- 1Department of Animal Production, Faculty of Agriculture and Veterinary Medicine, Thamar University, Dhamar, Yemen
- 2Institute of Animal Nutrition, Department of Veterinary Medicine, Free University of Berlin, Berlin, Germany
Livestock production in developing countries faces several difficulties such as a general shortage of feed resources, regional availability, and quality. Climate change further exacerbates these problems, leading to a massive reduction in ruminant productivity. Therefore, there is a need for the use of adaptable and resilient forage plants that can also contribute to reducing greenhouse gases. The Moringa oleifera tree is well known as an agroforestry tree and has adapted to growing in harsh conditions. It produces a high amount of biomass in a short period and contains high levels of nutrients and biologically active components. All parts of the Moringa tree are valuable and have multiple benefits and applications. Therefore, Moringa oleifera has great potential and can be used as a forage crop, storing carbon dioxide (CO2) and improving ruminant performance and the livelihoods of farmers in the tropics. This article aimed to present the results and findings of studies related to the use of Moringa in ruminant feed (cattle, sheep, and goats) and its contribution to climate protection. Several studies highlighted that M. oleifera can be used as green fodder either individually or in combination with other crops or concentrate feeds to improve the performance of ruminants, such as the growth rate, milk yield, and milk constituents, without negatively impacting animal health. This improvement in performance could be attributed to the favorable nutrient content in M. oleifera, delivering proteins in conjunction with bioactive compounds such as alkaloids, flavonoids, phenolics, glucosinolates, carotenoids, sterols, saponins, phenolic acids, tannins, and isothiocyanates. Furthermore, it has been shown that this plant can be produced in high yields and thus might be an excellent carbon dioxide sink to absorb and utilize carbon dioxide, reducing the anthropogenic load of carbon dioxide in the atmosphere. In addition, feeding cattle and other ruminants with M. oleifera leaves or seeds significantly decreases ruminal methane emissions, which could contribute to adapting to climate-friendly farming. Thus, the use of Moringa can make a sustainable contribution to strengthening animal production, especially in countries with limited feed resources.
Introduction
Ruminant production is a very important sector for farmers in developing countries. The meat and milk from ruminants are relevant sources of nutrients such as proteins, essential amino acids, calcium, and vitamin D, contributing to a balanced diet in humans. Ruminant production faces multiple and global challenges such as limited arable land, feed availability due to ongoing reduction in pasture and cultivable areas for crop production, water deficiency, and accelerating climate change (Halmemies-Beauchet-Filleau et al., 2018). Climate change is a challenge for plant and animal production and it is expected to have important consequences on environmental performance, especially in tropical and semitropical regions. Drought periods, heat waves, and seasonal variation in rainfall impair crop production and thus can also threaten the requirements and supplies of adequate and high-quality feed for farm animals. In the past three decades, climate change has reduced global agricultural production by 1–5% per decade (Thornton et al., 2015). In addition, in developing countries, the cost of feed ingredients is volatile and constantly increasing, which are the main constraints in low-income countries (Devendra and Leng, 2011), further complicating animal production and making products unaffordable for the consumer. Therefore, mitigating and reducing these impacts requires creative and innovative efforts by scientists and specialists worldwide, which may also help to stabilize and increase livestock productivity. Studies show that the environmental and climate impact of livestock farming can be improved through sustainable animal feeding by selecting optimal and innovative feeds, as well as by using adaptive and resilient forage plants that also have a positive effect by limiting the CO2 footprint. Gedefaw (2015) reported that there is an urgent need to implement climate-friendly strategies that can build more resilient food and feed systems to combat climate change. Trees and shrubs in the tropics play very important roles in human and livestock nutrition (Smith, 1994). Moreover, reports showed that tree leaves are one of the most promising protein substitutes, with high nutritional value, medicinal benefits, and low market prices (Kakengi et al., 2005). In this context, the M. oleifera tree has great potential as a foliage plant to improve the livelihoods of farmers in the tropics (Gedefaw, 2015). The Moringa oleifera is a fast-growing softwood tree and is well adapted to growing in harsh conditions such as those in arid and semiarid areas. Moringa oleifera possesses not only a high nutritional profile (protein, fat, and minerals) but it is also an excellent source of biologically active compounds, and, furthermore, it has a low quantity of antinutritional factors such as tannins and saponins. All parts of Moringa (leaves, seeds, pods, flowers, bark, and roots) are valuable and have multiple benefits and uses. Its products have been traditionally used in medicine, human nutrition, feed for animals, water purification, fuel wood, dye, conservation, and green manure. Therefore, it is known as miracle or a multipurpose tree (Koul and Chase, 2015; Rizwan et al., 2022). In addition, it has been reported as one of the most widely used fodder crops, with potential as a browse plant in ruminant dietary supplementation (Makkar and Becker, 1996) and it is considered a beneficial and ideal tree for agroforestry (Horn et al., 2022). Moreover, M. oleifera is a suitable plant for climate protection due to its high use and adaptability in agriculture and the environment (Ndubuaku et al., 2015; Daba, 2016).
As mentioned above, the foliage of trees and shrubs can be used as feed or supplements to provide animals with nutrients (proteins and minerals). In addition, these trees contain secondary metabolites (tannins and saponins) that reduce methane production, with antimethanogenic properties (Kamra et al., 2008; Jayanegara et al., 2009; Delgado et al., 2012; Palangi and Lackner, 2022). In this context, this review highlights and discusses the main research findings related to M. oleifera as a readily available and cheap feed for ruminants (cattle, sheep, and goats) in combination with its effects on animal performance. Moreover, this review aims to explain the use of parts of this plant as feed or dietary supplements to reduce rumen methane formation and also to understand its contribution to climate change adaptation in livestock production in developing countries.
Biological characteristics of M. oleifera and its cultivation
Moringa oleifera is a slender softwood plant belonging to the monogeneric family Moringaceae which includes approximately 13 species. This tree, native to the foothills of the Himalayas, India, has been introduced and cultivated in many tropical and subtropical countries (Bosch, 2004; Price, 2007). Moringa is a fast-growing, deciduous tree and branches freely. This plant can withstand dry seasons. It is a perennial plant and can grow to a height of 10 m and a trunk diameter of 40 cm. Moringa oleifera is considered a small- to medium-sized tree that is well adapted to the temperature range 20–40°C. It grows in the wild and is also planted on farms and compounds. It can be grown in all kinds of soils. However, it is best suited to fertile and well-drained soils and it tolerates slight frosts (Price, 2007; Trigo et al., 2021). Moringa trees have large and deep taproots, are white in color, have a distinctive pungent odor, and very sparse lateral roots that allow them to grow in areas with little rainfall (Mohanty et al., 2020). Its leaves are tripinnate and compound, feathery and small in size, with green to dark green elliptical leaflets 1–2 cm long. The leaves grow on trees with drooping and hanging branches, mostly at the tips of the branches (Paliwal et al., 2011). The flowering of moringa occurs 4–12 months after planting. However, some varieties can flower at 4–5 months after planting. The flowers tend to have yellow-white petals with a pleasantly light fragrance. Its immature fruit (pods) are green in color, 10–60 cm in length, contain 15–20 seeds, and at maturity change color to brown. The shape of a dry seed is round or triangular and it is covered by a light woody shell with papery wings; each tree can produce between 15,000 and 25,000 seeds annually (Paliwal et al., 2011; Su and Chen, 2020; Amad and Zentek, 2022) depending on the variety. Moringa oleifera can be propagated by direct seedlings, cuttings, or by seeds under minimal care and with a wide range of management practices (Paliwal et al., 2011). Moringa trees grown from seeds start producing pods (fruit) in the first year. Similarly, trees grown from large cuttings typically begin producing fruit 12–18 months after planting. Moringa oleifera can be harvested several times during the growing season and has a high biomass yield ranging from 43 to 115 t/hectare annually (Makkar and Becker, 1996; Makkar and Becker, 1997; Kholif et al., 2016).
Nutrients and phytochemical compounds in M. oleifera
Nutrients
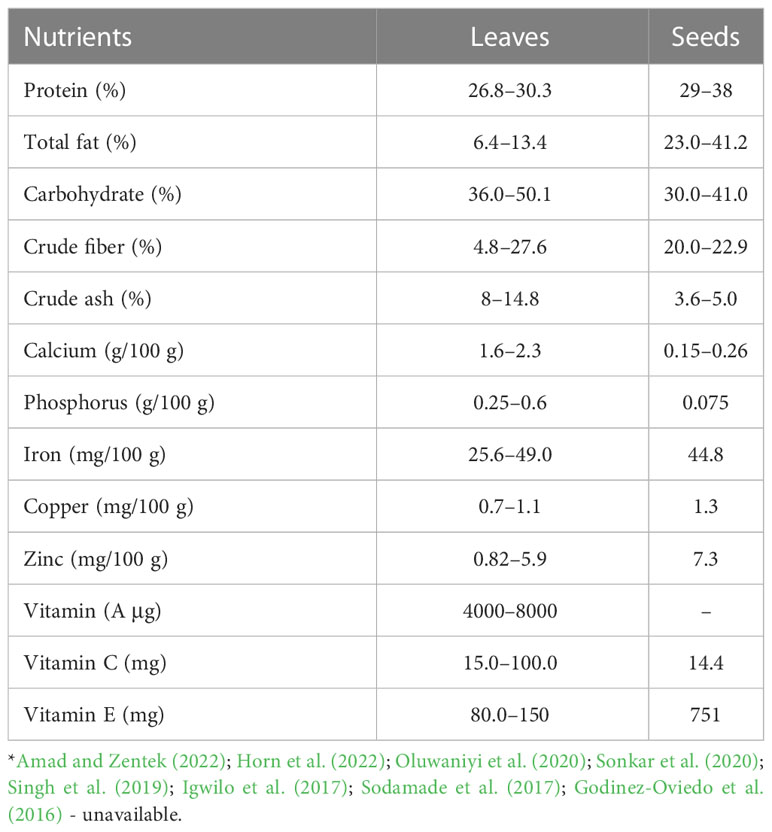
Moringa oleifera is known for its nutritional potential; therefore, all parts of M. oleifera are a storehouse of important and essential nutrients such as proteins, fats, carbohydrates, vitamins, and essential amino acids (Stadtlander and Becker, 2017). Previous studies reported the concentrations of organic nutrients and minerals in different parts of this plant (Table 1). It must be emphasized that stems and immature pods of M. oleifera have lower nutrient levels compared to leaves. The leaves of Moringa contain approximately 27–30% or slightly more protein on a dry matter (DM) basis, with significant quantities of all the essential amino acids (Amad and Zentek, 2022). The protein content of Moringa leaf meal is higher than that of other forage plants such as Medicago sativa (Alfalfa) and many commonly consumed green leafy vegetables (spinach and mint). In addition, it is equivalent to many pulses such as soybeans, which contain 22–24% protein DM (Joshi and Mehta, 2010). The leaves have high contents of calcium (Ca), potassium (K), magnesium (Mg), sodium (Na), chloride (Cl), and iron (Fe) among other minerals (Mulyaningsih and Yusuf, 2018). The crude fat content of Moringa leaves reaches an average of up to 9% on a dry matter basis, with polyunsaturated omega-3 and omega-6 fatty acids, in particular linoleic, linolenic, and oleic acid dominating. Moringa oleifera leaves have a high concentration of crude fiber (4.8–27.6% DM). The seeds of the Moringa plant have high levels of protein and lipids (Table 1). Dry seeds contain 18–25% protein and thus have approximately twice the content in cereals (Paliwal et al., 2011). An early study by Makkar and Becker (1997) mentioned that the protein content in untreated kernels reached 36.7% DM. The seeds of M. oleifera are rich in oil, with even higher contents than soybeans, ranging between 30–40%. The oil is highly resistant to rancidity and is similar to olive oil in its fatty acid profile (Fahey, 2005; Ben Salem and Makkar, 2009; Paliwal et al., 2011; Leone et al., 2015). In addition, Moringa flowers are rich in proteins, lipids, dietary fiber, carbohydrates, and minerals. The pods of Moringa contain high levels of dietary fiber but are low in crude protein and fat (Rizwan et al., 2022). The leaves, flowers, and pods of the Moringa tree are rich in provitamins A (ß-caroten), vitamin D, vitamin E, vitamin B (thiamine, riboflavin, niacin, pantothenic acid, vitamin B6, and folate B9), and vitamin C (Gopalakrishnan et al., 2016; Abbas et al., 2018). In fact, the Moringa tree is a source of highly digestible nutrients and every part of the Moringa is suitable and important for nutritional or therapeutic use in many tropical countries, as shown by Fahey (2005); Paliwal et al. (2011) and Su and Chen (2020).
Phytochemicals and antinutrients
Moringa oleifera is also a reservoir of functional phytochemicals or bioactive compounds, such as alkaloids, flavonoids, phenolics, glucosinolates, carotenoids, sterols, saponins, phenolic acids, tannins, and isothiocyanates, which are present in significant concentrations in various parts of the plant (Verma et al., 2009; Meireles et al., 2020; Mohanty et al., 2020). The diversity of these phytochemicals contributes to many functional traits, including pharmacological and therapeutic properties. Therefore, M. oleifera has been widely used in traditional medicine in tropical and subtropical regions for centuries. Scientific reports mentioned that the leaves of M. oleifera contain a total of 35 bioactive compounds, including high concentrations of flavonoids (21.8%), followed by tannins (14.3%), saponins (12.6%), and alkaloids (2.4%) (Maqsood et al., 2017). Flavonoids, glycosides, terpenoids, and phenols in the leaf extract of M. oleifera showed potent antibacterial activity against Staphylococcus aureus and Escherichia coli, as well as antifungal effects and antioxidant activities (Elangovan et al., 2015; Malhotra and Mandal, 2018). Anwar and Bhanger (2003) stated that Moringa leaves also contain flavonoids such as kaempferol and quercetin, which have higher antioxidant activities than ascorbic acid. Moringa oleifera leaves and seed meals act as valuable sources of vitamins, selenium, flavonoids, phenolics, and carotenoids, which makes them suitable as a nutritionally valuable and healthy ingredient, valuable for preventive medicine (Williams, 2013). Carotenoids play an important role in animal nutrition due to their strong antioxidant properties (Bhaskarachary et al., 1995). The flowers and roots of this plant also contain the antimicrobial compound Pterygospermin, which showed a potential effect against microbial species such as Fusarium solani, Bacillus subtilis, Staphylococcus aureus, and Pseudomonas areogunosa (Pandey, 2012). Aqueous extracts of mature and premature flowers studied by chromatographic analysis showed the presence of free sugar, D-galactose, D-glucose, O-mannose, polysaccharide, amino acids, alkaloids, and flavonoids such as rhamnetin, isoquercitrin, and kaempferitrin. The pods contained isothiocyanates, thiocarbamates, and nitriles. The fruits contained cytokines, whereas seeds contained high concentrations of benzylglucosinolate (Faizi et al., 1998; Pramanik and Islam, 1998; Bhattacharya et al., 2018).
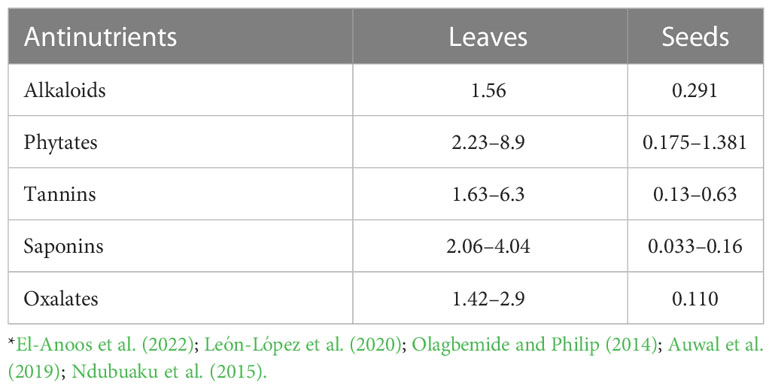
On the other hand, M. oleifera tree has numerous antinutritional factors or antinutrients including tannins, alkaloids, phytate, saponins, oxalates, cyanide hydrogen, and enzyme inhibitors (Table 2). The types and concentrations of these anti-nutritional factors seem to differ from one part of the plant to another, and also depend on growth conditions and cultivars (Stevens et al., 2015; Sultana et al., 2015b). The seeds tend to show lower concentrations of antinutrients than the leaves (Fayis, 2017). These anti-nutritional factors are responsible for the reduction in feed intake, digestibility, absorption, and utilization, and may produce other adverse effects that impact the health and productivity of animals (Akande and Fabiyi, 2010). Early reports from Noonan and Savage (1999) and Radek and Savage (2008) indicated that the levels of anti-nutritional factors found in M. oleifera are lower compared with those found in some other leafy vegetables. Oxalates and phytates are present in Moringa leaves at 10.5 and 21 g/kg DM, respectively (Rizwan et al., 2022). Oxalates are insoluble and not harmful to animals. Trypsin inhibitors are found at relatively low levels (Ogbe and Affiku, 2011) or are not present (Makkar and Becker, 1997; Balami et al., 2018). Hydrogen cyanide (HCN) does not exist or is present in low concentrations in leaves and has no harmful effects (Teixeira et al., 2014). Saponins and tannins also exist in Moringa leaves at high concentrations of approximately 4.7–5 g/kg and 12–20.6 g/kg DM, respectively (Moyo et al., 2011; Teixeira et al., 2014; Su and Chen, 2020). An increase in tannins and phytates can negatively affect digestibility and metabolism by reducing the rate of utilization of dietary nutrients, and this could lead to a negative growth rate in animals (Amad and Zentek, 2022). The seeds of Moringa contain 479 mg/kg DM tannins and 2.5 g/kg DM saponins (Fayis, 2017). Igwilo et al. (2014) reported that M. oleifera roots were rich in anti-nutritional factors, with higher concentrations of tannins and oxalates (450 mg/kg and 171 mg/kg, respectively), while those of saponins, phytates, and cyanogenic glycosides were lower (42 mg/kg, 0.7 mg/kg, and 27.2 mg/kg, respectively).
Use of Moringa oleifera in ruminant feeding
In developing countries, the use of Moringa tree leaves is common as a cheap and valuable alternative feedstuff and as a source of nutrients, aiming to overcome the limitations of other feed sources. The relatively low cost of feed helps farmers to maintain and improve animal production (Kholif et al., 2016). Ruminants can digest fibers such as cellulose, hemicelluloses, and other cell wall constituents because of their rumen microorganisms, which contain the necessary enzymes for breaking down these macromolecular substances. The high nutritional and bioactive composition of M. oleifera along with its low amounts of anti-nutrient compounds makes it suitable for ruminant nutrition. Debela and Tolera (2013) reported that Moringa fed as fresh forage or leaf meal can be considered a good potential source of supplementary protein in the diets of ruminant animals; moreover, it can be fed either as an extract or as dried forage.
Use in cattle feeding
The leaf, seed, and bark of Moringa are readily eaten by cattle as a constituent of their daily ration. Reports mentioned that supplementation with M. oleifera enhanced milk yield and milk composition (Babiker et al., 2017; Kholif et al., 2018; Brar et al., 2022). In one study, dairy cows (second lactation) fed a Moringa leaf supplement at a rate of 0 to 1.7 kg DM/d increased their milk production compared to those not fed. This increase in milk yield may be due to the positive effect of Moringa on the rumen environment, which leads to an increase in microbial production in the rumen. The protein in Moringa also has good rumen bypass properties (Sarwatt et al., 2004). Feeding dairy cows 2 kg or 3 kg DM of M. oleifera increased milk yield (4.9 and 5.1 kg/day) compared to cows fed only Brachiaria brizantha hay; this improvement was related to an increase in feed intake and in the digestibility of nutrients (dry matter, organic matter, crude protein, and dietary fiber) in M. oleifera (Sanchez et al., 2006). Moreover, it was concluded that ensiled Moringa can be fed in large quantities (10.40 kg DM/day) to dairy cows without any adverse effect on nutrient intake or digestibility, due to the high content of protein and fiber (Mendieta-Araica et al., 2011). Cows fed with M. oleifera (40 and 20% DM) had higher milk yields compared to those fed with alfalfa ration (40%). The milk constituents, including total solids, fat, protein, and ash, were significantly higher when Moringa forage was used, which correlated to higher nutrient digestibility levels, better nitrogen utilization, increased levels of rumen volatile fatty acids, microbial yield, and acetic acid concentration (Khalel et al., 2014). Similar results were obtained by Cohen-Zinder et al. (2016), who showed an increase in milk yield, milk fat, and protein content in lactating cows fed ensiled M. oleifera supplementation at 180 g/kg total mixed ration (TMR) DM. Likewise, the addition of Moringa (6% rachis and twig) to the diet of lactating multiparous cows improved milk production by 4.7% due to increased feed intake, nutrient digestibility, and ruminal fermentation (Zhang et al., 2018). In this study, increased milk fatty acid profiles, including mono-unsaturated and polyunsaturated fatty acids, were observed; however, the protein, glucose, and total solids in the milk were not affected, probably because the basal diet was high in fiber. Complete replacement of Berseem hay in the ration of lactating cows with 20% M. oleifera stems showed the best feed conversion and nutrient digestibility, leading to superior milk yield and composition (El-Esawy et al., 2018). Malik et al. (2019) concluded that the consumption and digestibility of dry matter and organic matter were improved by supplementation with M. oleifera in Bali cows. Shankhpal et al. (2019) found a similar tendency, and stated that cows fed 15 kg Moringa green fodder had higher milk yields, significantly more milk fat, lactose, and β-Carotene, but reduced cholesterol content compared to the control group (Figure 1). The authors showed that the b-carotene levels improved by 37%, while the level of cholesterol decreased by 17.6% in cows fed Moringa as green fodder. Kekana et al. (2019) mentioned that supplementation with M. oleifera at 60 g/cow/day markedly reduced oxidative stress in milk, measured with a modified Trolox equivalent antioxidant capacity (TEAC).
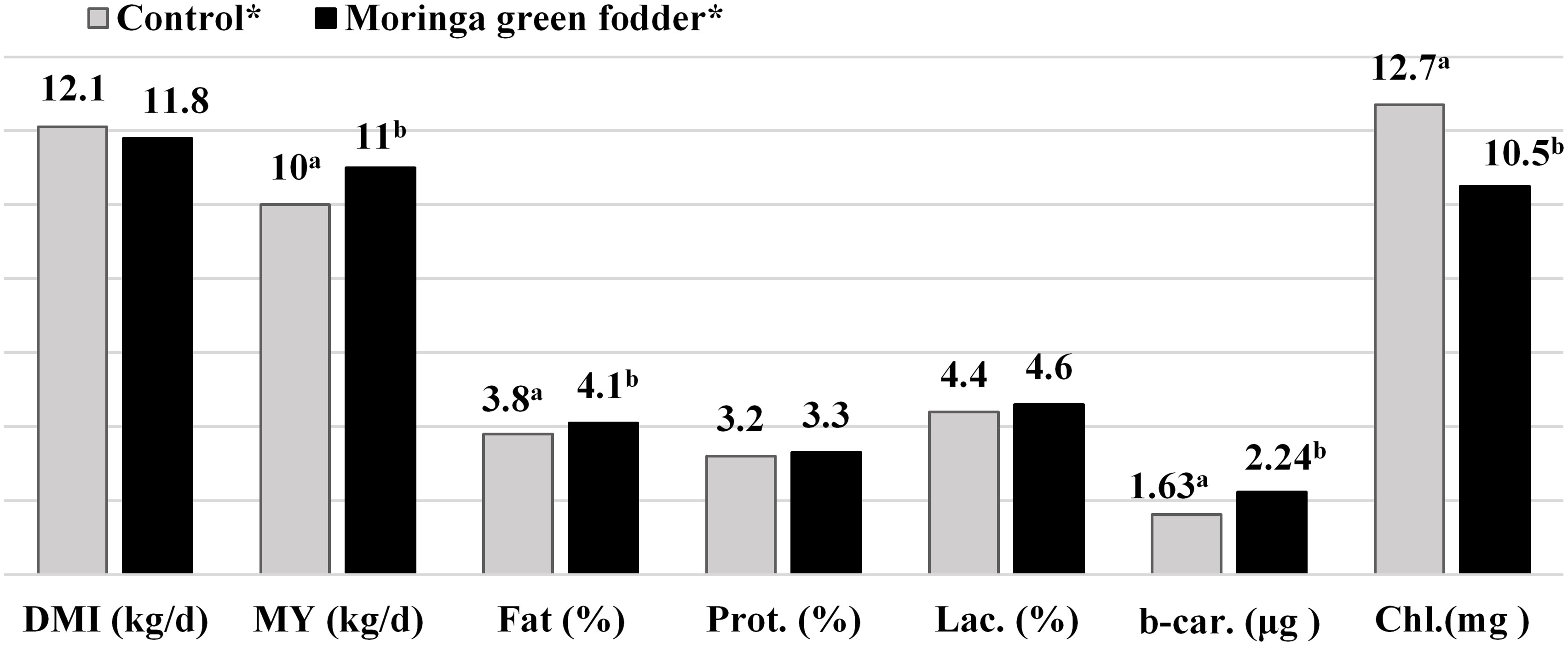
Figure 1 Effect of feeding Moringa oleifera on milk yield and its composition in crossbred cows (Shankhpal et al., 2019. Modified). DMI, Dry matter intake; MY, Milk yield; Prot, Protein; Lac, Lactose; B-Car, B-Carotene; Chl, Cholseterol. *15.0kg Moringa green fodder by replacing 15.0 kg hybrid Napier. ab Mean bearing different superscripts differ significantly (P < 0.01).
On the other hand, Mendieta-Araica et al. (2011) reported that when soybean meal was replaced with a Moringa leaf meal in dairy cow feed, a decrease in milk yield occurred, with no effect on milk composition, which was probably linked to increases in protein and energy intake in the soybean meal feed. Other studies showed that different levels of M. oleifera (0, 3, 6, and 9% DM of rachis and branch) in the diet resulted in similar feed intake, leading to the same milk production (Dong et al., 2019). Moringa leaf meal supplementation (0, 30, and 60 g/cow/day DM) had no effect on body weight or milk yield (Kekana et al., 2019). A recent study by Kekana et al. (2022) showed that supplementation with M. oleifera leaf meal at 16.6 g/100 kg body weight had no effect on the dry matter intake and body weight of cows, calf birth weight, or colostrum yield. In another experiment, the replacement of conventional concentrate ingredients (particularly ground corn, soybean meal, and wheat bran) with up to 50% mixtures of Moringa mash (leaves, twigs, and branches of Moringa) did not affect the DM intake or live weight gain of bulls (Sultana et al., 2021). The body weight, weight gain, and height at withers of crossbred calves fed TMR without and with 5% DM of M. oleifera meal were not affected (Sherasiya et al., 2022).
Based on the above, it can be stated that providing Moringa as green fodder, silage, or supplementary feed to cows in different proportions increases milk production and its components. It should be noted that the differences between the milk production results in different studies may be mainly due to breed type, age, production stage, parts of Moringa used (leaves, stem, seed, flowers, or a mix of these), and environmental and management conditions. The positive effects of Moringa on milk yield and higher milk fat could be attributed to a higher feed intake, apparent nutrient digestibility, and increased fermentation efficiency, which were enhanced by phytochemical-rich Moringa in conjunction with amino acids and minerals such as P, Ca, and Mg in M. oleifera leaves that are essential for high milk synthesis, as observed for other forage plants. It was shown that a small amount of tree leaves (leucaena hay) affected the rumen environment, leading to improved utilization of a low-quality roughage diet (Kabatange and Shayo, 1991). Moreover, moderate levels of phenols and tannins in M. oleifera (especially in the leaves) provide antioxidant and antimicrobial properties, which positively affect ruminant production (Verma et al., 2009; Cohen-Zinder et al., 2016). Moreover, rumen methanogenesis could be inhibited by phenols and tannins, resulting in inhibition of rumen CH4 production, as reflected in the improved fat yield and efficiency of milk production (Shaani et al., 2016; Dong et al., 2019). In addition, Moringa foliage is a good source of protein and amino acids, which can enhance the utilization of dietary N (Figure 2), boosting the productivity of dairy and beef cattle (Bashar et al., 2020; Sultana et al., 2021).
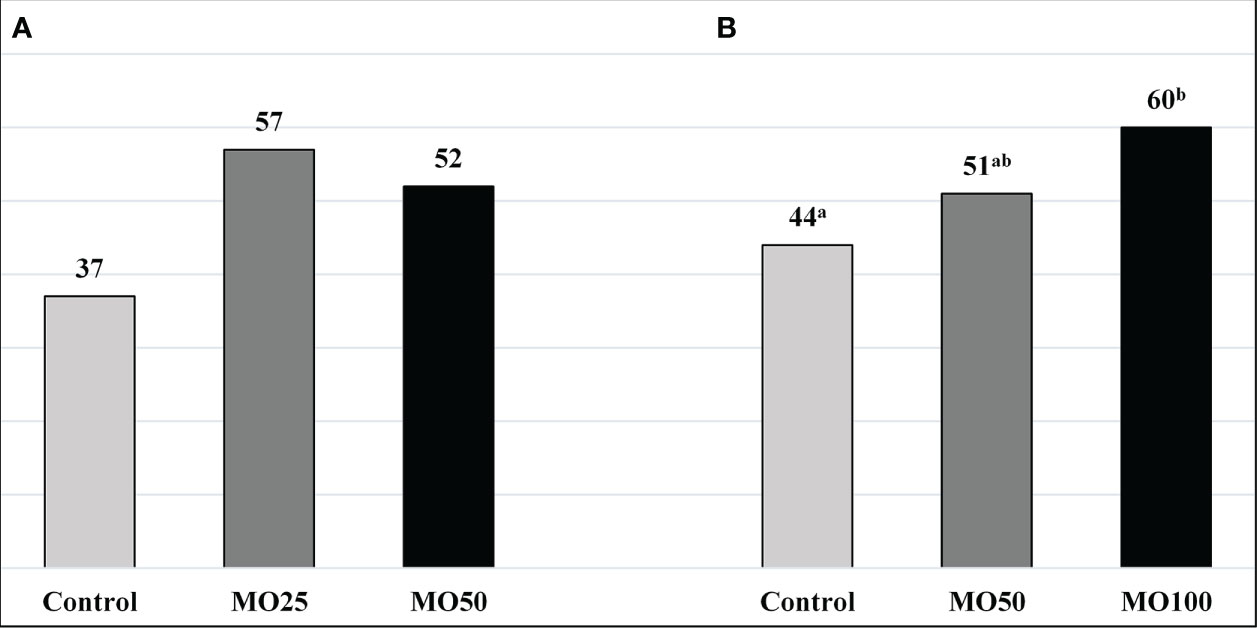
Figure 2 Effect of feeding Moringa oleifera on N-utilization in bull (A) (Sultana et al., 2021) and dairy cows (B) (Bashar et al., 2020) (in %). MO, Moringa oleifera; replacing concentrate mixture 25% or 50% (A) and 50 or 100% of concentrate mixture (B). ab Mean bearing different superscripts differ significantly (P < 0.05).
Use in sheep feeding
In an experiment replacing cottonseed cake with M. oleifera leaf meal (20% DM) in the diet of growing sheep (based on maize bran), an improvement in the digestibility of DM and an increased growth rate (20%) were observed; however, the feed conversion was poor (Murro et al., 2003). Feeding lambs with defatted M. oleifera seed meal at 2, 4, and 6 g DM/lamb/day had no effect on hay intake, diet digestibility, or N-balance; however, the highest daily gains were obtained for lambs fed intermediate levels of defatted M. oleifera seed meal (Ben Salem and Makkar, 2009). This was possibly due to the fact that 4 g/day of defatted M. oleifera seed meal in the diet increased the energy value of the diet. Lambs fed M. oleifera stems had higher feed efficiency; however, no significant difference was observed in the average daily and total body weight gain between animals in the control group fed clover hay and the experimental animals, which was due to the fact that clover hay had the highest nutrient digestibility and nutritive value (Mahmoud, 2013). In addition, a Moringa diet increased the average daily weight gain and milk yield of Najdi ewes compared to those fed alfalfa diets (Babiker et al., 2016; Babiker et al., 2017) (Figure 3). Lambs fed 75% and 100% Moringa foliage in a rice straw diet exhibited a positive growth performance, good carcass traits, and reduced subcutaneous fat (Sultana et al., 2017), similar to results obtained by Allam et al. (2015), who found that rations containing Moringa leaves improved the feed conversion and growth performance. Lambs fed 0, 25, and 50 g/kg DM M. oleifera root bark exhibited improvements in feed efficiency and body N retention, ultimately increasing the mean daily gain of these lambs (Soltan et al., 2018). Supplementation of male Barki sheep with Moringa seeds (4 g DM per day/head) significantly increased their final body weight and daily gain (EL-Hedainy et al., 2020). Moreover, a study on Deccani lambs showed a significantly higher body condition score when they were fed M. oleifera leaf meal at 25% in the concentrate mixture (Bhokre et al., 2020). These results and benefits were based on the excellent protein levels in Moringa forage and its positive effect on microbial protein formation in the rumen.
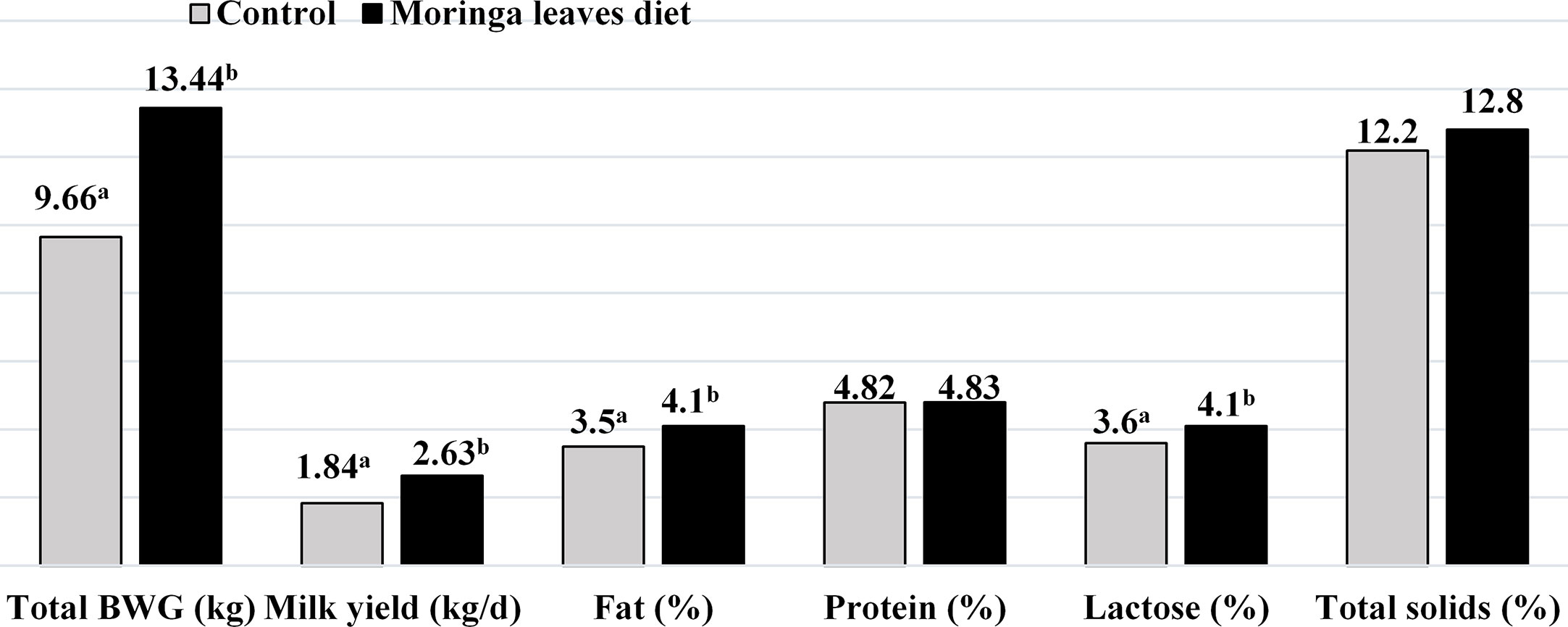
Figure 3 Effect of Moringa oleifera feeding on total live body weight gain, milk yield and composition Najdi ewes (Babiker et al., 2017). ab Mean bearing different superscripts differ significantly (P < 0.05).
Feeding Najdi ewes with 25% M. oleifera in their daily ration increased the milk yield compared to those fed alfalfa diets (40%), and there was a slight increase in milk fat, lactose, and energy output. An M. oleifera diet improved the milk yield due to its lower neutral detergent fiber (NDF) and acid detergent fiber (ADF), higher metabolizable energy contents, and excellent rumen bypass characteristics (Sarwatt et al., 2004; Babiker et al., 2016). In contrast, dietary supplementation with hydroalcoholic extracts of M. oleifera leaves at doses of 20, 40, or 60 mL/ewes/day in lactating ewes had no effect on milk yield and composition. Also, in this study there was no effect on ewe weaning efficiency, average daily gain, or litter weaning weight of lambs (Olvera-Aguirre et al., 2020). Early experiments indicated lower malondialdehyde values in the milk and serum of ewes fed M. oleifera and this might be attributed to the high total phenolic content and antioxidant capacity of an M. oleifera diet (Babiker et al., 2016; Babiker et al., 2017). In general, the improved milk yield and composition (fat, lactose, total energy, and energy output) is due to the lower NDF and ADF, high mineral contents (especially P, Ca, and Mg), high metabolizable energy, undegradable rumen protein, and adequate amino acid profile in M. oleifera feed (Zarkadas et al., 1995; Sarwatt et al., 2004; Babiker et al., 2016; El-Naggar et al., 2017), which led to improved nutrient utilization and body weight.
Use in goat feeding
Regarding feeding goats with M. oleifera, data indicated that dietary replacement of sunflower seed cake with 25, 75, and 100% Moringa meal did not significantly affect goat body weight gain (g/d) (Sarwatt et al., 2002). However, in the same study, goats that were fed 75 and 100% Moringa meal had greater dry matter intake compared to those that were not fed Moringa. Goats fed 20 and 50% Moringa leaves showed increased digestibility of dry matter, crude protein, and organic matter, resulting in an improvement in live weight gain (Aregheore, 2002). Similarly, results showed higher feed intake with improved mean daily weight gain in goats fed 200 g of dried M. oleifera (Moyo et al., 2011). The average animal weight gain improved and reached an average of 20.8 g/animal/day in African dwarf goats that were supplemented with Moringa compared to those fed a mixed concentrate, which can be attributed to the improvement in feed conversion and protein efficiency ratios (Asaolu et al., 2012). Goats fed a concentrate diet with 15% M. oleifera leaf meal had significantly higher rates of growth than those fed 5% and 10% M. oleifera (Tona et al., 2014). This indicated that a higher M. oleifera leaf content (15%) resulted in better feed utilization and nutrient digestibility, improving growth performance. Sultana et al. (2015b) found that the crude protein digestibility and final body live weight of experimental male goats increased linearly with increasing dietary Moringa leaf supplement content (25, 50, 75, and 100%). In addition, the replacement of a conventional concentrate mixture with 50 and 100% dried M. oleifera leaves improved the body weight and average daily body weight gain, without affecting the feed intake of Mehsana goat kids (Damor et al., 2017). One study indicated that kid goats fed 20 and 25% Moringa forage, included leaves, petioles, stems, and soft rachis, achieved a significantly superior final weight, total weight gain, and daily gain, which were linked to the better feed efficiency and higher digestibility of most nutrients (Ahmed and Shaarawy, 2019). Moreover, the daily supplementation of M. oleifera at 40, 60 and 80 g/day/head in goats on natural pasture had a significant impact on the overall body weight gain and final body weight of bucks; however, there was no significant difference among goats supplemented with leuceane Leucacephala and M. oleifera. The results also showed a significant improvement in all measured female reproduction efficiency parameters such as birth rate, twinning rate, and birth and weaning weight compared to control goats (Mataveia et al., 2019). Another study showed that supplementing the diet of goats with M. oleifera extract at different concentrations (30 and 60 ml extract/animal) improved the dry matter intake and feed conversion efficiency, which was reflected in a higher body weight and daily weight gain (Pedraza-Hernández et al., 2021). As shown in Figure 4A, Moringa oleifera supplementation improved the daily weight gain (g/day) and N-retention (%) in goat kids compared to both the control and 25% cowpea feed mixture groups (Wankhede et al., 2022). On the contrary, 75 and 100% M. oleifera leaf used as a replacement for concentrates had a negative impact on the final body weight and mean daily gain. These negative influences on growth rate parameters could be due to a reduction of crude protein digestibility, which is probably caused by the high content of anti-nutrient compounds such as phytates, tannins, and oxalate in M. oleifera (Zaher et al., 2020). Replacing a commercial concentrate mixture with 25 and 50% M. oleifera meal did not affect the overall body weight or biometry (body length and heart girth) of Surti kids, which may be due to the same protein content in all experimental groups (Pandey et al., 2022).
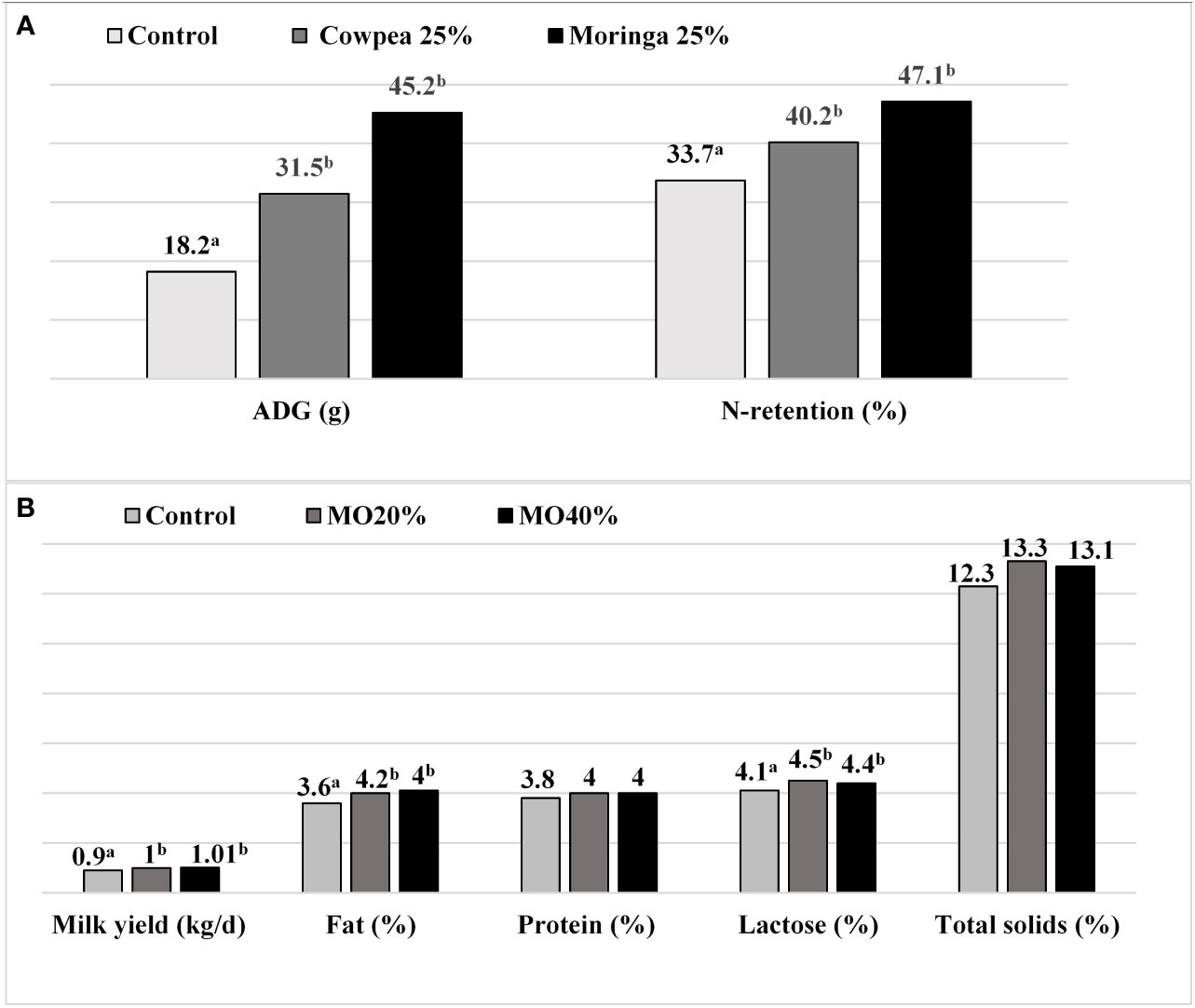
Figure 4 Effect of Moringa oleifera feeding on goats’ performance: (A) average daily body weight gain (ADG) and N-retention in goats kids, adapted from Wankhede et al. (2022); (B) milk yield and composition of Damascus Goats, adapted from Kholif et al. (2022). ab Mean bearing different superscripts differ significantly (P < 0.05).
Regarding milk production, a previous study mentioned that replacing sesame meal with M. oleifera leaf meal in the goat diet increased the milk yield by 10 to 15%, which was attributed to an increased feed intake, improved nutrient digestibility, and ruminal fermentation (Kholif et al., 2015). Other results showed that replacing alfalfa with air-dried M. oleifera leaves (25%) positively affected the milk yield of goats and improved the oxidative status of milk and serum (Babiker et al., 2017). The administration of an oral dose of M. oleifera leaf extract to lactating Nubian goats increased the milk production and composition as a result of improved feed intake (Kholif et al., 2019). Moreover, the replacement of a concentrate feed mixture with a mixture of M. oleifera (20 and 40 DM basis) improved the nutrient digestion, ruminal fermentation characteristics, milk yield, and milk composition, concentrations of milk fat and lactose, and feed efficiency compared to the control treatment in lactating goats (Kholif et al., 2022; Morsy et al., 2022) (Figure 4B). Other findings have shown that milk yield, weight gain (by kids), and reproductive performance were high in goats that were fed 2 and 3.5% M. oleifera leaf powder in comparison to the control group. The supplementation of M. oleifera leaf powder improved the plasma flavonoids (p < 0.05) and the total antioxidant capacity (TAC) by suppressing the malondialdehyde concentration and the total oxidant status values, which could be due to the synergistic effects of flavonoids, phenolics, selenium, and vitamin C present in M. oleifera leaves (Afzal et al., 2022). The positive effects of supplementation with M. oleifera on growth, milk performance, and reproduction (ovulation rate) in goats was due to the supply of high-quality energy and protein and greater palatability, digestion, and utilization of nutrients in these feed sources (Asaolu et al., 2012; Sultana et al., 2015a; Moyo et al., 2016; Damor et al., 2017; Kholif et al., 2018; Kholif et al., 2019; Mataveia et al., 2019). Furthermore, these positive effects could be linked to the beneficial effects on ruminal function (better rumen bypass characteristics) and digestibility resulting from the consumption of vital micronutrients in M. oleifera such as vitamins A, B, and C as well as amino acids (Kholif et al., 2015; Sayed-Ahmed et al., 2018; Ahmed and Shaarawy, 2019). Moreover, the bioactive compounds or secondary metabolites in Moringa might have enhanced the anthelmintic activity and improved the nutrient digestibility, which led to better growth performances in goats (Pedraza-Hernández et al., 2021; Kholif et al., 2022).
Contribution of M. oleifera to climate change mitigation
Climate change is primarily caused by greenhouse gas (GHG) emissions, resulting in warming of the atmosphere (Stocker et al., 2014), to which livestock farming, particularly ruminant meat and dairy, is a major contributor of global methane production and global warming (Palangi and Lackner, 2022). Global warming has led to increased land desertification, air and water pollution, and reduced biodiversity and agricultural productivity (Thornton and Gerber, 2010; Bellarby et al., 2013; Gerber et al., 2013). Climate change is expected to have severe consequences on small farms practicing animal production, which dominate the agriculture sector in developing countries (Verchot et al., 2005; Daba, 2016). Therefore, many adaptive measures are required, including agroforestry and improved feeding practices, to mitigate the climatic effects of animal husbandry. Agroforestry is an efficient and integrated land use system that creates and maximizes the use of feed crops, tree crops, and livestock on the same unit of land. The roles of agroforestry systems (trees and shrubs) are well known in the mitigation of and adaptation strategies for global climate change (Toppo and Raj, 2018; Horn et al., 2022). Trees and shrubs can modify a local climate by impacting the temperature, wind speed, humidity, water balance, and nutrient cycling. Trees and shrubs can further contribute to global carbon sequestration via carbon dioxide absorption from the atmosphere and the subsequent release of oxygen, with the type of tree planted having a significant influence on the environmental outcome (Betts et al., 2007; Jose, 2009; Smith et al., 2012). Improved feeding practices include the incorporation of agroforest plants as high-quality forages into animal diets (Thornton and Herrero, 2010a). An alternative is dietary modification to reduce methane production in cattle (Rendón-Huerta et al., 2018; Mangar et al., 2022). The forage intake of plants with high digestibility will generally reduce GHG emissions arising from rumen fermentation and stored manure (Hristov et al., 2013). Dietary manipulation offers dual benefits: the improvement of animal production and the reduction of GHG emissions (Haque, 2018). An early report stated that the utilization of deep-rooted plants can sequester more carbon than natural rangeland vegetation (Fisher et al., 1994). Moringa oleifera is a suitable plant in terms of climate protection because of its high adaptability and numerous nutritional, agricultural, medicinal, domestic, industrial, and environmental benefits (Ndubuaku et al., 2015; Daba, 2016). Moringa oleifera trees have deep roots that are well adapted to harsh environmental and soil conditions. They do not require much maintenance and can easily grow and yield high amount of biomass in different agro-ecological zones. Therefore, they are often considered as ideal and beneficial trees for agroforestry (Devkota and Bhusal, 2020; Horn et al., 2022). Desertification is a major problem caused by climate change, and the Moringa tree can play a role in combating this phenomenon. Moringa trees can grow quickly and efficiently in dry areas, and, due to their deep roots, can withstand prolonged drought as well as protecting the soil from erosion. Furthermore, M. oleifera trees could be used as a dual solution, providing an alternative source of food and feed, while also mitigating the impacts of climate change (Trigo et al., 2021). M. oleifera can sequester more atmospheric carbon dioxide through all its different parts (Gedefaw, 2015; Sahoo et al., 2020). This plant yields high biomass even during the dry season and acts as a good sink for carbon dioxide, thus reducing the level of carbon dioxide in the atmosphere, which is one of the main causes of ozone layer depletion and global warming (Ndubuaku et al., 2015; Daba, 2016; Sahoo et al., 2020). An early report showed that the capacity of the Moringa tree to absorb carbon dioxide was fifty times higher than that of the Japanese cedar tree and twenty times higher than that of general vegetation (Villafuerte and Villafurte-Abonal, 2009). In addition, an evaluation of the physiological parameters of three drought-tolerant tree species (Moringa oleifera, Colophospermum mopane, and Sclerocarya birrea), to determine their potential to mitigate climate change through carbon sequestration under semi-arid conditions, showed that M. oleifera was among the species with the highest capacity for carbon sequestration (Mabapa et al., 2018). The replacement of soybean meal with Moringa leaf meal decreased CH4 production in goats and steers (Elghandour et al., 2017). Other results asserted that the use of M. oleifera supplementation in the diet of dairy cows not only improved dairy production (milk yield) and quality of milk but also helped to regulate the microbial metabolic function and methane emissions (Dong et al., 2019). In in-vitro experiments, rumen emissions were significantly reduced when leaves or extracts of Moringa were used, through active modulation of the rumen microbiome (Sarkar et al., 2016; Akanmu and Hassen, 2018; Ebeid et al., 2020). Lins et al. (2019) examined the effects of increasing concentrations of raw, ground Moringa seeds on rumen fermentation and CH4 production, using the rumen stimulation technique (Rusitec). The results of this study indicated that methane production linearly decreased as the inclusion of Moringa seeds increased (0, 100, 200, and 400 g/kg concentrate dry matter). Increasing Moringa by 1 g/kg DM decreased CH4 emission by 6 g/kg gain and absorbed nitrogen loss by 0.069%, possibly due to improved energy efficiency (Sultana et al., 2021). Using M. oleifera extract as feed supplement reduced CH4 and CO2 production in goats (Pedraza-Hernández et al., 2019). Further studies provided evidence that M. oleifera can help to mitigate methane emissions in cattle (Soliva et al., 2005; Soltan et al., 2019; Mangar et al., 2022). Additionally, other results showed that feeding lambs Moringa oleifera root bark reduced the estimated CH4 produced per unit of body weight gain (Soltan et al., 2018). Supplementing diets with alternative protein sources from trees and shrubs increased the dry matter intake and improved microbial protein synthesis in the rumen and the efficiency of rumen fermentation, with a shift in fermentation towards propionate (Halmemies-Beauchet-Filleau et al., 2018). The Recent study by Kholif et al. (2023) showed that replacing a concentrate feed mixture with up to 30% M. oleifera silage positively impacted the ruminal fermentation, with an inhibition of CH4 production. The methane inhibitory effects of these foliage plants might be induced by secondary metabolites such as alkaloids, flavonoids, and tannins, which are capable of interacting with rumen microbes and influencing ruminal fermentation patterns, leading to inhibition of methanogen activity (Akanmu and Hassen, 2018; Dong et al., 2019; Akanmu et al., 2020; Ku-Vera et al., 2020). Extracts from leaves of diverse plants with increased flavonoids and tannins levels reduced CH4 emission and increased microbiota counts (Broudiscou et al., 2002). Tannin-rich forages can effectively reduce CH4 emission (Ramirez-Restrepo and Barry, 2005; Palangi et al., 2022), while higher concentrations of saponins could suppress CH4-producing microbes (Bodas et al., 2012). In addition, highly nutritious forage can reduce ruminant methane production since feed moves through the digestive system more rapidly (Knapp et al., 2014; Khusro et al., 2021).
Conclusion
In the tropics, leaves from forage trees and shrubs are good protein supplements for ruminants and have the potential to improve nutrient digestibility and reduce enteric methane emissions. Moringa oleifera is widespread in tropical and subtropical regions and possesses promising traits such as rapid growth, increased biomass, drought resistance, and minimal care requirements. Due to its high content of nutrients, especially proteins and bioactive compounds, it can be used as an alternative to conventional ruminant feed materials. Various results showed that using M. oleifera either individually or in combination with other feedstuff improved the production performance (growth rate, milk yield, and milk quality) in cattle, sheep, and goats. All parts of Moringa oleifera can sequester more atmospheric carbon dioxide, and, by feeding it to ruminants, can also reduce rumen methane emissions. This indicates that this plant can be used in agroforestry systems, which can supply animals with high value feed supplements and contribute to the adaptation and mitigation of climate change. Finally, future research should focus on the effects of different supplements of M. oleifera feed and extracts (leaves, seeds, flowers, roots, and bark) on ruminant performance and CH4 emission.
Author contributions
Both authors have made a substantial and direct contribution to this scientific article and approved it for publication.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Abbas R. K., Elsharbasy F. S., Fadlelmula A. A. (2018). Nutritional values of Moringa oleifera, total protein, amino acid, vitamins, minerals, carbohydrates, total fat and crude fiber, under the semi-arid conditions of Sudan. J. Microb. Biochem. Technol. 10 (2), 56–58. doi: 10.4172/1948-5948.1000396
Afzal A., Hussain T., Hameed A., Shahzad M., Mazhar M. U., Yang G. (2022). Dietary Moringa oleifera alters periparturient plasma and milk biochemical indicators and promotes productive performance in goats. Front. Vet. Sci. 8. doi: 10.3389/fvets.2021.787719
Ahmed M., Shaarawy A. (2019). Effect of feeding Moringa oleifera forage on productive performance of growing goat kids. Egypt. J. Sheep Goat Sci. 14 (1), 25–37. doi: 10.21608/ejsgs.2019.33232
Akande K. E., Fabiyi E. F. (2010). Effect of processing methods on some anti-nutritional factors in legume leeds for poultry peeding. Intern. J. Poult. Sci. 9 (10), 996–1001. doi: 10.3923/ijps.2010.996.1001
Akanmu A. M., Hassen A. (2018). The use of certain medicinal plant extracts reduced in vitro methane production while improving in vitro organic matter digestibility. Anim. Prod. Sci. 58, 900–908. doi: 10.1071/AN16291
Akanmu A. M., Hassen A., Adejoro F. A. (2020). Gas production, digestibility and efficacy of stored or fresh plant extracts to reduce methane production on different substrates. Anim 10, 146. doi: 10.3390/ani10010146
Allam S. M., Aboul-Fotouh G. E., El-Garhy G. M., Gamal O. (2015). Use of moringa leaves (Moringa oleifera) in fattening lambs rations. Egypt. J. Nutr. Feeds 18 (2 Special), 11–17. doi: 10.21608/ejnf.2015.104349
Amad A. A., Zentek J. (2022). Moringa (M. oleifera) leaf meal in diets for broilers and laying hens: a review. J. Agr. Sci. 14 (10), 12–33. doi: 10.5539/jas.v14n10p12
Anwar F., Bhanger M. (2003). Analytical characterization of Moringa oleifera seed oil grown in temperate regions of Pakistan. J. Agric. Food Chem. 51, 6558–6563. doi: 10.1021/jf0209894
Aregheore E. M. (2002). Intake and digestibility of Moringa oleifera-batiki grass mixtures by growing goats. Small Rum. Res. 46 (1), 23–28. doi: 10.1016/S0921-4488(02)00178-5
Asaolu V., Binuomote R., Akinlade J., Aderinola O., Oyelami O. (2012). Intake and growth performance of west African dwarf goats fed Moringa oleifera, gliricidia sepium and Leu-caena leucocephala dried leaves as supplements to cassava peels. J. Biol. Agric. Health Care 2, 76–88.
Auwal M. M., Yelwa J. M., Abubakar I., Umar J. B., Anchau H. G., Tanimu F. B. (2019). The levels of antinutritional factors in Moringa oleifera and vernomia amygdalina leaves found in some part of plateau state, Nigeria. Orient. J. Phys. Sci. 4 (2), 1–5. doi: 10.13005/OJPS04.02.06
Babiker E. E., Al Juhaimi F., Ghafoor K., Mohamed H. E., Abdoun K. A. (2016). Effect of partial replacement of alfalfa hay with moringa species leaves on milk yield and composition of najdi ewes. Trop. Anim. Health Prod. 48 (7), 1427–1433. doi: 10.1007/s11250-016-1111-9
Babiker E. E., Juhaimi F. A., Ghafoor K., Abdoun K. A. (2017). Comparative study on feeding value of moringa leaves as a partial replacement for alfalfa hay in ewes and goats. Livest. Sci. 195, 21–26. doi: 10.1016/j.livsci.2016.11.010
Balami A. G., Ndahi J. J., Gadzama J. J., Enam S. J., Chiroma M. A., Abdu P. A., et al. (2018). Effect of Moringa oleifera feed supplementation on the serum biochemical profile of broilers challenged with very virulent infectious bursal disease virus. J. Adv. Vet. Anim. Res. 5 (2), 155–165. doi: 10.5455/javar.2018.e260
Bashar M. K., Huque K. S., Sarker N. R., Sultana N. (2020). Quality assessment and feeding impact of moringa feed on intake, digestibility, enteric CH4 emission, rumen fermentation, and milk yield. J. Adv. Vet. Anim. Res. 7 (3), 521–529. doi: 10.5455/javar.2020.g449
Bellarby J., Tirado R., Leip A., Weiss F., Lesschen J. P., Smith P. (2013). Livestock greenhouse gas emissions and mitigation potential in Europe. Glob. Change Biol. 19, 3–18. doi: 10.1111/j.1365-2486.2012.02786.x
Ben Salem H., Makkar H. P. S. (2009). Defatted moringa oleifera seed meal as a feed additive for sheep. Anim. Feed Sci. Technol. 150 (1-2), 27–33. doi: 10.1016/j.anifeedsci.2008.07.007
Betts R., Boucher O., Collins M., Cox P. M., Falloon P. D., Gedney N., et al. (2007). Projected increase in continental runoff due to plant responses to increasing carbon dioxide. Nature 448, 1037–1041. doi: 10.1038/nature06045
Bhaskarachary K., Rao D. S. S., Deosthale Y. G., Reddy V. (1995). Carotene content of some common and less familiar foods of plant origin. Food Chemist. 54 (2), 189–193. doi: 10.1016/0308-8146(95)00029-I
Bhattacharya A., Tiwari P., Sahu P. K., Kumar S. (2018). A review of the phytochemical and pharmacological characteristics of Moringa oleifera. J. Pharm. Bioallied Sci. 10 (4), 181–191. doi: 10.4103/JPBS.JPBS_126_18
Bhokre S. M., Rajanna N., Ramana D. B. V., Nagalakshmi D., Kishan Kumar M., Sashi Kumar M. (2020). Effect of feeding of moringa (Moringa oleifera) leaf meal-based diets on the biometry and body condition score of deccani lambs. Int. J. Curr. Microbiol. App. Sci. 9 (4), 1089–1096. doi: 10.20546/ijcmas.2020.904.129
Bodas R., Prieto N., García-González R., Andrés S., Giráldez F. J., López S. (2012). Manipulation of rumen fermentation and methane production with plant secondary metabolites. Anim. Feed Sci. Technol. 176, 78–93. doi: 10.1016/j.anifeedsci.2012.07.010
Brar S., Haugh C., Robertson N., Owuor P. M., Waterman C., Fuchs G. J. III, et al. (2022). The impact of Moringa oleifera leaf supplementation on human and animal nutrition, growth, and milk production: a systematic review. Phytotherapy Res. 36 (4), 1600–1615. doi: 10.1002/ptr.7415
Broudiscou L. P., Papon Y., Broudiscou A. F. (2002). Effects of dry plant extracts on feed degradation and the production of rumen microbial biomass in a dual outflow fermenter. Anim. Feed Sci. Technol. 101, 183–189. doi: 10.1016/S0377-8401(02)00221-3
Cohen-Zinder M., Leibovich H., Vaknin Y., Sagi G., Shabtay A., Ben-Meir Y., et al. (2016). Effect of feeding lactating cows with ensiled mixture of Moringa oleifera, wheat hay and molasses, on digestibility and efficiency of milk production. Anim. Feed Sci. Technol. 211, 75–83. doi: 10.1016/j.anifeedsci.2015.11.002
Daba M. (2016). Miracle tree: a review on multi-purposes of Moringa oleifera and its implication for climate change mitigation. J. Earth. Sci. Clim. Change. 7, 366. doi: 10.4172/2157-7617.1000366
Damor S. V., Pawar M. M., Ankuya K. J., Gami Y. M., Srivastava A. K., Chauhan H. D., et al. (2017). Effect of feeding different levels of moringa (Moringa oleifera) leaves on growth performance of mehsana goat kids. Trends Biosci. 10, 3190–3193.
Debela E., Tolera A. (2013). Nutritive value of botanical fractions of Moringa oleifera and Moringa stenopetala grown in the mid-rift valley of southern Ethiopia. Agrofor. Syst. 87, 1147–1155. doi: 10.1007/s10457-013-9626-9
Delgado D. C., Galindo J., González R., González N., Scull I., Dihigo L., et al. (2012). Feeding of tropical trees and shrub foliages as a strategy to reduce ruminal methanogenesis: studies conducted in Cuba. Trop. Anim. Health Prod. 44 (5), 1097–1104. doi: 10.1007/s11250-011-0045-5
Devendra C., Leng R. A. (2011). Feed resources for animals in Asia: issues, strategies for use, intensification and integration for increased productivity. Asian-Aust. J. Anim. Sci. 24 (3), 303–321. doi: 10.5713/ajas.2011.r.05
Devkota S., Bhusal K. K. (2020). Moringa oleifera: a miracle multipurpose tree for agroforestry and climate change mitigation from the Himalayas–a review. Cogent Food Agric. 6 (1), 1–8. doi: 10.1080/23311932.2020.1805951
Dong L., Zhang T., Diao Q. (2019). Effect of dietary supplementation of Moringa oleifera on the production performance and fecal methanogenic community of lactating dairy cows. Anim 9 (5), 262. doi: 10.3390/ani9050262
Ebeid H. M., Mengwei L., Kholif A. E., Hassan F. U., Lijuan P., Xin L., et al. (2020). Moringa oleifera oil modulates rumen microflora to mediate in vitro fermentation kinetics and methanogenesis in total mix rations. Curr. micro 77, 1271–1282. doi: 10.1007/s00284-020-01935-2
El-Naggar S., Abou−ward G. A., Tawila M. A., Gad S. M., Ali A. M. (2017). Impact of incorporating moringa oleifera seed cake as protein source in growing lambs ration. Agric. Eng.Int. CIGR J., 289–292.
Elangovan M., Dhanarajan M. S., Elangovan I., Elangovan M. (2015). Determination of bioactive compounds from the petroleum ether leaf extract of Moringa oleifera and phyllanthus emblica using GC-MS analysis. World J. Pharm. Res. 4 (3), 1284–1298.
El-Anoos E. M., Fahmy H. A., Mahmoud E. A., Sharaf A. M. (2022). Effect of soaking process on chemical characteristics and anti-nutritional factors of some moringa seed varieties. J. Food Dairy Sci. 13 (1), 9–15. doi: 10.21608/jfds.2022.117048.1035
El-Esawy G. S., Reyad W. A. E. A., Ali M. F., Gaafar H. M. (2018). Effect of feeding Moringa oliefera stems on productive performance of lactating friesian cows. Egyp. J. Nutr. Feeds. 21 (3), 593–603. doi: 10.21608/ejnf.2018.63233
Elghandour M. M. Y., Vallejo L. H., Salem A. Z. M., Mellado M., Camacho L. M., Cipriano M., et al. (2017). Moringa oleifera leaf meal as an environmental friendly protein source for ruminants: biomethane and carbon dioxide production, and fermentation characteristics. J. Cleaner Prod. 165, 1229–1238. doi: 10.1016/j.jclepro.2017.07.151
EL-Hedainy D. K., El-Wakeel E., Rashad A. M. A. (2020). Effect of moringa seed meal as a feed additive on performance of fattening male barki sheep. Int. J. Vet. Sci. Res. 6 (2), 184–187. doi: 10.17352/ijvsr.000072
Fahey J. W. (2005). Moringa oleifera: a review of the medical evidence for its nutritional, therapeutic, and prophylactic properties. part 1. Trees Life J. 1 (5), 1–15.
Faizi S., Siddiqui B. S., Saleem R., Aftab K., Shaheen F., Gilani A. H. (1998). Hypotensive constituents from the pods of Moringa oleifera. Planta Med. 64, 225–228. doi: 10.1055/s-2006-957414
Fayis M. E. K. (2017). Comparative study of anti-nutritional factors in Moringa oleifera leaves and seed.
Fisher M. J., Rao I. M., Ayarza M. A., Lascano C. E., Sanz J. I., Thomas R. J., et al. (1994). Carbon storage by introduced deep-rooted grasses in the south American savannas. Nature 371 (6494), 236–238.
Gedefaw M. (2015). Environmental and medicinal value analysis of moringa (Moringa oleifera) tree species in sanja, north gondar, Ethiopia. Am. Int. J. Contemp. Sci. Res. 2 (9), 20–35.
Gerber P. J., Steinfeld H., Henderson B., Mottet A., Opio C., Dijkman J., et al. (2013). Tackling climate change through livestock: a global assessment of emissions and mitigation opportunities (Rome: FAO).
Godinez-Oviedo A., Guemes-Vera N., Acevedo-Sandoval O. A. (2016). Nutritional and phytochemical composition of Moringa oleifera lam and its potential use as nutraceutical plant: a review. Pakis. J. Nutr. 15 (4), 397.
Gopalakrishnan L., Doriya K., Kumar D. S. (2016). Moringa oleifera: a review on nutritive importance and its medicinal application. Food Sci. Hum. Well. 5, 49–56. doi: 10.1016/j.fshw.2016.04.001
Halmemies-Beauchet-Filleau A., Rinne M., Lamminen M., Mapato C., Ampapon T., Wanapat M., et al. (2018). Review: alternative and novel feeds for ruminants: nutritive value, product quality and environmental aspects. Anim 12(S2), 295–309. doi: 10.1017/S1751731118002252
Haque M. (2018). Dietary manipulation: a sustainable way to mitigate methane emissions from ruminants. J. Anim. Sci. Technol. 60, 15. doi: 10.1186/s40781-018-0175-7
Horn L., Shakela N., Mutorwa M. K., Naomab E., Kwaambwa H. M. (2022). Moringa oleifera as a sustainable climate-smart solution to nutrition, disease prevention, and water treatment challenges: a review. J. Agric. Food Res. 47, 100397. doi: 10.1016/j.jafr.2022.100397
Hristov A. N., Oh J., Lee C., Meinen R., Montes F., Ott T., et al. (2013). “Nutritional and management strategies to mitigate animal greenhouse gas emissions,” in Proceedings 2013; 24th. Ann Florida Ruminant Nutrition Symp, University of Florida. 90–98. doi: 10.4172/2167-0412.1000101
Igwilo I. O., Ezeonu F. C., Ezekwesili-Ofili J. O., Igwilo S. N., Nsofor C. I., Abdulsalami M. S., et al. (2014). Anti-nutritional factors in roots of a local cultivar of Moringa oleifera (Lam). Pak. J. Biol. Sci. 17, 114–117. doi: 10.3923/pjbs.2014.114.117
Igwilo I. O., Ugochukwu G. C., Ezekwesili C. N., Nwenyi V. (2017). Comparative studies on the nutrient composition and antinutritional factors in different parts of Moringa oleifera plant found in awka, Nigeria. Bioscientist 5 (1), 1–12. doi: 10.3923/pjbs.2014.114.117
Jayanegara A., Togtokhbayar N., Makkar H. P. S., Becker K. (2009). Tannins determined by various methods as predictors of methane production reduction potential of plants by an in vitro rumen fermentation system. Anim. Feed Sci. Tech. 150, 230–237. doi: 10.1016/j.anifeedsci.2008.10.011
Jose S. (2009). Agroforestry for ecosystem services and environmental benefits: an overview. Agrofor. Syst. 76, 1–10. doi: 10.1007/s10457-009-9229-7
Joshi P., Mehta D. (2010). Effect of dehydration on the nutritive value of drumstick leaves. J. Metabolo. Sys. Bio. 1 (1), 5–9.
Kabatange M. A., Shayo C. M. (1991). Rumen degradation of maize stover as influenced by leucaena hay supplementation. Livest. Res. Rural Devel. 3, 2.
Kakengi A. M. V., Shem M. N., Sarwatt S. V., Fujihara T. (2005). Can Moringa oleifera be used as a protein supplement for ruminants? Asian-Aus. J. Anim. Sci. 18 (1), 42–47. doi: 10.5713/ajas.2005.42
Kamra D. N., PatraA A. K., Chatterjee P. N., Kumar R., Agarwal N. and Chaudhary L. C. (2008). Effect of plant extracts on methanogenesis and microbial profile of the rumen of buffalo: a brief overview. Aust. J. Exp. Agric. 48 (2), 175–178. doi: 10.1071/EA07268
Kekana T. W., Marume U., Muya C. M., Nherera-Chokuda F. V. (2019). Lactation performance and blood metabolites in lactating dairy cows micro-supplemented with Moringa oleifera leaf meal. S. Afr. J. Anim. Sci. 49, 709–716. doi: 10.4314/sajas.v49i4.12
Kekana T. W., Marume U., Nherera-Chokuda F. V. (2022). Prepartum supplementation of Moringa oleifera leaf meal: effects on health of the dam, colostrum quality, and acquisition of immunity in the calf. J. Dairy Sci. 105, 5813–5821. doi: 10.3168/jds.2021-21535
Khalel M. S., Shwerab A. M., Hassan A. A., Yacout M. H., El-Badawi A. Y., Zaki M. S. (2014). Nutritional evaluation of Moringa oleifera fodder in comparison with Trifolium alexandrinum (berseem) and impact of feeding on lactation performance of cows. Life Sci. J. 11(10), 1040–1054.
Kholif A. E., Gouda G. A., Abu Elella A. A., Patra A. K. (2022). Replacing the concentrate feed mixture with Moringa oleifera leaves silage and chlorella vulgaris microalgae mixture in diets of damascus goats: lactation performance, nutrient utilization, and ruminal fermentation. Animals 12, 1589. doi: 10.3390/ani12121589
Kholif A. E., Gouda G. A., Galyean M. L., Anele U. Y., Morsy T. A. (2019). Extract of Moringa oleifera leaves increases milk production and enhances milk fatty acid profile of Nubian goats. Agroforest. Syst. 93, 1877–1886. doi: 10.1007/s10457-018-0292-9
Kholif A. E., Gouda G. A., Morsy T. A., Matloup O. H., Sallam S. M., Patra A. K. (2023). Associative effects between Chlorella vulgaris microalgae and Moringa oleifera leaf silage used at different levels decreased in vitro ruminal greenhouse gas production and altered ruminal fermentation. Environ. Sci. pollut. Res. 30 (3), 6001–6020. doi: 10.1007/s11356-022-22559-y
Kholif A. E., Gouda G. A., Morsy T. A., Salem A. Z. M., Lopez S., Kholif A. M. (2015). Moringa oleifera leaf meal as a protein source in lactating goat’s diets: feed intake, digestibility, ruminal fermentation, milk yield and composition, and its fatty acids profile. S. Rum. Res. 129, 129–137. doi: 10.1016/j.smallrumres.2015.05.007
Kholif A. E., Gouda G. A., Olafadehan O. A., Abdo M. M. (2018). Effects of replacement of Moringa oleifera for berseem clover in the diets of Nubian goats on feed utilization, and milk yield, composition and fatty acid profile. Animal 12, 964–972. doi: 10.1017/S1751731117002336
Kholif A. E., Morsy T. A., Gouda G. A., Anele U. Y., Galyean M. L. (2016). Effect of feeding diets with processed Moringa oleifera meal as protein source in lactating Anglo-Nubian goats. Anim. Feed Sci. Technol. 217, 45–55. doi: 10.1016/j.anifeedsci.2016.04.012
Khusro A., Aarti C., Elghandour M. M., Adegbeye M. J., Mellado M., Barbabosa-Pliego A., et al. (2021). “Dietary manipulation to mitigate greenhouse gas emission from livestock,” in Handbook of climate change mitigation and adaptation (Cham: Springer International Publishing), 2537–2575. doi: 10.1007/978-1-4614-6431-0_131-2
Knapp J. R., Laur G. L., Vadas P. A., Weiss W. P., Tricarico J. M. (2014). Invited review: enteric methane in dairy cattle production: quantifying the opportunities and impact of reducing emissions. J. Dairy Sci. 97, 3231–3261. doi: 10.3168/jds.2013-234
Koul B., Chase N. (2015). Moringa oleifera lam.: panacea to several maladies. J. Chem. Pharm. Res. 7 (6), 687–707.
Ku-Vera J. C., Jiménez-Ocampo R., Valencia-Salazar S. S., Montoya- Flores M. D., Molina-Botero I. C., Arango J., et al. (2020). Role of secondary plant metabolites on enteric methane mitigation in ruminants. Front. Vet. Sci. 7. doi: 10.3389/fvets.2020.00584
Leone A., Spada A., Battezzati A., Schiraldi A., Aristil J., Bertoli S. (2015). Cultivation, genetic, ethnopharmacology, phytochemistry and pharmacology of Moringa oleifera leaves: an overview. Intern. J. Mol. Sci. 16 (6), 12791. doi: 10.3390/IJMS160612791
León-López L., Escobar-Zúñiga Y., Milán-Carrillo J., Domínguez-Arispuro D. M., Gutiérrez-Dorado R., Cuevas-Rodríguez E. O. (2020). Chemical proximate composition, antinutritional factors content, and antioxidant capacity of anatomical seed fractions of Moringa oleifera. Acta Universitaria. 30, e2892. doi: 10.15174/au.2020.2892
Lins T. D. A., Terry S. A., Silva R. R., Pereira L. G. R., Jancewicz L. J., He M. L., et al. (2019). Effects of the inclusion of Moringa oleifera seed on rumen fermentation and methane production in a beef cattle diet using the rumen simulation technique (Rusitec). Animal 13 (2), 283–291. doi: 10.1017/S1751731118001428
Mabapa P. M., Ayisi K. K., Mariga I. K. (2018). Comparison of gas exchange in Moringa oleifera and other drought tolerant tree species for climate change mitigation under semi-arid condition of northern south Africa. Int. J. Agric. Biol. 20, 2669–2676. doi: 10.17957/IJAB/15.0808
Mahmoud A. E. M. (2013). Effect of feeding on Moringa oleifera stems on productive performance of growing lambs. Egyp. J. Nutt. feed 16 (2), 281–292.
Makkar H. P. S., Becker K. (1996). Nutritional value and antinutritional components of whole and ethanol extract of Moringa oleifera leaves. Anim. Feed Sci. Technol. 63 (1-4), 211–228. doi: 10.1016/S0377-8401(96)01023-1
Makkar H. P. S., Becker K. (1997). Nutrients and antiquality factors in different morphological parts of the Moringa oleifera tree. J. Agric. Sci. 128 (3), 311–322. doi: 10.1017/S0021859697004292
Malhotra S. P. K., Mandal T. K. (2018). Phytochemical screening and in vitro antibacterial activity of Moringa oleifera (Lam.) leaf extract. Arch. Agric. Env. Sci. 3 (4), 367–372. doi: 10.26832/24566632.2018.030406
Malik A., Gunawan A., Erlina S., Widaningsih R. E. (2019). Effect of Moringa oleifera (Moringa) supplementation via urea molasses multi-nutrient moringa block (um3b) on nutrient intake and utilization in bali cattle. J. Anim. Heal. Prod. 7 (2), 70–74. doi: 10.17582/journal.jahp/2019/7.2.70.74
Mangar A., Muetzel S., Malik A., Bhuker A., Mor V., Molenaar A., et al. (2022). Moringa oleifera l. a potential plant for greenhouse gas mitigation in temperate agriculture systems. Agri 12, 1116. doi: 10.3390/agriculture12081116
Maqsood M., Qureshi R., Arshad M., Ahmed M. S., Ikram M. (2017). Preliminary phytochemical screening, antifungal and cytotoxic activities of leaves extract of Moringa oleifera lam. from salt range, Pakistan. Pak. J. Bot. 49 (1), 353–359.
Mataveia G., Garrine C. M. L. P., Pondja A., Hassen A., Visser C. (2019). Impact of supplementation of Moringa oleifera and Leucaena leucacephala tree fodder on the production performance of indigenous goats in Mozambique. Black Sea J. Agric. 2 (2), 93–102.
Meireles D., Gomes J., Lopes L., Hinzmann M., Machado J. (2020). A review of properties, nutritional and pharmaceutical applications of Moringa oleifera: integrative approach on conventional and traditional Asian medicine. Adv. Tradit. Med. 1 (21), 495–515. doi: 10.1007/s13596-020-00468-0
Mendieta-Araica B., Spörndly E., Reyes-Sánchez N., Spörndly R. (2011). Feeding Moringa oleifera fresh or ensiled to dairy cows–effects on milk yield and milk flavor. Trop. Anim. Health Prod. 43 (5), 1039–1047. doi: 10.1007/s11250-011-9803-7
Mohanty M., Mohanty S., Bhuyan K. S., Bhuyan R. (2020). Phytoperspective of Moringa oleifera for oral health care: an innovative ethnomedicinal approach. Phytother. Res. 35 (03), 1345–1357. doi: 10.1002/ptr.6896
Morsy T. A., Hadhoud F. I., Kholif A. E., Abu Elella A. A., Olafadehan O. A. (2022). Potential of moringa oleifera silage to replace concentrate feed mixture in diet of lactating Damascus goats”. Ann. Anim. Sci. 22 (4), 1373–1383. doi: 10.2478/aoas-2022-0058
Moyo B., Masika P. J., Muchenje V. (2016). Potential use of Moringa oleifera leaf in animal feeding: a review. Int. J. Curr. Agric. Res. 4, 9187–9194.
Moyo B., Patrick J. M., Muchenje V. (2011). Effect of supplementing crossbred Xhosa lop-eared goat castrates with Moringa oleifera leaves on growth performance, carcass and non-carcass characteristics. Trop. Anim. Health Prod. 44, 801–809. doi: 10.1007/s11250-011-9970-6
Mulyaningsih T. R., Yusuf S. (2018). Determination of minerals content in leaves of Moringa oleifera by neutron activation analysis. Ganendra J. Nucl. Sci. Technol. 21 (1), 11–16. doi: 10.17146/gnd.2018.21.1.3683
Murro J. K., Muhikambele V. R. M., Sarwatt S. V. (2003). Moringa oleifera leaf meal can replace cottonseed cake in the concentrate mix fed with rhodes grass (Chloris gayana) hay for growing sheep. Livest. Res. Rural Dev. 15 (11).
Ndubuaku U. M., Uchenna N. V., Baiyeri K. P., Ukonze J. (2015). Anti-nutrient, vitamin and other phytochemical compositions of old and succulent moringa (Moringa oleifera lam) leaves as influenced by poultry manure application. Afr. J. Biotech. 14 (32), 2502–2509. doi: 10.5897/AJB2015.14848
Noonan S., Savage G. (1999). Oxalate content of food and its effect on humans. Asia Pac. J. Clin. Nutri. 8, 64–74. doi: 10.1046/j.1440-6047.1999.00038.x
Ogbe A. O., Affiku J. P. (2011). Proximate study, mineral and anti-nutrient composition of Moringa oleifera harvested from lafia, Nigeria: potential benefits for poultry nutrition and health. J. Microbiol. Biotechnol. Food Sci. 1 (3), 296–308.
Olagbemide P. T., Philip C. N. A. (2014). Proximate analysis and chemical composition of raw and defatted Moringa oleifera kernel. Adv. Life Sci. Technol. 24, 92–99.
Oluwaniyi O. O., Obi B. C., Awolola G. V. (2020). Nutritional composition and antioxidant capacity of Moringa oleifera seeds, stem bark and leaves. Ilorin J. Sci. 7 (1), 53–65. doi: 10.54908/iljs.2020.07.01.004
Olvera-Aguirre G., Mendoza-Taco M. M., Arcos-Álvarez D. N., Piñeiro-Vázquez A. T., Moo-Huchin V. M., Canul-Solís J. R., et al. (2020). Effect of feeding lactating ewes with Moringa oleifera leaf extract on milk yield, milk composition and preweaning performance of ewe/lamb pair. Anim. (Basel 10 (7), 1117. doi: 10.3390/ani10071117
Palangi V., Lackner M. (2022). Management of enteric methane emissions in ruminants using feed additives: a review. Animals 12 (24), 3452. doi: 10.3390/ani12243452
Palangi V., Taghizadeh A., Abachi S., Lackner M. (2022). Strategies to mitigate enteric methane emissions in ruminants: a review. Sustainability 14, 13229. doi: 10.3390/su142013229
Paliwal R., Sharma V., Pracheta J. (2011). A review on horse radish tree (Moringa oleifera): a multipurpose tree with high economic and commercial importance. Asian J. Biotechnol. 3 (4), 317–328. doi: 10.3923/ajbkr.2011.317.328
Pandey A. (2012). Moringa oleifera lam. (Sahijan)- a plant with a plethora of diverse therapeutic benefits: an updated retrospection. Med. Aroma. Plants. 01 (01), 1–8. doi: 10.4172/map.1000101
Pandey A., Modi R. J., Lunagariya P. M., Islam M. (2022). Effect of feeding Moringa oleifera meal on growth performance of growing surti kids under intensive system of management. Ind. J. Vet. Sci. Biotech. 18 (1), 72–75. doi: 10.21887/ijvsbt.18.1.14
Pedraza-Hernández J., Elghandour M. M., Khusro A., Camacho-Diaz L. M., Vallejo L. H., Barbabosa-Pliego A., et al. (2019). Mitigation of ruminal biogases production from goats using Moringa oleifera extract and live yeast culture for a cleaner agriculture environment. J. Clean. Prod. 234, 779–786. doi: 10.1016/j.jclepro.2019.06.126
Pedraza-Hernández J., Elghandour M. M. M. Y., Khusro A., Salem M. Z. M., Camacho-Diaz L. M., Barbabosa-Pliego A., et al. (2021). Assessment on bioactive role of Moringa oleifera leaves as anthelmintic agent and improved growth performance in goats. Trop. Anim. Health Prod. 53 (2), 318. doi: 10.1007/s11250-021-02745-9
Pramanik A., Islam S. S. (1998). Chemical investigation of aqueous extract of the mature and premature flowers of Moringa oleifera (Sajina) and structural studies of a polysaccharide isolated from its premature flowers. Indian J. Chem. 37B, 676–682.
Radek M., Savage G. P. (2008). Oxalates in some Indian green leafy vegetables. Int. J. Food Sci. Nutr. 59, 246–260. doi: 10.1080/09637480701791176
Ramirez-Restrepo C. A., Barry T. N. (2005). Alternative temperate forages containing secondary compounds for improving sustainable productivity in grazing ruminants. Anim. Feed Sci. Technol. 120, 179–201. doi: 10.1016/j.anifeedsci.2005.01.015
Rendón-Huerta J. A., Pinos-Rodríguez J. M., Kebreab E. (2018). Animal nutrition strategies to reduce greenhouse gas emissions in dairy cattle. Acta Universitaria. 28 (5), 34–41. doi: 10.15174/au.2018.1766
Rizwan N., Rizwan D., Banday M. T. (2022). Moringa oleifera: the miracle tree and its potential as non- conventional animal feed: a review. Agric. Rev. 1–11. doi: 10.18805/ag.R-2405
Sahoo J. P., Mohapatra U., Sahoo S., Samal K. C. (2020). Insights into the miracle plant Moringa oleifera. Pharma Inn. J. 9 (7), 473–479. doi: 10.22271/tpi.2020.v9.i7h.4978
Sanchez N. R., Sporndlyn E., Ledin I. (2006). Effect of feeding different levels of foliage of Moringa oleifera to creole dairy cows on intake, digestibility, milk production and composition. Livest. Sci. 101, 24–31. doi: 10.1016/j.livprodsci.2005.09.010
Sarkar S., Mohini M., Nampoothiri V. M., Mondal G., Pandita S., Mahesh M. S. E. (2016). “Effect of supplementation of Moringa oleifera leaves on in vitro methane emissions and rumen fermentation on roughage-based ration,” in Proceedings of the XVI Biennial Animal Nutrition Conference on Innovative Approaches for Animal Feeding and Nutritional Research, Karnal, India, 6–8 February.
Sarwatt S., Kapange S., Kakengi A. (2002). Substituting sunflower seed-cake with Moringa oleifera leaves as a supplemental goat feed in Tanzania. Agrofor. Sys. 56, 241–247. doi: 10.1023/A:1021396629613
Sarwatt S., Milang’ha M., Lekule F., Madalla N. (2004). Moringa oleifera and cottonseed cake as supplements for smallholder dairy cows fed Napier grass. Livest. Res. Rural Dev. 16, 12–18.
Sayed-Ahmed M. E., Farag M. E., El-Sayed F. A., Shaarawy A. M. (2018). Evaluation of using Moringa oleifera plant in goat rations and its impact on productive and reproductive performance. Egypt. J. Sheep Goat Sci. 13 (3), 1–21.
Shaani Y., Eliyahu D., Mizrahi I., Yosef E., Ben-Meir Y., Nikbachat M., et al. (2016). Effect of feeding ensiled mixture of pomegranate pulp and drier feeds on digestibility and milk performance in dairy cows. J. Dairy Res. 1, 35–41. doi: 10.1017/S0022029915000618
Shankhpal S., Waghela C., Sherasia P., Sridhar V., Srivastava A. K., Singh D. (2019). Effect of feeding moringa (Moringa oleifera) as green fodder on feed intake, milk yield, microbial protein synthesis and blood profile in crossbred cows. Ind. J. Anim. Nutr. 36 (3), 228–234. doi: 10.5958/2231-6744.2019.00038.0
Sherasiya A. N., Lunagariya P. M., Modi. R. J., Patel J. H., Chaudhary M. M., Wadhwani K. N. (2022). Effect of incorporation of Moringa oleifera meal in feed on growth performance of crossbred heifers. Ind. J. Vet. Sci. Biotech. 18 (2), 21–25. doi: 10.21887/ijvsbt.18.2.4
Singh L., Singh J., Singh J. (2019). Medicinal and nutritional values of drumstick tree (moringa oleifera): a review. Int. J. Curr. Micro. Appl. Sci. 8 (5), 1965–1974. doi: 10.20546/ijcmas.2019.805.228
Smith O. B. (1994). “Using fodder from trees and shrubs to feed livestock in the tropics,” in FAO better farming series. (Rome: FAO), vol. 42.
Smith J. P., Pearce B. D., Wolfe M. S. (2012). Reconciling productivity with protection of the environment: is temperate agroforestry the answer? Renewable Agric. Food Syst. 28 (1), 80–92.
Sodamade A., Owonikoko A., Owoyemi D. (2017). Nutrient contents and mineral composition of Moringa oleifera seed. Int. J. Commun. Sys. 5 (2), 205–207.
Soliva C., Kreuzer M., Foidl N., Foidl G., Machmüller A., Hess H. (2005). Feeding value of whole and extracted Moringa oleifera leaves for ruminants and their effects on ruminal fermentation in vitro. Anim. Feed. Sci. Technol. 118, 47–62. doi: 10.1016/j.anifeedsci.2004.10.005
Soltan Y. A., Hashem N. M., Morsy A. S., El-Azrak K. M., El-Din A. N., Sallam S. M. (2018). Comparative effects of moringa oleifera root bark and monensin supplementations on ruminal fermentation, nutrient digestibility and growth performance of growing lambs. Anim. Feed Sci. Technol. 235, 189–201. doi: 10.1016/j.anifeedsci.2017.11.021
Soltan Y. A., Morsy A., Hashem N. M., Sallam S. (2019). Impact of supplementary Moringa oleifera leaf extract on ruminal nutrient degradation and mitigating methane formation in vitro. Egypt. J. Nutr. Feed. 22, 55–62. doi: 10.21608/ejnf.2019.75840
Sonkar N., Singh N., Santra A. K., Mishra S., Prakash L., Soni A. (2020). Application of moringa (Moringa oleifera) in livestock feed: a review. Int. J. Chem. St. 8 (1), 1729–1735. doi: 10.22271/chemi.2020.v8.i1y.8513
Stadtlander T., Becker K. (2017). Proximate composition, amino and fatty acid profiles and element compositions of four different moringa species. J. Agric. Sci. 9, 46. doi: 10.5539/jas.v9n7p46
Stevens C. G., Ugese F. D., Otitoju G. T., Baiyeri K. P. (2015). Proximate and anti-nutritional composition of leaves and seeds of Moringa oleifera in Nigeria: a comparative study. Agro-Sci 14 (2), 9–17. doi: 10.4314/as.v14i2.2
Stocker T. F., Qin D., Plattner G. K., Tignor M. M., Allen S. K., Boschung J., et al. (2014). Climate change 2013: the physical science basis. contribution of working group I to the fifth assessment report of IPCC the intergovernmental panel on climate change.
Su B., Chen X. (2020). Current status and potential of Moringa oleifera leaf as an alternative protein source for animal feeds. Front. Vet. Sci. 7 (53). doi: 10.3389/fvets.2020.00053
Sultana N., Alimon A., Huque K., Baba M., Hossain J. (2015b). Evaluation of moringa foliage (Moringa oleifera) as goat feed. Iran. J. Appl. Anim. Sci. 4), 865–871.
Sultana N., Alimon A. R., Huque K. S., Sazili A. Q., Yaakub H., Hossain J., et al. (2015a). The feeding value of moringa (Moringa oleifera) foliage as replacement to conventional concentrate diet in Bengal goats. Adv. Anim. Vet. Sci. 3 (3), 164–173. doi: 10.14737/journal.aavs/2015/3.3.164.173
Sultana N., Das N. G., Kabir M. A., Deb G. K., Islam M. T. (2021). Metabolic benefit of bulls being fed moringa leaves twigs and branches as a major concentrate ingredient. Front. Anim. Sci. 2. doi: 10.3389/fanim.2021.712919
Sultana N., Rakib M. R. H., Hossain S. M. J., Ahmed S., Ershaduzamman M., Talukder M. A. I. (2017). Effect of replacement of conventional concentrate in a rice straw diet by moringa foliage on lamb production performances. J. Exp. Agric. Int. 5, 31329. doi: 10.9734/JEAI/2017/31329
Teixeira E. M. B., Carvalho M. R. B., Neves V. A., Silva M. A., Arantes-Pereira L. (2014). Chemical characteristics and fractionation of proteins from Moringa oleifera lam. leaves. Food Chem. 147, 51–54. doi: 10.1016/j.foodchem.2013.09.135
Thornton P. K., Boone R. B., Ramirez-Villegas J. (2015) Climate change impacts on livestock (Copenhagen, Denmark: CGIAR Research Program on Climate Change, Agriculture and Food Security (CCAFS). Available at: http://cgspace.cgiar.org/rest/bitstreams/54910/retrieve (Accessed 27 September 2022).
Thornton P. K., Gerber P. J. (2010). Climate change and the growth of the livestock sector in developing countries. Mitig. Adapt. Strateg. Glob. Change. 15, 169–184. doi: 10.1007/s11027-009-9210-9
Thornton P. K., Herrero M. (2010a). Potential for reduced methane and carbon dioxide emissions from livestock and pasture management in the tropics. Proc. Natl. Acad. Sci. U.S.A 107 (46), 19667–19672. doi: 10.1073/pnas.0912890107
Tona G., Ogunbosoye O. D. O., Bakare B. A. (2014). Growth performance and nutrient digestibility of West African dwarf goats fed graded levels of concentrate diet containing Moringa oleifera leaf meal. Int. J. Curr. Microbiol. Appl. Sci. 3 (8), 99–106.
Toppo P., Raj A. (2018). Role of agroforestry in climate change mitigation. J. Pharmcong. Phytochem. 7 (2), 241–243.
Trigo C., Castelló M. L., Ortolá M. D., García-Mares F. J., Soriano D. M. (2021). Moringa oleifera: an unknown crop in developed countries with great potential for industry and adapted to climate change. Foods 10, 31. doi: 10.3390/foods10010031
Verchot L. V., Mackensen J., Kandji S., van Noordwijk M., Tomich T., Ong C., et al. (2005). “Opportunities for linking adaptation and mitigation in agroforestry systems,” in Tropical forests and adaptation to climate change in search of synergies . (Jakarta, Indonesia: Center for International Forestry Research), 103–121.
Verma A. R., Vijayakumar M., Mathela C. S., Rao C. V. (2009). In vitro and in vivo antioxidant properties of different fractions of Moringa oleifera leaves. Food Chem. Toxic. 47, 2110–2196. doi: 10.1016/j.fct.2009.06.005
Villafuerte L. R., Villafurte-Abonal L. (2009). Data taken from the forestry agency of japan in moringa; malunggay phillippines (Singapore: apples of gold publishing), 240.
Wankhede S. D., Dutta N., Tambe M. B., Kaur N., Jadhav S. E., Pattanaik A. K. (2022). Effect of dietary inclusion of Moringa oleifera foliage on nutrient metabolism, metabolic profile, immunity and growth performance of goat kids. Emer. Anim. Spec. 3, 100005. doi: 10.1016/j.eas.2022.100005
Williams L. L. (2013). Moringa oleifera: could this be an answer to our need for an alternative to fighting drug-resistance and chronic infections? Med. Aroma. Plants. 02 (01), 1–3. doi: 10.4172/2167-0412.1000E142
Zaher H. A., Alawaash S. A., Tolba A. M., Swelum A. A., Abd El-Hack M. E., Taha A. E., et al. (2020). Impacts of moringa oleifera foliage substituted for concentrate feed on growth, nutrient digestibility, hematological attributes, and blood minerals of growing goats under Abu Dhabi conditions. Sustainability 12, 6096. doi: 10.3390/su12156096
Zarkadas C. G., Yu Z., Burrows V. D. (1995). Protein quality of three new Canadian-developed naked oat cultivars using amino acid compositional data. J. Agric. Food Chem. 43, 415–421. doi: 10.1021/jf00050a030
Keywords: ruminants, performance, agroforestry, climate change, methane emission, carbon dioxide mitigation
Citation: Amad AA and Zentek J (2023) The use of Moringa oleifera in ruminant feeding and its contribution to climate change mitigation. Front. Anim. Sci. 4:1137562. doi: 10.3389/fanim.2023.1137562
Received: 04 January 2023; Accepted: 25 April 2023;
Published: 26 May 2023.
Edited by:
Demin Cai, Yangzhou University, ChinaReviewed by:
Valiollah Palangi, Ege University, TürkiyeAhmed E. Kholif, National Research Centre, Egypt
Copyright © 2023 Amad and Zentek. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jürgen Zentek, anVlcmdlbi56ZW50ZWtAZnUtYmVybGluLmRl
†These authors have contributed equally to this work
 Abdulkarim Abdulmageed Amad
Abdulkarim Abdulmageed Amad Jürgen Zentek
Jürgen Zentek