al., 2003). To overcome the problem of lower transfection efficiencies, chitosan is combined with other biomaterials. For orthopedic applications, it can be incorporated into titanium films with pDNA for BMP-2 or even incorporated in alginate hydrogel as nanoparticles (Park et al., 2007). In addition to chitosan, alginate has also been utlized for gene delivery. It has many advantages such as; (I) it is non-toxic, (II) bacteriostatic, (III) anti-inflammatory, (IV) biocompatible, and (V) form of nanoparticles or be combined with other hydrogels (Krebs et al., 2010). The use of alginate-mediated transfections with pDNA was characterized by high transfection efficiency, slow release kinetics, in vitro osteogenic differentiation, and in vivo bone formation (Wegman et al., 2011, 2014). It has been applied in bone tissue-engineering applications both in vitro and in vivo (Bourgeat-Lami, 2002; Stevens et al., 2005). Gelatin as another well-known natural polymer that has been widely used in bone tissue engineering as a delivery system for DNA and growth factors (Kasper et al., 2005; Kasper et al., 2006; Chew et al., 2011). In general, natural polymers, are often easy to work with, are readily available and rarely trigger immune responses. Yet they are not widely utilized gene-delivery systems for tissue engineering. Apart from polyplexes or lipoplexes, these natural polymers are also often combined with other materials such as ceramics or synthetic polymers to be closer to biomechanical, osteoconductive, and osteoinductive properties of the targeted tissue.
New studies have demonstrated the use of inorganic nanoparticles as a NVGD method (Bourgeat-Lami, 2002; Chowdhury and Akaike, 2005). These methods consist mostly of coupling small material particles such as iron oxide, silica, gold, or even calcium phosphate (CaP) with plasmid DNA. These particles deliver the pDNA into the cell via endocytosis. CaPs particles are favored in bone regeneration for their capacity to increase the strength and stiffness of the constructs. CaPs possess numerous advantages, which include; (I) excellent stability, (II) are biodegradable and biocompatible, (III) good solubility, (IV) good resorbability, (V) good binding affinity to DNA, and (V) efficient cellular uptake (Olton et al., 2007). CaPs present lower toxicity than carbon nanotubes, silica, magnetic particles, or quantum dots (Olton et al., 2007). CaP nanoparticles have also been combined with shRNA (Olton et al., 2007). When applied to human osteoblasts, this system showed efficient bone formation (Olton et al., 2007). Related to CaPs and known as the mineral component of bone Hydroxyapatite (HA) can also be used as a component of the NVGD strategy (Uskokovic and Uskokovic, 2011). Another related example, the nanohydroxyapatite (nHA) vector can deliver pDNA encoding for VEGF and BMP-2 to MSCs, and as a result, can markedly enhance bone healing and tissue vascularisation (Curtin et al., 2015). While these methods demonstrate some limitations such as moderate transfection efficiency and retention within the circulation, they do show several advantages, such as; (I) easy storage ability, (II) low toxicity, and (III) reasonable shape control. As a consequence, more and more studies are utilizing inorganic nanoparticles (Parveen et al., 2012).
Physical transfection methods involve permeabilization of the cell membrane, allowing pDNA to enter the cells. Different methods are used to permeabilize the cell membrane “in a safe way,” such as electroporation, which uses a high-intensity electric pulse. This method is not very often used but can present interesting results in the context of bone tissue engineering (Lee et al., 2019). With a transfection efficiency reaching 70–75%, BMP2 gene transduction using electroporation for the functional enhancement has been shown to enhance the in vivo osteogenic potential of human bone-marrow-derived mesenchymal stromal cells (hBMSCs) and adipo-tissue-derived stromal cells (ASCs), alone, or in combination with other factors (Hsieh et al., 2018).
Sonoporation disrupts the cell membrane using ultrasound to induce transfection. However, this method is not very successful and is considered as a highly experimental procedure since cell death is high. To compensate for the lower efficacy of this NVGD method, a highly osteoinductive co-expression strategy was investigated using BMP 2 and BMP-7 with significant results (Feichtinger et al., 2014) (Table 3). When sonoporation was directly compared with passive gene delivery, it demonstrated an increased probability of gene expression and bone formation related to the ultrasound energy applied. However, bone-related gene expression levels and bone volumes were not increased.
All physical methods, however, destabilize the cell membrane temporarily, which in many cases leads to low cell survival. The problem of those techniques is to search out the optimal conditions. One more difficulty is to reach deep into the tissue. These techniques are mainly capable of penetrating the skin and might maybe reach the adipose tissue and muscle just under the skin. However, bone cannot be reached with non-invasive methods, making it less optimal for orthopedic applications.
For all the non-viral gene therapy technics/approaches described above and applied in bone tissue engineering for bone regeneration, many hurdles need to be overcome as most of the techniques are based on particle uptake and controlled cell membrane damage. After described the techniques above, we can say that the main disadvantages of in vivo application are; (I) low penetration depth, (II) high levels of cell death and tissue damage, (III) chances of off-target effects, and (IV) risk of particle migration. Doing ex vivo transfections could be one way to overcome those issues. In that case, the DNA is not directly transferred into the body to the cells of interest; however, in a multiple steps protocol, the desired host cells are (I) isolated from the body, (II) transfected in vitro followed by a selection, and (III) and “grafts” back to the host to act as protein factories or directly as bone-forming cells. The two main advantages compared to in vivo transfections are the step pre-selection of the cells of interest and the post-selection of the transfected cells. This step of quality control of the used cells increases the safety of this NVGD strategy. As safety is one of the main concerns in bone regenerative medicine, the ex vivo NVGD model seems to be more potent at the moment in the context of clinical applications (Sheyn et al., 2008b, 2011; Lai et al., 2011). However, the harvesting of autologous cells arises with a disadvantage, with additional surgery and time-spending (Aggarwal et al., 2010).
Peptides, as the NVGD method, are generally used to enhance membrane activity and targeting ability. We can notably cite as an example, a paper where a system using PEG synthetic hydrogel, functionalized with a collagen-mimetic peptide (aka. GFOGER) (Shekaran et al., 2014). In this study, the hydrogel was applied to murine bone critical-sized defects, and the authors demonstrated that this functionalized hydrogel provided increased osteoprogenitor localization in the defect site, sustained in vivo release of encapsulated molecules, enhanced bone formation, and induced defect bridging. With respect to these results, this system demonstrated great potential for gene delivery despite being developed initially for BMP-2 delivery. In another study, TGF-β1 was delivered by a novel NVGD vector called (K)16GRGDSPC chemically linked to a bone scaffold made with PLGA. Applying this TGF-β1 functionalized scaffold to rabbit critical size bone defects significantly increased bone regeneration compared to control groups (Pan et al., 2014).
To combine many of the beneficial effects of NVGD methods, hybrid delivery systems can be an attractive approach, in particular, lipid and polymer integrated materials. PEI modified with linoleic acid and combined with different scaffolds such as collagen and gelatine as vehicles was used to study the expression levels of FGF-2 and BMP-2 after implantation in rat subcutaneous pockets (Rose et al., 2012). Another example, consisting of an organic/inorganic hybrid of pDNA-Lipoplex complex co-precipitated within apatite and loaded onto PLGA sheets, was investigated to integrate both osteoconductivity and osteoinductivity (Luong et al., 2009). Results demonstrated that the organic/inorganic hybrid resulted in improved transfection efficiency in all groups. To conclude, the co-precipitation of the DNA-lipoplexes within apatite also resulted in higher stability and better spatial distribution of DNA delivery (Luong et al., 2009).
Another option could be to combine natural and synthesized polymers to optimize NVGD systems. A NVGD consisting of a positively charged gene vector within gelatine microspheres and combined with a hydrogel of a crosslinked oligo (PEG-fumarate) (OPF) was used to investigate the effects of pBMP-2 in a critical-size rat cranial defect model on bone formation (Kasper et al., 2005). Surprisingly, there was a lack of improvement in bone regeneration, possibly due to an insufficient release of the DNA from the hydrogel (Kasper et al., 2006). Another team investigated the delivery of pBMP-2 using a biodegradable branched triacrylate/amine polycationic polymer (TAPP) that was combined with gelatine microparticles loaded within a porous tissue-engineered scaffold. In this study, they investigated the interplay between gelatine degradation, TAPP degradation, pDNA release, and mineralized matrix production in a rat calvarial critical-size defect model. The data showed that the hybrid composite scaffolds did not generate an enhanced bone regeneration in a critical-size rat cranial defect, as analyzed by microcomputed tomography and histology. These results claim, however, those polycationic polymers with a slow degradation rate can prolong the release of pDNA from composite scaffolds and suggest that gelatin microparticles comprising biodegradable polycationic polymers could be established to release pDNA in an intact polyplex form (Chew et al., 2011).
New approaches were emerging recently using the engineered matrices as a vector for targeted DNA construct, most of the time in the form of a plasmid. Multiple studies have shown that in vivo implantation of gene activated scaffolds/hydrogels/matrices/complexes at sites of bone defect was linked with expression of pDNA and retention for at least 6 weeks. This was followed by the induction of newly formed bone in a reproducible, stable, time-dependent, and dose-dependent manner (Bonadio et al., 1999).
NVGD methods stay in the focus of current research because of promising results in various areas of orthopedic research. We have shown that the clinical trials registered until now are mainly based in the area of bone, followed by hip, shoulder, and tendon for musculoskeletal diseases. However, the number of publications on non-viral gene delivery is not directional proportional to the interest in clinical trials in the different joints and tissues. It seemed that most literature was found for bone repair, followed by cartilage, IVD, and ligament approaches.
A common problem of all non-viral methods seemed to find promising solutions to deliver DNA or RNA with musculoskeletal specific cells in connective tissues. Significant conceptual differences exist between gene delivery methods to isolated cells in vitro and to in vivo or ex vivo to tissue. The current literature demonstrates the enthusiasm and powerful approach of non-viral gene systems to the areas of bone and joint diseases. The limited number of clinical trials related to non-viral gene delivery may also reflect some of the challenges that the field of gene therapy has faced over the past decade due to safety concerns related to viral vectors. However, as this review demonstrates, many NVGD methods have significant potential but require further protocol optimization or longer-term animal studies to determine their efficacy. Indeed it appears that efficacy and efficiency of the therapeutic strategy whether it is cell proliferation or structural restoration of soft or hard tissue, remains one of the significant challenges of NVGD systems. It is likely that a “one-shoe fits all” approach will not work for all orthopedic tissues, and a more targeted approach dependent on cell type, tissue composition/structure, and disease state/defect size will be necessary.
Cytotoxicity of viral vectors and the risk of host integration of these genomes, which might cause unpredicted gene mutations of the host genome, are clear contra-indicators for viral gene therapy. Conversely, non-viral gene therapy methods are on the rise, and here a tremendous variety of delivery methods exist, as we have listed in this review. In terms of clinical translation from in vitro to in vivo, a significant hurdle is transducing an adequate number of cells to enhance the therapeutic parameters of interest in the target tissue of interest. This can be challenging for orthopedic tissues that are relatively acellular such as the IVD and cartilage but might not be as difficult for repairing bone that is more cellular and vascularized. However, transfection efficiency has to be optimized while also taking into account any effects of cytoxicty, which has been observed for NVGDs such as cationic polymers and electroporation. On the flip-side, increased cellularity and vascularization of bone could lead to off-target and even unwarranted responses in other tissues but is likely not a problem for disk or cartilage. Disease state also needs to be considered when transitioning from in vitro to in vivo and the ability of NVGD systems to transduce cells within a degenerate tissue environment that is often catabolic and inflammatory. This may result in increased turnover, degradation and clearance of the NVGD system, limiting overall efficacy of the therapeutic strategy and optimizing NVGD systems to take these parameters into account, for example, creating polyplexes within MMP-degradable hydrogels for therapeutic release (Feng et al., 2018) or EVs that can package multiple genes targeting both tissue regeneration and inflammation. Also, to direct in vivo translation, ex vivo culture offers an alternative route whereby cells can be extracted, manipulated in vitro with NVGD systems, and then reinserted back into the patient, similar to what is currently being done for autologous chondrocyte implantation therapy (Krill et al., 2018). This circumvents problems around transfection efficiency in vivo. However, it often involves harvesting cells/tissue from healthy regions and also significant expansion time ex vivo.
In our view, on the side of carrier-based NVGD, the future research and potential lie in the areas of EVs in the combination of miRNA or lncRNA transmission that influence the host cells with specific functions. Here, we have seen tremendous potential, with many groups that are interested in how OA or IVDD could be targeted by transient modification of BMPs and or inflammatory genes or genes of the ECM, depending on the application in orthopedics. One of the key attractive features of NVGD is safety and low immunogenicity. Lipid-based vectors can be readily endocytosed, tissue-nano-transfection offers a safe and specific method to transfect single cells with high efficiency, polymers, both natural and synthetic can be hybridized to increase the efficiency of delivery and EVs can be generated from autologous cells packaged with a number of gene vectors.
On the side of carrier-free and physical methods how to overcome the cellular membrane, we found that electroporation (nucleofection) has been applied by many studies with relatively high efficiencies, both in vitro and in vivo directly on tissue. EVs are attractive NGVD systems as they demonstrate minimal immunogenicity, can be readily generated from autologous human cells in large quantities, can be endocytosed, and loaded with gene vector of interest. Furthermore, an interesting and exciting area is the use of tissue nano-transfection, which has high clinical value with the ability to transfect single cells in vivo and, in turn generating endogenous EVs with genetic cargo (Gallego-Perez et al., 2017).
For the IVD, NVGD methods have been primarily investigated in vitro with some studies using organ culture or in vivo rat or rabbit models. The type of vectors that have been investigated range from anti-inflammatory/fibrotic agents, siRNA targeting anti-apoptotic/catabolic enzymes, or discogenic growth factors and transcription factors. For the disk, specific considerations that apply include transducing a relatively acellular tissue. These particular tissue regions may require different vectors (NP versus inner or outer AF), ECM (negatively charged proteoglycans), and disease state. Most in vivo studies have focused on utilizing synthetic polymers with some success and therefore highlighting these NVGD methods (Feng et al., 2018). However, emerging/future areas that could be used by the IVD could include EVs which could be readily injected or tissue nano-transfection that could be applied directly to the disk surface. Furthermore, to truly assessing the safety and efficacy of NVGD methods for treating painful IVD degeneration and regenerating the IVD, utilizing relevant animals and assessing parameters that include pain behaviors seems paramount.
BG initiated the review, performed the major literature search on PubMed, wrote the sections on cartilage repair, drafted major parts of the MS, painted and created the figures, and provided the funding. ST provided the sections on EVs. DP provided the chapters on lipofection and IVD regeneration, language in the “Discussion and Conclusion” section, provided the funding, and edited the manuscript. JG provided the section on bone regeneration. AG provided the text on the non-viral gene therapy methods and electroporation. AC approved and edited the text. NH-C and AS-P provided the sections on the advantages and disadvantages of NVGD and introduction to synthetic polymer-based gene vectors. All the authors approved the final version of the manuscript.
Financial support was received from iPSpine H2020 project under the grant agreement #825925, the Swiss National Science Foundation Project 310030E_192674/1, and by an International Training Network (ITN) “Disc4all” under the grant agreement #955735, all assigned to BG. Financial support was also received from National Institutes of Health via NIAMS R61AR0767861 award assigned to DP and NH-C.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
We thank Eva Roth and Andrea Oberli for laboratory support for the non-viral gene delivery studies.
AAV, adenoviral vector; ADAMTS5, A Disintegrin and Metalloproteinase with Thrombospondin motifs 5; ASCs, adipo-tissue-derived mesenchymal stromal cells; BMPs, bone morphogenic proteins; CAP, chondrocyte-affinity peptide; CGMS, composites of cationized gelatin microspheres; GAG, glycosaminoglycans; GMP, good manufacturing practice; GDS, gene delivery systems; HA, hyaluronic acid; hBMSCs, human bone-marrow-derived mesenchymal stromal cells; hTERT, human telomerase reverse transcriptase; EP, electroporation; ECM, extracellular matrix; EVs, extracellular vesicles; FITC, fluorescein isothiocyanate; IVD, intervertebral disk; IVDD, intervertebral disk degeneration disease; LNPs, lipid nucleic acid nanoparticles; LTI, lysine-tri-isocyanate; MiRNA, micro-RNA; MMP, matrix metalloproteinase; MSC, mesenchymal stromal cell; MPMs, mixed polyplex micelles; MVs, matrix vesicles; N.D., non-determined; NVGD, non-viral gene delivery; OA, osteoarthritis; PAMAM, polyamidoamine dendrimers; pBMP-2, plasmid containing BMP-2; PDGF, platelet-derived growth factor; pDNA, plasmid DNA; PEI, poly-ethylene-imine; PLGA, Poly(D,L-lactide-coglycolide) lactide:glycolide acid; PTH, parathyroid hormone; siRNA, silencing RNA; VEGF, vascular endothelial growth factor.
Abdallah, B., Hassan, A., Benoist, C., Goula, D., Behr, J. P., and Demeneix, B. A. (1996). A powerful nonviral vector for in vivo gene transfer into the adult mammalian brain: polyethylenimine. Hum. Gene Ther. 7, 1947–1954. doi: 10.1089/hum.1996.7.16-1947
Aggarwal, R., Pompili, V. J., and Das, H. (2010). Genetic modification of ex-vivo expanded stem cells for clinical application. Front. Biosci. 15, 854–871. doi: 10.2741/3650
Akinc, A., Thomas, M., Klibanov, A. M., and Langer, R. (2005). Exploring polyethylenimine-mediated DNA transfection and the proton sponge hypothesis. J. Gene. Med. 7, 657–663. doi: 10.1002/jgm.696
Al-Dosari, M. S., and Gao, X. (2009). Nonviral gene delivery: principle, limitations, and recent progress. AAPS J. 11:671. doi: 10.1208/s12248-009-9143-y
Ali, O. A., and Mooney, D. J. (2008). Sustained GM-CSF and PEI condensed pDNA presentation increases the level and duration of gene expression in dendritic cells. J. Control. Release 132, 273–278. doi: 10.1016/j.jconrel.2008.07.005
Andaloussi, E. L., Mäger, I., Breakefield, X. O., and Wood, M. J. (2013). Extracellular vesicles: biology and emerging therapeutic opportunities. Nat. Rev. Drug. Discov. 12, 347–357. doi: 10.1038/nrd3978
Anderson, H. C. (2003). Matrix vesicles and calcification. Curr. Rheumatol. Rep. 5, 222–226. doi: 10.1007/s11926-003-0071-z
Anderson, J. M., and Shive, M. S. (1997). Biodegradation and biocompatibility of PLA and PLGA microspheres. Adv. Drug Deliv. Rev. 28, 5–24. doi: 10.1016/s0169-409x(97)00048-3
Antoniou, J., Goudsouzian, N. M., Heathfield, T. F., Winterbottom, N., Steffen, T., Poole, A. R., et al. (1996). The human lumbar endplate. Evidence of changes in biosynthesis and denaturation of the extracellular matrix with growth, maturation, aging, and degeneration. Spine 21, 1153–1161. doi: 10.1097/00007632-199605150-00006
Arena, C. B., Mahajan, R. L., Nichole, R. M. R., and Davalos, R. V. (2013). An experimental and numerical investigation of phase change electrodes for therapeutic irreversible electroporation. J. Biomech. Eng. 135:111009. doi: 10.1115/1.4025334
Arvizo, R., Bhattacharya, R., and Mukherjee, P. (2010). Gold nanoparticles: opportunities and challenges in nanomedicine. Expert. Opin. Drug Deliv. 7, 753–763. doi: 10.1517/17425241003777010
Ayuni, E. L., Gazdhar, A., Giraud, M. N., Kadner, A., Gugger, M., Cecchini, M., et al. (2010). In vivo electroporation mediated gene delivery to the beating heart. PLos One 5:e14467. doi: 10.1371/journal.pone.0014467
Balmayor, E. R., and van Griensven, M. (2015). Gene therapy for bone engineering. Front. Bioeng. Biotechnol. 3:9. doi: 10.3389/fbioe.2015.00009
Banala, R. R., Vemuri, S. K., Dar, G. H., Palanisamy, V., Penkulinti, M., Surekha, M. V., et al. (2019). Efficiency of dual siRNA-mediated gene therapy for intervertebral disc degeneration (IVDD). Spine J. 19, 896–904. doi: 10.1016/j.spinee.2018.10.016
Basarkar, A., and Singh, J. (2007). Nanoparticulate systems for polynucleotide delivery. Int. J. Nanomed. 2, 353–360.
Benjaminsen, R. V., Mattebjerg, M. A., Henriksen, J. R., Moghimi, S. M., and Andresen, T. L. (2013). The possible “proton sponge” effect of polyethylenimine (PEI) does not include change in lysosomal pH. Mol. Ther. 21, 149–157. doi: 10.1038/mt.2012.185
Beshay, M., Gazdhar, A., Gugger, M., Reymond, M., and Schmid, R. A. (2009). The feasibility of non-viral gene transfer to the diaphragm in vivo. Dev. Growth Differ. 51, 547–553. doi: 10.1111/j.1440-169X.2009.01117.x
Boere, J., Malda, J., van de Lest, C. H. A., van Weeren, R. P., and Wauben, M. H. M. (2018). Extracellular vesicles in joint disease and therapy. Front. Immunol. 9:2575. doi: 10.3389/fimmu.2018.02575
Boissier, M. C., and Bessis, N. (1997). Gene therapy in rheumatology. From the laboratory to the patient. Rev. Rhum. Engl. Ed. 64, 1–3. doi: 10.1142/9789814354189_0001
Bonadio, J., Smiley, E., Patil, P., and Goldstein, S. (1999). Localized, direct plasmid gene delivery in vivo: prolonged therapy results in reproducible tissue regeneration. Nat. Med. 5, 753–759. doi: 10.1038/10473
Bono, N., Ponti, F., Mantovani, D., and Candiani, G. (2020). Non-viral in vitro gene delivery: it is now time to set the bar! Pharmaceutics 12:183. doi: 10.3390/pharmaceutics12020183
Boukany, P. E., Morss, A., Liao, W. C., Henslee, B., Jung, H., Zhang, X., et al. (2011). Nanochannel electroporation delivers precise amounts of biomolecules into living cells. Nat. Nanotechnol. 6, 747–754. doi: 10.1038/nnano.2011.164
Bourgeat-Lami, E. (2002). Organic-inorganic nanostructured colloids. J. Nanosci. Nanotechnol. 2, 1–24. doi: 10.1166/jnn.2002.075
Boussif, O., Lezoualc’h, F., Zanta, M. A., Mergny, M. D., Scherman, D., Demeneix, B., et al. (1995). A versatile vector for gene and oligonucleotide transfer into cells in culture and in vivo: polyethylenimine. Proc. Natl. Acad. Sci. U.S.A. 92, 7297–7301. doi: 10.1073/pnas.92.16.7297
Bruno, S., Collino, F., Deregibus, M. C., Grange, C., Tetta, C., and Camussi, G. (2013). Microvesicles derived from human bone marrow mesenchymal stem cells inhibit tumor growth. Stem Cells Dev. 22, 758–771. doi: 10.1089/scd.2012.0304
Bucher, C., Gazdhar, A., Benneker, L. M., Geiser, T., and Gantenbein-Ritter, B. (2013). Nonviral Gene delivery of growth and differentiation Factor 5 to human mesenchymal stem cells injected into a 3D bovine intervertebral disc organ culture system. Stem Cells Int. 2013:326828. doi: 10.1155/2013/326828
Cai, D., Mataraza, J. M., Qin, Z. H., Huang, Z., Huang, J., Chiles, T. C., et al. (2005). Highly efficient molecular delivery into mammalian cells using carbon nanotube spearing. Nat. Methods 2, 449–454. doi: 10.1038/nmeth761
Cappariello, A., Loftus, A., Muraca, M., Maurizi, A., Rucci, N., and Teti, A. (2018). Osteoblast-derived extracellular vesicles are biological tools for the delivery of active molecules to bone. J. Bone Miner. Res. 33, 517–533. doi: 10.1002/jbmr.3332
Chang, L., Gallego-Perez, D., Chiang, C. L., Bertani, P., Kuang, T., Sheng, Y., et al. (2016). Controllable large-scale transfection of primary mammalian cardiomyocytes on a nanochannel array platform. Small 12, 5971–5980. doi: 10.1002/smll.201601465
Chen, S., Tang, Y., Liu, Y., Zhang, P., Lv, L., Zhang, X., et al. (2019). Exosomes derived from miR-375-overexpressing human adipose mesenchymal stem cells promote bone regeneration. Cell. Prolif. 52:e12669. doi: 10.1111/cpr.12669
Cheng, X., Zhang, G., Zhang, L., Hu, Y., Zhang, K., Sun, X., et al. (2018). Mesenchymal stem cells deliver exogenous miR-21 via exosomes to inhibit nucleus pulposus cell apoptosis and reduce intervertebral disc degeneration. J. Cell. Mol. Med. 22, 261–276. doi: 10.1111/jcmm.13316
Chew, S. A., Kretlow, J. D., Spicer, P. P., Edwards, A. W., Baggett, L. S., Tabata, Y., et al. (2011). Delivery of plasmid DNA encoding bone morphogenetic protein-2 with a biodegradable branched polycationic polymer in a critical-size rat cranial defect model. Tissue Eng. Part. A 17, 751–763. doi: 10.1089/ten.TEA.2010.0496
Chowdhury, E. H., and Akaike, T. (2005). Bio-functional inorganic materials: an attractive branch of gene-based nano-medicine delivery for 21st century. Curr. Gene Ther. 5, 669–676. doi: 10.2174/156652305774964613
Chung, S. A., Wei, A. Q., Connor, D. E., Webb, G. C., Molloy, T., Pajic, M., et al. (2007). Nucleus pulposus cellular longevity by telomerase gene therapy. Spine 32, 1188–1196. doi: 10.1097/BRS.0b013e31805471a3
Corsi, K., Chellat, F., Yahia, L., and Fernandes, J. C. (2003). Mesenchymal stem cells, MG63 and HEK293 transfection using chitosan-DNA nanoparticles. Biomaterials 24, 1255–1264. doi: 10.1016/s0142-9612(02)00507-0
Curtin, C. M., Tierney, E. G., McSorley, K., Cryan, S. A., Duffy, G. P., and O’Brien, F. J. (2015). Combinatorial gene therapy accelerates bone regeneration: non-viral dual delivery. Adv. Healthc. Mater. 4, 223–227. doi: 10.1002/adhm.201400397
Dabkowska, A. P., Barlow, D. J., Hughes, A. V., Campbell, R. A., Quinn, P. J., and Lawrence, M. J. (2012). The effect of neutral helper lipids on the structure of cationic lipid monolayers. J. R. Soc. Interf. 9, 548–561. doi: 10.1098/rsif.2011.0356
Dang, J. M., and Leong, K. W. (2006). Natural polymers for gene delivery and tissue engineering. Adv. Drug Deliv. Rev. 58, 487–499. doi: 10.1016/j.addr.2006.03.001
Das, J., Choi, Y. J., Yasuda, H., Han, J. W., Park, C., Song, H., et al. (2016). Efficient delivery of C/EBP beta gene into human mesenchymal stem cells via polyethylenimine-coated gold nanoparticles enhances adipogenic differentiation. Sci. Rep. 6:33784. doi: 10.1038/srep33784
Daya, S., and Berns, K. I. (2008). Gene therapy using adeno-associated virus vectors. Clin. Microbiol. Rev. 21, 583–593. doi: 10.1128/CMR.00008-08
De Fry, J., Martínez-Martínez, P., Losen, M., Bode, G. H., Temel, Y., Steckler, T., et al. (2010). Low current-driven micro-electroporation allows efficient in vivo delivery of nonviral DNA into the adult mouse brain. Mol. Ther. 18, 1183–1191. doi: 10.1038/mt.2010.62
De Jong, O. G., Van Balkom, B. W. M., Schiffelers, R. M., Bouten, C. V. C., and Verhaar, M. C. (2014). Extracellular vesicles: potential roles in regenerative medicine. Front. Immunol. 5:608. doi: 10.3389/fimmu.2014.00608
De Laporte, L., Rea, J. C., and Shea, L. D. (2006). Design of modular non-viral gene therapy vectors. Biomaterials 27, 947–954. doi: 10.1016/j.biomaterials.2005.09.036
De Witte, T. M., Fratila-Apachitei, L. E., Zadpoor, A. A., and Peppas, N. A. (2018). Bone tissue engineering via growth factor delivery: from scaffolds to complex matrices. Regen. Biomater. 5, 197–211. doi: 10.1093/rb/rby013
Deng, R., Yue, Y., Jin, F., Chen, Y., Kung, H. F., Lin, M. C., et al. (2009). Revisit the complexation of PEI and DNA - how to make low cytotoxic and highly efficient PEI gene transfection non-viral vectors with a controllable chain length and structure? J. Control. Release 140, 40–46. doi: 10.1016/j.jconrel.2009.07.009
Dimitriou, R., Jones, E., McGonagle, D., and Giannoudis, P. V. (2011). Bone regeneration: current concepts and future directions. BMC Med. 9:66. doi: 10.1186/1741-7015-9-66
Ding, Y., Jiang, Z., Saha, K., Kim, C. S., Kim, S. T., Landis, R. F., et al. (2014). Gold nanoparticles for nucleic acid delivery. Mol. Ther. 22, 1075–1083. doi: 10.1038/mt.2014.30
Diomede, F., Gugliandolo, A., Cardelli, P., Merciaro, I., Ettorre, V., Traini, T., et al. (2018). Three-dimensional printed PLA scaffold and human gingival stem cell-derived extracellular vesicles: a new tool for bone defect repair. Stem Cell Res. Ther. 9:104. doi: 10.1186/s13287-018-0850-0
Ditto, A. J., Shah, P. N., and Yun, Y. H. (2009). Non-viral gene delivery using nanoparticles. Expert. Opin. Drug Deliv. 6, 1149–1160. doi: 10.1517/17425240903241796
Dwivedi, P. P., Anderson, P. J., and Powell, B. C. (2012). Development of an efficient, non-viral transfection method for studying gene function and bone growth in human primary cranial suture mesenchymal cells reveals that the cells respond to BMP2 and BMP3. BMC Biotechnol. 12:45. doi: 10.1186/1472-6750-12-45
Elangovan, S., D’Mello, S. R., Hong, L., Ross, R. D., Allamargot, C., Dawson, D. V., et al. (2014). The enhancement of bone regeneration by gene activated matrix encoding for. Biomaterials 35, 737–747. doi: 10.1016/j.biomaterials.2013.10.021
Eliyahu, H., Barenholz, Y., and Domb, A. J. (2005). Polymers for DNA delivery. Molecules 10, 34–64. doi: 10.3390/10010034
Epstein, S. L. (1991). Regulatory concerns in human gene therapy. Hum. Gene. Ther. 2, 243–249. doi: 10.1089/hum.1991.2.3-243
Evans, C. H., Ghivizzani, S. C., and Robbins, P. D. (2006). Will arthritis gene therapy become a clinical reality? Nat. Clin. Pract. Rheumatol. 2, 344–345. doi: 10.1038/ncprheum0215
Evans, C. H., Ghivizzani, S. C., and Robbins, P. D. (2012). Orthopedic gene therapy–lost in translation? J. Cell. Physiol. 227, 416–420. doi: 10.1002/jcp.23031
Evans, C. H., and Robbins, P. D. (1999). Gene therapy of arthritis. Intern. Med. 38, 233–239. doi: 10.2169/internalmedicine.38.233
Feichtinger, G. A., Hofmann, A. T., Slezak, P., Schuetzenberger, S., Kaipel, M., Schwartz, E., et al. (2014). Sonoporation increases therapeutic efficacy of inducible and constitutive BMP2/7. Hum. Gene Ther. Meth. 25, 57–71. doi: 10.1089/hgtb.2013.113
Felgner, P. L., Gadek, T. R., Holm, M., Roman, R., Chan, H. W., Wenz, M., et al. (1987). Lipofection: a highly efficient, lipid-mediated DNA-transfection procedure. Proc. Natl. Acad. Sci. U.S.A. 84, 7413–7417. doi: 10.1073/pnas.84.21.7413
Feng, G., Chen, H., Li, J., Huang, Q., Gupte, M. J., Liu, H., et al. (2015). Gene therapy for nucleus pulposus regeneration by heme oxygenase-1 plasmid DNA. Biomaterials 52, 1–13. doi: 10.1016/j.biomaterials.2015.02.024
Feng, G., Zha, Z., Huang, Y., Li, J., Wang, Y., Ke, W., et al. (2018). Sustained and bioresponsive two-stage delivery of therapeutic miRNA via polyplex micelle-loaded injectable hydrogels for inhibition of intervertebral disc fibrosis. Adv. Healthc. Mater. 7:e1800623. doi: 10.1002/adhm.201800623
Feng, G., Zhang, Z., Dang, M., Zhang, X., Doleyres, Y., Song, Y., et al. (2017). Injectable nanofibrous spongy microspheres for NR4A1 plasmid DNA transfection to reverse fibrotic degeneration and support disc regeneration. Biomaterials 131, 86–97. doi: 10.1016/j.biomaterials.2017.03.029
Foldvari, M., Chen, D. W., Nafissi, N., Calderon, D., Narsineni, L., and Rafiee, A. (2016). Non-viral gene therapy: gains and challenges of non-invasive administration methods. J. Control. Release 240, 165–190. doi: 10.1016/j.jconrel.2015.12.012
Fu, H., Hu, D., Zhang, L., and Tang, P. (2018). Role of extracellular vesicles in rheumatoid arthritis. Mol. Immunol. 93, 125–132. doi: 10.1016/j.molimm.2017.11.016
Gallego-Perez, D., Otero, J. J., Czeisler, C., Ma, J., Ortiz, C., Gygli, P., et al. (2016). Deterministic transfection drives efficient nonviral reprogramming and uncovers reprogramming barriers. Nanomedicine 12, 399–409. doi: 10.1016/j.nano.2015.11.015
Gallego-Perez, D., Pal, D., Ghatak, S., Malkoc, V., Higuita-Castro, N., Gnyawali, S., et al. (2017). Topical tissue nano-transfection mediates non-viral stroma reprogramming and rescue. Nat. Nanotechnol. 12, 974–979. doi: 10.1038/nnano.2017.134
Gao, H., and Hui, K. M. (2001). Synthesis of a novel series of cationic lipids that can act as efficient gene delivery vehicles through systematic heterocyclic substitution of cholesterol derivatives. Gene Ther. 8, 855–863. doi: 10.1038/sj.gt.3301471
Gao, K., Li, L., He, L., Hinkle, K., Wu, Y., Ma, J., et al. (2014). Design of a microchannel-nanochannel-microchannel array based nanoelectroporation system for precise gene transfection. Small 10, 1015–1023. doi: 10.1002/smll.201300116
Garcia-Fuentes, M., and Alonso, M. J. (2012). Chitosan-based drug nanocarriers: where do we stand? J. Control. Release. 161, 496–504. doi: 10.1016/j.jconrel.2012.03.017
Gazdhar, A., Bilici, M., Pierog, J., Ayuni, E. L., Gugger, M., Wetterwald, A., et al. (2006). In vivo electroporation and ubiquitin promoter–a protocol for sustained gene expression in the lung. J. Gene. Med. 8, 910–918. doi: 10.1002/jgm.911
Gazdhar, A., Fachinger, P., van Leer, C., Pierog, J., Gugger, M., Friis, R., et al. (2007). Gene transfer of hepatocyte growth factor by electroporation reduces bleomycin-induced lung fibrosis. Am. J. Physiol. Lung Cell. Mol. Physiol. 292:L529.
GBD 2017 Disease and Injury Incidence and Prevalence Collaborators (2018). Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990-2017: a systematic analysis for the global burden of disease study 2017. Lancet 392, 1789–1858. doi: 10.1016/S0140-6736(18)32279-7
Gehl, J. (2003). Electroporation: theory and methods, perspectives for drug delivery, gene therapy and research. Acta Physiol. Scand. 177, 222–229. doi: 10.1046/j.1365-201X.2003.01093.x
Gelse, K., von der Mark, K., and Schneider, H. (2003). Cartilage regeneration by gene therapy. Curr. Gene Ther. 3, 305–317. doi: 10.2174/1566523034578276
Geng, T., and Lu, C. (2013). Microfluidic electroporation for cellular analysis and delivery. Lab. Chip 13, 3803–3821. doi: 10.1039/c3lc50566a
Gerlach, J. Q., and Griffin, M. D. (2016). Getting to know the extracellular vesicle glycome. Mol. Biosyst. 12, 1071–1081. doi: 10.1039/c5mb00835b
Gill, D. R., Pringle, I. A., and Hyde, S. C. (2009). Progress and prospects: the design and production of plasmid vectors. Gene. Ther. 16, 165–171. doi: 10.1038/gt.2008.183
Glover, D. J., Lipps, H. J., and Jans, D. A. (2005). Towards safe, non-viral therapeutic gene expression in humans. Nat. Rev. Genet. 6, 299–310. doi: 10.1038/nrg1577
Godbey, W. T., Wu, K. K., and Mikos, A. G. (1999). Poly(ethylenimine) and its role in gene delivery. J. Control. Release 60, 149–160. doi: 10.1016/s0168-3659(99)00090-5
Goepfert, C., Gazdhar, A., Frey, F. J., and Frey, B. M. (2011). Effect of electroporation-mediated diphtheria toxin A expression on PSA positive human prostate xenograft tumors in SCID mice. Prostate 71, 872–880. doi: 10.1002/pros.21303
Gonzalez-Fernandez, T., Sathy, B. N., Hobbs, C., Cunniffe, G. M., McCarthy, H. O., Dunne, N. J., et al. (2017). Mesenchymal stem cell fate following non-viral gene transfection strongly depends on the choice of delivery vector. Acta Biomater. 55, 226–238. doi: 10.1016/j.actbio.2017.03.044
Goomer, R. S., Deftos, L. J., Terkeltaub, R., Maris, T., Lee, M. C., Harwood, F. L., et al. (2001). High-efficiency non-viral transfection of primary chondrocytes and perichondrial cells for ex-vivo gene therapy to repair articular cartilage defects. Osteoarthr. Cart. 9, 248–256. doi: 10.1053/joca.2000.0382
Gorth, D. J., Martin, J. T., Dodge, G. R., Elliott, D. M., Malhotra, N. R., Mauck, R. L., et al. (2014). In vivo retention and bioactivity of IL-1ra microspheres in the rat intervertebral disc: a preliminary investigation. J. Exp. Orthop. 1:15. doi: 10.1186/s40634-014-0015-8
Gowrishankar, T. R., Pliquett, U., and Lee, R. C. (1999). Dynamics of membrane sealing in transient electropermeabilization of skeletal muscle membranes. Ann. N. Y. Acad. Sci. 888, 195–210. doi: 10.1111/j.1749-6632.1999.tb07957.x
Guo, X., and Huang, L. (2012). Recent advances in nonviral vectors for gene delivery. Acc. Chem. Res. 45, 971–979. doi: 10.1021/ar200151m
Guo-ping, W., Xiao-chuan, H., Zhi-hui, Y., and Li, G. (2010). Influence on the osteogenic activity of the human bone marrow mesenchymal stem cells transfected by liposome-mediated recombinant plasmid pIRES-hBMP2-hVEGF165 in vitro. Ann. Plast. Surg. 65, 80–84. doi: 10.1097/SAP.0b013e3181b4bc5d
Ha, D., Yang, N., and Nadithe, V. (2016). Exosomes as therapeutic drug carriers and delivery vehicles across biological membranes: current perspectives and future challenges. Acta Pharm. Sin. B 6, 287–296. doi: 10.1016/j.apsb.2016.02.001
Haag, J., Voigt, R., Soeder, S., and Aigner, T. (2009). Efficient non-viral transfection of primary human adult chondrocytes in a high-throughput format. Osteoarthr. Cart. 17, 813–817. doi: 10.1016/j.joca.2008.11.004
Haberl, S., Kandušer, M., Flisar, K., Hodžić, D., Bregar, V. B., Miklavčič, D., et al. (2013). Effect of different parameters used for in vitro gene electrotransfer on gene expression efficiency, cell viability and visualization of plasmid DNA at the membrane level. J. Gene Med. 15:169. doi: 10.1002/jgm.2706
Hargrave, B., Strange, R., Navare, S., Stratton, M., Burcus, N., Murray, L., et al. (2014). Gene Electro transfer of plasmid encoding vascular endothelial growth factor for enhanced expression and perfusion in the ischemic swine heart. PLoS One 9:e0115235. doi: 10.1371/journal.pone.0115235
Harrison, B. S., and Atala, A. (2007). Carbon nanotube applications for tissue engineering. Biomaterials 28, 344–353. doi: 10.1016/j.biomaterials.2006.07.044
Heller, R., Jaroszeski, M., Atkin, A., Moradpour, D., Gilbert, R., Wands, J., et al. (1996). In vivo gene electroinjection and expression in rat liver. FEBS Lett. 389, 225–225. doi: 10.1016/0014-5793(96)00590-x
Heuer, F., Schmidt, H., and Wilke, H. J. (2008). Stepwise reduction of functional spinal structures increase disc bulge and surface strains. J. Biomech. 41, 1953–1960. doi: 10.1016/j.jbiomech.2008.03.023
Heyde, M., Partridge, K. A., Oreffo, R. O., Howdle, S. M., Shakesheff, K. M., and Garnett, M. C. (2007). Gene therapy used for tissue engineering applications. J. Pharm. Pharmacol. 59, 329–350. doi: 10.1211/jpp.59.3.0002
Hodgkinson, T., Shen, B., Diwan, A., Hoyland, J. A., and Richardson, S. M. (2019). Therapeutic potential of growth differentiation factors in the treatment of degenerative disc diseases. JOR Spine 2:e1045. doi: 10.1002/jsp2.1045
Hsieh, M. K., Wu, C. J., Chen, C. C., Tsai, T. T., Niu, C. C., Wu, S. C., et al. (2018). BMP-2 gene transfection of bone marrow stromal cells to induce osteoblastic. Mat. Sci. Eng. C. Mat. Biol. Appl. 91, 806–816. doi: 10.1016/j.msec.2018.06.004
Huang, Y. C., Riddle, K., Rice, K. G., and Mooney, D. J. (2005a). Long-term in vivo gene expression via delivery of PEI-DNA condensates from porous. Hum. Gene Ther. 16, 609–617. doi: 10.1089/hum.2005.16.609
Huang, Y. C., Simmons, C., Kaigler, D., Rice, K. G., and Mooney, D. J. (2005b). Bone regeneration in a rat cranial defect with delivery of PEI-condensed plasmid. Gene Ther. 12, 418–426. doi: 10.1038/sj.gt.3302439
Huizinga, T. W. (1999). Gene therapy of arthritis and related disorders–first international meeting. 2-3 December 1998, Bethesda, USA. IDrugs 2, 97–99.
Humzah, M. D., and Soames, R. W. (1988). Human intervertebral disc: structure and function. Anat. Rec. 220, 337–356. doi: 10.1002/ar.1092200402
Hyder, I., Eghbalsaied, S., and Kues, W. A. (2020). Systematic optimization of square-wave electroporation conditions for bovine primary fibroblasts. BMC Mol. Cell Biol. 21:9. doi: 10.1186/s12860-020-00254-5
Im, G. I. (2016). Gene transfer strategies to promote chondrogenesis and cartilage regeneration. Tissue Eng. Part. B Rev. 22, 136–148. doi: 10.1089/ten.TEB.2015.0347
Inoue, T., and Krumlauf, R. (2001). An impulse to the brain–using in vivo electroporation. Nat. Neurosci. 4(Suppl.), 1156–1158. doi: 10.1038/nn1101-1156
Itaka, K., Ohba, S., Miyata, K., Kawaguchi, H., Nakamura, K., Takato, T., et al. (2007). Bone regeneration by regulated in vivo gene transfer using biocompatible polyplex. Mol. Ther. 15, 1655–1662. doi: 10.1038/sj.mt.6300218
Jafari, S. M. S., Shafighi, M., Beltraminelli, H., Weber, B., Schmid, R. A., Geiser, T., et al. (2018). Efficacy of in vivo electroporation-mediated IL-10 gene delivery on survival of skin flaps. J. Membr. Biol. 251, 211–219. doi: 10.1007/s00232-017-9974-x
Ji, A. M., Su, D., Che, O., Li, W. S., Sun, L., Zhang, Z. Y., et al. (2009). Functional gene silencing mediated by chitosan/siRNA nanocomplexes. Nanotechnology 20:405103. doi: 10.1088/0957-4484/20/40/405103
Jones, C. H., Chen, C. K., Ravikrishnan, A., Rane, S., and Pfeifer, B. A. (2013). Overcoming nonviral gene delivery barriers: perspective and future. Mol. Pharm. 10, 4082–4098. doi: 10.1021/mp400467x
Joshi, C. R., Labhasetwar, V., and Ghorpade, A. (2017). Destination brain: the Past, Present, and Future of therapeutic gene delivery. J. Neuroimmun. Pharmacol. 12, 51–83. doi: 10.1007/s11481-016-9724-3
Jubeck, B., Gohr, C., Fahey, M., Muth, E., Matthews, M., Mattson, E., et al. (2008). Promotion of articular cartilage matrix vesicle mineralization by type I collagen. Arthritis. Rheum. 58, 2809–2817. doi: 10.1002/art.23762
Kaiser, J. (2007). Death prompts a review of gene therapy vector. Science 317, 580–580. doi: 10.1126/science.317.5838.580
Kakutani, K., Nishida, K., Uno, K., Takada, T., Shimomura, T., Maeno, K., et al. (2006). Prolonged down regulation of specific gene expression in nucleus pulposus cell mediated by RNA interference in vitro. J. Orthop. Res. 24, 1271–1278. doi: 10.1002/jor.20171
Karimi, M., Solati, N., Ghasemi, A., Estiar, M. A., Hashemkhani, M., Kiani, P., et al. (2015). Carbon nanotubes part II: a remarkable carrier for drug and gene delivery. Expert. Opin. Drug Deliv. 12, 1089–1105. doi: 10.1517/17425247.2015.1004309
Kasper, F. K., Kushibiki, T., Kimura, Y., Mikos, A. G., and Tabata, Y. (2005). In vivo release of plasmid DNA from composites of oligo(poly(ethylene glycol)fumarate) and cationized gelatin microspheres. J. Control. Release 107, 547–561. doi: 10.1016/j.jconrel.2005.07.005
Kasper, F. K., Young, S., Tanahashi, K., Barry, M. A., Tabata, Y., Jansen, J. A., et al. (2006). Evaluation of bone regeneration by DNA release from composites of oligo(poly(ethylene glycol) fumarate) and cationized gelatin microspheres in a critical-sized calvarial defect. J. Biomed. Mater. Res. A 78, 335–342. doi: 10.1002/jbm.a.30698
Katas, H., and Alpar, H. O. (2006). Development and characterisation of chitosan nanoparticles for siRNA delivery. J. Control. Release 115, 216–225. doi: 10.1016/j.jconrel.2006.07.021
Kawai, M., Kataoka, Y., Sonobe, J., Yamamoto, H., Maruyama, H., Yamamoto, T., et al. (2018). Analysis of mineral apposition rates during alveolar bone regeneration over three weeks following transfer of BMP-2/7 gene via in vivo electroporation. Eur. J. Histochem. 62:2947. doi: 10.4081/ejh.2018.2947
Khoury, M., Bigey, P., Louis-Plence, P., Noel, D., Rhinn, H., Scherman, D., et al. (2006). A comparative study on intra-articular versus systemic gene electrotransfer in experimental arthritis. J. Gene. Med. 8, 1027–1036. doi: 10.1002/jgm.922
Kim, M., Steinberg, D. R., Burdick, J. A., and Mauck, R. L. (2019). Extracellular vesicles mediate improved functional outcomes in engineered cartilage produced from MSC/chondrocyte cocultures. Proc. Natl. Acad. Sci. U.S.A. 116, 1569–1578. doi: 10.1073/pnas.1815447116
Kitagawa, T., Iwazawa, T., Robbins, P. D., Lotze, M. T., and Tahara, H. (2003). Advantages and limitations of particle-mediated transfection (gene gun) in cancer immuno-gene therapy using IL-10, IL-12 or B7-1 in murine tumor models. J. Gene Med. 5, 958–965. doi: 10.1002/jgm.441
Kobayashi, S., Dono, K., Tanaka, T., Takahara, S., Isaka, Y., Imai, E., et al. (2003). Gene transfer into the liver by plasmid injection into the portal vein combined with electroporation. J. Gene Med. 5, 201–218. doi: 10.1002/jgm.329
Kooijmans, S. A., Vader, P., Sm, V. D., Ww, V. S., and Schiffelers, R. M. (2012). Exosome mimetics: a novel class of drug delivery systems. Int. J. Nanomed. 7, 1525–1541. doi: 10.2147/IJN.S29661
Kotnik, T., Miklavcic, D., and Mir, L. M. (2001). Cell membrane electropermeabilization by symmetrical bipolar rectangular pulses. Part II. Reduced electrolytic contamination. Bioelectrochemistry 54, 91–95. doi: 10.1016/s1567-5394(01)00115-3
Kowal, J., Arras, G., Colombo, M., Jouve, M., Morath, J. P., Primdal-Bengtson, B., et al. (2016). Proteomic comparison defines novel markers to characterize heterogeneous populations of extracellular vesicle subtypes. Proc. Natl. Acad. Sci. U.S.A. 113, E968–E977. doi: 10.1073/pnas.1521230113
Krebs, M. D., Salter, E., Chen, E., Sutter, K. A., and Alsberg, E. (2010). Calcium phosphate-DNA nanoparticle gene delivery from alginate hydrogels induces. J. Biomed. Mater. Res. A 92, 1131–1138. doi: 10.1002/jbm.a.32441
Krill, M., Early, N., Everhart, J. S., and Flanigan, D. C. (2018). Autologous chondrocyte implantation (ACI) for knee cartilage defects: a review of indications, technique, and outcomes. JBJS Rev. 6:e5. doi: 10.2106/JBJS.RVW.17.00078
Lai, Q. G., Yuan, K. F., Xu, X., Li, D. R., Li, G. J., Wei, F. L., et al. (2011). Transcription factor osterix modified bone marrow mesenchymal stem cells enhance callus formation during distraction osteogenesis. Oral. Surg. Oral. Med. Oral. Pathol. Oral. Radiol. Endod. 111, 412–419. doi: 10.1016/j.tripleo.2010.05.012
Lamichhane, T. N., Sokic, S., Schardt, J. S., Raiker, R. S., Lin, J. W., and Jay, S. M. (2015). Emerging roles for extracellular vesicles in tissue engineering and regenerative medicine. Tissue Eng. Part. B Rev. 21, 45–54. doi: 10.1089/ten.TEB.2014.0300
Le Maitre, C. L., Pockert, A., Buttle, D. J., Freemont, A. J., and Hoyland, J. A. (2007). Matrix synthesis and degradation in human intervertebral disc degeneration. Biochem. Soc. Trans. 35, 652–655. doi: 10.1042/BST0350652
Lechardeur, D., and Lukacs, G. L. (2002). Intracellular barriers to non-viral gene transfer. Curr. Gene Ther. 2, 183–194. doi: 10.2174/1566523024605609
Lee, E., Ko, J. Y., Kim, J., Park, J. W., Lee, S., and Im, G. I. (2019). Osteogenesis and angiogenesis are simultaneously enhanced in BMP2-/VEGF-transfected adipose stem cells through activation of the YAP/TAZ signaling pathway. Biomater. Sci. 7, 4588–4602. doi: 10.1039/c9bm01037h
Lee, S. J., Kang, S. W., Do, H. J., Han, I., Shin, D. A., Kim, J. H., et al. (2010). Enhancement of bone regeneration by gene delivery of BMP2/Runx2 bicistronic vector into adipose-derived stromal cells. Biomaterials 31, 5652–5659. doi: 10.1016/j.biomaterials.2010.03.019
Li, L., He, Z. Y., Wei, X. W., Gao, G. P., and Wei, Y. Q. (2015). Challenges in CRISPR/CAS9 delivery: potential roles of nonviral vectors. Hum. Gene Ther. 26, 452–462. doi: 10.1089/hum.2015.069
Li, L., He, Z. Y., Wei, X. W., and Wei, Y. Q. (2016). Recent advances of biomaterials in biotherapy. Regen. Biomater. 3, 99–105. doi: 10.1093/rb/rbw007
Li, W., Liu, Y., Zhang, P., Tang, Y., Zhou, M., Jiang, W., et al. (2018). Tissue-engineered bone immobilized with human adipose stem cells-derived exosomes promotes bone regeneration. ACS. Appl. Mater. Interf. 10, 5240–5254. doi: 10.1021/acsami.7b17620
Lin, Y., Tian, W., Chen, X., Yan, Z., Li, Z., Qiao, J., et al. (2005). Expression of exogenous or endogenous green fluorescent protein in adipose tissue-derived stromal cells during chondrogenic differentiation. Mol. Cell. Biochem. 277, 181–190. doi: 10.1007/s11010-005-5996-2
Lin, Z., McClure, M. J., Zhao, J., Ramey, A. N., Asmussen, N., Hyzy, S. L., et al. (2018). MicroRNA contents in matrix vesicles produced by growth plate chondrocytes are cell maturation dependent. Sci. Rep. 8:3609. doi: 10.1038/s41598-018-21517-4
Lirong, X., Danian, C., and Naihong, Y. (2014). In vivo electroporation of newborn mouse retina. Yi Chuan 36:1173. doi: 10.3724/SP.J.1005.2014.1173
Liu, F., Heston, S., Shollenberger, L. M., Sun, B., Mickle, M., Lovell, M., et al. (2006). Mechanism of in vivo DNA transport into cells by electroporation: electrophoresis across the plasma membrane may not be involved. J. Gene Med. 8, 353–361. doi: 10.1002/jgm.851
Liu, Y., Wu, D. C., Zhang, W. D., Jiang, X., He, C. B., Chung, T. S., et al. (2005). Polyethylenimine-grafted multiwalled carbon nanotubes for secure noncovalent immobilization and efficient delivery of DNA. Angew. Chem. Int. Ed. Engl. 44, 4782–4785. doi: 10.1002/anie.200500042
Lu, K., Li, H. Y., Yang, K., Wu, J. L., Cai, X. W., Zhou, Y., et al. (2017). Exosomes as potential alternatives to stem cell therapy for intervertebral disc degeneration: in-vitro study on exosomes in interaction of nucleus pulposus cells and bone marrow mesenchymal stem cells. Stem. Cell. Res. Ther. 8, 1–11. doi: 10.1186/s13287-017-0563-9
Luo, J., Sun, M. H., Kang, Q., Peng, Y., Jiang, W., Luu, H. H., et al. (2005). Gene therapy for bone regeneration. Curr. Gene Ther. 5, 167–179. doi: 10.2174/1566523053544218
Luong, L. N., McFalls, K. M., and Kohn, D. H. (2009). Gene delivery via DNA incorporation within a biomimetic apatite coating. Biomaterials 30, 6996–7004. doi: 10.1016/j.biomaterials.2009.09.001
Luu, Y. K., Kim, K., Hsiao, B. S., Chu, B., and Hadjiargyrou, M. (2003). Development of a nanostructured DNA delivery scaffold via electrospinning of PLGA and PLA-PEG block copolymers. J. Control. Release 89, 341–353. doi: 10.1016/s0168-3659(03)00097-x
Lv, H., Zhang, S., Wang, B., Cui, S., and Yan, J. (2006). Toxicity of cationic lipids and cationic polymers in gene delivery. J. Control. Release 114, 100–109. doi: 10.1016/j.jconrel.2006.04.014
Maas, S. L. N., Breakefield, X. O., and Weaver, A. M. (2017). Extracellular vesicles: unique intercellular delivery vehicles. Trends Cell. Biol. 27, 172–188. doi: 10.1016/j.tcb.2016.11.003
Macdonald, K. K., Cheung, C. Y., and Anseth, K. S. (2007). Cellular delivery of TGFbeta1 promotes osteoinductive signalling for bone. J. Tissue Eng. Regen. Med. 1, 314–317. doi: 10.1002/term.31
Madeira, C., Mendes, R. D., Ribeiro, S. C., Boura, J. S., Aires-Barros, M. R., da Silva, C. L., et al. (2010). Nonviral gene delivery to mesenchymal stem cells using cationic liposomes for gene and cell therapy. J. Biomed. Biotechnol. 2010:735349. doi: 10.1155/2010/735349
Malda, J., Boere, J., Van de lest, C. H., Weeren, P. V., and Wauben, M. H. (2016). Extracellular vesicles — new tool for joint repair and regeneration. Nat. Rev. Rheumatol. 12, 243–249. doi: 10.1038/nrrheum.2015.170
Marolt Presen, D., Traweger, A., Gimona, M., and Redl, H. (2019). Mesenchymal stromal cell-based bone regeneration therapies: from cell transplantation and tissue engineering to therapeutic secretomes and extracellular vesicles. Front. Bioeng. Biotechnol. 7:352. doi: 10.3389/fbioe.2019.00352
Matsuda, T., and Cepko, C. L. (2004). Electroporation and RNA Interference in the Rodent Retina in Vivo and in Vitro. Proc. Natl. Acad. Sci. U.S.A. 101, 16–22. doi: 10.1073/pnas.2235688100
Matsumoto, T., Komori, K., Shoji, T., Kuma, S., Kume, M., Yamaoka, T., et al. (2001). Successful and optimized in vivo gene transfer to rabbit carotid artery mediated by electronic pulse. Gene. Ther. 8, 1174–1179. doi: 10.1038/sj.gt.3301502
May, R. D., Tekari, A., Frauchiger, D. A., Krismer, A., Benneker, L. M., and Gantenbein, B. (2017). Efficient non-viral transfection of primary intervertebral disc cells by electroporation for tissue engineering application. Tissue. Eng. Part. C Methods 23, 30–37. doi: 10.1089/ten.TEC.2016.0355
Mehier-Humbert, S., and Guy, R. H. (2005). Physical methods for gene transfer: improving the kinetics of gene delivery into cells. Adv. Drug Deliv. Rev. 57, 733–753. doi: 10.1016/j.addr.2004.12.007
Melancon, M. P., Fuentes, A. T., Fuentes, D. T., Tian, L., Qiao, Y., Gu, J., et al. (2018). Development of an electroporation and nanoparticle-based therapeutic platform for bone metastases. Radiology 286, 149–157. doi: 10.1148/radiol.2017161721
Mellott, A. J., Forrest, M. L., and Detamore, M. S. (2013). Physical non-viral gene delivery methods for tissue engineering. Ann. Biomed. Eng. 41, 446–468. doi: 10.1007/s10439-012-0678-1
Mendt, M., Rezvani, K., and Shpall, E. (2019). Mesenchymal stem cell-derived exosomes for clinical use. Bone Marrow Transpl. 54, 789–792. doi: 10.1038/s41409-019-0616-z
Mentkowski, K. I., Snitzer, J. D., Rusnak, S., and Lang, J. K. (2018). Therapeutic potential of engineered extracellular vesicles. AAPS J. 20:50. doi: 10.1208/s12248-018-0211-z
Mintzer, M. A., and Simanek, E. E. (2009). Nonviral vectors for gene delivery. Chem. Rev. 109, 259–302. doi: 10.1021/cr800409e
Mir, L. M., Moller, P. H., André, F., and Gehl, J. (2005). Electric pulse-mediated gene delivery to various animal tissues. Adv. Genet. 54, 83–114. doi: 10.1016/S0065-2660(05)54005-7
Molnar, M. J., Gilbert, R., Lu, Y., Liu, A. B., Guo, A., Larochelle, N., et al. (2004). Factors influencing the efficacy, longevity, and safety of electroporation-assisted plasmid-based gene transfer into mouse muscles. Mol. Ther. 10, 447–455. doi: 10.1016/j.ymthe.2004.06.642
Monteiro, N., Ribeiro, D., Martins, A., Faria, S., Fonseca, N. A., Moreira, J. N., et al. (2014). Instructive nanofibrous scaffold comprising runt-related transcription factor 2 gene delivery for bone tissue engineering. ACS Nano 8, 8082–8094. doi: 10.1021/nn5021049
Moradian, H., Fasehee, H., Keshvari, H., and Faghihi, S. (2014). Poly(ethyleneimine) functionalized carbon nanotubes as efficient nano-vector for transfecting mesenchymal stem cells. Coll. Surf. B Biointerf. 122, 115–125. doi: 10.1016/j.colsurfb.2014.06.056
Moreira, C., Oliveira, H., Pires, L. R., Simoes, S., Barbosa, M. A., and Pego, A. P. (2009). Improving chitosan-mediated gene transfer by the introduction of intracellular buffering moieties into the chitosan backbone. Acta Biomater. 5, 2995–3006. doi: 10.1016/j.actbio.2009.04.021
Morrey, M. E., Anderson, P. A., Chambers, G., and Paul, R. (2008). Optimizing nonviral-mediated transfection of human intervertebral disc chondrocytes. Spine J. 8, 796–803. doi: 10.1016/j.spinee.2007.05.010
Mulcahy, L. A., Pink, R. C., and Carter, D. R. (2014). Routes and mechanisms of extracellular vesicle uptake. J. Extracell Vesic. 3:jev.v3.24641. doi: 10.3402/jev.v3.24641
Neumann, E., Schaefer-Ridder, M., Wang, Y., and Hofschneider, P. H. (1982). Gene transfer into mouse lyoma cells by electroporation in high electric fields. EMBO J. 1, 841–845. doi: 10.1002/j.1460-2075.1982.tb01257.x
Nguyen, M. K., Jeon, O., Krebs, M. D., Schapira, D., and Alsberg, E. (2014). Sustained localized presentation of RNA interfering molecules from in situ forming hydrogels to guide stem cell osteogenic differentiation. Biomaterials 35, 6278–6286. doi: 10.1016/j.biomaterials.2014.04.048
NIH Report (2002). Assessment of adenoviral vector safety and toxicity: report of the national institutes of health recombinant DNA advisory committee. Hum. Gene Ther. 13, 3–13. doi: 10.1089/10430340152712629
Nishida, K., Doita, M., Takada, T., Kakutani, K., Miyamoto, H., Shimomura, T., et al. (2006). Sustained transgene expression in intervertebral disc cells in vivo mediated by microbubble-enhanced ultrasound gene therapy. Spine 31, 1415–1419. doi: 10.1097/01.brs.0000219945.70675.dd
Nolte-’t Hoen, E. N. M., and Wauben, M. H. (2012). Immune cell-derived vesicles: modulators and mediators of inflammation. Curr. Pharm. Des. 18, 2357–2368. doi: 10.2174/138161212800166013
Nomikou, N., Feichtinger, G. A., Redl, H., and McHale, A. P. (2016). Ultrasound-mediated gene transfer (sonoporation) in fibrin-based matrices: potential for use in tissue regeneration. J. Tissue Eng. Regen. Med. 10, 29–39. doi: 10.1002/term.1730
Nomura, T., Nishimura, Y., Gotoh, H., and Ono, K. (2016). Rapid and efficient gene delivery into the adult mouse brain via focal electroporation. Sci. Rep. 6:29817. doi: 10.1038/srep29817
O’Brien, J. A., and Lummis, S. C. (2006). Biolistic transfection of neuronal cultures using a hand-held gene gun. Nat. Protoc. 1, 977–981. doi: 10.1038/nprot.2006.145
O’Brien, K., Breyne, K., Ughetto, S., Laurent, L. C., and Breakefield, X. O. (2020). RNA delivery by extracellular vesicles in mammalian cells and its applications. Nat. Rev. Mol. Cell. Biol. 26, 1–22. doi: 10.1038/s41580-020-0251-y
Odabas, S., Feichtinger, G. A., Korkusuz, P., Inci, I., Bilgic, E., Yar, A. S., et al. (2013). Auricular cartilage repair using cryogel scaffolds loaded with BMP-7-expressing primary chondrocytes. J. Tissue Eng. Regen. Med. 7, 831–840. doi: 10.1002/term.1634
Oliveira, A. C., Ferraz, M. P., Monteiro, F. J., and Simoes, S. (2009). Cationic liposome-DNA complexes as gene delivery vectors: development and. Acta Biomater. 5, 2142–2151. doi: 10.1016/j.actbio.2009.02.019
Olton, D., Li, J., Wilson, M. E., Rogers, T., Close, J., Huang, L., et al. (2007). Nanostructured calcium phosphates (NanoCaPs) for non-viral gene delivery: influence of the synthesis parameters on transfection efficiency. Biomaterials 28, 1267–1279. doi: 10.1016/j.biomaterials.2006.10.026
Ong, S. G., and Wu, J. C. (2015). Exosomes as potential alternatives to stem cell therapy in mediating cardiac regeneration. Circ. Res. 117, 7–9. doi: 10.1161/CIRCRESAHA.115.306593
O’Reilly, M., Jambou, R., Rosenthal, E., Montgomery, M., Hassani, M., Gargiulo, L., et al. (2015). The national institutes of health oversight of human gene transfer research: enhancing science and safety. Adv. Exp. Med. Biol. 871, 31–47. doi: 10.1007/978-3-319-18618-4_2
Orozco, L., Soler, R., Morera, C., Alberca, M., Sánchez, A., and García-Sancho, J. (2011). Intervertebral disc repair by autologous mesenchymal bone marrow cells: a pilot study. Transplantation 92, 822–828. doi: 10.1097/TP.0b013e3182298a15
Pack, D. W., Hoffman, A. S., Pun, S., and Stayton, P. S. (2005). Design and development of polymers for gene delivery. Nat. Rev. Drug Discov. 4, 581–593. doi: 10.1038/nrd1775
Pan, H., Zheng, Q., Yang, S., Guo, X., Wu, B., Zou, Z., et al. (2014). A novel peptide-modified and gene-activated biomimetic bone matrix accelerating bone regeneration. J. Biomed. Mater. Res. A 102, 2864–2874. doi: 10.1002/jbm.a.34961
Park, D. J., Choi, J. H., Leong, K. W., Kwon, J. W., and Eun, H. S. (2007). Tissue-engineered bone formation with gene transfer and mesenchymal stem cells in a minimally invasive technique. Laryngoscope 117, 1267–1271. doi: 10.1097/MLG.0b013e31805f680e
Park, K. (2009). PEI-DNA complexes with higher transfection efficiency and lower cytotoxicity. J. Control. Release 140:1. doi: 10.1016/j.jconrel.2009.09.015
Park, T. G., Jeong, J. H., and Kim, S. W. (2006). Current status of polymeric gene delivery systems. Adv. Drug Deliv. Rev. 58, 467–486. doi: 10.1016/j.addr.2006.03.007
Parveen, S., Misra, R., and Sahoo, S. K. (2012). Nanoparticles: a boon to drug delivery, therapeutics, diagnostics and imaging. Nanomedicine 8, 147–166. doi: 10.1016/j.nano.2011.05.016
Pasquet, L., Chabot, S., Bellard, E., Markelc, B., Rols, M. P., Reynes, J. P., et al. (2018). Safe and efficient novel approach for non-invasive gene electrotransfer to skin. Sci. Rep. 8:16833. doi: 10.1038/s41598-018-34968-6
Patil, S., Gao, Y. G., Lin, X., Li, Y., Dang, K., Tian, Y., et al. (2019). The development of functional non-viral vectors for gene delivery. Int. J. Mol. Sci. 20:5491. doi: 10.3390/ijms20215491
Pereira, H. F., Cengiz, I. F., Silva, F. S., Reis, R. L., and Oliveira, J. M. (2020). Scaffolds and coatings for bone regeneration. J. Mater. Sci. Mater. Med. 31:27. doi: 10.1007/s10856-020-06364-y
Pi, Y., Zhang, X., Shi, J., Zhu, J., Chen, W., Zhang, C., et al. (2011). Targeted delivery of non-viral vectors to cartilage in vivo using a chondrocyte-homing peptide identified by phage display. Biomaterials 32, 6324–6332. doi: 10.1016/j.biomaterials.2011.05.017
Pirmardvand Chegini, S., Varshosaz, J., and Taymouri, S. (2018). Recent approaches for targeted drug delivery in rheumatoid arthritis diagnosis and treatment. Artif. Cells Nanomed. Biotechnol. 46, 502–514. doi: 10.1080/21691401.2018.1460373
Pizzicannella, J., Gugliandolo, A., Orsini, T., Fontana, A., Ventrella, A., Mazzon, E., et al. (2019). Engineered extracellular vesicles from human periodontal-ligament stem cells increase VEGF/VEGFR2 expression during bone regeneration. Front. Physiol. 10:512. doi: 10.3389/fphys.2019.00512
Pomatto, M. A. C., Bussolati, B., D’Antico, S., Ghiotto, S., Tetta, C., Brizzi, M. F., et al. (2019). Improved loading of plasma-derived extracellular vesicles to encapsulate antitumor miRNAs. Mol. Ther. Methods Clin. Dev. 13, 133–144. doi: 10.1016/j.omtm.2019.01.001
Pranatharthiharan, S., Patel, M. D., D’Souza, A. A., and Devarajan, P. V. (2013). Inorganic nanovectors for nucleic acid delivery. Drug Del. Transl. Res. 3, 446–470. doi: 10.1007/s13346-012-0116-9
Prokop, A., Kozlov, E., Moore, W., and Davidson, J. M. (2002). Maximizing the in vivo efficiency of gene transfer by means of nonviral polymeric gene delivery vehicles. J. Pharm. Sci. 91, 67–76. doi: 10.1002/jps.1171
Properzi, F., Logozzi, M., and Fais, S. (2013). Exosomes: the future of biomarkers in medicine. Biomark. Med. 7, 769–778. doi: 10.2217/bmm.13.63
Raftery, R., O’Brien, F. J., and Cryan, S. A. (2013). Chitosan for gene delivery and orthopedic tissue engineering applications. Molecules 18, 5611–5647. doi: 10.3390/molecules18055611
Raftery, R. M., Walsh, D. P., Castaño, I. M., Heise, A., Duffy, G. P., Cryan, S. A., et al. (2016). Delivering nucleic-acid based nanomedicines on biomaterial scaffolds for orthopedic tissue repair: challenges, progress and future perspectives. Adv. Mater. 28, 5447–5469. doi: 10.1002/adma.201505088
Ramamoorth, M., and Narvekar, A. (2015). Non viral vectors in gene therapy- an overview. J. Clin. Diagn. Res. 9, GE01–GE06. doi: 10.7860/JCDR/2015/10443.5394
Rebersek, M., Miklavcic, D., Bertacchini, C., and Sack, M. (2014). Cell membrane electroporation-Part 3: the equipment. IEEE. Electr. Ins. Mag. 30, 8–18. doi: 10.1109/MEI.2014.6804737
Reckhenrich, A. K., Koch, C., Egana, J. T., and Plank, C. (2012). The use of non-viral gene vectors for bioactive poly-(D,L-lactide) implant surfaces in bone tissue engineering. Eur. Cell Mater. 23, 441–448. doi: 10.22203/ecm.v023a34
Rilla, K., Mustonen, A. M., Arasu, U. T., Härkönen, K., Matilainen, J., and Nieminen, P. (2019). Extracellular vesicles are integral and functional components of the extracellular matrix. Matrix Biol. 75–76, 201–219. doi: 10.1016/j.matbio.2017.10.003
Rim, Y. A., Nam, Y., Park, N., Jung, H., Lee, K., Lee, J., et al. (2020). Chondrogenic differentiation from induced pluripotent stem cells using non-viral minicircle vectors. Cells 9:582. doi: 10.3390/cells9030582
Rose, L. C., Kucharski, C., and Uludag, H. (2012). Protein expression following non-viral delivery of plasmid DNA coding for basic. Biomaterials 33, 3363–3374. doi: 10.1016/j.biomaterials.2012.01.031
Rosenthal, A. K., Gohr, C. M., Mitton-Fitzgerald, E., Grewal, R., Ninomiya, J., Coyne, C. B., et al. (2015). Autophagy modulates articular cartilage vesicle formation in primary articular chondrocytes. J. Biol. Chem. 290, 13028–13038. doi: 10.1074/jbc.M114.630558
Rüwald, J. M., Randau, T. M., Hilgers, C., Masson, W., Irsen, S., Eymael, R. L., et al. (2020). Extracellular vesicle isolation and characterization from periprosthetic joint synovial fluid in revision total joint arthroplasty. J. Clin. Med. 9:516. doi: 10.3390/jcm9020516
Sakai, D., Mochida, J., Iwashina, T., Hiyama, A., Omi, H., Imai, M., et al. (2006). Regenerative effects of transplanting mesenchymal stem cells embedded in atelocollagen to the degenerated intervertebral disc. Biomaterials 27, 335–345. doi: 10.1016/j.biomaterials.2005.06.038
Santos, J. L., Pandita, D., Rodrigues, J., Pego, A. P., Granja, P. L., and Tomas, H. (2011). Non-viral gene delivery to mesenchymal stem cells: methods, strategies and. Curr. Gene Ther. 11, 46–57. doi: 10.2174/156652311794520102
Saraf, A., and Mikos, A. G. (2006). Gene delivery strategies for cartilage tissue engineering. Adv. Drug Deliv. Rev. 58, 592–603. doi: 10.1016/j.addr.2006.03.005
Satkauskas, S., Ruzgys, P., and Venslauskas, M. S. (2012). Towards the mechanisms for efficient gene transfer into cells and tissues by means of cell electroporation. Expert. Opin. Biol. Ther. 12, 275–286. doi: 10.1517/14712598.2012.654775
Schafer, J., Hobel, S., Bakowsky, U., and Aigner, A. (2010). Liposome-polyethylenimine complexes for enhanced DNA and siRNA delivery. Biomaterials 31, 6892–6900. doi: 10.1016/j.biomaterials.2010.05.043
Shapiro, G., Lieber, R., Gazit, D., and Pelled, G. (2018). Recent advances and future of gene therapy for bone regeneration. Curr. Osteoporos. Rep. 16, 504–511. doi: 10.1007/s11914-018-0459-3
Shea, L. D., Smiley, E., Bonadio, J., and Mooney, D. J. (1999). DNA delivery from polymer matrices for tissue engineering. Nat. Biotechnol. 17, 551–554. doi: 10.1038/9853
Shekaran, A., Garcia, J. R., Clark, A. Y., Kavanaugh, T. E., Lin, A. S., Guldberg, R. E., et al. (2014). Bone regeneration using an alpha 2 beta 1 integrin-specific hydrogel as a BMP-2 delivery vehicle. Biomaterials 35, 5453–5461. doi: 10.1016/j.biomaterials.2014.03.055
Sheyn, D., Kallai, I., Tawackoli, W., Cohn Yakubovich, D., Oh, A., Su, S., et al. (2011). Gene-modified adult stem cells regenerate vertebral bone defect in a rat model. Mol. Pharm. 8, 1592–1601. doi: 10.1021/mp200226c
Sheyn, D., Kimelman-Bleich, N., Pelled, G., Zilberman, Y., Gazit, D., and Gazit, Z. (2008a). Ultrasound-based nonviral gene delivery induces bone formation in vivo. Gene Ther. 15, 257–266. doi: 10.1038/sj.gt.3303070
Sheyn, D., Pelled, G., Zilberman, Y., Talasazan, F., Frank, J. M., Gazit, D., et al. (2008b). Nonvirally engineered porcine adipose tissue-derived stem cells: use in posterior spinal fusion. Stem Cells 26, 1056–1064. doi: 10.1634/stemcells.2007-0858
Shi, J., Ma, Y., Zhu, J., Chen, Y., Sun, Y., Yao, Y., et al. (2018). A review on electroporation-based intracellular delivery. Molecules 23:3044. doi: 10.3390/molecules23113044
Shi, B., Xue, M., Wang, Y., Li, D., Zhao, X., and Li, X. (2018). An improved method for increasing the efficiency of gene transfection and transduction. Int. J. Physiol. Pathophysiol. Pharmacol. 10, 95–104.
Simpson, R. J., Jensen, S. S., and Lim, J. W. (2008). Proteomic profiling of exosomes: current perspectives. Proteomics 8, 4083–4099. doi: 10.1002/pmic.200800109
Smith, D. K. (2008). Dendrimers and the double helix–from DNA binding towards gene therapy. Curr. Top. Med. Chem. 8, 1187–1203. doi: 10.2174/156802608785849030
Sokołowska, E., and Błachnio-Zabielska, A. U. (2019). A critical review of electroporation as a plasmid delivery system in mouse skeletal muscle. Int. J. Mol. Sci. 20:2776. doi: 10.3390/ijms20112776
Somiari, S., Glasspool-Malone, J., Drabick, J. J., Gilbert, R. A., Heller, R., Jaroszeski, M. J., et al. (2000). Theory and in vivo application of electroporative gene delivery. Mol. Ther. 2, 178–187. doi: 10.1006/mthe.2000.0124
Song, H., and Park, K. H. (2020). Regulation and function of SOX9 during cartilage development and regeneration. Semin. Cancer. Biol. S1044-579X(20)30096-1. doi: 10.1016/j.semcancer.2020.04.008
Song, Y., Zheng, J., Yan, M., Ding, W., Xu, K., Fan, Q., et al. (2015). The effect of irreversible electroporation on the femur: experimental study in a rabbit model. Sci. Rep. 5:18187. doi: 10.1038/srep18187
Stevens, M. M., Marini, R. P., Schaefer, D., Aronson, J., Langer, R., and Shastri, V. P. (2005). In vivo engineering of organs: the bone bioreactor. Proc. Natl. Acad. Sci. U.S.A. 102, 11450–11455. doi: 10.1073/pnas.0504705102
Stöve, J., Fiedler, J., Huch, K., Günther, K. P., Puhl, W., and Brenner, R. (2002). Lipofection of rabbit chondrocytes and long lasting expression of a lacZ reporter system in alginate beads. Osteoarthr. Cart. 10, 212–217. doi: 10.1053/joca.2001.0495
Su, C. H., Wu, Y. J., Wang, H. H., and Yeh, H. I. (2012). Nonviral gene therapy targeting cardiovascular system. Am. J. Physiol. Heart Circ. Physiol. 303, H629–H638. doi: 10.1152/ajpheart.00126.2012
Sudo, H., and Minami, A. (2011). Caspase 3 as a therapeutic target for regulation of intervertebral disc degeneration in rabbits. Arthrit. Rheum. 63, 1648–1657. doi: 10.1002/art.30251
Sugrue, A., Vaidya, V. R., Livia, C., Padmanabhan, D., Abudan, A., Isath, A., et al. (2020). Feasibility of selective cardiac ventricular electroporation. PLoS One 15:e022921. doi: 10.1371/journal.pone.0229214
Sun, X. D., Jeng, L., Bolliet, C., Olsen, B. R., and Spector, M. (2009). Non-viral endostatin plasmid transfection of mesenchymal stem cells via collagen scaffolds. Biomaterials 30, 1222–1231. doi: 10.1016/j.biomaterials.2008.10.020
Sutaria, D. S., Badawi, M., Phelps, M. A., and Schmittgen, T. D. (2017). Achieving the promise of therapeutic extracellular vesicles: the devil is in details of therapeutic loading. Pharm. Res. 34, 1053–1106. doi: 10.1007/s11095-017-2123-5
Tachibana, R., Harashima, H., Ide, N., Ukitsu, S., Ohta, Y., Suzuki, N., et al. (2002). Quantitative analysis of correlation between number of nuclear plasmids and gene expression activity after transfection with cationic liposomes. Pharm. Res. 19, 377–381. doi: 10.1023/a:1015162722295
Tang, W., Jiang, D., Li, Z., Zhu, L., Shi, J., Yang, J., et al. (2019). Recent advances in microfluidic cell sorting techniques based on both physical and biochemical principles. Electrophoresis 40, 930–954. doi: 10.1002/elps.201800361
Tang, S., Richards, J., Khan, S., Hoyland, J., Gallego-Perez, D., Higuita-Castro, N., et al. (2019). Nonviral transfection with Brachyury reprograms human intervertebral disc cells to a pro-anabolic anti-catabolic/inflammatory phenotype: a proof of concept study. J. Orthop. Res. 37, 2389–2400. doi: 10.1002/jor.24408
Tavakoli, R., Gazdhar, A., Pierog, J., Bogdanova, A., Gugger, M., Pringle, I. A., et al. (2006). Electroporation-mediated interleukin-10 overexpression in skeletal muscle reduces acute rejection in rat cardiac allografts. J. Gene Med. 8, 242–248. doi: 10.1002/jgm.859
Tian, F., Cui, D., Schwarz, H., Estrada, G. G., and Kobayashi, H. (2006). Cytotoxicity of single-wall carbon nanotubes on human fibroblasts. Toxicol. Vitro 20, 1202–1212. doi: 10.1016/j.tiv.2006.03.008
Tierney, E. G., Duffy, G. P., Hibbitts, A. J., Cryan, S. A., and O’Brien, F. J. (2012). The development of non-viral gene-activated matrices for bone regeneration using polyethyleneimine (PEI) and collagen-based scaffolds. J. Control. Release 158, 304–311. doi: 10.1016/j.jconrel.2011.11.026
Tkach, M., Kowal, J., Zucchetti, A. E., Enserink, L., Jouve, M., Lankar, D., et al. (2017). Qualitative differences in T-cell activation by dendritic cell-derived extracellular vesicle subtypes. EMBO J. 36, 3012–3028. doi: 10.15252/embj.201696003
Tkach, M., and Théry, C. (2016). Communication by extracellular vesicles: where we are and where we need to go. Cell 164, 1226–1232. doi: 10.1016/j.cell.2016.01.043
To, K., Romain, K., Mak, C., Kamaraj, A., Henson, F., and Khan, W. (2020). The treatment of cartilage damage using human mesenchymal stem cell-derived extracellular vesicles: a systematic review of in vivo studies. Front. Bioeng. Biotechnol. 8:580. doi: 10.3389/fbioe.2020.00580
Torchilin, V. P. (2005). Recent advances with liposomes as pharmaceutical carriers. Nat. Rev. Drug Discov. 4, 145–160. doi: 10.1038/nrd1632
Tran, T. H., Mattheolabakis, G., Aldawsari, H., and Amiji, M. (2015). Exosomes as nanocarriers for immunotherapy of cancer and inflammatory diseases. Clin. Immunol. 160, 46–58. doi: 10.1016/j.clim.2015.03.021
Trubiani, S. D., Piattelli, A., Diomede, F., and Pizzicannella, J. (2019). Human oral stem cells, biomaterials and extracellular vesicles: a promising tool in bone tissue repair. Int. J. Mol. Sci. 20:4987. doi: 10.3390/ijms20204987
Tschon, M., Salamanna, F., Ronchetti, M., Cavani, F., Gasbarrini, A., Boriani, S., et al. (2016). Feasibility of electroporation in bone and in the surrounding clinically relevant structures: a preclinical investigation. Technol. Cancer Res. Treat. 15, 737–748. doi: 10.1177/1533034615604454
Tsuchiya, S., Chiba, M., Kishimoto, K. N., Nakamura, H., Tsuchiya, M., and Hayashi, H. (2017). Transfer of the bone morphogenetic protein 4 gene into rat periodontal ligament by in vivo electroporation. Arch. Oral. Biol. 74, 123–132. doi: 10.1016/j.archoralbio.2016.11.013
Tzeng, S. Y., Yang, P. H., Grayson, W. L., and Green, J. J. (2011). Synthetic poly(ester amine) and poly(amido amine) nanoparticles for efficient DNA and siRNA delivery to human endothelial cells. Int. J. Nanomed. 6, 3309–3322. doi: 10.2147/IJN.S27269
Urban, J. P., Smith, S., and Fairbank, J. C. (2004). Nutrition of the intervertebral disc. Spine 29, 2700–2709. doi: 10.1097/01.brs.0000146499.97948.52
Uskokovic, V., and Uskokovic, D. P. (2011). Nanosized hydroxyapatite and other calcium phosphates: chemistry of formation and application as drug and gene delivery agents. J. Biomed. Mater. Res. B Appl. Biomater. 96, 152–191. doi: 10.1002/jbm.b.31746
Vajgel, A., Mardas, N., Farias, B. C., Petrie, A., Cimoes, R., and Donos, N. (2014). A systematic review on the critical size defect model. Clin. Oral Implants Res. 25, 879–893. doi: 10.1111/clr.12194
van Niel, G., D’Angelo, G., and Raposo, G. (2018). Shedding light on the cell biology of extracellular vesicles. Nat. Rev. Mol. Cell. Biol. 19, 213–228. doi: 10.1038/nrm.2017.125
Vroomen, L. G. P. H., Petre, E. N., Cornelis, F. H., Solomon, S. B., and Srimathveeravalli, G. (2017). Irreversible electroporation and thermal ablation of tumors in the liver, lung, kidney and bone: what are the differences? Diagn. Interv. Imaging 98, 609–617. doi: 10.1016/j.diii.2017.07.007
Wang, S., and Lee, L. J. (2013). Micro-/nanofluidics based cell electroporation. Biomicrofluidics 7:11301. doi: 10.1063/1.4774071
Wang, W., Li, W., Ma, N., and Steinhoff, G. (2013). Non-viral gene delivery methods. Curr. Pharm. Biotechnol. 14, 46–60.
Wegman, F., Bijenhof, A., Schuijff, L., Oner, F. C., Dhert, W. J., and Alblas, J. (2011). Osteogenic differentiation as a result of BMP-2 plasmid DNA based gene therapy in vitro and in vivo. Eur. Cell Mater. 21, 230–242. doi: 10.22203/ecm.v021a18
Wegman, F., Geuze, R. E., van der Helm, Y. J., Cumhur Öner, F., Dhert, W. J. A., and Alblas, J. (2014). Gene delivery of bone morphogenetic protein-2 plasmid DNA promotes bone formation. J. Tissue Eng. Regen. Med. 8, 763–770. doi: 10.1002/term.1571
Wells, D. J. (2004). Gene therapy progress and prospects: electroporation and other physical methods. Gene Ther. 11, 1363–1369. doi: 10.1038/sj.gt.3302337
Wheeler, J. J., Palmer, L., Ossanlou, M., MacLachlan, I., Graham, R. W., Zhang, Y. P., et al. (1999). Stabilized plasmid-lipid particles: construction and characterization. Gene Ther. 6, 271–281. doi: 10.1038/sj.gt.3300821
Wilson, J. M. (2009). Medicine. A history lesson for stem cells. Science 324, 727–728. doi: 10.1126/science.1174935
Winn, S. R., Chen, J. C., Gong, X., Bartholomew, S. V., Shreenivas, S., and Ozaki, W. (2005). Non-viral-mediated gene therapy approaches for bone repair. Orthod. Craniofac. Res. 8, 183–190. doi: 10.1111/j.1601-6343.2005.00332.x
Wittenauer, R., Smith, L., and Aden, K. (2013). Background Paper 6.12 Osteoarthritis. Geneva: World Health Organization.
Wong, T. K., and Neumann, E. (1982). Electric field mediated gene transfer. Biochem. Biophys. Res. Commun. 107, 584–587. doi: 10.1016/0006-291X(82)91531-5
Wu, L. N., Genge, B. R., and Wuthier, R. E. (1992). Evidence for specific interaction between matrix vesicle proteins and the connective tissue matrix. Bone Miner. 17, 247–252. doi: 10.1016/0169-6009(92)90745-y
Wu, X., Wang, Y., Xiao, Y., Crawford, R., Mao, X., and Prasadam, I. (2019). Extracellular vesicles: potential role in osteoarthritis regenerative medicine. J. Orthop. Translat. 21, 73–80. doi: 10.1016/j.jot.2019.10.012
Wu, Y., Terp, M. C., Kwak, K. J., Gallego-Perez, D., Nana-Sinkam, S. P., and Lee, L. J. (2013). Surface-mediated nucleic acid delivery by lipoplexes prepared in microwell arrays. Small 9, 2358–2367. doi: 10.1002/smll.201202258
Xie, H., Wang, Z., Zhang, L., Lei, Q., Zhao, A., Wang, H., et al. (2017). Extracellular vesicle-functionalized decalcified bone matrix scaffolds with enhanced pro-angiogenic and pro-bone regeneration activities. Sci. Rep. 7:45622. doi: 10.1038/srep45622
Xie, X., Xu, A. M., Leal-Ortiz, S., Cao, Y., Garner, C. C., and Melosh, N. A. (2013). Nanostraw-electroporation system for highly efficient intracellular delivery and transfection. ACS Nano 7, 4351–4358. doi: 10.1021/nn400874a
Yan, J., Zhang, C., Zhao, Y., Cao, C., Wu, K., Zhao, L., et al. (2014). Non-viral oligonucleotide antimiR-138 delivery to mesenchymal stem cell sheets and the effect on osteogenesis. Biomaterials 35, 7734–7749. doi: 10.1016/j.biomaterials.2014.05.089
Yáñez-Mó, M., Siljander, P. R., Andreu, Z., Zavec, A. B., Borràs, F. E., Buzas, E. I., et al. (2015). Biological properties of extracellular vesicles and their physiological functions. J. Extracell Vesic. 4:27066. doi: 10.3402/jev.v4.27066
Yang, Q. Q., Shao, Y. X., Zhang, L. Z., and Zhou, Y. L. (2018). Therapeutic strategies for flexor tendon healing by nanoparticle-mediated co-delivery of bFGF and VEGFA genes. Coll. Surf. B Biointerf. 164, 165–176. doi: 10.1016/j.colsurfb.2018.01.031
Yarborough, M., and Sharp, R. R. (2009). Public trust and research a decade later: what have we learned since Jesse Gelsinger’s death? Mol. Genet. Metab. 97, 4–5. doi: 10.1016/j.ymgme.2009.02.002
Yuan, Q., Shah, J., Hein, S., and Misra, R. D. (2010). Controlled and extended drug release behavior of chitosan-based nanoparticle carrier. Acta Biomater. 6, 1140–1148. doi: 10.1016/j.actbio.2009.08.027
Zhang, G., Budker, V., Williams, P., Subbotin, V., and Wolff, J. A. (2001). Efficient expression of naked DNA delivered intraarterially to limb muscles of nonhuman primates. Hum. Gene Ther. 12, 427–438. doi: 10.1089/10430340150504046
Zhang, X., Kovtun, A., Mendoza-Palomares, C., Oulad-Abdelghani, M., Fioretti, F., Rinckenbach, S., et al. (2010). SiRNA-loaded multi-shell nanoparticles incorporated into a multilayered film as a reservoir for gene silencing. Biomaterials 31, 6013–6018. doi: 10.1016/j.biomaterials.2010.04.024
Zhao, X., Li, Z., Pan, H., Liu, W., Lv, M., Leung, F., et al. (2013). Enhanced gene delivery by chitosan-disulfide-conjugated LMW-PEI for facilitating osteogenic differentiation. Acta Biomater. 9, 6694–6703. doi: 10.1016/j.actbio.2013.01.039
Zhou, R., and Dean, D. A. (2007). Gene transfer of interleukin 10 to the murine cornea using electroporation. Exp. Biol. Med. 232, 362–369.
Zhu, W., Huang, L., Li, Y., Zhang, X., Gu, J., Yan, Y., et al. (2012). Exosomes derived from human bone marrow mesenchymal stem cells promote tumor growth in vivo. Cancer Lett. 315, 28–37. doi: 10.1016/j.canlet.2011.10.002
Zhu, W., Thomas, C. E., and Sparling, P. F. (2004). DNA immunization of mice with a plasmid encoding Neisseria gonorrhea PorB protein by intramuscular injection and epidermal particle bombardment. Vaccine 22, 660–669. doi: 10.1016/j.vaccine.2003.08.036
Keywords: non-viral gene delivery, bone, tendon, cartilage, intervertebral disk, GDF5, FOXF1, BMP2
Citation: Gantenbein B, Tang S, Guerrero J, Higuita-Castro N, Salazar-Puerta AI, Croft AS, Gazdhar A and Purmessur D (2020) Non-viral Gene Delivery Methods for Bone and Joints. Front. Bioeng. Biotechnol. 8:598466. doi: 10.3389/fbioe.2020.598466
Received: 24 August 2020; Accepted: 26 October 2020;
Published: 19 November 2020.
Edited by:
Georg A. Feichtinger, University of Leeds, United KingdomReviewed by:
Ilia Bozo, Federal Medical and Biological Agency of Russia, RussiaCopyright © 2020 Gantenbein, Tang, Guerrero, Higuita-Castro, Salazar-Puerta, Croft, Gazdhar and Purmessur. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Devina Purmessur, RGV2aW5hLlB1cm1lc3N1ckBvc3VtYy5lZHU=; d2FsdGVyLjM2OEBvc3UuZWR1; Benjamin Gantenbein, QmVuamFtaW4uR2FudGVuYmVpbkBkYm1yLnVuaWJlLmNo; QmVuamFtaW4uR2FudGVuYmVpbkBnbWFpbC5jb20=
†These authors have contributed equally to this work
‡Orcid: Benjamin Gantenbein, orcid.org/0000-0002-9005-0655; Shirley Tang, orcid.org/0000-0001-8807-2348; Julien Guerrero, orcid.org/0000-0003-4130-4230; Natalia Higuita-Castro, orcid.org/0000-0002-3297-6281; Ana I. Salazar-Puerta, orcid.org/0000-0003-4954-6226; Andreas S. Croft, orcid.org/0000-0003-1472-1979; Amiq Gazdhar, orcid.org/0000-0002-4807-8785; Devina Purmessur, orcid.org/0000-0002-7445-1678
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.