
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Agron. , 02 August 2022
Sec. Plant-Soil Interactions
Volume 4 - 2022 | https://doi.org/10.3389/fagro.2022.969960
Chickpea growers in Australia have no post-emergent (POST) herbicides labeled for broadleaf weed control and they rely on pre-emergent herbicides for control of broadleaf weeds. The objective of this study was to evaluate chickpea tolerance to POST application of paraquat (0, 90, 180, 360, and 540 g ai ha-1) in a randomized complete block design. Paraquat (180 g ai ha-1) applied POST (up to 10 days after crop emergence) did not injure chickpeas and reduce plant height, and biomass when compared with nontreated control. When the crop just emerged, even the highest dose of paraquat (540 g ai ha-1) did not cause any injury or reduce chickpea biomass compared with nontreated control. Moderate toxicity to chickpea at paraquat 360 or 540 g ai ha-1 was observed when applied 20 days after crop emergence. This study suggests that paraquat POST has the potential to provide broad-spectrum weed control, including broadleaf weeds at an early stage of chickpea. However, the potential for crop injury to paraquat at higher doses, especially when applied at a late stage of the crop warrants further evaluation under field conditions. The study also suggests that tank mix applications of paraquat with residual herbicides may be useful for season-long weed control in chickpea and result in improved yield. The current study warrants further evaluation of the tank-mix application of paraquat with pre-emergent herbicides for season-long weed control under field conditions.
Chickpea (Cicer arietinum L.) is an important pulse crop and has great export potential in Australia. Chickpea is considered for nutritional quality and it is an important source of protein and minerals. Globally, it occupies an area of 14.6 M ha area and with a production of 14.8 Mt (Nair et al., 2014; Merga and Haji, 2019). In the conservation agriculture systems of Australia, the cultivation of chickpea helps in resource-saving and increases the fertility status of the soil by biologically fixing nitrogen (Nyanga, 2012).
Weeds are an important limiting factor to achieving a high yield in chickpea as they rob the soil nutrients and available stored moisture in the soil and compete for weeds (Al-Thahabi et al., 1994). The slow-growing nature of the crop in the winter season makes it vulnerable to weeds, especially when infested with broadleaf weeds (Taran et al., 2013). Being a broadleaf crop, herbicide options for control of broadleaf weeds are very limited in chickpea (Nath et al., 2018). Weed control in chickpea is essential to reduce the yield loss and further build-up of the weed seed bank in the soil (Mohammadi et al., 2005).
In chickpea, yield losses may vary from 24%-63%, depending upon weed infestation levels (Muhammad et al., 2011). Previous studies in Australia revealed that seed and straw yields of chickpea could be reduced by 81% and 63%, respectively, when the field remained unweeded compared with the weed-free situation (GRDC, 2008). Chickpea in Australia is planted at wide row spacing (50 cm to 100 cm), and complete ground cover is not attainable until 8 to 10 weeks after planting (GRDC, 2008). In chickpea, the critical period of weed competition has been suggested between 35 to 60 days after crop emergence (Al-Thahabi et al., 1994; Mohammadi et al., 2005). Pre-emergence herbicides, such as pendimethalin, trifluralin, and aclonifen, are recommended for weed control in chickpea, but these are unable to provide a broad spectrum of weed control (Singh et al., 2014; Barros et al., 2018). In Europe, where herbicide options are limited due to strict EU legislation on pesticides, recent studies have shown that aclonifen as PRE fb by POST applications of ACCase-inhibitors can provide broad spectrum weed control in chickpea especially when combined with the use of increased seeding rates (Kanatas and Gazoulis, 2022). Some studies have suggested the use of imazethapyr for broad-spectrum weed control in chickpea (Kachhadiya et al., 2009; Khope et al., 2011), but reduced efficacy in the soil and phytotoxicity to the crop restricts its wider adoption (Kumar et al., 2016).
Post-emergent (POST) herbicides, such as quiazalofop-p-ethyl and fenoxaprop-p-ethyl, are available for weed control in chickpea, but they provide control o only grass weeds (Ansar et al., 2010; Kumar et al., 2015). It has been reported that broadleaf weeds, such as Medicago polymorpha L., Vicia sativa L., Convolvulus arvensis L., Chenopodium album L., Melilotus indicus (L.) All., and Rumex dentatus L. caused severe yield losses in chickpea (Nath et al., 2018). In Australia, chickpea is mainly infested with weeds, such as Avena ludoviciana Durieu, Sonchus oleraceus L., Chenopodium album L., Raphanus raphanistrum L., Arctotheca calendula (L.) Levyns, etc. Any herbicide may be selective to a crop, depending upon the dose, time, stage, crop-dependant, or having natural tolerance (Das, 2008), suggesting that herbicide’s safety to crops can be manipulated (Susha et al., 2018). Paraquat effectively controls grass and broadleaf weeds due to its high efficiency and low cost (Fuerst and Vaughn, 1990). Paraquat is quickly absorbed by plant leaves and inhibits photosynthesis by accepting electrons from photosystem I (PSI) (Qian et al., 2009). It is a light-activated non-selective herbicide that belongs to the bipyridylium family, and it breaks down the cell membranes in plants (Anonymous, 2016).
Peanuts (Arachis hypogaea L.) have been reported to have a natural tolerance to paraquat when applied before pegging and fruit development (Grichar, 1998). The timing of application of paraquat within 28 days after crop emergence is often referred to as a crack (Qian et al., 2009). Evaluation of new herbicides in chickpea may increase the flexibility in herbicide rotation programs aimed to delay the evolution of herbicide resistance by avoiding the overuse of any one group of herbicides (Heap, 2022).
There is a research gap to understand if chickpea is tolerant to paraquat when applied as POST. With limited options of herbicides for season-long and broad-spectrum weed control in chickpea, it is important to evaluate POST herbicides, such as paraquat, that may provide season-long broad-spectrum weed control and reduce yield losses due to weed interference. Therefore, the present investigation was undertaken to assess the paraquat selectivity of chickpeas. We hypothesized that POST applications of paraquat may be selective to chickpea, depending upon the crop stage.
The study regarding the evaluation of paraquat for chickpea safety was conducted in two runs (June 2021 and August 2021) at the research facility of the weed science unit at the Queensland Alliance for Agriculture and Food Innovation, The University of Queensland, Gatton, Australia. The study was conducted in pots that were kept on benches in an open environment under natural conditions.
The treatments comprised five paraquat (Gramoxone® 360 g/L, Syngenta Australia Ltd.) doses (0, 90, 180, 360, and 540 g ai ha-1) that were tested at six stages (0, 3, 6, 8, 10, and 20 days after crop emergence) of the chickpea crop. An adjuvant (Hasten™ 900 g/L, Victorian Chemical Company Pty. Limited, Australia) at 1% was added with paraquat. Treatments were tested in a randomized complete block design in 10 replicates. The chickpea variety used in the study was PBA Seamer (Seamer, hereafter). Seamer was selected as it is widely grown by chickpea growers in the northern cropping region of Australia. The characteristics such as semi-erect habit, excellent lodging tendency, and resistance to ascochyta blight made Seamer variety well adapted to the high rainfall of northern regions of Australia (Dron et al., 2022). Seamer has also been reported to have a greater weed-competitive ability than PBA HatTrick (Mahajan et al., 2019)
In the first experimental run, chickpea was sown on June 25, and July 5, 7, 9, 12, and 14, 2021. In the second experimental run, chickpea was sown on August 6, 16, 18, 20, 23, and 25, 2021. Chickpea seeds were planted at different dates so that paraquat spray could be done on the same day for different plant stages. Initially, three seeds of chickpea per pot were sown and after emergence, one plant of chickpea per pot was maintained. Pots of 20 cm diameter were used and each pot was filled with potting mix (Centenary Landscape, Brisbane, Queensland, Australia).
Paraquat spray in the first and second experimental runs was done on July 26 and September 03, 2021, respectively. The spray was done using a research track sprayer equipped with Teejet XR 110015 flat fan nozzles calibrated to an output spray volume of 108 L ha-1. Pots were kept dry until 24 h after spray and thereafter, were watered with a sprinkler system. The average height of chickpea at paraquat spray was 15, 10, 7, 6, 2, and 1 cm, which corresponded with 20, 10, 8, 6, 3, and 0 days after crop emergence, respectively.
Plants were allowed to grow for 52 days after treatment (DAT) of paraquat application to determine herbicide efficacy. Plants were assumed dead if they did not have at least one new leaf at 52 DAT. Plant biomass was evaluated at 52 DAT. At harvesting, the height of the plant was measured from the base to the tip of the plant. Plants were harvested from the base of the plants and dried in an oven at 70 °C for biomass. The biomass of chickpea in each treatment was assessed based on the relative percentage of their respective control.
Experimental data were subjected to the analysis of variance (ANOVA) using statistical software (CPCS1-Punjab Agricultural University, Ludhiana, India). Experimental runs x treatments interactions were found nonsignificant for plant height and biomass; therefore, data were pooled over the two experimental runs (a total of 20 replications) for further analysis. Where the ANOVA found significant treatment effects, means were separated at P ≤ 0.05 using Fisher’s protected LSD test. Data were also validated to meet the assumptions of normality and variance before analysis.
The plant height of chickpea with varying doses of paraquat did not decrease compared with nontreated control when paraquat was applied at crop emergence or 3 days after crop emergence (Table 1; Figure 1). The height of chickpea did not reduce when paraquat was applied at 180 g ai ha-1 at 6, 8, and 10 days after crop emergence compared with nontreated control. However, the height of chickpea was reduced even with the lowest dose of paraquat (90 g ai ha-1) compared with nontreated control when applied 20 days after crop emergence.
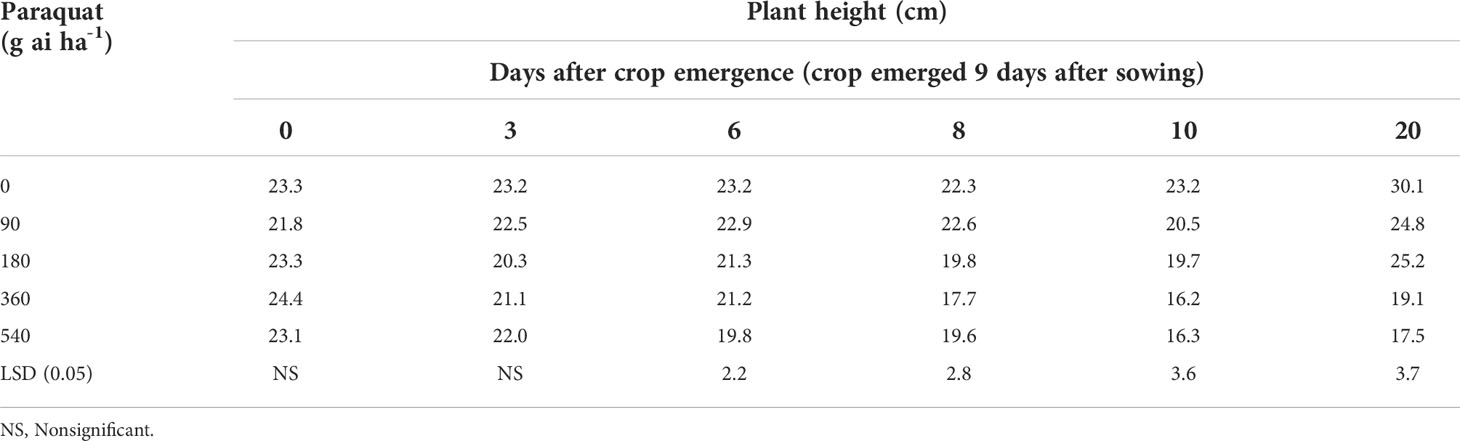
Table 1 Plant height of chickpea in relation to various doses of paraquat when applied at different crop stages (Data recorded 52 days after treatment).

Figure 1 Chickpea plants after paraquat spray done at different stages. Herbicide doses from left to right are 0, 90, 180, 360, and 540 g ai ha-1.
The biomass of chickpea did not decrease even at high doses of paraquat when applied at the crop emergence stage compared with nontreated control (Table 2; Figure 1). Paraquat 180 g ai ha-1 when applied at 3, 6, 8, and 10 days after crop emergence did not reduce the biomass of chickpea compared with nontreated control. However, the application of paraquat even at the lowest dose (90 g ai ha-1) reduced chickpea biomass compared with nontreated control when applied 20 days after crop emergence. No sign of visual toxicity to chickpea was observed when paraquat 180 g ai ha-1 was applied even after 20 days of crop emergence, although little suppression in biomass was there. However, signs of toxicity to chickpea were observed when treated with the highest dose of paraquat 540 g ai ha-1 at 6, 8, 10, and 20 days after crop emergence (Figure 1).
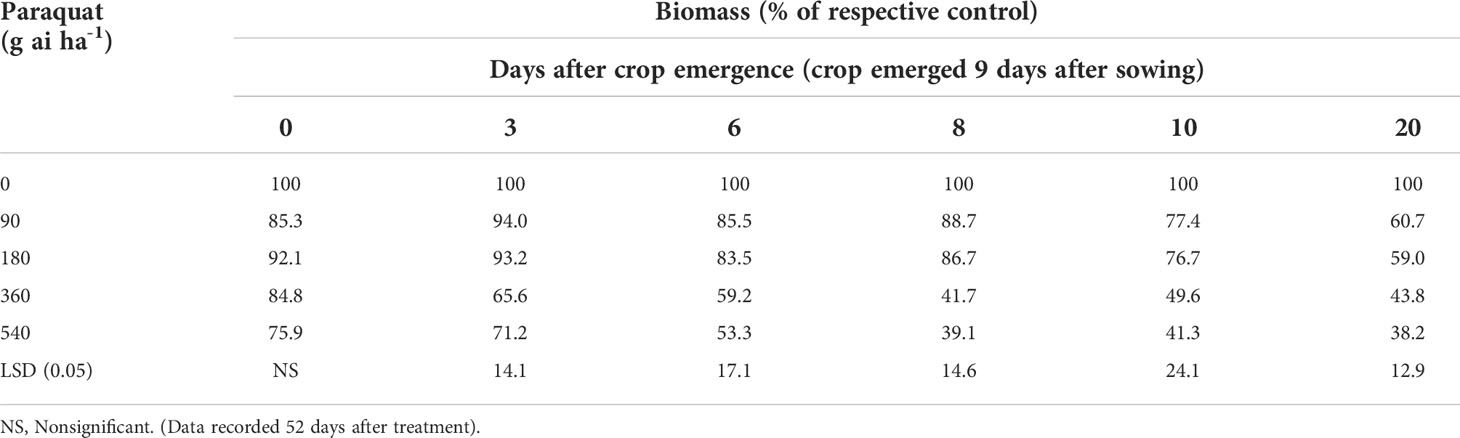
Table 2 Biomass of chickpea in relation to various doses of paraquat when applied at different crop stages.
This study implied that the application of paraquat 180 g ai ha-1 may prove useful for chickpea when applied up to 10 days after crop emergence. Paraquat application to chickpea even at the highest dose (540 g ai ha-1) did not reduce the plant height and biomass when applied just at the crop emergence stage. No sign of toxicity of paraquat to chickpea was observed when applied at 180 g ai ha-1 even after 20 days of crop emergence (visual observation). However, reduced height and biomass were observed with this treatment (180 g ai ha-1 applied 20 days after crop emergence) compared with nontreated control, which is likely to be recovered as there was no sign of toxicity.
Paraquat is a light-activated non-selective herbicide from the bipyridylium family, that breaks down cell membranes, therefore is useful for controlling grass, sedge, and broadleaf weeds. This study found that paraquat 180 g ai ha-1 applied up to 10 days after crop emergence was safe for chickpea. Therefore, paraquat at 180 g ai ha-1 can be used for broad-spectrum weed control in chickpea about 10 days after crop emergence. Chickpea is a slow-growing crop, taking 9-10 days for crop emergence in the winter season. Weed emergence is faster, therefore, paraquat application up to 20 days after crop planting (or 10 days after crop emergence) may control emerged weeds at that time without causing any damage to the crop.
Paraquat is a contact herbicide and can be tank-mixed with other herbicides that provide residual activity for season-long weed control. Residual herbicides, such as pendimethalin, trifluralin, triallate, isoxaflutole, prosulfocarb, and pyroxasulfone, are commonly used pre-emergent herbicides in Australian chickpea systems (GRDC, 2008). Residual activity from these herbicides when mixed with paraquat may help to control late cohorts of weeds, therefore, may provide season-long weed control. However, the tank mixing of paraquat with pre-emergent herbicides needs to be tested under field conditions for crop safety, season-long weed control, and high seed yield in chickpea. There is also a need to evaluate the effect of paraquat application on nodulation in chickpea.
We did not find a study, in which selectivity of chickpea to paraquat was reported. The selectivity of chickpea for paraquat was possibly due to the inherent tolerance of the crop against this herbicide. Paraquat resistance in weeds has been reported due to PAR 1 gene that caused a reduced uptake of paraquat through plasma membrane-localized transporters (Li et al., 2013). However, it is also possible that selectivity might have occurred due to the herbicide doses and stages of the crop as evident from the biomass and plant height data in this study. The leaves of chickpea are coated with 95% of malic acid and 5% of oxalic acid (Koundal and Sinha, 1981). In maize (Zea mays L.), paraquat (100 μM) caused injury by 69%-82% (Jang et al., 2019). However, leaf injury on maize plants did not appear when paraquat (100 μM) was mixed with malic acid or oxalic acid (1%) (Jang et al., 2019). This suggests that malic and oxalic acid contents in chickpea leaves might have nullified the activity of paraquat in chickpea and need further investigation.
It is quite possible that chickpea tolerance to paraquat in this study may be variety-specific as we conducted this study with one variety. It is also possible that tolerance to paraquat might be related to limited absorption/adsorption of herbicides by chickpea leaves when plants are small in size. Therefore, studies are required by including a greater number of varieties. There is also a need to study the adsorption/absorption of paraquat on chickpea leaves to fully understand the mechanism of chickpea tolerance to paraquat. However, in peanut cultivars, it was reported that foliar absorption and translocation of paraquat did not vary between cultivars (Wehtje et al., 1991).
Chickpea tolerance to paraquat was interesting, and registration of paraquat in chickpea may improve early weed control, especially of broadleaf weed species. Many grass and broadleaf weed species have been listed on the paraquat label (Anonymous, 2016). Although some toxicity was observed at the highest doses (360 or 540 g ai ha-1) of paraquat when applied 20 days after crop emergence, chickpea eventually recovered and may produce a higher yield than weed-infested crops, which need to be verified under field conditions. Growers may be willing to tolerate a moderate herbicide injury to chickpeas with paraquat if broadleaf weed control is improved, given that there are currently no broadleaf herbicides registered for chickpea in Australia. It has been reported that moderate levels of herbicide injury in chickpea did not cause yield loss (Taran et al., 2013). This first study was conducted in pots and therefore, we included time after crop planting/emergence. Future field studies should consider growing degree days in relation to chickpea stage and paraquat application timing.
Paraquat is registered for weed control in peanut within 28 days after emergence (Johnson et al., 1993). These authors argued that paraquat use later than 28 days after emergence is discouraged due to the potential for crop injury with less time for plant recovery. However, this fact is to be verified for chickpea under field conditions as it is a slow-growing and long-duration crop.
High broadleaf weed densities in chickpea may substantially reduce its yield and increase the weed seed bank in the soil if left uncontrolled. Ideally, broadleaf weed management in chickpeas includes the use of pre-emergent herbicides followed by hand-weeding if broadleaf weeds are not controlled by pre-emergent herbicides. In such a scenario, paraquat has the potential to control early flushes of broadleaf weeds in chickpeas.
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Conceptualization: BC, GM; Formal analysis: GM; Funding acquisition: BC; Investigation: BC and GM; Methodology: BC and GM; Project administration: BC; Writing – original draft: GM; Review & editing: BC. All authors contributed to the article and approved the submitted version.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fagro.2022.969960/full#supplementary-material
Al-Thahabi S. A., Yasin J. Z., Abu-Irmaileh B. E., Haddad N. I., Saxena M. C. (1994). Effect of weed removal on productivity of chickpea (Cicer arietinum l.) and lentil (Lens culinaris med.) in a Mediterranean environment. J. Agron. Crop Sci. 172, 333–334. doi: 10.1111/j.1439-037X.1994.tb00184.x
Anonymous. (2016). Gramoxone SL 2.0 herbicide label. EPA reg. no. 100-1431. syngenta pub. no. SCP 1431A-L1F 1115 (Greensboro, NC: Syngenta Crop Protection, LLC).
Ansar M., Anwar A., Arif M., Nadeem M., Zahid A. (2010). Screening of pre and post emergence herbicides against chickpea (Cicer arietinum l.) weeds under semi rainfed conditions of pothohar, Pakistan. Pak. J. Weed Sci. Res. 16, 421–430.
Barros J., Calado J., Carvalho M., Duarte I. (2018). Effect of different doses and spray volumes of the herbicide aclonifen for pre–emergence weed control in chickpea crop. Rev. Ciências Agrárias 41, 432–442.
Das T. K. (2008). Weed science: Basics and applications, first ed (New Delhi: Jain Brothers Publishers), 901.
Dron N., Merrill Ryan W. M., Forknall C., Hobson K., Sutton T., Bithell S. (2022). Phytophthora root rot and waterlogging in chickpeas–minimising risk and management options. Grains Res. Update, 100.
Fuerst E. P., Vaughn K. C. (1990). Mechanisms of paraquat resistance. Weed Technol. 4, 50–156. doi: 10.1017/S0890037X0002515X
Grichar W. J. (1998). Effects of paraquat application and timing on peanut (Arachis hypogaea) growth, yield and grade. Tex. J. Agric. Nat. Resour. 11, 41–47.
Heap I. M. The international survey of herbicide resistant weeds. Available at: http://www.weedscience.org/ (Accessed 22 January 2022).
Jang S. J., Kim S. S., Bak H. Y., Yun Y. B., Kuk Y. I. (2019). Effects of organic acids on paraquat activity in maize. S Afr J. Bot. 123, 286–292. doi: 10.1016/j.sajb.2019.03.034
Johnson W. C. III, Chamberlin J. R., Brenneman T. B., Todd J. W., Mullinix B. G. Jr, Cardina J. (1993). Effects of paraquat and alachlor on peanut (Arachis hypogaea) growth, maturity, and yield. Weed Technol. 7, 855–859. doi: 10.1017/S0890037X0003788X
Kachhadiya S. P., Savaliya J. J., Bhalu V. B., Pansuriya A. G., Savaliya S. G. (2009). Evaluation of new herbicides for weed management in chickpea (Cicer arietinum l.). Legume Res. 32, 293–297.
Kanatas P. J., Gazoulis I. (2022). The integration of increased seeding rates, mechanical weed control and herbicide application for weed management in chickpea (Cicer arietinum l.). Phytoparasitica 50 (1), 255–267. doi: 10.1007/s12600-021-00955-3
Khope D., Kumar S., Pannu R. K. (2011). Evaluation of post-emergence herbicides in chickpea (Cicer arietinum). Indian J. Weed Sci. 43, 92–93.
Koundal K. R., Sinha S. K. (1981). Malic acid exudation and photosynthetic characteristics in cicer arietinum. Phytochemistry 20, 1251–1252. doi: 10.1016/0031-9422(81)80015-5
Kumar N., Hazra K. K., Yadav S. L., Singh S. S. (2015). Weed dynamics and productivity of chickpea (Cicer arietinum) under pre- and post-emergence application of herbicides. Indian J. Agron. 6, 570–575.
Kumar N., Nath C. P., Hazra K. K., Sharma A. R. (2016). Efficient weed management in pulses for higher productivity and profitability. Indian J. Agron. 61, S93–S105.
Li J., Mu J., Bai J., Fu F., Zou T., An F., et al. (2013). Paraquat Resistant1, a golgi-localized putative transporter protein, is involved in intracellular transport of paraquat. Plant Physiol. 162, 470–483. doi: 10.1104/pp.113.213892
Mahajan G., McKenzie K., Chauhan B. S. (2019). Influence of row spacing and cultivar selection on annual ryegrass (Lolium rigidum) control and grain yield in chickpea (Cicer arietinum). Crop Pasture Sci. 70, 140–146. doi: 10.1071/CP18436
Merga B., Haji J. (2019). Economic importance of chickpea: production, value, and world trade. Cogent Food Agric. 5, 1615718. doi: 10.1080/23311932.2019.1615718
Mohammadi G., Javanshir A., Khooie F. R., Mohammadi S. A., Zehtab Salmasi S. (2005). Critical period of weed interference in chickpea. Weed Res. 45, 57–63. doi: 10.1111/j.1365-3180.2004.00431.x
Muhammad N., Sattar A., Ashiq M., Ahmad I. (2011). Efficacy of pre and post emergence herbicides to control weeds in chickpea (Cicer arietinum l.). Pakistan J. Weed Sci. Res. 17, 17–24.
Nair R., Schafleitner R., Easdown W., Ebert A., Hanson P., D’arros H. J., et al. (2014). Legume improvement program at AVRDC-the world vegetable center: impact and future prospects. Ratarstvo i povrtarstvo 51, 55–61. doi: 10.5937/ratpov51-5488
Nath C. P., Dubey R. P., Sharma A. R., Hazra K. K., Kumar N., Singh S. S. (2018). Evaluation of new generation post-emergence herbicides in chickpea (Cicer arietinum l.). Natl. Acad. Sci. Lett. 41, 1–5. doi: 10.1007/s40009-017-0604-z
Nyanga P. H. (2012). Food security, conservation agriculture and pulses: evidence from smallholder farmers in Zambia. J. Food Res. 1, 120. doi: 10.5539/jfr.v1n2p120
Qian H., Chen W., Sun L., Jin Y., Liu W., Fu Z. (2009). Inhibitory effects of paraquat on photosynthesis and the response to oxidative stress in chlorella vulgaris. Ecotoxicology 18, 537–543. doi: 10.1007/s10646-009-0311-8
Singh G., Aggarwal N., Ram H. (2014). Efficacy of post-emergence herbicide imazethapyr for weed management in different mungbean (Vigna radiata) cultivars. Indian J. Agric. Sci. 84, 540–543.
Susha V. S., Das T. K., Nath C. P., Pandey R., Paul S., Ghosh S. (2018). Impacts of tillage and herbicide mixture on weed interference, agronomic productivity and profitability of a maize–wheat system in the north-western indo-gangetic plains. Field Crop Res. 219, 180–191. doi: 10.1016/j.fcr.2018.02.003
Taran B., Holm F., Banniza S. (2013). Response of chickpea cultivars to pre- and post-emergence herbicide applications. Can. J. Plant Sci. 93, 279–286. doi: 10.4141/cjps2012-167
Keywords: gram, herbicide, post-emergence, toxicity, weed
Citation: Mahajan G and Chauhan BS (2022) The first report of chickpea (Cicer arietinum L.) tolerance to Paraquat in Australia. Front. Agron. 4:969960. doi: 10.3389/fagro.2022.969960
Received: 15 June 2022; Accepted: 14 July 2022;
Published: 02 August 2022.
Edited by:
Saddam Hussain, University of Agriculture, Faisalabad, PakistanReviewed by:
Ioannis Gazoulis, Agricultural University of Athens, GreeceCopyright © 2022 Mahajan and Chauhan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Gulshan Mahajan, Zy5tYWhhamFuQHVxLmVkdS5hdQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.