- 1Animal Behaviour Group, Division of Animals in Science and Society, Faculty of Veterinary Medicine, Department of Population Health Sciences, Utrecht University, Utrecht, Netherlands
- 2Behaviour and Welfare Group (Formerly: Emotion and Cognition Group), Faculty of Veterinary Medicine, Department of Population Health Sciences, Utrecht University, Utrecht, Netherlands
- 3University Medical Center (UMC) Utrecht Brain Centre, Utrecht University, Utrecht, Netherlands
Animal welfare is a multifaceted issue that can be approached from different viewpoints, depending on human interests, ethical assumptions, and culture. To properly assess, safeguard and promote animal welfare, concepts are needed to serve as guidelines in any context the animal is kept in. Several different welfare concepts have been developed during the last half decade. The Five Freedoms concept has provided the basis for developing animal welfare assessment to date, and the Five Domains concept has guided those responsible for safeguarding animal welfare, while the Quality of Life concept focuses on how the individual perceives its own welfare state. This study proposes a modified and extended version of an earlier animal welfare concept - the Dynamic Animal Welfare Concept (DAWCon). Based on the adaptability of the animal, and taking the importance of positive emotional states and the dynamic nature of animal welfare into account, an individual animal is likely in a positive welfare state when it is mentally and physically capable and possesses the ability and opportunity to react adequately to sporadic or lasting appetitive and adverse internal and external stimuli, events, and conditions. Adequate reactions are elements of an animal’s normal behavior. They allow the animal to cope with and adapt to the demands of the (prevailing) environmental circumstances, enabling it to reach a state that it perceives as positive, i.e., that evokes positive emotions. This paper describes the role of internal as well as external factors in influencing welfare, each of which exerts their effects in a sporadic or lasting manner. Behavior is highlighted as a crucial read-out parameter. As most animals under human care are selected for certain traits that may affect their behavioral repertoire it is crucial to have thorough ethograms, i.e., a catalogue of specific behaviors of the species/strain/breed under study. DAWCon highlights aspects that need to be addressed when assessing welfare and may stimulate future research questions.
Introduction
Animal welfare is a complex issue that can be approached from different viewpoints. People engaged with animal care and welfare, or with animal management in the broad sense, such as veterinarians and animal scientists, (industrial) farmers, consumers of animal-derived products, owners of companion animals, zoo keepers, and game keepers, may assume their own concepts of what animal welfare is and how to safeguard and improve animal welfare. (Nordquist et al., 2017). A broad range of concepts of ‘animal welfare’ have been proposed (Bousfield and Brown, 2010). The goal to safeguard and improve animal welfare calls for unifying concepts that are theoretically sound, objectifiable and quantifiable. Concepts approaching animal welfare from different perspectives may help to identify relevant aspects that might be overlooked if only one view were used, i.e. they may help to extend our manner of assessing and improving animal welfare (Fraser, 2008). We agree with Rushen (2003) that in defining ‘animal welfare’, scientists often address a limited range of aspects that inadequately address the multivariate and multidimensional nature of animal welfare.
To incorporate the dynamic and multifaceted nature of animal welfare, we propose the Dynamic Animal Welfare Concept (DAWCon). This study first summarizes a number of well-established current concepts of animal welfare, such as the Five Freedoms (FAWC - Farm Animal Welfare Council, 1979a, FAWC - Farm Animal Welfare Council, 1979b), the Five Domains (Mellor, 2017), and the Quality of Life concept (Yeates and Main, 2009; Yeates, 2016). We then expand on the DAWCon and elaborate on its different aspects, comparing DAWCon with well-established welfare concepts and outlining its potential merits.
Concepts of animal welfare
The five freedoms concept
The welfare of intensively farmed animals has been the subject of investigations since the work of a committee, headed by Brambell et al., 1965, more than half a century ago. This work was seminal for the concept of the Five Freedoms put forward by the Farm Animal Welfare Council (FAWC) (1979) of the United Kingdom, which has since then been used as a guide to assess animal welfare, in particular on commercial farms (Brambell et al., 1965), forming the basis for a number of welfare assessment tools (e.g., Blokhuis et al., 2007; Veissier et al., 2008; Blokhuis et al., 2010).
Four of the five freedoms primarily concentrate on aspects of husbandry that potentially compromise welfare (1: freedom from hunger or thirst; 2: freedom from discomfort; 3: freedom from pain, injury or disease; 5: freedom from fear and distress). These freedoms largely neglect factors that might promote animal welfare (McCulloch, 2013), i.e. the Five Freedoms concept has received criticism (McCulloch, 2013; Cornish et al., 2016; Lawrence et al., 2018). The fourth freedom, namely “freedom to express normal behavior - by providing sufficient space, proper facilities and company of the animal’s own kind.” (FAWC - Farm Animal Welfare Council 1979a, 1979b; FAWC - Farm Animal Welfare Committee, 2013) is the only one that focuses on positive factors, taking account of animal’s perspective and the animal’s wants.
Removal of negative factors (e.g., hunger, thirst, pain, fear, and distress), as explicitly stated in the first, second, third and fifth freedoms, are believed to improve welfare. This assumption is challenged by the biological function of these negative states: they may help an animal to cope with its environment and to survive (Ohl and van der Staay, 2012). For example, the experience of pain evokes, in interaction with cognitive processes, certain behavioral reactions such as avoidance of pain inducing stimuli or protection of affected body parts and has as such a protective character (e.g., Rutherford, 2002). Similarly, the stress response aids the animal in regaining a state of normal biological functioning (Moberg, 2000). Thus, negative (emotional) reactions should be considered as an indicator of an animal’s adaptive capacity to avoid ‘negative welfare’ (Ohl and van der Staay, 2012). Even though negative experiences can temporarily be neutralized by applying (one of) the 5 freedoms, this hardly can be considered an improvement of welfare, as negative experiences form the basis for the animal’s motivation to obtain resources or to avoid e.g. pain-inducing stimuli. Animals should be given the opportunities to perform behavior they experience as rewarding (e.g. searching for food) (e.g., Mellor, 2016a). Moreover, the absence of factors considered as negative does not guarantee per se that the animal experiences good welfare.
The five domains concept
The Five Domains concept was originally formulated in 1994 to assess the impact of procedures on the welfare of experimental animals (Mellor and Reid, 1994). It reformulated the Five Freedoms into Five Domains, namely 1, thirst/hunger/malnutrition, 2, environmental challenge, 3, disease/injury/functional impairment, 4, behavioral/interactive restriction, and 5, anxiety/fear/pain/distress, to guide those responsible for safeguarding animal welfare, i.e., owners, animal care takers, wildlife managers, etc. The Five Domains concept can be applied to animals inside and outside the experimental context, is continuously being updated (Mellor, 2016a; Mellor, 2016b), emphasizes the importance of positive affective experiences (Mellor, 2015), and takes human-animal interactions into account (Mellor et al., 2020).
The notion of the importance of positive experiences, rather than the mere absence of negative experiences, has been a major driver in animal welfare research. Consequently, the individual animal’s perception of its welfare state has become an important research focus in recent years.
The quality of life concept
The Quality of Life concept is inspired by human psychology (Green and Mellor, 2011) and medicine (in particular in relation to mental health, Berlim and Fleck, 2003), focuses on how the individual perceives its own welfare state. In animals, Quality of Life takes the balance between negative and positive experiences into account. The preponderance of positive experiences increases Quality of Life, at the same time individual variation in the impact of certain experiences needs to be taken into consideration (McMillan, 2005). Yeates (2016) added the important notion that Quality of Life needs to be considered over time, as a sum of experiences made by the individual. Consequently, he suggests considering Quality of Life and Animal Welfare over time as synonyms.
A dynamic concept of animal welfare: welfare as a function of adaptation
The Faculty of Veterinary Medicine at Utrecht University in the Netherlands applies a concept of animal welfare (Ohl and Hellebrekers, 2009) developed by Ohl & van der Staay (2012). Building on previous animal welfare concepts, this concept states “An individual is in a positive welfare state when it is able to actively adapt to its living conditions and to reach a state that it perceives as positive.” (Ohl and Hellebrekers, 2009, p. 754; translated from Dutch). Integrating the dynamic aspect of adaptation and the concepts of the Five Freedoms, Five Domains, and Quality of Life, we previously proposed a conceptual approach to animal welfare stating that an individual is in a positive welfare state when it has “the freedom adequately to react to [conditions that potentially compromise welfare and] display normal behavioral patterns that allow the animal to adapt to the demands of the prevailing environmental circumstances and enable it to reach a state that it perceives as positive.” (Ohl and van der Staay, 2012, p. 17). Here we present and discuss a modified and extended version of this approach, the Dynamic Animal Welfare Concept (DAWCon):
An individual is likely in a positive welfare state when it is mentally and physically capable and possesses the ability and opportunity to react adequately to sporadic or lasting appetitive and adverse internal and external stimuli, events, and conditions. Adequate reactions are elements of an animal’s normal behavior. They allow the animal to cope with and adapt to the demands of the (prevailing) environmental circumstances, enabling it to reach a state that it perceives as positive, i.e., that evokes positive emotions.
The dynamics of the individual animal’s capacity to adequately cope and adapt to its environment is central to the concept of welfare. Whereas the adaptive capacity of an animal includes both positive and negative emotional responses, attention in the animal welfare discussion is mostly directed at ‘negative’ emotions. In DAWCon, the continuum between positive and negative welfare is considered; it recognizes that the animal must have (or must be provided) the freedom and capacity to react appropriately, i.e., adaptively, to both positive as well as potentially harmful (negative) stimuli. Within this framework, it is of utmost relevance to assessing whether an animal is able to fulfill the demands of the respective environmental circumstances, given the limits of the animals’ capacity to adapt (see Box 1; Figure 1) (Ohl and van der Staay, 2012).
Box 1
Graphical representation of the net effects of internal and external factors and of the limit of adaptability on an individual’s welfare state.
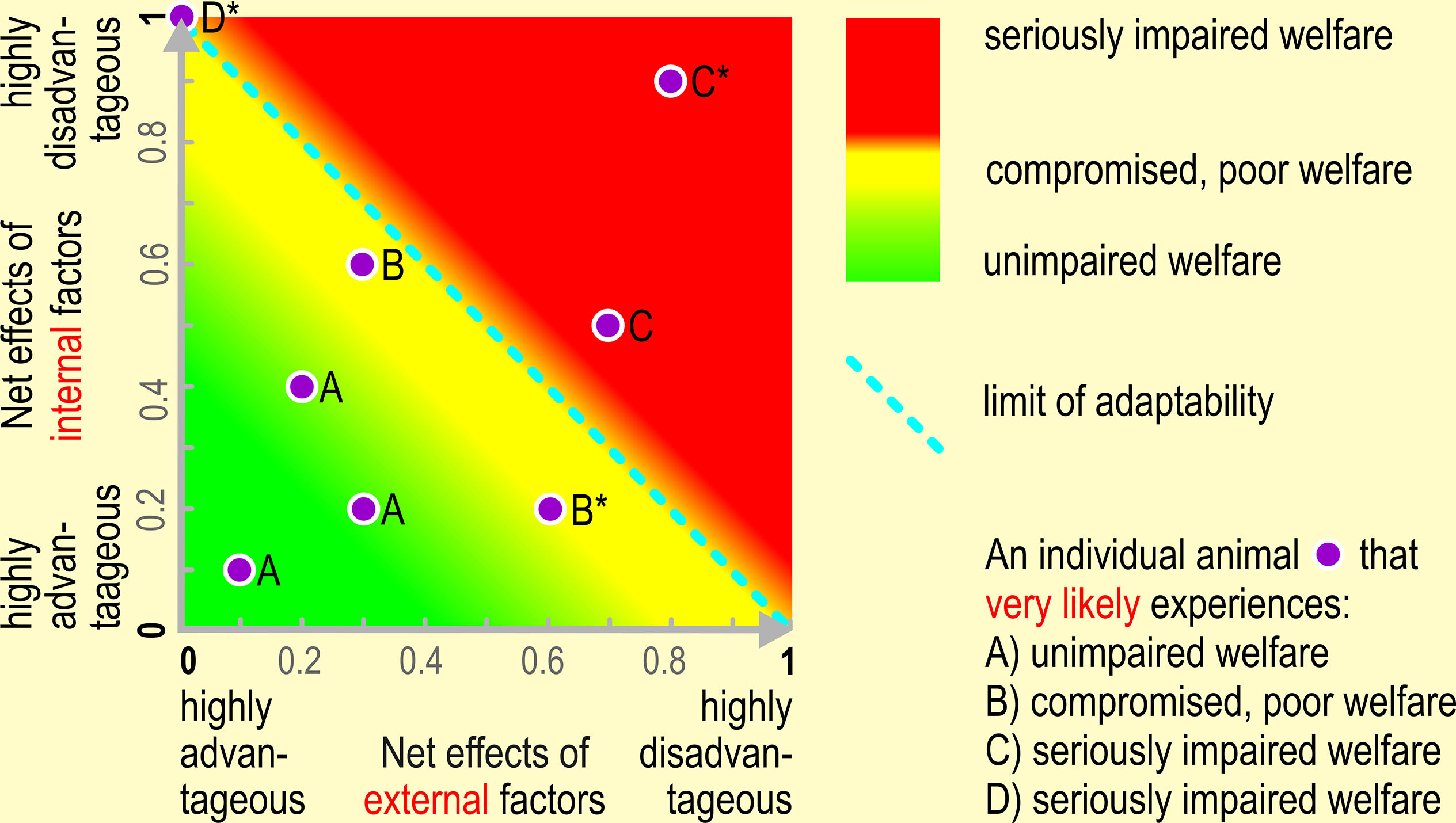
Figure 1 An animal must be able to adequately react and adapt to the net effect of internal and external factors in order to safeguard good welfare. The limit of adaptability does not dichotomously separate good from bad welfare. Instead, welfare may already be endangered or compromised/poor even if the limits of an animal’s adaptability have not yet been exceeded. The limits of adaptability, “a naturally selected design feature of the organism, extending within limits the range of circumstances under which it can survive and function” (Barnard and Hurst, 1996, p. 418) are set by the environment of evolutionary adaptation, or when the environmental demands exceed the regulatory range of allostatic mechanisms (Korte et al., 2007). The better the animal’s condition and the more advantageous the (prevailing) environmental circumstances, the more likely the animal is able to cope with and adapt to them. For illustration purposes, we chose an arbitrary scaling (0 –1) of the horizontal (x) and vertical (y) axis. Given this scaling of the x- and y-axis from value 0 (maximum advantageous net effects of the internal or external factors, respectively) to value 1 (maximum disadvantageous net effects of the internal or external factors), respectively, we assume that the net effect of internal and external factors are additive. In an earlier publication, we assumed a non-linear relationship, without explicitly defining the underlying relationship formally (Ohl and van der Staay, 2012, Figure 2). Additivity is the most simple conceivable relationship between the net effects of internal and external factors. However, further research might indicate that the relationship is much more complex. One of the aims of future research thus may be to determine appropriate scales, how the net effect of internal and external factors interact, and which relationship between these factors defines the limits of adaptability. Such an approach may become the basis for simulating the relationships between internal and external factors using appropriate formulas, and consequently, in the long run, to simulate the effects of manipulating internal and external factors on animal welfare. The insight derived from simulations must, however, be subjected to experimental scrutiny. The line (1.0), (0.1) defines the limit of adaptability, i.e. both the x- intercept and y-intercept have the scale value 1; If the sum of the net effects ≥ 1 (i.e. if the sum of the x- and y-coordinate ≥ 1) the animal’s limit of adaptation is exceeded, i.e. the animal is unable to adapt to its current condition. Examples: Animal B* (0.6, 0.2), sum score 0.8; below limit of adaptability. Note that the animal might experience compromised or poor welfare, even if it is still able to adapt to a certain degree; The animal might experience poorer welfare, the longer it must cope with conditions which are near its adaptation limit. Animal C* (0.8, 0.9), sum score 1.7; exceeding limit of adaptability. The animal is unable to adapt and will suffer from seriously impaired welfare Animal D* (0.0, 1.0), sum score 1.0; exceeding limit of adaptability. The highly disadvantageous net effects of the internal factors cannot be compensated anymore with even the most advantageous net effects of the external factors, and the animal will suffer from seriously impaired welfare. Also, this case should prompt those in charge of safeguarding animal welfare to think about, and eventually apply, a humane endpoint (Gauvin et al., 2018).
As the emotional state of an animal is the result of both negative and positive emotions, a lack of adaptation towards aversive stimuli may lead to either sensitization or generalization of such stimuli and may ultimately result in a dysfunctional, non-adaptive state (e.g. pathological anxiety, Salomons et al., 2010). An animal’s welfare may thus be compromised if the net impact of adverse internal or external factors challenges or exceeds the animal’s adaptability (Ohl et al., 2008) to the point that the animal is unable to adapt to the demands of the prevailing environmental circumstances. Consequently, it will not be able to reach a state that it perceives as positive.
Physiological and behavioral observations and measures might be aggregated into one or a few measures of the animal’s welfare (ranging from very poor to very good). This aggregate measure is supposed to roughly run in parallel with the animal’s adaptability (see examples in Box 1, Figure 1), i.e., conditions that do not exceed the animal’s adaptability are likely perceived as more favorable and more likely to induce a state that the animal perceives as positive. On the contrary, conditions that are close to an animal’s limit of adaptability, especially when exposure is frequent and/or long lasting and the animal is urged to invest highly in adaptation, or that exceed their ability to adapt most likely induce an emotional state that the animal perceives as (highly) negative (see Figure 2).

Figure 2 Perception of the inner (emotional) state and adaptability to internal and external factors. Left panel: An individual’s emotions and cognitive abilities and the resultant overt behavior and physiological reactions are controlled by its genotype, its environment, and the interaction of genotype and environment. We define welfare as a state that the animal perceives as positive (Ohl et al., 2009; Ohl and van der Staay, 2012). The perception of its inner state is determined by the interplay of positive and negative emotions and cognitive processes, where emotions may modulate cognition and vice versa (indicated by double-headed purple arrows: emotions modulate cognitive processes and vice versa). Cognitive or judgment bias tasks, for example, explicitly make use of the interaction between emotions and cognition to assess an animal’s emotional state (Bateson, 2016; Roelofs et al., 2016; Roelofs and van der Staay, 2017). Right panel: The net impact of internal and external factors in interaction with an animal’s specific genetic background, determine the adaptability needed by an individual. The limit of adaptability is, within certain bounds, dynamic and determined by, for example, hormonal changes, long-term effects of specific experiences, and, in social animals, the status/position in the group, herd, or flock.
In the following, we elaborate on the different aspects of DAWCon and how these are crucial to a state of good welfare.
The dynamic component in DAWCon
The proposed animal welfare concept considers the dynamics of the animal’s environment over time, of the animal’s behavior in response to changing environmental conditions, and the resulting interactions. The animal’s adaptive capacities and abilities, mental or physical, and opportunities [based on prevailing conditions provided by their environment (i.e., by their habitat or environment, or by humans who keep them)] are crucial for the success of these interactions. Coping with and adapting to these dynamics entails a continuous succession of positive and negative states [when, for example, the animal is limited in its coping abilities and/or opportunities to adapt. Such limitations might for example be due to (lasting) adverse health- and/or housing conditions that impede reaching a state perceived as positive (Ohl and van der Staay, 2012)] throughout the animals’ life.
Recognizing the dynamics of the animal interacting with a changing environment implies that acute negative states do not reflect negative or poor welfare per se (Browning and Veit, 2021). Negative states may, however, affect welfare in the long run through cumulative experience. Cumulative experience is defined as: “(…) the sum of all the events and effects including their quantity, intensity, duration, recovery and the way of amelioration, on the welfare of an animal over time.” (Pickard and members of the Animal Procedures Committee, 2013, p. 6). The animal’s mood state can therefore be seen as an integrative function of its acute emotional experiences over time (Nettle and Bateson, 2012).
As long as the animal can adequately react to negative states (e.g., through adequate coping with adverse and appetitive stimuli, events, and conditions and adaptation to those, or by being offered the opportunity to choose a positive alternative), welfare is not acutely at stake or might be compromised only transiently. Nevertheless, there might be long lasting consequences of acutely compromised welfare: the effects of these experiences on an animal’s emotional state might accumulate, if the animal cannot habituate, or if the time between adverse events does not allow the animal to recover (Pickard and members of the A P C, 2013; Bateson and Poirier, 2019). Acute states of severely compromised welfare may, as well, affect the animal’s future ability to cope with successive challenges, as in the case of experiences during sensitive phases (Lay Jr., 2020; Mason, 2000). Conclusions on an individual’s welfare can thus only be drawn based on measurements repeated over time. Measuring acute states, without any knowledge of how and why they evolved, does not allow us to draw robust conclusions on welfare. They may, however, provide information about the level of allostatic load and activation of the acute stress response.
Animal health and animal welfare
An individual is likely in a positive welfare state when it is mentally and physically capable and possesses the ability and opportunity …
Animal health is a physical condition that is a prerequisite for animal welfare (Broom, 2007; Animal health code commission, 2019). Compromised health conditions will limit the animals’ ability to adequately react to the demands of the (prevailing) environmental circumstances and are a source of pain and stress. Whether a positive welfare state, on the other hand, helps to improve physical and mental health remains an interesting topic for future research (Boyle et al., 2022).
Until today no universally accepted definition of (animal) health exists. Although explicitly referring to wildlife health, the definition proposed by Stephen (2014) proves useful in a broader context, as well. To adopt the definition for animal health in general, we replaced ‘wildlife health’ with ‘animal health’. Three features are emphasized: “1) health is the result of interacting biologic, social, and environmental determinants that promote and maintain health as a capacity to cope with change over time; 2) health cannot be measured solely by what is absent (i.e., lack of disease or hazards) but rather by characteristics of the animals and their ecosystem that affect their vulnerability and resilience to a suite of interacting social and environmental harms; and 3) animal health is not a biologic state but rather a dynamic human social construct based on social expectations and scientific knowledge.” (Stephen, 2014, pp. 429-430). Recently Huber and colleagues delineated health as “(…) resilience or capacity to cope and maintain and restore one’s integrity, equilibrium, and sense of wellbeing (…)”, or, in short. “(…) the ability to adapt and to self manage.” (Huber et al., 2011, p. 2). Note, that both definitions reflect a dynamic view of health in line with the DAWCon.
Reaction norms, robustness, and resilience
… to react adequately to …
Reaction norm curves are tools to detect gene by environment interactions in animal studies. A genotype may yield a different phenotype in a different environment [i.e., a Genotype by Environment (G x E) interaction], a relationship that can graphically be depicted in the norm of reaction or reaction norm curves (Fuller et al., 2005), where “(…) reaction norms refers to a set of phenotypes that can be produced by an individual genotype that is exposed to different environmental conditions.” (Schlichting and Pigliucci, 1998, p. 51) (see Figure 3). Reaction norm curves may be useful to assess the behavioral plasticity and adaptability of an animal and to guide the goals of breeding and selection programs, or of modifications in the animal’s environment to improve its welfare (see also paragraph Coping and adaptation). Reaction norms can be estimated by testing the phenotypic reaction of a genotype to known environmental covariates, and modeling the G x E interactions via reaction norm models (Oliveira et al., 2018; Chen et al., 2021). Knowledge about individual behavioral and physiological reaction norms could improve the efficiency of interventions aimed at promoting welfare (Linder et al., 2020).
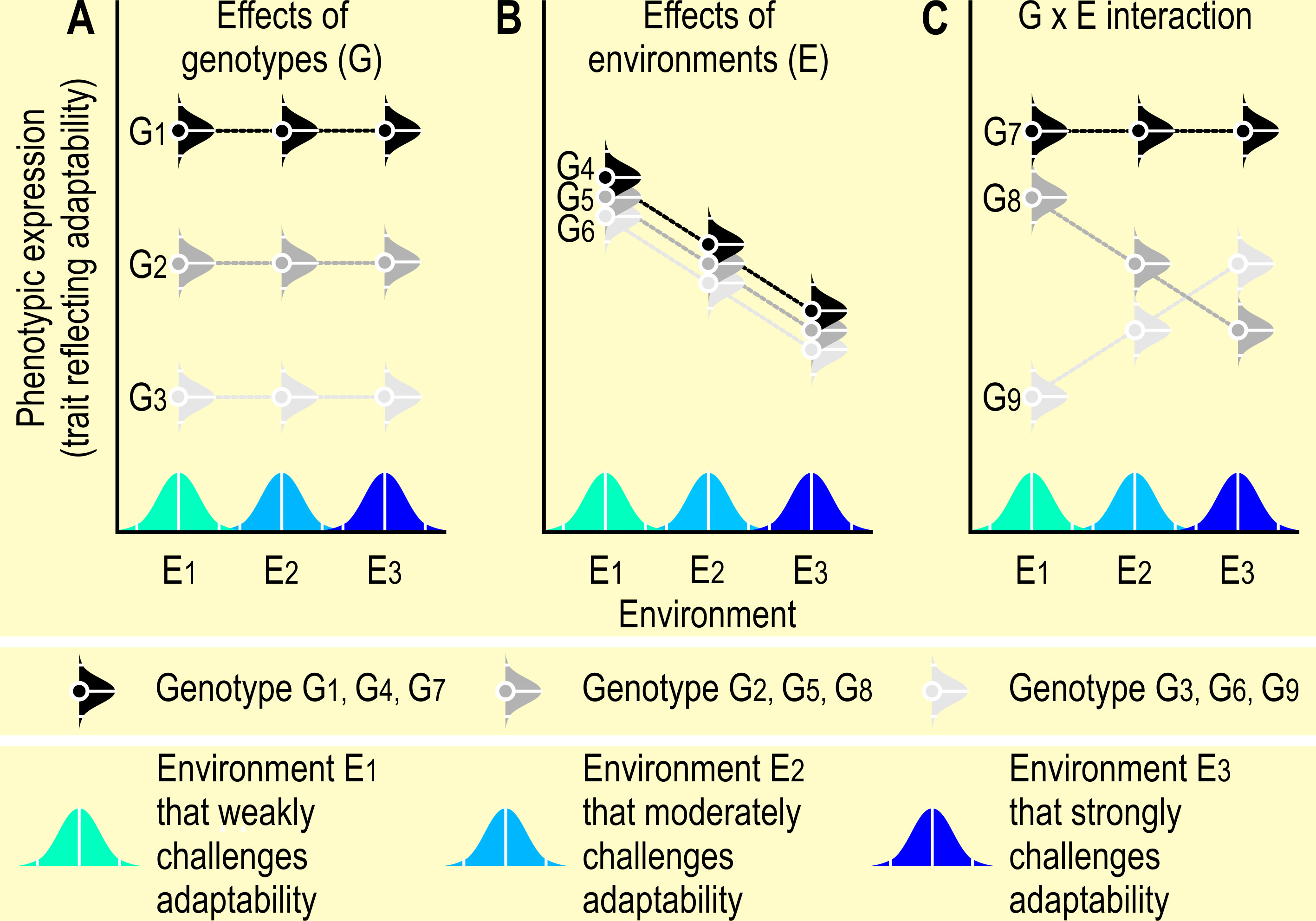
Figure 3 Reaction norm curves. Reaction norms are, the “set of phenotypes that a single genotype produces in a given set of environments” (Dingemanse et al., 2009, p. 81), i.e. they visualize the effects of environment (E1 –E3) on the phenotypic expression of a trait depending on genotype (G1 – G9). The bell-shaped curves in this figure indicate that the environments and the phenotypes are represented by a range of data around a mean environmental gradient (Voelkl and Würbel, 2016). In panel (A), phenotypic expression depends on genotype, whereas the different environments do not have a differential effect on the phenotype. The environments affect the phenotypic expression in panel (B), whereas genotype and environment interact in panel (C) (depicted as suggested by Fuller et al., 2005, p. 446, and inspired by S.M. Carr, https://www.mun.ca/biology/scarr/6390_Norm_of_Reaction.html). Note that in these examples, the plots show no change or a linear change across environments. However, the phenotypic expressions of a genotype across a range of environments may be non-linear. Also, the distributions of phenotypic expressions of a genotype may be narrower or wider than the standard normal distributions depicted in this graph. Consequently, knowledge of the reaction norms in one environment may not predict reaction norms in a different environment (Niemelä and Dingemanse, 2014).
Genetic diversity is one key to achieving the aim to safeguard the adaptability of animals to changing environmental conditions (Scherf, 2000). Even in captivity, animals may be exposed to dynamic changes across their lifetime, e.g., transport, social mixing, temperature changes, noise, outdoor access. Strict breed standards may lead to inbreeding, i.e. reduced genetic variation (Farrell et al., 2015). Unfortunately, a considerable number of pedigree breeds have a very small gene pool that inevitably leads to inbreeding (Collins et al., 2010; Leroy, 2011). Inbreeding may reduce the individual’s ability to adapt to various environmental appetitive and adverse conditions.
The animal’s resilience is “(…) the capacity (…) to cope with short-term perturbations in their environment and return rapidly to their pre-challenge status” (Colditz and Hine, 2016, p. 1961). Physiological and behavioral adaptations contribute to resilience and can be supported by cognitive processes (Parsons et al., 2016). The mechanisms enabling adaption to short-term perturbations – i.e., resilience – appear to differ from those enabling adaptation to lasting environmental conditions, i.e., robustness (Colditz and Hine, 2016).
Breeding for robustness is discussed especially in farm animals (Friggens et al., 2017). The effects of the environment may be reduced in robust animals, i.e., they may be able to adapt to a broad range of different environments (Figure 3A, in particular genotype G1 and G7, and to a lesser degree genotype G2, whereas genotype G3 only poorly adapts to any of the environments). Breeding for robustness can therefore be seen as breeding for a flat reaction norm (see Figure 3A), i.e. breeding for ‘generalists’ instead of ‘specialists’) (Strandberg, 2009). Generalists are animals that are able to adapt to a broad range of housing and management conditions, whereas specialists are selected to adapt to a very specific, narrow range of housing and management conditions. It is easier to define breeding goals for specialists than for generalists.
Internal and external factors
… to sporadic or lasting appetitive and adverse internal and external stimuli, events, and conditions. …
Animals are submitted to a large variety of exposure scenarios, comprising sporadic, acute, and lasting, chronic, factors in their environment. A factor might be considered as an element [event(s), condition(s)] that contributes to a particular result or situation. In animal welfare concepts (e.g., the Five Freedoms, FAWC - Farm Animal Welfare Council, 1979a, 1979b) and welfare research, the impact of adverse factors usually gains most attention, whereas appetitive and advantageous factors generally are all too often neglected, or only play a subordinate role. Animals will actively avoid adverse factors (e.g., noxious or punishing stimuli), and will actively approach/seek factors (e.g., appetitive stimuli) that are associated with or predict a positive outcome. Animal welfare may be improved by promoting the actions of appetitive factors (see, e.g., Baciadonna et al., 2018). Appetitive factors may neutralize, compensate for, or ameliorate the effects of adverse factors. In addition to removing the impact of adverse factors, promoting and introducing appetitive factors may be a strong strategy to improve animal welfare. Enhancing the predictability of the environment, and providing the animal with control may add appetitive aspects to its environment, even when exposed to stressors (Weiss, 1972). Thus, promoting appetitive factors may shift the net effects of appetitive and adverse factors to positive values more effectively compared to removing adverse factors. Consequently, in welfare evaluations and in strategies to improve welfare, the impact and potential of both appetitive and adverse factors and their interactions needs to be scientifically inventoried to be better understood (see, e.g., Krebs et al., 2018 as a recent example). Future research should focus on the potential of appetitive factors for improving animal welfare.
In terms of the internal factors that are determined by genetics, health and physiological characteristics, animals are also exposed to manifold external factors in their physical and social environment (Mellor et al., 2020). These factors comprise sporadic stimuli and events, as well as longer lasting conditions. The actions of lasting adverse internal and external factors create the basic frame conditions for an animal’s welfare (see Tables 1A, B for examples). They may determine the range of appetitive and adverse conditions to which an animal can adapt. Effects of sporadic adverse factors may add to the lasting ones. The net effects of lasting and sporadic appetitive and adverse actions determine the position of an individual as depicted in Figure 1. In some instances, an animal will already be unable to adapt due to the effects of lasting and/or frequently present adverse internal and external factors. In other instances, it will exceed its limit of adaptability through the additional action of sporadic adverse factors. The effects of lasting adverse factors should be visible on each successive welfare assessment time point (repeated welfare assessment), whereas the impact of sporadic adverse factors may be missed more easily or only be detected on a subset of the measurement timepoints (see also Assessing animal welfare based on DAWCon). External factors are usually under stronger control of the animal keeper and welfare improving actions may be realized easier and faster than if animal welfare is compromised by adverse internal factors.

Table 1 Examples of appetitive and adverse internal and external factors and their potential lasting and sporadic consequences for animal welfare.
It is, however, not always unambiguously possible to classify a factor as internal or external, and its impact as sporadic or lasting (Tables 1A, B). External conditions may lead to an acute response, but its impact may lead to lasting consequences in internal conditions. For example, inadequate socialization of a dog pup due to neglect by the keeper may be considered an adverse external factor. If, however, the puppy cannot properly be socialized (e.g., due to its anxious personality based on perinatal G x E interactions), this may be considered as an adverse internal factor. In both instances, the impact of the failed socialization is most likely lasting.
Another example might be highly aggressive behavior. If a dog is selected from an aggressive breed as a fighting dog and trained to behave highly aggressively (e.g. towards animals or other people) (Harding, 2013), meaning it is unable to act appropriately in social situations, this may be considered the result of both adverse internal and external factors. However, highly aggressive behavior can also be due to medical causes, such as pain or neurological disorders (Luescher and Reisner, 2008) and might then be considered as an internal factor. Also, here, in both instances, the impact is most likely lasting.
Changes in the perception of pain may serve as yet another example. An injection, for example for vaccination, must be seen as an external, pain inducing factor and its impact is most likely sporadic. However, due to medical conditions such as infections or due to gene defects or cognitive decline a normally non-painful factor might be perceived as painful (e.g., Tracey and Mantyh, 2007) by the animal and may then be seen as an internal factor. In the latter instance, the impact is most likely lasting.
In research on human health and wellbeing, the realization of the importance of a holistic life-course approach has led to the formulation of the exposome concept. The exposome represents the non-genetic drivers of health and disease across the life-course of an individual (Wild, 2005; Wild, 2012). Miller and Jones (2014, p. 2) formulated a broad definition of the exposome as “the cumulative measure of environmental influences and associated biological responses throughout the lifespan, including exposures from the environment, diet, behavior, and endogenous processes.” The exposome approach, which aims “(…) to provide a neutral description of the totality of an individual’s non-genetic exposure that can then be used to identify those specific exposures associated with well-being, health and disease.” (Bateson and Poirier, 2019, p. 42) shows similarities with the DAWCon concept, as it considers the life-course cumulative effect of factors influencing welfare and addresses the external and internal factors related to welfare in a system science approach (Kalia et al., 2020). Both the exposome and DAWCon concept, bridge the role of the environment in health over multiple continua including: from populations to individuals, from external to internal environments, from discrete exposures to life course, and from single stressors to multiple determinants.
It must be noted though, that the concepts of internal and external factors (Ohl and van der Staay, 2012; Mellor, 2017) and the concepts of internal and external exposomes (Zhang P. et al., 2021) are not fully congruent. For example, in Zhang’s schematic overview of internal versus external exposomes, stress belongs to the external exposomes (see Zhang P. et al., 2021, Figure 1, p. 840). Referring to external stressors inducing internal stress, or more appropriate, an internal stress response, would align more with the classification of internal and external factors in DAWCon. While also the exposome framework places factors into domains of internal and external, a sharp distinction cannot always be made (Wild, 2012). Therefore, in research on animal welfare, internal and external factors must be explicitly defined, using the DAWCon concept, eventually in combination with an exposome approach (Zhang H. et al., 2021). Although we chose to assume a simple relationship between the net effects of internal and external factors, further research eventually might prove that this relationship is far more complex. Therefore, we encourage future research to determine appropriate measurement scales, the manner in which the net effect of internal and external factors interact, and the exact position and shape of the line symbolizing the limits of adaptability.
Natural, innate, and normal behavior and the impact of domestication
Adequate reactions are elements of an animal’s normal behavior…
The ‘freedom to express natural behavior’ is often stressed as an important aspect of good welfare (Bracke and Hopster, 2006) or considered as fundamental for good animal welfare (see, Yeates, 2018; Browning, 2019). The concepts of natural and normal behaviors (Segerdahl, 2007; Yeates, 2018) are, however, not self-evident and natural behavior is difficult to define (Learmonth, 2019; for an in depth discussion of this concept see Segerdahl, 2007; Lerner, 2008). In the literature, the terms natural, innate, and normal behavior have been used interchangeably. They refer to behaviors that are inherent to animals and are considered to be components of an animal’s biological functioning as key issues of animal welfare (Dawkins, 2003).
Natural behaviors develop to enable an animal to adapt to the challenges of the natural environment. Animals are believed to be highly motivated to perform these behaviors which may help to reduce or to avoid types of stress that lead to deterioration of the animals’ adaptive capacities (i.e. distress, Kupriyanov and Renad Zhdanov, 2014). Recently, Yeates (2018) criticized that Bracke and Hopster (2006) limited their definition of natural behaviors to pleasurable behaviors, which may not reflect the entire spectrum of natural behavior animals are motivated to perform.
Yeates (2018) went even further and proposed to define natural behavior as behavior that is ‘unaffected by man’. According to Veasey and colleagues, the behavior of wild animals, i.e., “(…) ‘the behaviour expressed by an animal subject to environmental and evolutionary pressures with minimal human intervention’, is often used as a bench mark by which the welfare of captive animals can be assessed.” (Veasey et al., 1996, p. 13). This approach may, however, not be applicable to all captive animals. The environment in which domesticated animals are kept may substantially deviate from the environment in which the wild ancestor evolved (see also Figure 5A), and the animal may no longer be equipped with the behavioral repertoire to appropriately face environmental challenges (e.g., through the housing and management system).
Farm and companion animals are usually the result of strong genetic selection and continuous human interventions (in particular with respect to management and housing conditions, Nordquist et al., 2017), i.e., are the product of domestication. Domestication is the process of adaptation of the animal to live near/with humans (companion animals), or under the housing and management conditions created by humans (farm animals). According to Price (1999, 1984) “(…) domestication is defined as that process by which a population of animals becomes adapted to man and to the captive environment by some combination of genetic changes occurring over generations and environmentally induced developmental events reoccurring during each generation.” (Price, 1984, p. 3). Domestication may strongly affect the behavioral repertoire of an animal (Rooney and Bradshaw, 2014), i.e., domestication may alter the animal’s behavioral repertoire in response to the selection pressure (Fraser et al., 1997). Špinka (2006) incorporates the impact of domestication and states that an animal’s natural behavioral repertoire consists of “(…) behavioral elements and behavioral sequences that have evolved either during the evolution of the species or during its domestication ‘in order’ to increase the fitness (…) of the behaving animal.” (Špinka, 2006, p. 118). Nevertheless, domesticated animals may still possess (most of) the behavioral repertoire of their wild ancestors (e.g. pigs, Stolba and Wood-Gush, 1989).
Given the difficulty to assess the effects of domestication on animal behavior, we suggest using the term ‘normal behavior’ instead of ‘natural behavior’ for domesticated animals. Also, according to Browning (2019), “Those who advocate natural behavior appear to be using a “teleological” conception of welfare, in which naturalness is considered fundamental to welfare, outside of its effects in other areas. Others are instead considering animal welfare from a subjective standpoint – that is, consisting of the positive experience of life by the animal, and where only those factors that affect this experience are important in determining welfare.” (2019, p. 328).
Normal behaviors such as dustbathing and perching in chickens and rooting in pigs are innate, and “(…) are driven by internal factors, and are internally and physiologically regulated (…)” (Hartcher and Jones, 2017, p. 769). Also, normal behavior is predictable behavior, based on the knowledge of conspecifics, and animal keepers, about which behaviors and reactions should be expected under the prevailing conditions.
“Any definition of normal must remain open and available for change and continuous modification, as it is inevitably produced out of a necessity for a measurement or benchmark of something else — namely abnormal and disordered.” (Segura, 2015, p. 6). For example, the strength of motivation to perform certain behaviors (i.e. frequency and/or duration of performance) can depend on environmental conditions. The expression of rooting behavior in pigs, a behavior they are highly motivated to perform, has been found to be flexible in response to nutritional needs (Beattie and O’Connellt, 2002). Normal behavior thus might be contrasted with abnormal behavior, and it might be necessary to define normal in terms of the abnormal (Segura, 2015). We tentatively define abnormal behavior in animals as dysfunctional, aberrant, and non- or maladaptive, and unpredictable behavior, which all have the potential to evoke distress in the individual (see also Ramsden, 2013). Dysfunctional behavior is behavior that interferes with the ability to function effectively in daily life, i.e., that tends to be maladaptive. Aberrant behavior seriously deviates from what other animals recognize and interpret correctly. It deviates from behavior that can be expected and is considered adequate in the current situation (e.g. expressing defensive aggression while as friendly approached by an unfamiliar individual), triggering adequate responses in conspecifics (and eventually other species that are familiar with interacting with individuals of the species that show deviant behavior, e.g., dog owner – dog). Individuals’ behaviors are thus guided by what they anticipate being the expectations of their peers and of their social environment. Maladaptive behavior is behavior that is inadequate for coping with situational changes. The animal may fail to use an adequate coping strategy (Ramsden, 2013). Instead, it may engage in abnormal behavior, such as stereotypic behavior, or it may express a ‘zombie’-like behavior, i.e., it may become highly inactive or unreactive to external appetitive or aversive stimuli (D’Eath et al., 2010).
Abnormal behavior may become extreme and develop into a pathological state. Ohl et al. (2008), for example, defined pathological anxiety-related behavior in animals as lacking adaptive value and that is incommensurate with the actual situation. The transition from normal to abnormal and pathological behavior is fluent, due to a lack of well-defined threshold values. Consequently, it may be difficult to recognize and diagnose a) when behaviors become abnormal, and b) when they become pathological. In human societies, shared cultural values and world views may determine which behaviors are considered normal and acceptable, and as abnormal and unacceptable (Ramsden, 2013).
Cultural values may also affect classifying animal behavior as normal or abnormal. In the context of welfare assessment, the need to perform normal and/or natural behaviors, the amount of reward and level of satisfaction that is provided by performing these behaviors, and the amount of frustration caused by inhibiting these behaviors, can be assessed scientifically (Hartcher and Jones, 2017). We suggest principally using the criterion of deviant behavior for classifying behavior as normal or abnormal, and using the severity of distress, dysfunction maladaptation associated with specific behavior as additional criteria.
Welfare issues may emerge if the animal has a strong motivation to display behaviors that are no longer adaptive and desirable in its current situation (see e.g., Miller and Polack, 2018). Examples are: nest building behavior of sows (Yun and Valros, 2015), the rooting behavior of pigs on concrete floors without litter (Studnitz et al., 2007), and dust bathing in chickens kept on wire floors (Vestergaard, 1980). The inability to perform these behaviors for which an animal is highly motivated, and/or preventing the reward associated with successfully performing these behaviors, because the substrate needed is not provided, might lead to frustration, negative effects on maternal behavior (e.g. sow nest building, Cronin et al., 1996; Herskin et al., 1998; Jarvis et al., 1999; Thodberg et al., 2002) or body condition and health (e.g. dustbathing chicken, van Liere, 1992; Weeks and Nicol, 2006) and thus impair welfare. Notably, certain (natural) behavioral patterns such as e.g. rank-related aggression inducing injuries, are detrimental to animal welfare (Špinka, 2006). Not only the victim of aggression may experience impaired welfare, but also the aggressor if its motivation to express aggression is suppressed or prevented, e.g. through management measures (Segerdahl, 2007). Furthermore, some natural behaviors, such as aggression directed towards humans or to pen mates or soiling the house in companion animals may be ‘unwanted’ from the owner’s/animal caretaker’s point of view (Rydhmer and Canario, 2014). These conflicts have the potential to create welfare issues, e.g., if the companion animal is surrendered to a shelter.
The expression of nonadaptive and maladaptive behavior strongly indicates that the animal’s adaptive limits are exceeded and that its welfare is compromised or seriously impaired. Maladaptive behavior harms the animal, whereas an animal showing nonadaptive behavior will at best inadequately cope with the challenges with which it is confronted. Signs of compromised welfare are nonadaptive or maladaptive behaviors such as stereotypies (Mason and Latham, 2004), enduring symptoms of an activated stress physiology, distress, pathological anxiety (Ohl et al., 2008), apathy and/or depression (e.g. horses, Fureix et al., 2012), automutilations (e.g. in dogs, Ghaffari et al., 2007), pathological aggression (Natarajan and Caramaschi, 2010) and injurious behavior (e.g. severe feather pecking in chickens, Rodenburg et al., 2013). Note, that the absence of nonadaptive or maladaptive behavior cannot be taken as an indication of unimpaired welfare (McPhee and Carlstead, 2010), welfare may still be compromised.
Coping and adaption
… allow the animal to cope with and adapt to the demands of the (prevailing) environmental circumstances, …
Animals must be able to cope with and adapt to persistent/conditional (adverse) environmental conditions to ensure their welfare (Broom, 1986; Broom, 1991; Broom, 2001; Hill and Broom, 2009). Coping with and adapting to challenges depend to a large extent on the capacities of the animal, which may vary according to the genetic constitution, especially in heavily selected breeds and strains. Most concepts of animal welfare share the view that poor welfare is associated with exceeding the coping capacity of animals (McEwen and Wingfield, 2003).
The limits of adaptability are dynamic, i.e., our concept of animal welfare contains elements of allostasis (Figure 4). Under normal conditions, sound and unimpaired animals have a wide regulatory range of allostatic mechanisms at their disposal. If these mechanisms are activated outside their regulatory range, several problems may occur.
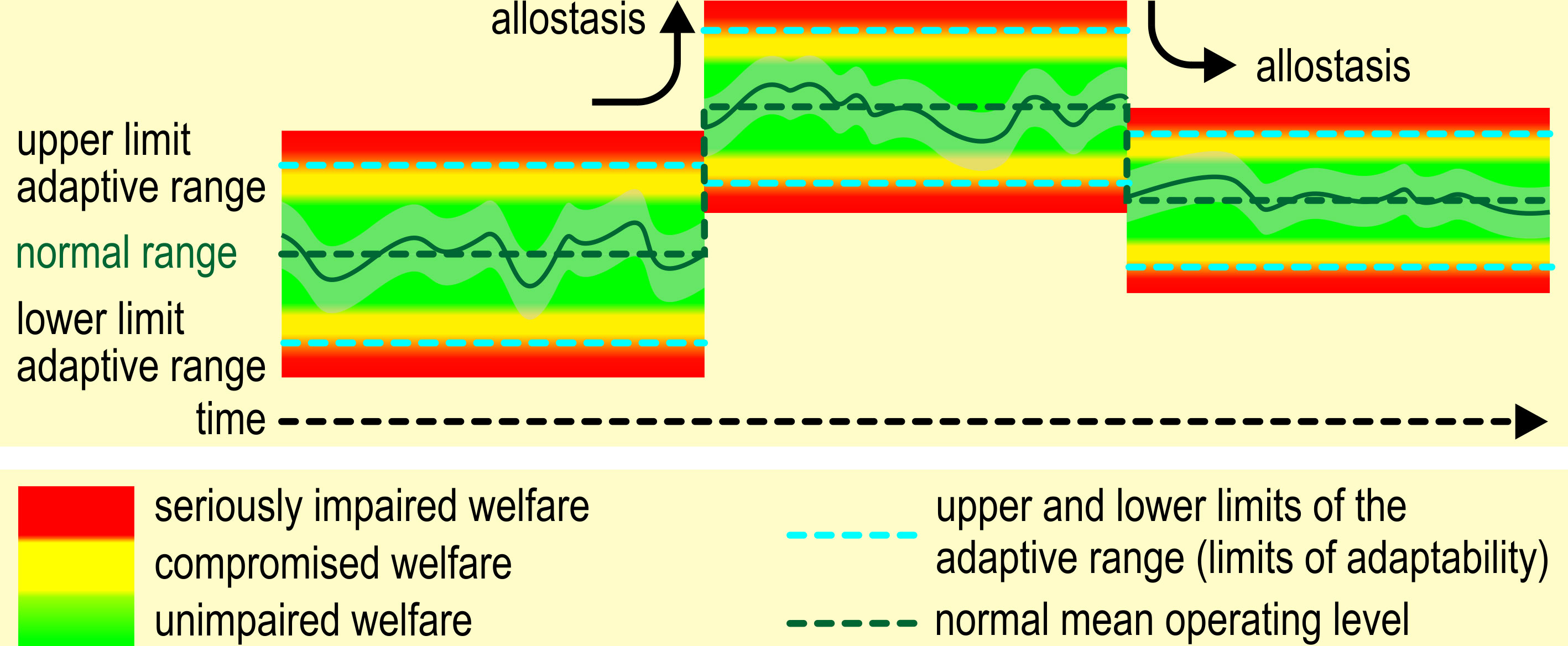
Figure 4 Homeostasis and allostasis in the context of animal welfare. Note that, if the animal reaches its limits of adaptability (blue dashed lines), its welfare becomes compromised (see also Figure 1). Values within the normal range will not impair the animal’s welfare, although values approaching the limits of adaptability may compromise welfare.
Repeated or chronic activation may lead to failure to adapt/habituate, failure to invoke adequate physiological responses, or to terminate the physiological responses with the termination of the challenge. Chronic deviation of the regulatory system from normal may induce an allostatic state, i.e. a chronic imbalance in the regulatory system (McEwen, 2004) that eventually may lead to a new equilibrium. These changes are not inevitably irreversible: “the body and the brain have a huge capacity for adaptive plasticity.” (Korte et al., 2005). Korte et al. (2007) conceive allostasis as a change of the internal set-points to meet environmental demands (stability through change). A new set-point (allostatic state) is thought to entail a narrowing of the regulatory range (Koob and Le Moal, 2001). Establishing a new equilibrium will come with costs: continued operation of the allostatic state or overactivation of allostatic responses (allostatic load) (McEwen, 2004) may exceed the capacity of the individual to cope and accumulate to allostatic overload (McEwen and Wingfield, 2003, p. 4).
Note, that we distinguish between coping and adaptation, although the distinction between these two terms is less sharp in the scientific literature. They may sometimes be used interchangeably. We use coping to describe a way of responding to an experienced impact with a shorter-term temporal horizon (immediate action/reaction to sporadic impact/effects). Adaptation is the process of adjusting to change with a longer-term temporal horizon [e.g., in response to lasting changes in the environment or of internal factors], eventually across generations as a result of genetic selection (see also Tables 1A, B). The ability to make these adjustments is called adaptive capacity, which depends on the animal’s ability and capability to adapt, as described in the DAWCon. Applying a coping strategy to deal with a specific condition may be considered as a form of adaptation.
Especially in captivity, certain behavioral adaptations may not be suitable anymore to face the environmental challenges (Figure 5A). In the worst case, the animal may still feel a strong motivation or need to exhibit certain behaviors, even though their functional consequences may no longer be required for survival (Duncan, 1998). If these behaviors do not help to adapt to the situation, or if they cannot be performed, the welfare of the animal may be compromised. On the other side, the animal may be faced with challenges for which it lacks adaptive behavior (Figure 5B). This mismatch is likely to result in compromised welfare.
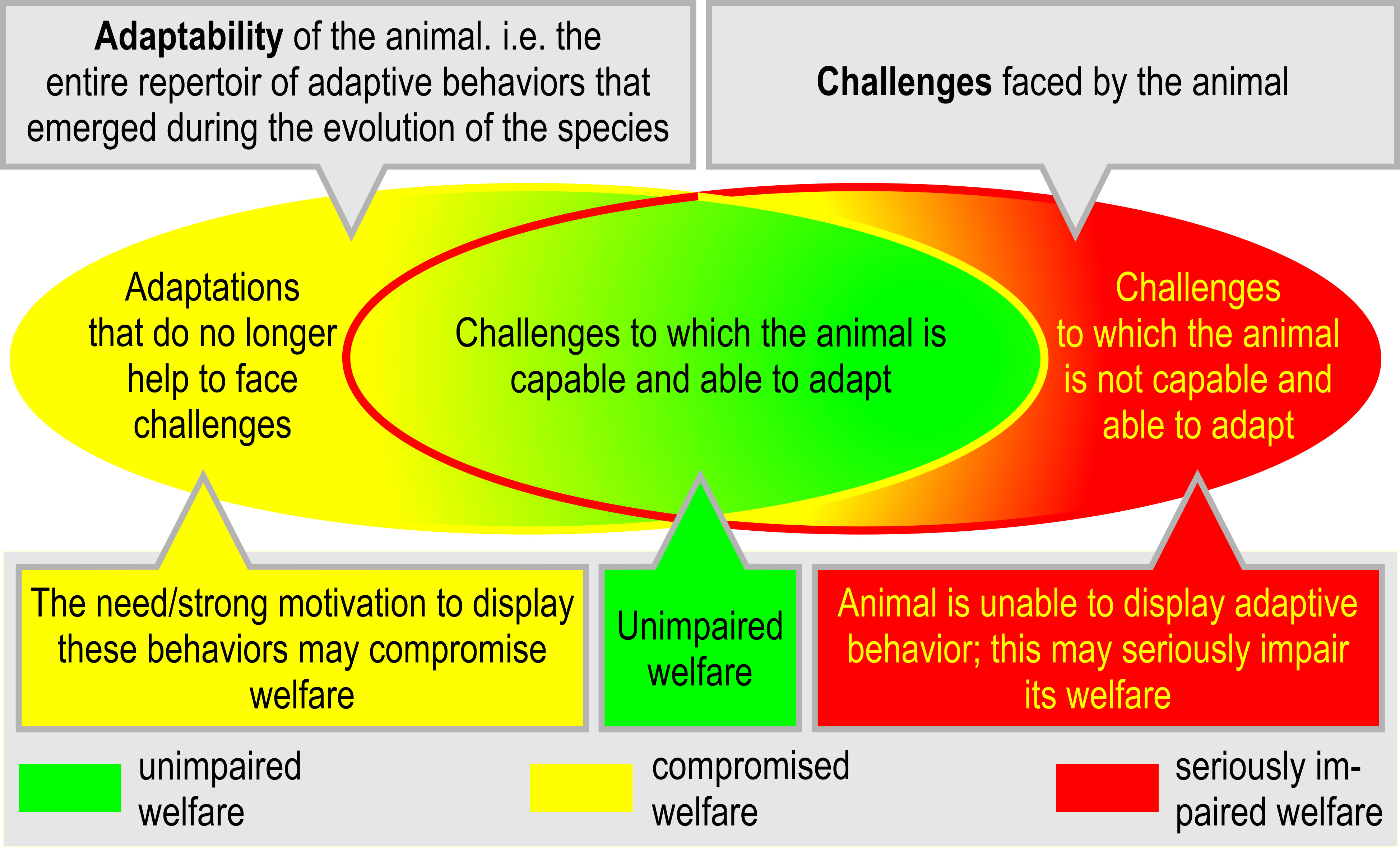
Figure 5 The mismatch between behavioral adaptability and challenges and the consequences of failing or succeeding to adapt. If an animal’s capacity and/or ability to adapt and cope with adverse internal and/or external factors is exceeded, the animal may be confronted with (unsolvable) problems (inspired by and modified from Fraser et al., 1997, p. 198).
The strategies to push the limits of adaptability and to improve the animal’s welfare are different for sporadic and lasting adverse factors. The limit of adaptability of an animal, or whether it will experience compromised welfare, may directly be affected by its degree of robustness. Robust breeds may have the required adaptability to cope with a broad range of net effects of internal and external factors, whereas the same constellation of factors may already exceed the limits of adaptability in less robust breeds.
The importance of positive emotions
…enabling it to reach a state that it perceives as positive, i.e., that evokes positive emotions.
DAWCon assumes that animals can experience negative as well as positive emotions. It is, however, valid to assume that most, if not all, non-human animal species perceive their environment, and depending on their cognitive abilities, process information differently from us. The capacity of subjective experiences and emotions might vary due to taxonomic affiliation (Fraser, 1999). Fraser and Duncan (1998) explicitly assign the capacity to experience positive emotions to higher vertebrates, however, this capacity may be much more widespread across the animal kingdom (Bliss-Moreau, 2017). Knowledge and appreciation of species differences is crucial to safeguarding animal welfare and calls for more research on animal taxa, about which we understand little in terms of emotional processing, cognitive abilities, and sentience (e.g., Perry et al., 2017). Challenges of an animal’s welfare may arise “(…) from anthropomorphism, particularly the misattribution of human cognitive abilities or emotions to animals, or from anthropocentrism, a failure to realize that their animal perceives the world through a different set of senses to their own” (Bradshaw and Casey, 2007, p. 149).
According to Anderson and Adolphs (2014) emotional behaviors represent internal emotional states. Usually, their frame of reference is the behavior and underlying neuroanatomy of humans. Whereas it is valid to assume that mammals possess the ability to experience positive emotions (Burgdorf and Panksepp, 2006), doubts have risen about whether species with a larger phylogenetic distance to mammals possess the ability, as well (see, e.g., the ongoing vivid discussion about this topic in the dedicated journal in ‘Animal Sentience’, ISSN: 2377-7478). Phylogenetic closeness is seen to be reflected in the degree of similarities/homology (structural and compositional correspondence) (e.g., Northcutt, 2012). An even stronger argument for this closeness might be analogy (serving similar functions, e.g., Panksepp, 1998, p. 1998). Both homology and analogy might thus serve as arguments for the likelihood that the degree of phylogenetic closeness reflects the capacity of non-human animals to experience emotions comparably to humans.
A large phylogenetic distance is traditionally believed to exist between vertebrates and invertebrates (reviewed by, e.g., Holland et al., 2015). There are, however, first indications that groups of invertebrates such as arthropods, like insects and vertebrates have a common evolutionary origin (e.g., Holland et al., 2015). This common history may be the foundation for the assumption that arthropods probably possess the ability to track experiences of reward and punishment, being relevant within processes of adaptation to environmental conditions (Bateson et al., 2011). It might be wise, as suggested by Mendl et al. (2011) to comparatively explore the possible adaptive benefits of conscious experiences in natural environments. Conscious experience needs to be proven for insects and for other species with a larger evolutionarily distance to humans, such as, for example, reptiles and amphibians. There is a chance that some species might possess the ability to successfully adapt to environmental conditions without, or with limited conscious experience (Dawkins, 2017). Thus, DAWCon may be restricted to species evolutionary close to humans. Consequently, there may be a need for new, additional concepts of animal welfare for species with a larger phylogenetic distance from humans.
The conceptual approach to welfare in the past decades moved from focusing on the absence of negative states (Five Freedoms) to promoting positive welfare (Mellor, 2015). Current welfare concepts agree that the animal’s mental states deserve increased attention, and that positive affect and emotions are a crucial feature of positive welfare (Boissy et al., 2007; Yeates and Main, 2008; Lawrence et al., 2019). The advance in research on animal consciousness and sentience, and the capacity to experience emotions surely supported this shift in the conceptual approach to animal welfare (Mellor and Beausoleil, 2015; Dawkins, 2017). Emotions have evolved along the process of adaptation, evoke behavioral or physiological processes, and function to maximize fitness (Revord et al., 2021). Negative emotions aid animals in avoiding adverse stimuli, positive emotions promote them to seek appetitive factors and rewards. The continuum between negative and positive emotions is part of an animal’s dynamic welfare. Note that, similar to behavioral adaptations, in a captive environment, not all emotions necessarily prove adaptive (Revord et al., 2021). To reach a state of unimpaired welfare, DAWCon explicitly emphasizes the importance that internal and external factors should enable an animal to experience positive emotions. The animal’s experiences are subjective and are shaped by its current and past experiences. To assess an individual’s perception of its emotional state requires a holistic view and ideally combines physiological and behavioral measures (Boissy et al., 2007; Mendl et al., 2010) (see also Figure 2). Cognitive processes may affect the subjective experience and the emotional reactivity of an individual, while vice versa, emotional experiences may affect cognitive processes (Boissy and Lee, 2014). The latter complex interplay is being investigated through behavioral testing paradigms such as cognitive bias and attention bias (Crump et al., 2018). In humans, coping ability and positive affect are shown to contribute to increased wellbeing (Fredrickson and Joiner, 2002), a promising process to pursue in animals, as well (Boissy and Lee, 2014).
Assessing animal welfare based on DAWCon
Behavior is an important read out and indicator of an animal’s welfare state and for identifying changes. While expression of normal behavior does not necessarily equal good welfare, the development of maladaptive and abnormal behavioral patterns may indicate compromised welfare. To recognize these aberrations, all-encompassing ethograms are the basis of any assessment tool for animal welfare. As Nordquist et al. (2017) pointed out, precise descriptions/definitions of the natural and normal behavioral repertoire of the species in an ethogram is a prerequisite for conclusions on the adaptive capacity of an animal. Notably, if animals are observed in an environment that does not provide the relevant resources to perform normal behavior, an ethogram may not reflect the full behavioral spectrum. Thus, comparative research under diverse environmental conditions is necessary. Referring to farm animal welfare, already Kilgour (1978) pointed to the exigency to obtain this basic information. To address specific scientific and welfare questions, however, ethograms for narrowly defined, delimited behavioral domains have been developed and are available. Unfortunately, comprehensive ethograms of many captive species are still missing.
Next to behavioral measures, the investigation of physiological parameters is crucial to be able to make assumptions about an animal’s emotional state and to follow coping processes. In search of ‘iceberg indicators’, i.e., single parameters that may serve as an overall index of animal welfare (Heath et al., 2014; Collins et al., 2015), a myriad of physiological indices of arousal or stress coping have been investigated and put forward as valuable proxies for welfare assessment, e.g., heart rate variability (von Borell et al., 2007; Kovács et al., 2014) or glucocorticoids (Otovic and Hutchinson, 2015).
Importantly, coping and adaptation are dynamic processes not at least due to the dynamics of an animal’s environment, therefore need adequate evaluation techniques. The assessment of the welfare state of an animal needs multiple measurements (multiple readouts) over time (repeated measurements) (Figure 6), as they reflect the dynamics of the individual’s interaction with its environment over time (Ohl and van der Staay, 2012), and the animal’s needs change over time (Millar, 2013). Measurement at one single time-point is of limited relevance: in particular because it does not provide measurements of reactions to, and recovery from adverse environmental interferences (Friggens et al., 2017).
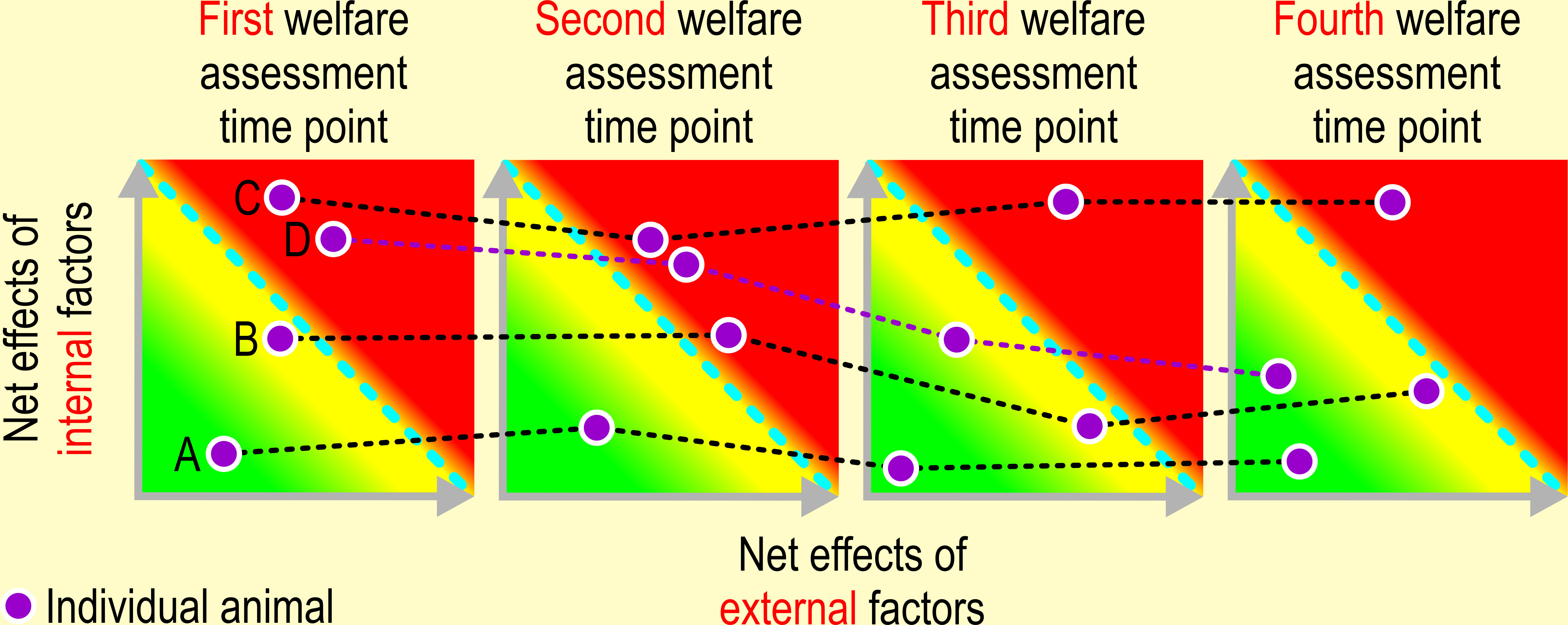
Figure 6 Repeated assessment of animal welfare. Drawing conclusions about animal welfare must be based on assessing an animal’s emotional and physiological state repeatedly over a longer period of time. Individual A: the welfare of this animal is not at stake at any of the four welfare assessment time points, i.e., the impact of net effects of adverse internal and external factors never exceeds its limit of adaptability. The net impact of appetitive and adverse internal and external factors exceeds the limit of adaptability of animal B at the second time point, whereas its welfare is compromised on time points 1, 3, and 4. The welfare of animal C is seriously impaired on all assessment time points, i.e., the impact of adverse internal and external factors always exceeds its limits of adaptability. Animal D, an overweight, brachycephalic dog underwent surgery to alleviate its dyspnea (reducing impact of an adverse internal factor) between the second and third welfare assessment time point. Also, the feeding regime was improved to induce significant weight loss (reducing the impact of an adverse external factor, namely malnutrition). The condition of this dog is slowly improving.
Ascertainment of the momentary condition of the animal provides an indication to eventually take immediate action for improving its condition, in particular, if welfare appears to be compromised or impaired. However, a single measurement is a snapshot that does not provide a valid indicator of an animal’s general welfare state and cannot detect persistent impairment of animal welfare (Temple et al., 2013).
The acute mental and physical state of an individual may well reflect successful earlier coping and/or adaptation. Integrated measures, such as concentration of glucocorticoids in hair, provide retrospective information. While not providing details on the acute reactions to adverse or appetitive factors, levels of glucocorticoid may indicate an overall higher exposure to adverse factors. Caution should be taken when interpreting relative levels of physiological markers, as the measured concentrations may also be the result of previous adaptation to a new allostatic set point, and may not indicate compromised welfare, per se.
Multiple measurements are furthermore necessary in order to develop reliable welfare assessment tools, providing results representative over longer periods of time (Waiblinger et al., 2001; Kirchner et al., 2014; Battini et al., 2016; Can et al., 2017; Diener, 2017), and across different phases of life (see. e.g., Munoz et al., 2018).
Promising developments regarding the longitudinal assessment of parameters, which may be, at least potentially, relevant to animal welfare, concern the use of cameras and sensor technologies (e.g., Li et al., 2020). Artificial intelligence and machine learning have recently gained momentum within livestock farming (e.g., Valletta et al., 2017; Van Hertem et al., 2017; Neethirajan et al., 2021) and for the behavioral phenotyping of rodents used in animal experiments (for a review see, e.g., Voikar and Gaburro, 2020). Technology can add to the (longitudinal) measurement of animal welfare. However, such technologies and the information they yield need to be thoroughly validated. It is thus of high importance to clearly define which parameters are considered to be of importance to animal welfare and to continue the search for novel parameters.
Many welfare assessment tools have been developed such as the Welfare Quality protocol (e.g., Czycholl et al., 2015), and assessment criteria according to the Five Domains (e.g. McGreevy et al., 2018), and the Quality of Life (e.g., Green and Mellor, 2011), approaching animal welfare from different points of view (see Webster, 2016). Unfortunately, the correlation between assessment tools derived from these (and other) concepts (e.g., Andreasen et al., 2013) and their consistency over time may be weak (Knierim and Winckler, 2009; van Eerdenburg et al., 2021), indicating the necessity for more research to derive well validated and useful welfare assessment tools.
Strategies to improve welfare based on DAWCon
Several strategies to safeguard and promote welfare come forward from the DAWCon (Figure 7). First, ensuring the animal’s health and the animal’s capacities and abilities to cope efficiently with its environment through preventive measures, such as regular health care, and via breeding programs and genetic selection is crucial to avoid internal lasting adverse states, e.g., as present in brachycephalic breeds (Packer and Tivers, 2015). Breeding goals should be adjusted to forestall adverse consequences on animal welfare (Rauw et al., 1998; Kanis et al., 2005). By providing the opportunity to perform normal behavior, and appropriate nutrition and cognitive stimulation, the animal’s capacity to cope and adapt, both physically and mentally, will be further supported. By doing so, welfare will likely not be severely compromised by sporadic adverse factors, though the exposure of the animals to these and especially lasting adverse factors should be minimized. Instead, appetitive stimuli should be provided, e.g., by structural, social, and foraging enrichment (Newberry, 1995). Interventions to enhance the possibility to experience positive states and positive welfare should aim at the following features (1) positive emotions; (2) positive affective engagement; (3) quality of life and (4) happiness (Lawrence et al., 2019). Adaptability may be enhanced, as well, by training an animal to cope with its current adverse circumstances/adverse conditions.
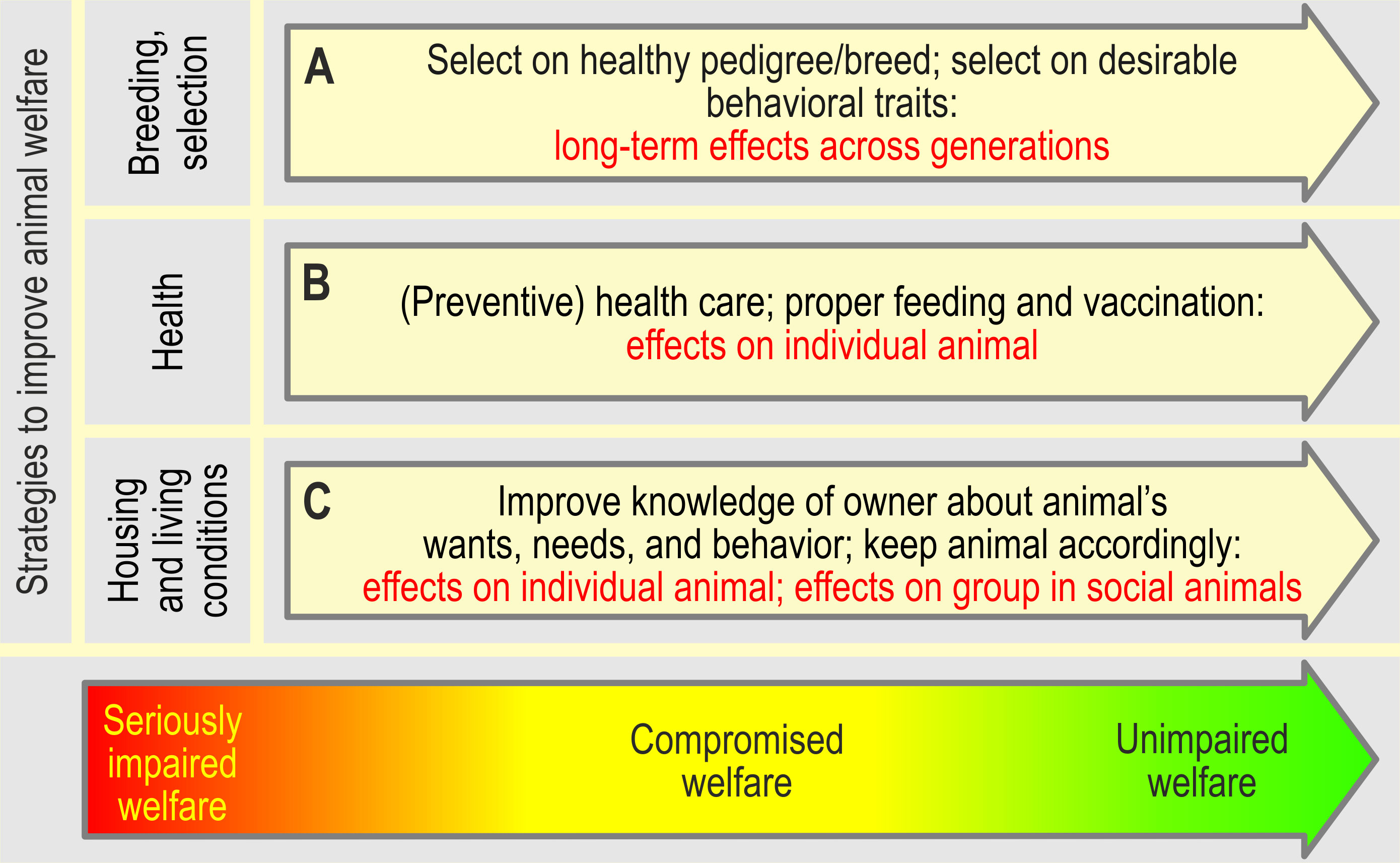
Figure 7 Strategies to improve, and the expected effects of the actions taken on the animal’s welfare (inspired by Kanis et al., 2004; Nordquist et al., 2017). Note that the effects of breeding and selection become visible only over several generations (i.e., it is not suited to improve the welfare state of an individual animal, but, if successful, will gradually improve animal welfare across successive generations). In farm animals, the consequences of genetic selection on production traits on the welfare of individual animals (e.g. selection for large litter sizes in pigs; increase of number of low weight piglets, which decreases viability; severe teat competition when the number of piglets exceeds the number of teats of the sow, Rutherford et al., 2013) should be evaluated. Breeding goals should eventually be adjusted to forestall adverse consequences on animal welfare (Rauw et al., 1998; Kanis et al., 2005). By contrast, improving the health (eventually by medical treatment) of an animal and improving its housing and living conditions, may instantly affect an animal’s welfare state. Similarly, instant positive effects on animal welfare may result from instructing owners and professional training of farmers about the animal’s needs and the animal’s behavior, if the acquired knowledge is put into practice.
We suggest that the causes of undesired traits and behaviors such as expression of gene mutations in response to breeding selection should be investigated as one of the most relevant starting points for research, because a detrimental genetic constitution may forestall or seriously constrain adaptability. It is of utmost importance to review and reassess breeding standards that may have detrimental effects on the animal’s welfare (Asher et al., 2009), i.e. to square up to the absurdity of some breeding standards (King et al., 2012). Breeding programs thus should focus on the internal causes of undesired traits and behaviors, rather than on eliminating these by external manipulations, because the latter approach may generate other, sometimes more urgent, welfare issues.
Adapting breeding aims, and the breeding practices to reach these aims, will not instantly lead to the amelioration of animal welfare, because their effects will take a few successive generations to become visible even in traits that respond quickly to selection. In particular, breeding aims should be re-evaluated and redefined, and breed standard regulations should abolish inbreeding, the main cause of the numerous genetic problems of many popular breeds (Arman, 2007; Jeppsson, 2014).
For instance, conditions that may increase the probability of a difficult birth (dystocia, i.e. a difficult birth or the inability to expel the fetus(es) from the birth canal during parturition) should be considered. Examples are breeding animals with a narrow birth canal (maternal origin), or with oversized fetuses (fetal origin), e.g. achondroplastic type breeds, and breeds selected for large heads (Eneroth et al., 1999; Ogbu et al., 2016). The cessation of such conditions will be beneficial for the welfare of potentially affected animals (i.e. the females that would have been used for breeding and their oversized puppies) and for the breed as a whole. Neutering of all animals that are at risk of dystocia may be an option (McKenzie, 2010).
An area of research that intrinsically is prone to (severe) animal welfare problems is the development and use of genetically modified animals as models of neurobehavioral disorders or other disabling disorders. These models target the disease or a subset of specific symptoms and/or disease pathogenesis (Brown and Murray, 2006), and are believed to offer highly relevant disease models. Their aim is to generate signs similar to those seen in the target species (usually humans) (Doyle et al., 2012). With the expression (of symptoms of) disorders, the animals used may experience dysfunctions that cause discomfort (van Zutphen and De Deyn, 2000; le Bars et al., 2001; Mertens and Rülicke, 2007). For this reason, animal welfare should be closely monitored during the different stages of model development and model application (Zintzsch et al., 2020).
The net effects of internal factors might be of special relevance for evaluating the welfare of genetically modified rodents (and other species, such as pigs, Perleberg et al., 2018; Tanihara et al., 2021). Providing external factors, in particular management and housing conditions, may be crucial in alleviating the welfare consequences of the induced genetic modifications. The impact of adverse internal factors due to the dysfunctions induced may, however, be too severe to compensate via favorable external conditions.
Approaches to improving animal welfare are shifting from adapting the animal to its environment (e.g., by selection) to adapting the environment to the animal, e.g., by designing appropriate husbandry and management conditions (Nordquist et al., 2017). This approach will likely prove the most effective because external factors are easier to control and adapt than internal factors. While improving welfare via breeding programs and genetic selection are ongoing activities (Lawrence and Wall, 2014; Rauw and Gomez-Raya, 2015), their effects will become visible only after generations of selective breeding.
Concluding remarks
Animal welfare is a complex matter (e.g., Webster, 1998) that has been defined in many different ways. Clearly defined concepts help to formulate (new) research hypotheses and direct animal welfare research. This study has presented a framework that revolves around the concept of the (limit of) adaptability of an animal and the combined effects of sporadic or lasting adverse internal and external factors over time. The Dynamic Animal Welfare Concept combines and adds onto existing welfare concepts, such as the Five Freedoms (FAWC - Farm Animal Welfare Council, 1979a, 1979b), the Five Domains (Mellor and Beausoleil, 2015; Mellor, 2017), and Quality of Life (Green and Mellor, 2011). The DAWCon assumes the viewpoint of the individual animal and defines welfare as a state that the animal perceives as positive and that evokes positive emotions. Though it is only indirectly possible to assess whether an animal perceives its state as positive (see Figure 2), the animal’s welfare state can be deduced using objectively derived data (Veasey, 2017).
The DAWCon explicitly addresses:
● The dynamic nature of animal welfare, and welfare not being a snapshot measurement, but needs to be assessed over time;
● The importance of good health for welfare; though compromised health might not automatically result in compromised welfare, and vice versa;
● The capacity and ability of an animal to react to challenges and the importance of internal factors such as genetics, and external factors, such as environmental circumstances, providing the opportunity to do so;
● The importance of normal behavior for the animal’s ability to cope and adapt, but also as a read-out parameter for the assessment of welfare, the latter requiring thorough ethograms;
● The consideration of emotions, and specifically positive emotions, as part of safeguarding animal welfare.
All these elements can be identified and distinguished as parameters in animal welfare research and help to formulate exact research questions and hypotheses. Importantly, an animal’s welfare is not simply the sum of all factors that we consider relevant. Instead, we must confirm animal welfare in each individual case via direct observations of the animal (behavioral, physiological, and physical measures), with special attention to identifying putative appetitive and adverse internal and external factors that may influence animal welfare. One needs, however, to keep in mind that also moral or ethical standards influence conceptual approaches towards animal welfare and that subjective ethical assessment determines whether a certain welfare status is accepted by society (Ohl and van der Staay, 2012). Concepts that approach animal welfare from different angles may help to direct attention to relevant aspects of this issue that might be overlooked if only one view were used, i.e., they extend our manner of assessing and improving animal welfare (Fraser, 2008). The utility of the DAWCon will be reflected by the research that it is able to stimulate and generate.
Author contributions
SA and FJS wrote the first draft of this manuscript. VG wrote sections of this manuscript and contributed to its further editing. FJS designed the figures. All authors contributed to the final article and approved the submitted version.
Acknowledgments
We like to thank Lisa Fijn and Roel Vermeulen for constructive comments on earlier versions of this manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Anderson D. J., Adolphs R. (2014). A framework for studying emotions across species. Cell 157, 187–200. doi: 10.1016/j.cell.2014.03.003
Anderson C., von Keyserlingk M. A. G., Lidfors L. M., Weary D. M. (2020). Anticipatory behaviour in animals: a critical review. Anim. welfare 29, 231–238. doi: 10.7120/09627286.29.3.231
Andreasen S. N., Wemelsfelder F., Sandøe P., Forkman B. (2013). The correlation of qualitative behavior assessments with welfare quality® protocol outcomes in on-farm welfare assessment of dairy cattle. Appl. Anim. Behav. Sci. 143, 9–17. doi: 10.1016/j.applanim.2012.11.013
Animal health code commission (2019). “Animal welfare (section 7),” in Terrestrial animal health code (Paris, France: World organisation for animal health), 333–491.
Arman K. (2007). A new direction for kennel club regulations and breed standards. Can. veterinary J. 48, 953–965.
Asher L., Diesel G., Summers J. F., McGreevy P. D., Collins L. M. (2009). Inherited defects in pedigree dogs. part 1: disorders related to breed standards. veterinary J. 182, 402–411. doi: 10.1016/j.tvjl.2009.08.033
Baciadonna L., Düpjan S., Briefer E. F., Padilla de la Torre M., Nawroth C. (2018). Looking on the bright side of livestock emotions–the potential of heir transmission to promote positive welfare. Front. veterinary Sci. - Anim. Behav. welfare 5 (218), 6. doi: 10.3389/fvets.2018.00218
Barnard C.J., Hurst J.L. (1996). Welfare by design: the natural selection of welfare criteria. Anim. Welf. 5, 405–433.
Bassett L., Buchanan-Smith H. M. (2007). Effects of predictability on the welfare of captive animals. Appl. Anim. Behav. Sci. 102, 223–245. doi: 10.1016/j.applanim.2006.05.029
Bateson M. (2016). Optimistic and pessimistic biases: a primer for behavioural ecologists. Curr. Opin. Behav. Sci. 12, 115–121. doi: 10.1016/j.cobeha.2016.09.013
Bateson M., Desire S., Gartside S. E., Wright G. A. (2011). Agitated honeybees exhibit pessimistic cognitive biases. Curr. Biol. 21, 1070–1073. doi: 10.1016/j.cub.2011.05.017
Bateson M., Poirier C. (2019). Can biomarkers of biological age be used to assess cumulative lifetime experience? Anim. welfare 28, 41–56. doi: 10.7120/09627286.28.1.041
Battini M., Barbieri S., Waiblinger S., Mattiello S. (2016). Validity and feasibility of human-animal relationship tests for on-farm welfare assessment in dairy goats. Appl. Anim. Behav. Sci. 178, 32–39. doi: 10.1016/j.applanim.2016.03.012
Beattie V. E., O’Connellt N. E. (2002). Relationship between rooting behaviour and foraging in growing pigs. Anim. welfare 11, 295–303.
Berlim M. T., Fleck M. P. A. (2003). “Quality of life”: a brand new concept for research and practice in psychiatry. Rev. Bras. psiquiatria 25, 249–252. doi: 10.1590/S1516-44462003000400013
Berny P., Caloni F., Croubels S., Sachana M., Vandenbroucke V., Davanzo F., et al. (2010). Animal poisoning in europe. part 2: companion animals. veterinary J. 183, 255–259. doi: 10.1016/j.tvjl.2009.03.034
Bliss-Moreau E. (2017). Constructing nonhuman animal emotion. Curr. Opin. Psychol. 17, 184–188. doi: 10.1016/j.copsyc.2017.07.011
Blokhuis H. J., Fiks van Niekerk T., Bessel W., Elson A., Guémené D., Kjaers J. B., et al. (2007). The LayWel project: welfare implications of changes in production systems for laying hens. World’s poultry Sci. J. 63, 101–104. doi: 10.1079/WPS2006132
Blokhuis H. J., Veissier I., Miele M., Jones B. (2010). The welfare quality® project and beyond: safeguarding farm animal well-being. Acta agriculturæ Scandinavica. Section A Anim. Sci. 60, 129–140. doi: 10.1080/09064702.2010.523480
Boissy A., Lee C. (2014). How assessing relationships between emotions and cognition can improve farm animal welfare. Rev. scientifique technique (int. office epizootics) 33, 103–110.
Boissy A., Manteuffel G., Jensen M. B., Oppermann Moe R., Spruijt B., Forkman B., et al. (2007). Assessment of positive emotions in animals to improve their welfare. Physiol. Behav. 92, 375–397. doi: 10.1016/j.physbeh.2007.02.003
Bousfield B., Brown R. (2010). Animal welfare. Vet. Bull. - Agric. Fish. Conserv. Dep. Newsl. 1, 1–12.
Boyle L. A., Edwards S. A., Bolhuis J. E., Pol F., Zupan Šemrov M., Schütze S., et al. (2022). The evidence for a causal link between disease and damaging behavior in pigs. Front. veterinary Sci. - Anim. Behav. welfare 8. doi: 10.3389/fvets.2021.771682
Bracke M. B. M., Hopster H. (2006). Assessing the importance of natural behavior for animal welfare. J. Agric. Environ. ethics 19, 77–89. doi: 10.1007/s10806-005-4493-7
Bradshaw J. W. S., Casey R. A. (2007). Anthropomorphism and anthropocentrism as influences in the quality of life of companion animals. Anim. welfare 16, S149–S154.
Brambell F. W. R., Barbour D. S., Barnett L., Ewer T. K., Hobson A., Pitchforth H., et al. (1965). Report of the technical committee to enquire into the welfare of animals kept under intensive livestock husbandry systems (No. cmnd. 2836) (London: Her Majesty’s stationary office).
Broom D. M. (1986). Indicators of poor welfare. Br. veterinary J. 142, 524–526. doi: 10.1016/0007-1935(86)90109-0
Broom D. M. (1991). Animal welfare: concepts and measurement. J. Anim. Sci. 69, 4167–4175. doi: 10.2527/1991.69104167x
Broom D. M. (2001). “Coping, stress and welfare,” in Coping with challenge: Welfare in animals including humans. Ed. Broom D. M. (Berlin: Proceedings of Dahlem Conference. Dahlem University Press), pp. 1–9.
Broom D. M. (2007). Quality of life means welfare: how is it related to other concepts and assessed? Anim. Welfare 16 (S1), 45–53.
Browning H. (2019). The natural behavior debate: two conceptions of animal welfare. J. Appl. Anim. Welfare Sci. 23, 325–337. doi: 10.1080/10888705.2019.1672552
Browning H., Veit W. (2021). Positive wild animal welfare. https://philsci-archive.pitt.edu/19608/1/browningveit2021positive_welfare.pdf (accessed 3-2-2021)
Brown M. J., Murray K. A. (2006). Phenotyping of genetically engineered mce: humane, ethical, environmental, and husbandry issues. ILAR J. 47, 118–123. doi: 10.1093/ilar.47.2.118
Burgdorf J., Panksepp J. (2006). The neurobiology of positive emotions. Neurosci. Biobehav. Rev. 30, 173–187. doi: 10.1016/j.neubiorev.2005.06.001
Can E., Vieira A., Battini M., Mattiello S., Stilwell G. (2017). Consistency over time of animal-based welfare indicators as a further step for developing a welfare assessment monitoring scheme: The case of the animal welfare indicators protocol for dairy goats. J. dairy Sci. 100, 9194–9204. doi: 10.3168/jds.2017-12825
Catherall D. R. (2003). How fear differs from anxiety. Traumatology 9, 76–92. doi: 10.1177/153476560300900202
Chen S.-Y., Freitas P. H. F., Oliveira H. R., Lázaro S. F., Huang Y. J., Howard J. T., et al. (2021). Genotype-by-environment interactions for reproduction, body composition, and growth traits in maternal-line pigs based on single-step genomic reaction norms. Genet. Sel. Evol. 53, 51. doi: 10.1186/s12711-021-00645-y
Colditz I. G., Hine B. C. (2016). Resilience in farm animals: biology, management, breeding and implications for animal welfare. Anim. prod. Sci. 56, 1961–1983. doi: 10.1071/AN15297
Collins L. M., Asher L., Summers J., Diesel G., McGreevy P. D. (2010). Welfare epidemiology as a tool to assess the welfare impact of inherited defects on the pedigree dog population. Anim. welfare 19, S67–S75.
Collins S., Burn C. C., Cardwell J. M., Bell N. J. (2015). Evaluating the concept of iceberg indicators for on-farm welfare assessment of dairy cattle by farmers. Cattle Pract. 23, 300–301.
Cornish A., Raubenheimer D., McGreevy P. (2016). What we know about the public’s level of concern for farm animal welfare in food production in developed countries. Animals 6, 74, 15. doi: 10.3390/ani6110074
Cronin G. M., Simpson G. J., Hemsworth P. H. (1996). The effects of the gestation and farrowing environments on sow and piglet behaviour and piglet survival and growth in early lactation. Appl. Anim. Behav. Sci. 46, 175–192. doi: 10.1016/0168-1591(95)00657-5
Crump A., Arnott G., Bethell E. J. (2018). Affect-driven attention biases as animal welfare indicators: review and methods. Animals 8, 136. doi: 10.3390/ani8080136
Czycholl I., Büttner K., Grosse Beilage E., Krieter J. (2015). “Review of the assessment of animal welfare with special emphasis on the “Welfare quality® animal welfare assessment protocol for growing pigs.” Arch. Anim. Breed. Arch. Tierz. doi: 10.5194/aab-58-237-2015
Dawkins M. S. (2003). Behaviour as a tool in the assessment of animal welfare. Zoology 106, 383–387. doi: 10.1078/0944-2006-00122
Dawkins M. S. (2017). Animal welfare with and without consciousness. J. zool. 301, 1–10. doi: 10.1111/jzo.12434
D’Eath R. B., Conington J., Lawrence A. B., Olsson I. A. S., Sandøe P. (2010). Breeding for behavioural change in farm animals: practical, economic and ethical considerations. Anim. welfare 19, 17–27.
Diener E. (2017). If, why, and when subjective well-being influences health, and future needed research. Appl. psychol.: Health well-being 9, 133–167. doi: 10.1111/aphw.12090
Dietz L., Arnold A.-M. K., Goerlich-Jansson V. C., Vinke C. M. (2018). The importance of early life experiences for the development of behavioural disorders in domestic dogs. Behaviour 155, 83–114. doi: 10.1163/1568539X-00003486
Dingemanse N. J., Kazem A. J. N., Réale D., Wright J. (2009). Behavioural reaction norms: animal personality meets individual plasticity. Trends Ecol. Evol. 25, 81–89. doi: 10.1016/j.tree.2009.07.013
Doyle A., McGarry M. P., Lee N. A., Lee J. J. (2012). The construction of transgenic and gene knockout/knockin mouse models of human disease. Transgenic Res. 21, 327–349. doi: 10.1007/s11248-011-9537-3
Edgar J. L., Mullan S. M., Pritchard J. C., McFarlane U. J. C., Main D. C. J. (2013). Towards a ‘Good life’ for farm animals: development of a resource tier framework to achieve positive welfare for laying hens. Animals 3, 584–605. doi: 10.3390/ani3030584
Eneroth A., Linde-Forsberg C., Uhlhorn M., Hall M. (1999). Radiographic pelvimetry for assessment of dystocia in bitches: a clinical study in two terrier breeds. J. small anmal. Pract. 40, 257–264. doi: 10.1111/j.1748-5827.1999.tb03076.x
Estevez I., Andersen I.-L., Nævdal E. (2007). Group size, density and social dynamics in farm animals. Appl. Anim. Behav. Sci. 103, 185–204. doi: 10.1016/j.applanim.2006.05.025
Farrell L. L., Schoenebeck J. J., Wiener P., Clements D. N., Summers K. M. (2015). The challenges of pedigree dog health: approaches to combating inherited disease. Canine Genet. Epidemiol. 2, 14. doi: 10.1186/s40575-015-0014-9
FAWC - Farm Animal Welfare Committee (2013). The farm animal welfare committee - annual review 2012-2013 (London).
FAWC - Farm Animal Welfare Council (1979a) “Five freedoms” (The national archives). Available at: https://webarchive.nationalarchives.gov.uk/20121010012427/http://www.fawc.org.uk/freedoms.htm (Accessed 10.20.16).
FAWC - Farm Animal Welfare Council (1979b) Press statement. Available at: https://webarchive.nationalarchives.gov.uk/20121010012428/http://www.fawc.org.uk/pdf/fivefreedoms1979.pdf (Accessed 1.3.19).
Fraser D. (1999). Animal ethics and animal welfare science: bridging the two cultures. Appl. Anim. Behav. Sci. 65, 171–189. doi: 10.1016/S0168-1591(99)00090-8
Fraser D. (2008). Understanding animal welfare (Acta veterinaria Scandinavica). doi: 10.1186/1751-0147-50-S1-S1
Fraser D., Duncan I. J. C. (1998). “Pleasure”, “pains” and animal welfare: toward a natural history of affect. Anim. welfare 7, 383–396.
Fraser D., Weary D. M., Pajor E. A., Milligan B. N. (1997). A scientific conception of animal welfare that reflects ethical concerns. Anim. welfare 6, 187–205.
Fredrickson B. L., Joiner T. (2002). Positive emotions trigger upward spirals toward emotional well-being. psychol. Sci. 13, 172–175. doi: 10.1111/1467-9280.00431
Friggens N. C., Blanc F., Berry D. P., Puillet L. (2017). Review: Deciphering animal robustness. a synthesis to facilitate its use in livestock breeding and management. Animal 11, 2237–2251. doi: 10.1017/S175173111700088X
Fuller T., Sarkar S., Crews D. (2005). The use of norms of reaction to analyze genotypic and environmental influences on behavior in mice and rats. Neurosci. Biobehav. Rev. 29, 445–456. doi: 10.1016/j.neubiorev.2004.12.005
Fureix C., Jego P., Henry S., Lansade L., Hausberger M. (2012). Towards an ethological animal model of depression. PloS One 7 (6), e39280. doi: 10.1371/journal.pone.0039280
Gauvin D. V., Craig L., Boley S. E. (2018). Statutory imposed terms of stress, distress, well-being and animal welfare: suggested guidelines for humane endpoints in animal studies. J. pharma. Pharm. Sci. 2, 14. doi: 10.24218/vjpps.2018.21
Ghaffari M. S., Khorami N., Marjani M., Aldavood S. J. (2007). Penile self-mutilation as an unusual sign of a separation-related problem in a crossbreed dog. J. small Anim. Pract. 48, 651–653. doi: 10.1111/j.1748-5827.2007.00338.x
Gough A., Thomas A., O’Neill D. (2018). Breed predispositions to disease in dogs and cats, 3rd ed (Hoboken, NJ, USA: John Wiley & Sons Ltd).
Green T. C., Mellor D. J. (2011). Extending ideas about animal welfare assessment to include ‘quality of life’ and related concepts. New Z. veterinary J. 59, 263–271. doi: 10.1080/00480169.2011.610283
Guitart R., Croubels S., Caloni F., Sachana M., Davanzo F., Vandenbroucke V., et al. (2010a). Animal poisoning in europe. part 1: farm livestock and poultry. Vet J. 183, 260–265. doi: 10.1016/j.tvjl.2009.03.033
Guitart R., Sachana M., Caloni F., Croubels S., Vandenbroucke V., Berny P. (2010b). Animal poisoning in Europe. Part 3: wildlife. Vet J. 183, 249–254. doi: 10.1016/j.tvjl.2009.03.002
Harding S. (2013). ‘Bling with bite’ – the rise of status and weapon dogs. Vet. Rec. 173, 261–263. doi: 10.1136/vr.f5374
Hartcher K. M., Jones B. (2017). The welfare of layer hens in cage and cage-free housing systems. World’s poultry Sci. J. 73, 767–782. doi: 10.1017/S0043933917000812
Heath C. A. E., Browne W. J., Mullan S., Main D. C. J. (2014). Navigating the iceberg: reducing the number of parameters within the welfare quality® assessment protocol for dairy cows. Animal :12, 1978–1986. doi: 10.1017/S1751731114002018
Herskin M. S., Jensen K. H., Thodberg K. (1998). Influence of environmental stimuli on maternal behaviour related to bonding, reactivity and crushing of piglets in domestic sows. Appl. Anim. Behav. Sci. 58, 241–254. doi: 10.1016/S0168-1591(97)00144-5
Hill S. P., Broom D. M. (2009). Measuring zoo animal welfare: theoryand practice. Zoo Biol. 28, 531–544. doi: 10.1002/zoo.20276
Holland N. D., Holland L. Z., Holland P. W. H. (2015). Scenarios for the making of vertebrates. Nature, 5205, 450–455. doi: 10.1038/nature14433
Howell T. J., King T., Bennett P. C. (2015). Puppy parties and beyond: the role of early age socialization practices on adult dog behavior. Vet. medicine: Res. Rep. 6, 143–153. doi: 10.2147/VMRR.S62081
Huber M., Knottnerus J. A., Green L., van der Horst H., Jadad A. R., Kromhout D., et al. (2011). How should we define health? Br. Med. J. 343, d4163, 3. doi: 10.1136/bmj.d4163
Indrebø A. (2008). Animal welfare in modern dog breeding. Acta veterinaria Scandinavica 50 (Suppl 1), S6, 6. doi: 10.1186/1751-0147-50-S1-S6
Jarvis S., McLean K. A., Calvert S. K., Deans L. A., Chirnside J., Lawrence A. B. (1999). The responsiveness of sows to their piglets in relation to the length of parturition and theinvolvement of endogenous opioids. Appl. Anim. Behav. Sci. 63, 195–207. doi: 10.1016/S0168-1591(99)00013-1
Jeppsson S. (2014). Purebred dogs and canine wellbeing. J. Agric. Environ. ethics 27, 417–430. doi: 10.1007/s10806-013-9470-y
Kalia V., Barouki R., Miller G. W. (2020). “The exposome: pursuing the totality of exposure,” in A new paradigm for environmental chemistry and toxicology - from concepts to insights. Eds. Jiang G., Li X. (Singapore: Springer Nature), 3–10. doi: 10.1007/978-981-13-9447-8
Kanis E., de Greef K. H., Hiemstra A., van Arendonk J. A. M. (2005). Breeding for societally important traits in pigs. J. Anim. Sci. 83, 948–957. doi: 10.2527/2005.834948x
Kanis E., van de Belt H., Groen A. F., Schakel J., de Greef K. H. (2004). Breeding for improved welfare in pigs: a conceptual framework and its use in practice. Anim. Sci. 78, 315–329. doi: 10.1017/S1357729800054102
Keeling L. J., De Oliveira D., Rustas B.-O. (2016). Use of mechanical rotating brushes in dairy cows – a potential proxy for performance and welfare?. in: Precision dairy farming Wageningen: Wageningen Acad. Publishers, 343–347.
Keshavarz K., Quimby F. W. (2002). An investigation of different molting techniques with an emphasis on animal welfare. J. Appl. poultry Res. 11, 54–67. doi: 10.1093/japr/11.1.54
Kilgour R. (1978). The application of animal behavior and the humane care of farm animals. J. Anim. Sci. 46, 1478–1486. doi: 10.2527/jas1978.4651478x
King T., Marston L. C., Bennett P. C. (2012). Breeding dogs for beauty and behaviour: why scientists need to do more to develop valid and reliable behaviour assessments for dogs kept as companions. Appl. Anim. Behav. Sci. 137, 1–12. doi: 10.1016/j.applanim.2011.11.016
Kirchner M. K., Schulze Westerat H., Knierim U., Tessitore E., Cozzi G., Winckler C. (2014). On-farm animal welfare assessment in beef bulls: consistency over time of single measures and aggregated welfare quality® scores. Animal 8:3, 461–469. doi: 10.1017/S1751731113002267
Knierim U., Winckler C. (2009). On-farm welfare assessment in cattle: validity, reliability and feasibility issues and future perspectives with special regard to the welfare quality® approach. Anim. Welf 18, 451–458.
Koob G. F., Le Moal M. (2001). Drug addiction, dysregulation of reward, and allostasis. Neuropsychopharmacology 24, 97–129. doi: 10.1016/S0893-133X(00)00195-0
Korte S. M., Koolhaas J. M., Wingfield J. C., McEwen B. S. (2005). The Darwinian concept of stress: benefits of allostasis and costs of allostatic load and the trade-offs in health and disease. Neurosci. Biobehav. Rev. 29, 3–38. doi: 10.1016/j.neubiorev.2004.08.009
Korte S. M., Olivier B., Koolhaas J. M. (2007). A new animal welfare concept based on allostasis. Physiol. Behav. 92, 422–428. doi: 10.1016/j.physbeh.2006.10.018
Kovács L., Jurkovich V., Bakony M., Szenci O., Póti P., Tőzsér J. (2014). Welfare implication of measuring heart rate and heart rate variability in dairy cattle: literature review and conclusions for future research. Animal :2, 316–330. doi: 10.1017/S1751731113002140
Krebs B. L., Marrin D., Phelps A., Krol L., Watters J. V. (2018). Managing aged animals in zoos to promote positive welfare: a review and future directions. Anim. 8 116, 22. doi: 10.3390/ani8070116
Kupriyanov R., Renad Zhdanov R. (2014). The eustress concept: problems and outlooks. World J. Med. Sci. 11, 179–185. doi: 10.5829/idosi.wjms.2014.11.2.8433
Laule G. (2010). “Positive reinforcement training for laboratory animals,” in The UFAW handbook on the care and management of laboratory and other research animals, vol. pp . Eds. Kirkwood J. K., Hubrecht R. (The Universities Federation for Animal Welfare), 206–218. doi: 10.1002/9781444318777
Lawrence A. B., Newberry R. C., Špinka M. (2018). “Positive welfare: What does it add to the debate over pig welfare?,” in Advances in pig welfare, herd and flock welfare. Ed. Špinka M. (Elsevier - Woodhead Publishing), 415–444. doi: 10.1016/B978-0-08-101012-9.00014-9
Lawrence A. B., Vigors B., Sandøe P. (2019). What is so positive about positive animal welfare–a critical review of the literature. Animals 9:783, 19. doi: 10.3390/ani9100783
Lawrence A. B., Wall E. (2014). Selection for ‘environmental fit’ from existing domesticated species. Rev. scientifique technique (int. office epizootics) 33, 171–179.
Lay D. C. Jr. (2020). “Consequences of stress during development,” in The biology of animal stress - basic principles and implications for animal welfare, vol. pp . Eds. Moberg G. P., Mench J. A. (United Kingdom: CABI Publishing, Oxon), 249–267.
Learmonth M. J. (2019). Dilemmas for natural living concepts of zoo animal welfare. Animals 318, 13. doi: 10.3390/ani9060318
le Bars D., Gozariu M., Cadden S. W. (2001). Animal models of nociception. Pharmacol. Rev. 53, 597–652.
Lerner H. (2008). “The concepts of health, well-being and welfare as applied to animals - a philosophical analysis of the concepts with regard to the differences between animals (Linköping studies in arts and science no. 438; dissertations on health and society no. 13),” in Linköpings universitet (Linköping, Sweden: Department of Medical and Health Sciences).
Leroy G. (2011). Genetic diversity, inbreeding and breeding practices in dogs: results from pedigree analyses. veterinary J. 189, 177–182. doi: 10.1016/j.tvjl.2011.06.016
Linder A. C., Gottschalk A., Lyhne H., Langbak M. G., Jensen T. H., Pertoldi C. (2020). Using behavioral instability to investigate behavioral reaction norms in captive animals: theoretical implications and future perspectives. Symmetry 12, 603. doi: 10.3390/sym12040603
Li N., Ren Z., Li D., Zeng L. (2020). Review: automated techniques for monitoring the behaviour and welfare of broilers and laying hens: towards the goal of precision livestock farming. Animal 14:3, 617–625. doi: 10.1017/S1751731119002155
Lloyd J. K. F. (2017). Minimising stress for patients in the veterinary hospital: why it is important and what can be done about it. Vet. Sci. 22, 19. doi: 10.3390/vetsci4020022
Luescher A. U., Reisner I. R. (2008). Canine aggression toward familiar people: a new look at an old problem. Vet. Clinics - small Anim. Pract. 38, 1107–1130. doi: 10.1016/j.cvsm.2008.04.008
Mason W. A. (2000). “Early developmental influences of experience on behaviour, temperament and stress,” in The biology of animal stress - basic principles and implications for animal welfare, vol. pp . Eds. Moberg G. P., Mench J. A. (Oxon, United Kingdom: CABI Publishing), 269–290.
Mason G. J., Latham N. R. (2004). Can’t stop, won’t stop - is stereotypy a reliable animal welfare indicator? Anim. welfare 13 (suppl. 1), S57–S69.
McCulloch S. P. (2013). A critique of FAWC’s five freedoms as a framework for the analysis of animal welfare. J. Agric. Environ. ethics 26, 959–975. doi: 10.1007/s10806-012-9434-7
McEwen B. S. (2004). “Chapter 2 - protective and damaging effects of the mediators of stress and adaptation: allostasis and allostatic load,” in Allostasis, homeostasis, and the costs of physiological adaptation. Ed. Schulkin J. (Cambridge: Cambridge University Press), 65–98. doi: 10.1017/CBO9781316257081.005
McEwen B. S., Wingfield J. C. (2003). The concept of allostasis in biology and biomedicine. Hormones Behav. 43, 2–15. doi: 10.1016/S0018-506X(02)00024-7
McGreevy P., Berger J., de Brauwere N., Doherty O., Harrison A., Fiedler J., et al. (2018). Using the five domains model to assess the adverse impacts of husbandry, veterinary, and equitation interventions on horse welfare. Animals 8:41, 27. doi: 10.3390/ani8030041
McKenzie B. (2010). Evaluating the benefits and risks of neutering dogs and cats. CAB reviews: perspectives in agriculture, veterinary science, nutrition and natural resources, Vol. 5. 18. doi: 10.1079/PAVSNNR20105045
McMillan F. D. (2005). “The concept of quality of life in animals (chapter 13),” in Mental Health and Well-being in Animals. Ed. McMillan F. D. (Oxford, United Kingdom: Blackwell Publishing), 183–200.
McPhee M. E., Carlstead K. (2010). “Chapter 25 - effects of captivity on the behavior of wild mammals,” in Wild mammals in captivity: Principles and techniques for zoo management, vol. pp . Eds. Kleima D. G., Thompson K., Kirk Baer C. (Chicago, U.S.A: University of Chicago Press), 303–313.
Mellor D. J. (2015). Positive animal welfare states and reference standards for welfare assessment. New Z. veterinary J. 63, 17–23. doi: 10.1080/00480169.2014.926802
Mellor D. J. (2016a). Updating animal welfare thinking: moving beyond the “five freedoms” towards “a life worth living. Animals 6:21, 20. doi: 10.3390/ani6030021
Mellor D. J. (2016b). Moving beyond the “five freedoms” by updating the “five provisions” and introducing aligned “animal welfare aims. Animals 6, 59. doi: 10.3390/ani6100059
Mellor D. J. (2017). Operational details of the five domains model and its key applications to the assessment and management of animal welfare. Animals 7:60, 20. doi: 10.3390/ani7080060
Mellor D. J., Beausoleil N. J. (2015). Extending the ‘Five domains’ model for animal welfare assessment to incorporate positive welfare states. Anim. welfare 24, 241–253. doi: 10.7120/09627286.24.3.241
Mellor D. J., Beausoleil N. J., Littlewood K. E., McLean A. N., McGreevy P. D., Jones B., et al. (2020). The 2020 five domains model: including human–animal interactions assessments of animal welfare. Animals 10, 1870. doi: 10.3390/ani1010187024
Mellor D. J., Reid C. S. W. (1994). “Concepts of animal well-being and predicting the impact of procedures on experimental animals,” in Department of physiology and anatomy (New Zealand: Massey University Palmerston North).
Mendl M., Burman O. H. P., Paul E. S. (2010). An integrative and functional framework for the study of animal emotion and mood. Proc. R. Soc. London Ser. B Biol. Sci. 277, 2895–2904. doi: 10.1098/rspb.2010.0303
Mendl M., Paul E. S., Chittka L. (2011). Animal behaviour: emotion in invertebrates? Curr.Biol 21, R463–R465. doi: 10.1016/j.cub.2011.05.028
Mertens C., Rülicke T. (2007). Welfare assessment and phenotype characterisation of transgenic mice. Alternatives to Anim. exp. 24, 46–48.
Millar L. N. (2013). Improving captive animal welfare through the application of cognitive enrichment (PhD thesis - psychology) (Exeter, United Kingdom: University of Exeter).
Miller G. W., Jones D. P. (2014). The nature of nurture: refining the definition of the exposome. Toxicol. Sci. 137, 1–2. doi: 10.1093/toxsci/kft251
Miller R. R., Polack C. W. (2018). Sources of maladaptive behavior in ‘normal’ organisms. Behav. processes 154, 4–12. doi: 10.1016/j.beproc.2017.12.017
Moberg G. P. (2000). “Biological response to stress: implications for animal welfare,” in The biology of animal stress. Eds. Moberg G. P., Mench J. A. (Wallingford, United Kingdom: CAB International), pp. 1–21. doi: 10.1079/9780851993591.0001
Munoz C., Campbell A., Barber S., Hemsworth P., Doyle R. (2018). Using longitudinal assessment on extensively managed ewes to quantify welfare compromise and risks. Animals :8, 13. doi: 10.3390/ani8010008
Natarajan D., Caramaschi D. (2010). Animal violence demystified. Front. Behav. Neurosci. 4:9. doi: 10.3389/fnbeh.2010.00009
Neethirajan S., Reimert I., Kemp B. (2021). Measuring farm animal emotions —sensor-based approaches. Sensors 21, 553, 22. doi: 10.3390/s21020553
Nettle D., Bateson M. (2012). The evolutionary origins of mood and its disorders. Curr. Biol. 22, R712–R721. doi: 10.1016/j.cub.2012.06.020
Newberry R. C. (1995). Environmental enrichment: increasing the biological relevance of captive environments. Appl. Anim. Behav. Sci. 44, 229–243. doi: 10.1016/0168-1591(95)00616-Z
Niemelä P. T., Dingemanse N. J. (2014). Artificial environments and the study of a’daptive’ personalities. Trends Ecol. Evol. 29, 245–247. doi: 10.1016/j.tree.2014.02.007
Nijdam E., Arens P., Lambooij E., Decuypere E., Stegeman J. A. (2004). Factors influencing bruises and mortality of broilers during catching, transport, and lairage. Poultry Sci. 83. doi: 10.1093/ps/83.9.1610
Nordquist R. E., van der Staay F. J., van Eerdenburg F. J. C. M., Velkers F. C., Fijn L., Arndt S. S. (2017). Mutilating procedures, management practices, and housing conditions that may affect the welfare of farm animals: implications for welfare research. Animals 7:12, 22. doi: 10.3390/ani7020012
Northcutt R. G. (2012). Evolution of centralized nervous systems: two schools of evolutionary thought. Proc. Natl. Acad. Sci. United States America 109, 10626–10633. doi: 10.1073/pnas.1201889109
Ogbu K. I., Ochai S. O., Danladi M. M. A., Abdullateef M. H., Agwu E. O., Gyengdeng J. G. (2016). A review of neonatal mortality in dogs. Int. J. Life Sci. 4, 451–460.
Ohl F., Arndt S. S., van der Staay F. J. (2008). Pathological anxiety in animals. veterinary J. 175, 18–26. doi: 10.1016/j.tvjl.2006.12.013
Ohl F., Endenburg N., Vaarkamp H., Pijpers A., Rothuizen J., Hellebrekers L. J., et al. (2009)Dierenwelzijn - de diergeneeskundige positie (Animal welfare - the veterinary position). In: Dierenwelzijn - de diergeneeskundige positie (Animal welfare - the veterinary position). Available at: https://www.google.com/url?sa=t&rct=j&q=&esrc=s&source=web&cd=1&ved=2ahUKEwiVq6y4stHfAhUCPFAKHYcoB4QQFjAAegQICBAC&url=https%3A%2F%2Fwww.uu.nl%2Ffiles%2Fdgk-organisatie-profiel-en-missie-dierenwelzijn-de-diergeneeskundige-positie-sept-2009pdf&usg=AOvVaw0qyGjSw7d9yxxqfbwIZBde (Accessed 1.3.19).
Ohl F., Hellebrekers L. (2009). ‘Dierenwelzijn’ – de diergeneeskundige positie. Tijdschrift. voor Diergeneeskunde. 134, 754–755.
Ohl F., van der Staay F. J. (2012). Animal welfare: at the interface between science and society. veterinary J. 192, 13–19. doi: 10.1016/j.tvjl.2011.05.019
Oliveira D. P., Lourenco D. A. L., Tsuruta S., Misztal I., Santos D. J. A., de Araújo Neto F. R., et al. (2018). Reaction norm for yearling weight in beef cattle using single-step genomic evaluation. J. Anim. Sci. 96, 27–34. doi: 10.1093/jas/skx006
Olsson I. A. S., Westlund K. (2007). More than numbers matter: the effect of social factors on behaviour and welfare of laboratory rodents and non-human primates§. Appl. Anim. Behav. Sci. 103, 229–254. doi: 10.1016/j.applanim.2006.05.022
Otovic P., Hutchinson E. (2015). Limits to using HPA axis activity as an indication of animal welfare. ALTEX: Alternativen. zu Tierexperimenten. 32, 4–50. doi: 10.14573/altex.1406161
Packer R. M. A., Tivers M. S. (2015). Strategies for the management and prevention of conformation-related respiratory disorders in brachycephalic dogs. Vet. medicine: Res. Rep. 6, 219–232. doi: 10.2147/VMRR.S60475
Panksepp J. (1998). Affective neuroscience - the foundations of human and animal emotions (Inc., New York, Oxford: Oxford University Press).
Parsons S., Kruijt A.-W., Fox E. (2016). A cognitive model of psychological resilience. J. Exp. Psychopathol. 7, 296–310. doi: 10.5127/jep.053415
Perleberg C., Kind A., Schnieke A. (2018). Genetically engineered pigs as models for human disease. Dis. Models Mech. 11 (1), dmm030783, 12. doi: 10.1242/dmm.030783
Perry C. J., Barron A. B., Chittka L. (2017). The frontiers of insect cognition. Curr. Opin. Behav. Sci. 16, 111–118. doi: 10.1016/j.cobeha.2017.05.011
PIckard and members or the Animal Procedures Committee(2013). “Animal procedures committee,” in Review of the assessment of cumulative severity and lifetime experience in non-human primates used in neuroscience research (Report of the animal procedures committee’s primate subcommittee working group). Available at: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/261687/cs_nhp_review_FINAL_2013_corrected.pdf.
Price E. O. (1984). Behavioral aspects of animal domestication. Q. Rev. Biol. 59, 1–32. doi: 10.1086/413673
Price E. O. (1999). Behavioral development in animals undergoing domestication. Appl. Anim. Behav. Sci. 65, 245–271. doi: 10.1016/S0168-1591(99)00087-8
Ramsden P. (2013). “Defining abnormal behaviour (chapter 1),” in Understanding abnormal psychology: Clinical and biological perspectives (London, Unidted Kingdom: SAGE Publications Ltd).
Ranchet M., Morgan J. C., Akinwuntan A. E., Devos H. (2017). Cognitive workload across the spectrum of cognitive impairments: a systematic review of physiological measures. Neurosci. Biobehav. Rev. 80, 516–537. doi: 10.1016/j.neubiorev.2017.07.001
Rauw W. M., Gomez-Raya L. (2015). Genotype by environment interaction and breeding for robustness in livestock. Front. Genet. 6. doi: 10.3389/fgene.2015.00310
Rauw W. M., Kanis E., Noordhuizen-Stassen E. N., Grommers F. J. (1998). Undesirable side effects of selection for high production efficiency in farm animals: a review. Livestock prod. Sci. 56, 15–33. doi: 10.1016/S0301-6226(98)00147-X
Revord J., Sweeny K., Lyubomirsky S. (2021). Categorizing the function of positive emotions. Curr. Opin. Behav. Sci. 39, 93–97. doi: 10.1016/j.cobeha.2021.03.001
Rodenburg T. B., van Krimpen M. M., de Jong I. C., de Haas E. N., Kops M. S., Riedstra B. J., et al. (2013). The prevention and control of feather pecking in laying hens: identifying the underlying principles. Poult Sci. 69, 361–373. doi: 10.1017/S0043933913000354
Roelofs S., Boleij H., Nordquist R. E., van der Staay F. J. (2016). Making decisions under ambiguity: judgment bias tasks for assessing emotional state in animals. Front. Behav. Neurosci. 10. doi: 10.3389/fnbeh.2016.00119
Roelofs S., van der Staay F. J. (2017). “Judgment bias,” in Encyclopedia of animal cognition and behavior. Eds. Vonk J., Shackelford T. K. (Springer Science Business Media), 7. doi: 10.1007/978-3-319-47829-6_1046-1
Rooney N., Bradshaw J. (2014). “Chapter 11 - canine welfare science: an antidote to sentiment and myth,” in Domestic dog cognition and behavior. Ed. Horowitz A. (Berlin, Heidelberg: Springer-Verlag), pp. 241–274. doi: 10.1007/978-3-642-53994-7_11
Rushen J. (2003). Changing concepts of farm animal welfare: bridging the gap between applied and basic research. Appl. Anim. Behav. Sci. 81, 199–214. doi: 10.1016/S0168-1591(02)00281-2
Rushen J., de Passillé A. M. (2010). “The importance of good stockmanship and its benefits for the animals,” in Improving animal welfare: A practical approach. Ed. Grandin T. (Wallingford, UK: CAB International), pp. 50–63.
Rutherford K. M. D., Baxter E. M., D’Eath R. B., Turner S. P., Arnott G., Roehe R., et al. (2013). The welfare implications of large litter size in the domestic pig I: biological factors. Anim. welfare 22, 199–218. doi: 10.7120/09627286.22.2.199
Rydhmer L., Canario L. (2014). Behavioral genetics in pigs and relations to welfare (Chapter 11), in: Grandin T., Deesing M.J. (Eds.), Genetics and the Behavior of Domestic Animals. London, United Kingdom: Elsevier, Academic Press. 397–434.
Salomons A. R., van Luijk J. A. K. R., Reinders N. R., Kirchhoff S., Arndt S. S., Ohl F. (2010). Identifying emotional adaptation: behavioural habituation to novelty and immediate early gene expression in two inbred mouse strains. Genes Brain Behav. 9, 1–10. doi: 10.1111/j.1601-183X.2009.00527.x
Scherf B. D. (2000). World watch list for domestic animal diversity. 3rd ed (Rome: Food and Agriculture Organization (FAO) of the United Nations).
Schlichting C. D., Pigliucci M. (1998). “Phenotypic evolution - a reaction norm perspective,” in Sinauer associates (Massachusetts, U.S.A: Inc. Publishers, Sunderland).
Segerdahl P. (2007). Can natural behaviour be cultivated? the farm as local human/animal culture. J. Agric. Environ. ethics 20, 167–193. doi: 10.1007/s10806-006-9028-3
Segura K. (2015) Defining normal. Available at: https://www.csustan.edu/sites/default/files/groups/University%20Honors%20Program/Journals/defining_normal_k._segura.pdf (Accessed 4.15.18).
Špinka M. (2006). How important is natural behaviour in animal farming systems? Appl. Anim. Behav. Sci. 100, 117–128. doi: 10.1016/j.applanim.2006.04.006
Stephen C. (2014). Toward a modernized definition of wildlife health. J. wildlife Dis. 50, 427–430. doi: 10.7589/2013-11-305
Stolba A., Wood-Gush D. G. M. (1989). The behaviour of pigs in a semi-natural environment. Anim. prod. 48, 419–425. doi: 10.1017/S0003356100040411
Strandberg E. (2009). “The role of environmental sensitivity and plasticity in breeding for robustness: lessons from evolutionary genetics,” in Breeding for robustness in cattle, vol. pp . Eds. Klopčič M., Reents R., Philipsson J., Kuipers A. (Wageningen: EAAP Publication. Wageningen Academic Publishers), 17–33.
Studnitz M., Bak Jensen M., Pedersen L. J. (2007). Why do pigs root and in what will they root? a review on the exploratory behaviour of pigs in relation to environmental enrichment. Appl. Anim. Behav. Sci. 107, 183–197. doi: 10.1016/j.applanim.2006.11.013
Sundburg C. R., Belanger J. M., Bannasch D. L., Famula T. R., Oberbauer A. M. (2016). Gonadectomy effects on the risk of immune disorders in the dog: a retrospective study. BMC veterinary Res. 12, 278. doi: 10.1186/s12917-016-0911-5
Tanihara F., Hirata M., Otoi T. (2021). Current status of the application of gene editing in pigs. J. Reprod. Dev. 67, 177–187. doi: 10.1262/jrd.2021-025
Temple D., Manteca X., Dalmau A., Velarde A. (2013). Assessment of test–retest reliability of animal-based measures on growing pig farms. Livestock Sci. 151, 35–45. doi: 10.1016/j.livsci.2012.10.012
Thodberg K., Jensen K. H., Herskin M. S. (2002). Nest building and farrowing in sows: relation to the reaction pattern during stress, farrowing environment and experience. Appl. Anim. Behav. Sci. 77, 21–42. doi: 10.1016/S0168-1591(02)00026-6
Tracey I., Mantyh P. W. (2007). The cerebral signature for pain perception and its modulation. Neuron 55, 377–391. doi: 10.1016/j.neuron.2007.07.012
Valletta J. J., Torney C., Kings M., Thornton A., Madden J. (2017). Applications of machine learning in animal behaviour studies. Anim. Behav. 124, 203–220. doi: 10.1016/j.anbehav.2016.12.005
van der Harst J. E., Spruijt B. M. (2007). Tools to measure and improve animal welfare: reward-related behaviour. Anim. welfare 6(S) 67-73, 67–73.
van Eerdenburg F. J. C. M., Hof T., Doeve B., Ravesloot L., Zeinstra E. C., Nordquist R. E., et al. (2021). The relation between hair-cortisol concen-tration and various welfare assessments of Dutch dairy farms. Anim. 11 821, 17. doi: 10.3390/ani11030821
van Hagen M. A. E. (2019). “Breeding short muzzled dogs - criteria for the enforcement of article 3.4. of the animal keepers decree (Besluit houders van dieren) – breeding companion animals,” in Department of animals in science and society and experise centre genetics of companion animals, Utrecht university; commissioned by the ministry of agriculture, nature and food quality, the Hague, the Netherlands. Available at: https://www.fecava.org/wp-content/uploads/2020/06/breeding-short-muzzled-dogs-in-the-netherlands_expertisecentre-genetics-of-companionanimals-2019-transl (access date 3-2-2022).
Van Hertem T., Rooijakkers L., Berckmans D., Peña Fernández A., Norton T., Berckmans D., et al. (2017). Appropriate data visualisation is key to precision livestock farming acceptance. Comput. Electron. Agric. 138, 1–10. doi: 10.1016/j.compag.2017.04.003
van Zutphen L. F. M., De Deyn P. P. (2000). Animal use in experimental neuropathology: provisions for animal welfare and ethics. Neurosci. Res. Commun. 26, 149–160. doi: 10.1002/1520-6769(200005/06)26:3<149::AID-NRC3>3.0.CO;2-4
Veasey J. S. (2017). In pursuit of peak animal welfare; the need to prioritize the meaningful over the measurable. Zoo Biol. 36, 413–425. doi: 10.1002/zoo.21390
Veasey J. S., Waran N. K., Young R. J. (1996). On comparing the behavior of zoo housed animals with wild conspecifics as a welfare indicator. Anim. Welf 5, 13–24.
Veissier I., Butterworth A., Bock B., Roe E. (2008). European Approaches to ensure good animal welfare. Appl. Anim. Behav. Sci. 113, 279–297. doi: 10.1016/j.applanim.2008.01.008
Vestergaard K. (1980). The regulation of dustbathing and other behaviour patterns in the laying hen: a lorenzian approach, in: Moss, r. (Ed.), the laying hen and its environment, current topics in veterinary medicine and animal science (Martinus Nijhoff Publishers, The Hague), pp. 101–113.
Viñuela-Fernández I., Jones E., Welsh E. M., Fleetwood-Walker S. M. (2007). Pain mechanisms and their implication for the management of pain in farm and companion animals. veterinary J. 174, 227–239. doi: 10.1016/j.tvjl.2007.02.002
Voelkl B., Würbel H. (2016). Reproducibility crisis: are we ignoring reaction norms? Trends Pharmacol. Sci. 37, 509–510. doi: 10.1016/j.tips.2016.05.003
Voikar V., Gaburro S. (2020). Three pillars of automated home-cage phenotyping of mice: novel findings, refinement, and reproducibility based on literature and experience. Front. Behav. Neurosci. 14. doi: 10.3389/fnbeh.2020.575434
von Borell E., Langbein J., Després G., Hansen S., Leterrier C., Marchant-Forde J., et al. (2007). Heart rate variability as a measure of autonomic regulation of cardiac activity for assessing stress and welfare in farm animals — a review. Physiol. Behav. 92, 293–316. doi: 10.1016/j.physbeh.2007.01.007
Waiblinger S., Knierim U., Winckler C. (2001). The development of an epidemiologically based on-farm welfare assessment system for use with dairy cows. Acta agric. Scandinavica section A — Anim. Sci. 51, S30, 73–77. doi: 10.1080/090647001316923108
Webster A. J. F. (1998). What use is science to animal welfare? Naturwissenschaften 85, 262–269. doi: 10.1007/s001140050496
Webster J. (2016). Animal welfare: freedoms, dominions and “a life worth living. Animals 6, 35. doi: 10.3390/ani6060035
Weeks C. A., Nicol C. J. (2006). Behavioural needs, priorities and preferences of laying hens. World’s poultry Sci. J. 62. doi: 10.1079/WPS200598
Weiss J. M. (1972). Psychological factors in stress and disease. Sci. Am. 226, 104–113. doi: 10.1038/scientificamerican0672-104
Wild C. P. (2005). Complementing the genome with an ‘“exposome”’: the outstanding challenge of environmental exposure measurement in molecular epidemiology. Cancer epidemiol. Biomarkers Prev. 14, 1847–1850. doi: 10.1158/1055-9965.EPI-05-0456
Wild C. P. (2012). The exposome: from concept to utility. Int. J. Epidemiol. 41, 24–32. doi: 10.1093/ije/dyr236
Yeates J. (2016). Quality of life and animal behaviour. Appl. Anim. Behav. Sci. 181, 19–26. doi: 10.1016/j.applanim.2016.04.018
Yeates J. W., Main D. C. J. (2008). Assessment of positive welfare: a review. veterinary J. 175, 293–300. doi: 10.1016/j.tvjl.2007.05.009
Yeates J., Main D. (2009). Assessment of companion animal quality of life in veterinary practice and research. J. small Anim. Pract. 50, 274–281. doi: 10.1111/j.1748-5827.2009.00755.x
Yun J., Valros A. (2015). Benefits of prepartum nest-building behaviour on parturition and lactation in sows — a review. Asian Australas. J. Anim. Sci. 28, 1519–1524. doi: 10.5713/ajas.15.0174
Zhang P., Carlsten C., Chaleckis R., Hanhineva K., Huang M., Isobe T., et al. (2021). Defining the scope of exposome studies and research needs from a multidisciplinary perspective. Environ. Sci. Technol. Lett. 8, 839–852. doi: 10.1021/acs.estlett.1c00648
Zhang H., Hu H., Diller M., Hogan W. R., Prosperi M., Guo Y., et al. (2021). Semantic standards of external exposome data. Environ. Res. 197, 111185. doi: 10.1016/j.envres.2021.11118520
Zintzsch A., Noe E., Grimm H. (2020). Navigating uncertainties: how to assess welfare and harm in genetically altered animals responsibly–a practical guideline. Animals 10, 857. doi: 10.3390/ani10050857
Ziv G. (2017). The effects of using aversive training methods in dogs - a review. J. veterinary Behav. 19, 50–60. doi: 10.1016/j.jveb.2017.02.004
Keywords: health, domestication, welfare assessment, behavior, emotions, animals under human care, adaptation, robustness
Citation: Arndt SS, Goerlich VC and van der Staay FJ (2022) A dynamic concept of animal welfare: The role of appetitive and adverse internal and external factors and the animal’s ability to adapt to them. Front. Anim. Sci. 3:908513. doi: 10.3389/fanim.2022.908513
Received: 30 March 2022; Accepted: 04 July 2022;
Published: 05 August 2022.
Edited by:
Jean-Loup Rault, University of Veterinary Medicine Vienna, AustriaReviewed by:
Jeremy N. Marchant, Livestock Behavior Research Unit (USDA-ARS), United StatesBrianna N. Gaskill, Novartis Institutes for BioMedical Research, United States
Copyright © 2022 Arndt, Goerlich and van der Staay. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Saskia S. Arndt, Uy5TLkFybmR0QHV1Lm5s
 Saskia S. Arndt
Saskia S. Arndt Vivian C. Goerlich
Vivian C. Goerlich F. Josef van der Staay
F. Josef van der Staay