- 1Programa de Pós-Graduação em Biologia Animal, Instituto de Biologia, Universidade Estadual de Campinas, Campinas, São Paulo, Brazil
- 2Programa de Pós-Graduação em Ecologia, Universidade Federal do Rio Grande do Sul, Porto Alegre, Brazil
- 3Departamento de Ecologia e Biologia Evolutiva, Universidade Federal de São Paulo, Diadema, São Paulo, Brazil
Background: Biological invasions pose a critical threat to biodiversity, affecting ecological balance and native species’ communication. Eleutherodactylus johnstonei, an exotic anuran in São Paulo, vocalizes at intensities that could interfere with native anuran species, potentially causing acoustic masking.
Methods: We evaluated the effects of E. johnstonei's calls on the vocalizations of two native species, Scinax imbegue and Physalaemus cuvieri, both with and without spectral overlap with the invasive species. Field playbacks were conducted using six versions of stimuli, including E. johnstonei's calls, the native Boana bischoffi (as a control), and white noise. We recorded response calls and behavioral changes of S. imbegue and P. cuvieri males.
Results: The calls of E. johnstonei did not affect the spectral or temporal parameters of the native species’ announcement calls. However, S. imbegue males displayed behavioral responses such as cessation of vocalization or movement away from the noise source. Additionally, B. bischoffi's calls and white noise influenced native species’ call parameters.
Discussion: Our findings reveal that exotic species’ vocalizations may disrupt native anurans’ acoustic behavior. This impact varies with species and context, underlining the need for further research on anuran acoustic interactions across different frequencies and acoustic environments to fully understand the effects of exotic acoustic interference.
1 Introduction
Biological invasions pose significant threats to biodiversity (Mack et al., 2000; McGeoch et al., 2010; Pyšek et al., 2020), reaching beyond conventional challenges like competition, predation, hybridization, disease transmission, and alterations in community composition (Mack et al., 2000; McGeoch et al., 2010; Blackburn et al., 2014). Exotic invasive species’ impact extends to intricate challenges that might affect ecosystem functioning, including interference through vocalizations within the acoustic niche of native species, such as amphibians and birds (Both and Grant, 2012; Farina et al., 2013; Bleach et al., 2015).
Urbanization further amplifies the influence of invasive species on ecosystems by generating new niches and resources for their establishment and spread (e.g. Cadotte et al., 2017; Borden and Flory, 2021). Human activities linked to urbanization, such as trade and transportation, facilitate the introduction and spread of invasive species across regions, fostering increased connectivity between urban and natural habitats (e.g. Fonseca et al., 2019, 2021). This accelerated potential for disruption in local ecosystems is compounded by altered ecological dynamics. Given the constant rise in the introduction of invasive species by accidental pathways or as pets, it becomes imperative to focus on introductions in urban areas and anticipate potential consequences on native species naturally inhabiting these environments.
Communication is a cornerstone of evolutionary biology, mediating reproductive, social, and territorial interactions (Narins, 2001; Brumm, 2013). Successful communication involves encoding information into a signal, transmitting it to a receiver, and relies on acoustic properties, distance, and environmental factors influencing signal transmission (Shannon et al., 2016; Forrest, 1994; Ryan and Rand, 1993; Castellano et al., 2003).
Animals adjust vocalization parameters to minimize spectral or temporal overlap with other species, maximizing signal transmission chances (Krause, 1987). Interference in transmission or reception can lead to declines in species density and distribution (Sun and Narins, 2005). Exotic species also contribute to environmental noise through acoustic signals during reproduction, resulting in disruptions to intra-specific acoustic communication when their signals overlap with those of native species (Brumm and Slabbekoorn, 2005). This degradation of emitted signals hampers the communication of native species, which rely on acoustic signals for various aspects of their behavior (Brumm and Slabbekoorn, 2005).
The acoustic niche hypothesis (Krause, 1987) suggests that animals adjust parameters of their vocalizations to minimize spectral or temporal overlap with others, thereby maximizing the chances of signal transmission. The acoustic niche refers to the frequency and temporal patterns that a species occupies to communicate effectively within on the environment (Mullet et al., 2017). When different species share the same habitat, they often adapt their vocalizations to avoid mutual interference, allowing an efficient communication. Signal interference that hinders inter- and intra-specific communication can lead to declines in species density and distribution (Sun and Narins, 2005).
Acoustic signal masking caused by new sounds can affect communication, abundance, reproduction, physiological stress, energy demands, and other behaviors (Wells, 1977; Bradbury and Vehrencamp, 1998; Rheindt, 2003). This masking occurs when sounds from other sources, natural or anthropogenic, interfere with a species’ vocalizations, disrupting its acoustic niche. In response, native species may quickly adjust their vocalizations (e.g., Cunnington and Fahrig, 2010) or may not immediately react to the acoustic niche interference, especially in the short term (e.g., Lengagne, 2008). Understanding the interaction between acoustic niche and acoustic masking is crucial for comprehending species’ adaptations to changes in their sound environment and the implications for their survival and behavior.
Anuran amphibians, which primarily communicate through acoustic signals, serve as excellent models to study invasion impacts on the acoustic niche due to their sensitivity to environmental changes and responses to new sounds (Wells, 2007; Starnberger et al., 2014). Additionally, anuran amphibians possess adaptations related to sound communication, allowing them to emit and perceive sounds within a wide frequency range (Narins, 1995). Even though, their ability to adapt to novel sounds varies widely, and behavioral and physiological negative consequences can follow (e.g., Lengagne, 2008; Tennessen et al., 2018; Caorsi et al., 2023).
Eleutherodactylus johnstonei, a terrestrial anuran native to the Lesser Antilles, has a history of human-mediated introductions and established populations in urban areas in Central and South America, including Brazil (Kaiser et al., 2002; Lever, 2003; Melo et al., 2014; Yuan et al., 2022). The calling activity of E. johnstonei males can represent a significant source of noise in the environment, disturbing residents (Melo et al., 2014). In this study, we assess the potential effects of acoustic niche invasion by E. johnstonei (Barbour, 1914) on two native species that produce vocalizations with and without spectral overlap with the exotic species. Our hypotheses are: (1) when there is spectral overlap, the call of E. johnstonei induces changes in temporal and spectral parameters of the native species males’ calls, negatively affecting the acoustic niche; (2) when there is no spectral overlap, the call of E. johnstonei induces changes in temporal parameters of the call, also impairing intra-specific communication of the native species.
2 Materials and methods
2.1 Study area
We conducted the experiments in the edges of artificial ponds within the visitor area of the Parque Estadual das Fontes do Ipiranga (PEFI), located in the city of São Paulo (23°38’32.7”S 46°37’32.0”W), Brazil. PEFI encompasses the São Paulo Zoo and the São Paulo Botanical Garden. Situated within the Atlantic Forest morphoclimatic domain (Ab’Saber, 1977), PEFI features vegetation at various successional stages (Gomes et al., 2003). The climate is characterized by a dry winter (April to September) and a rainy summer (October to March), with average temperatures ranging from 18°C in winter to 22°C in summer (Santos and Funari, 2002). Currently, PEFI has records of 22 anuran species, none of which are threatened or endemic (Lisboa et al., 2021). There are no records of E. johnstonei However, the site is situated just 6 km from the known established population of E. johnstonei and exhibits favorable conditions for its occurrence (Brasileiro et al., 2021).
2.2 Exotic species
Eleutherodactylus johnstonei successfully inhabits disturbed environments once introduced (e.g., Ortega et al., 2005; Bomford et al., 2009; Ernst et al., 2011) and occupies anthropized areas, forest edges, open spaces, and urban environments such as gardens and greenhouses (Schwartz, 1967; Kaiser et al., 2002; Leonhardt et al., 2019). In Brazil, individuals from the exotic population of E. johnstonei were introduced at least as early as 1995, when its vocalization were first recorded (Toledo and Measey, 2018). Currently, E. johnstonei can be found both on the ground (among ornamental garden vegetation and rocks) and climbing in vegetation (herbaceous, shrubby) as well as on walls and hedges (personal observation). Reproduction takes place during the warm and rainy months (November to April), coinciding with the reproductive period of the native species selected in this study.
Eleutherodactylus johnstonei displays a vocal repertoire consisting of four distinct calls (Flechas et al., 2018). The advertisement call is the most common (Figure 1A) and consists of two adjacent notes with distinct temporal and spectral parameters (Tárano and Fuenmayor, 2008; Melo and Brasileiro, 2022). On average, the first note has a dominant frequency of 1.77 kHz, and the second note of 3.42 kHz (Melo and Brasileiro, 2022; Table 1). Both notes are tonal, with frequency modulation at the end of the first note and the beginning of the second note (Figure 1A). The frequency range of the advertisement call varies from 1.6 kHz to 3.5 kHz (Melo and Brasileiro, 2022; Table 1).
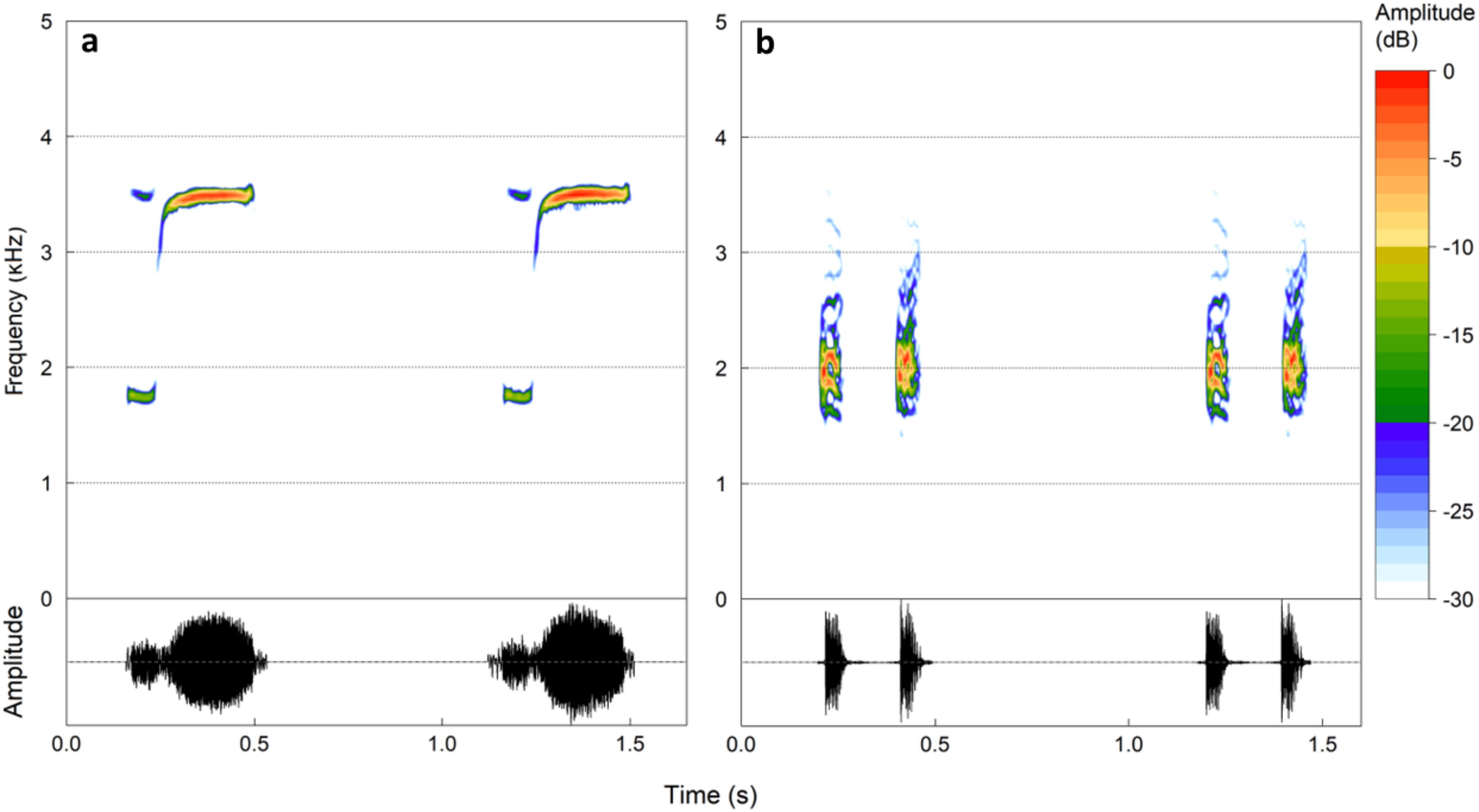
Figure 1. Spectrograms (above) and oscillograms (below) of the advertisement calls of the anurans that were used as stimuli, (A) Eleutherodactylus johnstonei and (B) Boana bischoffi. Spectrogram parameters: window size = 1,024, overlap = 90%, window type = “Hann”. Figure created using the R package ‘seewave’ (Sueur et al., 2008).
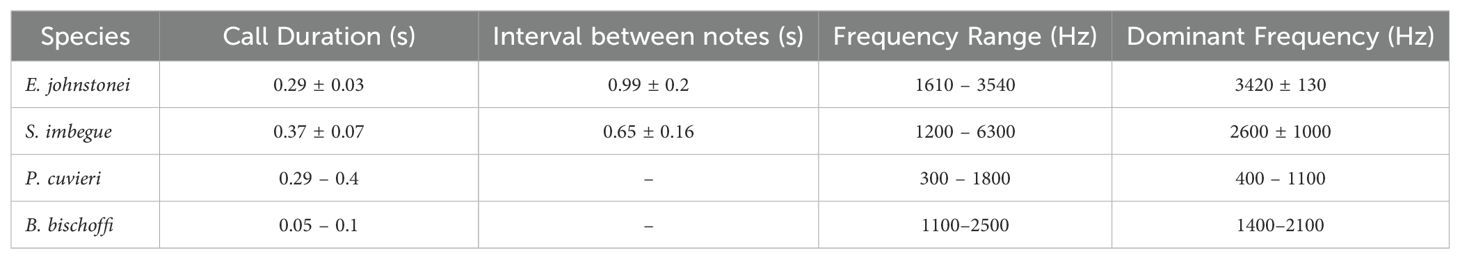
Table 1. Duration of note (s), interval between notes (s), frequency range (Hz), and dominant frequency (Hz) of the advertisement call of Eleutherodactylus johnstonei (exotic species) and native species from Fontes do Ipiranga State Park used in the study (Scinax imbegue (native species with overlap), Physalaemus cuvieri (native species without overlap), and Boana bischoffi (native species used as a control).
2.3 Native species
In our study, we opted for native species based on the dominant frequency of their advertisement calls, their abundance at the study site and with their reproductive cycles aligning with those of E. johnstonei, i.e., showing similar calling activity patterns. Specifically, we chose Scinax imbegue and Physalaemus cuvieri because they represent species with and without spectral overlap with E. johnstonei, respectively. As a heterospecific control, we chose the advertisement call of the native species Boana bischoffi, coexisting with others in PEFI. We collected the call parameters for S. imbegue, P. cuvieri, B. bischoffi, and E. johnstonei from the literature (Pombal, 2010; Gambale and Bastos, 2014; Conte et al., 2016). Detailed characteristics are provided in Table 1.
The control species, Boana bischoffi, is native to the southeastern and southern regions of Brazil (Teixeira et al., 2022), with a thriving population in PEFI (Lisboa et al., 2021). These individuals are commonly found in permanent ponds within or in proximity to forest fragments (Lisboa et al., 2021) and are observed climbing on ornamental vegetation (personal observation). Furthermore, they exhibit an extended reproductive period, with a reduction in vocalization activity during winter (Pombal, 2010). The advertisement call comprises one or two multi-pulsed notes, occupying a frequency range (Pombal, 2010) similar to E. johnstonei (Table 1; Figure 1B).
Scinax imbegue, native to the Atlantic Forest in the southeast and south of Brazil (Nunes et al., 2012), thrives in semi-open and open environments featuring lentic water bodies (Nunes et al., 2012; Fiorillo et al., 2018). The species engages in continuous reproduction throughout the year (Santos and Conte, 2014; Fiorillo et al., 2018), with heightened vocalization activity during the rainy season (personal observation). Within PEFI, these individuals exhibit habitat versatility, occupying artificial lakes and their peripheries (Lisboa et al., 2021) while vocalizing on the ground, climbing on rocks, branches, low plants, ornamental plants, and in aquatic environments (personal observation). Scinax imbegue’s acoustic repertoire comprises territorial, courtship, and advertisement calls. The advertisement call is characterized by a single multi-pulsed note, featuring a dominant frequency of 2.6 ± 1 kHz (Conte et al., 2016; Table 1; Figure 2A).
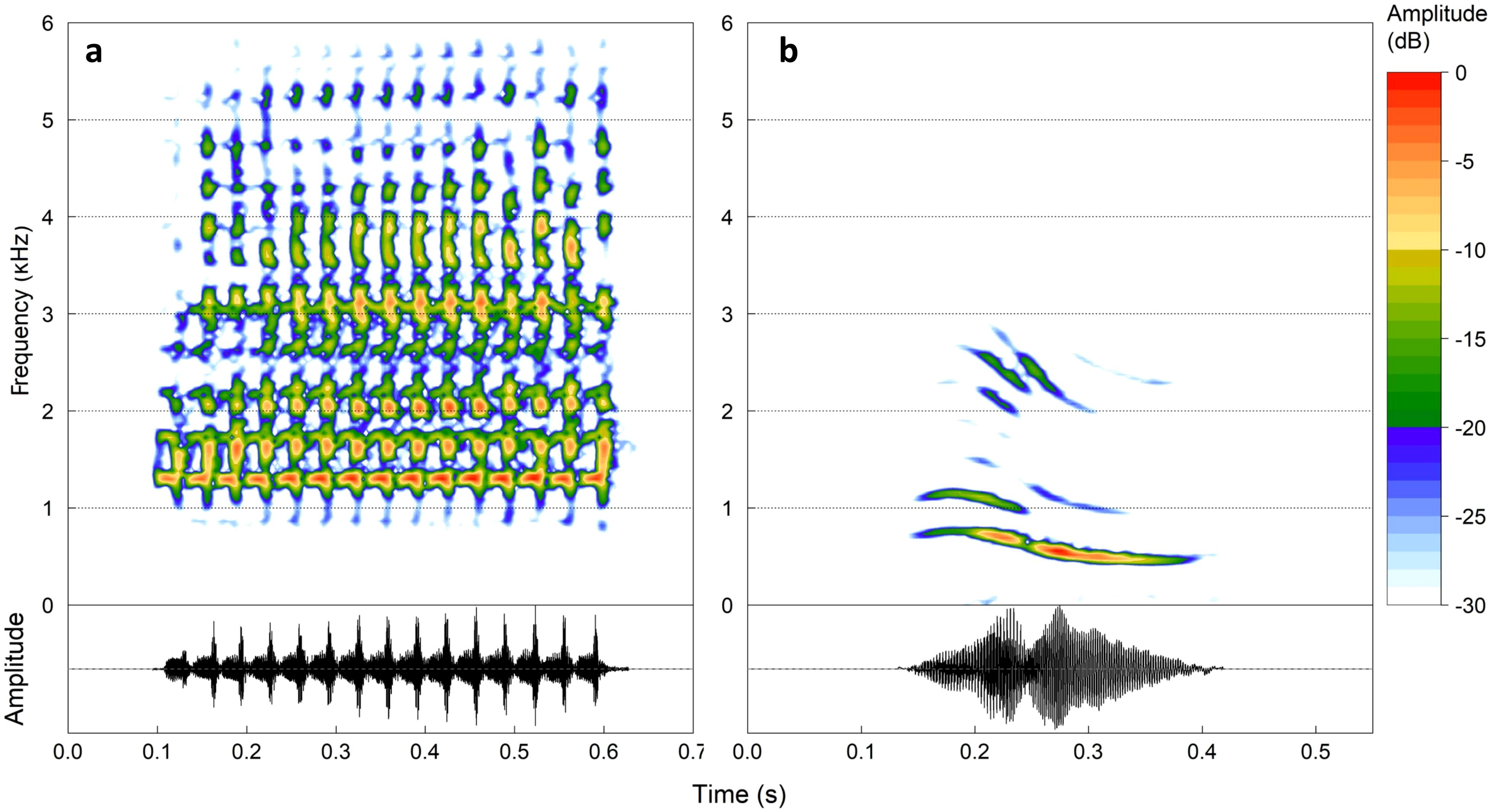
Figure 2. Spectrograms (above) and oscillograms (below) of the advertisement calls of native species exposed to playback stimuli. Species with spectral overlap with E. johnstonei, (A) Scinax imbegue, and species without spectral overlap (B) Physalaemus cuvieri. Spectrogram parameters: window size = 1,024, overlap = 90%, window type = “Hann”. Figure created using the R package ‘seewave’ (Sueur et al., 2008).
Physalaemus cuvieri is distributed across the Northeast, Midwest, and South of Brazil (Frost, 2024). Within PEFI, these individuals are situated in artificial lakes, along their peripheries, and in puddles within the forest (Lisboa et al., 2021). Reproduction occurs during the rainy season (November to March) in temporary ponds and puddles, with individuals typically located in sheltered spots, vocalizing in flooded environments, on the water surface, and on damp soil (Barreto and Andrade, 1995; Bastos et al., 2003; Brasileiro et al., 2005). The advertisement call of P. cuvieri comprises a single note with harmonic structures (Figure 2B) (Gambale and Bastos, 2014), featuring a dominant frequency ranging between 0.4 kHz and 1.1 kHz (Table 1; Figure 2B). The spectral overlap between the advertisement calls of the native and exotic species is showed in Figure 3.
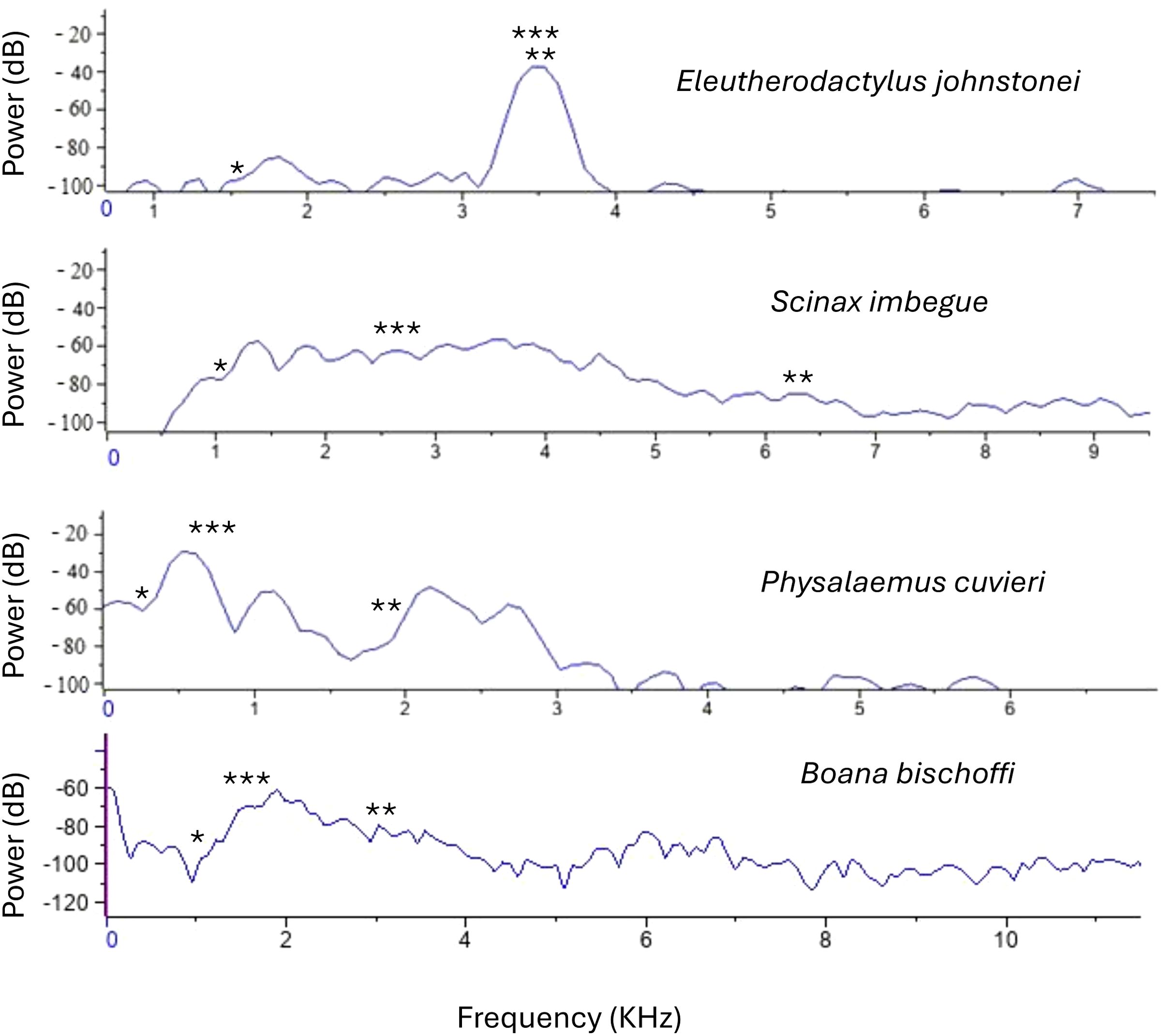
Figure 3. Power spectrum of the advertisement calls of Eleutherodactylus johnstonei, Scinax imbegue, Physalaemus cuvieri, and Boana bischoffi. *minimum frequency, **maximum frequency and ***dominant frequency.
2.4 Playback calls preparation
We recorded all advertisement calls used to prepare the playback calls in 2020 before conducting the experiments. All recordings were taken from solitary males. We recorded the advertisement calls of six solitary males of E. johnstonei and five solitary males of B. bischoffi to create stimulus playbacks. We conducted the recordings of E. johnstonei calls in the Jardim Cordeiro neighborhood, southern region of São Paulo city, Brazil, in March 2020, and the calls of B. bischoffi in PEFI in November 2020. We utilized a TASCAM DR-22WL portable recorder and a Sennheiser ME 66 unidirectional microphone to capture all calls. We use a portable decibel meter measured the average amplitude (dB) of the calls (INS-135, Introsul). In addition to the calls of E. johnstonei and B. bischoffi, we employed white noise as a stimulus to test whether the potential changes were a specific response to the vocalizations of E. johnstonei or a general response to noises (Medeiros et al., 2017). We have deposited our audio files used to organize the playbacks in the audio archive of Fonoteca Neotropical Jacques Vielliard (FNJV), Universidade Estadual de Campinas, State of São Paulo, Brazil (E. johnstonei FNJV 50605–50610; B. bischoffi FNJV 124328-124330, P. cuvieri FNJV124325-124326; S. imbegue FNJV 50617-50621).
We visually inspected the quality of the recordings of E. johnstonei and B. bischoffi in the RAVEN PRO v. 1.5 software (Bioacoustics Research Program, 2014) and subsequently randomly selected notes emitted by males of both species to compose the three-minute stimulus playbacks. We constructed the stimulus playbacks in the Audacity 2.4.1 program. We standardized the sound pressure level at 75 dB for all stimuli at approximately 1 m from the source. The E. johnstonei stimulus playback (n = 6; mean CRC = 22.24 mm; temperature = 25.3 ± 0.6°C; humidity = 64 ± 3%) has a mean call duration of 288 ± 30 seconds and a dominant frequency of 3.42 ± 0.13 kHz. The B. bischoffi stimulus playback (n = 5; temperature = 19.6 ± 2.8°C; humidity = 71 ± 1%) has a mean call duration of 0.07 ± 0.03 seconds and a dominant frequency of 1.72 ± 0.05 kHz.
We organized the playbacks according to the A-B-A protocol (McGregor et al., 1992) following the structure of three minutes of pre-stimulus silence (S1), nine minutes of stimulus (three minutes for each stimulus – E1, E2, E3), and three minutes of post-stimulus silence (S2), totaling 15 minutes. We created six different versions of the playback, only changing the order of the three stimuli (E1, E2, E3) between the three minutes of pre-stimulus silence (S1) and the three minutes of post-stimulus silence (S2). Stimuli E1, E2, and E3 correspond to the advertisement calls of E. johnstonei, the advertisement calls of B. bischoffi, and white noise, respectively. Each target male was exposed to only one version of the playback, with the first target individual assigned to version 1, the second to version 2, and so on (see Caorsi et al., 2017).
2.5 Playback experiments
We located males of the target species through active search and removed the nearby conspecific males to avoid interference in the recording. We positioned the directional microphone and the speaker at approximately 1 meter from the focal male (Figure 4). Before conducting the recordings, we stepped back and waited until the male resumed normal vocalization (5-10 min). We adjusted the field playbacks using a decibel meter, considering the distance between the focal male and the speaker, and played the playback. We measured the air temperature and relative humidity for each recording.
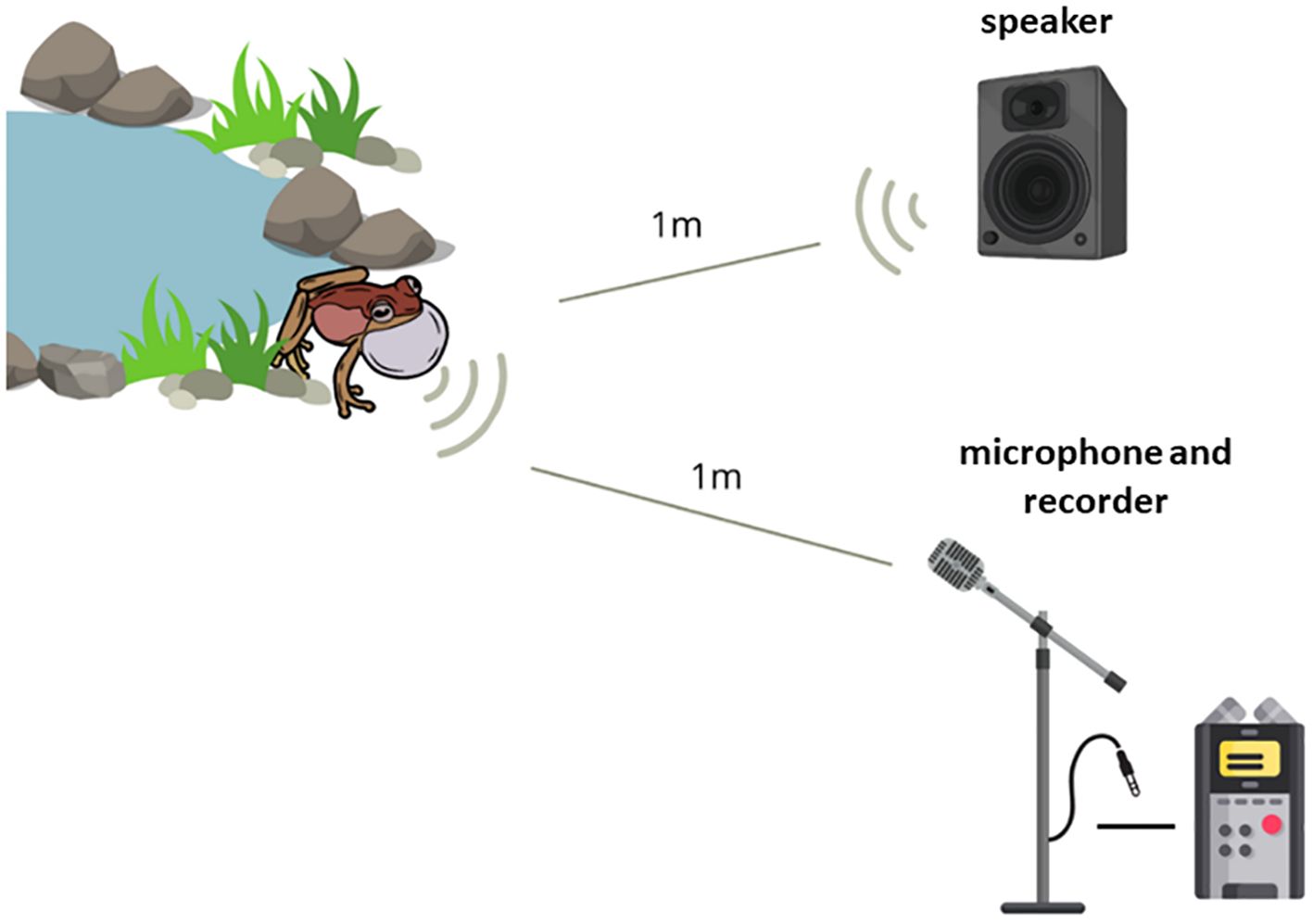
Figure 4. Field playback experiment setup during stimulus playback and recording of response from Scinax imbegue and Physalaemus cuvieri species.
At the end of the recording, we captured the focal male, measured the rostro-cloacal length (CRC, mm), and mass (g) with the aid of a digital caliper and a semi-analytical scale. To prevent males from being resampled, we marked individuals by amputating the third toe of the right foot (Phillott et al., 2007; Correa et al., 2013). We also maintained a minimum distance of 5 meters between individuals recorded on the same night. All experiments were approved by the Chico Mendes Institute for Biodiversity Conservation (ICMBIO - Permit No. 73346-2) and the Committee on Ethics in the Use of Animals (CEUA/Unifesp – No. 1005160320).
2.6 Acoustic analyses
We divided the 15-minute recordings into files of three minutes each for each individual according to the following segments: (1) pre-stimulus, (3) during the stimulus, and (1) post-stimulus, using the Audacity 2.4.1 program. We digitized the recordings at 44.1 kHz, with a resolution of 16 bits and a Fast Fourier Transform of 1024 points, at Raven Pro. We followed the note-centered approach and the concepts of notes, pulses, and calls as defined by Köhler et al. (2017). To calculate the calling rate (notes – 1)/min, we counted the calls emitted at each time segment during the three-minute recording. To measure other parameters of the advertisement call [note interval and duration (s), dominant frequency (Hz), maximum and minimum frequency (Hz)], we selected 9 notes in each time period, choosing the first three notes at the beginning of the recording, the three notes emitted from the middle of the period (starting from 1 minute and 30 seconds), and the last three notes. In cases where males emitted fewer than 9 notes, we used all observed notes in the period to measure the acoustic parameters.
2.7 Statistical analyses
We utilized a Permutational Multivariate Analysis of Variance with Distance Matrices to investigate the potential impact of stimuli on various call parameters within both species. Subsequently, post-hoc pairwise comparisons were conducted to evaluate distinctions between groups. The fixed factors encompassed the type of stimulus and the time periods (S1 – E1 – E2 – E3 – S2), with individuals serving as blocks. Also, the exposure order (e.g. E1-E2-E3; E1-E3-E2;…) was included as a fixed factor. We executed the analyses in the R program utilizing the “Vegan: Community Ecology” package (Oksanen et al., 2013), and employed the “Seewave” package (Sueur et al., 2008) for the generation of spectrograms and oscillograms.
3 Results
3.1 Scinax imbegue – species with spectral overlap with E. johnstonei
We recorded 21 males of Scinax imbegue. Seven individuals stopped calling when exposed to the stimulus playback (call of E. johnstonei) but resumed after approximately one minute. One individual stopped calling during the experiment, and six changed positions by moving away from the speaker but continued calling. We discarded the calls from three males due to the low quality of the recordings.
The treatment rate affected the calling (F=2.7; p < 0.05) but not the treatment order (p > 0.05). The males’ calling rate decreased from an average of 29.7 calls/minute during the white noise stimulus (E3) to 19.5 calls/minute during the post-stimulus silence (F = 1.7; p < 0.05) Males did not alter the note duration of the advertisement call (F = 1.4; p > 0.05) or the interval between notes (F = 0.6; p > 0.05) in response to the stimuli, and the treatment order was also not significant (p > 0.05 for all). The dominant frequency, minimum frequency, and maximum frequency did not change in response to the stimuli (p > 0.05), and the order was not significant (p > 0.05).
3.2 Physalaemus cuvieri – species without spectral overlap with E. johnstonei
We recorded 17 males of Physalaemus cuvieri. No individual changed its initial position, and all called throughout the experiment without interruptions. We discarded one call record of P. cuvieri, due to external interferences in one recording.
The treatment did not affect the calling rate (F = 2.06; p > 0.05) or the treatment order (p > 0.05). Males progressively increased the duration of notes during the experiment (F = 2.7; p < 0.05), with an average of 0.27 seconds during the pre-stimulus silence (S1) to 0.30 seconds during the post-stimulus silence (S2). P. cuvieri males did not change the note interval or dominant frequency of the call (p > 0.05 for all) in response to the stimuli. The maximum frequency significantly increased during the E2 stimulus (call of B. bischoffi) (F = 0.6; p < 0.05), and the minimum frequency changed during the stimuli (F = 3.02; p < 0.05), decreasing from an average of 416 Hz during the pre-stimulus silence (S1) to 405 Hz during the post-stimulus silence (S2).
4 Discussion
In this study, the advertisement call of the exotic species E. johnstonei did not induce changes in the spectral and temporal parameters of the advertisement calls of the native species Scinax imbegue and Physalaemus cuvieri. However, when exposed to the advertisement calls of Boana bischoffi and white noise, the target native species modified parameters of their advertisement calls. This is the first study to assess the effect of a high-frequency call of an exotic species on native species. Studies on the invasive bullfrog and the invasive Cuban treefrog Osteopilus septentrionalis have focused on frequencies lower than 2500 Hz (Capranica, 1968; Both and Grant, 2012; Tennessen et al., 2016; Medeiros et al., 2017), and frequencies lower than 1200 Hz for the invasive species Rhinella marina (Bleach et al., 2015). All these previous studies reported changes induced by the exotic species calls on native species. Our study suggests that to understand how acoustic species will respond to novel invasive sounds we should focus in the call features of both the invasive and the native species.
Males of S. imbegue decreased the calling rate after exposure to white noise but maintained the duration and interval between notes constant during playback. The calling rate is easily altered by individuals in the presence of acoustic interferences (Wong et al., 2009) and can be modulated by some species to avoid signal overlap or influenced by energy availability, social context, and vocalization strategies (Wells and Taigen, 1986; Köhler et al., 2017). Changes in the calling rate have been observed in response to invasive species (Bleach et al., 2015; Tennessen et al., 2016; Medeiros et al., 2017) and anthropogenic noises (Sun and Narins, 2005; Parris et al., 2009; Kaiser et al., 2011). Among these modifications, species exhibit either an increase or decrease in the calling rate, but they do not seem to follow a clear pattern and vary according to the species and noise characteristics (Caorsi et al., 2023).
Males of Scinax imbegue did not alter spectral parameters in the presence of acoustic stimuli. The advertisement call of S. imbegue covers a broad range of frequencies with relatively low spectral overlap with the high-frequency call of E. johnstonei. Furthermore, the population of S. imbegue in the study is located in an urban area, with constant presence of anthropogenic noises, and therefore, may be more tolerant to sound stimuli and consequently have higher fitness in disturbed environments (Ghalambor et al., 2007; Wells and Schwartz, 2007). These characteristics may explain the absence of changes in the spectral parameters of the advertisement calls of S. imbegue males. To understand the effects of the advertisement call of E. johnstonei on the spectral parameters of the calls of native species, we suggest testing species with higher frequency overlap and present in different acoustic landscapes.
When exposed to the calls of Boana bischoffi and white noise, males of Physalaemus cuvieri altered temporal and spectral parameters of the advertisement call. Physalaemus cuvieri males emitted longer notes after the playback, similar to males of the species Dendropsophus minutus and Boana leptolineata after exposure to the call of the bullfrog and white noise, respectively (Medeiros et al., 2017). This temporal alteration may be a generic response to noise, eliciting different effects on species. However, the maximum and minimum frequencies changed in response to the call of B. bischoffi and white noise, while the dominant frequency remained constant. Similar frequency variations were observed for Scinax nasicus in ponds near roads, where traffic noise is present (Leon et al., 2019). Variability in frequency range and dominant frequency is often related to an attempt to avoid signal overlap; however, the absence of modifications in these parameters during noise exposure has been documented (Lengagne, 2008). This demonstrates that the impact caused by noise is variable among species and contexts, requiring further studies with species vocalizing at different frequencies.
Regarding behavior, males of S. imbegue ceased vocalizing and/or moved away from the noise source during the experiment (pers. Obs). This behavior was observed for B. bischoffi and Boana leptolineata during experiments using anthropogenic noise (Caorsi et al., 2017). However we did not observe any behavior changes for P. cuvieri during the playback of stimuli. Reversible modifications in behavior are short-term mechanisms employed by signal emitters to reduce acoustic signal masking (Brumm and Slabbekoorn, 2005). For example, individuals may switch the emitted calls, causing their vocalizations to coincide with periods of low noise and thus avoiding masking by conspecifics (Zelick and Narins, 1982; Grafe, 1996), heterospecifics (Brumm, 2006; Wong et al., 2009), or abiotic noises (Sun and Narins, 2005; Vargas-Salinas and Amézquita, 2013). These behavioral results are consistent with the hypothesis that species with a higher degree of signal overlap with noise are more affected (Parris et al., 2009; Cunnington and Fahrig, 2010) and reinforce that individuals’ responses to new sounds in the environment (anthropogenic noise or calls of exotic species) vary among species and acoustic landscapes.
Finally, effects of novel invasive sounds might affect native species at metabolic and or physiological level, even when calling is not altered (Shine, 2014; Still et al., 2019; Mitchell et al., 2020). The results obtained in this study underscore the potential for new sounds in the environment to influence the acoustic behavior of individuals. Given the large populations of E. johnstonei in exotic habitats, the chorus produced by males could potentially amplify noise, but this effect remains untested. Moreover, this study aimed to assess the threat that E. johnstonei poses to native species, but it sets up artificial encounters between the invader and two pond-breeders, interactions may rare or never occur in natural settings. However, these stimulations provide valuable insights into possible ecological impacts under controlled conditions. When acoustic interferences disrupt the communication between individuals, species density and distribution can be reduced (Sun and Narins, 2005), since spectral and temporal parameters play a crucial role in partner selection and localization, and consequently, reproduction. (Gerhardt and Schwartz, 2001). Like anthropogenic noises, invasive species can induce changes in the calling parameters of native species, a mechanism that warrant further attention, as species introductions continue to increase globally (Pyšek et al., 2020).
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Ethics statement
The animal study was approved by Chico Mendes Institute for Biodiversity Conservation (ICMBIO - Permit No. 73346-2) and the Committee on Ethics in the Use of Animals (CEUA/Unifesp – No. 1005160320). The study was conducted in accordance with the local legislation and institutional requirements.
Author contributions
NM: Investigation, Methodology, Writing – review & editing, Data curation, Formal analysis, Writing – original draft. CB: Formal analysis, Methodology, Writing – review & editing, Conceptualization, Validation. CB: Conceptualization, Writing – review & editing, Methodology, Funding acquisition, Investigation, Resources, Supervision.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study was made possible through the funding provided by the São Paulo Research Foundation (FAPESP; #2013/50741-7; #2020/12866-6) and the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES– Finance Code 001 to NM).
Acknowledgments
We would like to thank the Parque Estadual das Fontes do Ipiranga and Concessionária Paulista administrations, for their assistance in facilitating field permits and logistics. Additionally, we are grateful to Dr. Elaine Gonsales, Dr. Felipe Toledo, and Dr. Thaís Guedes for their unwavering support and insightful revisions, as well as to Wellington Palhares (in memmorian), Amanda D’Ambrósio, Marcelo Melo, and Natália Catai for their invaluable contributions to both field and laboratory activities.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/famrs.2024.1457928/full#supplementary-material
References
Ab’Sáber A. N. (1977). Os domínios morfoclimáticos da América do Sul: primeira aproximação. Geomorfologia 52, 1–22.
Barreto L., Andrade G. V. (1995). Aspects of the reproductive biology of Physalaemus cuvieri (Anura: Leptodactylidae) in northeastern Brazil. Amphibia-Reptilia 16, 67–76. doi: 10.1163/156853895X00208
Bastos R. F., Motta J. A. O., Lima L. P., Guimarães L. D. (2003). Anfíbios da Floresta Nacional de Silvânia, Estado de Goiás [Amphibians of the Silvânia National Forest, Goiás State] (Goiânia: Stylo Gráfica e Editora).
Bioacoustics Research Program (2014). Raven Pro: Interactive Sound Analysis Software (Version 1.6) [Computer software] (Ithaca, NY: The Cornell Lab of Ornithology). Available at: http://www.birds.cornell.edu/raven.
Blackburn T. M., Essl F., Evans T., Hulme P. E., Jeschke J. M., Kühn I., et al. (2014). A unified classification of alien species based on the magnitude of their environmental impacts. PloS Biol. 12, e1001850. doi: 10.1371/journal.pbio.1001850
Bleach I. T., Beckmann C., Both C., Brown G. P., Shine R. (2015). Noisy neighbours at the frog pond: Effects of invasive cane toads on the calling behaviour of native Australian frogs. Beh. Ecol. Sociobiol 69, 675–683. doi: 10.1007/s00265-015-1879-z
Bomford M., Kraus F., Barry S. C., Lawrence E. (2009). Predicting establishment success for alien reptiles and amphibians: A role for climate matching. Biol. Invasions 11, 713–724. doi: 10.1007/s10530-008-9285-3
Borden J. B., Flory S. L. (2021). Urban evolution of invasive species. Front. Ecol. Environ. 19, 184–191. doi: 10.1002/fee.2295
Both C., Grant T. (2012). Biological invasions and the acoustic niche: The effect of bullfrog calls on the acoustic signals of white-banded tree frogs. Biol. Let. 8, 714–716. doi: 10.1098/rsbl.2012.0412
Bradbury J. W., Vehrencamp S. L. (1998). Principles of animal communication (Sunderland, MA: Sinauer Associates).
Brasileiro C. A., Fonseca E., Giovanelli J. G. R., Melo N. B. V., Both C. (2021). “Herpetofauna invasora no Brasil: Presente e futuro [Invasive herpetofauna in Brazil: Present and future],” in Herpetologia Brasileira Contemporânea [Contemporary Brazilian Herpetology]. Ed. Toledo L. F. (Anolis Book, São Paulo), 263–273.
Brasileiro C. A., Sawaya R. J., Kiefer M. C., Martins M. (2005). Amphibians of an open Cerrado fragment in southeastern Brazil. Biota Neotropica 5, 93–109. doi: 10.1590/S1676-06032005000300006
Brumm H. (2006). Signaling through acoustic windows: Nightingales avoid interspecific competition by short-term adjustment of song timing. J. Comp. Physiol. A 192, 1279–1285. doi: 10.1007/s00359-006-0158-x
Brumm H. (Ed.) (2013). Animal communication and noise (Vol. 2) (Springer Science and Business Media).
Brumm H., Slabbekoorn H. (2005). Acoustic communication in noise. Adv. Study Behav. 35, 151–209. doi: 10.1016/S0065-3454(05)35004-2
Cadotte M. W., Yasui S. L. E., Livingstone S., MacIvor J. S. (2017). Are urban systems beneficial, detrimental, or indifferent for biological invasion? Biol. Invasions 19, 3489–3503. doi: 10.1007/s10530-017-1586-y
Caorsi V. Z., Both C., Cechin S., Antunes R., Borges-Martins M. (2017). Effects of traffic noise on the calling behavior of two Neotropical hylid frogs. PloS One 12, e0183342. doi: 10.1371/journal.pone.0183342
Caorsi V. Z., Both C., Márquez R., Llusia D., Narins P., Debon M., et al. (2023). Effects of anthropogenic noise on anuran amphibians. Bioacoustics 32, 90–120. doi: 10.1080/09524622.2022.2070543
Capranica R. R. (1968). The vocal repertoire of the bullfrog (Rana catesbeiana). Behaviour 31, 302–324. doi: 10.1163/156853968X00306
Castellano S., Giacoma C., Ryan M. J. (2003). Call degradation in diploid and tetraploid green toads. Biol. J. Lin. Soc 78, 11–26. doi: 10.1046/j.1095-8312.2003.00119.x
Conte C. E., Vieira K., Crivellari L. B., Berneck B. (2016). A new species of Scinax Wagler (Anura: Hylidae) from Paraná, Southern Brazil. Zootaxa 4193, 245–265. doi: 10.11646/zootaxa.4193.2.3
Correa D. T., Guimarães M., Lopes Oliveira T. A., Martins M., Sawaya R. J. (2013). Toe-clipping vital to amphibian research. Nature 493, 305. doi: 10.1038/493305e
Cunnington G. M., Fahrig L. (2010). Plasticity in the vocalizations of anurans in response to traffic noise. Acta Oecologica 36, 463–470. doi: 10.1016/j.actao.2010.06.002
Ernst R., Massemin D., Kowarik I. (2011). Non-invasive invaders from the Caribbean: the status of Johnstone’s Whistling frog (Eleutherodactylus johnstonei) ten years after its introduction to Western French Guiana. Biol. Invasions 13, 1767–1777. doi: 10.1007/s10530-010-9930-5
Farina A., Pieretti N., Morganti N. (2013). Acoustic patterns of an invasive species: the Red-billed Leiothrix (Leiothrix lutea Scopoli 1786) in a Mediterranean shrubland. Bioacoustics 22, 175–194. doi: 10.1080/09524622.2012.761571
Fiorillo B. F., Faria C. S., Silva B. R., Martins M. (2018). Anurans from preserved and disturbed areas of Atlantic Forest in the region of Etá Farm, municipality of Sete Barras, state of São Paulo, Brazil. Biota Neotropica 18. doi: 10.1590/1676-0611-BN-2017-0509
Flechas S. V., Ortega-Chinchilla J. E., Arenas L. M., Amézquita A. (2018). The function of supplementary notes in the communication system of johnstone’s whistling frog, eleutherodactylus johnstonei. Herpetol. Rev. 49, 626–632.
Fonseca É., Both C., Cechin S. Z. (2019). Introduction pathways and socio-economic variables drive the distribution of alien amphibians and reptiles in a megadiverse country. Diver. Distrib 25, 1130–1141. doi: 10.1111/ddi.12920
Fonseca É., Both C., Cechin S. Z., Winck G. (2021). Pet distribution modelling: Untangling the invasive potential of Trachemys dorbigni (Emydidae) in the Americas. PloS One 16, e0259626. doi: 10.1371/journal.pone.0259626
Forrest T. G. (1994). From sender to receiver: propagation and environmental effects on acoustic signals. Amer. Zool. 34, 644–654. doi: 10.1093/icb/34.6.644
Frost D. R. (Ed.) (2024). Amphibian Species of the World: an Online Reference. Version 6.1 (09/01/2024) (New York, USA: American Museum of Natural History). Available at: https://amphibiansoftheworld.amnh.org/index.php.
Gambale P. G., Bastos R. P. (2014). Vocal repertoire and bioacoustic analyses in Physalaemus cuvieri (Anura, Leptodactylidae) from southern Brazil. Herpetol. J. 24, 31–39.
Gerhardt H. C., Schwartz J. J. (2001). “Auditory tuning and frequency preferences in anurans,” in Anuran Communication. Ed. Ryan M. J. (Washington and London: Smithsonian Institution Press), 73–85.
Ghalambor C. K., McKay J. K., Carroll S. P., Reznick D. N. (2007). Adaptive versus non-adaptive phenotypic plasticity and the potential for contemporary adaptation in new environments. Funct. Ecol. 21, 394–407. doi: 10.1111/j.1365-2435.2007.01283.x
Gomes E. P. C., Mantovani W., Kageyama P. Y. (2003). Mortality and recruitment of trees in a secondary montane rain forest in Southeastern Brazil. Braz. J. Biol. 63, 47–60. doi: 10.1590/S1519-69842003000100007
Grafe T. U. (1996). The function of call alternation in the African reed frog (Hyperolius marmoratus): precise call timing prevents auditory masking. Behav. Ecol. Sociobiol. 38, 149–158. doi: 10.1007/s002650050227
Kaiser H., Barrio-Amorós C. L., Trujillo J. D., Lynch J. D. (2002). Expansion of Eleutherodactylus johnstonei in northern South America: Rapid dispersal through human interactions. Herpetol. Rev. 33, 290–294.
Kaiser K., Scofield D. G., Alloush M., Jones R. M., Marczak S., Martineau K., et al. (2011). When sounds collide: the effect of anthropogenic noise on a breeding assemblage of frogs in Belize, Central America. Behaviour 148, 215–232. doi: 10.1163/000579510X551660
Köhler J., Jansen M., Rodríguez A., Kok P. J. R., Toledo L. F., Emmrich M., et al. (2017). The use of bioacoustic in anuran taxonomy: theory, terminology, methods and recommendations for best practice. Zootaxa 4251, 1–124. doi: 10.11646/zootaxa.4251.1.1
Krause B. (1987). The niche hypothesis: How animals taught us to dance and sing. Whole Earth Rev. 57, 14–16.
Lengagne T. (2008). Traffic noise affects communication behaviour in a breeding anuran, Hyla arborea. Biol. Conserv. 141, 2023–2031. doi: 10.1016/j.biocon.2008.05.017
Leon E., Peltzer P. M., Lorenzon R., Lajmanovich R. C., Beltzer A. H. (2019). Effect of traffic noise on Scinax nasicus advertisement call (Amphibia, Anura). Iheringia. Série Zoologia 109. doi: 10.1590/1678-4766e2019007
Leonhardt F., Jimenez-Bolaño J. D., Ernst R. (2019). Whistling invaders: Status and distribution of Johnstone’s Whistling frog (Eleutherodactylus johnstonei Barbour 1914), 25 years after its introduction to Colombia. NeoBiota 45, 39. doi: 10.3897/neobiota.45.33515
Lever C. (2003). Naturalized amphibians and reptiles of the world (New York: Oxford University Press).
Lisboa C. S., Vaz R. I., Malagoli L. R., Barbo F. E., Venturini R. C., Brasileiro C. A. (2021). Herpetofauna from an atlantic forest fragment in São Paulo, Brasil. Herpetol. Conserv. Biol. 16, 436–451.
Mack R. N., Simberloff D., Mark Lonsdale W., Evans H., Clout M., Bazzaz F. A. (2000). Biotic invasions: causes, epidemiology, global consequences, and control. Ecol. Applicat. 10, 689–710. doi: 10.1890/1051-0761(2000)010[0689:BICEGC]2.0.CO;2
McGeoch M. A., Butchart S. H., Spear D., Marais E., Kleynhans E. J., Symes A., et al. (2010). Global indicators of biological invasion: species numbers, biodiversity impact and policy responses. Divers. Distr. 16, 95–108. doi: 10.1111/j.1472-4642.2009.00633.x
McGregor P. K., Dabelsteen T., Shepherd M., Pedersen S. B. (1992). The signal value of matched singing in great tits: evidence from interactive playback experiments. Anim. Behav. 43, 987–998. doi: 10.1016/S0003-3472(06)80012-6
Medeiros C. I., Both C., Grant T., Hartz S. M. (2017). Invasion of the acoustic niche: variable responses by native species to invasive American bullfrog calls. Biol. Invasions 19, 675–690. doi: 10.1007/s10530-016-1327-7
Melo N. B. V., Brasileiro C. A. (2022). Advertisement call of Johnstone’s whistling frog Eleutherodactylus johnstonei in Brazil. Herpetol. Bul. 16, 1–6. doi: 10.33256/hb160.16
Melo M. A., Lyra M. L., Brischi A. M., Geraldi V. C., Haddad C. F. B. (2014). First record of the invasive frog Eleutherodactylus johnstonei (Anura: Eleutherodactylidae) in São Paulo, Brazil. Salamandra 50, 177–180.
Mitchell B. A., Callaghan C. T., Rowley J. J. (2020). Continental-scale citizen science data reveal no changes in acoustic responses of a widespread tree frog to an urbanisation gradient. J. Urb. Ecol. 6, juaa002. doi: 10.1093/jue/juaa002
Mullet T. C., Farina A., Gage S. H. (2017). The acoustic habitat hypothesis: an ecoacoustics perspective on species habitat selection. Biosemiotics 10, 319–336. doi: 10.1007/s12304-017-9288-5
Narins P. M. (1995). Frog communication. Sci. Am. 273, 78–83. doi: 10.1038/scientificamerican0895-78
Narins P. M. (2001). “Vibration communication in vertebrates,” in Ecology of Sensing (Berlin, Heidelberg, Springer), 127–148.
Nunes I., Kwet A., Pombal J. P. Jr. (2012). Taxonomic revision of the Scinax alter species complex (Anura: Hylidae). Copeia 2012, 554–569. doi: 10.1643/CH-11-088
Oksanen J., Blanchet F. G., Kindt R., Legendre P., Minchin P. R., O’Hara R. B., et al. (2013). Package “vegan. Community Ecol. Package Version. 2013, 2.
Ortega J. E., Serrano V. H., Ramírez-Pinilla M. P. (2005). Reproduction of an introduced population of Eleutherodactylus johnstonei at Bucaramanga, Colombia. Copeia 2005, 642–648. doi: 10.1643/CH-04-223R1
Parris K. M., Velik-Lord M., North J. M. (2009). Frogs call at a higher pitch in traffic noise. Ecol. Soc 14, 25. doi: 10.5751/ES-02687-140125
Phillott A. D., Skerratt L. F., McDonald K. R., Lemckert F. L., Hines H. B., Clarke J. M., et al. (2007). Toe-clipping as an acceptable method of identifying individual anurans in mark recapture studies. Herpetol. Rev. 38, 305–308.
Pombal J. P. Jr (2010). O espaço acústico em uma taxocenose de anuros (Amphibia) do Sudeste do Brasil. Arq. Mus. Nac. 68, 135–144.
Pyšek P., Hulme P. E., Simberloff D., Bacher S., Blackburn T. M., Carlton J. T., et al. (2020). Scientists’ warning on invasive alien species. Biol. Rev. 95, 1511–1534. doi: 10.1111/brv.12627
Rheindt F. E. (2003). The impact of roads on birds: does song frequency play a role in determining susceptibility to noise pollution? J. Für Ornithologie 144, 295–306. doi: 10.1046/j.1439-0361.2003.03004.x
Ryan M. J., Rand A. S. (1993). Species recognition and sexual selection as a unitary problem in animal communication. Evolution 47, 647–657. doi: 10.2307/2410076
Santos E. J., Conte C. E. (2014). Riqueza e distribuição temporal de anuros (Amphibia: Anura) em um fragmento de Floresta Ombrófila Mista. Iheringia Série Zoologia 104, 323–333. doi: 10.1590/1678-476620141043323333
Santos P. M., Funari F. L. (2002). “Clima local,” in Parque Estadual das Fontes do Ipiranga (PEFI): Unidade de Conservação que Resiste à Urbanização de São Paulo. Eds. Bicudo D. C., Forti M. C., Bicudo C. E. M. (Secretaria do Meio Ambiente do Estado de São Paulo, São Paulo, Brazil), 76–93.
Schwartz A. (1967). Frogs of the genus eleutherodactylus in the lesser antilles. Stud. Fauna Curacao Other Caribbean Islands 24, 1–62.
Shannon G., McKenna M. F., Angeloni L. M., Crooks K. R., Fristrup K. M., Brown E., et al. (2016). A synthesis of two decades of research documenting the effects of noise on wildlife. Biol. Rev. 91, 982–1005. doi: 10.1111/brv.12207
Shine R. (2014). A review of ecological interactions between native frogs and invasive cane toads in Australia. Austral Ecol. 39, 1–16. doi: 10.1111/aec.12066
Starnberger I., Preininger D., Hödl W. (2014). From uni-to multimodality: towards an integrative view on anuran communication. J. Comp. Physiol. A 200, 777–787. doi: 10.1007/s00359-014-0923-1
Still M. B., Lea A. M., Hofmann H. A., Ryan M. J. (2019). Multimodal stimuli regulate reproductive behavior and physiology in male túngara frogs. Horm. Behav. 115, 104546. doi: 10.1016/j.yhbeh.2019.06.010
Sueur J., Aubin T., Simonis C. (2008). Seewave, a free modular tool for sound analysis and synthesis. Bioacoustics 18, 213–226. doi: 10.1080/09524622.2008.9753600
Sun J. W., Narins P. M. (2005). Anthropogenic sounds differentially affect amphibian call rate. Biol. Conserv. 121, 419–427. doi: 10.1016/j.biocon.2004.05.017
Tárano Z., Fuenmayor E. (2008). Analysis of the vocalizations of johnstone’s whistling frog (Eleutherodactylus johnstonei: eleutherodactylidae) in Northern-central Venezuela. South Amer. J. Herpet. 3, 229–238. doi: 10.2994/1808-9798-3.3.229
Teixeira A. C., Marcelino V. R., Alexandrino J., Haddad C. F., Giaretta A. A. (2022). Populational differentiation in boana bischoffi (Anura, hylidae): revisiting the issue using molecular, morphological, and acoustic data. J. Herpetol. 56, 110–119. doi: 10.1670/20-121
Tennessen J. B., Parks S. E., Swierk L., Reinert L. K., Holden W. M., Rollins-Smith L. A., et al. (2018). Frogs adapt to physiologically costly anthropogenic noise. Proc. R. Soc B 285, 20182194. doi: 10.1098/rspb.2018.2194
Tennessen J. B., Parks S. E., Tennessen T. P., Langkilde T. (2016). Raising a racket: invasive species compete acoustically with native treefrogs. Anim. Behav. 114, 53–61. doi: 10.1016/j.anbehav.2016.01.021
Toledo L. F., Measey J. (2018). Invasive frogs in Sao Paulo display a substantial invasion lag. BioInvasions Records 7, 325–328. doi: 10.3391/bir.2018.7.3.15
Vargas-Salinas F., Amézquita A. (2013). Traffic noise correlates with calling time but not spatial distribution in the threatened poison frog Andinobates bombetes. Behaviour 150, 569–584. doi: 10.1163/1568539X-00003068
Wells K. D. (1977). “The courtship of frogs,” in The Reproductive Biology of Amphibians (Springer, Boston, MA), 233–262.
Wells K. D. (2007). The Ecology and Behavior of Amphibians (Chicago Press: University of Chicago Press).
Wells K. D., Schwartz J. J. (2007). “The behavioral ecology of anuran communication,” in Hearing and sound communication in amphibians (Springer, New York, NY), 44–86.
Wells K. D., Taigen T. L. (1986). The effect of social interactions on calling energetics in the gray treefrog (Hyla versicolor). Behav. Ecol. Sociobiol. 19, 9–18. doi: 10.1007/BF00303837
Wong S., Parada H., Narins P. M. (2009). Heterospecific acoustic interference: effects on calling in Oophaga pumilio. Biotropica 41, 74. doi: 10.1111/j.1744-7429.2008.00452.x
Yuan M. L., Frederick J. H., McGuire J. A., Bell R. C., Smith S. R., Fenton C., et al. (2022). Endemism, invasion, and overseas dispersal: the phylogeographic history of the Lesser Antillean frog, Eleutherodactylus johnstonei. Biol. Invasions 24, 1–16. doi: 10.1007/s10530-022-02803-9
Keywords: acoustic niche, acoustic communication, biological invasion, exotic species, environmental noise
Citation: Melo NBV, Both C and Brasileiro CA (2024) Novel sounds, native responses: exploring the acoustic consequences of Eleutherodactylus johnstonei’s invasion in urban areas. Front. Amphib. Reptile Sci. 2:1457928. doi: 10.3389/famrs.2024.1457928
Received: 01 July 2024; Accepted: 14 October 2024;
Published: 08 November 2024.
Edited by:
Joice Ruggeri, CONICET Institute of Subtropical Biology (IBS), ArgentinaReviewed by:
John Roger Downie, University of Glasgow, United KingdomMonica Jacinto-Maldonado, University of Sonora, Mexico
Copyright © 2024 Melo, Both and Brasileiro. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Cinthia A. Brasileiro, Y2ludGhpYS5icmFzaWxlaXJvQHVuaWZlc3AuYnI=
 Natalia Bispo Vieira Melo1
Natalia Bispo Vieira Melo1 Camila Both
Camila Both Cinthia A. Brasileiro
Cinthia A. Brasileiro