- African Centre for Crop Improvement, School of Agricultural, Earth and Environmental Sciences, University of KwaZulu-Natal, Pietermaritzburg, South Africa
The narrow genetic variation for drought adaptive traits and biomass allocation in wheat (Triticum aestivum L.) presents a major bottleneck for breeding. Induced mutagenesis creates genetic variation and complements conventional breeding for drought tolerance improvement. The aims of this study were to induce mutations in wheat genotype LM43 using three ethyl methanesulphonate (EMS) treatments, and to develop mutant populations for improving drought tolerance, biomass allocation and agronomic performance. Experiments were conducted under controlled and field conditions at the University of KwaZulu-Natal. Data on percentage germination (%G), days to 90% maturity (DTM), plant height (PH), shoot biomass (SB), root biomass (RB), root-shoot ratio (RSR), spike length (SL), spikelet count (SPS), thousand seed weight (TSW), and grain yield (GY) were collected from M1 to M4 generations. Significant (p < 0.001) differences among individuals and generations were observed for all the assessed traits and the generation × population interaction effects were significant (p < 0.01) for SB, TSW, and GY due to EMS treatments. The differences among the generations showed that the mutagenic effects were cumulative and exhibited clear segregations in subsequent generations. The new selections with unique biomass allocation, drought response and agronomic performance will be useful for wheat improvement programs.
Introduction
An estimated seven billion people across the world depend on bread wheat (Triticum aestivum L.; 2n = 6x = 42, AABBDD) for food, making it the second most important food crop globally (Tilman et al., 2011). Wheat is a source of fiber, carbohydrates and proteins (Mahajan and Tuteja, 2005). Globally, wheat was produced on ~218 million hectares with an output of 772 million tons of grain in the year 2017 [Food Agricultural Organization (FAO), 2018]. However, it is projected that a 70% increase in wheat production will be required to suffice human consumption by the year 2060 (Ortiz et al., 2008). Reports indicate that wheat production and productivity has declined by 5.5% in the last few decades due to climate change-induced drought and heat stresses (Daryanto et al., 2016). There is a need to develop wheat cultivars with improved yield potential and enhanced resistance to biotic and abiotic constraints to meet the projected demand for wheat.
Drought stress is one of the major constraints of wheat production and productivity. Daryanto et al. (2016) estimated that, on average, 21% loss in yield can be incurred in wheat when moisture availability decreases by 40%. The impact of drought on wheat production is influenced by genotype (Daryanto et al., 2016), the stress intensity and duration (Park et al., 2016; Sun et al., 2017), plant health status and nutrition (Lobell et al., 2008; Yu et al., 2018) and genotype-by-environment interactions. Supplemental irrigation is used to mitigate the impact of drought stress. However, this option is not sustainable. Average rainfall is declining and inadequate to replenish water reservoirs to meet human, industrial and agricultural uses, which may create conflict on water management and use. Developing drought adapted cultivars is among the most sustainable strategies to reduce water demand for agriculture and minimize the impact of drought stress on wheat production.
Several wheat breeding programs spearheaded by the International Maize and Wheat Improvement Centre (CIMMYT) and the International Centre for Agricultural Research in Dry Areas (ICARDA) in collaboration with various national organizations initiated the development of wheat breeding lines with improved drought tolerance. The breeding lines reportedly exhibited high yield potential and were adapted to dryland farming ecologies (Smale et al., 2002). The successful development of drought tolerant cultivars depends on identifying and exploiting wide genetic variation for drought adaptive traits in wheat. Drought adaptive traits include flowering and maturity periods, plant height and spike length, kernel weight, tillering capacity, and biomass allocation (Abdolshahi et al., 2013; Mehraban et al., 2018; Hooshmandi, 2019). Most adaptive traits have been investigated extensively in studies on drought tolerance and yield in wheat, while biomass allocation has been less reported. Assessing biomass allocation in plants involve quantifying biomass in the above and below ground parts. Root traits have been neglected in breeding program due to difficulties associated with root sampling and phenotyping (Den Herder et al., 2010; Fang et al., 2017). Conventional wheat varieties exhibit narrow genetic variation for root traits because most breeding programs primarily aim to improve harvest indices to increase yield potential. While this has led to increased grain yield potential, it has narrowed genetic variation for rooting ability, lowered root to shoot ratios and increased susceptibility to drought stress in modern varieties (White et al., 2015).
Genetic variation allows for selection of superior individuals. Breeding wheat populations for drought tolerance has been limited by several factors including large environmental variance encountered during phenotyping, lack of genetic variation and loss of genetic diversity in improved cultivars. The loss of genetic diversity has contributed to stagnant yields and high susceptibility of wheat to environmental stress (Keneni et al., 2012; Voss-Fels et al., 2015). The narrow genetic diversity in wheat is attributed to continuous directional selection within a narrow range of elite parental lines. Many spring wheat cultivars in developing countries were developed involving at least one elite parent bred by CIMMYT (Smale et al., 2002). Thus, there is a need to create new genetic variation for developing new cultivars with improved drought stress tolerance. Genetic variation is created after recombination of genes through controlled crosses. Recombination occurs through sexual reproduction when divergent and complementary parents are crossed. This process does not occur naturally in self-pollinating species such as wheat. Self-pollinating species require emasculation prior to crossing, which is tedious and expensive. Furthermore, conventional breeding by crossing of superior genotypes is a long-term process that takes about 12 years to develop distinct, stable, and uniform varieties (Shivakumar et al., 2018). There is a need to rapidly create genetic variation and develop superior cultivars within a shorter period to respond to the rapidly changing environment.
Induced mutagenesis, which involves exposing biological material to chemical or physical agents that induce genetic modification through mutations in the DNA, has been used in widening genetic variation in self-pollinated species such as rice, sorghum, and wheat [International Atomic Energy Agency (IAEA), 2020]. The resultant mutant varieties created through mutagenesis have improved productivity and quality (Kenzhebayeva et al., 2014). The use of induced mutagenesis has the potential to create new genetic variation that may not be created by conventional breeding strategies. For instance, the possible genetic recombination obtained by sexual reproduction after crossing is limited by the initial allelic diversity within the base breeding population (Voss-Fels et al., 2015). Mutagenesis broadens the possibilities of allelic diversity of the base population. The efficacy of the mutagenic agent can be manipulated by altering its dosage and treatment conditions. It is imperative to generate large mutant populations to enhance the efficiency of mutagenesis and increase the probability of obtaining superior mutant individuals. Various mutagens including ethyl methanesulphonate (EMS) have been used successfully to improve agronomic traits such as flowering and maturity period, reduced plant height, yield, grain quality and tolerance to abiotic and biotic stress (Maluszynski and Kasha, 2002; Kontz et al., 2009; Singh and Balyan, 2009; Dhaliwal et al., 2015; Nazarenko et al., 2018; Lethin et al., 2020).
The use of EMS mutagenesis requires less sophisticated equipment, which makes it appropriate for developing countries, and poses low health and environmental hazard risks (Anbarasan et al., 2013). However, mutations obtained after exposure to EMS are random and some may not be useful in developing fit-for-purpose varieties. There is a need to develop various populations and select superior mutant genotypes or families. The selected families can either be used as parental lines to develop breeding populations or released as mutant varieties. Reportedly, mutagenesis caused desirable genetic changes and improved agronomic performance in wheat, rice, and cowpea (e.g., Dhaliwal et al., 2015; Horn et al., 2016; Luz et al., 2016). However, there are no previous reports on development of breeding populations of wheat with optimized biomass allocation, drought tolerance and agronomic performance. There is a need to identify novel mutant breeding populations with enhanced biomass allocation for drought tolerance and agronomic performance in wheat. Early generation selection of mutant populations is important to advance desirable traits in wheat (OlaOlorun et al., 2020). In a preliminary study, OlaOlorun et al. (2019) established three ideal EMS treatment conditions in wheat genotype LM43. The three pre-determined EMS treatment conditions were suitable to induce mutation and to select ideotypes with high yield, improved drought tolerance and high root to shoot ratios (OlaOlorun et al., 2021). Biomass allocation to roots has been neglected in wheat breeding despite the importance of roots in nutrient cycling, water extraction, carbon retention to soil. Studies have reported that biomass allocation can be pivotal in drought tolerance (Griffiths and Paul, 2017; Mathew et al., 2019). Therefore, the objectives of this study were to induce mutations in a wheat genotype LM43 using three predetermined ethyl methanesulphonate treatments, and to develop breeding populations involving M1 to M4 generations for enhanced drought tolerance, biomass allocation and agronomic performance.
Materials and Methods
Plant Materials
Bread wheat genotype designated as LM43, was selected from a panel of germplasm obtained from CIMMYT. The genotype was selected after prior evaluation for its drought tolerance and yield potential (Mwadzingeni et al., 2016). A preliminary study to establish optimal conditions for effective mutagenesis with minimum biological damage was conducted prior to embarking on a large-scale mutagenesis (OlaOlorun et al., 2019).
Selection Procedure
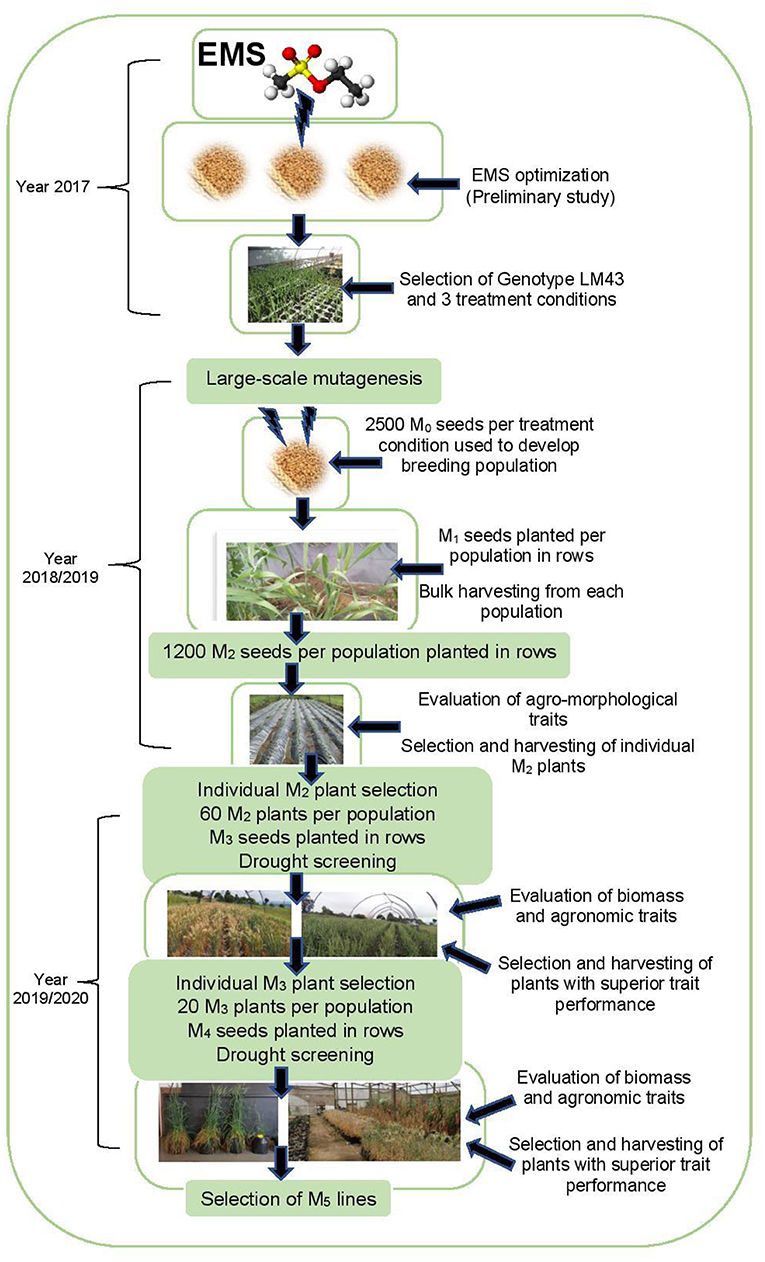
The selection procedure across generations is illustrated in Figure 1. Preliminary phenotypic variation analyses showed that EMS mutagenesis was effective on genotype LM43 (OlaOlorun et al., 2019). Hence this genotype was selected for large-scale mutagenesis under three EMS treatment conditions. Breeding populations were developed under four generations based on the three EMS treatment conditions (OlaOlorun et al., 2021). Fresh EMS treated M1 seeds were planted in the field between March and August 2018. The first breeding population (Population 1) was developed after the treatment of seeds at 0.1% v/v EMS for 1 h at 25°C. The second breeding population (Population 2) was derived after seeds were treated under 0.1% v/v EMS for 1 h at 30°C while the third breeding population (Population 3) involved seeds exposed to 0.7% v/v EMS for 1.5 h at 25°C. In addition, an untreated seed of the genotype LM43 was included as Population 4 and as a comparative control. M1 plants were grown to maturity and the grains were harvested and bulked according to their respective treatments and developed into populations. The M2 seed harvested from M1 plants were grown out as M2 plants. During the M2 generation, 180 individual plants were purposefully selected based on high biomass and yield potential and further evaluated at M3 and M4 generations. Selections made in the M3 and M4 generations were for improved agronomic performance, drought tolerance and biomass allocation under drought-stressed and non-stressed conditions.
Planting Sites and Establishment
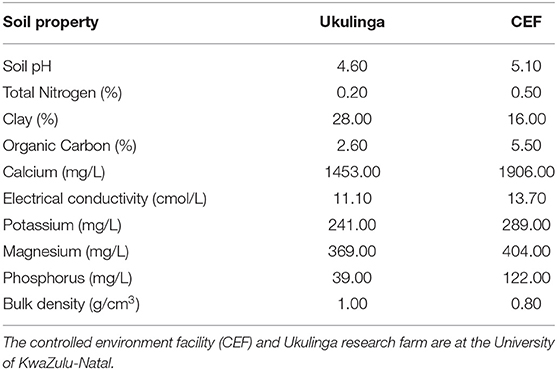
The M1 and M2 generations were evaluated at Ukulinga Research Farm of the University of KwaZulu-Natal (UKZN) (290 40'S, 300 24'E; 806 m above sea level) during the 2018/2019 cropping season. The M3 and M4 generations were established both at Ukulinga Research Farm and under greenhouse condition at the Controlled Environment Facility (CEF) at UKZN during the 2019/2020 cropping season. The meteorological data during the growing period and soil physiochemical properties at both sites are provided in Tables 1, 2, respectively. The M1 and M2 generations were planted under normal growing conditions with irrigation up to maturity, while the M3 and M4 were screened under drought-stressed and non-stressed conditions. Under field conditions, seeds were planted on 12 m long rows. The spacing between rows was 60 cm and plants were spaced 10 cm apart within a row. The spacing was consistent with normal field planting but was selected to optimize planting density within the available space and customized planting conditions. The experiments used custom-made plastic mulch as rain-out selection strategy. In the greenhouse, seeds were planted in 10-liter capacity plastic pots filled with pine bark. All experiments were laid out in a randomized complete block design with two replications. Drought stress was imposed by withholding irrigation water to 35% field capacity at anthesis, while the non-stressed treatment was well watered up to physiological maturity.
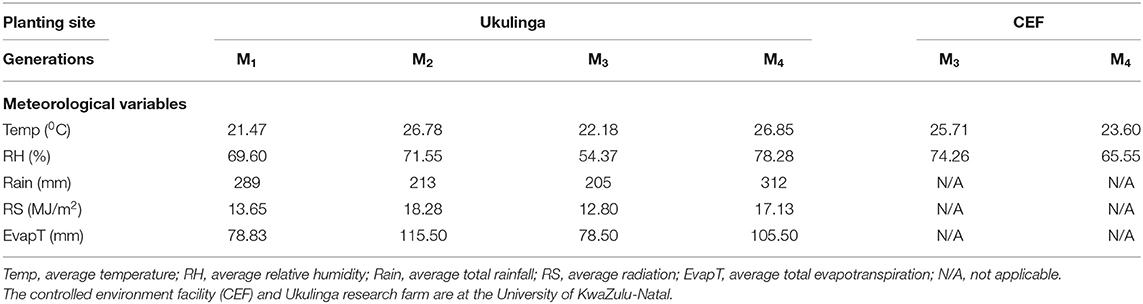
Table 1. Meteorological data recorded at the study sites during evaluation of the M1 to M4 generations of wheat.
Data Collection and Analysis
Quantitative data from 10 selected and tagged plants was collected during each generation to summarize the genetic variation and aid selection. The following data were collected during the M1 through M4: days to 90% maturity (DTM), plant height (PH), shoot biomass (SB), spike length (SL), 1000-seed weight (TSW) and grain yield (GY). In addition, percentage germination (%G) and number of spikelets per spike (SPS) were collected at M1 and M2 generations, while root biomass (RB) and root-shoot ratio (RSR) were measured at M3 and M4 generations. Data collection procedures were adapted from Mathew et al. (2019). The data were subjected to analysis of variance (ANOVA) and vital descriptive statistics were computed using GenStat 18th edition (Payne et al., 2017). The relationships among traits were quantified under each stress treatment using the Pearson correlations coefficient with the SPSS version 24 (IBM SPSS I, 2016). Trait correlation strengths were categorized into weak, moderate and strong following Zou et al. (2003).
Results
Analysis of Variance
The analysis of variance for M1 and M2 generations showed that the population × generation interaction effects were significant (p < 0.01) for SB, TSW, and GY (Table 3). Significant (p < 0.001) differences across the mutant generations were observed for all traits measured, while the population main effect showed significant (p < 0.05) impact on PH, RB, and GY.
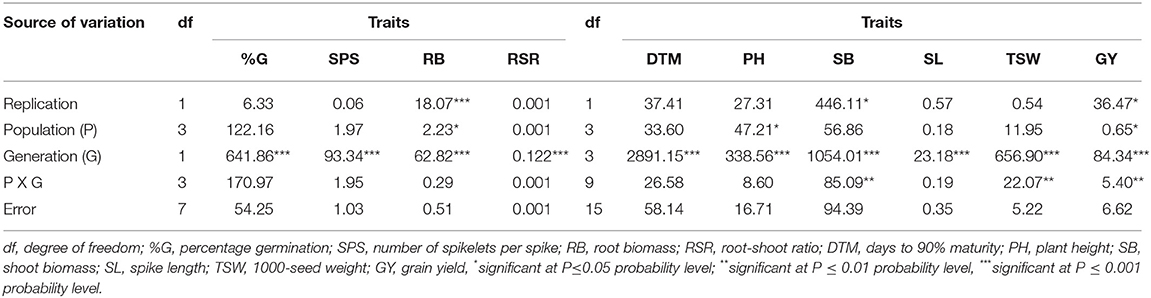
Table 3. Mean squares and significant tests for traits measured in three EMS-treated and control populations of wheat planted across two and four generations.
There were significant (p < 0.05) differences in PH and SB in response to the three-way population × generation × water regime interaction effects at M3 and M4 generations (Table 4). The effects of the interaction involving generation and population were significant (p < 0.01) for SB and TSW only. The generation × water regime, and population × water regime interaction effects resulted in significant (p < 0.05) differences in SB, SL, TSW, and GY among the M3 and M4 mutants. Significant (p < 0.05) differences were observed among the M3 and M4 mutants for most traits due to the main effect of the mutant generations and water regimes, while only SB and GY varied significantly (p < 0.05) among the populations.
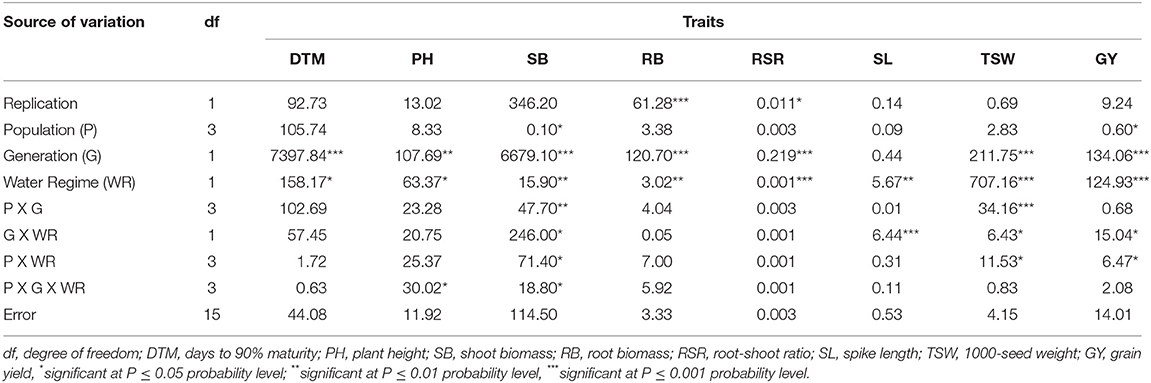
Table 4. Mean squares and significant tests for traits measured in three EMS-treated and control populations of wheat under two water regimes at M3 and M4 generations.
Quantitative Traits Measured During M1 to M4 Generations
Summaries of quantitative traits measured at each generation and from various breeding populations were presented in Figures 2, 3. M1 mutants from Population 2 recorded the shortest plant height (96.23 cm), highest shoot biomass (66.06 g/m2) and grain yield (21.75 g) compared with other breeding populations (Figure 2A). At the M2, the mutants from Population 3 recorded the highest SB (61.82 g/m2) while mutant plants developed in Population 2 maintained the highest GY (19.48 g). Mutants from Population 1 recorded the shortest PH (83.71 cm) and highest TSW (47.29 g) (Figure 2B).
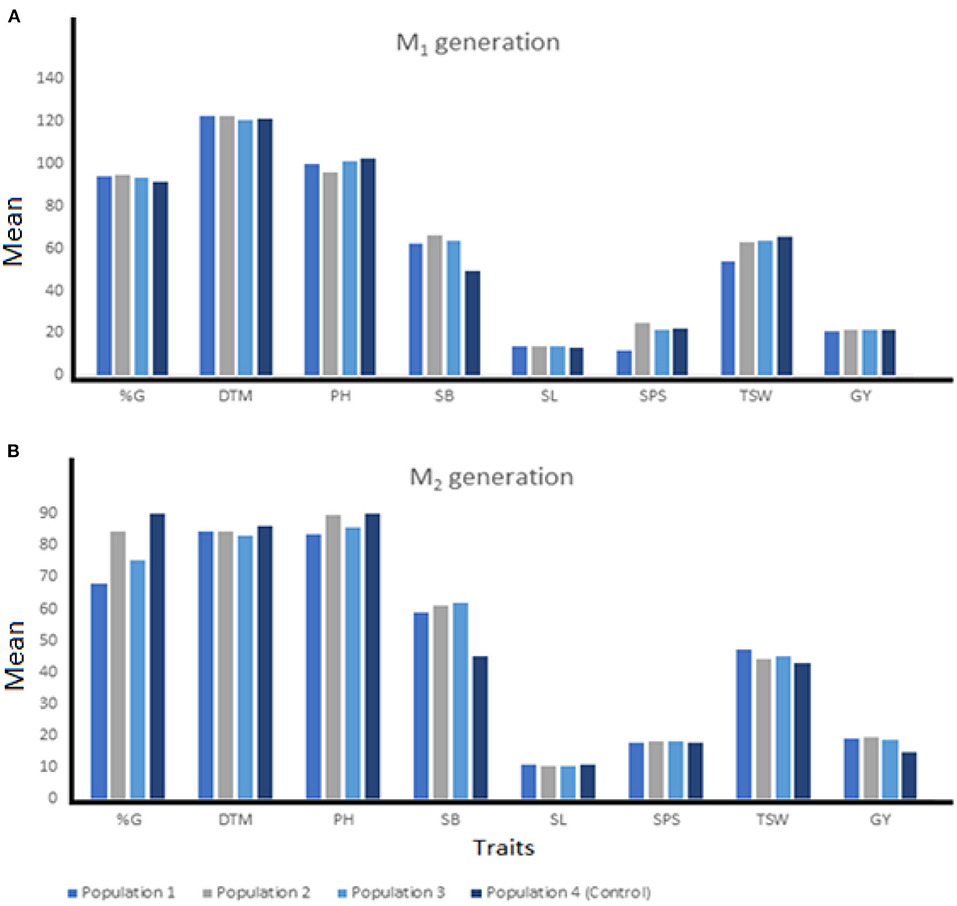
Figure 2. The means of individuals among three EMS-treated and control populations of wheat at M1 (A) and M2 (B) generation. %G, percentage germination; DTM, days to 90% maturity; PH, plant height; SB, shoot biomass; SL, spike length; SPS, number of spikelets per spike; TSW, 1000-seed weight; GY, grain yield.
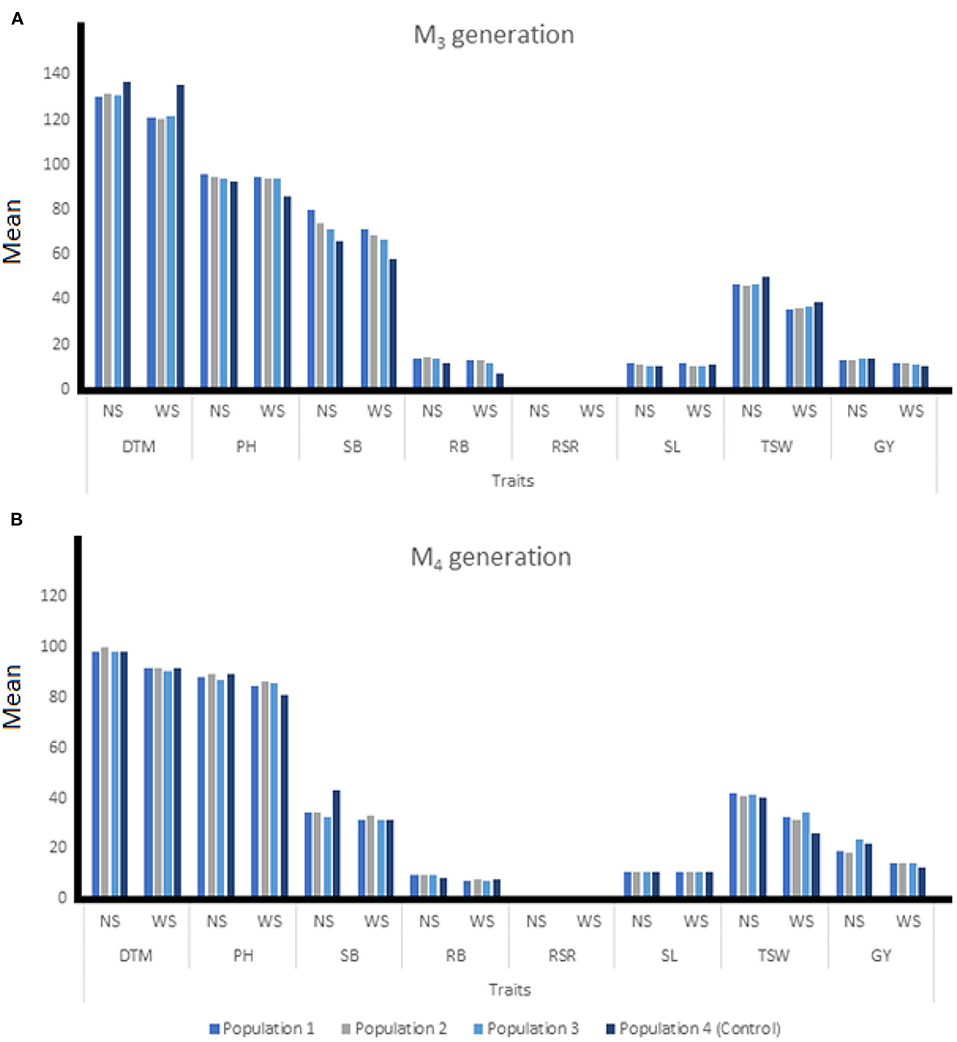
Figure 3. The means of individuals among three EMS-treated and control populations of wheat at M3 (A) and M4 (B) generation under two water regimes. NS, non-stressed condition; WS, water stressed condition; DTM, days to 90% maturity; PH, plant height; SB, shoot biomass; RB, root biomass; RSR, root-shoot ratio; SL, spike length; TSW, 1000-seed weight; GY, grain yield.
At M3, mutant plants developed in Population 2 produced the highest grain yield of 11.58 g under drought-stress condition (Figure 3A). The highest shoot biomass was produced under non-stress and water stressed conditions at 80.04 and 71.51 g/m2, respectively for mutants in Population 1. Mutants from Population 2 recorded the highest root biomass under non-stress and water stress conditions at 14.36 and 13.37 g/m2, respectively. During the M4 generation, mutant plants established in Population 1 produced the highest root biomass (9.38 g/m2) under non-stressed condition, while Population 2 recorded the highest RB (7.87 g/m2) under water stress. The highest GY (23.51 g) under non-stressed condition was recorded for mutants from population 3 while mutants from Population 1 had the highest GY of 14.53 g under water stressed conditions. Under water stress, mutants from Population 2 had the highest SB (32.93 g/m2) while mutant plants from Population 3 recorded the shortest PH of 87.33 cm (Figure 3B).
The mean performance of the three EMS-treated populations and the untreated control across four generations are presented in Figure 4. Mutants developed from Population 3 had the highest SB of 55.43 g/m2 while the highest GY (18.39 g) was recorded for mutant plants in Population 2. The SL and TSW were the highest for mutants from Population 2 (13.64 cm and 61.61 g, respectively) across the four generations. Figure 5 summarizes the differences among the M4 wheat Populations under water stressed and non-stressed conditions in two planting sites.
Figure 4. Mean performance of (A) shoot biomass, (B) spike length, (C) thousand seed weight and (D) gain yield for three EMS-treated and control populations of wheat during four selection generations. Different letters on error bars represent significant differences at the 0.05 probability level.

Figure 5. Differences between drought-stressed and non-stressed M4 wheat populations at (A) the controlled environment facility and (B) Ukulinga research farm of University of KwaZulu-Natal.
Variation Observed at M3 Generation
Many individual plants in the M3 generation were available for selection based on their breeding population and observed variation in spike and awn morphology (Figure 6). Individual plants with variable tiller number (Figure 7), plant height and shoot biomass production (Figure 8) and, biomass partitioning into roots and shoots (Figure 9) were also observed. Qualitative traits had limited variation in M3 generation when compared with the M2. However, segregation at M3 generation produced a wider range of variation (Figures 8, 9) making selection more efficient. Various spike mutants with high number of seeds from each breeding population were selected. Subsequently, abnormal, and deformed spikes with low number of seeds were discarded. Mutants with high root and shoot biomass and number of tillers were identified and advanced to M4 generation.

Figure 6. (A–T) show variations in spike and awn morphology in wheat mutant populations during the M3 generation under the controlled environment facility. (A–E) (Population 1), (F–M) (Population 2), (N–T) (Population 3) and (U) (Control).

Figure 7. Differences in tiller formation in wheat mutants during the M3 generation (A–F) at the controlled environment facility. (A,B) (Population 1), (C,D) (Population 2), and (E,F) (Population 3).

Figure 8. Variation in plant height and shoot biomass production among M3 wheat populations. (B,G) (Population 1), (C,D) (Population 2), (E,F,H) (Population 3), and (A) (Control).

Figure 9. Variation in biomass partitioning between roots and shoots among M3 wheat populations. (A,B) (Population 1), (C,D) (Population 2), (E–G) (Population 3), and (H) (Control).
Quantitative Traits Association
Grain yield showed positive and significant associations with SL (r = 0.71; p < 0.001), TSW (r = 0.41; p < 0.05), and PH (r = 0.49; p < 0.01). Plant height was positively associated with all traits measured in all the four mutant generations (Table 5). Shoot biomass exhibited positive and moderate correlations with DTM (r = 0.46; p < 0.01), PH (r = 0.43; p < 0.05), and TSW (r = 0.40; p < 0.05). Strong and positive correlations existed between TSW and PH (r = 0.79; p < 0.001), and between TSW and SL (r = 0.77; p < 0.001). Likewise, DTM had moderate correlation with TSW (r = 0.46; p < 0.01).
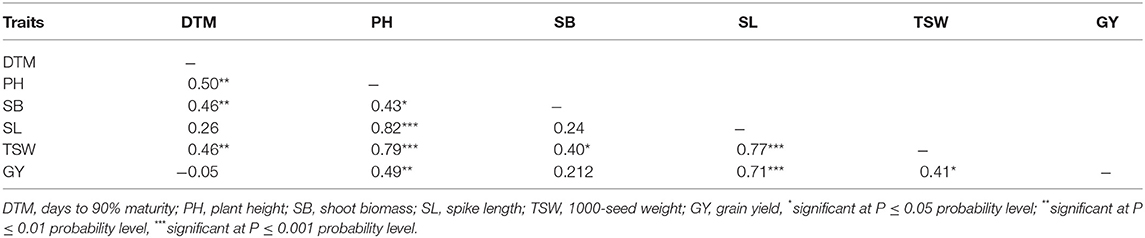
Table 5. Pairwise correlation coefficients among agronomic traits measured in three EMS-treated populations of wheat and control during four generations.
In Table 6, the upper diagonal shows correlations recorded under non-stressed conditions. There were strong correlations between GY and RSR (r = −0.72, p < 0.001), SL (r = 0.77, p < 0.001), and TSW (r = 0.65, p < 0.01). The secondary traits also exhibited interdependent associations. The RSR exhibited a negative and strong association with SL (r = −0.72, p < 0.001) while SB and RB (r = 0.83), SB and RSR (r = 0.62), and RB and RSR (r = 0.79) were significantly (p < 0.05) correlated. The correlations among traits measured under water stressed conditions were different. Root biomass exhibited stronger correlation with GY (r = 0.55, p < 0.05) than the correlation between SB and GY (r = 0.30, p < 0.05) under water stressed conditions (Table 6, lower diagonal). SB was correlated to all the other traits, while RB was only correlated to RSR, SB, and GY. Grain yield exhibited significant association (p <0.05) with all traits except PH. The RSR exhibited moderately to strong correlations with GY (r = 0.67, p < 0.05), SB (r = 0.74, p < 0.001), and RB (r = 0.94, p < 0.001).
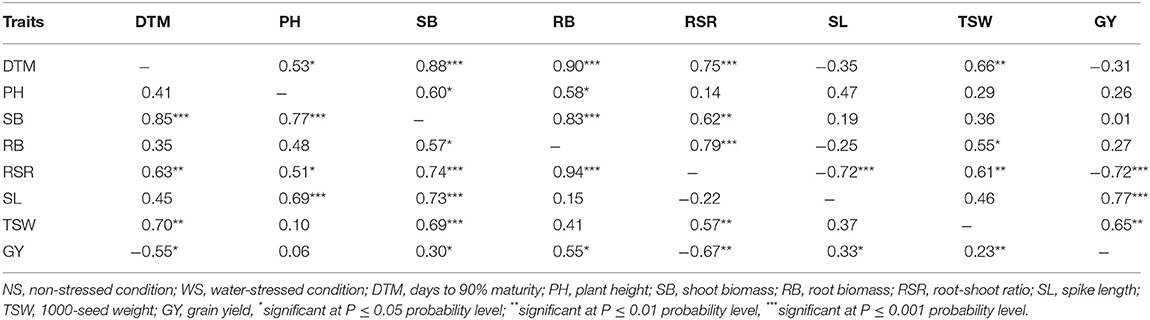
Table 6. Pair-wise correlation coefficients among agronomic traits measured in three EMS-treated and control populations of wheat evaluated under water-stressed (lower diagonal) and non-stressed (upper diagonal) conditions during the M3 and M4 generations.
Discussion
Genotypic Variation for Phenotypic Traits
The significant (p < 0.05) effects of generations, breeding populations and their interaction for most agronomic traits probably resulted from genetic segregation or cumulative mutagenic effects in subsequent generations. Each generation was self-pollinated to generate the subsequent generation and the variation in subsequent generations could be due to segregation at heterozygous loci caused by mutations in M1 generation. Similarly, Shorinola et al. (2019) found both superior and inferior mutants in later generations of wheat and supposed that the variation emanated from segregating heterozygous mutant phenotypes from the initial population. In other studies, the phenotypic variation between early and subsequent populations was attributed to the cumulative effects of the EMS. Hussain et al. (2018) asserted that the variation in subsequent generations is induced by non-lethal cumulative mutagenic effects. Singh et al. (2006) reported significant variation between M1 and M2 generations with reduced variation in M3 generation, which was attributed to homozygosity even at mutated loci in advanced generations. Expectedly, phenotypic expression in mutant generations was significantly (p < 0.05) affected by drought-stress. Traits such as SB, SL, TSW, and GY were significantly reduced under drought stress, which corroborated previous studies (Marchin et al., 2020). Soil moisture is vital for biological process and nutrient transport, and inadequate water supply interferes with essential processes leading to poor growth and development (Daryanto et al., 2016). Grain yield production under drought stress was likely supported by families that were able to maintain high shoot biomass production. It is reported that shoot-related traits influence grain production under water-limited environments by translocation of assimilates previously synthesized in the shoot before the onset of the detrimental drought stress (Abdolshahi et al., 2015).
Mean Performance of EMS Treated Populations
The lack of definite trends in the pattern of variation among the EMS-treated wheat populations point to the random nature of mutations induced by EMS and the wide variation created in subsequent segregating generations. The superior agronomic performance of EMS mutagenized populations compared to the untreated control for biomass, yield and yield-related traits measured under water stress during M3 and M4 generation indicates that EMS is efficient in creating potentially useful variation. It can be assumed that genetic modification through mutations induced by EMS improved drought tolerance. EMS is a potent mutagen and widely used in plant breeding programs (Talebi et al., 2012; Luz et al., 2016). Mutagenesis has potential to create genetic variation for exploitation in breeding for improved biomass and yield-related traits under water-limited environments (Addai and Salifu, 2016; Luz et al., 2016). Population 2 had the highest average biomass and yield performance across selection generations under the two water regimes. Selected individual mutants from Population 2 can be recommended for further screening for enhanced biomass and yield stability in water-limited environments.
Morphological Traits of M3 Mutants
Morphological variations reported in this study revealed the usefulness of chemical mutagenesis in wheat breeding. Detectable mutations result in traits that are morphologically distinct showing that such traits would be underpinned by heritable genetic changes (Gnanamurthy et al., 2012). The various types of spikes observed at the M3 generation suggested that genetic changes in the spikes were attributable to EMS mutagenesis. Mutations can occur as chromosomal breakage, disturbed auxin synthesis, disruption of mineral metabolism and accumulation of free amino acids leading to variation in spike morphology (Goyal and Khan, 2010). Reportedly, spike morphology affects the extent of seed set and threshability of wheat grains. Minor and major mutations were reported to affect spike morphology in hexaploid wheat (Nalam et al., 2007). Mutants with favorable spike morphology (i.e., longer spikes, adequate seed set, and heavier seed weight) are useful genetic resources to improve grain yield potential and enhance grain threshability (Sharma et al., 2019). Variations in spike mutants generated from an EMS mutagenized wheat population study were reported by Dhaliwal et al. (2015). The positive effect of EMS mutagen was also confirmed by the wide range of variation in biomass traits. Variation in biomass is important to develop a larger breeding parental population for subsequent drought improvement programs, since evaluating and optimizing biomass partitioning will indirectly improve yield especially for water-limited environments. Variation in agronomic performance is a product of genotype, environment, and genotype × environmental interaction effects. The genetic constitution of individuals is modified due to EMS mutagenesis leading to heritable genetic changes. Mutational events amongst induced plants vary due to inherent genotype differences, EMS dose and permeability, and reshuffling of genes on mutated genomic regions (Hussain et al., 2018). Therefore, variation present in agronomic performance is underpinned by genetic changes introduced through EMS mutagenesis. This will condition substantial changes in the genetic homeostasis and physiological functions including in the synthesis of hormones, growth regulators, protein coding and cell division. As a result, heritable and desirable genetic changes in agronomic performance and physiological responses are introduced in functional mutants for selection. Detection of mutations caused by major genes is feasible using diagnostic molecular markers in the early mutant generations. However, changes due to minor genetic effects are linked to involvement of a larger numbers of genes that condition phenotypic segregations in the early selection generation. Hence, marker-assisted selection should be made in the advanced selection generation when homozygous and homogenous stable mutants are identified. This will identify genetically stable and homozygous individuals due to the inherent self-fertilization system in wheat. Previous studies used molecular markers to select mutant individuals in the M3 or M4 generations (Kenzhebayeva et al., 2014; Hussain et al., 2018).
Trait Associations
The significant (p < 0.05) correlations observed among the measured traits suggest that the traits were interdependent and provide opportunities for simultaneous selection. The positive and significant association exhibited by GY and SB with the other yield related traits indicate the strong linkage between above ground traits. These traits can easily be selected simultaneously during yield improvement. Taller plants may be able to accumulate adequate photosynthates for attaining higher above ground biomass, which can directly increase grain yield (Zhang et al., 2009). Previously, the influence of above ground traits such as biomass production, spike morphology and kernel weight on grain yield was established (Reynolds et al., 2007; Kandić et al., 2009; Rahman et al., 2016). However, genotypes that accumulate excessive above ground biomass at the expense of developing extensive root systems may be susceptible to drought stress, especially in sub-Sahara Africa where wheat is grown under residual moisture and the rainfall is increasingly becoming erratic and inadequate (Haque et al., 2016). The stronger associations between the biomass traits and grain yield under water stressed conditions shows that biomass partitioning under drought is more critical for plant survival and attaining reasonable yield. For instance, a slight decrease in rooting capacity is likely to have higher influence on grain yield under drought stressed conditions compared to non-stressed conditions. Genotypes with potential to accumulate higher above ground biomass before the onset of drought stress have comparative advantage under terminal drought as they can translocate assimilates from shoot biomass to grains during grain filling (Kandić et al., 2009). The positive and significant correlations of RB and SB are favorable to develop cultivars with high extensive root biomass for water and nutrient extraction and shoot biomass for building adequate above ground biomass to support grain filling. Palta et al. (2011) asserted that a direct and positive relationship between root and shoot biomass is necessary for grain yield improvement. The negative association between RSR and GY regardless of moisture availability conditions indicates that there should be a balance between biomass allocation to above and below ground parts to avoid compromising grain production. Excessively large root systems have high maintenance costs that will limit amount of assimilates available for biomass accumulation in shoots or grain. On the other hand, shallow rooted plants with disproportionately large shoots have higher risk for lodging at anthesis, which increases chances of susceptibility to diseases and pests and reduces grain quantity and quality (Berry, 2013; Dahiya et al., 2018).
Conclusion
This study established the importance of EMS mutagenesis in creating genetic variation within and among wheat breeding populations. Wide phenotypic variation in mutants under each breeding population were identified for improving drought tolerance, biomass, yield, and yield-related traits. The differences in agronomic performance among the generations exhibited that segregation and cumulative mutagenic effects contributed to the genetic variation. There is a need to ensure that the favorable mutations are fixed in homozygous and homogenous states before cultivar release. Mutants with favorable agronomic performance can be selected as parental populations for crop improvement. Identified mutants need further screening for biomass and yield stability in diverse environments especially in drought stressed areas. Furthermore, the selected mutants are candidate training populations to identifying genomic regions controlling biomass allocation and yield components. This will contribute to marker-assisted selection for biomass allocation, and yield and yield related traits in wheat.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author Contributions
All authors listed have made a substantial, direct and intellectual contribution to the work, and approved it for publication.
Funding
This work was supported by African Centre for Crop Improvement (ACCI), University of KwaZulu-Natal, Pietermaritzburg Campus.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
The African Centre for Crop Improvement (ACCI) of the University of KwaZulu-Natal is acknowledged for providing financial and technical support.
References
Abdolshahi, A. R., Nazari, M., Safarian, A., Sadathossini, T. S., Salarpour, M., and Amiri, H. (2015). Integrated selection criteria for drought tolerance in wheat (Triticum aestivum L.) breeding programs using discriminant analysis. Field Crops Res. 174, 20–29.
Abdolshahi, R., Safarian, A., Nazari, M., Pourseyedi, S., and Mohamadi-Nejad, G. (2013). Screening drought-tolerant genotypes in bread wheat (Triticum aestivum L.) using different multivariate methods. Arch. Agron. Soil Sci. 59, 685–704. doi: 10.1080/03650340.2012.667080
Addai, I. K., and Salifu, B. (2016). Selection of mutants with improved growth and total grain yield in the M2 generation of pearl millet (Pennicetum glaceum L.) in the Northern region of Ghana. J. Agron. 15, 88–93. doi: 10.3923/ja.2016.88.93
Anbarasan, K., Sivalingam, H. D., Rajendran, R, Anbazhagan, M., and Chidambaram, A. A. (2013). Studies on the mutagenic effect of EMS on seed germination and seedling characters of Sesame (Sesamum indicum L.) Var.T MV3. Int. J. Biol. Sci. 3, 68–70.
Berry, P. M. (2013). “Lodging resistance in cereals,” in Sustainable Food Production, eds P. Christou, R. Savin, B. A. Costa-Pierce, I. Misztal, and C. B. A. Whitelaw (New York, NY: Springer), 1096–1110.
Dahiya, S., Kumar, S., Harender, and Chaudhary, C. (2018). Lodging: significance and preventive measures for increasing crop production. Int. J. Chem. 6, 700–705.
Daryanto, S., Wang, L., and Jacinthe, P. A. (2016). Global synthesis of drought effects on maize and wheat production. PLoS ONE 11:e0156362. doi: 10.1371/journal.pone.0156362
Den Herder, G., Van Isterdael, G., Beeckman, T., and De Smet, I. (2010). The roots of a new green revolution. Trends Plant Sci. 15, 600–607. doi: 10.1016/j.tplants.2010.08.009
Dhaliwal, A. K., Mohan, A., Sidhu, G., Maqbool, R., and Gill, K. S. (2015). An Ethylmethane sulfonate mutant resource in pre-green revolution hexaploid wheat. PLoS ONE 10:e0145227. doi: 10.1371/journal.pone.0145227
Fang, Y., Du, Y., Wang, J., Wu, A., Qiao, S., Xu, B., et al. (2017). Moderate drought stress affected root growth and grain yield in old, modern and newly released cultivars of winter wheat. Front. Plant Sci. 8:672. doi: 10.3389/fpls.2017.00672
Food and Agricultural Organization (FAO) (2018). World Food and Agriculture-Statistical Pocketbook. Rome:FAO. 1–254.
Gnanamurthy, S., Mariyammal, S., Dhanavel, D., and Bharathi, T. (2012). Effect of gamma rays on yield and yield components characters R3 generation in cowpea (Vigna unguiculata (L.). Walp.). Int. J. Res. Plant Sci. 2, 39–42.
Goyal, S., and Khan, S. (2010). Cytology of induced morphological mutants in Vigna mungo (L.) Hepper. Egypt. J. Biol. 12, 81–85.
Griffiths, C. A., and Paul, M. J. (2017). Targeting carbon for crop yield and drought resilience. J. Sci. Food Agric. 97, 4663–4671. doi: 10.1002/jsfa.8501
Haque, E., Osmani, A. A., Ahmadi, S. H., and Ban, T. (2016). Development of pre-breeding technology for root system study and selection of Kihara Afghan wheat landraces (KAWLR) to enhance wheat breeding in the rain-fed region. Breed. Sci. 66, 808–822. doi: 10.1270/jsbbs.16043
Hooshmandi, B. (2019). Evaluation of tolerance to drought stress in wheat genotypes. A. Artículos de Investigación. 37, 37–43. doi: 10.4067/S0718-34292019000200037
Horn, L. N., Ghebrehiwot, H. M., and Shimelis, H. A. (2016). Selection of novel cowpea genotypes derived through gamma irradiation. Front. Plant Sci. 7:262. doi: 10.3389/fpls.2016.00262
Hussain, M., Iqbal, M. A., Till, B. J., and Rahman, M. U. (2018). Identification of induced mutations in hexaploid wheat genome using exome capture assay. PLoS ONE. 13:e0201918. doi: 10.1371/journal.pone.0201918
IBM SPSS I (2016). IBM Corporation Released. IBM SPSS (Statistical Package for the Social Sciences) Statistics for Windows, Version 24.0. Armonk, NY: IBM Corp.
International Atomic Energy Agency (IAEA). (2020). IAEA Mutant Database. Available online at: http://mvd.iaea.org/ (accessed June 15, 2020).
Kandić, V., Dodig, D., Jović, M., Nikolić, B., and Prodanović, S. (2009). The importance of physiological traits in wheat breeding under irrigation and drought stress. Genetika 41, 11–20. doi: 10.2298/GENSR0901011K
Keneni, G., Bekele, E., Imtiaz, M., and Dagne, K. (2012). Genetic vulnerability of modern crop cultivars: causes, mechanism and remedies. Int. J. Plant Res. 2, 69–79. doi: 10.5923/j.plant.20120203.05
Kenzhebayeva, S., Turasheva, S., Doktyrbay, G., Buerstmayr, H., Atabayeva, S., and Alybaeva, R. (2014). Screening of mutant wheat lines to resistance for Fusarium head blight and using SSR markers for detecting DNA polymorphism. IERI Proc. 8, 66–76. doi: 10.1016/j.ieri.2014.09.012
Kontz, B., Franklin, S., and Brunel, C. (2009). Selection of winter wheat mutant lines resistant to drought stress. J. Undergrad. Res. 7, 85–90.
Lethin, J., Shakil, S. S. M., Hassan, S., Sirijovski, N., Töpel, M., Olsson, O., et al. (2020). Development and characterization of an EMS-mutagenized wheat population and identification of salt-tolerant wheat lines. BMC Plant Biol. 20:18. doi: 10.1186/s12870-019-2137-8
Lobell, D. B., Burke, M. B., Tebaldi, C., Mastrandrea, M. D., Falcon, W. P., and Naylor, R. L. (2008). Prioritizing climate change adaptation needs for food security in 2030. Science 319, 607–610. doi: 10.1126/science.1152339
Luz, K. V., Silveira, S. F., Magalhães da Fonseca, G., Groli, E. L., Figueiredo, R. G., Baretta, D., et al. (2016). Identification of variability for agronomically important traits in rice mutant families. Plant Breed. 75, 41–50. doi: 10.1590/1678-4499.283
Mahajan, S., and Tuteja, N. (2005). Cold, salinity and drought stresses: an overview. Arch. Biochem. Biophys. 444, 13–58. doi: 10.1016/j.abb.2005.10.018
Maluszynski, M., and Kasha, K. J., (eds.). (2002). Mutations, in vitro and molecular techniques for environmentally sustainable crop Improvement. Dordrecht; Boston, MA: Kluwer Academic Publishers. doi: 10.1007/978-94-015-9996-2
Marchin, R. M., Ossola, A., Leishman, M. R., and Ellsworth, D. S. (2020). A simple method for simulating drought effects on plants. Front. Plant Sci. 10:1715. doi: 10.3389/fpls.2019.01715
Mathew, I., Shimelis, H., Mutema, M., Clulow, A., Zengeni, R., Mbava, N., et al. (2019). Selection of wheat genotypes for biomass allocation to improve drought tolerance and carbon sequestration into soils. J. Agron. Crop Sci. 205, 385–400. doi: 10.1111/jac.12332
Mehraban, S. A., Tobe, A., Gholipouri, A., Amiri, E., Ghafari, A., and Rostaii, M. (2018). Evaluation of drought tolerance indices and yield stability of wheat cultivars to drought stress in different growth. World J. Environ. Biosci. 7, 8–14.
Mwadzingeni, L., Shimelis, H., Tesfay, S., and Tsilo, T. J. (2016). Screening of bread wheat genotypes for drought tolerance using phenotypic and proline analyses. Front. Plant Sci. 7, 1–12. doi: 10.3389/fpls.2016.01276
Nalam, V. J., Vales, M. I., Watson, C. J., Johnson, E. B., and Riera-Lizarazu, O. (2007). Map-based analysis of genetic loci on chromosome 2D that affect glume tenacity and threshability, components of the free-threshing habit in common wheat (Triticum aestivum L.). Theor. Appl. Genet. 116, 135–145. doi: 10.1007/s00122-007-0653-7
Nazarenko, M., Lykholat, Y., Grygoryuk, I., and Khromikh, N. (2018). Optimal doses and concentrations of mutagens for winter wheat breeding purposes. Part I. Grain productivity. J. Cent. Eur. Agric. 19, 194–205. doi: 10.5513/JCEA01/19.1.2037
OlaOlorun, B. M., Shimelis, H., Laing, M., and Matthew, I. (2021). Morphological variations of wheat (Triticum aestivum L. em. Thell.) under variable ethyl methanesulphonate mutagenesis. Cereal Res. Commun. 49, 301–310. doi: 10.1007/s42976-020-00092-3
OlaOlorun, B. M., Shimelis, H., and Matthew, I. (2020). Variability and selection among mutant families of wheat for biomass allocation, yield and yield-related traits under drought-stressed and non-stressed conditions. J Agron Crop Sci. 00, 1–18. doi: 10.1111/jac.12459
OlaOlorun, B. M., Shimelis, H., Matthew, I., and Laing, M. (2019). Optimizing the dosage of ethyl methanesulphonate mutagenesis in selected wheat genotypes. S. Afr J Plant Soil. 36, 1–10. doi: 10.1080/02571862.2019.1610808
Ortiz, R., Sayre, K. D., Govaerts, B., Gupta, R., Subbarao, G. V., Ban, T., et al. (2008). Climate change: can wheat beat the heat? Agric. Ecosyst. Environ. 126, 46–58 doi: 10.1016/j.agee.2008.01.019
Palta, J. A., Chen, X., Milroy, S. P., Rebetzke, G. J., Dreccer, M. F., and Watt, M. (2011). Large root systems: are they useful in adapting wheat to dry environments? Funct. Plant Biol. 38, 347–354. doi: 10.1071/FP11031
Park, S., Im, J., Jang, E., and Rhee, J. (2016). Drought assessment and monitoring through blending of multi-sensor indices using machine learning approaches for different climate regions. Agr. Forest Meteorol. 216, 157–169. doi: 10.1016/j.agrformet.2015.10.011
Payne, R. W., Murray, D. A., and Harding, S. A. (2017). An Introduction to the Genstat Command Language, 18th Edn. Hemel, Hempstead, UK: VSN International Ltd. 1–137.
Rahman, M., Barma, N. C. D., Biswas, B. K., Khan, A. A., and Rahman, J. (2016). Study on morpho-physiological traits in spring wheat (Triticum aestivum L.) under rainfed condition. Bangladesh J. Agr. Res. 41, 235–250. doi: 10.3329/bjar.v41i2.28227
Reynolds, M., Calderini, D., Condon, A., and Vargas, M. (2007). Association of source/sink traits with yield, biomass and radiation use efficiency among random sister lines from three wheat crosses in a high-yield environment. J. Agric. Sci. 145, 3–16. doi: 10.1017/S0021859607006831
Sharma, J. S., Running, K. L., Xu, S. S., Zhang, Q., Haugrud, A. R. P., Sharma, S., et al. (2019). Genetic analysis of threshability and other spike traits in the evolution of cultivated emmer to fully domesticated durum wheat. Mol. Genet. Genomics 294, 757–771. doi: 10.1007/s00438-019-01544-0
Shivakumar, M., Nataraj, V., Kumawat, G., Rajesh, V., Chandra, S., Gupta, S., and Bhatia, V. S. (2018). Speed breeding for Indian Agriculture: a rapid method for development of new crop varieties. Curr. Sci. 115:1241. doi: 10.18520/cs%2Fv115%2Fi7%2F1241-1241
Shorinola, O., Kaye, R., Golan, G., Peleg, Z., Kepinski, S., and Uauy, C. (2019). Genetic Screening for mutants with altered seminal root numbers in hexaploid wheat using a high-throughput root phenotyping platform. G3 (Bethesda) 9, 2799–2809. doi: 10.1534/g3.119.400537
Singh, N. K., and Balyan, H. S. (2009). Induced Mutations in Bread Wheat (Triticum aestivum L.) cv. ‘Kharchia 65' for Reduced Plant Height and Improve Grain Quality Traits. Adv. Biol. Res. 3, 215–221.
Singh, S. P., Singh, R. P., Prasad, J. P., Agrawal, R. K., and Shahi, J. P. (2006). Induced genetic variability for protein content, yield and yield components in microsperma lentil (Lens culinaris Medik). Madras Agric. J. 93, 155–159.
Smale, M., Reynolds, M. P., Warburton, M., Skovmand, B., Trethowan, R., Singh, R. P., et al. (2002). Dimensions of diversity in modern spring bread wheat in developing countries from 1965. Crop Sci. 42, 1766–1779. doi: 10.2135/cropsci2002.1766
Sun, P., Zhang, Q., Cheng, C., Singh, V. P., and Shi, P. J. (2017). ENSO-induced drought hazards and wet spells and related agricultural losses across Anhui Province, China. Nat. Hazards. 89, 963–983. doi: 10.1007/s11069-017-3002-4
Talebi, A. B., Talebi, A. B., and Shahrokhifar, B. (2012). Ethyl methane sulphonate (EMS) induced mutagenesis in malaysian rice (cv.MR219) for lethal dose determination. Am. J. Plant Sci. 3, 1661–1665. doi: 10.4236/ajps.2012.312202
Tilman, D., Balzer, C., Hill, J., and Befort, B. L. (2011). Global food demand and the sustainable intensification of agriculture. Proc. Natl. Acad. Sci. U. S. A. 108, 20260–20264. doi: 10.1073/pnas.1116437108
Voss-Fels, K., Frisch, M., Qian, L., Kontowski, S., Friedt, W., Gottwald, S., et al. (2015). Sub-genomic diversity patterns caused by directional selection in bread wheat gene pools. Plant Genome 8, 1–13. doi: 10.3835/plantgenome2015.03.0013
White, C. A., Sylvester-Bradley, R., and Berry, P. M. (2015). Root length densities of UK wheat and oilseed rape crops with implications for water capture and yield. J. Exp. Bot. 66, 2293–2303.
Yu, H., Zhang, Q., Sun, P., and Song, C. (2018). Impact of droughts on winter wheat yield in different growth stages during 2001–2016 in Eastern China. Int. Disaster Risk Sci. 9, 376–391. doi: 10.1007/s13753-018-0187-4
Zhang, G. H., Xu, Q., Zhu, X. D., Qian, Q., and Xue, H. W. (2009). Shallot-Like1 is a Kanadi transcription factor that modulates rice leaf rolling by regulating leaf abaxial cell development. Plant Cell 21:719–735. doi: 10.1105/tpc.108.061457
Keywords: agronomic performance, genetic variation, mutant generations, phenotypic variation, wheat, yield-related traits
Citation: OlaOlorun BM, Shimelis H, Laing M and Mathew I (2021) Development of Wheat (Triticum aestivum L.) Populations for Drought Tolerance and Improved Biomass Allocation Through Ethyl Methanesulphonate Mutagenesis. Front. Agron. 3:655820. doi: 10.3389/fagro.2021.655820
Received: 19 January 2021; Accepted: 29 July 2021;
Published: 24 August 2021.
Edited by:
Moez Amri, Mohammed VI Polytechnic University, MoroccoReviewed by:
Ahmad M. Alqudah, Martin Luther University of Halle-Wittenberg, GermanyLiang Chen, Northwest A&F University Herbarium, China
Copyright © 2021 OlaOlorun, Shimelis, Laing and Mathew. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Isack Mathew, aXNhY2ttYXRoZXcmI3gwMDA0MDtnbWFpbC5jb20=
 Boluwatife M. OlaOlorun
Boluwatife M. OlaOlorun Hussein Shimelis
Hussein Shimelis Mark Laing
Mark Laing Isack Mathew
Isack Mathew