
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CASE REPORT article
Front. Anesthesiol. , 30 November 2022
Sec. Cardiothoracic Anesthesiology
Volume 1 - 2022 | https://doi.org/10.3389/fanes.2022.963380
This article is part of the Research Topic Advancements and Challenges in Cardiothoracic Anesthesiology View all 5 articles
Emergent cardiac surgery in patients with acute coronary syndrome (ACS) is always a challenging task for surgeons, anesthesiologists and patients. As “time is muscle,” early revascularization by percutaneous coronary intervention (PCI) has been largely advocated to salvage myocardial ischemic cells. In cases judged not suitable for PCI, on-pump coronary artery bypass grafting (CABG) is indicated with cardioplegic arrest and eventually anesthetic preconditioning to enhance myocardial protection. In these high-risk emergent procedures, adjuvant interventions to cardioplegic arrest are aimed to maximize the chance of cardiac functional recovery. Although the infusion of glucose-insulin-potassium (GIK) has demonstrated interesting cardioprotective effects in animal models of myocardial ischemia and in patients undergoing elective cardiac surgery, this cardioprotective strategy has not yet been adopted largely and has been ignored so far in emergent myocardial revascularization procedures. In this case series, we describe the effects of GIK on left ventricular performance assessed by transesophageal echocardiography in four patients with ACS who required emergent CABG surgery. The GIK solution of 20 g glucose, 20 UI insulin and 10 mEq potassium chloride was infused twice over 30 min, first after anesthesia induction and later after aortic unclamping. The systolic performance was assessed after anesthesia induction and after each GIK infusion using the 3D left ventricular ejection fraction, as well as the global longitudinal and circumferential strain. The diastolic function was assessed based on mitral inflow patterns (E-and A-waves) as well as flow propagation velocity.
In patients with acute coronary syndromes (ACSs) presenting with unstable angina, with or without ST-segment elevation myocardial infarction (STEMI/NSTEMI), cardiac surgery has been largely replaced by percutaneous coronary intervention (PCI) as the primary reperfusion therapy [1–3]. Currently, emergent coronary artery bypass grafting (CABG) is performed in less of 10% of patients with ACS [4]. According to the European Society Cardiology (ESC) and European Association of Cardio-Thoracic Surgery (EACTS) guidelines, CABGS should be performed in cases of ongoing ischemia or hemodynamic instability, particularly in patients with complex multivessel coronary artery disease (CAD) or left main coronary disease (LMCA) with the infarct-related artery considered unsuitable for PCI. So far, the optimal timing and the best surgical revascularization strategy are a matter of vivid debates [5].
Compared with elective CABG surgery, emergent procedures are associated with larger blood losses due to anti-thrombotic treatment and with larger myocardial injuries resulting in heart failure requiring mechanical circulatory assistance as well as reduced long-term survival and quality of life [6, 7]. Hence, besides standard perioperative cardioprotective measures including hypothermic cardioplegic arrest and anesthetic preconditioning [8], innovative interventions such as the intravenous administration of glucose-insulin potassium (GIK) solutions may contribute to improve outcomes in patients with ACS undergoing CABG surgery. Indeed, GIK administration has demonstrated cardioprotective effects in experimental models of myocardial ischemia and in elective on-pump cardiac surgery, however, the variability of protocols and heterogeneity of results have precluded its wider clinical application [9, 10].
Herein, we describe the effects of GIK on left ventricular performance using transesophageal echocardiography (TEE) in four patients with ongoing myocardial ischemia and their early clinical outcome after emergent CABG.
Standardized perioperative management of patients undergoing emergent CABG included combined general anesthesia (propofol, sufentanil, dexmedetomidine supplemented with inhaled isoflurane), cardiopulmonary bypass (CPB), aortic cross-clamping and arrested heart with antegrade cardioplegia (Cardioplexol®). Inhaled isoflurane was used from anesthesia induction until the start of CPB as the main anesthetic agent (0.8–1.2 MAC).
During the weaning process from CPB, a protocolized approach was guided by TEE to optimize ventricular performances by adjusting cardiac preload with fluids, afterload with vasoactive agents and,–eventually-, ventricular contractility with inotropes [11]. A 60 ml solution of GIK (20 g glucose, 20 UI insulin and 10 mEq potassium chloride) was infused over 30 min twice, first after anesthesia induction and later following aortic unclamping and myocardial reperfusion. This GIK regimen was selected based on favorable results achieved in a randomized controlled trial including moderate-to-high risk patients undergoing valvular or coronary artery bypass surgery [12]. A comprehensive TEE examination was performed at three time periods (EPIQ Philips Healthcare): (1) before GIK administration and after anesthesia induction, (2) 10 min after the end of the GIK infusion and before starting CPB, (3) after completion of the second GIK infusion and weaning from CPB. A TEE examination before the second GIK infusion was not performed as the patient's circulation was still supported by bypass machine.
Left ventricular (LV) systolic function was assessed with the QLAB 3D-advanced quantification software package to compute 3D left ventricular ejection fraction (LVEF) as well as global longitudinal strain (GLS) and global circumferential strain (GCS) using speckle tracking imaging. To assess LV diastolic function, the mitral inflow was analyzed in terms of early and late filling (E-and A-waves) as well as flow propagation velocity (Vp) using color M-mode. All TEE exams were performed by the same experienced operator, limiting therefore the risk of interobserver variability. The results are summarized in Figure 1. Patient's data were collected and extracted from the electronic hospital data management system. At the University Hospital of Geneva, a “general consent form” was implemented since 2014 which allows the use of routinely collected health-related data/samples for future unspecified research projects and for its storage in databases and biobanks. All four patients reported herein agreed that their anonymous data could be used for research purpose and publication. The local ethics committee waived the need to submit a research protocol as the case series did not exceed 5 patients.
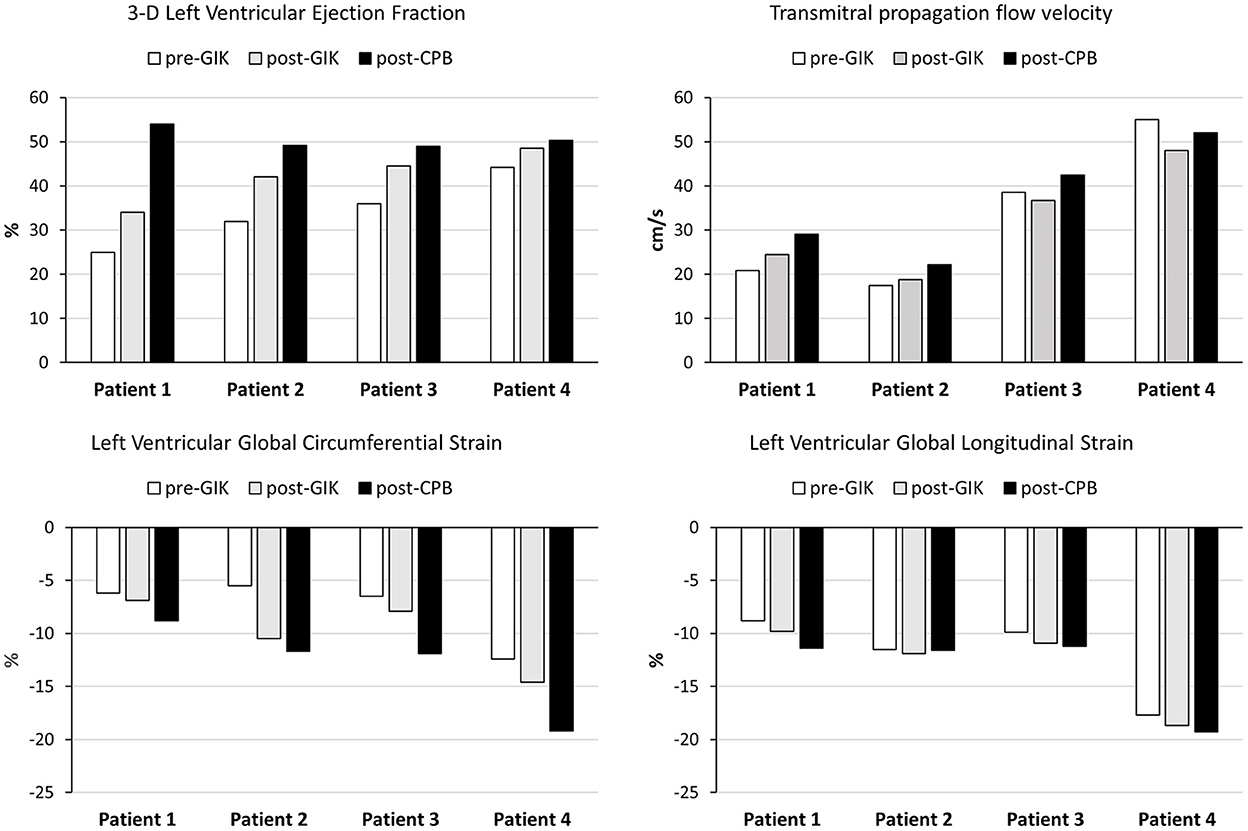
Figure 1. Assessment of left ventricular function by transesophageal echocardiography in four patients receiving glucose-insulin-potassium (GIK) before and after cardiopulmonary bypass (CPB); pre-GIK □; post-GIK  ; post-CPB ■.
; post-CPB ■.
A 58-year old caucasian female with a history of hypertension and dyslipidemia was admitted to the emergency department (ED) with chest pain at rest, shortness of breath and pulsed oxygen saturation (SpO2) of 90% on room air. The ECG revealed non-ST elevation myocardial infarction (NSTEMI). A low left ventricular ejection fraction (LVEF), estimated at 25–30% was documented by transthoracic echocardiography (TTE) and laboratory tests showed elevated plasma levels of high-sensitivity cardiac troponin T (hs-TnT 180 ng/ml). Coronarography demonstrated an occluded circumflex artery (CxA), critical stenosis of the LMCA as well as 70–90% stenoses of the right coronary artery (RCA) and first diagonal artery (D1). Continuous positive airway pressure (CPAP) and intravenous nitroglycerin were started and an intra-aortic balloon pump (IABP) was implanted to support the failing LV and improve gas exchanges. In this patient with severe three-vessel disease and an EuroScore-II of 20.9%, the decision was taken to proceed with emergent CABG.
Following the first infusion of GIK, TEE examination showed increases in 3-D LVEF (from 25 to 34%), GLS (from −8.8 to −9.8%), GCS (from −6.2 to −6.9%), Vp (from 20.8 to 24.4 cm/s) and E/A (from 0.5 to 0.8). Coronary revascularization was achieved using the left internal mammary artery (LIMA) to bypass the LAD artery and 3 venous grafts on the RCA, CxA and D1. Aortic cross-clamp and CPB durations were 70 min and 129 min, respectively. After aortic unclamping and verification of adequate flow in all coronary grafts with Doppler ultrasounds, weaning from CPB proceeded uneventfully with low doses of norepinephrine (0.02 mcg/kg/min) and optimal cardiac filling. TEE examination confirmed further improvements in 3-D LVEF (54%), GLS (−11.4%), GCS (−8.8%) and Vp (29.1 cm/s). The patient was transferred to the intensive care unit (ICU), extubated 8 h postoperatively and the IABP was removed with no need for inotropic support. Seven days after surgery, she was discharged to a rehabilitation center with medical treatment including ticagrelor, metoprolol, enalapril and atorvastatine.
A 79-year man, active smoker, with a history of hypertension, diabetes mellitus and dyslipidemia was transferred to our hospital with persisting chest pain at rest, hypoxemia (SpO2 88%) and documentation of ST segment elevation in the inferior ECG leads. Apical and lateral hypokinesia with a LVEF at 35% was reported on TTE. Under analgo-sedation, non-invasive ventilation was initiated and an IABP was inserted to maintain blood pressure. Plasma levels of hs-TnT were increased at 330 ng/ml. Coronarography demonstrated an occluded RCA, critical stenoses of the proximal CxA and the mid LDA including D1. Given the administration of a loading dose of prasugrel for STEMI and the risk of bleeding, CABG was delayed for 72 h in this patient with STEMI in compensated hemodynamic status and an EuroScore-II of 23%.
Before CPB and following the administration of GIK, TEE examination showed increases in 3-D LVEF (from 32 to 42%), GLS (from −11.5 to −11.9%), GCS (from −5.5 to −10.5%), Vp (from 17.4 to 18.8 cm/s) and E/A (from 0.9 to 1.1). A quadruple coronary bypass was performed using the LIMA to the LAD and D1 artery as well as venous grafts on the marginal artery RCA, CxA and D1. Aortic cross-clamp and CPB durations were 56 and 80 min, respectively. After aortic unclamping and verification of adequate flow in all coronary grafts, weaning from CPB proceeded under norepinephrine infusion (0.03 mcg/kg/min), optimal cardiac filling and atrio-ventricular electrical pacing. Sinus rhythm resumed spontaneously and TEE examination confirmed sustained improvements in 3-D LVEF (49.2%), GCS (−11.7%), Vp (22.2 cm/s) and E/A (1.2) whereas GLS was unchanged (-11.9%). The patient was transferred to the ICU, he was extubated 16 h thereafter and the IABP was removed on the 2d day after surgery. In the surgical ward, atrial fibrillation was treated with amiodarone and pneumonia required antibiotic therapy. Eighteen days after surgery, the patient was discharged home with medical treatment including aspirin, metoprolol, enalapril, metformin, insulin and atorvastatin.
A 71-year man with a history of lung cancer, IHD, hypertension, diabetes mellitus, dyslipidemia and peripheral vascular disease was admitted to the ED with chest pain refractory to sublingual nitrate. In 2013, he had presented a STEMI requiring an emergent PCI with a drug-eluting stent in the RCA. On admission, plasma levels of hs-TnT were above the upper limit (44 ng/ml) and ECG recordings showed elevated and inverted T waves on precordial leads (V3–V6). Moderately decreased LVEF (45%) and severe hypokinesia on mid- and basal inferior segments were reported by TTE. Coronary angiography showed a marked progression of coronary atheromatic lesions with occlusion of the stent in the RCA, critical stenosis of the LMCA, the marginal branch of the LDA and the interventricular posterior artery (IVPA). The long stenotic coronary lesions appeared best accessible to surgical revascularization with minimal delay given the persisting chest pain and the raising levels of hs-TnT. The calculated EuroScore-II was 24.2%.
Following pre-bypass GIK infusion, TEE examination showed increases in 3-D LVEF (from 36 to 44.5%), GLS (from −9.9 to −10.9%), and GCS (from −6.5 to −7.9%) whereas Vp and E/A were unchanged. A quadruple coronary bypass was performed using the LIMA to the LAD, the RIMA to the left marginal artery, as well as sequential venous grafts to the interventricular posterior artery. Aortic cross-clamp and CPB durations were 79 min and 102 min, respectively. After aortic unclamping and verification of adequate flow in all coronary grafts, CPB was discontinued, cardiac filling was optimized with no need to administer inotropes and TEE reported further improvement in LV function (3-D LVEF 49%, GLS −11.2%, GCS −11.9%, Vp 42.5cm/s and E/A ratio 1.1). The patient was transferred to the ICU and extubated 12 h thereafter. Postoperatively, bleeding at the femoral venous site required surgical hemostasis and drainage with red blood cells transfusion. Twelve days after surgery, the patient was discharged home with his usual medications.
A 42-year-old woman with a known uterine myoma and repeated gynecological bleeding, consulted our ED because of recurrent and severe chest pain irradiating in her left arm at rest. The ECG showed sinus tachycardia (94 beats/min) and abnormal T waves (V1–V4). Lab tests revealed raised hs-TnT (297 ng/ml) and low hemoglobin levels (80 g/L). TTE showed severe hypokinesia in the apical, antero-septal and anterior segments with a LVEF estimated at 45%. A single critical stenosis of the proximal LDA was visualized on coronary angiography. Surgical myocardial revascularization was advocated in this young patient with single-vessel disease, EuroScore-II at 4% and NSTEMI triggered by acute anemia due to uterine bleeding.
Following GIK infusion in the pre-CPB period, TEE examination showed slight increases in 3-D LVEF (from 44.2 to 48.5%), GLS (from −17.7 to −18.7%), and GCS (from −12.4 to −14.6%) whereas Vp and E/A remained unchanged. A single coronary bypass graft was performed using the LIMA to the LAD. Two units of red blood cells were given to maintain the hemoglobin level above the threshold of 90 g/L. Aortic cross-clamp and CPB durations were 19 and 32 min, respectively. After aortic unclamping and verification of adequate flow in the arterial graft, CPB was discontinued under low doses of norepinephrine (0.02 mcg/kg/min). TEE examination before sternal closure ascertained a satisfactory ventricular function (3-D LVEF of 50.3%, GLS of −19.3%, GCS of −19.2%, Vp of 52 cm/s and E/A ratio of 1.4. Within 1 h after the end of surgery, the patient was extubated and norepinephrine infusion was discontinued. The postoperative time course was uneventful, the patient being discharge home 7 days after surgery on aspirin and beta-blocker treatment.
In these four patients with ACS, pre-bypass administration of GIK resulted in immediate improvement in LV systolic and diastolic function as assessed by 2-D and 3-D TEE imaging that suggested the effectiveness of GIK in optimizing circulatory conditions. In contrast, after weaning from CPB, sustained or further improvement in LV followed by progressive withdrawal of pharmacological and mechanical circulatory support could be attributed to the effects of myocardial revascularization, GIK-induced myocardial protection or a combination of these factors.
In recent prospective cohort studies, emergent surgical revascularization represented 2–6% of all CABG procedures with an operative mortality ranging between 3 and 25%, accounting for more than 20% of all death after CABG [13–16]. Besides preexisting comorbidities, the presence of circulatory shock, elevated logistic EuroScore-II and raising blood levels of hs-TnT as well as prolonged aortic clamping time are strong predictors for postoperative cardiovascular complications and in-hospital mortality. Documentation of LV dysfunction in patients with ACS has significant implications regarding risk stratification and peri-procedural revascularization by PCI or CABG surgery [6]. Therefore, intraoperative TEE represent an unique tool to evaluate the effectiveness of potential cardioprotective interventions in patients undergoing CABG surgery [17].
All four patients presented refractory myocardial ischemia, three had EuroScore higher than 20% and two of them showed circulatory shock requiring IABP implantation, vasoactive infusions and non-invasive ventilatory support. In such critical hemodynamic conditions, early surgical revascularization was aimed to salvage ischemic cardiomyocytes and to limit further extension of myocardial injuries [18]. As an adjunct to this guideline-directed approach, pretreatment with GIK was aimed to enhance the tolerance to myocardial ischemia, to improve ventricular function through lesser energy demanding pathways and activate cellular pro-survival systems (Figure 2) [19].
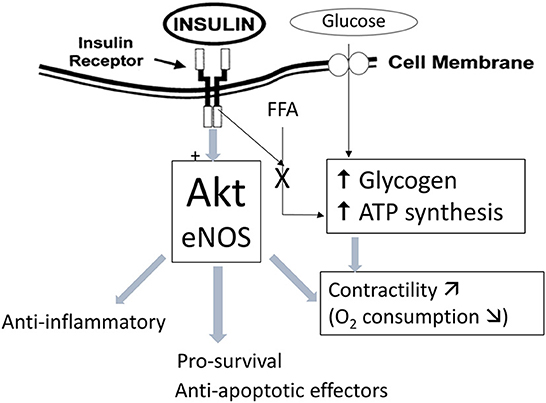
Figure 2. Mechanism of glucose-insulin-potassium (GIK) protection in myocardial cells. ATP, adenosine triphosphate; eNOS, endothelial nitric oxide synthase; FFA, free fatty acids; Akt, protein-kinase B/Akt signaling pathway.
In the failing heart and the early phase of ACS, a metabolic vicious circle involving low cardiac output, ongoing myocardial ischemia, chest pain and respiratory distress activates the sympathetic nervous system which in turn, aggravates myocardial damage via tachycardia and hypoxemia as well as indirectly by increasing free fatty acids (FFA) and glucose levels [20]. From animal studies, there is strong evidence that GIK infusion slows the progression of ischemia-reperfusion injuries by inhibiting FFA production and promoting more efficient glucose utilization to generate high-energy phosphate compounds [19]. The timing and dosage of such metabolic therapy is crucial and has resulted in contrasting clinical outcomes. Indeed, in randomized controlled trials involving patients with ACS, GIK infusion initiated within 2 h after the onset of symptoms was associated with lower in-hospital mortality [21] whereas later GIK infusion failed to improve survival and to limit the progression of MI [22]. Likewise, among patients undergoing emergent CABG for ACS and pretreated with GIK, the results of four small RCTs have demonstrated a lower release of cTnT/I [23], modulation of the inflammatory response [24, 25], as well as better hemodynamics and earlier discharge from the ICU and the hospital [26].
Our echocardiographic and clinical observations lend support to these experimental and clinical data. For the first time, we were able to quantitate improvements in LV function in patients with ongoing myocardial ischemia, using new imaging modalities, namely, three-dimensional LVEF measurements and speckle tracking analysis for LV myocardial strain. Preoperatively, moderate-to-severe systolic and diastolic impairment in LV function was reflected by low 3-D LVEF (25–35%), low LV GCS/GLS (−6–11%) and decreased Vp (< 40 cm/sec) in three patients with multiple-disease CAD whereas minor contractile alterations of the LV were associated with a single critical stenosis of the LAD in the last patient. Using full-volume acquisition, 3-D TEE has been shown to yield more reproducible volume measurements compared with 2-D TEE and it is therefore more suitable for serial evaluations. As the subendocardial longitudinal muscle fibers are more vulnerable to ischemia, GLS enables the clinicians to precisely evaluate the functional consequences of acute myocardial ischemia and the response to a potential protective therapy [27]. Regarding LV diastolic function, the easily performed and highly reproducible color M-mode derived Vp measurements are less dependent on loading conditions and heart rate and therefore, they best reflect the spatio-temporal distribution of LV relaxation and flow generated by atrio-ventricular pressure gradient in the early diastolic time [28].
In summary, metabolic preconditioning of the heart with GIK infusion is a promising cardioprotective adjunct to standard hypothermic arrest in patients with ACS undergoing emergent surgical revascularization. Although we provided preliminary evidence for GIK-induced enhancement of LV function based on TEE imaging, this evidence remains limited as it is based on a case series. Well-designed and powered clinical trials should be conducted to establish the effectiveness, the appropriate timing and dose regimen of GIK therapy in patients with acute myocardial ischemia.
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Ethical review and approval was not required for the study on human participants in accordance with the local legislation and institutional requirements. The patients/participants provided their written informed consent to participate in this study.
CE and ML: design. ML: manuscript draft. All authors: data collection, critical review of paper, and approval of final version.
Open access funding provided by University of Geneva.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Collet JP, Thiele H. The 'ten commandments' for the 2020 ESC guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation. Eur Heart J. (2020) 41:3495–7. doi: 10.1093/eurheartj/ehaa624
2. Ibánez B, James S, Agewall S, Antunes MJ, Bucciarelli-Ducci C, et al. 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation. Rev Esp Cardiol. (2017) 70:1082. doi: 10.1016/j.rec.2017.11.010
3. Roe MT, Messenger JC, Weintraub WS, Cannon CP, Fonarow GC, Dai D, et al. Treatments, trends, and outcomes of acute myocardial infarction and percutaneous coronary intervention. J Am Coll Cardiol. (2010) 56:254–63. doi: 10.1016/j.jacc.2010.05.008
4. ElBardissi AW, Aranki SF, Sheng S, O'Brien SM, Greenberg CC, Gammie JS. Trends in isolated coronary artery bypass grafting: an analysis of the society of thoracic surgeons adult cardiac surgery database. J Thorac Cardiovasc Surg. (2012) 143:273–81. doi: 10.1016/j.jtcvs.2011.10.029
5. Neumann FJ, Sousa-Uva M, Ahlsson A, Alfonso F, Banning AP, Benedetto U, et al. 2018 ESC/EACTS guidelines on myocardial revascularization. Eur Heart J. (2019) 40:87–165. doi: 10.1093/eurheartj/ehy394
6. Kurki TS, Kataja M, Reich DL. Emergency and elective coronary artery bypass grafting: comparisons of risk profiles, postoperative outcomes, and resource requirements. J Cardiothorac Vasc Anesth. (2003) 17:594–7. doi: 10.1016/S1053-0770(03)00202-7
7. Fukui T, Tabata M, Morita S, Takanashi S. Early and long-term outcomes of coronary artery bypass grafting in patients with acute coronary syndrome versus stable angina pectoris. J Thorac Cardiovasc Surg. (2013) 145:1577–83, 1583.e1. doi: 10.1016/j.jtcvs.2012.05.043
8. Annachhatre AS, Annachhatre SR. Preconditioning in cardiac anesthesia where are we? Ann Card Anaesth. (2019) 22:412–21. doi: 10.4103/aca.ACA_116_18
9. Fan Y, Zhang AM, Xiao YB, Weng YG, Hetzer R. Glucose-insulin-potassium therapy in adult patients undergoing cardiac surgery: a meta-analysis. Eur J Cardiothorac Surg. (2011) 40:192–9. doi: 10.1016/j.ejcts.2010.10.007
10. Li Q, Yang J, Zhang J, Yang C, Fan Z, Yang Y, et al. Effect of perioperative glucose-insulin-potassium therapy in patients undergoing on-pump cardiac surgery: a meta-analysis. Heart Surg Forum. (2020) 23:E063–9. doi: 10.1532/hsf.2735
11. Licker M, Diaper J, Cartier V, Ellenberger C, Cikirikcioglu M, Kalangos A, et al. Clinical review: management of weaning from cardiopulmonary bypass after cardiac surgery. Ann Card Anaesth. (2012) 15:206–23. doi: 10.4103/0971-9784.97977
12. Ellenberger C, Sologashvili T, Kreienbühl L, Cikirikcioglu M, Diaper J, Licker M. Myocardial protection by glucose-insulin-potassium in moderate- to high-risk patients undergoing elective on-pump cardiac surgery: a randomized controlled trial. Anesth Analg. (2018) 126:1133–41. doi: 10.1213/ANE.0000000000002777
13. Schumer EM, Chaney JH, Trivedi JR, Linsky PL, Williams ML, Slaughter MS. Emergency coronary artery bypass grafting: indications and outcomes from 2003 through 2013. Tex Heart Inst J. (2016) 43:214–9. doi: 10.14503/THIJ-14-4978
14. Ellenberger C, Sologashvili T, Cikirikcioglu M, Verdon G, Diaper J, Cassina T, et al. Risk factors of postcardiotomy ventricular dysfunction in moderate-to-high risk patients undergoing open-heart surgery. Ann Card Anaesth. (2017) 20:287–96. doi: 10.4103/aca.ACA_60_17
15. Rojas SV, Trinh-Adams ML, Uribarri A, Fleissner F, Iablonskii P, Rojas-Hernandez S, et al. Early surgical myocardial revascularization in non-ST-segment elevation acute coronary syndrome. J Thorac Dis. (2019) 11:4444–52. doi: 10.21037/jtd.2019.11.08
16. Liakopoulos OJ, Slottosch I, Wendt D, Welp H, Schiller W, Martens S, et al. Surgical revascularization for acute coronary syndromes: a report from the North Rhine-Westphalia surgical myocardial infarction registry. Eur J Cardiothorac Surg. (2020) 58:1137–44. doi: 10.1093/ejcts/ezaa260
17. Metkus TS, Thibault D, Grant MC, Badhwar V, Jacobs JP, Lawton J, et al. Transesophageal echocardiography in patients undergoing coronary artery bypass graft surgery. J Am Coll Cardiol. (2021) 78:112–22. doi: 10.1016/j.jacc.2021.04.064
18. Liakopoulos OJ, Schlachtenberger G, Wendt D, Choi YH, Slottosch I, Welp H, et al. early clinical outcomes of surgical myocardial revascularization for acute coronary syndromes complicated by cardiogenic shock: a report from the North-Rhine-Westphalia surgical myocardial infarction registry. J Am Heart Assoc. (2019) 8:e012049. doi: 10.1161/JAHA.119.012049
19. Grossman AN, Opie LH, Beshansky JR, Ingwall JS, Rackley CE, Selker HP. Glucose-insulin-potassium revived: current status in acute coronary syndromes and the energy-depleted heart. Circulation. (2013) 127:1040–8. doi: 10.1161/CIRCULATIONAHA.112.130625
20. Juszczyk A, Jankowska K, Zawiślak B, Surdacki A, Chyrchel B. Depressed cardiac mechanical energetic efficiency: a contributor to cardiovascular risk in common metabolic diseases-from mechanisms to clinical applications. J Clin Med. (2020) 9:2681. doi: 10.3390/jcm9092681
21. Selker HP, Beshansky JR, Sheehan PR, Massaro JM, Griffith JL, D'Agostino RB, et al. Out-of-hospital administration of intravenous glucose-insulin-potassium in patients with suspected acute coronary syndromes: the IMMEDIATE randomized controlled trial. JAMA. (2012) 307:1925–33. doi: 10.1001/jama.2012.426
22. Zhao YT, Weng CL, Chen ML, Li KB, Ge YG, Lin XM, et al. Comparison of glucose-insulin-potassium and insulin-glucose as adjunctive therapy in acute myocardial infarction: a contemporary meta-analysis of randomised controlled trials. Heart. (2010) 96:1622–6. doi: 10.1136/hrt.2010.194563
23. Shim JK, Yang SY, Yoo YC, Yoo KJ, Kwak YL. Myocardial protection by glucose-insulin-potassium in acute coronary syndrome patients undergoing urgent multivessel off-pump coronary artery bypass surgery. Br J Anaesth. (2013) 110:47–53. doi: 10.1093/bja/aes324
24. Celkan MA, Kazaz H, Daglar B, Celik A, Koruk S, Kocoglu H. Effects of glucose-insulin-potassium solution on cardiac cytokines and enzymes. Thorac Cardiovasc Surg. (2006) 54:532–6. doi: 10.1055/s-2006-924478
25. Koskenkari JK, Kaukoranta PK, Rimpiläinen J, et al. Anti-inflammatory effect of high-dose insulin treatment after urgent coronary revascularization surgery. Acta Anaesthesiol Scand. (2006) 50:962–9. doi: 10.1111/j.1399-6576.2006.01100.x
26. Lazar HL, Philippides G, Fitzgerald C, Lancaster D, Shemin RJ, Apstein C. Glucose-insulin-potassium solutions enhance recovery after urgent coronary artery bypass grafting. J Thorac Cardiovasc Surg. (1997) 113:354–60. doi: 10.1016/S0022-5223(97)70333-7
27. Klaeboe LG, Edvardsen T. Echocardiographic assessment of left ventricular systolic function. J Echocardiogr. (2019) 17:10–6. doi: 10.1007/s12574-018-0405-5
Keywords: glucose-insulin-potassium, myocardial protection, acute coronary syndrome, aortic cross-clamping, cardiopulmonary bypass
Citation: Ellenberger C, Hagerman A, Putzu A, Cikirikcioglu M and Licker M (2022) Myocardial protection with glucose-insulin potassium in patients with acute coronary syndromes requiring coronary artery bypass grafting: A case series. Front. Anesthesiol. 1:963380. doi: 10.3389/fanes.2022.963380
Received: 07 June 2022; Accepted: 25 August 2022;
Published: 30 November 2022.
Edited by:
Laura Pasin, University Hospital of Padua, ItalyReviewed by:
Pittarello Demetrio, University Hospital of Padua, ItalyCopyright © 2022 Ellenberger, Hagerman, Putzu, Cikirikcioglu and Licker. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Marc Licker, TWFyYy1qb3NlcGgubGlja2VyQGhjdWdlLmNo
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.