- 1Auckland Bioengineering Institute, The University of Auckland, Auckland, New Zealand
- 2Stanford Cardiovascular Institute, Stanford University, Stanford, CA, United States
- 3School of Natural and Computational Sciences, Massey University, Auckland, New Zealand
- 4Chongqing Health Center for Women and Children, Chongqing, China
Introduction
Despite mounting evidence of detrimental effects of maternal smoking on fetuses, including still birth, intrauterine growth restriction (IUGR), and sudden infant death syndrome (SIDS) among many others, the prevalence of smoking women exceeds 10% in a majority of developed countries (Dessı̀ et al., 2018). For example, in the USA, the prevalence is about 14% (Dessı̀ et al., 2018), and in Poland, this rate is 15–20% (Chełchowska et al., 2018). Pregnancy smoking rates can be much higher in certain sub-populations of these countries, such as for instance, among the indigenous NZ Māori population at rates of 34%—well above the national level of 10% (Humphrey et al., 2016). Smoking cessation is challenging due to multiple reasons, including nicotine addiction, cultural and social-economic status, and resistance to life-style change (Dessı̀ et al., 2018). Early interventions are required to reverse the pathological course exposing the fetus to hazards from smoking. This demands a precise understanding of the underlying etiologies and evaluating appropriate pharmaceutical targets. We are particularly interested in pathologies emerging in the fetal cardiovascular system (CVS) due to smoking exposure (Dikalov et al., 2019). Indeed, active and passive maternal smoking cause hemodynamic changes in major fetal blood vessels such as the umbilical arteries (UAs)—a biomarker for impaired feto-placental circulation (Westergaard et al., 2001).
Unfortunately, examining the molecular and hemodynamic mechanisms of fetuses in utero is difficult due to ethical and technical reasons. Instead, ex vivo and in vitro experiments are performed; e.g., investigating the endothelial nitric oxide synthase (eNOS) activity in response to maternal smoking, whereby endothelial cells (ECs) were harvested from fetal umbilical veins immediately after postpartum (Andersen et al., 2004; Hedengran et al., 2018). However, in vitro experiments are costly, and only a limited set of experimental parameters are financially feasible. By contrast, in silico modeling may yield useful insights into the key aspects of CVS risks still awaiting full characterization. In silico models include mathematical representations of hemodynamic changes, the mechanistic stimuli and transduction pathways in the CVS, the absorption and clearance of drugs, to name a few (Chen and Popel, 2006; Barrett et al., 2012). Furthermore, some seminal discoveries of CVS diseases were made possible due to substantial method developments in DNA sequencing and corresponding bioinformatics (Joubert et al., 2016). However, applications of in silico models to nicotine effects on fetuses are seriously lacking. We thus here sketch a roadmap for application of multiscale in silico models, suggesting how the power of mathematical and computational modeling methods may augment and illuminate this significant public health problem.
Doppler Ultrasound and Clinical Observations
Exposure to active smoking affects the systole/diastole (S/D) flow velocity ratio (S/D) in the fetal UA and the middle cerebral artery (MCA), as revealed from Doppler sonography ultrasound examinations (Albuquerque et al., 2004; Dicke et al., 2009; Milnerowicz-Nabzdyk and Bizon, 2015; Stampalija et al., 2017). In general, the S/D ratio in the UA and MCA is greater in smokers than in non-smokers, signifying a lower or absent end-diastolic flow. High S/D ratios are typical in fetuses with IUGR (Albuquerque et al., 2004). Hence, umbilical S/D ratio is used as a marker for adverse outcomes and placental abnormalities (Dicke et al., 2009). In addition, the S/D and PI (pulsatility index) in UAs are similarly reliable in fetuses >27 weeks gestation (Dicke et al., 2009).
Clinical ultrasound studies also show structural changes in the umbilical cord in pregnancies complicated by IUGR: the size of the Wharton's jelly is increased (101.60 ± 37.75 in the smoking IUGR group vs. 84.97 ± 54.16 in the control group), yet the diameters of umbilical vessels are reduced (Milnerowicz-Nabzdyk and Bizon, 2015).
In vitro Techniques for Studying Nicotine Effects
Endothelium-Dependent Vessel Relaxation
Endothelium-dependent vessel relaxation is mediated by nitric oxide (NO), which is synthesized from the amino acid L-arginine by eNOS (Chełchowska et al., 2018). The activity and concentration of the calcium-dependent eNOS were significantly lower in the umbilical vein ECs of smokers compared with non-smokers (Andersen et al., 2004). In addition, the NO and eNOS concentration in the serum of smokers were significantly lower than the non-smoking group. In contrast, inducible NOS and oxidative stress index values were higher in the smokers' group (Chełchowska et al., 2018).
Stem Cell Technology
The rapid development of stem cell technology brings new opportunities for nicotine mechanism studies to facilitate precise treatment (Takahashi and Yamanaka, 2006). In particular, the human induced pluripotent stem cells (hiPSCs) can be derived from patient- and ethnic-specific background and hence can reproduce different kinds of cell types of human organs. For example, the change in Ca2+ homeostasis due to nicotine exposure was recently demonstrated by using human embryonic stem cells (hESCs; Guo et al., 2019). The same group reported that e-cigarettes caused dysfunction of hiPSC-derived ECs (Lee et al., 2019). With next generation sequencing technologies, stem cell scientists may be able to predict nicotine-induced toxicity in human cells. Moreover, ethnic-specific studies are valuable for novel mechanism discovery in those sub-populations prone to smoking exposure.
DNA Sequencing
Development of CVS diseases is associated with up-regulation of miR-206 in smokers compared to non-smokers (Vrijens et al., 2015). In utero exposure to maternal smoking is also associated with DNA methylation changes and reduced neuronal development using DNA Methylation and Gene Expression Arrays (Chatterton et al., 2017). Dysregulation of key pathways critical to development is identified by using Methylation Arrays (Joubert et al., 2016). These seminal discoveries could not have been made possible without advances in bioinformatics methods.
In silico Models for the CVS
Hemodynamics Models
Numerous hemodynamics models have been developed targeting at different spatial scales of the CVS, ranging from a large vasculature with thousands of vessels (Muller et al., 2017), to a single vessel but with complex geometry such as a knotted UA (Wilke et al., 2018), or boundary conditions (van de Vosse and Stergiopulos, 2011). If the investigation focuses on the 3D wall shear stress (WSS) acting on blood vessels, then a 3D flow model may serve the purpose (Saw et al., 2017; Wilke et al., 2018). Investigating the blood flow in a vasculature proves more computationally feasible with 1D Navier-Stokes equations coupled with a wall constitutive equation (Formaggia et al., 2006; van de Vosse and Stergiopulos, 2011). To our knowledge, this method has not been applied to the fetal CVS yet, but a 3D-lumped parameter model coupled method has been used to simulate the blood flow in the ductus venosus (Leinan et al., 2013). To study pressure wave propagation along the elastic arterial wall, the flow equations are solved from the frequency domain, using methods such as the Transmission Line Theory (Muller et al., 2017), or Fourier series analysis for the velocity waveforms (Sled et al., 2019).
Calcium Signaling Models
In silico models have been proposed for the effects of WSS on ion channel flux in ECs (Comerford et al., 2008; Ho et al., 2011), and on the eNOS activation and subsequent NO production (Chen and Popel, 2006). Shear stress initiates a release of intracellular ATP leading to a cascade of events including calcium (Ca2+) influx through intracellular store-operated Ca2+ channels that are key to activating NO-producing eNOS (Andrews et al., 2014). These events occur in the ubiquitous Ca2+ micro-domains formed in sub-plasma membrane regions known as caveolae (Cohen et al., 2004) and are the focus of multiple modeling efforts in disparate cell types (Berridge, 2006). However, while Ca2+ mediation of eNOS activation and production of NOS are a ripe target for mathematical modeling, we are not aware of such a model when associated with nicotine effects as mentioned above in smokers and non-smokers. Its successful application would provide important insights into the complex mechanisms described above in the fetal CVS.
Pharmacokinetic (PK) Models
Pharmaceutical treatment of the fetal CVS requires drugs to cross the placenta barrier, then be metabolized and up-taken by target tissues. However, the fetal liver is quite limited in metabolic functions: the metabolism enzymes are simply not well-developed (Jiang et al., 2013). In vivo PK profile data of drugs, such as the vasodilators for treating IUGR (Sharp and Alfirevic, 2015), are very rare. Current clinical practice is by analyzing blood samples extracted from the umbilical cord but lacking any data from other organs (Huang et al., 2017). Thus, pregnant PK models which contain the maternal-placental-fetal unit can only be validated partially, i.e., in the blood compartment (Ke et al., 2012; Sharma et al., 2018). Nevertheless, predictions made from pregnant PK models for drug distribution, metabolism and clearance in fetuses are still valuable due to the use of nicotine replacement therapy in pregnant women (Benowitz and Dempsey, 2004). Notably, an in silico PK approach may be the only feasible option to estimate the nicotine and cotinine concentration in fetuses for ethical concerns. Nevertheless, the qualitative data of enzyme maturation (Ke et al., 2012) and the placenta transport kinetics (Prouillac and Lecoeur, 2010) are essential for any in silico PK model validation.
Bioinformatics
Genomic data along with clinical correlates are being increasingly used in clinical practices as therapeutic, prognostic and diagnostic biomarkers of numerous pathologies. A meta-data analysis across 13 cohorts (n = 6,685) found over 6,000 of total 450,000 CpG sites were differentially methylated in relation to maternal smoking at genome-wide statistical significance (Joubert et al., 2016). Robust linear regression was used in R to evaluate the association between maternal smoking during pregnancy and cord blood DNA methylation. Covariate-adjusted statistical models are run for each cohort. Specifically, inverse variance-weighted fixed-effects meta-analysis was performed with a bioinformatics tool Metal (Willer et al., 2010).
Discussion
In above sections, we have provided a mini-review of the current knowledge on the effects of maternal smoking on the CVS of fetuses. Although the presented data are fragmentary, they represent what we have learned from clinics (e.g., the velocity waveforms of UAs), from laboratories via tissue or blood sample analysis, and also from stem cell or DNA sequencing studies. In silico models, therefore, must necessarily be of a very diverse nature in order to integrate and interpret these data.
Hence, in proposing a roadmap for incorporating such different datasets and models for this challenging and complex topic, we suggest at first tackling the underlying biology; particularly analyzing bio-markers for fetal CVS risks. These risk factors due to maternal smoking are confounded as well by fetal sex, race, mother age, pregnancy stage, and family disease history, to name a few. Building on this understanding, we then assemble a validated mathematical and computational model for linkage of existing experiment data, and interrogate the model to reveal novel connections between underlying mechanisms (Figure 1). More specifically, since the absent end-diastolic flows and its associated S/D ratio are viewed as the a bio-marker for adverse birth outcomes (Dicke et al., 2009), simulations with an in silico model may be made for normal and abnormal blood flow patterns, similar to that performed for UAs (Wilke et al., 2018), and for ductus venosus (Leinan et al., 2013) but also incorporating nicotine effect data (Dicke et al., 2009). Moreover, it is essential to connect the disparate scales of WSS and signaling dynamics in an overarching model to explore how nicotine may affect vascular wall remodeling in fetuses (Milnerowicz-Nabzdyk and Bizon, 2015).
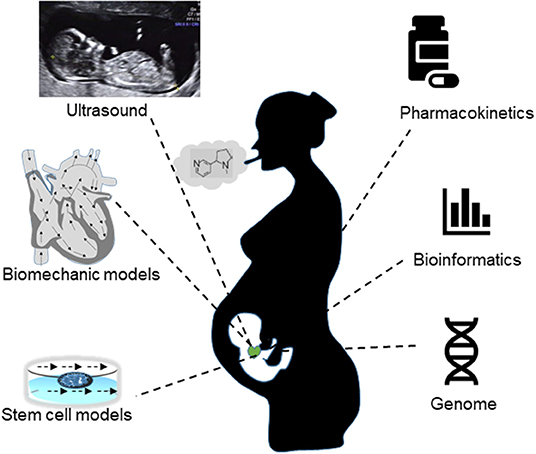
Figure 1. A synergy of in vivo measurements (ultrasound flow indices, drug concentrations), in vitro experiments (stem cell, DNA sequencing) and in silico methods (biomechanics models, pharmacokinetic models, informatics, etc.) are required to gain a better understanding of the CVS risks in fetuses exposed to active or passive maternal smoking.
Such a modeling framework would benefit from the philosophy of systems biology, where the analysis of physiological phenomena need to integrate the data and models from multiple spatial scales, i.e., from the molecular level to cell and organ levels (Hunter and Borg, 2003), and from bioinformatic analysis of genomic data (Joubert et al., 2016). For example, while the model for NO production and calcium signaling has to work from the protein level (Chen and Popel, 2006), a hemodynamic model for flow waveform would have to incorporate flow data in blood vessels at the organ level. To estimate the PK profile in response to nicotine, an organism level model is required for quantifying the mass exchanges between multiple compartments such as the blood, the fetus, and the placenta.
In summary, a synergy of in vivo clinical observations, in vitro experiments and in silico models is proposed here for revealing the underlying mechanistic mechanisms of fetal CVS risks due to maternal smoking. Collaborations from multiple research groups, and from multiple disciplines are, naturally, essential. The roadmap we sketched in this opinion paper points where the efforts—challenging as they will be—should be directed in order to tackle a health issue of such profound importance.
Author Contributions
HH, HG, and SM drafted the manuscript. JT and PH attended the conceptual discussion of the paper and reviewed the paper.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
This work was supported by the Scientific Innovation of Technological Innovation of New Zealand (UOAX1702). HH acknowledges the financial support from the University of Auckland foundation.
References
Albuquerque, C. A., Smith, K. R., Johnson, C., Chao, R., and Harding, R. (2004). Influence of maternal tobacco smoking during pregnancy on uterine, umbilical and fetal cerebral artery blood flows. Early Hum. Dev. 80, 31–42. doi: 10.1016/j.earlhumdev.2004.05.004
Andersen, M. R., Walker, L. R., and Stender, S. (2004). Reduced endothelial nitric oxide synthase activity and concentration in fetal umbilical veins from maternal cigarette smokers. Am. J. Obstet. Gynecol. 191, 346–351. doi: 10.1016/j.ajog.2003.12.040
Andrews, A. M., Jaron, D., Buerk, D. G., and Barbee, K. A. (2014). Shear stress-induced no production is dependent on ATP Autocrine signaling and capacitative calcium entry. Cell. Mol. Bioeng. 7, 510–520. doi: 10.1007/s12195-014-0351-x
Barrett, J. S., Alberighi, O. D. C., Läer, S., and Meibohm, B. (2012). Physiologically based pharmacokinetic (PBPK) modeling in children. Clin. Pharmacol. Ther. 92, 40–49. doi: 10.1038/clpt.2012.64
Benowitz, N., and Dempsey, D. (2004). Pharmacotherapy for smoking cessation during pregnancy. Nicotine Tob. Res. 6, 189–202. doi: 10.1080/14622200410001669169
Berridge, M. J. (2006). Calcium microdomains: organization and function. Cell Calcium 40, 405–412. doi: 10.1016/j.ceca.2006.09.002
Chatterton, Z., Hartley, B. J., Seok, M. H., Mendelev, N., Chen, S., Milekic, M., et al. (2017). In utero exposure to maternal smoking is associated with DNA methylation alterations and reduced neuronal content in the developing fetal brain. Epigenet. Chromatin 10:4. doi: 10.1186/s13072-017-0111-y
Chełchowska, M., Ambroszkiewicz, J., Gajewska, J., Mazur, J., Lewandowski, L., Reśko-Zachara, M., et al. (2018). Influence of active exposure to tobacco smoke on nitric oxide status of pregnant women. Int. J. Environ. Res. Public Health 15:2719. doi: 10.3390/ijerph15122719
Chen, K., and Popel, A. S. (2006). Theoretical analysis of biochemical pathways of nitric oxide release from vascular endothelial cells. Free Radic. Biol. Med. 41, 668–680. doi: 10.1016/j.freeradbiomed.2006.05.009
Cohen, A. W., Hnasko, R., Schubert, W., and Lisanti, M. P. (2004). Role of caveolae and caveolins in health and disease. Physiol. Rev. 84, 1341–1379. doi: 10.1152/physrev.00046.2003
Comerford, A., Plank, M. J., and David, T. (2008). Endothelial nitric oxide synthase and calcium production in arterial geometries: an integrated fluid mechanics/cell model. J. Biomech. Eng. 130, 011010–011013. doi: 10.1115/1.2838026
Dessì, A., Corona, L., Pintus, R., and Fanos, V. (2018). Exposure to tobacco smoke and low birth weight: from epidemiology to metabolomics. Expert Rev. Proteomics 15, 647–656. doi: 10.1080/14789450.2018.1505508
Dicke, J. M., Huettner, P., Yan, S., Odibo, A., and Kraus, F. T. (2009). Umbilical artery doppler indices in small for gestational age fetuses. J. Ultrasound Med. 28, 1603–1610. doi: 10.7863/jum.2009.28.12.1603
Dikalov, S., Itani, H., Richmond, B., Arslanbaeva, L., Vergeade, A., Rahman, S. M. J., et al. (2019). Tobacco smoking induces cardiovascular mitochondrial oxidative stress, promotes endothelial dysfunction, and enhances hypertension. Am. J. Physiol. Heart Circ. Physiol. 316, H639–H646. doi: 10.1152/ajpheart.00595.2018
Formaggia, L., Lamponi, D., Tuveri, M., and Veneziani, A. (2006). Numerical modeling of 1D arterial networks coupled with a lumped parameters description of the heart. Comput. Methods Biomech. Biomed. Engin. 9, 273–288. doi: 10.1080/10255840600857767
Guo, H., Tian, L., Zhang, J. Z., Kitani, T., Paik, D. T., Lee, W. H., et al. (2019). Single-Cell RNA sequencing of human embryonic stem cell differentiation delineates adverse effects of nicotine on embryonic development. Stem Cell Rep. 12, 772–786. doi: 10.1016/j.stemcr.2019.01.022
Hedengran, K. K., Andersen, M. R., Szecsi, P. B., Lindh, C., Uldbjerg, N., and Stender, S. (2018). Environmental tobacco smoke exposure during pregnancy has limited effect on infant birthweight and umbilical vein endothelial nitric oxide synthase. Acta Obstet. Gynecol. Scand. 97, 1309–1316. doi: 10.1111/aogs.13419
Ho, H., Suresh, V., Kang, W., Cooling, M. T., Watton, P. N., and Hunter, P. J. (2011). Multiscale modeling of intracranial aneurysms: cell signaling, hemodynamics, and remodeling. IEEE Trans. Biomed. Eng. 58, 2974–2977. doi: 10.1109/TBME.2011.2160638
Huang, K. H., Chou, A. K., Jeng, S. F., Ng, S., Hsieh, C. J., Chen, M. H., et al. (2017). The impacts of cord blood cotinine and glutathione-s-transferase gene polymorphisms on birth outcome. Pediatr. Neonatol. 58, 362–369. doi: 10.1016/j.pedneo.2016.08.006
Humphrey, G., Rossen, F., Walker, N., and Bullen, C. (2016). Parental smoking during pregnancy: findings from the growing up in New Zealand cohort. N. Z. Med. J. 129, 60–74.
Hunter, P. J., and Borg, T. K. (2003). Integration from proteins to organs: the physiome project. Nat. Rev. Mol. Cell Biol. 4, 237–243. doi: 10.1038/nrm1054
Jiang, X. L., Zhao, P., Barrett, J. S., Lesko, L. J., and Schmidt, S. (2013). Application of physiologically based pharmacokinetic modeling to predict acetaminophen metabolism and pharmacokinetics in children. CPT Pharmacometrics Syst. Pharmacol. 2:e80. doi: 10.1038/psp.2013.55
Joubert, B. R., Felix, J. F., Yousefi, P., Bakulski, K. M., Just, A. C., Breton, C., et al. (2016). DNA methylation in newborns and maternal smoking in pregnancy: genome-wide consortium meta-analysis. Am. J. Hum. Genet. 98, 680–696. doi: 10.1016/j.ajhg.2016.02.019
Ke, A., Nallani, S., Zhao, P., Rostami-Hodjegan, A., and Unadkat, J. (2012). A PBPK model to predict disposition of CYP3A-metabolized drugs in pregnant women: verification and discerning the site of CYP3A induction. CPT Pharmacometrics Syst. Pharmacol. 1, 1–10. doi: 10.1038/psp.2012.2
Lee, W. H., Ong, S. G., Zhou, Y., Tian, L., Bae, H. R., Baker, N., et al. (2019). Modeling cardiovascular risks of e-cigarettes with human-induced pluripotent stem cell–derived endothelial cells. J. Am. Coll. Cardiol. 73, 2722–2737. doi: 10.1016/j.jacc.2019.03.476
Leinan, P. R., Degroote, J., Kiserud, T., Skallerud, B., Vierendeels, J., and Hellevik, L. R. (2013). Velocity profiles in the human ductus venosus: a numerical fluid structure interaction study. Biomech. Model Mechanobiol. 12, 1019–1035. doi: 10.1007/s10237-012-0460-1
Milnerowicz-Nabzdyk, E., and Bizon, A. (2015). How does tobacco smoke influence the morphometry of the fetus and the umbilical cord?—Research on pregnant women with intrauterine growth restriction exposed to tobacco smoke. Reprod. Toxicol. 58, 79–84. doi: 10.1016/j.reprotox.2015.08.003
Muller, A., Clarke, R., and Ho, H. (2017). Fast blood-flow simulation for large arterial trees containing thousands of vessels. Comput. Methods Biomech. Biomed. Engin. 20, 160–170. doi: 10.1080/10255842.2016.1207170
Prouillac, C., and Lecoeur, S. (2010). The role of the placenta in fetus exposure to xenobiotics: importance of membrane transporters, human models for transfer studies. Drug Metab. Dispos. 38, 1623–1635. doi: 10.1124/dmd.110.033571
Saw, S. N., Dawn, C., Biswas, A., Mattar, C. N. Z., and Yap, C. H. (2017). Characterization of the in vivo wall shear stress environment of human fetus umbilical arteries and veins. Biomech. Model. Mechanobiol. 16, 197–211. doi: 10.1007/s10237-016-0810-5
Sharma, R. P., Schuhmacher, M., and Kumar, V. (2018). The development of a pregnancy PBPK model for bisphenol a and its evaluation with the available biomonitoring data. Sci. Total Environ. 624, 55–68. doi: 10.1016/j.scitotenv.2017.12.023
Sharp, A. N., and Alfirevic, Z. (2015). Vasodilators for treating intrauterine growth restriction. Cochrane Database Syst. Rev. CD011978. doi: 10.1002/14651858.CD011978
Sled, J. G., Stortz, G., Cahill, L. S., Milligan, N., Ayyathurai, V., Serghides, L., et al. (2019). Reflected hemodynamic waves influence the pattern of Doppler ultrasound waveforms along the umbilical arteries. Am. J. Physiol. Heart Circ. Physiol. 316, H1105–H1112. doi: 10.1152/ajpheart.00704.2018
Stampalija, T., Dowswell, T., and Alfirevic, Z. (2017). Fetal and umbilical Doppler ultrasound in high-risk pregnancies. Cochrane Database Syst. Rev. 6:CD007529. doi: 10.1002/14651858.CD007529.pub4.
Takahashi, K., and Yamanaka, S. (2006). Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 126, 663–676. doi: 10.1016/j.cell.2006.07.024
van de Vosse, F. N., and Stergiopulos, N. (2011). Pulse wave propagation in the arterial tree. Annu. Rev. Fluid Mech. 43, 467–499. doi: 10.1146/annurev-fluid-122109-160730
Vrijens, K., Bollati, V., and Nawrot, T. S. (2015). MicroRNAs as potential signatures of environmental exposure or effect: a systematic review. Environ. Health Perspect. 123, 399–411. doi: 10.1289/ehp.1408459
Westergaard, H. B., Langhoff-Roos, J., Lingman, G., Marsál, K., and Kreiner, S. (2001). A critical appraisal of the use of umbilical artery Doppler ultrasound in high-risk pregnancies: use of meta-analyses in evidence-based obstetrics. Ultrasound Obstet. Gynecol. 17, 466–476. doi: 10.1046/j.1469-0705.2001.00415.x
Wilke, D. J., Denier, J. P., Khong, T. Y., and Mattner, T. W. (2018). Pressure and flow in the umbilical cord. J. Biomech. 79, 78–87. doi: 10.1016/j.jbiomech.2018.07.044
Keywords: maternal smoking, nicotine, in silico model, umbilical artery, fetus
Citation: Ho H, Guo H, Means S, Tang J and Hunter P (2020) Maternal Smoking Induced Cardiovascular Risks in Fetuses: How Can in silico Models Help? Front. Bioeng. Biotechnol. 8:97. doi: 10.3389/fbioe.2020.00097
Received: 10 August 2019; Accepted: 03 February 2020;
Published: 19 February 2020.
Edited by:
Fengfeng Zhou, Jilin University, ChinaReviewed by:
Nagarajan Raju, Vanderbilt University Medical Center, United StatesVenkata Yellapantula, Memorial Sloan Kettering Cancer Center, United States
Copyright © 2020 Ho, Guo, Means, Tang and Hunter. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Harvey Ho, aGFydmV5LmhvJiN4MDAwNDA7YXVja2xhbmQuYWMubno=
 Harvey Ho
Harvey Ho Hongchao Guo
Hongchao Guo Shawn Means
Shawn Means Jing Tang4
Jing Tang4 Peter Hunter
Peter Hunter