
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Agron. , 20 July 2021
Sec. Pest Management
Volume 3 - 2021 | https://doi.org/10.3389/fagro.2021.676208
This article is part of the Research Topic The Use of Plant Extracts and Essential Oils as Biopesticides View all 12 articles
In the context of the major crop losses, pesticides will continue to play a key role in pest management practice in absence of practical and efficient alternatives; however, increasing awareness regarding environmental and human health impacts of conventional pesticides as well as the development of resistance and cross-resistance reduced their availability and promoted the search for alternative control strategies and reduced-risk pesticides. Among the various alternatives, a drastic re-emergence of interest in the use of plant-derived compounds, called allelochemicals, was noted and demand for an organic product is rising. Currently, azadirachtin, a tetranortriterpenoid derived from the neem seed of the Indian neem tree [Azadirachta indica A. Juss (Meliaceae)], is one of the prominent biopesticides commercialized and remains the most successful botanical pesticide in agricultural use worldwide. Azadirachtin is a powerful antifeedant and insect growth disruptor with exceptional low residual power and low toxicity to biocontrol agents, predators, and parasitoids. This review summarizes the state of the art on key azadirachtin insecticidal activities and risk assessment, identifies knowledge gaps that could serve as the basis for future research direction and highlights limitation in agricultural use and the development of novel strategies by the use of nanotechnology to control its release rate and improve its stability and sustainability.
The United Nations predicts that the global population will increase from 7.7 billion in 2019 to 9.7 billion in 2050 (United Nations, 2017), this evolution is the main factor that will increase the demand for food production which is expected to continue to grow and is projected to increase by 25–70% in 2050 to meet the increasing human demand (Hunter et al., 2017; Silva, 2018). Annual crop losses caused by insects, weeds, and diseases are estimated between 20 and 40 percent, similar to those of 50 years ago due to the intensification of agricultural production together with the effects of climate change (FAO, 2017). To safeguard and improve food security, crop protection from pests is required and aimed to avoid or prevent crop losses or to reduce them to an economically acceptable level (Karuppuchamy and Venugopal, 2016).
Over the years and since the 1950s, conventional synthetic insecticides have played a crucial role in increasing agricultural productivity (Aktar et al., 2009; Popp et al., 2013). In the context of the major crop losses, pesticides will continue to play a key role in pest management practice in absence of practical and efficient alternatives. Indeed, the beneficial outcome from the use of pesticides remains vital for avoiding hunger and food insecurity and meeting the demand of today and future generations especially in the developing countries (Deravel et al., 2014); however, the extensive use of pesticides generates human and environmental health risk and hazards (Carson, 1962; Aktar et al., 2009; Nicolopoulou-Stamati et al., 2016; Jars et al., 2018) and a growing resistance to targeted pests by exerting selection pressure on insect pests (Harrop et al., 2014; Helps et al., 2017).
After the publication of the Silent Spring by Rachel Carson (Carson, 1962), and to attenuate the negative impacts of pesticides in the environment and public health, search for alternative control strategies and reduced risk pesticides became a real challenge (Pimentel, 1997; Khater, 2012). Consequently, a drastic re-emergence of interest in the use of natural pesticides known as biopesticides was noted (Cantrell et al., 2012; Kumar, 2015; Mishra et al., 2018; Haddi et al., 2020). Although there is no formally agreed definition, biopesticides are eco-friendly pest management agents based on living organisms or natural products (Chandler et al., 2011). They may be derived from animals (ex: nematodes), microorganisms (ex: Bacillus thuringiensis), plants (ex: Azadirachta) as well as certain minerals (Damalas and Koutroubas, 2018). If biopesticides are gaining popularity as reduced environmental impact alternatives to conventional synthetic pesticides, the biopesticides market remains small (5%) to the worldwide pesticide market (Olson, 2015). However, this segment of the industry is experiencing rapid growth in recent years with a compound annual growth rate of 8.64% and is projected to outpace that of chemical pesticides (Olson, 2015; Damalas and Koutroubas, 2018).
The main advantages of biopesticides are that they are inherently less toxic than conventional pesticides by offering more targeted action against specific pests (Damalas and Koutroubas, 2018). Indeed, conventional pesticides which exert their effects on the nervous system of insects often affect a broad spectrum of pests along with bird and mammalian species (Thakora, 2006). Furthermore, biopesticides often are effective in very small quantities and decompose quickly, resulting in lower exposures and largely avoiding the pollution problems caused by conventional pesticides (FAO). When using as a component of integrated pest management (IPM) programs, biopesticides can supplement the conventional pesticides and greatly reduce their use and offer potentially higher crop yields (Thakora, 2006; Damalas and Koutroubas, 2018).
Recently, among the biopesticides, plants with pesticidal properties have been the subject of an increasing number of academic researches as a potential option for environment friendly pest management tools for developing sustainable agricultural practices and promote human and environmental safety (Isman, 2006; Cantrell et al., 2012; Hikal et al., 2017). Plants, the most common source of biopesticides, produce a great variety of secondary metabolites potentially applicable in IPM programs (Céspedes et al., 2014).
Growing attention has been given to the neem tree, Azadirachta indica A. Juss. (Meliaceae), as the most prominent biopesticide (Isman and Grieneisen, 2014; Aribi et al., 2020). In Asia, the neem tree is regarded as a wonder tree and has been used for centuries in Ayurvedic medicine as one of oldest medical systems in humanity (Biswas et al., 2002; Pasquoto-Stigliani et al., 2017). Among its many attributed properties, it acts as an antidiabetic, immunostimulant, antimicrobial, antiviral, cholesterol-lowering agents, contraceptive and anticancer remedy, and it has long been revered by ancient Indian people and is entitled “village drugtore” (Tinghui et al., 2001; Hummel et al., 2016; Moga et al., 2018; Blum et al., 2019). Additionally, aqueous extracts of powdered neem kernels have been used as an insecticide in India for about 2,000 years for the control of insect pests (Schmutterer, 1995). In recent time, and following the isolation of azadirachtin, the major active compound, that is mainly responsible for the insecticidal activity of neem, the use of neem-based insecticide has increased in the last 30 years (Chaudhary et al., 2017; Pasquoto-Stigliani et al., 2017). Currently, azadirachtin is one of the prominent biopesticides commercialized and remains the most successful botanical pesticide in agricultural use worldwide (Isman and Grieneisen, 2014; Chaudhary et al., 2017; Aribi et al., 2020); however, its mechanisms of action still unclear and remain to be clarified especially in relation to the neurophysiological and the possible long-term activities.
Neem is an evergreen fast-growing tree native to India and Burma, it grows in arid, semiarid, and tropical regions (Schmutterer, 2002). Today, the neem tree is widely distributed throughout tropical and subtropical Asia, Africa, Australia, and South America (Kumar et al., 2016). Neem products have been obtained from several species of neem trees belonging to the Meliaceae family. A. indica. Juss, is the most important species of this group considered a renewable resource of various useful domestic, medicinal, and agricultural products (Kumar et al., 2016). All parts of the tree (leaf, flower, seed kernel, wood, bark, and twig), are a source of biologically active ingredients, and the maximum of activity is associated with the seed kernel (Kumar et al., 2016). More than 300 different phytochemicals have been reported from different parts of the neem tree (Gupta et al., 2017) and over 130 of these compounds belongs to limonoid-type triterpenoids that are endowed with potent medicinal and insecticidal properties (Chen et al., 2018); However, the chemical composition of neem is far to be completely elucidated, as evidenced by the novel compounds reported each year (Nicoletti et al., 2016; Chen et al., 2018). The most important neem limonoids include azadirachtin, nimbolide, salannin, nimbin, deacetylnimbin, mahmoodin, epoxy-azadiradione, deacetylgedunin, and gedunin (Nagini, 2014; Gupta et al., 2017). These compounds have been shown to possess many useful properties of which, antifeedancy, insecticidal, and insect growth disruption are used in the management of pest (Schmutterer, 1995). Most of the triterpenoids of neem were found in very small quantities in various parts of the tree and account for the total bioactivity of the neem seed extract (Mordue et al., 2010). Azadirachtin A is the major active component and is responsible for 72 to 90% of the biological activity (Schmutterer, 1990; Mordue et al., 2010).
Azadirachtin is a complex tetranortriterpenoid with 16 chiral carbon centers, derived from the mevalonic acid pathway in the neem tree (Hansen et al., 1993; Aarthy et al., 2018). It is a highly oxidized tetranorterpenoid natural product related to limonin, the bitter principle of citrus fruits and known as limonoids (Benuzzi and Ladurner, 2018). Azadirachtin A is considered as the main constituent and azadirachtin commercial formulations, available on the world market for insect control in organic farming, contain a stated amount of azadirachtin A (Table 1) (Benuzzi and Ladurner, 2018). It has a complex molecular structure and following the determination of its correct structure in 1985 (Kraus et al., 1985), the first total synthesis of this molecule was published two decades after the discovery of the compound (Jauch, 2008). Azadirachtin is a broad-spectrum insecticide (Figure 1), its acts as a feeding deterrent, insect growth disruptor (IGD), and sterilant and is used to control various agricultural pest species, including Coleoptera, Heminoptera, Diptera, Orthoptera, and Isoptera (Morgan, 2009). The toxicity of azadirachtin varies among insect orders and is influenced by the different penetration rates and activities of detoxifying enzymes (Table 2).
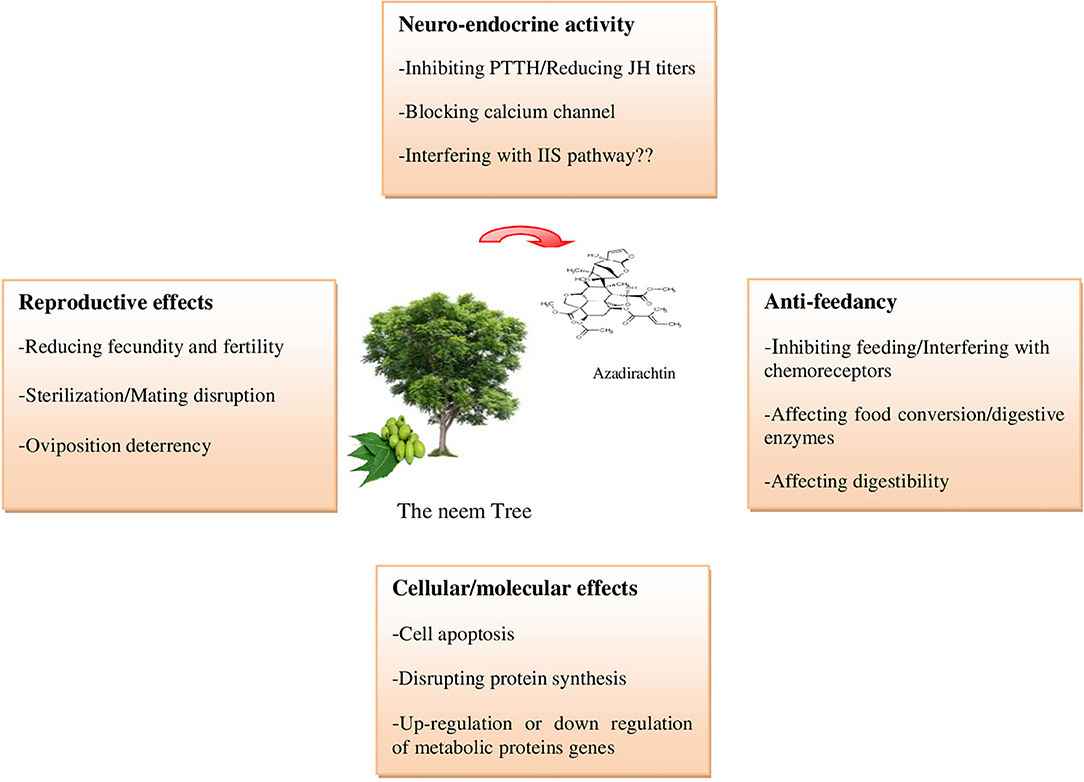
Figure 1. Principals azadirachtin action on insects (Photos: https://www.shutterstock.com/).
The chemical complexity of azadirachtin minimizes the potential risk of insect resistance (Mordue et al., 2010). Feng and Isman (1995) reported development of resistance to pure azadirachtin over 40 generations in the peach potato aphid Myzus persicae but no resistance was reported with neem seed extract. Bomford and Isman (1996) also showed habituation to pure azadirachtin in the tobacco cutworms with less sensitivity to the antifeedant properties of azadirachtin, but not to neem with the same absolute amount of azadirachtin. This might account for avoiding desensitization to commercial neem-based insecticides containing additional non- AZA-compounds (Bomford and Isman, 1996). Azadirachtin A is very well-received by the root system, and, subsequently, it is systematically distributed through the xylem into the green parts of plant tissues and stored in leaves in an unchanged form. In addition, a very low content of azadirachtin A in plant tissues may protect significantly plant damage against phytophagous pest larvae (Pavela, 2016).
In addition, azadirachtin has displayed remarkable selectivity with low mammalian toxicity (Mordue et al., 2010). According to Raizada et al. (2001), azadirachtin has shown an LD50 value of more than 5,000 mg/kg which falls into class U (Unlikely to present an acute hazard) of the WHO (2009) toxicity rating. Azadirachtin is registered in the United States as a general-use pesticide with a toxicological class Environmental Protection Agency (EPA) of IV (relatively non-toxic). Azadirachtin seems to be selective, non-mutagenic, and readily degradable and has also been reported as safer for non-target organisms and beneficial organisms (Medina et al., 2004; Cordeiro et al., 2010; Mordue et al., 2010; Celestino et al., 2014; Dai et al., 2019); however, the presumed safety of azadirachtin has been questioned, especially, in relation to natural enemies and pollinators (Barbosa et al., 2015; Lima et al., 2015; Xavier et al., 2015; Bernardes et al., 2017, 2018; Francesena and Schneider, 2018). Nevertheless, semi-field and field studies may enable to reliably predict potential side effects of azadirachtin on non-target insects. However, azadirachtin is still considered as one of the best alternatives to conventional insecticides in IPMprograms and considered as one of the most promising plant compounds for pest control organic agriculture (Tomé et al., 2013; Bezzar-Bendjazia et al., 2017). Despite the progress on the physiological and biological activities and agricultural application of azadirachtin, its exact mechanism of action, especially, at the molecular level is not yet fully understood (Lai et al., 2014; Dawkar et al., 2019).
In insects, 20-hydroxyecdysone (20E) and juvenile hormone (JH) play a central role in the regulation of growth and development (Bensebaa et al., 2015), and the hormonal balance determines the outcome of each developmental transition (Dubrovsky, 2005). Therefore, any interference with hormonal homeostasis leads to interrupted development and is considered as a potential specific target for pest control (Pener and Dhadialla, 2012). Azadirachtin is known as an antagonist of these two principles hormones; its major action was its ability to modify or suppress hemolymph ecdysteroid and JH titers through inhibition of the secretion of morphogenetic peptide hormone (PTTH) and allatotropins from the corpus cardiacum complex and this account for its well-documented IGD effects defined mostly as reduced pupation, malformation or a failure of adult emergence (Mordue and Blackwell, 1993; Bezzar-Bendjazia et al., 2017). Furthermore, this compound is known to cause degenerative structural changes of the nuclei in all endocrine glands (prothoracic gland, corpus allatum, and corpus cardiacum) responsible for controlling molting and ecdysis in insect which would contribute to a generalized disruption of neuroendocrine function (Mordue et al., 2010). Azadirachtin applied on the diet at 74 ppm affects the growth, suppresses ecdysis, and inhibits ecdysteroids synthesis in the larvae of Ostrinia furnacafis Guenée (Min-Li and Shin-Foon, 1987). In Tenebrio molitor, the injection of 1 μg of azadirachtin into freshly ecdysed pupae induced a significant depletion of levels of immunoreactive ecdysteroids affecting 20-hydroxyecdysone levels and suppressing the ecdysteroid peak that normally appears at the middle of the instar (Marco et al., 1990). A drastic reduction of hemolymph ecdysteroid titers was also reported in Rhodnius prolixus after a unique dose of azadirachtin (Garcia et al., 1990). In addition to its effects on morphogenetic PTTH, azadirachtin affects ecdysone 20-monooxygenase activity, the insect cytochrome P450-dependant hydroxylase responsible for the conversion of the steroid hormone ecdysone to its more active metabolite, and 20E (Smith and Mitchell, 1988). Indeed, in vitro analysis of three insect species, homogenates of wandering third instar larvae of Drosophila melanogaster, fat body or midgut from last instar larvae of Manduca sexta and abdomens from adult female Aedes aegypti, incubated with radiolabelled ecdysone and azadirachtin revealed inhibition of the ecdysone 20-monooxygenase with a dose-dependent relationship (Smith and Mitchell, 1988); however, ingested or injected azadirachtin had no effect on ecdysone 20-monooxygenase activity in Spodoptera frugiperda (Yu, 2000). Besides its negative effects on molting hormone, azadirachtin induced a delay or a reduction in JH titters, primarily by hindering the release of the allatotropins and thereby blocking the synthetic and release processes of the JH (Mordue et al., 2010; Dhra et al., 2018).
Azadirachtin is reported to impair the growth and molting process of insects and induced robust developmental delays in the larva-to-pupa and the pupae-to-adult transition compromising their survival (Hasan and Ansari, 2011; Tomé et al., 2013; Lai et al., 2014; Bezzar-Bendjazia et al., 2016). In addition, growth and nutrient intake are functionally linked processes in development and growth and body mass are directly affected by nutrient uptake principally governed by the insulin/insulin-like growth factor signaling (IIS) pathway (Tennessen and Thummel, 2011). Lai et al. (2014) reported that the inhibition of growth and development in D. melanogaster after azadirachtin treatment was similar to those caused by disruption of the IIS pathway. In addition, azadirachtin can inhibit the excitatory cholinergic transmission and block partly the calcium channel (Qiao et al., 2014), and this might interfere with different endocrinological and physiological actions in insects.
The negative effects of azadirachtin on reproduction were reported in several insect orders (Pineda et al., 2009; Tine et al., 2011; Tomé et al., 2013; Boulahbel et al., 2015; Er et al., 2017; Oulhaci et al., 2018). Reduced fecundity and fertility has been recorded in many insects including Spodoptera littoralis, D. melanogaster, Galleria mellonella, Dysdercus cingulatus, Tuta absoluta, and Helicoverpa armigera (Pineda et al., 2009; Pandey and Tiwari, 2011; Tomé et al., 2013; Ahmad et al., 2015; Er et al., 2017; Oulhaci et al., 2018) and could be due to the interference of azadirachtin with yolk protein synthesis and or its uptake into oocytes (Boulahbel et al., 2015). In leaf-cutting ant queens Atta sexdens, azadirachtin affects oviposition, decreases, and inhibits vitellogenin reserve, which impact negatively the egg development (Amaral et al., 2018).
Sterility effects in females due to interference with vitellogenin synthesis and uptake into oocytes were also reported. A single injection of 10 μg of azadirachtin resulted in sterilizing effect on Locusta migratoria migratorioides with an arrest of terminal oocytes maturation and oviposition (Rembold and Sieber, 1981). In Heteracris littoralis, ovaries in azadirachtin-treated females showed complete shrinkage with oocyte growth arrest with disintegration and destruction in follicular cells and mitochondria (Ghazawi et al., 2007). In males, azadirachtin decreases significantly the number of cysts and the apical nuclei within the cysts in D. melanogaster (Oulhaci et al., 2018). The inhibition of spermiogenesis was also reported in Mylabris indica (Vivekananthan and Selvisabhanayakam, 2014) and Heteracris littoralis (Ghazawi et al., 2007).
For the normal progress of oogenesis and spermatogenesis, a proper balance between JH and 20E is needed, antagonist action of azadirachtin on these two principal hormones account for the deleterious effects on reproductive parameters. Indeed, the application of exogenous 20E after azadirachtin treatment can compensate for its depressive effects on D. melanogaster and restored normal values of yolk protein content in the fat body and ovaries (Boulahbel et al., 2015).
In addition, azadirachtin was finding to alter reproductive behavior in D. melanogaster by reducing mating success (Aribi et al., 2017; Oulhaci et al., 2018). The impact of azadirachtin on sex behavior and mating response to sexual pheromones was also reported in Oncopeltus fasciatus (Dorn et al., 1987) and the predator Neoseiulus baraki (Lima et al., 2015). Oviposition sites treated with azadirachtin or other neem-based compounds induce an oviposition repellence, deterrence, or inhibition in several species of insects after a probable detection of the bioinsecticide on the treated surface (Schmutterer, 1990; Dhar et al., 1996, Cordeiro et al., 2010; Tomé et al., 2013). Pure azadirachtin was also reported to deter the oviposition in Nezara viridula (Riba et al., 2003). A single larval exposure to a commercial formulation of azadirachtin, the Neem Azal, was found to reduce fecundity in D. melanogaster and enhance avoidances to this compound (Bezzar-Bendjazia et al., 2016). These effects were observed in the next non-exposed generations which can be used as repellent strategies in pest management programs (Ferdenache et al., 2019).
Azadirachtin is usually associated with a marked antifeedant activity and even behavioral avoidance in a large number of insect species including hemipterans (Kumar and Poehling, 2007), lepidopterans (Charleston et al., 2006; Shannag et al., 2015), ortheopterans (Capinera and Froeba, 2007), coleopterans (Baumler and Potter, 2007), and dipterans (Kilani-Morakchi et al., 2017).
Insects use an olfaction system to search and locate potential food and thereafter contacting chemoreception, called primary antifeedancy, which could confirm its quality and provide a basis for food selection and discrimination (Lee et al., 2010). A signal to the brain provokes avoidance from further approach or feeding.
The primary antifeeding effect of azadirachtin seems to be mediated by gustatory chemosensillas and linked to inhibition on the rate of firing of sugar-sensitive cells of the gustatory chemoreceptors by activating bitter sensitive gustatory cells (Lee et al., 2010; Weiss et al., 2011; Delventhal and Carlson, 2016). Indeed, the sensitivity to primary antifeedancy of azadirachtin was reported in different species, which starve to death rather than ingest the biopesticide (Mordue and Nisbet, 2000). An internal feedback mechanism called secondary antifeedancy, including a long-term reduction in food intake, and deleterious effects on different insect tissues (muscles, fat body, gut epithelial cells), is also reported (Mordue et al., 2010; Khosravi and Sendi, 2013; Shannag et al., 2015). Third-instar larvae of S. littoralis orally treated with sublethal concentrations of azadirachtin display a reduction in food intake, conversion efficiency, and feeding behavior (Martinez and van Emden, 1999). In second instar larvae of Spodoptera eridania, short-term consumption (2 days) of food treated with Azatrol, a commercial formulation of azadirachtin, reduced relative consumption rate, the efficiency of conversion of ingested food, relative growth rate, approximate digestibility, and assimilation rate of food during the entire larval developmental period (Shannag et al., 2015). In D. melanogaster, a single topical application of azadirachtin on early third instars larvae decreased significantly the amount of larval food intake and disrupted the ability of the insect to digest food by interfering with digestive enzymes activities (Bezzar-Bendjazia et al., 2017). This effect is also observed in adults surviving the pre-imaginal treatment, which suggests a long-term antifeedancy and delayed effects through the developmental stage with a possible reinforcement of the insecticidal activity of azadirachtin (Kilani-Morakchi et al., 2017).
In addition, azadirachtin showed an agonistic effect on dopaminergic neurons and can induce aversive taste memory in D. melanogaster, and such memory is regulated by dopaminergic signals in the brain resulting in inhibition of proboscis extension response (PER) (Yan et al., 2017).
Besides the above mentioned effects, accounting for its broad-spectrum activities, azadirachtin was also shown to cause upregulation of p53, resulting in cell cycle mediated cells apoptosis induction and cell proliferation inhibition in S Spodoptera litura S1-1 cell line (Huang et al., 2011). In the same species, Shu et al. (2018) demonstrated that azadirachtin induced structural alteration in the larval midgut by apoptosis activation including increased expression of caspase family members and apoptosis-binding motif 1 and the release of cytochrome c from mitochondria to cytoplasm, which may affect the digestion and absorption of nutrients. The induction of apoptosis through caspase-dependent pathways by azadirachtin was also reported in S. frugiperda cultures cell line Sf9 (Shu et al., 2015). Based on proteomic studies, Sun et al. (2018) reported that the molecular response mechanism of male infertility induced by azadirachtin in S. litura may be linked to regulation of many proteins in the pathway of focal adhesion exerting influences in detachment of cell attachment, the loss of cell-cell interactions, and inducing apoptosis at the pupal stage. Furthermore, many proteins in the adenosine monophosphate-activated protein kinase (AMPK) pathway were also changed at the adult stage after azadirachtin treatment as larvae (Sun et al., 2018). In D. melanogaster, a depolymerization of actin causing a cell arrest and apoptosis caspase-independent was reported after azadirachtin treatment (Anuradha et al., 2007; Anuradha and Annadurai, 2008).
At the cellular level, azadirachtin disrupts protein synthesis and secretion. In Schistocerca gregaria, injections of 3 μg azadirachtin/g body weight induce an inhibitory effect of the incorporation of radiolabelled glycine into the protein of the whole locust (Paranagama et al., 2004). Roberston et al. (2007) reported that the heat-shock protein, hsp 60, in cultured Drosophila Kc 167 cells could bind to azadirachtin A which might be associated with a failure of protein synthesis and release.
At the molecular level, azadirachtin alters or prevents the transcription and/or expression of several proteins. Ingestion of 10 ppm of azadirachtin in third instars larvae of Ostrinia furnacalis significantly affected the fat body by interfering with protein expression related to hemolymph lipid (Huang et al., 2007). Lai et al. (2014) reported that azadirachtin downregulated expression of genes of cuticular protein and amylase and upregulated gene odorant-binding protein 99b (Obp99b) in D. melanogaster, which may be related to the development, molting defects, and antifeedancy action of the biopesticide. Azadirachtin treatment was shown to increase superoxide dismutase activity (SOD) and malondialdehyde contents (MDA) in D. melanogaster and induce antioxidant enzymes, such as SOD, catalase (CAT), and gluthation S-transferase (GST), by an upregulation of gene expression to protect against oxidative damage caused by elevated and accumulation of reactive oxygen species (ROS) triggered by a stress response to azadirachtin (Zhang et al., 2018). Azadirachtin also inhibits the expression of ferritin and thioredoxin peroxidase genes, in the sweet potato of whitefly Bemissia tabaci, related to protective roles against oxidative stress (Asaduzzaman et al., 2016).
Recently, azadirachtin was found to regulate the growth of S. frugiperda by affecting the insect chitin synthesis pathway by a downregulation of 31 cuticle proteins and several other genes encoding important enzymes involved in insect chitin and hormone biosynthesis, such as, trehalase, chitin-synthase, chitin deacetylase, chitinase (Shu et al., 2020). The suppressed expression of chitin biosynthesis and cuticle genes by azadirachtin might represent the molecular basis for the retardation of molting and growth.
Genes encoding enzymes responsible for key steps in hormone biosynthesis were also affected by azadirachtin. Azadirachtin also affected genes encoding key enzymes in hormone biosynthesis, such as genes encoding farnesol dehydrogenase, responsible for oxidization of farnesol, a precursor of JH named farnesal (Mayoral et al., 2009); the gene encoding an aldehyde dehydrogenase, which is responsible for converting farnesal into farnesoic acid and CYP15A1_C1, which converts the farnesoic acid to JH-III acid (Qu et al., 2015); the gene encoding JH epoxide hydrolase, responsible for JH degradation by hydrolyzing the epoxide of JH (Zhao et al., 2017); the gene encoding cytochrome oxidase-related proteins CYP307A1 and CYP314A1, which catalyze the 20-Hydroxyecdysone (Liu et al., 2019). All these changes in the expression levels of these key genes account for the disruption of the synthesis of JH and ecdysone, and therefore, interfere with the balance of these hormones, contributed to the growth inhibition.
Azadirachtin-based pesticides act on a wide range of pestiferous insects from different orders as well as some ectoparasites which present high sensitivity to these compounds. The major property of azadirachtin is the blockage of neurosecretory peptides, which regulate the synthesis and release of ecdysteroids and JH leading to disruption of endocrine events. The important roles of these hormones in arthropods physiology for normal development leave open the possibility that azadirachtin may pose a hazard to non-target species. Indeed, Barbosa et al. (2015) reported that long-term chronic exposure with azadirachtin may affect reproduction and behaviors of the bumblebee Bombus terrestris under laboratory conditions. Similarly, in vitro chronic exposure of azadirachtin affects stingless bee, Partamona helleri, by reducing the survival, development time, growth, and affecting reproductive organs but did not affect the larval food intake, the rate of emergence of queen and walking activity (Bernardes et al., 2018); however, the instability of azadirachtin and its low residual potential persistence makes these chronic conditions unexpected under semi-field and field situations. Azadirachtin was also found to be selective to the honeybee, Apis cerana, based on three essential risk assessment criteria [selectivity ratio, probit substitution method (%), and hazard ratio/risk quotient (Challa et al., 2019)].
In the case of predatory insects and parasitoids, azadirachtin, and neem-based insecticides show slight to moderate toxic effects and are considered to be harmless and with a certain degree of selectivity, especially for the adult insects (Raguraman and Kannan, 2014); however, pre-imaginal instars of beneficial organisms (nymphal/larval instars) are more susceptible to neem insecticides under laboratory conditions (Raguraman and Kannan, 2014). Hence, it is important to control the stage of parasitoids/predators used and the timing of application to avoid any toxicity in semi-field and field applications.
According to European Food Safety Authority (European Food Safety Authority, 2011), azadirachtin has moderate to high toxicity to aquatic organisms (acute LC50 = 0.048 mg azadirachtin A/L, chronic NOEC = 0.0047 mg azadirachtin A/L) and aquatic insects (chronic NOEC = 0.0016 mg azadirachtin A/L), with an aquatic half-life of around 30 days. The risk assessment for this compound focused on freshwater organisms as there are no marine or estuarine data. However, the risk values did not exceed the criteria and were predicted to be low when azadirachtin was used following the label instruction of the product (Goktepe et al., 2004; European Food Safety Authority, 2011).
Azadirachtin is not highly mobile in soil due to its oily composition. Its half-lives in soil are about few hours to 1 or 2 days reducing the risk to earthworms and soil macro-organisms. The hazard index of heavy metal contamination in vegetables after soil treatment with azadirachtin was <1 and does not exceed the WHO/FAO permissible limit in vegetables, suggesting it is safer for consumption (Egwu et al., 2019).
However, information regarding the fate, behavior, and toxicity of individual compounds, and the degradation of products are needed to complement its relatively favorable ecotoxicological profile (European Food Safety Authority, 2011). In general, European Food Safety Authority (2018) reported that the margin safety of the risk assessment performed for azadirachtin A is considered sufficient to estimate the risk from the whole azadirachtin. In addition, semi-field and field studies should be performed considering situations that may include acute and chronic exposure in the risk assessment setup.
Azadirachtin has a variety of physiological effects on many insect pests, such as antifeedancy (Qin et al., 2020), growth and development inhibition (Zhao et al., 2019), impairment of oocyte structure, inhibition of fecundity, and egg viability (Bezzar-Bendjazia et al., 2016; Amaral et al., 2018; Oulhaci et al., 2018; Ferdenache et al., 2019). Despite extensive studies of the mechanisms that highlight the physiological effects of azadirachtin, the behavioral effects remain more controversial (Charleston et al., 2006; Hasan and Ansari, 2011; Tomé et al., 2013).
The fitness and survival of insects strongly depends on successful localization of host plants, food source, mating partners, and oviposition sites. Many insect behaviors are heavily dependent on chemosensation, especially on the perception of olfactory and gustatory cues (Herrero, 2012; Depetris-Chauvin et al., 2015; Walker et al., 2016). In addition to these olfactory and gustatory cues, locomotion represents an integral part of insect behaviors as is essential for food-seeking, mating, and escape response (Zhu et al., 2020). The ability of insects to modify their behavior based on prior experience is essential for their survival (Chia and Scott, 2020). Increasing evidence has highlighted the critical role of early life experience in adult behavior in insects (Caubet et al., 1992; Bezzar-Bendjazia et al., 2016; Ferdenache et al., 2019). In addition, exposure to a stressor, such as pesticides, has been shown to prompt a range of behavioral effects which can be inherited to the next generation (Ferdenache et al., 2019; Lu et al., 2020). Recent work demonstrated for the first time that D. melanogaster can modulate its behavior based on previous experiences of early life (third instars larvae) with azadirachtin affecting oviposition site preference and food selection and enhancing avoidances of this compound in adults of parent generation as well as the non-exposed F1 generation (Bezzar-Bendjazia et al., 2016; Kilani-Morakchi et al., 2017; Ferdenache et al., 2019). These changes in insect behavioral responses are influenced by individual sensory experience and may leave an “imprinted” trace into adult life in accordance to experience-induced learning by changes in the neurophysiology of insects (Dukas, 2008; Little et al., 2019). Indeed, biogenic amines, octopamine (OA), serotonin (5-HT), and dopamine (DA) are known to convey the reinforcing cues for many different types of associative memory in Drosophila (Masek and Keene, 2016). Azadirachtin treatment was found to reduce OA, 5-HT, and DA levels in both the brain and the hemolymph of Acherontia styx (Awad et al., 1997). Furthermore, azadirachtin interferes with the amount of 5-HT in the endocrine organs and, mainly, in the brain of locusts (Banerjee and Rembold, 1992).
Moulin et al. (2020) reported that transient dysregulation of the dopaminergic signaling can produce behavioral alterations in D. melanogaster adults, which can then be carried to descendants. In addition, azadirachtin can excite different clusters of dopaminergic neurons, such as PPL1, and increase dopamine release inducing aversive taste memory in Drosophila (Yan et al., 2017); however, the neurophysiological actions of azadirachtin remain to be clarified. In addition, insecticides are known to be able to provoke epigenetic alterations, which can be inherited in the next generations (Vandegehuchte and Janssen, 2011); this possible epigenetic alteration induced by azadirachtin treatment was never investigated. The comprehension of the mechanisms that induce the transgenerational conservation of the aversive effects of azadirachtin may contribute to better use of this compound in IPM programs.
In addition, azadirachtin had the potential to be used in synergy with other botanical compounds. Indeed, azadirachtin and clarified neem oil can significantly synergize the pyrethrum activity while reducing or eliminating the need for pipronyl butoxide as an agent to augment pyrethrum activity, which represents a significant cost advantage when compared with existing pyrethrum/pipronyl butoxide formulations (Chang et al., 1996). On the other hand, phenol compounds in neem were suspected to synergize with the main component (azadirachtin) in increasing the antifeedant activity on S. litura (Prianto et al., 2019). The use of azadirachtin in synergy with B. thuringiensis (Bandyopadhyay et al., 2014) and karanj (Pongamia pinnata Pierre) was also reported (Kumar et al., 2007). Azadirachtin was found to enhance the efficacy of B. thuringiensis in Cydia pomonella, S. exigua, and Dendrolimus pini (Konecka et al., 2019). This synergistic effect was observed between azadirachtin and multicapsid nucleopolyhedrovirus (SfMNPV) on the mortality of S. frugiperda (Pineda et al., 2014).
More studies are needed for synergism between azadirachtin and other insecticides to find combinations that can effectively control pests. Essential oil or their main compounds, especially compounds (linalool, borneol) with antifeeding activities, might represent a good candidate.
If rapid degradation by sunlight and low persistence in the environment are considered as advantages of the use of azadirachtin and neem derived products, it also represents a problem for their use on a large scale and is disadvantageous from an agribusiness perspective, since they result in lower efficiency and necessitates a greater number applications (Pasquoto-Stigliani et al., 2017).
The chemical nature of the media containing azadirachtin formulation is important and influences its stability. Indeed, studies on the effect of various solvents on the stability of azadirachtin in extracts and formulations reported higher stability of azadirachtin in alcoholic and other aprotic solvents, which are neutral, as compared with protic solvents (Pereira et al., 2019). Furthermore, azadirachtin was most stable in mildly acidic solutions between pH 4 and 6 (Pereira et al., 2019).
The neem-based oil in water emulsion formulation by high shear mixing also improves stability and bio-efficacity of the biopesticide by a decrease of particle size of the emulsion with the increase of stirring time leading to excellent emulsion stability (Iqbal et al., 2020). In addition, the stability of neem oil-based microemulsion can be enhanced by the use of botanical synergists, such as aqueous extract of Prosopis Juliflora (Sharma et al., 2019).
The use of nanotechnology also represents a way to overcome such limitations, and the development of controlled-release formulations of botanical insecticides by polymeric encapsulation has been studied in recent years (Das et al., 2014; Pasquoto-Stigliani et al., 2017). Flores-Céspedes et al. (2015) reported that natural polymers, such as kraft lignin and alginate, protect azadirachtin against photodegradation and could be used to improve its stability and delivery to its site of action. These new procedures to encapsulate botanical pesticides provide several benefits including slow-release, enhanced stability of compounds, use of small dose, and masking of odor (Chaudhary et al., 2017). Poly(ε-caprolactone) nanocapsules loaded with neem oil are save to soil microbiota during 300 days of exposure and did not affect the net photosynthesis and stomatal conductance of maize plants, and present lower toxicity against non-target organisms (Pasquoto-Stigliani et al., 2017); however, the same nanocapsule containing a mixture of neem oil and oleic acid presented higher toxicity and led to negative effects. Recently, Shanmugapriya et al. (2019) demonstrated that azadirachtin loaded in silica nanoparticles at 500 ppm showed high mortality of adult Bemissia tabaci and can be used as an alternative to chemical pesticides.
Although nanotechnology is still at an early stage in the agricultural sector, it is clear that there is growing interest in its use; however, studying the toxicity of nano pesticides and understanding their mechanism of action in target organisms is a key factor in the selection of the best formulations for use in agricultural applications (Feng and Peng, 2012; Seugling et al., 2019; Jesser et al., 2020).
In addition, a sublethal dose of azadirachtin was reported to induce hormesis in the Mexican Bean Weevil, Zabrotes subfasciatus, with increased fecundity daily to compensate for azadirachtin-induced decreased longevity (Vilca Malqui et al., 2014). In addition, the population of Z. subfasciatus engendered from females exposed to azadirachtin present a higher rate of population increase and a higher net reproductive rate (Vilca Malqui et al., 2014). Similar results were reported in Myzus persicae exposed to sublethal concentrations of azadirachtin with a modest hormetic response under laboratory conditions (Cutler et al., 2007). Evidence-based toxicology under field conditions must be used to solidify the importance of hormesis to understand the risk of exposure to azadirachtin and neem-based compounds. In addition, new research tools, such as toxicogenomics and statistical modeling processes, must be designed to evaluate possible hormetic responses when devising pest management strategies.
Health and environmental concerns have influenced the use of safe and non-hazardous pest control measures. Azadirachtin-based insecticides have recently been promoted as an alternative pest control method, especially in agroecological farming and organic agricultural systems. Azadirachtin has broad-spectrum activity for combating numerous pests in different crops, and it has not yet reached most of its potential utilization. Currently, information is sparse on the possible long-term and transgenerational effects of azadirachtin on insects; a better comprehension of this phenomenon could improve its use in IPM programs by reducing the concentrations used, frequency of application and targeting the best time of application, which might enhance its ecotoxicological profile.
In addition, the nanoencapsulation of this biopesticide provides a novel way to enhance its stability and sustainability, since they protect it against degradation and modulate its release.
SK-M wrote the manuscript. SK-M, HM-G, and KS contributed to the collection of the information and the discussion and revised the manuscript. All authors approved the final version of the manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Aarthy, T., Mulani, F. A., Pandreka, A., Kumar, A., Nandikol, S. S., Haldar, S., et al. (2018). Tracing biosynthetic origin of limonoids and their functional groups through stable isotope labeling and inhibition in neem tree (Azadirachta indica) cell suspension. BMC. Plant. Biol. 18:230. doi: 10.1186/s12870-018-1447-6
Abdel-Gawad, R. M. (2018). Development rate and ultrastructure changes of puparia of Megaselia scalaris (Loew) (Diptera: Phoridae) induced by azadirachtin. Egypt. Acad. J. Biolog. Sci. 11,109–120. doi: 10.21608/eajb.2018.11984
Abedi, Z., Saber, M., Vojoudi, S., Mahdavid, V., and Parsaeyan, E. (2014). Acute, sublethal, and combination effects of azadirachtin and Bacillus thuringiensis on the cotton bollworm, Helicoverpa armigera. J. Insect. Sci. 14:30. doi: 10.1093/jis/14.1.30
Ahmad, S., Ansari, M. S., and Muslim, M. (2015). Toxic effects of neem based insecticides on the fitness of Helicoverpa armigera (Hübner). Crop. Prot. 68, 72–78. doi: 10.1016/j.cropro.2014.11.003
Aktar, M. W., Sengupta, D., and Chowdhury, A. (2009). Impact of pesticides use in agriculture their benefits and hazards. Interdisc. Toxicol. 2, 1–12. doi: 10.2478/v10102-009-0001-7
Aljedani, D. M. (2018). Assessment of effectiveness of the imidacloprid and azadirachtin on the black watermelon bug. Int. J. Zool. Res. 14, 61–70. doi: 10.3923/ijzr.2018.61.70
Amaral, K. D., Martinez, L. C., Lima, M. A. P., Serrão, J. E., and Castro Della Lucia, T. M. (2018). Azadirachtin impairs egg production in Atta sexdens leaf-cutting queens. Environ. Pollut. 243, 809–814. doi: 10.1016/j.envpol.2018.09.066
Anuradha, A., and Annadurai, R. S. (2008). Biochemical and molecular evidence of azadirachtin binding to insect actins. Curr. Sci. 95, 1588–1593. Available online at: https://www.jstor.org/stable/24105517
Anuradha, A., Annadurai, R. S., and Shashidhara, L. S. (2007). Actin cytoskeleton as a putative target of the neem limonoid azadirachtin A. Insect. Biochem. Mol. Biol. 37, 627–634. doi: 10.1016/j.ibmb.2007.03.009
Aribi, N., Denis, B., Kilani-Morakchi, S., and Joly, D. (2020). L'azadirachtine, un pesticide naturel aux effets multiples. Médecine/Sciences 36, 44–49. doi: 10.1051/medsci/2019268
Aribi, N., Oulhaci, M. C., Kilani-Morakchi, S., Sandoz, J. C., Kaiser, L., Denis, B., et al. (2017). Azadirachtin impact on mate choice, female sexual receptivity and male activity in Drosophila melanogaster (Diptera: Drosophilidae). Pestic. Biochem. Physiol. 143, 95–101. doi: 10.1016/j.pestbp.2017.09.002
Asaduzzaman, M., Shim, J. K., Lee, S., and Lee, K. Y. (2016). Azadirachtin ingestion is lethal and inhibits expression of ferritin and thioredoxin peroxidase genes of the sweetpotato whitefly Bemissia tabaci. J. Asia. Pac. Entomol. 19, 1–4. doi: 10.1016/j.aspen.2015.10.011
Awad, E. W., Saadé, F. E., and Amiri, M. H. (1997). Effect of azadirachtin on the nutrition, development and biogenic amine levels in the Eastern death's head hawk moth, Acherontia styx (Lepidoptera: Sphingidae). Exp. Biol. 2, 1–14. doi: 10.1007/s00898-997-0015-6
Bandyopadhyay, W. S., Gotyal, B. S., Satpathy, S., Selvaraj, K., Tripathi, A. N., and Ali, N. (2014). Synergistic effect of azadirachtin and Bacillus thuringiensis against Bihar hairy caterpillar, spilarctia obliqua walker. Biopestic. Int. 10, 71–76.
Banerjee, S., and Rembold, H. (1992). Azadirachtin A interferes with control of serotonin pools in the neuroendocrine system of locusts. Naturwissenschaften 79, 81–84. doi: 10.1007/BF01131808
Barbosa, W. F., De Meyer, L., Guedes, R. N., and Smagghe, G. (2015). Lethal and sublethal effects of azadirachtin on the bumblebee Bombus terrestris (Hymenoptera: Apidae). Ecotoxicology 24, 130–142 doi: 10.1007/s10646-014-1365-9
Baumler, R., and Potter, D. A. (2007). Knockdown, residual, and antifeedant activity of pyrethroids and home landscape bioinsecticides against japanese beetles (Coleoptera: Scarabaeidae) on linden foliage. J. Econ. Entomol. 100, 541–548. doi: 10.1093/jee/100.2.451
Bensebaa, F., Kilani-Morakchi, S., Aribi, N., and Soltani, N. (2015). Evaluation of pyriproxyfen, a juvenile hormone analog, on Drosophila melanogaster (Diptera: Drosophilidae) insecticidal activity, ecdysteroid contents and cuticle formation. Eur. J. Entomol. 112, 625–631. doi: 10.14411/eje.2015.084
Benuzzi, M., and Ladurner, E. (2018). “Plant protection tools in organic farming,” in Handbook of Pest Management in Organic Farming, eds V. Vacante, and S. Kreiter (Cesena, FC: CAB international), 24–59. doi: 10.1079/9781780644998.0024
Bernardes, R. C., Barbosa, W. F., Martins, G. F., and Lima, M. A. P. (2018). The reduced-risk insecticide azadirachtin poses a toxicological hazard to stingless bee Partamona helleri (Friese, 1990) queens. Chemosphere 201, 550–556. doi: 10.1016/j.chemosphere.2018.03.030
Bernardes, R. C., Tomé, H. V. V., Barbosa, W. F., Guedes, R. N. C., and Lima, M. A. P. (2017). Azadirachtin-induced antifeeding in neotropical stingless bees. Apidologie 48, 275–285. doi: 10.1007/s13592-016-0473-3
Bezzar-Bendjazia, R., Kilani-Morakchi, S., and Aribi, N. (2016). Larval exposure to azadirachtin affects fitness and oviposition site preference of Drosophila melanogaster. Pestic. Biochem. Physiol. 133, 85–90. doi: 10.1016/j.pestbp.2016.02.009
Bezzar-Bendjazia, R., Kilani-Morakchi, S., Ferdenache, M., and Aribi, N. (2017). Azadirachtin induces larval avoidance and antifeeding by disruption of food intake and digestive enzymes in Drosophila melanogaster (Diptera: Drosophilidae). Pestic Biochem. Physiol. 143, 135–140. doi: 10.1016/j.pestbp.2017.08.006
Biswas, K., Chattopadhyay, I., and Banerjee, R. K. (2002). Biological activities and medicinal properties of neem (Azadirachta indica). Curr. Sci. 82,1336–1345.
Blum, F. C., Singh, J., and Merrell, D. S. (2019). In vitro activity of neem (Azadirachta indica) oil extract against Helicobacter pylori. J. Entomopharmacol. 232, 236–243. doi: 10.1016/j.jep.2018.12.025
Bomford, M. K., and Isman, M. B. (1996). Desensitization of fifth instar Spodoptera litura to azadirachtin and neem. Entomol. Exp. Applic. 81, 307–313. doi: 10.1046/j.1570-7458.1996.00101.x
Boulahbel, B., Aribi, N., Kilani-Morakchi, S., and Soltani, N. (2015). Insecticidal activity of azadirachtin on Drosophila melanogaster and recovery of normal status by exogenous 20-hydroxyecdysone. Afr. Entomol. 23, 224–233. doi: 10.4001/003.023.0104
Cantrell, C. L., Dayan, F. E., and Duke, S. O. (2012). Natural products as sources for new pesticides. J. Nat. Prod. 75, 1231–1242. doi: 10.1021/np300024u
Capinera, J. L., and Froeba, J. G. (2007). Behavioral responses of Schistocerca americana (Orthoptera: Acrididae) to azadirex (neem)-treated host plants. J. Econ. Entomol. 100, 117–122. doi: 10.1603/0022-0493(2007)100[117:BROSAO]2.0.CO;2
Caubet, Y., Jaisson, P., and Lenoir, A. (1992). Preimaginal induction of adult behaviour in insects. Q. J. Exp. Psychol. Sect. 44, 165–178.
Celestino, D., Braoios, G. I., Ramos, R. G., Gontijo, L. M., and Guedes, R. N. C. (2014). Azadirachtin-mediated reproductive response of the predatory pirate bug Blaptostethus pallescens. Biol. Control. 59, 697–705. doi: 10.1007/s10526-014-9601-z
Céspedes, C., Salazar, J. R., Ariza-Castolo, A., Yamaguchi, L., Avila, J. G., Aqueveque, P., et al. (2014). Biopesticides from plants: Calceolaria integrifolia s.l. Environ. Res. 132, 391–406. doi: 10.1016/j.envres.2014.04.003
Challa, G. K., Firake, D. M., and Behere, G. T. (2019). Bio-pesticide applications may impair the pollination services and survival of goragers of honey bee, Apis cerana fabricius in oilseed brassica. Environ. Pollut. 249, 598–609. doi: 10.1016/j.envpol.2019.03.048
Chandler, D., Bailey, A. S., Tatchell, G. M., Davidson, G., Greaves, J., and Grant, W. P. (2011). The development, regulation and use of biopesticides for integrated pest management. Phil. Trans. R. Soc. B 366, 1987–1998. doi: 10.1098/rstb.2010.0390
Chang, P. F. Z., Walter, J. F., and Hartis, J. R. (1996). Word Intellectual Property Organization. WO1996039034A1. WIPO.
Charleston, D. S., Kafir, R., Dicke, M., and Vet, L. E. M. (2006). Impact of botanical extracts derived from Melia azedarach and Azadirachta indica on populations of Plutella xylostella and its natural enemies: a field test of laboratory findings. Biol. Control 39, 105–114. doi: 10.1016/j.biocontrol.2006.05.012
Chaudhary, S., Kanwar, R. K., Sehgal, A., Cahill, D. M., Barrow, C. J., Sehgal, R., et al. (2017). Progress on Azadirachta indica based biopesticides in replacing synthetic toxic pesticides. Front. Plant. Sci. 8:610. doi: 10.3389/fpls.2017.00610
Chen, J., Fan, X., Zhu, J., Song, L., Li, Z., Lin, F., et al. (2018). Limonoids from seeds of Azadirachta indica A. Juss. And their cytotoxic activity. Acta. Pharm. Sin. B 8, 639–644. doi: 10.1016/j.apsb.2017.12.009
Chia, J., and Scott, K. (2020). Activation of specific mushroom body output neurons inhibits proboscis extension and sucrose consumption. PLoS ONE 15:e0223034. doi: 10.1371/journal.pone.0223034
Cordeiro, E. M. G., Corrêa, A. S., Venzon, M., and Guedes, R. N. C. (2010). Insecticide survival and behavioral avoidance in the laccewings Chrysoperla externa and Ceraeochrysa cubana. Chemosphere 81, 1352–1357. doi: 10.1016/j.chemosphere.2010.08.021
Cutler, G. C., Ramanaidu, K., Astatkie, T., and Isman, M. B. (2007). Green peach aphid, Myzus persicae (Hemiptera: Aphididae), reproduction during exposure to sublethal concentrations of imidacloprid and azadirachtin. Pest. Manag. Sci. 65, 205–209. doi: 10.1002/ps.1669
Dai, W., Li, Y., Zhu, J., Ge, L.q., Yang, G.q., and Liu, F. (2019). Selectivity and sublethal effects of some frequently–used biopesticides on the predator Cyrtorhinus lividipennis reuter (Heminoptera: Miridae). J. Integr. Agr. 18, 124–133. doi: 10.1016/S2095-3119(17)61845-8
Damalas, C. A., and Koutroubas, S. D. (2018). Current statuts and recent developments in biopesticide use. Agriculture 8:13. doi: 10.3390/agriculture8010013
Das, R. K., Sarma, S., Brar, S. K., and Verma, M. (2014). Nanoformulation of insecticides: novel products. J. Biofertil. Biopestic. 5:e120. doi: 10.4172/2155-6202.1000e120
Dawkar, V. V., Barage, S. H, Barbole, R. S, Fatangare, A., Grimalt, S, Haldar, S., et al. (2019). Azadirachtin-A from Azadirachta indica impacts multiple biological targets in cotton bollworm Helicoverpa armigera. ACS Omega. 4, 9531–9541. doi: 10.1021/acsomega.8b03479
Delventhal, R., and Carlson, J. (2016). Bitter taste receptors confer diverse functions to neurons. eLife 5:e11181. doi: 10.7554/eLife.11181
Depetris-Chauvin, A., Galagovsky, D., and Grosjean, Y. (2015). Chemicals and chemoreceptors: ecologically relevant signals driving behavior in Drosophila. Front. Ecol. Evol. 3:41. doi: 10.3389/fevo.2015.00041
Deravel, J., Krier, F., and Jacques, Ph. (2014). Les biopesticides, alternatives aux produits phytosanitaires chimiques (synthèse bibliographique). Biotechnol. Agron. Soc. Environ. 18, 220–232.
Dhar, R., Dawar, H., Garg, S., Basir, S. F., and Talwar, G. P. (1996). Effect of volatiles from neem and other natural products on gonotrophic cycle and oviposition of Anopheles stephensi and An.Culicifacies (Diptera: Culicidae). J. Med. Entomol. 33,195–201 doi: 10.1093/jmedent/33.2.195
Dhra, G., Ahmad, M., Kumar, J., and Patanjali, P. K. (2018). Mode of action of azadirachtin: a natural insecticide. Int. Res. J. 7, 41–46.
Dorn, A., Rademacher, J. M., and Sehn, E. (1987). “Effects of azadirachtin on reproductive organs and fertility in the large milkweed bug, Oncopeltus fasciatus,” in Proceedings of the 3rd International Neem Conference, 273–288.
Dubrovsky, E. B. (2005). Hormonal cross talk in insect development. Trends Endocrinol. Metab. 16, 6–11. doi: 10.1016/j.tem.2004.11.003
Dukas, R. (2008). Evolutionary biology of insect learning. Annu. Rev. Entomol. 53, 145–160. doi: 10.1146/annurev.ento.53.103106.093343
Egwu, O. C., Dickson, M. A., Gabriel, O. T., Okai, I. R., and Amanabo, M. (2019). Risk assessment of heavy metals level in soil and jute leaves (Corchorus olitorius) treated with azadirachtin neem seed solution and organochlorine pesticides. Int. J. Envir. Agric. Biotech. 4, 756–766. doi: 10.22161/ijeab/4.3.24
Er, A., Taşkiran, D., and Sak, O. (2017). Azadirachtin-induced effects on various life history traits and cellular immune reactions of Galleria mellonella (Lepidoptera: Pyralidae). Arch. Biol. Sci. 69, 335–344. doi: 10.2298/A.B.S.160421108E
European Food Safety Authority (2011). Conclusion on the peer review of the pesticide risk assessment of the active substance azadirachtin. EFSA J. 9:1858. doi: 10.2903/j.efsa.2011.1858
European Food Safety Authority (2018). Peer review of the insecticide risk assessment of the active substance azadirachtin (margosa extract). EFSA J. 16:5234. doi/epdf/10.2903/j.efsa.2018.5234
Feng, B. H., and Peng, L. F. (2012). Synthesis and characterization of carboxymethyl chitosan carrying ricinoleic functions as an emulsifier for azadirachtin. Carbohydr. Polym. 88, 576–582. doi: 10.1016/j.carbpol.2012.01.002
Feng, R., and Isman, M. B. (1995). Selection for resistance to azadirachtin in the green peach aphid Myzus persicae. Experientia 51, 831–833. doi: 10.1007/BF01922438
Ferdenache, M., Bezzar-Bendjezia, R., Marion Poll, F., and Kilani-Morakchi, S. (2019). Transgenerational effects from single larval exposure to azadirachtin on life history and behavior traits of Drosophila melanogaster. Sci. Rep. 9:17015. doi: 10.1038/s41598-019-53474-x
Flores-Céspedes, F., Martínez-Domínguez, G. P., Villafranca-Sánchez, M., and Fernández-Pérez, M. (2015). Preparation and characterization of azadirachtin alginate-biosorbent based formulations: water release kinetics and photodegradation study. J. Agric. Food. Chem. 63, 8391–8398. doi: 10.1021/acs.jafc.5b03255
Francesena, N., and Schneider, M. I. (2018). Selectivity assessment of two biorational insecticides, azadirachtin and pyriproxyfen, in comparison to a neonicotinoid, acetamiprid, on pupae and adults of a Neotropical strain Eretmocerus mundus mercet. Chemosphere 206, 349–358. doi: 10.1016/j.chemosphere.2018.05.010
Garcia, E. S., Luz, N., Azambuja, P., and Rembold, H. (1990). Azadirachtin depresses the release of prothoracicotropic hormone in Rhodnius prolixus larvae: evidence from head transplantations. J. Insect. Physiol. 36, 679–682. doi: 10.1016/0022-1910(90)90073-O
Ghazawi, N. A., El-Shranoubi, E. D., El-Shazly, M. M., and Abdel Rahman, K. M. (2007). Effects of azadirachtin on mortality rate and reproductive system of the grasshopper Heteracris littoralis Ramb (Orthoptera: Acrididae). J. Orthopt. Res. 16, 57–65. doi: 10.1665/1082-6467(2007)16[57:EOAOMR]2.0.CO;2
Goktepe, I., Portier, R., and Ahmedna, M. (2004). Ecological risk assessment of neem-based pesticides. Pesticides, food contaminants, and agricultural wastes. J. Environ. Sci. Health B 39, 311–320. doi: 10.1081/PFC-120030244
Gupta, S. C., Prasad, S., Tyagi, A. K., Kunnumakkara, A. B., and Aggarwal, B. B. (2017). Neem (Azadirachta indica): an indian traditional panacea with modern molecular basis. Phytomedicine 34,14–20. doi: 10.1016/j.phymed.2017.07.001
Haddi, K., Turchen, L. M., Viteri Jumbo, L. O., Guedes, R. N. C., Pereira, E. J. G., Aguiar, R. W. S., et al. (2020). Rethinking biorational insecticides for pest management: unintended effects and consequences. Pest. Manag. Sci. 76, 2286–2293. doi: 10.1002/ps.5837
Hansen, D. J., Cuomo, J., Khan, M., Gallagher, R. T., and Ellenberge, W. P. (1993). “Advances in neem and azadirachtin chemistry and bioactivity,” in Natural and Engineered Pest Management Agents, ACS Symposium Series, ed P. Hedin (Washington, DC: American Chemical Society), 103–129. doi: 10.1021/bk-1994-0551.ch008
Harrop, T. W. R., Sztal, T., Lumb, C., Good, R. T., Daborn, P. J., Batterham, P., et al. (2014). Evolutionary changes in gene expression, coding sequence and copy-number at the Cyp6g1 locus contribute to resistance to multiple insecticides in Drosophila. PLoS ONE 9:e84879. doi: 10.1371/journal.pone.0084879
Hasan, F., and Ansari, M. S. (2011). Toxic effects of neem-based insecticides on Pieris brassicae (Linn.). Crop. Prot. 30, 502–507. doi: 10.1016/j.cropro.2010.11.029
Helps, J. C., Paveley, N. D., and Bosch, F. (2017). Identifying circumstances under which high insecticide dose increases or decreases resistance selection. J. Theor. Biol. 7, 153–167. doi: 10.1016/j.jtbi.2017.06.007
Herrero, P. (2012). Fruit fly behavior in response to chemosensory signals, Peptides 38, 228–237. doi: 10.1016/j.peptides.2012.09.019
Hikal, W. M., Baeshen, R. S., and Said-Al, A. H. A. (2017). Botanical insecticide as simple extractives for pest control. Cogent Biol. 3:1404274. doi: 10.1080/23312025.2017.1404274
Huang, J. F., Shui, K. J., Li, H. Y., Hu, M. Y., and Zhong, G. H. (2011). Antiproliferative effect of azadirachtin A on Spodoptera litura Sl-1 cell line through cell cycle arrest and apoptosis induced by up-regulation of p53. Pestic. Biochem. Phys. 99, 16–24. doi: 10.1603/00138746(2007)100245:EOAOHP2.0.CO;2
Huang, Z., Shi, P., Chen, G., and Du, J. (2007). Effects of azadirachtin on hemolymph protein expression in Ostrinia furnacalis (Lepidoptera: Crambidae). Ann. Entomol. Sci. Am. 100, 245–250.
Hummel, H. E., Langner, S., Hein, D. F., Sanguanpong, U., and Schmutterer, H. (2016). Unusually versatile plant genus Azadirachta with many useful and so far incompletely exploited properties for agriculture, medicine and industry. Acta. Fytotechn. Zootechn. 18, 169–175. doi: 10.15414/afz.2015.18.si.169-175
Hunter, M. C., Smith, R. G., Schipanski, M. E., Atwood, L. W., and Mortensen, D. A. (2017). Agriculture in 2050: recalibrating targets for sustainable intensification. BioScience 67, 386–391. doi: 10.1093/biosci/bix010
Iqbal, N., Kumar, N., Saini, M. K., Dubey, S., Agrawal, A., and Kumar, J. (2020). Role of high shear mixing in improving stability and bio-efficacy of botanical oil in water formulation for early stage mosquito eradication. Helion 6:e03380. doi: 10.1016/j.heliyon.2020.e03380
Irigaray, F. J., Moreno-Grijalba, F., Marco, V., and Pérez-Moreno, I. (2010). Acute and reproductive effects of align®, an insecticide containing azadirachtin, on the grape berry moth, Lobesia botrana. J. Insect. Sci. 10:33. doi: 10.1673/031.010.3301
Isman, M. B. (2006). Botanical insecticides, deterrents, and repellents in modern agriculture and an increasingly regulated world. Annu. Rev. Entomol. 51, 45–66. doi: 10.1146/annurev.ento.51.110104.151146
Isman, M. B., and Grieneisen, M. L. (2014). Botanical insecticide research: many publications, limited useful data. Trends. Plant. Sci. 19,140–145. doi: 10.1016/j.tplants.2013.11.005
Izadi, H., Sarnevesht, M., Sadeghi, R., Mahdian, K., and Jalai, M. A. (2012). Toxic effects of pyriproxyfen, neemarin, acetamiprid and Ferula assafoetida essential oil on the common pistachio psylla, Agonoscena pistaciae. Arch. Phytopat. Plant. Protect. 45, 2236–2242. doi: 10.1080/03235408.2012.724973
Jars, E., Neupane, D., and London, L. (2018). Pesticide poisonings in low- and middle-income countries. Environ. Health Insights 12, 1–3. doi: 10.1177/1178630217750876
Jauch, J. (2008). Total synthesis of azadirachtin-finally completed after 22 years. Angew. Chem. Int. Ed. 47, 34–37. doi: 10.1002/anie.200703814
Jesser, E., Yeguermanb, C., Gilia, V., Santillana, G., Murrayc, A. P., Dominic, C., et al. (2020). Optimization and characterization of essential oil nanoemulsions using ultrasound for new ecofriendly insecticides. ACS Sust. Chem. Eng. 8, 7981–7992. doi: 10.1021/acssuschemeng.0c02224
Karuppuchamy, P., and Venugopal, S. (2016). “Integrated pest management,” in Ecofriendly Pest Management for Food Security, ed Omkar (San Diego, CA: Academic Press), 651–684. doi: 10.1016/B978-0-12-803265-7.00021-X
Khater, H. F. (2012). Prospects of botanical biopesticides in insect pest management. Pharmacologia 3, 641–656. doi: 10.5567/pharmacologia.2012.641.656
Khosravi, R., and Sendi, J. J. (2013). Effect of neem pesticide (achook) on midgut enzymatic activities and selected biological compounds in the hemolymph of lesser mulberry pyralid, Glyphodes pyloalis walker (Lepidoptera: Pyralidae). J. Plant. Prot. Res. 5, 238–247. doi: 10.2478/jppr-2013-0036
Kilani-Morakchi, S., Bezzar-Bendjazia, R., Ferdenache, M., and Aribi, N. (2017). Preimaginal exposure to azadirachtin affects food selection and digestive enzymes in adults of Drosophila melanogaster (Diptera: Drosophilidae). Pestic. Biochem. Physiol. 140, 58–64. doi: 10.1016/j.pestbp.2017.06.004
Konecka, E., Kaznowski, A., and Tomkowiak, D. (2019). Insecticidal activity of mixtures of Bacillus thuringiensis crystals with plant oils of Sinapis alba and Azadirachta indica. Ann. Appl. Biol. 174, 364–371. doi: 10.1111/aab.12502
Kraus, W., Bokel, M., Klenk, A., and Pöhn, H. (1985). The structure of azadirachtin and 22,23-dihydro-23β-methoxyazadirachtin. Tetrahedron Lett. 26, 6435–6438. doi: 10.1016/S0040-4039(00)99020-8
Kumar, D., Rahal, A., and Malik, J. K. (2016). “Neem extract,” in Nutraceuticals, ed R. Gupta (London: Academic Press), 585–597. doi: 10.1016/B978-0-12-802147-7.00043-7
Kumar, P., and Poehling, H. M. (2007). Effects of azadirachtin, abamectin, and spinosad on sweetpotato white?y (Homoptera: Aleyrodidae) on tomato plants under laboratory and greenhouse conditions in the humid tropics. J. Econ. Entomol. 100, 411–420. doi: 10.1093/jee/100.2.411
Kumar, S. (2015). Biopesticide: an environment friendly pest management strategy. J. Biofertil. Biopestici. 6:1. doi: 10.4172/2155-6202.1000e127
Kumar, V., Chandrashekar, K., and Sidhu, O. P. (2007). Synergistic action of neem and karanj to aphids and mites. J. Ent. Res. 31, 121–124. doi: 10.1007/s11259-007-0078-4
Lai, D., Jin, X., Wang, H., Yuan, M., and Xu, H. (2014). Gene expression profile change and growth inhibition in Drosophila larvae treated with azadirachtin. Biotechnology 185, 51–56. doi: 10.1016/j.jbiotec.2014.06.014
Lee, Y., Kim, S., and Montell, C. (2010). Avoiding DEET through insect gustatory receptors. Neuron 67, 555–561. doi: 10.1016/j.neuron.2010.07.006
Lima, D. B., Melo, J. W. S., Guedes, N. M. P., Gontijo, L. M., Guedes, R. N. C., and Gondim, M. G. C. Jr. (2015). Bioinsecticide-predator interactions: Azadirachtin behavioral and reproductive impairment of the coconut mite predator Neoseiulus baraki. PLoS ONE 10:e0118343. doi: 10.1371/journal.pone.0118343
Little, C. M., Chapman, T. W., and Hillier, N. K. (2019). Considerations for insect learning in integrated pest management. J. Insect. Sci. 19:6. doi: 10.1093/jisesa/iez064
Liu, P. F., Wang, W., Ling, X., Lu, Q., Zhang, J., He, R., et al. (2019). Regulation hormone-related genes in Ericerus pela (Hemiptera: Coccidae) for dimorphic metamorphosis. J. Insect Sci. 19:16. doi: 10.1093/jisesa/iez092
Lu, Z., Dong, S., Li, C., Li, L., Yu, Y., Yin, S., et al. (2020). Sublethal and transgenerational effects of sulfoxaflor on the demography and feeding behaviour of the mirid bug Apolygus lucorum. PLoS ONE 15:e0232812. doi: 10.1371/journal.pone.0232812
Marco, M. P., Pascual, N., Bellès, X., Camps, F., and Messeguer, A. (1990). Ecdysteroid depletion by azadirachtin in Tenebrio molitor pupae. Pest. Biochem. Physiol. 38, 60–65. doi: 10.1016/0048-3575(90)90149-V
Martinez, S. S., and van Emden, H. F. (1999). Sublethal concentrations of azadirachtin affect food intake, conversion efficiency and feeding behaviour of Spodoptera littoralis (Lepidoptera: Noctuidae). Bull. Entomol. Res. 89, 65–71. doi: 10.1017/S0007485399000085
Masek, P., and Keene, A.C. (2016). Gustatory processing and taste memory in Drosophila. J. Neurogenet. 30, 112–121. doi: 10.1080/01677063.2016.1185104
Mayoral, J. G., Nouzova, M., Navare, A., and Noriega, F. G. (2009). NADP+-dependent farnesol dehydrogenase, a corpora allata enzyme involved in juvenile hormone synthesis. Proc. Natl. Acad. Sci. U.S.A. 106, 21091–2109. doi: 10.1073/pnas.0909938106
Medina, P., Budia, F., Del Estal, P., and Viñuela, E. (2004). Influence of azadirachtin, a botanical insecticide, on Chrysoperla carnea (Stephens) reproduction: toxicity and ultrastructural approach. J. Econ. Entomol. 97, 43–50. doi: 10.1093/jee/97.1.43
Min-Li, Z., and Shin-Foon, C. (1987). The effects of azadirachtin on the ecdysteroid titre in larvae of Ostrinia furnacafis Guenée. J. Appl. Ent. 103, 355–359. doi: 10.1111/j.1439-0418.1987.tb00995.x
Mishra, R. K., Bohra, A., Kamaal, N., Kumar, K., Gandhi, K., Sujayanand, G. K., et al. (2018). Utilization of biopesticides as sustainable solutions for management of pests in legume crops: achievements and prospects. Egypt. J. Biol. Pest. Control 28:3. doi: 10.1186/s41938-017-0004-1
Moga, M. A., Balan, A., Anastasiu, C. V., Dimienescu, O. G., Neculoiu, C. D., and Gavris, C. (2018). An overview on the anticancer activity of Azadirachta indica (neem) in gynecological cancers. Int. J. Mol. Sci. 19:3898. doi: 10.3390/ijms19123898
Mordue, A. J., Morgan, E.D., and Nisbet, A. J. (2010). “Azadirachtin, a natural product in insect control,” in Insect Control: Biological and synthetic agents, eds L. I. Gilbert and S. S. Gill (Elsevier; Academic), 185–203.
Mordue, A. J., and Nisbet, A. J. (2000). Azadirachtin from the neem tree (Azadirachta indica): its actions against insects. Ann. Soc. Entomol. Brasil. 29, 615–632. doi: 10.1590/S0301-80592000000400001
Mordue, L. A. J., and Blackwell, A. (1993). Azadirachtin: an update. J. Insect. Physiol. 39, 903–924. doi: 10.1016/0022-1910(93)90001-8
Morgan, E. D. (2009). Azadirachtin, a scientific goldmine. Bioorgan. Med. Chem. 17, 4096–4105. doi: 10.1016/j.bmc.2008.11.081
Moulin, T. C., Ferro, F., Berkins, S., Hoyer, A., Williams, M. J., and Schiöth, H. B. (2020). Transient administration of dopaminergic precursor causes inheritable overfeeding behavior in young Drosophila melanogaster adults. Brain Sci. 10:487. doi: 10.3390/brainsci10080487
Nagini, S. (2014). “Neem limonoids as anticancer agents: modulation of cancer hallmarks and oncogenic signaling,” in The Enzymes, Vol. 36 (Elsevier Inc), 131–147. doi: 10.1016/B978-0-12-802215-3.00007-0
Nicoletti, M., Murugan, K., Canale, A., and Benelli, G. (2016). Neem-borne molecules as eco-friendly control tools against mosquito vectors of economic importance. Curr. Org. Chem. 20, 2681–2689. doi: 10.2174/1385272820666160218233923
Nicolopoulou-Stamati, P., Maipas, S., Kotampasi, C., Stamatis, P., and Hens, L. (2016). Chemical pesticides and human health: the urgent need for a new concept in agriculture. Front. Public Health 4:148. doi: 10.3389/fpubh.2016.00148
Olson, S. (2015). An analysis of the biopesticide market now and where it is going. Outlooks Pest. Manag. 26, 203–206. doi: 10.1564/v26_oct_04
Oulhaci, M. C., Denis, B., Kilani-Morakchi, S., Sandoz, J. C., Kaiser, L., Joly, D., et al. (2018). Azadirachtin effects on mating success, gametic abnormalities and progeny survival in Drosophila melanogaster (Diptera). Pest. Manag. Sci. 74,174–180. doi: 10.1002/ps.4678
Pandey, J. P., and Tiwari, R. K. (2011). Neem based insecticides interaction with development fecundity of red cotton bug, Dysdercus cingulatus fab. Int. J. Agri. Res. 6, 335–346. doi: 10.3923/ijar.2011.335.346
Paranagama, P. A., Connolly, J. D., and Strang, R. H. C. (2004). Azadirachtin effects on proteins synthesized in fat body, haemolymph, ovary and mid-gut of locusts, Schistocerca Gregaria. J. Natn. Sci. Fondation. 32, 13–28. doi: 10.4038/jnsfsr.v32i1-2.2419
Pasquoto-Stigliani, T., Campos, E. V. R., Oliveira, J. L., Silva, C. M. G., Bilesky-José, N., Guilger, M., et al. (2017). Nanocapsules containing neem (Azadirachta Indica) oil: development, characterization, and toxicity evaluation. Sci. Rep. 7:5929. doi: 10.1038/s41598-017-06092-4
Pavela, R. (2016). History, presence and perspective of using plant extracts as commercial botanical insecticides and farm products for protection against insects – a review. Plant Protect. Sci. 52, 229–241. doi: 10.17221/31/2016-PPS
Pener, M. P., and Dhadialla, T. S. (2012). An overview of insect growth disruptors; applied aspects. Adv. Insect. Physiol. 43, 1–162. doi: 10.1016/B978-0-12-391500-9.00001-2
Pereira, V. V., Kumar, D., Agiwal, M., and Prasad, T. G. (2019). Stability of azadirachtin: a tetranortriterpenoid from neem tree. Int. J. Chem. Stud. 7, 412–419.
Pimentel, D. (1997). “Pest management in agriculture,” in Techniques for Reducing Pesticide Use: Economic and Environmental Benefits, eds D. Pimentel (Chichester: Wiley), 1–11.
Pineda, S., Martínez, A. M., Figueroa, J. I., Schneider, M. I., Estal, P. D., Viñuela, E., et al. (2009). Influence of azadirachtin and methoxyfenozide on life parameters of Spodoptera littoralis (Lepidoptera: Noctuidae). J. Econ. Entom. 102, 1490–1496. doi: 10.1603/029.102.0413
Pineda, S., Pérez-Robledo, C. A., Hernández, R. E., Figueroa de la Rosa, J. I., Chavarrieta, J. M., and Martínez, A. M. (2014). Combined and individual effects of a nucleopolyhedrovirus and azadirachtin on themortality and maize-leaf consumption of Spodoptera frugiperda. Phytoparasitica 42, 571–578 doi: 10.1007/s12600-014-0395-4
Popp, J., Petö, K., and Nagy, J. (2013). Pesticide productivity and food security: a review. Agron. Sust. Dev. 33, 243–255. doi: 10.1007/s13593-012-0105-x
Prianto, A. H., Budiawan, N. F. N., Yulizar, Y., and Simanjuntak, P. (2019). The synergy effect of azadirachtin and minor components of neem seed oil on antifeedant activity of Spodoptera litura. Bull. Penelit. Tanam. Rempah Obat. 30, 27–34. doi: 10.21082/bullittro.v30n1.2019.27-34
Qiao, J., Zou, X., Lai, D., Yan, Y., Wang, Q., Li, W., et al. (2014). Azadirachtin blocks the calcium channel and modulates the cholinergic miniature synaptic current in the central nervous system of Drosophila. Pest. Manag. Sci. 70, 1041–1047. doi: 10.1002/ps.3644
Qin, D., Zhang, P., Zhou, Y., Liu, B., Xiao, C., Chen, W., et al. (2020). Antifeeding effects of azadirachtin on the fifth instar Spodoptera litura larvae and the analysis of azadirachtin on target sensilla around mouthparts. Arch. Insect. Biochem. Physiol. 103:e21646. doi: 10.1002/arch.21646
Qu, Z., Kenny, N. J., Lam, H. M., Chan, T. F., Chu, K. H., Bendena, W. G., et al. (2015). How did arthropod sesquiterpenoids and ecdysteroids arise? Comparison of hormonal pathway genes in noninsect arthropod genomes. Genome Biol. Evol. 7, 1951–1959. doi: 10.1093/gbe/evv120
Raguraman, S., and Kannan, M. (2014). “Non-target effects of botanicals on beneficial arthropods with special reference to Azadirachta indica,” in Advances in Plant Biopesticides Chapter: Non-Target Effects of Botanicals on Beneficial Arthropods With Special Reference to Azadirachta Indica, ed D. Singh (Springer). doi: 10.1007/978-81-322-2006-0_10
Raizada, R. B., Srivastava, M. K., Kaushal, R. A., and Singh, R. P. (2001). Azadirachtin, a neem biopesticide: subchronic toxicity assessment in rats. Food Chem. Toxicol. 39, 477–448. doi: 10.1016/S0278-6915(00)00153-8
Rembold, H., and Sieber, K. P. (1981). Inhibition of oogenesis and ovarian ecdysteroid synthesis by azadirachtin in Locusta migratoria migratorioides (R. and F.). Z. Naturforsch. C 36, 466–469. doi: 10.1515/znc-1981-5-621
Riba, M., Marti, J., and Sans, A. (2003). Influence of azadirachtin on development and reproduction of Nezara viridula L, (Het., Pentatomidae). J. Appl. Entomol. 127, 37–41. doi: 10.1046/j.1439-0418.2003.00684.x
Roberston, S. L., Ni, W., Dhadialla, T. S., Nisbet, A. J., Mc Custer, C., Ley, S. V., et al. (2007). Identification of a putative azadirachtin-binding complex from Drosophila Kc167 cells. Arch. Insect. Biochem. Physiol. 64, 200–208. doi: 10.1002/arch.20171
Schmutterer, H. (1990). Properties and potential of natural pesticides from the neem tree, Azadirachta indica. Ann. Rev. Entomol. 35, 271–297. doi: 10.1146/annurev.en.35.010190.001415
Schmutterer, H. (1995). The Neem Tree: Source of Unique Natural Products for Integrated Pest Management, Medicine, Industry and Other Purposes. Wenheim: VCH, 1–696. doi: 10.1002/3527603980
Seugling, J., Kuhnen, S., de Barros, G. P., Velerinho, M. B., Mazzarino, L., and Bricarello, P. A. (2019). Development of Baccharis dracunculifolia (Asteraceae) essential oil nanoemulsion and its biological activity on pre-pupae of Cochliomyia hominivorax (Diptera: Calliphoridae). J. Pharm. Pharmacol. 7, 293–308. doi: 10.17265/2328-2150/2019.06.003
Shanmugapriya, S., Jeya Sundara Sharmila, D., Karthikeyan, G., and Subramanian, K. S. (2019). Bioassay of azadirachtin nanofomulation against bemisia tabaci, the vector of mungbean yellow mosaic virus. Madras. Agric. J. 106, 522–527. doi: 10.29321/MAJ.2019.000293
Shannag, H. K., Capinera, J. L., and Freihat, N. M. (2015). Effects of neem-based insecticides on consumption and utilization of food in larvae of Spodoptera eridania (Lepidoptera: Noctuidae). J. Insect. Sci. 15:152. doi: 10.1093/jisesa/iev134
Sharma, R., Kumari, A., Singh, N. S., Singh, M. K., Dubey, S., Iqbal, N., et al. (2019). Development and stability enhancement of neem oil based microemulsion formulation using botanical synergist. J. Mol. Liq. 296:112012. doi: 10.1016/j.molliq.2019.112012
Shu, B., Wang, W., Hu, Q., Huang, J., Hu, M., and Zhong, G. (2015). A comprehensive study on apoptosis induction by azadirachtin in Spodoptera frugiperda cultured cell line Sf9. Arch. Insect. Biochem. Physiol. 89, 153–168. doi: 10.1002/arch.21233
Shu, B., Yu, H., Li, Y., Zhong, H., Li, X., Cao, L., et al. (2020). Identification of azadirachtin responsive genes in Spodoptera frugiperda larvae based on RNA-seq. Pest. Biochem. Physiol. 172:104745. doi: 10.1016/j.pestbp.2020.104745
Shu, B., Zhang, J., Cui, G., Sun, R., Yi, X., and Zhong, G. (2018). Azadirachtin affects the growth of Spodoptera litura fabricius by inducing apoptosis in larval midgut. Front. Physiol. 9:137. doi: 10.3389/fphys.2018.00137
Silva, G. (2018). Feeding the World in 2050 and Beyond – Part 1: Productivity Challenges. Michigan State University Extension.
Smith, S. L., and Mitchell, M. J. (1988). Effects of azadirachtin on insect cytochrome P-450 dependent ecdysone 20-monooxygenase activity. Biochem. Biophys. Res. Commun. 154, 559–563. doi: 10.1016/0006-291X(88)90176-3
Sun, R., Cui, G., Chen, Y., Shu, B., Zhong, G., and Yi, X. (2018). Proteomic profiling analysis of male infertility in Spodoptera litura larvae challenged with azadirachtin and its potential regulated pathways in the following stages. Proteomics 18:e1800192. doi: 10.1002/pmic.201800192
Tennessen, J. M., and Thummel, C. S. (2011). Coordinating growth and maturation insights from Drosophila. Curr. Biol. 21, R750–R757. doi: 10.1016/j.cub.2011.06.033
Thakora, Y. (2006). The biopesticide market for global agricultural use. Indust. Biotechnol. 2, 194–208. doi: 10.1089/ind.2006.2.194
Tine, S., Aribi, N., and Soltani, N. (2011). Laboratory evaluation of azadirachtin against the oriental cockroach, Blatta orientalis L. (Dictyoptera, Blattellidae): Insecticidal activity and reproductive effects. Afr. J. Biotechnol. 10, 19816–19824. doi: 10.5897/AJB11.2497
Tinghui, X., Wegener, M., O'Shea, M., and Deling, M. (2001). “World distribution and trade in neem products with reference to their potential in China,” in AARES 2001 Conference of Australian Agricultural and Resource Economics Society (Adelaide, SA), 15.
Tomé, H. V. V., Martins, J. C., Correea, S., Galdino, T. V. S., Picançon, M. C., and Guedes, R. N. C. (2013). Azadirachtin avoidance by larvae and adult females of the tomato leafminer Tuta absoluta. Crop. Prot. 46, 63–69. doi: 10.1016/j.cropro.2012.12.021
United Nations. (2017). World Population Prospects: The 2017 Revision; United Nations, Department of Economic and Social Affairs. New York, NY: Population Division. Available online at: https://www.un.org/~development/desa/publications/world-population-prospects-the-2017-revision.html
Vandegehuchte, M. B., and Janssen, C. R. (2011). Epigenetics and its implications for ecotoxicology. Ecotoxicology 20, 607–624. doi: 10.1007/s10646-011-0634-0
Vilca Malqui, K. S., Vieira, J. L., Guedes, R. N. C., and Gontijo, L. M. (2014). Azadirachtin-induced hormesis mediating shift in fecundity longevity trade-off in the Mexican bean weevil (Chrysomelidae: Bruchinae). J. Econ. Entomol. 107, 860–866. doi: 10.1603/EC13526
Vivekananthan, T., and Selvisabhanayakam, S. N. (2014). Histopathological observations on testes of adult blister beetle, Mylabris indica (thunberg) (Coleoptera: Meloidae) treated with neem. J. Entomol. Res. 38, 45–52.
Walker, W. B. III., Gonzalez, F., Garczynski, S. F., and Witzgall, P. (2016). The chemosensory receptors of codling moth Cydia pomonella-expression in larvae and adults. Sci. Rep. 6:23518. doi: 10.1038/srep23518
Weiss, L. A., Dahanukar, A., Kwon, J. Y., Banerjee, D., and Carlson, J. R. (2011). The molecular and cellular basis of bitter taste in Drosophila. Neuron. 69, 258–272. doi: 10.1016/j.neuron.2011.01.001
WHO (2009). The WHO Recommended Classification of Pesticides by Hazard and Guidelines to Classification 2009.2010. Available online at: http://www.who.int/ipcs/publications/pesticides_2009.pdf (accessed May 6, 2018).
Xavier, V. M., Message, D., Picanço, M. C., Chediak, M., Júnior, P. A. S., Ramos, R., et al. (2015). Toxicity and sublethal effects of botanical insecticides to honey bees. J. Insect. Sci. 15:137. doi: 10.1093/jisesa/iev110
Yan, Y., Gu, H., Xu, H., and Zhang, Z. (2017). Induction of aversive taste memory by azadirachtin and its effects on dopaminergic neurons of Drosophila. J. South. China Agri. Univ. 38, 12–18.
Yu, S. J. (2000). Allelochemical induction of hormone-metabolizing microsomal monooxygenases in the fall armyworm. Zool. Stud. 39, 243–249.
Zada, H., Ahmad, B., Hassan, E., Saljoqi, A. U. R., Naheed, H., and Salim, M. (2018). Toxicity potential of different azadirachtin against Plutella Xylostella (Lepidoptera; Plutellidae) and its natural enemy, Diadegma Species. J. Agron. Agri. Sci. 1, 1–7. doi: 10.24966/AAS-8292/100003
Zhang, J., Suna, T., Suna, Z., Lia, H., Qia, X., Zhonga, G., et al. (2018). Azadirachtin acting as a hazardous compound to induce multiple detrimental effects in Drosophila melanogaster. J. Hazard. Mater. 359, 338–347. doi: 10.1016/j.jhazmat.2018.07.057
Zhao, J., Zhou, Y., Li, X., Cai, W., and Hua, H. (2017). Silencing of juvenile hormone epoxide hydrolase gene (Nljheh) enhances short wing formation in a macropterous strain of the brown planthopper, Nilaparvata lugens. J. Insect Physiol. 102, 18–26. doi: 10.1016/j.jinsphys.2017.08.012
Zhao, T., Lai, D., Zhou, Y., Xu, H., Zhang, Z., Kuang, S., et al. (2019). Azadirachtin A inhibits the growth and development of Bactrocera dorsalis larvae by releasing cathepsin in the midgut. Ecotoxicol. Environ. Saf. 183:109512. doi: 10.1016/j.ecoenv.2019.109512
Zhong, B., Lv, C., and Qin, W. (2017). Effectiveness of botanical Insecticide Azadirachtin Against Tirathaba rufivena (Lepidoptera: Pyralidae). Fla Entomol. 100, 215–218. doi: 10.1653/024.100.0215
Keywords: azadirachtin, Azadirachta indica, nanotechnology, alternative pest control, agroecosystems
Citation: Kilani-Morakchi S, Morakchi-Goudjil H and Sifi K (2021) Azadirachtin-Based Insecticide: Overview, Risk Assessments, and Future Directions. Front. Agron. 3:676208. doi: 10.3389/fagro.2021.676208
Received: 04 March 2021; Accepted: 27 April 2021;
Published: 20 July 2021.
Edited by:
Rachid Lahlali, Ecole Nationale d'Agriculture de Meknès, MoroccoReviewed by:
Roman Pavela, Crop Research Institute (CRI), CzechiaCopyright © 2021 Kilani-Morakchi, Morakchi-Goudjil and Sifi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Samira Kilani-Morakchi, c2FtaXJhLm1vcmFrY2hpQHVuaXYtYW5uYWJhLmR6 orcid.org/0000-0002-1662-4913
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.