
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Agron. , 16 April 2024
Sec. Climate-Smart Agronomy
Volume 6 - 2024 | https://doi.org/10.3389/fagro.2024.1376110
This article is part of the Research Topic Sustainable Nutrient Management under Climate Change View all 7 articles
Purpose: This study investigates the influence of incorporating Azolla, rice straw, and NPKS fertilizers on phosphorus use efficiency (PUE) and rice productivity in lowland paddy fields. Despite Azolla’s well-known role as a nitrogen-fixing aquatic fern in rice production, its specific impact on PUE remains unclear. The primary objective is to explore diverse treatment combinations to identify synergies that enhance both PUE and overall rice productivity.
Methods: The study was conducted at Mkula Irrigation Scheme in the Kilombero Valley, Tanzania; the field experiment employed a randomized complete block design with 13 treatments and three replications. Treatments comprised various combinations of Azolla, rice straw, and chemical fertilizers, incorporating 50% and 100% rates of nitrogen (N) applied with phosphorus (P), potassium (K), and sulfur (S).
Results: The study reveals the substantial impact of Azolla application on total nitrogen, available phosphorus, and exchangeable potassium levels in the soil. Particularly noteworthy were treatment combinations involving Azolla, rice straw, and reduced rates of synthetic nitrogen, along with specific P, K, and S applications, which exhibited the highest phosphorus uptake and PUE. Specifically, combining rice straw and Azolla with reduced N rates, alongside 30 kg P ha−1 + 30 kg K ha−1 + 20 kg S ha−1, resulted in the highest phosphorus uptake (73.57 kg/ha) and PUE (46.24%).
Conclusion: Integrated nutrient management, incorporating rice straw and Azolla alongside synthetic fertilizers, demonstrates synergistic effects on phosphorus uptake and efficiency while maintaining soil quality. The study underscores the potential of such integrated strategies to optimize PUE and contribute to sustainable rice production in lowland paddy fields.
Phosphorus (P) deficiency is among the major constraints on plant growth and crop production globally. This vital nutrient plays a crucial role in numerous biochemical and physiological processes, including energy transfer, genetic constituents’ relocation (DNA and RNA), and promoting vegetative growth in rice plants (Bird et al., 2001; Verzeaux et al., 2017; Azene et al., 2022; Mboyerwa et al., 2022). However, P availability is limited in many parent materials and becomes even more complicated in waterlogged conditions associated with high levels of iron, aluminum, and calcium, leading to low availability for plant uptake in both acidic and alkaline soils (Fageria et al., 2010, 2011; Rivaie et al., 2013; Lemanowicz, 2018). Additionally, several factors, such as pH, soil parent material, management practices, and climate change, influence P fractions and solubility of phosphorus (Azene et al., 2022). Phosphorus may be present in soil parent materials in relatively high amounts, yet more than 80% gets fixed as immobile stock and a fraction of P that is available to plant does not exceed 15%–20% (van de Wiel et al., 2016; Biswas Chowdhury and Zhang, 2021; Jiang et al., 2021). Previous studies demonstrated that fertilization of P into soil results in its distribution among the solid phase and clay-sized minerals. Increased clay content enhances P retention, with adsorption being reversible. Initial P fertilization sees low efficiency, but with additional P, efficiency improves. Equilibrium leads to equally rapid adsorption and desorption reactions. In highly calcareous soils, soluble P retention occurs mainly through precipitation, posing a challenge due to the availability of Ca for P precipitation. Organic matter retention of P is an inefficient process, causing P accumulation, but immobilization is reversible (Syers et al., 2008; Fixen et al., 2014). Therefore, for better sustainable soil management practices, there is a need for further research to optimize P use efficiency (PUE) and to explore the implications of these findings for global food security and environmental sustainability.
Soil organic matter serves as a significant reservoir of soil P, influencing nutrient availability, soil structure, cation exchange capacity, soil buffering capacity, and water holding capacity (Oyange et al., 2020). It was observed that Azolla compost and rice straw are common organic residues in rice cropping systems, but rice straw takes time to mineralize due to its wider C/N ratio (Wan et al., 2016). Co-application of straw with other crop residues with lower C:N ratio, such as milk vetch, green manure, and Azolla, can minimize this issue (Wan et al., 2016; Van Hung et al., 2020). Azolla not only acts as an eco-friendly amendment but also fixes nitrogen, enhancing nitrogen use efficiency and promoting rice growth and yield (Cabangon et al., 2015; Fosu-Mensah et al., 2015; Cissé and Vlek, 2022). However, more research is needed to explore the potential of Azolla in lowland rice production systems, especially its impact on PUE and enzyme activities.
Maintaining an appropriate level of plant-available P in soils is crucial. Over-application can lead to water eutrophication and disturbance in marine ecosystems while insufficient P levels reduce crop yield and limit the uptake of other essential nutrients like nitrogen (Ladha et al., 2000; Mandana et al., 2014; Zhang et al., 2017; Bhunia et al., 2021; Zhang et al., 2022b). Several strategies, such as improving soil structure, managing P sources, and using appropriate amounts of P and timing of its application can enhance PUE and solubilization (Syers et al., 2008).
Assessing soil health and quality through physical, chemical, and biological indicators is essential (Konare et al., 2010; Hengl et al., 2015). However, focusing solely on physiochemical indicators may not fully explain the effect of organic matter on nutrient availability through microbiological activities (Reddy et al., 2001; Yadav et al., 2020). Soil enzymes play an important role in the transformation of soil organic carbon (e.g., B-amylase, maltase, cellulase, β-galactosidase, and β-glucosidase), nitrogen (e.g., urease, protease, amidase, and nitrate reductase), P (phytases, acid phosphomonoestarases, and alkaline phosphomonoestarases), and S (sulfatase) cycle (Liang et al., 2014; Dotaniya and Meena, 2015; Bagheri Novair et al., 2020). Since enzymes are catalysts and mediators of various important soil functions (Abraham, 2010). Increased activity of these enzymes directly reflects proper availability of specific nutrients related to the enzymes (Gu et al., 2009). For example, soil urease catalyzes the hydrolysis of urea in the soil to CO2 and NH3/NH4+ (Saha et al., 2008; Fisher et al., 2017) (Equation 1).
This enzyme is released by many groups of microbes like bacteria, fungi, yeast, and algae, and they are responsible for the availability of N added through urea or free nitrogen fixed by microorganisms (Gu et al., 2009). Another important enzyme is phosphomonoestarases, which are responsible for hydrolysis of organic phosphorus compounds in either acidic or alkaline soils. Various types of phosphatases depend on the number of bonds hydrolyzed and optimum pH. Plant roots also release acid phosphatase at the P deficiency stage and mobilize available P in the soil. Fungi also produce acid phosphatase and most bacteria produce alkaline phosphatase. Other factors like soil organic carbon, total nitrogen, soil pH, clay content, and moisture can also influence soil enzymes (Gu et al., 2009; Kumari et al., 2017). Therefore, by utilizing enzyme activities as indicators, researchers can assess the effects of the application of organic materials on soil fertility, nutrient cycling, soil health, organic residue decomposition, and plant physiological processes in paddy fields. This information could contribute to a comprehensive understanding of the benefits and impacts of organic practices on paddy performances and help to guide sustainable agriculture practices.
Therefore, the objective of this study is to examine the effect of integrating chemical fertilizers (NPKS) with Azolla compost and rice straw having different C:N ratios on P uptake and PUE, as well as its implications on soil chemical fertility and enzyme activity in lowland paddy ecosystems.
The field layout was carried out at Mkula Irrigation Scheme (7°47′57.084′′ S, 36°54′47.592′′ E), Kilombero district. The district is located within agroecological zone E10 in “Eastern Plateaux and Mountain Blocks” in Morogoro Region-Tanzania. The climate is classified as a tropical savanna climate with a bimodal rainfall distribution pattern, having dry spells separating a short rainy season from October to December and a long rainy season from March to May (Kwesiga et al., 2020; Michael et al., 2023). The mean annual rainfall and temperature range from 1,200 to 1,400 mm and 22–23°C, respectively (Alaivasha et al., 2022; Marzouk et al., 2023). The site receives sufficient water drained from the forest reservoir on the eastern side of Udzungwa Mountain.
The field experiments were carried out for two consecutive rice-growing seasons from 2022 to 2023. Randomized complete block design (RCBD) was adopted with 13 treatment levels with half and full recommended levels of N (i.e., 50 kg N ha−1 and 100 kg N ha−1) applied with recommended levels of P and or K and S coupled with Azolla or rice straw incorporation in three replications. Details of the treatment combination are shown in Table 1. The dimensions of the individual experimental plot were 3 m × 6 m, and the space between replicate blocks was 1.5 m and that between plots within a replicate was 0.5 m. The ridges protruded 30 cm above the soil to prevent any fertilizer runoff and lateral contamination.
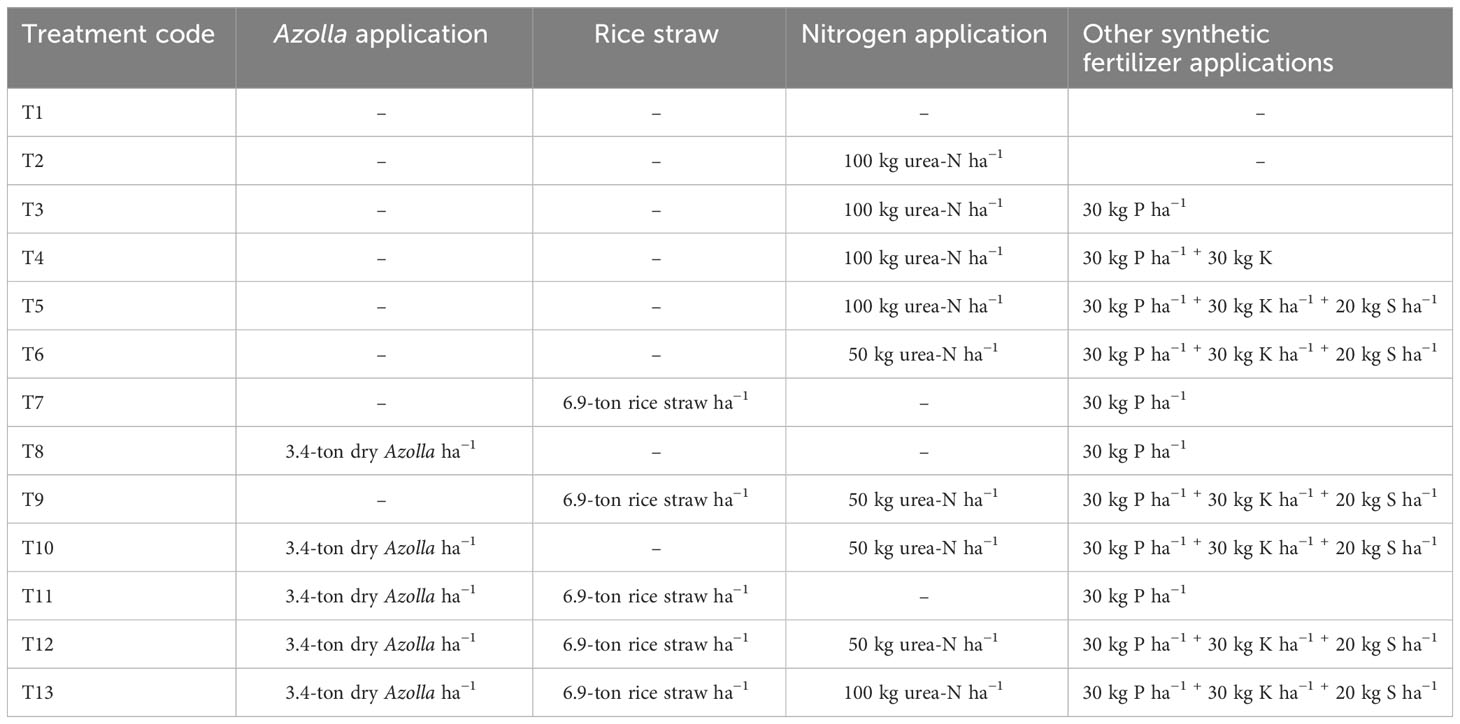
Table 1 Summary of the experimental treatments of chemical fertilizers, Azolla, and rice straw application.
Azolla plant was collected from the Aquaculture Unit of the Sokoine University of Agriculture (6°51′9.5′′ S, 37°38′59.7′′ E) in Tanzania. Before establishing culture, Azolla was harvested and analyzed for organic C, total N, P, and C/N ratio. Subsequently, 6 kg of fresh Azolla was multiplicated in the propagation pond (6 m × 5 m) of the same aquaculture unit at the university campus. During preparation, 7.5 kg 30 m−2 of cow dung and triple superphosphate [TSP, Ca3(PO4)2] fertilizer at 75 g P 30 m−2 were applied in three split doses at 4-day intervals for 22 days (Watanabe and Berja, 1983; Bagheri Novair et al., 2020). Nitrogen content of Azolla dry matter, surface water pH, water temperature, and electrical conductivity were analyzed by taking representative samples after every 5 days until the Azolla had accumulated maximum nitrogen in biomass (Table 2). Thereafter, Azolla was harvested, drained, and transferred to the field for inoculation (Figure 1). Rice straw was collected from farmers’ fields, cutting manually using a knife and incorporated into the soil for the respective straw treatments during land preparation using a hand hoe. By obtaining representative samples, the straw was dried and transported to the laboratory for chemical analysis of N through Kjeldahl and P by the wet digestion method (Okalebo et al., 2002).
Azolla in its fresh form was applied at a rate of 2 kg per 18 m2 and inoculated during nursery preparation and incorporated in the soil after 15 days. At this stage, Azolla had covered the surface of the water completely (see Figure 1). Fresh Azolla biomass at 36.4 kg 18 m−2 was incorporated to the soil using a hand hoe 3 days before transplanting of rice seedlings. Another 2 kg 18 m−2 was inoculated 6 days after transplanting (DAT) and incorporated at a rate of 29.2 kg 18 m−2 into the soil at 40 DAT. Before incorporation, Azolla was harvested within a 1 m × 1 m wooden frame (Watanabe et al., 1991), collected, dried, and analyzed for total N through Kjeldahl and P by the wet digestion method (Okalebo et al., 2002) (see Table 3). The fertilizers containing the primary (NPK) and S secondary macronutrients were applied. Fertilizers containing P, K, and S were applied uniformly in all experimental plots while N from urea (46% N) was applied at two rates of 100 kg N ha−1, being the recommended rate and half rate (50 kg N ha−1). Urea fertilizer for both rates of N was applied in two splits of 50% basal application at 7 DAT and another 50% was top-dressed at 45 DAT, which was close to the booting stage. The triple superphosphate fertilizer (30 kg P ha−1), muriate of potash (30 kg K ha−1), and ammonium sulfate (21.0% N and 24.0% S) at 20 kg S ha−1 were applied through broadcasting as basal fertilizers, except for the absolute control plots. A rice cultivar (c.v SARO-5 TXD 360) was used in this experiment, and it was obtained from the Tanzania Research Institute at Katrini-Ifakara (TARI-CATRINI). Rice seedlings (at 18 days old) were transplanted into well-puddled soils at a spacing of 20 cm × 20 cm in all treatments. Rice straw 11.3 kg 18 m−2 was spread evenly across the designated straw treatment plots and incorporated into the soil using a hand hoe during the farm preparation stage.
Fifteen randomly selected rice plants were harvested at the booting stage (75 DAT). All aboveground plant samples were taken, oven-dried at 70°C to a constant weight, and ground to pass through a 1-mm sieve and digested using H2SO4. P nutrient content was calorimetrically determined as described by Watanabe and Olsen (1965), Using the Micro Kjeldahl method, total N content was determined as described by Seleiman et al. (2022).
Phosphorus uptake (PU) (kg ha−1) was calculated by multiplying their nutrient concentration (%) by their biological weight (kg ha−1) (Assefa et al., 2021) (Equation 2).
PUE (%) was calculated using the balanced method (Equation 3) described by Fixen et al. (2014) and Syers et al. (2008).
The agronomic efficiency of applied P (AE) was calculated according to Andriamananjara et al. (2019) (Equation 4).
Before planting rice, soil samples were collected for the analyses of selected physicochemical properties. The composite soil samples were taken from the experimental site from a depth of 0–20 cm using augur randomly from 10 spots by walking in a zigzag pattern. At the end of each experiment, soil sampling was done in each treatment plot. After carefully mixing the composite samples, 2 kg of subsample was taken and brought to Sokoine University of Agriculture soil laboratory. The submitted sample was air-dried and grounded to pass a 2-mm mesh-sized sieve and analyzed using the standard procedure described in Table 3.
Three types of soil enzymes (urease, acid phosphatase, and alkaline phosphatase) were selected as indicators of microbial capacity to drive nutrient cycling (N and P, respectively). Soil urease activity was assessed by the method described by Tabatabai (1994). Five grams of oven-dry soil was placed in a 25-mL volumetric flask followed by the addition of 5 mL of 2 mg/mL urea solution and incubated at 37°C for 5 h using a Memmert 0214 incubator. After 5 h, 50 mL of 2 M potassium mercuric acetate solution (KCI-PMA) was added to stop the enzymatic reaction and the solution was shaken for 1 h. The solution was then filtered through filter paper Whatman no. 42. Then, 1 mL of aliquot was placed in a 50-mL volumetric flask followed by the addition of 10 mL of KCI-PMA solution and 30 mL of coloring reagents (25 mL of 2.5% diacetyl monoxime + 10 mL of 0.25% Thiosemicarbazide). The solution was placed in a water bath for 30 min to allow chemical reactions to proceed and the formation of a complex-colored compound. After cooling solution was diluted to 50 mL, a concentration of urea was determined by an AR-2000 spectrophotometer at a wavelength of 512 nm. Acid and alkaline phosphatases were analyzed spectrophotometrically by the method described by Kandeler et al. (1999). Oven-dry soil (1 g) was placed in a glass bottle followed by the addition of 0.2 mL of toluene to arrest microbial activities. Then, 4 mL of Modified Universal Buffer (MUB) of pH 6.5 was added. For acidic phosphatase and for alkaline phosphatase (pH 11), MUB was prepared by mixing 12.1 g of Tris (hydroxymethyl) aminomethane (THAM), 11.6 g of maleic acid, 14 g of citric acid, and 6.3 g of boric acid in 488 mL of 1 N sodium hydroxide and the solution was diluted to 1 L with distilled water. Then, 1 mL of 0.05 M P-nitrophenyl phosphate solution was mixed and incubated for 1 h. After incubation, 1 mL of 0.5 M calcium chloride and 4 mL of 0.5 M sodium hydroxide were added and mixed properly and then filtered through Whatman No. 42. A concentration of p-nitrophenol solution was determined by an AR-2000 spectrophotometer at a wavelength of 440 nm (Adams, 1992; Kandeler et al., 1999).
Data were analyzed using GenStat software version 15th edition. The experiment unit was arranged in an RCBD with three replications. Grain yield, grain P uptake, and available soil P were subjected to one-way ANOVA to examine the effects of treatments, and the mean was compared by Tukey test (p< 0.05). Before ANOVA, the normality of the data and homogeneity of variance were checked using the Shapiro–Wilk and Bartlett tests, respectively. Spearman rank correlation analysis among rice yield attributes, PUE, and soil properties was performed by GenStat software. The coefficient values and their associated significance was tested at 5% confidence level and reported under different p-values: *p< 0.05, **p< 0.01, and ***p< 0.001.
The soil in the experimental site has sandy clay loam texture topsoil (61.68% sand, 27.04% silt, and 11.28% clay). The topsoil (0–20 cm) has a pH (H2O) of 4.8, E.C 0.06 dS/m, and contains 1.35% organic C, 0.33% total N, 0.68 mg kg−1 available P, 58.70 ppm available K, 0.19 mg kg−1 exchangeable Ca, 3.75% exchangeable sodium percentage, and 1.6 cmol kg−1 cation exchange capacity. Details of organic amendments are shown in Table 4.
Data in Table 5 show that except soil pH, all other tested soil chemical parameters were significantly affected by the different treatment combinations of synthetic fertilizers (NPKS), Azolla, and rice straw incorporation. Soil pH at the end of the first and second season ranged from 4.87 to 5.3 and 4.2 to 4.7, respectively. There is no significant difference in pH among treatments (p = 0.84). However, significant effects of pH were noted between the seasons (p< 0.05). The experiment results indicate a marked increase in the mean of total N compared to the pretreatment levels. The total N of soil ranged from medium (0.18%) to high (0.46%) for the first season and medium (0.13) to very high (0.65) in the second season. There is a significant (p< 0.05) increase in total N between the first and second season. The treatments involving the application of organic amendments (Azolla and rice straw incorporation) significantly impact the change in soil TN reserve over sole synthetic fertilizers. The concentration of SOC increased significantly (p< 0.05) between seasons compared to the pretreatment levels. At the end of the first season, the highest concentration of SOC (2.9%) was recorded under the treatment of sole rice straw incorporation, which was statistically (p< 0.05) higher than other treatments. At the end of the second experiment, the highest value of SOC (5.79%) was recorded under the treatment involving Azolla and 50% reduced N along with 30 kg P ha−1, 30 kg K ha−1, and 20 kg S ha−1, which was statistically similar to other organic amended treatments and superior over sole synthetic treatments. The C:N ratio of soil at a depth of 0–20 cm varied between 4.37 to 11.33 and 4.6 to 29.3 for the first and second seasons, respectively. The highest C:N ratio was recorded under sole rice straw treatments in both seasons. There is a significant difference (p< 0.05) in C:N ratio between different treatments and between seasons. The exchangeable K+ concentrations in the soil at a depth of 0–20 cm ranged from 28.19 to 122.12 ppm in the first season and from 29.58 to 98.43 ppm in the second season. Treatments incorporating Azolla (T8, T10, T11, T12, and T13) consistently demonstrated increasing soil K+ levels. The results indicate that the total P content in the soil significantly (p< 0.05) varied with treatment and seasons. In the first and second seasons, the total P content ranged from 0.31 to 2.39 mg kg−1 and 0.15 to 2.95 mg kg−1, respectively. The highest P content was recorded under organic matter application (Azolla and rice straw) that was statistically higher over sole synthetic fertilizer treatments.
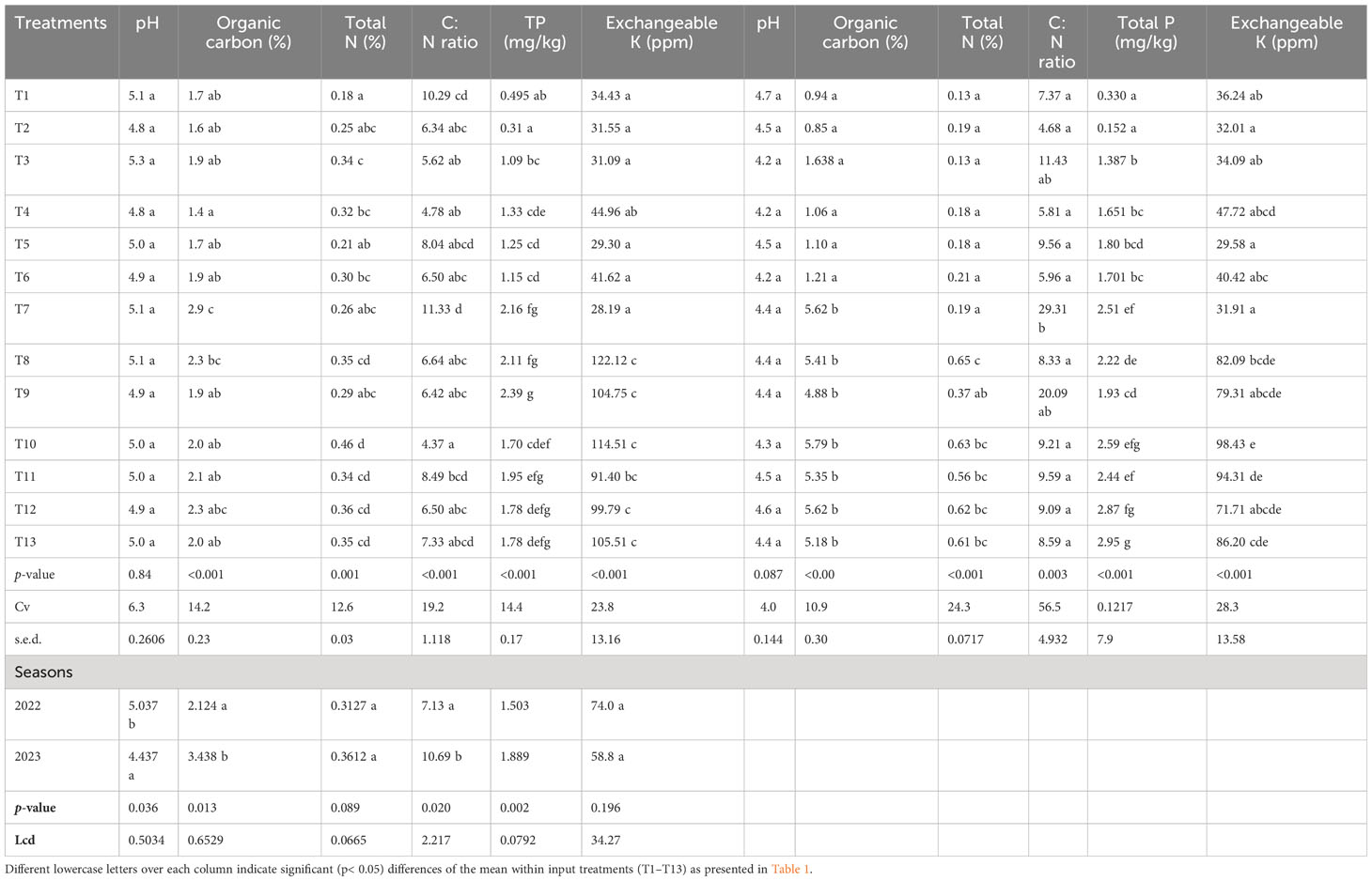
Table 5 The effect of applying Azolla, rice straw, and NPKS fertilizer combinations on soil chemical properties.
The data showed that the co-application of Azolla, rice straw, and synthetic fertilizers resulted in significantly higher enzymatic activities in both seasons compared to control and sole synthetic fertilizer treatments (Table 4). The rate of urea hydrolysis varied from 85.4 to 160.2 µg urea-N hydrolyzed g−1 dw soil 5 h−1 in the first season and 51.1 to 188.5 µg urea-N hydrolyzed g−1 dw soil 5 h−1 in the second season. The treatment involving Azolla combined with 50% reduced N, along with 30 kg P ha−1, 30 kg K ha−1, and 20 kg S ha−1, and co-treatment of Azolla, rice straw + 100 kg N ha−1, 30 kg P ha−1, 30 kg K ha−1, and 20 kg S ha−1 exhibited the highest urease activity for the first and second seasons, respectively. These two treatments significantly (p< 0.001) outperformed sole synthetic fertilizer combinations. No significant difference in urease activity was observed between different synthetic fertilizer applications. The treatments Azolla + 30 kg P ha−1 and Azolla + 50% reduced N, along with 30 kg P ha−1, 30 kg K ha−1, and 20 kg S ha−1, consistently resulted in statistically similar urea hydrolysis as per full recommended synthetic fertilizer application. The highest activities of acid phosphatase in the first season, 99.98 µg nitro phenol hydrolyzed g−1 DW soil 1 h−1, were recorded in rice straw + 50 kg N ha−1 + 30 kg P ha−1, 30 kg K ha−1, and 20 kg S ha−1. In the second season, the highest activity of acid phosphatase, 136.38 µg nitro phenol hydrolyzed g−1 dw soil 1 h−1, was recorded under Azolla + rice straw with 100 kg N ha−1 + 30 kg P ha−1, 30 kg K ha−1, and 20 kg S ha−1. Except for the sole application of 100 Kg N h−1, all other treatments significantly (p< 0.001) differed from the control. Interestingly, treatments involving co-application of P with Azolla and rice straw significantly (p< 0.001) outperformed synthetic fertilizer combination treatments. Despite the population being normally distributed as per the Shapiro–Wilk test for Normality (4.597), there is no significant difference (p< 0.001) observed in alkaline phosphatase between fertilized treatments and even control.
The data of rice grain yield, P uptake, PUE, and AE of two cropping seasons are listed in Table 5. Results of this study indicated that the total biomass and rice grain yield increased significantly (p< 0.001) under co-application of Azolla, rice straw, and synthetic fertilizer treatments (Figure 2). The highest effect of P applied fertilizer on rice grain yield was observed under co-application of Azolla and rice straw along with 100 kg N ha−1 + 30 kg P ha−1, 30 kg K ha−1, and 20 kg S ha−1. No significant difference was observed between rice straw application and 30 kg P ha−1 with control. However, the application of rice straw with 50% reduced N along with 30 kg P ha−1, 30 kg K ha−1, and 20 kg S ha−1 significantly increased rice grain yield over control. Interestingly, the application of Azolla with 50% reduced N along with + 30 kg P ha−1, 30 kg K ha−1, and 20 kg S ha−1 significantly (p< 0.001) maintained a higher grain yield as per full application of 100 kg N ha−1 + 30 kg P ha−1, 30 kg K ha−1, and 20 kg S ha−1 through synthetic fertilizers. The total P uptake, PUE, and AE of P were affected by co-application of organic and synthetic fertilizers, suggesting the residual effect of treatments on P uptake and PUE (Table 5). The total phosphorus concentration in the above rice plant biomass ranges from 2.6 to 73.57 kg ha−1 and from 2.37 to 81.45 kg ha−1 for the first and second seasons, respectively. The study found that co-application of Azolla and rice straw + 100 kg N ha−1 along with 30 kg P ha−1, 30 kg K ha−1, and 20 kg S ha−1 consistently resulted in higher P uptake and was significantly (p< 0.001) higher compared to other treatments. The sole application of 100 kg N ha−1 significantly (p< 0.001) increased aboveground P concentration over control. The application of Azolla + 30 kg P ha−1 and Azolla + 50% reduced N along with 30 kg P ha−1, 30 kg K ha−1, and 20 kg S ha−1 resulted in similar P uptake (p< 0.001) over the balanced recommended level of synthetic fertilizers. No significant difference was observed between control and sole rice straw + 30 kg P ha−1. According to the study, the application of rice straw along with 30 kg P ha−1 resulted in lower PUE and was significantly lower in the average PUE over other treatments. On the other hand, the application of Azolla, rice straw, and 100 kg N ha−1 along with 30 kg P ha−1, 30 kg K ha−1, and 20 kg S ha−1 consistently resulted in higher PUE that significantly outperformed other treatments. The treatment involving Azolla + 50% reduced N along with 30 kg P ha−1, 30 kg K ha−1, and 20 kg S ha−1 resulted in similar PUE with a balanced application of 100 kg N ha−1 + 30 kg P ha−1, 30 kg K ha−1, and 20 kg S ha−1. The mean values of AE generally increased with increased with increasing N rates in years 1 and 2. Co-application of Azolla, rice straw, and 100 kg N ha−1 along with 30 kg P ha−1, 30 kg K ha−1, and 20 kg S ha−1 consistently increased AE by 91.4% and 16.6% compared to full application of 100 kg N ha−1 along with 30 kg P ha−1, 30 kg K ha−1, and 20 kg S ha−1 through synthetic fertilizers for first and second seasons, respectively. The application of rice straw with 30 kg P ha−1 consistently resulted in low AE of P that was statistically (p< 0.001) lower compared to other treatments.
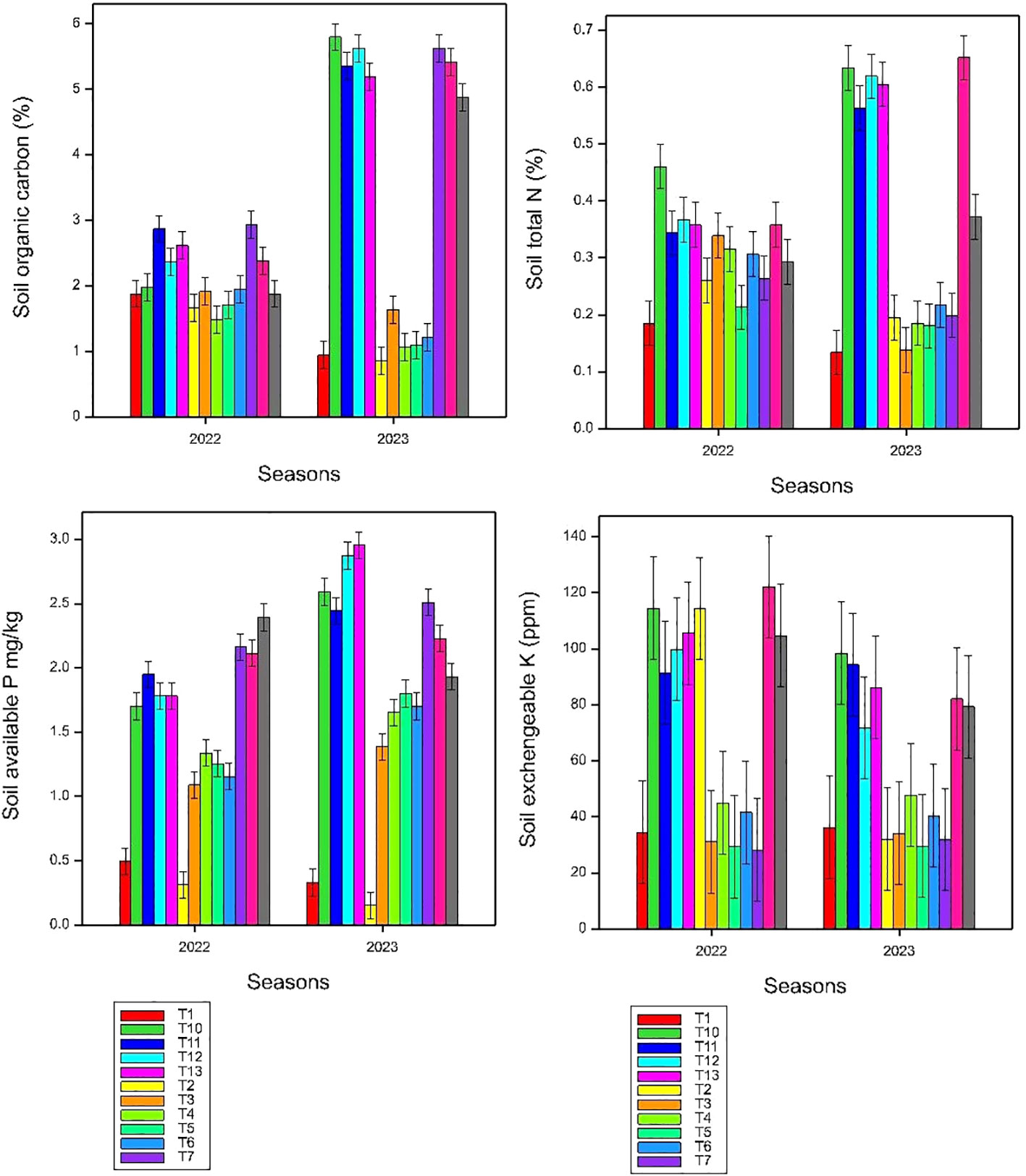
Figure 2 Effects of co-application of Azolla, rice straw, and NPKS fertilizer combination on some selected soil properties, small bars are standard errors at p< 0.05.
The results for Spearman rank correlation analysis among rice yield attributes, PUE, and soil properties are shown in Figure 3. There was significant positive correlation between rice grain yield vis-à-vis agronomic efficiency of P (AEP), nitrogen uptake (NU), PU, and PUE (r = 0.99***, 0.877***, 0.895*** and 0.895***, respectively). Soil total N (TN) has a very strong positive correlation with soil organic carbon, acid phosphatase, exchangeable K+ and soil urease (URE) (r = 0.616***, 0.704***, 0.620***, and 0.704***, respectively). It also moderately correlated with NU (r = 0.251*). URE has a strong positive correlation with acid phosphatase (Ac.PH) and organic carbon (r = 1.000*** and 0.448**, respectively). PUE has strong positive correlations with PU and NU (r = 0.875***, and 0.895***, respectively), and moderately correlated with acid and alkaline phosphatase (r = 0.354* and 0.273*, respectively).
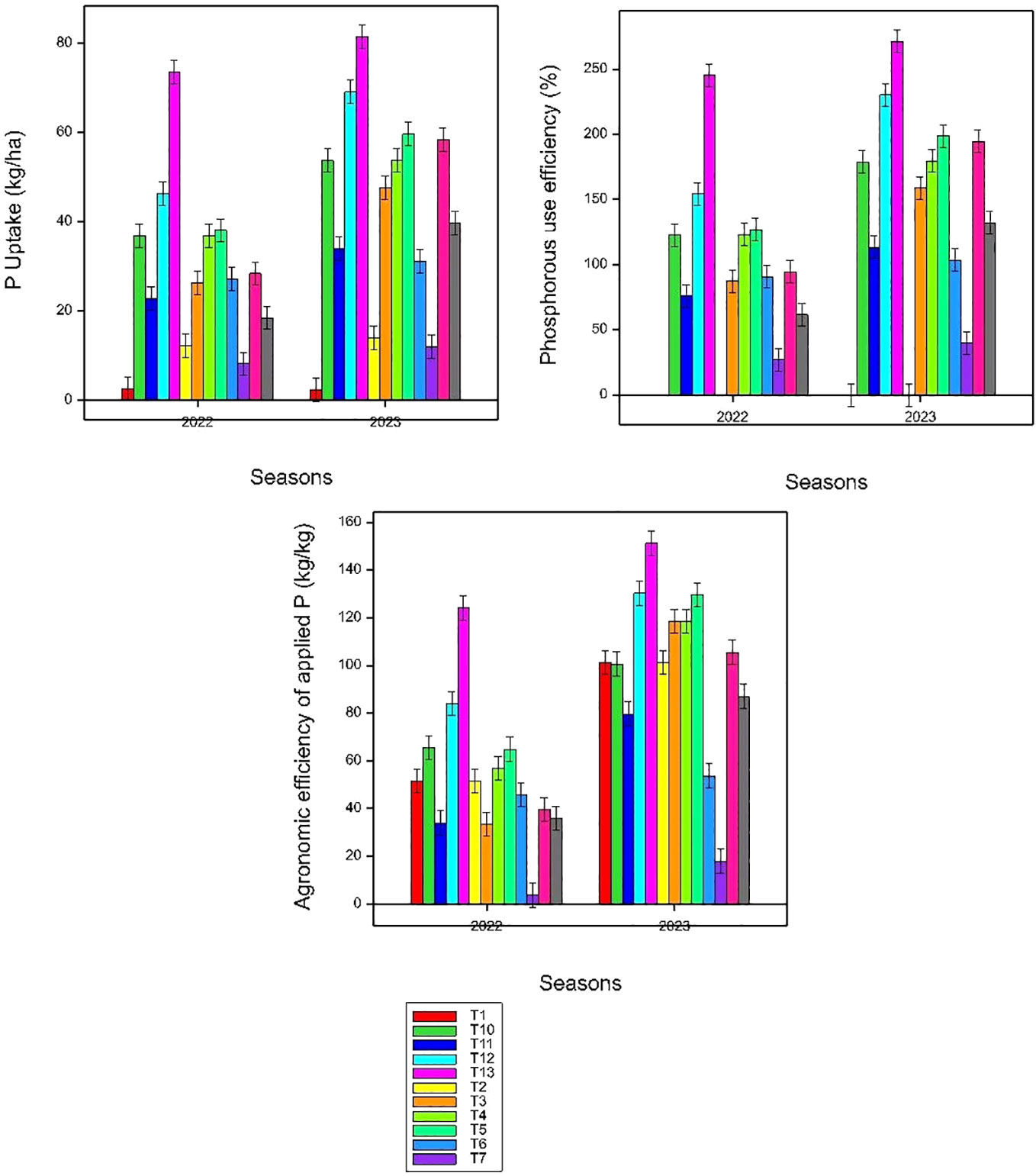
Figure 3 Effects of co-application of Azolla, rice straw, and NPKS fertilizer combination on soil urease, acid, and alkaline phosphatases; small bars are standard errors at p< 0.05.
The present study demonstrated that short-term application of Azolla and rice straw impact change in most chemical fertility (Figure 2). The stability of pH within certain ranges suggests that the treatments were effective in maintaining a suitable soil environment for rice cultivation. However, the observed seasonal disparity in pH emphasizes the dynamic nature of soil processes, influenced by both applied treatments and external factors associated with seasonal changes. Soil chemical fertility depends on several factors such as climate, topography, nature of the soil, and type of amendments (Körschens et al., 2013; Tian et al., 2015a). Similar results were reported by Zhou et al. (2015) in which application of chemical fertilizers alone or partially substituted with organic fertilizers increased the soil pH by 0.71 to 0.96 units over the control. This might be due to the severe microbial nitrification process that led to soil acidification, which is largely attributed to ammonium fertilizers applied in the soil (Tian et al., 2015b). Soil total nitrogen is the major determinant and indicator of soil fertility and quality in an agricultural ecosystem and is closely related to soil productivity (Li et al., 2022). The significant increase in total nitrogen (TN) levels observed in the soil, particularly in the Azolla-fertilized treatments, might be attributed to Azolla N fixing capacity. Azolla is known for its ability to fix atmospheric nitrogen with the help of nitrogen-fixing cyanobacteria present in its symbiotic relationship (Akhtar et al., 2020). This process results in an increase in available nitrogen in the soil (Dey and Datta, 2008). The combination of Azolla and rice straw incorporation might have synergistic effects on nitrogen availability. Azolla provides nitrogen through biological nitrogen fixation, while rice straw helps in accumulation of TN stocks. Together, they create a more comprehensive impact on soil TN levels. Rice straw incorporation significantly (p< 0.05) affected the soil organic carbon. This might be due to the wider C/N (59.1) ratio of straw that takes longer to decompose and thus improve soil aggregation and soil water retention and reduce bulk density of the soil, promoting crop growth and TN stocks (Ekawati and Purwanto, 2014; Li et al., 2022). The observed increase in SOC concentrations is statistically significant (p< 0.05), emphasizing the reliability of the results. This suggests that the changes in SOC are not due to random variability but are attributed to the applied treatments. This observed outcome highlights the importance of nutrient management strategies that integrate organic inputs such as Azolla and rice straw and the synergy between organic amendments and adjusted synthetic fertilizers contributes to the improvement of SOC, indicating a more sustainable and holistic approach to soil health. Soil organic carbon and its stoichiometric characteristics are important indicators for the quality and quantity of soil organic matter (Tong et al., 2023). The carbon-to-nitrogen (C:N) ratio in the soil is a crucial indicator of nutrient availability and microbial activity (Landon, 1991). A higher C:N ratio generally indicates slower decomposition of organic matter relative to nitrogen release. The consistently highest C:N ratio recorded under sole rice straw treatments in both seasons suggests that the decomposition of rice straw, which is rich in carbon, outpaced nitrogen release. This leads to a higher C:N ratio, indicating a relative abundance of carbon compared to nitrogen. This is in accordance with some results of Zhang et al. (2015). However, results demonstrated that the incorporation of synthetic fertilizers, Azolla, and rice straw each contributes differently to the carbon and nitrogen content of the soil, influencing the overall C:N ratio. The significant differences in C:N ratio between seasons suggest that continuous application of treatment combinations could enhance organic matter reserve and decomposition that subsequently impacts change in nutrient release. This might be a holistic approach to nutrient management and sustainable agricultural practices. Results of the experiments indicated that application of Azolla and rice straw enhances P content in soil. The rise in soil P content can be attributed to Azolla’s high P absorption capacity, directly promoting Azolla biomass growth. The elevated PUE in these treatment combinations provides clear evidence that upon the decomposition of Azolla plants, the organic nitrogen and phosphorus undergo rapid mineralization, releasing them as biofertilizers available for the thriving rice plants. The study by Chatterjee et al. (2021) demonstrated that long-term organic fertilization, including the application of Azolla, consistently yielded the highest P content in soil, surpassing the levels observed in treatments solely reliant on synthetic fertilizers. These findings emphasize the effectiveness of organic matter (Azolla and rice straw) in enhancing soil phosphorus levels, suggesting its potential as a sustainable alternative to synthetic fertilizers.
Soil microbial communities produce enzymes in response to soil changes that modify organic substrates and other soil factors (Lagos et al., 2015). Hence, it is possible to use enzymatic activity as an indicator of soil changes (Saha et al., 2008; Liang et al., 2014). This study showed clear response of enzyme activities by co-application of Azolla, rice straw, and synthetic fertilizers on URE activity over sole synthetic fertilizers (Figure 4). The higher urease activity in treatment involve Azolla combined with 50% reduced N, along with 30 kg P ha−1, 30 kg K ha−1, and 20 kg S ha−1, and co-treatment of Azolla, rice straw + 100 kg N ha−1, 30 kg P ha−1, 30 kg K ha−1, and 20 kg S ha−1 could be attributed to improvement of soil organic carbon content that directly enhances microbial community (Luo et al., 2016; Sharma et al., 2021; Singh et al., 2023). Generally, an increase in urease activity indicates an improvement in the ability of microorganisms in the soil to convert urea into ammonium and make it available to plants. This is crucial for NU by crops (Apoorva et al., 2018). Similar urease activities in rice straw treatment and 50% reduced level of N along with 30 kg P ha−1, 30 kg K ha−1, and 20 kg S ha−1 enhanced soil microbial population and increased soil N availability. A previous study by Sharma et al. (2021) demonstrated that the co-application of rice straw with N fertilizer significantly increases soil enzyme activity through enhanced substrate availability. Another study (Jat et al., 2019) found that if cereal residues are incorporated in soil, N immobilization might require higher levels of fertilizer N in the short term as compared with no residue. The return of crop residues may, however, lead to a net buildup of readily mineralized soil organic nitrogen, which may reduce crop fertilizer requirements in the future. Phosphomonoestarases are large groups of enzymes that increase the rate of organic phosphate hydrolysis (Sırt Çıplak and Akoğlu, 2020). The higher activities of acid phosphomonoestarases than alkaline phosphomonoestarases might be due to the pH nature of the soil, since the optimal activity of these enzymes is pH dependent (Nannipieri et al., 2012). Previous studies demonstrated that in adaptation to different environmental conditions, acidic soils tend to have higher levels of acid phosphomonoestarases, while alkaline soils tend to have higher levels of alkaline phosphomonoestarases; for this reason, neither enzyme is positively correlated with the other (Nannipieri et al., 2012; Dotaniya et al., 2018). Higher levels of acid phosphomonoestarases might also be influenced by wet soil conditions as observed by Grierson and Adams (2000) in moist winter and spring; acid phosphatase activity was between 30 and 40 mmol p-Nitrophenol g−1 h−1 that declined to less than 10 mmol p-Nitrophenol g−1 h−1 during the dry summer. The highest activities of acid phosphatase under rice straw + 50 kg N ha−1 + 30 kg P ha−1, 30 kg K ha−1, and 20 kg S ha−1 in the first season and Azolla + rice straw with 100 kg N ha−1 + 30 kg P ha−1, 30 kg K ha−1, and 20 kg S ha−1 in the second season might be due to enhancing a favorable environment for microbial activity and nutrient cycling, specifically benefiting acid phosphatase activity in the soil. The study by Nannipieri et al. (2012) demonstrated that acid phosphatase activities are correlated with the content of organic matter and decrease with soil depth. Another study (Mohamed et al., 2021) reported that incorporating rice straw stimulates soil microorganisms and accordingly enhances soil dehydrogenase, catalase, and acid phosphatase activities.
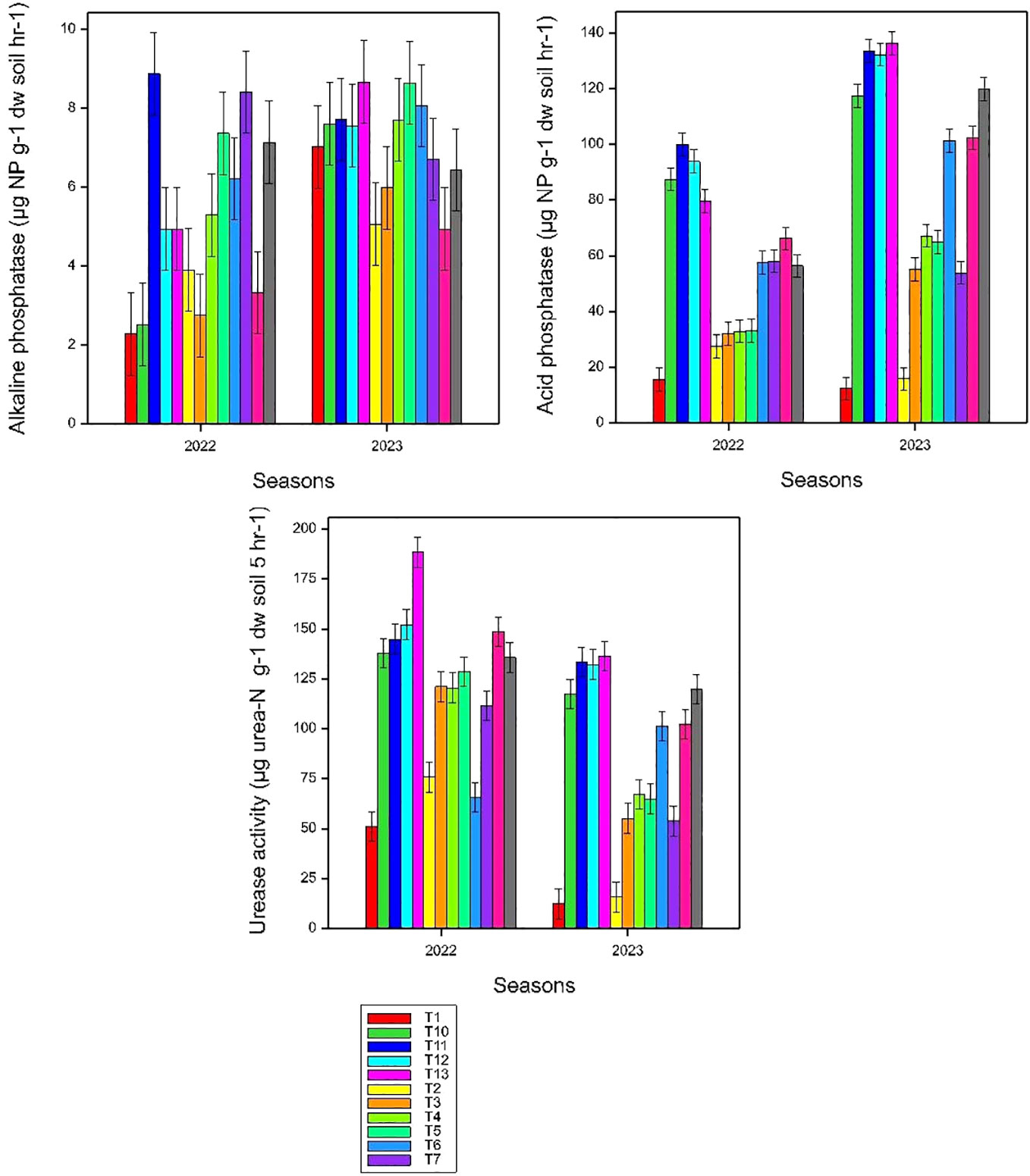
Figure 4 Effects of applying rice straw, Azolla, and NPKS fertilizer combinations (T1–T13) on total biomass (kg ha−1) and rice grain yield (kg ha−1); small bars are standard errors at p< 0.05.
The observed outcomes in rice grain yield based on different treatments suggest that the interaction between organic amendments (rice straw and Azolla) and synthetic fertilizers, along with variations in nutrient management, plays a crucial role in influencing crop productivity (Figure 5). The application of Azolla and rice straw along with 100 kg N ha−1, 30 kg P ha−1, 30 kg K ha−1, and 20 kg S ha−1 has a significant effect on the total biomass and grain yield. This effect may be attributed to the physicochemical changes in the rhizosphere soil, including (1) improved soil water holding capacity (Andriamananjara et al., 2019); (2) decreased soil bulk density, thereby improving root nutrient acquisition (Awodun, 2008); (3) enhance availability of N, P, and other essential nutrients (Division et al., 1995; Ali et al., 2014; Chawngthu et al., 2020; Liu et al., 2021); (4) decreased P sorption sites after interaction of organic anion released during Azolla and rice straw decomposition in the soil through complexation or precipitation of soluble Al or competitive sorption sites; and (5) promotion of soil microbial biomass and activity (Andriamananjara et al., 2019; Dhar et al., 2022; Khmelevtsova et al., 2022). Additionally, Azolla, being a nitrogen-fixing aquatic fern, may have contributed nitrogen to the soil, compensating for the reduced synthetic nitrogen input, fostering optimal conditions for rice growth. The maintenance of a higher grain yield in sole Azolla along with 30 kg P ha−1 compared to full synthetic fertilizer application suggests the efficiency of Azolla in nutrient provision. Because the application of Azolla, rice straw, and 50% reduced N along with 30 kg P ha−1, 30 kg K ha−1, and 20 kg S ha−1 did not significantly affect total biomass and rice grain yield over the full recommended level of NPKS through synthetic fertilizers, our results suggest that this combination may contribute to yield and long-term environmental and soil quality management.
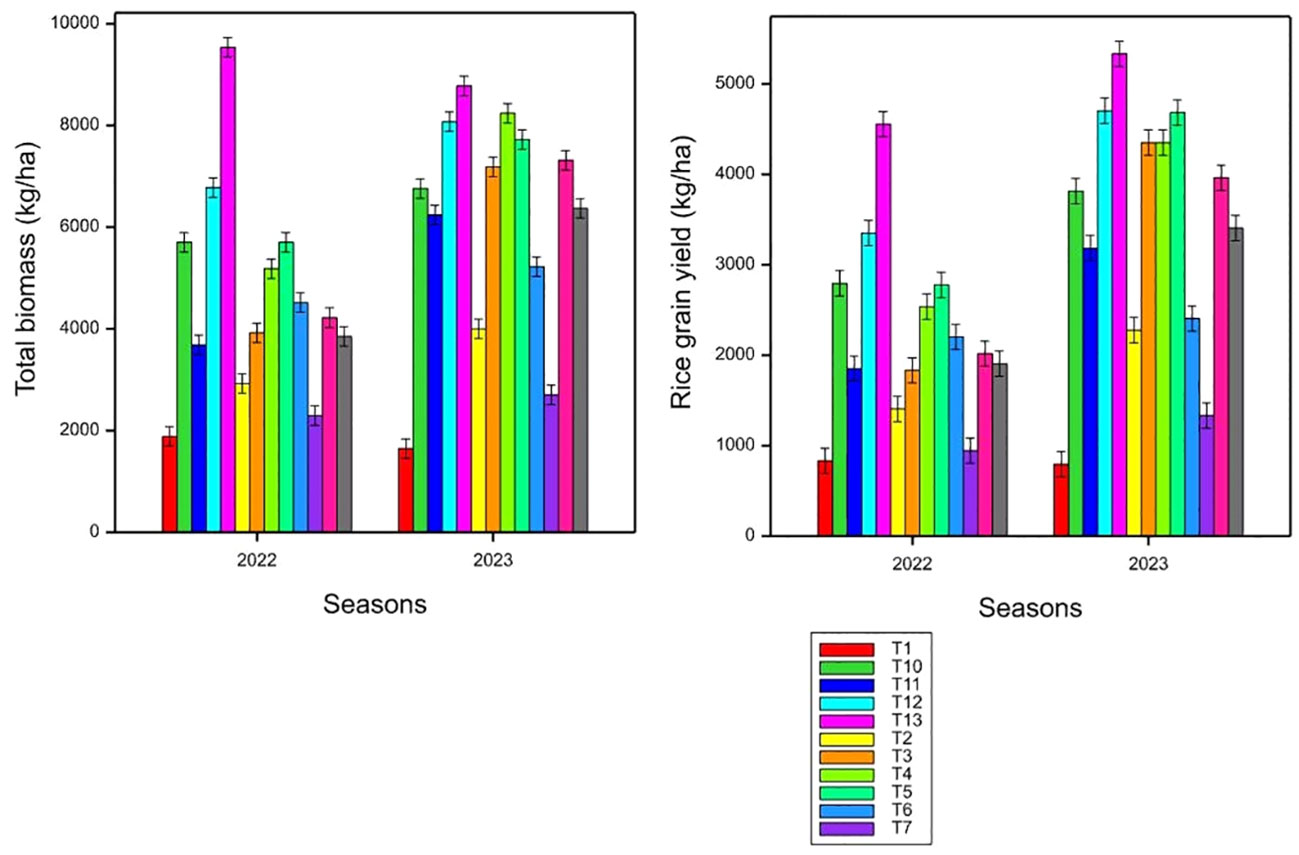
Figure 5 Effects of applying rice straw, Azolla, and NPKS fertilizer combinations (T1–T13) on P uptake, agronomic efficiency of P, and P use efficiency; small bars are standard errors at p< 0.05.
The study reveals interesting findings regarding phosphorus (P) uptake in plants under various treatment conditions (Table 6); higher P uptake under co-application of Azolla and rice straw along with 100 kg N ha−1, 30 kg P ha−1, 30 kg K ha−1, and 20 kg S ha−1 might be due to the synergistic effect of Azolla, rice straw, and synthetic fertilizers, which likely created an optimal environment for phosphorus availability and uptake by rice plants. Compared to the control using the full dose of chemical N fertilizer alone (100 kg N ha−1) seems to have a positive effect on phosphorus concentration possibly due to stimulating plant growth and biomass production, which can increase the demand and uptake of P by plants (Tang et al., 2021; Zhang et al., 2022a). However, the effect of N input on P uptake may depend on the type, amount, and timing of N input, the plant species and functional traits, the soil properties and P status, and the interactions with other factors (Zhang et al., 2022b; Chen et al., 2023). Therefore, the response of P uptake to N input may vary across different ecosystems and experimental conditions. The addition of rice straw alone with 30 kg P ha−1 may not provide a substantial boost in PU compared to the control, possibly due to the high carbon-to-phosphorus ratio of rice straw that may lead to microbial immobilization of phosphorus, reducing its availability for plant uptake (Mi et al., 2016). However, rice straw decomposition may be accelerated by the presence of organic amendments with low C:N ratio (Gummert et al., 2020; Van Hung et al., 2020). These results provide valuable insight into effective and environmentally conscious nutrient management practices, which can have positive implications for crop productivity, soil health, and agricultural sustainability. PUE was expressed using a balanced method to show how much P is being taken out of the system compared with the amount applied and the residual (Syers et al., 2008). Inorganic P was applied as triple super phosphate (TSP) at a constant rate (30 kg P ha−1). However, it was observed that different treatments had a significant effect (p ≤ 0.05) on PUE, indicating that phosphorus input does not solely determine PUE, but various other factors also affect it. Diverging responses of sole rice straw incorporation along with 30 kg P ha−1 with respect to PUE were observed. This suggests that, under the specific conditions of the study, the use of rice straw with 30 kg P ha−1 did not efficiently contribute to phosphorus utilization by the plants, possibly due to the high C/N ratio of straw that creates a nitrogen deficiency in the soil (Zheng et al., 2015). This was also confirmed by researchers (Nekir, 2019; Liu et al., 2020; Chen et al., 2021) in which nitrogen is required for proper P uptake and utilization, and its deficiency can limit the efficiency of P uptake. The higher PUE under the co-application of Azolla, rice straw, and 100 kg N ha−1 along with P, K, and S might be due to the synergistic effect of this treatment combination, particularly with added nitrogen and balanced nutrients, which appears to enhance PUE, possibly due to improved nutrient availability and microbial activity. There is substantial evidence that application of a high rate of N results in soil acidification, which decreases exchangeable Ca and Mg concentrations while mobilizing Fe and Al with significant effects on repressing soil microbial population and soil phosphatase activity (Kimani et al., 2020; Chen et al., 2021; Zhang et al., 2022b).
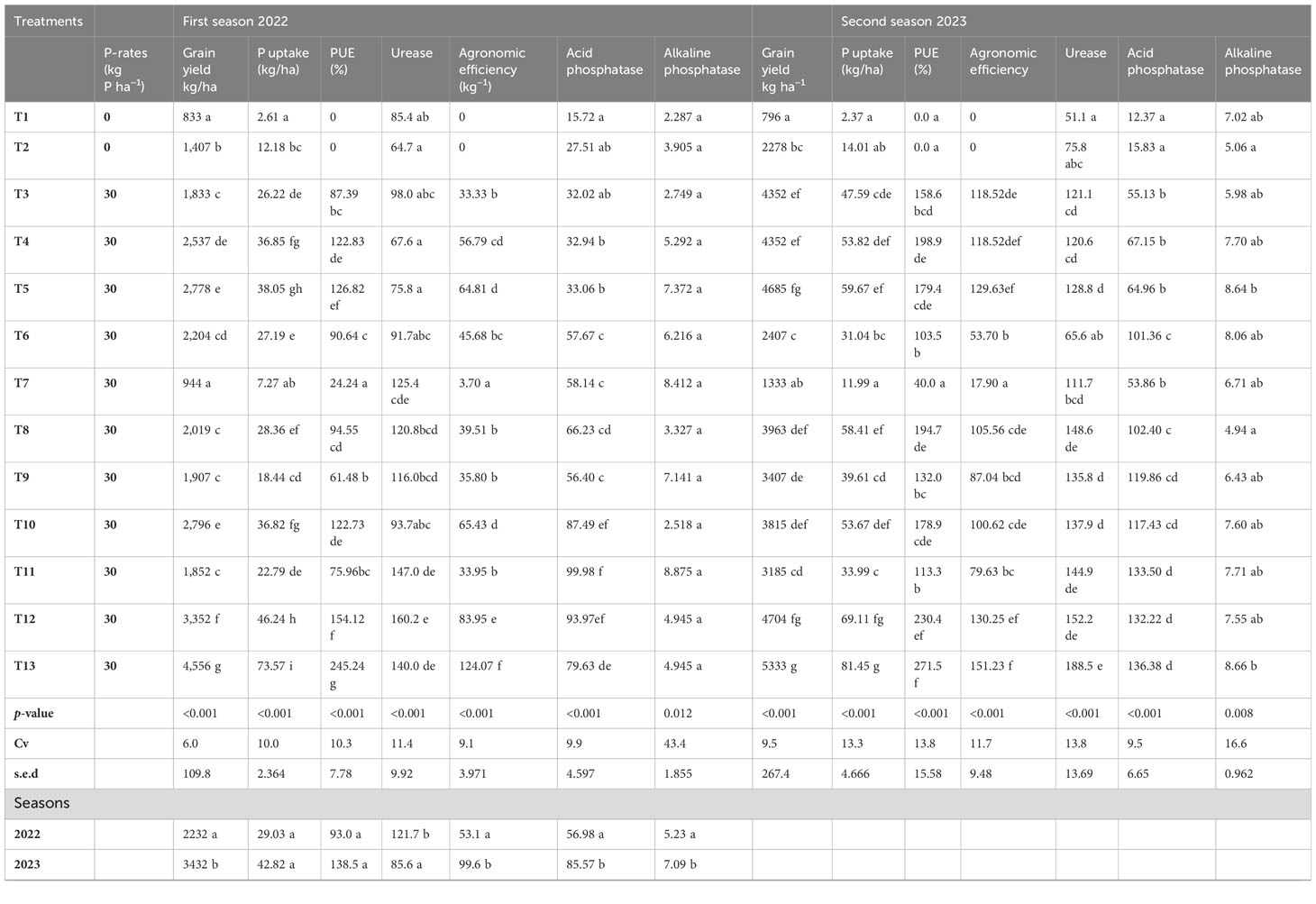
Table 6 Effects of Azolla, synthetic fertilizers, and rice straw on P use efficiency and enzyme activities.
The consistency increase in mean values of agronomic efficiency (AE) with higher nitrogen rates in both years suggests that, in the context of this study, increasing the nitrogen input positively influenced agronomic efficiency, likely leading to improved crop productivity. A similar observation was made by Biswas Chowdhury and Zhang (2021) and Chen et al. (2021). This indicates a more effective use of applied nutrients for crop yield. The high AE in the co-application of Azolla and rice straw along with 100 kg N ha−1, 30 kg P ha−1, 30 kg K ha−1, and 20 kg S ha−1 treatments was attributed to soil C status and total nutrients added that increase microbial activity and carbon availability, and caused indirect modifications of the soil. By adding organic matter and nutrients to paddy soils, organic amendment can enhance soil biological activity and sustain soil quality (Andriamananjara et al., 2019). The combination of Azolla, rice straw, and half the amount of N, along with 30 kg P ha−1, 30 kg K ha−1, and 20 kg S ha−1, may have improved the agronomic efficiency of P in this study. This may have achieved a better balance of nitrogen and phosphorus in the soil and promoted the soil nutrient cycling and microbial activity. Therefore, based on our research findings, we strongly recommend adopting alternative fertilization strategies to mitigate the negative impacts associated with overapplication of synthetic chemical fertilizers. Specifically, we propose the application of rice straw and Azolla with reduced nitrogen as a viable solution. This approach can be considered a form of slow-release fertilization, offering several benefits. Notably, it enhances phosphorus (P) recovery efficiency and contributes to better soil health management for the future.
In this study, the positive correlation coefficients indicate that as one variable increases, the other tends to increase as well. A coefficient value close to 1 suggests a strong linear relationship between the variables, implying that changes in one variable are highly predictive of changes in the other. The Spearman rank correlation analysis conducted on rice yield attributes, PUE, and soil properties revealed significant associations among them (Figure 6). Rice grain yield exhibits a remarkably strong positive correlation with agronomic efficiency of P (AEP), NU, PU, and PUE (r = 0.99***, 0.877***, 0.895***, and 0.895***, respectively). Nitrogen and phosphorus play an important role in supporting rice growth and yield (Fomba et al., 2020; Rajï, 2020; Rupngam et al., 2023). Proper utilization of N and P enhances root development, shoot growth, photosynthesis, effective tiller formation, and rice grain yield (Kalayu, 2019; Margalef et al., 2021; Aimen et al., 2022; Tariq et al., 2022), emphasizing the critical role of nutrient use efficiency in achieving higher yields. Soil total nitrogen (TN) demonstrates strong positive correlations with soil organic carbon, P uptake, PUE, acid phosphatase, exchangeable K+, and URE highlighting the intricate relationships between soil fertility indicators. These correlations suggest that a balanced availability of nitrogen might play a role in enhancing the efficiency of phosphorus utilization by rice plants (Marklein and Houlton, 2011; Arenberg and Arai, 2021). Optimal nitrogen levels might support metabolic processes that contribute to more efficient PU and utilization, leading to higher PUE (Hogan et al., 2010). Moreover, TN moderately correlates with NU, indicating its influence on soil N availability. However, the moderate correlation between TN and N uptake might be due to increasing N losses associated with excessive N application. Moreover, excess nitrogen prolongs the vegetative growth period, delays maturity, and attracts insect pests, leading to disease epidemics (Anas et al., 2020). The strong positive correlations exist between URE, acid phosphatase, and organic carbon, underscoring the interconnected nature of soil enzymatic activities and organic matter application. Soil organic matter inputs have been found to alter soil enzyme activity and allocation patterns (Weintraub et al., 2013). However, the correlation between soil enzymes and organic matter is intricate and dependent on the enzyme type, as well as the quality and quantity of organic matter (Margalef et al., 2021). Interestingly, PUE demonstrates strong positive associations with PU and NU, and moderate correlations with acid and alkaline phosphatase, illustrating the interplay between nutrient utilization efficiency and enzymatic processes in soil.
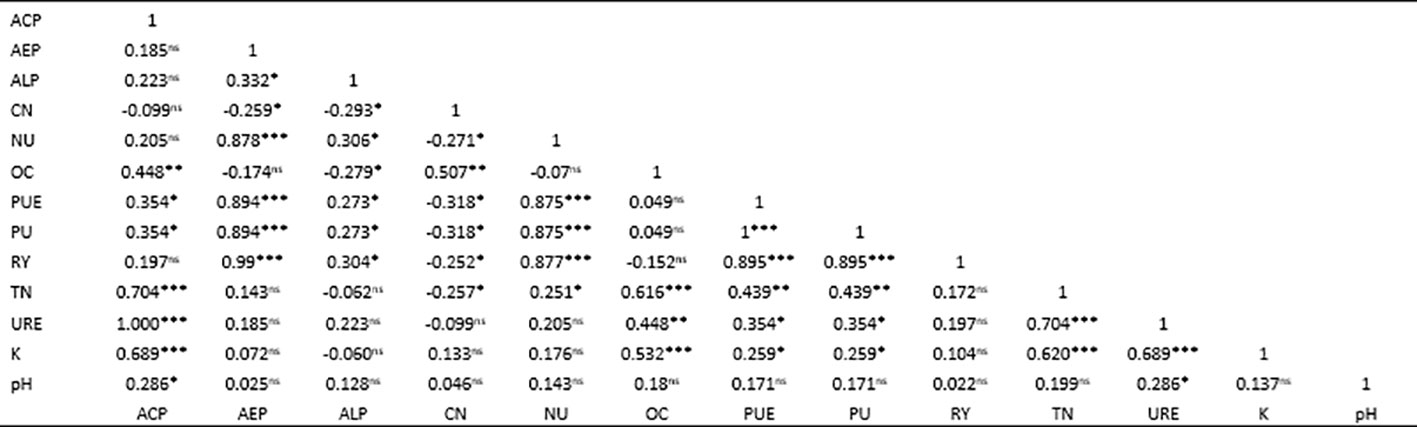
Figure 6 Correlation matrix displaying correlations among rice yield, phosphorus use efficiency, enzyme activity, and other soil properties. The values indicate coefficients and their associated significance *p< 0.05, **p< 0.01, ***p< 0.001 at 5% confidence level (ACP, acid phosphatase; AEP, agronomic efficiency of P; ALP, alkaline phosphatase; NU, nitrogen uptake; PUE, P use efficiency; PU, P uptake; RY, rice grain yield; TN, total N; URE, urease). NS, not significance.
The results suggest that the short-term application of Azolla and rice straw can induce significant soil chemical fertility and enzyme activity. In addition, the results indicated that the synergistic effect of Azolla and rice straw contributes to higher soil total nitrogen levels, maintaining pH stability for rice cultivation, and enhances soil total N, organic carbon, and total P highlight dynamic soil processes influenced by treatments and external factors. Total nitrogen increase, particularly in Azolla-treated soils, suggests its nitrogen-fixing capacity. Rice straw incorporation influences soil organic carbon, impacting soil health sustainably. The study further indicates that the co-application of Azolla and rice straw with 50% reduced N and balanced 30 kg P ha−1, 30 kg K ha−1, and 20 kg S ha−1 improves rice yield, PU, and PUE, demonstrating a sustainable practice for enhancing crop performance and soil health. The study underlines that short-term application of Azolla and rice straw with reduced N and balanced PKS enhances phosphorus content in soils and underscores Azolla’s high absorption capacity and its role in enhancing soil fertility and PUE. These findings emphasize the potential of organic amendments for holistic nutrient management and sustainable agriculture practices.
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
SM: Conceptualization, Methodology, Resources, Writing – original draft. HT: Methodology, Supervision, Writing – original draft. NA: Supervision, Writing – original draft. HC: Project administration, Resources, Writing – original draft. JS: Supervision, Writing – original draft.
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abraham G. (2010). Antioxidant enzyme status in Azolla microphylla in relation to salinity and possibilities of environmental monitoring. Thin Solid Films 519, 1240–1243. doi: 10.1016/j.tsf.2010.08.076
Adams M. A. (1992). Phosphatase activity and phosphorus fractions in Karri (Eucalyptus diversicolor F. Muell.) forest soils. Biol. Fertil. Soils 14, 200–204. doi: 10.1007/BF00346061
Aimen A., Basit A., Bashir S., Aslam Z., Faheem M., Amjad S., et al. (2022). Saudi Journal of Biological Sciences Sustainable phosphorous management in two different soil series of Pakistan by evaluating dynamics of phosphatic fertilizer source. Saudi J. Biol. Sci. 29, 255–260. doi: 10.1016/j.sjbs.2021.08.086
Akhtar M., Sarwar N., Ashraf A., Ejaz A., Ali S., Rizwan M. (2020). Beneficial role of Azolla sp. in paddy soils and their use as bioremediators in polluted aqueous environments: implications and future perspectives. Arch. Agron. Soil Sci. 1–14. (Taylor and Francis Ltd). doi: 10.1080/03650340.2020.1786885
Alaivasha E., Tumbo M., Senyangwa J., Mourice S. (2022). Influence of water management farming practices on soil organic carbon and nutrients : A case study of rice farming. Agronomy 12, 148. doi: 10.3390/agronomy12051148
Ali M. A., Sattar M. A., Islam M. N., Inubushi K. (2014). Integrated effects of organic, inorganic and biological amendments on methane emission, soil quality and rice productivity in irrigated paddy ecosystem of Bangladesh: Field study of two consecutive rice growing seasons. Plant Soil 378, 239–252. doi: 10.1007/s11104-014-2023-y
Anas M., Liao F., Verma K. K., Sarwar M. A., Mahmood A., Chen Z. L., et al. (2020). Fate of nitrogen in agriculture and environment: agronomic, eco-physiological and molecular approaches to improve nitrogen use efficiency. Biol. Res. 53 (1), 1–20. doi: 10.1186/s40659-020-00312-4
Andriamananjara A., Rakotoson T., Razafimbelo T., Rabeharisoa L., Razafimanantsoa M. P., Masse D. (2019). Farmyard manure improves phosphorus use efficiency in weathered P deficient soil. Nutrient Cycling Agroecosys. 115, 407–425. doi: 10.1007/s10705-019-10022-3
Apoorva M. R., Rao P. C., Padmaja G., Reddy R. S. (2018). Activity of soil urease, phosphatase and dehydrogenase as influenced by various sources of zinc in rice (Oryza sativa L.). Int. J. Curr. Microbiol. Appl. Sci. 7, 2640–2647. doi: 10.20546/ijcmas.2018.701.315
Arenberg M. R., Arai Y. (2021). Nitrogen species specific phosphorus mineralization in temperate floodplain soils. Sci. Rep., 1–12. doi: 10.1038/s41598-021-96885-5
Assefa S., Haile W., Tena W. (2021). Heliyon Effects of phosphorus and sulfur on yield and nutrient uptake of wheat (Triticum aestivum L.) on Vertisols, North Central, Ethiopia. Heliyon 7, e06614. doi: 10.1016/j.heliyon.2021.e06614
Awodun M. A. (2008). Effect of Azolla (Azolla species) on physiochemical properties of the soil. World J. Agric. Sci. 4, 157–160.
Azene B., Qiu P., Zhu R., Pan K., Sun X., Nigussie Y., et al. (2022). Response of soil phosphorus fractions to land use change in the subalpine ecosystems of Southeast margin of Qinghai-Tibet Plateau, Southwest China. Ecol. Indic. 144, 109432. doi: 10.1016/j.ecolind.2022.109432
Bagheri Novair S., Mirseyed Hosseini H., Etesami H., Razavipour T. (2020). Rice straw and composted azolla alter carbon and nitrogen mineralization and microbial activity of a paddy soil under drying–rewetting cycles. Appl. Soil Ecol. 154, 103638. doi: 10.1016/j.apsoil.2020.103638
Beretta A. N., Silbermann A. V., Paladino L., Torres D., Bassahun D., Musselli R., et al. (2014). Análisis de textura del suelo con hidrómetro: Modificaciones al método de Bouyoucus. Ciencia e Investigacion Agraria 41, 263–271. doi: 10.4067/S0718-16202014000200013
Bhunia S., Bhowmik A., Mallick R., Mukherjee J. (2021). Agronomic Efficiency of Animal-Derived Organic Fertilizers and Their Effects on Biology and Fertility of Soil : A Review. 1–25. doi: 10.3390/agronomy11050823
Bird J. A., Horwath W. R., Eagle A. J., Van Kessel C. (2001). Immobilization of fertilizer nitrogen in rice: effects of straw management practices. Soil Sci. Soc. America 65, 1143–1152. doi: 10.2136/sssaj2001.6541143x
Biswas Chowdhury R., Zhang X. (2021). Phosphorus use efficiency in agricultural systems: A comprehensive assessment through the review of national scale substance flow analyses. Ecol. Indic. 121, 107172. doi: 10.1016/j.ecolind.2020.107172
Bray R. H., Kurtz L. T. (2009). Determination of total, organic and available forms of phosphorus in soils. In Soil Sci. 59, 39–45.
Bremner M. (1996). “Chapter 37: Nitrogen-Total. Methods of Soil Analysis Part 3.,” in Chemical Methods-SSSA Book Series 5, vol. 5. , 1085–1121.
Cabangon R. J., Tuong T. P., Castillo E. G., Fosu-Mensah B. Y., Lumpkin A., Plueknett D. L., et al. (2015). Ammonia volatilization losses from paddy fields under controlled irrigation with different drainage treatments. Soil Sci. Plant Nutr. 2, 329–342. doi: 10.1155/2014/417605
Chatterjee D., Nayak A. K., Mishra A., Swain C. K., Kumar U., Bhaduri D., et al. (2021). Effect of long-term organic fertilization in flooded rice soil on phosphorus transformation and phosphate solubilizing microorganisms. J. Soil Sci. Plant Nutr. 21 (2), 1368–1381. doi: 10.1007/s42729-021-00446-8
Chawngthu L., Hnamte R., Lalfakzuala R. (2020). Isolation and characterization of rhizospheric phosphate solubilizing bacteria from wetland paddy field of Mizoram, India. Geomicrobiol. J. 37, 366–375. doi: 10.1080/01490451.2019.1709108
Chen S., Cade-Menun B. J., Bainard L. D., St. Luce M., Hu Y., Chen Q. (2021). The influence of long-term N and P fertilization on soil P forms and cycling in a wheat/fallow cropping system. Geoderma 404, 115274. doi: 10.1016/j.geoderma.2021.115274
Chen S., Zhang W., Ge X., Zheng X., Zhou X., Ding H., et al. (2023). Response of plant and soil N, P, and N:P stoichiometry to N addition in China: A meta-analysis. Plants 12. doi: 10.3390/plants12112104
Cissé M., Vlek P. L. G. (2022). Conservation of urea-N by immobilization-remobilization in a rice-Azolla intercrop Vol. 250. Eds. Cissé M., Vlek P. L.G. (Springer), 95–104. Available at: https://www.jstor.org/stable/24129397.
Dey M., Datta S. (2008). Nitrogen fixation in rice. Rice Improvement Genomics Era. (Elsevier B.V). doi: 10.1201/9781439822562.ch13
Dhar S., Vikram M., Rajawat S. (2022). Microbes-mediated integrated nutrient management for improved rhizo-modulation, pigeonpea productivity, and soil bio-fertility in a semi-arid. doi: 10.3389/fmicb.2022.924407
Division C., Industry P., Box G. P. O., Box P. O. (1995). Biological nitrogen fixation : An efficient source of nitrogen for s agricultural production? 3–28.
Dotaniya M. L., Aparna K., Dotaniya C. K., Singh M., Regar K. L. (2018). “Role of soil enzymes in sustainable crop production,” in Enzymes in Food Biotechnology: Production, Applications, and Future Prospects (Elsevier Inc). doi: 10.1016/B978-0-12-813280-7.00033-5
Dotaniya M. L., Meena V. D. (2015). Rhizosphere effect on nutrient availability in soil and its uptake by plants: A review. Proc. Natl. Acad. Sci. India Sect. B - Biol. Sci. 85, 1–12. doi: 10.1007/s40011-013-0297-0
Ekawati I., Purwanto Z. (2014). Application of immature rice straw compost, Azolla, and Urea for increasing rice fields production based on local wisdom. J. Basic. Appl. Sci. Res. 4 (12), 130–134.
Fageria N. K., Carvalho G. D., Santos A. B., Ferreira E. P. B., Knupp A. M. (2011). Chemistry of lowland rice soils and nutrient availability. Commun. Soil Sci. Plant Anal. 42, 1913–1933. doi: 10.1080/00103624.2011.591467
Fageria N. K., de Morais O. P., dos Santos A. B. (2010). Nitrogen use efficiency in upland rice genotypes. J. Plant Nutr. 33, 1696–1711. doi: 10.1080/01904167.2010.496892
Fisher K. A., Yarwood S. A., James B. R. (2017). Soil urease activity and bacterial ureC gene copy numbers: Effect of pH. Geoderma 285, 1–8. doi: 10.1016/j.geoderma.2016.09.012
Fixen P., Brentrup F., Bruulsema T., Garcia F., Norton R., Zingore S. (2014). Nutrient/fertilizer use efficiency: measurement, current situation and trends.
Fomba M., Coulibaly A. A., Konko Y., Fofana B., Coulibaly A. A., Diallo Y. (2020). Agronomic efficiency of deep urea placement technology in lowland rice cultivation in the ecological conditions of the sikasso region in Mali: case of the village of Dalabani. OALib 07, 1–23. doi: 10.4236/oalib.1106513
Fosu-Mensah B. Y., Vlek P. L. G., Manske G., Mensah M. (2015). The influenec of azolla pinnata on floodwater chemistry, grain yield and nitrogen uptake of rice in Dano, southwestern Burkina Faso. J. Agric. Sci. 7. doi: 10.5539/jas.v7n8p118
Grierson P. F., Adams M. A. (2000). Plant species affect acid phosphatase, ergosterol and microbial P in a Jarrah (Eucalyptus marginata Donn ex Sm.) forest in south-western Australia. Soil Biol. Biochem. 32, 1817–1827. doi: 10.1016/S0038-0717(00)00155-3
Gu Y., Wang P., Kong C. H. (2009). Urease, invertase, dehydrogenase and polyphenoloxidase activities in paddy soil influenced by allelopathic rice variety. Eur. J. Soil Biol. 45, 436–441. doi: 10.1016/j.ejsobi.2009.06.003
Gummert M., Van Hung N., Chivenge P., Douthwaite B. (2020). Sustainable Rice Straw Management. Eds. Gummert B.D.M., Van Hung N., Chivenge P. (Springer Cham). doi: 10.1007/978-3-030-32373-8
Hengl T., Heuvelink G. B. M., Kempen B., Leenaars J. G. B., Walsh M. G., Shepherd K. D., et al. (2015). Mapping soil properties of Africa at 250 m resolution: Random forests significantly improve current predictions. PloS One 10. doi: 10.1371/journal.pone.0125814
Hogan E. J., Minnullina G., Smith R. I., Crittenden P. D. (2010). Effects of nitrogen enrichment on phosphatase activity and nitrogen : phosphorus relationships in Cladonia portentosa 911–925. doi: 10.1111/j.1469-8137.2010.03222.x
Jat S. L., Parihar C. M., Dey A., Nayak H. S., Ghosh A., Parihar N., et al. (2019). Dynamics and temperature sensitivity of soil organic carbon mineralization under medium-term conservation agriculture as affected by residue and nitrogen management options. Soil Tillage Res. 190, 175–185. doi: 10.1016/j.still.2019.02.005
Jiang B., Shen J., Sun M., Hu Y., Jiang W., Wang J., et al. (2021). Soil phosphorus availability and rice phosphorus uptake in paddy fields under various agronomic practices. Pedosphere 31, 103–115. doi: 10.1016/S1002-0160(20)60053-4
Kalayu G. (2019). Phosphate solubilizing microorganisms: Promising approach as biofertilizers. Int. J. Agron. 2019. doi: 10.1155/2019/4917256
Kandeler E., Tscherko D., Spiegel H. (1999). Long-term monitoring of microbial biomass, N mineralisation and enzyme activities of a chernozem under different tillage management. Biol. Fertil. Soils 28, 343–351. doi: 10.1007/s003740050502
Khmelevtsova L. E., Sazykin I. S., Azhogina T. N., Sazykina M. A. (2022). Influence of agricultural practices on bacterial community of cultivated soils. Agric. (Switzerland) 12. doi: 10.3390/agriculture12030371
Kimani S. M., Bimantara P. O., Hattori S., Tawaraya K., Sudo S., Cheng W. (2020). Azolla incorporation and dual cropping influences CH4 and N2O emissions from flooded paddy ecosystems. Soil Sci. Plant Nutr. 66, 152–162. doi: 10.1080/00380768.2019.1705736
Konare H., Yost R. S., Doumbia M., Mccarty G. W., Jarju A., Kablan R. (2010). Loss on ignition: Measuring soil organic carbon in soils of the sahel, west africa. Afr. J. Agric. Res. 5, 3088–3095. doi: 10.5897/AJAR
Körschens M., Albert E., Armbruster M., Barkusky D., Baumecker M., Behle-Schalk L., et al. (2013). Effect of mineral and organic fertilization on crop yield, nitrogen uptake, carbon and nitrogen balances, as well as soil organic carbon content and dynamics: results from 20 European long-term field experiments of the twenty-first century. Arch. Agron. Soil Sci. 59, 1017–1040. doi: 10.1080/03650340.2012.704548
Kumari J. A., Rao P. C., Padmaja G., Madhavi M. (2017). Effect of physico-chemical properties on soil enzyme urease activity in some soils of ranga reddy district of Telangana State, India 6, 11, 1708–1714. doi: 10.20546/ijcmas
Kwesiga J., Grotelüschen K., Senthilkumar K., Neuhoff D., Döring T. F., Becker M. (2020). Rice yield gaps in smallholder systems of the kilombero floodplain in Tanzania. Agronomy 10, 1–14. doi: 10.3390/agronomy10081135
Ladha J. K., Dawe D., Ventura T. S., Singh U., Ventura W., Watanabe I. (2000). Long-term effects of urea and green manure on rice yields and nitrogen balance. Soil Sci. Soc. America J. 64, 1993–2001. doi: 10.2136/sssaj2000.6461993x
Lagos M. L., Maruyama F., Nannipieri P., Mora M. L., Ogram A., Jorquera M. A. (2015). Current overview on the study of bacteria in the rhizosphere by modern molecular techniques: a mini-review. J. Soil Sci. Plant Nutr. 15.
Landon J. R. (1991). Booker Tropical Soil Manual: A handbook for soil survey and agricultural land evaluation in the tropics and subtropics. Ed. Landon J. R. (Routledge Taylor & Francis Croup).
Lemanowicz J. (2018). Dynamics of phosphorus content and the activity of phosphatase in forest soil in the sustained nitrogen compounds emissions zone. Environ. Sci. pollut. Res. 25, 33773–33782. doi: 10.1007/s11356-018-3348-5
Li M., Han X., Li L. J. (2022). Total nitrogen stock in soil profile affected by land use and soil type in three counties of mollisols. Front. Environ. Sci. 10, 1–4. doi: 10.3389/fenvs.2022.945305
Liang Q., Chen H., Gong Y., Yang H., Fan M., Kuzyakov Y. (2014). Effects of 15 years of manure and mineral fertilizers on enzyme activities in particle-size fractions in a North China Plain soil. Eur. J. Soil Biol. 60, 112–119. doi: 10.1016/j.ejsobi.2013.11.009
Lindsay W. L., Norvell W. A. (1978). Development of DTPA soil test for Zn, Fe, Mn and Cu. Soil Sci. Soc Am. J. 42, 421–428.
Liu J., Han C., Zhao Y., Yang J., Cade-Menun B. J., Hu Y., et al. (2020). The chemical nature of soil phosphorus in response to long-term fertilization practices: Implications for sustainable phosphorus management. J. Clean. Product. 272, 123093. doi: 10.1016/j.jclepro.2020.123093
Liu H., Yang G., Ji H., Feng Y., Zhang Y., Chen L., et al. (2021). Nitrogen fertilizer reduction in combination with Azolla cover for reducing ammonia volatilization and improving nitrogen use efficiency of rice. PeerJ 9. doi: 10.7717/peerj.11077
Luo X., Fu X., Yang Y., Cai P., Peng S., Chen W., et al. (2016). Microbial communities play important roles in modulating paddy soil fertility. Sci. Rep. 6, 1–12. doi: 10.1038/srep20326
Mandana T., Akif G., Azin N. Z. (2014). Effect of nitrogen on rice yield, yield components and quality parameters. Afr. J. Biotechnol. 13, 91–105. doi: 10.5897/AJB
Margalef O., Sardans J., Maspons J., Molowny-Horas R., Fernández-Martínez M., Janssens I. A., et al. (2021). The effect of global change on soil phosphatase activity. Global Change Biol. 27, 5989–6003. doi: 10.1111/gcb.15832
Marklein A. R., Houlton B. Z. (2011). Nitrogen inputs accelerate phosphorus cycling rates across a wide variety of terrestrial ecosystems. doi: 10.1111/j.1469-8137.2011.03967.x
Marzouk S. H., Tindwa H. J., Massawe B. H. J., Amuri N. A., Semoka J. M. (2023). Pedological characterization and soil fertility assessment of the selected rice irrigation schemes, Tanzania 1–14. doi: 10.3389/fsoil.2023.1171849
Mattigod S. V., Zachara J. M. (1996). “METHODS OF SOIL ANALYSIS PART 3 Chemical Methods,” in Methods of Soil Analysis. Eds. Cheng H. H., Westerman R. L.
Mboyerwa P. A., Kibret K., Mtakwa P., Aschalew A. (2022). Rice yield and nitrogen use efficiency with system of rice intensification and conventional management practices in Mkindo irrigation scheme, Tanzania. Front. Sustain. Food Syst. 6. doi: 10.3389/fsufs.2022.802267
Mi W., Wu L., Brookes P. C., Liu Y., Zhang X., Yang X. (2016). Changes in soil organic carbon fractions under integrated management systems in a low-productivity paddy soil given different organic amendments and chemical fertilizers. Soil Tillage Res. 163, 64–70. doi: 10.1016/j.still.2016.05.009
Michael P. S., Sanga H. G., Shitindi M. J., Herzog M., Meliyo J. L., Massawe B. H. J. (2023). Uncovering spatiotemporal pattern of floods with Sentinel-1 synthetic aperture radar in major rice-growing river basins of Tanzania. Front. Earth Sci. 11. doi: 10.3389/feart.2023.1183834
Mohamed I., Bassouny M. A., Abbas M. H. H., Ming Z., Cougui C., Fahad S., et al. (2021). Rice straw application with different water regimes stimulate enzymes activity and improve aggregates and their organic carbon contents in a paddy soil. Chemosphere 274, 129971. doi: 10.1016/j.chemosphere.2021.129971
Nannipieri P., Giagnoni L., Renella G., Puglisi E., Ceccanti B., Masciandaro G., et al. (2012). Soil enzymology: Classical and molecular approaches. Biol. Fertil. Soils 48, 743–762. doi: 10.1007/s00374-012-0723-0
Nekir B. (2019). Effect of organic matter on rice nitrogen and phosphorus use efficiency under calcareous sodic soil of amibara district, Ethiopia. J. Agric. Crops 5, 178–185. doi: 10.32861/jac.14
Nelson D., Sommers L. (1996). “Chemical Methods Soil Science Society of America Book Series. In Soil Science Society of America,” in Methods of Soil Analysis. Part 3. Chemical Methods. Ed. Bigham J. M. (SSSA, Madison).
Okalebo J. R., Gathua K. W., Paul L. W. (2002). Laboratory Methods of Soil and Plant Analysis: A Working Manual. 2nd ed. (SACRED Africa, Kenya Any), 1–131.
Oyange W.A., Chemining’wa G. N., Kanya J. I., Nthakanio P. N. (2020). Effect of time of Azolla incorporation and inorganic fertilizer application on growth and yield of Basmati rice. Afr. J. Agric. Res. 15, 464–472. doi: 10.5897/AJAR
Rajï V. (2020). Impact of nitrogen and phosphorus on grain yield in winter triticale grown on degraded vertisol 1–15. doi: 10.3390/agronomy10060757
Reddy K. S., Singh M., Tripathi A. K., Swarup A., Dwivedi A. K. (2001). Changes in organic and inorganic sulfur fractions and S mineralisation in a Typic Haplustert after long-term cropping with different fertiliser and organic manure inputs. Aust. J. Soil Res. 39, 737–748. doi: 10.1071/SR00020
Rivaie A. A., Isnaini S., Maryati (2013). Changes in soil N, P, K, rice growth and yield following the application of Azolla pinnata. J. Biol. Agric. Healthc. 3, 112–117.
Rochette P., Bertrand N. (2007). Soil-surface gas emissions. 2nd ed. Ed. Carter M. R. (Taylor and Francis group LLC). doi: 10.1201/9781420005271.ch65
Rupngam T., Messiga A. J., Karam A. (2023). Solubility of soil phosphorus in extended waterlogged conditions: An incubation study. Heliyon 9. doi: 10.1016/j.heliyon.2023.e13502
Saha S., Prakash V., Kundu S., Kumar N., Mina B. L. (2008). Soil enzymatic activity as affected by long term application of farm yard manure and mineral fertilizer under a rainfed soybean-wheat system in N-W Himalaya. Eur. J. Soil Biol. 44, 309–315. doi: 10.1016/j.ejsobi.2008.02.004
Seleiman M. F., Elshayb O. M., Nada A. M., El-Leithy S. A., Baz L., Alhammad B. A., et al. (2022). Azolla compost as an approach for enhancing growth, productivity and nutrient uptake of Oryza sativa L. Agronomy 12, (2). doi: 10.3390/agronomy12020416
Sharma S., Singh P., Choudhary O. P. (2021). Nitrogen and rice straw incorporation impact nitrogen use efficiency, soil nitrogen pools and enzyme activity in rice-wheat system in north-western India. Field Crops Res. 266, 108131. doi: 10.1016/j.fcr.2021.108131
Singh V., Gupta R. K., Kalia A., Al-Ansari N., Alataway A., Dewidar A. Z., et al. (2023). Soil type and integrated nitrogen nutrient-rice straw residue management techniques affect soil microbes, enzyme activities and yield of wheat crop. Heliyon 9, e16645. doi: 10.1016/j.heliyon.2023.e16645
Sırt Çıplak E., Akoğlu K. G. (2020). Enzymatic activity as a measure of total microbial activity on historical stone. Heritage 3, 671–681. doi: 10.3390/heritage3030038
Syers J. K., Johston A. E., Curtin D. (2008). Efficiency of soil and fertilizer phosphorus use (Communication Division).
Tang X., Zhang C., Yu Y., Shen J., van der Werf W., Zhang F. (2021). Intercropping legumes and cereals increases phosphorus use efficiency; a meta-analysis. Plant Soil 460, 89–104. doi: 10.1007/s11104-020-04768-x
Tariq M. R., Shaheen F., Mustafa S., Sajid A. L. I., Fatima A., Shafiq M., et al. (2022). Phosphate solubilizing microorganisms isolated from medicinal plants improve growth of mint. PeerJ 10, 1–19. doi: 10.7717/peerj.13782
Tian W., Wang L., Li Y., Zhuang K., Li G., Zhang J., et al. (2015b). Responses of microbial activity, abundance, and community in wheat soil after three years of heavy fertilization with manure-based compost and inorganic nitrogen. Agricult. Ecosyst. Environ. 213, 219–227. doi: 10.1016/j.agee.2015.08.009
Tian K., Zhao Y., Xu X., Hai N., Huang B., Deng W. (2015a). Effects of long-term fertilization and residue management on soil organic carbon changes in paddy soils of China: A meta-analysis. Agricult. Ecosyst. Environ. 204, 40–50. doi: 10.1016/j.agee.2015.02.008
Tong R., Wu T., Jiang B., Wang Z., Xie B., Zhou B. (2023). Soil carbon, nitrogen, and phosphorus stoichiometry and its influencing factors in Chinese fir plantations across subtropical China. Front. Forests Global Change 5. doi: 10.3389/ffgc.2022.1086328
van de Wiel C. C. M., van der Linden C. G., Scholten O. E. (2016). Improving phosphorus use efficiency in agriculture: opportunities for breeding. Euphytica 207, 1–22. doi: 10.1007/s10681-015-1572-3
Van Hung N., Maguyon-Detras M. C., Migo M. V., Quilloy R., Balingbing C., Chivenge P., et al. (2020). “Rice Straw Overview: Availability, Properties, and Management Practices,” in Sustainable Rice Straw Management (Springer International Publishing), 1–13. doi: 10.1007/978-3-030-32373-8_1
Verzeaux J., Hirel B., Dubois F., Lea P. J., Tétu T. (2017). Agricultural practices to improve nitrogen use efficiency through the use of arbuscular mycorrhizae: Basic and agronomic aspects. Plant Sci. 264, 48–56. doi: 10.1016/j.plantsci.2017.08.004
Walkley A., Black I. A. (1934). An examination of the degtjareff method for determining soil organic matter, and a proposed modification of the chromic acid titration method. Soil Science 37, 29–38). doi: 10.1097/00010694-193401000-00003
Wan H., Jian-fu W. U., Xiao-hua P. A. N., Xue-ming T. A. N., Yong-jun Z., Qing-hua S. H. I., et al. (2016). Effects of long-term straw return on soil organic carbon fractions and enzyme activities in a double-cropped rice paddy in South China. J. Integr. Agric. 20, 236–247. doi: 10.1016/S2095-3119(20)63347-0
Watanabe F. S., Olsen S. R. (1965). Test of an ascorbic acid method for determining phosphorus in water and NaHCO 3 extracts from soil 1 A need exists for a more suitable method to determine. Soil Sci. Soc Proc., 677–678.
Watanabe I., Berja N. S. (1983). The growth of four species of azolla as affected by temperature. Aquat. Bot. 15. doi: 10.1016/0304-3770(83)90027-X
Watanabe I., Padre B., Ramirez C., Watanabe I. (1991). Mineralization of azolla n and its availability to wetland rice: I. Nitrogen Mineralization of Different Azolla Species as Affected by Their Chemical Composition. Soil Sci. Plant Nutr. 37, 679–688. doi: 10.1080/00380768.1991.10416936
Weintraub S. R., Wieder W. R., Cleveland C. C., Townsend A. R. (2013). Organic matter inputs shift soil enzyme activity and allocation patterns in a wet tropical forest. Biogeochemistry 114 (1–3), 313–326. doi: 10.1007/s10533-012-9812-2
Yadav S., Kumar R., Chandra M. S., Singh S., Yadav R. B., Kumar M. (2020). Soil organic carbon sequestration and carbon pools in rice based cropping systems in indo-gangetic plains: an overview. Int. Res. J. Pure Appl. Chem., 122–136. doi: 10.9734/irjpac/2020/v21i2430341
Zhang P., Wei T., Li Y., Wang K., Jia Z., Han Q., et al. (2015). Effects of straw incorporation on the stratification of the soil organic C, total N and C:N ratio in a semiarid region of China. Soil Tillage Res. 153, 28–35. doi: 10.1016/j.still.2015.04.008
Zhang W. P., Fornara D., Liu G. C., Peñuelas J., Sardans J., Sun J. H., et al. (2022a). Interspecific interactions affect N and P uptake rather than N:P ratios of plant species: Evidence from intercropping. J. Plant Ecol. 15, 223–236. doi: 10.1093/jpe/rtab084
Zhang Y., Ye C., Su Y., Peng W., Lu R., Liu Y., et al. (2022b). Soil Acidification caused by excessive application of nitrogen fertilizer aggravates soil-borne diseases: Evidence from literature review and field trials. Agricult. Ecosyst. Environ. 340, 108176. doi: 10.1016/j.agee.2022.108176
Zhang X., Zhang R., Gao J., Wang X., Fan F., Ma X., et al. (2017). Thirty-one years of rice-rice-green manure rotations shape the rhizosphere microbial community and enrich beneficial bacteria. Soil Biol. Biochem. 104, 208–217. doi: 10.1016/j.soilbio.2016.10.023
Zheng M., Huang J., Chen H., Wang H., Mo J. (2015). European Journal of Soil Biology Responses of soil acid phosphatase and beta-glucosidase to nitrogen and phosphorus addition in two subtropical forests in southern China. Eur. J. Soil Biol. 68, 77–84. doi: 10.1016/j.ejsobi.2015.03.010
Keywords: biofertilizers, crop nutrient recovery efficiency, improved food systems, nutrient omission, smallholder farming systems, sustainable environment
Citation: Marzouk SH, Tindwa HJ, Amuri NA, Chande HH and Semoka JM (2024) Enhancing phosphorus use efficiency and soil quality indicators in lowland paddy ecosystem through Azolla, rice straw, and NPKS fertilizers. Front. Agron. 6:1376110. doi: 10.3389/fagro.2024.1376110
Received: 24 January 2024; Accepted: 11 March 2024;
Published: 16 April 2024.
Edited by:
Hanuman Singh Jatav, Sri Karan Narendra Agriculture University, IndiaReviewed by:
Muhammad Madnee, Islamia University of Bahawalpur, PakistanCopyright © 2024 Marzouk, Tindwa, Amuri, Chande and Semoka. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Said H. Marzouk, YmlubWFyem91a0BnbWFpbC5jb20=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.