- Laboratory of Biotechnology for Food and Energy Security, Faculty of Nature and Life Sciences, Department of Biotechnology, University Oran 1 Ahmed Ben Bella, Oran, Algeria
Plant Growth Promoting Bacteria (PGPBs) are a strong ally for sustainable agriculture. They offer an interesting alternative to chemical fertilizers and pesticides. Many microorganisms have been widely documented for their PGPR traits, but actinobacterial microbes which have been increasingly documented only these two past decades for their ability to promote plant growth. Their action on plant health and yield could be either direct, indirect or both. This review will cover articles that have been published on Actinobacteria PGP traits, highlighting the involved mechanisms to reveal their strong potential as microbial fertilizers. Possible strategies to encourage Actinobacteria use as bioinoculants are also discussed.
Introduction
Sustainability is a significant challenge currently being faced by human beings. How can we nourish the ever-growing world population and at the same time offer viable soil for future crop production for the next generations? Agricultural ecosystems are fragile and excessive inputs, especially chemicals (nitrogen and phosphate fertilizers) and pesticides, which enable maximal yield, could work for a while, but plant survival is a tributary of soil health, which is, in turn, intimately linked to microbial diversity for nutrient turnover. It has been reported that excessive chemical inputs exert negative impacts on humans and environmental health (Glick, 2012). Moreover, with these incoming chemical inputs, the plant microbiome is modified and in accordance with hologenome theory, which outlines that microorganisms play a role in the evolution of animals and plants (Rosenberg et al., 2009), presumably, the evolution of plants and their associated symbiont microbiota (named holobiont) could be affected.
Plant microbiome are analogous to the gut microbiome: just as the gut microbiome plays a central role in human health (O'Hara and Shanahan, 2006), so too does the plant microbiome present the same properties in plant health. The microbiome is the entire microbial population inhabiting the plant with an extension to the rhizospheric microbiome, as there are important interactions between both (Rosenberg et al., 2009). Among these microbial populations, there are an important group named PGPR “Plant Growth Promoting Rhizobacteria” for those living in the rhizosphere, which ameliorate plant growth both directly and indirectly (Kloepper, 1978) as well as PGPB “Plant Growth Promoting Bacteria” including rhizospheric bacteria and those which are free-living in the soil or associated to plants in rhizoplane, phyllosphere and inside plants as endophytes (Bashan and De-Bashan, 2005). These microbes help plant growth by enhancing soil nutrient availability (Scagliola et al., 2016), the supply of phytohormones, and provide systemic resistance induction against phytopathogens. Thus, the employment of Plant Growth Promoting Bacteria (PGPB) is considered a promising alternative to conventional agricultural practices, in terms of chemical fertilizer and control of pathogenic agents (Bashan, 1998).
The use of Actinobacteria in agricultural practice has increased in recent years, due to their potential action as PGPR and their ubiquitous repartition in plants (Yadav et al., 2018). Actinobacteria are Gram positive bacteria with a high G+C content in their DNA, ranging from 51 to more than 70% (Ventura et al., 2007), well known for their metabolite production, mainly antibiotics (Saxena, 2014). They are present in the phyllosphere, endosphere (Lopez-Velasco et al., 2013), rhizosphere, and are free living in soil (Bulgarelli et al., 2013). In addition to their PGPB action, some other actinobacterial characteristics could encourage wider use as bioinoculant: many Actinobacteria, which generally represent an abundant proportion of soil microbiota, are particularly effective plant root system colonizers and by forming spores, they are able to endure unfavorable growth conditions (Alexander, 1977) and are more persistent in drought soils (Santos-Medellín et al., 2017). They play a critical role in organic matter recycling (Lacey, 1978) by increasing soil organic matter and nitrogen content along with essential macro and micro-elements, which in turn ameliorate plant growth, carbon metabolism, and allocation, and improve plant yield (AbdElgawad et al., 2020). Finally, their antagonistic and competitive characteristics permit them to colonize the rhizosphere with regards to other soil microorganisms (Bulgarelli et al., 2013). All the characteristics cited above designate Actinobacteria as an auspicious inoculant.
Many studies have proven the PGP action of the plant microbiome, but there is a gap between in vitro trials and efficiency in the field, particularly concerning their commercialization as a final bio-input product. The purpose of this review is to demonstrate the beneficial and protective impact of Actinobacterial on plant growth by highlighting the main direct or indirect PGPB traits. All genera of this important taxon were examined to identify their potential PGPB actions. On the other hand, the main impediments and future prospects of their use as biofertilizers are discussed.
Actinobacteria Diversity and Importance
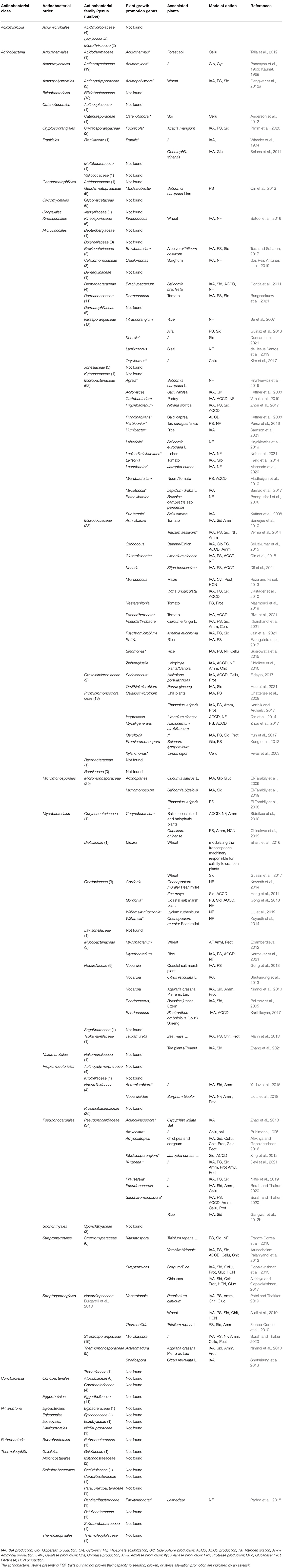
According to Ludwig et al. (2012), in terms of the number and variety of identified species, the phylum Actinobacteria represents one of the largest taxonomic units among the 18 major lineages currently recognized within the domain Bacteria, including 5 subclasses, 6 orders, and 14 suborders. Its genomic diversity reflects its biodiversity which could have great biotechnological applications (Ventura et al., 2007). But an update based on 16S rDNA trees done by Gao and Gupta (2012), eliminated the taxonomic ranks of subclasses and suborders, elevating the former subclasses and suborders to the ranks of classes and orders, respectively. The phylum “Actinobacteria” is thus divided into six classes: Acidimicrobiia (01 order) (Norris, 2012), Actinobacteria (include 20 orders) after the classification based on the whole genome (Nouioui et al., 2018), Coriobacteriia (02 order) (Gupta et al., 2013), Nitriliruptoria (02 orders) (Ludwig et al., 2012), Rubrobacteria (01 order) (Suzuki, 2012), and Thermoleophilia (02 orders) (Suzuki and Whitman, 2012). Based on Actinobacteria classification (Parte et al., 2020) (consulted 01/01/2022), the cited classes above include 73 families and 443 genera which are unequally distributed. The majority of them (394) are within the class Actinobacteria (Table 1).
Actinobacteria present various and different lifestyles including plant pathogens (e.g., Streptomyces scabiei, S. acidiscabies, and S. turgidiscabies) (Wanner, 2006), mammalian pathogens (e.g., Mycobacterium spp., Nocardia spp., Corynebacterium spp., Tropheryma spp., and Propionibacterium spp.), plant commensals (Leifsonia spp.), soil inhabitants (Streptomyces spp.) as reported by Ventura et al. (2007), nitrogen-fixing symbionts (Frankia) (Franche et al., 2009), and gastrointestinal tract (GIT) inhabitants (Bifidobacterium spp.) (Ventura et al., 2007; Barka et al., 2016). It should be noticed that members of Actinobacteria class are associated with plants growing in different habitats as well as under extreme environments (Goudjal et al., 2013; Singh et al., 2016; Sahay et al., 2017).
Rhizospheric Actinobacteria are predominant in nature, with economic importance to humans because both agricultural and forest fields depend on their contributions to soil systems (Yadav et al., 2017). They possess diverse physiological and metabolic properties, like extracellular enzyme production and the formation of a wide variety of secondary metabolites (Schrempf, 2001). Rhizosphere harbors diverse actinobacterial species which have been further exploited for secondary metabolites (Geetanjali, 2016; Yadav et al., 2018). It has been reported by Berdy (2003), that microorganisms produced about 23,000 bioactive secondary metabolites, over 10,000 of these compounds are produced by Actinobacteria, which represent 45% of all discovered bioactive microbial metabolites and 80% if we only consider those compounds used in a practical way. Among Actinobacteria, Streptomyces species produced around 7,600 compounds, and these statistical evaluations should increase, perhaps not in an exponential way, but with the continuous growth of the number of new microbial metabolites.
The genus Streptomyces dominated actinobacterial strains isolated from soil, representing over 95% (Williams and Vickers, 1988). These actinobacterial strains are considered Streptomyces and Non-Streptomyces. Among the bioactive compounds produced by Actinobacteria, antibiotics, which initially confer them competitiveness are the most important in terms of biotechnological application as they produce the majority of the naturally occurring antibiotics (Barka et al., 2016). Other actinobacterial metabolites possess biotechnological applications, including in antifungal (Hoshino et al., 2004), bioherbicide/biopesticide (Waldron et al., 2001), antiparasitic (Burg et al., 1979), antiviral (Farmer and Suhadolnik, 1972), antitumor agent (Igarashi et al., 2007), immunostimulatory (de Reijke et al., 1997), and immunosuppressive (Uyeda et al., 2001) products.
The endophytic trait has been described mostly in the class Actinobacteria (Singh and Dubey, 2018), but with the advances of molecular identification tools, other endophytic candidates have been revealed so far, as for Thermoleophilia class, e.g., Solirubrobacter phytolaccae (Solirubrobacterales order) (Wei et al., 2014) and Patulibacter (Solirubrobacterales order) (Ferrando et al., 2012); for Rubrobacteria class e.g., Rubrobacteria genus (Rubrobacterales order) (Girija et al., 2018) and Coriobacteria class (Ren et al., 2018).
The fact that Actinobacteria could survive mesophilic but also for some candidates at thermophilic conditions reaching 60°C is an encouraging trait for its inocula use (Edwards, 1993), a fortiori they are considered as aridity–winners by Marasco et al. (2021) who stated that aridity changes composition and interactions of the plant-microbial community, this by modulating the distribution of aridity-tolerant (winners) and aridity-susceptible (losers) bacterial taxa, which is in favor of the former in a dry environment. There have also been reports that actinobacterial inoculation could not only protect plants from the deleterious effects of drought but also show significant increases in their measured physiological parameters (Chukwuneme et al., 2020). Furthermore, acidophilic Actinobacteria could play a major role in the inoculation of plants living in acidic soil (Bull, 2011). Many halotolerant Actinobacteria have been isolated from saline environments and have proven to be useful crop protective agents to plants in stressful conditions (Siddikee et al., 2010; Zhou et al., 2017; Qin et al., 2018). The extremophile character of some actinobacterial strains could be a valuable tool for rehabilitating degraded areas under extreme environmental conditions and they can enhance crop production under multiple conditions of stress, such as extreme temperatures, pH, salinity, and drought (Qin et al., 2011).
PGP Traits of Actinobacteria
Bacterial strains are considered as PGPR if they can fulfill at least two of the three following criteria: aggressive colonization, plant growth stimulation, or biocontrol (Vessey, 2003). Globally, plant growth-promoting rhizobacteria (PGPR) are the rhizosphere bacteria that can enhance plant growth by a wide variety of mechanisms like phytohormones production, 1-Aminocyclopropane-1-carboxylate (ACC) deaminase production, induction of systemic resistance (ISR), phosphate solubilization, siderophore production, biological nitrogen fixation (BNF), rhizosphere engineering, quorum sensing (QS) signal interference and inhibition of biofilm formation, exhibiting antifungal activity, production of volatile organic compounds (VOCs), promoting beneficial plant-microbe symbioses, interference with pathogen toxin production, etc. (Bhattacharyya and Jha, 2012; Kumar and Singh, 2020). From a practical point of view, PGP-microbes could be used as biofertilizers by providing macro and micronutrients like biological nitrogen fixation (Vessey, 2003) and utilization of insoluble phosphorous (Chang and Yang, 2009), as biostimulants or phytostimulants by improving nutrient use and efficiency thanks to phytohormones production (Lugtenberg et al., 2002), as biocontronl by controlling plant pathogens using antibiotics or siderophores (Vessey, 2003), for rhizomediation by enhancing heavy metal solubility or decreasing the bioavailability of toxic compounds (Denton, 2007), and as biotisation agents by reducing chemical inputs in in vitro plant tissues culture (Diehdhiou et al., 2021). Regarding Actinobacteria, one of the major components of rhizosphere microbial populations, they showed a significant ecological role in soil nutrient cycling (Halder et al., 1991; Elliott and Lynch, 1995) as well as in plant growth-promoting activities (Merzaeva and Shirokikh, 2006), and numerous reports (Gomes et al., 2000; Sousa Cd et al., 2008; Goudjal et al., 2013; Kaur et al., 2013) are available on their potential as plant growth-promoting agents. To illustrate plant promoting ability among Actinobacteria phylum, we reviewed the 443 actinobacterial genera by associating each of them to term “plant growth promotion.” The most relevant results are reported in Table 1.
Among the mechanism of action, we will report below major processes of plant growth promotion related to Actinobacteria. But before, one should wonder if PGP traits, precisely, which one or how many should be accumulated to exert the maximum growth improvement of plants. For example, the most effective strain E108, identified as Curtobacterium flaccumfaciens, has increased barley growth up to 300% but has shown only two out of the six investigated plant growth promoting activities comparatively to two other strains, namely the Microbacterium natoriense strain E38 and Pseudomonas brassicacearum strain E8, which did not promote plant growth even though they showed many PGP traits (Cardinale et al., 2015).
Direct Action
Biological Nitrogen Fixation
Nitrogen is generally regarded to be one of the major limiting nutrients in plant growth (Franche et al., 2009). It is widely known that Frankia, the sole genus of Frankiales order fixes atmospheric nitrogen in symbiosis with actinorhizal plants, which play a major role in colonizing nitrogen-poor soils and initiation of ecological successions (Normand et al., 2007). Some studies on nitrogen-fixing properties among the Gram-positive Actinobacteria revealed that some non-symbiotic species of Agromyces, Arthrobacter, Corynebacterium, Micromonospora, Mycobacterium, Streptomyces, and Propionibacteria have nitrogen fixing capacity (Sellstedt and Richau, 2013). More particularly, the nitrogen fixing Arthrobacter humicola (Verma et al., 2014), Corynebacterium spp. (Verma et al., 2014), Microbacterium FS-01 (Karlidag et al., 2007). To complement these data, Table 1 summarizes the nitrogen fixation ability among Actinobacteria class and some representatives in Thermoleophilia class. From the former, Microbacteriaceae which is the largest family (62 genus) includes at least seven nitrogen fixers: Agreia (Hrynkiewicz et al., 2019), Curtobacterium (Vimal et al., 2019), Herbiconiux (Pérez et al., 2016), Labedella (Hrynkiewicz et al., 2019), Lacisediminihabitans (Noh et al., 2021), Leucobacter (Machado et al., 2020) and Rathayibacter (Poonguzhali et al., 2006).
Producing Phytohormones Like Auxins, Cytokinins, and Gibberellins
Phytohormones are involved in many physiological processes, they include auxins, gibberellins, cytokinins, ethylene, and abscisic acid, which are classified based on their function and structure composition. They are mainly produced by rhizospheric microorganisms, fungi, algae, and Actinobacteria (Mulani et al., 2021).
The auxins are a group of indole ring compounds that have the capacity to ameliorate plant growth by stimulating seed germination, root initiation and elongation, and seedling growth (El-Tarabily et al., 2008). Indole-3-Acetic Acid (IAA) is an auxin that is common and natural and is resulted from L-tryptophan metabolism in microorganisms (Davies, 2004), as we could observe from Table 1, IAA is widely produced by Actinobacteria. Even, it was reported a tryptophan- independent pathway operation in Micrococcus aloeverae (Ahmad et al., 2020).
Cytokinins are considered the second group of plant hormones biosynthesized by microbes. They mediate signal exchange from roots to shoots under environmental stresses. They also induce cell division, cell enlargement, and increase root surface area with the help of intense proliferation of adventitious and lateral roots (Jackson, 1993). These hormones have been reported for actinobacterial strains, but to a lesser extent than auxins as it was reported for Leifsonia soli (Kang et al., 2014) and Promicromonospora (Kang et al., 2012).
Gibberellins are plant hormones, that are considered ubiquitous. They generate the diverse metabolic functions necessary during plant growth steps such as seed germination, stem elongation, sex expression, flowering, formation of fruits, and senescence (Hedden, 1997). Gibberellins actinobacterial species production was reported in several studies, as for Streptomyces olivaceoviridis, S. rochei and S. rimosus cultures which were excellent producers of gibberellins-like substances, showing wheat plant growth promotion (Aldesuquy et al., 1998) and for Arthrobacter globiformis (Katznelson et al., 1962).
Solubilizing Minerals Like Phosphorus
Phosphorous limitation could prevent plant growth, first because phosphorous is vital and secondly, its bioavailability from the soil is often limited (Feng et al., 2004). Phosphate Solubilizing Bacteria (PSB) including Actinobacteria could increase the availability of soluble phosphate by various mechanisms like production of low molecular weight organic acid, along with the production of hydroxyl and carboxyl groups, serving as a chelating agent to chelate the cations (mainly Ca) bound to phosphate converting them into soluble forms (Kpomblekou-a and Tabatabai, 1994) or by enzymatic actions mostly phosphatase (Solans et al., 2019) and phytase (Sharma et al., 2017), although, it seems that the major action of Actinobacteria was enzymatic (Nimaichand et al., 2016). Phosphate Solubilizing Actinobacteria are often associated with the production of plant growth-promoting regulators, increasing biological nitrogen fixation effectiveness or enhancing the availability of other trace elements such as iron, zinc, etc. (Ponmurugan et al., 2006). This statement is correlated with the reported data in Table 1, mainly with IAA production for important crops such wheat (Allali et al., 2019), maize (Marín et al., 2013), rice (Susilowatia et al., 2015), and Phaseus vulgaris (Karthik and Arulselvi, 2017).
Production of Siderophores
Iron is often a limiting living growth factor, microorganisms developed siderophore production which relies on chelation phenomena, with a high affinity for iron (Fe3+) chelation and low molecular weight (500–1,000 Da) (Neilands, 1995). After chelation, the available ionic form (Fe+2) is easily absorbed by microorganisms (Kaszubiak, 1998). Microbial siderophores may act as plant promoters by dispensing iron to plants and as a biocontrol agent against phytopathogens by limiting its availability and thus killing pathogens (Anilkumar et al., 2017). In terms of siderophores production, Actinobacteria is one the most important group (Franco-Correa and Chavarro-Anzola, 2016) as illustrated in Table 1, which reports many siderophore productions by many species associated with grain crops, such as for Brevibacterium associated to Triticum aestivum (Tara and Saharan, 2017), Gordonia with Zea mays (Hong et al., 2011), Amycolatopsis with chickpea and sorghum (Alekhya and Gopalakrishnan, 2016), Kitasatospora with Trifolium repens L. (Franco-Correa et al., 2010), Streptomyces with Rice (Gopalakrishnan et al., 2013) and chickpea (Alekhya and Gopalakrishnan, 2017), Nocardiopsis with wheat (Allali et al., 2019), Thermobifida with Trifolium repens L. (Franco-Correa et al., 2010) and Micrococcus with Vigna unguiculata (Dastager et al., 2010). It has also been reported for halophyte plants as for Brachybacterium associated to Salicornia brachiate (Gontia et al., 2011), Micromonospora with Salicornia bigelovii (El-Tarabily et al., 2019); for medicinal plants as for Frigoribacterium with Nitraria sibirica (Zhou et al., 2017), Pseudarthrobacter with Curcuma longa L. (Kharshandi et al., 2021), Psychromicrobium with Arnebia euchroma (Jain et al., 2021), Ornithinimicrobium with Panax ginseng (Huo et al., 2021), Pseudonocardia with Camellia spp. (Borah and Thakur, 2020); for metal-accumulating plants as for Agromyces with Salix caprea (Kuffner et al., 2008), Nocardia and Actinomadura with Aquilaria crassna Pierre ex Lec (Gong et al., 2018; Nimnoi et al., 2010) respectively, Rhodococcus with Brassica juncea L. Czern (Belimov et al., 2005) and Dermacoccus (Rangseekaew et al., 2021) and Arthrobacter (Banerjee et al., 2010) associated to tomato.
1-Aminocyclopropane-1- Carboxylate Deaminase (ACC Deaminase)
Ethylene, which are the aging hormones of plants (Patel et al., 2018), are produced as a response to stress “stress ethylene,” meaning the development of the plant is slowed, to respond to this stress condition and promote plant growth, PGP bacteria produce 1-aminocyclopropane-1-carboxylate (ACC) deaminase (Glick, 2005). This (ACC) deaminase delivered by PGPR, will metabolize ACC into alpha-ketobutyrate, methionine, and ammonia and thus will regulate ethylene production (Mulani et al., 2021). Inoculation of ACC deaminase-producing bacteria immediately enhances plant root elongation and promotes shoot growth (Onofre-Lemus et al., 2009). It has been reported the presence of ACC deaminase genes among Actinobacteria such as Actinoplanes, Agreia, Arthrobacter, Austwickia, Brevibacterium, Streptomyces, Amycolatopsis, Mycobacterium, Nocardioidaceae, Rhodococcus, and others (Nascimento et al., 2014). Some halotolerant Actinobacteria showed ACC deaminase activities such as Corynebacterium variabile, Micrococcus yunnanensis, and Arthrobacter nicotianae, promoting canola plant growth under salt stress conditions (Siddikee et al., 2010).
Production of Hydrolytic Enzymes
Actinobacteria, as a dominant member of the saprophytic community, have been known to secrete a wide array of hydrolytic enzymes in natural conditions (Jog et al., 2016). Actinobacteria are considered as primary decomposers of dead organic matter, especially lignocellulosic biomass (Větrovsk et al., 2014). They show a remarkable ability to produce cellulase, xylanase, lignin peroxidase, and chitinase enzyme cocktail in addition to protease, lipase, pectinase, keratinase, amylase, invertase, and phytase that can trigger as a first step plant biomass degradation, thus processing it into simpler form for a second decomposition step initiated by secondary decomposers (Jog et al., 2016). Finally, complex nutrients are transformed into the simplest mineral forms, which act as natural fertilizers promoting plant health (Jog et al., 2012). These hydrolytic enzymes not only play a role in biomass turnover but also the biocontrol process, as described below.
Induction of Systemic Resistance
Induction of Systemic Resistance (ISR) is activated by non-pathogenic plant-associated microorganisms. Localized infection or treatment with microbial components, products, or a variety of structurally unrelated organic compounds and inorganic compounds caused systemic resistance (ISR) to infectious diseases and herbivorous insects (Ghiasian, 2020). Plant hormones Jasmonic acid (JA) and ethylene provide many contributions to the regulation of the group of inter-related signaling pathways required in ISR induction (Pieterse et al., 1998). Actinobacteria, reported as wheat endophyte, can induce defense pathways in Arabidopsis (Conn et al., 2008). Another report (Zhao et al., 2012) showed that culture filtrate from Streptomyces bikiniensis HD-087 was able to induce systemic resistance in cucumber against Fusarium wilt, caused by F. oxysporum f.sp. cucumerinum. Furthermore, Micromonospora spp. isolated from alfalfa nodules induced ISR through the jasmonate pathway (Martínez-Hidalgo et al., 2015). It seems that Actinobacteria are detected by the plant as “minor” pathogens because they do not have pathogenic determinant, as some endophytic actinobacterial strains possess the faculty to activate the plant defense genes at a low level in the absence of a pathogen (Coombs and Franco, 2003).
Indirect Action
Biocontrol
Biological control is the use of living organisms to modify the agricultural ecosystem to control a crop disease or prevent the establishment of a pest (Dowling and O'Gara, 1994). Bacteria that are involved in protecting plants are often referred to as biocontrol agents (Beattie, 2007).
Actinobacteria are widely recognized for their potential in biocontrol (El-Tarabily and Sivasithamparam, 2006; Hasegawa et al., 2006) because they are important producers of bioactive compounds (Qin et al., 2011). Over the past 50 years, there have been many studies on the mechanisms by which Actinobacteria might inhibit pathogens in soil, including antibiosis, nutrient competition, production of degradative enzymes (Subramanian et al., 2016), nitrous oxide production (Salwan and Sharma, 2020), and quorum quenching (Vesuna and Nerurkar, 2020).
Antibiosis is defined by the secretion of molecules that kill or reduce the growth of the target pathogen, this could be mediated by the secretion of specific or non-specific metabolites of microbial origin, by lytic agents, enzymes, volatile compounds, or other toxic substances (Fravel, 1988). Antibiotic-mediated inhibition of pathogens is generally the primary focus in efforts to suppress plant diseases. However, the diversity of secondary metabolites produced by Streptomyces and other Non- Streptomyces species foreshadows an interesting ability for suppressing fungal, bacterial, oomycete, and nematode pathogens (Barka et al., 2016).
Antibiotics are classified into two groups: volatile and non-volatile antibiotics. Volatile antibiotic substances like HCN, ammonia, aldehyde, alcohol, acetone, methane, 2-ethylethyl-1–hexanol, dimethyl sulfide, thioacetate, y-butyrolactones (Cellini et al., 2021) whereas phenazine, phenazine-1-carboxylic acid 2 -hydroxyphenazine, and pyrrolnitrin are some of the non-volatile antibiotic substances (Zhang et al., 2020). As it was reported further, Actinobacteria are widely known for their ability to produce antibiotics that allow them to inhibit pathogens in general and plant pathogens in particular (Berdy, 2005), especially Streptomyces genus, which have been the major producer for bioactive metabolites (Alexander, 1978) exhibiting an immense biocontrol action against a range of phytopathogens (Wang et al., 2013). They account for nearly 60% of the production of agriculturally important antibiotics (Ilic et al., 2007). But Non-Streptomyces antibiotic producers shouldn't be neglected as there are many reports about their potential antimicrobial production as for Actinoplanes sp. producing Xanthone (Cooper et al., 1992); Actinomadura madurae producing Simaomicin (Maiese et al., 1990); Micromonospora spp. producing Spartanamicins (Nair et al., 1992); Saccharothrix spp. producing Formamicin (Igarashi et al., 1997) and Streptosporangium albidum producing Aculeximycin (Ikemoto et al., 1983).
Among volatile compounds considered as antibiosis molecules, Hydrogen Cyanide Nitrogen HCN plays a role in biocontrol, by sequestering iron, thus, competing with phytopathogens (Gu et al., 2020) along with phosphate free for plant assimilation (Rijavec and Lapanje, 2016; Backer et al., 2018) and by inhibiting terminal “cytochrome c oxidase” in the respiratory chain and binding to metalloenzymes which confers it the property of suppressing phytopathogens (Gu et al., 2020). This metabolite production was reported for Streptomyces spp., Microbispora spp., Actinomadura spp., Micromonospora spp., Nocardia spp. (Dalal and Kulkarni, 2014), for many Streptomyces strains (Alekhya and Gopalakrishnan, 2017; Vijayabharathi et al., 2018) and Nocardiopsis (Allali et al., 2019). Another volatile compound, nitric oxide produced by Streptomyces has been suggested to activate plant defense against pathogen attack (Vaishnav et al., 2018).
In addition to producing antibiotics against a variety of pathogenic diseases in plants, hydrolytic enzymes, which are produced by antagonistic microbes, are capable of degrading fungal and bacterial cell walls, cell membranes, cell membrane proteins, and extracellular virulence factors which have been implicated in the biocontrol of plant diseases (Pal and Gardener, 2006). These hydrolytic enzymes include chitinase, cellulase, glucanase, protease, and phospholipase (Palaniyandi et al., 2013). Streptomyces are largely predominant in the suppression of plant disease by the production of chitinase, glucanase (Lee et al., 2012); and protease (Fróes et al., 2012); on another hand, Actinoplanes campanulatus was reported as β-glucanase producer (El-Tarabily et al., 2009); Micromonospora carbonacea produced chitinase; β-1,3-glucanase (El-Tarabily et al., 2000) and cellulase (El-Tarabily et al., 1996) and finally, Amycolatopsis secreted protease, glucanase and pectinase (Alekhya and Gopalakrishnan, 2016).
Quorum sensing (QS) could be defined as bacterial population density regulation and the regulation of their gene expression accordingly (Fuqua et al., 1994). On another hand, quorum quenching covers all processes implicated in (QS) disturbance (Dong and Zhang, 2005), this phenomenon opens many applications in medicine, aquaculture, crop production, and anti-biofouling (Grandclément et al., 2016). Over the last decade, a total of six Actinobacterialgenera: Arthrobacter (Flagan et al., 2003), Microbacterium (Wang et al., 2009), Mycobacterium (Chen and Xie, 2011), Nocardioid-es (Yoon et al., 2006), Rhodococcus (Latour et al., 2013), and Streptomyces (Ooka et al., 2013) have been reported for their quorum quenching activity.
Actinobacteria as Helper Bacteria
The most important symbiotic plant microorganisms namely mycoryzal, actinorhizal, and rhizobial symbiosis establishment are impacted by many biotic and abiotic factors. Several reports have shown the improvement of legume symbiosis and mycorrhizal symbiosis in dual inoculations with diverse PGPR (Barea et al., 2005); however, there is less information on this subject with Actinobacteria. There is a rising belief that Helper Bacteria could promote these symbioses. Rhodococcus, Streptomyces, and Arthrobacter are considered a Mycorrhizal Helper (Frey-Klett et al., 2007). Moreover, Schrey and Tarkka (2008) showed that the Streptomyces genus promotes the formation of symbioses between plant roots and microbes, and this is in part due to their direct positive influence on the symbiotic partner, expressed as, e.g., promotion of hyphal elongation of symbiotic fungi; furthermore, Franco-Correa et al. (2010) showed that co-inoculation of Streptomyces spp. MCR9 and MCR24 and Glomus mosseae produced synergic benefits on plant growth and P acquisition. The selected actinobacterial strains improved Arbuscular Mycorrhiza (AM) formation in clover plants. Concerning actinorhizal symbiosis, it was observed that saprophytic strains namely Streptomyces MM40, Actinoplanes ME3, and Micromonospora MM18 acted as helper bacteria (Solans, 2007). These actinobacterial strains clearly produced phytohormones (Solans et al., 2011) and had enzymatic activity for cellulose, hemicellulose, pectin, and lignocellulose (Solans and Vobis, 2003), but the real responsible metabolites are still unknown. The same saprophytic Actinobacteria used for actinorhizal symbiosis co-inoculation were co-inoculated to Medicago sativa–Sinorhizobium meliloti symbiosis (Solans, 2007). In these assays, the plants co-inoculated with Actinobacteria and rhizobium showed an increase in nodulation and plant growth compared with plants with single inoculations. In addition, Lotus tenuis plants co-inoculated with Mesorhizobium loti and saprophytic actinobacterial strains (MM40, ME3, and MM18) showed a promoting effect on nodulation and biomass. Another study reported that the combination of Streptomyces kanamyceticus and Bradyrhizobium japonicum increased nodulation and shoot nitrogen composition of soybeans by up to 55 and 41%, respectively (Gregor et al., 2003). Even if there is still scarce information on the potential of Actinobacteria as Symbiosis Helper, but the studies cited above could encourage this domain of investigation for improving symbiosis under diverse conditions.
Commercial Formulation of Actinobacterial Bioinoculants
From the reviewed information discussed above, it is clear that Actinobacteria possess some interesting characteristics, such as ubiquity, rhizosphere colonization ability (filamentous structure), competitiveness, the capacity to resist harsh conditions, in addition to high strength spores that allow them to survive for prolonged periods in soil and in storage containers in addition to high PGP activity and nutrient cycling capability (Jog et al., 2016). Actinobacteria have proven their value as PGPR and biocontrol agent, but few available commercial actinobacterial products are disposable. Among these commercial products, we could cite an active ingredient, Strptomyces lydicus WYEC108, which gave rise to multiple commercial products: “Actinovate® AG” (Elliott et al., 2009), “Actinovate® SP” and “Micro108® soluble” for biocontrol; “Micro108® Seed” and “Inoculant Action Iron®” (produced by Naturalindustries) for biocontrol and plant growth promotion. Streptomyces violaceusniger strain YCED9 microbial agent was named “Thatch Control” for biocontrol (produced by Naturalindustries); S. griseoviridis strain K61 “Mycostop®” for biocontrol (Suleman et al., 2002); Streptomyces saraceticus KH400 “YAN TEN Streptomyces saraceticus” for biocontrol (produced by Yanten). Metabolite Polyoxin D (produced by Streptomyces cacoi var. asoensis) named AFFIRMWDG (produced by Chlearychemical) and “PH-D® Fungicide” (produced by Arista-na) both used as a fungicide for turfgrass fungi. Some field trials tested the innocuity of actinobacterial bioinoculation as it was done for “Mycostop®,” which was used for the control of Fusarium wilt of carnation and root rot disease of cucumber, and has been used in greenhouse production to protect flowers from pathogens (White et al., 1990). Likewise, Actinovate®, a biocontrol formulation of Streptomyces lydicus registered from AgBio in the United States of America, has been suggested for a wide range of environments ranging from greenhouses to field conditions, similarly, for Streptomyces lydicus WYEC 108 (MicroPlus®), which has been reported to possess disease suppression against powdery mildew and several root decay fungi (Solanki et al., 2016).
In general terms, “innovation” should include academic research, governmental institutions, industry, and civil society. Overall scientists have made an effort to develop eco-friendly and safe living organisms such as bio-intrant for ameliorating crop yield and protecting plants from pathogens, but these efforts will be infructuous if industry is not interested. Secondly, if governments do not establish legislation supervising the biotechnological application of PGPBs and facilitating interaction between “university” and “industry” (for example spin-off projects from a university or research and development department in industry) and finally if society in general and farmers, in particular, do not adhere to the use of bioinoculation (Etzkowitz and Zhou, 2017).
This assessment being established, much effort should be made before commercializing Actinobacteria or actinobacterial products for the development of sustainable agricultural solutions, beyond laboratory trials, including field assays to evaluate bioinoculant facing plant species, soil nature, and environmental conditions, which are unique for each ecosystem. It is crucial to evaluate bio-input safety and prevent the spread of antibioresistance.
Conclusion and Perspectives
There are important fields of investigation in developing the use of PGPB for ameliorating plant growth, alleviating plants stress, and enhancing plant resistance to pests. Among these PGPB, Actinobacteria (PGPA) are increasingly studied (Nimaichand et al., 2016). Sustainable agriculture is well integrated in the roadmap of industrialized and developing countries. The former to minimize negative impacts on atmospheric greenhouse gas (GHG) concentrations and water quality caused by N and P losses following high fertilization rates (Haygarth et al., 2013) along with minimizing pesticide impact on environmental and human health (Bernardes et al., 2015). The latter, because they practice low-input agriculture where fertilizers, pesticides, and agro-technical machinery are not widely available (too expensive) and where the application of putative inexpensive bioinoculants is a great challenge (Bashan, 1998). The reviewed literature cited above clearly demonstrates the high potential of Actinobacteria in ameliorating plant growth, whether acting directly or indirectly, and/or as fighting tools against phytopathogens. Furthermore, much valuable research has highlighted the beneficial effects of Actinobacteria PGPB actions on crop yield outlining that these Actinobacterial strains could be candidates as microbial fertilizers. Future studies will deal with the next steps in terms of exploring the effect of these microorganisms on plants under greenhouse conditions (semi-controlled conditions), and then under field conditions (different soil characteristics, environmental conditions, agricultural practices…), and primarily, which formulations of these bioinoculants should be selected: liquid, organic, inorganic, polymeric or encapsulated. This “secret art” formulation will ensure compatibility with routine field practices, should be easy to use, environment-friendly, and have long storage quality (Bashan et al., 2014). In addition to these crucial scientific and bioprocessing stages, registration and regulatory approval of the product should be initiated once the bioinoculant proved its efficacy (Backer et al., 2018). It is obvious that the gap between in vitro trials and marketable final products necessitates investment, time, and multidisciplinary skills (Bashan et al., 2014). North America is considered a leader for bioinoculant production in terms of generating revenue, followed by Europe, Asia-Pacific, South America, and finally to a lesser extent Africa (Soumare et al., 2020). Based on the fact that the global biofertilizer market will reach US$1.66 billion by 2022 (Timmusk et al., 2017), government and industries should be confident of a return on any investment. Overall, the major applications of bioinoculation have used BNF bacteria (especially rhizobia of about 79%), followed by phosphate solubilizing bacteria (~15%) while other inoculants including mycorrhizal products make up the remaining percentage (Research, 2014). Based on the Actinobacteria PGP traits described in this review, which are in addition to their competitiveness, ubiquity, and tremendous potential for metabolite production, this large taxonomic group worth a special attention even if it is not considered as a best candidate presently. Ultimately, it should play a key role in formulating multi-strain inoculants with synergistic actions for promoting sustainable agriculture.
Author Contributions
All authors listed have made a substantial, direct, and intellectual contribution to the work and approved it for publication.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We are thankful for SNDL (Système National de Documentation en Ligne) affiliated to MESRS | DGRSDT | CERIST, which allowed us to access scientific literature.
References
AbdElgawad, H., Abuelsoud, W., Madany, M. M., Selim, S., Zinta, G., Mousa, A. S., et al. (2020). Actinomycetes enrich soil rhizosphere and improve seed quality as well as productivity of legumes by boosting nitrogen availability and metabolism. Biomolecules. 10, 1675. doi: 10.3390/biom10121675
Ahmad, E., Sharma, S. K., and Sharma, P. K. (2020). Deciphering operation of tryptophan-independent pathway in high indole-3-acetic acid (IAA) producing Micrococcus aloeverae DCB-20. FEMS Microbiol. Lett. 367, fnaa190. doi: 10.1093/femsle/fnaa190
Aldesuquy, H., Mansour, F., and Abo-Hamed, S. (1998). Effect of the culture filtrates ofStreptomyces on growth and productivity of wheat plants. Folia Microbiologica. 43, 465–470. doi: 10.1007/BF02820792
Alekhya, G., and Gopalakrishnan, S. (2016). Exploiting plant growth-promoting Amycolatopsis sp. in chickpea and sorghum for improving growth and yield. J. Food Legumes. 29, 225–231.
Alekhya, G., and Gopalakrishnan, S. (2017). Biological control and plant growth-promotion traits of Streptomyces species under greenhouse and field conditions in chickpea. Agricult. Res. 6, 410–420. doi: 10.1007/s40003-017-0278-2
Alexander, M. (1978). Introduction to soil microbiology. Soil Sci. 125, 331. doi: 10.1097/00010694-197805000-00012
Allali, K., Goudjal, Y., Zamoum, M., Bouznada, K., Sabaou, N., and Zitouni, A. (2019). Nocardiopsis dassonvillei strain MB22 from the Algerian Sahara promotes wheat seedlings growth and potentially controls the common root rot pathogen Bipolaris sorokiniana. J. Plant Pathol. 101, 1115–1125. doi: 10.1007/s42161-019-00347-x
Anderson, I., Abt, B., Lykidis, A., Klenk, H.-P., Kyrpides, N., and Ivanova, N. (2012). Genomics of aerobic cellulose utilization systems in actinobacteria. PloS One. 7, e39331. doi: 10.1371/journal.pone.0039331
Anilkumar, R. R., Edison, L. K., and Pradeep, N. (2017). Exploitation of fungi and actinobacteria for sustainable agriculture. Microbial Biotechnol. 17, 135–62. doi: 10.1007/978-981-10-6847-8_6
Arunachalam Palaniyandi, S., Yang, S. H., Damodharan, K., and Suh, J. W. (2013). Genetic and functional characterization of culturable plant-beneficial actinobacteria associated with yam rhizosphere. J. Basic Microbiol. 53, 985–995. doi: 10.1002/jobm.201200531
Backer, R., Rokem, J. S., Ilangumaran, G., Lamont, J., Praslickova, D., Ricci, E., et al. (2018). Plant growth-promoting rhizobacteria: context, mechanisms of action, and roadmap to commercialization of biostimulants for sustainable agriculture. Front. Plant Sci. 9, 1473. doi: 10.3389/fpls.2018.01473
Banerjee, S., Palit, R., Sengupta, C., and Standing, D. (2010). Stress induced phosphate solubilization by'Arthrobacter'Sp. And'Bacillus' sp. isolated from tomato rhizosphere. Austr. J. Crop Sci. 4, 378–383.
Barea, J.-M., Pozo, M. J., Azcon, R., and Azcon-Aguilar, C. (2005). Microbial co-operation in the rhizosphere. J. Experiment. Bot. 56, 1761–1778. doi: 10.1093/jxb/eri197
Barka, E. A., Vatsa, P., Sanchez, L., Gaveau-Vaillant, N., Jacquard, C., Klenk, H.-P., et al. (2016). Taxonomy, physiology, and natural products of Actinobacteria. Microbiol. Mol. Biol. Rev. 80, 1–43. doi: 10.1128/MMBR.00019-15
Bashan, Y. (1998). Inoculants of plant growth-promoting bacteria for use in agriculture. Biotechnol Adv. 16, 729–770. doi: 10.1016/S0734-9750(98)00003-2
Bashan, Y., and De-Bashan, L. (2005). Encyclopedia of Soils in the Environment. New York, NY: Elsevier.
Bashan, Y., de-Bashan, L. E., Prabhu, S., and Hernandez, J.-P. (2014). Advances in plant growth-promoting bacterial inoculant technology: formulations and practical perspectives (1998–2013). Plant Soil. 378, 1–33. doi: 10.1007/s11104-013-1956-x
Batool, F., Rehman, Y., and Hasnain, S. (2016). Phylloplane associated plant bacteria of commercially superior wheat varieties exhibit superior plant growth promoting abilities. Front. Life Sci. 9, 313–322. doi: 10.1080/21553769.2016.1256842
Beattie, G. A. (2007). Plant-associated bacteria: survey, molecular phylogeny, genomics and recent advances. Plant-Assoc. Bacteria 17, 1–56. doi: 10.1007/978-1-4020-4538-7_1
Belimov, A., Hontzeas, N., Safronova, V., Demchinskaya, S., Piluzza, G., Bullitta, S., et al. (2005). Cadmium-tolerant plant growth-promoting bacteria associated with the roots of Indian mustard (Brassica juncea L. Czern.) Soil Biol. Biochemistr. 37, 241–250. doi: 10.1016/j.soilbio.2004.07.033
Bernardes, M. F. F., Pazin, M., Pereira, L. C., and Dorta, D. J. (2015). Impact of pesticides on environmental and human health. Toxicol. Studies-cell Drugs Environ. 2015:195–233. doi: 10.5772/59710
Bharti, N., Pandey, S. S., Barnawal, D., Patel, V. K., and Kalra, A. (2016). Plant growth promoting rhizobacteria Dietzia natronolimnaea modulates the expression of stress responsive genes providing protection of wheat from salinity stress. Scientific reports. 6, 1–16. doi: 10.1038/srep34768
Bhattacharyya, P. N., and Jha, D. K. (2012). Plant growth-promoting rhizobacteria (PGPR): emergence in agriculture. World J. Microbiol. Biotechnol. 28, 1327–1350. doi: 10.1007/s11274-011-0979-9
Borah, A., and Thakur, D. (2020). Phylogenetic and functional characterization of culturable endophytic actinobacteria associated with camellia spp. for growth promotion in commercial tea cultivars. Front. Microbiol. 11, 318. doi: 10.3389/fmicb.2020.00318
Br hlmann, F. (1995). Purification and characterization of an extracellular pectate lyase from an Amycolata sp. Appl. Environ. Microbiol. 61, 3580–3585. doi: 10.1128/aem.61.10.3580-3585.1995
Bulgarelli, D., Schlaeppi, K., Spaepen, S., Van Themaat, E. V. L., and Schulze-Lefert, P. (2013). Structure and functions of the bacterial microbiota of plants. Ann. Rev. Plant Biol. 64, 807–838. doi: 10.1146/annurev-arplant-050312-120106
Burg, R. W., Miller, B. M., Baker, E. E., Birnbaum, J., Currie, S. A., Hartman, R., et al. (1979). Avermectins, new family of potent anthelmintic agents: producing organism and fermentation. Antimicrob. Agents Chemother. 15, 361–367. doi: 10.1128/AAC.15.3.361
Cardinale, M., Ratering, S., Suarez, C., Montoya, A. M. Z., Geissler-Plaum, R., and Schnell, S. (2015). Paradox of plant growth promotion potential of rhizobacteria and their actual promotion effect on growth of barley (Hordeum vulgare L.) under salt stress. Microbiol. Res. 181, 22–32. doi: 10.1016/j.micres.2015.08.002
Cellini, A., Spinelli, F., Donati, I., Ryu, C.-M., and Kloepper, J. W. (2021). Bacterial volatile compound-based tools for crop management and quality. Trends Plant Sci. 21, 528–569. doi: 10.1016/j.tplants.2021.05.006
Chang, C.-H., and Yang, S.-S. (2009). Thermo-tolerant phosphate-solubilizing microbes for multi-functional biofertilizer preparation. Bioresour. Technol. 100, 1648–1658. doi: 10.1016/j.biortech.2008.09.009
Chatterjee, S., Sau, G. B., and Mukherjee, S. K. (2009). Plant growth promotion by a hexavalent chromium reducing bacterial strain, Cellulosimicrobium cellulans KUCr3. World J. Microbiol. Biotechnol. 25, 1829–1836. doi: 10.1007/s11274-009-0084-5
Chen, J., and Xie, J. (2011). Role and regulation of bacterial LuxR-like regulators. J. Cellul. Biochemistr. 112, 2694–2702. doi: 10.1002/jcb.23219
Chinakwe, E., Nwogwugwu, N., Ibekwe, V., Chinakwe, P., Egbadon, E., and Adeleye, S. (2019). Isolation and Evaluation of Bacteria Exhibiting Multiple Plant Growth Traits in the Rhizosphere of Yellow Bell Pepper (Capsicum chinense). J. Adv. Microbiol. 2019, 1–6. doi: 10.9734/jamb/2019/v14i430070
Chukwuneme, C. F., Babalola, O. O., Kutu, F. R., and Ojuederie, O. B. (2020). Characterization of actinomycetes isolates for plant growth promoting traits and their effects on drought tolerance in maize. J. Plant Interact. 15, 93–105. doi: 10.1080/17429145.2020.1752833
Conn, V., Walker, A., and Franco, C. (2008). Endophytic actinobacteria induce defense pathways in Arabidopsis thaliana. Mol. Plant-Microbe Interact. 21, 208–218. doi: 10.1094/MPMI-21-2-0208
Coombs, J. T., and Franco, C. M. (2003). Isolation and identification of actinobacteria from surface-sterilized wheat roots. Appl. Environ. Microbiol. 69, 5603–5608. doi: 10.1128/AEM.69.9.5603-5608.2003
Cooper, R., Truumees, I., Gunnarsson, I., Loebenberg, D., Horan, A., Marquez, J., et al. (1992). Sch 42137, a novel antifungal antibiotic from an Actinoplanes sp. Fermentation, isolation, structure and biological properties. J. Antibiotics. 45, 444–453. doi: 10.7164/antibiotics.45.444
Dalal, J., and Kulkarni, N. (2014). Antagonistic and plant growth promoting potentials of indigenous endophytic actinomycetes of soybean (Glycine max (L) Merril). CIBTech J. Microbiol. 3, 1–12.
Dastager, S. G., Deepa, C., and Pandey, A. (2010). Isolation and characterization of novel plant growth promoting Micrococcus sp NII-0909 and its interaction with cowpea. Plant Physiol. Biochemistr. 48, 987–992. doi: 10.1016/j.plaphy.2010.09.006
Davies, P. J. (2004). Plant hormones: Biosynthesis, Signal Transduction, action!: New York, NY: Springer Science and Business Media;.
de Jesus Santos, A. F., Moreira, Z. P. M., de Souza, J. T., de Oliveira, L. M., Barbosa, H. R., de Souza Silva, E., et al. (2019). Culturable diazotrophic bacterial community associated with Agave sisalana P. plants from semi-arid regions in Brazil. Revista Brasileira de Ciências Agrárias. 14, 1–10. doi: 10.5039/agraria.v14i3a5666
de Reijke, T. M., De Boer, E., Schamhart, D., and Kurth, K. (1997). Immunostimulation in the urinary bladder by local application of Nocardia rubra cell wall skeleton preparation (Rubratin) for superficial bladder cancer immunotherapy—a phase I/II study. Urological Res. 25, 117–120. doi: 10.1007/BF01037926
Denton, B. (2007). Advances in phytoremediation of heavy metals using plant growth promoting bacteria and fungi. MMG 445 Basic Biotechnol. 3, 1–5.
Devi, T. S., Vijay, K., Vidhyavathi, R., Kumar, P., Govarthanan, M., and Kavitha, T. (2021). Antifungal activity and molecular docking of phenol, 2, 4-bis (1, 1-dimethylethyl) produced by plant growth-promoting actinobacterium Kutzneria sp. strain TSII from mangrove sediments. Archiv. Microbiol. 2021, 1–14. doi: 10.21203/rs.3.rs-291369/v1
Diehdhiou, A. G., Arora, N. K., Tawfeeq Al-Ani, L. K., Ngom, M., Fall, S., Hafidi, M., et al. (2021). Potential role and utilization of plant growth promoting microbes in plant tissue culture. Front. Microbiol. 12, 615. doi: 10.3389/fmicb.2021.649878
Dif, G., Belaouni, H. A., Goudjal, Y., Yekkour, A., Djemouai, N., and Zitouni, A. (2021). Potential for plant growth promotion of Kocuria arsenatis Strain ST19 on tomato under salt stress conditions. South Afric. J. Bot. 138, 94–104. doi: 10.1016/j.sajb.2020.12.014
Dong, Y.-H., and Zhang, L.-H. (2005). Quorum sensing and quorum-quenching enzymes. J. Microbiol. 43, 101–109.
dos Reis Antunes, G., Santana, S. R. A., Escobar, I. E. C., da Silva Brasil, M., de Araújo, G. G. L., Voltolini, T. V., et al. (2019). Associative diazotrophic bacteria from forage grasses in the Brazilian semi-arid region are effective plant growth promoters. Crop Pasture Sci. 70, 899–907. doi: 10.1071/CP19076
Dowling, D. N., and O'Gara, F. (1994). Metabolites of Pseudomonas involved in the biocontrol of plant disease. Trends Biotechnol. 12, 133–141. doi: 10.1016/0167-7799(94)90091-4
Duncan, T. R., Werner-Washburne, M., and Northup, D. E. (2021). Diversity of siderophore-producing bacterial cultures from carlsbad caverns national park caves, carlsbad, New Mexico. J. Cave Karst Stud. 83, 1. doi: 10.4311/2019ES0118
Edwards, C. (1993). Isolation properties and potential applications of thermophilic actinomycetes. Appl. Biochemistr. Biotechnol. 42, 161–179. doi: 10.1007/BF02788050
Egamberdieva, D. (2012). Colonization of Mycobacterium phlei in the rhizosphere of wheat grown under saline conditions. Turkish J. Biol. 36, 487–492. doi: 10.3906/biy-1012-4
Elliott, L., and Lynch, J. (1995). The international workshop on establishment of microbial inocula in soils: cooperative research project on biological resource management of the Organization for Economic Cooperation and Development (OECD). Am. J. Alternat. Agricult. 10, 50–73. doi: 10.1017/S0889189300006160
Elliott, M., Shamoun, S., Sumampong, G., James, D., Masri, S., and Varga, A. (2009). Evaluation of several commercial biocontrol products on European and North American populations of Phytophthora ramorum. Biocontrol Sci. Technol. 19, 1007–1021. doi: 10.1080/09583150903243870
El-Tarabily, K., Nassar, A., Hardy, G. S. J., and Sivasithamparam, K. (2009). Plant growth promotion and biological control of Pythium aphanidermatum, a pathogen of cucumber, by endophytic actinomycetes. J. Appl. Microbiol. 106, 13–26. doi: 10.1111/j.1365-2672.2008.03926.x
El-Tarabily, K. A., AlKhajeh, A. S., Ayyash, M. M., Alnuaimi, L. H., Sham, A., ElBaghdady, K. Z., et al. (2019). Growth promotion of Salicornia bigelovii by Micromonospora chalcea UAE1, an endophytic 1-aminocyclopropane-1-carboxylic acid deaminase-producing actinobacterial isolate. Front. Microbiol. 10, 1694. doi: 10.3389/fmicb.2019.01694
El-Tarabily, K. A., Nassar, A. H., and Sivasithamparam, K. (2008). Promotion of growth of bean (Phaseolus vulgaris L.) in a calcareous soil by a phosphate-solubilizing, rhizosphere-competent isolate of Micromonospora endolithica. Appl. Soil Ecol. 39, 161–171. doi: 10.1016/j.apsoil.2007.12.005
El-Tarabily, K. A., and Sivasithamparam, K. (2006). Non-streptomycete actinomycetes as biocontrol agents of soil-borne fungal plant pathogens and as plant growth promoters. Soil Biol. Biochemistr. 38, 1505–1520. doi: 10.1016/j.soilbio.2005.12.017
El-Tarabily, K. A., Soliman, M. H., Nassar, A. H., Al-Hassani, H. A., Sivasithamparam, K., McKenna, F., et al. (2000). Biological control of Sclerotinia minor using a chitinolytic bacterium and actinomycetes. Plant Pathol. 49, 573–583. doi: 10.1046/j.1365-3059.2000.00494.x
El-Tarabily, K. A., Sykes, M. L., Kurtböke, I. D., Hardy, G. E. S. J., Barbosa, A. M., and Dekker, R. F. (1996). Synergistic effects of a cellulase-producing Micromonospora carbonacea and an antibiotic-producing Streptomyces violascens on the suppression of Phytophthora cinnamomi root rot of Banksia grandis. Canad. J. Bot. 74, 618–624. doi: 10.1139/b96-078
Etzkowitz, H., and Zhou, C. (2017). The Triple Helix: University–industry–government innovation and Entrepreneurship. London: Routledge.
Evangelista, E. V., Garcia, F. C., and Cruz, J. A. (2017). Isolation, characterization and identification of plant growth-promoting rhizobacteria. Int J Agric Technol. 13, 715–727.
Farmer, P., and Suhadolnik, R. (1972). Nucleoside antibiotics biosynthesis of arabonofuranosyladenine by Streptomyces antibioticus. Biochemistry. 11, 911–916. doi: 10.1021/bi00755a034
Feng, K., Lu, H.-M., Sheng, H.-J., Wang, X.-L., and Mao, J. (2004). Effect of organic ligands on biological availability of inorganic phosphorus in soils. Pedosphere. 14, 85–92.
Ferrando, L., Mañay, J. F., and Scavino, A. F. (2012). Molecular and culture-dependent analyses revealed similarities in the endophytic bacterial community composition of leaves from three rice (Oryza sativa) varieties. FEMS Microbiol. Ecol. 80, 696–708. doi: 10.1111/j.1574-6941.2012.01339.x
Fidalgo, C. I. A. (2017). Endophytic Bacterial Communities of Halimione portulacoides. Portugal Universidade de Aveiro.
Flagan, S., Ching, W.-K., and Leadbetter, J. R. (2003). Arthrobacter strain VAI-A utilizes acyl-homoserine lactone inactivation products and stimulates quorum signal biodegradation by Variovorax paradoxus. Appl. Environ. Microbiol. 69, 909–916. doi: 10.1128/AEM.69.2.909-916.2003
Franche, C., Lindström, K., and Elmerich, C. (2009). Nitrogen-fixing bacteria associated with leguminous and non-leguminous plants. Plant Soil. 321, 35–59. doi: 10.1007/s11104-008-9833-8
Franco-Correa, M., and Chavarro-Anzola, V. (2016). “Actinobacteria as plant growth-promoting rhizobacteria,” in Actinobacteria: Basics and Biotechnological Applications BoD–Books on Demand., ed Dhanasekaran, Y, p. 131.
Franco-Correa, M., Quintana, A., Duque, C., Suarez, C., Rodríguez, M. X., and Barea, J.-M. (2010). Evaluation of actinomycete strains for key traits related with plant growth promotion and mycorrhiza helping activities. Appl. Soil Ecol. 45, 209–217. doi: 10.1016/j.apsoil.2010.04.007
Fravel, D. R. (1988). Role of antibiosis in the biocontrol of plant diseases. Ann. Rev. Phytopathol. 26, 75–91. doi: 10.1146/annurev.py.26.090188.000451
Frey-Klett, P., Garbaye, J., and Tarkka, M. (2007). The mycorrhiza helper bacteria revisited. New Phytol. 176, 22–36. doi: 10.1111/j.1469-8137.2007.02191.x
Fróes, A., Macrae, A., Rosa, J., Franco, M., Souza, R., Soares, R., et al. (2012). Selection of a Streptomyces strain able to produce cell wall degrading enzymes and active against Sclerotinia sclerotiorum. J. Microbiol. 50, 798–806. doi: 10.1007/s12275-012-2060-2
Fuqua, W. C., Winans, S. C., and Greenberg, E. P. (1994). Quorum sensing in bacteria: the LuxR-LuxI family of cell density-responsive transcriptional regulators. J. Bacteriol. 176, 269–275. doi: 10.1128/jb.176.2.269-275.1994
Gangwar, M., Rani, S., and Sharma, N. (2012a). Diversity of endophytic Actinomycetes from wheat and its potential as plant growth promoting and biocontrol agents. J. Adv. Lab. Res. Biol. 3, 13–19.
Gangwar, M., Rani, S., and Sharma, N. (2012b). Investigating endophytic actinomycetes diversity from rice for plant growth promoting and antifungal activity. Int. J. Adv. Life Sci. 1, 12.
Gao, B., and Gupta, R. S. (2012). Phylogenetic framework and molecular signatures for the main clades of the phylum Actinobacteria. Microbiol. Mol. Biol. Rev. 76, 66–112. doi: 10.1128/MMBR.05011-11
Geetanjali, J. P. (2016). Antibiotic production by rhizospheric soil microflora-a review. Int. J. Pharmaceuti. Sci. Res. 7, 4304–4314.
Ghiasian, M. (2020). Endophytic microbiomes: biodiversity, current status, and potential agricultural applications. Adv. Plant Microbiome Sustainable Agricult. 20, 61–82. doi: 10.1007/978-981-15-3208-5_3
Girija, D., Rajeevan, P., Balakrishnan, S., Panchami, P., and Mohan, M. (2018). 16S rRNA gene taxonomic profiling of endophytic bacteria associated with phylaenopsis roots. J. Horticult. Sci. 13, 103–107. doi: 10.24154/JHS.2018.v13i01.012
Glick, B. R. (2005). Modulation of plant ethylene levels by the bacterial enzyme ACC deaminase. FEMS Microbiol. Lett. 251, 1–7. doi: 10.1016/j.femsle.2005.07.030
Glick, B. R. (2012). Plant growth-promoting bacteria: mechanisms and applications. Scientifica. 1:401. doi: 10.6064/2012/963401
Gomes, R., Semedo, L., Soares, R., Alviano, C., And, L. L., and Coelho, R. (2000). Chitinolytic activity of actinomycetes from a cerrado soil and their potential in biocontrol. Lett. Appl. Microbiol. 30, 146–150. doi: 10.1046/j.1472-765x.2000.00687.x
Gong, Y., Bai, J.-L., Yang, H.-T., Zhang, W.-D., Xiong, Y.-W., Ding, P., et al. (2018). Phylogenetic diversity and investigation of plant growth-promoting traits of actinobacteria in coastal salt marsh plant rhizospheres from Jiangsu, China. Systemat. Appl. Microbiol. 41, 516–527. doi: 10.1016/j.syapm.2018.06.003
Gontia, I., Kavita, K., Schmid, M., Hartmann, A., and Jha, B. (2011). Brachybacterium saurashtrense sp. nov., a halotolerant root-associated bacterium with plant growth-promoting potential. Int. J. Systemat. Evol. Microbiol. 61, 2799–2804. doi: 10.1099/ijs.0.023176-0
Gopalakrishnan, S., Srinivas, V., Vidya, M. S., and Rathore, A. (2013). Plant growth-promoting activities of Streptomyces spp. in sorghum and rice. SpringerPlus. 2, 1–8. doi: 10.1186/2193-1801-2-574
Goudjal, Y., Toumatia, O., Sabaou, N., Barakate, M., Mathieu, F., and Zitouni, A. (2013). Endophytic actinomycetes from spontaneous plants of Algerian Sahara: indole-3-acetic acid production and tomato plants growth promoting activity. World J. Microbiol. Biotechnol. 29, 1821–1829. doi: 10.1007/s11274-013-1344-y
Grandclément, C., Tannières, M., Moréra, S., Dessaux, Y., and Faure, D. (2016). Quorum quenching: role in nature and applied developments. FEMS Microbiol. Rev. 40, 86–116. doi: 10.1093/femsre/fuv038
Gregor, A., Klubek, B., and Varsa, E. (2003). Identification and use of actinomycetes for enhanced nodulation of soybean co-inoculated with Bradyrhizobium japonicum. Canadian J. Microbiol. 49, 483–491. doi: 10.1139/w03-061
Gu, S., Wei, Z., Shao, Z., Friman, V.-P., Cao, K., Yang, T., et al. (2020). Competition for iron drives phytopathogen control by natural rhizosphere microbiomes. Nat. Microbiol. 5, 1002–1010. doi: 10.1038/s41564-020-0719-8
Guiñaz,ú, L. B, Andrés, J. A., Rovera, M., Balzarini, M., and Rosas, S. B. (2013). Evaluation of rhizobacterial isolates from Argentina, Uruguay and Chile for plant growth-promoting characteristics and antagonistic activity towards Rhizoctonia sp. and Macrophomina sp. in vitro. Euro. J. Soil Biol. 54, 69–77. doi: 10.1016/j.ejsobi.2012.09.007
Gupta, R. S., Chen, W. J., Adeolu, M., and Chai, Y. (2013). Molecular signatures for the class Coriobacteriia and its different clades; proposal for division of the class Coriobacteriia into the emended order Coriobacteriales, containing the emended family Coriobacteriaceae and Atopobiaceae fam. nov., and Eggerthellales ord. nov., containing the family Eggerthellaceae fam. nov. Int. J. Systemat. Evol. Microbiol. 63, 3379–97. doi: 10.1099/ijs.0.048371-0
Gusain, P., Paliwal, R., and Singh, V. (2017). Rhizoremediation of cadmium-contaminated soil associated with hydroxamate siderophores isolated from Cd-resistant plant growth–promoting Dietzia maris and Lysinibacillus strains. Int. J. Phytoremediat. 19, 290–299. doi: 10.1080/15226514.2016.1225281
Halder, A., Mishra, A., and Chakrabartty, P. (1991). Solubilization of inorganic phosphates by Bradyrhizobium. Indian J. Experiment. Biol. 29, 28–31.
Hasegawa, S., Meguro, A., Shimizu, M., Nishimura, T., and Kunoh, H. (2006). Endophytic actinomycetes and their interactions with host plants. Actinomycetologica. 20, 72–81. doi: 10.3209/saj.20.72
Haygarth, P., Bardgett, R., and Condron, L. (2013). “Phosphorus and nitrogen cycles and their management,” in Russell's Soil Conditions and Plant Growth, 12th ed Wiley-Blackwell, Hoboken, New Jersey, United States. pp. 132–158.
Hedden, P. (1997). The oxidases of gibberellin biosynthesis: their function and mechanism. Physiologia Plantarum. 101, 709–719. doi: 10.1111/j.1399-3054.1997.tb01055.x
Hong, S. H., Ryu, H., Kim, J., and Cho, K.-S. (2011). Rhizoremediation of diesel-contaminated soil using the plant growth-promoting rhizobacterium Gordonia sp. S2RP-17. Biodegradation. 22, 593-601. doi: 10.1007/s10532-010-9432-2
Hoshino, Y., Mukai, A., Yazawa, K., Uno, J., Ishikawa, J., Ando, A., et al. (2004). Transvalencin A, a thiazolidine zinc complex antibiotic produced by a clinical isolate of Nocardia transvalensis. J. Antibiotics. 57, 797–802. doi: 10.7164/antibiotics.57.797
Hrynkiewicz, K., Patz, S., and Ruppel, S. (2019). Salicornia europaea L. as an underutilized saline-tolerant plant inhabited by endophytic diazotrophs. J. Adv. Res. 19, 49–56. doi: 10.1016/j.jare.2019.05.002
Huo, Y., Kang, J. P., Ahn, J. C., Kim, Y. J., Piao, C. H., Yang, D. U., et al. (2021). Siderophore-producing rhizobacteria reduce heavy metal-induced oxidative stress in Panax ginseng Meyer. J. Ginseng Res. 45, 218–227. doi: 10.1016/j.jgr.2019.12.008
Igarashi, M., Kinoshita, N., Ikeda, T., Nakagawa, E., Hamada, M., and Takeuchi, T. (1997). Formamicin, a novel antifungal antibiotic produced by a strain of saccharothvix sp. I. taxonomy, production, isolation and biological properties. J. Antibiotics. 50, 926–931. doi: 10.7164/antibiotics.50.926
Igarashi, Y., Trujillo, M. E., Martínez-Molina, E., Yanase, S., Miyanaga, S., Obata, T., et al. (2007). Antitumor anthraquinones from an endophytic actinomycete Micromonospora lupini sp. nov. Bioorganic Med. Chemistr. Lett. 17, 3702–3705. doi: 10.1016/j.bmcl.2007.04.039
Ikemoto, T., Katayama, T., Shiraishi, A., and Haneishi, T. (1983). Aculeximycin, a new antibiotic from streptosporangium albidum ii. Isolation, physicochemical and biological properties. J. Antibiotics. 36, 1097–1100. doi: 10.7164/antibiotics.36.1097
Ilic, S., Konstantinovic, S., Todorovic, Z., Lazic, M., Veljkovic, V., Jokovic, N., et al. (2007). Characterization and antimicrobial activity of the bioactive metabolites in streptomycete isolates. Microbiology. 76, 421–428. doi: 10.1134/S0026261707040066
Jackson, M. (1993). Are plant hormones involved in root to shoot communication? Adv. Bot. Res. 19, 103–187. doi: 10.1016/S0065-2296(08)60204-9
Jain, R., Bhardwaj, P., Pandey, S. S., and Kumar, S. (2021). Arnebia euchroma, a plant species of cold desert in the Himalayas, harbors beneficial cultivable endophytes in roots and leaves. Front. Microbiol. 21, 12. doi: 10.3389/fmicb.2021.696667
Jog, R., Nareshkumar, G., and Rajkumar, S. (2012). Plant growth promoting potential and soil enzyme production of the most abundant S treptomyces spp. from wheat rhizosphere. J. Appl. Microbiol. 113, 1154–1164. doi: 10.1111/j.1365-2672.2012.05417.x
Jog, R., Nareshkumar, G., and Rajkumar, S. (2016). Enhancing soil health and plant growth promotion by actinomycetes. Plant Growth Promot. actInobacteria. 16, 33–45. doi: 10.1007/978-981-10-0707-1_3
Kang, S.-M., Khan, A. L., Hamayun, M., Hussain, J., Joo, G.-J., You, Y.-H., et al. (2012). Gibberellin-producing Promicromonospora sp. SE188 improves Solanum lycopersicum plant growth and influences endogenous plant hormones. J Microbiol. 50, 902–9. doi: 10.1007/s12275-012-2273-4
Kang, S.-M., Khan, A. L., You, Y.-H., Kim, J.-G., Kamran, M., and Lee, I.-J. (2014). Gibberellin production by newly isolated strain Leifsonia soli SE134 and its potential to promote plant growth. J. Microbiol. Biotechnol. 24, 106–112. doi: 10.4014/jmb.1304.04015
Karlidag, H., Esitken, A., Turan, M., and Sahin, F. (2007). Effects of root inoculation of plant growth promoting rhizobacteria (PGPR) on yield, growth and nutrient element contents of leaves of apple. Scientia Horticulturae. 114, 16–20. doi: 10.1016/j.scienta.2007.04.013
Karmakar, J., Goswami, S., Pramanik, K., Maiti, T. K., Kar, R. K., and Dey, N. (2021). Growth promoting properties of Mycobacterium and Bacillus on rice plants under induced drought. Plant Sci. Today. 8, 49–57. doi: 10.14719/pst.2021.8.1.965
Karthik, C., and Arulselvi, P. I. (2017). Biotoxic effect of chromium (VI) on plant growth-promoting traits of novel Cellulosimicrobium funkei strain AR8 isolated from Phaseolus vulgaris rhizosphere. Geomicrobiol. J. 34, 434–442. doi: 10.1080/01490451.2016.1219429
Karthikeyan, M. (2017). Rhodococcus globerulus colonizing the Medicinal Plant Plectranthus. Int J Cur Res Rev| Vol. 9, 7.
Kaszubiak, H. (1998). Mutual antagonism between fluorescent pseudomonads and soil actinomycetes. Polish J. Environ. Stud. 7, 207–212.
Katznelson, H., Sirois, J., and Cole, S. E. (1962). Production of a gibberellin-like substance by Arthrobacter globiformis. Nature. 196, 1012–1013. doi: 10.1038/1961012b0
Kaunat, H. (1969). Bildung von Indolderivaten durch rhizospharenspezifische Bakterien und Aktinomyzeten. Zentralb Bakteriol Parasitenk Infektionskr Hyg Abt I Orig.
Kaur, T., Sharma, D., Kaur, A., and Manhas, R. K. (2013). Antagonistic and plant growth promoting activities of endophytic and soil actinomycetes. Archiv. Phytopathol. Plant Protect. 46, 1756–1768. doi: 10.1080/03235408.2013.777169
Kayasth, M., Kumar, V., and Gera, R. (2014). Gordonia sp.: a salt tolerant bacterial inoculant for growth promotion of pearl millet under saline soil conditions. Biotech. 4, 553–557. doi: 10.1007/s13205-013-0178-5
Kharshandi, F., Khyllep, A., and Kayang, H. (2021). Plant Growth-Promoting Rhizobacteria of Curcuma longa L. and Their Impact on its Growth. Proceedings of the National Academy of Sciences, India Section B. Biologic. Sci. 91, 769–776. doi: 10.1007/s40011-021-01268-5
Kim, D.-U., Kim, S.-G., Lee, H., Park, A.-Y., and Ka, J.-O. (2017). Oryzihumus soli sp. nov., isolated from soil and emended description of the genus Oryzihumus. Int. J. Systemat. Evol. Microbiol. 67, 3960–3964. doi: 10.1099/ijsem.0.002231
Kloepper, J. W. (1978). “Plant growth-promoting rhizobacteria on radishes,” in Proc of the 4th Internet Conf on Plant Pathogenic Bacter, Station de Pathologie Vegetale et Phytobacteriologie, Angers: INRA.
Kpomblekou-a, K., and Tabatabai, M. (1994). Effect of organic acids on release of phosphorus from phosphate rocks1. Soil Sci. 158, 442–453. doi: 10.1097/00010694-199415860-00006
Kuffner, M., Puschenreiter, M., Wieshammer, G., Gorfer, M., and Sessitsch, A. (2008). Rhizosphere bacteria affect growth and metal uptake of heavy metal accumulating willows. Plant Soil. 304, 35–44. doi: 10.1007/s11104-007-9517-9
Kumar, A., and Singh, J. (2020). Biofilms Forming Microbes: Diversity and Potential Application. Plant Microbiomes Sustain. Agricult. 25, 173. doi: 10.1007/978-3-030-38453-1_6
Lacey, J. (1978). “Ecology of actinomycetes in fodders and related substrates,” in Zentralblatt fur Bakteriologie, Parasitenkunde, Infektionskrankheiten und Hygiene.
Latour, X., Barbey, C., Chane, A., Groboillot, A., and Burini, J.-F. (2013). Rhodococcus erythropolis and its γ-lactone catabolic pathway: an unusual biocontrol system that disrupts pathogen quorum sensing communication. Agronomy. 3, 816–838. doi: 10.3390/agronomy3040816
Lee, S. Y., Tindwa, H., Lee, Y. S., Naing, K. W., Hong, S. H., Nam, Y., et al. (2012). Biocontrol of Anthracnose in Pepper Using Chitinase, $(Fróes) $-1, 3 Glucanase, and 2-Furancarboxaldehyde Produced by Streptomyces cavourensis SY224. J. Microbiol. Biotechnol. 22, 1359–1366. doi: 10.4014/jmb.1203.02056
Liotti, R. G., da Silva Figueiredo, M. I., da Silva, G. F., de Mendonça, E. A. F., and Soares, M. A. (2018). Diversity of cultivable bacterial endophytes in Paullinia cupana and their potential for plant growth promotion and phytopathogen control. Microbiol. Res. 207, 8–18. doi: 10.1016/j.micres.2017.10.011
Liu, Y.-H., Wei, Y.-Y., Mohamad, O. A. A., Salam, N., Zhang, Y.-g, Guo, J.-W., et al. (2019). Diversity, community distribution and growth promotion activities of endophytes associated with halophyte Lycium ruthenicum Murr. Biotech. 9, 1–12. doi: 10.1007/s13205-019-1678-8
Lopez-Velasco, G., Carder, P. A., Welbaum, G. E., and Ponder, M. A. (2013). Diversity of the spinach (Spinacia oleracea) spermosphere and phyllosphere bacterial communities. FEMS Microbiol. Lett. 346, 146–154. doi: 10.1111/1574-6968.12216
Ludwig, W., Euzéby, J., Schumann, P., Busse, H.-J., Trujillo, M. E., Kämpfer, P., et al. (2012). “Road map of the phylum Actinobacteria,” in Bergey's manual® of systematic bacteriology New York, NY: Springer; p. 1–28.
Lugtenberg, B. J., Chin-A-Woeng, T. F., and Bloemberg, G. V. (2002). Microbe–plant interactions: principles and mechanisms. Antonie Van Leeuwenhoek. 81, 373–383. doi: 10.1023/A:1020596903142
Machado, P. C., Andrade, P. H. M., de Sousa, C. P., de Souza, C. W. O., and Lacava, P. T. (2020). In vitro characterization of endophytic bacteria associated with physic nut (Jatropha curcas L.) and their potential for plant-growth promotion and biocontrol. Brazilian J. Develop. 6, 88572–88589. doi: 10.34117/bjdv6n11-326
Madhaiyan, M., Poonguzhali, S., Lee, J.-S., Lee, K.-C., Saravanan, V. S., and Santhanakrishnan, P. (2010). Microbacterium azadirachtae sp. nov., a plant-growth-promoting actinobacterium isolated from the rhizoplane of neem seedlings. Int. J. Systemat. Evol. Microbiol. 60, 1687–1692. doi: 10.1099/ijs.0.015800-0
Maiese, W., Korshalla, J., Goodman, J., Torrey, M., Kantor, S., Labeda, D., et al. (1990). Simaomicin (LL-D42067), a novel antibiotic from Actinomadura madurae. J. Antibiotics. 43, 1059–1063. doi: 10.7164/antibiotics.43.1059
Marasco, R., Fusi, M., Rolli, E., Ettoumi, B., Tambone, F., Borin, S., et al. (2021). Aridity modulates belowground bacterial community dynamics in olive tree. Environ. Microbiol. 23, 6275–6291. doi: 10.1111/1462-2920.15764
Marín, M., Wong, I., Mena, J., Morán, R., Pimentel, E., Sánchez, I., et al. (2013). Zea mays L. plant growth promotion by Tsukamurella paurometabola strain C-924. Biotecnol Apl. 30, 105-10.
Martínez-Hidalgo, P., García, J. M., and Pozo, M. J. (2015). Induced systemic resistance against Botrytis cinerea by Micromonospora strains isolated from root nodules. Front. Microbiol. 6, 922. doi: 10.3389/fmicb.2015.00922
Masmoudi, F., Abdelmalek, N., Tounsi, S., Dunlap, C. A., and Trigui, M. (2019). Abiotic stress resistance, plant growth promotion and antifungal potential of halotolerant bacteria from a Tunisian solar saltern. Microbiol. Res. 229, 126331. doi: 10.1016/j.micres.2019.126331
Merzaeva, O., and Shirokikh, I. (2006). Colonization of plant rhizosphere by actinomycetes of different genera. Microbiology. 75, 226–230. doi: 10.1134/S0026261706020184
Mulani, R., Mehta, K., Saraf, M., and Goswami, D. (2021). Decoding the mojo of plant-growth-promoting microbiomes. Physiolo. Mol. Plant Pathol. 115 <101687. doi: 10.1016/j.pmpp.2021.101687
Nafis, A., Raklami, A., Bechtaoui, N., El Khalloufi, F., El Alaoui, A., Glick, B. R., et al. (2019). Actinobacteria from extreme niches in morocco and their plant growth-promoting potentials. Diversity. 11, 139. doi: 10.3390/d11080139
Nair, M. G., Mishra, S. K., Putnam, A. R., and Pahdey, R. C. (1992). Antifungal anthracycline antibiotics, spartanamicins A and B from Micromonospora spp. J. Antibiotics. 45, 1738–1745. doi: 10.7164/antibiotics.45.1738
Nascimento, F. X., Rossi, M. J., Soares, C. R., McConkey, B. J., and Glick, B. R. (2014). New insights into 1-aminocyclopropane-1-carboxylate (ACC) deaminase phylogeny, evolution and ecological significance. PloS One. 9, 6. doi: 10.1371/journal.pone.0099168
Neilands, J. (1995). Siderophores: structure and function of microbial iron transport compounds. J. Biolo. Chemistr. 270, 26723–26726. doi: 10.1074/jbc.270.45.26723
Nimaichand, S., Devi, A. M., and Li, W.-J. (2016). “Direct plant growth-promoting ability of actinobacteria in grain legumes,” in Plant Growth Promoting Actinobacteria. New York, NY: Springer; p. 1–16.
Nimnoi, P., Pongsilp, N., and Lumyong, S. (2010). Endophytic actinomycetes isolated from Aquilaria crassna Pierre ex Lec and screening of plant growth promoters production. World J. Microbiol. Biotechnol. 26, 193–203. doi: 10.1007/s11274-009-0159-3
Noh, H.-J., Park, Y., Hong, S. G., and Lee, Y. M. (2021). Diversity and physiological characteristics of Antarctic lichens-associated bacteria. Microorganisms. 9, 607. doi: 10.3390/microorganisms9030607
Normand, P., Queiroux, C., Tisa, L. S., Benson, D. R., Rouy, Z., Cruveiller, S., et al. (2007). Exploring the genomes of Frankia. Physiologia Plantarum. 130, 331–343. doi: 10.1111/j.1399-3054.2007.00918.x
Norris, P. R. (2012). Class II. Acidimicrobiia class. nov. Bergey's Manual of Systematic Bacteriology. 1968.
Nouioui, I., Carro, L., García-López, M., Meier-Kolthoff, J. P., Woyke, T., Kyrpides, N. C., et al. (2018). Genome-based taxonomic classification of the phylum Actinobacteria. Front. Microbiol. 9, 2007. doi: 10.3389/fmicb.2018.02007
O'Hara, A. M., and Shanahan, F. (2006). The gut flora as a forgotten organ. EMBO Rep. 7, 688–693. doi: 10.1038/sj.embor.7400731
Onofre-Lemus, J., Hernández-Lucas, I., Girard, L., and Caballero-Mellado, J. (2009). ACC (1-aminocyclopropane-1-carboxylate) deaminase activity, a widespread trait in Burkholderia species, and its growth-promoting effect on tomato plants. Appl. Environ. Microbiol. 75, 6581–6590. doi: 10.1128/AEM.01240-09
Ooka, K., Fukumoto, A., Yamanaka, T., Shimada, K., Ishihara, R., Anzai, Y., et al. (2013). Piericidins, novel quorum-sensing inhibitors against Chromobacterium violaceum CV026, from Streptomyces sp. TOHO-Y209 and TOHO-O348. Open J. Med. Chemistr. 2013, 245. doi: 10.4236/ojmc.2013.34012
Padda, K. P., Puri, A., and Chanway, C. P. (2018). Isolation and identification of endophytic diazotrophs from lodgepole pine trees growing at unreclaimed gravel mining pits in central interior British Columbia, Canada. Canad. J. For. Res. 48, 1601–1606. doi: 10.1139/cjfr-2018-0347
Palaniyandi, S. A., Yang, S. H., Zhang, L., and Suh, J.-W. (2013). Effects of actinobacteria on plant disease suppression and growth promotion. Appl. Microbiol. Biotechnol. 97, 9621–9636. doi: 10.1007/s00253-013-5206-1
Panosyan, A., Marshavina, Z., Arutunyan, R., and Aslanyan, S. (1963). The nature of physiologically active substances of actinomycetes and the effect of their metabolites on plant growth. Plant Microbes Relation. 1963, 241–244.
Parte, A. C., Carbasse, J. S., Meier-Kolthoff, J. P., Reimer, L. C., and Göker, M. (2020). List of Prokaryotic names with Standing in Nomenclature (LPSN) moves to the DSMZ. Int. J. Systemat. Evol. Microbiol. 70, 5607. doi: 10.1099/ijsem.0.004332
Patel, D., Patel, A., Vora, D., Menon, S., Vadakan, S., Acharya, D., et al. (2018). A resourceful methodology to profile indolic auxins produced by rhizo-fungi using spectrophotometry and HPTLC. Biotech. 8, 1–13. doi: 10.1007/s13205-018-1428-3
Patel, K. B., and Thakker, J. N. (2019). Growth promotion and biocontrol activity of Nocardiopsis dassonvillei strain YM12: an isolate from coastal agricultural land of Khambhat. Vegetos. 32, 571–582. doi: 10.1007/s42535-019-00064-x
Pérez, M. L., Collavino, M. M., Sansberro, P. A., Mroginski, L. A., and Galdeano, E. (2016). Diversity of endophytic fungal and bacterial communities in Ilex paraguariensis grown under field conditions. World J. Microbiol. Biotechnol. 32, 61. doi: 10.1007/s11274-016-2016-5
Ph?m, H. T. T., Suwannapan, W., Koomsiri, W., Inahashi, Y., Také, A, Matsumoto, A., et al. (2020). Fodinicola acaciae sp. nov., an Endophytic Actinomycete Isolated from the Roots of Acacia mangium Willd. and its genome analysis. Microorganisms. 8, 467. doi: 10.3390/microorganisms8040467
Pieterse, C. M., Van Wees, S. C., Van Pelt, J. A., Knoester, M., Laan, R., Gerrits, H., et al. (1998). A novel signaling pathway controlling induced systemic resistance in Arabidopsis. Plant Cell. 10, 1571–1580. doi: 10.1105/tpc.10.9.1571
Ponmurugan, P., and Gopi, C. In vitro production of growth regulators phosphatase activity by phosphate solubilizing bacteria. Afr. J. Biotechnol. (2006) 5, 348-50.
Poonguzhali, S., Madhaiyan, M., and Sa, T. (2006). Cultivation-dependent characterization of rhizobacterial communities from field grown Chinese cabbage Brassica campestris ssp pekinensis and screening of traits for potential plant growth promotion. Plant and soil. 286, 167–180. doi: 10.1007/s11104-006-9035-1
Qin, S., Bian, G.-K., Zhang, Y.-J., Xing, K., Cao, C.-L., Liu, C.-H., et al. (2013). Modestobacter roseus sp. nov., an endophytic actinomycete isolated from the coastal halophyte Salicornia europaea Linn., and emended description of the genus Modestobacter. Int. J. Systemati. Evol. Microbiol. 63, 2197–202. doi: 10.1099/ijs.0.044412-0
Qin, S., Feng, W.-W., Zhang, Y.-J., Wang, T.-T., Xiong, Y.-W., and Xing, K. (2018). Diversity of bacterial microbiota of coastal halophyte Limonium sinense and amelioration of salinity stress damage by symbiotic plant growth-promoting actinobacterium Glutamicibacter halophytocola KLBMP 5180. Appl. Environ. Microbiol. 84, e01533–e01518. doi: 10.1128/AEM.01533-18
Qin, S., Xing, K., Jiang, J.-H., Xu, L.-H., and Li, W.-J. (2011). Biodiversity, bioactive natural products and biotechnological potential of plant-associated endophytic actinobacteria. Appl. Microbiol. Biotechnol. 89, 457–473. doi: 10.1007/s00253-010-2923-6
Qin, S., Zhang, Y.-J., Yuan, B., Xu, P.-Y., Xing, K., Wang, J., et al. (2014). Isolation of ACC deaminase-producing habitat-adapted symbiotic bacteria associated with halophyte Limonium sinense (Girard) Kuntze and evaluating their plant growth-promoting activity under salt stress. Plant Soil. 374, 753–766. doi: 10.1007/s11104-013-1918-3
Rangseekaew, P., Barros-Rodríguez, A., Pathom-Aree, W., and Manzanera, M. (2021). Deep-sea actinobacteria mitigate salinity stress in tomato seedlings and their biosafety testing. Plants. 8, 1687. doi: 10.3390/plants10081687
Raza, F. A., and Faisal, M. (2013). Growth promotion of maize by desiccation tolerant'Micrococcus luteus'-cp37-chp37 isolated from Cholistan desert, Pakistan. Austr. J. Crop Sci. 7, 1693–1698.
Ren, Z., Tang, S., Jiang, Y., Jiang, M., Zheng, S., Liu, W., et al. (2018). High-throughput sequencing analysis of endophytic bacteria diversity in fruits of white and red pitayas from three different origins. Polish J. Microbiol. 67, 27. doi: 10.5604/01.3001.0011.6139
Research, T. M. (2014). Biofertilizers (Nitrogen Fixing, Phosphate Solubilizing and Others) Market for Seed Treatment and Soil Treatment Applications–Global Industry Analysis, Size, Share, Growth, Trends and Forecast, 2013-2019. New York, NY: Transparency Market Research Albany.
Rijavec, T., and Lapanje, A. (2016). Hydrogen cyanide in the rhizosphere: not suppressing plant pathogens, but rather regulating availability of phosphate. Front. Microbiol. 7, 1785. doi: 10.3389/fmicb.2016.01785
Riva, V., Mapelli, F., Dragonetti, G., Elfahl, M., Vergani, L., Crepaldi, P., et al. (2021). Bacterial inoculants mitigating water scarcity in tomato: the importance of long-term in vivo experiments. Front. Microbiol. 12, 1328. doi: 10.3389/fmicb.2021.675552
Rivas, R., Sanchez, M., Trujillo, M. E., Zurdo-Pineiro, J. L., Mateos, P. F., Martínez-Molina, E., et al. (2003). Xylanimonas cellulosilytica gen. nov., sp. nov., a xylanolytic bacterium isolated from a decayed tree (Ulmus nigra). Int. J. Systemat. Evol. Microbiol. 53, 99–103. doi: 10.1099/ijs.0.02207-0
Rosenberg, E., Sharon, G., and Zilber-Rosenberg, I. (2009). The hologenome theory of evolution contains Lamarckian aspects within a Darwinian framework. Environ. Microbiol. 11, 2959–2962. doi: 10.1111/j.1462-2920.2009.01995.x
Sahay, H., Yadav, A. N., Singh, A. K., Singh, S., Kaushik, R., and Saxena, A. K. (2017). Hot springs of Indian Himalayas: potential sources of microbial diversity and thermostable hydrolytic enzymes. Biotech. 7, 1–11. doi: 10.1007/s13205-017-0762-1
Salwan, R., and Sharma, V. (2020). Molecular and biotechnological aspects of secondary metabolites in actinobacteria. Microbiol. Res. 231, 126374. doi: 10.1016/j.micres.2019.126374
Samad, A., Trognitz, F., Compant, S., Antonielli, L., and Sessitsch, A. (2017). Shared and host-specific microbiome diversity and functioning of grapevine and accompanying weed plants. Environ. Microbiol. 19, 1407–1424. doi: 10.1111/1462-2920.13618
Samson, M., Bez, C., Georges, H., Joseph, B., and Venturi, V. (2021). Characterization of bacterial strains from bacterial culture collection of rice sheath in Burundi highlights an Alcaligenes species strain with antibacterial activity against Pseudomonas fuscovaginae rice pathogen. Afric. J. Microbiol. Res. 15, 497–511. doi: 10.5897/AJMR2021.9513
Santos-Medellín, C., Edwards, J., Liechty, Z., Nguyen, B., and Sundaresan, V. (2017). Drought stress results in a compartment-specific restructuring of the rice root-associated microbiomes. MBio. 8, e00764–e00717. doi: 10.1128/mBio.00764-17
Saxena, S. (2014). Microbial metabolites for development of ecofriendly agrochemicals. Allelopath J. 33, 1–24.
Scagliola, M., Pii, Y., Mimmo, T., Cesco, S., Ricciuti, P., and Crecchio, C. (2016). Characterization of plant growth promoting traits of bacterial isolates from the rhizosphere of barley (Hordeum vulgare L.) and tomato (Solanum lycopersicon L.) grown under Fe sufficiency and deficiency. Plant physiol. Biochemistr. 107, 187–196. doi: 10.1016/j.plaphy.2016.06.002
Schrempf, H. (2001). Recognition and degradation of chitin by streptomycetes. Antonie Van Leeuwenhoek. 79, 285–289. doi: 10.1023/A:1012058205158
Schrey, S. D., and Tarkka, M. T. (2008). Friends and foes: streptomycetes as modulators of plant disease and symbiosis. Antonie Van Leeuwenhoek. 94, 11–19. doi: 10.1007/s10482-008-9241-3
Sellstedt, A., and Richau, K. H. (2013). Aspects of nitrogen-fixing Actinobacteria, in particular free-living and symbiotic Frankia. FEMS Microbiol. Lett. 342, 179–186. doi: 10.1111/1574-6968.12116
Selvakumar, G., Bhatt, R. M., Upreti, K. K., Bindu, G. H., and Shweta, K. (2015). Citricoccus zhacaiensis B-4 (MTCC 12119) a novel osmotolerant plant growth promoting actinobacterium enhances onion (Allium cepa L.) seed germination under osmotic stress conditions. World J. Microbiol. Biotechnol. 31, 833–839. doi: 10.1007/s11274-015-1837-y
Sharma, U., Kumari, S., Sinha, K., and Kumar, S. (2017). Isolation and molecular characterization of phytase producing actinobacteria of fruit orchard. The Nucleus. 60, 187–195. doi: 10.1007/s13237-017-0205-8
Shutsrirung, A., Chromkaew, Y., Pathom-Aree, W., Choonluchanon, S., and Boonkerd, N. (2013). Diversity of endophytic actinomycetes in mandarin grown in northern Thailand, their phytohormone production potential and plant growth promoting activity. Soil Sci. Plant Nutri. 59, 322–330. doi: 10.1080/00380768.2013.776935
Siddikee, M. A., Chauhan, P. S., Anandham, R., Han, G.-H., and Sa, T.-M. (2010). Isolation, characterization, and use for plant growth promotion under salt stress, of ACC deaminase-producing halotolerant bacteria derived from coastal soil. J. Microbiol. Biotechnol. 20, 1577–1584. doi: 10.4014/jmb.1007.07011
Singh, R., and Dubey, A. K. (2018). Diversity and applications of endophytic actinobacteria of plants in special and other ecological niches. Front. Microbiol. 9, 1767. doi: 10.3389/fmicb.2018.01767
Singh, R. N., Gaba, S., Yadav, A. N., Gaur, P., Gulati, S., Kaushik, R., et al. (2016). First high quality draft genome sequence of a plant growth promoting and cold active enzyme producing psychrotrophic Arthrobacter agilis strain L77. Standards Genom. Sci. 11, 1–9. doi: 10.1186/s40793-016-0176-4
Solanki, M. K., Malviya, M. K., and Wang, Z. (2016). Actinomycetes bio-inoculants: A modern prospectus for plant disease management. Plant Growth Promot. Actinobact. 16, 63–81. doi: 10.1007/978-981-10-0707-1_5
Solans, M. (2007). Discaria trinervis–Frankia symbiosis promotion by saprophytic actinomycetes. J. Basic Microbiol. 47, 243–50. doi: 10.1002/jobm.200610244
Solans, M., Messuti, M. I., Reiner, G., Boenel, M., Vobis, G., Wall, L. G., et al. (2019). Exploring the response of Actinobacteria to the presence of phosphorus salts sources: Metabolic and co-metabolic processes. J. Basic Microbiol. 59, 487–495. doi: 10.1002/jobm.201800508
Solans, M., and Vobis, G. (2003). Actinomycetes saprofíticos asociados a la rizósfera y rizoplano de Discaria trinervis. Ecología austral. 13, 097–107.
Solans, M., Vobis, G., Cassán, F., Luna, V., and Wall, L. G. (2011). Production of phytohormones by root-associated saprophytic actinomycetes isolated from the actinorhizal plant Ochetophila trinervis. World J. Microbiol. Biotechnol. 27, 2195–2202. doi: 10.1007/s11274-011-0685-7
Soumare, A., Diedhiou, A. G., Thuita, M., Hafidi, M., Ouhdouch, Y., Gopalakrishnan, S., et al. (2020). Exploiting biological nitrogen fixation: a route towards a sustainable agriculture. Plants. 9, 1011. doi: 10.3390/plants9081011
Sousa Cd, S., Soares, A. C. F., and Garrido Md, S. (2008). Characterization of streptomycetes with potential to promote plant growth and biocontrol. Scientia Agricola. 65, 50–55. doi: 10.1590/S0103-90162008000100007
Su, Y., Shinano, T., and Purnomo, E. O. S. A. K.I. M. (2007). Growth promotion of rice by inoculation of acid-tolerant, N2-fixing bacteria isolated from acid sulfate paddy soil in South Kalimantan, Indonesia. Tropics. 16, 261–274. doi: 10.3759/tropics.16.261
Subramanian, K., Muniraj, I., and Uthandi, S. (2016). Role of actinomycete-mediated nanosystem in agriculture. Plant Growth Promot. Actinobacteria. 16, 233–47. doi: 10.1007/978-981-10-0707-1_15
Suleman, P., Al-Musallam, A., and Menezes, C. (2002). The effect of biofungicide Mycostop on Ceratocystis radicicola, the causal agent of black scorch on date palm. BioControl. 47, 207–216. doi: 10.1023/A:1014519726573
Susilowatia, D. N., Sudianac, I. M., Mubarika, N., and Suwantoa, A. (2015). Species and functional diversity of rhizobacteria of rice plant in the coastal soils of Indonesia. Indonesian J. Agricult. Sci. 16, 39–50. doi: 10.21082/ijas.v16n1.2015.39-50
Suzuki, K. (2012). “Rubrobacteria class,” in Bergey's Manual of Systematic Bacteriology: The Actinobacteria, Vol.5, 2nd Edn, eds M. Goodfellow, P. Kampfer, H. -J. Busse, M. E. Trujillo, J. -I. Suzuki, W. Ludwig, and W. B. Whitman (New York, NY: Springer), 2004–2009.
Suzuki, K.-I., and Whitman, W. (2012). Class VI. Thermoleophilia class. nov. Bergey's Man. Systemat. Bacteriol. 5, 2010–2028.
Talia, P., Sede, S. M., Campos, E., Rorig, M., Principi, D., Tosto, D., et al. (2012). Biodiversity characterization of cellulolytic bacteria present on native Chaco soil by comparison of ribosomal RNA genes. Res. Microbiol. 163, 221–232. doi: 10.1016/j.resmic.2011.12.001
Tara, N., and Saharan, B. S. (2017). Plant growth promoting traits shown by bacteria Brevibacterium frigrotolerans SMA23 Isolated from Aloe vera rhizosphere. Agricult. Sci. Digest-A Res. J. 37, 226–231. doi: 10.18805/asd.v37i03.9223
Timmusk, S., Behers, L., Muthoni, J., Muraya, A., and Aronsson, A.-C. (2017). Perspectives and challenges of microbial application for crop improvement. Front. Plant Sci. 8, 49. doi: 10.3389/fpls.2017.00049
Uyeda, M., Mizukami, M., Yokomizo, K., and Suzuki, K. (2001). Pentalenolactone I and hygromycin A, immunosuppressants produced by Streptomyces filipinensis and Streptomyces hygroscopicus. Biosci. Biotechnol. Biochemistr. 65, 1252–1254. doi: 10.1271/bbb.65.1252
Vaishnav, A., Sharma, S. K., Choudhary, D. K., Sharma, K. P., Ahmad, E., Sharma, M. P., et al. (2018). Nitric oxide as a signaling molecule in plant-bacterial interactions. Plant Microbiom. Stress Respon. 14, 183–99. doi: 10.1007/978-981-10-5514-0_8
Ventura, M., Canchaya, C., Tauch, A., Chandra, G., Fitzgerald, G. F., Chater, K. F., et al. (2007). Genomics of Actinobacteria: tracing the evolutionary history of an ancient phylum. Microbiol. Mol. Biol. Rev. 71, 495–548. doi: 10.1128/MMBR.00005-07
Verma, P., Yadav, A. N., Kazy, S. K., Saxena, A. K., and Suman, A. (2014). Evaluating the diversity and phylogeny of plant growth promoting bacteria associated with wheat (Triticum aestivum) growing in central zone of India. Int J Curr Microbiol Appl Sci. 3, 432–447.
Vessey, J. K. (2003). Plant growth promoting rhizobacteria as biofertilizers. Plant Soil. 255, 571–586. doi: 10.1023/A:1026037216893
Vesuna, A. P., and Nerurkar, A. S. (2020). Biocontrol impact of AHL degrading actinobacteria on quorum sensing regulated virulence of phytopathogen Pectobacterium carotovorum subsp. carotovorum BR1. Plant Soil. 453, 371–388. doi: 10.1007/s11104-020-04623-z
Větrovský, T, Steffen, K. T., and Baldrian, P. (2014). Potential of cometabolic transformation of polysaccharides and lignin in lignocellulose by soil Actinobacteria. PloS One. 9, e89108. doi: 10.1371/journal.pone.0089108
Vijayabharathi, R., Gopalakrishnan, S., Sathya, A., Srinivas, V., and Sharma, M. (2018). Deciphering the tri-dimensional effect of endophytic Streptomyces sp. on chickpea for plant growth promotion, helper effect with Mesorhizobium ciceri and host-plant resistance induction against Botrytis cinerea. Microbial Pathogene. 122, 98–107. doi: 10.1016/j.micpath.2018.06.019
Vimal, S. R., Patel, V. K., and Singh, J. S. (2019). Plant growth promoting Curtobacterium albidum strain SRV4: an agriculturally important microbe to alleviate salinity stress in paddy plants. Ecolo. Indicat. 105, 553–562. doi: 10.1016/j.ecolind.2018.05.014
Waldron, C., Matsushima, P., Rosteck, P. R. Jr., Broughton, M. C., Turner, J., Madduri, K., et al. (2001). Cloning and analysis of the spinosad biosynthetic gene cluster of Saccharopolyspora spinosa. Chemistr. Biol. 8, 487–499. doi: 10.1016/S1074-5521(01)00029-1
Wang, C., Wang, Z., Qiao, X., Li, Z., Li, F., Chen, M., et al. (2013). Antifungal activity of volatile organic compounds from Streptomyces alboflavus TD-1. FEMS Microbiol. Lett. 341, 45–51. doi: 10.1111/1574-6968.12088
Wang, W.-Z., Morohoshi, T., Someya, N., and Ikeda, T. (2009). Diversity and distribution of N-acylhomoserine lactone (AHL)-degrading activity and AHL-lactonase (AiiM) in genus Microbacterium. Microbes Environ. 2009, 1203190368.
Wanner, L. A. (2006). A survey of genetic variation in Streptomyces isolates causing potato common scab in the United States. Phytopathology. 96, 1363–1371. doi: 10.1094/PHYTO-96-1363
Wei, L., Ouyang, S., Wang, Y., Shen, X., and Zhang, L. (2014). Solirubrobacter phytolaccae sp. nov., an endophytic bacterium isolated from roots of Phytolacca acinosa Roxb. Int. J. Systemat. Evol. Microbiol. 64, 858–62. doi: 10.1099/ijs.0.057554-0
Wheeler, C., Crozier, A., and Sandberg, G. (1984). The biosynthesis of indole-3-acetic acid by Frankia. Frankia Symbioses. New York, NY: Springer; p. 99–104.
White, J., Linfield, C., Lahdenpera, M., and Uoti, J. (1990). Mycostop-a novel biofungicide based on Streptomyces griseoviridis. London: British Crop Protection Council.
Williams, S., and Vickers, J. (1988). Detection of actinomycetes in the natural environment: problems and perspectives. Biol. Actinomycetes. 88, 265–270.
Xing, K., Bian, G.-K., Qin, S., Klenk, H.-P., Yuan, B., Zhang, Y.-J., et al. (2012). Kibdelosporangium phytohabitans sp. nov., a novel endophytic actinomycete isolated from oil-seed plant Jatropha curcas L. containing 1-aminocyclopropane-1-carboxylic acid deaminase. Antonie Van Leeuwenhoek. 101, 433-41. doi: 10.1007/s10482-011-9652-4
Yadav, A. N., Sachan, S. G., Verma, P., and Saxena, A. K. (2015). Prospecting cold deserts of north western Himalayas for microbial diversity and plant growth promoting attributes. J. Biosci. Bioeng. 119, 683–693. doi: 10.1016/j.jbiosc.2014.11.006
Yadav, A. N., Verma, P., Kour, D., Rana, K. L., Kumar, V., Singh, B., et al. (2017). Plant microbiomes and its beneficial multifunctional plant growth promoting attributes. Int. J. Environ. Sci. Nat. Resour. 3, 1–8. doi: 10.19080/IJESNR.2017.03.555601
Yadav, A. N., Verma, P., Kumar, S., Kumar, V., Kumar, M., Kumari Sugitha, T. C., et al. (2018). “Actinobacteria from Rhizosphere,” in: Elsevier, editor. In New and future developments in microbial biotechnology and bioengineering, p. 13–41.
Yoon, J.-H., Lee, J.-K., Jung, S.-Y., Kim, J.-A., Kim, H.-K., and Oh, T.-K. (2006). Nocardioides kongjuensis sp. nov., an N-acylhomoserine lactone-degrading bacterium. Int. J. Systemat. Evol. Microbiol. 56, 1783–1787. doi: 10.1099/ijs.0.64120-0
Yun, B.-R., Roh, S. G., and Kim, S. B. (2017). Diversity and physiological properties of soil actinobacteria in Ulleung Island. Korean J. Microbiol. 53, 242–250. doi: 10.7845/kjm.2017.705
Zhang, H., Han, L., Jiang, B., and Long, C. (2021). Identification of a phosphorus-solubilizing Tsukamurella tyrosinosolvens strain and its effect on the bacterial diversity of the rhizosphere soil of peanuts growth-promoting. World J. Microbiol. Biotechnol. 37, 1–14. doi: 10.1007/s11274-021-03078-3
Zhang, S., Gui, C., Shao, M., Kumar, P. S., Huang, H., and Ju, J. (2020). Antimicrobial tunicamycin derivatives from the deep sea-derived Streptomyces xinghaiensis SCSIO S15077. Nat. Product Res. 34, 1499–1504. doi: 10.1080/14786419.2018.1493736
Zhao, K., Li, J., Zhang, X., Chen, Q., Liu, M., Ao, X., et al. (2018). Actinobacteria associated with Glycyrrhiza inflata Bat. are diverse and have plant growth promoting and antimicrobial activity. Scientific Rep. 8, 1–13. doi: 10.1038/s41598-018-32097-8
Zhao, S., Du, C.-M., and Tian, C.-Y. (2012). Suppression of Fusarium oxysporum and induced resistance of plants involved in the biocontrol of Cucumber Fusarium Wilt by Streptomyces bikiniensis HD-087. World J. Microbiol. Biotechnol. 28, 2919–2927. doi: 10.1007/s11274-012-1102-6
Keywords: Actinobacteria, PGPR, PGPB, bioinoculant, biocontrol, sustainability
Citation: Boukhatem ZF, Merabet C and Tsaki H (2022) Plant Growth Promoting Actinobacteria, the Most Promising Candidates as Bioinoculants? Front. Agron. 4:849911. doi: 10.3389/fagro.2022.849911
Received: 06 January 2022; Accepted: 09 February 2022;
Published: 21 March 2022.
Edited by:
Mohamed Lazali, University of Khemis Miliana, AlgeriaReviewed by:
Adnane Bargaz, Mohammed VI Polytechnic University, MoroccoRim Maougal, Université Frères Mentouri Constantine 1, Algeria
Copyright © 2022 Boukhatem, Merabet and Tsaki. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zineb Faiza Boukhatem, Ym91a2hhdGVtLmZhaXphQHVuaXYtb3JhbjEuZHo=
†These authors have contributed equally to this work
 Zineb Faiza Boukhatem
Zineb Faiza Boukhatem Chahinez Merabet
Chahinez Merabet Hassini Tsaki†
Hassini Tsaki†