- 1Department of Entomology, University of California Riverside, Riverside, CA, United States
- 2University of California Kearney Agricultural Research and Extension Center, Parlier, CA, United States
Hemp is a newly (re)introduced crop to United States and California agriculture. A study was initiated in the summer of 2021 to survey the arthropods present in hemp in two regions of California: Fresno County in the Central Valley and Ventura County along the Central Coast. Eight hemp plots were sampled every two weeks from August to mid-October using a combination of D-vac samples, leaf collections, and visual observations. All samples were processed and ultimately recorded as the total number of specimens collected per morphospecies across all sampling dates, further broken down to express the number of specimens collected from the Central Valley and the Central Coast. D-vac sampling was the most reliable method for specimen collection and led to the recovery of arthropods from 11 orders, 69 families, and 157 morphospecies. Approximately 13,000 specimens were collected and processed, half of which were whiteflies (Hemiptera: Aleyrodidae). Of the specimens recovered, Hemiptera was the most representative order (with and without whiteflies), followed by Thysanoptera and then Hymenoptera. The most frequently collected specimen was Engytatus modestus (Hemiptera: Miridae). Very few pest species were recovered, cannabis aphid (Phorodon cannabis) being the only one that was observed in any noticeable density. Many generalist predators and parasitoid wasps were also collected. Findings from this survey provide baseline information on the arthropod species present in California hemp. This survey will be repeated and expanded in future growing seasons.
Introduction
Hemp (Cannabis sativa L., <0.3% THC) has recently had a revival in United States agriculture, and in California in particular. At the federal level, prohibition rendered hemp cultivation illegal for the greater part of the past century, but language in Section 7606 of the 2014 Farm Bill (U.S. H.R. 2642 – Agricultural Act of 2014 113th Congress [2013–2014]) gave justification for universities to conduct hemp research and for commercial production to occur in states where cultivation was allowed. With the subsequent passage of the 2018 Farm Bill (U.S. H.R. 2—115th Congress [115-334]), hemp was federally legalized for commercial production and as of 2022, cultivation is allowed in all 50 states. Effective January 1, 2017, the California Industrial Hemp Farming Act (Senate Bill 566, Chapter 398, Statutes of 2013) authorized commercial production of hemp in California. In 2021, California had the fifth highest acreage for hemp production in the United States (2,650 acres) and was second highest in acreage devoted to floral/cannabinoid hemp production (1,900 acres) (National Hemp Report, 2022).
Arthropod species present in hemp are poorly documented, with no published surveys from California and few for the rest of the United States. The most comprehensive texts thus far are from Cranshaw et al. (2019), which focused on hemp production in Colorado, Virginia, and Tennessee, and McPartland et al. (2000), which collected reports from various locations throughout the world. To date, the only state-specific studies to catalogue arthropods in Cannabis sativa have been from hemp in Colorado (Schreiner and Cranshaw, 2020) and cannabis (Cannabis sativa L., >0.3% THC) in Mississippi (Lago and Stanford, 1989). There are also several state level Extension guides (Britt et al., 2020; Hansen et al., 2020; Kesheimer et al., 2021), but these texts focus exclusively on pest species (arthropods, pathogens, and weeds) in hemp and guidelines for their management. Compilations of plant pathogens and viruses present in hemp also exist (Punja, 2018; Punja et al., 2019; Thiessen et al., 2020; Chiginsky et al., 2021). In California, Wilson et al. (2019) conducted an online survey of cannabis growers to solicit information about many aspects of production, including pest monitoring and management. However, accuracy of identification of arthropods reported in the survey is uncertain since respondents were self-reporting.
Here, a field study was conducted in the summer and fall of 2021 to catalogue the arthropod species present in hemp across two regions of California. With no prior records detailing this information, this study was intended to provide baseline data on the diversity, abundance, and potential pest status of arthropods found on hemp in California.
Materials and Methods
Field Sampling
Eight floral/cannabinoid hemp sites (Table 1) were sampled during the 2021 growing season to catalogue the arthropod community in California hemp. Specimens were collected from two general regions in California: 1) Fresno County in the Central Valley and 2) Ventura County on the Central Coast (Figure 1). Two sites at each location were research institutes and two sites were licensed commercial hemp growers. Sites were sampled twice monthly beginning in August (August 3, Central Valley; August 9, Central Coast) and sampling was terminated just prior to harvest, which was mid-October (October 5, Central Valley; October 12, Central Coast).
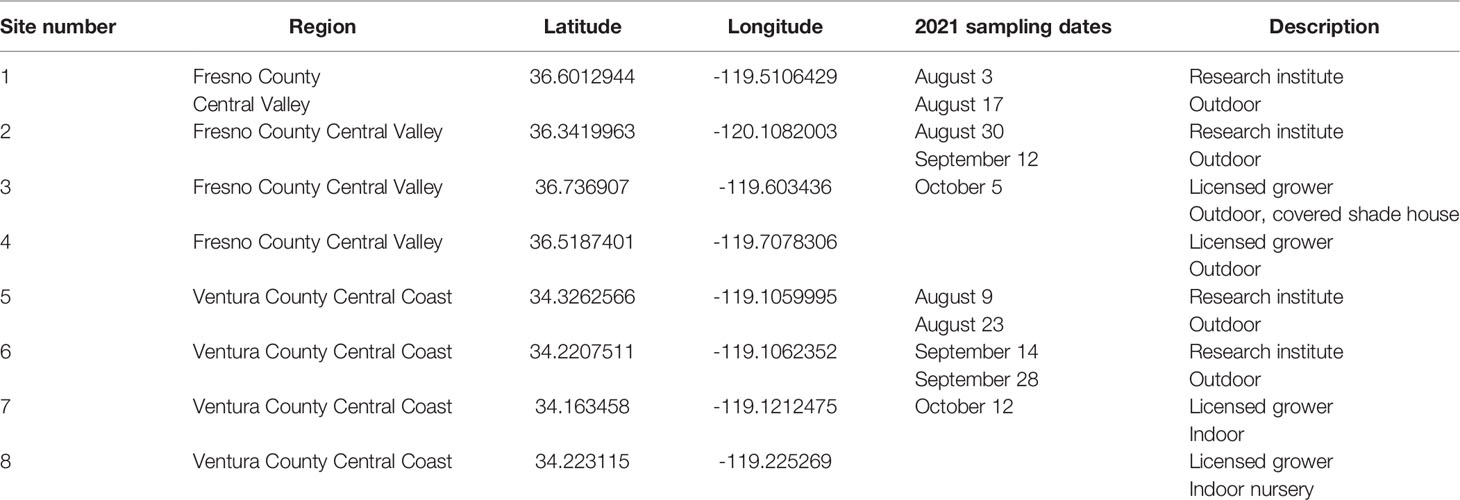
Table 1 Site number, region, latitude and longitude coordinates, sampling dates, and description of hemp sites sampled in California in 2021.
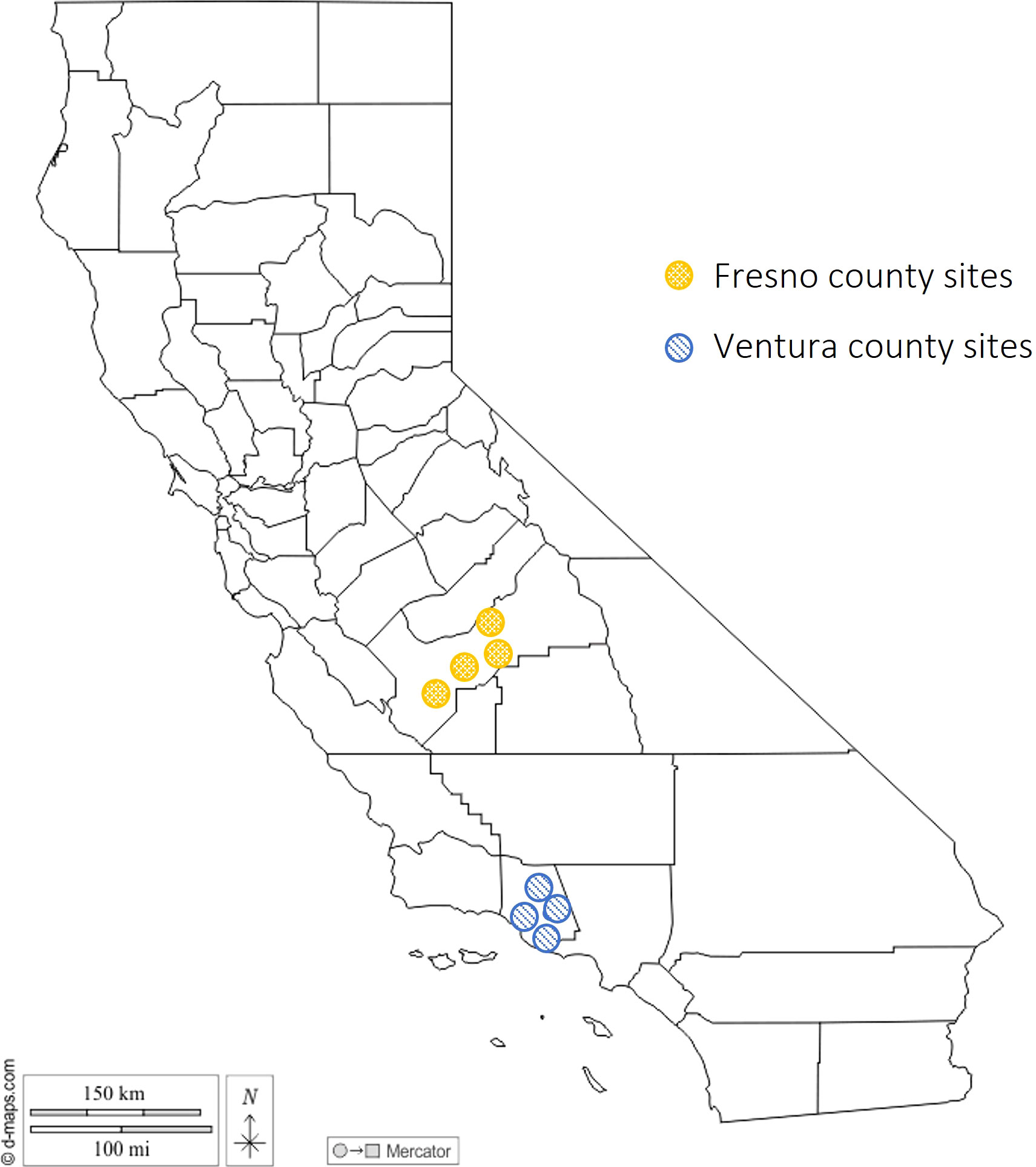
Figure 1 Location of hemp sites sampled in 2021. Yellow dots with checkerboard texture are indicative of Fresno county sites and blue dots with parallel line texture are indicative of Ventura county sites.
Sampling Techniques
D-vac type suction sampling was the primary sampling technique used. This consisted of vacuum suctions of hemp plants using a 25-cc gasoline blower/vacuum (Husqvarna, Stockholm, Sweden) fitted with a 5-gallon bucket on the vacuum tube (1 square-foot sampling cone) covered with a fine mesh collection bag. D-vac samples were conducted at three random sample points at each site. For each sample, a transect of hemp plants was vacuumed for a total of 60 seconds while moving at a walking pace; for this study, approximately 50 feet or 15.2 meters were travelled in each 60 second period. All collected arthropod specimens were transferred to a 1-gallon plastic freezer bag and held in a cooler with ice during transport to the laboratory.
Leaf collections were used to sample for mites (hemp russet mite, Aculops cannibicola [Acari: Eriophyidae]; twospotted spider mite, Tetranychus urticae [Trombidiformes: Tetranychidae]; and broad mite, Polyphagotarsonemus latus [Trombidiformes: Tarsonemidae]). At each site, ten leaves were collected at each of three random sample points. Leaves were processed with a mite brush upon returning to the lab.
Visual samples were used to document presence and injury resulting from species that have been classified as injurious to hemp (Cranshaw et al., 2019), including corn earworm (Helicoverpa zea, Lepidoptera: Noctuidae) (Britt et al., 2021), twospotted spider mite (Trombidiformes: Tetranychidae), and cannabis aphid, Phorodon cannabis (Hemiptera: Aphididae) (Cranshaw et al., 2018). At each site, apical sections of ten hemp buds were examined at each of three random sample points.
Lab Processing and Taxonomic Identification
In the laboratory, D-vac samples were sorted into morphologically distinct groupings, or morphospecies, and identified to family or lowest taxonomic level possible. Family level identifications were made using keys from Triplehorn et al. (2005) and Marshall (2006), followed by comparisons to specimen photographs from the web portals BugGuide1 and iNaturalist2. Voucher specimens of each morphospecies were pinned or preserved and are currently housed at the University of California’s Kearney Agricultural Research and Extension Center in Parlier, CA.
Results and Discussion
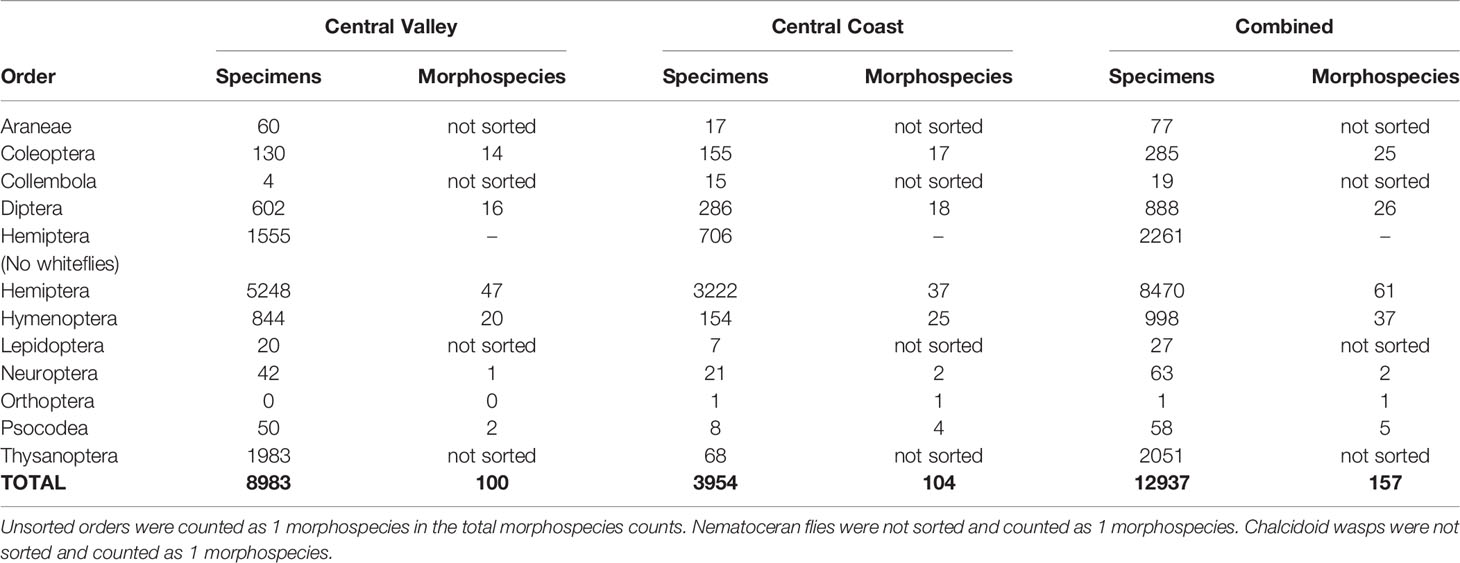
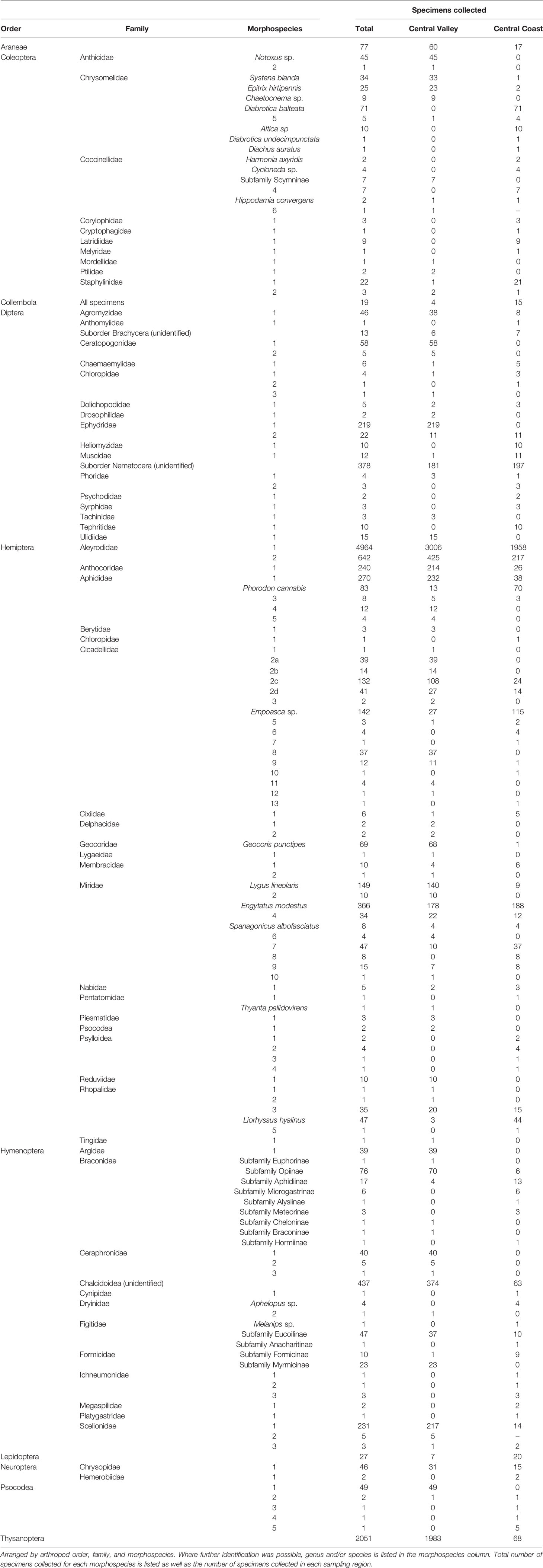
In total, the D-vac sampling effort led to the recovery of arthropods from 11 orders, 69 families, and 157 morphospecies (Table 2). A total of 12,937 specimens were processed, half of which were whiteflies (Hemiptera: Aleyrodidae) (6,209). Other than whiteflies, the most frequently collected specimens were: Engytatus modestus (Hemiptera: Miridae), 366 specimens; Aphididae morphospecies 1 (Hemiptera: Aphididae), 270 specimens; Anthocoridae morphospecies 1 (Hemiptera: Anthocoridae), 240 specimens; Scelionidae morphospecies 1 (Hymenoptera: Scelionidae), 231 specimens; Ephydridae morphospecies 1 (Diptera: Ephydridae), 219 specimens; Lygus lineolaris (Hemiptera: Miridae), 149 specimens; Empoasca sp. (Hemiptera: Cicadellidae), 142 specimens; and Cicadellidae morphospecies 2c (Hemiptera: Cicadellidae), 132 specimens (Table 3). Order Hemiptera had the greatest number of specimens collected overall (8,470), even when the whitefly specimens were excluded (2,261), followed by Thysanoptera (2,051), and Hymenoptera (998).
Potential Pests
A few major pests of hemp include corn earworm (Britt et al., 2021), cannabis aphid (Cranshaw et al., 2018), hemp russet mite (McPartland and Hillig, 2003), and twospotted spider mite (McPartland et al., 2000; Cranshaw et al., 2019). Cannabis aphid was found in both sampling regions and is the only one of the pest species that was observed in any kind of noticeable density. Recently, it has been confirmed that cannabis aphid is a vector of potato virus y (Pitt et al., 2022) in hemp in Colorado. Similarly, in the past, cannabis aphid was documented as a vector of alfalfa mosaic virus and confirmed to transmit cucumber mosaic virus (Schmidt and Karl, 1970). However, it should be noted that other aphid morphospecies were also found in both regions and in higher numbers.
Very few lepidopteran larvae were collected in D-vac samples and hardly any corn earworm larvae were documented with visual observations. Additionally, no sites had considerable presence of or suspected injury resulting from any of these species of concern, so there are no visual data to report. Very few to no mites were recovered from leaf collections in this study, so there are also no mite results to report.
Generalist Predators and Parasitoids
Notable in this study was the large number of natural enemies collected, including Anthocoridae (minute pirate bugs, Hemiptera), Reduviidae (assassin bugs, Hemiptera), Nabidae (damsel bugs, Hemiptera), Coccinellidae (lady beetles, Coleoptera), Chrysopidae (lacewings, Neuroptera). Additionally, a considerable number of Hymenopteran parasitoid families were also collected (Braconidae [107 specimens], Ceraphronidae [46 specimens], Cynipidae [1 specimen], Dryinidae [5 specimens], Figitidae [49 specimens], Ichneumonidae [5 specimens], Megaspilidae [2 specimens], Platygastridae [1 specimen], and Scelionidae [239 specimens]).
Beet Leafhopper
Several leafhopper morphospecies were collected during this survey. Although the species was not confirmed in this study, beet leafhopper, Circulifer tenellus (Hemiptera: Cicadellidae), is a species of concern for hemp in California since it can transmit beet curly top virus (Severin, 1931). So far, beet curly top virus has been confirmed in hemp in Arizona (Hu et al., 2020), Colorado (Giladi et al., 2020), and Oregon (Rivedal et al., 2021). Symptoms in hemp include stunted growth and yellowing of leaves (Giladi et al., 2020), potentially leading to yield loss. Beet curly top virus is problematic in California (Wintermantel et al., 2003) and several different strains have been confirmed in various California crops such as tomatoes (Chen et al., 2010), peppers (Chen et al., 2017), basil (Chen et al., 2014), and others. Signs and symptoms of beet curly top virus were noted at one of the research sites in the Central Valley, so this is something to monitor in future studies where leafhoppers (particularly beet leafhopper) are present.
Species Not Recovered
Bees have been observed in hemp, but these reports have been from pollen producing fiber or grain varieties of the crop (O’Brien and Arathi, 2019; Flicker et al., 2020). The floral/cannabinoid varieties sampled in this study do not produce pollen. Several species of stink bugs have been readily observed in hemp (Cranshaw et al., 2019) although crop injury resulting from feeding has not been documented (Britt et al., 2019). Only two stink bugs total were recovered from 2021 surveys.
Study Limitations
Lepidopteran larvae and mites are known to be associated with hemp (Lago and Stanford, 1989; McPartland et al., 2000; McPartland and Hillig, 2003; Cranshaw et al., 2019; Schreiner and Cranshaw, 2020; Britt et al., 2021) but this survey recovered very few of both these groups. D-vac sampling is unlikely to recover arthropods which strongly adhere to substrate or are embedded in plant tissue which could include larvae of lepidopterans and others, such as coleopterans (lady beetles) and neuropterans (lacewings). Aphids and other soft-bodied specimens were captured by the D-vac, so the suction was likely strong enough to collect mite specimens if they were present. Because visual sampling targeting these mites and lepidopteran larvae also recovered very few observations of either arthropod group, it is likely that there were not very many lepidopteran larvae or mites present at the study sites.
Conclusions and Future Directions
This study provided baseline information regarding the arthropod community present in California hemp. Many species were present, including a variety of generalist predators and parasitoids. This work will continue and expand in the coming years to incorporate sampling from more regions in California to additionally express seasonal phenology of each observed species. At most of the sites sampled, 2021 was the first year hemp was cultivated. It is likely that the arthropod community will change in the coming years as the crop is more regularly cultivated throughout California.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Author Contributions
KB and HW designed the study. KB arranged sampling with growers and research centers and conducted field sampling. SM and VM conducted specimen ID. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We are incredibly grateful to the growers who allowed us to sample from their hemp plots, as well as the agriculture operations personnel at the University of California research stations who handled initial planting and plot maintenance throughout the 2021 growing season. We also thank Dmitri A. Dmitriev who is currently assisting with Cicadellidae identification.
Footnotes
References
Britt K., Kuhar T. P., Cranshaw W., McCullough C. T., Taylor S. V., Arends B. R., et al. (2020) ‘Integrated Pest Management of Hemp in Virginia’ (Virginia Cooperative Extension). Available at: https://www.pubs.ext.vt.edu/content/dam/pubs_ext_vt_edu/ENTO/ento-349/ENTO-349.pdf (Accessed 4 January 2021).
Britt K. E., et al. (2021). Pest Management Needs and Limitations for Corn Earworm (Lepidoptera: Noctuidae), an Emergent Key Pest of Hemp in the United States. J. Integrated. Pest Manage. 12 (1), 1–11. doi: 10.1093/JIPM/PMAB030
Britt K. E., Pagani M. K., Kuhar T. P. (2019). First Report of Brown Marmorated Stink Bug (Hemiptera: Pentatomidae) Associated With Cannabis Sativa (Rosales: Cannabaceae) in the United States. J. Integrated. Pest Manage. 10 (1), 1–3. doi: 10.1093/JIPM/PMZ014
Chen L.-F., Brannigan K., Clark R., Gilbertson R. L. (2010). Characterization of Curtoviruses Associated With Curly Top Disease of Tomato in California and Monitoring for These Viruses in Beet Leafhoppers. Plant Dis. 94 (1), 99–108. doi: 10.1094/PDIS-94-1-0099
Chen L. F., Natwick E., Cabrera S., Gilbertson R. L. (2014). First Report of Curly Top Disease of Basil Caused by Beet Severe Curly Top Virus in California. Plant Dis. 98 (2), 286. doi: 10.1094/PDIS-07-13-0686-PDN
Chen L. F., Batuman O., Aegerter B. J., Willems J., Gilbertson R. L. (2017). First Report of Curly Top Disease of Pepper and Tomato in California Caused by the Spinach Curly Top Strain of Beet Curly Top Virus. Plant Dis. 101 (7), 1334. doi: 10.1094/PDIS-02-17-0206-PDN
Chiginsky J., Langemeier K., MacWilliams J., Albrecht T., Cranshaw W., Fulladolsa A. C., et al. (2021). First Insights Into the Virus and Viroid Communities in Hemp (Cannabis Sativa). Front. Agron. 3. doi: 10.3389/FAGRO.2021.778433/BIBTEX
Cranshaw W. S., Halbert S. E., Favret C., Britt K. E., Miller G. L. (2018). Phorodon Cannabis Passerini (Hemiptera: Aphididae), a Newly Recognized Pest in North America Found on Industrial Hemp. Insecta. Mundi. 0662, 1–12.
Cranshaw W., Schreiner M., Britt K., Kuhar T. P., McPartland J., Grant J., et al. (2019). Developing Insect Pest Management Systems for Hemp in the United States: A Work in Progress. J. Integrated. Pest Manage. 10 (1), 1–10. doi: 10.1093/jipm/pmz023
Flicker N. R., Poveda K., Grab H. (2020). The Bee Community of Cannabis sativa and Corresponding Effects of Landscape Composition, Environmental Entomology, 49, 197–202. doi: 10.1093/ee/nvz141
Giladi Y., Hadad L., Luria N., Cranshaw W., Lachman O. (2020). First Report of Beet Curly Top Virus Infecting Cannabis Sativa in Western Colorado. Plant Dis. 104 (3), 999. doi: 10.1094/PDIS-08-19-1656-PDN
Hansen Z., Bernard E., Grant J., Gwinn K., Hale F., Kelly H., et al. (2020) Hemp Disease and Pest Management (University of Tennessee Extension). Available at: https://extension.tennessee.edu/publications/Documents/W916.pdf (Accessed 4 January 2021).
Hu J., Masson R., Dickey L. (2020). First Report of Beet Curly Top Virus Infecting Industrial Hemp (Cannabis Sativa) in Arizona. Plant Dis. 105, 1233–1233. doi: 10.1094/PDIS-11-20-2330-PDN
Kesheimer K., Sikora E., Conner K., Kemble J.. (2021) Hemp: Insect, Weed, and Disease Control Recommendations for 2022 (Auburn, AL). Available at: https://www.aces.edu/wp-content/uploads/2021/12/ANR-2635_HempPestManagement_121621L.pdf (Accessed 16 March 2022).
Lago P. K., Stanford D. F. (1989). Phytophagous Insects Associated With Cultivated Marijuana, Cannabis Sativa in Northern Mississippi. J. Entomol. Sci. 24 (4), 437–445. doi: 10.18474/0749-8004-24.4.437
Marshall S. A. (2006). Insects: Their Natural History and Diversity: With a Photographic Guide to Insects of Eastern North America. 1st edn (Buffalo, NY: Firefly Books).
McPartland J. M., Clarke R. C., Watson D. P. (2000). Hemp Diseases and Pests. 1st edn. Eds. Mcpartland J. M., Clarke R. C., Watson D. P. (Wallingford, Oxon, UK: CABI Publishing).
McPartland J. M., Hillig K. W. (2003). The Hemp Russet Mite. J. Ind. Hemp. 8 (2), 107–112. doi: 10.1300/J237v08n02_10
National Hemp Report (2022). Available at: https://downloads.usda.library.cornell.edu/usda-esmis/files/gf06h2430/xd07hw825/v692v917t/hempan22.pdf (Accessed 15 March 2022).
O’Brien C., Arathi H. S. (2019). Bee Diversity and Abundance on Flowers of Industrial Hemp (Cannabis Sativa L.). Biomass Bioenergy 122, 331–335. doi: 10.1016/j.biombioe.2019.01.015
Pitt W. J, Kairy L., Villa E., Nalam V. J., Nachappa P. (2022). Virus Infection and Host Plant Suitability Affect Feeding Behaviors of Cannabis Aphid (Hemiptera: Aphididae), a Newly Described Vector of Potato Virus Y. Environ. Entomol 51 (2), 322–331. doi: 10.1093/EE/NVAC001
Punja Z. K. (2018). Flower and Foliage-Infecting Pathogens of Marijuana (Cannabis Sativa L.) Plants. Can. J. Plant Pathol. 40 (4), 514–527. doi: 10.1080/07060661.2018.1535467
Punja Z. K., Collyer D., Scott C., Lung S., Holmes J., Sutton D. (2019). Pathogens and Molds Affecting Production and Quality of Cannabis Sativa L. Front. Plant Sci. 10. doi: 10.3389/fpls.2019.01120
Rivedal H. M., Funke C., Frost K. (2021). An Overview of Pathogens Associated With Biotic Stresses in Hemp Crops in Oregon 2019-2020. Plant Dis 6 (105), 1334–1340. doi: 10.1094/PDIS-11-21-2415-SR
Schmidt H. E., Karl E. (1970). A Contribution to the Analysis of Virus Diseases on Hemp (C. Sativa) With Regard to the Hemp Aphid (P. Cannabis) as Virus Vector. Zentralblatt. fur. Bakteriologie. Parasitenkunde. Infektionskrankheiten. und. Hygiene. 125 (1), 16–22. https://www.cabdirect.org/cabdirect/abstract/19711103812.
Schreiner M., Cranshaw W. (2020). A Survey of the Arthropod Fauna Associated With Hemp (Cannabis Sativa L.) Grown in Eastern Colorado. J. Kansas. Entomol. Soc. 93 (2), 113–131. doi: 10.2317/0022-8567-93.2.113
Severin H. H. P. (1931). Modes of Curly-Top Transmission by the Beet Leafhopper, Eutettix Tenellus (Baker). Hilgardia 6 (8), 253–276. doi: 10.3733/hilg.v06n08p253
Thiessen L. D., Schappe T., Cochran S., Hicks K., Post A. R. (2020). Surveying for Potential Diseases and Abiotic Disorders of Industrial Hemp (Cannabis Sativa) Production. Plant Health Prog. 21, 321–332. doi: 10.1094/php-03-20-0017-rs
Triplehorn C. A., Johnson N. F., Borror D. J. (2005). Borror and Delong’s Introduction to the Study of Insects. 7th edn (Belmont, CA: Thompson Brooks/Cole).
Wilson H., Bodwitch H., Carah J., Daane K., Getz C., Grantham T. E., et al. (2019). First Known Survey of Cannabis Production Practices in California. California. Agric. 73 (3), 119–127. doi: 10.3733/CA.2019A0015
Wintermantel W. M., Mosqueda N. F., Cortez A. A., Anchieta A. G.. (2003) Beet Curly Top Virus Revisited: Factors Contributing to Re-Emergence in California (San Antonio, Texas: 1st Joint IIRB-ASSBT Congress). Available at: https://www.researchgate.net/publication/43264372 (Accessed 21 March 2022).
Keywords: arthropod, survey, hemp (cannabis sativa L), California (USA), first year
Citation: Britt KE, Meierotto S, Morelos V and Wilson H (2022) First Year Survey of Arthropods in California Hemp. Front. Agron. 4:901416. doi: 10.3389/fagro.2022.901416
Received: 21 March 2022; Accepted: 30 May 2022;
Published: 29 June 2022.
Edited by:
Zamir Punja, Simon Fraser University, CanadaReviewed by:
Sean Michael Prager, University of Saskatchewan, CanadaCynthia Diane Scott-Dupree, University of Guelph, Canada
Copyright © 2022 Britt, Meierotto, Morelos and Wilson. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Kadie E. Britt, a2FkaWViQHVjci5lZHU=
 Kadie E. Britt
Kadie E. Britt Sarah Meierotto1,2
Sarah Meierotto1,2 Victoria Morelos
Victoria Morelos Houston Wilson
Houston Wilson