- 1Department of Population Medicine, Ontario Veterinary College, University of Guelph, Guelph, ON, Canada
- 2Department of Large Animal Clinical Sciences, College of Veterinary Medicine, Michigan State University, East Lansing, MI, United States
- 3Department of Veterinary Diagnostic and Production Animal Medicine, College of Veterinary Medicine, Iowa State University, Ames, IA, United States
- 4York Health Economic Consortium, University of York, York, United Kingdom
This systematic review and network meta-analysis aimed to estimate the relative efficacy of dry cow antimicrobial therapies, registered in Canada and/or the United States, to cure existing intramammary infections (IMI) in dairy cattle. The controlled trials examining all-cause cures of existing IMI present at dry-off were eligible. Five databases and four conference proceeding platforms were searched. The risk of bias at the level of the outcome was assessed using the Cochrane 2.0 risk of bias instrument (Cochrane, Denmark), and the overall confidence in the findings from the network meta-analysis was assessed using the Confidence in Network Meta-Analysis (CINeMA) platform. Of 3,743 articles screened for eligibility by the two independent reviewers, 58 trials were included in the Bayesian network meta-analysis for the all-cause cure of existing IMI from dry-off to calving. No antimicrobial treatment (non-active control) was associated with a decreased risk of a cure compared with all other currently labeled antimicrobials in Canada and the United States; however, lack of replication trials for some antimicrobial products created large credibility intervals and, therefore, we were unable to identify meaningful comparisons between the products. Poor reporting of trial features, heterogeneity in outcome measurements, and high risk of bias in some domains further contributed to this inability to compare antimicrobials. Continued improvement in the reporting of animal trials is required to make recommendations for antimicrobial products on the basis of efficacy.
Systematic Review Registration: https://atrium.lib.uoguelph.ca/xmlui/bitstream/handle/10214/16236/Protocol_NMA_efficacy_dryoff_antibiotics_cure_IMI.pdf?sequence=3&isAllowed=y.
Introduction
Intramammary infections (IMI) that are left untreated during the dry period can develop into clinical mastitis (CM) in the subsequent lactation (Pantoja et al., 2009; Bhutto et al., 2011), which affects production and milk quality. The importance of curing and preventing IMI during the dry period has led to the development of indications, such as the Pan-European agreement on dry cow therapy (2017), which states that only animals likely to be infected should receive dry cow antimicrobial therapy, although herds at high-risk for infections should be considered for the treatment with antimicrobials in addition to internal teat sealant products (Bradley et al., 2018). However, the WHO and the Canadian Veterinary Medical Association recommend reducing the use of antimicrobials of importance to human health in livestock production animals (Canadian Veterinary Medical Association, 2017; World Health Organization, 2017).
Given competing guidelines, the evaluation of comparative efficacies of dry cow antimicrobial therapy options is important when selecting an antibiotic. The use of non-efficacious antimicrobials contributes to use without benefit to animal health or welfare. To gather the highest level of evidence for the efficacy of antimicrobials to cure existing IMI in a field setting, a systematic review methodology can be used to synthesize data from controlled trials (Sargeant and O'Connor, 2014). The addition of a network meta-analysis allows the comparison of all dry cow antimicrobial products, which compares the products beyond the traditional pairwise meta-analysis of two interventions (Li et al., 2011). Network meta-analyses have the added benefit of providing direct comparisons of interventions within the controlled trials and indirect comparisons of interventions across the trials that share a common comparison group (e.g., non-active control) (Li et al., 2011). When enough evidence is available, network meta-analyses provide a rigorous synthesis of all available treatments to aid in clinical decision-making.
Pairwise meta-analyses have compared the efficacy of antimicrobials for the prevention of new IMI (Robert et al., 2006; Halasa et al., 2009b) and cure of existing IMI (Halasa et al., 2009a), examined blanket vs. selective dry cow therapy (Winder et al., 2019e), examined non-antimicrobial products for cure and prevention of IMI (Rabiee and Lean, 2013; Dufour et al., 2019), and prevention of IMI in heifers (Naqvi et al., 2018). Network meta-analyses have been used to investigate antimicrobials to prevent new IMI and CM in the early lactation period (Winder et al., 2019b), the treatment of CM in lactating cattle (Winder et al., 2019c; Nobrega et al., 2020), and the use of teat sealants to prevent IMI and CM (Winder et al., 2019d). The objective of this systematic review and network meta-analysis was to investigate the comparative efficacy of dry cow antimicrobial therapy, registered in Canada and/or the United States, to cure existing IMI in dairy cattle, which till today has not been summarized using network meta-analytic approaches. Producers and veterinarians can use efficacy information to inform dry cow treatment decisions. For example, where indicated as less or equally efficacious, the antimicrobials deemed important for human health can be avoided.
Materials and Methods
Protocol and Registration
A review protocol was created a priori and can be accessed online1. Manuscript preparation followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses extension for Network Meta-Analyses (PRISMA-NMA) reporting guidelines (Hutton et al., 2015; RRID:SCR_018721).
Eligibility Criteria
The controlled trials with naturally occurring diseases and published in English were eligible. The trial must have examined dairy cattle with an IMI at the cessation of lactation, defined by laboratory confirmation of one or more pathogens or a somatic cell count (SCC) cut point. The eligible interventions were antimicrobial dry cow treatment intervention with a comparison to an antimicrobial treatment, non-antimicrobial treatment method, or non-active comparator. At least one of the following outcomes had to be reported: all-cause cure of existing IMI from dry-off to calving; incidence of clinical mastitis over the first 30 days in milk (DIM) in cows with an IMI at the cessation of lactation; or total antimicrobial use over the first 30 DIM in cows with an IMI at the cessation of lactation. There were no date or study location restrictions.
There was no restriction on the eligible antimicrobial products included in the systematic review or network meta-analysis; however, the reporting of comparative efficacy and risk of bias in the overall network was limited to the treatment protocols labeled for use in Canada and the United States in 2019, as outlined by the Compendium for Veterinary Products, the Canada and United States versions (Animalytix, 2020a,b). The products containing the same compounds, but administered via a different route or dose, were also eligible for inclusion in comparative efficacy reporting and risk of bias analysis.
Information Sources
The database search was conducted on June 14, 2019: Medline (via Ovid SP), CAB Abstracts (via CAB Interface), Science Citation Index (via Web of Science), Conference Proceedings Citation Index—Science (via Web of Science), and Agricola (via Proquest). The following conference proceedings were hand-searched from 1997 to 2019 for papers ≥500 words: Proceedings of the American Association of Bovine Practitioners, World Association for Buiatrics, National Mastitis Council Conference Proceedings, and IDF Mastitis Conference Proceedings. The Freedom of Information New Animal Drug Approvals (NADA) summaries from the Food and Drug Administration (FDA) website (US Food and Drug Administration, 2019) were searched by drug use (dry cow therapy). Contact with study authors was not conducted.
Search
The search string for Science Citation Index (via Web of Science) is provided in Table 1. The search results were downloaded into EndNote2 (EndNote X7, Clarivate Analytics, Philadelphia; RRID:SCR_014001) and deduplicated. The references were then uploaded into DistillerSR3 (Evidence Partners Inc., Ottawa, ON, Canada), and further deduplicated.

Table 1. Search strategy to identify the relevant articles for the network meta-analysis assessing the relative efficacy of antimicrobial treatment protocols to cure existing intramammary infections (IMI) during the dry period in dairy cattle, conducted on June 14, 2019, in Science Citation Index (Web of Science).
Study Selection
Study selection, data extraction, and assessment of the risk of bias were performed in DistillerSR. The title and abstract screening pretest involved four reviewers screening 100 articles. Two reviewers independently screened each title and abstract using three primary screening questions: (1) Is the title or abstract available in English?; (2) Is a primary research study described in the title or abstract?; and (3) Are dry-off antimicrobial treatments in dairy cattle with an existing IMI described within the title or abstract? A response of “no” from two reviewers to any of the above questions resulted in exclusion. The full-text screening pre-test used 10 full-text articles. Six secondary screening questions were applied: (1) Is the study available in English?; (2) Is this a primary research study?; (3) Are dry-off antimicrobial treatments in dairy cattle with an existing IMI reported within the article?; (4) Is an eligible comparison group reported within the article?; (5) Are one or more of the following outcomes reported in the article: cure of existing IMI from dry-off to calving, the incidence of CM in the first 30 DIM in cows with an existing IMI at the cessation of lactation, or metrics for total antimicrobial use in the first 30 DIM in cows with an existing IMI at the cessation of lactation?; and (6) Is the study a controlled trial with natural disease exposure? An answer of “no” by two reviewers resulted in exclusion. The conflicts were resolved by mediation with a third reviewer where needed.
Data Collection Process
Data extraction was conducted in DistillerSR. The forms were pre-tested using four references. Data extraction from the relevant studies was conducted independently two times, and a third reviewer was consulted if consensus could not be reached.
Data Items
Study Characteristics
Study-level data included the year of publication, year of study conduct, country, the number of herds enrolled in the study, herd setting (commercial or research), breed, lactation number, and inclusion criteria at herd- and cow-level.
Population
To be eligible for a cure, the cows must have been diagnosed with an existing IMI at the cessation of lactation. Bacteriologic culture of one or more pathogens or any SCC cut point (e.g., cows with SCC >200,000 cells/ml were considered infected) were acceptable definitions, and details surrounding definitions were collected.
Intervention and Comparators
Intervention and comparator data included allocation level for the intervention (quarter or cow), number of study units enrolled, antimicrobial or non-antimicrobial products administered, route and frequency of administration, dose, and any concurrent treatments.
Outcomes
The authors had to provide case definitions for the all-cause cure of IMI (e.g., bacteriologic culture, SCC cut point, California Mastitis Test) and for clinical mastitis (e.g., visual assessment, udder palpation). All-cause cure data included any of the following outcomes: major pathogen cure, minor pathogen cure, all pathogen cure, Streptococci and Staphylococci cure, or a combination of these definitions. If only species-specific, general Gram-negative or Gram-positive cures were reported without all-cause cure also reported, these trials were excluded. We prioritized which outcomes were extracted as follows: adjusted summary effect size [adjusted odds ratios (OR) or relative risks (RR)] for dichotomous outcomes or adjusted mean differences for continuous outcomes (proportion differences), unadjusted summary effect estimates, and arm-level risk data. The methods of controlling for non-independence of observations were extracted when reported.
Risk of Bias Within Individual Studies
Risk of bias was assessed at the outcome level for the cure of existing IMI in dairy cattle at dry-off using the Cochrane Risk of Bias 2.0 instrument (Cochrane, Denmark) (Higgins et al., 2016), with the assessment questions modified as described in Winder et al. (2019b). The risk of bias was assessed independently two times, with disagreement resolved by consensus and mediation by a third reviewer where needed.
Summary Measures
Outcome data were analyzed on the log OR scale. For reporting, the log OR were back-transformed to the RR using the baseline risk from the model data assuming the baseline prior distribution was approximately normal. The posterior mean and standard deviation (SD) of the baseline risk mean were −0.57393 and 0.17353. The posterior mean and SD of the baseline risk SD were 0.92931 and 0.13260.
Data Manipulation Prior to Analysis
An a priori plan to merge the treatment groups was not considered in the protocol; however, a great deal of heterogeneity exists in the antimicrobial compounds and dosing in the literature. A previous network meta-analysis indicated creating a unique treatment regimen for each product and dose would have led to a sparse network (Winder et al., 2019b). Therefore, a post-hoc decision was made to merge several treatments based on biological and clinical relevancy. The antimicrobial products were considered separately, except for penicillin-aminoglycosides, which were combined based on the published guidance (World Organisation for Animal Health, 2007). Different dosages of the same route and duration of use for an antimicrobial product were combined. Extended therapy treatment protocols (antimicrobial products used more than once at dry-off) remained separate from the single-therapy treatments. All non-antimicrobial products (aside from teat sealants), such as vitamins and minerals, were merged due to their relative unimportance in the cure of IMI during the dry period (Mullen et al., 2014). The active antimicrobial products were combined with their antimicrobial and teat sealant combinations (e.g., cloxacillin was combined with cloxacillin-teat-seal of the same dose) as the teat sealants are only approved for use in the prevention of IMI (Animalytix, 2020a,b), and were not considered influential on the cure. The antimicrobial products are further referred to as treatment protocols to encompass these groupings.
Network Meta-Analysis
Planned Methods of Analysis and Implementation
A Bayesian network meta-analysis was conducted for the outcome of all-cause cure of existing IMI as previously described (Dias et al., 2011; O'Connor et al., 2013; Hu et al., 2020) that includes assessment of the geometry of the network (Salanti et al., 2008). Vague priors [i.e., N (0, 10,000)] were used for all basic parameters (Hu et al., 2020). For this model, weakly informative priors for variance, such as σ ~ U (0,2) and σ ~ U (0,5) were assessed (Dias et al., 2011; Hu et al., 2020). The analysis suggested similar results using both priors, thus σ ~ U (0,2) was kept in the model.
Markov Chain Monte Carlo (MCMC) simulation was implemented in Just Another Gibbs Sampler (JAGS) software (version 4.3.0) (Plummer et al., 2019) to generate all posterior summaries. The statistical analyses were performed using R software (version 3.6.0; R Core Team, Austria; Planting of a Tree) in a Catalina OS system (R Core Team, 2019). The model was fit by calling JAGS from R through the RJAGS package (Plummer et al., 2019; RRID:SCR_017573). Three chains of 10,000 iterations were simulated and convergence was assessed through the visualization of basic parameters in a history plot. Five thousand “burn-in” iterations were run then discarded, and the inference was based on a further 10,000 iterations. Model output included all possible pairwise comparisons of the log OR for the all-cause cure of existing IMI for the assessment of consistency, relative risks, and mean treatment ranks.
Assessment of Model Fit and Consistency
Model fit was assessed using the method proposed by Dias et al. (2010). Assessing the consistency involved comparing direct and indirect evidence for each treatment comparison that contained direct evidence within the network, as previously described (Dias et al., 2010; Hu et al., 2020).
Risk of Bias in Overall Network
The confidence in cumulative evidence from the overall network was evaluated using a modification of the Confidence in Network Meta-Analysis (CINeMA) platform (Nikolakopoulou et al., 2020; Papakonstatinou et al., 2020). We assessed the contribution of the risk of bias attributed to the randomization process and the risk of bias attributed to blinding of caregivers on the overall estimates of the network meta-analysis because the trials that fail to report these domains often have exaggerated treatment effects (Moher et al., 1998; Sargeant et al., 2009). The risk of bias due to the randomization process was reported as “no concerns,” “some concerns,” or “major concerns,” following the Cochrane Risk of Bias 2.0 instrument flow diagram (Higgins et al., 2016). The risk of bias due to blinding of the caregivers was assessed as “no concerns” if the caregivers were blind to the treatment allocation, “some concerns” if blinding of the caregivers was not reported, and “major concerns” if the caregivers were not blind to the treatment allocation. The results for blinding of caregivers were presented rather than outcome assessors, as bias due to blinding of outcome assessors was determined to be “low” in all but three trials, as the assessment was typically objective (laboratory diagnosis). In three trials, a part of the IMI diagnosis involved the use of the California Mastitis Test, which was considered subjective, and therefore, bias risk was determined to be “high” as the outcome assessors were not blinded in these studies.
The impact of indirectness on the overall network, which refers to the generalizability of included studies, was not considered an issue for this review due to the eligibility criteria for the trials reflecting commercial settings. The impact of imprecision on the overall network was assessed using below 0.8 or above 1.25 as clinically important effect sizes. The credibility intervals that spanned these values in either or both directions (protective, no effect, or hazardous) could potentially lead to different clinical decisions. Heterogeneity was assessed using an OR of 0.8.
The risk of bias across the studies was not assessed for this review because none of the pairwise comparisons were evaluated in more than 10 trials (Sterne et al., 2000).
Additional Analyses
An additional analysis was conducted to evaluate the network of evidence using only trials published between 1990 and 2019. The eradication programs for Streptococcus agalactiae, a bacterium very susceptible to penicillin products, were implemented in the 1980s and have achieved low levels of this pathogen in dairy herds (Makovec and Ruegg, 2003; Keefe, 2012), such that the penicillin products could have an inflated measure of cure of existing IMI within the network in studies prior to 1990. This analysis was not pre-specified in the protocol, nor did it provide inferences that differed from the full network meta-analysis. Therefore, this analysis (1990 to 2019) is not further discussed, but the results are available online (McMullen et al., 2021). The results from the full analysis (all studies) are presented below.
Results
Study Selection
The flow of studies through the screening process is presented in Figure 1. Following both levels of eligibility screening, an all-cause cure of existing IMI from dry-off to calving was reported in 72 trials. Incidence of CM in the first 30 DIM in cows with an existing IMI was reported in six trials, and a metric for total antimicrobial use in the first 30 DIM in cows with an existing IMI was reported in one trial. Because the incidence of CM and antimicrobial use outcomes were reported in a limited number of trials, these outcomes were not further analyzed or discussed in this manuscript. Cure of Staphylococcus aureus was reported in an additional 27 trials, and cure of Gram-positive bacteria was reported in an additional two trials; these trials were excluded from the analyses because all-cause data were not reported. Following the treatment merging process, 13 trials were removed because the intervention and comparator treatment arms became the same. One trial was removed due to insufficient data available to calculate the variance of the log odds in one of the treatment arms. Therefore, 58 trials from 53 articles were included in the network meta-analysis.
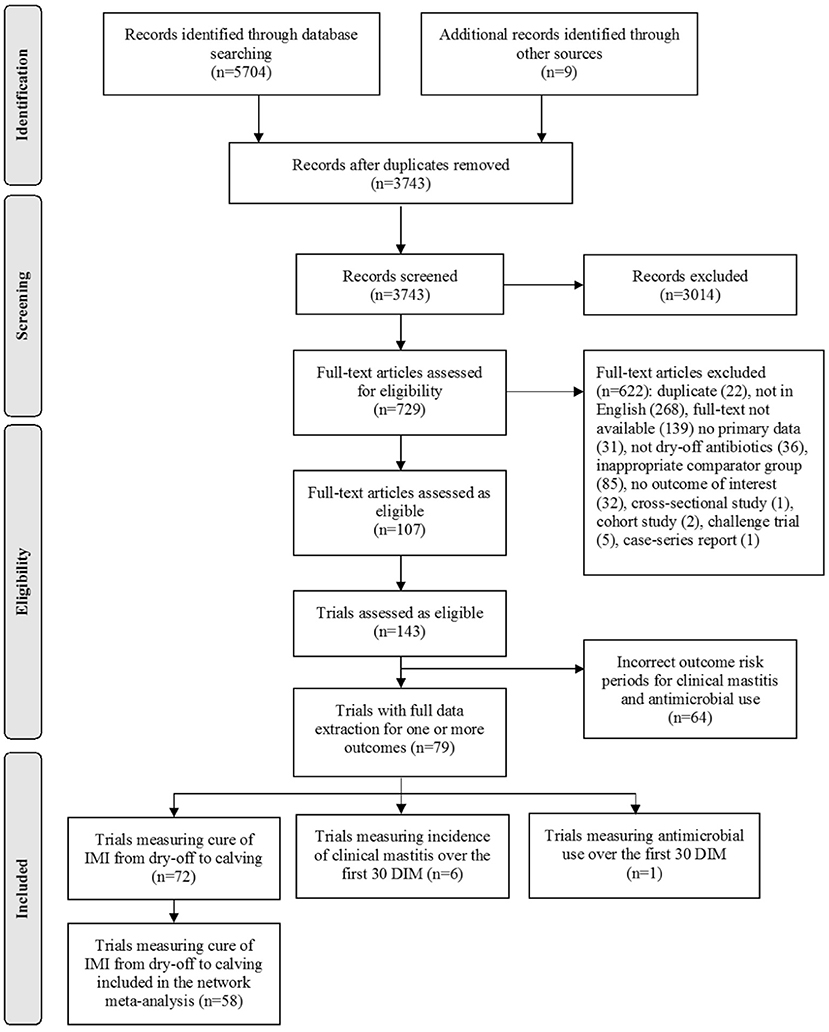
Figure 1. Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) flow diagram of the included studies and trials for the systematic review of dry-off antimicrobials to cure existing intramammary infections (IMI) in dairy cattle (Moher et al., 2009). The search, conducted by the researchers at the University of York, UK provided an update to a search used to identify the articles for a previous systematic review and network meta-analysis (Winder et al., 2019e).
Study Characteristics
The study characteristics for the 58 trials are included in Supplementary Table 1. The trials were conducted in 19 countries, the majority in the United States (n = 19), the United Kingdom (n = 7), or Ireland (n = 7). Most trials were conducted in commercial dairy herds (47/58; 81.0%), followed by research/university herds (4/58; 6.9%), and in both commercial and research/university herds (2/58; 3.5%). The type of study herd was not reported in three trials (5.2%). Twenty-eight trials were published prior to 1990, 28 trials were published in or following 1990, and the year of publication was not reported for two trials. The breed of dairy cattle studied was not reported in over half of the trials (33/58; 56.9%), but of remaining trials, the breed was Holstein cattle (14/58; 24.1%), multiple breeds (7/58; 12.1%), and one study each reporting Sahiwal, Norwegian Red, Lowland Black and White, and Brown Swiss cattle. The number of herds investigated in each trial was reported for 51 trials, the majority of which only included one herd (17/51; 33.3%). The number of herds within a trial ranged from 1 to 288.
Risk of Bias Within Individual Studies—The Cure of IMI From Dry-Off to Calving
The assessment of the within-study risk of bias resulted in all trials deemed to be of “high” risk (26/58; 44.8%) or “some concerns” (32/58; 55.2%).
We assessed 13 trials as “high” risk of bias for the randomization process, 42 trials with “some concerns,” and assessed three trials as “low” risk. The word “random” was used to describe allocation in almost half of the trials without details on sequence generation (22/58; 37.9%).
The risk of bias assessment due to deviations from the intended interventions resulted in 39 trials with “some concerns,” 19 trials with “low” risk, and no trials with “high” risk in this domain. Authors from five trials reported blinding of the caregivers (8.6%), and the majority of authors failed to report information for the management of study animals; therefore, deviations from the interventions could not be assessed (31/58; 53.5%). Most treatments were applied once at dry-off, which reduced the risk for deviations from the intended interventions; therefore, none of the trials were assessed as “high” risk bias in this domain.
We assessed five trials as “high” risk of bias due to missing outcome data, 15 as “some concerns,” and 38 as “low” risk of bias. The losses to follow-up were <5% in over half of the trials (30/58; 51.7%). When the losses to follow-up were >5%, the reasons for missing observations that likely affected the outcome (i.e., the cows were removed due to clinical mastitis) were reported in six trials (6/14; 42.9%). Of these six trials, the reasons for missing observations that likely affected the outcome were balanced among the intervention groups in two trials, which resulted in a low risk of bias for these trials. The fifth high risk trial was assessed as having missing outcome data that did not appear to be missing at random, but no information was provided for the loss to follow-up or reasons for missing outcome data.
The risk of bias assessment for measurement of the outcome resulted in a large number of “low” risk of bias trials (55/58; 94.8%), as cure of IMI was most often objectively assessed in the trials (i.e., bacteriologic culture or somatic cell count). The use of the California Mastitis Test as part of diagnosis was considered subjective, and reported in three trials, resulting in a “high” risk of bias (3/58; 5.2%).
The fifth domain, bias in the selection of the reported result, requires an a priori trial protocol to be published and available. A protocol created prior to the trial commencement was not reported in any trial, therefore, all the trials had “some concerns” in this domain.
Results of Individual Studies
Following the merge of treatment arms, a comparison of a non-active control to active treatment was made in about half of the included trials (30/58; 51.7%), of which 19 were two-arm trials and 11 were multi-arm (three or more) trials. Comparison of an active to active treatment was made in the rest of included trials, of which 21 were two-arm trials and seven were multi-arm trials.
Network Meta-Analysis: All-Cause Cure of Existing IMI From Dry-Off to Calving
The full network plot for the all-cause cure of existing IMI is shown in Figure 2. Forty unique treatment protocols from the 58 trials were included in the network, of which 40 were two-arm trials, 12 were three-arm trials, four were four-arm trials, and two were five-arm trials. In addition to non-active control and teat sealant, 31 treatment protocols were administered via intramammary route, six treatment protocols were administered via intramuscular route, and one protocol was a combination of intramammary and intramuscular treatment (Table 2).
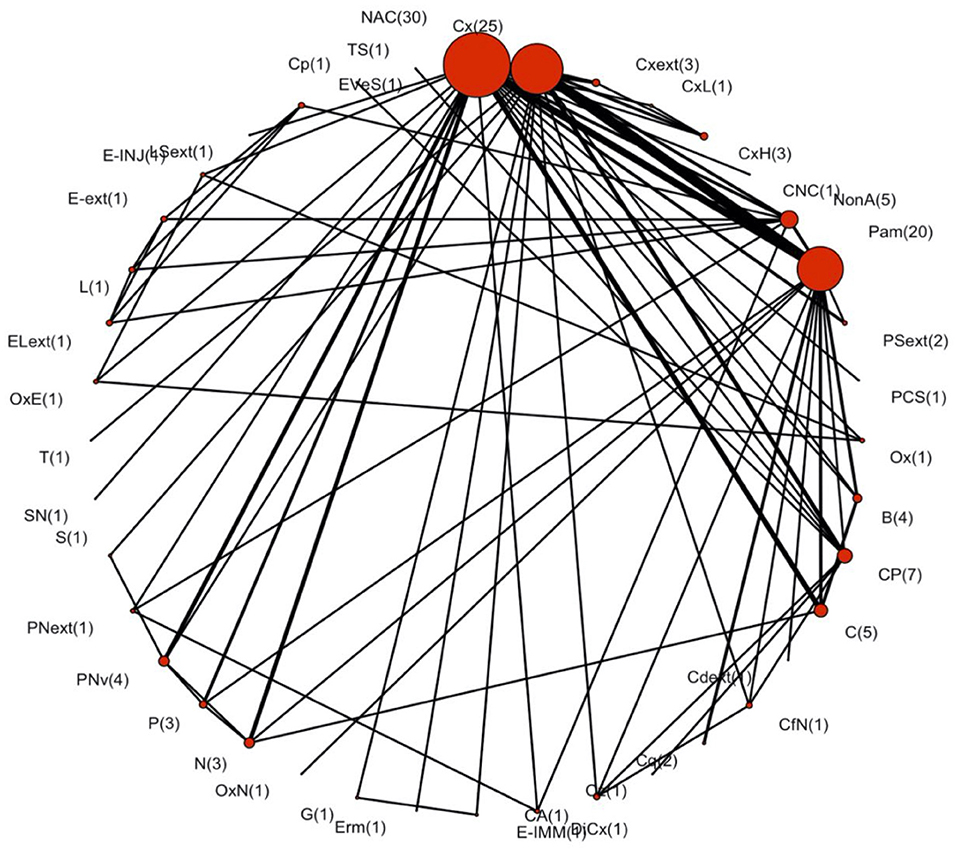
Figure 2. Network plot assessing the efficacy of dry cow antimicrobials, non-antimicrobial products, or placebos for the all-cause cure of intramammary infections during the dry period in dairy cattle. This plot contains 58 trials, with the number of treatment comparisons provided in parentheses. Non-active control had the most treatment comparisons (30 arms). Node size represents the number of times each treatment was used. Edge size represents the number of direct comparisons made between the two treatment protocols. The definitions of treatment acronyms are available in Table 2.

Table 2. Description of the treatment arms included in the network meta-analysis assessing the relative efficacy of antimicrobial dry cow products for cure of existing IMI during the dry period, and the corresponding tables and figures.
Summary of Network Geometry
The list of treatment protocols before and after the merging process is included in Supplementary Table 2. All the treatments were connected within the network. The geometry of the network was visually dominated by non-active control, cloxacillin, and penicillin-aminoglycosides, and the observed network co-occurrence score was 7.9 (95% CI: 7.4–7.8), which indicated a selective pattern of treatments used within the network comparisons (Salanti et al., 2008). The probability of interspecific encounter index was 0.9 indicating a diverse network (Salanti et al., 2008). The convergence of all the basic parameters was reached following 10,000 iterations.
Assessment of Model Fit and Consistency
The residual deviance of the network model was 82.9, and our data were comprised of 84 log OR suggesting no issues with model fit. None of the estimates resulting from the direct comparisons of treatment protocols were inconsistent with the estimates from indirect comparisons; therefore, no trials were removed from further analyses.
Mean Ranks and Probability Distribution for the Cure of IMI at Calving
The RR from the network meta-analysis for intramammary dry cow treatments currently labeled for use in Canada or the United States to cure existing IMI are provided in Figure 3. This figure provides a comparison of the risk of experiencing a cure of existing IMI from dry-off to calving when using the row treatment compared with the column treatment, for all the antimicrobial products included in this study. The left-hand section of the figure represents the 95% credibility intervals for each RR, in a mirrored fashion. For example, no antimicrobial treatment (non-active control; NAC) was associated with a decreased risk of experiencing a cure when compared with cloxacillin (Cx) (RR: 0.46, 95% CI: 0.22–0.80), penicillin-aminoglycosides (Pam) (RR: 0.51, 95% CI: 0.26–0.82), and ceftiofur (B) (RR: 0.52, 95% CI: 0.24–0.84), among others.
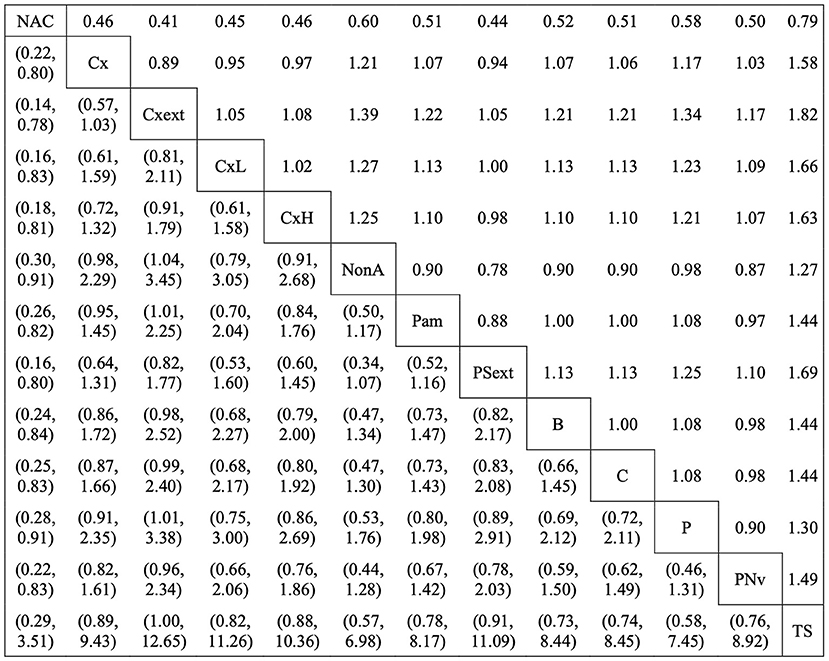
Figure 3. The relative risk ratios (RR) of currently labeled antimicrobial treatment protocols in Canada and the United States that were assessed in the network for the all-cause cure of existing intramammary infections during the dry period. The right-hand side of the matrix indicates the RR of row treatment compared with the column treatment (i.e., the risk of experiencing a cure with non-active control is 0.46 times the risk of experiencing a cure with cloxacillin). The left-hand side of the matrix indicates the 95% credibility intervals (CI) for each RR. The treatment acronyms are as follows: non-active control (NAC), cloxacillin (Cx), cloxacillin–extended therapy (Cxext), cloxacillin–low dosage (CxL), cloxacillin—high dosage (CxH), non-antimicrobial treatment (NonA), penicillin/aminoglycoside combinations (Pam), penicillin/streptomycin–extended therapy (PSext), ceftiofur (B), cefapyrin (C), penicillin (P), penicillin/novobiocin (PNv), teat sealant (TS) (Table 2).
The mean treatment ranks of the labeled protocols are provided in Figure 4 with corresponding 95% credibility intervals. Cloxacillin-extended therapy (Cxext) had the highest mean rank (mean rank: 8.5, 95% CI: 2.0–21.0), but due to a wide posterior distribution, the differences in all-cause cure of IMI during the dry period could not be established between the antimicrobial therapies.
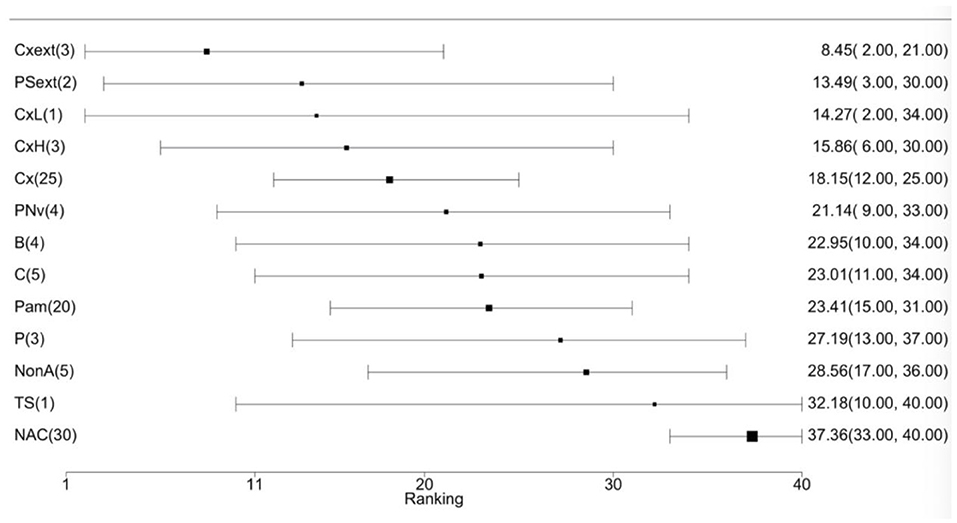
Figure 4. Forest plot of mean treatment rank for currently labeled antimicrobial treatment protocols in Canada and the United States to cure existing IMI during the dry period. The black squares indicate the mean rank of each treatment and its size reflects the precision (i.e., 1/variance) of the estimate. The values are reported as mean treatment rank with corresponding 95% CI. The number of treatment comparisons is provided in parentheses beside the treatment names. The treatment acronyms are as follows: cloxacillin–extended therapy (Cxext), penicillin/streptomycin–extended therapy (PSext), cloxacillin–low dosage (CxL), cloxacillin–high dosage (CxH), cloxacillin (Cx), penicillin/novobiocin (PNv), ceftiofur (B), cefapyrin (C), penicillin/aminoglycoside combinations (Pam), penicillin (P), non-antimicrobial treatment (NonA), teat sealant (TS), non-active control (NAC) (Table 2).
Risk of Bias in Overall Network
The contribution of trials to the estimates of the relative efficacy of the labeled antimicrobial treatment protocols for the all-cause cure of IMI based on the domain of risk of bias due to the randomization process is presented in Supplementary Figure 1, and the contribution of trials based on blinding of the caregivers in Supplementary Figure 2. Most pairwise comparisons (71/78) had a majority contribution from the trials where there were “some concerns” for bias due to the randomization process, and 7/78 pairwise comparisons had a majority contribution from the trials where there were “major concerns” for bias due to the randomization process. Although there was a “low” risk of bias due to the randomization process in a few trials (3/58), this was not the majority contribution for any pairwise comparisons of the labeled antimicrobial treatment protocols. Most pairwise comparisons had a majority contribution from the trials where authors provided no information for blinding of caregivers (71/78), followed by a small number with a majority contribution from the trials where authors did not blind caregivers (4/78), and a smaller number of trials with a majority contribution where authors reported blinding of caregivers (3/78).
Discussion
Summary of Evidence
Non-active control consistently resulted in relative risks for the cure of IMI <1 when compared with other treatment protocols, indicating each intramammary dry cow treatment currently labeled for use in Canada or the United States performed better than no treatment. The results of this research are consistent with the recommendations from the National Mastitis Council to include dry cow therapy as a component of an effective mastitis control program. Although this review used a novel methodology to assess the comparative efficacy of multiple antimicrobial options for the cure of IMI in dry cows, we were unable to find evidence of differences between the antimicrobial treatments. A low number of comparisons of each antimicrobial product created imprecise effect estimates. Further, poor reporting of trial features resulted in within-study bias that was assessed as “some concerns.” This is reflected in the risk of bias in the overall network where majority contributions of comparisons were majorly assessed as “some concerns.” These concerns with bias for the trials included in the network meta-analysis, as well as with bias in the overall network meta-analysis, would normally lead to a cautious interpretation of effect estimates (Li et al., 2011).
Interestingly, the incidence of clinical mastitis up to 30 DIM in cows with an existing IMI at the cessation of lactation and total antimicrobial use up to 30 DIM in cows with an existing IMI at the cessation of lactation were defined as critical outcomes to inform the antimicrobial dry cow therapy selection process within the present review, yet neither outcome was commonly reported. This suggests that both these outcomes are not considered relevant outcomes by mastitis researchers, or they are difficult to measure. There is a need to develop consistency among researchers in the use of outcomes of critical importance. The Core Outcome Measures in Effectiveness Trials Initiative was launched to guide the development of a set of core outcomes that should, at a minimum, be reported in all the clinical trials of specific topic areas (Kirkham et al., 2019). Currently, no such initiatives exist that are specific to the livestock species, but dairy researchers should consider adopting this approach to identify the core outcome measures that should be used in mastitis-related trials. Kelton et al. (1998) developed recommendations in the reporting of case definitions for clinical disease in dairy cattle, as well as guidelines to calculate and report the incidence rates for disease occurrence. Prior to this report, there was a lack of consistency in the definitions for clinical disease in dairy cattle used by the trialists (Kelton et al., 1998). These recommendations are an example of an early initiative employed to standardize reporting in the trials involving dairy cattle that can be built on further to include a minimum set of outcomes that could be used when investigating clinical diseases in dairy cows. In addition, standardized protocols have been developed by the National Mastitis Council and the International Dairy Federation, such as documents on procedures for collecting milk samples and guidance on the standardized methods for bacteriologic culture (National Mastitis Council, 2004; International Dairy Federation, 2020), that aid in the consistent classification of IMI. These guidance documents have helped to reduce the variability in diagnostic methods for outcome determination. Further, triplicate samples are the gold standard approach to sampling milk for bacteriologic culture (Andersen et al., 2010; Dohoo et al., 2011); however, in the present review, the bacteriologic cultures were performed using a single, duplicate, and triplicate samples. Dohoo et al. (2011) reported the best practices for collecting the milk samples that will be analyzed by bacteriologic culture, but the reporting of milk sampling methodologies continues to vary in dairy trials.
Another interesting finding relates to the definition of all-cause cure data, which varied among the authors—possibly as a result of the large range in the year of publication. For example, the all-cause cure was reported as a combination of Staphylococcal spp. and Streptococcal spp. in some trials, reported as only major pathogen cures in others, and reported as all major and minor pathogens in a few trials. A future approach for analyzing such data would be to evaluate pathogen-specific cures between the antimicrobial treatment protocols, especially with the large body of evidence investigating Staph aureus-infected cows (Nickerson et al., 1999; Østerås et al., 1999; Mendoza et al., 2016). Additionally, different antimicrobial products will be more efficacious against certain pathogens, thus pathogen-specific cure data will help reduce differing pathogen susceptibilities as a source of possible heterogeneity. However, the body of literature reporting cure of existing all-cause bacterial infections in dry cows is much larger than the body of literature reporting cure data for pathogen-specific infections; therefore, a network meta-analysis using pathogen-specific data likely would have led to an increasingly sparse network.
Lack of trial replication, meaning several evaluations of the same outcome for the same intervention, was an additional major limitation of the body of literature available for this topic. In addition to the issues in the reporting of outcomes and the risk period for measurement of these outcomes, lack of trial replication rendered us unable to form a solid foundation of evidence, which is consistent with the previous observations from mastitis network meta-analyses (Sargeant et al., 2019). Although all the treatment protocols were connected within our network, some treatment protocols were only used in one (e.g., gentamicin) or two (e.g., cefquinome) comparisons meaning the evaluation of these products for effectiveness to cure IMI were not well-replicated. Funding for replication of antimicrobial interventions in dairy science, and for developing consistent outcomes, is needed to take advantage of the benefits of network meta-analyses. Additionally, acknowledgment by publishing bodies to accept the literature that may not be novel would be beneficial to increase the body of evidence in the primary literature.
Limitations of the Review
In this study, 268 articles were excluded at full-text screening because they were not published in English, and translation was not a viable option. By using the trials that reported all-cause cure of existing IMI, the pathogen profiles could have differed by country, and thus so could have antimicrobial efficacies. Inclusion of these trials may have resulted in differing evidence on the basis of efficacy for these products to cure existing IMI; however, the inclusion of further relevant trials could have increased the precision of our summary estimates and led to a more meaningful interpretation of the results. Furthermore, the collapse of treatment protocols within our network meta-analysis could have resulted in some treatment protocols appearing more or less efficacious than in reality. However, the lack of available evidence necessitated combining the antimicrobial treatments to provide sufficient replication of interventions for analysis, and the attempt was made not to compromise the clinical relevancy for veterinarians and dairy producers.
The authors recognize the lack of external validity of our conclusions for dairy producers and veterinarians outside of Canada and the United States. However, the exclusion of antimicrobial products from countries other than Canada and the United States was done in the presentation of results; therefore, we know from the inclusion of all products in the network meta-analysis that the lack of replication trials for several antimicrobial products extends beyond Canada and the United States. Thus, the inferences likely would not have changed if all the products on a worldwide scale were included in the reporting of this network meta-analysis.
In addition, the limitations in precision of effect estimates of individual trials resulted in several pairwise comparisons within our network meta-analysis with “major concerns” for bias due to imprecision. Continued improvement in methodological reporting and replication of controlled trials in dairy science is needed to better inform cure decisions. Poor reporting is an issue that is recognized across the trials in dairy cattle science (Winder et al., 2019a), which can be mitigated using reporting guidelines, such as the Reporting Guidelines for Randomized Controlled Trials for Livestock and Food Safety (REFLECT) statement (O'Connor et al., 2010; Sargeant et al., 2010). Reporting of the key trial features has improved since the publication of REFLECT, which increases the transparency of trial methodologies (Moura et al., 2019). Therefore, the use of reporting guidelines should be mandatory for journal publication.
Conclusion
Antimicrobials most often reported in the trials evaluating the treatment of existing IMI during the dry period were cloxacillin, penicillin-aminoglycosides, and cefapyrin products. Non-active control was the most common comparator group. Although 58 trials were included in this analysis, the low number of trials contributing to each direct comparison created wide credibility intervals, which rendered us unable to comfortably differentiate the efficacy based on the ranked order of products. The consensus statements regarding a minimum set of required outcomes that should be reported in trials investigating the efficacy of antimicrobial products to improve udder health are needed and should be referenced in future dairy research. Further, trial replication evaluating antimicrobial treatments is required to avoid disparate network meta-analyses and form a solid evidence base for clinical decision-making. These major limitations of the body of evidence included in this network meta-analysis prevented us from evaluating the dry cow antimicrobial products on their basis of effectiveness to cure IMI during the dry period.
Data Availability Statement
The original contributions presented in this study are referenced and included in this article/Supplementary Material, further inquiries can be directed to the corresponding author.
Author Contributions
CM developed the review protocol, managed the research teams, conducted data screening, data extraction, and risk of bias assessment, conducted the analyses, interpreted the results, and developed the manuscript drafts. JS, DK, AO'C, and CW provided methodological support and content expertise, provided suggestions for edits of manuscript drafts, and approved the final manuscript. JG and HW developed the literature search strategies, conducted all searches, provided suggestions for edits of manuscript drafts, and approved the final manuscript. DH developed the user-defined functions in R for data analysis, provided methodological support, aided in risk of bias assessment, provided suggestions for edits of manuscript drafts, and approved the final manuscript. CR conducted data extraction, provided suggestions for edits of manuscript drafts, and approved the final manuscript. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
CM was awarded stipend support from the OVC Entrance Award and the Queen Elizabeth II Graduate Scholarship in Science and Technology from the University of Guelph and the Ministry of Training, Colleges and Universities. Additional acknowledgments to the donors of the Dr. Francis H.S. Newbould Award, the Dr. Casey Buizert Memorial Award, the Dr. R.A. McIntosh Graduate Award (OVC '45), and the Barbara Kell Gonsalves Memorial Scholarship for their funding support. Kineta Cousins, Katheryn Churchill, and Shannon Hookey provided support for all levels of screening and the assessment of the risk of bias of individual trials. The content of this manuscript has previously appeared online in the thesis of CM (McMullen et al., 2020).
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fanim.2021.726401/full#supplementary-material
Abbreviations
IMI, intramammary infections; CM, clinical mastitis; DIM, days in milk; SCC, somatic cell count; OR, odds ratio; RR, relative risk; JAGS, Just Another Gibbs Sampler; CINeMA, Confidence in Network Meta-Analysis.
Footnotes
1. ^https://atrium.lib.uoguelph.ca/xmlui/bitstream/handle/10214/16236/Protocol_NMA_efficacy_dryoff_antibiotics_cure_IMI.pdf?sequence=3&isAllowed=y
References
Andersen, S., Dohoo, I. R., Riekerink, R. O., and Stryhn, H. (2010). Diagnosing intramammary infections: evaluating expert opinions on the definition of intramammary infection using conjoint analysis. J. Dairy Sci. 93, 2966–2975. doi: 10.3168/jds.2009-2726
Animalytix (2020a). Product Uses – Dairy Cattle: Mastitis (Dry), Local Treatment and/or Prevention, Compendium of Veterinary Products. Available online at: https://bayerall.cvpservice.com/useindex/list/10017?spcsId=1530 (accessed August 4, 2020).
Animalytix (2020b). Product Use – Dairy Cattle: Mastitis (Dry), Local Treatment and/or Prevention, Compendium of Veterinary Products. Available online at: https://bam.cvpservice.com/useindex/list/10017?spcsId=1530 (accessed August 4, 2020).
Bhutto, A. L., Murray, R. D., and Woldehiwet, Z. (2011). The effect of dry cow therapy and internal teat-sealant on intra-mammary infections during the subsequent lactation. Res. Vet. Sci. 90, 316–320. doi: 10.1016/j.rvsc.2010.06.006
Bradley, A., De Vliegher, S., Farre, M., Jimenez, L. M., Peters, T., Schmitt-van de Leemput, E., et al. (2018). Pan-European agreement on dry cow therapy. Vet. Rec. 182:637. doi: 10.1136/vr.k2382
Canadian Veterinary Medical Association (2017). Veterinary Oversight of Antimicrobial Use – A Pan-Canadian framework of Professional Standards for Veterinarians. Available online at: https://www.canadianveterinarians.net/AMU-UAM (accessed August 4, 2020).
Dias, S., Welton, N. J., Caldwell, D. M., and Ades, A. E. (2010). Checking consistency in mixed treatment comparison meta-analysis. Stat. Med. 29, 932–944. doi: 10.1002/sim.3767
Dias, S., Welton, N. J., Sutton, A. J., and Ades, A. E. (2011). NICE DSU Technical Support Document 2: A Generalised Linear Modelling Framework for Pairwise and Network Meta-Analysis of randomised Controlled Trials. (Technical Support Document in Evidence Synthesis; No. TSD2). National Institute for Health and Clinical Excellence. Available online at: http://www.nicedsu.org.uk
Dohoo, I. R., Smith, J., Andersen, S., Kelton, D. F., and Godden, S. (2011). Diagnosing intramammary infections: evaluation of definitions based on a single milk sample. J. Dairy Sci. 94, 250–261. doi: 10.3168/jds.2010-3559
Dufour, S., Wellemans, V., Roy, J. P., Lacasse, P., Ordonez-Iturriaga, A., and Francoz, D. (2019). Non-antimicrobial approaches at drying-off for treating and preventing intramammary infections in dairy cows. Part 1. Meta-analyses of efficacy of using an internal teat sealant without concomitant antimicrobial treatment. Anim. Health Res. Rev. 20, 86–97. doi: 10.1017/S1466252319000070
Halasa, T., Nielen, M., Whist, A. C., and Østerås, O. (2009a). Meta-analysis of dry cow management for dairy cattle. Part 2. Cure of existing intramammary infections. J. Dairy Sci. 92, 3150–3157. doi: 10.3168/jds.2008-1741
Halasa, T., Østerås, O., Hogeveen, H, van Werven, T., and Nielen, M. (2009b). Meta-analysis of dry cow management for dairy cattle. Part 1. Protection against new intramammary infections. J. Dairy Sci. 92, 3134–3149. doi: 10.3168/jds.2008-1740
Higgins, J., Sterne, J., Savović, J, Page, M., Hróbjartsson, A., Boutron, I., et al. (2016). A revised tool for assessing risk of bias in randomized trials. Cochrane Database Syst. Rev. 10(Suppl. 1), 29–31. doi: 10.1002/14651858.CD201601
Hu, D., O'Connor, A. M., Wang, C., Sargeant, J. M., and Winder, C. B. (2020). How to conduct a Bayesian network meta-analysis. Front. Vet. Sci. 7:271. doi: 10.3389/fvets.2020.00271
Hutton, B., Salanti, G., Caldwell, D. M., Chaimani, A., Schmid, C. H., Cameron, C., et al. (2015). The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: checklist and explanations. Ann. Intern. Med. 162, 777–784. doi: 10.7326/M14-2385
International Dairy Federation (2020). 2020 – Milk – Bacterial count – Protocol for the Evaluation of Alternative Methods. Available online at: https://store.fil-idf.org/product/iso-16297-i-idf-161-2020-milk-bacterial-count-protocol-for-the-evaluation-of-alternative-methods/ (accessed August 4, 2020).
Keefe, G. (2012). Update on control of Staphylococcus aureus and Streptococcus agalactiae for management of mastitis. Vet. Clin. Food Anim. 28, 203–216. doi: 10.1016/j.cvfa.2012.03.010
Kelton, D. F., Lissemore, K. D., and Martin, R. E. (1998). Recommendations for recording and calculating the incidence of selected clinical diseases of dairy cattle. J. Dairy Sci. 81, 2502–2509. doi: 10.3168/jds.s0022-0302(98)70142-0
Kirkham, J. J., Gorst, S., Altman, D. G., Blazeby, J. M., Clarke, M., Tunis, S., et al. (2019). Core outcome Set-STAndardised protocol items: the COS-STAP statement. Trials 20, 1–7. doi: 10.1186/s13063-019-3230-x
Li, T., Puhan, M. A., Vedula, S. S., Singh, S., Dickersin, K., and The Ad Hoc Network Meta-analysis Methods Meeting Working Group (2011). Network meta-analysis- highlight attractive but more methodological research is needed. BMC Med 9:79. doi: 10.1186/1741-7015-9-79
Makovec, J. A., and Ruegg, P. L. (2003). Antimicrobial resistance of bacteria isolated from dairy cow milk samples submitted for bacterial culture: 8905 samples (1994-2001). J. Am. Vet. Med. Assoc. 222, 1582–1589. doi: 10.2460/javma.2003.222.1582
McMullen, C. K., Sargeant, J. M., Kelton, D. F., O‘Connor, A. M., Reedman, C. N., Hu, D., et al. (2021). Data From: Ancillary Analysis For: Relative Efficacy of Antimicrobial Treatments in Dairy Cattle to Cure Existing Intramammary Infections: A Systematic Review and Network Meta-Analysis (1990-2019). Scholars Portal Dataverse. doi: 10.5683/SP2/P62BXR
McMullen, C. K., Sargeant, J. M., Kelton, D. F., and Winder, C. B. (2020). Antimicrobial dry cow therapy and modifiable management in dry dairy cows to cure existing intramammary infections and improve udder health (Master's thesis). University of Guelph, Guelph, ON, Canada.
Mendoza, J., Martínez-Cortés, I., López-Ordaz, R., Gutiérrez, L., and Sumano, H. (2016). Concentrations of tilmicosin in mammary gland secretions of dairy cows following subcutaneous administration of one or two doses of an experimental preparation of tilmicosin and its efficacy against intramammary infections caused by Staphylococcus aureus. Am. J. Vet. Res. 77, 922–930. doi: 10.2460/ajvr.77.9.922
Moher, D., Liberati, A., Tetzlaff, J., Altman, D. G., and The PRISMA Group (2009). Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 6:e1000097. doi: 10.1371/journal.pmed.1000097
Moher, D., Pham, B., Jones, A., Cook, D. J., Jadad, A. R., Moher, M., et al. (1998). Does quality of reports of randomised trials affect estimates of intervention efficacy reported in meta-analyses? Lancet 352, 609–613. doi: 10.1016/S0140-6736(98)01085-X
Moura, C. A. A., Totton, S. C., Sargeant, J. M., O'Sullivan, T. L., Linhares, D. C. L., and O'Connor, A. M. (2019). Evidence of improved reporting of swine vaccination trials in the post-REFLECT statement publication period. J. Swine Health Prod. 27, 265–277. Available online at: https://lib.dr.iastate.edu/vdpam_pubs/142/
Mullen, K. A., Anderson, K. L., and Washburn, S. P. (2014). Effect of 2 herbal intramammary products on milk quantity and quality compared with conventional and no dry cow therapy. J. Dairy Sci. 97, 3509–3522. doi: 10.3168/jds.2013-7460
Naqvi, S. A., Nobrega, D. B., Ronksley, P. E., and Barkema, H. W. (2018). Invited review: effectiveness of precalving treatment on postcalving udder health in nulliparous dairy heifers: a systematic review and meta-analysis. J. Dairy Sci. 101, 4707–4728. doi: 10.3168/jds.2017-14301
National Mastitis Council (2004). Procedures for Collecting Milk Samples. Available online at: https://www.nmconline.org/wp-content/uploads/2016/09/Procedures-for-Collecting-Milk-Samples.pdf (accessed August 19, 2020).
Nickerson, S. C., Owens, W. E., Fox, L. K., Scheifinger, C. C., Shryock, T. R., and Spike, T. E. (1999). Comparison of tilmicosin and cephapirin as therapeutics for Staphylococcus aureus mastitis at dry-off. J. Dairy Sci. 82, 696–703. doi: 10.3168/jds.S0022-0302(99)75286-0
Nikolakopoulou, A., Higgins, J. P. T., Papakonstantinous, T., Chaimani, A., Del Giovane, C., Egger, M., et al. (2020). CINeMA: an approach for assessing confidence in the results of a network meta-analysis. PLoS Med. 17:e1003082. doi: 10.1371/journal.pmed.1003082
Nobrega, D. B., Naqvi, S. A., Dufour, S., Deardon, R., Kastelic, J. P., De Buck, J., et al. (2020). Critically important antimicrobials are generally not needed to treat nonsevere clinical mastitis in lactating dairy cows: results from a network meta-analysis. J. Dairy Sci. 103, 10585–10603. doi: 10.3168/jds.2020-18365
O'Connor, A. M., Coetzee, J. F., da Silva, N., and Wang, C. (2013). A mixed treatment comparison meta-analysis of antibiotic treatments for bovine respiratory disease. Prev. Vet. Med. 110, 77–87. doi: 10.1016/j.prevetmed.2012.11.025
O'Connor, A. M., Sargeant, J. M., Gardner, I. A., Dickson, J. S., Torrence, M. E., Dewey, C. E., et al. (2010). The REFLECT Statement: methods and processes of creating reporting guidelines for randomized controlled trials for livestock and food safety. Prev. Vet. Med. 93, 11–18. doi: 10.1016/j.prevetmed.2009.10.008
Østerås, O., Martin, S, W., and Edge, V. L. (1999). Possible risk factors associated with penicillin-resistant strains of Staphyloccous aureus from bovine subclinical mastitis in early lactation. J. Dairy Sci. 82, 927–938. doi: 10.3168/jds.S0022-0302(99)75311-7
Pantoja, J. C. F., Hulland, C., and Ruegg, P. L. (2009). Somatic cell count status across the dry period as a risk factor for the development of clinical mastitis in the subsequent lactation. J. Dairy Sci. 92, 139–148. doi: 10.3168/jds.2008-1477
Papakonstatinou, T., Nikolakopoulou, A., Higgins, J. P. T., Egger, M., and Salanti, G. (2020). CINeMA: software for semiautomated assessment of the confidence in the results of network meta-analysis. Campbell Syst. Rev. 16:e1080. doi: 10.1002/cl2.1080
Plummer, M., Stukalov, A., and Denwood, M. (2019). RJAGS: Bayesian Graphical Models Using MCMC. R Package, version 4.10. Available online at: http://CRAN.R-project.org/package=rjags
R Core Team (2019). R: A Language and Environment for Statistical Computing. Vienna: R Foundation for Statistical Computing. Available online at: https://www.R-project.org/
Rabiee, A. R., and Lean, I. J. (2013). The effect of internal teat sealant products (Teatseal and Orbeseal) on intramammary infection, clinical mastitis, and somatic cell counts in lactating dairy cows: a meta-analysis. J. Dairy Sci. 96, 6915–6931. doi: 10.3168/jds.2013-6544
Robert, A., Seegers, H., and Bareille, N. (2006). Incidence of intramammary infections during the dry period without or with antibiotic treatment in dairy cows – a quantitative analysis of published data. Vet. Res. 37, 25–48. doi: 10.1051/vetres:2005047
Salanti, G., Kavvoura, F. K., and Ioannidis, J. P. A. (2008). Exploring the geometry of treatment networks. Ann. Intern. Med. 148, 544–553. doi: 10.7326/0003-4819-148-7-200804010-00011
Sargeant, J. M., Elgie, R., Valcour, J., Saint-Onge, J., Thompson, A., Marcynuk, P., et al. (2009). Methodological quality and completeness of reporting in clinical trials conducted in livestock species. Prev. Vet. Med. 91, 107–115. doi: 10.1016/j.prevetmed.2009.06.002
Sargeant, J. M., and O'Connor, A. M. (2014). Introduction to systematic reviews in animal agriculture and veterinary medicine. Zoon Public Health 61, 3–9. doi: 10.1111/zph.12128
Sargeant, J. M., O'Connor, A. M., Gardner, I. A., Dickson, J. S., Torrence, M. E., Dohoo, I. R., et al. (2010). The REFLECT Statement: reporting guidelines for randomized controlled trials in livestock and food safety: explanation and elaboration. J. Food Prot. 73, 579–603. doi: 10.4315/0362-028X-73.3.579
Sargeant, J. M., O'Connor, A. M., and Winder, C. B. (2019). Editorial: systematic reviews reveal a need for more, better data to inform antimicrobial stewardship practices in animal agriculture. Anim. Health Res. Rev. 20, 103–105. doi: 10.1017/S1466252319000240
Sterne, J. A., Gavaghan, D., and Egger, M. (2000). Publication and related bias in meta-analysis: power of statistical tests and prevalence in the literature. J. Clin. Epidemiol. 53, 1119–1129. doi: 10.1016/s0895-4356(00)00242-0
US Food Drug Administration (2019). FOIA Drug Summaries. Available online at: https://animaldrugsatfda.fda.gov/adafda/views/#/foiDrugSummaries (accessed June 14, 2019).
Winder, C. B., Churchill, K. J., Sargeant, J. M., LeBlanc, S. J., O'Connor, A. M., and Renaud, D. L. (2019a). Invited review: completeness of reporting of experiments: REFLECTing on a year of animal trials in the Journal of Dairy Science. J. Dairy Sci. 102, 4759–4771. doi: 10.3168/jds.2018-15797
Winder, C. B., Sargeant, J. M., Hu, D., Wang, C., Kelton, D. F., Godkin, M. A., et al. (2019c). Comparative efficacy of antimicrobials for treatment of clinical mastitis in lactating dairy cattle: a systematic review and network meta-analysis. Anim. Health Res. Rev. 20, 229–246. doi: 10.1017/S1466252319000318
Winder, C. B., Sargeant, J. M., Hu, D., Wang, C., Kelton, D. F., Leblanc, S., et al. (2019b). Comparative efficacy of antimicrobial treatments in dairy cows at dry-off to prevent new intramammary infections during the dry period or clinical mastitis during early lactation: a systematic review and network meta-analysis. Anim. Health Res. Rev. 20, 199–216. doi: 10.1017/S1466252319000239
Winder, C. B., Sargeant, J. M., Hu, D., Wang, C., Kelton, D. F., Leblanc, S. J., et al. (2019d). Comparative efficacy of teat sealants given prepartum for prevention of intramammary infections and clinical mastitis: a systematic review and network meta-analysis. Anim. Health Res. Rev. 20, 182–198. doi: 10.1017/S1466252319000276
Winder, C. B., Sargeant, J. M., Kelton, D. F., LeBlanc, S., Duffield, T. F., Glanville, J., et al. (2019e). Comparative efficacy of blanket versus selective dry-cow therapy: a systematic review and pairwise meta-analysis. Anim. Health Res. Rev. 20, 217–228. doi: 10.1017/S1466252319000306
World Health Organization (2017). WHO Guidelines on Use of Medically Important Antimicrobials in Food-Producing Animals. Available online at: https://apps.who.int/iris/bitstream/handle/10665/258970/9789241550130-eng.pdf?sequence=1 (accessed August 4, 2020).
World Organisation for Animal Health (2007). OIE List of Antimicrobials of Veterinary Importance (OIE). Available online at: https://www.oie.int/fileadmin/Home/eng/Specific_Issues/docs/pdf/OIE_list_antimicrobials.pdf (accessed August 4, 2020).
Keywords: dairy cow, dry cow, antibiotic, subclinical mastitis (SCM), network meta-analysis (NMA)
Citation: McMullen CK, Sargeant JM, Kelton DF, O'Connor AM, Reedman CN, Hu D, Glanville J, Wood H and Winder CB (2021) Relative Efficacy of Dry-Off Antimicrobial Treatments in Dairy Cattle to Cure Existing Intramammary Infections: A Systematic Review and Network Meta-Analysis. Front. Anim. Sci. 2:726401. doi: 10.3389/fanim.2021.726401
Received: 13 July 2021; Accepted: 22 September 2021;
Published: 29 October 2021.
Edited by:
Erin E. Connor, University of Delaware, United StatesReviewed by:
Raghavendra G. Amachawadi, Kansas State University, United StatesJohn Lippolis, United States Department of Agriculture (USDA), United States
Antonio Barberio, Istituto Zooprofilattico Sperimentale delle Venezie, Italy
Copyright © 2021 McMullen, Sargeant, Kelton, O'Connor, Reedman, Hu, Glanville, Wood and Winder. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Charlotte B. Winder, d2luZGVyY0B1b2d1ZWxwaC5jYQ==
 Carrie K. McMullen
Carrie K. McMullen Jan M. Sargeant
Jan M. Sargeant David F. Kelton
David F. Kelton Annette M. O'Connor2
Annette M. O'Connor2 Cassandra N. Reedman
Cassandra N. Reedman Dapeng Hu
Dapeng Hu Hannah Wood
Hannah Wood Charlotte B. Winder
Charlotte B. Winder