
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
MINI REVIEW article
Front. Bioeng. Biotechnol. , 05 June 2020
Sec. Bioprocess Engineering
Volume 8 - 2020 | https://doi.org/10.3389/fbioe.2020.00545
Bacterial lysates, prepared from the microorganisms most frequently involved in human Respiratory Tract Infections (RTIs) have been in the market for several decades, and at present, several different brands are available in many countries worldwide. They all claimed to exert local and systemic immunomodulatory effects but different clinical trials show disparate results between them. The lack of consistency of predicted therapeutic effects has undermined their clinical use and hampered licensing in several countries. One explanation for such lack of consistency in the results is that their methods of preparation are also very different. Here, we review the available literature describing methods of preparation of bacterial lysates, including patent disclosure documents. We found a great variety of methodologies of preparation and a lack of standardized procedures among them. The main conclusion of our study is that there is a clear need for standardized protocols of production to obtain comparable results in clinical trials worldwide.
Respiratory tract infections (RTIs) such as acute bronchitis, community-acquired pneumonia (CAP), and others are the most prevalent infectious diseases in humans and cause millions of deaths annually worldwide (World Health Organization, 2017). Further, respiratory infections are the main comorbidity factor in chronic obstructive pulmonary disease (COPD). Although in the last couple of decades there have been significant improvements in treatment and control of the burden of these infections, there is still a lack of vaccines against most of the infectious agents responsible for RTIs; so other prophylactic strategies must be developed (Cazzola et al., 2008; Esposito et al., 2018). One strategy for treating these infections is the use of bacterial lysates (BLs) which were introduced in the 1970s as oral vaccines for the prevention and treatment of RTIs (Cazzola et al., 2012a; Hancock et al., 2012; Esposito et al., 2018). These lysates are mixtures of antigens derived from inactivated pathogens frequently involved in RTIs.
Over the last few decades, BLs have gained renewed attention because of their contribution to the reduction of recurrent RTIs in childhood (Gutiérrez-Tarango and Berber, 2001; Rozy and Chorostowska-Wynimko, 2008; Navarro et al., 2011). Positive outcomes have also been reported for treatment of chronic obstructive pulmonary disease (COPD) in adults (Cazzola et al., 2012b; Kearney et al., 2015). Studies have shown that BLs are effective immunostimulators, triggering specific responses in local areas of the mucosal immune system (Braido et al., 2007, 2011; Cazzola et al., 2012b; Kearney et al., 2015).
Despite extensive clinical use of BLs, their effects on the immune system are only partially known. The evidence from most studies suggests that BLs act as immunomodulators capable of inducing antibodies against specific pathogens as well as immunoregulatory responses at mucosal tissues (Braido et al., 2011; Cazzola et al., 2012b; Kearney et al., 2015; Esposito et al., 2018; Triantafillou et al., 2019). Particularly, it has been demonstrated that they can interact with different cells through bacterial wall components, such as proteoglycans or lipopolysaccharides, which interact with Toll-like receptors (TLR) on monocytes/macrophages, dendritic, or epithelial cells. These interactions stimulate the differentiation of monocytes into macrophages and activate immature dendritic cells, resulting in the production of selected chemokines and cytokines. Altogether, these responses will induce the recruitment of innate effector cells to the mucosal sites and induce lymphocyte activation that could help in the protection against invading pathogens. Some authors suggest that these responses do generate a state of “pre-alert” against infection (Kearney et al., 2015).
The lack of standardized protocols might be a barrier to the experimental reproducibility of immunostimulatory effects. Different authors have worked with different microorganism cell fractions as components of BLs. In particular, the various methods by which the lysates were prepared may have contributed to the inconsistencies between their reported biological effects. Possibly lack of rigor in experimental design, insufficient patient numbers, or other technical faults have led to some level of mistrust in the clinical trials performed (Cazzola et al., 2008). Standardized production protocols would be a useful step toward overcoming some of the current difficulties comparing the biological effects of BLs.
The two most common methods used for bacterial lysis are alkaline treatment and mechanical disruption, although heat or detergents have also been used (Bauer et al., 2008; Braido et al., 2011; Cazzola et al., 2012a). Alkaline lysis may produce protein denaturation of bacterial antigens, whereas mechanical disruption does not supposedly alter the antigenic structures in the BLs (Kearney et al., 2015; Jurkiewicz and Zielnik-Jurkiewicz, 2018; Triantafillou et al., 2019). Each bacterial strain is grown independently, harvested, inactivated by the selected procedure and then optionally lyophilized. Individual lysates are mixed in fixed proportions to give a polyvalent BL.
Commercial polyvalent bacterial lysates are available in the form of oral capsules or sublingual tablets (Table 1; European Medicines Agency, 2019). Among the best known, are Broncho-Vaxom® as an example of a bacterial immunostimulant obtained by chemical lysis and Ismigen® or Ribomunyl-D 53 composed of unaltered antigenic particles obtained by mechanical cell disruption (Braido et al., 2007; Cazzola et al., 2008). Other BLs commercially available are in use for treatment of other infections, such as urinary tract infections (Huber et al., 2000; Bauer et al., 2002, 2015; Aziminia et al., 2019; Wawrysiuk et al., 2019) or for tuberculosis relapse (Dyachyk, 2006), also with good results.
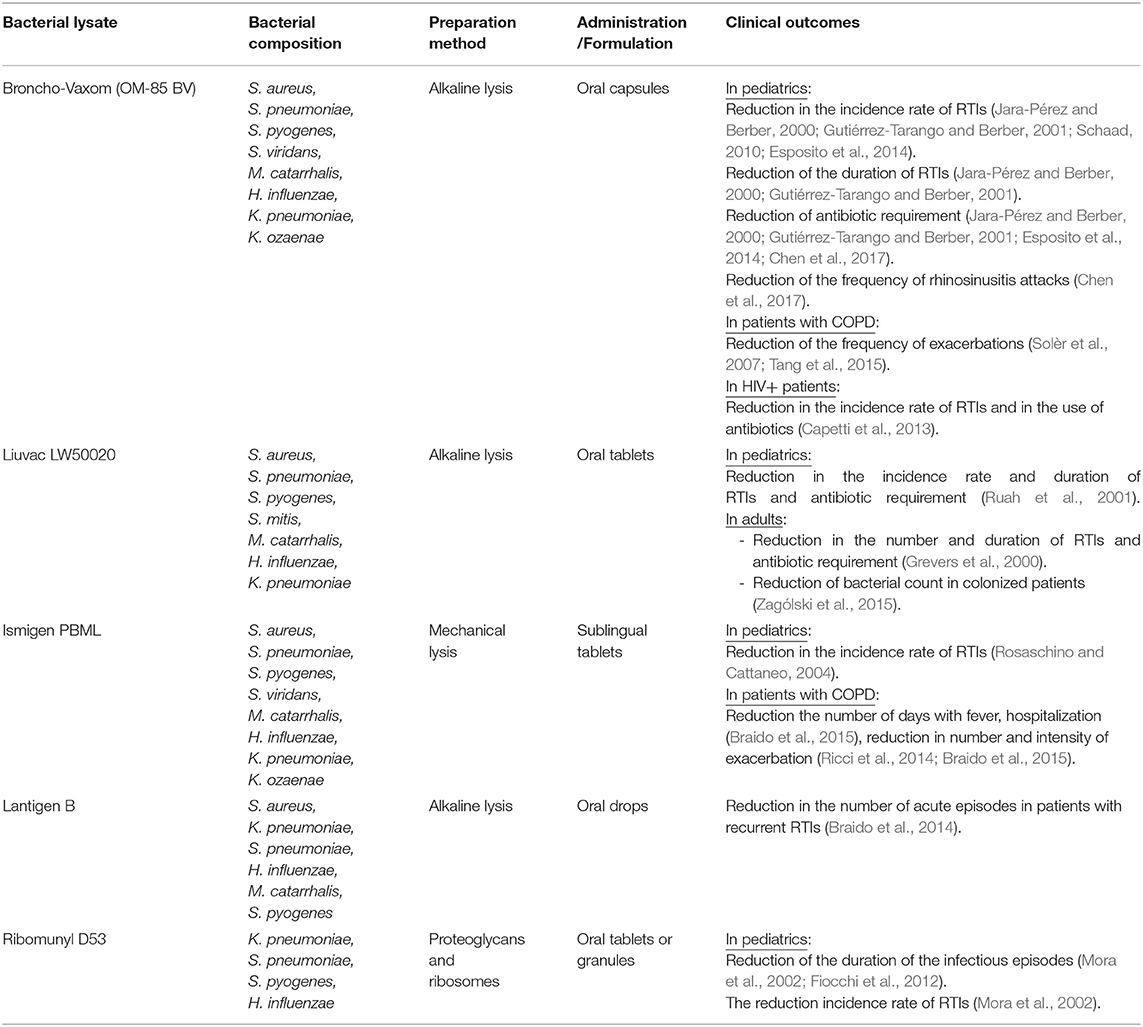
Table 1. Characteristics and clinical outcomes of commercial bacterial lysates used for treating RTIs.
Alkaline lysis uses sodium hydroxide ions to disrupt the cell membrane structure by changing pH to the range of 11.5–12.5.
One of the first bacterial lysates obtained by alkaline extraction was Lantigen B whose in vivo effects were first described in the 1970s (Tyrrell et al., 1972) and more recently demonstrated by Braido et al. (2014). Later on, Bauer et al. (1995) patented another process that included cultivation of Escherichia coli in an aqueous medium followed by alkaline extraction of bacterial proteins, in the presence of a “diluted aqueous source of OH- ions.” The alkaline lysis process used included concentration, ultrafiltration, diafiltration of the lysate, and lyophilization. The authors further described the extraction of lipopolysacharides (LPS) by ion-exchange chromatography and characterization of the lysate by biochemical analyses of amino acids racemized during the alkaline extraction. Additionally, other lysis processes for bacteria causing RTIs were included in the patent with variation in sodium hydroxide (NaOH) concentrations (Table 2; Bauer et al., 1995).
Bauer and other collaborators patented years later a bacterial extract for respiratory infections with a brief description of the process for its preparation. This was an alkaline lysate extracted from bacterial strains of Staphylococcus aureus, Moraxella catarrhalis, Klebsiella pneumoniae, Streptococcus pneumoniae, and Haemophilus influenzae (Bauer et al., 2008, 2010). The lysing process described in the patent covered a range of diverse possible conditions at the different stages of the process from bacteria fermentation to the resulting modified protein mixture. According to the authors, alkaline lysis of each strain or set of strains is suitable for all bacteria causing RTIs.
Among the commercially available formulations prepared by alkaline lysis are OM-85 BV (Broncho-Vaxom®) and Liuvac (LW-50020). OM-85 BV (Broncho-Vaxom®) is a mixture of H. influenzae, S. pneumoniae, K. pneumoniae, Klebsiella ozaenae, S. aureus, Streptococcus pyogenes, Streptococcus viridans, and M. catarrhalis. Liuvac is a mixture of bacterial lysates from S. aureus, S. pneumoniae, S. pyogenes, K. pneumoniae, M. catarrhalis, and H. influenzae that has been evaluated also for the treatment of chronic bronchitis and COPD (Cazzola et al., 2008, 2012b; Esposito et al., 2018).
For trademarked bacterial lysate formulations like OM-85 BV (Broncho-vaxom®) there is no access to a detailed preparation protocol stipulating, for example, NaOH concentrations, temperatures, or periods used for the cell lysis process.
The most widely used mechanical methods of bacterial cell lysis are ultra-sonication and high-pressure homogenization. For homogenization, different pressures are used depending on the cell type (Goldberg, 2008).
One of the first patents for mechanical bacterial lysate preparation found in the literature reported the use of desiccated and partially lysed bacterial antigens (Illiarten, 1971). The author described lysis of S. aureus, K. pneumoniae, Haemophilus influenzae, S. pneumoniae, and M. catarrhalis, by slow freezing followed by rapid thawing of the cell suspension at different temperatures ranges with ultrasonic treatment for different periods of time (Table 2).
Melioli and Fasani patented a mechanically lysed preparation of S. aureus, K. pneumoniae, S. pneumoniae, Haemophilus influenzae, and M. catarrhalis, separately or in combination (Melioli and Fasani, 2004). The particulate bacterial lysates were obtained by fragmentation of bacterial cells using a high-pressure valve, followed by separation of the unaltered antigenic particles fraction from soluble components by centrifugation, washing, and filtration (Table 2).
Later on, Coviello et al. (2014) described mechanically prepared lysates from S. pneumoniae, M.catarrhalis, K. pneumoniae, Micrococcus spp, Haemophilus influenza, and Streptoccocus spp. (i.e., Streptococcus anhemoliticus and S. viridans) by using sonication to disrupt the bacterial cell walls. In their particular lysis process, the bacterial material underwent cycles of temperature changes before the lysate was analyzed to confirm that no viable organisms remained (Table 2; Coviello et al., 2014).
The largest marketed representative brand of mechanical lysates preparations is Ismigen® which is a polyvalent mechanical bacterial lysate (PMBL) composed of lysates from S. aureus, S. pyogenes, S. viridans, K. ozaenae, H. influenzae type b, M. catarrhalis, and S. pneumoniae.
Another type of mechanical lysates are ribosomal-rich lysates. They are obtained by disrupting the cells with micro glass beads in a homogenizer, a technique first described in 1965 (Youmans and Youmans, 1965). Those authors showed that the ribosomal fraction obtained from ruptured myco-bacteria exhibited high immunogenicity. Since then, various authors have studied the protection conferred in animals using ribosomal fractions from bacteria such as S. pneumoniae, Salmonella Typhimurium, and Neisseria meningitidis (Thompson and Snyder, 1971; Venneman and Berry, 1971; Thomas and Weiss, 1972; Swendsen and Johnson, 1976). Then, Michel et al. (1978) described a ribosome preparation extracted from bacterial biomass after bacterial fermentation followed by mechanical disruption under pressure at low temperature, a series of centrifugations to remove unbroken cells and several filtration and sterilization steps to obtain the bacterial lysate (Table 2; Michel et al., 1978). Later, Dussourd d'Hinterland and colleagues marketed an intranasal polyvalent ribosomal vaccine for humans named Ribomunyl-D 53 composed of ribosomal preparations from K. pneumoniae, S. pneumoniae, S. pyogenes group A, and Haemophilus influenzae, with a membrane proteoglycan of a non-capsular strain of K. pneumoniae as an adjuvant (Dussourd d'Hinterland et al., 1980). Several studies have been performed showing its efficacy as immunostimulants (Lauener, 1994; Clot, 1997; Caliot et al., 2000; Bellanti et al., 2003; Bousquet and Fiocchi, 2006). As compared with alkaline bacterial extracts such as OM85 BV, D53 was shown to induce more antibody producing cells in the tonsils of treated children and the authors suggested that partially transcribed proteins present in the ribosomal enriched preparation could contain potent epitopes that would interact with immunocompetent cells more efficiently than large proteins present in the bacterial extract (Béné et al., 1993).
Altogether, mechanical cell disruption has proven to be an efficient method, achieving lysis of 80–100% of the bacteria (Cazzola et al., 2012a). As other authors have previously proposed, mechanical lysates contain particles that maintain several features of intact antigenic molecules, thus providing better interaction with Toll-like receptors which is one of the initial aims of these preparations (Villa et al., 2010; Cazzola et al., 2012a,b; Esposito et al., 2018; Triantafillou et al., 2019). However, the method has the limitation of heat generation which must be minimized to avoid protein denaturalization under the process and it is more expensive than alkaline lysis.
Bacterial lysates prepared by infecting bacteria with selected bacteriophages were patented in 2011 by Pillich and Balcarek who had been working with bacterial lysates since 2003 (Pillich and Balcarek, 2011). The initial idea dates back to 1917 when the use of bacteriophages for the treatment of bacterial diseases was proposed by D' Herelle (Fruciano and Bourne, 2007).
The authors prepared bacterial lysates from strains of S. aureus, K. pneumoniae, and Pseudomonas aeruginosa. They incubated the bacteriophages with the bacterial culture for a certain period. The resulting lysates containing cellular materials such as cell wall components, cellular membranes, proteins, ribosomal fractions, glycoproteins, DNA, and RNA, were then filtered and sterilized. The authors suggested that the use of bacteriophages is effective for all types of bacteria.
Because the protocol is patented the authors did not provide specific details on incubation times or bacteriophages doses. However, an approximation to bacteriophages culture concentration is given as well as approximate incubation times (from 3 to 48 h). The techniques used for antigenic pattern determination are given in Table 2 (Rothbard et al., 2014).
Many clinical studies have been carried out to evaluate the immunostimulatory effect of bacterial lysates, assessed as beneficial effects in patients susceptible to respiratory tract infections (RTIs) as children, elderly or COPD patients. Among all the bacterial lysates described in the literature, the largest number of clinical trials have been performed on OM-85 BV (Broncho-vaxom®) and Ismigen® (PMBL) as examples of chemical or mechanical lysis respectively (Bessler et al., 2010; Navarro et al., 2011; Lanzilli et al., 2013; Tang et al., 2015).
OM-85 has shown good safety and tolerance in many studies with reduction in recurrences of respiratory infections in children and adults (Jara-Pérez and Berber, 2000; Gutiérrez-Tarango and Berber, 2001; Solèr et al., 2007; Schaad, 2010). A meta-analysis of eight randomized controlled trials performed by Schaad et al., showed a reduction in the cases of recurrent RTIs among children treated with OM-85 (Schaad, 2010). Then, another meta-analysis also confirmed that data and showed the protective effect of OM-85 on recurrent RTIs in children (Yin et al., 2018; Esposito et al., 2019). Regarding COPD patients, a meta-analysis published by Pan et al., concluded that current evidence was inadequate for supporting a beneficial effect of OM-85BV for COPD patients, in terms of duration of hospitalization, the severity of acute exacerbation and total adverse events (Pan et al., 2015).
In general, authors agree that there are some limitations among the trials already performed so, there is a need for high-quality, large, multicenter, double-blind, placebo-controlled randomized clinical trials in order to confirm the role of immunostimulants in preventing RTIs in children (Del-Rio-Navarro et al., 2012) as well as adults or COPD patients (Pan et al., 2015).
On the other hand, there are also many clinical studies using BLs prepared by mechanical disruption showing promising results. Rosaschino and Cattaneo (2004) evaluated efficacy and tolerance in pediatric patients treated with PMBL and demonstrated that the treatment was effective with excellent tolerability of the BLs (Rosaschino and Cattaneo, 2004). Braido and Melioli studied another lysate preparation named Lantigen B in patients with recurrent RTIs and demonstrated a significant reduction of the number of acute episodes in these patients (Braido et al., 2014).
Results for the activity of BLs as vaccine have been described as depending partly on the pathogenic strain used, partly (in the case of ribosomal BLs) on the degree of purity of subcellular fraction, and partly on the presence or absence of other cell constituents such as polysaccharides (Michel et al., 1978). Different types of immune responses (cell-mediated or humoral) have been induced in different studies. Administration routes have included nasal, sublingual as well as oral (Schaad, 2010; Cazzola et al., 2012a; Rial et al., 2016).
In summary, several studies have shown the beneficial effects of treatment with bacterial extracts, either obtained through mechanical or chemical procedures. As already mentioned, some authors have stated that mechanical disruption could be a better alternative to chemical lysis due to the preservation of the antigens. However, no study has been performed comparing the biological effects of polyvalent bacterial lysates, prepared from the same bacterial cultures, but using mechanical or chemical lysate procedures (mechanical vs. chemical). This comparative studies could give some light for choosing the best method for preparing immunostimulant lysates.
Several methods have been used for years in the preparation of cell lysates (e.g., alkaline treatment or mechanical disruption). Since mechanical disruption is suggested to be the most promising way to lyse the cells, significant technological progress has been made in this field by some companies (Cazzola et al., 2012a; Jurkiewicz and Zielnik-Jurkiewicz, 2018; Esposito et al., 2019). They have developed focused-ultrasonication, an advanced computer-controlled technology that can control the dosage of energy delivered to gently disrupt the cell membranes of mammalian cells or abruptly disrupt the cell walls of bacteria. This technology could address the challenges of standardizing procedures for producing mechanical bacterial lysates (Wenger et al., 2008; Bláha et al., 2017).
This review summarizes the most prominent publicly available information for production and use of bacterial lysates. Despite their importance in public health and the number of studies done at the clinical level, there is a need for more standardized protocols for the preparation of these bacterial antigens because the literature does not describe lysis procedures in detail or the procedures are not openly available to the research community. The fact that the selected inactivation method is essential for the efficacy of the bacterial lysates is an important aspect that deserves in-depth exploration, especially as some of these treatments can lead to denaturation of the antigens. The lack of standardized protocols leads to different extract performance across laboratories and is a barrier to reproducibility. In turn, this can result in misleading evaluations of the effects produced by these lysates. Standard production protocols would be a useful step toward overcoming some of the current difficulties in comparing the immunological and clinical effects of BLs and their use as immunotherapy for the prevention and treatment of RTIs.
NS participated in the conception, information search and assisted in drafting the manuscript. FF and AR participated in the information search, assisted in drafting of the manuscript. VD assisted in language correction. JC assisted in drafting and correction of the manuscript.
This work was supported by PEDECIBA-Química, Uruguay.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Aziminia, N., Hadjipavlou, M., Philippou, Y., Pandian, S. S., Malde, S., and Hammadeh, M. Y. (2019). Vaccines for the prevention of recurrent urinary tract infections: a systematic review. BJU Int. 123, 753–768. doi: 10.1111/bju.14606
Bauer, H. W., Rahlfs, V. W., Lauener, P. A., and Bleßmann, S. S. (2002). Prevention of recurrent urinary tract infections with immuno-active E. coli fractions: a meta-analysis of five placebo-controlled double- blind studies. Int. J. Antimicrob. Agents 19, 451–456. doi: 10.1016/S0924-8579(02)00106-1
Bauer, J., Hirt, P., and Schulthess, A. (1995). Extract of Bacterial Macromolecules, A Process for Its Preparation and a Pharmaceutical Composition Containing the Same [Internet]. US 00 5424287A. Available online at: https://www.google.com/patents/US5424287
Bauer, J. A., Salvagni, M., Vigroox, J.-P. L., Chalvet, L., and Chiavaroli, C. (2008). Bacterial Extract for Respiratory Disorders and Process for iths Preparation [Internet]. WO 2008/109669 A2. Washington, DC: World Intellectual Property Organization. Available online at: http://www.freepatentsonline.com/8697154.html
Bauer, J. A., Salvagni, M., Vigroox, J.-P. L., Chalvet, L., and Chiavaroli, C. (2010). Bacterial Extract for Respiratory Disorders and Process for its Preparation. U.S. Patent 0227013 A1. Washington, DC: U.S. Patent and Trademark Office.
Bauer, J. A., Salvagni, M., Vigroux, J.-P. L., Chalvet, L., and Chiavaroli, C. (2015). Bacterial Extract for Digestive or Urinary Tract Disorders and Process for its Preparation. U.S. Patent 9,198,961 B2. Washington, DC: U.S. Patent and Trademark Office.
Bellanti, J. A., Olivieri, D., and Serrano, E. (2003). Ribosomal immunostimulation: assessment of studies evaluating its clinical relevance in the prevention of upper and lower respiratory tract infections in children and adults. Biodrugs 17, 355–367. doi: 10.2165/00063030-200317050-00005
Béné M, C., Kahl, L., Perruchet, A. M., Hermes, H., Mosges, M., Normier, G., et al. (1993). Bacterial lysates and ribosomes as inducers of specific immune responses: a comparative study. Scand. J. Immunol. 38, 496–498. doi: 10.1111/j.1365-3083.1993.tb02594.x
Bessler, W. G., Vor dem Esche, U., and Masihi, N. (2010). The bacterial extract OM-85 BV protects mice against influenza and Salmonella infection. Int. Immunopharmacol. 10, 1086–1090. doi: 10.1016/j.intimp.2010.06.009
Bláha, B. A. F., Morris, S. A., Ogonah, O. W., and Crescente, V. (2017). Development of a microscale cell disruption platform for Pichia pastoris in rapid bioprocess design. Biosep. Downstr. Process. 35, 130–140. doi: 10.1002/btpr.2555
Bousquet, J., and Fiocchi, A. (2006). Prevention of recurrent respiratory tract infections in children using a ribosomal immunotherapeutic agent: a clinical review. Paediatr. Drugs 8, 235–243. doi: 10.2165/00148581-200608040-00003
Braido, F., Melioli, G., Candoli, P., Cavalot, A., Di Gioacchino, M., Ferrero, V., et al. (2014). The bacterial lysate Lantigen B reduces the number of acute episodes in patients with recurrent infections of the respiratory tract: the results of a double blind, placebo controlled, multicenter clinical trial. Immunol. Lett. 162, 185–193. doi: 10.1016/j.imlet.2014.10.026
Braido, F., Melioli, G., Cazzola, M., Fabbri, L., Blasi, F., Moretta, L., et al. (2015). Sub-lingual administration of a polyvalent mechanical bacterial lysate (PMBL) in patients with moderate, severe, or very severe chronic obstructive pulmonary disease (COPD) according to the GOLD spirometric classification: a multicentre, double-blind, ran. Pulmon. Pharmacol. Ther. 33, 75–80. doi: 10.1016/j.pupt.2015.03.006
Braido, F., Schenone, G., Pallestrini, E., Reggiardo, G., Cangemi, G., Canonica, G. W., et al. (2011). The relationship between mucosal immunoresponse and clinical outcome in patients with recurrent upper respiratory tract infections treated with a mechanical bacterial lysate. J. Biol. Regul. Homeost. Agents 25, 477–485.
Braido, F., Tarantini, F., Ghiglione, V., Melioli, G., and Canonica, G. W. (2007). Bacterial lysate in the prevention of acute exacerbation of COPD and in respiratory recurrent infections. Int. J. Chron. Obstruct. Pulmon. Dis. 2, 335–345.
Caliot, E., Libon, C., Kernéis, S., and Pringault, E. (2000). Translocation of ribosomal immunostimulant through an in vitro-reconstituted digestive barrier containing M-like cells. Scand. J. Immunol. 52, 588–594. doi: 10.1046/j.1365-3083.2000.00819.x
Capetti, A., Cossu, M. V., Carenzi, L., and Rizzardini, G. (2013). Four years of immunization with OM-85 BV to prevent respiratory infections in HIV+ patients. Hum. Vaccines Immunother. 9, 1849–1851. doi: 10.4161/hv.25104
Cazzola, M., Anapurapu, S., and Page, C. P. (2012a). Polyvalent mechanical bacterial lysate for the prevention of recurrent respiratory infections: a meta-analysis. Pulmon. Pharmacol. Ther. 25, 62–68. doi: 10.1016/j.pupt.2011.11.002
Cazzola, M., Capuano, A., Rogliani, P., and Matera, M. G. (2012b). Bacterial lysates as a potentially effective approach in preventing acute exacerbation of COPD. Curr. Opin. Pharmacol. 12, 300–308. doi: 10.1016/j.coph.2012.01.019
Cazzola, M., Rogliani, P., and Curradi, G. (2008). Bacterial extracts for the prevention of acute exacerbations in chronic obstructive pulmonary disease: a point of view. Respir. Med. 102, 321–327. doi: 10.1016/j.rmed.2007.11.002
Chen, J., Zhou, Y., Nie, J., Wang, Y., Zhang, L., Shi, Q., et al. (2017). Bacterial lysate for the prevention of chronic rhinosinusitis recurrence in children. J. Laryngol. Otol. 131, 523–528. doi: 10.1017/S0022215117000524
Clot, J. (1997). Pharmacology of ribosomal immunotherapy. Drugs 54(Suppl. 1), 33–36. doi: 10.2165/00003495-199700541-00009
Coviello, S., Wimmenauer, V., Polack, F. P., and Irusta, P. M. (2014). Bacterial lysates improve the protective antibody response against respiratory viruses through Toll-like receptor 4. Hum. Vaccines Immunother. 10, 2896–2902. doi: 10.4161/hv.29784
Del-Rio-Navarro, B. E., Espinosa-Rosales, F. J., Flenady, V., and Sienra-Monge, J. J. L. (2012). Immunostimulants for preventing respiratory tract infection in children. Evid. Based Child Heal. 7, 629–717. doi: 10.1002/ebch.1833
Dussourd d'Hinterland, L., Normier, G., and Durand, J. (1980). Preparation of subcellular fractions. Arzneimittel Forschung Drug Res. 30, 126–132.
Dyachyk, N. (2006). Use of a Lyophilised Bacterial Lysate for the Prevention of Tuberculosis Relapse [Internet].WO 2006/084477 A1. Available online at: https://patentimages.storage.googleapis.com/7b/e3/87/4058f3c530e809/WO2006084477A1.pdf
Esposito, S., Bianchini, S., Polinori, I., and Principi, N. (2019). Impact of OM-85 given during two consecutive years to children with a history of recurrent respiratory tract infections: a retrospective study. Int. J. Environ. Res. Public Health 16, 1–8. doi: 10.3390/ijerph16061065
Esposito, S., Marchisio, P., Prada, E., Daleno, C., Porretti, L., Carsetti, R., et al. (2014). Impact of a mixed bacterial lysate (OM-85 BV) on the immunogenicity, safety and tolerability of inactivated influenza vaccine in children with recurrent respiratory tract infection. Vaccine 32, 2546–2552. doi: 10.1016/j.vaccine.2014.03.055
Esposito, S., Soto-Martinez, M. E., Feleszko, W., Jones, M. H., Shen, K.-L., and Schaad, U. B. (2018). Nonspecific immunomodulators for recurrent respiratory tract infections, wheezing and asthma in children. Curr. Opin. Allergy Clin. Immunol. 18, 198–209. doi: 10.1097/ACI.0000000000000433
European Medicines Agency (2019). Assessment Report : Bacterial Lysates-Containing Medicinal Products for Respiratory Conditions [Internet]. Vol. 31. Available online at: www.ema.europa.eu/how-to-find-us
Fiocchi, A., Omboni, S., Mora, R., Macchi, A., Nespoli, L., Arrigoni, S., et al. (2012). Efficacy and safety of ribosome-component immune modulator for preventing recurrent respiratory infections in socialized children. Allergy Asthma Proc. 33, 197–204. doi: 10.2500/aap.2012.33.3516
Fruciano, E., and Bourne, S. (2007). Phage as an antimicrobial agent: d'Herelle's heretical theories and their role in the decline of phage prophylaxis in the West. Can. J. Infect. Dis. Med. Microbiol. 18, 19–26. doi: 10.1155/2007/976850
Goldberg, S. (2008). Mechanical/physical methods of cell disruption and tissue homogenization. Methods Mol Biol. 424, 3–22. doi: 10.1007/978-1-60327-064-9_1
Grevers, G., Palacios, O., Rodriguez, B., Abel, S. V., and van Aubel, A. (2000). Treatment of recurrent respiratory tract infections with a polyvalent bacterial lysate: results of an open, prospective, multinational study. Adv. Ther. 17, 103–116. doi: 10.1007/BF02854843
Gutiérrez-Tarango, M. D., and Berber, A. (2001). Safety and efficacy of two courses of OM-85 BV in the prevention of respiratory tract infections in children during 12 months. Chest 119, 1742–1748. doi: 10.1378/chest.119.6.1742
Hancock, R. E. W., Nijnik, A., and Philpott, D. J. (2012). Modulating immunity as a therapy for bacterial infections. Nat. Rev. Microbiol. 10, 243–254. doi: 10.1038/nrmicro2745
Huber, M., Krauter, K., Winkelmann, G., Bauer, H. W., Rahlfs V. W., Lauener, P. A. A., et al. (2000). Immunostimulation by bacterial components : II. Efficacy studies and meta-analysis of the bacterial extract OM-89. Int. J. Immunopharmacol. 22, 1103–1111. doi: 10.1016/S0192-0561(00)00070-9
Illartein, P.-R. (1971). Pharmaceutical Preparation Based on Bacterial Antigens. U.S. Patent 003608066. U.S. Patent and Trademark Office.
Jara-Pérez, J. V., and Berber, A. (2000). Primary prevention of acute respiratory tract infections in children using a bacterial immunostimulant: a double-masked, placebo-controlled clinical trial. Clin. Ther. 22, 748–759. doi: 10.1016/S0149-2918(00)90008-0
Jurkiewicz, D., and Zielnik-Jurkiewicz, B. (2018). Bacterial lysates in the prevention of respiratory tract infections. Otolaryngol. Pol. 72, 1–8. doi: 10.5604/01.3001.0012.7216
Kearney, S. C., Dziekiewicz, M., and Feleszko, W. (2015). Immunoregulatory and immunostimulatory responses of bacterial lysates in respiratory infections and asthma. Ann. Allergy Asthma Immunol. 114, 364–369. doi: 10.1016/j.anai.2015.02.008
Lanzilli, G., Traggiai, E., Braido, F., Garelli, V., Folli, C., Chiappori, A., et al. (2013). Administration of a polyvalent mechanical bacterial lysate to elderly patients with COPD: effects on circulating T, B and NK cells. Immunol. Lett. 149, 62–67. doi: 10.1016/j.imlet.2012.11.009
Lauener, P. A. (1994). Bacterial lysates and ribosomes as inducers of specific immune responses: a comparative study. Scand. J. Immunol. 40, 466–467. doi: 10.1111/j.1365-3083.1994.tb03489.x
Melioli, G., and Fasani, R. (2004). Immunomodulating Composition Comprising a Particulate Fraction of Bacterial Mechanical Lysates. WO 2004/096270 A1. Rome: World Intellectual Property Organization.
Michel, F., Dussourd D'Hinterland, L., Bousquet, J., Pinel, A., and Normier, G. (1978). Immuno-stimulation by a ribosomal vaccine associated with a bacterial cell wall adjuvant in humans. Infect. Immun. 20, 760–769. doi: 10.1128/IAI.20.3.760-769.1978
Mora, R., Barbieri, M., Passali, G., Sovatzis, A., Mora, F., and Cordone, M. (2002). A preventive measurefor otitis media in children with upper respiratory tract infections. Int. J. Pediatr. Otorhinolaryngol. 63, 111–118. doi: 10.1016/S0165-5876(01)00649-8
Navarro, S., Cossalter, G., Chiavaroli, C., Kanda, A., Fleury, S., Lazzari, A., et al. (2011). The oral administration of bacterial extracts prevents asthma via the recruitment of regulatory T cells to the airways. Mucosal Immunol. 4, 53–65. doi: 10.1038/mi.2010.51
Pan, L., Jiang, X. G., Guo, J., Tian, Y., and Liu, C. T. (2015). Effects of OM-85 BV in patients with chronic obstructive pulmonary disease: a systematic review and meta-analysis. J. Clin. Pharmacol. 55, 1086–1092. doi: 10.1002/jcph.518
Pillich, J., and Balcarek, J. C. (2011). Composition and Methods for Treating Microbial Infections. U.S. Patent 008007817B2. Birmingham, AL: U.S. Patent and Trademark Office.
Rial, A., Ferrara, F., Suárez, N., Scavone, P., Marqués, J. M., and Chabalgoity, J. A. (2016). Intranasal administration of a polyvalent bacterial lysate induces self-restricted inflammation in the lungs and a Th1/Th17 memory signature. Microbes Infect. 18, 747–757. doi: 10.1016/j.micinf.2016.10.006
Ricci, R., Palmero, C., Bazurro, G., Riccio, A. M., Garelli, V., Di Marco, E., et al. (2014). The administration of a polyvalent mechanical bacterial lysate in elderly patients with COPD results in serological signs of an efficient immune response associated with a reduced number of acute episodes. Pulmon. Pharmacol. Ther. 27, 109–113. doi: 10.1016/j.pupt.2013.05.006
Rosaschino, F., and Cattaneo, L. (2004). Strategies for optimizing compliance of paediatric patients for seasonal antibacterial vaccination with sublingually administered Polyvalent Mechanical Bacterial Lysates (PMBL). Acta. BioMed. 75, 171–178. Available online at: https://www.mattioli1885journals.com/index.php/actabiomedica/article/view/2114
Rothbard, J. B., Mcgrane, P. L., Engleman, E. G., Fathman, G. C., and Kreider, E. (2014). Compositions and Methods for Treating Diseases [Internet]. United States Patent 8629115. 12/925357. Available online at: http://www.freepatentsonline.com/8629115.html
Rozy, A., and Chorostowska-Wynimko, J. (2008). Bacterial immunostimulants–mechanism of action and clinical application in respiratory diseases. Pneumonol. Alergol. Pol. 76, 353–359.
Ruah, S. B., Ruah, C., Van Aubel, A., Abel, S., and Elsasser, U. (2001). Efficacy of a polyvalent bacterial lysate in children with recurrent respiratory tract infections. Adv. Ther. 18, 151–162. doi: 10.1007/BF02850109
Schaad, U. B. (2010). OM-85 BV, an immunostimulant in pediatric recurrent respiratory tract infections: a systematic review. World J. Pediatr. 6, 5–12. doi: 10.1007/s12519-010-0001-x
Solèr, M., Mütterlein, R., and Cozma, G. (2007). Double-blind study of OM-85 in patients with chronic bronchitis or mild chronic obstructive pulmonary disease. Respiration 74, 26–32. doi: 10.1159/000093933
Swendsen, C. L., and Johnson, W. (1976). Humoral immunity to Streptococcus pneumoniae induced by a pneumococcal ribosome protein fraction. Infect. Immun. 14, 345–354. doi: 10.1128/IAI.14.2.345-354.1976
Tang, H., Fang, Z., Saborío, G., and Xiu, Q. (2015). Efficacy and safety of OM-85 in patients with chronic bronchitis and/or chronic obstructive pulmonary disease. Lung 193, 513–519. doi: 10.1007/s00408-015-9737-3
Thomas, D. W., and Weiss, E. (1972). Response of mice to injection of ribosomal fraction from group B Neisseria meningitidis. Infect. Immun. 6, 355–363. doi: 10.1128/IAI.6.3.355-363.1972
Thompson, H. C. W., and Snyder, I. S. (1971). Protection against pneumococcal infection by a ribosomal preparation. Infect. Immun. 3, 16–23. doi: 10.1128/IAI.3.1.16-23.1971
Triantafillou, V., Workman, A. D., Patel, N. N., Maina, I. W., Tong, C. C. L., Kuan, E. C., et al. (2019). Broncho-Vaxom® (OM-85 BV) soluble components stimulate sinonasal innate immunity. Int. Forum Allergy Rhinol. 9, 370–377. doi: 10.1002/alr.22276
Tyrrell, D. A., Nolan, P. S., Reed, S. E., and Healy, M. J. (1972). Trial of an oral bacterial antigen against common colds. Br. J. Prev. Soc. Med. 26, 129–131. doi: 10.1136/jech.26.2.129
Venneman, M. R., and Berry, L. J. (1971). Cell-mediated resistance induced with immunogenic preparations of Salmonella typhimurium. Infect. Immun. 4, 381–387. doi: 10.1128/IAI.4.4.381-387.1971
Villa, E., Garelli, V., Braido, F., Melioli, G., and Canonica, G. W. (2010). May we strengthen the human natural defenses with bacterial lysates? World Allergy Org. J. 3(Suppl. 8), S17–S23. doi: 10.1186/1939-4551-3-S2-S17
Wawrysiuk, S., Naber, K., Rechberger, T., and Miotla, P. (2019). Prevention and treatment of uncomplicated lower urinary tract infections in the era of increasing antimicrobial resistance—non-antibiotic approaches: a systemic review. Arch. Gynecol. Obstet. 300, 821–828. doi: 10.1007/s00404-019-05256-z
Wenger, M. D., DePhillips, P., and Bracewell, D. G. (2008). A microscale yeast cell disruption technique for integrated process development strategies. Biotechnol. Prog. 24, 606–614. doi: 10.1021/bp070359s
Yin, J., Xu, B., Zeng, X., and Shen, K. (2018). Broncho-Vaxom in pediatric recurrent respiratory tract infections: a systematic review and meta-analysis. Int. Immunopharmacol. 54, 198–209. doi: 10.1016/j.intimp.2017.10.032
Youmans, A. S., and Youmans, G. P. (1965). Immunogenic activity of a ribosomal fraction obtained from Mycobacterium tuberculosis. J. Bacteriol. 89, 1291–1297. doi: 10.1128/JB.89.5.1291-1298.1965
Keywords: respiratory tract infections (RTIs), immunomodulator, bacterial lysates, mechanical, alkaline, bacteriophage, vaccine
Citation: Suárez N, Ferrara F, Rial A, Dee V and Chabalgoity JA (2020) Bacterial Lysates as Immunotherapies for Respiratory Infections: Methods of Preparation. Front. Bioeng. Biotechnol. 8:545. doi: 10.3389/fbioe.2020.00545
Received: 08 January 2020; Accepted: 06 May 2020;
Published: 05 June 2020.
Edited by:
Joseph Boudrant, Centre National de la Recherche Scientifique (CNRS), FranceReviewed by:
Frederico C. Martinho, University of Maryland, Baltimore, United StatesCopyright © 2020 Suárez, Ferrara, Rial, Dee and Chabalgoity. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Norma Suárez, bnN1YXJlenN0ZXJAZ21haWwuY29t; Jose A. Chabalgoity, Y2hhYmFsZ29pdHkuam9zZUBnbWFpbC5jb20=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.