
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Anim. Sci., 07 July 2021
Sec. Animal Welfare and Policy
Volume 2 - 2021 | https://doi.org/10.3389/fanim.2021.669692
 Yara Slegers1
Yara Slegers1 Yoska Oolbekkink1,2
Yoska Oolbekkink1,2 Sanne Roelofs1,3,4
Sanne Roelofs1,3,4 F. Josef van der Staay1,3
F. Josef van der Staay1,3 Rebecca E. Nordquist1,3,4*
Rebecca E. Nordquist1,3,4*In pigs, higher birth order is associated with higher pre-weaning mortality. However, knowledge on the effect of birth order on welfare of surviving piglets is limited. The aim of this study was to explore the possible link between birth order and both newborn piglet performance and later affective state. Firstly, the following data were collected in 393 piglets from 27 litters: stillbirth, intactness of the umbilical cord and birth weight. Subsets of piglets were used to measure suckling latency (n = 67) and teat order (n = 21). Secondly, a subset of first-born (n = 9) and last-born (n = 7) piglets were trained to perform an active-choice judgement bias task (JBT). During discrimination training preceding the JBT, the pigs learned to associate two tone cues with the availability of either a large (4 M&M's® Milk Chocolate candies) or small (1 M&M's) reward, provided at two different locations. After training, ambiguous intermediate tones were introduced and the pig's choice of location was scored as either optimistic or pessimistic. Results showed that last-born piglets had a higher birth weight than middle-born piglets, while first-born piglets did not significantly differ from last- or middle-born piglets. They also latched to more caudal teats than first-born piglets. The last-born and first-born piglets showed a similar learning rate during discrimination training, and a similar latency to approach reward locations, and had a similar optimistic bias in the JBT.
The pre-and perinatal environment and events during this period can have major effects on the further development of organisms. In pigs, intrauterine environment and events surrounding farrowing can both affect future health and welfare outcomes. The impact of pre-, peri-, and neonatal factors may vary between piglets within the same litter. For instance, sibling pigs born into a litter of 14 or of 13 live piglets, which is the average size of a litter in the Netherlands (Wientjes, 2013) and Spain (Koketsu et al., 2021), respectively, may have been exposed to variation in pre- or perinatal events. This is seen in pigs which are born later in birth order, which have a higher chance of dying within the first days after birth (Hartsock and Graves, 1976; Tuchscherer et al., 2000; Baxter et al., 2008; Cabrera et al., 2012; Panzardi et al., 2013). Death of piglets pre-weaning is an extensive problem in swine husbandry, exemplified in a pre-weaning mortality rate of 13.3% in the Netherlands in 2017 (Agrovision, 2017) and 13.1% in a study covering a large cohort of Spanish pig farms (Koketsu et al., 2021).
Birth is the most stressful for the last-born piglet in a litter, since it has to endure the most uterine contractions. Each contraction causes a decline in blood flow and reduces oxygen delivery to the fetus (Alonso-Spilsbury et al., 2005). Moreover, the number of broken umbilical cords increases with birth order (Rootwelt et al., 2012). This is caused by the uterine contractions in combination with the longer distance which the cord of the last-born piglet, being in the most cranial part of the uterine horn, needs to stretch during parturition (Rootwelt et al., 2012). The blood flow to the fetus is badly compromised when the umbilical cord is ruptured (Alonso-Spilsbury et al., 2005). One result of this faltering oxygen supply following umbilical cord rupture can be irreversible brain damage (Hoeger et al., 2000; Alonso-Spilsbury et al., 2005; Castillo-Melendez et al., 2013). In humans, perinatal asphyxia is associated with cognitive impairment later in life (Armstrong-Wells et al., 2010; Herrera-Marschitz et al., 2014). In addition, rats with perinatal asphyxia show impaired spatial learning and less interest in novel environments (Galeano et al., 2011). It is thus a plausible hypothesis that last-born piglets show more cognitive impairments than their first-born siblings.
Last-born piglets are also at risk of receiving insufficient amounts of colostrum. Colostrum is important for energy uptake and the immune system. Because antibodies are not transported through the diffuse epitheliochorial placenta of pigs, piglets depend on acquiring maternal antibodies via colostrum (Baxter et al., 2008). The quantity and quality of colostrum piglets receive is not equal, since the anterior teats secrete more colostrum with higher concentrations of IgA and IgG than the posterior teats (Kim et al., 2000; Ogawa et al., 2014) and the amount of protein and immunoglobulins decreases by 50% in the first 6 h of suckling (Cabrera et al., 2012). Piglets nursing the anterior and middle teats have a greater average daily gain than those nursing the posterior teats (Kim et al., 2000; Sommavilla et al., 2015). A significant effect of birth order on the intake of IgG has been found (Klobasa et al., 2004; Cabrera et al., 2012), albeit not in all studies (Nguyen et al., 2013). This effect has two possible explanations. Firstly, last-born piglets are the last to arrive at the teat, when the anterior teats have already been claimed and colostrum quality is decreasing. Secondly, asphyxiated piglets might have more difficulty moving to and finding the udder (Alonso-Spilsbury et al., 2005; Castillo-Melendez et al., 2013).
The welfare of last-born pigs is likely to be compromised. Recent definitions of animal welfare include the concept that an animal should be able to adequately adapt to negative stimuli and that this adaptation should enable the animal to reach a state which it perceives as positive (Ohl and van der Staay, 2012). Last-born pigs are more likely to experience hypoxia, hunger, weakness and, due to impaired intake of maternal antibodies, possibly sickness, while potentially being less able to adapt to their environment. Hypoxia results in mild to moderate welfare compromise, while hunger and sickness would lead to moderate to severe welfare compromise (Mellor and Stafford, 2004). Hypoxia, hunger, and sickness can be measured, though sometimes indirectly. For example, the time to reach the udder and the weight and growth of a piglet can give an indication of hunger. Hypoxia can be measured in the blood, but this is an invasive method. The presence of a broken umbilical cord can also give an indication of hypoxia, since this is the most important cause of hypoxia in piglets (Langendijk et al., 2018). Additionally, a judgement bias test (JBT) can be a useful indicator of welfare, since this test can be used to detect both negative and positive emotional states (Düpjan et al., 2013) of an animal. A positive emotional state is a vital component of animal welfare.
JBTs have been used for a variety of animal species, including companion animals (Starling et al., 2014; McGuire et al., 2018), farm animals (Daros et al., 2014; Baciadonna et al., 2016), captive wild animals (Matheson et al., 2008; Keen et al., 2014), rodents (Parker et al., 2014), and insects (Bateson et al., 2011). The test is preceded by a period of discrimination training in which the animal learns to associate one cue with a highly valued positive outcome and another cue with a negative (or less positively valued) outcome. After the training period, the animal is confronted with one or more ambiguous cues which lie somewhere between the positive and negative cue. The reaction of the animal to the ambiguous cue(s) depends on their emotional state and personality traits (Asher et al., 2016). Animals in a positive emotional state are assumed to respond to the ambiguous cue(s) as if they expect a positive outcome, which is called an “optimistic” bias, while animals in a more negative emotional state are assumed to respond as if expecting a negative outcome, which is called a “pessimistic” bias (Murphy et al., 2014; Asher et al., 2016). Examples of factors that affect judgement bias of pigs are housing conditions (Douglas et al., 2012) and birth weight (Murphy et al., 2015).
The effect of birth order on the affective state of pigs has, to the authors' knowledge, not yet been studied. Moreover, the effect of birth order on piglet performance is not completely understood. The aim of this study was therefore to investigate (1) the possible effect of birth order on multiple variables indicative of viability and on weaning weight, and (2) performance of first- and last-born piglets in a JBT and its preceding discrimination training. The JBT allows to assess both the cognitive and emotional performance of piglets. We hypothesize that being last-born in a litter will negatively affect measures of suckling latency, including teat latching, and measures of JBT.
This study was reviewed and approved by the local ethics committee of Utrecht University.
The births of 29 litters with a total of 461 piglets [(Terra × Finnish Landrace) × Duroc] were attended live on the commercial breeding farm of the Faculty of Veterinary Science in Utrecht, the Netherlands. The inclusion criteria for the study were a full-term birth and a litter size of more than 10 piglets. Two litters were excluded due to a smaller litter size (n = 4 and n = 9). For recording of variables after birth, 393 piglets of which the birth order was known were included in the study. Human intervention was necessary for one sow. The sow was given a sedative (Stresnil 1 ml/20 kg; 40 mg/ml; Elanco) seven hours before parturition and injection of 1 cc (10 IU) of oxytocin (Oxytocin®, Dechra) after the second piglet was manually delivered.
Sows and litters were housed in a farrowing unit until weaning at 4 weeks of age (see in Roelofs et al., 2019 for details of sow housing prior to farrowing and details of movement of the sows from group housing to farrowing room). Briefly, the farrowing unit consisted of a mechanically ventilated, thermostatically controlled room containing 10 farrowing pens. Temperature inside the farrowing unit was maintained at 24°C until 1 week post-farrowing, after which it was maintained at 20°C. Each pen (2.4 × 1.8 m) was fitted with a centrally positioned farrowing crate (1.8 × 0.6 m). Pens had partially slatted floors with floor heating for the piglets. Sows were fed twice-daily, with water available ad libitum. Each sow was provided with a length of rope as chewing substrate.
After weaning, the 20 piglets which took part in the JBT (see section Judgement Bias Task) were moved to the research facilities. Pigs were housed in two straw-bedded pens of ~4 × 5 m. Each pen housed five first-born and five last-born piglets. Siblings were divided randomly over the two pens. The pens contained a covered nest area with plastic transparent slabs for insulation and heat lamps, which were removed after 8 weeks. Enrichment in the form of toys (a chain with chewing sticks and balls) was also present. The average daily temperature in the stable varied between −6 and 32°C. Water was available ad libitum via a drinking nipple and the pigs were fed twice daily.
Immediately after the birth of each live piglet, the following variables were recorded: birth order, time of expulsion and intactness of the umbilical cord. Latency to suckling was defined as the time from birth until reaching a teat and holding it for more than 2 s. After measuring suckling latency, the piglet received an ear tag and was weighed. Identification of the piglet had priority over suckling latency, so when a piglet was in risk of being misidentified, the ear was tagged before first suckling occurred and latency to suckling was not recorded. This resulted in the recording of latency to suckling of 67 piglets from 12 litters. If the piglet had still not latched to a teat after 1 h, the piglet was brought to an available teat; these piglets were then excluded from analysis for suckling latency (n = 5, of which 3 from beginning, 1 from middle, and 1 from end of farrowing). Other possible interventions were removing the membranes, clearing the airway and pulling the piglet away to prevent crushing by the sow. Moreover, some piglets were cross-fostered shortly after birth within litters born within the same week to create litters of roughly the same size, according to standard procedure at the breeding farm. When a piglet was stillborn, only birth order and time of expulsion was recorded. Additionally, pathological examination was performed on mummified piglets from one litter. These piglets were sent to the Veterinary Pathology Diagnostic Centre of Utrecht University directly after birth. Two to three days after birth, piglets were offered artificial milk (Milkiwean BabyMilk, Trouw Nutrition, Nutreco N.V., the Netherlands) in a feeder placed on the ground, according to standard procedure.
In the first week after birth, the teat which a piglet latched onto was observed for a subgroup of 10 first-born and 11 last-born piglets from 11 randomly chosen litters. During this measure the piglets were 1–4 days of age. Of the first-born piglets, 6 piglets were 1 day of age, 3 piglets were 2 days of age, and 1 piglet was 4 days of age; of the last-born piglets, 6 piglets were 1 day of age, 3 piglets were 2 days of age, and 2 piglets were 4 days of age. The teat pairs were numbered in ascending order from cranial to caudal. The observations were made twice in total, during two latching sessions on the same day. Suckling position was recorded when the piglets in a litter latched on to a teat. A latching session was excluded when two or more piglets were not latched to a teat. Scoring of suckling position did not take place specifically during the milk let-down phase. In the week before weaning, teat order was observed for 18 first-born and 19 last-born piglets from 19 randomly chosen litters, of which 10 were also used for recording of teat order in the week after birth and 9 were new litters. During this measure, the following ages were included: for first-born piglets: 2 piglets of 15 days of age, 2 piglets 17 days days of age, 3 piglets 18 days of age, 5 piglets 20 days of age, 4 piglets 22 days of age, and 2 piglets 23 days of age. For the last-born piglets, the following ages were included: 2 piglets of 15 days of age, 2 piglets 17 days of age, 4 piglets 18 days of age, 5 piglets 20 days of age, 4 piglets 22 days of age, 2 piglets 23 days of age.
The day before weaning, a subset of 10 first- and last-born piglets chosen for the JBT (as described in the next paragraph) were weighed. They were weighed again at the end of the JBT, when they were ~5 months old. One selected first-born piglet was weighed at weaning but could not be identified at follow-up, when all selected piglets were moved to the research facility (see section Housing). This piglet was replaced by its next-born sibling for the JBT and weighing afterwards.
Twenty pigs from nine litters, born in the same week, were selected based on birth order. Ideally, the first-born and last-born of a litter were chosen. First- and last-born piglets that had died before weaning were replaced by littermates, provided that the number of piglets born between the two piglets was never <9. These piglets are further referred to as first- or last-born. Of the resulting 10 first- and last-born pairs, nine were sibling pairs, while one pair consisted of a first-born and a last-born piglet from different litters. An overview of the subjects is given in Table 1. The selected pigs were moved to the research facility after weaning (at around 4 weeks of age).
The judgement bias apparatus (Figures 1A,B) consisted of a test arena (3.6 × 4.2 m) with two identical goal-boxes, an antechamber and a start-box (for dimensions see Figure 1B). Entrance from the start-box to the antechamber and access to the goal-boxes could be controlled by pulley-operated guillotine doors, whereas swing doors provided access to the test arena. Both goal-boxes contained a food bowl with a false bottom. M&M's® Milk Chocolate candies were used as rewards in the goal-boxes. The central food bowl was locked and was not used. M&M's® were also placed underneath the false bottoms of the food bowls in the goal-boxes to avoid discrimination through scent. These food bowls were covered with plastic balls to mask the reward from view. Tone cues were generated using Online Tone Generator software (Online Tone Generator) and played on speakers (Logitech z-313, Logitech Europe S.A., Lausanne, Switzerland) placed on the outer wall of the arena, between the two goal-boxes.
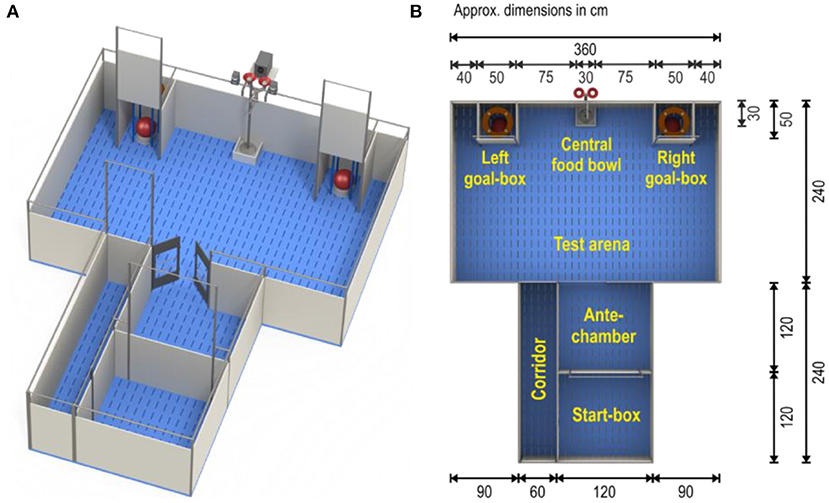
Figure 1. Judgement bias test apparatus (A) and its dimensions (B) (Illustration by Yorrit van der Staay). Food bowls pictured are dog bowl “Road Refresher” (Prestige Pets, Essex, UK), Size Large, Color Gray. Diameter of bowl base: 242 mm. Height of bowl: 102 mm. The ball covering the bowl is the “Push ‘N Play” (Jolly Pets, Streetsboro OH, USA), size large (diameter 25 cm); color red.
An overview of all steps in JBT training and testing is provided in Table 2. First, the pigs were habituated to the pen and the researcher for ~2 weeks, until they all approached the researcher voluntarily. The researcher sat in the pen for 30–60 min, two times a day (once in the morning and once in the afternoon). Next, they were habituated to the hallway that led to the test arena in sessions of the same length, then to the arena itself. The habituation in the test arena started with free exploration in groups of five. Rewards were placed in both goal-boxes. A session lasted 15 min and there were two sessions per day. When all pigs could lift the balls of the goal-boxes, group size was gradually decreased until the pigs were comfortable exploring the test arena individually.
Following habituation, the pigs' training continued with forced trials. In a forced trial, only one goal-box was open per trial. The pig entered the arena from the start-box and walked to the open goal-box to obtain a reward. The number of trials per training session (corresponding to 1 day) started at 8, but was gradually increased to 12 trials. After 3.5 weeks of forced trials, tone cues were introduced. Tones with a frequency of 200 and 1,000 Hz were used as positive and negative tones, i.e., corresponding to a large or small reward, respectively. Which tone was positive and which negative was counterbalanced across all piglets and within birth order group, as were the location of the large and small rewards (left or right goal-box). The small reward consisted of one M&M's® candy and the large reward of four candies.
Four pigs showed little interest in the single piece of candy as a reward; for these pigs, the amount of reward was increased to three (small reward) and eight (large reward) candies. The pigs again performed forced trials in which only one goal-box was open per trial. The tone corresponding to the open goal-box started when the pig was in the start-box and stopped when the pig lifted the ball. Each pig performed six positive and six negative trials in pseudo-random order for 4 days. Then, open-choice trials were performed in which both goal-boxes were open, but only the goal-box corresponding to the tone was baited. When the pig lifted the ball of the correct goal-box, it was rewarded with a clicker in addition to the food reward present in this goal-box. When it made a wrong choice, the incorrect goal-box was closed and the pig could still visit the correct goal-box. These open-choice trials were executed for 2 days, after which discrimination training started.
A session started with three forced trials as described above, of which two were always negative. The order of these trials was either negative-negative-positive or negative-positive-negative and was alternated every session. The three forced trials were followed by 10 open trials: 5 positive and 5 negative in a pseudo-random order which changed every session. In this pseudo-random order, no more than two trials of the same valence (positive or negative) succeeded each other. In an open trial, both goal-boxes were open. A correct choice was still rewarded with access to the food reward and a clicker, but the difference with open-choice trials (as used during pre-training) was that both goal-boxes were closed after a wrong choice and the pig had to return to the start-box without a reward. Every fifth session, the first six open trials were replaced by open-choice trials. In addition, from the tenth session onward, a partial reinforcement schedule was introduced: the correct goal-box was empty for one positive and one negative trial every session and a correct choice was only rewarded with a clicker. This was done to maintain responsiveness of pigs during testing, when ambiguous trials would go unrewarded (see section Testing). Discrimination training was performed until the pig either reached the criterion of four out of five correct choices in both the positive and negative trials for three consecutive sessions, or reached the maximum of 35 discrimination training sessions. This criterion was based on previous JBT experiments with pigs (Murphy et al., 2015; Roelofs et al., 2017).
Four testing sessions followed discrimination training when a pig had reached the criterion of 80% correct choices or after 35 training sessions. A test session was equivalent to discrimination training, save the addition of three ambiguous trials, inserted in the test session at trial 6, 11, and 16. Trial type and order of negative, positive, and ambiguous trials in the test sessions is shown in Supplementary Table 1. During those trials, one of the following ambiguous tones was played: a middle tone, a near-negative tone (intermediate between the middle and negative tone), or near-positive tone (intermediate between the middle and the positive tone). The ambiguous tones were of frequencies at equal intervals between the negative and positive tone on a logarithmic scale: 299.97, 447.21, and 668.74 Hz. Which frequency corresponded to the near-negative or near-positive valence depended on which tone the pig had learned to associate with a large or small reward. During the 6th trial, the middle tone was played, while the order of the near-positive and near-negative tones at the 11th or 16th trial alternated every session. The valence of the trials preceding the ambiguous trials (positive or negative) was counterbalanced across sessions to neutralize the effect of prior trials on judgement bias. During the ambiguous trials, the goal-boxes were empty. However, all choices were rewarded with a clicker to prevent the pigs from associating the new tones with the absence of a reward.
Statistical analyses were performed using R (R Core Team, 2020). The significance threshold was set at p = 0.05. Unless indicated otherwise, results are presented as mean ± SEM. The normal distribution of data was verified using visual inspection of residual plots (for linear mixed-effect models) and the Shapiro-Wilk test for normality (for t-tests). Group sizes and sex distributions (% males) used for measuring suckling latency, teat order, and birth weight are listed in Supplementary Table 2.
For analyses of the number of stillbirths, damaged umbilical cords, and piglets born inside the membranes, piglets were divided into two groups (first and second half) based on birth order relative to total litter size. Freshly stillborn and mummified piglets were included in litter size. In case of odd-numbered litter sizes, the median piglet was classified as second half. Results were analyzed using Fisher's exact test.
For analyses of suckling latency, a division into three groups (beginning, middle, and end of parturition) was made based on birth order relative to total litter size. To make these groups, litter size (n) was divided by three. If this division gave a remainder of 2, the number of piglets in the “beginning” group was equal to n/3 rounded down, while the number of piglets in the other two groups was n/3 rounded up. If the remainder was 1, the number of piglets in the “beginning” and “middle” group was n/3 rounded down, while the number of piglets in the “end” group was n/3 rounded up. For analysis of birth weight, only the first, middle, and last-born piglets were used. In an odd-numbered litter size (n), the middle piglet was n/2 rounded up. In an even-numbered litter size, both piglet n/2 and n/2+1 were considered middle. The weaning and end weights of the first- and last-born piglets were compared.
Suckling latency, teat order, birth weight, weaning weight, and end weight were analyzed using a linear mixed-effect model (lme in R), using the following method: firstly, the most suitable random effect structure was chosen based on study design. Secondly, fixed effect structure was chosen using the Aikaike Information Criterion (AIC). Maximum likelihood models with different combinations of variables as fixed effects were created. Assessed fixed effects were Birth Order; Litter Size; Birth Weight (only for analysis of suckling latency); Week (the week after birth or before weaning, only for analysis of teat order), Sex (for analysis of birth weight, weaning weight, and end weight) and possible interactions. The model with the lowest AIC was selected. Variables not included as fixed effects in the final model were assumed not to have explanatory value. See Supplementary Materials for full data sets and models. Thirdly, residual plots were visually inspected to check assumptions of linearity and homoscedasticity. A log10-transformation was performed on suckling latency and a square root-transformation on teat order to improve distribution of residuals.
For suckling latency, the best model, with Litter as random effect, included Birth order as fixed effect. For teat order, Piglet nested in Litter was used as random effect and Birth order as fixed effect. For birth weight, Litter was used as random effect and Birth order and Litter Size as fixed effects. For weaning and end weight, models with Litter as random effect and Birth order and Sex as fixed effects were used.
The following variables were calculated:
• Sessions until criterion, i.e., number of discrimination training sessions necessary to reach the criterion of four out of five correct choices (i.e., 80% correct) in the positive and negative trials;
• Correct choices, i.e., number of total correct choices per session block (three consecutive training sessions), forming a learning curve of discrimination training;
• Optimistic choice (OC) percentage, i.e., the percentage of choices for the goal-box normally containing the large reward during test trials of the JBT;
• Latency to respond, i.e., time from the pig's first step out of the start-box until one of the balls in a goal-box was lifted during test trials of the JBT.
Results from one pig were excluded from analysis of the learning curve because it failed to perform 13 trials per session in the beginning of discrimination training. Session 34 and 35 were also not included in the learning curve because of the low number of piglets that had not yet reached the criterion. To assess whether the increased rewards used in the training of four pigs affected their learning rate, the number of correct choices was analyzed using a model with Piglet nested in Litter as random effect and Session and Reward (normal or increased) as fixed effects.
Six pigs did not reach criterion task performance within 35 training sessions. To assess whether these pigs' ability to discriminate between the positive and negative stimulus was equal to that of the other pigs, OC percentage was analyzed using a model with Piglet nested in Session as random effect and Cue type, Criterion (reached or not reached) and Cue type X Criterion interaction as fixed effects.
Analysis of sessions until criterion, correct choices, and latency to respond was performed using a linear mixed-effect model. OC was analyzed using a generalized linear model. The assessed fixed effects for analysis of sessions until criterion were Birth Order, Reward Location and interactions between these two. The assessed fixed effects for analysis of correct choices, OC and latency were Birth Order; Sex; Cue Type (only for analysis of OC and latency); Session (only for analysis of correct choices); Birth Weight (only for analysis of latency) and all possible interactions. The model for sessions until criterion, with Litter as random effect, included Location as fixed effect. For correct choices, Piglet nested in Litter was used as random effect and Session block as fixed effect. The best model for OC, with Piglet nested in Session as random effect, had only Cue type as fixed effect. Latency to respond was analyzed using Piglet nested in Session as random effect and Sex, Cue type, Sex X Cue type interaction as fixed effects. Sex X Cue type interaction was analyzed by also running the model on separate datasets for each cue type.
Additionally, the effect of repeated testing on OC percentage was analyzed by creating a generalized linear model with Piglet nested in Session as random effect and Cue type and Session as fixed effects.
The 27 sows included in the study had a median parity of 4 [interquartile range (IQR) 1–5] and a mean litter size of 17 ± 3.0 (range 11–23). The median time between birth of the first and last piglet was 211 min (IQR 132–244), with a median birth interval of 9 min (IQR 5–17.75). Total stillbirth rate was 11.2%, consisting of freshly stillborn (4.6%) and mummified (6.6%) piglets. No cause of death was determined with pathological examination of mummified piglets. Five piglets (1.3%) were born inside the membranes; 7.2% was born with a damaged umbilical cord. Mean birth weight was 1.30 ± 0.32 kg (range 0.42–2.20 kg, n = 337). Median latency to suckling was 18 min (IQR 11–31, n = 67). Sixty minutes was the maximal value, which was recorded for five piglets. The percentage of piglets that latched to the same teat twice was 67% in the first week and 90% in the week before weaning. One last-born piglet was not observed to latch during either latching session.
The numbers of stillbirths, piglets born inside membranes or damaged umbilical cords did not differ between the first and second half of farrowing (Table 3). The analysis of stillbirths was repeated with exclusion of mummified piglets.
Suckling latency for piglets born in the beginning, middle and end of farrowing was similar [F(2,48) = 1.8, p = 0.18; Figure 2A]. Figure 2B illustrates that last-born piglets latched to more caudal teats than first-born piglets [F(1, 11) = 5.4, p = 0.04]. Last-born piglets were shown to have a higher birth-weight than middle-born piglets [t(42) = 2.7, p = 0.01], while the first-born piglets did not differ from the middle-born piglets [t(42) = −1.4, p = 0.18] or from the last-born piglets [t(42) = 1.3, p = 0.2]. At weaning and at the end of JBT training, first- and last-born piglets did not differ in body weight [Birth order group: F(1, 9) = 2.3, p = 0.4; F(1, 9) = 2.3, p = 0.2; Figure 3].
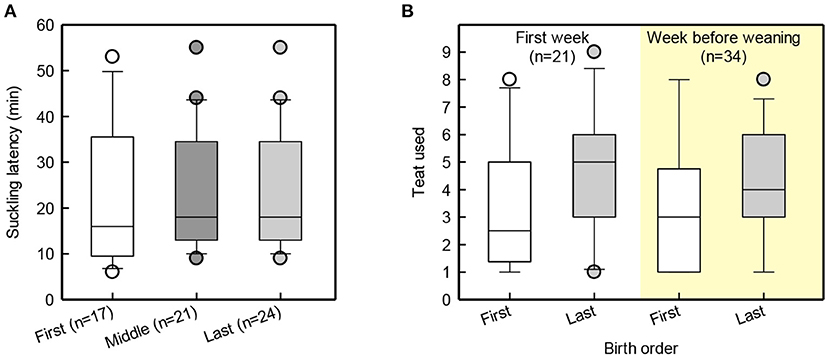
Figure 2. Box and whisker plot of suckling latency of piglets born in the beginning, middle and end of farrowing (A), and teat order of first and last-born piglets during the first week and in the week before weaning (B).
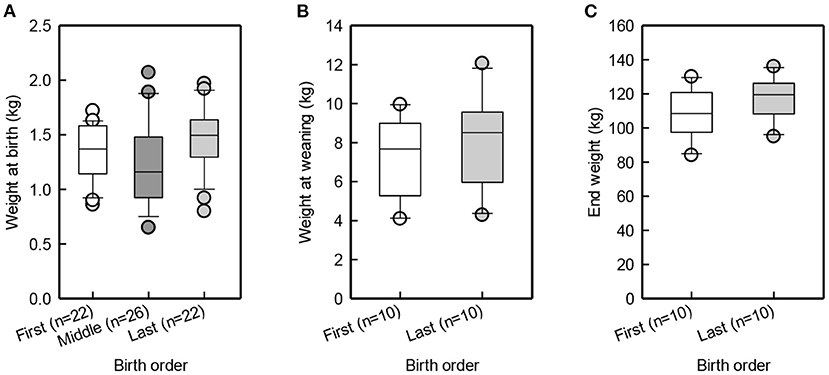
Figure 3. Box and whisker plot of birth weight of first, middle, and last-born piglets (A), weaning weight (B), and end weight (C) of first and last-born pigs.
Five first-born and five last-born piglets reached the training criterion of 80% correct choices within 35 sessions. Birth order did not affect the number of sessions required to reach the criterion (first-born 24.2 ± 2.4 sessions, last-born 24.1 ± 2.8 sessions), nor did the location of the large reward [Location: F(1, 6) = 5.8, p = 0.053]. Figure 4 shows the learning curves of first- and last-born pigs in the discrimination training phase. The number of correct choices increased with test sessions [Session: F(10, 144) = 50, p < 0.001]. Birth order was not included in the model with the lowest AIC, suggesting this factor did not influence performance during discrimination training.
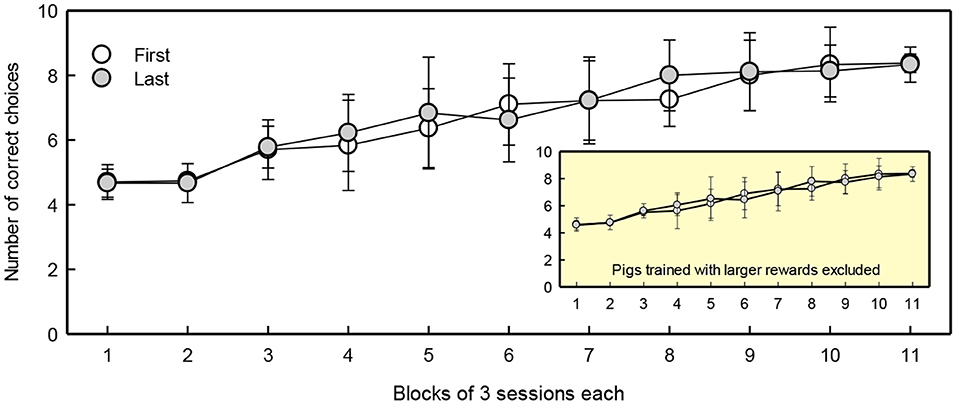
Figure 4. Number of correct choices per block of three sessions of first and last-born pigs. The learning curve with exclusion of pigs trained with a larger reward is provided in the right lower corner as insert. The means ± SEMs are depicted.
There were four pigs that were trained with increased rewards as they did not respond to the small reward of one candy (see section Pre-Training). These pigs made more correct choices during training compared to the rest of the animals [Reward: F(1, 17) = 6.8, p = 0.02]. Given that training the pigs with a larger reward caused differences in response compared to the rest of the group, and that the number of animals was not evenly distributed across the groups, these four pigs (one first-born and three last-born) were excluded from further analysis.
Of the six pigs that did not reach the criterion, one pig made 80% correct choices once, two pigs 3 times and three pigs 5 times, albeit not on consecutive days. Because of this, it was decided to test all pigs after 35 sessions. No difference in OC percentage between pigs that had or had not reached the criterion within 35 training sessions was found (χ2 = 1.6, df = 1, p < 0.3). Pigs that had not reached the criterion were therefore included in the analyses of OC and latency.
When cue type was more similar to the positive cue, optimistic bias increased (Cue: χ2 = 12, df = 1, p < 0.001; Figure 5A). Birth order was not included in the best model, suggesting it did not affect OC percentage. OC percentage did not differ between test sessions (Session: χ2 = 3.4, df = 3, p = 0.3). The OC percentage (mean ± SEM) for all pigs combined was 48 ± 0.073%, 54 ± 0.073%, 29 ± 0.066%, and 52 ± 0.073% during test session 1, 2, 3, and 4, respectively.
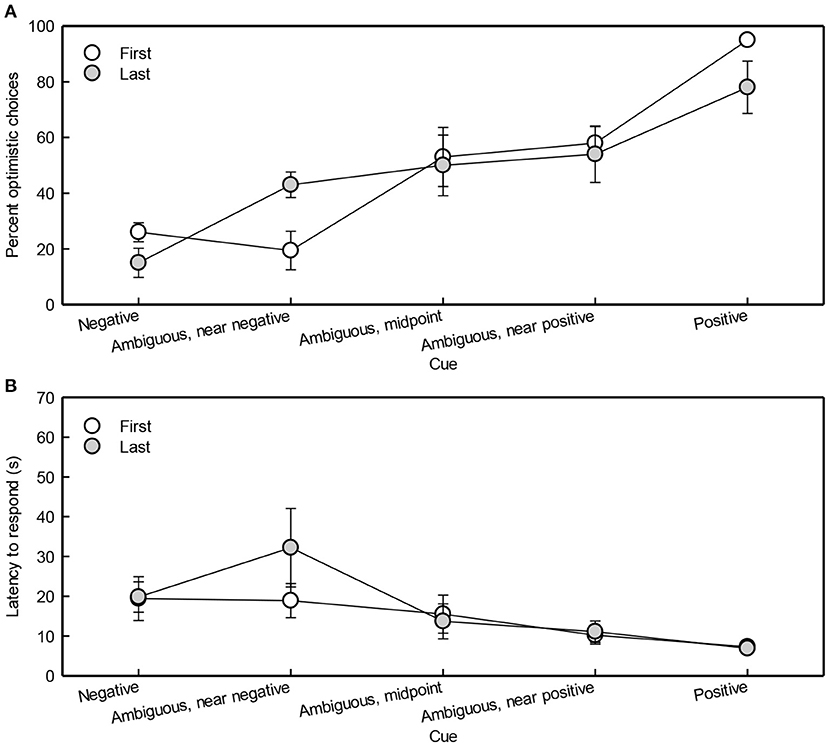
Figure 5. Optimistic choices (A) and latencies of first and last-born pigs (B) during testing in the judgment bias task. The means ± SEMs are depicted.
The latency to respond was affected by cue type (Figure 5B). Latency to respond decreased when cue type was more similar to the positive cue [Cue: F(1, 248) = 110, p < 0.0001]. Male pigs had a higher latency than females in response to the negative and near-negative cue [F(1, 14) = 12, p = 0.004; F(1, 14) = 8, p = 0.02, respectively]. Piglet end weight had no significant effect on latency to respond [Weight: F(1, 14) = 2, p = 0.2]. Birth order was not included in the model with the lowest AIC, suggesting it did not affect pigs' latency to respond.
The present study used non-invasive methods to examine the effect of birth order on the performance and welfare of commercially housed pigs. Of the variables measured in weaning piglets, birth order affected birth weight, with last-born piglets showing higher birth weight than piglets born mid-parturition. Last-born piglets were also seen latched to more caudal teats than first-born piglets. Last-born piglets were expected to have experienced a more difficult birth in addition to more competition during nursing, resulting in more stress and therefore negative effects on their affective state. The last-born and first-born piglets showed a similar learning rate during discrimination training, and a similar latency to approach reward locations, and had a similar optimistic bias in the JBT, thus did not support our hypothesis that last-born piglets would show more negative affect.
The total stillbirth rate of 11.3% in the present study is higher than the range of 6.2–9.2% found in other studies (Oliviero et al., 2010; Rootwelt et al., 2012; Panzardi et al., 2013; Langendijk et al., 2018). The high number of mummified piglets, 6.7% vs. ~2% in other studies (Rootwelt et al., 2012; Panzardi et al., 2013), accounts for this difference. Although pathological examination was performed on the mummified piglets from one litter, no specific cause of death could be identified. Other research has shown that stillbirth rate and either birth order (Rootwelt et al., 2012; Langendijk et al., 2018) or the duration of farrowing (Oliviero et al., 2010) are positively correlated. However, the present study did not find this relationship, neither with inclusion nor exclusion of mummified piglets. This could be a difference between pig farms, caused by differences in management or genetics, for example. Further research is necessary to determine which factors exactly cause this difference.
Intactness of the umbilical cord was measured to obtain an indication of asphyxia in a non-invasive way. A broken umbilical cord is one of the most important causes of asphyxia (Langendijk et al., 2018). In this study, no effect of birth order on either condition could be demonstrated. Langendijk et al. (2018) also found no relationship between birth order and intactness of the umbilical cord, whereas Rootwelt et al. (2012) did find an association. In the latter study, the same division into three birth order groups was used as was made in the present study. A reason for the difference between birth groups not reaching significance in the present study could be that the intactness of the umbilical cord was only recorded for live-born piglets. Since damage to the umbilical cord increases the chance of stillbirth (Langendijk et al., 2018), this could have affected the results. Langendijk et al. (2018) and Rootwelt et al. (2012) assessed the umbilical cords of both live-born and stillborn piglets, but they found contradictory results. The effect of birth order on damaged umbilical cords therefore remains unclear. In future research, stillborn piglets should be included in the assessment of umbilical cord intactness.
The average birth weight of the piglets in this study is comparable with other recent studies (Douglas et al., 2013; Bovey et al., 2014; Declerck et al., 2016). Middle-born piglets were shown to have a lower birth weight than last-borns, while the birth weight of first-born piglets did not differ significantly from middle or last-born piglets. Rootwelt et al. (2012) compared three birth order groups and found a comparable distribution of birth weight along the groups. They observed an additional difference between birth weight of the first and middle birth order group. Beaulieu et al. (2010) discovered a weak positive correlation between birth rank (i.e., relative birth order) and birth weight. Together, these studies support our finding of last-born piglets having a higher birth weight than middle-born piglets, which would be beneficial for last-born piglet survival (Quiniou et al., 2002; Baxter et al., 2008).
Colostrum and milk intake play an important role in piglet performance. Piglets can receive less colostrum than their siblings by either drinking later (Cabrera et al., 2012) or from more caudal teats (Kim et al., 2000; Ogawa et al., 2014). In the present study, the time it took piglets to latch for the first time did not differ between birth order groups. However, even when the time interval between birth and first suckling is the same, last-born piglets still begin latching later than first-born piglets simply because they are born later, on average 3.5 h after the first-born piglets. Therefore, their absolute time spent consuming colostrum is lower compared to first-born piglets. Moreover, a first-born has the opportunity to choose the most productive teat before the last piglet is born. In the present study, last-born piglets latched to more caudal teats than first-born piglets. Interestingly, this teat order was not reflected in weaning weight, as no significant differences were seen between the groups in weaning weight. This was an unexpected finding, as piglets nursing the cranial teats were expected to show a greater average daily gain based on other studies (Kim et al., 2000; Sommavilla et al., 2015), which would have led to higher weaning weight in first- compared to last-born piglets. The piglets nursing the caudal teats might have compensated a lesser milk intake by drinking more artificial milk, which was provided in the farrowing crate in the present study. Other studies provided energy-dense feed, which piglets eat very little of in the first weeks of life (Sommavilla et al., 2015), or provided no additional feed (Kim et al., 2000). It is also possible that the last-born piglets received more milk from the more caudal teats than expected, since we did not measure milk intake directly.
In the first week, one third of piglets changed their preferred teat between two subsequent drinking sessions. In the week before weaning, this was decreased to 10%, indicating that a stable teat order develops after the first week. This is in accordance with previous findings (Hemsworth et al., 1976). It should be noted that some piglets were cross-fostered before the first observation of teat order. The effect of cross-fostering on teat order is yet undetermined.
Ten pigs reached the criterion of 80% correct choices in both the positive and negative trials during three consecutive sessions. This success rate is lower than that found in previous studies with the same test design (Murphy et al., 2015; Roelofs et al., 2016, 2017). Pigs that did not reach the criterion responded equally well in response to the trained cues during testing as pigs that reached criterion performance, supporting the notion that they had learned to discriminate between the high and low tone.
Asphyxiated neonates show learning impairment in humans (Armstrong-Wells et al., 2010) and rats (Galeano et al., 2011). Because the last-born piglets are more at risk of oxygen deprivation during birth as they are in parturition the longest, we expected that they would need more training sessions to reach the criterion. However, the results of this study do not support this notion. As mentioned before, the effect that birth order has on asphyxia is still unclear. It is possible that we found no effect of birth order on learning rate because the last-born piglets did not experience more asphyxia. Perhaps fast farrowing prevented asphyxia, although farrowing times in the present study were comparable or even shorter than previously published studies (Baxter et al., 2008; Langendijk et al., 2018). Measuring the blood oxygen concentration after birth is a more sensitive method for measuring asphyxia and could be useful in future studies.
Pigs reacted faster to the cue more similar to the positive cue, showing that they preferred the large reward. This was also found in previous studies (Murphy et al., 2015; Roelofs et al., 2017). A preference for the high reward is essential in judgement bias testing. Males reacted slower than females in response to the near-negative and negative cue. Piglet weight was added to the model, to assess the hypothesis that males were heavier and therefore slower. However, this hypothesis was not supported. Another study found no difference in latency between male and female pigs (Roelofs et al., 2017).
Pigs responded optimistically to the intermediate ambiguous tone in 52% of trials. This optimistic bias is lower than Roelofs et al. (2017) and Murphy et al. (2015) reported, but higher than Murphy et al. (2013). Because the pigs are believed to have experienced good welfare in the relatively enriched housing, a higher optimistic bias was expected. Group housing with straw bedding is believed to enhance piglet welfare (Parrott and Misson, 1989). The lack of optimistic bias might be explained by the heat: the temperature in the stable suddenly increased to a maximum of 32°C (due to a sudden heat wave) around the time of testing. Although the effect of heat stress on the emotion of pigs has not been studied, heat is presumed to negatively affect the emotional state and to decrease motivation to consume food.
Birth order had no effect on optimistic choice percentage. The small sample size of this study, after exclusion of four piglets, could be an explanation why no difference was found. Another potential confounder is the enriched environment in which all pigs were housed in the present study. Pigs housed in enriched environments indeed have been found to show more optimistic bias than pigs in barren environments (Douglas et al., 2012); this could potentially obscure group differences if these are small. Housing pigs in a barren environment, more comparable to commercial housing settings, and then examining effects of birth order in a JBT could be a valuable addition for future research. To further validate the results of the present study, JBT results could be complemented by other tests measuring emotions (Roelofs et al., 2016), such discussed in a recent review of possibilities for testing emotions in pigs (Murphy et al., 2014).
One of the obstacles of the JBT is loss of ambiguity when pigs perform multiple test sessions (Roelofs et al., 2016). For example, Roelofs et al. (2017) reported a decrease in optimistic choice percentage when comparing the first and last two test sessions, suggesting the pigs learned that the ambiguous tones were unrewarded. To prevent this effect of repeated testing, partial reinforcement (unrewarded correct choices) during training and secondary reinforcement (voice and clicker) can be used. Neave et al. (2013) successfully used a partial reinforcement schedule during training of dairy calves, while Keen et al. (2014) used a clicker as secondary reinforcement in their study with grizzly bears. In the present study, partial reinforcement was implemented by not rewarding one negative and one positive trial every training session, starting from the tenth session. This way, the pigs learned that correct choices were not always rewarded with food. In addition, all correct choices during training and testing and all responses to the ambiguous tones were followed by secondary reinforcement with a clicker. On the one hand, secondary reinforcement prevents the pigs from learning that their choice of goal-box in response to the ambiguous tone is an incorrect choice. In the present study, no effect of repeated testing on optimistic choice percentage was found, which supports the usefulness of applying a partial and secondary reinforcement schedule. On the other hand, loss of ambiguity is also a risk of secondary reinforcement, because the pigs will perceive their choice as a correct choice. If loss of ambiguity resulting from secondary reinforcement is present, the pigs will show a decreased changing of goal-box choice, which cannot be detected by comparing OC percentage of the first and last two test sessions. However, the optimistic choice per session would then remain almost constant from the first session. In the present study, the overall optimistic choice per session varied from 29 to 54%, which makes it unlikely that loss of ambiguity occurred.
This study shows that piglets born later in birth order latch onto more caudal teats. No differences were seen in weaning weight. Results from discrimination training and the subsequent judgement bias test revealed no difference in discrimination learning or affective state between first- and last-born pigs. Conducting additional behavioral tests with piglets in poorer living conditions, mimicking the conditions on commercial farms more closely, is proposed.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
This study was reviewed and approved by the local animal welfare body of Utrecht University and according to the European Directive 2010/63/EU and the Dutch Experiments on Animals Act (Wod) as amended on December 18, 2014. Upon review and approval of the protocol by the local committee, it was determined that the potential distress to the animals was less than injection with a needle by a competent person; therefore further review and approval by the national body was deemed not necessary, in accordance with the relevant EU and national directives.
YS, RN, and SR contributed to study design. YS and YO acquired the data. YS performed statistical analysis and wrote the first draft of the manuscript. FS provided the graphs. RN, SR, and FS revised the manuscript. All authors have read and approved the final version.
This study was fully funded by the Department of Farm Animal Health, Faculty of Veterinary Science, University Utrecht.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The authors would like to thank Jan van Mourik, Dirk van der Heide, and Jan Adriaan den Hertog for their assistance in taking care of the pigs. They would further like to thank Vivian Witjes and Puck Eicher for their assistance in attending piglet births.
Preliminary results of this study were presented at the ISAE Benelux conference, 2018, and the abstract of this presentation has been published in: Slegers, Y., Oolbekkink, Y. & Nordquist, R.E. (2018). Effect of birth order on performance and affective state of pigs. Proceedings of the ISAE Benelux conference 2018, Geel, Belgium, October 10, 2018, p. 6; available online at: https://www.applied-ethology.org/res/Booklet ISAE Benelux 2018.pdf.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fanim.2021.669692/full#supplementary-material
Alonso-Spilsbury, M., Mota-Rojas, D., Villanueva-García, D., Martínez-Burnes, J., Orozco, H., Ramírez-Necoechea, R., et al. (2005). Perinatal asphyxia pathophysiology in pig and human: a review. Anim. Reprod. Sci. 90, 1–30. doi: 10.1016/j.anireprosci.2005.01.007
Armstrong-Wells, J., Bernard, T. J., Boada, R., and Manco-Johnson, M. (2010). Neurocognitive outcomes following neonatal encephalopathy. NeuroRehabilitation 26, 27–33. doi: 10.3233/NRE-2010-0533
Asher, L., Friel, M., Griffin, K., and Collins, L. M. (2016). Mood and personality interact to determine cognitive biases in pigs. Biol. Lett. 12:20160402. doi: 10.1098/rsbl.2016.0402
Baciadonna, L., Nawroth, C., and McElligott, A. G. (2016). Judgement bias in goats (Capra hircus): investigating the effects of human grooming. PeerJ. 4:e2485. doi: 10.7717/peerj.2485
Bateson, M., Desire, S., Gartside, S. E., and Wright, G. A. (2011). Agitated honeybees exhibit pessimistic cognitive biases. Curr. Biol. 21, 1070–1073. doi: 10.1016/j.cub.2011.05.017
Baxter, E. M., Jarvis, S., D'Eath, R. B., Ross, D. W., Robson, S. K., Farish, M., et al. (2008). Investigating the behavioural and physiological indicators of neonatal survival in pigs. Theriogenology 69, 773–783. doi: 10.1016/j.theriogenology.2007.12.007
Beaulieu, A. D., Aalhus, J. L., Williams, N. H., and Patience, J. F. (2010). Impact of piglet birth weight, birth order, and litter size on subsequent growth performance, carcass quality, muscle composition, and eating quality of pork. J. Anim. Sci. 88, 2767–2778. doi: 10.2527/jas.2009-2222
Bovey, K. E., Widowski, T. M., Dewey, C. E., Devillers, N., Farmer, C., Lessard, M., et al. (2014). The effect of birth weight and age at tail docking and ear notching on the behavioral and physiological responses of piglets1. J. Anim. Sci. 92, 1718–1727. doi: 10.2527/jas.2013-7063
Cabrera, R. A., Lin, X., Campbell, J. M., Moeser, A. J., and Odle, J. (2012). Influence of birth order, birth weight, colostrum and serum immunoglobulin G on neonatal piglet survival. J. Anim. Sci. Biotechnol. 3:42. doi: 10.1186/2049-1891-3-42
Castillo-Melendez, M., Baburamani, A. A., Cabalag, C., Yawno, T., Witjaksono, A., Miller, S. L., et al. (2013). Experimental modelling of the consequences of brief late gestation asphyxia on newborn lamb behaviour and brain structure. PLoS ONE 8:e77377. doi: 10.1371/journal.pone.0077377
Daros, R. R., Costa, J. H. C., von Keyserlingk, M. A. G., Hötzel, M. J., and Weary, D. M. (2014). Separation from the dam causes negative judgement bias in dairy calves. PLoS ONE 9:e98429. doi: 10.1371/journal.pone.0098429
Declerck, I., Dewulf, J., Sarrazin, S., and Maes, D. (2016). Long-term effects of colostrum intake in piglet mortality and performance. J. Anim. Sci. 94, 1633–1643. doi: 10.2527/jas.2015-9564
Douglas, C., Bateson, M., Walsh, C., Bédu,é, A, and Edwards, S. A. (2012). Environmental enrichment induces optimistic cognitive biases in pigs. Appl. Anim. Behav. Sci. 139, 65–73. doi: 10.1016/j.applanim.2012.02.018
Douglas, S. L., Edwards, S. A., Sutcliffe, E., Knap, P. W., and Kyriazakis, I. (2013). Identification of risk factors associated with poor lifetime growth performance in pigs. J. Anim. Sci. 91, 4123–4132. doi: 10.2527/jas.2012-5915
Düpjan, S., Ramp, C., Kanitz, E., Tuchscherer, A., and Puppe, B. (2013). A design for studies on cognitive bias in the domestic pig. J. Vet. Behav. Clin. Appl. Res. 8, 485–489. doi: 10.1016/j.jveb.2013.05.007
Galeano, P., Blanco Calvo, E., Madureira de Oliveira, D., Cuenya, L., Kamenetzky, G. V., Mustaca, A. E., et al. (2011). Long-lasting effects of perinatal asphyxia on exploration, memory and incentive downshift. Int. J. Dev. Neurosci. 29, 609–619. doi: 10.1016/j.ijdevneu.2011.05.002
Hartsock, T. G., and Graves, H. B. (1976). Neonatal behavior and nutrition-related mortality in domestic swine. J. Anim. Sci. 42, 235–241. doi: 10.2527/jas1976.421235x
Hemsworth, P. H., Winfield, C. G., and Mullaney, P. D. (1976). A study of the development of the teat order in piglets. Appl. Anim. Ethol. 2, 225–233. doi: 10.1016/0304-3762(76)90054-7
Herrera-Marschitz, M., Neira-Pena, T., Rojas-Mancilla, E., Espina-Marchant, P., Esmar, D., Perez, R., et al. (2014). Perinatal asphyxia: CNS development and deficits with delayed onset. Front. Neurosci. 8:47. doi: 10.3389/fnins.2014.00047
Hoeger, H., Engelmann, M., Bernert, G., Seidl, R., Bubna-Littitz, H., Mosgoeller, W., et al. (2000). Long term neurological and behavioral effects of graded perinatal asphyxia in the rat. Life Sci. 66, 947–962. doi: 10.1016/S0024-3205(99)00678-5
Keen, H. A., Nelson, O. L., Robbins, C. T., Evans, M., Shepherdson, D. J., and Newberry, R. C. (2014). Validation of a novel cognitive bias task based on difference in quantity of reinforcement for assessing environmental enrichment. Anim. Cogn. 17, 529–541. doi: 10.1007/s10071-013-0684-1
Kim, S. W., Hurley, W. L., Hant, I. K., and Easter, R. A. (2000). Growth of nursing pigs related to the characteristics of nursed mammary glands. J. Anim. Sci. 78, 1313–1318. doi: 10.2527/2000.7851313x
Klobasa, F., Schröder, C., Stroot, C., and Henning, M. (2004). [Passive immunization in neonatal piglets in natural rearing–effects of birth order, birth weight, litter size and parity]. Berl. Munch Tierarztl Wochenschr. 117, 19–23.
Koketsu, Y., Iida, R., and Piñeiro, C. (2021). A 10-year trend in piglet pre-weaning mortality in breeding herds associated with sow herd size and number of piglets born alive. Porc. Health Manag. 7:4. doi: 10.1186/s40813-020-00182-y
Langendijk, P., Fleuren, M., van Hees, H., and van Kempen, T. (2018). The course of parturition affects piglet condition at birth and survival and growth through the nursery phase. Animals 8:60. doi: 10.3390/ani8050060
Matheson, S. M., Asher, L., and Bateson, M. (2008). Larger, enriched cages are associated with ‘optimistic' response biases in captive European starlings (Sturnus vulgaris). Appl. Anim. Behav. Sci. 109, 374–383. doi: 10.1016/j.applanim.2007.03.007
McGuire, M. C., Johnson-Ulrich, Z., Robeson, A., Zeigler-Hill, V., and Vonk, J. (2018). I say thee ‘neigh': rescued equids are optimistic in a judgment bias test. J. Vet. Behav. 25, 85–91. doi: 10.1016/j.jveb.2018.03.009
Mellor, D. J., and Stafford, K. J. (2004). Animal welfare implications of neonatal mortality and morbidity in farm animals. Vet. J. 168, 118–133. doi: 10.1016/j.tvjl.2003.08.004
Murphy, E., Kraak, L., Broek van den, J., Nordquist, R. E., and Staay van der, F. J. (2015). Decision-making under risk and ambiguity in low-birth-weight pigs. Anim, Cogn. 18, 561–572. doi: 10.1007/s10071-014-0825-1
Murphy, E., Nordquist, R. E., and van der Staay, F. J. (2013). Responses of conventional pigs and Göttingen miniature pigs in an active choice judgement bias task. Appl. Anim. Behav. Sci. 148, 64–76. doi: 10.1016/j.applanim.2013.07.011
Murphy, E., Nordquist, R. E., and van der Staay, F. J. (2014). A review of behavioural methods to study emotion and mood in pigs, Sus scrofa. Appl. Anim. Behav. Sci. 159:9–28. doi: 10.1016/j.applanim.2014.08.002
Neave, H. W., Daros, R. R., Costa, J. H. C., Keyserlingk von, M. A. G., and Weary, D. M. (2013). Pain and pessimism: dairy calves exhibit negative judgement bias following hot-iron disbudding. PLoS ONE 8:e80556. doi: 10.1371/journal.pone.0080556
Nguyen, K., Cassar, G., and Friendschip, R. (2013). An investigation of the impacts of induced parturition, birth weight, birth order, litter size, and sow parity on piglet serum concentrations of immunoglobulin G. J. Swine Health Prod. 21, 139–143. Available online at: http://www.aasv.org/shap.html
Ogawa, S., Tsukahara, T., Tsuruta, T., Nishibayashi, R., Okutani, M., Nakatani, M., et al. (2014). Evaluation of secretion volume and immunoglobulin A and G concentrations in sow colostrum from anterior to posterior teats. Anim. Sci. J. Nihon. Chikusan Gakkaiho 85, 678–682. doi: 10.1111/asj.12211
Ohl, F., and van der Staay, F. J. (2012). Animal welfare: at the interface between science and society. Vet. J. Lond. Engl. 192, 13–19. doi: 10.1016/j.tvjl.2011.05.019
Oliviero, C., Heinonen, M., Valros, A., and Peltoniemi, O. (2010). Environmental and sow-related factors affecting the duration of farrowing. Anq24im. Reprod. Sci. 119, 85–91. doi: 10.1016/j.anireprosci.2009.12.009
Online Tone Generator - Free Simple Easy to Use. (2011). Available online at: http://onlinetonegenerator.com/ (accessed November 18, 2019).
Panzardi, A., Bernardi, M. L., Mellagi, A. P., Bierhals, T., Bortolozzo, F. P., and Wentz, I. (2013). Newborn piglet traits associated with survival and growth performance until weaning. Prev. Vet. Med. 110, 206–213. doi: 10.1016/j.prevetmed.2012.11.016
Parker, R. M. A., Paul, E. S., Burman, O. H. P., Browne, W. J., and Mendl, M. (2014). Housing conditions affect rat responses to two types of ambiguity in a reward-reward discrimination cognitive bias task. Behav. Brain Res. 274, 73–83. doi: 10.1016/j.bbr.2014.07.048
Parrott, R. F., and Misson, B. H. (1989). Changes in pig salivary cortisol in response to transport simulation, food and water deprivation, and mixing. Br. Vet. J. 145, 501–505. doi: 10.1016/0007-1935(89)90110-3
Quiniou, N., Dagorn, J., and Gaudré, D. (2002). Variation of piglets' birth weight and consequences on subsequent performance. Livest. Prod. Sci. 78, 63–70. doi: 10.1016/S0301-6226(02)00181-1
R Core Team (2020). R: A Language and Environment for Statistical Computing (version 4.0.0). Vienna: R Foundation for Statistical Computing. Available online at: https://www.R-project.org/
Roelofs, S., Boleij, H., Nordquist, R. E., and van der Staay, F. J. (2016). Making decisions under ambiguity: judgment bias tasks for assessing emotional state in animals. Front. Behav. Neurosci. 10:119. doi: 10.3389/fnbeh.2016.00119
Roelofs, S., Godding, L., de Haan, J. R., van der Staay, F. J., and Nordquist, R. E. (2019). Effects of parity and litter size on cortisol measures in commercially housed sows and their offspring. Physiol. Behav. 201:83–90. doi: 10.1016/j.physbeh.2018.12.014
Roelofs, S., Nordquist, R., and Josef van der Staay, F. (2017). Female and male pigs' performance in a spatial holeboard and judgment bias task. Appl. Anim. Behav. Sci. 191, 5–16. doi: 10.1016/j.applanim.2017.01.016
Rootwelt, V., Reksen, O., Farstad, W., and Framstad, T. (2012). Associations between intrapartum death and piglet, placental, and umbilical characteristics. J. Anim. Sci. 90, 4289–4296. doi: 10.2527/jas.2012-5238
Sommavilla, R., Costa, O. A. D., Honorato, L. A., Cardoso, C. S., and Hötzel, M. J. (2015). Teat order affects postweaning behaviour in piglets. Ciênc. Rural. 45, 1660–1666. doi: 10.1590/0103-8478cr20141512
Starling, M. J., Branson, N., Cody, D., Starling, T. R., and McGreevy, P. D. (2014). Canine sense and sensibility: tipping points and response latency variability as an optimism index in a canine judgement bias assessment. PLoS ONE 9:e107794. doi: 10.1371/journal.pone.0107794
Tuchscherer, M., Puppe, B., Tuchscherer, A., and Tiemann, U. (2000). Early identification of neonates at risk: traits of newborn piglets with respect to survival. Theriogenology 54, 371–388. doi: 10.1016/S0093-691X(00)00355-1
Keywords: animal welfare, pig, judgement bias, birth weight, suckling, pig behavior
Citation: Slegers Y, Oolbekkink Y, Roelofs S, van der Staay FJ and Nordquist RE (2021) Effects of Birth Order on Performance and Affective State of Pigs. Front. Anim. Sci. 2:669692. doi: 10.3389/fanim.2021.669692
Received: 19 February 2021; Accepted: 01 June 2021;
Published: 07 July 2021.
Edited by:
Manja Zupan Šemrov, University of Ljubljana, SloveniaReviewed by:
Karolina Noworyta, Polish Academy of Sciences (IF PAS), PolandCopyright © 2021 Slegers, Oolbekkink, Roelofs, van der Staay and Nordquist. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Rebecca E. Nordquist, ci5lLm5vcmRxdWlzdDFAdXUubmw=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.