- 1Ontario Aquaculture Research Centre, Office of Research, University of Guelph, Guelph, ON, Canada
- 2Department of Animal Biosciences, University of Guelph, Guelph, ON, Canada
Introduction: Cultured fish species are increasingly exposed to fungi and bacteria in the rearing environment, often causing disease and mortality causing aquaculture producers to rely on the use of chemical therapeutants which may have negative consequences for both human and environmental health. This study investigated the effect of humic acid as a treatment to reduce fungal and bacterial infections to increase survival during the incubation of rainbow trout (Oncorhynchus mykiss) eggs.
Methods: Humic acid, an aqueous extract of oxidized lignite was added to water entering both stacked tray and jar type incubators at a dosage rate of 5 mg/L from fertilization until hatching.
Results: The water treatment eliminated observable fungus and significantly improved survival until hatching in the treated incubator trays (77.2%) compared to the untreated controls (55.5%) (p ≤ 0.001). Egg survival was not improved in the incubator jars. The humic acid reduced bacterial diversity, but only in the stacked tray incubators and altered bacterial composition of the water after 20 days of treatment.
Discussion: The treatment increased the bacterial abundance of Burkholderiales, positively associated with healthy fish eggs and decreased the abundance of Flavobacterium and Aeromonas, known fish pathogens. These findings support the topical treatment of humic acid as a potential alternative treatment to prevent fungal infections and reduce mortality during incubation of rainbow trout eggs.
1 Introduction
Mortality during the incubation of eggs from a contamination by water molds (Saprolegnia spp.) and common aquatic pathogens ubiquitous in water results in annual production losses in the hatchery production of rainbow trout (Oncorhynchus mykiss) and other freshwater fishes (Heikkinen et al., 2016; Ozdemir et al., 2021). The management of fungal and bacterial infections has historically relied on the use of chemical treatments which may have negative effects on human and environmental health. Historically, malachite green was used as effective disinfection treatment against water molds and bacterial infections in both fish eggs and adults, but this product was banned in 1991 in United States and 2001 in European Union for safety reasons (Heikkinen et al., 2016). In Canada, malachite green is approved for use in the treatment of aquarium fish, but the product has not been allowed to be used in food fish since 1992 (Canadian Food Inspection Agency, 2017). Alternatives have been investigated and the most common egg disinfection treatment since the 1990’s has been formalin (Schreier et al., 1996; Wagner et al., 2008; Heikkinen et al., 2016). However, there are safety and environmental concerns related to the use of formalin in aquaculture. Staff working with formalin must wear respirators, gloves and eye protection because the product will cause irritation in the eyes and respiratory system of humans. Hydrogen peroxide has also been tested as a control for infections caused by water molds, but the required concentration (500 mg/L or greater) has been found to cause increased egg mortality 7–11 days after fertilization (Wagner et al., 2012).
Recently, there has been growing interest in the development of non-toxic and environmentally friendly products to safely disinfect incubating salmonid eggs, which would reduce risks to human and environmental health (Lieke et al., 2020). One possible solution may be humic substances, such as humic acid, fulvic acid and humins, which form from decaying debris and makes up to 95% of dissolved organic matter found in aquatic ecosystems (Thurman, 1985; Haitzer et al., 1998; Steinberg, 2003). These organic molecules of small molecular weight can interact with biological membranes (Meinelt et al., 2007) and exert both antibiotic and antifungal properties (Visser, 1985; Gryndler et al., 2005; Meinelt et al., 2007). Previous studies have found that treatment of rainbow trout eggs and larvae with humic substances increased defense against pathogenic fungi (Saprolegnia spp.) and pathogenic bacteria (Aeromonas spp.) (Schreckenbach et al., 1994; Meinelt et al., 2008). Several studies have also investigated humic acid as a feed additive in rainbow trout diets which demonstrated increased survival when fish were challenged with Yersinia ruckeri (Yilmaz et al., 2018a,b). However, there are no studies to date that have used next-generation sequencing to investigate the effects of humic substances on the bacterial community of water during the incubation of fish eggs.
The objective of this study was to test the effect of humic acid, derived from oxidized lignite coal, as an alternative treatment to reduce fungal and bacterial infections and improve survival of rainbow trout eggs during incubation. Additionally, the microbial community of filtered incubation water was assessed to determine how the bacterial community changed over time and between incubator systems (jar vs tray) when exposed to humic acid.
2 Materials and methods
2.1 Fish facilities
Gametes (eggs and sperm) were collected from mature rainbow trout held at the Ontario Aquaculture Research Centre (Elora, ON, Canada). Broodstock were maintained in accordance with animal utilization protocol #4582, reviewed and approved by the University of Guelph’s Animal Care Committee. For three consecutive weeks during the fall reproductive season, brood fish were sedated using a 75 mg/L solution of Syncaine (tricaine methanesulfonate; Syndel; Nanaimo, BC, Canada). Gametes were manually stripped from mature males and females. Only viable eggs were used in this study and poor quality eggs, identified as white eggs or those having an irregular shape, were discarded. Eggs from each female were fertilized using sperm from individual males. Fertilized eggs were water hardened for two hours. Eggs were enumerated by counting and weighing 100 eggs from each female to calculate the average egg weight. Fertilized egg batches collected from each female were weighed and then were divided by the average egg weight to estimate the total number of eggs fertilized.
After enumeration, fertilized eggs from all crosses were pooled and divided among two incubator types, stacked trays (12 L volume) or jars (6 L volume). During each of three consecutive weeks, fertilized eggs placed in the incubator trays were divided into four replicate groups of control (groundwater) and four replicate groups of treatment. The treatment incubator trays received groundwater continuously dosed with 5 mg/L humic acid (trade name: AC Aqua, MTS Environmental Inc., Exeter, ON, Canada) using a peristaltic pump according to the manufacturer’s recommendation.
Eggs were maintained in the dark and the flow to each incubator tray was calibrated to 10 L/min. Similarly, during each of three consecutive weeks, fertilized eggs placed in the incubator jars were divided into two replicate groups of control (groundwater) and two replicate groups of treatment (groundwater continuously dosed with humic acid at 5 mg/L). The flow to each incubator jar was calibrated to 3.0 L/min.
2.2 Survival
Survival was assessed at two important hatchery stages, the eyed stage and at hatch. When the pigmentation of the eyes was visible through the egg membrane (eyed stage), eggs were removed from the incubator trays. All dead eggs were removed and enumerated to assess the cumulative egg mortality from stocking to the eyed stage. Percent survival to the eyed stage was calculated as:
Live eyed eggs were returned to the incubator trays where they remained until hatch (42 days post-fertilization). At the time of hatching, all dead eggs and embryos were removed and enumerated to assess the cumulative mortality to the hatching stage in both incubator trays and jars. Percent survival to hatch was calculated as:
2.3 Sample type and collection
Prior to the initiation of the experiment, three different types of sample collection were tested in triplicate to determine the optimal sample type for this experiment. Type 1: Ten eggs were placed into a 20 mL collection vial and were homogenized using a hand-held homogenizer (SP Bel-Art ProCulture, South Wayne, NJ, USA). Type 2: Ten eggs were placed in a 20 mL collection vial and vortexed for 5 minutes to suspend bacteria which may have settled on the surface of the egg. Type 3: A 1L water sample collected from the discharge of the stacked tray incubator was concentrated through a microfiber filter. A 1 cm x 1 cm section of the filter was cut and placed in 400 μL of distilled water. The results indicated that DNA could be extracted from test sample type 1, the homogenized eggs. However, most of the sample was fish DNA. Test sample type 2 did not yield any detectable DNA whereas test sample type 3 had the greatest detectable level of bacterial DNA. Therefore type 3, the concentrated water sample, was selected for use in this study.
For the duration of the experiment, every 10 days from fertilization to hatch, 1L water samples were collected from the incubator trays and jars for a total of 5 time points (0, 10, 20, 30 and 40 days post-fertilization) using sterile glass beakers. The water sample was filtered through an autoclaved microfiber 1.5μm filter (Cytiva Whatman 934-AH). A 1 cm x 1 cm section was cut from the center of the filter paper using sterile scissors. The cut section of filter paper was resuspended in 400 μL of distilled water and frozen at -80°C.
Influent groundwater was analyzed weekly and had a mean (± standard deviation) temperature of 8.53 ± 0.04°C, dissolved oxygen of 10.69 ± 0.32 mg/L, pH of 7.90 ± 0.08 and total suspended solids of 0.29 ± 0.17 mg/L.
2.4 Extraction and sequencing of 16S rRNA amplicons
The DNA was extracted from the bacteria resuspended in water using a QIAamp® Fast DNA stool mini kit (Qiagen Inc, Toronto, ON, Canada) according to the manufacturer’s instructions. The DNA concentration was quantified using a Quibit 2.0 fluorimeter (Invitrogen, Thermo Fisher Scientific, Waltham, MA, USA). A two-stage PCR was performed to target the V3-V4 region of the 16S rRNA gene according to the 16S Library Preparation Guide (Illumina Inc, San Diego, CA, USA). The library was sequenced on the Illumina MiSeq platform at the University of Guelph (Guelph, ON, Canada) to produce 2x300 bp pair-end reads with a MiSeq Reagent kit v3 of 600 cycles (Illumina Inc). Quality of sequence reads was examined using MultiQC (https://multiqc.info/). Detailed methodology can be found in Huyben et al. (2021).
2.5 16S rRNA bioinformatics
The 16S rRNA gene sequences were analyzed using Mothur version 1.42.3 (Schloss et al., 2009) according to the MiSeq SOP [https://www.mothur.org/wiki/MiSeq_SOP] (Kozich et al., 2013). Sequence reads which were smaller than 300 bp, larger than 400 bp, had more than eight consecutive bp and were outside the V3-V4 region of the 16S rRNA were removed from the dataset. Filtered sequence reads were aligned to the SILVA reference database version 123 (Quast et al., 2012), pre-clustered to merge sequences with less than 2 bp difference and chimeras were removed using the open-source tool VSEARCH (Rognes et al., 2016). Sequences were classified using GreenGenes version 13_8_99 at a cut off of 80% (Cole et al., 2014) and taxon resembling chloroplasts, mitochondria, unknowns, archaea and eukaryotes were removed. All samples were normalized (subsampled) to the sample that had the lowest number of reads. All raw sequence data were deposited in the Sequence Read Archive (SRA) of NCBI under BioProject Accession PRJNA897011 (https://www.ncbi.nlm.nih.gov/bioproject/PRJNA897011).
2.6 Statistical analysis
Statistical analysis of survival was performed using SPSS software (version 26, IBM, NY, USA). Survival to the eyed stage and survival to hatch were reported as a percent. The replicates were assessed for normality using the Shapiro-Wilk test. Comparison of survival between treatment and control groups were assessed using the Mann-Whitney U-test. P-values below 0.05 were considered significant.
Normal distribution and homogeneity of each 16S rRNA gene sequence dataset were determined using Shapiro-Wilk and Levene tests in R (version 2022.07.1, R-Core-Team, 2015). When needed, data were normalized by log or square-root transformation and all data are presented as means ± standard error unless otherwise specified. A Student’s T-test was performed on normal data and a Wilcoxon test was performed on non-normal data. Alpha-diversity tables were created using the number of Operational Taxonomic Units (OTUs), Good’s Coverage, Shannon and Choa-1 indices based on the vegan package in R (Oksanen et al., 2018). Linear discriminant analysis effect size (Lefse) and ANOSIM were used to analyze the indicator OTU and beta-diversity. Galaxy was used to analyze and plot the Lefse analysis using Wilcoxon or Krukal-Wallis tests with a Linear Discriminant Analysis (LDA) cutoff of 3.0 [https://huttenhower.sph.harvard.edu/galaxy/]. P-values below 0.05 were considered significant.
3 Results
3.1 Fungus growth and egg survival
In the incubator trays, fungus was observed in the control replicates at 16 days post fertilization (dpf) for week 1 (W1) eggs, at 14 dpf for week 2 (W2) eggs and at 15 dpf for week 3 (W3) eggs. The mycelium was observed to expand to nearby live eggs in all control incubator trays across all fertilization dates within five days of the visual detection of fungus on dead eggs. Despite removing the dead and fungus-covered eggs at the eyed stage, fungus growth continued to compromise the eggs until hatch at 42 dpf. No observable fungus was detected in the treatment replicates. Due to the shape of the incubator jars and the constant movements of eggs, it was impossible to detect the first signs of fungus growth. However, once the mycelium was observed to expand to nearby live eggs, fungus growth expanded into mats, smothering the live eggs underneath.
In the incubator trays, the survival in the treatment groups exposed to humic acid (mean 95.5 ± 3.6%) was significantly greater than the control groups (mean 81.8 ± 4.6%) (p ≤ 0.001) at eye-up (24 dpf). At hatch (42 dpf), the survival in the treatment group (mean 77.2 ± 10.4%) was statistically significantly higher than the control group (mean 55.5 ± 16.1%) (p ≤ 0.001; Table 1). In the incubator jars, there was no difference in survival between the treatment (62.5 ± 27.6%) and control groups (70.9 ± 20.6%) (p = 0.699; Table 1) at hatch.
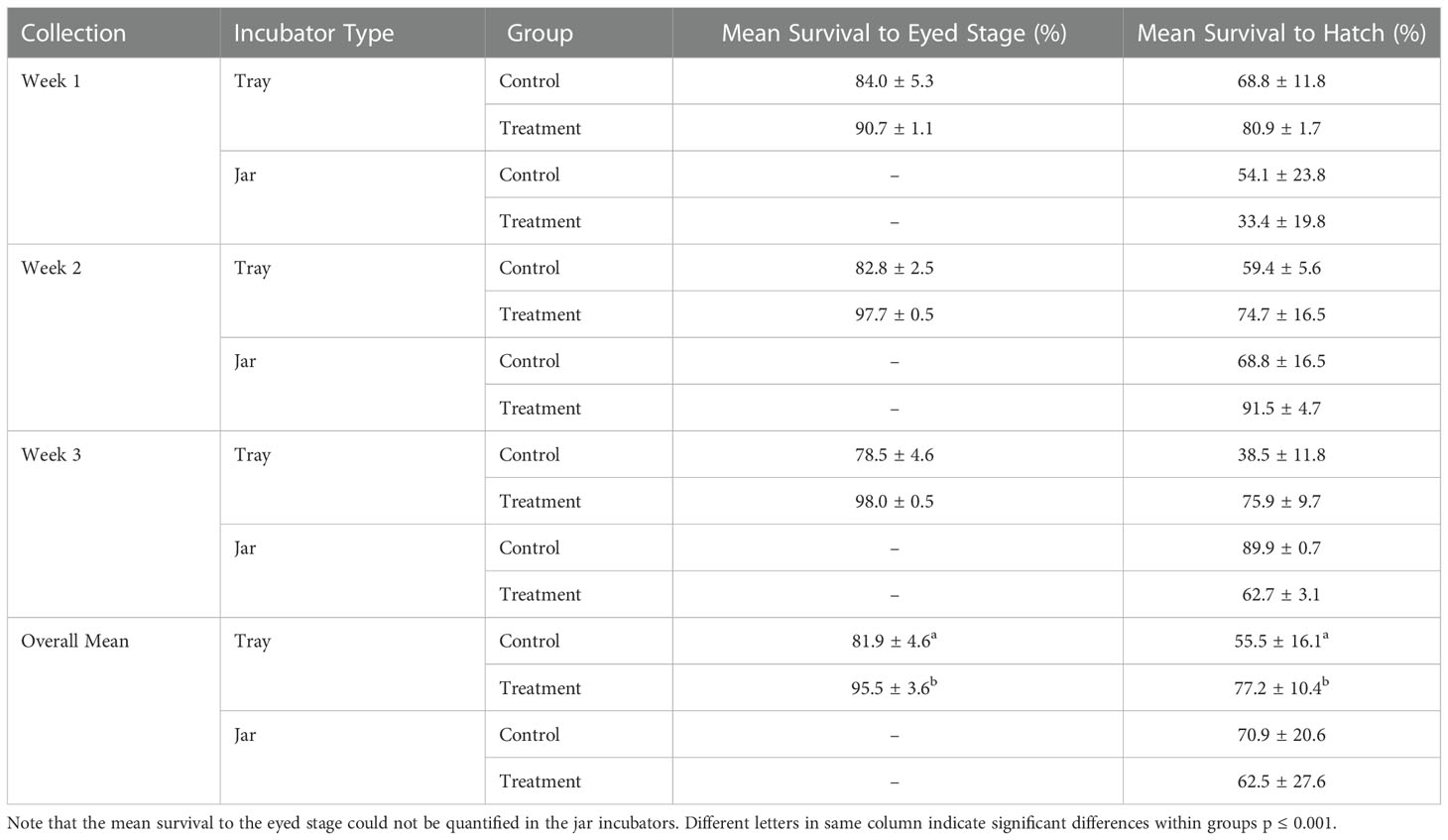
Table 1 Percent survival (± standard deviation) of rainbow trout eggs collected weekly for three weeks at the eyed stage (24 days post fertilization) and hatch (42 days post fertilization) incubated at 9°C in stacked incubator trays and incubating jars exposed to groundwater (control) and humic acid at 5 mg/L (treatment).
3.2 Alpha diversity of bacteria
Alpha diversity (i.e. Shannon) of OTUs were significant between treatment groups (p = 0.011) as well as over time (p< 0.001) (Figures 1, 2). Specifically, alpha diversity decreased over time between samples collected on day 0 compared to days 20, 30 and 40 (p< 0.01). Diversity between incubators trays and jars was not significant across all time points (p = 0.441) and the final three time points (p = 0.193), although diversity was numerically lower in the jars (Figure 3). Among the last three time points (day 20, 30 and 40), the diversity decreased in treated eggs compare to the control (p = 0.023), but only in water collected from the incubator trays (p = 0.032) (Figure 4).
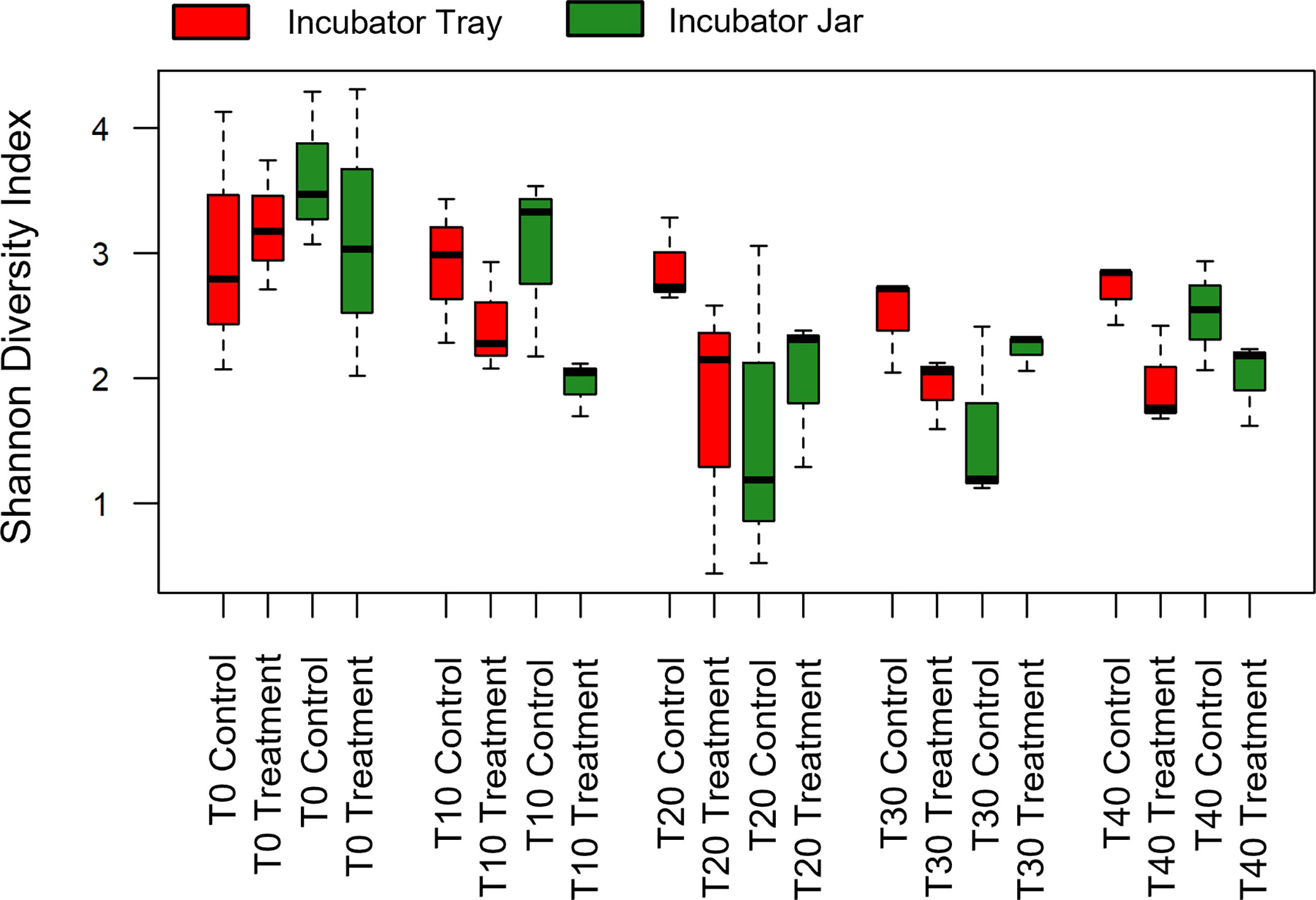
Figure 1 Alpha diversity (Shannon Diversity Index) of operational taxonomic units of all treatment groups using water filtered from rainbow trout eggs exposed to no topical agent (Control) or 5 mg/L humic acid (Treatment) and sampled over 0, 10, 20, 30 and 40 days using incubator trays or jars. (n = 9).
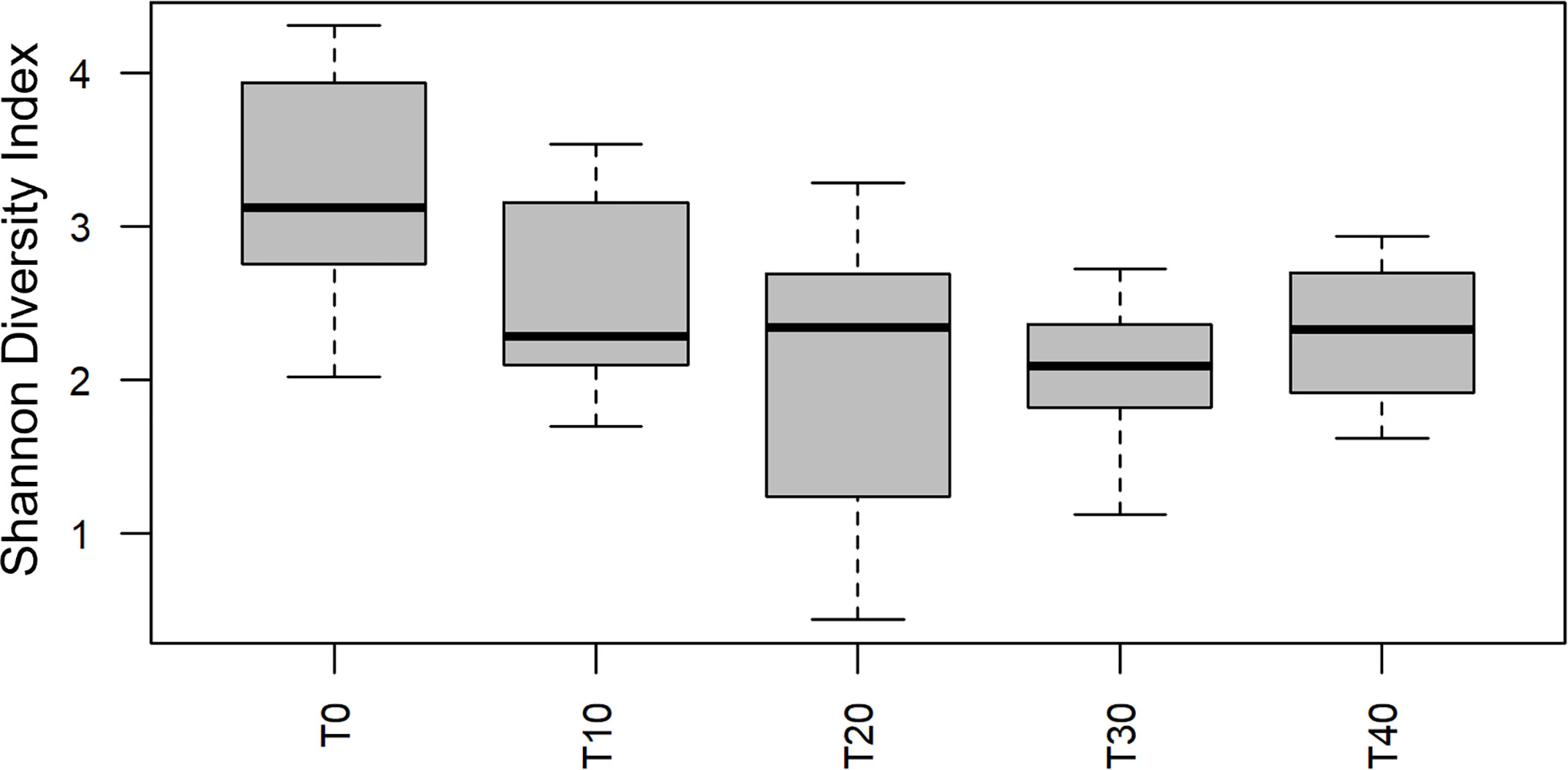
Figure 2 Alpha diversity (Shannon Diversity Index) of operational taxonomic units over time using water filtered from rainbow trout eggs exposed to no topical agent (Control) or 5 mg/L humic acid (Treatment) and sampled over 0, 10, 20, 30 and 40 days. (n = 9).
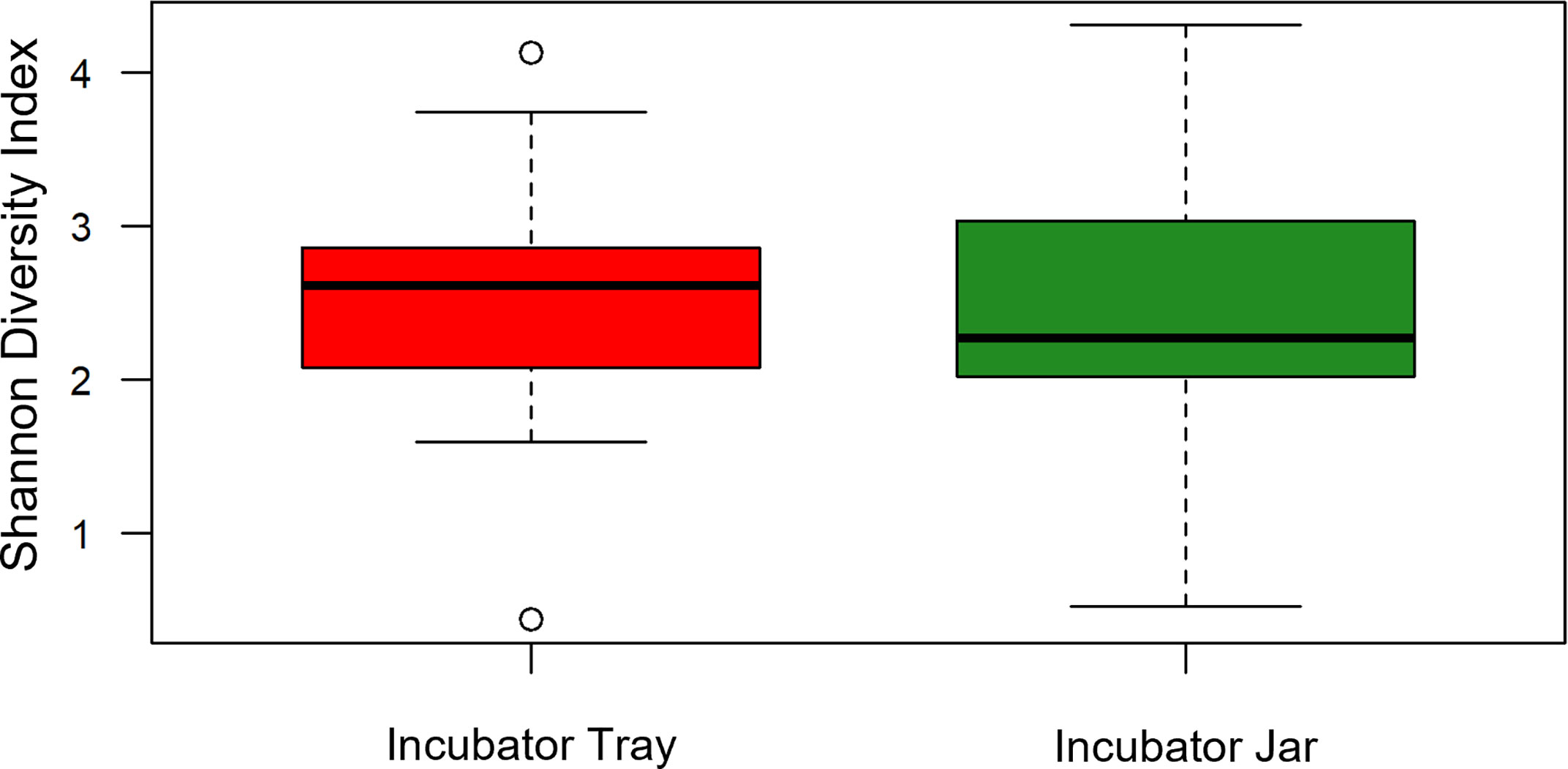
Figure 3 Alpha diversity (Shannon Diversity Index) of operational taxonomic units between incubator trays and jars using water filtered from rainbow trout eggs. In this case, all treatments and time points were pooled. (n = 9).
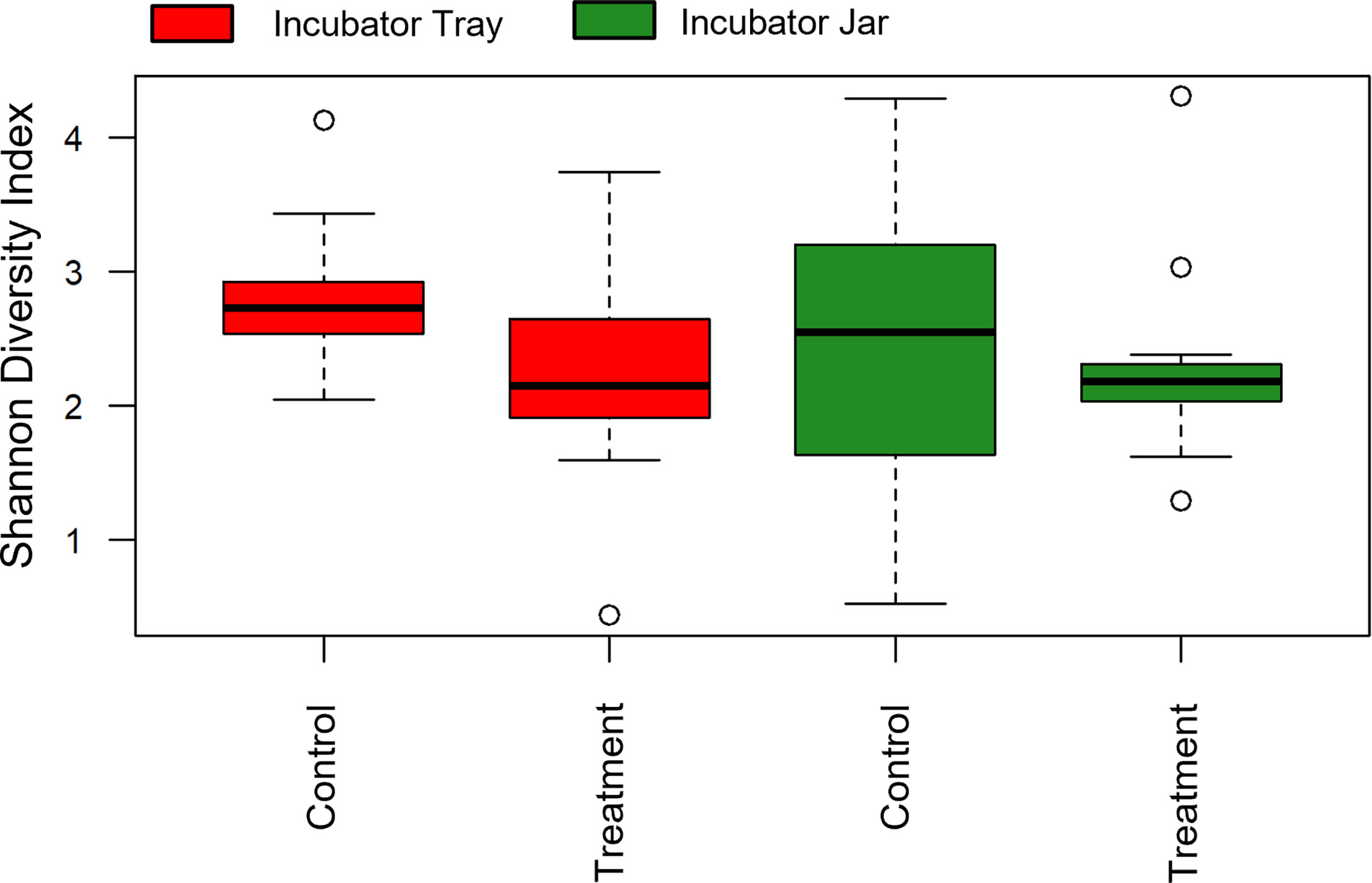
Figure 4 Alpha diversity (Shannon Diversity Index) of operational taxonomic units between incubator trays and jars as well as exposure to no topical agent (Control) or 5 mg/L humic acid (Treatment) using water filtered from rainbow trout eggs. In this case, time points were pooled, except 0, 10 and 20 day samples were removed to compare final effects. (n = 9).
3.3 Beta diversity of bacteria
Beta diversity was significantly affected by the treatment (p< 0.001), vessel (p = 0.021) and time (p< 0.001). Overlap was observed among the incubator tray and jar samples in the non-metric multidimensional scaling (NMDS) plots (Figure 5), whereas this was not observed in the treated vs untreated eggs (Figure 6).
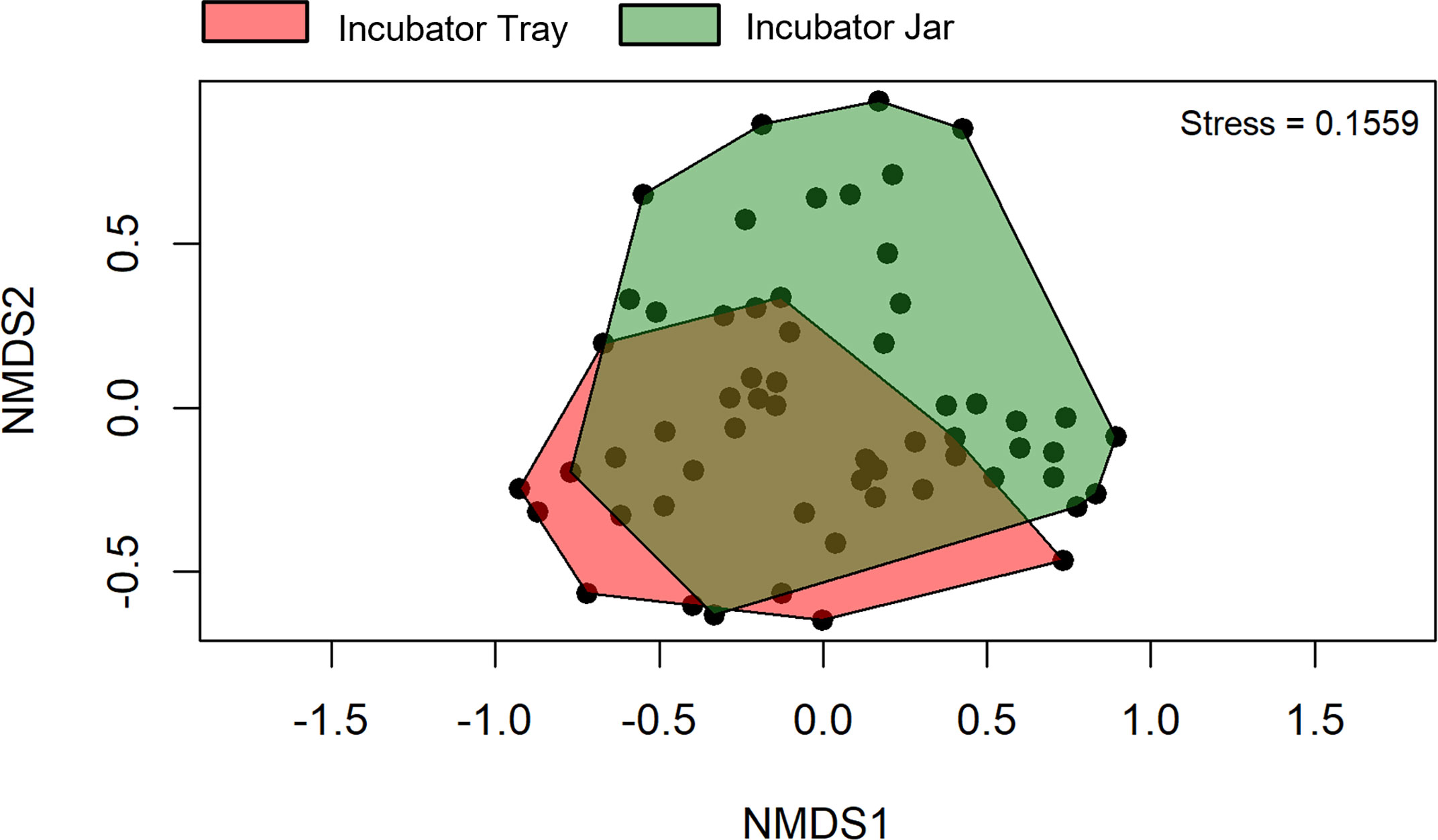
Figure 5 Beta diversity of operational taxonomic units between incubator trays and jars using water filtered from rainbow trout eggs. In this case, samples from all treatments and time points were pooled. NMDS, non-metric multidimensional scaling. (n = 9).
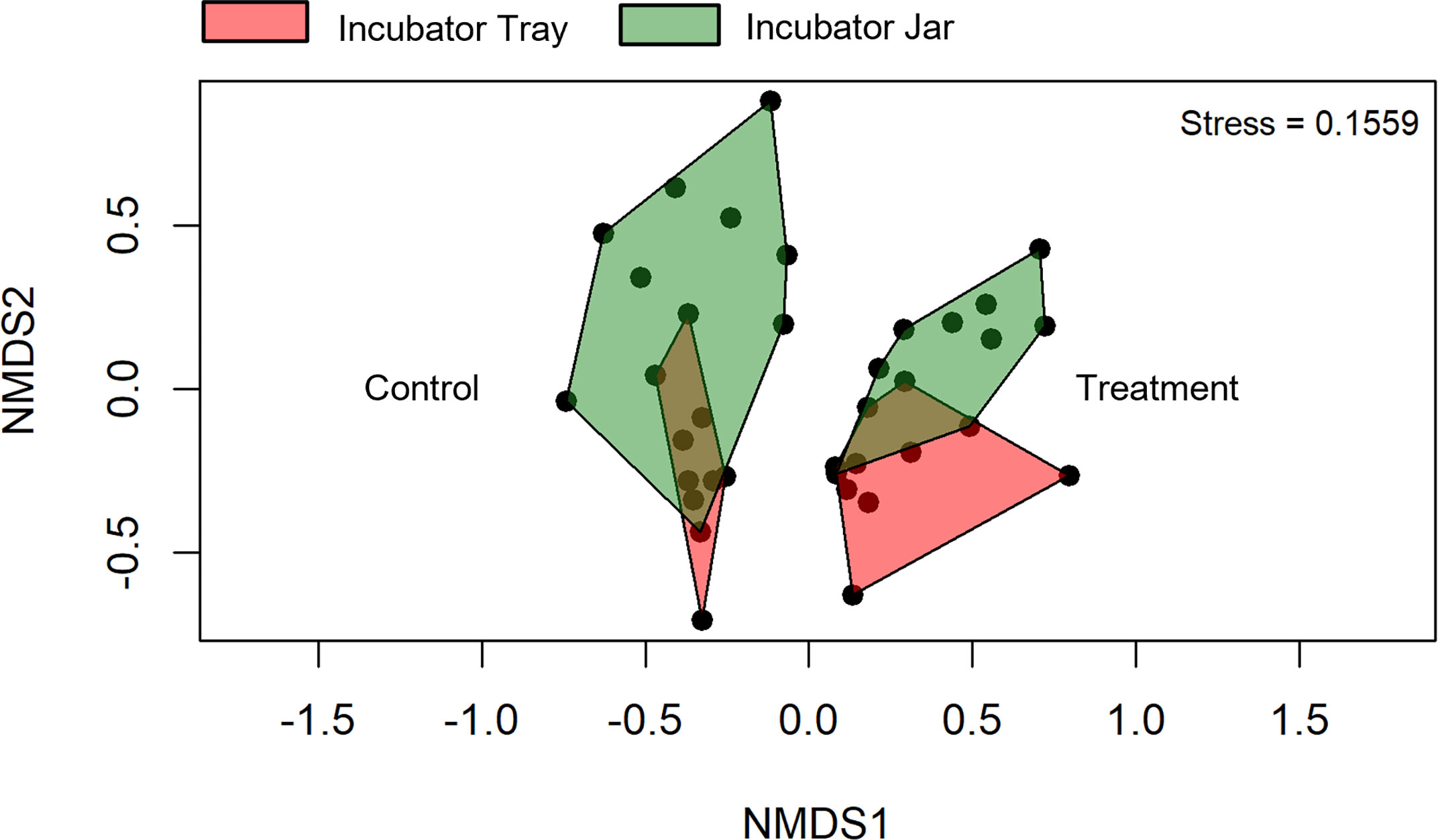
Figure 6 Beta diversity of operational taxonomic units between incubator trays and jars as well as exposure to no topical agent (Control) or 5 mg/L humic acid (Treatment) using water filtered from rainbow trout eggs. Time points were pooled, except 0, 10 and 20 day samples were removed to compare final effects. NMDS; non-metric multidimensional scaling. (n = 9).
At the phyla level, water from the rainbow trout eggs mainly contained Proteobacteria (Pseudomonadota; 69%) and Bacteroidetes (Bacteroidota; 19%) with lower abundance of Firmicutes (Bacillota), Actinobacteria (Actinomycetota) and Plantomycetes (2-4%) (Figure 7). At the genus level, samples mainly contained Comamonadaceae (17%), Flavobacterium (13%) and Pseudomonas (6%) with lower abundance of Acidovorax, Rhodoferax, Oxalobacteraceae, Massilia, Sphaerotilus and Chryseobacterium (3-5%) (Figure 8). Clostridium and Aeromonas spp. bacteria were also present at very low levels (0.4%). The Linear discriminant analysis effect size (LEfSe) analysis (Figure 9) found that the humic acid treatment increased the abundance of Comamonadaceae and decreased the abundance of Flavobacterium and Aeromonas spp. bacteria (Figure 10).
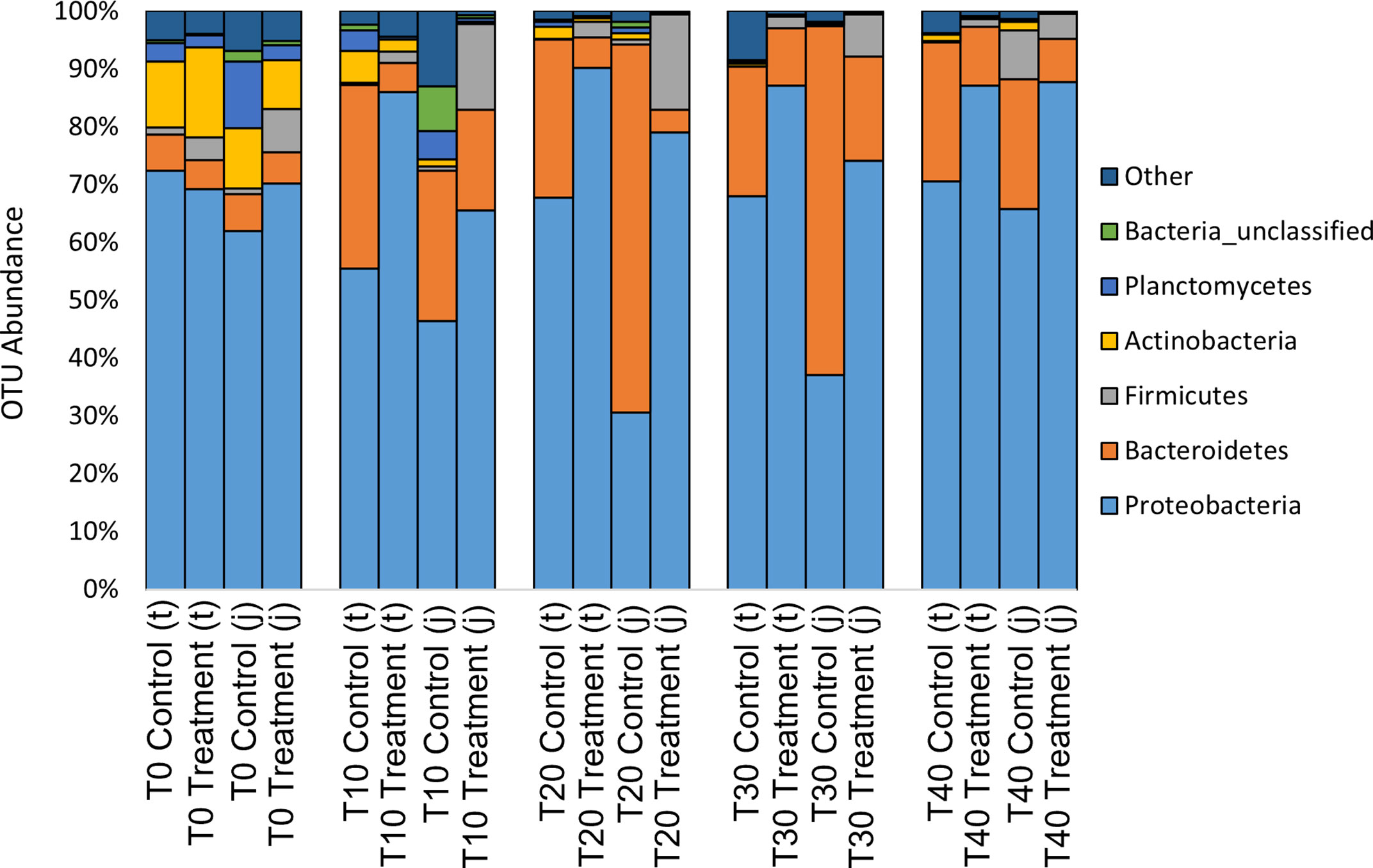
Figure 7 Relative abundance of operational taxonomic units (OTU) on the phyla level from water filtered from rainbow trout eggs exposed to no topical agent (Control) or 5 mg/L humic acid (Treatment) and sampled over 0, 10, 20, 30 and 40 days using incubator trays (t) or jars (j). (n = 9).
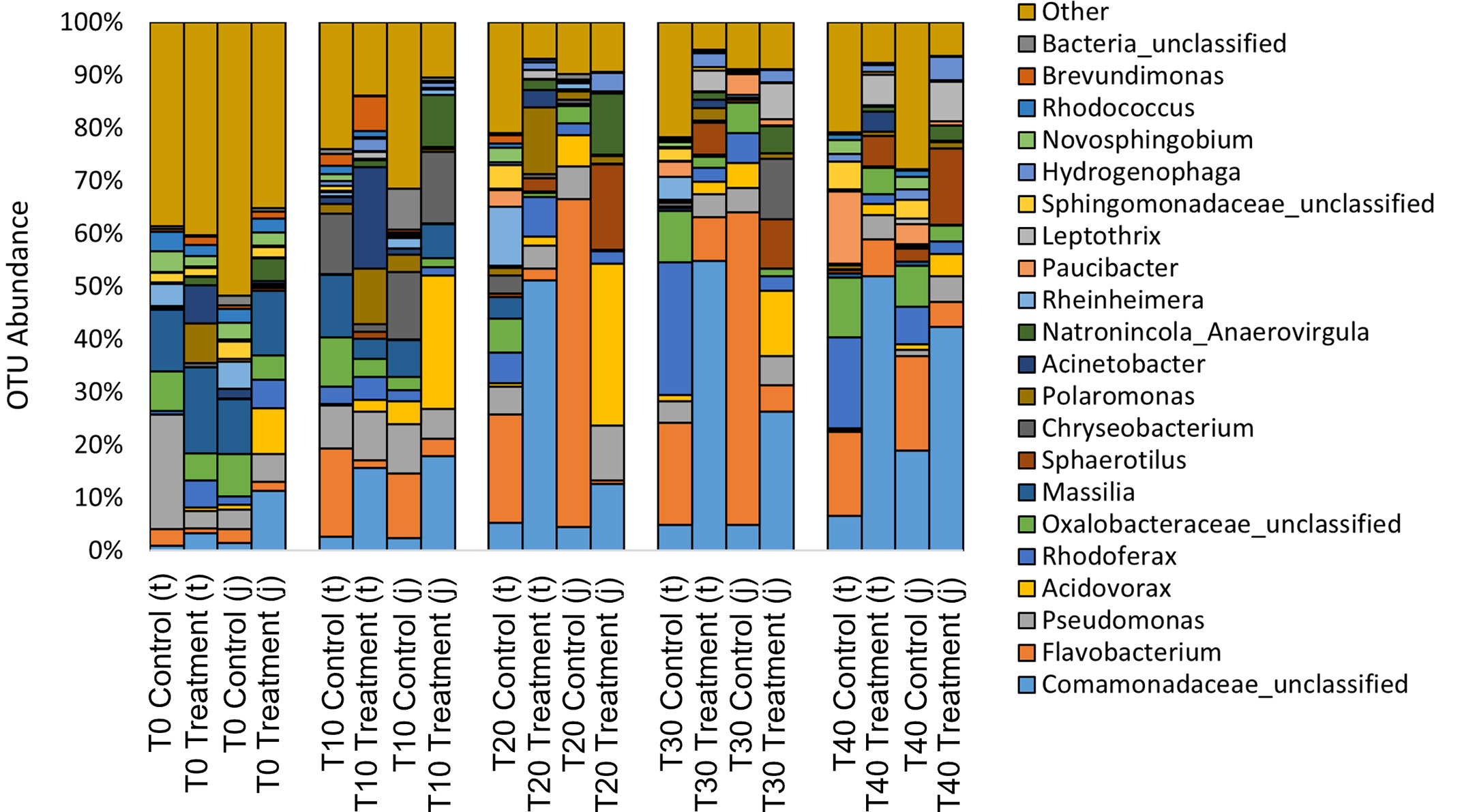
Figure 8 Relative abundance of operational taxonomic units (OTU) on the genus level from water filtered from rainbow trout eggs exposed to no topical agent (Control) or 5 mg/L humic acid (Treatment) and sampled over 0, 10, 20, 30 and 40 days using incubator trays (t) or jars (j). (n = 9).
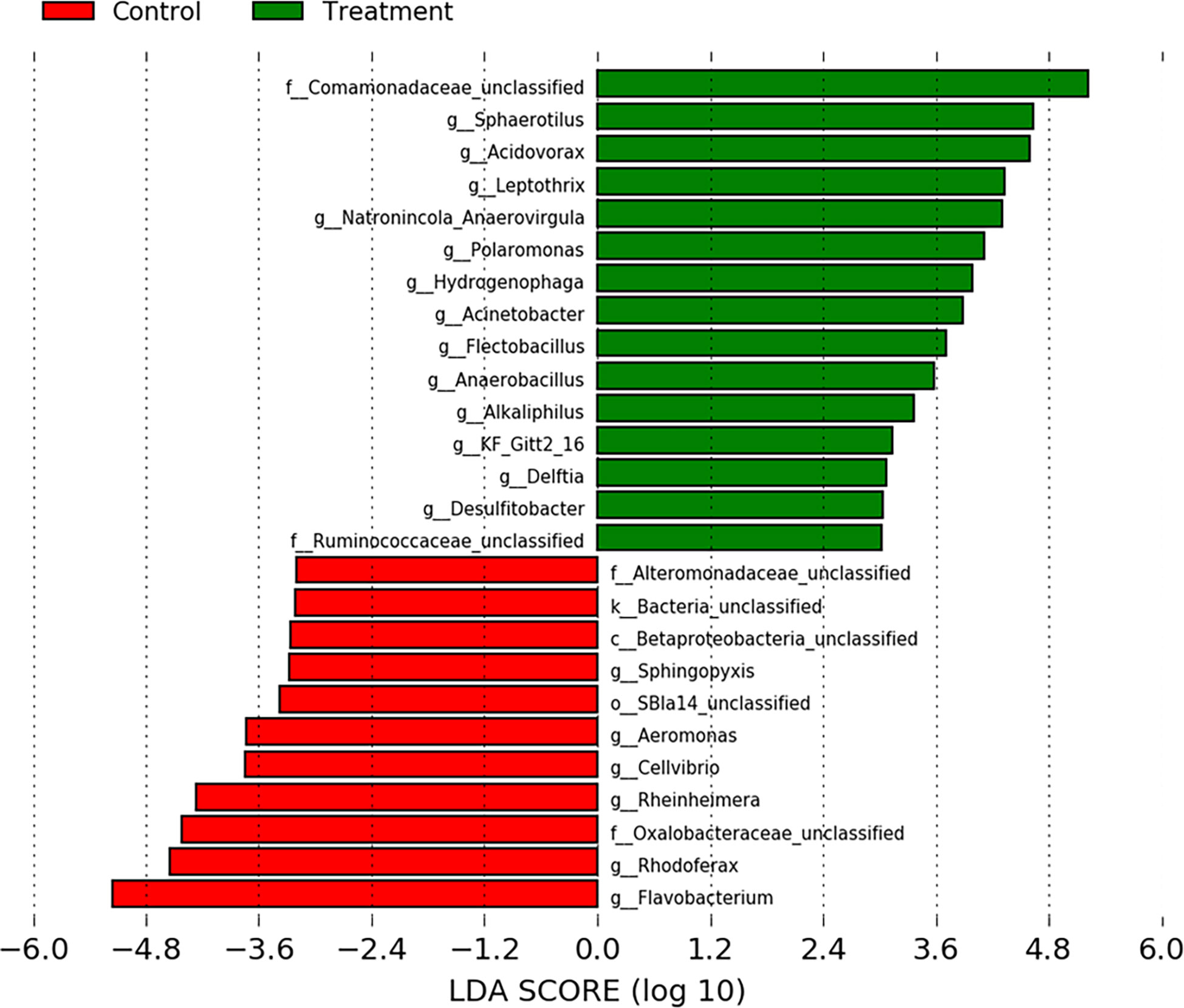
Figure 9 Linear discriminant analysis effect size (LEfSe) of indicator operational taxonomic units from water filtered from rainbow trout eggs exposed to no topical agent (Control) or 5 mg/L humic acid (Treatment). Samples over time and vessels were pooled and a Linear Discriminant Analysis (LDA) cutoff score of 3.0 was used. (n = 9).
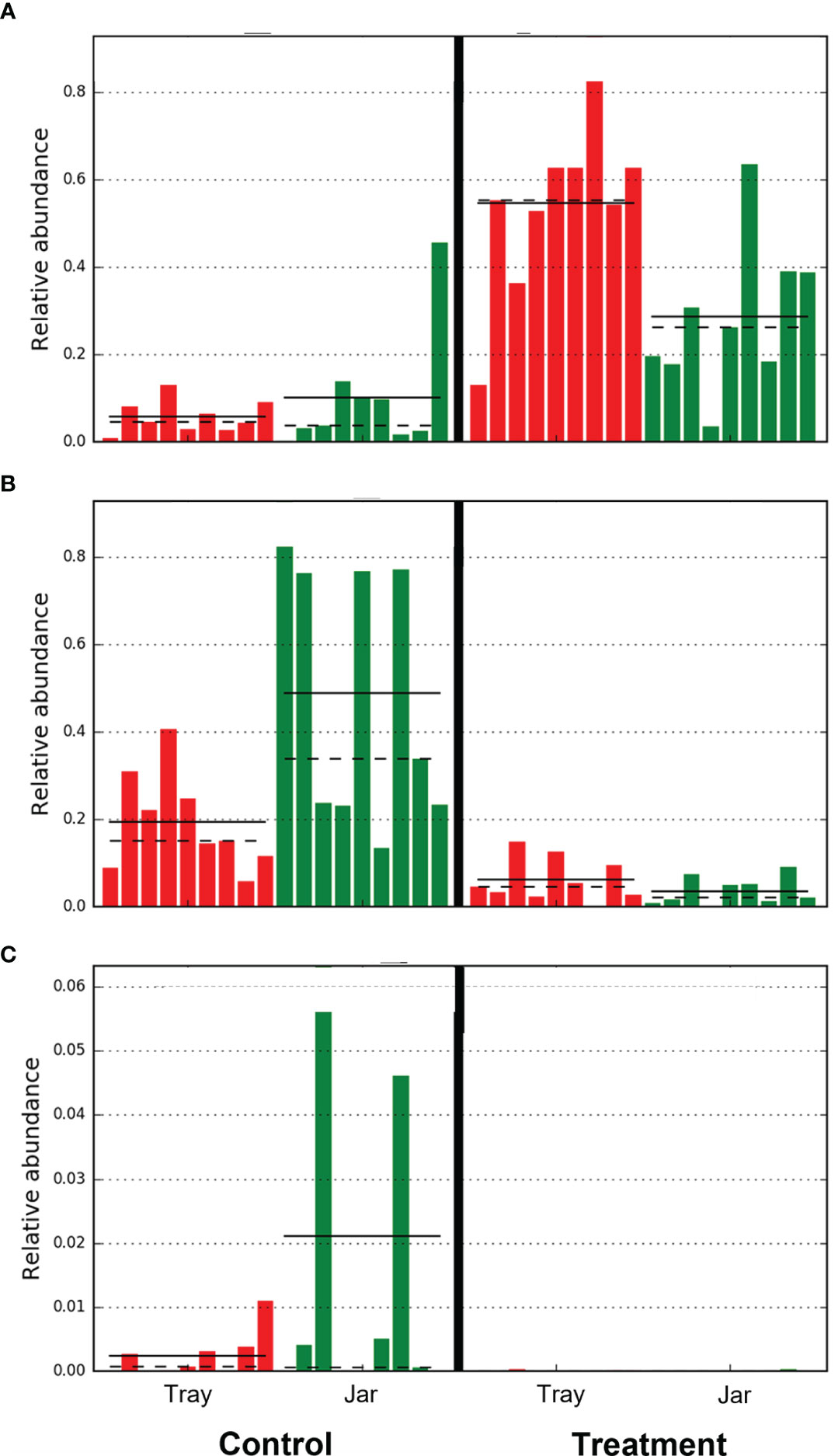
Figure 10 Relative abundance of operational taxonomic units of (A) Comamonadaceae (Burkholderiales order), (B) Flavobacterium, and (C) Aeromonas from water filtered from rainbow trout eggs exposed to no topical agent (Control) or 5 mg/L humic acid (Treatment). Comparison of incubator trays and jars, while pooled over time. (n = 9).
4 Discussion
In the present study, we evaluated the effect of humic acid as a microbial control agent during the incubation of rainbow trout eggs. In the absence of chemical or manual manipulation to reduce fungus growth, we expected the egg mortality in the control groups would be high. We predicted that exposure to humic acid in the water would reduce the growth of the fungal mycelium as observed by Meinelt et al. (2007). In the present study, exposure to humic acid in the water eliminated observable fungus and resulted in significantly improved survival compared to the control groups in the incubator trays.
Notably, there was no difference in survival between the control and treatment incubator jars. The authors believe this result is not an effect of the treatment, but rather the effect of the humic acid binding with particulates in the water. The influent ground water at the Ontario Aquaculture Research Centre is high in dissolved manganese (0.0942 mg/L) which forms a precipitate when the water is aerated. The humic acid was found to bind to the manganese and settle out in the incubation vessels. In the incubator trays, this did not cause a noticeable issue because the particulates settled to the bottom. In the incubator jars however, due to the constant upwelling of water, the bound particulates settled out on top of the hatched embryos and appeared to smother the alevins.
The filtered bacterial community of the water during the incubation of salmonid eggs is not well described, especially using next-generation sequencing methods that do not include culture dependent biases. As is the case with the gut microbiome of fishes, it is likely that the microbial community of the surface of fish eggs is strongly shaped by the rearing environment (Kim et al., 2021) rather than the species under culture. Kubilay et al. (2009) found the bacteria on rainbow trout eggs was varied and consisted primarily of Aeromonas, Corynebacterium, Enterobactericeae, and Acinetobacter while Moraxella, Flavobacterium and Cytophaga were also present to a lesser degree. Liu et al. (2014) found the most dominant bacterial phyla detected on healthy Atlantic salmon (Salmo salar) eggs were Proteobacteria (Pseudomonadota), Firmicutes (Bacillota), Actinobacteria (Actinomycetota) and Bacteroidetes (Bacteroidota). When evaluating the bacterial communities of Atlantic salmon eggs contaminated with Saprolegnia spp., Liu et al. (2014) found that Enterobacteriales, Clostridiales, Alteromonadales, Vibrionales and Aeromonadales were significantly more abundant in the diseased egg samples and several bacterial pathogens common to salmonid species, including Vibrio spp., Aeromonas spp. and Yersinia spp. were also identified.
In the present study, we characterized the bacterial communities from the rearing environment (rearing water in incubator trays versus incubator jars) and looked for changes over time from day 0 to day 40. Notably, due to the pore size of the filter paper used, only particles ≥ 1.5μm were captured, therefore the bacteria sampled by this method may not represent the complete microbiome of the water sample. Our results indicated that there was overlap in the microbial communities characterized in the incubator tray and jar samples (Figures 5, 6). At the phyla level, water from incubators mainly contained Proteobacteria (Pseudomonadota) and Bacteroidetes (Bacteroidota) with lower abundance of Firmicutes (Bacillota), Actinobacteria (Actinomycetota) and Plantomycetes (Figure 7), which is very similar to the results reported by Liu et al. (2014). At the genus level, water mainly contained Comamonadaceae, Flavobacterium and Pseudomonas with very low levels of Clostridium and Aeromonas (Figure 8). Species of Flavobacterium, Pseudomonas and Polaromonas are known to have a global distribution in many water bodies and have been found in over 50% of water samples taken from Arctic and Antarctic lakes (Michaud et al., 2012).
From day 0 to day 40, bacteria in the Actinomycetales order decreased over time whereas Burkholderiales, specifically the Comamonadaceae family, increased in the water treated with humic acid. The treatment reduced the bacterial diversity in the incubating water, but this was only observed in the incubator trays. Additionally, the humic acid altered the bacterial composition after 20 days of treatment and there was a clear distinction between the microbial communities among the eggs treated with humic acid compared to the controls which received untreated groundwater. Notably, the humic acid treatment increased the abundance of Comamonadaceae (Burkholderiales order), which has been positively associated with healthy fish eggs by Liu et al. (2014). A decrease in the abundance of known fish pathogens, Flavobacterium spp. and Aeromonas spp., was observed in the treatment incubators compared to controls (Figure 10). Reductions in Flavobacterium spp. and Aeromonas spp. bacteria have also been found on the skin of ayu fish (Plecoglossus altivelis) and intestine of juvenile loach (Paramisgurnus dabryanus) when fed 1% humus extract and 1.5% fulvic acid, respectively (Nakagawa et al., 2009; Gao et al., 2017). When rainbow trout were challenged with Yersinia ruckeri, fish fed diets supplemented with humic acid (0.6%) showed increased immune parameters, survival rates, and meat quality without negative impacts on growth performance (Yilmaz et al., 2018a). Similarly, another study by the same authors demonstrated that a diet supplemented with FARMARIN XP (Farmavet Group, Bucharest, Romania), a commercial product containing mainly humic acids along with fulvic, ulmic and fulfonic acids, increased the survival of rainbow trout following a challenge with Yersinia ruckeri (Yilmaz et al., 2018b). Both these studies suggest that humic acid may be used as a replacement for antibiotics in rainbow trout diets for the control of yersiniosis (Yilmaz et al., 2018a,b).
Finally, Liu et al. (2014) hypothesized that Actinobacteria (Actinomycetota) inhibited the attachment of the fungus causing agent, Saprolegnia spp., to salmon eggs after finding that a low incidence of fungus growth was strongly correlated with a high richness and abundance of Actinobacteria (Actinomycetota). The present study did not show similar results, Actinobacteria (Actinomycetota) abundance was greatest at 0 days post-fertilization and by day 20, appeared to be absent from the treated samples. It is possible that the differences observed in the present study may be due to differences in the microbial community on the surface of rainbow trout and Atlantic salmon eggs or differences in the rearing environment.
This study is limited by the large pore size of the filter paper and therefore some smaller bacteria in the water may not have been identified. This study should be repeated using a 0.2 µm pore size to fully characterise the microbiome of water during the incubation of rainbow trout eggs. Despite the limitations of the study, the results indicate that the bacterial flora of the water used to incubate rainbow trout eggs shared similarities and differences compared to previous research quantifying the microbial communities associated with incubating salmonid eggs. The application of humic acid as a water treatment during the incubation period eliminated observable fungus growth and improved survival in the incubator trays. When eggs were incubated in jars however, the humic acid treatment seemed to cause egg mortality due to the settling of particulates in the water. Additionally, the humic acid treatment increased the abundance of bacteria associated with healthy fish eggs and decreased the abundance of pathogenic bacteria. Therefore, it can be recommended that humic acid is a viable alternative for hatchery producers to use as a water treatment to reduce bacterial and fungal pathogens to improve the survival of rainbow trout eggs in incubator trays.
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found below: https://www.ncbi.nlm.nih.gov/, PRJNA897011.
Ethics statement
Ethical review and approval was not required for the animal study because fish eggs and alevins were used. This life stage is exempt from ethical review and approval as outlined by the guidelines established by the Canadian Council on Animal Care. The use and handling of the mature broodstock animals to generate eggs for this study was performed in accordance with animal utilization protocol #4582 and was reviewed and approved by the University of Guelph’s Animal Care Committee.
Author contributions
MC and MK conceived, planned and carried out the experiment. MC and MK collected samples and analyzed the survival data. DH analyzed the 16S data. Both MC and DH contributed to the final version of the manuscript. All authors contributed to the article and approved the submitted version.
Acknowledgments
The authors would like to acknowledge Scott Thompson from MTS Environmental Inc (Exeter, ON, Canada) for providing the humic acid product (AC Aqua) and Dr. Durda Slavic at the Animal Health Laboratory (Guelph, ON, Canada) for their assistance with 16S sequencing. The research took place at the Ontario Aquaculture Research Centre which is owned by the Government of Ontario through its agency, the Agricultural Research Institute of Ontario and managed by the University of Guelph through the Ontario Agri-Food Innovation Alliance.
Conflict of interest
This study received funding from MTS Environmental Inc. The funder provided the humic acid product but had no other involvement with the study.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Canadian Food Inspection Agency (2017) Malachite green - questions and answers. Available at: https://inspection.canada.ca/food-safety-for-consumers/fact-sheets/specific-products-and-risks/chemical-hazards/malachite-green/eng/1332268890141/1332268947157 (Accessed October 29, 2022).
Cole J. R., Wang Q., Fish J. A., Chai B., McGarrell D. M., Sun Y., et al. (2014). Ribosomal database project: Data and tools for high throughput rRNA analysis. Nucleic Acids Res. 42 (D1), D633–D642. doi: 10.1093/nar/gkt1244
Gao Y., He J., He Z., Li Z., Zhao B., Mu Y., et al. (2017). Effects of fulvic acid on growth performance and intestinal health of juvenile loach Paramisgurnus dabryanus (Sauvage). Fish Shellfish Immun. 62, 47–56. doi: 10.1016/j.fsi.2017.01.008
Gryndler M., Hrselova H., Sudova R., Gryndlerova H., Rezaova H., Merhautova V. (2005). Hyphal growth and mycorrhiza formation by the arbuscular mycorrhizal fungus Glomus claroideum BEG 23 is stimulated by humic substances. Mycorrhiza 15, 483–488. doi: 10.1007/s00572-005-0352-7
Haitzer M., Höss S., Traunspurger W., Steinberg C. E. W. (1998). Effects of dissolved organic matter (DOM) on the bioconcentration of organic chemicals in aquatic organisms - a review. Chemosphere 37 (7), 1335–1362. doi: 10.1016/S0045-6535(98)00117-9
Heikkinen J., Tiirola M., Mustonen S. M., Eskelinen P., Navia-Paldanius D., von Wright A. (2016). Suppression of Saprolegnia infections in rainbow trout (Oncorhynchus mykiss) eggs using protective bacteria and ultraviolet irradiation of the hatchery water. Aquac Res. 47, 925–939. doi: 10.1111/are.12551
Huyben D., Chiasson M., Lumsden J. S., Pham P. H., Chowdhury M. A. K. (2021). Dietary microencapsulated blend of organic acids and plant essential oils affects intestinal morphology and microbiome of rainbow trout (Oncorhynchus mykiss). Microorg 9, 2063. doi: 10.3390/microorganisms9102063
Kim P. S., Shin N. R., Lee J. B., Kim M. S., Whon T. W., Hyun D. W., et al. (2021). Host habitat is the major determinant of the gut microbiome of fish. Microbiome 9, 166. doi: 10.1186/s40168-021-01113-x
Kozich J. J., Westcott S. L., Baxter N. T., Highlander S. K., Schloss P. D. (2013). Development of a dual-index sequencing strategy and curation pipeline for analyzing amplicon sequence data on the MiSeq illumina sequencing platform. Appl. Environ. Microbiol. 79, 5112–5120. doi: 10.1128/AEM.01043-13
Kubilay A., Altun S., Savas S. (2009). A study on aerobic bacterial flora during incubation of rainbow trout (Oncorhynchus mykiss, Walbaum 1792) eggs in hatchery. J. FisheriesSciences.com. 3 (1), 5–9. doi: 10.3153/jfscom.2009002
Lieke T., Meinelt T., Hoseinifar S. H., Pan B., Straus D. L., Steinberg C. E. W. (2020). Sustainable aquaculture requires environmental-friendly treatment strategies for fish diseases. Rev. Aquacult. 12, 943–965. doi: 10.1111/raq.12365
Liu Y., de Bruijn I., Jack A. L. H., Drynan K., van den Berg A. H., Thoen E., et al. (2014). Deciphering microbial landscapes of fish eggs to mitigate emerging diseases. ISME J. 8, 2002–2014. doi: 10.1038/ismej.2014.44
Meinelt T., Paul A., My Phan T., Zwirnmanna E., Kruger A., Wienke A., et al. (2007). Reduction in vegetative growth of the water mold Saprolegnia parasitica (Coker) by humic substance of different qualities. Aquat Toxicol. 83, 93–103. doi: 10.1016/j.aquatox.2007.03.013
Meinelt T., Schreckenbach K., Pietrock M., Heidrich S., Steinberg C. E. W. (2008). Humic substances (review series). part 1: Dissolved humic substances (HS) in aquaculture and ornamental fish breeding. Env. Sci. pollut. Res. 15 (1), 17–22. doi: 10.1065/espr2007.08.448
Michaud L., Caruso C., Mangano S., Interdonato F., Bruni V., Lo Giudice A. (2012). Predominance of flavobacterium, pseudomonas, and polaromonas within the prokaryotic community of freshwater shallow lakes in the northern Victoria land, East Antarctica. FEMS Microbiol. Ecol. 82 (2), 391–404. doi: 10.1111/j.1574-6941.2012.01394.x
Nakagawa J., Iwasaki T., Kodama H. (2009). Protection against Flavobacterium psychrophilum infection (cold water disease) in ayu fish (Plecoglossus altivelis) by oral administration of humus extract. J. Vet. Med. Sci. 71 (11), 1487–1491. doi: 10.1292/jvms.001487
Oksanen J., Blanchet F., Friendly M., Kindt R., Legendre P., Mcglinn D., et al. (2018). “Vegan: community ecology package,” in R package version 2.5-1. Available at: https://cran.r-project.org/web/packages/vegan/vegan.pdf.
Ozdemir R. C., Tastan Y., Guney K. (2021). Prevention of saprolegniasis in rainbow trout (Oncorhynchus mykiss) eggs using oregano (Origanum onites) and laurel (Laurus nobilis) essential oils. J. Fish. Dis. 45, 51–58. doi: 10.1111/jfd.13531
Quast C., Pruesse E., Yilmaz P., Gerken J., Schweer T., Yarza P., et al. (2012). The SILVA ribosomal RNA gene database project: Improved data processing and web-based tools. Nucleic Acids Res. 41 (D1), D590–D596. doi: 10.1093/nar/gks1219
R-Core-Team (2015). “R: A language and environment for statistical computing,” in R foundation for statistical computing(Vienna, Austria). Available at: http://www.R-project.org.
Rognes T., Flouri T., Nichols B., Quince C., Mahé F. (2016). VSEARCH: A versatile open source tool for metagenomics. Peer J 4, e2584. doi: 10.7717/peerj.2584
Schloss P. D., Westcott S. L., Ryabin T., Hall J. R., Hartmann M., Hollister E. B., et al. (2009). Introducing mothur: Open-source, platform-independent, community-supported software for describing and comparing microbial communities. Appl. Environ. Microbiol. 75, 7537–7541. doi: 10.1128/AEM.01541-09
Schreckenbach K., Knösche R., Seubert B., Höke H. (1994). Klinische prüfung des synthesehuminstoffes HS 1500 bei eiern und larven von regenbogenforellen (Oncorhynchus mykiss). [English translation: Clinical testing of the synthetic humic substance HS 1500 in rainbow trout eggs and larvae (Oncorhynchus mykiss)] bericht, institute of inland fisheries e.V. Potsdam Sacrow.
Schreier T. M., Rach J. J., Howe G. E. (1996). Efficacy of formalin, hydrogen peroxide, and sodium chloride on fungal-infected rainbow trout eggs. Aquaculture 140, 323–331. doi: 10.1016/0044-8486(95)01182-X
Steinberg C. E. W. (2003). Ecology of humic substances in freshwaters: Determinants from geochemistry to ecological niches (Heidelberg: Springer Science & Business Media).
Thurman E. M. (1985). Organic geochemistry of natural waters (Dordrecht: Nijhoff, M./Junk, W. Publishers).
Visser S. A. (1985). Physiological action of humic substances on microbial cells. Soil Biol. Biochem. 17, 457–462. doi: 10.1016/0038-0717(85)90009-4
Wagner E. J., Arndt R. E., Billman E. J., Forest A., Cavender W. (2008). Comparison of the efficacy of iodine, formalin, salt, and hydrogen peroxide for control of external bacteria on rainbow trout eggs. N. Am. J. Aquacult. 70, 118–127. doi: 10.1577/A06-068.1
Wagner E., Oplinger R. W., Bartley M. (2012). Laboratory and production scale disinfection of salmonid eggs with hydrogen peroxide. N. Am. J. Aquacult. 74, 92–99. doi: 10.1080/15222055.2011.649888
Yilmaz S., Ergün S., Celik E. S., Yigit M. (2018a). Effects of dietary humic acid on growth performance, haemato-immunological and physiological responses and resistance of rainbow trout, Oncorhynchus mykiss to Yersinia ruckeri. Aquac Res. 49 (10), 3338–3349. doi: 10.1111/are.13798
Keywords: rainbow trout, microbial community, fungus, eggs, aquaculture, 16s rDNA sequencing
Citation: Chiasson M, Kirk M and Huyben D (2023) Microbial control during the incubation of rainbow trout (Oncorhynchus mykiss) eggs exposed to humic acid. Front. Aquac. 2:1088072. doi: 10.3389/faquc.2023.1088072
Received: 03 November 2022; Accepted: 09 January 2023;
Published: 19 January 2023.
Edited by:
Sevdan Yilmaz, Çanakkale Onsekiz Mart University, TürkiyeReviewed by:
Haitham Mohammed, Faculty of Veterinary Medicine, Assiut University, EgyptJonathan Roques, University of Gothenburg, Sweden
Murat Yigit, Çanakkale Onsekiz Mart University, Türkiye
Copyright © 2023 Chiasson, Kirk and Huyben. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Marcia Chiasson, bWFyY2lhY2hAdW9ndWVscGguY2E=
 Marcia Chiasson
Marcia Chiasson Michael Kirk1
Michael Kirk1 David Huyben
David Huyben