
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Anim. Sci., 11 February 2025
Sec. Animal Physiology and Management
Volume 6 - 2025 | https://doi.org/10.3389/fanim.2025.1502984
 Nicola Oosthuizen1
Nicola Oosthuizen1 Lucas M. Goncalves2
Lucas M. Goncalves2 Samir Burato2
Samir Burato2 Molly S. Smith2
Molly S. Smith2 Kelsey M. Harvey3
Kelsey M. Harvey3 Graham Cliff Lamb4,5
Graham Cliff Lamb4,5 Pedro L. P. Fontes2*
Pedro L. P. Fontes2*This study evaluated the use of two different gonadotropin-releasing hormone (GnRH) compounds in estrus synchronization protocols with or without presynchronization. Cows (n = 1,585) were enrolled in a completely randomized design with a 2×2 factorial arrangement of treatments. Within each location, cows were randomly assigned to one of four treatments: (1 and 2) cows were estrus synchronized using the 7-day CO-Synch + CIDR protocol (7D) wherein they received an injection of one of two GnRH compounds [gonadorelin hydrochloride (GH) or gonadorelin diacetate tetrahydrate (GDT)] and a controlled internal drug release (CIDR) device on day 0, an injection of prostaglandin F2α (PG) at CIDR removal on day 7, and a second injection of their respective GnRH compound at fixed-time artificial insemination (TAI) on day 10 (7D-GH and 7D-GHT); (3 and 4) cows were treated with the 7&7 Synch (7&7) protocol, wherein they were treated the same as treatments 1 and 2 but received their CIDR inserts on day -7 in conjunction with an injection of PG (7&7-GH and 7&7-GDT). Breeding indicator patches were applied to all cows at CIDR removal and were evaluated for activation at TAI. Follicle diameter differed by ovulation synchronization protocol on days 0, 7, and 10, where cows enrolled in the 7&7 had greater (P ≤ 0.008) dominant follicle diameters than those in the 7D. No differences (P ≥ 0.19) in ovarian parameters were determined between GH and GDT cows. Estrus expression differed (P < 0.001) by synchronization protocol and was greater in cows enrolled in the 7&7 than the 7D (80.4 ± 2.8 vs. 55.5 ± 4.1%, respectively), yet no differences (P = 0.32) in estrus expression were determined between GH- and GDT-treated cows. Pregnancy rates to TAI did not differ (P = 0.57) by GnRH compound but differed (P = 0.01) by synchronization protocol, where cows enrolled in the 7&7 had greater PR/AI when compared to those in the 7D (60.9 ± 2.5 vs. 53.9 ± 2.6%, respectively). In conclusion, fertility was improved through the use of presynchronization; however, no differences in fertility parameters were determined between GH and GDT in either synchronized or presynchronized beef cows.
Gonadotropin-releasing hormone (GnRH) products are commonly utilized during estrus synchronization of both beef and dairy females to induce ovulation; however, ovulation will only occur if a sufficiently large dominant follicle is present on the ovary (Sartori et al., 2001). When GnRH was administered at a random stage of the estrous cycle, only 66% of cows ovulated and had synchronized emergence of their subsequent follicular wave (Geary et al., 2000). Therefore, presynchronization strategies have been utilized to increase the response to the injection of GnRH at the beginning of estrus synchronization protocols (Perry et al., 2012; Bonacker et al., 2020). Perry and colleagues administered prostaglandin F2α (PG) 3 days prior to administration of GnRH and insertion of a controlled internal drug release (CIDR) device for 6 days (Perry et al., 2012). This strategy resulted in a greater number of beef cows responding to the initial injection of GnRH and increased pregnancy rates to fixed-time artificial insemination (PR/AI; TAI). Presynchronization with a CIDR insert and an injection of PG 7 days prior to GnRH administration also resulted in improved fertility outcomes in both beef heifers (Oosthuizen et al., 2020) and cows (Andersen et al., 2021). This strategy, commonly referred to as the 7&7 Synch (7&7), requires a CIDR to be in place for 14 days and an injection of GnRH to be administered 7 days after CIDR insertion to stimulate the formation of a new follicular wave and a dominant follicle for TAI. If females do not respond to this injection of GnRH, a persistent follicle could develop, which would have reduced fertility to TAI (Ahmad et al., 1995).
Currently, there are five GnRH products available in the United States, all of which contain the identical decapeptide, gonadorelin, yet differ by their salt and composition of dilutant, which could affect their ability to stimulate ovulation (Martínez et al., 2003; Souza et al., 2009; Luchterhand et al., 2019). These products are comprised of one of three gonadorelin compounds, namely gonadorelin hydrochloride (GH), gonadorelin acetate, or gonadorelin diacetate tetrahydrate (GDT). Numerous studies have been conducted to determine the efficacy of these GnRH compounds in comparison to one another in dairy cows. Research in both lactating and non-lactating dairy cows demonstrated differences in the percentage of females ovulating to the GnRH injection, where females that received GDT had a greater rate of ovulation than those that received GH (Martínez et al., 2003; Souza et al., 2009). Poock and colleagues evaluated PR/AI in postpartum dairy cows that were treated with either GDT or GH and reported that the GnRH product did not significantly affect PR/AI when dairy cows were treated with an Ovsynch-based protocol (Poock et al., 2015). Conversely, fewer lactating dairy cows synchronized with the Double-Ovsynch protocol ovulated to GH when compared with GDT, and PR/AI were lower in GH-treated females (Luchterhand et al., 2019). These studies all report that GDT is more effective at inducing ovulation than GH yet reports on PR/AI are mixed. Furthermore, the abovementioned research was only conducted in dairy cows using synchronization strategies without the use of a progestin, which may limit its applicability to estrus synchronization programs for beef females. Literature on gonadorelin compound efficacy in beef females is limited (Stevenson et al., 2000).
The objectives of the present experiment were to: 1) compare ovarian parameters, estrus expression, and PR/AI between GH and GDT in beef cows synchronized with progesterone, PG, and GnRH, and 2) determine if differences exist in these response variables between GnRH compounds when used during estrus synchronization with or without presynchronization. Based on the differences between GnRH compounds reported in the dairy cows, it was hypothesized that the use of GDT would result in greater PR/AI compared with GH in beef cows exposed to estrus synchronization. In addition, it was hypothesized that differences in PR/AI due to GnRH compound would be mitigated by exposing cows to presynchronization with the 7&7 Synch protocol.
All cows were handled in accordance with procedures approved by the University of Georgia’s Animal Care and Use Committee. A total of 1,585 suckled primiparous (n = 411) and multiparous (n = 1,174) Bos taurus beef cows (Angus, Angus × Hereford, Angus × Simmental) from 15 locations (Table 1) in five states (CO, GA, MS, NE, SD) were enrolled in a completely randomized design with a 2×2 factorial arrangement of treatments. Within location, cows were randomly assigned to one of four treatments (Figure 1): 1) cows were estrus synchronized using the 7-day CO-Synch + CIDR protocol (7D) wherein they received 100 µg of GH (2 mL im; Factrel; Zoetis Animal Health, Parsippany, NJ) and a CIDR insert (EAZI-BREED CIDR; 1.38 g progesterone; Zoetis Animal Health) on day 0, 25 mg of PG (2 mL im; dinoprost tromethamine; Lutalyse HighCon; Zoetis Animal Health) at CIDR removal on day 7, and 100 µg of GH (2 mL im; Factrel) on day 10 at TAI 66 ± 2 hours later (7D-GH; n = 389); 2) cows were treated the same as 7D-H but received 100 µg of GDT as their GnRH compound (2 mL im; Cystorelin; Boehringer Ingelheim, Ingelheim, Germany) on days 0 and 10 (7D-GDT; n = 397); 3) cows were treated the same as 7D-GH but received a CIDR insert on day -7 concurrent with an injection of PG (7&7-GH; n = 391); 4) cows treated the same as 7&7-GH but received 100 µg of GDT as their GnRH compound (2 mL im; Cystorelin) on days 0 and 10 (7&7-GDT; n = 408). Breeding indicator patches (Estrotect; Rockway Inc., Spring Valley, WI) were applied to all cows at CIDR removal and were evaluated for activation at TAI to determine if estrus had been expressed. Patches were considered activated when at least 50% of the rub-off coating was removed from the patch or when the patch was missing. On day -7, cow body weight (BW) and body condition score (BCS) were recorded. Body condition score was recorded on a scale of 1 to 9 as previously described by Wagner et al. (1988). Each location provided their own AI technician(s) and their own conventional semen. Clean-up bulls were introduced no less than 8 days after TAI at each location. Transrectal ultrasonography was performed at each location between 28 and 55 days after TAI to determine PR/AI.
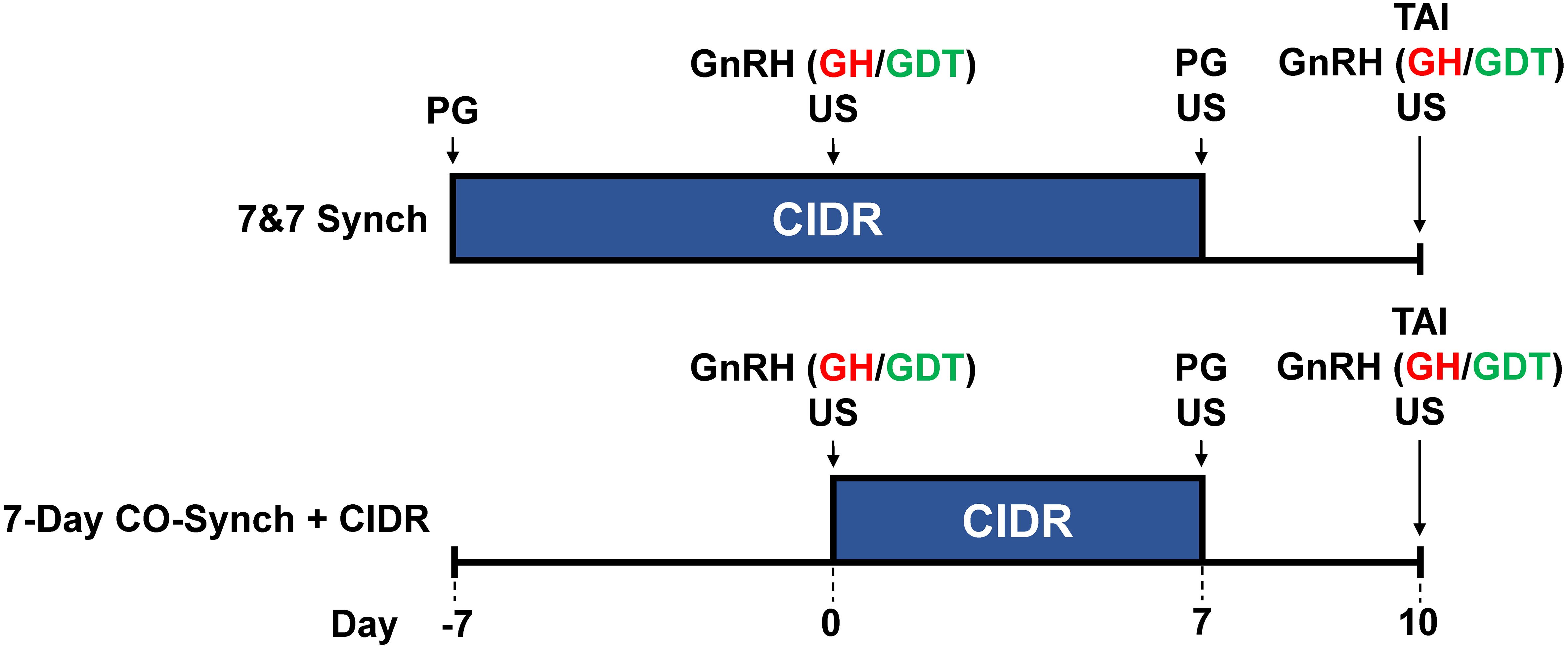
Figure 1. Schematic of treatments. CIDR, controlled internal drug release insert; GnRH, gonadotropin-releasing hormone; PG, prostaglandin F2α; TAI, fixed-time artificial insemination—performed 66 ± 2 hours after PG administration; US, ultrasound. At the time of GnRH administration, cows received either a GnRH product comprised of gonadorelin hydrochloride (GH) or a product comprised of gonadorelin diacetate tetrahydrate (GDT). Ultrasonography was performed on days 0, 7, and 10 to evaluate the presence and size of a dominant follicle and the presence of a corpus luteum. Breeding indicator patches were applied to all heifers at CIDR removal (day 7) and were evaluated for activation at the time of TAI (day 10).
Transrectal ultrasonography (Sonoscape S8EXP, Soundscape Medical Corp, Shenzhen, GD, China) was performed in a subset of multiparous cows (n = 160) at location O on days 0 (GnRH), 7 (PG), and 10 (TAI) to determine the presence of a dominant follicle, the dominant follicle diameter, and the presence and number of corpora lutea (CLs). Follicles with a diameter of ≥ 4 mm were recorded. The length and width of the largest follicle on each ovary were measured using electronic calipers. The first measurement was taken at the widest point of the follicle and the second was taken at the tallest point at a right angle to the first measurement. The mean of these two measurements was used to reflect the diameter of the follicle. Cows with a dominant follicle on day 7 (≥ 8.5 mm) and with no dominant follicle on day 10 were considered to have ovulated prior to TAI.
All data were analyzed as a completely randomized design with a 2×2 factorial arrangement of treatments using the SAS statistical package (version 9.4; SAS/STAT, SAS Inst. Inc., Cary, NC, USA). A cow was considered the experimental unit in all the analyses. The GLIMMIX procedure of SAS was used to analyze the binary response variables (follicles ≥ 10 mm on day 0, absence of a CL on days 0 and 7, presence of a single CL on days 0 and 7, presence of multiple CLs on days 0 and 7, estrus expression, PR/AI, and ovulation between days 7 and 10), whereas the MIXED procedure of SAS was used to analyze the continuous descriptive (BCS, BW, and days postpartum [DPP]) and response variables (follicle diameter on days 0, 7, and 10). The models for estrus expression and PR/AI included the fixed effect of synchronization protocol (7D or 7&7), GnRH compound (GH or GDT), parity (primiparous or multiparous), and all interactions, as well as the random effect of location. The models for BCS, BW, and DPP included the fixed effect of treatment. The models for ovarian parameters included the fixed effects of synchronization protocol, GnRH compound, and the interaction. For all models, when significance (P ≤ 0.05) was determined for a fixed effect, least squares means were separated using the PDIFF option in SAS. Insemination technician and sire were equally distributed among treatments and consequently were not included in any of the models. Statistical significance was declared at P ≤ 0.05, with 0.05 < P ≤ 0.10 considered a tendency. Least square means ± SEM are reported.
Cow BCS, BW, and DPP at each location are summarized in Table 1. No differences were determined for cow BCS [7D-GH (4.97 ± 0.09), 7D-GDT (5.01 ± 0.09), 7&7-GH (4.93 ± 0.09), 7&7-GDT (4.96 ± 0.09); P = 0.29], BW [7D-GH (564.68 ± 19.80 kg), 7D-GDT (566.22 ± 19.80 kg), 7&7-GH (557.86 ± 19.80 kg), 7&7-GDT (559.01 ± 19.78 kg); P = 0.31], or DPP [7D-GH (88.43 ± 3.89), 7D-GDT (88.06 ± 3.89), 7&7-GH (88.33 ± 3.89), 7&7-GDT (89.08 ± 3.89); P = 0.89] among treatments.
A summary of ovarian response variables recorded on days 0, 7, and 10 is presented in Table 2. At GnRH administration on day 0, follicle diameter differed (P < 0.001) by synchronization protocol, where cows enrolled in the 7&7 had a greater mean follicle diameter than those enrolled in the 7D (14.61 ± 0.37 vs. 11.94 ± 0.38 mm, respectively). Furthermore, a greater (P = 0.03) proportion of cows in the 7&7 had a dominant follicle ≥ 10 mm in diameter on day 0 compared with cows in the 7D (93.4 ± 2.9% vs. 80.7 ± 4.7%, respectively). No difference between GnRH compounds and no GnRH × synchronization protocol interaction was determined for follicle diameter on day 0 or the proportion of cows with a dominant follicle ≥ 10 mm in diameter on day 0 (P ≥ 0.56). The percentage of cows without a CL on day 0 differed (P < 0.001) by synchronization protocol, where a greater proportion of cows enrolled in the 7&7 protocol did not have a CL present compared with 7D cows (69.6 ± 5.2% vs. 31.9 ± 5.2%, respectively). Moreover, fewer (P = 0.0002) cows in the 7&7 protocol had a single CL present on Day 0 compared with 7D cows (26.6 ± 5.0% vs. 56.8 ± 5.5%, respectively), and there was a tendency (P = 0.095) for a greater proportion of cows enrolled in the 7D to have multiple CL present on day 0 compared with cows enrolled in the 7&7 (11.1 ± 3.5% vs. 3.6 ± 2.2%, respectively). No difference between GnRH compounds and no GnRH × synchronization protocol interaction was determined for the percentage of cows without a CL on day 0, the percentage of cows with a single CL on day 0, or the percentage of cows with multiple CLs on day 0 (P ≥ 0.38).
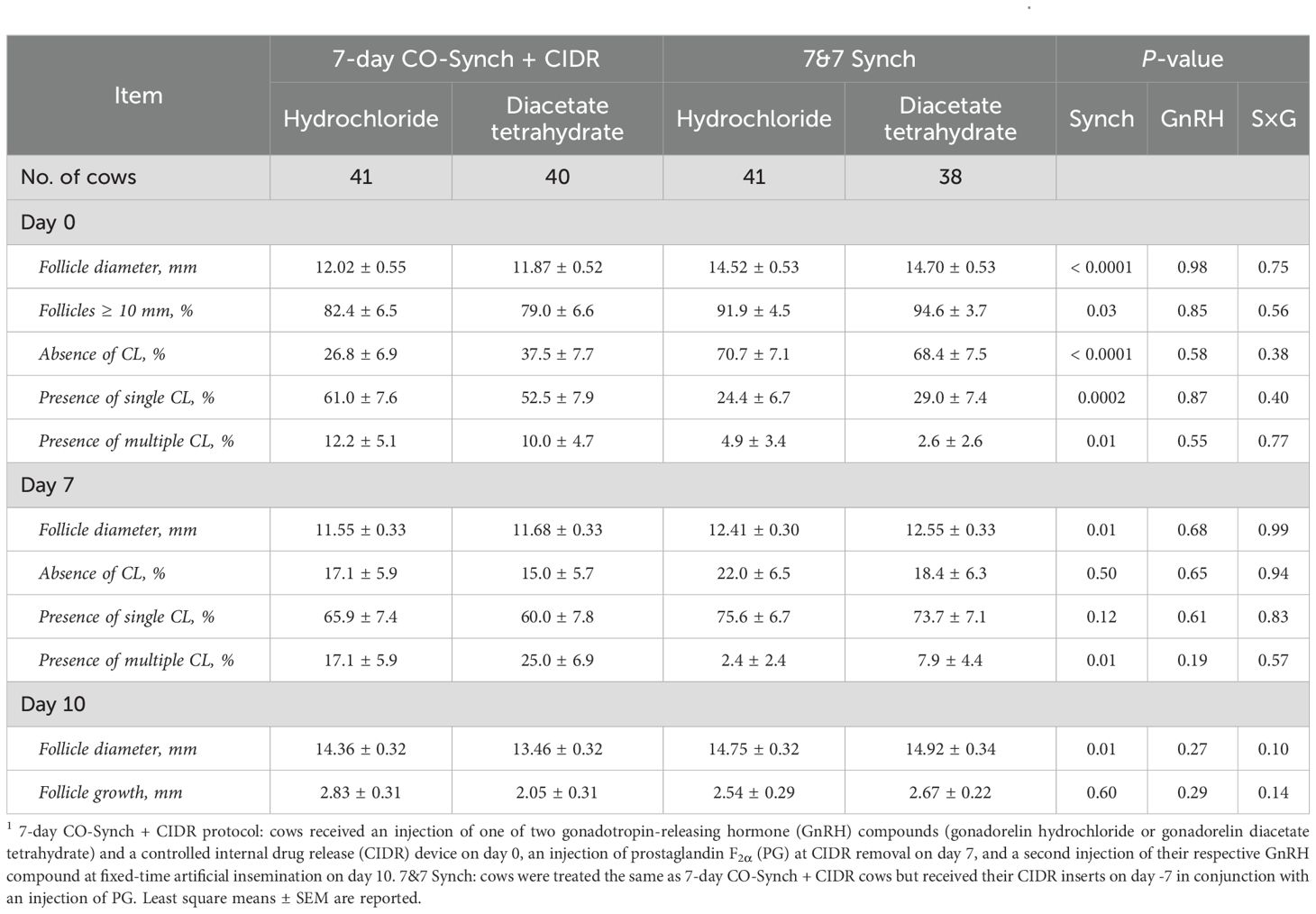
Table 2. Ovarian response variables by synchronization protocol and GnRH compound in a subset (n = 160) of cows1.
At CIDR removal on day 7, follicle diameter differed by synchronization protocol and was greater (P = 0.008) in the cows enrolled in the 7&7 when compared to cows enrolled in the 7D (12.48 ± 0.22 vs. 11.61 ± 0.23 mm, respectively). No difference between GnRH compounds and no GnRH × synchronization protocol interaction was determined for follicle diameter on day 7 (P ≥ 0.68). In addition, there was no effect of synchronization protocol or GnRH compound, and no GnRH × synchronization protocol interaction was determined for the percentage of cows without a CL on day 7 (P ≥ 0.50). Furthermore, no difference (P = 0.12) was determined between synchronization protocols for the presence of a single CL on day 7 (74.7 ± 4.9 vs. 63.0 ± 5.4%, respectively). No difference between GnRH compounds and no GnRH × synchronization protocol interaction was determined for the presence of a single CL on day 7 (P ≥ 0.61). Nevertheless, the proportion of cows with multiple CL on day 7 differed (P = 0.009) by synchronization protocol and was greater in cows enrolled in the 7D when compared to those enrolled in the 7&7 (20.8 ± 4.6 vs. 4.4 ± 2.5%, respectively). No difference between GnRH compounds and no GnRH × Synchronization protocol interaction was determined for the presence of multiple CL on day 7 (P ≥ 0.19).
On day 10, an effect of synchronization protocol was determined, where follicle diameter was greater (P = 0.01) in cows enrolled in the 7&7 than those in the 7D (14.83 ± 0.23 vs. 13.91 ± 0.23 mm, respectively). There was no impact of GnRH (P = 0.26) and no GnRH × synchronization protocol interaction (P = 0.10) was determined. Furthermore, no effect of synchronization protocol (P = 0.28) or GnRH compound (P = 0.92), and no GnRH × synchronization protocol interaction (P = 0.96) was determined for the percentage of cows that ovulated between days 7 and 10 [7D-GH (7.3 ± 4.1%), 7D-GDT (7.5 ± 4.2%), 7&7-GH (12.2 ± 5.1%), 7&7-GDT (13.2 ± 5.5%)]. Follicular growth between days 7 and 10 was not influenced by GnRH (P = 0.29) or synchronization protocol (P = 0.60), and no GnRH × synchronization protocol interaction was determined (P = 0.14).
Overall, 69.1% of cows expressed estrus and the percentage of cows expressing estrus ranged from 53.3% to 83.1% among locations (Table 1). The percentages of cows expressing estrus prior to TAI are depicted in Figure 2. Estrus expression differed by synchronization strategy, where cows enrolled in the 7&7 had greater (P < 0.001) expression of estrus than those enrolled in the 7D (80.4 ± 2.8 vs. 55.5 ± 4.1, respectively). No differences were determined for estrus expression between GnRH compounds (67.9 ± 3.7% and 70.8 ± 3.5%, for GH and GDT, respectively; P = 0.32) or between cow parity (67.1 ± 4.4% and 71.6 ± 3.3%, for primiparous and multiparous cows, respectively; P = 0.25). No interactions between synchronization strategy, GnRH compound, and parity were determined (P ≥ 0.28).
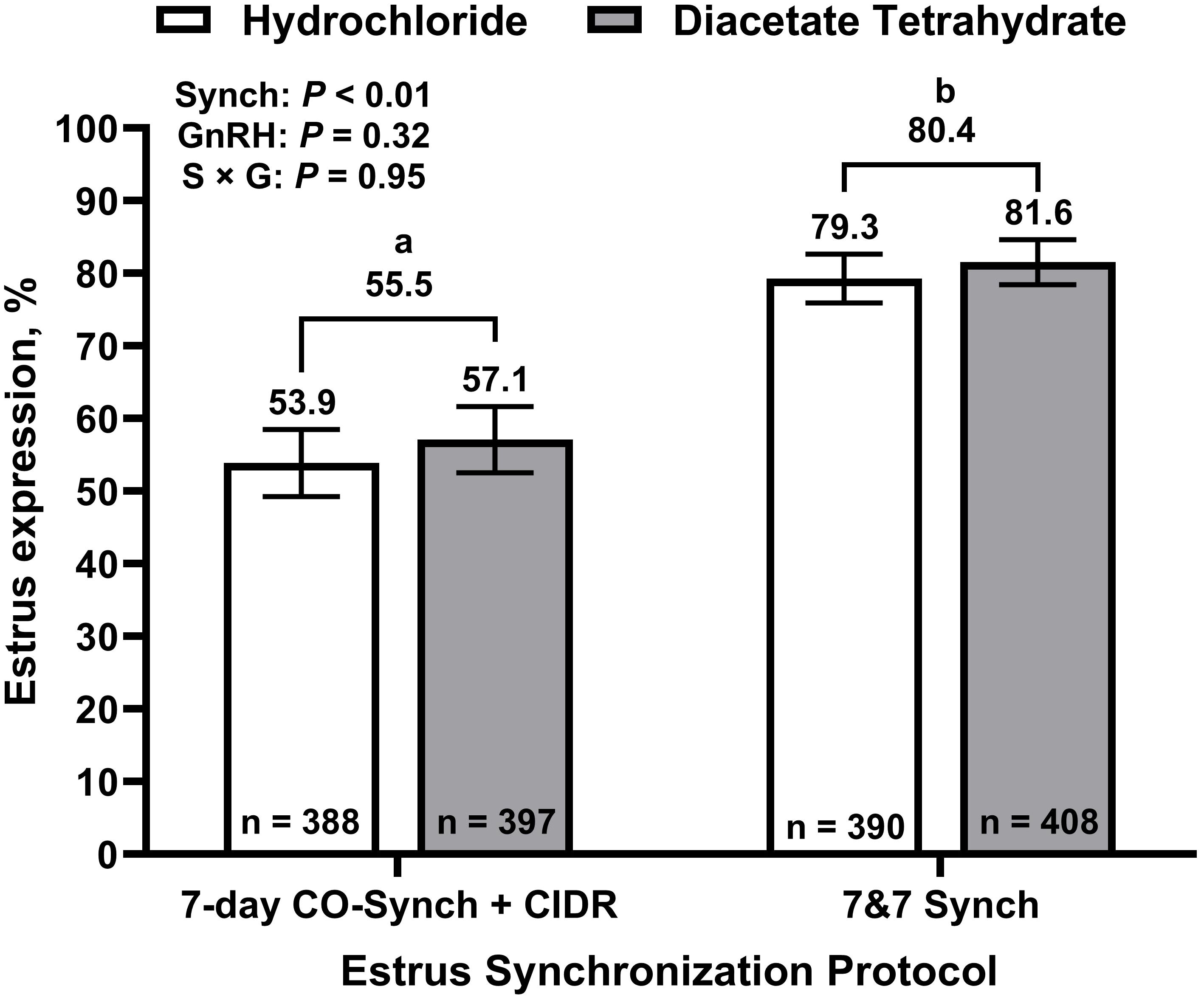
Figure 2. Estrus expression by treatment. 7-day CO-Synch + CIDR protocol: cows received an injection of one of two gonadotropin-releasing hormone (GnRH) compounds (gonadorelin hydrochloride or gonadorelin diacetate tetrahydrate) and a controlled internal drug release (CIDR) device on day 0, an injection of prostaglandin F2α (PG) at CIDR removal on day 7, and a second injection of their respective GnRH compound at fixed-time artificial insemination (TAI) on day 10. 7&7 Synch: cows were treated the same as 7-day CO-Synch + CIDR cows but received their CIDR inserts on day -7 in conjunction with an injection of PG. Breeding indicator patches were applied to all heifers at CIDR removal (day 7) and were evaluated for activation at the time of TAI (day 10). Least square means ± SEM are reported. a,bBars with different superscripts differ (P ≤ 0.05).
Overall, 58.8% of cows became pregnant to TAI, and PR/AI ranged from 48.0% to 73.8% among locations (Table 1). Pregnancy rates to TAI are depicted in Figure 3. An effect of synchronization strategy on PR/AI was determined, where cows in the 7&7 treatments had greater (P = 0.01) PR/AI than those in the 7D treatments (60.9 ± 2.5 vs. 53.9 ± 2.6, respectively). No differences were determined between GnRH compounds for PR/AI (56.7 ± 2.6% and 58.3 ± 2.5%, for GH and GDT, respectively; P = 0.57), or between cow parities (55.0 ± 3.2% and 59.9 ± 2.1%, for primiparous and multiparous cows, respectively; P = 0.17). No interactions between synchronization strategy, GnRH compound, and parity were determined (P ≥ 0.21).
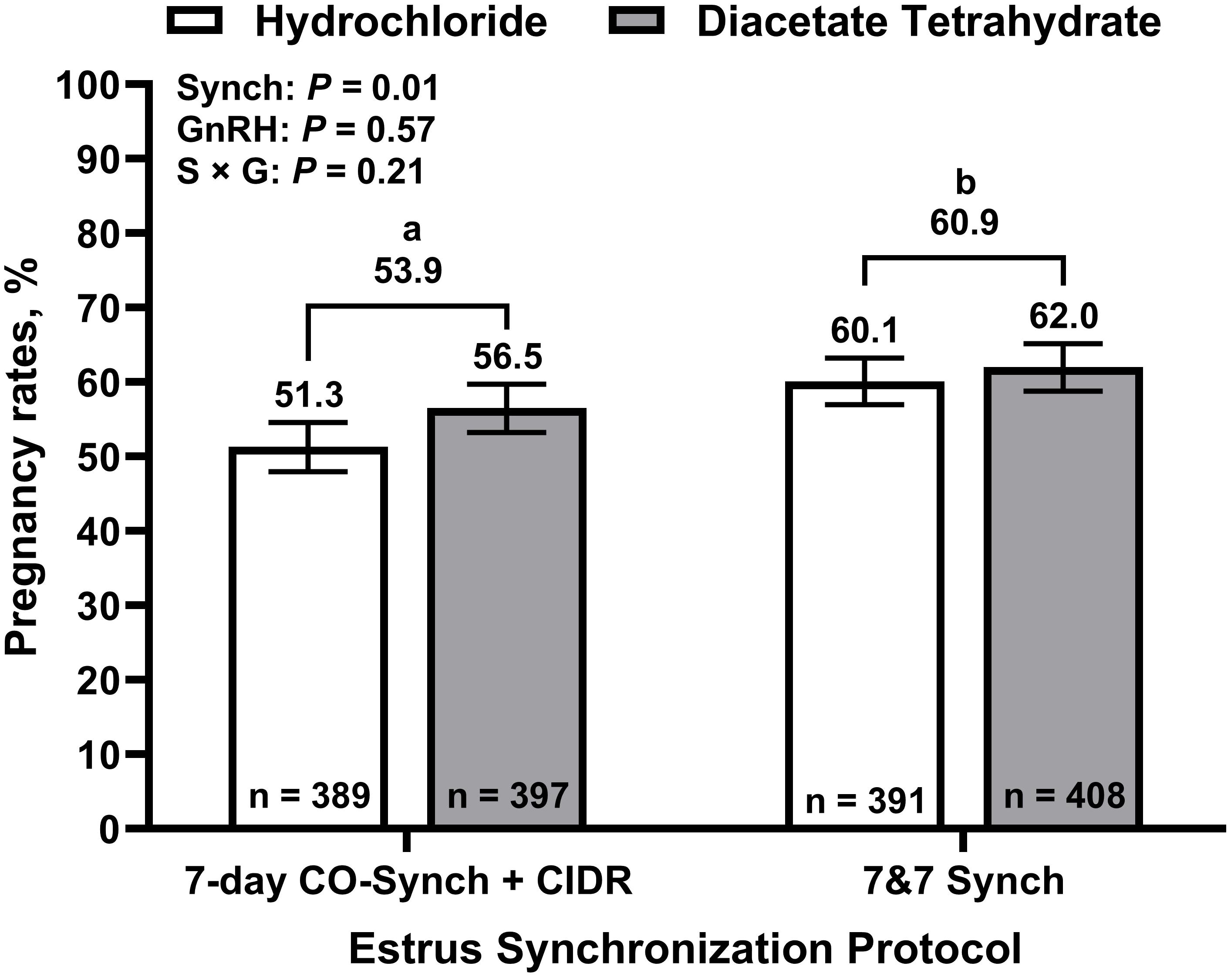
Figure 3. Pregnancy rates to fixed-time artificial insemination by treatment. 7-day CO-Synch + CIDR protocol: cows received an injection of one of two gonadotropin-releasing hormone (GnRH) compounds (gonadorelin hydrochloride or gonadorelin diacetate tetrahydrate) and a controlled internal drug release (CIDR) device on day 0, an injection of prostaglandin F2α (PG) at CIDR removal on day 7, and a second injection of their respective GnRH compound at fixed-time artificial insemination on day 10. 7&7 Synch: cows were treated the same as 7-day CO-Synch + CIDR cows but received their CIDR inserts on day -7 in conjunction with an injection of PG. Least square means ± SEM are reported. a,bBars with different superscripts differ (P ≤ 0.05).
The purpose of this experiment was to compare fertility outcomes of beef cows treated with either GH or GDT during an estrus synchronization protocol with or without presynchronization. Based on dairy cattle literature, wherein greater PR/AI (Luchterhand et al., 2019) and greater rates of ovulation (Martínez et al., 2003; Souza et al., 2009; Luchterhand et al., 2019) have been reported in GDT-treated females, it was hypothesized that PR/AI would be greater in GDT-treated beef cows. Furthermore, because presynchronization with the 7&7 has been reported as a potential strategy to increase the ovulatory response to the initial GnRH injection (Bonacker et al., 2020), we hypothesized that any differences in PR/AI present between GH and GDT would be mitigated through the use of presynchronization with the 7&7. Contrary to our hypotheses, the results indicate no differences in PR/AI when cows were treated with either GnRH compound during either synchronization or presynchronization.
Dominant follicles reportedly acquire the ability to undergo induced ovulation at a diameter of approximately 10 mm (Sartori et al., 2001; Perry et al., 2005); therefore, this threshold was utilized in the present study to indicate follicles capable of GnRH-induced ovulation. During the 7D, GnRH is administered at the time of CIDR device insertion to stimulate ovulation via an induced LH surge, yet only two-thirds of beef cows ovulate in response to GnRH administered at a random stage of their estrous cycle (Geary et al., 2000). Females that fail to ovulate to the initial GnRH have a less uniform follicular growth pattern during the period of the CIDR insert, and consequently, have a greater variability in follicle size at TAI (Perry et al., 2012). This variability could lead to a greater proportion of smaller follicles present at TAI, which are less likely to ovulate spontaneously or to the injection of GnRH, resulting in fewer oocytes available for fertilization (Sartori et al., 2001; Perry et al., 2005). In addition, follicle size is associated with follicle maturity, and GnRH-induced ovulation of physiologically immature follicles can result in reduced PR/AI and increased embryonic mortality (Perry et al., 2005). To overcome this issue, presynchronization strategies have been developed to increase the proportion of females with a dominant follicle capable of ovulation at the time of the initial injection of GnRH, and can thus increase the rate of ovulation to the GnRH injection and the synchrony of subsequent follicular wave emergence (Perry et al., 2007, 2012; Bonacker et al., 2020). When Perry et al. (2012) administered PG 3 days prior to CIDR insertion, a 17-percentage point (%pt) increase in the proportion of cows that responded to the initial injection of GnRH was observed. Moreover, a 40-%pt increase in follicular wave initiation was observed after the initial injection of GnRH when replacement heifers received PG 3 days prior to CIDR insertion (Grant et al., 2011). Presynchronization with PG 3 days prior to CIDR insertion also resulted in greater uniformity of follicular development and reduced variation in the interval between CIDR removal and estrus expression (Grant et al., 2011). Through the use of the 7&7, Bonacker et al. (2020) reported a 58-%pt increase in the proportion of cows with a follicle ≥ 10 mm at the initial GnRH administration and the follicle diameters were 4.7 mm larger. Similarly, in the present study, the proportion of cows with follicles ≥ 10 mm in diameter on day 0 was 12.6%pt greater for the 7&7 cows compared with the 7D cows, and these follicles were 2.7 mm larger on average, indicating that greater follicular maturity may have been achieved through the use of this presynchronization approach.
In the 7&7, PG is administered at the start of the protocol to induce luteolysis of a CL that may be present on the ovary; however, due to the presence of a CIDR insert, circulating concentrations of progesterone inhibit the expression of estrus and spontaneous ovulation prior to the first GnRH injection by suppressing the release of luteinizing hormone (Colazo et al., 2008). In the present study, 38%pt fewer cows in the 7&7 treatments had a CL present at GnRH administration on day 0 than in the 7D treatments, which indicates that the injection of PG on day -7 induced ovulation in a large proportion of females. Nevertheless, 30% of the 7&7 cows still had a CL present on day 0, which indicates that a proportion of females had either recently ovulated and did not have a CL present on their ovaries or had a young CL present that was not yet capable of responding to the injection of PG on day -7 (Rowson et al., 1972). The proportion of cows with a single CL present on day 7 did not differ between 7&7 and 7D cows and no differences were determined in the proportion of cows with a CL (single or multiple) on day 7. During the 7&7, a large proportion of cows will not have a CL present at CIDR removal unless ovulation has occurred to the initial injection of GnRH or if they fail to respond to the initial injection of PG. A proportion of 7&7 cows that fail to respond to the initial PG will have a CL present at the initial GnRH administration, and subsequently, those that ovulate to the GnRH will develop a secondary CL. Similarly, 7D cows that have a CL present at CIDR insertion and ovulate to the initial GnRH will develop a secondary CL by CIDR removal. Given the lesser proportion of 7&7 cows with a single CL on day 0 than 7D cows (approximately 27 vs. 57%), the 48% increase in the proportion of 7&7 cows with a single CL on day 7 compared with the 6% increase in the 7D, and the minor increases in the proportion of females with multiple CLs on day 7 in both the 7&7 and the 7D (approximately 1 and 10%), it is plausible that a greater proportion of 7&7 cows ovulated to the initial injection of GnRH, yet ovulation rate was not directly evaluated in the present study. Nevertheless, the results observed for ovarian response variables indicate a more uniform ovarian presentation in cows treated with presynchronization.
In dairy cattle, regardless of the presynchronization strategy, the type of GnRH compound has also been shown to affect the rate of ovulation to GnRH. Numerous studies have been conducted to compare the effects of different GnRH compounds on fertility parameters, where results demonstrated a lesser rate of ovulation in females treated with GH when compared with those treated with GDT (Martínez et al., 2003; Souza et al., 2009; Luchterhand et al., 2019). Research in non-lactating dairy cows indicated differences in the percentage of females ovulating to the injection of GnRH, where females treated with a GDT product had a 38%pt greater rate of ovulation than those treated with GH (Martínez et al., 2003). In lactating dairy cows, the treatment groups that received GDT had an 18 to 30%pt increase in ovulation rate compared with cows that received GH (Souza et al., 2009) and 11%pt fewer cows synchronized with the Double-Ovsynch protocol tended to ovulate to the third GnRH when treated with GH compared with cows treated with GDT (Luchterhand et al., 2019). Nevertheless, research reports comparing the efficacy of these different GnRH compounds in beef cattle are scarce (Stevenson et al., 2000), with no large-scale experiments conducted comparing the effectiveness of different sources of gonadorelin on ovulation in beef females. In the present study, no differences were determined between GH and GDT for the presence of follicles ≥ 10 mm on day 0, for follicle diameter on day 7, or for the presence of a single or multiple CLs on days 0 or 7, which when taken together, suggest no difference in the rate of ovulation to the initial GnRH between GnRH compounds.
Presynchronization with the 7&7 has been reported to increase the expression of estrus in beef females by 14 to 20%pt (Andersen et al., 2021; Pancini et al., 2022; Ketchum et al., 2024), therefore, it was unsurprising that estrus expression was 25%pt greater in cows exposed to the 7&7 in the present study. The increase in estrus expression in the 7&7 cows is likely due to greater synchrony of follicular wave emergence between days 0 and 7 and greater follicle size at CIDR removal than the 7D cows, as a result of an increased response to the initial GnRH. Another potential reason for greater estrus expression prior to TAI in 7&7 cows is reduced circulating concentrations of progesterone during ovulatory follicle development when compared with 7D cows. Cows in the 7&7 treatment were exposed to exogenous progesterone from their CIDR inserts for 14 days, yet less exogenous progesterone was released over time (Rathbone et al., 2002), resulting in decreased circulating concentrations of progesterone during the last 7 days of use (Chacher et al., 2017; Dias et al., 2021). While circulating concentrations of progesterone were not evaluated in the present study, Bonacker et al. (2020) reported that cows exposed to the 7&7 had lesser circulating concentrations of progesterone at CIDR removal compared with 7D cows; therefore, it is reasonable to speculate that 7&7 cows in the present study also had decreased circulating concentrations of progesterone on day 7 compared with 7D cows. This is further supported by the greater proportion of 7&7 cows that did not have a CL present on day 0 compared with 7D cows, and the greater proportion of 7D cows with multiple CLs present on day 7 compared with 7&7 cows. Reduced circulating concentrations of progesterone during ovulatory follicular development have been associated with hastened follicular development, increased estrogen production, and earlier onset of estrus expression (Mercadante et al., 2015; Fontes et al., 2019; Dias et al., 2021). Collectively, these findings suggest that the hormonal environment induced by the 7&7 creates conditions that favor enhanced follicular development and earlier expression of estrus. Although differences in estrus expression have been reported between synchronization protocols, no reports exist on estrus expression between different GnRH compounds in either beef or dairy females. In this study, no differences were determined between GH and GDT for estrus expression, which supports the notion that no differences in ovulation rate to the initial GnRH or differences in synchrony of the subsequent follicular wave were present between GnRH compounds.
Reports of PR/AI in beef females exposed to the 7&7 have varied (Oosthuizen et al., 2020; Andersen et al., 2021; Pancini et al., 2022). In beef heifers, PR/AI were increased by 7%pt in heifers treated with the 7&7 when compared with control heifers (Oosthuizen et al., 2020) and PR/AI tended to be 10%pt greater in 7&7 heifers when compared with 7D heifers (Mercadante et al., 2021). In beef cows, Andersen and colleagues reported an 11%pt increase in PR/AI when cows were treated with the 7&7 when compared with those treated with the 7D (Andersen et al., 2021), yet Pancini and colleagues reported no differences in PR/AI between 7&7 and 7D cows (Pancini et al., 2022). In the present study, PR/AI were 7%pt greater in 7&7 cows when compared with 7D cows, which is likely the result of the increased expression of estrus, as females with greater estrus expression are known to have greater PR/AI (Richardson et al., 2016). This increase in PR/AI was observed regardless of cow parity and corroborates a previous report (Andersen et al., 2021). Collectively, these results provide further evidence of the benefit of using the 7&7 as a reproductive management tool to improve fertility in beef females.
Research in dairy females supports a difference in ovulation rate among GnRH compounds yet reports on differences in PR/AI are inconsistent. Poock and colleagues reported no differences in PR/AI in postpartum dairy cows treated with either GDT or GH during an Ovsynch-based synchronization protocol (Poock et al., 2015), whereas Luchterhand and colleagues reported PR/AI were approximately 6%pt greater in GDT-treated cows compared with those treated with GH (Luchterhand et al., 2019). To our knowledge, only a single publication has investigated differences in fertility in suckled beef cows receiving GH or GDT during ovulation synchronization, wherein cows were administered either GH or GDT 7 days prior to PG administration with or without a norgestomet implant (Stevenson et al., 2000). No differences in fertility were observed between GH and GDT, yet animal numbers were limited. No large-scale reports on PR/AI among gonadorelin compounds in beef cattle have been published. In support of Poock et al. (2015), but in contrast to Luchterhand et al. (2019), no differences in PR/AI were determined between the GH- and GDT-treated cows in the present study, which is corroborated by the lack of differences in ovarian parameters or estrus expression between GnRH compounds. Differences in results among studies may be associated with intrinsic physiological differences between high-producing dairy cows and suckled beef cows. Lactating dairy cows have decreased circulating concentrations of progesterone compared with beef cows due to a greater rate of hepatic metabolism of progesterone (Sangsritavong et al., 2002). The negative feedback of progesterone on the hypothalamus plays an important role in modulating GnRH secretion, decreasing follicular development and ovulatory response to exogenous GnRH (Bergfeld et al., 1996; Dias et al., 2021; Pessoa et al., 2024). Moreover, the present study used exogenous progesterone to increase systemic concentrations of progesterone and modulate ovulatory follicle development during the ovulation synchronization protocols (Pessoa et al., 2024). Hence, intrinsic physiological differences between beef and dairy cows, and differences in ovulation synchronization protocols may have contributed to the discrepancies observed among the studies. Alternatively, contrary to our initial hypothesis, there could be no biological differences in the effectiveness between GnRH compounds or the differences in effectiveness may be minimal, limiting the ability of studies to consistently detect statistical differences between treatments when evaluating binary response variables.
In summary, no differences were determined in ovarian parameters, estrus expression, or PR/AI between females treated with GH or GDT when exposed to either synchronization with the 7D or presynchronization with the 7&7; however, estrus expression and PR/AI were greater in presynchronized versus synchronized cows. As a result, beef cattle producers have the flexibility to use GnRH products comprised of either GH or GDT without affecting their fertility outcomes in suckled beef cows. Although an additional animal handling event and injection of PG are required when making use of the 7&7 compared with the 7D, the use of the 7&7 in beef cows could result in greater PR/AI than the use of the 7D.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The animal studies were approved by University of Georgia’s Animal Care and Use Committee (AUP: A2023 11-016-Y1-A1). The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent was not obtained from the owners for the participation of their animals in this study because verbal consent was obtained and producers participated in a hands-on way during the project. Producers were present at all animal handling events.
NO: Conceptualization, Data curation, Investigation, Methodology, Project administration, Resources, Writing – original draft, Writing – review & editing. LG: Data curation, Writing – review & editing, Investigation. SB: Data curation, Writing – review & editing, Investigation. MS: Investigation, Writing – review & editing. KH: Investigation, Resources, Writing – review & editing, Data curation. GL: Conceptualization, Methodology, Resources, Writing – review & editing. PF: Conceptualization, Formal analysis, Investigation, Methodology, Resources, Writing – review & editing.
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
The authors thank Zoetis Animal Health (Parsippany, NJ) for their donation of PG (Lutalyse HighCon) and CIDR inserts (EAZI-BREED CIDR), and Estrotect (Rockway Inc., Spring Valley, WI) for the donation of breeding indicator patches. The authors also thank Stephanie Nelson, Bobby Strecker, the North West Georgia Research and Education Center, the Mississippi State Prairie Research Unit, and all of the cattle producers who allowed the use of their cows for this study.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declare that no Generative AI was used in the creation of this manuscript.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Ahmad N., Schrick F. N., Butcher R. L., Inskeep E. K. (1995). Effect of persistent follicles on early embryonic losses in beef cows. Biol. Reprod. 52, 1129–1135. doi: 10.1095/biolreprod52.5.1129
Andersen C. M., Bonacker R. C., Smith E. G., Spinka C. M., Poock S. E., Thomas J. M. (2021). Evaluation of the 7 & 7 Synch and 7-day CO-Synch + CIDR treatment regimens for control of the estrous cycle among beef cows prior to fixed-time artificial insemination with conventional or sex-sorted semen. Anim. Reprod. Sci. 235. doi: 10.1016/j.anireprosci.2021.106892
Bergfeld E. G. M., Kojima F. N., Cupp A. S., Wehrman M. E., Peters K. E., Mariscal V., et al. (1996). Changing dose of progesterone results in sudden changes in frequency of luteinizing hormone pulses and secretion of 17β-estradiol in bovine females. Biol. Reprod. 54, 546–553. doi: 10.1095/biolreprod54.3.546
Bonacker R. C., Stoecklein K. S., Locke J. W. C., Ketchum J. N., Knickmeyer E. R., Spinka C. M., et al. (2020). Treatment with prostaglandin F2α and an intravaginal progesterone insert promotes follicular maturity in advance of gonadotropin-releasing hormone among postpartum beef cows. Theriogenology 157, 350–359. doi: 10.1016/j.theriogenology.2020.08.018
Chacher M. F. A., Çolak A., Hayirli A. (2017). Efficacy of repeatedly used CIDR device in cattle reproduction: a metaanalysis review of progesterone concentration and conception rate. Turk J. Vet. Anim. Sci. 41, 692–697. doi: 10.3906/vet-1706-75
Colazo M. G., Kastelic J. P., Davis H., Rutledge M. D., Martinez M. F., Small J. A., et al. (2008). Effects of plasma progesterone concentrations on LH release and ovulation in beef cattle given GnRH Domest Anim Endocrinol. 34, 109–117. doi: 10.1016/j.domaniend.2006.11.004
Dias H. P., Poole R. K., Albuquerque J. P., dos Santos P. H., Castilho A. C. S., Pohler K. G., et al. (2021). Progesterone dose during synchronization treatment alters luteinizing hormone receptor and steroidogenic enzyme mRNA abundances in granulosa cells of Nellore heifers. Anim. Reprod. Sci. 225. doi: 10.1016/j.anireprosci.2020.106681
Fontes P. L. P., Cooke R. F., Oosthuizen N., Timlin C. L., Dias N. W., Currin J. F., et al. (2019). Impacts of administering prostaglandin F2α analogue 24 h prior to progesterone insert removal on expression of estrus in beef females. Livest Sci. 226, 82–86. doi: 10.1016/j.livsci.2019.06.007
Geary T. W., Downing E. R., Bruemmer J. E., Whittier J. C. (2000). Ovarian and estrous response of suckled beef cows to the select synch estrous synchronization protocol. Prof. Anim. Sci 16, 1–5. doi: 10.15232/S1080-7446(15)31653-3
Grant J. K., Abreu F. M., Hojer N. L., Fields S. D., Perry B. L., Perry G. A. (2011). Influence of inducing luteal regression before a modified controlled internal drug-releasing device treatment on control of follicular development. J. Anim. Sci. 89, 3531–3541. doi: 10.2527/jas.2011-3852
Ketchum J. N., Quail L. K., Epperson K. M., Guy C. P., Rich J. J. J., Zoca S. M., et al. (2024). Evaluation of two beef cow fixed-time AI protocols that utilize presynchronization. Theriogenology 213, 59–65. doi: 10.1016/j.theriogenology.2023.09.017
Luchterhand M., Gamarra C. A., Gennari R. S., Carvalho P. D., Barletta R. V., Souza A. H. (2019). Ovulation and fertility response to commercially available GnRH products in lactating cows synchronized with the Double-Ovsynch protocol. Anim. Reprod. Sci. 202, 42–48. doi: 10.1016/j.anireprosci.2019.01.006
Martínez M. F., Mapletoft R. J., Kastelic J. P., Carruthers T. (2003). The effects of 3 gonadorelin products on luteinizing hormone release, ovulation, and follicular wave emergence in cattle. Can. Vet. J. 44, 125–131.
Mercadante V. R. G., Kozicki L. E., Ciriaco F. M., Henry D. D., Dahlen C. R., Crosswhite M. R., et al. (2015). Effects of administration of prostaglandin F at initiation of the seven-day CO-Synch+controlled internal drug release ovulation synchronization protocol for suckled beef cows and replacement beef heifers. J. Anim. Sci. 93, 5204–5213. doi: 10.2527/jas.2015-8967
Mercadante V. R. G. R. G., Lamb G. C. C., Oosthuizen N., Wege Dias N. W., Pancini S., Haines H., et al. (2021). Estrus response and pregnancy rates of beef replacement heifers enrolled in two fixed-time artificial insemination protocols, with or without pre-synchronization. J. Anim. Sci. 99, 125–126. doi: 10.1093/jas/skab235.228
Oosthuizen N., Fontes P. L. P., Porter K., Lamb G. C. (2020). Presynchronization with prostaglandin F2a and prolonged exposure to exogenous progesterone impacts estrus expression and fertility in beef heifers. Theriogenology 146, 88–93. doi: 10.1016/j.theriogenology.2020.02.010
Pancini S., Dias N. W. W., Currin J., Clark S., Stewart J. L., Mercadante V. R. G. (2022). Estrus Response and Pregnancy Rates of Beef Cows Enrolled in two Fixed-Time Artificial Insemination Protocols, with or without pre-Synchronization. J. Anim. Sci. 100, 255–256. doi: 10.1093/jas/skac247.462
Perry G. A., Perry B. L., Krantz J. H., Rodgers J. (2012). Influence of inducing luteal regression before a modified fixed-time artificial insemination protocol in postpartum beef cows on pregnancy success. J. Anim. Sci. 90, 489–494. doi: 10.2527/jas.2011-4319
Perry G. A., Smith M. F., Lucy M. C., Green J. A., Parks T. E., MacNeil M. D., et al. (2005). Relationship between follicle size at insemination and pregnancy success. Proc. Natl. Acad. Sci. U.S.A. 102, 5268–5273. doi: 10.1073/pnas.0501700102
Perry G. A., Smith M. F., Roberts A. J., MacNeil M. D., Geary T. W. (2007). Relationship between size of the ovulatory follicle and pregnancy success in beef heifers. J. Anim. Sci. 85, 684–689. doi: 10.2527/jas.2006-519
Pessoa G. A., Fontes P. L. P., Junior I. C., Junior F. R. L., Alves N., Sa Filho O. G., et al. (2024). Fertility of predominantly Bos taurus beef cows exposed to fixed-time artificial insemination protocols with intravaginal inserts containing different amounts of progesterone. Theriogenology 234, 73–82. doi: 10.1016/j.theriogenology.2024.12.003
Poock S. E., Lamberson W. R., Lucy M. C. (2015). Effect of different gonadorelin (GnRH) products used for the first or resynchronized timed artificial insemination on pregnancy rates in postpartum dairy cows. Theriogenology 84, 504–508. doi: 10.1016/j.theriogenology.2015.04.002
Rathbone M. J., Bunt C. R., Ogle C. R., Burggraaf S., Macmillan K. L., Burke C. R., et al. (2002). Reengineering of a commercially available bovine intravaginal insert (CIDR insert) containing progesterone. J. Controlled Release 85, 105–115. doi: 10.1016/S0168-3659(02)00288-2
Richardson B. N., Hill S. L., Stevenson J. S., Djira G. D., Perry G. A. (2016). Expression of estrus before fixed-time AI affects conception rates and factors that impact expression of estrus and the repeatability of expression of estrus in sequential breeding seasons. Anim. Reprod. Sci. 166, 133–140. doi: 10.1016/j.anireprosci.2016.01.013
Rowson L., Tervit H. R., Brand A. (1972). The use of prostaglandins for synchronization of oestrus in cattle. J. Reprod. Fertil 29, 145. doi: 10.1530/jrf.0.0290145-a
Sangsritavong S., Combs D. K., Sartori R., Armentano L. E., Wiltbank M. C. (2002). High feed intake increases liver blood flow and metabolism of progesterone and estradiol-17β in dairy cattle. J. Dairy Sci. 85, 2831–2842. doi: 10.3168/jds.S0022-0302(02)74370-1
Sartori R., Fricke P. M., Ferreira J. C., Ginther O., Wiltbank M. C. (2001). Follicular deviation and acquisition of ovulatory capacity in bovine follicles. Biol. Reprod. 65, 1403–1409. doi: 10.1095/biolreprod65.5.1403
Souza A. H., Cunha A. P., Silva E. P. B., Gümen A., Ayres H., Guenther J. N., et al. (2009). Comparison of gonadorelin products in lactating dairy cows: Efficacy based on induction of ovulation of an accessory follicle and circulating luteinizing hormone profiles. Theriogenology 72, 271–279. doi: 10.1016/j.theriogenology.2009.02.016
Stevenson J. S., Thompson E., Forbes W. L., Lamb G. C., Grieger D. M., Corah L. R. (2000). Synchronizing estrus and (or) ovulation in beef cows after combinations of GnRH, norgestomet, and prostaglandin F2alpha with or without timed insemination. J. Anim. Sci. 78, 1747–1758. doi: 10.2527/2000.7871747x
Keywords: beef cows, GnRH, gonadorelin diacetate tetrahydrate, gonadorelin hydrochloride, presynchronization
Citation: Oosthuizen N, Goncalves LM, Burato S, Smith MS, Harvey KM, Lamb GC and Fontes PLP (2025) Comparison of different GnRH compounds on fertility outcomes in ovulation synchronized and presynchronized beef cows. Front. Anim. Sci. 6:1502984. doi: 10.3389/fanim.2025.1502984
Received: 27 September 2024; Accepted: 20 January 2025;
Published: 11 February 2025.
Edited by:
Gianni Battacone, University of Sassari, ItalyReviewed by:
Christian Hanzen, University of Liège, BelgiumCopyright © 2025 Oosthuizen, Goncalves, Burato, Smith, Harvey, Lamb and Fontes. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Pedro L. P. Fontes, cGVkcm9mb250ZXNAdWdhLmVkdQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.