
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Anim. Sci., 10 August 2023
Sec. Animal Welfare and Policy
Volume 4 - 2023 | https://doi.org/10.3389/fanim.2023.1215206
Pigs are widely acknowledged for their olfactory abilities. Research on pigs’ olfactory capacities has focused mainly on aspects of olfaction that directly impact production, such as palatability of feed and pheromones to stimulate reproduction. Several basic research questions remain unanswered, such as which odors do pigs like/dislike, and how may odors enrich their lives? This review aims to explore the currently available literature on pig olfaction to elucidate the current knowns and unknowns within the following topics: chemicals in a pig’s environment, the olfactory organs of pigs, olfactory detection and acuity, behavioral reactions to odors, aversive odors, reaction to novel odors, attractive odors, and odors as a management tool in commercial pig production. The review focuses on complex odors of non-social origin (e.g., ammonia), and when information on this topic is lacking we include information from research on other mammalian species. We found that the olfactory organs of pigs are already functional at birth and that piglets can recognize the smell of the sow within 12h postpartum. Compared with humans and several other mammalian species, the pig’s olfactory system is highly developed, and the use of their sense of smell is incorporated into their natural behavior. While sniffing is a well-known behavior in pigs’ exploratory behavioral repertoire, this review points to a lack of knowledge on pigs’ behavioral reactions specifically when exposed to odors. Some odors appear attractive to pigs, whereas others appear repellent. Depending on the properties of the odor, providing pigs access to odors may be a way to stimulate their sense of smell, and could possibly be used in commercial pig production to enrich their lives. The review lastly highlights potential risks for animal welfare caused from lacking knowledge about how pigs perceive odors in their environment, and proposes future research questions and ways to utilize pigs’ sense of smell in the daily management of these animals. Further research on the olfactory abilities of pigs could help to ensure a more sustainable pig production.
Pigs (Sus scrofa) are well known for using their highly developed sense of smell, for instance while hunting for truffles in the ground. Pigs’ motivation to sniff and root is even mentioned in various children’s books:
[Wilbur the pig]: “You know where I’d really like to be this evening?”
[Charlotte the spider]: “Where?”
[Wilbur the pig]: “In a forest looking for beechnuts and truffles and delectable roots, pushing leaves aside with my wonderful strong nose, searching and sniffing along the ground, smelling, smelling, smelling…” (White, 1952).
This quote is from the popular children’s novel Charlotte’s Web, and it emphasizes how many people, not only within the scientific community but also in the public, acknowledge the sense of smell in pigs and its importance in pigs’ lives. Pigs are omnivorous animals and their natural sources of food are found sporadically within large home range areas, and sometimes underground. Pigs will use their snouts to sniff and root in the substrate to locate edible parts and, to some degree, to satisfy a motivation to explore (termed inquisitive exploration) (Wood-Gush and Vestergaard, 1989; Day et al., 1995). Pigs, in addition, use their sense of smell to detect and differentiate between conspecifics (Meese and Baldwin, 1975; Mendl et al., 2002), and body odors are an important component in mating behavior and when interacting with (unknown or known) individuals (Stolba and Woodgush, 1989).
Pigs have been co-existing with humans for about 10,000 years (Giuffra et al., 2000; Amaral et al., 2011; Meiri et al., 2013) and though many parallels are drawn between humans and pigs, for instance when considering pigs as human models in biomedical research, it should be emphasized that the olfactory systems of pigs and humans differ (Pond and Houpt, 1978). For instance, the olfactory bulb of pigs constitutes 7% of their brain size (Brunjes et al., 2016) and pigs have 1,113 functional olfactory receptor genes (Nguyen et al., 2012; Paudel et al., 2015) as compared with 0.01% of the brain (Kavoi and Jameela, 2011) and 347 olfactory receptor genes in humans. Therefore, we must assume that the sense of smell differs between the two species and thus precautions should be taken with regard to the olfactory environment in which we house farmed pigs. This is particularly important when considering the large population of pigs living under human care within the worldwide farming industry. In 2020, there were 146 million farmed pigs in the EU alone (Eurostat, 2022).
Not surprisingly, with regard to olfaction, porcine research has mainly focused on studying aspects deemed relevant for enhancing commercial pig production, and thereby benefiting farm economy. One aspect is the importance of smell for the palatability of feed (i.e., combination of taste, smell, and texture of the feed, which is sensed before swallowing) (Jacela et al., 2010). This research is aimed at increasing feed intake in pigs (e.g., Jacela et al., 2010) and hence adds important information for optimizing production. Another aspect is the use and importance of odors for reproduction. Odors (boar smell/androsterone) may be utilized to stimulate estrus in sows, thereby facilitating reproduction (e.g., Booth and Signoret, 1992; Sørensen, 1996; Rekwot et al., 2001). A line of studies has examined how pheromones are detected and processed in pigs (e.g., McGlone, 1985; Dorries et al., 1997; Guiraudie et al., 2003; Salazar et al., 2003; Salazar et al., 2004). In the social context, studies show that pigs are able to use odors/pheromones (compounds not specified) when discriminating between conspecifics (Meese and Baldwin, 1975; Mendl et al., 2002), and boar odour or androsterone may be utilized to stimulate oestrus in sows (e.g., Rekwot et al., 2001; McGlone et al., 2019a; McGlone et al., 2019b). A line of studies has also investigated the use pf pig appeasing pheromone to reduce aggression in pigs (for review see: Peden et al., 2018). This is of interest due to the frequent mixing of pigs within the pig industry (e.g., piglets are weaned and later moved from the weaner to the finisher unit, and sows are mixed after weaning).
While knowledge of pigs’ sense of smell with regard to feed palatability and detection of pheromones is relatively well documented, research on the more general olfactory abilities of pigs is sparse. As a result, some on-farm practices and management procedures might not be adapted to accommodate this sensory modality of pigs, nor may it satisfy pigs’ motivation for using their sense of smell. This is first and foremost a welfare issue, as pigs may suffer from living in an environment with low levels of olfactory stimulation, which may lead to boredom or anhedonia (Figueroa et al., 2015), and exposure to high concentrations of potentially aversive gasses such as ammonia [NH3 (Koerkamp et al., 1998; Seedorf and Hartung, 1999; Jones et al., 2001)] and hydrogen sulfide [H2S (Beauchamp et al., 1984; Ni et al., 2021)]. Conversely, there may be several ways in which more knowledge of the olfactory abilities of pigs could be used for optimizing on-farm management. If pigs are given an outlet from boredom by stimulating their motivation to investigate through sniffing, odors could provide a means to reduce abnormal behaviors such as oral stereotypies (Lawrence and Terlouw, 1993) and tail/ear biting (e.g., Zonderland, 2010; Valros and Heinonen, 2015; Godyn et al., 2019) and possibly lower aggression (Godyn et al., 2019). If current concentrations of gasses such as ammonia and hydrogen sulfide are aversive and/or harmful to pigs, regulations should account for this, and means should be initiated to prevent exposing pigs to such concentrations. Improving the olfactory environment in commercial pig houses could benefit on-farm management as well as the welfare of the animals, ultimately resulting in a more sustainable pig production. If science is successful in mapping the odors that pigs can detect and differentiate and find aversive or attractive, the favorable chemicals might be used as enrichment, as conditioned stimuli, or to increase feed intake during critical periods in a pig’s life.
This review explores the available literature on pig olfaction, with the aim of elucidating the current knowns and unknowns within the topic. The review covers topics such as chemicals in the pig environment, olfactory organs of the pig, olfactory detection and acuity, behavioral reactions to odors, aversive odors, reaction to novel odors, attractive odors, and odors as a management tool in commercial pig production. Due to the limited available research, literature on feed palatability and taste preferences will be included when relevant but these are not the main focus of the review, and hence the authors refer to the original papers on feed palatability for readers who might wish for further details (e.g., Jacela et al., 2010). The review further seeks to elucidate potential risks in terms of animal welfare in the commercial pig industry that the lack of knowledge poses, for instance poor olfactory stimulation or potentially harmful effects of chemicals arising from a poor air quality. Lastly, we suggest potential ways to utilize odors in practical pig management and identify promising areas still in need of further research. Based on these unexplored areas, we propose the most central new research questions, which should be the focus in order to improve pig welfare and sustainability of the commercial production.
In their daily life, domestic pigs will encounter various types of substances, which may or may not be odorous. In commercial pig barns ammonia and hydrogen sulfide are present at varying concentrations (Hoff et al., 2006; Ni et al., 2021), and the buildings and inventory of a pig barn may also emit odors. As pigs are housed in groups, and typically with several groups within one barn section, each pig will be exposed to—and have to relate to—the smell of several conspecifics. The pig has evolved nine glands for the production of social odors [digital, preputial, vulvar, anal, mental, salivary, buccal, pre-orbital, and Harderian glands (Pond and Houpt, 1978; Watson, 2004)], illustrating the complexity of pigs’ social olfactory communication. Domestic pigs are also exposed to the smell of humans, as well as of straw, feed, and water (it is currently unknown if water is odorless or may contain odorants). Furthermore, when moved to a new barn section, pigs are exposed to the smell of disinfectants and cleaning agents, and in the farrowing barn there is often a smell of blood when piglets are newly tail docked and male piglets castrated. As pigs can encounter a broad variety of chemicals in their environment, definitions of the terms used to classify various chemicals and odorants in this review and within the scientific community are listed in Tables 1, 2. The tables include classifications of the chemicals and types of odors, as well as the definition and examples of such chemicals from the pigs’ environment.
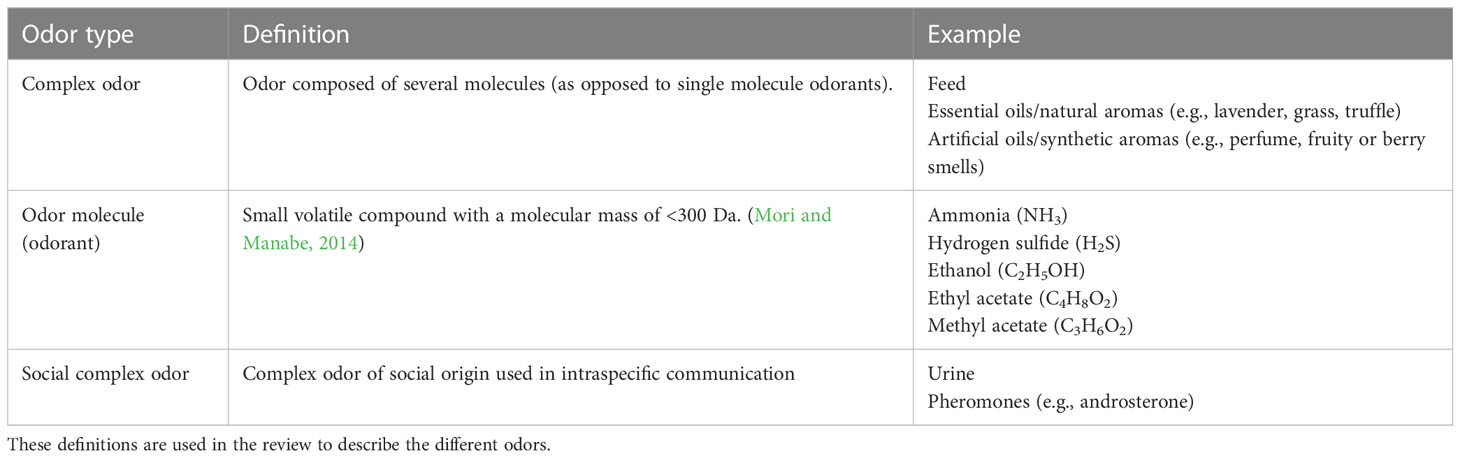
Table 2 Definitions of odor types based on their complexity (single molecule vs. several molecules) and origin (social vs. non-social).
In the next section, the review expands on the currently available knowledge on pig olfactory abilities and discusses the potential of using odors in commercial pig production and management. The review focuses on odors of non-social origin, meaning any odors arising from the pigs’ surroundings (for example, those listed as natural aromas in Table 2) and not from other pigs. In contrast to other odorants in the environment of the pig, non-social odors of complex structure are likely sparsely represented in commercial pig houses, whereas pigs are exposed to a variety of these under natural conditions. Based on the ubiquitous presence of such odors in the pig’s natural habitat, we hypothesize that these substances are of relevance to pigs—also when the animals are housed under commercial conditions. In addition, these types of substances are easily accessible and may be added to the pig environment without interfering with farm practices (e.g., by clotting the slurry system). Making use of such complex odors in management routines of commercial pig production is interesting with respect to improving or optimizing, for instance enrichment procedures to increase pig welfare. Knowledge about pheromones and complex odors from a social origin will be included when deemed necessary for the understanding of the proposed research questions or implications on farm, but it is not the main focus of this review (we refer to McGlone et al. (2022) for a detailed review of odors of social origin).
Like the majority of mammals, the pig’s olfactory, gustatory, and trigeminal systems are involved in chemical sensing (i.e., the sense of taste, smell, and somatosensation). While chemosensitivity involves both taste and smell, most mammalian animals rely on olfaction as the primary chemosensory modality, which makes the olfactory systems prominent and well developed (Wackermannová et al., 2016). Although the pig has functional trigeminal sensory neurons (Salazar et al., 2000) that likely play a role in pig olfaction, the below sections will focus on the two most studied olfactory systems of the pig: the main olfactory system and the accessory olfactory system.
The nose is the main olfactory organ of the pig (Figure 1A), containing the olfactory epithelium, which lines the inner surface of the nasal cavity (Lledo et al., 2005). This neuroepithelium directly interacts with inhaled odors of varying shapes, sizes, and chemical functions, and thus recognizes thousands of different volatile molecules (i.e., odors) (Ebrahimi and Chess, 1998; Firestein, 2001). Nasal mucus lines the olfactory epithelium, and once odor molecules are dissolved in this mucus, binding is allowed between odor molecules and the receptors on the cilia of the sensory neurons. These sensory neurons are connected to the main olfactory bulb (Figures 2A, B) by an axon projected from the basal pole of the neuron (Lledo et al., 2005). Several axons merge into the densely packed fascicles that comprise the olfactory nerves, which transmit the electrical signal to the olfactory bulb (Firestein, 2001).
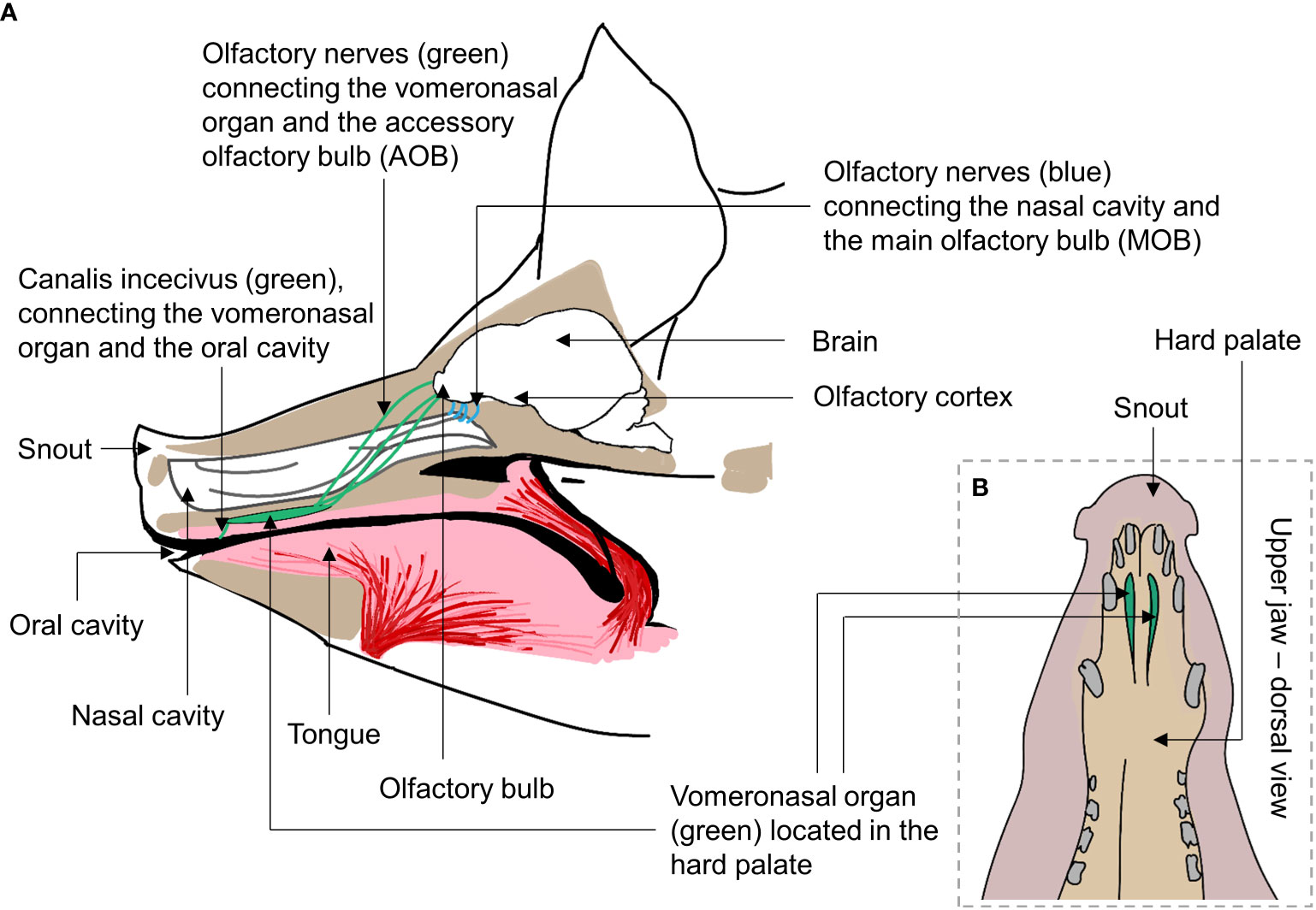
Figure 1 (A) Simple schematic view of olfactory systems of the domestic pig (sagittal section of the pig head). The nasal and oral cavity of the pig are illustrated, and the long and narrow nasal cavity is opened while breathing. The olfactory sensory neurons (blue) project to the olfactory bulb in the front of the pig brain. The vomeronasal organ (VNO) of the pig is situated in the upper jaw, in the hard palate with the incisive canals (canalis incecivus) connecting the VNO and the nasal cavity (highlighted by the green areas). (B) The VNO is located behind the front teeth in the upper jaw. This organ is thought to be especially used to detect poorly volatile organic compounds, although this is currently being debated (McGlone et al., 2022), and may trigger the flehmen response. Altogether, these form the first part of the main and accessory olfactory systems of the pig. (Line art based on: A (Døving and Trotier, 1998; Conrad et al., 2014; Kyllar et al., 2014; Brunjes et al., 2016; John et al., 2020) and B (Døving and Trotier, 1998; Kyllar et al., 2014)).
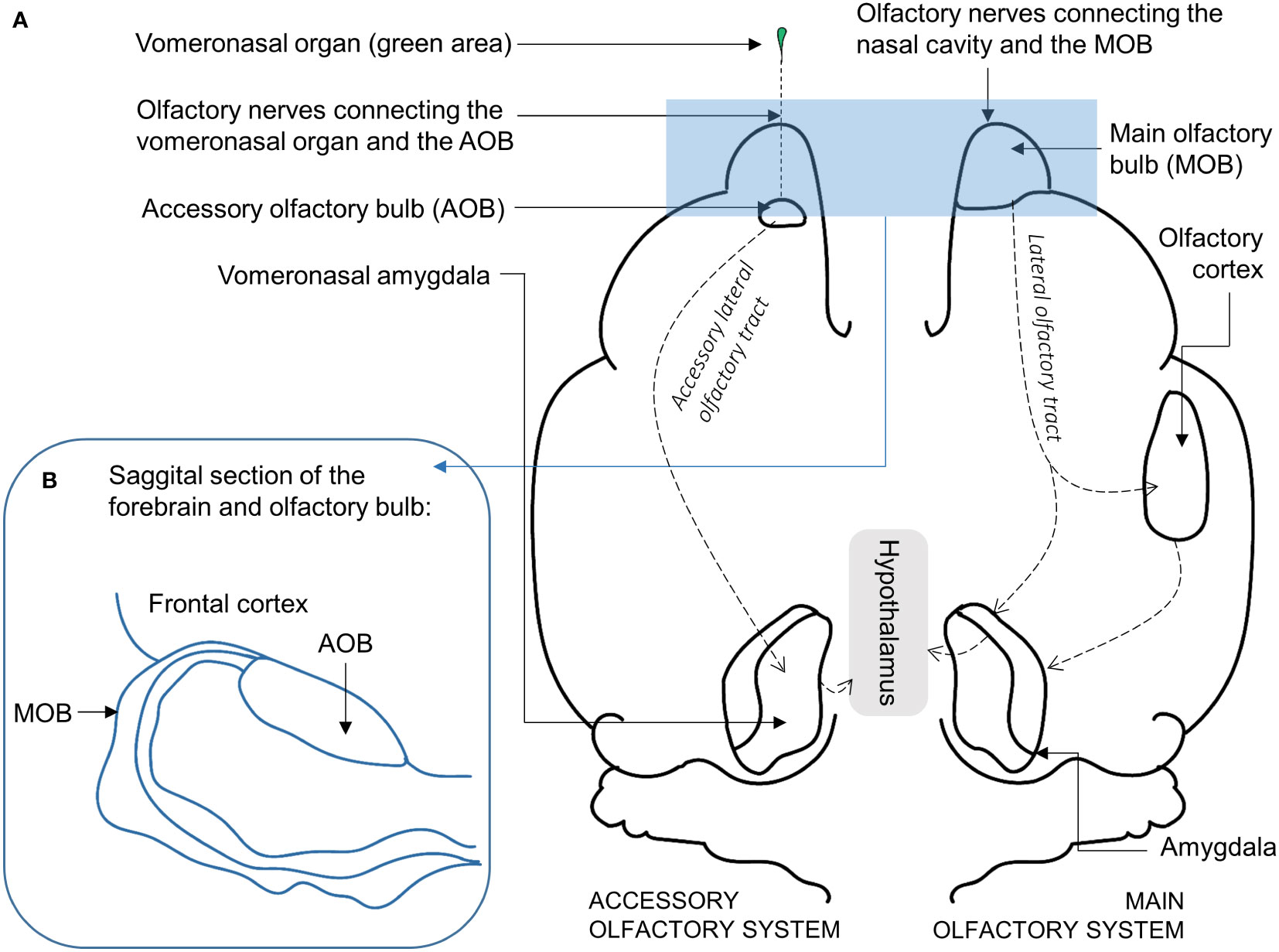
Figure 2 (A) Basal view of the pig brain illustrating a simplified organization of the main olfactory system (MOS) on the left and the accessory olfactory system (AOS) on the right, with emphasis on the olfactory bulb (OB). The olfactory nerves connect the nasal cavity to the main olfactory bulb (MOB) in the fore brain. The olfactory nerves from the vomeronasal organ (VNO) connect to the accessory olfactory bulb (AOB). (B) Sagittal section of the OB, illustrating the placement of the MOB relative to the AOB inside the OB. (Line art based on: A (Meisami and Bhatnagar, 1998; Sauleau et al., 2009; Brunjes et al., 2016) and B (Døving and Trotier, 1998; Meisami and Bhatnagar, 1998).
The olfactory bulb (OB) is located at the rostral end of the brain (Figures 1A, 2A, B) and the structure is relatively large, weighing approximately 3 g and having a volume of 3 mL (Brunjes et al., 2016). For comparison, the brain of the adult domestic pig measures 10 cm from the rostral tip to the dorsal part of the cerebellum and weighs about 90 g. The OB of the pig thus accounts for approximately 7% of their brain size (Brunjes et al., 2016), whereas in humans it accounts for only approximately 0.01% (Kavoi and Jameela, 2011). Pigs, in addition, have one of the largest olfactory receptor (OR) repertoires in the animal kingdom, with 1,113 functional olfactory receptor genes and 188 pseudogenes (Nguyen et al., 2012; Paudel et al., 2015). The estimate is much higher than that reported for humans (about 339 intact OR genes and 297 OR pseudogenes (Malnic et al., 2004)) and comparable to that of dogs (about 1,100 olfactory receptor genes (not counting incomplete genes) and 20.3% pseudogenes (Rouquier and Giorgi, 2007)) and mice (~1,200 functional olfactory genes (Rouquier and Giorgi, 2007; Zhang et al., 2007) and 20% pseudogenes (Rouquier and Giorgi, 2007)).
In addition to the main olfactory system, the domestic pig also has a functional vomeronasal organ (VNO) (Figures 1A, B) (Salazar et al., 2000). The VNO is, among other functions, involved in the detection of pheromones [although not androsterone in pigs (Dorries et al., 1997)]. The VNO is believed to have a central function in the expression of sexual behavior by releasing the LHRH (luteinizing-hormone-releasing hormone), which stimulates mounting behavior (e.g., Døving and Trotier, 1998) and studies suggest that the VNO of domestic pigs is already functional at birth (Horrell and Eaton, 1984; Salazar et al., 2004). The VNO of pigs has only V1R receptors and no V2R or FPR receptors (Liberles et al., 2009; Dinka et al., 2016), as opposed to, for instance, the VNOs of rats and mice, which have all families of receptors (Herrada and Dulac, 1997; Rodriguez et al., 2002). This indicates that the VNO of pigs may not be as capable of detecting many different pheromones as the rodent VNO, but it is still comparable to that of dogs, which also has only V1R receptors (Coli et al, 2016). In contrast to domestic dogs (Coli et al, 2016), there is no indication of the VNO of pigs undergoing involution (Barrios et al., 2014). Consequently, unlike for dogs, there is no morphological signs that pigs’ sense of smell has regressed following domestication. Recent studies have also illustrated an important role of the VNO in the chemical communication and social behavior of pigs. Asproni et al. (2022) showed an association between aggression (measured as wounds on the body of the pigs) and vomeronasalitis (i.e., inflammation of the VNO), and Mechin et al. (2022) later found that this condition led to a reduction in the thickness of the sensory epithelium, possibly explaining why vomeronasalitis seems to disrupt chemical communication, leading to aggression.
Collectively, these studies illustrate that the olfactory organs of the pig are well developed, large, and well organized, doubtlessly due to the central role that olfaction plays in the biology of the pig. The next section will review and discuss the currently available definitions of the various chemicals that commercially housed pigs may encounter in their life.
Only a few studies have sought to demonstrate the olfactory acuity in pigs. Jones et al. (2001) found that juvenile Duroc × Landrace crossbreds had an olfactory detection threshold for butanol at 2.09 parts per trillion, and Sondergaard et al (2010) found a detection threshold for Göttingen minipigs of 0.05 parts per million (ppm) for ethanol and 0.01 ppm for ethyl acetate. As for other animal species, results of the study indicated inter-individual variability in the sensitivity (Sondergaard et al., 2010). For comparison, the detection threshold for methyl and ethyl acetate in humans are around 103 and 102 ppm, respectively (Cometto-Muniz and Cain, 1991). In a study by Laska and Seibt (2002), squirrel monkeys and pigtail macaques could detect ethanol at 368.55 ppm (110.56 for the best individual). In another study, Croney et al. (2003) tested whether pigs (so-called micro pigs) could utilize visual (colors orange and green) or olfactory (odors coconut and almond) cues to locate a food source in novel surroundings. Pigs provided with either a visual or an olfactory cue performed equally well in the test and the authors thus suggested that olfactory cues are as salient as visual cues for pigs in learning and foraging situations. Collectively these studies imply a high olfactory acuity in pigs.
Pigs are omnivorous animals and their natural sources of food are found sporadically within large home range areas. Hence, under natural conditions, pigs spend a substantial amount of their active time searching for food (Stolba and Woodgush, 1989). When pigs explore their environment, they rely on their sensory apparatus, which includes sight, hearing, taste, touch, and smell. To allow their olfactory organ to detect odorants of potential interest, pigs will direct their snout toward the odor, and sniff the air near the source to allow their olfactory organ to detect volatile odorants (see details about the pig olfactory organ and odor detection and acuity above). Sniffing behavior is a well-known part of pig exploratory behavior (Studnitz et al., 2007), and since pigs are already able to utilize olfaction at birth (Morrow-Tesch and McGlone, 1990a), sniffing/smelling is an innate part of pigs’ behavioral repertoire. Pigs display sniffing in several situations, for instance when exploring odors and new environments, and sniffing is also an important part of feed-seeking behavior (see: Studnitz et al., 2007). The pig will use its snout to sniff and root in the substrate to locate edible parts, and, to some degree, to satisfy a motivation to explore (termed inquisitive exploration) (Wood-Gush and Vestergaard, 1989; Day et al., 1995). Sniffing and rooting are thus often connected. Explorative behavior may, moreover, include licking and/or biting the substrate. While solely inhaling the air near an odor may not allow for the detection of less-volatile substrates, rooting, licking, and biting behavior may allow the less-volatile substrates to be detected by the VNO (Figure 1). In addition to sniffing, rooting, licking, and biting behavior, pigs may also express rubbing and rolling behavior when exposed to certain odors (Rørvang et al., 2023b.). These behaviors were recently described as a response to odors in pigs for the first time, and, although the mechanisms underlying the behaviors are not fully understood, the behaviors emphasize that pigs’ behavioral repertoire when exposed to odors includes more than just sniffing and rooting. However, it remains to be shown if rubbing and rolling behaviors imply that physical access to odors is important to pigs, and if, when given access, these behaviors will increase or decrease. Nonetheless, some odors seem to evoke specific types of behavioral reactions in pigs (as, e.g., rubbing and rolling), emphasizing the need for further studies of how pigs perceive the chemicals in their surroundings.
The abovementioned illustrates that, when conducting pig olfactory research, it is important to consider which behaviors are relevant to include with regard to the study aim. For example, if investigating the suitability of a given odor as enrichment for pigs, measuring only sniffing behavior would likely not paint the full picture of pigs’ interest in, or preference for, the odor. We therefore recommend that researchers consider the full odor exploration behavioral repertoire by (1) allowing pigs physical access to the odors studied to allow for the display of licking, biting, rooting, rubbing, and rolling, as well as other relevant behaviors not currently mapped, and (2) including the aforementioned behaviors in the ethograms when studying pig odor exploration. If, on the other hand, the study aims at clear-cut olfactory research as, for instance, pigs’ ability to detect a given odor, then pigs should not be allowed physical contact to the odor, to avoid confounding smell and taste of the substrate being tested. It is important to consider the health status of the olfactory organs of the pigs, as recent studies on the pig VNO have shown that inflammation of the VNO leads to increased aggression (see above, and Asproni et al., 2022; Mechin et al., 2022) and thus this will affect the results if pigs are tested/housed in a social environment. Lastly, it may also be important to consider the previous housing conditions of the animals studied, as Jones et al. (2001) found indications that prolonged exposure to 40 ppm NH3 was damaging to the olfactory apparatus of pigs.
In the below sections the review considers potentially aversive and attractive odors and discusses the implications these may have on the welfare and production of pigs in a commercial environment. Unfortunately, so far, no studies have sought to clarify why pigs appear to perceive some odors as aversive and others as attractive. Therefore, it is not known if attractive/aversive odors share some specific structural/chemical properties or whether the reactions are elicited by, for example, the concentration or potency of the odors.
One of the major challenges in modern commercial pig production is the inevitable gas emissions from the production. Aerial contaminants constitute a concern for the environment, for the health of the humans working in the buildings, and for the animals being housed in these buildings (Wathes and Charles, 1994). Ammonia (NH3) and hydrogen sulfide (H2S) are the most well-known gas emissions arising from the manure of pigs (Donham et al., 1977; Bottcher, 2001). While NH3 is a result of the incomplete feed nitrogen conversion into animal product (meat and milk), H2S is produced under anaerobic conditions, and thus mainly arises from manure storage (Donham et al., 1977). Several studies document that high NH3 concentrations may occur in indoor pig production systems (Figure 3), with temporary levels rising to levels that cause concern for animal health (average 4.5–17.8 ppm for sows, weaners, and finishers (recorded in the Netherlands (maximum level 59.8 ppm), Germany (maximum level 43.7 ppm), England (maximum level 58.6 ppm), and Denmark (maximum level 43.8 ppm) (Koerkamp et al., 1998)): average 9.1–15.4 ppm for sows, weaners, and finishers; 16% recordings above 20 ppm (Seedorf and Hartung, 1999); 3.9–14.4 ppm in the outlet air from a Swedish finisher barn (Jeppsson et al., 2021a; Jeppsson et al., 2021b)). Ammonia concentrations above 100 ppm cause a loss in feed consumption and thus weight gain in pigs (Stombaugh et al., 1969) and concentrations of 50–75 ppm have a negative impact on pigs’ ability to clear bacteria from their lungs (Drummond et al., 1978). Jones et al. (2001) investigated the effect of acute and chronic exposure of juvenile pigs to NH3 (~40 ppm). Although acute exposure did not appear to affect rooting behavior or olfactory acuity, the authors found indications that prolonged exposure to 40 ppm NH3 was damaging to the olfactory apparatus and hence to the sensory capabilities of pigs. In another study, chronic exposure to NH3 (43 ppm) did not impair the pigs’ ability to perceive a similar NH3 concentration in an open field test (Jones et al., 2000). Furthermore, when exposed to a similar NH3 concentration (43 ppm) in the open field test, the pigs showed a reduced latency to approach the center of the open field and increased general activity (Jones et al., 2000). The results of (Jones et al., 2000; Jones et al., 2001) can seem contradictory and it remains unclear how chronic exposure to high and low concentrations of NH3, and low concentrations combined with acute peaks in the NH3 concentration (as is common in commercial indoor systems), affect the animals and their olfactory abilities.
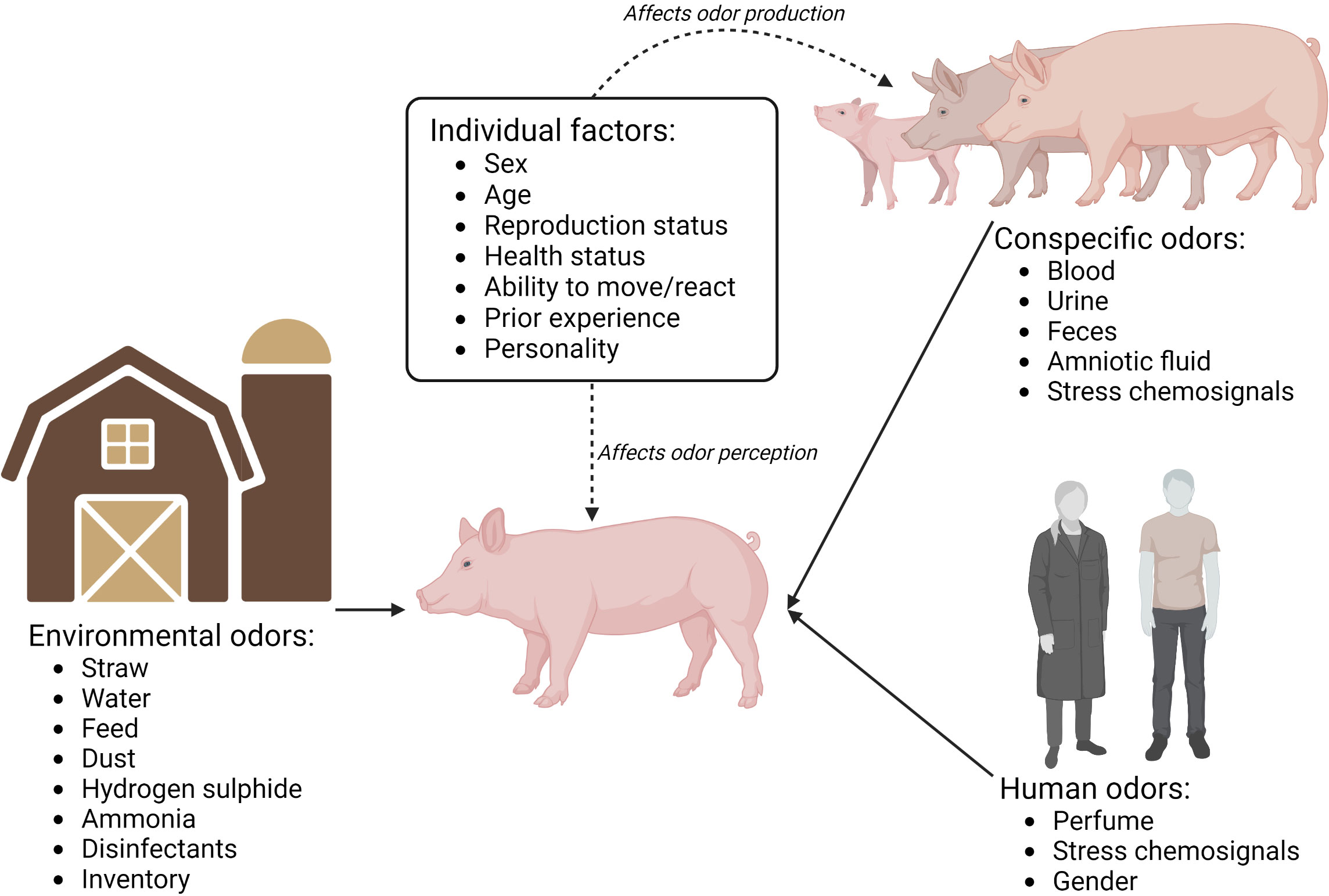
Figure 3 A theoretical overview of odors in commercial pig housing systems, which pigs may be exposed to. Odors can arise from conspecifics, humans, and the environment/housing itself. Dashed arrows represent how individual factors (sex, age, reproduction status, ability to move or react when detecting the odor, prior experience with the odor, and perhaps personality) can affect the perception and production of odors. Future research should focus on the study of such aspects to expand our knowledge about pig olfaction. Created with BioRender.com.
H2S is commonly present in pig houses (Donham, 2000) (Figure 3), although often at lower concentrations. The odor of this gas has a very low odor detection threshold (0.01–0.3 ppm for humans) and studies found that the smell of H2S is often perceived as aversive to, and provokes physical reactions (e.g., nausea) in, humans. At concentrations below 10 ppm, H2S has no adverse effects on pig growth (Curtis et al., 1975), but exposure at > 50 ppm causes chronic intoxication (Smith and Gosselin, 1979). Furthermore, at a concentration of 5 ppm, which can be noxious to humans, symptoms of anxiety have been observed in humans and, with respect to the lethality and harmful effects of H2S concentration, the specific exposure is more important than the duration of exposure (Guidotti, 2010). Thus, although no studies have shown direct adverse effects on pig welfare from exposure to H2S, there is reason to believe that pigs might suffer from exposure to H2S. Future research is needed to clarify whether this is the case.
A complicating factor in the spread of the above-described harmful gases is their ability to get absorbed by airborne dust particles. Most such dust particles arise from the feedstuffs on farms (Bottcher, 2001) and can lead to an increased concentration of NH3 and H2S absorbed in the respiratory system (Donham et al., 1986). In commercial pig housing systems, studies report that as many as 50% of the workers at pig farms experience respiratory diseases (Reynolds et al., 1996). NH3 exposure has proven to cause ophthalmia and to raise the apoptosis rate of neuron cells (Yang et al., 2003) and spleen cells (An et al., 2019) in humans. In pigs, recent research found that high levels of NH3 induced a series of abnormal physiological responses (Wang et al., 2020a; Wang et al., 2020b). In addition, NH3 exposure has also been shown to cause chronic irritation of both the olfactory and VNO epithelium of rabbits (Gaafar et al., 1998), and it is thus likely that NH3 (and other emission gases such as H2S) have a similar impact in pig olfaction and thereby negatively affect their welfare. In Sweden, national regulations state the minimum air quality standards for pig barns (minimum air quality requirements: maximum 10 ppm NH3, 3,000 ppm CO2, 0.5 ppm H2S, organic dust 10 mg/m3 (Jordbruksverk, 2019)), although the formulated levels have not yet been supported by scientific studies. Furthermore, no specific levels or legal requirements for air quality have been formulated in relation to the welfare of animals in the EU (Union, E, 2009), although the 1998 Council Directive states that “Air circulation, dust levels, temperature, relative air humidity and gas concentrations must be kept within limits, which are not harmful to the animals” (Union, E, 1998). Due to the potential damaging effects that elevated NH3 and H2S concentrations (and other gases) may have on the olfactory apparatus of pigs, further studies are needed to establish the detailed need for minimum air quality requirements in commercial pig houses throughout the EU.
As mentioned above, commercially housed pigs are exposed to a variety of olfactory inputs, some of which may be perceived by the pigs as aversive or unpleasant. Many of the odors that pigs are exposed to are substances that pigs would not encounter in the wild (e.g., the smell of disinfectants), and some are present in a much larger scale or variety than they would be in nature (e.g., pheromones from more than 100 conspecifics at the same time). Some odors may be odors that pigs would attempt to avoid in nature (e.g., the smell of conspecifics’ blood and humans). It is currently not well established how exposure to these odors and odor concentrations affect the animals, as only a few studies exist. In their line of experiments of nursing piglets, Morrow-Tesch and Mcglone (1990b) exposed litters of nursing piglets to novel odors (NI-217, amyl acetate). The authors then compared the piglets’ behavioral reaction to that being elicited by odors originating from the sow (such as urine, milk, and feces). NI-217 is a commercially available mixture of terpene-type citrus oils, which has an orange-like smell, and amyl acetate is a commonly used single molecule odorant with a characteristic banana smell. The piglets reacted with repulsion (i.e., avoidance behavior) to the smells of amyl acetate and NI-217. The aversive reaction may be, however, not only caused by the odors per se but a reaction to the novelty or the high concentration of the odors. Krebs (2007) also found that pigs were repulsed by sour milk, and in a field test pigs did not come within 0.5 m of control or sour milk odors. Again, this may be a reaction to the novelty of the odor (particularly where the novel odor was combined with a field test) rather than pigs in general being repulsed by the smell of sour milk. Earlier, before regulations regarding food hazards and biosecurity were implemented, it was common to feed pigs with leftovers from the kitchen, including spoiled/sour milk, which pigs voluntarily consumed. It thus seems unlikely that pigs, in the study by Krebs (2007), avoided the smell of milk due to health hazards and/or an unwillingness to consume it. A contributing factors influencing how pigs react toward novel odors is age. Docking et al. (2008) found a shorter latency to approach novel enrichment objects in growers (4–20 weeks of age, > 5 kg) than in weaners and sucklers (0–4 weeks of age, < 5 kg). In our recent study, finisher pigs exposed to a variety of novel (natural origin) odors did not show aversion/repulsion to the odors of study and some odors, although novel, even evoked further explorative behavior (sniffing, biting, and licking) (Rørvang et al., 2023a). It may thus be a valid argument to use growing/finishing pigs to limit the potential effect of novelty expressed by younger pigs when testing pigs’ reactions to odors, but it may be difficult to completely avoid novelty effects while also ensuring no prior experience with the odors tested.
In their pioneering study, Morrow-Tesch and McGlone (1990a) demonstrated that piglets learn to recognize the odor of their mother within the first 12h after birth. Before that time, a maternal–neonatal pheromone is controlling nursing behavior (Morrow-Tesch and McGlone, 1990a) (Figure 3). The authors further suggest that the olfactory abilities of piglets should be considered when aiming to modify pig behavior to improve survival. Attractive or controlling odors are thus a central aspect of the life of pigs. In this section we focus on complex odors of non-social origin, as studies of pig pheromones and the effects of these are covered elsewhere (e.g., McGlone et al., 2022).
Only a few studies have assessed pigs’ interest in odors of non-social origin. These studies have mainly used complex odors and only a few controlled for taste (i.e., pigs are restricted from licking/eating the odors) and thereby purely tested for the effect of smell. Blackie and de Sousa (2019) investigated if weaner pigs (housed in a semi-barren environment) showed a preference for an odorized rope with garlic to a non-odorized rope. The pigs showed a preference for the odorized rope and although the authors noted habituation over time (2-week testing period), interest was reinstated by re-spraying the odor onto the rope. It is unclear if the pigs preferred the odorized rope due to the taste or smell, or a combination of the two, as pigs had physical access to biting and licking the ropes.
In conformity with this, Nowicki et al. (2015) found that pigs spent more time near natural odors (in the Nowicki article termed “aromas”)—moist soil, grass, and dried mushrooms—than synthetic odors—vanilla, orange, and strawberry, with strawberry being the most popular synthetic aroma. In a second part of their experiments, Nowicki et al. (2015) further showed that pigs used odorized objects (aromas of natural origin) for longer than identical odorless objects, although the detected difference was insignificant. Moreover, the authors found that, although the interest in the objects decreased over time, interest in the odorized objects remained at a higher level for the duration of the experiment (14 days). Changing the odor after 14 days resulted in a significant rise in interest in the odorized object in comparison to odorless objects. These results suggest that a given enrichment object or material may have increased value to pigs if odors are added to it, and, further, that odors in general may constitute a useful tool to increase the attractiveness and durability of enriching objects and materials.
In a recent study, we and our co-authors investigated pigs’ interest in a range of complex odors of non-social origin (Rørvang et al., 2023a). The study was an attempt at mapping pigs’ ability to detect and distinguish between such odors and to evaluate pigs’ interest in each odor. The pigs were free to explore and sniff the odor but did not have access to physically bite/lick/touch the odor source (i.e., not able to taste the odor). The study showed that pigs were able to detect and differentiate between all odors and that sniffing, licking, biting, and rooting were common parts of pig odor exploration behavior (as pigs directed these behaviors toward the odor, rather than an odorless control) even without having physical access to reach the odor. None of the odors, however, elicited more sniffing (as a measure of interest) than others, although non-significant differences were found. Moreover, since pigs were tested in pairs, social behavior was observed when pigs were exploring the odors. Pushing of the other pig while sniffing the odor, bite aggressions, and displacements of the other pig from the odor were observed, and these were almost exclusively expressed while exploring the odors as opposed to the odorless controls. The occurrence of these social behaviors was not dependent on the specific odor (Rørvang et al., 2023a). Collectively, the results of the above-described studies imply that odors are perceived as a resource to pigs, and that access to odors may constitute enrichment either directly via sniffing or when applied to other existing enrichment materials. However, odors should be selected with care as some may result in aggression/resource guarding. Further studies on the use of odors as enrichment materials are needed.
As emphasized by Andersen et al. (2020), being able to control pig elimination (defecation) behavior is of major interest due to the potential of improving animal welfare and farmer working conditions and reducing pen soiling, collectively resulting in a reduced environmental footprint from this animal production system. Controlling pig elimination behavior seems possible as pigs do not eliminate in random places. In their review, Andersen et al. (2020) found that pigs prefer to eliminate away from their nest. Piglets less than 24h old have even been observed moving away from the farrowing nest to eliminate (Petherick, 1983; Stangel and Jensen, 1991). Although there is an age-dependent development in the elimination behavior of pigs, no clear pattern in the behavior has emerged (Andersen et al., 2020). However, studies do show that exploratory behavior and sniffing may play a role in pig elimination behavior (Wechsler and Bachmann, 1998; Guo et al., 2015), and studies demonstrated that sniffing or exploring preceded 50%–70% of all elimination observations (Hartsock and Barczewski, 1997; Guo et al., 2015). Therefore, it is relevant to speculate if odors may be utilized to manage pig eliminations, and a question for future studies to explore is whether odors play a role in this behavior. Several farmers experience problems with pigs eliminating in parts of the pen not intended for this purpose. Thus, identifying odors that pigs relate to elimination could hold potential for directing the behavior to the intended pen areas.
Research on potential calming effects of odors on animal stress is sparse, but the few studies available show promising results. The most commonly used odors in these studies are essential oils (i.e., of pure natural origin). Some of these odors appear to encourage relaxation and alleviate stress. The ambient odor of lavender, for instance, has repeatedly been shown to decrease motility in laboratory-housed rodents (Buchbauer et al., 1991; Shaw et al., 2007), and to reduce activity and vocalizations in dogs housed in rescue shelters (Graham et al., 2005). Bradshaw et al. (1998) provided pigs with access to lavender straw in an attempt to reduce stress (salivary cortisol) and travel sickness (indicators, e.g., foaming, repetitive chomping, retching, and vomiting) during transit. Lavender straw did not affect the overall levels of salivary cortisol, but the authors suggested that lavender straw may lower the incidents and severity of travel-induced sickness. In their study, Oostindjer et al. (2011) explored whether feeding sows with an anethol diet (a very small-sized molecule, thought to be able to pass from the placenta to the fetus; personal communication with the authors) late postpartum and during lactation would result in lowered stress in their piglets post weaning. Post-weaning piglets received anethol either in their feed or in the air. Piglets exposed to anethol pre weaning tended to have lower cortisol levels post weaning and to vocalize less on day 1 after weaning. The piglets with anethol also displayed more play behavior in the first week post weaning. The authors concluded that perinatal flavor learning may result in lowered stress when individuals are exposed to a familiar flavor or odor during a challenging situation (Oostindjer et al., 2011). This suggests that the odors could be used to calm the animals/reduce stress when pigs enter unfamiliar areas or situations. In another study, Oostindjer et al. (2009) found behavioral changes in piglets exposed prenatally to flavor (anise) through the maternal diet, suggesting flavor recognition when re-exposed to the flavor postnatally.
Studies on rodents show that rats can learn to associate an unrelated odor with a pleasant human interaction (e.g., tickling) (Bombail et al., 2019), and that male rats prefer to mate with females that smell of almond (or even cadaverine) if they have previously copulated with females doused with these odors (Kippin and Pfaus, 2001; Pfaus et al., 2001). Dudink et al. (2006) showed that ringing a bell (neutral stimulus) while distributing seeds (unconditioned stimulus) to nursing pigs in the farrowing crates led to long-lasting conditioning. There is thus reason to suggest that pigs (and other farm animal species) may be trained to form a positive association between an odor and a pleasant experience. From studies of humans, we also know that specific odors can elicit certain memories (Jellinek, 2004); the same appears to be true for dogs (Quaranta et al., 2020).
Thus, if pigs could be conditioned to associate certain odors with a pleasant experience or memory, this could be beneficial in several situations within pig production, particularly in relation to stressful events. If odors could be used to attract pigs to a certain place (e.g., barn section) it would limit the need for physically driving/moving the animals, a practice that is associated with a high risk of injuries and stress in the animals (e.g., Brandt and Aaslyng, 2015; Lindahl et al., 2016) and risk of worker injuries (Langley and Morrow, 2010). Therefore, it could be relevant for future studies to investigate whether pigs are able to associate odors with a pleasant experience or memory, and how this may be utilized in practice.
The olfactory systems are the first among the sensory systems to develop in the mammalian (including human) fetuses, and evolutionarily the olfactory bulb gave rise to the limbic system (Joseph, 2013). The olfactory bulb is thus connected to the limbic system (the brain areas controlling emotions, instincts, and memory), which has been referred to as the “nose-brain” (Lledo et al., 2005). The primary olfactory cortex (located in the piriform cortex) projects to the hypothalamus, the hippocampus, and the amygdala (Figure 2A); the amygdala induces emotions and facilitates coding of memories (Soudry et al., 2011). The olfactory system thus shares a close anatomical link to the brain circuits involved in memory (Savic et al., 2000), learning (Soudry et al., 2011), and emotion (Anderson et al., 2003), which explains why olfactory stimuli can evoke emotional memory (as described above in section 8.2). Structural and functional imagery techniques have recently allowed for the identification of overlapping brain areas involved in olfactory processing and depression in humans (Soudry et al., 2011; Höflich et al., 2012; Naudin and Atanasova, 2014). A close link between olfaction and depression is further emphasized by the lower olfactory performance displayed by people affected by depression, as well as the reciprocal effect in patients with olfactory dysfunction, in which the depression commonly worsens with the severity of olfactory impairment. On a more optimistic note, a recent study showed that a rich olfactory environment can improve olfactory abilities in humans, potentially also enhancing mental health (Oleszkiewicz et al., 2021). Research on urban stress-relieving gardens/forests further show that, when separating the impact of visual, auditory, and olfactory nature stimuli on stress reduction (such as feeling calm and relaxed), odors seem to have a more profound effect on stress reduction than visual and auditory stimuli (Hedblom et al., 2019). Hence, being a macrosmatic animal, the impact of odors on pigs’ affective states could be even greater. In this case, exposing pigs to complex odors of non-social origin could improve their welfare via the direct link between olfactory processing and wellbeing described above. The volatile molecules of the non-social complex odors will be inhaled by the pigs, and once dissolved in the nasal mucus the molecules will bind to the respective receptors, allowing the electrical stimulation of the olfactory bulb (Figure 2A). In this case, the MOB is stimulated, which projects through the lateral olfactory tract to many basal areas of the brain, but, importantly, to the primary olfactory cortex (Figure 2A), which projects to the limbic system (Lledo et al., 2005). In case of involvement of the accessory olfactory system, the odor molecules would instead enter the VNO via the incisive canals from the mouth and, upon binding to receptors, stimulate the AOB (Døving and Trotier, 1998; Brunjes et al., 2016). The AOB projects through the accessory lateral olfactory tract to, among other brain areas, the medial region of the amygdala (vomeronasal amygdala), from which tertiary projections target certain regions of the hypothalamus (Brunjes et al., 2016). The accessory olfactory system is known to control innate endocrine and behavioral reactions, whereas the main olfactory system modulates conscious odor perceptions linked to emotions and memory (Meisami and Bhatnagar, 1998). Although it is not possible to elucidate specifically which olfactory system is involved when pigs sense complex odors of non-social origin (it is also likely that both are involved), the high volatility of these chemicals implies that the MOB plays a central role. Exposure to non-social, complex odors may thus directly affect the emotions and memories and thus the welfare of pigs, and it is possible that repeated exposures could be linked to form positive experiences or odor memories (see section 8.2) if done with the right positive stimulus and the right timing between unconditioned odor and positive stimulus (e.g., food). More research on this topic should be a future focus of animal welfare research.
In this section, we want to highlight the five areas identified for future study into pig olfaction, identified as the most urgent for the sustainability of pig production and for improving the welfare of commercially housed pigs:
(1) Ammonia and hydrogen sulfide are a major concern in animal welfare as these gases are present at temporarily high concentrations in all types of pig house units and barn sections. Future studies should aim to clarify what levels of ammonia and hydrogen sulfide are acceptable when considering the welfare of the pigs, and the potential effects on the pigs’ olfactory abilities. Currently, the regulations and/or recommendations stating a maximum acceptable level of emissions are merely based on a concern for the humans working in the barn rather than a concern for animal welfare. Additionally, the standards are set from a human health perspective and do not consider how the animals (or human workers) may perceive the constant exposure to aversive odors. Future studies should thus focus on defining at which level or concentration these gases become unpleasant, aversive, and damaging to pigs (and humans).
(2) It may be argued that the smell of feed is attractive to pigs, but, considering restrictively fed pregnant sows, it may be that the constant smell of non-accessible feed (especially in the increasingly used automatic feeding systems (e.g., electronic sow feeders (ESFs)) is a stressor to the animals. Whether this is the case, however, is currently not known, and thus warrants further investigation to safeguard the welfare of sows.
(3) A large number of pigs are usually housed in the same barn section, and this arrangement means that each pig has to process and relate to odors of social origin arising from many different individuals. Whether this arrangement causes stress remains unexplored and deserves further studies, but it is also worth analyzing if pigs perceive the many different origins, or if pigs may habituate or only relate to the social odors in their very near vicinity.
(4) During management practices such as tail docking and castration, the smell of blood can become intense in the farrowing unit, at least to the human nose (personal perception, and personal communication with numerous Scandinavian pig farmers and staff). To the best of our knowledge there are currently no studies on how this exposure affects the animals—with respect to the smell of blood from both unfamiliar animals and siblings and offspring—and hence it is unknown if measures should be taken to limit or cover up the smell. As a first step, it is necessary to investigate if pigs detect the smell of conspecific blood, and secondly whether this odor is perceived negatively.
(5) A variety of odors is present in pig houses, but usually the odor composition is rather constant throughout the life of the pig. Hence, the olfactory environment lacks complexity in terms of odor variation. Considering the macrosmatic pig, it would be worth looking into if a lack of olfactory stimulation can contribute to boredom, and if odor stimulation could reduce symptoms of boredom. Additionally, it would be relevant to unravel if olfactory stimulation of pigs affects pigs’ sense of smell, thereby improving pigs’ olfactory abilities; the equivalent has been documented in humans (Oleszkiewicz et al., 2021). Since odors do not interfere with the inventory or housing system and could readily be applied by, for instance, spraying odors onto existing enrichment material, it may be relatively easy to implement “odor provision” and thereby stimulate these macrosmatic animals. These are all central future research questions in order to refine and optimize commercial pig production management and systems.
Olfaction is a central part of the life of pigs. However, pigs’ highly developed sense of olfaction combined with the conditions of commercial pig production gives rise to concerns for animal welfare. Various emissions from pig barns and chemicals used inside the barns may be perceived as aversive by the animals, which could be detrimental to their welfare. Contrarily, other odors, rarely present in pig facilities, appear attractive and could hold enriching properties for pigs. Considering the central role of olfaction, improvements to the olfactory environment for commercial pig-producing facilities could be a major step forward in improving the welfare of our farmed pigs and the sustainability of the production system.
Author contributions according to CRediT in the order authors appear on the paper: S-LS: conceptualization, investigation, writing—original draft, writing—review and editing, and funding acquisition. MR: conceptualization, methodology, investigation, resources, writing—original draft, writing—review and editing, visualisation, project administration and funding acquisition.
Funding for conducting this review for MR was provided by the Swedish University of Agricultural Sciences, Department of Biosystems and Technology, Alnarp, Sweden, and the Department of Animal Environment and Health, Skara, Sweden, and for S-LS by the Innovation Center for Organic Agriculture, Aarhus, Denmark.
The authors would like to thank Dr. Heidi Mai-Lis Andersen, Innovation Center for Organic Farming, Aarhus N, Denmark, and Dr. Anna Wallenbeck, Swedish University of Agricultural Sciences, Department of Animal Environment and Health, Skara, Sweden, for valuable input, and the reviewers for very helpful comments and suggestions.
First author S-LS is employed by the Innovation Center for Organic Farming. The company is a non-political institution and thus the political interest of its owner organizations are not influencing the decision-making or scientific work of the company. Funding for working hours for S-LS was funded by the Innovation Center for Organic Farming, Denmark.
The remaining author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Amaral A. J., Ferretti L., Megens H. J., Crooijmans R. P. M. A., Nie H. S., Ramos-Onsins S. E., et al. (2011). Genome-Wide Footprints of Pig Domestication and Selection Revealed through Massive Parallel Sequencing of Pooled DNA. PloS One 6, e14782. doi: 10.1371/journal.pone.0014782
An Y., Xing H. J., Zhang Y., Jia P. C., Gu X. H., Teng X. H. (2019). The evaluation of potential immunotoxicity induced by environmental pollutant ammonia in broilers. Poultry Sci. 98, 3165–3175. doi: 10.3382/ps/pez135
Andersen H. M. L., Kongsted A. G., Jakobsen M. (2020). Pig elimination behavior-A review. Appl. Anim. Behav. Sci. 222, 104888. doi: 10.1016/j.applanim.2019.104888
Anderson A. K., Christoff K., Stappen I., Panitz D., Ghahremani D. G., Glover G., et al. (2003). Dissociated neural representations of intensity and valence in human olfaction. Nat. Neurosci. 6, 196–202. doi: 10.1038/nn1001
Asproni P., Mainau E., Cozzi A., Carreras R., Bienboire-Frosini C., Teruel E., et al. (2022). Is There a Link between Vomeronasalitis and Aggression in Stable Social Groups of Female Pigs? Animals 12, 303. doi: 10.3390/ani12030303
Barrios A. W., Sanchez-Quinteiro P., Salazar I. (2014). Dog and mouse: toward a balanced view of the mammalian olfactory system. Front. Neuroanat. 8. doi: 10.3389/fnana.2014.00106
Beauchamp R., Bus J. S., Popp J. A., Boreiko C. J., Andjelkovich D. A., Leber P. (1984). A critical review of the literature on hydrogen sulfide toxicity. CRC Crit. Rev. Toxicol. 13, 25–97. doi: 10.3109/10408448409029321
Blackie N., de Sousa M. (2019). The Use of Garlic Oil for Olfactory Enrichment Increases the Use of Ropes in Weaned Pigs. Animals 9z, 148. doi: 10.3390/ani9040148
Bombail V., Jerome N., Lam H., Muszlak S., Meddle S. L., Lawrence A. B., et al. (2019). Odour conditioning of positive affective states: Rats can learn to associate an odour with being tickled. PloS One 14, e0212829. doi: 10.1371/journal.pone.0212829
Booth W. D., Signoret J. P. (1992). Olfaction and reproduction in ungulates. Oxf Rev. Reprod. Biol. 14, 263–301.
Bottcher R. W. (2001). An Environmental Nuisance: Odor Concentrated and Transported by Dust. Chem. Senses 26, 327–331. doi: 10.1093/chemse/26.3.327
Bradshaw R. H., Marchant J. N., Meredith M. T., Broom D. M. (1998). Effects of lavender straw on stress and travel sickness in pigs. J. Altern. Complementary Med. 4, 271–275. doi: 10.1089/acm.1998.4.3-271
Brandt P., Aaslyng M. D. (2015). Welfare measurements of finishing pigs on the day of slaughter: a review. Meat Sci. 103, 13–23. doi: 10.1016/j.meatsci.2014.12.004
Brunjes P. C., Feldman S., Osterberg S. K. (2016). The Pig Olfactory Brain: A Primer. Chem. Senses 41, 415–425. doi: 10.1093/chemse/bjw016
Buchbauer G., Jirovetz L., Jager W., Dietrich H., Plank C., Karamat E. (1991). Aromatherapy - Evidence for Sedative Effects of the Essential Oil of Lavender after Inhalation. Z. Fur Naturforschung C-a J. Biosci. 46, 1067–1072. doi: 10.1515/znc-1991-11-1223
Coli A., Stornelli M. R., Giannessi E. (2016). The dog vomeronasal organ: A review. Dog Behav. 1, 24–31. doi: 10.4454/db.v2i1.27
Cometto-Muniz J. E., Cain W. S. (1991). Nasal pungency, odor, and eye irritation thresholds for homologous acetates. Pharmacol. Biochem. Behav. 39, 983–989. doi: 10.1016/0091-3057(91)90063-8
Conrad M. S., Sutton B. P., Dilger R. N., Johnson R. W. (2014). An In Vivo Three-Dimensional Magnetic Resonance Imaging-Based Averaged Brain Collection of the Neonatal Piglet (Sus scrofa). PloS One 9, e107650. doi: 10.1371/journal.pone.0107650
Croney C. C., Adams K. M., Washington C. G., Stricklin W. R. (2003). A note on visual, olfactory and spatial cue use in foraging behavior of pigs: indirectly assessing cognitive abilities. Appl. Anim. Behav. Sci. 83, 303–308. doi: 10.1016/S0168-1591(03)00128-X
Curtis S., Drummond J., Simon J. (1975). Effects of aerial ammonia, hydrogen sulfide and swine-house dust on rate of gain and respiratory-tract structure in swine. J. Anim. Sci. 41 (3), 735–739. doi: 10.2527/jas1975.413735x
Day J. E. L., Kyriazakis I., Lawrence A. B. (1995). The Effect of Food-Deprivation on the Expression of Foraging and Exploratory-Behavior in the Growing Pig. Appl. Anim. Behav. Sci. 42, 193–206. doi: 10.1016/0168-1591(95)93889-9
Dinka H., Le M. T., Ha H., Cho H., Choi M. K., Choi H., et al. (2016). Analysis of the vomeronasal receptor repertoire, expression and allelic diversity in swine. Genomics 107, 208–215. doi: 10.1016/j.ygeno.2015.10.003
Docking C. M., de Weerd H. A. V., Day J. E. L., Edwards S. A. (2008). The influence of age on the use of potential enrichment objects and synchronisation of behaviour of pigs. Appl. Anim. Behav. Sci. 110, 244–257. doi: 10.1016/j.applanim.2007.05.004
Donham K. J. (2000). The concentration of swine production - Effects on swine health, productivity, human health, and the environment. Veterinary Clinics North America-Food Anim. Pract. 16, 559–597. doi: 10.1016/S0749-0720(15)30087-6
Donham K. J., Rubino M., Thedell T. D., Kammermeyer J. (1977). Potential health hazards to agricultural workers in swine confinement buildings. J. Occup. Environ. Med. 19, 383–387.
Donham K. J., Scallon L. J., Popendorf W., Treuhaft M. W., Roberts R. C. (1986). Characterization of dusts collected from swine confinement buildings. Am. Ind. Hygiene Assoc. J. 47, 404–410. doi: 10.1080/15298668691389955
Dorries K. M., Adkins-Regan E., Halpern B. P. (1997). Sensitivity and behavioral responses to the pheromone androstenone are not mediated by the vomeronasal organ in domestic pigs. Brain Behav. Evol. 49, 53–62. doi: 10.1159/000112981
Døving K. B., Trotier D. (1998). Structure and function of the vomeronasal organ. J. Exp. Biol. 201, 2913–2925. doi: 10.1242/jeb.201.21.2913
Drummond J. G., Curtis S. E., Simon J. (1978). Effects of atmospheric ammonia on pulmonary bacterial clearance in the young pig. Am J Vet Res 39 (2), 211–212.
Dudink S., Simonse H., Marks I., de Jonge F. H., Spruijt B. M. (2006). Announcing the arrival of enrichment increases play behaviour and reduces weaning-stress-induced behaviours of piglets directly after weaning. Appl. Anim. Behav. Sci. 101, 86–101. doi: 10.1016/j.applanim.2005.12.008
Ebrahimi F. A., Chess A. (1998). The specification of olfactory neurons. Curr. Opin. Neurobiol. 8, 453–457. doi: 10.1016/S0959-4388(98)80031-7
Eurostat (2022) Agricultural Production - Livestock and Meat - Statistics Explained. Available at: https://ec.europa.eu/eurostat/statistics-explained/index.php?title=Agricultural_production_-_livestock_and_meat&oldid=549389#Livestock_population (Accessed 20 January 2022).
Figueroa J., Sola-Oriol D., Manteca X., Perez J. F., Dwyer D. M. (2015). Anhedonia in pigs? Effects of social stress and restraint stress on sucrose preference. Physiol. Behav. 151, 509–515. doi: 10.1016/j.physbeh.2015.08.027
Firestein S. (2001). How the olfactory system makes sense of scents. Nature 413, 211–218. doi: 10.1038/35093026
Gaafar H., Tantawy A., Hamza M., Shaaban M. (1998). The effect of ammonia on olfactory epithelium and vomeronasal organ neuroepithelium of rabbits: a histological and histochemical study. ORL 60 (2), 88–91. doi: 10.1159/000027571
Giuffra E., Kijas J. M. H., Amarger V., Carlborg O., Jeon J. T., Andersson L. (2000). The origin of the domestic pig: Independent domestication and subsequent introgression. Genetics 154, 1785–1791. doi: 10.1093/genetics/154.4.1785
Godyn D., Nowicki J., Herbut P. (2019). Effects of Environmental Enrichment on Pig Welfare-A Review. Animals 9, 383. doi: 10.3390/ani9060383
Graham L., Wells D. L., Hepper P. G. (2005). The influence of olfactory stimulation on the behaviour of dogs housed in a rescue shelter. Appl. Anim. Behav. Sci. 91, 143–153. doi: 10.1016/j.applanim.2004.08.024
Guidotti T. L. (2010). Hydrogen Sulfide : Advances in Understanding Human Toxicity. Int. J. Toxicol. 29, 569–581. doi: 10.1177/1091581810384882
Guiraudie G., Pageat P., Cain A. H., Madec I., Nagnan-Le Meillour P. (2003). Functional characterization of olfactory binding proteins for appeasing compounds and molecular cloning in the vomeronasal organ of pre-pubertal pigs. Chem. Senses 28, 609–619. doi: 10.1093/chemse/bjg052
Guo Y. G., Lian X. M., Yan P. S. (2015). Diurnal rhythms, locations and behavioural sequences associated with eliminative behaviours in fattening pigs. Appl. Anim. Behav. Sci. 168, 18–23. doi: 10.1016/j.applanim.2015.01.011
Hartsock T. G., Barczewski R. A. (1997). Prepartum behavior in swine: Effects of pen size. J. Anim. Sci. 75, 2899–2904. doi: 10.2527/1997.75112899x
Hedblom M., Gunnarsson B., Iravani B., Knez I., Schaefer M., Thorsson P., et al. (2019). Reduction of physiological stress by urban green space in a multisensory virtual experiment. Sci. Rep. 9, 10113. doi: 10.1038/s41598-019-46099-7
Herrada G., Dulac C. (1997). A novel family of putative pheromone receptors in mammals with a topographically organized and sexually dimorphic distribution. Cell 90, 763–773. doi: 10.1016/S0092-8674(00)80536-X
Hoff S. J., Bundy D. S., Nelson M. A., Zelle B. C., Jacobson L. D., Heber A. J., et al. (2006). Emissions of ammonia, hydrogen sulfide, and odor before, during, and after slurry removal from a deep-pit swine finisher. J. Air Waste Manage. Assoc. 56, 581–590. doi: 10.1080/10473289.2006.10464472
Höflich A., Baldinger P., Savli M., Lanzenberger R., Kasper S. (2012). Imaging treatment effects in depression. Rev. Neurosci. 23, 227–252. doi: 10.1515/revneuro-2012-0038
Horrell R. I., Eaton M. (1984). Recognition of Maternal Environment in Piglets - Effects of Age and Some Discrete Complex Stimuli. Q. J. Exp. Psychol. Section B-Comparative Physiol. Psychol. 36, 119–130. doi: 10.1080/14640748408402198
Jacela J. Y., DeRouchey J. M., Tokach M. D., Goodband R. D., Nelssen J. L., Renter D. G., et al. (2010). Feed additives for swine: Fact sheets - flavors and mold inhibitors, mycotoxin binders, and antioxidants. J. Swine Health Production 18, 27–32. doi: 10.4148/2378-5977.7069
Jellinek J. S. (2004). Proust remembered: has Proust’s account of odor-cued autobiographical memory recall really been investigated? Chem. Senses 29, 455–458. doi: 10.1093/chemse/bjh043
Jeppsson K.-H., Olsson A.-C., Nasirahmadi A. (2021a). Increased air velocity in the lying area improves pen hygiene and reduces ammonia emissions from houses with partly slatted pens for growing/finishing pigs. Livestock Sci. 251, 104607. doi: 10.1016/j.livsci.2021.104607
Jeppsson K. H., Olsson A. C., Nasirahmadi A. (2021b). Cooling growing/finishing pigs with showers in the slatted area: Effect on animal occupation area, pen fouling and ammonia emission. Livestock Sci. 243, 104377. doi: 10.1016/j.livsci.2020.104377
John J. M., Edgar O. A.-R., Courtney A., Meyer M. W., Karlee D. J., Elaina M. M., et al. (2020). “Understanding Sow Sexual Behavior and the Application of the Boar Pheromone to Stimulate Sow Reproduction,” in Animal Reproduction in Veterinary Medicine. Eds. Faruk A., Rita P. C., Miguel Q. (Rijeka: IntechOpen), 2.
Jones J. B., Wathes C. M., Persaud K. C., White R. P., Jones R. B. (2001). Acute and chronic exposure to ammonia and olfactory acuity for n-butanol in the pig. Appl. Anim. Behav. Sci. 71, 13–28. doi: 10.1016/S0168-1591(00)00168-4
Jones J. B., Wathes C. M., White R. P., Jones R. B. (2000). Do pigs find a familiar odourant attractive in novel surroundings? Appl. Anim. Behav. Sci. 70, 115–126. doi: 10.1016/S0168-1591(00)00149-0
Jordbruksverk S. (2019). SJVFS2019:20: Statens Jordbruksverks Föreskrifter och Allmänna Råd om Grishållning inom Lantbruket m.M., Statens Jordbruksverks Författningssamling, Jönköping. Jönköping, Sweden: Statens Jordbruksverk.
Joseph R. (2013). The naked neuron: Evolution and the languages of the body and brain. (New York, USA: Springer). 1, 437. doi: 10.1007/978-1-4899-6008-5
Karlson P., Lüscher M. (1959). The proposed biological term ‘pheromone’. Nature 183, 1835. doi: 10.1038/1831835b0
Kavoi B. M., Jameela H. (2011). Comparative Morphometry of the Olfactory Bulb, Tract and Stria in the Human, Dog and Goat. Int. J. Morphology 29, 939–946. doi: 10.4067/S0717-95022011000300047
Kippin T. E., Pfaus J. G. (2001). The development of olfactory conditioned ejaculatory preferences in the male rat I. Nat. unconditioned stimulus. Physiol. Behav. 73, 457–469. doi: 10.1016/S0031-9384(01)00484-X
Koerkamp P. W. G. G., Metz J. H. M., Uenk G. H., Phillips V. R., Holden M. R., Sneath R. W., et al. (1998). Concentrations and emissions of ammonia in livestock buildings in Northern Europe. J. Agric. Eng. Res. 70, 79–95. doi: 10.1006/jaer.1998.0275
Krebs N. (2007). Odors and Pheromones: Influences of Olfaction on Behavior, Physiology, and Performance to Reduce Stress in Pigs (Texas, USA: Texas Tech University). Available at: http://hdl.handle.net/2346/15793.
Kyllar M., Stembirek J., Putnova I., Stehlik L., Odehnalova S., Buchtova M. (2014). Radiography, computed tomography and magnetic resonance imaging of craniofacial structures in pig. Anat Histol. Embryol. 43, 435–452. doi: 10.1111/ahe.12095
Langley R. L., Morrow W. E. M. (2010). Livestock Handling-Minimizing Worker Injuries. J. Agromedicine 15, 226–235. doi: 10.1080/1059924X.2010.486327
Laska M., Seibt A. (2002). Olfactory sensitivity for aliphatic alcohols in squirrel monkeys and pigtailmacaques. J. Exp. Biol. 205, 1633–1643. doi: 10.1242/jeb.205.11.1633
Lawrence A. B., Terlouw E. M. C. (1993). A Review of Behavioral-Factors Involved in the Development and Continued Performance of Stereotypic Behaviors in Pigs. J. Anim. Sci. 71, 2815–2825. doi: 10.2527/1993.71102815x
Liberles S. D., Horowitz L. F., Kuang D. H., Contos J. J., Wilson K. L., Siltberg-Liberles J., et al. (2009). Formyl peptide receptors are candidate chemosensory receptors in the vomeronasal organ. Proc. Natl. Acad. Sci. U.S.A. 106, 9842–9847. doi: 10.1073/pnas.0904464106
Lindahl C., Pinzke S., Herlin A., Keeling L. J. (2016). Human-animal interactions and safety during dairy cattle handling-Comparing moving cows to milking and hoof trimming. J. Dairy Sci. 99, 2131–2141. doi: 10.3168/jds.2014-9210
Lledo P. M., Gheusi G., Vincent J. D. (2005). Information processing in the mammalian olfactory system. Physiol. Rev. 85, 281–317. doi: 10.1152/physrev.00008.2004
Malnic B., Godfrey P. A., Buck L. B. (2004). The human olfactory receptor gene family (vol 101, pg 2584, 2004). Proc. Natl. Acad. Sci. U.S.A. 101, 7205–7205. doi: 10.1073/pnas.030788210
McGlone J. J. (1985). Olfactory cues and pig agonistic behavior: evidence for a submissive pheromone. Physiol. Behav. 34, 195–198. doi: 10.1016/0031-9384(85)90105-2
McGlone J. J., Devaraj S., Garcia A. (2019a). A novel boar pheromone mixture induces sox estrus behaviors and reproductive success. Appl. Anim. Behav. Sci. 219, 104832. doi: 10.1016/j.appanim.2019.104832
McGlone J. J., Garcia A., Rakhshandeh A. (2019b). Multi-farm analyses indicate a novel pheromone improves sow reproductive performance. Animals 9, 37–47. doi: 10.3390/ani9020037
McGlone J. J., Archer C., Henderson M. (2022). Interpretive review: Semiochemicals in domestic pigs and dogs. Front. Vet. Sci. 9. doi: 10.3389/fvets.2022.967980
Mechin V., Asproni P., Bienboire-Frosini C., Cozzi A., Chabaud C., Arroub S., et al. (2022). Inflammation interferes with chemoreception in pigs by altering the neuronal layout of the vomeronasal sensory epithelium. Front. Vet. Sci. 9. doi: 10.3389/fvets.2022.936838
Meese G., Baldwin B. (1975). The effects of ablation of the olfactory bulbs on aggressive behaviour in pigs. Appl. Anim. Ethology 1, 251–262. doi: 10.1016/0304-3762(75)90018-8
Meiri M., Huchon D., Bar-Oz G., Boaretto E., Horwitz L. K., Maeir A. M., et al. (2013). Ancient DNA and Population Turnover in Southern Levantine Pigs- Signature of the Sea Peoples Migration? Sci. Rep. 3, 3035. doi: 10.1038/srep03035
Meisami E., Bhatnagar K. P. (1998). Structure and diversity in mammalian accessory olfactory bulb. Microsc Res. Tech. 43, 476–499. doi: 10.1002/(SICI)1097-0029(19981215)43:6%3C476::AID-JEMT2%3E3.0.CO;2-V
Mendl M., Randle K., Pope S. (2002). Young female pigs can discriminate individual differences in odours from conspecific urine. Anim. Behav. 64, 97–101. doi: 10.1006/anbe.2002.3040
Mori K., Manabe H. (2014). Unique Characteristics of the Olfactory System (Tokyo, Japan: Springer Japan), 1–18. doi: 10.1007/978-4-431-54376-3_1
Morrow-Tesch J., McGlone J. J. (1990a). Sensory systems and nipple attachment behavior in neonatal pigs. Physiol. Behav. 47, 1–4. doi: 10.1016/0031-9384(90)90034-2
Morrow-Tesch J., Mcglone J. J. (1990b). Sources of Maternal Odors and the Development of Odor Preferences in Baby Pigs. J. Anim. Sci. 68, 3563–3571. doi: 10.2527/1990.68113563x
Naudin M., Atanasova B. (2014). Olfactory markers of depression and Alzheimer’s disease. Neurosci. Biobehav. Rev. 45, 262–270. doi: 10.1016/j.neubiorev.2014.06.016
Nguyen D. T., Lee K., Choi H., Choi M. K., Le M. T., Song N., et al. (2012). The complete swine olfactory subgenome: expansion of the olfactory gene repertoire in the pig genome. BMC Genomics 13, 584. doi: 10.1186/1471-2164-13-584
Ni J. Q., Erasmus M. A., Croney C. C., Li C., Li Y. (2021). A critical review of advancement in scientific research on food animal welfare-related air pollution. J. Hazard. Mater. 408, 124468. doi: 10.1016/j.jhazmat.2020.124468
Nielsen B. L., Rampin O., Meunier N., Bombail V. (2015). Behavioral responses to odors from other species: introducing a complementary model of allelochemics involving vertebrates. Front. Neurosci. 9. doi: 10.3389/fnins.2015.00226
Nowicki J., Swierkosz S., Tuz R., Schwarz T. (2015). The influence of aromatized environmental enrichment objects with changeable aromas on the behaviour of weaned piglets. Veterinarski Arhiv. 85, 425–435.
Oleszkiewicz A., Heyne L., Sienkiewicz-Oleszkiewicz B., Cuevas M., Haehner A., Hummel T. (2021). Odours count: human olfactory ecology appears to be helpful in the improvement of the sense of smell. Sci. Rep. 11, 16888. doi: 10.1038/s41598-021-96334-3
Oostindjer M., Bolhuis J. E., Simon K., van den Brand H., Kemp B. (2011). Perinatal Flavour Learning and Adaptation to Being Weaned: All the Pig Needs Is Smell. PloS One 6, e25318. doi: 10.1371/journal.pone.0025318
Oostindjer M., Bolhuis J. E., van den Brand H., Kemp B. (2009). Prenatal Flavor Exposure Affects Flavor Recognition and Stress-Related Behavior of Piglets. Chem. Senses 34, 775–787. doi: 10.1093/chemse/bjp063
Paudel Y., Madsen O., Megens H. J., Frantz L. A. F., Bosse M., Crooijmans R. P. M. A., et al. (2015). Copy number variation in the speciation of pigs: a possible prominent role for olfactory receptors. BMC Genomics 16, 330. doi: 10.1186/s12864-015-1449-9
Peden R. S. E., Turner S. P., Boyle L. A., Camerlink I. (2018). The translation of animal welfare research into practice: The case of mixing aggression between pigs. Appl. Anim. Behav. Sci. 204, 1–9. doi: 10.1016/j.applanim.2018.03.003
Petherick J. C. (1983). A Note on the Space Use for Excretory Behavior of Suckling Piglets. Appl. Anim. Ethology 9, 367–371. doi: 10.1016/0304-3762(83)90016-0
Pfaus J. G., Kippin T. E., Centeno S. (2001). Conditioning and sexual behavior: A review. Hormones Behav. 40, 291–321. doi: 10.1006/hbeh.2001.1686
Pond W. G., Houpt K. A. (1978). The Biology of The Pig. 1st ed (Cornell University), ISBNISBN: 9780801411373.
Quaranta A., d’Ingeo S., Siniscalchi M. (2020). Odour-Evoked Memory in Dogs: Do Odours Help to Retrieve Memories of Food Location? Animals 10, 1249. doi: 10.3390/ani10081249
Rekwot P. I., Ogwu D., Oyedipe E. O., Sekoni V. O. (2001). The role of pheromones and biostimulation in animal reproduction. Anim. Reprod. Sci. 65, 157–170. doi: 10.1016/S0378-4320(00)00223-2
Reynolds S. J., Donham K. J., Whitten P., Merchant J. A., Burmeister L. F., Popendorf W. J. (1996). Longitudinal evaluation of dose-response relationships for environmental exposures and pulmonary function in swine production workers. Am. J. Ind. Med. 29, 33–40. doi: 10.1002/(SICI)1097-0274(199601)29:1%3C33::AID-AJIM5%3E3.0.CO;2-%23
Rodriguez I., Del Punta K., Rothman A., Ishii T., Mombaerts P. (2002). Multiple new and isolated families within the mouse superfamily of V1r vomeronasal receptors. Nat. Neurosci. 5, 134–140. doi: 10.1038/nn795
Rørvang M. V., Schild S.-L. A., Stenfelt J., Grut R., Gadri M. A., Valros A., et al. (2023a). Odor exploration behavior of the domestic pig (Sus scrofa) as indicator of enriching properties of odors. Front. Behav. Neurosci. 17. doi: 10.3389/fnbeh.2023.1173298
Rørvang M. V., Schild S. L. Aa., Wallenbeck A., Stenfelt J., Grut R., Valros A., et al. (2023b). Rub ‘n roll – pigs express rubbing and rolling behaviour when exposed to odours. Appl. Anim. Behav. Sci. 2023, 106022. doi: 10.1016/j.applanim.2023.106022
Rouquier S., Giorgi D. (2007). Olfactory receptor gene repertoires in mammals. Mutat. Research-Fundamental Mol. Mech. Mutagenesis 616, 95–102. doi: 10.1016/j.mrfmmm.2006.11.012
Salazar I., Lombardero M., Cifuentes J. M., Quinteiro P. S., Aleman N. (2003). Morphogenesis and growth of the soft tissue and cartilage of the vomeronasal organ in pigs. J. Anat. 202, 503–514. doi: 10.1046/j.1469-7580.2003.00183.x
Salazar I., Quinteiro P. S., Lombardero M., Aleman N., de Troconiz P. F. (2004). The prenatal maturity of the accessory olfactory bulb in pigs. Chem. Senses 29, 3–11. doi: 10.1093/chemse/bjh001
Salazar I., Sanchez-Quinteiro P., Lombardero M., Cifuentes J. M. (2000). A descriptive and comparative lectin histochemical study of the vomeronasal system in pigs and sheep. J. Anat. 196, 15–22. doi: 10.1046/j.1469-7580.2000.19610015.x
Sauleau P., Lapouble E., Val-Laillet D., Malbert C. H. (2009). The pig model in brain imaging and neurosurgery. Animal 3, 1138–1151. doi: 10.1017/S1751731109004649
Savic I., Gulyas B., Larsson M., Roland P. (2000). Olfactory functions are mediated by parallel and hierarchical processing. Neuron 26, 735–745. doi: 10.1016/S0896-6273(00)81209-X
Seedorf J., Hartung J. (1999). Survey of ammonia concentrations in livestock buildings. J. Agric. Sci. 133, 433–437. doi: 10.1017/S0021859699007170
Shaw D., Annett J. M., Doherty B., Leslle J. C. (2007). Anxiolytic effects of lavender oil inhalation on open-field behaviour in rats. Phytomedicine 14, 613–620. doi: 10.1016/j.phymed.2007.03.007
Sondergaard L. V., Holm I. E., Herskin M. S., Dagnaes-Hansen F., Johansen M. G., Jorgensen A. L., et al. (2010). Determination of Odor Detection Threshold in the Gottingen Minipig. Chem. Senses 35, 727–734. doi: 10.1093/chemse/bjq076
Sørensen P. W. (1996). Biological responsiveness to pheromones provides fundamental and unique insight into olfactory function. Chem. Senses 21, 245–256. doi: 10.1093/chemse/21.2.245
Soudry Y., Lemogne C., Malinvaud D., Consoli S.-M., Bonfils P. (2011). Olfactory system and emotion: common substrates. Eur. Ann. Otorhinolaryngology Head Neck Dis. 128, 18–23. doi: 10.1016/j.anorl.2010.09.007
Stangel G., Jensen P. (1991). Behavior of Semi-Naturally Kept Sows and Piglets (except Suckling) during 10 Days Postpartum. Appl. Anim. Behav. Sci. 31, 211–227. doi: 10.1016/0168-1591(91)90006-J
Stolba A., Woodgush D. G. M. (1989). The Behavior of Pigs in a Semi-Natural Environment. Anim. Prod. 48, 419–425. doi: 10.1017/S0003356100040411
Stombaugh D., Teague H., Roller W. (1969). Effects of atmospheric ammonia on the pig. J. Anim. Sci. 28, 844–847. doi: 10.2527/jas1969.286844x
Studnitz M., Jensen M. B., Pedersen L. J. (2007). Why do pigs root and in what will they root? A review on the exploratory behaviour of pigs in relation to environmental enrichment. Appl. Anim. Behav. Sci. 107, 183–197. doi: 10.1016/j.applanim.2006.11.013
Union, E (1998). Council Directive 98/58/EC of 20 July 1998 concerning the protection of animals kept for farming purposes. Off. J. Eur. Comission.
Union, E (2009). Council directive 2008/120/EC of 18 December 2008 Laying down Minimum Standards for the Production of Pigs. Off. J. Eur. Uninion 47, 5–13.
Valros A., Heinonen M. (2015). Save the pig tail. Porcine Health Manage. 1, 2. doi: 10.1186/2055-5660-1-2
Wackermannová M., Pinc L., Jebavý L. (2016). Olfactory sensitivity in mammalian species. Physiol. Res. 65, 369. doi: 10.33549/physiolres.932955
Wang X. T., Wang M. Y., Chen S. Z., Wei B. X., Gao Y., Huang L. H., et al. (2020b). Ammonia exposure causes lung injuries and disturbs pulmonary circadian clock gene network in a pig study. Ecotoxicology Environ. Saf. 205, 111050. doi: 10.1016/j.ecoenv.2020.111050
Wang H., Zhang Y., Han Q., Xu Y. M., Hu G. H., Xing H. J. (2020a). The inflammatory injury of heart caused by ammonia is realized by oxidative stress and abnormal energy metabolism activating inflammatory pathway. Sci. Total Environ. 742, 140532. doi: 10.1016/j.scitotenv.2020.140532
Wathes C. M., Charles D. (1994). Livestock housing (Wallingford (UK: CAB International), 1994, ISBN: ISBN: 0851987745y.
Wechsler B., Bachmann I. (1998). A sequential analysis of eliminative behaviour in domestic pigs. Appl. Anim. Behav. Sci. 56, 29–36. doi: 10.1016/S0168-1591(97)00075-0
Wood-Gush D. G. M., Vestergaard K. (1989). Exploratory behavior and the welfare of intensively kept animals. J. Agric. Ethics 2, 161–169. doi: 10.1007/BF01826929
Wyatt T. D. (2014). Pheromones and animal behavior: chemical signals and signatures (Cambridge, New York, USA: Cambridge University Press), ISBN: ISBN: 978-0-521-11290-1.
Yang L., Omori K., Omori K., Otani H., Suzukawa J., Inagaki C. (2003). GABAC receptor agonist suppressed ammonia-induced apoptosis in cultured rat hippocampal neurons by restoring phosphorylated BAD level. J. Neurochem. 87, 791–800. doi: 10.1046/j.1471-4159.2003.02069.x
Zhang X., Zhang X., Firestein S. (2007). Comparative genomics of odorant and pheromone receptor genes in rodents. Genomics 89, 441–450. doi: 10.1016/j.ygeno.2007.01.002
Zonderland J. J. (2010). Talking tails: quantifying the development of tail biting in pigs ( Wageningen, The Netherlands: Wageningen University). Available at: https://www.proquest.com/openview/a872ac30862c6ed04e51cbeb89b4a577/1?pq-origsite=gscholar&cbl=2026366&diss=y.
Keywords: pig management, ammonia, sense, behavior, animal welfare, scent, sensory enrichment, positive welfare
Citation: Schild S-LA and Rørvang MV (2023) Pig olfaction: the potential impact and use of odors in commercial pig husbandry. Front. Anim. Sci. 4:1215206. doi: 10.3389/fanim.2023.1215206
Received: 01 May 2023; Accepted: 30 June 2023;
Published: 10 August 2023.
Edited by:
E. Tobias Krause, Institute for Animal Welfare and Animal Husbandry, Friedrich-Loeffler-Institute, GermanyReviewed by:
Marc Gilles, Bielefeld University, GermanyCopyright © 2023 Schild and Rørvang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Maria Vilain Rø;rvang, bWFyaWF2LnJvcnZhbmdAc2x1LnNl
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.