- Department of Plant Ecophysiology, Faculty of Agriculture, University of Tabriz, Tabriz, Iran
Introduction: Biofertilizers and intercropping are two main components in sustainable production systems.
Materials and methods: A two-year (2020–2021) study was conducted in East Azarbaijan, Iran, to evaluate the effects of arbuscular mycorrhizal fungi (AMF), growth-promoting bacteria (GPB) and chemical fertilizer (CF) on fenugreek (Trigonella foenum-graecum L.) (F) oil yield and compositions in intercropping with Moldavian balm (Dracocephalam mobdavica L.) (MB). The cropping patterns included MB sole cropping, fenugreek sole cropping (F) and replacement intercropping ratios consisted of Moldavian balm : fenugreek (MB:F (1:1)), MB:F (2:2) and MB:F (4:2) and additive intercropping of MB:F (100:50).
Results: For both years, among the intercropping patters, the highest seed and oil yields were obtained in MB:F (100:50) intercropping pattern treated with CF and AMF+GPB. In all cropping patterns except MB:F (4:2), the highest anthocyanin, total flavonoid, and mucilage contents were observed in plants received AMF+GPB. At all treatments, the linoleic, oleic, and linolenic acid were the main components of fenugreek oil. In MB:F (1:1), (2:2), (4:2), and (100:50) intercropping patterns, the linoleic acid content in AMF+GPB treatment, increased by 9.45%, 6.63%, 15.20%, and 7.82%, respectively, compared with sole fenugreek. The highest total land equivalent ratio (LERT) values were obtained in 2021 and MB:F (100:50) intercropping pattern treated with CF (1.70) and AMF+GPB (1.63).
Conclusions: In general, it could be concluded that MB:F (100:50) intercropping pattern treated with AMF+GPB improved the oil yield and unsaturated fatty acid contents of fenugreek compared with sole cropping and could be recommended in sustainable production systems.
1 Introduction
Fenugreek (Trigonella foenum-graecum L.) is one of the oldest medicinal plants in the world, which belongs to the Fabaceae family (Zandi et al., 2017). This medicinal plant is native to an area extending from Iran to northern India but is now cultivated in China, Greece, Ukraine, and north and east Africa (Petropoulos, 2002). Also, in central regions of Iran, different species of this plant are used for traditional Persian medicine (Jhajhria and Kumar, 2016). This plant is recommended for arid and semi-arid regions of Asia, sub-Saharan Africa, and Latin America as a low input and annual dryland legume. In Iran, fenugreek could be used for commercial production for small-scale farms with low capacity for investment (Basu et al., 2017). It has been reported that fenugreek is useful for humans in the treatment of a number of diseases, including reducing blood glucose, blood cholesterol, hair loss, liver ailments, and skin eruptions (Camlica and Yaldiz, 2024), because it contains trigonelline, diosgenin, flavonoid, and other compounds (Zandi et al., 2017). Moldavian balm (MB) (Dracocephalam mobdavica L.) is an herbaceous and annual medicinal plant, native to Central Asia and domesticated in Central and Eastern Europe (Vafadar-Yengeje et al., 2019; Amini et al., 2020). All organs of this plant contain essential oil, and their content varies depending on organ type, nutrients availability, and ecological factors (Hussein et al., 2006).
One of the main goals of agricultural systems is to achieve production stability and increase the productivity of agricultural ecosystem, through intercropping different compatible crops (Banik and Sharma, 2009). Intercropping system is aimed at creating an ecological balance, using more resources; reducing the damage of pests, diseases, and weeds; and reducing soil erosion and economic risk of production by increasing the quantity and quality of performance against time and place (Marastoni et al., 2019). The differences in nutrient uptake by different plants is important when designing intercropping systems and the use of legumes in intercropping is an effective way to compensate for nitrogen deficiency in the soil and increase production (Raza et al., 2021; El-Mehy et al., 2023). Hence, the implementation of the intercropping system of medicinal plants, one of its components is nitrogen fixation, can play a more effective role in using environmental resources and increase the productivity of the cropping system (Yaseen et al., 2014; Sakhavi et al., 2017a, Sakhavi et al., 2017b). Few studies have shown that intercropping system can affect the production, qualitative aspects, and chemical compositions of medicinal plants (Weisany et al., 2015; Vafadar-Yengeje et al., 2019; Amini et al., 2020; Rezaei-Chiyaneh et al., 2021).
In sustainable agricultural systems, one of the solutions to improve and maintain soil fertility is the use of internal (in-farm) inputs, including beneficial soil microorganisms that are known as biofertilizers (Sharma et al., 2013; Amini et al., 2017). These microorganisms are of special importance in sustainable agriculture with the aim of stimulating the nutrients cycle and reducing the need for chemical fertilizers (CFs) (Turan et al., 2010; Sarikhani and Amini, 2020). Among the biological fertilizers are arbuscular mycorrhizal fungi (AMF), which are able to increase the effective surface of the roots by creating a wide network and provide access to a large volume of soil (de Assis et al., 2020). Earlier studies have shown that mycorrhizal fungi cause significant changes in the quantity and quality of secondary metabolites of medicinal plants (Merlin et al., 2020). Using the mycorrhizal fungi in intercropping of dill (Anethum graveolens L.) with common bean (Phaseolus vulgaris L.) increased the essential oil yield of dill (Weisany et al., 2015). Also, the positive effect of mycorrhizal fungus on essential oil yield of dill and carum (Trachyspermum ammi Sprague) (Kapoor et al., 2002) and chamomile (Matricaria chamomilla L.) (de Almeida et al., 2020) have been reported. Atmospheric N2 can be fixed in the form of nitrate and ammonium ion by certain strains of Azospirillum, Azotobacter, and Rhizobium, which can be taken up by the plants, thereby improving growth (Sahoo et al., 2012). Azotobacter serves as a biofertilizer for important crops, such as wheat, barley, sesame, rice, maize, and sunflower. In addition to N2 fixation, Azotobacter is as a rich source of phytohormones such as gibberellins (GA) and indole acetic acid (IAA) (Dar et al., 2021). Azospirillum can enhance plant growth, development, and yield by increasing N2 status of the plant that could be attributed to different mechanisms, such as auxin synthesis and biological N2 fixation (Sahoo et al., 2012). Therefore, in production of medicinal plants, using biofertilizers could improve the quantity and quality of oil constituents, which is compatible with the goals of sustainable agricultural. Due to the necessity of evaluating the ecological dimensions of intercropping in sustainable production, this experiment was conducted with the aim of evaluating the oil yield and compositions of fenugreek in sole and intercropping with MB under biofertilizer [growth-promoting bacteria (GPB) and mycorrhiza] treatments.
2 Materials and methods
2.1 Experimental site, design, and field practice
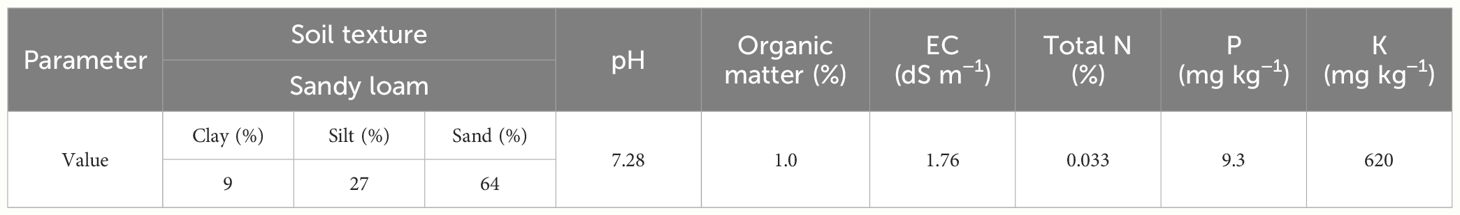
This research was conducted in Maragheh City in East Azarbaijan province, Iran (latitude 37˚4 N, longitude 46˚26 E, altitude 1478 m above sea level) in 2020 and 2021 growth seasons. The climatic data of monthly total precipitation and mean temperature of the experimental site during the growth seasons of 2020 and 2021 are presented in Table 1. The soil characteristics of the experimental field at a depth of 0–30 cm are presented in Table 2.

Table 1 Monthly total precipitation and mean temperature in 2020 and 2021 growing seasons in the experimental area.
The 5 × 3 factorial experiments were carried out based on randomized complete block design with three replications in 2020 and 2021. The cropping pattern (first factor) consisted of five levels: MB sole cropping, fenugreek sole cropping (F), and replacement intercropping ratios including 1 row of MB + 1 row of fenugreek (MB:F (1:1)), 2 rows of MB + 2 rows of fenugreek (MB:F (2:2)), and 4 rows of MB + 2 rows of fenugreek (MB:F (4:2)) and additive intercropping of MB + fenugreek MB:F (100: 50) (100% density of MB + 50% density of fenugreek planted between MB rows). The fenugreek is dominated crop, and the MB is dominating crop. The fertilizer treatment (second factor) consisted of three levels: 100% CF, application of AMF, and combined application of AMF and GPB (AMF+GPB). CF treatment was 50 kg ha−1 urea and 80 kg ha−1 triple superphosphate (according to soil test results), which were applied at planting time. Myco-Root bio-fertilizer contains arbuscular mycorrhiza fungi (AMF) of Glomus mosseae, Glomus intraradices, and Glomus etunicatum with count 107 to 108 CFU/g is provided by Zist Fanavar Pishtaz Varian Company, Karaj, Iran. This bio-fertilizer is an easy-to-use powder form that is used for crops as seed inoculation. According to the manufacturer’s instructions, 1 kg of MB and fenugreek seeds were placed in the shade on a clean surface, and after spraying a small amount of water on them, 40 g of AMF bio-fertilizer was added and mixed thoroughly, so that all the seeds were covered with a layer of bio-fertilizer. For inoculating the GPB, Biofarm bio-fertilizer used contained Azospirillum brasilense and Azotobacter chroococcum bacteria with a population of 2 × 107 CFU/g and was provided by Nature Biotechnology Company (Biorun) Karaj, Iran. According to the manufacturer’s instructions, 1 kg of MB and fenugreek seeds were inoculated with 40 mL of Biofarm and then planted. Also, the seeds of fenugreek were inoculated with Sinorhizobium meliloti for nitrogen fixing through symbiosis.
The deep mouldboard ploughing (25–30 cm) was used in the spring for seedbed preparation, which was followed by disk harrowing. The seeds of fenugreek and MB were planted manually at densities of 500,000 and 320,000 plants ha−1, respectively. In sole cropping and intercropping patterns, both crops were planted with 25 cm row space on 4 May 2020 and 15 May 2021. The size of the experimental plots in sloe fenugreek, sole MB, replacement intercropping patterns of MB:F (1:1), MB:F (2:2)) and additive intercropping of MB:F (100: 50) were 3 m (12 rows) wide × 3 m long. In replacement intercropping of MB:F (4:2), the size of the experimental plot was 4 m (16 rows) wide × 3 m long. The furrow irrigation was done after planting of both crops with 5-day intervals till seed maturity. During the growing season, the weeds in experimental plots were removed 3, 5, 7, 9, and 11 weeks after sowing by hand weeding. There was no need for pesticide application in the experimental field.
2.2 Fenugreek growth, seed, and oil yield
In each plot, 10 plants were randomly selected after removing the marginal effects (side rows and half a meter from the sides of the middle rows), and the selected plants were tagged before flowering stage (40 days after sowing). In both years, at maturity stage on 8 August 2020 (96 days after sowing) and 22 August 2021 (99 days after sowing), the fenugreek height was measured with a steel rule with the least count of 0.5 mm. To measure leaf chlorophyll content index (SPAD), chlorophyll content Meter SPAD-502 (Konica Minolta) device was used in vegetative growth stage (30 and 32 days after sowing in 2020 and 2021, respectively) and flowering stage (49 and 51 days after sowing in 2020 and 2021, respectively). Five plants were randomly selected from each plot, and the chlorophyll content index was recorded in three new full expanded leaves from upper, middle, and lower part of each plant and the average of the recorded values for two stages were used in data analysis (Vafadar-Yengeje et al., 2019). To determine fenugreek seed yield, in maturity stage, the plants in the middle rows of 1 m−2 area of each plot were harvested and dried at room temperature for 48h, and after threshing, the seed yield was determined. In order to extract the oil, 10 g of crushed seeds of each treatment were packed in Whatman paper, and then oil extraction was done using Soxhlet apparatus and 400 mL n-hexane solvent for 2.5h at 70°C. After 8h, the solvent was evaporated from the oil using a rotary and by measuring the amount of oil; it was measured as a percentage (oil content) (Fotohi Chiyaneh et al., 2022). Finally, the oil samples were stored at 4°C until the identification of chemical compounds with a gas chromatography–mass spectrometry (GC–MS) device. Oil yield was calculated using Equation 1:
2.3 Gas chromatography–mass spectrometry
The seed oil of fenugreek was analyzed using a GC–MS (Agilent 6890N, USA) with HP-5 MS column (30-mm diameter of tubular column, 0.25-mm internal diameter, and 0.25-lm thickness of film) as described with Fotohi Chiyaneh et al. (2022). The fatty acid methyl esters were prepared using the method described by Heidari et al. (2020). Two hundred microliter of the 2.0 M solution of methanolic potassium hydroxide was added to 50 mg of the sample in 2 mL n-hexane. The mixture was vigorously vortexed for 1 min and allowed to stand in a dark place until it becomes separate into two phases. After the upper hexane layer became transparent, 1 µL was injected into the GC–MS column. The identification of the chemical compounds of the oil was done by matching the mass spectra obtained of the sample through comparison with the mass spectrum report provided by Wiley 7.0 and Adams (Adams, 2001). The GC–MS analysis was done for fenugreek seed oil obtained in all treatments.
2.4 Anthocyanin, total flavonoid, and mucilage contents
In order to measure the amount of anthocyanin in fenugreek oil, 0.02 g of dry seeds obtained in all experimental plots (treatments) were ground with 4 mL of 1 M hydrochloric acid solution containing methanol in a porcelain mortar and, after 24h of storage in the refrigerator, the obtained solution was centrifuged for 10 min at 13,000 rpm. Then the upper phase was removed and the absorbance of the solutions was measured at 530 and 657 nm with a spectrophotometer (Mita et al., 1997). One molar hydrochloric acid of methanol solution was also used as a control and the amount of anthocyanin was obtained using Equation 2:
where A is the absorption of the solution and subscript numbers indicate the wavelengths in which the absorption was measured.
To measure the total flavonoid content of fenugreek, 0.1 g of dry seeds obtained in all experimental plots (treatments) were ground with 5 mL of ethanol in a porcelain mortar and then centrifuged at 10,000 rpm for 5 min. Then, 500 µL was removed from the upper phase and 1.5 mL of ethanol, 100 µL of 10% aluminum chloride, 100 µL of 1 M potassium acetate, and 2.8 mL of distilled water were added, and then was kept for 40 min at room temperature. Then the absorbance of the solutions was measured at 415 nm compared to the control without herbal extract (Chang et al., 2002). Finally, by placing the absorption value of the samples in the standard curve equation of quercetin, the amount of total flavonoid was measured in terms of mg of quercetin per g of seeds dry weight. The mucilage content was measured by the method of Kalyanasundaram et al. (1982). In all experimental plots (treatments), the combination of 1 g of dry seed and 10 mL of 0.1 normal hydrochloric acid solution was heated until the color of the seed shell changed, and after adding 60 mL of 96% ethyl alcohol, it was kept in the refrigerator for 5h. After filtering, the sediment was placed in a 50°C oven for 12h. Finally, after weighing, the mucilage content in fenugreek seeds was measured as a percentage.
2.5 Land equivalent ratio
In fenugreek–MB intercropping patterns the land equivalent ratio (LER) values were evaluated using Equations 3 and 4:
Where YF and YFI are the fenugreek seed yields in sole cropping and intercropping patterns, respectively, and YMB and YMBI are the MB dry herbage yields in sole cropping and intercropping patterns, respectively. Also, LERF and LERMB represent the partial LER of fenugreek and MB, respectively, and LERT is the total LER.
2.6 Statistical analysis
For analysis of variance (ANOVA), the SAS version 9.0.3 package was used. For two growing seasons of 2020 and 2021 and all traits, the combined ANOVA was done based on complete randomized block design with 15 treatments and three replicates. The data of LERF, LERMB, and LERT were not subjected to analysis of variance. The experimental data met the assumptions of normality and variance homogeneity, and no transformation was needed. For comparison of the means, the Duncan’s multiple range test was used at p ≤ 0.05.
3 Results
3.1 Fenugreek plant height
The effects of year, cropping pattern, year × cropping pattern, and fertilizer treatment were significant (p ≤ 0.05) on fenugreek plant height (Table 3). The interaction effect of year × cropping pattern (Figure 1) indicated that, in sole fenugreek, MB:F (4:2) and MB:F (100:50) intercropping patterns, the plants in 2020 were taller than those in 2021, while in MB:F (1:1) intercropping, the plants were taller in 2021. In MB:F (2:2) intercropping, the plants heights in 2020 and 2021 were not significantly different. Also the plants that received CF were taller than those in AMF and AMF+GPB treatments (Table 4).
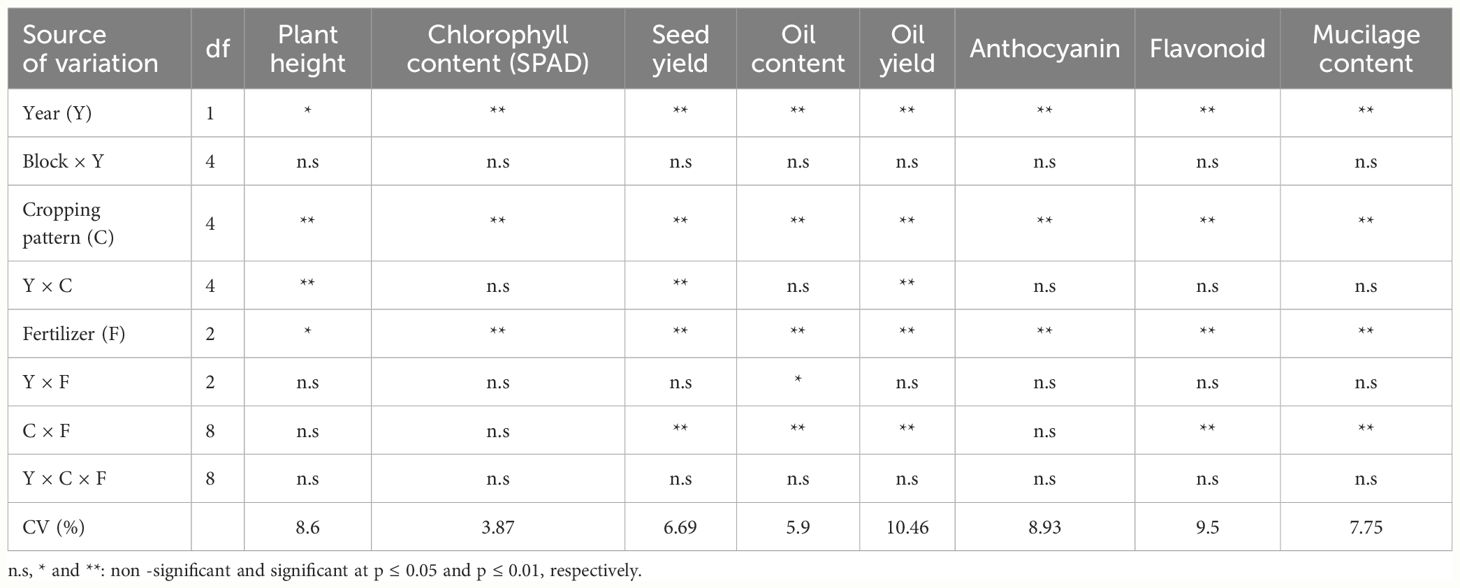
Table 3 Analysis of variance for effect of cropping system on selected traits of fenugreek under different fertilizer treatments.
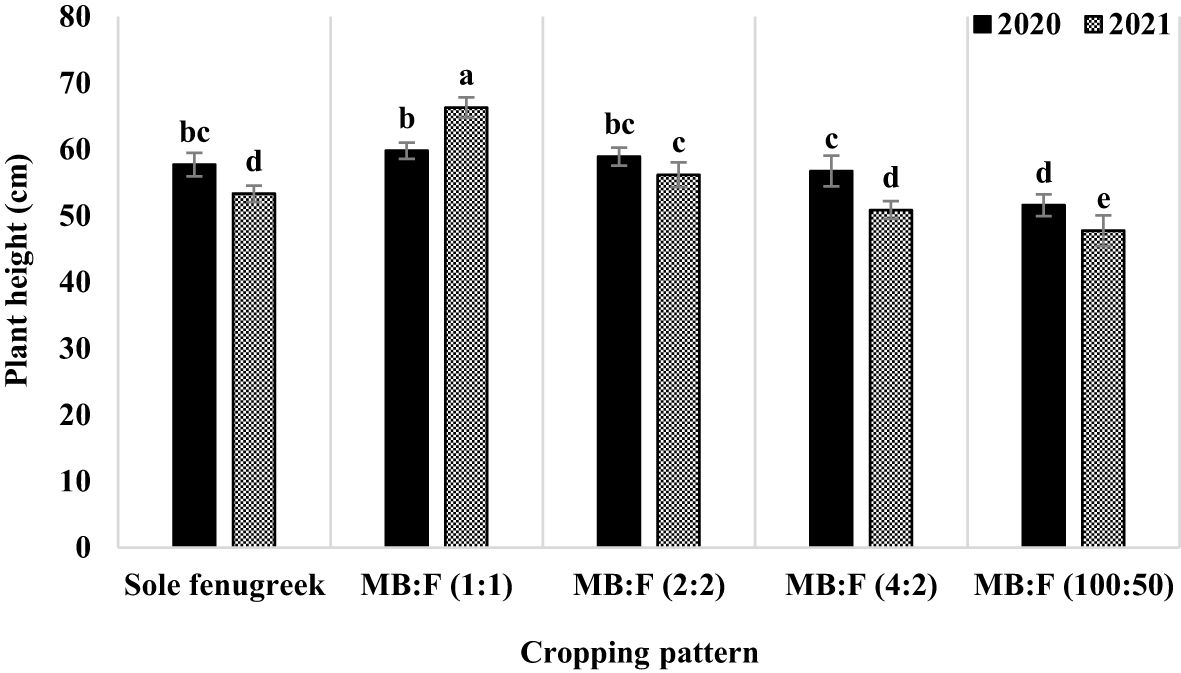
Figure 1 Plant height of Moldavian balm as influenced by year and cropping pattern. Different letters indicate significant differences at p ≤ 0.05.
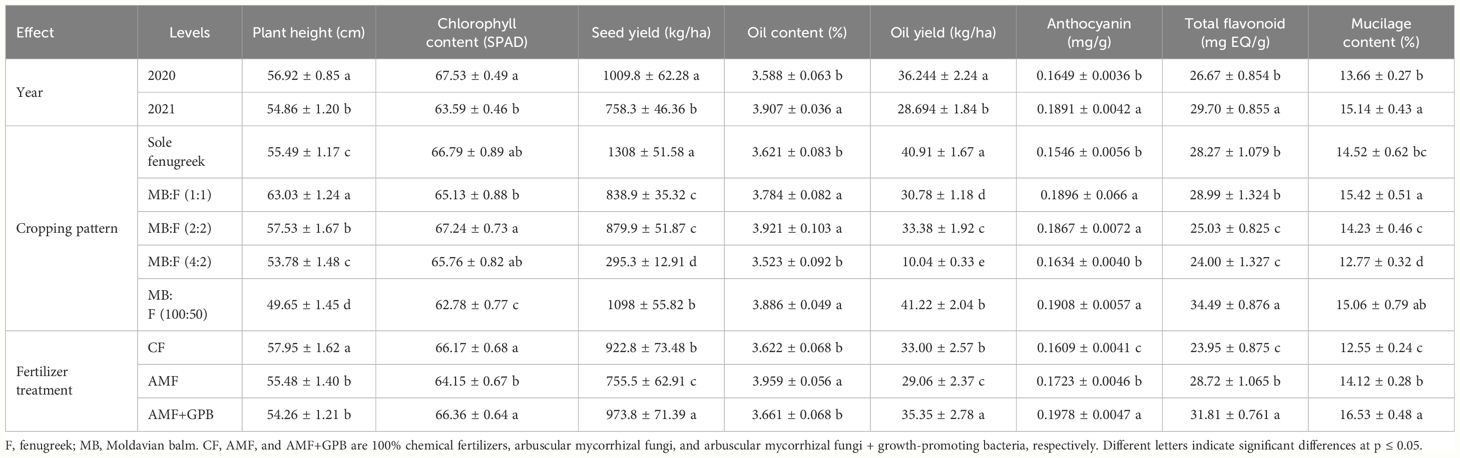
Table 4 The effect of year, cropping pattern, and fertilizer treatment on fenugreek height, chlorophyll content, seed yield, oil content, oil yield, anthocyanin, total flavonoid, and mucilage.
3.2 Leaf chlorophyll content index (SPAD)
The effects of year, cropping pattern, and fertilizer treatment were significant (p ≤ 0.01) on fenugreek SPAD (Table 3). The SPAD value in 2020 was higher than 2021 (Table 4). The highest (67.24) and lowest (62.78) SPAD values were observed in MB:F (2:2) and MB:F (100:50) intercropping patterns, respectively (Table 4). The SPAD values in plants treated with AMF+GPB and CF treatments increased significantly compared with that in AMF treatment.
3.3 Seed yield (kg/ha)
Fenugreek seed yield was influenced by year, cropping pattern, year × cropping pattern, fertilizer treatment, and cropping pattern × fertilizer treatments (p ≤ 0.01) (Table 3). The interaction effect of year × cropping pattern (Figure 2) showed that, in all cropping patterns except the MB:F (4:2), the seed yields in 2020 were higher than those in 2021. In MB:F (4:2) intercropping, the seed yields in 2020 and 2021 were not significantly different. The interaction of cropping pattern × fertilizer (Figure 3) showed that, in sole fenugreek, MB:F (4:2) and MB:F (100:50) intercropping patterns, the seed yields of plants received CF and AMF+GPB treatments were not significantly different, while in MB:F (1:1) and MB:F (2:2) intercropping patterns, the highest seed yields were observed in plants treated with AMF+GPB. Among the intercropping patterns, the highest seed yield was produced in MB:F (100:50).
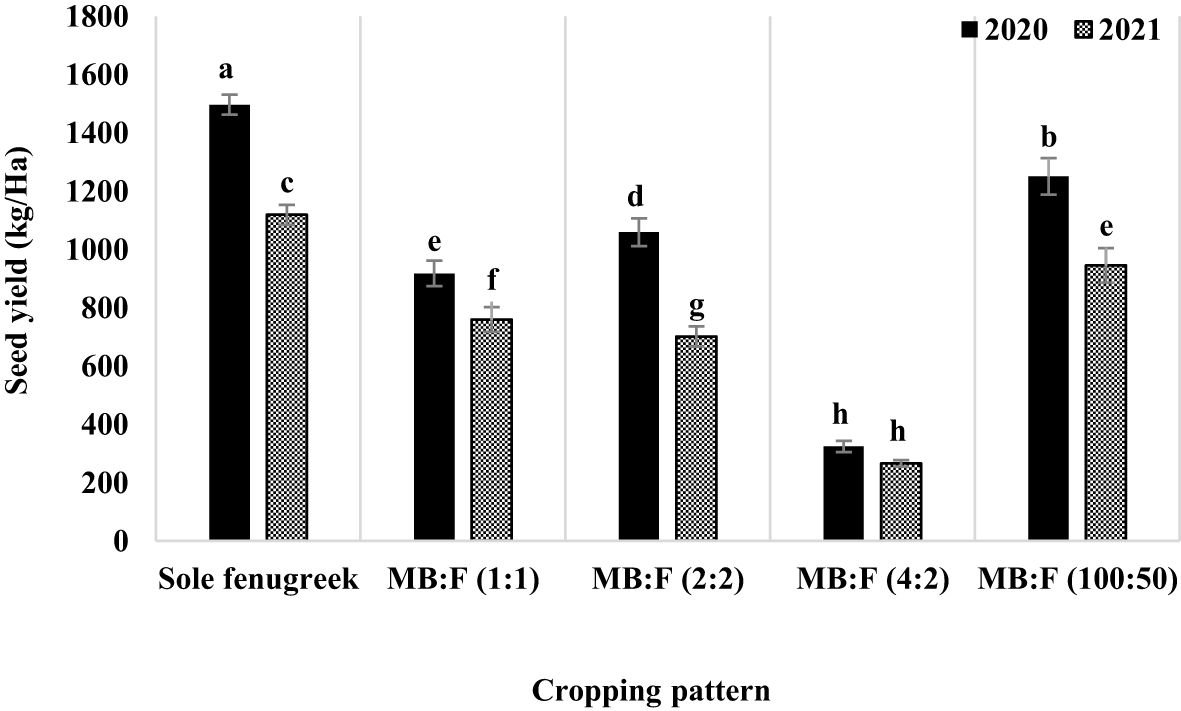
Figure 2 Fenugreek seed yield as influenced by year and cropping pattern. Different letters indicate significant differences at p ≤ 0.05.

Figure 3 Fenugreek seed yield as influenced by cropping pattern and fertilizer treatments. Different letters indicate significant differences at p ≤ 0.05.
3.4 Seed oil content (%)
The effects of year, cropping pattern, fertilizer treatment, and interaction effects of year × fertilizer treatment and cropping pattern × fertilizer treatment were significant (p ≤ 0.01) on fenugreek seed oil content (Table 3). The interaction effect of year × fertilizer treatment (Figure 4) indicated that, in plants treated with CF and AMF+GPB, the oil contents in 2020 were lower than those in 2021. The oil contents of plants treated with AMF were not significantly different in 2020 and 2021. The interaction effect of cropping pattern × fertilizer (Figure 5) indicated that, in sole fenugreek, the lowest oil content was observed in plants treated with CF, while in MB:F (1:1), MB:F (4:2) and MB:F (100:50) intercropping patterns, the oil contents of plants received CF and AMF+GPB treatments were not significantly different. In MB:F (4:2) intercropping pattern, the plants treated with AMF had higher oil content than those in CF and AMF+GPB treatments (Figure 5).
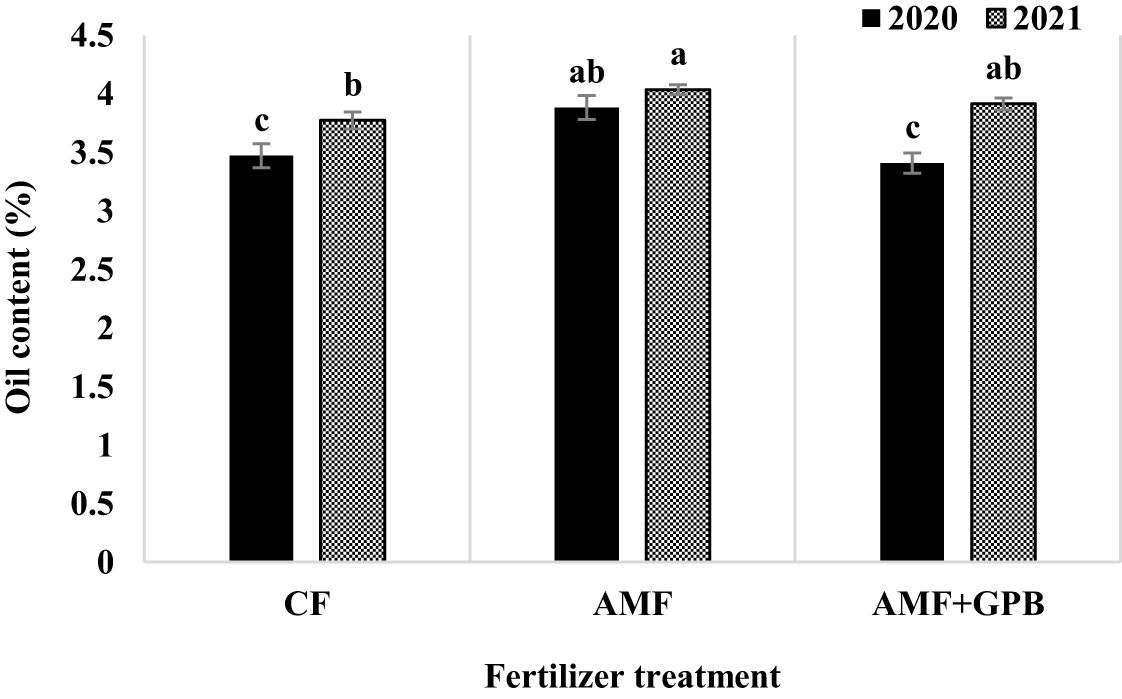
Figure 4 Fenugreek oil content as influenced by year and fertilizer treatments. Different letters indicate significant differences at p ≤ 0.05.
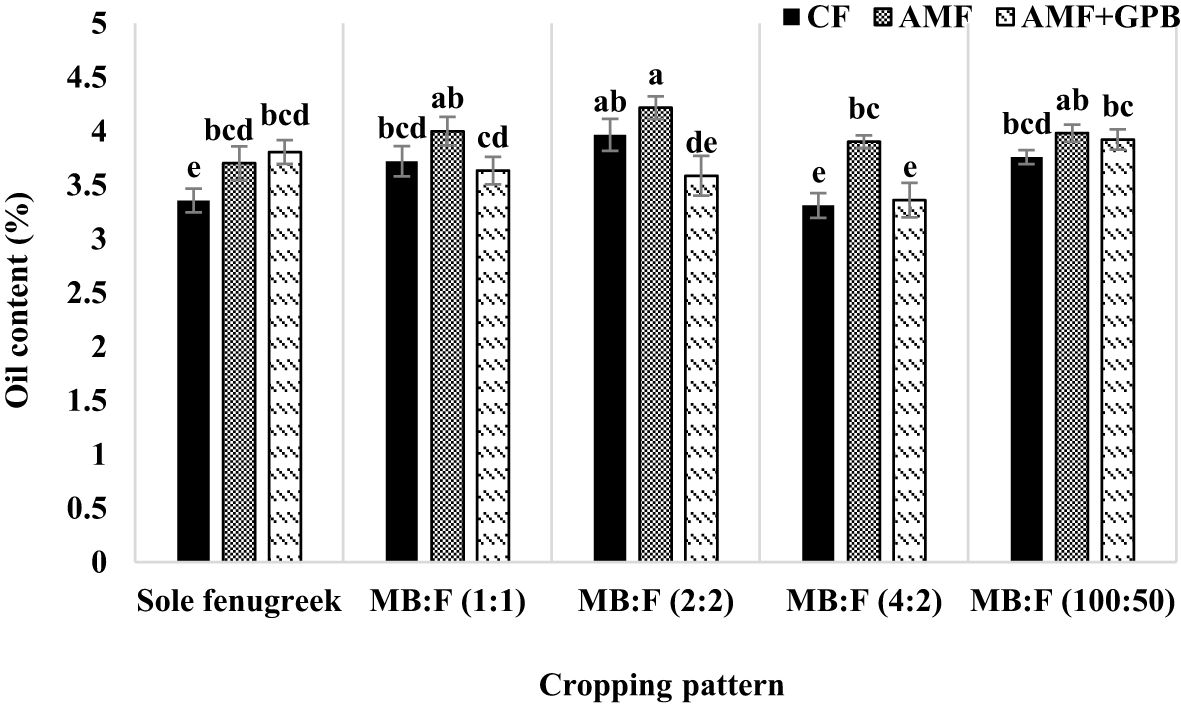
Figure 5 Fenugreek oil content as influenced by cropping pattern and fertilizer treatments. Different letters indicate significant differences at p ≤ 0.05.
3.5 Oil yield (kg/ha)
The effects of year, cropping pattern, year × cropping pattern, fertilizer treatments, and cropping pattern × fertilizer treatment were significant (p ≤ 0.01) on oil yield (Table 3). The interaction effect of year × cropping pattern (Figure 6) indicated that, in all cropping patterns except the MB:F (4:2), the oil yields in 2020 were higher than those in 2021. The mean comparison of interaction effect of cropping pattern × fertilizer treatment (Figure 7) showed that, in sole fenugreek, the highest oil yield was observed in plants treated with AMF+GPB, while in all intercropping patterns, the oil yields in plants received CF and AMF+GPB were not significantly different. Among the intercropping patterns, the highest oil yields were observed in MB:F (100:50).
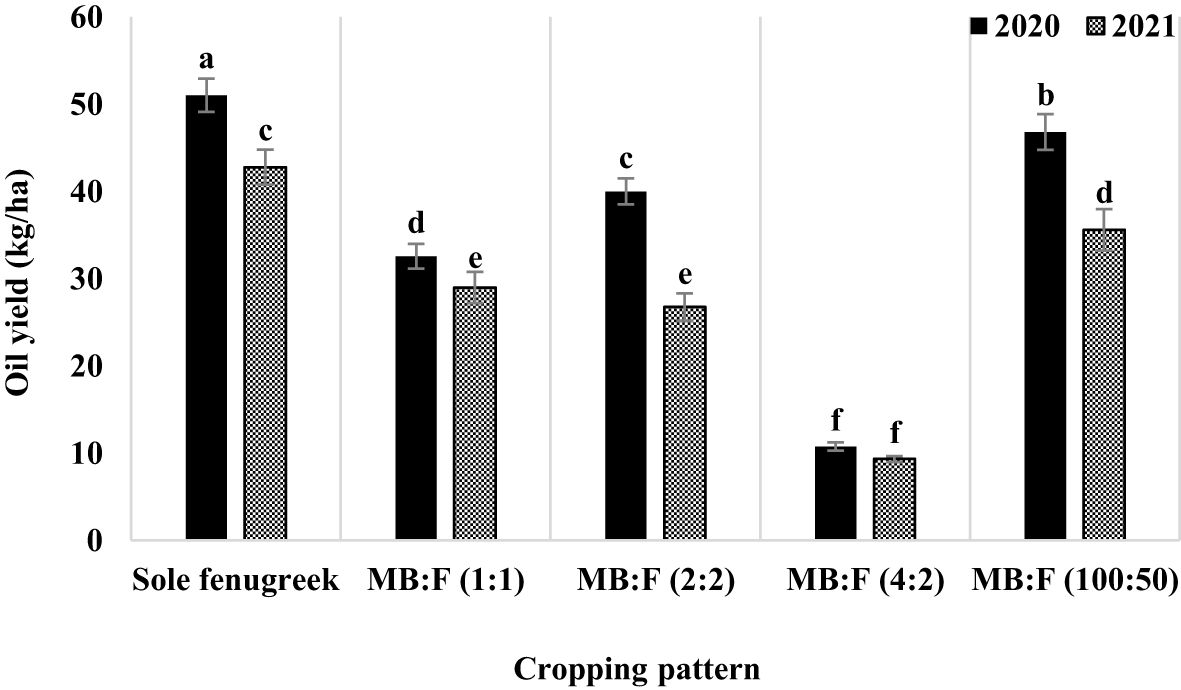
Figure 6 Fenugreek oil yield as influenced by year and fertilizer treatments. Different letters indicate significant differences at p ≤ 0.05.
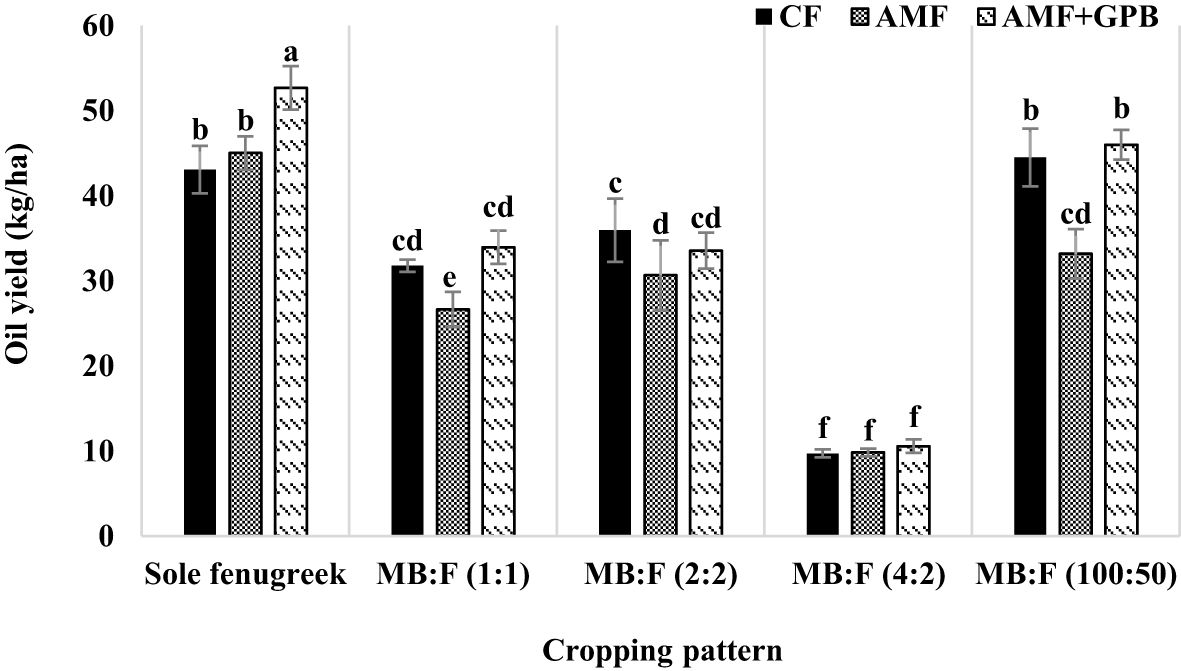
Figure 7 Fenugreek oil yield as influenced by cropping pattern and fertilizer treatments. Different letters indicate significant differences at p ≤ 0.05.
3.6 Oil composition (GC–MS)
The effects of intercropping patterns and fertilizer treatments on oil composition were evaluated through GC–MS analysis, and it was observed that the fenugreek seed oil contains eight main fatty acids, which constitute 92.6%–97.29% of total composition of the oil (Table 5). The main components of fenugreek seed oil were linoleic acid (39.21%–21.21%), oleic acid (23.65%–18.79%), linolenic acid (31.21%–21.17%), palmitic acid (12.14%–7.65%) and stearic acid (13.7%–25.25%), respectively. In all fertilizer treatments, the lowest content of linoleic acid was obtained in sole crop, and increased in all intercropping patterns and the highest value was observed in plants treated with MB:F (4:2) (Table 5). In all cropping patterns except MB:F (1:1), the plants received AMF and AMF+GPB fertilizer treatments had higher linoleic acid contents than CF treatment.
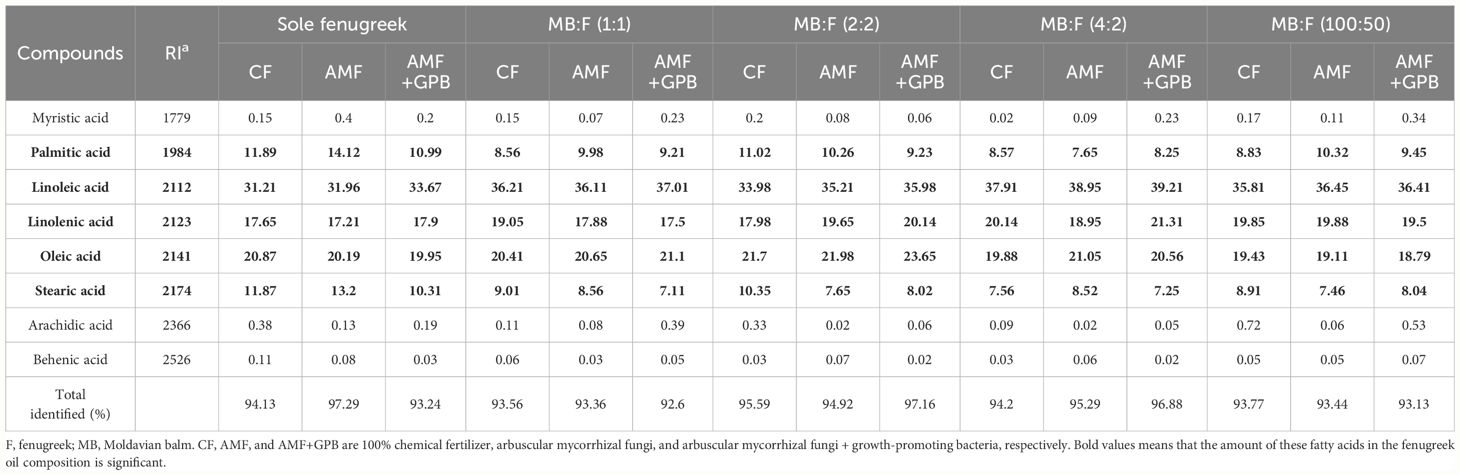
Table 5 Proportion of fenugreek oil constituents under different cropping patterns and fertilizer treatments (an average over the two years.
In all fertilizer treatments, the contents of oleic acid improved in MB:F (2:2) compared to other cropping patterns, so that the highest value was observed in this cropping pattern when treated with AMF+GPB (23.65%). The lowest content of oleic acid was observed in plants received AMF+GPB and MB:F (100:50) intercropping. In intercropping patterns except MB:F (100:50), the oleic acid contents under AMF and AMF+GPB treatments were higher than those in CF treatment. The highest content of linolenic acid was observed in MB:F (4:2) intercropping treated with AMF+GPB, which increased by 17.39% than sole crop. Also, the lowest content of linolenic acid was related to the plants received AMF+GPB in MB:F (1:1). The linolenic acid contents under AMF+GPB treatment was higher than those in CF and AMF fertilizer treatments except for MB:F (1:1) and (100:50) intercropping patterns (Table 5).
The highest contents of palmitic acid were obtained in sole crop (11.89, 14.12, and 10.99% under CF, AMF and AMF+GPB treatments, respectively). The lowest content of palmitic acid (7.56%) was obtained in plants received AMF and MB:F (4:2) intercropping, which decreased by 59.44% compared with that in sole fenugreek. In sole fenugreek, MB:F (1:1) and (100:50) cropping patterns, plants received AMF had higher palmitic acid contents compared to those in AMF+GPB and CF treatments, while in MB:F (2:2) and (4:2), the plants treated with CF had higher contents of palmitic acid. The highest contents of stearic acid were obtained in sole fenugreek and among the fertilizer treatments the highest value (13.2%) was observed under AMF treatment. In MB:F (1:1), (2:2) and (100:50) intercropping patterns, the content of stearic acid under CF treatment was higher than those in other fertilizer treatments, while in MB:F (4:2), the highest content of stearic acid was obtained in plants treated with AMF.
3.7 Anthocyanin (mg/g)
The content of anthocyanin in fenugreek seeds was significantly affected by year, cultivation pattern, and fertilizer treatments (p ≤ 0.01) (Table 3). The anthocyanin content in 2021 was higher than 2020. In MB:F (100:50), (1:1), and (2:2) cropping patterns, the anthocyanin contents increased significantly compared with sole fenugreek and MB:F (4:2). Among the fertilizer treatments, the plants treated with AMF+GPB had the highest content of anthocyanin (Table 4).
3.8 Total flavonoid (mg EQ/g)
The effects of year, cropping pattern, fertilizer treatments, and the interaction effect of cropping pattern × fertilizer treatment were significant (p ≤ 0.01) on total flavonoid content of fenugreek seeds (Table 3). The content of total flavonoid in 2021 was higher than 2020 (Table 4). The mean comparison for interaction effect of cropping pattern × fertilizer treatment (Figure 8) showed that, in all cropping patterns, except in MB:F (4:2), the highest contents of total flavonoid were observed in plants treated with AMF and AMF+GPB. In MB:F (4:2) intercropping, the total flavonoid content in plants that received AMF+GPB was higher than those of CF and AMF.
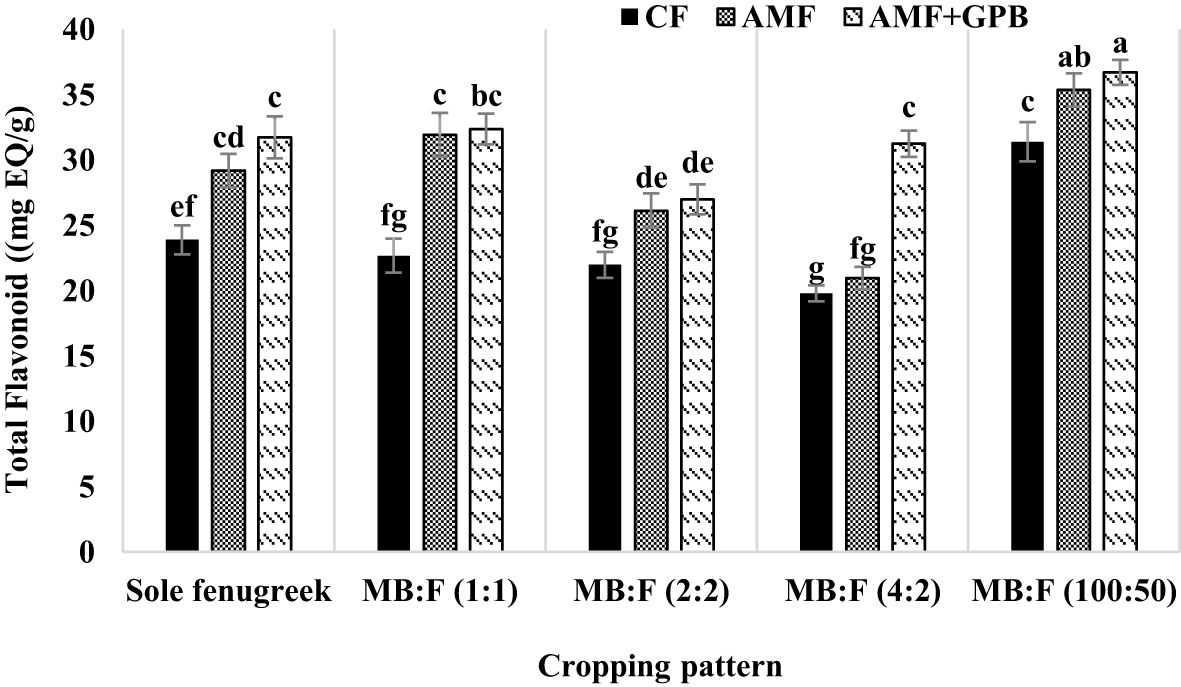
Figure 8 Total flavonoid content of fenugreek seeds as influenced by cropping pattern and fertilizer treatments. Different letters indicate significant differences at p ≤ 0.05.
3.9 Mucilage content (%)
The mucilage content of fenugreek seed was also affected by year, cropping pattern, fertilizer treatment, and interaction effect of cropping pattern × fertilizer treatments (p ≤ 0.01) (Table 3). The mucilage content increased in 2021 compared with that in 2020 (Table 4). In all cropping patterns (except in MB:F (4:2), the mucilage contents increased significantly in plants treated with AMF+GPB, compared with those in CF and AMF (Figure 9). In MB:F (4:2) intercropping, the mucilage contents in plants received AMF and AMF+GPB were not significantly different.
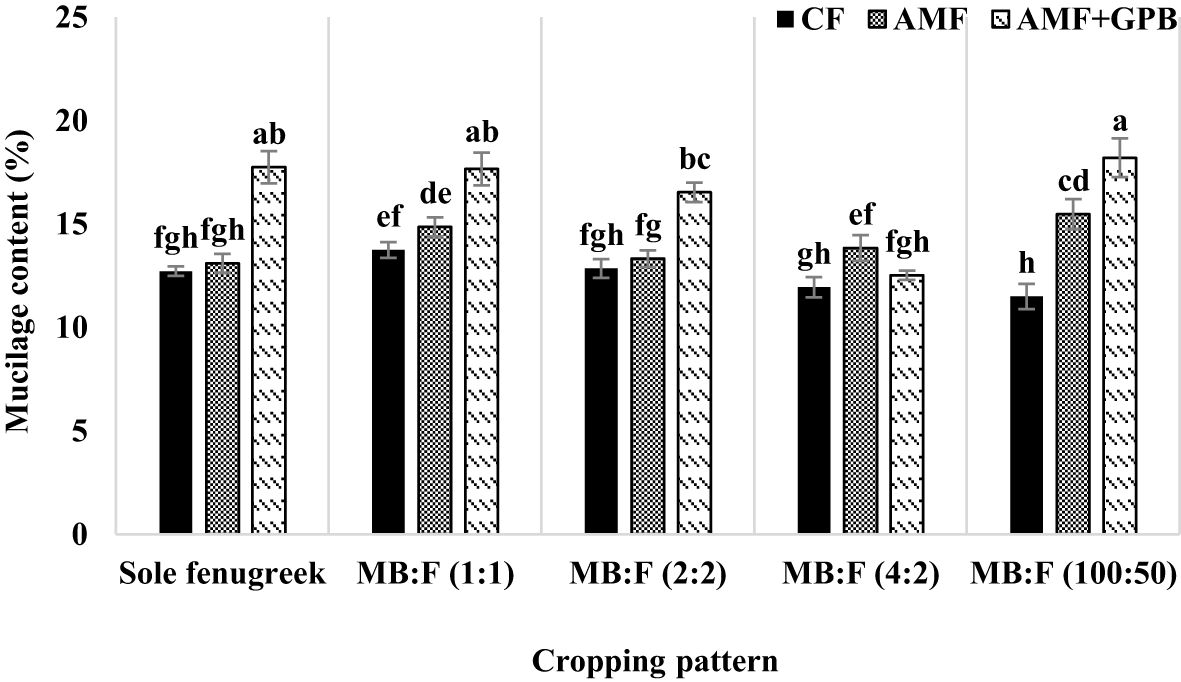
Figure 9 Mucilage content of fenugreek seed as influenced by cropping pattern and fertilizer treatments. Different letters indicate significant differences at p ≤ 0.05.
3.10 LER of intercropping patterns
The LERT index of all intercropping patterns were higher than 1.0 in both experimental years (Table 6). In general, LERT values in MB:F (100:50) were higher than those in MB:F (1:1), (2:2) and (4:2) intercropping patterns. In both years, the MB:F (100:50) intercropping pattern treated with CF had the highest LERT (1.58 and 1.70 for 2022 and 2021, respectively) and the AMF+GPB fertilizer treatment was the next. Comparison of LERF and LERMB (partial LERS) showed that, in most treatments [except MB:F (4:2)], LERF values were higher than those of LERMB, which indicates that the intercropping had positive effect on fenugreek.
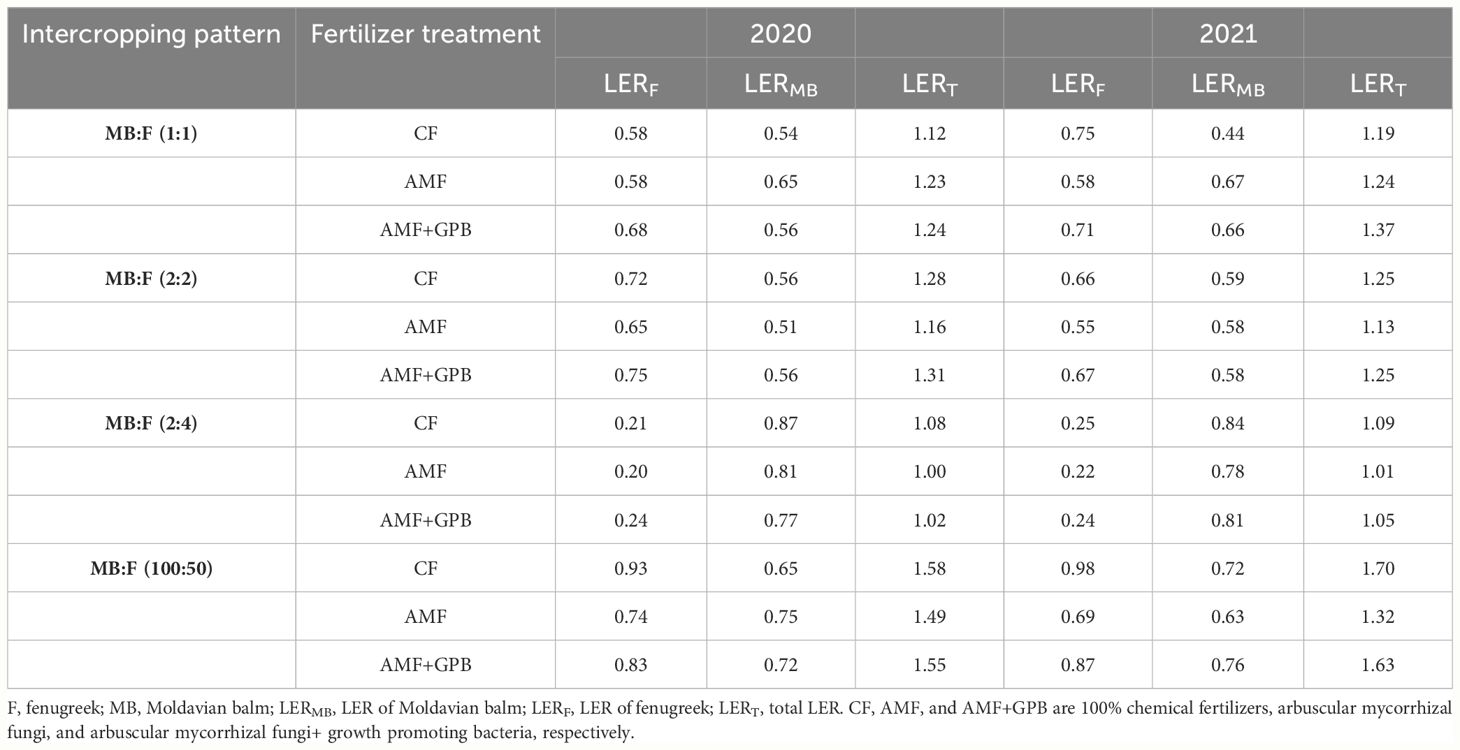
Table 6 Land equivalent ratio (LER) values at different intercropping patterns and fertilizer treatments in 2020 and 2021.
4 Discussion
4.1 Fenugreek plant height
The plant height is one of the characteristics that is affected by the plant growth conditions, and higher precipitation in 2020 could be the reason for greater fenugreek height in this year compared with 2021. The fenugreek heights in MB:F (1:1) and (2:2) cropping patterns were higher than sole fenugreek, which could be due to increase in competition between plants for light in intercropping compared to sole cropping. Shading of MB likely increased the auxin concentration in fenugreek plants and increased the plant height (Agegnehu et al., 2006). The reason for decrease in plant height in MB:F (100:50) intercropping could be attributed to competition between plants for limited resources (water, nutrients, and light), which has caused a decrease in growth and plant height in this intercropping pattern. Agegnehu et al. (2006) also reported that, in barley–faba bean intercropping, the faba bean plant height decreased significantly due to interspecific competition. The plant height was higher under CF treatment than those in AMF and AMF+GPB treatments. By increasing the possibility of quick access of fenugreek to a higher nitrogen level, the plant height increased due to increase in plant green area, photosynthetic capacity, and internodes length (López-Bellido et al., 2004).
4.2 Chlorophyll content index (SPAD)
Presumably, in MB:F (2:2) intercropping, the chlorophyll content index in leaves of fenugreek increased in high density and shading conditions of MB to absorb more light and produce photoassimilate (Agegnehu et al., 2006; Singh et al., 2013). The SPAD values of plants treated with AMF+GPB and CF were not significantly different. The effect of biofertilizers on increasing the amount of leaf chlorophyll content is related to better and more plant access to nutrients, such as potassium and nitrogen, provides chlorophyll precursors and increases protein and amino acids as the main precursors of chloroplast structure and activity (Rosas et al., 2006). Huang et al. (2004) also reported that nitrogen plays an essential role in the structure of photosynthetic pigments, including chlorophyll, and it is obvious that the amount of chlorophyll content index will improve with the use of chemical and biofertilizers which increase the N availability.
4.3 Seed yield
All cropping patterns produced higher seed yields in 2020 than those in 2021. The higher precipitation in 2020 growth season could be the main reason for increase in seed yields of all cropping patterns in 2020. Saseendran et al. (2015) reported that climatic variables (mainly precipitation) can have an intensifying effect on crop yield. Similar result is reported by Vafadar-Yengeje et al. (2019) in intercropping of faba bean (Vicia faba L.) with MB. After sole fenugreek, the highest seed yield was obtained in MB:F(100:50) that may be attributed to increase in yield in additive intercropping pattern due to higher density of fenugreek compared to replacement intercropping patterns, reduction in weed infestation, proper stratification and better use of environmental resources (Hauggaard-Nielsen et al., 2006; Vrignon-Brenas et al., 2016). Also, more soil coverage in additive intercropping patterns could increase water use efficiency by reducing the evaporation in soil moisture evacuation (Iqbal et al., 2017), so the soil moisture is spent on transpiration of crops, photosynthesis, and yield increase. Among the fertilizer treatments, the highest seed yield was obtained in plants received AMF+GPB treatment. Biofertilizers increase seed yield by creating a cycle of nutrients and making them available and by increasing available water and improving plant growth and development conditions (Grageda-Cabrera et al., 2018). Alizadeh et al. (2019) also reported that, in intercropping of linseed (Linum usitatissimum L.) and faba bean, the combined application of PGPR and mycorrhizal fungi, increased the seed yield of both crops. In fact, mycorrhizal symbiosis causes the osmotic regulation of the host plant and increases the contact of the root with soil particles, and then it increases soil nutrients and solubilizes soil minerals due to an increase of microbial activities and lead to improve in absorption of micro- and macro-elements by roots (Kothe and Turnau, 2018). Additionally it has a positive effect on symbiosis of plant with Rhizobium (Cardoso and Kuyper, 2006), which in this case can be expected to increase the yield of host plant (fenugreek).
4.4 Oil content (%)
The highest oil content of fenugreek was recorded in MB:F (2:2) intercropping, which could be due to increase in plant’s ability to use environmental resources in this cropping pattern (Agegnehu et al., 2006). The interaction effect of cropping pattern × fertilizer treatment showed that, in all cropping patterns, fenugreek plants treated with AMF had the highest oil content. In general, mycorrhizal fungi improve the plant–soil association by forming hyphae around the plant root, increase the absorption of nutrients such as nitrogen and phosphorus, and consequently improve the fatty acids biosynthesis and oil content (Chen et al., 2017; Fatiha, 2019). It was also observed that the plants treated with CF fertilizer had lower oil content than those in AMF and AMF+GPB. Beaudette et al. (2010) also reported that the use of nitrogen fertilizer in tree-based intercropping system, reduced the oil content of canola (Brassica napus L.). The decrease in oil content with the use of CFs has been reported to be related to the inverse relationship between oil content and protein content, in such a way that with increase of nitrogen, the potential production of hydrocarbon substances is reduced and a greater proportion of photosynthetic substances is allocated to the protein synthesis, and as a result, the amount of seed oil decreased (Khan et al., 2002).
4.5 Oil yield
The fenugreek seed yields in 2020 were higher than those in 2021, while the seed oil contents in 2020 were lower. Considering the high correlation between seed yield and oil yield, the fenugreek oil yields in 2020 were higher than those in 2021. The highest seed oil yield was produced in sole fenugreek, since the oil yield is a function of seed yield and oil content. In replacement intercropping patterns, the density of fenugreek is reduced compared to sole cropping; therefore, a decrease in seed yield and consequently in oil yield is expected (Yan et al., 2014). Because of higher seed yield in plants treated with AMF + PGPB and CF, the oil yields in these treatments were also higher. Alizadeh et al. (2019) also reported that the use of biofertilizers, increased the linseed oil yield in intercropping with faba bean. Although, the plants received CF had the lowest oil content among the fertilizer treatments but, in intercropping patterns, the oil yields under CF treatments were not significantly different with those under AMF+GPB. Khan et al. (2018) also reported that using the CFs containing nitrogen had a negative and significant effect on oil content, but due to the positive effect on seed yield, it ultimately increased oil yield.
4.6 Oil composition (GC–MS)
Oil quality depends on the fatty acids composition and the ratio of unsaturated to saturated fatty acids (Fotohi Chiyaneh et al., 2022). It was found that fenugreek seed oil is a rich source of unsaturated fatty acids such as linoleic acid, oleic acid and linolenic acid that are among the essential fatty acids with beneficial effects on human health (Calder, 2015). Our results are in agreement with the report of Ciftci et al. (2011), Ali et al. (2012), Al-Jasass and Al-Jasser (2012) and Sulieman et al. (2008), as they reported that the unsaturated fatty acids (linoleic acid, oleic acid and linolenic acid) make up most of the fatty acids of fenugreek seed oil. The oil content and fatty acids composition are influenced by factors such as genotype, planting date, soil fertility, planting density, and cropping pattern (Sabzalian et al., 2008). The unsaturated fatty acids contents in intercropping patterns were higher than sole fenugreek and the saturated fatty acids (palmitic acid, stearic acid, etc.) contents in sole fenugreek were higher than all intercropping patterns. It could be concluded that the intercropping patterns improved the environmental conditions for the synthesis of unsaturated fatty acid in fenugreek by increasing the nutrients availability (Gitari et al., 2018; Fotohi Chiyaneh et al., 2022). In most of the intercropping patterns, the contents of unsaturated fatty acids in plants treated with AMF+GPB were higher than those of CF treatment, while the contents of saturated fatty acids were higher in plants received CF and AMF treatments. In previous studies, the effectiveness of biofertilizers on increasing the quality of safflower (Carthamus tinctorius L.) oil in intercropping with faba bean (Vicia faba L.) (Saeidi et al., 2018), olive (Olea europaea L.) oil in intercropping with legumes (Chehab et al., 2019) and black cumin (Nigella sativa L.) oil in intercropping with fenugreek (Rezaei-Chiyaneh et al., 2021) has been reported. It was found that the use of CFs caused a decrease in unsaturated fatty acids and oil quality in oilseeds (Sharma, 2005). The use of biofertilizers improves access to nutrients by improving soil microbial activity and root development (Dawood et al., 2019), and production of fatty acid precursor compounds, which leads to an increase in unsaturated fatty acids contents and oil composition (Shu-tian et al., 2018). When nitrogen is available in a sufficient amount to the plant, leaf senescence occurs later and the plant can remobilization photoassimilate to its leaves for a longer time (Diacono et al., 2013); therefore, plant GPB cause the continuation of plant growth and improve the oil quality by supplying the nitrogen needed by the plant in reproductive stages. In this study, AMF+GPB treatment improved the quality of fenugreek oil (increased the contents of unsaturated fatty acids) due to the synergistic effects of GPB in nitrogen absorption and mycorrhizal fungi in providing suitable conditions for absorption of water, micro- and macro-elements.
4.7 Total flavonoid and anthocyanin
Flavonoid compounds are the result of the phenylpropanoid pathway, and the phenylalanine ammonia lyase (PAL) enzyme is the initiator of this pathway, which plays an essential role in formation of phenolic compounds and is raised as one of the indicators sensitive to environmental changes such as planting density and climate changes (light, temperature, humidity) (Vogt, 2010; Miranda et al., 2012). Therefore, it seems that the higher air temperature and lower precipitation in 2021 were effective in synthesis of the mentioned enzyme and in this way increased the synthesis of flavonoids through phenylpropanoid pathway. Also, the increase in biosynthesis of flavonoids in intercropping patterns [especially in MB:F (100:50)] may be due to activation of the plant’s defense strategy against competitive stress (Winyard et al., 2005). Dehghani Mashkani et al. (2011) also reported that biofertilizer treatments caused a significant increase in flavonoid content in chamomile (Matricaria recutita L.). Since flavonoids and other secondary metabolites are by-products of photosynthesis, application of biofertilizers increased their synthesis by improving the leaf area and nutrients availability (Mona and Khalil, 2006). The lower contents of total flavonoid and anthocyanin in plants treated with CF could be explained by protein competition model or growth differentiation balance. According to this theory, when the biomass increases in response to more availability of nitrogen, the concentration of phenolic compounds decreases, because the increase in plant’s need for protein for growth reduces the phenolic compounds, as well as biomass accumulation dilutes the concentration of phenolic compounds (Ibrahim et al., 2010).
4.8 Mucilage content (%)
Mucilage compounds are insoluble hydrocarbons in fenugreek seed and part of plant secondary metabolites (Wu et al., 2009). Increasing the mucilage content in 2021 could be attributed to lower precipitating and higher temperatures. Also, higher mucilage contents in MB:F (1:1) and (100:50) intercropping patterns may be due to the increase in interspecific competition (Miranda et al., 2012). The higher mucilage contents in biofertilizer treatments (AMF+GPB and AMF) at all cropping patterns indicates that mucilaginous compounds as one of the secondary metabolites can be influenced by increasing the availability of water and nutrients for plant caused by inoculation of biofertilizers (Yousefi et al., 2011).
4.9 Land equivalent ratio of intercropping
The partial LERs for fenugreek (LERF) were higher than those of MB (LERMB), which indicates that intercropping has a positive effect on fenugreek than MB. Monti et al. (2016) reported that the increase in partial LER higher than 0.5 depends on complementary degree of the intercropping components. Also, LERT higher than 1.0 obtained in all intercropping patterns and fertilizer treatments indicate that intercropping is more advantage than sole cropping (Amini et al., 2020). The superiority in intercropping is due to different morphological and growth properties and the tendency of intercropping components to make optimum use of resources such as soil moisture, light and nutrient elements, and there are differences in root structure, distribution of the canopy cover, and nutritional needs of plants in the intercropping (Hauggaard-Nielsen et al., 2008). The role of morphological differences in achieving higher LERS, have been reported in intercropping of soybean–sugar cane (Morsy et al., 2017), wheat–fenugreek (Wasaya et al., 2013), maize–pea (Mao et al., 2012), and faba bean–MB (Vafadar-Yengeje et al., 2019). The results of some studies have also shown that, when the legume species beside the other species are planted as an intercropping, due to the complementary effect, nitrogen stabilization is stimulated, which increases the growth and yield of the legume species due to the increase in the number of active nodes (Zhao et al., 2017). Although the presence of species together increases competitiveness to absorb environmental resources, if one species has nitrogen fixation ability, competitive pressure will be reduced, because the legume species will have less competition with other species in nitrogen absorption as one of the main and most restrictive factors (Karpenstein-Machan and Stuelpnagel, 2000).
5 Conclusions
Among the intercropping patterns, the highest seed and oil yield of fenugreek were observed in MB:F (100:50) pattern and the lowest ones in MB:F (4:2). In all intercropping patterns, the oil yields in plants received AMF+GPB and CF were not significantly different. The GC–MS analysis of fenugreek oil indicated that the contents of unsaturated fatty acids (linoleic and linolenic acids) increased in intercropping patterns compared with sole cropping. Also, in sole cropping of fenugreek and all intercropping patterns, the linoleic acid content increased in plants treated with AMF+GPB, compared with that in CF. The anthocyanin, total flavonoid, and mucilage contents were improved in plants under MB:F (100:50) intercropping pattern and AMF+GPB treatment. The highest LERT values were observed in MB: F (100:50) intercropping pattern (CF = 1.70, AMF+ GPB = 1.63). Generally, we can conclude that, in sustainable production systems, the fenugreek sole cropping and CF application could be replaced with additive intercropping of MB:F (100:50) and inoculation with AMF + GPB (AMF+GPB). These strategies will help the growers to improve the fenugreek oil yield a composition and reduce the harmful effects of CFs on agro-ecosystems.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author contributions
ZA: Formal analysis, Investigation, Methodology, Data curation, Software, Writing – original draft. RA: Formal analysis, Investigation, Methodology, Conceptualization, Project administration, Supervision, Validation, Writing – review & editing. AD: Conceptualization, Investigation, Methodology, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Acknowledgments
We thank the University of Tabriz for their technical support.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Adams R. P. (2001). Identification of essential oil components by gas chromatography/quadrupole mass spectroscopy (Carol Stream, USA: Allured publishing corporation). doi: 10.5555/20013153493
Agegnehu G., Ghizaw A., Sinebo W. (2006). Yield performance and land-use efficiency of barley and faba bean mixed cropping in Ethiopian highlands. Eur. J. Agron. 25, 202–207. doi: 10.1016/j.eja.2006.05.002
Ali M. A., Sayeed M. A., Alam M. S., Yeasmin M. S., Khan A. M., Muhamad I. I. (2012). Characteristics of oils and nutrient contents of Nigella sativa Linn. and Trigonella foenum-graecum seeds. Bull. Chem. Soc Ethiop 26, 55–64. doi: 10.4314/bcse.v26i1.6
Alizadeh K., Rezaei-Chiyaneh E., Amirnia R., Barin M. (2019). The effect of combined application of pgpr and mycorrhizal fungi in intercropping of linseed (Linum usitatissimum L.) and faba bean (Vicia faba L.) on growth characteristics and seed yield. Iran. J. Field Crops Res. 17, 123–140. doi: 10.22067/gsc.v17i1.71955
Al-Jasass F. M., Al-Jasser M. S. (2012). Chemical composition and fatty acid content of some spices and herbs under Saudi Arabia conditions. Sci. World J., 1–5. doi: 10.1100/2012/859892
Amini R., Choubforoush Khoei B., Dabbagh Mohammadi Nasab A., Raei Y. (2020). Effects of intercropping sugar beet (Beta vulgaris L.) with millet, soybean and Moldavian balm on yield and quality in an organic production system. Biol. Agric. Hortic. 36, 141–155. doi: 10.1080/01448765.2020.1739556
Amini R., Dabbagh MohammadiNasab A., Mahdavi Sh. (2017). Effect of organic fertilizers in combination with chemical fertilizer on tuber yield and some qualitative characteristics of potato (Solanum tuberosum L.). J. Agroecol. 9, 734–748. doi: 10.22067/jag.v9i3.47608
Banik P., Sharma R. C. (2009). Yield and resource utilization efficiency in baby corn—legume-intercropping system in the Eastern Plateau of India. J. Sustain Agr. 33, 379–395. doi: 10.1080/10440040902834970
Basu S. K., Zandi P., Cetzal L. X. (2017). Opportunities for fenugreek (Trigonella foenumgraecum L.) as a chemurgic crop in the emergent global nutraceutical and functional food industries. Int. J. Agric. Sci. 8, 9–13.
Beaudette C., Bradley R. L., Whalen J. K., McVetty P. B. E., Vessey K., Smith D. L. (2010). Tree-based intercropping does not compromise canola (Brassica napus L.) seed oil yield and reduces soil nitrous oxide emissions. Agric. Water Manage. 139, 33–39. doi: 10.1016/j.agee.2010.06.014
Calder P. C. (2015). Functional roles of fatty acids and their effects on human health. JPEN J. Parenter. Enteral Nutr. 39, 18S–32S. doi: 10.1177/0148607115595980
Camlica M., Yaldiz G. (2024). Comparison of twenty selected fenugreek genotypes grown under irrigated and dryland conditions: Morphology, yield, quality properties and antioxidant activities. Agronomy 14, 713. doi: 10.3390/agronomy14040713
Cardoso I. M., Kuyper T. W. (2006). Mycorrhizas and tropical soil fertility. Agric. Ecosyst. Environ. 116, 72–84. doi: 10.1016/j.agee.2006.03.011
Chang C. C., Yang M. H., Wen H. M., Chern J. C. (2002). Estimation of total flavonoid content in propolis by two complementary colorimetric methods. J. Food Drug Anal. 10, 178–182. doi: 10.38212/2224-6614.2748
Chehab H., Tekaya M., Ouhibi M., Gouiaa M., Zakhama H., Mahjoub Z., et al. (2019). Effects of compost, olive mill wastewater and legume cover cropson soil characteristics, tree performance and oil quality of olive trees cv. Chemlali grown under organic farming system. Sci. Hortic. 253, 163–171. doi: 10.1016/j.scienta.2019.04.039
Chen S., Zhao H., Zou C., Li Y., Chen Y., Wang Z., et al. (2017). Combined inoculation with multiple arbuscular mycorrhizal fungi improves growth, nutrient uptake and photosynthesis in cucumber seedlings. Front. Microbiol. 8. doi: 10.3389/fmicb.2017.02516
Ciftci O. N., Przybylski R., Rudzinska M., Acharya S. (2011). Characterization of fenugreek (Trigonella foenum-graecum) seed lipids. J. Am. Oil Chem. Soc. 88, 1603–1610. doi: 10.1007/s11746-011-1823-y
Dar S. A., Bhat R. A., Dervash M. A., Dar Z. A., Dar G. H. (2021). Azotobacter as biofertilizer for sustainable soil and plant health under saline environmental conditions. Microbiota Biofertilizers: A Sustain. Continuum Plant Soil Health, 231–254. doi: 10.1007/978–3-030–48771-3_14
Dawood M. G., Sadak M. S., Abdallah M. M. S., Bakry B. A., Darwish O. M. (2019). Influence of biofertilizers on growth and some biochemical aspects of flax cultivars grown under sandy soil conditions. Bull. Natl. Res. Cent 43, 1–13. doi: 10.1186/s42269-019-0122-x
de Almeida D. J., Alberton O., Otenio J. K., Carrenho R. (2020). Growth of chamomile (Matricaria chamomilla L.) and production of essential oil stimulated by arbuscular mycorrhizal symbiosis. Rhizosphere. 15, 100208. doi: 10.1016/j.rhisph.2020.100208
de Assis R. M. A., Carneiro J. J., Medeiros A. P. R., de Carvalho A. A., da Cunha Honorato A., Carneiro M. A. C., et al. (2020). Arbuscular mycorrhizal fungi and organic manure enhance growth and accumulation of citral, total phenols, and flavonoids in Melissa officinalis L. Ind. Crops Prod. 158, 112981. doi: 10.1016/j.indcrop.2020.112981
Dehghani Mashkani M. R., Naghdi Badi H., Darzi M. T., Mehrafarin A., Rezazadeh S., Kadkhoda Z. (2011). The effect of biological and chemical fertilizers on quantitative and qualitative yield of Shirazian Babooneh (Matricaria recutita L.). J. Med. Plants 10, 35–48. doi: 10.5555/20113396783
Diacono M., Rubino P., Montemurro F. (2013). Precision nitrogen management of wheat. A review. Agron. Sustain. Dev. 33, 219–241. doi: 10.1007/s13593-012-0111-z
El-Mehy A. A., Shehata M. A., Mohamed A. S., Saleh S. A., Suliman A. (2023). Relay intercropping of maize with common dry beans to rationalize nitrogen fertilizer. Front. Sustain. Food Syst. 7. doi: 10.3389/fsufs.2023.1052392
Fatiha A. I. D. (2019). Plant lipid metabolism. Adv. Lipid Metab., 1–16. doi: 10.5772/intechopen.81355
Fotohi Chiyaneh S., Rezaei-Chiyaneh E., Amirnia R., Keshavarz Afshar R., Siddique K. H. M. (2022). Changes in the essential oil, fixed oil constituents, and phenolic compounds of ajowan and fenugreek in intercropping with pea affected by fertilizer sources. Ind. Crops Prod. 178, 114587. doi: 10.1016/j.indcrop.2022.114587
Gitari H. I., Karanja N. N., Gachene C. K., Kamau S., Sharma K., Schulte-Geldermann E. (2018). Nitrogen and phosphorous uptake by potato (Solanum tuberosum L.) and their use efficiency under potato-legume intercropping systems. Field Crop Res. 222, 78–84. doi: 10.1016/j.fcr.2018.03.019
Grageda-Cabrera O. A., González-Figueroa S. S., Vera-Nuñez J. A., Aguirre-Medina J. F., Peña-Cabriales J. J. (2018). Effect of biofertilizers on the assimilation of nitrogen by the wheat crop. Rev. Mex. Cienc. Agríc 9, 281–289. doi: 10.29312/remexca.v9i2.1071
Hauggaard-Nielsen H., Andersen M. K., Joernsgaard B., Jensen E. S. (2006). Density and relative frequency effects on competitive interactions and resource use in pea–barley intercrops. Field Crop Res. 95, 256–267. doi: 10.1016/j.fcr.2005.03.003
Hauggaard-Nielsen H., Jornsgaard B., Kinane J., Jensen E. S. (2008). Grain legume cereal intercropping: the practical application of diversity, competition and facilitation in arable and organic cropping systems. Renew. Agric. Food Syst. 23, 3–12. doi: 10.1017/S1742170507002025
Heidari M., Talebpour Z., Abdollahpour Z., Adib N., Ghanavi Z., Aboul-Enein H. Y. (2020). Discrimination between vegetable oil and animal fat by a metabolomics approach using gas chromatography–mass spectrometry combined with chemometrics. J. Food Sci. Technol. 57, 3415–3425. doi: 10.1007/s13197-020-04375-9
Huang Z. A., Jiang D. A., Yang Y., Sun J. W., Jin S. H. (2004). Effects of nitrogen deficiency on gas exchange, chlorophyll fluorescence, and antioxidant enzymes in leaves of rice plants. Photosynthetica 42, 357–364. doi: 10.1023/B:PHOT.0000046153.08935.4c
Hussein M. S., El-sherbeny S. E., Khalil M. Y., Naguib N. Y., Aly S. M. (2006). Growth characters and chemical constituents of Dracocephalum moldavica L. plants in relation to compost fertilizer and planting distance. Sci. Hortic. 108 3), 322–331. doi: 10.1016/j.scienta.2006.01.035
Ibrahim M. H., Jaafar H. Z., Rahmat A., Rahman Z. A. (2010). The relationship between phenolics and flavonoids production with total non structural carbohydrate and photosynthetic rate in Labisia pumila Benth. under high CO2 and nitrogen fertilization. Molecules. 16, 162–174. doi: 10.3390/molecules16010162
Iqbal J., Abbasi B. A., Mahmood T., Kanwal S., Ali B., Shah S. A., et al. (2017). Plant-derived anticancer agents: A green anticancer approach. Asian Pac. J. Trop. Biomed. 7, 1129–1150. doi: 10.1016/j.apjtb.2017.10.016
Jhajhria A., Kumar K. (2016). Fenugreek with its medicinal applications. Int. J. Pharm. Sci. Rev. Res. 41, 194–201. Available at: https://globalresearchonline.net/journalcontents/v41-1/37.pdf.
Kalyanasundaram N. K., Patel P. B., Dalal K. C. (1982). Nitrogen need of Plantago ovata Forsk. in relation to the available nitrogen in soil [drug plant]. Indian J. Agric. Sci. 52, 240–242.
Kapoor R., Giri B., Mukerji K. G. (2002). Glomus macrocarpum: a potential bioinoculant to improve essential oil quality and concentration in dill (Anethum graveolens L.) and carum (Trachyspermum ammi (Linn.) Sprague). World J. Microb. Biotech. 18, 459–463. doi: 10.1023/A:1015522100497
Karpenstein-Machan M., Stuelpnagel R. (2000). Biomass yield and nitrogen fixation of legumes monocropped and intercropped with rye and rotation effects on a subsequent maize crop. Plant Soil. 218, 215–232. doi: 10.1023/A:1014932004926
Khan S., Anwar S., Kuai J., Noman A., Shahid M., Din M., et al. (2018). Alteration in yield and oil quality traits of winter rapeseed by lodging at different planting density and nitrogen rates. Sci. Rep. 8, 634. doi: 10.1038/s41598–017-18734–8
Khan N., Jan A., Khan I. A., Khan N. (2002). Response of canola to nitrogen and sulphur nutrition. Asian J. Plant Sci. 1, 516–518. doi: 10.3923/ajps.2002.516.518
Kothe E., Turnau K. (2018). Mycorrhizosphere communication: Mycorrhizal fungi and endophytic fungus-plant interactions. Front. Microbiol. 9. doi: 10.3389/fmicb.2018.03015
López-Bellido L., López-Bellido R. J., Castillo J. E., López-Bellido F. J. (2004). Chickpea response to tillage and soil residual nitrogen in a continuous rotation with wheat: I. Biomass and seed yield. Field Crop Res. 88, 191–200. doi: 10.1016/j.fcr.2004.01.011
Mao L., Zhang L., Li W., van der Werf W., Sun J., Spiertz H., et al. (2012). Yield advantage and water saving in maize/pea intercrop. Field Crop Res. 138, 11–20. doi: 10.1016/j.fcr.2012.09.019
Marastoni L., Sandri M., Pii Y., Valentinuzzi F., Brunetto G., Cesco S., et al. (2019). Synergism and antagonisms between nutrients induced by copper toxicity in grapevine rootstocks: Monocropping vs. intercropping. Chemosphere. 214, 563–578. doi: 10.1016/j.chemosphere.2018.09.127
Merlin E., Melato E., Lourenço E. L. B., Jacomassi E., Junior A. G., da Cruz R. M. S., et al. (2020). Inoculation of arbuscular mycorrhizal fungi and phosphorus addition increase coarse mint (Plectranthus amboinicus Lour.) plant growth and essential oil content. Rhizosphere 15, 100217. doi: 10.1016/j.rhisph.2020.100217
Miranda M., Vega-Gálvez A., Quispe-Fuentes I., Rodríguez M. J., Maureira H., Martínez E. A. (2012). Nutritional aspects of six quinoa (Chenopodium quinoa Willd.) ecotypes from three geographical areas of Chile. Chil. J. Agric. Res. 72, 175.
Mita S., Hirano H., Nakamura K. (1997). Negative regulation in the expression of a sugar-inducible gene in arabidopsis thaliana (A recessive mutation causing enhanced expression of a gene for β-amylase). Plant Physiol. 114, 575–582. doi: 10.1104/pp.114.2.575
Mona Y. K., Khalil Y. (2006). How-far would Plantago afra L. respond to bio and organic manures amendments. Res. J. Agr. Bio. Sci. 2, 12–21.
Monti M., Pellicanò A., Santonoceto C., Preiti G., Pristeri A. (2016). Yield components and nitrogen use in cereal-pea intercrops in Mediterranean environment. Field Crop Res. 196, 379–388. doi: 10.1016/j.fcr.2016.07.017
Morsy A. S., Elwan A., Eissa N. (2017). Studies on intercropping soybean with sugar cane under different nitrogen levels. Egypt. J. Agron. 39, 221–237. doi: 10.21608/agro.2017.848.1061
Petropoulos G. A. (2002). Fenugreek-the genus trigonella (London and New York: Taylor and Francis). doi: 10.1201/9780203217474
Raza M. A., Gul H., Wang J., Yasin H. S., Qin R., Khalid M. H. B., et al. (2021). Land productivity and water use efficiency of maize-soybean strip intercropping systems in semi-arid areas: A case study in Punjab Province, Pakistan. J. Cleaner Prod. 308, 127282. doi: 10.1016/j.jclepro.2021.127282
Rezaei-Chiyaneh E., Battaglia M. L., Sadeghpour A., Shokrani F., Dabbagh Mohammadi Nasab A., Raza M. A., et al. (2021). Optimizing intercropping systems of black cumin (Nigella sativa L.) and fenugreek (Trigonella foenum-graecum L.) through inoculation with bacteria and mycorrhizal fungi. Adv. Sustain. Syst. 5, 2000269. doi: 10.1002/adsu.202000269
Rosas S. B., Andrés J. A., Rovera M., Correa N. S. (2006). Phosphate-solubilizing Pseudomonas putida can influence the rhizobia–legume symbiosis. Soil Biol. Biochem. 38, 3502–3505. doi: 10.1016/j.soilbio.2006.05.008
Sabzalian M. R., Saeidi G., Mirlohi A. (2008). Oil content and fatty acid composition in seeds of three safflower species. J. Am. Oil Chem. Soc. 85, 717–721. doi: 10.1007/s11746-008-1254-6
Saeidi M., Yaghoub R. A. E. I., Amini R., Taghizadeh A., Pasban-Eslam B. (2018). Changes in fatty acid and protein of safflower as response to biofertilizers and cropping system. Turk J. Field Crop 23, 117–126. doi: 10.17557/tjfc.471666
Sahoo R. K., Bhardwaj D., Tuteja N. (2012). “Biofertilizers: a sustainable eco-friendly agricultural approach to crop improvement,” in Plant acclimation to environmental stress (Springer New York, New York, NY), 403–432. doi: 10.1007/978–1-4614–5001-6_15
Sakhavi S., Amini R., Shakiba M. R., Dabbagh Mohammadi-Nasab A. (2017a). Advantage of faba bean (Vicia faba L.) and cumin (Cuminum cyminum L.) intercropping under organic, biological and chemical fertilizer treatments. J. Agric. Sci. Sustain. Prod. 26, 17–32.
Sakhavi S., Amini R., Shakiba M. R., Dabbagh Mohammadi-Nasab A. (2017b). Effect of bio- and chemical fertilizers on grain and essential oil yield of cumin (Cuminum cyminum L.) in intercropping with faba bean (Vicia faba L.). J. Agric. Sci. Sustain. Prod. 27, 49–63.
Sarikhani M. R., Amini R. (2020). Biofertilizer in sustainable agriculture: Review on the researches of biofertilizers in Iran. J. Agric. Sci. Sustain. Production 30, 329–3365.
Saseendran S. A., Ahuja L. R., Ma L., Trout T. J., McMaster G. S., Nielsen D. C., et al. (2015). Developing and normalizing average corn crop water production functions across years and locations using a system model. Agric. Water Manage. 157, 65–77. doi: 10.1016/j.agwat.2014.09.002
Sharma P. B. (2005). Fertilizer management in sesame (Sesamum indicum L.) based intercropping system in Tawa command area. J. Oilseeds Res. 22, 63–65.
Sharma R. C., Sarkar S., Das D., Banik P. (2013). Impact assessment of arbuscular mycorrhiza Azospirillum and chemical fertilizer application on soil health and ecology. Commun. Soil Sci. Plant Anal. 44, 1116–1126. doi: 10.1080/00103624.2012.750335
Shu-tian L., Yu D., Tian-wen G., Ping-liang Zh., Ping H., Majumdar K. (2018). Sunflower response to potassium fertilization and nutrient requirement estimation. J. Integr. Agric. 17, 2802–2812. doi: 10.1016/S2095-3119(18)62074-X
Singh M., Singh U. B., Ram M., Yadav A., Chanotiya C. S. (2013). Biomass yield, essential oil yield and quality of geranium (Pelargonium graveolens L. Her.) as influenced by intercropping with garlic (Allium sativum L.) under subtropical and temperate climate of India. Ind. Crops Prod. 46, 234–237. doi: 10.1016/j.indcrop.2013.01.032
Sulieman A. M. E., Ali A. O., Hemavathy J. (2008). Lipid content and fatty acid composition of fenugreek (Trigonellafoenum-graecum L.) seeds grown in Sudan. Int. J. Food Sci. Technol. 43, 380–382. doi: 10.1111/j.1365-2621.2006.01446.x
Turan M., Gulluce M., Cakmakci R., Oztas T., Sahin F., Gilkes R. J., et al. (2010). “The effect of PGPR strain on wheat yield and quality parameters,” in Proceedings of the 19th World Congress of Soil Science: Soil solutions for a changing world, Brisbane, vol. 209. (Brisbane, Australia: International Union of Soil Sciences (IUSS), c/o Institut für Bodenforschung, Universität für Bodenkultur), 212. doi: 10.5555/20113345967
Vafadar-Yengeje L., Amini R., Nasab A. D. M. (2019). Chemical compositions and yield of essential oil of Moldavian balm (Dracocephalum moldavica L.) in intercropping with faba bean (Vicia faba L.) under different fertilizers application. J. Cleaner Prod. 239, 118033. doi: 10.1016/j.jclepro.2019.118033
Vrignon-Brenas S., Celette F., Amossé C., David C. (2016). Effect of spring fertilization on ecosystem services of organic wheat and clover relay intercrops. Eur. J. Agron. 73, 73–82. doi: 10.1016/j.eja.2015.10.011
Wasaya A., Ahmad R., Hassan F. U., Ansar M., Manaf A., Sher A. (2013). Enhancing crop productivity through wheat (Triticum aestivum L.)-fenugreek intercropping system. J. Anim. Plant Sci. 23, 210–215. doi: 10.5555/20133226794
Weisany W., Raei Y., Pertot I. (2015). Changes in the essential oil yield and composition of dill (Anethum graveolens L.) as response to arbuscular mycorrhiza colonization and cropping system. Ind. Crops Prod. 77, 295–306. doi: 10.1016/j.indcrop.2015.09.003
Winyard P. G., Moody C. J., Jacob C. (2005). Oxidative activation of antioxidant defence. Trends Biochem. Sci. 30, 453–461. doi: 10.1016/j.tibs.2005.06.001
Wu F. Z., Bao W. K., Zhou Z. Q., Wu N. (2009). Carbon accumulation, nitrogen and phosphorus use efficiency of sophora davidii seedlings in response to nitrogen supply and water stress. J. Arid Environ. 73 (12), 1067–1073. doi: 10.1016/j.jaridenv.2009.06.007
Yan S., Du X., Wu F., Li L., Li C., Meng Z. (2014). Proteomics insights into the basis of interspecific facilitation for maize (Zea mays) in faba bean (Vicia faba)/maize intercropping. J. Proteomics. 109, 111–124. doi: 10.1016/j.jprot.2014.06.027
Yaseen M., Singh M., Ram D. (2014). Growth, yield and economics of vetiver (Vetiveria zizanioides L. Nash) under intercropping system. Ind. Crops Prod. 61, 417–421. doi: 10.1016/j.indcrop.2014.07.033
Yousefi A. A., Khavazi K., Moezi A. A., Rejali F., Nadian H. A. (2011). Phosphate solubilizing bacteria and arbuscular mycorrhizal fungi impacts on inorganic phosphorus fractions and wheat growth. World Appl. Sci. J. 15, 1310–1318.
Zandi P., Basu S. K., Cetzal-Ix W., Kordrostami M., Chalaras S. K., Khatibai L. B. (2017). Fenugreek (Trigonella foenum-graecum L.): an important medicinal and aromatic crop. Act. Ingredients Aromat. Med. Plants., 207–224. doi: 10.5772/66506
Zhao M., Jones C. M., Meijer J., Lundquist P. O., Fransson P., Carlsson G., et al. (2017). Intercropping affects genetic potential for inorganic nitrogen cycling by root-associated microorganisms in Medicago sativa and Dactylis glomerata. Appl. Soil Ecol. 119, 260–266. doi: 10.1016/j.apsoil.2017.06.040
Keywords: fenugreek, fatty acid composition, legume-based intercropping, total flavonoid, mucilage
Citation: Amiriyan Chelan Z, Amini R and Dabbagh Mohammadi Nasab A (2024) Optimizing fenugreek (Trigonella foenum-graecum L.) oil yield and compositions in intercropping through growth-promoting bacteria and mycorrhiza. Front. Agron. 6:1422236. doi: 10.3389/fagro.2024.1422236
Received: 23 April 2024; Accepted: 31 May 2024;
Published: 18 June 2024.
Edited by:
Moritz Von Cossel, University of Hohenheim, GermanyReviewed by:
Victor Idowu Olugbemiga Olowe, Federal University of Agriculture, Abeokuta, NigeriaSaid Abdelhalim Saleh, National Research Centre, Egypt
Copyright © 2024 Amiriyan Chelan, Amini and Dabbagh Mohammadi Nasab. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Rouhollah Amini, cl9hbWluaUB0YWJyaXp1LmFjLmly; cmFtaW5pNThAZ21haWwuY29t
 Zahra Amiriyan Chelan
Zahra Amiriyan Chelan Rouhollah Amini
Rouhollah Amini Adel Dabbagh Mohammadi Nasab
Adel Dabbagh Mohammadi Nasab