- 1Ontario Agricultural College, Department of Animal Biosciences, University of Guelph, Guelph, ON, Canada
- 2College of Agriculture and Natural Resources, Department of Animal Science, Michigan State University, Michigan, MI, United States
- 3Research Centre, Shriners Hospitals for Children, Montreal, QB, Canada
- 4Faculty of Dental Medicine and Oral Health Sciences, McGill University, Montreal, QB, Canada
Introduction: To prepare laying hens for life in cage-free aviaries, they must be reared in aviaries that accustom them to the challenges of navigating a complex three-dimensional structure. Rearing aviaries vary in design and contain a brooding compartment in which chicks are confined during the first six weeks of age. These compartments vary greatly in their size and complexity. The present study aimed to examine the impact of environmental complexity during early life and birds’ genetic strain on their space use and forms/types of exercise.
Methods: Four consecutive flocks of brown and white chicks were raised in three styles of rearing aviary with low, intermediate, or high complexity. Behavioral observations were performed at three ages during the brooding phase (weeks 1, 3, and 5) and the open phase when the brooding compartments were opened (weeks 7, 11, and 17). Behaviors observed were categorized as aerial locomotion, perching, dynamic load-bearing behavior (DLB), and wing-involved load-bearing behavior (WLB).
Results: During the brooding phase, chicks in aviaries of high complexity exercised most frequently (e.g., DLB events/minute: 1.75 in High vs 1.11 in Mid and 0.10 in Low, p<0.0001), and the effect remained for whites, but not the browns, during the open phase. White pullets exercised more than brown pullets both in brooding (e.g., 1.47 vs 1.28 DLB events/minute, p<0.0001) and open phases in High and Mid (e.g., 1.17 vs 0.93 DLB events/minute, housing x stain interaction p=0.009). Throughout rearing, whites had higher odds of perching than browns (brooding: 0.52 vs 0.45, p=0.04, open: 0.27 vs 0.17, p=0.0007).
Discussion: We concluded that rearing aviary design can affect behavior during the brooding phase; however, once the brooding compartments were opened, housing differences almost exclusively affected white pullets. The data suggest that genetic strain of birds must be considered in the design of pullet housing with the goal of maximizing space use and musculoskeletal development of laying hen pullets.
1 Introduction
As an alternative to conventional cages, aviary housing is a concession towards improving laying hen welfare as it allows hens to perform a broader range of behaviors. Aviary systems promote behaviors that hens are highly motivated to perform and consist of elevated structures that accommodate perching (including night-time roosting), litter areas for dustbathing and foraging, and enclosed areas for nesting (Weeks and Nicol, 2006; Widowski et al., 2017). However, successful use of these 3-dimensional (3-D) spaces requires locomotion behavior including navigation (León et al., 2021), balancing (LeBlanc et al., 2016), and the proficient use of perches and ramps (Scott et al., 1997; LeBlanc et al., 2018). As land fowl (Galliformes), chickens are not inherently proficient in vertical locomotion, and the use of 3-D space must be learned (Gunnarsson et al., 2000). Additionally, hens housed in aviaries are known to have a high prevalence of keel bone fractures (KBF), some presumably from unsuccessful navigation attempts (Wilkins et al., 2011; Harlander-Matauschek et al., 2015; Toscano et al., 2018; Rufener and Makagon, 2020; Toscano et al., 2020). There is ample evidence for the negative impact of KBF on hen welfare, such as pain (Nasr et al., 2013), impaired mobility (Nasr et al., 2012; Rentsch et al., 2019), and potential signs of long-term negative affective states (Armstrong et al., 2020). The widespread occurrence of osteoporosis in laying hens likely contributes to their susceptibility to KBF.
Bipedal locomotion in chicks develops during their first two weeks of life, with running seemingly easier than walking due to a top-heavy body proportion that complicates balancing on a single leg (Muir et al., 1996). Exercise restriction through spatial limitations (cage: 8x8 cm, 20 cm2/bird) in the first week of life shortened stride lengths and decreased the maturation rate of head-bobbing behavior (Muir and Chu, 2002). The use of perches and platforms by chicks vary by strain (see Habinski et al., 2017a) but starts as early as two weeks of age (Kozak et al., 2016b). Early access to 3-D structures, such as elevated perches, platforms, and ramps/ladders, has been reported to alter space use (Heikkilä et al., 2006; Colson et al., 2008; Brantsæter et al., 2016), improve spatial locomotion (Gunnarsson et al., 2000; Norman et al., 2021), and decrease the risk of injury later in life (Norman et al., 2021).
Several lines of research support aviaries in promoting musculoskeletal health in chickens (Fleming et al., 1997; Heerkens et al., 2015). Bone strength increased with access to vertical space (short-term: Enneking et al., 2012; long-term: Hester et al., 2013), but not in response to running (in roosters Judex and Zernicke, 2000) or after providing them with additional floor space (Whitehead and Wilson, 1992 as cited in; Whitehead, 2004). Hens housed in enriched cages during the lay period had fewer KBF when reared in aviaries compared to conventional cages (Casey-Trott et al., 2017), demonstrating the long-term effects of early life environment. Indeed, it is proposed that bone responds to mechanical stimuli as the difference between a newly imposed versus habitual mechanical loading (Frost, 1987).
Laying hen development is also influenced by genetic factors. White- and brown-feathered strains have different behavioral (Dudde et al., 2018; Nelson et al., 2020; Peixoto et al., 2020; Peixoto et al., 2021) and physiological (Habig et al., 2012; Dudde et al., 2018; Nelson et al., 2020; Peixoto et al., 2021) phenotypes. Brown-feathered egg-layers are known to be less active (hens: Hewlett and Nordquist, 2019; pullets: Pufall et al., 2021), perform fewer aerial transitions as adults (Ali et al., 2016; Garant et al., 2022) and pullets (Kozak et al., 2016a; Chew et al., 2021b; Pufall et al., 2021), and roost in lower places than white-feathered birds (Ali et al., 2016; Ali et al., 2019). Due to their larger body sizes, brown strains occupy more horizontal space than whites (pullets: Giersberg et al., 2017; hens: Riddle et al., 2018), while white hens use more space for wing flapping (Riddle et al., 2018).
During early rearing, laying hens are housed in environments with no structures for egg collection (e.g., nest-boxes or tilted floors) and with furnishings (e.g., waterlines or perches) that can be adjusted as birds grow (Nicol et al., 2017). The designs of commercial rearing aviaries vary significantly in their spatial arrangement and complexity, particularly during brooding when chicks may be confined in compartments (Widowski and Torrey, 2018). This brooding phase lasts from day-old to 3-13 weeks, depending on manufacturer recommendations and producer preference (Pufall et al., 2021 supplementary material), after which pullets are released to the entire rearing aviary (open phase). Pullets stay in the rearing aviary until they are grown (15-18 weeks old) and moved into adult housing set up for egg collection (Janczak and Riber, 2015). Commercial rearing aviaries can be generally grouped into three basic designs, with total space and complexity (number and arrangement of perches and platforms, see Figure 1) being low, intermediate, or high during the initial brooding phase (Widowski and Torrey, 2018; Pufall et al., 2021). While the design still differs during the open phase, the spatial complexity is more comparable with similar structural resources in all three aviary designs. In an exploratory study on commercial farms, Pufall and colleagues (2021) found that pullets housed in high complexity rearing aviaries during the brooding phase showed more locomotory behavior and aerial transitions than pullets in aviaries of low and intermediate complexity designs.
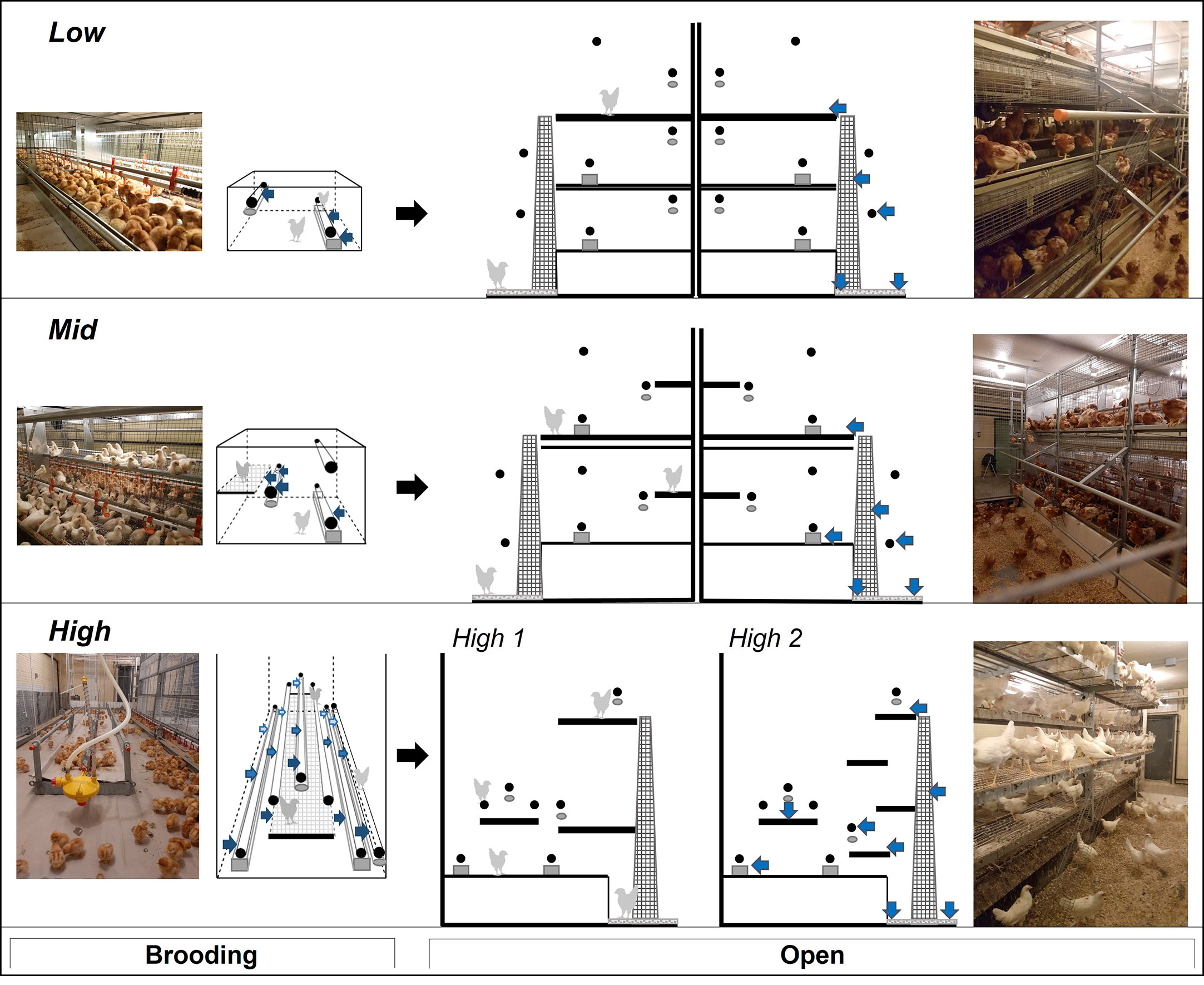
Figure 1 Photos and schematics of housing treatments varying in environmental complexity. Aviary style 1 (Low: Natura Primus), style 2 (Mid: Natura Filia), and style 3 (High: NivoVaria, ‘High.1’; Pullet Portal ‘High.2’). Design of brooding compartments (left) and design of aviaries during the open phase (right), black arrows indicate the transition from brooding to open housing. Elevated spatial structures are in black: perches (circles) and platforms (bars). Drinkers (ovals) and feeders (squares) are in grey. Arrows (blue) point to labels at pre-determined locations used for behavioural observations; focal birds were chosen based on proximity to labels.
In this study we investigate the effect of rearing aviary design on the amount and type of exercise chicks/pullets perform throughout the rearing phase. Lohmann Selected Leghorn-lite and Lohmann Brown-lite laying hen pullets were reared in low, intermediate, and high complexity models of commercial rearing aviaries. We hypothesized that 1) chicks in brooding compartments of higher complexity would perform more exercise including dynamic load-bearing exercise (locomotion) and wing-involved load bearing activity, and make more use of perches during the brooding phase, 2) pullets in rearing aviaries with high complexity, particularly during brooding, would exercise and perch more during the open phase than those in low or intermediate complexity, and 3) the amount of exercise and perching behavior would be higher in white than brown feathered chicks/pullets in all housing systems.
2 Materials and methods
2.1 Ethical statement
All use of animals and procedures in this study were considered and approved by the University of Guelph Animal Care Committee (Animal Utilization Protocol #4127).
2.2 Animals and management
Between the summer of 2019 and 2021, four consecutive flocks of Lohmann Selected Leghorn lite (white) and Lohmann Brown Lite (brown) were hatched at a commercial hatchery (Archer’s Poultry Farm Ltd., Ontario, Canada), beak treated with an infrared laser, and vaccinated against Infectious Bronchitis, Marek’s, Infectious Bursal disease, Newcastle disease, and Coccidiosis before transport to the research station. Both strains are commonly used in the Canadian egg industry (van Staaveren et al., 2018). For each flock, approximately 1,378 chicks of both strains were hatched and transported simultaneously and immediately placed into their housing treatments. Chicks were fed ad libitum from chain feeders with a standard layer chick starter crumble diet for six weeks [wk], then switched to a standard laying hen grower crumble diet. An intermittent lighting schedule was used for the first four days after hatch (4 h light: 2 h dark) followed by 16 h light: 8 h dark until five weeks of age and then 10 h light: 14 h dark for the remainder of the trial. LED lights provided a light intensity of 40 lux on day one and decreased steadily to 10 lux by five wk of age. Temperature was set to 34° C at placement and gradually decreased to 20° C by six wk of age. Chicks were further vaccinated against Newcastle disease and Infectious Bronchitis at three, 11, and 17 weeks of age and against Fowl Pox, Infectious Laryngotracheitis, and Avian Encephalomyelitis at six wk of age. Illustrations of bi-weekly body weights and mortality rates by rearing housing system and strain can be found in the appendix of the PhD thesis by Rentsch (2023).
2.3 Housing
The white-and brown-feathered strains within each flock were randomly divided into three different rearing aviaries (n=8/aviary style). Each aviary represented one of three general styles of commercial rearing aviaries (Figure 1; Table 1). The major difference between aviaries was the design of the brooding compartments (low, intermediate, or high complexity, left column in Figure 1), where chicks were confined for the first few weeks of life, henceforth called the brooding phase. Following the brooding phase, pullets were given access to a litter area, multiple tiers, additional perches, and ramps during what will, from now on, be called the open phase (Figure 1 right column), where the amount and arrangement of the various resources (i.e., complexity) also differ but to a lesser extent than during brooding. In this study, the brooding phase lasted six weeks for all pullets in all rearing systems. Space allowances and resource allocations for all three aviary styles are detailed in Table 1.
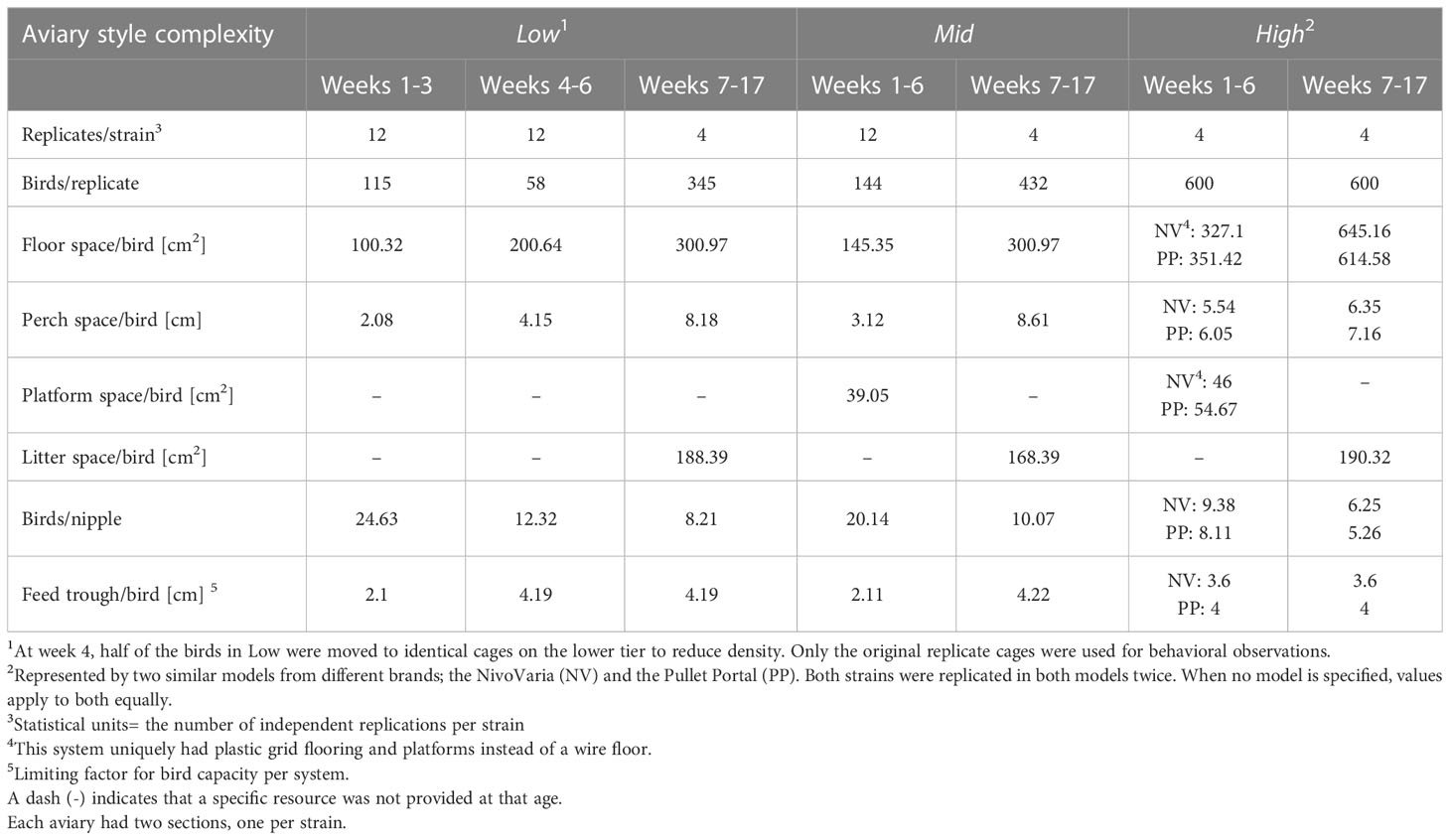
Table 1 Bird numbers, replication for behavioral observations (statistical unit), and resource allowances per strain for the three rearing aviaries.
Aviary Style 1 (low early complexity ‘Low’) was represented by the Natura Primus system (Big Dutchman North America, Holland, Michigan), a three-tiered system located in a single room but divided in the center creating two separate aviary sections, each facing a wall (Figure 1). The two strains were housed separately, one per section, and were visually but not auditorily divided. The brooding compartment consisted of three cages on the middle tier with 115 chicks per cage (L: 122 cm x W: 94 cm x H: 50 cm). The cages were furnished with an automated feeder, a drinker line with nipples, and two perches (Figure 1). One perch was mounted on the drinker line and was raised with the drinker as chicks grew (max H: 40 cm). The other was mounted in a fixed position (H: 14 cm). Halfway through the brooding phase (at 3 wk of age), half of the chicks were moved to cages in the lower tier to decrease the stocking density. At 6 wk of age, the cages were opened so that pullets had access to all three tiers, litter floor (W: 176.53 cm x L: 368.3 cm), external perches (H: 77 cm and 150 cm), and ramps connecting the three tiers (Figure 1). At that time, pullets from specific cages became indistinguishable and were treated as one population per strain.
Aviary Style 2 (intermediate early complexity ‘Mid’) was represented by the Natura Filia system (Big Dutchman North America, Holland, Michigan), a two-tier system located in a single room, but divided in the center creating two separate aviary sections, each facing a wall (Figure 1). The two strains were housed separately, one per section, and were visually but not auditorily divided. Each system had three brooding compartments, with 141 chicks per cage (L: 147 cm x W: 111.5 cm x H: 90 cm). The three cages offered an automated feeder, a drinker line of nipple drinkers, an elevated platform (L: 147 cm x W: 30.5 cm at H: 40 cm), and three perches. One of the perches was mounted to the drinker line and raised with the drinker as the chicks grew (max H: 40 cm), the other two were stationary (H: 19 cm and 68 cm). In the open phase, pullets could access a litter area (W: 161.5 cm x L: 442 cm) and two system tiers. Both tiers had three perches internal to the aviary structure (H: 19 cm, 40 cm, and 68 cm), two perches external over the litter area (H: 40 cm and 103 cm), and an external ramp that ran from the litter area to the second tier.
Aviary Style 3 (high early complexity ‘High’) aviaries were represented by two commercial aviaries, each located in a separate room (Figure 1). The Pullet Portal by Farmer Automatic (PP, Farmer Automatic, Georgia USA) was previously built at the research farm, but has since been discontinued. Therefore, a different but spatially similar model was chosen as the second replicate for High; the NivoVaria by Jansen (NV, VDL Jansen, formerly known as Jansen Poultry Equipment, Barneveld Netherland). Across the four consecutive flocks, the two strains were raised in both systems twice. Both High systems offered an open concept brooding compartment that held 600 chicks and spanned the length of the room (PP: L: 724 cm x W: 245 cm, NV: L: 726.5 cm x W: 236 cm, Figure 1). The brooding compartment was furnished with six perches and a swinging platform (PP: L: 567.5 cm x W: 58 cm, NV: L: 472.5 cm x W: 58.5 cm) which was raised as the chicks grew. Two perches were mounted over the automatic feeder (H: PP: 21 cm, NV: 26 cm), two were fixed over the edge of the platform (H: 26 cm), and two over drinker lines that were raised as the chicks grew (max H: 40 cm). In the open phase, chicks were given access to one more drinker line and perch (max H: 35 cm), a litter area (L: 726.5 cm x W: 157.5 cm), and either two or four tiers depending on the manufacturer (PP: L: 693.5cm x W: 127 cm H: 26 cm, 86 cm, 131 cm, and 176 cm, NV: L: 701 cm x W: 277 cm H: 60 cm, 170 cm, Figure 1).
2.4 Behavioral observations
Locomotory behavior and perching were quantified in all four flocks using focal animal sampling during live observations conducted at six time points: (wk 1 (days 3-4), wk 3 (days 14-18), wk 5 (days 28-32), wk 7 (days 42-46), wk 11 (days 70-74), and wk 17 (days 112-116)). Pre-determined locations were marked by taping labels to the housing structures and the number of labels was proportional to the number of birds in each system (during brooding: 9 in Low, 12 in Mid, 15 in High; in the open phase: 10 in Low, 12 in Mid, 16 in High per strain). Locations were chosen as evenly as possible between the different systems, with at least one at each of the following locations: the feeder, drinker, on a perch, on the platform (if available) or tier, and by the litter area (Figure 1). For each focal sample, the bird closest to the pre-determined location was selected and observed continuously for five minutes. Behavior was recorded by two observers using a behavior coding program (Pocket Observer 3.2 by 2012 Noldus Information Technology bv) on digital tablets (Samsung Galaxy Tab4 SM-T230NU) that allowed the recording of locations and all occurrences of behavior (Table 2) in real-time. During the brooding phase, the number of observations was doubled (per strain: Low: 18, Mid: 24, High: 30). To this end, each observer recorded the behaviour of a bird at every location, resulting in two observations per location. During the open phase, the observers were stationed at either end of the aviary where half the labels were visible, resulting in one observation per location. In wk 3, 5, 11 (flock 3 only), and 17, each aviary system was visited twice with two days between visits. No observations were recorded for flock 2 during wk 11 due to a COVID-19 research pause. Out of the 2880 focal bird observations planned during the brooding phase, 7.6% were lost to unforeseen circumstances such as observer illness or technical malfunctions. No observations were lost in the open phase. Overall, 2661 focal bird observations were logged during the brooding phase and 1216 during the open phase. All observations were conducted in the morning (09h-12h) or the afternoon (14h-17h). Morning and afternoon observations were balanced across ages and housing systems over all four flocks. During live observations, blinding was not possible, as both housing type and bird strain were visually distinguishable and only one observer was blinded to the hypotheses as the other was involved in designing the experiment. To assess inter-observer reliability, 15 individuals spread over all four flocks and different age groups were marked with livestock paint and observed simultaneously by both observers for the designated five minutes.
Aerial locomotion (AL) was analyzed as a frequency. Perching was analyzed as frequency and duration. Perching duration (in seconds; maximum 300sec) was analyzed with a reduced data set, only including observations when perching occurred: 1,367 out of 2,661 observations for the brooding phase, and 385 out of 1,216 observations for the open phase. Behavioral data were pooled into dynamic load-bearing behavior (DLB) and wing-involved load-bearing behavior (WLB) and analyzed as count data. At this point, there is still uncertainty about which forms of exercise load specific bones and to what degree (Tobalske, 2007; Vitienes et al., 2023). Our decision to pool types of exercise into DLB and WLB was based on the assumption that using specific muscles would load the bone said muscle is anchored to (Willie et al., 2020). The two groups were not mutually exclusive; DLB was analyzed to assess the relationship between spatial complexity and locomotion, assuming there would be a link to skeletal development in general. WLB, on the other hand, exclusively included behaviors that exercise the wings (a distinct subgroup of exercise (Gatesy and Dial, 1996)), which might have relevance for keel bone development specifically. DLB included walking, running, aerial transitioning, stepping up/down, and wing-assisted locomotion. WLB included wing flapping, aerial locomotion, wing-assisted locomotion, and sparring.
2.5 Statistical analysis
Analyses were done in R and R Studio (R Core Team, 2021) version 4.1.0 using packages ‘lme4’, ‘DHARMa’, ‘tidyverse’, ‘car’, ‘emmeans’, and ‘oddsratio’. The brooding and open phases were analyzed separately. As birds housed together cannot be treated as independent units, the statistical unit was brooding compartment for the brooding phase analyses (N= 24 for Low and Mid, N= 8 for High) and aviary system for the open phase analyses (N= 8 per aviary style). Data were analyzed by fitting (generalized-) linear mixed effect models (LMM and GLMM binomial, GLMM Poisson) depending on data distribution (Tables 3 and 4). LMM: DLB was normally distributed in the brooding and the open phase. GLMM binomial: Aerial locomotion and perching were analyzed as probabilities (binary) due to zero-inflation of the data and so were WLB during brooding and perching duration (the probability of exceeding 60 sec) during the open phase. GLMM Poisson: Perching duration during brooding was analyzed after rounding up or down to full minutes and WLB during open was analyzed as counts per minute. Fixed effects were aviary style, strain, and age, as well as all interactions. To make sure that birds living in the same housing unit would not be considered as independent from each other, the following random effects structure were incorporated into the analysis: i) start location (label number) nested in aviary style and flock to account for repeated measures in each compartment or aviary, ii) brooding compartment (for analyses of brooding phase) nested in aviary style and flock to account for the replication within each flock, iii) age nested in flock to account for the repeated measures taken at different timepoints within the same flock. All models were evaluated through visual assessment of model assumptions (homoscedasticity: Tukey-Anscombe plot and normalcy of residuals: Q-Q plot). Explanatory variables were tested with a Wald Chi-Squared test to establish the main effects explained in the model (Tables 3 and 4). The significance level was set at p<0.05. Post-hoc analyses were done for relevant pairwise comparisons using Tukey tests with Bonferroni corrections of p-values for multiple testing and calculating odds ratios (OR) for binomial models. OR were considered significant if the 95%-confidence interval (CI) did not include ‘1’.
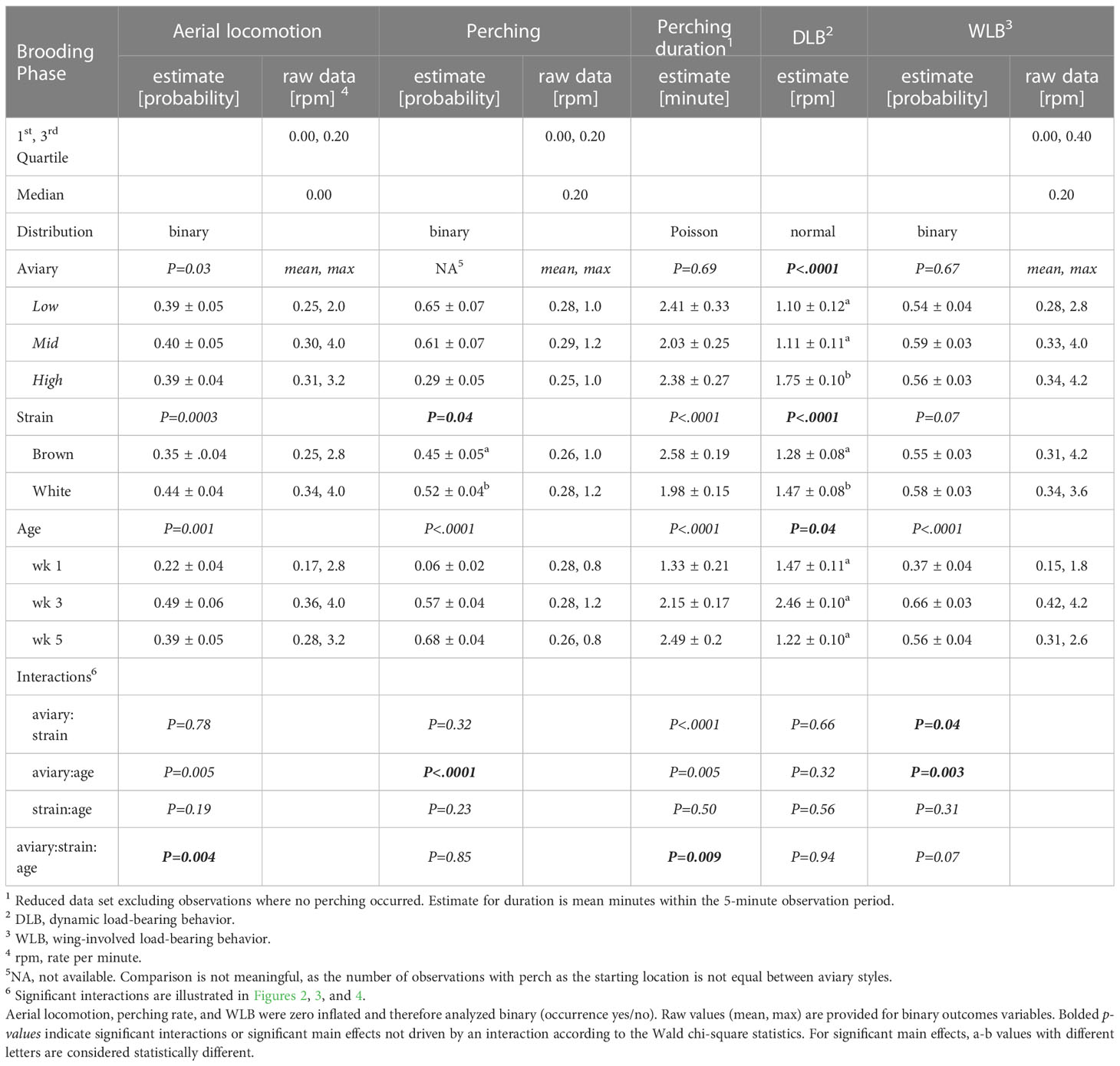
Table 3 Model estimates ± SEM, and p-values of generalized-/linear mixed models fitted to behavior variables in the brooding phase.
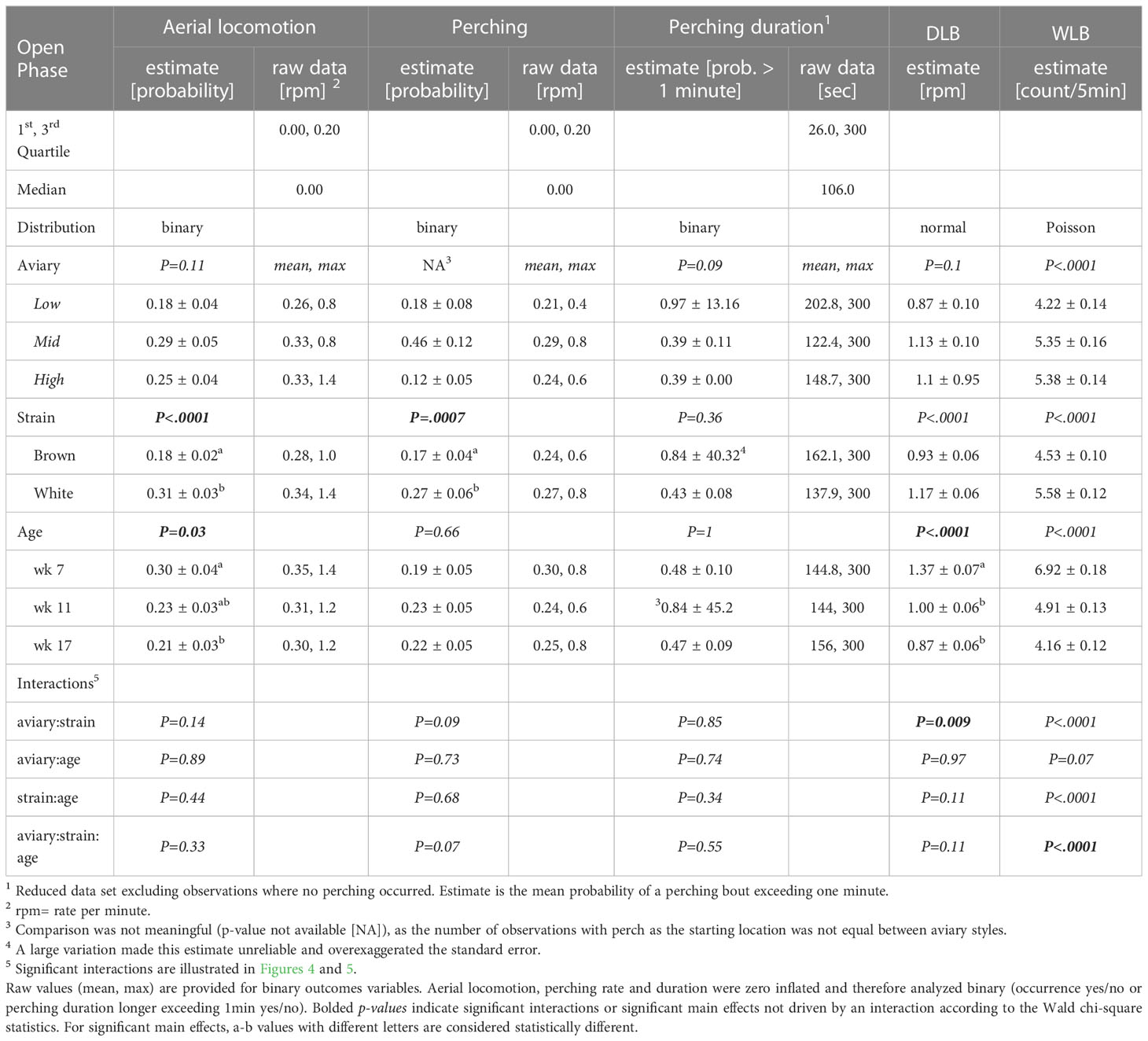
Table 4 Model estimates ± SEM, and p-values of generalized-/linear mixed models fitted to behavior variables in the open phase.
Aviary style was not included in the model for perching frequency, as the proportion of total pre-determined starting locations on perches varied between aviary styles (Figure 1), although it was considered in interactions when assessing effects of strain based on housing type. Perching duration followed a bimodal distribution with one narrow mode ranging from one to 60 seconds, peaking at five seconds (152 observations) and the second narrow mode at 300 seconds (233 observations). Hence, data were analyzed as the probability of perching duration exceeding 60 seconds (binary: perching duration = ‘0’ ≤ 60 sec > ‘1’). Due to the rarity of group-runs in aviaries of Low or intermediate (Mid) complexity, a meaningful statistical analysis was not possible, but descriptive statistics for the High complexity aviary are provided.
Inter-observer reliability for focal bird sampling was assessed by calculating kappa statistics with Observer (The Observer® XT by Noldus, Version: 14.2) for behavior durations and frequencies.
3 Results
Inter-observer reliability for behavioral observations was almost perfect for behavior durations (mean kappa=0.93) and moderate for frequencies (mean kappa=0.78) (interpretation based on McHugh, 2012).
3.1 Brooding phase (weeks 1-6)
Model outputs and main effect estimates are described in Table 3.
3.1.1 Aerial locomotion
The probability of aerial locomotion was best explained by a three-way interaction of aviary style, strain, and age (p = 0.004, interaction Figure 2). White birds were more likely to perform aerial locomotion than browns in High during wk 1 (p<0.0001) and in Low during wk 5 (p = 0.008). Generally, the probability of aerial locomotion increased with age from wk 1 to 3, then decreased for browns from wk 3 to wk 5 in Low and Mid and stayed the same for whites (see Figure 2). During wk 1, whites were more likely to perform aerial locomotion in High than in Mid (p= 0.03).
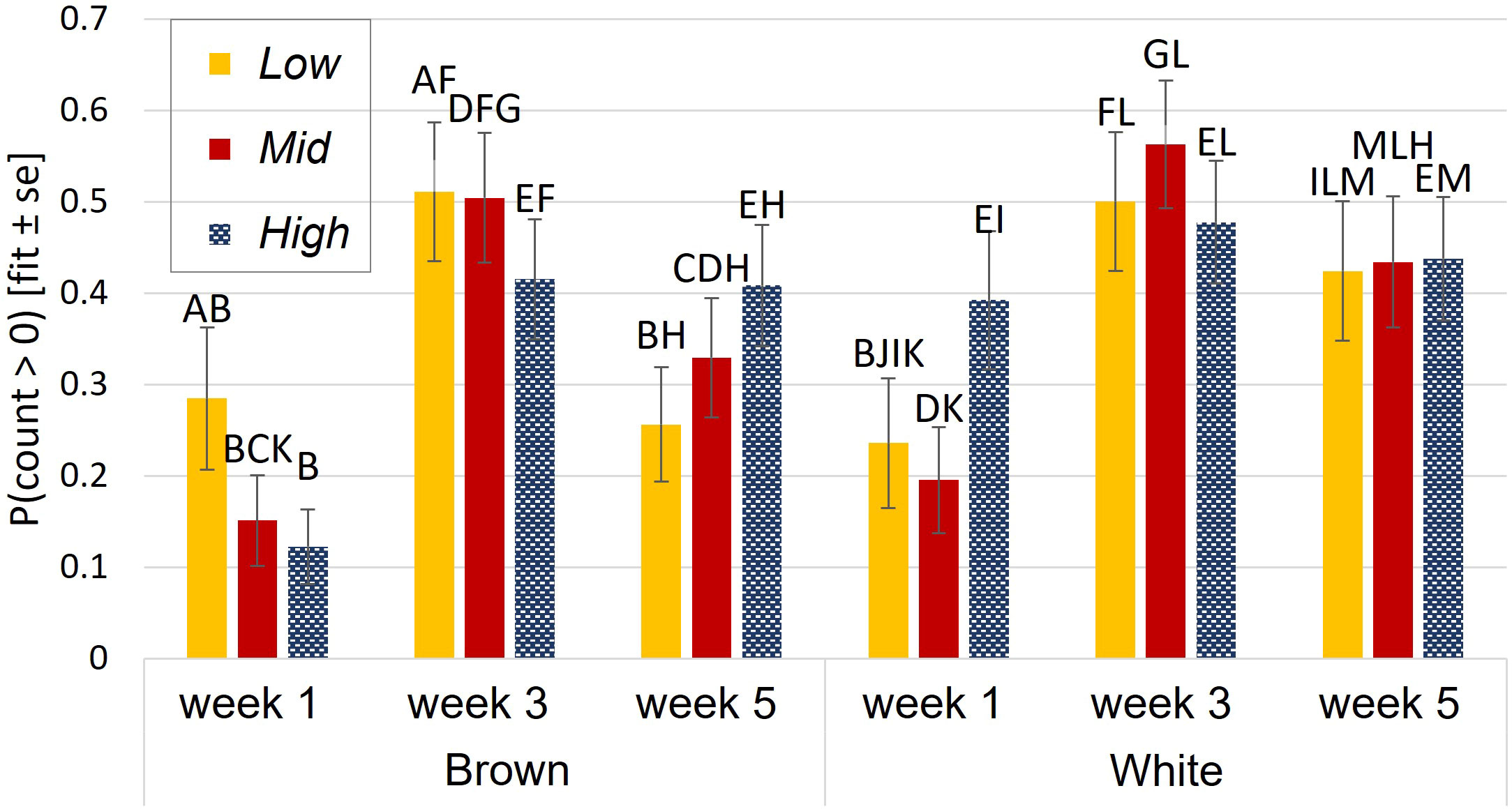
Figure 2 Aerial locomotion during brooding. Probability of aerial locomotion was affected by an interaction of aviary style complexity, strain, and age. Y-axis: the estimated probability of aerial locomotion and standard errors of the estimate. Different letters indicate statistical differences with p<0.05.
3.1.2 Perching
Chicks’ probability of being on the perch was affected by an interaction of aviary style and age (p<0.0001, Figure 3A), and by a strain effect (p= 0.04). White chicks had higher odds of perching than brown chicks, although the 95%-CI of the OR indicates that the odds are only marginally different (OR=1.18, CI= 1, 1.4). Perching probability increased with in Mid and High (p<0.01) but in Low, odds of perching increased between wk 1 and wk 3 (p<0.0001) and then decreased between wk 3 and wk 5 (p=0.001). During wk 1 and wk 3, High chicks were less likely to perch than chicks in Low (wk1: p= 0.0001, wk3: p<0.0001) or Mid (wk1: p= 0.0004, wk3: p= 0.004). Perching duration was explained by a three-way interaction of aviary style, strain, and age (p= 0.009). Generally, perching duration increased with age and was longer in browns than whites (see Figure 3B).
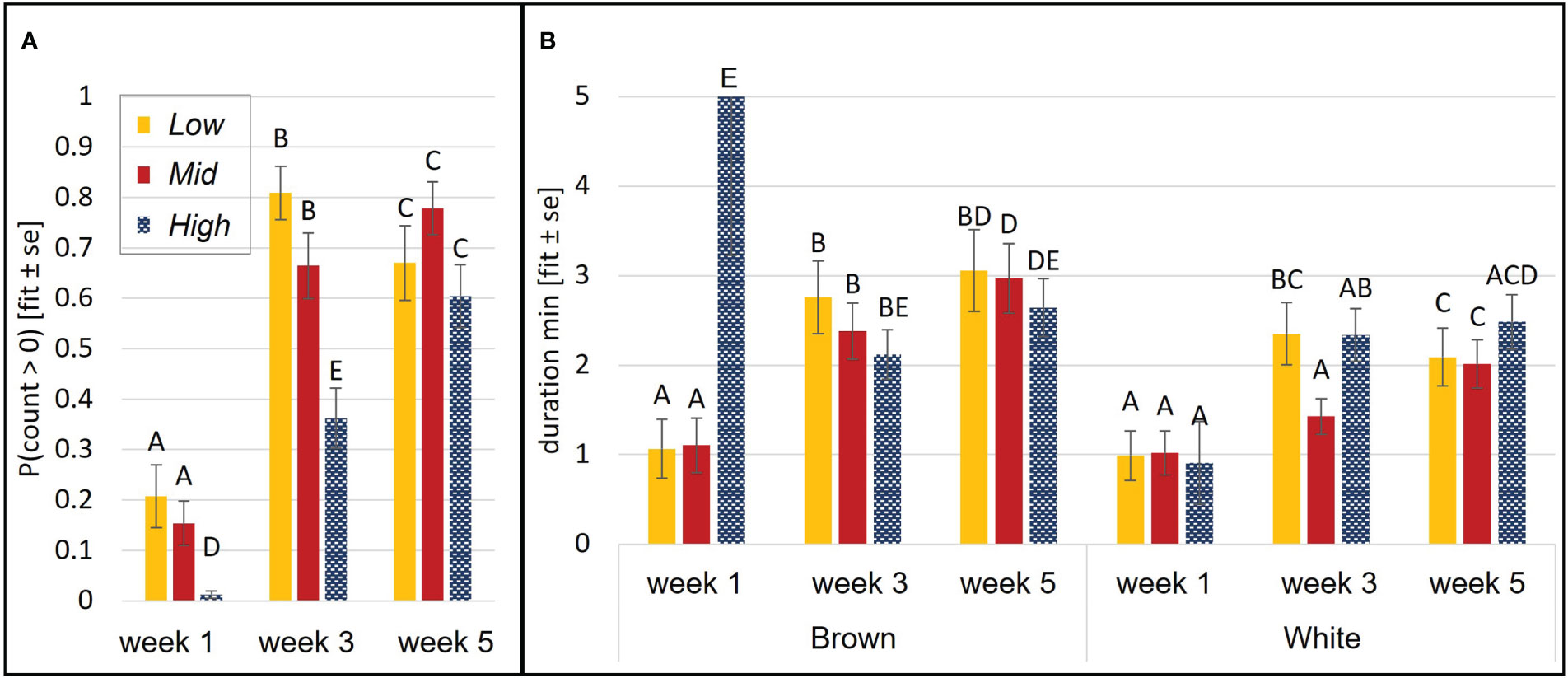
Figure 3 Perching behavior during brooding. Probability of perching was affected by an aviary style complexity by age interaction (A). Duration was affected by an interaction of aviary style complexity, strain, and age (B). Perching duration of brown High chicks in week 1 is likely overestimated due to a low n and large variance in this group. Y-axes: model estimates and standard errors. Different letters indicate a statistical difference with p<0.05.
3.1.3 Load bearing behavior
DLB frequency was affected by aviary style (p<0.0001), strain (p<0.0001), and age (p= 0.04). Chicks from High complexity aviaries performed more DLB than Low (p=0.006) and Mid chicks (p= 0.009). White chicks performed more DLB than browns. There were no statistical differences in DLB between the different ages in the post-hoc analysis (p>0.1). The probability of WLB was affected by an interaction of aviary style and age (p= 0.003) and an interaction of aviary style and strain (p= 0.04). WLB became more frequent by wk 3 relative to wk 1 but decreased in Low birds in wk 5 (Figure 4A). In High, white chicks had higher odds of WLB than browns (p= 0.02), while no strain differences were observed in Low (p=0.1) or Mid (p= 0.4, Figure 4B).
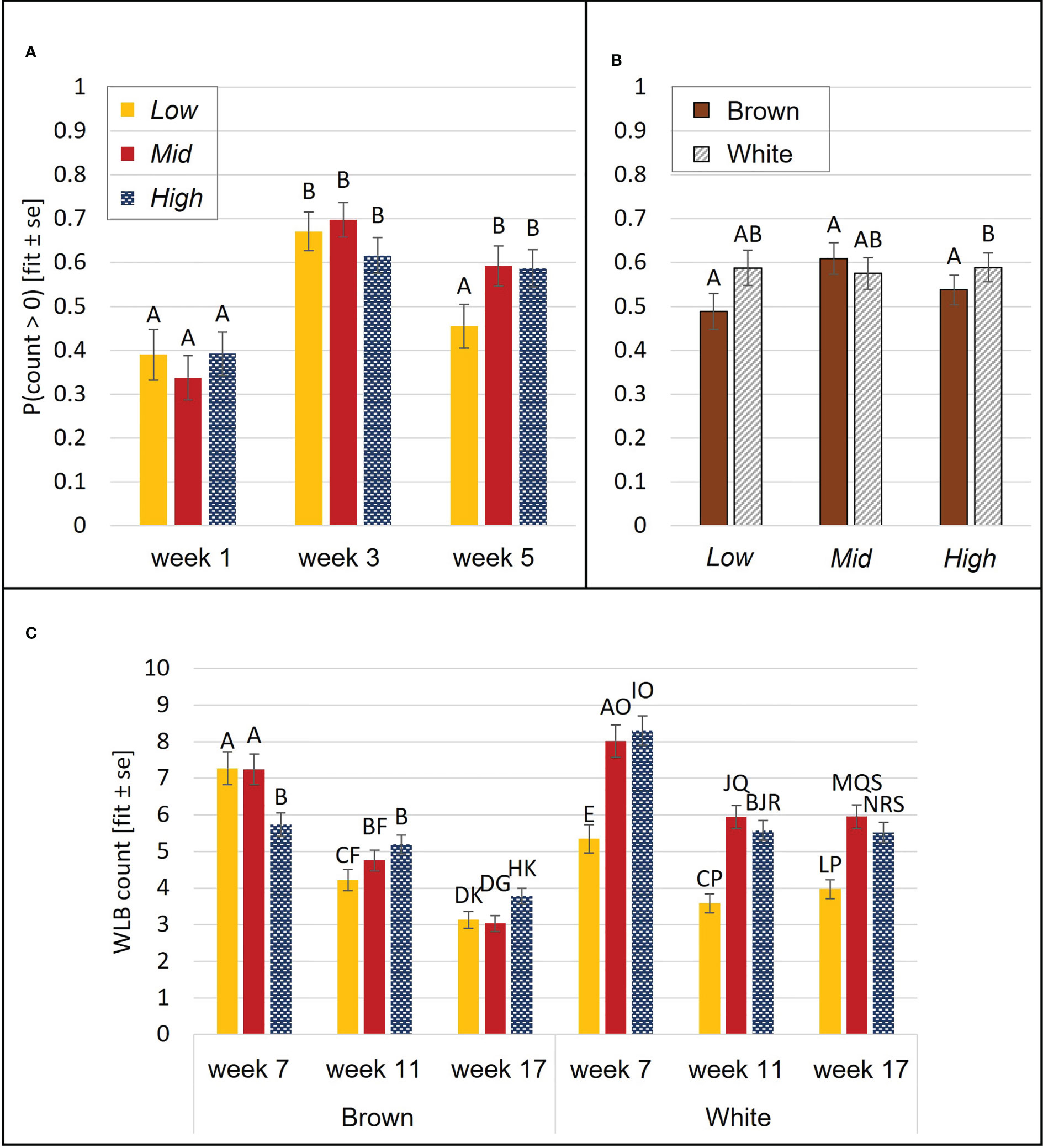
Figure 4 Wing-involved load bearing (WLB). During brooding, the probability of WLB was affected by an aviary style complexity by age interaction (A) and an aviary style complexity by strain interaction (B). During the open phase, count (per 5 min observation) of WLB was affected by an interaction of aviary style complexity, strain, and age (C). Y-axes: model estimates and standard errors. Different letters indicate a statistical difference with p<0.05.
3.1.4 Group runs
During the brooding phase, 258 group run participations were recorded. No brown chicks in Low were observed in a group run, and this behavior was only observed in 0.3% of the total observations in the white chicks. In Mid, group runs were recorded in 2.5% of the observations of browns and 2.3% of white chicks. In High, 39.9% and 38.7% of total observations included group runs in the browns and white chicks respectively.
3.2 Open phase (weeks 7-17)
Model outputs and main effect estimates are described in Table 4.
3.2.1 Aerial locomotion
The data show that strain (p<0.0001) and age (p= 0.03) impact the probability of observing aerial locomotion. White pullets had 1.95-fold higher odds of performing aerial locomotion than brown pullets (CI = 1.51, 2.53). Pullets had lower odds of performing aerial locomotion during wk 17 compared to wk 7 (OR = 0.68, CI = 0.49,0.95).
3.2.2 Perching
There was a strain effect on perching probability (p = 0.0007), with white pullets having 1.41-fold higher odds of perching than brown pullets (CI = 1.1,1.82). Perching duration was not affected by any of the main effects or their interactions in this phase.
3.2.3 Load bearing behavior
DLB was affected by an interaction between aviary style and strain (p= 0.009, Figure 5) and by age (p<0.0001). White pullets in Mid and High performed DLB at higher rates per minute (rpm) than browns (Mid: p= 0.0003, High: p= 0.0001, Low: p= 0.55 Figure 5) and more than Low white pullets (Mid: p= 0.02, High: p= 0.03). There was no aviary style effect on DLB rpm in browns (p>0.9). Pullets also performed more DLB during wk 7 than in wk 11 (p= 0.006) and wk 17 (p=0.0005). WLB frequency was explained by a three-way interaction of aviary style, strain, and age (p<0.0001). White pullets performed less WLB in Low than in Mid or High (p<0.0001 for all pairwise comparisons) (Figure 4C). Brown pullets housed in Low and Mid performed more WLB than High during wk 7 (Low: p= 0.01; Mid: p= 0.008). During wk 11, this trend was reversed, whereby High pullets exhibited higher WLB than Low (p= 0.03, Figure 4C), and in wk 17, with more WLB in High than Mid (p= 0.04). The frequencies of WLB behavior generally decreased across all ages for brown pullets in all systems. For white pullets, frequencies of WLB generally decreased from wk 7 to wk 11 in all systems with similar values during wk 11 and wk 17 (Figure 4C).
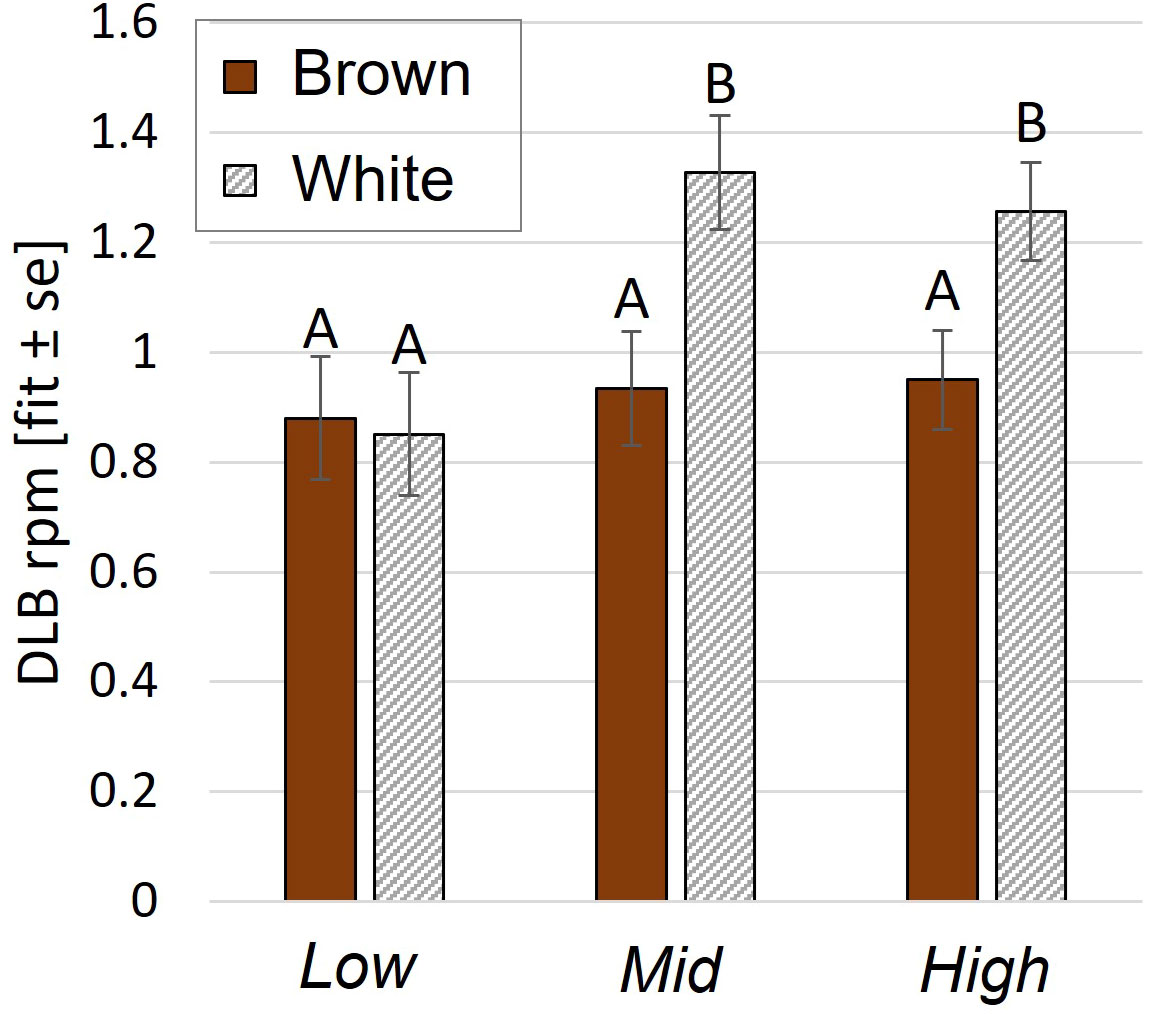
Figure 5 Dynamic load bearing behavior during the open phase. Rate per minute (rpm) of DLB behavior was affected by an aviary style complexity by strain interaction. Y-axis: Model estimates and standard errors. Different letters indicate a statistical difference with p<0.05.
3.2.4 Group runs
During the open phase, group runs were indiscriminately low with 23 occurrences in total (browns: Low=0.6%, Mid=0.5%, High=2.7%, whites: Low=0%, Mid=1.6%, High=4.3%).
4 Discussion
The present study aimed to assess whether chicks housed in spacious and complex brooding compartments exercised and perched more compared to chicks housed in less spacious and complex compartments during the brooding phase. The data demonstrate that increasing the brooding compartment complexity increased some forms of exercise, namely dynamic load-bearing behavior (DLB) and wing-involved load-bearing behavior (WLB); however, increasing complexity decreased the use of perches. Pullets raised in high-complexity brooding compartments were hypothesized to perform more forms of exercise and use perches more frequently during the open phase, and the data partially support this hypothesis for white birds (most WLB in High) but not for brown birds. Finally, whites generally exercised and perched more frequently than brown-feathered chicks and pullets in all three styles of rearing aviaries.
Group runs were assessed with descriptive statistics only, but effects were clearly apparent as group runs were almost exclusively observed in the brooding compartment with High complexity, similar to data reported from commercial systems (Pufall et al., 2021). Since running requires less balancing skills compared to walking (Muir et al., 1996), chicks are better suited to run due to disproportionally large heads and short, thin legs. The benefits of group running in chicks are unclear, but it does appear to be the most vigorous form of locomotion at that age, and as it is usually accompanied by wing flapping, it has the potential to strain long bones and the keel simultaneously.
Aerial locomotion increased after the first week and was generally highest during week three. There was a decrease in aerial locomotion for browns in Low complexity by the end of brooding, which could possibly be attributed to the birds growing and space becoming limiting. In the open phase, white-feathered birds performed more aerial locomotion than brown-feathered birds, but it decreased over time in all housing systems. The literature corroborates these age (Kozak et al., 2016b; Pufall et al., 2021) and strain effects (Kozak et al., 2016a; Chew et al., 2021b; Pufall et al., 2021), and on commercial rearing farms, aerial locomotion was observed least in Low complexity systems (Pufall et al., 2021).
Perching was observed more often in Low and Mid complexity brooding compartments than in High in weeks one and three of brooding which was unexpected. A possible explanation could be that the High complexity proved to be too difficult to navigate for the young chicks though further investigation would be required to confirm this hypothesis. In addition, fewer but longer perching bouts were observed in browns than whites during brooding and whites perched more often in the open phase. Greater use of perches in white pullets than browns was also found in furnished cages (Habinski et al., 2017b), indicating consistent strain differences in perching independent of housing system.
Load bearing and potential skeletomuscular consequences
Similar to aerial locomotion, DLB and WLB increased during brooding (albeit not significantly for DLB) and decreased towards the end of rearing. As pullets age, space for locomotory and loading behavior becomes restricted at commercial densities. At the end of the brooding phase, Mid and High complexity brooding compartments allowed for more WLB than Low and chicks in High complexity compartments exhibited more DLB than chicks in Low or Mid for the whole of brooding. White birds performed more WLB than browns in High throughout brooding and more WLB and DLB in High and Mid during most of the open phase. Consequently, during the open phase, it was predominantly the white pullets that took advantage of the opportunities for WLB in High and Mid aviaries. This corroborates data from commercial farms, where the highest prevalence of locomotory behavior was between 9-10 wk of age, with more locomotion observed in High systems and in white versus brown pullets (Pufall et al., 2021).
Muscle and bone strength is expected to increase in complex aviaries which encourage more exercise. Based on the current data, a more pronounced effect is expected in white pullets based on greater exercise levels, unless they reached a plateau in the response to bone loading where they no longer benefited from more bone loading behavior (Frost, 1987). On commercial farms, pullets from High had stronger leg bones than those raised in Low with no differences in leg or breast muscle mass or keel bone (KB) size (Pufall et al., 2021). Ross (2021) studied skeletal profiles of the birds used in the present study and found that white and brown pullets had stronger humeri (i.e., wing bones) in High complex brooding compartments relative to Low complex ones. Interestingly, no effect of aviary design was observed on the KB area (Pufall et al., 2021; Ross, 2021). It is possible that aviary-reared pullets may experience sufficient keel loading independently of aviary design. Unexpectedly, Ross (2021) reports that, at the end of the brooding phase, white pullets had larger length and radius of long bones (femur, tibia, humerus, radius) in Mid compared to Low and High. They hypothesized that the restricted horizontal space in Mid brooding compartments compared to High might force chicks to perform more jumping (e.g., aerial locomotion) to make use of the platform, however, this was not confirmed by the observational data on aerial locomotion presented here.
Based on differences in activity levels observed in the present study, white and brown pullets are expected to exhibit distinct skeletal characteristics, specifically, stronger keel and long bones in whites than in browns. The literature reports that in comparison to brown pullets, white pullets having stronger leg bones and larger keel areas in proportion to their body weight in all three rearing aviary designs (Pufall et al., 2021) and furnished cages (Fawcett et al., 2020), and they have proportionally thicker and longer tibiae in floor pens (Chew et al., 2021a). Post-mortem analysis of pullets from the present study at the end of brooding and open phases revealed skeletal differences between brown and white pullets, with whites having stronger femurs at the end of rearing and proportionally larger keel areas (Ross, 2021). However, browns had a higher bone mineral density (Ross, 2021). The WLB observed in these studies was positively associated with the skeletal development of the humerus, and strain differences emulated differences found in keel size.
Early sensitive window? Previous work suggests an early-life sensitive window for developing spatial tasks, such as perching behavior (Appleby and Duncan, 1989) and navigation in 3-D space (Gunnarsson et al., 2000). Our data support this theory for white feathered laying hen development. We propose that the opportunity for engaging in spatial tasks (via complex housing during brooding) and birds’ genetic background influence space use and behaviors during this sensitive developmental window. To confirm this hypothesis, candidate traits must be assessed at the end of rearing or during lay to test whether the design of the brooding compartment influences behavioral development.
4.1 Limitations and future directions
A more refined analysis of some recorded behaviors may have elucidated impacts of aviary design, bird strain and age. For example, distinguishing between upwards and downwards transitions and aerial locomotion with or without wing use (e.g., jumps) may demonstrate housing impact on a subset of behaviors. In the present study, WLB may be overestimated as it included aerial transitions without wing-use by default. Furthermore, separating upwards from downwards transitions, would indicate whether birds use wing-assisted locomotion predominantly for one or the other.
The unequal population sizes and system dimensions between the different aviary designs could not be accounted for in the statistical models and therefore was another constraint of the study by limiting comparison between treatment groups with differing space allowances and total floor space. It is notable, however, that such differences are also found in commercial settings that use the three aviary designs, so even if these factors cannot be disentangled, we would still argue that our findings are applicable to commercial settings. A strength of this paper is that it followed a field trial, thereby allowing comparison of a controlled experiment to a practical commercial setting, lending external validity to the conclusions drawn. Birds form this study were also used for further skeletomuscular analysis (Ross, 2021).
5 Conclusion
Rearing aviary design was most influential during the brooding phase, where brooding compartments differed substantially in their size and complexity. Once the brooding compartments were opened, housing design almost exclusively affected the behavior of white pullets. Overall, white chicks and pullets made more use of the available space. The data indicate that the optimal rearing aviary design depends on the genetic strain, as brown pullets appear not to utilize the spatial opportunities of high-complexity aviaries to their full extent. Further investigation of effect of the brooding compartment and open phase design on the development of pullet’s spatial skills and their response to specific bone strains is needed to inform pullet rearing practices aimed at improving laying hen welfare in aviary housing.
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found below: https://doi.org/10.5683/SP3/NAHIK3, borealis.
Ethics statement
The animal study was reviewed and approved by University of Guelph Animal Care Committee.
Author contributions
Study conception and design: TW, AR. Data acquisition and analysis: AR. Interpretation of data: All authors. Drafting of Manuscript: AR. All authors contributed to the article and approved the submitted version.
Funding
This research is part of the 2018-2023 Poultry Science Cluster which is supported by Agriculture and Agri-Food Canada as part of the Canadian Agricultural Partnership (grant # 054159), a federal-provincial-territorial initiative (OMAFRA grant UG/T2/2019/27372). Additional funding was provided by Egg Farmers of Canada (grant # 054009), the Ontario Argi-Food Innovation Alliance, and the Canadian Poultry Research Council (grant # 054159). We also thank Egg Farmers of Alberta, Fédération des producteurs d’oeufs du Québec, NSERC (RGPIN-2019-05374), and FRQS Programme de bourses de chercheur (BW).
Acknowledgments
We would like to acknowledge the Widowski-lab and in particular Nicole Bermingham whose helped made this data collection possible. Furthermore, we would like to thank the staff at the Arkell Poultry Research Station Guelph, Ontario, where birds were housed, managed, and cared for expertly, and with great consideration for the logistical challenges of a project this big.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Ali A. B. A., Campbell D. L. M., Karcher D. M., Siegford J. M. (2016). Influence of genetic strain and access to litter on spatial distribution of 4 strains of laying hens in an aviary system. Poult. Sci. 95, 2489–2502. doi: 10.3382/ps/pew236
Ali A. B. A., Campbell D. L. M., Karcher D. M., Siegford J. M. (2019). Nighttime roosting substrate type and height among 4 strains of laying hens in an aviary system. Poult. Sci. 98, 1935–1946. doi: 10.3382/ps/pey574
Armstrong E. A., Rufener C., Toscano M. J., Eastham J. E., Guy J. H., Sandilands V., et al. (2020). Keel bone fractures induce a depressive-like state in laying hens. Sci. Rep. 10, 1–14. doi: 10.1038/s41598-020-59940-1
Brantsæter M., Nordgreen J., Rodenburg T. B., Tahamtani F. M., Popova A., Janczak A. M. (2016). Exposure to increased environmental complexity during rearing reduces fearfulness and increases use of three-dimensional space in laying hens (Gallus gallus domesticus). Front. Vet. Sci. 3. doi: 10.3389/fvets.2016.00014
Casey-Trott T. M., Korver D. R., Guerin M. T., Sandilands V., Torrey S., Widowski T. M. (2017). Opportunities for exercise during pullet rearing, part II: long-term effects on bone characteristics of adult laying hens at the end-of-lay. Poult. Sci. 96, 2518–2527. doi: 10.3382/ps/pex060
Chen D. H., Bao J. (2012). General behaviors and perching behaviors of laying hens in cages with different colored perches. Asian-Australas. J. Anim. Sci. 25, 717–724. doi: 10.5713/ajas.2011.11366
Chew J., Widowski T., Herwig E., Shynkaruk T., Schwean-Lardner K. (2021a). The effect of light intensity on the body weight, keel bone quality, tibia bone strength, and mortality of brown and white feathered egg-strain pullets reared in perchery systems. Poult. Sci. 100, 1–19. doi: 10.1016/j.psj.2021.101464
Chew J., Widowski T., Herwig E., Shynkaruk T., Schwean-Lardner K. (2021b). The effect of light intensity, strain, and age on the behavior, jumping frequency and success, and welfare of egg-strain pullets reared in perchery systems. Animals 11, 1–14. doi: 10.3390/ani11123353
Colson S., Arnould C., Michel V. (2008). Influence of rearing conditions of pullets on space use and performance of hens placed in aviaries at the beginning of the laying period. Appl. Anim. Behav. Sci. 111, 286–300. doi: 10.1016/j.applanim.2007.06.012
Dudde A., Schrader L., Weigend S., Matthews L. R., Krause E. T. (2018). More eggs but less social and more fearful? differences in behavioral traits in relation to the phylogenetic background and productivity level in laying hens. Appl. Anim. Behav. Sci. 209, 65–70. doi: 10.1016/j.applanim.2018.08.017
Enneking S. A., Cheng H. W., Jefferson-Moore K. Y., Einstein M. E., Rubin D. A., Hester P. Y. (2012). Early access to perches in caged white leghorn pullets. Poult. Sci. 91, 2114–2120. doi: 10.3382/ps.2012-02328
Fawcett D. L., Casey-Trott T. M., Jensen L., Caston L. J., Widowski T. M. (2020). Strain differences and effects of different stocking densities during rearing on the musculoskeletal development of pullets. Poult. Sci. 99, 4153–4161. doi: 10.1016/j.psj.2020.05.046
Fleming R. H., Mccormack H. A., Mctier L., Whitehead C. C. (1997). Relationships between genetic, environmental and nutritional factors influencing osteoporosis in laying hens. Br. Poult. Sci. 47, 442–755. doi: 10.1080/00071669708418012
Frost H. M. (1987). Bone “mass” and the “mechanostat”: a proposal. Anat. Rec. 219, 1–9. doi: 10.1002/ar.1092190104
Garant R., Tobalske B. W., BenSassi N., van Staaveren N., Tulpan D., Widowski T., et al. (2022). Effects of clipping of flight feathers on resource use in gallus gallus domesticus. R. Soc. Open Sci. 9, 1–10. doi: 10.1098/rsos.211561
Gatesy S. M., Dial K. P. (1996). Locomotor modules and the evolution of avian flight. Evol. (N. Y). 50, 331–340. doi: 10.1111/j.1558-5646.1996.tb04496.x
Giersberg M. F., Kemper N., Hartung J., Schrader L., Spindler B. (2017). Determination of body width in brown and white layer pullets by image analyses. Br. Poult. Sci. 58, 230–235. doi: 10.1080/00071668.2017.1293230
Gunnarsson S., Yngvesson J., Keeling L. J., Forkman B. (2000). Rearing without early access to perches impairs the spatial skills of laying hens. Appl. Anim. Behav. Sci. 67, 217–228. doi: 10.1016/S0168-1591(99)00125-2
Habig C., Geffers R., Distl O. (2012). Differential gene expression from genome-wide microarray analyses distinguishes lohmann selected leghorn and lohmann brown layers. PloS One 7, 1–8. doi: 10.1371/journal.pone.0046787
Habinski A. M., Caston L. J., Casey-Trott T. M., Hunniford M. E., Widowski T. M. (2017a). Animal well-being and behavior: development of perching behavior in 3 strains of pullets reared in furnished cages. Poult. Sci. 96, 519–529. doi: 10.3382/ps/pew377
Habinski A. M., Caston L. J., Casey-Trott T. M., Hunniford M. E., Widowski T. M. (2017b). Development of perching behavior in 3 strains of pullets reared in furnished cages. Poult. Sci. 96, 519–529. doi: 10.3382/ps/pew377
Harlander-Matauschek A., Rodenburg T. B., Sandilands V., Tobalske B. W., Toscano M. J. (2015). Causes of keel bone damage and their solutions in laying hens. Worlds. Poult. Sci. J. 71, 461–472. doi: 10.1017/S0043933915002135
Heerkens J. L. T., Delezie E., Rodenburg T. B., Kempen I., Zoons J., Ampe B., et al. (2015). Risk factors associated with keel bone and foot pad disorders in laying hens housed in aviary systems. Poult. Sci. 95, 482–488. doi: 10.3382/ps/pev339
Heikkilä M., Wichman A., Gunnarsson S., Valros A. (2006). Development of perching behaviour in chicks reared in enriched environment. Appl. Anim. Behav. Sci. 99, 145–156. doi: 10.1016/j.applanim.2005.09.013
Hester P. Y., Enneking S. A., Haley B. K., Cheng H. W., Einstein M. E., Rubin D. A. (2013). The effect of perch availability during pullet rearing and egg laying on musculoskeletal health of caged White Leghorn hens. Poult. Sci. 92, 1972–1980. doi: 10.3382/ps.2013-03008
Hewlett S. E., Nordquist R. E. (2019). Effects of maternal care during rearing in white leghorn and brown nick layer hens on cognition, sociality and fear. Animals 9, 1–17. doi: 10.3390/ani9070454
Janczak A. M., Riber A. B. (2015). Review of rearing-related factors affecting the welfare of laying hens. Poult. Sci. 94, 1454–1469. doi: 10.3382/ps/pev123
Judex S., Zernicke R. F. (2000). High-impact exercise and growing bone: relation between high strain rates and enhanced bone formation. J. Appl. Physiol. 88, 2183–2191. doi: 10.1152/jappl.2000.88.6.2183
Kozak M., Tobalske B., Martins C., Bowley S., Wuerbel H., Harlander-Matauschek A. (2016a). Use of space by domestic chicks housed in complex aviaries. Appl. Anim. Behav. Sci. 181, 115–121. doi: 10.1016/j.applanim.2016.05.024
Kozak M., Tobalske B., Springthorpe D., Szkotnicki B., Harlander-Matauschek A. (2016b). Development of physical activity levels in laying hens in three-dimensional aviaries. Appl. Anim. Behav. Sci. 185, 66–72. doi: 10.1016/j.applanim.2016.10.004
Kruijt J. P. (1964). Ontogeny of social behaviour in Burmese red junglefowl (Gallus gallus spadiceus). Behav. Supplement 12, I–201. Available at: http://www.jstor.org/stable/30039152.
LeBlanc C., Tobalske B., Bowley S., Harlander-Matauschek A. (2018). Development of locomotion over inclined surfaces in laying hens. Animal 12, 585–596. doi: 10.1017/S1751731117001896
LeBlanc S., Tobalske B., Quinton M., Springthorpe D., Szkotnicki B., Wuerbel H., et al. (2016). Physical health problems and environmental challenges influence balancing behaviour in laying hens. PloS One 11, 1–16. doi: 10.1371/journal.pone.0153477
León B. M., Tobalske B. W., Sassi N.B., Garant R., Powers D. R., Harlander-Matauschek A. (2021). Domestic egg-laying hens, gallus gallus domesticus, do not modulate flapping flight performance in response to wing condition. R. Soc. Open Sci. 8, 1–12. doi: 10.1098/rsos.210196
McHugh M. L. (2012). Interrater reliability: the kappa statistic. Biochem. Med. (Zagreb). 22, 276–282. doi: 10.11613/BM.2012.031
Muir G. D., Chu T. K. (2002). Posthatching locomotor experience alters locomotor development in chicks. J. Neurophysiol. 88, 117–123. doi: 10.1152/jn.2002.88.1.117
Muir G. D., Gosline J. M., Steeves J. D. (1996). Ontogeny of bipedal locomotion: walking and running in the chick. J. Physiol. 493, 589–601. doi: 10.1113/jphysiol.1996.sp021406
Nasr M. A. F., Browne W. J., Caplen G., Hothersall B., Murrell J. C., Nicol C. J. (2013). Positive affective state induced by opioid analgesia in laying hens with bone fractures. Appl. Anim. Behav. Sci. 147, 127–131. doi: 10.1016/j.applanim.2013.04.015
Nasr M. A. F., Murrell J., Wilkins L. J., Nicol C. J. (2012). The effect of keel fractures on egg-production parameters, mobility and behaviour in individual laying hens. Anim. Welfare. 21, 127–135. doi: 10.7120/096272812799129376
Nelson J. R., Settar P., Berger E., Wolc A., O’Sullivan N., Archer G. S. (2020). Brown and white egg-layer strain differences in fearfulness and stress measures. Appl. Anim. Behav. Sci. 231, 1–7. doi: 10.1016/j.applanim.2020.105087
Nicol C. J., Bouwsema J., Caplen G., Davies A. C., Hockenhull J., Lambton S. L., et al. (2017). Farmed bird welfare science review (Melbourne, Victoria: Department of Economic Development, Jobs, Transport and Resources). Available at: https://agriculture.vic.gov.au/:data/assets/pdf_file/0008/529829/Farmed-Bird-Welfare-Science-Review.pdf.
Norman K. I., Weeks C. A., Tarlton J. F., Nicol C. J. (2021). Rearing experience with ramps improves specific learning and behaviour and welfare on a commercial laying farm. Sci. Rep. 11, 1–14. doi: 10.1038/s41598-021-88347-9
Peixoto M. R. L. V., Karrow N. A., Newman A., Head J., Widowski T. M. (2021). Effects of acute stressors experienced by five strains of layer breeders on measures of stress and fear in their offspring. Physiol. Behav. 228, 1–9. doi: 10.1016/j.physbeh.2020.113185
Peixoto M. R. L. V., Karrow N. A., Newman A., Widowski T. M. (2020). Effects of maternal stress on measures of anxiety and fearfulness in different strains of laying hens. Front. Vet. Sci. 7. doi: 10.3389/fvets.2020.00128
Pickel T., Scholz B., Schrader L. (2010). Perch material and diameter affects particular perching behaviours in laying hens. Appl. Anim. Behav. Sci. 127, 37–42. doi: 10.1016/j.applanim.2010.08.005
Pufall A., Harlander-matauschek A., Hunniford M., Widowski T. M. (2021). Effects of rearing aviary style and genetic strain on the locomotion and musculoskeletal characteristics of layer pullets. Animals 11, 1–20. doi: 10.3390/ani11030634
R Core Team (2021) R: a language and environment for statistical computing. Available at: https://www.R-project.org/ (Accessed May 2, 2023).
Rentsch A. K. (2023) Raising laying hens: the effect of early life environmental complexity and genetic strain on behavioural development. Available at: https://hdl.handle.net/10214/27422.
Rentsch A. K., Rufener C. B., Spadavecchia C., Stratmann A., Toscano M. J. (2019). Laying hen’s mobility is impaired by keel bone fractures and does not improve with paracetamol treatment. Appl. Anim. Behav. Sci. 216, 19–25. doi: 10.1016/j.applanim.2019.04.015
Riddle E. R., Ali A. B. A., Campbell D. L. M., Siegford J. M. (2018). Space use by 4 strains of laying hens to perch, wing flap, dust bathe, stand and lie down. PloS One 13, 1–16. doi: 10.1371/journal.pone.0190532
Ross E. (2021) Effect of early rearing environment on musculoskeletal traits and proximate composition in laying hen pullets. Available at: https://hdl.handle.net/10214/26363.
Rufener C., Makagon M. M. (2020). Keel bone fractures in laying hens: a systematic review of prevalence across age, housing systems, and strains. J. Anim. Sci. 98, S36–S51. doi: 10.1093/jas/skaa145
Scott G. B., Lambe N. R., Hitchcock D. (1997). Ability of laying hens to negotiate horizontal perches at different heights, separated by different angles. Br. Poult. Sci. 38, 48–54. doi: 10.1080/00071669708417939
Tobalske B. W. (2007). Biomechanics of bird flight. J. Exp. Biol. 210, 3135–3146. doi: 10.1242/jeb.000273
Toscano M., Booth F., Richards G., Brown S., Karcher D., Tarlton J. (2018). Modeling collisions in laying hens as a tool to identify causative factors for keel bone fractures and means to reduce their occurrence and severity. PloS One 13, 1–21. doi: 10.1371/journal.pone.0200025
Toscano M. J., Dunn I. C., Christensen J. P., Petow S., Kittelsen K., Ulrich R. (2020). Explanations for keel bone fractures in laying hens: are there explanations in addition to elevated egg production? Poult. Sci. 99, 4183–4194. doi: 10.1016/j.psj.2020.05.035
van Staaveren N., Decina C., Baes C. F., Widowski T. M., Berke O., Harlander-Matauschek A. (2018). A description of laying hen husbandry and management practices in Canada. Animals 8, 1–18. doi: 10.3390/ani8070114
Vitienes I., Mikolajewicz N., Hosseinitabatabaei S., Bouchard A., Julien C., Graceffa G., et al. (2023). Breed and loading history influence in vivo skeletal strain patterns in pre-pubertal female chickens. Bone 173, 1–15. doi: 10.1016/j.bone.2023.116785
Weeks C. A., Nicol C. J. (2006). Behavioural needs, priorities and preferences of laying hens. Worlds. Poult. Sci. J. 62, 296–307. doi: 10.1079/WPS200598
Whitehead C. C. (2004). Overview of bone biology in the egg-laying hen. Poult. Sci. 83, 193–199. doi: 10.1093/ps/83.2.193
Whitehead C. C., Wilson S. A. (1992). Characteristics of osteopenia in hens. Poult. Sci. Symposium 23, 265–280.
Widowski T. M., Casey-Trott T. M., Morrissey K. (2017). “Welfare of laying hens: an overview,” in Achieving sustainable production of eggs volume 2: animal welfare and sustainability. Ed. Roberts J. (Cam: Burleigh Dodds Science Publishing Limited), 57–84. doi: 10.19103/AS.2016.0012.22
Widowski T., Torrey S. (2018). “Rearing young birds for adaptability,” in Advances in poultry welfare. Ed. Mench J. A. (Duxforn, United Kingdom: Woodhead Publishing), 49–76. doi: 10.1016/B978-0-08-100915-4.00003-8
Wilkins L. J., McKinstry J. L., Avery N. C., Knowles T. G., Brown S. N., Tarlton J., et al. (2011). Influence of housing system and design on bone strength and keel bone fractures in laying hens. Vet. Rec. 169, 414. doi: 10.1136/vr.d4831
Keywords: laying hen, rearing aviary, brooding compartment, locomotion, load bearing
Citation: Rentsch AK, Harlander A, Siegford JM, Vitienes I, Willie BM and Widowski TM (2023) Rearing laying hens: the effect of aviary design and genetic strain on pullet exercise and perching behavior. Front. Anim. Sci. 4:1176702. doi: 10.3389/fanim.2023.1176702
Received: 28 February 2023; Accepted: 12 May 2023;
Published: 31 May 2023.
Edited by:
Rafael Freire, Charles Sturt University, AustraliaReviewed by:
Victoria Sandilands, Scotland’s Rural College, United KingdomMarkus Freick, Hochschule für Technik und Wirtschaft Dresden, Germany
Copyright © 2023 Rentsch, Harlander, Siegford, Vitienes, Willie and Widowski. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Tina M. Widowski, dHdpZG93c2tAdW9ndWVscGguY2E=
 Ana K. Rentsch
Ana K. Rentsch Alexandra Harlander
Alexandra Harlander Janice M. Siegford
Janice M. Siegford Isabela Vitienes
Isabela Vitienes Bettina M. Willie
Bettina M. Willie Tina M. Widowski
Tina M. Widowski