- 1Clinic for Anesthesiology and Intensive Therapy, University Clinical Center Nis, Niš, Serbia
- 2School of Medicine, University of Niš, Niš, Serbia
The porphyrias are a group of disorders related to deficient heme biosynthesis, caused by malfunction of certain enzymes in the synthesis pathway. Erythropoietic porphyrias present with cutaneous symptoms and do not affect the nervous system. Hepatic porphyrias develop acute attacks with mild to severe neurovisceral symptoms, dramatic course, and rare, but possibly lethal outcomes. Anesthetic management of patients suffering from hepatic porphyria is challenging regarding the possibility of triggering or worsening the acute attack with medications that induce or maintain anesthesia. The medications are labeled as safe or unsafe according to laboratory experiments, clinical studies, case reports and experience. In this paper, we discuss underlying pathophysiology, presentation, therapy recommendations and anesthetic implications related to porphyrias.
1. Introduction
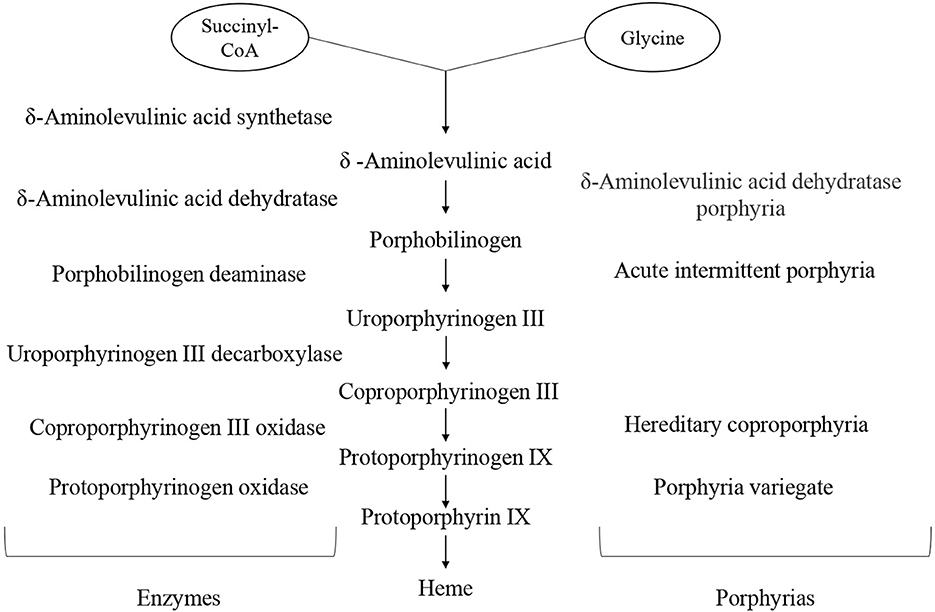
The porphyrias are a group of disorders related to a deficient heme biosynthesis, caused by malfunction of certain enzymes in the eight-step synthesis pathway. They can be inherited as autosome-dominant, autosome-recessive, or X-linked mutations hitting a specific enzyme [1]. All porphyrias can be classified according to the localization of the greatest enzymatic dysfunction, the deficient enzyme, and whether they develop acute attacks or not [2]. Considering localization, porphyrias can be erythropoietic and hepatic. Erythropoietic porphyrias present with cutaneous symptoms and do not affect the nervous system. Usually, they appear as photosensitivity and various chronic skin changes ranging from itching and erythema to bullae [3]. These are Porphyria Cutanea Tarda, Hepatoerythropoietic Porphyria, Congenital Erythropoietic Porphyria, Erythropoietic Protoporphyria, and X-linked Protoporphyria. The hepatic porphyrias can develop acute attacks that present with severe, sometimes life-threatening, neurovisceral symptoms, which are followed by asymptomatic periods [4]. According to the deficient enzyme, hepatic porphyrias are classified into Acute Intermittent Porphyria (AIP), Hereditary Coproporphyria (HCP), Variegate Porphyria (VP), and δ-Aminolevulinic Acid Dehydratase (ALAD) Porphyria (ADP). According to American Porphyria Organization, ~1–100 people per 50,000 are affected [5].
Anesthetic management of patients suffering from hepatic porphyria is challenging regarding the possibility of triggering or worsening the acute attack with medications that induce or maintain anesthesia [6]. A special concern is undiagnosed patients who develop their first attack while undergoing anesthesia. Affiliated factors in those patients are stress, hormone disturbances, hunger, and dehydration, all common in urgent surgical patients or patients preparing for an elective procedure. Nevertheless, the attack may trail in the presence of the triggering drug [7], which implies a complex background beyond porphyrias. All the patients suspected to develop porphyria crisis have to be carefully examined and well-prepared before entering the operating room.
2. Pathophysiology
The heme is metalloporphyrin made from a pyrrole ring and iron ion. It has several roles in the body: it is an oxygen and carbon dioxide transporter, it is a part of the electron transport chain, and it is a cofactor of several enzymes, including some in the microsomal hepatic system. The heme synthesis pathway takes place in mitochondria and cytosol. It begins with the binding of succinyl coenzyme A and glycine, the reaction catalyzed by aminolevulinic acid (ALA) synthetase. Intermediary products are called porphyrinogens and each step is catalyzed by a specific enzyme [8]. The whole process is shown in Figure 1.
In porphyria, the mutation can affect any enzyme in the pathway, resulting in a decreased, never completely absent activity, since the absence of heme is incompatible with life [9]. This leads to the deficient synthesis of the final product, heme, and cumulation of precursors above the impaired enzyme. The rate-limiting enzyme in the chain of hem biosynthesis is ALA synthetase. In physiological conditions, heme has a negative feedback loop effect on ALA synthetase activity, limiting the formation of porphyrinogens [10]. Consequently, the suppression is lacking in porphyrias, resulting in enhanced enzyme activity.
The neurotoxic effect of ALA synthetase has been confirmed earlier [11]. The autonomic ganglia, anterior horns of the spine, peripheral neurons, nuclei of the medulla oblongata, and axons in the brain are directly affected, while demyelination develops as a secondary effect. This results in neuropathy and autonomic decompensation. Also, low intracellular heme concentration has a cytotoxic effect itself. The third contributing pathophysiological factor is accumulated porphyrinogens which spontaneously turn into potent oxidative molecules, porphyrins [12, 13].
Often, the genetic mutation is not sufficient to induce the porphyria's onset, since the enzymatic activity is limited by some level, resulting in compromised, yet adequate biosynthesis. Symptoms appear only in presence of provoking factors and conditions causing rapid heme consumption, like infections, hormone fluctuations (puberty, pregnancy), starvation, dehydration, alcohol, and certain drug administration [6, 14, 15].
3. Clinical presentation
The erythropoietic porphyrias manifest with mild skin symptoms due to porphyrin accumulation in the skin. They never affect the nervous system and are not life-threating [16]. So far, no drugs were shown to trigger this type of porphyrias.
On the other hand, hepatic porphyrias develop acute attacks which present with mild to severe neurovisceral symptoms, dramatic course, and rare, but possibly lethal outcomes [17]. Usually, the attack starts as a non-specific abdominal pain coupled with sickness, vomiting, and constipation. Then, peripheral weakness and pain appear due to peripheral neuropathy and involvement of the spinal cord. The irritation of the autonomic ganglia leads to hypertension, palpitations, and arrhythmia. Severe attacks cause CNS symptoms, likewise confusion, hallucinations, and convulsions. Smooth muscle paralysis often leads to urine retention. Diarrhea and vomiting can cause dehydration and electrolyte disbalance, which lead to further neurological deterioration. The most severe manifestation is bulbar muscle paralysis, leading to acute respiratory arrest [4].
AIP presents with the most severe symptomatology and the highest mortality rate among porphyries [18]. It is a result of porphobilinogen deaminase (PBGD) deficiency. Although it is transmitted in an autosomal dominant manner, women develop symptoms more often than men. There is a wide range of triggers, including barbiturates, diazepam, oral contraceptives, steroid hormones, and some antibiotics. Acute seizures in pregnancy reach up to 42% mortality rate [19], therefore the recommendation is to wait at least 1 year after the last crisis before the pregnancy.
Hereditary coproporphyria is caused by coproporphyrinogen-III oxidase. Apart from neurovisceral symptoms, skin photosensitivity can also be seen in approximately one-fifth of the patients. Usually, it is asymptomatic and the onset is related to concomitant hepatic disease [20].
VP results from protoporphyrinogen oxidase deficiency. It manifests with acute neurovisceral symptoms and chronic skin alterations, like vesicles, bullae, erosions, hypertrichosis, and hyperpigmentation. It is more common in women, provoked by hormone fluctuation during the menstrual cycle [21].
ADP is extremely rare, since it is transmitted as autosomal recessive mutation in the δ-aminolevulinic acid dehydratase gene.
4. Therapeutical approach
Porphyria management should always start with the elimination of provoking factors, then continue with initial supportive care and specific therapy aiming to increase heme concentration and decrease ALA synthetase activity.
Symptomatic therapy depends on the current symptoms which vary in each attack. Paracetamol and opioid analgesics are safe options for pain relief [22, 23]. The cardiovascular symptoms are successfully treated with beta-blockers. Even more, propranolol showed an inhibitory effect on ALA synthetase [24]. Diazepam can induce the crisis and it is contraindicated in patients suffering from porphyria, however, it can be administrated in case of convulsions. Also, clonazepam and magnesium-sulfate are allowed but are less potent. Nausea is well-treated with prochlorperazine, ondansetron, promazine, and cyclizine, while metoclopramide should be avoided [25, 26]. Monitoring and correction of metabolic status are of great importance, including hydration, electrolytes, and caloric intake [27]. Oral intake is always preferable or per nasogastric tube. Parenteral nutritive solutions are a good alternative when gastrointestinal symptoms disable enteral nutrition.
The only indication for hem supplementation is neuropathy. Intravenous hem solutions are available in two forms, hematin and hemin (hem arginate), which is taking the lead lately because it was shown to cause fewer side effects [28]. It should be administrated in a dose of 3 mg/kg, dissolved in 100 ml 0.9% NaCl, for 4 days consequently. The solution is given as a slow, intravenous infusion, preferably through the central vein to avoid common peripheral thrombophlebitis. Since carbohydrates decrease ALA synthetase activity, the 5% or 10% Glucose solution is recommended, but concomitant administration with hem solution is not necessary according to new guidelines. Repeated heme infusions can lead to chronic hepatic inflammation due to iron accumulation. For those patients, the final option is liver transplantation [29]. FDA has recently approved Givosiran, a siRNA antagonist of ALA synthetase, which is expected to prolong asymptomatic periods and delay acute crises of the disease [30–32].
Porphyria is a rear disease with unspecific symptoms, therefore diagnosis can be difficult, especially in patients with negative family anamnesis. Laboratory testing of blood, urine, and stool is required since the diagnosis is confirmed with elevated ALA and porphyrinogens in the samples during an acute attack. Another diagnostic method is the measurement of certain enzyme activity in erythrocytes and hepatocytes, but this is not always a precise method. Genetic assays are the most certain diagnostic procedures, and they are recommended in families with positive anamnesis [30]. Note that the mutation is not equal to the disease, since 2/3 of mutation carriers do not develop symptoms in a lifetime.
5. Anesthetic management
The anesthetic approach is different during a remission period and an acute attack, regarding concomitant symptoms and metabolic disorders. A patent in remission can safely undergo any type of anesthesia. In the acute attack, however, it is preferable to choose general over regional anesthesia, since the regional approach has a greater risk of peripheral neuropathy.
Medications are labeled as safe or unsafe mainly according to the experience in the clinical practice and case reports. There are plenty of cell culture and animal studies, but the experience showed that the results obtained in laboratories often do not match outcomes in patients. Theoretically, if the mechanism of action includes increased engagement of the hepatic microsomal system and cytochrome synthesis, the drug is expected to cause a deterioration of the disease.
Preoperative management has an important role, especially during the acute attack. A special note should be on hydration, appropriate calorie intake, and electrolyte status [33]. Also, the autonomic nervous system should be examined, together with the cardiorespiratory function. The precise estimation of the patient is necessary in order to prevent undesirable outcomes. Premedication tends to achieve good anxiolysis and pain relief. Benzodiazepines showed mixed results in patients with acute porphyria. Midazolam and lorazepam are well tolerated [34, 35], while diazepam can provoke an acute attack and should be avoided. The only indication for diazepam in AIP is life-threatening convulsions in severe attacks [36]. Analgesia in acute porphyria crisis is safely achieved with morphine, fentanyl, alfentanil, and sufentanil. Among non-opioid drugs, aspirin, paracetamol, indomethacin, and naproxen are considered safe [37]. Although data about pentazocine are controversial, Rudowski and colleagues achieved rapid and safe analgesia in five patients suffering from abdominal pain in the acute porphyria crisis [38].
If general anesthesia is indicated, the drug of choice for induction is propofol. In the animal model for AIP, propofol did not increase ALA synthetase activity [39]. Also, urine ALA levels remained in the referent range after propofol administration in people suffering from AIP, even with repeated administration [40–42]. There was a debate earlier since a case report by Weir and Hodkinson showed increased porphyrins in the urine of a patient suffering from VP [43], but he did not develop the acute attack following propofol administration. An acute attack of HCP is described by Kasraie and Cousins [44] after induction and maintenance with propofol, but the patient has also received several medications which later were labeled as porphyrinogenic. Therefore, propofol is considered safe in patients with porphyrias, during the asymptomatic and symptomatic periods. Etomidate and ketamine showed certain impact on porphyria crisis onset in animal models, however, in clinical practice, they are considered safe in therapeutic doses [45–47]. Still, one should be careful with repeated etomidate usage, since Harrison at al. demonstrated the dose-dependent toxic effect in animal model [48]. Barbiturates are confirmed precipitating drugs in hepatic porphyria, therefore their administration is contraindicated. The pathophysiology mechanism includes enhanced synthesis of cytochrome P450 in the liver, resulting in increased hem consumption and decompensation of the condition. Although a retrospective study by Mustajoki et Heinonen [49] showed low risk in asymptomatic patients, the usage of barbiturates is limited regarding the availability of drugs with confirmed safety.
General anesthesia is maintained with volatile anesthetics. Halothane, nitrous-oxide, and isoflurane did not cause any adverse effects in porphyria patients [35, 50]. Enflurane showed porphyrinogenc action as reported by Buzaleh et al. [51], and should be avoided. Sevoflurane and desflurane are short-acting drugs, both safe even in prolongated and repeated administration [52, 53].
Myorelaxants and their antagonist are well tolerated and successfully applied during general anesthesia [54]. The safety of succinylcholine has been proven in laboratory conditions and clinical practice [55, 56]. Atracurium has been safely administrated during mitral valve replacement in a patent diagnosed with AIP, as Stevens and Kneeshaw reported [57]. Also, Hiseih at al. reported safe use of rocuronium in an AIP patient [58]. A general opinion is that muscle relaxants are safe for short-time usage, while the safety of administration during prolonged ventilation in ICU should be further examined [6].
6. Conclusion
The porphyrias are a special concern in anesthesiology regarding non-specific symptoms triggered by drugs that are being used to induce and maintain anesthesia. A great effort has been made so far to examine the safety of drugs and classify them as safe or unsafe. However, the practice has shown that under different circumstances, the same drug may give different outcomes. This is why some authors suggest creating a unique model for diagnosed and suspected porphyria patients, instead of further drug safety examination. The screening and diagnosis of asymptomatic mutation carriers are of great importance, in order to prevent crises and avoid complications. Further, preanesthetic stabilization focusing on the adequate assessment and optimization of metabolic status is crucial. Finally, the administration of well-known, safe drugs will prevent the onset and deterioration of the crisis.
Author contributions
MR wrote the paper, while MS, JR, and MV collaborated. RJ was the mentor of the project. All authors were involved in research.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The reviewer IB declared a shared affiliation with the author RJ to the handling editor at the time of review.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Elder G. Enzymatic defects in porphyria: an overview. Semin Liver Dis. (1982) 2:87–99. doi: 10.1055/s-2008-1040699
2. Muschalek W, Hermasch MA, Poblete-Gutiérrez P, Frank J. The porphyrias. J Deutsche Derma Gesell. (2022) 20:316–31. doi: 10.1111/ddg.14743
3. DeLeo VA, Poh-Fitzpatrick M, Mathews-Roth M, Harber LC. Erythropoietic protoporphyria 10 years experience. Am J Med. (1976) 60:8–22. doi: 10.1016/0002-9343(76)90528-3
4. Elder GH, Hift RJ, Meissner PN. The acute porphyrias. Lancet. (1997) 349:1613–7. doi: 10.1016/S0140-6736(96)09070-8
5. American Porphyria Organization. Supporting Individuals and Families Impacted by Porphyria. Bethesda: American Porphyria Organization (2022). Available online at: https://porphyriafoundation.org/ (accessed October 24, 2022).
6. Jensen NF, Fiddler DS, Striepe V. Anesthetic considerations in porphyrias. Anesth Anal. (1995) 80:591–9. doi: 10.1213/00000539-199503000-00028
7. Slavin SA, Christoforides C. Thiopental administration in acute intermittent porphyria without adverse effect. Anesthesiology. (1976) 44:77–9. doi: 10.1097/00000542-197601000-00021
8. Nilsson R, Schultz IJ, Pierce EL, Soltis KA, Naranuntarat A, Ward DM, et al. Discovery of genes essential for heme biosynthesis through large-scale gene expression analysis. Cell Metabol. (2009) 10:119–30. doi: 10.1016/j.cmet.2009.06.012
9. Moore MR, Wintrobe MM, editors. Disorders of Porphyrin Metabolism. New York, NY: Plenum Medical Book Co (1987). p. 374.
10. Phillips JD. Heme biosynthesis and the porphyrias. Mol Genet Metabol. (2019) 128:164–77. doi: 10.1016/j.ymgme.2019.04.008
11. Bissell DM, Lai JC, Meister RK, Blanc PD. Role of delta-aminolevulinic acid in the symptoms of acute porphyria. Am J Med. (2015) 128:313–7. doi: 10.1016/j.amjmed.2014.10.026
12. Dutt S, Hamza I, Bartnikas TB. Molecular mechanisms of iron and heme metabolism. Annu Rev Nutr. (2022) 42:311–35. doi: 10.1146/annurev-nutr-062320-112625
13. Monteiro H, Abdalla DulcineiaSP. Free radicals involvement in neurological porphyrias and lead poisoning. Mol Cell Biochem. (1991) 103:595. doi: 10.1007/BF00229595
14. Disler PB, Moore MR. Drug therapy in the acute porphyrias. Clin Dermatol. (1985) 3:112–24. doi: 10.1016/0738-081X(85)90037-9
15. Thadani H. Regular review: diagnosis and management of porphyria. BMJ. (2000) 320:1647–51. doi: 10.1136/bmj.320.7250.1647
16. Frank J, Poblete-Gutiérrez P. Delayed diagnosis and diminished quality of life in erythropoietic protoporphyria: results of a cross-sectional study in Sweden: Editorial: cross-sectional study on erythropoietic protoporphyria. J Intern Med. (2011) 269:270–4. doi: 10.1111/j.1365-2796.2010.02283.x
17. Karim Z, Lyoumi S, Nicolas G, Deybach JC, Gouya L, Puy H. Porphyrias: a 2015 update. Clin Res Hepatol Gastroenterol. (2015) 39:412–25. doi: 10.1016/j.clinre.2015.05.009
18. Ma Y, Teng Q, Zhang Y, Zhang S. Acute intermittent porphyria: focus on possible mechanisms of acute and chronic manifestations. IRDR. (2020) 9:187–95. doi: 10.5582/irdr.2020.03054
19. Farfaras A, Zagouri F, Zografos G, Kostopoulou A, Sergentanis TN, Antoniou S. Acute intermittent porphyria in pregnancy: a common misdiagnosis. Clin Exp Obstet Gynecol. (2010) 37:256–60.
20. Chen B, Solis-Villa C, Hakenberg J, Qiao W, Srinivasan RR, Yasuda M, et al. Acute intermittent porphyria: predicted pathogenicity of HMBS variants indicates extremely low penetrance of the autosomal dominant disease: HUMAN MUTATION. Hum Mutat. (2016) 37:1215–22. doi: 10.1002/humu.23067
21. Singal AK, Anderson KE. Variegate porphyria. In:Adam MP, Everman DB, Mirzaa GM, Pagon RA, Wallace SE, Bean LJ, et al., editors. GeneReviews®. Seattle (WA): University of Washington (1993).
22. Lambrecht RW, Gildemeister OS, Williams A, Pepe JA, Tortorelli KD, Bonkovsky HL. Effects of selected antihypertensives and analgesics on hepatic porphyrin accumulation. Biochem Pharmacol. (1999) 58:887–96. doi: 10.1016/S0006-2952(99)00154-9
23. Cardenas JL, Guerrero C. Acute intermittent porphyria: general aspects with focus on pain. Curr Med Res Opin. (2018) 34:1309–15. doi: 10.1080/03007995.2018.1435521
24. Menawat AS, Panwar RB, Kochar DK, Joshi CK. Propranolol in acute intermittent porphyria. Postgrad Med J. (1979) 55:546–7. doi: 10.1136/pgmj.55.646.546
25. Stein P, Badminton M, Barth J, Rees D, Stewart MF. Best practice guidelines on clinical management of acute attacks of porphyria and their complications. Ann Clin Biochem. (2013) 50:217–23. doi: 10.1177/0004563212474555
26. Zhao L, Wang X, Zhang X, Liu X, Ma N, Zhang Y, et al. Therapeutic strategies for acute intermittent porphyria. IRDR. (2020) 9:205–16. doi: 10.5582/irdr.2020.03089
27. Handschin C, Lin J, Rhee J, Peyer AK, Chin S, Wu PH, et al. Nutritional regulation of hepatic heme biosynthesis and porphyria through PGC-1alpha. Cell. (2005) 122:505–15. doi: 10.1016/j.cell.2005.06.040
29. Yasuda M, Erwin AL, Liu LU, Balwani M, Chen B, Kadirvel S, et al. Liver transplantation for acute intermittent porphyria: biochemical and pathologic studies of the explanted liver. Mol Med. (2015) 21:487–95. doi: 10.2119/molmed.2015.00099
30. Anderson KE, Bloomer JR, Bonkovsky HL, Kushner JP, Pierach CA, Pimstone NR, et al. Recommendations for the diagnosis and treatment of the acute porphyrias. Ann Intern Med. (2005) 142:439–50. doi: 10.7326/0003-4819-142-6-200503150-00010
31. Yasuda M, Gan L, Chen B, Kadirvel S, Yu C, Phillips JD, et al. RNAi-mediated silencing of hepatic Alas1 effectively prevents and treats the induced acute attacks in acute intermittent porphyria mice. Proc Natl Acad Sci U S A. (2014) 111:7777–82. doi: 10.1073/pnas.1406228111
32. Syed YY. Givosiran: a review in acute hepatic porphyria. Drugs. (2021) 81:841–8. doi: 10.1007/s40265-021-01511-3
34. Lambrecht RW, Gildemeister OS, Pepe JA, Tortorelli KD, Williams A, Bonkovsky HL. Effects of antidepressants and benzodiazepine-type anxiolytic agents on hepatic porphyrin accumulation in primary cultures of chick embryo liver cells. J Pharmacol Exp Ther. (1999) 291:1150–5.
36. Moore MR. International review of drugs in acute porphyria–1980. Int J Biochem. (1980) 12:1089–97. doi: 10.1016/0020-711X(80)90218-9
37. GCFC. SAFE LIST: Drugs that are Considered to be SAFE for Use in the Acute Porphyrias. Spanish Town: GCFC (2022). Available online at: http://www.wmic.wales.nhs.uk/porphyria_info.php (accessed January 17, 2023).
38. Rudowski W, Gregor A, Kostrzewska E, Kocylowski M, Zienkiewicz K. Analgesic effect of fortral (pentazocine) in acute intermittent porphyria. J Int Med Res. (1974) 2:284–8. doi: 10.1177/030006057400200406
39. Bohrer H, Schmidt H. Porphyrinogenicity and metabolic effects of propofol in am AIA-primed rat model. Eur J Anaesth. (1991) 8:486.
40. Mclouglin C. Use of propofol in a patient with porphyria. Br J Anaesth. (1989) 62:114. doi: 10.1093/bja/62.1.114
41. Mitterschiffthaler G, Theiner A, Hetzel H, Fuith LC. Safe use of propofol in a patient with acute intermittent porphyria. Br J Anaesth. (1988) 60:109–11. doi: 10.1093/bja/60.1.109
42. Meissner PN, Harrison GG, Hift RJ. Propofol as an IV anaesthetic induction agent in variegate porphyria. Br J Anaesth. (1991) 66:60–5. doi: 10.1093/bja/66.1.60
43. Weir PM, Hodkinson BP. Is propofol a safe agent in porphyria? Anaesthesia. (2007) 43:1022–3. doi: 10.1111/j.1365-2044.1988.tb05699.x
44. Kasraie N, Cousins TB. Propofol and the patient with hereditary coproporphyria. Anesth Anal. (1993) 77:862–3. doi: 10.1213/00000539-199310000-00038
45. Famewo CE. Induction of anaesthesia with etomidate in a patient with acute intermittent porphyria. Can Anaesth Soc J. (1985) 32:171–3. doi: 10.1007/BF03010045
46. Rizk SF, Jacobson JH, Silvay G. Ketamine as an induction agent for acute intermittent porphyria. Anesthesiology. (1977) 46:305–6. doi: 10.1097/00000542-197704000-00017
47. Capouet V, Dernovoi B, Azagra JS. Induction of anaesthesia with ketamine during an acute crisis of hereditary coproporphyria. Can J Anaesth. (1987) 34:388–90. doi: 10.1007/BF03010140
48. Harrison GG, Moore MR, Meissner PN. Porphyrinogenicity of etomidate and ketamine as continuous infusions. Br J Anaesth. (1985) 57:420–3. doi: 10.1093/bja/57.4.420
49. Mustajoki P, Heinonen J. General anesthesia in “inducible” porphyrias. Anesthesiology. (1980) 53:15–20. doi: 10.1097/00000542-198007000-00004
50. Harrison GG, Meissner PN, Hift RJ. Anaesthesia for the porphyric patient. Anaesthesia. (1993) 48:417–21. doi: 10.1111/j.1365-2044.1993.tb07018.x
51. Buzaleh AM, de Salamanca RE, del Batlle AM. Porphyrinogenic properties of the anesthetic enflurane. Gen Pharmacol Vasc Syst. (1992) 23:665–9. doi: 10.1016/0306-3623(92)90145-A
52. Evans PR, Graham S, Kumar CM. The use of sevoflurane in acute intermittent porphyria: correspondence. Anaesthesia. (2001) 56:388–9. doi: 10.1046/j.1365-2044.2001.01976-28.x
53. Messmer M, Gerheuser F, Forst H. Desfluran bei akute intermittierender Porphyrie. Anaesthesist. (2004) 53:244–8. doi: 10.1007/s00101-003-0615-7
54. Blekkenhorst Gerry H, Eales L. Drug safety in Porphyria. Lancet. (1980) 316:1250. doi: 10.1016/S0140-6736(80)92511-8
55. Bonkowsky H, Schady W. Neurologic manifestations of acute porphyria. Semin Liver Dis. (1982) 2:108–24. doi: 10.1055/s-2008-1040701
56. Findley H, Philips A, Cole D, Nair A. Porphyrias: implications for anaesthesia, critical care, and pain medicine. Contin Educ Anaesth Crit Care Pain. (2012) 12:128–33. doi: 10.1093/bjaceaccp/mks009
57. Stevens JJWM, Kneeshaw JD. Mitral valve replacement in a patient with acute intermittent porphyria. Anesth Anal. (1996) 82:416–8. doi: 10.1213/00000539-199602000-00036
Keywords: porphyria, anesthesia, preoperative management, propofol, volatile anesthetics
Citation: Randjelovic M, Stojanovic M, Radeka J, Vasilijic M and Jankovic R (2023) Anesthetic implications in porphyrias. Front. Anesthesiol. 2:1149949. doi: 10.3389/fanes.2023.1149949
Received: 23 January 2023; Accepted: 03 February 2023;
Published: 27 February 2023.
Edited by:
Biljana Kuzmanovska, Saints Cyril and Methodius University of Skopje, North MacedoniaReviewed by:
Ivana Budic, University of Niš, SerbiaMaja Mojsova Mijovska, University Clinic of Anesthesia, North Macedonia
Copyright © 2023 Randjelovic, Stojanovic, Radeka, Vasilijic and Jankovic. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Milica Randjelovic,  bWlyYW5kamVsb3ZpYzkxQGdtYWlsLmNvbQ==
bWlyYW5kamVsb3ZpYzkxQGdtYWlsLmNvbQ==
 Milica Randjelovic
Milica Randjelovic Milena Stojanovic
Milena Stojanovic Jovan Radeka
Jovan Radeka Milena Vasilijic1
Milena Vasilijic1 Radmilo Jankovic
Radmilo Jankovic