
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Anim. Sci., 15 March 2023
Sec. Animal Physiology and Management
Volume 4 - 2023 | https://doi.org/10.3389/fanim.2023.1050170
This article is part of the Research TopicAnimal Production in Agroecological ApproachView all 3 articles
 Stella Dokou1
Stella Dokou1 Ifigeneia Mellidou2
Ifigeneia Mellidou2 Soumela Savvidou3
Soumela Savvidou3 Ioanna Stylianaki4
Ioanna Stylianaki4 Nikolas Panteli5
Nikolas Panteli5 Efthimia Antonopoulou5
Efthimia Antonopoulou5 Jing Wang6
Jing Wang6 Katerina Grigoriadou2
Katerina Grigoriadou2 Athina Tzora7
Athina Tzora7 Lizhi Jin8
Lizhi Jin8 Ioannis A. Skoufos7
Ioannis A. Skoufos7 Ilias Giannenas1*
Ilias Giannenas1*The present trial examined the effects of diet supplementation with an extract including Greek oregano, garlic, rock samphire, and camelina, administered either in aqueous form or encapsulated in cyclodextrin, on broiler chickens. The duration of the trial was 35 days. Mixed broiler chicks (Ross-308, 120 individuals, 1 day old) were randomly allocated to one of three groups, each with four replicates. Control group A (CONTROL) was fed a basal diet consisting of maize and soybean. The diet of the AQORGCC and CDORGCC groups was further supplemented with aqueous and cyclodextrin-encapsulated herbal extracts, respectively. Levels of lipid and protein oxidation were determined in breast and thigh meat samples. Furthermore, to address cellular stress and signaling responses, the expression patterns of heat shock proteins (Hsp60, Hsp70, and Hsp90), mitogen-activated protein kinases (P38 and P44/42 MAPKs), and apoptotic-related proteins (Bcl-2/Bad ratio) were investigated in breast and thigh tissues using Western blot analysis. The intestinal morphometry of the duodenum, jejunum, and ileum was also assessed. To investigate ileal and cecal bacterial community diversity, 16S rRNA gene high-throughput amplicon sequencing on the V3–V4 hypervariable region was performed. The results showed that the herbal extract in cyclodextrin delayed meat lipid oxidation. According to the protein expression patterns, the formulated diets elicited tissue-specific cellular responses. Compared with the CONTROL group, dietary supplementation with the encapsulated form resulted in significant Hsp induction and MAPK activation, whereas, in the group whose diet was supplemented with the aqueous form, the expression of most of the examined proteins decreased or was maintained at a constant level. Villus height and lamina propria width were mostly affected by the aqueous herbal extract, whereas the number of goblet cells remained unchanged among the groups. Firmicutes, Proteobacteria, and Bacteroidota were the major phyla in mean relative abundance in all diets in both cecal and ileal samples. Alpha-diversity indices highlighted higher species richness and diversity in the cecum than in the ileum, as well as in chicks treated with the aqueous extract of the herbal mixture, but only in the cecum. Cecal beta-diversity differed between the cyclodextrin and the CONTROL groups, while ileal beta-diversity varied only between the aqueous-treated group and the CONTROL group. In conclusion, the dietary mixtures of herbal extracts (particularly those encapsulated in cyclodextrin) improved protein and lipid oxidation and increased the number of beneficial lactic acid-producing bacteria in the cecum, whereas the aqueous herbal extract mostly affected bacterial activity in the proximal part of the chicken intestine. Similarly, intestinal morphometry in the duodenum, jejunum, and ileum was mostly affected by the aqueous herbal extract, which seems to inhibit proteins associated with stress signaling in meat.
In the last few decades, aromatic and medicinal plants have been exploited in the last few decades as an efficient alternative or ecofriendly and cost-effective solution offering support to the efforts made to reduce the use of in-feed antimicrobial drugs (Wang et al., 2022). Because of the increased interest in the incorporation of phytobiotics in broiler chickens’ diets, to date both the scientific community and the production industry have explored different herbal feed additives and their mixtures, aiming to improve animal health, and thus performance parameters. Brenes and Roura (2010) suggested that the interactions of herbal mixtures need to be examined because of the complexity resulting from the vast number and variability of their bioactive compounds. It has also been suggested that herbal mixtures should be investigated in depth to identify possible special synergistic effects on chicken performance, which until now have not received much attention (Giannenas et al., 2018). Herbal extracts comprise plants, essential oils, plant extracts, and the by-products of herbal or crop processing. The use of herbal extracts in the feed industry has increased in recent years, as their biologically active compounds (polyphenols and essential oils) exert antimicrobial and antioxidant effects in food-producing animals (Anastasiou et al., 2019; Christaki et al., 2020; Mandalakis et al., 2021).
Previous studies on the antibacterial effects of plant essential oils, including oregano essential oil, suggest that hydrophobic bioactive compounds cause cellular pH disturbance and damage to the cell membrane, increase cell permeability, affect ATP production and protein synthesis, induce cytoplasmic changes, and interfere with quorum sensing (Lambert et al., 2001; Hyldgaard et al., 2012). Greek oregano essential oil (GOEO), extracted mainly from the leaves and inflorescences of Origanum vulgare L, using ecofriendly methods, has been reported to exhibit broad antibacterial activity, showing significant synergistic antibacterial properties with various compounds (Betancourt et al., 2012). In addition, other biological activities, such as antifungal, antiviral, antioxidant, and anticancer activities, have been documented for GOEO (Cervato et al., 2000; Kalemba and Kunicka, 2003; Gautam et al., 2014). Allium sativum L. (garlic) and Crithmum maritimum L. (rock samphire) extracts have also been reported to have beneficial antibacterial, antiviral, antifungal, antioxidant, and immunomodulatory effects, which have been attributed to their bioactive compounds (Meot-Duros and Magné, 2009; Chandran et al., 2022). In addition, Camelina sativa (camelina) seed extract has been reported to have both antioxidant and anti-inflammatory activity and has been proposed as a natural alternative to synthetic drugs (Kumar et al., 2017). Recently, a few high-throughput 16S rRNA sequencing studies have investigated the effect of herbal extracts on the microbial compositions and metabolic profiles of weaned piglets (Li et al., 2018) and chickens (Zhu et al., 2019), revealing that such extracts can be used as antibiotic alternatives to reduce the accumulation of pathogenic bacteria, and hence improve the intestinal health of these animals.
Differences in animals’ dietary regimes can often result in nutrient deficiencies, and the bioavailability of nutrients is susceptible to feed processing techniques (Oliva-Teles, 2012). Deficient dietary intake has been reported to affect chickens at morphological, biochemical, and immunological levels (Uni et al., 2000; Khoso et al., 2019). In addition, alterations in diet may result in oxidative stress induction, as has been previously reported in several animals, including fish (Antonopoulou et al., 2013; Feidantsis et al., 2014; Antonopoulou et al., 2017; Feidantsis et al., 2022), rats (Dhibi et al., 2011), and chickens (Liu et al., 2012). Mitogen-activated protein kinases (MAPKs) are highly conserved modules that mediate extracellular signal transduction to intracellular targets, thus regulating multiple cell responses, such as proliferation, differentiation, and apoptosis, to diverse stimuli including hormones, growth factors, and environmental stresses (e.g., nutritional changes) (Fischer et al., 2009; Kyriakis and Avruch, 2012; Antonopoulou et al., 2017; Feidantsis et al., 2022). Similarly, several stressors induce heat shock proteins (Hsps), a family of intracellular proteins that act as molecular chaperones and are involved in protein homeostasis, the prevention of protein misfolding, and aggregation and cell protection (Jäättelä, 1999; Figueiredo et al., 2007). In addition, apoptotic machinery, a programmed cell death procedure, may be recruited during stress conditions, including nutrient deficiency (Huang et al., 2016), to eliminate damaged or abnormal cells and ensure tissue homeostasis (Watson and Pritchard, 2000).
The gastrointestinal tract of chickens is considerably smaller than that of mammals. Consequently, the physical space available for digestion and absorption (Pan and Yu, 2014); thus, the maintenance of overall gut health is important. Gut health is based on the physical and physiological status of a living organism’s gastrointestinal tract, framed up by a list of different parameters, such as diet, mucosa, microorganisms, and immune system, that together are important in sustaining homeostasis (Wickramasuriya et al., 2022). Intestinal mucus is an adhesive material that is produced by a specific type of enteric cells, the goblet cells, and surrounds the gastrointestinal tract. In broiler chickens, mucus has been found to protect against intestinal colonization by pathogenic microorganisms; thus, the abundance of goblet cells is linked to health benefits for chickens (Broom, 2018).
In the context of dietary supplementation with novel herbal mixtures in broiler chickens, the aim of the present study was to evaluate the influence of a special phytobiotic mixture extract containing Origanum vulgare subsp. hirtum, Allium sativum, Crithmum maritimum, and Camelina sativa supplemented in two forms (i.e., aqueous extract or cyclodextrin encapsulation) on meat oxidation, cellular responses, intestinal morphology, abundance of goblet cells, and proximal and hindgut microbiota in broiler chickens.
Husbandry, euthanasia by electrical stunning prior to slaughter, experimental procedures, and the biosecurity precautions in place were in accordance with Greek legislation governing experimental animals and were approved by the local Public Veterinary Service (Reg. 489181(3254)/07.02.2018) for research experimental facilities. All institutional and national guidelines for the care and use of laboratory animals were followed.
Plant material of native Greek Origanum vulgare subsp. hirtum L. [oregano, IPEN (International Plant Exchange Network) accession number GR-1-80 BBGK- 03,2107], and Crithmum maritimum (sea fennel, IPEN accession number GR-1-80 BBGK- 17,5685) are ex-situ maintained at the collection of the Balkan Botanic Garden of Kroussia 84 (41°05′44.3″N 23°06′33.7″E) of the Institute of Plant Breeding and Genetic Resources, Hellenic Agricultural Organization-DEMETER, in Greece. Young plants were produced asexually by cuttings and were cultivated in the fields of the Institute. Leaves and flowers were collected, dried, and then used for the production of the extracts (Vasilopoulos et al., 2022). Allium sativum bulbs of the Greek traditional variety ‘Vyssa’ were kindly provided by the local agricultural cooperation of the village of Vyssa, north-east Greece. Camelina sativa as cultivated in the north-east of Greece, near the river Evros, and the seeds used for experimentation were also provided by the local Union of Agricultural Cooperation. All the plants were submitted to solvent extraction with either β-cyclodextrin aqueous solution or double-distilled water and were used in dried and powdered form. The concentration of Greek oregano, garlic, and rock samphire was 50 g per liter of solvent, whereas the concentration of camelina was 10 g per liter.
A total of 120 1-day-old hatched Ross-308 chicks, donated by PINDOS APSI hatchery, were randomly allocated into three equal groups, with four replicates of 10 birds each. The duration of the experimental trial was 35 days. All treatment replicates were housed in separate floor pens, each equipped with an infrared lamp for heating, in a specially designed experimental room at the Research Institute of Animal Science, Hellenic Agricultural Organisation-DEMETER, Paralimni (40°45′N, 22°27′E), Giannitsa, Greece, in which the temperature, relative humidity and lighting conditions were controlled, following the recommendations of the breeding company (Aviagen®, Huntsville, AL, USA), throughout September 2020 and October 2020. The chicks’ health was evaluated twice daily by a veterinarian surgeon. On day 1 in the hatchery, all birds were vaccinated against Newcastle disease (ND) and infectious bronchitis (IB) through spray vaccination, as well as against infectious bursal disease (IBD) through subcutaneous vaccination. The CONTROL diet was formulated in accordance with the breeding company’s recommendations (Aviagen, 2019), and comprised a maize and soybean meal in mash form. After the chicks were fed the basal diet, additional diets were prepared by incorporating Origanum vulgare subsp. hirtum L., Allium sativum L., Crithmum maritimum L., and Camelina sativa L. Crantz either as aqueous extract (AQORGCC group) at a concentration of 0.1% per kg of dry matter (DM) or as cyclodextrin extract (CDORGCC group) at the same concentration. Table 1 provides a detailed composition of the CONTROL group’s diet.
The preparation of herbal extracts was carried out in accordance with the method detailed by Vasilopoulos et al. (2022). We also determined diet oxidative aspects along with total phenolic content, in accordance with the methodology described by Singleton et al. (1999) expressed as gallic acid equivalents (GAEs) in mg/g using the Folin–Ciocalteu assay, while the determination of the antiradical activity was based on the assessment of 2,2-diphenyl-1-picrylhydrazyl (DPPH) reagent.
Two male and two female chickens from each of the four replicates, amounting to 16 birds per treatment, were chosen to meet the mean body weight of the pen, identified by plastic tie-up leg tags and were thereafter slaughtered, at day 35, so that both breast and thigh meat and intestinal samples could be collected.
The breast and thigh meat samples of two male and two female birds from each replicate were collected for further analysis regarding their oxidative status on days 1, 3, and 5 of refrigerated storage. Subsequently, meat samples from the same birds were placed in an oven at 90°C for 45 min on day 1 to determine the lipid oxidative stability of the cooked meat. The levels of thiobarbituric acid-reactive substances (TBARS) in broiler chicken meat samples were determined in accordance with the methodology described by Ahn et al. (1999), with minor modifications. To determine protein carbonyl levels, the method of Patsoukis et al. (2004) was applied to the same birds’ meat samples.
To investigate chicken cellular responses to dietary changes, the levels of Hsp60, Hsp70, and Hsp90 and of MAPKs (p38, p44/42), and the Bcl-2/Bad ratio were determined through SDS-PAGE/immunoblot analysis in accordance with well-established protocols. The homogenization of tissue samples, collected as described above, for total protein extraction was carried out in cold lysis buffer in accordance with the method described by Antonopoulou et al. (2020). The protein concentration of homogenized samples was measured using the colorimetric BioRad protein assay. Subsequently, equivalent amounts of protein (80 μg) were separated on 10% (w/v) acrylamide and 0.275% (w/v) bisacrylamide slab gels and electrophoretically transferred onto a 0.45-μm-thick nitrocellulose membrane filter (Schleicher & Schuell BioScience GmbH, Keene, NH, USA). Equal protein loading and transfer efficiency were assured by Ponceau staining (data not shown). Following the blocking of non-specific binding sites in 5% (w/v) non-fat milk in Tris-buffered saline–Tween (TBST) [20 mM Tris-HCl, 137 mM NaCl, 0.1% (v/v) Tween 20, pH 7.5], membranes were incubated overnight at 4°C with specific primary antibodies. The antibodies used were as follows: monoclonal rabbit anti-heat shock protein, 60 kDa (Cat. No. 12165, Cell Signaling, Beverly, MA, USA), polyclonal rabbit anti-heat shock protein, 70 kDa (Cat. No. 4872, Cell Signaling, Beverly, MA, USA), polyclonal rabbit anti-heat shock protein, 90 kDa (Cat. No. 4874, Cell Signaling, Beverly, MA, USA), monoclonal rabbit anti-phospho-p44/42 MAPK (Thr202/Tyr204) (Cat. No. 4370, Cell Signaling, Beverly, MA, USA), monoclonal rabbit anti-p44/42 MAPK (Cat. No. 4695, Cell Signaling, Beverly, MA, USA), polyclonal rabbit anti-phospho-p38 MAPK (Thr180/Tyr182) (Cat. No. 9211, Cell Signaling, Beverly, MA, USA), polyclonal rabbit anti-p38 MAPK (Cat. No. 9212, Cell Signaling, Beverly, MA, USA), monoclonal rabbit anti-Bcl2 (Cat. No. 3498, Cell Signaling, Beverly, MA, USA), and polyclonal rabbit anti-Bad (Cat. No. 9292, Cell Signaling, Beverly, MA, USA). Thereafter, blots were incubated with horseradish peroxidase (HRP)-conjugated secondary antibody [anti-rabbit IgG, HRP-linked antibody (Cat. No. 7074, Cell Signaling, Beverly, MA, USA)]. Before and after incubation, blots were washed in TBST (three times, for 5 min each time). The bands were detected through enhanced chemiluminescence (Cat. No. 7003, Cell Signaling, Beverly, MA, USA), with exposure to Fuji Medical X-ray films. Bands quantification was conducted in Image Studio Lite (LI-COR Biosciences, Lincoln, NE, USA).
On day 35 of the trial, following euthanasia (2–5 min), tissue samples were collected, as described above, from a standard area of duodenum, jejunum, and ileum and fixed in 10% neutral-buffered formalin, embedded in paraffin, cut into 3- to 4-μm-thick sections, and stained with hematoxylin and eosin. Alcian Blue pH 2.5/PAS staining (staining kit Bio-Optica, 04-163802A) was also performed to detect goblet cells. The ImageJ image processing and analysis program (National Institutes of Health, Bethesda, MD, USA, J 1.53k) was used for the histomorphometric analysis (Gava et al., 2015). The histomorphometric features that were evaluated in each image were villus height, crypt depth, lamina propria width, and the number of goblet cells per villus. To determine villus height and crypt depth, three images of favorably orientated sections cut vertically from the villus enterocytes to the muscularis mucosa of each sample were captured. Subsequently, the villus height was assessed by measuring the perpendicular distance from the villus tip to the villus–crypt junction level for 10 villi per section. Accordingly, crypt depth was measured for 10 corresponding crypts per section.
Approximately 250 mg (wet weight) of homogenized pooled samples of ileal or cecal digesta obtained from 16 broiler chickens in each group (as mentioned above, to provide a satisfactory and representative sample quantity) was analyzed. Thereafter, 12 separate CONTROL group samples (six ileal and six cecal) and 16 separate experimental group samples (eight ileal and eight cecal) were subjected to genomic DNA extraction using the Qiagen DNeasy PowerSoil Pro Kit (Qiagen, Carlsbad, CA, USA), following the manufacturer’s protocol. The quantity and quality of the extracted DNA were measured using a NanoDrop™ spectrophotometer (Thermo Fisher Scientific, Waltham, MA, USA), and agarose gel electrophoresis, respectively, to meet the requirements for subsequent sequencing. DNA samples were stored at −80°C prior to further analysis.
The V3–V4 hypervariable region of the bacterial 16S rRNA was amplified by PCR using the primer pair 341F/806R (341F: 5′-CCTAYGGGRBGCASCAG-3′, 806R: 5′-GGACTACCVGGGTATCTAAT-3′) (Cui et al., 2019). PCR amplification of targeted region was performed by using the above-mentioned primers connecting with individual barcodes. The amplified 16S rRNA amplicons from each sample were paired-end sequenced (2 × 250) on the Illumina NovaSeq 6000 platform in accordance with the standard protocol. The raw sequence data were submitted to the NCBI SRA database (NCBI BioProject PRJNA871958).
The raw paired-end reads were assigned to samples based on their unique barcodes and were truncated by cutting off the barcode and primer sequences. Paired-end reads were merged using FLASH (V1.2.7) (Magoč and Salzberg, 2011). Amplicon reads were filtered to obtain high-quality clean tags using the Quantitative Insights Into Microbial Ecology (QIIME, http://qiime.org/index.html ) software package (V1.7.0) (Caporaso et al., 2010). The tags were then compared with the reference database (SILVA138 database) using the UCHIME algorithm (version 4.2.40) (Edgar et al., 2011) to detect and remove chimera sequences. The remaining high-quality sequences were clustered into operational taxonomic units (OTUs) at 97% sequence identity using the UPARSE (version 7.0.1090, http://drive5.com/uparse/ ) pipeline (Edgar, 2013). Representative sequences for each OTU were screened for further annotation.
For each representative sequence, species annotation was developed using the Mothur method and the SSU rRNA database of SILVA138 (with a threshold of 0.8–1) at each taxonomic rank (Wang et al., 2007; Quast et al., 2012). The abundance information of the OTUs was normalized prior to the alpha- and beta-diversity analyses using a standard of sequence number corresponding to the sample with the least sequences.
Alpha-diversity (Chao1 and Shannon) indices were calculated using QIIME software (version 1.7.0) and displayed with the “phyloseq” package (McMurdie and Holmes, 2013) in R software (version 2.15.3). Rarefaction metrics were computed using the alpha_rarefaction.py script in the QIIME software package (Kuczynski et al., 2012). A heatmap based on the relative abundance of OTUs was generated using R software (Ling et al., 2014).
Beta-diversity was also assessed with QIIME using principal coordinates analysis (PCoA) based on weighted (assessment of community structure by considering the abundances of OTUs) and unweighted (assessment of community membership by considering the presence/absence of OTUs) UniFrac distance matrices (Lozupone and Knight, 2005). Analysis of similarities (ANOSIM) was performed to assess the overall similarity among the different groups by testing the significance of spatial separation in PCoA. The R-value indicated the extent to which the groups were separate in terms of their microbial communities (R > 0.75, good separation; R > 0.5, overlapping; and R < 0.25, no separation) (VAN, 1993).
To characterize the microbial differences between the different groups, and to identify potential biomarkers for each diet, linear discriminant analysis (LDA) effect size (LEfSe) analysis was employed (Segata et al., 2011) using Galaxy online tools (http://huttenhower.sph.harvard.edu/lefse/ ). Furthermore, a linear discriminant analysis (LDA) effect size (LEfSe) algorithm was applied to identify the significant microbial difference among the different diets groups, using an LDA score >3.
Prior to the onset of the experiment, the minimum required total sample size was calculated using the “power analysis for one-way ANOVA” methodology with G*Power 3.1.9.2 software and power ≥ 0.80. The basic study design was a random complete block (RCB) design, and the replication (pen) was considered as the experimental unit. Changes in lipid oxidation, the level of protein carbonyls, the activation of MAPKs, the expression patterns of Hsps, and the Bcl-2/bad ratio in response to the dietary treatments were tested for significance at the 5% level using one-way analysis of variance (ANOVA), and post-hoc comparisons were performed using Tukey’s test. Statistical analysis was conducted using the SPSS program (SPSS Statistics 25).
The idea of supporting the breeding of commercial broiler chickens by avoiding the use of antibiotic growth factors emerged long ago, owing to well-documented concerns about antibiotic resistance, and after public demand for natural products. The capacity of plant extracts to facilitate the achievement of this goal is continuously being investigated, and previous reports have investigated the effects of plant metabolic compounds on broiler chickens’ intestinal microbiota with hortatory results (Wang et al., 2022). Phytobiotics produced by green technologies may be further exploited as feed additives with enhanced properties either to support growth performance (Vasilopoulos et al., 2022) or to increase antimicrobial potency. The innovative technology of extract encapsulation may serve the aim of rearing broiler chickens in the absence of antibiotics by reaching targets in different directions, as phytobiotics act by various mechanisms. Among the advantages of encapsulation for plant-extracted ingredients are the increased bioavailability and solubility of core compounds, the prevention of ingredient degradation during processing or while in storage, and the masking of undesirable ingredient properties, such as unpleasant odor or flavor (Christaki et al., 2022). In addition, it has been proven that microencapsulation by spray drying has a beneficial effect on oregano essential oil, by enhancing the speed and level of its release of bioactive compounds (Partheniadis et al., 2017).
In the present study, a mixture of herbs, including oregano, garlic, rock samphire, and camelina, was chosen, and these herbs were extracted either with cyclodextrin or with water to be used as phytobiotic solution; subsequently, their effect as feed supplements in the diet of broiler chickens was evaluated by determining the levels of lipid and protein oxidation and the expression of stress-related and apoptosis proteins in breast meat, as well as changes in the chickens’ intestinal morphology and microbiota. It has been hypothesized that feed supplementation with plant extracts could impact the gastrointestinal function of chickens, and may also positively influence protein and lipid oxidation of chickens. For this reason, several indicators commonly applied at the experimental and industrial levels were evaluated. Furthermore, the nutrient profile of broiler chicken meat was assessed, focusing primarily on protein and lipid oxidation in both raw and cooked samples of breast meat. Our study results indicate that the tested extracts’ combination could be used as a feed supplement to improve the quality of broiler chicken meat. However, as poultry meat quality can also be affected by other dietary factors, further research is needed regarding the effect of tested phytobiotics. For example, phytobiotics should be incorporated in diets alongside other ingredients to evaluate the possible interactions between different ingredient components, as well as in diets with ingredients of different fat content for the evaluation of antioxidant activity (Vasilopoulos et al., 2022). It has already been stated that the same phytobiotic mixture in the cyclodextrin encapsulated form significantly increased the final mean body weight of broiler chickens at day 35 (final mean body weights: CONTROL 2,287.75 g; AQORGCC 2,264.5 g; and CDORGCC: 2,295.2 g), whereas feed intake and the feed conversion ratio were unaffected among the chickens fed either the CONTROL diet or a diet supplemented with the herbal mixture encapsulated in cyclodextrin/in its aqueous form (Dokou et al., 2023).
The total phenolic content of the extracts was 152.5 ± 1.46 mg GAE/g of plant material for the aqueous quadruple extract and 206.6 ± 2.77 mg GAE/g of plant material for the cyclodextrin quadruple extract. A small difference was observed between the extracts regarding their total phenolic content, with the cyclodextrin extract having the highest value. The total phenolic content of the CONTROL feed was 0.51 ± 0.15 mg GAE/g of dry matter. The results regarding the antiradical activity presented a different and interesting pattern. More specifically, the value for the aqueous extract was 690.38 ± 0.68 μmol Trolox equivalent/g of plant material, whereas the value for the cyclodextrin extract was equal to 620.87 ± 0.07 μmol Trolox equivalent/g of plant material. Although the cyclodextrin extract had a slightly higher total phenolic content than the aqueous extract, its antiradical activity was lower. Based on these results, it seems that the yield of components presenting scavenging activity against DPPH· is higher when water, rather than cyclodextrin solution, is used as an extraction solvent. The antiradical activity of the CONTROL feed was 41.5 ± 2.24 μmol Trolox equivalent/g of feed.
The effects of herbal dietary supplementation on both lipid and protein oxidation status in breast and thigh meat are presented in Table 2. Regarding breast meat, TBARS values on day 1 of meat preservation in the refrigerator were significantly lower in both herbal supplementation groups than in the CONTROL group (p < 0.001), and the AQORGCC group had the lowest value (p < 0.001). On day 3, the TBARS measurements had similar values among all groups (p = 0.546), while on days 1 and 5, for the cooked samples, both supplemented groups had lower values than the CONTROL (p = 0.005). Similarly, the levels of protein carbonyls in breast meat were found to be significantly lower in the supplemented groups, with the CDORGCC group having the lowest value (p < 0.001). In relation to thigh meat, the CDORGCC and CONTROL groups exhibited the lowest lipid oxidation values on days 3 and 5 (p < 0.001), while on day 1 the results were similar for all groups (p = 169). In terms of protein oxidation, the AQORGCC and CONTROL groups had decreased values (p < 0.001). Several aromatic plants have been identified as potential means to mediate tissue oxidative susceptibility owing to their rich polyphenols content (Mahfuz et al., 2021). An established action mechanism of substances rich in polyphenols is their attachment to free radicals, which is proven to cause tissue oxidation. In our investigation, both herbal supplemented feeds were found to have a significant phenolic content, which we can assume contributed to the lowering of lipid and protein oxidation levels. Similar results have been reported by several researchers (Giannenas et al., 2005; Chamorro et al., 2015; Puvača et al., 2015). These findings are in agreement with levels of antiradical activity being higher in the group receiving the experimental diets than in the group receiving the CONTROL diets.
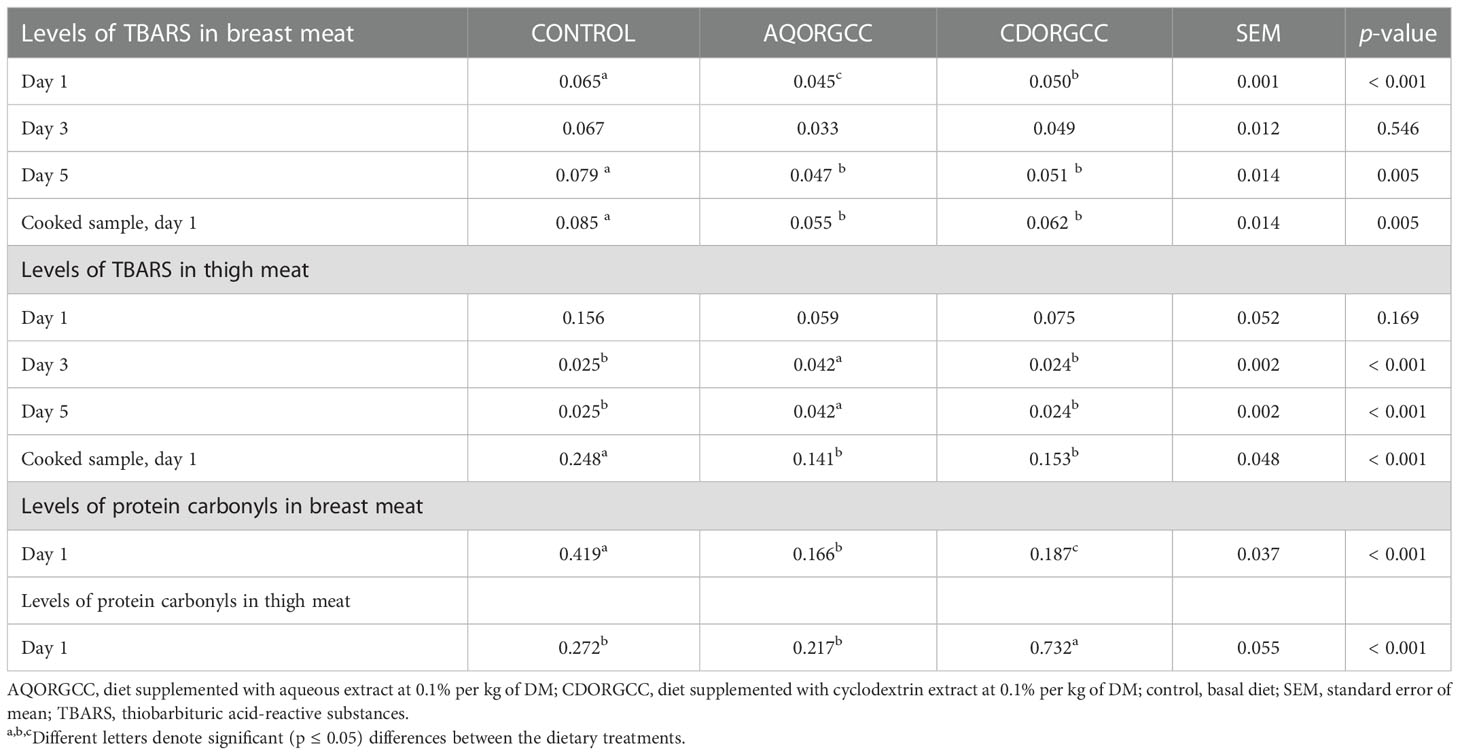
Table 2 Effect of herbal dietary supplementation on the lipid and protein oxidation levels in the treated breast and thigh meat of 16 chickens.
The induction of Hsps in response to dietary diversification has been previously reported in several animals (Kotzamanis et al., 2021; Bousdras et al, 2022), including chickens (Liu et al., 2015; Zhao et al., 2016). In this study, the effects of dietary use of herbal extracts in either encapsulated or aqueous form on the expression levels of Hsp60, Hsp70, and Hsp90 in chicken breast and thigh meat are depicted in Figure 1. The results obtained showed distinct tissue-specific patterns in regard to Hsps induction, which is in agreement with previous studies in chickens (Xie et al., 2014; Surai and Kochish, 2017). Compared with the CONTROL group, Hsp60 expression levels in both types of tissue exhibited a significant induction (p < 0.05) in response to the CDORGCC diet. By contrast, the AQORGCC diet resulted in differentiation of Hsp60 expression patterns between the two tissues, that is elevated Hsp60 levels were observed in thigh tissue, whereas a significant reduction in Hsp60 levels was apparent in breast tissue. Differences in the heat shock response between the two types of tissue were also observed in regard to Hsp70 levels. Specifically, Hsp70 expression levels were significantly induced (p < 0.05) in the chicken thigh tissue of the CDORGCC dietary group compared with the CONTROL group, whereas Hsp70 expression levels were increased in the breast tissue of the AQORGCC dietary group. In terms of Hsp90, the CDORGCC diet significantly induced protein levels in breast tissue, while a reduction was apparent in expression levels in thigh tissue. However, no differentiation in Hsp90 levels in both types of tissue was observed in the AQORGCC dietary group compared with the CONTROL group. In accordance with the present results, previous studies have reported that supplementation with plant polyphenolic compounds differentiates the expression of Hsps in a tissue-specific manner (Liu et al., 2014; Shirai et al., 2021). Dietary resveratrol supplements attenuated the heat stress-induced Hsps upregulation in the bursa of Fabricius and spleen of black-boned chickens, while elevated Hsp27 and Hsp90 mRNA expression was apparent in the thymus (Liu et al., 2014). Similarly, Shirai et al. (2021) observed the in vitro induction of Hsps in murine liver hepatoma cells incubated with zerumbone, a Zingiber zerumbet Smith rhizome extract. However, mice fed a diet containing the latter phytochemical displayed differentiation in Hsps expression pattern in the liver and the soleus muscle, whereas zerumbone seems to facilitate thermotolerance via Hsp70 expression (Shirai et al., 2021). The recruitment of Hsps is vital for the reversal or inhibition of protein denaturation or unfolding during stress conditions (Jäättelä, 1999; Surai and Kochish, 2017), including nutritional deficiencies. For instance, previous studies in chickens have shown that dietary selenium deficiency leads to an increase in Hsps mRNA and protein levels (Liu et al., 2015; Zhao et al., 2016). However, the induction of Hsps may occur as a response to variations in nutrient composition, mediating several protein metabolic processes, including protein synthesis (Hendrick and Hartl, 1993). In the present study, supplementation with phytobiotic extract in the aqueous form resulted in fewer detrimental effects in regard to Hsps recruitment than didCDORGCC treatment, which can perhaps be attributed to the antioxidant activity of herbal compounds, including radical scavenging.
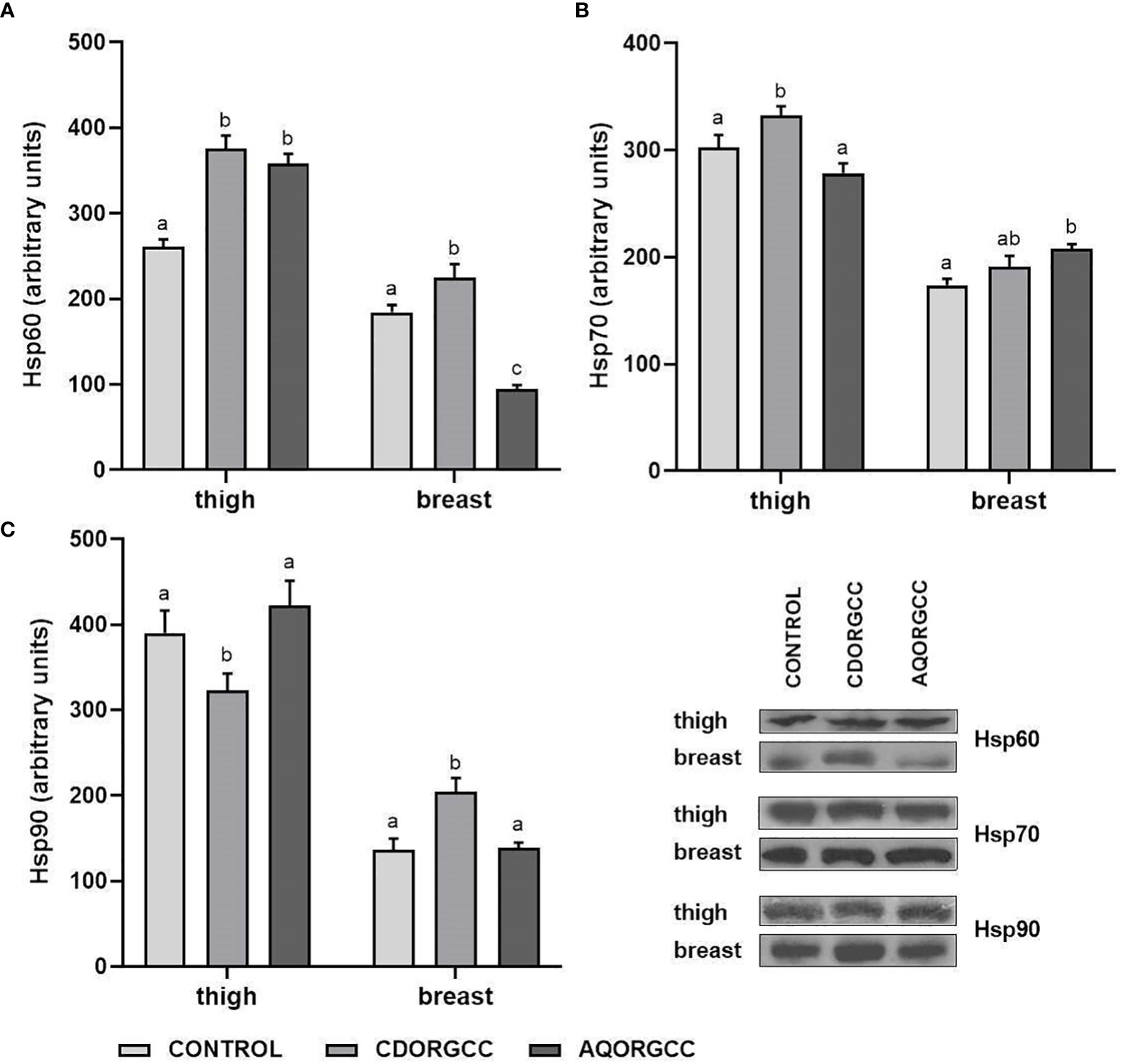
Figure 1 Expression levels of (A) Hsp60, (B) Hsp70, and (C) Hsp90 in the thigh and breast tissue of broiler chickens following the three different dietary treatments, i.e., control, basal diet; CDORGCC, diet supplemented with cyclodextrin extract at 0.1% per kg of DM; or AQORGCC, diet supplemented with aqueous extract at 0.1% per kg of DM. Representative protein bands are shown in the bottom right panel. Data represent means ± SD; n = 3 biological replicates. Different letters (a, b, c) denote significant (i.e., p ≤ 0.05) differences between the dietary treatments.
Similarly, supplementation with CDORGCC propelled significant (p ≤ 0.05) activation of p38 MAPK in both types of tissue compared with the CONTROL, while p44/42 MAPK activation was apparent in thigh tissue (Figure 2). By contrast, AQORGCC significantly induced p38 MAPK and p44/42 MAPK activation in breast and thigh tissue, respectively, and a reduction in the latter was observed in breast tissue. The activation of MAPKs due to changes in dietary regime has been little researched in chickens; most studies have focused on the role of MAPK/NF-κB pathway implications in necroptosis (Jiayong et al., 2020; Liu et al., 2022). The supplementation of rutin, a dietary bioflavonoid, alleviated the cadmium-induced MAPK protein levels in chicken liver due to treatment with cadmium (Liu et al., 2022). In this study, we found that AQORGCC reduced p44/42 MAPK activation levels in breast tissue, but that no effect was apparent on p38 MAPK activation levels in thigh tissue. Thymol, a natural phenolic compound found in thyme- and oregano-scented plants and which has strong anti-inflammatory properties, has been previously shown to interfere in the activation of the MAPK signaling pathway (Liang et al., 2014). In accordance with the present results, dietary supplementation with oregano essential oil has been reported to inhibit the activation of p44/42 MAPK, but not that of p38, in pig jejunum, while simultaneously improving the integrity of the intestinal barrier (Zou et al., 2016). However, it must be noted that MAPK signaling is involved in several cellular processes, including proliferation, differentiation, and development (Seger and Krebs, 1995); thus, the activation of MAPKs may not be entirely due to nutrient stressors. Nevertheless, phytochemicals seem to activate MAPKs to regulate the antiestrogenic effects of phytochemicals (Collins-Burow et al., 2012), as well as the transcription of genes involved in the response to oxidative stress (Loo, 2003). Thus, with regard to MAPK activation, supplementation with phytobiotic extract in aqueous form seems to improve animal welfare, although further investigation is required because of the variations between the examined tissues.
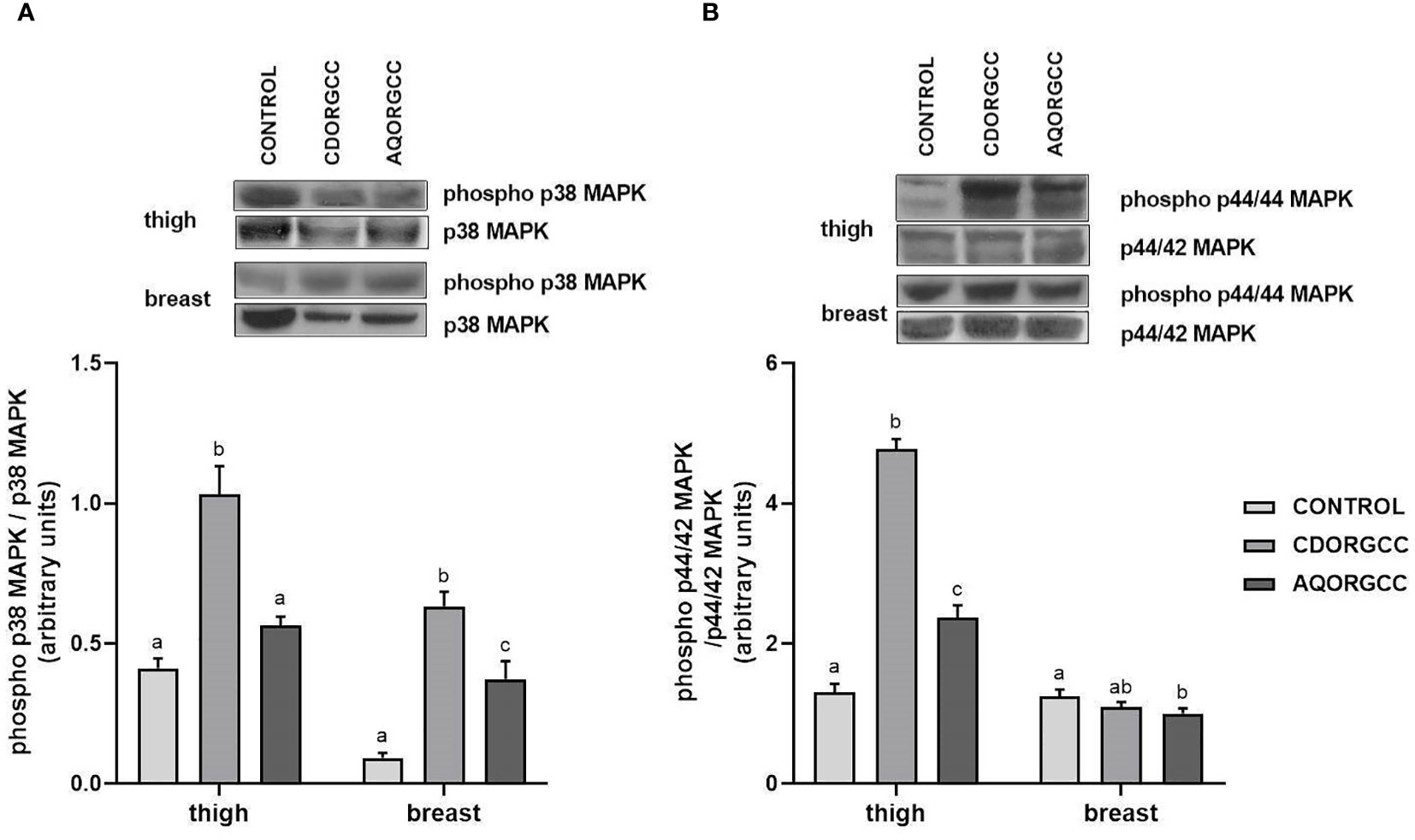
Figure 2 Phosphorylation of (A) p38 and (B) p44/42 MAPKs in the thigh and breast of broiler chickens following the three different dietary treatments, i.e., control, basal diet; CDORGCC, diet supplemented with cyclodextrin extract at 0.1% per kg of DM; AQORGCC, diet supplemented with aqueous extract at 0.1% per kg of DM. Representative protein bands are demonstrated in the top panels. Data represent means ± SD; n = 3 biological replicates. Different letters (a, b, c) denote significant (i.e., p ≤ 0.05) differences between the dietary treatments.
In terms of apoptotic machinery, the two types of tissue exhibited contradictory results following the dietary inclusion of herbal extracts (Figure 3). Specifically, the Bcl-2/Bad ratio was significantly reduced (p ≤ 0.05) in thigh tissue in both herbal extract groups compared with the CONTROL group. However, both diets resulted in a significant increase in the Bcl-2/Bad ratio in breast tissue. Cellular anti-apoptotic and pro-apoptotic proteins, including Bcl-2 and Bad, respectively (both members of the Bcl-2 family), are involved in the regulation of apoptosis, in which lessened sensitivity to cell death is indicated by a high Bcl-2/Bad ratio (Levine et al., 2008). The elevation of cytosolic cytochrome c is pivotal in cells undergoing apoptosis, while Bcl-2 overexpression prevents the release of cytochrome c from mitochondria to cytosols and, consequently, cell apoptosis in response to several stimuli, including metabolic stressors (Levine et al., 2008; Liu et al., 2013). For instance, dietary selenium deficiency has been shown to increase the mRNA levels of Bax and induce testicular apoptosis in chickens (Huang et al., 2016). Furthermore, cinnamon extract has been shown to exert antioxidant, anti-inflammatory, and anti-apoptotic effects against copper-induced nephrotoxicity in broiler chickens, restoring the mRNA levels of Bax, another pro-apoptotic member of the Bcl-2 family, and Bcl-2 to normal (Elazab et al., 2021). In accordance with the aforementioned results, the herbal mixture in the present study exhibited anti-apoptotic properties in breast tissue.
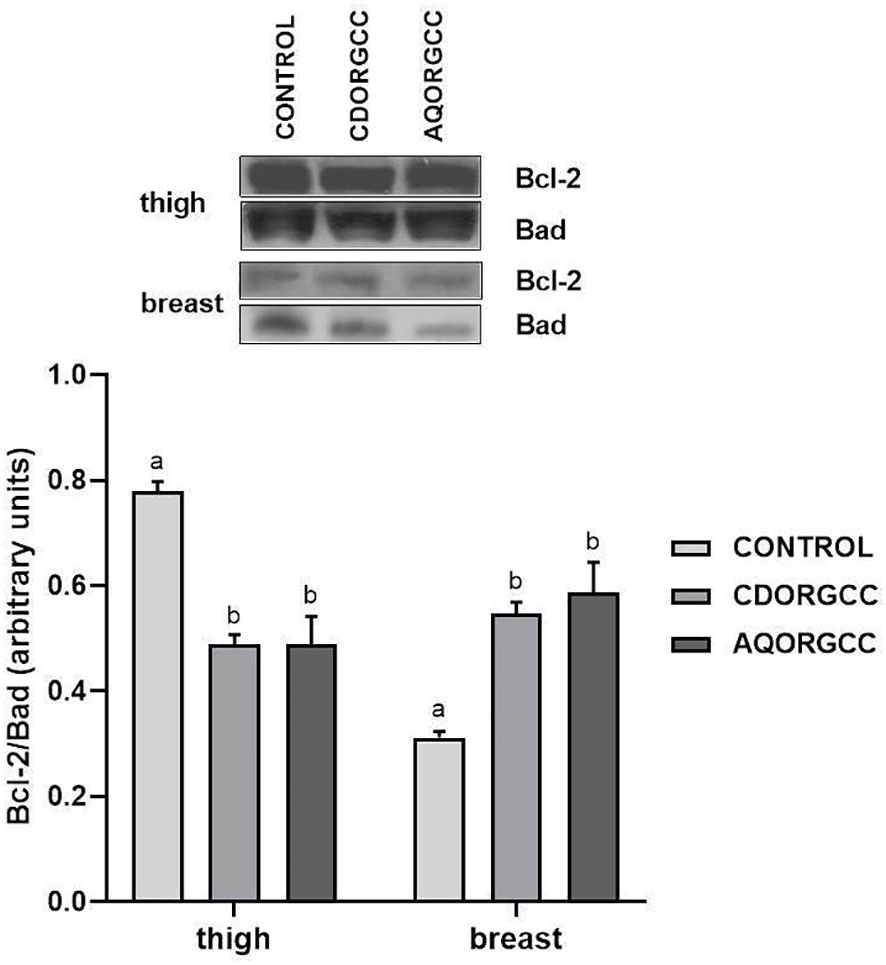
Figure 3 Bcl-2/Bad ratio in the thigh and breast of broiler chickens following the three different dietary treatments, i.e., control, basal diet; CDORGCC, diet supplemented with cyclodextrin extract at 0.1% per kg of DM; AQORGCC, diet supplemented with aqueous extract at 0.1% per kg of DM. Representative protein bands are shown in the top panel. Data represent means ± SD; n = 3 biological replicates. Different letters (a, b, c) denote significant (i.e., p ≤ 0.05) differences between the dietary treatments.
Intestinal morphometry was affected in different ways in different parts of the intestine and depending on the feed additive (Table 3). Villus height in the duodenum was greater in the AQORGCC dietary group than in the CONTROL and CDORGCC groups. Similarly, the lamina propria was wider in the AQORGCC group than in the other two groups. The number of goblet cells remained unaffected in all intestinal tissues. Villus height to crypt depth ratio was higher in the jejunum and ileum of the AQORGCC dietary group than in the other two groups, suggesting more efficient intestinal functionality with less energy demand in this group. In jejunum, villus height did not differ among groups, as the AQORGCC and CDORGCC groups were possibly not more potent in activity compared with the CONTROL diet group. Ileum morphology was however, affected by the dietary supplementation, where CONTROL group presented the highest value (p < 0.001). Crypt depth was also higher in the AQORGCC group, and lamina propria was also increased in this group (p < 0.001).

Table 3 Effect of dietary extract supplementation on the intestinal morphology and goblet cell number of broiler chickens.
In general, the birds fed the CONTROL diet had a shorter villus height, a higher crypt depth, and a lower villus height to crypt depth ratio than birds fed the herbal-enriched diets, and this difference was particularly notable in the small intestine of birds in the aqueous extract dietary group, and in the hindguts of those in the cyclodextrin dietary group. In the present study, we observed an increase in villus height, without a difference in the number of goblet cells, in the groups fed the herbal-enriched diets compared with the CONTROL group. Villus height is an important indicator of the digestive function of chickens because it is correlated with the absorptive efficiency of the intestinal mucous membrane, i.e., a higher villus height is indicative of better absorption in the small intestine (Wickramasuriya et al., 2022). In the absence of disease challenge or stress conditions (as indicated both by the equal numbers of goblet cells and by the fact that the lamina propria was higher in the group with greater villus height), it can be extrapolated that herbal extracts support growth and improve gut functionality, even in healthy chickens that are kept in sterile environments. The presence of toxins, even at low levels, or of bacteria lipopolysaccharides, can severely compromise several crucial intestinal functions and morphology, which is associated with an impairment of growth and performance (Ghareeb et al., 2015; Wang et al., 2015). Goblet cells secrete a sticky protein that has a protective effect on the intestinal epithelium (Antoni et al., 2014). Supplementation with a blend of essential oils (containing 15 mg/kg carvarcol) significantly increased villus length and goblet cells count in broiler chickens (Reisinger et al., 2011). Similarly, a combination of oregano with organic acids and clay minerals improved intestinal villus height, goblet cell counts, and intestinal cell functionality (Tzora et al., 2017).
Microbiota within the intestine contribute to nutrient digestion, fermentation of energy substrates, breakdown of non-starch polysaccharides, and prevention of disease through alternately bacteriostatic and bactericidal activity (Sergeant et al., 2014). The direct enhancement of broiler chickens’ productivity and health can be the outcome of favorable intestinal microbiota alteration (Sergeant et al., 2014). In this study, in an attempt to elucidate the effect of herbal extracts on the intestinal microbiota of broiler chickens, a high-throughput 16S rRNA amplicon sequencing analysis was performed. A total of 2,420,381 amplicons merged from paired-end reads were obtained from the 28 ileal and cecal samples, with an average of 84,442 sequences per sample. After data trimming and quality filtering, 1,723,713 high-quality sequences were acquired, with an average of 61,561 sequences per sample (Supplementary Table S1). These high-quality sequences for all the samples, after chimera removal, were clustered into 2,132 individual operational taxonomic units (97% identity), representing independent species belonging to 372 genera, 165 families, 99 orders, 39 classes, and 24 phyla (Supplementary Table S2). The cecal samples had a higher number of OTUs than the ileal samples (average numbers for the three diets were 656 and 530, respectively), whereas no statistically significant differences were observed between the different diet groups (Supplementary Table S3). The results pertaining to the number of observed species were similar, with 529 and 423 species identified in the cecum and the ileum, respectively.
In line with previous results (Wang et al., 2015), the two different intestinal tissues, cecum and ileum, seemed to be associated with distinct microbial communities, in terms of composition and richness. Overall, microbial communities in intestinal tissues were dominated by Firmicutes, Bacteroidota, Campilobacterota, and Proteobacteria, with distinct differences in species distribution between the ileum and caecum (Figure 4A; Supplementary Table S2). Firmicutes and Bateroidota are the most dominant bacterial phyla in chickens (Wen et al., 2019), whereas a higher ratio of Firmicutes to Bacteroidota, in terms of relative abundance, has been related to a greater percentage of body fat (Turnbaugh et al., 2006). Dietary supplementation with herbal mixtures resulted in this ratio being increased in cecal samples, whereas, in ileum samples, a decrease was observed, associated with body fat accumulation. Furthermore, Firmicutes made up 41.3% of cecal microbiota, while this proportion was increased to 69.7% in ileum, regardless of the diet. In addition, Euryarchaeota and Bacteroidota were more abundant in the cecum, whereas Campilobacterota, Verrucomicrobiota, and Actinobacteriota were more abundant in the ileum. In recent literature, a significantly lower fat deposition was observed in birds with lower Methanobrevibacter abundance than in birds with a high Methanobrevibacter abundance, despite there being no difference in the body weight of these birds, which is consistent with the results of previous studies (Hyldgaard et al., 2012; Sobral et al., 2014). Methanobrevibacter is a common and important methanogenic archaeon that primarily inhabits the cecum of chickens (Elazab et al., 2021). Methane-producing microorganisms can improve fermentation efficiency by consuming any excess hydrogen and formate in the bowel, which subsequently improves acetate production and allows the body to absorb more nutrients and calories (Salaheen et al., 2017).
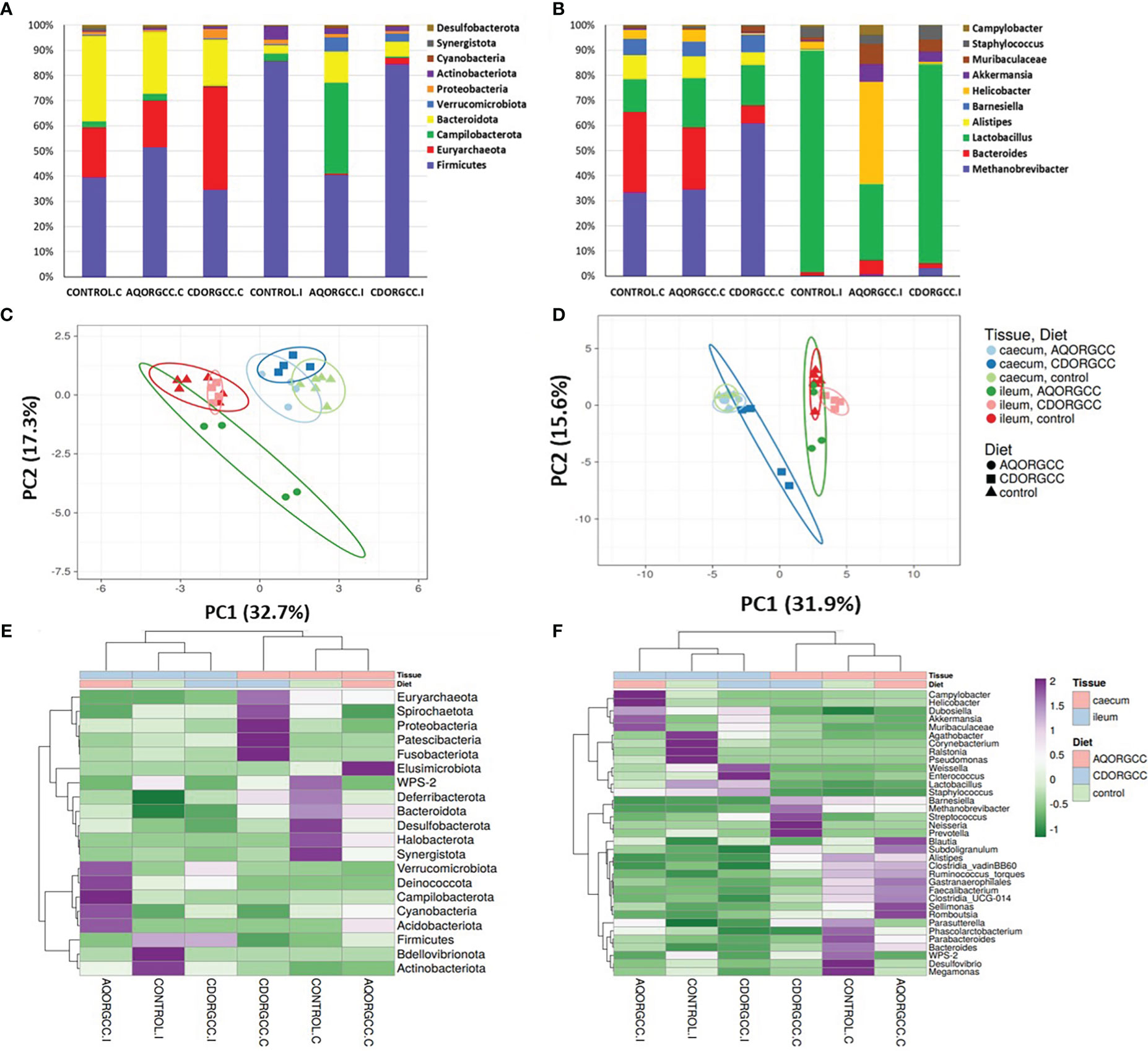
Figure 4 Distribution of the top 10 most abundant taxa of intestinal microbiota in ileum and cecum at the levels of phylum (A) and genus (B). Principal component analysis (PCA) based on OTU level microbiota composition of samples at the levels of phylum (C) and genus (D). The colors denote different groups. Heatmaps illustrate the correlations (purple, positive; green, negative) between phylogenetic groups at the levels of phylum (E) and genus (F).
Major bacterial compositional differences arising as a result of differences in diet were also detected. In cecum, for example, the cyclodextrin herbal mixture increased the abundance of Proteobacteria (3.2-fold), and decreased the abundance of Campilobacterota (5.4-fold), compared with the CONTROL diet (Figures 4A, E; Supplementary Table S2). By contrast, in the ileum, the abundance of Campilobacterota was significantly increased (10.4-fold) in birds fed the aqueous herbal mixture-supplemented diet compared with those fed the CONTROL diet. In addition, in both the aqueous and cyclodextrin herbal mixture groups, the abundances of Bacteroidota (4.2- and 1.9-fold, respectively) and Verrucomicrobiota (15.1- and 8.5-fold, respectively) were increased, whereas the abundance of Actinobacteriota (2.6- and 2.8-fold, respectively) was decreased in those groups compared with the CONTROL group. Several studies have demonstrated that plant extracts can stimulate the relative abundance of Firmicutes (Salaheen et al., 2017), Clostridiales and Lachnospiraceae (Diaz Carrasco et al., 2018), or Bacteroidetes (Wen et al., 2019). The inconsistency in these results clearly suggests that the use of herbal extracts as additives in the broiler diets can have a dominant role in modulating the composition of intestinal microbial communities.
At the genus level, Methanobrevibacter and Bacteroides dominated the cecal microbiota, whereas Lactobacillus was the most prevalent genus governing ileal microbiota (Figure 4B; Supplementary Table S2). Specifically, Methanobrevibacter accounted for 33.2% and 34.5% of cecal microbiota in the CONTROL and the aqueous herbal diet groups, respectively, but this proportion was remarkably enhanced (60.8%) in the cyclodextrin herbal mixture diet group. By contrast, in the cyclodextrin herbal mixture diet group, the abundance of Bacteroides was decreased compared with the CONTROL group (1.5-fold lower) and the aqueous herbal diet group (3.6-fold lower), accounting for less than 7% of the total intestinal microbiota. In the ileum, the aqueous herbal mixture diet increased the abundance of Helicobacter (20.8-fold) and Bacteroides (6.8-fold) compared with the CONTROL group. To date, Bacteroides has been found to be abundant in the cecal microbiota of chickens (Wei et al., 2013) and is known to provide nutrients for the host by metabolizing carbohydrates (Wexler, 2007), or by helping some (facultative) anerobic bacteria, including Escherichia coli, protecting against phagocytosis, to survive (Rotstein et al., 1989). Conversely, some other species of Bacteroides have been implicated in the pathogenesis of severe ulcerative diseases in humans and animals, including ulcerative colitis and Crohn’s disease (Wexler, 2007; Bloom et al., 2011).
It has been well established that several strains of lactobacilli exert a beneficial effect on their host’s intestinal health and performance (Huang et al., 2004). In particular, some selected lactobacilli present in the gut microbial communities of several animals, exhibit probiotic properties, including digestion capability, antagonistic activity against pathogenic microbes or the ability to inhibit the activity of pathogens (Musikasang et al., 2009; Panteli et al., 2021). The cecal abundance of Lactobacillus was slightly increased in the groups fed with aqueous (24.6%) and cyclodextrin (34.7%) herbal-supplemented diets compared with the CONTROL group (Figures 4B, F). This is in agreement with previous reports demonstrating that lactobacilli are more abundant in treatments with plant extracts (Li et al., 2018). Nevertheless, consistent with other studies (Placha et al., 2014; Wen et al., 2019), herbal extracts decreased the abundance of lactobacilli in the ileum studied herein.
The two genera of Akkermansia and Muribaculaceae showed a discernible increase in abundance in the diets with herbal mixture compared with the CONTROL group (Figures 4B, F). Another interesting finding is the fact that the cyclodextrin herbal mixture seemed to diminish the abundance of Campylobacter mostly in the ileum, but to a lesser extent in the caecum. Campylobacter bacteria are very diverse microorganisms, with a small genome size, and are often abundant in the environments surrounding poultry farms (Ellis-Iversen et al., 2012). As there is an urgent need to develop novel strategies to CONTROL the abundance of Campylobacter species across the poultry production chain, the addition of herbal extracts as feed additives for broiler chickens represents a promising approach. On the other hand, the Muribaculaceae family contains Gram-negative health-associated bacteria, initially reported by Lagkouvardos et al. (2019). Thus, diet supplementation with herbal extracts may also exert a beneficial impact on the intestinal microflora of broiler chickens.
Principal coordinates analysis also showed significant differences in microbial composition clusters among the three groups, with a distinct difference between the cecum and ileum (Figures 4C–F). At both the phylum and genus levels, ileal microbiota displayed the highest diversity in the aqueous herbal mixture group, while the lowest diversity was observed in the cyclodextrin herbal mixture group. A hierarchically clustered heatmap depicts the distinct difference in intestinal microbiota between the different groups. It is noteworthy that the aqueous herbal mixture formed a separate group in ileum, while the cyclodextrin herbal mixture in cecum, indicating that aqueous extracts are mainly active at proximal intestinal sites, up to the ileum, whereas cyclodextrin-encapsulated extracts are mostly active in the lower parts of the intestine, such as the cecum. Encapsulation could therefore be an effective form of administering herbal extracts, preventing their rapid breakdown to their metabolites and, consequently, their absorption in the first part of the intestinal tract.
The alpha-diversity, as indicated by the Chao1 index, the Shannon index, and the rarefaction of the observed species, highlighted greater species richness and diversity in cecal microbiota than in the ileal microbiota (Figure 5; Supplementary Table S3). Among cecal samples, the Chao1 diversity index revealed that community richness was substantially increased by diets containing herbal mixtures, but there was little difference between the groups fed diets supplemented with the two herbal mixtures in terms of the number of bacterial species (Figure 5A). In the ileum, no particular differences were observed in species richness between the different diet groups. Conversely, the Shannon and Simpson indices, which show species diversity within a group, were remarkably lower in the cyclodextrin herbal mixture diet group, indicating a biased community structure, with fewer dominant species present in the cecum (Figure 5B; Supplementary Table 3). This was consistent with previous results, which demonstrated that feeding with plant-derived extracts decreases diversity indices (Yin et al., 2017; Wen et al., 2019). A different pattern was evident regarding the ileum, in which species diversity and evenness were higher in the cyclodextrin herbal mixture diet group, indicating a higher abundance of dominant species.

Figure 5 Box plots of alpha-diversity of microbiota residing in the ileum and cecum of broiler chickens in the three different dietary groups, i.e., control, AQORGCC, and CDORGCC. (A) Chao1 value, (B) Shannon index, (C) Simpson index. AQORGCC, aqueous herbal extract diet group; CDORGCC, cyclodextrin herbal extract diet group; control, CONTROL group.
Beta-diversity was assessed by unweighted and weighted principal coordinates analysis (PCoA). Unweighted UniFrac considers the presence or absence of OTUs and therefore emphasizes rare or uncommon species, while weighted also weighs up the abundance of OTUs (Jovel et al., 2016). It was clear that the ileal microbiota clustered together to form a separate group from the cecal microbiota (Figure 6). The separation between the different diet groups was more apparent with regard to weighted UniFrac distances, with PCoA explaining 71.9% of the total variance. In the cecum, in particular, the cyclodextrin herbal mixture group showed distinct clustering, while in the ileum, distance between clusters was greater in the aqueous herbal mixture group than in the other two diet groups. Based on unweighted UniFrac distances, the inter-individual variation in microbial communities was higher in the ileum than in the cecum, suggesting that there is a greater presence of rare or uncommon species in this tissue. That is, while microbial communities were scattered in ileal samples, those of the cecum were relatively closely grouped. These results indicated a clear diversification in the bacterial communities of the cecum and ileum, as well as between the CONTROL group and herbal-supplemented diet groups, signifying that the clustering distances between samples were highly dependent not only on the tissue type, but also on the diet.
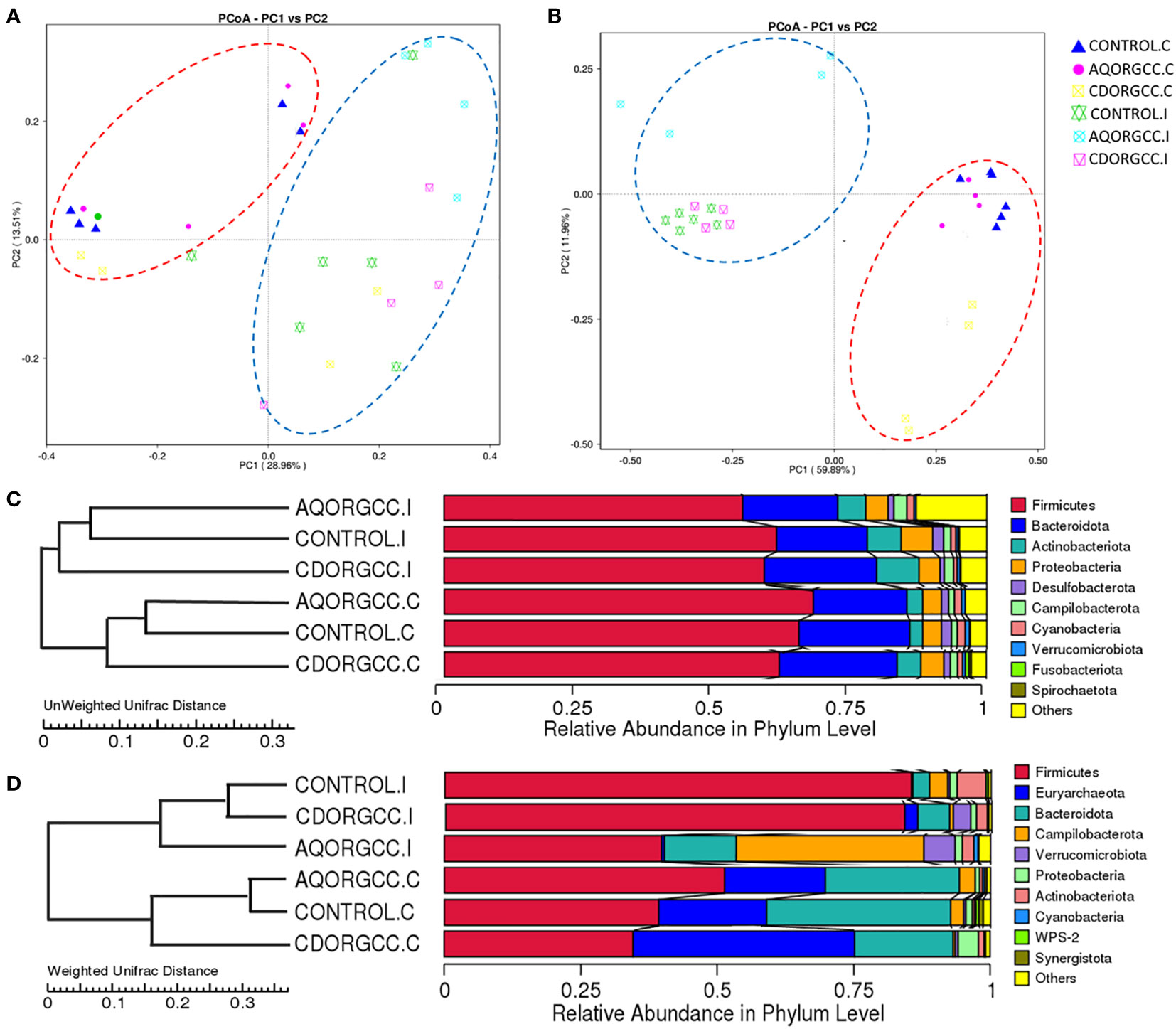
Figure 6 PCoA plots based on unweighted (A) and weighted (B) UniFrac distances of intestinal microbiota in the three different dietary groups, i.e., CONTROL, AQORGCC, and CDORGCC. The UPGMA cluster tree was based on the unweighted (C) and weighted (D) Unifrac distance of the relative abundance of each sample by phylum. The UPGMA cluster tree structure is presented on the left, while species relative abundance distribution at the phylum level for each sample is displayed on the right. C, cecum; I, ileum. AQORGCC, aqueous herbal extract diet group; CDORGCC, cyclodextrin herbal extract diet group; control, CONTROL group; UPGMA, unweighted pair-group method with arithmetic mean.
To investigate the similarity between different samples, we also performed a cluster analysis and constructed a sample cluster tree on the basis of unweighted (C) and weighted (D) UniFrac distances using the UPGMA method (unweighted pair-group method with arithmetic mean). The clustering results were integrated with a species relative abundance column chart at the phylum level for each sample. The phylogenetic tree showed that the bacterial communities were divided into two main clusters in a tissue-specific manner, with each cluster containing samples from all three diets (Figure 6C, D). The relatively small distance between the aqueous herbal mixture and CONTROL diet groups in the cecum, as well as between the cyclodextrin herbal mixture and CONTROL diet groups in the ileum, indicates that these groups have a similar microbial community structure. With regard to weighted UniFrac distances for ileal microbiota, it was obvious that the abundance, primarily of Campilobacterota, and secondly of Verrucomicrobiota, distinguished the aqueous herbal mixture diet group from the cyclodextrin herbal mixture and CONTROL groups, which seemed to be closely related. By contrast, by looking at the abundances of Euryarcheota and Proteobacteria in cecal microbiota, we were able to distinguish the cyclodextrin herbal mixture group from the other two diet groups. Conversely, the aqueous and the cyclodextrin herbal mixture diet groups had the farthest distance, and thus the largest community difference, regardless of the tissue examined.
A one-way analysis of similarity (ANOSIM) was performed to determine the differences in bacterial communities among the different diet groups (Figure 7). An R-value close to 1.0 suggests dissimilarity between groups, while an R-value close to 0 suggests an even distribution of high and low ranks “within” and “between” groups. The results of the ANOSIM test confirmed the structural dissimilarity between the ileal and cecal microbiota. In particular, for all groups, the R-value was very high (R > 0.8), showing that differences “within” groups were significantly greater than differences “between” groups (p-value of < 0.01). Regarding cecal microbiota, the AQORGCC (R = 0.825) or CDORGCC (R = 0.893) groups were both well separated from the CONTROL diet group. The results for ileal microbiota were similar, with R > 0.8, indicating a strong separation of the two herbal supplement groups from the CONTROL group.
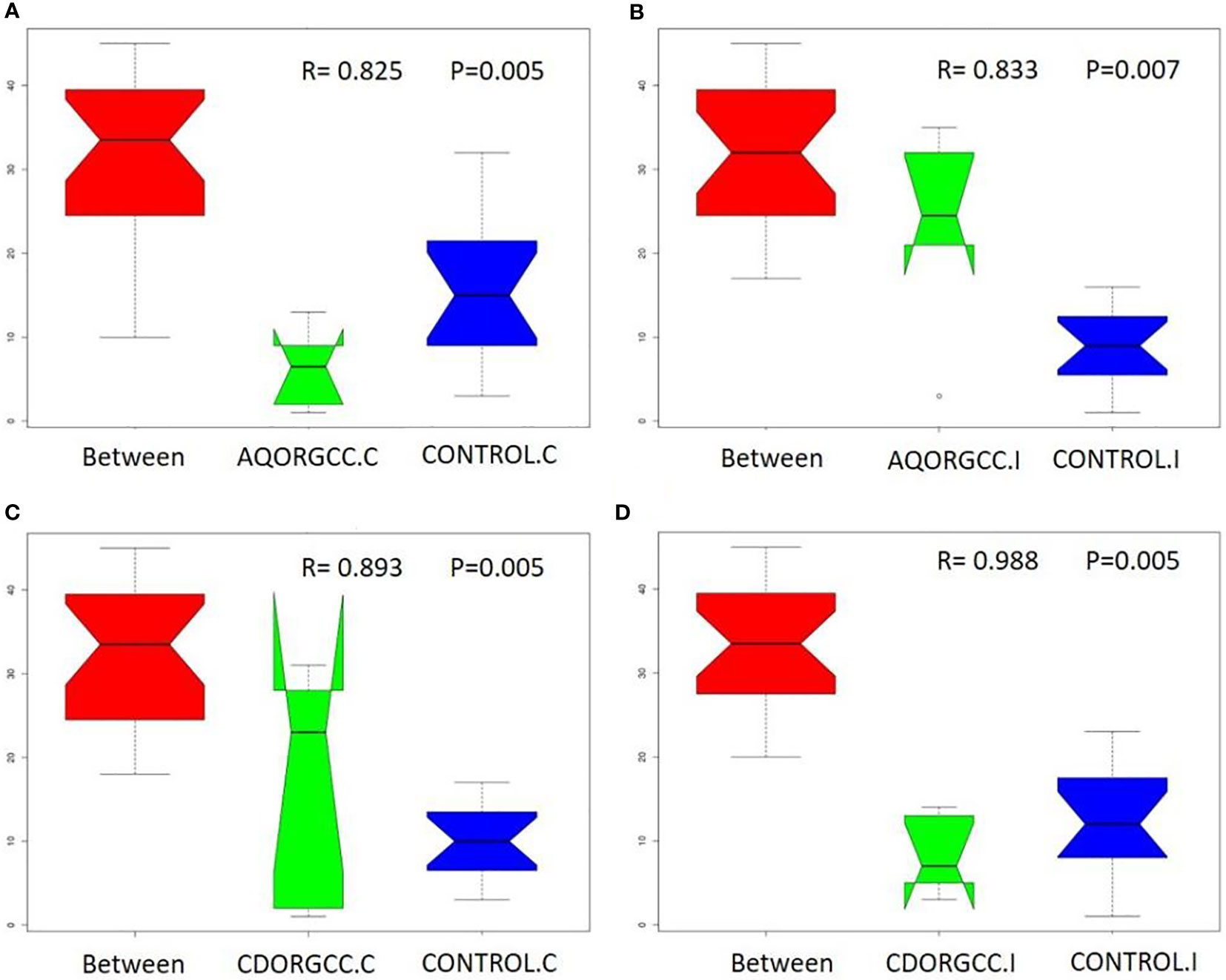
Figure 7 ANOSIM analysis of beta distance based on unweighted UniFrac distances of microbial communities in the three different dietary groups, i.e., (A) beta distance of AQORGCC.C and control.C; (B) beta distance of AQORGCC.I and CONTROL.I; (C) beta distance of CDORGCC.C and control.C; and (D) beta distance of CDORGCC.I and control.I “Between” refers to the comparison of the means of ranked dissimilarities between groups with the means of ranked dissimilarities within groups in each graph. The x-axis represents the “between” and “within” grouping information, and the y-axis represents the beta distance of “between” and “within” groups calculated by unweighted Unifrac. The R-value refers to the differences “between” and “within” groups, while the p-value represents the confidence level of the statistical analysis. ANOSIM, a one-way analysis of similarity; AQORGCC, aqueous herbal extract diet group; C, cecum; CDORGCC, cyclodextrin herbal extract diet group; control, CONTROL group; I, ileum.
Ternary plots were used to depict generalist (circles in the middle of the triangle) and group-specific (circles in the vertices) bacterial taxa in the three dietary groups (Supplementary Figure S1). At the genus level, we observed that Lactobacillus and Alistipes were generally present in cecal samples from all groups, and were thus considered as generalists. Both genera are generally considered beneficial to the host gut (Abe et al., 2012). One OTU identified as Bacteroides seemed to be specific for the CONTROL diet, while the abundance of Helicobacter seemed to distinguish the cyclodextrin herbal mixture diet group (very low abundance) from the other two diet groups (high abundance). Furthermore, in the cyclodextrin herbal mixture diet group, we observed high levels of Methanobrevibacter, whereas high levels of Faecalibacterium were observed in the other two diet groups. Members of the genus Faecalibacterium, which contains anerobic Gram-positive bacteria with immunoregulatory properties, are known to be able to produce short-chain fatty acids by fermenting dietary fiber in the host gut (Martín et al., 2017). In the ileum, Lactobacillus was generally abundant in the CONTROL and cyclodextrin herbal mixture diet groups, while Helicobacter seemed to be specific to the aqueous herbal mixture diet group.
The analysis of alpha-diversity indices in different dietary groups, as the initial step, revealed significant differences in the overall bacterial community structure between the various groups. As a further step, species accounting for the observed microbial diversification, thus representing potential group biomarkers, were detected using LefSe analysis. The results were plotted as a histogram of the distribution of LDA values among species (with a threshold of > 4), while a graph depicting phylogenetic distribution in the different groups is also provided (Figure 8). In the cecal microbiota of broiler chickens fed the CONTROL diet, the LDA scores of 10 taxa were greater than 4: the genera Bacteroides and Megamonas, and the species Bacteroides gallinaceum and Bacteroides uniformis. Similarly, based on LefSe analysis, the genera Methanobrevibacter and Streptococcus, and the genera Blautia, Faecalibacterium, Ruminococcus, and Romboutsia were associated with the cyclodextrin herbal mixture and the aqueous herbal mixture diet, respectively. Concerning the ileal microbiota, 37 taxa were found to be enriched in the different diets. In particular, the genera Lactobacillus and Corynebacterium were most abundant in the CONTROL diet, while Clostridium, Trichococcus, Parvibacter, Methanobrevibacter, and Weissella were most abundant in the cyclodextrin herbal mixture diet and, finally, Helicobacter and Campylobacter were most abundant in the aqueous herbal mixture diet and, finally, Helicobacter and Campylobacter most abundant in the aqueous herbal mixture diet. Overall, the number of biomarkers identified was slightly higher in the ileal than in the cecal microbial communities, as well as in the cyclodextrin herbal mixture of the ileum, compared to the other diets. The biomarker abundance comparison chart showing the difference in abundances among the dietary groups is presented in Supplementary Figure S2.

Figure 8 Significance analysis of species differences between groups. Histogram of LDA value distribution of species with significant differences in abundance between groups in the cecum (A) and in the ileum (B). Phylogenetic clade of species with significant differences in abundance between the different dietary groups in the cecum (C) and in the ileum (D) i.e., control, AQORGCC, and CDORGCC. AQORGCC, aqueous herbal extract diet group; C, cecum; CDORGCC, cyclodextrin herbal extract diet group; control, CONTROL group; I, ileum; LDA, linear discriminant analysis.
The incorporation of an extract derived from a mixture composed of Origanum vulgare subsp. hirtum, Allium sativum, Crithmum maritimum, and Camelina sativa in the diet of broiler chickens beneficially affected the antioxidative status and levels of protein carbonyls in breast meat samples by delaying lipid oxidation in raw and cooked samples, and this was seen at all time points except for day 3. In thigh samples, the CDORGCC extract delayed lipid oxidation only in cooked samples, and the AQORGCC extract resulted in lower protein oxidation than the CDORGCC extract. The dietary mixtures of herbal extracts sustained intestinal morphometry at different segments, which in turn suggests that they facilitate superior nutrient absorption. In addition, the dietary mixtures of herbal extracts increased levels of beneficial lactic acid-producing bacteria in the cecum. The two different intestinal tissues, that is the cecum and ileum, seemed to have distinct microbial communities, in terms of both composition and abundance. In the cecum, the abundance of lactobacilli, which exhibit probiotic properties, was enhanced with both herbal diets. On the other hand, in the ileum, the cyclodextrin herbal mixture decreased the abundance of the pathogenic Campylobacterota, whereas the aqueous herbal mixture decreased the abundance of Lactobacillus and increased the abundance of Helicobacter. Supplementation with herbal mixtures in both forms increased the abundances of the health-associated taxa of Akkermansia and Muribaculaceae, in turn highlighting the positive effect of herbal extract additives in the diets of broiler chickens. Moreover, the herbal mixture modulated Hsp induction and MAPK activation in a tissue-specific manner and displayed anti-apoptotic properties in breast tissue, whereas the herbal extract in the aqueous form exerted fewer detrimental effects with regard to stress proteins.
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found below: https://www.ncbi.nlm.nih.gov/, BioProject PRJNA871958.
The animal study was reviewed and approved by University Research Committee AUTH.
SS, SD, and IG performed the trial. IM performed the Microbiota analysis. IS performed the intestinal evaluation. NP and EA performed the stress protein tests. KG prepared and evaluated the tested herbs. JW, LJ, AT, and IAS evaluated the microbiota analysis. IG, LJ, and IAS acquired the research fund. All authors contributed to the article and approved the submitted version.
The authors declare that this study received funding from EU (European Regional Development Fund) and national Greek Funds in context of Operational Program “Competitiveness, Entrepreneurship and Innovation (EPAnEK)”, NSRF, Bilateral R&T Cooperation Greece-China 2014– 2020. Project Code: T7DKI-00313(MIS-5050735)- Acronym “Green Pro”. The funder was not involved in the study design, collection, analysis, and interpretation of data, the writing of this article or the decision to submit it for publication.
Author LJ was employed by Guangzhou Meritech Bioengineering Co. Ltd.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fanim.2023.1050170/full#supplementary-material
Supplementary Table 1 | Sequencing statistics and quality CONTROL of the acquired reads. Raw tags represents tags merged from paired-end reads; clean tags represents tags after filtering; effective tags represents tags after filtering chimera and can be finally used for subsequent analysis; AvgLen represents average length of effective tags; Q20 and Q30 are the percentages of bases whose quality value in effective tags is greater than 20 (sequencing error rate is less than 1%) and 30 (sequencing error rate is less than 0.1%); GC (%) represents GC content in effective tags.
Supplementary Table 2 | OTU identification and abundance table.
Supplementary Table 3 | Number of OTUs, number of observed species, and the alpha-diversity indices (Chao1, Shannon, Simpson) for each of the 28 samples. The total and average number of OTUs and average values of the alpha-diversity estimators are also shown.
Supplementary Figure 1 | Ternary plots reveal the relative abundance of OTUs (dot size) at (A, B) family and (C, D) genus levels among the three diet groups in the cecum and ileum. Generalist taxa are represented by circles in the middle of the triangle, whereas sample-specific bacterial taxa are represented by circles in the summit or along the edges of the triangle.
Supplementary Figure 2 | The relative biomarker abundance comparison chart showing the difference in abundances in the cecum and ileum among the three different diet groups.
Abe K., Ueki A., Ohtaki Y., Kaku N., Watanabe K., Ueki K. (2012). Anaerocella delicata gen. nov., sp. nov., a strictly anaerobic bacterium in the phylum bacteroidetes isolated from a methanogenic reactor of cattle farms. J. Gen. Appl. Microbiol. 58 (6), 405–412. doi: 10.2323/jgam.58.405
Ahn D. U., Olson D. G., Jo C., Love J., Jin S. K. (1999). Volatiles production and lipid oxidation in irradiated cooked sausage as related to packaging and storage. J. Food Sci. 64 (2), 226–229. doi: 10.1111/j.1365-2621.1999.tb15870.x
Anastasiou T. I., Mandalakis M., Krigas N., Vézignol T., Lazari D., Katharios P., et al. (2019). Comparative evaluation of essential oils from medicinal-aromatic plants of Greece: Chemical composition, antioxidant capacity and antimicrobial activity against bacterial fish pathogens. Molecules 25 (1), 148. doi: 10.3390/molecules25010148
Antoni L., Nuding S., Wehkamp J., Stange E. F. (2014). Intestinal barrier in inflammatory bowel disease. World J. Gastroenterol. 20 (5), 1165. doi: 10.3748/wjg.v20.i5.1165
Antonopoulou E., Chatzigiannidou I., Feidantsis K., Kounna C., Chatzifotis S. (2020). Effect of water temperature on cellular stress responses in meagre (Argyrosomus regius). Fish Physiol. Biochem. 46 (3), 1075–1091. doi: 10.1007/s10695-020-00773-0
Antonopoulou E., Chouri E., Feidantsis K., Lazou A., Chatzifotis S. (2017). Effects of partial dietary supplementation of fish meal with soymeal on the stress and apoptosis response in the digestive system of common dentex (Dentex dentex). J. Biol. Res. -Thessaloniki 24 (1), 1–10. doi: 10.1186/s40709-017-0071-1
Antonopoulou E., Kentepozidou E., Feidantsis K., Roufidou C., Despoti S., Chatzifotis S. (2013). Starvation and re-feeding affect hsp expression, MAPK activation and antioxidant enzymes activity of European Sea bass (Dicentrarchus labrax). Comp. Biochem. Physiol. 165 (1), 79–88. doi: 10.1016/j.cbpa.2013.02.019
Betancourt L., Phandanauvong V., Patiño R., Ariza-Nieto C., Afanador-Téllez G. (2012). Composition and bactericidal activity against beneficial and pathogenic bacteria of oregano essential oils from four chemotypes of origanum and lippia genus. Rev. Med. Vet. Zoot 59 (1), 21–31.
Bloom S. M., Bijanki V. N., Nava G. M., Sun L., Malvin N. P., Donermeyer D. L., et al. (2011). Commensal bacteroides species induce colitis in host-genotype-specific fashion in a mouse model of inflammatory bowel disease. Cell Host Microbe 9 (5), 390–403. doi: 10.1016/j.chom.2011.04.009
Bousdras T., Feidantsis K., Panteli N., Chatzifotis S., Piccolo G., Gasco L., et al. (2022). Dietary tenebrio molitor larvae meal inclusion exerts tissue-specific effects on cellular, metabolic, and antioxidant status in European Sea bass (Dicentrarchus labrax) and gilthead seabream (Sparus aurata). Aquac. Nutr. doi: 10.1155/2022/9858983
Brenes A., Roura E. (2010). Essential oils in poultry nutrition: Main effects and modes of action. Anim. Feed Sci. Technol. 158 (1-2), 1–14. doi: 10.1016/j.anifeedsci.2010.03.007
Broom L. J. (2018). Gut barrier function: effects of (antibiotic) growth promoters on key barrier components and associations with growth performance. Poult. Sci. 97 (5), 1572–1578. doi: 10.3382/ps/pey021
Caporaso J. G., Kuczynski J., Stombaugh J., Bittinger K., Bushman F. D., Costello E. K., et al. (2010). QIIME allows analysis of high-throughput community sequencing data. Nat. Methods 7 (5), 335–336. doi: 10.1038/nmeth.f.303
Cervato G., Carabelli M., Gervasio S., Cittera A., Cazzola R., Cestaro B. (2000). Antioxbdant properties of oregano (Origanum vulgare) leaf extracts. J. Food Biochem. 24 (6), 453–465. doi: 10.1111/j.1745-4514.2000.tb00715.x
Chamorro S., Viveros A., Rebolé A., Rica B. D., Arija I., Brenes A. (2015). Influence of dietary enzyme addition on polyphenol utilization and meat lipid oxidation of chicks fed grape pomace. Int. Food Res. J. 73, 197–203. doi: 10.1016/j.foodres.2014.11.054
Chandran D., Emran T. B., Nainu F., Sharun K., Kumar M., Mitra S., et al. (2022). Beneficial effects of dietary allium sativum (Garlic) supplementation on health and production of poultry: A mini-review. Toxicol. Rep. 9, 821–824.
Christaki E., Giannenas I., Bonos E., Florou-Paneri P. (2020). “Innovative uses of aromatic plants as natural supplements in nutrition,” in Feed additives. Eds. Florou-Paneri P., Christaki E., Giannenas I. (London, San Diego, CA: Academic Press), 19–34.
Christaki S., Moschakis T., Hatzikamari M., Mourtzinos I. (2022). Nanoemulsions of oregano essential oil and green extracts: Characterization and application in whey cheese. Food Control 141, 109190. doi: 10.1016/j.foodcont.2022.109190
Collins-Burow B. M., Antoon J. W., Frigo D. E., Elliott S., Weldon C. B., Boue S. M., et al. (2012). Antiestrogenic activity of flavonoid phytochemicals mediated via the c-jun n-terminal protein kinase pathway. cell-type specific regulation of estrogen receptor alpha. J. Steroid Biochem. Mol. Biol. 132 (1-2), 186–193. doi: 10.1016/j.jsbmb.2012.05.004
Cui K., Wang Q., Wang S., Diao Q., Zhang N. (2019). The facilitating effect of tartary buckwheat flavonoids and lactobacillus plantarum on the growth performance, nutrient digestibility, antioxidant capacity, and fecal microbiota of weaned piglets. Animals 9 (11), 986. doi: 10.3390/ani9110986
Dhibi M., Brahmi F., Mnari A., Houas Z., Chargui I., Bchir L., et al. (2011). The intake of high fat diet with different trans fatty acid levels differentially induces oxidative stress and non alcoholic fatty liver disease (NAFLD) in rats. Nutr. Metab. 8 (1), 1–12. doi: 10.1186/1743-7075-8-65
Diaz Carrasco J. M., Redondo E. A., Pin Viso N. D., Redondo L. M., Farber M. D., Fernandez Miyakawa M. E. (2018). Tannins and bacitracin differentially modulate gut microbiota of broiler chickens. BioMed. Res. Int. 7, 374. doi: 10.1155/2018/1879168
Dokou S., Vasilopoulou K., Bonos E., Grigoriadou K., Savvidou S., Stefanakis M., et al. (2023). Effects of dietary supplementation with phytobiotic encapsulated plant extracts on broilers’ performance parameters, welfare traits and meat characteristics. Ann. Anim. Sci.
Edgar R. C. (2013). UPARSE: highly accurate OTU sequences from microbial amplicon reads. Nat. Methods 10 (10), 996–998. doi: 10.1038/nmeth.2604
Edgar R. C., Haas B. J., Clemente J. C., Quince C., Knight R. (2011). UCHIME improves sensitivity and speed of chimera detection. Bioinformatics 27 (16), 2194–2200. doi: 10.1093/bioinformatics/btr381
Elazab S. T., Elshater N. S., Kishaway A. T., Ei-Emam H. A. (2021). Cinnamon extract and probiotic supplementation alleviate copper-induced nephrotoxicity via modulating oxidative stress, inflammation, and apoptosis in broiler chickens. Animals 11 (6), 1609. doi: 10.3390/ani11061609
Ellis-Iversen J., Ridley A., Morris V., Sowa A., Harris J., Atterbury R., et al. (2012). Persistent environmental reservoirs on farms as risk factors for campylobacter in commercial poultry. Epidemiol. Infect. 140 (5), 916–924. doi: 10.1017/S095026881100118X
Feidantsis K., Kaitetzidou E., Mavrogiannis N., Michaelidis B., Kotzamanis Y., Antonopoulou E. (2014). Effect of taurine-enriched diets on the hsp expression, MAPK activation and the antioxidant defence of the European sea bass (Dicentrarchus labrax). Aquac. Nutr. 20 (4), 431–442. doi: 10.1111/anu.12096
Feidantsis K., Soumalevris A., Panteli N., Chatzifotis S., Antonopoulou E. (2022). Synergistic effect of long-term feed deprivation and temperature on the cellular physiology of meagre (Argyrosomus regius). J. Therm. Biol. 105, 103207. doi: 10.1016/j.jtherbio.2022.103207
Figueiredo D., Gertler A., Cabello G., Decuypere E., Buyse J., Dridi S. (2007). Leptin downregulates heat shock protein-70 (HSP-70) gene expression in chicken liver and hypothalamus. Cell Tissue Res. 329 (1), 91–101. doi: 10.1007/s00441-007-0414-6
Fischer A. J., Scott M. A., Tuten W. (2009). Mitogen-activated protein kinase-signaling stimulates müller glia to proliferate in acutely damaged chicken retina. Glia 57 (2), 166–181. doi: 10.1002/glia.20743
Gautam N., Mantha A. K., Mittal S. (2014). Essential oils and their constituents as anticancer agents: a mechanistic view. BioMed. Res. Int. 2014, 154106. doi: 10.1155/2014/154106
Gava M. S., Moraes L. B., Carvalho D., Chitolina G. Z., Fallavena L. C. B., Moraes H. L. D. S., et al. (2015). Determining the best sectioning method and intestinal segment for morphometric analysis in broilers. Braz. J. Poult. Sci. 17, 145–149. doi: 10.1590/1516-635x1702145-150
Ghareeb K., Awad W. A., Böhm J., Zebeli Q. (2015). Impacts of the feed contaminant deoxynivalenol on the intestine of monogastric animals: poultry and swine. J. Appl. Toxicol. 35 (4), 327–337. doi: 10.1002/jat.3083
Giannenas I., Bonos E., Skoufos I., Tzora A., Stylianaki I., Lazari D., et al. (2018). Effect of herbal feed additives on performance parameters, intestinal microbiota, intestinal morphology and meat lipid oxidation of broiler chickens. Br. Poult. Sci. 59 (5), 545–553. doi: 10.1080/00071668.2018.1483577
Giannenas I. A., Florou-Paneri P., Botsoglou N. A., Christaki E., Spais A. B. (2005). Effect of supplementing feed with oregano and/or alpha-tocopheryl acetate on growth of broiler chickens and oxidative stability of meat. J. Anim. Feed Sci. 14 (3), 521. doi: 10.22358/jafs/67120/2005
Hendrick J. P., Hartl F. U. (1993). Molecular chaperone functions of heat-shock proteins. Annu. Rev. Biochem. 62 (1), 349–384. doi: 10.1146/annurev.bi.62.070193.002025
Huang M. K., Choi Y. J., Houde R., Lee J. W., Lee B., Zhao X. (2004). Effects of lactobacilli and an acidophilic fungus on the production performance and immune responses in broiler chickens. Poult. Sci. 83 (5), 788–795. doi: 10.1093/ps/83.5.788
Huang Y., Li W., Xu D., Li B., Tian Y., Zan L. (2016). Effect of dietary selenium deficiency on the cell apoptosis and the level of thyroid hormones in chicken. Biol. Trace Elem. Res. 171 (2), 445–452. doi: 10.1007/s12011-015-0534-x
Huang Z., Zhou L., Chen Z., Nice E. C., Huang C. (2016). Stress management by autophagy: Implications for chemoresistance. Int. J. Cancer 139 (1), 23–32. doi: 10.1002/ijc.29990
Hyldgaard M., Mygind T., Meyer R. L. (2012). Essential oils in food preservation: mode of action, synergies, and interactions with food matrix components. Front. Microbiol. 3, 12. doi: 10.3389/fmicb.2012.00012
Jäättelä M. (1999). Heat shock proteins as cellular lifeguards. Ann. Med. 31 (4), 261–271. doi: 10.3109/07853899908995889
Jiayong Z., Shengchen W., Xiaofang H., Gang S., Shiwen X. (2020). The antagonistic effect of selenium on lead-induced necroptosis via MAPK/NF-κB pathway and HSPs activation in the chicken spleen. Ecotoxicol. Environ. Saf. 204, 111049. doi: 10.1016/j.ecoenv.2020.111049
Jovel J., Patterson J., Wang W., Hotte N., O’Keefe S., Mitchel T., et al. (2016). Characterization of the gut microbiome using 16S or shotgun metagenomics. Front. Microbiol. 7, 459. doi: 10.3389/fmicb.2016.00459
Kalemba D. A. A. K., Kunicka A. (2003). Antibacterial and antifungal properties of essential oils. Curr. Med. Chem. 10 (10), 813–829. doi: 10.2174/0929867033457719
Khoso P. A., Zhang Y., Yin H., Teng X., Li S. (2019). Selenium deficiency affects immune function by influencing selenoprotein and cytokine expression in chicken spleen. Biol. Trace Elem. Res. 187 (2), 506–516. doi: 10.1007/s12011-018-1396-9
Kotzamanis Y., Fawole F. J., Brezas A., Kumar V., Fontanillas R., Antonopoulou E., et al. (2021). Dietary lysine requirement of greater amberjack juvenile (Seriola dumerili, risso, 1810). Aquac. Nutr. 27 (6), 2107–2118. doi: 10.1111/anu.13344
Kuczynski J., Stombaugh J., Walters W. A., González A., Caporaso J. G., Knight R. (2012). Using QIIME to analyze 16S rRNA gene sequences from microbial communities. Curr. Protoc. Microbiol. 27 (1), 1E–15. doi: 10.1002/9780471729259.mc01e05s27
Kumar K., Gupta S. M., Arya M. C., Nasim M. (2017). In vitro antimicrobial and antioxidant activity of camelina seed extracts as potential source of bioactive compounds. Proc. Natl. Acad. Sci. India. Sect. B 87 (2), 521–526. doi: 10.1007/s40011-015-0631-9
Kyriakis J. M., Avruch J. (2012). Mammalian MAPK signal transduction pathways activated by stress and inflammation: a 10-year update. Physiol. Rev. 92 (2), 689–737. doi: 10.1152/physrev.00028.2011
Lagkouvardos I., Lesker T. R., Hitch T. C., Gálvez E. J., Smit N., Neuhaus K., et al. (2019). Sequence and cultivation study of muribaculaceae reveals novel species, host preference, and functional potential of this yet undescribed family. Microbiome 7 (1), 1–15. doi: 10.1186/s40168-019-0637-2
Lambert R. J. W., Skandamis P. N., Coote P. J., Nychas G. J. (2001). A study of the minimum inhibitory concentration and mode of action of oregano essential oil, thymol and carvacrol. J. Appl. Microbiol. 91 (3), 453–462. doi: 10.1046/j.1365-2672.2001.01428.x
Levine B., Sinha S. C., Kroemer G. (2008). Bcl-2 family members: dual regulators of apoptosis and autophagy. Autophagy 4 (5), 600–606. doi: 10.4161/auto.6260
Liang D., Li F., Fu Y., Cao Y., Song X., Wang T., et al. (2014). Thymol inhibits LPS-stimulated inflammatory response via down-regulation of NF-κB and MAPK signaling pathways in mouse mammary epithelial cells. Inflammation 37 (1), 214–222. doi: 10.1007/s10753-013-9732-x
Li Y., Fu X., Ma X., Geng S., Jiang X., Huang Q., et al. (2018). Intestinal microbiome-metabolome responses to essential oils in piglets. Front. Microbiol. 9, 1988. doi: 10.3389/fmicb.2018.01988
Ling Z., Li Z., Liu X., Cheng Y., Luo Y., Tong X., et al. (2014). Altered fecal microbiota composition associated with food allergy in infants. Appl. Environ. Microbiol. 80 (8), 2546–2554. doi: 10.1128/AEM.00003-14
Liu J., Cui H., Liu X., Peng X., Deng J., Zuo Z., et al. (2012). Dietary high vanadium causes oxidative damage-induced renal and hepatic toxicity in broilers. Biol. Trace Elem. Res. 145 (2), 189–200. doi: 10.1007/s12011-011-9185-8
Liu J., Cui H., Peng X., Fang J., Zuo Z., Wang H., et al. (2013). Dietary high fluorine induces apoptosis and alters bcl-2, bax, and caspase-3 protein expression in the cecal tonsil lymphocytes of broilers. Biol. Trace Elem. Res. 152 (1), 25–30. doi: 10.1007/s12011-012-9595-2
Liu C. P., Fu J., Xu F. P., Wang X. S., Li S. (2015). The role of heat shock proteins in oxidative stress damage induced by Se deficiency in chicken livers. Biometals 28 (1), 163–173. doi: 10.1007/s10534-014-9812-x
Liu L. L., He J. H., Xie H. B., Yang Y. S., Li J. C., Zou Y. (2014). Resveratrol induces antioxidant and heat shock protein mRNA expression in response to heat stress in black-boned chickens. Poult. Sci. 93 (1), 54–62. doi: 10.3382/ps.2013-03423
Liu L., Zhao L., Liu Y., Yu X., Qiao X. (2022). Rutin ameliorates cadmium-induced necroptosis in the chicken liver via inhibiting oxidative stress and MAPK/NF-κB pathway. Biol. Trace Elem. Res. 200 (4), 1799–1810. doi: 10.1007/s12011-021-02764-5
Loo G. (2003). Redox-sensitive mechanisms of phytochemical-mediated inhibition of cancer cell proliferation. J. Nutr. Biochem. 14 (2), 64–73. doi: 10.1016/S0955-2863(02)00251-6
Lozupone C., Knight R. (2005). UniFrac: un nuevo método filogenético para comparar comunidades microbianas. Appl. Reinar. Microbiol. 71, 8228–8235. doi: 10.1128/AEM.71.12.8228-8235.2005
Magoč T., Salzberg S. L. (2011). FLASH: fast length adjustment of short reads to improve genome assemblies. Bioinformatics 27 (21), 2957–2963. doi: 10.1093/bioinformatics/btr507
Mahfuz S., Shang Q., Piao X. (2021). Phenolic compounds as natural feed additives in poultry and swine diets: A review. J. Anim. Sci. Biotechnol. 12 (1), 1–18. doi: 10.1186/s40104-021-00565-3
Mandalakis M., Anastasiou T. I., Martou N., Keisaris S., Greveniotis V., Katharios P., et al. (2021). Antibacterial effects of essential oils of seven medicinal-aromatic plants against the fish pathogen aeromonas veronii bv. sobria: To blend or not to blend? Molecules 26 (9), 2731. doi: 10.3390/molecules26092731
Martín R., Miquel S., Benevides L., Bridonneau C., Robert V., Hudault S., et al. (2017). Functional characterization of novel faecalibacterium prausnitzii strains isolated from healthy volunteers: a step forward in the use of f. prausnitzii as a next-generation probiotic. Front. Microbiol. 8, 1226. doi: 10.3389/fmicb.2017.01226
McMurdie P. J., Holmes S. (2013). Phyloseq: an r package for reproducible interactive analysis and graphics of microbiome census data. PloS One 8, e61217. doi: 10.1371/journal.pone.0061217
Meot-Duros L., Magné C. (2009). Antioxidant activity and phenol content of crithmum maritimum l. leaves. Plant Physiol. Biochem. 47 (1), 37–41. doi: 10.1016/j.plaphy.2008.09.006
Musikasang H., Tani A., H-kittikun A., Maneerat S. (2009). Probiotic potential of lactic acid bacteria isolated from chicken gastrointestinal digestive tract. World J. Microbiol. Biotechnol. 25, 1337–1345. doi: 10.1007/s11274-009-0020-8
Oliva-Teles A. (2012). Nutrition and health of aquaculture fish. J. Fish Dis. 35 (2), 83–108. doi: 10.1111/j.1365-2761.2011.01333.x
Panteli N., Mastoraki M., Lazarina M., Chatzifotis S., Mente E., Kormas K. A., et al. (2021). Configuration of gut microbiota structure and potential functionality in two teleosts under the influence of dietary insect meals. Microorganisms 9 (4), 699. doi: 10.3390/microorganisms9040699
Pan D., Yu Z. (2014). Intestinal microbiome of poultry and its interaction with host and diet. Gut Microbes 5 (1), 108–119. doi: 10.4161/gmic.26945
Partheniadis I., Karakasidou P., Vergkizi S., Nikolakakis I. (2017). Spectroscopic examination and release of microencapsulated oregano essential oil. ADMET DMPK 5 (4), 224–233. doi: 10.5599/admet.5.4.426
Patsoukis N., Zervoudakis G., Panagopoulos N. T., Georgiou C. D., Angelatou F., Matsokis N. A. (2004). Thiol redox state (TRS) and oxidative stress in the mouse hippocampus after pentylenetetrazol-induced epileptic seizure. Neurosci. Lett. 357 (2), 83–86. doi: 10.1016/j.neulet.2003.10.080
Placha I., Takacova J., Ryzner M., Cobanova K., Laukova A., Strompfova V., et al. (2014). Effect of thyme essential oil and selenium on intestine integrity and antioxidant status of broilers. Br. Poult. Sci. 55 (1), 105–114. doi: 10.1080/00071668.2013.873772
Puvača N., Kostadinović L., Popović S., Lević J., Ljubojević D., Tufarelli V., et al. (2015). Proximate composition, cholesterol concentration and lipid oxidation of meat from chickens fed dietary spice addition (Allium sativum, piper nigrum, capsicum annuum). Anim. Prod. Sci. 56 (11), 1920–1927. doi: 10.1071/AN15115
Quast C., Pruesse E., Yilmaz P., Gerken J., Schweer T., Yarza P., et al. (2012). The SILVA ribosomal RNA gene database project: improved data processing and web-based tools. Nucleic Acids Res. 41 (D1), D590–D596. doi: 10.1093/nar/gks1219
Reisinger N., Steiner T., Nitsch S., Schatzmayr G., Applegate T. J. (2011). Effects of a blend of essential oils on broiler performance and intestinal morphology during coccidial vaccine exposure. J. Appl. Poult. Res. 20 (3), 272–283. doi: 10.3382/japr.2010-00226
Rotstein O. D., Vittorini T., Kao J., McBurney M. I., Nasmith P. E., Grinstein S. (1989). A soluble bacteroides by-product impairs phagocytic killing of escherichia coli by neutrophils. Infect. Immun. 57 (3), 745–753. doi: 10.1128/iai.57.3.745-753.1989
Salaheen S., Kim S. W., Haley B. J., Van Kessel J. A. S., Biswas D. (2017). Alternative growth promoters modulate broiler gut microbiome and enhance body weight gain. Front. Microbiol. 8, 2088. doi: 10.3389/fmicb.2017.02088
Segata N., Izard J., Waldron L., Gevers D., Miropolsky L., Garrett W. S., et al. (2011). Metagenomic biomarker discovery and explanation. Genome Biol. 12 (6), 1–18. doi: 10.1186/gb-2011-12-6-r60
Seger R., Krebs E. G. (1995). The MAPK signaling cascade. FASEB J. 9 (9), 726–735. doi: 10.1096/fasebj.9.9.7601337
Sergeant M. J., Constantinidou C., Cogan T. A., Bedford M. R., Penn C. W., Pallen M. J. (2014). Extensive microbial and functional diversity within the chicken cecal microbiome. PloS One 9 (3), e91941. doi: 10.1371/journal.pone.0091941
Shirai Y., Kobayashi H., Ueda S., Soon Y. S., Kushiya A., Tsumagari R., et al. (2021). Protective effect of zingiber zerumbet smith extract on thermotolerance. Bioact. compd. Health Dis. 4 (1), 1–8. doi: 10.31989/bchd.v4i1.777
Singleton V., Orthofer R., Lamuela-Raventios R. M. (1999). Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent. Methods Enzymol. 299, 152–178. doi: 10.1016/S0076-6879(99)99017-1
Sobral M. V., Xavier A. L., Lima T. C., de Sousa D. P. (2014). Antitumor activity of monoterpenes found in essential oils. Sci. World J. 2014, 1-35. doi: 10.1155/2014/953451
Surai P. F., Kochish I. I. (2017). “Antioxidant systems and vitagenes in poultry biology: heat shock proteins,” in Heat shock proteins in veterinary medicine and sciences (Switzerland: Springer, Cham), 123–177.
Turnbaugh P. J., Ley R. E., Mahowald M. A., Magrini V., Mardis E. R., Gordon J. I. (2006). An obesity-associated gut microbiome with increased capacity for energy harvest. Nature 444 (7122), 1027–1031.
Tzora A., Giannenas I., Karamoutsios A., Papaioannou N., Papanastasiou D., Bonos E., et al. (2017). Effects of oregano, attapulgite, benzoic acid and their blend on chicken performance, intestinal microbiology and intestinal morphology. J. Poult. Sci. 54 (3), 218–227. doi: 10.2141/jpsa.0160071
Uni Z., Zaiger G., Gal-Garber O., Pines M., Rozenboim I., Reifen R. (2000). Vitamin a deficiency interferes with proliferation and maturation of cells in the chicken small intestine. Br. Poult. Sci. 41 (4), 410–415. doi: 10.1080/713654958
VAN Z. (1993). Germination and seedling development of two mistletoes, amyema preissii and lysiana exocarpi: Host specificity and mistletoe-host compatibility. Austral. Ecol. 18 (4), 419–429. doi: 10.1111/j.1442-9993.1993.tb00469.x
Vasilopoulos S., Dokou S., Papadopoulos G. A., Savvidou S., Christaki S., Kyriakoudi A., et al. (2022). Dietary supplementation with pomegranate and onion aqueous and cyclodextrin encapsulated extracts affects broiler performance parameters, welfare and meat characteristics. Poultry 1 (2), 74–93. doi: 10.3390/poultry1020008
Wang Q., Garrity G. M., Tiedje J. M., Cole J. R. (2007). Naive Bayesian classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. Appl. Environ. Microbiol. 73 (16), 5261–5267. doi: 10.1128/AEM.00062-07
Wang X., Li Y., Shen J., Wang S., Yao J., Yang X. (2015). Effect of astragalus polysaccharide and its sulfated derivative on growth performance and immune condition of lipopolysaccharide-treated broilers. Int. J. Biol. Macromol 76, 188–194. doi: 10.1016/j.ijbiomac.2015.02.040
Wang L., Zhang Y., Guo X., Gong L., Dong B. (2022). Beneficial alteration in growth performance, immune status, and intestinal microbiota by supplementation of activated charcoal-herb extractum complex in broilers. Front. Microbiol. 13, 856634–856634. doi: 10.3389/fmicb.2022.856634
Watson A. J., Pritchard D. M. (2000). VII. apoptosis in intestinal epithelium: lessons from transgenic and knockout mice. Am. J. Physiol. 278 (1), G1–G5. doi: 10.1152/ajpgi.2000.278.1.G1
Wei S., Morrison M., Yu Z. (2013). Bacterial census of poultry intestinal microbiome. Poult. Sci. 92 (3), 671–683. doi: 10.3382/ps.2012-02822
Wen C., Yan W., Sun C., Ji C., Zhou Q., Zhang D., et al. (2019). The gut microbiota is largely independent of host genetics in regulating fat deposition in chickens. ISME J. 13 (6), 1422–1436. doi: 10.1038/s41396-019-0367-2
Wexler H. M. (2007). Bacteroides: the good, the bad, and the nitty-gritty. Clin. Microbiol. Rev. 20 (4), 593–621. doi: 10.1128/CMR.00008-07
Wickramasuriya S. S., Park I., Lee K., Lee Y., Kim W. H., Nam H., et al. (2022). Role of physiology, immunity, microbiota, and infectious diseases in the gut health of poultry. Vaccines 10 (2), 172. doi: 10.3390/vaccines10020172
Xie J., Tang L., Lu L., Zhang L., Xi L., Liu H. C., et al. (2014). Differential expression of heat shock transcription factors and heat shock proteins after acute and chronic heat stress in laying chickens (Gallus gallus). PloS One 9 (7), e102204. doi: 10.1371/journal.pone.0102204
Yin D., Du E., Yuan J., Gao J., Wang Y., Aggrey S. E., et al. (2017). Supplemental thymol and carvacrol increases ileum lactobacillus population and reduces effect of necrotic enteritis caused by clostridium perfringes in chickens. Sci. Rep. 7 (1), 1–11. doi: 10.1038/s41598-017-07420-4
Zhao J., Xing H., Liu C., Zhang Z., Xu S. (2016). Effect of selenium deficiency on nitric oxide and heat shock proteins in chicken erythrocytes. Biol. Trace Elem. Res. 171 (1), 208–213. doi: 10.1007/s12011-015-0527-9
Zhu N., Wang J., Yu L., Zhang Q., Chen K., Liu B. (2019). Modulation of growth performance and intestinal microbiota in chickens fed plant extracts or virginiamycin. Front. Microbiol. 10, 1333. doi: 10.3389/fmicb.2019.01333
Keywords: herbal extract, apoptosis, stress response, broilers, intestinal morphometry, intestinal microbiota
Citation: Dokou S, Mellidou I, Savvidou S, Stylianaki I, Panteli N, Antonopoulou E, Wang J, Grigoriadou K, Tzora A, Jin L, Skoufos IA and Giannenas I (2023) A phytobiotic extract, in an aqueous or in a cyclodextrin encapsulated form, added in diet affects meat oxidation, cellular responses and intestinal morphometry and microbiota of broilers. Front. Anim. Sci. 4:1050170. doi: 10.3389/fanim.2023.1050170
Received: 21 September 2022; Accepted: 30 January 2023;
Published: 15 March 2023.
Edited by:
Erin E. Connor, University of Delaware, United StatesReviewed by:
Guofeng Han, Jiangsu Academy of Agricultural Sciences (JAAS), ChinaCopyright © 2023 Dokou, Mellidou, Savvidou, Stylianaki, Panteli, Antonopoulou, Wang, Grigoriadou, Tzora, Jin, Skoufos and Giannenas. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ilias Giannenas, aWdpYW5uZW5hc0B2ZXQuYXV0aC5ncg==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.