
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Agron., 28 September 2021
Sec. Pest Management
Volume 3 - 2021 | https://doi.org/10.3389/fagro.2021.725895
This article is part of the Research TopicThe Use of Plant Extracts and Essential Oils as BiopesticidesView all 12 articles
Despite the cornucopia of agricultural, economic and ecological ramifications of invasive alien plant species (IAPs) in sub-Saharan Africa, studies on their potential use as bio-insecticides have not received adequate attention compared to the burgeoning plethora of literature on their use in ethnomedicine. In the current study, we review the existing, but scattered literature on the insecticidal activity of different parts of some IAPs; specifically those invasive in sub-Saharan Africa but with published literature from Africa and elsewhere. From our literature survey, we found that 69 studies from four continents (Africa, Asia, North America and South America) reported the insecticidal activity of 23 plant species from 13 families (Asteraceae = 6 species; Solanaceae = 3 species; Apocynacee, Fabaceae and Euphorbiaceae 2 species each; Araceae, Bignoniaceae, Chenopodiaceae, Meliaceae, Mimosaceae, Myrtaceae, Papaveraceae, and Verbenaceae = 1 species each) that are invasive in, and alien to Africa. The highest number of published case studies were from India (n = 19) and Nigeria (n = 15). We found that varying concentrations of extracts or powders from different plant parts caused 50–100% mortality against a myriad of insect pests of agriculture and environmental importance. Our review discussed the prospects for exploiting IAPs as pesticidal plants in African countries especially among resource-poor small-holder farmers and locals to improve agricultural productivity and livelihoods. Finally, we highlighted safety concerns and challenges of using IAPs as bio-insecticides in Africa and formulates appropriate recommendations for future research.
Invasive alien plant species (IAPs) are among species whose naturalization threatens the biological biodiversity and functions of the ecosystem in their new geographic region (Richardson and Pyšek, 2012; Mostert et al., 2017; O'Connor and van Wilgen, 2020). These plants are among significant ecosystem drivers that degrades the quality of grazing, agricultural and natural lands (Richardson and van Wilgen, 2004; Davis, 2006). Due to the immense ecological and social pressures exerted by these plants, governments have announced the management of IAPs and millions of dollars are invested toward the management of these plants in South Africa and elsewhere in the world (McConnachie et al., 2010; Van Wilgen and Lange, 2011; Hoffmann and Broadhurst, 2016; Morokong et al., 2016; Hanley and Roberts, 2019).
Regardless of the efforts made toward minimizing the densities of invasion and the spread of these IAPs, follow-up treatments may be required to keep the populations of these non-native species at a level that prevents spread and harm to human health or the environment (Marais et al., 2004; Klein, 2011; Mukwevho and Mphephu, 2020). Although manual clearing of IAPs yields temporal relief on the intensity of invasion, continuous clearing alone favors the expansion of the invasion by species that are propagated vegetatively (Radtke et al., 2013). To minimize the further spread of IAPs through plant propagules, the cut plant materials from the above- and below-ground may be further processed to be used for socio-economic and ecological benefits in sub-Saharan Africa (Shackleton et al., 2007, 2018; Ngorima and Shackleton, 2019; Mugwedi, 2020).
The potential use of IAPs in ethnomedicine and various aspects of ethnobotany in Africa have received a great deal of attention (e.g., Omokhua et al., 2016, 2018a,b) however, studies on the use and potential of invasive alien plants as pesticidal plants to manage agricultural and environmental pests is only beginning to gain recognition (e.g., Midega et al., 2016; Mkindi et al., 2017; Stevensona et al., 2017; Uyi et al., 2018a,b). Since some IAP's contain some novel secondary phytochemicals, the harvested materials may be processed to be used against microorganisms, insects and weeds and other undesired plants (Deressa et al., 2015; Amir et al., 2017; Mkindi et al., 2017; Das et al., 2018; Zerihun and Ele, 2018; Mugwedi, 2020). Like other pesticides, biopesticides may repel insect pests, disrupt their development, affect reproduction or kill live organisms on contact (Mogg et al., 2008; Litt et al., 2014; Uyi and Adetimehin, 2018). Although different scientists consider IAPs as a threat to agriculture and biodiversity, dozens of IAPs have insecticidal properties that have been rigorously screened toward major pests, pollinators and wasps (including some parasitoids) around the globe (Isman, 2008; Mkenda et al., 2015; Mkindi et al., 2017; Stevensona et al., 2017).
Due to the cost of synthetic chemicals (Dougoud et al., 2019), impacts on non-target species (Theiling and Croft, 1988; Mulè et al., 2017), target pest's genetic drift (REX consortium, 2010; Khayatnezhad and Nasehi, 2021) and ecotoxicological impacts (Pimentel, 1995; Kankam, 2021), the United Nations (UN) promotes the use of environmentally safe products, such as aqueous extracts to minimize the impact of pests on crops (Phillips and Throne, 2010; Bommarco et al., 2013; Oliveira et al., 2014). Sustainable and eco-friendly biopesticides may be easily accessible by the resource-poor small-holder farmers and locals in countries where there is greater food insecurity, particularly in Africa (Sasson, 2012). Further processing of plant propagules also curbs the further distribution of IAPs through vegetative materials, hence also benefiting the livelihoods though reducing pressures by the agricultural pests on various crops. In this paper, we review the existing, but scattered literature on the insecticidal activity of different parts of some IAPs; specifically, those that are invasive in the sub-Saharan Africa. We discuss the prospects and opportunities for using IAPs as bio-insecticides of insect pests of agricultural and environmental importance. Finally, the paper highlights the safety concerns, research gaps, the challenges of using IAPs as bio-insecticides and formulates appropriate recommendations for future research.
The information presented in this review was obtained from journal articles that are relevant to the topic. Only literature on insecticidal (not repellence) properties of IAPs that are invasive in Africa were included. Plant like Azadirachta indica A. Juss (Miliaceae) that have wide usage and is already well-established for over 100 years were not considered in this review. The scientific papers analyzed were obtained from different sources such as Google Scholar, Science-Direct, PubMed, SciFinder, and Scopus. Systematically used keywords include invasive alien plants, insecticidal, pesticidal, insect pest, efficacy, mortality, with the scientific name of each plant reported to possess insecticidal properties in journal articles. We used Boolean operators (and, or, not or and not) to combine or exclude keywords in our search to obtain a more focused and productive results. The literature search was conducted between June 2019 and April 2020, and more than 120 published papers were identified. Among the excluded research papers were those that assessed the insecticidal properties of forest trees, plants that have not been declared as invasive in Africa and studies that did not include control treatments. The mean percentage of insect mortality reported here was recorded from either of the text, tables, graphs and/figures. Among the information derived from the research papers was the country in which different studies were conducted, name of the IAP's, the harvested/used plant part(s), the formulations, the target insect, developmental stages at which the formulation was applied, and the percentage mortality reported after application of the formulation. Only articles that reported data with means, sample size and a measurement of variance (standard deviation, standard error or confidence intervals) for all treatments with a clear indication of replication were considered. The scoring system of 0–4 was used to rate the insecticidal properties of IAPs against insects in Africa. The percentage mortality of 1–25, 26–50, 51–75, and 76–100% were ranked as 1, 2, 3, and 4, respectively, but the formulation that recorded zero percent mortality was ranked as 0.
Invasive alien plant species are identified as the plants that are intentionally or accidentally introduced to the regions beyond their native ranges (Richardson and Pyšek, 2012). Naturalized alien plant species are among significant ecosystem drivers that pose major threats to the native communities (e.g., plants and arthropods) in natural and agricultural ecosystems (Van Hengstum et al., 2013; Litt et al., 2014). The increase in the intensity of invasion aggravates the degree of threat to biodiversity and ecosystem function (Valone and Weyers, 2019). The distribution and problems of the IAPs reviewed in this paper are detailed in Tables 1A–E. Among the common impacts of the IAPs is the degradation of grazing land, competition with native species and cultivated crops for natural resources, supporting agricultural pests between cropping seasons, presenting health hazard to humans and poisoning of livestock (Aigbokhan et al., 2010; Alagesaboopathi and Deivanai, 2011; Park et al., 2012; Van Hengstum et al., 2013; Litt et al., 2014; Shackleton et al., 2017; Dandurand et al., 2019; O'Connor and van Wilgen, 2020). Although there is sufficient literature that documents the impacts of these plants, the global efforts on mapping the distribution of the plants in their non-native ranges is insufficient (Witt et al., 2018).
The current distribution of invasive alien plants has been recorded for various plants invading the landscapes of different countries in Africa (Henderson, 2001; Shackleton et al., 2017; Witt and Luke, 2017; Witt et al., 2018, 2019; Catarino et al., 2019), whilst other studies also predicted the future distribution of these weeds (McConnachie et al., 2010; Taylor et al., 2012; Tererai and Wood, 2014; Obiakara and Fourcade, 2018). Further, surveys on the distribution of agents associated with these IAPs contribute to the continuous update on the change of the invasion intensities (Mukwevho et al., 2018). Despite the remarkable efforts by the Centre for Agriculture and Bioscience International (CABI, sometimes also referred to as CAB International) to describe the international distribution of IAPs, insufficient records of plant distribution in other African countries result in fragmented distribution maps.
From the literature survey, we found 69 studies across the globe that reported insecticidal activities of 23 plant species that are invasive in, and alien to Africa. The identified species were from 13 plant families and comprised six species from Asteraceae, three species from Solanaceae, two species from Apocynaceae, Fabaceae and Euphorbiaceae, and one species each from Araceae, Bignoniaceae, Chenopodiaceae, Meliaceae, Mimosaceae, Myrtaceae, Papaveraceae, and Verbenaceae (Tables 2A–I). These reports showing the insecticidal activities of alien plants that are problematic in Africa originated from Africa, Asia, North America and South America. The highest number of published case studies were from India and Nigeria with 19 and 15, respectively, whilst countries such as Algeria, Argentina, Brazil, Colombia, Chile, China, Egypt, Ethiopia, Ghana, Kenya, Malawi, Mexico, Pakistan, Sudan, Tanzania, Togo, Tunisia, Turkey, and the United States of America have less than 6 reports each. We hypothesized that the large number of research papers from India, Nigeria and other developing countries may be due to the fact that scientists in these countries are aware of the limited availability of synthetic insecticides by the resource-poor small-holder farmers; locals in these countries are keen on identifying IAPs to control and manage insect pests of agricultural, environmental and medical importance. Due the ecotoxicological effects and high cost of synthetic insecticides, the use of plants with pesticidal properties to control insect pests in agro-ecosystems among resource-poor small-holder farmers has been historically widespread and adopted in Africa (Belmain and Stevenson, 2001; Midega et al., 2016). Despite the widespread use of these biorational methods, pest control in some ecosystems in Africa continues to rely on the use of synthetic insecticides when alternative biopesticides are unavailable (Isman, 2006, 2015; Isman and Grieneisen, 2014). Although a plethora of empirical research has demonstrated the insecticidal properties of weeds in general, our literature found evidence that some invasive alien plants in Africa possessed insecticidal properties against a range of insect pests.
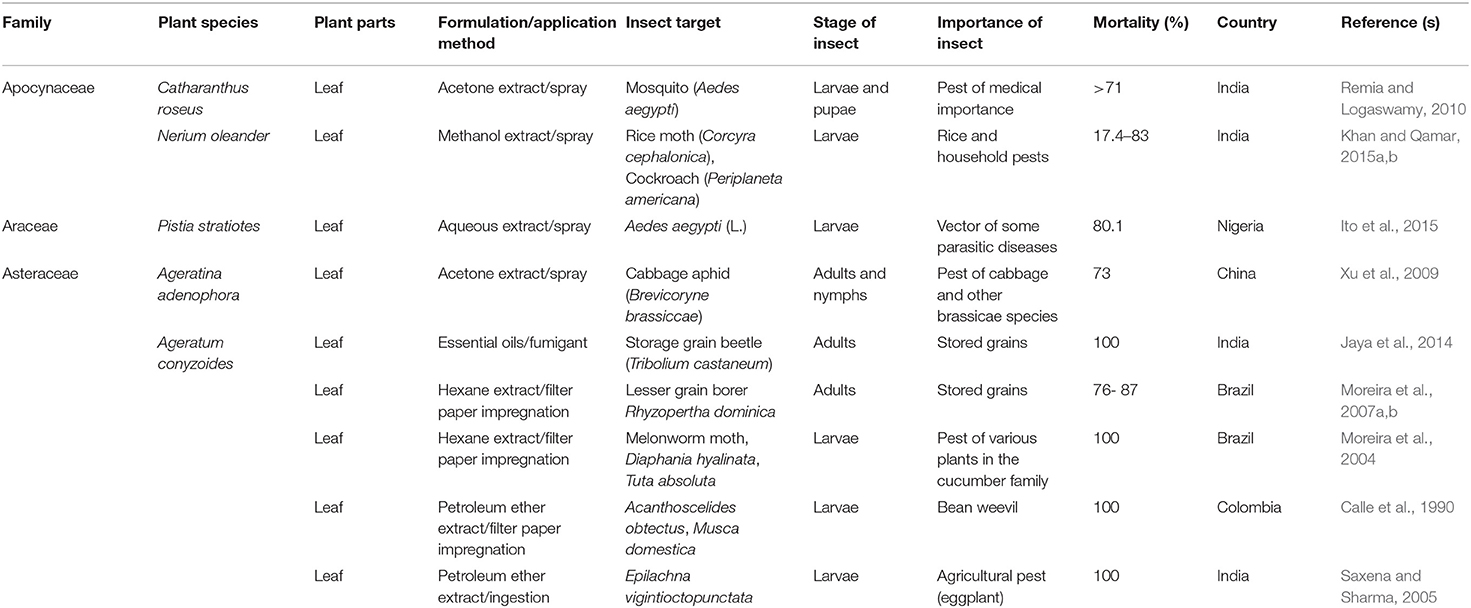
TABLE 2A. Published reports on the insecticidal activities of some plant species with invasive potentials in sub-Saharan Africa.
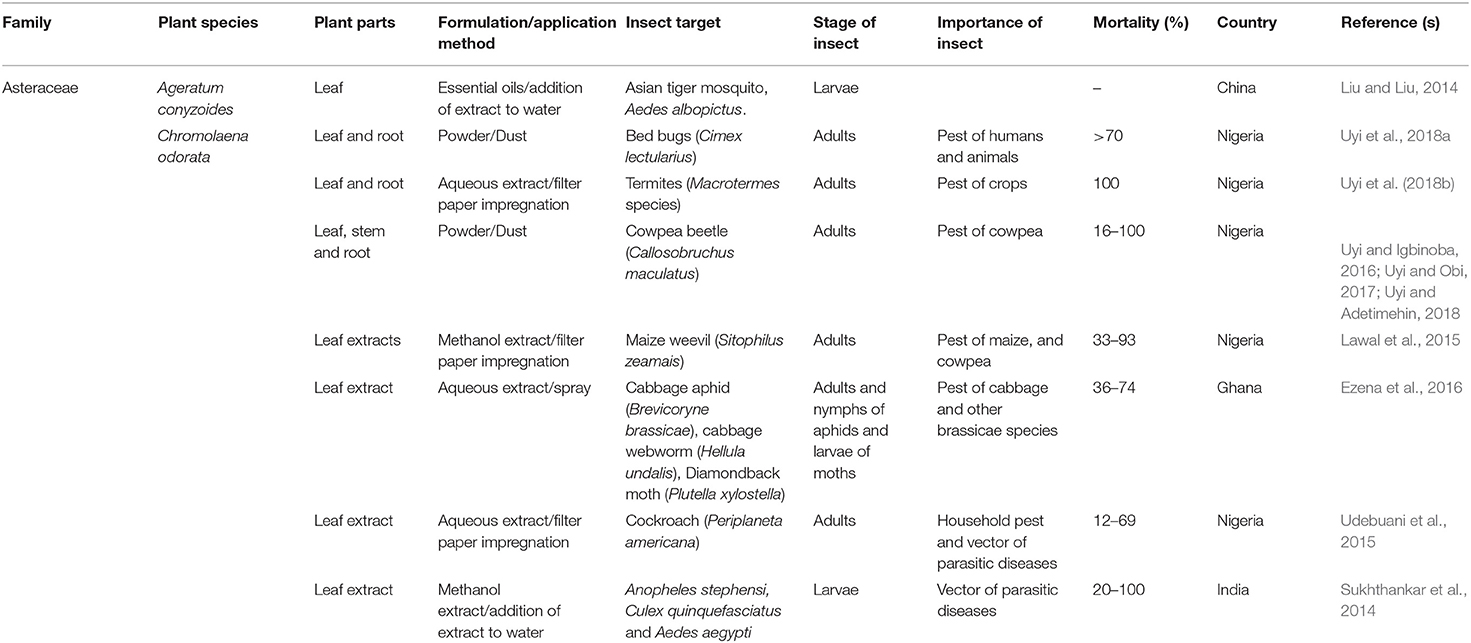
TABLE 2B. Published reports on the insecticidal activities of some plant species with invasive potentials in sub-Saharan Africa.

TABLE 2C. Published reports on the insecticidal activities of some plant species with invasive potentials in sub-Saharan Africa.
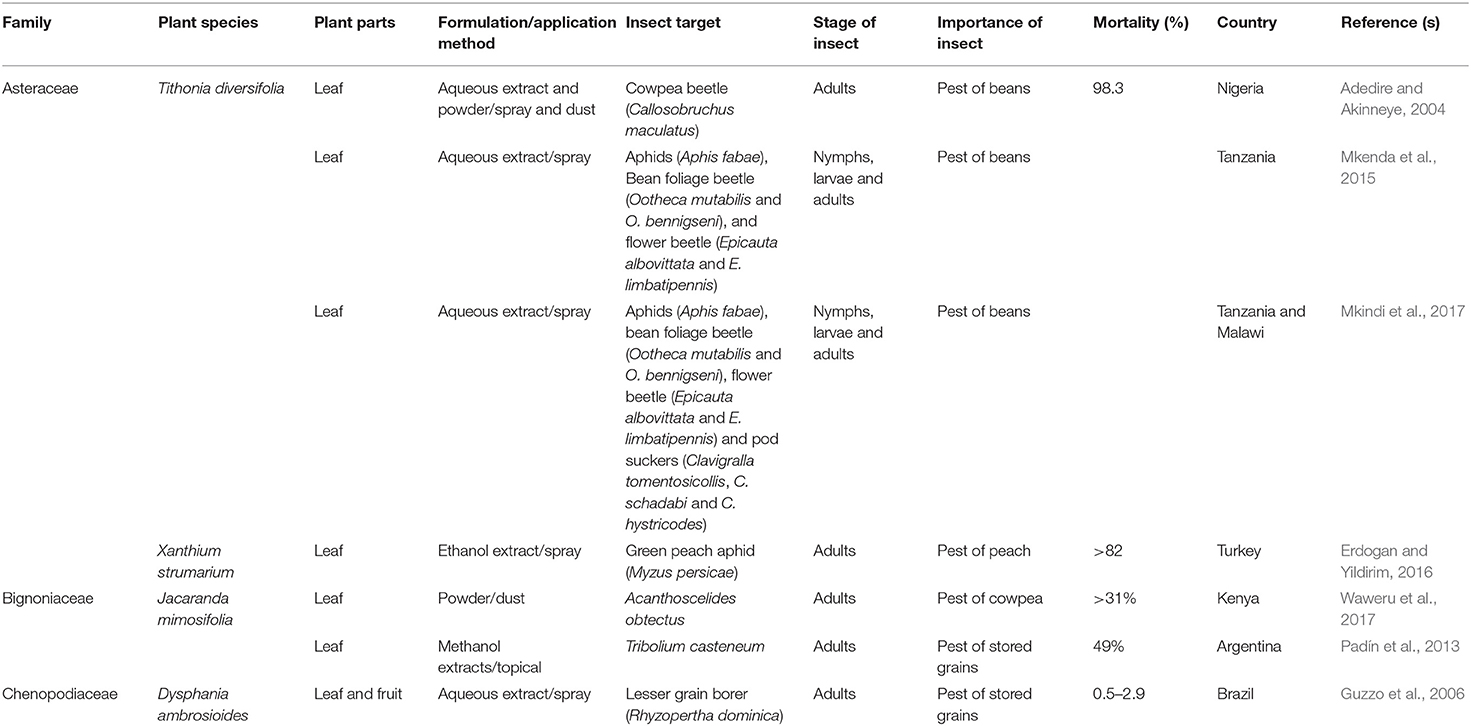
TABLE 2D. Published reports on the insecticidal activities of some plant species with invasive potentials in sub-Saharan Africa.
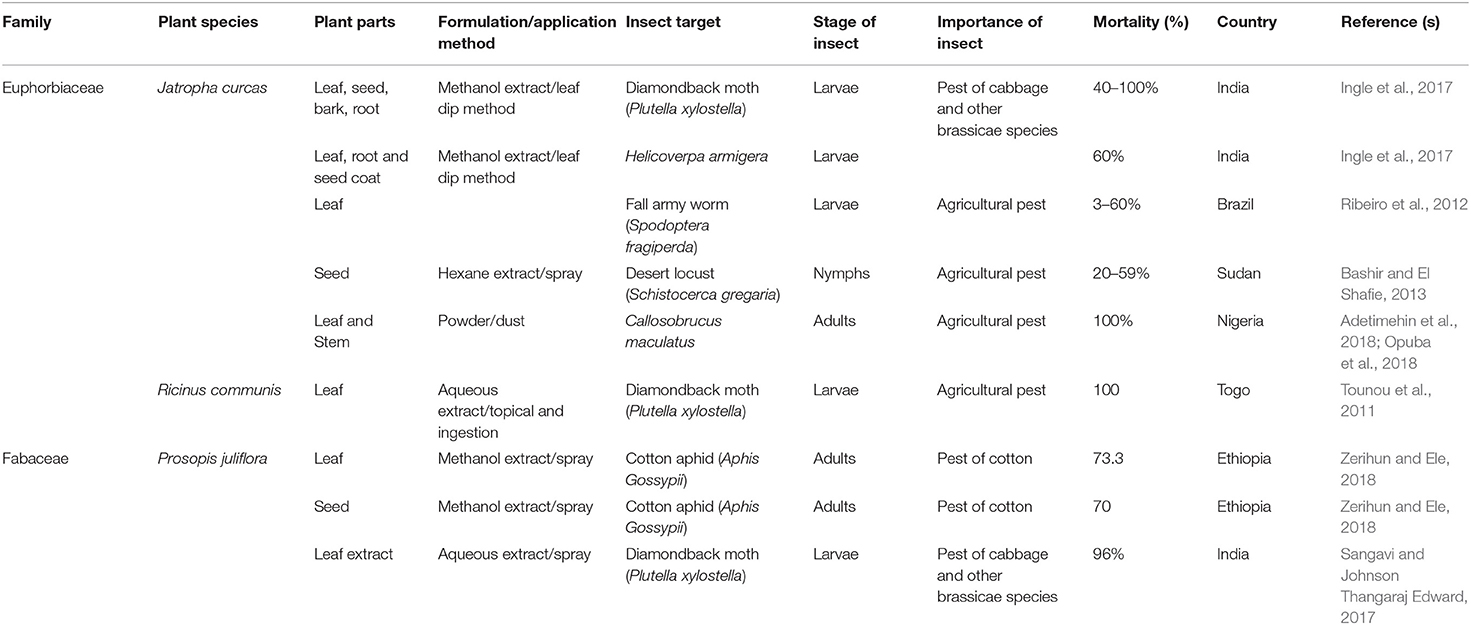
TABLE 2E. Published reports on the insecticidal activities of some plant species with invasive potentials in sub-Saharan Africa.

TABLE 2F. Published reports on the insecticidal activities of some species with invasive potentials in sub-Saharan Africa.

TABLE 2G. Published reports on the insecticidal activities of some plant species with invasive potentials in sub-Saharan Africa.
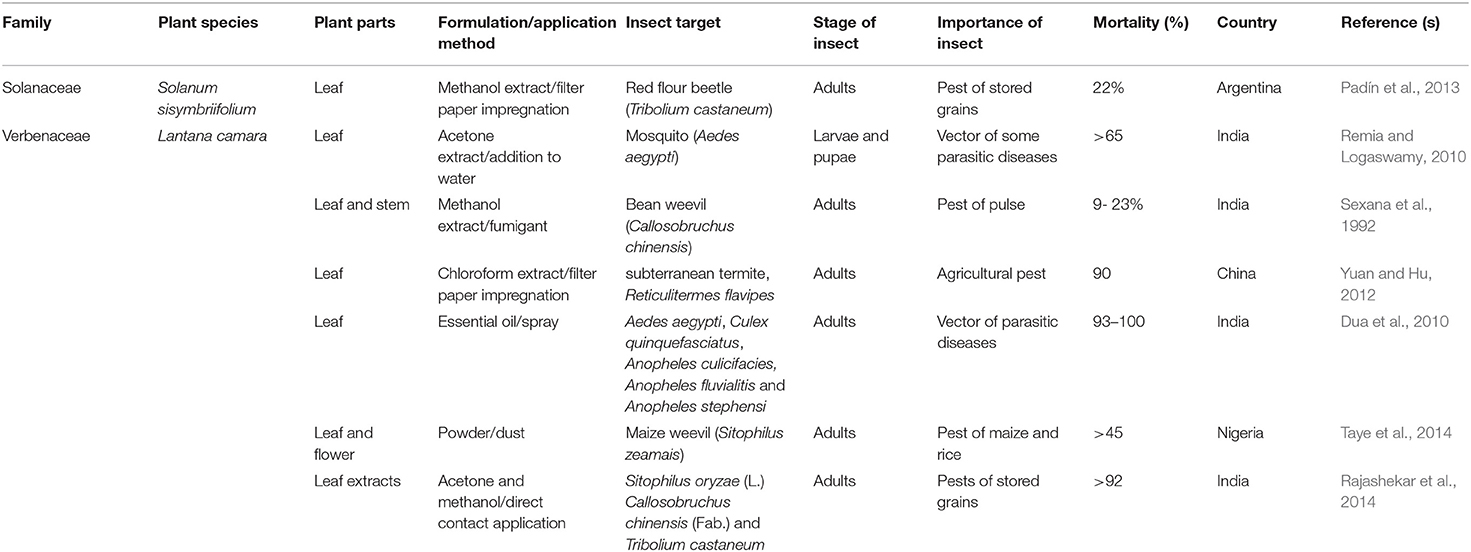
TABLE 2H. Published reports on the insecticidal activities of some plant species with invasive potentials in sub-Saharan Africa.
Several biological assays have been conducted to ascertain the efficacy of invasive alien plants against a myriad of insect pests with varying levels of insect mortality (Tables 2A–I). The survey demonstrated that leaf extracts were frequently used for bioassays, compared to other parts (i.e., roots, stems, inflorescences, fruits or seeds) of the plant (Figure 1). A majority of studies were conducted on members of the Asteraceae which represented 25 out of 69 studies and accounted for 38% of the total studies recorded in this review (Figure 2). Mean mortality rank of insect pests caused by the Asteraceae ranged from 50 to 100% (Figure 3).

Figure 1. Percentage prevalence of case studies on the parts of plant used to test for insecticidal activities of invasive alien plants. Data was obtained from 69 published case studies. IL, Inflorescence and leaf; ILS, Inflorescence; leaf and stem; ILR, Inflorescence; leaf and root; ILSR, Inflorescence; leaf, stem and root; LS, Leaf and stem; LSR, Leaf, stem, root.
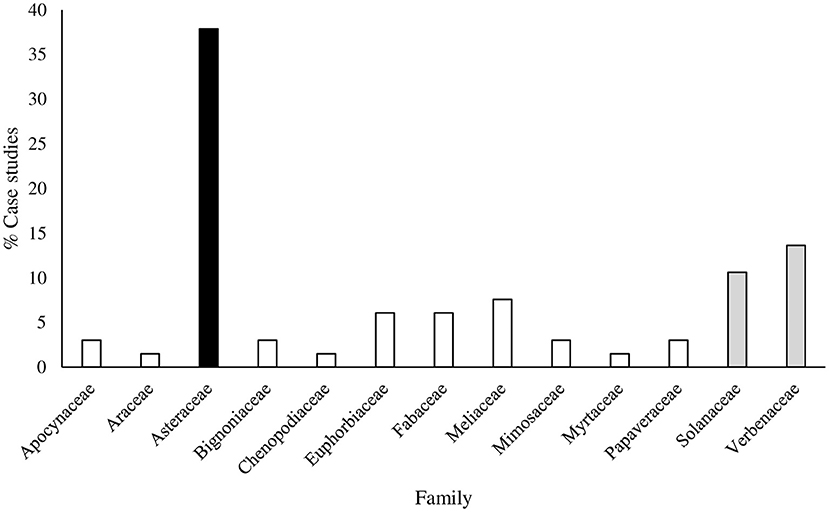
Figure 2. Prevalence of case studies on different families of invasive alien plant species tested for insecticidal activities. Data was obtained from 69 published case studies.
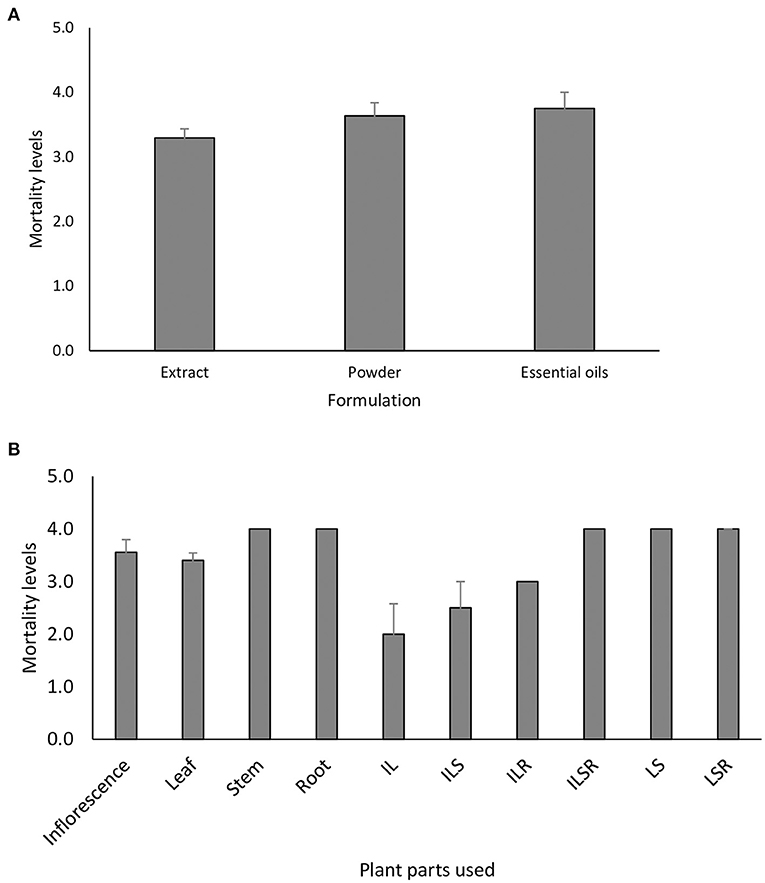
Figure 3. Mean mortality rank (±se) of insect pests of agriculture and medical importance caused by (A) different formulations from, and (B) different plant parts or combinations plant parts of invasive alien plants. Data was obtained from 69 published case studies. IL, Inflorescence and leaf; ILS, Inflorescence, leaf and stem; ILR, Inflorescence, leaf and root; ILSR, Inflorescence, leaf, stem and root; LS, Leaf and stem; LSR, Leaf, stem, root.
Six species in the family Asteraceae were reported effective against a number of insect pest species. In a laboratory and field study conducted by Xu et al. (2009), the acetone leaf extract of Ageratina adenophora caused up to 73% mortality in Brevicoryne brassicae after a 3-day exposure. Although the use of the essential oils of A. adenophora has been suggested for controlling aphids, ants and weevils in stored grains, there are no reports on the insecticidal use of this plant in invaded areas in Africa. Jaya et al. (2014) observed that essential oils from Ageratum conyzoides leaves caused 100% mortality against Tribolium castenum. Moreira et al. (2007a,b) isolated compounds including (5,6,7,8,3′,4′,5′-heptamethoxyflavone, 5,6,7,8,3′-pentamethoxy-4′, 5′-methylenedioxyflavone and coumarin) from the hexane extract of A. conyzoides leaves and tested the efficacy of the compounds against Rhyzopertha dominica and Diaphania hyalinata. Following a 24-h exposure, varying concentrations of the isolated compounds caused between 76 and 87% mortality in adults of R. dominica and 100% mortality in the larvae of D. hyalinata (Moreira et al., 2004, 2007a,b). The leaf extracts of A. conyzoides have also been reported to possess strong insecticidal activities (100% mortality) against the larvae of Acanthoscelides obtectus, Musca domestica and Epilachna vigintioctopunctata (Calle et al., 1990; Saxena and Sharma, 2005). Liu and Liu (2014) evaluated the larvicidal activity of the essential oil of A. conyzoides aerial parts against Aedes albopictus. The authors identified the principal constituents of the essential oils of A. conyzoides and concluded that the oils have insecticidal and larvicidal activities. Despite the burgeoning plethora of papers on the pesticidal activity of A. conyzoides against a myriad of arthropod pests (see Rioba and Stevenson, 2017), studies on the indigenous use of this plant in the control and management of insect pests are scarce. The increasing reports of the use of A. conyzoides in ethnomedicine for the treatment of a wide range of diseases in Africa (e.g., Nwauzoma and Dappa, 2013) suggest that the locals are exploiting the potential of the plant. Whether or not the plant has found use among the locals in its invasive range in Africa remains to be documented.
In a bioassay where Cimex lectularius adults were exposed to 2.0 g of Chromolaena odorata leaf powder, 70% mortality was reported after 5 days (Uyi et al., 2018a). Depending on concentrations, the leaf, stem and root powders of C. odorata were reported to cause between 16 and 100% mortality against adults of the Callosobruchus maculatus (Uyi and Igbinoba, 2016; Uyi and Obi, 2017; Uyi and Adetimehin, 2018). In Nigeria, Lawal et al. (2015) reported that the leaf extracts of C. odorata displayed a strong insecticidal activity by causing between 33 and 93% mortality in Sitophilus zeamais. In a field experiment in Ghana, Ezena et al. (2016) reported that varying concentrations of the leaf extract caused between 36 and 77% mortality in nymphs and adults of the Brevicoryne brassicae and Hellula undalis and Plutella xylostella. Udebuani et al. (2015) tested the efficacy of C. odorata leaf extract against Periplaneta americana by exposing the adults to different concentrations of the leaf extract and reported 12 to 69% mortality. Sukhthankar et al. (2014) investigated the insecticidal activity of different concentrations of methanolic leaf extract of C. odorata against the larvae of Anopheles stephensi, Culex quinquefasciatus and Aedes aegypti and found up to 100% mortality in these larvae after 24 h of exposure. Similar to A. conyzoides, studies documenting the indigenous use of C. odorata are scarce (but see Cobbinah et al., 1999). The authors conducted ethnobotanical surveys on plants used for the protection of stored cereals in Ghana and reported that cowpea treated with C. odorata leaf powder were free of insect infestation for 4 months and that the locals attributed this to the insecticidal or repellent activities of C. odorata.
Tesfu and Emana (2013) studied the insecticidal properties of different parts of Parthenium hysterophorus powders against Callosobruchus chinensis over 48 h and found that the highest dose (2/50 g seeds) of inflorescence, leaf and stem powder caused 77, 73, and 57% mortality, respectively. The leaf, stem and root extracts of P. hysterophorus have been reported to be effective against Ae. aegypti; larval mortality of 40 to 100% was recorded after exposure to the aqueous leaf extracts of P. hysterophorus (Kumar S. et al., 2012; Amir et al., 2017). In an investigation of the insecticidal efficacy of the leaf extract of P. hysterophorus against the larvae of the rice moth, Corcyra cephalonica, Khan and Qamar (2015a) reported 81% mortality of larvae. In another experiment, Khan and Qamar (2015b) recorded 14.4% adult mortality in P. americana. Reddy et al. (2018) investigated the insecticidal activity of P. hysterophorus against P. xylostella and Aphis craccivora in a field experiment and found that P. hysterophorus leaf extract showed promising toxicity (LC50 = 1140.68 mg L−1) to larvae of P. xylostella and A. craccivora (LC50 = 839 mg L−1) after 96 h of treatment. The authors did not report any specific mortality rates. Although several studies (see references in Tables 2A–I) have recommended the use of P. hysterophorus as a pesticidal plant in its invasive ranges in Asia and Africa, there is no evidence to show that the locals especially the resource-poor small-holder farmers are exploiting it as yet.
In Nigeria and Tanzania, the leaf and stem bark extracts of Tithonia diversifolia have been reported to cause 100% mortality of adult C. maculatus (Obembe and Kayode, 2013; Green et al., 2017). Similarly, studies on the insecticidal activity of the leaf extract of T. diversifolia against S. zeamais showed 43% mortality in adults (Obembe and Kayode, 2013). Babarinde et al. (2008) and Adedire and Akinneye (2004) demonstrated that the leaf powder of T. diversifolia caused 90 and 99% mortality of S. zeamais and C. maculatus, respectively. In a field experiment, Mkenda et al. (2015) showed that the leaf extracts of T. diversifolia significantly reduced the population of the nymphs/larvae and adults of Aphis fabae, Ootheca mutabilis, O. bennigseni, Epicauta albovittata and E. limbatipennis. The authors further showed that the control offered by the leaf extracts were comparable to lambda-cyhalothrin, a commonly used synthetic pyrethroid. Although without mortality figures, Mkindi et al. (2017) reported some insecticidal activity of T. diversifolia leaf extract against some important pests (Aphis fabae, Ootheca mutabilis and O. bennigsen, Epicauta albovittata, E. limbatipennis, Clavigralla tomentosicollis, C. schadabi, and C. hystricodes) of beans in Tanzania and Malawi. The authors reported that T. diversifolia offered effective control of key pest species that was comparable in terms of harvested bean yield to a synthetic pyrethroid. The leaf extract of another species of Asteraceae, Xanthium strumarium caused more than 82% mortality in green peach aphid, Myzus persicae (Erdogan and Yildirim, 2016). In Uganda, farmers used the leaf extract and powder of T. diversifolia for the management of field and stored product pests (Mugisha-Kamatenesi et al., 2008; Mwine et al., 2011). Tithonia diversifolia is known to contain sesquiterpene lactones and diterpenoids (Chagas-Paula et al., 2012), some of which have biological activities against insects such as termites (Adoyo et al., 1997). However, there is no specific information about which compounds are responsible for its insecticidal effect. Despite the traditional use of Xanthium strumarium in ethnomedicine for treating a variety of diseases (Fan et al., 2019), its use by locals in the management of insect pests of agricultural and medical importance have not been documented.
Three species in the family, Solanaceae were reported effective against a number of important field and stored product insect pests. Zapata et al. (2006) investigated the insecticidal efficacy of the leaf extract of Cestrum parqui against the Mediterranean fruit fly, Ceratitis capitata and recorded 55% mortality in the adults of this pest. Investigations on the insecticidal activity of Solanum elaeagnifolium showed that the leaf and seed extracts of this plant accounted for 88 and 84% mortality, respectively against the larvae of T. castenum in Tunisia (Ben Hamouda et al., 2015a). The leaf and seed extracts of S. elaeagnifolium offered effective control against the Spodoptera littoralis (Ben Hamouda et al., 2015b). The authors found that leaf and seed extracts, respectively caused 80 and 100% mortality in the larvae of S. littoralis. Ben Hamouda et al. (2015c) reported the mortality rate of up to 5 and 43% caused by the leaf and seed aqueous extract of Solanum elaeagnifolium against M. persicae. In an investigation into the insecticidal activity of S. sisymbriifolium leaf extract against T. castenum, Padín et al. (2013) reported 22% mortality in adult beetles. The traditional use of the leaf extract of C. parqui, S. sisymbriifolium, and S. elaeagnifolium for the control and management of insect pests in their invasive ranges in Africa have not been documented and therefore requires some ethnobotanical studies.
Two species each in the family, Apocynaceae, Euphorbiaceae, and Fabaceae were reported effective against some insect pests of medical, environmental and agricultural importance. Remia and Logaswamy (2010) studied the insecticidal activity of the leaf extract of Catharanthus roseus against Ae. aegypti and reported over 71% mortality in the larvae and pupae of this mosquito species. Khan and Qamar (2015a,b) investigated the efficacy of Nerium oleander against the larvae of a rice moth, Corcyra cephalonica and P. americana and found up to 83% mortality in the larvae of the rice moth and P. americana. Despite the usage of Apocynaceae species in ethnomedicine (CABI, 2020a), their use as pesticides by locals is yet to be reported.
The leaf, seed, stem bark and root extracts of Jatropha curcas have been found effective (i.e., with 40 to 100% mortality) against the nymphs and larvae of P. xylostella, Helicoverpa armigera, Spodoptera frugiperda, and Schistocerca gregaria (Ribeiro et al., 2012; Bashir and El Shafie, 2013; Ingle et al., 2017). Opuba et al. (2018) and Adetimehin et al. (2018) showed that 3.0 g of the leaf and stem bark powders of J. curcas caused 100 percent mortality in C. maculatus in a laboratory test. The leaf extract of Ricinus communis reportedly caused 100% mortality on the larvae of P. xylostella (Tounou et al., 2011). Investigations into the insecticidal efficacy of the leaf, seed and fruit extracts of Prosopis juliflora caused up to 73% mortality in adult cotton aphid, A. gossypii (Zerihun and Ele, 2018). Sangavi and Johnson Thangaraj Edward (2017) reported between 73 and 96% mortality in P. xylostella when the larvae were treated with the leaf extract of P. juliflora and Sesbania grandiflora. While the use of R. communis for the management of insect pests by locals is not known, J. curcas is used by farmers in Uganda for the control and management of both field and storage pests (Mugisha-Kamatenesi et al., 2008). The ethnopesticidal usage of P. juliflora and S. grandiflora is yet to be documented and therefore warrant some ethnobotanical investigation.
Insecticidal activity of at least one species from the following families: Araceae, Bignoniaceae, Chenopodiaceae, Meliaceae, Mimosaceae, Myrtaceae, Papaveraceae, and Verbenaceae was investigated. Ito et al. (2015) investigated the insecticidal activity of Pistia stratiotes and found that the leaf powder of this aquatic weed reduced the population of Ae. aegypti by 80%. Our survey also found that the leaf powder of Jacaranda mimosifolia caused 30% mortality in adults of A. obtectus (Waweru et al., 2017), while the leaf extract caused 49% mortality in adults of T. castenum (Padín et al., 2013). Guzzo et al. (2006) reported that the leaf and fruit extracts of Dysphania ambrosioides only caused low adult mortality (<5%) in R. dominica. The fruit extract of Melia azedarach has been reported to be effective in the control of several pests. For example. The fruit extract of this weed caused 44% larval mortality in Liriomyza huidobrensis and 100% larval mortality in S. frugiperda and S. littoralis (Hammad and McAuslane, 2010; Scapinello et al., 2014). Chiffelle et al. (2011) documented 86% mortality when the adults of the Elm leaf beetle, Xanthogaleruca luteola were treated with the fruit extract of M. azedarach. Similarly, Selvaraj and Mosses (2011) reported over 88% larval mortality in An. stephensi, Cx. quinquefasciatus and Ae. aegypti when larvae were treated with the fruit extracts. Although we found no traditional usage of the Araceae, Bignoniaceae, Chenopodiaceae species as pesticidal plants, we found that in Ghana, the leaves of M. azedarach were used as a bioinsecticide to minimize the impact of Ephestia cautella on cocoa beans (CABI, 2020b).
In a laboratory experiment on the efficacy of the root extract of Mimosa diplotricha, Uyi et al. (2018b) reported 100% mortality in worker termites, Macrotermes species when exposed to different concentrations for 12 h. In a different experiment on the efficacy of the leaf and root powders of M. diplotricha against C. lectularius and C. maculatus, Uyi et al. (2018a, 2020) reported more than 67% mortality for both insects. Nia et al. (2015) reported 53% mortality in the nymphs and adults of M. persicae when the leaf extract of Eucalyptus camaldulensis was used to treat infestations of this pest. Khan and Qamar (2015a,b) found significant mortalities (15–76%) in C. cephalonica and P. americana when the larvae of the moth and nymphs of the cockroach were exposed to the leaf extracts of Argemone mexicana. We found no reports on the ethnopesticidal usage of M. diplotricha and E. camaldulensis, but for A. mexicana, von Weizsäckerl (1995) reported that the leaf extract is used in parts of India to prepare antifeedant sprays for the management of insect pests.
From the Verbenaceae family, Lantana camara was reported active against some mosquito species and major pests of crops due to the insecticidal potential of the plant. Remia and Logaswamy (2010) investigated the efficacy of the leaf extract of L. camara against Ae. aegypti in the laboratory and found more than 65% larval and pupal mortality. The essential oils from the leaves of L. camara caused between 93 and 100% in Ae. aegypti, Cx. quinquefasciatus, An. culicifacies, An. fluvialitis and An. Stephensi when adults were exposed for 24 h (Dua et al., 2010). Leaf powders and extracts of L. camara were also reported effective against a number of stored product pests (S. zeamais, S. oryzae, S. granaries, C. chinensis, T. castenum) where it caused 9–100% mortality depending on the concentration (of the extract/powder) and period of exposure (Sexana et al., 1992; Zoubiri and Baaliouamer, 2012; Rajashekar et al., 2014; Taye et al., 2014). In a laboratory experiment in China, the leaf extract of L. camara caused 90% mortality in the subterranean termite, Reticulitermes flavipes, when the workers were exposed for 24 h. The leaf extract of L. camara was reported to possess some insecticidal activities against some field pests (e.g., A. fabae, Ootheca mutabilis, O. bennigseni, Epicauta albovittata, E. limbatipennis, Clavigralla tomentosicollis, C. schadabi, and C. hystricodes) of beans in Tanzania and Malawi (Mkindi et al., 2017). Despite the ethnomedicinal uses of L. camara in Africa and the numerous studies on its pesticidal properties, there is surprisingly only one report (Mugisha-Kamatenesi et al., 2008) on the use of the plant for the management of insect pest species in the invasive range of the plant in Africa.
Due to the associated non-target effects and cost of synthetic insecticides in Africa, many resource-poor small-holder farmers on the continent rely on the use of crude plant-based materials collected from the wild and locally prepared (using the available technology or crude methods) to control and manage insect pests problems in subsistence farming, which is wide spread on the continent (Cobbinah et al., 1999; Belmain and Stevenson, 2001; Isman, 2008; Nyirenda et al., 2011; Kamanula et al., 2017). Despite the demonstrated laboratory and field efficacy of botanicals from many invasive alien plants against a myriad of agricultural, medical and environmental insect pests (Tables 2A–I), only a few studies have documented the indigenous use of these IAPs as botanical pesticides by the locals and small-holder farmers in Africa (e.g., Cobbinah et al., 1999; Mugisha-Kamatenesi et al., 2008). Therefore, there is an urgent need to conduct ethnobotanical surveys to identify and document the IAPs used for the control and management of insect pest by locals and small-holder farmers in Africa. Although, assessing efficacy under field condition remains a serious challenge in the use of botanicals to control insect pests of crops, recent field trials on bean and cabbage pests suggest that some plant extracts are as effective as synthetic pesticides; however, botanicals tend to be much less harmful to natural enemies (Amoabeng et al., 2013; Mkenda et al., 2015). Such findings are crucial in convincing the policy makers and other relevant stakeholders to support the use of botanicals to control pests. Therefore, field studies on the insecticidal efficacy of IAPs with botanical pesticides should be prioritized and such study may receive generous funding from stakeholders in the agricultural sector because of the direct impact of such research.
Despite their efficacy against pests, botanical pesticides are often less harmful to beneficial insects and are therefore more compatible with other pest management strategies (Stevensona et al., 2017). For example, Mkenda et al. (2015) showed that Tithonia diversifolia (an invasive alien plant species) and other three pesticidal plant species were able to control a several of agricultural pests attacking Phaseolus vulgaris (common beans), but were also less harmful to beneficial insects (i.e., lady beetle and spider mites) compared to a synthetic pesticide. In similar field study, Ezena et al. (2016) investigated the insecticidal potential of three concentrations (10, 20, and 30 g/L) of the invasive C. odorata in the management of the major pests of cabbage (B. brassicae and P. xylostella) and their natural enemies in southern Ghana. The authors found that the three concentrations of C. odorata significantly reduced (by more than 30%) the number of B. brassicae and P. xylostella than tap water and conventional insecticide, lambda-cyhalothrin. The authors also found that plots sprayed with 20 g/L of C. odorata extract supported the highest number of insect natural enemies (Diaretiell rapae, Cotesia plutellae (Hymentoptera: Braconidae) and hoverflies compared to plots treated with lambda-cyhalothrin. Research to demonstrating compatibility of botanical pesticides with other pest management strategies is needed. Such research should also focus on determination of the underlying mechanisms that reduce the impact of pesticidal IAPs on beneficial insects and understands if this is due to selective toxicity or lower persistence. Due to their high efficacy and low toxicity to beneficial insects (e.g., Mkenda et al., 2015), there is the prospect to inform locals, small-holder farmers, and other relevant stakeholders of the potential usage of the IAPs listed in Tables 2A–I. This will allow for the exploitation of IAPs by harvesting and using them to control insect pests and alternately minimizing the invasion intensities and impact of IAPs in ecosystems. This will give the small-holder farmers and locals who are typically resource poor access to technologies and information to control insect pests and diseases that limit crop production and successful storage of agricultural produce.
A key priority in the widely popular subsistence farming system in Africa is to prevent stored product insects from reducing the market and nutritional values of the harvested produce. Many small-holder farmers (peasants) in Africa use botanical pesticides, locally derived from either indigenous or IAPs to protect stored commodities (Cobbinah et al., 1999; Belmain and Stevenson, 2001; Isman, 2008; Nyirenda et al., 2011; Midega et al., 2016; Kamanula et al., 2017). The use of botanical pesticides to protect stored products may directly or indirectly expose farmers and/or consumers to potentially toxic chemicals from the plant materials used. It is important to note that naturally occurring plant chemicals are not necessarily safe. For example, some compounds (e.g., Aconitum, aconitine, nicotine, rotenone, and strychnine) of plant origin are known to be highly poisonous to mammals and fish (Kolev et al., 1996; Neuwinger, 2004).
Although the use of pesticidal plants to control pests in agro-ecosystems and other modified ecosystems is perceived to be safer than conventional pesticides, care must be exercised in the use of some of plants (especially invasive alien plants with novel biochemicals) for pest management. Invasive alien plants with potential toxicity to aquatic fauna or mammals should be restricted and discouraged. Plant scientists and entomologists should conduct special bioassays not only to show the efficacy of botanical pesticides from alien invasive plants but also to demonstrate the safety of these locally manufactured pesticides on mammals and aquatic fauna. The results of such safety and risk assessment studies should be communicated to various stakeholders including small-holder farmers who rely heavily on exploring new plant species for various purposes including to manage pests and for ethnomedicinal purpose. Although the likelihood of acute toxicity from handling plants is substantially lower than the risk from handling synthetic pesticides (Coats, 1994; Isman, 2006), the use of appropriate personal protective equipment should be encouraged when processing and handling powders and extracts from invasive alien plant materials.
Despite the acceptance and increasing usage of the biopesticides by the global communities, the lack of government published regulatory framework impedes the rigorous research processes and hampers the adoption of the compounds [Gahukar, 2011; AATF (African Agricultural Technology Foundation), 2013; Ivase et al., 2017; Damalas and Koutroubas, 2018]. Like synthetic insecticides, the international and national regulations should be developed to govern the development of bio-insecticides and alternately protect the consumers and the natural ecosystems from the hazardous compounds (Chandler et al., 2011). Although the natural resources extracted from nature are generally regarded as safe to humans and the environment, risk assessment protocols and registration portfolio of bio-insecticides follow conventional insecticides (Damalas and Koutroubas, 2018; Marrone, 2019). The procedures are somewhat time-consuming and expensive for the bio-insecticide development companies. Furthermore, the costs of production of these natural compounds decelerate the commercialization processes of the products and once commercialized, the prices are inflated (Marrone, 2014; Ivase et al., 2017; Damalas and Koutroubas, 2018). The prospects of developing biopesticides include the distinct development of legislations that govern the screening and commercialization of the products (AATF, 2013; Seiber et al., 2014; Kumar and Singh, 2015; Ivase et al., 2017; Damalas and Koutroubas, 2018). Government's ability to subsidize the research on the development of compounds that are safer to use may accelerate the bio-insecticide development and commercialization processes (Marrone, 2014). Furthermore, the efficiency of bioinsecticides with limited efficacy may be integrated with compatible pest management practices to optimize the efficiency of the pest management program (Chandler et al., 2011). Further investigations on the persistence and efficiency of biopesticides derived from IAPs need to be prioritized to measure the overall cost of the benefit of the pest management products. Public and private sectors should also be encouraged to participate (i.e., technically or financially) on the development and production of this economical and environmentally friendly alternative, especially in the developing countries.
The diversity of invasive alien plant species (in Africa) with numerous examples of their insecticidal efficacy against important pests listed in this paper suggest that opportunity exist for using invasive alien plants in Africa as pesticides in agro-ecosystems and other managed ecosystems. This will result in small-holders spending less on synthetic insecticides, substantially reduction in crop production or pest management costs and increase productivity and quality of life. Despite the rise of research interest in plant pesticides from native plants and IAPs over the last decade in Africa (Isman and Grieneisen, 2014; Isman, 2015), surprisingly little time is invested in assessing efficacy under field conditions. The lack of meaningful chemical data (i.e., elucidation of bioactive compounds) reported alongside efficacy trials remains a major concern. Some of the published works on the effects of pesticides from native plants or IAPs are not repeatable for various reasons and adds little to our knowledge about mechanisms, efficacy or scope to use plant materials in pest management. Although the efficacy of the botanical pesticides from 23 invasive alien plant species in this study have been documented, further investigations on; (1) their efficacy under field conditions, mode of action and chemical data, (2) their compatibility with other pest management strategies, (3) the economic benefits of using pesticidal plants over synthetic products and (4) how to effectively commercialize the production of botanical insecticides from IAPs. For the first time, our review elucidates the insecticidal efficacy of the invasive alien plants in Africa and highlights the prospects for the use of these IAPs as pesticidal plants in African countries especially among resource-limited small-holder farmers and locals. It remains to be seen whether stakeholders (governments, research institutions, scientists, agriculturists, farmers, locals, extension workers, etc.) can effectively explore the safe use of botanically based insecticides (extracts, powders or other formulations) from IAPs in their regions for the control and management of insect pests in agro-ecosystems and other modified environments. This paper serves as a veritable reference for researchers and stakeholders who are interested in advancing, the science, technology or our understanding of the use of invasive alien plant to control and manage insect pests of agricultural, environmental medical importance.
OU and LM conceptualized the study and wrote the manuscript. OU, LM, AE, and MT interpreted the results and critically reviewed and amended the manuscript. All authors contributed to the article and approved the submitted version.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
We thank our respective institutions for availing us the needed time to write this review. We also thank Glory Dickson for providing some of the literature used in this review.
AATF (African Agricultural Technology Foundation). (2013). A Guide to the Development of Regulatory Frameworks for Microbial Biopesticides in Sub-Saharan Africa. Nairobi: African Agricultural Technology Foundation. Available online at: https://www.aatf-africa.org/wp-content/uploads/2018/11/Microbial-biopesticides.pdf
Abdulahi, M. M., Ute, J. A., and Regasa, T. (2017). Prosopis juliflora L: distribution, impacts and available control methods in Ethiopia. Trop. Subtrop. Agroecosyst. 20, 75–89.
Adedire, C. O., and Akinneye, J. O. (2004). Biological activity of tree marigold, Tithonia diversifolia, on cowpea bruchid, Callosobruchus maculatus (Coleoptera: Bruchidae). Ann. Appl. Biol. 144, 185–189. doi: 10.1111/j.1744-7348.2004.tb00332.x
Adetimehin, A. D., Opuba, S. K., Iloba, B. N., and Uyi, O. O. (2018). Insecticidal and anti-ovipositional activities of the stem-bark powder of Jatropha curcas (L.) (Euphorbiaceae) against Callosobruchus maculatus (Fab.) (Coleoptera: Chrysomelidae). Adv. Sci. Technol. 12, 27–34.
Adoyo, F., Mukulama, J. B., and Enyola, M. (1997). Using Tithonia concoctions for termite control in Busia District, Kenya. Ileia Newslett. 13, 24–25
Aigbokhan, E. I., Osazuwa-Peters, O. L., and Ilubon, K. O. (2010). Range and distribution of Mimosa diplotricha in Nigeria and effects of fire on seed germination. Nigerian J. Bot. 241, 141–151.
Alagesaboopathi, C., and Deivanai, M. (2011). Allelopathic potential of Sesbania grandiflora Pers. on germination of Cajanus cajan Mill. Sp. (Redgram) varieties. Int. J. Biosci. 1, 51–55.
Amir, H., Butt, B. Z., and Vehra, S. E. (2017). Evaluation of larvicidal activity of Parthenium hysterophorus against Aedes aegypti. Int. J. Mosquito Res. 4, 1–4.
Amoabeng, B. W., Gurr, G. M., Gitau, C. W., Nicol, H. I., Munyakazi, L., and Stevenson, P. C. (2013). Tri-trophic insecticidal effects of African plants against cabbage pests. PLoS ONE 8:e78651. doi: 10.1371/annotation/f0351003-b6f8-4249-ace5-bcd84dead916
Babarinde, S. A., Olabode, O. S., Akanbi, M. O., and Adeniran, O. A. (2008). Potential of Tithonia diversifolia with pirimiphos methyl in control of Sitophilus zeamais (Coleoptera: Curculionidae). Afr. J. Plant Sci. Biotechnol. 2, 77–80.
Bashir, E., and El Shafie, H. (2013). Insecticidal and antifeedant efficacy of Jatropha oil extract against the Desert Locust, Schistocerca gregaria (Forskal) (Orthoptera: Acrididae). Agric. Biol. J. N. Am. 4, 260–267. doi: 10.5251/abjna.2013.4.3.260.267
Belmain, S. R., and Stevenson, P. C. (2001). Ethnobotanicals in Ghana: reviving and modernising an age-old practise. Pesticide Outlook 6, 233–238. doi: 10.1039/b110542f
Ben Hamouda, A., Boussadia, O., Bedis, K., Laarif, A., and Braham, M. (2015c). Studies on insecticidal and deterrent effects of olive leaf extracts we Myzus persicae and Phthorimaea operculella. J. Entomol. Zool. Stud. 3, 294–297.
Ben Hamouda, A., Chaieb, I., Zarrad, K., and Laarif, A. (2015a). Insecticidal activity of methanolic extract of Silverleaf nightshade against Tribolium castaneum. Int. J. Entomol. Res. 3, 23–28.
Ben Hamouda, A., Zarrad, K., Chaieb, K., and Laarif, A. (2015b). Antifeedant and insecticidal properties of Solanum elaeagnifolium extracts on the African Cotton Leafworm. Azarian J. Agric. 2, 71–74.
Bommarco, R., Kleijn, D., and Potts, S. G. (2013). Ecological intensification: harnessing ecosystem services for food security. Trends Ecol. Evol. 28, 230–238. doi: 10.1016/j.tree.2012.10.012
CABI (2020a). Nerium oleanda (Oleander). Invasive Species Compendium. Available online at: https://www.cabi.org/isc/datasheet/36220 (accessed December 23, 2020).
CABI (2020b). Melia azedarach (Chinaberry). Invasive Species Compendium. Available online at: https://www.cabi.org/ISC/datasheet/33144 (accessed November 2, 2020).
Calle, J., Rivera, A., Luis, G. J., Agular, Z. E., Niemeyer, H. M., and Joseph-Nathan, P. (1990). Insecticidal activity of the petroleum ether extract of Ageratum conyzoides L. ReBioinvasions Recv. Colombiana Química Rev. Col. Quim. 19:91.
Catarino, L., Indjai, B., Duarte, M. C., and Monteiro, F. (2019). Chromolaena odorata invasion in Guinea-Bissau (West Africa): first records and trends of expansion. Bioinvasions Rec. 8, 190–198. doi: 10.3391/bir.2019.8.1.20
Chagas-Paula, D. A., Oliveira, R. B., Rocha, B. A., and Da Costa, F. B. (2012). Ethnobotany, chemistry, and biological activities of the genus Tithonia (Asteraceae). Chem. Biodivers. 9, 210–235. doi: 10.1002/cbdv.201100019
Chandler, D., Bailey, A. S., Tatchell, G. M., Davidson, G., Greaves, J., and Grant, W. P. (2011). The development, regulation and use of biopesticides for integrated pest management. Philos. Transac. R. Soc. B Biol. Sci. 366, 1987–1998. doi: 10.1098/rstb.2010.0390
Chiffelle, I., Huerta, A., Azua, F., Puga, K., and Araya, J. E. (2011). Antifeeding and insecticide properties of aqueous and ethanolic fruit extracts from Melia azedarach L. on the Elm leaf beetle Xanthogaleruca luteola Müller. Chilean J. Agric. Res. 71, 218–225. doi: 10.4067/S0718-58392011000200006
Coats, J. R. (1994). Risks from natural versus synthetic insecticides. Ann. Rev. Entomol. 39, 489–515. doi: 10.1146/annurev.en.39.010194.002421
Cobbinah, J. R., Moss, C., Golob, P., and Belmain, S. R. (1999). Conducting Ethnobotanical Surveys: An Example From Ghana on Plants Used for the Protection of Stored Cereals and Pulses (NRI Bulletin 77).
Damalas, C. A., and Koutroubas, S. D. (2018). Current status and recent developments in biopesticide use. Agriculture 8:13. doi: 10.3390/agriculture8010013
Dandurand, L. M., Zasada, I. A., and LaMondia, J. A. (2019). Effect of the trap crop, Solanum sisymbriifolium, on Globodera pallida, Globodera tabacum, and Globodera ellingtonae. J. Nematol. 51, 1–11. doi: 10.21307/jofnem-2019-030
Das, M., Acharya, B., Saquib, M., and Chettri, M. (2018). Effect of aqueous extract and compost of invasive weed Ageratina adenophora on seed germination and seedling growth of some crops and weeds. J. Biodivers. Conserv. Bioresour. Manage. 4, 11–20. doi: 10.3329/jbcbm.v4i2.39843
Davis, M. A. (2006). Invasion biology 1958-2005: the pursuit of science and conservation in Conceptual Ecology and Invasion Biology: Reciprocal Approaches to Nature, eds M.W. Cadotte, S. M. Mcmahon, and T. Fukami (Dordrecht: Springer), 1–27.
Deressa, T., Lemessa, F., and Wakjira, M. (2015). Antifungal activity of some invasive alien plant leaf extracts against mango (Mangifera indica) anthracnose caused by Colletotrichum gloeosporioides. Int. J. Pest Manage. 61, 99–105. doi: 10.1080/09670874.2015.1016135
Dougoud, J., Toepfer, S., Bateman, M., and Jenner, W. H. (2019). Efficacy of homemade botanical insecticides based on traditional knowledge. A review. Agron. Sustain. Dev. 39, 1–22. doi: 10.1007/s13593-019-0583-1
Dua, V. K., Pandey, A. C., and Dash, A. P. (2010). Adulticidal activity of essential oil of Lantana camara leaves against mosquitoes. Indian J. Med. Res. 131, 434–439.
Ekhator, F., Uyi, O. O., Ikuenobe, C. E., and Okeke, C. E. (2013). The distribution and problems of the invasive alien plant, Mimosa diplotricha C. Wright ex Sauvalle (Mimosaceae) in Nigeria. Am. J. Plant Sci. 4, 866–877. doi: 10.4236/ajps.2013.44107
Erdogan, P., and Yildirim, A. (2016). Insecticidal activity of three different plant extracts on the green peach aphid [(Myzus persicae Sulzer) (Hemiptera: Aphididae)]. J. Entomol. Res. Soc. 18, 27–35.
Ezena, G. N., Akotsen-Mensah, C., and Fening, K. O. (2016). Exploiting the insecticidal potential of the invasive siam weed, Chromolaena odorata L. (Asteraceae) in the management of the major pests of cabbage and their natural enemies in southern Ghana. Adv. Crop Sci. Technol. 4:1000230. doi: 10.4172/2329-8863.1000230
Fan, W., Fan, L., Peng, C., Zhang, Q., Wang, L., Li, L., et al. (2019). Traditional uses, botany, phytochemistry, pharmacology, pharmacokinetics and toxicology of xanthium strumarium L.: a review. Molecules 24:359. doi: 10.3390/molecules24020359
Farag, M., Ahmed, M. H. M., Yousef, H., and Abdel-Rahman, A. A. H. (2011). Repellent and insecticidal activities of Melia azedarach L. against cotton leafworm, Spodoptera littoralis (Boisd.). Zeitschrift fur Naturforschung. J. Biosci. 66, 129–135. doi: 10.1515/znc-2011-3-406
Foxcroft, L. C., Henderson, L., Nichols, G. R., and Martin, B. W. (2003). A revised list of alien plants for the Kruger National Park. Koedoe 26, 21–44. doi: 10.4102/koedoe.v46i2.54
Gahukar, R. T. (2011). Use of neem and plant-based biopesticides in floriculture: current challenges and perspectives – a review. J. Horticult. Sci. Biotechnol. 86, 203–209. doi: 10.1080/14620316.2011.11512748
Gillett, J. B. (1963). Sesbania in Africa (excluding Madagascar) and southern Arabia. Kew Bull. 17, 91–157. doi: 10.2307/4118710
Green, P. W. C., Belmain, S. R., Ndakidemi, P. A., Farrell, I. W., and Stevenson, P. C. (2017). Insecticidal activity of Tithonia diversifolia and Vernonia amygdalina. Ind. Crops Prod. 110, 15–21. doi: 10.1016/j.indcrop.2017.08.021
Guzzo, E. G., Tavares, M. A. G. C., and Vendramim, J. D. (2006). Evaluation of insecticidal activity of aqueous extracts of Chenopodium spp. in relation to Rhyzopertha dominica (Fabr.) (Coleoptera: Bostrichidae) in 9th International Working Conference on Stored Product Protection PS7-37 – 6333 (São Paulo).
Hammad, E. A. F., and McAuslane, H. (2010). Effect of Melia azedarach L. extract on Liriomyza sativae (Diptera: Agromyzidae) and its biocontrol agent Diglyphus isaea (Hymenoptera: Eulophidae). J. Food Agric. Environ. 8, 1247–1252.
Hanley, N., and Roberts, M. (2019). The economic benefits of invasive species management. People Nat. 1, 124–137. doi: 10.1002/pan3.31
Henderson, L. (2001). Alien Weeds and Invasive Plants: A Complete Guide to Declared Weeds and Invaders in South Africa Handjournal No. 12. Pretoria: ARC-PPRI.
Henderson, L. (2007). Invasive, naturalized and casual alien plants in southern Africa: a summary based on the Southern African Plant Invaders Atlas (SAPIA). Bothalia 37, 215–248. doi: 10.4102/abc.v37i2.322
Henderson, L., and Cilliers, C. J. (2002). Invasive Aquatic Plants, Plant Protection Research Institute Handjournal No. 16. Pretoria: Agricultural Research Council, 1–88.
Hoffmann, B. D., and Broadhurst, L. M. (2016). The economic cost of managing invasive species in 320 Australia. NeoBiota 31, 1–18. doi: 10.3897/neobiota.31.6960
Ingle, K. P., Deshmukh, A. G., Padole, D. A., Dudhare, M. S., Moharil, M. P., and Khelurkar, V. C. (2017). Screening of insecticidal activity of Jatropha Curcas (L.) against diamond back moth and Helicoverpa Armigera. J. Entomol. Zool. Stud. 5, 44–50.
Isman, M. B. (2006). Botanical insecticides in an increasingly regulated world. Annu. Rev. Entomol. 51, 45–66. doi: 10.1146/annurev.ento.51.110104.151146
Isman, M. B. (2008). Perspective botanical insecticides: for richer, for poorer. Pest Manag. Sci. 64, 8–11. doi: 10.1002/ps.1470
Isman, M. B. (2015). A renaissance for botanical insecticides? Pest Manag. Sci. 71, 1587–1590. doi: 10.1002/ps.4088
Isman, M. B., and Grieneisen, M. L. (2014). Botanical insecticide research: many publications, limited useful data. Trends Plant Sci. 19, 140–145. doi: 10.1016/j.tplants.2013.11.005
Ito, E. E., Nmor, J. C., Ake, J. E. G., and Utebor, K. (2015). Larvicidal activity of Pistia stratiotes (Water Lettuce) against Larvae of Aedes aegypti. Adv. Res. 3, 589–595. doi: 10.9734/AIR/2015/13667
Ivase, T. J.-P., Nyakuma, B. B., Ogenyi, B. U., Balogun, A. D., and Hassan, M. N. (2017). Current status, challenges, and prospects of biopesticide utilization in Nigeria. Agric. Environ. 9, 95–106. doi: 10.1515/ausae-2017-0009
Jaya, P. S., Prakash, B., and Dubey, N.K. (2014). Insecticidal activity of Ageratum conyzoides L., Coleus aromaticus Benth. and Hyptis suaveolens (L.) Poit essential oils as fumigant against storage grain insect Tribolium castaneum Herbst. J. Food Sci. Technol. 51, 2210–2215. doi: 10.1007/s13197-012-0698-8
Kamanula, J. F., Belmain, S. R., Hall, D. R., Farman, D. I., Goyder, D. J., Mvumi, B. M., et al. (2017). Chemical variation and insecticidal activity of Lippia javanica (Burm. F.) Spreng essential oil against Sitophilus zeamais Motschulsky. Industr. Crops Prod. 110, 75–82. doi: 10.1016/j.indcrop.2017.06.036
Kankam, F. (2021). Causes and management of pesticides contamination in agriculture: a review. Ghana J. Sci. 7:2. doi: 10.47881/265.967x
Khan, I., and Qamar, A. (2015a). Comparative bioefficacy of selected plant extracts and some commercial biopesticides against important household pest, Periplaneta americana. J. Entomol. Zool. Stud. 3, 219–224.
Khan, I., and Qamar, A. (2015b). Evaluation of antifeedant and larvicidal activity of some commercial biopesticides and plant extracts on Rice Moth, Corcyra cephalonica (Stainton). Eur. J. Exp. Biol. 5, 61–68.
Khayatnezhad, M., and Nasehi, F. (2021). Industrial pesticides and a methods assessment for the reduction of associated risks: a review. Adv. Life Sci. 8, 202–210.
Kiran, B. R., and Prasad, M. N. V. (2017). Ricinus communis L. (Castor bean), a potential multi-purpose environmental crop for improved and integrated phytoremediation. EuroBiotech J. 1, 101–116. doi: 10.24190/ISSN2564-615X/2017/02.01
Klein, H. (2011). A catalogue of the insects, mites and pathogens that have been used or rejected, or are under consideration, for the biological control of invasive alien plants in South Africa. Afr. Entomol. 19, 515–549. doi: 10.4001/003.019.0214
Kolev, S. T., Leman, P., Kite, G. C., Stevenson, P. C., Shaw, D., and Murray, V. S. G. (1996). Toxicity following accidental ingestion of Aconitum containing Chinese remedy. Hum. Exp. Toxicol. 15, 839–842. doi: 10.1177/096032719601501008
Kumar, S., Nair, G., Singh, A. P., Batra, S., Wahab, N., and Warikoo, R. (2012). Evaluation of the larvicidal efficiency of stem, roots and leaves of the weed, Parthenium hysterophorus (Family: Asteraceae) against Aedes aegypti. Asian Pacific J. Trop. Dis. 2, 395–400. doi: 10.1016/S2222-1808(12)60086-3
Kumar, S., and Singh, A. (2015). Biopesticides: present status and the future prospects. J. Fertilizers Pesticides 6:2. doi: 10.4172/2471-2728.1000e129
Kumar, S. D., Masarrat, H., and Muntaha, Q. (2012). Comparative potential of different botanicals and synthetic insecticides and their economics against Leucinodes orbonalis in eggplant. J. Plant Protect. Res. 52, 35–39. doi: 10.2478/v10045-012-0006-7
Lawal, O. A., Opoku, A. R., and Ogunwande, I. A. (2015). Phytoconstituents and insecticidal activity of different solvent leaf extracts of Chromolaena odorata L., against Sitophilus zeamais (Coleoptera: Curculionidae). Eur. J. Med. Plants 5, 237–247. doi: 10.9734/EJMP/2015/6739
Litt, A. R., Cord, E. E., Fulbright, T. E., and Shuster, G. L. (2014). Effects of invasive plants on arthropods. Conserv. Biol. 28, 1532–1549. doi: 10.1111/cobi.12350
Liu, X. C., and Liu, Z. L. (2014). Evaluation of larvicidal activity of the essential oil of Ageratum conyzoides L. aerial parts and its major constituents against Aedes albopictus. J. Entomol. Zool. Stud. 2, 345–350.
Macdonald, A. W., Reaser, J. K., Bright, C., Neville, L. E., Howard, G. W., Murphy, S. J., et al. (2003). Invasive Alien Species in South Africa: National Reports and Directory of Resources. GISP, Cape Town, SA.
Marais, C., van Wilgen, B. W., and Stevens, D. (2004). The clearing of invasive alien plants in South Africa: a preliminary assessment of costs and progress. S. Afr. J. Sci. 100, 97–103.
Marrone, P. G. (2014). The market and potential for biopesticides, in Biopesticides: State of the Art and Future Opportunities in ACS Symposium Series, Vol. 1172, eds A. D. Gross, J. R. Coats, S. O. Duke, and J. N. Seiber (Washington, DC: American Chemical Society), 245–258.
Marrone, P. G. (2019). Pesticidal natural products – status and future potential. Pest Manag. Sci. 75, 2325–2340. doi: 10.1002/ps.5433
McConnachie, A. J., Strathie, L. W., Mersie, W., Gebrehiwot, L., Zewdie, K., Abdurehim, A., et al. (2010). Current and potential geographical distribution of the invasive plant Parthenium hysterophorus (Asteraceae) in eastern and southern Africa. Weed Res. 51, 71–84. doi: 10.1111/j.1365-3180.2010.00820.x
Midega, C. A. O., Murage, A. W., Pittchar, J. O., and Khan, Z. R. (2016). Managing storage pests of maize Farmers' knowledge, perceptions and practices in western Kenya. Crop Prot. 90, 142–149. doi: 10.1016/j.cropro.2016.08.033
Mkenda, P. A., Mwanauta, R., Stevenson, P. C., Ndakidemi, P., Mtei, K., and Belmain, S. R. (2015). Field margin weeds provide economically viable and environmentally benign pest control compared to synthetic pesticides. PLoS ONE 10:e0143530. doi: 10.1371/journal.pone.0143530
Mkindi, A., Mpumi, N., Tembo, Y., Stevenson, P. C., Ndakidemi, P. A., Mtei, K., et al. (2017). Invasive weeds with pesticidal properties as potential new crops. Ind. Crops Prod. 110, 113–122. doi: 10.1016/j.indcrop.2017.06.002
Mogg, C., Petit, P., Cappuccino, N., Durst, T., McKague, C., Foster, M., et al. (2008). Tests of the antibiotic properties of the invasive vine Vincetoxicum rossicum against bacteria, fungi and insects. Biochem. Syst. Ecol. 36, 383–391. doi: 10.1016/j.bse.2008.01.001
Moreira, D. M., Picanço, C. M., Barbosa, L. C. A., Guedes, R. N. C., and Silver, E. M. (2004). Toxicity of leaf extracts of Ageratum conyzoides to Lepidoptera pests of horticultural crops. Biol. Agric. Hortic. 22, 251–260. doi: 10.1080/01448765.2004.9755288
Moreira, M. D., Picanco, M. C., and Barbosa, L. C. (2007a). Compounds from Ageratum conyzoides: isolation, structural elucidation, and insecticidal activity. Pest Manag. Sci. 63, 615–621. doi: 10.1002/ps.1376
Moreira, M. D., Picanço, M. C., Barbosa, L. C., Guedes, R. N. C., de Campos, R., Silva, G. A., et al. (2007b). Plant compounds insecticide activity against Coleoptera pests of stored products. Pesq. Agropec. Bras. Brasília 42, 909–915. doi: 10.1590/S0100-204X2007000700001
Morokong, T., Blignaut, J., Nkambule, N., Mudhavanhu, S., and Vundla, T. (2016). Clearing invasive alien plants as a cost-effective strategy for water catchment management: the case of the Olifants river catchment, South Africa. South Afr. J. Econ. Manage. Sci. 19, 774–787. doi: 10.4102/sajems.v19i5.1594
Mostert, E., Gaertner, M., Holmes, P. M., Rebelo, A. G., and Richardson, D. M. (2017). Impacts of invasive alien trees on threatened lowland vegetation types in the Cape Floristic Region, South Africa. South Afr. J. Bot. 108, 209–222. doi: 10.1016/j.sajb.2016.10.014
Mugisha-Kamatenesi, M., Deng, A. L., Ogendo, J. O., Omolo, E. O., Mihale, M. J., Otim, M., et al. (2008). Indigenous knowledge of field insect pests and their management around Lake Victoria Basin in Uganda. Afr. J. Environ. Sci. Technol. 2, 342–348.
Mugwedi, L. (2020). Harnessing opportunities provided by the invasive Chromolaena odorata to keep it under control. Sustainability 12:6505. doi: 10.3390/su12166505
Mukwevho, L., and Mphephu, T. E. (2020). The role of the flower-galling mite, Aceria lantanae, in integrated control of the light pink 163LP variety of Lantana camara (L.) in South Africa. Biol. Control 49:104309. doi: 10.1016/j.biocontrol.2020.104309
Mukwevho, L., Olckers, T., and Simelane, D. O. (2018). Occurrence of different Lantana camara varieties across four South African provinces and their susceptibility to a biotype of the gall-forming mite Aceria lantanae. Biocontrol Sci. Technol. 28, 377–387. doi: 10.1080/09583157.2018.1450490
Mulè, R., Sabella, G., Robba, L., and Manachini, B. (2017). A systematic review of the effects of chemical insecticides on four common butterfly families. Front. Environ. Sci. 5:32. doi: 10.3389/fenvs.2017.00032
Muniappan, R., Reddy, G., and Lai, P. Y. (2005). Distribution and biological control of Chromolaena odorata in Invasive Plants: Ecological and Agricultural Aspects, ed Inderjit (Basel: Birkhäuser), 223–233.
Mwine, J., Pvan, D., Kamoga, G., Kudamba, P., Nasuuna, M., and Jumba, F. (2011). Ethnobotanical survey of pesticidal plants used in South Uganda: case study of Masaka district. J. Med. Plants Res. 5, 1155–1163.
Negussie, A., Nacro, S., Achten, W. J., Norgrove, L., Kenis, M., Hadgu, K., et al. (2014). Insufficient evidence of Jatropha curcas L. Invasiveness: experimental observations in Burkina Faso, West Africa. BioEnergy Res. 8, 1–11. doi: 10.1007/s12155-014-9544-3
Neuwinger, H. D. (2004). Plants used for poison fishing in tropical Africa. Toxicon 44, 417–430. doi: 10.1016/j.toxicon.2004.05.014
Ngorima, A., and Shackleton, C. M. (2019). Livelihood benefits and costs from an invasive alien tree (Acacia dealbata) to rural communities in the Eastern Cape, South Africa. J. Environ. Manage. 229, 158–165. doi: 10.1016/j.jenvman.2018.05.077
Nia, B., Frash, A., and Azou, I. (2015). Insecticidal activity of three plants extracts against Myzus persicae (Sulzer, 1776) and their phytochemical screening. Acta Agric. Slov. 105, 261–267. doi: 10.14720/aas.2015.105.2.09
Nwauzoma, A. B., and Dappa, M. S. (2013). Ethnobotanical studies of Port Harcourt metropolis, Nigeria. ISRN Bot. 2013:829424. doi: 10.1155/2013/829424
Nyirenda, S. P. N., Sileshi, G., Belmain, S. R., Kamanula, J. F., Mvumi, B., Sola, P., et al. (2011). Farmers' ethno-Ecological knowledge of vegetable pests and their management using pesticidal plants in northern Malawi and eastern Zambia. Afr. J. Agric. Res. 6, 1525–1537.
Obembe, O. M., and Kayode, J. (2013). Insecticidal activity of the aqueous extracts of four under-utilized tropical plants as protectant of cowpea seeds from Callosobruchus maculatus infestation. Pakistan J. Biol. Sci. 16, 175–179. doi: 10.3923/pjbs.2013.175.179
Obiakara, M. C., and Fourcade, Y. (2018). Climatic niche and potential distribution of Tithonia diversifolia (Hemsl.) A. Gray in Africa. PLoS ONE 13:9e0202421. doi: 10.1371/journal.pone.0202421
O'Connor, T. G., and van Wilgen, B. W. (2020). The impact of invasive alien plants on rangelands in South Africa in Biological Invasions in South Africa. Invading Nature - Springer Series in Invasion Ecology, Vol. 14, eds B. van Wilgen, J. Measey, D. Richardson, J. Wilson, T. Zengeya (Cham: Springer), 459–487.
Oliveira, G. L. S., Oliveira, F. R. A. M., de Alencar, M. V. O. B, Junior, A. L. G., de Souza, A. A., Cavalcante, A. A. C. M., et al. (2014). Evaluation of antioxidant capacity of the aqueous extract of Cynara scolymus L. (Asteraceae) in vitro and in Saccharomyces cerevisiae. Afr. J. Pharm. Pharmacol. 8, 136–147. doi: 10.5897/AJPP2013.3836
Omokhua, A. G., Abdalla, M. A., Van Staden, J., and McGaw, L. J. (2018a). A comprehensive study of the potential phytomedicinal use and toxicity of invasive Tithonia species in South Africa. BMC Complement. Altern. Med. 18:272. doi: 10.1186/s12906-018-2336-0
Omokhua, A. G., Madikizela, A., Aro, A., Uyi, O. O., Van Staden, J., and McGaw, L. J. (2018b). Noxious to ecosystems, but relevant to pharmacology: four South African alien invasive plants with pharmacological potential. South Afr. J. Bot. 117, 41–49. doi: 10.1016/j.sajb.2018.04.015
Omokhua, A. G., McGaw, L. J., Finnie, J. F., and Van Staden, J. (2016). Chromolaena odorata (L.) R.M. King and H. Rob. (Asteraceae) in sub-Saharan Africa: a synthesis and review of its medicinal potential. J. Ethnopharmacol. 183, 112–122. doi: 10.1016/j.jep.2015.04.057
Opuba, S. K., Adetimehin, A. D., Iloba, B. N., and Uyi, O. O. (2018). Insecticidal and anti-ovipositional activities of the leaf powder of Jatropha curcas (L.) (Euphorbiaceae) against Callosobruchus maculatus (F.) (Coleoptera: Chrysomelidae). Anim. Res. Int. 15, 2971–2978.
Padín, S. B., Fusé, C., Urrutia, M. I., and Dal Bello, G. M. (2013). Toxicity and repellency of nine medicinal plants against Tribolium castaneum in stored. Bull. Insectol. 66, 45–49.
Park, M. J., Cho, S. E., Wolcan, S., and Shin, H. D. (2012). First report of powdery mildew caused by Erysiphe betae on the invasive weed Dysphania ambrosioides in Korea. Plant Dis. 96, 592–596. doi: 10.1094/PDIS-11-11-1003
Phillips, T. W., and Throne, J. E. (2010). Biorational approaches to managing stored-product insects. Annu. Rev. Entomol. 55, 375–397. doi: 10.1146/annurev.ento.54.110807.090451
Pimentel, D. (1995). Amounts of pesticides reaching target pests: environmental impacts and ethics. J. Agric. Environ. Ethics 8, 17–29. doi: 10.1007/BF02286399
Radtke, A., Ambra, S., Zerbe, S., Tonon, G., Fontana, V., and Ammer, C. (2013). Traditional coppice forest management drives the invasion of Ailanthus altissima and Robinia pseudoacacia into deciduous forests. For. Ecol. Manage. 291, 308–317. doi: 10.1016/j.foreco.2012.11.022
Rajashekar, Y., Ravindra, K. V., and Bakthavatsalam, N. (2014). Leaves of Lantana camara Linn. (Verbenaceae) as a potential insecticide for the management of three species of stored grain insect pests. J. Food Sci. Technol. 51, 3494–3499. doi: 10.1007/s13197-012-0884-8
Reddy, S. G. E., Dolma, S. K., Verma, P. K., and Singh, B. (2018). Insecticidal activities of Parthenium hysterophorus L. extract and parthenin against diamondback moth, Plutella xylostella (L.) and aphid, Aphis craccivora Koch. Toxin Rev. 37, 161–165. doi: 10.1080/15569543.2017.1339281
Remia, K. M., and Logaswamy, S. (2010). Larvicidal efficacy of leaf extract of two botanicals against the mosquito vector Aedes aegypti (Diptera: Culicidae). Indian J. Nat. Prod. Resour. 1, 208–212.
REX consortium (2010). The skill and style to model the evolution of resistance to pesticides and drugs. Evol. Appl. 3, 375–390. doi: 10.1111/j.1752-4571.2010.00124.x
Ribeiro, S. S., Silva, T. B., Moraes, V. R. S., and Nogueira, P. C. L. (2012). Chemical constituents of methanolic extracts of Jatropha curcas L and effects on Spodoptera frugiperda (J.E. Smith) (Lepidoptera: Noctuidae). Quim. Nova 35, 2218–2221. doi: 10.1590/S0100-40422012001100022
Richardson, D. M., and Pyšek, P. (2012). Naturalization of introduced plants: ecological drivers of biogeographical patterns. New Phytol. 196, 383–396. doi: 10.1111/j.1469-8137.2012.04292.x
Richardson, D. M., and van Wilgen, B. W. (2004). Invasive alien plants in South Africa: how well do we understand the ecological impacts? S. Afr. J. Sci. 100, 45–52.
Rioba, N. B., and Stevenson, P. C. (2017). Ageratum conyzoides L. for the management of pests and diseases by small holder farmers. Industr. Crops Prod. 110, 22–29. doi: 10.1016/j.indcrop.2017.06.068
Sangavi, R., and Johnson Thangaraj Edward, Y. S. (2017). Anti-Insect activities of plant extracts on the diamondback moth, Plutella xylostella (L.). Int. J. Curr. Microbiol. Appl. Sci. 6, 28–39. doi: 10.20546/ijcmas.2017.612.004
Sasson, A. (2012). Food security for Africa: an urgent global challenge. Agric. Food Secur. 1:2. doi: 10.1186/2048-7010-1-2
Saxena, R., and Sharma, A. K. (2005). Insecticidal potentialities of Ageratum conyzoides and Nerium indicum leaves extracts against Epilachna 28-punctata (F.). Vegetos 18, 43–45.
Scapinello, J., de Oliveira, J. V., Chiaradia, L. A., Tomazelli Junior, O., Niero, R., and Magro, J. D. (2014). Insecticidal and growth inhibiting action of the supercritical extracts of Melia azedarach on Spodoptera frugiperda. Rev. Brasil. Engenharia Agrícola Ambiental 18, 866–872. doi: 10.1590/1807-1929/agriambi.v18n08p866-872
Seiber, J. N., Coats, J., Duke, S. O., and Gross, A. D. (2014). Biopesticides: state of the art and future opportunities. J. Agric. Food Chem. 62, 11613–11619. doi: 10.1021/jf504252n
Selvaraj, M., and Mosses, M. (2011). Efficacy of Melia azedarach on the larvae of three mosquito species Anopheles stephensi, Culex quinquefasciatus and Aedes aegypti (Diptera: Culicidae). Eur. Mosquito Bull. 29, 116–121.
Sexana, R. C., Dixit, P., and Harshan, V. (1992). Insecticidal action of Lantana camara against Callosobruchus chinensis (Coleoptera: Bruchidae). J. Stored Prod. Res. 28, 279–281. doi: 10.1016/0022-474X(92)90009-F
Shackleton, C., McGarry, D., Fourie, S., Gambiza, J., Shackleton, S., and Fabricius, C. (2007). Assessing the effects of invasive alien species on rural livelihoods: case examples and a framework from South Africa. Hum. Ecol. 35, 113–127. doi: 10.1007/s10745-006-9095-0
Shackleton, R. T., Shackleton, C. M., and Kull, C. A. (2018). The role of invasive alien species in shaping local livelihoods and human well-being: a review. J. Environ. Manage. 229, 145–157. doi: 10.1016/j.jenvman.2018.05.007
Shackleton, R. T., Witt, A. B., Aool, W., and Pratt, C. F. (2017). Distribution of the invasive alien weed, Lantana camara, and its ecological and livelihood impacts in eastern Africa. Afr. J. Range Forage Sci. 34, 1–11. doi: 10.2989/10220119.2017.1301551
Stevensona, P. C., Isman, M. B., and Belmain, S. R. (2017). Pesticidal plants in Africa: a global vision of new biological control products from local uses. Ind. Crops Prod. 110, 2–9. doi: 10.1016/j.indcrop.2017.08.034
Sukhthankar, J. H., Kumar, H., Godinho, M. H. S., and Ashwani, K. (2014). Larvicidal activity of methanolic leaf extracts of plant, Chromolaena odorata L. (Asteraceae) against vector mosquitoes. Int. J. Mosquito Res. 1, 33–38.
Taye, W., Asefa, W., and Woldu, M. (2014). Insecticidal activity of Lantana camara on maize weevils (Sitophilus zeamais Motsch.). Int. J. Res. Agric. Sci. 1, 43–46.
Taylor, S., Kumar, L., Reid, N., and Kriticos, D. J. (2012). Climate change and the potential distribution of an invasive shrub, Lantana camara L. PLoS ONE 7:e35565. doi: 10.1371/journal.pone.0035565
Tererai, F., and Wood, A. R. (2014). On the present and potential distribution of Ageratina adenophora (Asteraceae) in South Africa. South Afr. J. Bot. 95, 152–158. doi: 10.1016/j.sajb.2014.09.001
Tesfu, F., and Emana, G. (2013). Evaluation of Parthenium hysterophorus L. powder against Callosobruchus chinensis L. (Coleoptera: Bruchidae) on chickpea under laboratory conditions. Afr. J. Agric. Res. 8, 5405–5410.
Theiling, K. M., and Croft, B. A. (1988). Pesticide side-effects on arthropod natural enemies: a database summary. Agric. Ecosyst. Environ. 21, 191–218. doi: 10.1016/0167-8809(88)90088-6
Tounou, A. K., Mawussi, G., and Amadou, S. (2011). Bio-insecticidal effects of plant extracts and oil emulsions of Ricinus communis L. (Malpighiales: Euphorbiaceae) on the diamondback, Plutella xylostella L. (Lepidoptera: Plutellidae) under laboratory and semi- field conditions. J. Appl. Biosci. 43, 2899–2914.
Udebuani, A. C., Abara, P. C., Obasi, K. O., and Okuh, S. U. (2015). Studies on the insecticidal properties of Chromolaena odorata (Asteraceae) against adult stage of Periplaneta americana. J. Entomol. Zool. Stud. 3, 318–321.
Uyi, O. O. (2020). Mimosa diplotricha: a review and synthesis of its problem and control options. CAB Rev. 15:14. doi: 10.1079/PAVSNNR202015014
Uyi, O. O., and Adetimehin, A. D. (2018). Efficacy of the stem powder of an invasive alien plant, Chromolaena odorata (L) (Asteraceae) against Callosobruchus maculatus (Fab.) (Coleoptera: Chrysomelidae). J. Appl. Sci. Environ. Manage. 22, 379–385. doi: 10.4314/jasem.v22i3.15
Uyi, O. O., Adetimehin, A. D., and Ogu, O. P. (2018a). Repellent and insecticidal activities of the root extracts of Chromolaena odorata and Mimosa diplotricha against Macrotermes species. J. Entomol. 15, 135–142. doi: 10.3923/je.2018.135.142
Uyi, O. O., Adetimehin, A. D., Uyamasi, E. E., and Ejomah, A. J. (2018b). Insecticidal activities of the leaf powders of Chromolaena odorata and Mimosa diplotricha against the common bed bug, Cimex lectularius (L.). Int. J. Zool. Res. 14, 37–42. doi: 10.3923/ijzr.2018.37.42
Uyi, O. O., and Igbinoba, O. G. (2016). Repellence and toxicological activity of the root powder of an invasive alien plant, Chromolaena odorata (L.) (Asteraceae) against Callosobruchus maculatus (Fab.) (Coleoptera: Chrysomelidae). Anim. Res. Int. 13, 2510–2517.
Uyi, O. O., and Obi, B. N. (2017). The evaluation of the repellent and insecticidal activities of the leaf, stem and root powders of Siam weed (Chromolaena odorata) against the cowpea beetle, Callosobruchus maculatus. J. Appl. Sci. Environ. Manage. 21, 511–518. doi: 10.4314/jasem.v21i3.12
Uyi, O. O., Udeogwu, C. C., and Rotimi, J. (2020). Phytochemical constituents and insecticidal efficacy of the root and leaf powders of Mimosa diplotricha and Aspilia africana against Callosobruchus maculatus (Fab.) (Coleoptera: Chrysomelidae). J. Appl. Sci. Environ. Manage. 24, 645–652. doi: 10.4314/jasem.v24i4.16
Valone, T. J., and Weyers, D. P. (2019). Invasion intensity influences scale-dependent effects of an exotic species on native plant diversity. Sci. Rep. 9:18769. doi: 10.1038/s41598-019-55165-z
Van der Westhuizen, L., and Mpedi, P. (2011). The Initiation of a biological control programme Against Argemone mexicana L. and Argemone ochroleuca Sweet subsp. ochroleuca (Papaveraceae) in South Africa. Afr. Entomol. 19, 223–229. doi: 10.4001/003.019.0226
Van Hengstum, T., Hooftman, D. A. P., Oostermeijer, J. G. B., and van Tienderen, P. H. (2013). Impact of plant invasions on local arthropod communities: a meta-analysis. J. Ecol. 102, 4–11. doi: 10.1111/1365-2745.12176
Van Wilgen, B. W., and Lange, W. J. D. (2011). The costs and benefits of biological control of invasive alien plants in South Africa. Afr. Entomol. 19, 504–514. doi: 10.4001/003.019.0228
Waweru, W. R., Wambugu, F. K., and Mbabazi, R. (2017). Bioactivity of Jacaranda mimosifolia and Bougainvillea spectabilis leaves powder against Acanthoscelides obtectus. J. Entomol. Zool. Stud. 5, 110–112.
Witt, A., Beale, T., and van Wilgen, B. W. (2018). An assessment of the distribution and potential ecological impacts of invasive alien plant species in eastern Africa. Transac. R. Soc. South Afr. 73, 217–236. doi: 10.1080/0035919X.2018.1529003
Witt, A., and Luke, Q. (Eds.). (2017). Guide to the Naturalized and Invasive Plants of Eastern Africa. Wallingford: CABI. Available online at: http://www.cabi.org/cabejournals/ejournal/20173158959
Witt, A. B. R., Shackleton, R. T., Beale, T., Nunda, W., and Van Wilgen, B. W. (2019). Distribution of invasive alien Tithonia (Asteraceae) species in eastern and southern Africa and the socio-ecological impacts of Tithonia diversifolia in Zambia. Bothalia 49:a2356. doi: 10.4102/abc.v49i1.2356
Xu, R., Wu, D., Zhang, W. D., Yin, F., and Kuang, R. P. (2009). Efficacy of Ageratina adenophora extract and biogas fermentation residue against the cabbage aphid, Brevicoryne brassicae and an assessment of the risk to the parasitoid Diaeretiella rapae. Int. J. Pest Manage. 55, 151–156. doi: 10.1080/09670870802604062
Yuan, Z., and Hu, X. P. (2012). Repellent, antifeedant, and toxic activities of Lantana camara leaf extract against Reticulitermes flavipes (Isoptera: Rhinotermitidae). J. Econ. Entomol. 105, 2115–2121 doi: 10.1603/EC12026
Zachariades, C., Hoffmann, J. H., and Roberts, A. P. (2011a). Biological control of Mesquite (Prosopis species) (Fabaceae) in South Africa. Afr. Entomol. 19, 402–415. doi: 10.4001/003.019.0230
Zachariades, C., Strathie, L. W., Retief, E., and Dube, N. (2011b). Progress towards the biological control of Chromolaena odorata (L.) R.M.King and H.Rob. (Asteraceae) in South Africa. Afr. Entomol. 19, 282–302. doi: 10.4001/003.019.0229
Zapata, N., Budia, F., Vinuela, E., and Medina, P. (2006). Insecticidal effects of various concentrations of selected extractions of Cestrum parquion adult and immature Ceratitis capitata. J. Econ. Entomol. 99, 359–365. doi: 10.1093/jee/99.2.359
Zerihun, M., and Ele, E. (2018). Insecticidal activities of leaf, seed and stem bark extracts of Prosopis juliflora against the cotton aphid (Aphis Gossypii). Acad. Res. J. Agric. Sci. Res. 6, 202–221.
Keywords: invasive alien plant species, Africa, botanical insecticide, insect pest control, resource poor farmers
Citation: Uyi O, Mukwevho L, Ejomah AJ and Toews M (2021) Invasive Alien Plants in Sub-Saharan Africa: A Review and Synthesis of Their Insecticidal Activities. Front. Agron. 3:725895. doi: 10.3389/fagro.2021.725895
Received: 16 June 2021; Accepted: 27 August 2021;
Published: 28 September 2021.
Edited by:
Rachid Lahlali, Ecole Nationale d'Agriculture de Meknès, MoroccoReviewed by:
Barbara Manachini, University of Palermo, ItalyCopyright © 2021 Uyi, Mukwevho, Ejomah and Toews. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Osariyekemwen Uyi, b3Nhcml5ZWtlbXdlbi51eWlAdW5pYmVuLmVkdQ==
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.