- Agriculture and Food, Commonwealth Scientific and Industrial Research Organisation (CSIRO), Armidale, NSW, Australia
Early life experiences can have lasting impacts on an animal’s development. Extensive research evidence aligns across both human and non-human rodent and primate laboratory animals showing negative impacts of early life adversity such as impairments in neurological and behavioural development. Farmed animals experience a range of adversities across their production lifetimes, often early in life, including species atypical social groupings, invasive husbandry procedures, and transport. Correspondingly, farmed animals also demonstrate a wide range of impairments such as stereotypic, injurious, and other abnormal behaviours. An individual, however, needs to experience adversity to be able to develop resilience and coping mechanisms that facilitate dealing with challenges later in life. Not all individuals will experience stress vulnerability following adversity, with some individuals instead developing stress resilience. This mini review collates evidence on the positive effects of early life adversity on improving adaptability in farmed species, both terrestrial and aquatic. While evidence across farmed animal species is currently much less than for humans, laboratory rodents or non-human primates, similar patterns emerge where mild adversity early in life, can improve the adaptability of the animal in the face of future stressors. Many views of optimised welfare posit that farmed animals should be housed in as natural environments as possible to limit many of the typical adversities they face. However, strategic mild exposure to early life adversity may facilitate improved animal welfare under intensive commercial farming conditions. Future research into this area could provide management tools to better predict and promote stress resilience over stress vulnerability.
1 Introduction
It is well established that what happens early in life can have long lasting impacts on how an animal develops, behaves, and performs later in life (Eyck et al., 2019; Veit and Browning, 2023). Early life developmental conditions can start to have impact as early as in ovo/in utero. For example, heat stress experienced during pregnancy in dairy cattle can reduce the growth and immunity of their offspring (Ghaffari, 2022). Or impacts can occur in critical periods after birth/hatching (Reh et al., 2020), through to reaching maturity such as demonstrated extensively in the emotional behavioural development of laboratory rodents (Callaghan et al., 2013; Schneider, 2013). Impacts may even occur via parental stock experiences as evidenced in breeding chickens that may be experiencing suboptimal environments and translate this experience through to their offspring (De Haas et al., 2021). If an individual has optimal conditions in which to develop and grow then there will be numerous physical and behavioural benefits to that individual across its lifetime (Bayne, 2018; Campbell et al., 2019; Zhang et al., 2022; Veissier et al., 2024). Conversely, early life stress, adversity, and trauma, can result in lasting negative consequences for an individual (Campbell and Roth, 2023; Dettmer and Chusyd, 2023).
When referring to ‘early life conditions’, this could encompass one or multiple factors that all contribute to the development of an organism. For example, this could include the social environment the animal is experiencing (e.g., pair versus single housing in calves: Bolt et al., 2017; maternal deprivation in piglets: Brückmann et al., 2020; Gimsa et al., 2022), their nutrition (e.g., malnutrition in sheep during gestation and post-weaning: Poore et al., 2014), the absence of desirable resources (e.g., perches for laying hen pullets: Gunnarsson et al., 2000) or degree of cognitive stimulation (e.g., rearing environmental complexity and cognitive task performance in piglets: Martin et al., 2015). If we look at what is characterised as adversity, Chelini et al. (2022), took three macro categories of human childhood adversities (household challenges, abuse, and neglect as defined in the renowned CDC-Kaiser ACE Study Felitti et al., 1998) and aligned them with corresponding categories in rodent studies. Limited bedding and nesting could fall under ‘household challenges’, resource scarcity would be ‘abuse’, and maternal separation was categorised as ‘neglect’ (Chelini et al., 2022). Based on this, farmed animals across different livestock and aquaculture industries are often routinely exposed to one or all of these adversities during early life with their restricted housing conditions, limited desirable resources, and frequent maternal deprivation. Evidence aligned across both human and non-human animal studies, shows early life adversity, of varying forms, can result in impairments in immune function, neurological development, and an increased risk for psychological and psychiatric disorders (De Bellis and Zisk, 2014; Cross et al., 2017; Danese and Lewis, 2017; Babicola et al., 2021; Dettmer and Chusyd, 2023; Lee and Jung, 2024). Unsurprisingly, farmed animals also demonstrate a wide range of behavioural and neurological impairments, such as stereotypic, injurious, and other abnormal behaviours and chronic health issues such as lameness and infectious pathogen susceptibility (Rodenburg and Koene, 2007; Palmer and O’Connell, 2015; Tatemoto et al., 2022). These impairments may result from their early life experiences as there is ever-growing evidence of optimised early developmental environments of farmed species alleviating negative behavioural and health impacts (e.g., poultry: Campbell et al., 2019; dairy calves: Costa et al., 2019). These long-term impacts in an organism following their early adverse experiences are facilitated by mechanistic changes in neurological, transcriptional and epigenetic pathways (reviewed in Basile et al., 2021; Burns et al., 2018; Short et al., 2020; Smith and Pollak, 2020).
An individual, however, also needs to experience adversity or stressors to be able to develop resilience and coping mechanisms that facilitate dealing with stressful experiences and challenges later in life (Meehan and Mench, 2007; Monaghan and Haussmann, 2015; Dhabhar, 2018; Jessop, 2019). Phenotypic development in response to the surrounding environment is an evolutionarily advantageous strategy (Langenhof and Komdeur, 2018). For all animals, Langenhof and Komdeur (2018) hypothesised four factors that would determine an individual’s successful adaptation to their environment; perceiving if there was a need for a response to a stimulus, evaluating what an effective response would be, the individual’s ability to deliver that response, and bearing the cost of that response. Circumstances that facilitate adjustment to adversity during the developmental phase would improve an individual’s ability to exhibit the correct (and least costly) responses to environmental triggers across their lifetime, as specific to the species and their behavioural ecology. In support of positive impacts of early life adversity, there is evidence across a range of animal taxa showing stressful experiences early in development, can result in more adaptive coping strategies later in life (e.g., mice: Bodden et al., 2015; Santarelli et al., 2017, rats: Oomen et al., 2010, fish: Fontana et al., 2021; Zare et al., 2024, monkeys: Parker et al., 2019). Given that there are both positive and negative impacts of early life adversity, there is also an extensive body of literature aimed at understanding what adverse early life experiences and/or what individual differences, such as personality traits, can lead to an outcome of improved biological functioning, versus long-term detriment (Parker and Maestripieri, 2011; Brenhouse and Bath, 2019; Hartmann and Schmidt, 2020). That is, what constitutes the tipping point between stress resilience, and stress vulnerability (Murthy and Gould, 2018; Kentner et al., 2019).
The background provided here alludes to the extent of the information around early life adversities, long-term impacts and mechanisms across many species. However, there is much less research on the positive impacts of early life adversity in farmed animal species (see Lucas et al., 2024 for a framework on early life experiences of pigs). This mini review is aimed at collating evidence on the positive effects of early life adversity on improving adaptability in farmed species, both terrestrial and aquatic. An understanding of the consequences of early adversity of varying degrees and forms as relevant to different species can contribute toward strategies for mitigating developmental risks of animals under our care, thus improving their welfare.
2 Positive effects of early life adversity
The literature to date in farmed animals on the impacts of early life adversity is not as abundant as that across laboratory rodents and primates. However, there are several studies across varying taxa detailing the improvements in adaptability resulting from stressors that highlight the potential for further research in this area. The studies detailed in this section are also summarised in Table 1. Looking at the evidence in birds, Japanese quail subjected to unpredictable stressors across 8 days including handling, noise, and restraint, showed a reduction in body weight across the stressor period relative to individuals not undergoing the stressors (Calandreau et al., 2011). However, in reversal learning of a spatial memory task, the stressed birds outperformed the controls suggesting this sub-chronic period of stressor application improved the behavioural flexibility of the birds (Calandreau et al., 2011). In a separate experiment with quails, Zimmer et al. (2013) simulated maternal stress programming through corticosterone egg injections and post-hatch adversity via random food deprivation. When the birds reached adulthood, there were long-term effects of both the pre- and post-hatch adversity treatments. Relative to controls, these birds showed reduced corticosterone reactivity to capture-handling-restraint, improved exploration of and increased willingness to feed in a novel environment (Zimmer et al., 2013). Using the same types of measures, a subsequent study showed these positive impacts of early adversity were transgenerational showing mothers were able to pass along their ‘stress-coping phenotype’ (Zimmer et al., 2017).
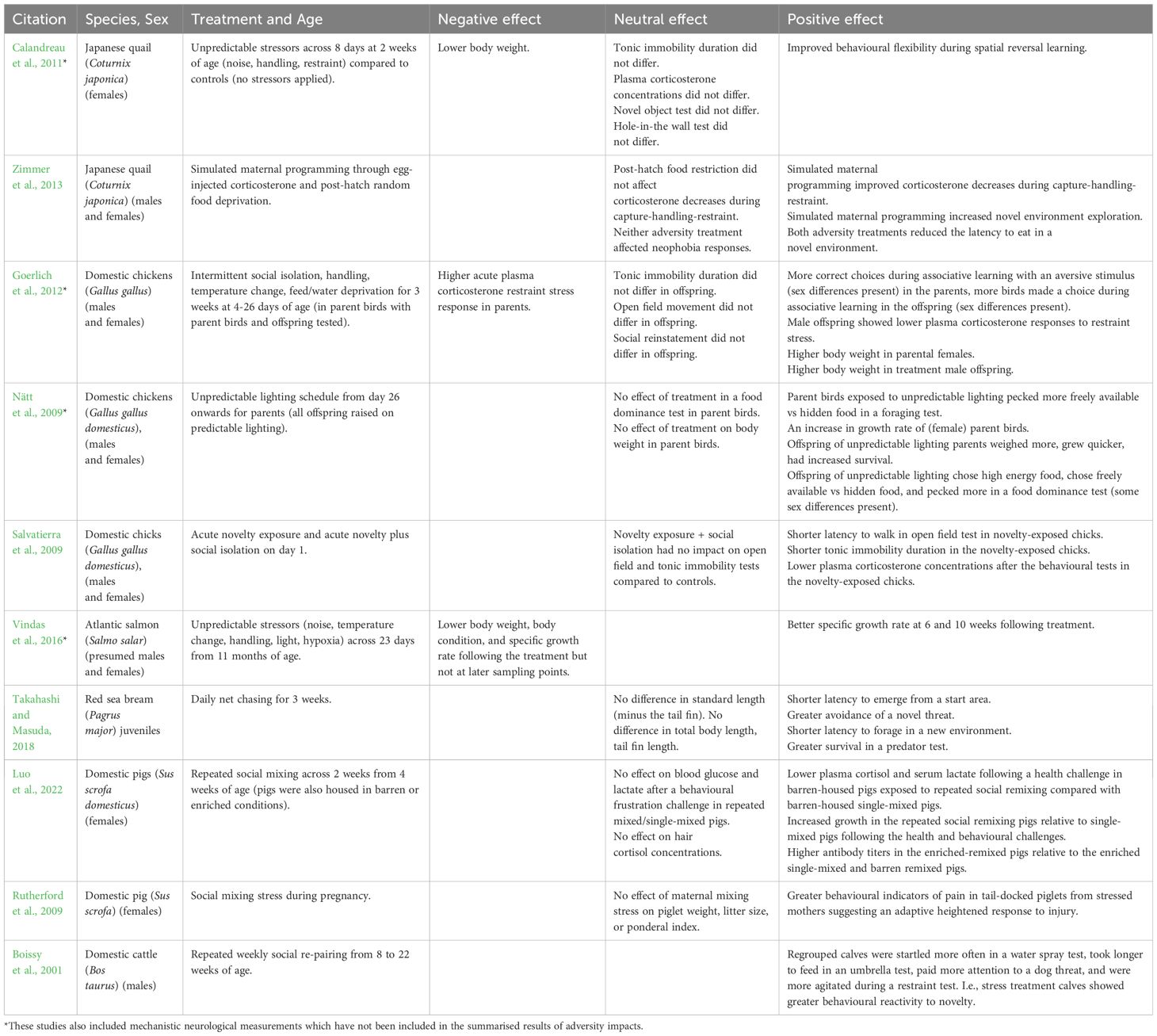
Table 1. A summary of the negative, neutral, and positive effects of early life adversity on growth, behaviour, and stress reactivity across farmed species.
In chickens, effects of stressors were shown in both parent chicks and later in their offspring demonstrating there are also transgenerational impacts of stressors in this species. Birds that had undergone an intermittent social isolation/handling/food and water deprivation stressor across 3 weeks showed better performance in an associative learning test using an aversive (bitter taste) stimulus and offspring showed a lower plasma corticosterone response to a restraint stress (Goerlich et al., 2012). There were differences in the significance of impacts between males and females, but results suggest an early stressor can improve coping ability (Goerlich et al., 2012). Further evidence for developmental environment impacts in chickens, including transgenerational transfer comes from a study that raised birds in an environment with unpredictable lighting, equating to unpredictable food supply (Nätt et al., 2009). The offspring of these parents showed adaptive foraging strategies and performed better compared with offspring of parent birds raised on standard predictable lighting (Nätt et al., 2009). Similarly, Salvatierra et al. (2009) found that acute exposure to a novel environment at one day of age in individually housed chicks, was able to reduce corticosterone responses and measures of fear two weeks later. However, if the chicks were exposed to the novel environment as well as social isolation (pair-housed chicks) they did not show a reduction in fear and stress measures relative to chicks that had not experienced the novel environment stressor, nor been social isolated. This suggests that the social isolation stressor ameliorated the positive effects of the novelty stressor but did not compound as these birds responded similarly to control chicks (Salvatierra et al., 2009).
Benefits of developmental stress have also been seen in fish species. Vindas et al. (2016) exposed Atlantic salmon to unpredictable stressors across a 3-week period and demonstrated that relative to controls, these fish showed improved growth rates 6 and 10 weeks after experiencing the stressor, even though their growth was negatively impacted immediately following the stressful treatment (Vindas et al., 2016). As part of typical husbandry processes, these fish had undergone a 6-week smoltification and a transfer to seawater for 4 weeks. The improved growth during these periods suggested the stress treatment improved their adaptability to the new conditions (Vindas et al., 2016). In red sea bream juveniles raised in aquaculture hatcheries for stock enhancement release, short-term daily net chasing across three weeks resulted in changes in adaptability as measured through various behavioural tests (Takahashi and Masuda, 2018). The fish that had been exposed to net chasing were quicker to emerge from a starting area indicating greater boldness, they foraged quicker after being transferred to a new environment, showed quicker avoidance/escape from a novel threat and had greater survival after predator exposure, relative to control fish (Takahashi and Masuda, 2018).
Improvement as a result of early stress has also been demonstrated in pigs that were housed in enriched or barren conditions, then subjected to a single or repeated stressful mixing events after weaning (Luo et al., 2022). Contrary to predictions that the repeated mixing may place too high an allostatic load on the pigs, there were some physiological stress-related measures that showed this mixing had benefits including better growth in these pigs relative to single-mixed pigs (Luo et al., 2022). Similar to transgenerational impacts illustrated in chickens and quails, social mixing to cause stress in sows during pregnancy was able to cause greater pain-related behaviours in their tail-docked piglets (Rutherford et al., 2009). The authors interpreted this result as an evolutionarily beneficial strategy to be more attuned to adversity in the environment, particularly when at risk of bodily harm (Rutherford et al., 2009). Aligned with this result, calves that were stressed via repeated social regrouping showed greater behavioural reactivity across a series of behavioural tests suggesting these calves were more attuned to novelty in their environment (Boissy et al., 2001). The authors interpreted this as allowing greater adaptation to environmental change (Boissy et al., 2001).
3 Chronic mild enrichment stress
Another angle to the positive effects of early life adversity is looking at the benefits of chronic mild stress, that may be imposed by environmental changes intended to benefit the animal. Specifically, environmental enrichment, typically through increased environmental complexity, is deemed a positive intervention. However, the presence of enrichment can increase the novelty the animals are exposed to, increase their activity levels, and potentially the social interactions they engage in. All of these may place mild chronic stress on the animal (Veissier et al., 2024). The stress-inoculation hypothesis, drawing on research from laboratory rodents, proposes a framework that posits environmentally enriched lab rodents are under a state of chronic mild stress, where they develop resilience to stress as a result of this early mild exposure (Crofton et al., 2015). Similar application of enrichment to specifically test stress inoculation has been applied in laying hens through exposure to novelty and change in their rearing environments. Experiencing periodic change during the rearing period of chicks and pullets, may reduce later responses to stress-inducing situations. In a study by Campbell et al. (2018), novelty was provided for laying hen chicks during their first 3 weeks of life in the form of novel objects, sound playbacks, flashing lights, and visual wall displays. The birds were transferred to a free-range system before sexual maturity and then tested for their responses to two induced stressors at 38-42 weeks of age. When daily range access was prevented completely, and then the available range area was reduced, the non-enriched birds showed a greater albumen corticosterone response to these imposed stressors relative to the enriched birds (Campbell et al., 2018). In another study with laying hen chicks, Skånberg et al. (2023) compared static environments with a single choice of perch or litter type to multiple changes in the perch and litter types during the first 3 weeks of life. Reduced freezing by the treatment birds in a novel environment test suggests the exposure to the environment changes reduced fear responses in a typically stress-inducing situation (Skånberg et al., 2023).
Conversely, there are some livestock studies, aligning with rodent research (Crofton et al., 2015), demonstrating enrichment actually results in increased anxiety compared with non-enriched animals. For example, Dickson et al. (2024), provided enrichment objects (ball, chew rope, brush) during the weaning period of beef cattle with the intention of mitigating the stress that is experienced during this developmental phase of maternal separation, yarding, and social regrouping. However, in tests of attention bias, the enriched animals displayed measures that were interpreted as greater anxiety thus contradicting the original hypothesis (Dickson et al., 2024). Similarly, pigs tested in an attention bias test showed greater indicators of a negative state if they had been in enriched (substrate, toys) home pen housing versus impoverished home pens, contrasting with experimental predictions (Luo et al., 2019). A study by Backus et al. (2017) that also provided pigs with substrates and varying novel objects in their home pens as well as daily positive human contact and treats found measures that indicated, contrary to predictions, increased anxiety during novel object and human interaction tests. In a laying hen rearing enrichment trial, different novel objects were provided throughout 16 weeks of rearing compared with no enrichments or static perching structures (Bari et al., 2020). The implementation of a range area reduction stressor at 44 weeks of age resulted in an increase in albumen corticosterone in both the non-enriched and novel object treatment groups compared to a decrease in the hens that were exposed to the static perching structures (Bari et al., 2020). Thus, even with what could be considered chronic, mild early life adversity, there may be a tipping point between stress resilience and stress adaptability. Understanding what makes an individual resilient or vulnerable after adverse experiences and the mechanisms leading to this, is an extensive field of research in humans and laboratory species with great scope to widen to more farmed species.
4 Discussion and conclusions
Farmed animals experience a range of stressors across their production lifetimes, including, for example, species atypical social groupings or social transitions, invasive husbandry procedures, transport, and changes in housing environments. These processes can occur during both the early development stages and later when the animals reach maturity. For optimal animal welfare, individual animals need to be able to cope with and adapt to these adverse experiences to avoid long-term detriment. There are decades worth of literature on humans and non-human taxa (typically rodents, primates) around early life adversities that cause behavioural, neurological, and physiological consequences as well as the physiological, epigenetic, and neurological mechanisms that may be facilitating these impacts. However, not all individuals will experience detriment following adversity, instead adversity may lead to increased resilience. While the quantum of evidence across farmed animal species is comparatively less, we see similar patterns emerging. Some adversity early in life can improve the adaptability of the animal in the face of future stressors.
Moving forward, there is scope to better understand what adversities can promote adaptation in an individual so that we may apply interventions that could strategically improve how farmed species perform under the constraints of the commercial context. It has been proposed that the vulnerability or resilience of an individual could be determined by ‘three-hits’ (Daskalakis et al., 2013). An individual is going to be influenced by their (hit-1): genetic predisposition, (hit-2): early-life environment, and (hit-3): later-life environment. Furthermore, the exact features of the adversity, such as the type of stressor, its duration, the sensory modality it is experienced through, and the precise timing of exposure are all going to impact the short and longer-term outcome (Parker and Maestripieri, 2011). However, it is generally accepted that mild to moderate adversities have the potential to improve adaptability, whereas severe adversities will be detrimental (Parker and Maestripieri, 2011; Daskalakis et al., 2013). The outcomes are also likely to be impacted by the natural biology and ecological relevance of the adversity to the animal. For example, in squirrel monkeys, implementing maternal separation at an age when the young monkeys would naturally be temporarily separated from their mothers, leads to stress resilience. Comparatively, maternal separation in primates at young ages that would not be biologically relevant leads to stress vulnerability (Parker and Maestripieri, 2011). Many processes that farmed animals are subjected to fall outside the scope of what may be naturally expected for the species, which could contribute toward the extent of behavioural, neurological, and health impairments that are often seen. It may be possible to utilise ‘natural’ adversities for a particular species to strategically prepare the animal for future stressors. Or to expose the animals to adversities that will enable them to adapt to what is expected to impact them later in life, such as environmental change, or social regrouping. Many views of optimised welfare posit that farmed animals should be housed in as natural environments as possible to limit many of the typical adversities they face. However, strategic mild exposure to early life adversity may facilitate improved animal welfare under intensive commercial farming conditions. Future research into this area could provide management tools to better predict and promote stress resilience over stress vulnerability.
Author contributions
DC: Writing – review & editing, Writing – original draft, Conceptualization.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. Open access fees were supported by a CSIRO Julius Career Award to DC.
Acknowledgments
The author wishes to acknowledge the Animal Behaviour and Welfare Team at CSIRO (both past and current members) whose discussions and support have contributed to the evolution of this text.
Conflict of interest
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Babicola L., Ventura R., D’Addario S. L., Ielpo D., Andolina D., Di Segni M. (2021). Long term effects of early life stress on HPA circuit in rodent models. Mol. Cell. Endocrinol. 521, 111125. doi: 10.1016/j.mce.2020.111125
Backus B. L., Sutherland M. A., Brooks T. A. (2017). Relationship between environmental enrichment and the response to novelty in laboratory-housed pigs. J. Am. Assoc. Lab. Anim. Sci. 56, 735–741.
Bari M. S., Downing J. A., Dyall T. R., Lee C., Campbell D. L. M. (2020). Relationships between rearing enrichments, range use, and an environmental stressor for free-range laying hen welfare. Front. Vet. Sci. 7, 480. doi: 10.3389/fvets.2020.00480
Basile F., Capaccia C., Zampini D., Biagetti T., Diverio S., Guelfi G. (2021). Omics insights into animal resilience and stress factors. Animals 11, 47. doi: 10.3390/ani11010047
Bayne K. (2018). Environmental enrichment and mouse models: Current perspectives. Anim. Models Exp. Med. 1, 82–90. doi: 10.1002/ame2.12015
Bodden C., Richter S. H., Schreiber R. S., Kloke V., Gerß J., Palme R., et al. (2015). Benefits of adversity?! How life history affects the behavioral profile of mice varying in serotonin transporter genotype. Front. Behav. Neurosci. 9, 47. doi: 10.3389/fnbeh.2015.00047
Boissy A., Veissier I., Roussel S. (2001). Behavioural reactivity affected by chronic stress: an experimental approach in calves submitted to environmental instability. Anim. Welf. 10, S175–S185. doi: 10.1017/S0962728600023605
Bolt S. L., Boyland N. K., Mlynski D. T., James R., Croft D. P. (2017). Pair housing of dairy calves and age at pairing: Effects on weaning stress, health, production and social networks. PloS One 12, e0166926. doi: 10.1371/journal.pone.0166926
Brenhouse H. C., Bath K. G. (2019). Bundling the haystack to find the needle: Challenges and opportunities in modelling risk and resilience following early life stress. Front. Neuroendocrinol. 54, 100768. doi: 10.1016/j.yfrne.2019.100768
Brückmann R., Tuchscherer M., Tuchscherer A., Gimsa U., Kanitz E. (2020). Early-life maternal deprivation predicts stronger sickness behaviour and reduced immune responses to acute endotoxaemia in a pig model. Int. J. Mol. Sci. 21, 5212. doi: 10.3390/ijms21155212
Burns S. B., Szyszkowicz J. K., Luheshi G. N., Lutz P.-E., Turecki G. (2018). Plasticity of the epigenome during early-life stress. Semin. Cell Dev. Biol. 77, 115–132. doi: 10.1016/j.semcdb.2017.09.033
Calandreau L., Bertin A., Boissy A., Arnould C., Constantin P., Desmedt A., et al. (2011). Effect of one week of stress on emotional reactivity and learning and memory performances in Japanese quail. Behav. Brain Res. 217, 104–110. doi: 10.1016/j.bbr.2010.10.004
Callaghan B. L., Graham B. M., Li S., Richardson R. (2013). From resilience to vulnerability: mechanistic insights into the effects of stress on transitions in critical period plasticity. Front. Psychiatry 4, 90. doi: 10.3389/fpsyt.2013.00090
Campbell D. L. M., de Haas E. N., Lee C. (2019). A review of environmental enrichment for laying hens during rearing in relation to their behavioral and physiological development. Poult. Sci. 98, 9–28. doi: 10.3382/ps/pey319
Campbell D. L. M., Hinch G. N., Downing J. A., Lee C. (2018). Early enrichment in free-range laying hens: effects on ranging behaviour, welfare and response to stressors. Animal 12, 575–584. doi: 10.1017/S1751731117001859
Campbell T. S., Roth T. L. (2023). “Chapter 12 – Modeling early-life adversity in the laboratory: animal models, their advantages, and future directions in extrapolating findings to humans,” in Translational Epigenetics, Perinatal and Developmental Epigenetics, vol. 32. Ed. Singh G. (Cambridge, Massachusetts, USA: Academic Press), 327–351. doi: 10.1016/B978-0-12-821785-6.00013-X
Chelini G., Pangrazzi L., Bozzi Y. (2022). At the crossroad between resiliency and fragility: A neurodevelopmental perspective on early-life experiences. Front. Cell. Neurosci. 16, 863866. doi: 10.3389/fncel.2022.863866
Costa J. H. C., Cantor M. C., Adderley N. A., Neave H. W. (2019). Key animal welfare issues in commercially raised dairy calves: social environment, nutrition, and painful procedures. Can. J. Anim. Sci. 99, 649–660. doi: 10.1139/cjas-2019-0031
Crofton E. J., Zhang Y., Green T. A. (2015). Inoculation stress hypothesis of environmental enrichment. Neurosci. Biobehav. Rev. 49, 19–31. doi: 10.1016/j.neubiorev.2014.11.017
Cross D., Fani N., Powers A., Bradley B. (2017). Neurobiological development in the context of childhood trauma. Clin. Psychol.: Sci. Pract. 24, 111–124. doi: 10.1111/cpsp.12198
Danese A., Lewis S. J. (2017). Psychoneuroimmunology of early-life stress: The hidden wounds of childhood trauma? Neuropsychopharmacology 42, 99–113. doi: 10.1038/npp.2016.198
Daskalakis N. P., Bagot R. C., Parker K. J., Vinkers C. H., de Kloet E. R. (2013). The three-hit concept of vulnerability and resilience: Toward understanding adaptation to early-life adversity outcome. Psychoneuroendocrinology 38, 1858–1873. doi: 10.1016/j.psyneuen.2013.06.008
De Bellis M. D., Zisk A. (2014). The biological effects of childhood trauma. Child Adolesc. Psychiatr. Clinics North America 23, 185–222. doi: 10.1016/j.chc.2014.01.002
De Haas E. N., Newberry R. C., Edgar J., Riber A. B., Estevez I., Ferrante V., et al. (2021). Prenatal and early postnatal behavioural programming in laying hens, with possible implications for the development of injurious pecking. Front. Vet. Sci. 8, 678500. doi: 10.3389/fvets.2021.678500
Dettmer A. M., Chusyd D. E. (2023). Early life adversities and lifelong health outcomes: A review of the literature on large, social, long-lived nonhuman mammals. Neurosci. Biobehav. Rev. 152, 105297. doi: 10.1016/j.neubiorev.2023.105297
Dhabhar F. S. (2018). The short-term stress response – Mother nature’s mechanism for enhancing protection and performance under conditions of threat, challenge, and opportunity. Front. Neuroendocrinol. 49, 175–192. doi: 10.1016/j.yfrne.2018.03.004
Dickson E. J., Monk J. E., Lee C., Campbell D. L. M. (2024). Environmental enrichment during yard weaning alters the performance of calves in an attention bias and novel object recognition test. Front. Anim. Sci. 5, 1364259. doi: 10.3389/fanim.2024.1364259
Eyck H. J. F., Buchanan K. L., Crino O. L., Jessop T. S. (2019). Effects of developmental stress on animal phenotype and performance: a quantitative review. Biol. Rev. 94, 1143–1160. doi: 10.1111/brv.12496
Felitti V. J., Anda R. F., Nordenberg D., Williamson D. F., Spitz A. M., Edwards V., et al. (1998). Relationship of childhood abuse and household dysfunction to many of the leading causes of death in adults. The Adverse Childhood Experiences (ACE) Study. Am. J. Prevent. Med. 14, 245–258. doi: 10.1016/s0749-3797(98)00017-8
Fontana B. D., Gibbon A. J., Cleal M., Sudwarts A., Pritchett D., Petrazzini M. E. M., et al. (2021). Moderate early life stress improves adult zebrafish (Danio rerio) working memory but does not affect social and anxiety-like responses. Dev. Psychobiol. 63, 54–64. doi: 10.1002/dev.21986
Ghaffari M. H. (2022). Developmental programming: prenatal and postnatal consequences of hyperthermia in dairy cows and calves. Domest. Anim. Endocrinol. 80, 106723. doi: 10.1016/j.domaniend.2022.106723
Gimsa U., Brückmann R., Tuchscherer A., Tuchscherer M., Kanitz E. (2022). Early-life maternal deprivation affects the mother-offspring relationship in domestic pigs, as well as the neuroendocrine development and coping behavior of piglets. Front. Behav. Neurosci. 16, 980350. doi: 10.3389/fnbeh.2022.980350
Goerlich V. C., Nätt D., Elfwing M., Macdonald B., Jensen P. (2012). Transgenerational effects of early experience in behavioral, hormonal and gene expression responses to acute stress in the precocial chicken. Hormones Behav. 61, 711–178. doi: 10.1016/j.yhbeh.2012.03.006
Gunnarsson S., Yngvesson J., Keeling L. J., Forkman B. (2000). Rearing without early access to perches impairs the spatial skills of laying hens. Appl. Anim. Behav. Sci. 67, 217–228. doi: 10.1016/S0168-1591(99)00125-2
Hartmann J., Schmidt M. V. (2020). “Chapter 11 – Stress resilience as a consequence of early life adversity,” in Stress Resilience. Ed. Chen A. (Cambridge, Massachusetts, USA: Academic Press), 149–164. doi: 10.1016/B978-0-12-813983-7.00011-2
Jessop D. S. (2019). The power of positive stress and a research roadmap. Stress 22, 521–523. doi: 10.1080/10253890.2019.1593365
Kentner A. C., Cryan J. F., Brummelte S. (2019). Resilience priming: Translational models for understanding resiliency and adaptation to early life adversity. Dev. Psychobiol. 61, 350–375. doi: 10.1002/dev.21775
Langenhof M. R., Komdeur J. (2018). Why and how the early-life environment affects development of coping behaviours. Behav. Ecol. Sociobiol. 72, 34. doi: 10.1007/s00265-018-2452-3
Lee S. H., Jung E.-M. (2024). Adverse effects of early-life stress: focus on the rodent neuroendocrine system. Neural Regen. Res. 19, 336–341. doi: 10.4103/1673-5374.377587
Lucas M. E., Hemsworth L. M., Hemsworth P. H. (2024). Review: early life piglet experiences and impacts on immediate and longer-term adaptability. Animal 18, 100889. doi: 10.1016/j.animal.2023.100889
Luo L., Reimert I., de Haas E. N., Kemp B., Bolhuis J. E. (2019). Effects of early and later life environmental enrichment and personality on attention bias in pigs (Sus scrofa domesticus). Anim. Cogn. 22, 959–972. doi: 10.1007/s10071-019-01287-w
Luo L., Zande L., Marwijk M., Knol E. F., Rodenburg T. B., Bolhuis J. E., et al. (2022). Impact of enrichment and repeated mixing on resilience in pigs. Front. Vet. Sci. 9. doi: 10.3389/fvets.2022.829060
Martin J. E., Ison S. H., Baxter E. M. (2015). The influence of neonatal environment on piglet play behaviour and post-weaning social and cognitive development. Appl. Anim. Behav. Sci. 163, 69–79. doi: 10.1016/j.applanim.2014.11.022
Meehan C. L., Mench J. A. (2007). The challenge of challenge: Can problem solving opportunities enhance animal welfare? Appl. Anim. Behav. Sci. 102, 246–261. doi: 10.1016/j.applanim.2006.05.031
Monaghan P., Haussmann M. F. (2015). The positive and negative consequences of stressors during early life. Early Hum. Dev. 91, 643–647. doi: 10.1016/j.earlhumdev.2015.08.008
Murthy S., Gould E. (2018). Early life stress in rodents: animal models of illness or resilience? Front. Behav. Neurosci. 12, 157. doi: 10.3389/fnbeh.2018.00157
Nätt D., Lindqvist N., Stranneheim H., Lundeberg J., Torjesen P. A., Jensen P. (2009). Inheritance of acquired behaviour adaptations and brain gene expression in chickens. PloS One 4, e6405. doi: 10.1371/journal.pone.0006405
Oomen C. A., Soeters H., Audureau N., Vermunt L., van Hasselt F. N., Manders E. M. M., et al. (2010). Severe early life stress hampers spatial learning and neurogenesis, but improves hippocampal synaptic plasticity and emotional learning under high-stress conditions in adulthood. J. Neurosci. 30, 6635–6645. doi: 10.1523/JNEUROSCI.0247-10.2010
Palmer M. A., O’Connell N. E. (2015). Digital dermatitis in dairy cows: A review of risk factors and potential sources of between-animal variation in susceptibility. Animals 5, 512–535. doi: 10.3390/ani5030369
Parker K. J., Buckmaster C. L., Hyde S. A., Schatzberg A. F., Lyons D. M. (2019). Nonlinear relationship between early life stress exposure and subsequent resilience in monkeys. Sci. Rep. 9, 16232. doi: 10.1038/s41598-019-52810-5
Parker K. J., Maestripieri D. (2011). Identifying key features of early stressful experiences that produce stress vulnerability and resilience in primates. Neurosci. Biobehav. Rev. 35, 1466–1483. doi: 10.1016/j.neubiorev.2010.09.003
Poore K. R., Hollis L. J., Murray R. J. S., Warlow A., Brewin A., Fulford L., et al. (2014). Differential pathways to adult metabolic dysfunction following poor nutrition at two critical developmental periods in sheep. PloS One 9, e90994. doi: 10.1371/journal.pone.0090994
Reh R. K., Dias B. G., Nelson C. A. III, Kaufer D., Werker J. F., Kolb B., et al. (2020). Critical period regulation across multiple timescales. Proc. Natl. Acad. Sci. 117, 38. doi: 10.1073/pnas.1820836117
Rodenburg T. B., Koene P. (2007). The impact of group size on damaging behaviours, aggression, fear and stress in farm animals. Appl. Anim. Behav. Sci. 103, 205–214. doi: 10.1016/j.applanim.2006.05.024
Rutherford K. M. D., Robson S. K., Donald R. D., Jarvis S., Sandercock D. A., Scott E. M. (2009). Pre-natal stress amplifies the immediate behavioural responses to acute pain in piglets. Biol. Lett. 5, 452–454. doi: 10.1098/rsbl.2009.0175
Salvatierra N. A., Cid M. P., Arce A. (2009). Neonatal acute stress by novelty in the absence of social isolation decreases fearfulness in young chicks. Stress 12, 328–335. doi: 10.1080/10253890802455433
Santarelli S., Zimmermann C., Kalideris G., Lesuis S. L., Arloth J., Uribe A., et al. (2017). An adverse early life environment can enhance stress resilience in adulthood. Psychoneuroendocrinology 78, 213–221. doi: 10.1016/j.psyneuen.2017.01.021
Schneider M. (2013). Adolescence as a vulnerable period to alter rodent behavior. Cell Tissue Res. 354, 99–106. doi: 10.1007/s00441-013-1581-2
Short A. K., Bolton J. L., Baram T. Z. (2020). “Mechanisms by which early-life experiences promote enduring stress resilience or vulnerability,” in Stress Resilience. Ed. Chen A. (Cambridge, Massachusetts, USA: Academic Press), 165–180. doi: 10.1016/B978-0-12-813983-7.00012-4
Skånberg L., Newberry R. C., Estevez I., Keeling L. J. (2023). Environmental change or choice during early rearing improves behavioural adaptability in laying hen chicks. Sci. Rep. 13, 6178. doi: 10.1038/s41598-023-33212-0
Smith K. E., Pollak S. D. (2020). Early life stress and development: potential mechanisms for adverse outcomes. J. Neurodev. Disord. 12, 34. doi: 10.1186/s11689-020-09337-y
Takahashi K., Masuda R. (2018). Net-chasing training improves the behavioral characteristics of hatchery-reared red sea bream (Pagrus major) juveniles. Can. J. Fish. Aquat. Sci. 75, 861–867. doi: 10.1139/cjfas-2017-0073
Tatemoto P., Broom D. M., Zanella A. J. (2022). Changes in stereotypies: effects over time and over generations. Animals 12, 2504. doi: 10.3390/ani12192504
Veissier I., Lesimple C., Brunet V., Aubé L., Botreau R. (2024). Review: Rethinking environmental enrichment as providing opportunities to acquire information. Animal 18, 101251. doi: 10.1016/j.animal.2024.101251
Veit W., Browning H. (2023). Developmental programming, evolution, and animal welfare: A case for evolutionary veterinary science. J. Appl. Anim. Welf. Sci. 26, 552–564. doi: 10.1080/10888705.2021.2014838
Vindas M. A., Madaro A., Fraser T. W. K., Höglund E., Olsen R. E., Øverli Ø., et al. (2016). Coping with a changing environment: the effects of early life stress. R. Soc. Open Sci. 3, 160382. doi: 10.1098/rsos.160382
Zare M., Esmaeili N., Hosseini H., Choupani S. M. H., Akhavan S., Salini M., et al. (2024). Do optimum dietary protein and early mild stress events prepare oscar (Astronotus ocellatus) for a stressful future? Aquacult. Rep. 34, 101854. doi: 10.1016/j.aqrep.2023.101854
Zhang Z., Gao L., Zhang X. (2022). Environmental enrichment increases aquatic animal welfare: A systematic review and meta-analysis. Rev. Aquacult. 14, 1120–1135. doi: 10.1111/raq.12641
Zimmer C., Boogert N. J., Spencer K. A. (2013). Developmental programming: Cumulative effects of increased pre-hatching corticosterone levels and post-hatching unpredictable food availability on physiology and behaviour in adulthood. Hormones Behav. 64, 494–500. doi: 10.1016/j.yhbeh.2013.07.002
Keywords: enrichment, aquaculture, stress, resilience, livestock, domestic animals
Citation: Campbell DLM (2024) Friend or foe? Early life adversity to improve farmed animal welfare. Front. Anim. Sci. 5:1484718. doi: 10.3389/fanim.2024.1484718
Received: 22 August 2024; Accepted: 30 September 2024;
Published: 16 October 2024.
Edited by:
Elena E. Terenina, Institut National de la Recherche Agronomique de Toulouse, FranceReviewed by:
Anette Wichman, Swedish University of Agricultural Sciences, SwedenCopyright © 2024 Campbell. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Dana L. M. Campbell, ZGFuYS5jYW1wYmVsbEBjc2lyby5hdQ==
 Dana L. M. Campbell
Dana L. M. Campbell