
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
BRIEF RESEARCH REPORT article
Front. Anim. Sci., 21 June 2022
Sec. Animal Physiology and Management
Volume 3 - 2022 | https://doi.org/10.3389/fanim.2022.914772
This article is part of the Research TopicAnimal Responses to Climatic Stress: Strategies for Coping with Harsh Climatic ConditionsView all 6 articles
 Kiriko Nakamura1
Kiriko Nakamura1 Ayumi Katafuchi1
Ayumi Katafuchi1 Saki Shimamoto2
Saki Shimamoto2 Goichiro Ogawa3
Goichiro Ogawa3 Nikhil Khandelwal4
Nikhil Khandelwal4 Kenji Tatsugawa5
Kenji Tatsugawa5 Yoshikazu Fujita5
Yoshikazu Fujita5 Akira Ohtsuka1
Akira Ohtsuka1 Daichi Ijiri1*
Daichi Ijiri1*We aimed to examine the effects of cyclical high ambient temperature (HT) and dried Neem (Azadirachta indica) leaf extract (DNE) supplementation on the growth performance, muscle lipid peroxidation level, and muscle drip loss of broiler chickens. Twenty-four 15-day old broiler chickens (Chunky strain ROSS 308) were divided into four treatment groups that were fed diets with or without 2.0% DNE under thermoneutral (25 ± 1°C) or cyclical HT (35 ± 1°C for 8 h/day) conditions. Supplementation of DNE did not affect the growth performance of the chicks, but HT reduced their feed intake, the weights of breast muscle and heart. In addition, supplementation with DNE ameliorated the negative effects of cyclical HT on feed intake and breast muscle mass. Furthermore, cyclical HT increased the malondialdehyde (MDA) concentration and drip loss over 48 h of storage of the breast muscle, and these effects were ameliorated by DNE. Collectively, we conclude that dietary supplementation with DNE reduces the muscle MDA concentration and drip loss of broiler chickens kept under HT conditions.
Air temperatures above the thermoneutral zone (defined as high ambient temperature, HT) induce environmental heat stress in animals. Since chickens lack sweat glands and have higher body temperatures, they are more vulnerable to heat stress than other domestic animals (Sahin et al., 2009). Under HT conditions, the generation of reactive oxygen species (ROS), such as singlet oxygen, hydrogen peroxide, and hydroxyl radicals, increases in various chicken tissues (Khan et al., 2012), causing the oxidation of lipids, proteins, and DNA, and impairing their function (Liu et al., 1999). Thus, heat is a major stressor for chickens, in which it causes a range of physiological alterations and marked reductions in growth performance, carcass yield, and meat quality (Whitehead and Keller, 2003; Zeferino et al., 2016).
The appearance is crucially important factor for initial selection by consumers and for final product satisfaction of poultry meat (Fletcher, 2002). Chicken muscle has a high polyunsaturated fatty acid in phospholipid of the subcellular membranes, and such the highly unsaturated phospholipid is considered to be easily undergone the oxidative degradation (i.e., lipid peroxidation) (Gray and Pearson, 1987). Therefore, chicken meat is considered to be more sensitive to oxidative deterioration during storage (Wilson et al., 1976; Igene and Pearson, 1979; Grashorn, 2007). The oxidation of these lipids in chicken meat deteriorates its quality (e.g., rancid odors, off-flavor development, drip loss, discoloration, loss of nutritional value, a decrease in shelf life, and the accumulation of toxic compounds) (Richards et al., 2002; Mapiye et al., 2012).
Neem (Azadirachta indica) has been used in traditional medicine (Saleem et al., 2018). However, since its leaves contain high concentrations of dietary fiber (Esonu et al., 2005; Bonsu et al., 2012), the dietary supplementation with 2.5%–7.5% of them resulted in a negative effect on the growth performance of chickens (Udedibie and Opara, 1998; Bonsu et al., 2012; Ubua et al., 2019). On the other hand, a dried extract of Neem leaves can be used in chicken feed without having negative effects on the growth performance or the serum biochemistry of the birds (Nnenna and Okey, 2013). Furthermore, Neem leaves have high antioxidant activities (Prakash et al., 2007; Heyman et al., 2017) due to high concentration of phenolic compounds, such as gallic acids and ferulic acids (Singh et al., 2005; Shewale and Rathod, 2018). In a previous study, we showed that dietary supplementation with an aqueous extract of dried neem leaves (DNE) at 2.0% increased the expression of genes encoding Cu/Zn superoxide dismutase, Mn superoxide dismutase, glutathione peroxidase 7, and catalase, and consequently reduced the drip loss and muscle lipid peroxidation of broiler chicken muscle (Nakamura et al., 2022).
In the present study, we examine the effects of dietary supplementation with DNE at 2.0% on the growth performance, meat yield, and meat quality (lipid peroxidation level and drip loss) of broiler chickens kept under prolonged HT conditions. To mimic realistic HT conditions, a cyclical HT environment (35 ± 1°C for 8 h/day) was created.
All the experimental protocols and procedures were approved by the Animal Care and Use Committee of Kagoshima University (approval number A22002). Twenty-four male broiler chicks (Chunky strain ROSS 308) at 1-day old were supplied from a commercial hatchery (Kumiai Hina Center, Kagoshima, Japan). The chicks were placed in a temperature-controlled room and provided with water and a commercial diet (23% crude protein, 3.1 Mcal/kg; Nichiwa Sangyou Company, Hyogo, Japan). On day 12, the chicks were housed individually in wire-bottomed aluminum cages (50 × 40 × 60 cm), and fed the basal diet (Table 1) for 3 days, until the start of the experimental period. The chicks were then randomly allocated to one of four groups using a 2 × 2 factorial design, with the main experimental factors being diet and ambient temperature. They were fed either the basal diet or the basal diet supplemented with 2.0% DNE, and exposed to either a thermoneutral environment (25 ± 1°C) or an HT environment (35 ± 1°C). DNE was prepared according to a method previously described (Nakamura et al., 2022). The experiment was conducted in a temperature-controlled room under 23 hours of light and 1 hour of darkness and at 50%–70% relative humidity. The chicks assigned to the HT treatment groups were kept at 35 ± 1°C for 8 h/day (9:00 AM to 17:00 PM) to mimic realistic summer condition. At 28 days of age, the chickens were weighed, anesthetized using carbon dioxide, and then killed by cervical dislocation. They were then dissected, and the masses of their breast muscles (pectoralis major muscles), leg muscles (thigh and drumstick), livers, hearts, and abdominal fat tissue depots were recorded.
Drip loss was determined according to the method described by Berri et al. (2008). The pectoralis major muscles were weighed immediately after dissection and then placed in a plastic bag and stored at 4°C for 48 hours. Afterwards, the breast muscles were wiped and weighed again. The difference in mass corresponded to the drip loss and was expressed as a percentage of the initial muscle mass.
MDA concentration is one of the most frequently used indicators of lipid peroxidation. To evaluate lipid peroxidation in chicken breast muscles, the MDA concentration was determined colorimetrically using 2-thiobarbituric acid reactive substance according to the method described by Ohkawa et al. (1979). Briefly, 0.3 g of each pectoralis major muscle was weighed and homogenized in 1 mL of 1.15% KCl and centrifuged at 20,000 × g for 5 minutes, then the supernatants were collected. Eighty microliters of each homogenate were mixed with 80 μL of 8.1% SDS, 220 μL of 20% acetic acid (pH 3.5), and 300 μL of 0.8% 2-thiobarbituric acid; the mixture was then vortexed, incubated at 95°C for 1 hour, and then transferred to ice. One milliliter of butanol-pyridine 15:1 (v/v) was then added to each sample, which was vortexed and centrifuged at 20,000 × g for 5 minutes. The absorbances of the supernatants (the butanol-pyridine layer) were measured using excitation at 535 nm and emission at 585 nm.
Data are presented as the mean ± standard error of the mean. The data were analyzed using two-way ANOVA, with individual comparisons being made using Tukey’s multiple comparison test. Correlation and regression analyses were performed using the General Linear Model procedure. These analyses were performed in R (R Core Team, Austria, 2020). Statistical significance was set at P < 0.05 for all the comparisons.
Under HT conditions, the oxidation of lipids, proteins, and DNA, impairing their function are occurred by increasing ROS generation in various chicken tissues (Liu et al., 1999; Khan et al., 2012). Therefore, ROS generation can have negative effects on the growth performance and meat quality of broiler chickens (Yunis and Cahaner, 1999; Zeferino et al., 2016). In this study, we confirmed that cyclical HT has significant effects on the final body mass, body mass gain, feed intake, and body temperature of broiler chicks (Table 2). In addition, although the leg muscle masses were not affected by the cyclical HT concitions, the breast muscle and heart masses of chickens kept under the HT conditions were lower (Table 2). These results are consistent with our previous findings (El-Deep et al., 2016; Shimamoto et al., 2020) and suggest that the cyclical HT conditions used in the present study (35°C for 8 h/day) cause the negative effects of heat stress that are commonly observed in broiler chickens (Yunis and Cahaner, 1999; Zeferino et al., 2016).
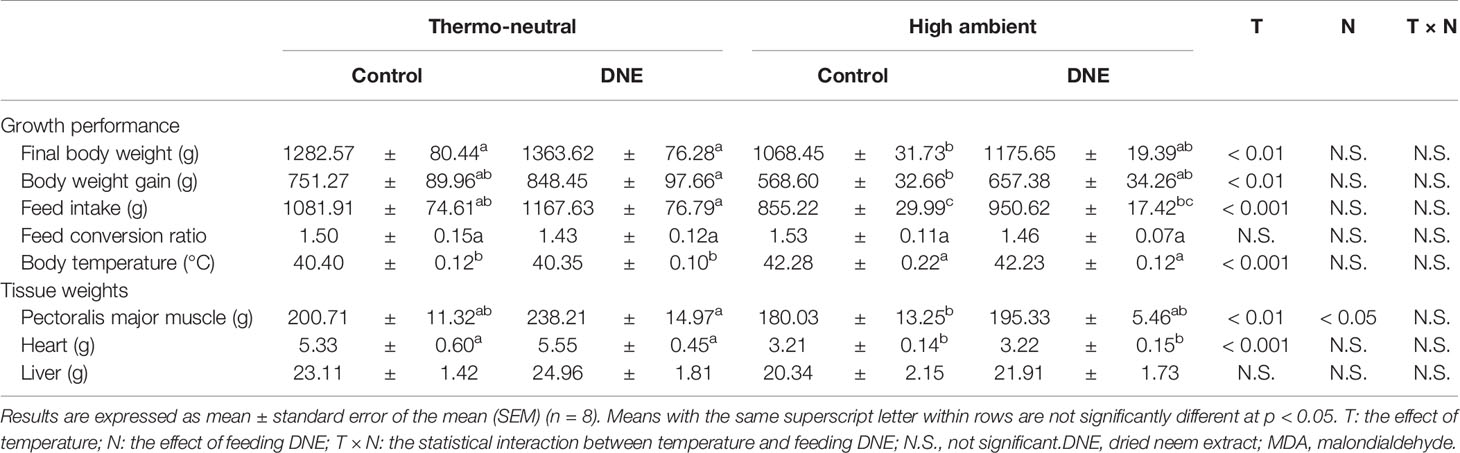
Table 2 Effects of dietary supplementation with DNE on growth performance and tissue weights of broiler chickens under high temperature conditions.
Although dietary supplementation with 2.0% of DNE did not affect the growth performance of the broiler chickens under thermoneutral conditions, it significantly affected their breast muscle mass. In addition, it ameliorated the negative effects of the cyclical HT. This might be explained by the high free radical-scavenging activity of the DNE (Nakamura et al., 2022). In our previous study, we found that dietary supplementation with 2.0% of DNE significantly increased the expression of genes encoding antioxidant enzymes and reduced the MDA concentration in the breast muscle of broiler chickens kept under thermo-neutral temperature (Nakamura et al., 2022). Consistent with this, in the present study, the MDA concentration was higher in the breast muscle of chickens kept under cyclical HT conditions than in that of chickens kept under thermoneutral conditions (Table 3). Dietary supplementation with DNE ameliorated the cyclical HT-induced increase in breast muscle MDA concentration, implying that the degradation of polyunsaturated fatty acids had been reduced. Consequently, dietary supplementation with DNE reduced muscle drip loss during storage (Table 3).
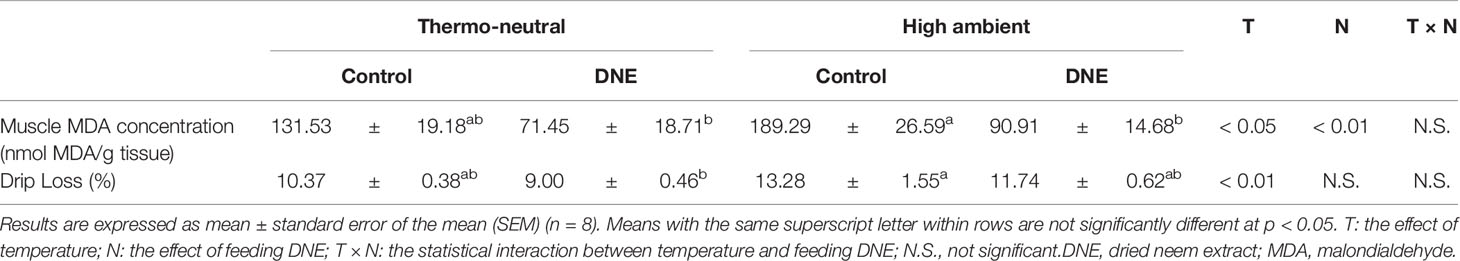
Table 3 Effects of dietary supplementation with DNE on MDA concentration and drip loss in the skeletal muscle of broiler chickens under high temperature conditions.
In conclusion, dietary supplementation with DNE ameliorates the negative effects of cyclical HT on the growth performance, meat yield, and meat quality (muscle lipid peroxidation and drip loss) from muscle of broiler chickens. Moreover, there was no significant difference, dietary supplementation with 2.0% of DNE increased body weight gain and decreased feed conversion ratio under either thermoneutral or the cyclical HT condition. And, dietary supplementation with 2.0% of DNE positively affected the weight of the pectoral muscle. Therefore, further studies on DNE are needed to determine its beneficial additive amount for broiler’s growth performance, meat yield and meat quality.
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
The animal study was reviewed and approved by The Animal Care and Use Committee of Kagoshima University.
KN, AK, SS, and DI conceived and designed the experiments. KN and AK performed the experiments. GO, NK, KT, and YF contributed reagents, materials, and analytical tools. KN, AK, SS, and DI wrote the paper. GO, NK, KT, YF, and AO reviewed and revised the paper. All authors contributed to the article and approved the submitted version.
This study was supported by a Grant-in-Aid for Scientific Research (C) (No. 19K06357) from The Japan Society for the Promotion of Science (JSPS) to DI.
Author GO is the president of Appli Co., Ltd, Japan. Author NK is a director of Bhaskar Herbaceuticals Pvt. Ltd. Authors KT and YF are employed by Shokkyo Co., Ltd.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
We are grateful to the Kagoshima Chicken Foods Company, Limited (Kagoshima, Japan) for the supply of broiler chicks. We thank Mark Cleasby, PhD from Edanz (https://jp.edanz.com/ac) for editing a draft of this manuscript.
Berri C., Besnard J., Relandeau C. (2008). Increasing Dietary Lysine Increases Final pH and Decreases Drip Loss of Broiler Breast Meat. Poult. Sci. 87, 480–484. doi: 10.3382/ps.2007-00226
Bonsu F. R. K., Kagya-Agyemang J. K., Kwenin W. K. J., Zanu H. K. (2012). Medicinal Response of Broiler Chickens to Diets Containing Neem (Azadirachta Indica) Leaf Meal, Haematology and Meat Sensory Analysis. World. Appl. Sci. J. 19, 800–805. doi: 10.5829/idosi.wasj.2012.19.06.827
El-Deep M. E., Ijiri D., Ebeid T. A., Ohtsuka A. (2016). Effects of Dietary Nano-Selenium Supplementation on Growth Performance, Antioxidative Status, and Immunity in Broiler Chickens Under Thermoneutral and High Ambient Temperature Conditions. J. Poult. Sci. 53, 274–283. doi: 10.2141/jpsa.0150133
Esonu B. O., Emenalom O. O., Udedibie A. B. I., Anyanwu A., Madu U., Inyang A. O. (2005). Evaluation of Neem (Azadirachta Indica) Leaf Meal on Performance, Carcass Characteristics and Egg Quality of Laying Hens. Inter. J. Agric. Rural. Dev. 6, 208–212. doi: 10.4314/ijard.v6i1.2611
Fletcher D. L. (2002). Poultry Meat Quality. Worlds Poult. Sci. J. 58, 131–145. doi: 10.1079/WPS20020013
Grashorn M. A. (2007). Functionality of Poultry Meat. J. Appl. Poult. Res. 16, 99–106. doi: 10.1093/japr/16.1.99
Heyman L., Houri-Haddad Y., Heyman S. N., Ginsburg I., Gleitman Y., Feuerstein O. (2017). Combined Antioxidant Effects of Neem Extract, Bacteria, Red Blood Cells and Lysozyme: Possible Relation to Periodontal Disease. BMC Complement. Altern. Med. 17, 399. doi: 10.1186/s12906-017-1900-3
Igene J. O., Pearson A. M. (1979). Role of Phospholipids and Triglycerides in Warmed-Over Flavor Development in Meat Model Systems. J. Food. Sci. 44, 1285–1290. doi: 10.1111/j.1365-2621.1979.tb06420.x
Khan R. U., Naz S., Nikousefat Z., Selvaggi M., Laudadio V., Tufarelli V. (2012). Effect of Ascorbic Acid in Heat-Stressed Poultry. Worlds Poult. Sci. J. 68, 477–489. doi: 10.1017/S004393391200058X
Liu D., Wen J., Liu J., Li L. (1999). The Roles of Free Radicals in Amyotrophic Lateral Sclerosis: Reactive Oxygen Species and Elevated Oxidation of Protein, DNA, and Membrane Phospholipids. FASEB J. 13, 2318–2328. doi: 10.1096/fasebj.13.15.2318
Mapiye C., Aldai N., Turner T. D., Aalhus J. L., Rolland D. C., Kramer J. K., et al. (2012). The Labile Lipid Fraction of Meat: From Perceived Disease and Waste to Health and Opportunity. Meat. Sci. 92, 210–220. doi: 10.1016/j.meatsci.2012.03.016
Nakamura K., Shishido M., Shimamoto S., Ogawa G., Khandelwal N., Tatsugawa K., et al. (2022). Effects of Supplementation With Dried Neem Leaf Extract on Lipid Peroxidation and Antioxidant Enzyme mRNA Expression in the Pectoralis Major Muscle of Broiler Chickens. J. Poult. Sci. 59, 75–80. doi: 10.2141/jpsa.0200120
Nnenna O. P., Okey A. A. (2013). Toxicity and Nutritional Assessment of Aqueous Azadirachta Indica (Neem) Leaf Extract in Broiler Chicks. Inter. J. Biosci. 3, 172–180. doi: 10.12692/ijb/3.6.172-180
Ohkawa H., Ohishi N., Yagi K. (1979). Assay for Lipid Peroxides in Animal Tissues by Thiobarbituric Acid Reaction. Anal. Biochem. 95, 351–358. doi: 10.1016/0003-2697(79)90738-3
Prakash D., Suri S., Upadhyay G., Singh B. N. (2007). Total Phenol, Antioxidant and Free Radical Scavenging Activities of Some Medicinal Plants. Inter. J. Food. Sci. Nutr. 58, 18–28. doi: 10.1080/09637480601093269
R Core Team (2020) R: A Language and Environment for Statistical Computing (Vienna, Austria: R Foundation for statistical computing). Available at: https://www.R-project.org/ (Accessed 25 March 2022).
Richards M. P., Modra A. M., Li R. (2002). Role of Deoxyhemoglobin in Lipid Oxidation of Washed Cod Muscle Mediated by Trout, Poultry and Beef Hemoglobins. Meat. Sci. 62, 157–163. doi: 10.1016/s0309-1740(01)00242-x
Sahin K., Sahin N., Kucuk O., Hayirili A., Prasad A. S. (2009). Role of Dietary Zinc in Heat Stressed Poultry: A Review. Poult. Sci. 88, 2176–2183. doi: 10.3382/ps.2008-00560
Saleem S., Muhammad G., Hussain M. A., Bukhari S. N. A. (2018). A Comprehensive Review of Phytochemical Profile, Bioactives for Pharmaceuticals, and Pharmacological Attributes of. Azadirachta indica. Phytother. Res. 32, 1241–1272. doi: 10.1002/ptr.6076
Shewale S., Rathod V. K. (2018). Extraction of Total Phenolic Content From Azadirachta Indica or (Neem) Leaves: Kinetics Study. Prep. Biochem. Biotechnol. 48, 312–320. doi: 10.1080/10826068.2018.1431784
Shimamoto S., Nakamura K., Tomonaga S., Furukawa S., Ohtsuka A., Ijiri D. (2020). Effects of Cyclic High Ambient Temperature and Dietary Supplementation of Orotic Acid, a Pyrimidine Precursor, on Plasma and Muscle Metabolites in Broiler Chickens. Metabolites 10, 189. doi: 10.3390/metabo10050189
Singh U. P., Maurya S., Singh D. P. (2005). Phenolic Acids in Neem (Azadirachta Indica) a Major Pre-Existing Secondary Metabolites. J. Herb. Pharmacother. 5, 35–43.
Ubua J. A., Ozung P. O., Inagu P. G. (2019). Dietary Inclusion of Neem (Azadirachta Indica) Leaf Meal can Influence Growth Performance and Carcass Characteristics of Broiler Chickens. Asian. J. Biol. Sci. 12, 180–186. doi: 10.3923/ajbs.2019.180.186
Udedibie A. B. I., Opara C. C. (1998). Responses of Growing Broilers and Laying Hens to the Dietary Inclusion of Leaf Meal From Alchornia Cordifolia. Anim. Feed. Sci. Technol. 71, 157–164. doi: 10.1016/S0377-8401(97)00120-X
Whitehead C. C., Keller T. (2003). An Update on Ascorbic Acid in Poultry. Worlds Poult. Sci. J. 59, 161–184. doi: 10.1079/WPS20030010
Wilson B. R., Pearson A. M., Shorland F. B. (1976). Effect of Total Lipids and Phospholipids on Warmed-Over Flavor in Red and White Muscle From Several Species as Measured by Thiobarbituric Acid Analysis. J. Agric. Food. Chem. 24, 7–11. doi: 10.1021/jf60203a040
Yunis R., Cahaner A. (1999). The Effects of Naked Neck (Na) and Frizzle (F) Genes on Growth and Meat Yields of Broilers and Their Interactions With Ambient Temperatures and Potential Growth Rate. Poult. Sci. 78, 1347–1352. doi: 10.1093/ps/78.10.1347
Keywords: Azadirachta indica, broiler chicken, heat stress, lipid peroxidation, Neem
Citation: Nakamura K, Katafuchi A, Shimamoto S, Ogawa G, Khandelwal N, Tatsugawa K, Fujita Y, Ohtsuka A and Ijiri D (2022) Effects of a Dried Neem Leaf Extract on the Growth Performance, Meat Yield and Meat Quality in Skeletal Muscle of Broiler Chickens Under High-Temperature Conditions. Front. Anim. Sci. 3:914772. doi: 10.3389/fanim.2022.914772
Received: 07 April 2022; Accepted: 16 May 2022;
Published: 21 June 2022.
Edited by:
Vishwajit S. Chowdhury, Kyushu University, Fukuoka, JapanReviewed by:
Hatem Eltahan, Agricultural Research Center, EgyptCopyright © 2022 Nakamura, Katafuchi, Shimamoto, Ogawa, Khandelwal, Tatsugawa, Fujita, Ohtsuka and Ijiri. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Daichi Ijiri, aWppcmlAYWdyaS5rYWdvc2hpbWEtdS5hYy5qcA==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.