- 1Department of Animal Science, Faculty of Agriculture and Technology, Rajamangala University of Technology Isan Surin Campus, Surin, Thailand
- 2Department of Animal Science, Faculty of Agriculture, Tropical Feed Resources Research and Development Center, Khon Kaen University, Khon Kaen, Thailand
- 3Division of Animal Science, Faculty of Agricultural Technology, Rajamangala University of Technology Thanyaburi, Pathum Thani, Thailand
The search for alternative modifiers of rumen fermentation to improve the production efficiency of livestock production is highly essential. This in vitro fermentation experiment was conducted using a factorial arrangement of two ratios of roughage to concentrate and seven levels of red amaranth (Amaranthus cruentus L.) leaf powder (RALP) as a percentage of total substrate in a completely randomized design (CRD). There were two factors: factor A was two ratios of roughage (R) to concentrate (C) at 60:40 and 40:60 and factor B was levels of RALP supplementation at 0, 2, 4, 6, 8, 10, and 12% dry matter (DM) of total dietary substrate. The results revealed that a R:C ratio at 40:60 increased rumen fermentation and reduced methane production (p < 0.05). The RALP incorporation as a feed additive was highly promising in enhancing propionate (C3) concentration, reducing acetate (C2) to (C3) ratio, and the protozoal population, while mitigating methane (CH4) production. Furthermore, DM degradation percentages were remarkably enhanced by increasing the RALP levels and R:C ratio at 40:60. In conclusion, plants rich in phytonutrients and minerals such as RALP and the lower R:C ratio showed a promising role in modulating rumen fermentation, mitigating methane production, as well as increasing substrate DM degradability.
Introduction
“Feeding the bugs, feeding the cows” has been stated for more than 50 years in order to provide the rumen to effectively perform the anaerobic fermentation process yielding end-products [volatile fatty acids (VFAs), ammonia nitrogen (NH3-N), carbon dioxide (CO2), hydrogen (H2), and methane (CH4)], which are absorbed, used for synthetic purposes in the target issues (Hungate, 1966). Wolin (1960) and De Souza et al. (2020) reported the stoichiometric balance in the rumen, CH4 production via the metabolic pathway of acetic production, while capturing the hydrogen in the propionate formation via succinate or randomizing pathway. The use of chemicals and/or antibiotics as an additive has resulted in the improvement of rumen fermentation efficiency by increasing propionate while mitigating methane production (McAllister et al., 2011; Wanapat et al., 2021). Rumen pH has been demonstrated to greatly influence rumen microorganisms and their fermentation end-products (Ørskov et al., 1974; Wanapat et al., 2014). By shifting levels of concentrate ratio, as well as supplementation, the rumen pH was dramatically reduced. Buffering rumen pH is therefore a necessity when the ruminants were fed with high level of concentrate or cereal grain supplementation. Herremans et al. (2020) used a meta-analysis, which confirms the beneficial effect of dietary tannins especially condensed tannins (CTs) on improving nitrogen utilization in ruminants by decreasing rumen protein degradation, NH3-N concentration, blood urea nitrogen, milk urea nitrogen, while digestibility of protein was significantly reduced.
Feed resources and plant extracts containing phytonutrients have been increasingly receiving more attention, as they affect rumen microorganisms and fermentation, especially the CT and saponins (SP). More attention has been paid to their biological activities and the antimicrobial properties (protozoa and methanogens) in the rumen (Castro-Montoya et al., 2011). Many kinds of plants and fruit wastes that contain phytonutrients have exhibited their effects in modulating the rumen fermentation, especially mitigating methane emission (Singh et al., 2018; Ampapon and Wanapat, 2021; Wanapat et al., 2021).
Amaranth, namely, red amaranth (Amaranthus cruentus, L.), has been reported to contain high concentration of phytonutrients, in which they could exert a significant effect on human health (Pulipati et al., 2017). Amaranth is a nutritious crop containing high levels of crude protein (CP), minerals, vitamins, as well as polyphenols and flavonoids especially in the leaf and seeds (Barba de la Rosa et al., 2009; Karamać et al., 2019). The main phenolic compounds in both leaf and seeds were gallic acid, vanillic acid, and p-coumaric acid (Paśko et al., 2008). A preliminary study by Chairatanayuth (1992) showed that amaranth leaf, especially in A. cruentus L., contained high CP (16.5%), lower NDF (41.5%), when harvested at 45 days of growth. Khandaker et al. (2010) reported the efficacy of polyphenols of red amaranth on the antioxidant activity, and the high level of total polyphenols in leaf was closely correlated (p < 0.05, R2 = 0.82) with the antioxidant activity. The red amaranth leaf contains high phytonutrients, proteins, and mineral contents, and no research work on its influence on rumen fermentation characteristics and nutrient digestibility in ruminants has been revealed.
The hypothesis of this experiment was utilizing red amaranth (Amaranthus cruentus, L.) leaf with two feed ratios which could improve the in vitro fermentation and mitigate methane production. Therefore, this experiment was conducted to investigate the effect of red amaranth (Amaranthus cruentus, L.) on in vitro fermentation end-products, gas production kinetics, protozoal population, and CH4 production using in vitro gas fermentation technique.
Materials and Methods
Experimental Design and Dietary Treatments
The red amaranth seeds (Chia Tai seed company, Bangkok, Thailand) were bought from the local market. The experiment was randomly assigned in a factorial arrangement of two factors of roughage to concentrate and seven levels of RALP percentage of total substrate in a completely randomized design (CRD). There were two factors: factor A was two levels of roughage to concentrate ratio (R:C) at 60:40 and 40:60 of dietary substrate at 0.20 g, whereas factor B was level of red amaranth (Amaranthus cruentus, L.) leaf powder (RALP) supplementation at 0, 2, 4, 6, 8, 10, and 12% of total dietary substrate.
The RALP was harvested from the plant after 25 days of growth. The red amaranth was planted in the experimental farm of Khon Kaen University, Khon Kaen, Thailand. The plots (1 × 3 m2) were prepared, sowing seeds, and watered morning at 7 a.m. and afternoon at 5 p.m. It was sun-dried, chopped, and ground to achieve the 1 mm length. Standard chemical analyses were employed to analyze for dry matter (DM), organic matter (OM), CP (AOAC, 2012), neutral-detergent fiber (NDF), and acid-detergent fiber (ADF) (Van Soest et al., 1991). Additional chemical procedures on CT (Burns, 1971) and SP (Kwon et al., 2003) were used. Macro-minerals were determined using wet digestion (nitric-perchloric digestion), atomic absorption spectrophotometry (novAA® 350 AAS) for total Ca, K, Mg, Zn, and Fe (Uddin et al., 2016).
Rumen and Substrate Inocula
As a source of rumen inocula, two rumen-fistulated crossbreds Holstein Friesian dairy steer [75% Holstein Friesian and 25% Thai native breed, about 370 kg body weight (BW)] were used, as rumen fluid donors. The rumen fluid donors were fed with concentrate (14% DM of CP) at 0.5% of BW, and rice straw was offered ad libitum to maintain normal rumen ecology for 21 days before rumen collection. Before the morning feeding, 1,000 ml of rumen fluid was collected from each animal to mixed, filtered, and then moved to the laboratory. The medium combining of reduced medium with rumen fluids (2:1) into hot plate for mixed under stirring at 39°C, under CO2. The ruminal fluid mixture filled 40 ml to all bottom and incubated in a water bath at 39°C. The in vitro incubation procedure was carried out according to the study of Menke et al. (1979), as modified and described by Kang et al. (2016).
In vitro Fermentation and Gas Production
The in vitro fermentation kinetics and gas production of all treatment samples were run intervally, starting from 1, 2, 4, 6, 8, 12, 24, 48, 72, to 96 h post-incubation (42 bottles: 3 bottles/treatment × 14 treatments). The 56 bottles [2 bottles/treatment × 14 treatments × 2 sampling time (4 and 8 h of incubation)] were separately prepared for pH, protozoa count, NH3-N, and VFA analysis. Measurement of fermentation gas production was recorded at each time, pH was measured at 4 and 8 h while the fluid was collected and was then divided into two parts. The first part of rumen fluid (18 ml) was collected and kept in a plastic bottle to which 2 ml of 1 M H2SO4 was added to stop fermentation process for later analyses of NH3-N by Kjeltec Auto 1030 Analyzer (AOAC, 2012), VFA (acetic; C2, propionic; C3, and butyric acids; C4) using HPLC, Instruments by Water and Nova Pak model 600E; water mode l484 UV detector; column nova Pak C18; column size 3.9 mm × 300 mm; mobile phase 10 m MH2PO4 [pH 2.5] according to Samuel et al. (1997), and the second portion of 1 ml rumen fluid was collected and kept in a plastic bottle to which 9 ml of 10% formalin solution for measuring of protozoal population using total direct count by hemocytometer (Boeco, Hamburg, Germany) (Galyean, 1989). Methane production [126 bottles: 3 bottles/treatment × 14 treatments × 3 sampling time (4, 8, and 12 h of incubation)] was determined from samples collected starting from 4, 8, to 12 h post-incubation. The 10 ml of gas production was collected using a 10-ml syringe, injected into 25-ml airtight serum bottles closed with a rubber lid and aluminum cap, covered with parafilm, and then measured using gas chromatography (Instruments by GC-17A System, Shimadzu; TCD detector; column shin carbon; column size 3 mm × 3 mm, activated charcoal 60/80 mesh) (Sittijunda et al., 2010). The gas production kinetic was performed based on the Ørskov and McDonal (1979) model:
where a = gas production from immediately soluble fraction, b = production of gas from the insoluble fraction, c = constant gas production rate for the insoluble fraction (b), t = time for incubation, (a + b) = the potential gas production. y = gas produced at the time “t.” The in vitro DM degradability (%) was calculated at both 12 and 24 h post-incubation [56 bottles: 2 bottles/treatment × 14 treatments × 2 sampling time (12 and 24 h of incubation)].
Statistical Analysis
All the obtained data were subjected to the general linear model (GLM) [SAS (Statistical Analysis System), 2013]. Differences among treatment means were compared by the Tukey's multiple comparison test (Crichton, 1999). The statistical parameters were R:C ratios, RALP levels, and R:C ratios × RALP levels interactions. Differences among statistical treatment parameters with p < 0.05 and p < 0.001 were taken as significant differences.
Results
Diet Compositions and the Nutritive Values
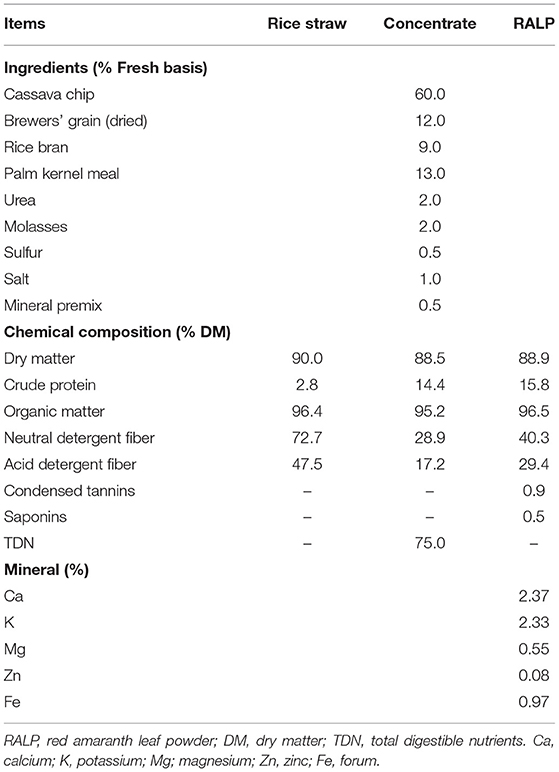
Table 1 presents details of feeds and their nutritive values on DM basis. Rice straw composited of 2.8% CP, 72.7% NDF, and 47.5% ADF, respectively. Concentrate was formulated and analyzed to contain 14.4% CP, 75% TDN, 28.9% NDF, and 17.2% ADF. The nutritive values of RALP were 15.8% CP, 40.3% NDF, 29.4% ADF, and 2.37% Ca, 2.33% K, and with 0.9% CT, 0.5% SP, respectively.
In vitro Gas Production and DM Degradability
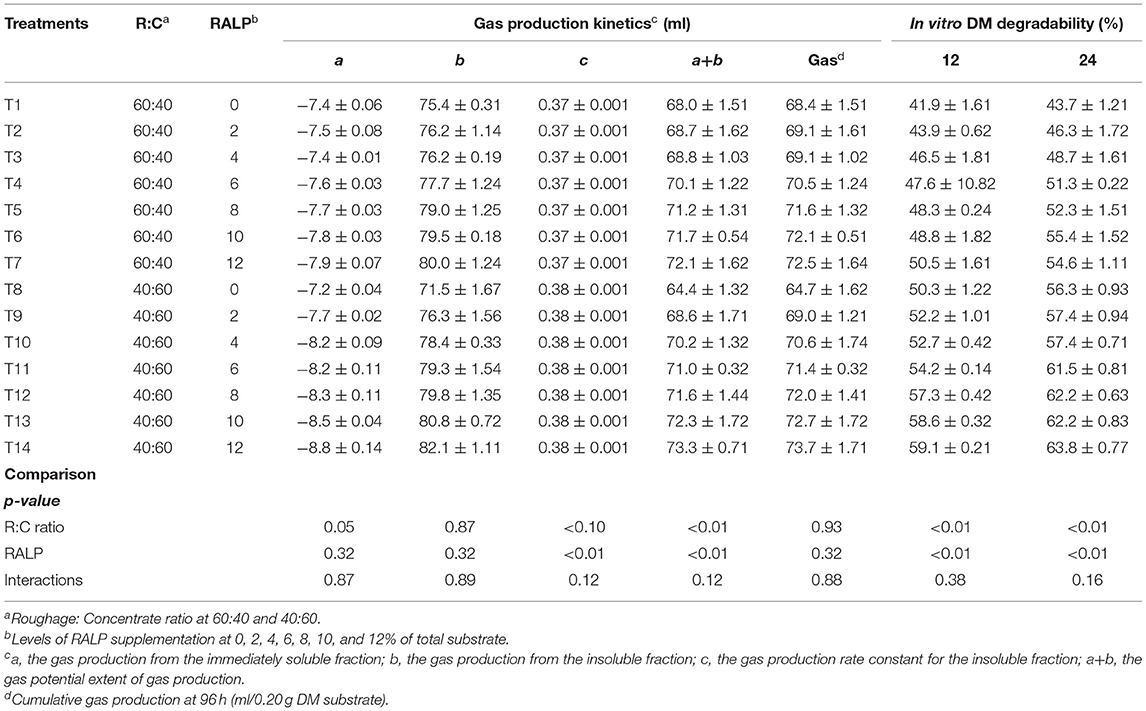
The gas production kinetic parameters are presented in Table 2. Ratio of roughage to concentrate and percentage of RALP had an effect on the fermentation process and subsequently on the gas production kinetics (c, a+b). The soluble fractions of gas production (a) and insoluble fraction of gas production (b) were not affected by R:C ratio, RALP, and interaction. The c was high in R:C ratio at 40:60 while there was no difference among treatment and interaction. The a+b values for both R:C ratio and RALP were significantly increased (p < 0.01), and there were no differences in the interaction. However, the cumulative production of gas was similar among treatment by R:C ratio, RALP, and interaction. As the level of RALP supplementation increased, regardless of R:C, the values were enhanced. In vitro DM degradability at both 12 and 24 h was significantly increased for both R:C and level of RALP supplementation (p < 0.05), and there was no difference in the interaction.
Rumen Fermentation Parameters
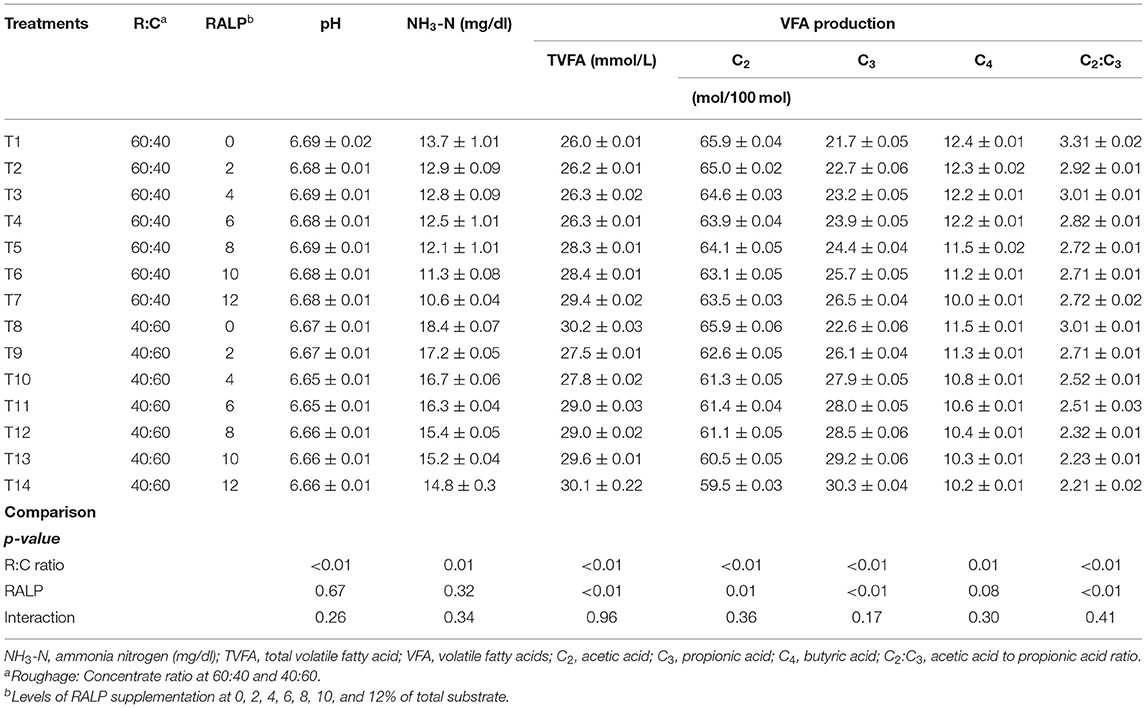
Table 3 presents the findings of pH, NH3-N, and VFA production. The rumen pH slightly dropped (6.69 to 6.56) by ratio of R:C (40:60), as compared to 60:40 (p < 0.05), while in RALP groups, they were no changes and interactions. Notable changes to increase on rumen NH3-N were obtained when R:C at 40:60 (p < 0.05) but did not affect the RALP groups, and there was no difference in the interaction. Remarkable changes on rumen total VFA and propionate production were obtained by R:C ratio at 40:60, as compared to 60:40, and the level of RALP increased for both levels of R:C ratio (p < 0.05) while there was no interaction. The acetic and butyric production were reduced by R:C ratio (40:60), as compared to 60:40 and RALP groups, and there obtains the interaction (p < 0.05), thus lowering the C2:C3 ratio correspondingly.
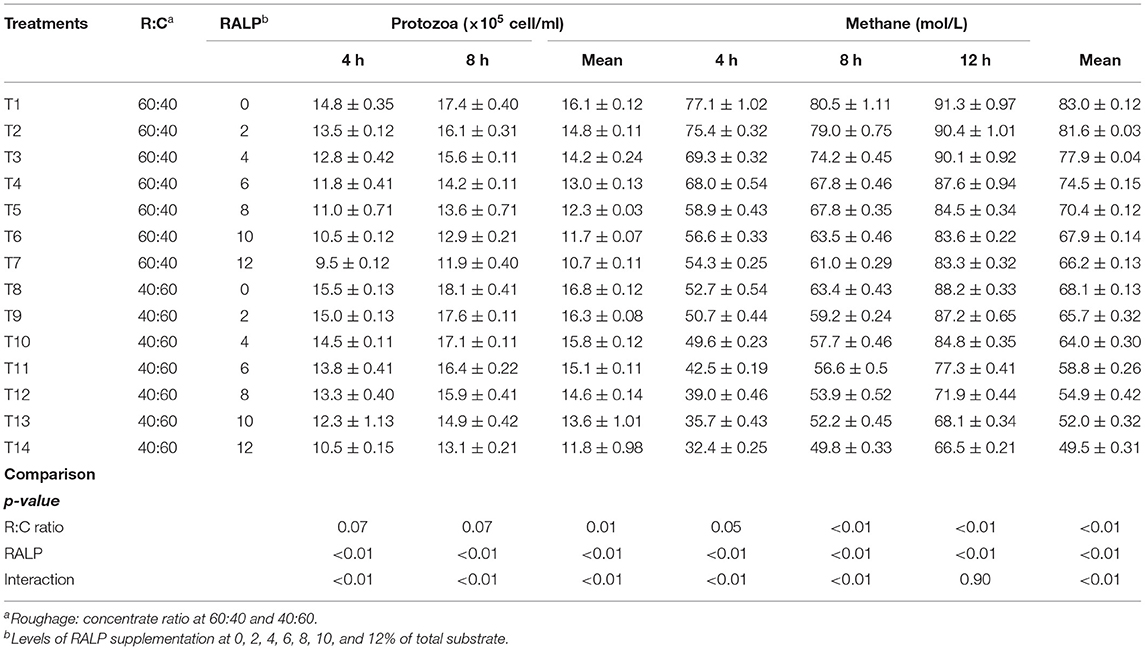
Table 4 shows in vitro protozoal population and methane production. The rumen protozoal population (×105 cell/ml) and methane production (mol/L) were reduced by the R:C and RALP supplementation, and there were significant interactions for both factors (p < 0.05). The CH4 production resulted in a remarkable decline of CH4 for both R:C and RALP supplementation but more dramatically for R:C at 40:60 (p < 0.05), respectively.
Discussion
Feed Ingredients and Feeding Value
Both roughage and concentrate sources can affect rumen fermentation characteristics when ingested. Roughage and its fibrous characteristics will stimulate rumination and fermentation by the activity of fibrolytic bacteria, while carbohydrate sources will be additionally degraded by amylolytic bacteria. It has been reported that rumen pH could be buffered to higher than 6.2 for efficient fiber degradation and to prevent incidence of sub-acute rumen acidosis (Zebeli et al., 2010). Ørskov et al. (1974) and Wanapat et al. (2014) reiterated the importance of R:C ratio on impacting the rumen pH, VFA production, and the variation in rumen microbiome especially fibrolytic bacteria group, namely, R. albus, R. flavefaciens, and Fibrobacter succinogenes, respectively (Koike and Kobayashi, 2001; Wanapat et al., 2014). Under this experiment, the R:C ratio of 40:60 was used and greatly influenced to fermentation characteristics. Supplementation of RALP additionally enhanced the production of propionic acid. Earlier work of Kang et al. (2014) stated the impact of banana flower powder which contained phytonutrients and high concentration of minerals which could lift up the rumen pH under high concentrate supplementation level in both in vitro and in vivo feeding experiments. Under this trial, RALP that consisted of high CP (15.8% CP), 0.9% CT, and 0.5% SP, along with high concentrations of macro-minerals especially Ca, K, and Mg. RALP could enhance rumen pH and fermentation.
In vitro Gas Production and DM Degradability
The R:C ratio and RALP supplementation level did not significantly impact on the a, b, and c. The R:C ratio at 40:60 yielded higher c constant than the R:C at 60:40. The a+b data for both R:C ratio were significantly increased by the RALP supplementation level, and there obtained the interactions. The accumulated total production has no significant difference among treatments.
In vitro DM degradability (%) was measured at both 12 and 24 h of incubation. Higher RALP supplementation level remarkably increased the DM degradability for both the R:C and was more pronounced for R:C at 40:60. There were no significant differences for both R:C and RALP interactions of the supplementation level. This occurrence could be due to more available carbohydrate for R:C at 40:60 and from the incremental RALP supplementation with combined phytonutrients (CT and SP) which could enrich the fermentation process. Furthermore, higher concentration of minerals could help buffer pH especially for R:C at 40:60. Aslam et al. (1991) pointed out that the use of rumen buffer such as NaHCO3 could be beneficial when more concentrate feed was fed. It was indicative that RALP supplementation could enhance the DM degradability; nevertheless, suitable level of supplementation level should be further investigated in in vivo trials.
Rumen Fermentation Parameters
Rumen fermentation end-products, namely, VFAs and NH3-N concentration, were synthesized by rumen microbiome. Russell and Rychlik (2001), Wallace et al. (2015), and Huws et al. (2018) have emphasized the close relationship of rumen microbiome and their fermentation utilization efficiency. As these fermentation end-products will serve as important substrates for the host ruminants. Under this work, the rumen pH was not changed but maintained higher for R:C at 60:40, as compared to 40:60, as the roughage level could have attributed to the result. It was notable that rumen NH3-N concentrations were higher for R:C at 40:60 and were declined when RALP supplementation level was increased for both R:C at 60:40 and 40:60, respectively, but there were no significant interactions. Total VFAs, C2, C3, C4, and C2:C3 ratio were greatly impacted. The C3 concentrations were clearly shown and enhanced by both R:C and RALP supplementation level, being more explicit for R:C at 40:60 and with increasing supplementation level of RALP, while C2:C3 were greatly lowered. The starch fed at high level in the diet appeared to improve the total VFA and C3 and decreased C2 and the C2:C3 ratio (Kim et al., 2012). Dietary source of RALP under this experiment has enormously supported the fermentation in which the beneficial outcomes were obtained. It was further speculated that level of RALP supplementation should be evaluated. Plants and fruit wastes that contain phytonutrients have shown their effects in modulating the rumen fermentation especially increased C3 concentration and mitigated methane production. The CH4 production reduced by inhibiter usually the hydrogen would be increased, which is rechanneled to other hydrogen sinks, such as propionic acid, which results in an increasing C3 concentration (Bodas et al., 2012; Kim et al., 2012; Singh et al., 2018).
Fermentation gas such as CH4 has been produced during anaerobic fermentation in the rumen. Johnson and Johnson (1995) reported the loss of metabolizable energy in the form of CH4 up to 15% digestible energy. Furthermore, CH4 is one of the greenhouse gases and it has a global warming potential 23 times that of CO2. Hence, the mitigation of CH4 via rumen fermentation has been the major concern. Dietary manipulation in the rumen has been receiving more interests (Hook et al., 2011). The protozoal population enumerated at 4 and 8 h of incubation and were reduced for both R:C ratio and by higher RALP supplementation level, and there was greatly interactive. As shown by many researchers that the reduction of protozoal population in the rumen has a direct effect on CH4 production since some methanogens adhered on protozoa. In addition, the presence of flavonoid extract in the feeds could also attribute to the mitigation of rumen CH4 by inhibiting cytoplasmic membrane function and cell wall synthesis contained in protozoal and methanogens (Sommai et al., 2021). As presented under this work, the rumen CH4 production was mitigated by increasing level of RALP supplementation for both R:C ratio at 60:40 and 40:60, respectively. This result could very well-support that RALP which could be highly promising to be employed in feeding to ruminant in order to improve rumen fermentation efficiency and mitigate CH4 production. Ampapon and Wanapat (2021) reported that supplementation of fruit peels' powder containing phytonutrients can reduce protozoa and methane production in dairy cows. Similarly, Gunun et al. (2018) reported that in vitro CH4 production was lower in the rambutan peel supplementation.
Under this investigation, plants rich in phytonutrients and minerals such as RALP have a promising role to modulate rumen fermentation in maintaining pH, which enhances propionate production, mitigates CH4 production, as well as increases DM degradability. In addition, the potential use of the R:C ratio at 40:60 could enhance gas production kinetic, nutrient degradability, and rumen fermentation. RALP supplementation demonstrated potential use as a rumen enhancer and deserves further in vivo trial investigation.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author/s.
Ethics Statement
The animal study was reviewed and approved by the Animal Care and Use Committee of Khon Kaen University and the Institute of Animals for Scientific Purpose Development (IAD), Thailand U1-06878-2560.
Author Contributions
TA planned and conducted the experiment feed preparation, samplings, and drafted the manuscript. BV feed preparation, samplings, and chemical analyses. PT chemical analyses and tabulation. MM statistical analysis interaction of data. MW supervised the design and execution of the experiment, interpretation of data, comment and supervised the writing manuscript, and handing of submission of the paper. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the Thailand Research Fund (TRF) through the Thailand Science Research and Innovation (TSRI) (TRF-IRN57W0002 and TRF-IRG5980010) and further supported by the Tropical Feed Resources Research and Development Center (TROFREC), Department of Animal Science, Faculty of Agriculture, Khon Kaen University, Thailand, and the Thailand Research Fund (TRF) through the Thailand Science Research and Innovation and Fundamental Fund (FF) project no. 65A103000130.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The reviewer AC declared a shared affiliation with several of the authors BV, MM, PT, and MW to the handling editor at the time of the review.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
Special appreciation was extended to the National Research Council of Thailand (NRCT) through the Basic Research Fund (record no. 2564A10302002).
References
Ampapon, T., and Wanapat, M. (2021). Mitigating rumen methane and enhancing fermentation using rambutan fruit peel powder and urea in lactating dairy cows. J. Anim. Physiol. and Anim. Nutr. 105, 1014–1023. doi: 10.1111/jpn.13526
AOAC. (2012). Official Methods of Analysis, 19th Edn. Gaithersburg, MD: Association of Official Analytical Chemists.
Aslam, M., Tucker, W. B., Hogue, J. F., Vernon, A. K., and Adams, G. D. (1991). Controlled ruminal infusion of sodium bicarbonate: effects of dietary and infused buffer on ruminal milieu. J. Dairy Sci. 74, 3496–3504. doi: 10.3168/jds.S0022-0302(91)78541-X
Barba de la Rosa, A. P., Fomsgaard, I. S., Laursen, B., Mortensen, A. G., Olvera-Martínez, L., Silva-Sánchez, C., et al. (2009). Amaranth (Amaranthus hypochondriacus) as an alternative crop for sustainable food production: phenolic acids and flavonoids with potential impact on its nutraceutical quality. J. Cereal. Sci. 49, 117–121. doi: 10.1016/j.jcs.2008.07.012
Bodas, R., Prieto, N., García-González, R., Andrés, S., Giráldez, F. J., and López, S. (2012). Manipulation of rumen fermentation and methane production with plant secondary metabolites. Anim. Feed. Sci. Technol. 176, 78–93. doi: 10.1016/j.anifeedsci.2012.07.010
Burns, R. E. (1971). Method for estimation of tannin in grain sorghum. Agron. J. 63, 511–512. doi: 10.2134/agronj1971.00021962006300030050x
Castro-Montoya, J. M., Makkar, H. P. S., and Becker, K. (2011). Chemical composition of rumen microbial fraction and fermentation parameters as affected by tannins and saponins using an in vitro rumen fermentation system. Can. J. Anim. Sci. 91, 433–448. doi: 10.4141/cjas2010-028
Chairatanayuth, P. (1992). Inclusion of amaranth crop residues in diet for cattle. Food Rev. Int. 8, 159–164. doi: 10.1080/87559129209540934
De Souza, J., Leskinen, H., Lock, A. L., Shingfield, K. J., and Huhtanen, P. (2020). Between-cow variation in milk fatty acids associated with methane production. PLoS One 15:e0235357. doi: 10.1371/journal.pone.0235357
Galyean, M. L. (1989). Laboratory Procedure in Animal Nutrition Research. Department of Animal and Life Science, New Mexico State University.
Gunun, P., Gunun, N., Cherdthong, A., Wanapat, M., Polyorach, S., Sirilaophaisan, S., et al. (2018). In vitro rumen fermentation and methane production as affected by rambutan peel powder. J. Appl. Anim. Res. 46, 626–631. doi: 10.1080/09712119.2017.1371608
Herremans, S., Vanwindekens, F., Decruyenaere, V., Beckers, Y., and Froidmont, E. (2020). Effect of dietary tannins on milk yield and composition, nitrogen partitioning and nitrogen use efficiency of lactating dairy cows: a meta-analysis. J. Anim. Physiol. Anim. Nutr. 104, 1209–1218. doi: 10.1111/jpn.13341
Hook, S. E., Steele, M. A., Northwood, K. S., Dijkstra, J., France, J., Wright, A. D. G., et al. (2011). Impact of subacute ruminal acidosis (SARA) adaptation and recovery on the density and diversity of bacteria in the rumen of dairy cows. FEMS Microbiol. Ecol. 78:275–284. doi: 10.1111/j.1574-6941.2011.01154.x
Huws, S. A., Creevey, C. J., Oyama, L. B., Mizrahi, I., Denman, S. E., Popova, M., et al. (2018). Addressing global ruminant agricultural challenges through understanding the rumen microbiome: past, present, and future. Front. Microb. 9:2161. doi: 10.3389/fmicb.2018.02161
Johnson, K. A., and Johnson, D. E. (1995). Methane emissions from cattle. J. Anim. Sci. 73, 2483–2492. doi: 10.2527/1995.7382483x
Kang, S., Wanapat, M., and Cherdthorng, A. (2014). Effect of banana flower powder supplementation as a rumen buffer on rumen fermentation efficiency and nutrient digestibility in dairy steers fed a high-concentrate diet. Anim. Feed. Sci. Technol. 196, 32–41. doi: 10.1016/j.anifeedsci.2014.07.003
Kang, S., Wanapat, M., and Viennasay, B. (2016). Supplementation of banana flower powder pellet and plant oil sources on in vitro ruminal fermentation, digestibility, and methane production. Trop. Anim. Health Prod. 48, 673–1678. doi: 10.1007/s11250-016-1142-2
Karamać, M., Gai, F., Longato, E., Meineri, G., Janiak, M. A., Amarowicz, R., et al. (2019). Antioxidant activity and phenolic composition of Amaranth (Amaranthus caudatus) during plant growth. Antioxidants (Basel) 8:173. doi: 10.3390/antiox8060173
Khandaker, L., Akond, A. M., Ali, M. B., and Oba, S. (2010). Biomass yield and accumulations of bioactive compounds in red amaranth (Amaranthus tricolor, L.) grown under different colored shade polyethylene in spring season. Sci. Hortic. 123, 289–294. doi: 10.1016/j.scienta.2009.09.012
Kim, J. M., Park, J. A., and Kim, D. H. (2012). Comparative proteomic analysis of chestnut blight fungus, Cryphonectria parasitica, under tannic-acid-inducing and hypovirus-regulating conditions. Can. J. Microbiol. 58, 863–871. doi: 10.1139/w2012-065
Koike, S., and Kobayashi, Y. (2001). Development and use of competitive PCR assays for the rumen cellulolytic bacteria: Fibrobacter succinogenes, Ruminococcus albus and Ruminococcus flavefaciens. FEMS Microb. Lett. 204, 361–366. doi: 10.1111/j.1574-6968.2001.tb10911.x
Kwon, J. H., Belanger, J. M., Pare, J. J., and Yaylayan, V. A. (2003). Application of the microwave-assisted process (MAP™) to the fast extraction of ginseng saponins. Food Res. Int. 36, 491–498. doi: 10.1016/S0963-9969(02)00197-7
McAllister, T. A., Beauchemin, K. A., Alazzeh, A. Y., Baah, J., Teather, R. M., and Stanford, K. (2011). The use of direct fed microbial to mitigate pathogens and enhance production in cattle. Can. J. Anim. Sci. 91, 193–211. doi: 10.4141/cjas10047
Menke, K. H., Raab, L., Salewski, A., Steingass, H., Fritz, D., and Schneider, W. (1979). The estimation of the digestibility and metabolizable energy content of ruminant feedstuffs from the gas production when they are incubated with rumen liquor in vitro. J. Agric. Sci. 92, 217–222. doi: 10.1017/S0021859600086305
Ørskov, E. R., Fraser, C., and Gordon, J. G. (1974). Effect of processing of cereals on rumen fermentation, digestibility, rumination time, and firmness of subcutaneous fat in lambs. Br. J. Nutr. 32, 59–69. doi: 10.1079/BJN19740058
Ørskov, E. R., and McDonal, I. (1979). The estimation of protein degradability in the rumen from incubation measurements weighted according to rate of passage. J. Agric. Sci. 92, 499–503. doi: 10.1017/S0021859600063048
Paśko, P., Sajewicz, M., Gorinstein, S., and Zachwieja, Z. (2008). Analysis of selected phenolic acids and flavonoids in Amaranthus cruentus and Chenopodium quinoa seeds and sprouts by HPLC. Acta. Chromatog. 20, 661–672. doi: 10.1556/AChrom.20.2008.4.11
Pulipati, S., Babu, P. S., Naveena, U., Parveen, S. R., Sumaya, S. K., and Nausheen, M. (2017). Determination of total phenolic, tannin, flavonoid contents and evaluation of antioxidant property of Amaranthus tricolor (L). Int. J. Pharm. Phytoch. Res. 9, 814–819. doi: 10.25258/phyto.v9i6.8184
Russell, J. B., and Rychlik, J. L. (2001). Factors that alter rumen microbial ecology. Science 292, 1119–1122. doi: 10.1126/science.1058830
Samuel, M., Sagathevan, S., Thomas, J., and Mathen, G. (1997). An HPLC method for estimation of volatile fatty acids in ruminal fluid. Indian J. Anim. Sci. 67, 805–807.
SAS (Statistical Analysis System). (2013). User's Guide: Statistic, Version 9, 4th Edn. Cary, NC: SAS Institute Inc.
Singh, I. Q. B. A.L., Hundal, J. S., Wadhwa, M., and Lamba, J. S. (2018). Assessment of potential of some tannins and saponins containing herbs on digestibility of nutrients, fermentation kinetics and enteric methane production under different feeding systems: an in vitro study. Indian J. Anim. Sci. 88, 443–452.
Sittijunda, S., Reungsang, A., and O-thong, S. (2010). Biohydrogen production from dual digestion pretreatment of poultry slaughterhouse sludge by anaerobic self-fermentation. Int. J. Hydro. Energy 35, 13427–13434. doi: 10.1016/j.ijhydene.2009.11.116
Sommai, S., Cherdthong, A., Suntara, C., So, S., Wanapat, M., and Polyorach, S. (2021). In vitro fermentation characteristics and methane mitigation responded to flavonoid extract levels from alternanthera sissoo and dietary ratios. Fermentation 7:109. doi: 10.3390/fermentation7030109
Uddin, A. H., Khalid, R. S., Alaama, M., Abdualkader, A. M., Kasmuri, A., and Abbas, S. A. (2016). Comparative study of three digestion methods for elemental analysis in traditional medicine products using atomic absorption spectrometry. J. Anal. Sci. Technol. 7:6. doi: 10.1186/s40543-016-0085-6
Van Soest, P. J., Robertsonand, J. B., and Lewis, B. A. (1991). Methods for dietary fiber neutral detergent fiber, and nonstarch polysaccharides in relation to animal nutrition. J. Dairy Sci. 74, 3583–3597. doi: 10.3168/jds.S0022-0302(91)78551-2
Wallace, R. J., Rooke, J. A., McKain, N., Duthie, C. A., Hyslop, J. J., Ross, D. W., et al. (2015). The rumen microbial metagenome associated with high methane production in cattle. BMC Genomics 16:839. doi: 10.1186/s12864-015-2032-0
Wanapat, M., Gunun, P., Anantasook, N., and Kang, S. (2014). Changes of rumen pH, fermentation and microbial population as influenced by different ratios of roughage (rice straw) to concentrate in dairy steers. J. Agric. Sci. (Cambridge) 152, 675–685. doi: 10.1017/S0021859613000658
Wanapat, M., Viennasay, B., Matra, M., Totakul, P., Phesatcha, B., Ampapon, T., et al. (2021). Supplementation of fruit peel pellet containing phytonutrients to manipulate rumen pH, fermentation efficiency, nutrient digestibility and microbial protein synthesis. J. Sci. Food Agric. 101, 4543–4550. doi: 10.1002/jsfa.11096
Wolin, M. J. (1960). A theoretical rumen fermentation balance. J. Dairy Sci. 43, 1452–1459. doi: 10.3168/jds.S0022-0302(60)90348-9
Keywords: phytonutrients, rumen ecology, leaf supplementation, degradability, ruminant
Citation: Ampapon T, Viennasay B, Matra M, Totakul P and Wanapat M (2022) Phytonutrients in Red Amaranth (Amaranthus cruentus, L.) and Feed Ratios Enhanced Rumen Fermentation Dynamics, Suppress Protozoal Population, and Methane Production. Front. Anim. Sci. 3:741543. doi: 10.3389/fanim.2022.741543
Received: 14 July 2021; Accepted: 25 February 2022;
Published: 11 April 2022.
Edited by:
Vivian Fischer, Federal University of Rio Grande do Sul, BrazilReviewed by:
Anusorn Cherdthong, Khon Kaen University, ThailandHalima Sultana, University of Florida, United States
Neeta Agarwal, Indian Veterinary Research Institute (IVRI), India
Hui Yin Tan, Tunku Abdul Rahman University College, Malaysia
Copyright © 2022 Ampapon, Viennasay, Matra, Totakul and Wanapat. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Metha Wanapat, bWV0aGFAa2t1LmFjLnRo
 Thiwakorn Ampapon
Thiwakorn Ampapon Bounnaxay Viennasay2
Bounnaxay Viennasay2 Metha Wanapat
Metha Wanapat