
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
PERSPECTIVE article
Front. Anim. Sci., 05 December 2023
Sec. Animal Welfare and Policy
Volume 4 - 2023 | https://doi.org/10.3389/fanim.2023.1284419
Individual laying hens within the same group show variation in their temperament traits and cognitive learning abilities, which can be affected by both their early rearing experiences and housing environments. Hens also have distinct individual patterns of movement within housing systems that may correlate with temperament and cognition. Individual behavioral tests can measure treatment impacts, but social dynamics may impact on an individual’s behavior. The aims of this perspective piece are to provide further evidence of pen-level variation using original data on social ranging patterns and fear assessment of free-range hens exposed to different, enriched rearing environments; and to encourage more studies to consider pen replicate variation as a means to better understand causes and mechanisms. A literature review showed that, while most published studies over the past decade assessing individual laying hen behavior included group-level replication (i.e., 83% of 54 articles reviewed), almost none considered inter-pen variation. The original data analysis of individual hens’ range use recordings showed significant treatment pen replicate variation in pop-hole following movements and hen–pair associations in the time spent together inside or outside. Significant inter-pen variation was also seen in tonic immobility tests on a subset of hens from the same study. Pen-level replication is important for scientific validity and for improving our understanding of why commercial flocks in the same environment can be so variable in their behavior to inform management practices. Further research could help to understand the mechanisms behind why groups of hens reared and housed in the same environments will show significant inter-group variation.
Laying hens within the same group can show individual variation in temperament traits such as fear, anxiety, and boldness, as well as individual variation in their cognitive learning abilities (de Haas et al., 2017a, b; Campbell et al., 2019; Ghareeb et al., 2008; Rentsch et al., 2023). Individual hens within a loose housing system will have distinct, consistent behavioral patterns of movement and resource use, such as where they spend their time across the day in an aviary system (Campbell et al., 2016a; Montalcini et al., 2023a, b) or whether or not they range outside in a free-range system (Campbell et al., 2020; Gómez et al., 2022). These individual resource use patterns have been shown to correlate with temperament traits and cognition (Campbell et al., 2016b; Campbell et al., 2018a; Kolakshyapati et al., 2020; Campbell et al., 2021). However, it is still not clear as to whether pre-existing traits cause variation in, for example, ranging behavior, or if increased range use can then modify an individual hen’s temperament and cognition. Temperament traits and cognitive processes are manipulable. They can vary depending on the history of the individual hen, such as the complexity of the developmental environment they were exposed to, early stressful experiences, access to specific environmental resources, and lighting conditions (Sobotik et al., 2020; Hedlund et al., 2021; Dumontier et al., 2023; Skånberg et al., 2023). Behavioral tests of temperament and cognition can be valid measures of the impacts of housing and management variation on these individual laying hen traits. However, laying hens are social animals and this must also be considered when interpreting individual behavioral assessments.
Laying hens engage in conspecific interactions, such as gentle and aggressive pecking (Michel et al., 2022), and exhibit group-level synchronous behaviors, such as dust bathing and ranging (Campbell et al., 2018b; Grebey et al., 2020). They may establish dominance hierarchies in certain group sizes. Aggression was shown toward unfamiliar over familiar individuals in a group size of 10, but not when the group held 120 birds (D’Eath and Keeling, 2003). In contrast, Grethen et al. (2023) found similar social hierarchies in groups of 20 and 120 individuals, and these changed across bird age. Large groups (thousands of individuals) may not form hierarchies due to the energetic costs of aggression toward many unfamiliar individuals (Zimmermann et al., 2006). However, subgroups of birds have been observed in localized areas of housing systems holding several hundred individuals (Odén et al., 2000), and there is evidence of preferential conspecific social associations across time (Campbell et al., 2018b; Gómez et al., 2022). The social dynamic of a particular group of laying hens may thus have an impact on the behavioral patterns and resource use exhibited by the hens, or vice versa.
Social dynamics can also affect the aggression and stress experienced by individuals within a group (Nicol et al., 1999; de Haas et al., 2012; Carvalho et al., 2018). The presence of a fearful individual can increase the stress experienced by other individuals in the group (de Haas et al., 2012), and strain mixing showed individual fear was increased when a higher fear strain was mixed with a lower fear strain (Uitdehaag et al., 2008). Social contagion of behavioral and physiological arousal has been demonstrated in chicks exposed to a mild stressor (Edgar and Nicol, 2018). Thus, pens and flocks of hens may vary in temperament and cognitive traits because of social influences. Detailing how group social interactions may impact an individual hen’s behavioral patterns, temperament traits, and cognitive abilities could improve our understanding of why group- or flock-based variation exists. This has implications for research validity as well as managing flocks in a commercial setting.
The aims of this perspective piece are twofold. Firstly, to provide further evidence of pen level variation using original data on social ranging patterns in an experimental free-range system, including how the social patterns were affected by rearing conditions as well as pen level variation in a temperament trait assessment. Secondly, to encourage more studies in the future to consider the variation among pen replicates both in their experimental design and results interpretations. This will build greater evidence and understanding of what may cause behavioral, temperament, and cognitive variation among pens and ensure the validity of future research.
A literature review was first conducted to determine how many published studies assessing laying hen behavior at the individual level included replication at the group (i.e., flock or pen) level. The summary of layer behavioral test studies was compiled across 54 articles, which were found through multiple online database searches across a time frame from January 2013 to June 2023 (further selection details are provided in the Supplementary Material). The selected articles investigated a treatment effect on laying hen behavior, as measured across individual behavioral tests of fear, anxiety, coping styles, or cognitive processes (learning and cognitive bias). The papers included only studies that housed birds of any age in groups of at least three. The papers were each reviewed to detail the treatment effect being tested, the age of the birds, the behavioral tests applied, flock/pen group size, whether or not replicates were included (simultaneously or sequentially), and, if replicates were present, how they were accounted for in the analyses (i.e., as random or fixed effects in the models or not included) and thus in the results interpretation.
This animal experimental research was approved by the Animal Ethics Committee (AEC17–092) of the University of New England, Armidale, NSW, Australia.
The social movement pattern data were obtained from radiofrequency identification (RFID) range-use recordings from a flock of free-range hens that had been reared in different enriched environments. The full experimental housing details for this study have been reported elsewhere (Campbell et al., 2020; Bari et al., 2021) and so are presented in brief here.
The Hy-Line® Brown layers (n = 1,386) were reared for 16 weeks indoors and then subsequently housed in a free-range facility until 65 weeks of age. The commercially obtained, beak-trimmed, 1-day-old chicks (n = 1,700) were reared in nine visually isolated floor-litter pens (6.2 m long × 3.2 m wide) across three separate rooms that differed only in the enrichment treatments (one replicate/room): (1) a “control” floor-litter-only group; (2) a “novelty” group, with weekly changed novel objects (e.g., balls, bottles, bricks, and strings); and (3) a “structural” group with five custom-designed H-shaped perching structures (0.60 m long, wide, and high) comprising two solid panels and one open-framed side that could be placed in different orientations. At 16 weeks, the pullets were transferred to one free-range shed containing nine pens and socially remixed within rearing treatment pen replicates (n = 154/pen; three replicates/rearing treatment). The indoor floor-litter pens were of the same configuration and visually isolated from each other (see Campbell et al., 2020 for a pen schematic, including the range area). Each indoor pen was connected to a visually separated outdoor range accessible via two pop-hole openings (18 cm wide × 36 cm high). The pop-holes first opened when the hens were 25 weeks of age thus allowing daily outdoor access from 09:15 to until after sunset.
All hens were banded with microchips [Trovan® Unique ID 100 (FDX-A), with an operating frequency of 128 kHz] fixed into adjustable leg bands (Roxan Developments Ltd., Selkirk, UK). The RFID systems were set up in the indoor pens (see Campbell et al., 2017 for further details). The systems recorded the time and date of each hen passing through the pop-hole, including the travel direction (onto the range or into the pen). The daily individual ranging data from 25 weeks to 64 weeks of age (272 days) were grouped into the first 50 days of available ranging and the last 100 days. The data were used to measure pop-hole following behavior and hen pair associations. The ranging data across the 45 days from 56 weeks to 62 weeks of age were used to select individuals for tonic immobility testing (see Tonic immobility test protocol and data).
Pop-hole following behavior was observed by Campbell et al. (2018b). The authors reported that hens were more likely to follow the movement of other hens when moving outside to the range or back inside the shed. Using the first 50 days and the last 100 days of available ranging, pop-hole following behavior was quantified for individual birds based on the protocol used by Campbell et al. (2018b). Pop-hole following was quantified as the number of movements in the same direction as the previous moving hen, which was calculated as a percentage of the total number of movements that the hen exhibited. These percentages of pop-hole following are presented as median values per pen within each rearing treatment group separately for the first 50 days and the last 100 days, as well as the percentage of hens within the group showing following behavior more frequently than what would be expected randomly. The following behavior was strongly skewed, partly due to some hens having many more movements than others, so the comparison of pens and treatments in percentages of pop-hole following was carried out using a Kruskal–Wallis test in R (R Core Team, 2015).
Based on the analysis protocol detailed in Campbell et al. (2018b), the daily individual bird data across the first 50 days and the last 100 days, were used to measure four variables around the time spent together for every possible pair of hens, A and B.
1. Total time when both hens were outside as a % of the time when A was outside.
2. Total time when both hens were outside as a % of the time when B was outside.
3. Total time when both hens were inside as a % of the time when A was inside.
4. Total time when both hens were inside as a % of the time when B was inside.
These time measurements when combined provide an accurate measure of the mean percentage of overlap time for each pair of hens, accounting for hens that may spend a lot of time inside or outside, and, thus, would have high levels of overlap with many other hens. The mean association percentages are displayed for each pen within each rearing treatment separately for the first 50 days and the last 100 days. The time together for pairs of hens followed an approximately normal distribution, so pens within treatments and treatments were compared using Student’s t-tests in R (R Core Team, 2015) for each pair of groups.
From 62 weeks to 63 weeks of age, a subset of birds from each pen and each rearing treatment were selected for behavioral testing, as described in Bari et al. (2021). Across all pens, 135 individuals were selected as “indoor” (no ranging) or “outdoor” (daily ranging for the longest time) across all treatment groups (44 control group hens; 45 novelty group hens; and 46 structural group hens). The individual testing of tonic immobility by a single, trained experimenter occurred within a separate room at the same facility. The variation between the individual pens in tonic immobility duration is presented in this article (treatment means were presented in Bari et al., 2021). The log10-transformed durations were analyzed using a general linear model, with the fixed effect of “pen” in JMP® 17.0.0 software (SAS Institute, Cary, NC, USA).
Of the 54 articles that were reviewed (see Supplementary Material for the full results of the review), a range of treatments were assessed, such as rearing environmental enrichment/environmental complexity (e.g., cage versus aviary housing, music playback, and dark brooders), strain effects, maternal effects, range use, hatchery stress, and lighting environment (all treatments are listed in the Supplementary Material). The testing ages ranged throughout pullet rearing and adulthood, with some studies including both male and female chicks, or the presence of male adults as a treatment condition. The group size per replicate was three birds up to 18,000 birds for a study that replicated flocks. Different individual tests were applied such as tonic immobility, manual restraint, open field, novel object, novel arena, human approach, social isolation, spatial navigation, and judgment bias. Across the 54 articles, there were seven that had no pen replication at all, two studies with no replicates but the strains were mixed within the single pen, and then a further four studies with partial replication across the study period. Thus, in total, 83% of articles reviewed did include some or full pen replication. However, only one study statistically investigated differences between the pens in behavioral test results with all other studies accounting for the pen as a random factor (if that information was explicitly stated).
Table 1 shows the median percentages of pop-hole following for the first 50 days when the hens were adapting to the pens, and for the last 100 days, when the hen movements were relatively stable in each pen. Random movements would generate pop-hole following of 50.5% and thus the results indicate that the hens were exhibiting following in their ranging patterns. Random following behavior is slightly greater than 50% because all of the first and last movements each day must be in the same direction, as hens move out to the range at the start of the day, and inside at the end of the day. For the first 50 days, several groups had a very low number of movements for some hens, resulting in 0% or 100% following for these hens. Therefore, the medians and the percentage of the hens with greater than 50.5% following give a better indication of the behavior of the majority of hens. Over the last 100 days about 90% of all hens exhibited this following behavior, with more than half of their movements following a previous hen, illustrating the change in pop-hole following across time. Within the rearing enrichment treatment groups, there were significant differences between the pen replicates in the pop-hole following values (all p-values were < 0.001 except for control pens in the last 100 days when the p-value was > 0.05). Across the first 50 days, 7-C and 9-S showed the most pop-hole following, with 8-N and 9-S showing the most in the last 100 days (Table 1).
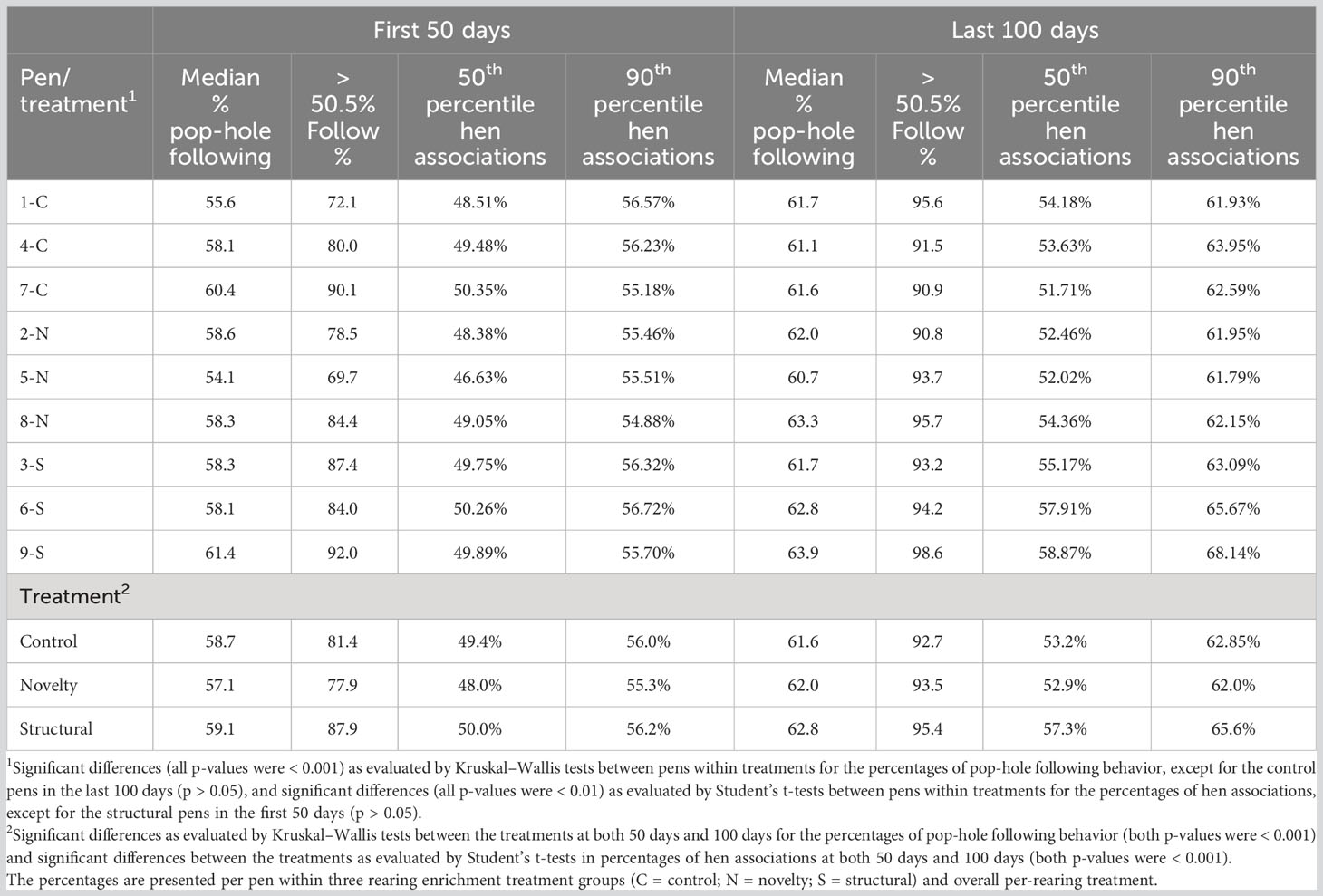
Table 1 The median percentages of pop-hole following behavior, percentages of the hens in the pen showing greater than 50.5% pop-hole following, and the 50th and 90th percentile of time spent together inside and outside for all pairs of hens for the first 50 days and the last 100 days of radiofrequency identification tracking of individual hen range usage across a flock cycle.
Table 1 also shows the median percentage of time that hens spent with other specific hens, based on the time both inside and outside for the first 50 days and the last 100 days. Random movements result in time together of very close to 50%, which aligns with the 50th percentile values shown within the first 50 days. The 90th percentile of time together indicates pairs of hens that chose to spend time together, both inside and outside. Across the first 50 days, the values suggest there were few pairs of hens that spent time together. However, for the last 100 days, the median time together is above 50% and the 90th percentile is higher, indicating that there were pairs of hens that spent time together, both inside and outside. There were significant differences (all p-values were < 0.01) between pens within the treatments for percentage of hen associations, except for the structural pens in the first 50 days (p > 0.05). The values between the pens within the rearing enrichment treatment groups showed the lowest 90th percentile value in 8-N across the first 50 days, and the highest 90th percentile value in 9-S across the last 100 days (Table 1). There were also significant differences between the treatments in percentages of hen associations at both 50 days and 100 days (both p-values were < 0.001). The least significant difference between the pens within the treatments was 1.8% and between the treatments was 1.1%.
In the results comparing rearing treatment and ranging behavior presented in Bari et al. (2021), there were no significant effects of either variable or their interaction on the duration of tonic immobility. Figure 1 illustrates the variation at the pen level showing the significant differences between flocks of birds (F(8,8) = 2.49, p = 0.02).
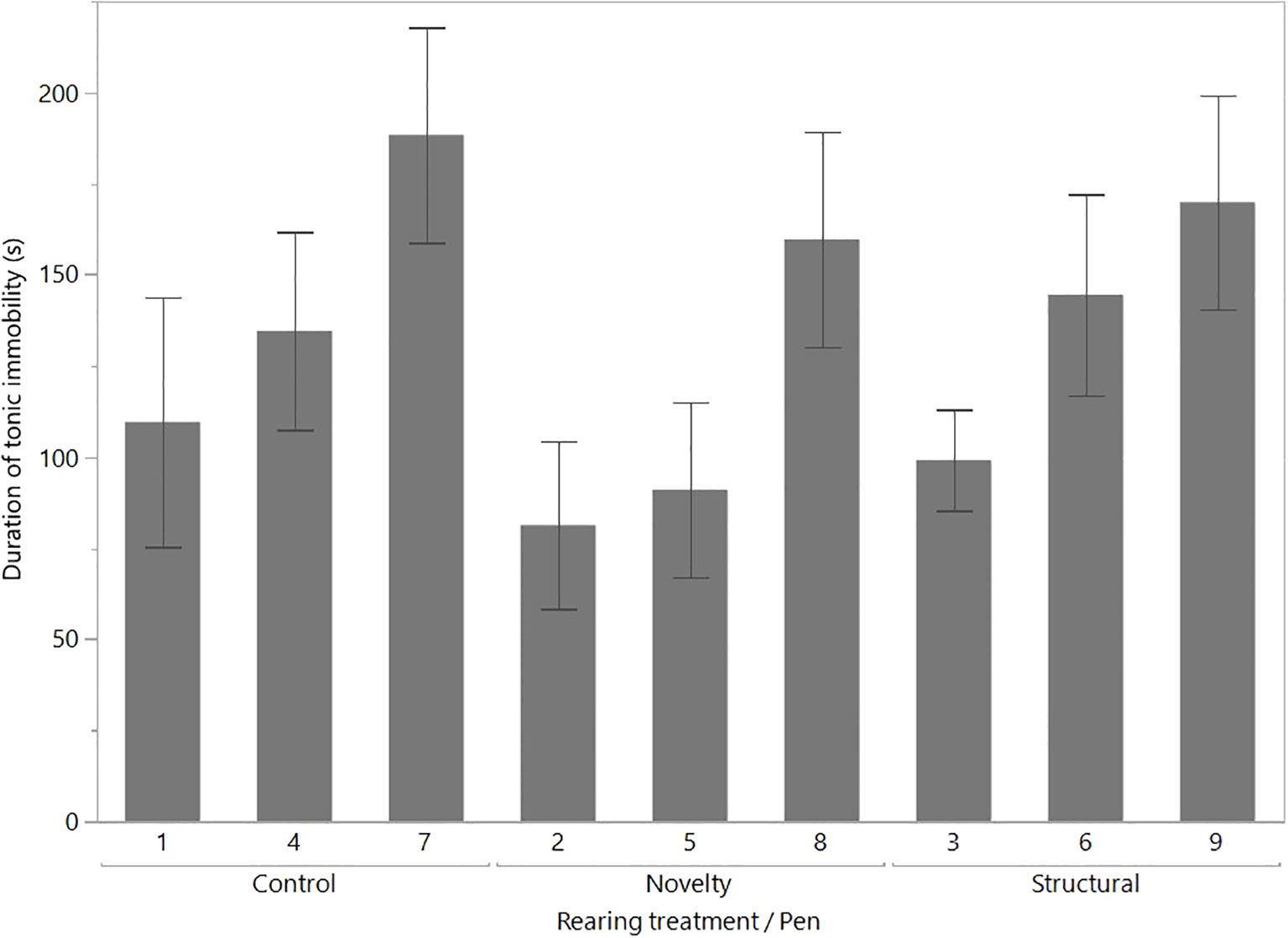
Figure 1 The significant (p < 0.02) pen-level variation for the mean (± SEM) duration of tonic immobility. The pens were from the three rearing enrichment treatment groups (i.e., control, novelty, and structural) and comprised outdoor (ranging) and indoor (no ranging) hens. The raw data are presented with the analyses conducted on transformed data. There were no significant effects of rearing treatment or ranging on tonic immobility duration, as presented in Bari et al. (2021).
This perspective considers the importance of pen replication in the assessment of individual laying hen behavior due to the potential group-level social dynamic impacts. The individual range use patterns from hen groups in an experimental free-range setting illustrated the different dynamics within a specific group of hens, including how it may change over time and be influenced by developmental conditions. Inter-pen variation was also seen in a behavioral test of fear that was conducted on a subset of the birds. Scanning published articles over the past decade showed that, although the majority of behavioral test studies replicated pens and/or flocks, there were still studies that considered individual-level differences to quantify treatment effects with only a single replicate per treatment. It is suggested that a single replicate cannot be an accurate representation of a particular treatment, given the potential for social influences on the behavior, temperament, and cognitive processes of the birds.
The results of the inter-flock differences in behavioral patterns illustrated in the data presented in this perspective are supported by inter-pen differences that were highlighted in some of the authors’ previous studies looking at hen social ranging interactions and range usage (Campbell et al., 2017; Campbell et al., 2018a, c). In the behaviors analyzed, including the mean daily time spent on the range by individual hens, mean daily number of visits to the range, mean visit duration, number of hens on the range simultaneously, and percentage of available days that the range was accessed, there were significant inter-pen differences more often than treatment differences (Campbell et al., 2017; Campbell et al., 2018c). Furthermore, inter-pen variation was also previously shown in the same measures as applied in the current study in the percentages of the time hen pairs spent together inside or outside and pop-hole following behavior (Campbell et al., 2018b). Thus, laying hens in groups comprise individuals that also contribute to the collective dynamic. Pen-level variation in tonic immobility and ranging behavior was also analyzed in Armstrong et al. (2020), but, overall, the consideration of inter-pen variation in the literature is limited. Many studies may replicate at the pen and/or flock level, but the scanning of the literature showed that it is almost exclusively for scientific integrity (i.e., accounted for as a random effect in the analyses). We argue the opportunity to consider inter-pen differences in laying hen research and factors that may then contribute to this variation. This could ultimately facilitate commercial farm management where inter-flock differences are common but the causes of these differences often unknown.
The mechanisms that lead to these inter-group differences in birds reared and housed in the same conditions warrant further research. Behavioral variation might be modulated through epigenetic changes where life experiences can have lasting impacts (Ericsson et al., 2016; Guerrero-Bosagna et al., 2020). Phenotypic variation can change a group’s social dynamic (Campderrich et al., 2017), as well as modulate the stress responses of individuals (Nazar et al., 2015). Tracking technologies to automate data collection at the individual level and allow modeling of larger datasets (e.g., Campbell et al., 2018b; Montalcini et al., 2023a, b) facilitate understanding of how individuals within a group may affect social patterns, and vice versa. The future research avenues to consider, for example, could be whether hens in a loose-housed system with more environmental choice, such as a free-range or aviary system, will show greater inter-pen variation in social dynamics than those in a floor-based indoor barn. Or the extent to which different types of enrichment can change social patterns and why. Or how the dynamic of a group may change with age, flock size, and experiences such as environmental stressors and disease. The ambient environment may also affect bird behavior, such as pen location within a facility or a tier of a cage unit. These changes in social dynamics within the group can then be further assessed for impacts on individual traits, including whether the addition or removal of individuals with known temperament or cognitive traits could modify the existing dynamic.
In conclusion, the group effect must be considered when designing and interpreting treatment effects on individual laying hen behavior. Further research could help to understand the mechanisms behind why groups reared and housed in the same environments will show significant inter-group variation.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The animal study was approved by the Animal Ethics Committee (AEC17-092) of the University of New England, Armidale, NSW, Australia. The study was conducted in accordance with the local legislation and institutional requirements.
DC: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Visualization, Writing – original draft. BH: Conceptualization, Data curation, Formal analysis, Methodology, Writing – review & editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This research was in part funded by Poultry Hub Australia (2017–20). Open-access fees were supported by a CSIRO Julius Career Award to DC.
Thank you to all technical staff at CSIRO who assisted with animal husbandry and data collection and to H. Ford for valuable discussions on the article pitch.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fanim.2023.1284419/full#supplementary-material
Armstrong E. A., Voelkl B., Voegeli S., Gebhardt-Henrich S. G., Guy J. H., Sandilands V., et al. (2020). Cell proliferation in the adult chicken hippocampus correlates with individual differences in time spent in outdoor areas and tonic immobility. Front. Vet. Sci. 7, 587. doi: 10.3389/fvets.2020.00587
Bari M. S., Allen S. S., Mesken J., Cohen-Barnhouse A. M., Campbell D. L. M. (2021). Relationship between range use and fearfulness in free-range hens from different rearing enrichments. Animals 11, 300. doi: 10.3390/ani11020300
Campbell D. L. M., Dickson E. J., Lee C. (2019). Application of open field, tonic immobility, and attention bias tests to hens with different ranging patterns. PeerJ 7, e8122. doi: 10.7717/peerj.8122
Campbell D. L. M., Dyall T. R., Downing J. A., Cohen-Barnhouse A. M., Lee C. (2020). Rearing enrichments affected ranging behavior in free-range laying hens. Front. Vet. Sci. 7. doi: 10.3389/fvets.2020.00446
Campbell D. L. M., Hinch G. N., Downing J. A., Lee C. (2016b). Fear and coping styles of outdoor-preferring, moderate-outdoor and indoor-preferring free-range laying hens. Appl. Anim. Behav. Sci. 185, 73–77. doi: 10.1016/j.applanim.2016.09.004
Campbell D. L. M., Hinch G. N., Downing J. A., Lee C. (2018c). Early enrichment in free-range laying hens: effects on ranging behaviour, welfare and response to stressors. Animal 12, 575–584. doi: 10.1017/S1751731117001859
Campbell D. L. M., Hinch G. N., Dyall T. R., Warin L., Little B. A., Lee C. (2017). Outdoor stocking density in free-range laying hens: radio-frequency identification of impacts on range use. Animal 11, 121–130. doi: 10.1017/S1751731116001154
Campbell D. L. M., Horton B. J., Hinch G. N. (2018b). Using radio-frequency identification technology to measure synchronised ranging of free-range laying hens. Animals 8, 210. doi: 10.3390/ani8110210
Campbell D. L. M., Karcher D. M., Siegford J. M. (2016a). Location tracking of individual laying hens housed in aviaries with different litter substrates. Appl. Anim. Behav. Sci. 184, 74–79. doi: 10.1016/j.applanim.2016.09.001
Campbell D. L. M., Talk A. C., Loh Z. A., Dyall T. R., Lee C. (2018a). Spatial cognition and range use in free-range laying hens. Animals 8, 26. doi: 10.3390/ani8020026
Campbell D. L. M., Whitten J. M., Slater E., Lee C. (2021). Rearing enrichments differentially modified hen personality traits and reduced prediction of range use. Anim. Behav. 179, 97–109. doi: 10.1016/j.anbehav.2021.06.024
Campderrich I., Liste G., Estevez I. (2017). The looks matter; aggression escalation from changes on phenotypic appearance in the domestic fowl. PloS One 12, e0188931. doi: 10.1371/journal.pone.0188931
Carvalho R. R., Palme R., da Silva Vasconcellos A. (2018). An integrated analysis of social stress in laying hens: The interaction between physiology, behaviour, and hierarchy. Behav. Process. 149, 43–51. doi: 10.1016/j.beproc.2018.01.016
D’Eath R. B., Keeling L. J. (2003). Social discrimination and aggression by laying hens in large groups: from peck orders to social tolerance. Appl. Anim. Behav. Sci. 84, 197–212. doi: 10.1016/j.applanim.2003.08.010
de Haas E. N., Kops M. S., Bolhuis J. E., Groothuis T. G. G., Ellen E. D., Rodenburg T. B. (2012). The relation between fearfulness in young and stress-response in adult laying hens, on individual and group level. Physiol. Behav. 107, 433–439. doi: 10.1016/j.physbeh.2012.08.002
de Haas E. N., Lee C., Hernandez C. E., Naguib M., Rodenburg T. B. (2017a). Individual differences in personality in laying hens are related to learning a colour cue association. Behav. Process. 134, 37–42. doi: 10.1016/j.beproc.2016.11.001
de Haas E. N., Lee C., Rodenburg T. B. (2017b). Learning and judgment can be affected by predisposed fearfulness in laying hens. Front. Vet. Sci. 4, 113. doi: 10.3389/fvets.2017.00113
Dumontier L., Janczak A. M., Smulders T. V., Nordgreen J. (2023). Effects of the rearing environment complexity on laying hens’ spatial cognition: A holeboard test approach. Appl. Anim. Behav. Sci. 260, 105878. doi: 10.1016/j.applanim.2023.105878
Edgar J. L., Nicol C. J. (2018). Socially-mediated arousal and contagion within domestic chick broods. Sci. Rep. 8, 10509. doi: 10.1038/s41598-018-28923-8
Ericsson M., Henriksen R., Bélteky J., Sundman A.-S., Shionoya K., Jensen P. (2016). Long-term and transgenerational effects of stress experienced during different life phases in chickens (Gallus gallus). PloS One 11, 4, e0153879. doi: 10.1371/journal.pone.0153879
Ghareeb K., Awad W. A., Niebuhr K., Böhm J., Troxler J. (2008). Individual differences in fear and social reinstatement behaviours in laying hens. Int. J. Poultry Sci. 7, 843–851. doi: 10.3923/ijps.2008.843.851
Gómez Y., Berezowski J., Jorge Y. A., Gebhardt-Henrich S. G., Vögeli S., Stratmann A., et al. (2022). Similarity in temporal movement patterns in laying hens increases with time and social association. Animals 12, 555. doi: 10.3390/ani12050555
Grebey T. C., Ali A. B. A., Swanson J. C., Widowski T. M., Siegford J. M. (2020). Dust bathing in laying hens: strain proximity to, and number of conspecifics matter. Poult. Sci. 99, 4103–4112. doi: 10.1016/j.psj.2020.04.032
Grethen K. J., Gómez Y., Toscano M. J. (2023). Coup in the coop: Rank changes in chicken dominance hierarchies over maturation. Behav. Process. 210, 104904. doi: 10.1016/j.beproc.2023.104904
Guerrero-Bosagna C., Pértille F., Gomez Y., Rezaei S., Gebhardt-Henrich S. G., Vögeli S., et al. (2020). DNA methylation variation in the brain of laying hens in relation to differential behavioral patterns. Comp. Biochem. Physiol. – Part D 35, 100700. doi: 10.1016/j.cbd.2020.100700
Hedlund L., Palazon T., Jensen P. (2021). Stress during commercial hatchery processing induces long-time negative cognitive judgement bias in chickens. Animals 11, 1083. doi: 10.3390/ani11041083
Kolakshyapati M., Taylor P. S., Hamlin A., Sibanda T. Z., de Souza Vilela J., Ruhnke I. (2020). Frequent visits to an outdoor range and lower areas of an aviary system is related to curiosity in commercial free-range laying hens. Animals 10, 1706. doi: 10.3390/ani10091706
Michel V., Berk J., Bozakova N., van der Eijk J., Estevez I., Mircheva T., et al. (2022). The relationship between damaging behaviours and health in laying hens. Animals 12, 986. doi: 10.3390/ani12080986
Montalcini C. M., Petelle M. B., Toscano M. J. (2023a). Commercial laying hens exhibit long-term consistent individual differences and behavioural syndromes in spatial traits. R. Soc. Open Sci. 10, 230043. doi: 10.1098/rsos.230043
Montalcini C. M., Toscano M. J., Gebhardt-Henrich S. G., Petelle M. B. (2023b). Intra-individual variation of hen movements is associated with later keel bone fractures in a quasi-commercial aviary. Sci. Rep. 13, 2377. doi: 10.1038/s41598-023-29587-9
Nazar F. N., Marin R. H., Liste G., Campderrich I., Estevez I. (2015). Manipulation of the phenotypic appearance of individuals in groups of laying hens: effects on stress and immune-related variables. Stress 18, 710–717. doi: 10.3109/10253890.2015.1078306
Nicol C. J., Gregory N. G., Knowles T. G., Parkman I. D., Wilkins L. J. (1999). Differential effects of increased stocking density, mediated by increased flock size, on feather pecking and aggression in laying hens. Appl. Anim. Behav. Sci. 65, 137–152. doi: 10.1016/S0168-1591(99)00057-X
Odén K., Vestergaard K. S., Algers B. (2000). Space use and agonistic behaviour in relation to sex composition in large flocks of laying hens. Appl. Anim. Behav. Sci. 67, 307–320. doi: 10.1016/S0168-1591(99)00123-9
R Core Team (2015). 'R: A language and environment for statistical computing (Vienna, Austria: ' R Foundation for Statistical Computing).
Rentsch A. K., Harlander A., Niel L., Siegford J. M., Widowski T. M. (2023). Rearing laying hens: Environmental complexity and genetic strain affect pullet but not chick performance in a T-maze learning task. Appl. Anim. Behav. Sci. 265, 105997. doi: 10.1016/j.applanim.2023.105997
Skånberg L., Newberry R. C., Estevez I., Keeling L. J. (2023). Environmental change or choice during early rearing improves behavioural adaptability in laying hen chicks. Sci. Rep. 13, 6178. doi: 10.1038/s41598-023-33212-0
Sobotik E. B., Nelson J. R., Archer G. S. (2020). How does ultraviolet light affect layer production, fear, and stres. Appl. Anim. Behav. Sci. 223, 104926. doi: 10.1016/j.applanim.2019.104926
Uitdehaag K. A., Rodenburg T. B., van Hierden Y. M., Bolhuis J. E., Toscano M. J., Nicol C. J., et al. (2008). Effects of mixed housing of birds from two genetic lines of laying hens on open field and manual restraint responses. Behav. Process. 79, 13–18. doi: 10.1016/j.beproc.2008.04.004
Keywords: chicken, poultry, behavior test, individual variation, group, replication
Citation: Campbell DLM and Horton BJ (2023) The necessity of pen replication to account for and understand the impacts of social dynamics on individual laying hen behavior. Front. Anim. Sci. 4:1284419. doi: 10.3389/fanim.2023.1284419
Received: 28 August 2023; Accepted: 17 November 2023;
Published: 05 December 2023.
Edited by:
Manja Zupan Šemrov, University of Ljubljana, SloveniaReviewed by:
Ingrid De Jong, Wageningen University and Research, NetherlandsCopyright © 2023 Campbell and Horton. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Dana L. M. Campbell, ZGFuYS5jYW1wYmVsbEBjc2lyby5hdQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.