- 1Chair of Plant Health, Institute of Agricultural and Environmental Sciences, Estonian University of Life Sciences, Tartu, Estonia
- 2Department of Plants and Crops, Faculty of Bioscience Engineering, Ghent University, Ghent, Belgium
Plant essential oils are being increasingly studied as a potential environmentally friendly alternative to synthetic insecticides. The insecticidal efficacy of essential oils on the cabbage seedpod weevil (Ceutorhynchus obstrictus), an important oilseed rape pest, has not been previously tested. We examined the impact of six essential oils on C. obstrictus via contact with dry residues on leaf and flower surfaces. We also examined the effect of these essential oils on a model non-target parasitoid wasp, Nasonia vitripennis. Exposure to dry residues of cumin (Cuminum cyminum) and cinnamon (Cinnamomum verum) essential oils (applied to oilseed rape leaves) resulted in significant loss of mortality and immobility in C. obstrictus adults. Treatment with C. cyminum essential oil at 1.5% resulted in 50.71% mortality and 87.3% combined mortality and immobility in C. obstrictus. Cinnamomum verum oil, at 1.5% concentration, resulted in 88.8% mortality and immobility among C. obstrictus 24 h post-treatment. All treatments studied with essential oil dry residues at 0.3% concentration caused high mortality and immobility in N. vitripennis. The greatest mortality and immobility were observed at 0.3% concentration in F. vulgare and C. verum treatments (54 and 53% loss respectively). At 0.1% concentration, F. vulgare and T. vulgaris significantly reduced parasitoids mobility and at 1.5% concentration all essential oils resulted in 100% mortality of N. vitripennis after 3 h. Our study revealed that C. cyminum and C. verum essential oils may have potential in the management of C. obstrictus. However, their impact on non-target organisms, including parasitoids, needs to be studied more thoroughly to determine the potential of essential oil main compounds in integrated pest management.
1 Introduction
The cabbage seedpod weevil (Ceutorhynchus obstrictus Marsham) is widely distributed and one of the most important oilseed rape pests in Europe (Williams, 2010) and North America (Buntin, 1998; Dosdall et al., 2006; Dosdall and Mason, 2010). Adult C. obstrictus feeds on flower buds and young pods of oilseed rape plants, but the main economic loss is caused by larvae feeding within the seedpods (Bonnemaison, 1957; Williams and Free, 1978). Management of C. obstrictus is mostly based on synthetic insecticides that have harmful effects on biodiversity, pollute the environment (Geiger et al., 2010) and may leave pesticide residues on products (Yigit and Velioglu, 2020). Broad-spectrum insecticides have fatal effects on naturally occurring predatory arthropods such as lacewings, spiders, ladybirds, carabid beetles, rove beetles and parasitoids, which otherwise can effectively control the abundance of agricultural pests and reduce the need to apply insecticides (Tschumi et al., 2016; Begg et al., 2017; Albrecht et al., 2020). For example, key parasitoids of C. obstrictus can substantially contribute to biocontrol services, as the parasitism rate of C. obstrictus can reach up to 90% (Veromann et al., 2011; Kovács et al., 2019). In the light of the European Union’s Farm to Fork Strategy which aims to diminish the negative impacts of agriculture on the environment, there is a great need to find environmentally sustainable pest control measures (European Commission, 2020).
Essential oils are of interest in pest science, as a possible alternative to synthetic plant protection products (Menossi et al., 2021; Devrnja et al., 2022). Several studies have highlighted their insecticidal effects on important agricultural pests (Das et al., 2021; Devrnja et al., 2022). An added complexity of essential oil studies is due to the variable chemical composition, even within the same plant species, as a result of different cultivars, growing conditions, production methods, plant parts used and harvesting times (Figueiredo et al., 2008; Baser and Buchbauer, 2009; Turek and Stintzing, 2013). Previously, several studies have examined the potential of essential oils to control another oilseed rape pest – pollen beetle (Brassicogethes aeneus Fabricius) (Mauchline et al., 2005; Pavela, 2011; Mauchline et al., 2013; Dorn et al., 2014; Willow et al., 2020). Pavela (2011) found that Carum carvi L., Thymus vulgare L. and Foeniculum vulgare Miller had an insecticidal effect against the pollen beetle, while Willow et al. (2020) showed only a slight insecticidal effect of residual exposure using very high dosage of Cinnamomum verum J. Presl oil; Mauchline et al. (2005); Mauchline et al. (2013) and Dorn et al. (2014) found repellent and lethal efficacy with Lavandula angustifolia Miller while Cook et al. (2007a) indicated no behavioural response from the main parasitoids Phradis interstitialis Thomson and P. morionellus Holmgren. However, it is unknown whether these essential oils are effective against another oilseed rape pest, C. obstrictus, as well which could potentially aid in the control of these two major pests simultaneously. While botanical insecticides are considered to be less toxic to humans and the environment compared to synthetic pesticides, their impact on the natural enemies of pests should be assessed, as they can interfere with their behavior or biology (Rampelotti-Ferreira et al., 2017; Parreira et al., 2018; Lima et al., 2020; Stenger et al., 2021).
Biosafety of plant protection products is a major concern in agriculture. Ideally, plant protection product applications should not be at the expense of non-target organism populations, especially those contributing to biological control of the target pest. Some essential oils have been evidenced as safe for such beneficial insects. For instance, essential oil residues of Origanum vulgare L. and T. vulgaris showed no sublethal or lethal effects in the parasitoid Trissolcus basalis Wollaston (Platygastridae) in direct contact and fumigation bioassays (Werdin González et al., 2013). Essential oil from Piper aduncum L. showed promising results to control the stink bug Euschistus heros Fabricius, while parasitism and emergence of the egg parasitoids Telenomus podisi Ashmead (Platygastridae) and Trissolcus urichi Crawford (Scelionidae) were unaffected (Turchen et al., 2016). However, decreases in parasitism rate have been observed in other species (Boeke et al., 2003; Stenger et al., 2021), leaving the biosafety profile of essential oils questionable with regard to parasitoids.
In this study, we aimed to investigate whether the dry residues of six plant essential oils affect the mortality and immobility of C. obstrictus, as well as mortality and immobility, and next generation development of the model parasitoid Nasonia vitripennis Walker (Hymenoptera: Pteromalidae). We show that Cuminum cyminum L. and C. verum essential oils have potential for use in C. obstrictus management, but also that their impact on parasitoids needs further study to determine the actual potential of these essential oils within integrated oilseed rape protection.
2 Materials and methods
2.1 Insects
We collected C. obstrictus adults from an untreated oilseed rape field (58.36377°N, 26.66145°E, Tartu County, Estonia) using a plant tapping method and collecting insects into a ventilated plastic bottle. In the laboratory we identified C. obstrictus via Morris (2008) and allowed weevils to feed ad libitum on oilseed rape leaves and flowers also collected from the same field. As the key parasitoids of C. obstrictus are of the family Pteromalidae, we used N. vitripennis as a model non-target biocontrol species in this study. Nasonia vitripennis were reared in a climate chamber (Sanyo MLR-351H, Japan) at 20 ± 2°C, 60% RH and 16:8 h light:dark cycle, using blow fly (Calliphora sp.) pupae as the host. Blow fly larvae were bought from a commercial fishing store and were allowed to pupate in the laboratory. To produce new N. vitripennis adults, we placed approximately 20 blow fly pupae into transparent, polystyrene, ventilated insect breeding dishes (diameter 10 cm x height 4 cm; SPL Life Sciences, Gyeonggi-do, South Korea; hereafter referred to as cages), and introduced approximately 20–50 fast moving (a proxy for insect health) N. vitripennis adults into the cages. Prior to introducing the parasitoids to the cages, parasitoids were fed 50% sugar water to optimize their reproductive potential.
2.2 Examining the effect of six essential oils on C. obstrictus via contact with dry treatment residues on leaf and flower surfaces
To examine the effect of essential oil applications on C. obstrictus’ mortality and immobility, we treated oilseed rape leaf and flower surfaces with T. vulgaris, F. vulgare, C. cyminum, C. verum, C. carvi and Cannabis sativa L. essential oils. Pure essential oils were ordered from Talia (Rome, Italy; www.taliaessenze.com) in 2019, and once received stored in a refrigerator at +4°C, in small separate boxes in darkness. Details regarding the origin of plants, plant parts used, extraction method, and the major relevant compounds in each essential oil used, are described in detail in Willow et al. (2020). The gas chromatography–mass spectrometry (GC-MS) used for analyzing the essential oils, is described in detail in Kännaste et al. (2014). In the present study, essential oils were used at a concentration of 1.5%, with acetone as the solvent and polysorbate Tween80 (0.05%) as a wetting agent. The negative control treatment contained only acetone and Tween80; the positive control treatment was analytical grade lambda-cyhalothrin applied at the recommended field concentration (7.5 g active compound/ha) in acetone and Tween80. For each treatment, oilseed rape leaves were individually placed on a petri dish and using a pipette, at 1000 µl of treatment solution were applied onto each oilseed rape leave (~ 12 cm x 9 cm). In each petri dish we included four oilseed rape flowers dipped in the respective [acetone + Tween80 + essential oil] solution. After that, treated leaves and flowers were allowed to air dry for 1 h, and then one leaf and four flowers were placed into each cage, followed by the introduction of eight C. obstrictus adults into each cage. Five cages per each treatment were prepared. Cages were kept in a ventilated room with an ambient air temperature of 22 ± 2°C, away from direct sunlight. Survival and mobility of weevils were assessed after 3 h and 24 h of exposure to dry residues of each treatment. In each sample, the number of weevils displaying immobility effects, including erratic movements or loss of mobility, was recorded, and all dead weevils were counted. This experiment was repeated twice, in total ten cages per treatment (N=10), total of 80 weevils per treatment.
The two most effective essential oils from the abovementioned tests were then evaluated for C. obstrictus control efficacy at four different concentrations. Here, we examined the dry residues of C. verum and C. cyminum essential oils applied at 0.5%, 1%, 1.5% and 2% concentrations. The experimental setup was the same as previously described, but the experiment was not repeated (N=5), total of 40 weevils per treatment.
2.3 Examining non-target effects of six essential oils on N. vitripennis
To examine the effects of essential oils on N. vitripennis, we treated filter paper, via pipette, with the same six plant essential oils as were tested on C. obstrictus, using acetone as the solvent, as well as Tween80 (0.05%). The positive controls were the same as used in the C. obstrictus assays. Here, all six essential oils were applied at four concentrations: 0.1%, 0.3%, 0.5% and 1.5%. For each treatment, we pipetted 1000 µL of treatment solution onto five pieces of filter paper (~ 7 cm x 3 cm) individually on a petri dish. After treatment, the filter papers were allowed to air dry for 1 h, and subsequently placed into cages, one piece of filter paper per cage. Eight N. vitripennis adults were introduced to each cage, and the cages were kept in a ventilated room with an ambient temperature of 22 ± 2°C. At 3 h and 24 h post-exposure to treatment residues, N. vitripennis mortality and immobility were monitored. This experiment was repeated twice, in total ten cages per treatment (N=10), total of 80 parasitoids per treatment.
To assess the potential impact of essential oils on the developmental success of N. vitripennis, we used already-parasitized blow fly pupae. For that, the parasitoids we allowed freely to lay eggs into the blow fly pupae for 7 days. After 7 days, adult parasitoids were removed, and blow fly pupae were dipped into the treatment solutions for 2 seconds, allowed to air dry for 1 h, and then placed into cages, 10 pupae per cage. Cages were kept in a ventilated room with an ambient temperature of 22 ± 2°C, away from direct sunlight, for three weeks. After that, all emerged parasitoids were counted, and all pupae were dissected to indicate the presence of unemerged parasitoids. The experiment was repeated twice and in total ten cages per treatment (N=10), total of 80 pupae per treatment.
2.4 Statistical analysis
Statistical analyses were performed in R v3.6.1 (R Core Team, 2018), using the R packages “car”, “emmeans”, “MASS”, “DHARMa” and “dunn.test” (Venables et al., 2002; Dinno and Dinno, 2017; Hartig and Hartig, 2017; Fox and Weisberg, 2019; Lenth, 2022). For C. obstrictus analyses, Generalized Linear Models (GLMs) with Poisson distribution and log link function and Wald statistics Type III empirical standard error were used. For the post-hoc comparisons, the Tukey test was used. To analyze N. vitripennis data, as the residuals of the model were not normally distributed, we used the nonparametric Kruskal-Wallis test, followed by Bonferroni-Dunn’s test for post-hoc pairwise comparisons.
3 Results
3.1 Effect of essential oil residues on C. obstrictus mortality and immobility
After 3 h of contact with dry residues of essential oil treatments, survival rates did not differ from the negative control treatment (Figure 1A). At 24 h post-treatment, however, we observed a significant effect on survival (χ2 = 132.41, df=7, p<0.0001). The highest mortality (82.5 ± 6.8%) was observed in the C. verum oil treatment, followed by the positive control (53.75 ± 3.75%) and C. cyminum oil (50.71 ± 8.77%) treatment, while the mortality of weevils exposed to T. vulgaris, C. carvi and C. sativa essential oil treatments did not differ significantly from the negative control (Figure 1A). Negative control treatment had no effect on survival.
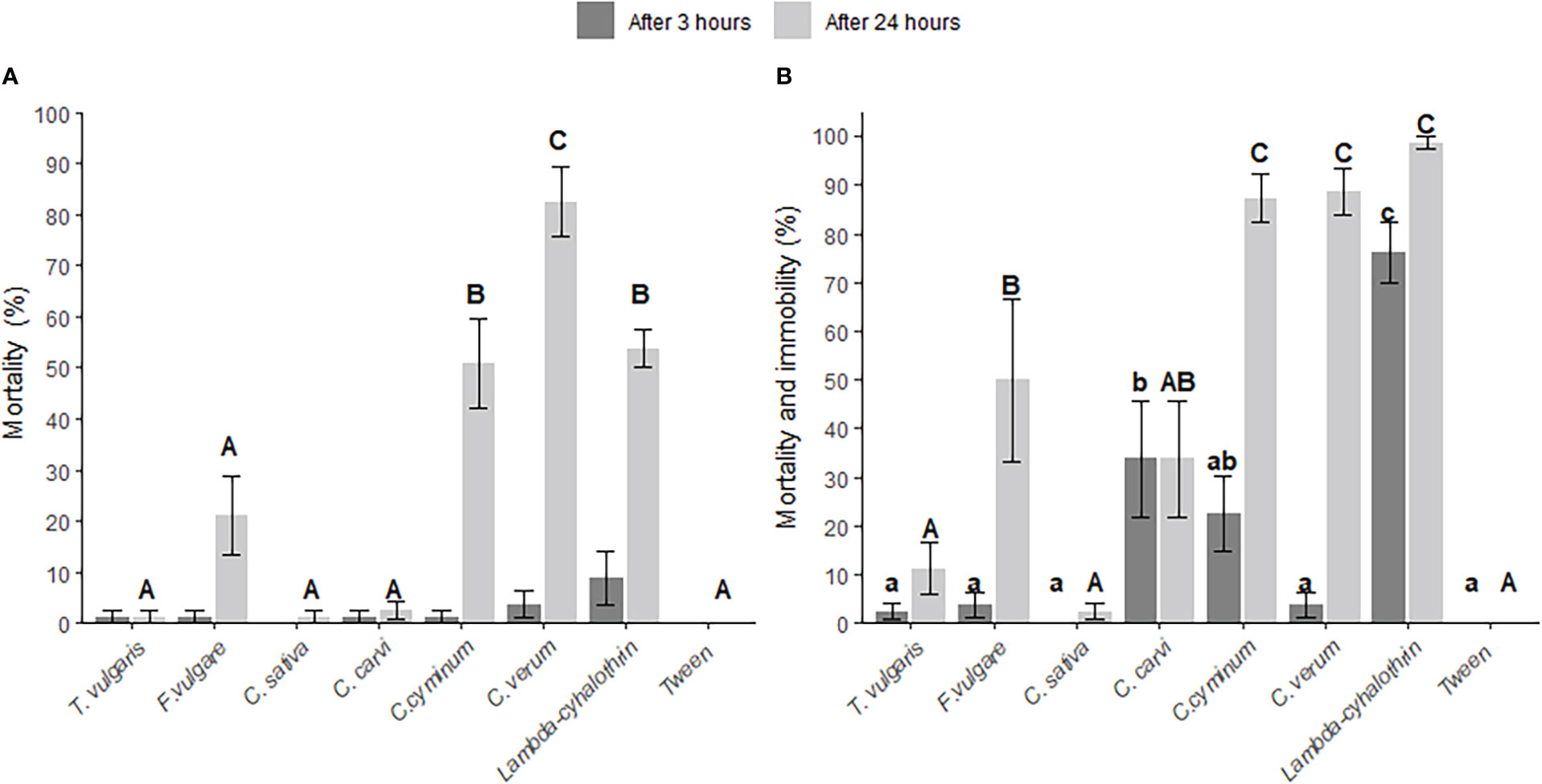
Figure 1 Effect of six plant essential oils (Thymus vulgaris, Foeniculum vulgare, Cuminum cyminum, Cinnamomum verum, Carum carvi and Cannabis sativa), lambda-cyhalothrin (positive control) and Tween80 (negative control) on Ceutorhynchus obstrictus mortality (A) and mortality and immobility (B), at 3 h and 24 h post-exposure to treated oilseed rape leaves and flowers, N=10 (80 weevils per treatment). All treatments were compared using Generalized Linear Models (GLMs) with Wald statistic Type III, post-hoc comparisons with Tukey test, error bars: ± SE. Different lowercase and uppercase letters indicate significant differences (p<0.05) between treatments at 3 h and 24 h post-exposure, respectively.
Treatment with essential oils had a significant impact on C. obstrictus mortality and immobility rates after 3 h (χ2 = 90.32, df=7, p <0.0001; Figure 1B). The greatest effect on C. obstrictus mortality and immobility was observed in the positive control treatment (76.3 ± 6.3%), followed by C. carvi oil (33.8 ± 11.9%) and C. cyminum oil (22.5 ± 7.9%). The mortality and immobility rate increased at 24 h post-exposure to dry residues, in all treatments except C. carvi. The greatest losses of mortality and immobility in C. obstrictus were observed in the C. cyminum (87.3 ± 4.9%) and C. verum (88.8 ± 4.7%) treatments, which did not differ significantly from the positive control treatment (98.8 ± 1.3%) with lambda-cyhalothrin. Treatments with T. vulgaris and C. sativa did not differ from the negative control (p>0.05), but F. vulgare differed significantly from both the positive and negative control (p<0.05). No mortality nor immobility effects were observed in the negative control treatment.
3.2 Effect of different concentrations of C. cyminum and C. verum essential oil residues on C. obstrictus mortality and immobility
As C. cyminum and C. verum represented the most effective essential oils against C. obstrictus, we examined the effects of their residues against C. obstrictus after applying these two essential oils at increasing concentrations (0.5%, 1%, 1.5% and 2%) (Figures 2A-D). At 3 h C. verum showed a significant effect on C. obstrictus survival (χ2 = 12.93, df=4, p=0.012) (Figure 2A), where concentrations of 1% and higher caused mortality; however no significant effect on C. cyminum was observed after 3 h (Figure 2B). At 24 h post-treatment, both C. cyminum and C. verum essential oil residues significantly increased C. obstrictus mortality (χ2 = 126.49, df=4, p<0.0001; χ2 = 87.29, df=4, p<0.0001, respectively). Ceutorhynchus obstrictus mortality rates in C. cyminum treatments were significantly greater in 1.5% and 2% compared to the 0.5% and 1% solutions (Figure 2B). The mortality rates of C. obstrictus treated with C. verum essential oil at 1%, 1.5% and 2% solutions exceeded 80% (82.5 ± 3.1%, 90 ± 10% and 90 ± 4.7%, respectively), each differing significantly from the C. verum 0.5% and control treatments. Mortality and immobility rates of C. obstrictus reached up to 90% at 24 h post-exposure to dry residues of C. cyminum essential oil applied at 1.5% and 2.0% concentrations (χ2 = 300,77, df=4, p<0.0001; Figure 2D).
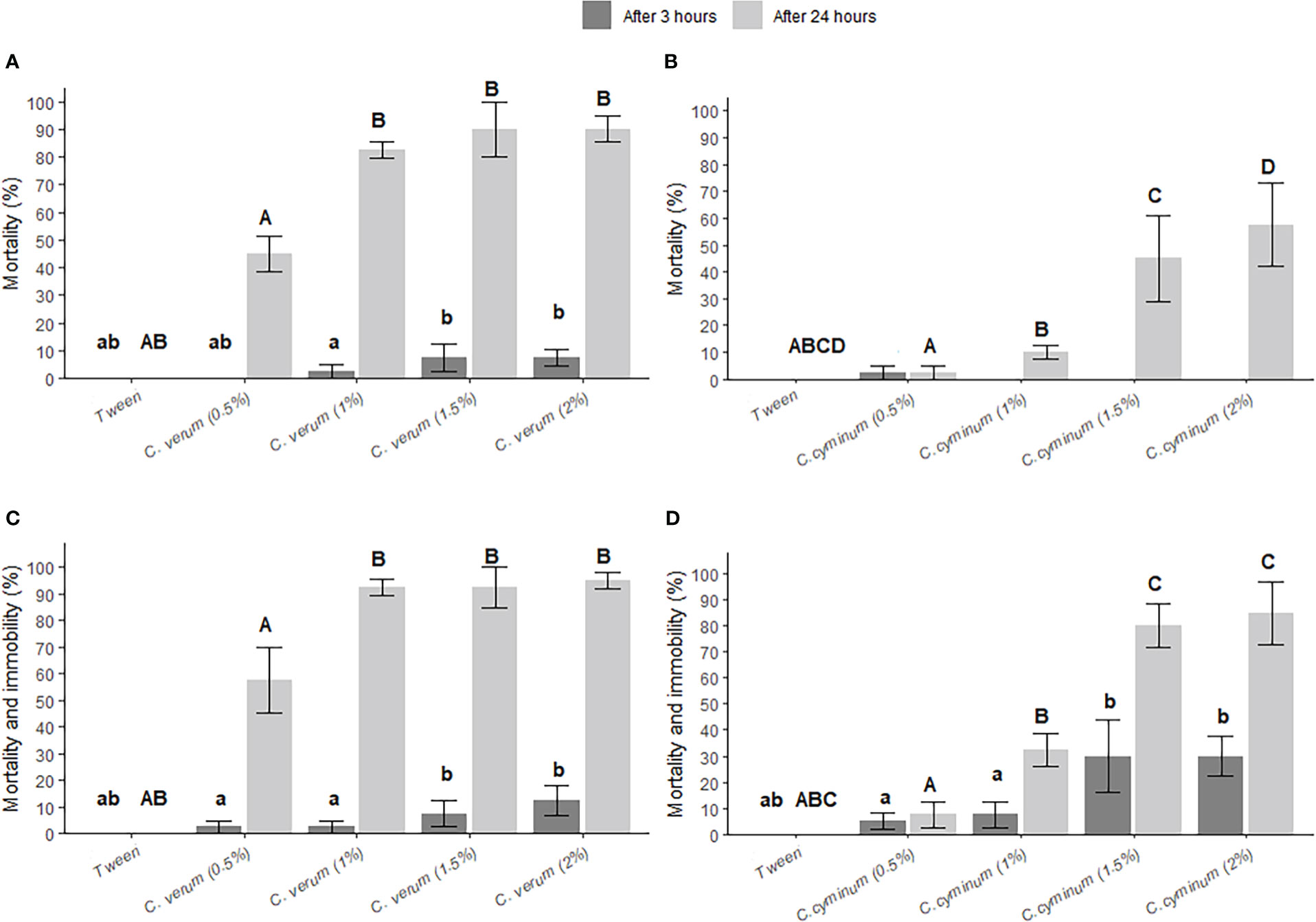
Figure 2 Effect of Cuminum cyminum essential oil residues, at different concentrations, on Ceutorhynchus obstrictus mortality ( ± SE) (A) and mortality and immobility ( ± SE) (C), at 3 h and 24 h post-exposure to treated oilseed rape leaves and flowers. Effect of Cinnamomum verum essential oil residues, at different concentrations, on C obstrictus mortality ( ± SE) (B) and mortality and immobility ( ± SE) (D), at 3 h and 24 h post-exposure to treated oilseed rape leaves and flowers, N=10 (80 weevils per treatment). All treatments were compared using Generalized Linear Models (GLMs) with Wald statistic Type III and post-hoc comparisons with Tukey test. Different lowercase and uppercase letters indicate significant differences (p<0.05) between treatments at 3 h and 24 h post-exposure, respectively. No significant difference was observed for C cyminum mortality after 3 h.
3.3 Effect of essential oil residue concentrations on N. vitripennis mortality and immobility
After 3 h of exposure to essential oil residues applied at 0.1% concentration, there was a significant effect on N. vitripennis mortality and immobility (χ2 = 20.96, df=6, p=0.0019), more parasitoids were affected in T. vulgaris and F. vulgare treatments, 43.5 ± 11.9% and 15 ± 8.3%, respectively (Figure S1I). After 3 h we observed under 10% mortality and immobility for C. sativa (2 ± 0.42%), C. carvi (0%), C. verum (7.0 ± 1.89%), C. cyminum (6 ± 1.07%). For insecticide (lambda-cyhalothrin) treated group, a small mortality was observed (1.5 ± 0.26%), same for negative control (tween) (4.5 ± 0.63%). At 24 h post-exposure of dry treatment residues, no significant effect on mortality and immobility was observed for any of the essential oil treatments applied at 0.1% concentration (χ2 = 7.49, df=6, p=0.28). The lowest mortality and immobility rates were observed in the C. carvi treatment (3 ± 1.5%) and the highest in the T. vulgaris treatment (29 ± 12.5%), but no significant differences between treatments were found. The mortality and immobility rates at 24 h post-exposure to T. vulgaris oil residues were lower than at 3 h post-exposure, indicating that some specimens were able to recover from knockdown effects.
In all essential oil treatments applied at 0.3% concentration, N. vitripennis mortality and immobility were significantly decreased at both 3 h and 24 h (χ2 = 24.98, df=6, p<0.001; χ2 = 27.78, df=6, p<0.0001, respectively) post-exposure to essential oil dry residues, compared to the control treatment. At 3 h, the F. vulgare treatment at 0.3% concentration resulted in the greatest mortality and immobility (54 ± 14.3%), followed by the C. verum treatment (53 ± 15.7%), T. vulgaris (50 ± 5.27%), C. sativa (3 ± 0.48%), C. carvi (15 ± 2.06%), C. cyminum (10 ± 3.16%), compared to the insecticide treatment lambda-cyhalothrin (1.5 ± 0.26%) and negative control (tween) (4.5 ± 0.63%). At 24 h, C. verum oil at 0.3% concentration resulted in the greatest loss of mobility (63.5 ± 4.1%), followed by T. vulgaris oil (60 ± 4.8%) (Figure 3A).
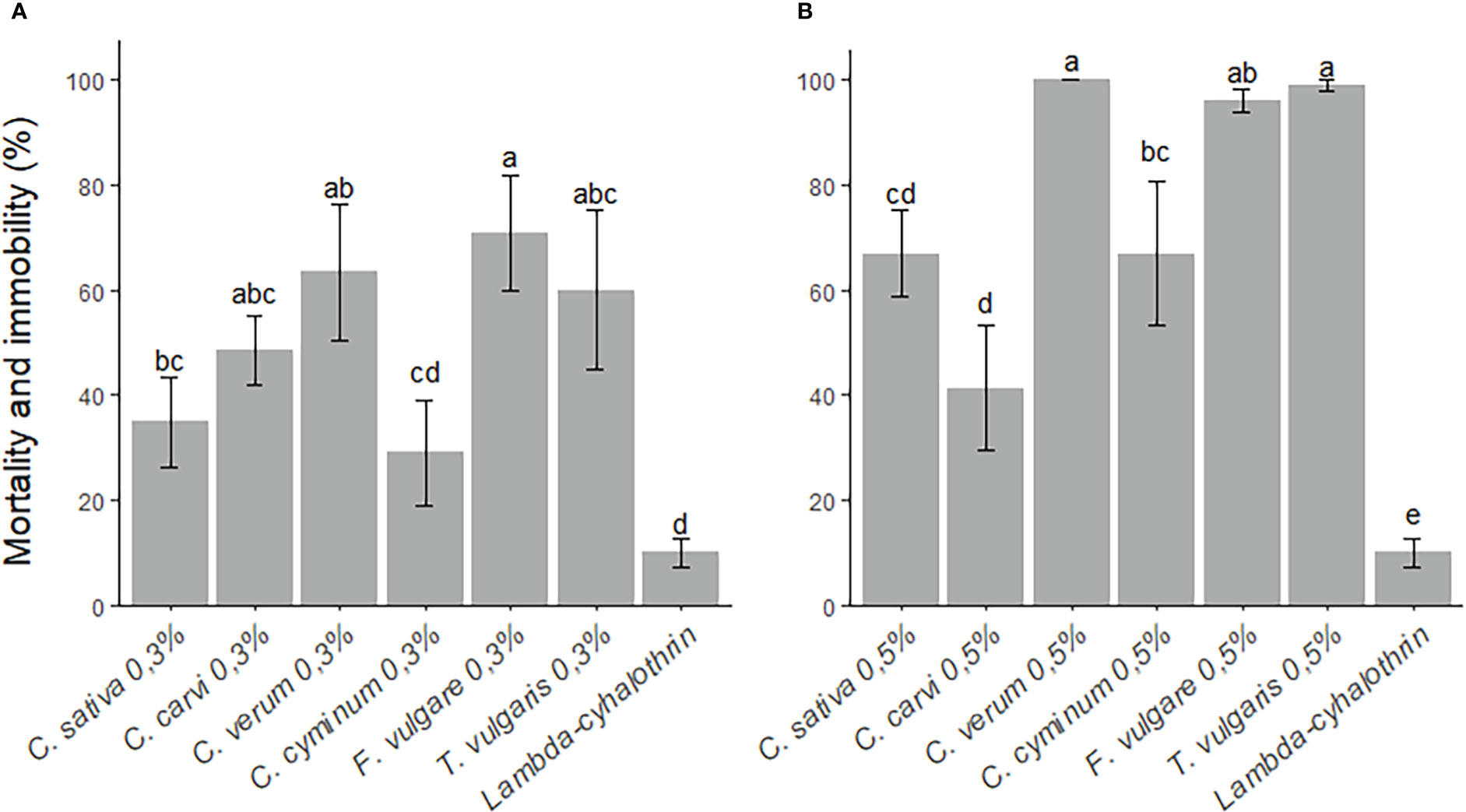
Figure 3 Mortality and immobility rate ( ± SE) of Nasonia vitripennis adults at 24 h post-exposure to essential oil (Thymus vulgaris, Foeniculum vulgare, Cuminum cyminum, Cinnamomum verum, Carum carvi and Cannabis sativa) dry residues [(A) 0.3% and (B) 0.5% concentrations] on filter paper. All treatments were compared using Kruskal-Wallis test, followed by Bonferroni-Dunn’s test for post-hoc pairwise comparisons. Different letters indicate significant differences (p<0.05) between treatments.
In all essential oil treatments applied at 0.5% concentration, N. vitripennis mortality and immobility were significantly decreased at both 3 h (χ2 = 59.54, df=6, p<0.0001) and 24 h (χ2 = 53.84, df=6, p<0.0001). At 3 h post-exposure to the C. verum treatment at 0.5% concentration, the mortality and immobility rate of N. vitripennis was 100%, followed by the T. vulgaris and F. vulgare treatments (100% and 97 ± 2.1%, respectively), C. cyminum (44 ± 4.48%), C. carvi (21 ± 3.31%) and C. sativa (24 ± 2.95%). At 24 h, there was a significant loss of mortality and immobility in all 0.5% concentration essential oil treatments. The greatest loss of mortality and immobility at 24 h was observed in the C. verum treatment (100%) (Figure 3B). Contact with dry residues of the insecticide lambda-cyhalothrin did not result in a significant loss of N. vitripennis mortality and immobility, compared to the negative control treatment. All essential oils, at 1.5% concentration, resulted in 100% mortality of N. vitripennis after 3 h.
3.4 Effect of essential oils on the number of next generation N. vitripennis
After allowing N. vitripennis to parasitize untreated blow fly pupae for 7 days, we treated the parasitized pupae to determine the post-parasitism mortality of developing parasitoids. Compared to the untreated control group, the average number of next generation N. vitripennis adults that emerged was greatest in the group consisting of untreated pupae (223 ± 16.3 specimens), followed by pupae treated with C. verum oil (176 ± 11.3 specimens) and F. vulgare oil (171.8 ± 12.5 specimens), although the differences were not significant (Figure 4). Compared to untreated pupae, significantly less parasitoids emerged from C. sativa, C. cyminum and T. vulgaris treatments decreasing the number of emerging parasitoids similar to lambda-cyhalothrin where only 85.7 ± 16 next generation parasitoids emerged.
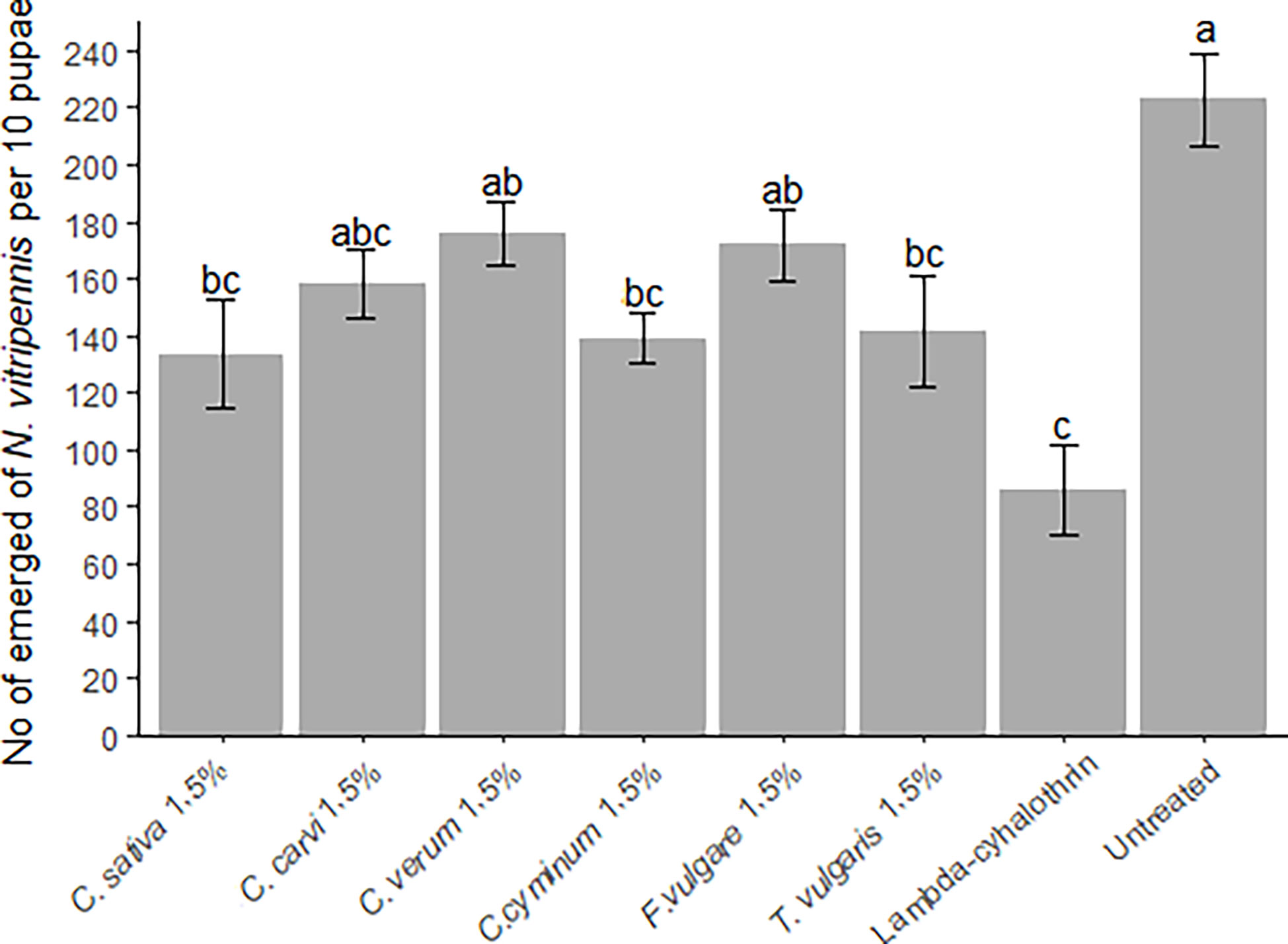
Figure 4 Mean ( ± SE) number Nasonia vitripennis specimens emerged from ten blow fly (Calliphora sp.) pupae treated with different essential oils (Thymus vulgaris, Foeniculum vulgare, Cuminum cyminum, Cinnamomum verum, Carum carvi and Cannabis sativa) (1.5% concentration) and lambda-cyhalothrin (positive control), as well as in untreated pupae, 7 days after first generation N. vitripennis were introduced to their hosts. Differences between treatments were compared using Kruskal-Wallis test, followed by Bonferroni-Dunn’s test for post-hoc pairwise comparisons. Different letters indicate significant differences between treatments (p<0.05).
4 Discussion
The present study showed that contact with essential oil residues via treated oilseed rape leaves and flowers caused both mortality and immobility in C. obstrictus adults. In addition to C. obstrictus, essential oil treatments showed an impact on the mortality and immobility of a model pteromalid parasitoid, N. vitripennis, and furthermore influenced the number of next generation adult parasitoids that emerged from their hosts. Our results showed that at 24 h post-treatment with C. cyminum and C. verum essential oils at 1.5% concentration they were as effective as the synthetic insecticide lambda-cyhalothrin, mortality and immobility of C. obstrictus adults reached 82.5% for C. verum and 50.7% for C. cyminum. There is an overlap in the occurrence of B. aeneus and C. obstrictus in oilseed rape fields (Veromann et al., 2006; Sulg et al., Under Review); B. aeneus arrives a little bit earlier than C. obstrictus, as its flight threshold temperature is 12°C (Williams, 2010), whereas for C. obstrictus, it is 13–15°C (Free and Williams, 1979; Lerin, 1991). However, they are both present in oilseed rape fields from the green bud stage (BBCH 51) (Veromann et al., 2006; Veromann et al., 2012). Therefore, it is possible that treatments targeting B. aeneus may also contribute to C. obstrictus control and vice versa. Our new findings show the potential of C. verum essential oil to manage C. obstrictus, but as it greatly exceeds that of previously reported for B. aeneus (17.5% combined immobility and mortality) (Willow et al., 2020), the two species are unlikely to be managed simultaneously using only C. verum. Based on previous results and our new findings, the treatment of oilseed rape with C. verum affects two of its main pests to some extent, therefore indicating the need for further investigations. The essential oils used in our study were almost the same (excluding anise) as in Willow et al. (2020). Gas chromatography–mass spectrometry results of the C. verum oil used in the present study are reported in detail in Willow et al. (2020). The primary active compound in the C. verum oil used was reported to be (E)-cinnamaldehyde (46%), followed by caryphyllene (15%), linalool (12%) and D-limonene (8%).
According to our best knowledge, the toxicities of essential oils for C. obstrictus, or other species in the genus Ceutorhynchus, have not previously been assessed. However, there are previous studies examining other members of the family Curculionidae, where essential oil treatment efficiencies have been examined. For instance, essential oils isolated by hydrodistilling the dried fruit of Trachyspermum ammi (L.) Sprague ex Turrill (Apicaceae) and Nigella sativa L. (Ranunculaceae) have shown repellent activity and toxic effects against the rice weevil (Sitophilus oryzae L.) (Chaubey, 2012). The rice weevil was also examined by Saad et al. (2018), where they found that, from all examined compounds contributing to acetylcholinesterase inhibition, the most promising was trans-cinnamaldehyde. Different essential oils, including C. verum, were studied against the stored product pest Sitophilus zeamais Motschulsky by Ramlal et al. (2020). They observed repellent and lethal effects of C. verum oil, resulting in 78% and 97% mortality at concentrations of 75 and 100 µL/mL, respectively. Similar to our study, the main constituent of C. verum essential oil in their study was cinnamaldehyde (62%) confirming that this compound can be potentially exploited against weevil pests.
The mortality rates in other essential oil treatments in our study were under 50% at 24 h, suggesting that their efficacy was insufficient for use in controlling C. obstrictus abundance in oilseed rape crops. In the present study, we did not examine other effects than mortality and immobility of these essential oils on C. obstrictus adults, e.g. repellence etc. For instance, previous studies have shown the repellent effects of essential oils on insects (reviewed in Lee (2018)) and also their potential to be used in storage facilities as pest management approaches (Cook et al., 2007b; Nerio et al., 2010; Campolo et al., 2018; Xu et al., 2018; Bandeira et al., 2021), but the use for managing agricultural pests have gained less attention. Essential oils can also be used to manage agricultural pests, but in order to use them in agricultural fields, the essential oils need to be more stable, preventing them from evaporating or biodegrading (Oladipupo et al., 2022). Future studies on C. obstrictus should examine behavioral effects in addition to the effects examined in the present study as exposure to essential oils, as well as treatment residuals, can result in behavioral changes, for example deterring egg laying or acting as antifeedant (Lazarević et al., 2020; Magierowicz et al., 2020; Stenger et al., 2021). Examining our two most effective (against C. obstrictus) essential oils, C. verum and C. cyminum, at four different concentrations, showed C. verum oil at 1% concentration to be the most efficient, resulting in almost 90% mortality and immobility at 24 h post-exposure, whereas C. cyminum oil at 2% concentration resulted in 90% morality and immobility at 24 h post-exposure. We presented mortality and immobility rates, since in nature, immobility is likely to result in mortality. Immobile insects are easier prey, as well as they may also die of dehydration, starvation, cold or heat stress, etc., as a result of being in contact with toxic substances.
Similar to other insecticides, the effects of essential oils on non-target organisms, including economically beneficial insects, should always be assessed. It is necessary to assess the impact of essential oils on the natural enemies of target pest species (e.g. relevant model parasitoids). The present study demonstrated that C. verum essential oil has the potential to control abundance of C. obstrictus, but at the same time it resulted in almost 100% mortality in the model pteromalid parasitoid N. vitripennis at 3 h post-exposure to treatments at 1.5% concentration of all studied essential oils except C. sativa. Testing the oils at 0.1% concentration showed that at 3 h post-exposure, T. vulgaris oil residues resulted in the highest mortality and immobility rates in N. vitripennis, although some parasitoids were able to recover from immobility by the 24 h time point. Similar to our results, Werdin González et al. (2013) found that one day old residues of T. vulgaris essential oil resulted in 100% mortality in parasitoid Trissolcus basalis Wollaston (Platygastridae) while one week old residues did not result in any mortality. When targeting a pest species, knowledge of parasitoid distribution in or arrival to the crop is crucial, as pesticide application times can be planned in a manner where the pesticide residues represent an insignificant threat to target pest-relevant biocontrol agents. Essential oil residues of C. verum show promising results for controlling C. obstrictus. However, C. verum oil treatment, at 0.1% concentration, resulted in an immobility rate of 17% in N. vitripennis. Increasing the concentration of C. verum oil to 0.3% resulted in almost four times this immobility rate in N. vitripennis, and residues from a 0.5% concentration application of C. verum oil resulted in 100% immobility. Thus, residues of C. verum essential oil, even when applied in low concentrations, are not safe for the parasitoid N. vitripennis. It remains unclear whether the essential oils could cause side effects in the next generation of N. vitripennis.
When treating parasitized pupae with C. sativa and C. cyminum it lowered hatching of the new generation of N. vitripennis to the same level as treatment with insecticide. But as direct exposure to essential oil residues, in the concentration of 1.5%, caused 100% mortality after 3 h among N. vitripennis, we can only assume that fly pupae served as protective shield mitigating the toxic effect of treatments. It has been reported that lambda-cyhalothrin can alter the ability of the parasitoids to find and infest their hosts, even when mortality is not observed among next generation female parasitoids (Desneux et al., 2004). Even though parasitoids developed in our study, their following parasitism efficacy remains unknown, and should be investigated in future studies. Whether the pods concealing parasitoids of C. obstrictus provide similar protection from developing parasitoids needs investigating. It also remains unknown whether the essential oil treatments in the present study had sublethal effects on next generation adult parasitoids, representing a crucial knowledge gap that is in need of assessment. Undetected sublethal effects, could result in death or decreased fecundity or jeopardize host location abilities. Our results show the potential of essential oils use in controlling C. obstrictus. However, much more research is needed before essential oils could be recommended for pest control.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author contributions
SS, RK, and EV conceived the study. SS, TK, and RK performed the experiments. SS performed data analyses and visualization. SS and EV wrote the first draft of the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the Estonian Research Council grant (PRG1056). This work was supported by the European Union, European Regional Development Fund (Estonian University of Life Sciences ASTRA project „Value-chain based bio-economy“).
Acknowledgments
We thank Mariette Sakkool and Merlyn Paltsmar for help with collecting data and Jonathan Willow for constructive comments and English editing.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fagro.2023.1107201/full#supplementary-material
References
Albrecht M., Kleijn D., Williams N. M., Tschumi M., Blaauw B. R., Bommarco R., et al. (2020). The effectiveness of flower strips and hedgerows on pest control, pollination services and crop yield: a quantitative synthesis. Ecol. Lett. 23, 1488–1498. doi: 10.1111/ele.13576
Bandeira P. T., Fávaro C. F., Francke W., Bergmann J., Zarbin P. H. G. (2021). Aggregation pheromones of weevils (Coleoptera: Curculionidae): Advances in the identification and potential uses in semiochemical-based pest management strategies. J. Chem. Ecol. 47, 968–986. doi: 10.1007/s10886-021-01319-1
Baser K. H. C., Buchbauer G. (2009). Handbook of essential oils: science, technology, and applications (Boca Raton: CRC press).
Begg G. S., Cook S. M., Dye R., Ferrante M., Franck P., Lavigne C., et al. (2017). A functional overview of conservation biological control. Crop Prot. 97, 145–158. doi: 10.1016/j.cropro.2016.11.008
Boeke S. J., Sinzogan A. A. C., De Almeida R. P., De Boer P. W. M., Jeong G., Kossou D. K., et al. (2003). Side-effects of cowpea treatment with botanical insecticides on two parasitoids of Callosobruchus maculatus: Side-effects of cowpea treatment with botanical insecticides. Entomol. Exp. Appl. 108, 43–51. doi: 10.1046/j.1570-7458.2003.00066.x
Bonnemaison L. (1957). Le charançon des siliques (Ceuthorrhynchus assimilis payk.). Biologie et méthodes de lutte. Ann. Epiphyt. 4, 387–543.
Buntin G. D. (1998). Cabbage seedpod weevil (Ceutorhynchus assimilis, paykull) management by trap cropping and its effect on parasitism by trichomalus perfectus (Walker) in oilseed rape. Crop Prot. 17, 299–305. doi: 10.1016/S0261-2194(98)00015-5
Campolo O., Giunti G., Russo A., Palmeri V., Zappalà L. (2018). Essential oils in stored product insect pest control. J. Food Qual. 2018, e6906105. doi: 10.1155/2018/6906105
Chaubey M. K. (2012). Biological effects of essential oils against rice weevil Sitophilus oryzae l. (Coleoptera: Curculionidae). J. Essent. Oil Bear. Plants 15, 809–815. doi: 10.1080/0972060X.2012.10644124
Cook S. M., Jönsson M., Skellern M. P., Murray D. A., Anderson P., Powell W. (2007a). Responses of phradis parasitoids to volatiles of lavender, lavendula angustifolia–a possible repellent for their host, meligethes aeneus. BioControl 52, 591–598. doi: 10.1007/s10526-006-9057-x
Cook S. M., Khan Z. R., Pickett, J. A. (2007b). The use of push-pull strategies in integrated pest management. Annu. Rev. Entomol. 52, 375–400. doi: 10.1146/annurev.ento.52.110405.091407
Das S., Singh V. K., Dwivedy A. K., Chaudhari A. K., Dubey N. K. (2021). Insecticidal and fungicidal efficacy of essential oils and nanoencapsulation approaches for the development of next generation ecofriendly green preservatives for management of stored food commodities: an overview. Int. J. Pest Manage., 1–32. doi: 10.1080/09670874.2021.1969473
Desneux N., Pham-Delègue M.-H., Kaiser L. (2004). Effects of sub-lethal and lethal doses of lambda-cyhalothrin on oviposition experience and host-searching behaviour of a parasitic wasp, Aphidius ervi: Effects of lambda-cyhalothrin on Aphidius ervi. Pest Manage. Sci. 60, 381–389. doi: 10.1002/ps.822
Devrnja N., Milutinović M., Savić J. (2022). When scent becomes a weapon–plant essential oils as potent bioinsecticides. Sustainability 14, 6847. doi: 10.3390/su14116847
Dorn B., Jossi W., Humphrys C., Hiltbrunner J. (2014). Screening of natural products in the laboratory and the field for control of pollen beetles. J. Appl. Entomol. 138, 109–119. doi: 10.1111/jen.12086
Dosdall L. M., Mason P. G. (2010). “Key pests and parasitoids of oilseed rape or canola in north America and the importance of parasitoids in integrated management,” in Biocontrol-based integrated management of oilseed rape pests. Ed. Williams I. H. (Dordrecht: Springer Netherlands), 167–213. doi: 10.1007/978-90-481-3983-5_6
Dosdall L. M., Ulmer B. J., Gibson G. A. P., Cárcamo H. A. (2006). The spatio-temporal distribution dynamics of the cabbage seedpod weevil, ceutorhynchus obstrictus (Coleoptera: Curculionidae), and its larval parasitoids in canola in western Canada. Biocontrol Sci. Technol. 16, 987–1006. doi: 10.1080/09583150600828320
European Commission (2020). Farm to fork strategy: for a fair, healthy and environmentally-friendly food system. Commun. Commun. Eur. Parliam. Counc. Eur. Econ. Soc Commun. Commun. Reg.
Figueiredo A. C., Barroso J. G., Pedro L. G., Scheffer J. J. (2008). Factors affecting secondary metabolite production in plants: volatile components and essential oils. Flavour Fragr. J. 23, 213–226. doi: 10.1002/ffj.1875
Fox J., Weisberg S. (2019). An r companion to applied regression (Thousand Oaks, CA: Sage publications).
Free J., Williams I. H. (1979). The infestation of crops of oil-seed rape (Brassica napus l.) by insect pests. J. Agric. Sci. 92, 203–218. doi: 10.1017/S0021859600060652
Geiger F., Bengtsson J., Berendse F., Weisser W. W., Emmerson M., Morales M. B., et al. (2010). Persistent negative effects of pesticides on biodiversity and biological control potential on European farmland. Basic Appl. Ecol. 11, 97–105. doi: 10.1016/j.baae.2009.12.001
Kännaste A., Copolovici L., Niinemets Ü. (2014). Gas chromatography-mass spectrometry method for determination of biogenic volatile organic compounds emitted by plants. Methods Mol. Biol. Clifton NJ 1153, 161–169. doi: 10.1007/978-1-4939-0606-2_11
Kovács G., Kaasik R., Lof M. E., van der Werf W., Kaart T., Holland J. M., et al. (2019). Effects of land use on infestation and parasitism rates of cabbage seed weevil in oilseed rape. Pest Manage. Sci. 75, 658–666. doi: 10.1002/ps.5161
Lazarević J., Jevremović S., Kostić I., Kostić M., Vuleta A., Manitašević Jovanović S., et al. (2020). Toxic, oviposition deterrent and oxidative stress effects of thymus vulgaris essential oil against acanthoscelides obtectus. Insects 11, 563. doi: 10.3390/insects11090563
Lee M. Y. (2018). Essential oils as repellents against arthropods. BioMed. Res. Int. 2018, e6860271. doi: 10.1155/2018/6860271
Lenth R. V. (2022) Emmeans: Estimated marginal means, aka least-squares means. Available at: https://CRAN.R-project.org/package=emmeans.
Lerin J. (1991). Influence of host plant phenology on the reproduction of the rape weevil, ceuthorhynchus assimilis payk. J. Appl. Entomol. Ger. Fr. 111 (3), 303–310.
Lima A. P. S., Santana E. D. R., Santos A. C. C., Silva J. E., Ribeiro G. T., Pinheiro A. M., et al. (2020). Insecticide activity of botanical compounds against spodoptera frugiperda and selectivity to the predatory bug podisus nigrispinus. Crop Prot. 136, 105230. doi: 10.1016/j.cropro.2020.105230
Magierowicz K., Górska-Drabik E., Golan K. (2020). Effects of plant extracts and essential oils on the behavior of acrobasis advenella (Zinck.) caterpillars and females. J. Plant Dis. Prot. 127, 63–71. doi: 10.1007/s41348-019-00275-z
Mauchline A. L., Cook S. M., Powell W., Osborne J. L. (2013). Effects of non-host plant odour on meligethes aeneus during immigration to oilseed rape. Entomol. Exp. Appl. 146, 313–320. doi: 10.1111/eea.12030
Mauchline A. L., Osborne J. L., Martin A. P., Poppy G. M., Powell W. (2005). The effects of non-host plant essential oil volatiles on the behaviour of the pollen beetle meligethes aeneus. Entomol. Exp. Appl. 114, 181–188. doi: 10.1111/j.1570-7458.2005.00237.x
Menossi M., Ollier R. P., Casalongué C. A., Alvarez V. A. (2021). Essential oil-loaded bio-nanomaterials for sustainable agricultural applications. J. Chem. Technol. Biotechnol. 96, 2109–2122. doi: 10.1002/jctb.6705
Morris M. G. (2008). Handbooks for the identification of British insects: True weevils. (Coleoptera: Curculionidae, ceutorhynchinae) (London: Royal Entomological Soc).
Nerio L. S., Olivero-Verbel J., Stashenko E. (2010). Repellent activity of essential oils: A review. Bioresour. Technol. 101, 372–378. doi: 10.1016/j.biortech.2009.07.048
Oladipupo S. O., Hu X. P., Appel A. G. (2022). Essential oils in urban insect management–a review. J. Econ. Entomol. 115, 1375–1408. doi: 10.1093/jee/toac083
Parreira D. S., Alcántara-de la Cruz R., Zanuncio J. C., Lemes P. G., da Silva Rolim G., Barbosa L. R., et al. (2018). Essential oils cause detrimental effects on biological parameters of trichogramma galloi immatures. J. Pest Sci. 91, 887–895. doi: 10.1007/s10340-017-0945-x
Pavela R. (2011). Insecticidal and repellent activity of selected essential oils against of the pollen beetle, meligethes aeneus (Fabricius) adults. Ind. Crops Prod. 34, 888–892. doi: 10.1016/j.indcrop.2011.02.014
Ramlal S., Khan A., Ramsewak R., Mohammed F. (2020). Bioactivity of essential oils from five spices against sitophilus zeamais motschulsky (Coleoptera: Curculionidae). Trop. Agric. 97, 67–81.
Rampelotti-Ferreira F. T., Coelho A., Parra J. R. P., Vendramim J. D. (2017). Selectivity of plant extracts for trichogramma pretiosum Riley (Hym.: Trichogrammatidae). Ecotoxicol. Environ. Saf. 138, 78–82. doi: 10.1016/j.ecoenv.2016.12.026
R Core Team (2018) R core team r: A language and environment for statistical computing r foundation for statistical computing. Available at: https://www.R-project.org.
Saad M. M. G., Abou-Taleb H. K., Abdelgaleil S. A. M. (2018). Insecticidal activities of monoterpenes and phenylpropenes against sitophilus oryzae and their inhibitory effects on acetylcholinesterase and adenosine triphosphatases. Appl. Entomol. Zool. 53, 173–181. doi: 10.1007/s13355-017-0532-x
Stenger L. D., Abati R., Pawlak I. G., Varpechoski G. O., De Souza Vismara E., Barbosa L. R., et al. (2021). Toxicity of essential oil of Eugenia uniflora (L.) to thaumastocoris peregrinus (Hemiptera: Thaumastocoridae) and selectivity to the parasitoid cleruchoides noackae (Lin & Hubert) (Hymenoptera: Mymaridae). Crop Prot. 147, 105693. doi: 10.1016/j.cropro.2021.105693
Sulg S., Kovács G., Willow J. M., Kaasik R., Smagghe G., Lövei G. L., et al. Spatiotemporal distancing of crops reduces pest pressure while maintaining conservation biocontrol in oilseed rape fields. (Under Review).
Tschumi M., Albrecht M., Bärtschi C., Collatz J., Entling M. H., Jacot K. (2016). Perennial, species-rich wildflower strips enhance pest control and crop yield. Agric. Ecosyst. Environ. 220, 97–103. doi: 10.1016/j.agee.2016.01.001
Turchen L. M., Piton L. P., Dall’Oglio E. L., Butnariu A. R., Pereira M. J. B. (2016). Toxicity of piper aduncum (Piperaceae) essential oil against euschistus heros (F.) (Hemiptera: Pentatomidae) and non-effect on egg parasitoids. Neotrop. Entomol. 45, 604–611. doi: 10.1007/s13744-016-0409-7
Turek C., Stintzing F. C. (2013). Stability of essential oils: A review: Stability of essential oils. Compr. Rev. Food Sci. Food Saf. 12, 40–53. doi: 10.1111/1541-4337.12006
Venables W., Ripley B., Venables W. (2002). Modern applied statistics with s (NY: Springer New York). doi: 10.1007/978-0-387-21706-2
Veromann E., Metspalu L., Williams I. H., Hiiesaar K., Mand M., Kaasik R., et al. (2012). Relative attractiveness of brassica napus, brassica nigra, eruca sativa and raphanus sativus for pollen beetle (Meligethes aeneus) and their potential for use in trap cropping. Arthropod-Plant Interact. 6, 385–394. doi: 10.1007/s11829-012-9191-6
Veromann E., Tarang T., Kevvai R., Luik A., Williams I. (2006). Insect pests and their natural enemies on spring oilseed rape in Estonia: impact of cropping systems. Agric. Food Sci. 15, 61–72. doi: 10.2137/145960606777245579
Veromann E., Williams I. H., Kaasik R., Luik A. (2011). Potential of parasitoids to control populations of the weevil Ceutorhynchus obstrictus (Marsham) on winter oilseed rape. Int. J. Pest Manage. 57, 85–92. doi: 10.1080/09670874.2010.539714
Werdin González J. O., Laumann R. A., da Silveira S., Moraes M. C. B., Borges M., Ferrero A. A. (2013). Lethal and sublethal effects of four essential oils on the egg parasitoids trissolcus basalis.Chemosphere 92, 608–615. doi: 10.1016/j.chemosphere.2013.03.066
Williams I. H. (2010). “The major insect pests of oilseed rape in Europe and their management: An overview,” in Biocontrol-based integrated management of oilseed rape pests. Ed. Williams I. H. (Dordrecht: Springer Netherlands), 1–43. doi: 10.1007/978-90-481-3983-5_1
Williams I. H., Free J. B. (1978). The feeding and mating behaviour of pollen beetles (Meligethes aeneus fab.) and seed weevils (Ceutorhynchus assimilis payk.) on oil-seed rape (Brassica napus l.). J. Agric. Sci. 91, 433–459. doi: 10.1017/S0021859600046554
Willow J., Sulg S., Kaurilind E., Silva A. I., Kaasik R., Smagghe G., et al. (2020). Evaluating the effect of seven plant essential oils on pollen beetle (Brassicogethes aeneus) survival and mobility. Crop Prot. 134, 105181. doi: 10.1016/j.cropro.2020.105181
Xu Q., Hatt S., Lopes T., Zhang Y., Bodson B., Chen J., et al. (2018). A push–pull strategy to control aphids combines intercropping with semiochemical releases. J. Pest Sci. 91, 93–103. doi: 10.1007/s10340-017-0888-2
Keywords: Ceutorhynchus obstrictus, Hymenoptera, biopesticide, biosafety, endoparasitoid, Brassica napus, IPM
Citation: Sulg S, Kaasik R, Kallavus T and Veromann E (2023) Toxicity of essential oils on cabbage seedpod weevil (Ceutorhynchus obstrictus) and a model parasitoid (Nasonia vitripennis). Front. Agron. 5:1107201. doi: 10.3389/fagro.2023.1107201
Received: 24 November 2022; Accepted: 17 January 2023;
Published: 26 January 2023.
Edited by:
Agnieszka Synowiec, University of Agriculture in Krakow, PolandReviewed by:
Mirza Abdul Qayyum, Muhammad Nawaz Shareef University of Agriculture, PakistanJozsef Kiss, Szent István University, Hungary
Copyright © 2023 Sulg, Kaasik, Kallavus and Veromann. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Silva Sulg, c2lsdmEuc3VsZ0BlbXUuZWU=; Eve Veromann, ZXZlLnZlcm9tYW5uQGVtdS5lZQ==
 Silva Sulg
Silva Sulg Riina Kaasik
Riina Kaasik Triin Kallavus
Triin Kallavus Eve Veromann
Eve Veromann