- 1Department of Science, Vidyadayini Institute of Science, Management and Technology, Sajjan Singh Nagar, Bhopal, India
- 2Department of Microbiology, College of Basic Sciences and Humanities, G.B. Pant University of Agriculture & Technology, Pantnagar, India
- 3Department of Soil Science, College of Agriculture, G.B. Pant University of Agriculture & Technology, Pantnagar, India
- 4Department of Agronomy, College of Agriculture, G.B. Pant University of Agriculture & Technology, Pantnagar, India
- 5Institute of Applied Sciences & Humanities, GLA University, Mathura, India
The poor agriculture practices, fragmented land holdings, fluctuating climatic conditions, and minimal external inputs lead to nutrient deficiency in the Himalayan agroecosystems. Because of the risks associated with chemical fertilizers, their implication is a big question mark. Therefore, two previously characterized plant growth-promoting rhizobacteria Pseudomonas jesenii MP1 and Pseudomonas palleroniana N26 were employed to enhance kidney bean productivity and soil health at farmer’s fields of Harsil and Chakrata regions of Uttarakhand Himalayas. The study revealed that MP1 and N26 treatment resulted in 25.62% and 37.23% higher grain yield than respective uninoculated controls at the trial fields of Harsil and Chakrata regions, respectively. Further, the bacterial treatments have significantly increased nitrogen, phosphorus, and potassium levels in the soils. The soil diversity analysis revealed the dominance of Proteobacteria and Actinobacteria at Harsil and Chakrata, respectively. Further, the MP1 treatment had increased Firmicutes percentage over uninoculated control at both locations. Conclusively, the application of cold adaptive Pseudomonas jesenii MP1 and Pseudomonas palleroniana N26 improved the grain yield and soil health status of the Himalayan agroecosystems. Therefore, they can be explored as an eco-friendly alternative for the commercial production of kidney beans.
Introduction
Agriculture is a prodigious private enterprise in India and will continue to be the lifeline of the Indian economy in the future. It is estimated that overall food demand will rise in proportion to the world population. The global population is continuously increasing at the rate of above 1.8 percent annually and it will reach the point from today’s calculated 7.4 billion to an anticipated demographical data of 9.6 billion by 2050 (United Nations, 2013). Current agricultural practices are neither economically nor environmentally sustainable. After the green revolution, chemical-based fertilizers and pesticides have enormously boosted agricultural production. However, their indiscriminate use, besides imposing a detrimental effect on the atmosphere has developed resistance in insects against common pesticides. The fertilizer consumption in India is increasing day by day and has reached 133 Kg ha-1 in the year 2019-20 in comparison to 94 Kg ha-1 in 2004-2005 (Agricultural Statistics, 2019). These trends can’t be neglected because they will lead to serious agricultural, environmental, and health issues.
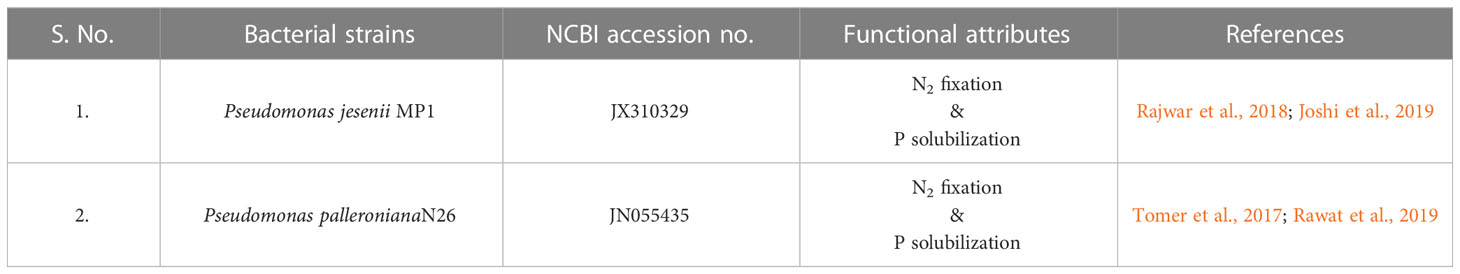
Kidney bean is a high-value cash crop in the Indian Himalayan regions. The local cultivar is known for its premium quality, unique taste, and nutritional value. However, due to nutritional deficiency, habitat degradation, and anthropogenic contamination, the commercialization of kidney beans could not be done properly. Therefore, an eco-friendly and cost-effective farming approach is urgently needed to enhance kidney bean productivity and achieve sustainable agriculture goals. In this regard, plant growth-promoting rhizobacteria (PGPR) seem better alternatives than others (Sahu et al., 2021). As compared to chemical fertilizers, they are safer with reduced environmental damage, have more targeted activity, and are effective in smaller quantities. Therefore, the present study employs previously characterized cold adaptive PGPR strains for the production of Phaseolus vulgaris (Kidney bean or Rajmash). These strains have already been tested on several pulse crops under net-house and natural field conditions (Suyal et al., 2017; Rajwar et al., 2018; Joshi et al., 2019; Rawat et al., 2019). Therefore, taking a step forward they were applied in the farmers’ fields at two different locations in Uttarakhand Himalaya. This study discusses the impact of the above-mentioned bioinoculants on the Rajmash crop yield and soil bacterial diversity during the first year of field trials. Moreover, the participation of the local farmers in the study has motivated them for adopting this technology. Therefore, besides promoting sustainable agriculture, this study will contribute to the socio-economic upliftment of the local marginal farmers. Although, consecutive studies are recommended to increase the efficiency and persistence of these bio-formulations under natural field conditions.
Materials and methods
Bacterial strain and growth conditions
Cold adaptive bacterial strains P. jesenii MP1 and P. palleroniana N26 were obtained from the culture collection of Department of Microbiology, GBPUAT Pantnagar, Uttarakhand [Table 1 near here]. Both the strains have already been characterized for their nitrogen fixation as well as phosphate-solubilizing potential (Suyal et al., 2017; Tomer et al., 2017; Rajwar et al., 2018; Joshi et al., 2019; Rawat et al., 2019). The strains were grown aerobically in a nutrient agar medium at 28 ± 1°C during the experiment.
Experimental sites and field trials
A field experiment was performed on farmer’s fields in the Indian Central Himalayan region at Harsil (31.0383˚N, 78.7377˚E) and Chakrata (30.7016˚N, 77.8696˚E) villages of Uttarakhand, India during 2019. In order to conduct the experiment in a such hilly region where agriculture fields are fragmented and terraced, a farmer’s field with a dimension area equal to or higher than 150m2 was selected at both sites. Afterward, potential bioinoculants containing bio-formulations (1x109 CFU g-1) were prepared. At the time of sowing, bioformulation was mixed in the Jaggrey solution subsequently kidney bean seeds were treated at the rate of 10 g Kg-1 seeds (Germination efficiency- >86%) followed by shade drying for 30 min. Subsequently, seeds were sown in the fields at both Harsil and Chakrata locations. Apart from this untreated seeds sown in the fields served as uninoculated farmers’ practices or control. With respect to each treated field, a control was set up. Both the sites belong to a temperate zone with an average temperature of 8-29˚C from May to September. A total of 3 treatments (P. jesenii MP1, P. palleroniana N26, and uninoculated controls) in each site with triplicates were laid down.
A total of six treatments comprising P. jesenii MP1, P. palleroniana N26, and uninoculated controls in both Harsil and Chakrata soils were laid out in triplicates.
Seed bacterization and sowing
The seeds of the local variety of Rajmah were surface sterilized by soaking in 0.1% HgCl2 for 2 min and then thoroughly washed with autoclaved distilled water. Later, the seeds were mixed with 0.1% charcoal and overnight grown culture having 109 CFU mL-1. The seeds were then dried for 1 h followed by the sowing in respective fields with plant to plant distance of 10 cm and row to row distance of 15 cm.
Soil sampling, soil nutrient analysis, and crop yield
The soil samples were collected before sowing and after harvesting the crop by using a sterilized trowel at a depth of 0-20cm. All the soil samples were collected in triplicates within sterilized polybags and transported to the laboratory under cold and sterile conditions. Soil samples were analyzed for available nitrogen (Subbiah and Asija, 1956), phosphorous (Olsen, 1954), and potassium (Jackson, 1973) content. Moreover, the crop yields were analyzed to compare the treatments with the respective controls.
Soil DNA extraction and quantification of gene copy numbers
Rhizospheric soil composite was subjected to total DNA extraction through Mo Bio’s Power Soil DNA isolation kit. The total genomic DNA present in the soil was extracted from 0.5gm composite as per the manufacturer’s protocol. The quantity and quality of extracted DNA were checked through NanoDrop at 260nm, following storage at -80˚C till further analysis. Copy numbers of 16S rRNA were quantified using iCycleriQ™ Multicolor (Bio-Rad Lab, Hercules, USA) qPCR machine as per the earlier reports (Suyal et al., 2015; Tomer et al., 2017; Rajwar et al., 2018; Suyal et al., 2019).
Metagenome sequencing and Bioinformatic analysis
The bacterial diversity was determined by sequencing V3-V4 segments using Illumina Hiseq sequencing machine along with Agilent Technologies 2100 Bioanalyzer. The quality of the raw sequences was analyzed through FastQC (v0.11.7) and TrimGalore (v0.5.0). Further, the QIIME software package (v. 1.9.0) was used for removing singletons, assigning operational taxonomy units (OTU) to the remaining sequences, and preparation of the Venn diagram (Kumar et al., 2019; Suyal et al., 2021a). The Rarefaction curve and diversity indices were analyzed by using Mothur (v.1.21.1) software.
Statistical analysis
The results were statistically analyzed through one-way ANOVA using R 3.6.1 at P<0.005 level of significance.
Results
Agronomical parameters and grain yield
The relative performance of cold adaptive bacteria i.e., P. jesenii MP1 and P. palleroniana N26 over altitudinal variation i.e. Chakrata and Harsil was established through field trial experiment. Overall results of the experiment demonstrated the positive consequence of both bacteria on agronomical and associated attributes of kidney beans over uninoculated control. At the Harsil site, the effect of P. jesenii MP1 on agronomical parameters such as root, shoot, and pod length, no. of gains per pod were found prominent. Similarly, at the Chakarta site, all agronomical parameters were highest positively influenced by P. jesenii MP1 [Table 2 near here]. Both the bacterial strains showed a significant increase in grain yield over respective controls at each experimental site. At Harsil, the maximum percent average grain yield was observed in the seeds inoculated with Pseudomonas jesenii MP1 (25.62% higher than the uninoculated control). Further, treatment of N26 had improved by 19.28% higher grain yield over the control. Contrary to it, at Chakrata, N26 treatment had provided maximum grain yield (37.23% higher than uninoculated control) followed by MP1 (11.70%). It indicates remarkable growth promotion potential of P. jesenii MP1 and P. palleroniana N26 at higher altitudes (Table 2).
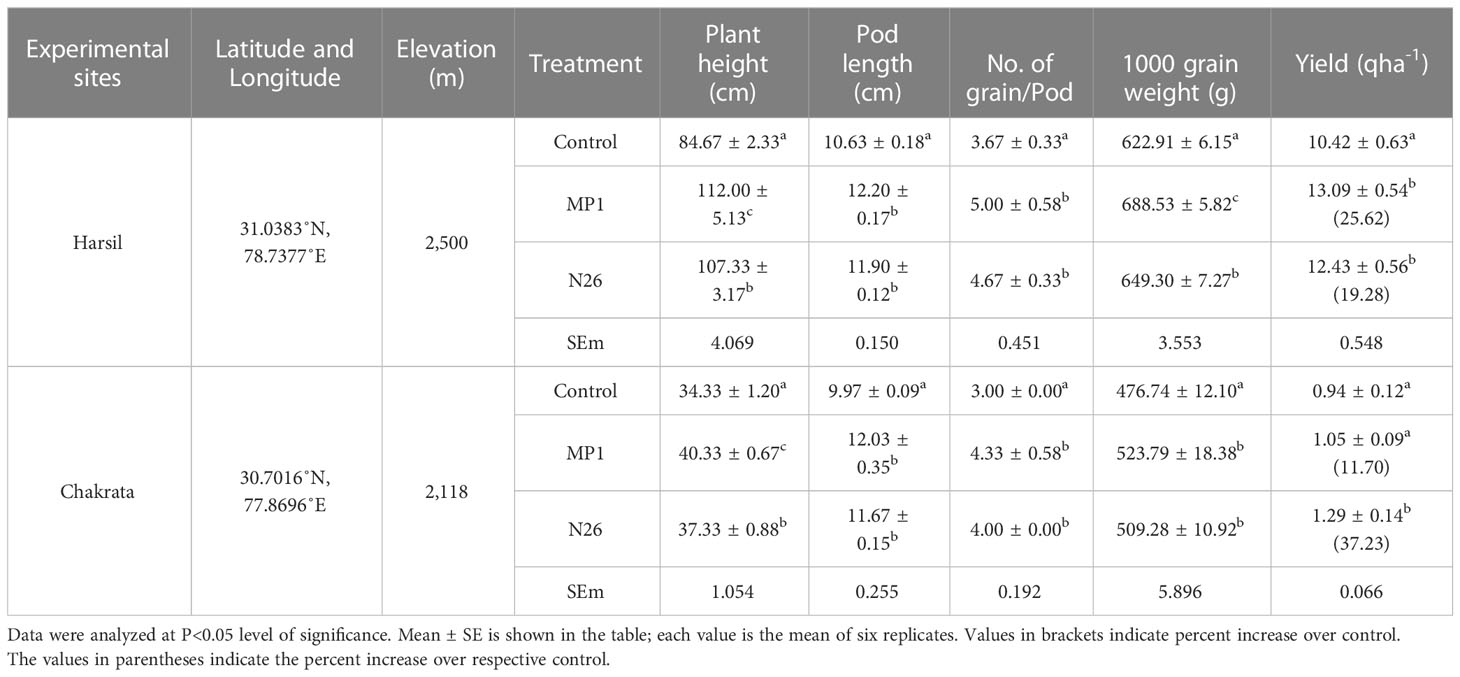
Table 2 Effect of Pseudomonas jesenii MP1 and Pseudomonas palleroniana N26 on grain yield of Kidney bean.
Soil nutrient status
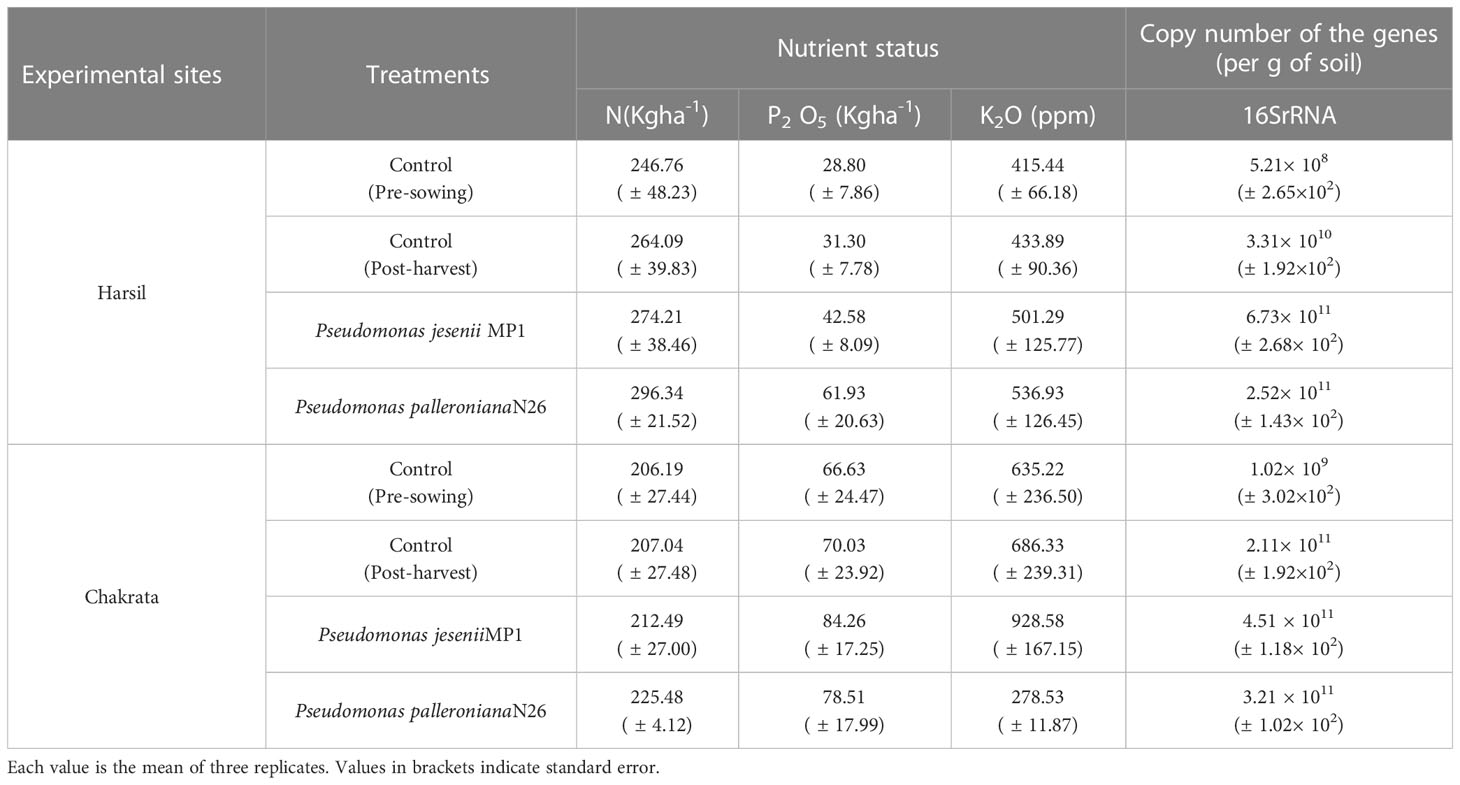
Under pre-sowing conditions, the soil nitrogen content of Harsil and Chakrata was 246.76 ± 48.23 kg ha-1 and 206.19 ± 27.44 kg ha-1, respectively [Table 3 near here]. After harvesting it became 264.09 ± 39.83 kg ha-1 (Harsil) and 207.04 ± 27.48 kg ha-1 (Chakrata). Further, the application of Pseudomonas jesenii MP1 improved it to 274.21 ± 38.46 kg ha-1 and 212.49 ± 27.00 kg ha-1 at Harsil and Chakrata, respectively. Similarly, Pseudomonas palleroniana N26 also enhanced soil nitrogen content to 296.34 ± 21.52 kg ha-1 and 225.48 ± 4.12 kg ha-1 at Harsil and Chakrata, respectively.
In the case of Phosphorus, Harsil, and Chakrata had 28.80 ( ± 7.86)kg ha-1 and 66.63 ( ± 24.47) kg ha-1 P2O5 in pre-sowing soils. After harvesting, it was found 31.30 ( ± 7.78) kg ha-1, and 70.03 ( ± 23.92) kg ha-1, respectively. When treated to the MP1, it reached 42.58 ( ± 8.09) kg ha-1 and 84.26 ( ± 17.25) kg ha-1 in Harsil and Chakrata, respectively. Furthermore, N26 treatment had improved it to 61.93 ± 20.63 and 78.51 ± 17.99, respectively.
Similarly, potassium content was 415.44 ± 66.18 ppm (Harsil) and 635.22 ± 236.50 ppm (Chakrata) in pre-sowing soils and 433.89 ± 90.36 ppm (Harsil) and 686.33 ± 239.31 ppm (Chakrata) in post-harvested soils. After treatment with MP1 and N26, it reached 501.29 ± 125.77 ppm and 536.93 ± 126.45 at Harsil and 928.58 ± 167.15 and 278.53 ± 11.87 ppm at Chakrata.
Bacterial diversity composition
The 16S rRNA gene abundance was maximum in MP1-treated soils at both sites followed by N26-treated soils with respect to the controls (Table 3). Based on the preliminary results obtained through gene abundances and grain yield, MP1-treated soils were selected for metagenome sequencing. Thus, four soil metagenomes viz. Uninoculated post-harvest soil of Harsil (HRC), Uninoculated post-harvest soil of Chakrata (CHC), MP1-treated soil of Harsil (HRM), and MP1-treated soil of Chakrata (CHM) was sequenced and analyzed. A sum of 31, 04,626.00 purified 16S rRNA amplicon sequences were retrieved from the sequencing. Filtered sequences were allocated to 85041 OTUs. A Venn diagram was plotted to find out the distribution of unique and shared bacterial species among the groups [Figure 1 near here]. The number of species groups present in CHC, CHM, HRC, and HRM is 38559, 35768, 36437, and 34644 respectively. The number of OTUs shared between CHC and CHM was 13231, while the common number of OTUs present between HRC and HRM was 11202. Control groups of both sites shared 13633 OTUs and treated groups had 11724 common OTUs. The total number of OTUs shared by all four groups was only 4318.
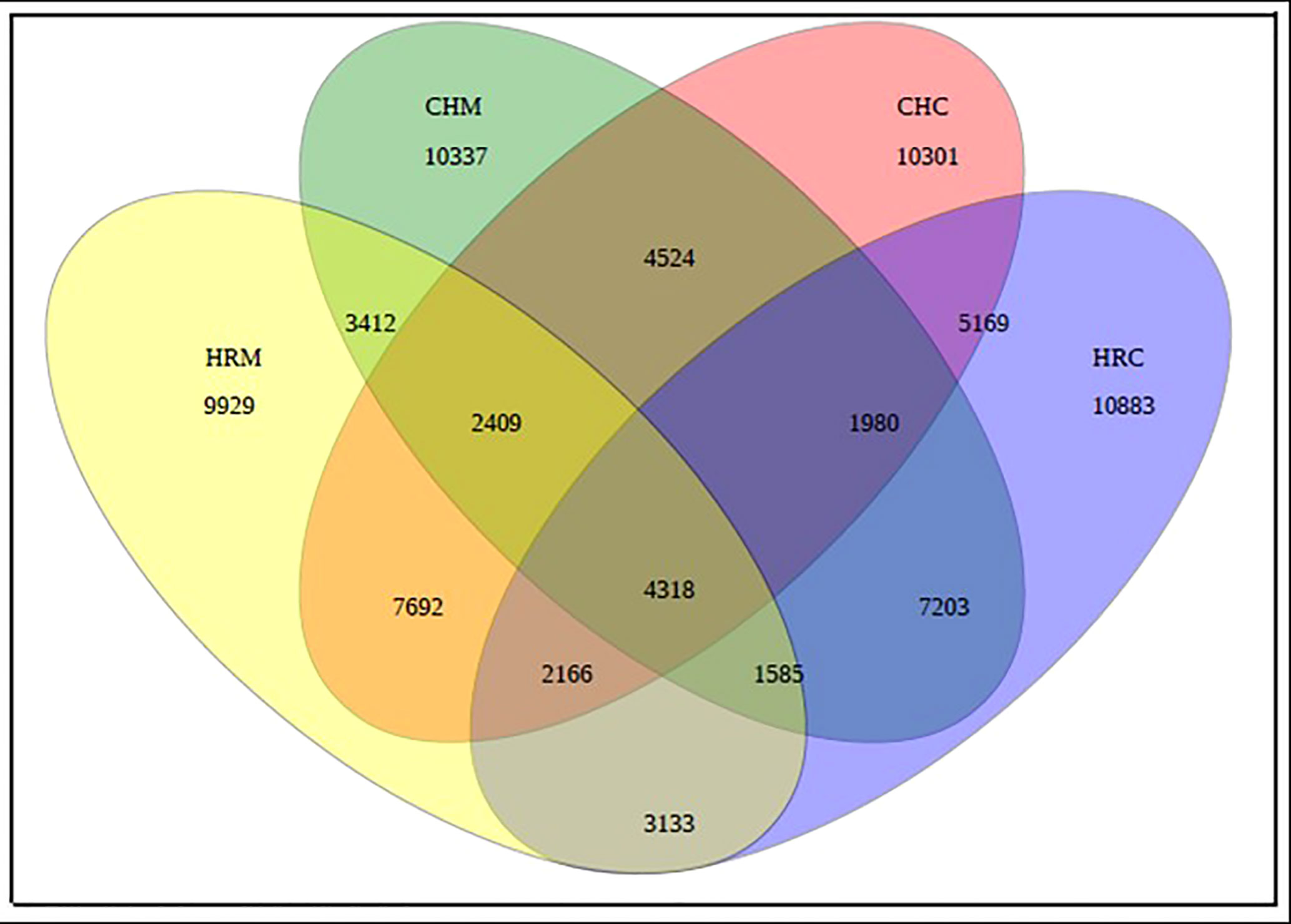
Figure 1 Venn diagram showing the total number of OTUs observed in the soil metagenomes. Where, CHC, CHM, HRC, and HRM represent the Uninoculated post-harvest soil of Chakrata, MP1 treated soil of Chakrata (CHM), Uninoculated post-harvest soil of Harsil, and MP1 treated soil of Harsil, respectively.
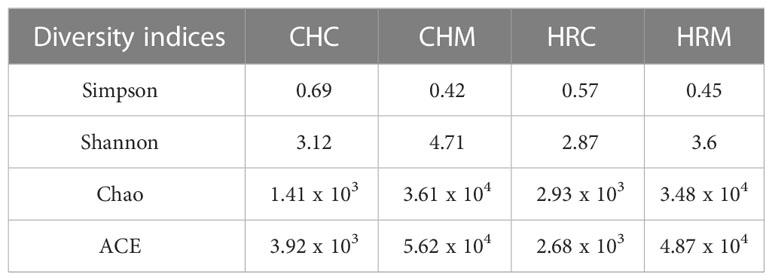
The diversity indices revealed the lower value of Simpson in bioinoculant treated soils (0.42 and 0.45 in CHM and HRM, respectively) in comparison to their respective controls (0.69 and 0.57, respectively) (Table 4 near here). Further, Shanaon values were higher in CHM (4.71) and HRM (3.6) than in respective controls i.e. CHC (3.12) and HRC (2.87). Similarly, species richness estimates- Chao and ACE were higher in CHM (3.61 x 104 and 5.62 x 104, respectively) and HRM (3.48 x 104 and 4.87 x 104, respectively) than in CHC (1.41 x 103 and 3.92 x 103, respectively) and HRC (2.93 x 103 and 2.68 x 104, respectively).
Taxonomic distribution and effect of treatment on soil diversity
Chakrata soil
The major phyla in CHC soil belonged to Actinobacteria (23%), Proteobacteria (21%), Cyanobacteria (14%), Acidobacteria (10%), and Planctomycetes (8%). While, dominating phyla of CHM soil were Actinobacteria (27%), Proteobacteria (23%), Firmicutes (20%), Acidobacteria (8%), and Planctomycetes (7%) [Figure 2 near here]. Further, CHC and CHM metagenomes had shown 8% and 6% of assigned OTUs, respectively. At the family level, Bacillaceae dominated in CHM (9%) in comparison to CHC (2%). The other major family was Hyphomicrobiaceae having 4% and 5% of OTUs in CHC and CHM, respectively. Furthermore, the dominating genera in CHC and CHM were Rhodoplanes(3%) and Bacillus (9%), respectively [Figure 3 near here].
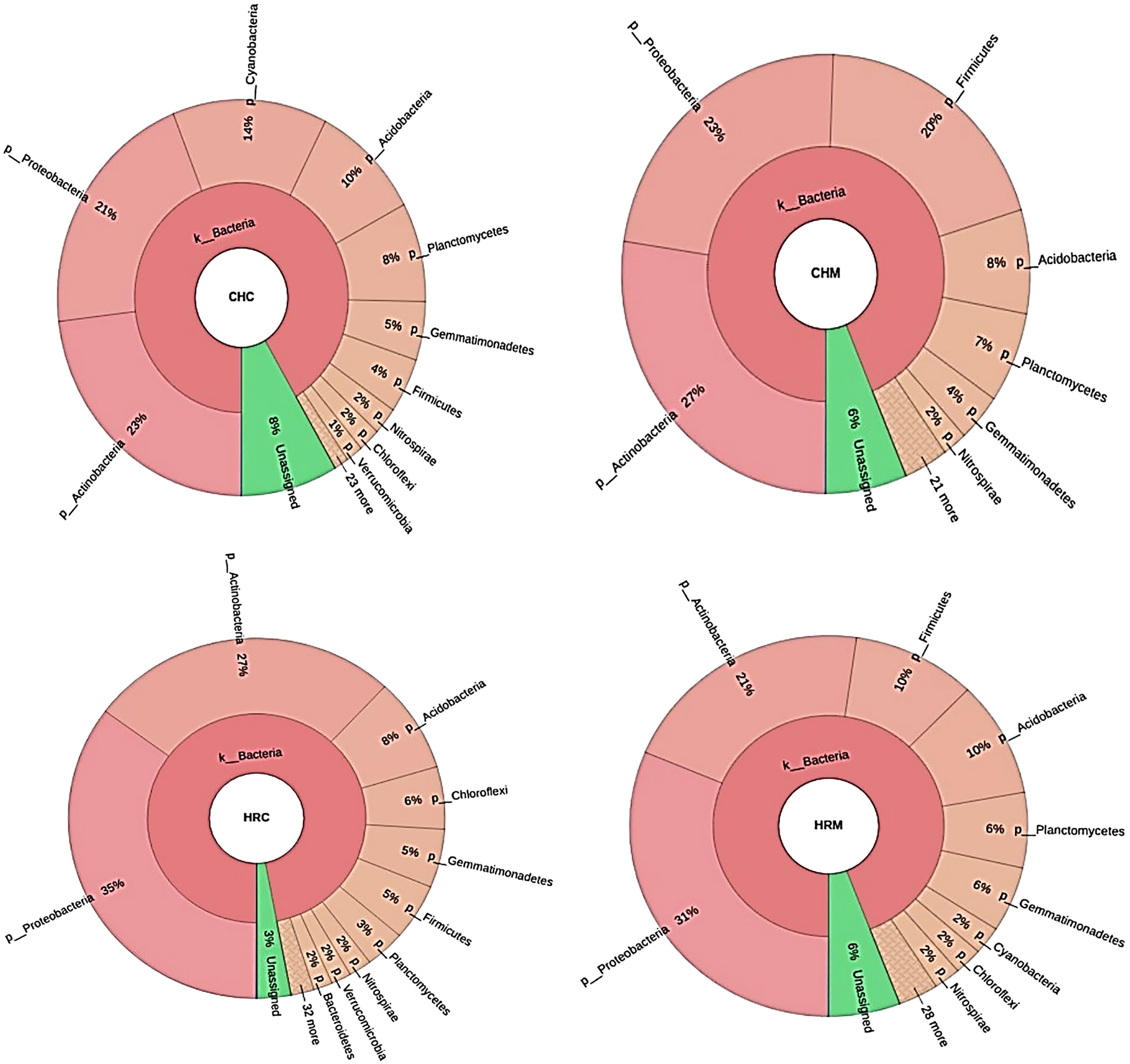
Figure 2 Distribution of the phylum among the soil metagenomes. Where, CHC, CHM, HRC, and HRM represent the Uninoculated post-harvest soil of Chakrata, MP1 treated soil of Chakrata (CHM), Uninoculated post-harvest soil of Harsil, and MP1 treated soil of Harsil, respectively.
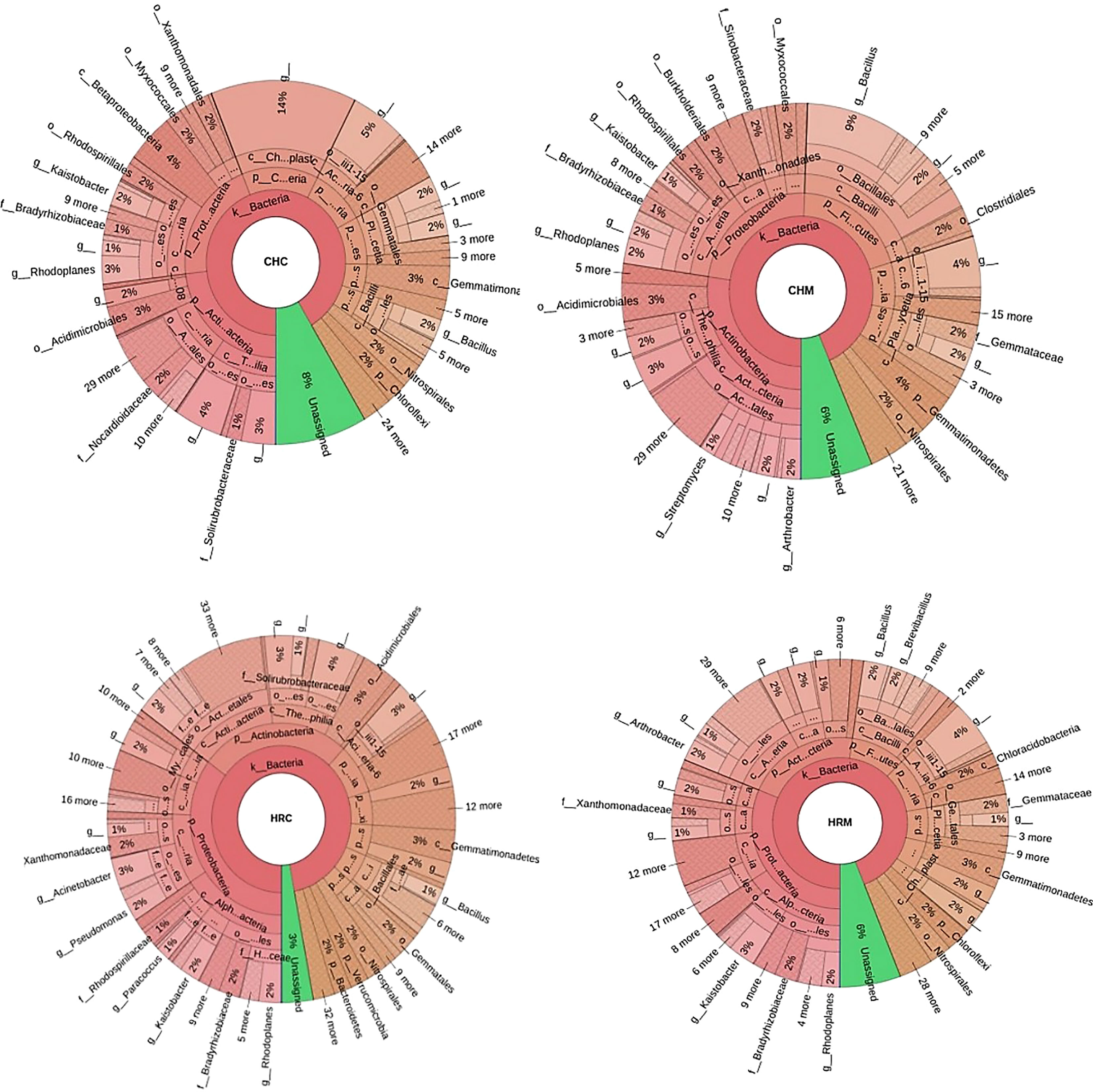
Figure 3 The abundance of the genera among the soil metagenomes. Where, CHC, CHM, HRC, and HRM represent the Uninoculated post-harvest soil of Chakrata, MP1 treated soil of Chakrata (CHM), Uninoculated post-harvest soil of Harsil, and MP1 treated soil of Harsil, respectively.
Harsil soil
Proteobacteria (35% and 31%) was the major phylum in HRC and HRM followed by Actinobacteria (27% and 21%), respectively (Figure 2). Other abundant phyla in HRM are Acidobacteria (10%), Firmicutes (10%), Planctomycetes (6%), and Gemmatimonadetes (6%); while HRC comprised of OTUs assigned with Acidobacteria (8%), Chloroflexi (6%), Gemmatimonadetes (5%) and Firmicutes (5%). Moreover, both had 6% and 3% assigned OTUs, respectively. More specifically, the dominant members in HRC and HRM were from Nocardioidaceae and Sphingomonadaceae families, respectively. The most abundant genus (2% in both) was Rhizoplane in both soils. The other abundant genus was Bacillus in both HRM (2%) and HRC (1%)(Figure 3).
Discussion
The cold adaptive Pseudomonas jesenii MP1 and Pseudomonas palleroniana N26 were originally isolated from the Himalayan soils and have already been tested in several pulse crops (Tomer et al., 2017; Rajwar et al., 2018; Joshi et al., 2019; Rawat et al., 2019). Both potential bacterial isolates i.e., Pseudomonas jesenii MP1 and Pseudomonas palleroniana N26 initially isolate from kidney bean rhizosphere and further their plant nutrient solubilizing potential under in vitro conditions on various mediums were explored by Tomer et al. (2017). Further, Tomer et al. (2017) and Rawat et al. (2019) determined bacterial seed germination and yield-enhancing potential through in vitro and greenhouse experiments. Afterward, Rajwar et al. (2018) and Joshi et al. (2019) documented the nutrient solubilizing potential and thereby crop growth and yield-improving potential of Pseudomonas jesenii MP1 for Chickpea under field conditions at the Crop research center, Pantnagar. Apart from this, Khan et al. (2023) also documented the yield, soil health, and nutrient biofortifying ability of both bacterial isolates under field conditions. Both bacterial isolates have significantly established their crop-improving potential therefore in the present study, they were directly applied to the farmer’s field to evaluate their real-time performance.
Nutrient status is the well-recognized health indicator of the soils. The present study revealed that inoculation of Pseudomonas jesenii MP1 and Pseudomonas palleroniana N26 significantly improved the nitrogen and phosphorus content in the soils. Moreover, a remarkable difference was observed in the case of potassium content which was found higher in bioinoculant-treated soils at both Harsil and Chakrata (23% and 20% higher, respectively). These results indicate that the unavailable nutrients of the soils were made available to the plants by the bio-inoculants. Further, it is also evident from the grain yield data, that showed MP1 and N26 treatment had improved 25.62 and 19.28% higher crop yield at Harsil and 11.70 and 37.23% higher yield at Chakrata over their respective controls. It shows that bioinoculants were able to sustain and perform better at higher altitudes. Recently, our group analyzed the agronomical parameters and soil microbial diversity for the second consecutive cropping year too of the present study (Khan et al., 2023). They have observed similar results in which MP1 and N26 have enhanced approx 23% of grain yield during the second consecutive year of cropping. Moreover, a significant increase in zinc and protein content has also been reported by them.
The metagenome sequencing revealed that both soils were rich in bacterial diversity as revealed through the presence of diversified taxa. It can be evident from the values of diversity indices too. Further, a higher Shannon index indicates additional unique species (Shannon, 1948) and a lower Simpson index indicates heterogeneous ecosystems (Simpson, 1949). Similarly, higher values of species richness indicate higher diversity in a given system. Therefore, in the present study, bioinoculant-treated soils have higher bacterial diversity with respect to their respective untreated controls. A large number of sequences were unassigned OTUs in each of the samples which confirms the existence of novel microbes and suggests the need for culture-dependent studies in the future. The metagenome analysis showed the dominance of phylum Proteobacteria at Harsil while phylum Actinobacteria at Chakrata. These results are in agreement with the previous studies that showed that higher altitude soils are dominated by proteobacteria, actinobacteria, and acidobacteria (Lugtenberg and Kamilova, 2009; Dai et al., 2018; Kumar et al., 2019). In addition, it has been observed that MP1 treatment altered the microbial diversity with an increment in Firmicutes percentage over uninoculated control at both locations. Previous studies have positively correlated the proteobacterial and firmicute abundance with higher nutrient mobilization and improved soil fertility(Pattnaik et al., 2021; Suyal et al., 2021a). Further, in accordance with the present study, cold adaptive Cyanobacteria, Acidobacteria, Planctomycetes, Gemmatimonadetes, Firmicutes, and Nitrospirae phylum were also reported from higher altitude agroecosystems (Suyal et al., 2015; Suyal et al., 2021b).
Conclusion
The present study revealed that the bioinoculant’s application has improved soil health and kidney bean production in the Himalayan farmer’s field. Besides promoting organic farming among marginal farmers, the study will help in the commercial production of the crop. Conclusively, Pseudomonas jesenii MP1 and Pseudomonas palleroniana N26 are strongly recommended to attain agro-environmental sustainability in the Himalayas and the socio-economic development of the associated farmers.
Data availability statement
The metagenomic data presented in this article has been submitted to the NCBI database under the accession number PRJNA607339.
Author contributions
DS: Experimental work, analysis of the results. AK: Experimental work, analysis of the results. AVS: Conceptualization, field arrangements. AA: Analysis of the results. NP: Conceptualization, field arrangements. VS: Conceptualization, field arrangements. RG: Conceptualization, manuscript editing. All authors contributed to the article and approved the submitted version.
Funding
This work was funded by the National Mission on Himalayan Studies (NMHS) grant no. GBPNI/NMHS-2019-20/MG to RG. However, this project did not have any funds received for open-access publication fees. Moreover, we did not receive any such grant from our institution or any other funding agency too.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Agricultural Statistics (2019) Government of India, ministry of agriculture & farmers welfare, department of agriculture, cooperation & farmers welfare, directorate of economics and statistics (Accessed 01/12/2022).
Dai B., Chen C., Long Y., Zheng L., Zhao H., Bai X., et al. (2018). Neural mechanisms for selectively tuning in to the target speaker in a naturalistic noisy situation. Nat.Commun 9, 2405. doi: 10.1038/s41467-018-04819-z
Joshi D., Chandra R., Suyal D. C., Kumar S. (2019). Impacts of bioinoculants Pseudomonas jesenii MP1 and Rhodococcusqingshengii S10107 on chickpea (Cicer arietinum l.) yield and soil nitrogen status. Pedosphere 29 (3), 388–399.
Khan A., Singh A. V., Pareek N., Arya P., Upadhayay V. K., Kumar Jugran A., et al. (2023). Credibility assessment of cold adaptive Pseudomonas jesenni MP1 and p. palleroniana N26 on growth, rhizosphere dynamics, nutrient status, and yield of the kidney bean cultivated in Indian central himalaya. Front. Plant Sci. 14. doi: 10.3389/fpls.2023.1042053
Kumar S., Suyal D. C., Yadav A., Shouche Y., Goel R. (2019). Microbial diversity and soil physiochemical characteristic of higher altitude. PloS One 14 (3), e0213844. doi: 10.1371/journal.pone.0213844
Lugtenberg B., Kamilova F. (2009). Plant-growth-promoting rhizobacteria. Ann. Rev.Microbiol. 63, 541–556. doi: 10.1146/annurev.micro.62.081307.162918
Olsen S. R. (1954). Estimation of available phosphorus in soils by extraction with sodium bicarbonate (Washington, D.C: Circular (United States. Department of Agriculture), no. 939. US Department of Agriculture).
Pattnaik S., Mohapatra B., Gupta A. (2021). Plant growth-promoting microbe mediated uptake of essential nutrients (Fe, p, K) for crop stress management: microbe–Soil–Plant continuum. Front.Agron 3, 689972. doi: 10.3389/fagro.2021.689972
Rajwar J., Chandra R., Suyal D. C., Tomer S., Kumar S., Goel R. (2018). Comparative phosphate solubilizing efficiency of psychrotolerantPseudomonas jesenii MP1 and acinetobacter sp. ST02 against chickpea for sustainable hill agriculture. Biologia 73 (8), 793–802.
Rawat N., Sharma M., Suyal D. C., Singh D. K., Joshi D., Singh P., et al. (2019). Psyhcrotolerant bio-inoculants and their co-inoculation to improve Cicer arietinum growth and soil nutrient status for sustainable mountain agriculture. J. Soil Sci. Plant Nutr. 19 (3), 639–647. doi: 10.1007/s42729-019-00064-5
Sahu B., Suyal D. C., Prasad P., Kumar V., Singh A. K., Kushwaha S., et al. (2020). Microbial Diversity of Chickpea Rhizosphere, In. Sharma S. K., Singh U. B., Sahu P. K.. (eds) Rhizosphere Microbes. Microorganisms for Sustainability, (Singapore: Springer Nature) 23, 483–502. doi: 10.1007/978-981-15-9154-9_20
Shannon C. (1948). A mathematical theory of communication. Bell. Syst. Tech. J. 27, 379–423. doi: 10.1002/j.1538-7305.1948.tb01338.x
Subbiah B., Asija G. L. (1956). Alkaline permanganate method of available nitrogen determination. Curr. Sci. 25, 259.
Suyal D. C., Joshi D., Kumar S., Bhatt P., Narayan A., Giri K., et al. (2021b). Himalayan Microbiomes for agro-environmental sustainability: current perspectives and future challenges. Microb. Ecol. 84 (3), 643–675. doi: 10.1007/s00248-021-01849-x
Suyal D. C., Kumar S., Joshi D., Yadav A., Shouche Y., Goel R. (2019). Comparative overview of red kidney bean (Phaseolus valgaris) rhizospheric bacterial diversity in perspective of altitudinal variations. Biologia 74 (10), 1405–1413. doi: 10.2478/s11756-019-00292-1
Suyal D. C., Kumar S., Yadav A., Shouche Y., Goel R. (2017). Cold stress and nitrogen deficiency affected protein expression of psychrotrophicDyadobacterpsychrophilus B2 and Pseudomonas jessenii MP1. Front.Microbiol 8, 430.
Suyal D. C., Soni R., Singh D. K., Goel R. (2021a). Microbiome change of agricultural soil under organic farming practices. Biologia 76 (4), 1315–1325. doi: 10.2478/s11756-021-00680-6
Suyal D. C., Yadav A., Shouche Y., Goel R. (2015). Bacterial diversity and community structure of Western Indian Himalayan red kidney bean (Phaseolus vulgaris l.) rhizosphere as revealed by 16S rRNA gene sequences. Biologia 70/3, 305—313.
Tomer S., Suyal D. C., Rajwar J., Yadav A., Shouche Y., Goel R. (2017). Isolation and characterization of phosphate solubilizing bacteria from Western Indian Himalayan soils. 3Biotech 7 (2), 1–8. doi: 10.1007/s13205-017-0738-1
Keywords: community structure, soil metagenome, cold adaptive microorganisms, kidney bean, bioinoculants
Citation: Suyal DC, Khan A, Singh AV, Agarwal A, Pareek N, Sah VK and Goel R (2023) Impact assessment of cold-adapted Pseudomonas jesenii MP1 and Pseudomonas palleroniana N26 on Phaseolus vulgaris yield and soil health. Front. Agron. 5:1121757. doi: 10.3389/fagro.2023.1121757
Received: 12 December 2022; Accepted: 19 May 2023;
Published: 31 May 2023.
Edited by:
Kanika Khanna, Guru Nanak Dev University, IndiaReviewed by:
Ishwar Prakash Sharma, Patanjali Research Foundation, IndiaKrishna Giri, Indian Council of Forestry Research and Education (ICFRE), India
Damini Maithani, IFTM University, India
Copyright © 2023 Suyal, Khan, Singh, Agarwal, Pareek, Sah and Goel. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Reeta Goel, cmVldGEuZ29lbEBnbGEuYWMuaW4=
†These authors have contributed equally to this work and share first authorship
 Deep Chandra Suyal
Deep Chandra Suyal Amir Khan2†
Amir Khan2† Ajay Veer Singh
Ajay Veer Singh Navneet Pareek
Navneet Pareek Reeta Goel
Reeta Goel