- 1Anesthesia Surgery Center of the First Affiliated Hospital of Gannan Medical University, Ganzhou, China
- 2Gannan Medical University, Ganzhou, China
- 3Department of Neurology, The First Affiliated Hospital of Gannan Medical University, Ganzhou, China
- 4Department of Oncology, The First Affiliated Hospital of Gannan Medical University, Ganzhou, China
- 5Department of Anesthesiology, The Ganzhou People's Hospital, Ganzhou, China
- 6Department of Anesthesiology, The First Affiliated Hospital of Xiamen University, Xiamen, China
Objective: Serratus anterior plane block (SAPB) is a new perioperative analgesia for patients undergoing thoracic and breast surgery. The primary purpose of this systematic review and meta-analysis was to investigate whether ultrasound-guided SAPB combined with general anesthesia provides safer and more effective postoperative analgesia than general anesthesia alone or general anesthesia combined with incisional local infiltration anesthesia in patients receiving thoracic and breast surgery.
Methods: We systematically searched PubMed, Embase, Web of Science and the Cochrane Library databases for clinical randomized controlled trials (RCTs) of SAPB for postoperative analgesia in thoracic and breast surgery. The primary outcome was the postoperative pain score. Secondary outcomes included intraoperative opioid consumption, 24-h postoperative opioid consumption, time to first use of analgesics, number of patients requiring urgent additional analgesics, opioid complications (postoperative nausea, vomiting, respiratory depression, constipation, dizziness, sedation) and length of hospital stay. The risk of bias was assessed using the Cochrane method and Jadad score.
Results: A total of 29 RCTs with 1,978 patients were included. Twelve studies included thoracic surgery, and 17 studies included breast surgery. The results of the meta-analysis showed that the rest or movement pain scores of the SAPB group were significantly lower than those of the control group at each postoperative time point. In addition, morphine consumption was significantly reduced in the SAPB group at 24 h postoperatively (standardized mean differences [SMD], −2.77; 95% confidence interval [CI], −3.56 to −1.97; P < 0.01). Intraoperative opioid consumption was significantly reduced in the SAPB group (SMD, −0.66; 95% CI, −1.03 to −0.28; P < 0.01); and the number of patients requiring urgent additional pain medication postoperatively (risk ratio [RR], 0.34; 95% CI, 0.27 to 0.42; P < 0.01) was significantly lower; and the time to first use of analgesics was significantly longer (SMD, 3.49; 95% CI, 2.23 to 4.74; P < 0.01); and the incidence of postoperative nausea and vomiting (PONV) (RR, 0.43; 95% CI, 0.34 to 0.54; P < 0.01), constipation (RR, 0.12; 95% CI, 0.03 to 0.52; P < 0.01; I2 = 0), dizziness (RR, 0.24; 95% CI, 0.06 to 0.92; P < 0.05; I2 = 0) and sedation (RR, 0.07; 95% CI, 0.01 to 0.52; P < 0.01; I2 = 0) were significantly lower; the length of hospital stay was significantly shorter (SMD, −0.28; 95% CI, −0.46 to −0.09; P < 0.01) and the SAPB group have a significantly reduced the incidence of postoperative pain syndrome at 3 months.
Conclusions: Compared with no SAPB block, ultrasound-guided SAPB provides superior postoperative analgesia by reducing postoperative pain scores, the incidence of postoperative pain syndrome at 3 months and perioperative opioid consumption in patients after thoracic and breast surgery. At the same time, SAPB reduces the incidence of side effects of opioids and shortens the length of hospital stay. SAPB can be used as a feasible technique for multimodal analgesia in the perioperative period.
Introduction
Due to the rich nerve distribution of the chest wall, postoperative pain is particularly pronounced after thoracic and breast surgery. Studies have found that approximately 78% of patients undergoing thoracotomy experience moderate to severe postoperative pain [1], ~50% of breast surgery patients experience varying degrees of postoperative pain [2]. In recent years, with the rapid development of minimally invasive surgery, most operations are performed under minimally invasive procedures, and the surgical incision is significantly smaller, but postoperative incision pain still exists, and postoperative pain control is still challenging, especially in major surgeries such as thoracic surgery. Severe postoperative pain seriously affects the postoperative recovery of patients and prolongs the length of hospital stay [3]. In addition, acute pain after surgery is at risk for progression to chronic pain, which also affects psychological changes, quality of life, and satisfaction of patients [4, 5]. At present, the analgesic effect is usually improved by increasing the use of opioids. However, drug-related side effects such as respiratory depression and PONV that come at any time cannot be ignored. Especially for elderly patients, repeated high-dose opioids may lead to cognitive impairment and even coma, which seriously affects the prognosis of elderly patients [6]. Generally, pain is caused by rib, muscle, and soft tissue injury at the incision in the chest [7]. In recent years, many articles have reported the application of various nerve tissues in postoperative analgesia after thoracic and breast surgery, such as thoracic epidural analgesia, intercostal nerve blocks, erector spinae plane blocks and paravertebral nerve blocks, which can relieve severe postoperative pain. However, each has its own disadvantages. For example, paravertebral nerve blocks have complications such as pneumothorax, spinal cord block, and neuronal damage [8]. Intercostal block provides significant analgesic effect, however, due to the limited diffusion of the local anesthetic after a single injection, multiple injections must be administered to increase pain relief. As a result, this approach often results in increased pain, prolonged procedure time, and an increased incidence of pneumothorax [9]. Thoracic epidural analgesia also has some disadvantages, such as inadvertently causing high block, local anesthesia toxicity, general spinal anesthesia, hypotension, vomiting, etc. [10].
Ultrasound-guided serratus anterior plane block (SAPB) is a new ultrasound-guided interfascial plane block technique. The serratus anterior originates from the surface of the anterior eight ribs and attaches to the medial border of the scapula and posterior to the latissimus dorsi. Therefore, SAPB can be achieved by blocking the lateral cutaneous branches of the T2-T9 spinal nerves by injecting a local anesthetic of a certain concentration and volume between the latissimus dorsi and serratus anterior muscles under linear ultrasound guidance [11]. It is a safe procedure under ultrasound-guided, and there is no possibility of neurological complications like epidural hematoma [12]. Due to its technical simplicity and relative safety, SAPB can be used for regional nerve blocks for intraoperative and postoperative lateral chest wall analgesia [5, 7]. In 2013, Blanco et al. [13] first described analgesia by SAPB after breast cancer surgery, and later, SAPB rapidly gained popularity for different types of operations, such as thoracic surgery [14], breast surgery [15], rib fracture surgery [16], and liver surgery [17]. Several studies have shown that SAPB can be used as a topical analgesic technique to reduce pain after surgery [18–20]. Therefore, SAPB may be an attractive option for pain relief after thoracic and breast surgery. Given the large number of thoracic and breast surgeries performed around the world and the varying levels of postoperative pain [21, 22], it is important to determine the analgesic effect of SAPB. However, there is no comprehensive meta-analysis to evaluate the analgesic efficacy and safety of SAPB after thoracic and breast surgery. Therefore, we conducted a systematic review and meta-analysis of eligible clinical randomized controlled trials (RCTs) to evaluate the analgesic efficacy and safety of perioperative ultrasound-guided SAPB combined with general anesthesia in patients undergoing thoracic and breast surgery.
Methods
This systematic review and meta-analysis was conducted according to the criteria of the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) statement [23]. This meta-analysis has been prospectively registered in the PROSPERO database (registration number: CRD42022322904).
Search strategy
We conducted a comprehensive database search of PubMed, Embase, the Web of Science and the Cochrane Library for RCTs that met the listed inclusion criteria. The last update time is March 2022. The search keywords were as follows: (“serratus anterior plane block” OR “serratus anterior block” OR “SAPB” OR “SAP block” OR “sap block”) and (“thoracic surgery” OR “thoracoscopic surgery” OR “thoracotomy” OR “ lobectomy” OR “rib fractures” OR “modified radical mastectomy” OR “mastectomy” OR “breast surgery” OR “lumpectomy” OR “postoperative pain” OR “postoperative analgesia”). Specific search strategies are detailed in the Supplementary material for terms. We also searched the gray literature by supplementary hand searching because SAPB is a new regional anesthesia technique first introduced in 2013.
Study selection criteria
Studies identified in the above databases were screened, and after the removal of duplicates, all references were further screened according to the title and abstract of the reference, with the following screening criteria: (1) all patients >18 years of age, placed under general anesthesia and undergoing studies involving thoracic and breast surgery; (2) use of ultrasound-guided SAPB for postoperative analgesia; (3) controls consisting of studies with no intervention, sham block, or incision infiltration; (4) studies reporting opioid consumption, postoperative visual analog scale (VAS) or numeric rating scale (NRS) assessment of pain scores; (5) study design: RCTs. Studies were excluded in the following cases: (1) letters, case reports, reviews, technical reports; (2) animal trials or studies involving body anatomy; (3) unavailable outcome data and authors who could not be contacted, (4) articles devoted to other types of regional blocks; (5) trials that were unpublished or ongoing, or were reported in conference abstracts only. All published full-text clinical RCTs were eligible for inclusion without language restrictions. Subsequently, full-text manuscripts of eligible studies were reviewed for inclusion, and the articles were independently assessed for inclusion by two authors (WFZ and YTW).
Data extraction
Two different authors checked the authenticity of the article titles and abstracts and carefully assessed the full texts to ensure that the articles met the eligibility criteria for this study, and any disagreements in opinions were resolved and discussed with a third reviewer (WDL) after the search. The data collected included the first author's name, publication time, sample size, ASA classification, injection site of interventional SAPB and use of local anesthetics (type, volume and concentration of local anesthetic), interventions used in the control group, etc. Extracted primary outcome measures were as follows: pain scores at rest and during movement 1, 2, 4, 6, 8, 12, 24, and 48 h after surgery in both groups. Secondary outcome measures were as follows: intraoperative opioid consumption (morphine equivalent), 24 h postoperative opioid consumption (morphine equivalent), time to first use of analgesics, number of patients requiring emergency additional analgesics, opioid complications (postoperative nausea, vomiting, respiratory depression, constipation, dizziness, sedation), length of hospital stay and the incidence of postoperative pain syndrome at 3 months. To facilitate meta-analysis, the Australian and New Zealand College of Anesthetists Opioid Calculator was used to convert various opioid doses to analgesic doses, such as oral morphine equivalents, and to normalize the doses analyzed [24, 25]. For incomplete data, the reviewer attempted to contact the author of the original article by email to request additional and complete data, and if the data values were represented in a graph format, the numerical data were extracted from the graph using WebPlotDigitizer [26]. If the data were presented as the median and quartile, Hozo's validation formula was used to convert the data to the mean and standard deviation [27]. The included studies assessed pain scores using the VAS or NRS, and the results were converted to a 0–10 scale for statistical evaluation.
Quality assessment
Methodological quality assessments were assessed independently by two authors, and any disagreements were resolved by a third author using the Cochrane risk of bias tool and Jadad scores. The Cochrane risk of bias tool provides descriptions, comments, and a judgment of “high”, “unclear” or “low” risk of bias for each included study: random sequence generation; allocation concealment; double-blind, outcome-assessed blinding; incomplete outcome data; selective outcome reporting; and other biases [28]. Each study was analyzed independently by two reviewers and was divided into three groups: low risk, unclear risk, and high risk. Studies with a high risk of bias in any one or more key areas were considered to be at high risk of bias. Studies with a low risk of bias in all key areas were considered to have a low risk of bias. Otherwise, they were considered to have unclear bias risks. The Jadad score (total 7 points) uses a score based on appropriate randomization (0–2), allocation concealment (0–2), double-blinding (0–2), and possible withdrawal (0-1) standards [29]. We considered studies to be moderate to high quality if they scored 3 or higher on these criteria.
Statistical analysis
We performed meta-analysis using Review Manager (version 5.3; the Nordic Cochrane Centre, the Cochrane Collaboration, Copenhagen, Denmark, 2014). Standardized mean differences (SMDs) and corresponding 95% confidence intervals (CIs) were calculated for continuous data using a random-effects model, while risk ratios (RRs) for dichotomous data were analyzed using the Mantel–Haenszel method with 95% confidence intervals. We calculated the I2 statistic to assess heterogeneity, and an I2 value >50% was considered the cutoff point for significant heterogeneity. Where significant heterogeneity was observed, a random-effects model was used; otherwise, a fixed-effects model was used. Sensitivity analyses were performed by the leave-one-out method to assess whether the results varied significantly. Subgroup analyses were also used to assess heterogeneity. Potential publication bias was determined by funnel plots. A P value < 0.05 with 95% CI was considered statistically significant.
Results
Results of the literature search
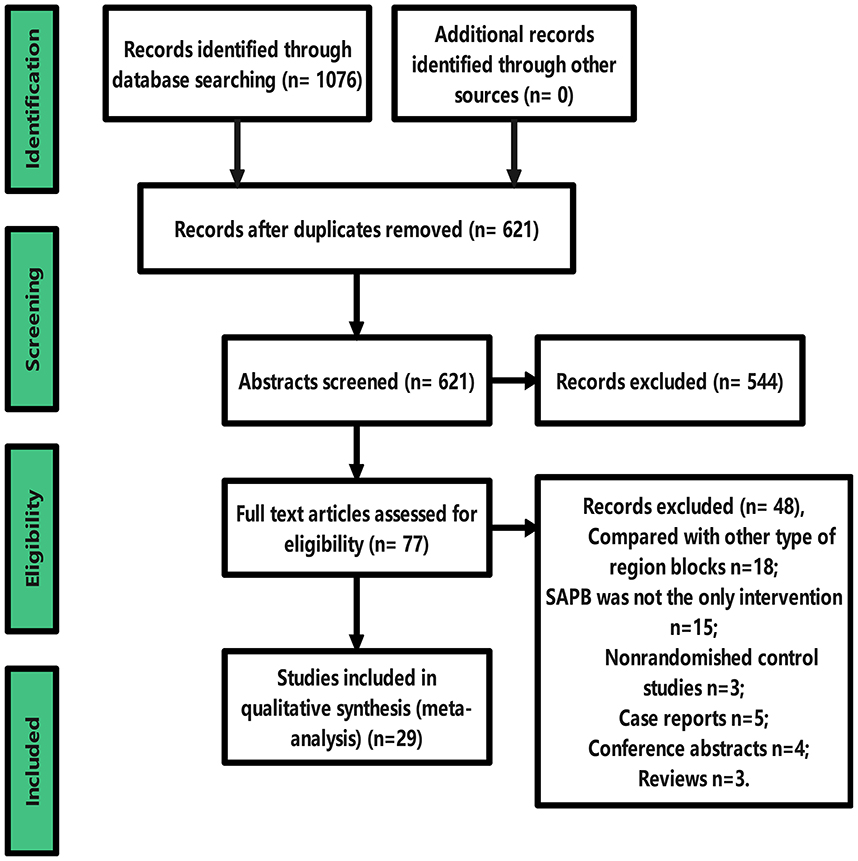
Our study selection process is shown in the PRISMA flow chart (Figure 1). The literature search initially retrieved 1076 studies of SAPB related to thoracic and breast surgery; 621 remained after excluding duplicates, and 77 remained after reviewing titles and abstracts after careful reading of the full text. Twenty-nine articles were finally included for systematic review and meta-analysis [4, 5, 14–16, 22, 30–52]. Forty-eight studies were excluded, including 5 case reports, 4 conference abstracts, 3 reviews, 3 nonrandomized controlled studies, 18 with SAPB compared to other types of regional blocks, and 15 in which SAPB was not the only intervention.
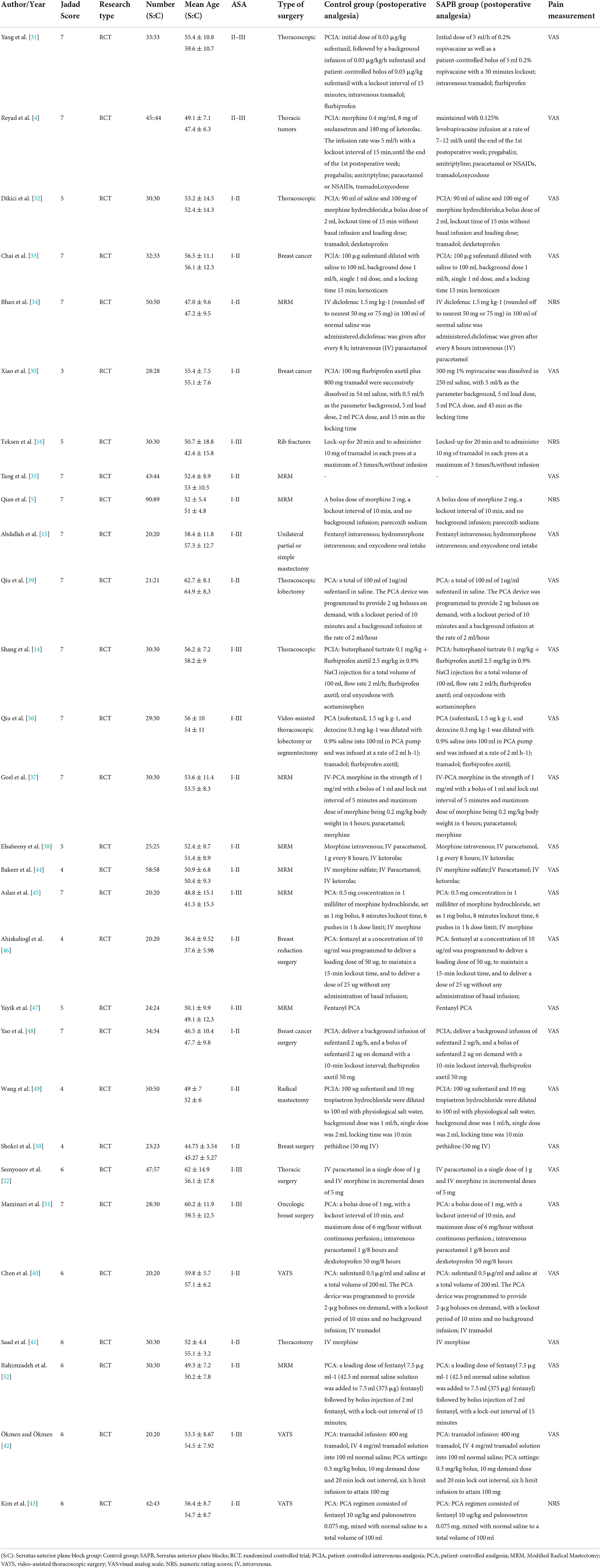
We analyzed 29 studies involving thoracic and breast surgery with a total of 1,978 patients, of whom 982 patients were randomly assigned to the SAPB group and the remaining 996 to the control group. Surgical procedures performed in 29 studies included 12 thoracic surgeries [4, 14, 16, 22, 31, 32, 36, 39–43] and 17 breast surgeries [5, 15, 30, 33–35, 37, 38, 44–52]. Most studies accepted single-segment techniques at the level of the T4 or T5 vertebral body, with only two studies using a catheter placed and connected to a patient-controlled device for continuous peripheral nerve block [4, 31]. In 4 studies, the control group used local incision infiltration [14, 32, 40, 50]; in 5 studies, the control group received no intervention. The level of SAPB blocking was deep in the serratus anterior in 10 studies [4, 5, 15, 31, 34, 38, 42, 44, 50, 51] and in the superficial serratus anterior in 16 studies [14, 16, 30, 33, 35–37, 39–41, 43, 45–49], and one used double-point injection (superficial + deep) [32]. In addition, 25 studies used VAS for pain scores [4, 14, 15, 22, 30–33, 35–42, 44–52], and 4 studies used NRS [5, 16, 34, 43]. All studies were identified as moderate to high quality according to the Jadad score (Table 1). Details of all studies included in this meta-analysis are shown in Tables 1, 2.
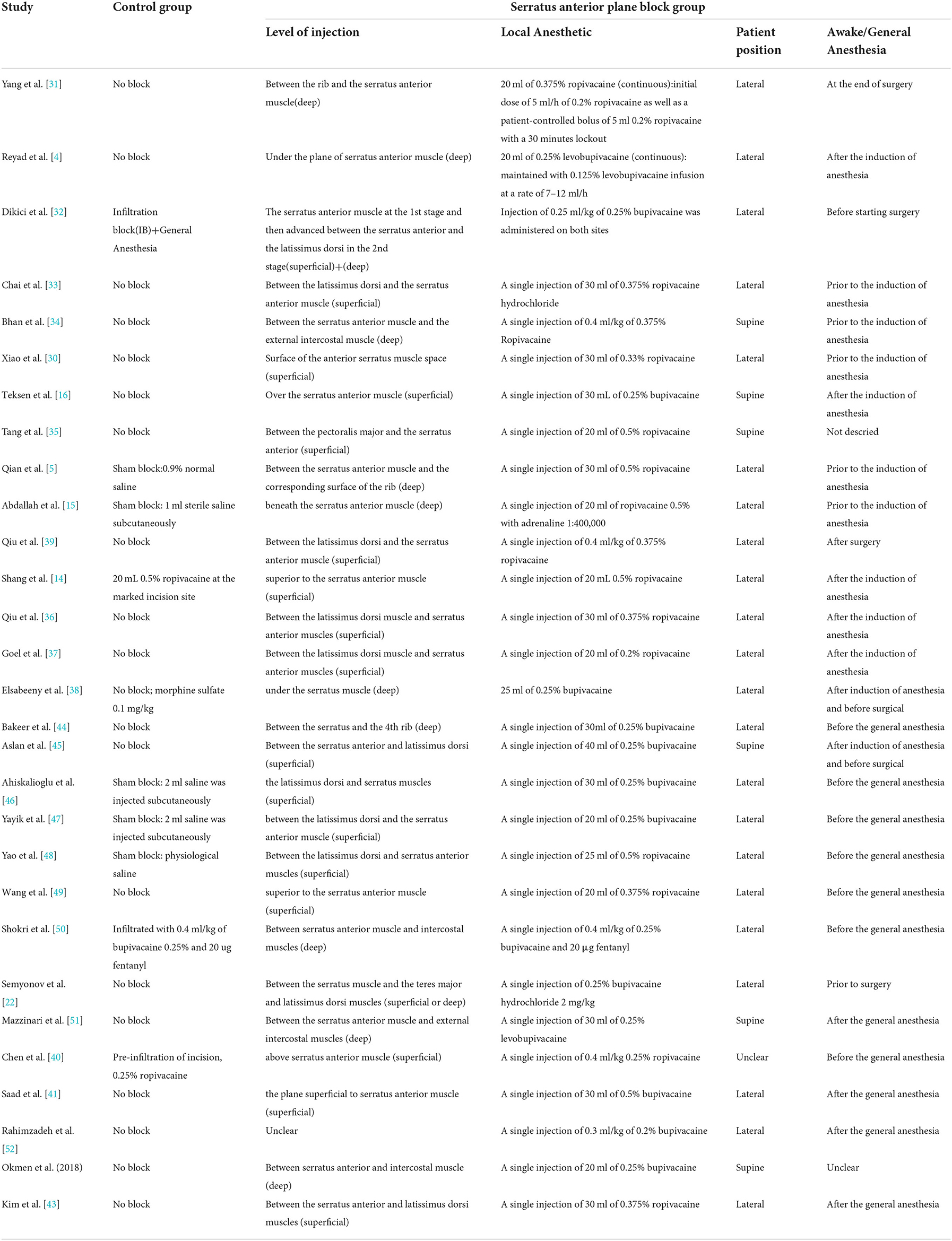
Table 2. Block characteristics of included studies.(between latissimus dorsi and serratus anterior (superficial plane) or between serratus anterior and intercostal muscles (deep plane).
Assessment of methodological quality
The risk assessment of the included studies is shown in Figure 2. All included studies in the analysis were clinical RCTs, 10 studies were judged as having a low risk of bias [5, 14, 15, 22, 31, 33, 35, 36, 46, 51], 3 studies were judged as having a high risk of bias [30, 34, 50], and the remaining 16 studies were judged to have an unclear risk of bias. Additionally, 4 studies did not provide enough information about allocation concealment [16, 30, 47, 49]. In terms of participant and personnel blinding, 3 studies were not blinded [30, 34, 50], and 13 were not described [4, 16, 32, 37–42, 44, 45, 49, 52]. Among the result-blinding methods, one item was not blinded [49], and 7 studies did not specify whether they have blinded [16, 30, 32, 38, 45, 47, 49]. All studies were not selectively reported. There were 3 studies with other bias [42, 43, 49]. Overall, the quality of the included studies was good.
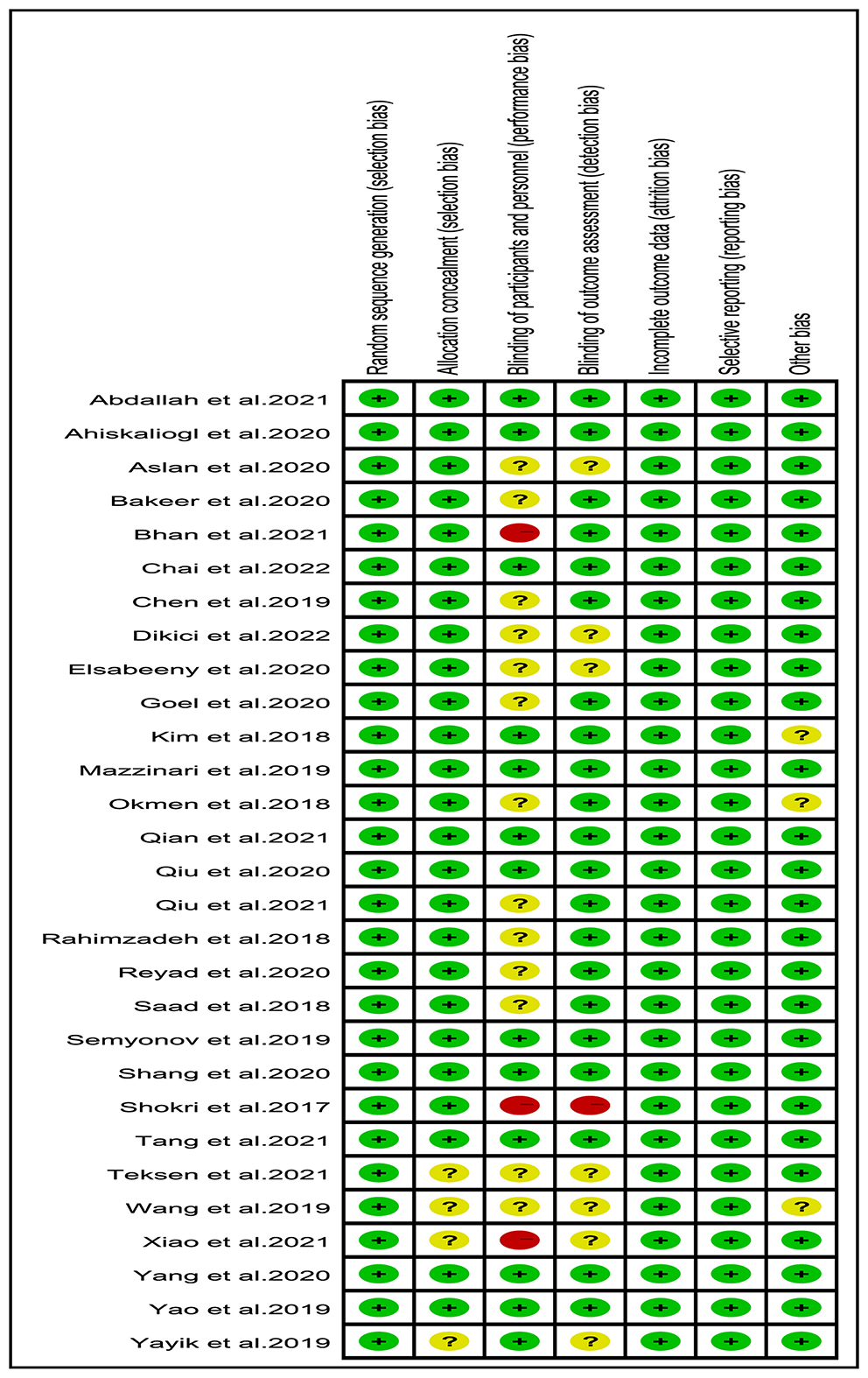
Figure 2. Risk of bias summary: authors' judgments about each risk of bias item for each included study. Green, red, and yellow circles indicate low, high, and unclear risk of bias, respectively.
Study outcomes
Postoperative pain scores
Of all studies, 12 analyzed postoperative resting pain scores in patients undergoing thoracic or breast surgery [4, 5, 31, 32, 34–37, 40, 44, 47, 49]. Compared with the control group, the SAPB group had significantly lower resting pain scores at different time points except for 6 h postoperatively (Figures 3, 4), indicated as follows: at 1 h (SMD, −1.54; 95% CI, −2.14 to −0.94; P < 0.01; I2 = 90%); at 2 h (SMD, −1.57; 95% CI, −2.32 to −0.82; P < 0.01; I2 = 92%); at 4 h (SMD, −1.43; 95% CI, −2.18 to −0.67; P < 0.01; I2 = 93%); at 6 h (SMD, −0.91; 95% CI, −2.14 to 0.33; P = 0.15; I2 = 92%); at 8 h (SMD, −1.46; 95% CI, −2.06 to −0.86; P < 0.01; I2 = 91%); at 12 h (SMD, −0.32; 95% CI, −0.46 to −0.17; P < 0.01; I2 = 5%); at 24 h (SMD, −0.80; 95% CI, −1.31 to −0.28; P < 0.01; I2 = 91%); and at 48 h (SMD, −0.49; 95% CI, −0.91 to −0.08; P < 0.05; I2 = 56%). No significant difference was shown 6 h after surgery, probably due to the limited number of included studies. Additionally, 24 studies analyzed postoperative movement or cough pain scores with the use of SAPB in patients undergoing thoracic or breast surgery [4, 5, 15, 16, 30–38]. Meta-analysis showed that compared with the control group, the SAPB group showed significantly reduced movement or cough pain scores at different postoperative time points, except for 48 h postoperatively (Figures 5–7), as follows: 1 h (SMD, −1.25; 95% CI, −1.76 to – 0.74; P < 0.01; I2 = 91%); 2 h (SMD, −1.33; 95% CI, −1.67 to −0.98; P < 0.01; I2 = 81%); 4 h (SMD, −1.37; 95% CI, −1.84 to −0.91; P < 0.01; I2 = 88%); 6 h (SMD, −1.04; 95% CI, −1.43 to −0.65; P < 0.01; I2 = 78%); 8 h (SMD, −1.18; 95% CI, −1.57 to −0.80; P < 0.01; I2 = 84%): 12 h (SMD, −0.76; 95% CI, −1.01 to −0.51; P < 0.01; I2 = 81%); 24 h (SMD, −0.71; 95% CI, −0.97 to −0.45; P < 0.01; I2 = 84%); and 48 h (SMD, −0.38; 95% CI, −0.84 to 0.08; P = 0.1; I2 = 80%).
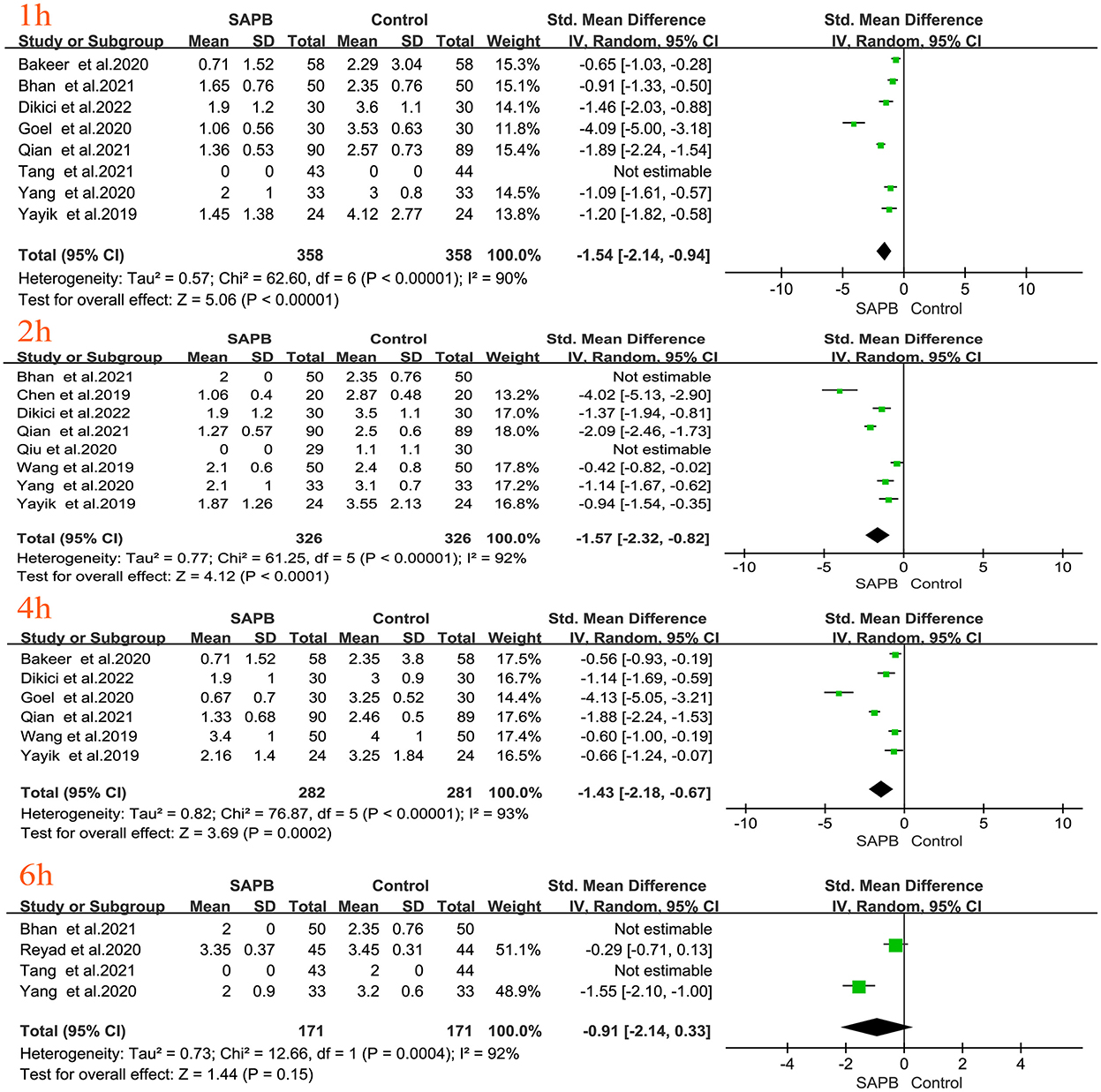
Figure 3. Forest plot of VAS pain scores at rest in the serratus anterior plane block group vs. the nonblock group at 1 h to 6 h after surgery. VAS, visual analog scale; SAPB, serratus anterior plane block; h, hour.
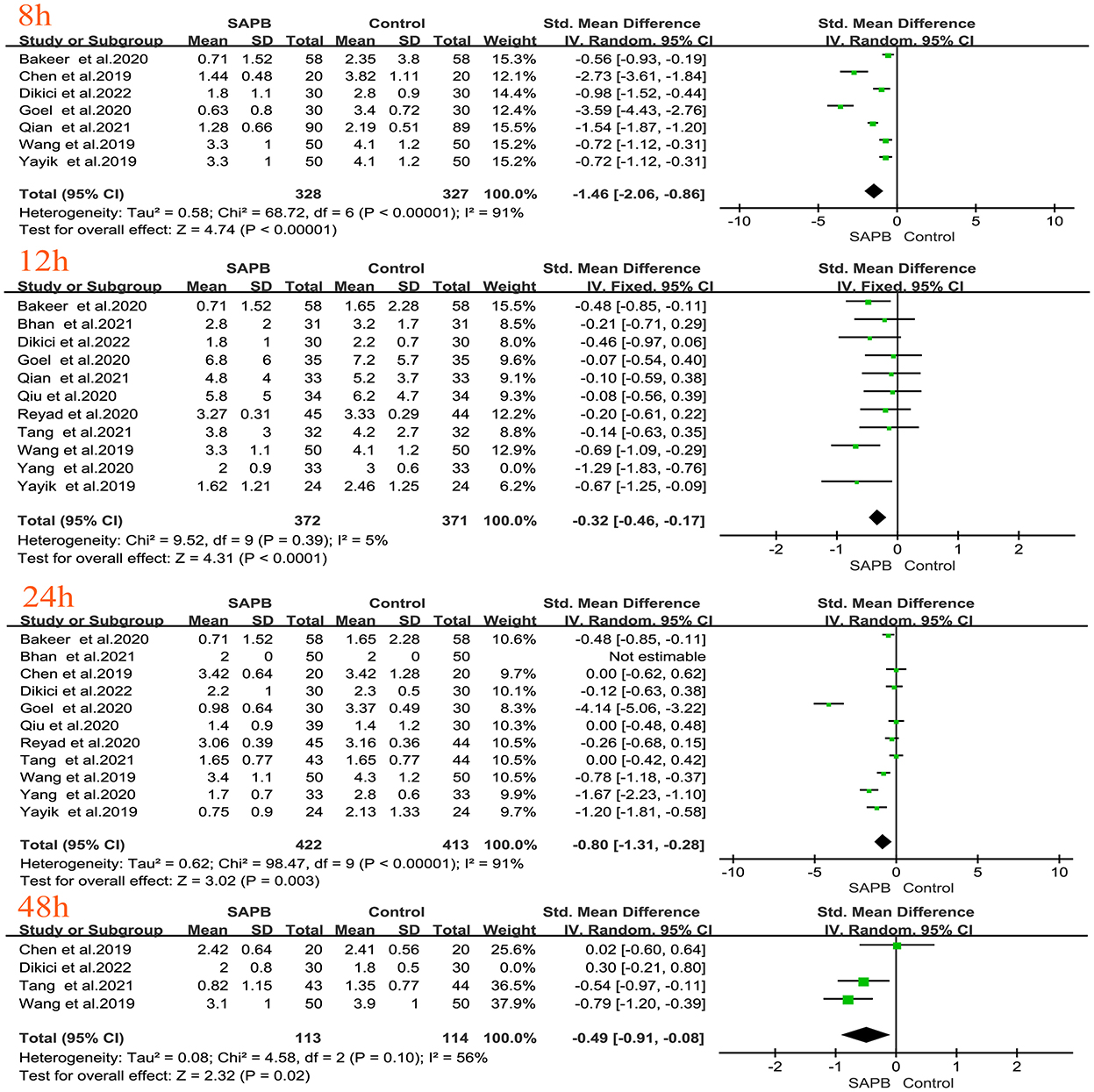
Figure 4. Forest plot of VAS pain scores at rest in the serratus anterior plane block group vs. the nonblock group at 8–48 h after surgery. VAS, visual analog scale; SAPB, serratus anterior plane block; h, hour.
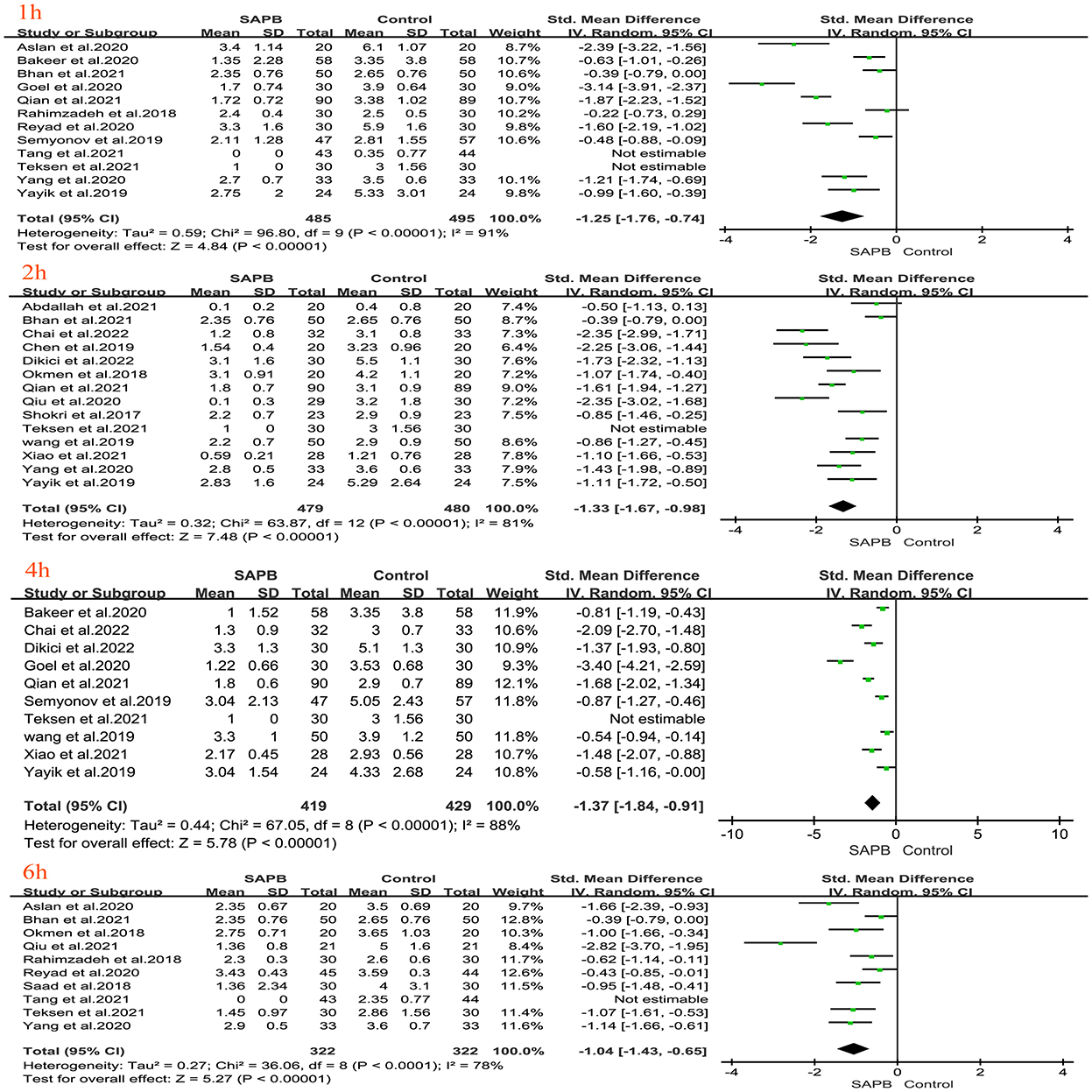
Figure 5. Forest plot of VAS pain scores at movement in the serratus anterior plane block group vs. the nonblock group at 1 h to 6 h after surgery. VAS, visual analog scale; SAPB, serratus anterior plane block; h, hour.
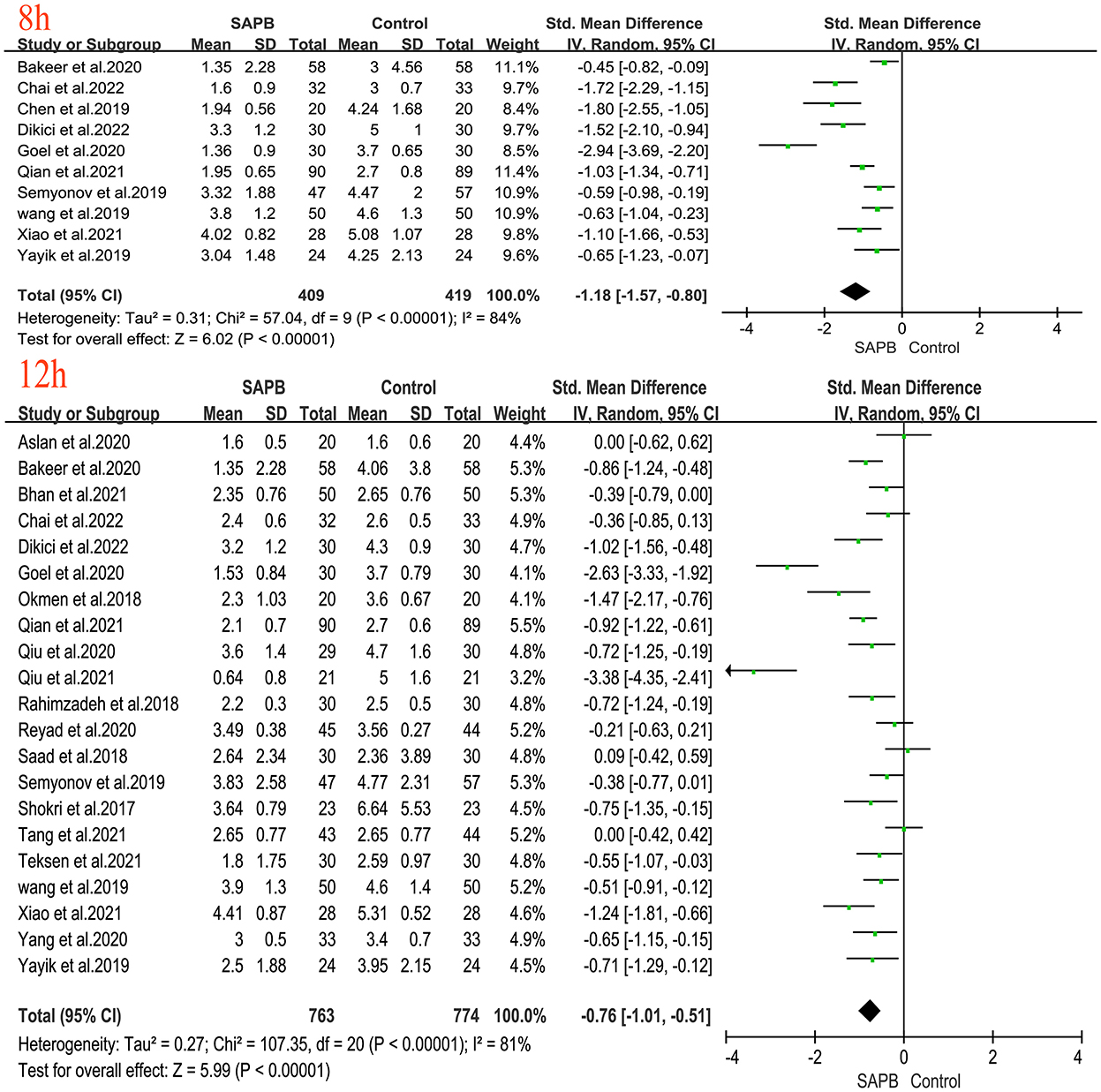
Figure 6. Forest plot of VAS pain scores at movement in the serratus anterior plane block group vs. the nonblock group at 8–12 h after surgery. VAS, visual analog scale; SAPB, serratus anterior plane block; h, hour.
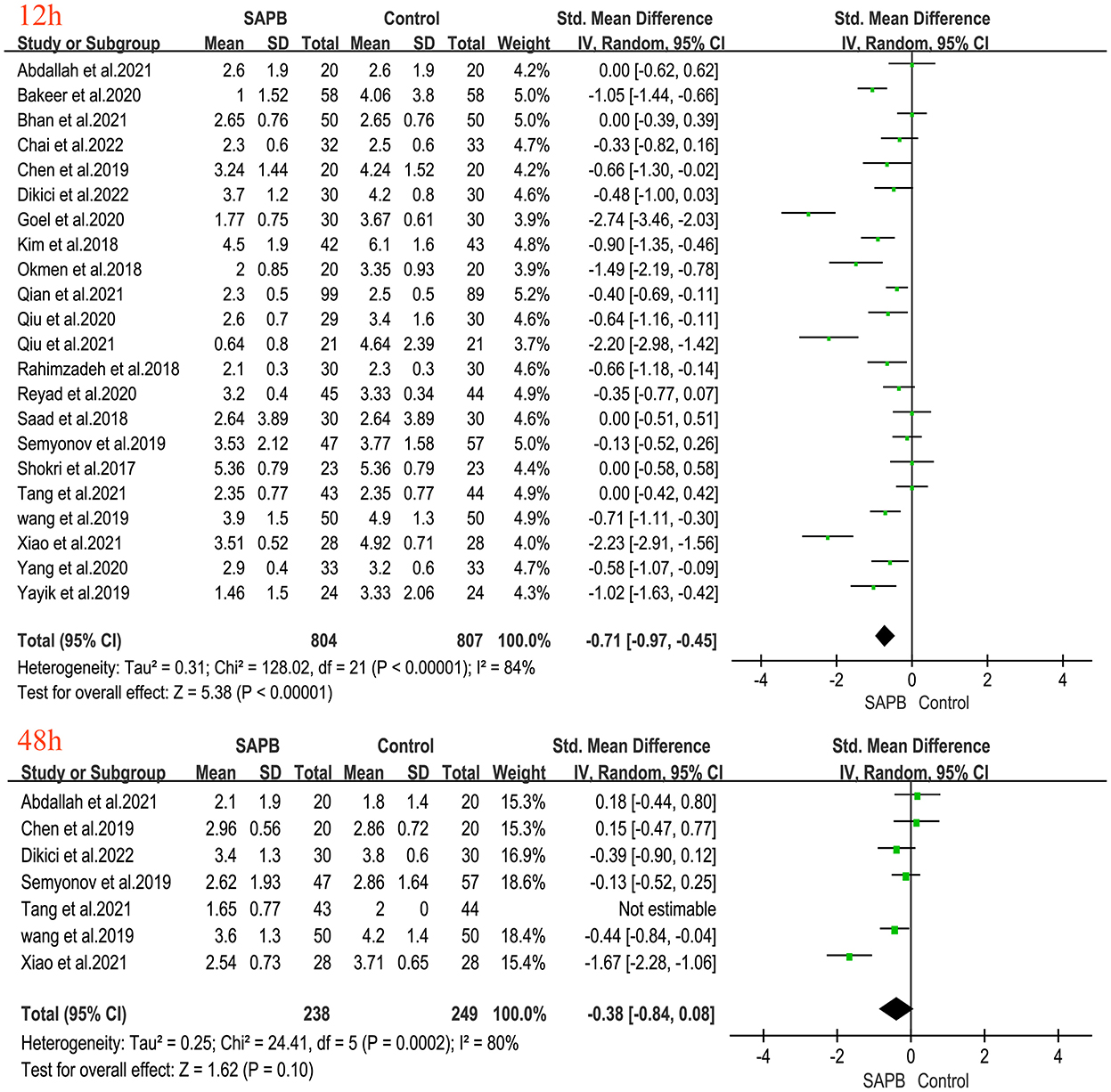
Figure 7. Forest plot of VAS pain scores at movement in the serratus anterior plane block group vs. the nonblock group at 24 h to 48 h after surgery. VAS, visual analog scale; SAPB, serratus anterior plane block; h, hour.
Postoperative opioid consumption at 24h
Nineteen studies reported 24 h postoperative opioid consumption in patients undergoing thoracic or breast surgery [5, 15, 16, 32, 33, 36–38, 45–48]. Meta-analysis showed that SAPB significantly reduced 24 h postoperative opioid consumption compared with the control group (Figure 8) (SMD, −2.77; 95% CI, −3.56 to −1.97; P < 0.01; I2 = 97%). We performed subgroup analyses, thoracic surgery (SMD, −0.98; 95% CI, −1.40 to −0.55; P < 0.01; I2 = 75%) and breast surgery (SMD, −3.79; 95% CI, −5.01 to −2.56; P < 0.01; I2 = 97%), and the results of the subgroup analysis were consistent.
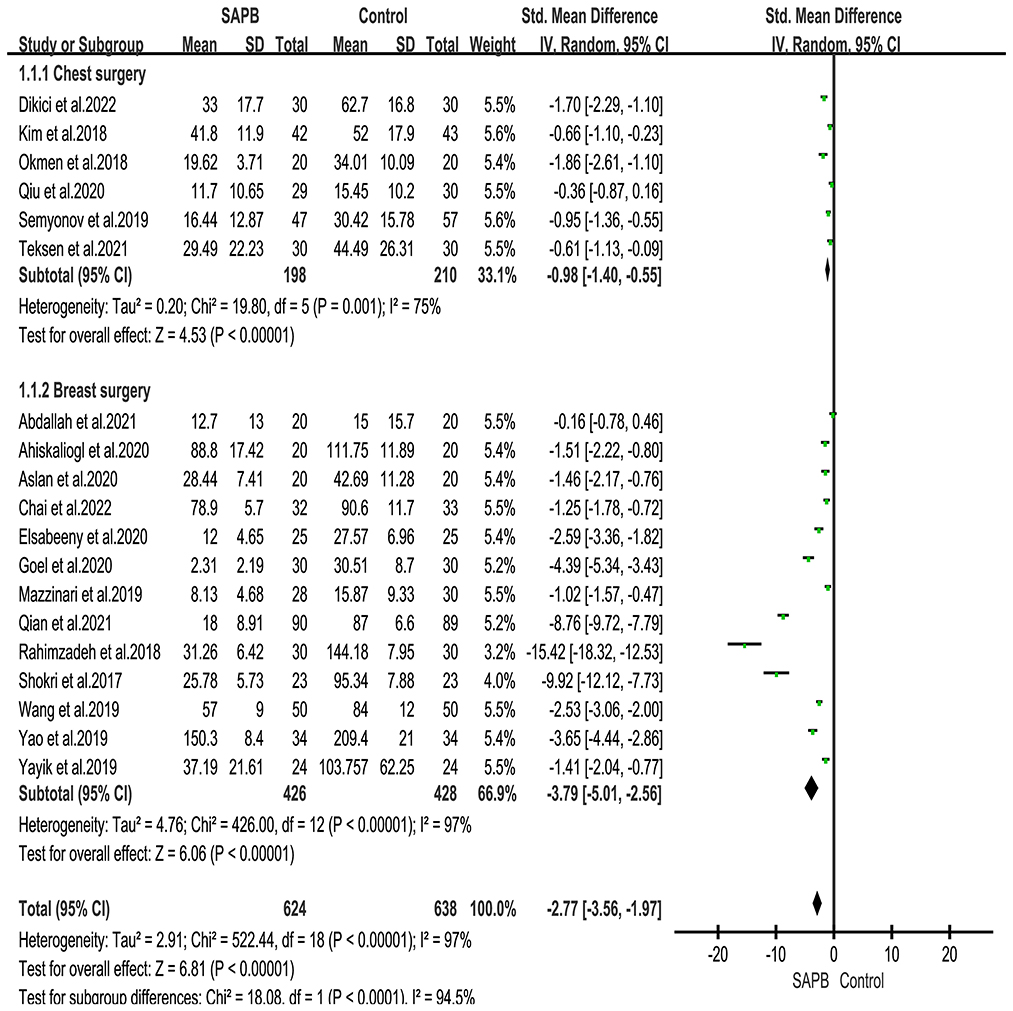
Figure 8. Forest plot for the comparison of oral morphine equivalents (mg) in the first 24 h after surgery. SAPB, serratus anterior plane block; h,hour.
Intraoperative opioid consumption
Seventeen studies reported intraoperative opioid consumption in patients undergoing thoracic or breast surgery [5, 14, 15, 30, 32, 33, 35, 36, 38, 39, 44, 49]. Meta-analysis showed that SAPB significantly reduced intraoperative opioid consumption compared with that in the control group (Supplementary Figure 1) (SMD, −0.66; 95% CI, −1.03 to −0.28; P < 0.01; I2 = 90%). We performed subgroup analyses of thoracic surgery (SMD, −0.37; 95% CI, −0.73 to −0.00; P = 0.05; I2 = 76%) and breast surgery (SMD, −0.91; 95% CI, −1.46 to −0.36; P < 0.01; I2 = 92%). The subgroup analysis results showed that there was no significant difference in intraoperative opioid consumption after SAPB in patients undergoing thoracic surgery, but the overall results showed that there was a significant difference between the two groups.
Number of patients requiring urgent additional analgesia after surgery
Thirteen studies including 781 patients [14, 31, 32, 34, 36, 38, 39, 42, 44–47, 52] reported the number of patients who required emergency additional analgesia after surgery for thoracic or breast surgery. Meta-analysis showed that SAPB significantly reduced the number of patients requiring rescue analgesia compared with the control group (Supplementary Figure 2) (RR, 0.34; 95% CI, 0.27 to 0.42; P < 0.01; I2 = 0). We performed subgroup analyses, including thoracic surgery (RR, 0.30; 95% CI, 0.21–0.44; P < 0.01; I2 = 17%) and breast surgery (RR, 0.36; 95% CI, 0.27–0.48; P < 0.01; I2 = 0), and the subgroup analysis results were consistent.
Time to first use of analgesics
Eleven studies including 685 patients [31, 32, 34, 36, 38, 44–47, 50, 52] reported the time to first use of analgesics in patients undergoing thoracic or breast surgery. Meta-analysis revealed that SAPB significantly prolonged the time to first use of analgesics compared with the corresponding time in controls (Supplementary Figure 3) (SMD, 3.49; 95% CI, 2.23 to 4.74; P < 0.01; I2 = 97%). We performed subgroup analyses, thoracic surgery (SMD, 0.80; 95% CI, 0.23 to 1.38; P < 0.01; I2 = 72%) and breast surgery (SMD, 4.65; 95% CI, 2.67 to 6.63; P < 0.01; I2 = 98%), and the subgroup analysis results were consistent.
Side effects of opioids and block-related complications
Twenty-one studies reported postoperative nausea and vomiting (PONV) [4, 5, 14–16, 22, 30–34, 36, 39, 40, 42, 43, 45, 46, 48–50]. Forest plots showed a significantly lower incidence of PONV in the SAPB group (as shown in Supplementary Figure 4) (RR, 0.43; 95% CI, 0.34–0.54; P < 0.01; I2 = 16%). We performed subgroup analyses, including thoracic surgery (RR, 0.47; 95% CI, 0.34–0.65; P < 0.01; I2 = 41%) and breast surgery (RR, 0.40; 95% CI, 0.29–0.55; P < 0.01; I2 = 0), and the subgroup analysis results were consistent. In addition, the incidence of respiratory depression was reported in three studies [31, 32, 42]. The forest plot showed no significant difference between the two groups (as shown in Supplementary Figure 4) (RR, 0.25; 95% CI, 0.05 to 1.14; P = 0.07; I2 = 0); the incidence of constipation was reported in three studies [4, 32, 45]. The forest plot showed a significant difference between the two groups (as shown in Supplementary Figure 5) (RR, 0.12; 95% CI, 0.03–0.52; P < 0.01; I2 = 0); the incidence of dizziness was reported in three studies [5, 43, 48]. The forest plot showed a significant difference between the two groups (as shown in Supplementary Figure 5) (RR, 0.24; 95% CI, 0.06–0.92; P < 0.05; I2 = 0); the incidence of sedation was reported in two studies [4, 32]. The forest plot showed a significant difference between the two groups (as shown in Supplementary Figure 5) (RR, 0.07; 95% CI, 0.01–0.52; P < 0.01; I2 = 0). In the trials included in this analysis, complications related to SAPB (e g., pleural puncture, pneumothorax, local anesthesia toxicity, or puncture site infection) were not reported.
Length of hospital stay and the incidence of postoperative pain syndrome at 3 months
Five studies reported the length of hospital stay [4, 5, 31, 34, 40]. The forest plot showed that the SAPB group have a significantly shortened length of hospital stay, and there was no heterogeneity between the two groups (as shown in Supplementary Figure 6) (SMD, −0.28; 95% CI, −0.46 to −0.09; P < 0.01; I2 = 0).Two studies reported the incidence of postoperative pain syndrome at 3 months [4, 33].The forest plot showed that the SAPB group have a significantly reduced the incidence of postoperative pain syndrome at 3 months (as shown in Supplementary Figure 7).
Sensitivity analysis and subgroup
We performed sensitivity analyses by one-by-one exclusion. For the resting pain score, heterogeneity decreased after excluding one study (except at the 12 h time point), and the heterogeneity of the rest and movement pain scores remained high at the other time points. In addition, the heterogeneity of intraoperative opioid consumption, 24 h postoperative opioid consumption, and time to first use of analgesics did not change significantly after one-by-one exclusion and subgroup analysis. Some publication bias was found among the included studies by visual inspection of the funnel plot (Supplementary Figure 8).
Discussion
This systematic review and meta-analysis demonstrates the potential postoperative analgesic effect of SAPB in patients after thoracic and breast surgery. The results of the analysis showed that compared with the control group, first, the SAPB group was able to obtain a significantly reducd pain score (VAS/NRS) within 48 h after the operation, except for 48 h postoperatively at movement, second, the SAPB group was able to obtain a significantly reduced the incidence of postoperative pain syndrome at 3 months. In addition, SAPB significantly reduced the consumption of intraoperative and postoperative opioids and the need for emergency additional analgesia after surgery; and significantly prolonged the time of first use of analgesics and shortened the length of hospitalization of patients. In terms of side effects of opioids, there was no statistically significant difference in the incidence of postoperative respiratory depression, but in the incidence of PONV, constipation, dizziness and sedation, SAPB can reduce the incidence of side effects of opioids. There was no incidence of SAPB-related complications (e g., pleural puncture, pneumothorax, local anesthesia toxicity, or puncture site infection) in all trials. Our results are similar to those of Chong et al. [53]. SAPB can reduce patients' pain scores and reduce intraoperative and postoperative opioids consumption, and this block appeared safe with no study reporting any block- related complications. Compared to Chong et al. 's article, we mainly compared SAPB to non-block care for postoperative analgesia, And we collected postoperative pain scores at 1, 2, 4, 6, 8, 12, 24, and 48 h and more information on opioid adverse reactions. These results confirm that ultrasound-guided SAPB can provide safe and effective postoperative analgesia for patients undergoing thoracic and breast surgery.
In recent years, with the development of multimodal analgesia, several strategies, including regional and peripheral nerve blocks, may reduce postoperative pain. As a new analgesic method, SAPB was first used to block the lateral cutaneous branch of the thoracic intercostal nerve in breast cancer surgery in 2013 and achieved a satisfactory regional block effect [13]. Anatomically, there may be two potential gaps on the surface of the serratus anterior, the superficial serratus anterior plane between the serratus anterior and the latissimus dorsi and the deep serratus anterior plane between the serratus anterior and the intercostal muscles. Intercostal nerve blockage achieved by the operator injecting a dose of local anesthetic in these two planes provides effective analgesia and reduces the surgical stress response as well as postoperative chest wall pain [34]. The mechanism of postoperative pain is currently unclear and may be related to persistent inflammation during surgery (through the release of histamine, prostaglandins, bradykinin, and cytokines) and injury-induced peripheral nerve sensitization [54]. Preoperative SAPB can significantly reduce postoperative pain by blocking the transmission of noxious stimuli, reducing the risk of central and peripheral nerve sensitization, and reducing the release of inflammatory pain-causing factors in the blocked area [55]. Ultrasound-guided SAPB is a promising mode of postoperative analgesia due to its obvious anatomical and bony landmark positioning under ultrasound and easy operation under ultrasound guidance [56].
In addition, as a new regional nerve block technology, the specific effect of SAPB in various surgeries is still being explored. Different types of surgery also have different effects on patients' postoperative pain and postoperative recovery. For example, most lobectomy procedures are performed thoracoscopically, which is associated with lower pain intensity and shorter recovery times than traditional thoracotomy [19, 40]. There have been relevant meta-analyses comparing the effectiveness of SAPB in different surgical types. For example, a meta-analysis by Zhang et al. [57] compared anterior serratus muscle block combined with general anesthesia for perioperative analgesia in patients undergoing video-assisted thoracoscopic surgery (VATS). The results showed that ultrasound-guided SAPB reduced postoperative pain scores after VATS and analgesic consumption after general anesthesia. The meta-analysis by Liu et al. [58] evaluated the analgesic effect of SAPB after thoracotomy. The results also showed that SAPB can reduce postoperative pain scores and opioid consumption at 24 h after surgery, and reduce the incidence of PONV after surgery. Regarding breast surgery, a meta-analysis by Hu et al. showed that SAPB can reduce opioid consumption, relieve pain after breast surgery, and decrease the incidence of PONV to a certain extent [53, 59]. Although the above studies have shown that SAPB can produce certain analgesic effects in different types of surgery, due to the prevalence of thoracic and breast surgery and the high heterogeneity between studies, it is necessary to further consider the effect of SAPB on postoperative pain in different types of surgery. The results of our meta-analysis suggest that SAPB can provide good analgesia and reduce intraoperative and postoperative opioid consumption in both thoracic and breast surgery. Studies have shown that common side effects of opioids include dizziness, PONV, constipation, and respiratory depression. Opioid-induced respiratory depression is due to activation of mu-opioid receptors, which are expressed on respiratory control neurons in the brainstem [60, 61]. In addition, the meta-analysis results showed that the incidence of PONV was significantly lower in patients treated with SAPB. This may have been due to less opioid use after surgery. Effective prevention of PONV can not only promote postoperative recovery but also shorten the discharge time of patients [55].
Although strict inclusion and exclusion criteria were used to standardized the included studies, this meta-analysis was highly heterogeneous. In terms of sensitivity analysis, analysis by omitting one study or subgroup analysis did not change the final conclusions of the combined analysis. The main reasons for the heterogeneity may include the following: First, different anesthesiologists skilled in the use of SAPB may achieve different analgesic effects, and this evidence comes from the study itself. Second, there were different levels of local anesthetic block; different concentrations, different volumes, and different types of local anesthetics; and different pain thresholds of patients. The types and optimal doses of local anesthetics in SAPB for thoracic and breast surgery are currently unknown, and well-designed RCTs are still needed. Third, we found that the higher heterogeneity was mainly in the pain score, which may be related to the inconsistency of the pain score scale, as well as the type and duration of surgery. Finally, in terms of opioid consumption, despite the eventual switch to equivalent doses of oral morphine, the use of analgesics for postoperative analgesia in each study was inconsistent, and some studies used nonsteroidal anti-inflammatory drugs such as paracetamol to supplement analgesics, which makes comparing opioids across trials more difficult and may also lead to significant heterogeneity.
Limitations
Although the above mentioned factors inevitably present heterogeneity in the above mentioned results, our results still support the good analgesic effect of SAPB in thoracic and breast surgery. However, our meta-analysis has other potential limitations. First, we did not perform other subgroup analyses, such as different procedures (e.g., lung resection, thoracic trauma, etc) and evaluation of the effect of different local anesthetics and analysis of the depth of block. Second, all included studies were performed only on thoracic and breast surgery, not all surgical procedures, and consequently selection bias may have overestimated the analgesic effect of SAPB. Third, we did not compare SAPB with other regional anesthesia techniques and cannot reflect the analgesic effect of SAPB in other regional blocks. Fourth, most of the literature consisted of small sample sizes, so it is necessary to expand the sample size to make the evidence more robust. Finally, A considerable number of included studies did not report other secondary outcomes, such as SAPB and opioid side effects (time to first exhaust, dizziness, postoperative delirium, etc.), duration of postanesthesia care unit (PACU) stay, and length of hospital stay. In addition, the incidence of postoperative pulmonary complications (e.g., pneumonia, atelectasis, respiratory failure) and mortality, and the incidence of postoperative pain syndrome (e.g., 3, 6 months or more) are very important, future studies should also focus on the incidence of pulmonary complications and mortality and postoperative pain syndromes. In the process of data collection, we did not collect pulmonary complications and mortality, which are critical to the prognosis of patients. Only 2 studies reported the incidence of postoperative pain syndrome. Acute postoperative pain is not controlled, and this pain can easily turn into chronic pain. The mechanism may be that pain leads to peripheral sensitization, which leads to abnormal spinal cord regulation, leading to central sensitization and further pain [55, 62]. Therefore, future studies should conduct more analyses of SAPB, which will provide a further basis for developing guidelines for perioperative pain management.
In contrast to these limitations, our systematic review has several strengths. First, we chose an important primary outcome (VAS pain scores). Our VAS pain scores included more time points, including 48 h after surgery, which would be more conducive to observe the pain degree of patients after surgery. Second, we also collected other adverse reactions to opioids, including constipation, dizziness, and sedation. These make our systematic review more comprehensive.
Conclusion
In conclusion, our systematic review and meta-analysis showed that SAPB has shown promise as a beneficial pain-relief technique. Compared with no block, ultrasound-guided SAPB provided better postoperative pain control by reducing VAS or NRS pain scores and reducing perioperative opioid consumption in patients following thoracic and breast surgery. At the same time, SAPB reduced the incidence of side effects of opioids and shortened the length of hospital stay. SAPB can be used as a feasible technique for multimodal analgesia in the postoperative perioperative period. However, due to the high degree of heterogeneity among studies, our results should be interpreted with caution, and more large-scale and high-quality RCTs are needed to validate and strengthen our results in the future.
Author contributions
WFZ, SHH, and WDL designed the meta-analysis and independently evaluated the methodological quality of included studies. MLZ, RPZ, and QHL performed the screening process for titles and abstracts, while ZGQ, RGL, YFW, and JLL performed the screening process for full texts. WFZ and YTW supervised the acquisition and extracted data. WFZ and WDL conducted the statistical analysis of data. WFZ wrote the manuscript. All authors contributed to the article and approved the submitted manuscript.
Funding
This work was supported by the Science and Technology Project of Jiangxi Provincial Health Commission, No. 202210876.
Acknowledgments
We would like to thank American Journal Experts (AJE) for his help with language-editing.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fanes.2022.980483/full#supplementary-material
Supplementary Figure 1. Forest plot for the comparison of oral morphine equivalents (mg) in the intraoperative opioid consumption. SAPB, serratus anterior plane block.
Supplementary Figure 2. Forest plot of the number of patients requiring rescue analgesia after surgery in the serratus anterior plane block group vs. the nonblock group. SAPB, serratus anterior plane block.
Supplementary Figure 3. Forest plot of the time to first use of analgesics in the serratus anterior plane block group vs. the nonblock group. SAPB, serratus anterior plane block.
Supplementary Figure 4. Forest plots showing the serratus anterior plane block-related side effects of opioids (nausea and vomiting and respiratory depression). PONV, postoperative nausea and vomiting; SAPB, serratus anterior plane block.
Supplementary Figure 5. Forest plots showing the serratus anterior plane block-related side effects of opioids (constipation, dizziness and sedation). SAPB, serratus anterior plane block.
Supplementary Figure 6. Forest plot of the hospital length of stay in the serratus anterior plane block group vs. the nonblock group. SAPB, serratus anterior plane block.
Supplementary Figure 7. Forest plot of the incidence of postoperative pain syndrome at 3 months in the serratus anterior plane block group vs. the nonblock group. SAPB, serratus anterior plane block.
Supplementary Figure 8. Funnel plot (A) oral morphine equivalents (mg) in the first 24 h after surgery; (B) oral morphine equivalents (mg) in the intraoperative opioid consumption; (C) number of patients requiring rescue analgesia after surgery; (D) time to first use of analgesics; (E) incidence of postoperative nausea and vomiting.
References
1. Marshall K, McLaughlin K. Pain management in thoracic surgery. Thorac Surg Clin. (2020) 30:339–46. doi: 10.1016/j.thorsurg.2020.03.001
2. Tait RC, Zoberi K, Ferguson M, Levenhagen K, Luebbert RA, Rowland K, et al. Persistent post-mastectomy pain: risk factors and current approaches to treatment. J Pain. (2018) 19:1367–83. doi: 10.1016/j.jpain.2018.06.002
3. Bendixen M, Jørgensen OD, Kronborg C, Andersen C, Licht PB. Postoperative pain and quality of life after lobectomy via video-assisted thoracoscopic surgery or anterolateral thoracotomy for early stage lung cancer: a randomised controlled trial. Lancet Oncol. (2016) 17:836–44. doi: 10.1016/S1470-2045(16)00173-X
4. Reyad RM, Shaker EH, Ghobrial HZ, Abbas DN, Reyad EM, Abd Alrahman AAM, et al. The impact of ultrasound-guided continuous serratus anterior plane block vs. intravenous patient-controlled analgesia on the incidence and severity of post-thoracotomy pain syndrome: a randomized, controlled study. Eur J Pain. (2020) 24:159–70. doi: 10.1002/ejp.1473
5. Qian B, Huang S, Liao X, Wu J, Lin Q, Lin Y, et al. Serratus anterior plane block reduces the prevalence of chronic postsurgical pain after modified radical mastectomy: a randomized controlled trial. J Clin Anesth. (2021) 74:110410. doi: 10.1016/j.jclinane.2021.110410
6. Daoust R, Paquet J, Moore L, Gosselin S, Gélinas C, Rouleau DM, et al. Incidence and risk factors of long-term opioid use in elderly trauma patients. Ann Surg. (2018) 268:985–91. doi: 10.1097/SLA.0000000000002461
7. Chen JQ, Yang XL, Gu H, Chai XQ, Wang D. The role of serratus anterior plane block during in video-assisted thoracoscopic surgery. Pain Ther. (2021) 10:1051–66. doi: 10.1007/s40122-021-00322-4
8. D'Ercole F, Arora H, Kumar PA. Paravertebral block for thoracic surgery. J Cardiothorac Vasc Anesth. (2018) 32:915–27. doi: 10.1053/j.jvca.2017.10.003
9. Turhan Ö, Sivrikoz N, Sungur Z, Duman S, Özkan B, Sentürk M, et al. Thoracic paravertebral block achieves better pain control than erector spinae plane block and intercostal nerve block in thoracoscopic surgery: a randomized study. J Cardiothorac Vasc Anesth. (2021) 35:2920–7. doi: 10.1053/j.jvca.2020.11.034
10. Gabriel RA, Swisher MW, Sztain JF, Curran BP, Said ET, Abramson WB, et al. Serratus anterior plane versus paravertebral nerve blocks for postoperative analgesia after non-mastectomy breast surgery: a randomized controlled non-inferiority trial. Reg Anesth Pain Med. (2021) 46:773–8. doi: 10.1136/rapm-2021-102785
11. Xie C, Ran G, Chen D, Lu Y. A narrative review of ultrasound-guided serratus anterior plane block. Ann Palliat Med. (2021) 10:700–6. doi: 10.21037/apm-20-1542
12. Gautam S, Agarwal A. Serratus plane block in the management of post-operativethoracotomy pain. Curr Anesthesiol Rep. (2019) 9:459–63. doi: 10.1007/s40140-019-00359-4
13. Blanco R, Parras T, McDonnell JG, Prats-Galino A. Serratus plane block: a novel ultrasound-guided thoracic wall nerve block. Anaesthesia. (2013) 68:1107–13. doi: 10.1111/anae.12344
14. Shang LH, Xiao ZN, Zhao YL, Long B. Analgesic Effect of serratus anterior plane block after thoracoscopic surgery: a randomized controlled double-blinded study. Ther Clin Risk Manag. (2020) 16:1257–65. doi: 10.2147/TCRM.S285244
15. Abdallah FW, Patel V, Madjdpour C, Cil T, Brull R. Quality of recovery scores in deep serratus anterior plane block vs. sham block in ambulatory breast cancer surgery: a randomised controlled trial. Anaesthesia. (2021) 76:1190–7. doi: 10.1111/anae.15373
16. Tekşen S, Öksüz G, Öksüz H, Sayan M, Arslan M, Urfalioglu A, et al. Analgesic efficacy of the serratus anterior plane block in rib fractures pain: a randomized controlled trial. Am J Emerg Med. (2021) 41:16–20. doi: 10.1016/j.ajem.2020.12.041
17. Wu Y, Yang W, Cai Z, Zhang Z. The effect of ultrasound-guided low serratus anterior plane block on laparoscopic cholecystectomy postoperative analgesia: a randomized clinical trial. Medicine (Baltimore). (2021) 100:e27708. doi: 10.1097/MD.0000000000027708
18. Elsabeeny WY, Ibrahim MA, Shehab NN, Mohamed A, Wadod MA. Serratus anterior plane block and erector spinae plane block vs. thoracic epidural analgesia for perioperative thoracotomy pain control: a randomized controlled study. J Cardiothorac Vasc Anesth. (2021) 35:2928–36. doi: 10.1053/j.jvca.2020.12.047
19. Park MH, Kim JA, Ahn HJ, Yang MK, Son HJ, Seong BG, et al. A randomised trial of serratus anterior plane block for analgesia after thoracoscopic surgery. Anaesthesia. (2018) 73:1260–4. doi: 10.1111/anae.14424
20. Edwards JT, Langridge XT, Cheng GS, McBroom MM, Minhajuddin A, Machi AT, et al. Superficial vs. deep serratus anterior plane block for analgesia in patients undergoing mastectomy: a randomized prospective trial. J Clin Anesth. (2021) 75:110470. doi: 10.1016/j.jclinane.2021.110470
21. Jonczyk MM, Jean J, Graham R, Chatterjee A. Surgical trends in breast cancer: a rise in novel operative treatment options over a 12 year analysis. Breast Cancer Res Treat. (2019) 173:267–74. doi: 10.1007/s10549-018-5018-1
22. Semyonov M, Fedorina E, Grinshpun J, Dubilet M, Refaely Y, Ruderman L, et al. Ultrasound-guided serratus anterior plane block for analgesia after thoracic surgery. J Pain Res. (2019) 12:953–60. doi: 10.2147/JPR.S191263
23. Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ (Clinical research ed). (2009) 339:b2535. doi: 10.1136/bmj.b2535
24. Svendsen K, Borchgrevink P, Fredheim O, Hamunen K, Mellbye A, Dale O, et al. Choosing the unit of measurement counts: the use of oral morphine equivalents in studies of opioid consumption is a useful addition to defined daily doses. Palliat Med. (2011) 25:725–32. doi: 10.1177/0269216311398300
25. Nielsen S, Degenhardt L, Hoban B, Gisev N. A synthesis of oral morphine equivalents (OME) for opioid utilisation studies. Pharmacoepidemiol Drug Saf. (2016) 25:733–7. doi: 10.1002/pds.3945
26. Drevon D, Fursa SR, Malcolm AL. Intercoder Reliability and Validity of WebPlotDigitizer in Extracting Graphed Data. Behav Modif. (2017) 41:323–39. doi: 10.1177/0145445516673998
27. Hozo SP, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol. (2005) 5:13. doi: 10.1186/1471-2288-5-13
28. Moher D, Shamseer L, Clarke M, Ghersi D, Liberati A, Petticrew M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev. (2015) 4:1. doi: 10.1186/2046-4053-4-1
29. McCormick F, Cvetanovich GL, Kim JM, Harris JD, Gupta AK, Abrams GD, et al. An assessment of the quality of rotator cuff randomized controlled trials: utilizing the Jadad score and CONSORT criteria. J Shoulder Elbow Surg. (2013) 22:1180–5. doi: 10.1016/j.jse.2013.01.017
30. Xiao YK, She SZ, Xu LX, Zheng B. Serratus anterior plane block combined with general analgesia and patient-controlled serratus anterior plane block in patients with breast cancer: a randomized control trial. Adv Ther. (2021) 38:3444–54. doi: 10.1007/s12325-021-01782-y
31. Yang XL, Gu H, Hu JC, Wang S, Wei X, Shu SH, et al. Operation, effectiveness, and limitations of continuous serratus anterior plane blocks for thoracoscopic surgery in adults. J Pain Res. (2020) 13:2401–10. doi: 10.2147/JPR.S264139
32. Dikici M, Akesen S, Yavaşcaoglu B, Bayram AS, Kaya FN, Gurbet A, et al. Comparison of intraoperative and post-operative effects of serratus anterior plane block performed with ultrasound and infiltration block in patients undergoing video-assisted thoracoscopic surgery. Agri. (2022) 34:23–32.
33. Chai B, Yu H, Qian Y, Chen X, Zhu Z, Du J, et al. Comparison of postoperative pain in 70 women with breast cancer following general anesthesia for mastectomy with and without serratus anterior plane nerve block. Med Sci Monit. (2022) 28:e934064. doi: 10.12659/MSM.934064
34. Bhan S, Mishra S, Gupta N, Garg R, Vig S, Thulkar S, et al. A prospective randomised study to assess the analgesic efficacy of serrates anterior plane (SAP) block for modified radical mastectomy under general anaesthesia. Turk J Anaesthesiol Reanimation. (2021) 49:124–9. doi: 10.5152/TJAR.2020.13
35. Tang W, Luo, G, Lu, Y, Chen, C, Liu, H, Li. Y, et al. Application of a new serratus anterior plane block in modified radical mastectomy under ultrasound guidance: a prospective, randomized controlled trial. J Clin Anesth. (2021) 74:110377. doi: 10.1016/j.jclinane.2021.110377
36. Qiu Y, Wu J, Huang Q, Lu Y, Xu M, Yang D, et al. Acute pain after serratus anterior plane or thoracic paravertebral blocks for video-assisted thoracoscopic surgery: A randomised trial. Eur J Anaesthesiol. (2020) 37:1–7. doi: 10.1097/eja.0000000000001196
37. Goel A, Palta S, Saroa R, Saxena P. Efficacy of serratus anterior muscle block as a part of multimodal analgesic regimen in patients undergoing modified radical mastectomy. Sri Lankan J Anaesthesiol. (2020) 28:125–30. doi: 10.4038/slja.v28i2.8547
38. Elsabeeny WY, Shehab NN, Wadod MA, Elkady MA. Perioperative analgesic modalities for breast cancer surgeries: a prospective randomized controlled trial. J Pain Res. (2020) 13:2885–94. doi: 10.2147/JPR.S274808
39. Qiu L, Bu X, Shen J, Li M, Yang L, Xu Q, et al. Observation of the analgesic effect of superficial or deep anterior serratus plane block on patients undergoing thoracoscopic lobectomy. Medicine (Baltimore). (2021) 100:e24352. doi: 10.1097/MD.0000000000024352
40. Chen G, Li Y, Zhang Y, Fang X. Effects of serratus anterior plane block for postoperative analgesia after thoracoscopic surgery compared with local anesthetic infiltration: a randomized clinical trial. J Pain Res. (2019) 12:2411–7. doi: 10.2147/JPR.S207116
41. Saad FS, El Baradie SY, Abdel Aliem MAW, Ali MM, Kotb TAM. Ultrasound-guided serratus anterior plane block versus thoracic paravertebral block for perioperative analgesia in thoracotomy. Saudi J Anaesth. (2018) 12:565–70. doi: 10.4103/sja.SJA_153_18
42. Ökmen K, Metin Ökmen B. Evaluation of the effect of serratus anterior plane block for pain treatment after video-assisted thoracoscopic surgery. Anaesth Crit Care Pain Med. (2018) 37:349–53. doi: 10.1016/j.accpm.2017.09.005
43. Kim DH, Oh YJ, Lee JG, Ha D, Chang YJ, Kwak HJ, et al. Efficacy of ultrasound-guided serratus plane block on postoperative quality of recovery and analgesia after video-assisted thoracic surgery: a randomized, triple-blind, placebo-controlled study. Anesth Analg. (2018) 126:1353–61. doi: 10.1213/ANE.0000000000002779
44. Bakeer AH, Kamel KM, Galil ASA, Ghoneim AA, Soud AHA, Hassan ME, et al. Modified pectoral nerve block vs. serratus block for analgesia following modified radical mastectomy: a randomized controlled trial. J Pain Res. (2020) 13:1769–75. doi: 10.2147/JPR.S252539
45. Aslan G, Avci O, Gündogdu O, Isbir AC, Özdemir Kol I, Kaygusuz K, et al. The effect of postoperative serratus anterior plane block on postoperative analgesia in patients undergoing breast surgery. Turk J Surg. (2020) 36:374–81. doi: 10.47717/turkjsurg.2020.4744
46. Ahiskalioglu A, Yayik AM, Demir U, Ahiskalioglu EO, Celik EC, Ekinci M, et al. Preemptive analgesic efficacy of the ultrasound-guided bilateral superficial serratus plane block on postoperative pain in breast reduction surgery: a prospective randomized controlled study. Aesthetic Plast Surg. (2020) 44:37–44. doi: 10.1007/s00266-019-01542-y
47. Yayik AM, Ahiskalioglu A, Sulak MM, Oral Ahiskalioglu E, Karakaya MA, Çelik EC, et al. The effect of ultrasound guided superficial serratus plane block for acute post mastectomy pain after modified radical mastectomy and axillary lymph node dissection: a randomized controlled study. J Anesthesiol. (2019) 27:121–7. doi: 10.5222/jarss.2019.85570
48. Yao Y, Li J, Hu H, Xu T, Chen Y. Ultrasound-guided serratus plane block enhances pain relief and quality of recovery after breast cancer surgery: a randomised controlled trial. Eur J Anaesthesiol. (2019) 36:436–41. doi: 10.1097/EJA.0000000000001004
49. Wang HJ, Liu Y, Ge WW, Bian LD, Pu LF, Jiang Y, et al. [Comparison of ultrasound-guided serratus anterior plane block and erector spinae plane blockperioperatively in radical mastectomy]. Zhonghua yi xue za zhi. (2019) 99:1809–13. doi: 10.3760/cma.j.issn.0376-2491.2019.23.012
50. Shokri H, Kasem AA. Efficacy of postsurgical ultrasound guided serratus intercostal plane block and wound infiltration on postoperative analgesia after female breast surgeries. A comparative study. Egypt J Anaes. (2019) 33:35–40. doi: 10.1016/j.egja.2016.11.006
51. Mazzinari G, Rovira L, Casasempere A, Ortega J, Cort L, Esparza-Minana JM, et al. Interfascial block at the serratus muscle plane vs. conventional analgesia in breast surgery: a randomized controlled trial. Reg Anesth Pain Med. (2019) 44:52–8. doi: 10.1136/rapm-2018-000004
52. Rahimzadeh P, Imani F, Faiz SHR, Boroujeni BV. Impact of the ultrasound-guided serratus anterior plane block on post-mastectomy pain: a randomised clinical study. Turk J Anaesthesiol Reanim. (2018) 46:388–92. doi: 10.5152/TJAR.2018.86719
53. Chong M, Berbenetz N, Kumar K, Lin C. The serratus plane block for postoperative analgesia in breast and thoracic surgery: a systematic review and meta-analysis. Reg Anesth Pain Med. (2019). doi: 10.1136/rapm-2019-100982
54. Menna C, De Falco E, Teodonio L, Andreetti C, Maurizi G, Ciccone AM, et al. Surgical wound-site inflammation: video-assisted thoracic surgery vs. thoracotomy. Interact Cardiovasc Thorac Surg. (2019) 28:240–6. doi: 10.1093/icvts/ivy231
55. Huang W, Wang W, Xie W, Chen Z, Liu Y. Erector spinae plane block for postoperative analgesia in breast and thoracic surgery: a systematic review and meta-analysis. J Clin Anesth. (2020) 66:109900. doi: 10.1016/j.jclinane.2020.109900
56. Kim S, Bae CM, Do YW, Moon S, Baek SI, Lee DH, et al. Serratus anterior plane block and intercostal nerve block after thoracoscopic surgery. Thorac Cardiovasc Surg. (2021) 69:564–9. doi: 10.1055/s-0040-1705152
57. Zhang X, Zhang C, Zhou X, Chen W, Li J, Wang H, et al. Analgesic effectiveness of perioperative ultrasound-guided serratus anterior plane block combined with general anesthesia in patients undergoing video-assisted thoracoscopic surgery: a systematic review and meta-analysis. Pain Med. (2020) 21:2412–22. doi: 10.1093/pm/pnaa125
58. Liu X, Song T, Xu HY, Chen X, Yin P, Zhang J, et al. The serratus anterior plane block for analgesia after thoracic surgery: a meta-analysis of randomized controlled trails. Medicine (Baltimore). (2020) 99:e20286. doi: 10.1097/MD.0000000000020286
59. Hu NQ, He QQ, Qian L, Zhu JH. Efficacy of ultrasound-guided serratus anterior plane block for postoperative analgesia in patients undergoing breast surgery: a systematic review and meta-analysis of randomised controlled trials. Pain Res Manag. (2021) 2021:7849623. doi: 10.1155/2021/7849623
60. Bateman JT, Levitt ES. Evaluation of G protein bias and beta-arrestin 2 signaling in opioid-induced respiratory depression. Am J Physiol Cell Physiol. (2021) 321:C681–C3. doi: 10.1152/ajpcell.00259.2021
61. Soffin EM, Lee BH, Kumar KK, Wu CL. The prescription opioid crisis: role of the anaesthesiologist in reducing opioid use and misuse. Br J Anaesth. (2019) 122:e198–208. doi: 10.1016/j.bja.2018.11.019
62. Pérez Herrero MA, López Álvarez S, Fadrique Fuentes A, Manzano Lorefice F, Bartolomé Bartolomé C, González J, et al. Quality of postoperative recovery after breast surgery. General anaesthesia combined with paravertebral vs. serratus-intercostal block. Rev Esp Anestesiol Reanim. (2016) 63:564–71. doi: 10.1016/j.redar.2016.03.006
Keywords: serratus anterior plane block, thoracic surgery, breast surgery, postoperative analgesia, meta-analysis
Citation: Liang W, Zhang W, Wu Y, Liu R, Qiu Z, Zhong R, Lan Q, Wang Y, Liu J, Zhong M and Hu S (2022) Efficacy and safety of ultrasound-guided serratus anterior plane block for postoperative analgesia in thoracic surgery and breast surgery: A systematic review and meta-analysis of randomized controlled studies. Front. Anesthesiol. 1:980483. doi: 10.3389/fanes.2022.980483
Received: 28 June 2022; Accepted: 26 July 2022;
Published: 30 November 2022.
Edited by:
Giovanni Landoni, San Raffaele Hospital (IRCCS), ItalyReviewed by:
Krisztina Tóth, Semmelweis University, HungaryManuel Granell, Independent Researcher, Valencia, Spain
Copyright © 2022 Liang, Zhang, Wu, Liu, Qiu, Zhong, Lan, Wang, Liu, Zhong and Hu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wenfu Zhang, MTE3OTUwNzk4MjJAcXEuY29t
 Weidong Liang
Weidong Liang Wenfu Zhang
Wenfu Zhang Yingting Wu2
Yingting Wu2 Shuhui Hu
Shuhui Hu