
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Anesthesiol. , 22 January 2025
Sec. Cardiothoracic Anesthesiology
Volume 3 - 2024 | https://doi.org/10.3389/fanes.2024.1483837
 Grace E. Namirembe1,†
Grace E. Namirembe1,† Jamie Sparling1,†
Jamie Sparling1,† Alexis Novak1
Alexis Novak1 Ariel Mueller1
Ariel Mueller1 Julia Bertsch1
Julia Bertsch1 Kwame Wiredu1
Kwame Wiredu1 Jason Z. Qu1
Jason Z. Qu1 M. Brandon Westover2
M. Brandon Westover2 Timothy T. Houle1
Timothy T. Houle1 Oluwaseun Akeju1*
Oluwaseun Akeju1*
Objectives: This study aimed to assess the enduring impact of cross-clamp duration on postoperative sleep disturbance and functional outcomes (up to 180 days) in cardiac surgery patients.
Design: This is a secondary analysis of data from a randomized, double-blind trial comparing dexmedetomidine to placebo for delirium prevention (Minimizing ICU Neurological Dysfunction with Dexmedetomidine-induced Sleep).
Setting: Data from patients recruited at a tertiary medical center in Boston, Massachusetts, between March 2017 and February 2022 were analyzed in January 2024.
Participants: The study included 394 patients aged ≥60 who underwent cardiac surgery with cardiopulmonary bypass.
Interventions: The primary exposure was cross-clamp time, while secondary exposures included surgical type [isolated coronary artery bypass graft (CABG) or not] and dexmedetomidine randomization.
Measurements and main results: The primary outcome was sleep quality, assessed using the PROMIS Sleep Disturbance questionnaire at 30, 90, and 180 days postoperatively. Secondary outcomes encompassed cognitive function and health-related quality of life in various domains. Sleep quality, measured by PROMIS scores, showed improvement over time, and did not differ based on cross-clamp duration (MD 0.74 points, 95% CI: −0.57, 2.07), procedure type (MD 2.14 points, 95% CI: 0.29, 3.99), or dexmedetomidine (MD 0.9 points, 95% CI: −1.33, 1.5). However, isolated CABG patients reported sleep disturbance at all time points. Notably, extended cross-clamp time (>90 min) significantly worsened the trajectories of mental health (90-day: MD −2.37 points, 95% CI: −4.35, −0.39; 180-day: MD −2.68 points, 95% CI: −4.62, −0.73) and applied cognition (180-day: MD: −2.59 points, 95% CI: −4.49, −0.68).
Conclusion: Regardless of the duration of the cross-clamp, sleep quality tends to improve over time following cardiac surgery. However, cross-clamp times that last longer than 90 min have been identified as a risk factor for self-reported declines in mental health and applied cognition.
Elderly surgery patients face a unique set of challenges during recovery (1). Specific to cardiac surgery, the duration of cross-clamp has been associated with adverse outcomes such as prolonged requirement for ventilatory support, renal dysfunction, extended hospitalization, and elevated mortality risk (2–5). Looking beyond the immediate postoperative phase, the impact of cross-clamp time is unclear. Further, it is also unclear what role cross-clamp time may play in patient reported outcomes in the postoperative period. A characterization of the association between prolonged cross-clamp time and patient-reported outcomes is needed to further our understanding of modifiable outcomes.
The Minimizing ICU Neurological Dysfunction with Dexmedetomidine-induced Sleep (MINDDS) trial aimed to investigate whether a nighttime loading dose of dexmedetomidine could reduce the occurrence of postoperative delirium among elderly cardiac surgery patients who were extubated within twelve hours of being admitted to the ICU (6, 7). The trial reported a significant decrease in the incidence of postoperative delirium on the day after surgery (7). Data on patient-reported outcomes and cognitive function were gathered from MINDDS trial participants for a duration of up to six months post-surgery. Consequently, this dataset provides an opportunity to explore hypotheses regarding the trends in patient-reported outcomes.
Therefore, this post hoc analysis of the MINDDS trial aimed to evaluate whether the trajectories of patient-reported outcome measures were different based on the duration of cardiopulmonary bypass. It was hypothesized that longer cross-clamp times would both be associated with heterogeneity in the trajectory of sleep disturbance over time, and differences in sleep disturbance scores at each time point.
This was a secondary analysis of the MINDDS clinical trial. Secondary use of this data, consistent with the parent trial aims, was approved by the Mass General Brigham Institutional Review Board. The MINDDS trial was a randomized placebo-controlled, double-blinded, single-site, parallel-arm superiority trial. Patients aged 60 years or older, who underwent cardiac surgery with planned cardiopulmonary bypass and planned postoperative admission to the intensive care unit (ICU) for at least 24 h were enrolled and randomized to receive either dexmedetomidine or placebo. Briefly, patients were excluded if they were allergic to dexmedetomidine, had renal or liver failure, were on antipsychotic or chronic benzodiazepine therapy, recently underwent cardiac surgery or were admitted to the ICU, underwent a procedure requiring total circulatory arrest, or those in whom follow up could not be performed reliably (e.g., blind, deaf, unable to communicate, etc.). Specific details of the inclusion and exclusion criteria were reported elsewhere (6). All patients provided written informed consent for the primary trial and subsequent use of their data.
In the parent trial, enrolled patients were randomized to receive either dexmedetomidine or placebo, which was administered intravenously after extubation and every night that the patient was in the ICU for up to three days. A dosage of 1 ug/kg over 40 min was administered at each time point. Delirium cases were identified with twice-daily use of the confusion assessment method, which was designed based on DSM-IV diagnostic criteria (8).
In the present analysis, cross-clamp time was considered the exposure of primary interest. Exposures of secondary interest were surgical type [isolated coronary artery bypass graft (CABG) vs. valvular surgery] and treatment assignment (dexmedetomidine or placebo). The randomization definition of exposure (rather than per-protocol administration) was utilized given the infrequency of non-adherence while leveraging the robust randomization used in the modified intention-to-treat design. Exploratory analyses were performed considering patient characteristics, including age, sex, and postoperative delirium status, defined as any instance of delirium within the first three post-surgical days.
The primary outcome of the present analysis was sleep quality, given previous findings that major surgery is associated with sleep and circadian phase disruption (9, 10). Sleep quality was assessed using the patient-reported outcome measures (PROMIS) Sleep Disturbance SF 4A V.1 at 30, 90 and 180 days. The PROMIS Sleep Disturbance – which can be administered via telephone - is an eight-item assessment of the patient's sleep disturbance over the prior seven days, with lower scores indicating better sleep quality. The assessment is scored on a range of 8–40, which was translated to a T-score for analysis, with a mean of 50 and a standard deviation of 10.
Secondary outcomes including telephonic Montreal Cognitive Assessment (t-MOCA), health-related quality of life in several domains, namely global physical and mental health, physical function, and pain, were also assessed using validated patient-reported outcome measures (PROMIS) at 30, 90 and 180 days. This included the PROMIS Global Health SF V.1.1 (which results in both a physical and mental score), PROMIS Physical Function SF 8b V.1.2, and PROMIS Pain Interference SF 8a V.1.0. As above, PROMIS measures were elicited from patients via phone and translated to a T-score for analysis. For physical and mental health PROMIS measures, higher scores were indicative of better function, whereas lower scores on the PROMIS Pain Interference short form was indicative of better outcomes.
For this analysis, continuous data are reported as mean (± standard deviation) or median [interquartile range] depending on their distribution, and as frequency counts (percentages) for categorical variables. Normality of continuous data was assessed visually using histograms. For all analyses, generalized linear mixed effect models were utilized, with a gaussian distribution and identify link. Separate models were constructed to evaluate each exposure and outcome of interest. In this model framework, a random intercept was included for each subject to account for the repeated outcomes collected within the same subject. In these models fixed effects were included for timepoint of measure, baseline scores and the exposure of interest, and the interaction of exposure with time. By including an interaction term, this allowed evaluation of whether or not the observed associations with the exposure varied at each time point or followed a consistent trajectory. In the event that no interaction was present, interaction terms were removed from the final model to allow evaluation of the main model effects. Final model results were interpreted, with values reported as a mean difference (MD) and its associated 95% confidence interval. Given the exploratory nature of this secondary analysis, evidence of an interaction was considered present if the p-value for the omnibus test for an interaction was less than 0.10. For all other analyses p-values <0.05 were considered statistically significant. All analyses were conducted in SAS version 9.4 (SAS Institute Inc., Cary NC). No imputation for missing data or adjustment for multiple testing was performed given the exploratory nature of this secondary analysis.
Given the design within the context of a larger randomized controlled trial, no a priori power calculation was performed for this analysis. Instead, all available data from the modified intention-to-treat cohort was utilized.
A total of 394 subjects were included in the modified intention-to-treat cohort and are included in this analysis. The median baseline score on the PROMIS Sleep Disturbance was 51 [IQR 44, 56], with only 26.9% of the cohort being female (Table 1). Overall, 197 (50.0%) participants underwent a surgical procedures with cross clamp time exceeding 90 min and 75 (19.0%) underwent an isolated CABG procedure. Of the 394 included participants, 188 (47.7%) were randomized to receive dexmedetomidine. Ultimately 275 patients could be contacted at 30 days, as well as 237 at 90 days and 242 subjects at 180 days. For each patient reported PROMIS functional outcomes, including sleep disturbance, scores improved from 30 to 90 days (Table 2).
Over time patients reported a decrease in PROMIS Sleep Disturbance scores, representing improved sleep quality (Figure 1). Despite this, the trajectory of sleep disturbance throughout the six-month period was not modified by the duration of cross-clamp (p = 0.69; Figure 1A) or by the type of procedure (p = 0.81; Figure 1B). Although there was a trend suggesting that the randomization assignment modified the trajectory of sleep disturbance over time, particularly driven by the observations at 90 days, this was not statistically significant (p = 0.13; Figure 1C).
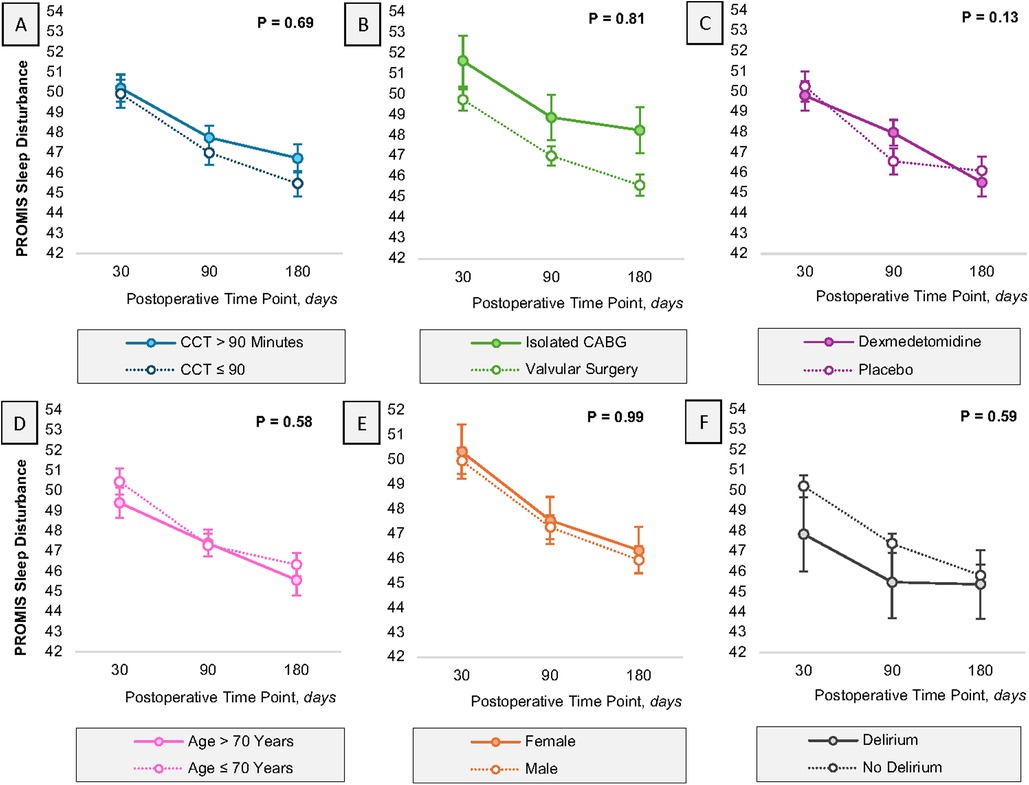
Figure 1. Heterogeneity time. Heterogeneity in the trajectory of sleep, as defined with the PROMIS sleep disturbance questionnaire, is reported for several covariates of interest, including cross clamp time (A), procedure type (B), randomization assignment (C), age (D), sex (E) and delirium status (F) error bars represent the standard error of the estimate. All estimates have been adjusted for baseline PROMIS Sleep Disturbance scores. P-values in the figure represent the omnibus test evaluating the presence of an interaction between the exposure of interest and time. CABG, coronary artery bypass graft; CCT, cross clamp time.
In models adjusting only for the time and the baseline sleep disturbance score, patients with cross-clamp time greater than 90 min experienced no difference in their sleep quality as compared to patients with shorter cross-clamp times (MD 0.74 points, 95% CI: −0.57, 2.07; p = 0.27). On the contrary, isolated CABG patients exhibited worse sleep quality at all time points as compared to patients who underwent a valvular procedure (MD 2.14 points, 95% CI: 0.29, 3.99; p = 0.02). Patients randomized to dexmedetomidine experienced similar sleep quality levels as compared to placebo (MD 0.09 points, 95% CI: −1.33, 1.50; p = 0.90). These data are summarized in Table 3. These results were conserved in sensitivity analyses adjusting for delirium occurring within the first three days postoperatively (Supplementary Table S1).
Over time, the trajectory of t-MOCA and patient-reported outcomes in various domains improved after surgery (Figure 2). However, cross-clamp time greater than 90 min significantly modified the trajectory of PROMIS Global Mental Health (p = 0.02). At 90 and 180 days, mental health scores were more than two points lower in those experiencing a longer cross-clamp time (90-day: MD −2.37 points, 95% CI: −4.35, −0.39; 180-day: MD −2.68 points, 95% CI: −4.62, −0.73; Figure 2G). Similarly, cross-clamp time greater than 90 min significantly modified the trajectory of PROMIS Applied Cognition, with those undergoing longer cross-clamp times experiencing a decrease in their applied cognition by 2.59 additional points at 180 days as compared to those with shorter durations at 180 days (MD: −2.59 points, 95% CI: −4.49, −0.68; Figure 2P).
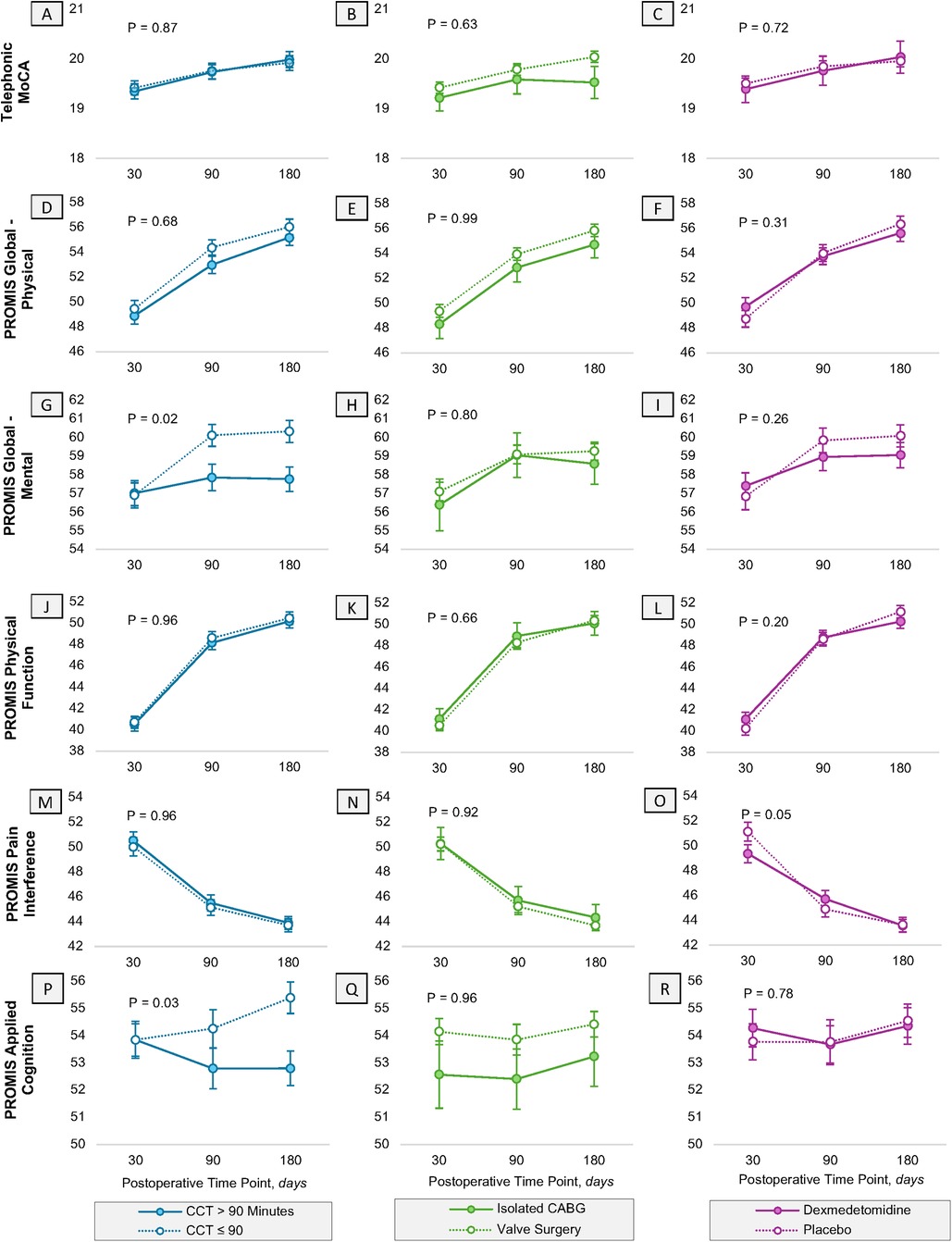
Figure 2. Heterogeneity in the trajectory of PROMIS functional outcomes and neurocognition. Heterogeneity in the trajectory of Patient-Reported Outcomes Measurement Information System (PROMIS) and cognition variables are assessed in the first 180 days postoperatively for (A–C) telephonic Montreal Cognitive Assessment, (D–F) global physical and (G–I) mental health, (J–L) physical function, (M–O) pain interference, and (P–R) applied cognition. This is reported separately for cross clamp time (blue), procedure type (green) and randomization assignment (purple). Error bars represent the standard error of the estimate. All estimates have been adjusted for their baseline scores, respectively. PROMIS, patient-reported outcomes measurement information system; CABG, coronary artery bypass graft; CCT, cross clamp time.
No heterogeneity in the trajectory of other functional outcomes was observed based off the procedure type (isolated CABG or not) or treatment assignment, with one exception. After adjusting for baseline function, randomization to dexmedetomidine modified the trajectory of pain interference over time, such that at 30 days patients who received dexmedetomidine had less pain interference as compared to patients who received placebo (MD −1.77, 95% CI: −3.83, 0.30), however this direction and magnitude did not persist through 90 and 180 days (Figure 2O).
Results for the exploratory analyses considering age, sex, and delirium status are shown in Supplementary Figure S1. Both age and sex significantly modified the trajectory of global physical function over time (p = 0.06 and 0.02, respectively), with similar scores through three months postoperatively, but with younger participants (Supplementary Figure S1D) and females (Supplementary Figure S1E) reporting higher scores at 180 days. Sex was also noted to modify the trajectory of pain and mental health over time (Supplementary Figures S1H, N, respectively). No other interactions were observed.
At each time point, patients who experienced delirium reported worse scores (applied cognition, tMOCA, physical function) than patients who did not. However, these associations were not modified over time (all p-values >0.52).
In this secondary analysis of the MINDDS trial, we explored whether sleep disturbance at each time point and its trajectory over time would be affected by longer cross-clamp time, procedural type, and nighttime dexmedetomidine. While overall sleep quality improved for all patients over time, the hypotheses that cross-clamp time exceeding 90 min would be associated with heterogeneity in the trajectory of sleep disturbance, and differences in sleep disturbance were not supported. This finding aligns with the general improved postoperative recovery trajectory after cardiac surgery (11). However, despite the improved sleep quality trajectories, patients undergoing isolated coronary CABG procedures consistently reported poor sleep quality at all time points compared to those undergoing valvular procedures.
Sleep is a state of altered arousal that offers cardiovascular, immune, and cognitive benefits (12, 13). However, few studies have reported on sleep disturbance after cardiac surgery. Overall, these studies suggest an increase in sleep disruption after CABG, with improvement as early as one month afterward (14–16). Patients who reported low-quality sleep after CABG were more likely to self-report worse quality of life (11). In the present study, patients who underwent isolated CABG did not report worse outcomes in the cognitive function and health-related quality of life evaluated. Consequently, the reason for the self-reported increase in sleep disturbance following isolated CABG procedures in this study remains unclear. Given objective and subjective sleep quality may differ (17), future polysomnography studies may provide additional valuable insights.
Our investigation went beyond studying sleep disturbance to evaluate various other functional outcomes. We found that patients who had cross-clamp times of greater than 90 min showed noticeably different patterns in their PROMIS Global Mental Health and Applied Cognition scores. These patients had lower scores in later time points, which indicates that longer cross-clamp times may lead to mental and cognitive health difficulties during the recovery phase. Considering the potential overlap between mental health and subjective cognitive items, further research would be advantageous to elucidate the particular constructs under scrutiny, such as anxiety and depression. Should these findings be validated through broader investigations, they could enhance our comprehension of the biological underpinnings of mental health. For instance, investigating the reasons behind significant variances observed at the 90-day mark and uncovering the biological mechanisms responsible for this delay, as well as determining whether they are modifiable, may substantially enrich our understanding of perioperative stressors an how they affect these outcomes. It is important to note however that reasons for an increased cross-clamp time are likely multifactorial. It is possible this could represent differences in the surgical approach, potential intraoperative characteristics or commplications, or even differences in technical skills. Thus, cross-clamp time may serve as proxy for these different scenarios and their association with mental and subjective cognitive abilities.
In this study, despite the observed associatons for mental health and applied cognition, no heterogenety was observed in physical function for those with increased cross-clamp times or different surgery types (e.g., valve vs. isolated CABG surgeries). It is possible that this is simply the result of an underpowered association, though the magnitude of any effect is likely not clinically meaningful. The rationale for this finding is less clear, particularly as there is some evidence that age and sex may play a role in global physical function, particularly at a year after surgery. Future studies may be required to replicate these findings, or to aid in our undstanding of this surprising finding.
Postoperative delirium, characterized by acute confusion and cognitive dysfunction following surgery (18–25), has been previously associated with impaired cognitive recovery (26–29). Despite dexmedetomidine's demonstrated role in reducing postoperative delirium incidence in the parent trial (7) and in other populations (30–36), the reported findings in this manuscript indicate that nighttime dexmedetomidine did not improve post-hospital discharge quality of life. It is possible that patients experiencing delirium may have had underlying vulnerabilities affecting their quality of life long before their hospital admission and postoperative delirium diagnosis, given that current evidence implies a lack of a direct causal association.
This study is not without limitations, including those inherent to the primary trial design and the study design as a nested cohort analysis within the context of a randomized controlled trial, including the possibility of residual confounding from other surgical or anesthetic characteristics that were not included in the exploratory analysis. Additionally, this was a secondary analysis of the MINDDS trial, so it was not powered for assessing long-term sleep disturbance or other functional outcomes. While it was possible that the results we observed are a result of an underpowered interaction analysis, we did identify some evidence of an interaction, and those lacking evidence were not likely of a clinically meaningful magnitude. Further limiting this analysis was the presence of missing data, in which follow-up patient interviews were not completed for various reasons, which could have created bias if patients with missing data were worse off than those retained in the study. Lastly, this analysis is limited to assessing function to six months after surgery, therefore we are unable to comment on longer term follow up.
In conclusion, our findings highlight the nuanced nature of postoperative recovery after cardiac surgery patients. While sleep disturbance improved over time for all patients, procedural factors such as cross-clamp time and type of surgery had varying impacts on sleep quality and broader functional outcomes such as mental health. These findings emphasize the significance of conducting prospective studies that aim to better comprehend the underlying associations between surgical factors and long-term patient-related outcomes. Additionally, these studies should explore the potential modifiability of these associations through preoperative interventions, such as comprehensive perioperative mental health intervention bundles (37).
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving humans were approved by Mass General Brigham Institutional Review Board. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
GN: Conceptualization, Data curation, Formal Analysis, Methodology, Writing – original draft, Writing – review & editing. JS: Formal Analysis, Investigation, Methodology, Writing – original draft, Writing – review & editing. AN: Data curation, Formal Analysis, Writing – original draft, Writing – review & editing. AM: Conceptualization, Data curation, Formal Analysis, Investigation, Supervision, Writing – original draft, Writing – review & editing. JB: Data curation, Formal Analysis, Writing – original draft, Writing – review & editing. KW: Data curation, Formal Analysis, Methodology, Supervision, Writing – original draft, Writing – review & editing. JQ: Investigation, Supervision, Writing – original draft, Writing – review & editing. MB: Data curation, Formal Analysis, Supervision, Writing – original draft, Writing – review & editing. TH: Conceptualization, Data curation, Formal Analysis, Investigation, Supervision, Writing – original draft, Writing – review & editing. OA: Conceptualization, Data curation, Funding acquisition, Investigation, Resources, Supervision, Writing – original draft, Writing – review & editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study was supported by a National Institutes of Health National Institute on Aging (NIA) grant (R01AG053582). The sponsor played no role in the study design, collection, analysis or interpretation of the data, writing of the manuscript or the decision to publish.
AM reports receiving funding from Roche Diagnostics, the Preeclampsia Foundation and the University of Chicago for statistical consulting projects related to biomarkers in preeclampsia. TTH reports receiving personal fees from Anesthesiology and Headache. OA is listed as an inventor on brain monitoring patents assigned to Massachusetts General Hospital.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fanes.2024.1483837/full#supplementary-material
1. Baquero GA, Rich MW. Perioperative care in older adults. J Geriatr Cardiol. (2015) 12(5):465–9. doi: 10.11909/j.issn.1671-5411.2015.05.018
2. Al-Sarraf N, Thalib L, Hughes A, Houlihan M, Tolan M, Young V, et al. Cross-clamp time is an independent predictor of mortality and morbidity in low- and high-risk cardiac patients. Int J Surg. (2011) 9(1):104–9. doi: 10.1016/j.ijsu.2010.10.007
3. Doenst T, Berretta P, Bonaros N, Savini C, Pitsis A, Wilbring M, et al. Aortic cross-clamp time correlates with mortality in the mini-mitral international registry. Eur J Cardiothorac Surg. (2023) 63(6):ezad147. doi: 10.1093/ejcts/ezad147
4. Ruggieri VG, Bounader K, Verhoye JP, Onorati F, Rubino AS, Gatti G, et al. Prognostic impact of prolonged cross-clamp time in coronary artery bypass grafting. Heart Lung Circ. (2018) 27(12):1476–82. doi: 10.1016/j.hlc.2017.09.006
5. Swinkels BM, Ten Berg JM, Kelder JC, Vermeulen FE, Van Boven WJ, de Mol BA. Effect of aortic cross-clamp time on late survival after isolated aortic valve replacement. Interact Cardiovasc Thorac Surg. (2021) 32(2):222–8. doi: 10.1093/icvts/ivaa244
6. Shelton KT, Qu J, Bilotta F, Brown EN, Cudemus G, D'Alessandro DA, et al. Minimizing ICU neurological dysfunction with dexmedetomidine-induced sleep (MINDDS): protocol for a randomised, double-blind, parallel-arm, placebo-controlled trial. BMJ Open. (2018) 8(4):e020316. doi: 10.1136/bmjopen-2017-020316
7. Qu JZ, Mueller A, McKay TB, Westover MB, Shelton KT, Shaefi S, et al. Nighttime dexmedetomidine for delirium prevention in non-mechanically ventilated patients after cardiac surgery (MINDDS): a single-centre, parallel-arm, randomised, placebo-controlled superiority trial. EClinicalMedicine. (2023) 56:101796. doi: 10.1016/j.eclinm.2022.101796
8. Inouye SK, van Dyck CH, Alessi CA, Balkin S, Siegal AP, Horwitz RI. Clarifying confusion: the confusion assessment method: a new method for detection of delirium. Ann Intern Med. (1990) 113(12):941–8. doi: 10.7326/0003-4819-113-12-941
9. Su X, Wang DX. Improve postoperative sleep: what can we do? Curr Opin Anaesthesiol. (2018) 31(1):83–8. doi: 10.1097/ACO.0000000000000538
10. van Zuylen ML, Meewisse AJG, Ten Hoope W, Eshuis WJ, Hollmann MW, Preckel B, et al. Effects of surgery and general anaesthesia on sleep-wake timing: CLOCKS observational study. Anaesthesia. (2022) 77(1):73–81. doi: 10.1111/anae.15564
11. Hunt JO, Hendrata MV, Myles PS. Quality of life 12 months after coronary artery bypass graft surgery. Heart Lung. (2000) 29(6):401–11. doi: 10.1067/mhl.2000.110578
12. Nedergaard M, Goldman SA. Glymphatic failure as a final common pathway to dementia. Science. (2020) 370(6512):50–6. doi: 10.1126/science.abb8739
13. Winkelman JW. Clinical practice. Insomnia disorder. N Engl J Med. (2015) 373(15):1437–44. doi: 10.1056/NEJMcp1412740
14. Yang PL, Huang GS, Tsai CS, Lou MF. Sleep quality and emotional correlates in Taiwanese coronary artery bypass graft patients 1 week and 1 month after hospital discharge: a repeated descriptive correlational study. PLoS One. (2015) 10(8):e0136431. doi: 10.1371/journal.pone.0136431
15. Redeker NS, Mason DJ, Wykpisz E, Glica B. Sleep patterns in women after coronary artery bypass surgery. Appl Nurs Res. (1996) 9(3):115–22. doi: 10.1016/S0897-1897(96)80206-0
16. Edell-Gustafsson UM, Hetta JE, Aren CB. Sleep and quality of life assessment in patients undergoing coronary artery bypass grafting. J Adv Nurs. (1999) 29(5):1213–20. doi: 10.1046/j.1365-2648.1999.01006.x
17. Akeju O, Hobbs LE, Gao L, Burns SM, Pavone KJ, Plummer GS, et al. Dexmedetomidine promotes biomimetic non-rapid eye movement stage 3 sleep in humans: a pilot study. Clin Neurophysiol. (2018) 129(1):69–78. doi: 10.1016/j.clinph.2017.10.005
18. Aldecoa C, Bettelli G, Bilotta F, Sanders RD, Audisio R, Borozdina A, et al. European Society of anaesthesiology evidence-based and consensus-based guideline on postoperative delirium. Eur J Anaesthesiol. (2017) 34(4):192–214. doi: 10.1097/EJA.0000000000000594
19. Bickel H, Gradinger R, Kochs E, Forstl H. High risk of cognitive and functional decline after postoperative delirium. A three-year prospective study. Dement Geriatr Cogn Disord. (2008) 26(1):26–31. doi: 10.1159/000140804
20. Eertmans W, De Deyne C, Genbrugge C, Marcus B, Bouneb S, Beran M, et al. Association between postoperative delirium and postoperative cerebral oxygen desaturation in older patients after cardiac surgery. Br J Anaesth. (2020) 124(2):146–53. doi: 10.1016/j.bja.2019.09.042
21. Hughes CG, Boncyk CS, Culley DJ, Fleisher LA, Leung JM, McDonagh DL, et al. American society for enhanced recovery and perioperative quality initiative joint consensus statement on postoperative delirium prevention. Anesth Analg. (2020) 130(6):1572–90. doi: 10.1213/ANE.0000000000004641
22. Koster S, Hensens AG, van der Palen J. The long-term cognitive and functional outcomes of postoperative delirium after cardiac surgery. Ann Thorac Surg. (2009) 87(5):1469–74. doi: 10.1016/j.athoracsur.2009.02.080
23. O'Keeffe ST, Ni Chonchubhair A. Postoperative delirium in the elderly. Br J Anaesth. (1994) 73(5):673–87. doi: 10.1093/bja/73.5.673
24. Saczynski JS, Marcantonio ER, Quach L, Fong TG, Gross A, Inouye SK, et al. Cognitive trajectories after postoperative delirium. N Engl J Med. (2012) 367(1):30–9. doi: 10.1056/NEJMoa1112923
25. Zhang WY, Wu WL, Gu JJ, Sun Y, Ye XF, Qiu WJ, et al. Risk factors for postoperative delirium in patients after coronary artery bypass grafting: a prospective cohort study. J Crit Care. (2015) 30(3):606–12. doi: 10.1016/j.jcrc.2015.02.003
26. Namirembe GE, Baker S, Albanese M, Mueller A, Qu JZ, Mekonnen J, et al. Association between postoperative delirium and long-term subjective cognitive decline in older patients undergoing cardiac surgery: a secondary analysis of the minimizing intensive care unit neurological dysfunction with dexmedetomidine-induced sleep trial. J Cardiothorac Vasc Anesth. (2023) 37(9):1700–6. doi: 10.1053/j.jvca.2023.04.035
27. Marcantonio ER, Flacker JM, Michaels M, Resnick NM. Delirium is independently associated with poor functional recovery after hip fracture. J Am Geriatr Soc. (2000) 48(6):618–24. doi: 10.1111/j.1532-5415.2000.tb04718.x
28. Newman MF, Kirchner JL, Phillips-Bute B, Gaver V, Grocott H, Jones RH, et al. Longitudinal assessment of neurocognitive function after coronary-artery bypass surgery. N Engl J Med. (2001) 344(6):395–402. doi: 10.1056/NEJM200102083440601
29. Sotaniemi KA, Mononen H, Hokkanen TE. Long-term cerebral outcome after open-heart surgery. A five-year neuropsychological follow-up study. Stroke. (1986) 17(3):410–6. doi: 10.1161/01.STR.17.3.410
30. Riker RR, Shehabi Y, Bokesch PM, Ceraso D, Wisemandle W, Koura F, et al. Dexmedetomidine vs midazolam for sedation of critically ill patients: a randomized trial. JAMA. (2009) 301(5):489–99. doi: 10.1001/jama.2009.56
31. Pandharipande PP, Pun BT, Herr DL, Maze M, Girard TD, Miller RR, et al. Effect of sedation with dexmedetomidine vs lorazepam on acute brain dysfunction in mechanically ventilated patients: the MENDS randomized controlled trial. JAMA. (2007) 298(22):2644–53. doi: 10.1001/jama.298.22.2644
32. Reade MC, Eastwood GM, Bellomo R, Bailey M, Bersten A, Cheung B, et al. Effect of dexmedetomidine added to standard care on ventilator-free time in patients with agitated delirium: a randomized clinical trial. JAMA. (2016) 316(7):773–4. doi: 10.1001/jama.2016.8602
33. Maldonado JR, Wysong A, van der Starre PJ, Block T, Miller C, Reitz BA. Dexmedetomidine and the reduction of postoperative delirium after cardiac surgery. Psychosomatics. (2009) 50(3):206–17. doi: 10.1176/appi.psy.50.3.206
34. Su X, Meng ZT, Wu XH, Cui F, Li HL, Wang DX, et al. Dexmedetomidine for prevention of delirium in elderly patients after non-cardiac surgery: a randomised, double-blind, placebo-controlled trial. Lancet. (2016) 388(10054):1893–902. doi: 10.1016/S0140-6736(16)30580-3
35. Hughes CG, Mailloux PT, Devlin JW, Swan JT, Sanders RD, Anzueto A, et al. Dexmedetomidine or propofol for sedation in mechanically ventilated adults with sepsis. N Engl J Med. (2021) 384(15):1424–36. doi: 10.1056/NEJMoa2024922
36. Shehabi Y, Howe BD, Bellomo R, Arabi YM, Bailey M, Bass FE, et al. Early sedation with dexmedetomidine in critically ill patients. N Engl J Med. (2019) 380(26):2506–17. doi: 10.1056/NEJMoa1904710
Keywords: cardiac surgery, cross-clamp time, dexmedetomidine, PROMIS, postoperative delirium, sleep, valvular surgery
Citation: Namirembe GE, Sparling J, Novak A, Mueller A, Bertsch J, Wiredu K, Qu JZ, Brandon Westover M, Houle TT and Akeju O (2025) Longitudinal impact of cross-clamp duration on postoperative sleep disturbance and quality of life in elderly cardiac surgery patients: a secondary analysis of the MINDDS trial. Front. Anesthesiol. 3:1483837. doi: 10.3389/fanes.2024.1483837
Received: 20 August 2024; Accepted: 16 December 2024;
Published: 22 January 2025.
Edited by:
Stefano Turi, San Raffaele Hospital (IRCCS), ItalyReviewed by:
Valentina Ajello, Policlinico Tor Vergata, ItalyCopyright: © 2025 Namirembe, Sparling, Novak, Mueller, Bertsch, Wiredu, Qu, Brandon Westover, Houle and Akeju. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Oluwaseun Akeju, b2x1d2FzZXVuLmFrZWp1QG1naC5oYXJ2YXJkLmVkdQ==
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.