- 1Department of Entomology, Kansas State University, Manhattan, KS, United States
- 2USDA-ARS Center for Grain and Animal Health Research, Manhattan, KS, United States
- 3Department of Entomology and Plant Pathology, University of Tennessee, Knoxville, Knoxville, TN, United States
Two major stored products pests of maize are the Prostephanus truncatus (Horn) (larger grain borer) and Sitophilus zeamais (Motschulsky) (maize weevil). Under climate change, P. truncatus may be expected to shift its distribution northward farther into the United States (US). Thus, there is a critical need to develop diversified chemical control tools in the post-harvest supply chain for these two species. In this study, we investigated the efficacy of a novel insecticide formulation, containing the insect growth regulator, S-methoprene, combined with the pyrethroid, deltamethrin and the synergist piperonyl butoxide (Central Life Science, Schaumberg, IL, US), compared to the existing commercial standard formulation without synergist, and controls at inducing direct mortality and sublethal changes in movement on treated grains as a grain protectant, and on concrete as a surface treatment. Mortality of adults was assessed visually, while movement was tracked with a network camera coupled with Ethovision software that automatically recorded velocity and distance moved by both species, after continuous exposure on treated material for 4–168 h. The novel formulation significantly induced mortality while reducing distance and velocity moved by multiple-fold compared to controls for exposed adults even after relatively brief exposure periods. In fact, the novel formulation was just as effective as the older formulation, but used only a fraction of the active ingredients, thus it may be more cost-effective. Overall, the novel insecticide formulation is a promising tool for controlling S. zeamais and P. truncatus in bulk storage and around other food facilities.
Introduction
Cereal grains comprise 30–60% of the daily caloric intake for humans around the globe. The top three most important cereal crops are maize, rice and wheat (Awika, 2011). Maize is a major staple crop and preferred in southern and eastern Africa, Central America, and Mexico (Awika, 2011; Ranum et al., 2014). It was reported that persons in Mexico consume 267 g of maize per day (Awika, 2011).
However, 10–30% of these important cereal crops, such as maize, are lost to insect pests post-harvest as these grains are harvested, stored, processed, and marketed to consumers (Pimentel, 1991). The major stored product pests of maize are the larger grain borer, Prostephanus truncatus (Horn) (Coleoptera: Bostrichidae) and the maize weevil, Sitophilus zeamais (Motschulsky) (Coleoptera: Curculionidae). Prostephanus truncatus is a long-time pest of stored maize in Mexico, the US, and parts of Central and South America (reviewed in Quellhorst et al., 2021), and was first described in 1878 (Hodges, 1986). However, the insect has also become an invasive species in Africa from 1981 onwards (Golob and Hodges, 1982), where it is estimated by Boxall (2002) to cause 34–40% in losses after only 3 months of storage.
The Southern US is at the very edge of the current range of P. truncatus, but the species represents an emerging biosecurity threat for maize production in the country (Arthur et al., 2019). In fact, Arthur et al. (2019) documented P. truncatus in three Mexican states directly adjacent to the US, including Nuevo Leon, Sonora, and Tamaulipas, with the closest site located <200 km (or a 3 h drive) from Arizona. While P. truncatus has been documented in maize facilities in southern states like Texas (e.g., records found in the research collection at Texas A&M; pers. comm. D. Ludwick), as well as California and Arizona (Cotton and Good, 1937; Dormond, 1998), it has not yet been detected in the breadbasket of the US, such as Kansas and adjacent areas (Quellhorst et al., 2021). The distributions of agricultural insects are predicted to shift under climate change (Macfadyen et al., 2018), and this is often in a polewards direction. As a result, under a changing climate, P. truncatus may be expected to become increasingly problematic at higher latitudes in the US. As it does so, it will encounter endemic S. zeamais with increasing frequency, and both may contribute to maize damage (Quellhorst et al., 2020).
Given the threat posed by P. truncatus and S. zeamais to the biosecurity of the US and thus the world's maize supply, it is critical that we expand the integrated pest management chemical toolbox to combat them. Traditionally, stored product insect control has focused on the use of fumigants to reduce consumer complaints, such as phosphine. However, the worker safety in applying fumigants is a concern, and consumers are increasingly demanding products with fewer insecticide inputs (Batte et al., 2007). This issue coupled with a stark rise in the global resistance to phosphine (Nayak et al., 2020) has made the search for additional alternative chemical tools an important focus.
Methoprene was first registered for use after harvest in the US under the name Diacon® in the 1980s (Arthur, 2004). The first commercial formulation of deltamethrin plus methoprene used in the US was Diacon IGR+ ® (Central Life Sciences, Schaumberg, IL, US). Diacon IGR+ contains 0.05 kg of active ingredient [AI]/L of deltamethrin and 0.12 kg [AI]/L of the insect growth regulator (IGR) methoprene. It can be applied at two commercially labeled rates, 0.5 ppm deltamethrin + 1.25 ppm methoprene, or 1.0 ppm deltamethrin + 2.5 ppm methoprene. For species such as P. truncatus and Rhyzopertha dominica (Fabricius) (Coleoptera, Bostrichidae), methoprene alone is enough to control these insect species because the eggs are laid outside the grain kernel by mated females (Athanassiou et al., 2010a,b). Thus, there is a brief time where the larva is exposed to the insecticide. However, for species like S. zeamais, the addition of deltamethrin is needed to control adults because this species lays eggs inside the grain kernels, thereby protecting them from methoprene exposures (Athanassiou et al., 2010a,b).
Gravista® (Central Life Sciences, Schaumberg, IL, US) is a new formulation of the Diacon IGR+ that consists of an IGR (2.85% methoprene, 0.027 kg [AI]/L), combined with the pyrethroid, 1.2% deltamethrin (0.012 kg [AI]/L) and a synergist, 33.3% piperonyl butoxide (0.32 kg [AI]/L). The main difference between the two insecticides (IGR + and Gravista) is the addition of a synergist. Synergists such as piperonyl butoxide (a cytochrome P450 detoxification enzyme inhibitor) allow for less active ingredient (deltamethrin and methoprene) to be incorporated in the product, as in this case, and may also help to control resistant insect populations.
While mortality is a common measurement for assessing the efficacy of an insecticide, it provides an incomplete picture of the full efficacy of a compound. In addition, Guedes et al. (2011) noted the importance of assessing the sublethal effects of exposure to insecticides in order to develop a comprehensive assessment of exposure to insecticides. This can be done using a variety of methodology, but one standard methodology is to use video-tracking coupled with Ethovision® software (Noldus et al., 2001, 2002; Morrison et al., 2018). Prior work has shown deltamethrin, when applied as an emulsifiable concentrate, can significantly decrease the walking ability of S. zeamais (Vélez et al., 2019), while no work to our knowledge has assessed sublethal effects of deltamethrin on P. truncatus. Further, little work has specifically been done with Diacon IGR+ or Gravista. Understanding the effects of sublethal toxicity on the behavior of insects may help to provide a better understanding of the efficacy of the insecticide in question and its ability to prevent movement and further damage after contact. Thus, our aims were to (i) characterize sublethal changes in movement over time after exposure to an existing formulation (IGR+®) and newer formulation (Gravista®) as a grain protectant on maize and residual spray on concrete surfaces, and (ii) quantify direct mortality from these compounds.
Materials and Methods
Insects
Adult P. truncatus and S. zeamais were obtained from insect colonies kept at the United States Department of Agriculture (USDA) Center for Grain and Animal Health Research facility in Manhattan, KS. Both insects were reared on whole kernels of maize and held in incubators under constant conditions at 27 ± 0.1°C (mean ± SE), 64 ± 1% RH, and 14:10 (L:D) h photoperiod for S. zeamais and at 27.2 ± 0.1°C, 37 ± 1% RH, and continuous darkness for P. truncatus as mandated by the applicable USDA-APHIS PPQ-approved permit (P526-191021-020).
Insecticide Formulations and Spray Rates on Concrete
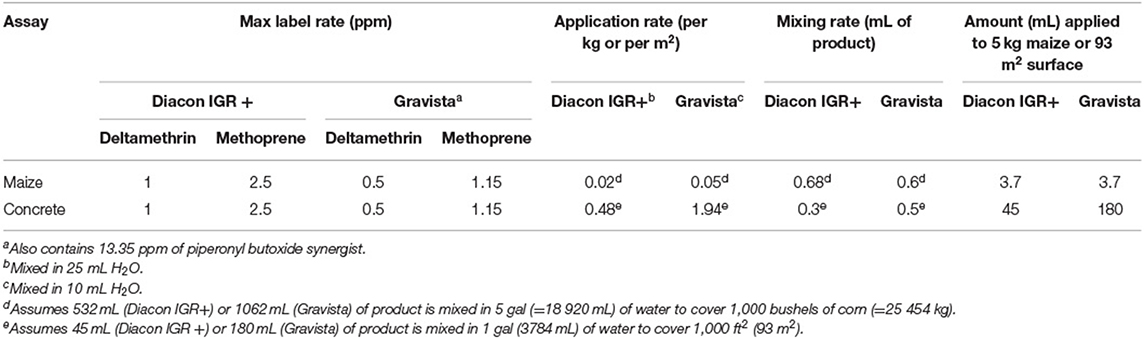
There were two insecticides in this study: an existing formulation (tradename: Diacon IGR+ ® Central Life Sciences, Schaumberg, IL, USA), and a new formulation with synergist and less concentrated active ingredients (tradename: Gravista®). Diacon IGR+ contains 11.4% methoprene and 4.75% deltamethrin, labeled for application at 0.12 kg AI/L and 0.05 kg AI/L, respectively. Label directions for application as a residual surface treatment give a range of 28.5 mL AI/L−171 mL AI/L H2O to cover 94 m2 for both compounds. To obtain the maximum possible effect, we employed the maximum labeled rate in this study (Table 1), namely 24 mg AI/m2 for deltamethrin and 57 mg AI/m2 for methoprene. This corresponded to 0.3 ml of the formulation in 25 ml H2O, sprayed at the rate of 0.3 ml per 50.3 cm2 arena, using separate artist's air brush (Badger 100 series, Badger Corporation, Franklin Park, IL, US) for each treatment. Each replicate was evenly applied to the concrete dish. The new Gravista formulation has one labeled rate, namely 684 ml formulation/L H2O to cover 92.9 m2. Scaling down, we mixed 0.5 ml of the new formulation in 10 ml H2O. This was sprayed at the same rate as the other compound. Distilled water was used for the control arenas at 0.3 mL per arena. The arenas were allowed to dry overnight (~8 h) prior to use in experiments.
Direct Mortality on Concrete
Concrete arenas were prepared for testing by first mixing a slurry of water and Rockite cement in a large pitcher (Rockite, Cleveland, OH, US; as published prior in Arthur, 2008). Briefly, water was added until a thick paste consistency was achieved. The mixture then sat for 1 min before it was stirred vigorously, making sure any remaining clumps of dry Rockite were hydrated. Once the mixture was a loose, batter-like consistency, 8.5 ml of the slurry was poured into plastic 100 × 20 mm Petri dishes (area of the bottom: 62 cm2; hereafter, arenas). The slurry was poured to approximately 1.27 cm thick. The cement dishes were then left at room temperature to cure for 24 h. The three treatments were sprayed onto the concrete arenas included: control (distilled water), an existing insecticide (Diacon IGR+: 11.40% S-methoprene + 4.75% deltamethrin), and a novel formulation (Gravista: 2.85% S-methoprene + 1.20% deltamethrin + Synergist, 33.30% piperonyl butoxide). There were 6 exposure times: 4, 8, 24, 48, 72, 168 h. A total of 270 individuals per species (P. truncatus and S. zeamais) were collected, placed in cohorts of 15 individuals per concrete-filled Petri dish. There were n = 3 replicate arenas per treatment combination.
After exposure, individual P. truncatus and S. zeamais were removed and placed into clean Petri dish arenas and evaluated for condition. Using a stereomicroscope (SMZ-18, Nikon Inc., Tokyo, Japan) under 60× magnification, P. truncatus and S. zeamais were classified as alive (moving normally, is able to right itself when flipped over, no twitching), affected (moving sluggishly or erratically, unable to right itself, twitching of antennae or legs may be present), or dead (completely immobile even after prodding) according to prior published definitions (Morrison et al., 2018).
Spray Rates on Treated Maize
Untreated, clean, and uninfested yellow maize was used in the experiments. Grain was sourced from a local farmer in northeast Kansas, US. The highest labeled rate for Diacon IGR+ on maize is 1 ppm deltamethrin and 2.5 ppm of methoprene, which is what we used in this study by adding 0.70 ml of the insecticide to 25 ml of H2O. After mixing, 0.5 mL was applied to 700 g lots of maize, and this was done three separate times, one for each replicate. For Gravista, the labeled rate on maize is 0.5 ppm deltamethrin and 1.15 ppm of methoprene, which is mixed by adding 0.56 mL of insecticide to 10 mL of H2O. After mixing, 0.5 mL was applied to 700 g lots of maize, using separate artist's air brush (Badger 100 series, Badger Corporation, Franklin Park, IL, US; designed to spray a line 3.2–50.8 mm wide with materials of high viscosity) at a pressure of 0.70 kg/cm2 for each treatment, and this was repeated three separate times, one for each replicate.
Insect Mortality on Treated Maize
To assess direct mortality on treated maize for application in bulk storage, we followed the same protocol as above for the concrete arenas with a few modifications. Specifically, the experiment was conducted with three bioassay replicates (n = 3); each with 18 combinations of three treatments (IGR+, Gravista, and H2O Control) and six exposure times (4, 8, 24, 48, 72, 168 h) of each treatment. For each bioassay replicate, 700 g of maize were treated with each insecticide or H2O (e.g., control) as described above. Each of 18 vials (120-mL volume) was filled with 100 g of treated maize and 30 adult insects of either species, and the insects were maintained at 27 ± 0.02°C. Only 15 of the 30 insects in each vial were randomly selected and evaluated for direct toxicity at each of six exposure times. Extra insects were provided in these experiments to account for a subset of individuals that may bore completely into the kernel (e.g., especially P. truncatus), which would prevent them from being classified and counted. To recover the insects, the grain was sieved (No. 10, 2.0 x 2.0 mm mesh, W.S Tyler, Mentor, OH, US), and shaken to maximize the number of insects falling into the collection pan.
Insect Mobility on Concrete Arenas and in Treated Maize
To assess insecticide effect on insect mobility in both of the bioassays above, a random subset individuals classified as either alive or affected were tracked with a network camera (Basler AG, Ahrensburg, Germany) coupled with Ethovision XT (v. 14.0, Noldus Software, Leesburg, VA, US). Individuals of each species were placed individually into arenas consisting of 90 mm Petri dishes lined with 85 mm Whatman #1 filter (GE Healthcare, Chicago, IL, USA). The camera was placed 80 cm above the arenas, and trials lasted 30 min. At the end of the trial, the total distance moved (cm) and the average instantaneous velocity (i.e., speed with a direction, cm/s) was recorded. To account for cursor bounce, an input filter was applied that discarded the accumulated distance if it was >5 cm per s. Each trial was manually checked for irregularities, and any with suspicious accumulations of distance were re-run. There were a total of n = 15 replicate individuals per treatment combination, totaling to 234 h of video for 468 individuals per species and assay.
Statistical Analysis
A generalized linear model (GLM) based on a quasibinomial to account for overdispersion in the dataset (Aho, 2017) was used to assess differences in percentage of mortality, with a separate model used for each species and assay. Fixed, explanatory variables included insecticide (Diacon IGR+, Gravista, and control), exposure time (0, 4, 8, 24, 48, 72, 168 h), and their interaction. Likelihood ratio tests were conducted to calculate the χ2-value and determine significance of each variable in a model with (alternative hypothesis) or without the variable (null hypothesis) in question. Upon a significant result from the GLM, Tukey HSD was used for multiple comparisons and implemented with the glht function from the multcomp package (Hothorn et al., 2008). Unless otherwise specified, α = 0.05, and R Statistical Software was used (R Core Team, 2019).
To analyze the data from the insecticide effect on insect mobility, a special case of a GLM was used based on a Gaussian distribution (e.g., general linear model) with distance moved and velocity as the response variables to account for the fact that each response was continuous, unbounded, and normally distributed (Bolker et al., 2009). A single model was created for each assay and species. The model used insecticide, exposure time, and their interaction as fixed, explanatory variables. Upon a significant result from the general linear model, Tukey HSD was used for multiple comparisons.
Results
Mortality on Concrete
On concrete, the insecticide had a significant overall effect on mortality (percent of individuals affected and dead) compared to the control for both S. zeamais and P. truncatus (Table 2, Figure 1). However, mortality was not significantly different for the two insecticide formulations, IGR+ and Gravista. The exposure time also significantly affected the condition of adults of both S. zeamais and P. truncatus, with more adults dead at 72 and 168 h than at other periods (Figure 2). Prostephanus truncatus was slightly more susceptible to both insecticide formulations with no “alive” individuals (all affected or dead) compared to some S. zeamais individuals remaining alive. Mortality reached 33–37% for insecticide-exposed S. zeamais, while it was 40–47% for insecticide-exposed P. truncatus by 168 h. Nonetheless, the majority of individuals were simply still affected. There was no significant interaction between exposure time and insecticide among the health conditions and species for the concrete assay, except for the percent of affected adult S. zeamais, where there were initially more alive adults after treatment with Gravista (Figure 2).
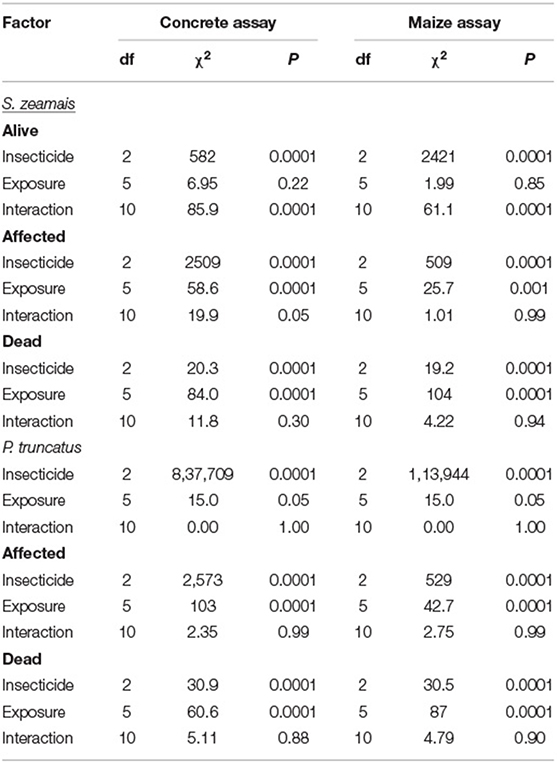
Table 2. Summary of statistical GLM model results for mortality of S. zeamais and P. truncatus in the concrete assay and maize assay.
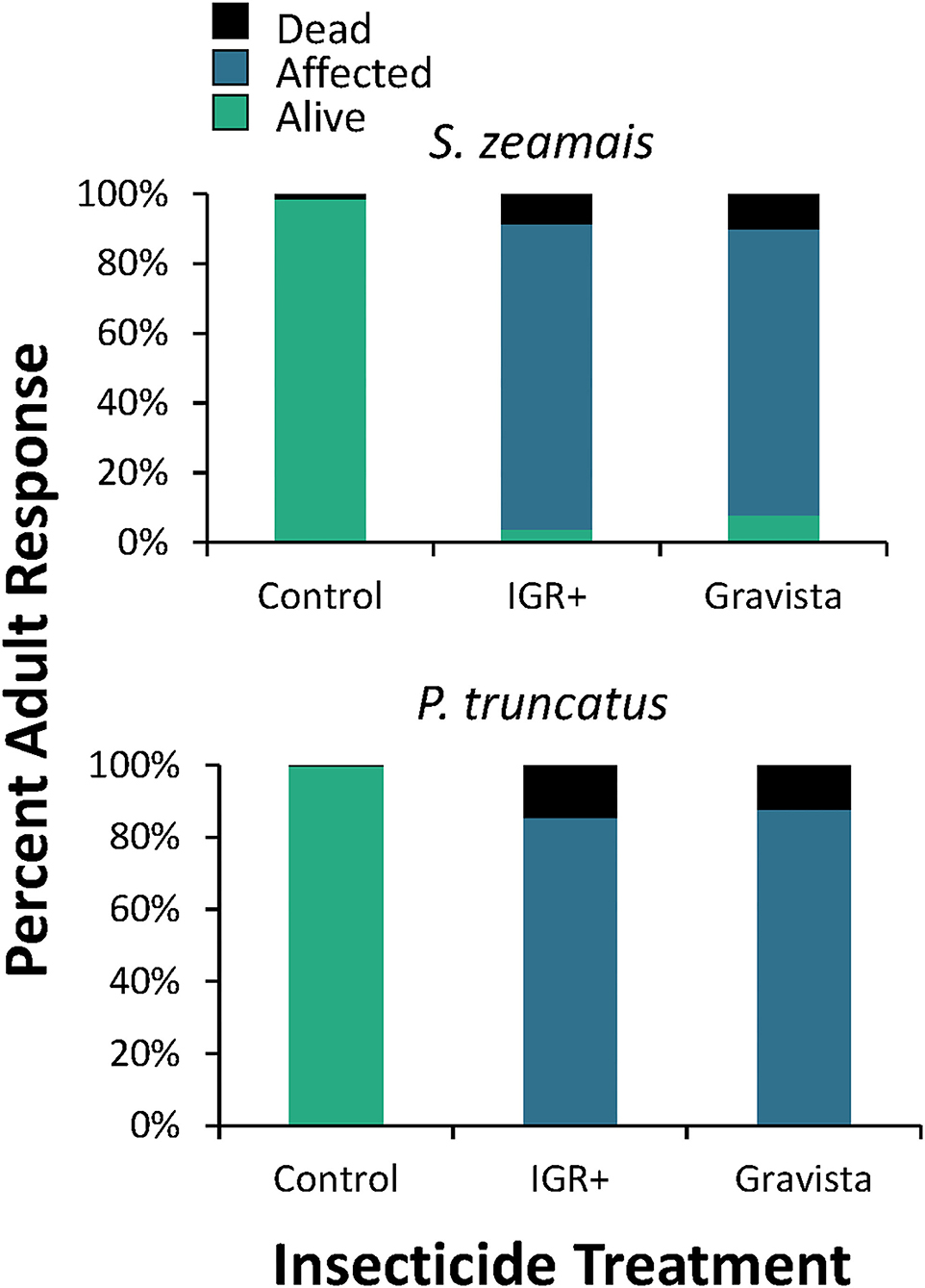
Figure 1. Overall percent mortality induced by an existing (methoprene + deltamethrin, IGR+) and novel (methoprene + deltamethrin + synergist, Gravista) commercial formulation of insecticide when applied against S. zeamais (top panel) and P. truncatus (bottom panel) on concrete arenas after exposure for 4–168 h, resulting in alive (green), affected (blue), or dead (black) individuals.
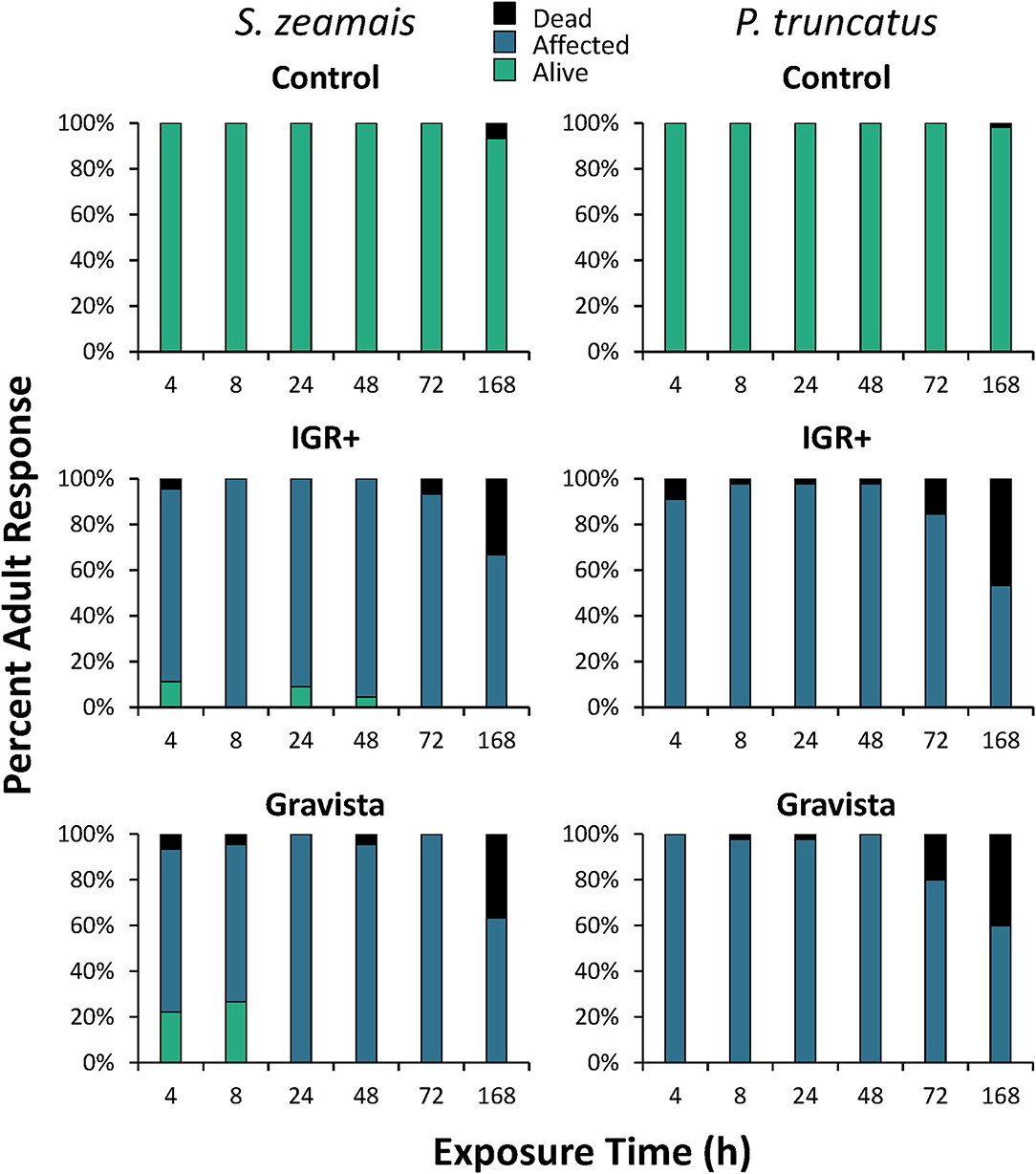
Figure 2. Percent mortality induced by an existing (methoprene + deltamethrin, IGR+) and novel (methoprene + deltamethrin + synergist, Gravista) commercial formulation of insecticide when applied against S. zeamais (left column) and P. truncatus (right column) on concrete arenas broken down by exposure time, resulting in alive (green), affected (blue), or dead (black) individuals.
Mobility Effects on Concrete
On concrete, the distance moved by S. zeamais was significantly affected by exposure to both insecticide formulations (IGR+ and Gravista; Table 3). IGR+-exposed S. zeamais showed a nearly 5-fold decrease in movement compared to the control, while Gravista-exposed S. zeamais exhibited a nearly 7-fold decrease (Figure 3). Exposure time significantly affected the distance moved by S. zeamais, with adults moving 1.8-fold less 168 h after exposure compared to 24 h after exposure. The interaction between insecticide and exposure time was not significant for S. zeamais.
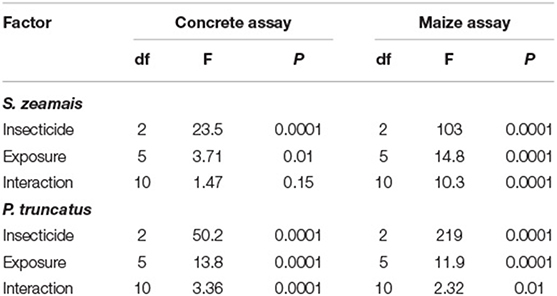
Table 3. Summary of statistical model results for GLM of distance moved by S. zeamais and P. truncatus in the after exposure in the concrete assay and maize assay.
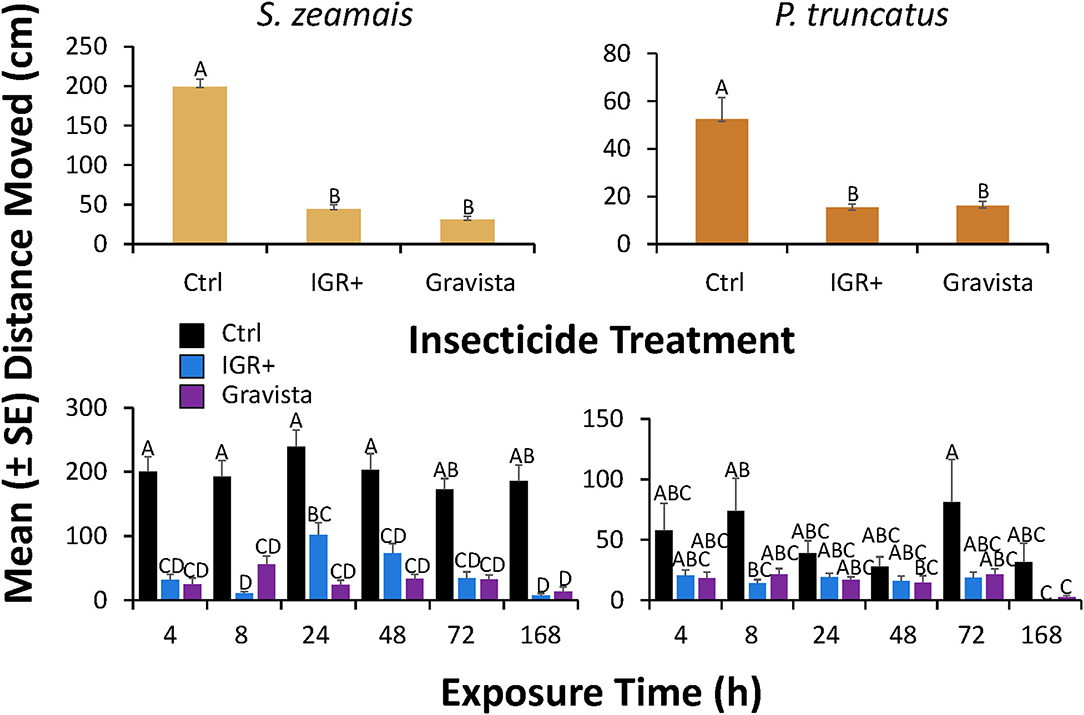
Figure 3. Distance moved (± SE) by S. zeamais (left column) and P. truncatus (right column) after application of an existing (methoprene + deltamethrin, IGR+) and novel (methoprene + deltamethrin + synergist, Gravista) commercial formulation of insecticide to concrete compared to a control overall (top panels) or broken down by exposure time (bottom panels). Bars with shared letters are not significantly different from each other (Tukey HSD, α = 0.05).
Moreover, on concrete, the distance moved by P. truncatus was also significantly affected by exposure to both insecticide formulations (Table 3). IGR+-exposed P. truncatus saw a nearly 4-fold decrease in distance moved compared to the control. Gravista-exposed P. truncatus saw a 3-fold decrease in movement compared to the control (Figure 3). Sitophilus zeamais moved an average of 200 cm compared to 53 cm by P. truncatus. Thus, a greater decrease in movement can be seen for both insecticide formulations for S. zeamais. Exposure time significantly affected the distance moved by P. truncatus, with adults moving 3.6-fold farther 72 h after exposure than 168 h after exposure. The interaction between insecticide and exposure time was significant (Table 3), with less movement in control individuals at 24 and 48 h compared to 4, 8, and 72 h (Figure 3).
On concrete, the velocity by S. zeamais was significantly affected by exposure to both insecticide formulations (IGR+ and Gravista) compared to controls (Table 4). IGR+-exposed S. zeamais exhibited a nearly 5-fold decrease in velocity compared to the control, while Gravista-exposed S. zeamais showed a nearly 7-fold decrease (Figure 4). Furthermore, exposure time significantly altered the velocity by S. zeamais, with adults moving 1.8-fold slower 168 h after exposure compared to 72 h afterwards. The interaction between insecticide and exposure time had a significant effect on the velocity (Table 4), with S. zeamais moving 1.4–14-fold faster 24 h after exposure to IGR+ compared to 4 and 168 h (Figure 4).
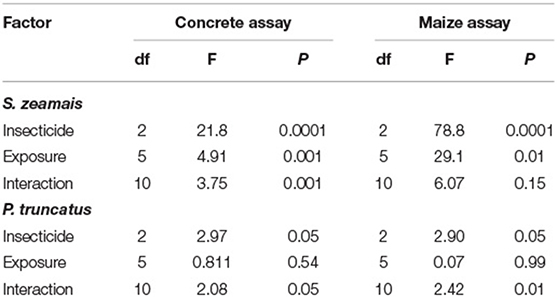
Table 4. Summary of statistical model results for GLM of velocity by S. zeamais and P. truncatus in the after exposure in the concrete assay and maize assay.
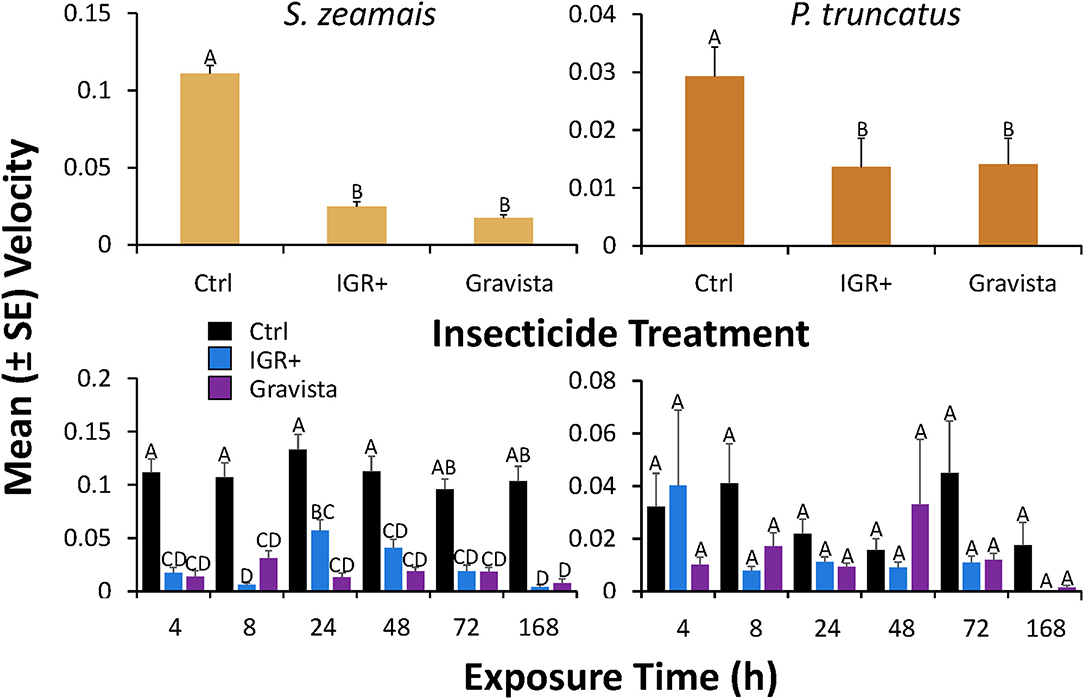
Figure 4. Velocity (± SE) by S. zeamais (left column) and P. truncatus (right column) after application of an existing (methoprene + deltamethrin, IGR+) and novel (methoprene + deltamethrin + synergist, Gravista) commercial formulation of insecticide to concrete compared to a control overall (top panels) or broken down by exposure time (bottom panels). Bars with shared letters are not significantly different from each other (Tukey HSD, α = 0.05).
Similarly, the velocity by P. truncatus was significantly affected by exposure to IGR+ and Gravista compared to a control (Table 4). IGR+- and Gravista-exposed P. truncatus showed a 2-fold decrease in velocity compared to the control. Again, it should be noted that, S. zeamais is a much more mobile species compared to P. truncatus, with control S. zeamais velocity on average 0.11 cm/s compared to 0.03 cm/s by P. truncatus. Thus, the greater decrease in velocity can be seen for both insecticide formulations for S. zeamais. By contrast, exposure time did not significantly affect the distance moved by P. truncatus. There was no significant interaction between the insecticide and exposure time on velocity.
Mortality on Maize
The overall mortality on maize was significantly different after exposure to both IGR+ and Gravista compared to the control for both S. zeamais and P. truncatus (Table 2, Figure 5). However, mortality (percent dead and percent affected) was not significantly different for the two insecticide formulations, IGR+ and Gravista. Exposure time significantly affected mortality by both species also (Figure 6), but there was no significant interaction between insecticide and exposure time on the percent of affected and percent of dead adults for either species (Table 2). As exposure time increased, so too did the number of dead adults, which reached 47–67% and 42–53% for insecticide-exposed S. zeamais and P. truncatus by 168 h, respectively. Prostephanus truncatus was slightly more susceptible to both insecticide formulations with no “alive” individuals (all affected or dead) compared to the S. zeamais. Sitophilus zeamais was more susceptible on maize compared to exposure on concrete with <1% left “alive” compared to nearly 8% “alive” on concrete.
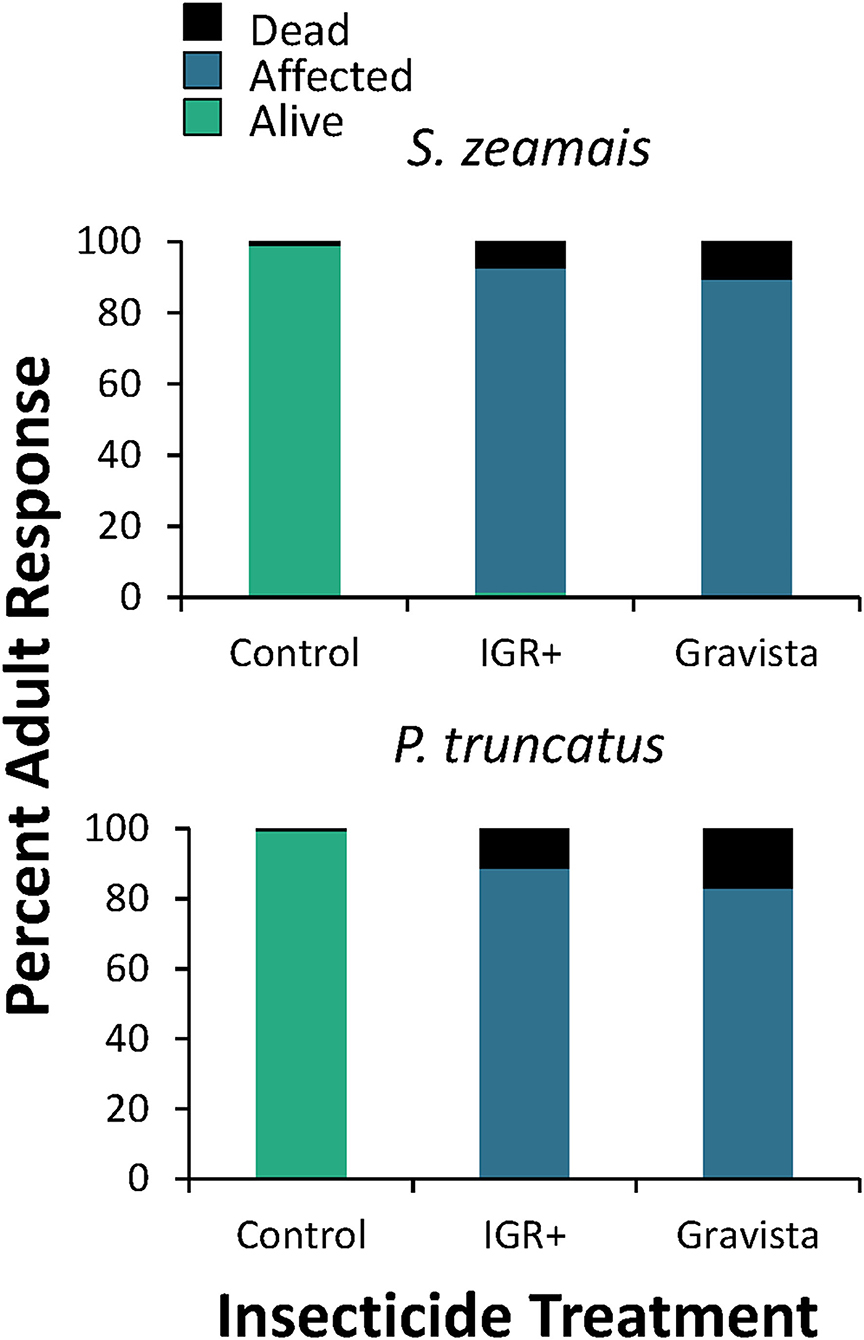
Figure 5. Overall percent mortality induced by an existing (methoprene + deltamethrin, IGR+) and novel (methoprene + deltamethrin + synergist, Gravista) commercial formulation of insecticide when applied against S. zeamais (top panel) and P. truncatus (bottom panel) as a grain protectant on maize after exposure for 4–168 h, resulting in alive (green), affected (blue), or dead (black) individuals.
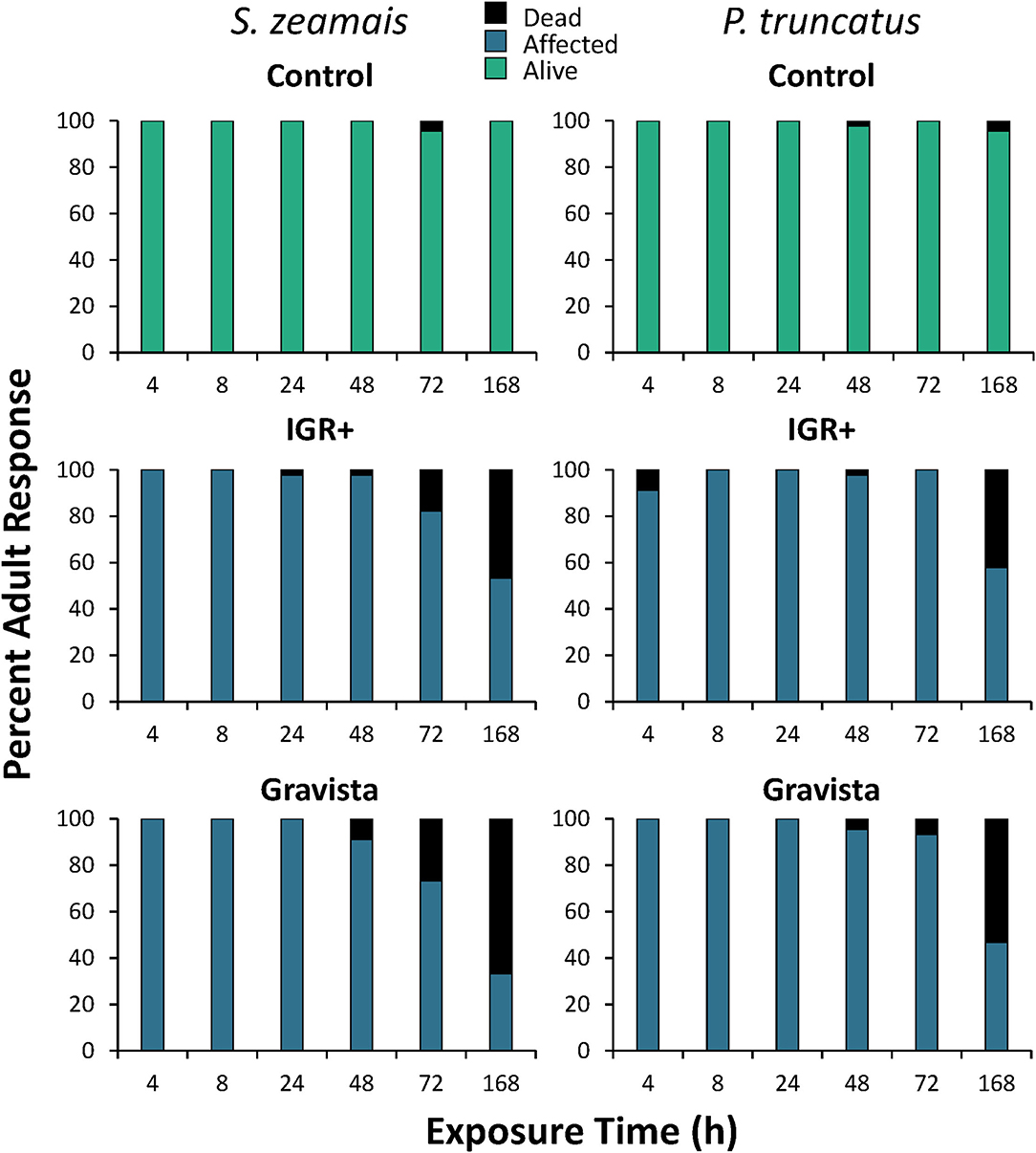
Figure 6. Percent mortality induced by an existing (methoprene + deltamethrin, IGR+) and novel (methoprene + deltamethrin + synergist, Gravista) commercial formulation of insecticide when applied against S. zeamais (left column) and P. truncatus (right column) on maize broken down by exposure time, resulting in alive (green), affected (blue), or dead (black) individuals.
Mobility Effects on Maize
On maize, the distance moved by S. zeamais was significantly affected by exposure to IGR+ and Gravista compared to the control (Table 3). IGR+-exposed and Gravista-exposed S. zeamais showed a 26-fold and 148-fold decrease, respectively, in distance moved compared to the control (Figure 7). Exposure time significantly affected the distance moved by S. zeamais, with adults 4 h after exposure moving 2-fold more than those 24 h afterwards. There was a significant interaction between insecticide and exposure time on the distance moved by S. zeamais, with control adults moving the least 24 h after exposure to the insecticide treatments and the most 4 and 48 h afterwards (Figure 7).
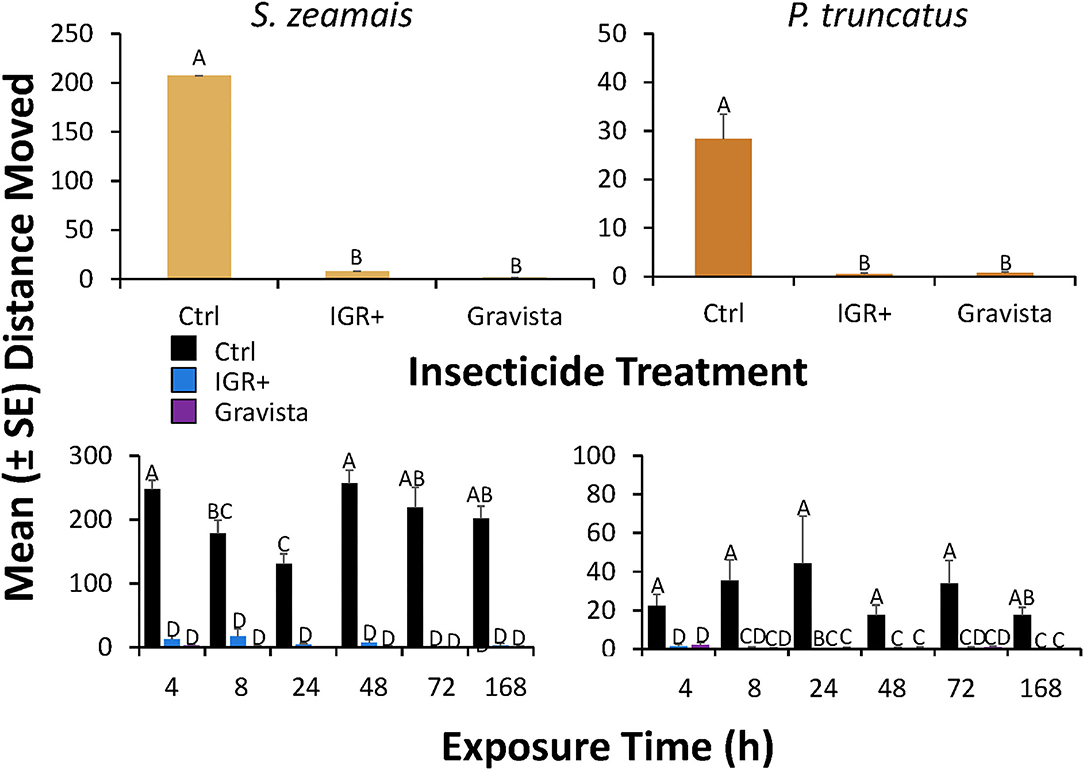
Figure 7. Distance moved (± SE) by S. zeamais (left column) and P. truncatus (right column) after application of an existing (methoprene + deltamethrin, IGR+) and novel (methoprene + deltamethrin + synergist, Gravista) commercial formulation of insecticide to maize as a grain protectant compared to a control overall (top panels) or broken down by exposure time (bottom panels). Bars with shared letters are not significantly different from each other (Tukey HSD, α = 0.05).
In addition, on maize, the distance moved by P. truncatus was significantly affected by exposure to both insecticide formulations (Table 4). IGR+-exposed P. truncatus saw a 49-fold decrease in movement compared to the control, while Gravista-exposed S. zeamais exhibited a 39-fold decrease in movement compared to the control. Exposure time did not significantly affected the distance moved by P. truncatus, with adults moving 2.5-fold more after 24 h exposure compared to 168 h afterwards. There was a significant interaction between insecticide and exposure time on distance moved by P. truncatus, with movement less at later exposure times for the control adults (Figure 7). It should be noted that both S. zeamais and P. truncatus were more susceptible to both insecticide formulations when exposed on maize vs. concrete, as both species saw nearly zero movement after exposure compared to the controls. When exposed on concrete, S. zeamais moved on average as much as 44 cm after exposure and P. truncatus 16 cm after exposure.
Similarly, on maize, the velocity by S. zeamais was significantly affected by exposure to both insecticide formulations (Table 4). IGR+-exposed and Gravista-exposed S. zeamais saw a 30-fold and 150-fold decrease, respectively, in velocity compared to the control (Figure 8). Exposure time significantly affected the velocity by S. zeamais, with adults moving 1.8-fold more 48 h after exposure compared to 24 h afterwards. The interaction between insecticide and exposure time did not have a significant effect on velocity moved by S. zeamais.
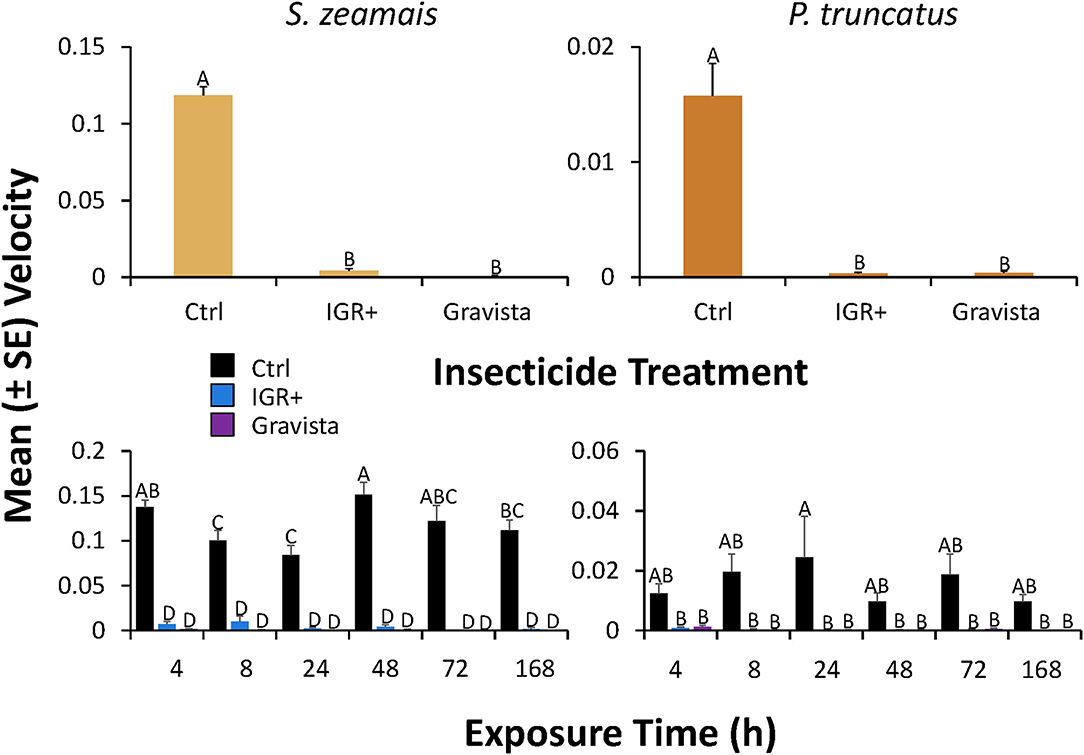
Figure 8. Velocity (± SE) by S. zeamais (left column) and P. truncatus (right column) after application of an existing (methoprene + deltamethrin, IGR+) and novel (methoprene + deltamethrin + synergist, Gravista) commercial formulation of insecticide to maize as a grain protectant compared to a control overall (top panels) or broken down by exposure time (bottom panels). Bars with shared letters are not significantly different from each other (Tukey HSD, α = 0.05).
Finally, the velocity by P. truncatus on maize was significantly affected by exposure to both insecticide formulations (IGR+ and Gravista) (Table 4). IGR+-exposed P. truncatus showed a nearly 67-fold decrease in movement compared to the control, while Gravista-exposed P. truncatus exhibited a 50-fold decrease (Figure 8). Exposure time did not significantly affect the distance moved by P. truncatus. Baseline mobility was greater for Sitophilus zeamais, with S. zeamais control individuals moving an average of 0.12 cm/s compared to 0.02 cm/s by P. truncatus.
Discussion
We examined both mortality and sublethal effects by the novel insecticide (Gravista) compared to IGR+ for two stored products pests. Up to this point, no one has evaluated the baseline mobility or sublethal effects of insecticides on the invasive P. truncatus. Importantly, we found that both compounds significantly reduced the movement of P. truncatus and S. zeamais compared to the controls, even after brief 4-h exposures. This is the first reported experiment using Ethovision coupled with video-tracking to capture sublethal reduction in movement (e.g., distance moved and velocity) related to an insecticide for P. truncatus. Baseline movement of P. truncatus decreased from 28–52 cm in 30 min in the controls to 0.56–16 cm, on average, while S. zeamais distance moved decreased from 199–207 cm to 7–44 cm in the same period. The reduction in movement by S. zeamais (5–27-fold) is in alignment with prior results on sublethal exposure of the species to deltamethrin (Morrison et al., 2021). Prostephanus truncatus movement was reduced by 3–50-fold compared to control individuals. The use of IGR+ and Gravista has the potential to manage both P. truncatus and S. zeamais and prevent their further movement. As Guedes et al., 2011 pointed out, understanding the sublethal effects of insecticide exposure allows for a more comprehensive picture of efficacy compared to solely focusing on direct lethality. Our study adds to a growing body of literature with different species of stored products insects that document sublethal exposure to insecticides (Morrison et al., 2018, 2019, 2021; Arthur et al., 2020; Wilkins et al., 2020).
Both IGR+ and Gravista provided effective control of both P. truncatus and S. zeamais, but the latter does it with 4-fold less active ingredient because of the addition of a synergist. Both insecticides caused direct mortality in the two pests, though markedly greater in P. truncatus compared to S. zeamais. It was clear P. truncatus was the more susceptible species, with a mortality of 40–47% by 168 h on concrete, whereas S. zeamais mortality reached 33–37%. Interestingly, mortality was higher when insects were exposed on grain instead of concrete. Mortality reached 47–67% and 42–53% for insecticide-exposed S. zeamais and P. truncatus by 168 h on maize. By contrast, immediately after treatment with Gravista in a residual study, S. oryzae mortality was 91% after 168 h (Arthur and Morrison, 2020). The differences in the mortality for the two weevils could be the result of the recent range expansion of S. zeamais (Corrêa et al., 2017), and its greater resistance to insecticides more generally (Ribeiro et al., 2003). Regardless, both IGR+ and Gravista can be considered effective at inducing mortality in both species even over the brief exposure times evaluated in this study.
In our study, we evaluated the relative efficacy of IGR+ and Gravista as a residual treatment on concrete or as a grain protectant on maize. We found efficacy was greater when insects were placed on maize than on concrete. There have been many studies that examine how the efficacy of insecticides varies on different substrates. These studies often compared concrete to varnished wood, metal, tile or laminated paper (Williams et al., 1983; White and Leesch, 1996; Arthur, 1997, 2008; Hagstrum and Subramanyam, 2006; Jenson et al., 2009; Wijayaratne et al., 2012). Generally, insecticides are found to have the greatest efficacy on non-porous substrates such as metal or tile, followed by porous substrates like concrete or wood. In addition, residual efficacy of insecticides was found to be greatest when applied on metal surfaces (Jankov et al., 2013). Concrete generally absorbs much of the formulation applied to it. In a few cases, depending on the insecticide and the insect species, efficacy was actually greater on concrete compared to other substrates. For example, when insecticidal pyrrole chlorfenapyr was applied to concrete, vinyl tile, and plywood surfaces, control of two Tribolium species was greater on concrete compared to vinyl tile and plywood (Arthur, 2008). As Chadwick (1985) noted, substrate type and its porosity may impact the absorption of the insecticide into the substrate and subsequent degradation of the insecticide. It would appear that at least in this study, IGR+ and Gravista were more effective on maize kernels that had been sprayed with insecticide compared with concrete, which had likely absorbed more of the insecticide below the surface, sequestering it from contact with the insects. It is possible that only the legs come into contact with the residual insecticides when insects move on the treated but flat concrete surface whereas the rest of the body can also come into contact with the residual insecticides when insects move around the treated maize kernels. However, both IGR+ and Gravista seem useful both as a residual surface treatment and as a grain protectant despite their varying efficacy in those forms. Both residual treatments around a food facilities and grain protectants for bulk storage situations have an role to play in comprehensive integrated pest management programs (Arthur and Subramanyam, 2012), with both of the compounds assessed in this study showing promise for incorporation.
Another aspect that may affect efficacy is the development of resistance to deltamethrin by one or both species. The use of deltamethrin in the US is relatively new, and to date, there has been no documented instance of resistance. However, there has been documented deltamethrin resistance in a variety of other countries, including Australia (Daglish and Nayak, 2018) and Brazil (Guedes et al., 2009). Incomplete dosing is known to induce resistance, but this is not likely to occur with the lower amounts of active ingredients in Gravista, because there is a much higher amount of synergist, which often acts to break resistance to pyrethroids in a variety of systems (Forrester et al., 1993).
Currently, S. zeamais is one of the most important pests of stored maize grains in the US and elsewhere. Despite the significance of the S. zeamais as a pest of maize grains globally, there is relatively little information compiled about the life history and biology of the insect pest. This could be due to the fact that S. zeamais is nearly indistinguishable from S. oryzae and was often referred to as “Sitophilus species” in the literature. Sitophilus zeamais is less selective when it comes to the commodities it attacks, feeding on commodities as diverse as apples, rice, wheat, and maize (Longstaff, 1981). The damage caused on maize by S. zeamais has been estimated by Boxall (2002) to be 10–20% after only 3 months in storage.
While S. zeamais is already present in the US, P. truncatus may invade under shifting distributions from climate change. Both are important and pernicious pests of maize (Quellhorst et al., 2021). Thus, the use of IGR+ and Gravista as crack-and-crevice treatments in or around food facilities in the US where grains are typically kept and grain protectant treatments of kernels stored for extended periods in bulk storage may be especially beneficial. Most food facilities lose some maize in both quantity and quality over the time if stored for extended periods. Both compounds appear like feasible options. Gravista, a formulation with much less active ingredient, may be an option to delay resistance development or overcome existing resistance problems for food facilities, because of the synergist.
While we have assessed both direct and sublethal effects of two compounds on two economically important species in stored products using two application methods, in the future it will be important to (1) determine whether exposure to these compounds affects dispersal to novel food patches, (2) evaluate changes in flight capacity after exposure to insecticides (e.g., Morrison et al., 2017), and (3) elucidate molecular mechanisms of stress after exposure to these compounds. While prior work has documented that S. zeamais and P. truncatus prefer different ends of the temperature spectrum (Quellhorst et al., 2020), it is unknown how temperature mediates response especially to the new formulation with synergist (e.g., Gravista). Follow-up studies on these topics will help more fully characterize important new compounds coming onto the market for stored product protection, while providing information on potential new chemical tools for stakeholders.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author Contributions
HQ, FA, and WM conceived of the experiments and methodology. AB, FA, and HQ set up the experiments and collected the data. HQ and WM wrote a first draft of the MS and jointly performed the statistical analysis. FA, HQ, WM, and KZ obtained funding for the research. FA, KZ, and WM provided supervision and facilities. All authors reviewed and edited drafts of the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This work was funded, in part, by a USDA NIFA CPPM Grants #2017-70006-27262, #2020-70006-33000, and a USDA NIFA Pre-doctoral Fellowship 2021-67011-35126.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We would like to thank the excellent technical assistance of Alex Bruce, Chloe Albin, and Robert Grosdidier (USDA-ARS) and the rest of the Morrison Lab. The use of trade names is for the purposes of providing scientific information only, and does not constitute endorsement by the Kansas State University or the United States Department of Agriculture. The Kansas State University and USDA are equal opportunity employers. This manuscript is contribution No. 22-212-J from the Kansas Agricultural Experiment Station, Kansas State University, Manhattan, Kansas.
References
Aho, K. A. (2017). Foundational and Applied Statistics for Biologists Using R. CRC Press: Boca Raton. p. 618. doi: 10.1201/b16126
Arthur, F. H. (1997). Differential effectiveness of deltamethrin dust on plywood, concrete, and tile surfaces against three stored-product beetles. J. Stored Prod. Res. 33, 167–173. doi: 10.1016/S0022-474X(96)00041-0
Arthur, F. H. (2004). Evaluation of methoprene alone and in combination with diatomaceous earth to control Rhyzopertha dominica (Coleoptera: Bostrichidae) on stored wheat. J. Stored Prod. Res. 40, 485–498. doi: 10.1016/S0022-474X(03)00060-2
Arthur, F. H. (2008). Efficacy of chlorfenapyr against Tribolium castaneum and Tribolium confusum (Coleoptera: Tenebrionidae) adults exposed on concrete, vinyl tile, and plywood surfaces. J. Stored Prod. Res. 44, 145–151. doi: 10.1016/j.jspr.2007.08.005
Arthur, F. H., Athanassiou, C. G., and Morrison, III W. R. (2020). Mobility of stored product beetles after exposure to a combination insecticide containing deltamethrin, methoprene, and a piperonyl butoxide synergist depends on species, concentration, and exposure time. Insects. 11, 151. doi: 10.3390/insects11030151
Arthur, F. H., Morrison, W.R. III, and Morey, A. C. (2019). Modeling the potential range expansion of larger grain borer, Prostephanus truncatus (Coleoptera: Bostrichidae). Sci. Rep. 9:6862. doi: 10.1038/s41598-019-42974-5
Arthur, F. H., and Morrison, III W. R. (2020). Methodology for assessing progeny production and grain damage on commodities treated with insecticides. Agron. 10, 804. doi: 10.3390/agronomy10060804
Arthur, F. H., and Subramanyam, B. (2012). “Chemical control in stored products”, in Stored Prod Protection, Hagstrum, D. W., Phillips, T. W., and Cuperus, G. W. (eds.). Manhattan, KS: Kansas State University. p. 95–100.
Athanassiou, C. G., Arthur, F. H., and Throne, J. E. (2010a). Efficacy of layer treatment with methoprene for control of Rhyzopertha dominica (Coleoptera: Bostrychidae) on wheat, rice and maize. Pest Manag. Sci. 67, 380–384. doi: 10.1002/ps.2064
Athanassiou, C. G., Arthur, F. H., and Throne, J. E. (2010b). Efficacy of methoprene for control of five species of psocids (Psocoptera) on wheat, rice, and maize. J. Food Prot. 73, 2244–2249. doi: 10.4315/0362-028X-73.12.2244
Awika, J. M. (2011). Major cereal grains production and use around the world. In Advances in cereal science: implications to food processing and health promotion. Amer Chem Soc. 1–13. doi: 10.1021/bk-2011-1089.ch001
Batte, M. T., Hooker, N. H., Haab, T. C., and Beaverson, J. (2007). Putting their money where their mouths are: Consumer willingness to pay for multi-ingredient, processed organic food products. Food Policy. 32, 145–159. doi: 10.1016/j.foodpol.2006.05.003
Bolker, B. M., Brooks, M. E., Clark, C. J., Geange, S. W., Poulsen, J. R, Stevens, M. H. H., et al. (2009). Generalized linear mixed models: a practical guide for ecology and evolution. Trends in Ecol Evol. 24, 127–135. doi: 10.1016/j.tree.2008.10.008
Boxall, R. A. (2002). Damage and loss caused by the larger grain borer Prostephanus truncatus. Integrated Pest Manag. Rev. 7, 105–121. 1026397115946. doi: 10.1023/A:1026397115946
Chadwick, P. R. (1985). Surfaces and other factors modifying the effectiveness of pyrethroids against insects in public health. Pestic. Sci. 16, 383–391. doi: 10.1002/ps.2780160413
Corrêa, A. S., Vinson, C. C., Braga, L. S., Guedes, R. N. C., and De Oliveira, L. O. (2017). Ancient origin and recent range expansion of the maize weevil Sitophilus zeamais, and its genealogical relationship to the rice weevil S. oryzae. Bulletin of Entomol Res. 107, 9–20. doi: 10.1017/S0007485316000687
Cotton, R. T., and Good, N. E. (1937). Annotated List of the Insects and Mites Associated With Stored Grain and Cereal Products, and of Their Arthropod Parasites and Predators. Washington, DC: U.S. Department of Agriculture; Government Printing Office. Misc. p. 81.
Daglish, G. J., and Nayak, M. K. (2018). Prevalence of resistance to deltamethrin in Rhyzopertha dominica (F.) in eastern Australia. J. Stor. Prod. Res. 78, 45–49. doi: 10.1016/j.jspr.2018.06.003
Dormond, M. (1998). Intraspecific variability and biogeographical patterns in the larger grain borer, Prostephanus truncatus, (Coleoptera: Bostrichidae). Berkeley, California: Doctorate, Department of Entomology, University of Berkeley.
Forrester, N. W., Cahill, M., Bird, L. J., and Layland, J. K. (1993). Section 10. Pyrethroid resistance: resistance breaking pyrethroids. Bull. Entomol. Res. Suppl. Series. 1, 83–96. doi: 10.1017/S1367426900000163
Golob, P., and Hodges, R. (1982). Study of an outbreak of Prostephanus truncatus (Horn) in Tanzania. Slough: Tropical Produ Institute. p. G164.
Guedes, N. M. P., Guedes, R. N. C., Ferreira, G. H., and Silva, L. B. (2009). Flight take-off and walking behavior of insecticide-susceptible and – resistant strains of Sitophilus zeamais exposed to deltamethrin. Bull. Entomol. Res. 99, 393–400. doi: 10.1017/S0007485309006610
Guedes, R. N. C., Guedes, N. M. P., and Rosi-Denadai, C. A. (2011). Sub-lethal effects of insecticides on stored-product insects: current knowledge and future needs. Stewart Postharvest Review. 7. doi: 10.2212/spr.2011.3.5
Hagstrum, D. W., and Subramanyam, B. (2006). Fundamentals of Stored-Product Entomology. St. Paul, MN: AACC International. doi: 10.1016/B978-1-891127-50-2.50018-1
Hodges, R. J. (1986). The biology and control of Prostephanus truncatus (Horn)(Coleoptera: Bostrichidae)—a destructive storage pest with an increasing range. J. Stored Prod. Res. 22, 1–14. doi: 10.1016/0022-474X(86)90040-8
Hothorn, T., Bretz, F., and Westfall, P. (2008). Simultaneous inference in general parametric models. Biometrical J. 50, 346–363. doi: 10.1002/bimj.200810425
Jankov, D., Indić, D., Kljajić, P., Almaši, R., Andrić, G., Vuković, S., et al. (2013). Initial and residual efficacy of insecticides on different surfaces against rice weevil Sitophilus oryzae (L.). J. Pest. Sci. 86, 211–216. doi: 10.1007/s10340-012-0469-3
Jenson, E. A., Arthur, F. H., and Nechols, J. R. (2009). Efficacy of methoprene applied at different temperatures and rates on surface substrates to control eggs and fifth instars of Plodia interpunctella. J. Econ. Entomol. 102, 1992–2002 doi: 10.1603/029.102.0533
Longstaff, B. C. (1981). Biology of the grain pest species of the genus Sitophilus (Coleoptera: Curculionidae): a critical review. Protec. Ecol. 3, 83–130.
Macfadyen, S., McDonald, G., and Hill, M. P. (2018). From species distributions to climate change adaptation: Knowledge gaps in managing invertebrate pests in broad-acre grain crops. Agric. Ecosyst. Environ. 253:208–219. doi: 10.1016/j.agee.2016.08.029
Morrison, W.R. III, Wilkins, R.V., Gerken, A.R., Scheff, D.S., Zhu, K.Y., Arthur, F.H., et al. (2018). Mobility of adult Tribolium castaneum (Coleoptera: Tenebrionidae) and Rhyzopertha dominica (Coleoptera: Bostrichidae) after exposure to long-lasting insecticide-incorporated netting. J. Econ. Entomol. 111, 2443–2453.
Morrison, W. R. III, Arthur, F. H., and Bruce, A. (2021). Characterizing and predicting sublethal shifts in mobility by multiple stored product insects over time to an old and novel contact insecticide in three key stored commodities. Pest Manag. Sci. 77, 1990–2006. doi: 10.1002/ps.6228
Morrison, W. R. III, Larson, N. L., Brabec, D., and Zhang, A. (2019). Methyl benzoate as a putative alternative, environmentally friendly fumigant for the control of stored product insects. J. Econ. Entomol. 112, 2458–2468. doi: 10.1093/jee/toz179
Morrison, W. R. III, Poling, B., and Leskey, T. C. (2017). The consequences of sublethal exposure to insecticide on the survivorship and mobility of Halyomorpha halys (Hemiptera: Pentatomidae). Pest Manag. Sci. 73, 389–396. doi: 10.1002/ps.4322
Nayak, M. K., Daglish, G. J., Phillips, T. W., and Ebert, P. R. (2020). Resistance to the fumigant phosphine and its management in insect pests of stored products: A global perspective. Annu. Rev. Entomol. 65:333–350. doi: 10.1146/annurev-ento-011019-025047
Noldus, L. P. J. J., Spink, A. J., and Tegelenbosch, R. A. J. (2001). EthoVision: a versatile video tracking system for automation of behavioral experiments. Behav. Res. Meth. Instrum. Comput. 33, 398–414. doi: 10.3758/BF03195394
Noldus, L. P. J. J., Spink, A. J., and Tegelenbosch, R. A. J. (2002). Computerised video tracking, movement analysis and behaviour recognition in insects. Comp. Electric. Agric. 35, 201–227. doi: 10.1016/S0168-1699(02)00019-4
Quellhorst, H. E., Athanassiou, C. G., Bruce, A., Scully, E. D., and Morrison, III W. R. (2020). Temperature-mediated competition between the invasive larger grain borer (Coleoptera: Bostrichidae) and the cosmopolitan maize weevil (Coleoptera: Curculionidae). Environ. Entomol. 49, 255–264. doi: 10.1093/ee/nvz151
Quellhorst, H. E., Athanassiou, C. G., Zhu, K. Y., and Morrison, III W. R. (2021). The biology, ecology and management of the larger grain borer, Prostephanus truncatus (Horn) (Coleoptera: Bostrichidae). J. Stored Prod. Res. 94, 101860. doi: 10.1016/j.jspr.2021.101860
R Core Team (2019). R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. Available online at: https://www.R-project.org/
Ranum, P., Peña-Rosas, J. P., and Garcia-Casal, M. N. (2014). Global maize production, utilization, and consumption. Ann N. Y. Acad Sci. (1312). 105–112. doi: 10.1111/nyas.12396
Ribeiro, B. M., Guedes, R. N. C., Oliveira, E. E., and Santos, J. P. (2003). Insecticide resistance and synergism in Brazilian populations of Sitophilus zeamais (Coleoptera: Curculionidae). J. Stored Prod. Res. 39, 21–31. doi: 10.1016/S0022-474X(02)00014-0
Vélez, M., Bernardes, R. C., Barbosa, W. F., Santos, J. C., and Guedes, R. N. C. (2019). Walking activity and dispersal on deltamethrin- and spinosad-treated grains by the maize weevil Sitophilus zeamais. Crop Protect. 118, 50–56. doi: 10.1016/j.cropro.2018.12.013
White, N. D. G., and Leesch, J. G. (1996). “Chemical control”, in Integr manag of insects in stored prod, Marcel Dekker, B., Subramanyam, D., Hagstrum, W., (eds.). New York. p 287–330 doi: 10.1201/9780203750612-7
Wijayaratne, L. W., Fields, P. G., and Arthur, F. H. (2012). Residual efficacy of methoprene for control of Tribolium castaneum (Coleoptera: Tenebrionidae) larvae at different temperatures on varnished wood, concrete, and wheat. J. Econ. Entomol. 105, 718–725. doi: 10.1603/EC11375
Wilkins, R., Zhu, K. Y., Campbell, J. F., and Morrison, III W. R. (2020). Mobility and dispersal of two cosmopolitan stored product insects are adversely affected by long-lasting insecticide netting in a life stage-dependent manner. J. Econ. Entomol. 113, 1768–1779. doi: 10.1093/jee/toaa094
Keywords: deltamethrin, methoprene, larger grain borer, maize weevil, integrated pest management
Citation: Quellhorst HE, Arthur FH, Bruce A, Zhu KY and Morrison WR III (2022) Exposure to a Novel Insecticide Formulation on Maize and Concrete Reduces Movement by the Stored Product Pests, Prostephanus truncatus (Horn) and Sitophilus zeamais (Motschulsky). Front. Agron. 4:868509. doi: 10.3389/fagro.2022.868509
Received: 02 February 2022; Accepted: 28 February 2022;
Published: 24 March 2022.
Edited by:
Cesar Rodriguez-Saona, Rutgers, The State University of New Jersey, United StatesReviewed by:
Panagiotis John Skouras, University of Peloponnese, GreeceNik G. Wiman, Oregon State University, United States
Copyright © 2022 Quellhorst, Arthur, Bruce, Zhu and Morrison. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hannah E. Quellhorst, aHF1ZWxsaG9Aa3N1LmVkdQ==
 Hannah E. Quellhorst
Hannah E. Quellhorst Frank H. Arthur2
Frank H. Arthur2 Kun Yan Zhu
Kun Yan Zhu