
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Audiol. Otol. , 11 March 2024
Sec. Technology and Innovation in Auditory Implants and Hearing Aids
Volume 2 - 2024 | https://doi.org/10.3389/fauot.2024.1347437
This article is part of the Research Topic Novel Technologies in the Field of Hearing Restoration where Over-the-Counter Hearing Aids are Permitted View all 3 articles
After decades of effort by shareholders, including government agencies, patient advocacy groups, and professional organizations, the U.S. Food and Drug Administration (FDA) established a new medical device category for over-the-counter (OTC) hearing aids on October 17, 2022. This FDA regulation allows adults aged 18 years or older with perceived mild-to-moderate hearing loss to purchase OTC hearing aids without a prescription or even a hearing test. The goal is to increase hearing aid accessibility, potentially leading to improved hearing and a better quality of life. In our analysis of the FDA Establishment Registration & Device Listing database, we found that the current OTC hearing aid market is still dominated by traditional hearing aid manufacturers, with limited disruptor from major consumer electronics and startup companies. Our technological analyses showed that the relatively high-level output specification without gain limitation allows sufficient amplification even for people with severe-to-profound hearing loss. Additionally, borrowing from the cochlear implant mapping strategy, we propose novel amplification algorithms for fitting OTC hearing aids without an audiogram. We argue that smartphones and true-wireless-stereo earbuds can functionally serve as OTC hearing aids, further increasing accessibility and affordability while reducing the stigma associated with hearing aids, especially in low- and mid-income countries. By treating more people at a younger age with less hearing loss than traditional prescription hearing aids, OTC hearing aids can potentially have a significant impact beyond hearing care, such as delaying or preventing cognitive decline in the elderly.
The World Health Organization estimated that approximately 20% of the world's population, or around 1.6 billion people, have some degree of hearing loss, with roughly 90% of them experiencing mild-to-moderate hearing loss (WHO, 2021). The number of people with hearing loss is expected to reach ~2.5 billion by 2050. As a result, hearing loss stands as the third-largest cause of years lived with disability, and untreated hearing loss imposes an annual global economic burden of US$1 trillion. This amount includes personal costs and additional costs related to health care, education support, lost wages and reduced quality of life (McDaid et al., 2021; Wilson and Tucci, 2021).
Hearing aids have been in existence for over a century, dating back to the invention of telephones. Modern digital prescription hearing aids, recognized as safe and effective medical devices, play a crucial role in mitigating the personal and societal burdens associated with hearing loss (e.g., Ferguson et al., 2017; Holman et al., 2021; Nixon et al., 2021). However, the adoption rate of these prescription hearing aids has remained relatively low, varying from 1 to 2% in low-income countries (Chadha et al., 2021) to 38% in the USA (Carr and Kihm, 2022). Several factors contribute to this low adoption rate of hearing aids, including limited awareness of the importance of hearing, restricted access to hearing care, and the high cost of hearing aids (Valente and Amlani, 2017; Jorgensen and Novak, 2020; Zheng et al., 2023).
To enhance public access to hearing aids and improve hearing, the FDA introduced a new category of over-the-counter (OTC) hearing aids on October 17, 2022, specifically designed for adults aged 18 or older with perceived mild-to-moderate hearing loss (https://www.fda.gov/medical-devices/hearing-aids/otc-hearing-aids-what-you-should-know). The OTC hearing aid is a medical device that utilizes air-conduction to amplify soft sounds, may feature a user-adjustable volume control, and, if inserted in the ear canal, must remain at least 10 mm away from the tympanic membrane. For the OTC hearing aid, FDA registration is required with clear package labeling specifying user age (18 or older). In addition, FDA listed the symptoms of perceived mild-to-moderate hearing loss as: “You have trouble hearing speech in noisy places, you have trouble hearing on the phone, you find it is hard to follow speech in groups, listening makes you tired, you need to turn up the volume on the TV or radio, and other people complain it's too loud”. Importantly, FDA listed warning signs that indicate the need to seek professional health care services (e.g., inability to hear speech even in a quiet room, observation of blood, pus, or fluid coming out of the ear). Finally, note that, unlike the OTC hearing aid, all other types of hearing aids necessitate a prescription from a licensed hearing health care professional.
Undoubtedly, the OTC hearing aid stands as a landmark achievement in hearing healthcare policy, ushering in an alternative option in hearing aid delivery for a large percentage of individuals with hearing loss and potentially transforming the entire hearing care and service market (Tucci and Califf, 2023). In this article, we will first examine the one-year-old market trends of OTC hearing aids. Subsequently, we will analyze the technical implementations of OTC hearing aids, focusing on amplification and fitting. Finally, we will explore the opportunities for OTC hearing aids, considering not only technical perspectives such as alternative smartphone implementations and the application of artificial intelligence but also their potential impact on global hearing healthcare and dementia.
It has now been a year since the FDA OTC hearing aid regulation went into effect. To assess its impact, let's first examine the supply side. A search on the FDA Establishment Registration and Device Listing database revealed a total of 113 entries related to OTC hearing aids (see Table 1). Among these entries, 74 manufacturers were identified, with 21 based in the USA, two in Europe, 49 in Asia, one in Mexico, and one in South Africa.
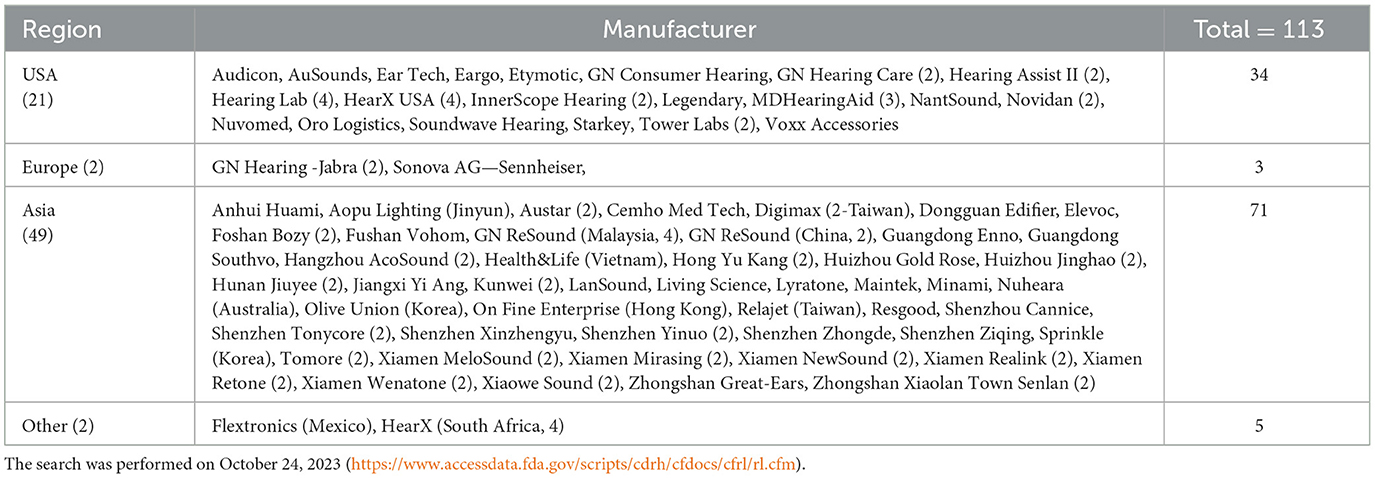
Table 1. FDA registered OTC hearing aids in terms of region (left column), manufacturers (middle column, number in the parentheses represents more than one entries from the same manufacturer) and the number of entries (right column).
Significantly, four of the “Big Five” traditional hearing aid manufacturers have ventured into the OTC hearing aid market. At present, the “Big Five” (Sonova, Demant, Sivantos/Widex, GN ReSound, and Starkey) controls the majority of the global market share for prescription hearing aids. GN ReSound and Sonova are selling OTC hearing aids through the acquisition of former audio brand names, Jabra and Sennheiser, respectively. Starkey, on the other hand, introduced a new line named “Start Hearing One.” Additionally, Widex, through a merger with Signia and agreements with SONY, is marketing several OTC hearing aids (https://electronics.sony.com/otc-hearing-aids), although none could be found on the FDA database. Demant is the only one among the “Big Five” yet to enter the OTC market. While several mid-sized companies like Eargo are also making a presence, most registrants are startups, particularly from Asia, striving to establish themselves in this emerging market.
Curiously absent from the OTC hearing aid database are USA consumer electronics manufacturers. Bose, the first company selling the OTC hearing aid, sold its self-fitting hearing aid technology to Lexie (part of HearX) and totally exited the hearing aid market. Apple's absence in the OTC hearing aid market is particularly surprising, because not only do they own more hearing aid-related patents than any hearing aid manufacturer (Zeng, 2015), but also are more than capable of producing a premium-quality hearing aid (Lin et al., 2022).
Second, the OTC hearing aids have brought about some small but desired changes on the demand side. When compared to new prescription hearing aid users, new OTC hearing aid users, on average, were 2 years younger (64 vs. 62) and have milder hearing loss, but more meaningfully, they had a significantly shorter duration of hearing loss [20.9 vs. 14.4 years reported by Knoetze et al. (2023)]. Note that the duration of 14.4 years was obtained from a population in South Africa, which was much longer than the 4- to 8.9-year delay reported in the USA (Simpson et al., 2019; Powers and Carr, 2022). Nevertheless, the overall uptake of OTC hearing aids is still low. Only 2% of American adults aged 40 and older with hearing difficulties reported purchasing an OTC hearing aid in the last 6 months, and just 4% may buy one in the next year (ASHA, 2023). An independent survey also indicated a negligible impact of OTC hearing aids on existing audiological practices (LaMartina, 2023).
Third, a reputable pair of OTC hearing aids costs between $800 and $3,000, but most consumers are only willing to pay around $200 for them (ASHA, 2023). Apple seems to have discovered this desirable price point for the AirPods, pricing them between $129 and $249. This price contributed to Apple's sales of over 80 million AirPods, or $15 billion in revenue in 2022, which was more than the combined revenue of $12 billion from all hearing aid and cochlear implant companies worldwide. Unless the OTC hearing aid label can increase the unit price, there is no incentive for companies like Apple and other consumer electronics giants to enter the OTC hearing aid market. The potential return does not outweigh the risks associated with the liabilities of a medical device. Both technical and business innovations are required for the OTC hearing aids to make the intended impact on the market.
One key technical ruling for the OTC hearing aid was to specify the maximum output level without limiting the gain (FDA, 2022). To ensure safety, the FDA set the maximum output level at 111 dB SPL at any frequency for OTC devices with linear amplification or 117 dB SPL for that with compression and a user-adjusted volume control when measured in a 2-cc coupler. To improve efficacy and broaden the range of intended users, the FDA declined to set the gain limit for it “reduces the ability to adequately amplify soft sound inputs in some cases” and may “constrain device design and innovation”. Here we demonstrate from two different perspectives that defining the maximum output level in a 2-cc coupler without limiting the gain allows amplification far more than what is needed for people with mild-to-moderate hearing loss.
We first examined the maximum output level at the ear drum that can be produced by an OTC hearing aid. Since the FDA ruling defined the maximum output level that is measured in a 2-cc coupler, the actual output level at the ear drum can exceed this maximum level because the effective ear canal volume is likely reduced by the presence of a hearing aid in the ear canal.
Table 2 shows our analysis results. For the linear OTC hearing aid, the FDA ruling set the maximum output level to be 111 dB SPL (Row 1) at any frequency using pure tones. The American National Standards Institute characterized the average real-ear-to-coupler difference or RECD (Row 2) between a 2-cc coupler (e.g., HA1) and the ear canal at octave frequencies from 250 to 8,000 Hz (ANSI, 2013). The addition of 111 dB SPL and the RECD gives the actual maximum output in dB SPL at the ear drum (Row 3). Assuming that the loudspeakers of OTC hearing aids have an insertion depth similar to that of an ear pod, the 0-dB HL can be estimated in terms of dB SPL (i.e., the reference equivalent threshold sound pressure level, or RETSPL, Row 4, Ho et al., 2017). The difference between Row 3 and Row 4 gives the maximum hearing loss in dB HL that a linear OTC hearing aid can potentially compensate in terms of sound awareness at thresholds (Row 5). The averaged degree of hearing loss is 114 dB HL, ranging from 102 dB HL at 250 Hz to 121 dB HL at 2,000 Hz. Because the FDA ruling allows compression OTC hearing aids to have a maximum output of 117 dB SPL (Row 6), they can produce 6-dB higher maximum output level at the ear drum (Row 7) and compensate for 6-dB more hearing loss than the linear counterpart, accordingly (Row 8). In either case, the >102 dB HLs are much more than the amplification needed to compensate for mild-to-moderate hearing loss (20–49 dB HL by WHO or 26–55 dB HL by ASHA).
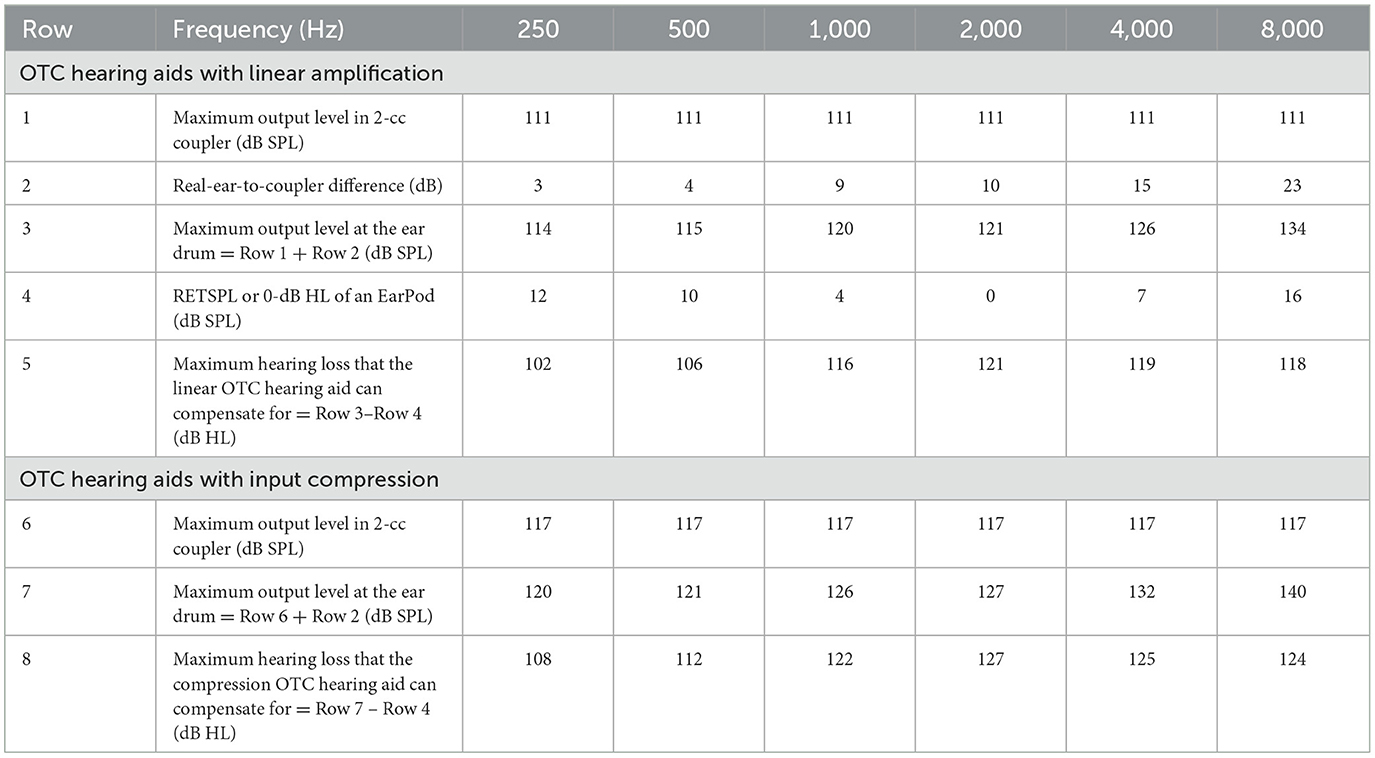
Table 2. Derivation of the maximum hearing level for an OTC hearing aid with linear amplification (rows 1–5) or input compression (Row 6–8).
The function of a hearing aid is not to provide sound awareness at the threshold, but rather to improve speech intelligibility at suprathreshold levels. Therefore, we derived a typical input-output function and gain associated with a compression hearing aid that produced a maximum output level of 117 dB SPL in a 2-cc coupler. Figure 1 shows the input-output function (blue line), which has an expansion region (1:2.5) between 0 and 20 dB SPL to reduce environmental and circuit noise or pumping noise associated with fast release times, a linear (1:1) and low-compression (3:1) region at moderate input levels for conversational speech, and a high-compression region (10:1) at high levels to limit output level (e.g., Villchur, 1973; Keidser et al., 2012). The gain function (orange line) is the difference between input and output levels. The maximum gains for soft (50 dB SPL), conversational (65 dB SPL), and loud (80 dB SPL) speech are 56, 42, and 31 dB, respectively. The maximum gain in the linear amplification region is 56 dB for input levels between 20 and 45 dB SPL.
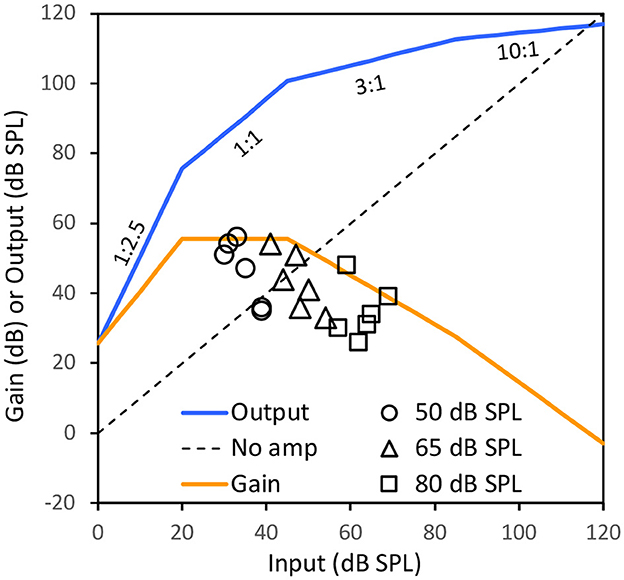
Figure 1. An input-output (blue line) and the gain (orange line) function of a hypothetical compression hearing aid that produces a 117-dB SPL maximum output level. The dotted diagonal line represents no amplification. The circles, triangles and squares represent the gains for a person with 80 dB HL flat hearing loss prescribed by NAL-NL2 at six octave frequencies from 250 to 8,000 Hz for soft (50 dB SPL), conversational (65), and loud (80) speech levels in a monaural hearing aid fitting (see Supplementary Table S1 for details). Note that the output and gain functions would be higher if RECD is added to the 117 dB SPL at different frequencies and the prescribed gain would be lower if binaural hearing aid fitting is used.
We then used NAL-NL2 to derive the amount of gain required for a hypothetic person with a severe 80-dB HL flat hearing loss (see Supplementary Table S1 for details). Figure 1 shows the gain required for such a person at six octave frequencies from 250 to 8,000 Hz at soft (circles), conversational (triangles) and loud (squares) speech. Regardless of speech levels and frequency channels, the prescribed gain for a person with 80-dB HL monaural hearing loss is at or below that is available for a compression OTC hearing aid with the 117-dB SPL maximum level even without the adjustment for the RECD. Also, when a binaural hearing aid fitting is assumed, the prescribed gains would be 2–7 dB lower than the prescribed gain for monaural hearing aid fitting, suggesting that the fitting range of OTC hearing aids can be even higher than an 80-dB HL flat hearing loss.
The above analyses showed that while OTC hearing aids are intended for people with mild-to-moderate hearing loss, the current FDA guideline in fact allows sufficient amplification for people with severe or profound hearing loss. On the one hand, the OTC hearing aids may present an opportunity for providing amplification for people with a wide range of hearing loss. On the other hand, the OTC hearing aid manufacturers and users need to be aware of the possibility and the danger of overamplification.
Another key technical ruling was loosely defined specifications to differentiate between customizing and fitting the OTC hearing aids (FDA, 2022). The differentiator between customizing and fitting is “the number of presets” in the OTC hearing aids. The “presets” can be frequency-dependent amplification, noise reduction, scene selection or other parameters in typical prescription hearing aids. If the number of presets for a parameter is limited, “for example, three”, then this process would be considered as customization not self-fitting, thus belonging to the OTC hearing aid category. If the number of presents is large, or controlled by software, apps, or similar tools, then the OTC hearing aids would become “self-fitting hearing aids” that must “comply with applicable requirements, including FDA 510(k) requirements and compliance with special controls”.
In practice, all “self-fitting” hearing aids would require FDA clearance even if they would be marketed as OTC hearing aids. Interestingly, FDA explicitly stated that for “devices intended fitting based off of a user-supplied audiogram, a requirement for the involvement of a licensed person to produce the audiogram may cause the device not to be an OTC hearing aid” (FDA, 2022). We feel that this explicit exclusion of a licensed person's produced audiogram seemed to be more about politics than technicality because the device itself is the same. Nevertheless, these rules pose serious technical challenges in designing the OTC hearing aids to meet an individual user's needs. For example, can we fit a hearing aid without an audiogram? Can a limited number of hearing aid presets meet the needs of most people with mild-to-moderate hearing loss?
Twenty years ago, we proposed that cochlear implant manufacturers should implement advanced hearing-aid front-end processing from directional microphones to multi-channel noise reduction algorithms to improve cochlear-implant performance (Chung et al., 2004, 2006). Today, we argue that hearing aid manufacturers should borrow the “mapping” algorithm in cochlear implants to fit the OTC hearing aids. In cochlear implants, each electrode may require different minimal and maximal electric current levels to reach hearing threshold (T level) and most comfortable loudness (M or C level). The cochlear implant speech processor has to compress a 50–60 dB sound dynamic range into a 10–20 dB electric dynamic range between the T and M levels (Zeng et al., 2002). Because only two of the three “mapping” parameters, namely, compression, T and M levels, are independent, cochlear implant manufacturers do not use the T level in their mapping, rather simply setting the T level to be either 0 or 10% of the M level (Vaerenberg et al., 2014). This mapping strategy is equivalent to fitting a hearing aid without an audiogram.
In fact, the FDA rulings have made the OTC hearing aid fitting easier and simpler than the cochlear implant mapping. Figure 2 illustrates how this borrowed idea can fit a compression OTC hearing aid for its targeted users. According to the FDA, the maximal level is either 111 dB SPL for a linear device or 117 dB SPL for a compressive device, which is considered here. The minimal level is either mild or moderate hearing loss (=blue or red triangles on the y-axis, corresponding to 35 or 50 dB SPL threshold, respectively). The overall input-output function includes four regions: (1) an expansion region at low input levels (e.g., 0 to 30 dB SPL), (2) a compression-amplification region at low-to-moderate levels, (3) a no-amplification region at high levels, and (4) a hard limit at 117 dB SPL output or above.
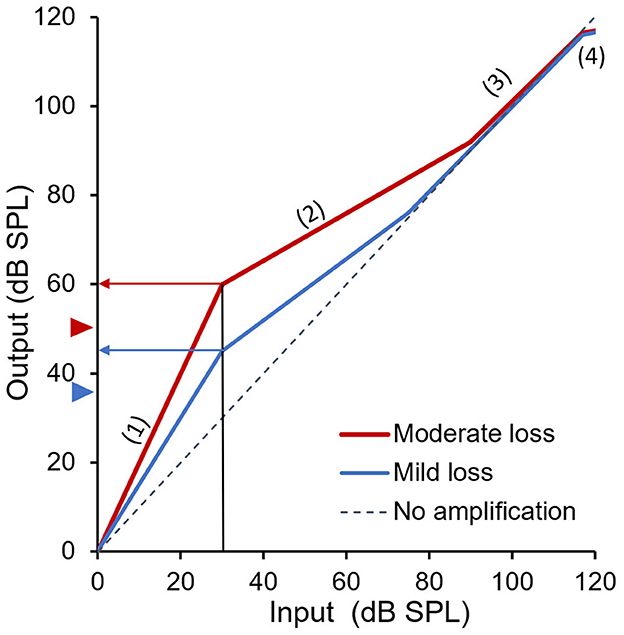
Figure 2. Two preset fittings of an OTC hearing aid for a person with mild (blue) or moderate (red) hearing loss. The gray dashed diagonal line represents no amplification. The blue and red triangles next to the y-axis represent the hearing threshold of mild and moderate hearing loss, respectively. The arrowed horizontal blue and red lines show restored “soft” loudness to an otherwise barely or not audible 30-dB SPL sound in a person with mild or moderate hearing loss.
First, the expansion region minimizes the effects of background noise or pumping sounds associated with amplification. Second, the compression region provides audibility to soft sounds, especially consonants that are critical to speech intelligibility. For example, without amplification, a 30-dB SPL normally soft sound (vertical black line) would be barely or not audible to a person with mild-to-moderate hearing loss. With proper amplification, the 30-dB SPL sound is not only audible but also at approximately “soft” loudness level for a person with mild (arrowed horizontal blue line or 10 dB above the 35 dB SPL threshold) or moderate hearing loss (arrowed horizontal red line or 10 dB above the 50 dB SPL threshold). In other words, as Gain = Output - Input, the hearing aid provided 45–30 = 15 dB of gain to the user with mild hearing loss and 60–30 = 30 dB of gain to the user with moderate hearing loss to make low-level sounds audible for them.
For high-level input sounds, because of loudness recruitment, there is no need to provide any amplification for individuals with mild-to-moderate hearing loss (Figure 2). Additionally, the output limiting region not only conforms to the FDA's OTC hearing aid guideline, but also helps reduce exposure to loud sounds. We note that this four-stage fitting is similar to an early implementation (Killion, 1993), which provides more amplification for low-level sounds and no gain or linear amplification for loud sounds. Our proposed fitting method (Figure 2) improves Killion's implementation and uses expansion to reduce the gain applied to very soft sounds that may not carry much linguistic information.
Most importantly, our proposed method allows a simple and quick OTC hearing aid fitting process. Specifically, the user only needs to adjust the gain at 30 dB SPL input level to make sure they can hear soft sounds and then the gains for conversational level sounds and loud sounds are automatically set. This adjustment can be performed at different frequency channels to suit the individuals' configuration of hearing loss and listening needs. Should a volume control be installed, our proposed fitting method can be used for individuals with more than mild-to-moderate hearing loss.
The next question we address is: can one design an OTC hearing aid with a limited set of frequency responses without requiring an audiogram? In response, we propose a straightforward solution involving high-frequency compression amplification to cater to the needs of most, if not all, OTC hearing aid users. Figure 3 illustrates two levels of one-channel amplification for sounds above 1,500 Hz, providing 20 dB or less amplification for soft sounds in mild loss cases (top panel) and 40 dB or less amplification for soft sounds in moderate loss cases (second panel). This high-frequency amplification is designed to be effective not only for individuals with mild-to-moderate high-frequency loss but also for those with the same degree of flat loss. Previous studies have demonstrated that the exact frequency response is not a critical parameter as long as speech sounds fall within a listener's dynamic range, without too much low or high frequency gains (van Buuren et al., 1995, 1996).
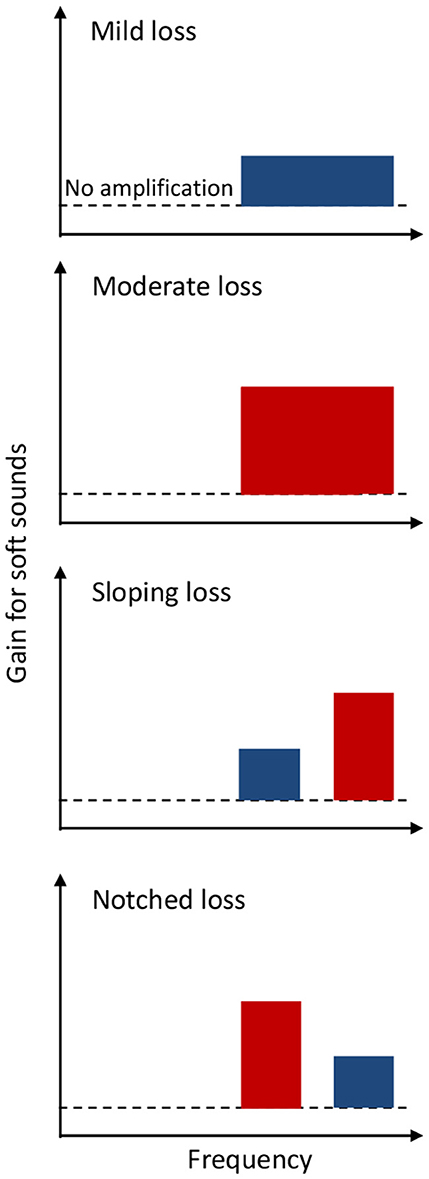
Figure 3. One-channel (top two panels) or two-channel (bottom two panels), high-frequency amplification for an OTC hearing aid to meet the needs of people with four different mild (blue) or moderate (red) hearing loss configurations. The dashed line represents no amplification.
While most potential OTC hearing aid users have high-frequency or flat hearing loss, some may have relatively steep sloping or notched hearing loss (Cruickshanks et al., 2020; Parthasarathy et al., 2020; Wang et al., 2021). To further address the need for those with sloping or notched hearing loss, additional two-channel amplification can be provided at high frequencies (bottom two panels in Figure 3). Together, these four preset amplification profiles can not only satisfy the FDA OTC hearing aid requirements, but more importantly likely improve both speech intelligibility and sound quality for people with different configurations of mild-to-moderate hearing loss. Future investigation is needed to compare performance between hearing aids using these four presets and that fitted with an audiogram in the targeted population.
The FDA's establishment of the OTC hearing aid category opens up a myriad of opportunities for applying innovative technologies to enhance ear and hearing healthcare, not only in the USA but also globally, particularly in low-middle-income countries (LMICs). In this discussion, we explore opportunities related to smartphones and True-Wireless-Stereo (TWS) devices, delve into the role of artificial intelligence, and assess the potential impact of OTC hearing aids on hearing care in LMICs and their role in delaying or reducing cognitive decline.
While the adoption rate is low for hearing aids, the smartphone penetration rate is high at 68%, with 6.3 billion smartphone subscriptions worldwide (https://www.statista.com/statistics/203734/global-smartphone-penetration-per-capita-since-2005/). Not only do smartphones have more computing power than hearing aid chips, but also an output level similar to the 117 dB SPL output limit specified for the OTC hearing aids (Shim et al., 2018). The challenge is to tackle combinations of different earbuds and smartphones, which may have different input and output specifications. Different combinations of the earbuds and smartphones would affect the noise floor, maximum output level, and frequency responses, rendering acoustic dynamic range and frequency response of the app system unpredictable. Here we show that a simple change in paradigm from measuring an individual's absolute hearing thresholds to her or his auditory dynamic range can turn an arbitrary smartphone-earbud combination into an OTC-like hearing aid.
An individual's relative dynamic range can be estimated based on a particular smartphone and earbud combination (Chung, 2021). For this user-specific smartphone-earbud system, hearing threshold and loudness discomfort levels, or the lower and upper boundaries of the dynamic range can be measured and recorded as the earbud loudspeaker's output in voltage, rather than in dB SPL, at audiometric frequencies from 250 to 8,000 Hz. Once the dynamic range of the user is known for the smartphone-earbud combination, wide-dynamic-range-compression functions similar to those depicted in Figures 1, 2 can be applied to amplify the incoming sounds, effectively turning a smartphone into a compression hearing aid for users with any configuration of hearing loss.
If the microphone sensitivity of the earbud-smartphone combination is unknown, the app can be designed to monitor the incoming sounds, and automatically adjust the gain so that most incoming sounds, e.g., 10–90% distribution of the instantaneous amplitudes, are mapped into the user's auditory dynamic range. Such an amplification paradigm has been implemented as adaptive dynamic range optimization for cochlear implants and hearing aids to regulate the device output (Blamey, 2005). A similar concept can be applied to the smartphone-earbud combination, without the need to know its input scaling.
TWS is a radio-frequency-based, audio transmission technology that requires no wired connection between the earbud and the source (e.g., a smartphone), nor the left and right earbuds. Because the wide usage of the TWS in high-definition audio, 3D sounds, noise cancellation and even heart rate sensing, the global TWS earbud market was valued at US$51 billion in 2022 and expected to grow 10 times more by 2030 (https://finance.yahoo.com/news/global-true-wireless-stereo-earbuds-093800105.html). Many consumer electronics companies, most notably, Apple, have been incorporating hearing aid functions in their TWS earbuds. The potential for making TWS earbuds into an OTC hearing aid is high, but pricing seems to discourage these manufacturers from entering the OTC hearing aid market (see Section 1). Here we discuss potential technological impacts of TWS on the OTC hearing aids.
First, many matured front-end signal processing algorithms in TWS can be implemented to improve safety, efficacy and satisfaction of the OTC hearing aids. One example is noise reduction algorithms. The noise reduction algorithms implemented in smartphones generally work well for low-frequency, steady-state noise such as engine noise.
Second, TWS can be used to take advantage of the wireless link between the microphones and the earbuds. For example, a lapel microphone app can turn the smartphone into a lapel microphone placed close to a sound source (e.g., television) or into a directional microphone pointing toward a speaker in a large room to improve signal-to-noise ratios and reduce the reverberation interference for the user. Similar to prescription hearing aids, the wireless connection can also stream the microphone input, a cellphone output or any audios to the OTC hearing aids. These algorithms can greatly reduce the degradation of speech due to background noise, distance, and reverberation and improve speech understanding and sound quality.
Bluetooth, the key technology underlying TWS, is being advanced rapidly, making TWS-based hearing aids a reality. For example, a concern has been raised about the relatively long delay time in Bluetooth links, i.e., 30–40 ms delay in the low-energy audio Bluetooth transmission (https://www.telink-semi.com/low-latency-headsets-tws-earbuds). Such long delays cannot be considered as “real-time” processing because they would be noticed by people with mild-to-moderate hearing loss as “disturbing” (Stone and Moore, 1999). Recent development of the low-complexity communication codec plus (LC3plus) protocol can reduce the delay to 2.5 ms, which is shorter than the 13 ms acceptable range for individuals with hearing loss (Stone and Moore, 2005). The LC3plus or similar technologies open the door to developing TWS earbuds, or even the microphone-smartphone-earbud combination into viable OTC hearing aids.
AI is poised to transform the overall technological landscape, including health care and service. As of October 19, 2023; FDA has authorized 171 AI-enabled medical devices, with an overwhelming majority (>80%) being in radiology and only one in otolaryngology (an image-guided planning and navigation system to enable ENT procedures, see https://www.fda.gov/medical-devices/software-medical-device-samd/artificial-intelligence-and-machine-learning-aiml-enabled-medical-devices). Starting in 2018, manufacturers introduced “smart” hearing aids that contained AI features. Some AI-related “low-hanging fruits” can be easily incorporated into the OTC hearing aids. For example, automatic soundscape analysis identifies sounds as either speech or music, applying compression or linear amplification to balance the needs between speech intelligibility and music quality. The OTC hearing aids may incorporate AI-based active noise control that suppresses not only background noise but also other sounds such as annoying environmental sounds or even speech from a different talker. The OTC hearing aids can be used in combination with many other AI technologies from automatic speech recognition to sign language translation (Lesica et al., 2021).
The greatest opportunity is to use AI to solve the “cocktail party” problem, or improving speech understanding in noise backgrounds, especially when the background contains multiple competing talkers (Wilson et al., 2022). Different from existing technologies such as directional microphones or FM systems, the AI-based algorithms have an ambitious goal to restore normal hearing to people with hearing loss, at least to those with mild-to-moderate hearing loss (Lesica, 2018). The rapid advances in AI technology, combined with the relatively friendly regulatory environment, should allow manufacturers to implement AI algorithms in OTC hearing aids and potentially benefit their users.
Although the OTC hearing aids were introduced by FDA in the USA, they can impact the global hearing care market in several important ways. First, this new hearing aid category will make future hearing aids more affordable than they are today. Affordable price would likely encourage people to try hearing aids, especially those who are relatively young and have short duration of hearing loss, but experience difficulty in listening to soft sounds and speech in noisy backgrounds (e.g., Knoetze et al., 2023). However, the cost of current OTC hearing aids is high, with a decent pair ranging from US$800 to 3000, which are still prohibitively expensive to many people, particularly those in LMICs, where ~80% of the world's population with hearing loss reside and where people have an annual income < US$1,000. A 10-time reduction in price will make the hearing aid cost an insignificant factor in global hearing health care. The prescription hearing aids will not achieve this cost reduction, but the OTC hearing aids may, when consumer electronics and startup companies directly compete against the traditional hearing aid manufacturers (Zeng, 2023).
One lesson learned from cochlear implants was that the device cost was the initial hurdle in China (Zeng, 1995), but limited or inconvenient accessibility is now mainly responsible for the relatively low cochlear implant adoption rate (Rebscher et al., 2018). The same lesson applies to the hearing aid market. Multiple appointments, long wait for service, and time away from work are some of the barriers for potential users in even high-income countries, where heavily deducted or even free hearing aids are available (Jorgensen and Novak, 2020; Powers and Bisgaard, 2022). The direct access to the OTC hearing aids should help remove these barriers, allowing those who have perceived mild-to-moderate hearing loss to try and hopefully benefit from hearing aids. Ideally and hopefully, the OTC hearing aids would enlarge the potential customer base of the prescription hearing aids, rather than competing for their market share. The emergency of tele-audiology and artificial intelligence will facilitate this market-shaping transformation, especially in LMICs where hearing healthcare infrastructure is limited or lacking (D'Onofrio and Zeng, 2021; Lesica et al., 2021).
The lack of awareness and stigma associated with hearing loss contribute to the globally low hearing aid uptake. For example, even for people in high-income countries, there is an average delay of 4–8.9 years between first notice of hearing loss and the adoption of hearing aids (Simpson et al., 2019; Powers and Carr, 2022). Moreover, disabilities in some communities in LMICs are associated with superstition or shame to the family. The prevalence of smartphones and TWS earbuds (see Sections 5.1 and 5.2) will make OTC hearing aids more discrete than traditional ones, raising awareness while reducing the stigma associated with hearing loss.
The risk for ~40% dementia can be potentially modified, with hearing loss in midlife accounting for the most, or 8.2-percentage points (Livingston et al., 2020). Indeed, recent studies have shown that hearing aids reduce the cognitive decline and dementia in certain high-risk populations (Lin et al., 2023; Yeo et al., 2023). Although it remains unclear whether the relationship between hearing loss and dementia is associative or causative, there is a widely-held assumption that early detection and intervention of hearing loss in adults might not only improve hearing and cognitive processing but also reduce social isolation and delay or prevent dementia in the elderly (e.g., Doherty and Desjardins, 2015; Powell et al., 2022; Huang et al., 2024). The OTC hearing aids target relatively young populations with perceived mild-to-moderate hearing loss, potentially producing a greater long-term cognitive benefit than the traditional prescription hearing aids, which are used by much older populations with greater hearing loss. This long-term benefit can be potentially the most important contribution of the OTC hearing aids.
The OTC hearing aid category, established on October 17, 2022, is designed for adults aged 18 or older with perceived mild-to-moderate hearing loss. Our market and technology analysis has led to the following key conclusions:
• Although there are many newly registered OTC hearing aids, the current OTC hearing aid market is dominated by the traditional hearing aid manufacturers, without apparent disruptors from consumer electronics or startup companies.
• Because of the high-level output without a gain limitation, the OTC hearing aids defined by FDA can provide sufficient amplification for people with severe-to-profound hearing loss.
• Technological innovations are lacking but needed to provide customized amplification for people with mild-to-moderate hearing loss.
• Smartphones and TWS earbuds can function like OTC hearing aids, presenting opportunities to further improve awareness, accessibility and affordability of hearing aids, particularly for users in low-and-mid-income countries.
• Artificial intelligence holds significant potential in enhancing the functionality and performance of OTC hearing aids.
• The OTC hearing aids are likely adopted by more people at younger ages with less hearing loss than the prescription hearing aids, potentially helping reduce or prevent cognitive decline and dementia.
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
KC: Writing – original draft, Writing – review & editing. F-GZ: Writing – original draft, Writing – review & editing.
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
The work was supported in part Center for Hearing Research, University of California, Irvine.
KC has a patent pending on the strategies to provide individualized amplification using earbuds and other sound modifiers. F-GZ owns stock in Axonics, DiaNavi, Neocortix, Nurotron, Syntiant, Velox, and Xense.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fauot.2024.1347437/full#supplementary-material
ANSI (2013). ANSI/ASA S3.46-2013 Methods of Measurement of Real-Ear Performance Characteristics of Hearing Aids. Washington, D.C.: American National Standards Institute.
ASHA (2023). Over-the-Counter Hearing Aids (OTCs) 1 Year Later. Rockville, MD: American Speech-Language-Hearing Association.
Blamey, P. J. (2005). Adaptive dynamic range optimization (ADRO): a digital amplification strategy for hearing aids and cochlear implants. Trends Amplif. 9, 77–98. doi: 10.1177/108471380500900203
Carr, K., and Kihm, J. (2022). MarkeTrak-tracking the pulse of the hearing aid market. Semin Hear 43, 277–288. doi: 10.1055/s-0042-1758380
Chadha, S., Kamenov, K., and Cieza, A. (2021). The world report on hearing, 2021. Bullet. World Health Organiz. 99, 242. doi: 10.2471/BLT.21.285643
Chung, K. (2021). A Universal Method to Turn Any Portable Electronic Devices and Sound Transducers into Personalized Environmental Sound Modifiers. United States Patent & Trademark Office.
Chung, K., Zeng, F. G., and Acker, K. N. (2006). Effects of directional microphone and adaptive multichannel noise reduction algorithm on cochlear implant performance. J. Acoust. Soc. Am. 120, 2216–2227. doi: 10.1121/1.2258500
Chung, K., Zeng, F. G., and Waltzman, S. (2004). Utilizing advanced hearing aid technologies as pre-processors to enhance cochlear implant performance. Cochlear Implants Int. 5, 192–195. doi: 10.1002/cii.227
Cruickshanks, K. J., Nondahl, D. M., Fischer, M. E., Schubert, C. R., and Tweed, T. S. (2020). A novel method for classifying hearing impairment in epidemiological studies of aging: the wisconsin age-related hearing impairment classification scale. Am. J. Audiol. 29, 59–67. doi: 10.1044/2019_AJA-19-00021
Doherty, K. A., and Desjardins, J. L. (2015). The benefit of amplification on auditory working memory function in middle-aged and young-older hearing impaired adults. Front. Psychol. 6, 721. doi: 10.3389/fpsyg.2015.00721
D'Onofrio, K. L., and Zeng, F. G. (2021). Tele-audiology: current state and future directions. Front. Digit. Health 3, 788103. doi: 10.3389/fdgth.2021.788103
FDA (2022). Medical Devices; Ear, Nose, and Throat Devices; Establishing Over-the- Counter Hearing Aids Federal Register. Available online at: https://www.federalregister.gov/documents/2022/08/17/2022-17230/medical-devices-ear-nose-and-throat-devices-establishing-over-the-counter-hearing-aids
Ferguson, M. A., Kitterick, P. T., Chong, L. Y., Edmondson-Jones, M., Barker, F., and Hoare, D. J. (2017). Hearing aids for mild to moderate hearing loss in adults. Cochrane Database Syst. Rev. 9, CD012023. doi: 10.1002/14651858.CD012023.pub2
Ho, C. Y., Li, P. C., and Young, S. T. (2017). Reference equivalent threshold sound pressure levels for Apple EarPods. J Acoust Soc Am 141, EL115. doi: 10.1121/1.4976110
Holman, J. A., Drummond, A., and Naylor, G. (2021). Hearing aids reduce daily-life fatigue and increase social activity: a longitudinal study. Trends Hear. 25, 23312165211052786. doi: 10.1177/23312165211052786
Huang, A. R., Reed, N. S., Deal, J. A., Arnold, M., Burgard, S., Chisolm, T., et al. (2024). Loneliness and social network characteristics among older adults with hearing loss in the ACHIEVE study. J. Gerontol. Series A Biol. Sci. Medical Sci. 79:glad196. doi: 10.1093/gerona/glad196
Jorgensen, L., and Novak, M. (2020). Factors influencing hearing aid adoption. Semin Hear 41, 6–20. doi: 10.1055/s-0040-1701242
Keidser, G., Dillon, H., Carter, L., and O'Brien, A. (2012). NAL-NL2 empirical adjustments. Trends Amplif 16, 211–223. doi: 10.1177/1084713812468511
Killion, M. C. (1993). The 3 types of sensorineural hearing loss: Loudness and intelligibility considerations. Hearing J. 46, 31–36.
Knoetze, M., Manchaiah, V., and Swanepoel, D. (2023). Hearing aid user perspectives: reasons and recommendations for prescription and over-the-counter device uptake. Hearing J. 76, 18–20. doi: 10.1097/01.HJ.0000919772.00462.3e
LaMartina, J. (2023). Audiology's not-so-scary future with OTC hearing aids. Hear. J. 76, 23–24. doi: 10.1097/01.HJ.0000991280.79328.30
Lesica, N. A. (2018). Why do hearing aids fail to restore normal auditory perception? Trends Neurosci 41, 174–185. doi: 10.1016/j.tins.2018.01.008
Lesica, N. A., Mehta, N., Manjaly, J. G., Deng, L., Wilson, B. S., and Zeng, F. G. (2021). Harnessing the power of artificial intelligence to transform hearing healthcare and research. Nature Mach. Intellig. 3, 840–849. doi: 10.1038/s42256-021-00394-z
Lin, F. R., Pike, J. R., Albert, M. S., Arnold, M., Burgard, S., Chisolm, T., et al. (2023). Hearing intervention versus health education control to reduce cognitive decline in older adults with hearing loss in the USA (ACHIEVE): a multicentre, randomised controlled trial. Lancet 402, 786–797. doi: 10.1016/S0140-6736(23)01406-X
Lin, H. Y. H., Lai, H. S., Huang, C. Y., Chen, C. H., Wu, S. L., Chu, Y. C., et al. (2022). Smartphone-bundled earphones as personal sound amplification products in adults with sensorineural hearing loss. Iscience 25, 12. doi: 10.1016/j.isci.2022.105436
Livingston, G., Huntley, J., Sommerlad, A., Ames, D., Ballard, C., Banerjee, S., et al. (2020). Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. Lancet 396, 413–446. doi: 10.1016/S0140-6736(20)30367-6
McDaid, D., Park, A.-L., and Chadha, S. (2021). Estimating the global costs of hearing loss. Int. J. Audiol. 60, 162–170. doi: 10.1080/14992027.2021.1883197
Nixon, G., Sarant, J., Tomlin, D., and Dowell, R. (2021). Hearing aid uptake, benefit, and use: the impact of hearing, cognition, and personal factors. J. Speech Lang. Hear. Res. 64, 651–663. doi: 10.1044/2020_JSLHR-20-00014
Parthasarathy, A., Romero Pinto, S., Lewis, R. M., Goedicke, W., and Polley, D. B. (2020). Data-driven segmentation of audiometric phenotypes across a large clinical cohort. Sci. Rep. 10, 6704. doi: 10.1038/s41598-020-63515-5
Powell, D. S., Oh, E. S., Reed, N. S., Lin, F. R., and Deal, J. A. (2022). Hearing Loss and Cognition: What We Know and Where We Need to Go. Front. Aging Neurosci. 13, 769405. doi: 10.3389/fnagi.2021.769405
Powers, T. A., and Bisgaard, N. (2022). MarkeTrak and eurotrak: what we can learn by looking beyond the U.S. Market. Semin Hear 43, 348–356. doi: 10.1055/s-0042-1758361
Powers, T. A., and Carr, K. (2022). MarkeTrak 2022: Navigating the chaning landscape of hearing healthcare. Hear. Rev. 29, 12–17.
Rebscher, S., Zhou, D. D., and Zeng, F. G. (2018). Development and clinical introduction of the nurotron cochlear implant electrode array. J. Int. Adv. Otol. 14, 392–400. doi: 10.5152/iao.2018.6285
Shim, H., Lee, S., Koo, M., and Kim, J. (2018). Analysis of output levels of an MP3 player: effects of earphone type, music genre, and listening duration. J. Audiol. Otol. 22, 140–147. doi: 10.7874/jao.2017.00339
Simpson, A. N., Matthews, L. J., Cassarly, C., and Dubno, J. R. (2019). Time from hearing aid candidacy to hearing aid adoption: a longitudinal cohort study. Ear Hear 40, 468–476. doi: 10.1097/AUD.0000000000000641
Stone, M. A., and Moore, B. C. (1999). Tolerable hearing aid delays. I. Estimation of limits imposed by the auditory path alone using simulated hearing losses. Ear Hear. 20, 182–192. doi: 10.1097/00003446-199906000-00002
Stone, M. A., and Moore, B. C. J. (2005). Tolerable hearing-aid delays: IV. effects on subjective disturbance during speech production by hearing-impaired subjects. Ear. Hear. 26, 225–235. doi: 10.1097/00003446-200504000-00009
Tucci, D. L., and Califf, R. M. (2023). Over-the-counter Hearing Aids Reply. JAMA. 329, 1226–1226. doi: 10.1001/jama.2023.1798
Vaerenberg, B., Smits, C., De Ceulaer, G., Zir, E., Harman, S., Jaspers, N., et al. (2014). Cochlear implant programming: a global survey on the state of the art. Scien.World J. 2014, 501738. doi: 10.1155/2014/501738
Valente, M., and Amlani, A. M. (2017). Cost as a barrier for hearing aid adoption. JAMA Otolaryngol.-Head Neck Surg. 143, 647–648. doi: 10.1001/jamaoto.2017.0245
van Buuren, R. A., Festen, J. M., and Houtgast, T. (1996). Peaks in the frequency response of hearing aids: evaluation of the effects on speech intelligibility and sound quality. J. Speech Hear. Res. 39, 239–250. doi: 10.1044/jshr.3902.239
van Buuren, R. A., Festen, J. M., and Plomp, R. (1995). Evaluation of a wide range of amplitude-frequency responses for the hearing impaired. J. Speech Hear. Res. 38, 211–221. doi: 10.1044/jshr.3801.211
Villchur, E. (1973). Signal processing to improve speech intelligibility in perceptive deafness. J Acoust. Soc. Am. 53, 1646–1657. doi: 10.1121/1.1913514
Wang, Q., Qian, M., Yang, L., Shi, J., Hong, Y., Han, K., et al. (2021). Audiometric phenotypes of noise-induced hearing loss by data-driven cluster analysis and their relevant characteristics. Front. Med. 8, 662045. doi: 10.3389/fmed.2021.662045
Wilson, B. S., and Tucci, D. L. (2021). Addressing the global burden of hearing loss. Lancet 397, 945–947. doi: 10.1016/S0140-6736(21)00522-5
Wilson, B. S., Tucci, D. L., Moses, D. A., Chang, E. F., Young, N. M., Zeng, F.-G., et al. (2022). Harnessing the power of artificial intelligence in otolaryngology and the communication sciences. J. Assoc. Res. Otolaryngol. 1–31. doi: 10.1007/s10162-022-00846-2
Yeo, B. S. Y., Song, H., Toh, E. M. S., Ng, L. S., Ho, C. S. H., Ho, R., et al. (2023). Association of hearing aids and cochlear implants with cognitive decline and dementia: a systematic review and meta-analysis. JAMA Neurol. 80, 134–141. doi: 10.1001/jamaneurol.2022.4427
Zeng, F. (2023). Over-the-counter hearing aids: hype or hope. Hear. J. 76, 6–7. doi: 10.1097/01.HJ.0000997260.29689.33
Zeng, F.-G. (2015). Do or die for hearing aid industry. Hear. J. 68, 6. doi: 10.1097/01.HJ.0000475871.58830.72
Zeng, F. G., Grant, G., Niparko, J., Galvin, J., Shannon, R., Opie, J., et al. (2002). Speech dynamic range and its effect on cochlear implant performance. J. Acoust. Soc. Am. 111, 377–386. doi: 10.1121/1.1423926
Keywords: hearing aids, over-the-counter, amplification, fitting, customization, smartphone, earbuds, artificial intelligence
Citation: Chung K and Zeng F-G (2024) Over-the-counter hearing aids: implementations and opportunities. Front. Audiol. Otol. 2:1347437. doi: 10.3389/fauot.2024.1347437
Received: 30 November 2023; Accepted: 21 February 2024;
Published: 11 March 2024.
Edited by:
Sumitrajit Dhar, Northwestern University, United StatesReviewed by:
Melanie Ferguson, Curtin University, AustraliaCopyright © 2024 Chung and Zeng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: King Chung, a2NodW5nMUBtZ2hpaHAuZWR1; Fan-Gang Zeng, ZnplbmdAdWNpLmVkdQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.