Rare genetic diseases collectively affect around 300 million people worldwide and a staggering 74% of them are childhood-onset conditions with neurological manifestations (Lee et al., 2020). Correction or replacement of malfunctioning genes using viral vectors showed outstanding pre-clinical and clinical benefits for inherited pediatric diseases, in particular those for which non-cell autonomous mechanisms such as cross-correction is effective (i.e., X-linked adrenoleukodystrophy or Batten's disease) (Naldini, 2015; Aubourg, 2016).
However, this field still faces important challenges in particular for conditions caused by genes encoding for non-secreted proteins and whose dysfunction affect large regions of the brain that cannot be covered by intra-cerebral delivery. This includes a plethora of rare genetic conditions such as mitochondrial diseases, lysosomal storage diseases, polyglutamine diseases, and neuromuscular diseases.
Gene delivery to the brain via systemic injection can be successfully achieved during a short period of time after birth where the blood-brain barrier (BBB) remains permeable. This entails establishing a diagnosis within this privileged time-window. Unfortunately, despite being early-onset diseases, many pediatric conditions manifest the first clinical signs when the BBB is no longer penetrable by viral vectors. Because of the limitations of our current toolbox, alternative strategies had to be designed in order to allow transgene delivery to the central nervous system (CNS) when the BBB has acquired its selective permeability. Methods to engineer the viral capsid such as peptide insertion, chemical modifications, shuffled genome libraries, directed evolution, or rationally targeted mutagenesis led to the generation of tenth of artificial variants among which some have been designed with the purpose to cross more efficiently the BBB when delivered into the bloodstream (Castle et al., 2016).
By combining the insertion of randomized sequences in a lox sites-containing cap gene with multiple selection rounds in Cre transgenic mice, Deverman and colleagues created series of brain-penetrating adeno-associated viral (AAV) vector variants. One of these, namely the AAV-PHP.B vector, induced a transgene expression in the CNS at least 40-fold greater than the parental AAV9 capsid when injected intravenously, and it retained a cellular tropism for peripheral organs (Deverman et al., 2016). In a follow-up study, the same group further evolved the PHP.B variant for better neuronal transduction. By applying the same Cre recombinase-based AAV target evolution strategy to the PHP.B capsid, they isolated the AAV-PHP.eB variant that showed enhanced CNS infectivity, enabling the reduction of the viral load delivered intravenously (Chan et al., 2017).
The generation of these novel brain-penetrant viral vectors opened a unique opportunity to overcome the obstacle that represents the BBB in the development of gene therapy for conditions with CNS pathology. Recent reports show that this idea was indeed quickly picked up by several groups and applied to a wide range of genetic diseases. They confirmed that the engineered AAV-PHP.B and AAV-PHP.eB variants efficiently transduced the CNS following injection in the vascular milieu. These tools were then used to restore the expression of defectives genes in several mouse models of mitochondrial diseases (i.e., mitochondrial hyper-fusion, and Leigh syndrome), cytoskeleton dysfunction (i.e., CDKL5 deficiency disorder), Rett syndrome and lysosomal disease (i.e., Pompe disease and Sandhoff disease). In all of these animal models, reinstatement of gene expression in the CNS and peripheral organs afforded robust therapeutic effects. The broad correction of pathological signs resulted in behavioral improvements and increased lifespan (Table 1). Notably, for some of these severe conditions, the use of these brain-penetrant AAV vectors provided the first demonstration that their respective pathological phenotypes can be rescued by gene therapy. This is for instance the case for the Ndufs4−/− mouse model of Leigh syndrome for which previous attempts of gene replacement therapy using an AAV9-Ndusf4 vector in neonates did not show significant effects (Di Meo et al., 2017). In addition, the in vivo studies listed in Table 1 have been performed in KO mice which is a paradigm that does not accurately mimic the genetic defects observed in patients. Mutations often result in the synthesis of a dysfunctional protein that can exert a dominant negative effect potentially hampering the therapeutic efficacy of the transgene. This question started to be addressed by Gao and colleagues who extended their findings in iPSC-derived neurons from a patient with CDKL5 disorder and confirmed the phenotypic improvement (Gao et al., 2020).
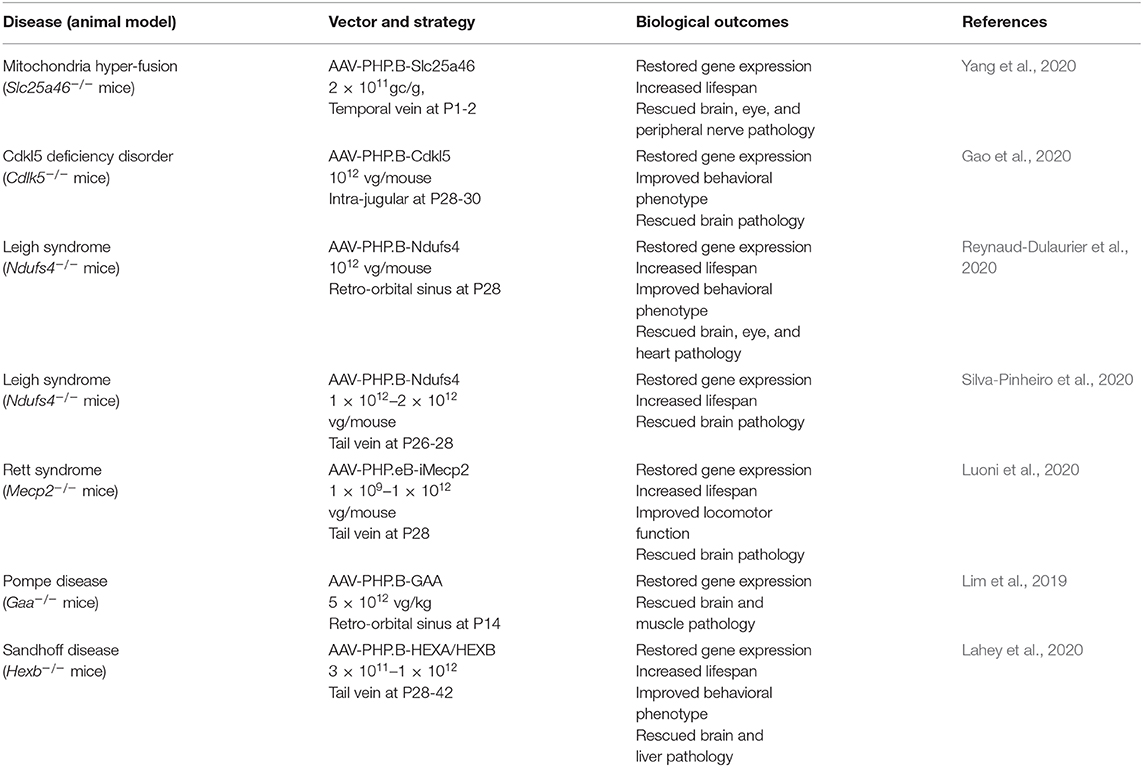
Table 1. Listing the preclinical studies of gene replacement therapies using the PHP.B or PHP.eB vectors in mouse models of rare genetic diseases with CNS pathology.
One can anticipate that this first set of pre-clinical studies will pave the way for similar strategies in other models of severe inherited conditions for which no curative options are currently available. In addition, the positive outcomes observed in these models of fatal neurological disorders make the PHP variants attractive tools for other brain conditions with late-onset. It this line, other groups have already extended the therapeutic repertoire of these new viral vectors by successfully tested them in models of Parkinson's and Alzheimer's disease (Morabito et al., 2017; Jin et al., 2020). Targeted delivery of AAV-PHP.B vectors also provided benefits in mouse models of deafness and retinitis pigmentosa (Giannelli et al., 2018; Gyorgy et al., 2019).
In parallel to these gene therapy studies, other research groups examined the versatility of the PHP variants. Since these artificial serotypes were isolated after several rounds of in vivo selection in C57BL6 mice, the brain-penetrant property of the AAV-PHP.B vector was tested in other genetic backgrounds. These experiments revealed that its ability to cross the BBB is strain-dependent and genetic approaches identified the lymphocyte antigen 6 complex locus A (Ly6a), a glycolsylphosphatidylinositol-anchored protein expressed by endothelial cells, as a key determinant for this differential efficacy (Hordeaux et al., 2018, 2019; Challis et al., 2019; Huang et al., 2019; Matsuzaki et al., 2019; Batista et al., 2020). Moreover, when tested in non-human primates, the AAV-PHP.B vector showed lower transduction efficiency than that observed in mice, highlighting inter-species variability in the BBB penetrance of this novel AAV serotype (Matsuzaki et al., 2018; Liguore et al., 2019). These observations suggest that while being powerful tools to achieve genetic manipulations in the rodent brain (e.g. gene replacement, editing, optogenetics), the first generation of PHP variants are unlikely to be used in clinic. This being said, Kumar and colleagues performed a multiplexed screening including a selection step in various mouse strains and they isolated a new capsid (i.e., PHP.C2) that induced good brain transduction even in genetic backgrounds expressing low levels of Ly6a. Yet, this novel variant needs to be tested in other species as well as in disease models in order to determine its potential transferability to humans.
This strategy of capsid engineering does not offer infinite possibilities and because of the shortage in AAV capsids, alternative approaches must be developed in order to overcome the limitations of these novel vectors in particular regarding their use in clinic. Capsid engineering also goes along with changes in the selectivity of tissue infection and cellular tropism. While the PHP.B serotype retains a transduction of peripheral tissues similar to that of the AAV9 parental capsid, the improved brain penetrance of the PHP.eB vector is at the expense of peripheral organs (Chan et al., 2017). This makes the PHP.eB serotype an interesting tool for conditions with dominant CNS pathology but less attractive for multisystem diseases.
Also, despite PHP vectors showed great efficacy in preclinical disease models, they are unlikely to be a solution for all brain disorders. Their region-dependent infectivity as well as their heterogenous tropism for neural cells represents a limitation for some diseases where specific cell types are affected (Deverman et al., 2016; Chan et al., 2017; Challis et al., 2019). This is for instance the case for multiple system atrophy in which alpha-synuclein-rich aggregates accumulate in oligodendrocytes, a cellular population that is poorly infected by these vectors in comparison to neurons and astrocytes (Deverman et al., 2016). Consequently, a “one-size-fits-all” strategy is unlikely to work and similar to the development of personalized medicine, some pathologies may require tailor-made vector constructs with features driven by the disease characteristics.
In summary, recent efforts in designing brain-penetrant AAV-capsids have made an important step forward by generating several tools. Their valuable features have already been challenged in animal models of severe diseases and they demonstrated remarkable therapeutic effects. Regardless of their applicability in a clinical setting, these emerging tools offer a unique opportunity to provide the proof-of-concept that many genetic pediatric diseases with CNS pathology could be rescued by gene delivery when suitable viral vectors will be available. This important piece of information brings new hope for patients affected by these conditions.
Author Contributions
All authors contributed to the preparation and writing of this paper and agreed on its final version.
Funding
MD was supported by an IDEX Chair of Excellence from the University of Grenoble-Alpes, the Edmond J. Safra Foundation, the Agence Nationale de la Recherche (ANR-JCJC program, grant #ANR-17-CE37-0008-01, and ANR-15-IDEX-02 NeuroCoG in the framework of the Investissement d'Avenir).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
MD laboratory is member of the Grenoble center of Excellence in Neurodegeneration.
References
Aubourg, P. (2016). Gene therapy for rare central nervous system diseases comes to age. Endocr. Dev 30, 141–146. doi: 10.1159/000439339
Batista, A. R., King, O. D., Reardon, C. P., Davis, C., Shankaracharya, P. V., Gray-Edwards, H., et al. (2020). Ly6a differential expression in blood-brain barrier is responsible for strain specific central nervous system transduction profile of AAV-PHP.B. Hum. Gene Ther. 31, 90–102. doi: 10.1089/hum.2019.186
Castle, M. J., Turunen, H. T., Vandenberghe, L. H., and Wolfe, J. H. (2016). Controlling AAV tropism in the nervous system with natural and engineered capsids. Methods Mol. Biol. 1382, 133–149. doi: 10.1007/978-1-4939-3271-9_10
Challis, R. C., Ravindra Kumar, S., Chan, K. Y., Challis, C., Beadle, K., Jang, M. J., et al. (2019). Systemic AAV vectors for widespread and targeted gene delivery in rodents. Nat. Protoc. 14, 379–414. doi: 10.1038/s41596-018-0097-3
Chan, K. Y., Jang, M. J., Yoo, B. B., Greenbaum, A., Ravi, N., Wu, W. L., et al. (2017). Engineered AAVs for efficient noninvasive gene delivery to the central and peripheral nervous systems. Nat. Neurosci. 20, 1172–1179. doi: 10.1038/nn.4593
Deverman, B. E., Pravdo, P. L., Simpson, B. P., Kumar, S. R., Chan, K. Y., Banerjee, A., et al. (2016). Cre-dependent selection yields AAV variants for widespread gene transfer to the adult brain. Nat. Biotechnol. 34, 204–209. doi: 10.1038/nbt.3440
Di Meo, I., Marchet, S., Lamperti, C., Zeviani, M., and Viscomi, C. (2017). AAV9-based gene therapy partially ameliorates the clinical phenotype of a mouse model of Leigh syndrome. Gene Ther. 24, 661–667. doi: 10.1038/gt.2017.53
Gao, Y., Irvine, E. E., Eleftheriadou, I., Naranjo, C. J., Hearn-Yeates, F., Bosch, L., et al. (2020). Gene replacement ameliorates deficits in mouse and human models of cyclin-dependent kinase-like 5 disorder. Brain 143, 811–832. doi: 10.1093/brain/awaa028
Giannelli, S. G., Luoni, M., Castoldi, V., Massimino, L., Cabassi, T., Angeloni, D., et al. (2018). Cas9/sgRNA selective targeting of the P23H Rhodopsin mutant allele for treating retinitis pigmentosa by intravitreal AAV9.PHP.B-based delivery. Hum. Mol. Genet. 27, 761–779. doi: 10.1093/hmg/ddx438
Gyorgy, B., Meijer, E. J., Ivanchenko, M. V., Tenneson, K., Emond, F., Hanlon, K. S., et al. (2019). Gene transfer with AAV9-PHP.B rescues hearing in a mouse model of usher syndrome 3A and transduces hair cells in a non-human primate. Mol. Ther. Methods Clin. Dev. 13, 1–13. doi: 10.1016/j.omtm.2018.11.003
Hordeaux, J., Wang, Q., Katz, N., Buza, E. L., Bell, P., and Wilson, J. M. (2018). The neurotropic properties of AAV-PHP.B are limited to C57BL/6J mice. Mol. Ther. 26, 664–668. doi: 10.1016/j.ymthe.2018.01.018
Hordeaux, J., Yuan, Y., Clark, P. M., Wang, Q., Martino, R. A., Sims, J. J., et al. (2019). The GPI-linked protein LY6A drives AAV-PHP.B transport across the blood-brain barrier. Mol. Ther. 27, 912–921. doi: 10.1016/j.ymthe.2019.02.013
Huang, Q., Chan, K. Y., Tobey, I. G., Chan, Y. A., Poterba, T., Boutros, C. L., et al. (2019). Delivering genes across the blood-brain barrier: LY6A, a novel cellular receptor for AAV-PHP.B capsids. PLoS ONE 14:e0225206. doi: 10.1371/journal.pone.0225206
Jin, J., Guo, J., Cai, H., Zhao, C., Wang, H., Liu, Z., et al. (2020). M2-like microglia polarization attenuates neuropathic pain associated with Alzheimer's disease. J. Alzheimers Dis. 76:1255–1265. doi: 10.3233/JAD-200099
Lahey, H. G., Webber, C. J., Golebiowski, D., Izzo, C. M., Horn, E., Taghian, T., et al. (2020). Pronounced therapeutic benefit of a single bidirectional AAV vector administered systemically in sandhoff mice. Mol. Ther. 28:1–11. doi: 10.1016/j.ymthe.2020.06.021
Lee, C. E., Singleton, K. S., Wallin, M., and Faundez, V. (2020). Rare genetic diseases: nature's experiments on human development. iScience 23:101123. doi: 10.1016/j.isci.2020.101123
Liguore, W. A., Domire, J. S., Button, D., Wang, Y., Dufour, B. D., Srinivasan, S., et al. (2019). AAV-PHP.B administration results in a differential pattern of CNS biodistribution in non-human primates compared with mice. Mol. Ther. 27, 2018–2037. doi: 10.1016/j.ymthe.2019.07.017
Lim, J. A., Yi, H., Gao, F., Raben, N., Kishnani, P. S., and Sun, B. (2019). Intravenous injection of an AAV-PHP.B vector encoding human acid alpha-glucosidase rescues both muscle and CNS defects in murine pompe disease. Mol. Ther. Methods Clin. Dev. 12, 233–245. doi: 10.1016/j.omtm.2019.01.006
Luoni, M., Giannelli, S., Indrigo, M. T., Niro, A., Massimino, L., Iannielli, A., et al. (2020). Whole brain delivery of an instability-prone Mecp2 transgene improves behavioral and molecular pathological defects in mouse models of Rett syndrome. Elife 9:1–30. doi: 10.7554/eLife.52629
Matsuzaki, Y., Konno, A., Mochizuki, R., Shinohara, Y., Nitta, K., Okada, Y., et al. (2018). Intravenous administration of the adeno-associated virus-PHP.B capsid fails to upregulate transduction efficiency in the marmoset brain. Neurosci. Lett. 665, 182–188. doi: 10.1016/j.neulet.2017.11.049
Matsuzaki, Y., Tanaka, M., Hakoda, S., Masuda, T., Miyata, R., Konno, A., et al. (2019). Neurotropic properties of AAV-PHP.B are shared among diverse inbred strains of mice. Mol. Ther. 27, 700–704. doi: 10.1016/j.ymthe.2019.02.016
Morabito, G., Giannelli, S. G., Ordazzo, G., Bido, S., Castoldi, V., Indrigo, M., et al. (2017). AAV-PHP.B-mediated global-scale expression in the mouse nervous system enables GBA1 gene therapy for wide protection from synucleinopathy. Mol. Ther. 25, 2727–2742. doi: 10.1016/j.ymthe.2017.08.004
Naldini, L. (2015). Gene therapy returns to centre stage. Nature 526, 351–360. doi: 10.1038/nature15818
Reynaud-Dulaurier, R., Benegiamo, G., Marrocco, E., Al-Tannir, R., Surace, E. M., Auwerx, J., et al. (2020). Gene replacement therapy provides benefit in an adult mouse model of Leigh syndrome. Brain 143, 1686–1696. doi: 10.1093/brain/awaa105
Silva-Pinheiro, P., Cerutti, R., Luna-Sanchez, M., Zeviani, M., and Viscomi, C. (2020). A single intravenous injection of AAV-PHP.B-hNDUFS4 ameliorates the phenotype of Ndufs4 (-/-) mice. Mol. Ther. Methods Clin. Dev. 17, 1071–1078. doi: 10.1016/j.omtm.2020.04.026
Keywords: gene therapy (GT), rare disease (RD), viral vector, CNS—central nervous system, blood-brain barrier
Citation: Reynaud-Dulaurier R and Decressac M (2020) PHP.B/eB Vectors Bring New Successes to Gene Therapy for Brain Diseases. Front. Bioeng. Biotechnol. 8:582979. doi: 10.3389/fbioe.2020.582979
Received: 13 July 2020; Accepted: 07 September 2020;
Published: 15 October 2020.
Edited by:
Georg A. Feichtinger, University of Leeds, United KingdomReviewed by:
Chengwen Li, University of North Carolina at Chapel Hill, United StatesCopyright © 2020 Reynaud-Dulaurier and Decressac. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Michael Decressac, bWljaGFlbC5kZWNyZXNzYWNAdW5pdi1ncmVub2JsZS1hbHBlcy5mcg==
 Robin Reynaud-Dulaurier
Robin Reynaud-Dulaurier Michael Decressac
Michael Decressac