- 1AgriServices Program, Syngenta Foundation for Sustainable Agriculture, Basel, Switzerland
- 2Sustainable Agroecosystem Group, Department of Environmental Systems Science, ETH Zürich, Zürich, Switzerland
- 3Department of Agricultural Research Services, Tanzania Agricultural Research Institute, Mwanza, Tanzania
- 4Regenerative Agriculture, Sustainable Agriculture Initiative Impact Platform, Basel, Switzerland
Introduction: Whitefly (Bemisia tabaci) is a pest of cassava (Manihot esculenta Crantz) and the vector for two of the crop’s major viral diseases – cassava mosaic disease (CMD) and cassava brown streak disease (CBSD), causing severe economic losses for farmers. In this context, we conducted an on-farm experiment to study the efficiency of a seed treatment technology containing thiamethoxam, fludioxonil, and metalaxyl for rapid multiplication of superior cassava genotypes and early protection of the crop against whitefly at Salima and Nkhotakota, central Malawi in the 2019/2020 and 2020/2021 cropping seasons, respectively.
Methods: The trials were conducted using a randomized complete block design with four replicates. The effect of the application of the seed treatment on stake germination, whitefly population, CMD and CBSD incidences, and stem and storage root yields of three cassava varieties (Kalawe, Mbundumali, and Sauti) at three stake sizes (8 cm, 16 cm, and 25 cm) was compared with control – a 25 cm stake size of each of the cassava variety without seed treatment. A benefit-cost analysis was conducted to determine the profitability of the seed treatment technology for each stake size under certified and non-certified stem scenarios.
Results and discussion: Regardless of stake size and variety, plant germination was highest (96% by 16%) and mean whitefly population lowest (adult 0.4 vs. 3.0 plant-1; nymph 1.0 vs. 3.3 plant-1) with seed treatment application than without. Disease incidence measurements showed no significant effect of seed treatment on CBSD control (p = 0.31), but it reduced CMD incidence by 17% vs. 20% in the untreated. Stem and storage root yields across stake size and cassava variety were highest with seed treatment; 489 bundles ha-1 and 10 (DM) Mg ha-1, respectively. Using the 8 cm stake regardless of variety resulted in the highest average benefit-cost ratio for certified (18.3 USD USD-1) and non-certified (7.8 USD USD-1) cassava stem scenarios.
Conclusion: We conclude that the application of the seed treatment tested in this study would offer protection to planted stakes, increase their germination, reduce whitefly population, increase stem and storage root yields in areas experiencing whitefly pressure, and result in high economic profits.
1 Introduction
Approximately 700 million people in sub-Saharan Africa depend on cassava (Manihot esculenta Crantz) (Szyniszewska, 2020). Cassava is cultivated in more than 40 countries in Africa (FAO and IFAD, 2020) including Nigeria, the Democratic Republic of Congo, Tanzania, Malawi, and Uganda (FAOSTAT, 2019). In Malawi, cassava is the second most important staple crop after maize, providing approximately 161 kcal person−1 day−1 (representing 7% of total daily caloric intake) and it is the main staple crop in the lake shore districts (FAO, 2023). Annual cassava production in Malawi is 5.6 million tons, which accounts for 3% of Africa’s total production, and 1.8% of global production (Adebayo, 2023). Among its important adaptive characteristics that endear the crop to Malawian farmers is its ability to withstand erratic rainfall and drought (Weigand, 2018). Other important characteristics of cassava include tolerance to nutrient-limited soils (Howeler, 2002), compatibility at intercropping with short-duration crops (Nwokoro et al., 2021), and its tolerance to several economic pests and diseases (NACGRAB, 2005).
Cassava is clonally propagated; thus, its stems are a potential source of revenue for farmers and other actors in the dominant informal seed sector, and, for seed production entrepreneurs in the rapidly emerging formal seed sector (Kilwinger et al., 2021), yet it facilitates the spread of diseases, caused by bacteria, fungi, and viruses that cause two of the crop’s most devastating diseases: cassava mosaic disease (CMD) and cassava brown streak disease (CBSD) (Legg et al., 2014). Whitefly (Bemisia tabaci) is the vector for CMD caused by cassava mosaic geminiviruses (belonging to the genus Begomovirus, family Geminiviridae) and CBSD caused by cassava brown streak Ipomoviruses (belonging to the genus Ipomovirus, family Potyviridae) (Legg et al., 2015; Winter et al., 2010). In East Africa, CMD and CBSD are known to be the major causes of seed degeneration and yield losses (Alonso Chavez et al., 2021).
The availability of pest and disease-free planting materials is a prerequisite for improving cassava productivity (FAO, 2010). However, the focus of insecticide and varietal improvement research is mostly aimed at other crops with a bigger commercial relevance to the input sector than cassava, such as grains, cotton, and ornamental crops (Slakie et al., 2013). Despite over a decade of breeding investments for CMD- and CBSD-resistant varieties, an end to yield losses from whiteflies and the diseases they transmit is yet to be achieved (Rey and Vanderschuren, 2017). Yield losses among small-scale farmers resulting from CBSD in Malawi were conservatively estimated at 18%–25% (Gondwe et al., 2003). This makes imperative the need for an affordable yet complementary crop protection technology for cassava producers, especially where the impact of whiteflies is prevalent. Studies conducted in Brazil and Uganda have shown positive impacts from treating planting materials with formulations of protective systemic compounds before planting (de Oliveira et al., 2020; Omongo et al., 2022). Results from both studies show that protective compounds, such as fungicides or insecticides, or a combination of both, prevented damage by pests and diseases, resulting in better crop performance: plant germination, vigor, development, and yields regardless of stake sizes (de Oliveira et al., 2020; Omongo et al., 2022). This presents an opportunity for further studies on the potential judicious use of systemic insecticides as seed treatments for the control of whitefly populations and, by extension, the diseases they transmit in Malawi.
To understand the potential of treating cassava planting materials with a formulation of Cruiser (thiamethoxam, a systemic insecticide) and Maxim XL (fungicides: fludioxonil and mefenoxam) before planting in vector and disease control, we evaluated its efficiency on three cassava varieties (Kalawe, Mbundumali, and Sauti) using three different stake sizes of each in two farming regions in Malawi—Nkhotakota and Salima. The specific objectives were to study the effects of the seed treatment application on (i) stake germination, termite damages on planted stakes, whitefly population, and CMD and CBSD incidences; (ii) stem and storage root yields of the cassava varieties under three stake sizes (8 cm, 16 cm, and 25 cm); and (iii) economic benefit of the seed treatment over certified and non-certified cassava stems. We hypothesized that application of the seed treatment will (i) protect planted stakes against termite attack and improve stake germination, (ii) offer protection to cassava against whiteflies thereby reducing CMD and CBSD incidences, and (iii) improve stem and storage root yields yet profitable. Our aspiration is that this work will guide future initiatives to increase the efficiency of the delivery of clean planting material and improved, pest and disease-tolerant cassava varieties to smallholder farmers across sub-Saharan Africa, increasing the resilience and productivity of farming systems and supporting food security across the sub-continent.
2 Materials and methods
2.1 Experimental site
The trials were conducted on-farm at Salima in the 2019/2020 cropping season (first season) and repeated in the 2020/2021 cropping season (second season) at Nkhotakota. Both Salima and Nkhotakota are districts in the central region of Malawi (Figure 1). Salima is located 13°78′ S, 34°45′ E, at an elevation of 538 masl, approximately 98 km east of Lilongwe. It has an annual mean temperature of 24°C and 1,266 mm of rainfall. Rainfall in Salima is bimodal (Figure 2) with most rain falling in the months between December and March. Nkhotakota, on the other hand, is located 12°93′ S, 34°28′ E, at an elevation of 472 masl, approximately 200 km northeast of Lilongwe. It has an annual mean temperature and rainfall of 23°C and 1,650 mm, respectively. Rainfall is bimodal in Nkhotakota (Figure 2) with the main rainy season spanning between December and April. The field trials in both seasons were planted in the second week of January when rainfall had stabilized and harvested after 11 months in the second week of December. The host farmers are potential cassava seed entrepreneurs identified for field evaluation of the MandiPlus seed treatment and subsequent scale-up in the country. Both farmers had been farming cassava for decades and were motivated by the potential of the MandiPlus seed treatment in achieving superior cassava seed pieces and improving cassava resilience and productivity.
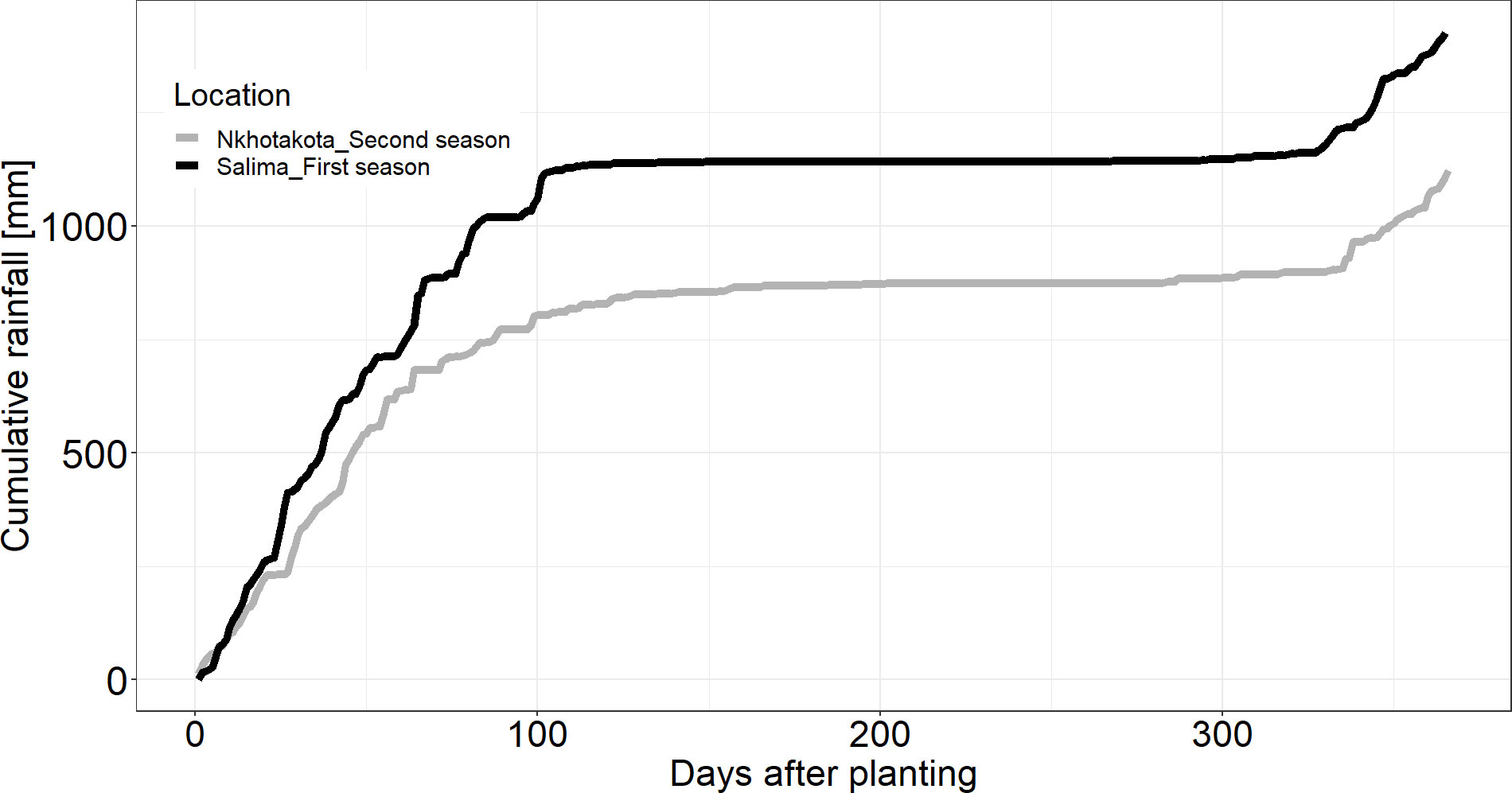
Figure 2 Cumulative rainfall at Nkhotakota and Salima from planting until crop harvest in the first and second seasons. Source: (CHIRPS, 2023).
2.2 Experimental design and treatment
A randomized complete block design with four replicates was used at both sites. We compared the effects of two seed treatments—a protective chemical solution versus control without chemical protection, on three stake sizes (8 cm, 16 cm, and 25 cm) of three cassava varieties (MBD: Mbundumali; KLW: Kalawe; and SUT: Sauti). The stake protective chemical solution called MandiPlus in this study comprised a slurry of vinyl white paint, an aqueous solution of Cruiser (active ingredient thiamethoxam at 350 g L−1), an insecticide [3-(2-Chloro-thiazol-5-ylmethyl(1,3,5) oxadiazinane-4-ylidene-N-nitroamine)], and Maxim XL (active ingredients metalaxyl-M at 10 g L−1 and fludioxonil at 25 g L−1), a fungicide. The protective chemical solution was compared to a control (CT) with neither a chemical solution nor a paint slurry applied. A comprehensive factor combination and the resulting number of treatments implemented in each field are shown in Table 1. The dimensions of the experimental plot were 5 m × 7 m (35 m2). Treatment plots and blocks were each separated by 2 m and 3 m pathways, respectively.
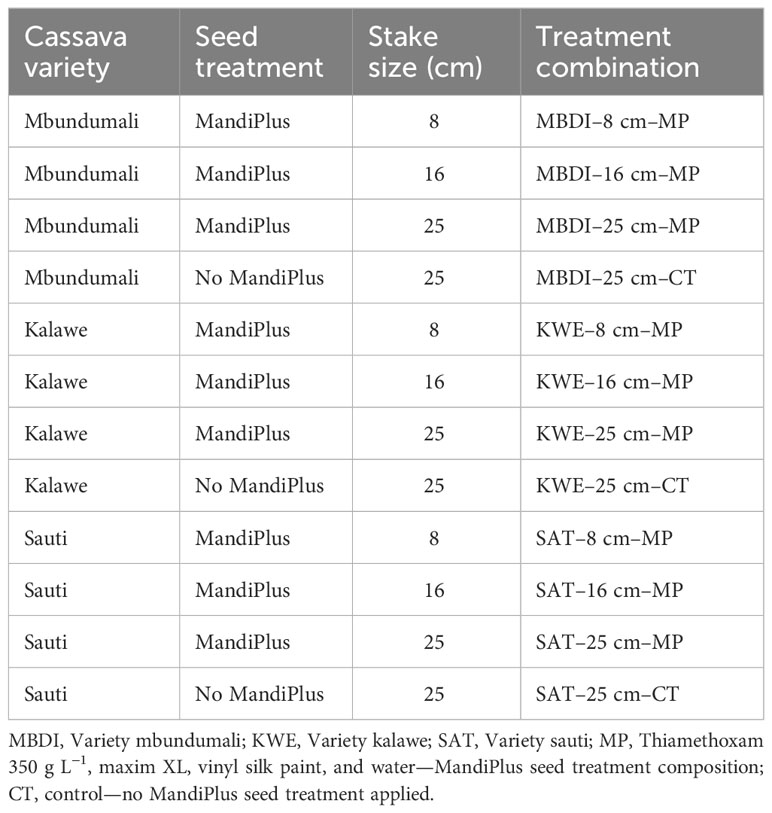
Table 1 Combination of the levels of studied factors in the MandiPlus field trials at Salima and Nkhotakota in Malawi.
2.3 MandiPlus seed treatment formulation and application
As per recommendations by Conceicão et al. (2023) and de Oliveira et al. (2020), the dosage of the agrochemicals used in the formulation of MandiPlus seed treatment was fixed. However, the stakes’ absorption volume for different sizes was pre-determined before mixture formulation and stake treatments. The formulation procedure involved the dipping of pieces of the desired stake length in water for 3 min to determine absorption volume over the period. Thereafter, the volume of absorption was calculated for the number of stakes required to plant a specified area of land, depending on the desired planting population density, i.e., the number of stakes per area × absorption per stake. Thus, the total volume of product required (TVPR) and the desired slurry was determined as follows:
Based on the above calculation procedure, the absorbed volume estimate per cutting was established. To increase seed treatment adhesion to stake cut surfaces, a binding agent (2% latex) was used. It is a recommendation that all the agrochemicals be applied with a water-based slurry. This seeks to provide uniform coverage of the stake, particularly the cut surfaces. The slurry comprised in sequential order: (i) water, (ii) vinyl white paint—a binding agent, (iii) fungicides, and (iv) insecticide. To avoid hydrolysis of some chemicals, the pH value was adjusted to between 6.5 and 7.0. After the preparation of the MandiPlus solution, the stakes were packed in a netted polypropylene bag and dipped into the solution for approximately 3 min. Immediately after this, the dipped cuttings were removed and placed on a plain surface to allow excess chemical drip off at room temperature for approximately 8 h before planting.
2.4 Site management, stake preparation, and planting
Both sites were disc-plowed and ridged by a tractor. The ridges were made 1 m apart, perpendicular to the fields’ gentle slopes. The plots were arranged such that they faced the direction of the ridges. Vigorous and healthy-looking stems of each cassava variety, showing no signs of disease infection or visible pest attack, between 12 and 15 months old, were obtained from the Department of Agricultural Research Services (DARS), Chitedze research center in Malawi and used for planting. The stems were cut into stake sizes of 8 cm, 16 cm, and 25 cm using sharp bush machetes. Farmers in Malawi use 25-cm stakes for planting. This informed the decision to use 25-cm stakes, without MandiPlus application, as a control treatment in this study. After the stems were cut into the required stake sizes, they were packed in netted polypropylene bags and dipped into a basin containing a slurry of MandiPlus seed treatment for 3 to 5 min. Treating the stakes with the slurry followed the treatment design (Tables 1, 2), whereby stakes used in the control plots were left untreated with the seed treatment slurry. Post-treatment, the stakes were placed on manila bags or coconut fronds under shades to air-dry for at least 24 h before planting. The stakes were planted on the ridge crests, in a slanting position, approximately at 45°C by inserting about ¾ of its length into the soil at a spacing of 1 m × 1 m for a plant population density of 10,000 plants ha−1. The fields were kept weed-free by manual weeding to prevent weed interference with crops.
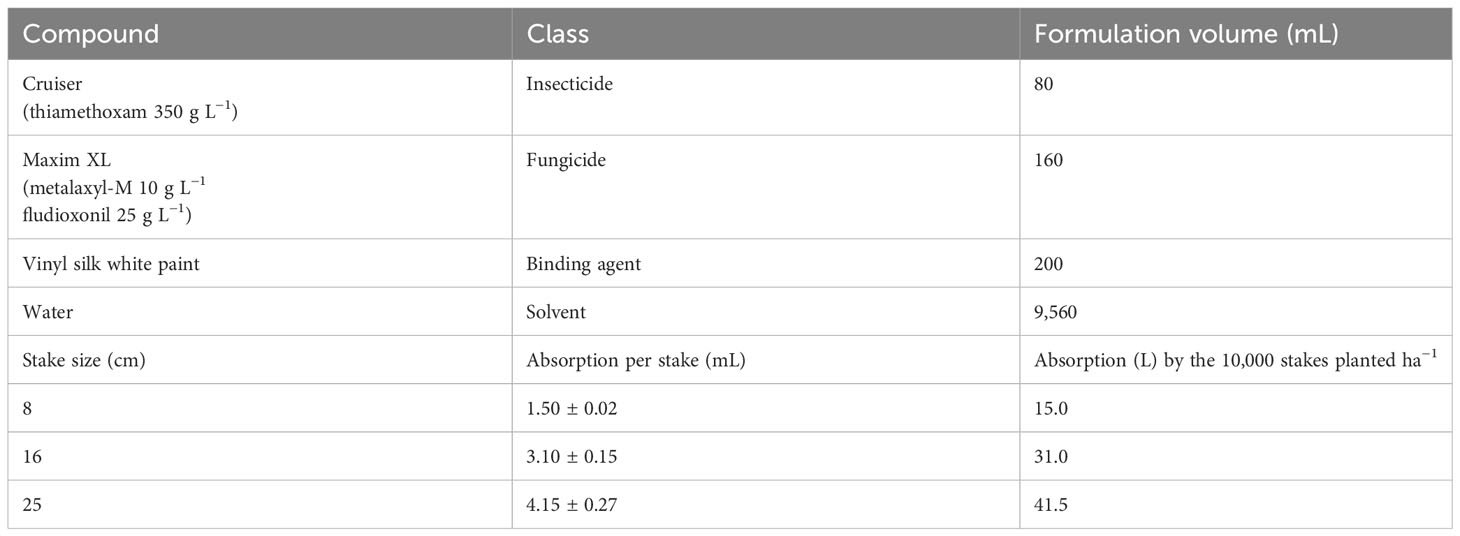
Table 2 Chemical compounds and volume used for the seed treatment formulation and the absorption by the different stake sizes used in the experiment.
2.5 Growth measurement, pest population, and disease incidence
The following agronomic measurements, pest (nymph and adult whitefly count), and disease (CMD and CBSD) incidence were measured on 10 sampled and tagged plants per treatment plot: (i) Germinated stakes and stakes damaged by termite count at 2 months after planting (MAP). This was done by counting and recording the number of sprouted and un-sprouted cassava stakes. To separate termite damage from other causes, only the stakes with termite activities such as mud tubes, frass, shallow, and hollowed-out stakes were counted as termite-damaged. (ii) The number of nymphs and adult whiteflies per plant. These were done at two monthly intervals starting at 2 MAP until the 6th MAP. Counting adult whiteflies was done on the abaxial of the first five fully expanded leaves from the apex of a sampled plant (or on the tallest branched on branched plants). For the nymphs, the counts were taken on the 10th to 12th leaves of the same plants. Usually, whitefly instars are found on older cassava leaves. (iii) CMD and CBSD incidences once at 3 MAP. Foliar incidence of CMD or CBSD was estimated by determining the number of symptomatic plants expressed as a percentage of the total number of sampled plants (Fargette et al., 1985; Mware et al., 2009; Musopole et al., 2023). (iv) Plant height (cm) at 2-month intervals beginning from 2 MAP until the 6th MAP. Plant height was measured from the plant base to the shoot apex, following one apex on branched plants.
2.6 Harvesting and yield assessments
Field trials of both seasons were harvested at 11 MAP. Four central cassava rows in each experimental plot were harvested for the yield assessments. Only the asymptomatic stems and fresh storage roots were used. The cassava stands on border rows were excluded to control border effects. Stems of the cassava stand within the four central rows marked out as net plots were cut at approximately 40 cm from their bases. The harvested stems were each cut into 100-cm stakes and arranged in a group of 50. A bundle of cassava stems comprises 50 stems, each 1 m. The stem yield for each treatment was counted and recorded. Stem yield is expressed in bundles ha−1. Marketable storage roots (≥3 cm top diameter) were harvested from every cassava stand used for stem yield assessment. The storage roots were harvested by pulling each stand and collecting the underground storage roots. The mass of storage root yields per plot is expressed in Mg ha−1.
2.7 Benefit–cost ratio analysis of MandiPlus seed treatment
Benefit–cost ratio (BCR), an economic indicator for cost–benefit analysis, was performed to assess the profitability of the MandiPlus cassava seed treatment relative to control (without MandiPlus application). The BCR was carried out for two categories of cassava seeds based on seed quality segmentation in Malawi, i.e., certified versus non-certified cassava planting stake pieces. In Malawi, the market value for certified cassava stem stakes used for propagation is higher than the non-certified stem stakes. This warranted the assessment of a BCR for the two categories of stem stakes. While the non-certified stems are sold for 0.7 USD per bundle, a bundle of certified stems is sold for 1.7 USD. Information on the prices of the stem categories was obtained from a local seed entrepreneur in Salami. Prices of the protective chemicals used for the formulation of the MandiPlus solution were obtained from a local agro-dealer that operates in the Salima district of central Malawi. A liter of Cruiser sold for 27.0 USD, a liter of Maxim XL sold for 32.0 USD, and a liter of vinyl silk white paint sold for 4.0 USD. The cost of water was insignificant and was therefore not included in the BCR calculations. Because MandiPlus is a cassava seed system technology, only the stem yield was considered in the BCR analysis. The revenue derived from the increase in asymptomatic stem yields in response to the MandiPlus application relative to the control treatment was regarded as the gross benefit. The BCR for each stake size treated with MandiPlus was calculated as the ratio of gross benefit to the total cost of the chemical solution requirement per stake size. The general rule of thumb is that for a technology to be profitable for smallholders, it has to have a BCR value >2.0 USD USD−1 invested, especially in a developing economy such as Malawi. A BCR value between 1.0 and 2.0 USD USD−1 is considered a risky investment, and a value<1.0 USD USD−1 indicates a loss.
2.8 Statistical analysis
All statistical analyses were performed in the Rstudio environment (R Core Team, 2020) using the linear mixed-effects model “lme4”. The repeated measurement operational procedure was only implemented in analyzing whitefly counts (adults and nymphs). In this case, the cassava variety, seed treatment, observation time (MAP), and factor interactions were the fixed factor variables, and the experimental blocks were the random factor variables. When analyzing CMD and CBSD disease incidences, sprouted stakes, stakes damaged by termites, and stem and storage root yields, the repeated measurement factor (MAP) was dropped in the model because they are non-repeated observations. Except for stem and storage root yields, other parameters were analyzed across stake sizes and seasons. After the BCR was computed, it was subjected to a simple analysis of variance test, whereby the BCR was the response variable, and the stem sizes and seed grades (the certified vs. non-certified seed) were the predictive variables. The significance of differences was evaluated at p ≤ 0.05. Data visualization was done in the Rstudio environment using the package “ggplot2()” (Gómez-Rubio, 2017).
3 Results
3.1 Effects of seed treatment and cassava variety on stake sprouting and damages by termites
The percentage of sprouted stakes differed significantly (p< 0.001) between both seasons. No significant effect of termite damage on the planted stakes (p = 0.61) was observed between the two seasons (Table 3). Sprouted stakes were higher in the first (90%) than in the second season (85%). A significant effect of seed treatment was observed on sprouted stakes (p< 0.001) and the stakes damaged by termites (p< 0.001; Figure 3). MandiPlus application increased stake sproutings compared with control. In contrast, termite-damaged stakes were highest in the control treatment in both seasons. Sprouted stakes and the stakes damaged by termites differed significantly between the cassava varieties over both seasons. While variety Mbundumali was the least damaged by termites compared with Sauti and Kalawe in both seasons, it had the highest sprouted stakes (92%) in both seasons. There were significant interaction effects between seed treatment and cassava variety on sprouted and damaged stakes by termites. The application of MandiPlus solution on the stakes of any of the cassava varieties improved sprouting and reduced termite damage in both seasons (Figure 3).
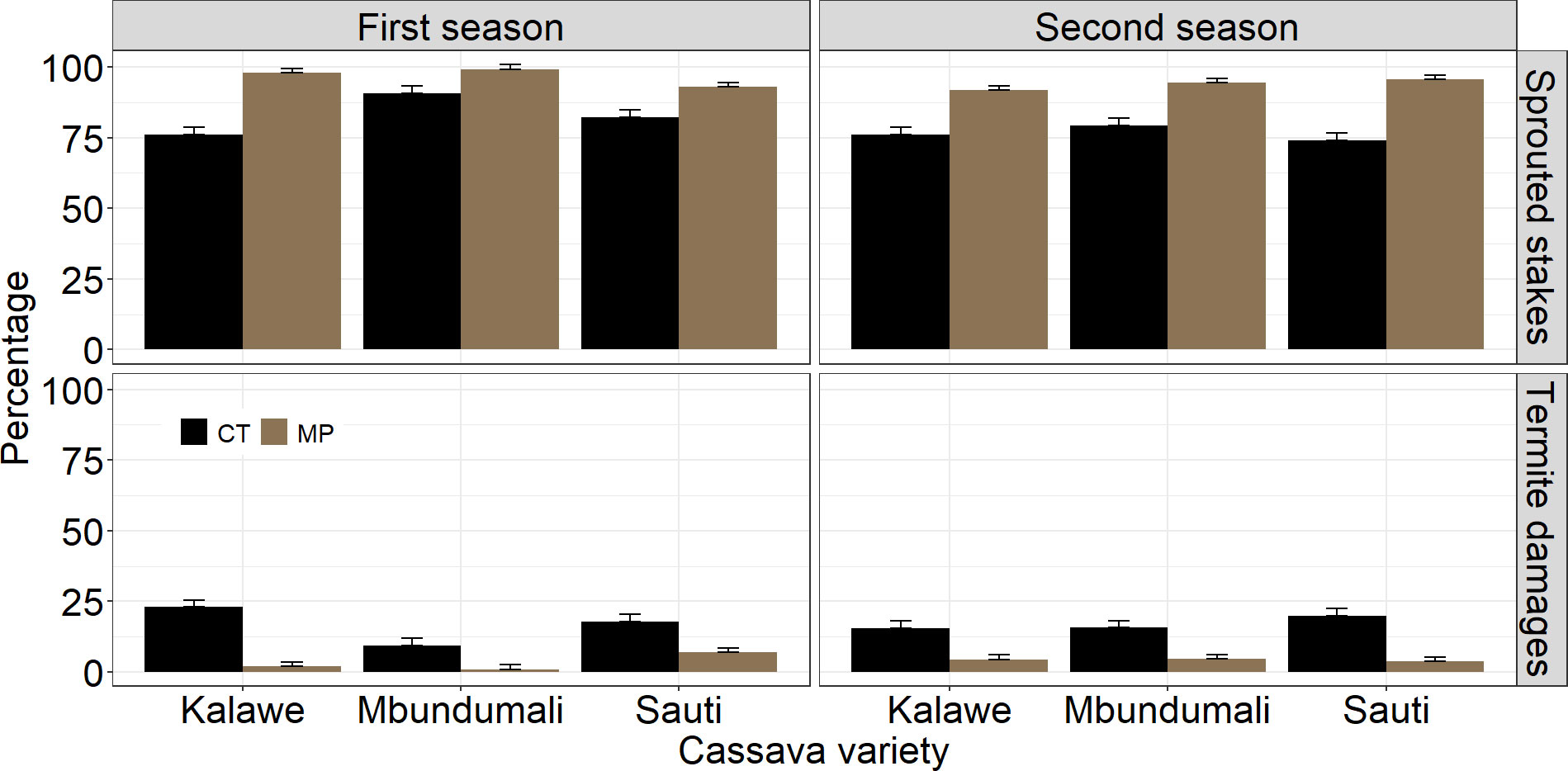
Figure 3 Effects of seed treatment and cassava variety on sprouted stakes and stakes damaged by termites in the first and second seasons of MandiPlus field experiments in Malawi. CT, control without MandiPlus treatment; MP, MandiPlus seed treatment. Error bars represent standard errors of the mean.
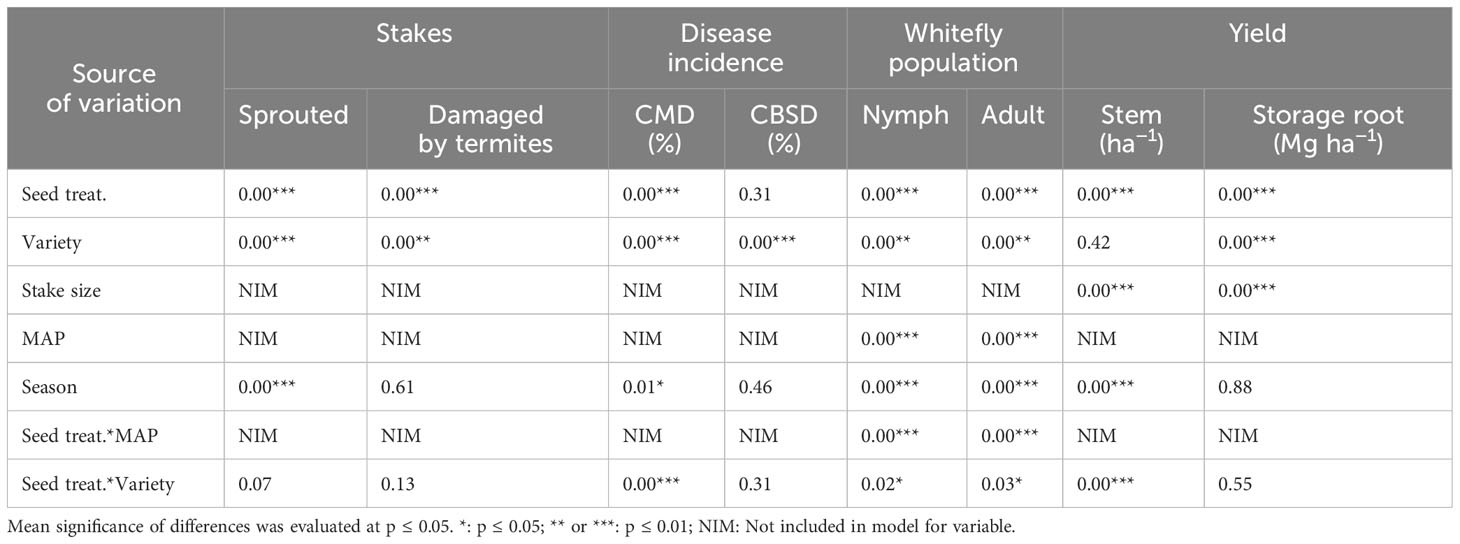
Table 3 Analysis of variance of treatments in MandiPlus application experiment in Malawi over two seasons.
3.2 Effects of seed treatment and cassava variety on whitefly nymph and adult populations
Nymph and adult whitefly populations were significantly different between both seasons (Table 3). The average nymph count per plant was lower in the first than in the second season (Figure 4A). In contrast, the adult whitefly population per plant was highest in the first season. Both the nymph and adult whitefly populations were highest without the MandiPlus application (Figure 4A). A significant effect of cassava variety was observed on the two parameters: nymph and adult whitefly populations were higher on variety Sauti than on either Mbundumali or Kalawe (Figure 4B). Adult and nymph whitefly populations increased on cassava leaves with plant age. Each was highest at 6 MAP of 2.3 per plant (nymphs) and 2.2 per plant (adults). A significant interaction effect was observed between seed treatment and cassava variety on nymph and adult whitefly populations on cassava leaves. Treating the planting stakes of each variety with MandiPlus solution before planting reduced mean nymph and adult whitefly populations on the plants compared with stakes planted without applying MandiPlus solution over both seasons (Figure 4B).
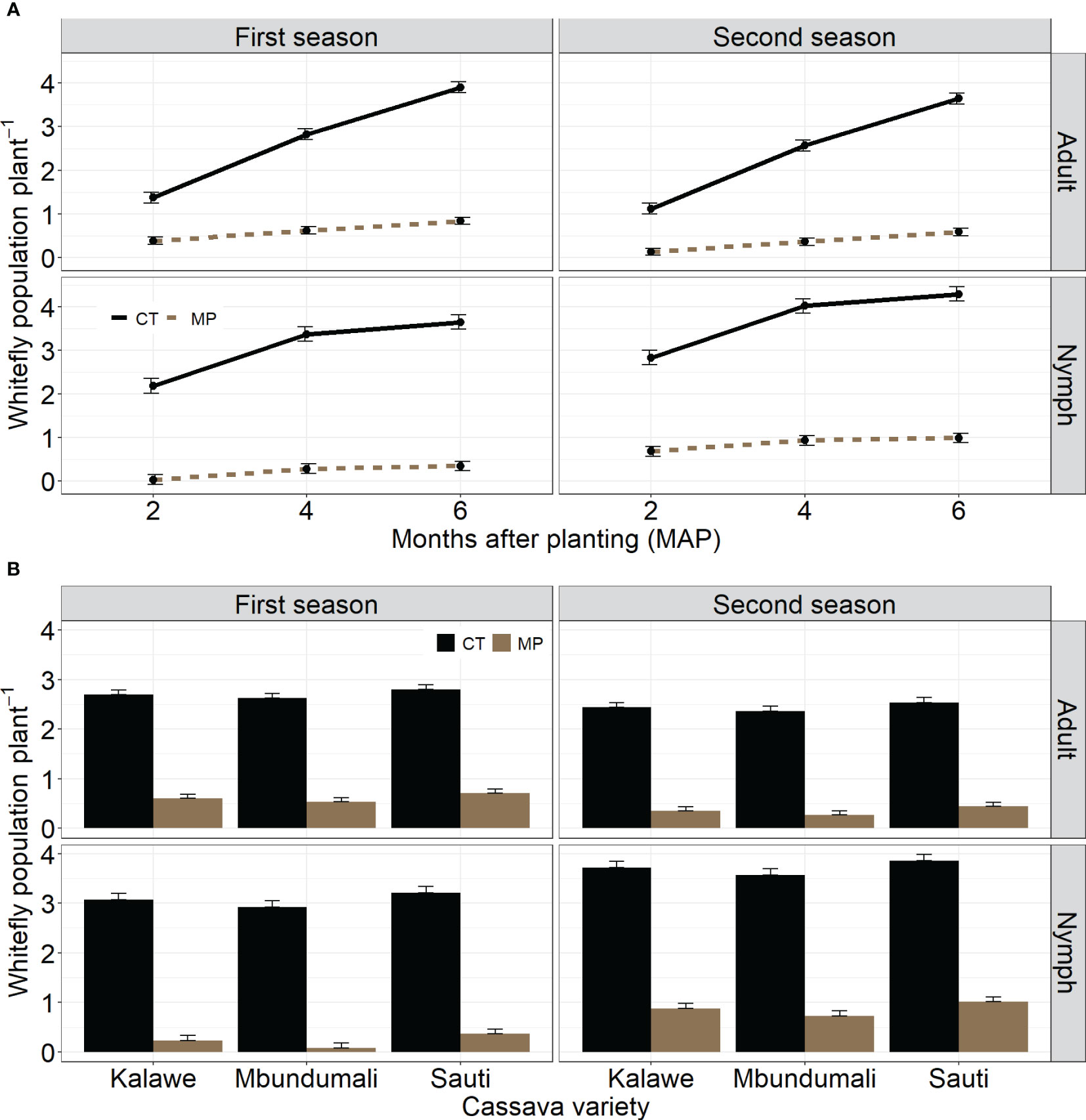
Figure 4 (A). Effects of seed treatment on whitefly nymph and adult populations per plant over time in the first and second seasons of MandiPlus field experimentations in Malawi. CT, control without MandiPlus treatment; MP, MandiPlus seed treatment. Error bars represent standard errors of the mean. (B) Effects of seed treatment and cassava variety on whitefly nymph and adult populations per plant in the first and second seasons of MandiPlus field experimentations in Malawi. CT, control without MandiPlus treatment; MP, MandiPlus seed treatment. Error bars represent standard errors of the mean.
3.3 Effects of seed treatment and cassava variety on CMD and CBSD incidences
The incidence of CMD was significantly different (p< 0.05) between the two seasons; however, CBSD was not (Table 3). Cassava mosaic disease incidence was highest in the first season (Figure 5). The application of the MandiPlus seed treatment solution significantly reduced CMD incidence compared with control in both seasons. No differences in CBSD incidence were observed between the control and MandiPlus treatments in either season. The variety Mbundumali had the highest CMD and CBSD incidences compared with either Kalawe or Sauti over both seasons (Figure 5). No significant interaction effect on CMD incidence was observed between cassava variety and seed treatment over both seasons. However, with or without the application of the MandiPlus formulation, the variety Mbundumali expressed the highest CBSD incidence symptoms compared with either Sauti or Kalawe over both seasons.
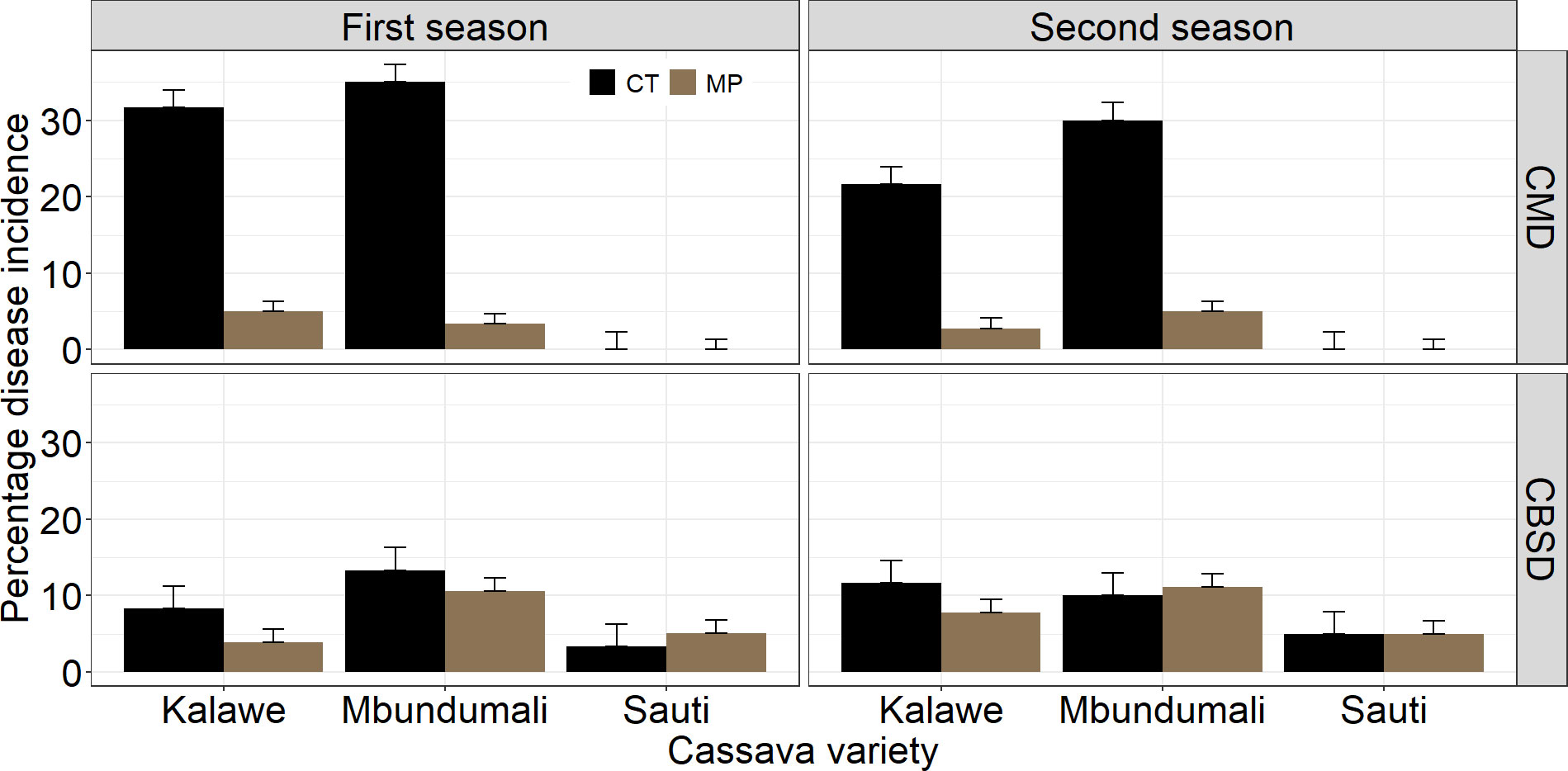
Figure 5 Effects of seed treatment and cassava variety on cassava mosaic disease (CMD) and cassava brown streak disease (CBSD) at 3 MAP in the first and second seasons of MandiPlus field experimentations in Malawi. CT, control without MandiPlus treatment; MP, MandiPlus seed treatment. Error bars represent standard errors of the mean.
3.4 Effects of seed treatment and cassava variety on cassava stem and storage root yields
Only stem yields differed significantly (p< 0.001) between both seasons. No significant differences in storage root yields were observed between the two seasons (p = 0.88; Table 3). Stem yield was higher in the first season (474 bundles ha−1) than in the second (428 bundles ha−1; Figure 6). Seed treatment significantly affected (p< 0.01) stem and storage root yields over both seasons: stem and storage root yields were highest with MandiPlus application in both seasons (Figures 6, 7). There was no significant effect of cassava variety on stem yield. However, storage root yield was higher by 1.2 Mg ha−1 from either Kalawe or Mbundumali to Sauti. The interaction of seed treatment and stake size was significant for stem and storage root yields in both seasons. Across stake sizes, stem yield increased by 152 bundles ha−1 from control (25 cm stake without MandiPlus) to MandiPlus treatment across seasons (Figure 6). Similarly, storage root yield increased by 3.0 Mg ha−1 from control to Mandiplus treatment across stake sizes over both seasons (Figure 7).
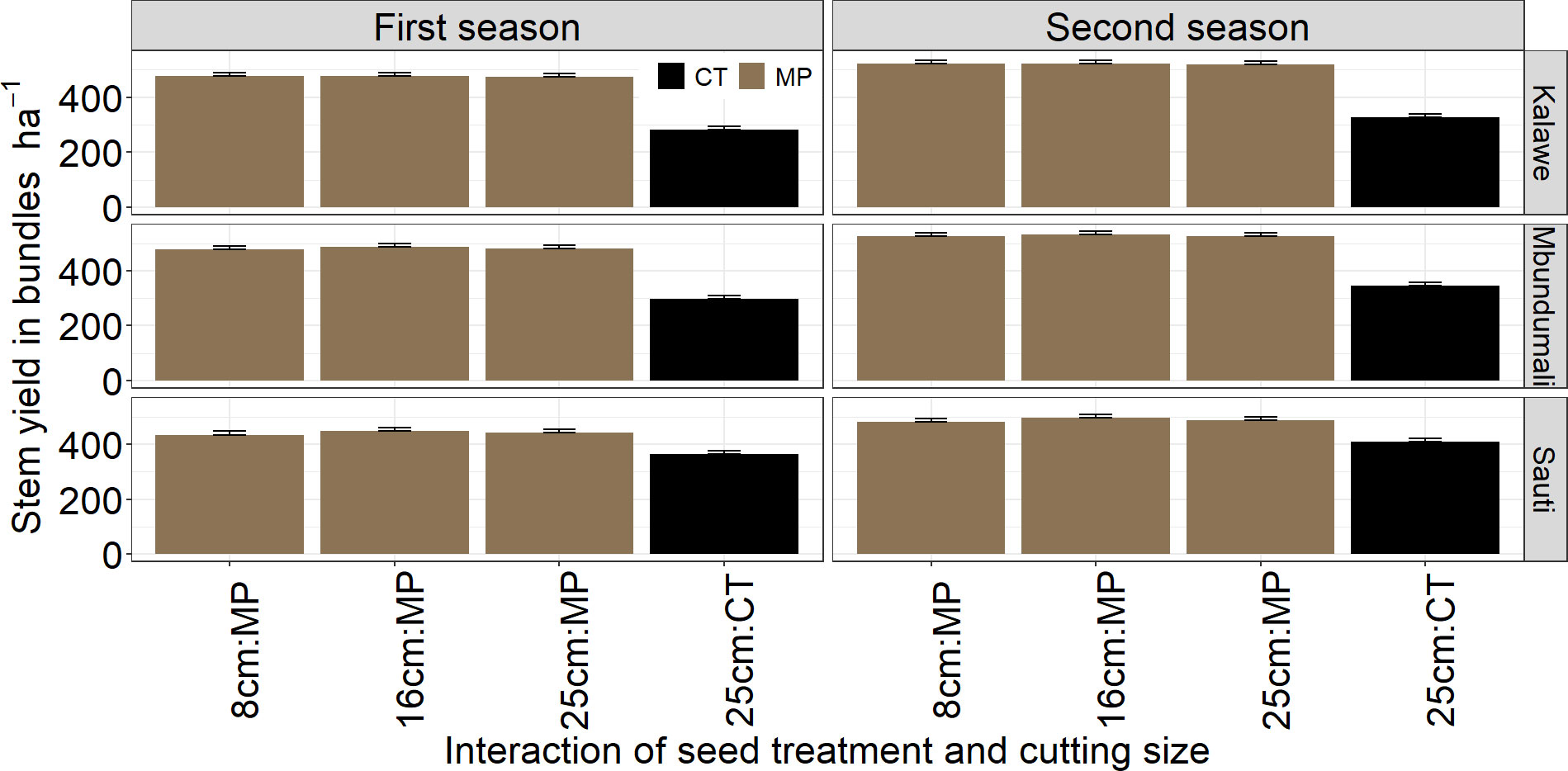
Figure 6 Effects of seed treatment, stake size, and cassava variety on stem yields in bundles ha−1 in the first and second seasons of MandiPlus field experimentations in Malawi. CT, control without MandiPlus treatment; MP, MandiPlus seed treatment. Error bars represent standard errors of the mean.
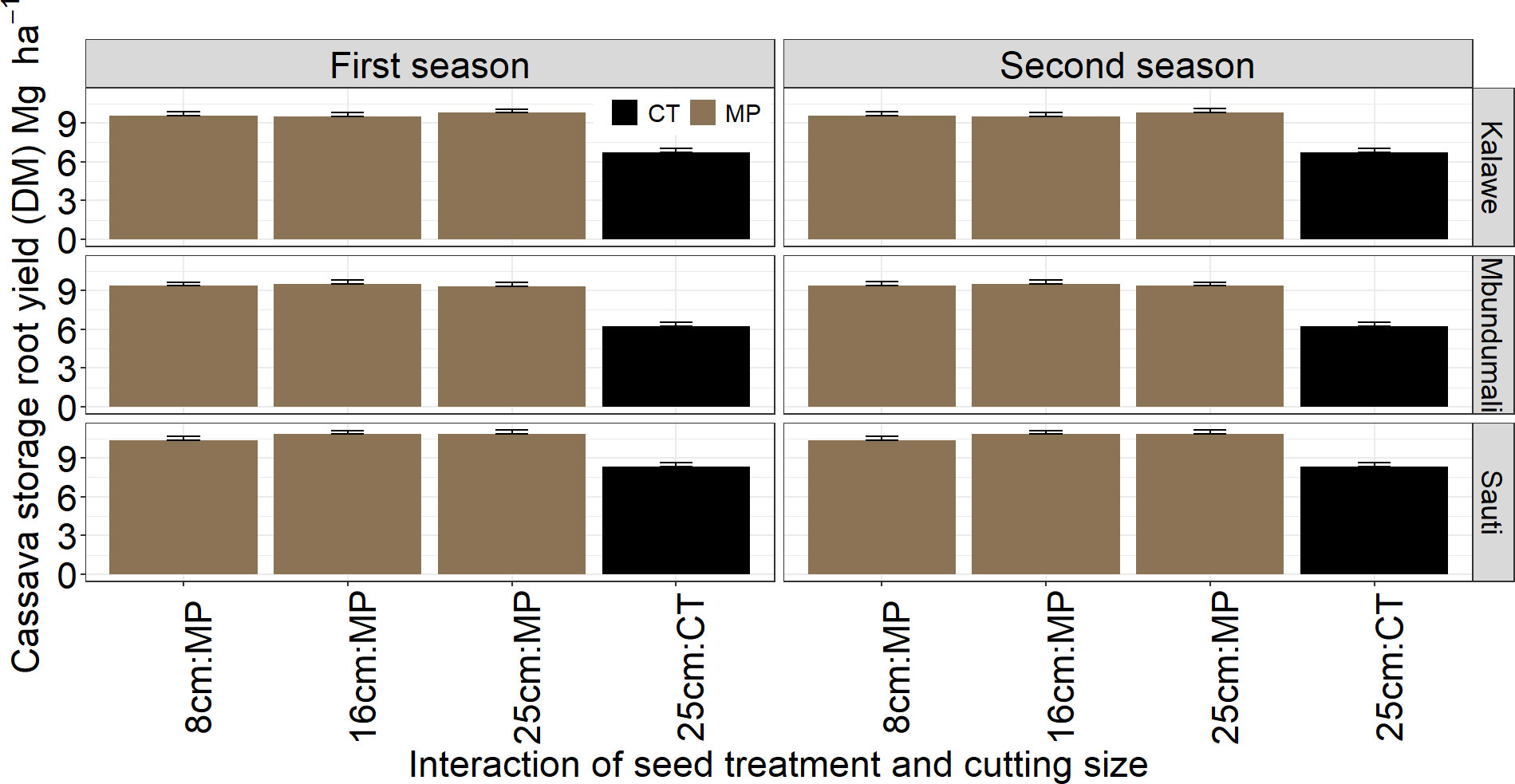
Figure 7 Effects of seed treatment, stake size, and cassava variety on storage root yields (Mg ha−1) in the first and second seasons of MandiPlus field experimentations in Malawi. CT, control without MandiPlus treatment; MP, MandiPlus seed treatment. Error bars represent standard errors of the mean.
3.5 Benefit–cost ratio of MandiPlus seed treatment application
The BCR analysis showed a higher BCR in the first season than in the second (p = 0.04). Comparing the certified with the non-certified stems scenarios shows a highly significant difference (p< 0.001) in BCR: average BCR was higher by 7.0 USD USD−1 from non-certified to certified stems (Figure 8). While a BCR for non-certified stems across stake size averaged 5.0 USD USD−1, BCR for certified stems averaged 11.4 USD USD−1. The result of the statistical analysis for the BCR between stem stake sizes shows that the MandiPlus application with 8-cm stakes produced the highest average BCR (13 USD USD−1) compared with either the 16-cm or 25-cm stakes. Using stem yields from 8-cm stakes, the average BCR was 18.3 USD USD−1 for the certified stem scenario and 8.0 USD USD−1 for the non-certified stems; with 16-cm stakes, the average was 9.2 USD USD−1 (certified) and 4.0 USD USD−1 (non-certified); and with 25-cm stakes, the average was 7.0 USD USD−1 (certified) and 3.0 USD USD−1 (non-certified) (Figure 8).
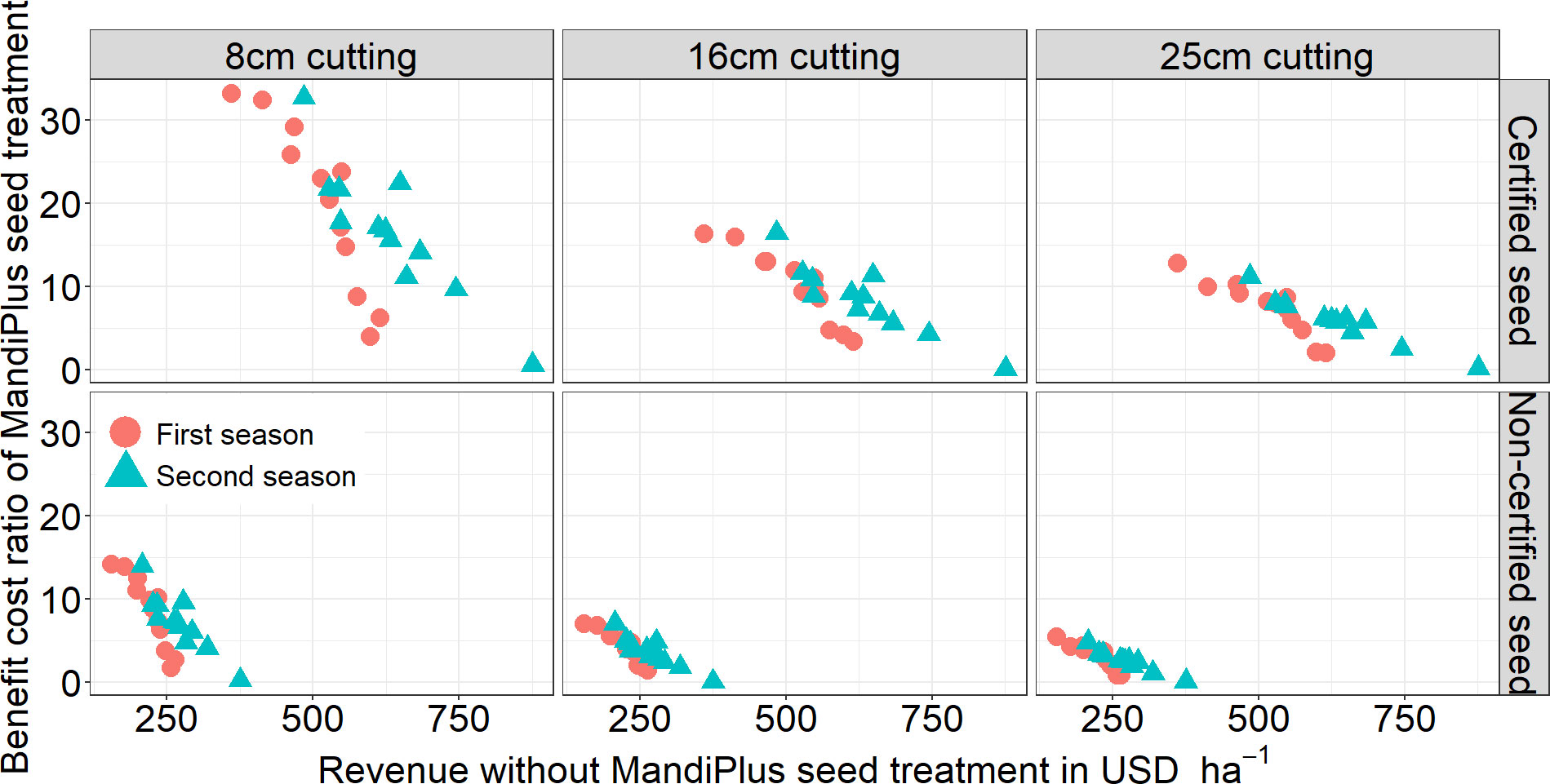
Figure 8 The relationship between the effect of MandiPlus treatment on the benefit–cost ratio (BCR) from stem yield in bundles (ha−1) from different stake sizes and revenue from stem yield without MandiPlus application. The BCR is shown for certified and non-certified cassava stem scenarios for the first and second seasons’ MandiPlus field evaluation in Malawi.
4 Discussion
4.1 Impacts of MandiPlus seed treatment application on stake germination, whiteflies, and disease incidence
Data from two trial sites and using three different cassava varieties in central Malawi show that systemic insecticides can prevent termite attacks on planted stem cuttings and improve germination regardless of the variety used and using any of the stake sizes tested in this study. Similar to our result, de Oliveira et al. (2020) and Omongo et al. (2022) reported reduced termite damage and better cassava performance with the use of systemic pesticide: plant germination, vigor, development, and yields regardless of stake sizes. This is aligned with similar results for the compounds tested in this study for other crops (Bass and Field, 2018).
The presence of cassava whiteflies threatens cassava production and farmer livelihoods across East and Southern Africa. This is through both direct feeding damage, as well as the vector-borne transmission of viral diseases. Whereas advances in resistance breeding have been achieved, the release and distribution of improved cultivars remain slow and inadequate to address this urgent challenge requiring a complementary solution. Our research now shows that the judicious application of seed treatment formulations that contain systemic insecticides can help control whitefly pest pressure and result in a reduced whitefly population and transmitted cassava viral diseases. The findings corroborate the report by Mware et al. (2009) that there exists a significant and positive correlation between the number of adult whiteflies and CBSD incidence, depicting a possible role of whiteflies in the spread of cassava viral diseases. This implies that any measure that controls whitefly population would effectively control whitefly-transmitted diseases.
Whereas our data provide no strong evidence for controlling CBSD, the use of systemic insecticides provides an opportunity to control CMD, strengthening existing varietal resistance. The different responses by the diseases to the seed treatment can be explained by the different infection mechanisms of the two viruses, with CBSD only requiring short contact between vector and host for successful infection (Calvert and Thresh, 2002) or varying tolerance to the diseases by the different cassava varieties used in this study (Perez-Fons et al., 2020) or the responsible virus species (Winter et al., 2010). Furthermore, Legg et al. (2011) reported that CMD pandemic spread is closely linked to the appearance of super-abundant Bemisia tabaci whitefly vector populations, in contrast to CBSD, where outbreaks occurred 3–12 years after whitefly population increases. This might explain the reduced CMD incidence but not CBSD with a reduced whitefly population with the seed treatment than without. However, some new cultivars and landraces show substantial levels of CBSD resistance (Masinde et al., 2018; Sheat and Winter, 2023), opening the opportunity for a complementary solution of genetic resistance and seed-applied solutions to control both pests and disease. Our result is an indicator for deliberate varietal selection for achieving the best result with the seed treatment for cassava whiteflies and disease controls. On the other hand, the potential to control CMD offers new applications for this technology in other parts of the world that experienced a recent rise in the disease, such as South and Southeast Asia (Hareesh et al., 2023).
4.2 Impacts of MandiPlus seed treatment application on the economics of cassava seed systems
Additionally, we show that the assessed seed treatment formulation not only reduces pest and disease prevalence but also holds the potential to increase the efficiency and business case of certified cassava seed production through increased multiplication rates. This has the potential to help increase the production of quality, certified, disease-free cassava planting material and also the production and efficient handling and distribution of planting material of improved cultivars, for instance, with increased resistance against viruses (de Oliveira et al., 2020).
5 Conclusion and recommendations
The hereby described seed treatment technology offers the potential to control cassava pests and vector-borne diseases. The seed treatment was effective against termites on planted stakes. This resulted in improved stake germination, stem, and storage root yields regardless of cassava variety and the stake size used: 8 cm, 16 cm, or 25 cm, offering an opportunity to increase the multiplication rates of certified cassava planting material with existing infrastructure and production procedures. The seed treatment solution also opens opportunities for the commercial production of certified planting material and the accelerated distribution of improved cultivars through an increase in the multiplication rate. In addition, applying the seed treatment reduced whitefly populations on cassava, thereby reducing CMD incidence in our study. However, the effect was variety-specific and would therefore require further investigation with more varieties, especially with varieties that are tolerant to whitefly and CBSD. The highest BCR obtained under certified stems scenario with the seed treatment regardless of stake size and variety is evidence of its potential to increase the business case of certified cassava seed production. We, therefore, propose integrating this technology into existing cassava seed systems in countries and regions that are heavily affected by yield loss caused by cassava whiteflies and whitefly-borne viral diseases.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author contributions
CN: Conceptualization, Data curation, Formal analysis, Methodology, Software, Visualization, Writing – original draft. DKa: Methodology, Supervision, Validation, Writing – review & editing. MC: Investigation, Validation, Writing – review & editing. DKl: Conceptualization, Funding acquisition, Investigation, Methodology, Project administration, Resources, Supervision, Validation, Writing – review & editing. MR: Funding acquisition, Project administration, Writing – review & editing. RB: Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. The authors declare financial support was received from the Bill and Melinda Gates Foundation (BMGF) for the research under grant number OPP 10589338 - funding to phase II of the African Cassava Whitefly Project (ACWP II).
Acknowledgments
The authors greatly appreciate the commitment and dedication of all the farmers who provided their fields for the trials and supported the management activities. We also appreciate the technical and management staff of the Tanzania Agricultural Research Institute, Tanzania for their various contributions. We appreciate the technical and administrative guidance from John Colvin and other members of the African Cassava Whitefly Project II. We appreciate the efforts of Aleksandra Sapala in putting together the materials on which this paper was further developed.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Adebayo W. G. (2023). Cassava production in Africa: A panel analysis of the drivers and trends. Heliyon 9 (9). doi: 10.1016/j.heliyon.2023.e19939
Alonso Chavez V., Milne A. E., van den Bosch F., Pita J., McQuaid C. F. (2021). “Modeling cassava production and pest management under biotic and abiotic constraints,” in Plant molecular biology (Springer Science and Business Media B.V), 325–349. Available at: https://link.springer.com/content/pdf/10.1007/s11103-021-01170-8.pdf.
Bass C., Field L. M. (2018). “Neonicotinoids,” in R772 current biology, vol. 28 (Elsevier Ltd.), R761–R783. Available at: https://www.cell.com/current-biology/pdf/S0960-9822(18)30697-3.pdf.
Calvert L. A., Thresh J. M. (2002). The viruses and virus diseases of cassava. Eds. Hillocks R. J., Thresh J. M., & Bellotti A. C. (CAB International), 237–259. Available at: http://ciat-library.ciat.cgiar.org/articulos_ciat/cabi_15ch12.pdf.
CHIRPS. (2023). Climate hazards group infrared precipitation with station data (SERVIR ClimateSERV). Available at: https://climateserv.servirglobal.net/.
Conceicão L. V., Cortes D. F. M., Klauser D., Robinson M., Oliveira E. J. (2023). New protocol for rapid cassava multiplication in field conditions: a perspective on speed breeding. Front. Plant Sci. 14. doi: 10.3389/fpls.2023.1258101/full
de Oliveira E. J., de Oliveira S. A. S., Otto C., Alicai T., De Freitas J. P. X., Cortes D. F. M., et al. (2020). A novel seed treatment-based multiplication approach for cassava planting material. PloS One 15 (3). doi: 10.1371/journal.pone.0229943
FAO. (2010). Cassava diseases in Africa - a major threat to food security. Strategic Programme Framework 2010 - 2015. Available at: https://www.fao.org/3/i1460e/i1460e.pdf&clen=3221781.
FAO. (2023). Strengthening linkages between small actors and buyers in the Roots and Tubers sector in Africa. Available at: https://www.fao.org/in-action/african-roots-and-tubers/countries/malawi/ru/#:~:text=Cassava%20is%20the%20second%20most,in%20the%20lake%20shore%20districts.
FAO and IFAD. (2020). The world cassava economy. The World Cassava Economy. Available at: https://www.fao.org/3/x4007e/X4007E00.htm#TOC.
FAOSTAT. (2019). Countries by commodity (Food and Agriculture Organization of the United Nations). Available at: http://www.fao.org/faostat/en/#rankings/countries_by_commodity.
Fargette D., Fauquet C., Thouvenel J.-C. (1985). Field studies on the spread of African cassava mosaic. Ann. Appl. Biol. 106 (2), 285–294. doi: 10.1111/j.1744-7348.1985.tb03118.x
Gómez-Rubio V. (2017). ggplot2 - elegant graphics for data analysis (2nd edition). J. Stat. Software 77 (Book Review 2), 2–5. doi: 10.18637/jss.v077.b02
Gondwe F., Mahungu N., Hillocks R., Moyo C., Sok M., Chipungus F., et al (2003). “Economic Losses Experienced by Small Scale Farmers in Malawi due to Cassava Brown Streak Virus Disease,” in Proceedings of an international workshop on cassava brown streak virus disease: past, present and future. Eds. Legg J. P., Hillock R. J. (Mombasa, Kenya), 28–38. Available at: https://assets.publishing.service.gov.uk/media/57a08cfded915d3cfd001752/R7563CBSDproceedings.pdf.
Hareesh P. S., Resmi T. R., Sheela M. N., Makeshkumar T. (2023). Cassava mosaic disease in South and Southeast Asia: current status and prospects. Front. Sustain. Food Syst. 7. doi: 10.3389/fsufs.2023.1086660
Howeler R. H. (2002). “Cassava: cassava mineral nutrition and fertilisation biology,” in Biology, production and utilisation. Eds. Hillocks R. J., et al. (New YorkCABI Publishing), 115–147. doi: 10.1079/9780851995243.0115
Kilwinger F., Mugambi S., Manners R., Schut M., Tumwegamire S., Nduwumuremyi A., et al. (2021). Characterizing cassava farmer typologies and their seed sourcing practices to explore opportunities for economically sustainable seed business models in Rwanda. Outlook Agric. 50 (4), 441–454. doi: 10.1177/00307270211045408
Legg J. P., Jeremiah S. C., Obiero H. M., Maruthi M. N., Ndyetabula I., Okao-Okuja G., et al. (2011). Comparing the regional epidemiology of the cassava mosaic and cassava brown streak virus pandemics in Africa. Virus Res. 159 (2), 161–170. doi: 10.1016/j.virusres.2011.04.018
Legg J. P., Lava Kumar P., Makeshkumar T., Tripathi L., Ferguson M., Kanju E., et al. (2014). Cassava virus diseases: biology, epidemiology, and management. Adv. Virus Res. 91 (1), 85–142. doi: 10.1016/BS.AIVIR.2014.10.001
Legg J. P., Lava Kumar P., Makeshkumar T., Tripathi L., Ferguson M., Kanju E., et al. (2015). “Cassava virus diseases: Biology, epidemiology, and management,” in Advances in virus research, vol. 91. (Academic Press Inc), 85–142. Available at: https://www.sciencedirect.com/science/article/abs/pii/S0065352714000025?via%3Dihub.
Masinde E. A., Mkamillo G., Ogendo J. O., Hillocks R., Mulwa R. M. S., Kimata B., et al. (2018). Genotype by environment interactions in identifying cassava (Manihot esculenta Crantz) resistant to cassava brown streak disease. Field Crops Res. 215, 39–48. doi: 10.1016/j.fcr.2017.10.001
Musopole H., Mtonga A., Maruthi M. N. (2023). Resistance levels of cassava landraces to CMD, CBSD, and vector whiteflies in Malawi. Available at: https://www.researchsquare.com/article/rs-2563018/v1.
Mware B., Miinda Ateka E., Songa J. M., DEVI Narla R. (2009) Transmission and distribution of cassava brown streak virus disease in cassava growing areas of Kenya. Available at: file:///C:/Users/s1094188/Downloads/Transmission_and_distribution_of_cassava_brown_str.pdf.
NACGRAB. (2005). Crop varieties released and registered in Nigeria catalogue. Available at: https://www.nacgrab.gov.ng/images/Varieties_Released_Catalogue.pdf.
Nwokoro C. C., Kreye C., Necpalova M., Adeyemi O., Busari M., Tariku M., et al. (2021). Developing recommendations for increased productivity in cassava-maize intercropping systems in Southern Nigeria. Field Crops Res. 272, 108283. doi: 10.1016/j.fcr.2021.108283
Omongo C. A., Opio S. M., Bayiyana I., Otim M. H., Omara T., Wamani S., et al. (2022). African cassava whitefly and viral disease management through timed application of imidacloprid. Crop Prot. 158, 106015. doi: 10.1016/j.cropro.2022.106015
Perez-Fons L., Ovalle T. M., Maruthi M. N., Colvin J., Becerra Lopez-Lavalle L. A., Fraser P. D. (2020). The metabotyping of an East African cassava diversity panel: A core collection for developing biotic stress tolerance in cassava. PloS One 15 (11 November). doi: 10.1371/journal.pone.0242245
R Core Team. (2020). The R project for statistical computing (3.4.4). Available at: https://www.r-project.org/.
Rey C., Vanderschuren H. (2017). The Annual Review of Virology is online at virology. Annu. Rev. Virol. 4, 429–452. doi: 10.1146/annurev-virology
Sheat S., Winter S. (2023). Developing broad-spectrum resistance in cassava against viruses causing the cassava mosaic and the cassava brown streak diseases. Front. Plant Sci. 14. doi: 10.3389/fpls.2023.1042701
Slakie E., McKee C., Gaffney A., Leigh Anderson C., Kay Gugerty M. (2013) Control strategies for whitefly as a vector for cassava viral diseases. Available at: https://epar.evans.uw.edu/sites/default/files/EPAR_UW_Request_233_Whitefly%2520Cassava%2520Control%2520Strategies_08.28.13.pdf&clen=278444&chunk=true.
Szyniszewska A. M. (2020). CassavaMap, a fine-resolution disaggregation of cassava production and harvested area in Africa in 2014. Sci. Data. doi: 10.1038/s41597-020-0501-z
Weigand J. M. (2018) Diversifying Malawi’s food security: Cassava’s promise as a dual-purpose crop A case study from the Lilongwe District. Available at: https://www.duo.uio.no/bitstream/handle/10852/64459/Jana-Weigand_-Final-version.pdf?sequence=1&isAllowed=y.
Keywords: whitefly, cassava, seed systems, seed treatment, cassava mosaic disease, cassava brown streak disease
Citation: Nwokoro CC, Kachigamba D, Chiipanthenga M, Klauser D, Robinson M and Berlin R (2024) Effects of seed treatment on cassava stake performance, whitefly population, disease incidence, and yield performance of cassava (Manihot esculenta Crantz) in Malawi. Front. Agron. 5:1303869. doi: 10.3389/fagro.2023.1303869
Received: 28 September 2023; Accepted: 20 December 2023;
Published: 15 January 2024.
Edited by:
Pei Li, Kaili University, ChinaReviewed by:
Jiban Shrestha, Nepal Agricultural Research Council, NepalPatrick Chiza Chikoti, Zambia Agriculture Research Institute (ZARI), Zambia
Copyright © 2024 Nwokoro, Kachigamba, Chiipanthenga, Klauser, Robinson and Berlin. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Charles Chigemezu Nwokoro, Q2hhcmxlcy5ud29rb3JvQHN5bmdlbnRhLmNvbQ==
 Charles Chigemezu Nwokoro
Charles Chigemezu Nwokoro Donald Kachigamba3
Donald Kachigamba3 Dominik Klauser
Dominik Klauser Robert Berlin
Robert Berlin