- 1WorldFish, Bayan Lepas, Penang, Malaysia
- 2WorldFish, Cuttack, Odisha, India
- 3WorldFish, Guwahati, Assam, India
- 4Biswal Aquatech, Jagatsinghpur, Odisha, India
- 5Department of Agricultural, Food, and Resource Economics, Michigan State University, East Lansing, MI, United States
Introduction: Small indigenous fish species (SIS) can be particularly rich in micronutrients and make a crucial contribution toward improving human nutrition. The introduction of mola (Amblypharyngodon mola), an SIS, which is particularly rich in vitamin A, into smallholder carp aquaculture systems has been widely promoted over the past decade as a promising nutrition-sensitive innovation. However, standardized techniques for the hatchery mass production of mola do not yet exist. We hypothesized that the lack of commercial hatchery mass-production techniques for mola seed is the key barrier limiting potential for widespread adoption of carp–SIS polyculture.
Methods: To address this gap, we conducted breeding trials at a private hatchery in Odisha, India, from July to September 2022, to identify standardized methods for the hatchery-based mass production of mola seed. Breeding was induced using a synthetic gonadotropin-releasing hormone analog (SGnRHa) at 0.5 mL and 0.25 mL per kg of body weight of female fish and male fish, respectively. Fish spawned in double hapas in breeding tanks.
Results: The average fertilization, spawning, and hatching rates over 10 breeding cycles were 81%, 82%, and 85%, respectively. A total of 8.5 million fertilized eggs and 6.4 million hatchlings were produced. The survival of fry during larval rearing trials at a stocking rate of 500 hatchlings/m2 was 58% after 22 days. The mola hatchlings and fry were sold to 29 farmers at prices comparable to those of Indian major carp.
Discussion: This article makes a unique contribution to the literature by documenting the entire process of hatchery-based mass mola seed production, including broodfish collection and maintenance, hormone dose optimization, breeding arrangements, breeder characteristics, breeding behavior and performance fecundity, larval rearing, and seed sales to farmers. This information is intended to serve as a protocol to be followed by any individual or institution with an interest in mola breeding and represents an important contribution to the development of nutrition-sensitive aquaculture.
1 Introduction
Fish is an irreplaceable source of micronutrients in diets in the Global South, where large numbers of vulnerable people belong to “fish-dependent” populations. Previous studies from South Asia have demonstrated that some small indigenous fish species—collectively called SIS1—can be particularly rich in micronutrients, including iron, calcium, zinc, and iodine, and vitamins A, B12, and D, and make a crucial contribution toward human nutrition (Bogard et al., 2017).
Historically in South Asia, SIS sourced from inland capture fisheries were abundant and cheap, but the productivity of freshwater aquatic resources has declined sharply in recent decades in response to overexploitation and habitat degradation, making once plentiful and affordable SIS increasingly scarce and expensive (Toufique and Belton, 2014).
Carp2 polyculture systems are the most widespread form of aquaculture throughout the Indian subcontinent. Introducing SIS into conventional carp polyculture has been promoted over the past decade as a promising nutrition-sensitive innovation, with the potential to substantially increase the micronutrient intakes of farming households, especially for women and children. Nutrition-sensitive aquaculture is defined as a food-based approach to aquaculture development that prioritizes the production of nutrient-dense SIS alongside conventional carp polyculture to support beneficial nutritional outcomes (Thilsted et al., 2016).
The promotion of nutrition-sensitive carp–SIS polyculture has centered around introducing mola carplet (Amblypharyngodon mola, hereafter referred to as “mola”), a small fish species which is particularly rich in vitamin A, into smallholder managed carp aquaculture systems in Bangladesh and India (Roos et al., 2002; Roos et al., 2007; Dubey et al., 2022). These efforts have been successful in producing significant quantities of mola in grow-out ponds with no additional inputs or management, and no reduction in carp yields (Wahab et al., 2003; Milstein et al., 2009). Subsequent studies have demonstrated that including mola in a carp polyculture system can increase the consumption of micronutrient-rich mola by women and children (Castine et al., 2017; Karim et al., 2017) and can be a cost-effective approach to reducing the burden of micronutrient malnutrition (Fiedler et al., 2016).
However, to date, efforts to promote nutrition-sensitive aquaculture have been dependent on the efforts of project or scheme extension workers to coordinate the harvesting of wild mola broodfish, maintain them in dedicated “brood ponds”, and then distribute them to the ponds in target communities. The harvesting process for collecting mola broodfish from the wild also destroys small-sized mola and other SIS. The high costs, difficulties in sourcing and transporting wild mola broodfish, and the lack of incentives for adoption by the private sector associated with this approach make its long-term sustainability following the withdrawal of project support questionable.
In contrast, the development of most farmed aquatic species that are now cultured at scale in Asia (e.g., carps, tilapias, and catfishes) has followed a trajectory from reliance on the seed sourced from the wild, to the hatchery-based mass production of seed using wild broodfish and closure of the lifecycle, to the domestication of broodfish and the creation of seed multiplication and distribution networks by dynamic private-sector actors, including hatcheries, nurseries, and fish seed traders (Belton, 2012).
Standardized hatchery techniques for the mass production of mola and other SIS seed do not yet exist. We hypothesized that a lack of commercial hatchery reproduction techniques for mola seed is a key bottleneck that limits the potential for widespread adoption of carp–SIS polyculture. The project reported on in this study was designed to overcome this key technical constraint to scaling up nutrition-sensitive aquaculture, by developing protocols for hatchery-based seed production of mola in Assam and Odisha, India.
This article reports the results of successful mola seed production trials, conducted in partnership with a private hatchery in Odisha, India, in 2022, which were designed to crack the code of mola seed mass production to facilitate the scaling up of nutrition-sensitive carp–SIS polyculture throughout South Asia. The article’s methodology and results are intended to serve as a road map, to be replicated by any individual or institution with an interest in mola seed production, including hatchery design, induced breeding techniques, and larval rearing.
The article is organized into five sections. Section 1 contains the introduction and background. Section 2 contains a review of previous literature on the nutrient content, reproductive biology, and artificial reproduction of mola. Section 3 details the materials and methods used in the study, including those relating to broodfish collection and maintenance, hormone dose optimization, and breeding arrangements. Section 4 presents results relating to breeder characteristics, breeding behavior and performance, fecundity, larval rearing, and seed sales to farmers. Section 5 concludes by reviewing the implications of the results for the wider promotion of mola as a farmed species, and the potential for the mass production of other candidate SIS in hatcheries.
2 Nutrient content, reproductive biology, and reproduction of mola
Mola fish are distributed throughout Bangladesh, India, Myanmar, and Pakistan. It inhabits rivers, streams, lakes, ponds, ditches, and other stagnant waters (Froese and Pauly, 2022). Its maximum recorded length is 20 cm (Talwar and Jhingran, 1991), although Rahman (1989) reported its maximum length as only 15 cm. The average lifespan of male and female fish is 13 months and 15 months, respectively, as reported in the Payra River of Southern Bangladesh by Ahamed et al. (2017).
It was discovered decades ago that mola contains high contents of calcium, iron, vitamin A, and vitamin B12 (Zafri and Ahmad, 1981), and, therefore, has high nutritional value; since then, it has been one of the most studied SIS. Mola is particularly rich in vitamin A, with 2,503 µg of vitamin A present per a 100-g edible portion (Bogard et al., 2015). Mola also contains high levels of essential amino acids. Mustafa et al. (2015) found that the lipid content of mola is 4.5%, and that the percentage of omega-3 polyunsaturated fatty acids (PUFAs) in total fatty acids is 6.31% ± 1.4% in the head and 5.93% ± 0.75% in the body. Mola has a higher lipid content than and similar protein content to most carps (Bogard et al., 2015).
Mola has small gill rakers that are connected with a membrane to filter plankton, and is predominantly a phytoplankton feeder. Mola is herbivorous, and Chlorophyceae are its most preferred food source (Gupta and Banerjee, 2013).
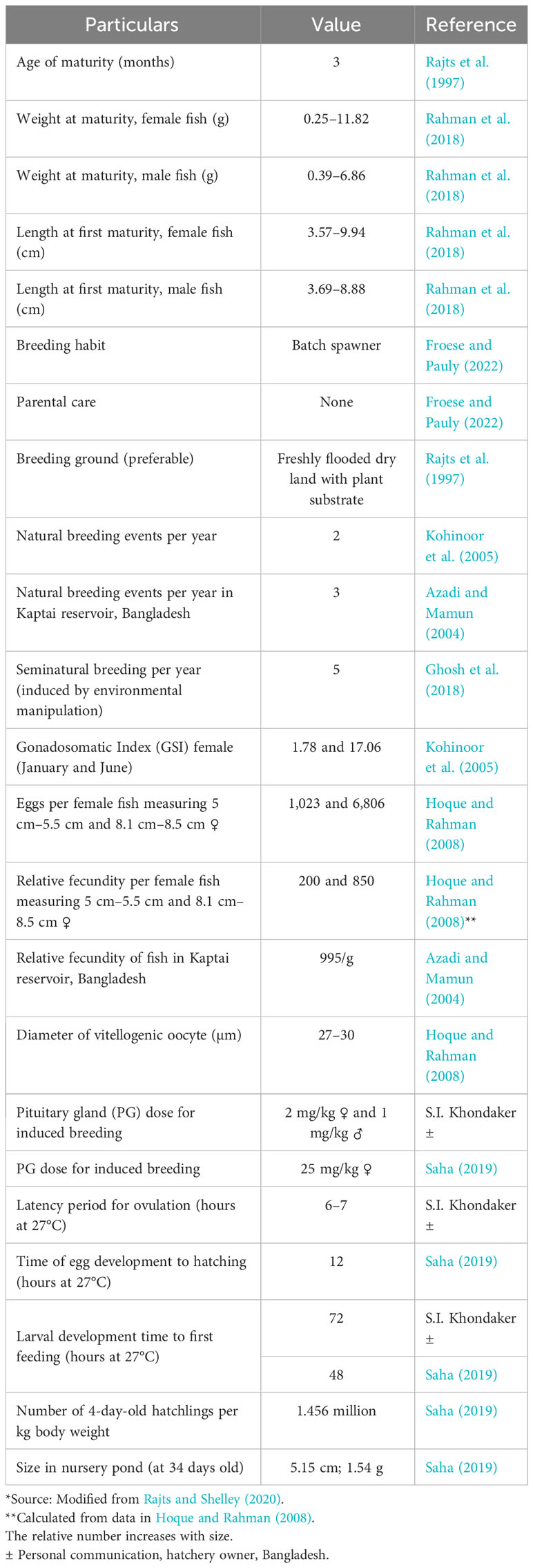
Mola is a prolific breeder, with first maturity reported to occur at 3 months of age (Rajts et al., 1997). Mola is a fractional spawner (Gupta and Banerjee, 2013). The reproductive biology of mola varies with the climate, water quality, food availability, size, and habitat. The present state of knowledge on the reproductive biology of mola is summarized in Table 1.
Mola eggs are slightly adhesive, and an appropriate substrate on which broodfish lay eggs is needed for successful spawning. Mola breeding can be stimulated by introducing fresh water (particularly rainwater), increasing water levels, and the provision of previously dry but freshly inundated land with substrate for the slightly adhesive eggs. The substrate can be rice plants, remaining rice straw, or artificial grass.
During spawning, the rice straw can serve as a substrate for the eggs and helps to generate infusoria3 and other zooplankton that provide a food source for young fry (Thilsted et al., 2016; Saha, 2019).
Kohinoor et al. (2005) studied the reproductive biology of mola for 1 year and found that the gonadosomatic index (GSI) values for female mola ranged from 1.78 ± 0.88 to 17.06 ± 2.66. The highest GSI values for female mola were observed in July. Monthly observation of the diameter of the ova indicated that the fish spawned at least two times per year, once between May and July, and again between September and October (Kohinoor et al., 2005).
The possibility of repeated induced breeding of mola has been demonstrated by several authors. Ghosh et al. (2018) carried out a successful breeding trial in West Bengal. They found that mola can be induced to breed five times in 1 year through environmental manipulation. They did so by flushing ponds with water of different origins. The spawning of mola was observed every time pond flushing occurred and also after heavy rain.
Mondal et al. (2020) maintained mola breeders in hapas in ponds throughout the year to study their breeding habits. Ripe ova were found for 9 months, that is, from April to December. The development of oocytes was identified at different stages in each batch, and natural breeding occurred several times during the culture period. This is consistent with the findings of Ghosh et al. (2018), which were that mola could be bred in captivity several times per year using environmental stimuli.
Saha (2019) reported success in hormonal-induced breeding with the use of carp pituitary gland (PG) extract. He reported that the best dose of PG extract for inducing mola to spawn in the hatchery was 25 mg per kg. The latency time was 6 h–8 h, and hatching occurred 17 h–18 h after fertilization, at a temperature of approximately 27°C. Saha estimated the average weight of 4-day-old hatchlings as 0.7 mg. The hatchlings were grown in a nursery pond and reached 5.14 cm± 0.42 cm in total length and 1.54 g± 0.33 g in weight in 34 days. Saha et al. (2014) also reported techniques for the successful transportation of live mola breeders, which are very sensitive to handling.
Despite a substantial body of work on the reproductive biology of mola, the efforts to conduct induced breeding have been largely ad hoc in nature. No comprehensive, standardized, easily replicable protocol for the mass breeding and larval rearing of mola under hatchery conditions has been published to date. In the rest of the article, we address this knowledge gap by reporting detailed results from our successful breeding and larval rearing trials of mola in Odisha, India, in 2022, which serve as a protocol for the future replication of mass mola seed production.
3 Materials and methods
3.1 Broodfish collection and maintenance
The induced breeding trial for the mass production of mola seed was conducted at the project partner hatchery complex located in Tulunga village (Biswal Aquatech; latitude 20°12′45.84'N/longitude 86°20′3.32'E), Jagatsighpur District, Odisha, India. A sample of wild mola weighing 40 kg was collected between March 2022 and April 2022 from three different sources—community water bodies, private ponds, and canals—to ensure high levels of genetic variation, and was transported to the partner hatchery in 10-L oxygenated plastic bags. On arrival, all live mola were treated with a 10 ppm potassium permanganate (KMnO4) solution and were stocked in two separate broodfish ponds (area per pond: 400 m2, depth: 1 m–1.5 m) at a density of 20 kg per pond (50 g/m2), and maintained for 3 months.
The broodfish ponds were prepared in accordance with standard protocols (Horvath et al., 2002; Rajts et al., 2022). The ponds were semidried and, after cleaning, the wet bottom was treated with powdered hydrated lime [Ca(OH)2], at the rate of 200 g/m2. Basal organic fertilization was conducted using mustard oil cake (MOC) at a rate of 20 g/m2, and water was gradually filled up to a depth of 1 m from a borewell. Following this, an initial application of the inorganic fertilizers urea and single superphosphate (SSP) was made at rates of 10 g/m2 and 20 g/m2, respectively, to augment pond productivity.
Mola broodfish were stocked when water transparency (Secchi disc reading) reached the optimum level of 25 cm–30 cm. The broodfish were fed to satiation twice daily with floating pelleted feed (Natpro feed; 1 mm in size; 42% protein). To maintain the healthy growth of plankton in the ponds, weekly applications of urea and SSP were made at rates of 2 g/m2 and 4 g/m2, respectively.
The water quality of broodfish ponds was monitored periodically between 08:00 and 10:00 using a HANNA multiparameter kit. The average physicochemical parameters of the broodfish ponds were: temperature 30°C, pH 7.5, dissolved oxygen 6.2 mg L–1, alkalinity 190 mg L–1, hardness 85 mg L–1, nitrite (NO2-N) 0.0 mg L–1, and ammonia (NH4-N) 0.6 mg L–1.
It is very difficult to distinguish between male and female mola during the early stages of maturity, but sex can be distinguished during the breeding season (Rajts and Shelley, 2020). When setting up the induced breeding, mature mola broodfish were identified based on their secondary sexual characteristics. Male fish are comparatively brighter than female fish, whereas female fish are larger in size and lighter in color than male fish. Mature female fish have smooth pelvic fins and a deeply forked caudal fin, and can be identified during the spawning season by their soft and conspicuously distended abdomen. Matured male fish have yellowish-colored caudal fins (Figure 1).
3.2 Induced breeding arrangements
The mature and healthy mola broodfish were collected from the broodfish ponds by repeated drag netting, followed by manual segregation by sex, and transferred to a concrete conditioning cum breeding tank (L × W × H: 3.9 m × 2.35 m × 1.10 m; capacity: 10 m3) with a constant water showering for approximately 6 h–8 h, for acclimatization and stimulating spawning readiness. On average, a total of 3.36 kg of male and female mola broodfish were conditioned and injected together. The fish were not fed before the breeding commenced. A combination of hormone administration and environmental manipulation was used to increase seed production.
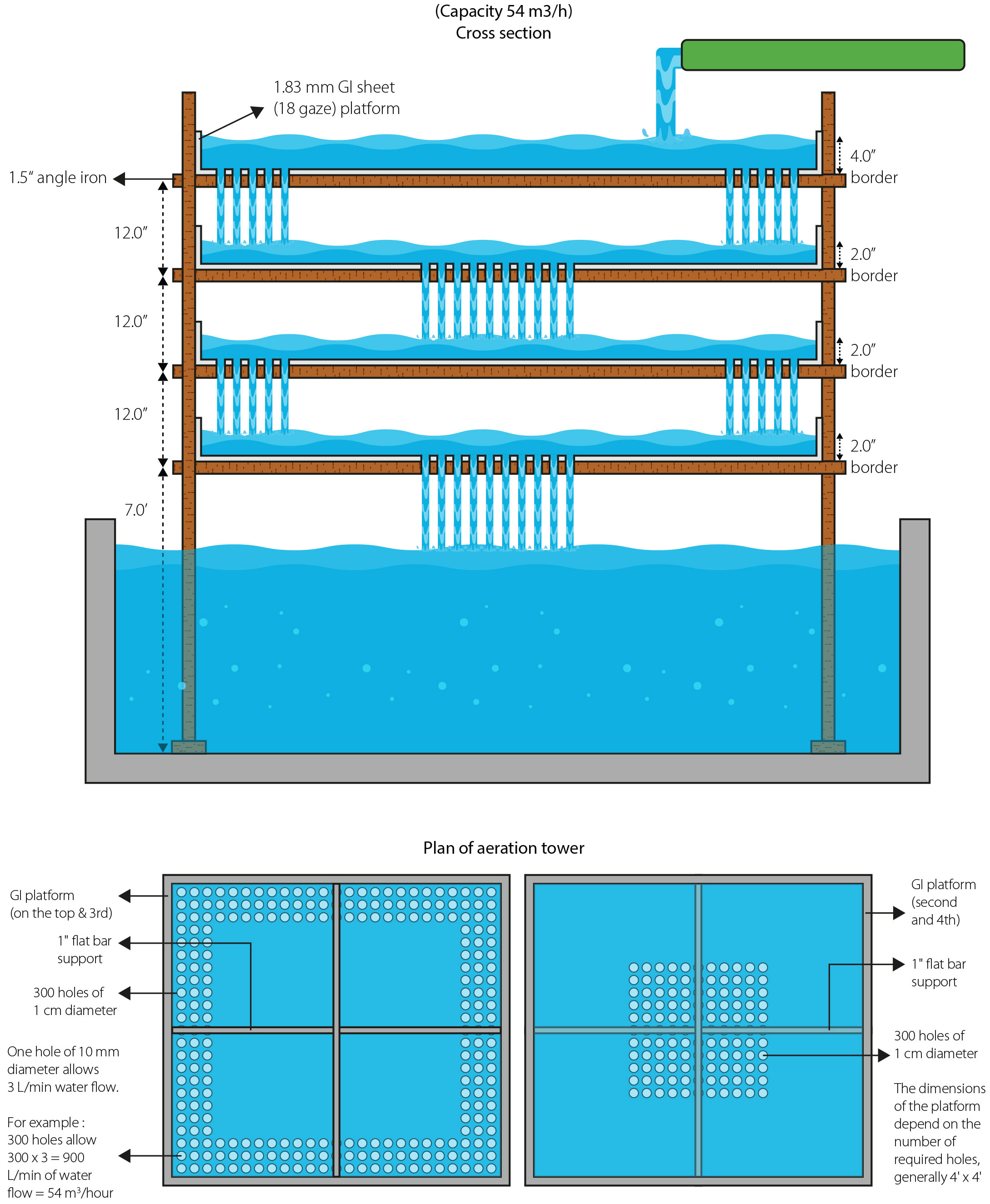
Two breeding tanks were prepared with double hapas. First, a 250-μm nylon mesh outer hapa was installed within the tank. Subsequently, with the aid of a galvanized iron frame, an inner hapa with a mesh size of 8 mm was installed inside the outer hapa. Throughout the trial, the breeding tank was exposed to a constant shower of oxygen-rich water from an overhead tank equipped with an aeration tower (Figure 2). A diagram illustrating the construction of an aeration tower is shown in Figure 3.
3.2.1 Preparation, dose optimization, and administration of inducing hormone
Synthetic gonadotropin-releasing hormone analog (SgnRHa with domperidone) (trade name—WOVA-FH™, manufactured by Biostadt India Limited, Mumbai, India) was used to induce spawning. To optimize the inducing hormone dose, we initially chose five different treatment doses, i.e., 0.1 mL to 0.5 mL per kg body weight of female fish (Table 2). Male fish were administered with half the dose given to female fish.
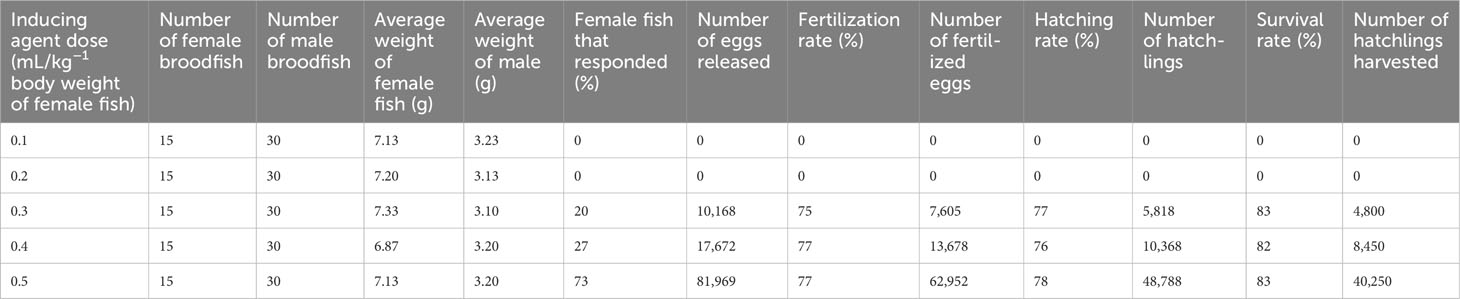
Table 2 Various doses of inducing agents (WOVA-FH) utilized for optimizing the best dose for mass seed production of mola (Amblypharyngodon mola).
The hormone dose was diluted 15 times in 0.65% sterile sodium chloride (NaCl) solution. Dilution was necessary because of the high viscosity of the product and the very small dose required for SIS. The inducing solution was administered into the peritoneal cavity of mola broodfish using an insulin diabetic syringe of 1-mL capacity with 40 graduations. In brief, doses of 0.5 mL and 0.25 mL per kg body weight of female fish and male fish, respectively, yielded the highest hatchling production among the five treatments, and these doses were selected for the mass production of mola seed.
The inducing solution was prepared and injected according to the individual body weight of the fish with an average 2: 1 male-to-female ratio. For female mola breeders weighing 1 kg, 8 mL of inducing solution (0.5 mL of hormone + 7.5 mL of water) was used. The following formula was used to determine the individual doses for breeders:
In which, G represents the number of graduations on the syringe, having the required volume of solution to be injected; H the desired dose of hormone to be applied (original hormone, mL kg –1); W the weight of fish to be injected (g); D the dilution rate of hormone; and V the volume of the diluted solution in one unit of syringe graduation.
For instance, 0.04 mL (5 × 0.008) of inducing solution is required for a single female mola weighing 5 g. One graduation of insulin syringe contains 0.025 mL (1/40 mL). Therefore, for a 5-g female, the volume of solution contained in 1.6-graduations (0.04/0.025) needs to be injected. Immediately after administering the inducing solution, the broodfish were returned to the 10-mm mesh inner hapa, which was covered with nylon mosquito nets to prevent the fish from jumping out.
Table 3 details the characteristics of mola broodfish used over 10 production cycles. A sample of mola broodfish weighing 34 kg in total (female broodfish weighed 21 kg in total and male broodfish weighed 13 kg in total) was injected. Breeding took place over 16 h–22 h during the peak breeding season (i.e., June 2022–September 2022), with a natural photoperiod of 12 h light: 12 h dark. The water quality of the breeding tanks was monitored, and the average values for temperature, pH, and dissolved oxygen were 29.53°C ± 0.39°C, 8.03 ± 0.12, and 6.86 mg/L–1± 0.31 mg/L–1, respectively, over the 10 production cycles.
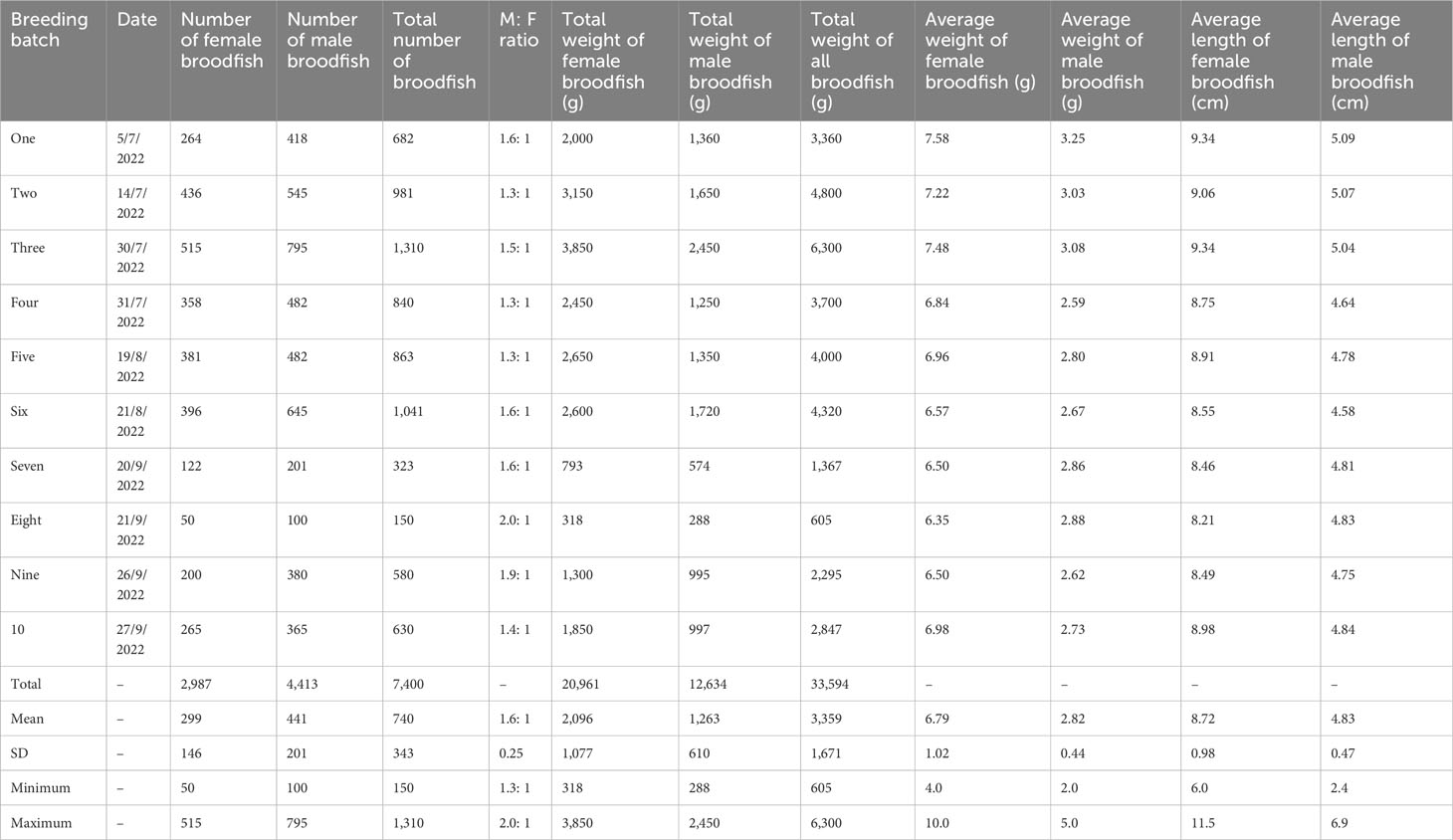
Table 3 Morphometric characteristics and sex ratio of mola broodfish (Amblypharyngodon mola) used for mass seed production.
3.3 Evaluation of breeding performance
The breeding performance of hatchery-based mass production of mola was evaluated in terms of spawning rates (percentage of female fish that responded), fertilization rate (%), hatching rate (%), hatchling production, and survival (%). The numbers of eggs and hatchlings were calculated using the volumetric method and the following equations (Sarkar et al., 2005; Kumar et al., 2018; Kumar et al., 2021):
3.4 Nursery rearing of hatchery-produced mola
Three-day-old hatchlings were stocked from July 2022 to August 2022 in three different nursery ponds that were 400 m2 in size (depth 1 m–1.5 m), at stocking densities of 500, 625, and 750 individuals per m2, and reared for 21 days. The nursing ponds were located inside the partner hatchery complex, at a distance of approximately 200 m from the breeding tanks.
3.4.1 Nursery pond preparation
The nursery ponds were dewatered, and the pond bottom soil was kept moist until lime application. Hydrated lime in powder form was applied at a rate of 200 g/m2 and left to dry on the pond bottom for a week. Mola hatchlings are sensitive to predation from copepods and some insects and insect larvae such as backswimmers and dragonfly larvae. Liming on wet soil followed by drying the pond bottom will eliminate most predators during pond preparation (Rajts et al., 2022).
Fermented MOC was sprayed on the pond bottom as organic fertilizer, after diluting in water at a rate of 20 g/m2. The ponds were gradually filled from a borewell to a depth of 1 m 3–4 days before mola seed was stocked. The inorganic fertilizers urea and SSP were applied by dilution in pondwater at rates of 10 g/m2 and 20 g/m2, respectively, when water filling started. Fermented MOC was sprayed on the pond surface daily thereafter, mainly as an organic fertilizer, at a rate of 1.25 g/m2.
During the initial days of rearing, hatchlings require plenty of small zooplankton because their mouths are small and they are unable to catch fast-moving prey (Rajts et al., 2022). This type of zooplankton consists of protozoans and rotifers. Dried grass was placed all-around the shallow areas of the pond to develop Paramecium and rotifer populations, which are the best source of food for the small mouths of hatchlings. The decomposing grass was removed after 7 days.
Before stocking mola hatchlings, the nursery ponds were netted several times with a mosquito net to remove other predatory insects such as backswimmers and their larvae. To repel predator copepods at the time of stocking, mola hatchlings were stocked within 3–4 days of filling with water. The stocking was conducted in the morning, with special attention paid to the gradual adjustment of the physicochemical characteristics of the carrying water and the pond water.
The hatchlings were fed with microencapsulated chicken eggs at a rate of five eggs per 100,000 hatchlings per day. This dose was divided across four separate feeding events per day. Feeding with the microencapsulated eggs was stopped after 5 days, but MOC application was continued. From day 6 onwards, commercial powdered feed (Natpro feed; 1 mm in size; 42% protein) was scattered on the water surface, ad libitum.
To enhance natural plankton productivity, urea and SSP were applied each week at rates of 2 g/m2 and 4 g/m2, respectively, depending on phytoplankton density (Secchi disc reading 25 cm–30 cm). Water quality deteriorates easily in nursery ponds when excess fertilizers are applied; therefore, 5 cm–6 cm of fresh water was added daily until the ponds were filled to a maximum depth of 1.5 m. After 3 weeks (i.e., on day 22), mola fry were harvested. Feeding was stopped 1 day prior to harvesting.
3.4.2 Survival and growth performance
To assess growth performance, weekly sampling was conducted to measure the length and weight of broodfish using ruler scales and a digital weighing balance (precision 1 mg; Saffron), respectively. Mortality and other behaviors were monitored daily. The growth performance was calculated using the following formulas:
Daily weight gain (mg) = final weight (mg)−initial weight (mg)/rearing duration. (10)
Specific growth rate (% day–1) = [Ln (final weight (mg))−Ln (initial weight (mg))]/rearing duration × 100. (11)
Survival rate (%) = final number of fry/initial number of hatchlings × 100. (12)
3.4.3 Water quality
The water quality parameters of nursery ponds were monitored 3 days before stocking and subsequently at 3-day intervals using a HANNA multiparameter kit. The parameters measured were temperature (°C), pH, dissolved oxygen (mg/L−1), ammonia-nitrogen (NH3-N) (mg/L−1), nitrite-nitrogen, (NO2-N) (mg/L−1), total alkalinity [as CaCO3 (mg/L−1)], and total hardness [as CaCO3) (mg/L−1)].
4 Results
4.1 Breeding behavior, latency, induced spawning, and egg production
Spawning was achieved within an average latency period of 6 h to 8 h after hormone administration and at an average water temperature of 29.5°C (range 28.5°C–30°C). The reproductive behavior of the mola fish was closely observed at regular intervals. Within 3 h–4 h after hormone administration, male mola initiated courtship by exhibiting active movements around female mola, with occasional synchronized swimming alongside female mola, followed by frequent coordinated movements. We found that each female fish paired with a single male fish. After pairing, the male fish increased the intensity of their movements, and aggressively and repeatedly chased and contacted the female fish’s abdominal area near the urogenital aperture. The frequency of coupling and twisting activity by male fish intensified during spawning.
Batch-specific information on average spawning and fertilization rates during mass production of mola seed is presented in Table 4. The spawning rate ranged from 74% to 87%, with a mean value of 82% ± 4.99%. The average absolute fecundity was recorded as 9,018 ± 3,120, with a range between 5,076 and 14,167, and the numbers of spawned eggs ranged between 515 and 778 eggs per g–1 female fish body weight, with a mean value of 609 ± 85 eggs per g–1 female fish body weight. Over the 10 production cycles, approximately 8.5 million fertilized eggs were produced. The fertilization rate ranged between 77% and 86%, with a mean value of 81% ± 2.39%, and the number of fertilized eggs per g body weight of female fish ranged between 415 and 618, with a mean value of 499 ± 73.
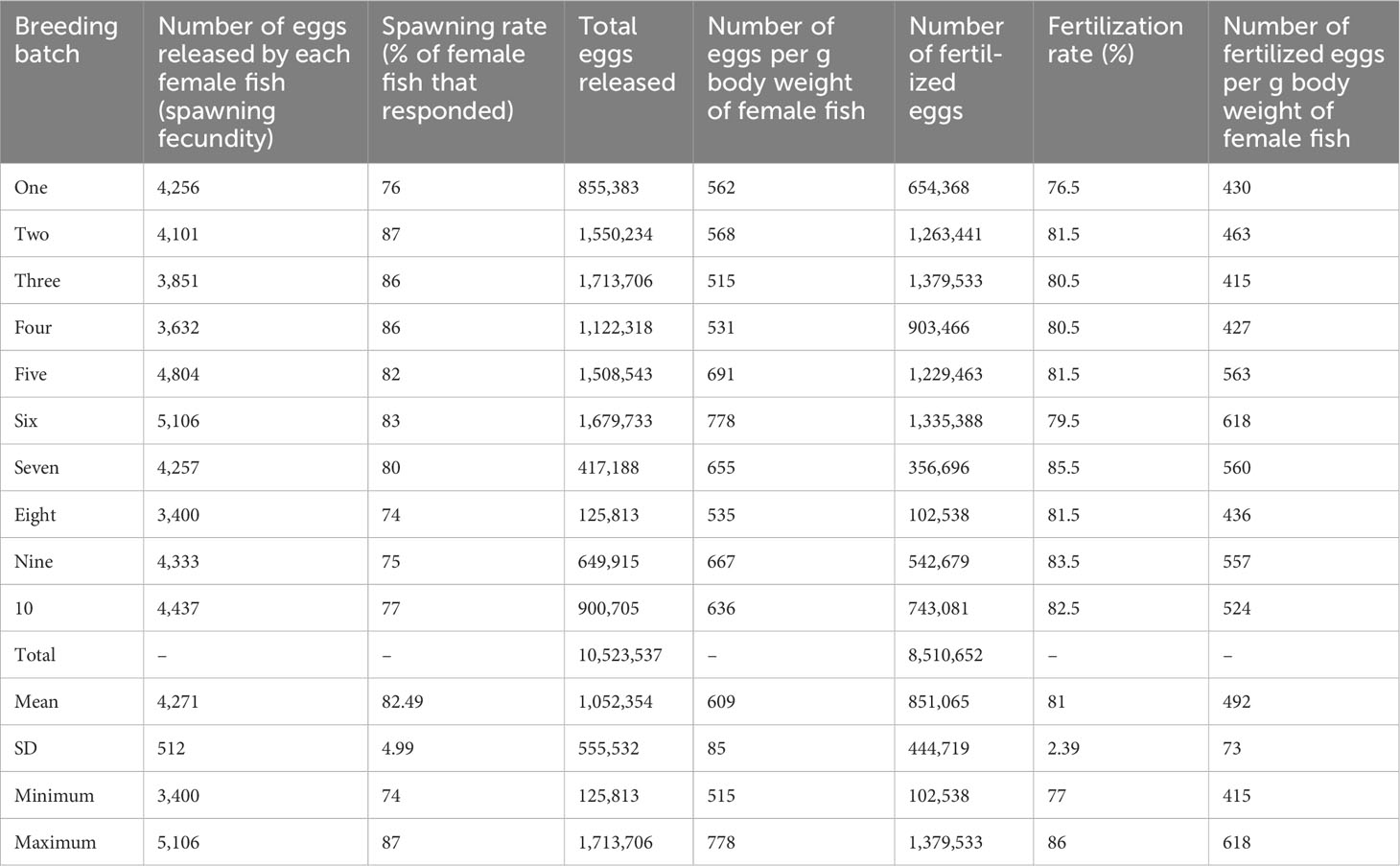
Table 4 Information (by batch) on spawning fecundity and fertilization rate during hatchery-based mass seed production of mola (Amblypharyngodon mola).
The fertilized eggs were light brown to yellowish in color, clear in appearance, demersal, and semi-adhesive (Figure 4). The eggs were found to be attached to the lower bottom part and lower lateral side of the 250-μm mesh outer hapa. The following morning, between 09:00 and 11:00, all breeders were removed from the inner hapa and relocated to a designated spent brood pond. The average numbers of dead male and female mola per spawning were 16.20 ± 10.24 and 9.90 ± 6.97, respectively, with mortality rates of between 5.54% ± 2.53% and 2.25% ± 1.07%, respectively.
4.2 Hatchling production and survival
Table 5 details information, by batch, on hatchling production during hatchery-based mass production of mola seed. Larvae were observed to hatch 12 h–14 h after fertilization, at a water temperature of 28.5°C–30°C. The hatching rate ranged from 76%–91%, with mean values of 85% ± 4.66%. Over the 10 production cycles, approximately 6.36 million hatchlings were harvested, with an average of 635,820 ± 335,171 hatchlings harvested per batch. The average number of hatchlings harvested per female fish ranged from 2,065 to 2,980, with a mean value of 2,580 ± 351. The total number of hatchlings produced per kg body weight of female fish ranged from 272,547 to 458,399, with a mean value of 375,979 ± 62,793.
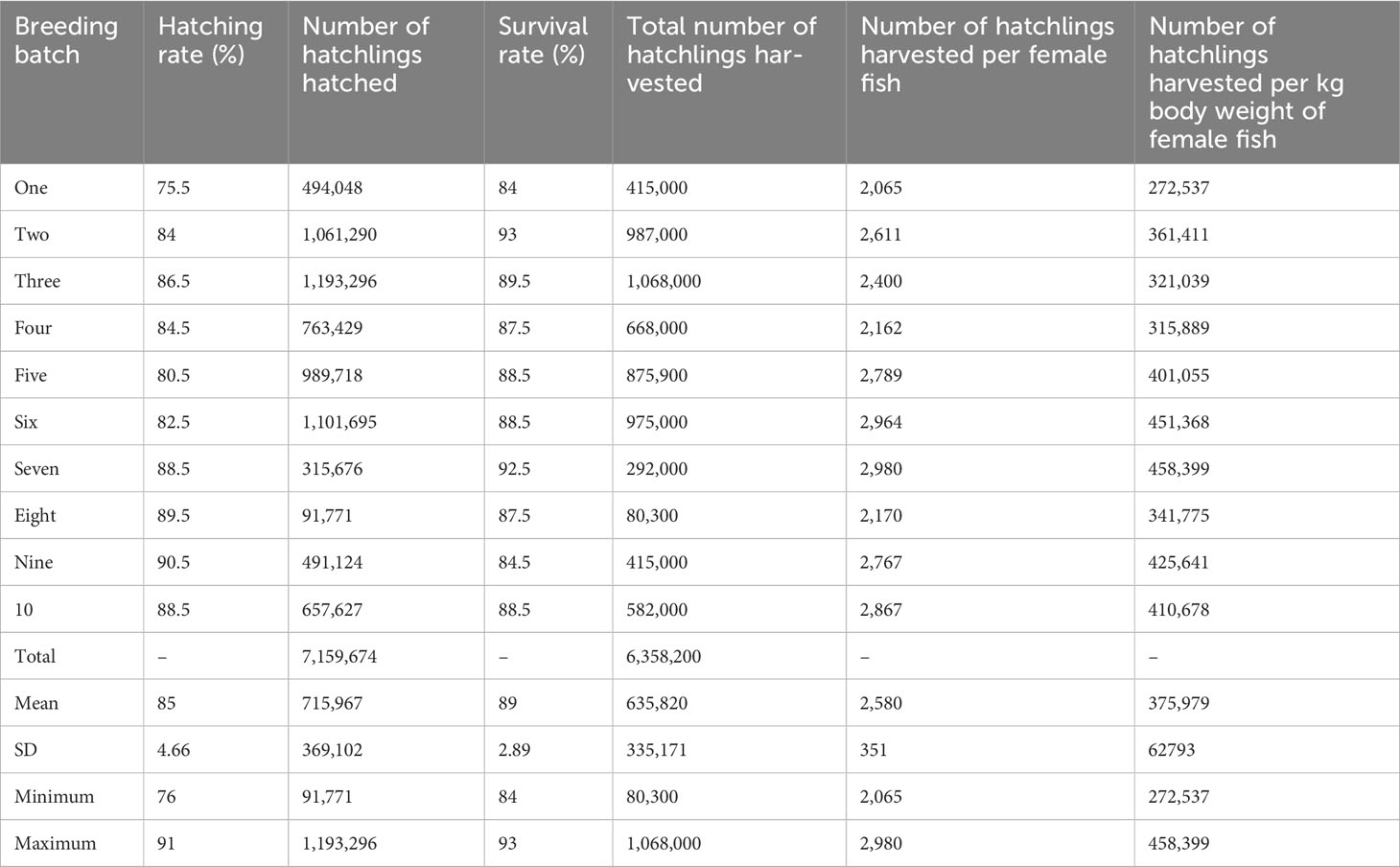
Table 5 Information (by batch) on the production and survival rate of mola (Amblypharyngodon mola) hatchlings during hatchery-based mass seed production.
The embryos began a twisting movement inside the egg capsule 5–7 times per minute before hatching. The twisting movement broke down the eggshell, and the embryo finally emerged tail first.
One-day-old hatchlings exhibit sluggish movement and tend to remain attached to the wall of the hapa (Figure 4). From the second day onwards, hatchlings move toward the water column and remain close to the side wall of the hapa while exhibiting resting swimming behavior. Beyond 48 h after hatching, hatchlings exhibit active swimming behavior, and the eyes and mouth become prominent. The yolk sac is completely absorbed within 72 h.
The newly hatched larvae are completely transparent, slender, and lack pigmentation. Rudimentary eye and otic vesicles are visible. A large yolk sac is present, which is bulbous in the anterior area and narrow in the posterior area.
From the third day onwards, the air bladder became pigmented black. The larva had three black spots when viewed from above; one of them was an air bladder, and the other two were eyeballs. Larval movements became faster and more frequent, and the larvae were found to be swimming casually.
4.3 Larval rearing of hatchlings
The average growth performance during the 3-week larval rearing period in terms of daily weight gain (mg day–1), specific growth rate (SGR; % day–1), and survival are presented in Table 6. The highest growth and survival were recorded at the lowest stocking density. After 21 days of rearing, the mean SGRs were 30.71% ± 2.75%, 30.26% ± 2.70%, and 29.21% ± 2.71% at stocking densities of 500/m2, 625/m2, and 750/m2, respectively. The respective survival values were 58%, 53%, and 48%, respectively.
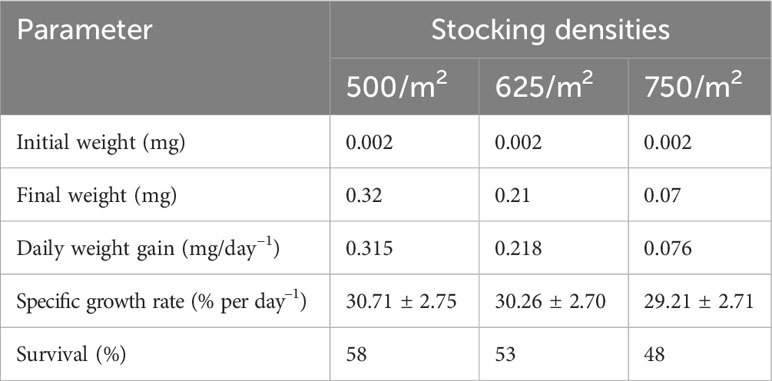
Table 6 Survival and growth performance of mola (Amblypharyngodon mola) hatchling at different stocking densities after 21 days of nursery rearing.
The average water quality parameters of the three nursery ponds during the nursery rearing period are presented in Table 7. The temperature ranged between 29.70°C and 34.5°C, and the pH ranged between 7.60 and 8.90 throughout the study period. The level of dissolved oxygen varied from 5.21 mg/L to 8.95 mg/L, but the averages remained comparable in the three ponds. As expected, nitrite (NO2-N) and ammonia (NH3-N) were found to be highest in the pond with the highest stocking density.
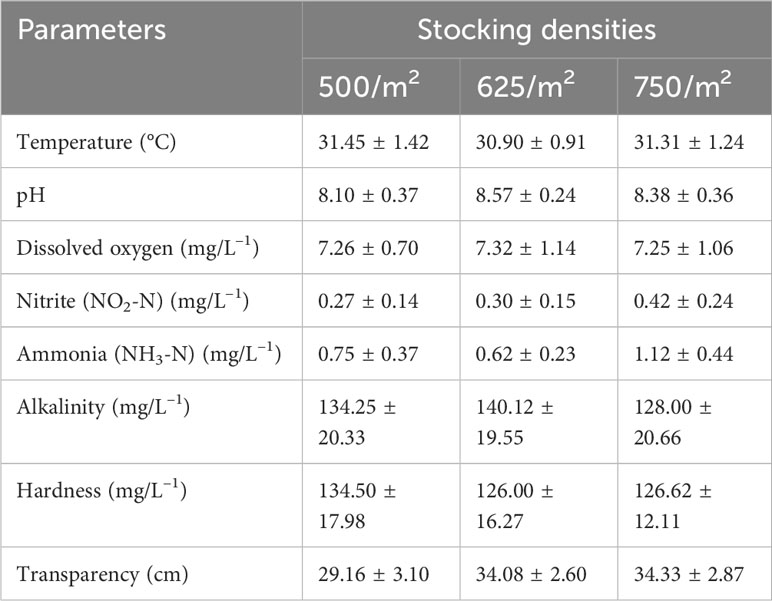
Table 7 Different water quality parameters during nursery rearing of mola (Amblypharyngodon mola) hatchlings at different stocking densities.
4.4 Dissemination of mola seed
Mola hatchlings and fry were either sold or distributed on a complimentary basis to carp farmers at regular intervals who had come to purchase carp seed from the project partner hatchery. From July 2022 to October 2022, 29 farmers took mola seed from the hatchery, of which nine took 3-day-old hatchlings and 20 purchased mola fry that were 1–1.5 inches in size. These farmers came from five districts in Odisha (Jagatshingpur, Bhadrak, Jajpur, Cuttack, and Kendrapara). A summary of mola seed sales is given in Table 8.

Table 8 Details of mola (Amblypharyngodon mola) seed sales from the partner hatchery of Odisha during 2022.
Cumulatively, 2.13 million hatchlings were sold directly, with an average price of 1,043 INR per 100,000 fry, and 70,500 mola fry were sold at an average rate of 0.28 INR per 100 fry. Seed were sold in well-oxygenated plastic bags, filled with clean aerated groundwater. By October 2022, the partner hatchery had earned a total of 41,650 INR (around USD 510) by selling mola seed originating from the project.
The basic details of all the farmers who purchased mola seeds were recorded. Most of the farmers stocked mola seed into pond-based carp polyculture systems, which had an average pond area of 0.3 ha. The mola seed were also purchased by village committees to stock in two Gram Panchayat (GP) tanks4, and by two private entrepreneurs for stocking biofloc aquaculture systems. Approximately 15,000 fry were distributed to women’s self-help groups (WSHGs)5 as part of promotional activities for stocking in GP tanks. The remaining unsold seed were stocked in carp polyculture ponds at the partner hatchery for grow-out and broodfish rearing.
5 Discussion
This article reports on the first systematic breeding methods aimed at developing a technical protocol for hatchery-based mass production of mola seed, a popular nutrient-rich SIS from South Asia. SIS are small and delicate to handle, making it difficult to produce seed from them in large quantities using conventional hatchery methods. Induced breeding, therefore, needs species-specific innovation and specially modified technology (Rajts et al., 2022).
In the present study, average absolute fecundity and spawning fecundity were found to be 9,018 and 4,271, respectively, which is comparable to the findings reported in the study by Saha (2019). Previous studies have also reported that mola is a highly fecund fish.
The average latency and incubation periods observed in the present study were 6 h–8 h and 12 h–14 h, respectively, which corresponds with the findings of Saha (2019). The latency period of mola is also similar to that of the endemic cyprinid fish Dawkinsia rohani (7 h–8 h at 22°C–25°C with 0.5 mL–0.7 mL of WOVA-FH per kg body weight of female fish and Barilius barila (6 h–7 h at 25°C–28°C with 0.5 mL of WOVA-FH per kg body weight of female fish) (Dey et al., 2016; Mariappan et al., 2021). However, the latency and incubation periods of mola are shorter than those of Indian major carps (Chattopadhyay, 2017).
In the present study, administering synthetic GnRH-based hormone through the peritoneal cavity at 0.5 mL kg–1 body weight of female mola resulted in a fertilization rate of 77%–86% (mean 81%), and hatching rate of 76%–91% (mean 85%). The number of hatchlings harvested per female fish ranged from 2,065 to 2,980 (mean: 2,580). This approach yielded better results than those previously reported by Saha (2019), which were observed in fish in their responses to PG hormone administration through the pectoral fin cavity [at 25 mg/kg−1 body weight of female mola (fertilization rate 84%, hatching rate 63%, and 678 hatchlings per female fish)].
This is supported by the fact that various cyprinids, such as Indian major carps, minor carps Labeo bata, and Pengba Osteobrama belangeri, performed better during breeding with synthetic hormone doses of 0.4 mL/kg−1 to 0.5 mL/kg−1 body weight for female fish and 2 kg−1 to 2.5 kg−1 body weight for male fish (Behera et al., 2007; Rath et al., 2007; Das et al., 2016). However, trials are required to explore the possibilities of higher doses of inducing agents for the mass seed production of SIS.
The efficacy of the synthetic GnRH-based hormones has been reported to be greater than that of PG hormone in cases of other cyprinid and carp species, as GnRHa works at a higher level of the brain–pituitary–gonadal axis, which can facilitate more balanced control of reproductive and physiological events for oocyte maturation and spawning (Rath et al., 2007; Das et al., 2016; Kumar et al., 2021). Commercial synthetic hormones are convenient for mass production of SIS seed because they are easily accessible in the market, in contrast to the time-consuming and labor-intensive process required to produce inducing solutions from carp pituitary extracts (hypophysation).
The present study was conducted with the intent of maintaining the sex ratio of male and female broodfish at 2: 1 (male > female), but in practice the ratio averaged 1.6: 1 over 10 production cycles. A male-biased sex ratio of two male fish to one female fish has been reported for many species to lead to a significantly higher fertilization rate, hatching rate, and larval survival (Mandal et al., 2016; Kumar et al., 2021).
With the same tanks set up for conditioning and spawning, the induced breeding protocol developed for the study eliminates the need for a separate breeding pool, hatching pool, tray, or incubator. Instead of a continuous flow of water, the current study used controlled showering, which also serves as a water-saving strategy. In addition, in contrast to Saha (2019), no substratum (grass, water, hyacinth, etc.) was used during the breeding trials; instead, eggs were attached to and gathered within the 250-μm outer hapa, which could be handled easily.
The hatchery setup included an aeration tower in the overhead water reservoir tank to maintain optimal levels of dissolved oxygen and degas groundwater during hatchery operations. The concept was first introduced in Bangladesh during the 1980s by Rajts (1986). Toxic gases are removed from the groundwater by the aeration tower as inflowing water pumped from the borewell passes through it on its way to the overhead tank. In addition, this process dissolves oxygen, ensuring that the water supply is almost completely saturated with oxygen, which increases larval survival Rajts (1986). By automatically aerating the water before it enters the overhead tank, this tool has the benefit of eliminating the need for additional aeration during breeding operation and is thus an energy-saving strategy. According to Avery and Steeby (2004), pretreating the water with degassing-aeration towers is a critical step in the enhancement of dissolved oxygen and the removal of unwanted gases, which are essential for effective hatching.
The spawning and hatching success of fish is influenced by factors that are related to water quality, especially water temperature. In our study, the water temperature in the breeding tanks was 29.53°C and the dissolved oxygen concentration was 6.86 mg/L–1, both of which are favorable for breeding (Chattopadhyay, 2017). Although it has been reported that most small indigenous fish breed at a water temperature of 26.0°C–28.5°C under captivity (Das et al., 2016; Mariappan et al., 2021), in slightly higher water temperatures, the embryos hatch faster, and have a better survival rate (Olaniyi and Omitogun, 2013).
For SIS, such as mola, spontaneous spawning may also be achieved by manipulating water quality parameters, alone or in combination with hormone induction. This might be accomplished by maintaining a constant level of dissolved oxygen; implementing constant showering and flow from groundwater or pond water; adding soft water, such as rainwater; and adding substratum to mimic the situation in spawning ponds (Rajts et al., 2022). This approach might stimulate fish to spawn for an extended period, even outside of their typical spawning season. Therefore, rainwater storage and use could be a critical additional step to stimulate the spontaneous spawning of SIS and extend the duration of spawning beyond the normal breeding season. In this regard, field trials are needed to better understand the interactions between environmental stimuli and reproductive parameters during the mola breeding cycle.
As mola is a prolific breeder, the sex-specific separation of mola broodfish should be maintained during both the off-season and breeding season to eliminate the possibility of auto-breeding. However, in the present study, sex-specific broodfish ponds were not maintained as it is very difficult to distinguish between male and female mola during the early-maturity stage, and sex can be determined only during the breeding season (Rajts and Shelley, 2020). It is also challenging to maintain the sex segregation of mola because of handling stress and injury to the fish during sorting.
During nursery rearing, the survival of mola fry depended on pond preparation, the presence of selectively cultured plankton in nursery ponds, and stocking density. For the first 3–4 days of nursery rearing, mola fry require that a sufficient number of rotifers (their food source) is present, but they are also at risk of being eaten by large copepod zooplankton. Therefore, management is necessary for the selective elimination of zooplankton, and also the selective development of protozoans and rotifers.
In the current study, ponds stocked with 500 individuals per m2 yielded the highest survival and growth rates after 3 weeks of culture. However, from preliminary observations during the study, it appears advisable for farmers to stock mola hatchlings at a lower stocking density during nursery rearing, such as 200 individuals per m2.
To prevent disease outbreaks and plankton shortages, fry should be harvested after 3 weeks of culture. They can then be stocked into a carp–mola polyculture grow-out pond at a rate of 5–10 individuals/m2. Further focused study is required to optimize the stocking density of mola fry during nursery rearing, and also in grow-out ponds.
Stocking hatchery-produced mola seed offers several potential advantages over the previous practice of stocking broodfish harvested from natural water bodies. Stocking wild breeders in seasonal ponds where water is available for a short period only can be challenging because ponds may dry up before their progeny reach market size. Moreover, ponds stocked with wild breeders contain SIS populations comprising a mix of age groups, resulting in the production of fish of variable sizes, which are less marketable.
In contrast, hatchery-produced SIS seed are of uniform size and age, and can be stocked at the optimum time and density to maximize survival and growth. Moreover, hatchery-produced seed can be conditioned and packed in clean well-oxygenated water before it is delivered to farmers, which would reduce the number of mortalities during transport and stocking. Stocking hatchery-produced fry may also ensure a uniform size of SIS at harvest, making them more marketable and thus more likely to be sold at better prices. Hatchery-based seed production also offers the possibility of stimulating spawning much earlier than in the wild, giving farmers a head start over nature.
The project is monitoring sales of mola seed by the partner hatchery (WorldFish, 2022). The average selling price of mola hatchlings (INR 1,043 per 100,000 pieces) and fry (INR 0.28 per piece) was comparable to that of carp seed sold by the hatchery. For example, rohu hatchlings typically sell for INR 900 per 100,000 pieces, whereas mixed carp fry (rohu, mrigal, and catla) cost INR 0.20–0.25 per piece. Even so, further thorough economic analysis and modeling are crucial to determining the cost–benefit ratio and setting the mola seed price.
Micronutrient deficiencies are a major public health issue in the Global South. Therefore, it is crucial that forms of aquaculture that produce vital micronutrients without depleting underlying environmental resources are developed (Gephart et al., 2020). The higher levels of key micronutrients in mola than in carp, the compatibility of mola with carp polyculture systems, and the opportunity to obtain multiple harvests per year make mola an ideal species for nutrition-sensitive aquaculture.
We hope that this groundbreaking commercial mass mola seed production trial will facilitate the large-scale adoption of nutrition-sensitive carp–mola polyculture, which will boost farm incomes, increase the consumption of micronutrient-dense fish, and support the livelihoods and well-being of vulnerable populations who currently experience nutritional inadequacies in regions of India and South Asia.
6 Conclusion
To conclude, the mass seed production of the nutrient-dense SIS mola can be achieved through induced breeding using GnRH-based synthetic hormone (Wova-FH) at a dose of 0.5 mL and 0.25 mL per kg body weight of female and male fish, respectively, combined with environmental manipulation using oxygen-rich water. The breeding protocol is simple and can be adopted by small-scale hatchery operators.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Ethics statement
Ethical approval was not required for the study involving animals in accordance with the local legislation and institutional requirements because this project was considered to be a description of husbandry procedures and, thus, animal ethics approval was not required.
Author contributions
FR: Formal Analysis, Investigation, Methodology, Visualization, Writing – review & editing. SD: Formal Analysis, Investigation, Methodology, Project administration, Writing – original draft, Writing – review & editing. KG: Formal Analysis, Investigation, Methodology, Writing – original draft. RD: Formal Analysis, Investigation, Writing – original draft. SB: Investigation, Resources, Writing – review & editing. AP: Supervision, Writing – review & editing. SR: Supervision, Writing – review & editing. ST: Funding acquisition, Writing – review & editing. CM: Supervision, Writing – review & editing. BB: Funding acquisition, Project administration, Supervision, Visualization, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work received financial support from the German Federal Ministry for Economic Cooperation and Development (BMZ) commissioned by the Deutsche Gesellschaft für Internationale Zusammenarbeit (GIZ) through the Fund International Agricultural Research (FIA) (grant number: 81260866).
Acknowledgments
The authors are thankful to hatchery technician Mr. BB Das and other staff of hatchery and Seafood Solutions LLP, Andhra Pradesh for their support. The authors are also sincerely indebted to the Fisheries and Animal Resources Development Department, Government of Odisha, for its active support and collaboration. Sections of this study were presented at the World Aquaculture 2022 Singapore conference. Sections of this study have also been released in the form of a popular blog on the WorldFish website (WorldFish, 2022).
Conflict of interest
SB is employed by Biswal Auqatech.
The remaining authors declare that the project was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) BB, ST declared that they were editorial board members of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Footnotes
- ^ SIS are characterized by their diminutive size (they grow to a maximum length of approximately 25 cm) and largely inhabit freshwater habitats, such as floodplains, rivers, streams, canals, ponds, rice fields, and wetlands.
- ^ Carp species: Indian major carp (rohu, Labeo rohita; catla, Gibelion catla; mrigal, Cirrhinus mrigala), Indian minor carp (bata, Labeo bata, Labeo gonius; calbasu, Labeo calbasu), and exotic carp (grass carp, Ctenopharyngodon idella; silver carp, Hypophthalmichthys molitrix; common carp, Cyprinus carpio; bighead carp, Aristichthys nobilis; black carp, Mylopharyngodon piceus; silver barb, Barbonymus gonionotus).
- ^ Infusoria are live food organisms (mostly composed of microscopic protozoans) that are eaten by fish hatchlings.
- ^ In Odisha, numerous earthen tanks or ponds have been excavated under government schemes and community programmes for water conservation through rainwater harvesting and multipurpose utilization. These tanks are managed by the decentralized governance system of Gram Panchayats under the Panchayati Raj and Drinking Water Department, the Government of Odisha. In Odisha there are approximately 62,000 multipurpose GP tanks, which have a total water area of 54,000 ha.
- ^ Women’s self-help groups are informal community groups led by women that are often used as a means of reducing poverty and empowering women to undertake many livelihood-generating activities using credit facilities. Currently, the Government of Odisha nurtures approximately 600,000 WSHGs, which have approximately 7 million women in total who participate in village-level socioeconomic activities and promote women’s empowerment.
References
Ahamed F., Ahmed Z. F., Hossain M., Ohtomi J. (2017). Growth and longevity of the mola carplet Amblypharyngodon mola (Cyprinidae) in the Payra River, southern Bangladesh. Egypt. J. Aquat. Res. 43 (4), 291–295. doi: 10.1016/j.ejar.2017.11.002
Avery J. L., Steeby J. A. (2004). “Hatchery management,” in Biology and culture of channel catfish. Developments in aquaculture and fisheries science-34. Eds. Tucker C. S., Hargreaves J. S. (Netherlands: Elsevier B.V.), 145–165. doi: 10.1016/s0167-9309(04)80009-7
Azadi M. A., Mamun A. (2004). Reproductive biology of the cyprinid, Amblypharyngodon mola (Hamilton) from the Kaptai Reservoir, Bangladesh. Pak. J. Biol. Sci. 7, 1727–1729. doi: 10.3923/pjbs.2004.1727.1729
Behera B. K., Das P., Singh N. S., Sahu A. K. (2007). Observation on the induced breeding of Labeo bata (Hamilton) with Ovaprim and Ovatide as inducing agents with a note to its development. J. Aquac. 15, 11–17.
Belton B. (2012). Culture, social relations and private sector development in the Thai and Vietnamese fish hatchery sectors. Asia Pac. Viewp. 53 (2), 133–146. doi: 10.1111/j.1467-8373.2012.01487.x
Bogard J. R., Farook S., Marks G. C., Waid J., Belton B., Ali M., et al. (2017). Higher fish but lower micronutrient intakes: Temporal changes in fish consumption from capture fisheries and aquaculture in Bangladesh. PloS One 12 (4), e0175098. doi: 10.1371/journal.pone.0175098
Bogard J. R., Thilsted S. H., Marks G. C., Wahab M., Hossain M. A. R., Jakobsen J., et al. (2015). Nutrient composition of important fish species in Bangladesh and potential contribution to recommended nutrient intakes. J. Food Compos. Anal. 42, 120–133. doi: 10.1016/j.jfca.2015.03.002
Castine S. A., Bogard J. R., Barman B. K., Karim M., Hossain M., Kunda M., et al. (2017). Homestead pond polyculture can improve access to nutritious small fish. Food Secur. 9, 785–801. doi: 10.1007/s12571-017-0699-6
Chattopadhyay N. R. (2017). Induced fish breeding-A practical guide for hatcheries (UK: Elesvier and Academic Press), 351.
Das P., Behera B. K., Meena D. K., Singh S. K., Mandal S. C., Das S. S., et al. (2016). Comparative efficacy of different inducing agents on breeding performance of a near threatened cyprinid Osteobrama belangeri in captivity. Aquac. Rep. 4, 178–182. doi: 10.1016/j.aqrep.2016.11.001
Dey A., Nur R., Sarkar D., Barat S. (2016). Captive breeding through synthetic hormone induced breeding of an eastern Himalayan hill stream fish, Barilius barila (Hamilto). Int. J. Fish. Aquat. Stud. 4 (5), 354–358.
Dubey S. K., Padiyar A. P., Shenoy N., Gaikawd A., Mohanty B., Baliarsingh B. K., et al. (2022). “Climate-smart and nutrition sensitive aquaculture in Odisha, India: a new horizon in sustainability, adaptation, and mitigation,” in Handbook on climate change and disasters. Ed. Shaw R. (UK: Edward Elgar Publishing Limited), 574–593. doi: 10.4337/9781800371613.00054
Fiedler J. L., Lividini K., Drummond E., Thilsted S. H. (2016). Strengthening the contribution of aquaculture to food and nutrition security: The potential of a vitamin A-rich, small fish in Bangladesh. Aquaculture 452, 291–303. doi: 10.1016/j.aquaculture.2015.11.004
Froese R., Pauly D. (2022). FishBase (World Wide Web electronic publication). Available at: www.fishbase.org.
Gephart J. A., Golden C. D., Asche F., Belton B., Brugere C., Froehlich H. E., et al. (2020). Scenarios for global aquaculture and its role in human nutrition. Rev. Fish. Sci. Aquac. 29 (1), 122–138. doi: 10.1080/23308249.2020.1782342
Ghosh A. S., Ghosh S. K., Ghosh M., Ali A. (2018). Studies on biodiversity of selected indigenous fish species, in Beels and Baors of South Bengal and their breeding potential through habitat modification. Int. J. Fish. Aquac. Stud. 6 (4), 479–483.
Gupta S., Banerjee S. (2013). Studies on some aspects of reproductive biology of Amblypharyngodon mola (Hamilton-Buchana). Int. Res. J. Biol. Sci. 2 (2), 69–77.
Hoque A. S. M. M., Rahman M. R. (2008). Reproductive ecology of mola (Amblypharyngodon mola). J. Agric. Rur. Dev. 6 (1&2), 165–174.
Horvath L., Tamás G., Seagrave C. (2002). Carp and pond fish culture (London, UK: Fishing News Books, Blackwell Scientific Publications Ltd).
Karim M., Ullah H., Castine S., Islam M. M., Keus H. J., Kunda M., et al. (2017). Carp–mola productivity and fish consumption in small-scale homestead aquaculture in Bangladesh. Aqua. Int. 25, 867–879. doi: 10.1007/s10499-016-0078-x
Kohinoor A. H. M., Islam M. S., Thilsted S. H., Wahab M. A. (2005). Reproductive biology of three important indigenous small fish viz., mola (Amblypharyngodon mola), Chela (Chela cachius) and punti (Puntius sophore). Iran. J. Fish. Sci. 5 (1), 29–48.
Kumar P., Biswas G., Ghoshal T. K., Kailasam M., Vijayan K. K. (2018). Embryonic and larval developments of brackish water catfish, Mystus gulio (Hamilton and Buchana) induced with human chorionic gonadotropin and consequent larval rearing. Aqua. Res. 49 (7), 2466–2476. doi: 10.1111/are.13706
Kumar P., Kailasam M., Biswas G., Christina L., Ghoshal T. K. (2021). Effect of different inducing hormones, sex ratio and oocyte diameter on breeding performance of brackish water catfish, Mystus gulio. Aquaculture 530, 735–821. doi: 10.1016/j.aquaculture.2020.735821
Mandal B., Kumar R., Jayasankar P. (2016). Efficacy of exogenous hormone (GnRHa) for induced breeding of climbing perch Anabas testudineus (Bloc) and influence of operational sex ratio on spawning success. Anim. Reprod. Sci. 171, 114–120. doi: 10.1016/j.anireprosci.2016.06.006
Mariappan P., Antony C., Subramaniam B., Nagarahalli M., Bhosale M. M. (2021). Successful breeding of the endemic cyprinid fish Dawkinsia rohani in controlled condition—First report. Aqua. Res. 52 (10), 4693–4700. doi: 10.1111/are.15303
Milstein A., Wahab M. A., Kadir A., Sagor M. F. H., Islam M. A. (2009). Effects of intervention in the water column and/or pond bottom through species composition on polyculture of large carps and small indigenous species. Aquaculture 286 (3–4), 246–253. doi: 10.1016/j.aquaculture.2008.09.036
Mondal S., Wahab A., Barman B. K., Abdulla A. A. (2020). Breeding biology of Mola carplet, (Amblypharyngodon mola, Hamilton 1822) in semi-natural condition. Asian J. Anim. Sci. 14, 111–120. doi: 10.3923/ajas.2020.111.120
Mustafa T., Naser N., Murshed S., Zeba F., Akter M., Ali L. (2015). Fatty acid composition of three small indigenous fishes of Bangladesh. Bangladesh J. Zool. 43 (1), 85–93. doi: 10.3329/bjz.v43i1.26141
Olaniyi W. A., Omitogun O. G. (2013). Stages in the early and larval development of the African catfish Clarias gariepinus (Teleostei, Clariidae). Zygote 22, 314–330. doi: 10.1017/S0967199413000063
Rahman A. K. A. (1989). Freshwater fishes of Bangladesh. 1st Edn. (Bangladesh: Zoological Society of Bangladesh, Department of Zoology, University of Dhaka).
Rahman M., Hossain M., Tumpa A. S., Hossain M., Billah M., Ohtomi J. (2018). Size at sexual maturity and fecundity of the mola carplet Amblypharyngodon mola (Hamilton 1822) (Cyprinidae) in the Ganges River, Bangladesh. Zool. Ecol. 28 (4), 429–436. doi: 10.1080/21658005.2018.1537906
Rajts F., Ahmed K. K., Khan A. M., Kaiya M. K. (1997). “Pond management and controlled breeding of Amblypharyngodon mola - a small indigenous fish species in Bangladesh,” in Proceedings of National Workshop on small indigenous fish culture in Bangladesh (Dhaka, Bangladesh: Integrated food assisted development project SP-2 (IFADEP SP 2), 71–79.
Rajts F., Belton B., Thilsted S. H. (2022). Guidelines for setting up breeding experiments for small indigenous species (SIS). (Penang, Malaysia: WorldFish). Program Report: 2022-03.
Rajts F. (1986). Summaries of inspection reports of fish farms and hatcheries of Bangladesh Technical Report, (Rome, Italy: Food and Agriculture Organization). 371 pp.
Rajts F., Shelley C. C. (2020). Mola (Amblypharyngodon mola) aquaculture in Bangladesh: Status and future needs. (Penang, Malaysia: WorldFish). Program Report: 2020–2045.
Rath S. C., Sarkar S. K., Gupta S. D., Sarangi N. (2007). Comparative account of induced breeding of Indian major carps with Ovaprim, Ovatide, WOVA- FH and carp pituitary extract. Indian J. Anim. Sci. 77 (10), 1057–1060.
Roos N., Leth T., Jakobsen J., Thilsted S. H. (2002). High vitamin A content in some small indigenous fish species in Bangladesh: perspectives food-based strategies to reduce vitamin A deficiency. Int. J. Food Sci. Nutr. 53 (5), 425–437. doi: 10.1080/0963748021000044778
Roos N., Wahab M. A., Hossain M. A. R., Thilsted S. H. (2007). Linking human nutrition and fisheries: incorporating micronutrient-dense, small indigenous fish species in carp polyculture production in Bangladesh. Food Nutr. Bull. 28 (2 Suppl.), S280–S293. doi: 10.1177/15648265070282S207
Saha M. K. (2019). Studies on morphometry, breeding and larval development of Amblypharyngodon mola (Hamilto) from different regions of Bangladesh (Mymensingh, Bangladesh: PhD thesis, Bangladesh Agricultural University).
Saha M. K., Eunus A. T. M., Barman B. K. (2014). “Transportation of mola (Amblypharyngodon mola) brood fish for stocking in the homestead ponds of Northwest Bangladesh,” in Advances in fisheries research in Bangladesh. Eds. Wahab M. A., M.S. S., Hossain M. A. R., Barman B. K., Hoq M. E. (Dhaka, Bangladesh: Bangladesh Fisheries Research Forum), 97–104.
Sarkar U. K., Deepak P. K., Kapoor D., Negi R. S., Paul S. K., Singh S. (2005). Captive breeding of climbing perch Anabas testudineus (Bloc) with Wova-FH for conservation and aquaculture. Aqua. Res. 36 (10), 941–945. doi: 10.1111/j.1365-2109.2005.01281.x
Talwar P. K., Jhingran A. G. (1991). Inland fishes of India and adjacent countries (New Delhi, India: Oxford-IBH Publishing Co. Pvt. Ltd.).
Thilsted S. H., Thorne-Lyman A., Webb P., Bogard J. R., Subasinghe R., Phillips M. J., et al. (2016). Sustaining healthy diets: The role of capture fisheries and aquaculture for improving nutrition in the post-2015 era. Food Pol. 61, 126–131. doi: 10.1016/j.foodpol.2016.02.005
Toufique K. A., Belton B. (2014). Is aquaculture pro-poor? Empirical evidence impacts fish consumption Bangladesh. World Dev. 64, 609–620. doi: 10.1016/j.worlddev.2014.06.035
Wahab M. A., Alim M. A., Milstein A. (2003). Effects of adding the small fish punti (Puntius sophore Hamilton) and/or mola (Amblypharyngodon mola Hamilton) to a polyculture of large carp.2003. Aqua. Res. 24 (2), 149–163. doi: 10.1046/j.1365-2109.2003.00784.x
WorldFish (2022). Cracking the code of mola mass production boosts nutrition-sensitive aquaculture in India. (Penang, Malaysia: WorldFish).
Keywords: small indigenous fish species (SIS), mola (Amblypharyngodon mola), induced breeding, environmental manipulation, mass seed production, nutrition-sensitive aquaculture
Citation: Rajts F, Dubey SK, Gogoi K, Das RR, Biswal SK, Padiyar AP, Rajendran S, Thilsted SH, Mohan CV and Belton B (2023) Cracking the code of hatchery-based mass production of mola (Amblypharyngodon mola) seed for nutrition-sensitive aquaculture. Front. Aquac. 2:1271715. doi: 10.3389/faquc.2023.1271715
Received: 02 August 2023; Accepted: 15 September 2023;
Published: 16 October 2023.
Edited by:
Jingzhen Wang, Beibu Gulf University, ChinaReviewed by:
Vikash Kumar, Central Inland Fisheries Research Institute (ICAR), IndiaChristopher Brown, Pukyong National University, Republic of Korea
Yongkai Tang, Chinese Academy of Fishery Sciences (CAFS), China
Copyright © 2023 Rajts, Dubey, Gogoi, Das, Biswal, Padiyar, Rajendran, Thilsted, Mohan and Belton. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Sourabh Kumar Dubey, cy5kdWJleUBjZ2lhci5vcmc=
†Present address: Ben Belton, International Food Policy Research Institute, Dhaka, Bangladesh
 Francois Rajts
Francois Rajts Sourabh Kumar Dubey
Sourabh Kumar Dubey Kalpajit Gogoi3
Kalpajit Gogoi3 Shakuntala Haraksingh Thilsted
Shakuntala Haraksingh Thilsted Chadag Vishnumurthy Mohan
Chadag Vishnumurthy Mohan Ben Belton
Ben Belton