- Department of Otorhinolaryngology-Head and Neck Surgery, Changi General Hospital, Medical Center 2B, Singapore, Singapore
Introduction: Patients with chronic dizziness often have an unremarkable laboratory vestibular examination and have medical clearance from other specialties. However, functional impairments are still significant and affect patients' quality of life. Recent diagnostic criteria and identification of persistent postural perceptual dizziness (PPPD) have helped us to better understand the psychological-somatic manifestations of organic disorders. As the literature suggests good efficacy using a combination of cognitive behavior therapy (CBT) and vestibular rehabilitation, we compared the efficacy of a hybrid protocol using dizziness handicap inventory (DHI) as an outcome measure amongst the different sub-types of chronic dizziness.
Methods: This was an observational study with 35 participants allocated to three different groups: those who strictly fulfilled the PPPD criteria, those with spontaneous episodic vestibular syndrome, and participants with non-specific dizziness. We compared the DHI total and sub-domain scores at baseline and 6 months post-intervention for differences. All participants undertook three sessions in 6 months.
Results: The total DHI scores were reduced in all three groups. However, the DHI total on average was 11 points higher in the episodic vestibular syndrome group. The emotional sub-domain scores were also reduced in all three groups, but the functional and physical scores were significantly higher in the episodic vestibular group.
Conclusion: A hybrid protocol worked best for typical PPPD patients who strictly fulfilled the criteria followed by participants in the non-specific dizziness group. When symptoms were episodic in spontaneous vestibular syndrome, only emotional handicap was reduced at 6 months, but functional and physical handicap scores remained high. Regardless, the DHI total scores in all groups were significantly reduced, possibly due to CBT reducing the anxiety that results from a lack of understanding of the clinical diagnoses.
1 Introduction
Although the majority of vestibular conditions are clinical diagnoses and can be diagnosed bedside with proper examination techniques (Baloh et al., 2011), most of the participants with dizziness at the outpatient otorhinolaryngology clinic are usually seen in the chronic phase and are also usually harder to manage. These participants commonly present with more than 3 months of dizziness history, and despite maximal medical management, still have complaints of functional dizziness. Often, further laboratory vestibular assessment, neurodiagnostic imaging, and other medical specialty investigations are unremarkable. In 2017, the diagnostic criteria for persistent postural perceptual dizziness (PPPD) reached a consensus (Staab et al., 2017) and described participants with symptoms of dizziness or unsteadiness present on most days for a duration of 3 months or longer. These symptoms are usually made worse in an upright posture, with any active or passive movement, and when exposed to provocative visual stimuli or complex visual patterns. The precipitating underlying condition may or may not be vestibular in origin and can include general medical illness or psychological distress, with PPPD leading to significant functional impairment and distress over time. Symptoms in PPPD are usually not pathognomonic and have many differentials to consider. Some participants may also not fit the typical diagnostic criteria leading to diagnostic challenges in the population. However, the diagnosis of classic PPPD may not be as important as formulating evidenced-based management plans for this group of participants, as the functional impairments (Chua, 2020) affecting participants' quality of life should be the focal point. A recent study by Powell et al. (2020) suggested that a high level of PPPD symptoms exists in the general nonclinical population and is not uncommon. As PPPD is a recently defined disorder of functional dizziness, less evidence is present for the management plans. While the evidence for selective serotonin reuptake inhibitors (SSRIs) is weak (Popkirov et al., 2018), psychologically informed cognitive behavior therapy (CBT) with relaxation techniques has recently shown promising evidence in a randomized controlled trial (Herdman et al., 2022). Our study aimed to recruit PPPD participants through a case-series design to investigate the effects of a hybrid CBT and vestibular rehabilitation. Our primary objective was to establish if there was a greater proportion of PPPD participants with a reduction in the Dizziness Handicap Index (DHI) by at least one category of severity, as compared to other dizziness groups. A secondary outcome was to analyze the emotional, functional, and physical aspects of DHI. We hypothesized that PPPD participants would have a significant reduction in all three domains, especially in the emotional aspects, as compared to other dizziness groups. This is because PPPD is characterized as a functional disorder and outcomes are heavily influenced by patients' psychological reactance to the organic problem. Hence, the emotional response from the limbic system could potentially be reduced. Whereas, in other diagnoses of dizziness, the organic problem itself may take precedence over any emotional reactance. DHI was considered an outcome measure as it is a commonly used and validated tool for exploring one's self-perceived dizziness handicap across different domains, including emotional responses. There is good evidence to suggest that DHI directly correlates with the quality of life in patients experiencing chronic dizziness.
2 Materials and methods
This study had a single-center, prospective observational design. The study was approved by the Singhealth Centralised Institute Board (CIRB) 2020/2250. Participants who presented to the Department of Otorhinolaryngology-Head and Neck Surgery between October 2020 to April 2021 and who had a clinical diagnosis after bedside examination by an ear, nose, and throat (ENT) physician were included. All participants undertook laboratory vestibular examination and neuro-diagnostic imaging to exclude any active/acute or uncompensated peripheral vestibular involvement. The participants who strictly fulfilled the PPPD diagnostic criteria as determined by the Barany Society (Staab et al., 2017) were allocated to one group, while the rest with atypical PPPD features were in the other group. In the atypical PPPD group, participants are further split into the clinical diagnosis sub-types, namely spontaneous episodic vestibular syndrome or non-specific dizziness. All participants had their baseline DHI recorded and were subjected to a hybrid CBT and vestibular rehabilitation protocol at our local institution. Participants returned in the 3rd month with progression to the protocol administered and DHI was measured again in the 6th month.
Spontaneous-episodic vestibular syndrome is defined by the recent GRACE-3 guidelines for emergency care when patients present with vertigo (Edlow et al., 2023) to consider a differential between vestibular migraine and Meniere's disease when a peripheral vestibular etiology is suspected. As not all patients will strictly fulfill the Barany Society's diagnostic criteria, spontaneous episodic vestibular syndrome may be a more appropriate term to use. In the PPPD group, participants either had a previously identified unilateral vestibulopathy or resolved BPPV. While the non-specific dizziness group had unremarkable bedside vestibular examination supported by a normal complete laboratory vestibular investigation and diagnosis of exclusion from other medical specialties such as neurology and cardiology. All patients had central causes of vertigo excluded prior to their recruitment and had no psychotropics or centrally sedating medications.
2.1 Cognitive restructuring
Cognitive restructuring is a technique used in CBT where negative thoughts and hypotheses about the person and the world are replaced by an alternative logical belief formulation. The goal is to grant cognitive access to an alternate belief when negative thought patterns surrounding a situation are identified (Clark, 2013). For example, a negative thought about fall risks may ensue in a patient with dizziness such as “I might fall if I cross the road.” An automatic incorrect assumption would be “If I go out, I might have to cross the road and hence, I might fall.” Taken in context, for a patient with Benign Paroxysmal Positional Vertigo (BPPV) and chronic dizziness, for example, the negative thought could be “My dizziness was triggered with quick head movements and hence moving head too quickly will cause BPPV.” We know that this is not true and psychoeducation about the pathophysiology of BPPV will help empower the patient to understand the disease process better. With extensive counseling, the patient may start to exhibit a good understanding of evidence-based beliefs that no post-maneuver restrictions are needed for BPPV. A correction to this cognitive bias could also be to suggest that if quick head movements induce BPPV, we should expect many sporting athletes and ballerinas with repeated BPPV yet we know this is untrue.
2.2 Cognitive relaxation
Areas of the brain, especially that of the limbic system mediating mood and regulating emotions can influence autonomic regulation. In participants with chronic persistent dizziness, it is not uncommon for them to experience autonomic dysregulation (Lee and Kim, 2014), which includes variation in heart rate and blood pressure. Since there are bi-directional neuronal pathways between vestibular and limbic systems (Rajagopalan et al., 2017), it is not unreasonable to suggest that vestibular stimulation or exercises that help to regulate anxiety, stress, and mood can help with chronic dizziness (Ferrè et al., 2012; Doll et al., 2016). As there are high levels of anxiety and depression seen in participants with dizziness (Kim et al., 2016) and evidence to suggest an interaction of stress and anxiety with central vestibular compensation (Saman et al., 2012), cognitive relaxation exercises such as deep diaphragmatic breathing, progressive muscle relaxation, and guided imagery (Toussaint et al., 2021) can target stress and anxiety levels. This may then reduce dizziness perception in participants with functional dizziness. The patients were taught deep diaphragmatic breathing and progressive muscle relaxation in all three sessions. They were expected to perform both cognitive relaxation exercises as a warmup, leading on to the actual vestibular rehabilitation protocol and ending off with the same relaxation exercises as a cooldown.
The patients were taught how to perform deep breathing through the nose for 4 sec and release the air through the mouth for another 4 sec. They were also asked to close their eyes during the exercise to minimize any sensory distraction and perform deep breathing for 10–15 cycles, three times daily.
Progressive muscle relaxation was paired with deep breathing, where patients were asked to extend both arms in a seated position and progressively clench their fist and forearm muscles, right up to shrugging of their shoulders when they breathed in. The patients relaxed all forearm muscles when they breathed out to release muscle tension. These two validated CBT techniques were carried out by a vestibular audiologist with good conceptual and procedural knowledge of vestibular disorders and who was trained by a psychologist in CBT.
2.3 Vestibular rehabilitation
The fundamental concept of vestibular rehabilitation comprises gaze adaptation, habituation, and substitution. Gaze adaptation consists of a series of repeated head movements while focusing on a target to help with vestibular ocular reflex (VOR) compensation and oscillopsia after a unilateral vestibular hypofunction (Schubert et al., 2008). This may be enhanced with dual tasking as there is evidence to suggest that participants with vestibular hypofunction also often complain about cognitive problems. Since there are bi-directional neuronal projections between vestibular and cortical areas, top-down cognitive inputs are also important in vestibular compensation (Danneels et al., 2020). We outline the protocol below which is adapted from an established evidenced-based approach (Gans, 2010).
2.3.1 Target tracking and head rotations
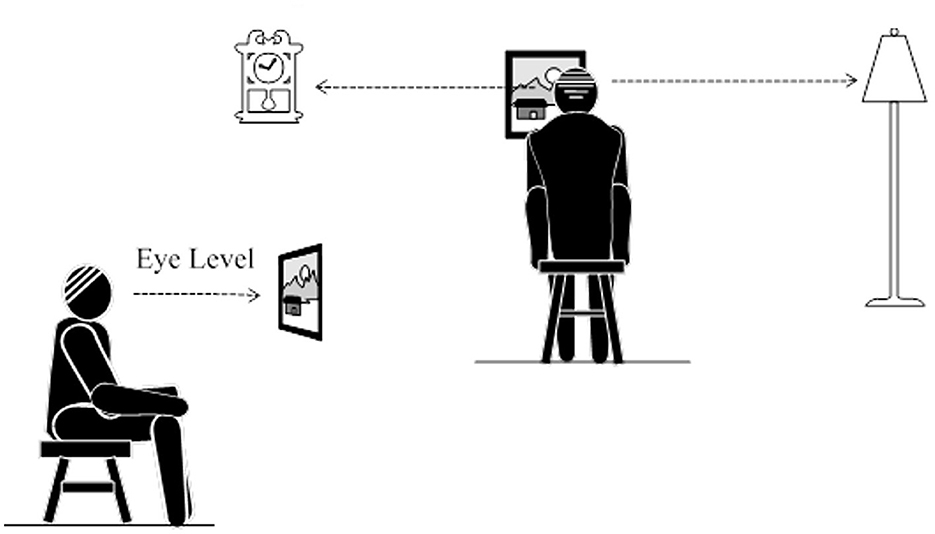
Participants were instructed to find a comfortable position on a sofa or chair at home. They were asked to locate three targets in their room at eye level. One should be over the left shoulder, one in front, and one over the right shoulder. Next, the participants moved their heads looking at the left target, then the center before moving to the right. They repeated this 10–15 times, turning their head without stopping before repeating it another 10–15 times but stopping at each target briefly for 2 s. All participants were instructed to perform this task three times daily for a duration of about 20 min each time based on evidence-based updated vestibular rehabilitation guidelines (Hall et al., 2022). An example of the instruction is shown in Figure 1.
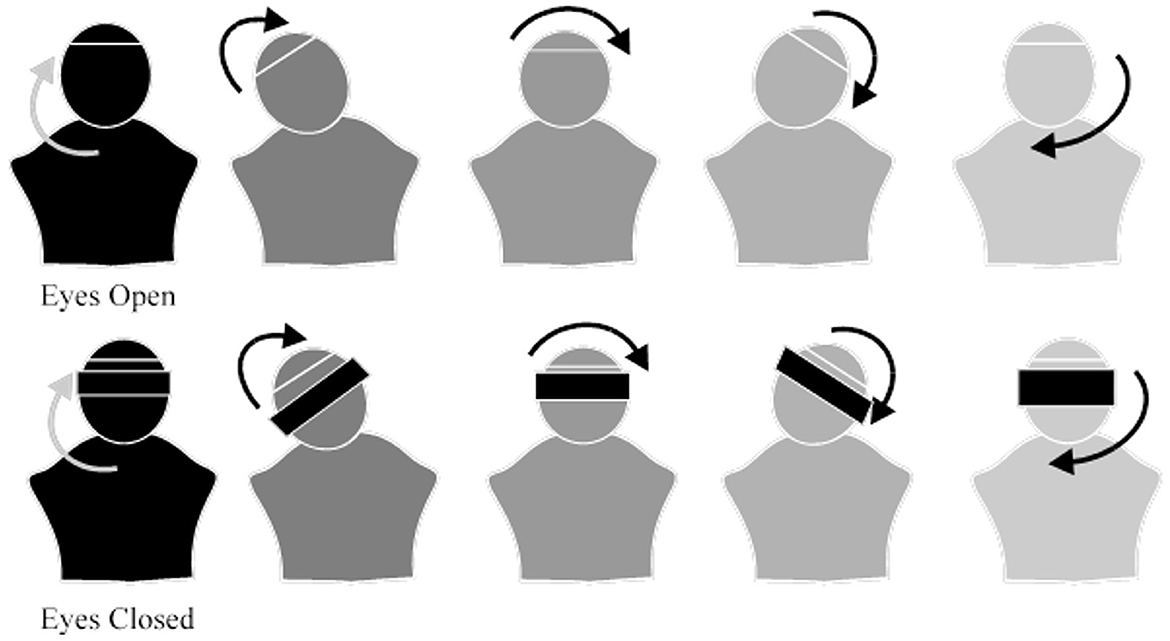
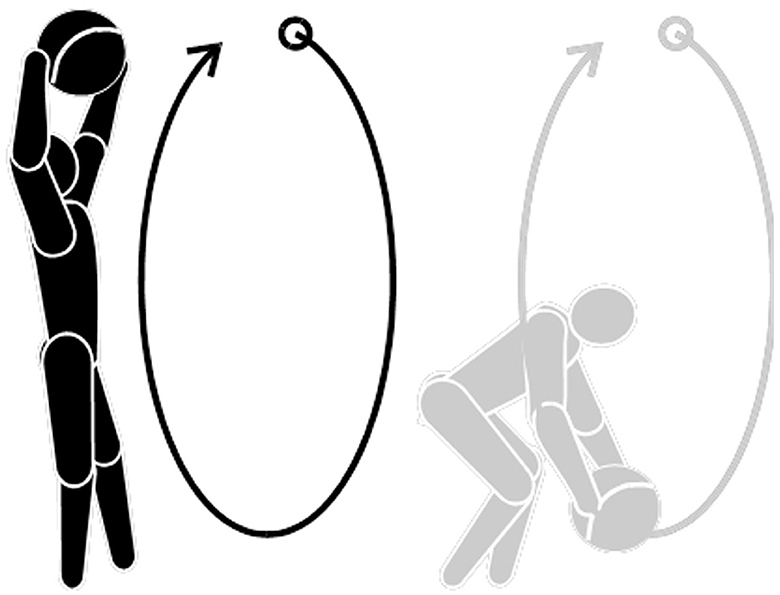
Next, the participants began moving their heads in a circular motion starting clockwise from their own perspective and with their eyes open. They will then repeat the motion with their eyes closed and repeat the exercise in the counterclockwise direction. The participants were asked to perform this circular motion in both directions for a total of 15–20 times, three times daily, as shown in Figure 2.
2.3.2 Gaze adaption: part one
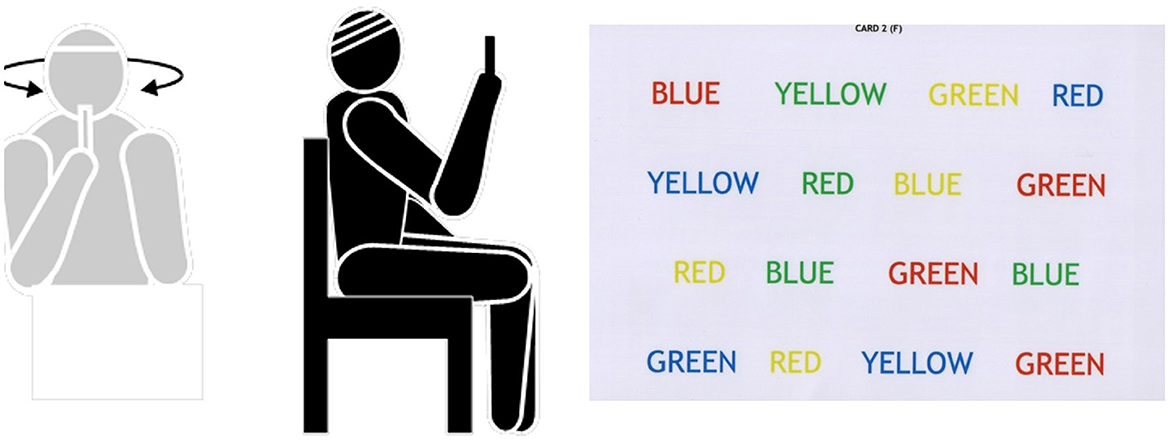
In a seated position, participants were asked first to focus on their fingers while turning their heads side to side at 60 beats per second. A free metronome service found on the Internet was used to guide the speed of head movements. After this, participants were given a cognitive task and were asked to perform the exercise in a dual-tasking paradigm. This was to be repeated 15–20 times, three times daily. Refer to Figure 3 for an example.
2.3.3 Gaze adaptation: part two
Participants progressed from VOR X1 at 60 beats per second (1 Hz) to VOR X2 at 120 beats per second (2 Hz) while seated. They were also instructed on VOR X2 with target moving in anti-phase (opposite direction) of head movements at 1 Hz. Participants then progressed from a seated position to standing upright, to standing on a dynamic foam surface and walking.
2.3.4 Sensory integration with gait
As participants with PPPD require grounding and sensory integration for reassurance due to their exaggerated belief of a loss of postural control, we utilized Peter Levine's framework for somatic therapy (Payne et al., 2015). The participants were taught interoceptive self-holding techniques that originated from treating participants with post-traumatic stress disorders. They were asked to place one hand under the opposite arm and then place the other hand over the upper part of the other arm as if they were giving themselves a hug. While self-hugging, participants were asked to walk while keeping themselves centered with deep breathing techniques. Participants could also modify such techniques with both hands firmly on their hips or on top of their heads, compressing the vertex while walking. There is recent evidence to suggest that sensory integration helps participants with balance dysfunction especially when integrated with dynamic posturography training and assessment (Gonzalez Eslait et al., 2023).
2.3.5 Ankle, circle sway, and ball circles
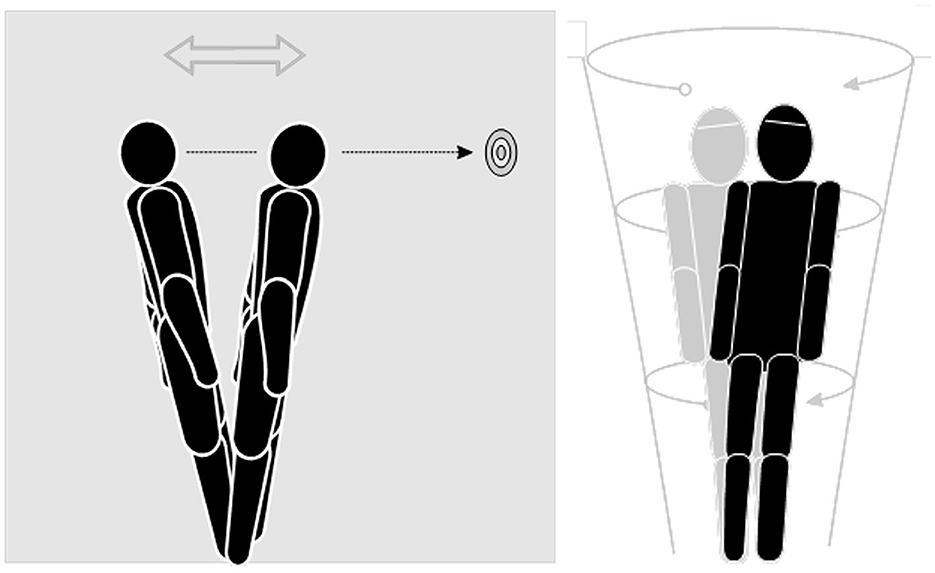
With wall support, the participants were asked to stand with their feet shoulder distance apart, with equal weight on both feet and their arms relaxed at their sides. They looked straight ahead, closed their eyes, and slowly shifted their weight forward and backward without bending their hips. As all movements were at the ankle, this helped to train limits of stability and is a form of habituation without visual inputs. This swaying was then extended to a circular motion by swaying forward, to the right side, to the rear, and then to the left and forward again in a circle sway. Lastly, an object such as a lightweight ball was used to make large circular movements by lifting the ball high overhead and low to the ground, bending their knees to touch the ground with the ball. The participants were asked to always keep their eyes on the ball and move smoothly and continuously. This helped to train VOR suppression (Figures 4, 5).
All participants went through cognitive restructuring, relaxation, and vestibular rehabilitation exercises including target tracking, head rotations, and gaze adaptation part one. They returned after 2 months with progression to gaze adaptation part two and sensory integration with gait. At the 4th month mark, participants were taught habituation exercises including ankle, circle sway, and ball circles. DHI was administered at baseline and at 6 months during the final follow-up review.
2.4 Statistical analyses
Exploratory data analyses were performed with SPSS version 29.0.1.0, IBM and non-parametric tests were used. Multivariate analysis and the Wilcoxon-signed ranked test for related samples were conducted.
3 Results
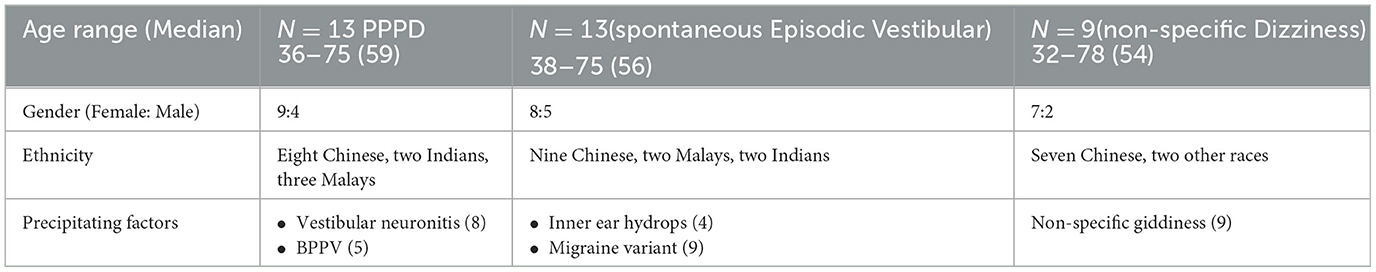
A total of 48 participants were recruited for this study, with a median age of 56 and an age range between 28 and 85 years old. Of the 48 participants, 13 had at most a mild degree of dizziness handicap when recorded at baseline and hence were not included in the analyses. Of the remaining 35 participants, 13 fulfilled the strict PPPD diagnostic criteria as defined by the Barany Society (Staab et al., 2017), while 22 had atypical symptoms of PPPD. Of the 22 non-PPPD patients, they were further categorized by their dizziness sub-type, namely non-specific dizziness or spontaneous episodic vestibular syndrome. Hence, we analyzed the results among the three groups by examining the precipitating factors in the PPPD, spontaneous episodic vestibular syndrome, and non-specific dizziness groups and comparing their DHI scores. The demographic and clinical characteristics of all participants are shown in Table 1. The majority (62%) of the PPPD participants had a previously identified unilateral vestibulopathy as the underlying precipitating factor while 38% had a history of BPPV. Nine patients had non-specific giddiness while in the spontaneous episodic vestibular group, 70% (9/13) had a vestibular migraine variant, and 30% (4/13) had inner ear hydrops. There were no significant differences in the median age among all three groups and there were consistently more females compared to males in each group, respectively.
3.1 Proportion of DHI severity reduction
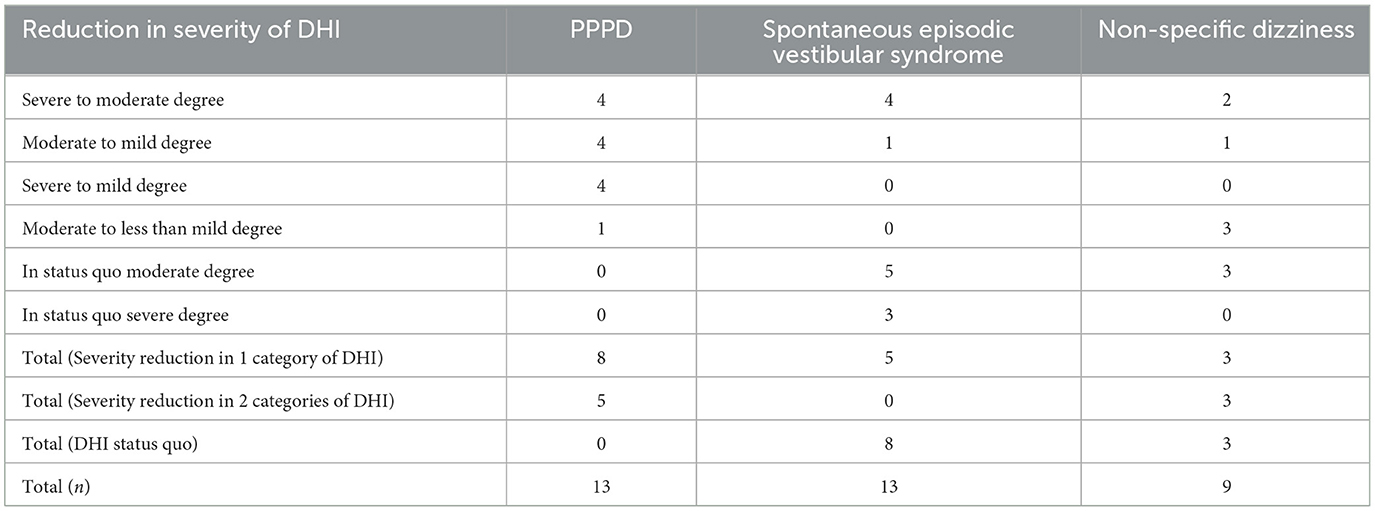
Of the 13 participants in the PPPD group, eight had a reduction in one category of severity in the DHI, four participants each from a Severe to Moderate Handicap and from a Moderate to Mild Handicap degree, respectively. Five participants had a reduction in two categories of severity in the DHI, with four participants going from a Severe to Mild Handicap degree and one participant from a Moderate to less than Mild Handicap. All 13 participants (100%) achieved the primary end objective of a reduction in at least one category of severity in the DHI post-intervention at 6 months. In the spontaneous episodic vestibular syndrome group, only 5 of 13 participants (38.5%) achieved a reduction in one category of severity in the DHI. The rest of the participants (8/13) had dizziness handicap status quo post-intervention. Lastly, in the non-specific dizziness group, six of nine participants (66.7%) had a reduction in at least one category of severity in the DHI. The results are further elucidated in Table 2.
3.2 Dizziness handicap index total
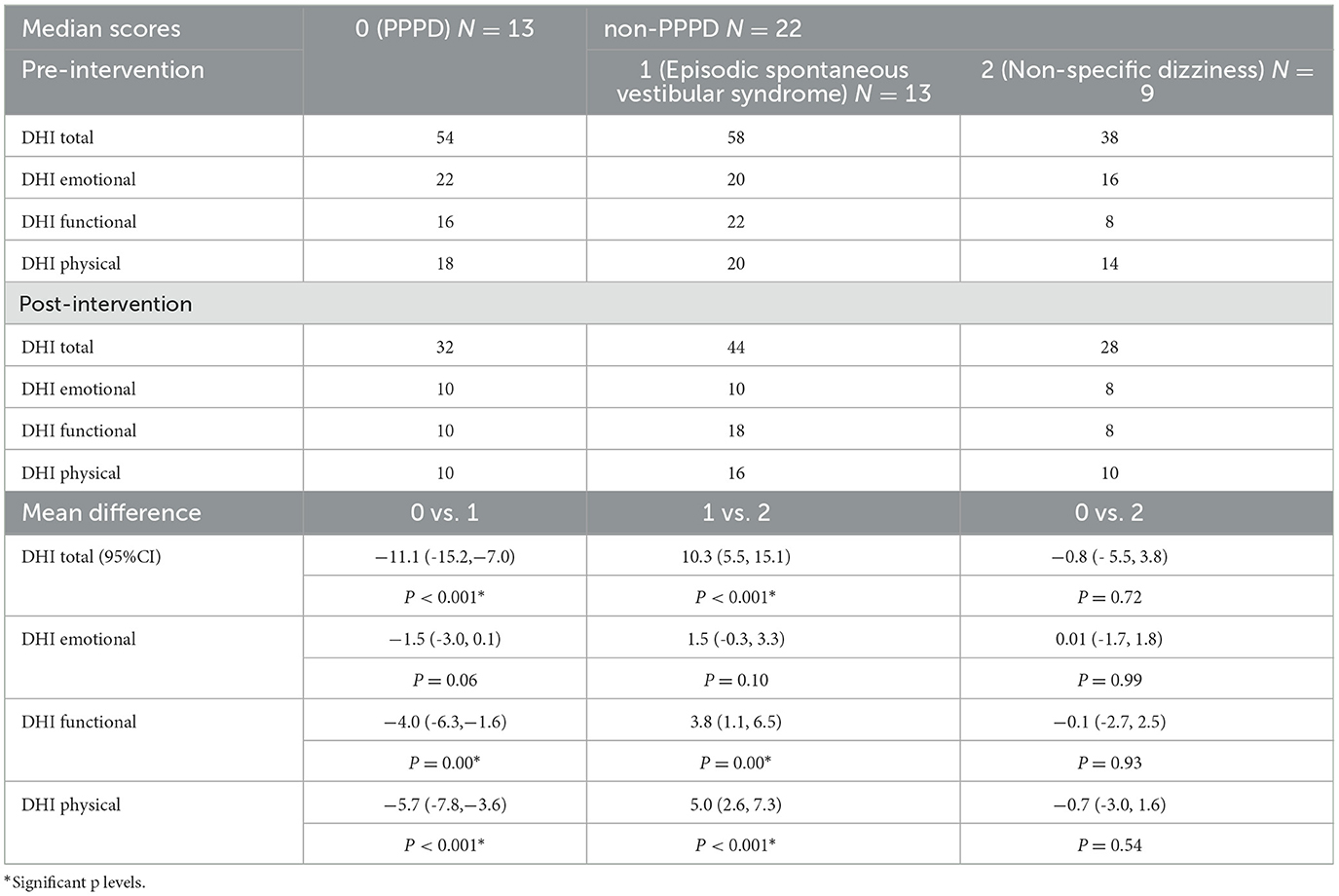
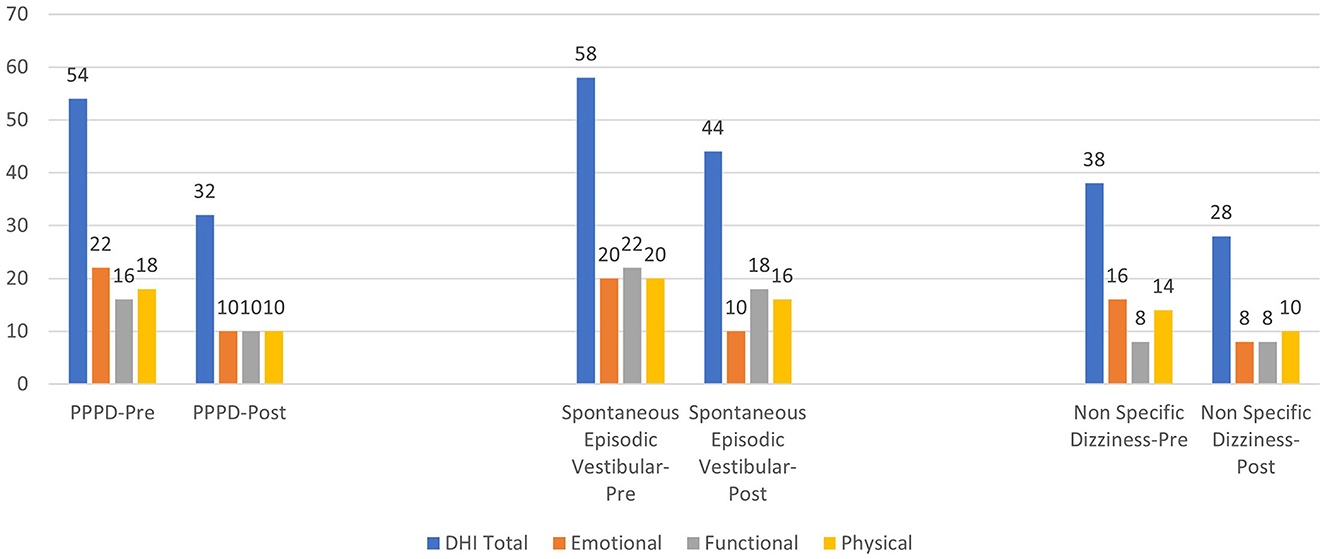
Adjusted for baseline DHI scores, multivariate analyses suggested no significant difference in the DHI total scores post-intervention between the non-specific dizziness group and the participants with PPPD. However, there were significant differences between the participants with spontaneous episodic vestibular syndrome and the other two groups (p < 0.001). On average, the participants with episodic vestibular syndrome had 10 and 11 points higher post-intervention DHI total scores as compared to the non-specific dizziness and PPPD groups, respectively (p < 0.001) (Table 3). Clinically, this is also significant as it could represent a reduction in one category of severity in the DHI, for example, from severe to moderate or from moderate to mild.
3.3 Dizziness handicap index sub-domains
3.3.1 Emotional scores
The post-intervention emotional scores for all groups were generally not significantly different from each other. However, the slight significance observed (p = 0.06) between the PPPD group and the participants with spontaneous episodic vestibular syndrome suggested a 1.5 points lower score in the PPPD group. This was not clinically significant. Hence, in all three groups, the post-intervention emotional DHI scores were quite similar.
3.3.2 Functional scores
The functional scores were significantly different with an average of four points difference between the PPPD group and the participants with spontaneous episodic vestibular syndrome. The findings were also similar when we compared the group with non-specific dizziness to the participants with spontaneous episodic vestibular syndrome.
3.3.3 Physical scores
Lower scores by an average of five to six points were observed post-intervention in the PPPD and non-specific dizziness groups as compared to the participants with episodic vestibular syndrome. This was statistically significant. A summary of the DHI scores compared among the three groups can be seen in Figure 6.
4 Discussion
All the participants in the PPPD group and ~67% of the participants in the non-specific dizziness group achieved a reduction in at least one severity category in the DHI. When organic precipitating vestibular factors were well identified in the PPPD group and no longer active or acute, the combined psychologically informed hybrid vestibular rehabilitation intervention appeared to be effective in treating all 13 participants with PPPD. We see an overall average of 10 to 11 points lower DHI total scores and between 4 and 6 points lower DHI physical and functional scores, at 6 months follow-up in the PPPD group as compared to the participants with spontaneous episodic vestibular syndrome. The emotional DHI subdomain scores were reduced in all three groups which suggests that the significantly higher DHI total scores in the episodic group were likely due to higher functional and physical handicap. This is not unreasonable as participants may fluctuate between the interictal and active phases of their episodic condition and, at their 6 month follow-up, may have had vertigo attacks in between resulting in higher physical and functional handicaps. Hence, despite vestibular rehabilitation, participants will be decompensated whenever there's an active attack. However, the psychological reaction can still be attenuated as evidenced by the reduction of emotional scores in all three groups. This suggests the effects of psychoeducation and dizziness counseling regardless of precipitating clinical diagnoses or even when one does not fulfill the typical criteria for PPPD as in the non-specific dizziness group.
There were also no significant differences observed between the PPPD and non-specific dizziness groups in all DHI domains and total score post-intervention. This suggests that although PPPD criteria may not be strictly fulfilled in the non-specific dizziness group, the current diagnostic criteria may not be granular enough to identify the atypical cases and sub-types of PPPD. As clinical diagnoses such as PPPD, including vestibular migraine and Meniere's Disease, are not guided by any objective laboratory investigations but by symptomology phenotypes and clinical history, there may be variants that are not well identified yet to capture all sub-types.
Hybrid dizziness counseling with vestibular rehabilitation works best when underlying precipitating factors are mostly resolved. When recurrent, as in the case of episodic vestibular syndrome, DHI scores will fluctuate overall and hence intervention will be less effective. Achieving good control of vertigo is key for the rehabilitation of patients in this group. Nevertheless, psychoeducation, cognitive restructuring, and relaxation can help to improve mood, reduce cognitive biases, and reduce DHI overall handicap scores, especially in the emotional subdomain. As emotions are linked to vestibular compensation, we believe that de-stressing and improving anxiety and mood through empowerment with a combination of education and vestibular exercises can help with reducing dizziness perception. However, the limitations of this study include a small sample size that has to be further validated in future studies. Furthermore, such a hybrid technique may not be transferrable to different demographics, including the pediatric population or when cultural differences apply and language becomes a barrier for counseling. Prospective studies in the future should also include a control group to better elucidate the effects of hybrid CBT and VRT.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by Singapore Health Centralised Institute Research. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
KC: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Resources, Supervision, Writing—original draft, Writing—review & editing. JD: Data curation, Validation, Writing—review & editing. HY: Supervision, Writing—review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Baloh, R. W., Honrubia, V., and Kerber, K. A. (2011). Baloh and Honrubia's Clinical Neurophysiology of the Vestibular System, 4 edn., Contemporary Neurology Series. New York, NY: Oxford Academic (accessed August 7, 2023).
Chua, K. W. D. (2020). The focus on functional impairments in the rehabilitation of participants with unexplained functional dizziness. Int. Phys. Med. Rehab. J. 5, 122–123. doi: 10.15406/ipmrj.2020.05.00243
Clark, D. A. (2013). “Cognitive restructuring,” in The Wiley Handbook of Cognitive Behavioral Therapy, ed S. G. Hofmann (Hoboken, NJ: Wiley-Blackwell). doi: 10.1002/9781118528563.wbcbt02
Danneels, M., Van Hecke, R., Leyssens, L., Degeest, S., Cambier, D., van de Berg, R., et al. (2020). 2BALANCE: a cognitive-motor dual-task protocol for individuals with vestibular dysfunction. BMJ Open 10:e037138. doi: 10.1136/bmjopen-2020-037138
Doll, A., Hölzel, B. K., Mulej Bratec, S., Boucard, C. C., Xie, X., Wohlschläger, A. M., et al. (2016). Mindful attention to breath regulates emotions via increased amygdala-prefrontal cortex connectivity. Neuroimage 134, 305–313. doi: 10.1016/j.neuroimage.2016.03.041
Edlow, J. A., Carpenter, C., Akhter, M., Khoujah, D., Marcolini, E., Meurer, W. J., et al. (2023). Guidelines for reasonable and appropriate care in the emergency department 3 (GRACE-3): acute dizziness and vertigo in the emergency department. Acad. Emerg. Med. 30, 442–486. doi: 10.1111/acem.14728
Ferrè, E. R., Bottini, G., and Haggard, P. (2012). Vestibular inputs modulate somatosensory cortical processing. Brain Struct. Funct. 217, 859–864. doi: 10.1007/s00429-012-0404-7
Gans, R. E. (2010). Vestibular Rehabilitation: Protocols and Programs. Tampa, FL: AIB Education Foundation Press.
Gonzalez Eslait, F. J., Escudero Triviño, P. A., Giraldo Vergara, Y. V., Morales García, M. A., and Lucero Gutiérrez, V. F. (2023). Implementation outcomes of a sensory integration therapy program with computerized dynamic posturography in participants with balance and sensory dysfunction. J. Otol. 18, 26–32. doi: 10.1016/j.joto.2022.12.001
Hall, C. D., Herdman, S. J., Whitney, S. L., Anson, E. R., Carender, W. J., Hoppes, C. W., et al. (2022). Vestibular rehabilitation for peripheral vestibular hypofunction: an updated clinical practice guideline from the academy of neurologic physical therapy of the american physical therapy association. J. Neurol. Phys. Ther. 46, 118–177. doi: 10.1097/NPT.0000000000000382
Herdman, D., Norton, S., Murdin, L., Frost, K., Pavlou, M., and Moss-Morris, R. (2022). The INVEST trial: a randomised feasibility trial of psychologically informed vestibular rehabilitation versus current gold standard physiotherapy for people with persistent postural perceptual dizziness. J. Neurol. 269, 4753–4763. doi: 10.1007/s00415-022-11107-w
Kim, S. K., Kim, Y. B., Park, I. S., Hong, S. J., Kim, H., and Hong, S. M. (2016). Clinical analysis of dizzy participants with high levels of depression and anxiety. J. Audiol. Otol. 20, 174–178. doi: 10.7874/jao.2016.20.3.174
Lee, H., and Kim, H. A. (2014). Autonomic dysfunction in chronic persistent dizziness. J. Neurol. Sci. 344, 165–170. doi: 10.1016/j.jns.2014.06.048
Payne, P., Levine, P. A., and Crane-Godreau, M. A. (2015). Somatic experiencing: using interoception and proprioception as core elements of trauma therapy. Front. Psychol. 6:93. doi: 10.3389/fpsyg.2015.00093
Popkirov, S., Stone, J., and Holle-Lee, D. (2018). Treatment of Persistent Postural-Perceptual Dizziness (PPPD) and related disorders. Curr. Treat. Options Neurol. 20, 50. doi: 10.1007/s11940-018-0535-0
Powell, G., Derry-Sumner, H., Rajenderkumar, D., Rushton, S. K., and Sumner, P. (2020). Persistent postural perceptual dizziness is on a spectrum in the general population. Neurology 94, e1929–e1938. doi: 10.1212/WNL.0000000000009373
Rajagopalan, A., Jinu, K. V., Sailesh, K. S., Mishra, S., Reddy, U. K., and Mukkadan, J. K. (2017). Understanding the links between vestibular and limbic systems regulating emotions. J. Nat. Sci. Biol. Med. 8, 11–15. doi: 10.4103/0976-9668.198350
Saman, Y., Bamiou, D. E., Gleeson, M., and Dutia, M. B. (2012). Interactions between stress and vestibular compensation - a review. Front. Neurol. 3:116. doi: 10.3389/fneur.2012.00116
Schubert, M. C., Migliaccio, A. A., Clendaniel, R. A., Allak, A., and Carey, J. P. (2008). Mechanism of dynamic visual acuity recovery with vestibular rehabilitation. Arch. Phys. Med. Rehabil. 89, 500–507. doi: 10.1016/j.apmr.2007.11.010
Staab, J. P., Eckhardt-Henn, A., Horii, A., Jacob, R., Strupp, M., Brandt, T., et al. (2017). Diagnostic criteria for persistent postural-perceptual dizziness (PPPD): consensus document of the committee for the classification of vestibular disorders of the Bárány Society. J. Vestib. Res. 27, 191–208. doi: 10.3233/VES-170622
Toussaint, L., Nguyen, Q. A., Roettger, C., Dixon, K., Offenbächer, M., Kohls, N., et al. (2021). Effectiveness of progressive muscle relaxation, deep breathing, and guided imagery in promoting psychological and physiological states of relaxation. Evid. Based Complement Alternat. Med. 2021:5924040. doi: 10.1155/2021/5924040
Keywords: dizziness, handicap, vertigo, counseling, rehabilitation
Citation: Chua KWD, Deng J and Yuen HW (2023) Hybrid dizziness counseling with vestibular rehabilitation in participants with chronic dizziness: a comparison of dizziness handicap. Front. Audiol. Otol. 1:1277872. doi: 10.3389/fauot.2023.1277872
Received: 15 August 2023; Accepted: 25 October 2023;
Published: 30 November 2023.
Edited by:
Eugen Constant Ionescu, Hospices Civils de Lyon, FranceReviewed by:
Viviana Mucci, Western Sydney University, AustraliaDavid Tomé, Polytechnic of Porto (P.Porto), Portugal
Copyright © 2023 Chua, Deng and Yuen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Kenneth Wei De Chua, kenneth_chua@cgh.com.sg
 Kenneth Wei De Chua
Kenneth Wei De Chua Jing Deng
Jing Deng