- 1Centre for Veterinary Epidemiological Research, Atlantic Veterinary College, University of Prince Edward Island, Charlottetown, PE, Canada
- 2Department of Health Management, Atlantic Veterinary College, University of Prince Edward Island, Charlottetown, PE, Canada
- 3eFishery, Pte. Ltd., Bandung, Jawa Barat, Indonesia
Current information on biosecurity measures implemented by shrimp farmers in Indonesia is limited. This study describes farmer demographic characteristics, on-farm biosecurity practices, farm production and disease status, among small and medium holder shrimp farms on Java Island, Indonesia. A questionnaire-based survey was conducted from November 2019 to May 2020 to collect data from shrimp farms operating in various regions of the Java Island. A numerical score was assigned for each of the assessed biosecurity practices, based on whether it was a conventional risk factor or a protective factor. Based on responses from 90 shrimp farmers, who volunteered to participate in the study, mean overall biosecurity scores ranged from 32 to 54 (out of a maximum score of 100). Most farms (88.9%) either shared common water sources with other aquaculture farms or were connected to other farms via water channel. Farm equipment sharing was common both within (91.1%) and between (41%) farms. Water pre-treatment was common (99%), but approximately a third of the farms did not practice any routine quality assessment for post larvae before stocking. On average, farms produced 1.6 kg/m2 (95% CI: 1.2, 2.0) of shrimp with a harvest size of 43 shrimp/kg (95% CI: 37, 49) or an average weight of 23.3 g at time of harvest. An increasing trend in harvest weight per pond area and shrimp size at harvest was noted with increasing overall biosecurity score. These results indicated that farms with better biosecurity practices tended to have a higher production yield.
1 Introduction
Indonesia is currently one of the five largest exporters of shrimp in the world (Henriksson et al., 2019). Shrimp aquaculture plays a vital role, particularly in the country’s rural coastal economy (Yi, 2013). Infectious diseases pose a significant risk to the shrimp industry both globally (Lightner, 2007; Asche et al., 2021) and in Indonesia (Lestariadi and Yamao, 2018).
Infectious disease outbreaks in shrimp farms are affected by many host, environmental, and husbandry factors. Successful farm management reduces the risk of introduction of harmful pathogens into a farming site. However, if introduction of such pathogens cannot be averted, addressing factors that promote their spread within farms minimizes the potential impact on shrimp morbidity and mortality and consequential economic losses.
Infectious shrimp diseases may be transmitted between farms and ponds through several pathways (Lee et al., 2022). Pathogens may be transmitted vertically through brood stock or horizontally between neighboring ponds or farms. Other animal species, such as pets (e.g., dogs, cats), wild birds, wild crustaceans (e.g., crabs, shrimp) or pests, may also act as vectors for the introduction of pathogens into a shrimp farm. Pathogens may also be introduced into shrimp farms via personnel, through contaminated feed, farm equipment and vehicles, water supplied to the shrimp pond, and shrimp carcasses.
Among other farm management practices, on-farm biosecurity i.e., measures undertaken to exclude specific pathogens from the farm, can mitigate the risk of disease introduction to the farm, within-farm spread, and potential release and transmission to other shrimp farms (Lightner, 2007). If farmers or farm managers can make informed decisions and distinguish higher risk activities from lower or minimal risk activities in their farm, they can optimize their efforts and resource allocation towards implementing practical and cost-effective methods to address those high-risk activities. This has the potential to promote a reduction of economic losses attributable to outbreaks of infectious diseases.
To our knowledge, there have been limited studies describing current biosecurity practices implemented by shrimp farmers in Indonesia. A recent study described biosecurity practices across grow-out and intensive shrimp farms in Java, Lampung, and Banyuwangi regions of Indonesia, based on results from a questionnaire survey, which sampled JALA™’s clientele of shrimp farms (Delphino et al., 2022). That study identified distinct categories of farms in different regions based on descriptions of biosecurity practices, farm management, and characteristics of data structure (such as missing information or incomplete records). The present study differed from that of Delphino et al. (2022) in geographical location of the farms; farms comprising the study population (eFishery Pte. Ltd.) were also distinct, in terms of farm size, farm type and management practices.
The specific objectives of this study were to describe the current biosecurity practices using data from questionnaire survey and to identify association between these practices and production performance on shrimp farms on Java Island, Indonesia.
2 Materials and methods
2.1 Study area
This study was conducted in the Java Island region of Indonesia. This island is a national economic hub and houses over half of the total population in the country (Shvili, 2021) and shrimp farming is practiced in areas close to the coastline. Figure S1 illustrates the study area, which included West Java, East Java, North Coast, and South Coast regions.
2.2 Study design and data collection
In-person interviews of farmers serviced by eFishery Pte. Ltd™. were conducted between November 2019 and February 2020 using a standardized questionnaire. eFishery Pte. Ltd™ is an aquaculture company that provides data science and information technology services to over 30,000 aquaculture farmers across 24 provinces in Indonesia. The questionnaire (see Supplementary Material) used in the study was developed using methods described in Delphino et al., 2022. Briefly, references to develop questions suitable for the study included the “Basic Principles of Biosecurity Guidelines” from the Canadian Food Inspection Agency (CFIA, 2012) and previously validated questions from a biosecurity study in aquaculture in Vietnam (Boerlage et al., 2017). Revisions to these questions were made based on feedback from local industry partners, following initial testing with a subset of farmers. The original version in English was then translated to Bahasa language to create the final version of the questionnaire, which was administered to the respondents by local field workers.
Farmer’s demographic information and additional data on farm management practices were collected from voluntarily participating farmers. This study focused on farm practices known to be higher risk from a biosecurity perspective. The questionnaire encompassed 58 questions from seven broad categories pertinent to on-farm biosecurity practices, viz: farm access management, equipment and staff sharing, seed and stocking management, pond preparation, water management, control of pests and other wild species, and record-keeping systems (Table S1). Production and disease occurrence data in participating farms were based on previous production cycles and collected by phone interview between March and May 2020. Data from in-person and phone interviews were then used to assess the association between biosecurity practices and overall shrimp production.
Production data included total harvest weight per pond (kg), pond size (m2), and shrimp size at harvest. Shrimp size at harvest represented the total number of shrimp units per kilogram of product at harvest. A lower number of shrimps per kg represented a larger shrimp size at harvest and vice versa. Shrimp size at harvest was also converted to average shrimp weight (g) at harvest.
2.3 Data analysis
A numerical score was assigned for each item of on-farm biosecurity practice, based on whether the practice was conventionally a risk or a protective factor for infectious disease spread. Higher risk practices were assigned lower scores. A dichotomous variable (yes/no) was assigned a score of zero if no intervention measure was present and a score of one otherwise. Furthermore, for variables with four response categories, viz., never, sometimes/once a month, usually/once a week, always/every day, assigned scores were zero, one, two and three, respectively.
Biosecurity scores were summed for each category and converted to a total score of 100 to standardize the score for comparison purposes. An average of standardized scores for all categories was then calculated to determine the overall score, which ranged from 0 to 100; a higher score represented a more comprehensive performance in the category of the biosecurity practices assessed. It is noted that biosecurity scores calculated this way rather represented ordinal data instead of a continuous data and therefore, distances between categories does not represent a standard unit of measure.
Production performance was measured in terms of harvest weight per pond area in square meter (kg/m2) and shrimp size at harvest (number of shrimp/kg). The former was calculated by dividing harvest weight per pond (kg) by pond area (m2).
Descriptive analyses were performed in Stata 17 (StataCorp, 2019) and carried out for farmers’ demographics, farm characteristics, on-farm biosecurity practices, farm production performance, and disease occurrence. Due to a high number of missing data points for production data, correlations between farm production performance and biosecurity scores of different on-farm practices were only visually assessed using scatter plots.
3 Results
3.1 Characteristics of farms and farmers
A total of 90 shrimp farms from five regions (Banten, East Java, North Coast, Subang, and South Coast regions) of Java Island, Indonesia, participated in the study. A third of all shrimp farms (36.7%) were in Subang region (Table S2). Demographic information and other characteristics of the farm, farmer and/or farm worker, such as aquaculture experience, and water connectivity status is presented in Table S2. All participants of the survey were males, of which over 60% had completed postsecondary education (associated degree or higher, Table S2). Approximately a quarter of the respondents reported they had received formal training in aquaculture management. Median years of experience in aquaculture was six years for farm owners, and five years for workers (Table S2). Each farmer managed a median of seven shrimp ponds with 90% of the ponds being active (shrimp present at the time of sampling). Approximately 88.9% of these farms shared common water sources with other aquaculture farms and 65.6% of them were connected to other farms via water channel. Most of the surveyed farms (83%) were located within one-km distance to their closest neighboring aquaculture farm.
3.2 On-farm biosecurity practices
Details of on-farm biosecurity practices of the surveyed shrimp farms are presented in Tables 1, S4.
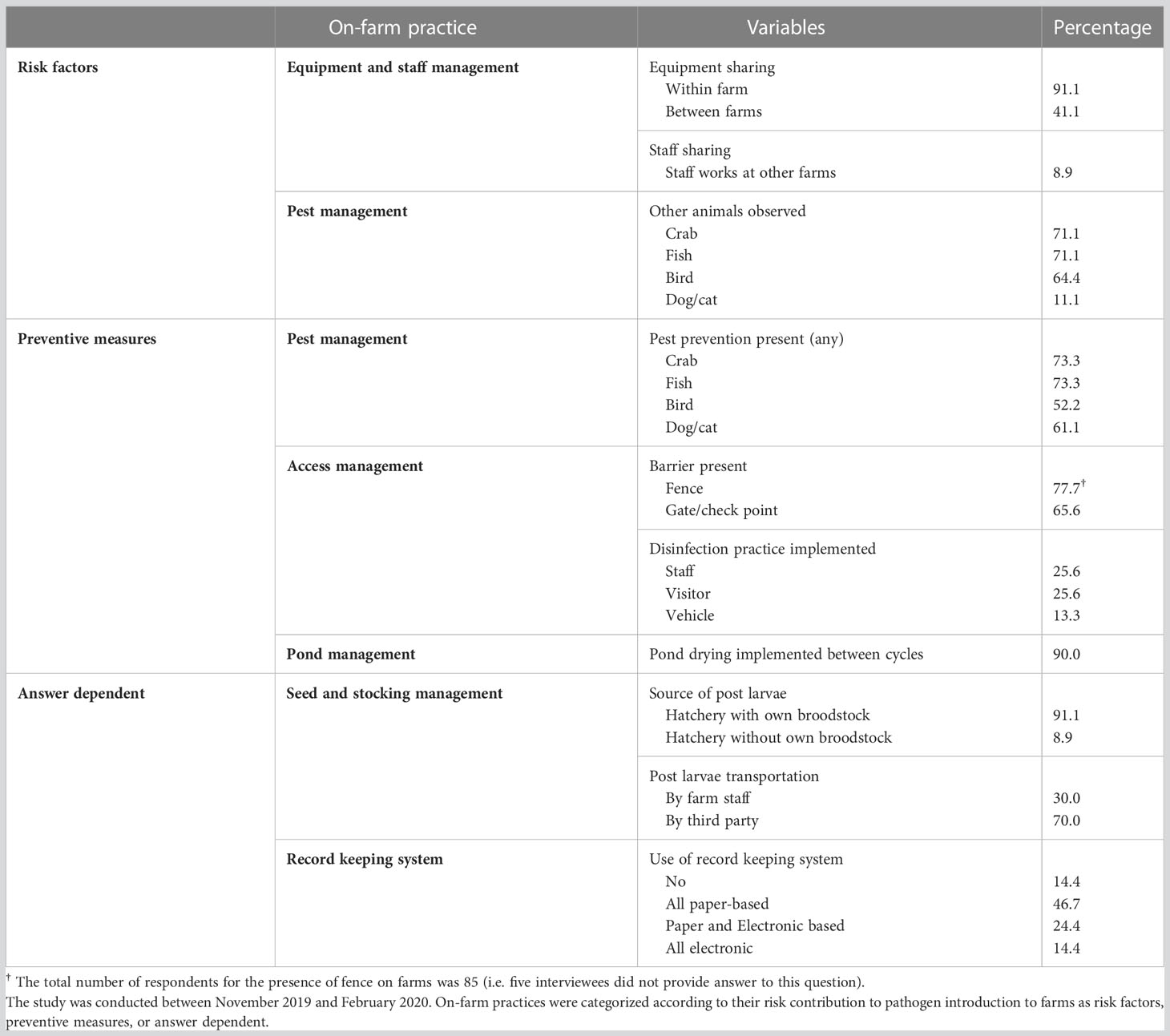
Table 1 Frequency distribution of on-farm biosecurity practices of surveyed shrimp farms (n = 90) on Java Island, Indonesia.
Most farms reported access to the farm was managed via fencing (77.7%) and installation of gate/check points (65.6%) to prevent unsolicited person or vehicle entry to their farm. Only 25.6% of farms practiced disinfection of staff and visitors before entry was allowed into their premise. Even lesser number of farms (13.3%) practiced vehicle pre-entry disinfection as a biosecurity measure (Table 1). In 8.9% of the farms, staff were employed at more than one farm at the same time (Table 1).
Most farms (91.1%) obtained their post larvae (PL) from external hatcheries with own broodstock (Table 1). PL quality was not assessed in 37.8% of the farms. For routine practices of PL quality assessment, approximately a third of the farms always screened PL hepatopancreas and gut health before stocking their ponds, while 46.1% of the farms always performed a stress test, which is typically applied as an abrupt 30-minute to two hours exposure of shrimp postlarvae (PL) to low salinity with subsequent assessments of survival to determine quality of PL batches (Palacios and Racotta, 2007) before stocking (Table S3).
In most cases (90.0%), farms practiced pond drying (mean 18 days; SD 14 days) before restocking (Table 1). Most farms prepared their ponds before stocking new PLs by treating with lime or calcium carbonate (85.6%). Ammonium sulphate and urea was used, to some degree, for pond preparation by 8.9% and 15.6% of the farms respectively (Table S3).
Over 65% of farms reported having observed other animal species (crab, fish, and bird) on their farms. Dogs or cats were present in 11.1% of the farms (Table 1). 73.3% of the farms used physical barriers such as fences, nets, screens or ropes, or chemical treatment of water to prevent entry of wild crabs and fish from their shrimp ponds. Measures to prevent birds and dogs and cats from accessing shrimp ponds were practiced by 52.2% and 61.1% of the farms respectively. (Table 1).
Most farms (99%) pre-treated water before using it for initial filling of their ponds. Considering farms that always used chemical for water preparation, chlorine (46.7%), lime (63.3%), and saponin (63.3%) were commonly used to pre-treat water in the water preparation process in these farms. Iodine, calcium cyanamide, sodium hydroxide and hydrogen peroxide were rarely used (71.1% to 97.8% reported never used; Table S3).
Mean and 95% confidence intervals of scores for each category of biosecurity practice are presented in Figure 1. Mean overall biosecurity scores varied across regions, with the lowest mean score of 33 (95% CI: 24, 42) for East Java region and the highest mean score of 54 (95% CI: 43, 64) for South Coast region (Table S4). Among the seven biosecurity categories, pest management (across all regions) scored lowest with a mean score of 20 (Figure 1). The mean score of pest management by region ranged from 19 to 33 (Table S4). Record keeping system across all regions scored highest with a mean score of 50 (Figure 1) and the mean score by region for this category ranged from 45 to 74 (Table S4). Visual assessment did not indicate any correlation between overall biosecurity score and farmer’s experience (Figure S2).
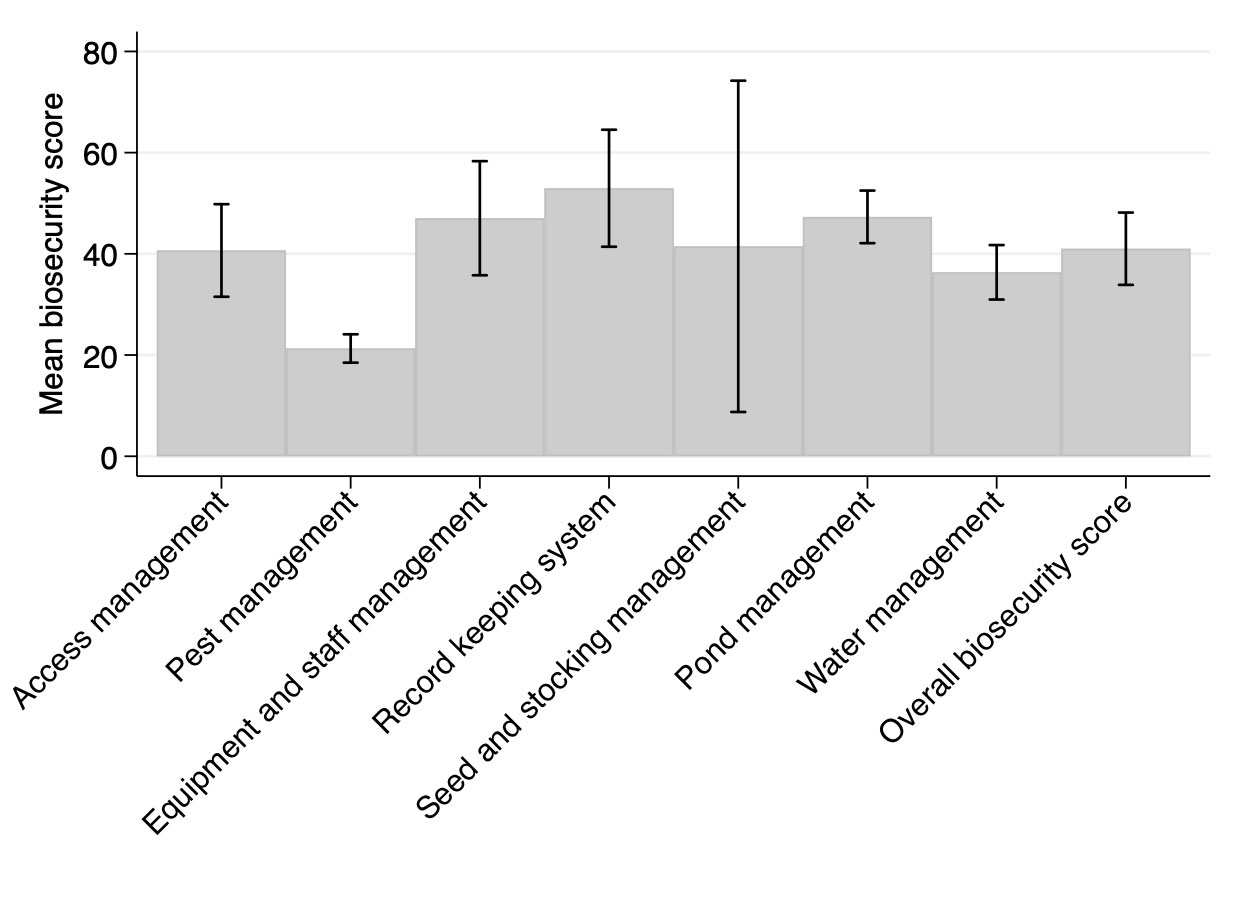
Figure 1 Mean with 95% confidence interval, adjusted for region clustering, for score of each biosecurity category and overall biosecurity score of shrimp farms (n = 90) on Java Island, Indonesia. The study was conducted between November 2019 and February 2020. Scores ranged from 0 to 100%; a higher score represents a more comprehensive performance in the biosecurity practices assessed.
3.3 Production performance
Production performances were measured in terms of shrimp harvest weight per pond area (kg/m2) and shrimp size at harvest (number of shrimp/kg) based on available records (n = 31 and n = 36, respectively). On average, farms produced 1.6 kg/m2 (95% CI: 1.2, 2.0) of shrimp with a size of 43 shrimp/kg (95% CI: 37, 49), which is equivalent to 23.3 g/shrimp at time of harvest.
3.4 Correlation between production performance and biosecurity score
Based on visual assessment, harvest weight per pond area (kg/m2) and shrimp size at harvest (shrimp units/kg) were found to have a positive correlation with higher biosecurity scores for access management and record keeping (Figures 2, 3) respectively. Most farms with higher access management scores (over 60) had larger shrimp sizes at harvest (25 g/shrimp or more) (Figure 3). An increasing trend in harvest weight per pond area and reduction in shrimp size at harvest was noted with increasing overall biosecurity score (Figure S3).
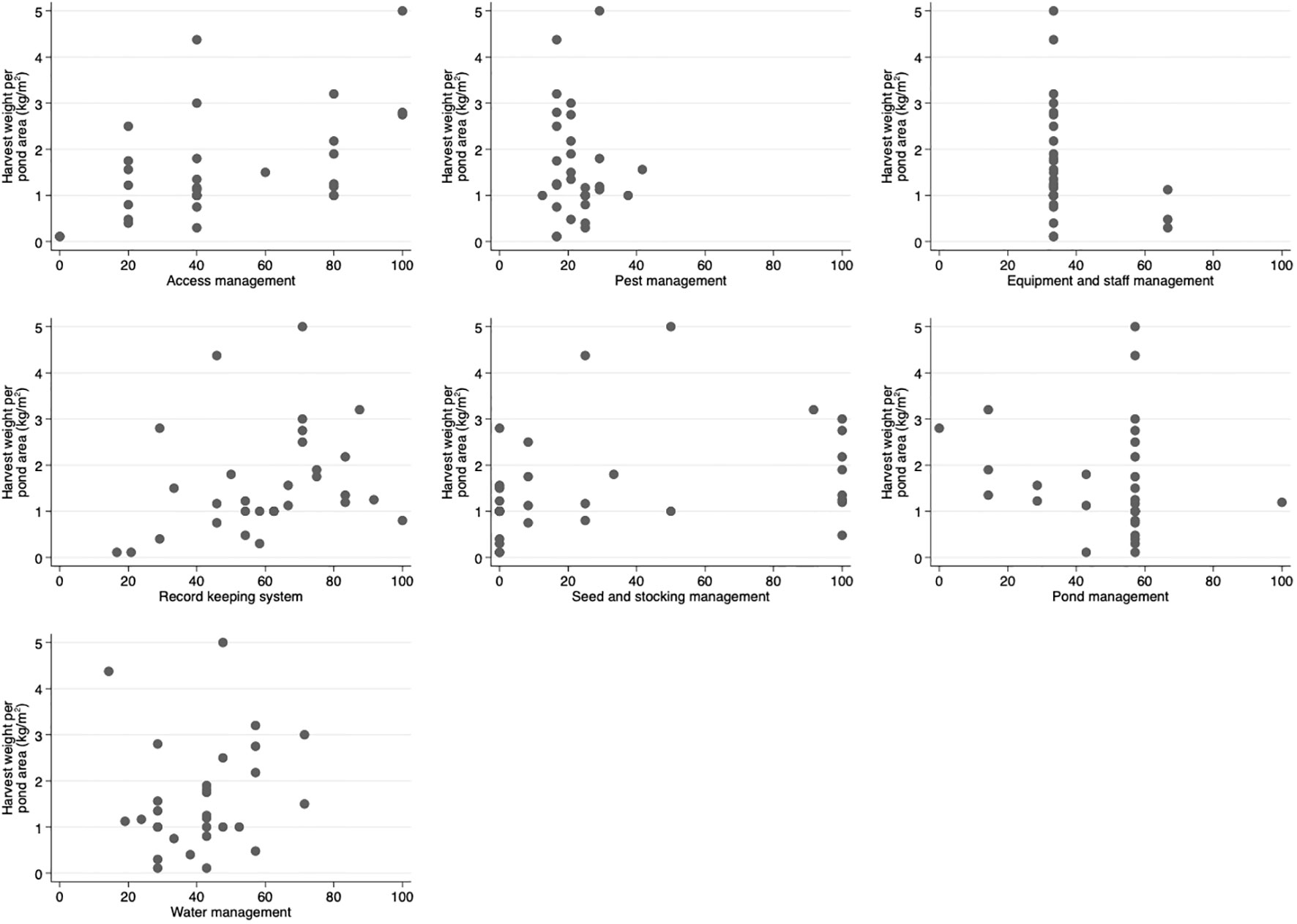
Figure 2 Scatter plots of harvested weight per pond area (kg/m2) and score for each biosecurity category for shrimp farms (n = 31) on Java Island, Indonesia. The study was conducted between November 2019 and February 2020. Score ranged from 0 to 100. A higher score represented a more comprehensive performance in the biosecurity practices assessed.
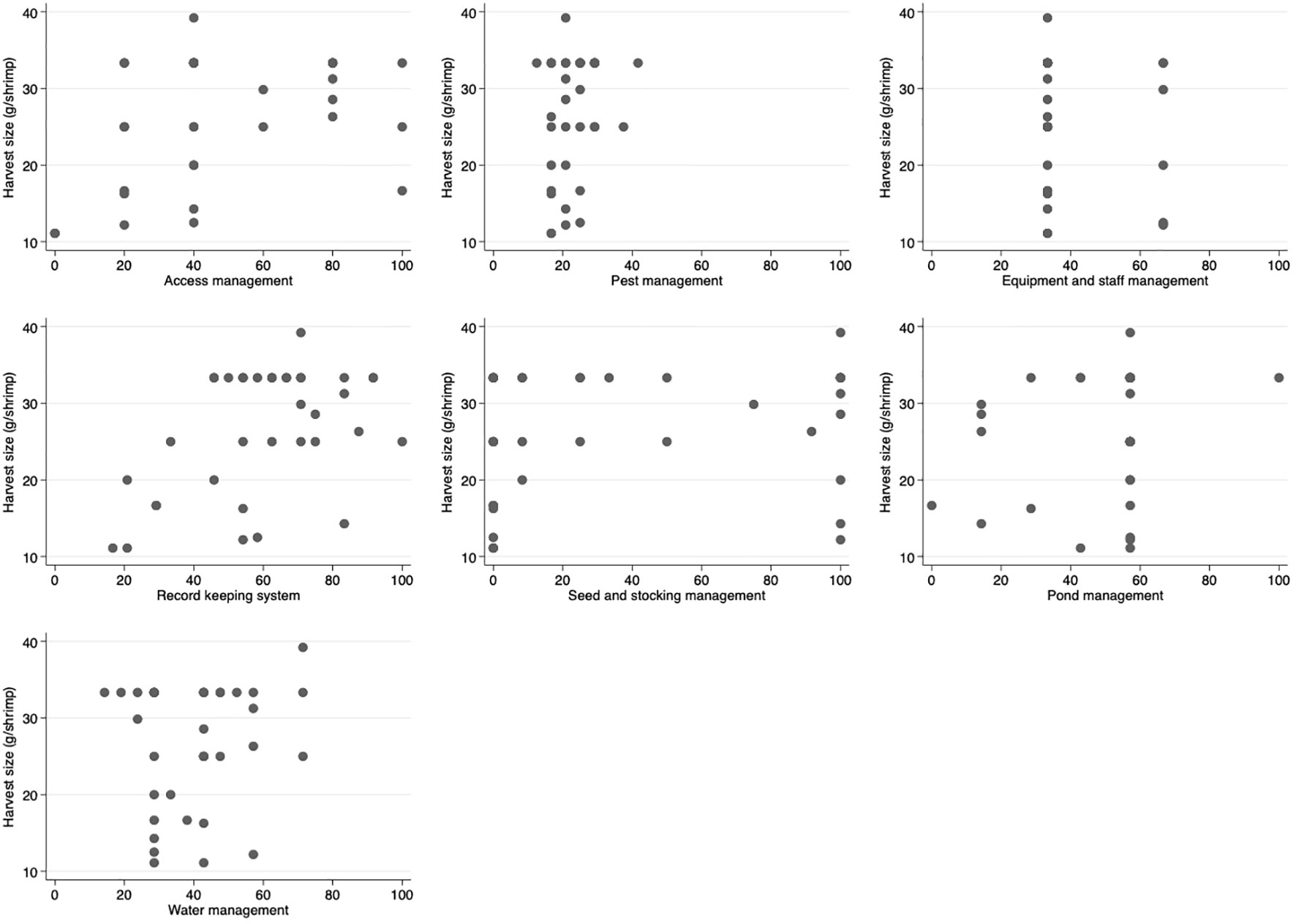
Figure 3 Scatter plots of shrimp size at harvest (g/shrimp) and score for each biosecurity category for shrimp farms (n = 36) on Java Island, Indonesia. Score ranged from 0 to 100%. The study was conducted between November 2019 and February 2020. A higher score represented a more comprehensive performance in the biosecurity practices assessed.
4 Discussion
This study describes farm and farmer characteristics pertinent to on-farm biosecurity practices of small to medium holder shrimp farms in Java Island, Indonesia. Additionally, it summarizes production performances in these farms and examines how biosecurity practices are related to the overall production in these farms. These results inform prioritization and focus of preventative procedures as shrimp farmers in Indonesia design farm biosecurity plans. Additionally, future studies addressing cost-benefit analyses of implementing biosecurity practices would benefit from knowledge provided by this study. While a conclusion regarding causal links was not possible with this study design, our results indicated that farms with better biosecurity practices tend to have a higher production yield. It is important to recognize that correlations identified between farm production performance and biosecurity scores represent a trend in data (because of the sample size limitations and not accounting for other potential confounders), but more comprehensive studies would be needed to establish an association between biosecurity practices and production indices.
It was beyond the scope of our study to identify factors affecting the adoption of biosecurity practices at shrimp farms. Since farmer demographics are known to generally influence biosecurity practices, our finding that a higher number of years spent in aquaculture farming was not associated with better biosecurity practices implemented at the farm was unexpected. Factors that influence farmers’ decisions should be investigated to help understand constraints faced by Indonesian shrimp farmers. A Sri Lankan study identified that difference in economic status of small holder shrimp farmers was a major factor resulting in disproportionate implementation of basic farm biosecurity practices (Munasinghe et al., 2010). While we did not determine the socioeconomic characteristics of the participating farms in our study, the role of this factor in biosecurity adoption cannot be disregarded (Can and Atlug, 2014) and requires further exploration.
All farms that participated in this study were serviced by eFishery Pte. Ltd™. As the studied farms were conveniently selected and only voluntarily participating farms were included in the study, we acknowledge that our sampling approach may have introduced selection bias into the study. However, this approach was chosen given the time, logistic, and financial constraints available for the study and our sample fairly represents overall shrimp farm population on the Java Island, which is one of the major farmed shrimp production areas in Indonesia.
Our questionnaire included questions regarding shrimp disease occurrence during past production cycles. White spot disease (WSD), infectious myonecrosis (IMNV), white feces disease (WFD), and Enterocytozoon hepatopenaei (EHP) were the major infectious diseases reported. However, some respondents apparently reported lists of infectious diseases for which they were aware rather than diseases that occurred at their farms. Due to this ambiguity in the responses, we excluded the disease occurrence data from our final analyses.
In this study we found that it was common for water sources to be shared between farms and also that equipment was shared within and between farms. However, the effect of these practices on the potential for spread of infectious disease agents could not be quantified. A study based on 110 production cycle records in 2021 from 26 shrimp farms in Jawa Timur province in Banyuwangi district, Indonesia, reported that farms with better biosecurity practices tended to have fewer infectious pathogen detections and better production estimates (Laurin et al., 2022). A different study in Vietnam (Boerlage et al., 2017) identified several primary factors as being potentially very influential on the probability of pathogen introduction into an aquaculture farm. These included: the source and infection status of juvenile stock in ponds; exposure to wild crustaceans in the water source; exposure to water sources shared with other farms; exposure to pathogens carried over in the environment from the last crop cycle (i.e., untreated pond bottoms); and exposure to fomites, equipment, or people inadvertently transporting pathogens between ponds or sites.
The lack of reliable data recording systems was identified as one of the limiting factors to assess the impacts of changes in risk activities on disease occurrence outcomes. Since only 14.4% farms in this study had electronic records, complete access to a recorded history of biosecurity practices and disease or production outcomes became cumbersome and inefficient to facilitate analytical processes. Introduction of electronic records that are reliably complete and repeatable between cycles and farming sites is a crucial step forward in disease risk identification and management.
While biosecurity practices are not the only elements to consider when providing advice on the improvement of farm management practices, they have a direct and measurable impact on the occurrence and spread of pathogens within and between farms (Tung et al., 2020). Ultimately, this impacts the overall productivity of the farm. This causal relationship between biosecurity inadequacies leading to decreased production implies a temporal sequence of events. For this reason, concurrent collection of both farm production and disease occurrence data and farm biosecurity data are equally important. These data should be collected for all farms to be studied and linked at the farm level to describe the impact of biosecurity practices on farm production.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Author contributions
TP: Questionnaire design, initial draft of manuscript and data analysis. MG: Initial draft of manuscript and final revision. KH: Design of the study, supervision of the project and critical revision of the article. DG: Design of the study and data collection. MD: Data analysis, manuscript revision. HB: Project management and writing. NS: Data gathering and data cleaning. KT: Design of the study, supervision of the project and critical revision of the article. All authors contributed to the article and approved the submitted version.
Funding
This research was cofunded by IDH, the Sustainable Trade Initiative, and eFishery Pte. Ltd™. (Indonesia). The funders were not involved in the study design, collection, analysis, interpretation of data, the writing of this article, or the decision to submit it for publication.
Acknowledgments
We thank shrimp farmers and their staff who participated in the study and assisted with data acquisition.
Conflict of interest
Authors DG and NS are employed by eFishery, Pte. Ltd, an aquaculture company that provides data science and information technology services to over 30,000 aquaculture farmers across 24 provinces in Indonesia, which has been declared in the institutional affiliation.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author KT declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/faquc.2023.1169149/full#supplementary-material
References
Asche F., Anderson J. L., Botta R., Kumar G., Abrahamsen E. B., Nguyen L. T., et al. (2021). The economics of shrimp disease. J. Invertebrate Pathol. 186. doi: 10.1016/j.jip.2020.107397
Boerlage A., Nguyen K. V., Davidson J., Phan V. T., Bui T. N., Dang L. T., et al. (2017). Finfish marine aquaculture in northern Vietnam: factors related to pathogen introduction and spread. Aquaculture 466, 1–8. doi: 10.1016/j.aquaculture.2016.09.037
Can M. F., Altuğ N. (2014). Socioeconomic implications of biosecurity practices in small-scale dairy farms. Veterinary Q. 34 (2), 67–73. doi: 10.1080/01652176.2014.951130
Canadian Food Inspection Agency (2012) Basic principles of biosecurity. Available at: https://inspection.canada.ca/animal-health/terrestrial-animals/biosecurity/tools/basic-principles/eng/1344821232793/1344821360235.
Delphino M. K. V. C., Laurin E., Thitiwan P., Rahardjo R. B., Wildan L. H., Zulfikar G., et al. (2022). Description of biosecurity practices on shrimp farms in Java, lampung, and banyuwangi, Indonesia. Aquaculture 556. doi: 10.1016/j.aquaculture.2022.738277
Henriksson P. J. G., Banks L. K., Suri S. K., Pratiwi T. Y., Fatan N. A., Troell M. (2019). Indonesian Aquaculture futures–identifying interventions for reducing environmental impacts. Environ. Res. Lett. 14. doi: 10.1088/1748-9326/ab4b79
Laurin E., Delphino M. K. V. C., Rahardio R. B., Hakim L., Zulfikar W. G., Burnley H., et al. (2022). Infectious disease detection associated with trends in production, environmental and biosecurity factors for shrimp (Litopenaeus vannamei and Penaeus monodon) production systems in banyuwangi, Indonesia. J. Fish Dis. 45, 1231–1236. doi: 10.1111/jfd.13621
Lee D., Yu Y.-B., Choi J.-H., Jo A.-H., Hong S.-M., Kang J.-C., et al. (2022). Viral shrimp diseases listed by the OIE: a review. Viruses 14 (585). doi: 10.3390/v14030585
Lestariadi R. A., Yamao M. (2018). Where do risks in shrimp farming come from? empirical results from small farmers in East Java Indonesia. J. Agribusiness Rural Dev. 1 (47), 39–47. doi: 10.17306/J.JARD.2018.00366
Lightner D. V. (2007). Biosecurity in shrimp farming: pathogen exclusion through use of SPF stock and routine surveillance. J. World Aquaculture Soc. 36 (3), 229–248. doi: 10.1111/j.1749-7345.2005.tb00328.x
Munasinghe M. N., Stephen C., Abeynayake P., Abeygunawardena I. S. (2010). Shrimp farming practices in the puttallam district of Sri Lanka: implications for disease control, industry sustainability, and rural development. Veterinary Med. Int. 2010. doi: 10.4061/2010/679130
Palacios E., Racotta I. S. (2007). Salinity stress test and its relation to future performance and different physiological responses in shrimp postlarvae. Aquaculture 268, 123–135. doi: 10.1016/j.aquaculture.2007.04.034
Shvili J. (2021) Java Island. world atlas. Available at: https://www.worldatlas.com/islands/java-island.html.
Tung D. X., Tuan H. A., Minh N. T. T., Padungtod P. (2020). Economic analysis of enhanced biosecurity practices in three types of chicken farms in northern Vietnam. Livestock Res. Rural Dev. 32 (54).
Yi D. (2013) Modern variety adoption and intensification in Indonesian shrimp aquaculture: are poor farmers included? Available at: http://www.ifpri.org/cdmref/p15738coll2/id/127966/filename/128177.pdf.
Keywords: biosecurity, farm management, Penaeus vannamei, epidemiology, whiteleg shrimp
Citation: Patanasatienkul T, Gautam M, Hammell KL, Gilang D, Delphino MKVC, Burnley H, Salsabila NA and Thakur KK (2023) Survey of farm management and biosecurity practices on shrimp farms on Java Island, Indonesia. Front. Aquac. 2:1169149. doi: 10.3389/faquc.2023.1169149
Received: 18 February 2023; Accepted: 09 June 2023;
Published: 06 July 2023.
Edited by:
Beatriz Novoa, Institute of Marine Research, Spanish National Research Council (CSIC), SpainReviewed by:
Mengqiang Wang, Ocean University of China, ChinaSubhendu K. Otta, Central Institute of Brackishwater Aquaculture (ICAR), India
Copyright © 2023 Patanasatienkul, Gautam, Hammell, Gilang, Delphino, Burnley, Salsabila and Thakur. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Milan Gautam, bWlsYW5nYXV0YW0yMDQwQGdtYWlsLmNvbQ==
 Thitiwan Patanasatienkul
Thitiwan Patanasatienkul Milan Gautam1,2*
Milan Gautam1,2* K. Larry Hammell
K. Larry Hammell Dimas Gilang
Dimas Gilang Marina K. V. C. Delphino
Marina K. V. C. Delphino Krishna K. Thakur
Krishna K. Thakur