- 1Centre for Animal Science, Queensland Alliance for Agriculture and Food Innovation, The University of Queensland, St Lucia, QLD, Australia
- 2SunPork Group, Eagle Farm, QLD, Australia
- 3Animal Welfare Science Centre, The University of Melbourne, Parkville, VIC, Australia
- 4Centre for Animal Science, Queensland Alliance for Agriculture and Food Innovation and the School of Veterinary Science, The University of Queensland, Gatton QLD, Australia
Introduction: Early life interactions with the sow or humans can have lifelong consequences on welfare and performance in pigs. It was hypothesised that piglets that received increased maternal contact and positive human contact would display improved responses to stressful events and improved performance.
Methods: Ninety-eight litters were allocated to a 2 x 2 factorial design for maternal contact (MC+)/restricted maternal contact (MC-); and positive human contact (HC+)/no additional human contact (HC-). Modified farrowing crates were used to restrict maternal contact (MC-) and litters in the HC+ treatment received five minutes of daily positive human interaction (stroking). Behavioural and physiological responses were assessed during processing at three days of age and during a behaviour test in which piglets were introduced to an empty arena, novel object, human hand and human standing in the arena at 18 days of age. Observations of behaviour in the home pen and growth and survival of piglets to weaning were also assessed.
Results: At processing, MC- piglets had more squeals (P = 0.015), tended to have more grunts (P = 0.063) and struggle attempts (P = 0.079), and had increased plasma cortisol concentrations (P = 0.009) after processing than MC+ piglets. During the behaviour test, HC+ piglets were more active (P = 0.014) and had more vocalisations (P < 0.05) in the empty arena than HC piglets. Furthermore, HC+ piglets were more likely to approach a human standing in the arena (P = 0.006) than HC- piglets. MC- piglets were less active (P = 0.008) and were less likely to approach the novel object (P = 0.049) than MC+ piglets. MC+ piglets tended to have higher weaning weights (P = 0.055) and more MC+ piglets were successfully weaned (P = 0.022) than MC- piglets. There was no significant effect of HC on piglet performance.
Discussion: While positive handling did not affect behavioural and physiological stress response to processing, HC+ reduced fear of novelty and humans. These findings highlight the importance of early life experiences has on stress resilience early in life.
1 Introduction
Livestock, particularly in intensive settings, are exposed to potential stressors such as routine husbandry and housing practices. These stressors can impair animal welfare and performance [reviewed in Colditz and Hine (2016)]. Stress resilience refers to the ability of an individual to cope and recover from stressors (Colditz and Hine, 2016). Evidence of improved stress resilience would be a decrease or more controlled physiological stress response following a stressor and reduced behaviours indicative of stress, particularly during perceived stressful situations. It is also suggested that animals with improved stress resilience would be more robust to illness and have improved growth and reproductive performance. Increasing stress resilience is important for the livestock industries because individuals that cope and recover from exposure to routine husbandry and housing are likely to have improved welfare and productivity. The early life period has been shown to be the most influential time for brain development related to how an individual responds to stress and subsequent stress resilience (Romeo et al., 2009).
The response to stress is not always straightforward. Most empirical research on stress resilience has examined extreme disruptions of the parent–offspring relationship in laboratory species, which might have some relevance for the impact of commercial changes to animal management on stress resilience. For instance, Parker and Maestripieri (2011) concluded that “empirical studies suggest that early life stress exposure may be best conceptualized as a quadratic, rather than linear, function.” Too much stress is certainly a bad thing, but so is too little, because young animals then lack the experience of learning to deal independently with stress. Rather, facing an intermediate amount of stress in early life can be beneficial by inducing an inoculating effect on behaviour [reviewed in Lyons et al. (2009)].
There is extensive research in rodents and non-human primates showing that early maternal contact affects brain development, resulting in long-term alterations in behavioural and physiological stress responses [reviewed in Levine (2002); McEwen (2007); Parker and Maestripieri (2011)]. Offspring born to dams that provide more tactile interactions, such as licking and grooming, are more explorative, less reactive and produce a more contained glucocorticoid response to novel stressors. Conversely, poor mothering leads to increased emotional reactivity and reduced cognition and hypothalamo pituitary adrenal (HPA) axis activity in their offspring. These early interactions can have lasting effects on behavioural and physiological stress responses to challenges and socio-positive behaviours (Coutellier et al., 2008).
There have been many studies investigating the use of loose housing farrowing pens to promote the sow-piglet bond. In particular Chidgey et al. (2016) found that loose farrowing pens allows for more interactions between sows and their piglets compared to farrowing crates. In terms of stress resilience, piglets reared in farrowing crates compared to farrowing pens, had reduced escape behaviour during routine husbandry procedures early in life, reduced fear behaviour to human and novel stimuli pre- and post-weaning, reduced injuries at two weeks of age and post-weaning and reduced cortisol concentrations post-weaning and post-isolation later in life (Hayes et al., 2021; Lucas, 2022). Reduced maternal contact through imposed gradual weaning reduced piglet stress at weaning as measured by reduced cortisol response (de Ruyter et al., 2017; Turpin et al., 2017; Escribano et al., 2019) and improved post-weaning performance by increasing growth and feed intake in the week following weaning (Turpin et al., 2017). However, in pigs, maternal contact is thought to stem from nose-to-nose contact between the sow and her piglets (Portele et al., 2019). A limitation of comparing farrowing pens to crates is that it cannot be determined if any treatment effects observed are due to increased sow interaction or due to having a more complex housing environment for the piglets. Considering that in Australia, sows and their litters are commonly housed in farrowing crates for the duration of lactation, it is important to investigate the impact of maternal contact within a crate system. Research on the topic of farrowing housing has also been reported in a recent review (Lucas et al., 2023). While the methods of the previous research have implied that maternal contact is important for the development of stress resilience, directly determining if obstructing nose-to-nose contact between sows and piglets affects development of stress resilience is yet to be investigated.
Since the 1980s, evidence has accumulated about the effects of human interactions on the fear behaviour and physiological stress responses of farm animals. Handling studies indicate that negative or aversive handling of pigs, imposed briefly but regularly, will increase the fear of humans and reduce the growth, feed conversion efficiency, reproduction and health of these animals [reviewed in Hemsworth et al. (2015)]. Alternatively, brief but regular positive handling, particularly during early life, is effective in reducing fear responses to humans later in life (Hemsworth et al., 1986; Hemsworth and Barnett, 1992). In addition, some authors have proposed that positive human contact may provide a source of enrichment that confers stress resilience for farm animals in challenging situations (Hemsworth, 2003; Hemsworth, 2018; Rault et al., 2020).
There is limited evidence in pigs that positive human contact encourages an ongoing positive affective state with broader stress resilience. Brief daily positive human contact has been shown to reduce the magnitude of cortisol response resulting from confinement housing of sows (Pedersen et al., 1998). Furthermore, there is evidence that positive human contact during early life is effective at improving stress resilience. Brief daily positive contact of piglets reduced escape behaviour during routine husbandry procedures, fear behaviour to human and novel stimuli pre- and post-weaning, injuries post-weaning and cortisol concentrations post-weaning (Hayes et al., 2021; Lucas, 2022). Muns et al. (2015) also showed that positive human contact of piglets on the first day of life reduced escape behaviour during tail docking on the subsequent day. Finally, forced positive handling of piglets also reduced fear of humans, increased play behaviour and reduced vocalisations in a novel arena, and increased body weight at 12 weeks of age (de Oliveira et al., 2015; Zupan et al., 2016).
The aim of this experiment was to examine the effects of maternal contact in farrowing crates and positive human contact in early life on the behavioural and physiological stress responses of piglets to routine stressors under commercial piggery conditions. It was hypothesised that piglets that received maternal contact and positive human contact pre-weaning would display decreased responses to well-recognised stressful events and therefore improve growth, mortality and successful weaning.
2 Materials and methods
All animal procedures were conducted with prior institutional ethical approval under and in accordance with the ‘Australian code for the care and use of animals for scientific purposes 8th edition’ (National Health and Medical Research Council: Canberra, 2013) and approved by the PIRSA animal ethics committee (Approval #24-20).
2.1 Animals and management
This experiment was conducted at a large commercial breeder unit in South Australia (SunPork Pty Ltd., Wasleys, SA, Australia) during summer 2021. The breeder unit housed 7,500 sows and their sucking piglets that operated on a continuous farrowing basis with progeny finished offsite. Sows used in this experiment included 98 mixed parity (3.5 s.d. 2.1) Large White x Landrace sows (Camborough 42, PIG AU, Grong Grong NSW, Australia) and their offspring with average litter size 11.7 s.d. 1.1. In total, 1150 piglets were used throughout the experiment. The experiment was conducted within two rooms in the farrowing house. There were three rows within each room and each row had 30 crates.
Apart from treatment application, usual commercial management was applied. In summary, sows entered the farrowing house at approximately day 110 of gestation and were housed in naturally ventilated sheds with a temperature activated dripper system (28°C) used for evaporative cooling. Housing for all sows during parturition and lactation consisted of a standard farrowing crate, 1.8 × 2.4 metres in size, with an uncovered creep area of 0.6 × 1.2 meters heated via lamp for piglets, an ad libitum feeder, two water nipples for the sow and one water nipple for the piglets. Flooring of crates consisted entirely of plastic slatted tiles. Sows were delivered 2 kg of a transition diet twice daily, at 0700 h and 1600 h prior to farrowing, with ad libitum access to a standard lactation diet post farrowing. Sows that had not farrowed by day 115 of gestation were induced to farrow with 0.8ml of a prostaglandin analogue (Juramate, Jurox Animal Health, Rutherford, NSW, Australia) to reduce the farrowing spread as per farm practice. At 24 h of age, piglets were cross fostered within maternal contact treatment (refer to section 2.2.1 Maternal contact treatment) based on the sow’s rearing capacity (functional teat number). At three days of age, piglets underwent processing where piglets were removed from the home crate and placed in a solid sided trolley and were tail docked, orally drenched with coccidiostat (Baycox, Bayer, Auckland, New Zealand), received an intramuscular iron injection (Feron 200 + B12, Bayer, Auckland, New Zealand) and were ear tagged, prior to being placed back into the creep area. Health and welfare checks were conducted twice daily and, if required, sows and piglets were treated with approved medications. Animals not responding to the medication regimen were removed from the experiment. Piglets were weaned from 20 to 26 days of age and piglets weighing less than 4kg were retained in the farrowing house and removed from the experiment. There were 108 piglets removed from the experiment, due to morbidity or mortality and an additional 45 piglets that were not weaned due to low liveweight.
2.2 Experimental design
The experiment was a 2 maternal contact during farrowing/lactation treatment (MC-, MC+) by 2 human contacts during lactation treatment (HC-, HC+) factorial in 5 randomised blocks. Thus, there were 20 experimental units, with one experimental unit of each of the four treatment combinations in each block. Most of the 20 experimental units contained five adjacent sows and their litters, although 2 experimental units contained four adjacent sows and their litters. Three central litters of each experimental unit were selected as focal litters, so as to minimise emotional contagion effects of adjacent sows and litters in a different experimental unit that might have a different treatment combination (Reimert et al., 2013). Two piglets (one male, one female; n=24 per treatment) from focal litters were selected for physiological sampling and four piglets (two male, two female, n=48 per treatment) selected to participate in a behavioural test. Focal piglets were selected at random, however, obvious runts were excluded. Blocking was associated with the spatial arrangement of farrowing crates. Sows were stratified by parity to treatments within blocks.
2.2.1 Maternal contact treatment
The MC+ sows were housed in standard farrowing crates (Figure 1A). For MC- sows, restrictions were put in place two days prior to expected farrowing date. The restrictions were made from 60 × 70 × 0.3 cm poly belt (PolyBelt Australia, Townsville, QLD, Australia) sheets. A sheet was attached near the front of the stall as shown in Figure 1B. The restriction reduced piglet access to the head of the sow while still allowing full piglet access to the udder.
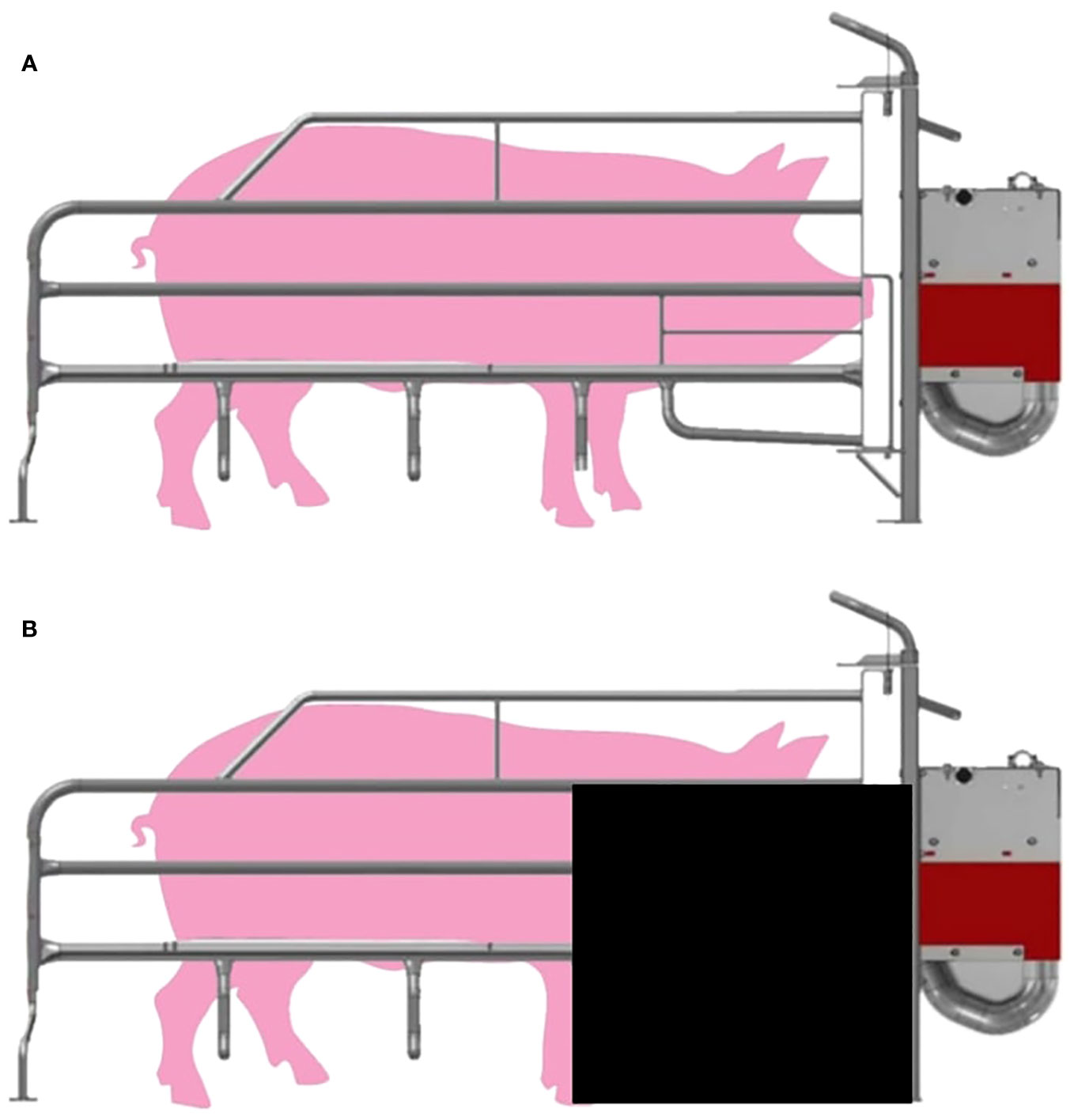
Figure 1 Diagram of (A) sow farrowing crate (MC+) and (B) sow farrowing crate with restriction (MC-) which was made from 60 × 70 × 0.3 cm poly belt sheet attached to the front of the stall.
2.2.2 Human contact treatment
Piglets from the HC- treatment only received routine contact with stock people associated with regular husbandry and management. The HC+ piglets received daily positive human interactions from 2 d of age until weaning. The HC+ treatment involved an experimenter sitting in the creep area of the pen for five minutes and gently patting, stroking, or scratching piglets that approached the experimenter, similar to Lucas (2022). The same interactions were imposed on HC+ piglets if they were sucking at the udder or sleeping at the time of treatment imposition The positive human contact treatment was implemented, at a time between 0700-1400 h, by five female experimenters. The allocation of experimenters to litters was rotated between days, so that the litter became accustomed to multiple people providing positive handling.
2.3 Measurements
2.3.1 Behaviour
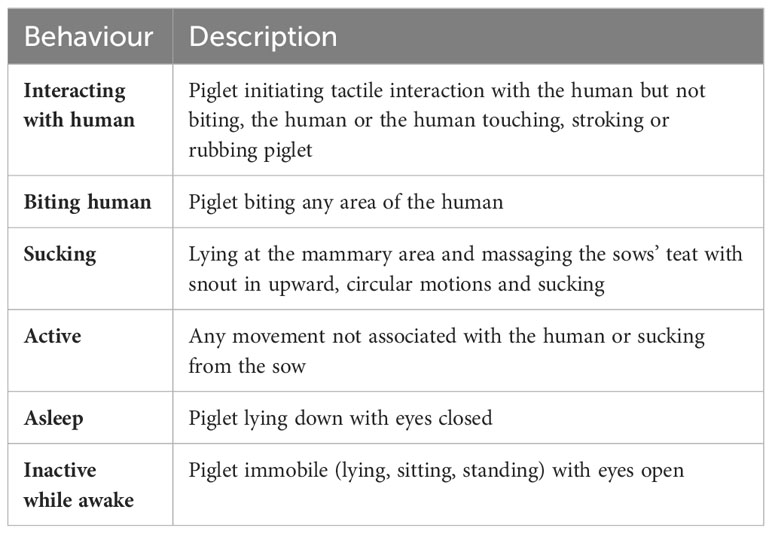
2.3.1.1 Piglet behaviour during positive human contact
While positive human contact was being applied, piglet behaviour was assessed daily in all litters in the HC+ treatment. Instantaneous scan sampling occurred at 1-minute intervals during each 5-minute daily handling bout. Behaviours recorded were interacting with the human, biting the human, sucking, active, asleep and inactive while awake (Table 1). For each litter and each week for 3 weeks, the percentage of time in each behaviour was estimated by averaging over the daily estimates of percentage of time in each behaviour. The daily estimates of percentages were obtained by summing the percentage of piglets exhibiting a behaviour at each scan in the 5-minute period of HC+, and then dividing by the number of scans in the 5-minute period.
2.3.1.2 Behavioural observations the home pen
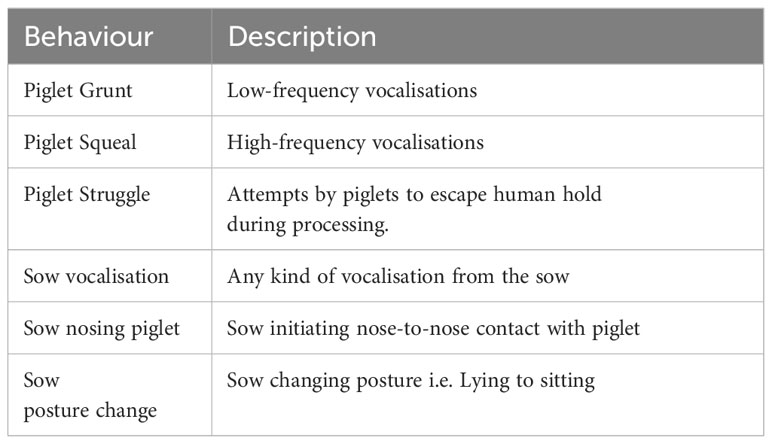
Behavioural observations of piglets and sows occurred in all focal pens over a 4-hour period at approximately 1 week (7.7 s.d. 1.2 d) and 3 weeks (19.7 s.d. 0.5 d) of lactation from 1000h-1400h. Instantaneous scan sampling occurred with 1-minute intervals. GoPro (Hero 4 Black, GoPro Inc, San Mateo, CA, USA) cameras were placed with a top-down view of the crate. Behaviours were assessed using the ethogram in Table 2. The number of piglets in view was calculated from the total number of piglets in the pen minus the number of piglets out of view. The percentage of piglets in view was calculated as the average, over scans, percentage of piglets in view at each scan. The percentage of piglets in view of each behaviour was obtained by summing the percentage of piglets exhibiting a behaviour at each scan and then dividing by the number of scans over the 4-hour period.

Table 2 Ethogram of piglet and sow behaviour in the home pen during lactation, adapted in part from Martin et al. (2015).
2.3.1.3 Piglet and sow behaviour during processing
At piglet processing, behaviours from focal litters and sows were assessed directly by two observers, one scoring sow behaviour and one scoring piglet behaviour (Table 3). The observation period commenced when all piglets were removed from the farrowing crate and concluded when the last piglet was placed back into the creep area.
2.3.1.4 Behaviour during novelty and human test at 18 days
At 18 days of age, 4 piglets (2 males, 2 females) from each focus litter were tested together in a novel arena. The test was conducted for 4 minutes with 1 minute exposure to the following conditions: empty arena, novel object (orange traffic cone) in arena, human hand in arena, and human standing inside arena. The novel object, human hand and human standing were all placed in the same area of the arena (square 9, Figure 2). The human used in the test was familiar to the piglets but wore different overalls and boots compared to their normal attire. The arena measured 2m x 2m x 0.6m with solid sides and contained lines marked on the floor of the arena to divide the arena into nine 0.45m2 squares, with each square numbered (Figure 2). There was also a GoPro (Hero 4 Black, GoPro Inc, San Mateo, CA, USA) camera mounted above the arena to record piglet behaviour. Piglets were transported in a solid sided trolley to a separate building where the test arena was located. Removal from the home crate to the start of the test was standardised to 2 minutes. At the start of the test, all piglets were placed simultaneously in the same square (square 7). Behaviour of piglets were assessed after the test through video footage; however all vocalisations were recorded in real time. Behaviours are outlined in Table 4. All measurements were collected on a litter basis, without individual identification of piglets.
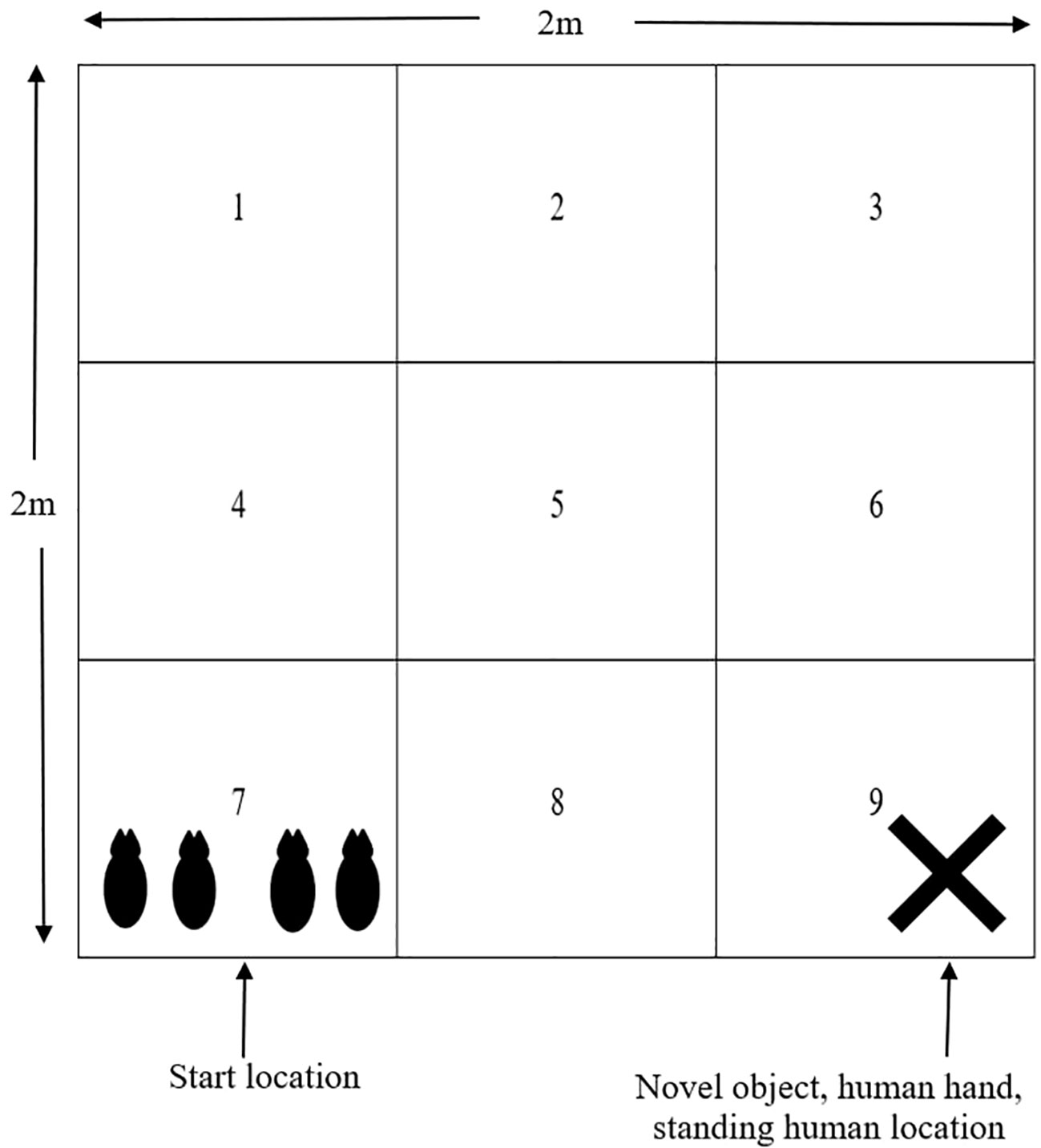
Figure 2 Diagram of the novel arena used at 18 days of age. The arena was divided into 9 0.45m2 squares indicated by the solid lines within the arena. All piglets were placed into square 7 at the start of the test and the novel object, human hand and human were all located in square 9, as shown by the cross.
2.3.2 Physiology
Blood samples were collected from focus piglets one hour after processing. Piglets were taken out of the home crate and restrained for less than 2 minutes for sample collection. Samples were collected using 21G needle and 5mL syringe by jugular venipuncture. Samples were collected into lithium heparin vacutainers (BD Vacutainer, Plymouth, UK) and were kept on ice until they were centrifuged at 1500 × g for 20 minutes. Samples were then aliquoted in duplicate into 0.5ml plasma samples and frozen and stored at -80° C until analysis. When thawed prior to analysis, samples were pooled based on experimental unit. Plasma cortisol concentration was quantified using a commercial radioimmunoassay kit (ImmuChemTM CT cortisol kit, MP Biomedicals, Orangeburg, NY, USA) according to manufacturer’s instructions. Haptoglobin and Immunoglobulin A (IgA) were quantified using a commercially available ELISA kit (CUSABIO pig haptoglobin ELISA kit, CUSABIO Technology LLC, Houston, TX, USA; CUSABIO pig IgA ELISA kit, CUSABIO Technology LLC, Houston, TX, USA) according to manufacturer’s instructions. The average inter-assay CV was 8.20% for cortisol. Inter-assay CV was 10% and the intra-assay CV was 8% for both haptoglobin and IgA.
2.3.3 Performance
Each piglet in all pens was individually weighed at day 3 and prior to weaning using a purpose-built weigh cart that was calibrated to 0.05kg. Individual deaths and removals were recorded once piglets were ear tagged at three days of age. Piglets that failed to wean due to illness, injury or weighing less than 4kg were also recorded. The sum of deaths, removals and failed to wean equated to the total of pigs not weaned.
2.4 Statistical analysis
A single summary value of each analysed measurement was calculated for each experimental unit of 4-5 sows and their litter. Average piglet weights and losses which were calculated from all piglets in the experimental unit. For measurements taken only on a focal pen, the single summary value was an average of the focal pen results for each experimental unit. Prior to statistical analysis, many measurements were transformed, to maintain homoscedastic residuals as means changed and to reduce distributional skewness. In particular, behaviour observations conducted during the positive human contact treatment, at rest in the home pen at 1 and 3 weeks and in the novelty and human tests at 18 days underwent angular transformation and grunts during the novelty and human test at 18 days were log10(y - 8) transformed. Squeals (except for in empty area) were log10 transformed. Squeals in empty arena were log10(y + 0.01) transformed. The percentage of piglets to approach and to enter the same square as the object were angularly transformed. The physiological measures at processing were also log10 transformed. Transformations were empirically determined by examining fitted values versus residual graphs, both prior and after transformations.
Each measurement was analysed using a 5 block, 2 MC by 2 HC factorial, randomised block analysis of variance (ANOVA). The analyses of piglet behaviour during handling were restricted to the HC+ treatment, and thus the only treatment examined was the MC main effect. For measurements that had many experimental units with the same value (many zero values for biting human whilst human contact was being applied, along with several sow behaviours during behavioural observations in the home pen, but also many pigs having the maximum imputed time of 61s for interacting with a human in the arena test), P values were calculated using non-parametric permutation tests of treatments to ‘plots within blocks’ for the usual main effect and interaction F statistics. There were no missing values in any analysis, and no outliers were removed from the analyses.
All analyses were conducted in GenStat for Windows 21st Edition (VSN International, 2022), using the ANOVA directive and the APERMTEST procedure. Data are deemed significant when P < 0.05 and a trend when P < 0.1.
3 Results
Of the 60 reported measurements that were calculated on all four treatment combinations (all measurements except the piglet behaviour measurements conducted on the HC+ piglets during treatment imposition), two had statistically significant maternal contact by human contact interaction at the 5% level and no interaction was statistically significant at the 1% level (Supplementary Table 1). This is less than what would be expected by chance (expected number significant at 5% = 0.05 × 60 = 3; expected number significant at 1% level = 0.6), and thus tables of means are presented as the main effect for maternal contact and the main effect of human contact.
3.1 Piglet behaviour while applying positive human contact
The percentage of piglets interacting with the human, at any time point while the human contact treatment was being applied, regardless of the maternal contact treatment, increased from 2.8% in week 1 to 10.6% in week 2 and then to 29.0% in week 3 (Table 5). The percentage of piglets that were biting the human increased from 0.1% in week 1 to 2.8% in week 3. These increases over time, in the percentages of piglets interacting with, or biting, the human, were associated with corresponding reductions in percentage piglets active, asleep, inactive while awake and sucking. None of the human experimenters observed any avoidance of the piglets when applying the human contact treatment.
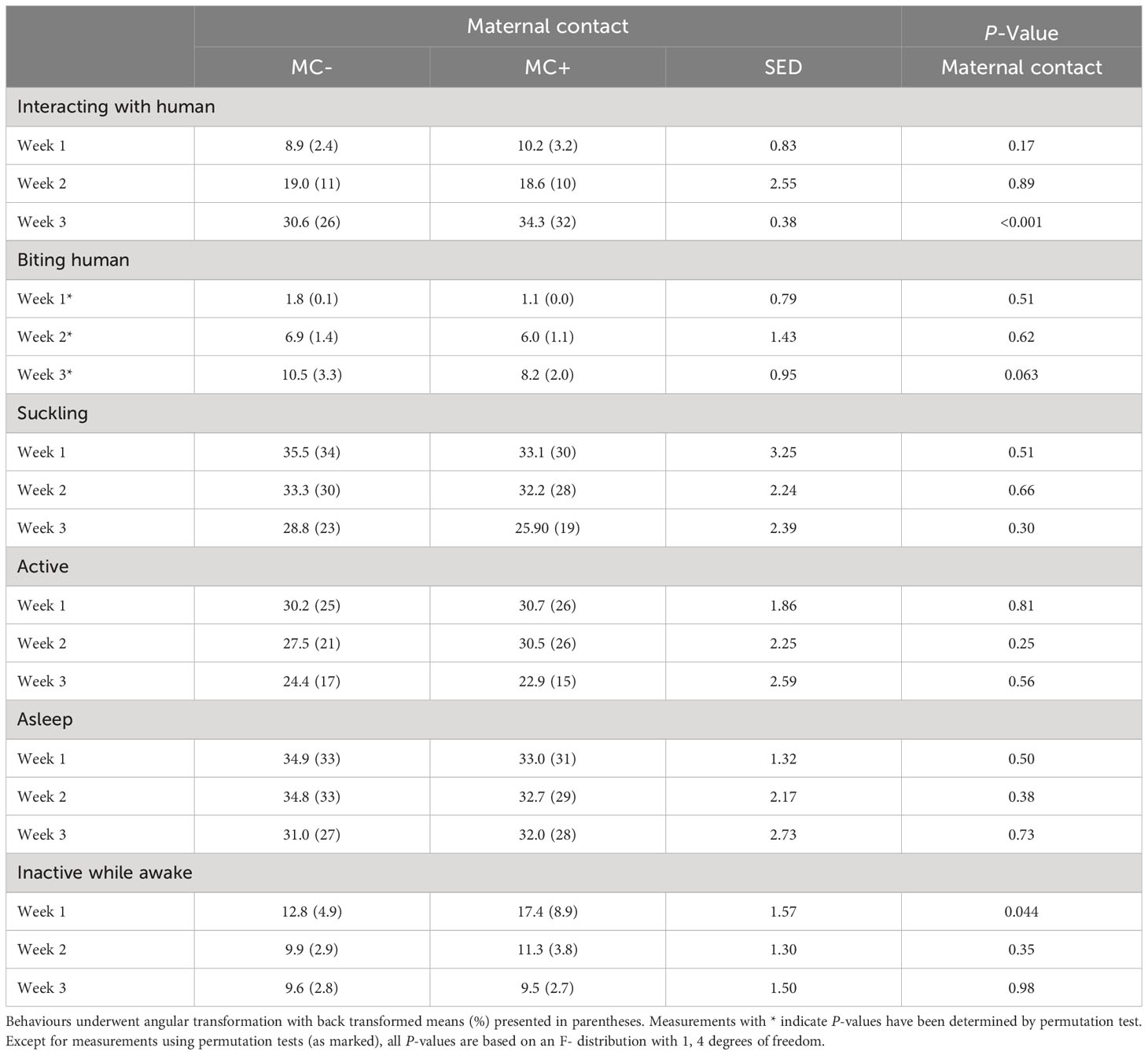
Table 5 Effect of maternal contact on percentage of piglets in each behaviour category while the experimenter was applying the positive human contact treatment averaged over days in each of the three-weeks of the lactation period.
There were no effects of the maternal contact treatment on piglets’ active, asleep, or sucking behaviour at any time point while the human contact treatment was being applied to the MC+ piglets, however, in week 1, there was some evidence that more MC+ piglets were inactive while awake (8.9% vs 4.9%; P = 0.044) compared to MC- piglets (Table 5). Furthermore, in week 3, there was statistical evidence that MC+ piglets spent a higher percentage of time interacting with the human compared to MC- piglets (31.7% vs 25.9%, P < 0.001; Table 5), although the standard error of difference (0.38) on the angular scale was unusually small compared to other measures in Table 5. There was a trend that MC- piglets were more likely to bite the human in week 3 compared to MC+ piglets (3.3% vs 2.0%, P = 0.063).
3.2 Behavioural observations in the home pen
During the first week, MC- piglets displayed less nose-to-nose contact with the sow (P = 0.031; Table 6) than MC+ piglets. There was no human contact treatment effect on piglet behaviour at 1 week of age (P > 0.05). For sows in the first week of lactation, MC- sows had an increase in the incidence of bar biting compared to MC+ treatment (P = 0.023; Table 6). Furthermore, HC+ sows tended to display less bar biting compared to HC- sows (P = 0.097). There was also a tendency for MC+ sows to drink more (P = 0.097). In week 3 about 8% of piglets were not in view in MC- compared to about 4% of piglets not in view in MC+ (P = 0.009; Table 7) although, in both maternal contact treatments, only about 3% of piglets were out of view in week 1 (Table 6). MC- piglets spent less time near the sow while inactive compared to MC+ piglets (P = 0.017). There was also a trend that HC- piglets spent more time initiating nose to nose contact with the sow compared to HC+ piglets (P = 0.078) and HC-piglets displayed less social contact with pen mates (P = 0.090). During the third week, HC- sows expressed more bar biting behaviour compared to HC+ sows (P = 0.043; Table 7). There were no effects of the maternal contact treatments on sow behaviour in week 3 of lactation (P > 0.05).
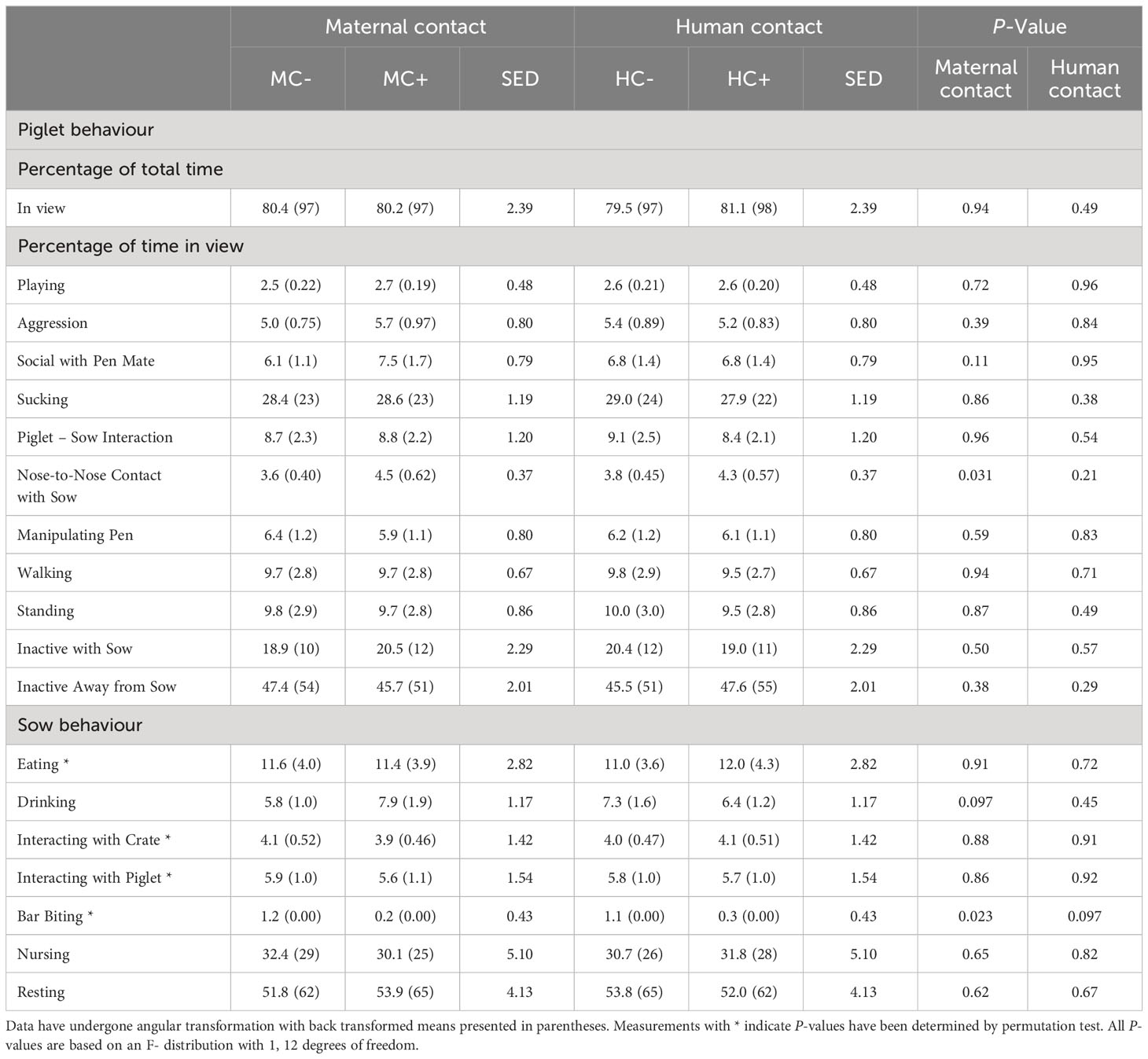
Table 6 Piglet and sow behaviour observed in the home pen during a 4-hour period in week 1 post-farrowing.
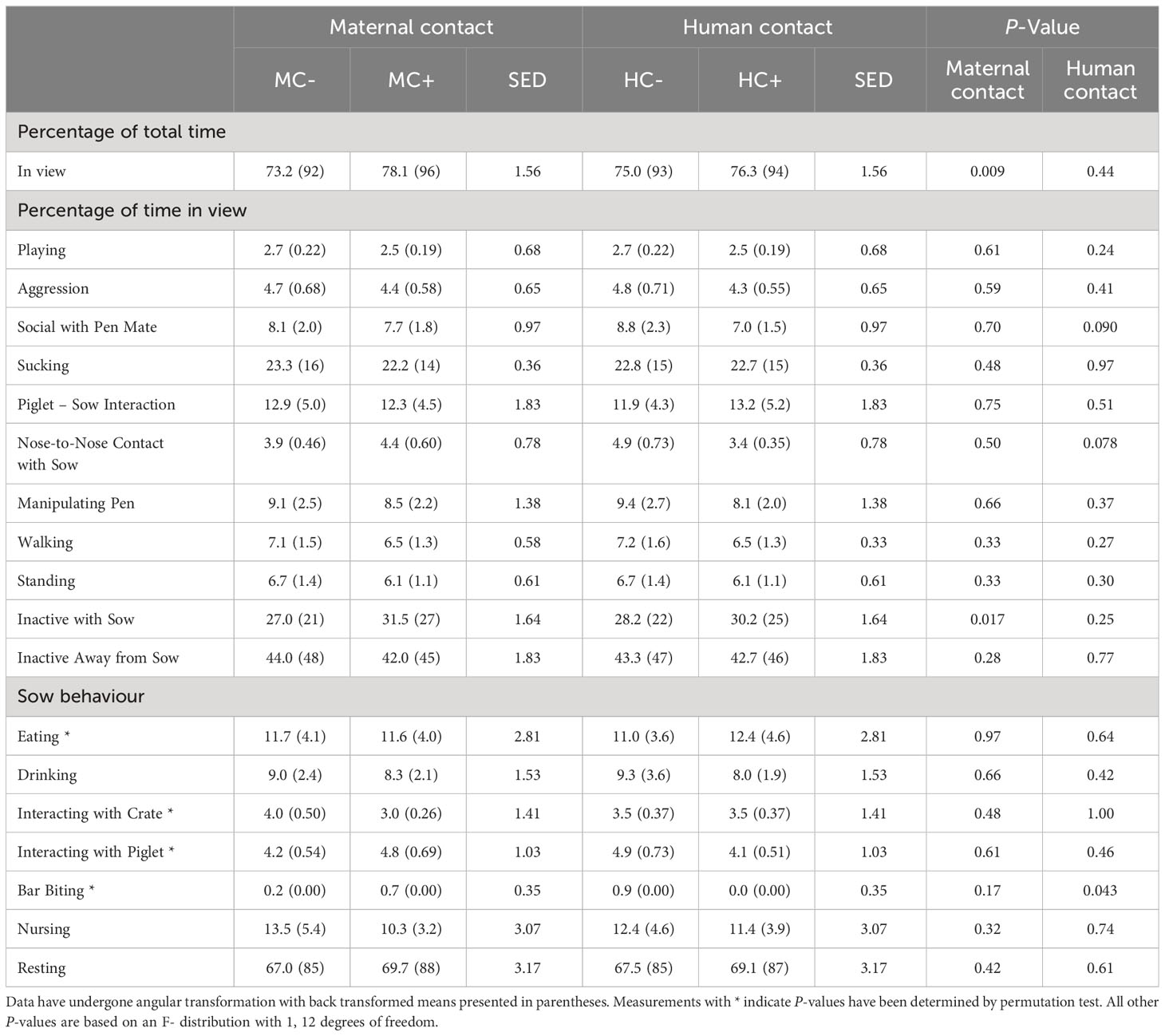
Table 7 Piglet and sow behaviour observed in the home pen during a 4-hour period in week 3 post-farrowing.
3.3 Processing
MC- piglets exhibited approximately a third more squeals than MC+ piglets during piglet processing (33 vs. 24 squeals per litter; sed = 3.1, P = 0.015, Table 8). This response was also observed for MC+ piglets tending to display increased piglet grunts (47 vs. 37 grunts per litter, sed = 5.2; P = 0.063) and piglet struggle attempts (9.4 vs 7.9 struggles per litter, sed = 1.25 P = 0.079). MC+ sows also tended to initiate nose to nose contact with piglets more than MC- sows (2.1 vs. 1.0 attempts per litter, sed = 0.66; P = 0.069). There was no evidence of human contact effects on piglet or sow behaviour during processing (P > 0.05).
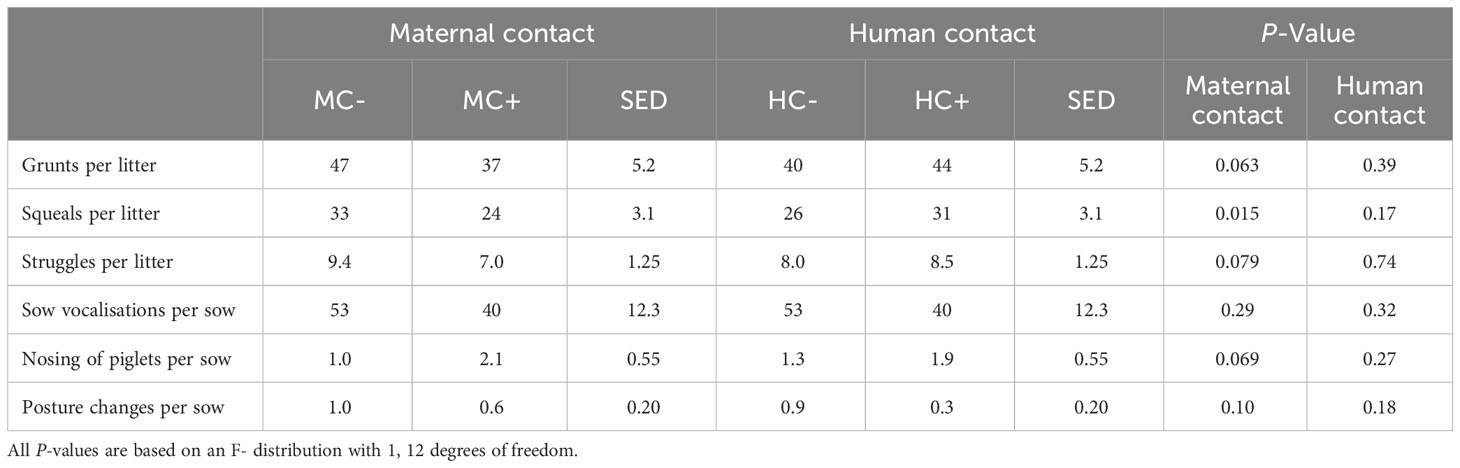
Table 8 Effect of maternal contact (MC) and human contact (HC) on piglet and sow behaviours during piglet processing at three days post-farrowing.
Plasma cortisol concentration one hour after processing increased in MC- piglets by 61% (95% confidence interval = (15%, 125%), P = 0.009, Table 9) compared to MC+ piglets. There was no human contact treatment effect (P > 0.05) on plasma cortisol concentration. There was no evidence of any human contact or maternal contact treatment effects on haptoglobin concentrations (P > 0.05 for all main effects), however HC+ piglets tended (P = 0.085) to have a lower IgA concentration compared to HC- piglets.
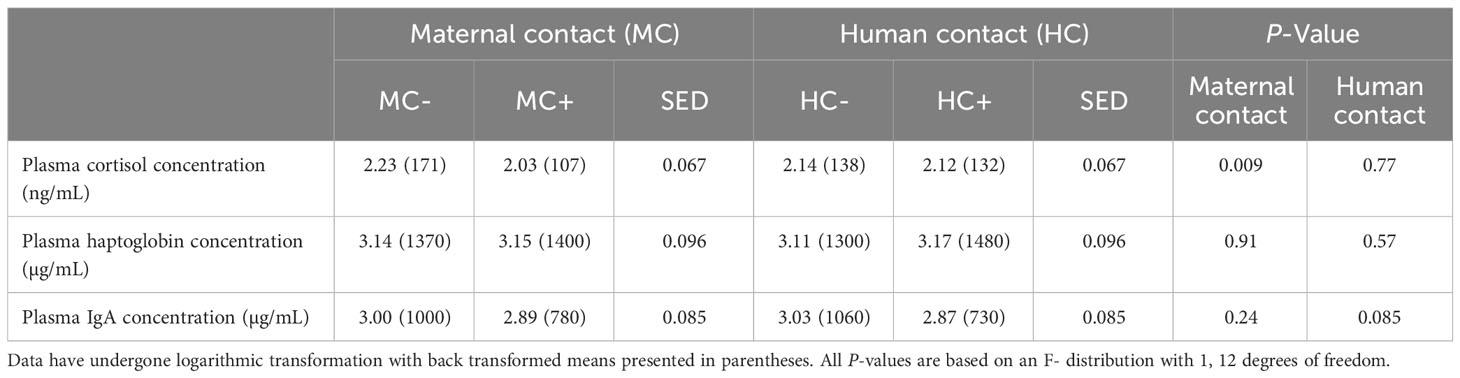
Table 9 Effect of maternal contact (MC) and human contact (HC) on piglet physiology one hour post processing.
3.4 Novelty and human test at 18 days
The number of lines crossed, number of grunts and number of squeals decreased as the test progressed (Figure 3). Piglets from the HC+ treatment were more active (P = 0.014) and had more vocalisations which included both grunts (P = 0.008) and squeals (P = 0.031), than HC- in an empty arena (Figure 3). There was no evidence (P > 0.05) of human contact effects on vocalisations and activity in response to the novel object, human hand and human presence.
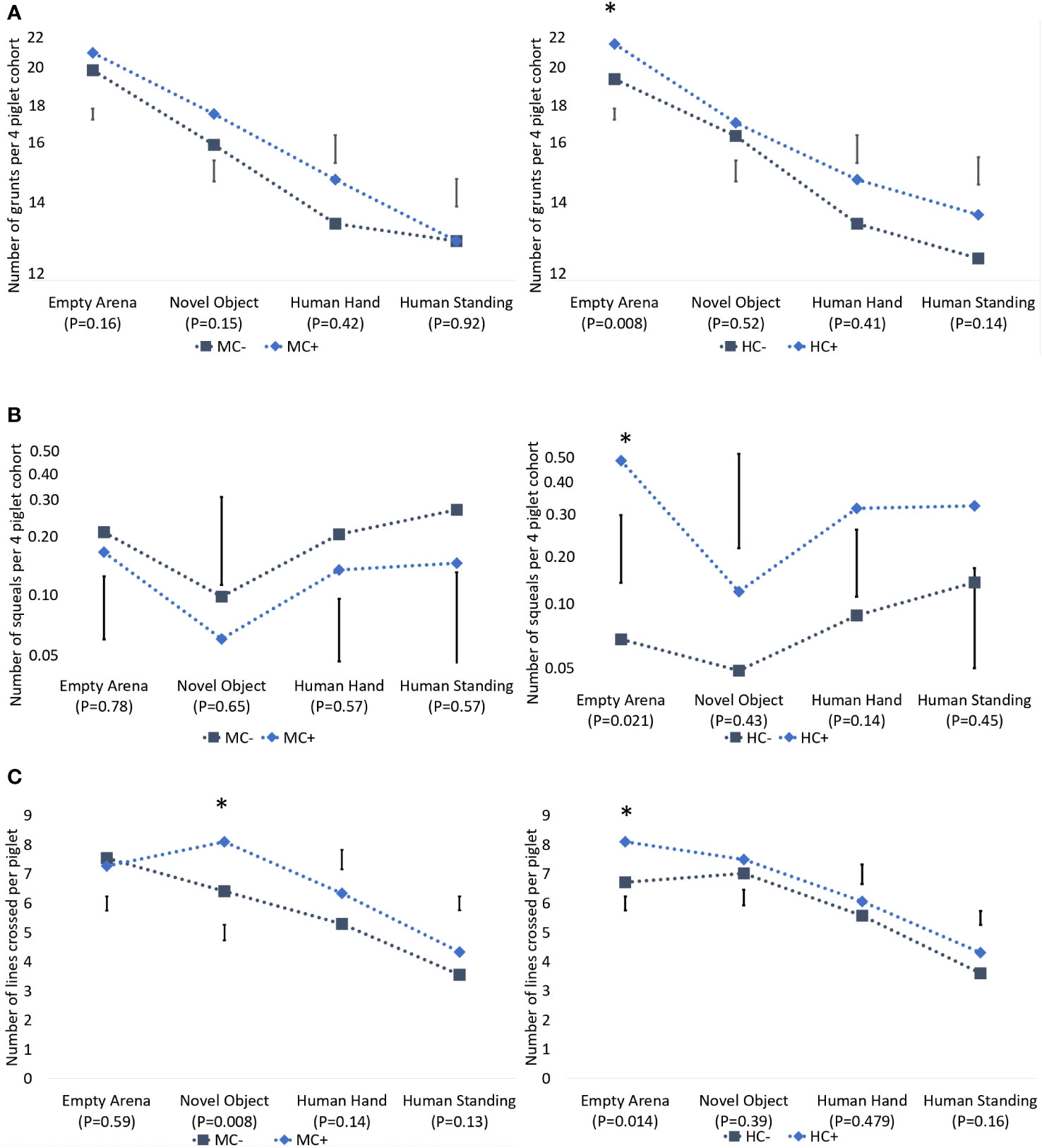
Figure 3 Effect of maternal contact (MC) and human contact (HC) on grunts per litter (A), squeals per litter (B) and number of lines crossed per piglet (C) during the novelty and human test at 18 days of age. Grunts were log (y - 8) transformed and squeals were log(y + 0.01) transformed, and thus the y-axes are on log(y - 8) and log(y + 0.01) scales, respectively. Error bars denote standard error of difference. The * indicates P < 0.05. All P-values are based on an F- distribution with 1, 12 degrees of freedom.
The only observed maternal contact effect on these piglet behaviour measurements was during exposure to the novel object. Piglets from the MC+ treatment crossed more lines than the MC- piglets (8.1 vs 6.4 lines crossed per pig; sed = 0.54; P = 0.008; Figure 3), but there was no difference (P > 0.05) in vocalisations (grunts or squeals). There were no significant (P > 0.05) differences due to maternal contact in these measurements when piglets were exposed to an empty arena, to a human hand and to a human standing in the arena.
With respect to approach behaviour, MC+ piglets were more likely to approach the novel object than the MC- piglets (99% vs 92%; P = 0.049; Table 10). There were no significant (P > 0.05) maternal contact effects when piglets were exposed to a human hand and to a human standing in the arena. When exposed to human presence, about 90% of HC+ piglets approached the standing human despite only about two thirds of HC- piglets approaching (91% vs 67%; P = 0.006; Table 10). No other human contact effects (P > 0.05) were detected on these measurements.
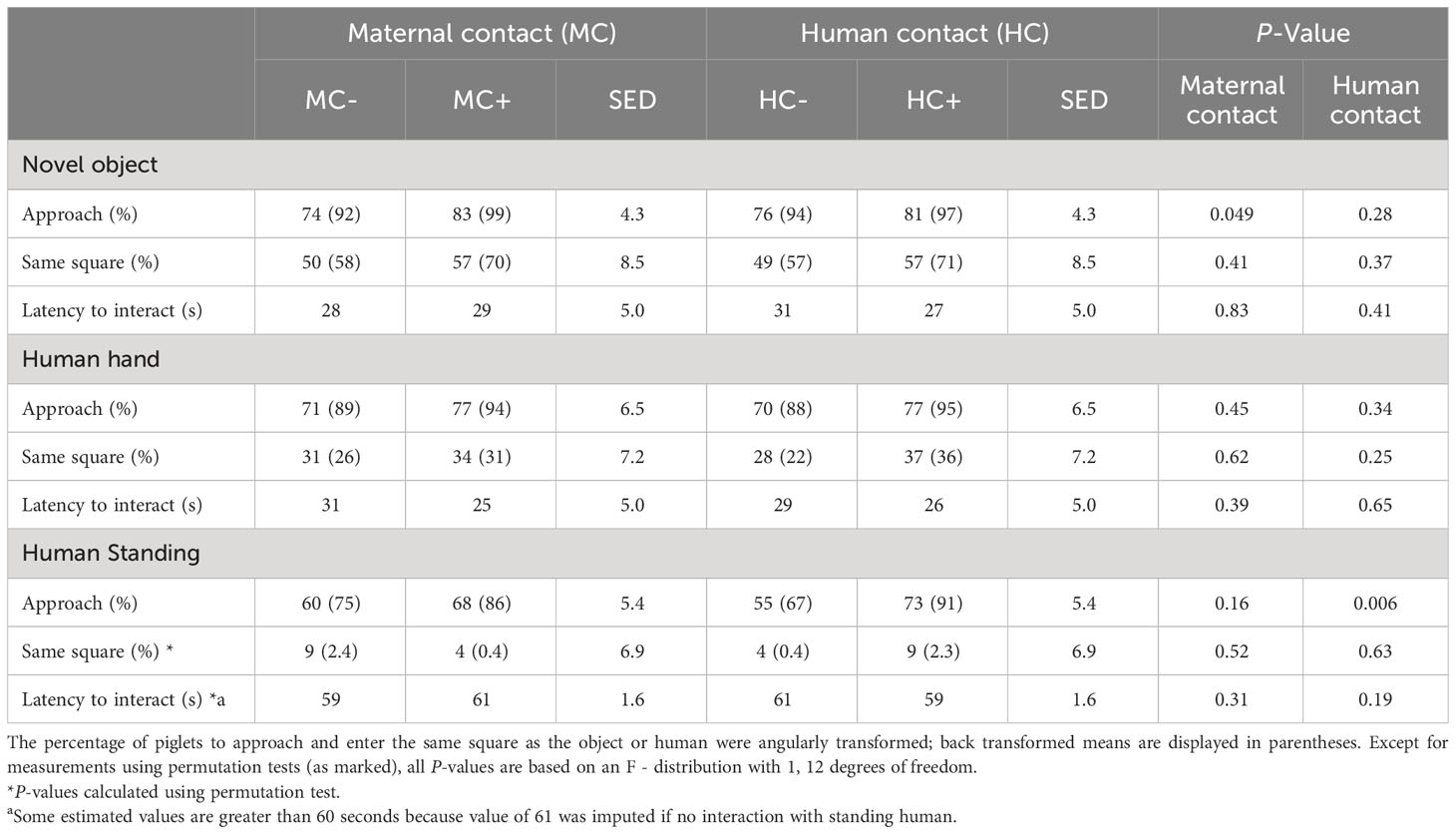
Table 10 Effect of human contact (HC) and maternal contact (MC) on behavioural responses related to level of interaction with novelty and humans at 18 days of age.
3.5 Performance
The total number of pigs not weaned (either due to death, removal or failed 4kg weight limit) was 5% greater in MC- than in MC+ (P = 0.022), although the statistical analysis could not determine which components (death, removal or failed to wean; all with P > 0.05) contributed to this (Table 11). The MC- piglets tended to have reduced weaning weights compared to MC+ piglets (difference = -0.35 kg, sed = 0.163; P = 0.055; Table 11). There was no significant (P > 0.05) effect of maternal contact on piglet weight at 3 d of age, and no significant (P > 0.05) effects of human contact on piglet weight at day three or weaning, and losses from day three to weaning.
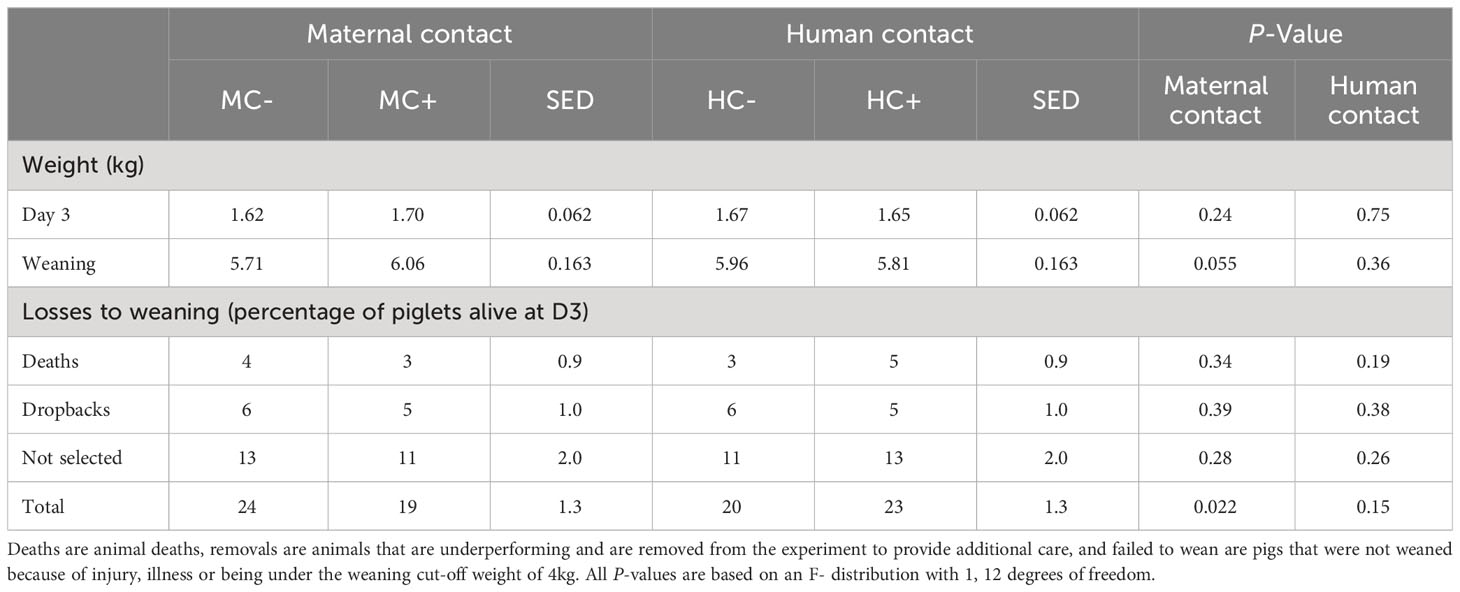
Table 11 Effect of maternal contact (MC) and human contact (HC) on piglet weight at three days of age and weaning along with piglet losses.
4 Discussion
This experiment tested the impact of maternal contact and positive human contact on the early development of stress resilience in piglets. In early lactation, piglets receiving increased maternal contact displayed less squeals during processing and lower plasma cortisol concentration one hour after processing compared to piglets that received reduced maternal contact. Later in lactation, however, there were effects of both increased maternal contact and positive human contact that support the development of stress resilience in piglets. Increased maternal contact appeared to increase explorative behaviour (as indicated by increased lines crossed) in the presence of a novel object and reduce fear in piglets to a novel object (as indicated by increased approaches to the object). Based on approach behaviour, increased maternal contact reduced fear of novelty while positive human contact reduced fear of a standing human. Performance of piglets to weaning was improved with increased maternal contact. Thus, these findings together with previous studies support our hypothesis that increased maternal contact and positive human contact can improve stress resilience during stressful events during lactation.
The restricted maternal contact treatment imposed in this experiment was constructed, as much as possible, to differ from the MC+ only in the level of restriction of maternal bonding that could occur. Nose-to nose contact has previously been identified to facilitate the sow-piglet bond (Portele et al., 2019). Most of the previous research has investigated maternal care within pen-based systems which allow for greater variety of behaviour and freedom of interactions compared to farrowing crates later in the lactation period (Singh et al., 2017). Loose housing farrowing systems provide greater environmental and social complexity to piglets than farrowing crates. This extra complexity can confound the effects of maternal contact on the development of stress resilience. Piglets born to sows in loose housing systems, with greater maternal contact have previously been found to display changes in behavioural and physiological responses to stressors consistent with decreased stress resilience compared to farrowing crates with less maternal contact (Lucas, 2022). These authors explain that the observed decrease in stress resilience might be caused by differences in environmental complexity, rather than with changes in maternal bond. With the current experiment, we successfully reduced the nose-to-nose contact during early lactation while maintaining all other aspects of routine animal housing and husbandry. Bar biting in sows is considered an abnormal behaviour and may develop in response to stressful conditions (Mason, 1991; Hemsworth, 2018). The observations on MC- sows in the present experiment indicate an increase in bar biting in week 1 (4-fold increase relative to MC+ sows, although still occurring less than 0.1% of the time observed) but not in week 3. This finding suggests that the MC- treatment may have been stressful for some sows and thus there is the possibility that this may have contributed to the behaviour and stress physiology of the MC- piglets.
Rault et al. (2019) reported that electrocorticogram (EEG) changes in pigs have been linked to behavioural shifts indicative of a positive welfare state during belly rubbing, suggesting that pigs viewed these types of interactions with humans as positive. It has been suggested that human contact of a positive nature during early life, up to weaning, has been shown to have persistent effects of fear of humans in many animals, including pigs (Hemsworth, 2018). What potentially makes this early life period so effective is that pigs may initially view the contact as a stressor that they learn to overcome and therefore build resilience. Muns et al. (2015) conducted positive human contact in conjunction with sucking bouts to condition the piglet to an already positive event. In this study, the human contact was successful in reducing struggle attempts in response to the processing event at three days of age, however, this method of positive human contact is difficult to replicate in a large, commercial production setting. In agreement with de Oliveira et al. (2019), we have shown that piglets become accustomed to positive human contact over time as the number of piglets interacting with the human increased 100 fold from early to late lactation.
During the third week of positive human contact, the maternal effect was statistically significant at the 0.1% level (P < 0.001) for increasing interaction with the human. However, this was associated with a much lower standard error of difference than any other measurement and the difference between angularly transformed means of the maternal effect at week 3 was insufficient to obtain statistical significance at the 5% level if a typical standard error of difference had been used. This indicates caution is needed before interpreting evidence of a maternal contact effect on interaction with humans at week 3. However, it still indicates that MC- piglets become less responsive to handling, potentially due to the stress of reduced contact with the sow. The maternal contact treatment was only significant at the 5% level on one of the other measurements. Overall, evidence that maternal contact affected piglet behaviour while the positive contact treatment was being applied, was weak.
Other than time in view, there was little evidence of behavioural differences between the treatments when litters were observed in the home pen. We expected that there may be changes in play and social behaviour as previous research has shown a link between improved stress resilience and play behaviour (Spinka et al., 2001). Play behaviours in particular are generally displayed when all other needs are met when a pig is in a positive welfare state (Hemsworth et al., 2015). Other studies have shown that maternal contact increases play behaviour in piglets, however most of this involved using loose housing farrowing systems which naturally have a more complex environment for piglets to explore which increases play and explorative behaviours when in the home pen (Martin et al., 2015; Lucas, 2022). In the current experiment, only a very small percentage of piglets were engaged in play, or social behaviour, potentially indicating that environmental complexity is more important for play and social behaviour to develop, and farrowing crates might be insufficiently complex to be conducive for piglet play in any treatment.
Animal vocalisations have recently been suggested to be used to indicate positive welfare states, particularly in farm animals (Laurijs et al., 2021). In pigs, vocalisations can be categorised as low or high frequency and can be further divided by modulated, stable or tonal (Tallet et al., 2013). Grunts are generally considered to be low frequency vocalisations and can occur when eating, communicating, exploring, or during negative situations that have similar arousal levels as positive situations (Algers and Jensen, 1985; Friel et al., 2019). Squeals and screams are both high frequency vocalisations and are generally expressed when pigs are experiencing pain or social isolation (Tallet et al., 2013). In the present experiment, piglets that received restricted maternal contact had an increase in squeals at processing. This is in accord with reduced maternal contact increasing the aversiveness of the piglet to processing at three days of age. During the empty arena stage of the novelty and human tests at 18 days, piglets that received positive human contact had increased grunts and squeals, admittedly at a very low level of squeals. The squeals during the test may be associated with arousal or excitement to the new environment, as also observed by Marchant et al. (2001), rather than being associated with a negative or stressful situation. Recording frequency and duration of vocalisations would allow for further analysis to categorise piglet vocalisations to help understand the welfare state of the pig (Leliveld et al., 2016).
Husbandry procedures, such as piglet processing are performed to provide the pig with the best outcome later however these procedures are likely to elicit physiological and behavioural indicators of pain (Morrison and Hemsworth, 2020; Prunier et al., 2021). In the current experiment, maternal contact was successful in reducing squealing during processing and plasma cortisol concentration post processing, compared to piglets that had restricted maternal contact. This, together with the reduction of grunts and squeals with reduced maternal contact, may be evidence of the importance of nose-to-nose contact with the sow in developing stress resilience in young piglets. Caution is required when interpreting the cortisol results since these were taken on piglets at a single timepoint at rest or following a behavioural test. Previous experiments investigating positive human contact and the impact on piglet behaviour during processing within the first few days of life have found that positively handled piglets displayed decreased struggle attempts (Muns et al., 2015; Hayes et al., 2021) but we found no effect of positive handling on the behavioural and physiological responses of piglets to processing. Fewer than 3% of piglets engaged with the human during the first week in the positive human contact treatment in the current experiment, and thus it is unlikely there was sufficient exposure to humans during this early time frame to materially impact stress resilience to the processing at day. In contrast, Muns et al. (2015) stroked piglets during suckling events which may have provided piglets the opportunity to associate the positive event of feeding with human interaction at a very young age. However, this method of positive human contact is difficult to replicate in a large, production setting. Differences in response, possibly associated with subtle differences in husbandry and management, indicate that there is still a lot to learn about the mechanism of positive handling on stress resilience.
Arena tests are well known tools for assessing animal emotions and welfare. Exploration and locomotion in open field tests are often measured by number of lines crossed and can be useful to indicate anxiety whereas latency to approach and interact with novel objects or humans often indicate fear responses (Murphy et al., 2014). In the present experiment, piglets with restricted maternal contact were less likely to approach the object, indicating an increased fear response. Nearly every tested piglet in MC+ approached the novel object (back-transformed mean was 99%), while about one in twelve piglets in MC- did not approach the object (back-transformed mean for approach was 92%). This indicates more fear of novelty for piglets with restricted maternal contact.
Positive human contact increased the number of lines crossed in the first minute of the empty unfamiliar arena. Care is required in interpreting lines crossed in a novel arena test. While well validated in some farm animal species, open field tests are less well validated in pigs [reviewed in Forkman et al. (2007)]. Based on squares entered in the novel arena test, Donald et al. (2011) reported that azaperone-treated pigs appeared to be less fearful than saline-treated controls (Donald et al., 2011). In contrast, activity in the arena was found to be unaffected by social manipulation and was reduced in pigs exposed to the arena a second time. Thus, increased activity by the HC+ piglets in the empty arena in the present study may be indicative of reduced fear of novel environments.
Positive human contact piglets were also more likely to approach the standing human. However, it was surprising there was no human contact treatment effect on entering the same square as the human and latency to physically interact with the human hand or standing human. Only two piglets throughout the test touched the standing human. It was expected that piglets that had previously had positive human interactions would be more likely to interact with the human. In two similar studies, piglets that received positive human contact were faster to approach and interact with both the hand and the standing human (Hayes et al., 2021; Lucas, 2022). However, the arena used in the two previous studies was smaller than the one used in the current experiment which may have kept the piglets more focused on the novelty of the arena. Even though the arena test is an artificial test of behavioural responses to novelty and humans, pigs in commercial settings are exposed to new environments and interact with humans daily, and therefore our results indicate that both maternal contact and positive human contact aid stress resilience. However, a better understanding of human contact that is rewarding for piglets as well as the longer-term effects of positive handling on fear in pigs are required.
Piglets receiving increased maternal contact displayed evidence of improved stress resilience measured at processing and during the arena test, and this improved stress resilience may have positively improved performance. The trend in decreased piglet weaning weight in MC- piglets is likely stronger than observed as piglets that failed to wean due to being smaller than 4kg were not given a weaning weight. There was initial concern that the restricted maternal contact may have inhibited colostrum and milk intake, but the fact that piglets were not different in weight at day three and there was no difference in sucking behaviour would discount this notion. One mechanism that could explain the improved performance is that stress is known to stimulate an immune response which decreases the amount of nutrition that can be absorbed in the gut, resulting in poor growth and health (Moeser et al., 2007). While there was no difference in IgA or haptoglobin at three days of age that would indicate this, it would be valuable to see if there is an impact later in lactation to explain the improved performance. There was no evidence that positive human contact affected productivity in in the current experiment. While the piglets may differ in their stress responses to processing and humans, the HC- piglets may not have been physiologically stressed by the routine contact with stockpeople managing them to the extent that they did not experience discernible differences in growth performance or mortality compared to HC+ piglets.
In conclusion, this experiment provides evidence that both maternal contact and positive human contact may affect stress resilience, but in different ways. Restricting maternal contact may reduce stress resilience in piglets, as seen by increased squeals and increased plasma cortisol concentration around processing and reduced number of piglets successfully weaned. Positive human contact may reduce fear of both novelty and a standing human, which is supported by previous research.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The animal studies were approved by Department of Primary Industries and Regions, South Australia (PIRSA). The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent was obtained from the owners for the participation of their animals in this study.
Author contributions
KT: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Writing – original draft. JS: Conceptualization, Investigation, Methodology, Project administration, Writing – review & editing. KP: Conceptualization, Investigation, Methodology, Project administration, Supervision, Writing – review & editing. DD’S: Supervision, Writing – review & editing. KB: Conceptualization, Formal Analysis, Methodology, Writing – review & editing. PH: Conceptualization, Funding acquisition, Methodology, Supervision, Writing – review & editing. AT: Conceptualization, Funding acquisition, Methodology, Supervision, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This research was supported in part by the Australian Research Council (LP180100218) and Australasian Pork Research Institute Limited. The funder was not involved in the study design, collection, analysis, interpretation of data, the writing of this article or the decision to submit it for publication.
Acknowledgments
The authors would like to thank Jess Zemitis, Dannielle Glencorse, Serena Barnes and Gemma Balog for their technical assistance, Megan Lucas, Lauren Hemsworth and Rebecca Morrison for their input and the farm staff at SunPork.
Conflict of interest
Authors JS, KP and DD'S were employed by the company SunPork Group.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fanim.2023.1289518/full#supplementary-material
References
Algers B., Jensen P. (1985). Communication during suckling in the domestic pig. Effects of continuous noise. Appl. Anim. Behav. Sci. 14 (1), 49–61. doi: 10.1016/0168-1591(85)90037-1
Chidgey K. L., Morel P. C. H., Stafford K. J., Barugh I. W. (2016). Observations of sows and piglets housed in farrowing pens with temporary crating or farrowing crates on a commercial farm. Appl. Anim. Behav. Sci. 176, 12–18. doi: 10.1016/j.applanim.2016.01.004
Colditz I. G., Hine B. C. (2016). Resilience in farm animals: biology, management, breeding and implications for animal welfare. Anim. Production Sci. 56 (12), 1961–1983. doi: 10.1071/AN15297
Coutellier L., Friedrich A. C., Failing K., Würbel H. (2008). Variations in the postnatal maternal environment in mice: effects on maternal behaviour and behavioural and endocrine responses in the adult offspring. Physiol. Behav. 93 (1-2), 395–407. doi: 10.1016/j.physbeh.2007.09.008
de Oliveira D., Keeling L. J., Paranhos da Costa M. J. R. (2019). Individual variation over time in piglet´s reactions to early handling and its association to weight gain. Appl. Anim. Behav. Sci. 215, 7–12. doi: 10.1016/j.applanim.2019.04.005
de Oliveira D., Paranhos Da Costa M. J. R., Zupan M., Rehn T., Keeling L. J. (2015). Early human handling in non-weaned piglets: Effects on behaviour and body weight. Appl. Anim. Behav. Sci. 164, 56–63. doi: 10.1016/j.applanim.2015.01.002
de Ruyter E. M., van Wetter W. H. E. J., Lines D. S., Plush K. J. (2017). Gradually reducing sow contact in lactation is beneficial for piglet welfare around weaning. Appl. Anim. Behav. Sci. 193, 43–50. doi: 10.1016/j.applanim.2017.03.011
Donald R. D., Healy S. D., Lawrence A. B., Rutherford K. M. D. (2011). Emotionality in growing pigs: Is the open field a valid test? Physiol. Behav. 104, 906–913. doi: 10.1016/j.physbeh.2011.05.031/j.physbeh.2011.05.031
Escribano D., Ko H.-L., Chong Q., Llonch L., Manteca X., Llonch P. (2019). Salivary biomarkers to monitor stress due to aggression after weaning in piglets. Res. Veterinary Sci. 123, 178–183. doi: 10.1016/j.rvsc.2019.01.014
Forkman B., Boissy A., Meunier-Salaün M. C., Canali E., Jones R. B. (2007). A critical review of fear tests used on cattle, pigs, sheep, poultry and horses. Physiol. Behav. 92 (3), 340–374. doi: 10.1016/j.physbeh.2007.03.016
Friel M., Kunc H. P., Griffin K., Asher L., Collins L. M. (2019). Positive and negative contexts predict duration of pig vocalisations. Sci. Rep. 9 (1), 1–7. doi: 10.1038/s41598-019-38514-w
Hayes M. E., Hemsworth L. M., Morrison R. S., Tilbrook A. J., Hemsworth P. H. (2021). Positive human contact and housing systems impact the responses of piglets to various stressors. Animals 11 (6), 1619. doi: 10.3390/ani11061619
Hemsworth P. H. (2003). Human–animal interactions in livestock production. Appl. Anim. Behav. Sci. 81, 185–198. doi: 10.1016/S0168-1591(02)00280-0
Hemsworth P. H. (2018). Key determinants of pig welfare: Implications of animal management and housing design on livestock welfare. Anim. Production Sci. 58 (8), 1375–1386. doi: 10.1071/AN17897
Hemsworth P. H., Barnett J. L. (1992). The effects of early contact with humans on the subsequent level of fear of humans in pigs. Appl. Anim. Behav. Sci. 35 (1), 83–90. doi: 10.1016/0168-1591(92)90018-7
Hemsworth P. H., Barnett J. L., Hansen C., Gonyou H. W. (1986). The influence of early contact with humans on subsequent behavioural response of pigs to humans. Appl. Anim. Behav. Sci. 15 (1), 55–63. doi: 10.1016/0168-1591(86)90022-5
Hemsworth P. H., Mellor D. J., Cronin G. M., Tilbrook A. J. (2015). Scientific assessment of animal welfare. New Z. Veterinary J. 63 (1), 24–30. doi: 10.1080/00480169.2014.966167
Laurijs K. A., Briefer E. F., Reimert I., Webb L. E. (2021). Vocalisations in farm animals: A step towards positive welfare assessment. Appl. Anim. Behav. Sci. 236, 105264. doi: 10.1016/j.applanim.2021.105264
Leliveld L. M. C., Düpjan S., Tuchscherer A., Puppe B. (2016). Behavioural and physiological measures indicate subtle variations in the emotional valence of young pigs. Physiol. Behav. 157, 116–124. doi: 10.1016/j.physbeh.2016.02.002
Levine S. (2002). Regulation of the hypothalamic-pituitary-adrenal axis in the neonatal rat: The role of maternal behavior. Neurotoxicity Res. 4 (5-6), 557–564. doi: 10.1080/10298420290030569
Lucas M. E. (2022). Positive human-Animal Interactions, Early Life experiences and Stress Resilience in Pigs (The University of Melbourne).
Lucas M. E., Hemsworth L. M., Hemsworth P. H. (2023). Review: Early life piglet experiences and impacts on immediate and longer-term adaptability. animal 100889. doi: 10.1016/j.animal.2023.100889
Lyons D. M., Parker K. J., Katz M., Schatzberg A. F. (2009). Developmental cascades linking stress inoculation, arousal regulation, and resilience. Front. Behav. Neurosci. 3 (32). doi: 10.3389/neuro.08.032.2009
Marchant J. N., Whittaker X., Broom D. M. (2001). Vocalisations of the adult female domestic pig during a standard human approach test and their relationships with behavioural and heart rate measures. Appl. Anim. Behav. Sci. 72 (1), 23–39. doi: 10.1016/S0168-1591(00)00190-8
Martin J. E., Ison S. H., Baxter E. M. (2015). The influence of neonatal environment on piglet play behaviour and post-weaning social and cognitive development. Appl. Anim. Behav. Sci. 163, 69–79. doi: 10.1016/j.applanim.2014.11.022
Mason G.J. (1991). Stereotypies: a critical review. Animal Behaviour 41 (6), 1015–1037. doi: 10.1016/S0003-3472(05)80640-2
McEwen B. S. (2007). Physiology and neurobiology of stress and adaptation: Central role of the brain. Psycological Rev. 87 (3), 873–904. doi: 10.1152/physrev.00041.2006
Moeser A. J., Ryan K. A., Nighot P. K., Blikslager A. T. (2007). Gastrointestinal dysfunction induced by early weaning is attenuated by delayed weaning and mast cell blockade in pigs. Am. J. Physiol. - Gastrointestinal Liver Physiol. 293 (2), G413–G421. doi: 10.1152/ajpgi.00304.2006
Morrison R., Hemsworth P. (2020). Tail docking of piglets 1: Stress response of piglets to tail docking. Animals 10 (1701), 1–10. doi: 10.3390/ani10091701
Muns R., Rault J.-L., Hemsworth P. (2015). Positive human contact on the first day of life alters the piglet's behavioural response to humans and husbandry practices. Physiol. Behav. 151, 162–167. doi: 10.1016/j.physbeh.2015.06.030
Murphy E., Nordquist R. E., van der Staay F. J. (2014). A review of behavioural methods to study emotion and mood in pigs, Sus scrofa. Appl. Anim. Behav. Sci. 159, 9–28. doi: 10.1016/j.applanim.2014.08.002
National Health and Medical Research Council (2013) Australian code for the care and use of animals for scientific purposes, 8th edition. Canberra: National Health and Medical Research Council
Parker K. J., Maestripieri D. (2011). Identifying key features of early stressful experiences that produce stress vulnerability and resilience in primates. Neurosci. Biobehav. Rev. 35 (7), 1466–1483. doi: 10.1016/j.neubiorev.2010.09.003
Pedersen V., Barnett J. L., Hemsworth P. H., Newman E. A., Schirmer B. (1998). The effects of handling on behavioural and physiological responses to housing in tether-stalls among pregnant pigs. Anim. Welfare 7 (2), 137–150. doi: 10.1017/S0962728600020467
Portele K., Scheck K., Siegmann S., Feitsch R., Maschat K., Rault J. L., et al. (2019). Sow-piglet nose contacts in free-farrowing pens. Animals 9 (8), 513. doi: 10.3390/ani9080513
Prunier A., Tallet C., Sandercock D. (2021). “Evidence of pain in piglets subjected to invasive management procedures,” in Understanding the behaviour and improving the welfare of pigs. Ed. Edwards S. (Cambridge, UK: Burleigh Dodds Science Publishing).
Rault J.-L., Truong S., Hemsworth L., Le Chevoir M., Bauquier S., Lai A. (2019). Gentle abdominal stroking (‘belly rubbing’) of pigs by a human reduces EEG total power and increases EEG frequencies. Behav. Brain Res. 374, 111892. doi: 10.1016/j.bbr.2019.04.006
Rault J.-L., Waiblinger S., Boivin X., Hemsworth P. (2020). The power of a positive human–animal relationship for animal welfare. Front. Veterinary Sci. 7. doi: 10.3389/fvets.2020.590867
Reimert I., Bolhuis J. E., Kemp B., Rodenburg T. B. (2013). Indicators of positive and negative emotions and emotional contagion in pigs. Physiol. Behav. 109, 42–50. doi: 10.1016/j.physbeh.2012.11.002
Romeo R. D., Tang A. C., Sullivan R. M. (2009). “Early-life experiences: enduring behavioral, neurological, and endocrinological Consequences,” in Hormones, Brain and Behavior (Second Edition). Eds. Pfaff D. W., Arnold A. P., Etgen A. M., Rubin S. E. F. &R. T. (San Diego: Academic Press), 1975–2006.
Singh C., Verdon M., Cronin G. M., Hemsworth P. H. (2017). The behaviour and welfare of sows and piglets in farrowing crates or lactation pens. Animal 11 (7), 1210–1221. doi: 10.1017/S1751731116002573
Spinka M., Newberry R. C., Bekoff M. (2001). Mammalian Play: training for the unexpected. Q. Rev. Biol. 76 (2), 141–168. doi: 10.1086/393866
Tallet C., Linhart P., Policht R., Hammerschmidt K., Šimeček P., Kratinova P., et al. (2013). Encoding of situations in the vocal repertoire of piglets (Sus scrofa): A comparison of discrete and graded classifications. PloS One 8 (8), e71841. doi: 10.1371/journal.pone.0071841
Turpin D. L., Langendijk P., Plush K., Pluske J. R. (2017). Intermittent suckling with or without co-mingling of non-littermate piglets before weaning improves piglet performance in the immediate post-weaning period when compared with conventional weaning. J. Anim. Sci. Biotechnol. 8 (14), 1–12. doi: 10.1186/s40104-017-0144-x
VSN International (2022). The guide to the Genstat Command Language (Release 22), Part 2 Statistics (Hemel Hempstead, UK: VSN International).
Keywords: pig welfare, stress resilience, early life experiences, handling, production
Citation: Tomas K, Savaglia J, Plush KJ, D’Souza DN, Butler KL, Hemsworth PH and Tilbrook AJ (2024) Maternal contact and positive human interactions during lactation impacts piglet performance and behaviour during lactation. Front. Anim. Sci. 4:1289518. doi: 10.3389/fanim.2023.1289518
Received: 06 September 2023; Accepted: 12 December 2023;
Published: 04 January 2024.
Edited by:
Anastasia S. Tsingotjidou, Aristotle University of Thessaloniki, GreeceReviewed by:
Sebastien Goumon, ETH Zürich, SwitzerlandEmma Fàbrega Romans, Institute of Agrifood Research and Technology (IRTA), Spain
Copyright © 2024 Tomas, Savaglia, Plush, D’Souza, Butler, Hemsworth and Tilbrook. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Katelyn Tomas, ay50b21hc0B1cS5lZHUuYXU=
 Katelyn Tomas
Katelyn Tomas Jemma Savaglia
Jemma Savaglia Kate J. Plush
Kate J. Plush Darryl N. D’Souza
Darryl N. D’Souza Kym L. Butler
Kym L. Butler Paul H. Hemsworth
Paul H. Hemsworth Alan J. Tilbrook
Alan J. Tilbrook