- 1Department of Animal Sciences, University of Illinois, Urbana, IL, United States
- 2Department of Animal Biology, University of California, Davis, Davis, CA, United States
- 3Department of Animal and Food Sciences, Oklahoma State University, Stillwater, OK, United States
Social conflict is inevitable among group-housed sows and may contribute to poorer welfare among those sows experiencing more social stress. The degree of individual welfare is associated with social position within the group. Therefore, this study examined the effects of social status on behavior, immune, endocrine, and productivity of group-housed pregnant sows fed a diet supplemented with 30% wheat middlings and 15% soybean hulls (MID-SH) or 30% distillers dried grains with solubles and 30% corn germ meal (DDGS-GM) and in pens with individual feeding places made from short (58.4 cm) or long (203.2 cm) barriers. A 2 × 2 factorial design resulted in 4 experimental treatment groups (n = 9 sows/diet-length-block combination): (1) MID-SHshort; (2) MID-SHlong; (3) DDGS-GMshort; (4) DDGS-GMlong. Groups of sows equally representing all diet-length combinations across 4 blocks (n = 36 sows/block) were subjected to a feeding competition test to identify highest (dominant) and lowest (subordinate) ranked sows within each group resulting in 64 sows (n = 16 sows/treatment; n = 32 sows/social status). Data revealed 2- and 3-way interactive effects on aggressive behavior (P < 0.005), postural (P < 0.01), oral (P < 0.0001), and eating (P < 0.005) behaviors, sow mean body weights and gains (P < 0.05) and litter weaning weights (P < 0.05), especially among subordinates in pens with long barriers. Subordinates in pens with long barriers received 21% less aggression and were 73% less likely to be displaced than subordinates in pens with short ones (P < 0.0001). Dietary treatment also influenced some of these measures among the subordinates in pens with long barriers. For example, subordinates in DDGS-GMlong received 64 and 67% less aggression than subordinates in DDGS-GMshort and MID-SHshort (P < 0.005). Eat bouts were greatest among subordinates in MID-SHlong, and sitting and sham-chewing were less. However, those in DDGS-GMlong spent less time standing and laying, and their litters were 15.28 kg heavier (P = 0.01), but overall subordinates fed DDGS-GM diet were lightest and gained less total body weight than those fed MID-SH (P < 0.05). Other measures such as neutrophil-to-lymphocyte ratio were elevated among dominants in MID-SHlong (P < 0.05); whereas, cortisol (P = 0.06) was lowest and glucose (P = 0.09) highest for subordinates in DDGS-GMlong. These data imply that subordinates benefited from being housed in pens with long barriers, but the type of dietary fiber consumed differentially influenced behavioral budget and several sow- or litter-related traits among subordinates in pens with long barriers. In contrast, the subordinates in pens with short barriers had poorer welfare regardless of diet. Collectively, these data imply that social status is a crucial factor contributing to variation in individual well-being among group-housed sows and that sows of different social positions within a group may evoke different biological responses in an attempt to cope.
Introduction
Group housing of gestating sows provides the opportunity for sows to exercise, express normal behaviors, and socially interact with conspecifics. Unfortunately, the social interactions are not always positive, and for some sows in the group, it may result in chronic stress. The initial aggression occurs upon mixing, which is inevitable due to the innate need to establish and reinforce the group's hierarchical relationships (McGlone, 1985). Despite an established social hierarchy, sows still engage in social conflicts to gain access to feed and other resources (Spoolder et al., 2009; Maes et al., 2016). Sows kept in group pens experience acute stress due to inevitable social conflicts. In contrast, others experience chronic stress often associated with prolonged social stress or aggression, resulting in compromised well-being. Social relationships affect individuals' ability to access resources, which, in turn, affects the level of aggression and, ultimately, individual welfare, especially lower-ranked sows (Verdon et al., 2015). Previous research implies that subordinate sows are more likely to receive more aggression and be displaced more often from resources; thus, they are most vulnerable and more likely to experience poorer welfare than higher-rank sows (Hoy et al., 2009; Zhao et al., 2013).
The aggression related to feeding competition is often frequent and short in duration, but the aggression level varies with the group-housing and feeding systems in place (Gonyou, 2005). The use of full-body length feeding stalls, dietary modifications, and foraging materials can minimize aggressive behavior and improve satiety (Andersen et al., 1999; de Leeuw et al., 2005; Sapkota et al., 2016), but with inconsistent outcomes, especially improving sow well-being. For example, it has been reported that feeding high-fiber diets to group-housed gestating sows improves satiety and reduces aggression and stereotypic behaviors (de Leeuw et al., 2005; Sapkota et al., 2016). Others have found no effect of feeding high-fiber diets on the frequency of stereotypic behaviors (Holt et al., 2006). The contradictory findings on satiety and stereotypic behaviors may be due to fiber type, such that soluble fibers may improve feeding motivation. In contrast, bulky or highly fermentable fibers do not affect these behaviors (da Silva et al., 2013). Still, others found that sham-chewing was reduced, and resting behavior increased when sows were fed a high-fiber diet with 19.1% soybean hulls but not beet pulp (Sapkota et al., 2016). Contrarily, sows fed soybean hulls and wheat middlings fiber diet spent less time performing oral-nasal-facial and sham-chew behaviors than sows fed a low fiber diet (Kranendonk et al., 2007). Although the type of dietary fiber-fed and feeding system used may partly explain the inconsistent effects on aggression and stereotypical behaviors, these factors alone do not explain individual welfare variation among group-housed sows due to social conflicts. Not all sows within the group benefit; some sows are attacked and displaced more than others, resulting in them becoming more fearful and less competitive over time due to these social conflicts (O'Connell et al., 2003; Elmore et al., 2011).
Assessing stress is often challenging due to inter-animal variability and other factors such as genetics, physiological state, and previous experiences (Moberg, 2004). Although genetics plays a role in expressing an aggressive phenotype in pigs (Lovendahl et al., 2005; Stukenborg et al., 2011), group dynamics and social experiences may also contribute to the development and expression of aggression (Verdon et al., 2017). Social status differentially affects the biological response organized in response to a stressor and may partially determine the biological consequences (Salak-Johnson and McGlone, 2007). Sows are highly social animals, but when unfamiliar sows are mixed into groups, negative consequences for sow welfare are a major concern; thus, reducing aggression remains a priority among welfare scientists. Although intraspecific variation in aggressive behavior among group-housed sows has been well-documented (Verdon et al., 2016), the role social status plays on aggressive, behavioral, and immunological outcomes indicative of well-being among group-housed sows fed fiber diets using a competitive individual feeding stall system are limited. We hypothesize that minimizing aggressive behavior toward subordinates during feeding and providing them a place to retreat may be beneficial, resulting in improved well-being. Therefore, this study aimed to assess the effects of social status on behavior, immune status, and well-being of group-housed sows fed two different dietary fiber types and housed in pens with either short or long feeding barriers throughout gestation.
Materials and Methods
The animal protocol was reviewed and approved by the University of Illinois Institutional Animal Care and Use Committee (protocol no. 13097).
Animals and Experimental Design
The study was conducted at the University of Illinois Swine Research Center, Urbana, Illinois, from September 2013 to June 2015. Sixty-four primiparous (n = 21) and multiparous (n = 43) gestating sows used in this study were from a large-scale study of a 144 sows (n = 36 sows/group). Once confirmed pregnant, groups of 9 sows were randomly allotted to one of two dietary treatments and a pen equipped with individual feeding stall spaces made from different length barriers. The dietary fiber treatments were 30% wheat middlings and 15% soybean hulls (MID-SH) or 30% distillers dried grains with solubles and 30% corn germ meal (DDGS-GM). The barrier lengths were either 58.4 cm (short; width = 48.3 cm) or 203.2 cm (long; width = 57.2 cm) in length. Diet treatments were initiated 2-days before sows were moved into their group pens to facilitate acceptance of the treatment diets without competition.
At d 37 post-breeding, sows were moved from individual gestation stalls (0.61 × 2.13 m) to one of four experimental treatment pens (n = 9 sows/diet-length-block combination): (1) MID-SHshort; (2) MID-SHlong; (3) DDGS-GMshort; (4) DDGS-GMlong. Before moving sows into group pens, they were subjected to a feeding competition test to identify social status. Social position was determined post-hoc, and only the highest (dominant, n =32) and lowest (subordinates = 32) ranked sows within each treatment pen were used in this analysis. Therefore, all sows remained in their assigned treatment pens until gestational day 108. Each pen was equipped with 9 individual feeding places. The total floor-space allowance within the pens was constant at 1.7 m2/sow, but the available open floor space beyond the feeding places differed due to the difference in the length of the barriers. The pens with short barriers had more available pen space outside the feeding area than the pens with long barriers. Sows were fed in the same order at 6.30 am each day. Water was provided ad libitum. Individual nipple drinkers were fixed on the left side of each barrier within each feeding place (9 nipples/pen).
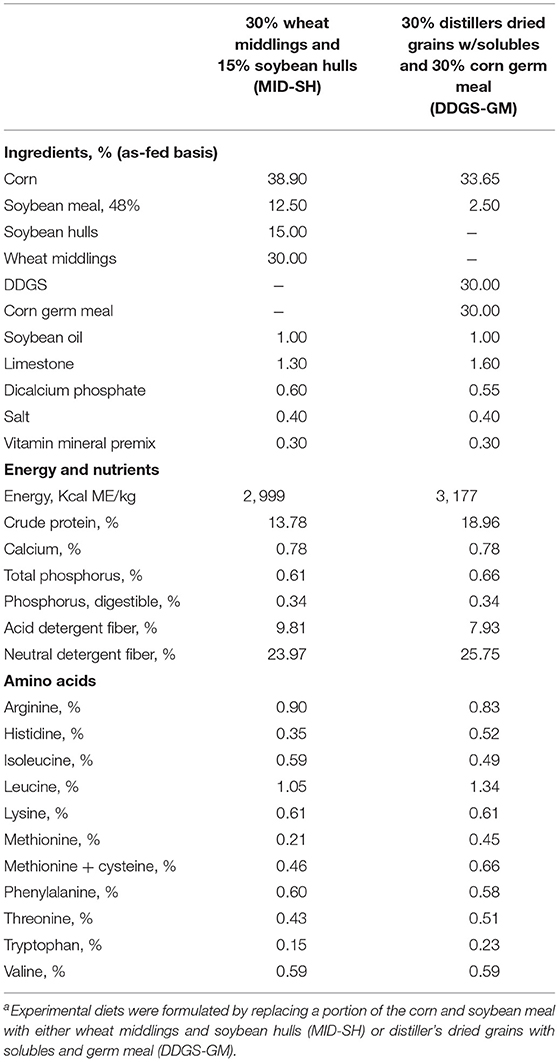
Shown in Table 1 are the ingredients for the treatment diets where the fiber ingredients replaced a portion of the shelled corn and soybean meal. Both diets were formulated to meet or exceed NRC requirements for gestating sows (NRC, 2012). From gestational day (GD) 35 through GD 90, sows were offered 2.3 kg/d of MID-SH or 2.1 kg/d of DDGS-GM diet, and from GD 91 to GD 108 sows were offered 3.6 kg/d of MID-SH or 3.4 kg/d of DDGS-GM diet to adjust for the differences in energy between the two diets. Diets had a calculated composition (as fed basis) of 13.8 and 19% crude protein (CP), respectively, and both diets provided a calculated ME/d of 6,700 kcal and 10,720 kcal for the two different gestational periods.
Social Status and Other Behaviors
Social status was unknown when sows were assigned and moved to treatment pens. Status was determined after the feeding competition test and video-record viewing. Initially, groups of 9 sows were placed in a non-experimental pen (4.10 × 4.10 m) equipped with one feeder to determine social status using the feeding competition test previously described by Parent et al. (2012). After a 5-min acclimation period to the experimental pen, 4 kg of the treatment diet was added to the feeder. The 30-min test was captured using EverFocus EQ120/AEN colored cameras (EverFocus Co., LTD., Duarte, CA) located above each pen and recorded using Geovision GVd1240 video capture card (Geovision, Inc., Irvine, CA). After the test, all sows were moved to their assigned treatment pens, where they remained until GD 108.
Definitions for aggressive behaviors registered during the feeding competition test are shown in Table 2 and are similar to those reported by Parent et al. (2012) with modifications. All aggressive interactions were registered, including the “initiator” and “receiver” of each aggressive encounter using video records. Based on these interactions, a dominance value (DV) was calculated for each sow (n = 9 sows per treatment group). The equation was: DV = aggressive encounters initiated ÷ (aggressive encounters initiated + encounters received). The sows with the highest DV were classified as dominant, and the lowest DV were subordinate, resulting in a subpopulation of 64 sows (n = 16 sows/treatment).
Live behavioral observations were also registered during feeding at various time points, including first feeding post-grouping and then at 3-week intervals (3-, 6-, 9-weeks post-grouping) until sows moved to the farrowing facility. Live postural and behavioral observations were taken using instantaneous sampling directly before glucose measurement. The observational period included 30-min before feed delivery and then at 30-min increments up to 120 min post-feeding. Frequencies and durations of each aggressive encounter and other postural and maintenance behaviors were registered during the observational period similar to DeDecker et al. (2014) with minor modifications (Pacheco, 2015; Table 2). The observer was blinded to sow social status; therefore, behaviors were collected on all animals within the group, but only data for the dominant (n = 32) and subordinate (n = 32) sows in each treatment pen were analyzed.
Cell Counts, Differentials, and Isolation
Sows were nose-snared, and 15 mL of blood was collected via jugular venipuncture using syringes containing sodium heparin on GD 30, 70, 90, 104, and again at the end of lactation. All measures, including cell counts, differentials, and cell isolation, were performed as previously reported (Sutherland et al., 2005; Salak-Johnson et al., 2012). Briefly, before centrifugation of blood samples, whole blood smears were made, fixed in methanol, stained with Hema-3 staining system (Fisher Scientific, Houston, TX), and then viewed under a light microscope to determine leukocyte differential counts. Total white blood cell counts (WBC) were made electronically using a Coulter Z1 particle counter (Beckman Coulter). Ten μL of whole blood was added to Isoflow (10 mL; Beckman Coulter, Beckman, FL), and red blood cells were lysed with Zap-o-globin (Beckman Coulter).
Whole blood was diluted in RPMI medium (Gibco, Carlsbad, CA), layered over Histopauqe-1077 (density:1.077 g/ml; Sigma) and−1119 (density:1.119 g/ml; Sigma) and centrifuged at 700 × g for 30 min. Lymphocytes were collected from the 1077 layer, washed twice in RPMI, and resuspended, and counted. Neutrophils were isolated from the 1119 layer and washed in RMPI. Red blood cells were lysed using cold endotoxin-free water, and isotonicity was restored using 10 × phosphate buffer saline. Neutrophils were centrifuged at 475 × g for 10 min, the supernatant decanted, and the pellet was washed and then resuspended in RPMI. Cell concentrations were adjusted based on immune-assay requirements.
Glucose, Cortisol, and Interleukin-12
Sow blood glucose levels were measured 2-days before feeding the diet treatments (baseline), then every 3-days for the first 2 weeks post-grouping, and then again on a bi-weekly basis until sows were moved to the farrowing room. Samples were taken 30-min before feeding and then 30, 60, 90, and 120 min post-feeding at each measurement day after sow behavior was registered. A drop of blood was obtained from the ear vein using a small 20 g needle and added to a glucose strip. Blood glucose levels were measured immediately by inserting the strip into the portable electronic glucose monitor (Precision Xtra Monitor Abbott, Alameda, CA), as previously described by de Leeuw et al. (2005), with minor modifications described by Pacheco (2015). Samples not obtained in <5 min were excluded from the analysis.
Total plasma cortisol and interleukin-12 (IL-12) were measured at gestational days 30 (baseline) and 90. Cortisol was measured using a commercially available radioimmunoassay following the manufacture's instructions (MP Biomedicals, Santa Ana, CA). The intra- and inter-assay CVs were 8.3 and 9.7%, respectively, and the minimal detectable concentration was 3 ng/mL. Plasma IL-12 was measured using an enzyme-linked immunosorbent assay (R&D Systems, Minneapolis, MN). The intra- and inter-assay CVs were 4.9 and 7.7%, respectively, and the minimal detectable concentration was 9 pg/mL.
Lymphocyte Proliferation Assay
A mitogen-induced lymphocyte proliferation assay was performed as previously described by Sutherland et al. (2005). Briefly, isolated porcine lymphocytes were used at a concentration of 5 × 106 cells/mL and placed in triplicate on a sterile 96-well flat-bottom plate. Concanavalin A (ConA; Sigma Aldrich) and lipopolysaccharide (LPS; Sigma Aldrich) were used as mitogens (ConA: 0, 2, and 20 μg/mL; LPS: 0, 5, and 50 μg/mL) to stimulate T and B cells, respectively. Plates were incubated, the reaction stopped, and then read at a wavelength of 550 nm with reference wavelength 690 nm using a microplate reader (Thermo Scientific Instruments). Results were expressed as a proliferation index: Optical density(550/690nm) of stimulated cells ÷ Optical density(550/690nm) of nonstimulated cells.
Sow- and Litter-Related Traits
Sow body weight (BW) was taken on GD 30, 70, 90, and 104, and at the end of lactation (135 days post-breeding), ± 1day. Sow backfat depth was measured using a longitudinal imaging ultrasound scan (Aloka-500V machine, Hitachi Aloka, Wallingford, CT) cranial to the last rib on the same days as BW measures excluding GD 70. Litter-related traits included the total number of piglets born and born alive and the number of females, males, stillborn, mummified, laid on, euthanized, total mortality (no. stillborn + no. mummified + no. laid on + no. euthanized), and piglets weaned. Calculated litter traits included litter weight at birth, adjusted litter weight at birth (number of piglets born), litter wean weight, adjusted litter wean weight (number of piglets weaned), and mean piglet weaning weight. All measures were taken and calculated as previously reported (Salak-Johnson et al., 2007; DeDecker et al., 2014).
Statistical Analyses
Post-hoc analysis was conducted on social status classification. All data were analyzed using the mixed model procedure of SAS (SAS Inst. Inc., Cary, NC), with repeated measures utilizing a first-order autoregressive structure. Except for lesion scores, all traits were tested for normality using Kolmogorov-Smirnov and Shapiro-Wilk tests, and the transformation was applied to traits deviating from a normal distribution. A linear mixed-effects model was used to analyze these measurements. The main fixed effects were diet (MID-SH and DDGS-GM), barrier length (short and long), and social status (dominant and subordinate), and all second and third-order interactions between these factors. A random effect of the block was included in the model. The physiologic measures model also included the gestational day that blood samples were taken, and the model for behavior included days post-grouping. All measurements were from a single sow: thus, the experimental unit was the sow (Salak-Johnson et al., 2007; Hanson et al., 2011). The least-square means were generated and separated statistically with pairwise t-tests (PDIFF option). Significance was set at P < 0.05, whereas trends were discussed at P < 0.10.
Results
Aggressive Behaviors and Encounters
The only 3-way interactive effect that occurred was for mean number of aggressive encounters toward subordinates (P < 0.005). Subordinates fed DDGS-GM and in pens with long feeding barriers (DDGS-GMlong) were the least likely to be on the receiving end of an aggressive encounter (2.81 ± 0.99, no.) compared to subordinates in DDGS-GMshort (7.80 ± 1.1, no.), MID-SHshort (8.50 ± 1.0, no.) or MID-SHlong (6.56 ± 1.1, no.) treatments.
Table 3 shows the interactive effect of social status and barrier length on aggressive behaviors, including the total number of aggressive encounters and displacements and the initiator and receiver of each attack between dominant and subordinate sows housed in group pens. In general, social status and barrier length had the greatest impact on these measures. The subordinates in pens with short barriers were four times more likely to be displaced (P < 0.0001), and most often, they were displaced by dominant sows (P < 0.001) during feeding than subordinates in pens with long barriers. The dominant sows housed in pens with short barriers initiated more aggressive encounters (P < 0.005) during feeding and displaced (P < 0.0001) others more often than the dominants in pens with long barriers (Table 3).
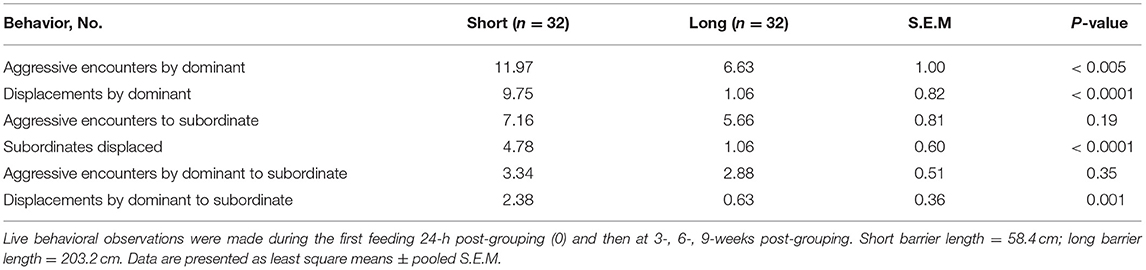
Table 3. Numbers of aggressive encounters and displacements displayed by dominant and subordinate gestating sows housed in small group pens equipped with short and long length feeding barriers.
Postural, Maintenance, and Oral Behaviors
In Table 4, the interactive effects for social status × barrier length × diet that occurred on postural, maintenance, and oral behaviors during the behavioral observational period included pre-and post-feeding. The subordinates in the DDGS-GMlong treatment spent 38.8 and 72.2% less time standing and laying, respectively, compared to subordinates in the DDGS-GMshort treatment and subordinates and dominants in other treatment groups; the exception was for dominants in the DDGS-GMlong they spent the least time standing (P < 0.01). Conversely, the subordinates in the DDGS-GMshort treatment spent the most time laying compared to subordinates and dominants in other treatments (Table 4). The dominant sows in the DDGS-GMlong treatment also spent 100% less time sitting than dominant and subordinate sows in all other treatments (P < 0.005). Conversely, subordinates in the DDGS-GMlong spent the most time sitting (Table 4). Walk and drink behaviors were not affected (P > 0.70).
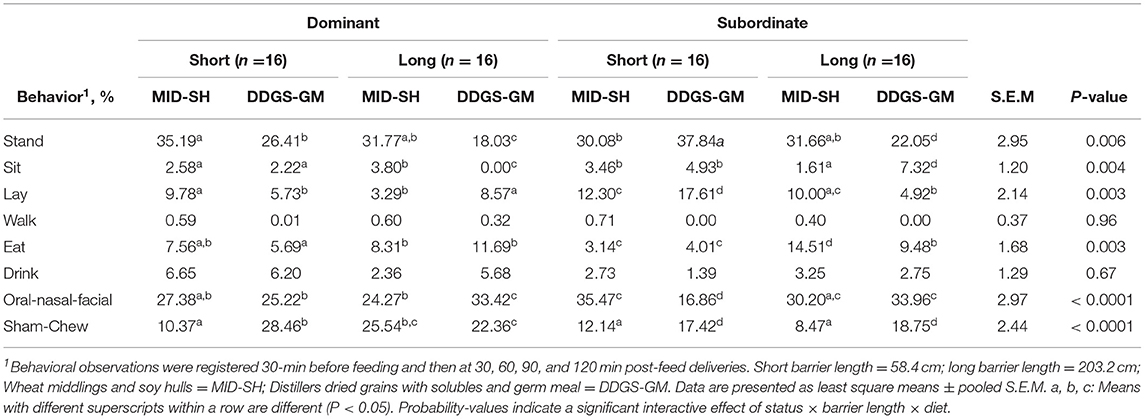
Table 4. Interactive effects of social status on mean postural, maintenance, and oral behaviors for group-housed gestating sows fed one of two dietary fiber treatments and housed in pens equipped with short and long feeding barriers (n = 64 total sows).
Interestingly, the subordinates in the MID-SHlong treatment spent 52% more time eating than subordinates in DDGS-GMlong and 74% more time than dominants in the same treatment (P < 0.005). They also spent significantly more time eating than subordinates in MID-SHshort or DDGS-GMshort treatments (Table 4). Whereas, the dominant sows in the DDGS-GMlong spent more time eating than dominants in DDGS-GMshort. Although the subordinates in the DDGS-GMshort treatment spent less time eating, they expressed the lowest percentage of oral-nasal-facial (ONF) behaviors compared to either subordinates or dominants in all other treatments; in fact, they spent 52% less time displaying ONF behavior compared to subordinates in the MID-SHshort treatment (P < 0.0001). At the same time, subordinates in the MID-SHlong treatment spent 60 and 41% less time sham-chewing than subordinates in either DDGS-GMlong or MID-SHshort treatments, respectively (P < 0.0001; Table 4). Overall, the dominant sows in the DDGS-GMlong treatment expressed the most ONF behavior, while those in the DDGS-GMshort the most sham-chew behavior (Table 4).
Moreover, it is important to note that the differences between dominants and subordinates for standing (P < 0.015) and sitting (P < 0.0001) occurred between sows fed DDGS-GM diet, and eating was different when sows were fed MID-SH diet (P < 0.0035). Specifically, subordinates fed the DDGS-GM spent more time standing (dominant: 28.6 vs. subordinate: 38.9, S.E.M = 3.8) and sitting (dominant: 1.16 vs. subordinate: 11.1, S.E.M = 1.6), and those fed the MID-SH diet spent more eating (dominant: 9.1 vs. subordinate: 14.2, S.E.M = 1.4).
Sow- and Litter-Related Measures
No 3-way interactive effects occurred for any of the sow-related measures. However, several social status × barrier length or social status × diet did occur for sow body weights and weight gains and losses (Table 5). The subordinate sows in pens with short barriers gained 33.5 and 21.4% less body weight from GD 30 to GD 70 than dominants in pens with short barriers and subordinates in pens with long, respectively (P < 0.05); whereas, dominants and subordinates in pens with long barriers had a similar body weight gain. Overall, subordinate sows fed the DDGS-GM diet mean body weight was 3.5 and 6.0% lighter (P = 0.01) than the subordinates fed the MID-SH diet and dominants fed the DDGS-GM diet, respectively (Table 5). They also gained 39 and 41% less (P = 0.02) total body weight than their subordinates fed the MID-SY diet and dominants fed the DDGS-GM diet.
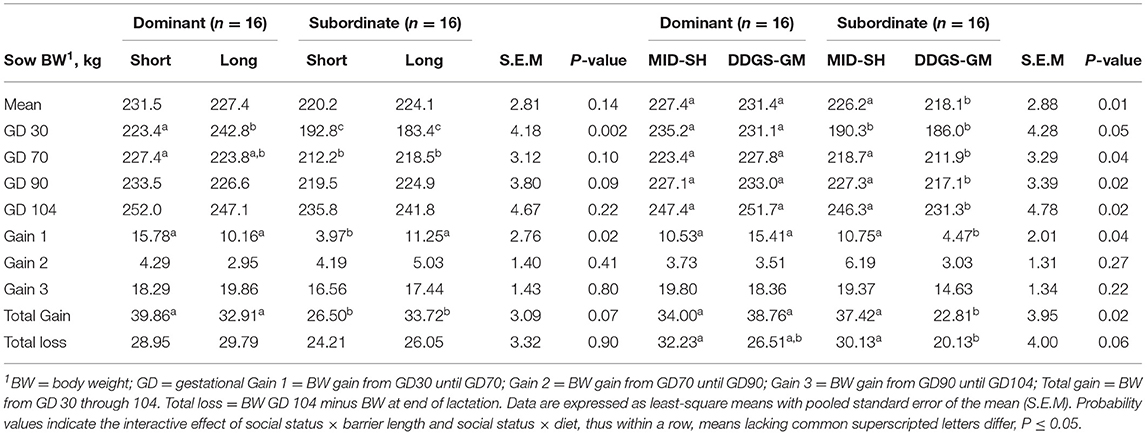
Table 5. Interactive effects of social status and feeding barrier length or dietary treatment on live body weights, weight gain, and weight loss throughout gestation for group-penned sows (n = 64 total sows).
There were no interactive effects of social status × barrier length × diet that occurred for litter-related traits, except for litter weaning weight and average piglet weaning weight (Table 6). There was a tendency for the dominants in the DDGS-GMshort and MID-SHlong treatments to farrow more piglets than dominants and subordinates in other treatments (P = 0.10), but the number of piglets born alive was not different (P = 0.13). The subordinates in the DDGS-GMlong treatment litter weaning weight was 15.82 and 10.45 kg heavier than subordinates in DDGS-GMshort and MID-SHlong treatments, respectively (P = 0.01). Their average weaned piglet weight was also 1.67 and 1.18 kg heavier than subordinates in the DDGS-GMshort and MID-SHlong treatments, respectively (P = 0.005). While litter and average piglet wean weights were similar among dominants regardless of treatment (Table 6).
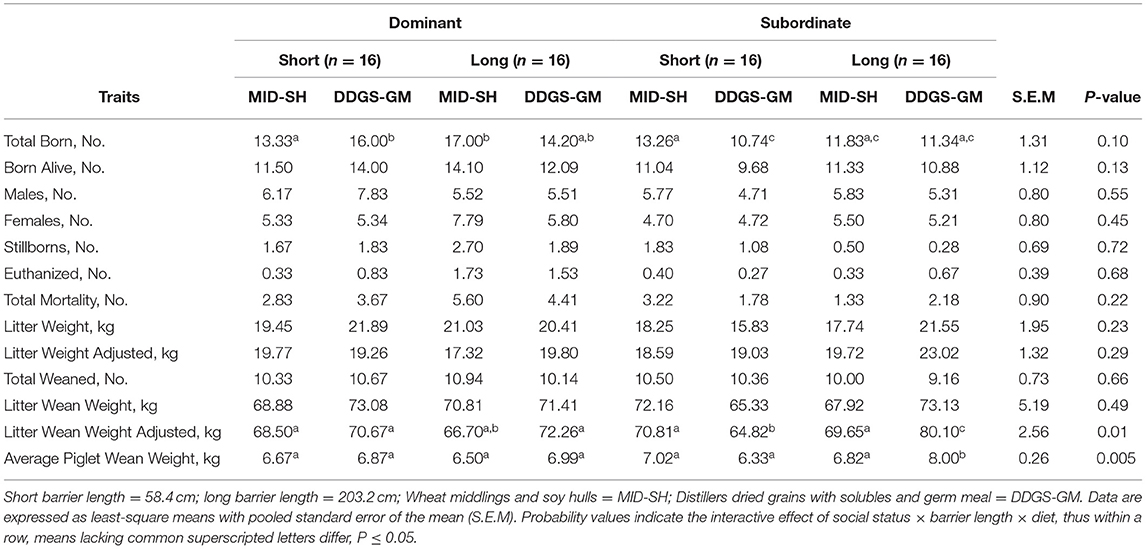
Table 6. Interactive effects of social status on mean litter-related traits for group-housed gestating sows fed one of two dietary fiber treatments and housed in pens equipped with either short or long length feeding barriers (n = 64 total sows).
Immune, Cortisol, and Glucose Measures
Shown in Table 7 are the interactive effects for social status × barrier length × diet that occurred for immune, cortisol, and glucose measures. The only interactive effect that occurred were on mean neutrophil-to-lymphocyte (N:L) ratio (P < 0.05), percentage of lymphocytes (P = 0.06), and plasma cortisol concentrations (P = 0.06). The dominants in the MID-SHshort treatment had the highest N:L ratio and lowest lymphocyte percentages compared to subordinates in the same treatment and sows in all other treatments, regardless of status. Interestingly, subordinates in the DDGS-GMlong treatment tended to have the lowest mean cortisol concentration. However, the highest mean glucose concentrations were compared to both subordinates and dominants in other treatment groups (Table 7). No other immune measures, including lymphocyte proliferation and interleukin-12, were different among dominant and subordinate sows in either treatment (P > 0.20).
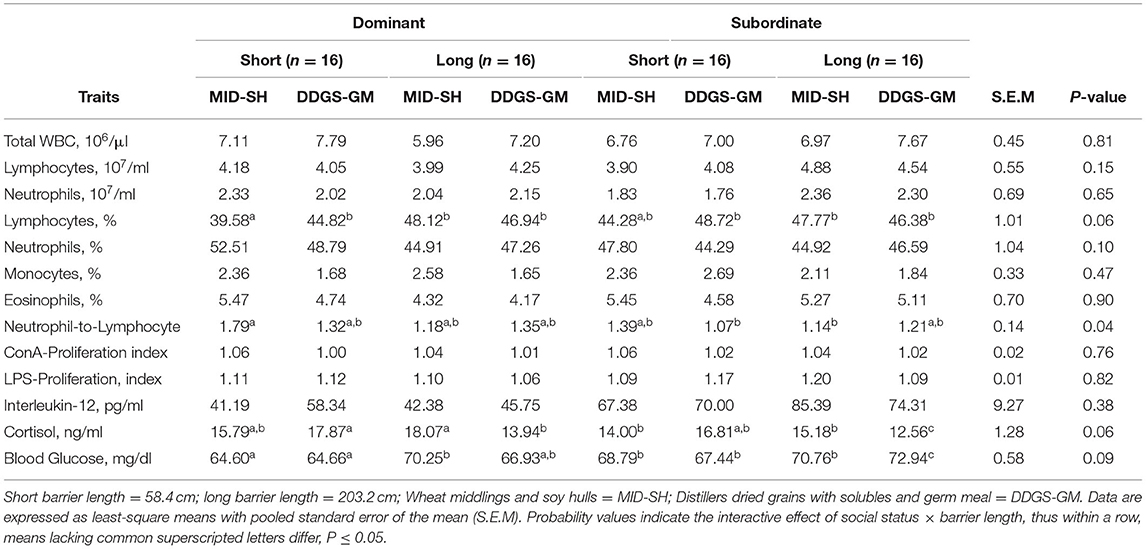
Table 7. Interactive effects of social status, barrier length, and diet on mean immune, cortisol, and glucose measures for pregnant sows housed in small group pens throughout gestation (n = 64 total sows).
It is important to note that several social status × barrier length effects occurred for a few immune measures and glucose. The subordinate sows (6.88 ± 0.22, No. 107/10 μl) in pens with short barriers had lower total WBC counts than dominant sows (7.50 ± 0.22, No. 107/10 μl) in the same pens and subordinates (7.2 ± 0.22, No. 107/10 μl) in pens with long barriers, but counts were similar to dominants (6.5 ± 0.22, No. 107/10 μl) in pens with long barriers (social status × barrier length; P < 0.05). Total neutrophil numbers were higher among subordinate sows (2.33 ± 0.17, No. 107/ml) in pens with long barriers compared to subordinates (1.77 ± 0.17, No. 107/ml) in pens with short barriers, but similar to dominants sows in pens with either short (2.19 ± 0.17, No. 107/ml) or long (2.17 ± 0.17, No. 107/ml) barriers (social status × barrier length; P < 0.05). Also, the subordinates (80.82 ± 6.3, pg/ml) in pens with long barriers IL-12 levels were 17.9% greater than subordinates (68.56 ± 6.3, pg/ml) in pens with short barriers and more than 62.0% greater than dominant sows in pens with either long (48.81 ± 6.3, pg/ml) or short (49.78 ± 6.3, pg/ml) barriers (social × barrier length; P = 0.06). Moreover, subordinate sows in pens with long barriers had greater mean blood glucose levels (72.85 ± 0.59, mg/dL) compared to their contemporaries in pens with short (68.0 ± 0.59, mg/dL) and dominant sows in pens with either long (68.51 ± 0.59, mg/dL) or short (64.63 ± 0.59, mg/dL) barriers (social status × barrier length; P < 0.05). Subordinates (70.64 ± 0.67, mg/dL) fed the DDGS-GM diet had greater glucose than dominants (66.49 ± 0.67, mg/dL) fed same diet whereas subordinate (68.22 ± 0.67, mg/dL) and dominant (68.39 ± 0.67, mg/dL) sows fed the MID-SH diet had similar concentrations (social status × diet; P = 0.07).
Discussion
Unfortunately, aggression is inevitable among group-housed sows. It can be exacerbated by social disruption and the competitive ability of the individual within the group resulting in some sows experiencing more stress than others resulting in compromised well-being. Animal welfare reflects the individual's successful adaptation, not the population (Ohl and van der Staay, 2012). Within a group, the degree of individual welfare is associated with social status (Li et al., 2017). Nevertheless, sow social status may be one of the most critical and overlooked factors influencing welfare variation among group-housed gestating sows. These results revealed that the biological consequences of adapting to social conflict and the degree of individual welfare among loose-housed pregnant sows are partly influenced by social status. Social status may also explain the differential behavioral and immunological responses and productivity outcomes between sows in the same pen environments. More specifically, these data imply that dominant and subordinate sows use different coping mechanisms to adapt to their pen environment constraints, indicating that the stress they experienced was different due to their social position within the group or individual perception. Collectively, these results validate that the interactive role of social status, the housing infrastructure, and fiber diets can differentially affect the individual welfare of gestating sows, but reducing competition around feeding, especially for the subordinate sows, may be beneficial.
Pregnant sows are limited-fed, which results in more aggression among pen mates for resources (e.g., feed, water, lying space). The more dominant sows are often the most competitive and more likely to be the aggressor and displacer, whereas subordinate sows are more likely to be on the receiving end of the aggressive encounter and most often displaced during feeding (Spoolder et al., 2009; Li et al., 2012; Wang and Li, 2016). Often, the subordinates experience poorer welfare as indicated by higher skin lesions, lower feed intake, less body weight gain, and a greater fear response (Elmore et al., 2011; Pacheco and Salak-Johnson, 2016; Li et al., 2017). Previously, O'Connell et al. (2003) found lower-ranked sows received less aggression when housed in pens with extended-length feeding barriers than shoulder-length ones. This was certainly the case here. In general, subordinate sows benefited the most from being housed in pens with long barriers. They experienced the least number of aggressive encounters and were less likely to be displaced, and had longer eating bouts than subordinates in pens with short ones. Subordinates in these pens and fed the DDGS-GM diet experienced the least social stress. The extended length served as a physical barrier that made it more difficult for more dominant sows to displace lower-rank sows; therefore, the subordinates could consume more of their daily feed allotment—maximizing feed intake. They also expressed a higher percentage of satiety-like behaviors (stand, sit, and lay), less oral-nasal-facial and sham-chew behaviors, and heavier live body weights and gains. They weaned heavier piglets resulting in better well-being than subordinates in pens with short barriers.
Interesting, there were differential dietary effects among the subordinates in pens with long barriers on behavior, sow body weight and gain, and litter weaning weight. However, this dietary effect was not found among subordinates in pens with short barriers. Overall the subordinates fed high-fiber diets and housed in pens with feeding places made from the longer length barriers had a behavioral profile indicative of contentment and possibly less stressed and more satiated than did subordinates in pens with short barriers. More specifically, subordinates in the DDGS-GMlong were 70% less likely to be on the receiving end of an aggressive encounter during feeding than those in MID-SHlong treatment. Those fed the MID-SH diet also spent 53% longer eating than those in DDGS-GMlong. At the same time, subordinates in DDGS-GMlong treatment spent 23 and 50% less time standing and laying during the observational period than those in fed MID-SH. While subordinates fed the MID-SH diet gained 64% more total body weight but lost 50% more body than those fed the DDGS-GM diet. The increased body weights and gains among the subordinates fed the MID-SH diet may be due to it being bulkier than the DDGS-GM resulting in a feeling of fullness and contentment as resulting in slower digestion and prolonged postprandial peak in blood glucose (de Leeuw et al., 2004) and increased gastric distension (DeDecker et al., 2014). However, the subordinates in DDGS-GMlong treatment had longer eating bouts than those in short and had increased satiety behaviors.
Nevertheless, both diets elicited higher, more stable mean glucose concentrations, but subordinates in DDGS-GMlong treatment had the highest glucose concentrations. Also, both diets are mostly insoluble, but the MID-SH diet is 5 to 10% more soluble (Jaworski and Stein, 2017), but unlikely enough to explain the differences. Especially since the litter and average piglet weaning weights among the subordinates in the DDGS-GMlong were 10.45 and 1.18 kg heavier than those fed MID-SH. Their litters were also 15.28 kg heavier than subordinates in the DDGS-GMshort treatment. The subordinates fed the DDGS-GM also had longer eating bouts and displayed a higher percentage of satiety behaviors, but they gained less total body weight. Lopez et al. (2021) also found that sows fed the DDGS-GM diet had deeper backfat depth and lost less body weight at the end of lactation than those fed MID-SH. The difference in the physicochemical properties between the two diets may partly explain the difference. Maybe the sows fed the DDGS-GM diet differentially partitioned energy during gestation due to experiencing different constraints but compensated during lactation, which was evident by increased feed consumption (Lopez et al., 2021). This may also partly explain the improved weaning weights among the subordinates in the DDGS-GMlong treatment, but unlikely. Lopez found no interactive effects of barrier length × diet on any measures; only main effects. They found that sows fed the DDGS-GM weaned pigs were 0.5 kg heavier than those fed the MID-SH diet. Thus, these data imply that the differential differences between subordinates in the MID-SHlong and DDGS-GMlong treatments are more likely driven by social position within the group.
Moreover, these data also support that dominant and subordinates experienced different contraints within their environments, thus evoking different biological responses to adapt, especially the dominants in pens with long barriers. Overall, dominants were more competitive and initiated aggressive encounters, and displaced others from the feeding places. However, by 3-weeks post-grouping, dominants in pens with long barriers were less successful in attacking and displacing subordinates which may have been frustrating. Conversely, the number of aggressive encounters and displacement by dominants toward the subordinates in pens with short barriers increased by 190 and 184% (Pacheco, 2015), indicating that the subordinates in these pens were continuously experiencing social stress. Limit-fed sows tend to be more frustrated, and merely increasing the amount of feed offered (Li et al., 2012) or feeding fiber (Pacheco, 2015) can reduce stereotypic behaviors and alter satiety-related behaviors (lay, stand, sit, and exploratory). In general, dominants displayed the highest percentage of oral-nasal-facial behaviors, and sham-chewing post-feeding and high-fiber diets had minimal effects on these behaviors until social status and length were considered. Dominants in DDGS-GMshort treatment had the highest percentage of oral-nasal-facial behaviors, but the lowest percentage of sham-chewing when fed the MID-SH diet. At the same time, subordinates displayed less oral-nasal-facial behaviors in DDGS-GMshort and less sham-chewing in the MID-SHlong treatment. These differences may reflect different coping mechanisms required to adapt to the constraints of the pen environment. The dominant sows in pens with longer length barriers could have been frustrated due to the inability to displace others and steal feed. The shorter barrier made it easier for dominants to acquire extra feed by displacing others from the feeder, resulting in higher feed intake, compensating for the extra energy needed to maintain social status.
In contrast, subordinates in pens with short barriers may have been more stressed initially but eventually adapted to the pen environment. The dominant sows housed in pens with short barriers were more aggressive and more successful, displacing other sows from the feeder, especially subordinates, throughout the entire gestational period. They received more aggressive attacks and displacements during feeding, restricting their ability to consume their feed allotment, which was evident by having the shortest eating bouts. Also, the subordinates may be more fearful, especially early on, which may explain the longer bouts of standing and laying during the observational period, contributing to their shorter eat bouts. Initial aggression can lead to subordinates being fearful of further conflicts while attempting to obtain feed (Kranendonk et al., 2007), while higher-ranked sows defend their access to feed, resulting in them being heavier during gestation and lactation (Zhao et al., 2013). Lower-ranked sows may suffer from fear and behavioral restriction in the presence of more dominant sows when kept in pens with an unprotected feeding system (Chapinal et al., 2010). Over time, subordinates spent 30% less time eating and 49% more time laying than subordinates in pens with long barriers. We postulate that this behavioral budget change among subordinates in pens with short barriers may imply that they learned to eat faster, and once they were displaced, they retreated to avoid further conflict. These sows were often observed laying in the open-pen area away from the feeding places, which may explain their shorter eating bouts, a higher percentage of time spent laying and standing during the observational period, and lower body weight gain. It is plausible that this may have been a compensatory response evoked by a lack of satiety experienced by sows in the pens with short barriers that had reduced feed intake due to feeding competition during gestation; however, it is more likely this is due to the social status and not diet treatment alone since for the most part subordinates in the pens with short barriers had similar responses, regardless of diet.
Finally, social stress has been shown to influence immune systems such as lymphocyte proliferation and natural killer cell cytotoxicity (Morrow-Tesch et al., 1994; Salak-Johnson and Webb, 2018). An individual's social rank often plays a more significant role in the stress responsiveness within the group than the stressor itself, thus contributing to individual welfare variation, implying that animals of different social statuses may evoke different biological responses in an attempt to cope (Salak-Johnson and McGlone, 2007). For example, 24-h after moving sows into a new pen environment, the acute stress response was evoked regardless of social rank (DeDecker and Salak-Johnson, 2020). Nevertheless, the magnitude of change for several immune traits was affected by social rank compared to the baseline, resulting in differential effects on the immune measures of various social ranks coping differently. However, stress does not always suppress the immune system, and it may enhance or have no effect. Social rank-related differences in the distribution of leukocyte populations imply that high and low social ranks are not simply a persistent stressful situation. For example, a higher percentage of neutrophils in subordinates may indicate that subordinates occupy a more stressful position (Widowski et al., 1989). In contrast, a higher neutrophil-to-lymphocyte ratio among dominants may indicate that they occupy a more stressful situation (Sutherland et al., 2006). Surprisingly, there were limited and inconsistent 3-way interactive effects on immune measures and cortisol; thus, it is more likely that social status (or social environment) partly explains the immunological differences between treatments. Subordinates fed the MID-SH diet had the lowest neutrophil-to-lymphocyte ratio, whereas dominants fed the same diet but in pens with short barriers had the highest. However, as evident by heavier body weights and gain, the higher N:L ratio among the dominants in MID-SHshort treatment does not indicate poorer welfare. Thus, the social rank had differential modulating effects on these parameters to cope with the constraints, and the appropriate physiological response was initiated.
Conclusion
Herein we demonstrate that social status or social position within a group-pen differentially affects behavior, immune status, and productivity measures among sows in pens with short or long barriers and fed different fiber diets. These differential profiles between dominant and subordinates support that social status indicates an individual's welfare within a group. Collectively, subordinate sows in pens with long barriers experienced less social stress. The longer barriers provided them protection and allowed them to consume their daily feed allotment and dietary fiber differentially affected behavioral budgets between the subordinates in these pens. We also demonstrate that dominant and subordinates used different coping mechanisms to adapt to the constraints of their pen environments, either by altering behavioral budgets or physiological responses without a welfare cost per se. Social status is a crucial factor that contributes to variation in individual well-being among group-housed sows. Finally, reducing competition around feeding and minimizing social conflict among lower-ranked sows may be beneficial.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The animal study was reviewed and approved by The University of Illinois Institutional Animal Care and Use Committee (protocol no. 13097).
Author Contributions
JS-J conceptualized and conceived studies, designed experiments, supervised field and lab experiments and analyses, obtained funding, administrated the project, and reviewed and edited the manuscript. EP and ML performed experiments and contributed to investigation and data collection. EP wrote the original version of the manuscript and analyzed data. All authors contributed to the article and approved the submitted version.
Funding
This research was funded by the National Pork Board, grant number 12-200, the Illinois Agricultural Experiment Station, and Oklahoma Agricultural Experiment Station.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
The authors gratefully acknowledge Dr. S. Rodriquez-Zas for statistical advice and Dr. H. Stein for technical assistance with diet formulation, and Mr. G. Bressner for technical assistance with daily animal care.
References
Andersen, I. L., Boe, K. E., and Kristiansen, A. L. (1999). The influence of different feeding arrangements and food type on competition at feeding in pregnant sows. Appl. Anim. Behav. Sci. 65, 91–104. doi: 10.1016/S0168-1591(99)00058-1
Chapinal, N., Ruiz-De-La-Torre, J. L., Cerisuelo, A., Gasa, J., Baucells, M. D., and Manteca, X. (2010). Aggressive behavior in two different group-housing systems for pregnant sows. J. Appl. Anim. Welf. Sci. 13, 137–153. doi: 10.1080/10888700903579846
da Silva, C. S., van den Borne, J. J., Gerrits, W. J., Kemp, B., and Bolhuis, J. E. (2013). Effects of dietary fibers with different physicochemical properties on feeding motivation in adult female pigs. Physiol. Behav. 107, 218–230. doi: 10.1016/j.physbeh.2012.07.001
de Leeuw, J. A., Jongbloed, A. W., and Verstegen, M. W. A. (2004). Dietary fiber stabilizes blood glucose and insulin levels and reduces physical activity in sows (Sus scrofa). J. Nutri. 134, 1481–1486. doi: 10.1093/jn/134.6.1481
de Leeuw, J. A., Zonderland, J. J., Altena, H., Spoolder, H. A. M., Jongbloed, A. W., and Verstegen, M. W. A. (2005). Effects of levels and sources of dietary fermentable non-starch polysaccharides on blood glucose stability and behaviour of group-housed pregnant gilts. Appl. Anim. Behav. Sci. 94, 15–29. doi: 10.1016/j.applanim.2005.02.006
DeDecker, A. E., Hanson, A. R., Walker, P. M., and Salak-Johnson, J. L. (2014). Space allowance and high fiber diet impact performance and behavior of group-kept gestating sows. J. Anim. Sci. 92, 1666–1674. doi: 10.2527/jas.2013-6776
DeDecker, A. E., and Salak-Johnson, J. L. (2020). Effect of social rank on well-being and space utilization of dry sows kept in a free access stall-pen housing environment. Open J. Anim. Sci. 10, 287–283. doi: 10.4236/ojas.2020.102017
Elmore, M. R. P., Garner, J. P., Johnson, A. K., Kirkden, R. D., Richert, B. T., and Pajor, E. A. (2011). Getting around social status: Motivation and enrichment use of dominant and subordinate sows in a group setting. Appl. Anim. Behav. Sci. 133, 154–163. doi: 10.1016/j.applanim.2011.05.017
Gonyou, H. W. (2005). Experiences with alternative methods of sow housing. J. Am. Vet. Med. Assoc. 226, 1336–1340. doi: 10.2460/javma.2005.226.1336
Hanson, A. R., DeDecker, A. E., Salak-Johnson, J. L., and Walker, P. M. (2011). A comparison of using pen versus individual sow as the experimental unit when evaluating data from housing studies. J. Anim. Sci. 2:5.
Holt, J. P., Johnston, L. J., Baidoo, S. K., and Shurson, G. C. (2006). Effects of a high-fiber diet and frequent feeding on behavior, reproductive performance, and nutrient digestibility in gestating sows. J. Anim. Sci. 84, 946–955. doi: 10.2527/2006.844946x
Hoy, S., Bauer, J., Borberg, C., Chonsch, L., and Weirich, C. (2009). Impact of rank position on fertility of sows. Livest. Sci. 126, 69–72. doi: 10.1016/j.livsci.2009.05.018
Jaworski, N. W., and Stein, H. H. (2017). Disappearance of nutrients and energy in the stomach and small intestine, cecum and colon of pigs fed corn-soybean meal diets containing distillers dried grains with solubles, wheat middlings, or soybean hulls. J. Anim. Sci. 95, 727–739. doi: 10.2527/jas2016.0752
Kranendonk, G., Van Der Mheen, H., Fillerup, M., and Hopser, H. (2007). Social rank of pregnant sows affects their body weight gain and behavior and performance of the offspring. J. Anim. Sci. 85, 420–429. doi: 10.2527/jas.2006-074
Li, Y. Z., Wang, L. H., and Johnston, L. J. (2012). Sorting by parity to reduce aggression toward first parity sows in group-gestation housing systems. J. Anim. Sci. 90, 4514–4522. doi: 10.2527/jas.2011-4869
Li, Y. Z., Wang, L. H., and Johnston, L. J. (2017). Effects of social rank on welfare and performance of gestating sows housed in two group sizes. J. Swine Health Prod. 25, 290–298.
Lopez, M., Pacehco, E., and Salak-Johnson, J. (2021). Dietary fiber source and length of feeding partitions differentially affected behavior, immune status, and productivity of group-housed dry sows. Agriculture 11, 34–48. doi: 10.3390/agriculture11010034
Lovendahl, P., Damgaard, L. H., Nielsen, B. L., Thodberg, K., Su, G., and Rydhmer, L. (2005). Aggressive behaviour of sows at mixing and maternal behaviour are heritable and genetically correlated traits. Livest. Prod. Sci. 93, 73–85. doi: 10.1016/j.livprodsci.2004.11.008
Maes, D., Pluym, L., and Peltoniemi, O. (2016). Impact of group housing of pregnant sows on health. Porcine Health Manage. 2, 17–23. doi: 10.1186/s40813-016-0032-3
McGlone, J. J. (1985). A quantitative ethogram of aggressive and submissive behaviors in recently regrouped pigs. J. Anim. Sci. 61, 559–565. doi: 10.2527/jas1985.613556x
Moberg, G. P. (2004). “Biological response to stress: implications for animal welfare,” in The Biology of Animal Stress: Basic Principles and Implications for Animal Welfare, eds G. P. Moberg and J. A. Mench (London: CABI Publishing), 1–21.
Morrow-Tesch, J. L., McGlone, J. J., and Salak-Johnson, J. L. (1994). Heat and social stress effects on pig immune measures. J. Anim. Sci. 72, 2599–2609. doi: 10.2527/1994.72102599x
O'Connell, N. E., Beattie, V. E., and Moss, B. W. (2003). Influence of social status on welfare of sows in static and dynamic groups. Anim. Welf. 12, 239–249.
Ohl, F., and van der Staay, F. J. (2012). Animal welfare: at the interface between science and society. Vet. J. 192, 13–19. doi: 10.1016/j.tvjl.2011.05.019
Pacheco, E., and Salak-Johnson, J. L. (2016). Social status affects welfare metrics of group-housed gestating sows. J. Vet. Res. Anim. Husb. 1, 103–108.
Pacheco, E. A. (2015). Assessing the Well-Being of Gestating Submissive Sows in Group Pens Using Multiple Welfare Metrics (Master Thesis). University of Illinois, Urbana-Champaign, IL.
Parent, J. P., Meunier-Salaun, M.-C., Vasseur, E., and Bergeron, R. (2012). Stability of social hierarchy in growing female pigs and pregnant sows. Appl. Anim. Behav. Sci. 142, 1–10. doi: 10.1016/j.applanim.2012.09.011
Salak-Johnson, J. L., DeDecker, A. E., Horsman, M. J., and Rodriquez-Zas, S. L. (2012). Space allowance for gestating sows in pens: behavior and immunity. J. Anim. Sci. 90, 3232–3242. doi: 10.2527/jas.2011-4531
Salak-Johnson, J. L., and McGlone, J. J. (2007). Making sense of apparently conflicting data: stress and immunity in swine and cattle. J. Anim. Sci. 85, E81–E88. doi: 10.2527/jas.2006-538
Salak-Johnson, J. L., Niekamp, S. R., Rodriguez-Zas, S. L., Ellis, M., and Curtis, S. E. (2007). Space allowance for dry, pregnant sows in pens: body condition, skin lesions, and performance. J. Anim. Sci. 85, 1758–1769. doi: 10.2527/jas.2006-510
Salak-Johnson, J. L., and Webb, S. R. (2018). Pig social status and chronic cold or crowd stressors differentially impacted immune response. Open J. Anim. Sci. 8, 280–293. doi: 10.4236/ojas.2018.83021
Sapkota, A., Marchant-Forde, J. N., Richert, B. T., and Lay, D. C. (2016). Including dietary fiber and resistant starch to increase satiety and reduce aggression in gestating sows. J. Anim. Sci. 94, 2117–2127. doi: 10.2527/jas.2015-0013
Spoolder, H. A. M., Geudeke, M. J., Van der Peet-Schwering, C. M. C., and Soede, N. M. (2009). Group housing of sows in early pregnancy: a review of success and risk factors. Livest. Sci. 125, 1–14. doi: 10.1016/j.livsci.2009.03.009
Stukenborg, A., Traulsen, I., Puppe, B., Presuhn, U., and Krieter, J. (2011). Agonistic behaviour after mixing in pigs under commercial farm conditions. App. Anim. Behav. Sci. 129, 28–35. doi: 10.1016/j.applanim.2010.10.004
Sutherland, M. A., Niekamp, S. R., Rodriguez-Zas, S. L., and Salak-Johnson, J. L. (2006). Impacts of chronic stress and social status on various physiological and performance measures in pigs of different breeds. J. Anim. Sci. 84, 588–596. doi: 10.2527/2006.843588x
Sutherland, M. A., Rodriguez-Zas, S. L., Ellis, M., and Salak-Johnson, J. L. (2005). Breed and age affect baseline immune traits, cortisol, and performance in growing pigs. J. Anim. Sci. 83, 2087–2095. doi: 10.2527/2005.8392087x
Verdon, M., Hansen, C. F., Rault, J. L., Jongman, E., Hansen, L. U., Plush, K., et al. (2015). Effects of group housing on sow welfare: a review. J. Anim. Sci. 93, 1999–2017. doi: 10.2527/jas.2014-8742
Verdon, M., Morrison, R. S., Rice, M., Butler, K. L., and Hemsworth, P. H. (2017). The short-term behavioural response of sows, but not gilts, to a social stimulus is related to sow aggressiveness in groups. Behav. Proc. 140, 216–225. doi: 10.1016/j.beproc.2017.04.013
Verdon, M., Morrison, R. S., Rice, M., and Hemsworth, P. H. (2016). Individual variation in sow aggressive behavior and its relationship with sow welfare. J. Anim. Sci. 94, 1203–1214. doi: 10.2527/jas.2015-0006
Wang, L. J. H., and Li, Y. Z. (2016). Effect of continuous access to feeding stalls during mixing on behavior, welfare, and performance of group-housed gestating sows in different social ranks. Can. J. Anim. Sci. 96, 386–396. doi: 10.1139/cjas-2015-0054
Widowski, T. M., Curtis, S. E., and Graves, C. N. (1989). The neutrophil:lymphocyte ratio in pigs fed cortisol. Can. J. Anim. Sci. 69:501. doi: 10.4141/cjas89-058
Keywords: aggression, behavior, immune, social status, well-being
Citation: Pacheco E, Lopez M and Salak-Johnson JL (2021) Social Status Differentially Affects Behavioral and Immunological Outcomes of Group-Kept Sows Fed Different Dietary Fiber Using Different Length Feeding Barriers. Front. Anim. Sci. 2:719136. doi: 10.3389/fanim.2021.719136
Received: 01 June 2021; Accepted: 02 August 2021;
Published: 06 September 2021.
Edited by:
Pasquale De Palo, University of Bari Aldo Moro, ItalyReviewed by:
Takashi Bungo, Hiroshima University, JapanHalima Sultana, University of Florida, United States
Copyright © 2021 Pacheco, Lopez and Salak-Johnson. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Janeen L. Salak-Johnson, amFuZWVuLmpvaG5zb24mI3gwMDA0MDtva3N0YXRlLmVkdQ==
 Eridia Pacheco1,2
Eridia Pacheco1,2 Janeen L. Salak-Johnson
Janeen L. Salak-Johnson