- 1Department of Anesthesiology and Pain Medicine, Inselspital, Bern University Hospital, University of Bern, Bern, Switzerland
- 2Department for Anesthesia, Prehospital Emergency Medicine and Pain Therapy, University Hospital Basel, Basel, Switzerland
- 3Department of Cardiothoracic Anesthesia and ICM, Manchester University Foundation Trust, Manchester, United Kingdom
- 4Department of Cardiovascular Surgery, Inselspital, Bern University Hospital, University of Bern, Bern, Switzerland
Study objective: Perioperative transfusion is associated with reduced survival and increased morbidity and mortality. Several studies report wide variation in clinical transfusion practice. However, the influence of simple, practical factors–such as which blood products are available, and when - is often disregarded. We hypothesized that these practical issues are relevant confounders of transfusion decisions.
Design: Web-based survey.
Setting: Multi-institutional.
Participants: Members of the European Association of Cardiothoracic Anesthesiology and Intensive Care (EACTAIC) society reached by monthly newsletter in November 2020.
Interventions: No interventions.
Measurements: Survey responses.
Main results: The newsletter was opened by 429 members. We collected 51 complete surveys, resulting in a response rate of 11.9%. 72% of participants reported having a local algorithm for the use of blood products and coagulation factors. Latency in the time of blood product delivery / availability and the possibility to store or return unused products were most often reported as having an influence on transfusion practice. For point-of-care test availability, 86% of addressees reported rotational thromboelastometry / -elastography, 76% hemoglobin tests, 24% international normalized ratio (INR) measurement and 22% platelet function testing. Six percent of the respondents did not have access to point-of-care tests. The majority of addressees reported that they were able to obtain more than 10 allogeneic blood products simultaneously (63%). Packed red blood cells were available with a delay of 10–15 min and platelets with a delay of 15–20 min.
Conclusions: Our survey indicates a wide variability in the logistics of perioperative transfusion practice. The information gained could provide a solid basis for future improvements of the guidelines, but also in local transfusion practices.
Introduction
Perioperative red blood cell (RBC) transfusion has been associated with increased morbidity and reduced survival in cardiac surgery [1–3]. As objective measures to determine global tissue hypoxia are lacking and the identification of patients needing RBC transfusion is difficult (based solely on age, hematocrit or co-morbidities), comprehensive recommendations have been proposed to guide peri-operative RBC transfusion in this setting [4–6]. Although recent data from the Society of Thoracic Surgeons show declining blood product use over the past years [7], several studies have reported poor adoption of guidelines and wide variation in daily clinical practice [8–12].
The influence of the following simple factors is usually disregarded in related studies and guidelines: (1) which blood products are available to the transfusing physician, and when; (2) what are the factors influencing the internal algorithms, and (3) what kind of transfusion behavior exists among physicians?
We hypothesize that local practical issues are an important contributor to transfusion decisions and may explain some of the variability in transfusion guideline adoption. A better understanding of these issues would allow to address them in upcoming guidelines and to reduce the incidence of unnecessary transfusions.
Materials and methods
In cooperation with the European Association of Cardiothoracic Anesthesiology and Intensive Care (EACTAIC) society, the authors developed a web-based survey addressing local factors in logistics and organization that may have an influence on transfusion practice. The survey consisted of 34 questions on transfusion practice and three additional questions on place of work, clinical experience of the responsible physician, and main field of practice. These were optional, to maintain privacy in small centers if desired. Survey questions were set up by two authors (Daniel Gerber and Gabor Erdoes) and reviewed by a senior consultant with more than 30 years practice in cardiac anesthesia. The estimated completion time was <7 min. The link to the survey was sent to all EACTAIC members via the association's monthly newsletter in November 2020. A reminder was sent 4 weeks later, in December 2020. The survey was conducted using the Survey Monkey web platform [13] and responses were collected between November 2020 and January 2021. Based on a population size of 500 subjects, a confidence level of 95% and a margin of error of 10% we aimed to collect at least 81 responses. No incentive was offered for completion of the survey. In the questionnaire, the term “labile blood products” was used for packed red blood cells [PRBC], fresh-frozen plasma [FFP] and platelet concentrates [PLTC]. For better international understandability, the term “allogeneic blood products” will be used in this article. “Coagulation factor concentrates” was the category name used for fibrinogen concentrate, prothrombin complex concentrate [PCC], and recombinant FVIIa. This nomenclature will be used throughout the article. Results are reported as percentage or median and interquartile range (IQR).
Results
Demographic data
We received 66 survey responses including 51 complete surveys. Fifteen responses were incomplete, mostly consisting of only two answers, suggesting a technical problem, as there was a skip logic after question 2 in the survey. Since 429 members opened the newsletter, this resulted in a response rate of 11.9%. This results in a margin of error of 13% with a 95% confidence level.
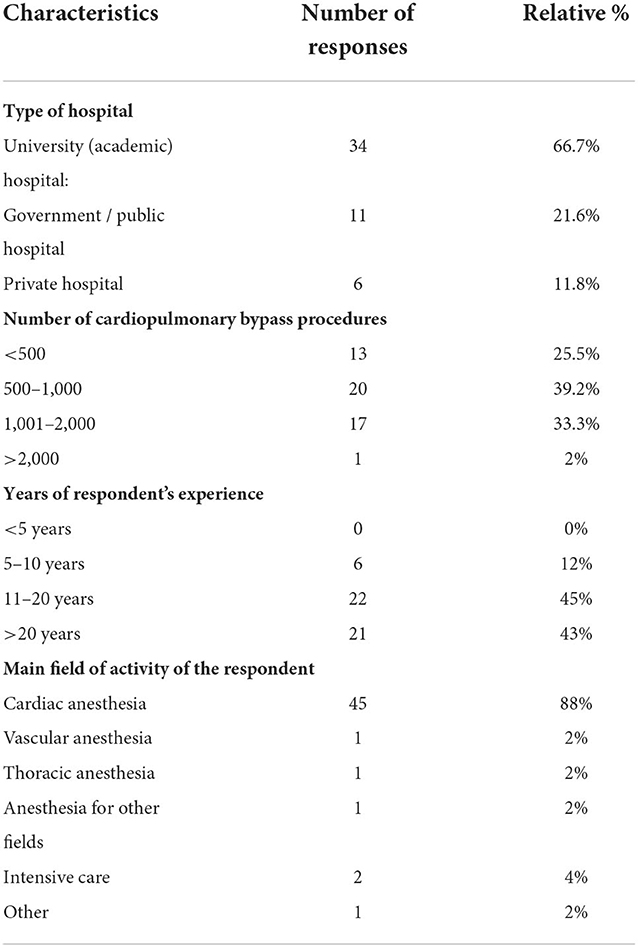
Most responses were collected from participants working in cardiac surgery (88%). Other respondents came from intensive care medicine (4%), vascular anesthesia, thoracic anesthesia and other fields of anesthesia (2% each) (Table 1).
Responses came from 25 countries across Europe with the majority of responses from hospitals in Germany, Switzerland (12% each) and the United Kingdom (10%). Place of work has been a voluntary answer, of two hospitals, double answers have been collected.
Most respondents were employed in hospitals, with 67% working in a university hospital and 22% in a non-academic hospital. 12% of responses came from EACTAIC members working in private hospitals. Experience performing cardiac surgery with cardiopulmonary bypass (CPB) was distributed as follows: 25% of centers performed fewer than 500 CPB procedures per year, 40% between 500 and 1,000 procedures, and 33% between 1,000 and 2,000 procedures. 12% of respondents had between 5 and 10 years of work experience, 45% between 11 and 20 years, and 43% more than 20 years (Table 1).
Transfusion algorithms and practice
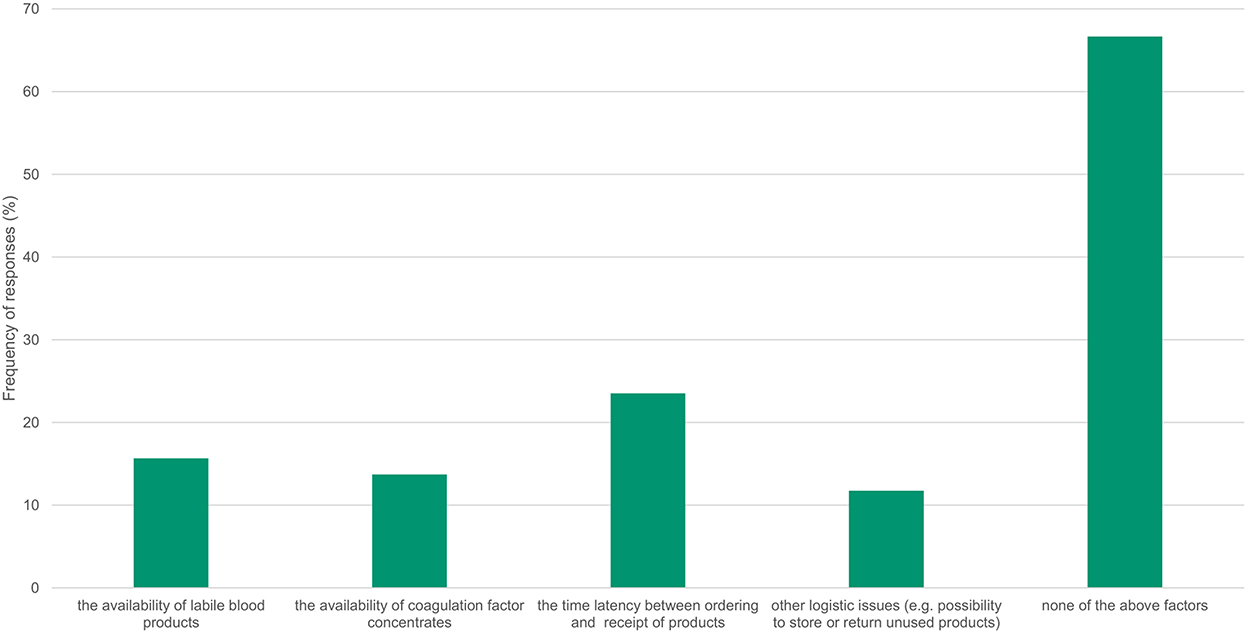
72% of participants reported having a local algorithm to administer blood products and coagulation factors. Several features were reported as having an influence on the local transfusion algorithm (Figure 1): the availability of allogeneic blood products (16%), the availability of coagulation factors (14%), the latency of time between ordering and receipt of products (24%) and other logistical issues, such as the possibility to store or return unused products (12%). 67% of respondents reported that these factors had no influence on their local transfusion algorithm.
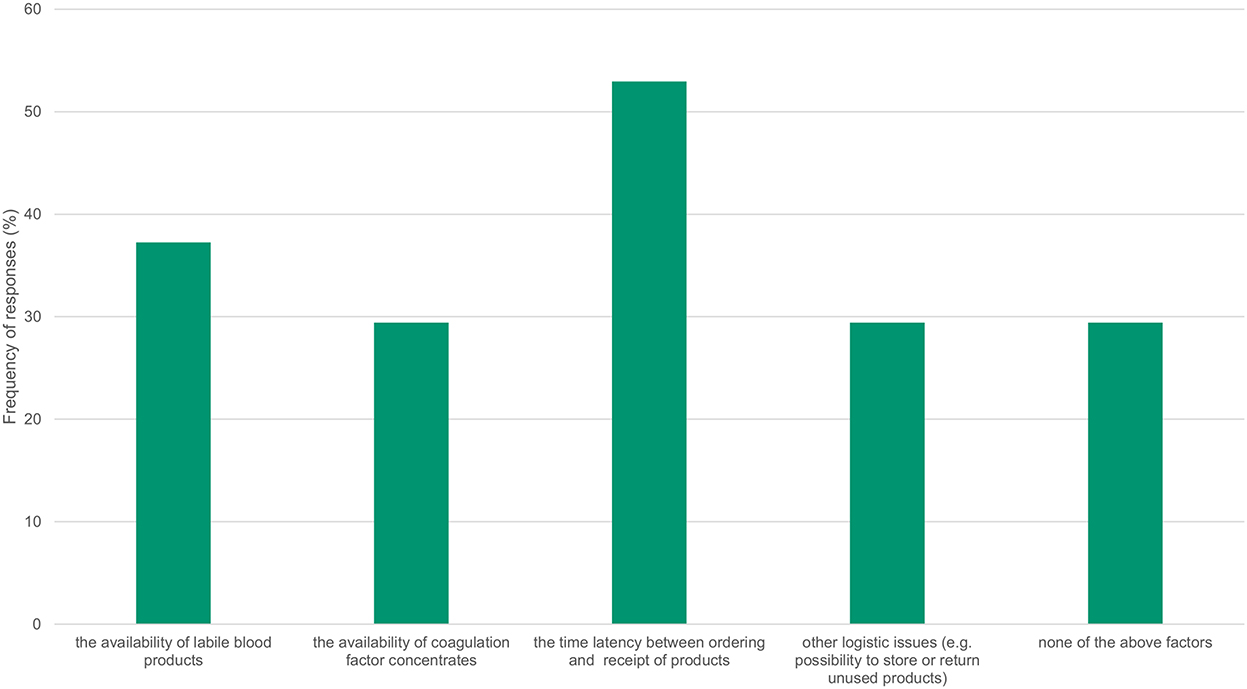
The influence on transfusion practice was also investigated (Figure 2), with 37% indicating that the availability of allogeneic blood products had an impact on practice, and 30% naming factor concentrates. For 53% of respondents, the time between ordering and receiving products, and for 29% other logistical aspects, such as the ability to store or return unused products, had an impact on their transfusion practice. Only 29% of respondents indicated that these factors had no influence on their transfusion practice.
Regarding point-of-care (POC) test availability, 86% reported that rotational thromboelastometry/-elastography was available at their institutions (14% for both), 76% had access to POC hemoglobin measurements, 24% had POC-PT (INR) measurements, and 22% had POC platelet function testing. 6% of respondents did not have any of the above-mentioned POC devices. Due to the low return rate, we did not group the answers according to level of care (academic/non-academic) or hospital size.
Ordering and delivery
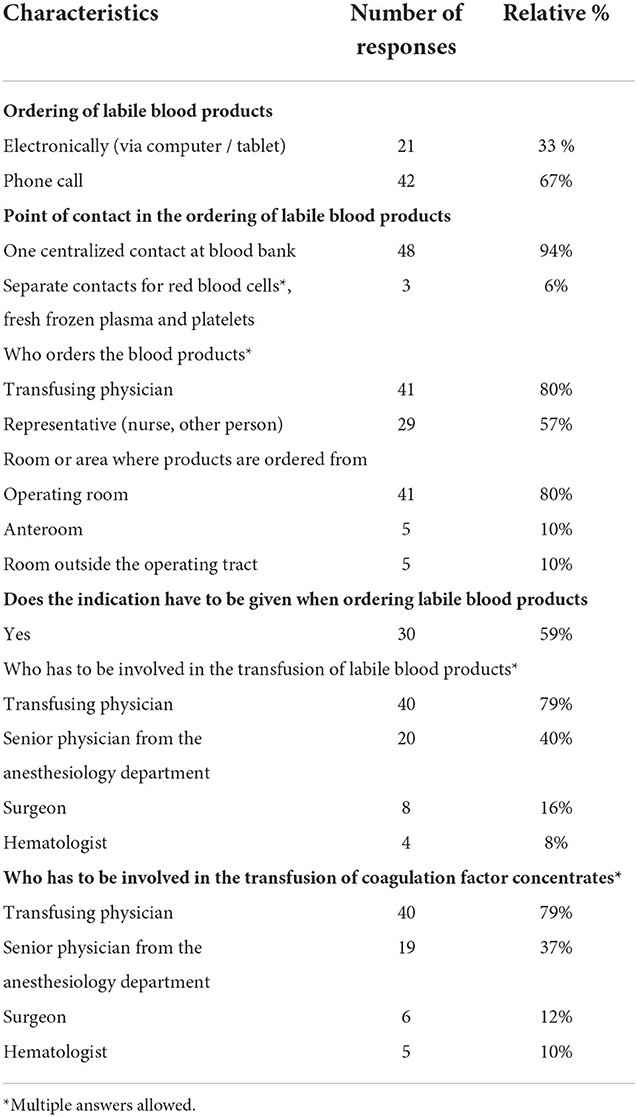
The majority of respondents stated that ordering of allogeneic blood components was handled by calling the blood bank (67% of responses, Table 2). 33% reported using an electronic system. Both options were associated with a high level of satisfaction (80% [IQR 59.5–89] and 80% [IQR 60–89], respectively). For telephone ordering, 81% of respondents indicated that the telephone contact was always available. A lab technician was most likely to answer the phone (81%), and language barriers were cited as a problem in 12% of calls. In most centers, a central contact person in the blood bank was responsible for receiving orders for allogeneic blood products (94%). In most institutions, the transfusing physician himself ordered blood products (82%), but other staff members (e.g., nurses) also played an important role in ordering for 56% of the respondents. Allogeneic blood products were most frequently ordered from the operating room (80%), followed by an anteroom within the operating room (10%) or a location outside the operating room (10%). The indication for use had to be stated in 59% of the responses when ordering allogeneic blood products.
Delivery
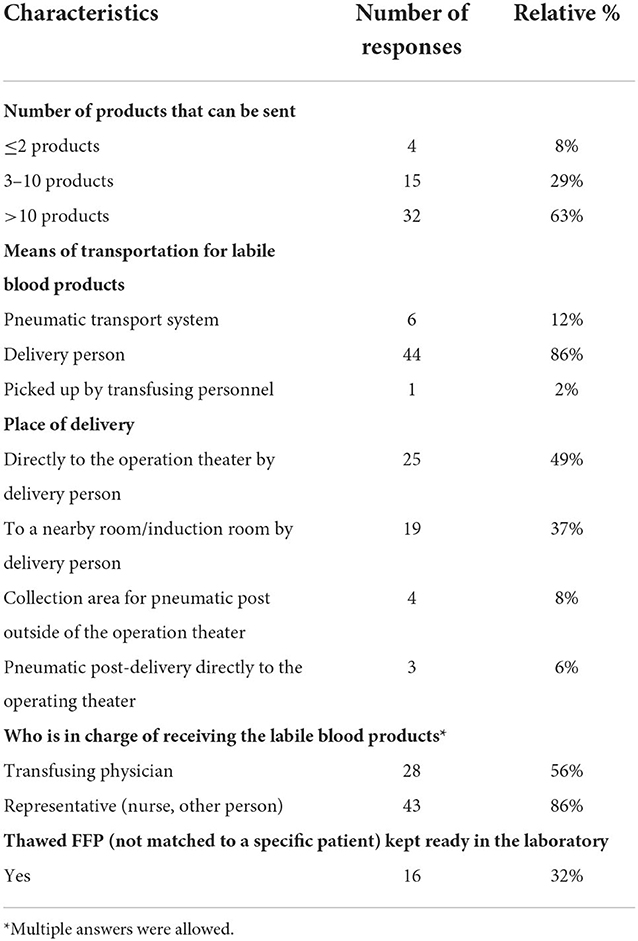
Regarding the delivery of blood products, the majority of respondents indicated that they could receive more than 10 allogeneic blood products at one time (63%). About one third of respondents reported receiving only 3–10 products (29%) or 2 products or less (8%) as shown in Table 3.
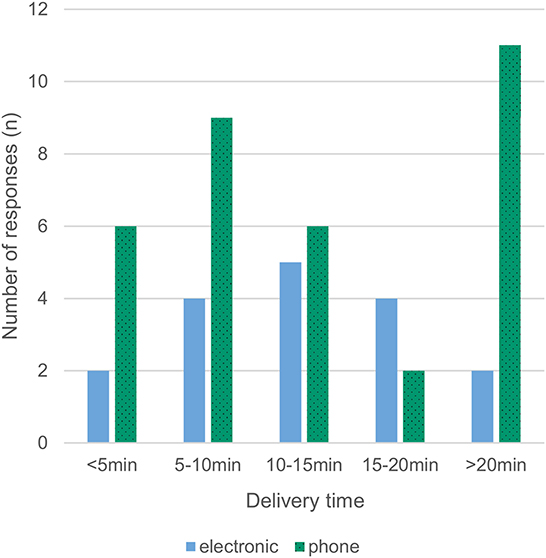
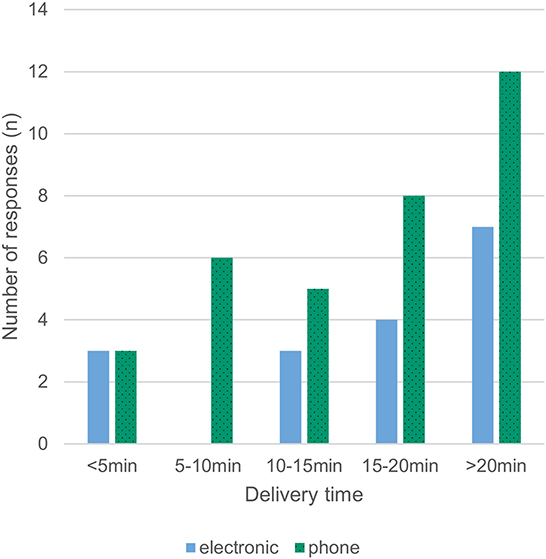
Most respondents (86%) reported that a delivery person transported the allogeneic blood products to the operating room. Pneumatic transport systems were used in 12% of cases, and only a minority picked up the products from the blood bank themselves (2%). RBCs were available with a median delay of 10–15 min, with 16% of respondents reporting availability in <5 min and 25% reporting a waiting time of more than 20 min (Fig 3). For platelets, a median delay of 15–20 min was reported between order and delivery, with 12% of respondents reporting availability in <5 minutes and 37% reporting a waiting time of more than 20 min (Figures 3, 4). Satisfaction with delivery times was a median of 80 on a visual analog scale of 0 (dissatisfied) to 100 (completely satisfied) [14].
Control, storage, and disposal
Post-delivery management of allogeneic blood products was heterogeneous: 33% transfused immediately, 25% used storage in a nearby room (e.g., anesthesia induction room), 29% stored the products refrigerated in the operating room (OR), and 12% stored unrefrigerated products in the operating room. 39% of the respondents performed a traditional bedside test, while 10% implemented electronic bedside scanning and 51% relied on dual control to verify patient compatibility prior to transfusion. Even after testing the products, significant heterogeneity exists: 47% transfused immediately, 27% kept products unrefrigerated in the OR until transfusion, and 25% stored products in an OR refrigerator. Coagulation factor concentrates were available in the OR for 43% of respondents, 35% stored them in the central laboratory, and 22% stored them at another collection point in the hospital (e.g., in the emergency department or in a central storage area outside the OR). 70% of the responders dispose of blood products in special waste and 30% do so in normal garbage.
Discussion
Transfusion practice for cardiac surgery is known to vary between centers as well as between physicians [15, 16]. We set up this survey together with EACTAIC to evaluate whether practical issues and logistic factors have an influence on transfusion behavior. As main finding, the data from our survey show wide variability in the logistics of perioperative transfusion and a relevant subjective influence of these factors mainly on transfusion practice and to a lesser extent on local transfusion algorithms. Time to delivery of allogeneic blood products was the most frequently cited factor influencing transfusion behavior. The reported variability might explain part of the inconsistencies and mixed effects of interventions aiming to reduce transfusion rate.
To our knowledge, this is the first survey to assess basic/logistical factors affecting transfusion practices. As the survey was sent to all EACTAIC members, the targeted study population (patients undergoing cardiothoracic and vascular procedures) had high exposure to perioperative transfusion.
Studies have shown reduced blood transfusion with a multidisciplinary approach in cardiac surgery, but logistic issues have not been addressed [17]. Delivery times have been reduced by using pneumatic delivery systems [18], but the small proportion of respondents using a pneumatic delivery system did not appear to experience clinically relevant shorter delivery times. This might be an indicator that the transportation system is only a small contributor to delivery latency. A further reduction of transfusion incidence was shown with the use of viscoelastic tests, which are fortunately well-available according to our results (86%). The use of point-of-care tests might as well-have significant effects on the delay between “bleeding due to coagulation disorders” and “administering the correct products,” this has not been considered in our survey or in a previous work.
We found a high rate of 67% ordering by phone call. Separating delivery times depending on electronic ordering vs. ordering by phone call as in Figure 4 suggests that delivery times might be shorter with electronic ordering, but drawing firm conclusions is prohibited by the low overall response rate. The delivery times for coagulation factor concentrates where not queried, but assuming shorter availability times when the products are available in the OR (43% in our survey) compared to storage in other locations seems reasonable. This assumed benefit has to be weighed against more challenging stock management, place requirements and sometimes billing issues too.
Since a considerable amount of blood products are stored unrefrigerated in the operation theater (12% before testing, 27% after testing), the use of an indicator on the products to reveal long unrefrigerated storage times should be considered.
We find it reasonable that logistical factors would have a major influence on transfusion practice. In many situations, the transfusing physician (and of course the patient) bears the brunt of a delayed or canceled transfusion (e.g., greater blood loss, hemodynamic instability, re-do surgery due to bleeding, hemodynamic instability) and has a high awareness therefore, while the consequences of unnecessary transfusions (increased morbidity and mortality, higher hospital costs) remain hidden.
Although this is pure interpretation, we think that the subjective benefits of transfusion might lead to risk-averse behavior, a concept borrowed from economics and sociology [19]. In combination with (perceived) long delivery times and absent possibilities for storage and return of unused products, this might be a significant factor influencing the incidence of transfusions. In cardiac surgery, the interaction with the perfusionist has a high potential for altering transfusion requirements through different perfusion techniques, transfusion triggers and transfusion cultures across the professional guilds. Increasing physicians' awareness and education have been proposed by others [20] as a way to improve transfusion strategies.
The results of this survey shed light on the potential benefits of adjusting institutional conditions and processes to improve adherence to transfusion guidelines. This has the potential to reduce mortality and morbidity and improve cost efficacy. Approaches must be tailored to very specific logistical issues and needs, and input is necessary from the person responsible for facility transfusions. Benchmarks of transfusion incidence compared to other hospitals as well as a monitoring tool for transfusion frequency on an individual basis might be helpful, although care must be taken to ensure that baseline factors are appropriately corrected, because different case mixes, patient factors, and surgeons have a major impact on transfusion behavior [15].
A strength of this study is the focus on the practical and logistical aspects of perioperative transfusion – an issue poorly studied in the past. Transfusion variability is well-known and also other factors might be relevant – e.g., awareness of the costs, seasonal differences in view of shortages of blood products or different individual triggers for transfusion [11, 12, 15, 16]. In order to keep the time required to complete short, this survey did not cover these issues. The main limitation is, however, the low response rate, which was calculated from the total number of newsletters opened in which the survey was originally embedded. This is probably a very generous but honest calculation - to the disadvantage of the reported response rate - especially as not all members who read the newsletter also want to answer the survey. A further reason for the low response rate could be explained by “survey fatigue,” with large numbers of surveys sent out during the COVID-19 pandemic, as well as the fact that we decided against offering an incentive for survey completion, an intervention known to improve response rates [21]. Voluntary surveys have an inherent bias because primarily people who are interested in the topic answer them.
We consider 51 fully completed response forms from 25 countries in combination with the authors experience to be a solid basis - even if not representative–for drawing initial conclusions from transfusion practice in our subspecialty. The responses will help us to plan further studies in this area with the aim to improve patient care in cardiovascular and thoracic surgery.
Conclusions
Significant differences in transfusion logistics were found in European cardiovascular and thoracic centers. The information obtained in this survey highlights deviations from the most recent transfusion guidelines. They could provide a solid basis for future improvements of the guidelines, but also in local transfusion practices.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author/s.
Ethics statement
Ethical review and approval was not required for the study on human participants in accordance with the local legislation and institutional requirements. The patients/participants provided their written informed consent to participate in this study.
Author contributions
DG and GE: conception and design of the work, acquisition, analysis, interpretation of data, and drafting the work. DB, SA, and FZ: acquisition, analysis, and interpretation of data. All authors contributed to the article and approved the submitted version.
Acknowledgments
The authors thank Ms. Noemi Albanese, AIM Group, EACTAIC Association Management Company, for assistance in programming, conducting, and collecting the responses of the present survey. The authors acknowledge Jeannie Wurz, Medical Editor from the Bern University Hospital, for careful reading and editing of the manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fanes.2022.995963/full#supplementary-material
References
1. Loop FD, Starr NJ. et al. Transfusion in coronary artery bypass grafting is associated with reduced long-term survival. Annals Thoracic Surg. (2006) 81:1650–7. doi: 10.1016/j.athoracsur.2005.12.037
2. Paone G, Likosky DS, Brewer R, Theurer PF, Bell GF, Cogan CM, et al. Transfusion of 1 and 2 units of red blood cells is associated with increased morbidity and mortality. Annals Thoracic Surg. (2014) 97:87–93. doi: 10.1016/j.athoracsur.2013.07.020
3. Murphy GJ, Reeves BC, Rogers CA, Rizvi SI, Culliford L, Angelini GD. Increased mortality, postoperative morbidity, and cost after red blood cell transfusion in patients having cardiac surgery. Circulation. (2007) 116:2544–52. doi: 10.1161/CIRCULATIONAHA.107.698977
4. Pagano D, Milojevic M, Meesters MI, Benedetto U, Bolliger D, von Heymann C, et al. (2017 EACTS/EACTA guidelines on patient blood management for adult cardiac surgery. Eu J Cardio Thoracic Surg. (2018) 53:79–111. doi: 10.1093/ejcts/ezx325
5. Raphael J, Mazer CD, Subramani S, Schroeder A, Abdalla M, Ferreira R, et al. Society of cardiovascular anesthesiologists clinical practice improvement advisory for management of perioperative bleeding and hemostasis in cardiac surgery patients. J Cardiothorac Vasc Anesth. (2019) 33:2887–99. doi: 10.1053/j.jvca.2019.04.003
6. Tibi P, McClure RS, Huang J, Baker RA, Fitzgerald D, Mazer CD, et al. STS/SCA/AmSECT/SABM update to the clinical practice guidelines on patient blood management. J Cardiothorac Vasc Anesth. (2021) 35:2569–91. doi: 10.1053/j.jvca.2021.03.011
7. D'Agostino RS, Jacobs JP, Badhwar V, Fernandez FG, Paone G, Wormuth DW, et al. The society of thoracic surgeons adult cardiac surgery database: 2019 update on outcomes and quality. Ann Thorac Surg. (2019) 107:24–32. doi: 10.1016/j.athoracsur.2018.10.004
8. Cabana MD, Rand CS, Powe NR, Wu AW, Wilson MH, Abboud PA, et al. Why don't physicians follow clinical practice guidelines? A framework for improvement. Jama. (1999) 282:1458–65. doi: 10.1001/jama.282.15.1458
9. Stover EP, Siegel LC, Parks R, Levin J, Body SC, Maddi R, et al. Variability in transfusion practice for coronary artery bypass surgery persists despite national consensus guidelines: a 24-institution study. Institutions of the multicenter study of perioperative ischemia research group. Anesthesiology. (1998) 88:327–33. doi: 10.1097/00000542-199802000-00009
10. Likosky DS, FitzGerald DC, Groom RC, Jones DK, Baker RA, Shann KG, et al. The effect of the perioperative blood transfusion and blood conservation in cardiac surgery clinical practice guidelines of the society of thoracic surgeons and the society of cardiovascular anesthesiologists upon clinical practices. J Extra Corpor Technol. (2010) 42:114–21.
11. Snyder-Ramos SA, Möhnle P, Weng YS, Böttiger BW, Kulier A, Levin J, et al. The ongoing variability in blood transfusion practices in cardiac surgery. Transfusion. (2008) 48:1284–99. doi: 10.1111/j.1537-2995.2008.01666.x
12. Magruder JT, Blasco-Colmenares E, Crawford T, Alejo D, Conte JV, Salenger R, et al. Variation in red blood cell transfusion practices during cardiac operations among centers in Maryland: results from a state quality-improvement collaborative. Ann Thorac Surg. (2017) 103:152–60. doi: 10.1016/j.athoracsur.2016.05.109
13. SurveyMonkey Inc,. San Mateo, California, USA. Available online at: http://www.surveymonkey.com (accessed January 30, 2022).
14. McCormack HM, Horne DJ, Sheather S. Clinical applications of visual analogue scales: a critical review. Psychol Med. (2009) 18:1007–19. doi: 10.1017/S0033291700009934
15. Cote C, MacLeod JB, Yip AM, Ouzounian M, Brown CD, Forgie R, et al. Variation in transfusion rates within a single institution: exploring the effect of differing practice patterns on the likelihood of blood product transfusion in patients undergoing cardiac surgery. J Thorac Cardiovasc Surg. (2015) 149:297–302. doi: 10.1016/j.jtcvs.2014.09.004
16. Fitzgerald DC, Simpson AN, Baker RA, Wu X, Zhang M, Thompson MP, et al. Determinants of hospital variability in perioperative red blood cell transfusions during coronary artery bypass graft surgery. J Thorac Cardiovasc Surg. (2020). doi: 10.1016/j.jtcvs.2020.04.141
17. Brevig J, McDonald J, Zelinka ES, Gallagher T, Jin R, Grunkemeier GL. Blood transfusion reduction in cardiac surgery: multidisciplinary approach at a community hospital. Ann Thorac Surg. (2009) 87:532–9. doi: 10.1016/j.athoracsur.2008.10.044
18. Kok JHJ, Lam CWL, Lee MYJ. Reducing delivery times of emergency blood products through pneumatic tube systems. Asian J Transfus Sci. (2019) 13:3–9. doi: 10.4103/ajts.AJTS_52_18
19. Kahneman D, Tversky A. Prospect theory: an analysis of decision under risk. Econometrica. (1979) 47:263–91. doi: 10.2307/1914185
20. Ranucci M, Aronson S, Dietrich W, Dyke CM, Hofmann A, Karkouti K, et al. Patient blood management during cardiac surgery: do we have enough evidence for clinical practice? J Thorac Cardiovasc Surg. (2011) 142:249–32. doi: 10.1016/j.jtcvs.2011.04.007
Keywords: transfusion, logistics, EACTAIC survey, transfusion algorithm, transfusion variability
Citation: Gerber D, Bolliger D, Agarwal S, Zulauf F and Erdoes G (2022) Blood and coagulation product disposition in the modern era: An international multicenter survey endorsed by the European Association of Cardiothoracic Anesthesiology and Intensive Care (EACTAIC). Front. Anesthesiol. 1:995963. doi: 10.3389/fanes.2022.995963
Received: 16 July 2022; Accepted: 06 September 2022;
Published: 30 November 2022.
Edited by:
Vladimir Lomivorotov, Novosibirsk Scientific Research Institute of Pathology, RussiaReviewed by:
Paolo Bianchi, Royal Brompton Hospital, United KingdomTae-Yop Kim, Konkuk University Medical Center, South Korea
Copyright © 2022 Gerber, Bolliger, Agarwal, Zulauf and Erdoes. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Daniel Gerber, ZGFuaWVsLmdlcmJlckBpbnNlbC5jaA==
†ORCID: Daniel Gerber orcid.org/0000-0001-6267-2409
 Daniel Gerber
Daniel Gerber Daniel Bolliger
Daniel Bolliger Seema Agarwal3
Seema Agarwal3 Gabor Erdoes
Gabor Erdoes