
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Audiol. Otol., 04 August 2023
Sec. Tinnitus
Volume 1 - 2023 | https://doi.org/10.3389/fauot.2023.1238164
This article is part of the Research TopicTinnitus Tools and Protocols for Effective Clinical PracticeView all 11 articles
Objective: This interventional study tested the hypothesis that hearing aids with a tinnitus sound support feature would reduce the impact of tinnitus for both new and experienced hearing aid users over a 12-week trial period.
Methods: A total of 19 experienced hearing aid users and 21 participants with no previous hearing aid experience completed the study. Hearing aids were fitted and dispensed with tinnitus masking sounds adjusted to individual preferences. The primary outcome measure was the Tinnitus Functional Index (TFI) score change between baseline and the end of the 12-week trial. This trial was registered on the Australian New Zealand Clinical Trials Registry, trial ID: ACTRN12621001754831.
Results: The TFI scores and secondary measures indicated significant improvements (reductions in tinnitus impact) at the end of the trial compared to the baseline for both experienced and new hearing aid users. Since no group differences were observed, pooled data are presented in this study. The median TFI total score before treatment was 49.0 (IQR = 40.0), and the median TFI total score after treatment was 26.0 (IQR = 26.0). A significant reduction (p = 0.0001) in the total TFI score of 24 points was observed after treatment, producing a large effect size (d = 0.60).
Conclusions: The results confirm previous findings that hearing aids assist in reducing the impact of tinnitus on daily life. The Oticon miniRITE R combination hearing aids used in this study resulted in similar improvements for both new and existing hearing aid users. This suggests that the tinnitus-reducing effects of these aids were greater than those already being used by participants.
The onset of tinnitus and its psychoacoustic characteristics have a strong association with hearing loss (Roberts et al., 2008; Savastano, 2008). The benefit of hearing aids (HAs) in mitigating tinnitus has been recognized for decades (Saltzman and Ersner, 1949; Bentzen, 1958; Shekhawat et al., 2013). The quality and variability in tinnitus sound therapy research using HAs have been criticized (Sereda et al., 2018; Kikidis et al., 2021). Kikidis et al. (2021) reviewed 34 studies and found that 50% of the studies had fewer than 40 participants, with a wide range of inclusion criteria and follow-up periods to measure outcomes. However, there has been a growth in the volume of research, and the weight of evidence supports HA use for tinnitus (Shekhawat et al., 2013; Kikidis et al., 2021; Jacquemin et al., 2022). HAs are recommended for patients with persistent bothersome tinnitus and hearing loss according to the guidelines of the American Academy of Otolaryngology-Head and Neck Surgery (Tunkel et al., 2014). They are also important tools in tinnitus masking (Vernon and Schleuning, 1978; McNeill et al., 2012), tinnitus retraining therapy (Jastreboff and Jastreboff, 2000), tinnitus activities treatment (Tyler et al., 2021), and progressive tinnitus management (Henry et al., 2010). As the HA technology has improved, so have the outcomes (Trotter and Donaldson, 2008).
HAs may reduce tinnitus through the psychological benefit of assisting hearing, with less attention being paid to hearing problems and to tinnitus, through the masking of sound, and through counseling accompanying HA fitting (Coles, 1985; Møller et al., 2011). Counseling is not the primary reason for the success of HAs in reducing tinnitus. Those who receive HAs and counseling do better than those receiving only counseling (Searchfield et al., 2010; Lee et al., 2022). HA fitting without any counseling also improves tinnitus (Shekhawat et al., 2014). Psychosocial improvement in hearing handicap is not the sole reason for tinnitus reduction. HA users' improvement in their tinnitus-related quality of life was predicted by the degree of masking achieved at the fitting appointment (McNeill et al., 2012). The greatest long-term benefit was observed if total masking was achieved at the first fitting; no masking resulted in no long-term reduction in tinnitus handicap. HA use in individuals with tinnitus may also improve sleep and concentration (Zarenoe et al., 2017). Depending on an individual's tinnitus and hearing characteristics, their acute and chronic improvement may be the result of different mechanisms including improved communication, redirected attention, and reduced auditory gain (Searchfield, 2020). The behavioral benefits of the HA fitting process on attention and cognition are mirrored in distributed network changes shown in imaging following HA use for tinnitus. A positron emission tomography (PET) study compared the glycolytic metabolism after 6 months of HA use in persons with and without tinnitus (Simonetti et al., 2022). The PET study showed that metabolism increases in the frontal and temporal regions and decreases in the parietal lobe and cerebellum (Simonetti et al., 2022). Simply stated, amplified sound drives the networks of auditory and associated systems to change tinnitus positively.
Soon after wearable tinnitus maskers were developed, they were combined with amplifiers and called combination aids (Tyler and Bentler, 1987; British Society of Audiology, 2020). At present, many HAs have some form of tinnitus sound therapy as a programmable option. The value of adding therapeutic therapy sounds to amplification is uncertain (Sereda et al., 2018; Tutaj et al., 2018). The sounds generated by HAs have usually been some form of broadband noise (Kim et al., 2014), but, currently, fractal tones (Sweetow and Sabes, 2010) and synthesized ocean wave sounds (Sereda et al., 2017) have also become available. HAs can also wirelessly stream sounds from smartphone applications (via Bluetooth). Until fairly recently, the research investigating HAs and additional therapy sounds as tinnitus management tools has been limited. The benefits of combined sound and amplification and amplification alone are similar (Tutaj et al., 2018). Although different therapy sounds do not affect outcomes greatly (Barozzi et al., 2016; Henry et al., 2017), nature sounds are more pleasant to listen to than broadband noise but appear to be less effective in reducing tinnitus (Durai and Searchfield, 2017). A feasibility study, with 8 participants, of an earlier generation of the Oticon HAs tested in the present study, found broadband noise to be the most effective masker, while simulated ocean wave sounds provided distraction and/or some relaxation (Sereda et al., 2017).
This research was undertaken to further understand the effectiveness of recent HA-based sound therapy developments from one HA manufacturer and to inform clinical practice on how to optimize different forms of tinnitus sound support (TSS).
Our hypotheses were that (1) Tinnitus Functional Index (TFI) and Tinnitus Handicap Inventory (THI) scores would be similar or significantly better than baseline after fitting and 3 months of use of the study devices and (2) the clinical performance of TSS will be the same or better in comparison to the current tinnitus solution, if any (i.e., pre-study HAs).
All participants gave written informed consent for inclusion before participation in the study. The study was conducted in accordance with the Declaration of Helsinki. The protocol was approved by the Northern B Health and Disability Ethics Committee (21/NTB/233). This trial was registered with the Australian New Zealand Clinical Trials Registry (ACTRN12621001754831).
A pre-post interventional design with two groups (new and experienced HA users) was implemented. Participants attended three scheduled appointments at the University of Auckland Clinics, Auckland, New Zealand: baseline assessment and HA fitting (week 0), follow-up (week 3), and final appointment (week 12). Repeated outcome measures were obtained at baseline (study screening) and 12 weeks after the initial fitting appointment via online questionnaires. The study ran from 21 January 2022 until 12 December 2022.
Participants were recruited by advertising on the University of Auckland's research website and through the University of Auckland Tinnitus Research Participant Database mailing list. The inclusion criteria were as follows: adults aged 18 years or above; chronic tinnitus (at least 6 months since onset); new and experienced HA users with a slightly symmetric (16 dB HL) to moderately severe (70 dB HL) binaural symmetric (PTA4 difference between ears ≤15 dB) sensorineural or mixed flat or sloping hearing loss; HA fitting level for the 60- or 85-dB speaker and domes or custom molds including all types and configurations of hearing loss; and scores in the range of “Normal to Severe” in each of the three categories on the Depression Anxiety and Stress Scale−21 Items (DASS 21) (Lovibond and Lovibond, 1995; Antony et al., 1998). The exclusion criteria were as follows: a score of “Extremely Severe” in any one of the three categories of the DASS 21 and objective, pulsatile tinnitus. The flow of participants is shown in Figure 1. The mean participant characteristics for the sample are summarized in Table 1. Audiogram data for the sample are plotted in Figures 2A, B. A total of 40 participants were included in this study, of which 21 of them were new hearing aid users and 19 were experienced hearing aid users. All but one of the experienced users had used their hearing aid for at least 6 months.
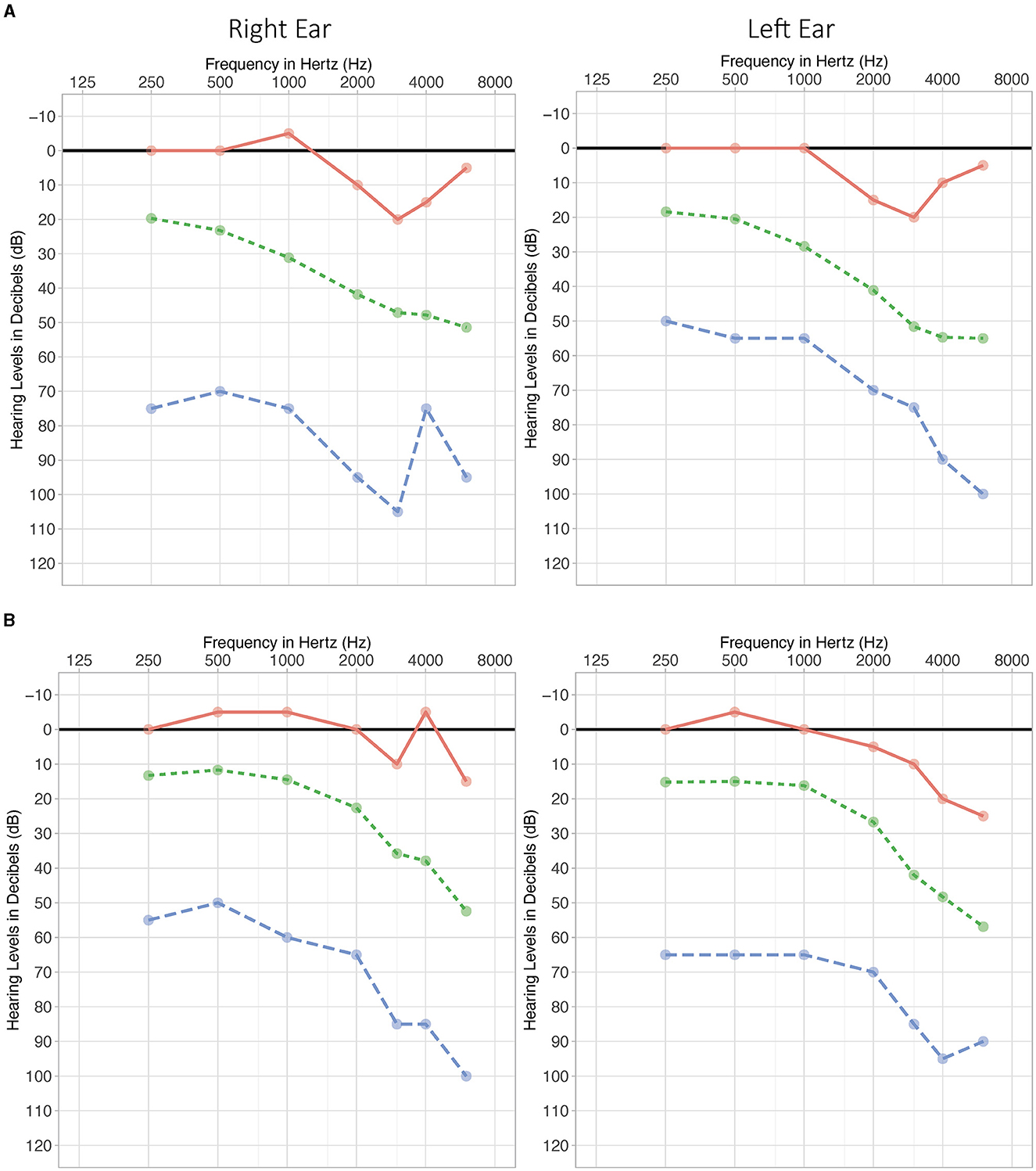
Figure 2. Audiogram for experienced (A) and new users (B). Mean (green, dotted line), maximum hearing loss (blue dashed line), and minimum hearing loss (red solid line).
Participants were instructed to use the provided HAs (study devices) as needed to assist hearing and help with their tinnitus. They were encouraged to use them for several hours per day at least. Participants were asked to explore all four of the programs provided. Participants received verbal counseling on the use of sound therapies for tinnitus according to their needs. All participants were provided with a written information sheet about tinnitus, its pathology, and management strategies.
After contacting the researchers, participants were given an information sheet outlining the background and aims of the trial and details of study measurements. After providing written informed consent, participants were assigned a unique identifier code so that the data could be managed and analyzed in a de-identified manner. Participants were provided with a link to online questionnaires coded, stored, and collated using the University of Auckland's Research Electronic Data Capture (REDCap) account. De-identified data from eligible participants was inputted into the clinical data collection cloud service, SMART-TRIAL, by the researchers to enable monitoring and management by the study sponsors and funder's research team. These systems are compliant with data safety laws as well as good clinical practice.
Background and screening: Participants completed the Tinnitus Sample Case History Questionnaire (TSCHQ) (Langguth et al., 2007) and the DASS 21. These participants provided eligibility data and background that could be used to help guide tinnitus counseling.
Tinnitus: The TFI (Meikle et al., 2012), which has been validated in New Zealand (Chandra et al., 2018), served as the primary tinnitus outcome measure in this trial. The TFI assesses the impact of tinnitus across various dimensions. The THI (Newman et al., 1996, 1998) served as a secondary measure.
The Client Oriented Scale of Improvement on Tinnitus (COSIT) (Searchfield, 2019) goals were established for each participant through discussion with the researcher about how tinnitus was affecting their life and what they hoped to achieve with the intervention. Participants could choose up to five personal goals to be evaluated at the end of their participation in the study.
Participants also rated on numerical scales how much of a problem their tinnitus was (0 not a problem; −5 very big problem) and the severity of tinnitus perception across five dimensions: strength, intrusiveness, discomfort, unpleasantness, and how easy it was to ignore (0 not a problem; −10 extreme problem).
Pure-tone audiometry (MEdRX, AVANT Stealth Audiometer, 0.25–8 kHz) was conducted in a sound-treated room (ISO 8253-1:2010) and employed the modified Hughson–Westlake procedure (Carhart and Jerger, 1959). Tinnitus psychoacoustic outcomes were measured using tinnitus testing software (MEdRX, Tinnometer). The tinnitus pitch-matching was assessed throughout the test frequency range of 0.25–16 kHz using a two-alternative forced-choice method. The measurement continued until two repeated responses were obtained.
The study devices, Oticon More 1 miniRITE R HAs, were dispensed and coupled to the prescribed settings provided by the Genie fitting software, individually adjusted to match the NAL-NL2 targets using a real ear measurement (REM)-based procedure, presented and recorded via an Audioscan Verifit 2 device (version 4.24.6), and programmed via a Noah Link Wireless device. This protocol was based on international state-of-the-art principles for best practices for HA fittings. HA fittings were made by a final-year University of Auckland Audiology intern under the supervision of two of the authors (GDS and PJS).
Four program settings were provided to all participants: (A) Amplification alone (for normal/quiet situations), (B) Enhanced noise reduction settings (for noisy situations), (C) Amplification plus TSS masking sound (quiet with masker), and (D) Enhanced noise reduction settings plus TSS masking sound (noisy with masker). Program 1 on the study device was always Amplification alone (setting A). On programs 2–4, the order of settings on B–D was counterbalanced between participants. In cases where spouses/partners were both enrolled in the study, they were given the same program in order to avoid confusion. One participant received an order that was not correctly assigned according to the counterbalancing allocation.
The TSS masking sound was delivered binaurally through the HAs. Participants were able to choose between modulated or unmodulated white, red, and pink noise or the personalized “Fit to audiogram” sound. Alternatively, three “Ocean” options were available (amplitude modulation of white, red, or pink noise to simulate ocean wave sounds). Default TSS levels were measured using the REM equipment. If required, the researcher then adjusted the TSS to a preferred level indicated by the participant and a second REM measure was recorded.
Counseling was delivered by two of the authoring researchers (GDS and/or PJS), along with the audiology intern, and was based on a psycho-educational and goal-based approach (Searchfield et al., 2011) that was standardized for the study.
A follow-up visit was scheduled for approximately 3 weeks after the initial fitting visit. This allowed participants to request adjustments to the fitting and TSS settings based on their experience with the study HAs. Participants were also able to request a change to the program order (i.e., the allocated program order could be rearranged according to the participant's preference).
Twelve weeks after the fitting appointment, participants again completed the TFI, THI, DASS 21, and severity scales, as well as the COSIT outcomes form. An informal end-of-trial interview was conducted to better understand participant experiences, and any changes to fitting, programs, and TSS settings were made according to the participant's preferences.
Power analysis (G*Power 3.1.9.4) was based on two-tailed t-tests. Power analyses indicated that larger sample sizes were required to detect meaningful effect sizes on the SSQ-12 (hearing-related measures outside the scope of this article) (Gatehouse and Noble, 2004; Noble et al., 2013) than for the tinnitus-related measures. Therefore, sample sizes were based on the SSQ-12 power analyses that indicated that at least 19 participants were required to be current HA users (for an effect size of 0.7, alpha = 0.05, and power = 0.8) and that at least 9 participants were required to have not used HAs prior to enrolling in the study (for an effect size of 1.15, alpha = 0.05, and power = 0.8). Therefore, the minimum sample size was estimated to be N = 28. However, during the recruitment, we aimed to enroll 40 participants to account for dropout and large variations in parameters around the test subject in tinnitus studies (e.g., severity of symptoms, psychological stress among participants, HA use and experience, hearing loss configurations).
Data analysis was conducted using the statistical programming language R. Data distribution was assessed visually using Q–Q plots and histograms and statistically using the Shapiro–Wilk normality test for each outcome measure. Most outcome measures were revealed to be non-normally distributed; therefore, the results are presented as the median and interquartile range (IQR), unless indicated otherwise. Non-parametric two-sample Wilcoxon signed-rank tests were applied to investigate differences between groups (experienced and new HA users) and measured at different time points (baseline and end of 12-week intervention period).
The movement between the severity categories based on the TFI and THI scores and pre- and post-interventions was evaluated. Bins were created to determine the number of participant scores that fell in the following category ranges. The severity categories of TFI were low = 0–18, lower moderate = 19–42, upper moderate = 43–65, and high = 66–100 (Gos et al., 2020) and THI (tinnitus handicap) were slight = 0–16, mild = 18–36, moderate = 38–56, severe = 58–76, and catastrophic = 78–100 (Newman et al., 1998; McCombe et al., 2001).
The COSIT degree of change score ranges from “With the therapy my tinnitus is…”: 1, worse; 2, no different; 3, slightly better; 4, better; and 5, much better. The COSIT final score ranges from “I am annoyed by the tinnitus…”: 1, almost always; 2, most of the time; 3, half of the time; 4, occasionally; and 5, hardly ever. Descriptive statistics are presented in the Results section.
Program usage time for each setting was calculated as a proportion of total usage time. Descriptions are presented in the Results section.
Baseline and final TFI total scores for the experienced and new user groups are illustrated in a box and whisker plot in Figure 3. As no significant difference in the TFI total score was observed between the groups, scores were pooled for statistical analysis. The baseline total TFI score ranged from 12.4 to 85 points, and the final total TFI score ranged from 2.4 to 80 points (see Supplementary Figure 1 for distribution). Table 2 shows the median, IQR, median change score, and effect size of the TFI total and the subscale scores before and after treatment. The median TFI total score was 49 (IQR = 40) before treatment and 26 (IQR = 26) after treatment. A statistically significant reduction (p = 0.0001) in the total TFI score of 24 points was observed after treatment, with a large effect size (d = 0.60). The Auditory and the Relaxation subscales had the highest change scores of 32 points after treatment with the combination aids (Auditory: p = 0.0004, Relaxation: p = 0.004; Table 2). Significant reductions in the other TFI subscales (Intrusive, Sense of control, Cognitive, Sleep, Quality of life, and Emotional) were also observed after treatment, with the change scores ranging from 1.6 to 27 points (Table 2; Supplementary Figure 2). The largest effect sizes were observed for the Emotional, Auditory, Quality of Life, Sleep, and Cognitive scales (Table 2). The least improvement was observed for the Intrusiveness subscale, with a minimal reduction in score. However, the change was statistically significant and produced a medium effect size (Table 2).
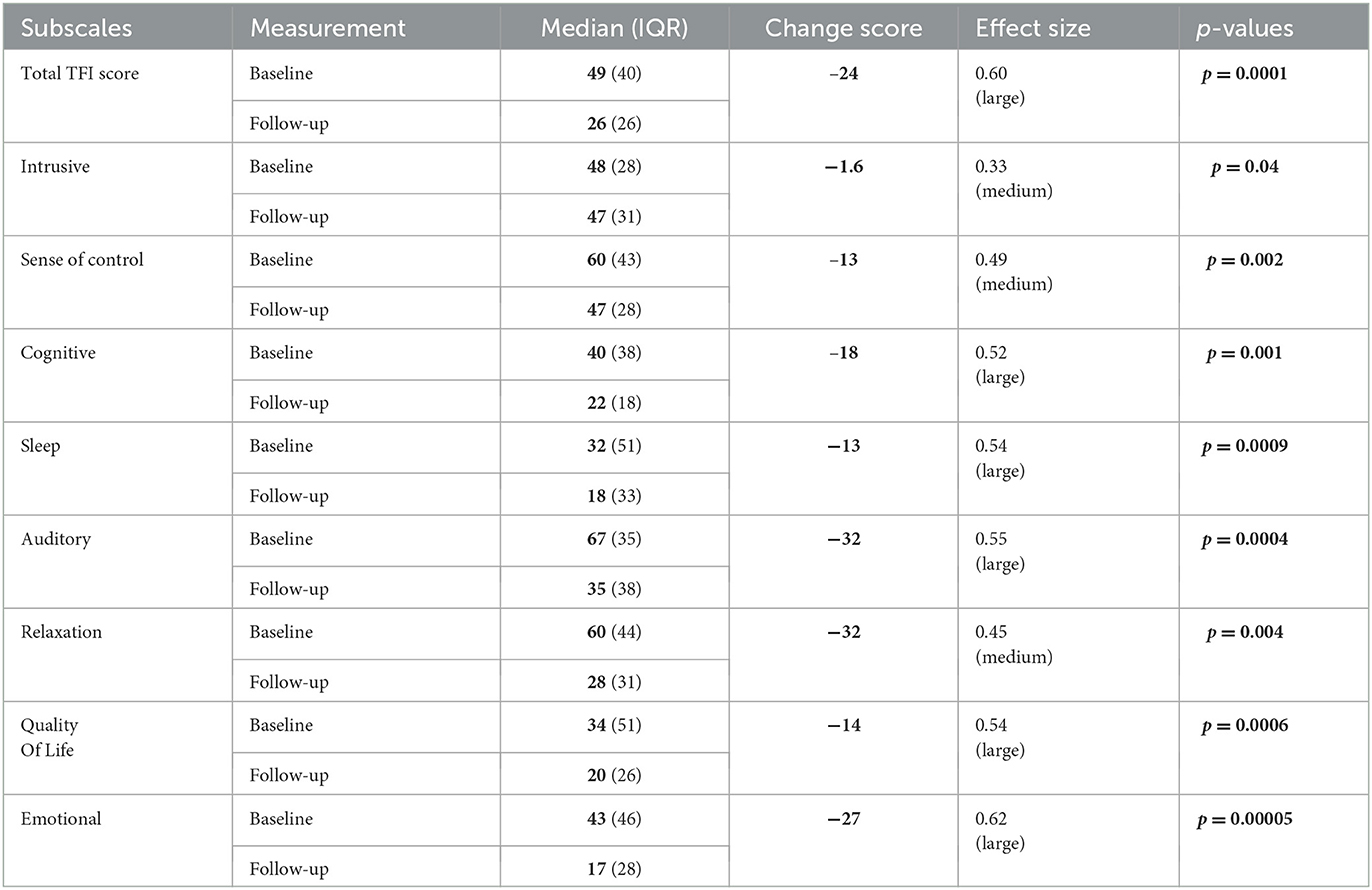
Table 2. Median, interquartile range, change scores, effect sizes and p-values of the TFI and TFI subscales before and after treatment with the combination aids.
Fewer participants were categorized as having upper moderate or high severity tinnitus and more as having lower moderate or low severity tinnitus after treatment than at baseline (Figure 4).
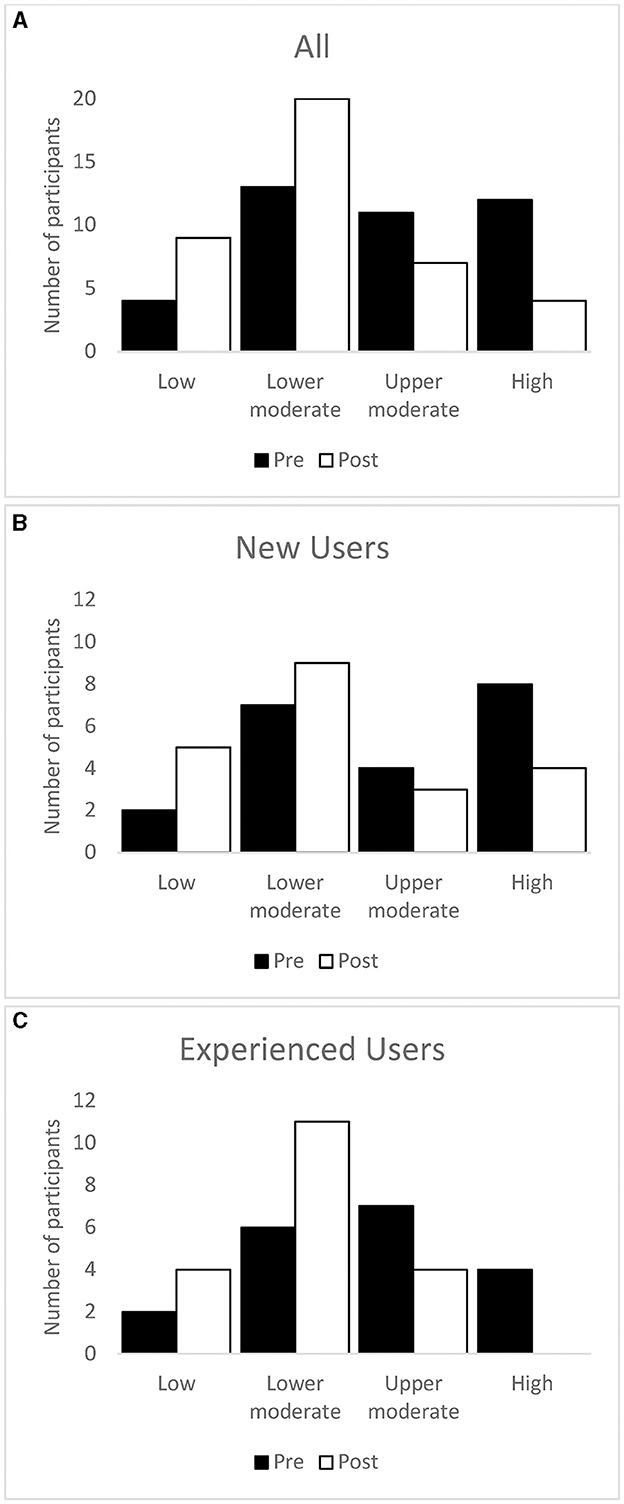
Figure 4. Number of participants in each TFI severity category at baseline and after the intervention period. Whole sample (A), new users (B), and experienced users (C).
Baseline and final THI total scores for the experienced and new user groups are illustrated in a box and whisker plot in Figure 5. As no significant difference in the THI total score was observed between the groups, scores were pooled for statistical analysis. The baseline total THI score ranged from 6 to 98 points, and the final total THI score ranged from 2 to 82 points (see Supplementary Figure 3 for distribution). Table 3 shows the median, IQR, median change score, and effect size of the THI total and the subscale scores before and after treatment. The median THI total score was 40 (IQR = 33) before treatment and 23 (IQR = 27) after treatment. A statistically significant reduction (p = 0.0001) total THI score of 17 points was observed after treatment, with a large effect size (d = 0.61). Significant reductions in the three subscales (Functional, Emotional, and Catastrophic response) were also observed with effect sizes ranging from d = 0.46 to 0.62 and change scores ranging from 3 to 8 points (Table 3; Supplementary Figure 4).
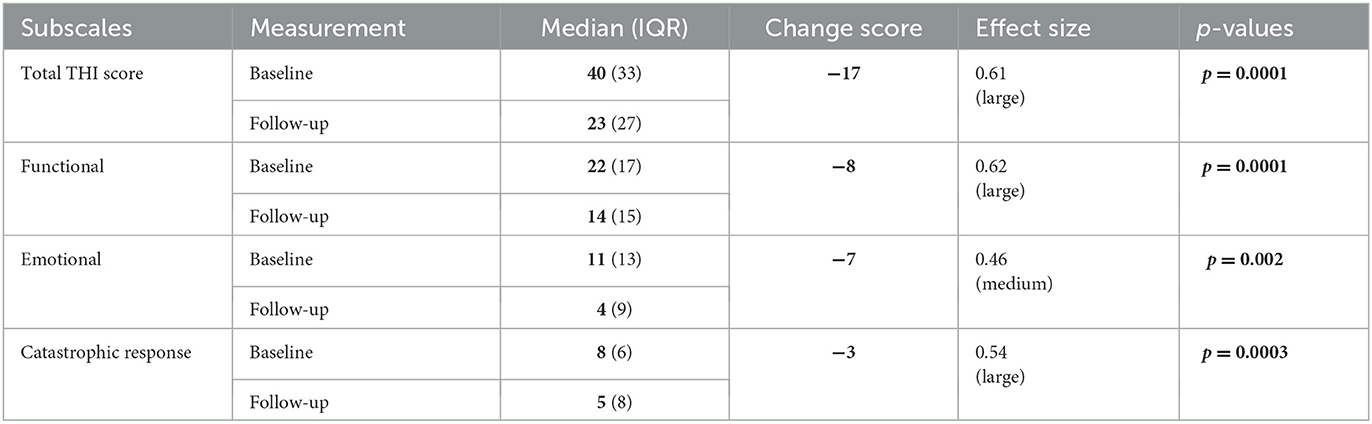
Table 3. Median, interquartile range, change scores, effect size, and p-values of the THI and THI subscales before and after treatment with the combination aids.
Scores indicated that fewer participants were categorized as having a catastrophic or severe tinnitus handicap and more as having a moderate, mild, or slight tinnitus handicap after treatment than at baseline (Figure 6).
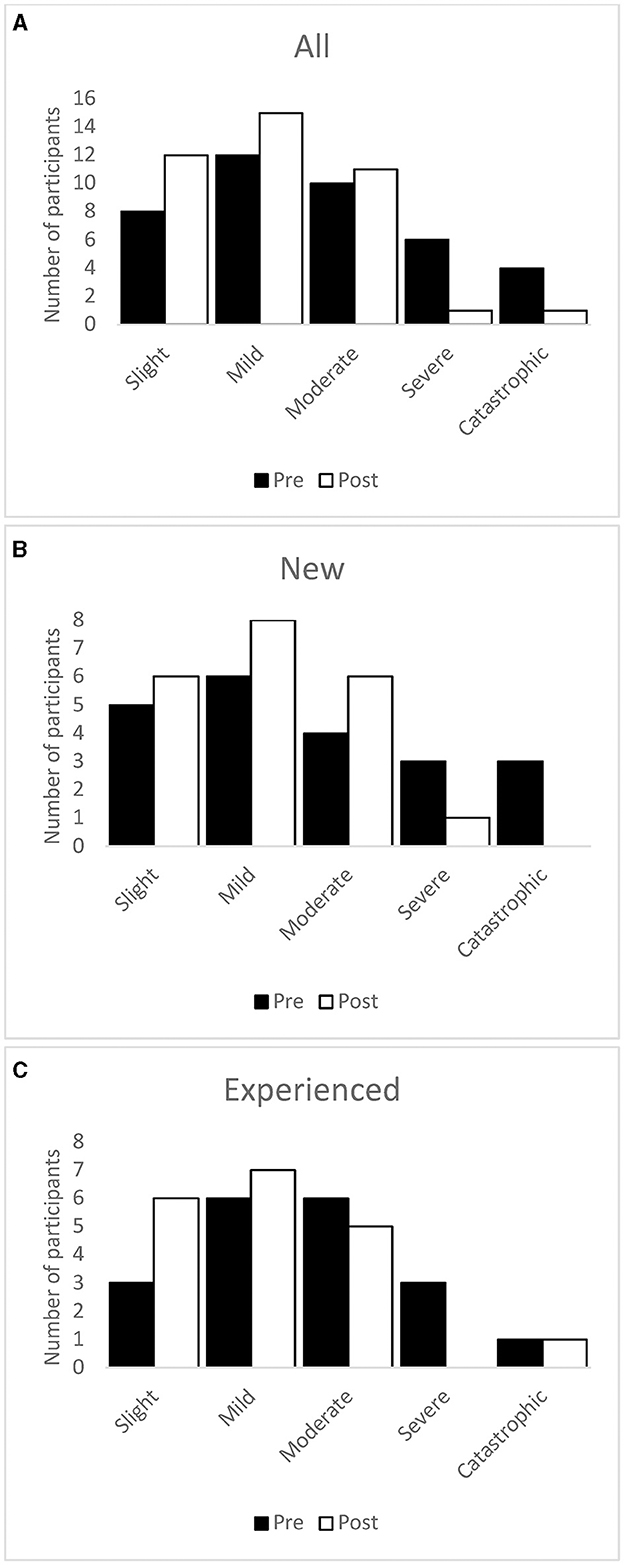
Figure 6. Number of participants in each THI severity category at baseline and after the intervention period. Whole sample (A), new users (B), and experienced users (C).
The means and standard deviations for Change and Final Scores are presented in Table 4 and indicated improvements in tinnitus in relation to personal goals after treatment. Table 5 shows the proportion of the whole sample, new users, and experienced users who rated their improvement at “3–Slightly Better” or higher on at least one of their goals, on half or more of their goals, and on all their goals. The majority of participants improved on at least 50% of their goals, and 50% of the participants reported improvement on 100% of their goals. Experienced users were more likely to report improvements than new users (see Discussion for explanation).
Experienced and new users showed a similar pattern of program use. Figure 7 shows the usage pattern for the entire sample. The Amplification only setting (setting A) was the most used setting, followed by Amplification plus TSS masking sound (setting C). Enhanced Noise Reduction plus TSS (setting D) was used more than Enhanced Noise Reduction alone (setting B). Experienced users spent proportionally more time on setting A and less time on the other settings than new users.
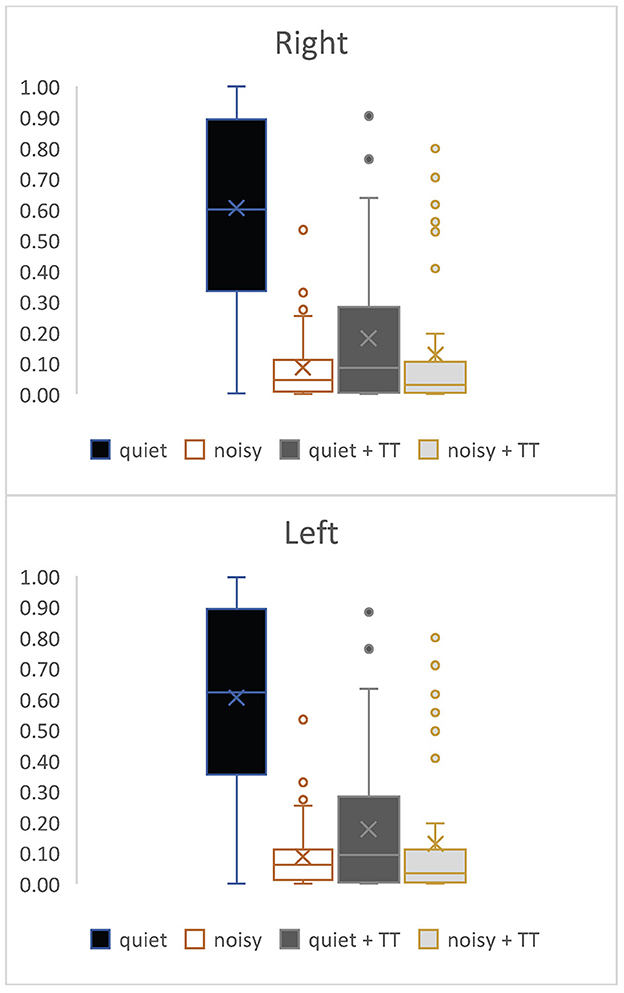
Figure 7. The proportion of individual program use as a function of total usage for right and left ears.
The results of this study confirm previous findings that HAs assist in reducing the impact of tinnitus on daily life. The Oticon miniRITE HAs used in this study resulted in similar improvements for both new and existing hearing aid users. This suggests that the tinnitus-reducing effects of these aids was greater than those of the aids already being used by participants.
The TFI is one of the most widely used self-report tinnitus measures for documenting treatment effects as pre-post change scores (Henry et al., 2010). Studies suggest that a reduction in the total TFI score of at least 13 points is clinically meaningful (Meikle et al., 2012). The THI has been used alone or in combination with other tinnitus self-report measures for evaluating treatment effects across a wide range of treatment modalities. A change score of 20 points suggests that the treatment is clinically meaningful (Newman et al., 1998). Participants in our study completed both the TFI and the THI at two points in time, before the treatment started and 12 weeks after treatment with the combination aids. Our first hypothesis that, after 3 months of the use of the study device, the impact of tinnitus would be reduced, was confirmed; there were statistically significant reductions in total THI and TFI scores and all their subscales over the course of the study. The movement of participants from more severe to less severe categories provided further evidence for the benefit gained from the use of the study devices. Our results suggested that participants in our sample experienced improvements in emotional, auditory, quality of life, sleep, and cognitive aspects of their lives, highlighting the wide-ranging benefits of the combination study devices in tackling this heterogeneous condition.
The COSIT results provided converging evidence that personally meaningful improvements were achieved for most participants. Interestingly, while both new and experienced HA user groups reported improvements and no statistical differences were observed between them, COSIT results and TFI/THI category movements indicated that experienced users were more likely to report improvements at the end of the trial than new users. This result was surprising because one would expect naive users to experience greater effects than those who would ostensibly be receiving some benefit from their existing devices and, therefore, would have less room for improvement. This may be the result of those with more experience having more realistic expectations. However, it also indicates that the study devices provided benefits beyond that provided by the HAs they were using prior to the study, supporting our second hypothesis that the clinical performance of the study devices would be the same or better than the current tinnitus solution (previous HAs).
Many participants reported that they were not attending as many social events or visiting loud places such as restaurants compared to their normal activities prior to the COVID-19 pandemic. This means that our examination of program use may not reflect usage under more demanding, normal circumstances.
The main limitation of this study was that no control group or control condition was included in the design. This makes it difficult to determine which elements of the intervention (tinnitus counseling, TSS, and amplification) contributed to the benefits observed. Counseling is unlikely to have accounted for the entire effect as it has been shown that HAs can provide benefits even when no counseling is provided (Shekhawat et al., 2014). In the present study, both naive and experienced users showed benefit, so it is unlikely that simple amplification of sound accounted for the entire effect; otherwise, the experienced users would be expected to show less pronounced effects than naive users. It is possible that amplification and/or settings may have been superior with the study devices. Indeed, Amplification alone was the most used program setting. However, the next most used program setting was Amplification plus TSS masking sound. This indicates that people used TSS as needed and that the benefit did not necessarily require constant use. It is our opinion that the effects observed were due to the combination of counseling and the technologies offered through study devices.
A no-intervention period between enrollment and baseline may have been useful to assess the stability of TFI and THI scores, as we have previously found that within-subject scores can vary dramatically over time in some people prior to any intervention (Searchfield and Sanders, 2022). If those with unstable scores between enrollment and baseline are excluded, this type of design can provide reassurance that any effects are a result of the study intervention. However, our large effect sizes and consistent improvement across the whole sample, regardless of experience, give us confidence that the benefits observed were due to the study devices rather than chance or confounding factors.
The strengths of this study included a relatively large sample size for this type of research and the inclusion of both new and experienced HA users, meaning that our findings generalize to both populations. Individual needs and preferences were considered for fitting and TSS settings, and the follow-up appointment in the third week of the study allowed for flexibility after a period of familiarization and adaptation to the devices.
Future studies could investigate the contributions of counseling, amplification, and TSS to reductions in tinnitus severity through controlled studies. While blinding is difficult in these types of studies, controls can be included through the staggered introduction of intervention components after a no-intervention period.
The datasets presented in this article are not readily available because ethical permission was granted specifically for the purposes of this study. There was no approval for public data sharing. Requests to access the datasets should be directed to RN, email: cmV1ckBvdGljb24uY29t.
The studies involving human participants were reviewed and approved by Northern B Health and Disability Ethics Committee (21/NTB/233). The patients/participants provided their written informed consent to participate in this study.
GS was the principal investigator, oversaw the project, and carried out some data collection. PS carried out the bulk of data collection, administered the project, and contributed to data analysis. RN carried out the bulk of data analysis. JJ wrote the original protocol and designed the study. All authors contributed to writing and editing the manuscript.
This research was funded by Demant A/S.
The authors acknowledge Ruth Doran and Oscar Cañete (Oticon), who aided in project administration and data analysis, respectively, and Jane Jeong (University of Auckland), who contributed to study procedures and data entry.
RN is employed by Oticon as a Senior Researcher. JJ was employed by Oticon as a Post-Market Clinical Researcher during the start of the study. GS is a founder and director of Tinnitus Tunes and TrueSilence Therapeutics tinnitus treatment companies. The authors declare that this study received funding from Demant A/S. The funder had the following involvement in the study: study design, and data analysis.
PS and GS declared that they were editorial board members of Frontiers at the time of submission. This had no impact on the peer review process and the final decision.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fauot.2023.1238164/full#supplementary-material
Antony, M. M., Bieling, P. J., Cox, B. J., Enns, M. W., and Swinson, R. P. (1998). Psychometric properties of the 42-item and 21-item versions of the Depression Anxiety Stress Scales in clinical groups and a community sample. Psychol. Assess 10, 176–181. doi: 10.1037/1040-3590.10.2.176
Barozzi, S., Del Bo, L., Crocetti, A., Dyrlund, O., Passoni, S., Zolin, A., et al. (2016). A comparison of nature and technical sounds for tinnitus therapy. Acta Acustica United Acustica 102, 540–546. doi: 10.3813/AAA.918971
Bentzen, O. (1958). Audiology and hearing-aid therapy. Acta Otolaryngol. 49, 24–32. doi: 10.3109/00016485809124396
British Society of Audiology (2020). Practice guidance Fitting of combination hearing aids for subjects with tinnitus. Available online at: https://www.thebsa.org.uk/resources/practice-guidance-fitting-of-combination-hearing-aids-for-subjects-with-tinnitus/ (accessed July 11, 2023).
Carhart, R., and Jerger, J. (1959). Preferred method for clinical determination of pure-tone thresholds. J. Speech Hear. Disor. 24, 330–345. doi: 10.1044/jshd.2404.330
Chandra, N., Chang, K., Lee, A., Shekhawat, G. S., and Searchfield, G. D. (2018). Psychometric validity, reliability, and responsiveness of the tinnitus functional index. J. Am. Acad. Audiol. 29, 609–625. doi: 10.3766/jaaa.16171
Coles, R. R. A. (1985). “Tinnitus and its management,” in Scott-Brown's Otolaryngology, ed. A. G. Kerr (London: CRC Press) 368–414.
Durai, M., and Searchfield, G. D. (2017). A mixed-methods trial of broad band noise and nature sounds for tinnitus therapy: group and individual responses modeled under the adaptation level theory of tinnitus. Front. Aging Neurosci. 9, 44. doi: 10.3389/fnagi.2017.00044
Gatehouse, S., and Noble, W. (2004). The speech, spatial and qualities of hearing scale (SSQ). Int. J. Audiol. 43, 85–99. doi: 10.1080/14992020400050014
Gos, E., Rajchel, J. J., Dziendziel, B., Kutyba, J., Bienkowska, K., Swierniak, W., et al. (2020). How to interpret tinnitus functional index scores: a proposal for a grading system based on a large sample of tinnitus patients. Ear. Hear. 42, 654–661. doi: 10.1097/AUD.0000000000000967
Henry, J. A., McMillan, G., Dann, S., Bennett, K., Griest, S., Theodoroff, S., et al. (2017). Tinnitus management: randomized controlled trial comparing extended-wear hearing aids, conventional hearing aids, and combination instruments. J. Am. Acad. Audiol. 28, 546–561. doi: 10.3766/jaaa.16067
Henry, J. A., Zaugg, T. L., Myers, P. J., and Kendall, C. J. (2010). Progressive Tinnitus Management: Clinical Handbook for Audiologists Appendixes. Long Beach, CA, USA: VA Employee Education System.
Jacquemin, L., Gilles, A., and Shekhawat, G. S. (2022). Hearing more to hear less: a scoping review of hearing aids for tinnitus relief. Int. J. Audiol. 61, 887–895. doi: 10.1080/14992027.2021.2007423
Jastreboff, P. J., and Jastreboff, M. M. (2000). Tinnitus retraining therapy (TRT) as a method for treatment of tinnitus and hyperacusis patients. J. Am. Acad. Audiol. 11, 162–177. doi: 10.1055/s-0042-1748042
Kikidis, D., Vassou, E., Markatos, N., Schlee, W., and Iliadou, E. (2021). Hearing aid fitting in tinnitus: a scoping review of methodological aspects and effect on tinnitus distress and perception. J. Clin. Med. 10, 2896. doi: 10.3390/jcm10132896
Kim, B. J., Chung, S.-W., Jung, J. Y., and Suh, M.-W. (2014). Effect of different sounds on the treatment outcome of tinnitus retraining therapy. Clin. Exp. Otorhinolaryngol. 7, 87. doi: 10.3342/ceo.2014.7.2.87
Langguth, B., Goodey, R., Azevedo, A., Bjorne, A., Cacace, A., Crocetti, A., et al. (2007). Consensus for tinnitus patient assessment and treatment outcome measurement: Tinnitus Research Initiative meeting, Regensburg, July 2006. Progr. Brain Res. 166, 525–536. doi: 10.1016/S0079-6123(07)66050-6
Lee, H., Kang, D., Yeo, S., and Kim, S. (2022). Hearing aid effects and satisfaction in patients with tinnitus. J. Clin. Med. 11, 1096. doi: 10.3390/jcm11041096
Lovibond, P. F., and Lovibond, S. H. (1995). The structure of negative emotional states: Comparison of the depression anxiety stress scales (DASS) with the beck depression and anxiety inventories. Behav. Res. Ther. 33, 335–343. doi: 10.1016/0005-7967(94)00075-U
McCombe, A., Baguley, D., Coles, R., McKenna, L., McKinney, C., and Windle-Taylor, P. (2001). Guidelines for the grading of tinnitus severity: the results of a working group commissioned by the British Association of Otolaryngologists, Head and Neck Surgeons, 1999. Clin. Otolaryngol. All. Sci. 26, 388–393. doi: 10.1046/j.1365-2273.2001.00490.x
McNeill, C., Távora-Vieira, D., Alnafjan, F., Searchfield, G. D., and Welch, D. (2012). Tinnitus pitch, masking, and the effectiveness of hearing aids for tinnitus therapy. Int. J. Audiol. 51, 914–919. doi: 10.3109/14992027.2012.721934
Meikle, M. B., Henry, J. A., Griest, S. E., Stewart, B. J., Abrams, H. B., McArdle, R., et al. (2012). The tinnitus functional index: Development of a new clinical measure for chronic, intrusive tinnitus. Ear. Hear. 33, 443. doi: 10.1097/AUD.0b013e3182597b3e
Møller, A. R., Langguth, B., De Ridder, D., and Kleinjung, T. (2011). Textbook of Tinnitus. New York: Springer. doi: 10.1007/978-1-60761-145-5
Newman, C. W., Jacobson, G. P., and Spitzer, J. B. (1996). Development of the tinnitus handicap inventory. Arch. Otolaryngol. Head Neck Surg. 122, 143–148. doi: 10.1001/archotol.1996.01890140029007
Newman, C. W., Sandridge, S. A., and Jacobson, G. P. (1998). Psychometric adequacy of the Tinnitus Handicap Inventory (THI) for evaluating treatment outcome. J. Am. Acad. Audiol. 9, 153–60.
Noble, W., Jensen, N. S., Naylor, G., Bhullar, N., and Akeroyd, M. A. (2013). A short form of the Speech, Spatial and Qualities of Hearing scale suitable for clinical use: The SSQ12. Int. J. Audiol. 52, 409–412. doi: 10.3109/14992027.2013.781278
Roberts, L. E., Moffat, G., Baumann, M., Ward, L. M., and Bosnyak, D. J. (2008). Residual inhibition functions overlap tinnitus spectra and the region of auditory threshold shift. J. Assoc. Res. Otolaryngol. 9, 417–435. doi: 10.1007/s10162-008-0136-9
Saltzman, M., and Ersner, M. S. (1949). Tinnitus aurium in otosclerosis. Arch. Otolaryngol. Head Neck Surg. 50, 440–442. doi: 10.1001/archotol.1949.00700010452007
Savastano, M. (2008). Tinnitus with or without hearing loss: are its characteristics different? Eur. Arch. Oto-Rhino-Laryngol. 265, 1295–1300. doi: 10.1007/s00405-008-0630-z
Searchfield, G. D. (2019). A client oriented scale of improvement in tinnitus for therapy goal planning and assessing outcomes. J. Am. Acad. Audiol. 30, 327–337. doi: 10.3766/jaaa.17119
Searchfield, G. D. (2020). “Sense and sensibility: a review of the behavioral neuroscience of tinnitus sound therapy and a new typology,” in The Behavioral Neuroscience of Tinnitus, eds. J. Searchfield Grant, and D., Zhang (Cham: Springer International Publishing) 213–247. doi: 10.1007/7854_2020_183
Searchfield, G. D., Kaur, M., and Martin, W. H. (2010). Hearing aids as an adjunct to counseling: Tinnitus patients who choose amplification do better than those that don't. Int. J. Audiol. 49, 574–579. doi: 10.3109/14992021003777267
Searchfield, G. D., Magnusson, J., Shakes, G., Biesinger, E., and Kong, O. (2011). “Counseling and psycho-education for tinnitus management,” in Textbook of Tinnitus, eds. A. R. Møller, B. Langguth, D. De Ridder, and T. Kleinjung (New York, NY: Springer New York) 535–556. doi: 10.1007/978-1-60761-145-5_70
Searchfield, G. D., and Sanders, P. J. (2022). A randomized single-blind controlled trial of a prototype digital polytherapeutic for tinnitus. Front. Neurol. 13, 958730. doi: 10.3389/fneur.2022.958730
Sereda, M., Davies, J., and Hall, D. A. (2017). Pre-market version of a commercially available hearing instrument with a tinnitus sound generator: feasibility of evaluation in a clinical trial. Int. J. Audiol. 56, 286–294. doi: 10.1080/14992027.2016.1254822
Sereda, M., Xia, J., El Refaie, A., Hall, D. A., and Hoare, D. J. (2018). Sound therapy (using amplification devices and/or sound generators) for tinnitus. Cochr. Datab. System. Rev. 2018, CD013094. doi: 10.1002/14651858.CD013094.pub2
Shekhawat, G. S., Searchfield, G. D., and Stinear, C. M. (2013). Role of hearing aids in tinnitus intervention: a scoping review. J. Am. Acad. Audiol. 24, 747–762. doi: 10.3766/jaaa.24.8.11
Shekhawat, G. S., Searchfield, G. D., and Stinear, C. M. (2014). Randomized trial of transcranial direct current stimulation and hearing aids for tinnitus management. Neurorehabil. Neur. Rep. 28, 410–419. doi: 10.1177/1545968313508655
Simonetti, P., Ono, C. R., Godoi Carneiro, C., de Ali Khan, R., Shahsavarani, S., Husain, F. T., et al. (2022). Evaluating the efficacy of hearing aids for tinnitus therapy – A Positron emission tomography study. Brain Res. 1775, 147728. doi: 10.1016/j.brainres.2021.147728
Sweetow, R. W., and Sabes, J. H. (2010). Effects of acoustical stimuli delivered through hearing aids on tinnitus. J. Am. Acad. Audiol. 21, 461–473. doi: 10.3766/jaaa.21.7.5
Trotter, M. I., and Donaldson, I. (2008). Hearing aids and tinnitus therapy: a 25-year experience. J. Laryngol. Otol. 122, 1052–1056. doi: 10.1017/S002221510800203X
Tunkel, D. E., Bauer, C. A., Sun, G. H., Rosenfeld, R. M., Chandrasekhar, S. S., Cunningham, E. R., et al. (2014). Clinical practice guideline: tinnitus. Otolaryngol. Head Neck Surg. 151, S1–S40. doi: 10.1177/0194599814545325
Tutaj, L., Hoare, D. J., and Sereda, M. (2018). Combined amplification and sound generation for tinnitus: a scoping review. Ear. Hear. 39, 412–422. doi: 10.1097/AUD.0000000000000516
Tyler, R., and Bentler, R. (1987). Tinnitus maskers and hearing aids for tinnitus. Semin. Hear. 8, 49–60. doi: 10.1055/s-0028-1089904
Tyler, R. S., Stocking, C., Ji, H., Witt, S., and Mancini, P. C. (2021). Tinnitus activities treatment with total and partial masking. J. Am. Acad. Audiol. 32, 501–509. doi: 10.1055/s-0041-1731698
Vernon, J., and Schleuning, A. (1978). Tinnitus: a new management. Laryngoscope 88, 413–419. doi: 10.1288/00005537-197803000-00005
Keywords: tinnitus, hearing aid, masking, sound therapy, clinical study, combination devices
Citation: Sanders PJ, Nielsen RM, Jensen JJ and Searchfield GD (2023) Hearing aids with tinnitus sound support reduce tinnitus severity for new and experienced hearing aid users. Front. Audiol. Otol. 1:1238164. doi: 10.3389/fauot.2023.1238164
Received: 10 June 2023; Accepted: 18 July 2023;
Published: 04 August 2023.
Edited by:
Adriana L. Smit, University Medical Center Utrecht, NetherlandsReviewed by:
David Tomé, Polytechnic of Porto (P.Porto), PortugalCopyright © 2023 Sanders, Nielsen, Jensen and Searchfield. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Grant D. Searchfield, Zy5zZWFyY2hmaWVsZEBhdWNrbGFuZC5hYy5ueg==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.