
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Bioeng. Biotechnol. , 26 September 2019
Sec. Computational Genomics
Volume 7 - 2019 | https://doi.org/10.3389/fbioe.2019.00236
 Christian Ndekezi1†
Christian Ndekezi1† Joseph Nkamwesiga1
Joseph Nkamwesiga1 Sylvester Ochwo1
Sylvester Ochwo1 Magambo Phillip Kimuda1,2
Magambo Phillip Kimuda1,2 Frank Norbert Mwiine1
Frank Norbert Mwiine1 Robert Tweyongyere3
Robert Tweyongyere3 Wilson Amanyire1
Wilson Amanyire1 Dennis Muhanguzi1,4*†
Dennis Muhanguzi1,4*†Ticks are arthropod vectors of pathogens of both Veterinary and Public health importance. Acaricide application, which is currently the mainstay of tick control, is hampered by high cost, the need for regular application and a selection of multi-acaricide resistant tick populations. In light of this, future tick control approaches are poised to rely on the integration of rational acaricide application and other methods, such as vaccination. To contribute to systematic research-guided efforts to produce anti-tick vaccines, we carried out an in-silico analysis of tick aquaporin-1 (AQP1) protein in order to identify tick-specific AQP1 peptide motifs that can be used in future peptide anti-tick vaccine development. We carried out multiple sequence alignment (MSA), motif analysis, homology modeling, and structural analysis to identify tick-specific AQP1 peptide motifs. BepiPred, Chou and Fasman-Turn, Karplus and Schulz Flexibility, and Parker-Hydrophilicity prediction models were used to predict these motifs' potential to induce B cell mediated immune responses. The tick AQP1 (GenBankID: QDO67142.1) protein was largely similar to the bovine AQP1 (PDB:1J4N) (23 % sequence similarity; Structural superimposition of the homology model and 14JN homotetramers gave an RMSD = 3.269 while superimposition of a single chain gave an RMSD = 1.475). Tick and bovine AQP1 transmembrane domains were largely similar while their extracellular and cytoplasmic domain loops showed variation. Two tick-specific AQP1 peptide motifs; M7 (residues 106–125, p = 5.4e-25), and M8 (residues 85–104, p = 3.3e-24) were identified. These two motifs are located on the extra-cellular AQP1 domain. Motifs; M7 and M8 showed the highest Parker-Hydrophilicity prediction immunogenicity scores of 1.784 and 1.536, respectively. These two motifs can be a good starting point for the development of potential tick AQP1 peptide-based anti-tick vaccines. Further analyses such as molecular dynamics, in vitro assays, and in vivo immunization assays are required to validate these findings.
Ticks are arthropod vectors of pathogens of both Veterinary and Public health importance in tropical and sub-tropical regions of the world (Jongejan and Uilenberg, 2004; Wikel, 2018). Ticks are associated with both direct and indirect constraints to livestock health and production (Jongejan and Uilenberg, 2004; Wikel, 2018). Heavy tick infestation (Figure 1) is often associated with direct constraints to livestock production including blood loss (anemia), discomfort, skin-and-hide quality loss, and tick paralysis (Eskezia and Desta, 2016). Their indirect constraints to livestock production and health relate to transmission of diseases of veterinary importance including: anaplasmosis (Anaplasma spp.), babesiosis (Babesia spp.), theileriosis (Theileria spp.), and heartwater (Cowdria ruminatium) (Jongejan and Uilenberg, 2004). In addition, ticks transmit zoonotic pathogens belonging to Babesia spp., Borrelia spp., Rickettsia spp., Ehrlichia spp., Francisella tularensis, Coxiella burnetii, and viruses such as Nairovirus (Bunyaviridae) that causes Crimean-Congo Hemorrhagic Fever (CCHF) and tick-borne encephalitis virus (Flaviviridae) (Sambri et al., 2004; Telmadarraiy et al., 2015).
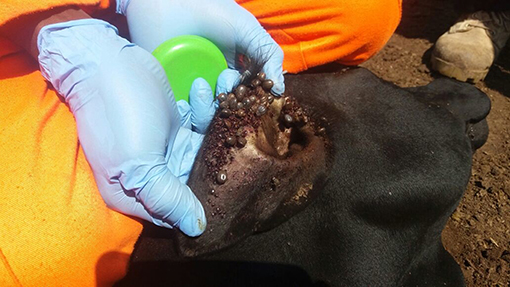
Figure 1. Heavily tick-infested short horn East African Zebu heifer from Serere District, South-eastern Uganda.
In order to reduce the impact of ticks on livestock health and production as well as withdraw their negative public health effects, acaricides, tick hand-picking, animal movement control, and vaccination are often applied to control ticks (Chizyuka and Mulilo, 1990; Jongejan, 1999). Acaricides are the most effective tick and tick-borne diseases (TBDs) control measure (Domingos et al., 2013). However, the ever increasing cost of acaricides in less developed countries, which are the most affected by ticks and TBDs, implies that acaricides cannot be regularly applied for tick control (Chizyuka and Mulilo, 1990). Moreover, acaricide overuse and misuse are associated with a selection of multi-acaricide resistant tick populations (Abbas et al., 2014); a problem that has been on the rise in Uganda (Vudriko et al., 2016). In light of this, future tick control strategies will have to depend on the integration of economically effective acaricide application, vaccination, breeding livestock for tick resistance, and other available tick control methods such as controlled animal movements (Ghosh and Azhahianambi, 2007). To date, anti-tick vaccine development has been slow. For example, the only commercially available recombinant vaccine against R. microplus was developed more than 2 decades ago (Rodríguez et al., 1995; Parizi et al., 2012). There is, therefore, a need to rekindle systematic research-guided efforts to evaluate crucial tick proteins and biological pathways for vaccine development, hence the motivation to evaluate tick specific AQP1 peptide motifs.
Tick aquaporins belong to the Membrane Intrinsic Protein (MIP) superfamily which are known to play a key role in the transportation of water, glycerol and urea across the cell membrane. MIP has three subfamilies; classical aquaporin (cAQP), aquaglyceroporin, and Super aquaporin (S-aquaporin). The major difference between the three subfamilies is in their sequence signature motifs around the protein pore. Classical aquaporins allow only the passage of water while aquaglyceroporins conduct glycerol, water, and small uncharged solutes. On the other hand, the role of S-aquaporin is currently not well known but it is believed to be involved in cellular differentiation, apoptosis, organogenesis, mating, and intercellular communication; activities that are important in multicellular organism (Ishibashi et al., 2011). There are two types of tick aquaporins. Both Aquaporins (AQP1 and AQP2) help in concentrating blood meals by facilitating the excretion of excess water back into the host through the salivary glands (Knepper, 1994; Ball et al., 2009). AQP1 is expressed in tissues such as the gut, rectal sac, and most abundantly in the tick salivary glands while AQP2 is only expressed in the salivary glands (Campbell et al., 2010; Hussein et al., 2015). Tick AQP1 recombinant antigens have previously been shown to be effective against different I. ricinus and R. microplus stages, highlighting the potential of using AQP1 as a candidate antigen for anti-tick vaccine development (Guerrero et al., 2014; Contreras and de la Fuente, 2017). It is against this background that tick AQP1 is localized in more tissues and is a more efficient water channel than tick AQP2 that we chose to assess tick AQP1 peptide motifs and not those of tick AQP2.
The development of an AQP1-derived anti-tick vaccine remains a challenge mostly due to the high sequence and structural similarities between the tick and host (humans and Bovine) AQP1 orthologs (Guerrero et al., 2014). However, this can potentially be overcome by identifying tick specific immunogenic regions within the AQP1 protein that will allow for a more selective immunogenic response. Furthermore, tick species isolated from different tick endemic regions are required to assess the conservation of these tick AQP1 immunogenic regions. In the study herein, we analyzed tick AQP1 from Ixodid ticks isolated from three different agro-ecological zones in Uganda and bovine AQP1 proteins derived from the Protein Data Bank (PDB) in order to identify tick-specific AQP1-peptide motifs (Berman et al., 2000). The tick-specific AQP1 peptide motifs were mapped onto the modeled 3-dimensional (3D) protein structure and further analyzed to determine their B cell stimulation potential. These results present peptides that might be of value in the development of potential anti-tick AQP1 peptide-based vaccines.
This study involved identification and confirmation of representative tick species collected from cattle sampled from districts along the main cattle keeping agro-ecological zones of Uganda. The AQP1 gene of these ticks was amplified by PCR and confirmed by Sanger sequencing. We used Multiple Sequence Alignment (MSA), motif analysis, homology modeling and structural analysis to identify tick-specific AQP1 peptide motifs. We then used Immune Epitope Database Analysis Resource (IEDB) to assess the immunogenicity of the tick-specific AQP1 peptide motifs (Figure 2).
Ticks were collected from cattle in four districts of Uganda, namely Serere (south-eastern Uganda), Kotido (north-eastern Uganda), Mbarara, and Kiruhuura (south-western Uganda) (Figure 3). The choice of these four districts was 2-fold; they represent the main cattle keeping Agro-ecological zones and have the highest tick burden in the country (MAAIF and UBOS, 2008; Kabi et al., 2014; Vudriko et al., 2016).
Cattle in each village were gathered at a central cattle holding ground where the sampled cattle were physically restrained before a half body tick collection was carried out. Ticks were collected from each of the five predilection sites, namely (i) the inner and outer fore-legs, (ii) hind-legs and abdomen, (iii) tail and anal areas, (iv) head and neck, (v) lateral and dorsal areas, shoulders to tail base and ears. Collected tick specimens were put in different customized tick collection containers covered with gauze. The tick specimens were transferred to Makerere University, College of Veterinary Medicine, Animal Resources and Bio-security, Molecular Biology Laboratory for morphological and molecular identification to species level (Labruna et al., 2009; Farkas et al., 2013; Walker et al., 2014).
Total RNA from homogenized tick samples was extracted using GE Healthcare RNA extraction kit (Chicago, USA). Complementary DNA (cDNA) was synthesized using a GoScript Reverse Transcriptase (Promega, Madison, Wisconsin, USA). Briefly, a mixture of 1 μl of RNA, 5 μl of poly T primers and 14 μl of nuclease-free water were preheated at 70°C for 10 min. The mixture was then placed on ice pending further steps. The enzyme mix (1 × first strand buffer, 4 μl of MgCl2, 2 mM dNTP, 0.51 μl of SUPERase inhibitor, 1 μl of superscript II, 5 μl of the RNA mix, and RNase-free water) was then incubated in a thermocycler according to the manufacturer's cycling conditions; one cycle of 10 min at 25°C, 45 min at 37°C, 45 min at 42°C, followed by 15 min at 70°C. The cDNA was used immediately or stored at −20°C until it was needed for further analysis.
Tick species were confirmed by PCR and Sanger sequencing of 12S ribosomal RNA (12S rRNA) as previously described by Labruna et al. (2009). The 12S PCR was carried out using a single pair of primers (12S-F-AAACTAGGATTAGATACCCT) and (12SR-AATGAGAGCGACGGGCGATGT) that amplify a 300 bp fragment of the 12S ribosomal RNA gene. Briefly, PCR reactions were prepared in 50 μl final reaction volumes that each contained 1.56 U Taq DNA polymerase (New England Biolabs, USA), 1 × standard buffer, 0.25 mM of each dNTP, 0.25 mM of each forward and reverse primer, 1.25 mM MgCl2, PCR-grade water and 5 μl of the cDNA template. The 12S rRNA and tick AQP1 gene PCRs were completed in an S1000 Thermal Cycler (BIO-RAD, California, USA) with an initial denaturation of 94°C for 5 min followed by 30 cycles of 94°C for 30 s, 52°C for 45 s, 72°C for 45 s and a final extension of 72°C for 7 min.
We designed a single pair of primers (AQP1-1F- 5′GCGTGAAGATCAAGAACGCC3′ and AQP1-1R 5′GCCAATTGGAATCGAGGTGC3′) to amplify an 842 bp fragment spanning the entire tick AQP1 gene. The tick AQP1 PCR reactions were performed in an S1000 Thermal Cycler in 50 μl final reaction volume containing 1 × standard buffer, 0.25 mM of each dNTP, 0.25 mM each of forward and reverse primers, 1.56 U Taq DNA polymerase, 1.25 mM MgCl2, 32.18 μl of PCR-grade water and 5 μl of the cDNA template. The thermocycling conditions were; 95°C for 5 min, followed by 30 cycles of 95°C for 30 s, 50°C for 30 s, and 72°C for 1 min, and a final extension step at 72°C for 10 min.
All PCR amplicons were resolved on 2% agarose (Bioline, Memphis, USA) gels stained with GelRed® Nucleic Acid Gel Stain (Biotium, California, USA) and visualized on an ultraviolet transilluminator (Wagtech International, Thatcham, UK) for fragment size determination. The amplicons were sized against a 50 bp DNA molecular ladder (Bioline, Memphis, USA). PCR products were purified from agarose gels using QIAquick® PCR purification kit (Hilden, Germany) according to manufacturer's instructions prior to sequencing. The PCR amplicons were commercially Sanger sequenced at Inqaba Biotechnical Industries (Pty) Ltd (Pretoria, South Africa).
A standard nucleotide BLAST (BLASTn) search was performed for both the tick AQP1 and 12S gene sequences using NCBI against the non-redundant nucleotide DataBank (Altschul et al., 1990). The tick AQP1 gene sequences (S1 Data) were translated into 6 open reading frames using ExPASy translate tool (Gasteiger et al., 2003). Protein-protein BLAST algorithm (BLASTp) was used to search for similar tick AQP1 amino acid sequences against PDB using a BLOSUM62 matrix (Berman et al., 2000; Rédei, 2008). Tick AQP1 amino acids and 12S nucleotide sequences from this study and their respective homologous sequences were retrieved the GenBank (Benson et al., 1999; Consortium, 2009). Multiple Sequence Analyses were carried out using MUSCLE (Edgar et al., 2004). Phylogenetic analyses were performed on both datasets using the maximum likelihood method with 1000 bootstrap in MEGA software version X (Tamura et al., 2013). A Pearson correlation matrix of AQP1 homologs was drawn using R studio using Sequir, ggplot2, and reshape2 packages (R Development Core Team, 2010).
Bovine and tick AQP1 amino acid sequences in FASTA format were analyzed in Multiple Em for Motif Elicitation (MEME) tool Version 5.0.5 (Bailey et al., 2009) using the default settings (http://meme-suite.org/tools/meme). This motif-based sequence analysis tool was set to pick at most 50 motifs which appear in at least 2 amino acid sequences. A motif heatmap was generated using a mast text file from MEME and an in-house python script. Tick-specific AQP1 motifs were analyzed for their conservation among different tick species using WebLogo (Crooks et al., 2004).
The tick AQP1 homotetrameric homology model was calculated utilizing MODELLER version 9.22 using the “automodel” class (Sali and Blundell, 1993). We used a representative tick AQP1 protein sequence from the study (GenBank: MK334178) as the protein query sequence. AQP1 protein structure templates with sequence identities higher than 30% were selected as suitable templates for homology modeling. A total of 100 homology models were calculated for the tick AQP1 homotetrameric protein. The best four models were selected based on the lowest normalized Discrete Optimized Protein Energy (z-DOPE) score (Eswar et al., 2007). The model structures were evaluated using Protein Structure Analysis (ProSA-web) and Ramachandran plot using RAMPAGE in order to select the best model (Sippl, 1993; Kleywegt and Jones, 1996; Lovell et al., 2003; Wiederstein and Sippl, 2007). The tick-specific AQP1 peptide motifs were mapped onto the tick AQP1 homology model using PyMol, Version 1.7.4 (DeLano, 2002; Forlemu et al., 2017). The structure super-imposition of the tick and bovine AQP1 proteins was done using PyMol (DeLano, 2002).
The antigenicity of the tick-specific peptide motifs was analyzed semi-empirically based on methods which use physicochemical properties of amino acids residues and their frequencies of occurrence (Chou and Fasman, 1978; Parker et al., 1986; Kolaskar and Tongaonkar, 1990). Antigenicity analyses were completed using the Immune Epitope Database Analysis Resource (IEDB) (http://tools.iedb.org/main/). Seven IEDB methods were used to evaluate the antigenicity of the tick-specific AQP1 peptide motifs:
i. BepiPred-1.0 linear epitope prediction which predicts linear B-cell epitopes based on Hidden Markov Models (HMM) and a propensity scale. Residues with a score above a 0.350 default threshold were considered to have a high probability of being part of an epitope (Jespersen et al., 2017)
ii. BepiPred-2.0: Sequential B-Cell Epitope Predictor which uses a Random Forest algorithm optimized on epitopes and non-epitope amino acids determined from protein crystal structures. Residues with a score above a 0.500 default threshold were considered to have a high probability of being part of an epitope (Jespersen et al., 2017)
iii. Chou and Fasman Beta-turn prediction which uses the prediction of turns in order to predict epitopes. Residues with a score above a 0.924 threshold were considered to have a high probability of being part of an epitope (Chou and Fasman, 1974)
iv. Emini surface accessibility scale which is calculated based on surface accessibility. A score >1.000 indicates a high probability of the peptide being found on the protein surface (Emini et al., 1985)
v. Karplus and Schulz that determines the flexibility of protein segments based on the B-factors of 31 protein structures. Residues with a score above a 0.962 threshold were considered to have a high probability of being part of an epitope (Karplus and Schulz, 1985)
vi. Kolaskar and Tongaonkar antigenicity scale which uses a semi-empirical method that is based on the physicochemical probabilities of amino acid residues of the protein of interest and their frequencies of occurrence in experimentally known epitopes from other proteins. Residues with a score above a 1.067 threshold were considered to have a high probability of being part of an epitope (Kolaskar and Tongaonkar, 1990)
Parker-hydrophobicity prediction that relies on a hydrophilic scale based on peptide retention times during high-performance liquid chromatography (HPLC) on a reversed-phase column. Residues above −0.326 threshold were considered to have a high probability of being part of an epitope (Parker et al., 1986).
This study was approved by the Makerere University, School of Biosecurity, Biotechnology and Laboratory Sciences Research and Ethics Committee (SBLS/REC/16/136) and Uganda National Council for Science and Technology (A 513). Informed consent was obtained from cattle owners before their cattle were enrolled into the study. All efforts were made to minimize animal stress during cattle restraint and tick collection. These activities were conducted by qualified veterinarians.
The amino acid sequence numbering used in this section is based on tick AQP1 (Accession: MK334178).
Six tick species were morphologically identified and confirmed by 12S rRNA gene sequencing. The 12S rRNA gene sequences formed two distinct clusters with GenBank sequences of the genera Rhipicephalus and Amblyomma (Figure 4). The tick species included Rhipicephalus turanicus, Rhipicephalus muhsamae, and Amblyomma lepidum for Kotido district isolates. Rhipicephalus appendiculatus for Mbarara, Serere, and Kiruhuura district isolates. Rhipicephalus microplus and Amblyomma Variegatum for Serere district isolates.
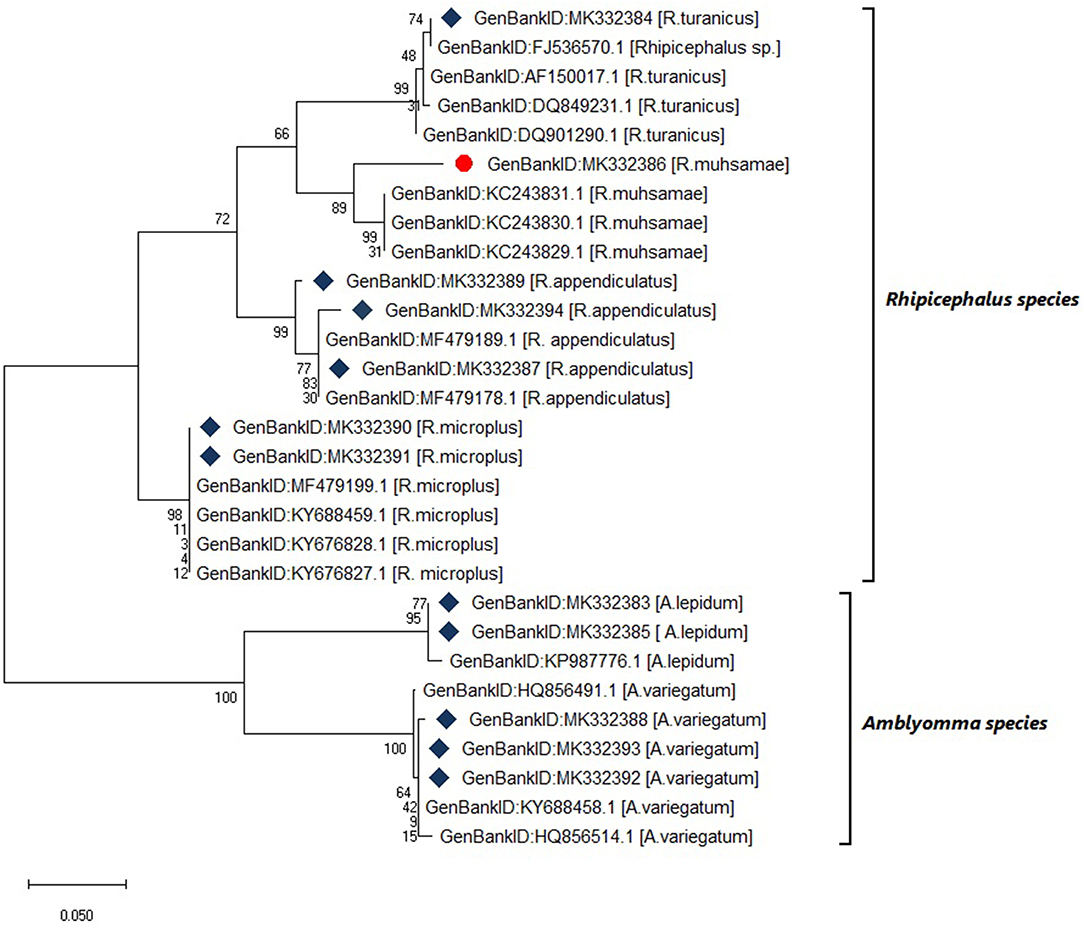
Figure 4. Maximum likelihood phylogenetic tree of tick species based on 12S rDNA gene sequences. Key:  indicates 12S rDNA gene sequences from this study
indicates 12S rDNA gene sequences from this study  and indicates a tick species identified for the first time in Uganda by this study. The phylogenetic tree was constructed using MEGA X software with the maximum likelihood model with 1000 bootstrap. The scale indicates the rate of nucleotide substitution per node.
and indicates a tick species identified for the first time in Uganda by this study. The phylogenetic tree was constructed using MEGA X software with the maximum likelihood model with 1000 bootstrap. The scale indicates the rate of nucleotide substitution per node.
Within the tick AQP1 amino acid sequences, we observed substitution rates ranging between 0.025 and 0.800. Amino acid substitution rates close to 0 indicate high sequence similarity. In contrast, a comparison of ortholog AQP1 and tick AQP1 amino acid sequences showed higher substitution rates ranging between 1.110 and 1.270. The tick AQP1 amino acid sequences grouped in a distinct clade separate from other orthologous AQP1 amino acid sequences (Figure 5).
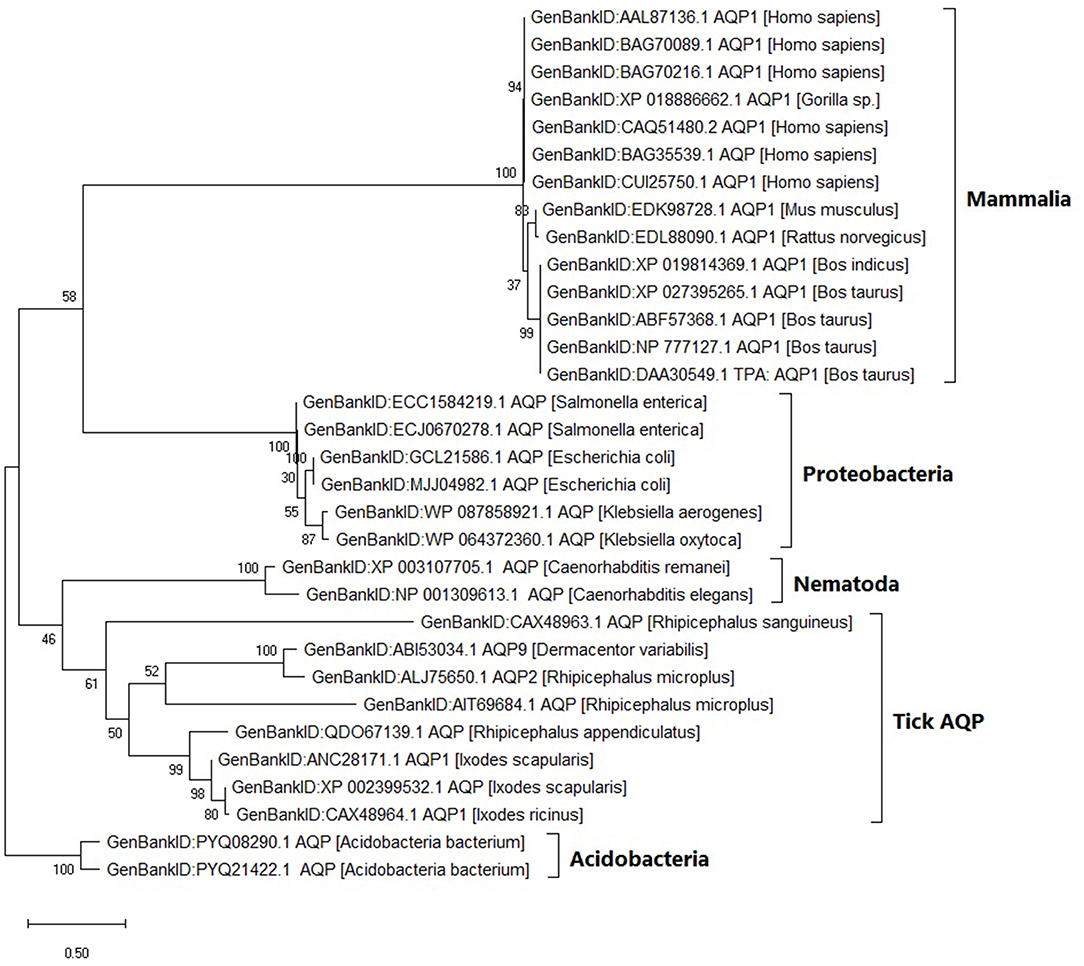
Figure 5. Maximum likelihood phylogenetic tree of AQP1 amino acid sequences from various tick species and other organisms. The tree is drawn to scale, with branch lengths measured in the number of substitutions per site. This analysis involved 32 amino acid sequences. There were a total of 346 positions in the final dataset. Evolutionary analyses were conducted in MEGA X.
The tick AQP1 amino acid sequences and their orthologs were further analyzed using a protein dissimilarity matrix that showed that the tick AQP1 amino acid sequences had positive Pearson correlation coefficients (r) ranging from 0.160 to 1.000 (Figure 6). Tick AQP1 amino acid sequences were negatively correlated to those of bovines and humans (r = −0.4 to −0.6) (Figure 6). Altogether, the phylogenetic and Pearson correlation matrix analyses show that much as AQP1 is conserved among different species, some differences exist between tick AQP1 and bovine AQP1 protein sequences.
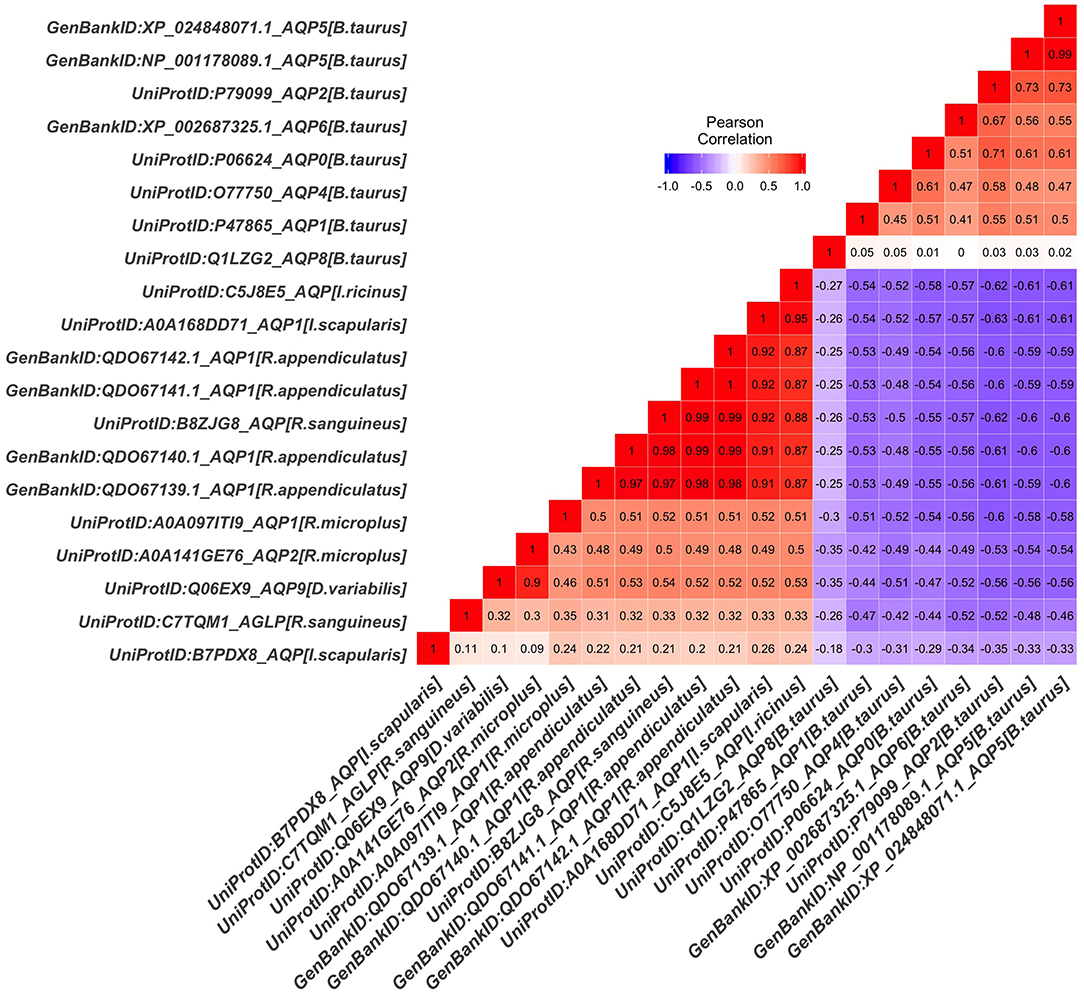
Figure 6. Protein dissimilarity matrix of tick AQP1 amino acid sequences and their orthologs. Blue and red color ranges denote negative and positive correlation respectively The function scale fill gradient2 was used with the argument limit, c (−1,1).
MSA analysis of the tick AQP1 and its bovine orthologs showed local regions with high sequence conservation. The Asparagine-Proline-Alanine (NPA) motif (NPA1: residues 49-51, NPA2: residues 180-181) was conserved in all amino acid sequence orthologs. The NPA is a highly conserved hydrophobic motif that forms part of the pore for each AQP1 monomer (Finn and Cerda, 2015). In addition, tick AQP1 motifs contained an aspartic acid (D) residue after the second NPA motif; a signature for aquaglyceroporins (Figure 7).
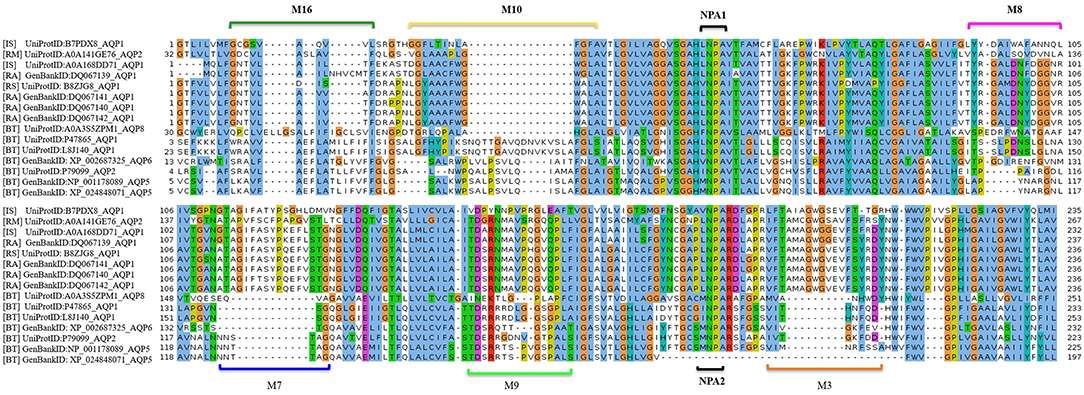
Figure 7. Multiple Sequence Alignment (MSA) of tick and bovine AQP1 amino acids. The NPA1 and NPA2 motifs were conserved among the AQP1 orthologs. Also shown are the tick specific peptide motifs: M3, M7, M8, M9, M10, and M16. The MSA was completed using MUSCLE.
MEME analysis was used to identify tick-specific AQP1 peptide motifs. The tick-specific AQP1 motifs were conserved among the four different tick species (Ixodes scapularis, R. appendiculatus, R. sanguineus, and R. microplus) with E-values ranging from 1.6e-108 to 7.6e-013. These motifs included: M3 (residues 193-212), M7 (residues 106-125), M8 (residues 85-104), M9 (residues 149-168), M10 (residues 12-31), and M16 (residues 2-11) (Figure 8).
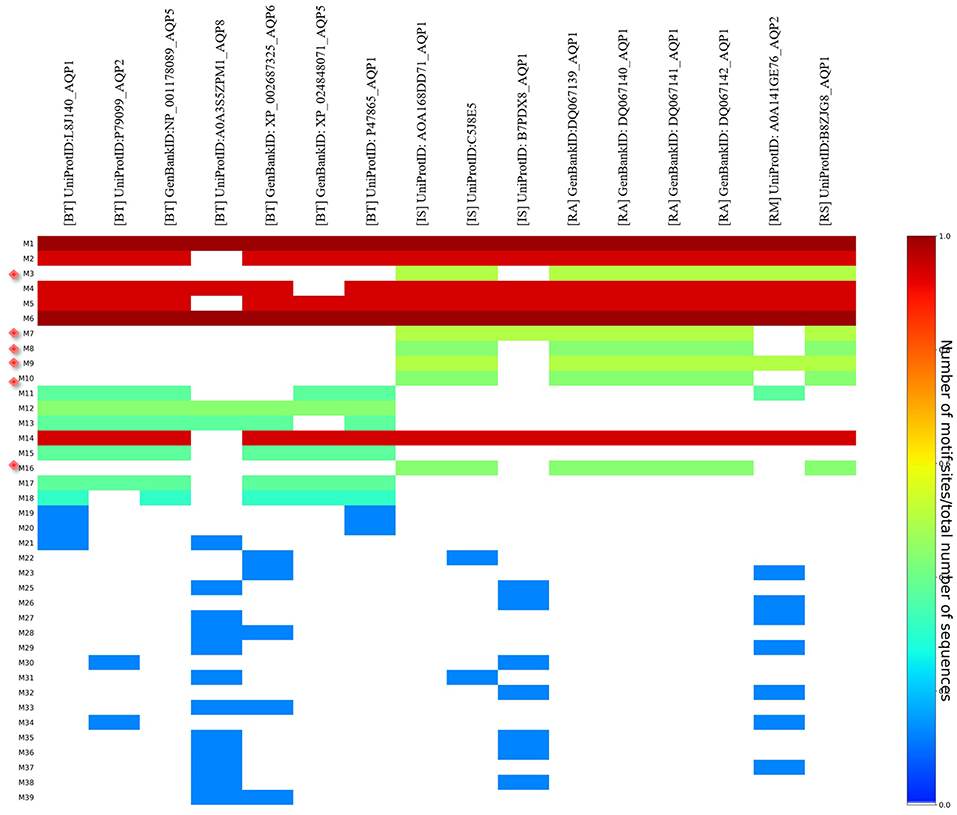
Figure 8. Heat map of tick and bovine AQP1 peptide motifs. Tick specific AQP1 peptide motifs: M3, M7, M8, M9, M10, and M16 are denoted by red diamonds. Six AQP1 peptide motifs; M1, M6, M2, M4, M5, and M14 were common to both tick and bovine AQP1. Key: [BT] stands for B. taurus, [IS] stands or I. scapularis, [RA] stands for R. appendiculatus, [RS] stands for R. sanguineus and [RM] stands for R. microplus.
The position p-value (the probability of a single motif appearing in the observed consensus sequence) of the consensus tick AQP1 sequence (MK334178) further showed this conservation level across all the six motifs. Position p-values <0.0001 were considered significant. All the tick-specific AQP1 peptide motifs had lengths ≥ 19 amino acid residues except M16 whose length was 10 amino acid residues. The motif conservation level was biased to amino acids with similar physicochemical properties. For instance, motifs M3, M9, M10 consisted of a high number of conserved hydrophobic amino acids (residues 16–20, 9–20, and 11–19 respectively) which are colored in black. The most highly conserved motif was M16 with over 70% of its residues being hydrophobic. Most of this motif was located in the transmembrane domain. Motif M7 contained a high number of substitutions all of which were observed in one tick species (I. scapularis: NCBI nucleotide sequence XP002399532) with a p-value of 5.4e-25. Motifs M7 and M8 were largely made up of hydrophilic residues (Figure 9).
Two Escherichia coli strain K12 AQP1 structures, namely 1LDF and 1FX8 were selected as template structures from PDB after a sequence similarity search. Homology model04 which had a z-DOPE score of −0.358 and a ProSA z-score of −3.400 was selected for use in further analyses (S12 Figure). A z-DOPE score equal to or < -1 indicates that the protein model is close to the native structure (Bastianelli et al., 2011) (Table 1). A Ramachandran plot analysis using RAMPAGE showed that model04 had 884 (92.9 %) of its residues in the favored region, 45 (4.7 %) in the allowed region and 23 (2.4 %) in the outlier region (Table 1). See alignment file (PIR) and atomic coordinates of the top four homology models generated in S2–S6 Data.
The NPA1 (chain A residues 49–51) and NPA2 (chain A residues 180–181) motifs are located within the transmembrane domain (Figure 10A). The aromatic-arginine (ar/R) filter was made of residues Trp33, Gly136, Ile171, and Asp188 (Figure 10B). Mapping of the six motifs onto the modeled tick AQP1 protein structure showed that motifs M9, M10, and M16 are located within the transmembrane protein domain while motifs M3, M7, and M8 are located in the extracellular protein domain (Figures 10C,D). Motif M10 was more localized in the inner part of the homotetrameric protein while motif M3, M7, and M8 were located on the extracellular surface of the quaternary structure of the AQP1 protein model (Figures 10C,D). The structural superimposition of the tick AQP1 homology model04 and bovine AQP1 (PDB: 1J4N) showed that the proteins were structurally similar with an RMSD value of 3.269 for the homotetrameric structures and RMSD = 1.475 for similar chain monomers (S13 Figure). The structural similarities were mainly observed in the transmembrane domain while variations were observed in the loops located on the extracellular and cytoplasmic domains of the protein (S13 Figure).
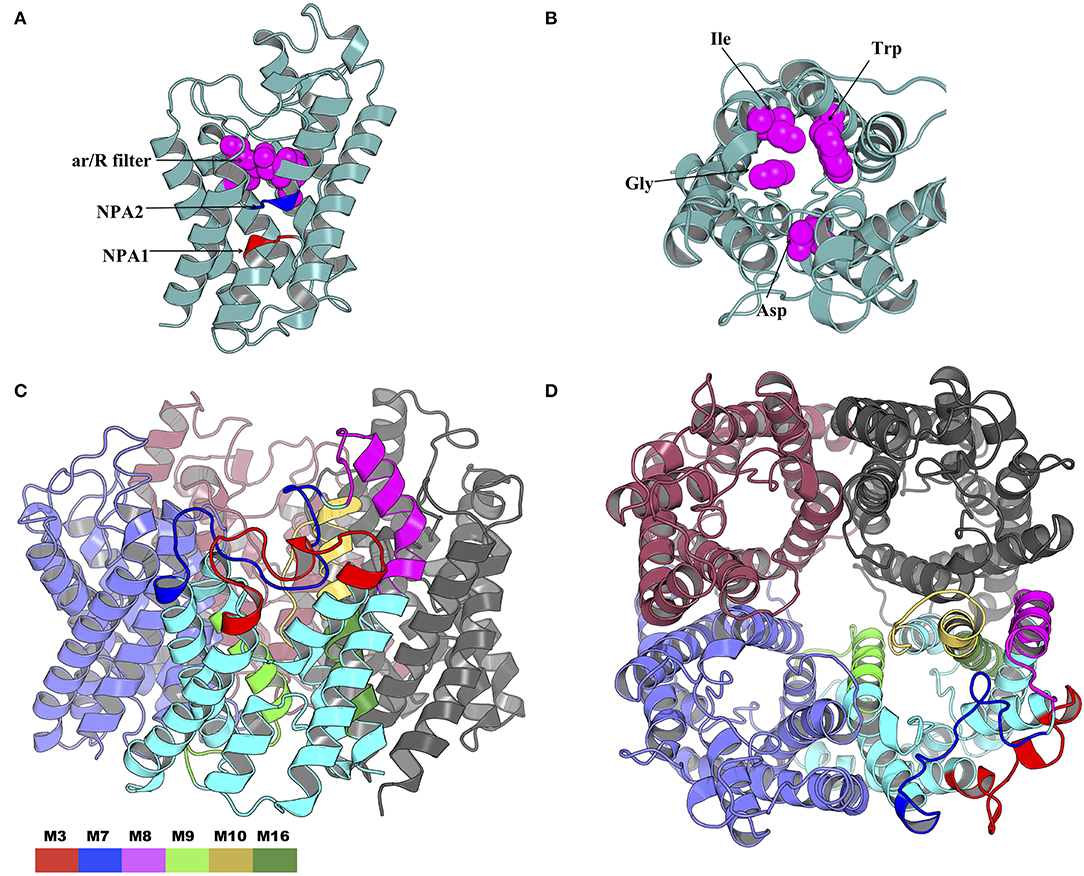
Figure 10. Cartoon rendering of the tick AQP1 homology model and motif mapping. (A) A side-view of the modeled tick AQP1 monomer showing the position of the two Asparagine-Proline-Alanine (NPA) motifs and the aromatic-arginine (ar/R) filter. (B) A top view of the modeled tick AQP1 monomer showing the amino acid sequences that constitute the ar/R filter (chain A residues: Trp33, Gly136, Ile171, and Asp188). (C) A cartoon rendering of the tick AQP1 protein and (D). A surface view rendering of the tick AQP1 protein. Both (C,D) show the mapped tick-specific peptide motifs. Motifs M7 and M8 were located on the protein surface and in the extra-cellular domain while M9, M10, and M6 were located in the transmembrane domain. The motifs were colored as follows: M3- red, M7- blue, M8- purple, M9- light green, M10- yellow, and M16- dark green.
The Immune Epitope Database (IEDB) methods were used to evaluate whether the motifs were located in potential B cell epitopes. The results indicate that motifs M7 and M8 might be potential antigenic peptide epitopes. Motifs M7 and M8 had Mean BepiPred 2.0 Linear Epitope Prediction scores 0.551 and 0.528 that were above the 0.5 threshold value (Figure 11 and Table 2). Motifs M7 and M8 also had mean Parker hydrophilicity scores of 1.784 and 1.536, respectively, that were above the −0.326-threshold score (Figure 12). The two motifs also showed mean Chou and Fasman Beta-Turn Predictions and Karplus and Schulz Flexibility predictions that were slightly above their respective thresholds Motif M7 had a mean BepiPred 1.0 Linear Epitope Prediction of 0.464 that was above the 0.350 threshold value unlike motif M8 that had a mean score of −0.494. Motif M16 which was the shortest peptide motif (Figure 9) and located in the transmembrane region of the protein (Figure 10) was below the thresholds for most of the methods used indicating it was a poor epitope candidate. Other transmembrane peptide motifs such as M9 and M10 were also predicted to be poor epitope candidates (Table 2). Extra supporting information on B cell peptide motif antigenicity has been provided as S7–S11 Figures.
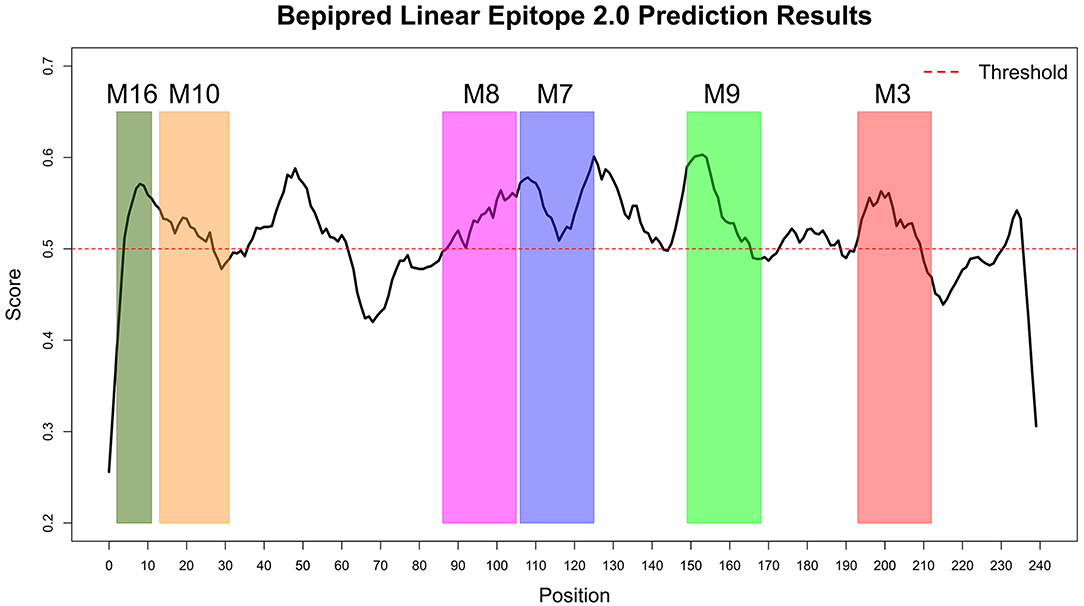
Figure 11. BepiPred 2.0 Linear Epitope Prediction method. All the motif did show potential of being part of the epitope, however motif M7 and M8 were the only motifs with all the residues above the threshold (0.5). Residues with scores above this threshold were considered to have a high probability of being part of an epitope.
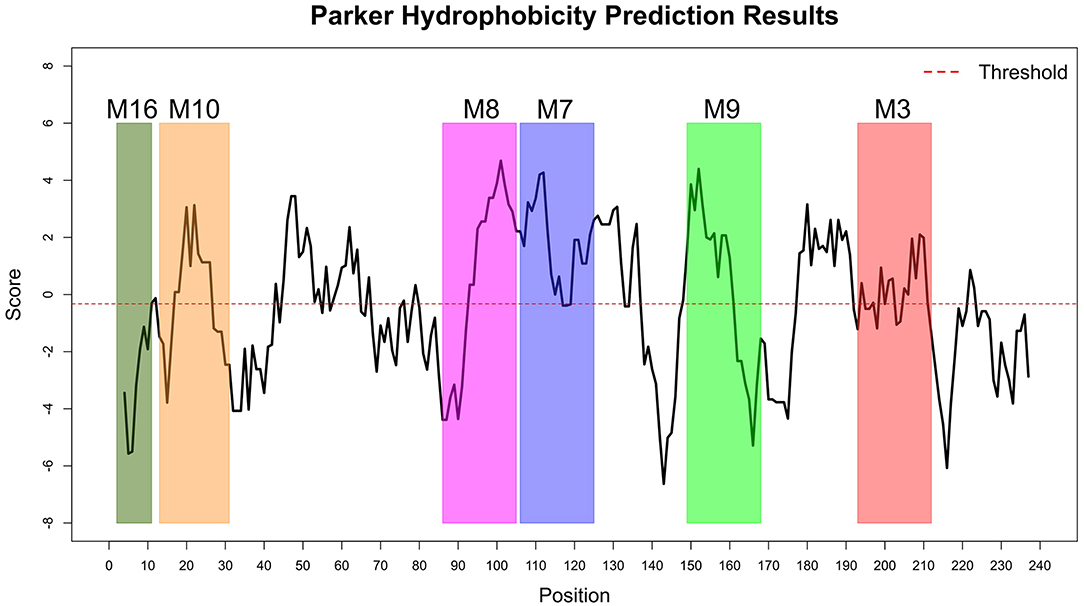
Figure 12. Parker Hydrophilicity Prediction methods. Motif M7, C terminal end of M8, N terminal end of M9, part of M9 and M10 were potentially part of the epitopes with residue scored above the threshold (-0.326). However, motif M16 residues-scores were below the threshold level. Residues with scores above threshold were considered to have a high probability of being part of an epitope.
In this study, a combination of DNA sequencing and in silico-based methods were used to predict tick-specific AQP1 peptide motifs. This involved comparing tick AQP1 protein sequences isolated from four districts in Uganda and AQP1 ortholog sequences from GenBank and PDB. We identified a total of 6 tick-specific AQP1 peptide motifs that were mapped onto the tick AQP1 protein structure (homology model). The predicted motifs were also assessed for their potential of being candidate peptide epitopes using IEDB tools.
The study-tick AQP1 protein sequences showed regions of similarity with AQP1 orthologs (Figure 7). This is due to the fact that AQP1 plays similar functions in the different species. In addition, all AQP proteins have 6 transmembrane alpha helical domains, a signature characteristic to all Major Intrinsic Protein (MIP) superfamily proteins (Finn and Cerda, 2015). The highest AQP1 sequence similarity within the AQP1 orthologs was observed mainly around the Asparagine-Proline-Aline (NPA) motif and aromatic/Arginine (ar/R) selectivity filters (Beitz et al., 2006; Gonen and Walz, 2006; Yakata et al., 2007; Figure 7). These filters slow down the flow of molecules across the protein pore by a Grotthuss mechanism (Beitz et al., 2006; Gonen and Walz, 2006; Yakata et al., 2007). Similar studies have indicated that the arrangement of the ar/R residues directly correlates with the functional properties of the channel (Beitz et al., 2006; Gonen and Walz, 2006; Yakata et al., 2007). Classical water-selective aquaporins (CAQPs) such as AQP1 usually show tight ar/R clusters within which passage of water is allowed while ions and glycerol are blocked (Yakata et al., 2007; Finn and Cerda, 2015; Ishibashi et al., 2017) (Figures 10A,B). The tick AQP1 from this study contained an aspartic acid residue (D) after the second NPA motif which is the signature sequence of Aquaglyceroporin (AQGP) (Figures 7, 10A,B). This finding is in conformity with previous studies which looked at AQP1 proteins from different tick species (Bowman and Sauer, 2004; Guerrero et al., 2014; Évora et al., 2017; Ishibashi et al., 2017). An aspartic acid residue always coexists with a longer loop which increases the pore's permeability to larger molecules such as glycerol (Ishibashi et al., 2011). Despite the presence of a D residue, this functional characterization of tick AQP1 does not warrant transportation of glycerol or urea (Ball et al., 2009). Such tight water transport mechanisms seen in tick AQP1 helps them to concentrate the blood meal component in the midgut by removing only water back into the host via the salivary gland (Bowman and Sauer, 2004).
The high sequence similarity within the AQP1 from all different tick species further emphasizes its potential for use as a catch-all anti-tick vaccine candidate (Contreras and de la Fuente, 2017). Whole tick AQP1 structure super-imposition to the bovine AQP1 protein indicated that the two proteins were structurally similar (RMSD = 3.269 for the homotetramers and RMSD = 1.475 for similar chain monomers). Such similarities to the host protein can affect vaccine efficacy and potentially cause adverse autoimmune effects if vaccinated with the whole AQP1 recombinant protein (Overwijk et al., 1999). This challenge could have been the reason for failure of the recombinant AQP1 protein as vaccine against I. ricinus (Campbell et al., 2010). Future AQP1-anti-tick vaccines therefore should at least be based on reverse vaccinology methods focusing on tick-specific AQP1 peptide motifs that might constitute vaccine development pipeline (Article, 2016). Peptide based vaccines use specific peptide fragments that induce high specific immune responses, thereby increasing vaccine efficacy and reducing potential adverse effects caused by whole protein vaccination (Li et al., 2014). A number of peptide-derived vaccines have been developed, some of these are in clinical trials. A good example is the discovery of neutralizing epitopes to HIV and influenza viruses (Liu et al., 2007; Sui et al., 2009).
MSA of tick AQP1 amino acid sequences and their bovine orthologs highlighted some differences between them (Figure 7). These regions of difference were observed in the loops, particularly those located in the extracellular domain (S13 Figure). Structural mapping of peptide motifs onto the AQP1 homology model protein showed that motifs M9, M16, part of M10 and M3 were all located on tick AQP1 transmembrane domains (Figure 10). Motif modeling and sequence logo identification showed that these motifs contained high number of hydrophobic residues which usually encode for most transmembrane alpha helices (Sonnhammer and Krogh, 1998). This phenomenon is due to the fact that hydrophobic residues are non-polar, thus, they have to fold in such a way that they are buried in the lipid bilayer of the cell membrane (Sonnhammer and Krogh, 1998). Moreover, these motifs (M3, M9, M10, and M16) showed relatively lower potential of being B cell epitope region compared to motifs M7 and M8 (Table 2).
Both motif M7 and M8 were linear epitopes, yet most B cell peptide motif are discontinuous in nature. Nevertheless continuous epitopes can evoke antibodies that can recognize and bind to antigenic proteins, albeit with lower binding affinities (Chen et al., 2009). The motifs identified by this study require further evaluation using wet-lab animal models and in vitro tests in order to determine their immunogenicity.
We identified two motifs (assigned names M7 and M8) that can potentially be incorporated into AQP1 peptide anti-tick vaccine development efforts. The peptide motifs were highly conserved among tick AQP1 and differed from bovine and human AQP1. This indicates that they are an interesting potential targets against a wide range of tick species. We envisage future studies where we can apply the approaches used in this study on the entire tick proteome. This will enable us to identify a larger set of potentially antigenic peptides. We also require wet-lab in vivo and in vitro studies to determine the immunogenicity and efficacy of the tick-specific M7 and M8 peptide motifs. The wet-lab studies can also help determine the conformations the peptides will take in vaccine formulations and the possibility of cross-reactivity with other bovine proteins.
The datasets generated for this study can be found in the NCBI data base under the following accession numbers: MK332384, MK332386, MK332389, MK332394, MK332387, MK332390, MK332391, MK332383, MK332385, MK332388, MK332393, MK332392, MK334175, MK334178, MK334176, MK334177.
The animal study was reviewed and approved by Makerere University, School of Biosecurity, Biotechnology and Laboratory Sciences Research and Ethics Committee (SBLS/REC/16/136) and Uganda National Council for Science and Technology (A 513). Written informed consent was obtained from the owners for the participation of their animals in this study.
Author Magambo Phillip Kimuda is a member of the Trypanogen Consortium (www.trypanogen.net).
CN and DM designed and implemented the study. CN, DM, JN, WA, FM, RT, SO, and MK performed the experiments and data analysis. All authors participated in writing and proofreading the manuscript and approved the final manuscript for publication.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
We are grateful to the different veterinary officers in the 4 districts who helped the core study team in collecting tick samples.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fbioe.2019.00236/full#supplementary-material
S1 Data. Fasta file of tick AQP1 sequences MK334175- MK334178 (R. appendiculatus).
S2 Data. PIR alignment file of MK334178 query sequence and templates (1FX8 and 1LDF).
S3-6 Data. Atom coordinate files for tick AQP1 homology models (model01-model04).
S7 Figure. Chou and Fasman Beta-turn Prediction method. Motif M7, M8, and M9 had the high number of residues above the threshold score (0.924). While Motif M16 had no residues above the threshold. Residues with scores above threshold were considered to have a high probability of being part of an epitope.
S8 Figure. Karplus and Schulza Flexibility Prediction method. All the Motif had high residues above the threshold (1) except M3 and M16 which had higher residues below the threshold. Residues with scores above threshold were considered to have a high probability of being part of an epitope.
S9 Figure. Bepipred 1.0 Linear Epitope Prediction method. Motif M7 and the C-terminal end of M8 shown potential of being part of the peptide with antigenicity score above the threshold level (0.35). residues with scores above threshold were considered to have a high probability of being part of an epitope.
S10 Figure. Emini Surface Accessibility Prediction method. All the motif except motif M16 had high number of residues above the threshold score (0.924). Residues with scores above threshold were considered to have a high probability of being part of an epitope.
S11 Figure. Kolaskar and Tongaonkar Antigenicity Prediction method. Motif M16 followed by part of motif M10 and M8 at the C terminal end had values above the threshold (−0.326). Motif M7, M9, and M3 had a high number of residues below the threshold. Residues with scores above threshold were considered to have a high probability of being part of an epitope.
S12 Figure. Evaluation of the top tick AQP1 homology model (model04) using ProSA. (A) Model04 had a ProSA z-score of−3.400 that clustered well with experimentally determined models, and (B) A plot of residue scores of model04.
S13 Figure. Structural superimposition of tick AQP1 homology model and bovine AQP1 (PDB: 1J4N). Structural superimposition of homotetrameric structures gave an RMSD = 3.269 while superimposition of a single chain monomer gave an RMSD = 1.475.
Abbas, R. Z., Zaman, M. A., Colwell, D. D., Gilleard, J., and Iqbal, Z. (2014). Acaricide resistance in cattle ticks and approaches to its management: the state of play. Vet. Parasitol. 203, 6–20. doi: 10.1016/j.vetpar.2014.03.006
Altschul, S. F., Gish, W., Miller, W., Myers, E. W., and Lipman, D. J. (1990). Basic local alignment search tool. J. Mol. Biol. 215, 403–410. doi: 10.1016/S0022-2836(05)80360-2
Article, C. R. (2016). Strategies for new and improved vaccines against ticks and tick-borne diseases. Parasite Immunol. 38, 754–769. doi: 10.1111/pim.12339
Bailey, T. L., Boden, M., Buske, F. A., Frith, M., Grant, C. E., Clementi, L., et al. (2009). MEME S UITE : tools for motif discovery and searching. Nucleic Acids Res. 37, 202–208. doi: 10.1093/nar/gkp335
Ball, A., Campbell, E. M., Jacob, J., Hoppler, S., and Bowman, A. S. (2009). Identification, functional characterization and expression patterns of a water-specific aquaporin in the brown dog tick, Rhipicephalus sanguineus. Insect Biochem. Mol. Biol. 39, 105–112. doi: 10.1016/j.ibmb.2008.10.006
Bastianelli, G., Bouillon, A., Nguyen, C., Crublet, E., Pêtres, S., Gorgette, O., et al. (2011). Computational reverse-engineering of a spider-venom derived peptide active against plasmodium falciparum SUB1. PLoS ONE 6:e21812. doi: 10.1371/journal.pone.0021812
Beitz, E., Wu, B., Holm, L. M., Schultz, J. E., and Zeuthen, T. (2006). Point mutations in the aromatic arginine region in aquaporin 1 allow passage of urea, glycerol, ammonia, and protons. Proc. Natl. Acad. Sci. U.S.A. 103, 269–274. doi: 10.1073/pnas.0507225103
Benson, D. A., Boguski, M. S., Lipman, D. J., Ostell, J., Ouellette, B. F. F., Rapp, B. A., et al. (1999). GenBank. Nucleic Acids Res. 27, 12–17. doi: 10.1093/nar/27.1.12
Berman, H. M., Westbrook, J., Feng, Z., Gilliland, G., Bhat, T. N., Weissig, H., et al. (2000). The protein data bank. Nucleic Acids Res. 28, 235–242. doi: 10.1093/nar/28.1.235
Bowman, A. S., and Sauer, J. R. (2004). Tick salivary glands: function, physiology and future. Parasitology 129, S67–81. doi: 10.1017/S0031182004006468
Campbell, E. M., Burdin, M., Hoppler, S., and Bowman, A. S. (2010). Role of an aquaporin in the sheep tick Ixodes ricinus: assessment as a potential control target. Int. J. Parasitol. 40, 15–23. doi: 10.1016/j.ijpara.2009.06.010
Chen, S. W., Regenmortel, M. H. V., and Van Pellequer, J. (2009). Structure-activity relationships in peptide-antibody complexes: implica- tions for epitope prediction and development of synthetic peptide vaccines. Curr. Med. Chem. 16, 953–964. doi: 10.2174/092986709787581914
Chizyuka, H. G., and Mulilo, J. B. (1990). Methods currently used for the control of multi-host ticks: their validity and proposals for future control strategies. Parassitologia 32, 127–132.
Chou, P., and Fasman, G. (1978). Prediction of the secondary structure of proteins from their amino acid sequence. Adv. Enzym. Relat. Areas Mol. Biol. 47, 45–148. doi: 10.1002/9780470122921.ch2
Chou, P. Y., and Fasman, G. D. (1974). Prediction of protein conformation. Biochemistry. 13, 222–45. doi: 10.1021/bi00699a002
Consortium, U. (2009). The universal protein resource (UniProt) 2009. Nucleic Acids Res. 37, D169–74. doi: 10.1093/nar/gkn664
Contreras, M., and de la Fuente, J. (2017). Control of infestations by Ixodes ricinus tick larvae in rabbits vaccinated with aquaporin recombinant antigens. Vaccine 35, 1323–1328. doi: 10.1016/j.vaccine.2017.01.052
Crooks, G., Hon, G., Chandonia, J., and Brenner, S. (2004). NCBI GenBank FTP Site nWebLogo: a sequence logo generator. Genome Res. 14, 1188–1190. doi: 10.1101/gr.849004
DeLano, W. L. (2002). The PyMOL Molecular Graphics System. Schrödinger LLC. Available online at: wwwpymolorg Version 1; https://pymol.org/2/
Domingos, A., Antunes, S., Borges, L., and Do Rosário, V. E. (2013). Approaches towards tick and tick-borne diseases control. Rev. Soc. Bras. Med. Trop. 46, 265–269. doi: 10.1590/0037-8682-0014-2012
Edgar, R. C., Drive, R. M., and Valley, M. (2004). MUSCLE : multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 32, 1792–1797. doi: 10.1093/nar/gkh340
Emini, E. A., Hughes, J. V., Perlow, D. S., and Boger, J. (1985). Induction of hepatitis A virus-neutralizing antibody by a virus-specific synthetic peptide. J. Virol. 55, 836–839.
Eskezia, B. G., and Desta, A. H. (2016). Review on the impact of ticks on livestock health and productivity. J. Biol. Agric. Healthc. 6, 1–7.
Eswar, N., Webb, B., Marti-Renom, M. A., Madhusudhan, M. S., Eramian, D., Shen, M., et al. (2007). “Comparative protein structure modeling using MODELLER,” in Current Protocols in Bioinformatics, 50, 2.9.1–2.9.31. doi: 10.1002/0471140864.ps0209s50
Évora, P. M., Sanches, G. S., Guerrero, F. D., León, A. P., and Bechara, G. H. (2017). Immunogenic potential of Rhipicephalus (Boophilus) microplus aquaporin 1 against Rhipicephalus sanguineus in domestic dogs. Vet. Parasitol. 26, 60–66. doi: 10.1590/S1984-29612017005
Farkas, R., Estrada-Peña, A., Jaenson, T. G. T., Pascucci, I., and Madder, M. (2013). “Basic biology and geographical distribution of tick species involved in the transmission of animal pathogens, including zoonoses,” in Ticks and Tick-Borne Diseases: Geographical Distribution and Control Strategies in the Euro-Asia Region, eds M. Salman and J. Tarrés-Call (CABI Publishing), 6–26.
Finn, R. N., and Cerda, J. (2015). Evolution and functional diversity of aquaporins. Biol. Bull. 229, 6–23. doi: 10.1086/BBLv229n1p6
Forlemu, N., Watkins, P., and Sloop, J. (2017). Molecular docking of selective binding affinity of sulfonamide derivatives as potential antimalarial agents targeting the glycolytic enzymes : GAPDH, Aldolase and TPI. Open J. Biophys. 7, 41–57. doi: 10.4236/ojbiphy.2017.71004
Gasteiger, E., Gattiker, A., Hoogland, C., Ivanyi, I., Appel, R. D., and Bairoch, A. (2003). ExPASy: the proteomics server for in-depth protein knowledge and analysis. Nucleic Acids Res. 31, 3784–3788. doi: 10.1093/nar/gkg563
Ghosh, S. P., and Azhahianambi, M. P. Y. (2007). Upcoming and future strategies of tick control: a review. J. Vector Borne Dis. 44, 79–89. Available online at: http://quod.lib.umich.edu/cgi/p/pod/dod-idx?c=icmc;idno=bbp2372.2000.228
Gonen, T., and Walz, T. (2006). The structure of aquaporins. Q. Rev. Biophys. 39, 361–396. doi: 10.1017/S0033583506004458
Guerrero, F. D., Andreotti, R., Bendele, K. G., Cunha, R. C., Miller, R. J., Yeater, K., et al. (2014). Rhipicephalus (Boophilus) microplus aquaporin as an effective vaccine antigen to protect against cattle tick infestations. Parasit. Vectors 7:475. doi: 10.1186/PREACCEPT-7072367031355619
Hussein, H. E., Scoles, G. A., Ueti, M. W., Suarez, C. E., Adham, F. K., Guerrero, F. D., et al. (2015). Targeted silencing of the aquaporin 2 gene of Rhipicephalus (Boophilus) microplus reduces tick fitness. Parasit. Vectors 8:618. doi: 10.1186/s13071-015-1226-2
Ishibashi, K., Kondo, S., Hara, S., and Morishita, Y. (2011). The evolutionary aspects of aquaporin family. Am. J. Physiol. Regul. Integr. Comp. Physiol. 300, 566–576. doi: 10.1152/ajpregu.90464.2008
Ishibashi, K., Morishita, Y., and Tanaka, Y. (2017). “The evolutionary aspects of aquaporin family,” in Aquaporins (Advances in Experimental Medicine and Biology), Vol. 969, ed B. Yang (Dordrecht: Springer). doi: 10.1007/978-94-024-1057-0_2
Jespersen, M. C., Peters, B., Nielsen, M., and Marcatili, P. (2017). BepiPred-2.0: improving sequence-based B-cell epitope prediction using conformational epitopes. Nucleic Acids Res. 45, W24–W29. doi: 10.1093/nar/gkx346
Jongejan, F., and Uilenberg, G. (2004). The global importance of ticks. Parasitology 129, S3–14. doi: 10.1017/S0031182004005967
Kabi, F., Masembe, C., Muwanika, V., Kirunda, H., and Negrini, R. (2014). Geographic distribution of non-clinical Theileria parva infection among indigenous cattle populations in contrasting agro-ecological zones of Uganda: implications for control strategies. Parasit. Vectors 7:414. doi: 10.1186/1756-3305-7-414
Karplus, P. A., and Schulz, G. E. (1985). Prediction of chain flexibility in proteins - A tool for the selection of peptide antigens. Naturwissenschaften 72, 212–213. doi: 10.1007/BF01195768
Kleywegt, G. J., and Jones, T. A. (1996). Phi/Psi-chology: Ramachandran revisited. Structure 4, 1395–1400. doi: 10.1016/S0969-2126(96)00147-5
Knepper, M. A. (1994). The aquaporin family of molecular water channels. Proc Natl Acad Sci U.S.A. 91, 6255–6258. doi: 10.1073/pnas.91.14.6255
Kolaskar, a, S., and Tongaonkar, P. C. (1990). A semi-empirical method for prediction of antigenic detetermininants on protein antigens. Febbs Lett. 276, 172–174. doi: 10.1016/0014-5793(90)80535-Q
Labruna, M. B., Naranjo, V., Mangold, A. J., Thompson, C., Estrada-Pẽa, A., Guglielmone, A. A., et al. (2009). Allopatric speciation in ticks: genetic and reproductive divergence between geographic strains of Rhipicephalus (Boophilus) microplus. BMC Evol. Biol. 9:46. doi: 10.1186/1471-2148-9-46
Li, W., Joshi, M., Singhania, S., Ramsey, K., and Murthy, A. (2014). Peptide vaccine: progress and challenges. Vaccines 2, 515–536. doi: 10.3390/vaccines2030515
Liu, Y., McNevin, J., Zhao, H., Tebit, D. M., Troyer, R. M., McSweyn, M., et al. (2007). Evolution of human immunodeficiency virus type 1 cytotoxic T-lymphocyte epitopes: fitness-balanced escape. J. Virol. 81, 12179–12188. doi: 10.1128/jvi.01277-07
Lovell, S. C., Davis, I. W., Iii, W. B. A., de Bakker, P. I. W., Word, J. M., Prisant, M. G., et al. (2003). Structure validation by Calpha geometry: Phi,psi and Cbeta deviation. Proteins 50, 437–450. doi: 10.1002/prot.10286
Overwijk, W. W., Lee, D. S., Surman, D. R., Irvine, K. R., Touloukian, C. E., Chan, C. C., et al. (1999). Vaccination with a recombinant vaccinia virus encoding a “self” antigen induces autoimmune vitiligo and tumor cell destruction in mice: requirement for CD4(+) T lymphocytes. Proc. Natl. Acad. Sci. U.S.A. 96, 2982–2987. doi: 10.1073/pnas.96.6.2982
Parizi, L. F., Githaka, N. W., Logullo, C., Konnai, S., Masuda, A., Ohashi, K., et al. (2012). The quest for a universal vaccine against ticks: cross-immunity insights. Vet. J. 194, 158–165. doi: 10.1016/j.tvjl.2012.05.023
Parker, J., Guo, D., and Hodges, R. (1986). New hydrophilicity scale derived from high-performance liquid chromatography peptide retention data: correlation of predicted surface residues with antigenicity and X-ray-derived accessible sites. Biochemistry 25, 5425–5432. doi: 10.1021/bi00367a013
R Development Core Team (2010). R: A Language and Environment for Statistical Computing. Available online at: http://www.r-project.org/
Rédei, G. P. (2008). “BLASTP,” in Encyclopedia of Genetics, Genomics, Proteomics and Informatics (Dordrecht: Springer Netherlands), 221. doi: 10.1007/978-1-4020-6754-9_1881
Rodríguez, M., Massard, C. L., da Fonseca, A. H., Ramos, N. F., Machado, H., Labarta, V., et al. (1995). Effect of vaccination with a recombinant Bm86 antigen preparation on natural infestations of boophilus microplus in grazing dairy and beef pure and cross-bred cattle in Brazil. Vaccine 13, 1804–1808. doi: 10.1016/0264-410X(95)00119-L
Sali, A., and Blundell, T. L. (1993). Comparative protein modelling by satisfaction of spatial restraints. Unit Struct. Mol. Biol. 234, 779–815. doi: 10.1006/jmbi.1993.1626
Sambri, V., Marangoni, A., Storni, E., Cavrini, F., Moroni, A., Sparacino, M., et al. (2004). [Tick borne zoonosis: selected clinical and diagnostic aspects]. Parassitologia 46, 109–113.
Sippl, M. J. (1993). Recognition of errors in the three-dimensional structures. Proteins Struct. Funct. Genet. 17, 355–362. doi: 10.1002/prot.340170404
Sonnhammer, E. L. L., and Krogh, A. (1998). A hidden Markov model for predicting transmembrane helices in protein sequences. Proc. Int. Conf. Intell. Syst. Mol. Biol. 6, 175–182.
Sui, J., Hwang, W. C., Prez, S., and Ge, W. (2009). Structural and functional bases for broad-spectrum neutralization of avian and human influenza A viruses. Nat. Struct. Mol. Biol. 16, 256–273. doi: 10.1038/nsmb.1566
Tamura, K., Stecher, G., Peterson, D., Filipski, A., and Kumar, S. (2013). Molecular evolutionary genetics analysis version 6.0. Mol. Biol. Evol. 30, 2725–2729. doi: 10.1093/molbev/mst197
Telmadarraiy, Z., Chinikar, S., Vatandoost, H., and Faghihi, F. (2015). Vectors of crimean congo hemorrhagic fever virus in Iran A historical perspective of CCHF disease in Iran. J. Arthropod. Borne Dis. 9, 137–147.
Vudriko, P., Okwee-Acai, J., Tayebwa, D. S., Byaruhanga, J., Kakooza, S., Wampande, E., et al. (2016). Emergence of multi-acaricide resistant Rhipicephalus ticks and its implication on chemical tick control in Uganda. Parasit. Vectors 9:4. doi: 10.1186/s13071-015-1278-3
Walker, A. R., Bouattor, A., Camicas, J. L., Estrada-Pena, A., Horak, I. G., Latiff, A. A, et al. (2014). Ticks of Domestic Animals in Africa: A Guide to Identification of Species. Bioscience Reports. Available online at: https://books.google.co.ug/books/about/Ticks_of_domestic_animals_in_Africa.html?id=XZMOAQAAMAAJ&pgis=1 (accessed March 8, 2016).
Wiederstein, M., and Sippl, M. J. (2007). ProSA-web: interactive web service for the recognition of errors in three-dimensional structures of proteins. Nucleic Acids Res. 35, W407–410. doi: 10.1093/nar/gkm290
Wikel, S. K. (2018). Ticks and tick-borne infections: complex ecology, agents, and host interactions. Vet. Sci. 5:60. doi: 10.3390/vetsci5020060
Keywords: antigenicity, aquaporin-1 protein, tick control, peptide motifs, cattle
Citation: Ndekezi C, Nkamwesiga J, Ochwo S, Kimuda MP, Mwiine FN, Tweyongyere R, Amanyire W and Muhanguzi D (2019) Identification of Ixodid Tick-Specific Aquaporin-1 Potential Anti-tick Vaccine Epitopes: An in-silico Analysis. Front. Bioeng. Biotechnol. 7:236. doi: 10.3389/fbioe.2019.00236
Received: 13 June 2019; Accepted: 10 September 2019;
Published: 26 September 2019.
Edited by:
Harinder Singh, J. Craig Venter Institute, United StatesReviewed by:
Hifzur Rahman Ansari, King Abdullah International Medical Research Center KAIMRC, Saudi ArabiaCopyright © 2019 Ndekezi, Nkamwesiga, Ochwo, Kimuda, Mwiine, Tweyongyere, Amanyire and Muhanguzi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Dennis Muhanguzi, bHVja3lkZW5ub0Bjb3ZhYi5tYWsuYWMudWc=
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.