- 1Department of Biology, Acadia University, Wolfville, NS, Canada
- 2Department of Chemistry, Acadia University, Wolfville, NS, Canada
The two-spotted spider mite, Tetranychus urticae Koch, is a globally distributed polyphagous agricultural pest that is resistant to a variety of synthetic chemical pesticides. Plant essential oils have been recognized as a novel natural source of pest control that have a reduced impact to the environment and human health relative to synthetic pesticides, and which may provide a viable alternative for managing this pest. The present study focuses on assessing the potential of mite semiochemicals and essential oil constitutes as repellents and miticides. We developed an innovative electrophysiological approach (electrotarsography) to record olfactory sensitivity of T. urticae. Additionally, a novel two-choice behavioural assay was designed to determine whether T. urticae is attracted or repelled by selected compounds. Using Gas Chromatography-linked Electrotarsal detection (GC-ETD), we determined that T. urticae can sense many terpenoids commonly found in plant essential oils, such as eucalyptol, thymol, and linalool. In addition, T. urticae responded to carboxylic acids and aldehydes, which are known to be detected by ionotropic receptors. In two-choice behavioural assays, T. urticae was repelled by various essential oil active ingredients, as well as carboxylic acids and aldehydes. The study provides much needed insight to identify behaviourally relevant chemical cues for the development of mite control strategies.
Introduction
Tetranychus urticae Koch (Trombidiformes: Tetranychidae) (two-spotted spider mite) is an agricultural pest with a host range of over 1100 species of plants that is resistant to many pesticides (Xu, 2018). Tetranychus urticae is active mostly on the underside of the leaves, feeding by puncturing the leaf cells with its cheliceral stylets and aspirate the cell contents, which causes leaf chlorosis and can lead to plant death (Riga, 2014; Wang et al., 2015; Xu, 2018). Infestations of T. urticae can lower the overall photosynthetic rate of plants and total chlorophyll content (Park and Lee, 2002). For these reasons, infestations of T. urticae are correlated with reduced crop yield and plant death for various plant species (Archer and Bynum, 1993). Mites can rapidly evolve resistance to many synthetic pesticides due to their high fecundity and short generation time (Grbic, 2007).
Plant-derived products are a viable alternative compared to synthetic pesticides. Plant essential oils have been recognized as a natural source of biologically-active compounds with novel pesticidal properties that are environmentally sound and safe for human health (Tripathi et al., 2009). Essential oils are complex secondary metabolite mixtures extracted from a variety of aromatic plant and tree parts, such as leaves, roots, flowers, bark, and wood, and contain volatile, highly aromatic compounds, including monoterpenes, phenols, and sesquiterpenes. These oils may also play a key role in attracting some beneficial insects for the dispersal of pollen and seeds (Tripathi et al., 2009). In addition, they play an important role in protecting plants against pathogens, and herbivorous arthropod pests (Faraone et al., 2015).
The foretarsi of mites are the primary site of olfaction, and possess up to 28 sensilla (Carr and Roe, 2016). Additionally, mites have chemoreceptors located in the sensilla on each palp. In acarines, chemosensory receptors on the palps and the foretarsi are responsible for the detection of food, pheromones, host kairomones, plant and fungal allomones, and arthropod repellents (Faraone et al., 2019; Faraone et al., 2020; Renthal, 2022). There are two types of olfactory receptors known for arthropods: (1) G-protein coupled odorant receptors (GPCRs) with seven membrane-spanning regions and a co-receptor which pairs with odorant receptors (Wanner, 2007; Wanner and Robertson, 2010); and (2) ionotropic receptors (IRs) which respond to ligand-binding by opening ion channels (Croset, 2010). The mechanism of chemoreception in Acari is different from that observed in insects; Acari lack GCPRs to aid in olfaction, which are highly prevalent in insects (Wanner, 2007; Wanner and Robertson, 2010; Ngoc, 2016; Josek et al., 2018). Furthermore, T. urticae lacks the TRPA1 gene, which plays a role in modulating olfactory sensitivity (Peng, 2015) and repellency (Melo et al., 2021).
Mites respond to immature female pheromones, female sex pheromones, and alarm pheromones (Carr and Roe, 2016). An attractive behavioural response is usually indicated by movement towards the source of compound(s) whereas a repellent behavioural response is usually indicated by movement away from the source of compound(s) (Carr and Roe, 2016). Based on electrophysiological recordings of the honeybee ectoparasitic mite, Varroa destructor Anderson and Trueman (Mesostigmata: Varroidae), sensory neurons housed on the foretarsi of mites respond to volatile stimuli (dialkoxybenzenes and 5(2'-hydroxyethyl) cyclopent-2-en-1-ol ether derivatives) (Eliash, 2014; Singh, 2015). Previous electrophysiological studies have demonstrated that V. destructor is responsive to components of the yarrow essential oil and several methyl-alkane groups identified from volatile collections of honeybees (Light et al., 2020a; Light et al., 2021). Additionally, a study done by Light et al. (2020b) found electrophysiological responses to methyl palmitate, ethyl palmitate, and 2-hexanol – common in-hive volatiles. Electrophysiological work on T. urticae is still developing, as there are many challenges associated with their relatively small size and ensuing difficulty mounting specimens (Carr and Roe, 2016)
The main goal of this study was to investigate compounds with different functional groups as potential repellents of T. urticae. In order to screen the olfactory response of T. urticae towards essential oils components, we developed an innovative electrophysiological approach. Additionally, a novel two-choice behavioural assay was designed to determine whether T. urticae is attracted or repelled by selected compounds.
Methods and Materials
Animal and plant care
Spider mites were provided by Vineland Research and Innovation Centre (Vineland Station, ON, Canada) to establish a colony at Acadia University, Wolfville (NS, Canada). All mites used in this study were adult females: they are easily identifiable by their large size and blunt abdomen relative to males (Meena et al., 2013). The colony was maintained on common bean plants (Phaseolus vulgaris L.) in wire mesh cages (100 x 100 x 100 cm) located in a growth chamber (25 ± 2°C, 16:8 L:D, 70 ± 5 % RH). Bean plants were changed every 2 days, replacing several of the oldest plants with 3-week-old plants. Bean plants were grown in phytotrons in the Harriet Irving Botanical Gardens (K.C. Irving Environmental Science Centre, Acadia University, NS, Canada), kept between 18 and 25°C on a 12:12 light/dark photoperiod.
Chemicals
A variety of essential oil components, organic acids and aldehydes were chosen for testing. The chemicals and their sources include: alpha-phellandrene, linalool (97%) (+/-) racemic mixture, nonanal (95%), thymol (≥98%), eucalyptol (99%), propionic acid (≥99.5%, FCC, FG), isovaleric acid (≥99%), hexanoic acid (≥99%), octanoic acid (>99%), pentanal (≥99%), hexanal (98%), and octanal (≥99%), which were all purchased from Sigma-Aldrich (Saint Louis, MO, USA). Butyric acid (≥98%), Cis-3-hexen-1-ol (>99%), and Cis-3-hexenyl-acetate (>99%) were bought from Bedoukian (Danbury, CT, USA). Beta-phellandrene (80%) was purchased from Foreverest (Xiamen, Fujian, China). Farnesene (mixture of isomers) and heptanal (92%) were boughtfrom SAFC (Steinheim, Germany). HPLC-grade hexane was purchased from Fisher Scientific (Fair Lawn, NJ, USA). Eucalyptus, coriander essential oil, and neem oil were bought from Ultra International B.V. (LA Spijkenisse, Netherlands). Lavender essential oil was purchased from New Directions Aromatics (Mississauga, ON, Canada). Freshly extracted basil essential oil was provided by Dr. Faraone (Chemistry Department, Acadia University). Tween-80 was purchased from Sigma-Aldrich (Saint Louis, MO, USA).
Electrotarsogram studies
A novel electrophysiological approach was developed to record olfactory sensitivity of female T. urticae using a Syntech EAG fork electrode (Ockenfels Syntech, Buchenbach, Germany). A small amount of electrode gel (approximately 0.1mL) (Signagel®, Parker Laboratories Inc, NJ, United States) was placed on the base electrode, and a single female mite was mounted dorsally within the gel, with her gnathosoma facing the recording electrode (Figure 1). The foretarsi were attached underneath the recording electrode with additional electrode gel. Then the tips of the foretarsi were cut with a scalpel (Sigma-Aldrich, Saint Louis, MO, USA) to help establish a connection for the electrotarsography recordings. Given the small size of T. urticae (an adult female is approximately 0.5mm), all mounting preparations required the use of a dissecting microscope (AmScope SM-1BSX-64S, Irvine, CA, USA).
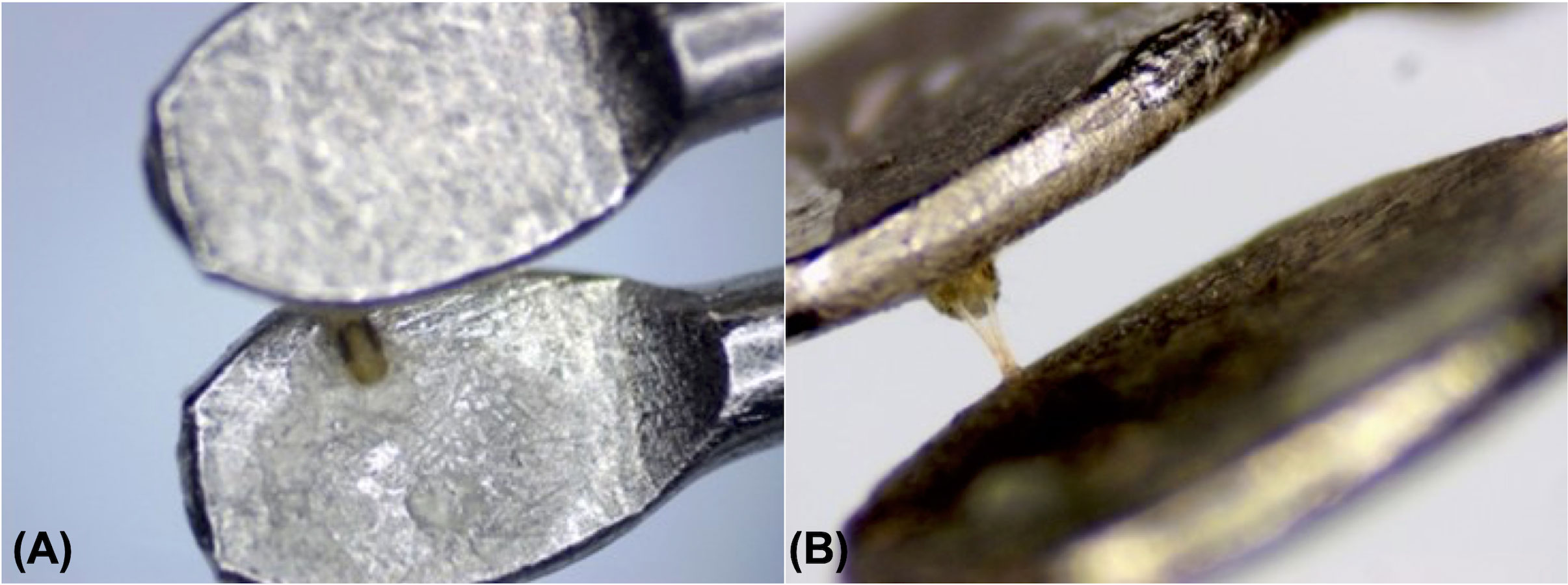
Figure 1 Female Tetranychus urticae mounted dorsally on a fork electrode, where (A) shows the body on the base electrode, and (B) shows foretarsi attached to the recording electrode with electrode gel.
Electrotarsography (ETG) was used to screen our selected compounds to which T. urticae can sense. We focused on screening compounds known to be detected by ionotropic rectors, such as carboxylic acids and aldehydes (Ni, 2021), and various active ingredients of essential oils known to be potentially effective for pest management. All ETG and GC-ETD recordings were amplified via the Intelligent Data Acquisition Controller-2 (IDAC-2) (Syntech, Kirchzarten, Germany) and recorded through Data acquisition for Gas Chromatograph with EAD (GcEad 2012 v1.2.4 (2012-06-24), Syntech, Kirchzarten, Germany).
ETG measured the stimulation of the olfactory sensilla of a female mite specimen to a given compound within a stimulus cartridge when a puff of air is directed through the cartridge to the mounted specimen. The voltage recorded in response to specific stimuli corresponded to a specimen’s sensitivity to a particular compound.
Stimulus cartridges contained a single piece of filter paper (7 mm x 35 mm Fisherbrand, Fisher Scientific, ON, Canada) loaded with 10 μL of the compound of interest. The loaded filter paper was placed into a disposable borosilicate glass pipette (Fisherbrand, Fisher Scientific, ON, Canada). Then the glass pipette was capped with a 1-mL disposable pipette tip (Fisherbrand, Fisher Scientific, ON, Canada) and sealed with dental wax (Electron Microscopy Sciences, Hatfield, PA, USA). The following compounds were tested: α-phellandrene, β-phellandrene, butyric acid, farnesene, linalool, (Z)-3-hexen-1-ol, (Z)-3- hexen-1-yl acetate, and nonanal. Compounds of interest were diluted in HPLC-grade hexane and tested at 10 ng, 100 ng, 1 μg, and 10 μg. All prepared stimulus cartridges were stored at -20°C until use. Stimulus cartridges were reloaded with diluted compounds after 1 month or a maximum of 20 puffs based on preliminary data.
All compounds were tested in randomized order with increasing dosages (10 ng to 10 μg). A single odorant puff lasted 0.3 s controlled by a Syntech stimulus controller CS-55 V2.7 (Ockenfels Syntech). The flow rate of air for each puff is 3.54 L/min. Each stimulus cartridge was puffed in intervals of 30 s to allow for preparation recovery (time to recover from last stimulus and be prepared for next stimulus).
After screening both essential oil active ingredients, carboxylic acids, and aldehydes with ETG, three different master mixes were prepared based on combination of essential oils, carboxylic acids, or aldehydes. We test master mixes (e.g., selected individual compounds all dissolved in the same vial using the same solvent) to minimize the number of spider mite preps required for the acquisition of the recordings. The different master mixes were screened using the Varian 450-GC with a flame ionization detector (GC-FID) and a non-polar DB-5 Column (30m, 0.25mm, 0.25μm film thickness) (Varian Inc., Lake Forest, CA, USA) linked to an electrotarsal detector (GC-ETD), to detect responses to individual components of the mixtures. Each compound added to the essential oil master mix had a final concentration of 100 ng/μL. However, it is important to recognize that the individual compounds of each essential oil mixture vary in concentration, and that some compounds exist in more than one essential oil mixture, which increases the overall concentration. This may affect the recorded peak amplitude based on a dose-response relationship. Since the objective of the electrotarsogram study was only to identify through a qualitative analysis which compounds the mites would respond to, and not necessarily a concentration dependency, we tested the essential oils together as a master mix. Additionally, we wanted to test the various individual components by using them from their direct source (e.g., essential oil). Carboxylic acid and aldehyde master mixes were prepared at a concentration of 1 μg/μL. All master mixes were prepared using hexane as solvent. The essential oil master mix contained lavender, eucalyptus, basil, neem, and coriander. However, preliminary data demonstrated that the mites were only responding to some of the active ingredients (linalool, thymol, and eucalyptol), which became the composition of chemicals used for the essential oil master mix at a final concentration of 100 ng/μL. The carboxylic acid series consisted of propionic acid, butyric acid, isovaleric acid, hexanoic acid, and octanoic acid. The aldehyde master mix contained propionaldehyde, pentanal, hexanal, heptanal, octanal, and nonanal.
The GC-FID linked to an GC-ETD was used to record the olfactory response of female T. urticae. The FID reached a temperature of 320°C before ignition. Oven temperature was initially held at 50°C for 5 minutes, then increased to 186°C at a rate of 8°C/min, and then up to 280°C at a rate of 40°C/min where it was held for 2 minutes. The column had a flow rate of 1.2 mL/min, and helium was the carrier gas. Samples were injected in splitless mode. The GC-ETD response was recorded in concert with the GC output using GC-EAD software (GcEad 2012 v1.2.4; Ockenfels Syntech, Buchenbach, Germany) for the duration of the GC run.
Behavioural assays
After determining which compounds elicited olfactory response in female T. urticae, a novel behavioural assay was developed to determine whether perceived compounds induced attraction or repellency (Figure 2). For each replicate, a leaf from a bean plant was dipped halfway into the compound horizontally (at a concentration of either 10 ng/μL or 100 ng/μL) so that one half from the primary vein on the leaf was treated with compound of interest and the other half treated with the solvent only (i.e., control). Compounds were dissolved in Tween-80 (aqueous) at 0.1% v/v dissolved in water. Concentrations of either 10 ng/μL or 100 ng/μL were tested based on the concentrations to which female T. urticae responded in ETG experiments. A piece of filter paper with a width of 5 mm was adhered along the primary vein of the leaf with a small amount of petroleum jelly (Vaseline® Petroleum Jelly Unscented, Toronto, Canada). The filter paper strip divided the treatment and control sides of the leaf, and provided a ‘neutral’ launch area for the mite, as well as making it easier to see the mite when transferring to the filter paper. The treated leaf with attached filter paper was then placed between two pieces of plexiglass and held together with a bulldog clip. The top piece of plexiglass had a circular hole cut out with a diameter of 2 cm and a depth of 0.5 cm. A single adult female mite was placed on the filter paper through the hole of the plexiglass and observed for twenty minutes. After twenty minutes the location of the mite was recorded. If the mite was on the control side, the response was recorded as repellent; if the mite was on the treatment side, it was recorded as attraction. In case the mite was still on the filter paper, or escaped the plexiglass, it was recorded as non-responding. Mites had a maximum of twenty minutes to make a choice as it was observed in this study that female T. urticae typically choose a specific location to feed after fifteen minutes.
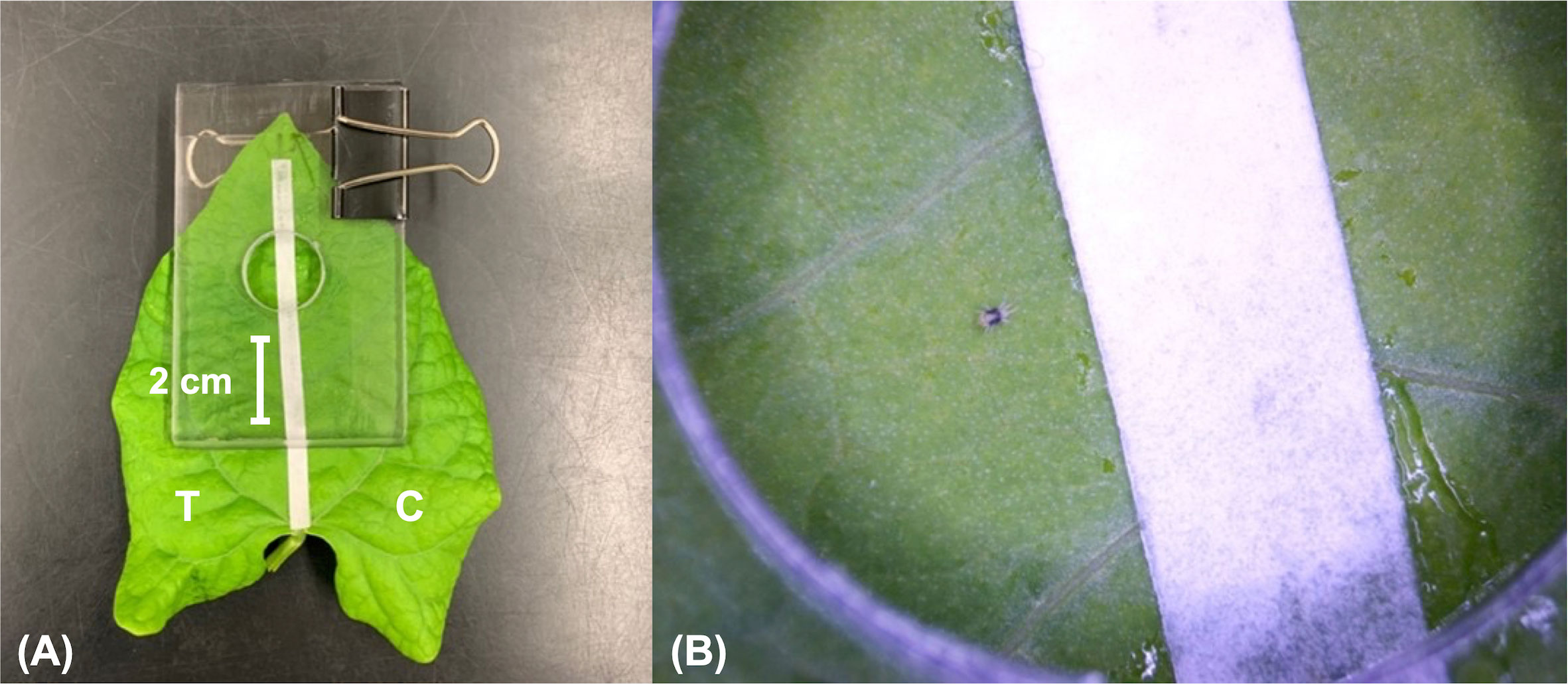
Figure 2 (A) Setup for Tetranychus urticae behavioural assays, in which half of the bean plant leaf (Phaseolus vulgaris L.) is treated with a compound of interest, and half of the leaf is treated with the solvent only. The treatment (T) and control (C) side of the leaf is divided by the filter paper. (B) Behavioural assay in progress.
Solvents
Prior to testing the compounds of interest, different solvents were tested to determine a viable carrier for all compounds to be dissolved in. A viable solvent would be one to which female T. urticae was neither attracted nor repelled. The potential solvents tested were hexane, ethanol, acetone, and Tween-80 (aqueous) at 0.1% v/v dissolved in water.
Our protocol addressed previous issues with the design of T. urticae behavioural assays, including: (1) we only tested one mite at a time to avoid social behaviour from occurring; (2) we tested potential solvents for repellency prior to testing compounds of interest; (3) we used whole leaves to avoid plant damage-related compounds.
Data analyses
ETG and ETD peak amplitudes were measured on GcEad software (GcEad 2012 v1.2.4 (2012-06-24), Syntech, Kirchzarten, Germany) and exported to Excel (Microsoft Excel for Mac, Version 16.54). To compare sensitivity of female T. urticae to various stimuli, a Shapiro-Wilk normality test was performed on ETD peak amplitudes for each compound at each of the 4 doses (10 ng, 100 ng, 1 μg, and 10 μg). A Two-way Analysis of Variance (ANOVA) with Tukey test was performed to determine if there was a significant difference in peak amplitude between compounds and/or dose (α = 0.05).
To determine potential repellency of compounds through two-choice behavioural assays, binomial tests (performed using the R Stats Package) were performed for each compound at each concentration to determine whether the proportion of mites that choose treatment side was greater than 0.5 (one-tailed test with α = 0.05). All statistical analyses were done using R version 4.0.3 (R Core Team 2020).
Results
Screening for ETG-active compounds
Peak ETG amplitudes were significantly different among compounds, as determined from results of two-way ANOVA (F7,20 = 5.76, p < 0.001) in which peak amplitude of butyric acid was significantly higher than all other compounds (Figure 3). The mean standardized peak amplitude for butyric acid at 10000 ng was 0.923 ± 1.011 mV. Adjusted p-values of butyric acid compared to all other compounds are shown in Table 1. Peak ETG amplitudes were significantly different between concentrations, as determined from results of two-way ANOVA (F3,20 = 3.709, p < 0.05). There was a significant interaction between compound and concentration for peak ETG amplitudes, as determined from results of two-way ANOVA (F21,20 = 3.328, p < 0.001).
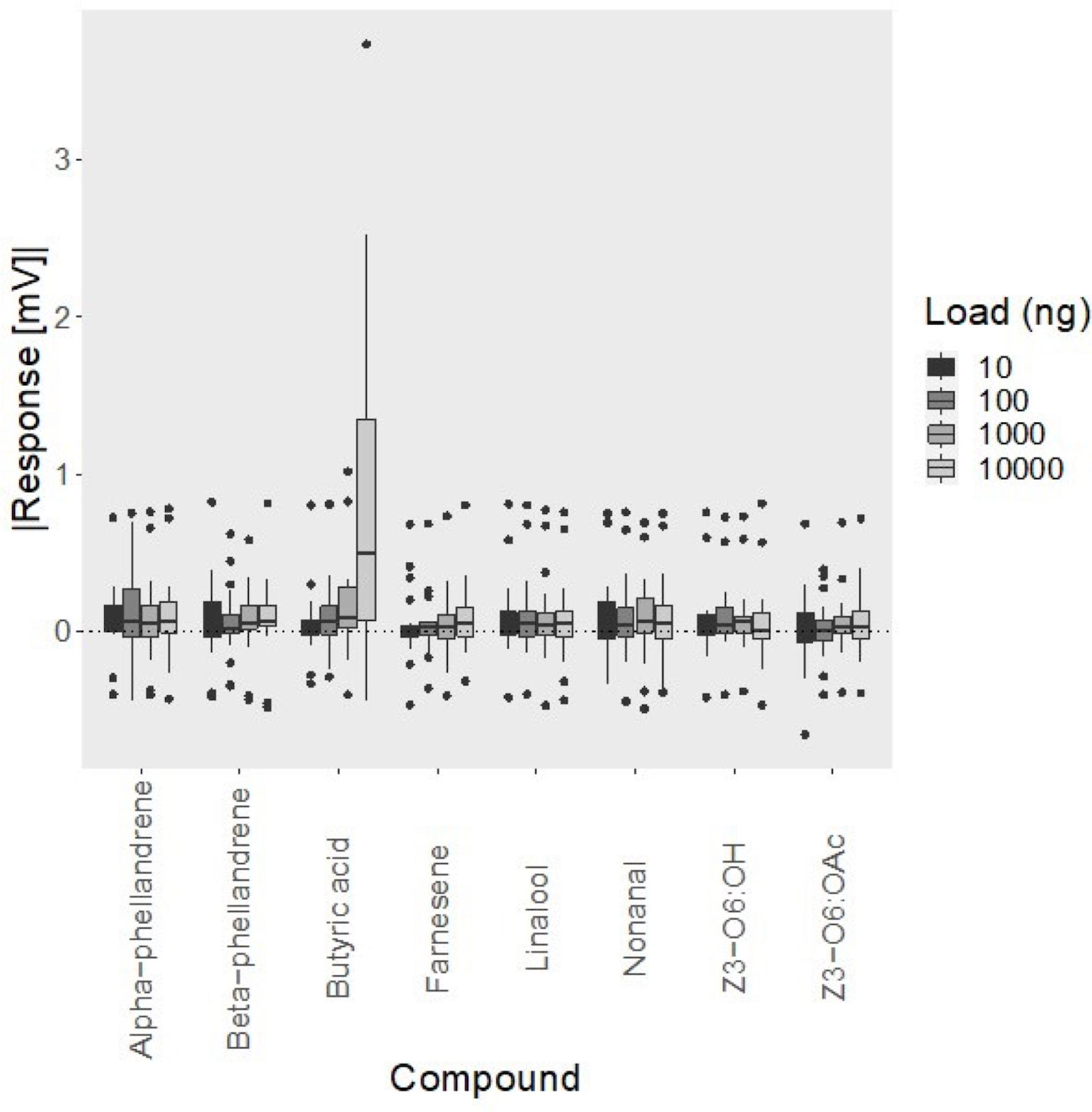
Figure 3 Electrotarsal response of adult female Tetranychus urticae to compounds with different functional groups, at 10 ng, 100 ng, 1 μg, 10 μg (n = 20). All responses are standardized to hexane by subtracting the response of hexane from the response of the compound. The bar represents the median, the box represents the interquartile range, and the whiskers represent the maximum and minimum values that are not outliers.
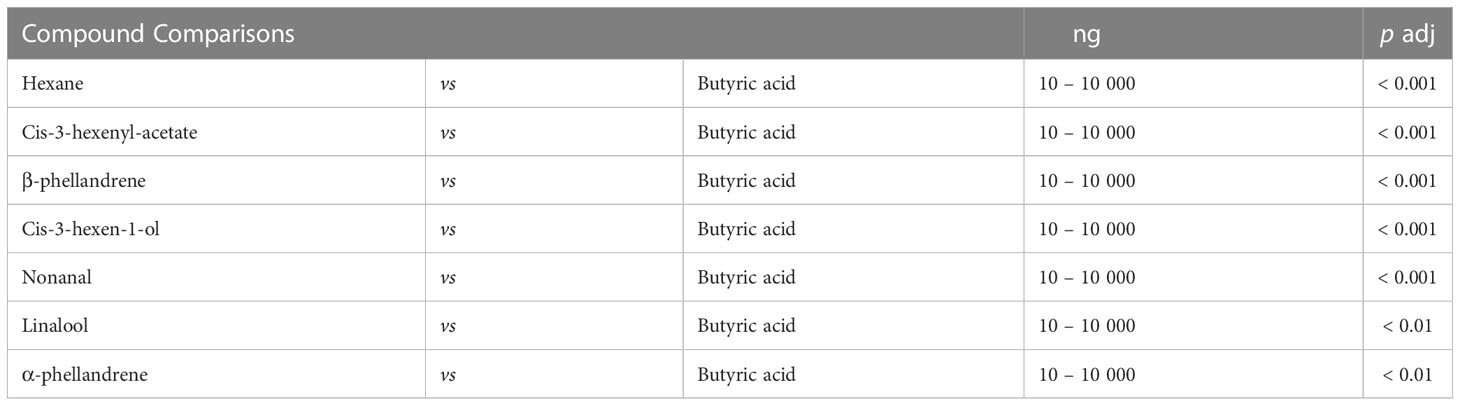
Table 1 Adjusted p-values from Tukey multiple comparisons of means for the butyric acid response of adult female Tetranychus urticae at all tested concentrations (10 ng, 100 ng, 1 μg, 10 μg) compared to 7 other compounds at the same concentrations (n = 20).
Gas chromatography-linked electrotarsal detection
Two-spotted spider mites responded to a series of carboxylic acids via stimulation from GC effluent (Figures 4A, 5A), including propionic acid, butyric acid, isovaleric acid, hexanoic acid, and octanoic acid. Butyric acid, a 4-carbon carboxylic acid, had a mean peak amplitude of 0.132 mV ± 0.0223. Carboxylic acids with 4 carbons had a smaller mean peak amplitude. Two-spotted spider mites also responded to a series of aldehydes (Figures 4B, 5B), including pentanal, hexanal, heptanal, octanal, and nonanal. Heptanal and octanal had a mean peak amplitude of 0.127 mV ± 0.0354 and 0.123 mV ± 0.00901 respectively, and all other aldehydes had a smaller mean peak amplitude. Finally, the mites responded to 3 different essential oil active ingredients (e.g., eucalyptol, linalool, and thymol) (Figures 4C, 5C). Eucalyptol had a mean peak amplitude of 0.0973 mV ± 0.0197. Other essential oil active ingredients had a smaller mean peak amplitude. Overall, the mites had the strongest responses to the carboxylic acid series, then the aldehyde series; the weakest response was recorded toward the essential oil active ingredients.
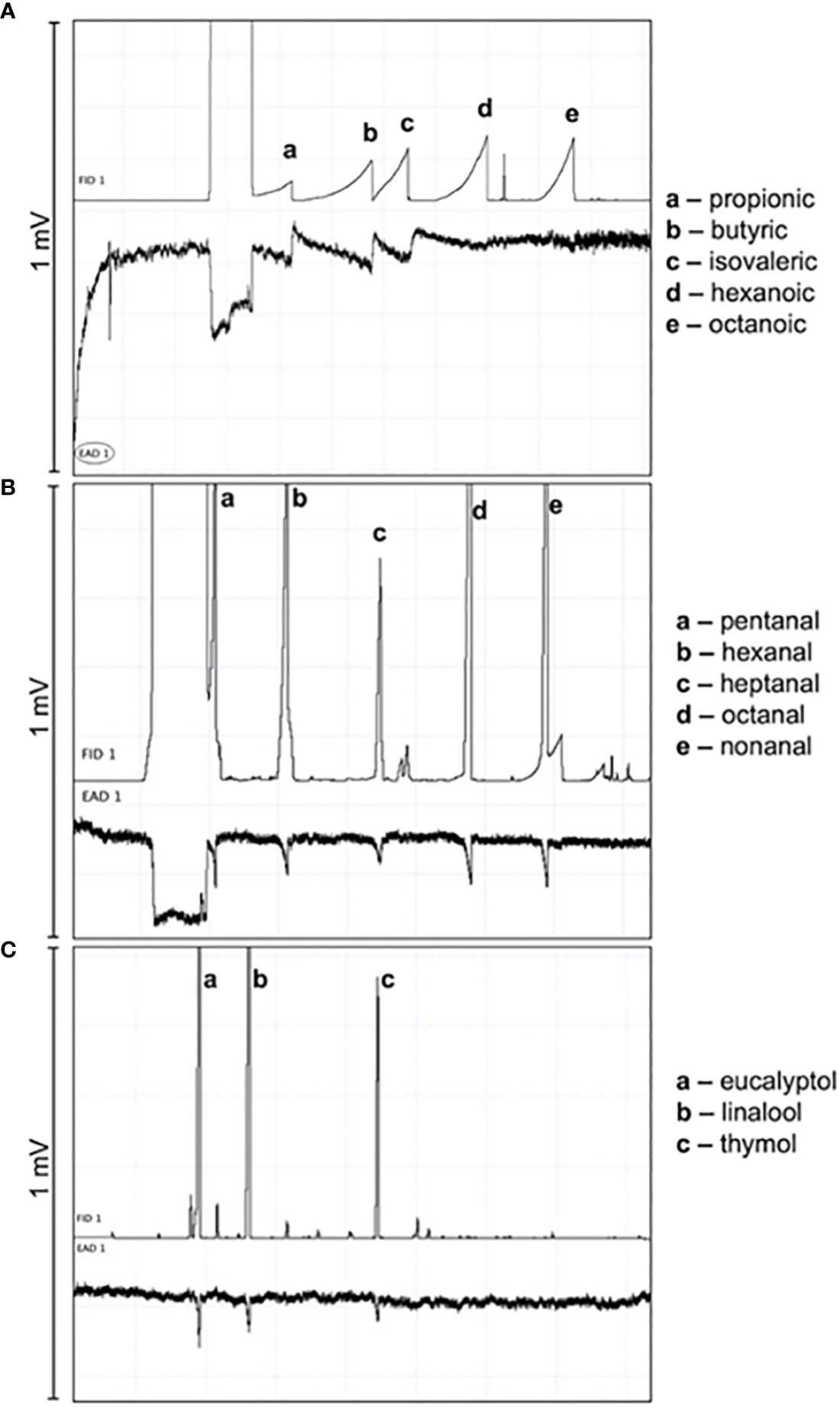
Figure 4 Gas Chromatography-linked Electrotarsal Detection displaying electrotarsal responses of Tetranychus urticae to a master mix of (A) carboxylic acids at a concentration of 1 μg/μL, (B) aldehydes at a concentration of 1 μg/μL, (C) essential oil active ingredients at a concentration of 100 ng/μL (volume injected: 1µl).
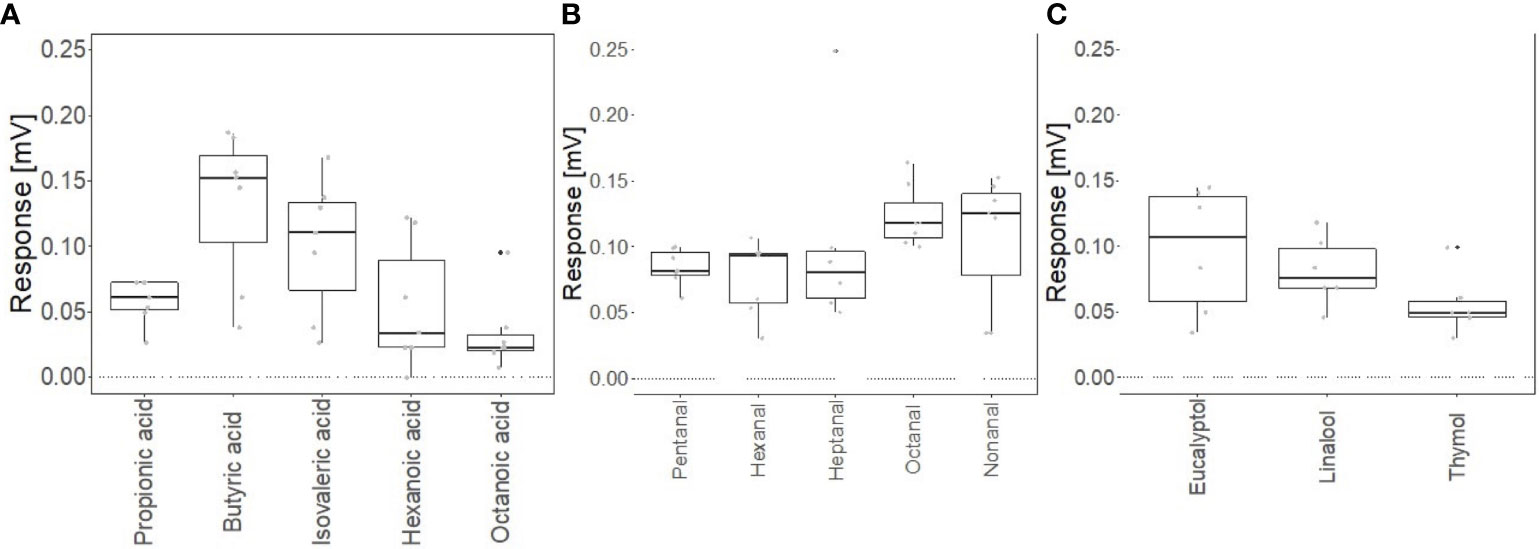
Figure 5 GC-Electrotarsal response of adult female Tetranychus urticae to a master mix of (A) carboxylic acids at a concentration of 1 μg/μL (n = 7), (B) aldehydes at a concentration of 1 μg/μL (n = 7), (C) essential oil active ingredients at a concentration of 100 ng/μL (n = 6) presented by 1µl injection on to a Gas Chromatogram and recorded via Electrotarsographic detection (GC-ETD). The bar represents the median, the box represents the interquartile range, and the whiskers represent the maximum and minimum values that are not outliers.
Solvents
When testing potential solvents for two-choice behavioural assay (Figure 6), female T. urticae was significantly repelled by hexane (p < 0.0001), ethanol (p < 0.0001), and acetone (p < 0.01), as determined from results of exact binomial test. Tetranychus urticae was not significantly repelled by Tween-80 (0.1% v/v), as determined from results of exact binomial test (p = 0.1), so this was determined to be a suitable solvent to use for behavioural assays.
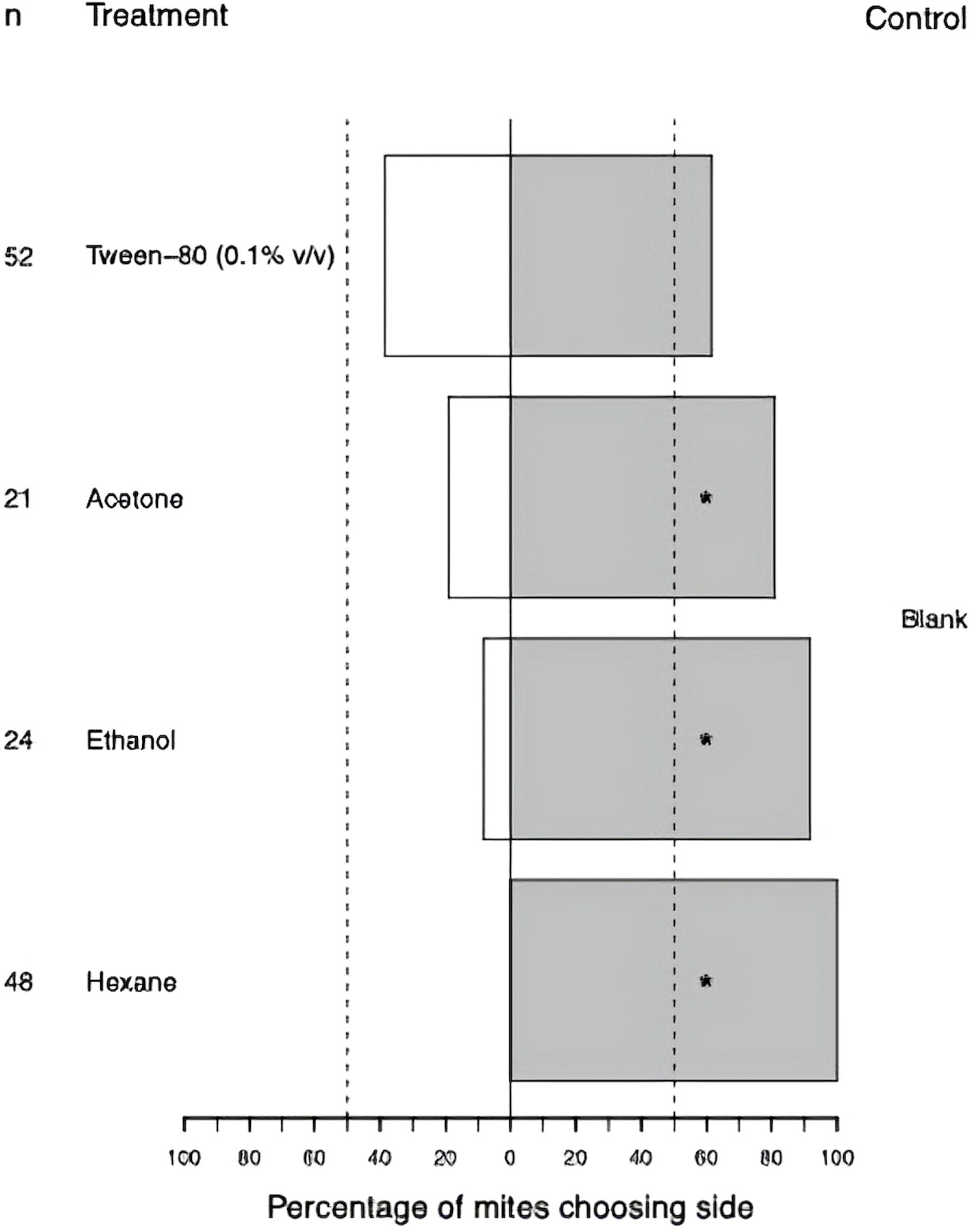
Figure 6 Percentage of adult female Tetranychus urticae choosing to feed on treated side of bean leaf (Phaseolus vulgaris L.) after 20 minutes in two-choice behavioural assay (n = 50). The dashed lines indicate the null expectation of 50%. Non-responding mites are not included in the figure. * indicate significantly repelled according to binomial test.
Behavioural assays
The results of the exact binomial tests determined that T. urticae females were significantly repelled by octanal at 10 ng/μL (p < 0.01), and at 100 ng/μL (p < 0.0001). Tetranychus urticae was repelled by butyric acid at 100 ng/μL (p < 0.001), but not at 10 ng/μL (p = 0.1). Isovaleric acid was repellent at 10 ng/μL (p < 0.0001), but not at 100 ng/μL (p = 0.7). Tetranychus urticae was repelled by heptanal at 100 ng/μL (p < 0.0001), but not at 10 ng/μL (p = 1). Tetranychus urticae was not significantly repelled by linalool at 10 ng/μL (p = 0.4), but was repelled at 100 ng/μL (p < 0.01). Tetranychus urticae was not significantly repelled by thymol at 10 ng/μL (p = 0.6) but was repelled at 100 ng/μL (p < 0.01). Tetranychus urticae was not significantly repelled by eucalyptol at 10 ng/μL or at 100 ng/μL (p = 0.4; p = 0.2). The results of the behavioural assays demonstrate that there was a concentration dependency for the repellency of T. urticae females to various compounds (Figure 7).
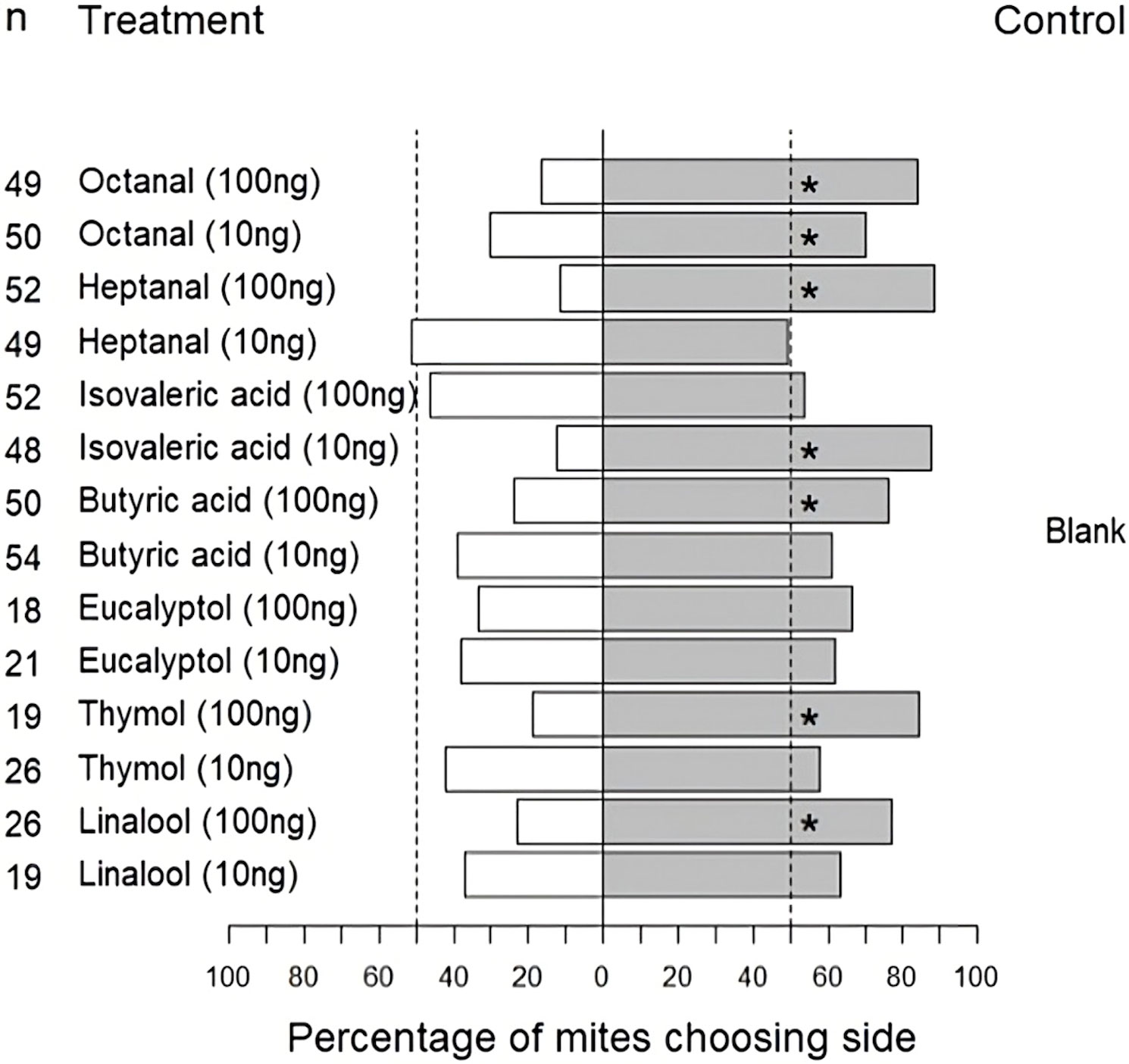
Figure 7 Percentage of adult female Tetranychus urticae choosing to feed on treated side of bean leaf (Phaseolus vulgaris L.) after 20 minutes in two-choice behavioural assay. The dashed lines indicate the null expectation of 50% of mites choosing each side. Non-responding mites are not included in the figure. * indicate significantly repelled according to binomial test.
Discussion
Our novel electrophysiological approach demonstrated that T. urticae females detects a variety of compounds that have potential to be used for the management of this species. Compounds which elicited an electrophysiological response by the receptors on the foretarsi and palps were carboxylic acids, aldehydes, and several active ingredients of essential oil mixtures. Receptors involved in the detection are probably ionotropic receptors, as they have been widely reported to be responsible for the detection of chemical compounds with a carbonyl group in acarines (Ngoc, 2016). Additionally, our two-choice behavioural assay was used to determine whether detected compounds repelled female T. urticae. This is an innovative approach to studying the electrophysiological and behavioural response of T. urticae and other tetranychids given the many past challenges associated with their small size and the level of difficulty mounting the specimen.
A study done by Tak and Isman, 2017 investigated the repellency of various essential oil components to T. urticae, including linalool, eucalyptol, and thymol. Among them, only thymol showed consistent repellent activity when using a bridge assay design. Our study also demonstrated that at 100 ng/μL thymol repels T. urticae using our leaf-dip assay.
We determined that linalool significantly repels T. urticae females, which is inconsistent with the results reported by by Tak and Isman, 2017. The lack of repellency by linalool in that study may be related to the different experimental design used. The main three experimental design elements of the set-up used by Tak et al. contributing to conflicting results compared to our study, are: (1) Tak et al. tested multiple mites within the same assay at same time; this allows for the confounding effect of several mite interaction with repellent behaviour; (2) their study used acetone for solvent; our study demonstrated that acetone repels mites and (3) the use of cut leaf discs may allow damage-related plant compounds to be released and detected by the mites, confounding the test. To our knowledge, no electrophysiological studies have been conducted on T. urticae looking at their olfactory response. With our new mite-mounting technique, we have demonstrated for the first time that female T. urticae can detect three common essential oil components through GC-ETD.
Tak and Isman, 2017 also investigated compounds with various functional groups for toxicity and repellency to T. urticae. Their results demonstrate that the most acaricidal compounds tested on bean leaves were aldehydes (trans-cinnamaldehyde), phenols (i.e., carvacrol and thymol), acids (i.e., geranic acid), and ketones (i.e., camphor), and that effective repellent compounds are alcohols (e.g., α-terpineol and borneol), aldehydes (trans-cinnamaldehyde), phenols (e.g., carvacrol, eugenol, and thymol), and acids (e.g., geranic acid) emitted by bean or cabbage plants. In our study we have also determined that aldehydes (e.g., heptanal and octanal) and acids (e.g., butyric acid and isovaleric acid) repel T. urticae females when applied on bean plants.
A different study done by Kheradmand et al. (2015) also demonstrated that T. urticae is repelled by various essential oils, including Cuminum cyminum L. (cumin), Syzgium aromaticum L. (clove), and Mentha spicata L. (spearmint) in a dose-dependent manner. Kheradmand et al. found that increasing concentrations of essential oils consistently increased repellency to T. urticae, whereas we demonstrated an optimal concentration for repellency effect. Furthermore, essential oils of Foeniculum vulgare Miller and Lavandula angustifolia Miller have shown a significant toxicity on the adult females of T. urticae in both contact and fumigant assays (Ebadollahi et al., 2015).
Vapor pressure deficit interactions between tested compounds and surface characteristics of the leaf can impact the results of behavioural studies. The chemical nature of the solvent may also influence mite response and how each mite engages with the carried test compound. We found that several solvents exert a repellent effect on T. urticae females and influences the mite’s behavioural response. We selected the optimal solvent (i.e., aqueous solution with Tween-80) that carried the active test compound(s) without altering the mite’s response.
The association between the insecticidal activities of thymol and carvacrol and the ability to block ionotropic GABA receptors has previously been documented (Melo, 2010; Rattan, 2010; Costa, 2011). Tested monoterpenes (such as eucalyptol, (-)-citronellal, limonene, alpha-pinene, pulegone, and 4-terpineol) were the most promising miticide alternatives for possible use against T. urticae due to the low doses required to produce a high mortality in both techniques of direct contact and fumigation. These compounds also displayed high acaricidal activity that may be due to their different binding modes with acetylcholinesterase and GABA-T, and they interacted with more amino acid residues (Abdelgaleil et al., 2009). As described above, previous studies (Melo, 2010; Rattan, 2010; Costa, 2011) suggest a potential acaricidal mechanism of action for the essential oil active ingredients detected by T. urticae (i.e., thymol, linalool, and eucalyptol). Future research could target other candidate compounds, investigating the detection of monoterpenes such as (-)-citronellal, limonene, alpha-pinene, pulegone, and 4-terpineol.
Previous studies suggest that Acari have only ionotropic receptors to aid in olfaction (Wanner, 2007; Wanner and Robertson, 2010; Josek et al., 2018)., and lack olfactory receptors typically found in insects. Mite electrophysiological responses revealed that the strongest responses were elicited when a carboxylic acid functional group was present. Receptors involved in the detection of carboxylic acids are likely ionotropic receptors, as these compounds have been widely reported for detection of carbonyl groups in acarines, including T. urticae (Ngoc, 2016). In addition, the number of hydrocarbons in the compound impacts sensitivity; butyric acid (4C hydrocarbons) was significantly more responsive than other organic acids tested. A previous study performed in our lab (Faraone et al., 2020) revealed that Ixodes scapularis Say (Ixodida: Ixodidae) (black-legged tick) also responds to carboxylic acids and aldehydes, which further suggests that there are specific functional groups being detected by ionotropic receptors in acarines. We developed an innovative approach to screen compounds for detected by T. urticae females, including a new mite-mounting procedure to record the electrophysiological response of mites to selected chemicals and mixtures. We designed an improved behavioural assay to evaluate the behavioural response induced by compounds detected through ionotropic receptors on the foretarsi. This approach is a valuable tool to select volatile compounds with promising repellent properties to be used for the management of T. urticae. Moreover, electrophysiological and behavioural methods developed will allow future examination of molecules with various functional groups that may affect T. urticae, which will aid in development of new products to control mites. Known ligands of ionotropic receptors are potential targets for development of mite attractants or repellents. Our evaluation of several solvents demonstrated that the chemical nature of the solvent can impact the mite response to the tested compound, and therefore the result of the induced behaviour.
Data availability statement
The original contributions presented in the study are included in the article. Further inquiries can be directed to the corresponding author.
Author contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by KG. The first draft of the manuscript was written by KG, and all other authors commented and revised previous versions of the manuscript. All authors contributed to the article and approved the submitted version.
Funding
We acknowledge the financial support of the Acadia University, the Natural Sciences and Engineering Research Council of Canada (RGPIN-2017-04319 and CRDPJ-54633-19 to NKH), and Canada Foundation for Innovation (22087 to NKH).
Acknowledgments
We would like to thank Sarah Hobbs, Jacob Ouellette and the entire INSECTA lab for their assistance and support. Additionally, we thank the K.C. Irving Environmental Science Centre for the use of growth chambers, phytotrons and research space.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Abdelgaleil S. A. M., Mohamed M. I. E., Badawy M. E. I., El-arami. S. A. A. (2009). Fumigant and contact toxicities of monoterpenes to Sitophilus oryzae (L.) and Tribolium castaneum (Herbst) and their inhibitory effects on acetylcholinesterase activity. J. Chem. Ecol. 35, 518–525. doi: 10.1007/s10886-009-9635-3
Archer T. L., Bynum E. D. (1993). Yield loss to corn from feeding by the banks grass mite and two-spotted spider mite (Acari: tetranychidae. Exp. Appl. Acarol. 17, 895–903. doi: 10.1007/BF02328066
Carr A. L., Roe M. (2016). Acarine attractants: chemoreception, bioassay, chemistry and control. Pestic Biochem. Physiol. 131, 60–79. doi: 10.1016/j.pestbp.2015.12.009
Costa B. (2011). Anxiolytic properties of a 2-phenylindolglyoxylamide TSPO ligand: stimulation of in vitro neurosteroid production affecting GABAA receptor activity. Psychoneuroendocrinology 36, 463–472. doi: 10.1016/j.psyneuen.2010.07.021
Croset V. (2010). Ancient protostome origin of chemosensory ionotropic glutamate receptors and the evolution of insect taste and olfaction. PloS Genet. 6, e1001064. doi: 10.1371/journal.pgen.1001064
Ebadollahi A., Jalali sendi J., Aliakbar A., Razmjou J. (2015). Acaricidal activities of essential oils from Satureja hortensis (L.) and Teucrium polium (L.) against the two spotted spider mite, Tetranychus urticae Koch (Acari: tetranychidae). Egyptian J. Pest control 25, 171–176.
Eliash N. (2014). Can we disrupt the sensing of honey bees by the bee parasite varroa destructor? PloS One 9, e106889. doi: 10.1371/journal.pone.0106889
Faraone N., Hillier N. K., Cutler. G. C. (2015). Plant essential oils synergize and antagonize toxicity of different conventional insecticides against Myzus persicae (Hemiptera: aphididae. PloS One 10, 0127774. doi: 10.1371/journal.pone.0127774
Faraone N., Light M., Scott C., MacPherson S., Hillier. N. K. (2020). Chemosensory and behavioural responses of Ixodes scapularis to natural products: role of chemosensory organs in volatile detection. Insects 11, 502. doi: 10.3390/insects11080502
Faraone N., MacPherson S., Hillier N. K. (2019). Behavioral responses of Ixodes scapularis tick to natural products: development of novel repellents. experimental and applied acarology. 79, 195–207. doi: 10.1007/s10493-019-00421-0
Grbic M. (2007). Mity model: Tetranychus urticae, a candidate for chelicerate model organism. Bioessays 29, 489–496. doi: 10.1002/bies.20564
Josek T., Walden K. K. O., Allan B. F., Alleyne M., Robertson. H. M. (2018). A foreleg transcriptome for Ixodes scapularis ticks: candidates for chemoreceptors and binding proteins that might be expressed in the sensory haller’s organ. Ticks Tick-borne Dis. 9, 1317–1327. doi: 10.1016/j.ttbdis.2018.05.013
Kheradmand K., Beynaghi S., Asgari S., Garjan. A. (2015). Toxicity and repellency effects of three plant essential oils against two-spotted spider mite, Tetranychus urticae (Acari: tetranychidae. J. Agric. Sci. Technology. 17, 1223–1232.
Light M., Faraone N., Shutler D., Cutler G. C., Hillier. N. K. (2021). Varroa destructor (Mesostigmata: varroidae) electrophysiological activity towards common yarrow (Asteraceae) essential oil and its components. Can. Entomol. 153, 211–221. doi: 10.4039/tce.2020.65
Light M., Shutler D., Cutler G. C., Hillier. N. K. (2020a). Electrotarsogram responses to synthetic odorants by Varroa destructor, a primary parasite of western honey bees (Apis mellifera. Exp. Appl. Acarol. 81, 515–530. doi: 10.1007/s10493-020-00525-y
Light M., Shutler D., Cutler G. C., Hillier. N. K. (2020b). Varroa destructor mite electrophysiological responses to honey bee (Apis mellifera) colony volatiles. Exp. Appl. Acarol. 81, 495–514. doi: 10.1007/s10493-020-00519-w
Meena N. K., Rampal B., Medhi R. P. (2013). Biology and seasonal abundance of the two-spotted spider mite, tetranychus urticae, on orchids and rose. Phytoparasitica 41 597–609. doi: 10.1007/s12600-013-0320-2
Melo S. A. (2010). A genetic defect in exportin-5 traps precursor microRNAs in the nucleus of cancer cells. Cancer Cell. 18, 303–315. doi: 10.1016/j.ccr.2010.09.007
Melo N., Capek M., Arenas O. M., Afify A., Yilmaz A., Potter C. J., et al. (2021). The irritant receptor TRPA1 mediates the mosquito repellent effect of catnip. Curr. Biol. 31 (9), 1988–1994. doi: 10.1016/j.cub.2021.02.010
Ngoc P. C. T. (2016). Complex evolutionary dynamics of massively expanded chemosensory receptor families in an extreme generalist chelicerate herbivore. Genome Biol. Evolution. 8, 3323–3339. doi: 10.1093/gbe/evw249
Ni L. (2021). The structure and function of ionotropic receptors in Drosophila. Front. Mol. Neurosci. 13. doi: 10.3389/fnmol.2020.638839
Park Y.-L., Lee J.-H. (2002). Leaf cell and tissue damage of cucumber caused by two-spotted spider mite (Acari: tetranychidae. J. Econ Entomol. 95, 952–957. doi: 10.1093/jee/95.5.952
Peng G. (2015). Plant-derived tick repellents activate the honey bee ectoparasitic mite TRPA1. Cell Rep. 12, 190–202. doi: 10.1016/j.celrep.2015.06.025
Rattan R. S. (2010). Mechanism of action of insecticidal secondary metabolites of plant origin. Crop Protection. 29, 913–920. doi: 10.1016/j.cropro.2010.05.008
Renthal R. (2022). “CHAPTER9 - arthropod repellents and chemosensory reception,” in Advances in arthropod repellents. Eds. Corona C., Debboun M., Coats J. (London, UK; Academic Press), 141–162.
Riga M. (2014). Abamectin is metabolized by CYP392A16, a cytochrome P450 associated with high levels of acaricide resistance in Tetranychus urticae. Insect Biochem. Mol. Biol. 46, 43–53. doi: 10.1016/j.ibmb.2014.01.006
Singh N. K. (2015). The effect of DEET on chemosensing of the honey bee and its parasite Varroa destructor. Apidologie 46, 380–391. doi: 10.1007/s13592-014-0330-1
Tak J.-H., Isman M. (2017). Acaricidal and repellent activity of plant essential oil-derived terpenes and the effect of binary mixtures against Tetranychus urticae Koch (Acari: tetranychidae. Ind. Crops Products. 108, 786–792. doi: 10.1016/j.indcrop.2017.08.003
Tripathi A. K., Upadhyay S., Bhuiyan M., Bhattacharya. P. R. (2009). A review on prospects of essential oils as biopesticide in insect-pest management. Pharmacog. Phytother. 1, 12.
Wang L., Zhang Y., Xie W., Wu Q., Wang. S. (2015). A bioassay for evaluation of the resistance of Tetranychus urticae (Acari: tetranychidae) to selected acaricides. saaa 20, 579–590. doi: 10.11158/saa.20.6.1
Wanner K. W. (2007). A honey bee odorant receptor for the queen substance 9-oxo-2-decenoic acid. PNAS 104, 14383–14388. doi: 10.1073/pnas.0705459104
Wanner K., Robertson H. (2010). Lepidopteran chemoreceptors in molecular biology and genetics of the Lepidoptera (Boca Raton, Fl, USA; CRC Press).
Keywords: behavioural response, carboxylic acids, electrotarsography, essential oils, twospotted spider mite
Citation: Gaudet K, Faraone N and Hillier NK (2023) Investigating chemoreception and behavioural responses of Tetranychus urticae (Trombidiformes: Tetranychidae) to organic acids, aldehydes and essential oil components. Front. Agron. 5:1212705. doi: 10.3389/fagro.2023.1212705
Received: 26 April 2023; Accepted: 14 June 2023;
Published: 04 July 2023.
Edited by:
Jeremy Dean Allison, Canadian Forest Service, CanadaReviewed by:
Alex Mangini, United States Department of Agriculture (USDA), United StatesGerhard Johannes Gries, Simon Fraser University, Canada
Copyright © 2023 Gaudet, Faraone and Hillier. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Nicoletta Faraone, bmljb2xldHRhLmZhcmFvbmVAYWNhZGlhdS5jYQ==
 Kayla Gaudet
Kayla Gaudet Nicoletta Faraone
Nicoletta Faraone Neil Kirk Hillier
Neil Kirk Hillier