- 1School of Food Science and Engineering, Foshan University, Foshan, China
- 2Sustainable Biochemical and Biosynthetic Engineering Center, Foshan Wu-Yuan Biotechnology Co., Ltd., Guangdong Biomedical Industrial Base, Foshan, China
A Commentary on
Preparation, characterization and application of rod-like chitin nanocrystal
by using p-toluenesulfonic acid/choline chloride deep eutectic solvent as a hydrolytic media by Cao, S.-L., Gu, W.-M., Ou-Yang, W.-D., Chen, D.-C., Yang, B.-Y., Lai, L.-H., et al. (2019). Carbohydr. Polym. 213, 304–310. doi: 10.1016/j.carbpol.2019.02.092
Introduction
Chitin nanocrystal (ChiNC) is a unique bio-based nanomaterial extracted from chitin. This nano-biopolymer is biocompatible, biodegradable, and antimicrobial. In recent decades, ChiNC exhibits great potential in several fields, such as bio-medicine (Sahraee et al., 2017) and biocatalysis (Huang et al., 2018). In the recent publication from our research group, a green preparation method for ChiNC was established by using deep eutectic solvent (DES) as media and the relative ChiNC product was proved to be an excellent enzyme carrier for lipase immobilization. In this commentary, we would like to add some information for the design of the recyclable ChiNC nanocrystal enzyme carriers by using DES as media, which provides insight in enzyme immobilization.
Challenges in Developing Mild and Green Methods in Preparing CHINC Enzyme Carriers
According to the previous studies, acid hydrolysis (Gopalan and Dufresne, 2003; Goodrich and Winter, 2007) and oxidation (Fan et al., 2008) are common ways of producing ChiNC. However, there are some drawbacks in these methods.
In the acid hydrolysis, abundant acid medias (mineral acids and/or organic acids) were used. Unfortunately, these acid medias were difficult to recycle. This leads to waste of resources and pollution. For example, during a typical acid hydrolysis process of ChiNC, treating 1 g of chitin materials would consume about 30 mL 3 mol/L HCl at first. In the following process, about 5–10 times of water (150 to 300 mL) was required for dilution (thus, the HCl media cannot be reused). In addition, this process needs to be repeated for more than three times. Moreover, 3.6 g of NaOH was used to neutralize the HCl media. In general, to treat 1 kg chitin, about 30 L 3M HCl solution, 3.6 kg solid NaOH, and more that 600 L water was consumed. Besides, as for TEMPO(2,2,6,6-Tetramethyl-1-piperidinyloxy)-oxidation, to fabricate the ChiNC with abundant carboxyl groups, 1 mol TEMPO and more than 100 L water are needed to treat 1 kg of chitin. Thus, designing the ChiNC preparation process by using hydrolytic media with low volatility and reusability is urgently needed to solve this problem.
Deep eutectic solvents (DESs) are mixtures consisting of hydrogen bond donor and acceptor pair with low volatility. Thus, DESs exhibit great potential as recyclable reaction medias. Moreover, it is important to choose a good diluent. The following requirements need to be met as a suitable diluent: (1) the diluent should be completely miscible with DES to form a homogeneous mixture solution; (2) since the high viscosity of the DES resulted in lower sedimentation efficiency, the diluent should have relatively low viscosity to reduce the viscosity of the mixture media; (3) in order to separate and recycle the dilute and DESs from the mixture media, the dilute should be volatile with a suitable boiling point. In our previous experiments, ethanol was found to fulfill all the above requirements. Also, it is noteworthy that the ChiNC is easy to sedimentate in the ethanol media.
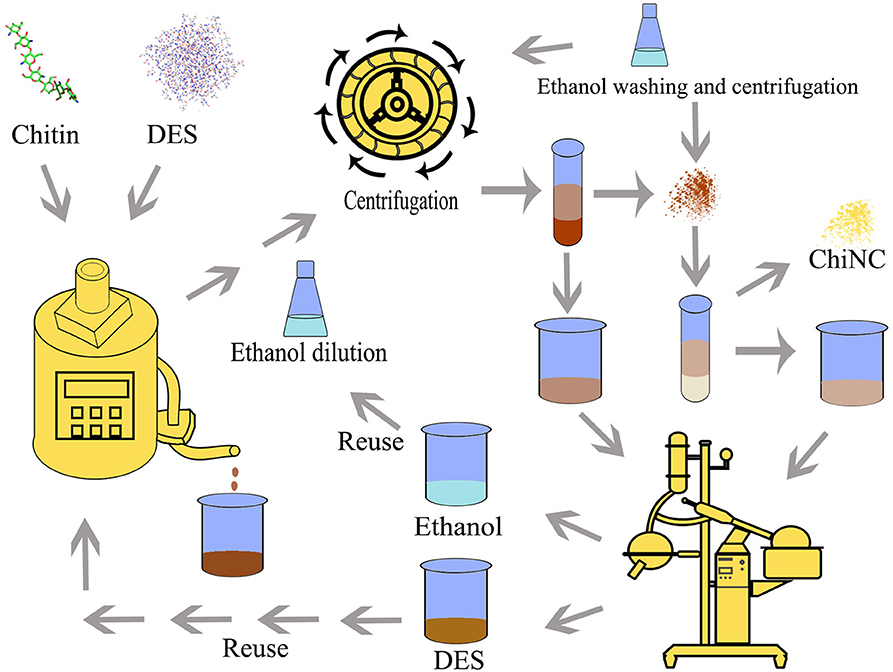
Thus, in our latest publication, a type of acidic DESs (p-toluenesulfonic acid/choline chloride, PTA-ChCl) was used. The preparation process was designed as follows (Figure 1): (1) chitin raw material was treated with PTA-ChCl DES to form ChiNC; (2) the mixture was diluted by about absolute ethanol and centrifuged to separate the ChiNC product and mixture media (containing PTA-ChCl and absolute ethanol); (3) the ChiNC product was furtherly washed with absolute ethanol; (4) PTA-ChCl and absolute ethanol was separated from the mixture media obtained in step (2) by distillation or rotational evaporation. In these preparation process, the PTA-ChCl and ethanol can be reused.
Future Perspective
There are several aspects that need further exploration in using DESs to prepare ChiNC nanocarriers. Firstly, the effects of different DESs treatments on the physical and chemical properties of the ChiNC nanocarriers need to be further investigated. This can help researchers to design more efficient and greener ChiNC preparation processes. Secondly, a clear understanding of the interaction between ChiNC enzyme carriers and enzyme is lacking. Until now, the mechanism of the interaction of the enzyme and carriers at the atomic and molecular level still require further investigation. Thus, molecular dynamic simulation and quantum chemistry is a promising way to better explain the underlying mechanism at the atomic and/or molecular level.
Author Contributions
S-LC, W-MG, W-HJ, M-JX, K-PH, X-YX, W-XZ, R-FS, Y-SL, and L-HL wrote the manuscript. S-LC and W-MG edited the manuscript. W-MG manufactured the Figure.
Funding
This research was funded by Project of Department of Education of Guangdong Province (Young Creative Talents, Natural Science, No. 2017KQNCX217), Guangdong Basic and Applied Basic Research Foundation (2019A1515110621), High-Level Talent Start-Up Research Project of Foshan University (GG07016), College Students' Innovation and Entrepreneurship Training Program (National 201911847023, Guangdong-S201811847101, S201911847097, S201911847091, Foshan University-XJ2018101, XJ2018241, XJ2019213, Special Funds for the Cultivation of Guangdong College Students' Scientific and Technological Innovation (pdjh2020b0627 Climbing Program Special Funds).
Conflict of Interest
S-LC was part-time employed by the company Foshan Wu-Yuan Biotechnology Co., Ltd.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
Cao, S.-L., Gu, W.-M., Ou-Yang, W.-D., Chen, D.-C., Yang, B.-Y., Lai, L.-H., et al. (2019). Preparation, characterization and application of rod-like chitin nanocrystal by using P-toluenesulfonic acid/choline chloride deep eutectic solvent as a hydrolytic media. Carbohydr. Polym. 213, 304–310. doi: 10.1016/j.carbpol.2019.02.092
Fan, Y., Saito, T., and Isogai, A. (2008). Chitin nanocrystals prepared by TEMPO-mediated oxidation of alpha-chitin. Biomacromolecules 9, 192–198. doi: 10.1021/bm700966g
Goodrich, J. D., and Winter, W. T. (2007). Alpha-chitin nanocrystals prepared from shrimp shells and their specific surface area measurement. Biomacromolecules 8, 252–257. doi: 10.1021/bm0603589
Gopalan, N. K., and Dufresne, A. (2003). Crab shell chitin whisker reinforced natural rubber nanocomposites. 1. Processing and swelling behavior. Biomacromolecules 4, 666–674. doi: 10.1021/bm020127b
Huang, W.C., Wang, W., Xue, C., and Mao, X. (2018). Effective enzyme immobilization onto a magnetic chitin nanofiber composite. ACS Sustain. Chem. Eng. 6, 8118–8124. doi: 10.1021/acssuschemeng.8b01150
Keywords: enzyme immobilization, chitin nanocrystal, deep eutectic solvent, lipase, biocatalysis
Citation: Jiang W-H, Gu W-M, Xiong M-J, He K-P, Xu X-Y, Zhang W-X, Shen R-F, Lai L-H, Lv Y-S and Cao S-L (2020) Commentary: Preparation, characterization and application of rod-like chitin nanocrystal by using p-toluenesulfonic acid/choline chloride deep eutectic solvent as a hydrolytic media. Front. Bioeng. Biotechnol. 8:505. doi: 10.3389/fbioe.2020.00505
Received: 27 February 2020; Accepted: 29 April 2020;
Published: 12 June 2020.
Edited by:
Wen-Yong Lou, South China University of Technology, ChinaReviewed by:
Xiangzhao Mao, Ocean University of China, ChinaYongqin Lv, Beijing University of Chemical Technology, China
Copyright © 2020 Jiang, Gu, Xiong, He, Xu, Zhang, Shen, Lai, Lv and Cao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shi-Lin Cao, c2VlbGFtLnNoaWxpbi5jYW8mI3gwMDA0MDtnbWFpbC5jb20=; c2hpbGluLmNhbyYjeDAwMDQwO2Zvc3UuZWR1LmNu
†ORCID: Wei-Ming Gu orcid.org/0000-0001-6954-0468
Lin-Hao Lai orcid.org/0000-0002-1329-2378
‡These authors share first authorship
 Wen-Hao Jiang
Wen-Hao Jiang Wei-Ming Gu
Wei-Ming Gu Mei-Jie Xiong1
Mei-Jie Xiong1 Kang-Ping He
Kang-Ping He Xiao-Ying Xu
Xiao-Ying Xu Wen-Xi Zhang
Wen-Xi Zhang Shi-Lin Cao
Shi-Lin Cao