
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Bioeng. Biotechnol., 14 May 2020
Sec. Nanobiotechnology
Volume 8 - 2020 | https://doi.org/10.3389/fbioe.2020.00425
This article is part of the Research TopicAntioxidant NanomedicineView all 6 articles
 Zeinab Nouri1
Zeinab Nouri1 Marziyeh Hajialyani2
Marziyeh Hajialyani2 Zhila Izadi2
Zhila Izadi2 Roodabeh Bahramsoltani3,4
Roodabeh Bahramsoltani3,4 Mohammad Hosein Farzaei2,5*
Mohammad Hosein Farzaei2,5* Mohammad Abdollahi6,7*
Mohammad Abdollahi6,7*Metabolic syndrome includes a series of metabolic abnormalities that leads to diabetes mellitus and cardiovascular diseases. Plant extracts, due to their unique advantages like anti-inflammatory, antioxidant, and insulin sensitizing properties, are interesting therapeutic options to manage MetS; however, the poor solubility and low bioavailability of lipophilic bioactive components in the herbal extracts are two critical challenges. Nano-scale delivery systems are suitable to improve delivery of herbal extracts. This review, for the first time, focuses on nanoformulations of herbal extracts in MetS and related complications. Included studies showed that several forms of nano drug delivery systems such as nanoemulsions, solid lipid nanoparticles, nanobiocomposites, and green-synthesized silver, gold, and zinc oxide nanoparticles have been developed using herbal extracts. It was shown that the method of preparation and related parameters such as temperature and type of polymer are important factors affecting physicochemical stability and therapeutic activity of the final product. Many of these formulations could successfully decrease the lipid profile, inflammation, oxidative damage, and insulin resistance in in vitro and in vivo models of MetS-related complications. Further studies are still needed to confirm the safety and efficacy of these novel herbal formulations for clinical application.
Metabolic syndrome (MetS), also known as “syndrome X” and “insulin-resistance syndrome,” is characterized by several metabolic abnormalities, including insulin resistance, type 2 diabetes, obesity, hypertension, and dyslipidemia (Kaur, 2014; Dalvand et al., 2017; Ebrahimi-Mameghani et al., 2018). About 20–30% of the world population is diagnosed with MetS, which makes the disease as a global health issue (Beltrán-Sánchez et al., 2013; Xi et al., 2013; Vishram et al., 2014; Pucci et al., 2017). MetS is the result of a series of genetic and environmental factors; however, the exact etiology is not yet understood (Feldeisen and Tucker, 2007). The underlying mechanisms encompass insulin resistance, elevated plasma free fatty acids, chronic inflammation, and oxidative stress (Bergman et al., 2001; Pan and Kong, 2018). The increased level of free fatty acids results in suppression of insulin clearance and is closely associated with insulin resistance in obese individuals. To overcome the resistance, pancreas secretes more insulin, leading to hyperinsulinemia (Oh et al., 2018). Free fatty acids cause induction and suppression of protein kinase in the liver and the muscle cells, respectively, which subsequently increases gluconeogenesis in liver and diminishes glucose uptake in muscles (Rochlani et al., 2017). Chronic inflammation is implicated in visceral obesity and exacerbates insulin resistance, which is characterized by the abnormal production of adipocytokines such as tumor necrosis factor-α (TNF-α), interleukin-1 (IL-1), IL-6, leptin, and plasminogen activator inhibitor-1 (PAI-1) (Vaziri et al., 2005; Di Lorenzo et al., 2013). Oxidative stress induces insulin resistance and also abrogates the adiponectin production by adipocytes (Furukawa et al., 2017). Adiponectin is an important anti-inflammatory and anti-atherogenic adipokine and is considered as a protective factor against the development and progression of chronic diseases related to metabolic disorders and oxidative stress including diabetes, hypertension, and cardiovascular diseases (Becic et al., 2018).
Secretions of adipose tissue stimulate mineralocorticoid release from adrenal cells and promote the renin angiotensin aldosterone system activity. Consequently, an elevation in renal sodium retention and vascular tone, as well as an inhibition of norepinephrine reuptake occur, which leads to hypertension. So, there is a direct relationship between obesity and the pathogenesis of hypertension (Ehrhart-Bornstein et al., 2003; Cabandugama et al., 2017). The pathophysiological mechanisms of MetS are schematically summarized in Figure 1. Management of MetS involves lifestyle modification, which consists of particular recommendations on physical activity and dietary interventions to achieve a normal weight, modulation of glycemic and lipid profile, as well as a decrease in blood pressure (Grundy, 2016).
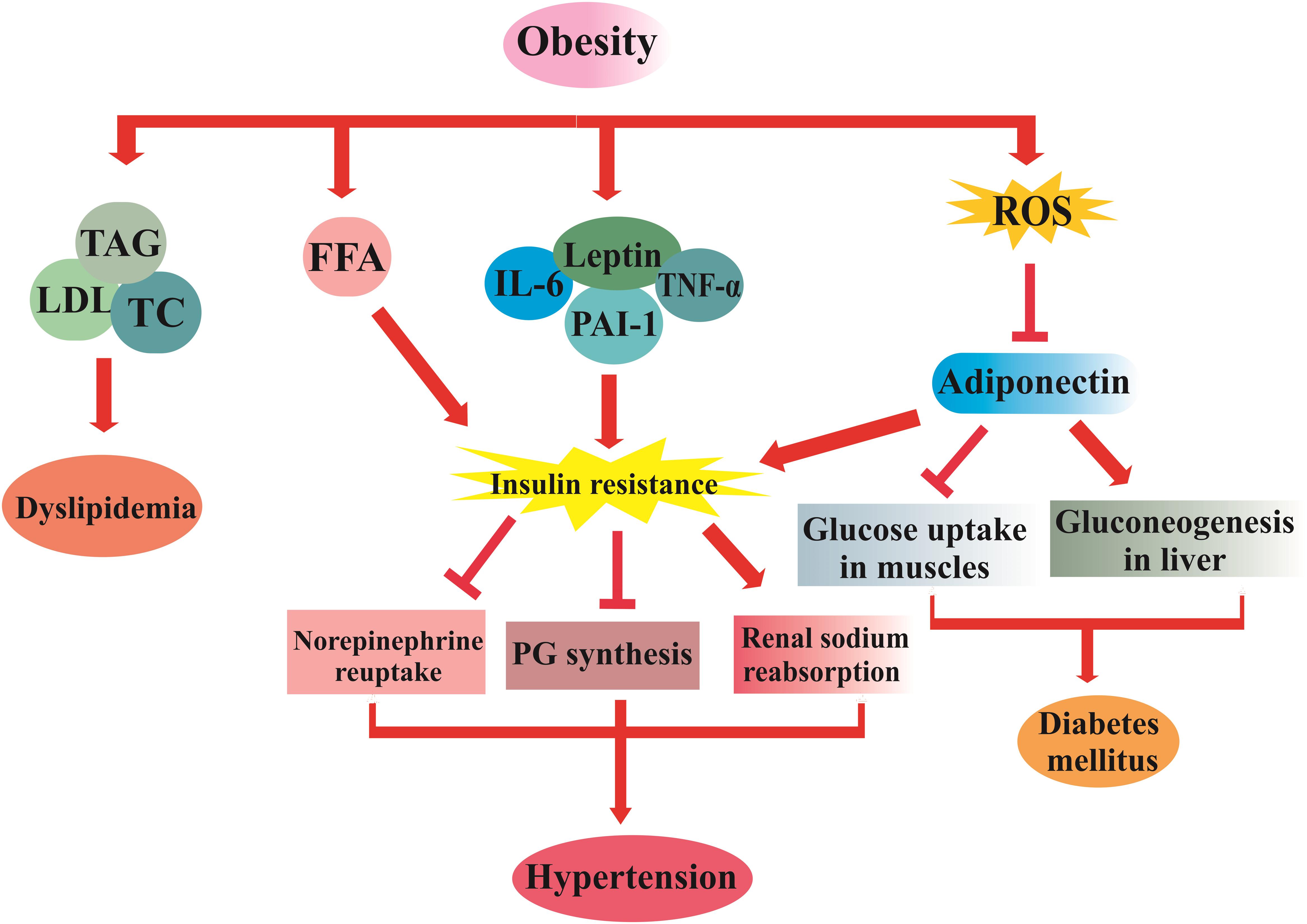
Figure 1. The pathophysiological mechanism of MetS. TNFα, tumor necrosis factor; PAI-1, plasminogen activator inhibitor-1; IL-6, interleukin 6; FFA, free fatty acid; PG, prostaglandin; TAG, triacylglycerol; LDL-C, low density lipoprotein; TC, total cholesterol; ROS, reactive oxygen species.
Several studies have shown that medicinal plants and their isolated compounds possess beneficial therapeutic effects like anti-inflammatory, antioxidant, and insulin-sensitizing properties (Naseri et al., 2018). Oral administration of plant extracts is shown to be effective on MetS and its complication by reducing visceral obesity, systolic and diastolic blood pressure, and blood glucose. Additionally, medicinal plants enhance insulin secretion and cardiovascular function, and suppress gluconeogenesis, inflammation and oxidative stress (Naseri et al., 2018; Payab et al., 2019). The main mechanisms of medicinal plants for managing MetS are presented in Figure 2. In spite of the promising effects of medicinal plants to manage MetS and its complications, due to a low bioavailability, their bioactivity is diminished (El-Far et al., 2016; Hassani et al., 2016; Cardozo et al., 2018; Firouzi et al., 2018; Hosseini et al., 2018; Tajmohammadi et al., 2018). One of the promising ways for the improvement of bioavailability, stability, solubility, and biodistribution of natural products is formulating these compounds in nanostructured forms (Gera et al., 2017).
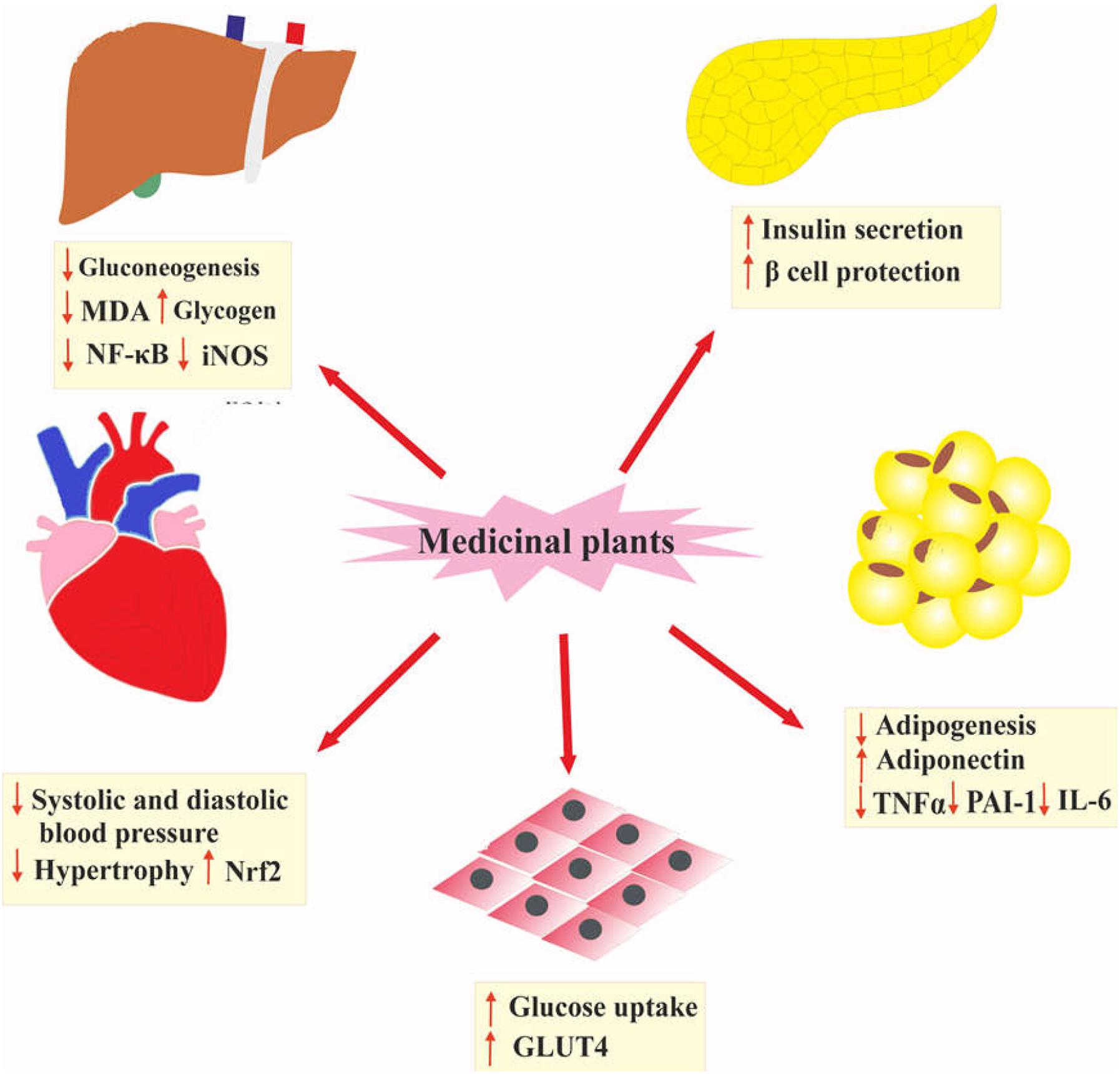
Figure 2. The main mechanisms of medicinal plants for managing MetS. Nrf2, nuclear factor erythroid 2–related factor 2; GLUT4, glucose transporter type 4; TNFα, tumor necrosis factor; PAI-1, plasminogen activator inhibitor-1; IL-6, Interleukin 6; MDA, malondialdehyde; NF-κB, nuclear factor-κB; iNOS, inducible nitric oxide synthase.
Various nanostructured formulations such as green-synthesized nanoparticles (NPs), nanoemulsions, solid lipid nanoparticles (SLNs), nanostructured lipid carriers (NLCs), nanoliposomes, and polymeric NPs have been developed using natural products (Taghipour et al., 2019). Plant extracts mediated synthesis of NPs is one of the best and environment friendly method for the green synthesis of metal NPs (Khan et al., 2017).
Nanoemulsions are stable colloidal systems that are favorable and suitable vehicles for controlled delivery of lipophilic ingredients (Aswathanarayan and Vittal, 2019). SLNs are lipid-based NPs that can be easily fabricated by biodegradable and biocompatible solid lipids (Ghasemiyeh and Mohammadi-Samani, 2018). NLCs are another type of lipid-based nano carrier systems with colloidal particles composed of both solid and liquid lipids (Madane and Mahajan, 2016). Nanoliposomes provide a useful technology for delivering and targeting both hydrophilic and lipophilic phytobioactive constituents (Khorasani et al., 2018). Biodegradable polymeric NPs offer numerous advantages, since they protect bioactive constituents from degradation, enhance solubility, and provide controlled delivery and targeting (Pereira et al., 2018).
Ganesan et al. (2017) reviewed the beneficial effects of nanostructured formulations of phytochemicals to counteract diabetes. In our previous study, we reviewed the beneficial effects of nanoformulation originated from phytochemicals to combat MetS and its related complications (Taghipour et al., 2019). There is no comprehensive review about the potential use of various nanostructured formulations fabricated from herbal extracts, as promising future drugs to treat MetS and its associated complications. The present study, for the first time, provides a comprehensive review on the beneficial effects of nanoformulated herbal extracts on MetS and related disorders considering the in vitro and in vivo experiments.
Administration of nano-based drug delivery systems is one of the main strategies to enhance targeting capability and also to improve the safety and efficacy of drugs (Kesharwani et al., 2018). Conventional drug delivery systems are often accompanied with some critical limitations such as high dosage, low efficacy, low bioavailability, lack of target specificity, and dose-dependent side effects (Surendiran et al., 2009; Subramani et al., 2012). In vitro and in vivo investigations showed that nano-drug delivery systems such as nanomicelles, liposomes, and hydrogel-based nanocomposites can provide drug targeting to a specific site (Ponnappan and Chugh, 2015; Kesharwani et al., 2018). In case of diabetes and diabetes-associated dysfunctions, effective delivery of insulin via oral route by these nanoformulations is highly preferred compared to the available parenteral preparations, due to a higher patient compliance (Wilczewska et al., 2012; Fangueiro et al., 2015; Maity et al., 2017). In spite of the recent advancements of insulin delivery by NPs, there is still a challenge regarding the low bioavailability and poor gastric absorption of insulin. Some strategies have been utilized to overcome this challenge. For example, liposomes are mostly used to entrap lipophilic and hydrophilic drugs, to achieve higher efficacy and bioavailability, as well as fewer side effects. Liposomes modified with targeted ligand biotin were found to be effective in facilitating the delivery of insulin within oral route with limited leakage of insulin from inner aqueous parts and also facilitated insulin uptake through intestinal epithelia by receptor-mediated endocytosis (Zhang et al., 2014). The nanoliposomes had a longer gastric residence due to a higher resistance to enzymatic degradation by the proteolytic enzymes such as pepsin and trypsin. Conjugation of NPs with cell-penetrating peptides is another solution to improve the bioavailability (Wu et al., 2004). For example, peptide-protamine was used by Sheng et al. (2016), conjugated with insulin and encapsulated in poly (lactic-co-glycolic) acid (PLGA) NPs (as a mucoadhesive nanoformulation), and coated with N-trimethyl chitosan chloride. After oral administration of the formulation to diabetic rats, the onset of hypoglycemic effect was found to appear faster and remained longer. The bioavailability of this formulation was considerably increased, corroborating that the prepared nanoformulation could be internalized in cells easier than insulin (Sheng et al., 2016). NPs can also be coated with some protective agents such as chitosan, to prevent or decrease enzymatic digestion (Wu et al., 2004). Also, their mucus layer permeation can be promoted using coatings like N-(2-hydroxypropyl) methacrylamide copolymer (pHPM) (Qu et al., 2016).
SLNs are other nano-drug delivery systems which can potentially enhance the intestinal absorption and protect both peptide-based and organic hypoglycemic agents against enzymatic degradation (Sarmento et al., 2007).
Conclusively, nanoformulation of conventional drugs used for the management of MetS results in improved efficacy/bioavailability and reduced side effects; thus, the same approach can be employed to enhance delivery of plant-derived natural compounds to manage MetS which is discussed as follows.
Diabetes mellitus is a metabolic disorder which is caused by insulin resistance or progressive pancreatic beta cell failure and lack of insulin secretion, which cause a disturbance in the metabolism of carbohydrates, lipids, and proteins that consequently leads to micro- and macrovascular complications (Choudhari et al., 2017). Various plant extracts have been introduced to control diabetes and its complications. Nanostructured formulations of herbal extracts can potentiate their antidiabetic properties through the regulation of pharmacokinetics and increment of bioavailability (Ganesan et al., 2017). NLCs are drug delivery systems containing both solid and liquid lipids as a core matrix and can be used as drug carriers for lipophilic drugs, to increase their solubility and bioavailability (Ong et al., 2016). Marrubiin, a diterpenoid in the Leonotis leonurus (L.) R.Br., has a preventive and therapeutic effect in diabetes in experimental studies (Nakhlband et al., 2018). Marrubiin can induce insulin secretion, increase insulin sensitivity and upregulate glucose transporter type (GLUT)-2 gene (Mnonopi et al., 2012). This phytochemical is poorly soluble in water. NLCs based on acetonic leaf extract of L. leonurus were fabricated by high-pressure homogenization method and were evaluated regarding antidiabetic effects in an in vitro model. Under hyperglycemic condition, the nanoformulation stimulated insulin release in INS1 pancreatic β cells and elevated glucose uptake in Chang liver cells compared to the extract. Extract-loaded NPs had an average particle size of 220 nm and were stable in different storage temperatures (Odei-Addo et al., 2017). Therefore, NLCs might be a suitable candidate to be evaluated in an animal model of diabetes.
Ficus religiosa L., commonly known as Peepal tree, possesses several pharmacological effects including antioxidant, anti-inflammatory, antidiabetic and neuroprotective activity (Singh et al., 2011). SLNs, formulated using an ethanolic stem bark extract of F. religiosa, were assessed in streptozotocin (STZ) and fructose-induced animal model of diabetes. Results showed that extract loaded SLNs had pronounced hypoglycemic and insulin sensitizing effects compared with the extract suspension. The nanoformulations based on SLNs provided an initial burst release followed by sustained release (Priyanka et al., 2018). SLNs offer attractive properties including easy production, low particle size, low toxicity, and good loading capacity of active molecules (Gordillo-Galeano and Mora-Huertas, 2018). In spite of various advantages of SLNs, the initial burst release makes the SLNs delivery systems unfavorable for oral delivery of several natural products that can improve chronic diseases (Ganesan et al., 2018). One of the promising ways to overcome this drawback is surface modification of the SLNs (Ganesan et al., 2018). Therefore, F. religiosa extract loaded surface modified SLNs can be the subject of future studies.
Plicosepalus acaciae (Zucc.) Wiens & Polhill and P. curviflorus (Benth. ex Oliv.) Tiegh. are medicinal plants which demonstrated antidiabetic activity, probably due to their antioxidant effects (Al-Taweel et al., 2012). Three SLNs based on methanolic extract of P. acacia and P. curviflorus were prepared with emulsion solvent evaporation method and their antidiabetic activity was evaluated in STZ+ high-fat diet (HFD)-induced diabetes. It was revealed that the proportion of lipid used in NPs directly correlates with pharmacological activity, so that the formulation with higher content of lipids had a better effect to reduce blood glucose, insulin resistance, and glycated hemoglobin compared with the simple extract or pioglitazone. Also, a remarkable decrease in malondialdehyde (MDA), as well as an increase in the endogenous antioxidant mediators, including glutathione (GSH), superoxide dismutase (SOD), and catalase (CAT) were observed with the SLNs having the highest lipid ratio (Aldawsari et al., 2014). A further area of research is needed to elucidate the hypoglycemic mechanisms of P. acaciae and P. curviflorus extracts-loaded SLNs formulations.
Alpha-eleostearic acid is a typical conjugated trienoic fatty acid isomer of conjugated linolenic acid (Tsuzuki et al., 2006). Previous research demonstrated the presence of a high level of α-eleostearic acid in bitter gourd (Momordica charantia L.) seed oil, which displays strong antioxidant and anti-inflammatory activity in an animal model of diabetes (Saha and Ghosh, 2012). The bioavailability and efficacy of α-eleostearic acid is low due to slow transport across the gastrointestinal (GI) tract (Plourde et al., 2006). Nanoemulsion colloidal systems can increase GI absorption of bioactive hydrophobic molecules (Kumar Dey et al., 2012). Nanoemulsion containing bitter gourd oil diminished blood sugar and enhanced antioxidant enzymes (CAT, SOD, and glutathione peroxidase (GPx) in alloxan (ALX)-induced diabetic rats. The nanoemulsion system in comparison to the conventional emulsion showed higher physical stability and remained mono-phasic during 12 weeks of storage, which is one of the advantages of nano-sized droplets of the emulsion. This nanoformulation improved bioavailability and decreased the required dose (Paul et al., 2014).
Syzygium cumini (L.) Skeels. [Synonym: Syzygium jambolanum (Lam.) DC.] is an Indian medicinal plant with previously demonstrated antidiabetic activity (Samadder et al., 2011). A high content of polyphenols in leaf extract of S. cumini is responsible for antidiabetic and anti-inflammatory effects (Ajiboye et al., 2018). The hypoglycemic effect of S. cumini is related to upregulation of GLUT4, phosphatidylinositol 3 kinase (PI3K), and peroxisome proliferator activator receptor gamma (PPAR-γ) (Anandharajan et al., 2006). The ameliorative effects of S. cumini can be augmented by encapsulation in polymeric NPs. PLGA, a biodegradable and biocompatible polymer made from lactic acid and glycolic acid monomers (Danhier et al., 2012), was used to prepare S. cumini nanoformulation by the solvent displacement method. The prepared nanoformulation, caused a significant increase in glucose uptake, glucokinase activity, and GLUT4 protein expression in L6 rat skeletal muscle cells. Additionally, reactive oxygen species (ROS) production, nuclear factor-κB (NF-κB), a key contributor to cellular inflammatory cascades (Baker et al., 2011), and inducible nitric oxide synthase (iNOS), the enzyme that synthesizes NO, were significantly reduced in the in vitro model. In the rat model of arsenic-induced diabetes, blood sugar and glycosylated hemoglobin levels in the extract- and nanoformulation-treated groups were significantly decreased, but the decrease by nanoformulation was more remarkable than that of the simple extract. The authors claimed that formulating this extract in form of NPs could improve its penetration into blood brain barrier (Samadder et al., 2012). Considering the central nervous system (CNS) complications of diabetes, such antidiabetic formulation may have a dual action to control both hyperglycemia and CNS effects of the disease which can be the subject of future studies.
Recently, a growing attention has been payed to the use of herbal extracts to produce metal-based biocompatible NPs due to its cost-effectiveness and eco-friendly nature, as well as high efficacy and stability (Ovais et al., 2016). Active phyto-constituents, adhered to metal NPs, are responsible for their reduction and stabilization (Mittal et al., 2013).
Argyreia nervosa (Burm. f.) Bojer, from the family Convolvulaceae has been used in traditional Indian medicine for several therapeutic indications such as antidiabetic, anti-inflammatory and diabetic wound healing (Singhal et al., 2011; Paulke et al., 2013). Silver NPs (AgNPs) prepared using an aqueous leaf extract of A. nervosa showed in vitro inhibitory effects on α-amylase and α-glucosidase, which are important enzymes in carbohydrate metabolism with IC50 values of 55.5 and 51.7 μg/ml, respectively. Adherence of the functional groups of the phytochemicals to AgNPs enhanced surface area and significantly increased the entrapment of free radicals compared with the simple extract (Saratale et al., 2017). Thus, the formulation may be a suitable candidate to be evaluated in an animal model of diabetes.
Eysenhardtia polystachya (Ortega) Sarg. is commonly known as “palo azul” and has shown beneficial effects on the alleviation of bladder disorders and kidney stone (Perez et al., 1998). According to previous studies, flavonoid enriched E. polystachya extract has diminished oxidative damage in an animal model of diabetes (Perez-Gutierrez et al., 2016). Campoy et al. biosynthesized Ag NPs using a hydromethanolic extract of E. polystachya bark. The nanoformulation elevated pancreatic β cells survival and ameliorated insulin resistance and hyperglycemia, as well as dyslipidemia in glucose-induced diabetes in zebrafish. Also, in INS1 pancreatic β cell line intoxicated with hydrogen peroxide (H2O2), nanoformulated extract could significantly restore the insulin secretory ability of cells, which indicates antidiabetic activity of extract to be, at least in part, attributed to its antioxidant properties (Campoy et al., 2018).
Pouteria sapota (Jacq.) with the common name of “sapote” is found in Mexico and South America. The highest concentration of polyphenols in the fruit of this plant are responsible for its antioxidant activity (Ma et al., 2004). AgNPs were green-synthesized using the aqueous leaf extract of P. sapota and evaluated regarding the antidiabetic activity in cellular and animal models. In vitro antidiabetic properties of the AgNPs was corroborated by decreasing non-enzymatic glycosylation of hemoglobin, inhibition of α-amylase, and enhancement of glucose uptake by yeast cells. In STZ-induced animal model of diabetes, biosynthesized AgNPs and extract significantly improved SOD and CAT activity, decreased blood glucose, and enhanced plasma insulin level (Prabhu et al., 2018). P. sapota extract and its biosynthesized AgNPs can be considered as an effective agent in the management of diabetes.
Some preclinical and clinical studies found that cinnamon (the genus Cinnamomum spp.) can improve insulin resistance, decrease blood glucose concentrations and hemoglobinA1c (HbA1c) (Cao et al., 2007; Crawford, 2009). Cinnamomum litseifolium Thwaites was collected from two different regions in India and the antidiabetic effects of the leaf essential oil, formulated as nanoemulsions, were evaluated. The essential oil sample, containing β-phellandrene as the major component (66%), showed a higher inhibitory effect on α-amylase and α- glucosidase enzymes rather than the other sample with methyl eugenol, (E)-caryophyllene, epi-α-muurolol, α-cadinol, and shyobunol as the main ingredients (totally constitute about 60% of the essential oil). It should be mentioned that both nanoformulations showed lower inhibition compared to acarbose (Sriramavaratharajan and Murugan, 2018). Further in vivo investigations are needed to confirm the efficiency of these preparations as hypoglycemic agents.
Costus speciosus (J. Koenig) Sm. (synonym Cheilocostus speciosus (J. Koenig) C. D. Specht) has shown an antidiabetic effect via induction of insulin secretion and improvement in insulin sensitivity (Ali et al., 2014). Ethanolic leaf extract of C. speciosus was loaded in PLGA NPs to enhance bioactivity and provide sustained release of active constituents. PLGA NPs increased the expression of insulin (I&II), and GLUT4 genes; while decreased the expression of GLUT2 gene. In addition, the nanoformulation diminished blood sugar, enhanced high density lipoprotein cholesterol (HDL-C) and decreased total cholesterol (TC), triacylglycerol (TAG), and low density lipoprotein (LDL-C) in STZ-induced diabetic rats. The nanoformulated extract was more effective in controlling the lipid profile and blood glucose in comparison to the simple extract (Alamoudi et al., 2014).
Stevioside is a glycoside isolated from Stevia rebaudiana (Bertoni). Both in vitro and in vivo studies have indicated that stevioside and the extract of S. rebaudiana can be used as an alternative treatment for diseases associated with the MetS (Singla et al., 2017a; Ahmad and Ahmad, 2018). To provide a continuous-release formulation, titanium dioxide (TiO2) nanomaterial is a suitable choice due to the numerous pores which supplying a controlled release preparation (Lopez et al., 2010; López et al., 2011). Hydroethanolic leaf extract of S. rebaudiana was formulated using TiO2 nanomaterial by sol-gel method and was evaluated in ALX-induced diabetic rats. After 31 days of treatment, there was a significant and permanent decrease in blood glucose, glycosylated hemoglobin, TC, and TAG, along with a higher insulin concentration (P < 0.01) in animals treated with extract-loaded nanomaterial (20 and 30 μM) compared to the diabetic group treated with TiO2 nanomaterial alone. TiO2 nanomaterial could provide a sustained and controlled release of the plant extract which resulted in prolonged stimulation of insulin secretion (Langle et al., 2015). The previous study has shown nanoencapsulated stevioside in pluronic-F-68-poly-lactic acid (PLA) interestingly increased intestinal absorption, bioavailability, and provided a controlled release of the active component (Barwal et al., 2013). Perumal et al. (2016) have investigated hydromethanolic leaf extract of S. rebaudiana-loaded chitosan NPs prepared through the nanoprecipitation method. Chitosan is an eco-friendly, non-toxic and cost-effective polymer which is used as an excipient for drug delivery (Yadav et al., 2019). In the STZ-induced diabetic rats treated with the extract-loaded NPs, a considerable body weight gain and increase in SOD, CAT, and GSH, as well as a decrease in LPO were observed. In vivo antidiabetic evaluation showed a remarkable decrease in blood sugar and HbA1c in the group treated with nanoformulated extract (Perumal et al., 2016).
Gymnemic acid is the main active constituent of Gymnema sylvestre (Retz.) R. Br. ex Sm. with antidiabetic properties (Ota and Ulrih, 2017). Gold NPs (AuNPs), synthesized using the aqueous extract of G. sylvestre, significantly abated inflammatory mediators, including TNF-α, IL-6 and C-reactive protein in ALX induced diabetic animal model. In addition, lower blood glucose, HbA1c, TAG, LDL-C, as well as a higher HDL-C level were achieved in AuNPs-treated rats compared with the diabetic control group. The nanoformulated extract could also show some improvement in the histology of islet cells. It should be mentioned that there was no significant improvement in the insulin level and body weight in diabetic rats; therefore, the plant seems to have an insulin-stimulating effect rather than an insulin-mimetic effect in the slight improvement of insulin level (Karthick et al., 2014).
Marsilea quadrifolia L. is known as “aquatic fern” with antidiabetic and antioxidant activities (Zahan et al., 2011). Nanosized particles can penetrate efficiently into the cells and regulate its function. Biosynthesized AuNPs with methanolic extract of M. quadrifolia elevated glucose utilization in 3T3 L1 adipocytes, as an in vitro model of diabetes, in a dose depended manner. Moreover, the high dose of AuNPs (30 μg/ml) and pioglitazone showed a similar effect. Polyphenols and flavonoids of the extract attached to the surface of AuNPs improved bioavailability, induced transmitted GLUT4 vesicle to the cell membrane and probably increased glucose uptake in adipocytes (Chowdhury et al., 2017).
Sambucus nigra L. belongs to the Caprifoliaceae family. The fruit extract of S. nigra contains several polyphenols which are responsible for antidiabetic activity (Badescu et al., 2015). Biogenic AuNPs were formulated with acetonic fruit extract of S. nigra. There was no significant difference in insulin level between the STZ-induced diabetic rats treated with the extract or AuNPs compared with the diabetic control group. The extract failed to reduce blood sugar also. Although AuNPs reduced blood glucose, the change was not statistically significant. The biogenic AuNPs abolished expression of the cyclooxygenase (COX)-2, which produces prostaglandins that promote inflammation, and enhanced antioxidant activity in the diabetic animals. It can be concluded that the AuNPs can be considered as an adjuvant therapy in diabetes due to augmentation of antioxidant competence, as well as mitigation of inflammation (Opris et al., 2017).
Vaccinium arctostaphylos L. is a well-known medicinal plant in Iran. Previous studies indicated that several species of the genus Vaccinium have beneficial effects on diabetes (Cignarella et al., 1996; Martineau et al., 2006). Chemically synthesized ZnO NPs possess antioxidant, antimicrobial and antidiabetic activities (Das et al., 2013; Alkaladi et al., 2014; Nagajyothi et al., 2014). Functionalizing NPs with natural products can enhance their bioactivity and biocompatibility (Opris et al., 2017). Ethanolic fruit extract of V. arctostaphylos was used for green synthesis of ZnO NPs by microwave-assisted method. The biosynthesized ZnO NPs abolished fasting blood sugar and promoted HDL-C level. A remarkable decrease in TC was observed in the group treated with biogenic ZnO NPs. Overall, the biofabricated ZnO NPs revealed better treating efficacies on ALX-induced diabetes vs. the chemically synthesized ZnO NPs. Since both ZnO as well as the extract, have antidiabetic effects the synergistic effect caused greater efficiency of biogenic ZnO NP to control diabetes (Bayrami et al., 2018).
Musa paradisiaca L. is commonly known as “plantain” and belongs to the Musaceae family. Previous data showed a 38.13% decrease in blood glucose in animal treated with extract of unripe plantain (Eleazu et al., 2013). Stem juice extract of M. paradisiaca was used for green synthesis of AgNPs. A remarkable increase in insulin levels as well as a decrease in blood sugar and HbA1c was observed in the STZ-induced diabetic rats treated with AgNPs. Increase in glucose uptake and the induction of insulin secretion are the possible mechanisms responsible for these observations (Anbazhagan et al., 2017).
Chamaecostus cuspidatus (Nees & Mart.) C.D.Specht & D.W.Stev. is known as “insulin plant” with antidiabetic and antioxidant activities (Ponnanikajamideen et al., 2016). AuNPs green-synthesized using aqueous leaf extract of C. cuspidatus were evaluated in STZ-induced diabetic rats. AuNPs elevated body weight, decreased TC and abolished lipid peroxidation, hydroxyl radicals, and nitric oxide. Additionally, AuNPs slightly decreased blood glucose and increased glycogen and insulin level. Nanoformulation with the mentioned plant was cost-effective and reduced the required dose (Ponnanikajamideen et al., 2018). Active phyto-constituents adhered to metal NPs which are responsible for their reduction and stabilization need in-depth clarification in future studies.
Previous data have shown that the extract of Hibiscus subdariffa L. calyx (family Malvaceae), containing various polyphenols, can be potentially used for improving insulin resistance and diabetes-related nephropathy (Peng et al., 2014). ZnO NPs were formulated using an aqueous leaf extract of H. subdariffa prepared at 60 and 100°C and were investigated in STZ-induced diabetic mice. Remarkable decrease in blood sugar to 59.58 and 48.27% was observed in the group treated with ZnO NPs synthesized at 60 and 100°C, respectively. A lower level of pro-inflammatory cytokines (TNF-α, IL-1β, IL-6) which is associated with Th1, and a higher level of anti-inflammatory cytokines (IL-4 and IL-10) which is linked with Th2 (Charrad et al., 2016), was observed in the diabetic group treated with ZnO NPs produced at 60°C compared to 100°C. Moreover, ZnO NPs increased the expression of glucokinase (GK), insulin receptor A (IRA), and GLUT2 genes as well as decreased the expression of Pyruvate Kinase L/R gene. Spherical-shape particles with lower size were observed in ZnO NPs synthesized at 60°C vs. 100°C. Generally, biosynthesized NPs at 60°C showed a higher antidiabetic effect vs. 100°C due to lower particle size, and the higher bioactive compounds used as a stabilizer (Bala et al., 2015).
ZnO NPs were synthesized using aqueous leaf extracts of different plants, such as Azadirachta indica A. Juss., Hibiscus rosa-sinensis L., Murraya koenigii (L.) Spreng, Moringa oleifera Lam, Tamarindus indica L., and were evaluated regarding antidiabetic and antioxidant activities in an in vitro model. All of the plants listed are medicinal plants which are widely used as traditional and folk remedies in India (Tachibana et al., 2001). The extract of these plants is rich in carbohydrates, glycosides, phenolic compounds, flavonoids, saponins, and tannins, with previously demonstrated antidiabetic and antioxidant activities (Kuppusamy et al., 2015). All of the extract-mediated ZnO NPs showed antioxidant effects, but the highest activity was observed with T. indica and M. oleifera, possibly due to the presence of proteins and amino acids that do not exist in other plants. ZnO NPs which were chemically prepared exhibited no antioxidant effect. Also, T. indica showed the highest α-amylase and α-glucosidase inhibition compared to ZnO NPs prepared with other plant extracts and chemically synthesized ZnO NPs (Rehana et al., 2017). Hence, phytosynthesized NPs seems to be a safe and efficient alternative for therapeutic use.
Cassia Fistula L. has shown antioxidant, antidiabetic and protective effect on diabetic nephropathy (Agnihotri and Singh, 2013). Biosynthesized AuNPs were prepared with aqueous stem bark extract of C. fistula. The extract is rich in lupeol, β-sitosterol, and hexacosanol that are responsible for the reduction of gold ions into AuNPs which occurs more quickly after adding the plant extract. Although oral administration of both extract and biosynthesized AuNPs significantly increased body weight and HDL-C level, and ameliorated blood glucose and HbA1c, the nanoformulation was more effective. Improvement of lipid profile probably occurred due to the presence of β-sitosterol in the extract. In addition, biogenic AuNPs were more effective in reduction of urea, creatinine, and uric acid level vs. extract alone. This result showed beneficial effects of biogenic AuNPs as a promising agent in the management of complications associated with diabetes (Daisy and Saipriya, 2012).
Silybum marianum L., belonging to Asteraceae family, is one of the oldest medicinal plants and has been widely used for treating liver and gallbladder illnesses (Kosina et al., 2019). Silymarin, extracted from the S. marianum fruits, is a multicomponent extract and has demonstrated antioxidant and antidiabetic activities (Heidari Khoei et al., 2019). ZnO NPs biosynthesized using S. marianum seed extract reduced fasting blood sugar, TC, TAG and augmented HDL-C and insulin level in ALX-induced diabetic rats. On the other hand, ZnO NPs exerted antibacterial activity against Escherichia coli (Arvanag et al., 2019). Similarly, ZnO NPs prepared by Nasturtium officinale R.Br. leaf extract exhibited antidiabetic and antibacterial performances against the most common bacteria found in diabetic foot ulcer including Staphylococcus aureus and E. coli (Aris et al., 2019; Bayrami et al., 2019). Therefore, future studies should evaluate the beneficial effect of ZnO NPs prepared by S. marianum and N. officinale against diabetic wound infection.
The ginger extracted from the Zingiber officinale is considered as an antidiabetic agent through interfering with NF-κB cascade pathway and displaying anti-inflammatory and antioxidant properties (Saedisomeolia et al., 2019). The application of nanotechnology provides a useful delivery system for ginger compounds and enhance their biological activities (Zhang et al., 2016, 2018). Nano transdermal delivery for ginger ingredient, gingerol, increased its bioavailability ratio (Baskar et al., 2012). In an in vivo study performed by Garg et al. (2016) fabricated AgNPs using ethanolic extract of ginger rhizome exhibited notable hypoglycemic properties. Further investigations are needed in order to understand the clarification of the molecular mechanisms of ginger extract based NPs on diabetes.
Altogether, several findings revealed that nanostructured formulations of plant extracts were able to overcome drawbacks of chemical counterparts, affecting the promising effects of plant extracts in diabetes. Considering the critical role of herbal-based nanoformulations in combating inflammation and oxidative damages, future studies should focus on other aspects of their uses in the management of oxidative and inflammation related diseases. Almost all of the current evidence focused on the efficacy of plant extracts-loaded NPs in in vitro and/or in vivo models of diabetes. Future preclinical and clinical trials should be conducted to confirm their safety and efficacy in diabetic patients. Toxicological aspects, plausible side effects as well as main molecular mechanisms of plant extracts-loaded NPs need appropriate assessment before conducting clinical trials. Additionally, more profound investigations and engineered methods are required to design surface modified nanostructures of plant extracts to achieve optimized drug delivery systems.
Delayed diabetic wound healing is associated with impaired angiogenesis and bacterial infection. Also, delayed treatment of diabetic foot ulcer can lead to foot amputation (Muniandy et al., 2018). Hence, due to the advantages of medicinal plants for wound healing, natural product-based nanoformulations can be used for amelioration of the wound. A nanobiocomposite hydrogel was prepared as cellulose nanocrystals using Dendrocalamus hamiltonii Nees & Arn. ex Munro, and Bambusa bambos (L.) Voss with previously demonstrated antimicrobial and wound healing effects (Singla et al., 2017b). Cellulose nanocrystals were then impregnated with AgNPs and were evaluated in the excision wound in STZ-induced diabetic mice. The nanoformulation abolished the level of TNF-α and IL-6, which are suppressors of fibroblast proliferation and epithelialization (Dinh et al., 2012). In the last stage of wound healing, the nanobiocomposite enhanced the level of transforming growth factor β (TGF-β), which stimulates the formation of granulation tissue and increases wound closure rate (Wang et al., 2013). Additionally, nanoformulation increased the level of platelet-derived growth factor (PDGF), fibroblast growth factors (FGF), and vascular endothelial growth factor (VEGF), participating in wound healing process by induction of angiogenesis and epithelization (Losi et al., 2013). Nanoformulation-treated group elevated density of collagen fibril and promoted complete wound healing (98–100%). Both cellulose nanocrystals and AgNPs possess anti-inflammatory and antimicrobial effects. A synergistic effect caused faster wound healing. Also, cellulose nanocrystals create a moist environment and reduce wound healing time (Singla et al., 2017c). Nanobiocomposite hydrogel can be considered as a suitable dressing material for wound healing purpose and seems as a favorable choice for skin drug delivery system.
Long-term hyperglycemia associated with inflammation and oxidative damage contributes to the development of diabetic complications such as retinopathy, neuropathy, nephropathy, and cardiovascular disease (Volpe et al., 2018).
The earliest manifestation of cardiomyopathy is cardiac diastolic impairment followed by systolic dysfunction that consequently leads to heart failure (Dai et al., 2018). Among various pathophysiological mechanisms, ROS-related hyperglycemia caused oxidative stress in cardiomyocytes, and seems to play a critical role in diabetic cardiomyopathy (Xu Z. et al., 2016). Herbal remedies can have an important role in the amelioration of diabetic cardiomyopathy. Atale et al. (2017) evaluated the cardioprotective effects of green synthesized AgNPs prepared by methanolic seed extract of S. cumini on H9C2 cells derived from embryonic rat heart. Both extract and biogenic AgNPs ameliorated the size of glucose-stressed cells and decreased lipid peroxidation, but the decrease by AgNPs was more remarkable than that of the extract. The methanolic extract of S. cumini seems to be rich of polyphenolic compounds with antioxidant activity (Neha et al., 2013). Both AgNPs as well as the extract have antioxidant properties; therefore, the synergistic effect caused greater efficiency of biogenic Ag NPs vs. extract (Atale et al., 2017).
Diabetic nephropathy (DN) is considered as a major cause of end stage renal disease. DN is characterized by progressive proteinuria and decrease in glomerular filtration rate resulting in the loss of renal function (Bhattacharjee et al., 2016). It has been demonstrated that pomegranate (Punica granatum L.) has promising therapeutic effects against diabetes, cardiovascular complications and DN (Zarfeshany et al., 2014; Shehab et al., 2018). Most of the phytobioactive compounds are unstable and susceptible to enzymatic/non-enzymatic hydrolysis (Volf et al., 2014). In spite of widespread promising effects, pomegranate peel extract has remained underused. AuNPs produced by pomegranate peel extract ameliorated STZ-induced glomerular sclerosis and renal fibrosis. From a mechanistic point of view, AuNPs abrogated pro-inflammatory cascade in nephritic tissue through the regulation of the MAPK/NF-κB/STAT3 pathway. Additionally, AuNPs increased antioxidant performance by activation of nuclear factor erythroid 2–related factor 2 (Nrf2). AuNPs diminished protein glycation through the suppression of RAGE- NOX-4/p47phox activation followed by the mitigation of the ROS generation (Manna et al., 2019). Considering the results, the biogenic AuNPs prepared by pomegranate extract may be a desirable agent to combat DN.
Mulberry leaf (Morus alba L.) is a traditional medicine used for treating diabetes. Mulberry leaf extract displayed hypoglycemic and insulin sensitizing effects through the activation of IRS-1/PI3K/Glut-4 signaling pathway (Cai et al., 2016). Diabetic retinopathy is considered as a common complication of diabetes which caused visual dimming and blindness (Rübsam et al., 2018). Inflammation, oxidative stress and hyperglycemia play a key role in diabetes-mediating retinopathy (Yeh et al., 2016). AgNPs biosynthesized using mulberry leaf extract alleviated deterioration in the retinal cell layer in diabetes or Aluminum-intoxicated rats (Xu et al., 2019). Further mechanistic studies are needed to confirm the beneficial effect of AgNPs fabricated by mulberry leaf extract to combat diabetic retinopathy.
Obesity is a major global health concern and is characterized by an imbalance between food intake and energy expenditure which causes excessive accumulation of fat in blood, adipose tissue, and liver (de Freitas Junior and de Almeida, 2017; Lee et al., 2018). Obesity is an important risk factor for type 2 diabetes, cancer, and cardiovascular diseases (Grigoraş et al., 2018). Phytosome is a phyto-phospholipid complex, which is produced by forming a hydrogen bond between polar part of phospholipids and phytochemicals. Phytosome is more stable than liposome which results from the hydrogen bond formation (Ghanbarzadeh et al., 2016). Phytosome is considered as an important approach to overcome the poor absorption and low bioavailability of phyto-constituents (Chi et al., 2020). Soybean, Glycine max (L.), is an example of a plant that has been investigated as an alternative choice for common chemical anti-obesity medications due to its content of different bioactive peptides (Singh et al., 2014). Methanolic extract of soybean was used for the synthesis of nanosized phytosome-based thermogel and evaluated for pharmacological effects in HFD-induced obese rats. Solvent evaporation, co-solvency and salting out methods were used for the preparation of phytosomes, as well as a cold method for preparation of a thermogel. Thermogel possesses thixotropic behavior, i.e., the transient viscosity of the fluid depends on its shearing (Wei et al., 2019). The gel transformation temperature was optimized at 31.5°C. The nanophytosome reduced weight gain, adipose tissue weight and daily intake of food. In addition, topical treatment with nanophytosomal thermogel significantly reduced TC, TAG, LDL-C and very low-density lipoprotein (VLDL)-C compared to the negative control group. Nanosized particles and high skin penetration of soy extract-loaded phytosomes seem to be responsible for the systemic effect. Nanophytosome formulation of soybean caused better release (92.50% within 2 h), which has a direct and reverse relation with increased percentage of extract and phosphatidylcholine in the phytosome, respectively. The nanophytosome revealed high stability and perfect entrapment efficiency due to the formation of a hydrogen bond between OH groups in the extract and phosphatidylcholine in the phytosome (El-Menshawe et al., 2018). Therefore, it can be concluded that nanophytosomal thermogel of soybean may be a useful formulation in treatment of obesity. In another study, AuNPs prepared by aqueous extract of Smilax glabra Roxb. Decreased body weight, blood glucose, and liver marker enzymes in HFD and STZ-induced obese diabetic rats. From the mechanistic point of view, AuNPs alleviated inflammatory markers including TNFα and IL-β, abolished leptin and resitin, and increased adiponectin in obese diabetic rats (Ansari et al., 2019). From the results, AuNPs represented an outstanding performance in alleviating both diabetes and obesity. It could possibly be used in future as a promising agent to improve obesity-related complications.
Dyslipidemia is a serious metabolic disorder accompanied by lipid abnormalities such as elevated TAG, VLDL-C, TC, LDL-C and decreased HDL-C (Sun et al., 2018). Management of dyslipidemia is important since it is a risk factor for cardiovascular diseases (Buşilă et al., 2017). One possible mechanism by which dyslipidemia leads to cardiovascular disorders is the generation of free radicals and oxidative stress (Singh et al., 2016). Previous studies demonstrated the antioxidant and antihyperlipidemic potential of both garlic oil and kenaf seed oil (Ragavan et al., 2017; Cheong et al., 2018). Garlic (Allium sativum L.) oil mostly constitutes sulfur-containing compounds such as alliin, allicin, diallyl sulfide, diallyl disulfide, and diallyl trisulfide which are responsible for antidiabetic, antihyperlipidemic and anti-atherogenic effects (Zheng et al., 2013; Sambu et al., 2015; Ebrahimzadeh-Bideskan et al., 2016). The use of garlic oil is limited due to its strong odor, stomach irritation, low stability, poor solubility and bioavailability (Gao et al., 2013). Nanoemulsion of garlic oil was prepared with a proportion of 1:2 (oil: surfactant) and evaluated regarding its preventive and therapeutic effects on HFD-induced dyslipidemia in an animal model. In the preventive method, HFD and drugs (garlic oil, nanoformulation, and standard drug) were administrated simultaneously and at the end of the 6 weeks, serum biochemical parameters were measured; whereas in the curative method, HFD was administrated during 10 weeks and drugs (garlic oil, nanoformulation, and standard drug) were given during the last 6 weeks. Nano-sized droplets had a spherical shape and were stable without forming sedimentation or phase separation. In the animal study, body weight gain was significantly lower in nanoformulation, standard drug, and garlic oil-treated rats in comparison to HFD control rats. Garlic oil nanoemulsion reduced lipid profile dose-dependently in both preventive and curative studies. Dyslipidemia causes liver injury and induces alteration in the hepatic enzymes such as ALT, AST, and ALP (Cheraghpour et al., 2019). These markers were significantly increased in rats treated with atorvastatin, garlic oil and nanoformulation of garlic oil at a concentration of 0.65 mg/kg; while they were decreased in rats treated with nano-encapsulated garlic oil at concentrations of 0.18 and 0.46 ml/kg. Lymphocytic infiltration and necrosis, which indicates liver damage (Huang et al., 2019), were not observed in animals treated with 0.18 and 0.46 ml/kg of nanoemulsion. Hematoxylin and Eosin staining revealed a decrease in vesicular steatosis (presence of numerous vesicle of fat in the liver) at 0.65 ml/kg of the nanoformulation. Moreover, 0.18 and 0.46 ml/kg of nanoemulsion were more effective in prevention and treatment of hepatic steatosis induced by HFD. Nanoformulation of garlic oil had higher efficiency, stability, and lower liver toxicity than direct intake of garlic oil (Ragavan et al., 2017). This nanostructured formulation of garlic oil could be an advantageous formulation for treatment of dyslipidemia.
According to the previous studies, natural ingredients of Kenaf (Hibiscus cannabinus L.) seed oil have a cholesterol-lowering effect. On the other hand, because of its poor solubility in water, its bioavailability is low (Cheong et al., 2018). Kenaf seed oil was prepared as a nanoemulsion and a macroemulsion and was investigated in high cholesterol diet (HCD)-induced dyslipidemia in rats. Nanoemulsion showed higher stability than macroemulsion due to its smaller droplet size. Beta-cyclodextrin in the emulsifier mixtures has a cholesterol-lowering effect by inhibiting the absorption of cholesterol in the small intestine (García-Mediavilla et al., 2003). Also, natural compounds present in the kenaf seed oil such as phytosterols and saponins have cholesterol-lowering effect (Shi et al., 2004; Lu et al., 2010). Synergistic effect between phytochemicals of kenaf seed oil and sodium caseinate as well as beta-cyclodextrin caused the lowest level of TC and LDL-C in nanoemulsion treated rats. Moreover, prepared nanoemulsion alleviated atherogenic and coronary risk index and increased antioxidant performance corroborated by reducing MDA and elevating GSH. In addition, nanoemulsion diminished accumulation of fat droplets in the liver and enhanced binucleate cells, which shows that hepatic cells can be regenerated after damage. Body weight gain was remarkably decreased in emulsifier mixtures and nanoemulsion treated rats, while simple kenaf seed oil revealed the lowest effect on weight gain. Therefore, weight loss probably occurs due to the presence of sodium caseinate, as well as beta-cyclodextrin in the emulsifier mixtures. Casein is the main protein of milk that has been reported to have a weight loss effect (Anderson et al., 2007). Overall, this nanoformulating method increased the bioavailability, stability, and efficacy of kenaf seed oil (Cheong et al., 2018).
Pulmonary arterial hypertension (PAH) is a progressive medical condition characterized by disturbance in the pulmonary vascular function, increase in vascular resistance and obstruction of the pulmonary artery, which eventually result in right ventricular hypertrophy as well as right-sided heart failure (Kikuchi et al., 2018). Oxidative stress, nitric oxide, and inflammation participate in the development of PAH (Xu et al., 2017). Antioxidant and anti-inflammatory effects of phytochemicals make them a promising option for treating PAH (Xu D. et al., 2016; Meghwani et al., 2018; Xiang et al., 2018). Copaiba oil is an oil-resin that is produced from different species of the genus of Copaifera and is used in Brazilian traditional medicine (Kanis et al., 2012). β-caryophyllene is the major constituent of copaiba oil with antioxidant and anti-inflammatory effects (Ames-Sibin et al., 2018). In addition, this compound is a calcium channel blocker and has an inhibitory effect on cell growth (Rasheed et al., 2015). Nanoencapsulated copaiba oil was investigated in Monocrotaline (MCT)-induced PAH. Both free oil and NPs enhanced sulfhydryl groups (SH), SOD, GPx, and Nrf2, as well as abolished oxidized glutathione (GSSG) concentration, but nanocapsule was more effective. Nrf2 is an antioxidant transcription factor that plays a pivotal role in the protection of cells against oxidative stress (Hu et al., 2019). Both oil and nanoformulation possessed significant effect on the decrease of right ventricular hypertrophy index. Treatment with free oil significantly increased acceleration time/ejection time ratio, which indicates a decrease of PAH; whereas nanocapsules had no significant effect on pulmonary vascular resistance. Thus, the authors concluded that nanoformulation is more effective on heart tissue than pulmonary circulation. Moreover, the preparation of nanocapsules using pectin aqueous solution with antioxidant effect could have a synergistic effect with copaiba oil and increased pharmacological effect (Campos et al., 2017). Therefore, nanoencapsulation has been known effective in favorable delivery and better efficiency of copaiba oil.
Considering the complications of MetS and different mechanisms involved in its pathophysiology, successful strategies are necessary for prevention and treatment of the disease. Despite the evidence provided over the past decades regarding the therapeutic effects of the plant-derived compounds or herbal extracts on the quality of life and human health, their delivery is always problematic. In the last years, nano-based drug delivery systems have been introduced as one of the main strategies to overcome these problems to improve the efficiency of herbal extracts in the treatment of MetS and its related complications (Figure 3). Diabetes is recognized as a challenging metabolic disease and its management is always problematic due to its complexity. As presented, nanoformulations of herbal extracts as NLCs, SLNs, nanoemulsions colloidal systems, and other formulations have shown a significant increase in the antidiabetic effects of the extracts compared with the conventional formulations. Also, the type of nanoformulation and their preparation method had clearly a direct role in their antioxidant activity which depends on the chemical characteristics and the degree of solubility of these compounds. For example, nanoemulsions due to high stability can be a good candidate to deliver the hydrophobic extracts to improve bioavailability and decrease the required dose. On the other hand, the green synthesis of metal NPs such as Zn, Ag, and Au supports this conclusion that Au NPs can be more effective in improving diabetes-related complications.
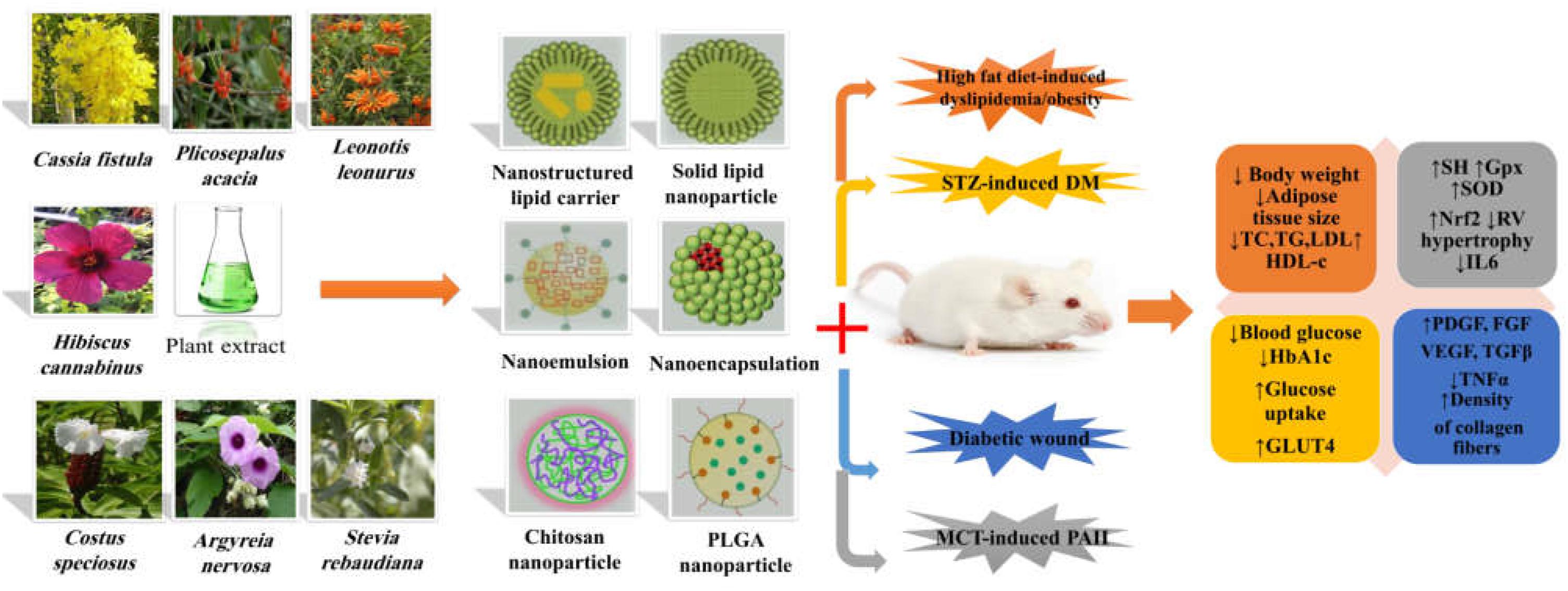
Figure 3. The role of extract-loaded nano-delivery systems in management of metabolic syndrome and its complications. STZ, streptozotocin; MCT, monocrotaline; PAH, pulmonary arterial hypertension; TC, total cholesterol; TAG, triacylglycerol; LDL-C, low-density lipoprotein-cholestrol; VLDL, very-low-density lipoprotein; HDL-C, high-density lipoprotein; SH, sulfhydryl group; GPx, glutathione peroxidase; SOD, superoxide dismutase; Nrf2, nuclear factor erythroid 2–related factor 2; RV, right ventricular; IL-6, interleukin 6; PDGF, platelet-derived growth factor; FGF, fibroblast growth factors; VEGF, vascular endothelial growth factor; TGFβ, transforming growth factor β; TNFα, tumor necrosis factor; GLUT4, glucose transporter type 4; HbA1c, hemoglobin A1c; PLGA poly lactic-co-glycolic acid.
In case of delayed diabetic wound healing, which is one of the complications of diabetic patients, the antibacterial properties of nano-formulated plant extracts have provided excellent advantages for improving diabetic wound healing and its associated problems. Considering the included studies in this review, cellulose based-biocomposites along with AgNPs showed the successful recovery even in the last stage of wound healing in a mouse model. Other characteristics of herbal extracts are antioxidant and anti-inflammatory properties, which make them promising therapeutic agents in the management of diabetic cardiomyopathy and obesity. Studies on the delivery of extracts with AgNPs were presented as great examples to decrease oxidative stress in cardiomyocytes and nanophytosome formulations in anti-obesity therapy.
Another unique property of medicinal plants, especially sulfur-containing herbal oils, is antihyperlipidemic potential which has demonstrated the highest effect on dyslipidemia in the nanoemulsion formulation. In addition, nanoemulsion and nanoencapsulation of these compounds demonstrated beneficial therapeutic effects on PAH.
Our purpose in the current review was to criticize a collection of pharmaceutical and biopharmaceutical studies on the effect of nanoformulation of plant extracts and comparison of different nanostructures such as lipid-based carriers (SLNs and NLCs), nanoemulsions and green synthesized metal NPs on metabolic disorders through in vitro and in vivo experiments (Table 1).
The findings of these studies clearly confirm that most of phytomedicines can be successfully formulated by various nano-delivery approaches and thus successfully delivered to induce the required therapeutic effect. In addition to the proven role of nano-delivery systems, various loading methods, which are also discussed here, seem to be a critical factor. Moreover, targeted delivery of nano-formulated phytomedicines can pave the way to link traditional medicine with modern pharmaceutical techniques to be used in a wide range of diseases, including metabolic disorders.
ZN, MH, and ZI did a literature review and prepared the first draft of the manuscript. ZN, RB, and MH edited the manuscript and proposed and included some vital modifications. MF and MA design throughout the work and did the final edition of the manuscript. ZN, MH, MF, and RB revised the manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Agnihotri, A., and Singh, V. (2013). Effect of Tamarindus indica Linn. and Cassia fistula Linn. stem bark extracts on oxidative stress and diabetic conditions. Acta Pol. Pharm. 70, 1011–1019.
Ahmad, U., and Ahmad, R. S. (2018). Anti diabetic property of aqueous extract of Stevia rebaudiana Bertoni leaves in Streptozotocin-induced diabetes in albino rats. BMC Complement. Altern. Med. 18:179. doi: 10.1186/s12906-018-2245-2
Ajiboye, B. O., Ojo, O. A., Akuboh, O. S., Abiola, O. M., Idowu, O., and Amuzat, A. O. (2018). Anti-Hyperglycemic and anti-inflammatory activities of polyphenolic-rich extract of Syzygium cumini Linn leaves in alloxan-induced diabetic rats. J. Evid. Based Integr. Med. 23:2515690x18770630. doi: 10.1177/2515690X18770630
Alamoudi, E. F., Khalil, W. K., Ghaly, I. S., Hassan, N. H., and Ahmed, E. S. (2014). Nanoparticles from of Costus speciosus extract improves the antidiabetic and antilipidemic effects against STZ-induced diabetes mellitus in Albino rats. Int. J. Pharm. Sci. Rev. Res. 29, 279–288.
Aldawsari, H. M., Hanafy, A., Labib, G. S., and Badr, J. M. (2014). Antihyperglycemic activities of extracts of the mistletoes Plicosepalus acaciae and P. curviflorus in comparison to their solid lipid nanoparticle suspension formulations. Z. Naturforsch. C 69, 391–398. doi: 10.5560/znc.2014-0047
Ali, H. A., Almaghrabi, O. A., and Afifi, M. E. (2014). Molecular mechanisms of anti-hyperglycemic effects of Costus speciosus extract in streptozotocin-induced diabetic rats. Saudi Med. J. 35, 1501–1506.
Alkaladi, A., Abdelazim, A. M., and Afifi, M. (2014). Antidiabetic activity of zinc oxide and silver nanoparticles on streptozotocin-induced diabetic rats. Int. J. Mol. Sci. 15, 2015–2023. doi: 10.3390/ijms15022015
Al-Taweel, A. M., Perveen, S., Fawzy, G. A., Alqasoumi, S. I., and El Tahir, K. E. J. F. (2012). New flavane gallates isolated from the leaves of Plicosepalus curviflorus and their hypoglycemic activity. Fitoterapia 83, 1610–1615. doi: 10.1016/j.fitote.2012.09.010
Ames-Sibin, A. P., Barizão, C. L., Castro-Ghizoni, C. V., Silva, F. M., Sá-Nakanishi, A. B., Bracht, L., et al. (2018). β-Caryophyllene, the major constituent of copaiba oil, reduces systemic inflammation and oxidative stress in arthritic rats. J. Cell. Biochem. 119, 10262–10277. doi: 10.1002/jcb.27369
Anandharajan, R., Jaiganesh, S., Shankernarayanan, N., Viswakarma, R., and Balakrishnan, A. J. P. (2006). In vitro glucose uptake activity of Aegles marmelos and Syzygium cumini by activation of Glut-4, PI3 kinase and PPARγ in L6 myotubes. Phytomedicine 13, 434–441. doi: 10.1016/j.phymed.2005.03.008
Anbazhagan, P., Murugan, K., Jaganathan, A., Sujitha, V., Samidoss, C. M., Jayashanthani, S., et al. (2017). Mosquitocidal, antimalarial and antidiabetic potential of Musa paradisiaca-synthesized silver nanoparticles: in vivo and in vitro approaches. J. Clust. Sci. 28, 91–107.
Anderson, J. W., Fuller, J., Patterson, K., Blair, R., and Tabor, A. J. M. (2007). Soy compared to casein meal replacement shakes with energy-restricted diets for obese women: randomized controlled trial. Metabolism 56, 280–288. doi: 10.1016/j.metabol.2006.10.013
Ansari, S., Bari, A., Ullah, R., Mathanmohun, M., Veeraraghavan, V. P., Sun, Z., et al. (2019). Gold nanoparticles synthesized with Smilax glabra rhizome modulates the anti-obesity parameters in high-fat diet and streptozotocin induced obese diabetes rat model. J. Photochem. Photobiol. B Biol. 201:111643. doi: 10.1016/j.jphotobiol.2019.111643
Aris, F. A. F., Fauzi, F. N. A. M., Tong, W. Y., and Abdullah, S. S. S. (2019). Interaction of silver sulfadiazine wıth bacterial cellulose via ex-situ modification method as an alternative diabetic wound healing. Biocatal. Agric. Biotechnol. 21:101332.
Arvanag, F. M., Bayrami, A., Habibi-Yangjeh, A., and Pouran, S. R. (2019). A comprehensive study on antidiabetic and antibacterial activities of ZnO nanoparticles biosynthesized using Silybum marianum L seed extract. Mater. Sci. Eng. C 97, 397–405. doi: 10.1016/j.msec.2018.12.058
Aswathanarayan, J. B., and Vittal, R. R. (2019). Nanoemulsions and their potential applications in food industry. Front. Sustain. Food Syst. 3:95. doi: 10.3389/fsufs.2019.00095
Atale, N., Saxena, S., Nirmala, J. G., Narendhirakannan, R., Mohanty, S., and Rani, V. J. (2017). Synthesis and characterization of Sygyzium cumini nanoparticles for its protective potential in high glucose-induced cardiac stress: a green approach. Appl. Biochem. Biotechnol. 181, 1140–1154. doi: 10.1007/s12010-016-2274-6
Badescu, M., Badulescu, O., Badescu, L., and Ciocoiu, M. (2015). Effects of Sambucus nigra and Aronia melanocarpa extracts on immune system disorders within diabetes mellitus. Pharm. Biol. 53, 533–539. doi: 10.3109/13880209.2014.931441
Baker, R. G., Hayden, M. S., and Ghosh, S. J. C. M. (2011). NF-κB, inflammation, and metabolic disease. Cell Metab. 13, 11–22.
Bala, N., Saha, S., Chakraborty, M., Maiti, M., Das, S., Basu, R., et al. (2015). Green synthesis of zinc oxide nanoparticles using Hibiscus subdariffa leaf extract: effect of temperature on synthesis, anti-bacterial activity and anti-diabetic activity. RSC Adv. 5, 4993–5003.
Barwal, I., Sood, A., Sharma, M., Singh, B., and Yadav, S. C. (2013). Development of stevioside Pluronic-F-68 copolymer based PLA-nanoparticles as an antidiabetic nanomedicine. Coll. Surf. B Biointerfaces 101, 510–516. doi: 10.1016/j.colsurfb.2012.07.005
Baskar, V., Selvakumar, K., Madhan, R., Srinivasan, G., and Muralidharan, M. (2012). Study on improving bioavailability ratio of anti-inflammatory compound from ginger through nano transdermal delivery. Asian J. Pharm. Clin. Res. 5, 241–246.
Bayrami, A., Ghorbani, E., Pouran, S. R., Habibi-Yangjeh, A., Khataee, A., and Bayrami, M. J. U. S. (2019). Enriched zinc oxide nanoparticles by Nasturtium officinale leaf extract: joint ultrasound-microwave-facilitated synthesis, characterization, and implementation for diabetes control and bacterial inhibition. Ultrason. Sonochem. 58:104613. doi: 10.1016/j.ultsonch.2019.104613
Bayrami, A., Parvinroo, S., Habibi-Yangjeh, A., and Rahim Pouran, S. (2018). Bio-extract-mediated ZnO nanoparticles: microwave-assisted synthesis, characterization and antidiabetic activity evaluation. Artif. Cells Nanomed. Biotechnol. 46, 730–739. doi: 10.1080/21691401.2017.1337025
Becic, T., Studenik, C., and Hoffmann, G. (2018). Exercise increases adiponectin and reduces leptin levels in prediabetic and diabetic individuals: systematic review and meta-analysis of randomized controlled trials. Med. Sci. 6:97. doi: 10.3390/medsci6040097
Beltrán-Sánchez, H., Harhay, M. O., Harhay, M. M., and Mcelligott, S. (2013). Prevalence and trends of metabolic syndrome in the adult US population, 1999–2010. J. Am. Coll. Cardiol. 62, 697–703. doi: 10.1016/j.jacc.2013.05.064
Bergman, R. N., Van Citters, G. W., Mittelman, S. D., Dea, M. K., Hamilton-Wessler, M., Kim, S. P., et al. (2001). Central role of the adipocyte in the metabolic syndrome. J. Investig. Med. 49, 119–126. doi: 10.2310/6650.2001.34108
Bhattacharjee, N., Barma, S., Konwar, N., Dewanjee, S., and Manna, P. J. (2016). Mechanistic insight of diabetic nephropathy and its pharmacotherapeutic targets: an update. Eur. J. Pharmacol. 791, 8–24. doi: 10.1016/j.ejphar.2016.08.022
Buşilă, C., Stuparu-Creţu, M., Barna, O., and Balan, G. (2017). Dyslipidemia in children as a risk factor for cardiovascular diseases. Biotechnol. Biotechnol. Equip. 31, 1192–1197. doi: 10.1016/j.ijpam.2019.05.003
Cabandugama, P. K., Gardner, M. J., and Sowers, J. R. (2017). The renin angiotensin aldosterone system in obesity and hypertension: roles in the cardiorenal metabolic syndrome. Med. Clin. 101, 129–137. doi: 10.1016/j.mcna.2016.08.009
Cai, S., Sun, W., Fan, Y., Guo, X., Xu, G., Xu, T., et al. (2016). Effect of mulberry leaf (Folium Mori) on insulin resistance via IRS-1/PI3K/Glut-4 signalling pathway in type 2 diabetes mellitus rats. Pharm. Biol. 54, 2685–2691. doi: 10.1080/13880209.2016.1178779
Campos, C., De Castro, A. L., Tavares, A. M. V., Fernandes, R. O., Ortiz, V. D., Barboza, T. E., et al. (2017). Effect of free and nanoencapsulated copaiba oil on monocrotaline-induced pulmonary arterial hypertension. J. Cardiovasc. Pharmacol. 69, 79–85. doi: 10.1097/FJC.0000000000000442
Campoy, A. H. G., Gutierrez, R. M. P., Manriquez-Alvirde, G., and Ramirez, A. M. (2018). Protection of silver nanoparticles using Eysenhardtia polystachya in peroxide-induced pancreatic β-cell damage and their antidiabetic properties in zebrafish. Int. J. Nanomedicine 13, 2601–2612. doi: 10.2147/IJN.S163714
Cao, H., Polansky, M. M., and Anderson, R. A. (2007). Cinnamon extract and polyphenols affect the expression of tristetraprolin, insulin receptor, and glucose transporter 4 in mouse 3T3-L1 adipocytes. Arch. Biochem. Biophys. 459, 214–222. doi: 10.1016/j.abb.2006.12.034
Cardozo, C., Inada, A., Marcelino, G., Figueiredo, P., Arakaki, D., Hiane, P., et al. (2018). Therapeutic potential of brazilian cerrado campomanesia species on metabolic dysfunctions. Molecules 23:2336. doi: 10.3390/molecules23092336
Charrad, R., Berraïes, A., Hamdi, B., Ammar, J., Hamzaoui, K., and Hamzaoui, A. J. I. (2016). Anti-inflammatory activity of IL-37 in asthmatic children: correlation with inflammatory cytokines TNF-α, IL-β, IL-6 and IL-17A. Immunobiology 221, 182–187. doi: 10.1016/j.imbio.2015.09.009
Cheong, A. M., Jessica Koh, J. X., Patrick, N. O., Tan, C. P., and Nyam, K. L. (2018). Hypocholesterolemic effects of kenaf seed oil, macroemulsion, and nanoemulsion in high-cholesterol diet induced rats. J. Food Sci. 83, 854–863. doi: 10.1111/1750-3841.14038
Cheraghpour, M., Imani, H., Ommi, S., Alavian, S. M., Karimi-Shahrbabak, E., Hedayati, M., et al. (2019). Hesperidin improves hepatic steatosis, hepatic enzymes, and metabolic and inflammatory parameters in patients with nonalcoholic fatty liver disease: a randomized, placebo-controlled, double-blind clinical trial. Phytother. Res. 33, 2118–2125. doi: 10.1002/ptr.6406
Chi, C., Zhang, C., Liu, Y., Nie, H., Zhou, J., and Ding, Y. (2020). Phytosome-Nanosuspensions for silybin-phospholipid complex with increased bioavailability and hepatoprotection efficacy. Eur. J. Pharm. Sci. 144:105212. doi: 10.1016/j.ejps.2020.105212
Choudhari, V. P., Gore, K. P., and Pawar, A. T. (2017). Antidiabetic, antihyperlipidemic activities and herb–drug interaction of a polyherbal formulation in streptozotocin induced diabetic rats. J. Ayurveda Integr. Med. 8, 218–225. doi: 10.1016/j.jaim.2016.11.002
Chowdhury, A., Kunjiappan, S., Bhattacharjee, C., Somasundaram, B., and Panneerselvam, T. (2017). Biogenic synthesis of Marsilea quadrifolia gold nanoparticles: a study of improved glucose utilization efficiency on 3T3-L1 adipocytes. In Vitro Cell Dev. Biol. Anim. 53, 483–493. doi: 10.1007/s11626-017-0136-3
Cignarella, A., Nastasi, M., Cavalli, E., and Puglisi, L. (1996). Novel lipid-lowering properties of Vaccinium myrtillus L. leaves, a traditional antidiabetic treatment, in several models of rat dyslipidaemia: a comparison with ciprofibrate. Thromb. Res. 84, 311–322. doi: 10.1016/s0049-3848(96)00195-8
Crawford, P. (2009). Effectiveness of cinnamon for lowering hemoglobin A1C in patients with type 2 diabetes: a randomized, controlled trial. J. Am. Board Fam. Med. 22, 507–512. doi: 10.3122/jabfm.2009.05.080093
Dai, B., Li, H., Fan, J., Zhao, Y., Yin, Z., Nie, X., et al. (2018). MiR-21 protected against diabetic cardiomyopathy induced diastolic dysfunction by targeting gelsolin. Cardiovasc. Diabetol. 17:123. doi: 10.1186/s12933-018-0767-z
Daisy, P., and Saipriya, K. (2012). Biochemical analysis of Cassia fistula aqueous extract and phytochemically synthesized gold nanoparticles as hypoglycemic treatment for diabetes mellitus. Int. J. Nanomedicine 7:1189. doi: 10.2147/IJN.S26650
Dalvand, S., Bakhshi, E., Zarei, M., Asl, M. T., and Ghanei, R. (2017). Prevalence of metabolic syndrome in Iran: a systematic review and meta-analysis. Med. Surg. Nurs. J. 5, 1–14.
Danhier, F., Ansorena, E., Silva, J. M., Coco, R., Le Breton, A., and Préat, V. J. (2012). PLGA-based nanoparticles: an overview of biomedical applications. J. Control Release 161, 505–522. doi: 10.1016/j.jconrel.2012.01.043
Das, D., Nath, B. C., Phukon, P., Dolui, S. K. J. C., and Biointerfaces, S. B. (2013). Synthesis of ZnO nanoparticles and evaluation of antioxidant and cytotoxic activity. Coll. Surf B Biointerfaces 111, 556–560. doi: 10.1016/j.colsurfb.2013.06.041
de Freitas Junior, L. M., and de Almeida, E. B. Jr. (2017). Medicinal plants for the treatment of obesity: ethnopharmacological approach and chemical and biological studies. Am. J. Transl. Res. 9:2050.
Di Lorenzo, C., Dell’agli, M., Colombo, E., Sangiovanni, E., and Restani, P. (2013). Metabolic syndrome and inflammation: a critical review of in vitro and clinical approaches for benefit assessment of plant food supplements. Evid. Based Complement. Alternat. Med. 2013:105212. doi: 10.1155/2013/782461
Dinh, T., Tecilazich, F., Kafanas, A., Doupis, J., Gnardellis, C., Leal, E., et al. (2012). Mechanisms involved in the development and healing of diabetic foot ulceration. Diabetes Metab. Res. Rev. 61, 2937–2947. doi: 10.2337/db12-0227
Ebrahimi-Mameghani, M., Asghari-Jafarabadi, M., and Rezazadeh, K. (2018). TCF7L2-rs7903146 polymorphism modulates the effect of artichoke leaf extract supplementation on insulin resistance in metabolic syndrome: a randomized, double-blind, placebo-controlled trial. J. Integr. Med. 16, 329–334. doi: 10.1016/j.joim.2018.05.006
Ebrahimzadeh-Bideskan, A.-R., Hami, J., Alipour, F., Haghir, H., Fazel, A.-R., and Sadeghi, A. (2016). Protective effects of ascorbic acid and garlic extract against lead-induced apoptosis in developing rat hippocampus. Metab. Brain Dis. 31, 1123–1132. doi: 10.1007/s11011-016-9837-7
Ehrhart-Bornstein, M., Lamounier-Zepter, V., Schraven, A., Langenbach, J., Willenberg, H., Barthel, A., et al. (2003). Human adipocytes secrete mineralocorticoid-releasing factors. Proc. Natl. Acad. Sci. U.S.A. 100, 14211–14216. doi: 10.1073/pnas.2336140100
Eleazu, C. O., Iroaganachi, M., and Eleazu, K. (2013). Ameliorative potentials of cocoyam (Colocasia esculenta L.) and unripe plantain (Musa paradisiaca L.) on the relative tissue weights of streptozotocin-induced diabetic rats. J. Diabetes Res. 2013:160964. doi: 10.1155/2013/160964
El-Far, Y. M., Zakaria, M. M., Gabr, M. M., El Gayar, A. M., El-Sherbiny, I. M., and Eissa, L. A. (2016). A newly developed silymarin nanoformulation as a potential antidiabetic agent in experimental diabetes. Nanomedicine 11, 2581–2602. doi: 10.2217/nnm-2016-0204
El-Menshawe, S. F., Ali, A. A., Rabeh, M. A., and Khalil, N. M. (2018). Nanosized soy phytosome-based thermogel as topical anti-obesity formulation: an approach for acceptable level of evidence of an effective novel herbal weight loss product. Int. J. Nanomedicine 13:307. doi: 10.2147/IJN.S153429
Fangueiro, J. F., Silva, A. M., Garcia, M. L., and Souto, E. B. (2015). Current nanotechnology approaches for the treatment and management of diabetic retinopathy. Eur. J. Pharm. Biopharm. 95, 307–322. doi: 10.1016/j.ejpb.2014.12.023
Feldeisen, S. E., and Tucker, K. L. (2007). Nutritional strategies in the prevention and treatment of metabolic syndrome. Appl. Physiol. Nutr. Metab. 32, 46–60. doi: 10.1139/h06-101
Firouzi, S., Malekahmadi, M., Ghayour-Mobarhan, M., Ferns, G., and Rahimi, H. R. (2018). Barberry in the treatment of obesity and metabolic syndrome: possible mechanisms of action. Diabetes Metab. Syndr. Obes. 11, 699–704. doi: 10.2147/DMSO.S181572
Furukawa, S., Fujita, T., Shimabukuro, M., Iwaki, M., Yamada, Y., Nakajima, Y., et al. (2017). Increased oxidative stress in obesity and its impact on metabolic syndrome. J. Clin. Invest. 114, 1752–1761. doi: 10.1172/JCI21625
Ganesan, P., Arulselvan, P., and Choi, D.-K. (2017). Phytobioactive compound-based nanodelivery systems for the treatment of type 2 diabetes mellitus–current status. Int. J. Nanomedicine 12, 1097–1111. doi: 10.2147/IJN.S124601
Ganesan, P., Ramalingam, P., Karthivashan, G., Ko, Y. T., and Choi, D.-K. (2018). Recent developments in solid lipid nanoparticle and surface-modified solid lipid nanoparticle delivery systems for oral delivery of phyto-bioactive compounds in various chronic diseases. Int. J. Nanomedicine 13, 1569–1583. doi: 10.2147/IJN.S155593
Gao, C., Jiang, X., Wang, H., Zhao, Z., and Wang, W. (2013). Drug metabolism and pharmacokinetics of organosulfur compounds from garlic. J. Drug Metab. Toxicol. 4:159. doi: 10.2133/dmpk.dmpk-10-rg-053
García-Mediavilla, V., Villares, C., Culebras, J. M., Bayón, J. E., and González-Gallego, J. (2003). Effects of dietary β-cyclodextrin in hypercholesterolaemic rats. Pharmacol. Toxicol. 92, 94–99. doi: 10.1034/j.1600-0773.2003.920206.x
Garg, A., Pandey, P., Sharma, P., and Shukla, A. K. (2016). Synthesis and characterization of silver nanoparticle of ginger rhizome (Zingiber officinale) extract: synthesis, characterization and anti diabetic activity in streptozotocin induced diabetic rats. Eur. J. Biomed. Pharm. Sci. 3, 605–611.
Gera, M., Sharma, N., Ghosh, M., Huynh, D. L., Lee, S. J., Min, T., et al. (2017). Nanoformulations of curcumin: an emerging paradigm for improved remedial application. Oncotarget 8:66680. doi: 10.18632/oncotarget.19164
Ghanbarzadeh, B., Babazadeh, A., and Hamishehkar, H. (2016). Nano-phytosome as a potential food-grade delivery system. Food Biosci. 15, 126–135.
Ghasemiyeh, P., and Mohammadi-Samani, S. (2018). Solid lipid nanoparticles and nanostructured lipid carriers as novel drug delivery systems: applications, advantages and disadvantages. Res. Pharm. Sci. 13, 288–303. doi: 10.4103/1735-5362.235156
Gordillo-Galeano, A., and Mora-Huertas, C. E. (2018). Solid lipid nanoparticles and nanostructured lipid carriers: a review emphasizing on particle structure and drug release. Eur. J. Pharm. Biopharm. 133, 285–308. doi: 10.1016/j.ejpb.2018.10.017
Grigoraş, A., Amalinei, C., Balan, R. A., Giuşcă, S. E., Avădănei, E. R., Lozneanu, L., et al. (2018). Adipocytes spectrum–from homeostasia to obesity and its associated pathology. Ann. Anat. 219, 102–120. doi: 10.1016/j.aanat.2018.06.004
Grundy, S. M. (2016). Metabolic syndrome update. Trends Cardiovasc. Med. 26, 364–373. doi: 10.1016/j.tcm.2015.10.004
Hassani, F. V., Shirani, K., and Hosseinzadeh, H. (2016). Rosemary (Rosmarinus officinalis) as a potential therapeutic plant in metabolic syndrome: a review. Naunyn Schmiedebergs Arch. Pharmacol. 389, 931–949. doi: 10.1007/s00210-016-1256-0
Heidari Khoei, H., Fakhri, S., Parvardeh, S., Shams Mofarahe, Z., Ghasemnejad-Berenji, H., Nazarian, H., et al. (2019). Testicular toxicity and reproductive performance of streptozotocin-induced diabetic male rats: the ameliorating role of silymarin as an antioxidant. Toxin Rev. 38, 223–233.
Hosseini, A., Razavi, B. M., and Hosseinzadeh, H. (2018). Saffron (Crocus sativus) petal as a new pharmacological target: a review. Iran J. Basic Med. Sci. 21:1091. doi: 10.22038/IJBMS.2018.31243.7529
Hu, T., Schreiter, F. C., Bagchi, R. A., Tatman, P. D., Hannink, M., and Mckinsey, T. A. (2019). HDAC5 catalytic activity suppresses cardiomyocyte oxidative stress and NRF2 target gene expression. J. Biol. Chem. 294, 8640–8652. doi: 10.1074/jbc.RA118.007006
Huang, Z.-B., Zheng, Y.-X., Li, N., Cai, S.-L., Huang, Y., Wang, J., et al. (2019). Protective effects of specific cannabinoid receptor 2 agonist GW405833 on concanavalin A-induced acute liver injury in mice. Acta Pharmacol. Sin. 40, 1404–1411. doi: 10.1038/s41401-019-0213-0
Kanis, L. A., Prophiro, J. S., Da Silva Vieira, E., Do Nascimento, M. P., Zepon, K. M., Kulkamp-Guerreiro, I. C., et al. (2012). Larvicidal activity of Copaifera sp.(Leguminosae) oleoresin microcapsules against Aedes aegypti (Diptera: Culicidae) larvae. Parasitol. Res. 110, 1173–1178. doi: 10.1007/s00436-011-2610-2
Karthick, V., Kumar, V. G., Dhas, T. S., Singaravelu, G., Sadiq, A. M., and Govindaraju, K. (2014). Effect of biologically synthesized gold nanoparticles on alloxan-induced diabetic rats—an in vivo approach. Coll. Surf. B Biointerfaces 122, 505–511. doi: 10.1016/j.colsurfb.2014.07.022
Kaur, J. (2014). A comprehensive review on metabolic syndrome. Cardiol. Res. Pract. 2014:943162. doi: 10.1155/2014/943162
Kesharwani, P., Gorain, B., Low, S. Y., Tan, S. A., Ling, E. C. S., Lim, Y. K., et al. (2018). Nanotechnology based approaches for anti-diabetic drugs delivery. Diabetes Res. Clin. Pract. 136, 52–77. doi: 10.1016/j.diabres.2017.11.018
Khan, Z. U. H., Khan, A., Chen, Y., Shah, N. S., Muhammad, N., Khan, A. U., et al. (2017). Biomedical applications of green synthesized Nobel metal nanoparticles. J. Photoch. Photobiol. B 173, 150–164. doi: 10.1016/j.jphotobiol.2017.05.034
Khorasani, S., Danaei, M., and Mozafari, M. R. (2018). Nanoliposome technology for the food and nutraceutical industries. Trends Food Sci. Tech. 79, 106–115. doi: 10.3390/molecules25030638
Kikuchi, N., Satoh, K., Kurosawa, R., Yaoita, N., Elias-Al-Mamun, M., Siddique, M. A. H., et al. (2018). Selenoprotein P Promotes the development of pulmonary arterial hypertension. Circulation 138, 600–623. doi: 10.1161/CIRCULATIONAHA.117.033113
Kosina, P., Paloncıová, M., Rajnochová Svobodová, A., Zálešák, B., Biedermann, D., Ulrichová, J., et al. (2019). Dermal delivery of selected polyphenols from silybum marianum. Theoretical and experimental study. Molecules 24:61. doi: 10.3390/molecules24010061
Kumar Dey, T., Ghosh, S., Ghosh, M., Koley, H., and Dhar, P. (2012). Comparative study of gastrointestinal absorption of EPA & DHA rich fish oil from nano and conventional emulsion formulation in rats. Food Res. Int. 49, 72–79.
Kuppusamy, P., Yusoff, M. M., Parine, N. R., and Govindan, N. (2015). Evaluation of in-vitro antioxidant and antibacterial properties of Commelina nudiflora L. extracts prepared by different polar solvents. Saudi J. Biol. Sci. 22, 293–301. doi: 10.1016/j.sjbs.2014.09.016
Langle, A., González-Coronel, M. A., Carmona-Gutiérrez, G., Moreno-Rodríguez, J. A., Venegas, B., Muñoz, G., et al. (2015). Stevia rebaudiana loaded titanium oxide nanomaterials as an antidiabetic agent in rats. Rev. Bras. Farmacogn. 25, 145–151.
Lee, Y.-S., Kim, S.-H., Yuk, H., Lee, G.-J., and Kim, D.-S. (2018). Tetragonia tetragonoides (Pall.) Kuntze (New Zealand Spinach) Prevents Obesity and Hyperuricemia in High-Fat Diet-Induced Obese Mice. Nutrients 10:1087. doi: 10.3390/nu10081087
López, T., Bata-Garcia, J. L., Esquivel, D., Ortiz-Islas, E., Gonzalez, R., Ascencio, J., et al. (2011). Treatment of Parkinson’s disease: nanostructured sol–gel silica–dopamine reservoirs for controlled drug release in the central nervous system. Int. J. Nanomedicine 6:19. doi: 10.2147/IJN.S13223
Lopez, T., Ortiz, E., Alvarez, M., Navarrete, J., Odriozola, J. A., Martinez-Ortega, F., et al. (2010). Study of the stabilization of zinc phthalocyanine in sol-gel TiO2 for photodynamic therapy applications. Nanomedicine 6, 777–785. doi: 10.1016/j.nano.2010.04.007
Losi, P., Briganti, E., Errico, C., Lisella, A., Sanguinetti, E., Chiellini, F., et al. (2013). Fibrin-based scaffold incorporating VEGF-and bFGF-loaded nanoparticles stimulates wound healing in diabetic mice. Acta Biomater. 9, 7814–7821. doi: 10.1016/j.actbio.2013.04.019
Lu, B., Xia, D., Huang, W., Wu, X., Zhang, Y., and Yao, Y. J. (2010). Hypolipidemic effect of bamboo shoot oil (P. pubescens) in Sprague–Dawley rats. J. Food Sci. 75, H205–H211. doi: 10.1111/j.1750-3841.2010.01716.x
Ma, J., Yang, H., Basile, M. J., and Kennelly, E. J. (2004). Analysis of polyphenolic antioxidants from the fruits of three Pouteria species by selected ion monitoring liquid chromatography- mass spectrometry. J. Agric. Food Chem. 52, 5873–5878. doi: 10.1021/jf049950k
Madane, R. G., and Mahajan, H. S. (2016). Curcumin-loaded nanostructured lipid carriers (NLCs) for nasal administration: design, characterization, and in vivo study. Drug Deliv. 23, 1326–1334. doi: 10.3109/10717544.2014.975382
Maity, S., Mukhopadhyay, P., Kundu, P. P., and Chakraborti, A. S. (2017). Alginate coated chitosan core-shell nanoparticles for efficient oral delivery of naringenin in diabetic animals—An in vitro and in vivo approach. Carbohydr. Polym. 170, 124–132. doi: 10.1016/j.carbpol.2017.04.066
Manna, K., Mishra, S., Saha, M., Mahapatra, S., Saha, C., Yenge, G., et al. (2019). Amelioration of diabetic nephropathy using pomegranate peel extract-stabilized gold nanoparticles: assessment of nf-κb and nrf2 signaling system. Int. J. Nanomedicine 14, 1753–1777. doi: 10.2147/IJN.S176013
Martineau, L. C., Couture, A., Spoor, D., Benhaddou-Andaloussi, A., Harris, C., Meddah, B., et al. (2006). Anti-diabetic properties of the Canadian lowbush blueberry Vaccinium angustifolium Ait. Phytomedicine 13, 612–623. doi: 10.1016/j.phymed.2006.08.005
Meghwani, H., Prabhakar, P., Mohammed, S. A., Dua, P., Seth, S., Hote, M. P., et al. (2018). Beneficial effect of Ocimum sanctum (Linn) against monocrotaline-induced pulmonary hypertension in rats. Medicines 5:34. doi: 10.3390/medicines5020034
Mittal, A. K., Chisti, Y., and Banerjee, U. C. (2013). Synthesis of metallic nanoparticles using plant extracts. Biotechnol. Adv. 31, 346–356. doi: 10.1016/j.biotechadv.2013.01.003
Mnonopi, N., Levendal, R.-A., Mzilikazi, N., and Frost, C. (2012). Marrubiin, a constituent of Leonotis leonurus, alleviates diabetic symptoms. Phytomedicine 19, 488–493. doi: 10.1016/j.phymed.2011.12.008
Muniandy, K., Gothai, S., Tan, W. S., Kumar, S. S., Mohd Esa, N., Chandramohan, G., et al. (2018). In Vitro wound healing potential of stem extract of alternanthera sessilis. Evid. Based Complement. Alternat. Med. 2018:3142073. doi: 10.1155/2018/3142073
Nagajyothi, P., Sreekanth, T., Tettey, C. O., Jun, Y. I., Mook, S. H. J. B., and Letters, M. C. (2014). Characterization, antibacterial, antioxidant, and cytotoxic activities of ZnO nanoparticles using Coptidis Rhizoma. Bioorg. Med. Chem. Lett. 24, 4298–4303. doi: 10.1016/j.bmcl.2014.07.023
Nakhlband, A., Eskandani, M., Saeedi, N., Ghafari, S., Omidi, Y., Barar, J., et al. (2018). Marrubiin-loaded solid lipid nanoparticles’ impact on TNF-α treated umbilical vein endothelial cells: a study for cardioprotective effect. Coll. Surf. B Biointerfaces 164, 299–307. doi: 10.1016/j.colsurfb.2018.01.046
Naseri, R., Farzaei, F., Haratipour, P., Nabavi, S. F., Habtemariam, S., Farzaei, M. H., et al. (2018). Anthocyanins in the management of metabolic syndrome: a pharmacological and biopharmaceutical review. Front. Pharmacol. 9:1310. doi: 10.3389/fphar.2018.01310
Neha, A., Vibha, R., and Sciences, B. (2013). GC-MS analysis of bioactive components in the ethanolic and methanolic extract of Syzygium cumini. Int. J. Pharma Bio Sci. 4, 296–304.
Odei-Addo, F., Shegokar, R., Muller, R. H., Levendal, R. A., and Frost, C. (2017). Nanoformulation of Leonotis leonurus to improve its bioavailability as a potential antidiabetic drug. Biotech 7:344. doi: 10.1007/s13205-017-0986-0
Oh, Y. S., Baek, D. J., Park, E.-Y., and Jun, H.-S. (2018). Fatty acid-induced lipotoxicity in pancreatic beta-cells during development of type 2 diabetes. Front. Endocrinol. 9:384. doi: 10.3389/fendo.2018.00384
Ong, Y. S., Yazan, L. S., Ng, W. K., Noordin, M. M., Sapuan, S., Foo, J. B., et al. (2016). Acute and subacute toxicity profiles of thymoquinone-loaded nanostructured lipid carrier in BALB/c mice. Int. J. Nanomedicine 11:5905. doi: 10.2147/IJN.S114205
Opris, R., Tatomir, C., Olteanu, D., Moldovan, R., Moldovan, B., David, L., et al. (2017). The effect of Sambucus nigra L. extract and phytosinthesized gold nanoparticles on diabetic rats. Coll. Surf. B Biointerfaces 150, 192–200. doi: 10.1016/j.colsurfb.2016.11.033
Ota, A., and Ulrih, N. P. (2017). An overview of herbal products and secondary metabolites used for management of type two diabetes. Front. Pharmacol. 8:436. doi: 10.3389/fphar.2017.00436
Ovais, M., Khalil, A. T., Raza, A., Khan, M. A., Ahmad, I., Islam, N. U., et al. (2016). Green synthesis of silver nanoparticles via plant extracts: beginning a new era in cancer theranostics. Nanomedicine 12, 3157–3177. doi: 10.2217/nnm-2016-0279
Pan, Y., and Kong, L.-D. (2018). High fructose diet-induced metabolic syndrome: pathophysiological mechanism and treatment by traditional Chinese medicine. Pharmacol. Res. 130, 438–450. doi: 10.1016/j.phrs.2018.02.020
Paul, D., Dey, T. K., Mukherjee, S., Ghosh, M., and Dhar, P. (2014). Comparative prophylactic effects of α-eleostearic acid rich nano and conventional emulsions in induced diabetic rats. J. Food Sci. Technol. 51, 1724–1736. doi: 10.1007/s13197-014-1257-2
Paulke, A., Kremer, C., Wunder, C., Achenbach, J., Djahanschiri, B., Elias, A., et al. (2013). Argyreia nervosa (Burm. f.): receptor profiling of lysergic acid amide and other potential psychedelic LSD-like compounds by computational and binding assay approaches. J. Ethnopharmacol. 148, 492–497. doi: 10.1016/j.jep.2013.04.044
Payab, M., Hasani-Ranjbar, S., Shahbal, N., Qorbani, M., Aletaha, A., Haghi-Aminjan, H., et al. (2019). Effect of the herbal medicines in obesity and metabolic syndrome: a systematic review and meta-analysis of clinical trials. Phytother. Res. 34, 526–545. doi: 10.1002/ptr.6547
Peng, C.-H., Yang, Y.-S., Chan, K.-C., Wang, C.-J., Chen, M.-L., Huang, C.-N., et al. (2014). Hibiscus sabdariffa polyphenols alleviate insulin resistance and renal epithelial to mesenchymal transition: a novel action mechanism mediated by type 4 dipeptidyl peptidase. J. Agric. Food Chem. 62, 9736–9743. doi: 10.1021/jf5024092
Pereira, M. C., Oliveira, D. A., Hill, L. E., Zambiazi, R. C., Borges, C. D., Vizzotto, M., et al. (2018). Effect of nanoencapsulation using PLGA on antioxidant and antimicrobial activities of guabiroba fruit phenolic extract. Food Chem. 240, 396–404. doi: 10.1016/j.foodchem.2017.07.144
Perez, G. R. M., Vargas, S. R., Perez, G. S., and Zavala, S. M. (1998). Antiurolithiatic activity of Eysenhardtia polystachya aqueous extract on rats. Phytother. Res. 12, 144–145.
Perez-Gutierrez, R. M., Garcia-Campoy, A. H., and Muñiz-Ramirez, A. (2016). Properties of flavonoids isolated from the bark of eysenhardtia polystachya and their effect on oxidative stress in streptozotocin-induced diabetes mellitus in mice. Oxid. Med. Cell Longev. 2016:9156510. doi: 10.1155/2016/9156510
Perumal, V., Manickam, T., Bang, K.-S., Velmurugan, P., and Oh, B.-T. (2016). Antidiabetic potential of bioactive molecules coated chitosan nanoparticles in experimental rats. Int. J. Biol. Macromol. 92, 63–69. doi: 10.1016/j.ijbiomac.2016.07.006
Plourde, M., Sergiel, J.-P., Chardigny, J.-M., Grégoire, S., Angers, P., and Sébédio, J.-L. (2006). Absorption and metabolism of conjugated α-linolenic acid given as free fatty acids or triacylglycerols in rats. Nutr. Metab. 3:8. doi: 10.1186/1743-7075-3-8
Ponnanikajamideen, M., Rajeshkumar, S., and Annadurai, G. (2016). In Vivo antidiabetic and in vitro antioxidant and antimicrobial activity of aqueous leaves extract of Chamaecostus cuspidatus. Res. J. Pharm. Technol. 9:1204.
Ponnanikajamideen, M., Rajeshkumar, S., Vanaja, M., and Annadurai, G. (2018). In-Vivo anti-diabetic and wound healing effect of antioxidant gold nanoparticles synthesized using insulin plant (Chamaecostus Cuspidatus). Can. J. Diabetes 43, 82–89.
Ponnappan, N., and Chugh, A. (2015). Nanoparticle-mediated delivery of therapeutic drugs. Pharm. Med. 29, 155–167.
Prabhu, S., Vinodhini, S., Elanchezhiyan, C., and Rajeswari, D. (2018). Evaluation of antidiabetic activity of biologically synthesized silver nanoparticles using Pouteria sapota in streptozotocin-induced diabetic rats. J. Diabetes 10, 28–42. doi: 10.1111/1753-0407.12554
Priyanka, K., Sahu, P. L., and Singh, S. (2018). Optimization of processing parameters for the development of Ficus religiosa L. extract loaded solid lipid nanoparticles using central composite design and evaluation of antidiabetic efficacy. J. Drug Deliv. Sci. Technol. 43, 94–102.
Pucci, G., Alcidi, R., Tap, L., Battista, F., Mattace-Raso, F., and Schillaci, G. (2017). Sex-and gender-related prevalence, cardiovascular risk and therapeutic approach in metabolic syndrome: a review of the literature. Pharmacol. Res. 120, 34–42. doi: 10.1016/j.phrs.2017.03.008
Qu, Z., Zheng, N., Zhang, Y., Zhang, L., Liu, J., Wang, Q., et al. (2016). Preventing the BDNF and NGF loss involved in the effects of cornel iridoid glycoside on attenuation of experimental autoimmune encephalomyelitis in mice. Neurol. Res. 38, 831–837. doi: 10.1080/01616412.2016.1200766
Ragavan, G., Muralidaran, Y., Sridharan, B., Ganesh, R. N., and Viswanathan, P. (2017). Evaluation of garlic oil in nano-emulsified form: optimization and its efficacy in high-fat diet induced dyslipidemia in Wistar rats. Food Chem. Toxicol. 105, 203–213. doi: 10.1016/j.fct.2017.04.019
Rasheed, H. M., Khan, T., Wahid, F., Khan, R., and Shah, A. J. (2015). Chemical composition and vasorelaxant and antispasmodic effects of essential oil from Rosa indica L. petals. Evid. Based Complement. Alternat. Med. 2015:279247. doi: 10.1155/2015/279247
Rehana, D., Mahendiran, D., Kumar, R. S., and Rahiman, A. K. (2017). In vitro antioxidant and antidiabetic activities of zinc oxide nanoparticles synthesized using different plant extracts. Bioprocess Biosyst. Eng. 40, 943–957. doi: 10.1007/s00449-017-1758-2
Rochlani, Y., Pothineni, N. V., Kovelamudi, S., and Mehta, J. L. (2017). Metabolic syndrome: pathophysiology, management, and modulation by natural compounds. Ther. Adv. Cardiovasc. Dis. 11, 215–225. doi: 10.1177/1753944717711379
Rübsam, A., Parikh, S., and Fort, P. E. (2018). Role of inflammation in diabetic retinopathy. Int. J. Mol. Sci. 19:942.
Saedisomeolia, A., Arzati, M. M., Abdolahi, M., Sedighiyan, M., Rangel, A., Muench, G., et al. (2019). Mechanisms of action of ginger in nuclear factor-kappaB signaling pathways in diabetes. J. Herb. Med. 16:100239.
Saha, S. S., and Ghosh, M. (2012). Antioxidant and anti-inflammatory effect of conjugated linolenic acid isomers against streptozotocin-induced diabetes. Br. J. Nutr. 108, 974–983. doi: 10.1017/S0007114511006325
Samadder, A., Chakraborty, D., De, A., Bhattacharyya, S. S., Bhadra, K., and Khuda-Bukhsh, A. R. (2011). Possible signaling cascades involved in attenuation of alloxan-induced oxidative stress and hyperglycemia in mice by ethanolic extract of Syzygium jambolanum: drug-DNA interaction with calf thymus DNA as target. Eur. J. Pharm. Sci. 44, 207–217. doi: 10.1016/j.ejps.2011.07.012
Samadder, A., Das, S., Das, J., Paul, A., and Khuda-Bukhsh, A. R. (2012). Ameliorative effects of Syzygium jambolanum extract and its poly (lactic-co-glycolic) acid nano-encapsulated form on arsenic-induced hyperglycemic stress: a multi-parametric evaluation. J. Acupunct. Meridian Stud. 5, 310–318. doi: 10.1016/j.jams.2012.09.001
Sambu, N. K., Kashinath, R., and Ambekar, J. (2015). Effect of diallyl disulphide on diabetes induced dyslipidemia in male albino rats. J. Clin. Diagn. Res. 9:BF01. doi: 10.7860/JCDR/2015/13374.5860
Saratale, G. D., Saratale, R. G., Benelli, G., Kumar, G., Pugazhendhi, A., Kim, D.-S., et al. (2017). Anti-diabetic potential of silver nanoparticles synthesized with Argyreia nervosa leaf extract high synergistic antibacterial activity with standard antibiotics against foodborne bacteria. J. Clust. Sci. 28, 1709–1727.
Sarmento, B., Martins, S., Ferreira, D., and Souto, E. B. (2007). Oral insulin delivery by means of solid lipid nanoparticles. Int. J. Nanomedicine 2:743.
Shehab, G. M., Alblihed, M. A., Albarakati, A. Y., and El Awady, M. A. M. (2018). The protective effect of pomegranate (Punica granatum) against oxidative stress and nephropathy induced by diabetes in male rats: a biochemical, molecular and histopathological study. Annu. Res. Rev. Biol. 26, 1–13. doi: 10.1080/0886022X.2016.1207053
Sheng, J., He, H., Han, L., Qin, J., Chen, S., Ru, G., et al. (2016). Enhancing insulin oral absorption by using mucoadhesive nanoparticles loaded with LMWP-linked insulin conjugates. J. Control. Release 233, 181–190. doi: 10.1016/j.jconrel.2016.05.015
Shi, J., Arunasalam, K., Yeung, D., Kakuda, Y., Mittal, G., and Jiang, Y. (2004). Saponins from edible legumes: chemistry, processing, and health benefits. J. Med. Food 7, 67–78. doi: 10.1089/109662004322984734
Singh, B. P., Vij, S., and Hati, S. (2014). Functional significance of bioactive peptides derived from soybean. Peptides 54, 171–179. doi: 10.1016/j.peptides.2014.01.022
Singh, D., Singh, B., and Goel, R. K. (2011). Traditional uses, phytochemistry and pharmacology of Ficus religiosa: a review. J. Ethnopharmacol. 134, 565–583. doi: 10.1016/j.jep.2011.01.046
Singh, S. V., Shrivastava, A., Chaturvedi, U., Singh, S. C., Shanker, K., Saxena, J. K., et al. (2016). A mechanism-based pharmacological evaluation of efficacy of Flacourtia indica in management of dyslipidemia and oxidative stress in hyperlipidemic rats. J. Basic Clin. Physiol. Pharmacol. 27, 121–129. doi: 10.1515/jbcpp-2015-0017
Singhal, A., Gupta, H., and Bhati, V. (2011). Wound healing activity of Argyreia nervosa leaves extract. Int. J. Appl. Basic Med. Res. 1, 36–39. doi: 10.4103/2229-516X.81978
Singla, R., Singla, N., and Jaitak, V. (2017a). Stevia rebaudiana targeting α-amylase: an in-vitro and in-silico mechanistic study. Nat. Prod. Res. 33, 548–555. doi: 10.1080/14786419.2017.1395433
Singla, R., Soni, S., Kulurkar, P. M., Kumari, A., Mahesh, S., Patial, V., et al. (2017b). In situ functionalized nanobiocomposites dressings of bamboo cellulose nanocrystals and silver nanoparticles for accelerated wound healing. Carbohydr. Polym. 155, 152–162. doi: 10.1016/j.carbpol.2016.08.065
Singla, R., Soni, S., Patial, V., Kulurkar, P. M., Kumari, A., Mahesh, S., et al. (2017c). In vivo diabetic wound healing potential of nanobiocomposites containing bamboo cellulose nanocrystals impregnated with silver nanoparticles. Int. J. Biol. Macromol. 105, 45–55. doi: 10.1016/j.ijbiomac.2017.06.109
Sriramavaratharajan, V., and Murugan, R. (2018). Evaluation of chemical composition, antioxidant and anti-hyperglycemic activities of the essential oil based nanoemulsions of Cinnamomum litseifolium. Nat. Prod. Res. 33, 2430–2433. doi: 10.1080/14786419.2018.1446137
Subramani, K., Pathak, S., and Hosseinkhani, H. (2012). Recent trends in diabetes treatment using nanotechnology. Dig. J. Nanomater. Biostruct. 7, 85–95.
Sun, Y.-E., Wang, W., and Qin, J. (2018). Anti-hyperlipidemia of garlic by reducing the level of total cholesterol and low-density lipoprotein: a meta-analysis. Medicine 97:e0255. doi: 10.1097/MD.0000000000010255
Surendiran, A., Sandhiya, S., Pradhan, S., and Adithan, C. (2009). Novel applications of nanotechnology in medicine. Indian J. Med. Res. 130, 689–701.
Tachibana, Y., Kikuzaki, H., Lajis, N. H., and Nakatani, N. (2001). Antioxidative activity of carbazoles from Murraya koenigii leaves. J. Agric. Food Chem. 49, 5589–5594. doi: 10.1021/jf010621r
Taghipour, Y. D., Hajialyani, M., Naseri, R., Hesari, M., Mohammadi, P., Stefanucci, A., et al. (2019). Nanoformulations of natural products for management of metabolic syndrome. Int. J. Nanomedicine 14:5303. doi: 10.2147/IJN.S213831
Tajmohammadi, A., Razavi, B. M., and Hosseinzadeh, H. (2018). Silybum marianum (milk thistle) and its main constituent, silymarin, as a potential therapeutic plant in metabolic syndrome: a review. Phytother. Res. 32, 1933–1949. doi: 10.1002/ptr.6153
Tsuzuki, T., Kawakami, Y., Abe, R., Nakagawa, K., Koba, K., Imamura, J., et al. (2006). Conjugated linolenic acid is slowly absorbed in rat intestine, but quickly converted to conjugated linoleic acid. J. Nutr. 136, 2153–2159. doi: 10.1093/jn/136.8.2153
Vaziri, N. D., Xu, Z.-G., Shahkarami, A., Huang, K. T., Rodruguez-Iturbe, B., and Natarajan, R. (2005). Role of AT-1 receptor in regulation of vascular MCP-1, IL-6, PAI-1, MAP kinase, and matrix expressions in obesity. Kidney Int. 68, 2787–2793. doi: 10.1111/j.1523-1755.2005.00750.x
Vishram, J. K., Borglykke, A., Andreasen, A. H., Jeppesen, J., Ibsen, H., Jørgensen, T., et al. (2014). Impact of age and gender on the prevalence and prognostic importance of the metabolic syndrome and its components in Europeans. The MORGAM Prospective Cohort Project. PLoS One 9:e107294. doi: 10.1371/journal.pone.0107294
Volf, I., Ignat, I., Neamtu, M., and Popa, V. I. J. C. P. (2014). Thermal stability, antioxidant activity, and photo-oxidation of natural polyphenols. Chem. Pap. 68, 121–129.
Volpe, C. M. O., Villar-Delfino, P. H., Dos Anjos, P. M. F., and Nogueira-Machado, J. A. (2018). Cellular death, reactive oxygen species (ROS) and diabetic complications. Cell Death Dis. 9, 1–9. doi: 10.1038/s41419-017-0135-z
Wang, X., Qian, Y., Jin, R., Wo, Y., Chen, J., Wang, C., et al. (2013). Effects of TRAP-1-like protein (TLP) gene on collagen synthesis induced by TGF-β/Smad signaling in human dermal fibroblasts. PLoS One 8:e55899. doi: 10.1371/journal.pone.0055899
Wei, Y., Solomon, M. J., and Larson, R. G. (2019). Time-dependent shear rate inhomogeneities and shear bands in a thixotropic yield-stress fluid under transient shear. Soft Matter 15, 7956–7967. doi: 10.1039/c9sm00902g
Wilczewska, A. Z., Niemirowicz, K., Markiewicz, K. H., and Car, H. (2012). Nanoparticles as drug delivery systems. Pharmacol. Rep. 64, 1020–1037.
Wu, Z., Ping, Q., Wei, Y., and Lai, J. (2004). Hypoglycemic efficacy of chitosan-coated insulin liposomes after oral administration in mice. Acta Pharmacol. Sin. 25, 966–972.
Xi, B., He, D., Hu, Y., and Zhou, D. (2013). Prevalence of metabolic syndrome and its influencing factors among the Chinese adults: the China Health and Nutrition Survey in 2009. Prev. Med. 57, 867–871. doi: 10.1016/j.ypmed.2013.09.023
Xiang, L. L., Li, Y., Deng, X., Kosanovic, D., Schermuly, R., and Li, X. H. (2018). EXPRESS: natural plant products in treatment of pulmonary arterial hypertension. Pulm Circ. 8:2045894018784033. doi: 10.1177/2045894018784033
Xu, D., Li, Y., Zhang, B., Wang, Y., Liu, Y., Luo, Y., et al. (2016). Resveratrol alleviate hypoxic pulmonary hypertension via anti-inflammation and anti-oxidant pathways in rats. Int. J. Med. Sci. 13, 942–954. doi: 10.7150/ijms.16810
Xu, Z., Wang, S., Ji, H., Zhang, Z., Chen, J., Tan, Y., et al. (2016). Broccoli sprout extract prevents diabetic cardiomyopathy via Nrf2 activation in db/db T2DM mice. Sci. Rep. 6:30252. doi: 10.1038/srep30252
Xu, L., Li, W., Shi, Q., Li, H., Yang, Z., Liao, D., et al. (2019). Synthesis of mulberry leaf extract mediated gold nanoparticles and their ameliorative effect on Aluminium intoxicated and diabetic retinopathy in rats during perinatal life. J. Photochem. Photobiol. B Biol. 196:111502. doi: 10.1016/j.jphotobiol.2019.04.011
Xu, T., Liu, S., Ma, T., Jia, Z., Zhang, Z., and Wang, A. (2017). Aldehyde dehydrogenase 2 protects against oxidative stress associated with pulmonary arterial hypertension. Redox Biol. 11, 286–296. doi: 10.1016/j.redox.2016.12.019
Yadav, T. C., Saxena, P., Srivastava, A. K., Singh, A. K., Yadav, R. K., Prasad, R., et al. (2019). “Potential applications of chitosan nanocomposites: recent trends and challenges,” in Advanced Functional Textiles and Polymers: Fabrication, Processing and Applications, eds S. Islam and B. S. Butola (Hoboken, NY: Wiley), 365–403.
Yeh, P.-T., Huang, H.-W., Yang, C.-M., Yang, W.-S., and Yang, C.-H. (2016). Astaxanthin inhibits expression of retinal oxidative stress and inflammatory mediators in streptozotocin-induced diabetic rats. PLoS One 11:e0146438. doi: 10.1371/journal.pone.0146438
Zahan, R., Ripa, F. A., Alam, M. B., Haque, M. A., Mosaddik, M., and Nahar, L. (2011). Hypoglycemic and in vitro antioxidant effects of methanolic extract of Marsilea quadrifolia plant. Pharmacogn. J. 3, 86–92.
Zarfeshany, A., Asgary, S., and Javanmard, S. H. (2014). Potent health effects of pomegranate. Adv. Biomed. Res. 3:100. doi: 10.4103/2277-9175.129371
Zhang, M., Viennois, E., Prasad, M., Zhang, Y., Wang, L., Zhang, Z., et al. (2016). Edible ginger-derived nanoparticles: a novel therapeutic approach for the prevention and treatment of inflammatory bowel disease and colitis-associated cancer. Biomaterials 101, 321–340. doi: 10.1016/j.biomaterials.2016.06.018
Zhang, M., Xu, C., Liu, D., Han, M. K., Wang, L., Merlin, D., et al. (2018). Oral delivery of nanoparticles loaded with ginger active compound, 6-shogaol, attenuates ulcerative colitis and promotes wound healing in a murine model of ulcerative colitis. J. Crohns Colitis 12, 217–229. doi: 10.1093/ecco-jcc/jjx115
Zhang, X., Qi, J., Lu, Y., Hu, X., He, W., and Wu, W. (2014). Enhanced hypoglycemic effect of biotin-modified liposomes loading insulin: effect of formulation variables, intracellular trafficking, and cytotoxicity. Nanoscale Res. Lett. 9:185. doi: 10.1186/1556-276X-9-185
Keywords: medicinal plants, nanoparticles, diabetes, metabolic syndrome, nanophytomedicines, phytotherapy
Citation: Nouri Z, Hajialyani M, Izadi Z, Bahramsoltani R, Farzaei MH and Abdollahi M (2020) Nanophytomedicines for the Prevention of Metabolic Syndrome: A Pharmacological and Biopharmaceutical Review. Front. Bioeng. Biotechnol. 8:425. doi: 10.3389/fbioe.2020.00425
Received: 21 January 2020; Accepted: 14 April 2020;
Published: 14 May 2020.
Edited by:
Chiara Martinelli, Centro per Microboriobotici, Istituto Italiano di Tecnologia, ItalyReviewed by:
Murat Yavuz, Dicle University, TurkeyCopyright © 2020 Nouri, Hajialyani, Izadi, Bahramsoltani, Farzaei and Abdollahi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mohammad Hosein Farzaei, bWguZmFyemFlaUBnbWFpbC5jb20=; Mohammad Abdollahi, TW9oYW1tYWRAVFVNUy5BYy5Jcg==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.