
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Agron. , 31 October 2024
Sec. Disease Management
Volume 6 - 2024 | https://doi.org/10.3389/fagro.2024.1470194
 Ramaraju Manasa1,2
Ramaraju Manasa1,2 R. Sarada Jayalakshmi Devi3
R. Sarada Jayalakshmi Devi3 Kuruba Vemana2
Kuruba Vemana2 K. John4
K. John4 G. Rama Rao5
G. Rama Rao5 P. J. Anubhava1,6
P. J. Anubhava1,6 L. K. Vidyashree1,6
L. K. Vidyashree1,6 Kurella Sri Ananth1,7
Kurella Sri Ananth1,7 Kale Santosh8
Kale Santosh8 Gajanan Sawargaonkar8
Gajanan Sawargaonkar8 Hari Kishan Sudini1*
Hari Kishan Sudini1*Due to the pathogen’s ability to survive in the soil for longer durations, soil-borne diseases are often difficult to control. This study investigates the multifaceted impacts of biochar on the management of stem rot disease in groundnut and its influence on soil properties and microbial communities. The effects of biochar at different concentrations, such as 0%, 1%, 3%, and 5% on groundnut stem rot disease incited by Sclerotium rolfsii were evaluated thoroughly. Under laboratory conditions, biochar exhibited no direct inhibitory effects on S. rolfsii at varying concentrations but revealed an indirect suppression of sclerotial body production, suggesting a concentration-dependent influence on pathogen resting structures. Further, it was observed that biochar treatments effectively delayed symptom onset and reduced disease progression in groundnut plants, with significant variation observed among genotypes and biochar concentrations. Notably, interactions involving genotypes ICGV 171002 and ICGV 181035 with BC2 + Sr (3% conc. of biochar + S. rolfsii) and BC3 + Sr (5% conc. of biochar + S. rolfsii) treatments showed superior efficacy in disease reduction under controlled conditions. Field evaluations confirmed these findings, highlighting genotype-specific responses to biochar treatments. However, no significant difference was observed between BC2 + Sr (3%) and BC3 + Sr (5%) treatments in managing stem rot disease compared to controls. Biochar application significantly increased soil nutrient levels, including nitrogen, phosphorus, and potassium, and increased soil organic matter content, EC, pH, emphasizing its potential to improve soil fertility. Overall, these findings highlight the potential benefits of biochar for sustainable agriculture through disease management, soil nutrient enrichment, and microbial modulation, warranting further investigation into optimal application strategies across different agricultural contexts.
Sclerotium rolfsii, a soil-borne fungus, is a severe hindrance to various crops in warm and humid climates. It causes stem and pod rot disease in groundnuts, which has a severe impact on productivity. Stem rot, also known as Sclerotium blight, southern blight, or white mould, is a severe concern in groundnut cultivation, resulting in significant crop losses despite preventive measures. Stem rot typically reduces groundnut yields by 10 to 40%, but in severely affected fields, the reduction might reach 80% (Bera et al., 2014). Pod and peg rots from S. rolfsii are widespread in various countries, causing significant pod losses at harvest. Stem rot thrives in warm and damp conditions, while pod rots are more common in drier soils. In dense soils, the fungus damages surface-level plants, whereas in lighter soils, it can harm pegs and pods deeply (Mehan et al., 1994). The fungus survives in soil as sclerotia and in crop remains as mycelium, with sclerotia enduring for years without a host. Currently, effective preventative and control measures are inadequate to ensure sustainable protection for all crops. Conventional management approaches such as resistant cultivars, crop rotation, soil solarization techniques, etc., may not consistently effectively combat S. rolfsii, which can thrive in adverse soil conditions and infect diverse host species (Tian et al., 2021). While a variety of chemical and biological methods are used for disease control, they face numerous limitations (Wang et al., 2024). Furthermore, continuous application of chemical pesticides contributes to significant environmental pollution and promotes the emergence of pesticide-resistant pathogens (Chen et al., 2020). Hence, there is an urgent need to explore alternative environmentally friendly approaches for effectively controlling the disease. The use of soil amendments like biochar might be a promising approach, as their suppressive activity has been shown for a wide range of soil-borne diseases (Akhter et al., 2015).
The use of biochar as a soil amendment has increased significantly, with diverse applications in the fields of agriculture, energy, industry, and environment (Wang et al., 2024). Biochar, a carbon-rich material, is produced by the pyrolysis of organic wastes like manure, wood, and crop residues under low oxygen levels (Pasumarthi et al., 2024; Akhter et al., 2015; Chen et al., 2020; Tian et al., 2021). Biochar is composed of variety of nutrients, including carbon, nitrogen, phosphorus, potassium, calcium, and magnesium, as well as its physical and chemical properties like bulk density (BD), water holding capacity (WHC), nutrient retention, soil acidity, electrical conductivity (EC) and cation exchange capacity (CEC) etc., is determined by the type of organic material, the pyrolysis temperature and the retention time employed (Antal and Gronli, 2003; Gaskin et al., 2008). Besides enhancing soil properties, biochar can sequester carbon, mitigate greenhouse gas emissions, adsorb heavy metals & organic pollutants (Zhang et al., 2013) and improve plant growth (Kumar et al., 2024; Zhang et al., 2016). It also fosters soil microbial activity, diversity, and composition (Elad et al., 2010; Graber et al., 2010; Lehmann et al., 2011).
Biochar shows remarkable stability in soil, with a lifespan extending up to thousands of years. Several recent studies have shown that biochar effectively suppresses various soil-borne plant diseases caused by different pathogens and can even strengthen plant defenses against foliar pathogens like Botrytis cinerea and Leveillula taurica in crops such as tomato and pepper, and in strawberries against Botrytis cinerea, Colletotrichum acutatum, and Podosphaera aphanis (Elad et al., 2010; Meller Harel et al., 2012). Further, biochar can influence the progression of diseases caused by soil-borne pathogens through several mechanisms, including the induction of plant defenses, alterations in soil micro-environment, enhancement of beneficial microbe populations, direct inhibition of pathogen growth via toxic compounds, and changes in rhizosphere exudate chemistry (Graber and Elad, 2013; Graber et al., 2014). For example, the application of rice straw biochar reduced the frequency and severity of tobacco bacterial wilt caused by Ralstonia solanacearum, attributed to improved soil conditions and bacterial diversity (Zhang et al., 2017). Furthermore, biochar application suppresses diseases by directly adsorbing pathogens and indirectly by adsorbing root exudates (Gu et al., 2017). Hence, biochar application emerges as an intriguing approach to plant disease management (Jaiswal et al., 2017). However, most of the studies regarding biochar application have been conducted under laboratory conditions over short durations. The long-term impacts, specifically exceeding 12 months of biochar application on soil microbial communities, soil biochemical properties, and disease remain poorly understood in field experiments. Biochar’s efficiency in disease suppression varies according to the dosage applied (Jaiswal et al., 2014, Jaiswal et al., 2015). It is hypothesized that biochar application would modify soil properties and increase microbial population, particularly beneficial bacteria, thereby reducing stem rot incidence. It was further conjectured that different biochar dosages would have varying degrees of efficacy in controlling the disease. The purpose of this study is to determine the optimal biochar dosage for effectively controlling stem rot disease in groundnut.
The experiment was conducted during the rainy season (June/July to September/October) of 2023 under field and glasshouse conditions at ICRISAT (17° 5’ N lat.; 78° 2’E long.), Patancheru, India. Four groundnut genotypes, such as ICGV 171025, 181035, 171002, and 211107 were included in the study based on their moderate resistance reaction to stem rot disease under artificial epiphytotic conditions in the field and glasshouse (data not shown). Two standard checks, one tolerant (CS 319) (Mahatma et al., 2018; Nogiya et al., 2021) and one susceptible (TMV 2) (Rani et al., 2022; Sunkad et al., 2016) were also included.
The stem rot pathogen, S. rolfsii used in this study was obtained from the culture collection of the Groundnut Pathology laboratory, ICRISAT, Patancheru. The pathogen was initially isolated from infected groundnut plants with characteristic symptoms of stem rot at Patancheru fields and maintained at 25°C on potato dextrose agar (PDA; HiMedia). Before this, Koch’s postulates were used to confirm the pathogenicity of the fungal culture. Sclerotial bodies were harvested from the media plates, cultured for 14 days, and stored at 4°C for further studies.
After being maintained on a PDA medium, the fungus was mass multiplied on sterilized and autoclaved sorghum grains for inoculating the pots under glasshouse and field conditions. Sorghum grains that had been soaked overnight were sterilized in glass flasks (100 g in each flask) and polythene bags (about 500 g each) and were inoculated with mycelial discs (using a borer of 10mm diameter) taken from the margins of actively growing cultures in PDA media. The inoculated flasks and polythene bags were incubated for 10–15 days at 28 ± 2°C (Bera et al., 2016; Jacob et al., 2018). After 30 days after sowing (DAS) for both conditions, the field soils and the sterilized soil in the pots were artificially inoculated with the pathogen inoculum, which was then added to the field soils a second time after 45 days.
A cost-effective, locally made portable kiln with a single barrel was utilized for biochar preparation. The kiln dimensions are 88 cm in length, 81 cm in circumference, and 58 cm in diameter. It features an opening on the top surface with a lid for adding feedstock, measuring 31×31 cm. To assist in the burning process, the bottom of the kiln has approximately 20 openings with a diameter of 3.2 ± 0.1 cm. A wire mesh was placed at the bottom, and groundnut shells were weighed and inserted into the kiln. Groundnut shells were used in the study to produce the biochar through pyrolysis (slow) at 450 - 500°C for 60 minutes. After being produced, the biochar is ground into a fine powder and stored in an airtight container till further use (Figure 1). The analysis of biochar samples involved determining various physio-chemical parameters, which include organic carbon -OC (Walkley-Black method), (Nelson and Sommers, 1982), total nitrogen (N), phosphorus (P) (Setter et al., 2020), potassium (K) (Olsen and Sommers, 1982; Helmke and Sparks, 1996), sulfur (S) (Sparks et al., 1996), water holding capacity (WHC) (Klute, 1986), pH (Thomas, 1996), electrical conductivity (EC) (Rhoades, 1996), bulk density (BD), particle density (PD), and exchangeable cations like calcium (Ca), magnesium (Mg), sodium (Na) (Okalebo et al., 2002) (Table 1).

Figure 1. Groundnut shell biochar prepared at 450-500°C through slow pyrolysis. (A) Clean groundnut shells (B) Bio-charred groundnut shells (C) Powdered biochar.
In this study, biochar was incorporated at concentrations of 1%, 3% and 5%. These concentrations were selected based on prior research that demonstrated their efficacy in controlling soil-borne diseases and promoting plant health. Wang et al. (2024) used biochar concentrations up to 3%, Jin et al. (2023) applied concentrations ranging from 1% to 5%, and Luigi et al. (2022) used biochar concentrations between 1% and 5% for controlling bacterial soil-borne diseases, Fusarium wilt in tomatoes and viral infections in tomato seedlings respectively. Based on these studies, the 3% concentration was identified as the optimal level, with additional 1% and 5% concentrations included to evaluate a broader range of effects.
The biochar samples derived from groundnut shells were analyzed using SEM. The Quanta FEG 250 SEM model from Eindhoven, Netherlands was used with a resolution range capacity of a minimum of 1 nm to examine the surface morphology of biochar samples. The instrument supported with xT microscope control V6 2.8 software was used to examine the pore size of the biochar samples. The surface morphology and micropores of the biochar prepared under pyrolysis conditions are shown in Figure 2. An increased prevalence of interconnected micropores suggests an enhancement in biochar porosity.
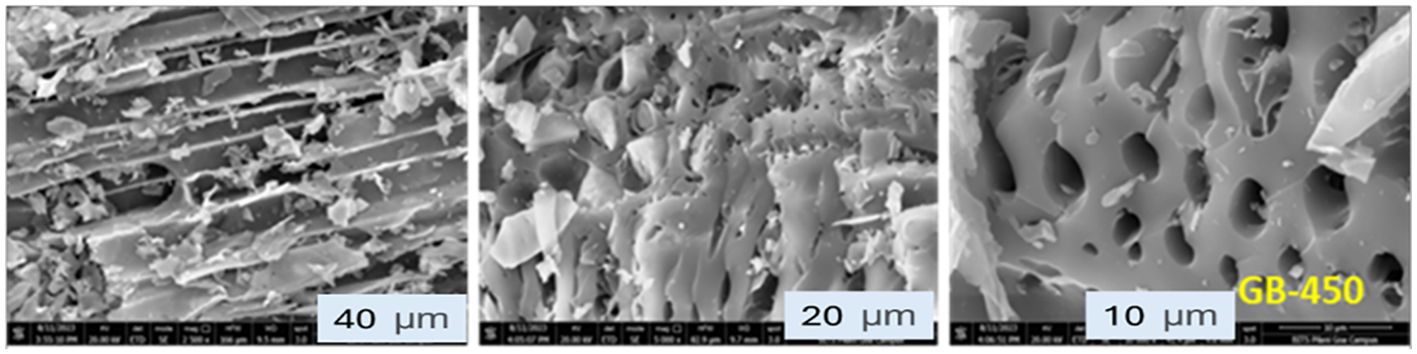
Figure 2. Scanning electron microscopy (SEM) of groundnut shell-derived biochar samples at temperature 450 °C. The structure shown slightly denser with few visible cavities and a combination of oval to slightly irregular macropore shapes were observed.
The biochar bio-assay test was carried out using a poisoned food technique in the laboratory to assess the effectiveness of varying concentrations of biochar against the pathogen. Potato Dextrose Agar (PDA) medium was prepared and poisoned with biochar at levels of 1%, 3%, and 5% (with 1g, 3g, and 5g of biochar added to 100 ml of PDA in a 250ml conical flask, which was subsequently autoclaved) and unpoisoned PDA medium served as control (0%). Approximately 20 mL of the poisoned medium was poured into 90 mm sterilized petri plates and allowed to cool, and all plates were inoculated with a 5 mm mycelial disc of actively growing S. rolfsii. Each treatment was replicated three times These plates were then placed in an incubator at 26 ± 1°C for five days, and the colony diameter was measured, recorded and compared with control (Schmitz, 1930).
The experiment was laid out in a completely randomized design (CRD) for controlled conditions and a split-plot design for field conditions. Four treatments were assessed in these experiments. Each treatment was replicated three times, encompassing the application of 0% (no biochar + Stem rot pathogen inoculation -Sr), 1% (biochar + Stem rot pathogen inoculation – BC1 + Sr), 3% (biochar + Stem rot pathogen inoculation – BC2 + Sr), and 5% (biochar + Stem rot pathogen inoculation – BC3 + Sr) biochar with pathogen inoculation. Biochar was added to the soil two days and thirty days before sowing under controlled and field conditions respectively. Under controlled conditions, the test plants were cultivated in six-inch plastic pots filled with sterilized soil (2 kg per pot), comprising a mixture of one part sand to two parts clay soil, augmented with the specified biochar concentrations. The temperature was maintained at 26 ± 2°C. Nine seeds of each line were sown in three pots, having three seeds in each pot, comprising three replications. The pots were inoculated with pathogen inoculum with an inoculum rate of 8-10 g per plant. Disease incidence (DI) was recorded for every 15 days interval after inoculation and expressed in percentage. The pots were irrigated whenever required.
Under field conditions, the main plot was divided into four sub-plots. Each advanced breeding line (ABL) was sown in three lines on 3 meters width and with a spacing of 0.5 meters between lines and 10 cm between the plants in each subplot. Three blocks were maintained, and each block contained six main plots (12 meters in length x 3 meters in width) with three single lines. Blocks were sufficiently irrigated before the application of inoculum and inoculated on the following day with an inoculum rate of 1.3 kg per 12 m2 plot. DI was recorded for every 30-day interval after inoculation and expressed in percentage.
Stem rot disease severity was measured under glasshouse conditions and expressed in percentage at 60 DAI based on a 1-5 scale wherein, 1= Healthy plant; 2= Lesions on stem only; 3= Up to 25% of the plant symptomatic (wilted, dead, or decaying); 4 = 26- 50% of the plant symptomatic and 5= >50% of the plant symptomatic (Divya Rani et al., 2018) (Figure 3).

Figure 3. Scoring of inoculated plants after application of biochar under controlled conditions. The arrows indicate the level of extension of infection on the plant. 1= Healthy plant; 2= Lesions on stem only; 3= Up to 25% of the plant symptomatic (wilted, dead, or decaying); 4 = 26- 50% of the plant symptomatic and 5= >50% of the plant symptomatic (Divya Rani et al., 2018).
Where, a = Number of disease plants having the same degree of infection, b = Degree of infection, A = Total number of plants and K = Highest degree of infection
At the end of the experiment, the soil was collected from the rhizosphere region from each treatment in the field and analyzed to determine soil pH, OC, EC, available nitrogen, available phosphorous, available potassium, and enzyme activities such as dehydrogenase, urease and sucrase following standard protocols. The urease activity was assessed using urea as a substrate following the method of Yao et al. (2006). Five grams of moist soil was incubated with 1 ml of methylbenzene, 10 ml of 10% urea solution, and 20 ml of citrate buffer (pH 6.7) at 37°C for 24 hours. After incubation, 1 ml of the filtered soil extract, 1 ml of sodium phenolate, and 3 ml of sodium hypochlorite were added, and the mixture was diluted to 50 ml. The absorbance was then measured at 578 nm using a spectrophotometer (Shimadzu corp. UV-spectrophotometer), and urease activity was expressed as NH3-N g⁻¹ h⁻¹ at 37°C.
Dehydrogenase activity was evaluated using triphenyl tetrazolium chloride (TTC) as a substrate, following Thalmann (1968). A TTC solution (0.3–0.4 g/100 ml) was mixed with 5 g of moist soil and incubated for 24 hours at 30°C. After incubation, 40 ml of acetone was added, and absorbance was measured at 546 nm with a spectrophotometer (Shimadzu corp. UV-spectrophotometer). Dehydrogenase activity was expressed as μg TTC g⁻¹ h⁻¹.
The sucrase activity was determined by measuring glucose content after incubation for 24 h at 37°C with sucrose as a substrate. To determine the sucrase activity, 5 g of air-dried soils were incubated for 24 h at 37°C with 15 mL of 8% sucrose, 5 mL of phosphate buffer at pH 5.5, and 0.1 mL of toluene. The solution was filtered, and 3, 5-dinitrosalicylic acid monohydrate solution was then added. The glucose released by sucrase reacted with 3-5-dinitrosalicylic acid and then was measured based on the absorbance at 508 nm with a spectrophotometer (Shimadzu corp. UV-spectrophotometer). The results were expressed as mg glucose g−1h−1 (Wu et al., 2020).
Four replicates of each sub-sample for three enzymes were analyzed. Moreover, substrate-free and soil-free controls were added for each sample to account for non-enzymatic substrate hydrolysis.
The quantity of S. rolfsii present in the soil was assessed using a method adapted from Wang et al., 2013. At 30 days following the inoculation of the pathogen, 10 grams of freshly collected soil were added to flasks with 90 mL of sterile water, then diluted to a concentration of 10-6. The soil suspension was spread onto a PDA medium and incubated in an incubator for two days. A plate culture counting technique was used to determine the quantity of S. rolfsii present in the soil. Similarly, the soils were collected at the time of harvesting and followed the same procedure to know the percent reduction of S. rolfsii population in the soil.
The population densities of bacteria and fungi were assessed using Martin’s dilution method (Martin, 1950). PDA was employed as a growth medium for bacteria and fungi. 10 grams of fresh soil were collected and added to flasks with 90 mL of sterile water, then agitated for 30 minutes at 150 rpm using a shaker. Following this, 100 µL of supernatant from each sample was combined with 900 µL of sterile water in a 2 mL sterile centrifuge tube. Vortex the centrifuge tube to mix the solution thoroughly. Subsequently, the solution was diluted to concentrations of 10-3 and 10-7 for bacterial and fungal analyses, respectively. These dilutions were spread onto the corresponding growth media and then placed in an incubator, maintained at 30°C. Bacterial cultures were incubated for 2 days, and fungal cultures for 5 days as well. Following the incubation period, the number of colonies was recorded to determine the densities of different microbial populations.
Two factor Completely Randomized Design (CRD) and Split-plot design were used for laboratory and field experiments respectively. In both cases, three replications were included. The data were statistically analyzed using SPSS 16 software. Prior to conducting ANOVA, the assumptions of normality and homogeneity of variance were tested using Shapiro wilk and Barlett’s test, respectively. Post-hoc analysis was conducted using Duncan’s Multiple Range Test (DMRT) and non-parametric test Kruskal-Wallis test to identify significant differences among treatment means, with further mean separation performed using critical difference (CD) method.
In the poison food technique conducted under laboratory conditions, different concentrations of biochar (0%, 1%, 3%, and 5%) were investigated against stem rot pathogen. Interestingly, there were no apparent direct inhibitory effects of biochar observed across any concentration level against stem rot pathogen when compared with the control (0% conc.). However, upon closer examination, an indirect suppression of sclerotial body production became evident with increasing concentrations of biochar when compared with control. These findings suggest a complex interplay between biochar concentration and its influence on sclerotial bodies formation of the stem rot pathogen (Figure 4).
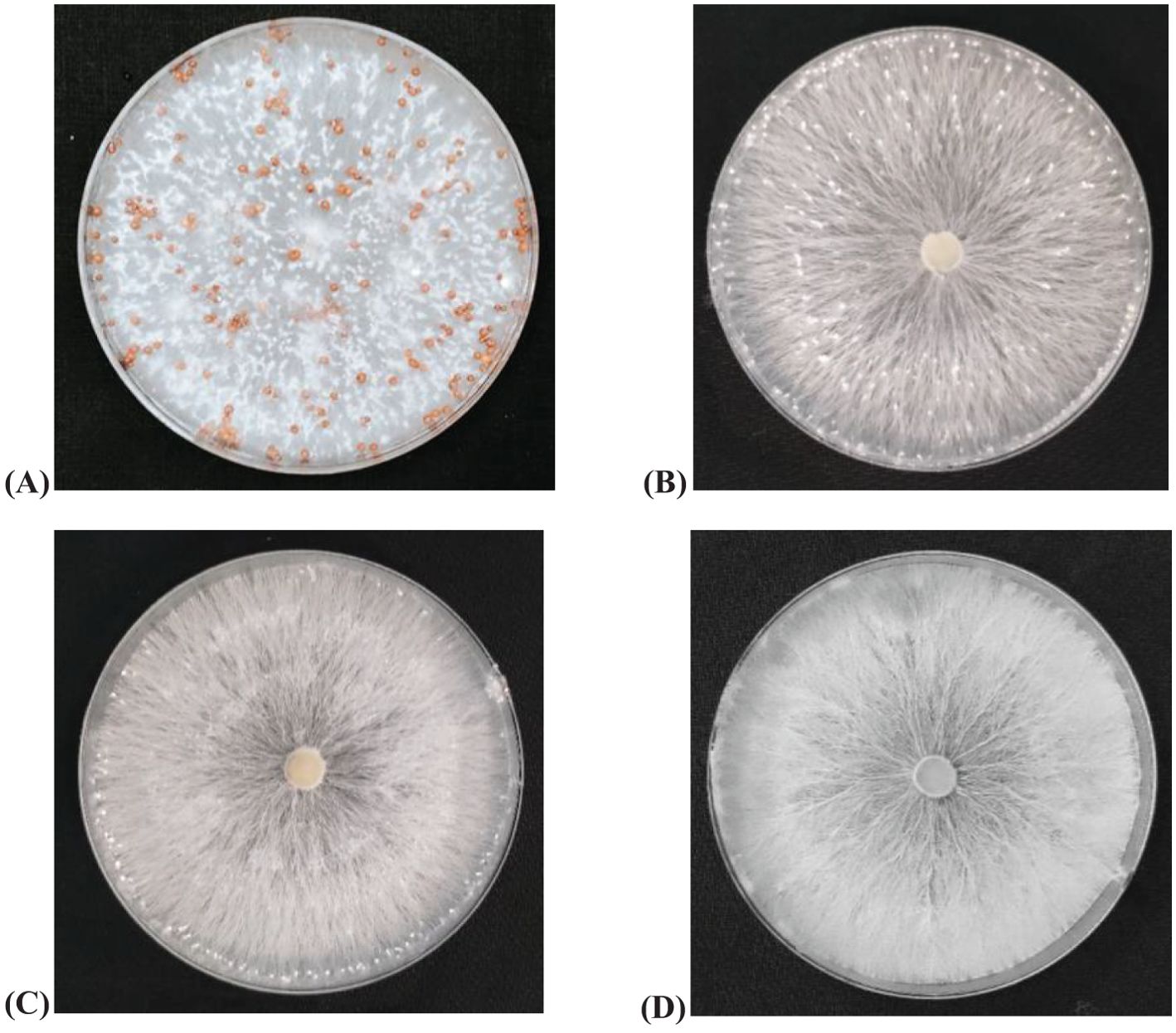
Figure 4. Efficacy of different concentrations of biochar against stem rot pathogen under in vitro evaluation (A) Control (B) 1% conc. (C) 3% conc. (D) 5% conc. The photographs were taken 14 days after observations.
Symptoms of stem rot were evident in the S. rolfsii-inoculated group (Sr) seven days post-inoculation (dpi). However, in the biochar-treated group, these symptoms were delayed until ten days after inoculation, indicating a biochar-induced slowdown in pathogen proliferation. Both biochar treatments effectively restrained the disease progression and enhanced the resistance of groundnut plants to the disease. Out of 4 ABLs evaluated, ICGV 171002 showed the least PDI followed by ICGV 181035, ICGV 211107 and ICGV 171025 when compared with the checks (DMRT test, p <0.05). Of the four different biochar concentrations applied, BC3 + Sr treatment is found to be effective, followed by BC2 + Sr, BC1 + Sr when compared with Sr alone. Among the interactions between genotypes and BC, ICGV 171002 x BC2 + Sr and BC3 + Sr, ICGV 181035 x BC2 + Sr and ICGV 211107 x BC3 + Sr have shown 11.11% incidence followed by ICGV 181035 x BC2+ Sr with 14.81% incidence, ICGV 171002 x BC1 + Sr, ICGV 181035 x BC2+ Sr, ICGV 211107 x BC2+ Sr and BC3 + Sr, ICGV 171025 and CS 319 x BC2 + Sr and BC3 + Sr with 22.22% incidence at 60dpi. These findings suggest that the Spanish and Virginia types with long durations (ICGV 181035 and ICGV 171002 respectively) and with interactions of BC2 +Sr and BC3 + Sr are more effective than other interactions in significantly reducing stem rot disease in groundnut under controlled conditions (Figures 5, 6). Photos of inoculated control pots are not included in Figure 6.
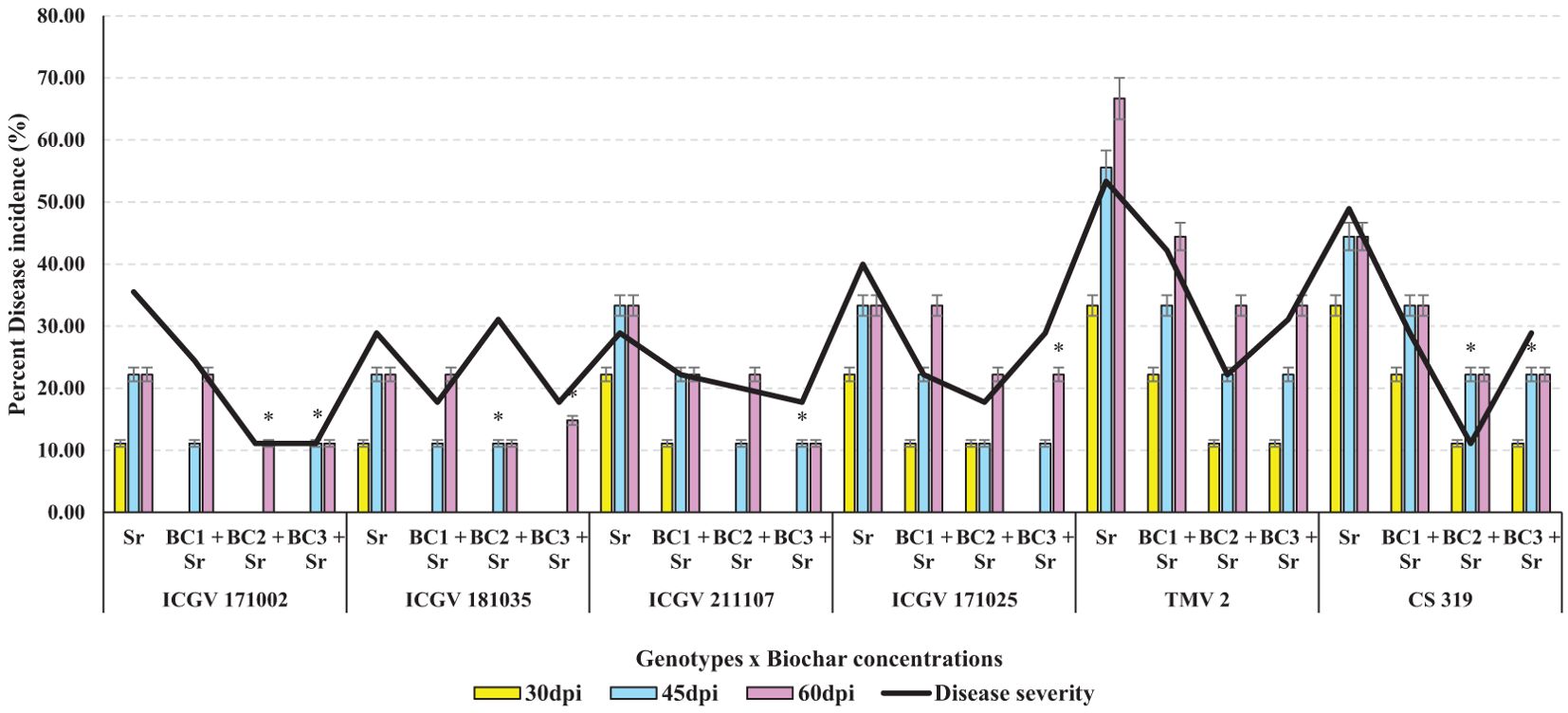
Figure 5. Performance of groundnut ABLs and different rates of application of biochar against S. rolfsii as evaluated under controlled conditions during 2023. (*p < 0.05 - significant).

Figure 6. Performance of different concentrations of groundnut shell biochar in selected ABLs, (A) ICGV 171002, (B) ICGV 181035, (C) CS 319 and (D) TMV 2 against stem rot pathogen. Healthy plant, no biochar and no S. rolfsii inoculation (Sr); 1% concentration, 1% concentration of biochar and S. rolfsii inoculation (BC1+Sr); 3% concentration, 3% concentration of biochar and S. rolfsii inoculation (BC2+Sr); 5% concentration, 5% concentration of biochar and S. rolfsii inoculation (BC3+Sr). The photographs were documented at the end of the experiment (120 DAS).
In field conditions, among the four assessed ABLs, ICGV 171002 displayed the lowest PDI, followed by ICGV 181035, ICGV 171025, and ICGV 211107, compared to checks. Within the tested BC variants, there was no discernible difference between the effectiveness of BC2 + Sr and BC3 + Sr treatments in managing stem rot disease in comparison to Sr alone. Regarding genotype-BC interactions, the incidence of disease was 16.62% for ICGV 171002 x BC3+ Sr, 19.79% for ICGV 181035 x BC3 + Sr, 19.91% for ICGV 171002 x BC2 + Sr, 23.51% for ICGV 181035 x BC2 + Sr, 27.51% for ICGV 171002 x BC1 + Sr and 27.46% for ICGV 181035 x BC1 + Sr when compared to controls and inoculated checks at 90 dpi (Figure 7).
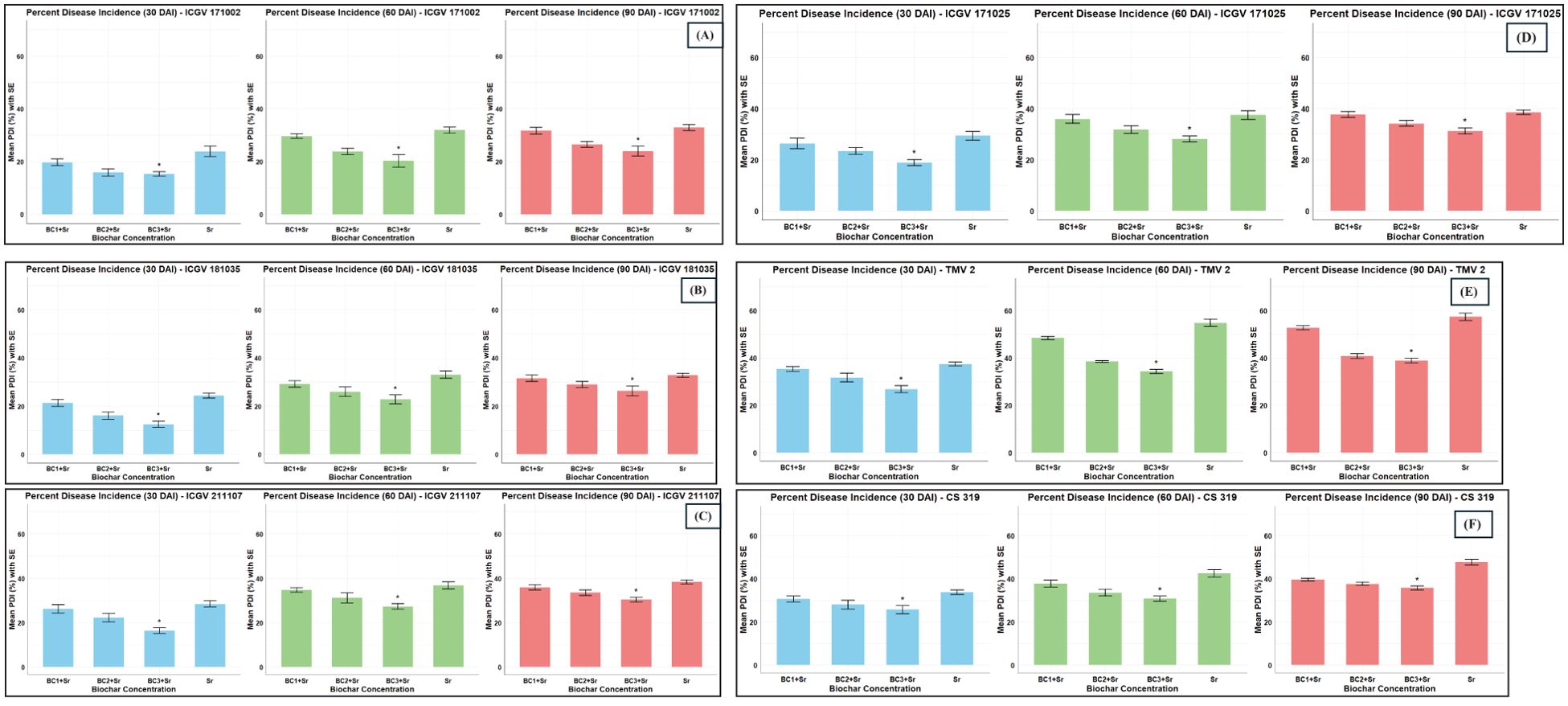
Figure 7. Disease progression over time for different ABLs (A) ICGV 171002 (B) ICGV 181035 (C) ICGV 211107 (D) ICGV 171025 (E) TMV 2 (F) CS 319. Suppressive effects of biochar amendment (at four different concentrations namely Sr, no biochar and no S. rolfsii application; BC1+Sr, 1% concentration of biochar and S. rolfsii inoculation; BC2+ Sr, 3% concentration of biochar and S. rolfsii application; BC3+Sr, 5% concentration of biochar and S. rolfsii inoculation) on S. rolfsii of groundnut ABLs at three different intervals of post inoculation. (*p < 0.05 - significant).
The physical and chemical parameters of the soil were significantly affected (P < 0.05) by the application of biochar amendments. The results unequivocally show that biochar additions greatly improve the physical and chemical characteristics of soil across various groundnut genotypes, particularly at higher concentrations (BC3+Sr). Increased pH, electrical conductivity, organic carbon, and available nutrients (N, P, and K) are some of these enhancements. These alterations imply that biochar might be a useful soil supplement for raising crop productivity and soil fertility, especially in soils with low organic matter or nutrient deficits. Furthermore, although there are genotype-specific reactions to biochar, the general pattern suggests that the advantages of biochar are universal for many types of groundnuts. By raising the pH, biochar inputs reduce the acidity of soils in all genotypes. The pH rises vary based on genotype and level of biochar, from around 0.2 to 1.0 unit. Higher concentrations of biochar show a steady increase in EC, indicating increased ionic activity in the soil that may have an impact on salinity and nutrient availability. Because biochar contains a lot of carbon and can enhance soil organic matter, its content grows dramatically as biochar content increases. Biochar enhances nitrogen availability, which is beneficial for plant growth. This might be the result of enhanced circumstances for bacteria that fix nitrogen and higher nitrogen retention. The increased availability of phosphorus implies that biochar either releases phosphorus or enhances soil properties that facilitate phosphorus uptake and solubilization. A direct contribution from the biochar or better nutrient retention is suggested by the increased potassium availability with biochar. This suggests that adding biochar to soil at 3-5% rates can improve its chemical and physical characteristics (Table 2).
Regarding soil enzyme activities, the urease, sucrase, and dehydrogenase activities in soil treated with biochar (BC2 and BC3) in all genotypes increased significantly (P < 0.05), suggesting that biochar applications ranging from 3-5% can enhance soil urease, sucrase, and dehydrogenase activity when compared with 1% and control. The activities of these are direct indicators of soil microbial activity. High activities of these enzymes signify active microbial metabolism, reflecting healthy and fertile soil conditions. Monitoring these enzyme activities provides valuable information on soil health, the effectiveness of soil management practices, and the progress of soil remediation efforts. Thus, enzymes serve as a key link in understanding and assessing soil microbial dynamics (Figure 8).
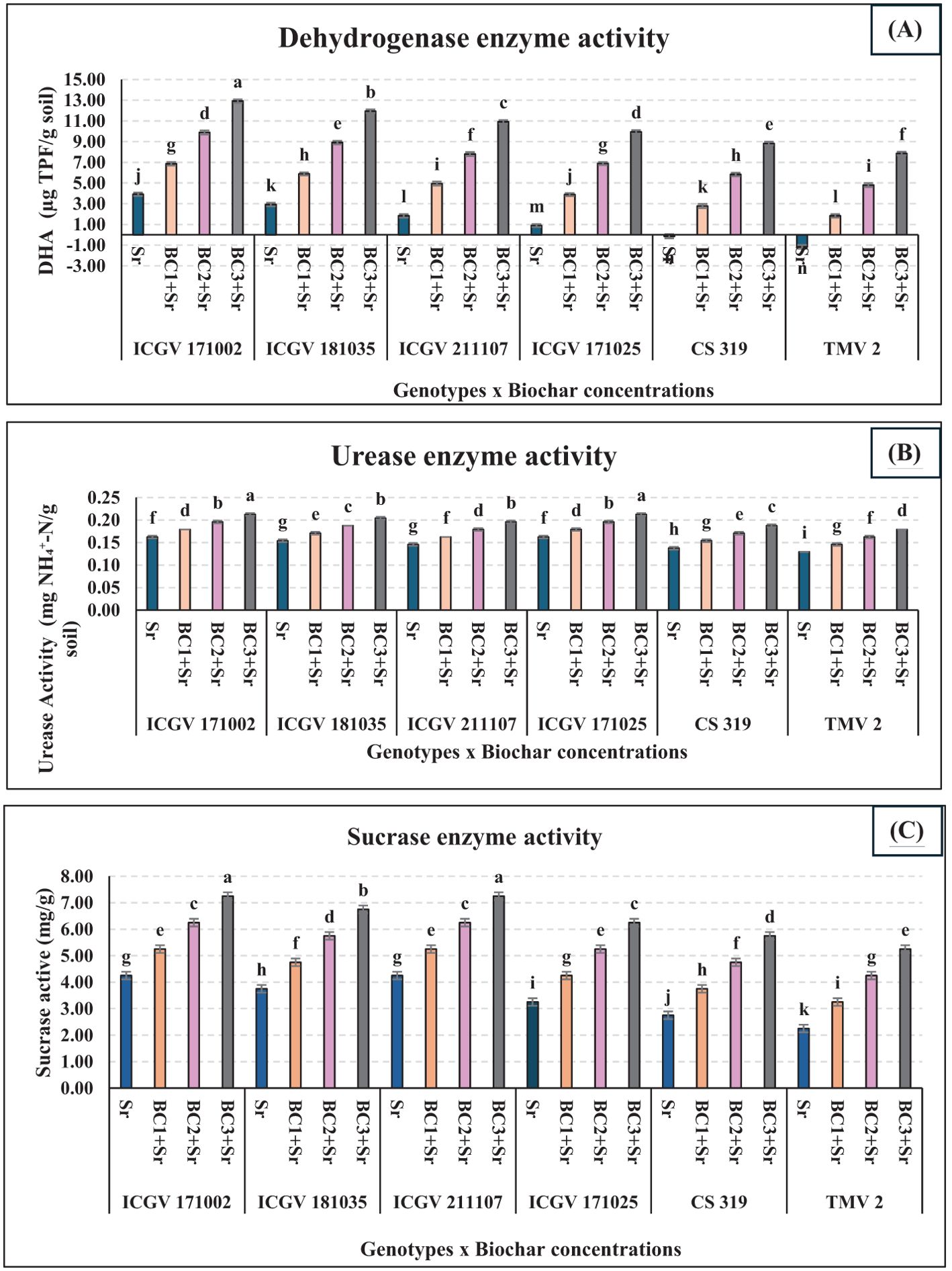
Figure 8. Efficacy of groundnut shells biochar on soil enzyme activities (A) Dehydrogenase enzyme activity (B) Urease enzyme activity (C) Sucrase enzyme activity. In the graph, the letters (a–d, etc.) assigned to each bar represents the results of statistical comparisons, typically from an ANOVA test followed by a post-hoc analysis. Each letter marks groups that do not significantly differ from each other; bars that share the same letter indicate no significant difference in the measured parameter between those groups, while bars with different letters signify statistically significant differences.
This analysis highlights biochar’s influence on the abundance of fungi and bacteria in soil treated with S. rolfsii. The data suggests that biochar application shifts the microbial balance by significantly increasing bacterial populations in soil, potentially enhancing soil health and reducing fungal populations, which may help control fungal pathogens like S. rolfsii. Bacteria thrive in soil with higher biochar concentrations, showing a significant increase from 0% to 3% and stabilizing at 5% (DMRT test, p <0.05). Further studies are recommended to explore the long-term effects of biochar on soil microbial dynamics and to optimize biochar concentrations for various soil types and crops (Figure 9).
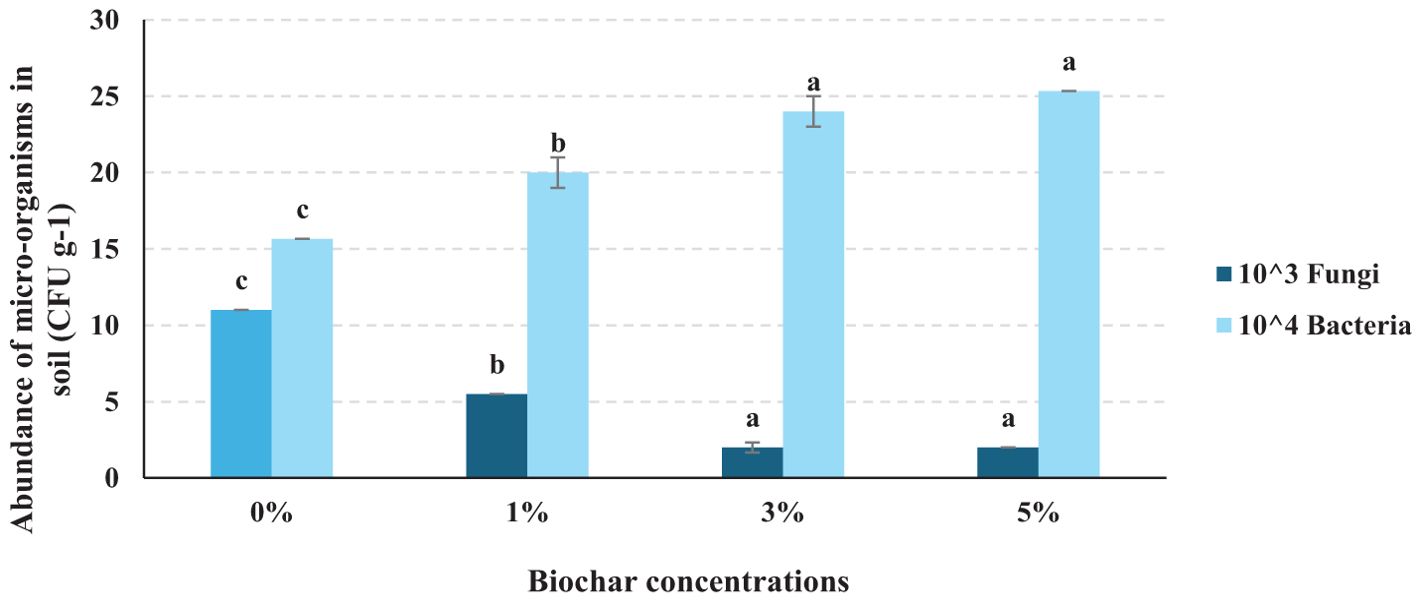
Figure 9. Abundance of soil microbial populations in soil after biochar application and S. rolfsii inoculation to the soil. 10^3 and 10^4indicated at which dilutions, the fungal and bacterial populations were abundant in different biochar concentrations. In the graph, the letters (a–c) assigned to each bar represents the results of statistical comparisons, typically from an ANOVA test followed by a post-hoc analysis. Each letter marks groups that do not significantly differ from each other; bars that share the same letter indicate no significant difference in the measured parameter between those groups, while bars with different letters signify statistically significant differences. .
S. rolfsii inoculum application has significantly increased the populations of S. rolfsii in the soil by 58.33%. However, the soils treated with biochar had significantly lower quantities of S. rolfsii compared to control (Sr). The populations of S. rolfsii in soil were decreased by 28.57% in BC1+Sr, 52.38% in BC2+Sr and 57.14%% in BC3+Sr. The pathogen succumbs in soil with increasing concentrations of biochar, showing a significant decrease from 0% to 3% and stabilizing at 5% (DMRT test, p <0.05) (Figure 10).
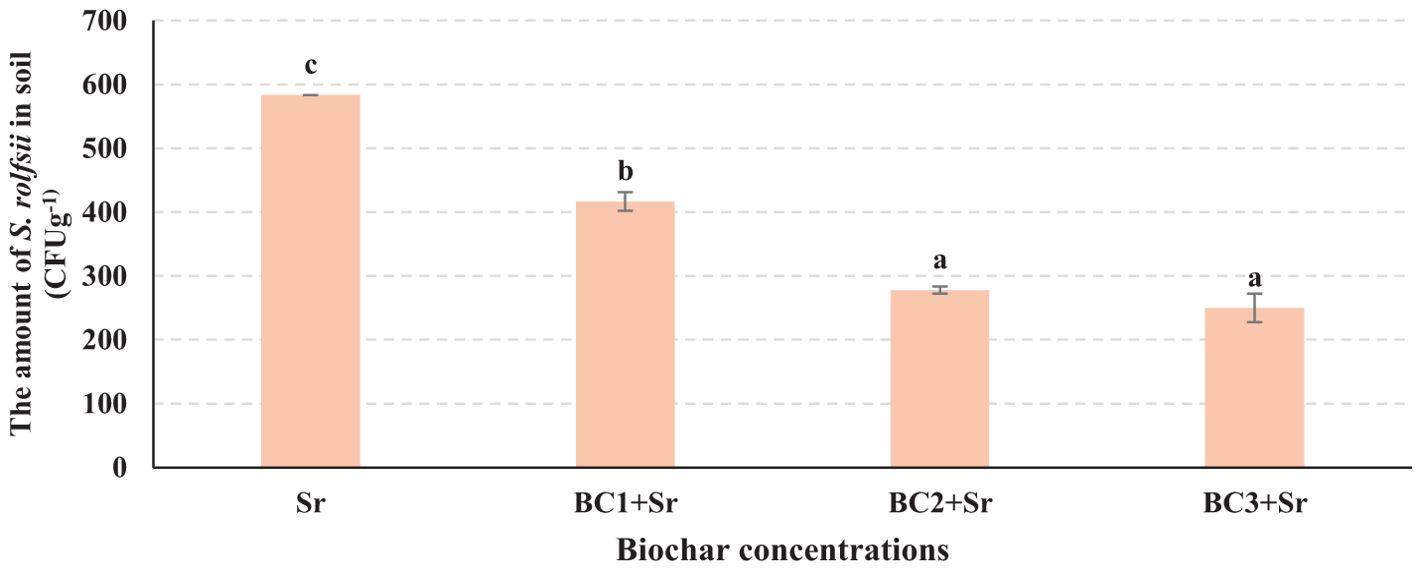
Figure 10. Sclerotium rolfsii population in soil treated with biochar. Serial dilution of pooled soil samples from six groundnut genotypes, mixed according to biochar concentration (0%, 1%, 2%, and 4%). Samples were prepared by combining soil from all genotypes at each concentration level. In the graph, the letters (a–c) assigned to each bar represents the results of statistical comparisons, typically from an ANOVA test followed by a post-hoc analysis. Each letter marks groups that do not significantly differ from each other; bars that share the same letter indicate no significant difference in the measured parameter between those groups, while bars with different letters signify statistically significant differences.
The management of stem rot pathogen in groundnut is a critical concern for sustainable agriculture. Conventional methods include crop rotation, chemical fungicides, and biological control agents for the management of this soil-borne pathogen. Nevertheless, these methods often have limitations, such as their environmental impact, resistance to development, and cost. Therefore, innovative and eco-friendly approaches are necessary to increase disease management and crop productivity. In recent years, biochar has gained attention due to its multifaceted benefits. Research has demonstrated that biochar, a carbon-rich product obtained from the pyrolysis of organic materials improves soil health, enhances nutrient retention, and influences soil microbial communities. Based on recent studies, biochar can indirectly suppress soil-borne pathogens by altering soil pH, enhancing beneficial microbial populations, and improving soil structure. In this context, our study explored the use of groundnut shell biochar as a novel approach for controlling stem rot in groundnut plants. Groundnut shells, an abundant agricultural byproduct, offer a valuable resource for biochar production. Employing groundnut shell biochar recycles waste material and provides a sustainable solution to disease management.
Our in vitro evaluations of biochar concentrations (0%, 1%, 3%, and 5%) against the stem rot pathogen, conducted using the poison food technique, revealed an intriguing pattern. Contrary to initial expectations, there was no direct inhibitory effect on the pathogen across all biochar concentrations compared to the control. This result implies that biochar’s primary mode of action might not involve direct antimicrobial activity. Instead, our study identified as biochar concentrations increased, sclerotial bodies production was indirectly suppressed. The SEM analysis of biochar in our study revealed highly porous surface, with interconnected micropores. This morphology likely plays a crucial role in the indirect suppression of pathogen by increasing surface for microbial colonization. The micropores not only retain moisture and nutrients but also become a habitat for beneficial microbes, which can out compete the pathogen populations. This suppression indicates a complex interaction where biochar might be affecting the environmental conditions or nutrient dynamics in a way that impairs the pathogen’s resting structures. Similar findings were reported by Jaiswal et al. (2014) and Lehmann et al. (2011), who found that biochar amendments in soil may alter nutrient availability and microbial activity, which influences pathogen viability indirectly. Lehmann and colleagues noted that biochar can strengthen the metabolomics process, enhance soil structure, nutrient retention, and foster beneficial microbial communities, all of which can create an environment less favorable to pathogen survival and reproduction. This supports the hypothesis that biochar’s efficacy in suppressing stem rot pathogens is due to its ability to modify the soil micro-environment, enhancing the competitive advantage of beneficial microbes over pathogens.
The fact that our study revealed an indirect inhibition of sclerotial body development emphasizes how crucial it is to comprehend the intricate relationships that exist between microbial communities, soil nutrients, and biochar. Many soil-borne diseases, including S. rolfsii, depend on sclerotia for their long-term survival and propagation. By reducing sclerotial production, biochar may effectively diminish the pathogen’s ability to endure in the soil, thereby reducing the frequency of disease in subsequent crops. This mechanism of action indicates the potential of biochar as a sustainable soil amendment that not only improves soil health but also provides a long-term strategy for managing soil-borne diseases.
When translating these findings to field conditions, it is essential to consider additional variables such as soil composition, weather patterns, and plant-microbe interactions that are inherently present under field conditions. Field experiments provide evidence of how biochar’s indirect effects on the pathogen’s reproductive structures impact disease incidence and plant health over a growing season. Our field studies corroborated the laboratory findings, demonstrating that biochar applications (especially at concentrations of 3% and 5%) effectively delayed symptom onset and reduced disease incidence. This supports the notion that biochar creates less permissive conditions for pathogen proliferation, enhancing plant resistance.
Moreover, our study assessed four ABLs of groundnut, among which ICGV 171002 exhibited the lowest PDI, followed by ICGV 181035, ICGV 211107, and ICGV 171025. These findings highlight the genetic variability in disease resistance among groundnut varieties. The effectiveness of biochar treatments varied with different concentrations (BC3 + Sr, BC2 + Sr, BC1 + Sr, Sr), where BC3 + Sr and BC2 + Sr treatment showed the highest efficacy in lowering the disease incidence compared to the control under control conditions. This aligns with studies indicating biochar’s potential to enhance soil health and plant vigor, thereby reducing disease susceptibility (Jeffery et al., 2011). Under field conditions, both BC2 + Sr and BC3 + Sr treatments effectively controlled stem rot disease compared to controls, although there was no significant difference between their efficacies. However, specific genotype-biochar interactions influenced disease outcomes, with ICGV 171002 and ICGV 181035 consistently exhibiting the lowest PDI across environments, suggesting intrinsic genetic traits that enhance their resistance to the pathogen. This could be due to the differential expression of defense-related genes, where certain genotypes upregulate pathogenesis-related proteins (PR proteins), phenolic compounds, or other defense mechanisms in response to pathogens. Additionally, these genotypes may have better-developed root systems, along with biochar’s ability to improve soil nutrient retention, strengthen plant defenses (Kaur et al., 2017). The greater efficacy treatments also indicate that biochar concentration significantly influences disease suppression by creating optimal rhizosphere environment for resistant genotypes. Differences in genotype responses to biochar treatments may also be linked to ability to modulate soil microbiota and respond to biochar’s influence on plant hormonal signaling pathways, further enhancing their defense responses. These findings emphasize the importance of selecting appropriate groundnut genotypes and optimizing biochar application strategies to maximize disease management benefits in real world field settings. The observed discrepancies between laboratory and field results, particularly regarding the efficacy of different biochar concentrations between BC2+Sr and BC3+Sr, can be attributed to several factors. Field conditions introduce a more complex environment where fluctuating moisture levels, temperature variations and diverse microbial interactions may influence performance of biochar. Additionally, soil properties in the field, including organic matter content, pH and microbial diversity differ from those in controlled conditions, potentially altering how biochar interacts with soil and pathogens. Furthermore, laboratory conditions can be optimized while field environments often present competitive pressures for other micro-organisms, which can affect controlling the pathogen.
Biochar has been shown to alter the abundance and diversity of microbial populations in the soil. In our study, the density of bacteria was increased, and that of fungi was decreased as the biochar concentration increased. Previous studies demonstrated that soil with a pH of neutral and slightly alkaline had a promoting effect on the growth of bacteria but had an inhibitory effect on fungi. Additionally, the pH can indirectly affect the microbiome by modifying the molecular structure of native soil organic matter. Soil microbial activity quantifies soil function, especially in the carbon and nitrogen cycles and the breakdown of organic matter (Ameloot et al., 2013). The biological breakdown of the organic and mineral components of soil is directly mediated by soil enzymes. Soil-specific enzyme activities, such as urease, sucrase, phosphatase, and dehydrogenase activities, are essential to the cycle of nutrients in the soil and can be used to gauge soil microbial activity and assess soil health. Measuring soil function involves measuring soil microbial activity, particularly in the nitrogen and carbon cycles and organic matter decomposition. Certain enzymes, such as hydrolase and glucosidase, aid in organic substances decomposition. Meanwhile, enzymes like amidase, urease, phosphatase, and sulfatase are responsible for nutrient mineralization. Urease, phosphatase, and aryl-sulphatase play a crucial role in the breakdown of nitrogen, phosphorous, and sulfur compounds. In addition, there may be a significant correlation between soil enzymes and disease suppression. Certain enzymes, such as chitinases and glucanases, can enhance plant resistance to infections by breaking down polysaccharides, chitin, and 𝛽-glucans that contribute to fungal cell wall strength. This process ultimately leads to the destruction of the cell wall’s integrity. In their study, Baek et al. (1999) discovered a good correlation between the activity of the chitinase enzyme and the ability to control cotton seedling disease caused by Rhizoctonia solani. According to Woo et al. (1999), the ability of biocontrol to combat B. cinerea on bean leaves was diminished when there was a disruption in chitinase activity. Previous studies demonstrated that biotic stresses could cause activation of plant immunity (systemic resistance), which may act to reshape the microbiome, enriching microbes that likely benefit plant defense. Therefore, we speculate that the higher positive effect of 3% and 5% biochar than other doses on groundnut ABLs and stem rot suppression was linked to their various effects on the diversity and composition of rhizosphere microbial communities, especially the higher promoting influence on some bacteria that possess plant-growth stimulating and plant pathogen-suppressing potentials. The mechanism behind the indirect suppression of the pathogen following the application of biochar to soil is briefly elaborated in the Figure 11.
In conclusion, our study provides compelling evidence that groundnut shell biochar can effectively suppress stem rot disease by modifying soil conditions and enhancing plant resistance mechanisms. Future research should further explore determining the optimal application rates for different crops and soils, evaluating the impact of application timing, and exploring how different types of biochar affects soil health. Additionally, understanding how biochar interacts with different soil conditions and other soil amendments, assessing its long-term effects, whether re-application is needed if so, how often and evaluating its economic and environmental impacts.
The overall results of our study indicate that biochar application indirectly inhibits the growth of stem rot pathogen, increases plant disease resistance, improves soil fertility, and brings a favourable change in soil microbial communities. Biochar’s complex interactions with the soil and pathogens underscore the need for further research to optimize its use across different agricultural settings and soil types. Going forward, studies should focus on the long-term impacts of biochar application, its effects on wide range of pathogens, and the mechanisms by which biochar influences plant-microbe-pathogen interactions. By leveraging biochar’s multifaceted benefits, sustainable agricultural practices can be developed to improve crop health and yield while reducing disease pressures.
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
RM: Data curation, Formal analysis, Investigation, Writing – original draft. RD: Conceptualization, Supervision, Writing – review & editing. KV: Supervision, Writing – review & editing. KJ: Supervision, Writing – review & editing. GR: Supervision, Writing – review & editing. PA: Writing – review & editing. LV: Methodology, Writing – review & editing. KSA: Methodology, Writing – review & editing. KS: Writing – review & editing. GS: Methodology, Writing – review & editing. HS: Conceptualization, Funding acquisition, Project administration, Resources, Supervision, Writing – review & editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. The work has been undertaken as part of the ICAR-ICRISAT Collaborative Projects (2019-23). Partial funding support of the One CGIAR “Plant Health Initiative” is gratefully acknowledged. The first author would like to thank ANGRAU and the Government of Andhra Pradesh for their financial support in the form of stipend.
The authors are thankful to the Groundnut Breeding Unit at ICRISAT, Patancheru, for providing the seed material required for the research.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fagro.2024.1470194/full#supplementary-material
Akhter A., Hage-Ahmed K., Soja G., Steinkellner S. (2015). Compost and biochar alter mycorrhization, tomato root exudation, and development of Fusarium oxysporum f. sp. lycopersici. Front. Plant Science. 6. doi: 10.3389/fpls.2015.00529
Ameloot N., Graber E. R., Verheijen F. G., De Neve S. (2013). Interactions between biochar stability and soil organisms: review and research needs. Eur. J. Soil Science. 64, 379–390. doi: 10.1111/ejss.12064
Antal M. J., Gronli M. (2003). The art, science, and technology of charcoal production. Ind. Eng. Chem. Res. 42, 1619–1640. doi: 10.1021/ie0207919
Baek J. M., Howell C. R., Kenerley C. M. (1999). The role of an extracellular chitinase from Trichoderma virens Gv29-8 in the biocontrol of Rhizoctonia solani. Curr. Genet. 35, 41–50. doi: 10.1007/s002940050431
Bera S. K., Kamdar J. H., Kasundra S. V., Thirumalaisami P. P. (2016). Identification of groundnut genotypes and wild species resistant to stem rot using an efficient field screening technique. Electronic J. Plant Breeding. 7, 61–70. doi: 10.5958/0975-928X.2016.00009.0
Bera S. K., Kasundra S. V., Kamdar J. H., BC A., Lal C., Thirumalasmy P. P., et al. (2014). Variable response of interspecific breeding lines of groundnut to Sclerotium rolfsii infection under field and laboratory conditions. Electronic J. Plant Breeding. 5, 22–29.
Chen S., Qi G., Ma G., Zhao X. (2020). Biochar amendment controlled bacterial wilt through changing soil chemical properties and microbial community. Microbiological Res. 231, 126373. doi: 10.1016/j.micres.2019.126373
Divya Rani V., Sudini H., Narayan Reddy P., Vijay Krishna Kumar K., Uma Devi G. (2018). Resistance screening of groundnut advanced breeding lines against collar rot and stem rot pathogens. Int. J. Pure Appl. Bioscience. 6, 467–474. doi: 10.18782/2320-7051.5494
Elad Y., David D. R., Harel Y. M., Borenshtein M., Kalifa H. B., Silber A., et al. (2010). Induction of systemic resistance in plants by biochar, a soil-applied carbon sequestering agent. Phytopathology. 100, 913–921. doi: 10.1094/PHYTO-100-9-0913
Gaskin J. W., Steiner C., Harris K., Das K. C., Bibens B. (2008). Effect of low-temperature pyrolysis conditions on biochar for agricultural use. Trans. ASABE. 51, 2061–2069. doi: 10.13031/2013.25409
Graber E. R., Elad Y. (2013). Biochar impact on plant resistance to disease. Biochar Soil biota. 21, 278. doi: 10.1201/b14585-3
Graber E. R., Frenkel O., Jaiswal A. K., Elad Y. (2014). How may biochar influence severity of diseases caused by soilborne pathogens? Carbon Manage. 5, 169–183. doi: 10.1080/17583004.2014.913360
Graber E. R., Meller Harel Y., Kolton M., Cytryn E., Silber A., Rav David D., et al. (2010). Biochar impact on development and productivity of pepper and tomato grown in fertigated soilless media. Plant soil. 337, 481–496. doi: 10.1007/s11104-010-0544-6
Gu Y., Hou Y., Huang D., Hao Z., Wang X., Wei Z., et al. (2017). Application of biochar reduces Ralstonia solanacearum infection via effects on pathogen chemotaxis, swarming motility, and root exudate adsorption. Plant Soil. 415, 269–281. doi: 10.1007/s11104-016-3159-8
Helmke P. A., Sparks D. L. (1996). “Lithium, sodium, potassium, rubidium, and cesium,” in Methods of soil analysis: Part 3 chemical methods. (Madison: Soil Science Society) 5, 551–574. doi: 10.2136/sssabookser5.3.c19
Jacob S., Sajjalaguddam R. R., Sudini H. K. (2018). Streptomyces sp. RP1A-12 mediated control of peanut stem rot caused by Sclerotium rolfsii. J. Integr. agriculture. 17, 892–900. doi: 10.1016/S2095-3119(17)61816-1
Jaiswal A. K., Elad Y., Graber E. R., Frenkel O. (2014). Rhizoctonia solani suppression and plant growth promotion in cucumber as affected by biochar pyrolysis temperature, feedstock and concentration. Soil Biol. Biochem. 69, 110–118. doi: 10.1016/j.soilbio.2013.10.051
Jaiswal A. K., Elad Y., Paudel I., Graber E. R., Cytryn E., Frenkel O. (2017). Linking the belowground microbial composition, diversity and activity to soilborne disease suppression and growth promotion of tomato amended with biochar. Sci. Rep. 7, 44382. doi: 10.1038/srep44382
Jaiswal A. K., Frenkel O., Elad Y., Lew B., Graber E. R. (2015). Non-monotonic influence of biochar dose on bean seedling growth and susceptibility to Rhizoctonia solani: the “Shifted R max-Effect. Plant soil. 395, 125–140. doi: 10.1007/s11104-014-2331-2
Jeffery S., Verheijen F. G., van der Velde M., Bastos A. C. (2011). A quantitative review of the effects of biochar application to soils on crop productivity using meta-analysis. Agriculture Ecosyst. environment. 144, 175–187. doi: 10.1016/j.agee.2011.08.015
Jin X., Zhou X., Wu F., Xiang W., Pan K. (2023). Biochar amendment suppressed Fusarium wilt and altered the rhizosphere microbial composition of tomatoes. Agronomy. 13, 1811. doi: 10.3390/agronomy13071811
Kaur H., Salh P. K., Singh B. (2017). Role of defense enzymes and phenolics in resistance of wheat crop (Triticum aestivum L.) towards aphid complex. J. Plant Interactions. 12, 304–311. doi: 10.21203/rs.3.rs-2779843/v1
Klute A. (1986). “Water retention: Laboratory methods,” in Methods of soil analysis: Part 1-Physical and mineralogical methods. (Madison: American Society of Agronomy, Soil Science Society of America), 635–662. doi: 10.2136/sssabookser5.1.2ed.c26
Kumar N. V., Sawargaonkar G., Rani C. S., Pasumarthi R., Kale S., Prakash T. R., et al. (2024). Harnessing the potential of pigeon pea and maize feedstock biochar for carbon sequestration, energy generation, and environmental sustainability. Bioresources Bioprocessing. 11, 5. doi: 10.1186/s40643-023-00719-3
Lehmann J., Rillig M. C., Thies J., Masiello C. A., Hockaday W. C., Crowley D. (2011). Biochar effects on soil biota–a review. Soil Biol. Biochem. 43, 1812–1836. doi: 10.1016/j.soilbio.2011.04.022
Luigi M., Manglli A., Dragone I., Antonelli M. G., Contarini M., Speranza S., et al. (2022). Effects of biochar on the growth and development of tomato seedlings and on the response of tomato plants to the infection of systemic viral agents. Front. Microbiol. 13. doi: 10.3389/fmicb.2022.862075
Mahatma M. K., Thawait L. K., Jadon K. S., Thirumalaisamy P. P., Bishi S. K., Jadav J. K., et al. (2018). Metabolic profiles of groundnut (Arachis hypogaea L.) genotypes differing in Sclerotium rolfsii reaction. Eur. J. Plant Pathology. 151, 463–474. doi: 10.1007/s10658-017-1387-2
Martin J. P. (1950). Use of acid, rose bengal, and streptomycin in the plate method for estimating soil fungi. Soil science. 69, 215–232. doi: 10.1097/00010694-195003000-00006
Mehan V. K., Mayee C. D., McDonald D. (1994). Management of Sclerotium rolfsii-caused stem and pod rots of groundnut—a critical review. Int. J. Pest Manag. 40, 313–320. doi: 10.1080/09670879409371906
Meller Harel Y., Elad Y., Rav-David D., Borenstein M., Shulchani R., Lew B., et al. (2012). Biochar mediates systemic response of strawberry to foliar fungal pathogens. Plant Soil. 357, 245–257. doi: 10.1007/s11104-012-1129-3
Nelson D. W., Sommers L. E. (1982). “Total carbon, organic carbon, and organic matter,” in Methods of soil analysis: Part 2 chemical and microbiological properties. (Madison: American Society of Agronomy, Soil Science Society of America), 9. 539–579. doi: 10.2134/agronmonogr9.2.2ed.c29
Nogiya R., Mahatma M. K., Gowda A., Raval S. S., Rathod V., Gajera H. P., et al. (2021). Changes in expression of polyamines and ethylene biosynthesis genes in groundnut (Arachis hypogaea L.) genotypes during Sclerotium rolfsii infection. Indian J. Exp. Biol. (IJEB). 59, 476–483. doi: 10.56042/ijeb.v59i07.53158
Okalebo J. R., Gathua K. W., Woomer P. L. (2002). Laboratory methods of soil and plant analysis: a working manual. 2nd ed. Vol. 21 (Nairobi: Sacred Africa), pp.25–pp.26.
Olsen S. R., Sommers E. L. (1982). “Phosphorus soluble in sodium bicarbonate,” in Methods of soil analysis, part, 2. (Madison: American Society of Agronomy, Soil Science Society of America), 404–430.
Pasumarthi R., Sawargaonkar G., Kale S., Kumar N. V., Choudhari P. L., Singh R., et al. (2024). Innovative bio-pyrolytic method for efficient biochar production from maize and pigeonpea stalks and their characterization. J. Cleaner Production. 448, 141573. doi: 10.1016/j.jclepro.2024.141573
Rani V. D., Sudini H., Reddy P. N., Devi G. U., Kumar K. V. K. (2022). Integrated Management of Stem Rot and Collar Rot Diseases of Groundnut incited by Aspergillus Niger and Sclerotium rolfsii. Biol. Forum 14, 1524–1530.
Rhoades J. D. (1996). “Salinity: Electrical conductivity and total dissolved solids,” in Methods of soil analysis: Part 3 Chemical methods. (Madison: Soil Science Society of America), 5, 417–435. doi: 10.2136/sssabookser5.3.c14
Setter C., Borges F. A., Cardoso C. R., Mendes R. F., Oliveira T. J. P. (2020). Energy quality of pellets produced from coffee residue: Characterization of the products obtained via slow pyrolysis. Ind. Crops Products. 154, 112731. doi: 10.1016/j.indcrop.2020.112731
Sparks D. L., Page A. L., Helmke P. A., Loeppert R. H., Soltanpour P. N., Tabatabai M. A., et al. (1996). Methods of soil analysis, part 3: Chemical methods (Madison: Soil Science Society of America) 14. doi: 10.2136/sssabookser5.3
Sunkad G., Poornima S. H., Naik M. (2016). Diagnosis of stem and pod rot of groundnut and their management. Indian Phytopathology. 69, 38–40.
Thalmann A. (1968). Zur Metodik der Bestimmung der De-hydrogenaseaktivität im Boden mittels Triphenyltetrazo-liumchlorid (TTC). Landwirtsch Forsch. 21, 249–258.
Thomas G. W. (1996). “Soil pH and soil acidity,” in Methods of soil analysis: part 3 chemical methods. (Madison: American Society of Agronomy, Soil Science Society of America), 5, 475–490. doi: 10.2136/sssabookser5.3.c16
Tian J. H., Shuang R. A. O., Yang G. A. O., Yang L. U., Cai K. Z. (2021). Wheat straw biochar amendment suppresses tomato bacterial wilt caused by Ralstonia solanacearum: Potential effects of rhizosphere organic acids and amino acids. J. Integr. Agriculture. 20, 2450–2462. doi: 10.1016/S2095-3119(20)63455-4
Wang L., Cai K., Chen Y., Wang G. (2013). Silicon-mediated tomato resistance against Ralstonia solanacearum is associated with modification of soil microbial community structure and activity. Biol. Trace element Res. 152, 275–283. doi: 10.1007/s12011-013-9611-1
Wang S., Wang L., Li S., Zhang T., Cai K. (2024). The win–win effects of an invasive plant biochar on a soil–crop system: controlling a bacterial soilborne disease and stabilizing the soil microbial community network. Microorganisms. 12, 447. doi: 10.3390/microorganisms12030447
Woo S. L., Donzelli B., Scala F., Mach R., Harman G. E., Kubicek C. P., et al. (1999). Disruption of the ech42 (endochitinase-encoding) gene affects biocontrol activity in Trichoderma harzianum P1. Mol. Plant-Microbe Interactions. 12, 419–429. doi: 10.1094/MPMI.1999.12.5.419
Wu J., Wang H., Li G., Ma W., Wu J., Gong Y., et al. (2020). Vegetation degradation impacts soil nutrients and enzyme activities in wet meadow on the Qinghai-Tibet Plateau. Sci. Rep. 10, 21271. Available at: https://www.nature.com/articles/s41598-020-78182-9.
Yao X.-H., Min H., Lu Z.-H., Yuan H.-P. (2006). Influence of acetamiprid on soil enzymatic activities and respiration. Eur. J. Soil Biol. 42, 120–126. doi: 10.1016/j.ejsobi.2005.12.001
Zhang C., Lin Y., Tian X., Xu Q., Chen Z., Lin W. (2017). Tobacco bacterial wilt suppression with biochar soil addition associates to improved soil physiochemical properties and increased rhizosphere bacteria abundance. Appl. Soil Ecology. 112, 90–96. doi: 10.1016/j.apsoil.2016.12.005
Zhang D., Yan M., Niu Y., Liu X., van Zwieten L., Chen D., et al. (2016). Is current biochar research addressing global soil constraints for sustainable agriculture? Agriculture Ecosyst. environment. 226, pp.25–pp.32. doi: 10.1016/j.agee.2016.04.010
Keywords: groundnut, shells, biochar, S. rolfsii, stem rot incidence
Citation: Manasa R, Devi RSJ, Vemana K, John K, Rao GR, Anubhava PJ, Vidyashree LK, Sri Ananth K, Santosh K, Sawargaonkar G and Sudini HK (2024) Biochar as a strategy to manage stem rot disease of groundnut incited by Sclerotium rolfsii. Front. Agron. 6:1470194. doi: 10.3389/fagro.2024.1470194
Received: 25 July 2024; Accepted: 09 October 2024;
Published: 31 October 2024.
Edited by:
Aziz Aziz, Université de Reims Champagne-Ardenne, FranceReviewed by:
Aria Dolatabadian, University of Western Australia, AustraliaCopyright © 2024 Manasa, Devi, Vemana, John, Rao, Anubhava, Vidyashree, Sri Ananth, Santosh, Sawargaonkar and Sudini. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hari Kishan Sudini, aGFyaWtpc2hhbi5zdWRpbmlAaWNyaXNhdC5vcmc=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.