
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Agron. , 05 July 2022
Sec. Disease Management
Volume 4 - 2022 | https://doi.org/10.3389/fagro.2022.911969
This article is part of the Research Topic Cocoa Diseases and their Management View all 5 articles
The vascular disease Verticillium wilt of cacao (Theobroma cacao), caused by the soilborne fungus Verticillium dahliae, is often qualified as a “minor” disease. However, it can cause severe losses locally, for example, in western Uganda and northeast of the Democratic Republic of Congo. This disease is difficult to control, notably due to an extended host range and prolonged survival in the soil. Therefore, Verticillium wilt must be addressed through an integrated disease management strategy as employed for other tree crops such as olive. Few studies, nonetheless, have focused on how to confront this disease in cacao. This paper aims to provide an overview of our knowledge on Verticillium wilt on cacao and the integrated disease management strategies for preventing and controlling it. Promising avenues based on findings in other crops that could be adapted to cacao are also explored. Good agricultural practices, genetic resistance, biological control, induced resistance, and the use of organic amendments with or without biocontrol agents are discussed. Moreover, the potential benefits of some of these solutions toward the resistance to other cacao diseases, abiotic stress, and nutrition improvement are presented.
The cacao tree (Theobroma cacao L., Malvaceae) is an understory tree that occupies, in its natural habitat, the lower strata of the humid forests of tropical South America. It produces fruits (pods) with seeds (beans) used in the confectionery industry, notably for the production of chocolate. Cacao farming has increased from around 4.4 million hectares in the 1960s to over 11.8 million hectares in 2018 (FAO, 2022), producing today around 5 million tons of cocoa beans (ICCO, 2022). Cocoa production is essential for approximately 5.5 million smallholder cacao farmers and around 14 million rural workers (Fountain and Hütz-Adams, 2015). The West African cacao belt (from Ivory Coast to Cameroon) accounts for over 70% of the world’s cocoa production (ICCO, 2022), while the remainder comes from the Americas and Asia.
Diseases are the main reasons for production losses (Ploetz, 2016). The five most damaging diseases of cacao are black pod, witches’ broom, frosty pod, vascular streak dieback, and cacao swollen shoot virus, which, collectively, are responsible for annual losses of approximately 1.3 million tons of cacao beans, representing some 20% of total hypothetical yield (Ploetz, 2016). Other diseases can significantly impact cacao but on a local scale, such as Verticillium wilt (VW) in western Uganda and northeast of the Democratic Republic of Congo.
VW on cacao is caused by the soilborne fungus, Verticillium dahliae Kleb. Verticillium is a genus of Ascomycete fungi whose main morphological characteristic is the formation of verticillate conidiophores producing asexual conidia (Inderbitzin et al., 2011).
Besides cacao, V. dahliae causes disease on over 400 hosts worldwide, mainly dicotyledonous species, including many economically important crops (Malcolm et al., 2013). Weeds can be asymptomatic hosts and act as V. dahliae reservoirs (Resende, 1994; Malcolm et al., 2013). Verticillium dahliae can remain dormant in the soil for years through small and melanized resting structures that are extremely durable and will only germinate in the proximity of a suitable host (Inderbitzin et al., 2011).
Verticillium dahliae was recognized as a cacao pathogen for the first time in Uganda, causing a disease previously referred to as “sudden death disease” reported in this country since 1915 (Leakey, 1965). Verticillium wilt was indicated as the main disease affecting cacao in Uganda, with tree losses of up to 30% on some farms (Matovu, 1973) and considered to be a significant limitation to cacao production (Leakey, 1965). In Uganda, VW has also been reported on cotton (Gossypium hirsutum L.) (Emechebe et al., 1972; CFC and 21FT, 2003). Following this first report, Verticillium wilt on cacao was reported in Brazil, where it was identified as a severe problem in the States of Bahia and Espírito Santo (Oliveira, 1983; Resende, 1994). In certain areas, it was estimated to cause annual plant mortality of up to 10% (de Almeida et al., 1989). Verticillium dahliae has also been reported on cacao in other countries in South America (Table 1), notably Colombia (Granada, 1989; Resende et al., 1995) and Peru (Leon-Ttacca et al., 2019a; Leon-Ttacca et al., 2019b; Bouchon, 2020). In Ecuador, a pathogen causing wilt of cacao was also identified as being in the genus Verticillium but was not identified to species level (Zavala et al., 2010).
Recent studies showed that VW was found in five districts in Uganda, albeit with variable incidence, and in neighboring regions of the Democratic Republic of Congo: Ituri and North Kivu (Bouchon, 2020). Since cocoa production in Uganda and DR Congo is expanding, production in Uganda increased from 8.982 in 2008 to 30.752 metric tonnes in 2018 (UEPB, 2021) and in DR Congo from 5.431 in 2010 to 18.475 metric tonnes in 2018 (BCC, 2019) it means there is a risk that these new areas could be impacted by V. dahliae.
Although VW is generally considered a minor cacao disease globally, it can be a serious constraint to cocoa production locally. Given its status as a minor cacao disease, it is not surprising that relatively little is known about VW on cacao. Only a few studies were conducted on VW, mainly in Uganda during the 1960-1970s and in Brazil during the 1990-2000s. Limited knowledge has been generated on the biology of the disease; its epidemiology was studied mainly through observations under field conditions (Trocmé, 1972; Matovu, 1973). Only a few publications have addressed its control in cacao. Most of the current knowledge regarding Verticillium wilt control comes from cotton and olive (Olea europaea L. subsp. europaea var. europaea) (e.g., Montes-Osuna and Mercado-Blanco, 2020).
Verticillium wilt symptoms (Figure 1) can vary between hosts, and there are no unique symptoms that belong to all plants infected. Disease symptoms may comprise chlorosis, vein clearing, wilting, stunting and necrosis. Brown vascular discoloration may be observed in stem tissue sections (Fradin and Thomma, 2006) (Figures 1A, B). Through infection of the roots, V. dahliae causes a vascular disease that results in severe yield and quality losses (Inderbitzin et al., 2011). In cacao, when V. dahliae enters the xylem, the tree forms defensive barriers that inhibit transpiration, leading to discoloration, and generally sudden wilting of leaves and subsequent necrosis of leaves and fine branches, stunting or death of the plant (Emechebe et al., 1971; Resende et al., 1996b; Leon-Ttacca et al., 2019a; Leon-Ttacca et al., 2019b) (Figure 1C). In general, symptoms of V. dahliae infection appear when the cacao tree starts to bear pods (Matovu, 1973), although the pathogen most likely infected the tree beforehand. Severe attacks, following especially dry conditions or waterlogging, can cause the death of a cacao tree one week after a situation of apparent health and vigor (Leakey, 1965). In certain cases, natural recovery of an infected tree can occur; this has been reported for trees such as cacao (Emechebe et al., 1974), peach (Prunus persica (L.) Batsch) (Ciccarese et al., 1990), almond (P. dulcis (Mill.) Webb) (Ciccarese et al., 1990), apricot (P. armeniaca L.) (Taylor and Flentje, 1968; Vigouroux and Castelain, 1969), avocado (Persea americana Mill.) (Latorre and Allende, 1983), olive (Blanco-López et al., 1990), pistachio (Pistacia vera L.) (Hiemstra, 1998) and ash tree (Fraxinus excelsior L.) (Keykhasaber et al., 2018). For a given species, recovery may depend on the genotype. In cacao, recovery is rarely observed (Matovu, 1973) and only in more resistant varieties (Resende, 1994). For olive, it has been observed that the recovery of resistant and moderately resistant genotypes was three times more likely to occur than the recovery of susceptible plants (López-Escudero and Blanco-López, 2005). More details on the biology of Verticillium wilt in cacao are presented in Flood et al. (2016).

Figure 1 Symptoms of Verticillium wilt on cacao trees. (A) cross- and (B) longitudinal sections of cacao stems infected with V. dahliae, showing staining in xylem; (C): cacao tree showing wilt symptoms following infection with V. dahliae.
Two main pathotypes of V. dahliae have been reported on several crops, such as cotton and olive: defoliating and non-defoliating, based on their ability to induce either complete defoliation of the host or partial/no defoliation (Schnathorst and Mathre, 1966; Bejarano-Alcázar et al., 1996). However, a continuum of symptoms, rather than the occurrence of distinct pathotypes, was suggested by other authors (Ashworth Jr, 1983; Dervis et al., 2010). Cotton plants infected by defoliating pathotypes develop symptoms sooner and more rapidly than when infected by non-defoliating pathotypes (Bejarano-Alcázar et al., 1995; Bejarano-Alcázar et al., 1997). Only one isolate was characterized as defoliating in cacao; its origin was Colombia (Resende, 1994). It overcame the resistance of a tree considered to be resistant to non-defoliating isolates. Infection with defoliating isolates from cotton triggered increased symptoms in cacao, less pronounced water stress, and enhanced ethylene production compared with non-defoliating isolates (Resende et al., 1996).
To assess genetic variation in V. dahliae, vegetative compatibility, i.e., the ability to form a stable heterokaryon by fusion between separate isolates, has been used (Daayf et al., 1995; Jiménez-Díaz et al., 2006). In VW isolates from cacao in Uganda, three distinct genetic groups (i.e., vegetative compatibility groups, VCG) were found among 24 isolates. The only VCG found in the Democratic Republic of Congo (DR Congo, 5 isolates) is similar to one of the VCG found in Uganda. Verticillium dahliae from Peru (4 isolates studied) form a distinct genetic group (Bouchon, 2020). To improve understanding of Verticillium wilt etiology, it will be necessary to study V. dahliae genetic variation and its implications by assessing VCG groups from different geographic regions. Resistance of cacao to V. dahliae may vary depending on the VCG of the infecting isolate as it is in several other crops (e.g., Dervis et al., 2010; Göre et al., 2014; Jiménez-Díaz et al., 2017; Fan et al., 2018). Nonetheless, there is insufficient information on the symptoms’ severity triggered by different V. dahliae isolates towards cacao.
Verticillium dahliae can be isolated from the xylem of roots, stems, branches, twigs, leaves, and even seeds of many commercial crops. Diagnosis is often carried out following isolation of the fungus from excised vascular tissue on an NP-10 semi-selective medium (Kabir et al., 2004). Although serological tests have been developed to certify planting materials, efforts to detect and identify Verticillium species mainly use molecular diagnostic techniques that enable consistent and early in-planta detection, e.g., in olive (Mercado-Blanco et al., 2003; Mousavi et al., 2020).
These molecular diagnostic techniques are especially useful since they allow for detection even at (very) low inoculum densities. This is necessary since V. dahliae can damage crops at very low inoculum densities, e.g., starting at 2 CFU/g of soil for strawberry (Fragaria x ananassa Duch.) and cotton (Harris and Yang, 1996; Bejarano-Alcázar et al., 1996). Moreover, they can help determine the presence of V. dahliae in the soil of land potentially dedicated to cacao cultivation, thereby preventing problems later. These molecular techniques, notably real-time quantitative PCR, nested PCR, loop-mediated isothermal amplification (LAMP), or recombinase polymerase amplification combined with lateral-flow dipstick technology, are preferable to any plating technique (Pérez-Artés et al., 2005; Moradi et al., 2014; Borza et al., 2018; Ju et al., 2020) but might present problems in countries where resources are limited, and the correct research infrastructure is absent, as in numerous cocoa-producing countries. In these cases, plating techniques remain of interest.
Generally speaking, controlling Verticillium wilt in cacao is difficult and costly. Moreover, no curative solutions exist for VW. Although applying systemic fungicides for Verticillium wilt control, e.g., from the benzimidazoles’ family, has shown favorable results with annual crops such as strawberry, they are inefficient in controlling Verticillium wilt in tree crops such as cacao (Oliveira, 1983; Lawrence et al., 1991) and olive (Jiménez-Diaz et al., 2012). Fungicide treatments with benomyl and azoxystrobin showed some efficiency in reducing VW in avocado fields, but they were significantly less effective than a combination of cultural practices (Ramírez-Gil and Morales-Osorio, 2021). Also, economic and environmental costs were not considered – nor was the fact that the use of benomyl is not authorized in certain countries.
Verticillium wilt control in cacao has not received the attention it merits. It is clear that to reduce the losses due to VW and prevent its spread to new planting areas, integrated management strategies, including combinations of biological, chemical, physical, and cultural control measures, are needed (e.g., Mercado-Blanco and López-Escudero, 2012; Ramírez-Gil and Morales-Osorio, 2021). For example, in field conditions, an integrated approach for managing Verticillium wilt in avocado was the most efficient. The combination of pruning, solarization, drainage, application of Trichoderma sp. and mycorrhiza (Rhizoglomus fasciculatum Thaxt.), sucrose, organic matter, and mineral amendment led to a disease reduction of 80.3% at the end of a two-year field trial. In contrast, fungicide treatment led to a disease reduction of 62.3% and solarization to a 35.7% disease reduction (Ramírez-Gil and Morales-Osorio, 2021).
In light of the above, it is clear that there is a need to address the challenges posed by VW in cacao. Thus, this review aims to present an overview of current knowledge regarding VW in cacao and propose promising solutions for its control. The review considers the available literature on VW in cacao and looks at control strategies for VW in other crops to provide recommendations for future research.
Any successful cacao pest and disease management strategy starts with implementing good agricultural (control) practices. This is also valid for VW, and practices that aim to reduce the incidence, severity, and spread of V. dahliae are thus required.
As a first step, when planting new cacao, planting materials — cacao seedlings and potting soil — should come from disease-free regions and be pathogen-free (Pereira et al., 2008).
Planting in areas that could be contaminated should be avoided (Flood et al., 2016). Thus, replanting in areas previously affected by VW should be avoided unless the site has lain fallow for an extended period. However, since V. dahliae can survive up to 14 years in the field under non-host cropping (Mol et al., 1996), this is generally not a viable option. Detection of V. dahliae in soils before plantation is advised. Soil solarization could be a means to reduce fungal soilborne inocula in established orchards. However, this technique requires consideration of two factors. First, infections can occur deeper than the depth at which solarization is found efficient. Second, in shaded areas, the soil temperature is always lower, limiting the possibilities for pathogen eradication (Ten Hoopen and Krauss, 2006).
Sanitation practices are required: any dead trees should be uprooted and eliminated by burning (Oliveira and Luz, 2005) as they can provide a source of inoculum. Also, tools in contact with symptomatic trees and contaminated soils should be disinfected. When planting, care should be taken to avoid damage to cacao seedlings’ roots, as wounds can provide access to Verticillium. Similarly, damaging cacao trees should be avoided during plot maintenance practices such as weeding (Emechebe, 1975). However, weed management is crucial in cacao growing areas since certain weeds can act as a reservoir of V. dahliae (Resende, 1994; Malcolm et al., 2013). For example, among 12 common Brazilian weeds, 8 are hosts to V. dahliae (Resende et al., 1994).
Optimal shading is essential for cacao cultivation as it notably helps reduce excessive loss of water through evapotranspiration and contributes to mitigating stressful environmental conditions (Lahive et al., 2018). Also, once the disease is installed, adequate shading could improve VW management and extend the life of the plants (Trocmé, 1972; Oliveira and Luz, 2005; Pereira et al., 2008). Lack of shade and drought impact VW incidence and severity. Disease prevalence was found to be slightly higher in a relatively dry year (1235 mm of annual rainfall) in a natural infestation of cacao trees growing in fields surrounding Uganda’s National Institute of Cacao Research in Kituza compared to the two previous, more humid years (1617 and 1589 mm of annual rainfall, respectively) (Trocmé, 1972). In Brazil, increased incidence of Verticillium wilt was noted in dry areas in combination with a lack of shade (de Almeida et al., 1989), and shading has also been shown to reduce the incidence and severity of Verticillium wilt of cacao in Uganda (Trocmé, 1972; Matovu, 1973). Indeed, Trocmé noted in 1972 that 75% of symptomatic trees (out of 212 trees from various cacao fields) were under reduced or no shade; this percentage was 63% symptomatic trees out of 443 trees in the Kituza region, presumably due to a more humid microclimate. Matovu made similar observations in 1973 when he noticed from a study on 373 cacao farms that 74% of the cacao farms installed on previously forested fields had a VW incidence of less than 1%. In contrast, for cacao fields installed on lands previously occupied by banana plantations, the percentage was 62% (Matovu, 1973). The author hypothesized this difference to be due to shade and soil fertility. Emechebe (1975) also observed a significant reduction in the incidence of infection (21 days after inoculation of cacao seedlings with V. dahliae) in conditions of (artificial) high relative humidity, 57% compared to 77% under normal atmospheric humidity, and highlighted the role of transpiration in the systemic distribution of V. dahliae conidia. Taking into account Verticillium wilt into the agroforestry research efforts would help understand the ecology of this disease better and help design mitigation solutions.
An additional way to reduce VW incidence would be to fulfill cacao’s nutritional needs adequately. Indeed, Verticillium wilt is more severe in nutritionally stressed plants (Davis and Allen, 1984; Romanyà et al., 2019). Since soil fertility is low in some Ugandan soils (Van Asten et al., 2012), this could contribute to the disease’s prevalence in Uganda. Pegg and Brady (2002) reviewed several studies on different crops that showed that adding copper, cobalt, zinc, molybdenum, and manganese may help reduce V. dahliae incidence. In addition, sulfur may play an essential role in Verticillium wilt resistance. Indeed, resistant plants showed an accumulation of elemental sulfur in attacked xylem in cacao (Resende et al., 1996a) and tomato (Solanum lycopersicum L.) (Klug et al., 2015). Besides, enhanced fertilization with sulfur alleviated the incidence of Verticillium wilt in tomato (Bollig et al., 2013; Klug et al., 2015) and olive (Romanyà et al., 2019). Since cacao needs numerous nutritional elements to ensure high productivity, a well-reasoned fertilization regime could thus simultaneously increase production and reduce VW losses.
Selection for increased disease resistance in breeding programs is considered the most suitable way to deliver a long-term solution to the problem of Verticillium wilt of cacao (Resende, 1994; Oliveira and Luz, 2005; Pereira et al., 2008). Genetic resistance/tolerance to Verticillium wilt has been found in numerous crops (Fradin and Thomma, 2006).
Major genes for VW resistance are known in tomato (Ve1) (Fradin et al., 2009), potato (Jansky et al., 2004), lettuce (Lactuca sativa L.) (Sandoya et al., 2017), and sea-island cotton (G. barbadense L.) (Zhang et al., 2014). Resistance to V. dahliae is also associated with a major dominant gene in Arabidopsis thaliana (L.) Heynh. (Veronese et al., 2003). In cacao, the segregation ratio of resistance in seedlings from 5 crosses suggested monogenic heritability, with susceptibility being dominant (Braga and Silva, 1989). In contrast, polygenic control of resistance was identified in hop (Humulus lupulus L.) (Jakse et al., 2013) and olive (Trapero et al., 2015).
However, resistance/tolerance is often limited to a few genotypes such as, e.g., olive (Trapero et al., 2015), strawberry (Shaw et al., 2010), lettuce (Sandoya et al., 2017), tomato (Carrer Filho et al., 2016), sunflower (Helianthus annuus L.) (Creus et al., 2007) and cotton (Zhou et al., 2014; Abdelraheem et al., 2020). Resistance also depends on the virulence of Verticillium isolates (Resende, 1994). For example, most commercial olive cultivars are not resistant enough to a defoliating pathotype of V. dahliae (Carrero-Carrón et al., 2018), despite the significant amount of work done on genetic resistance/tolerance on olive for VW during the last 15 years (reviewed in Montes-Osuna and Mercado-Blanco, 2020).
The resistance of a limited number of cacao genotypes has been assessed with V. dahliae isolates from different origins (in vivo and in vitro: 6 and solely in vivo: 11 genotypes have been tested). Clones Pound 7, SIC 2, SIC 328, and PA 30 have been identified as resistant, clones PA 121, IMC 67, and SIC 802 as intermediate resistant, and clones BE 5, ICS 1, ICS 6, and ICS 8 as susceptible with non-defoliating isolates (Braga and Silva, 1989; Resende, 1994). Nonetheless, a defoliating Colombian isolate overcame the resistance of Pound 7 (Resende, 1994). In Uganda, the Amazonian and Amelonado types of cacao were less susceptible than the Trinatario ones in inoculation experiments (Emechebe, 1975). It is important to note that low genetic variability has been observed in farmed cacao in Uganda. Indeed, most cacao trees have an admixed status with mainly Marañon ancestry with significant contributions from Amelonado and Iquitos lineages (Gopaulchan et al., 2019). This low level of genetic variability presents a risk since there is little to no genetic basis for developing resistance to VW.
Breeding for VW resistance/tolerance in cacao would require more cacao genotypes to be assessed. In vivo inoculation of seedlings, followed by symptom observation, has been proven to be effective in identifying genotypes resistant to Verticillium wilt in trees such as Pistacia atlantica Desf., and Pistacia integerrima Stewart (Morgan et al., 1992), Acer platanoides L. (Chambers and Harris, 1997), A. rubrum L. (Townsend and Hock, 1973), O. europaea (Trapero et al., 2013) as well as T. cacao (Braga and Silva, 1989; Resende, 1994). On 15-day-old cacao seedlings, stem puncture inoculations have been shown to discriminate between resistant, moderate resistant, or susceptible cacao varieties to V. dahliae (Resende, 1994). Despite the young age at which it is possible to distinguish between resistance levels, this technique is demanding in cacao material and space. Moreover, such a set-up poses questions about how to contain the disease in the nursery, eliminate infected plants, disinfect soil, and presents difficulty in obtaining sufficient clonal material. In vitro evaluation of Verticillium wilt resistance using leaves is less demanding in space and plant material: it is a non-destructive method that allows promising seedlings to remain healthy, contrary to the in vivo method. Also, these leaf bioassays are relatively easy to perform and demand much less time than the in vivo tests using seedlings. Moreover, in hop and tomato, in vitro tests led to comparable outcomes to in vivo tests (Pegg and Street, 1984; Gold and Robb, 1995; Gold et al., 1996). In vitro cacao leaf bioassays have been used for screening cacao disease resistance or evaluating the variation in virulence for several cacao pathogens, e.g., Phytophthora megakarya (Brasier and Griffin) (Efombagn et al., 2011), Phytophthora palmivora (Butl.) (Tahi et al., 2000; Nyadanu et al., 2009), V. dahliae (Bouchon, 2020), Colletotrichum gloeosporioides (Penz. & Sacc.) (Maximova et al., 2006), Ceratocystis cacaofunesta (Engelbr. & Harr.) (Magalhães et al., 2016) and Lasiodiplodia theobromae (Pat.) Griff., and Maubl (Ali et al., 2020).
In vitro tests were designed to assess Verticillium wilt tolerance/resistance of cacao trees using leaves (blades and petioles) by Bouchon (2020). Two tests were developed: 1) electrolyte leakage on leaves and 2) fungal re-isolation following inoculation of leaves through petioles. Both tests are needed to decide between tolerance, resistance, or susceptibility. The results obtained were in agreement with those obtained from in vivo evaluation. However, further leaf sampling optimization needs to be carried out to enhance the test’s robustness and to be able to compare results between differently aged trees. Indeed, petiole sizes and leaf positions on trees need to be standardized (Bouchon, 2020).
In addition, efforts to identify VW resistance/tolerance could also benefit from a participatory breeding approach, especially since farmers can identify trees naturally recovered from Verticillium wilt, potentially providing a source of resistance (Resende, 1994). A relatively quick assessment of interesting trees could be realized by selecting leaves from resistant mother trees and assessing in vitro their VW resistance. Pollination could be used to create crosses between promising trees, and seedlings could be evaluated. Since Verticillium is a soilborne disease, once resistant rootstock has been identified, grafting could be proposed to manage Verticillium wilt as proposed for olive (Carrero-Carrón et al., 2018), avocado (Haberman et al., 2020) or, e.g., Ceratocystis wilt in cacao (Magalhães et al., 2016; dos Santos Fernandes et al., 2018).
The search for VW resistance in cacao is unfortunately still in its infancy. For example, genomic associations between SNP markers and Verticillium wilt resistance have only been addressed by Bouchon (2020). The marker that discriminates the most between Verticillium wilt resistance and susceptibility groups (TcSNP1111) is located at a putative resistance gene (nucleotide-binding site gene).
The use of antagonistic organisms (Biological Control agents, BCA) to reduce/suppress the activity of pathogens is an approach with many benefits in plant disease management. Indeed, it is compatible with organic certification, sustainable and durable (Ferreira and Musumeci, 2021), although nontarget effects should be controlled for.
Desirable BCAs for V. dahliae control can be selected from microorganisms in non-symptomatic plants present in infested fields or from suppressive soils or composts. The screening for biocontrol activity of potential BCA needs to be done in field conditions to increase their chances of success (Deketelaere et al., 2017). Microsclerotia are a major V. dahliae inoculum source and are the primary long-term survival structures and thus play an important role in the disease cycle (Fradin and Thomma, 2006). Therefore, desirable BCAs against V. dahliae need to have the “ability to affect the survival or germination of microsclerotia. Since the disease affects the xylem of the host plant, BCA should also be able to colonize the xylem and/or cortex and compete with the pathogen for nutrients and/or space”. Additional desirable characteristics of BCA include the capacity to “induce a resistance response in the plant and/or to promote plant growth.” The BCAs have to colonize the host quickly and “to adapt and endure the harsh (a)biotic conditions that they likely have to face after being released” (Ruano-Rosa et al., 2016; Deketelaere et al., 2017).
Several reviews have looked at the potential of biocontrol agents for Verticillium wilt management. Pseudomonas spp. and Bacillus spp. are the most common bacterial BCA reported for VW control (Deketelaere et al., 2017; Acharya et al., 2020). A few studies have looked at the potential of arbuscular mycorrhizal fungi to control Verticillium wilt (e.g., Mulero-Aparicio et al., 2020; Poveda and Baptista, 2021). Among the fungal BCA against VW, Trichoderma and non-pathogenic xylem-colonizing isolates of Fusarium and Verticillium have received the most attention (Deketelaere et al., 2017; Acharya et al., 2020).
The ubiquitous genus Trichoderma comprises over 375 fungal species (Cai and Druzhinina, 2021). They are found worldwide in a wide range of ecosystems. They are constitutive of the soil microflora. Also, they are common endophytes of annual and perennial plants (Pandey et al., 2021), including cacao. Several species of Trichoderma have been isolated as endophytes from cacao (Mejía et al., 2008; Bailey et al., 2008; Hanada et al., 2010; Almeida et al., 2018; Leon-Ttacca et al., 2019a; Leon-Ttacca et al., 2019b).
The potential of Trichoderma to alleviate biotic stress has been shown in several studies (Khana et al., 2020; Ferreira and Musumeci, 2021). Their antifungal activity relies on several mechanisms: mycoparasitism (suppression, parasitization, or killing of other fungi) (Druzhinina et al., 2011), antibiosis (production of toxins that are detrimental to other fungi), and competition (Khana et al., 2020). Trichoderma can induce resistance in plants (Hoitink et al., 2006; Nawrocka and Małolepsza, 2013) and enhance tolerance against abiotic stresses such as drought and extreme temperatures, salinity, and heavy metal accumulation (Khana et al., 2020). In addition, Trichoderma can act as plant growth promoters by, for example, improving nutrient availability and nitrogen use efficiency in plants (Carrero-Carrón et al., 2016; Khana et al., 2020; Meher et al., 2020).
Trichoderma spp. are the most studied BCA (Meher et al., 2020) and the most widely commercialized: 60% of fungal-based biocontrol agents (Singh et al., 2009). Thanks to a wide range of benefits, Trichoderma spp. are marketed worldwide as biofertilizers, biopesticides, stimulants of natural resistance, and growth promoters (Meher et al., 2020). In India, about 250 formulations comprising Trichoderma are destined for farmers’ application (Swain and Mukherjee, 2020), and over 50 Trichoderma-based biofungicides against many soilborne and foliar diseases are identified (Lorito et al., 2010; Woo et al., 2014). In the case of cacao diseases, Trichoderma is indeed the most studied BCA.
Numerous studies have shown the potential of Trichoderma to reduce Verticillium wilt in different annual crops such as strawberry (Mirmajlessi et al., 2016), cotton (Hanson, 2000; Naraghi et al., 2012), eggplant (Solanum melongena L.) (Mokhtari et al., 2018), and tomato (Jabnoun-Khiareddine et al., 2009) as well as in woody hosts such as olive, pistachio, and avocado (Supplementary Table 1). However, only one study has been conducted on the use of Trichoderma for VW control in cacao (Leon-Ttacca et al., 2019a; Leon-Ttacca et al., 2019b). Ten Trichoderma isolates from six species, all endophytes of cacao trees isolated in Peru, were assessed in vitro. Among them, isolates of T. asperellum (Samuels, Lieckf. & Nirenberg) and T. atroviride (Karst.) were the ones with the most antibiosis & mycoparasitism capacities (Leon-Ttacca et al., 2019a; Leon-Ttacca et al., 2019b). However, as Deketelaere et al. (2017) mentioned, for many BCAs, these traits are often not correlated with activity in vivo, and there are doubts that they play a role in planta. This means that field experiments using these Trichoderma isolates will be necessary to evaluate their potential for VW control in cacao.
Trichoderma has potential benefit in control strategies for other cacao diseases such as cacao black pod disease (Deberdt et al., 2008; Mpika et al., 2009; Hanada et al., 2010; Mbarga et al., 2014; Sriwati et al., 2019; Harni et al., 2020; Mbarga et al., 2020), frosty pod rot (Bailey et al., 2008; Mejía et al., 2008; Leiva et al., 2020); and witches’ broom (De Marco and Felix, 2002; De Souza et al., 2008; Loguercio et al., 2009).
Thanks to these potential benefits, using organic amendments fortified with Trichoderma would thus be an exciting avenue to explore. Using Trichoderma sp. in addition to compost has been shown to control disease (e.g., Rosmana et al., 2019) and increase cacao yield (Rafiuddin et al., 2020) (see also paragraph 2.5). Notwithstanding the potential benefits, BCA applications can have adverse or non-intended effects. Certain Trichoderma spp. can cause disease in humans, mainly immunocompromised humans, due to respiratory tract complications, and they can also cause allergic reactions. Also, some BCAs can negatively impact other fungi, causing losses in mushroom production (for more information, see, e.g., Kredics et al., 2021). Research efforts on developing biocontrol options for VW control should take these potential risks into account.
Several studies have looked at priming cacao plants against VW by inducing resistance (Resende et al., 2002; Ribeiro et al., 2006; Cavalcanti et al., 2008). Ribeiro et al. (2006) studied the use of potassium phosphite to induce resistance to V. dahliae in cacao seedlings. Unfortunately, although potassium phosphite presented antifungal effects, inhibiting the germination of V. dahliae conidia, no significant impacts were found in treated cacao seedlings. In the case of potatoes, the application of phosphites limited the growth of Verticillium spp. In vitro and in vivo, yet a high concentration is required (Borza et al., 2019).
Resende et al. (2002) studied the use of an S-methyl esther of the benzo-(1,2,3)-thiadiazole-7 carbotioic acid (acibenzolar-S-methyl, ASM), a compound in the benzothiadiazole group and an analog of salicylic acid, known to be involved in the acquisition of systemic resistance by plants (Görlach et al., 1996; Lawton et al., 1996), for the induction of resistance against V. dahliae and Moniliophthora perniciosa ((Stahel) Aime & Phillips-Mora) (previously Crinipellis perniciosa (Stahel) Singer), the causal agent of witches’ broom disease in cacao. Their findings showed that, independently of the dose used, spraying of ASM 15 days before inoculation with V. dahliae promoted the greatest reduction of disease severity (55.4%) compared to the inoculated control treatment. Similarly, for witches’ broom disease, ASM induced a reduction in the percentage of diseased seedlings ranging from 33.5 to 84.5%. Interestingly, Cavalcanti et al. (2008) tested the use of an M. perniciosa-based suspension for induction of resistance to V. dahliae in cacao and compared it with ASM. Although ASM conferred the best protection of cacao plants against V. dahliae, reaching 30.2% protection compared with water-pre-treated controls, the plants pre-treated with a heterogeneous fraction of chitosan from M. perniciosa mycelium yielded 24.3% protection (80% of the ASM performance). More on the preventive effect of ASM on plant pathogens is provided by Sandroni et al. (2020). However, it is important to note that ASM is not compatible with organic certifications.
Thus, although a certain level of resistance was obtained, none of the substances tested was found to protect cacao against V. dahliae fully. Nonetheless, these results indicate that inducing resistance to V. dahliae is a promising avenue for managing VW in cacao.
Organic amendments such as compost can significantly suppress the activity of pathogenic fungi, including V. dahliae (Bonanomi et al., 2007). In a review of 250 articles on the suppressive capacity of organic compost on soilborne diseases, it was found that V. dahliae was among the most responsive fungi to suppressive composts. Among the 17 articles on Verticillium disease, representing 84 experiments, in 72% of the cases, the amendments were suppressive, 21% had a non-significant effect, and only in 7% of the cases, organic amendments were found to increase disease incidence (Bonanomi et al., 2007). Composts were found to be suppressive against V. dahliae in several annual crops: tomato (Antoniou et al., 2017; Reddy, 2017; Kadoglidou et al., 2020), eggplant (Ikeda et al., 2015; Markakis et al., 2016; Kanaan et al., 2018; De Corato et al., 2018; De Corato et al., 2019), pepper (Capsicum annum L.) (Abada et al., 2018; Ojinaga et al., 2020; Tubeileh and Stephenson, 2020), cotton (Castaño and Avilés, 2013; Avilés and Borrero, 2017) and strawberry (Li et al., 2019).
For perennial crops, grape marc composts were efficient in reducing quite significantly the inoculum density of V. dahliae measured up to 30 cm depth in olive orchards (Mulero-Aparicio et al., 2020) and significantly reducing disease incidence in 1-year and 30-year-old trees in orchard (Mulero-Aparicio et al., 2020) as well as on five-month-old rooted olive cuttings from a highly susceptible cultivar (Varo‐Suárez et al., 2018). For avocado, in field conditions, application of organic matter (bovine manure and plant and mushroom residues) and a mineral amendment to 7-years-avocado reduced disease severity by 50%, and the V. dahliae inoculum levels in soils and leaves by 53% (Ramírez-Gil and Morales-Osorio, 2021).
Although the use of organic amendments for VW control has not (yet) been explored in cacao, the beforementioned shows the potential of organic amendments. Moreover, the development of such amendments for V. dahliae control could add value to agricultural waste products and diversify income sources. One requirement would be to use non-contaminated materials (plants and manure) to make compost (López-Escudero and Blanco-López, 1999) or to assure the elimination of any potential pathogens during the composting process.
Composts can also be used for the management of other cacao diseases. Studies on the use of compost for disease control in cacao are listed in Supplementary Table 2. These findings open interesting possibilities for multi-disease control in areas where cacao is affected by several diseases/pathogens (including VW).
The ability of composts to suppress plant diseases has been attributed to the chemical composition of composts and the presence of antagonistic soil microflora (Lazarovits et al., 2000; Pérez-Piqueres et al., 2006). However, one major problem with their use is the variability in their suppressive activity linked to difficulties replicating and standardizing compost quality over time (De Corato, 2020). One way to increase their effectiveness is to mix compost with different substances or biological control agents (BCA). Ruano-Rosa and Mercado-Blanco (2015) reviewed numerous studies in which BCAs were added to organic amendments against a diverse array of soilborne diseases. In the specific case of Verticillium wilt, the addition of BCA to compost might increase the disease reduction capacity, as observed in other crops. For example, adding Bacillus subtilis (Ehrenberg) Cohn or Pseudomonas fluorescens Migula to compost led to a 66% pepper yield increase compared to compost alone and up to a 450% yield increase compared to the control (no compost and no BCA) (Abada et al., 2018). Also, adding a non-pathogenic strain of Fusarium oxysporum to olive waste compost reduced by 1.6 times the inoculum density of the pathogen, about 50% compared to 30% (Mulero-Aparicio, 2019).
In a specific cropping system, compost efficiency and the suppressive response delay are also determined by the frequency and the application rate of compost amendment (De Corato, 2020). In addition, some essential oils, plant extracts, and algae have also proven to inhibit V. dahliae mycelial growth and reduce disease incidence; however, field use has not been reported (Acharya et al., 2020; Montes-Osuna and Mercado-Blanco, 2020). Therefore, studying the possibility of using enriched compost, either with BCA, essential oils, plant extracts, and/or algae, for V. dahliae control on cacao would be interesting.
Unfortunately, in many countries and especially in Africa, the availability of BCA is limited, complicating the use of enriched composts. Nonetheless, the use of plant extracts could be envisioned, especially since farmers themselves have developed the use of plant extracts for cacao pest control (e.g., Coulibaly et al., 2002).
Considering the ubiquitous availability of cacao pod husks (CPH) in cacao farming (they account for 70–75% of whole fruit weight), their use in compost is of particular interest. Composting diseased pods and pod husks, in general, is a sanitation practice. Indeed, it is an efficient way to reduce pathogen inoculum, as proposed, for example, for P. megakarya (Doungous et al., 2018). Furthermore, composting pods attacked by cacao pod borers (Conopomorpha cramerella Snellen) is an excellent way to eliminate these harmful insects (Fidelis and Rao, 2017).
Adding BCAs, such as Trichoderma spp., can also aid in getting rid of cacao pathogens and has been shown to effectively eliminate P. palmivora (Angraeni and Sriwati, 2020). It would be interesting to test for the suppressive capacity of composts made with cacao pod husks toward V. dahliae, as such composts have shown suppressive effects on other cacao pathogens.
Moreover, cacao pod husks can improve soil proprieties as well as soil fertility. CPHs are rich in minerals such as K, Ca, and P (Lu et al., 2018). CPH fertilizers demonstrated yield benefits for maize and tomato. In Nigeria, cacao farmers using CPH fertilizer achieved almost three times more profit than farmers that did not use CPH fertilizers (Agbeniyi et al., 2011). In addition, CPHs are rich in organic macromolecules such as cellulose, hemicellulose, and lignin; returning this matter to the soil without being burnt could help deal with soil organic matter depletion frequently encountered in cacao fields and associated with cacao yield decline (Lu et al., 2018). Apart from compost, other types of soil amendments can be made from CPH: biochar (Munongo et al., 2017) and ashes fertilizer (Ayeni et al., 2008; Kone et al., 2020). It could be interesting to study these, especially with regard to farmers’ acceptance. Indeed, some farmers reported barriers to compost utilization. For example, in Cameroon, lack of time and experience with compost were the major difficulties reported (48% and 22% respectively of the 120 farmers surveyed) (Essougong et al., 2020). In Nigeria, lack of knowledge was also identified as an important barrier. Out of 204 surveyed farmers, 63% had no knowledge regarding the usage of CPHs in amendments (Adeogun et al., 2013). The lack of time could be addressed through the participative development of protocols to do accelerated composts. Indeed, different biological organisms, fungi such as Trichoderma spp., degrade CPHs, accelerate composting, and improve compost quality (e.g., Rahim et al., 2018; Thaha et al., 2020).
CPH in decomposition can serve as a breeding site for cacao pollinators; placing fresh cacao fruit husks in different places of fields has proven to significantly increase the number of fruits per tree and consequently increase yield (Forbes and Northfield, 2017). Yet no study has regarded the impact of CPH compost or a combination of both composted and fresh CPHs on pollinators.
Verticillium wilt in cacao has not received the attention it merits. This disease can substantially impact yield and, consequently, the livelihoods of cacao farmers, notably in Uganda, DR Congo, Brazil, and Peru. This disease, like many soilborne diseases, is difficult to control. Unfortunately, relatively little research has been done on how to manage it on cacao.
Planting in contaminated areas should be avoided; testing for V. dahliae presence in soils before planting is advised. Other prevention measures such as using disease-free materials and avoiding wounding the trees are recommended. In addition, correct implementation of good agricultural practices such as shading, weed management, and satisfying nutritional cacao needs are required to prevent or reduce the incidence and severity of V. dahliae. In case of V. dahliae attacks, sanitation measures are to be adopted to avoid further spreading, such as removing and burning dead trees and disinfection of tools used in maintenance.
No curative measures exist yet. Using resistant cacao varieties would be the best way to cultivate cacao even in (low to medium) contaminated soils. Indeed, genetic resistance would provide long-lasting effects, but it will be long and costly to achieve, despite the possibility of using in vitro tests. Also, in Uganda, it would be limited by the critical local genetic bottleneck. Thus, the use of organic amendments possibly enriched with BCA or natural extracts capable of inducing resistance to VW in the cacao trees or directly interacting with the pathogen seems the best avenue to explore as it could bring low-cost solutions to farmers with moderate research efforts. The more so since it will also permit the delivery of necessary nutrition to the cacao tree, which helps boost productivity. To successfully develop this solution, it is needed to isolate and identify potential BCAs amongst local endophytes, epiphytes, soils, and available organic amendments. Organic amendments need to be evaluated for their suppressive capacity, resistance induction, and growth promotion. Promising farmer innovations for VW also need to be looked at. Measures to be included in integrated strategies for controlling Verticillium wilt in cacao trees are summarized in Figure 2.
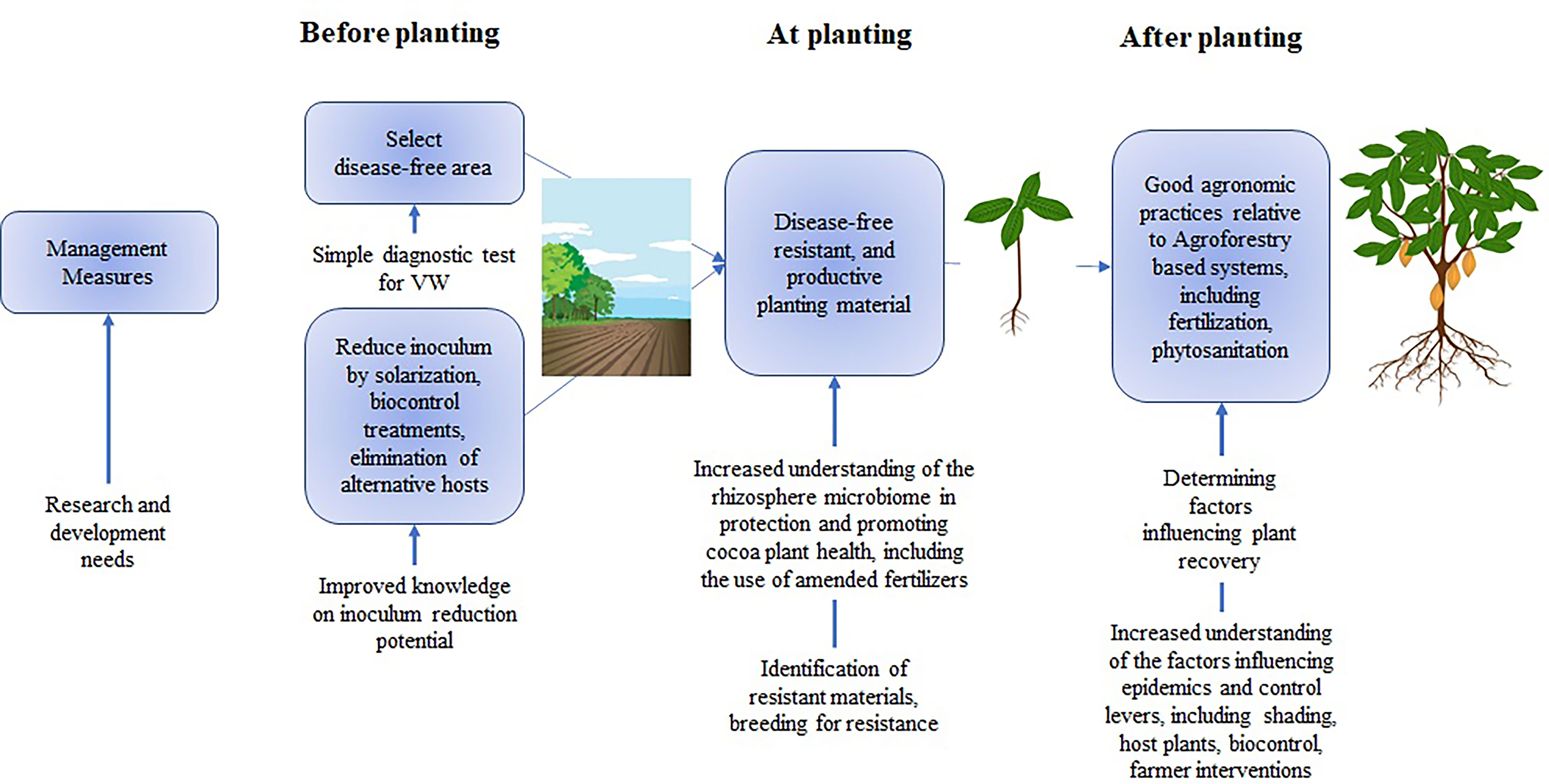
Figure 2 Measures to inlude a management strategy for cacao and research gaps (adapted from Keykhasaber et al., 2018). Image credits: Havryliuk-Kharzhevska/Shutterstock.com.
Given the current cacao dynamics in Uganda and, more generally speaking, Central Africa, with increasing areas under cacao cultivation, the risk of further spread of VW is present. Therefore, more attention to its control is urgently needed to prevent this pathogen from impacting cacao cultivation further.
A-SB drafted the manuscript; GH critically and substantially revised the manuscript. All authors contributed to the article and approved the submitted version.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
We thank David Guest for his helpful comments on this article. The genetic part of this review comes from the Ph.D. “Vascular wilt disease of Theobroma cacao in Uganda and DR Congo caused by Verticillium dahliae: studies on management using a genetic approach” funded by ESCO Kivu and CRUK, for which we thank them again. We acknowledge the financial support for the publication by CIRAD.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fagro.2022.911969/full#supplementary-material
DR Congo, Democratic Republic of Congo; BCA, Biocontrol agent; CPH, Cacao Pod Husk.
Abada K., Attia A., Zyton M. (2018). Management of Pepper Verticillium Wilt by Combinations of Inducer Chemicals for Plant Resistance, Bacterial Bioagents and Compost. J. Appl. Biotechnol. Bioeng. 5 (2), 117–127. doi: 10.15406/jabb.2018.05.00126
Abdelraheem A., Elassbli H., Zhu Y., Kuraparthy V., Hinze L., Stelly D., et al. (2020). A Genome-Wide Association Study Uncovers Consistent Quantitative Trait Loci for Resistance to Verticillium Wilt and Fusarium Wilt Race 4 in the US Upland Cotton. Theor. Appl. Genet. 133 (2), 563–577. doi: 10.1007/s00122-019-03487-x
Acharya B., Ingram T. W., Oh Y., Adhikari T. B., Dean R. A., Louws F. J. (2020). Opportunities and Challenges in Studies of Host-Pathogen Interactions and Management of Verticillium Dahliae in Tomatoes. Plants 9 (11), 1622. doi: 10.3390/plants9111622
Adeogun S. O., Fapojuwo E. O., Oyeyinka R. A., Adamu C. O., Abiona B. J. (2013). Training Needs Assessment of Cocoa Farmers Association Members on Soil Management Techniques in Cross River State of Nigeria. Ethiop. J. Environ. Stud. Manag. 6 (5), 551−560. doi: 10.4314/ejesm.v6i5.13
Agrianual (2009). Anuário da Agricultura Brasileira. São Paulo, Brazil : FNP Consultoria & Agroinformativos
Agbeniyi S., Oluyole K., Ogunlade M. (2011). Impact of Cocoa Pod Husk Fertilizer on Cocoa Production in Nigeria. World J. Agric. Sci. 7 (2), 113–116.
Ali S. S., Asman A., Shao J., Balidion J. F., Strem M. D., Puig A. S., et al. (2020). Genome and Transcriptome Analysis of the Latent Pathogen Lasiodiplodia Theobromae, an Emerging Threat to the Cacao Industry. Genome 63 (1), 37–52. doi: 10.1139/gen-2019-0112
Almeida K. A., Armesto C., Monteiro F. P., de Souza J. T. (2018). Diversity of Trichoderma Species Isolated From Dead Branches and Sapwood of Theobroma Cacao Trees. Trop. Plant Biol. 43 (1), 90–94. doi: 10.1007/s40858-017-0191-z
Angraeni L., Sriwati R. (2020). Application of Various Species of Trichoderma Spp. In Composting Cocoa Pod Husk Contaminated by Phytophthora Palmivora. Paper Presented at the IOP Conference Series:. Earth Environ. Sci. 515 (1), 12069. doi: 10.1088/1755-1315/515/1/012069
Antoniou A., Tsolakidou M., Stringlis I. A., Pantelides I. S. (2017). Rhizosphere Microbiome Recruited From a Suppressive Compost Improves Plant Fitness and Increases Protection Against Vascular Wilt Pathogens of Tomato. Front. Plant Sci. 8. doi: 10.3389/fpls.2017.02022
Arici S. E., Demirtas A. E. (2019). The Effectiveness of Rhizosphere Microorganisms to Control Verticillium Wilt Disease Caused by Verticillium Dahliae Kleb. In Olives. Arab. J. Geosci. 12 (24), 1–9. doi: 10.1007/s12517-019-4962-3
Ashworth Jr L. (1983). Aggressiveness of Random and Selected Isolates of Verticillium Dahliae From Cotton and the Quantitative Relationship of Internal Inoculum to Defoliation. Phytopathology, 73 (9), 1292–1295. doi: 10.1094/Phyto-73-1292
Avilés M., Borrero C. (2017). Identifying Characteristics of Verticillium Wilt Suppressiveness in Olive Mill Composts. Plant Dis. 101 (9), 1568–1577. doi: 10.1094/PDIS-08-16-1172-RE
Ayeni L., Adetunji M., Ojeniyi S., Ewulo B., Adeyemo A. (2008). Comparative and Cumulative Effect of Cocoa Pod Husk Ash and Poultry Manure on Soil and Maise Nutrient Contents and Yield. Am.-Eurasian J. Sustain. Agric. 2 (1), 92–97.
Bailey B. A., Bae H., Strem M., Crozier J., Thomas S., Samuels G., et al. (2008). Antibiosis, Mycoparasitism, and Colonization Success for Endophytic Trichoderma Isolates With Biological Control Potential in Theobroma Cacao. Biol. Control 46 (1), 24–35. doi: 10.1016/j.biocontrol.2008.01.003
BCC (2019). Available at: http://www.bcc.cd/downloads/pub/rapann/rapport_annuel_2019.pdf.
Bejarano-Alcázar J., Blanco-López M., Melero-Vara J., Jiménez-Díaz R. M. (1996). Etiology, Importance, and Distribution of Verticillium Wilt of Cotton in Southern Spain. Plant Dis. 80 (11), 1233–1238. doi: 10.1094/PD-80-1233
Bejarano-Alcázar J., Blanco-López M., Melero-Vara J., Jiménez-Díaz R. M. (1997). The Influence of Verticillium Wilt Epidemics on Cotton Yield in Southern Spain. Plant Pathol. 46 (2), 168−178. doi: 10.1046/j.1365-3059.1997.d01-221.x
Bejarano-Alcázar J., Melero-Vara J., Blanco-López M., Jiménez-Díaz R. M. (1995). Influence of Inoculum Density of Defoliating and Nondefoliating Pathotypes of Verticillium Dahliae on Epidemics of Verticillium Wilt of Cotton in Southern Spain. Phytopathology 85 (12), 1474−1481. doi: 10.1094/Phyto-85-1474
Blanco-López M., Rodríguez-Jurado D., Jiménez-Díaz R. M. (1990). Incidence and Seasonal Variation of Verticillium Wilt in Olive Orchards, Paper presented at the 5th International Verticillium Symposium, Leningrad, ex-URSS,5.
Bollig K., Specht A., Zahn M., Horst W. J. (2013). Sulphur Supply Impairs Spread of Verticillium Dahliae in Tomato. Eur. J. Plant Pathol. 135 (1), 81–96. doi: 10.1007/s10658-012-0067-5
Bonanomi G., Antignani V., Pane C., Scala F. (2007). Suppression of Soilborne Fungal Diseases With Organic Amendments. J. Plant Pathol. 89 (3), 311–324. doi: 10.2307/41998409
Borza T., Beaton B., Govindarajan A., Gao X., Liu Y., Ganga Z., et al. (2018). Incidence and Abundance of Verticillium Dahliae in Soil From Various Agricultural Fields in Prince Edward Island, Canada. Eur. J. Plant Pathol. 151 (3), 825830. doi: 10.1007/s10658-017-1408-1
Borza T., Peters R. D., Gao X., Wang-Pruski G. (2019). Effects of Phosphite on the In Vitro Growth of Verticillium Nonalfalfae and Verticillium Dahliae and on Their In Vivo Ability to Infect Potato Plants. Eur. J. Plant Pathol. 155 (4), 1333–1344. doi: 10.1007/s10658-019-01859-z
Bouchon A. S. (2020). Vascular Wilt Disease of Theobroma Cacao in Uganda and DR Congo Caused by Verticillium Dahliae: Studies on Management Using a Genetic Approach PhD Thesis. (Aberdeen, UK: University of Aberdeen). doi: 10.13140/RG.2.2.36237.87523
Braga M., Silva S. (1989). Resistência do Cacaueiro (Theobroma Cacao L.) a Verticillium Dahliae Kleb [Resistance of Cacao (Theobroma Cacao L.) to Verticillium Dahliae Kleb]. Agrotrópica (Brasil) 1 (2), 116–121.
Bubici G., Cirulli M. (2012). Control of Verticillium Wilt of Olive by Resistant Rootstocks. Plant Soil 352 (1), 363–376. doi: 10.1007/s11104-011-1002-9
Cai F., Druzhinina I. S. (2021). In Honor of John Bissett : Authoritative Guidelines on Molecular Identification of Trichoderma. Fungal Divers. 107 (1), 1−69. doi: 10.1007/s13225-020-00464-4
Carrer Filho R., Oliveira R. M., Dias V. D., Rocha G. A., Dianese É.D.C., Cunha M. G. D. (2016). Selection of Tomato Accessions Resistant to Verticillium Wilt. Pesq. Agropec. Trop. 46 (4), 429–433. doi: 10.1590/1983-40632016v4643106
Carrero-Carrón I., Rubio M., Niño-Sánchez J., Navas-Cortés J., Jiménez-Díaz R. M., Monte E., et al. (2018). Interactions Between Trichoderma Harzianum and Defoliating Verticillium Dahliae in Resistant and Susceptible Wild Olive Clones. Plant Pathol. 67 (8), 1758–1767. doi: 10.1111/ppa.12879
Carrero-Carrón I., Trapero-Casas J. L., Olivares-García C., Monte E., Hermosa R., Jiménez-Díaz R. M. (2016). Trichoderma Asperellum is Effective for Biocontrol of Verticillium Wilt in Olive Caused by the Defoliating Pathotype of Verticillium Dahliae. Crop Prot. 88, 45–52. doi: 10.1016/j.cropro.2016.05.009
Castaño R., Avilés M. (2013). Factors That Affect the Capacity of Growing Media to Suppress Verticillium Wilt. Acta Hortic. 1013, 465–471. doi: 10.17660/ActaHortic.2013.1013.57
Cavalcanti F., Resende M., Ribeiro P. M. Jr., Pereira R., Oliveira J. (2008). Induction of Resistance Against Verticillium Dahliae in Cacao by a Crinipellis Perniciosa Suspension. J. Plant Pathol. 90 (2), 273–280. doi: 10.2307/41998504
CFC I., 21FT (2003). Assessment of the Impact and Main Dynamics of Cotton Diseases Affecting in Particular Small-Scale Production Systems in Southern and Eastern Africa. (South Africa: CFC, ICAC, 21FT).
Chambers D. A., Harris D. C. (1997). Methods of Screening Acer Platanoides L. Seedlings for Resistance to Wilt (Verticillium Dahliae Kleb.). J. Hortic. Sci. 72 (4), 601–608. doi: 10.1080/14620316.1997.11515549
Ciccarese F., Frisullo S., Cirulli M. (1990). Natural Disease Recovery From Verticillium Wilt in Peach. Paper Presented at the 5th International Verticillium Symposium (ex-URSS 6: Leningrad).
Coulibaly O., Mbila D., Sonwa D. J., Adesina A., Bakala J. (2002). Responding to Economic Crisis in Sub-Saharan Africa: New Farmer-Developed Pest Management Strategies in Cocoa-Based Plantations in Southern Cameroon. Integr. Pest Manage. Rev. 7 (3), 165–172. doi: 10.1023/b:ipmr.0000027500.24459.fe
Creus C., Bazzalo M., Grondona M., Andrade F., León A. J. (2007). Disease Expression and Ecophysiological Yield Components in Sunflower Isohybrids With and Without Verticillium Dahliae Resistance. Crop Sci. 47 (2), 703–708. doi: 10.2135/cropsci2006.05.0307
Daayf F., Nicole M., Geiger J. (1995). Differentiation of Verticillium Dahliae Populations on the Basis of Vegetative Compatibility and Pathogenicity on Cotton. Eur. J. Plant Pathol. 101 (1), 69–79. doi: 10.1007/BF01876095
Davis J., Allen T. (1984). Relationships of Defined PVX Infection Levels to Verticillium Wilt, Yield, and Quality of the Russet Burbank Potato. Am. Potato J. 61 (11), 669–682. doi: 10.1007/BF02852930
de Almeida O., de Almeida L., de Figueiredo J. (1989). Obtencao, Em Meio De Cultura, De Propágulos De Verticillium Dahliae Kleb., Causador Da Murcha De-Verticillium Em Cacaueiro (Theobroma Cacao L.). Agrotrópica (Brasil) 1 (3), 213–215.
Deberdt P., Mfegue C. V., Tondje P. R., Bon M., Ducamp M., Hurard C., et al. (2008). Impact of Environmental Factors, Chemical Fungicide and Biological Control on Cacao Pod Production Dynamics and Black Pod Disease (Phytophthora Megakarya) in Cameroon. Biol. Control 44 (2), 149–159. doi: 10.1016/j.biocontrol.2007.10.026
De Corato U. (2020). Disease-Suppressive Compost Enhances Natural Soil Suppressiveness Against Soil-Borne Plant Pathogens: A Critical Review. Rhizosphere 13, 100192. doi: 10.1016/j.rhisph.2020.100192
De Corato U., Patruno L., Avella N., Lacolla G., Cucci G. (2019). Composts From Green Sources Show an Increased Suppressiveness to Soilborne Plant Pathogenic Fungi: Relationships Between Physicochemical Properties, Disease Suppression, and the Microbiome. Crop Prot. 124, 104870. doi: 10.1016/j.cropro.2019.104870
De Corato U., Salimbeni R., De Pretis A., Patruno L., Avella N., Lacolla G., et al. (2018). Microbiota From ‘Next-Generation Green Compost’ Improves Suppressiveness of Composted Municipal-Solid-Waste to Soil-Borne Plant Pathogens. Biol. Control 124, 1–17. doi: 10.1016/j.biocontrol.2018.05.020
Deketelaere S., Tyvaert L., França S. C., Höfte M. (2017). Desirable Traits of a Good Biocontrol Agent Against Verticillium Wilt. Front. Microbiol. 8. doi: 10.3389/fmicb.2017.01186
De Marco J. L., Felix C. R. (2002). Characterization of a Protease Produced by a Trichoderma Harzianum Isolate Which Controls Cocoa Plant Witches’ Broom Disease. BMC Biochem. 3 (1), 1–7. doi: 10.1186/1471-2091-3-3
Dervis S., Mercado-Blanco J., Erten L., Valverde-Corredor A., Pérez-Artés E. (2010). Verticillium Wilt of Olive in Turkey: A Survey on Disease Importance, Pathogen Diversity and Susceptibility of Relevant Olive Cultivars. Eur. J. Plant Pathol. 127 (2), 287–301. doi: 10.1007/s10658-010-9595-z
De Souza J., Bailey B., Pomella A., Erbe E., Murphy C., Bae H., et al. (2008). Colonization of Cacao Seedlings by Trichoderma Stromaticum, a Mycoparasite of the Witches’ Broom Pathogen, and its Influence on Plant Growth and Resistance. Biol. Control 46 (1), 36–45. doi: 10.1016/j.biocontrol.2008.01.010
dos Santos Fernandes L., Royaert S., Correa F. M., Mustiga G. M., Marelli J., Correa R. X., et al. (2018). Mapping of a Major QTL for Ceratocystis Wilt Disease in an F1 Population of Theobroma Cacao. Front. Plant Sci. 9. doi: 10.3389/fpls.2018.00155
Doungous O., Minyaka E., Longue E. A. M., Nkengafac N. J. (2018). Potentials of Cocoa Pod Husk-Based Compost on Phytophthora Pod Rot Disease Suppression, Soil Fertility, and Theobroma Cacao L. Growth. Environ. Sci. pollut. Res. 25 (25), 25327–25335. doi: 10.1007/s11356-018-2591-0
Druzhinina I. S., Seidl-Seiboth V., Herrera-Estrella A., Horwitz B. A., Kenerley C. M., Monte E., et al. (2011). Trichoderma: The Genomics of Opportunistic Success. Nat. Rev. Microbiol. 9 (10), 749–759. doi: 10.1038/nrmicro2637
Efombagn M. I. B., Bieysse D., Nyassé S., Eskes A. (2011). Selection for Resistance to Phytophthora Pod Rot of Cocoa (Theobroma Cacao L.) in Cameroon: Repeatability and Reliability of Screening Tests and Field Observations. Crop Prot. 30 (2), 105–110. doi: 10.1016/j.cropro.2010.10.012
Emechebe A. (1975). Some Host Factors Affecting Inoculation of Cacao Seedlings With Verticillium Dahliae. E. Afr. Agric. For. J. 40 (3), 271–277. doi: 10.1080/00128325.1975.11662744
Emechebe A., Leakey C. L., Banage W. (1971). Verticillium Wilt of Cacao in Uganda: Symptoms and Establishment of Pathogenicity. Ann. Appl. Biol. 69 (3), 223–227. doi: 10.1111/j.1744-7348.1971.tb04674.x
Emechebe A., Leakey C. L., Banage W. (1972). Verticillium Wilt of Cacao in Uganda: The Relationship Between Verticillium Dahliae and Cacao Roots. Ann. Appl. Biol. 70 (2), 157–162. doi: 10.1111/j.1744-7348.1972.tb04699.x
Emechebe A., Leakey C. L., Banage W. (1974). Verticillium Wilt of Cacao in Uganda: Wilt Induction by Mechanical Vessel Blockage and Mode of Recovery of Diseased Plants. E. Afr. Agric. For. J. 39 (4), 337–343. doi: 10.1080/00128325.1974.11662658
Essougong U. P. K., Slingerland M., Mathé S., Vanhove W., Ngome P. I. T., Boudes P., et al. (2020). Farmers’ Perceptions as a Driver of Agricultural Practices: Understanding Soil Fertility Management Practices in Cocoa Agroforestry Systems in Cameroon. Hum. Ecol. 48, 709–720. doi: 10.1007/s10745-020-00190-0
Fan R., Cockerton H. M., Armitage A. D., Bates H., Cascant-Lopez E., Antanaviciute L., et al. (2018). Vegetative Compatibility Groups Partition Variation in the Virulence of Verticillium Dahliae on Strawberry. PloS One 13 (2), e0191824. doi: 10.1371/journal.pone.0191824
FAO (2022). Available at: http://www.fao.org/faostat/en/#data/QC.
Ferreira F. V., Musumeci M. A. (2021). Trichoderma as Biological Control Agent: Scope and Prospects to Improve Efficacy. World J. Microbiol. Biotechnol. 37 (5), 1–17. doi: 10.1007/s11274-021-03058-7
Fidelis C., Rao B. R. (2017). Enriched Cocoa Pod Composts and Their Fertilising Effects on Hybrid Cocoa Seedlings. Int. J. Recycl. Org. Waste. Agricult. 6 (2), 99–106. doi: 10.1007/s40093-017-0156-8
Flood J., Ten Hoopen G. M., Krauss U., Akrofi A. (2016). “Root-Infecting Fungi Attacking Theobroma Cacao,” in Cacao Diseases. Eds. Bailey B., Meinhardt L. (Cham, Switzerland: Springer), (pp. 449–480.
Forbes S. J., Northfield T. D. (2017). Increased Pollinator Habitat Enhances Cacao Fruit Set and Predator Conservation. Ecol. Appl. 27 (3), 887–899. doi: 10.1002/eap.1491
Fotoohiyan Z., Rezaee S., Bonjar G. H. S., Mohammadi A. H., Moradi M. (2017). Biocontrol Potential of Trichoderma Harzianum in Controlling Wilt Disease of Pistachio Caused by Verticillium Dahliae. J. Plant Prot. Res. 57 (2), 185–193. doi: 10.1515/jppr-2017-0025
Fradin E. F., Thomma B. P. (2006). Physiology and Molecular Aspects of Verticillium Wilt Diseases Caused by V. Dahliae and V. Albo-Atrum. Mol. Plant Pathol. 7 (2), 71–86. doi: 10.1111/j.1364-3703.2006.00323.x
Fradin E. F., Zhang Z., Juarez Ayala J. C., Castroverde C. D., Nazar R. N., Robb J., et al. (2009). Genetic Dissection of Verticillium Wilt Resistance Mediated by Tomato Ve1. Plant Physiol. 150 (1), 320–332. doi: 10.1104/pp.109.136762
Gold J., Lee B., Robb J. (1996). Colonization of Tomatoes by Verticillium Dahliae: Determinative Phase II. Can. J. Bot. 74 (8), 1279–1288. doi: 10.1139/b96-155
Gold J., Robb J. (1995). The Role of the Coating Response in Craigella Tomatoes Infected With Verticillium Dahliae, Races 1 and 2. Physiol. Mol. Plant Pathol. 47 (3), 141–157. doi: 10.1006/pmpp.1995.1048
Gopaulchan D., Motilal L. A., Bekele F., Clause S., Ariko J. O., Ejang H. P., et al. (2019). Morphological and Genetic Diversity of Cacao (Theobroma Cacao L.) in Uganda. Physiol. Mol. Plant Pathol. 25 (2), 361–375. doi: 10.1007/s12298-018-0632-2
Göre M., Erdoğan O., Caner Ö, Aydın M., Berk S. (2014). VCG Diversity and Virulence of Verticillium Dahliae From Commercially Available Cotton Seed Lots in Turkey. Eur. J. Plant Pathol. 140 (4), 689–699. doi: 10.1007/s10658-014-0500-z
Görlach J., Volrath S., Knauf-Beiter G., Hengy G., Beckhove U., Kogel K., et al. (1996). Benzothiadiazole, a Novel Class of Inducers of Systemic Acquired Resistance, Activates Gene Expression and Disease Resistance in Wheat. Plant Cell 8 (4), 629–643. doi: 10.1105/tpc.8.4.629
Granada G. (1989). Marchitez Del Cacao Por Verticillium Dahliae Vol. 12 (Colombia: Cacaotero Colombiano), 17–28.
Haberman A., Lazare S., Hazanovsky M., Lebiush S., Zipori I., Busatn A., et al. (2020). Management of Verticillium Wilt of Avocado Using Tolerant Rootstocks. Plants 9 (4), 531. doi: 10.3390/plants9040531
Hanada R. E., Pomella A. W. V., Costa H. S., Bezerra J. L., Loguercio L. L., Pereira J. O. (2010). Endophytic Fungal Diversity in Theobroma Cacao (Cacao) and T. Grandiflorum (Cupuaçu) Trees and Their Potential for Growth Promotion and Biocontrol of Black-Pod Disease. Fungal Biol. 114 (11-12), 901–910. doi: 10.1016/j.funbio.2010.08.006
Hanson L. E. (2000). Reduction of Verticillium Wilt Symptoms in Cotton Following Seed Treatment With Trichoderma Virens. J. Cotton. Sci. 4 (4), 224–231.
Harni R., Amaría W., Ferry Y., Marhaeni L. (2020). Effect of Trichoderma Spp. And Potassium Fertilizer on Phytophthora Palmivora Infection in Cacao Seedlings. Paper Presented at the IOP Conference Series. Earth Environ. Sci. 418 (1), 012015. doi: 10.1046/j.1365-3059.1996.d01-96.x
Harris D., Yang J. (1996). The Relationship Between the Amount of Verticillium Dahliae in Soil and the Incidence of Strawberry Wilt as a Basis for Disease Risk Prediction. Plant Pathol., 45 (1), 106–114. doi: 10.1046/j.1365-3059.1996.d01-96.x
Hiemstra J. (1998). A Compendium of Verticillium Wilts in Tree Species. (Wageningen, Netherlands: CPRO).
Hoitink H., Madden L., Dorrance A. (2006). Systemic Resistance Induced by Trichoderma Spp.: Interactions Between the Host, the Pathogen, the Biocontrol Agent, and Soil Organic Matter Quality. Phytopathology 96 (2), 186–189. doi: 10.1094/PHYTO-96-0186
ICCO (2022). Available at: https://www.icco.org/wp-content/uploads/Production_QBCS-XLVIII-No.-1.pdf.
Ikeda K., Banno S., Furusawa A., Shibata S., Nakaho K., Fujimura M. (2015). Crop Rotation With Broccoli Suppresses Verticillium Wilt of Eggplant. J. Gen. Plant Pathol. 81 (1), 77–82. doi: 10.1007/s10327-014-0559-6
Inderbitzin P., Bostock R. M., Davis R. M., Usami T., Platt H. W., Subbarao K. V. (2011). Phylogenetics and Taxonomy of the Fungal Vascular Wilt Pathogen Verticillium, With the Descriptions of Five New Species. PloS One 6 (12), e28341. doi: 10.1371/journal.pone.0028341
Jabnoun-Khiareddine H., Daami-Remadi M., Ayed F., El Mahjoub M. (2009). Biological Control of Tomato Verticillium Wilt by Using Indigenous Trichoderma Spp. Afr. J. Plant Sci. Biotechnol. 3 (1), 26–36.
Jakse J., Cerenak A., Radisek S., Satovic Z., Luthar Z., Javornik B. (2013). Identification of Quantitative Trait Loci for Resistance to Verticillium Wilt and Yield Parameters in Hop (Humulus Lupulus L.). Theor. Appl. Genet. 126 (6), 1431–1443. doi: 10.1007/s00122-013-2062-4
Jamdar Z., Mohammadi A., Mohammadi S. (2013). Study of Antagonistic Effects of Trichoderma Species on Growth of Verticillium Dahliae, the Causal Agent of Verticillium Wilt of Pistachio Under Laboratory Condition. J. Nuts 4 (4), 53–56.
Jansky S., Rouse D., Kauth P. (2004). Inheritance of Resistance to Verticillium Dahliae in Diploid Interspecific Potato Hybrids. Plant Dis. 88 (10), 1075–1078. doi: 10.1094/PDIS.2004.88.10.1075
Jimenez-Diaz R. M., Cirulli M., Bubici G., del Mar Jimenez-Gasco M., Antoniou P. P., Tjamos E. C. (2012). Verticillium Wilt, a Major Threat to Olive Production: Current Status and Future Prospects for its Management. Plant Dis. 96 (3), 304−329. doi: 10.1094/PDIS-06-11-0496
Jiménez-Díaz R. M., Mercado-Blanco J., Olivares-Garcia C., Collado-Romero M., Bejarano-Alcázar J., Rodriguez-Jurado D., et al. (2006). Genetic and Virulence Diversity in Verticillium Dahliae Populations Infecting Artichoke in Eastern-Central Spain. Phytopathology 96 (3), 288–298. doi: 10.1094/PHYTO-96-0288
Jiménez-Díaz R. M., Olivares-García C., Trapero-Casas J. L., Jiménez-Gasco M., Navas-Cortés J. A., Landa B. B., et al. (2017). Variation of Pathotypes and Races and Their Correlations With Clonal Lineages in Verticillium Dahliae. Plant Pathol. 66 (4), 651–666. doi: 10.1111/ppa.12611
Ju Y., Li C., Shen P., Wan N., Han W., Pan Y. (2020). Rapid and Visual Detection of Verticillium Dahliae Using Recombinase Polymerase Amplification Combined With Lateral Flow Dipstick. Crop Prot. 136, 105226. doi: 10.1016/j.cropro.2020.105226
Kabir Z., Bhat R., Subbarao K. (2004). Comparison of Media for Recovery of Verticillium Dahliae From Soil. Plant Dis. 88 (1), 49–55. doi: 10.1094/PDIS.2004.88.1.49
Kadoglidou K., Chatzopoulou P., Maloupa E., Kalaitzidis A., Ghoghoberidze S., Katsantonis D. (2020). Mentha and Oregano Soil Amendment Induces Enhancement of Tomato Tolerance Against Soilborne Diseases, Yield and Quality. Agronomy 10 (3), 406. doi: 10.3390/agronomy10030406
Kanaan H., Hadar Y., Medina S., Krasnovsky A., Mordechai-Lebiush S., Tsror L., et al. (2018). Effect of Compost Properties on Progress Rate of Verticillium Dahliae Attack on Eggplant (Solanum Melongena L.). Compost. Sci. Util. 26 (2), 71–78. doi: 10.1080/1065657X.2017.1366375
Keykhasaber M., Thomma B. P., Hiemstra J. A. (2018). Verticillium Wilt Caused by Verticillium Dahliae in Woody Plants With Emphasis on Olive and Shade Trees. Eur. J. Plant Pathol. 150, 21–37. doi: 10.1007/s10658-017-1273-y
Khana M. R., Parveena G., Zaidb A., Wanic S. H., Jogaiahd S. (2020). “Potential of Trichoderma Species in Alleviating the Adverse Effects of Biotic and Abiotic Stresses in Plants,” in Biocontrol Agents and Secondary Metabolites: Applications and Immunization for Plant Growth and Protection. Ed. Jogaiah S. (Sawston, UK: Woodhead Publishing), (pp. 85–112).
Klug K., Hogekamp C., Specht A., Myint S. S., Blöink D., Küster H., et al. (2015). Spatial Gene Expression Analysis in Tomato Hypocotyls Suggests Cysteine as Key Precursor of Vascular Sulfur Accumulation Implicated in Verticillium Dahliae Defense. Physiol. Plantarum 153 (2), 253–268. doi: 10.1111/ppl.12239
Konam J. K., Guest D. I. (2002). Leaf Litter Mulch Reduces the Survival of Phytophthora Palmivora Under Cocoa Trees in Papua New Guinea. Australas. Plant Pathol. 31 (4), 381–383. doi: 10.1071/AP02043
Kone K., Akueson K., Norval G. (2020). On the Production of Potassium Carbonate From Cocoa Pod Husks. Recycling 5 (3), 23. doi: 10.3390/recycling5030023
Kredics L., Naeimi S., Hatvani L., Vágvölgyi C., Cai F., Druzhinina I. S., et al. (2021). ‘The Good, the Bad and the Ugly’ in the Shades of Green: The Genus Trichoderma in the Spotlight. Indian Phytopathol. 74, 403–411. doi: 10.1007/s42360-021-00352-0
Kuswinanti T., Junaid M., Surapati U. (2019). A Promising Microbial Use on Cocoa: Decomposing Cocoa Waste and Controlling Lasiodiplodia Theobromae in-Vitro. Earth Environ. Sci. 343 (1), 12256. doi: 10.1088/1755-1315/343/1/012256. Paper presented at the IOP Conference Series.
Lahive F., Hadley P., Daymond A. J. (2018). The Physiological Responses of Cacao to the Environment and the Implications for Climate Change Resilience. A Review. Agron. Sustain. Dev. 39 (1), 5. doi: 10.1007/s13593-018-0552-0
Latorre B., Allende P. (1983). Occurrence and Incidence of Verticillium Wilt Chilean Avocado Groves. Plant Dis. 67 (4), 445–447. doi: 10.1094/PD-67-445
Lawrence J., Campêlo A., de Figueiredo J. (1991). Diseases of Cocoa. III-Vascular and Root Fungal Diseases. [Enfermidades do Cacaueiro. III- Doenças Fúngicas Vasculares E Radiculares.]. Agrotrópica (Brasil) 3 (2), 65–73.
Lawton K. A., Friedrich L., Hunt M., Weymann K., Delaney T., Kessmann H., et al. (1996). Benzothiadiazole Induces Disease Resistance in Arabidopsis by Activation of the Systemic Acquired Resistance Signal Transduction Pathway. Plant J. 10 (1), 71–82. doi: 10.1046/j.1365-313X.1996.10010071.x
Lazarovits G., Conn K., Tenuta M. (2000). Control of Verticillium Dahliae With Soil Amendments: Efficacy and Mode of Action. Advances in Verticillium Research and Disease Management (St Paul, USA: APS), (pp. 274–291).
Leakey C. (1965). Sudden Death Disease of Cacao in Uganda Associated With Verticillium Dahliae Kleb. E. Afr. Agric. For. J. 31 (1), 21–24. doi: 10.1080/00128325.1965.11662020
Leiva S., Oliva M., Hernández E., Chuquibala B., Rubio K., García F., et al. (2020). Assessment of the Potential of Trichoderma Spp. Strains Native to Bagua (Amazonas, Perú) in the Biocontrol of Frosty Pod Rot (Moniliophthora roreri). Agronomy 10 (9), 1376. doi: 10.3390/agronomy10091376
Leon-Ttacca B., Arévalo-Gardini E., Bouchon A. S. (2019a). Sudden Death of Theobroma Cacao L. Caused by Verticillium Dahliae Kleb. In Peru and its In Vitro Biocontrol. Cienc. Tecnol. Agropecuaria 20 (1), 133–148. doi: 10.21930/rcta.vol20_num1_art:1251
Leon-Ttacca B., Arévalo-Gardini E., Bouchon A. S. (2019b). Muerte Repentina De Theobroma Cacao L. Causado Por Verticillium Dahliae Kleb. En El Perú Y Su Biocontrol In Vitro. Cienc. Tecnol. Agropecuaria 20 (1), 117–132. doi: 10.21930/rcta.vol20num1art:1251
Li X., Wang X., Shi X., Wang Q., Li X., Zhang S. (2019). Compost Tea-Mediated Induction of Resistance in Biocontrol of Strawberry Verticillium Wilt. J. Plant Dis. Prot. 127, 257–268. doi: 10.1007/s41348-019-00290-0
Loguercio L. L., de Carvalho A. C., Niella G. R., De Souza J. T., Pomella A. W. V. (2009). Selection of Trichoderma Stromaticum Isolates for Efficient Biological Control of Witches’ Broom Disease in Cacao. Biol. Control 51 (1), 130–139. doi: 10.1016/j.biocontrol.2009.06.005
López-Escudero F. J., Blanco-López M. A. (1999). First Report of Transmission of Verticillium Dahliae by Infested Manure in Olive Orchards in Andalucia (Southern Spain). Plant Dis. 83, 1178–1178. doi: 10.1094/PDIS.1999.83.12.1178B
López-Escudero F. J., Blanco-López M. A. (2005). Recovery of Young Olive Trees From Verticillium Dahliae. E. J. Plant Pathol. 113 (4), 367–375. doi: 10.1007/s10658-005-3145-0
Lorito M., Woo S. L., Harman G. E., Monte E. (2010). Translational Research on Trichoderma: From ‘Omics to the Field. Annu. Rev. Phytopathol. 48, 395–417. doi: 10.1146/annurev-phyto-073009-114314
Lu F., Rodriguez-Garcia J., Van Damme I., Westwood N. J., Shaw L., Robinson J. S., et al. (2018). Valorisation Strategies for Cocoa Pod Husk and its Fractions. Curr. Opin. Green Sustain. Chem. 14 14, 80–88. doi: 10.1016/j.cogsc.2018.07.007
Magalhães D. M. A., Lopes U. V., Niella A. R. R., Damaceno V. O. (2016). Leaf Disc Method for Screening Ceratocystis Wilt Resistance in Cacao. Trop. Plant Pathol. 41 (3), 155–161. doi: 10.1007/s40858-016-0081-9
Malcolm G. M., Kuldau G. A., Gugino B. K., Jiménez-Gasco M. (2013). Hidden Host Plant Associations of Soilborne Fungal Pathogens: An Ecological Perspective. Phytopathology 103 (6), 538–544. doi: 10.1094/PHYTO-08-12-0192-LE
Maridueña-Zavala M. G., Freire-Peñaherrera A., Espinoza-Lozano R. F., Villavicencio-Vasquez M., Jimenez-Feijoo M., Cevallos-Cevallos J. M. (2019). Genetic Characterization of Moniliophthora Perniciosa From Ecuador and In Vitro Sensitivity to Compost Tea. Eur. J. Plant Pathol. 154 (4), 943–959. doi: 10.1007/s10658-019-01714-1
Markakis E. A., Fountoulakis M. S., Daskalakis G. C., Kokkinis M., Ligoxigakis E. K. (2016). The Suppressive Effect of Compost Amendments on Fusarium Oxysporum F. Sp. Radicis-Cucumerinum in Cucumber and Verticillium Dahliae in Eggplant. Crop Prot. 79, 70–79. doi: 10.1016/j.cropro.2015.10.015
Matovu S. (1973). A Survey of Cocoa Diseases in Uganda. E. Afr. Agric. For. J. 38 (3), 218–228. doi: 10.1080/00128325.1973.11662584
Maximova S. N., Marelli J., Young A., Pishak S., Verica J. A., Guiltinan M. J. (2006). Over-Expression of a Cacao Class I Chitinase Gene in Theobroma Cacao L. Enhances Resistance Against the Pathogen, Colletotrichum Gloeosporioides. Planta 224 (4), 740–749. doi: 10.1007/s00425-005-0188-6
Mbarga J. B., Begoude B., Ambang Z., Meboma M., Kuaté J., Ewbank W., et al. (2020). Field Testing an Oil-Based Trichoderma Asperellum Formulation for the Biological Control of Cacao Black Pod Disease, Caused by Phytophthora Megakarya. Crop Prot. 132, 105134. doi: 10.1016/j.cropro.2020.105134
Mbarga J. B., Begoude B., Ambang Z., Meboma M., Kuaté J., Schiffers B., et al. (2014). A New Oil-Based Formulation of Trichoderma Asperellum for the Biological Control of Cacao Black Pod Disease Caused by Phytophthora Megakarya. Biol. Control 77, 15–22. doi: 10.1016/j.biocontrol.2014.06.004
Meher J., Rajput R. S., Bajpai R., Teli B., Sarma B. K. (2020). “Trichoderma: A Globally Dominant Commercial Biofungicide. In C. Manoharachary,” in Trichoderma: Agricultural Applications and Beyond. Eds. Singh H. B., Ajit V. (Cham, Switzerland: Springer), (pp. 195–208).
Mejía L. C., Rojas E. I., Maynard Z., Van Bael S., Arnold A. E., Hebbar P., et al. (2008). Endophytic Fungi as Biocontrol Agents of Theobroma Cacao Pathogens. Biol. Control 46 (1), 4–14. doi: 10.1016/j.biocontrol.2008.01.012
Mercado-Blanco J., López-Escudero F. J. (2012). Verticillium Wilt of Olive and its Control: The Heat is on. Plant Soil 355 (1), 17–21. doi: 10.1007/s11104-011-1091-5
Mercado-Blanco J., Rodríguez-Jurado D., Parrilla-Araujo S., Jiménez-Díaz R. M. (2003). Simultaneous Detection of the Defoliating and Nondefoliating Verticillium Dahliae Pathotypes in Infected Olive Plants by Duplex, Nested Polymerase Chain Reaction. Plant Dis. 87 (12), 1487–1494. doi: 10.1094/PDIS.2003.87.12.1487
Mirmajlessi S. M., Mänd M., Najdabbasi N., Larena I., Loit E. (2016). Screening of Native Trichoderma Harzianum Isolates for Their Ability to Control Verticillium Wilt of Strawberry. Zemdirbyste-Agriculture 103 (4), 397–404. doi: 10.13080/z-a.2016.103.051
Mokhtari W. M., Achouri M., Remah A., Boubaker H. (2018). Verticillium Dahliae-Eggplant as the Pathosystem Model to Reveal Biocontrol Potential of Three Trichoderma Spp in Green House Conditions. Atlas J. Biol., 417–421. doi: 10.5147/ajb.v0i0.172
Mol L., Van Halteren J. M., Scholte K., Struik P. C. (1996). Effects of Crop Species, Crop Cultivars and Isolates of Verticillium Dahliae on the Population of Microsclerotia in the Soil, and Consequences for Crop Yield. Plant Pathol. 45 (2), 205–214. doi: 10.1046/j.1365-3059.1996.d01-125.x
Montes-Osuna N., Mercado-Blanco J. (2020). Verticillium Wilt of Olive and its Control: What did We Learn During the Last Decade? Plants 9 (6), 735. doi: 10.3390/plants9060735
Moradi A., Almasi M., Jafary H., Mercado-Blanco J. (2014). A Novel and Rapid Loop-Mediated Isothermal Amplification Assay for the Specific Detection of Verticillium Dahliae. J. Appl. Microbiol. 116 (4), 942−954. doi: 10.1111/jam.12407
Morán-Diez M. E., Carrero-Carrón I., Rubio M. B., Jiménez-Díaz R. M., Monte E., Hermosa R. (2019). Transcriptomic Analysis of Trichoderma Atroviride Overgrowing Plant-Wilting Verticillium Dahliae Reveals the Role of a New M14 Metallocarboxypeptidase CPA1 in Biocontrol. Front. Microbiol. 10. doi: 10.3389/fmicb.2019.01120
Morgan D., Epstein L., Ferguson L. (1992). Verticillium Wilt Resistance in Pistachio Rootstock Cultivars: Assays and an Assessment of Two Interspecific Hybrids. Plant Dis. 76 (3), 310–313. doi: 10.1094/PD-76-0310
Mousavi S. A., Keykhasaber M., Fahmideh L., Aran M. (2020). A Robust Method for Identification and in-Planta Detection of Verticillium Dahliae in the Infected Olive Trees, Using Real-Time PCR and Nested PCR. Physiol. Mol. Plant Pathol. 112, 101559. doi: 10.1016/j.pmpp.2020.101559
Mpika J., Kébé I., Issali A., N’guessan F., Druzhinina S., Komon-Zélazowska M., et al. (2009). Antagonist Potential of Trichoderma Indigenous Isolates for Biological Control of Phytophthora Palmivora the Causative Agent of Black Pod Disease on Cocoa (Theobroma Cacao L.) in Côte D’ivoire. Afr. J. Biotechnol. 8 (20), 5280–5293.
Mulero-Aparicio A. (2019). Biological Control of Verticillium Wilt of Olive With the Nonpathogenic Strain of Fusarium Oxysporum FO12 and the Grape Marc Compost CGR03 PhD Thesis. (Cordoba Spain: University of Córdoba).
Mulero-Aparicio A., Varo A., Agustí-Brisach C., López-Escudero F., Trapero A. (2020). Biological Control of Verticillium Wilt of Olive in the Field. Crop Prot. 128, 104993. doi: 10.1016/j.cropro.2019.104993
Munongo M. E., Nkeng G. E., Njukeng J. N. (2017). Production and Characterisation of Compost Manure and Biochar From Cocoa Pod Husks. Int. J. Adv. Scient. Res. Manage 2 (2), 26–31.
Naraghi L., Heydari A., Ahmadi A., Sarkari S., Maleki N. (2012). Investigation of the Effect of Antagonistic Fungi on the Incidence of Cotton Verticillium Wilt and Seedling Damping-Off Diseases. Appl. Entomol. Phytopathol. 79 (2), 251–272. doi: 10.22092/JAEP.2012.107224
Nawrocka J., Małolepsza U. (2013). Diversity in Plant Systemic Resistance Induced by Trichoderma. Biol. Control 67 (2), 149–156. doi: 10.1016/j.biocontrol.2013.07.005
Nyadanu D., Assuah M., Adomako B., Asiama Y., Opoku I., Adu-Ampomah Y. (2009). Efficacy of Screening Methods Used in Breeding for Black Pod Disease Resistance Varieties in Cocoa. Afr. Crop Sci. J. 17 (4), 175–186. doi: 10.4314/acsj.v17i4.54298
Ogunlade M. O., Orisajo S. B. (2020). Integrated Soil Fertility Management for Smallholder Cocoa Farms: Using Combination of Cocoa Pod Husk Based Compost and Mineral Fertilizers. I. J. P. S 32 (2), 68–77. doi: 10.9734/IJPSS/2020/v32i230248
Ojinaga M., Gandariasbeitia M., Orbegozo E., Ortíz A., Guerrero M., Lacasa C., et al. (2020). Biodisinfestation for Meloidogyne and Verticillium Control in Commercial Protected Crops in the Basque Country Atlantic Area (Northern Spain). Acta Hortic. 1270, 327–336. doi: 10.17660/ActaHortic.2020.1270.40
Oliveira M. (1983). Sensibilidade De Verticillium Dahliae Kleb., Agente Causal Da Murcha De Verticillium do Cacaueiro (Theobroma Cacao L.), a Fungicidas In Vitro. Rev. Theobroma (Brasil) 13 (1), 35–39.
Oliveira M., Luz E. (2005). Identificação E Manejo Das Principais Doenças do Cacaueiro No Brasil (Ilhéus, Brasil: CEPLAC/CEPEC/SEFIT).
Pandey R., Jaisani P., Yadav D. (2021). Trichoderma Spp. In the Management of Stresses in Plants and Rural Prosperity. Indian Phytopathol. 74, 453–467. doi: 10.1007/s42360-021-00373-9
Pegg G., Street P. (1984). Measurement of Verticillium Albo-Atrum in High and Low Resistance Hop Cultivars. Trans. Br. Mycol. Soc. 82 (1), 99–106. doi: 10.1016/s0007-1536(84)80216-8
Pereira R. B., Resende M., Ribeiro P. M. Jr., Amaral D. R., Lucas G. C., Cavalcanti F. R. (2008). Activation of Defence Responses on Cocoa Against Verticillium Wilt by Natural Extracts and Acibenzolar-S-Methyl. [Ativação De Defesa Em Cacaueiro Contra a Murcha-De- Verticílio Por Extratos Naturais E Acibenzolar-S-Metil]. Pesqui. Agropecu. Bras. 43 (2), 171–178. doi: 10.1590/S0100-204X2008000200003
Pérez-Artés E., Mercado-Blanco J., Ruz-Carrillo A. R., Rodríguez-Jurado D., Jiménez-Díaz R. M. (2005). Detection of the Defoliating and Nondefoliating Pathotypes of Verticillium Dahliae in Artificial and Natural Soils by Nested PCR. Plant Soil 268 (1), 349–356. doi: 10.1007/s11104-004-0378-1
Pérez-Piqueres A., Edel-Hermann V., Alabouvette C., Steinberg C. (2006). Response of Soil Microbial Communities to Compost Amendments. Soil Biol. Biochem. 38 (3), 460–470. doi: 10.1016/j.soilbio.2005.05.025
Ploetz R. (2016). “The Impact of Diseases on Cacao Production: A Global Overview,” in Cacao Diseases. Eds. Bailey B., Meinhardt L. (Cham, Switzerland: Springer), (pp. 33–59).
Poveda J., Baptista P. (2021). Filamentous Fungi as Biocontrol Agents in Olive (Olea Europaea L.) Diseases: Mycorrhizal and Endophytic Fungi. Crop Prot. 146, 105672. doi: 10.1016/j.cropro.2021.105672
Rafiuddin S., Nasaruddin N., Osmar H. (2020). Growth of Cacao Seedlings on Application of Several Sawdust Compost Composition Enriched With Trichoderma Sp. Earth Environ. Sci. 575 (1), 12147. doi: 10.1088/1755-1315/575/1/012147
Rahim I., Nasruddin A., Kuswinanti T., Asrul L., Rasyid B. (2018). Utilization of Cocoa Pod Husk Waste Composting by Tremella Sp and Pleurotus Sp as a Medium to Growth of Cocoa Seedling. Earth Environ. Sci. 156 (1), 12012. doi: 10.1088/1755-1315/156/1/012012
Ramírez-Gil J. G., Morales-Osorio J. G. (2021). Proposal for Integrated Management of Verticillium Wilt Disease in Avocado Cultivar Hass Crops. Agronomy 11 (10), 1932. doi: 10.3390/agronomy11101932
Reddy P. P. (2017). “Cover/Green Manure Crops,” in Sustainable Intensification of Crop Production. Ed. Reddy P. P. (Singapore: Springer), (pp. 55–67).
Resende M. (1994). Vascular Wilt of Cocoa (Theobroma Cacao L.) Caused by Verticillium Dahliae Kleb: Studies on Pathogenicity and Resistance (PhD Thesis (UK: University of Bath).
Resende M., Flood J., Cooper R. M. (1994). Host Specialization of Verticillium Dahliae, With Emphasis on Isolates From Cocoa (Theobroma Cacao). Plant Pathol. 43 (1), 104–111. doi: 10.1111/j.1365-3059.1994.tb00559.x
Resende M., Flood J., Cooper R. M. (1995). Effect of Method of Inoculation, Inoculum Density and Seedling Age at Inoculation on the Expression of Resistance of Cocoa (Theobroma Cacao L.) to Verticillium Dahliae Kleb. Plant Pathol. 44 (2), 374–383. doi: 10.1111/j.1365-3059.1995.tb02790.x
Resende M., Flood J., Ramsden J. D., Rowan M. G., Beale M. H., Cooper R. M. (1996a). Novel Phytoalexins Including Elemental Sulphur in the Resistance of Cocoa (Theobroma Cacao L.) to Verticillium Wilt (Verticillium Dahliae Kleb.). Physiol. Mol. Plant Pathol. 48 (5), 347–359. doi: 10.1006/pmpp.1996.0028
Resende M., Mepsted R., Flood J., Cooper R. M. (1996b). Water Relations and Ethylene Production as Related to Symptom Expression in Cocoa Seedlings Infected With Defoliating and non-Defoliating Isolates of Verticillium Dahliae. Plant Pathol. 45 (5), 964–972. doi: 10.1111/j.1365-3059.1996.tb02907.x
Resende M., Nojosa G., Cavalcanti L., Aguilar M., Silva L., Perez J., et al. (2002). Induction of Resistance in Cocoa Against Crinipellis Perniciosa and Verticillium Dahliae by Acibenzolar-S-Methyl (ASM). Plant Pathol. 51 (5), 621–628. doi: 10.1046/j.1365-3059.2002.00754.x
Ribeiro P. M. Jr, de Resende M. L., Pereira R. B., Cavalcanti F. R., Amaral D. R., de Pádua M. A. (2006). Fosfito De Potássio Na Indução De Resistência a Verticillium Dahliae Kleb., Em Mudas De Cacaueiro (Theobroma Cacao L.). [Effect of Potassium Phosphite on the Induction of Resistance in Cocoa Seedlings (Theobroma Cacao L.) Against Verticillium Dahliae Kleb]. Ciênc. Agrotec. 30 (4), 629–636. doi: 10.1590/S1413-70542006000400006
Romanyà J., Sancho-Adamson M., Ortega D., Trillas M. I. (2019). Early Stage Effects of Verticillium Wilt of Olive (WVO) on Nutrient Use in Young Olive Trees Grown in Soils Amended With Compost and Mineral Fertilisation. Plant Soil 436 (1), 193–209. doi: 10.1007/s11104-018-03923-9
Rosmana A., Kuswinanti T., Asman A., Mandy Y. I., Muhayang M. T., Kesia A. M. (2018). Composted Plant Residues Improve Control Capability of Trichoderma Asperellum Against Vascular Streak Dieback Disease on Cacao. Int. J. Agric. Biol. 20, 1795–1800. doi: 10.17957/IJAB/15.0694
Rosmana A., Taufik M., Asman A., Jayanti N. J., Hakkar A. A. (2019). Dynamic of Vascular Streak Dieback Disease Incidence on Susceptible Cacao Treated With Composted Plant Residues and Trichoderma Asperellum in Field. Agronomy 9 (10), 650. doi: 10.3390/agronomy9100650
Ruano-Rosa D., Mercado-Blanco J. (2015). “Combining Biocontrol Agents and Organics Amendments to Manage Soil-Borne Phytopathogens,” in Organic Amendments and Soil Suppressiveness in Plant Disease Management. Eds. Meghvansi M. K., Varma A. (Cham, Switzerland: Springer), (pp. 457–478).
Ruano-Rosa D., Prieto P., Rincón A. M., Gómez-Rodríguez M. V., Valderrama R., Barroso J. B., et al. (2016). Fate of Trichoderma Harzianum in the Olive Rhizosphere: Time Course of the Root Colonization Process and Interaction With the Fungal Pathogen Verticillium Dahliae. BioControl 61 (3), 269–282. doi: 10.1007/s10526-015-9706-z
Sandoya G. V., Gurung S., Short D. P., Subbarao K. V., Michelmore R. W., Hayes R. J. (2017). Genetics of Resistance in Lettuce to Races 1 and 2 of Verticillium Dahliae From Different Host Species. Euphytica 213 (1), 1–12. doi: 10.1007/s10681-016-1813-0
Sandroni M., Liljeroth E., Mulugeta T., Alexandersson E. (2020). Plant Resistance Inducers (PRIs): Perspectives for Future Disease Management in the Field. CAB Rev. 15, 1–10. doi: 10.1079/PAVSNNR202015001
Schmidt J. E., DuVal A., Isaac M. E., Hohmann P. (2022). At the Roots of Chocolate: Understanding and Optimizing the Cacao Root-Associated Microbiome for Ecosystem Services. A Review. Agron. Sustain. Dev. 42, 14. doi: 10.1007/s13593-021-00748-2
Schnathorst W., Mathre D. (1966). Host Range and Differentiation of a Severe Form of Verticillium Albo-Atrum in Cotton. Phytopathology 56 (10), 1155–1161.
Shaw D., Gordon T., Larson K., Gubler W., Hansen J., Kirkpatrick S. (2010). Strawberry Breeding Improves Genetic Resistance to Verticillium Wilt. Calif. Agric. 64 (1), 37–41. doi: 10.3733/ca.v064n01p37
Singh H., Singh B., Singh S., Singh S., Sarma B. (2009). “Biological Control of Plant Diseases: Current Status and Future Prospects,” in Recent Advances in Biopesticides: Biotechnological Applications. Ed. Johri J. K. (New Delhi, India: New India Pub), (pp. 193–304).
Sriwati R., Chamzurn T., Soesanto L., Munazhirah M. (2019). Field Application of Trichoderma Suspension to Control Cacao Pod Rot (Phytophthora Palmivora). AGRIVITA J. Agric. Sci. 41 (1), 175–182. doi: 10.17503/agrivita.v41i1.2146
Swain H., Mukherjee A. K. (2020). “Host–pathogen–Trichoderma Interaction,” in Trichoderma. Eds. Sharma A. K., Sharma P. (Singapore: Springer), (pp. 149–165).
Tahi M., Kebe I., Eskes A., Ouattara S., Sangare A., Mondeil F. (2000). Rapid Screening of Cacao Genotypes for Field Resistance to Phytophthora Palmivora Using Leaves, Twigs and Roots. Eur. J. Plant Pathol. 106, 87–94. doi: 10.1023/A:1008747800191
Taylor J., Flentje N. (1968). Infection, Recovery From Infection and Resistance of Apricot Trees to Verticillium Albo-Atrum. New Zeal. J. Bot. 6 (4), 417–426. doi: 10.1080/0028825X.1968.10428580
Tchameni S. N., Sameza M. L., O’donovan A., Fokom R., Mangaptche Ngonkeu E. L., Wakam Nana L., et al. (2017). Antagonism of Trichoderma Asperellum Against Phytophthora Megakarya and its Potential to Promote Cacao Growth and Induce Biochemical Defence. Mycology 8 (2), 84–92. doi: 10.1080/21501203.2017.1300199
Ten Hoopen G. M., Krauss U. (2006). Biology and Control of Rosellinia Bunodes, Rosellinia Necatrix and Rosellinia Pepo: A Review. Crop Prot. 25 (2), 89–107. doi: 10.1016/j.cropro.2005.03.009
Thaha A. R., Umrah U., Asrul A., Rahim A., Fajra F., Nurzakia N. (2020). The Role of Local Isolates of Trichoderma Sp. As a Decomposer in the Substrate of Cacao Pod Rind (Theobroma Cacao L.). AIMS Agric. Food 5 (4), 825−834. doi: 10.3934/agrfood.2020.4.825
Townsend A., Hock W. (1973). Tolerance of Half-Sib Families of Red Maple to Verticillium Wilt. Phytopathology 63 (6), 673–676. doi: 10.1094/Phyto-63-673
Trapero C., Díez C. M., Rallo L., Barranco D., López-Escudero F. J. (2013). Effective Inoculation Methods to Screen for Resistance to Verticillium Wilt in Olive. Sci. Hortic. 162, 252–259. doi: 10.1016/j.scienta.2013.08.036
Trapero C., Rallo L., López-Escudero F. J., Barranco D., Díez C. (2015). Variability and Selection of Verticillium Wilt Resistant Genotypes in Cultivated Olive and in the Olea Genus. Plant Pathol. 64 (4), 890–900. doi: 10.1111/ppa.12330
Trocmé O. (1972). Contribution À L'étude D'une Maladie Du Cacaoyer En Ouganda: Le Dessèchement Éco-Fongique Des Branches. Café Cacao Thé 16 (3), 219–235.
Tubeileh A. M., Stephenson G. T. (2020). Soil Amendment by Composted Plant Wastes Reduces the Verticillium Dahliae Abundance and Changes Soil Chemical Properties in a Bell Pepper Cropping System. Curr. Plant Biol. 22, 100148. doi: 10.1016/j.cpb.2020.100148
UEPB (2021). Available at: https://ugandaexports.go.ug/uploads/stats/Formal_and_Informal_Exports_by_Commodity_and_Quantity,_Calender_Year_hbc608mb.xls.
Van Asten P., Wanyama I., Mukasa D., Nansamba R., Kisaakye J., Sserubiri I., et al. (2012). Mapping and Evaluating Improved Intercrop and Soil Management Options for Ugandan Coffee Farmers. (No. 90) (Nigeria: IITA/LEAD). Available at: https://pdf.usaid.gov/pdf_docs/PA00HT7Z.pdf.
Varo-Suárez A., Raya-Ortega M., Agustí-Brisach C., García-Ortiz-Civantos C., Fernández-Hernández A., Mulero-Aparicio A., et al. (2018). Evaluation of Organic Amendments From Agro-Industry Waste for the Control of Verticillium Wilt of Olive. Plant Pathol. 67 (4), 860–870. doi: 10.1111/ppa.12798
Veronese P., Narasimhan M. L., Stevenson R. A., Zhu J., Weller S. C., Subbarao K. V., et al. (2003). Identification of a Locus Controlling Verticillium Disease Symptom Response in Arabidopsis Thaliana. Plant J. 35 (5), 574–587. doi: 10.1046/j.1365-313X.2003.01830.x
Vigouroux A., Castelain C. (1969). La Verticilliose De L'abricotier. I. Premières Observations Sur Les Symptômes Et L'évolution De La Maladie. Hypothèses Sur Quelques Facteurs De Variation. Ann. Phytopathol. 1, 427–448.
Vitullo D., Altieri R., Esposito A., Nigro F., Ferrara M., Alfano G., et al. (2013). Suppressive Biomasses and Antagonist Bacteria for an Eco-Compatible Control of Verticillium Dahliae on Nursery-Grown Olive Plants. Int. J. Environ. Sci. Technol. 10 (2), 209–220. doi: 10.1007/s13762-012-0145-4
Woo S. L., Ruocco M., Vinale F., Nigro M., Marra R., Lombardi N., et al. (2014). Trichoderma-Based Products and Their Widespread Use in Agriculture. Open Mycol. J. 8 (1), 71–126. doi: 10.2174/1874437001408010071
Zavala M. G. M., Feijoo M. I. J., García E. L. P. (2010). Actualización De La Micobiota Patogénica Del Cacao “Arriba” (Theobroma Cacao) Presente En La Costa Ecuatoriana. Rev. Tecnol. ESPOL 23 (1), 21–26.
Zhang J., Fang H., Zhou H., Sanogo S., Ma Z. (2014). Genetics, Breeding, and Marker-Assisted Selection for Verticillium Wilt Resistance in Cotton. Crop Sci. 54 (4), 1289–1303. doi: 10.2135/cropsci2013.08.0550
Keywords: integrated management, Verticillium wilt, cacao, organic amendment, biocontrol agents, induced resistance, genetic resistance, sustainability
Citation: Bouchon AS and Hoopen GM (2022) Integrated Management of Verticillium Wilt of Cacao. Front. Agron. 4:911969. doi: 10.3389/fagro.2022.911969
Received: 03 April 2022; Accepted: 30 May 2022;
Published: 05 July 2022.
Edited by:
David Ian Guest, The University of Sydney, AustraliaReviewed by:
John M. Maingi, Kenyatta University, KenyaCopyright © 2022 Bouchon and Hoopen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Anne-Sophie Bouchon, YW5uZV9zb3BoaWUuYm91Y2hvbkB5YWhvby5mcg==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.