- School of Software, Henan Polytechnic University, Jiaozuo, China
The identification of cancer subtypes plays a very important role in the field of medicine. Accurate identification of cancer subtypes is helpful for both cancer treatment and prognosis Currently, most methods for cancer subtype identification are based on single-omics data, such as gene expression data. However, multi-omics data can show various characteristics about cancer, which also can improve the accuracy of cancer subtype identification. Therefore, how to extract features from multi-omics data for cancer subtype identification is the main challenge currently faced by researchers. In this paper, we propose a cancer subtype identification method named CAEM-GBDT, which takes gene expression data, miRNA expression data, and DNA methylation data as input, and adopts convolutional autoencoder network to identify cancer subtypes. Through a convolutional encoder layer, the method performs feature extraction on the input data. Within the convolutional encoder layer, a convolutional self-attention module is embedded to recognize higher-level representations of the multi-omics data. The extracted high-level representations from the convolutional encoder are then concatenated with the input to the decoder. The GBDT (Gradient Boosting Decision Tree) is utilized for cancer subtype identification. In the experiments, we compare CAEM-GBDT with existing cancer subtype identifying methods. Experimental results demonstrate that the proposed CAEM-GBDT outperforms other methods. The source code is available from GitHub at https://github.com/gxh-1/CAEM-GBDT.git.
1 Introduction
As a branch of the disease, cancer is a complex genomic disease that can occur in various parts of the human body and has a great impact on human life. Therefore, cancer treatment and prognosis are very important, but accurate identification of cancer subtypes is an important prerequisite when cancer treatment and prognosis are carried out (Guo et al., 2023). Patients with the same subtype of cancer may behave differently (Alexander et al., 2022), but they share common characteristics that can be used to identify them. Accurate identification of cancer subtypes is conducive to exploring the pathogenesis of cancer, plays a decisive role in treatment and prognosis (Zhang et al., 2022), greatly increases the cure rate of patients, and promotes the further development of research on cancer subtype identification (Wang et al., 2020).
The mainstream bioinformatics data used by researchers is multiple types of omics data based on tumors. These data express cancer from different perspectives. Multi-omics data include genome, transcriptome, epigenome, proteome, exposome, and microbiome (Ao et al., 2022). Multi-omics data analysis can reveal different aspects of the same sample and provide additional beneficial information for research. Therefore, compared with a single type of gene expression data, methods based on multi-omics data can more accurately identify cancer subtypes.
Gene expression data in bioinformatics refers to the data that describe the expression levels of genes under specific conditions or in specific cell types. Gene expression is the process by which genetic information is transcribed into RNA and translated into proteins. miRNA molecules participate in the regulation of gene expression mainly by binding to target mRNA, leading to their degradation or translation inhibition, thereby regulating gene expression. DNA methylation data provides information about the methylation status in the genome. These data can reveal which genes or gene regions are methylated under specific conditions or in different types of cells. DNA methylation data plays a crucial role in studying gene regulation. The relationship between these three types of omics data is inseparable. They influence each other, and the relationship between them can be extracted through models to achieve the identification of cancer subtypes. Therefore, ultimately, these three types of omics data are adopted as inputs to the model.
The main challenge currently is how to extract the information related with cancer subtypes from multi-omics data. Meanwhile, the dataset about cancer patients usually includes a small number of samples, and the samples have very high dimensions (Brigham et al., 2012; Hammerman et al., 2012; Muzny et al., 2012).
Most of the existing methods are based on unsupervised learning clustering or supervised learning classification, using multi-omics data to achieve cancer subtype identification. It can be divided into early integration, intermediate integration and late integration (Lipkova et al., 2022) according to the different focus of the identification method. Pre-integration simply links mult-omics data into one matrix, and then processes the unified matrix. These methods commonly exacerbated the problem of dimensionality explosion, making imbalanced phenomenon of sample size and dimensionality even more serious. The representative methods include K-means, Spectral clusting and LRAcluster (low-rank-approximation-based multi-omics data clustering) (Wu et al., 2015). The characteristic of this type of method is that it is easy to ignore the local characteristics of some omics data (Zhao et al., 2023).
The method of post-integration is different from the method of pre-integration. Post-integration processes each omics data separately and integrates the clustering results to obtain the final clustering solution. Post-integration effectively avoids the mistakes of pre-integration. Clustering is performed on each omics data to ensure the influence of weak signals. Such as moBRCA-net (Choi and Chae, 2023) and Subtype-WESLR (Song et al., 2022), but post-integration also has its own shortcomings. When multiple omics data have different contributions to the clustering results, it will have a great impact on the performance of these method. So both pre-integration and post-integration have a common characteristic, they are unable to express interactions between different omics data. Therefore, mid-integration has gradually become a favored object among researchers (Kang et al., 2022).
Mid-integration achieves data merging and dimensionality reduction by establishing an unified model. Not simply linked data or linked feature matrices. iCluster Bayes (Mo et al., 2018) and Cascade Deep Forest (El-Nabawy et al., 2021) present statistical ensemble methods to solve ensemble challenges. These methods model the distribution of each data type and then maximize the likelihood of multi-omics data based on joint latent variables. However, due to the complexity of multi-omics data, traditional statistical or mathematical models still face huge challenges in accurately modeling high-dimensional multi-omics data. The earliest method SNF (Wang et al., 2014), ERDCN (Lei et al., 2022) process the multi-omics data by constructing a sample similarity network about the co-expression patterns of cancer genes. However, these methods are susceptible to data noise and feature heterogeneity.
In recent years, deep learning has become more and more widely used in the field of medical care and has become a popular method favored by researchers (Dai et al., 2021). Many of these models have achieved good results in the field of cancer subtype identification, such as HI-DFN Forest (Xu et al., 2019), Subtype-GAN (Yang et al., 2021) and SADLN (Sun et al., 2023). The HI-DFN Forest employs a stacked autoencoder to learn advanced representations from each omics data, and then integrates all the learned representations into an autoencoder layer to learn complex representations. The DFN Forest model classifies (Zhou and Feng, 2019) patients into different cancer subtypes. Subtype-GAN utilizes a generative adversarial network to extract features from omics data through relatively independent layers and simultaneously input the extracted information into a shared layer for data integration. Consensus GMM clustering is used to predict cancer subtype outcomes. SADLN combines the encoder, self-attention, decoder, and discriminator into a unified framework, leveraging the integrated representations learned from the network, and utilizes Gaussian mixture models to identify cancer subtypes.
In recent years, convolutional autoencoders have shown good performance in reducing the dimensionality of high-dimensional data (Naderan and Zaychenko, 2020). applied convolutional autoencoders to the field of cancer subtype identifying and conducted comparative experiments with regular autoencoders on image datasets. The experimental results indicate that convolutional autoencoders achieve better dimensionality reduction compared to traditional autoencoders.
In this paper, we propose a method called CAEM-GDBT, which combines a convolutional autoencoder, a convolutional self-attention module, and a GBDT classifier to accomplish the classification of cancer subtypes. CAEM-GBDT is dedicated to establishing an integrated framework that includes an encoder, decoder, attention module, and identification module, enabling joint training and optimization. Compared to existing methods, it requires fewer computational resources and offers higher accuracy. The limitation of early integration is that the feature extraction module and the subsequent clustering module are relatively independent. In contrast, in CAEM-GBDT, the feature extraction module is tightly connected with the identification module. The drawback of late integration is that when extracting features, weights are introduced to process multi-omics data, which can easily overlook the interactions between different types of omics data. CAEM-GBDT employs a convolutional attention module mechanism, processing features from both channel and spatial perspectives, fully leveraging the relationships between different omics data. It treats multi-omics data as a whole for feature extraction. In summary, CAEM-GBDT can overcome the limitations of both early and late integration.
2 Methods
We divided the entire method into three modules: data preprocessing module, feature extraction module, and identifying module. As shown in Figure 1, in the data preprocessing module, we preprocess multi-omics data and use it as input for the next module. In the feature extraction module, we use a combination of a convolutional autoencoder and a convolutional attention module for extracting important features about cancer subtypes. In the convolutional autoencoder. We use one-dimensional convolutional layers to replace some of the fully connected layers in a regular encoder. By employing one-dimensional convolution, we extract low-dimensional key features from the data. This feature is then connected to the output of the decoder to form a matrix, and this combined input is fed into the classifier section. In the identifying module, a GBDT classifier is used for cancer subtype identifying. This entire process is referred to as CAEM-GBDT.
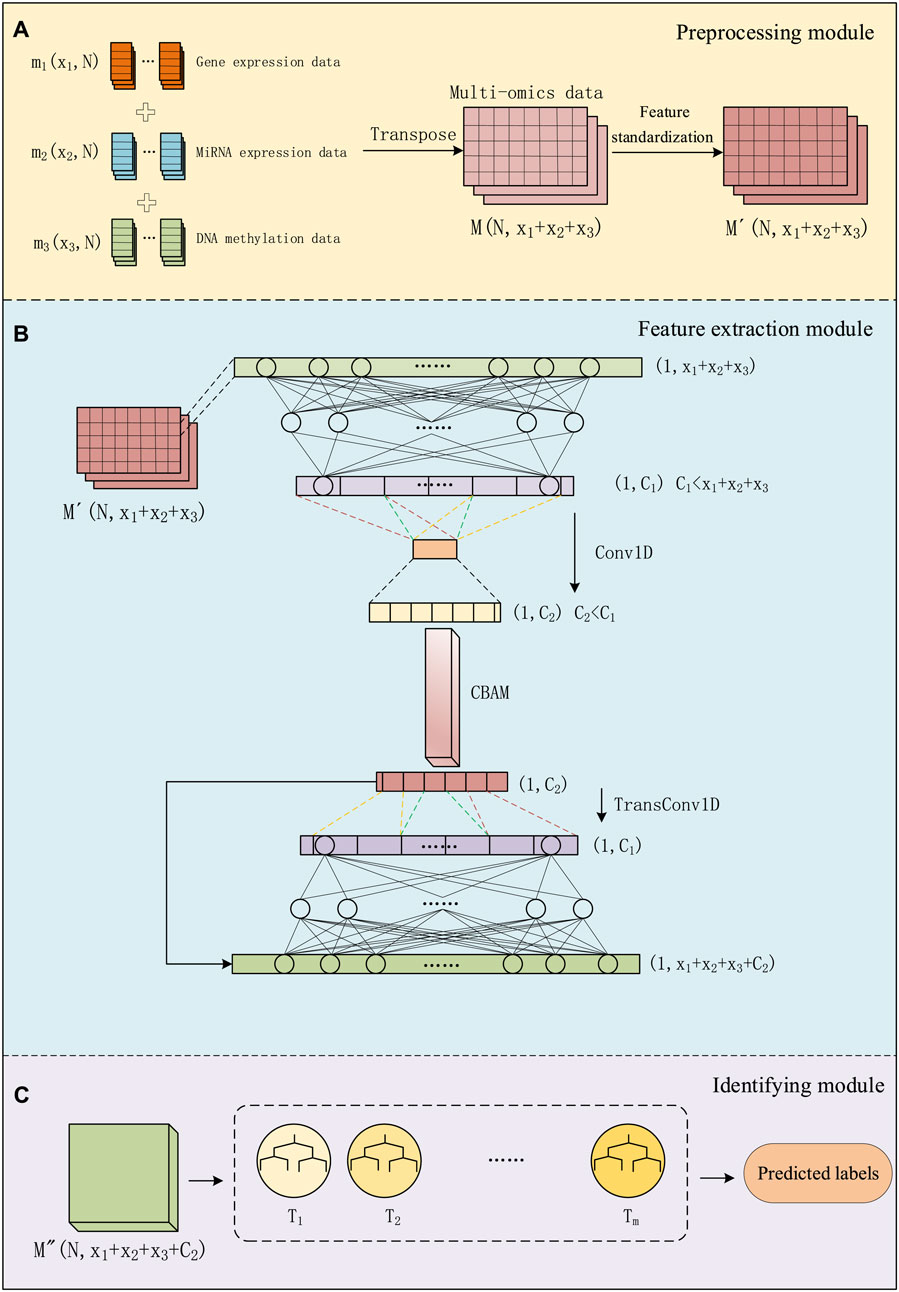
Figure 1. The process of CAEM-GBDT, (A) represents the data Preprocessing module, (B) represents the Feature extraction module, (C) represents the Identifying module.
2.1 Preprocessing module
We use three types of omics data as inputs to the model, including the gene expression data matrix m1(x1, N), miRNA expression data matrix m2(x2, N) and DNA methylation data matrix m3(x3, N), Each column represents a sample, with a total of N samples, and rows x1, x2 and x3 represent the dimensions of different data types. We transpose and concatenate these data matrices into a multi-omics data matrix M.
For multi-omics data with different dimensions, we concatenated the multi-omics feature matrices from the perspective of sample quantity. Although different omics data within the same dataset have different sample dimensions, they have the same number of samples. We concatenate the multi-omics data from the perspective of sample number and, when inputting to the model, designate different encoder networks for data with different dimensions. This approach enables the concatenation of multi-omics data.
As shown in Figure 1A, before training the model, the data matrix undergoes feature standardization aiming to map features to a distribution with a mean of 0 and a standard deviation of 1. The multi-omics matrix after standardization is denoted as
the calculation is applied to each row, where
2.2 Feature extraction module
2.2.1 Convolutional autoencoder
The autoencoder, operates by mapping input data through a multi-layer network. In this process, we first process the matrix by an encoding step and extract intermediate features. The features are reconstructed with the same size as the input. The process involves compressing the data (encoding), followed by using this complex representation to reconstruct the data (decoding). Throughout the learning process, optimization of the loss function occurs to minimize it, aiming to faithfully reconstruct the input. Through the autoencoder, the system learns meaningful representations of the input data, which is valuable for tasks such as feature learning and data reconstruction.
As shown in Figure 1B, Given the input sample
where (
Next, the high-level representation h from several fully connected layer is processed through several Conv1D layers. In the calculation process of convolution, the output data
W1 represents the width of the input data, and H1 represents the height of the input data. The convolution kernel size
Finally, the input sample
In the decoder section, the low-dimensional feature
where Insize represents the input height or width, padding represents the padding size. In transposed convolution, the purpose of padding is to remove the outermost layer. Stride represents the step size, kernel_size is the size of the convolutional kernel, and Outsize is the height or width output after transposed convolution. The upsampling layer, in contrast to the max-pooling layer, repeats each time step size times along the time axis, restoring the dimensions scaled by max-pooling. The data processed by the transposed convolutional layers is denoted as
Following, the high-level representation
where
We chose the GELU (Gaussian Error Linear Unit) activation function for both the convolutional layers and transposed convolutional layers. GELU can be seen as a combination of the relu and dropout concepts. For high-dimensional biological data, an abundance of features may impact feature learning. In such cases, if there is a desire to discard unimportant information, the GELU activation function is employed for non-linear transformation of features. When x is relatively large, y is more likely to be retained, and as x decreases, y is more likely to be set to 0. However, when x is less than 0, there is a certain probability that y is not set to 0. The mathematical formula for the approximate computation of GELU is Formula 8:
The structure of the layered convolutional encoder involves constructing a CAE for each type of omics data. The hyperparameters of each CAE are optimized separately to make the reconstructed input of each CAE as similar as possible to its original input. Ultimately, the goal is to find a model that performs well on a combination of various omics data types. The cost function for each CAE is formulated as Formula (9):
Where the first term represents mean squared error, and the second term is L2 regularization,
2.2.2 Convolutional attention
The convolutional block attention module consists of two sequential parts, as shown in Figure 2: the Channel Attention Module (Figure 2A) and the Spatial Attention Module (Figure 2B). The input data typically includes multiple channels. The Channel Attention Module’s main function is to assign individual weights to each channel, enhancing the influence of important channels and reducing the proportion of irrelevant channel information. Two types of pooling layers are used to pool the input feature F in terms of width and height. The pooling results are then processed using a shared fully connected layer (MLP). Afterward, the results are integrated through addition, and the channel attention map C is obtained through a mapping function ∫. Finally, the weights are multiplied to channel-wise weight C and applied to F.
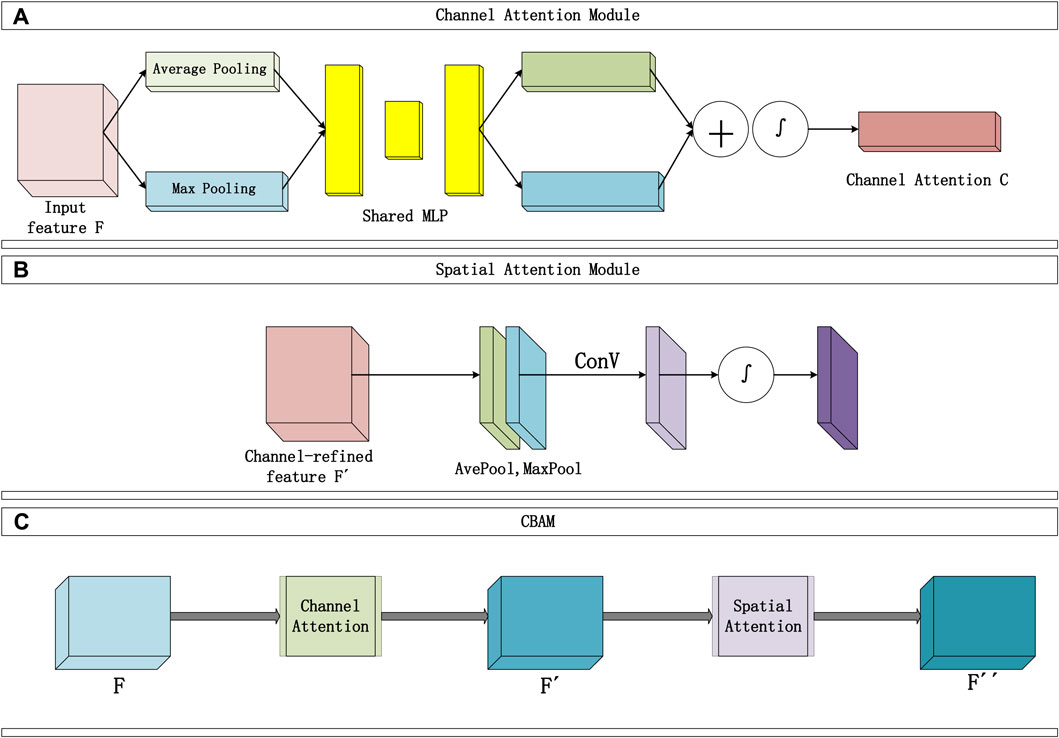
Figure 2. Convolutional attention module structure diagram, (A) represents the Channel Attention module, (B) represents the Spatial Attention module.
The Channel Attention Module constructs two shared fully connected layers (MLP) in the attention module, namely, ‘shared_layer_one’ and ‘shared_layer_two’. The specific structure is as follows: ‘shared_layer_one’ is the first fully connected layer, with an output dimension equal to the number of channels divided by the ratio, and uses the relu activation function. ‘Shared_layer_two’ is the second fully connected layer, with an output dimension equal to the number of input feature channels, and has no activation function. These shared layers are used to process the results of average pooling and max pooling, respectively calculating the weights of each channel.
In order to obtain an effective channel attention map, the dual pooling computation process enhances the feature representation compressed by this module. The specific computation process is illustrated in Figure 2A, and the calculation formula can be expressed as Formula 10:
Where ∫ represents the Sigmoid activation function, W1、W0 are the weight matrices shared by the MLP, and C(F) represents the channel attention map (weights).
Moreover, the use of shared layers ensures that the same set of weights is applied to different pooling results, thereby introducing consistency in the computation. The two shared fully connected layers are applied sequentially to the results of average pooling and max pooling, meaning that these fully connected layers are shared in both pooling paths. In this way, the average pooling and max pooling results are processed into the same dimensions and transformed by the same network weights. This shared mechanism ensures consistency when the model calculates the attention weights of each channel, avoiding the introduction of different parameters in different pooling paths, thereby enhancing the model’s generalization ability.
The Spatial Attention Module focuses on which information in the input data is more crucial and can further complement features that were not previously noticed. Specifically, this module sequentially performs two types of pooling computations along the channel direction, then stacks them to form a numerical matrix. Subsequently, it undergoes a standard convolutional layer (with one channel) to concatenate, adjusting the previously disordered channels in the matrix. Following that, an attention map S is calculated using an activation function, and finally, S is multiplied with the input of this module. The Spatial Attention Module is illustrated in Figure 2B, and the detailed calculation Formula 11 is as follows:
In the equation, ∫ represents the Sigmoid function,
Finally, the overall process of the Convolutional Attention Module is illustrated in Figure 2B and can be summarized as follows: The input F passes through the first channel module to obtain the channel attention map C, and the matrix
CBAM combines the spatial attention map
2.3 Identifying module
This study employed the GBDT classifier for cancer subtype identifying. GBDT is one of the excellent algorithms in the Boosting series of ensemble learning. Boosting imparts a strong dependency relationship among individual learners of the same kind, integrating them sequentially. The Gradient Boosting Tree algorithm organizes weak classifier decision trees through the addition principle, connecting each weak classifier. It optimizes residuals using gradient descent. In GBDT, the next weak classifier is trained using the gradient of the loss from the previous weak classifier. In this way, each iteration develops in the direction of reducing the loss, seeking an optimal solution. GBDT is an additive model of decision trees (as shown in Figure 1C), and the general calculation process can be represented as Formula 14:
GBDT adopts the forward distribution algorithm. The first step is to initialize the boosting tree.
In Formula 16, the parameters of the next tree are chosen to minimize the bias as much as possible:
“L ()" refers to the loss function. The loss function for GBDT classification algorithm is different from regression algorithms. It calculates the training loss using the formula of log-likelihood loss function, as shown in Eq. 17:
Where N is the number of samples,
GBDT adopts a one-vs-all strategy to predict multi-class targets. M decision trees are trained as weak classifiers for each different category. Assuming there are K categories, after training, there will be a total of M*K trees. The training objective of GBDT is to optimize gradients to reduce bias, thereby improving the final classifier’s results. Each decision regression tree has a limited depth. Finally, GBDT weights and sums the weak classifiers obtained in each round of training to obtain the predicted labels for cancer subtypes.
To address the potential overfitting issue in GBDT, we adopted the following two methods to reduce the risk: Limiting the number of weak classifiers: Generally, increasing the number of weak classifiers enhances the model’s fit to the training data but also increases the risk of overfitting. In our study, we limited the number of weak classifiers to 300, which helps reduce the overfitting risk. Early stopping strategy: By monitoring the model’s performance on the validation set, we save the parameters and stop training when the model reaches its optimal performance. This effectively prevents the model from overfitting the training data.
To ensure the model’s generalization ability, we integrated parameters from two datasets, enabling GBDT to adapt to both the BRCA and GBM datasets, thereby improving the model’s generalization capability. Additionally, the weak classifiers in GBDT consider only a subset of features each time, which, while reducing the correlation between features, enhances the model’s generalization ability.
In CAEM, the feature matrix obtained through the Convolutional Autoencoder and Convolutional Attention modules is input into the GBDT module to obtain the final prediction results for cancer subtypes.
3 Experiment and analysis
3.1 Datasets
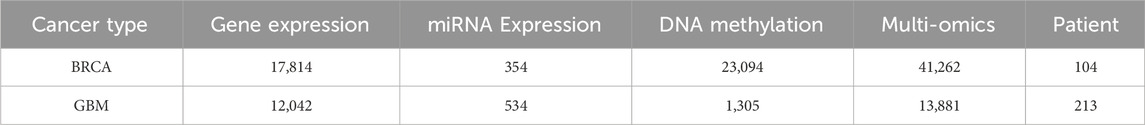
To demonstrate the effectiveness of the proposed method CAEM-GBDT, this study considered two different datasets. One dataset is from TCGA, including 104 samples of Invasive Breast Carcinoma (BRCA), the another dataset includes 213 samples of Glioblastoma Multiforme (GBM). We randomly divide each dataset into five equally sized parts, with four parts used as the training set and the remaining one part as the test set. However, in the BRCA dataset, due to the small number of samples in the test set, we changed the ratio of the training set to the test set to 3:1 when the input data was multi-omics data. We set 100 epochs for training the model. For each cancer type, we utilized gene expression data, miRNA expression data, DNA methylation data. Table 1 shows detailed information about the two datasets.
3.2 The encoder network structure and parameter settings
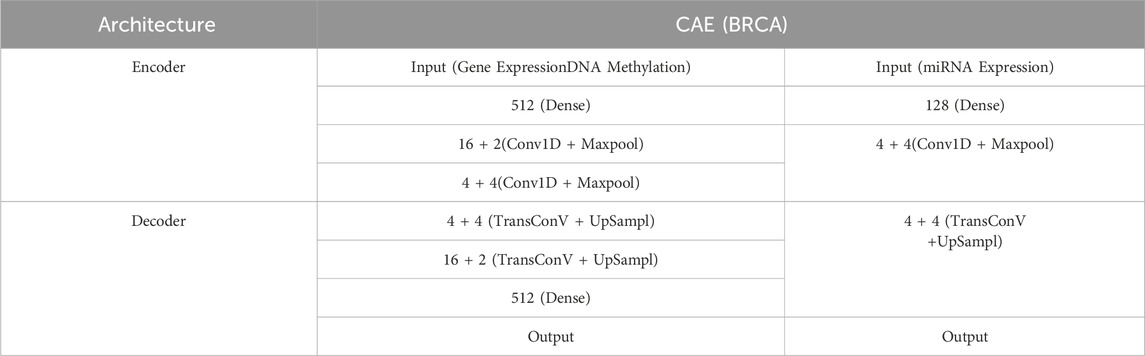
In order to choose a CAE structure with better performance, this study trained CAEs with different numbers of hidden layers and hidden units for different data types. Tables 2, 3 shows the corresponding network structures and parameters for CAE.
Where the convolutional layers have a kernel size of 3, padding mode is set to ‘same’, and the stride is 2. The activation function for the convolutional layers is chosen as the Gelu function.
In the GBM dataset, we have adopted the same encoder structure for three different types of omics data. The main reason for this is that the dimensionalities of multiple omics data in the GBM dataset are not significantly different from those in the BRCA dataset. Therefore, in the BRCA dataset, we set the same encoder network for gene expression data and DNA methylation data, which have larger dimensions, while a different encoder network is set for miRNA data. This approach ensures that the convolutional autoencoder is better suited to each omics data type, leading to more efficient feature extraction.
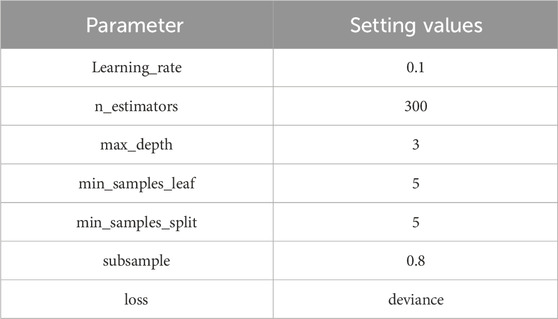
In addition to CAE, for the GBDT module, this study employs five-fold cross-validation to calculate the minimum mean squared error for evaluating the performance of different parameter settings in GBDT. Table 4 presents the specific parameter settings for the GBDT module.
3.3 Ablation experiment
3.3.1 Ablation experiment of the CAEM
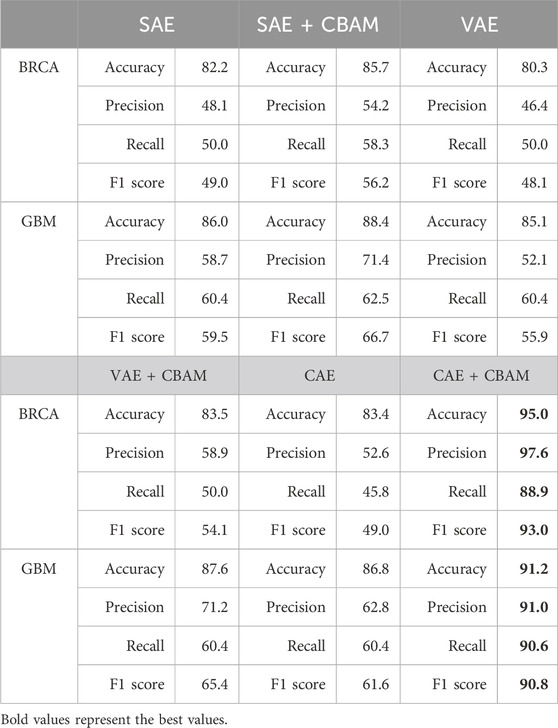
This study conducts ablation experiments to determine the contribution of the CAEM module. In the field of dimensionality reduction, deep learning offers many outstanding methods, including representative ones such as Stacked Autoencoders (SAE), Variational Autoencoders (VAE), and the Convolutional Autoencoder (CAE). These dimensionality reduction methods are combined with Convolutional Block Attention Module (CBAM) in various configurations for ablation experiments. The performance is ultimately evaluated based on accuracy. The experimental results are shown in Table 5.
From Table 5, it can be observed that both in terms of individual accuracy and overall F1 score, CBAM demonstrates significantly superior performance across both datasets. Particularly noteworthy is its improvement in CAE, where it achieves nearly a 10% increase in performance on the BRCA dataset. On the BRCA dataset, the accuracy of the other five models is below 90%, while CAEM achieves an accuracy of up to 95%. In contrast, the proposed CAEM model exhibits superior performance. On the GBM dataset, except for the high accuracy achieved by the combination of SAE and CBAM, the accuracy of the other models is significantly lower than that of the CBAM model. Although the combination of SAE and CBAM achieves an accuracy of 88.4%, our proposed CAEM model demonstrates significantly better performance, achieving an accuracy of 91.2%. Furthermore, the CAEM module exhibits good robustness on both datasets. Therefore, the CBAM module can enhance the classification performance of the model to some extent. These experiments above serve as evidence for the effectiveness of the CBAM mechanism.
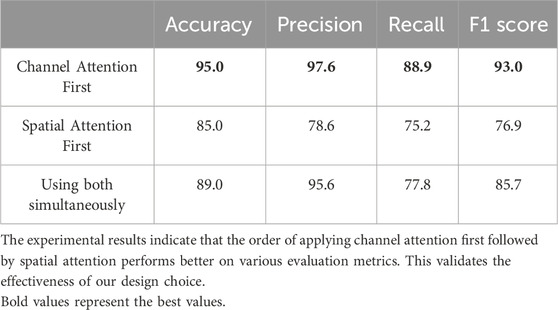
3.3.2 Ablation study of the attention mechanism
The channel attention module aims to enhance specific channel features by assigning weights to each channel. By applying channel attention first, we can effectively enhance the channel features that are more important for the task. On this basis, the spatial attention module can further focus on important regions in the spatial dimension, thereby further improving the quality of feature representation. We have experimentally demonstrated the effectiveness of this order. Using multi-omics data from the BRCA dataset as an example and taking accuracy, precision, recall, and F1 score as evaluation criteria, the experimental results are shown in Table 6.
3.4 Comparison experiment of dimensionality reduction methods
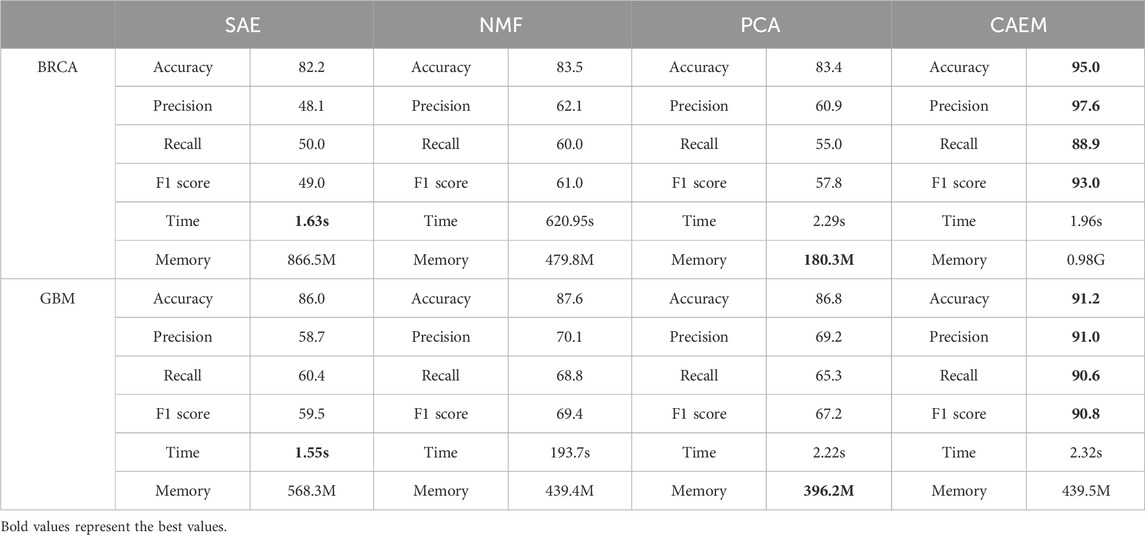
In CAEM-GBDT, the hierarchical structure CAEM serves as the input for the GBDT classification model. This paper compares it with three dimensionality reduction methods: SAE, NMF, and PCA. The evaluation is performed using these two datasets as input to assess the performance of CAEM in learning features. Classification accuracy is used as the criterion for judging the effectiveness of the dimensionality reduction methods.
From Table 7, it can be seen that in terms of runtime, the training time for one epoch is similar among the three methods except for NMF. Although CAEM-GBDT requires a larger amount of memory, it does not consume more runtime. Moreover, it achieves significantly better results compared to other dimensionality reduction methods. The accuracy of the proposed CAEM dimensionality reduction method is significantly higher than the other three methods. In BRCA dataset, CAEM’s accuracy is notably higher than other dimensionality reduction methods, reaching 95.0%. Similarly, CAEM performs exceptionally well on the GBM dataset. Therefore, this demonstrates the superiority of CAEM for dimensionality reduction. In terms of learning features and achieving dimensionality reduction, CAEM demonstrates the best performance, followed by SAE, NMF, and PCA. The deep learning CAEM dimensionality reduction method can effectively integrate multiple omics data, which will be beneficial for the classification of cancer subtypes.
3.5 Comparison experiment with other models
To demonstrate the performance of GBDT, this study employs four different classification models as the final classifiers. The low-dimensional data representation processed by the CAEM module is used as input for each of the four classifiers, with accuracy serving as the evaluation metric.
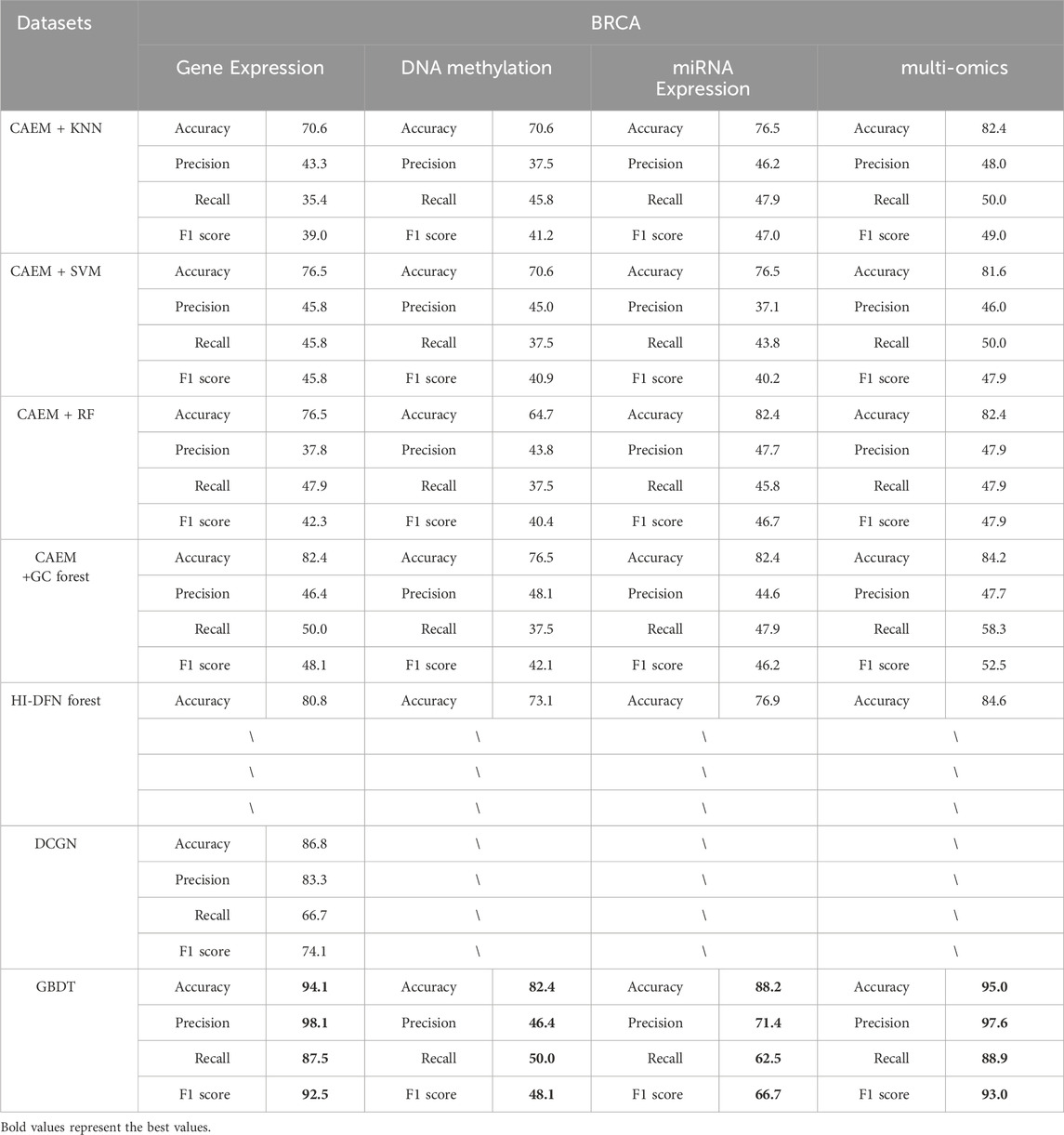
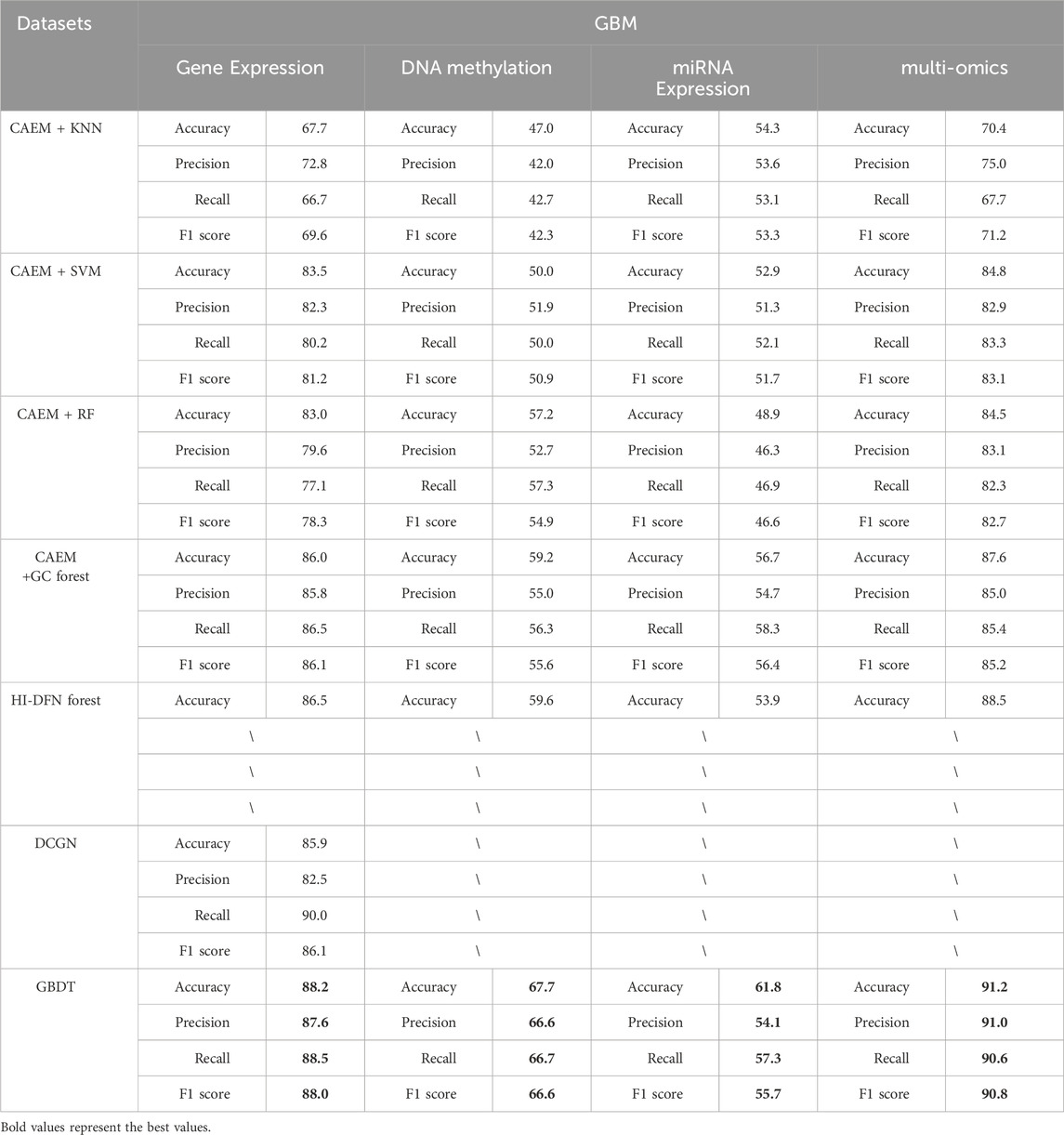
Furthermore, CAEM-GBDT compared its classification accuracy with other methods, namely, the HI-DFN forest method, which is compatible with multi-omics data as model input, and the DCGN (Shen et al., 2022) method, which is not compatible with multi-omics data as input. In this paper, We evaluated the performance of these two methods as well as four classifiers using the same dataset, and the experimental results are shown in Tables 8, 9.
As shown in Tables 8, 9, it is evident that GBDT outperforms other classifiers. In the BRCA dataset shown in Table 6, the accuracy of GBDT using multi-omics data is 95.0%, whereas KNN is 82.4%, SVM is 81.6%, RF is 82.4%, and GC forest is 84.2%. Similarly, in Table 7, the best-performing model in the GBM dataset is also GBDT, particularly achieving an accuracy of 91.2% on multi-omics data, significantly surpassing other classifiers. Therefore, GBDT is selected as the final classifier module in the model.
First, regarding single-omics data, CAEM-GBDT achieves high accuracy across all three omics data types in both datasets. Particularly in the BRCA dataset, whether using single-omics or multi-omics data, CAEM-GBDT improves accuracy by nearly 10% compared to other methods. Meanwhile, the F1 score is also far higher than other methods, demonstrating CAEM-GBDT’s robust performance. In the GBM dataset, CAEM-GBDT also shows improvements over existing methods, especially in DNA methylation data, where the accuracy improvement reaches 8.1%.
In comparison with the HI-DFN forest model, the results of the model were provided by their paper, so evaluation indicators such as F1 scores were not obtained. CAEM-GBDT exhibited high accuracy in all aspects, especially on the multi-omics data of the BRCA dataset, where the accuracy reaches 95%, nearly 10% higher than the HI-DFN forest. In comparison with the DCGN method, DCGN is a cancer subtype identification method exclusively designed for gene expression data. This method exhibits good accuracy and stability in gene expression datasets but cannot handle multi-omics data or other omics data as input. Whether using single-omics data or multi-omics fused data, CAEM-GBDT demonstrates superior performance and better robustness.
From the overall trend in the tables, it can be observed that when the model uses single-omics data as input, the accuracy and F1 score are not as high as those with multi-omics data. Although the accuracy on gene expression data in the BRCA dataset reaches 94.1%, it still improves to 95% when combined with the other two omics data types. This phenomenon is particularly evident in the GBM dataset, where the accuracies of gene expression data, DNA methylation data, and miRNA expression data are 88.2%, 67.7%, and 61.8% respectively. Each omics data type alone has low accuracy, but when combined, the accuracy reaches 91.2%. This demonstrates the impact of multi-omics data on cancer subtype identification results. This trend is not only observed in CAEM-GBDT but also in other models. Therefore, it can be concluded that multi-omics data, compared to single-omics data, enables the model to learn complementary information between omics data, achieving higher accuracy and F1 scores for cancer subtype identification. Moreover, the results are more convincing as they integrate various omics data, benefiting cancer subtype identification.
4 Discussion
With the advancement of sequencing technologies, integrating multi-omics data for cancer subtype identification is a major challenge faced by researchers. This paper proposes a cancer subtype classification method, named-GBDT, based on Convolutional Autoencoder and Convolutional Attention. It aims to accurately extract crucial information contained in complex multi-omics data. The Convolutional Attention Mechanism is employed to further enhance features in the high-level representation, enabling the classifier to more effectively identify important hidden information in the data and perform cancer subtype classification. Compared to other deep learning methods published at the current stage, CAEM-GBDT has the following characteristics:1. CAEM-GBDT designs different encoder networks for different data settings. It can handle individual omics data as well as fuse multiple omics data as input to the model, achieving high accuracy in both scenarios.2. CAEM-GBDT attempts to learn relationships between multiple omics data, fully leveraging multi-omics information among samples. This enhances the model’s robustness, making the identification results more convincing.
This paper conducted encoder network ablation experiments, dimensionality reduction method comparison experiments, classifier comparison experiments, and comparative experiments with the HI-DFN forest model, demonstrating the powerful capabilities of CAEM-GBDT. Experimental results indicate that the fusion of multi-omics data is effective and necessary. Multi-omics data describe the data from different perspectives, providing the model with more stable performance and enhanced robustness.
Although CAEM-GBDT has demonstrated promising results on the BRCA and GBM datasets, there are still some limitations. CAEM-GBDT is tailored specifically for supervised learning in classification. In the research on unsupervised learning and clustering methods, CAEM-GBDT’s performance is not as satisfactory. Therefore, addressing this limitation will be a key focus of our next research steps.
5 Conclusion
This paper proposes a cancer subtype classification method, CAEM-GBDT, based on Convolutional Autoencoder and Convolutional Attention Mechanism. The integration of multiple omics data improves the accuracy of cancer subtype classification. Addressing the challenges posed by high-dimensional and sparse biological datasets, this approach combines Convolutional Autoencoder (CAE) and Convolutional Block Attention Module (CBAM) to obtain effective data representations from cancer data. The results on two TCGA datasets demonstrate that CAEM-GBDT outperforms other methods, showing its superior performance.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Ethics statement
The manuscript presents research on animals that do not require ethical approval for their study.
Author contributions
JS: Conceptualization, Investigation, Methodology, Writing–original draft, Writing–review and editing. XG: Methodology, Software, Writing–original draft, Writing–review and editing. HB: Software, Writing–review and editing, Formal Analysis, Investigation, Validation. JL: Investigation, Writing–review and editing, Conceptualization, Funding acquisition, Methodology, Supervision.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This research was supported by the National Natural Science Foundation of China (Grant Nos 62372156, 61972134), Young Backbone Teachers of Henan Province (Grant No. 2020GGJS050), Doctoral Fund of Henan Polytechnic University (Grant No. B2018-36), Innovative Research Team of Henan Polytechnic University (Grant No. T2021-3), and Henan Provincial Department of Science and Technology Research Project (Grant No. 242102210097).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Alexander, J., Mariani, O., Meaudre, C., Fuhrmann, L., Xiao, H., Naidoo, K., et al. (2022). Assessment of the molecular heterogeneity of E-cadherin expression in invasive lobular breast cancer. Cancers 14 (2), 295. doi:10.3390/cancers14020295
Ao, C., Gao, L., and Yu, L. (2022). Research progress in predicting DNA methylation modifications and the relation with human diseases. Curr. Med. Chem. 29 (5), 822–836. doi:10.2174/0929867328666210917115733
Brigham, W. s. (2012). Comprehensive molecular portraits of human breast tumours. Nature 490 (7418), 61–70. doi:10.1038/nature11412
Choi, J. M., and Chae, H. (2023). moBRCA-net: a breast cancer subtype classification framework based on multi-omics attention neural networks. BMC Bioinforma. 24 (1), 169–215. doi:10.1186/s12859-023-05273-5
Dai, W., Yue, W., Peng, W., Fu, X., Liu, L., and Liu, L. (2021). Identifying cancer subtypes using a residual graph convolution model on a sample similarity network. Genes. 13 (1), 65. doi:10.3390/genes13010065
El-Nabawy, A. a., Belal, N. A., and El-Bendary, N. (2021). A cascade deep forest model for breast cancer subtype classification using multi-omics data. Mathematics 9 (13), 1574. doi:10.3390/math9131574
Guo, L., Lei, X., Chen, M., and Pan, Y. (2023). MSResG: using GAE and residual GCN to predict drug–drug interactions based on multi-source drug features. Interdiscip. Sci. Comput. Life Sci. 15, 171–188. doi:10.1007/s12539-023-00550-6
Hammerman, P. S., Voet, D., Lawrence, M. S., Voet, D., Jing, R., Cibulskis, K., et al. (2012). Comprehensive genomic characterization of squamous cell lung cancers. Nature 489 (7417), 519–525. doi:10.1038/nature11404
Kang, M., Ko, E., and Mersha, T. B. (2022). A roadmap for multi-omics data integration using deep learning. Briefings Bioinforma. 23 (1), bbab454. doi:10.1093/bib/bbab454
Lei, S., Lei, X., and Liu, L. (2022). Drug repositioning based on heterogeneous networks and variational graph autoencoders. Front. Pharmacol. 13, 1056605. doi:10.3389/fphar.2022.1056605
Lipkova, J., Chen, R. J., Chen, B., Lu, M. Y., Barbieri, M., Shao, D., et al. (2022). Artificial intelligence for multimodal data integration in oncology. Cancer Cell. 40 (10), 1095–1110. doi:10.1016/j.ccell.2022.09.012
Mo, Q., Shen, R., Guo, C., Vannucci, M., Chan, K. S., and Hilsenbeck, S. G. (2018). A fully Bayesian latent variable model for integrative clustering analysis of multi-type omics data. Biostatistics 19 (1), 71–86. doi:10.1093/biostatistics/kxx017
Muzny, D. M., Bainbridge, M. N., Chang, K., Dinh, H. H., Drummond, J. A., Fowler, G., et al. (2012). Comprehensive molecular characterization of human colon and rectal cancer. Nature 487 (7407), 330–337. doi:10.1038/nature11252
Naderan, M., and Zaychenko, Y. (2020). “Convolutional autoencoder application for breast cancer classification,” in 2020 IEEE 2nd international conference on system analysis and intelligent computing (SAIC) (Germany: IEEE), 1–4.
Shen, J., Shi, J., Luo, J., Zhai, H., Liu, X., Wu, Z., et al. (2022). Deep learning approach for cancer subtype classification using high-dimensional gene expression data. BMC Bioinforma. 23 (1), 430–517. doi:10.1186/s12859-022-04980-9
Song, W., Wang, W., and Dai, D.-Q. (2022). Subtype-WESLR: identifying cancer subtype with weighted ensemble sparse latent representation of multi-view data. Briefings Bioinforma. 23 (1), bbab398. doi:10.1093/bib/bbab398
Sun, Q., Cheng, L., Meng, A., Ge, S., Chen, J., Zhang, L., et al. (2023). SADLN: self-attention based deep learning network of integrating multi-omics data for cancer subtype recognition. Front. Genet. 13, 1032768. doi:10.3389/fgene.2022.1032768
Wang, B., Mezlini, A. M., Demir, F., Fiume, M., Tu, Z., Brudno, M., et al. (2014). Similarity network fusion for aggregating data types on a genomic scale. Nat. methods 11 (3), 333–337. doi:10.1038/nmeth.2810
Wang, F., Lei, X., and Wu, F.-X. (2020). A review of drug repositioning based chemical-induced cell line expression data. Curr. Med. Chem. 27 (32), 5340–5350. doi:10.2174/0929867325666181101115801
Wu, D., Wang, D., Zhang, M. Q., and Gu, J. (2015). Fast dimension reduction and integrative clustering of multi-omics data using low-rank approximation: application to cancer molecular classification. BMC genomics 16 (1), 1022–1110. doi:10.1186/s12864-015-2223-8
Xu, J., Wu, P., Chen, Y., Meng, Q., Dawood, H., and Dawood, H. (2019). A hierarchical integration deep flexible neural forest framework for cancer subtype classification by integrating multi-omics data. BMC Bioinforma. 20 (1), 527–611. doi:10.1186/s12859-019-3116-7
Yang, H., Chen, R., Li, D., and Wang, Z. (2021). Subtype-GAN: a deep learning approach for integrative cancer subtyping of multi-omics data. Bioinformatics 37 (16), 2231–2237. doi:10.1093/bioinformatics/btab109
Zhang, Y., Lei, X., Pan, Y., and Wu, F.-X. (2022). Drug repositioning with GraphSAGE and clustering constraints based on drug and disease networks. Front. Pharmacol. 13, 872785. doi:10.3389/fphar.2022.872785
Zhao, J., Zhao, B., Song, X., Lyu, C., Chen, W., Xiong, Y., et al. (2023). Subtype-DCC: decoupled contrastive clustering method for cancer subtype identification based on multi-omics data. Briefings Bioinforma. 24 (2), bbad025. doi:10.1093/bib/bbad025
Keywords: cancer subtype, cancer subtype identification, convolutional autoencode, convolutional block attention module, multi-omics
Citation: Shen J, Guo X, Bai H and Luo J (2024) CAEM-GBDT: a cancer subtype identifying method using multi-omics data and convolutional autoencoder network. Front. Bioinform. 4:1403826. doi: 10.3389/fbinf.2024.1403826
Received: 20 March 2024; Accepted: 13 June 2024;
Published: 15 July 2024.
Edited by:
Chunhou Zheng, Anhui University, ChinaReviewed by:
Lun Hu, Chinese Academy of Sciences (CAS), ChinaHongdong Li, Central South University, China
Copyright © 2024 Shen, Guo, Bai and Luo. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Junwei Luo, bHVvanVud2VpQGhwdS5lZHUuY24=
 Jiquan Shen
Jiquan Shen Xuanhui Guo
Xuanhui Guo Hanwen Bai
Hanwen Bai Junwei Luo
Junwei Luo